The Integument
Structure
The skin is the largest organ in the body and has haired and hairless portions (Figs. 17-1 and 17-2). The skin consists of epidermis, dermis, subcutis, and adnexa (hair follicles and sebaceous, sweat, and other glands). The histologic structure varies greatly by anatomical site and among different species of animals. The haired skin is thickest over the dorsal aspect of the body and on the lateral aspect of the limbs and is thinnest on the ventral aspect of the body and the medial aspect of the thighs. Haired skin has a thinner epidermis, whereas nonhaired skin of the nose and pawpads has a thicker epidermis (see Figs. 17-1 and 17-2). The skin of large animals is generally thicker than the skin of small animals. The subcutis, consisting of lobules of adipose tissue and fascia, connects the more superficial layers (epidermis and dermis) with the underlying fascia and musculature.
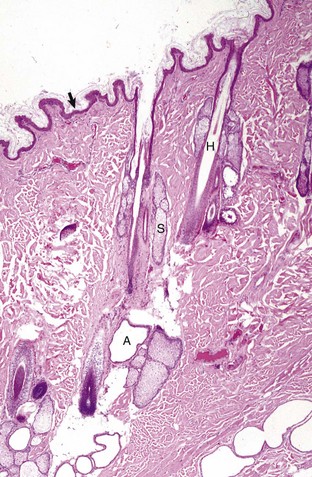
Fig. 17-1 Normal skin, haired, thorax, dog.
The epidermis (arrow) in haired skin has an undulating surface but lacks rete ridges. The epidermis in haired skin has fewer nucleated cell layers than the epidermis in nonhaired (hairless) skin such as that on the nose and pawpads (see Fig. 17-2); thus it is referred to as “thin” skin. Hair follicles (H), apocrine glands (A), and sebaceous glands (S) are present. The haired skin is thickest over the dorsal aspect of the body and on the lateral aspect of the limbs, and it is thinnest on the ventral aspect of the body and the medial aspect of the thighs. H&E stain. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
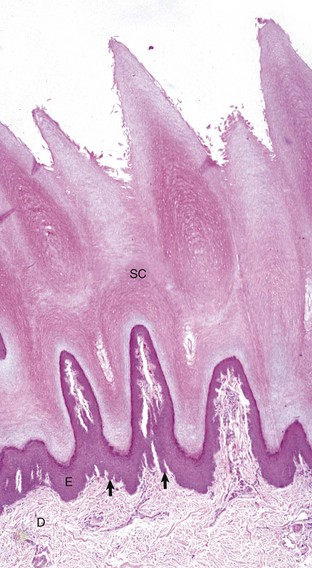
Fig. 17-2 Normal skin, hairless, pawpad, dog.
The epidermis (E) in hairless (nonhaired) skin has more numerous nucleated cell layers and more abundant stratum corneum than the epidermis in haired skin; thus it is referred to as “thick” skin. Note the dense zone of compact stratum corneum (SC) over the surface. The epidermis and dermal papillae in the superficial dermis (D) interdigitate to form rete ridges (arrows). The rete ridges strengthen the attachment between the epidermis and dermis. In the dog, the contour of the epidermal surface follows the epidermal ridges and thus is papillated. H&E stain. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
Epidermis
The epidermis is divided into layers based on the morphology of the keratinocyte, the major cell type of the epidermis. The epidermis of haired skin consists of four basic layers: stratum corneum, stratum granulosum, stratum spinosum, and stratum basale (Fig. 17-3). The epidermis of hairless skin consists of five layers; the fifth layer is the stratum lucidum, which is located between the stratum granulosum and stratum corneum. Keratinocytes originate from germinal cells in the stratum basale of the epidermis, ascend through the layers of the epidermis, changing in appearance and other characteristics in each layer until they reach the stratum corneum as fully keratinized, dead corneocytes. Keratinocytes are continuously shed from the stratum corneum. The transit time for a keratinocyte from the stratum basale to shed in the stratum corneum is approximately 1 month, although this time can be accelerated in some disorders such as primary seborrhea characterized clinically by scaling.
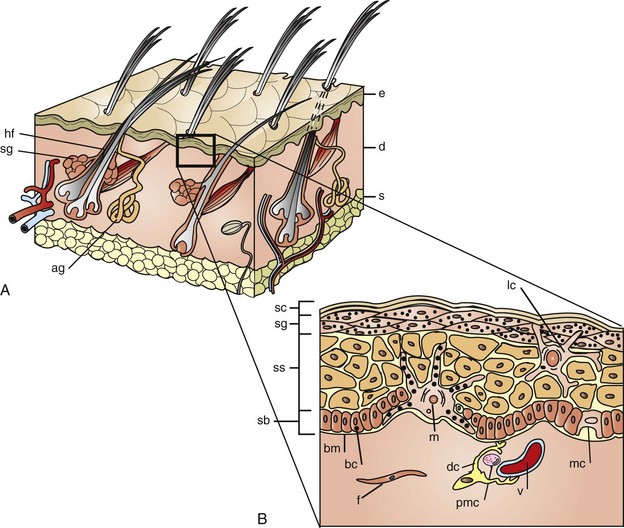
Fig. 17-3 Schematic diagram of the structure of the skin.
A, The skin is composed of epidermis (e), dermis (d), and subcutis (s), with adnexa consisting of hair follicles (hf), sebaceous glands (sg), and apocrine glands (ag). B, This projection of the epidermis demonstrates the progressive upward maturation of basal cells (bc) in the stratum basale (sb) through the stratum spinosum (ss), stratum granulosum (sg) into the cornified squamous epithelial cells of the stratum corneum (sc). Melanocytes (m), midepidermal dendritic Langerhans’ cells (lc), and Merkel cells (mc) are also present. The subjacent dermis contains small vessels (v), fibroblasts (f), perivascular mast cells (pmc), and dendrocytes (dc), potentially important in dermal immunity and repair. (Adapted from Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders; Gawkrodger DJ: Dermatology: an illustrated colour text, ed 2, New York, 1997, Churchill Livingstone.)
The outermost layer of the epidermis is the stratum corneum, which consists of many sheets of flattened, keratinized cells termed corneocytes. Keratin is an intracellular fibrous protein that is in part responsible for the toughness of the epidermis, enabling the epidermis to form a protective barrier. The next layer is the stratum granulosum, which consists of effete cells with basophilic keratohyalin granules. In nonhaired skin, the stratum corneum and stratum granulosum are separated by an additional layer of compacted, fully keratinized cells, the stratum lucidum, best seen in the pawpad. Deep to the stratum granulosum is the stratum spinosum, a layer of polyhedral-shaped cells attached to one another by desmosomes. During fixation and processing for microscopic examination, the cells of the stratum spinosum contract, except for the desmosomal attachments. These attachment sites create the appearance of “spines” or intercellular bridges, leading to the name of this layer. The visibility of the intercellular bridges is enhanced when there is intercellular edema of the epidermis. The stratum spinosum in haired areas is thinner in dogs and cats and is thicker in horses, cattle, and pigs. The innermost layer of the epidermis is the germinal layer or stratum basale, which consists of a single layer of cuboidal cells resting on a basement membrane. Intermixed within the basal cell layer are melanocytes, Langerhans’ cells, and Merkel cells.
Melanocytes, embryologically derived from neural crest cells, are also present in lower layers of the stratum spinosum and produce melanin pigment, giving skin and hair their color. Melanocytic granules are transferred to and distributed in keratinocytes as a caplike cluster of granules between the nucleus and the external surface of the skin to help protect the nucleus from UV light–induced injury. Langerhans’ cells are bone marrow–derived cells of monocyte-macrophage lineage that process and present antigen to sensitized T lymphocytes, thereby modulating immunologic responses of the skin. Langerhans’ cells are present in the basal, spinous, and granular layers of the epidermis but have a preference for a suprabasal position. Merkel cells are located in the basal layer and join with keratinocytes via desmosomal junctions. Merkel cells are located in haired and hairless skin, particularly in regions of the body with high tactile sensitivity (digits and lips), and in the outer portion of hair follicles. When Merkel cells are associated with an axon, they form a Merkel cell–neurite complex and function as a slowly adapting mechanoreceptor. The specialized areas of the skin containing these Merkel cell–neurite complexes are known as tylotrich pads (hair discs, tactile pads). The axon associated with the Merkel cell is myelinated but near the epidermis, the myelin sheath is lost, and the nerve fibers terminate at the basal aspect of the Merkel cell. Merkel cells have granules that contain chemical mediators (met-enkephalin, vasoactive intestinal peptide, chromogranin A, acetylcholine, calcitonin gene-related peptide, neuron-specific enolase, and synaptophysin). The specific role or pathophysiologic mechanism that these chemical mediators have in nerve transduction or in paracrine influence of keratinocytes or hair follicle epithelial cells remains unknown. The origin of Merkel cells also remains unknown.
Basement Membrane Zone
The epidermis and dermis are separated by a basement membrane. In hairless areas, such as the pawpads and nasal planum, this junction is irregular because of epidermal projections that interdigitate with dermal papillae (e.g., rete ridges/also known as rete pegs), thus strengthening the epidermal-dermal attachment by providing resistance to shearing. In densely haired areas, the junction is smoother and has an undulating appearance as the epidermal-dermal attachment is strengthened by the hair follicles. The more sparsely haired skin of pigs has more epidermal-dermal interdigitations (rete ridges) and fewer hair follicles. The basement membrane zone is composed of hemidesmosomes of basal cells (i.e., keratin intermediate filaments and attachment plaques), the lamina lucida (plasma membrane, subdesmosomal dense plate, and anchoring filaments), and the lamina densa (i.e., type IV collagen), which also serve to anchor the epidermis to dermis (Fig. 17-4). The importance of the basement membrane in anchoring function is noted in some immune-mediated diseases in which antibodies target, bind, and ultimately damage a component in the basement membrane and result in the formation of bullae (see the discussion on reactions characterized grossly by vesicles or bullae as the primary lesion and histologically by vesicles or bullae within the basement membrane [bullous dermatoses] section on Selected Autoimmune Reactions). The basement membrane zone also serves as a scaffold for migration of epidermal cells in wound healing and as an initial barrier to invasion of the dermis by neoplastic keratinocytes.
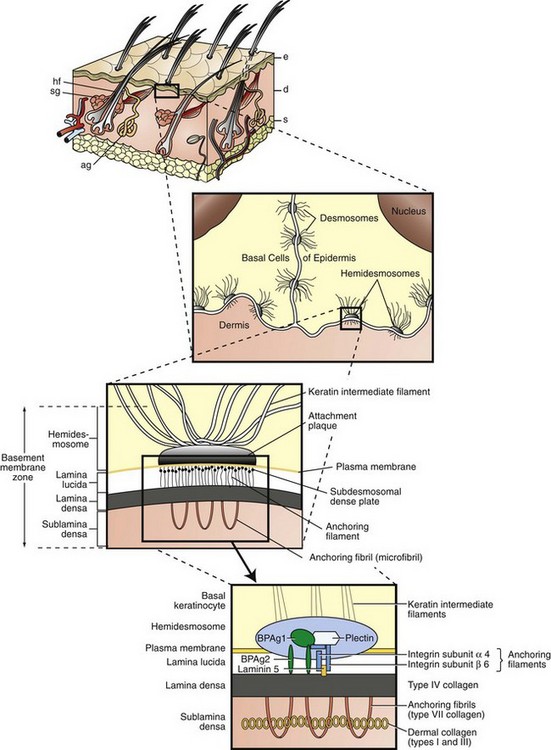
Fig. 17-4 Schematic diagram of basement membrane structure of the skin.
Keratinocytes attach to each other via desmosomes and to the basement membrane via hemidesmosomes. The projection of the basement membrane zone illustrates the multiple, interconnecting layers of this zone. The most superficial layer consists of the basal layer hemidesmosomes (keratin intermediate filaments and attachment plaques). The next layer, the lamina lucida, is an electron-lucent zone composed of the plasma membrane, subdesmosomal dense plate, and anchoring filaments. The deepest layer is the lamina densa, an electron-dense zone that consists of type IV collagen. Anchoring fibrils (type VII collagen), serve to attach the lamina densa and epidermis to the papillary dermis. The interconnecting layers of the basement membrane zone provide an important function in epidermal-dermal adherence, are the site of immune reactant deposition in cutaneous disease (see Table 17-11), and serve as a barrier to invasion by malignant epidermal tumors. ag, Apocrine glands; d, dermis; e, epidermis; hf, hair follicles; s, subcutis; sg, sebaceous glands. (Adapted from Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders; Rubin E, Farber JL: Pathology, ed 3, Philadelphia, 1999, Lippincott-Raven; and Elder DE: Lever’s histopathology of the skin, ed 10, Philadelphia, 2009, Lippincott Williams & Wilkins.)
Dermis
The dermis (corium), consisting of collagen and elastic fibers in a glycosaminoglycan ground substance, supports hair follicles, glands, vessels, and nerves. By convention, the dermis is generally subdivided into superficial and deep layers that blend together without a clear line of demarcation. The superficial dermis conforms to the contour of the epidermis and generally supports the upper portion of the hair follicle and sebaceous glands. It is composed of fine collagen fibers and is wider in the skin of cattle and horses than in the skin of dogs and cats. The deep dermis supports the lower portion of the hair follicle and apocrine glands and is composed of collagen bundles larger than those in the superficial dermis. Smooth muscle fibers of the arrector pili muscle attach the connective tissue sheath of the hair follicle to the epidermis and are responsible for causing the hair to stand erect. Skeletal muscle fibers from the cutaneous muscle extend into the lower dermis and are responsible for voluntary skin movement. Mast cells, lymphocytes, plasma cells, macrophages, and rarely eosinophils and neutrophils can be found in normal dermis. These cells are bone marrow derived–cells and arrive via the blood vascular system, thus they are typically concentrated around small superficial blood vessels.
Vessels and Nerves
Cutaneous arteries give rise to three vascular plexuses: deep, middle, and superficial. The deep plexus supplies the subcutis and deep portions of follicles and apocrine glands; the middle plexus supplies the sebaceous glands, midportion of follicles, and arrector pili muscles; and the superficial plexus supplies the superficial portions of follicles and epidermis. Lymph capillaries arise in the superficial dermis and connect with a subcutaneous plexus. The lymph vessels then converge to form larger channels that eventually reach peripheral lymph nodes.
The skin is an important sensory organ containing millions of microscopic nerve endings that perceive itch, pain, temperature, pressure, and touch (Fig. 17-5). The nerve endings consist of Meissner’s corpuscles, pacinian corpuscles (Pacini’s corpuscles), free sensory nerve endings, and mucocutaneous end organs (similar to Meissner’s corpuscles but located in mucocutaneous skin). These nerve endings are minute, and the free sensory nerve endings are so delicate that they require special staining techniques, such as silver impregnation, to be visualized microscopically. The sensations of itch, pain, touch, temperature, and displacement of body hair are detected by the free sensory nerve endings. Itching, a form of mild pain that promotes the desire to scratch, is one of the most common reasons animals are presented to veterinarians. The sensations of touch and pressure are detected by Meissner’s and Pacini’s corpuscles. Sensations detected by free sensory nerve endings and by the corpuscles are transmitted to the spinal cord via the dorsal root ganglia. Sensory fibers to the facial area are supplied by the trigeminal nerve. Motor fibers (adrenergic and cholinergic) are supplied by the sympathetic component of the autonomic nervous system (see Fig. 17-5). Adrenergic fibers travel from the spinal cord through postganglionic fibers in peripheral nerves and arborize into plexuses that innervate blood vessels, arrector pili muscles, and apocrine sweat glands. Stimulation by these adrenergic fibers causes vasoconstriction and piloerection (raising of the hair shafts). Cholinergic fibers travel from the spinal cord and arborize into plexuses that innervate the eccrine sweat glands. Stimulation of these fibers in humans causes widespread eccrine sweating, important in thermoregulation and recognized clinically as “beads of sweat” on the skin. Because the eccrine ducts open directly on the surface of the skin, the secretion is more easily seen clinically. This phenomenon does not occur in dogs or cats because they lack eccrine glands in haired skin. However, cholinergic and to a lesser degree adrenergic fiber stimulation in dogs and cats causes sweating of the eccrine glands of pawpads at times of excitement or agitation. In the haired skin, equine sweat glands are considered to be the epitrichial (apocrine) type, where the duct opens into the follicular canal near the skin surface, but less commonly the duct may open in a depression near the follicle opening or directly on the skin surface. As in humans, sweating in the horse is important in thermoregulation. However, the precise mechanisms that control sweating in the horse are unknown. The horse has a rich supply of vessels and nerves around sweat glands. It appears that equine sweat gland secretion is controlled by an interaction among neural, humoral, and paracrine factors. The only other domestic animal in which apocrine gland secretion is thought to play a thermoregulatory role is cattle, but sweating is not typically clinically visible except in horses.
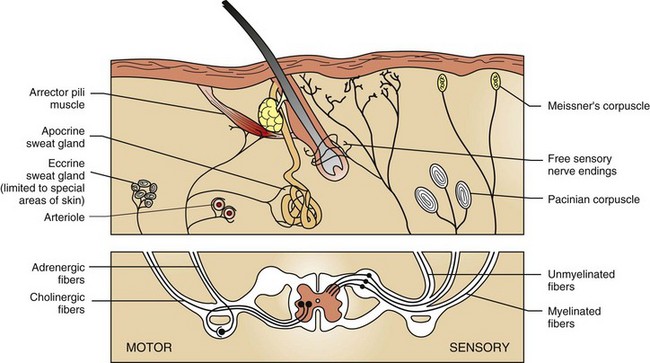
Fig. 17-5 Schematic diagram of cutaneous innervation.
Cutaneous nerve endings transmit sensations of touch, pressure, temperature, pain, and itch (pruritus) via dorsal root ganglia to the central nervous system. Motor fibers in skin are supplied by the autonomic nervous system. Adrenergic fibers activate arterioles, arrector pili muscles, and apocrine glands; cholinergic fibers stimulate eccrine glands. (Adapted from Ackerman AB: Histologic diagnosis of inflammatory skin diseases, Philadelphia, 1978, Lea and Febiger.)
Subcutis (Panniculus, Hypodermis)
The subcutis attaches the dermis to subjacent muscle or bone and consists of adipose tissue and collagenous and elastic fibers, which provide flexibility. Adipose tissue insulates against temperature variation and in the case of pawpads, serves in shock absorption. Adipose tissue also stores calories as triglycerides. In addition, there is recent evidence that fat cells secrete via autocrine, paracrine, and endocrine mechanisms a variety of cytokines, chemokines, and hormone-like factors such as adiponectin, leptin, resistin, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and acute-phase proteins. These factors have been called adipokines and are thought to play a role in metabolism, and some may also contribute to adverse events associated with obesity.
Adnexa
The growth of hair occurs within hair follicles in a sequence of stages (Fig. 17-6). These stages include hair genesis, growth, maturation, and loss. In the anagen stage of the hair cycle, mitotic activity and growth occur. The catagen stage is a transitional phase during which cellular proliferation ceases. The follicle then enters a resting stage, telogen, after which mitotic activity and new hair production resumes. The exogen stage is the phase in which the old hair is shed. In many animals hair follicle growth occurs in cycles, resulting in periodic loss or shedding of the hair coat. The reason the hair growth occurs in cycles is not clear. Some hypotheses include that the cycle provides the ability to: (1) shed fur to cleanse the body surface, (2) adapt and change body cover in response to changing environment (winter to summer) or social conditions, or (3) protect against malignant transformation that might occur in a rapidly dividing tissue. The regulation of hair cycling is exceedingly complex and incompletely understood. Factors that play a role include genetics, photoperiod, temperature, nutrition, hormones, health status, and neural mechanisms. Genetic factors determine the hair shaft length (e.g., short-haired versus long-haired breeds of dogs). The exact signals that control the hair cycle have not been identified; however, growth factors, such as epidermal growth factor, fibroblast growth factor (FGF), hepatocyte growth factor, platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and insulin-like growth factor (ILGF), have been localized to the skin and hair follicles. These growth factors probably play a crucial role in regulation of the hair cycle and follicle growth.
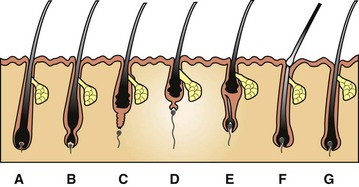
Fig. 17-6 Schematic diagram of the hair cycle.
A, Anagen. During this growing stage, hair is produced by mitosis in epithelial cells covering the apex of the follicular papilla (dermal papilla), which is enveloped by hair matrix cells at the hair bulb. B, Early catagen. In this transitional stage, a constriction occurs at the hair bulb, and the hair shaft above this becomes a “club hair.” C, Catagen. The distal follicle becomes thick and corrugated and pushes the hair outward. D, Telogen. This is the resting stage in which the follicular papilla separates and an epithelial strand shortens to form a secondary germ. E, Early anagen. The secondary germ grows down to enclose the follicular papilla, and a new hair bulb forms. F, Exogen. This refers to the shedding of the old hair shaft. G, Anagen. The hair elongates as growth continues. Note that the anagen hair bulbs are located deeper in the dermis than the catagen and telogen hair bulbs. (Adapted from Scott DW, Miller WH Jr, Griffin CE: Muller and Kirk’s small animal dermatology, ed 6, Philadelphia, 2001, Saunders.)
Photoperiod acts via the hypothalamus, pituitary, and pineal glands, which secrete tropic hormones, such as melatonin and the gonadal, thyroid, and adrenal hormones, that also influence hair growth. Some hormones, such as thyroid and growth hormone, stimulate hair growth, whereas excessive levels of estrogen or glucocorticoids suppress hair growth. The cells in the follicular papilla (sometimes called dermal papilla) regulate epithelial growth and are probably the target of the tropic hormones. Nutrition and health status have a significant influence on hair growth and quality. Hair is largely composed of protein. Thus diets low in protein or disease states associated with severe protein loss result in poor quality hair coat. Animals in poor health have larger numbers of hair follicles in telogen, and because telogen hairs are more easily shed than anagen hairs, animals in poor health tend to shed more heavily than healthy animals. Also, in disease states, cuticle formation can be defective, resulting in a dull or dry hair coat. It is also thought that the hair cycle is influenced by the nervous system. Evidence of such is implied by the abundant nerve supply of the hair follicle, the high density of Merkel cells in the hair follicle epithelium, and facts that keratinocytes express several neurotransmitter and neuropeptide receptors whose stimulation can alter keratinocyte proliferation and differentiation. Indirect influence by autonomic nerves could also be mediated by alteration of hair follicle blood flow and thus of oxygen and nutrient supply.
Forms of hair follicles vary in different animals (Fig. 17-7). Horses and cattle have evenly distributed simple follicles with one large (i.e., primary) follicle, usually with sebaceous and apocrine glands and arrector pili muscles. Pigs have simple follicles grouped in clusters. Goats, dogs, and cats have compound follicles that consist of primary follicles and smaller secondary follicles. Sheep have simple follicles in hair-growing areas and compound follicles in wool-growing areas. Primary follicles have the hair bulb rooted more deeply in the dermis than secondary follicles. The depth of the hair bulbs varies with species. In dogs and cats, the anagen hair bulbs of primary follicles are at the dermal-subcutaneous junction, whereas in horses and cattle the anagen hair bulbs are in the mid-dermis. In all species, the bases of telogen follicles are more superficially located than the bases of anagen follicles. Typically, primary and secondary hair shafts emerge through a common follicular opening. Tactile hairs include sinus and tylotrich hairs. Sinus hairs, also termed vibrissae, arise in simple follicles with a blood-filled sinus located between the inner and outer layers of the dermal sheath. Sinus hairs generally occur on the nose, above the eyes, on the lips and throat, and on the palmar aspect of the carpus of cats. Sinus hairs function as mechanoreceptors (i.e., touch receptors). Tylotrich hairs also function as mechanoreceptors and are scattered among the regular body hairs. The arrector pili muscles extend from the connective tissue sheath of the hair follicles at the junction of the middle and inferior portion of the follicle and attach to the superficial dermis. The arrector pili smooth muscles are oriented almost perpendicularly to the wall of the follicle and are well developed on the back of animals, especially dogs. Muscle contraction causes erection of hairs and expression of the contents of sebaceous glands.
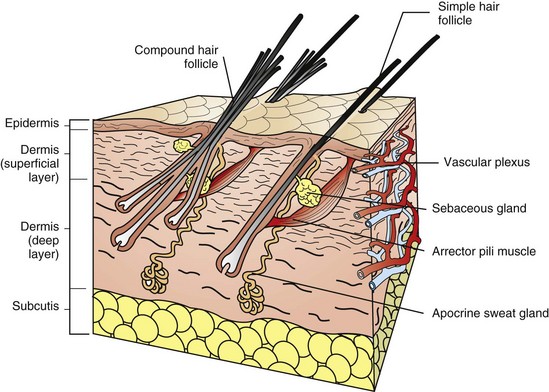
Fig. 17-7 Schematic diagram of the skin and simple and compound hair follicles.
For the purpose of simplification, the vascular supply is illustrated on one face only. Note the simple hair follicle (right) and the compound hair follicle (left). Simple follicles consist of one large primary follicle with the hair bulb in the dermis or subcutis (depth varies with species), and with sebaceous and apocrine glands, and arrector pili muscles. Compound follicles consist of a large primary follicle and smaller secondary follicles. The hair bulbs of secondary follicles are located more superficially in the dermis than the hair bulbs of primary follicles. Secondary follicles may have sebaceous glands but lack apocrine glands and arrector pili muscles. (From Dellman DH, Brown EM: Textbook of veterinary histology, ed 3, Philadelphia, 1987, Lea and Febiger.)
Sweat Glands
There are two basic types of sweat glands: apocrine glands and eccrine glands. Apocrine glands are located throughout haired areas of skin in domestic animals and are tubular- or saccular-coiled glands (see Fig. 17-7). The ducts of the apocrine glands open in the superficial portion of the hair follicle; thus these glands are also called epitrichial glands. The glands are lined by secretory cuboidal to low columnar epithelium surrounded by contractile myoepithelial cells. Other apocrine glands include the interdigital glands of small ruminants, glands of the external ear canal and eyelids of domestic animals, anal sac glands of dogs and cats, and the mental organ of pigs. Eccrine glands are merocrine in secretion and in contrast to ducts of apocrine glands, the ducts open directly onto the surface of the epidermis. Thus eccrine glands are also called atrichial glands. They are tubular glands lined by cuboidal epithelium surrounded by myoepithelium and are confined mainly to pawpads of dogs and cats, frog region of ungulates, carpus of pigs, and nasolabial region of ruminants and pigs.
Sebaceous Glands
Sebaceous glands are simple, branched, or compound alveolar glands that undergo holocrine secretion, with ducts opening into hair follicles except at some mucocutaneous junctions where the glands open on the surface of the skin (e.g., meibomian gland/also known as tarsal gland). Well-developed sebaceous glands are found in the supracaudal gland of dogs and cats; infraorbital, inguinal, and interdigital regions of sheep; the base of the horn of goats; the anal sac glands of cats; the preputial glands of horses; and the submental organ of cats.
Specialized Structures
Anal sacs are specialized cutaneous structures that are especially prone to develop lesions. Anal sacs are bilateral diverticula located between internal and external anal sphincter muscles in dogs and cats and have ducts that open onto the anus at the level of the anocutaneous junction. Ducts and sacs are lined by stratified squamous epithelium. In cats, the sac wall has sebaceous and apocrine glands, but in dogs, the wall has only apocrine glands. The anal sacs can become distended with secretory products, rupture after trauma, and cause bacterial infection and chronic inflammation (foreign body reaction) in contiguous tissues. Carcinomas of the apocrine gland of the anal sac in dogs are often associated with tumor cell production of parathyroid hormone-related protein (PTHrP) and humoral hypercalcemia of malignancy.
Hepatoid (i.e., circumanal or perianal) glands occur most commonly in the skin around the anus and are also present in skin near the prepuce, tail, flank, and groin. These glands are modified sebaceous gland that have nonpatent ducts and are composed of peripheral reserve cells that surround lobules of differentiated cells resembling hepatocytes, resulting in the name “hepatoid” glands. Adenomas of the perianal glands in male dogs are often testosterone dependent.
The claws of dogs and cats shield the third phalanx and consist of a wall (i.e., dorsal and lateral sides) and sole (i.e., ventral side), both of which are stratified squamous keratinizing epithelium. The wall consists of hard keratin and the sole of softer keratin. The dermis of the claw consists of dense collagen, elastic tissue, and blood vessels that can bleed profusely if the claw is trimmed too short. The claw fold is a fold of skin that covers the wall laterally and dorsally for a short distance. Hooves consist of the wall, sole, and frog in solipeds; and a wall, sole, and prominent bulb in ruminants and pigs. The hoof wall comprises three structurally distinct layers (i.e., stratum externum, stratum medium, and stratum internum), which are formed by the proliferation and downward movement of epidermal cells arising from a specialized junction of the epidermis and dermis, a region known as the coronary band or coronet. The stratum internum of the inner hoof wall interdigitates with the dermal lamina of the corium, thereby anchoring the inner hoof wall to the dermis that covers the third phalanx. If the attachment of the stratum internum to the dermal lamina fails, the shearing forces of body weight and movement lead to vascular damage in this region and ultimate damage to the corium of the sole and coronet. The separation of the third phalanx from the inner hoof wall is the primary pathologic process leading to the painful condition of laminitis most often seen in horses and cattle.
The digital pads of dogs and cats have a thick epidermis composed of all layers, including the stratum lucidum. The surface is covered by compacted layers of stratum corneum and is smooth in the cat; however, in the dog, the surface is covered by conical papillae that conform to the outline of the epidermal surface (see Fig. 17-2). The epidermis and dermis interdigitate via rete ridges and dermal papillae, thus providing resistance to shear forces. Eccrine (atrichial) glands are present in the dermis and the adipose tissue. Lobules of adipose tissue that act as a cushion are subdivided by collagenous stroma and elastic tissue.
The chestnuts and ergots of the horse are considered to be vestiges of the first, second, and fourth digits. Chestnuts are located in the supracarpal and tarsal area on the medial surface of the limbs, and the ergot is located at the flexion of the fetlocks (metacarpophalangeal articulation). Chestnuts and ergots are histologically similar and consist of compact stratum corneum covering thick cellular layers of the epidermis. The rete ridges are long and interdigitate with long dermal papillae.
Function
The skin is not only the largest organ in the body, but one of the most important. Without the skin, terrestrial mammalian life could not exist. The skin has numerous functions, which are listed in Box 17-1. The skin prevents significant loss of fluid and electrolytes (e.g., the stratum corneum barrier), protects against physical and chemical injury (e.g., the stratum corneum barrier, keratin filaments, desmosomal and hemidesmosomal junctions, collagen, and elastic fibers), participates in temperature and blood pressure regulation (e.g., the hair coat, sweat glands, and vascular supply), produces vitamin D (e.g., ultraviolet [UV] light photolysis of dehydrocholesterol), serves as a sensory organ (e.g., tactile hairs, Merkel cells, and nerves), and stores fat, water, vitamins, carbohydrates, protein, and other nutrients (e.g., subcutaneous fat). Absorption, although not a primary function, also occurs. In addition, the keratinocyte, a major source of cytokines and antimicrobial peptides, is now considered to be an integral part of the innate and adaptive immune systems protecting against microbial injury and participating in inflammation and tissue repair.
Portals of Entry
Normal intact skin has many natural defenses and barriers that render it impenetrable to most organisms and protect the body from a variety of other types of insults that include pressure, friction, mild mechanical trauma, temperature extremes, UV light exposure, and chemical absorption.
The route by which an infectious agent gains entry into the body is called the portal of entry (Box 17-2). Many pathogens can only cause disease when entering the body via their specific portal of entry. A few pathogens, such as hookworm larvae, are able to penetrate intact normally functioning skin. Dermatophytes are able to colonize the cornified structures (hair, claws) and the stratum corneum and cause disease without ever entering living tissue. Clinical disease in a dermatophyte infection is the result of the host’s reaction to the organism and its by-products. The skin only becomes an efficient portal of entry for microorganisms when the barrier is damaged by trauma, excessive moisture, heat or cold, or by disruption of the normal flora of the integument. A number of microorganisms (e.g., Staphylococcus intermedius, Streptococcus sp., Corynebacterium pseudotuberculosis, Pasteurella sp., Proteus sp., Pseudomonas sp., and Escherichia coli) gain entrance to the body by either entering through natural pores, such as hair follicles or glands with ducts that traverse the epidermis, or by the parenteral route, which includes all types of breaks in the skin, including injections, insect bites, and other types of wounds. Organisms that are able to inhabit hair follicles, such as mites or bacteria, gain entry to the body when the wall of the follicle is ruptured, leading to emptying of follicular contents into the dermis. Similarly, rupture of glands or ducts can lead to entry of microorganisms. From here, infectious agents can stimulate a robust host immune response or possibly spread to other areas of the body by gaining entry to the bloodstream or traveling to regional and distant lymph nodes via lymph flow.
Intact skin with its waterproof barrier provides some protection against weak acids and alkali substances and water-soluble compounds, but certain lipid-soluble compounds can be absorbed directly through intact skin as can some artificially engineered gases developed for chemical warfare. UV radiation (UVR) can damage the skin by direct exposure if the body’s natural defenses, such as the hair coat and melanin pigments, are not present or are inadequate. The lesions of solar (actinic) dermatitis (see the section on Disorders of Physical, Radiation, or Chemical Injury: Solar (Actinic) Dermatosis, Keratosis, and Neoplasia) typify the effects of chronic exposure to UVR. In addition to solar dermatitis, squamous cell carcinomas, hemangiomas, and hemangiosarcomas have an increased tendency to develop in skin chronically damaged by UVR.
The dermal capillaries can be a portal of entry to the skin via the hematogenous route. Embolization of infectious agents, such as bacteria (Erysipelothrix rhusiopathiae [diamond skin disease]) or fungi (systemic infection with Blastomyces dermatitidis) can damage the skin via this route during hematogenous dissemination. Tumor cells (hemangiosarcoma) can also embolize to the skin and lead to metastatic tumor foci or possible cutaneous infarction. The hematogenous route is also most often the delivery system for drugs (adverse cutaneous reactions to the administration of trimethoprim-potentiated sulfonamides; photosensitization dermatitis that occurs with phenothiazine ingestion) and toxins (gangrenous ergotism caused by the mycotoxin of Claviceps purpurea) to reach the skin.
Rarely, an infectious agent that is neurotropic can migrate from a ganglion along sensory nerves via axonal flow to the skin (reactivation of feline herpesvirus 1 (FHV-1) infection in cats resulting in ulcerative facial dermatitis). The skin can also be secondarily infected or traumatized or damaged by extension of pathologic processes affecting adjacent support structures such as bone, muscle, lymph nodes, or glands (locally invasive mammary gland carcinoma resulting in cutaneous ulceration).
Defense Mechanisms
The skin is a complex organ composed of many integrated components structurally and functionally designed to protect the host. Host defenses against injury principally consist of three broad mechanisms: (1) physical defense, (2) immunologic defense, and (3) repair mechanisms. The most critical defense is the barrier derived from the more superficial layers of the skin, which include the stratum corneum, epidermis, basement membrane, and superficial dermis. Without these outer layers of the skin, animals cannot survive (consider, for example, the deleterious effects of extensive burns and immune-mediated diseases such as pemphigus vulgaris). One of the most important cells in the skin is the keratinocyte. The keratinocytes terminally differentiate to form the stratum corneum, the outermost barrier of the skin. The keratinocytes produce keratin filaments, desmosomes, and hemidesmosomes, providing structural integrity to the cytoplasm and an interconnecting network that anchors the keratinocytes to each other and the basement membrane. Keratinocytes produce cytokines (including IL-1, IL-6, IL-8, IL-3, TNF-α, colony-stimulating factors) and growth factors (including TGF-α, TGF-β, PDGF, FGF), thus participating in innate and adaptive immunity and in the communication between the two. Keratinocytes also dissolve desmosomes and hemidesmosomes and form actin filaments so they can migrate to cover skin wounds and then proliferate to regenerate the wounded skin. Keratinocytes thus not only orchestrate the activities of the skin but also serve as many members of the orchestra.
Physical Defense Mechanisms
Barriers of the skin against physical injury are listed in Box 17-3. The hair coat, particularly the long dense hair coat of some dogs and cats, serves as a physical barrier to temperature extremes, UVR, and minor trauma. The hair coat also sheds water as a result of the lipids provided by sebaceous gland secretion. Vibrissae, or tactile hairs, and sensory neurons provide awareness of the physical environment, allowing the animal to make appropriate reactions for survival such as reflex responses to heat and other noxious stimuli. Claws, especially on cats, serve as a quite effective barrier against predators by providing traction for climbing and serve as weapons to be used against aggressors. Horns of cattle, sheep, and goats also provide some physical defense capabilities.
The stratum corneum is an exceedingly important component of the barrier, imparting protection from the exterior and preventing water loss from the interior. The stratum corneum is composed largely of keratins, a family of proteins called intermediate filaments. Keratin proteins are the major structural proteins of the skin, hair, and claws. The stratum corneum is considered to be the “bricks and mortar” of the barrier. The bricks are the flattened cornified cells (corneocytes) with their resistant cell envelopes and keratin microfibrils, and the mortar consists of intercorneocyte lipids.
The bricks are formed at the level of the stratum granulosum when the keratinocytes are transformed into the flattened corneocytes. The transformation occurs when (1) the nucleus is digested, (2) the keratin intermediate filaments aggregate into microfibrils oriented parallel to the skin surface, (3) the lipids are released into the intercellular space, and (4) the cell membrane is replaced by a resilient cell envelope consisting of cross-linked protein with lipids covalently bound to its surface. Filaggrin (which is an acronym for filament-aggregating protein) from the keratohyalin granules in the stratum granulosum plays a significant role in formation of the bricks by participating in the aggregation of keratin filaments into tight bundles. The keratin intermediate filaments and filaggrin compose 80% to 90% of the protein mass of the epidermis. Later, filaggrin is digested by proteolytic enzymes to produce components of amino acids that form the “natural moisturizing factor” of the stratum corneum, which serves to help maintain hydration, flexibility, and proper desquamation. Concurrent with the aggregation of the keratin filaments, the resistant cornified envelope is transformed from the water-permeable phospholipid cell membrane of the keratinocyte when the membrane-bound enzymes (e.g., transglutaminases), cross-link proteins from the keratohyalin granules (e.g., loricrin) and the cytoplasm (e.g., involucrin) in isopeptide bonds. Other proteins (including trichohyalin and small proline-rich proteins) are similarly cross-linked and eventually the entire cell membrane consists of cross-linked proteins. The proteins of the cornified envelope compose 7% to 10% of the protein mass of the epidermis. The corneocytes are joined together by desmosomes that are modified from those joining keratinocytes in lower layers of the epidermis by the addition of a protein called corneodesmosine. These stratum corneum desmosomes are referred to as corneodesmosomes.
The mortar is formed when lipids, from the lamellar bodies in the stratum granulosum, are released into the intercellular space. These intercorneocyte lipids (glycosyl ceramides, cholesterol, cholesterol esters, and long-chain fatty acids) are hydrophobic and prevent transepidermal water loss. Lamellar bodies have other important functions: (1) they provide enzymes that generate ceramides and free fatty acids that are incorporated into the lipid membranes; (2) they provide proteases and antiproteases that regulate digestion of corneodesmosomes and shedding of cornified cells to the exterior; and (3) they secrete antimicrobial peptides including defensins into the intercellular compartment of the stratum corneum. The lipid component of the stratum corneum surrounds the protein component to which it is covalently bound and provides adhesion of the cornified cells (i.e., bricks) to the intercellular lipids (i.e., mortar). Layers of the corneocytes and their corneocyte envelope (i.e., bricks), and intercellular lipids (i.e., mortar) form a tough and resilient protective barrier. The waterproofing and repellency of the stratum corneum is in part provided by the keratinocyte and sebum-derived lipids.
Other barrier functions of the skin include defense against antioxidant injury provided by vitamin E in sebaceous gland secretion and defense against UV light provided by the hair coat and also by melanin pigment in keratinocytes. The cap of melanin pigment over the nucleus helps protect the nucleus (and its nucleic acid) against UV light–induced injury by scattering and absorbing UV light rays. The basement membrane zone serves as an initial barrier to invasion of the dermis by neoplastic epidermal cells. The panniculus through its insulating properties serves as a barrier against cold temperatures. Secretion from apocrine glands in cattle and horses provides defense against excessive heat.
Resistance to Mechanical Forces
Anatomic features of the skin that provide resistance to physical injury are listed in Box 17-3. Hair follicles help anchor the epidermis to the dermis, as do epidermal-dermal interdigitations, thus these interdigitations are most numerous in nasal planum and pawpad where hair follicles are absent and resistance to shearing force is necessary. Host defense against mechanical injury is also provided by the tightly bundled keratin filaments of the corneocyte, the resilience of the cornified envelope, the adhesion of the cornified envelope and intercellular lipids, and the corneodesmosomes. In addition, the keratinocytes contain keratin filaments and form desmosomal junctions with adjacent cells (see Fig. 17-4). The keratin filaments perform a structural role (i.e., cytoskeletal) in the cells, and the desmosomes promote adhesion of epidermal cells and resistance to mechanical stresses. The basement membrane anchors the epidermis to the dermis via hemidesmosomes providing structural integrity against trauma. Dermal collagen and elastic tissue provide resilience and strength to the skin and support for the vessels, nerves, and adnexa. The panniculus protects against surface trauma by providing some shock absorption (e.g., pawpads), by facilitating movement, and by anchoring the dermis to fascia. Thus the various components of the epidermis, dermis, adnexa, and panniculus provide a flexible interconnecting framework to protect the host against mechanical injury.
Immunologic Defense Mechanisms
Innate immunity is a primitive, highly conserved response that quickly detects and impairs pathogens and harmful environmental stimuli encountered daily in life and does not require antigen-specific receptors. Innate immunity protects the host during the first 7 days of exposure to a pathogen before development of an adaptive immune response, and also initiates and assists the adaptive immune response (see Web Table 17-1). The diversity of microorganisms requires equal diversity in host defense responses. The first phase of innate host defense consists of the barrier of the stratum corneum, which prevents pathogen adherence and provides an antimicrobial surface consisting of antimicrobial peptides and fatty acids. The antimicrobial peptides (e.g., β-defensins and cathelicidins) are effective against many organisms, including viruses, bacteria, protozoa, and insects, and probably kill some of these pathogens by damaging their lipid membranes. The surface barrier also includes normal flora of nonpathogenic bacteria that competes with pathogenic microorganisms for nutrients and for attachment sites on cells. The normal flora also produces antimicrobial substances that prevent pathogen colonization. Because the dry cornified surface is such an effective barrier to pathogens, a wound or abrasion is usually necessary for a pathogen to gain entrance. Web Fig. 17-1 provides an illustration of how the innate and adaptive (acquired) immune systems participate in host defense against bacterial pathogens that have gained entrance into the skin through a wound in the epidermis. When injured, keratinocytes release a variety of antimicrobial peptides, chemokines, and cytokines, which activate endothelial cells and attract macrophages, neutrophils, and lymphocytes to the site of injury. Early key cells that play a role in innate immunity are the tissue macrophages that contact, bind, phagocytose, and thereby eliminate many types of pathogens. Pathogen recognition is mediated by pattern-recognition receptors (PRRs), including Toll-like receptors and others, that recognize repeating patterns of molecular structures common to broad classes of pathogens and efficiently differentiate pathogen antigens from self-antigens. The repeating patterns of molecular structures on pathogens are called pathogen-associated molecular patterns (PAMPs).
WEB TABLE 17-1
Innate Immunity Host Defense Mechanisms
| Stratum corneum barrier | Prevents pathogen adherence and provides antimicrobial surface |
| Macrophages (dendritic cells) with pattern-recognition receptors | Recognize broad classes of pathogens, differentiate pathogen antigens from self antigens, initiate Toll signaling pathway, and secrete cytokines, facilitating inflammation and innate immunity. |
| Toll signaling pathway | Promotes expression of large numbers of genes, resulting in production of cytokines, chemokines, and adhesion molecules important in inflammation and innate immunity. |
| Macrophages and neutrophils | Recognize, ingest, and destroy pathogens. |
| Endothelial cells | Express adhesion molecules and trigger kinin and coagulation systems, facilitating influx of plasma proteins and migration of leukocytes (cell trafficking) necessary to control infection. |
| Coagulation system | Forms blood clot in case of injury to control blood loss and prevents microorganisms from entering the bloodstream. |
| Complement enzyme cascade | Recruits inflammatory cells, opsonizes pathogens, and kills some pathogens. |
| Lipid mediators | Increase vascular permeability and induce influx and activation of leukocytes sustaining inflammatory responses. |
Innate immunity protects the host in the first 7 days of exposure and does not require antigen-specific receptors but does not provide protection to later reexposure.
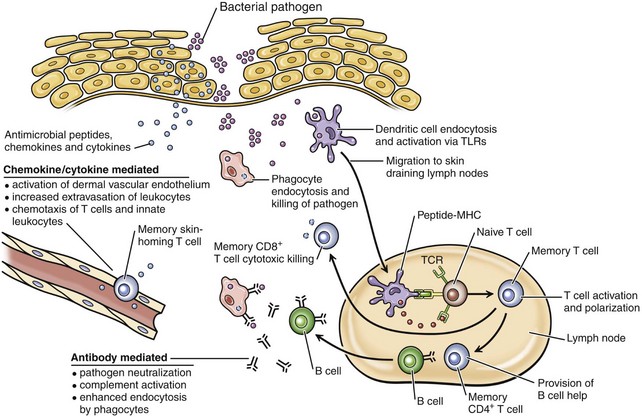
Web Fig. 17-1 Diagram of interactions between the innate and acquired immune systems in response to bacterial infection of the skin.
In response to bacteria that have breached the epithelial barrier, keratinocytes synthesize anti-microbial peptides, chemokines, and cytokines. These factors lead to activation of the dermal capillary endothelium, inducing the migration of innate leukocytes and memory T lymphocytes into the skin and additionally guiding these cells via chemotactic gradients. These factors and bacterial antigens activate innate phagocytes to kill ingested organisms and activate dendritic cells to migrate to the local skin lymph nodes. In the lymph nodes, dendritic cells present bacterial antigens to naive and central memory T lymphocytes, leading to stimulation of pathogen-specific lymphocytes. Effector CD8+ T lymphocytes exit the lymph node, home to inflamed skin, and kill pathogens. Helper CD4+ T lymphocytes provide help to B lymphocytes, inducing the production of antibodies that directly neutralize pathogens and lead to additional targeting of innate responses. Antibody-directed phagocytosis by innate cells leads to enhanced antigen presentation, further enhancing acquired responses. (From Clark R, Kupper T: J Invest Dermatol 125:629-637, 2005.)
A few examples of PAMPs include lipopolysaccharide (most Gram-negative bacteria), peptidoglycan (Gram-positive bacteria), CpG motifs (mostly bacterial pathogens), lipoarabinomannan (mycobacteria), mannans and zymosan (yeast), double and single stranded RNA (viruses), and heat shock proteins (bacteria, fungi, algae, protozoa). These PAMPs have been conserved during evolution and allow the innate immune system to broadly distinguish between self-antigens and pathogen antigens. The important outcomes of pathogen-receptor binding include the activation of phagocytic and other immune effector cells and release of cytokines, chemokines, adhesion molecules, and other inflammatory mediators initiating an acute-phase response. The acute-phase response proteins can opsonize a broad range of pathogens and can also activate the complement cascade, making pathogens more susceptible to phagocytosis and killing by macrophages and neutrophils. Once initiated, the innate immune response helps to start the antigen-specific immune response (i.e., adaptive immunity). Innate immunity is crucial to protecting the host in the early days of infection; however, pathogens can evade innate immunity and innate immunity does not lead to immunologic memory characteristic of adaptive immunity.
Acquired (Adaptive) Immunity
Whereas innate immunity works immediately to detect and destroy microorganisms, acquired or adaptive immunity develops later because the lymphocytes that contribute to adaptive immunity specific for the invading pathogen must increase in number by clonal expansion (see Web Table 17-2). The major components of the cutaneous adaptive immune system include keratinocytes, dendritic antigen-presenting cells (Langerhans’ cells and dermal dendritic cells), lymphocytes, and endothelial cells (see Web Fig. 17-1).
WEB TABLE 17-2
Adaptive Immunity Host Defense Mechanisms
| Langerhans’ cells in epidermis and dendritic cells in dermis | Ingest and process antigen, present antigen to naïve T lymphocytes in lymph nodes, present antigen to sensitized T lymphocytes at site of injury, and produce cytokines that upregulate inflammation and immune responses. |
| T lymphocytes | After stimulation by antigen-presenting cells in lymph node, migrate back to the site of injury. |
| CD8+ (cytotoxic lymphocytes) | Recognize antigen expressed on the cell surface and kill the cell (cytotoxic lymphocytes); responsible for killing neoplastic cells, some bacteria and parasites, and all viruses that replicate inside cells. |
| CD4+ TH1 | Activate macrophages, helping to control infection by intracellular bacteria. |
| CD4+ TH2 | Activate B lymphocytes, helping eliminate extracellular pathogens. |
| B lymphocytes | Secrete immunoglobulin, providing defense against pathogens (often bacteria) in extracellular spaces. |
| Endothelial cells | Express adhesion molecules and bind to stimulated T lymphocytes. |
| Keratinocytes | Produce cytokines and growth factors upregulating or downregulating inflammation and immune responses. |
| Cytokines, chemokines, and adhesion molecules | Contribute as in innate immune response. |
Adaptive (acquired) immunity develops after innate immunity because the lymphocytes that contribute to adaptive immunity specific for the invading pathogen must increase in number by clonal expansion and provides protection on later reexposure to pathogen.
The adaptive-immune response is initiated by a stimulus (in this example, the epidermal injury, microbial invasion, and signals provided from the innate immune system), at which time the bone marrow–derived antigen processing and antigen-presenting cells (Langerhans’ cells in the epidermis and dendritic cells in the perivascular dermis) ingest and process the antigen. Pathogen recognition is mediated through PAMPs as described previously. The major function of Langerhans’ cells and dermal dendritic cells is antigen processing and presentation; thus these cells are referred to as “professional antigen processing and presenting cells.” Langerhans’ and dermal dendritic cells reexpress the ingested and processed antigen on their cell surfaces and migrate via afferent lymphatic vessels to the paracortical areas of skin-associated lymph nodes, where they arrive as mature and powerful antigen-presenting cells. These skin-derived dendritic cells then initiate a pathogen-specific protective immune response by presenting antigen to the naïve T lymphocytes. Langerhans’ cells also produce cytokines (e.g., IL-1 and TNF-α), thus participating in upregulation of inflammatory and immune responses in the skin.
The T lymphocytes activated by antigen presentation in the skin-associated lymph nodes are also known as sensitized or memory T lymphocytes. These memory T lymphocytes are subdivided into two types, central memory and effector memory T lymphocytes. The central memory cells generally circulate between the blood and lymph nodes, serve mostly as long-term reservoirs of immunologic memory, and when stimulated by antigen give rise to both central memory and effector memory T lymphocytes. The effector memory T lymphocytes express skin-associated homing receptors (e.g., cutaneous lymphocyte antigen [CLA]) that interact with adhesion molecules (E-selectin, P-selectin, vascular cell adhesion molecule 1 [VCAM-1], and intercellular adhesion molecule 1 [ICAM-1]) on cytokine-activated endothelial cells in the dermal vessels at the site of initial injury, thus providing a way for the effector memory T lymphocytes to find their way back to the site of the injury and pathogen entrance. Once in the skin and after receipt of a renewed antigenic stimulus by the professional antigen-presenting cells, the effector memory T lymphocytes undergo clonal expansion, resulting in the generation of protective effector mechanisms. Most of the lymphocytes in the skin are T-helper lymphocytes, but various types of T and B lymphocytes contribute to adaptive immunity.
Lymphocytes recognize pathogens (i.e., antigens) via cell surface receptors. The B lymphocytes have immunoglobulin molecules as the receptors for antigen, and on activation, B lymphocytes secrete immunoglobulin, which provides defense against pathogens (often bacteria) in the extracellular spaces. Antibody facilitates pathogen neutralization, complement activation, and enhanced endocytosis by phagocytes. In contrast, T lymphocytes have receptors that recognize foreign antigens expressed as peptide fragments bound to major histocompatibility complex (MHC) proteins (see Chapter 5 for a review). One class of T lymphocyte expresses the CD8 molecule on the surface (i.e., CD8+ T lymphocytes). These CD8+ T lymphocytes recognize peptide fragments bound to MHC I, then kill the cell, and thus are also called cytotoxic T lymphocytes.
Another class of T lymphocytes expresses the CD4 molecule on their surface. This class of T lymphocyte is divided into subclasses. One subclass, the CD4+ T lymphocyte subset (TH1 [helper]), recognizes peptide fragments (e.g., microbial antigen) bound to MHC II and releases cytokines, including interferon-γ (IFN-γ), resulting in an inflammatory response via macrophage activation. A second subclass, CD4+ T lymphocyte subset (TH2 [helper]), recognizes peptides (including allergens) bound to MHC II and releases cytokines, including IL-4, IL-5, and IL-13, resulting in inflammatory responses in which eosinophils predominate and stimulates B lymphocytes to secrete immunoglobulin. Another subclass, the T regulatory lymphocyte (Treg), acts to suppress responses of other T lymphocytes. Most antigens require an accompanying signal from helper T lymphocytes before they can stimulate B lymphocytes to proliferate and differentiate into antibody-secreting plasma cells. Thus T lymphocytes are crucial to adaptive immunity by destroying pathogen-infected cells, by activating macrophages, and by activating B lymphocytes.
Thus complex interactions between host cells, pathogens or other antigens, and inflammatory mediators of the innate and adaptive immune system typically result in appropriate host defenses, the removal of the inciting pathogen, and the generation of differentiated memory lymphocytes through clonal expansion, allowing faster specific immune responses in future encounters with the offending antigen. Impaired host defense mechanisms can lead to increased susceptibility to infection, to development of neoplasia, or to chronic inflammatory or autoreactive disorders such as atopic dermatitis, contact hypersensitivity, or lupus erythematosus.
Disease Example of Barrier Dysfunction
Atopic dermatitis (atopy) serves as an example of a common disease associated with impaired function of the epidermal barrier and immunity. It is a multifactorial, chronic and relapsing, often severely pruritic skin disease that affects humans, horses, dogs, and cats. It can cause severe discomfort including sleeplessness from pruritus, and is associated with secondary skin infections. Although the etiopathogenesis of atopic dermatitis has been studied most extensively in humans, many similarities with the human disease have been identified in atopic dogs. Advances in the knowledge of canine atopic dermatitis have occurred through the recent development and validation of animal models of the disease; these models have allowed more in-depth investigative studies in dogs and more precise comparisons of the disease between humans and dogs. Atopic dermatitis is a common problem in dogs; it is estimated to affect 10% to 15% of the population (Web Fig. 17-2). It is also a common human disease estimated to affect 5% to 20% of children. Similarities in the disease in humans and dogs include young age of onset; genetic inheritance; similar clinical lesion distribution; similar histopathologic lesions, including infiltration of immunoglobulin E (IgE+) CD1c+ dendritic cells; dry skin with increased transepidermal water loss; decreased stratum corneum ceramides (lipids); decreased epidermal filaggrin; increased colonization of surface staphylococci; positive atopy patch test; increased IgE-specific responses; and TH2-dominated immune responses. A major difference in the disease is that children with atopic dermatitis often develop asthma and allergic rhinitis, whereas dogs do not; the reason for this difference is currently unknown.
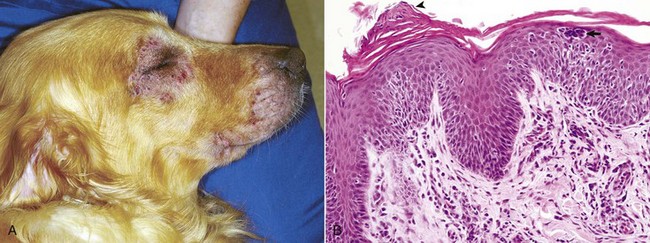
Web Fig. 17-2 Atopic dermatitis, skin, dog.
A, This Golden retriever has erythema, alopecia, and erosions in the skin around the eye and muzzle. The lesions are caused by self-trauma from rubbing and scratching as a result of pruritus. B, Photomicrograph from experimentally induced lesion of atopic dermatitis in the skin of a dog. The epidermis has acanthosis, mild spongiosis, and a few lymphocytes and clustered Langerhans’ cells (arrow). Focal parakeratosis (arrowhead) is also noted. H&E stain. (A courtesy Dr. David Duclos, Animal Skin and Allergy Clinic. B courtesy Dr. Thierry Olivry, College of Veterinary Medicine, North Carolina State University.)
Atopic dermatitis in humans is a multifactorial, heterogeneous genetic disease that arises in association with environmental factors. Defects in three groups of genes important in epidermal barrier function in atopic humans have suggested that in most cases alterations in the epidermal barrier contribute to the development of atopic dermatitis and may represent the primary event. The type and degree of genetic defect plus interaction with environmental factors appear to influence the severity or probability of developing the disease. A defective epidermal barrier facilitates penetration of allergens though the skin, as well as the interaction of the allergens with the local antigen-presenting and immune effector cells (Web Fig. 17-3). Changes in genes encoding for structural proteins (especially filaggrin), epidermal proteases, and protease inhibitors have been identified in humans with atopic dermatitis. These defects serve to reduce stratum corneum hydration and increase transepidermal water loss, increase cleavage of corneodesmosomes junctions, reduce lamellar body secretion and thus stratum corneum lipids, increase pH, and reduce antimicrobial properties of the stratum corneum. These alterations to the barrier favor population by pathogenic versus nonpathogenic bacteria and sustain allergen entrance through the barrier. The allergens are phagocytized by dendritic cells, which present the allergen to TH lymphocytes and recruit other CD4+ T lymphocytes to the site. The activated dendritic cells and the cytokines produced by the CD4+ T lymphocyte (particularly IL-4) lead to switching from a TH1 to TH2 response (in early lesions) and release of proinflammatory cytokines and production of IgE, which in the presence of allergen, activates mast cells. There is evidence suggesting that at least one of these proinflammatory cytokines, IL-31 produced by T lymphocytes that express CLA and localize in the skin, significantly contributes to pruritus, a hallmark of atopic dermatitis. Langerhans’ cells also contribute in early lesions as they prime naïve T lymphocytes into the TH2 type (with high IL-4 production). IL-4 may also be produced by mast cells, basophils, or eosinophils. Exogenous factors also contribute. For example, proteases from house dust mites can facilitate cleavage of corneodesmosomes and additionally contribute to epidermal barrier damage. The resulting inflammation causes pruritus, which stimulates scratching, further damaging the epidermal barrier (and keratinocytes resulting in release of additional proinflammatory cytokines, including IL-1), and creating a vicious cycle that perpetuates the disease and that is difficult to control. About 25% of humans with severe atopic dermatitis have IgE antibody directed toward self-proteins. These antibodies may develop after intracellular proteins from keratinocytes are released when the keratinocytes are damaged by scratching. These keratinocyte proteins may mimic microbial structure and induce IgE autoantibodies that further perpetuate the disease. In summary, atopic dermatitis is a complex, multifactorial, heterogeneous disease in which altered epidermal barrier function appears to contribute to the pathogenesis by facilitating penetration of allergens though the skin, as well as the interaction of the allergens with the local antigen-presenting and immune effector cells. It serves as an example of the importance of the epidermal barrier in protecting the host against injury from allergens, microbial infections, and autoreactive disorders.
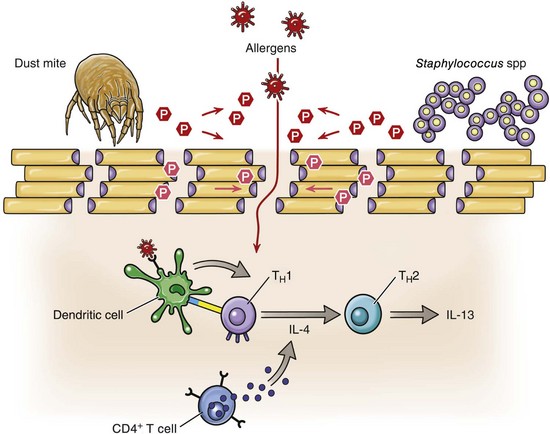
Web Fig. 17-3 Schematic diagram illustrating the defective epidermal barrier in individuals with atopic dermatitis.
The epidermal barrier is formed by the lower layers of the stratum corneum, and is composed of differentiated keratinocytes, termed corneocytes (beige rectangles), held together with corneodesmosomes (purple spheres). The hyperactivity of degradatory proteases (red hexagons) found within the epidermis, and contributed to by exogenous proteases (red hexagons), from house dust mites and Staphylococcus sp., for example, facilitate the cleavage of the corneodesmosome junctions. This is just one event in the breakdown of the epidermal barrier that permits the penetration of allergens. Dendritic cells (DC) (green) found in the dermis take up and present these allergens (red stars) to helper T (TH) lymphocytes and recruit more CD4+ T lymphocytes (blue). Activated DC and IL-4, expressed by CD4+ T lymphocytes, promote TH1 to TH2 switching with the subsequent release of proinflammatory cytokines and elevation of IgE levels. (Adapted from Cork MJ, Danby SG, Vasilopoulos Y, et al: J Invest Dermatol 129: 1892-1908, 2009.)
Regeneration and Repair
Regeneration and repair also constitute a host defense mechanism to injury (Web Box 17-1). The details of tissue regeneration and repair are covered in Chapter 3, whereas the basic mechanisms involved in healing of cutaneous wounds are summarized here. Two common types of cutaneous wounds are used as examples, and these include wounds with opposed edges, such as surgical incisions, and larger wounds in which the edges cannot be opposed, such as broad ulcers, necrosis associated with deep burns, or large areas of trauma in which portions of the skin have been lost (Web Fig. 17-4). Recall that regeneration and repair are a dynamic process involving multiple and overlapping stages. These stages include (1) blood clotting and inflammation (12 to 24 hours after injury), (2) reepithelization, fibroplasia, and angiogenesis (3 to 7 days after injury), and (3) wound contraction and collagen production (1 to 2 weeks after injury).
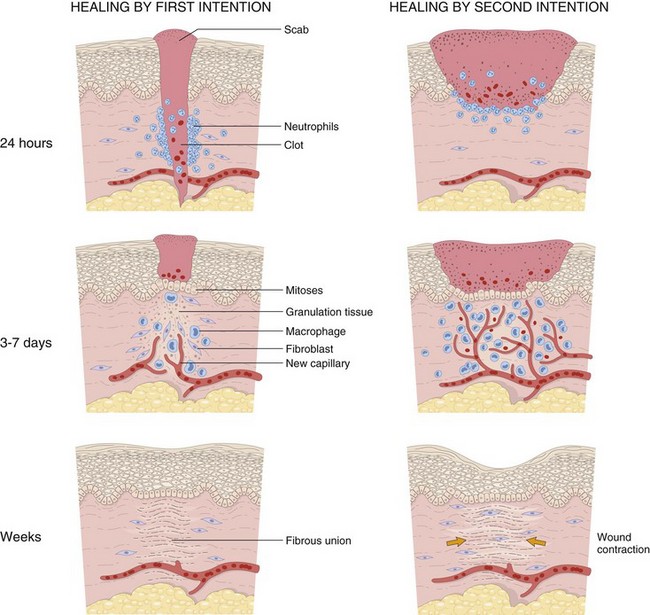
Web Fig. 17-4 Schematic diagram of the steps in wound healing by first intention (left) and second intention (right).
Note large amounts of granulation tissue and wound contraction in healing by second intention. (From Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
Healing of Wounds with Opposed Edges
The simplest healing involves the clean, uninfected surgical incision in which the edges of the wound are closely opposed by sutures so that the wound space is narrow (see Web Fig. 17-4). Healing of these wounds is called primary union or healing by first intention. These wounds cause minimal necrosis of cells of the epidermis, dermis, and adnexa and minimal disruption of the basement membrane. Thus they heal quickly without significant architectural change, although a thin scar remains and the adnexa destroyed by the incision are permanently lost.
The first stage of wound healing is blood clotting and inflammation and begins within the first 12 to 24 hours of injury. The process begins with blood vessel disruption, platelet aggregation, blood coagulation, and clot formation in the vessel and wound space. Dehydration of the surface of the clot forms the scab that covers the wound. The clot in the wound space provides the matrix for migration of inflammatory cells, endothelial cells, and fibroblasts. Platelet-derived factors and factors associated with the coagulation and complement cascades facilitate recruitment of inflammatory cells, such as neutrophils and macrophages, to phagocytose pathogens, foreign particles, and debris. Many of the cytokines and chemokines that govern inflammation in regeneration and repair are the same as those participating in inflammatory processes of other causes. Neutrophils, the first cells to arrive at the margins of the incision, phagocytose pathogens and debris, then either slough with desiccated tissue, are phagocytosed by macrophages, or undergo apoptosis. Macrophages replace neutrophils, secrete collagenase (facilitates tissue debridement), and release growth factors initiating the formation of granulation tissue.
The second stage of wound healing consists of reepithelialization, fibroplasia, and angiogenesis and is maximal between 3 and 7 days of injury. The process of reepithelialization begins within hours of injury from basal cell keratinocytes adjacent to the incision. For the basal cells to become mobile, they retract intercellular tonofilaments, dissolve desmosomes and hemidesmosomes, and form cytoplasmic actin filaments. In addition to mobility, within 24 to 48 hours from injury, there is mitosis of basal cells at the edges of the wound. The mitosis is induced by growth factors from epidermal cells, macrophages, and dermal parenchymal cells. Basal cells from both sides of the wound migrate along the cut edges of the dermis separating nonviable tissue from viable tissue by using the expression of various surface integrin receptors on viable cells and through the production of collagenase, which debrides dead tissue. The basal cells join at the midline of the wound, and as reepithelialization develops, the nonviable tissue above this newly united epidermis, is sloughed. Basal cells also produce extracellular matrix, such as fibronectin, to facilitate reestablishment of the basement membrane. The basal cells revert to a nonmigratory normal phenotype, form hemidesmosomes, and firmly reattach to the newly formed basement membrane, thus beginning the reestablishment of the epidermis over the wounded skin.
Fibroplasia and angiogenesis begin with the formation of granulation tissue, which is the term applied to a specialized type of tissue composed of proliferative fibroblasts and vascular endothelial cells produced in healing of soft tissue injury. Granulation tissue is the hallmark of healing and is named for its clinical appearance of soft pink to tan tissue with minute red foci consisting of capillaries (granules). However, it is the histologic appearance that is diagnostic, a latticework array of proliferative capillaries (angiogenesis) oriented perpendicular to proliferative fibroblasts (fibroplasia). The fibroblasts produce extracellular matrix, which is remodeled, ultimately resulting in scar formation (i.e., cicatrix formation).
The process of forming granulation tissue begins about 3 days after injury. Fibroplasia (fibrosis) is induced when cytokines (IL-1, TNF-α) and growth factors (TGF-β, PDGF, endothelial growth factor [EGF], FGF) from inflammatory cells (especially macrophages), platelets, and endothelial cells stimulate fibroblasts to proliferate, migrate, and ultimately produce extracellular matrix. As in reepithelialization, the ability of fibroblasts to migrate into the clot or provisional matrix requires alteration in expression of surface receptors and production of proteolytic enzymes to provide a path for migration. When fibroblasts have migrated into and filled the wound space, growth factors and cytokines (TGF-β, PDGF, FGF, IL-1, IL-13) direct fibroblasts to switch from migration to protein (collagen) production, providing the structural protein that eventually contributes to wound strength. Collagen production begins by days 3 to 5 and persists for several weeks, depending on the size of the wound.
Concurrent with fibroblast migration into the wound space, angiogenesis, which is the formation of new blood vessels in an area of tissue injury, is also occurring (see Chapters 2 and 3). Angiogenesis develops from preexisting vessels and also from endothelial precursor cells (angioblast-like cells) from the bone marrow. Many growth factors play a role in angiogenesis, but vascular endothelial growth factor (VEGF) is considered to be the most important. In angiogenesis from preexisting vessels, macrophages and injured cells in the wound site release cytokines (such as FGF and VEGF), causing endothelial cells to release proteinases (e.g., procollagenase). The proteinases degrade components of the endothelial cell basement membrane. The disruption of the endothelial cell basement membrane removes the barrier otherwise confining endothelial cells, thereby permitting endothelial cells to migrate into the injured site in response to cytokines and growth factors released from injured or stimulated cells (e.g., FGF from macrophages, VEGF from keratinocytes, and heparin from mast cells). The migratory endothelial cells form tubes that express αvβ3 integrin, facilitating endothelial cell adhesion and migration. The endothelial cells deposit provisional matrix of fibronectin and proteoglycans and eventually form new basement membrane.
The growth factors continue to stimulate endothelial cell proliferation, ensuring a supply of endothelial cells for the extension of capillary tubes so that blood flow to the area of soft tissue injury can be reestablished. In angiogenesis from endothelial precursor cells, VEGF stimulates the mobilization of endothelial cell precursors from the bone marrow and proliferation and differentiation of these cells at the site of injury; however, the mechanism whereby these cells are directed to the site of injury is unclear. Once at the site of injury, these endothelial cell precursors form a delicate capillary plexus that eventually links to existing capillaries, facilitating formation of a capillary network. The newly formed vessels, whether produced by sprouting from preexisting capillaries or from endothelial precursor cells, are provided structural support by pericytes (recruited by angiopoietin 1 interacting with Tie 2, an endothelial cell receptor) and smooth muscle cells (recruited by PDGF), and by production of extracellular matrix proteins (stimulated by TGF-β).
Angiogenesis is a well-regulated process. More than 20 molecules present in tissue act as angiogenesis inhibitors and modulate the reparative process. By the end of the second stage of wound healing, the incision is filled with granulation tissue, neovascularization is maximal, collagen fibrils bridge the incision, and reepithelialization has been completed. By day 7, the usual time to remove sutures, the tensile strength of the incision site is about 10% that of unwounded skin.
The third and final phase of wound repair involves collagen production and wound contraction. However, wound contraction principally affects large wounds that heal by second intention and is described later. Production of collagen and proliferation of fibroblasts (directed by cytokines, such as TGF-β) progress fairly rapidly in this phase of wound repair. In contrast, inflammation, edema, and increased vascularity disappear. The proliferation and maturation of endothelial cells, fibroblasts, and inflammatory cells that contribute to wound repair depend on complex feedback control mechanisms between cells, cytokines, enzymes, and the extracellular matrix microenvironment. This complex interaction has been called dynamic reciprocity. As the wound space is bridged by granulation tissue and fibroblasts produce collagen, the endothelial cells undergo apoptosis, thus reducing capillary numbers. Similarly, fibroblasts and macrophages also undergo apoptosis. The reduction of endothelial cells, fibroblasts, and inflammatory cells leads to formation of an acellular scar. By the third week of wound repair, the wound has about 20% the tensile strength of normal skin. Over the ensuing months, collagen production is reduced, but there is continued slow remodeling of the extracellular matrix leading to a healed wound that at maximum strength is only 70% to 80% that of unwounded skin.
Healing of Wounds with Separated Edges
Healing of wounds with separated edges (healing by second intention) occurs when there is more extensive loss of skin tissue. Healing by this process is more difficult and time consuming, and larger scars result and replace the cutaneous architecture. The principal differences between healing by primary intention and healing by second intention include the following: (1) in healing by second intention, inflammation is usually more extensive because there is more tissue damage that must be removed and secondary infection is more likely; (2) granulation tissue is more extensive because the wound has wider edges so there is a larger gap to fill; and (3) wound contraction occurs, reducing the size of the wound to a fraction of the original size (experimentally, it has been shown that large wounds in rabbits are reduced to 5% to 10% of their original size in about 6 weeks).
Wound contraction begins during the second week of healing when fibroblasts develop phenotypic characteristics of smooth muscle cells. These consist of cytoplasmic bundles of actin-containing microfilaments, and formation of cell-cell and cell-matrix linkages. Fibroblasts link to the extracellular fibronectin matrix and collagen matrix by fibronectin (e.g., α5β1) and collagen (e.g., α1β1, α2β1) receptors and to each other through direct adherens junctions. Also, newly synthesized collagen bundles form covalent cross-links with themselves and with collagen bundles at the wound edge. These linkages thus provide a conduit across the wound space so that the traction of fibroblasts on the matrix can contract the wound, substantially reducing its size and facilitating healing by second intention.
Responses to Injury
A Primer to Histologic Patterns
A number of “new” terms commonly used to classify diseases of the skin are used throughout this chapter. For convenience and to provide quick reference, these terms are listed online in Web Glossary 17-1. Although there are many new terms to learn, it should be realized that the system used to form these terms is similar to that used for other anatomic systems. Basically, prefixes and suffixes are added to word roots to create specific terms that define the pathology of the skin. For example, the prefix “epi” (meaning on or above) combined with the word root “dermat(o)” (referring to skin) creates the word “epidermis,” which simply means the portion of the skin above the dermis. Likewise, the term hypodermis refers to the portion of the skin below the dermis, also called the subcutis or panniculus. Suffixes are used in the same way. For example, the suffix “itis” (meaning inflammation) combined with the word root “dermat(o)” forms the word “dermatitis,” which simply means inflammation of the skin. Similarly, terms referring to inflammation predominantly within the epidermis, follicles, or the panniculus are epidermitis, folliculitis, and panniculitis, respectively. The suffix “osis” refers to a disease process, often noninflammatory. Thus combining dermat(o) and osis forms the word “dermatosis,” which means any disease of the skin, especially one not characterized by inflammation. Dermatoses are the plural of dermatosis and thus mean noninflammatory skin diseases.
Numerous endogenous and exogenous factors can potentially cause injury of the skin (Fig. 17-8). Determining a definitive diagnosis of a skin disorder depends on obtaining a complete history, including age, breed, and sex of the animal; conducting a thorough physical examination, paying particular attention to the distribution of skin lesions; and performing additional diagnostic tests, such as skin scrapings, surface cytology, a complete blood count, serum chemistry panel, skin biopsy sampling, and microbiologic cultures. Results from cutaneous biopsy sampling are often useful and can be necessary to establish a definitive diagnosis for skin diseases. Although the skin has a limited range of responses to injury, the distribution and types of inflammatory cells in the lesion often represent a recognizable pattern that can be used to (1) formulate a list of specific etiologic agents that could cause the lesion or (2) suggest categories of disease with similar lesions and a common pathogenesis. Algorithms (i.e., a set of directions for accomplishing some task that has a recognizable endpoint) have been developed for the recognition of histopathologic patterns in veterinary dermatopathology (Table 17-1). Recognition of patterns, both clinically and histologically, can facilitate differential diagnoses of skin disease (Table 17-2). Patterns of responses to injury are illustrated by changes in the epidermis, dermis, adnexa, and panniculus and are discussed in the next sections.
TABLE 17-1
Examples of Pattern Diagnosis in the Skin
A pattern consists of two parts: a component of the skin (e.g., epidermis) + a histologic reaction of that component to injury (e.g., hyperkeratosis) = pattern (hyperkeratotic diseases of the epidermis).
TABLE 17-2
Differential Diagnoses of Selected Patterns that Can Be Recognized Clinically and Histologically
Some of these disorders could be placed in different patterns. For instance, disorders characterized by vesicles or bullae may develop into an ulcerative pattern after the vesicles or bullae rupture.
*Hyperkeratotic disorders may also affect the nasal planum and pawpads; see Box 17-11.
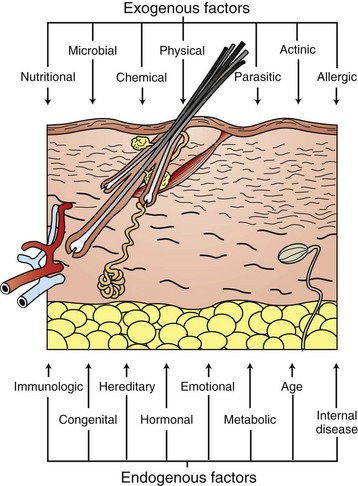
Fig. 17-8 Schematic diagram of the exogenous and endogenous factors that influence the skin.
A myriad of exogenous and endogenous factors influence the gross and microscopic appearance of the skin. Because the skin can respond to these factors in a limited number of ways, different skin disorders may have a similar appearance. Identification of the cause of a skin disorder therefore often requires not only histopathologic evaluation but also clinical history, including clinical lesion distribution and appearance. (Redrawn from Dr. Ann M. Hargis, DermatoDiagnostics.)
Responses of the Epidermis to Injury
Alterations in Epidermal Growth or Differentiation: The basal cells (i.e., basal keratinocytes) in their postmitotic state migrate outward from the basal layer, eventually forming the cornified layers (stratum corneum) of the epidermis. In the normal epidermis, balance is established between the rate of proliferation of the basal cells (germinal cells) and the rate of loss of differentiated cells (squamous epithelial cells) from the surface, resulting in the constant thickness of the epidermis and each of the layers. The orderly proliferation, differentiation, and cornification of epidermal cells is regulated by cytokines (e.g., epidermal growth factor, FGFs, ILGFs, ILs, and TNF), hormones (e.g., cortisol and vitamin D3), and nutritional factors such as protein, zinc, copper, fatty acids, and vitamin A and B vitamins. The cytokines that regulate keratinocyte growth and differentiation are produced by a variety of cell types in the skin, including endothelial cells, leukocytes, fibroblasts, and keratinocytes. Thus keratinocytes also have a self-regulatory role (i.e., autocrine) in their growth and differentiation, and inflammatory cells, among others, can influence keratinocyte growth and differentiation.
Disorders of Cornification: Disorders of cornification (i.e., alterations in the formation of the stratum corneum) can be primary, such as in primary seborrhea, but more often are secondary to a variety of factors such as inflammation, trauma, or metabolic or nutritional disorders. A disorder of cornification called hyperkeratosis is characterized by an increase in the thickness of the stratum corneum and occurs in two forms: orthokeratotic and parakeratotic hyperkeratosis. In orthokeratotic hyperkeratosis (also referred to as hyperkeratosis), squamous epithelial cells are anuclear, and in parakeratotic hyperkeratosis (also referred to as parakeratosis), the squamous epithelial cells have nuclei. Subtypes of hyperkeratosis include basket weave (exaggerated undulating pattern of layers of the normal stratum corneum), compact (compacted layers of basket weave stratum corneum), and laminated (layers of stratum corneum that are more linear and less undulating). Both parakeratosis and hyperkeratosis are common nonspecific responses to chronic stimuli (e.g., superficial trauma, inflammation, or sun exposure) and also occur as primary lesions. For example, hyperkeratosis is a feature of the cornification disorder, primary seborrhea, of the cocker spaniel (Fig. 17-9), ichthyosis, and vitamin A deficiency. Diffuse parakeratosis is a feature of zinc-responsive dermatosis and superficial necrolytic dermatopathy (hepatocutaneous syndrome) (Fig. 17-10). Hyperkeratosis and parakeratosis can be accompanied by alterations in the thickness of the granular cell layer (stratum granulosum). Generally, hyperkeratosis is associated with an increased thickness of the granular cell layer (hypergranulosis), and parakeratosis is associated with a decreased thickness of the granular cell layer (hypogranulosis).
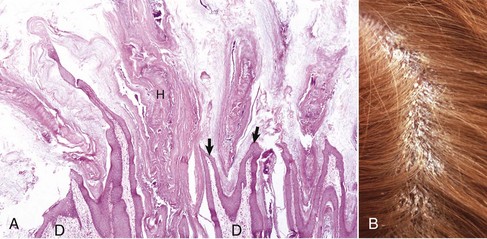
Fig. 17-9 Primary idiopathic seborrhea, skin, haired, dog.
A, Note the marked orthokeratotic hyperkeratosis (H). The stratum corneum within the hair follicles is increased in quantity, extends through the follicular opening to the external skin surface, and distends follicular openings (arrows), creating a papillomatous appearance in the epidermis. D, Dermis. H&E stain. B, Hair is parted to reveal excessive scaling, the result of orthokeratotic hyperkeratosis. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
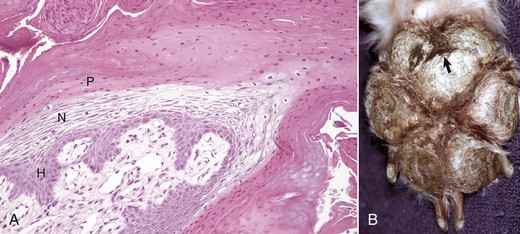
Fig. 17-10 Superficial necrolytic dermatitis, skin, dog.
A, Nuclei of the thickened stratum corneum have been retained (parakeratotic hyperkeratosis [parakeratosis]). The epidermis has a trilaminar pattern (red, white, and blue) created by three layers: (1) parakeratotic layer (P), (2) subparakeratotic edema/necrolytic layer (N), and (3) deep epidermal hyperplastic layer (H). The pathogenesis of superficial necrolytic dermatitis is not completely understood but is speculated in most cases to be the result of an underlying systemic disease (such as severe liver disease or diabetes mellitus) that interferes with the normal nutrient metabolism needed to form a healthy epidermis. H&E stain. B, Pawpad. Note the fissure (arrow) and crusts. The crusting is largely a result of the parakeratosis. Secondary infections by bacteria, yeast, and fungi can also contribute to the formation of crusts by causing fluid, leukocytes, and other cellular debris to accumulate on the surface. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
Epidermal Hyperplasia: Epidermal hyperplasia is an alteration in epidermal growth or differentiation characterized by an increase in the number of cells within the epidermis, most often within the stratum spinosum, and is also referred to as acanthosis. Hyperplasia is a response common to a variety of stimuli, often chronic, and occurs in a variety of types, including regular, irregular, papillated, and pseudocarcinomatous (pseudoepitheliomatous) (Fig. 17-11). Some forms of epidermal hyperplasia (regular, irregular, and pseudocarcinomatous) can develop in sequence. In early stages of epidermal hyperplasia, the epidermal dermal interface is mildly undulating, but as the hyperplasia progresses, there often is an elongation of the rete ridges (ridges that extend into the dermis and interdigitate with dermal papillae) that can be regular or irregular. In regular epidermal hyperplasia, the rete ridges are approximately evenly sized and shaped, whereas in irregular epidermal hyperplasia, the rete ridges are less uniform. Pseudocarcinomatous hyperplasia is a chronic and late stage of epidermal hyperplasia that develops after milder forms (regular or irregular). It refers to marked hyperplasia of the epidermis, resulting in many branching and anastomosing epidermal ridges that deeply interdigitate with dermal collagen fibers. Mitotic figures can be numerous in proliferating basal cells, but in contrast to squamous cell carcinoma, the keratinocytes maintain normal polarity, are not atypical, and do not penetrate the basement membrane. Pseudocarcinomatous hyperplasia develops after chronic injury, as seen with chronic dermal suppurative or granulomatous inflammation or at the edges of persistent, nonhealing ulcers. Psoriasiform epidermal hyperplasia is an exaggerated type of regular epidermal hyperplasia in which the epidermis forms elongated rete ridges that are of similar length and width and that interdigitate with similarly elongated dermal papillae. This type of hyperplasia has very regular or uniform histologic appearance and is a feature of certain disease syndromes, such as psoriasiform lichenoid dermatitis of the Springer spaniel and porcine juvenile pustular psoriasiform dermatitis (pityriasis rosea). Papillated epidermal hyperplasia is a unique form of epidermal hyperplasia in which fingerlike (papillary) projections of epidermis develop above the skin surface; it is a feature of some papillomas, hamartomas, and calluses.
Fig. 17-11 Schematic diagram of the patterns of epidermal hyperplasia.
Exaggerated regular epidermal hyperplasia is also called psoriasiform epidermal hyperplasia, named after the human condition psoriasis, which is characterized by this type of epidermal hyperplasia. (Adapted from Ackerman AB, Boer A, Bennin B, et al, eds: Histologic diagnosis of inflammatory skin diseases, ed 3, New York, 2005, Ardor Scribendi.)
Dyskeratosis: Dyskeratosis is an alteration in epidermal differentiation characterized by premature keratinization of cells in the viable layers of the epidermis. Dyskeratotic keratinocytes are shrunken, are separated from adjacent keratinocytes, and have a pyknotic nucleus and brightly eosinophilic cytoplasm because of the accumulation of keratin filaments. Dyskeratosis occurs when epidermal maturation is deranged such as in zinc-responsive dermatosis. Dyskeratosis is also a feature of epidermal dysplasia, which is a premalignant change in solar (actinic) keratoses.
Apoptosis: Apoptosis refers to programmed cell death. Apoptotic keratinocytes have the morphologic features of dyskeratotic cells. However, in apoptosis, the cytoplasmic eosinophilia is caused by condensation of cytoplasmic organelles. Apoptotic cells are phagocytosed by adjacent keratinocytes. Phagocytosis before cellular disintegration prevents the development of an acute inflammatory response that would be elicited by the liberated cellular constituents. Thus the process of apoptosis is significantly different from that of necrosis, in which cell lysis releases cellular contents into the extracellular space and causes an inflammatory response. Apoptosis is seen with diseases such as lupus erythematosus and erythema multiforme (Fig. 17-12).
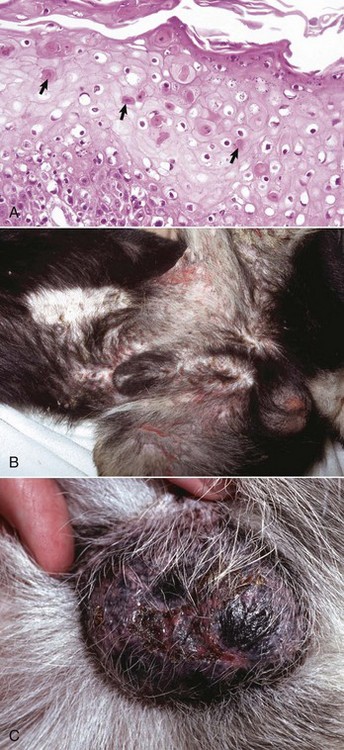
Fig. 17-12 Erythema multiforme, skin, dog.
A, Apoptotic keratinocytes (arrows) are present in the cellular layers of the epidermis. The increased staining intensity of apoptotic keratinocytes is a result of condensation of cytoplasmic organelles and nuclei. H&E stain. B, Skin of abdomen, inguinal region, and scrotum. Note the circular and linear erosions. The clinical lesions resulting from apoptotic keratinocytes depend on the prevalence and location of the apoptotic cells in the epidermal strata. Numerous deeply located apoptotic keratinocytes can lead to loss of part of the epidermis, resulting in erosions that develop into ulcers. C, Scrotum, erythema, ulceration, and crusting are present. Crusts form because the ulceration (injury) results in the release of inflammatory mediators, leading to the accumulations of fluid and cellular exudate that cover and dry on the ulcerated surface. (A courtesy Dr. Ann M. Hargis, DermatoDiagnostics. B courtesy Clinical Dermatology Service, College of Veterinary Medicine, University of Florida. C courtesy Dr. Alexander Werner, Valley Veterinary Specialty Service.)
Necrosis: Necrosis refers to the death of cells and is characterized by nuclear pyknosis (shrunken and dense nucleus), karyorrhexis (nuclear membrane rupture with fragmentation and release of contents), or karyolysis (complete dissolution of the nucleus with loss of chromatin material), organelle swelling, plasma membrane rupture, and release of cytoplasmic elements into the extracellular space accompanied by an acute inflammatory response. Causes of epidermal necrosis include physical injury (lacerations, thermal burns), chemical injury (irritant contact dermatitis), and injury as a result of ischemia and infarction (vasculitis, thromboembolism). Necrosis of the epidermis can result in erosion (loss of a superficial portion of the epidermis) or ulceration (loss of an area of the entire epidermis and portion of the dermis).
Dysplasia: Dysplasia is defined as abnormal development. Classically, it refers to alteration in size, shape, and organization of adult cells (keratinocytes). Dysplasia is a stage of abnormal development that precedes the formation of a noninvasive (in situ) carcinoma. This is the stage of carcinoma that occurs before the abnormal epidermal cells penetrate the basement membrane. The histologic features include irregular stratification of keratinocytes, variation in cell and nuclear size, increase in number of mitoses, dyskeratosis, and large hyperchromatic nuclei.
Epidermal Atrophy: Atrophy is a decrease in the number and size of the cells within the epidermis and occurs as a consequence of sublethal cell injury. Cutaneous atrophy can affect the epidermis, follicles, sebaceous glands, and dermal collagen and occurs in response to hormonal imbalances, such as hyperadrenocorticism in dogs and cats, partial ischemia, and in severe malnutrition.
Alterations in Epidermal Fluid Balance and Cellular Adhesion:
Edema and Intracellular Fluid Accumulation: Edema refers to fluid accumulation between cells. Intercellular edema of the epidermis is called spongiosis because as the intercellular space expands with fluid, the epidermis develops a “spongy” appearance (Fig. 17-13). The term “spongiosis” is used in other chapters of this book to describe responses to injury that are unique to other tissues or organ systems, and in those circumstances the term “spongiosis” has different meanings. Severe intercellular edema of the epidermis results in the formation of spongiotic vesicles, which are variably sized vesicular spaces in the epidermis. The spongiotic vesicles often blend with intercellular spaces that are also widened but to a lesser degree; thus intercellular bridges are often prominent between keratinocytes bordering spongiotic vesicles. Spongiosis is common in epidermal inflammation (epidermitis) caused by staphylococci or Malassezia sp.
Fig. 17-13 Edema, skin, dog.
A, Intercellular epidermal edema. Note the appearance of “spines” between keratinocytes (arrows) of the stratum spinosum caused by widening of the intercellular spaces by edema. The keratinocytes remain connected to each other via desmosomal attachment sites (see Fig. 17-4). H&E stain. B, Acute allergic otitis, ear. Note erythema from dermal congestion (hyperemia) and the moist glistening surface of the skin from dermal and epidermal edema. (A courtesy Dr. Ann M. Hargis, DermatoDiagnostics. B courtesy Dr. David Duclos, Animal Skin and Allergy Clinic.)
Intracellular fluid accumulation results in cytoplasmic swelling of keratinocytes, and if the swelling is severe, the swollen keratinocytes can burst, forming microvesicles supported by the walls of the ruptured cells. This type of epidermal damage is termed reticular degeneration. Intracellular fluid accumulation limited to the basal layer of the epidermis is termed hydropic or vacuolar degeneration and can result in the formation of intrabasilar vesicles. Hydropic degeneration is a consequence of damage to basal keratinocytes when the basal keratinocytes cannot maintain normal homeostasis, and fluid accumulates within the cells (Fig. 17-14). Examples of diseases commonly resulting in hydropic degeneration include lupus erythematosus, dermatomyositis, and drug eruptions. Ballooning degeneration, a form of intracellular fluid accumulation of keratinocytes in more superficial layers of the epidermis, such as the stratum spinosum, is characterized by swollen cells losing their intercellular attachments. This type of degeneration can result in the formation of a fluid-filled vesicle. Viruses that infect cells of the epidermis, such as the pox and parapox viruses, can cause lysis of cytoplasmic keratin and a buildup of excessive fluid, resulting in ballooning degeneration (see the section on Pathologic Reactions of the Entire Cutaneous Unit).
Fig. 17-14 Hydropic degeneration, skin, dog.
A, Note vacuolization of the cells of the basal layer (arrows). The vacuolated basal cells have resulted in a cleft (arrowhead) between the epidermis and dermis. A few lymphocytes are also present in the superficial dermis and lower layers of the epidermis. H&E stain. B, Dermatomyositis, periocular. Erythema, depigmentation, erosion, and crusting are the result of injury to basal cells. The vacuolar degeneration of basal cells weakens the epidermal dermal attachment and results in formation of vesicles, erosions, and ulcers. In addition, inflammatory mediators are released, resulting in fluid and cellular exudate, which dry on the surface and form crusts. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
Acantholysis: Acantholysis is the disruption of intercellular junctions (desmosomes) between keratinocytes of the epidermis. The process is initiated by damage to transmembrane glycoproteins belonging to the cadherin family of adhesion molecules and leads to splitting of the extracellular core of the desmosomes. Subsequently, the desmosomal plaques dissolve and intermediate filaments retract to the perinuclear region of the keratinocytes. Acantholysis occurs with immune-mediated injury, as seen in pemphigus (type II cytotoxic hypersensitivity) or with neutrophilic enzymatic destruction, as seen in superficial pyoderma or uncommonly with some Trichophyton sp. infections. The microscopic lesions vary with the location of acantholysis within the various layers of the epidermis. In pemphigus foliaceous, acantholysis occurs in the subcorneal epidermis, resulting in release of free-floating keratinocytes in subcorneal vesicles and pustules (Fig. 17-15). In pemphigus vulgaris, acantholysis occurs in the epidermis, just above the basal layer, resulting in the separation of the upper epidermis from the basal cells (which are often referred to as a row of tombstones) attached to the basal lamina (Fig. 17-16). Fluid accumulating between the separated layers of the epidermis forms vesicles of varied sizes and shapes.
Fig. 17-15 Pemphigus foliaceous, skin.
A, Horse. The subcorneal pustule contains neutrophils and acantholytic cells (arrows), which are epidermal cells separated from each other as a result of the loss of desmosomal attachments. In pemphigus foliaceous, the pustule is located in the superficial epidermis, resulting in clinical lesions that are superficial (pustules that can rupture to form erosions and crusts). The roof of the pustule is the stratum corneum, and the base of the pustule is the stratum spinosum. Forceful clipping or scrubbing the surface of the pustule can lead to rupture and thus can make the sample nondiagnostic. H&E stain. B, Inguinal region, dog. Multiple pustules (circumscribed accumulations of pus in the epidermis visible as irregularly ovoid, slightly elevated tan areas) are present in the sparsely haired skin of the inguinal region. The skin within the black ellipse has been injected with local anesthetic in preparation for biopsy sampling. C, Face, dog. Erythema, alopecia, focal erosion, crusting, and depigmentation are present on medial surface of the pinnae, periocular skin, and dorsum of the muzzle and the nasal planum. Crusts develop as the result of upward growth of the epidermis and disruption of pustules. Erosions develop as the result of loss of stratum corneum and pustular exudate, which exposes the stratum spinosum. Depigmentation can result from inflammation and damage to pigment containing epidermal cells. D, Pawpad, dog (same dog as in C). Erosions (arrows), depigmentation, and crusts are present. (A courtesy Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida. B courtesy Dr. David Duclos, Animal Skin and Allergy Clinic. C and D courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
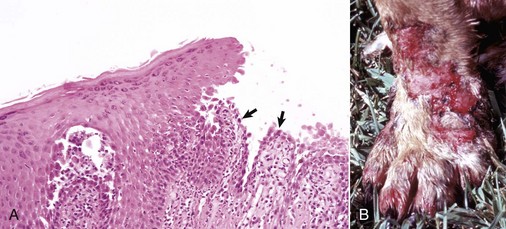
Fig. 17-16 Pemphigus vulgaris, skin, dog.
A, Suprabasilar clefting has left a row of basal cells (arrows) attached to the dermis. The single row of basal cells is fragile and easily damaged leading to formation of ulcers, with subsequent fluid loss and secondary bacterial infection. H&E stain. B, Leg. Note the erythema and large confluent areas of ulceration. In contrast to pemphigus foliaceous (more commonly characterized by erosions and crusts), pemphigus vulgaris is characterized by larger more confluent ulcers because the acantholysis in pemphigus vulgaris occurs deeper in the epidermis (in the cells of the lower epidermis). Vesicles are not frequently seen as they rapidly progress to ulcers, the more common clinical lesion. (A courtesy Dr. Ann M. Hargis, DermatoDiagnostics. B courtesy Dr. Alan Mundell, Animal Dermatology Service.)
Vesicles: Vesicles are fluid-filled cavities within or beneath the epidermis (Web Fig. 17-5). If the cavity is less than 1 cm in diameter, it is called a vesicle; if it is greater than 1 cm in diameter, it is called a bulla (pl. bullae). Vesicles can develop in any layer of the epidermis or beneath the epidermis and can form as the result of acantholysis, epidermal or dermal edema, degeneration of basal cells and keratinocytes, or other processes, such as immune mediated targeting of components of the basement membrane, or frictional trauma and burns that damage proteins and lead to a lack of cohesion between the epidermal cells or between the epidermis and dermis, resulting in the accumulation of fluid within a cavity. The location of vesicles or bullae within the layers of the epidermis is suggestive of certain diseases. For instance, intraepidermal vesicles can occur in viral infections, subcorneal vesicles in pemphigus foliaceous, suprabasilar vesicles in pemphigus vulgaris (see Fig. 17-16), and subepidermal vesicles in bullous pemphigoid or thermal burns (Fig. 17-17).
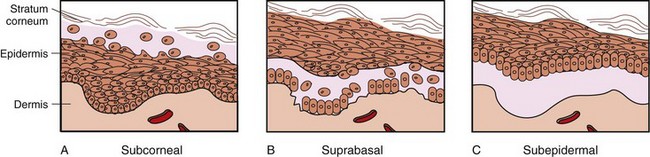
Fig. 17-17 Schematic diagram of the sites of vesicle formation in the skin.
A, In a subcorneal vesicle, the stratum corneum forms the roof of the vesicle (as in impetigo or pemphigus foliaceous). B, In a suprabasal vesicle, a portion of the epidermis (stratum spinosum) forms the roof (as in pemphigus vulgaris). C, In a subepidermal vesicle, the entire epidermis separates from the dermis and forms the roof (as in bullous pemphigoid). (From Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
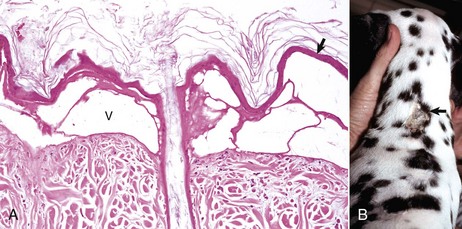
Web Fig. 17-5 Thermal burn, full thickness (third-degree) skin, dog.
A, There is necrosis of the epidermis (arrow), follicular epithelium, and dermis. Because of increased capillary permeability, fluid has accumulated between the dermis and epidermis, forming vesicles (V). H&E stain. B, The dry necrotic skin is the site of the burn (arrow). (Courtesy Blackwell Publishing.)
Inflammatory Lesions of the Epidermis: Acute inflammation of the epidermis actually begins in the dermis with active hyperemia, edema, and migration of leukocytes, often neutrophils (see inflammatory disorders of the dermis section). The edema fluid arises from dilated venules and can move intercellularly through the epidermis, widening the intercellular spaces and causing spongiosis. In thermal burns of the skin, larger quantities of fluid accumulate within or below the epidermis forming vesicles; the fluid reaching the epidermal surface dries to form a largely acellular crust. The leukocytes (often neutrophils in acute inflammation) migrate from the superficial dermal vessels, through the superficial dermis, and into the intercellular spaces of the deep and then superficial layers of the epidermis. The aggregation of migrating leukocytes in the epidermis is termed exocytosis. Exocytosis of leukocytes is common in inflammation and is usually accompanied by spongiosis. If the inflammation progresses, the migrating leukocytes form pustules within the epidermis or the stratum corneum. Pustules usually dry rapidly and become crusts (Fig. 17-18). The type of leukocyte recruited into the epidermis is influenced by complex interactions of cytokines involved in the pathogenesis of the disease and can be useful in classifying and ultimately diagnosing the disease. For instance, intraepidermal eosinophils can be seen in association with ectoparasite bites. Lymphocytic infiltrates into the epidermis are often seen with immune-mediated diseases such as lupus erythematosus. Malignant lymphoma affecting the epidermis is also characterized by intraepidermal lymphocytes. Erythrocytes can also be present in the epidermis, usually associated with trauma, or circulatory disturbances, such as marked vasodilation and vasculitis.
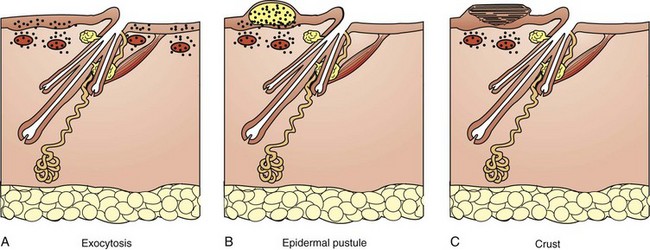
Fig. 17-18 Schematic diagram of the inflammatory patterns of the epidermis.
A, Leukocytes (black dots) migrate from the perivascular dermis into the epidermis, a process called exocytosis. B, Leukocytes migrate into the epidermis and accumulate to form a pustule. C, The pustule dries to form a crust. (Redrawn from Dr. Ann M. Hargis, DermatoDiagnostics; and Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida.)
Pustules: Pustules (microabscesses) are accumulations of inflammatory cells (pus) within the epidermis (see Fig. 17-15). Epidermal pustules vary in inflammatory cell content and location in the epidermis, depending on the pathogenesis of the disease. The pustules of superficial bacterial infections generally contain degenerate neutrophils and coccoid bacteria and are often located beneath the stratum corneum (subcorneal). In ectoparasitic hypersensitivity, pemphigus foliaceous, and feline eosinophilic plaque, the pustules can be filled with eosinophils. Small pustules containing neoplastic lymphocytes (Pautrier’s microabscesses) are present in epitheliotropic lymphoma.
Crusts: Crusts are composed of dried fluid and cellular debris (i.e., dried exudates) located on the epidermal surface; thus crusts are indicative of a previous exudative process. Crusts are not specifically diagnostic but can hold the key to diagnosis in some diseases. For example, in dermatophilosis, the most diagnostic portion of the skin sample is the crust, which is multilaminated and contains the Gram-positive, branching coccoid organism Dermatophilus congolensis. Similarly, crusts formed through aging of pustules in pemphigus foliaceous are multilaminated and frequently contain numerous acantholytic cells. Crusts can also contain hair shafts infected with spores and hyphae of dermatophytes.
Alterations in Epidermal Pigmentation: Pigmentary alterations include hyperpigmentation, hypopigmentation, and pigmentary incontinence. Melanin is produced by melanocytes located in the basal and lower spinous layers of the epidermis, in the external root sheath and hair matrix of follicles, and perivascularly in the dermis. Melanocytes have surface receptors for hormones, such as melanocyte-stimulating hormone, and these hormones regulate melanogenesis. Other factors that influence the amount of melanin pigment in skin and hair are genes, age, temperature, and inflammation.
Hyperpigmentation: Hyperpigmentation results from an increased production of melanin from existing melanocytes or an increase in the number of melanocytes. An example of hyperpigmentation caused by an increased number of melanocytes is a lentigo, a rare localized nonneoplastic proliferation of melanocytes confined to the epidermis and resulting in formation of a black macular circumscribed lesion that is usually less than 1 cm in diameter. Most hyperpigmentation of the epidermis results from increased production of melanin from existing melanocytes. Possible mechanisms of increased melanin production include increases in the rate of production of melanosomes (i.e., granules within melanocytes that contain tyrosinase and synthesize melanin), in melanosome size, in rates of melanosome transfer to keratinocytes and increased survival of melanosomes in keratinocytes. Examples of epidermal hyperpigmentation by increased production of melanin include chronic inflammatory diseases (most common cause), such as chronic allergic dermatitis, and endocrine dermatoses, such as hyperadrenocorticism. Hyperpigmentation secondary to inflammation is thought to result from release of melanocyte-stimulating factors from keratinocytes. It is thought that these factors are present in normal epidermis, but that their level or activity is increased in response to stimulation or keratinocyte stress.
Hypopigmentation: Hypopigmentation can be congenital or hereditary and develops because of a lack of melanocytes, failure of melanocytes to produce melanin, or failure of transfer of melanin to epidermal cells. Hypopigmentation can also be acquired via a loss of existing melanin or melanocytes (depigmentation). Because copper is a component of tyrosinase, production of melanin pigment depends on copper, and copper deficiency can result in reduced pigmentation.
Pigmentary Incontinence: Pigmentary incontinence refers to the loss of melanin pigment from the basal layer of the epidermis or outer root sheath or bulb of the hair follicles caused by damage to the cells of the basal layer or of the follicular components and the accumulation of the pigment in macrophages in the upper dermis or perifollicular regions, respectively. Basal layer pigmentary incontinence can be a nonspecific lesion associated with inflammation; however, it is also seen with diseases that specifically damage basal cells or melanocytes such as lupus erythematosus or vitiligo. Perifollicular pigmentary incontinence occurs in diseases in which inflammation targets the follicular wall, such as demodicosis, or there is abnormal growth or development of hair follicles, such as in some types of follicular dysplasia. Leukotrichia and leukoderma (Fig. 17-19) refer to decreased pigmentation of hair and skin, respectively.
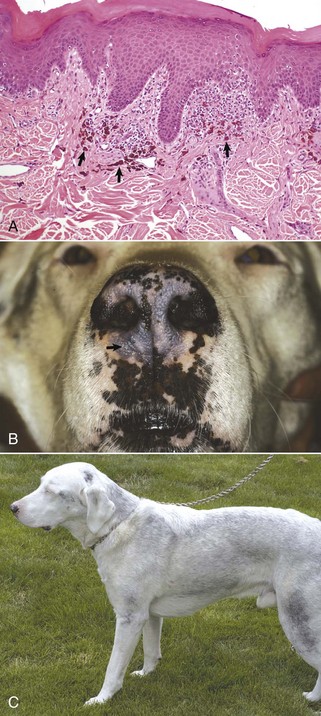
Fig. 17-19 Leukoderma, skin, dog.
A, Injury to the epidermal pigment containing cells (predominantly melanocytes) has caused loss of melanin pigment in the epidermis (leukoderma). The pigment from the damaged melanocytes has been phagocytosed by dermal macrophages (pigmentary incontinence) (arrows). Mild to moderate lymphocytic inflammation is present along the epidermal dermal interface. H&E stain. B, Nose and lips. Areas of the normally black skin of the planum nasale and lips have been partially (bluish-gray) or totally (pink) depigmented. The bluish-gray areas are in the process of becoming depigmented. Biopsy samples should be collected from the bluish-gray areas (arrow) to identify active inflammation and diagnostic lesions. Biopsy samples from black skin or from already totally depigmented skin will likely only show normal appearing black or nonpigmented skin, respectively, with no evidence of inflammation. C, Leukotrichia, body. This dog is the same one depicted in B. He was a black Labrador retriever mix. More than 90% of the black hair coat became white (leukotrichia). Biopsy samples from white-haired areas may be normal because the inflammatory event that caused the pigment loss is likely gone by the time nonpigmented hair shafts emerge from the hair follicles. (A courtesy Dr. Ann M. Hargis, DermatoDiagnostics. B and C courtesy Dr. David Duclos, Animal Skin and Allergy Clinic.)
Responses of the Dermis to Injury
Alterations in Growth, Development, or Tissue Maintenance:
Dermal Atrophy: Dermal atrophy results from a decrease in the quantity of collagen fibrils and fibroblasts in the dermis and leads to a decrease in the thickness of the dermis noted clinically by thin, translucent skin with more visible vasculature. The principal causes of dermal atrophy in domestic animals are catabolic diseases associated with protein degradation, such as hyperadrenocorticism (particularly in dogs and cats), and starvation. In cats with hyperadrenocorticism, collagen loss is sufficient to increase fragility of the skin, which tears with normal handling. Severe dermal atrophy can also be caused by repeated topical application of glucocorticoids.
Fibrosis: Fibrosis (fibroplasia) develops in response to various injuries, particularly ulceration of the epidermis. It consists of proliferation of fibroblasts and newly formed collagen fibrils (extracellular matrix). In the early stage of fibroplasia (called granulation tissue), the long axis of the fibroblasts and collagen fibrils are parallel to the surface of the skin and are oriented perpendicular to vertically aligned proliferative vessels. Clinically, the capillaries are seen on the surface as minute red dots that create a “granular” appearance, thus the name “granulation tissue.” Microscopically, the orientation pattern provides a “lattice-work” appearance to the tissue (Fig. 17-20). Fibrosis refers to the gradual deposition and maturation of collagen to form a scar. During fibrosis, collagen production increases and fibroblast and capillary numbers decrease, resulting in less cellular dense collagen oriented in thick hyaline bundles in parallel arrangement (e.g., a scar), which grossly appear white and glistening.
Fig. 17-20 Granulation tissue, skin, horse.
A, Note vertically oriented capillaries (C) and horizontally oriented fibroblasts and a few collagen fibers providing a “lattice-work” appearance to the granulation tissue. B, Leg. Note ulcerated area filled with granulation tissue. C, Exuberant granulation tissue, leg. Granulation tissue is a normal component of wound healing. However, excessive or exuberant granulation tissue can develop as a pathologic process. This condition occurs especially in horses, when an ulcer fails to become reepithelialized. Although this process is commonly called “exuberant granulation tissue,” at the stage depicted in this photograph, most of the granulation tissue has been converted to fibrous connective tissue. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Collagen Dysplasia: Collagen dysplasia is generally an inherited abnormality of collagen that results in decreased tensile strength and an increased ability of the skin to stretch beyond normal limits. Because the tensile strength is reduced, even minor trauma can cause the skin to tear. Healing results in formation of scars. Microscopic features vary among the different types of collagen dysplasia disorders, and in some the skin has no microscopic alterations. Collagen bundles can vary in size and shape and consist of tangled fibers with an abnormal organizational pattern.
Solar Elastosis: Solar elastosis is caused by chronic exposure of the skin to the UV spectrum of sunlight. Sunlight consists of visible, UV, and infrared light rays. The portion of the UV light most damaging to the skin is ultraviolet B (UVB) and is in the range of 290 to 320 nm. The amount of light reaching the skin is dependent on a variety of environmental and host factors that can greatly influence the geographic incidence and anatomic locations of solar damage. Environmental factors include quantity of ozone, smog, and cloud cover that tend to absorb and scatter some of the UV rays. Altitude and latitude are also very important. The atmosphere at high altitude is thinner, so there is less oxygen and particulate matter to absorb and scatter the UV rays. Latitude is also critically important. It has been estimated that the incidence of sunlight-induced cancer in humans doubles for every 265 miles closer to the equator that humans reside. At high latitudes, the path of sunlight through the ozone layer is longer than at lower latitudes, so the ozone absorbs more of the harmful UV light. The path of sunlight through the ozone layer is also one of the reasons that sunlight has more damaging UV rays in summer months and at midday. Increased wind velocity has also been shown to have an enhancing influence on damaging effects of UV light on the skin. Local environmental factors can influence the quantity of UV light reaching an animal’s skin. Such factors include availability of shelter from sunlight in the form of trees, shade panels, or indoor housing, and color of the ground material (lightly colored sand) that can increase exposure to UV light through reflection. Host factors include quantity of hair, degree of pigmentation, thickness of stratum corneum, and other difficult to define genetic factors. Therefore solar damage is more prevalent in high altitude, low latitude parts of the world and in animals residing outside for long periods of time. Lesions generally develop in poorly haired and lightly pigmented sites. Solar elastosis develops in the superficial dermis of chronically sun-exposed skin, and consists of increased numbers of thick, interwoven, basophilic fibers with the staining characteristics of elastin (thus the name, “solar elastosis”). In the nonpigmented abdominal skin of dogs, solar elastosis may develop intermixed with or below a linear band of scarring parallel to the epidermal surface (laminar fibrosis). The pathogenesis of the development of solar elastosis is complex and not fully understood and may vary with species. Current evidence involving studies in humans and mouse models supports the view that a majority of the elastotic material (elastin, fibrillin, and glycosaminoglycans) is newly formed as the result of altered function of sun-damaged fibroblasts, but that degradation of preexisting dermal matrix proteins, including elastin and also most likely collagen, seems to be involved as well. Solar elastosis is less prominent in the skin of domestic animals than in humans but is often prominent in lightly pigmented, poorly haired sun-exposed skin and eyelids of horses and in the lower eyelids of Hereford cattle residing in sunny locations.
Degenerative Collagen Disorders of the Dermis: The term collagen degeneration has been used to refer to an altered histologic appearance of collagen fibers in hematoxylin and eosin (H&E) stained sections, whereby there is brightly eosinophilic granular to amorphous material bordering the fibers and somewhat obscuring the fiber detail. The collagen fibers and bordering eosinophilic material have also been referred to as flame figures, a result in part to the irregular, sometimes radiating edges and brightly eosinophilic staining intensity. Electron microscopic studies in cats and humans indicate that the collagen fibers can be disrupted, but they are not “degenerate.” Instead, the brightly eosinophilic granular and amorphous material consists of aggregates of many eosinophils and eosinophil granules that border slightly disrupted but otherwise normal collagen fibers. These flame figures are seen in conditions in which eosinophils are prominent, including in reactions to insect bites, mast cell tumors, and eosinophilic granulomas (collagenolytic granulomas) (Fig. 17-21). Collagen lysis refers to dissolution of collagen fibrils morphologically consisting of amorphous, lightly eosinophilic material lacking fibrillar detail. Collagen lysis is likely a secondary event caused by proteolytic enzymes released by a variety of cells, including eosinophils (collagenase) and neutrophils (collagenolytic proteinase, collagenase).
Fig. 17-21 Eosinophilic granuloma, skin, cat.
A, Ulcerated skin (left) with fragmented collagen (arrows) is bordered by degranulated eosinophils. H&E stain. B, Fragmented collagen (arrows) is bordered by a row of macrophages (M), multinucleated giant cells (G), and degranulated eosinophils (E), somewhat resembling a flame (“flame figure”). H&E stain. C, Upper lip. Bilateral ulcers are present on the upper lips, but the ulcer on the right side of the photograph is more extensive (arrow). (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
Disorders Characterized by Abnormal Deposits in the Dermis:
Amyloid: Amyloid is an abnormal proteinaceous substance that can be deposited in a localized site in the body (organ limited) or can be “systemic” where multiple organs are involved. In the systemic form, amyloid deposition can be the result of a primary abnormality of plasma cells (primary amyloidosis), in which case the amyloid is derived from components of immunoglobulin light chains (AL amyloid), or as a result of chronic inflammatory conditions (secondary amyloidosis), in which case the amyloid is derived from serum amyloid-associated protein (SAA protein), an acute-phase reactant produced by the body in response to inflammation. Cutaneous amyloid deposition can occur in association with systemic amyloidosis, but visceral involvement is more common and clinically important. Cutaneous deposition of amyloid is rare but is seen in horses, dogs, and cats. In horses, it can occur in association with secondary systemic amyloidosis or in a localized (organ-limited) form. In organ-limited amyloidosis, the skin and/or upper respiratory tract are typically involved, the condition is not associated with recognized triggering causes, and the amyloid is produced from immunoglobulin light chains (AL amyloid). Clinical lesions vary from papules and plaques to nodules that usually are covered by a normal hair coat. Similar nodules can also develop in the respiratory mucosa and regional lymph nodes. Histologic lesions include nodular to diffuse granulomatous dermatitis and panniculitis with deposition of amyloid, an eosinophilic hyaline material. Cutaneous deposition of amyloid in dogs and cats is usually the localized type, and is seen in association with extramedullary plasma cell tumors (AL amyloid). However, cutaneous deposition of amyloid in dogs also can be triggered by a monoclonal gammopathy (AL amyloid). Clinical and histologic lesions reflect the triggering disease.
Mucin: Mucin (glycosaminoglycan [GAG]), a normal component of the ground substance of the dermis, consists of protein bound to hyaluronic acid and can be deposited in increased quantity in focal areas or diffusely. Because hyaluronic acid has a great affinity for binding water, the skin in cases of mucin deposition (mucinosis) has a thick, puffy appearance. In cases of severe mucinosis, the skin, when pricked with a needle, can exude mucin (a stringy fluid material). In histologic sections, much of the water is lost and mucin appears as fine amphophilic granules or fibrils that separate dermal collagen. Examples of disorders with dermal mucin deposition are termed myxedema in hypothyroidism and mucinosis in the Chinese Shar-Pei dog.
Calcium Deposits: Mineralization is a general term for the deposition of insoluble, inorganic minerals in the cutaneous tissue and usually consists of calcium in combination with phosphate or carbonate. Because the mineral most often deposited is calcium, the terms mineralization and calcification are often used interchangeably. Mineralization can occur in four basic forms: (1) dystrophic, (2) metastatic, (3) idiopathic, and (4) iatrogenic. Mineralization can also occur in tissues that have been traumatized, which may encompass more than one of these pathogenic mechanisms. Dystrophic mineralization occurs as a result of injury or degeneration of cellular and extracellular components of the skin, with normal serum calcium levels, and without abnormalities in calcium metabolism. Examples include deposition of mineral in granulomas and calcinosis cutis seen in some cases of hyperadrenocorticism. In metastatic mineralization, the calcium deposits develop without preceding tissue injury or degeneration and in association with hypercalcemia, usually the result of abnormal metabolism of calcium, phosphorus, or vitamin D. Examples include deposition of calcium salts in soft tissues in chronic renal disease and in poisoning with cholecalciferol (vitamin D3). Mitochondria have a high affinity for calcium and phosphate, and participate in both dystrophic and metastatic calcification by concentrating intracellular calcium and phosphate to levels that allow crystallization. Mechanisms leading to calcium deposition in dystrophic mineralization involve reduced pH of injured tissue, the influx of calcium into injured cells, and then into mitochondria. Metastatic calcification may be the result of increased extracellular calcium levels and the failure of the cell’s normal ability to strictly regulate intracellular calcium with resultant accumulation within mitochondria. Mineralization can affect individual or groups of collagen fibers, resulting in increased basophilia and fragmentation of fibers in H&E-stained sections. The causes of idiopathic mineralization are not known; it occurs in the absence of tissue injury or abnormalities in calcium or phosphorus metabolism. Calcinosis circumscripta is a form of tissue mineralization in which calcium is deposited as amorphous nodular aggregates in the soft tissues of the tongue and in the subcutis of the pawpads of dogs. The pathogenesis is not defined, but some cases are considered idiopathic, whereas others may be the result of tissue trauma. Calcium deposits can elicit a granulomatous inflammatory response, a foreign-body reaction to the deposits. Iatrogenic mineralization develops via direct exposure to calcium salts such as occurs with topical exposure to calcium chloride deicing solutions.
Inflammatory Disorders of the Dermis: Dermatitis is inflammation of the dermis. Acute dermatitis begins with active hyperemia (increased blood flow), edema, and migration of leukocytes and results from release of cytokines and other mediators of acute inflammation (see Chapter 3). Active hyperemia is caused by vasodilation of arterioles, which causes increased blood flow at reduced velocity to capillary beds and postcapillary venules. Edema is caused by increased vascular permeability. Fluid leaves vessels mostly through widened interendothelial cell junctions. With a mild increase in vascular permeability, the edema fluid is clear (serous) because there are very few plasma proteins in the fluid. With increasing vascular permeability or endothelial cell injury, larger protein molecules, such as fibrinogen, escape from vessels, and the edema fluid becomes more eosinophilic and amorphous to fibrillar (fibrinous). The next step in acute dermatitis is migration of leukocytes from vessels into the perivascular dermis. The slowing of the blood flow and the endothelial cell expression of adhesion molecules that bind circulating leukocytes permit the migration of leukocytes in acute inflammation. The slowing of the blood flow allows leukocytes to move from the center of the vessel, where blood flow is fastest, to the margin, where they contact and attach to the activated endothelial cells.
After attaching to activated endothelial cells, leukocytes migrate between the endothelial cells into the perivascular dermis. The type of leukocyte that migrates and the sequence of cellular influx depend on activation of different adhesion molecules and chemotactic factors in the different phases of inflammation. In many types of acute inflammation, neutrophils are one of the first cells to arrive. Neutrophils predominate in the first 6 to 24 hours of injury and are generally replaced by macrophages in 24 to 48 hours. This sequence of cellular exudation can vary. For example, in reactions mediated by IgE cross-linking, such as type I hypersensitivity responses, mast cells (located in the perivascular dermis) are stimulated to release contents of granules (occurs in seconds) and synthesize and release inflammatory mediators (prostaglandins, leukotrienes, and cytokines), resulting in the influx of eosinophils, basophils, CD4+ TH2 lymphocytes, and macrophages. Mast cell degranulation and TH2 lymphocyte activation cause eosinophils to accumulate in large numbers. Thus eosinophils often comprise the majority of leukocytes in inflammatory reactions against parasites and other allergic reactions.
Acute dermatitis typically results in one of four outcomes. First, there can be complete resolution, which occurs when the inciting stimulus has short duration and there is little tissue damage that is completely repaired. Second, an abscess can form, which occurs with pus-producing (pyogenic) bacterial infections. Third, healing occurs by replacement of the injured area by fibrous connective tissue (e.g., scarring), which occurs when there is significant tissue destruction (such as a deep burn) in which the parenchymal tissues are lost and thus cannot regenerate. Fourth, there is progression of acute dermatitis to chronic dermatitis.
Chronic dermatitis is inflammation of the skin that lasts weeks or months. Histologic features of chronic dermatitis include accumulation of macrophages, lymphocytes, and plasma cells; tissue destruction in part caused by the inflammatory cells; and a reparative host response of fibrosis and angiogenesis. Chronic dermatitis is usually caused by persistent infections often associated with delayed hypersensitivity and the formation of granulomas (e.g., Mycobacteria sp.), presence of foreign material in the skin (e.g., embedded suture), or autoimmune reactions in which self-antigens provoke an ongoing immunologic inflammatory response against host tissue (e.g., lupus erythematosus). Macrophages are a key cell in chronic dermatitis. They arise from monocytes in the peripheral blood and mature to macrophages whose primary function is phagocytosis. Macrophages also become activated by a variety of chemical mediators, including the cytokine IFN-γ secreted by sensitized T lymphocytes. When activated, macrophages also secrete many mediators of tissue injury (toxic oxygen metabolites, proteases, and coagulation factors) that contribute to chronic inflammation and fibrosis (growth factors, angiogenesis factors, and collagenases). The presence of lymphocytes and plasma cells in chronic inflammation is indicative of a host immune response.
The inflammatory milieu in the progression of acute and chronic dermatitis can be further complicated by other superimposed factors, notably physical injury from self-trauma, secondary bacterial infection of traumatized surface, injury from insect bites attracted by odor or exudate, and moderation by the host immune response or therapy. Thus dermal inflammatory responses to different stimuli often have overlapping histologic features, providing a diagnostic challenge for the dermatopathologist. Even so, the distribution of leukocytes often evolves into recognizable patterns that, when combined with the inflammatory cell type and other morphologic changes, suggest a group of differential diagnoses or the cause or pathogenesis of a specific disease (see Tables 17-1 and 17-2). Dermal patterns of inflammation that have been used in histologic diagnosis include perivascular dermatitis, interface dermatitis (inflammation affecting the basilar epidermis and superficial dermis that often obscures the epidermal dermal interface), nodular to diffuse dermatitis with infectious agents, and nodular to diffuse dermatitis without infectious agents (Fig. 17-22). For example, perivascular dermatitis with eosinophils is suggestive of hypersensitivity associated with parasites or other antigens; interface dermatitis with lymphocytes is suggestive of an immune response directed toward epidermal cells, such as lupus erythematosus or erythema multiforme; and nodular dermatitis with macrophages (granulomatous dermatitis) indicates a persistent stimulus, such as infection with acid-fast bacteria or fungi. Thus patterns of inflammation combined with cellular composition of infiltrates are useful in microscopic diagnosis.
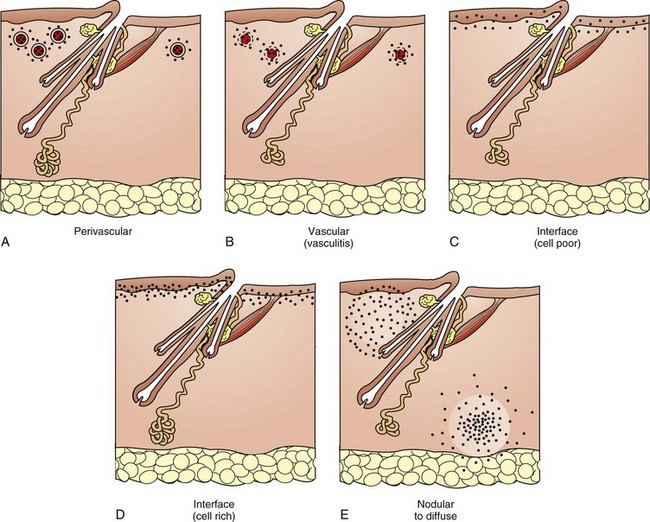
Fig. 17-22 Schematic diagram of the inflammatory patterns of the dermis.
A, Perivascular: Leukocytes (black dots) migrate from the dermal vessels into the perivascular dermis. This is the least specific pattern because all inflammatory patterns are at one stage, perivascular. B, Vascular (vasculitis): Leukocytes target the vessel wall, resulting in necrosis, inflammation, leakage of fibrin and red blood cells, and if severe, thrombosis and infarction. C, Interface, cell poor: Mild, often lymphocytic, inflammation located along the epidermal dermal interface with vacuolar or apoptotic degeneration of basal cells. D, Interface, cell rich: Dense band of inflammation along the epidermal dermal interface, obscuring the basilar layer of the epidermis, and with vacuolar or apoptotic degeneration of basal cells. E, Nodular to diffuse with or without microorganisms. Inflammation, typically granulomatous to pyogranulomatous, which partially effaces the architecture of the dermis may be associated with infectious agents or may be sterile. (Redrawn from Dr. Ann M. Hargis, DermatoDiagnostics; and Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida.)
Responses of the Adnexa to Injury
The term adnexa refers to appendages or adjunct parts, which in the skin includes hair follicles and glands. The major clinical and histologic changes involve the hair follicles, and thus follicular lesions are emphasized in the next discussion.
Alterations in Maintenance, Growth, and Development:
Atrophy: Atrophy refers to gradual reduction (involution) in size and can be physiologic or pathologic. Physiologic atrophy is related to the normal progression of the hair follicle cycle (i.e., the hair cycle stages of growth [anagen], transition [catagen], rest [telogen], and shed [exogen]). Pathologic atrophy occurs when the degree of atrophy is greater than that expected for a given stage of the hair cycle. Causes of follicular atrophy include hormonal abnormalities, nutritional abnormalities, inadequacy of blood supply, inflammation, and general state of health, including stressful events or systemic illness. Some types of pathologic atrophy can be reversed when the underlying cause is corrected. Damage to germinal epithelium can result in destruction or total loss of the adnexa with replacement by a scar. Examples include extensive inflammation and disruption of the follicle (folliculitis and furunculosis) and bordering glands and dermis that destroy a significant portion of the adnexal germinal epithelium, thermal burns sufficiently deep to involve the adnexa and dermal vessels, thrombosis causing infarction (i.e., diamond skin disease in pigs), and severe physical trauma such as lacerations that remove components of the skin, including adnexa.
Hypertrophy: Hypertrophy is an increase in the unit size of a structure or an individual cell. Follicular hypertrophy, which results in follicles that are longer and wider than normal for the site, develops secondary to repeated surface trauma such as in acral lick dermatitis. Hyperplasia is an increase in the number of cells in a structure. Enlargement of adnexa, a common response to injury, usually involves both hypertrophy and hyperplasia and is observed in follicles and sebaceous and apocrine glands associated with chronic allergic dermatitis.
Abnormalities of Hair Cycle Stages: Abnormalities of hair cycle stages occur when there is disruption in the normal progression from the anagen, catagen, telogen, and exogen stages of the hair cycle (see Fig. 17-6). Clinical and histologic lesions are diverse. Some animals have failure of hair to regrow after clipping (hypothyroidism, postclipping alopecia). Other animals have sudden shedding of hair coat (synchronous change of many follicles from telogen into anagen, resulting in loss of old telogen hair shafts as new hair shafts emerge, termed telogen effluvium). Yet other animals have gradual but progressive loss of hair shafts associated with endocrine disease such as hyperadrenocorticism.
Follicular Dysplasia: Follicular dysplasia refers to incomplete or abnormal development of follicles and hair shafts and is usually an inherited abnormality of the hair follicles in which there is failure of hair growth, production of defective hair shafts, or failure to maintain hair within follicles. The conditions may be congenital or tardive (late occurring). Different types of follicular dysplasia syndromes are described, but most are poorly characterized. Microscopic features vary but include abnormal keratinocytes in the hair matrix or abnormally formed follicles, which produce abnormal hair shafts, resulting clinically in a reduced or absent hair coat. The lesions of follicular dysplasia that are most easily recognized microscopically are color associated and include color mutant alopecia (color dilution follicular dysplasia) and black hair follicular dysplasia (Fig. 17-23), in which melanin pigment abnormalities serve as a marker for the dysplasia. These pigment abnormalities include abnormally clumped melanin pigment granules in melanocytes in the epidermis and follicular hair matrix cells; irregularly sized, shaped, and distributed melanin pigment clumps in hair shafts; and macrophages containing melanin pigment and located in the perifollicular dermis. The pathogenesis of the abnormal hair coat appears to involve breakage of hair shafts in areas of large pigment clumps, an abnormality of development of the cuticle, and also possibly an abnormality of hair shaft development caused by damaged hair matrix cells.
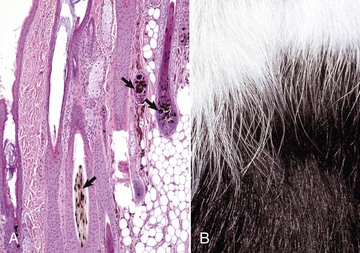
Fig. 17-23 Black hair follicular dysplasia, skin, dog.
A, Large and variably sized and shaped melanin pigment granules are present in hair shafts and hair matrix cells (arrows). Macrophages that have phagocytosed melanin pigment are present in the dermis, most prominently near hair bulbs. The melanin presumably comes from damaged hair matrix cells. H&E stain. B, Note alopecia in black-haired area and normal hair coat in white-haired area. The skin in the black-haired area is pigmented; thus alopecia is not easily recognized from a distance. (A courtesy Dr. Ann M. Hargis, DermatoDiagnostics. B courtesy Blackwell Publishing.)
Sebaceous Gland Dysplasia: Abnormal development of sebaceous glands is rare, is presumed to be genetic in origin, and has been reported in dogs and cats. The pathogenesis is unknown. Histologically, in early stages, there are increased numbers of small epithelial reserve cells compared with mature sebaceous gland cells. The number of mature sebaceous gland cells may be reduced, cytoplasmic vacuolization may be irregular, and the orderly differentiation of reserve cells to mature cells is missing. In the late stage, there is subtotal atrophy, where only a few reserve cells and a few mature sebaceous gland cells remain. Clinical lesions consist of adherent scales, poor hair coat, and progressive alopecia. The poor hair coat and alopecia are presumed to be the result of follicular atrophy and dysplasia that develop after or secondary to the sebaceous gland lesions.
Adnexal Inflammatory Disorders:
Folliculitis: Folliculitis is inflammation of a hair follicle. Folliculitis can be histologically classified according to the anatomic segment of the hair follicle affected and the type of leukocytes present in the inflammatory infiltrate (Fig. 17-24). The types of follicular inflammation include perifolliculitis, mural folliculitis, luminal folliculitis, and inflammation of the hair bulb (bulbitis). The inflammation of hair follicles begins in the perifollicular blood vessels with the same hemodynamic, permeability, and leukocytic changes that comprise dermal inflammation. Leukocytes migrate from perifollicular blood vessels to the dermis, resulting in perifolliculitis (inflammation around but not involving the hair follicle). Perifolliculitis is not specific for any category of disease but is an initial event in the development of folliculitis. Perifolliculitis also often coincides with folliculitis of a variety of causes. The perifollicular inflammatory cells then migrate into the follicular wall, resulting in mural folliculitis (inflammation limited to the wall of the follicle). Depending on the cause of the inflammatory process, the leukocytes can remain localized to the follicular wall or can progress into the follicular lumen.
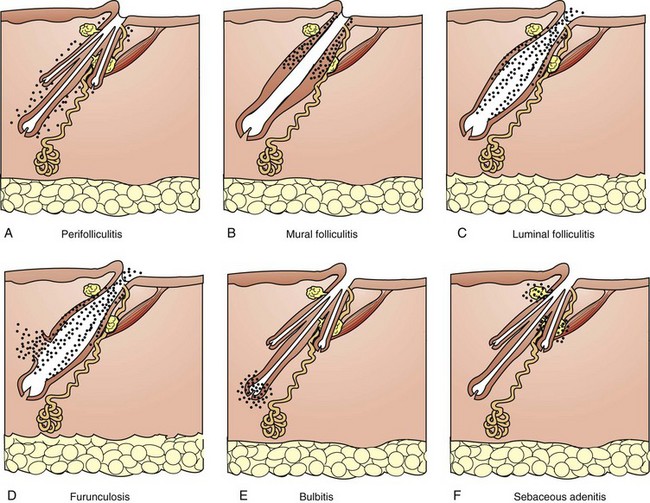
Fig. 17-24 Schematic diagram of the inflammatory patterns of the adnexa.
A, Perifollicular: Leukocytes (black dots) migrate from the dermal vessels near follicles into the perifollicular dermis. B, Mural folliculitis: Inflammation targets the follicular wall. There are subtypes of mural folliculitis that vary with level of involvement (superficial versus inferior), type of the inflammation (pustular versus necrotizing), and the degree or severity of penetration into the follicular wall (interface versus infiltrative). C, Luminal folliculitis: Inflammatory exudate is present in the follicular lumen, and inflammation also usually involves the wall, often a response to follicular infection. D, Furunculosis: Disruption of the follicular wall resulting in release of luminal contents into the bordering dermis. E, Bulbitis: Inflammation targeting the inferior segment of the hair follicle, also called the hair bulb. F, Sebaceous adenitis: inflammation targeting the sebaceous glands. For purposes of simplification, a simple hair follicle is illustrated in parts B, C, and D. (Redrawn from Dr. Ann M. Hargis, DermatoDiagnostics; and Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida.)
Mural: In mural folliculitis, leukocytes remain largely confined to the follicular wall. Mural folliculitis is further subdivided by the location, type, or severity of involvement of the follicular wall such as interface (outer aspect of the follicular wall), infiltrative (more infiltration into the follicular wall), pustular (presence of pustules in the follicular wall), or necrotizing (necrosis and disruption of the follicular wall). The subtype of mural folliculitis can provide insight into the pathogenesis of folliculitis (e.g., disease process). For example, pustular mural folliculitis is a feature of pemphigus foliaceous, and interface mural folliculitis is a feature of demodicosis (Fig. 17-25).
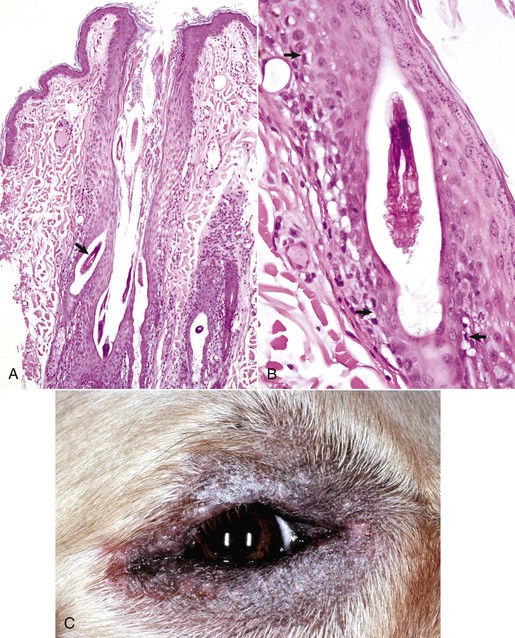
Fig. 17-25 Demodicosis, skin, dog.
A, Note mites deep in the lumens of follicles (arrow) and also inflammation in the outer wall of the follicle (interface mural folliculitis) and in the perifollicular dermis (perifolliculitis). H&E stain. B, Note the mite in the follicular lumen. There are a few lymphocytes bordering the follicular wall and mild vacuolar degeneration of basal cells (arrows) (lymphocytic interface mural folliculitis) of the follicular wall. H&E stain. C, Localized demodicosis, periocular. Skin is alopecic, lichenified, and hyperpigmented. Alopecia is likely caused by inflammation, initiated by an immune response to mite infestation and possibly secondary bacterial infection, that results in damage and sometimes disruption of the follicular wall (furunculosis). (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
Bulbitis: Bulbitis refers to inflammation directed at the deepest portion of the follicle, the hair bulb. Alopecia areata is a specific example of bulbitis. It develops in horses, cattle, dogs, and cats; is characterized by lymphocytic infiltrates around and in growing hair bulbs; and results in alopecia. In horses and dogs, antifollicular cell-mediated immunity and humoral immunity participate. Cellular infiltrates of the hair bulb are mostly cytotoxic CD8+ lymphocytes and CD1+ dendritic antigen-presenting cells, whereas CD8+ and CD4+ lymphocytes predominate in the peribulbar infiltrate. Autoantibodies target trichohyalin, hair keratins, and other components of the hair follicle. The damage to hair matrix cells within the hair bulb results in formation of dystrophic (abnormal) hair shafts. Alopecia areata can spontaneously resolve, but the newly growing hairs can be white instead of pigmented, probably as a result of damage to melanocytes in the hair bulb.
Luminal: Luminal folliculitis refers to inflammation involving the lumen of the follicle and usually the wall of the follicle (Fig. 17-26). Luminal folliculitis develops when the leukocytes in the follicular wall migrate into the lumen, usually as a result of a stimulus in the follicular lumen, such as a follicular infection with bacteria (staphylococci) or dermatophytes (Microsporum, Trichophyton), or infestation with parasites (Demodex, Pelodera). The inflammation can weaken the follicular wall leading to rupture, known as furunculosis (Fig. 17-27), and release of follicular contents into the dermis. There are other causes of furunculosis, including trauma to the surface of the skin resulting in epidermal hyperplasia at the opening of the follicle, plugging of the follicle by stratum corneum, and accumulation of follicular contents including glandular secretions (comedo formation). The gradual accumulation of this luminal material can cause distention of the follicle and thinning of the follicular wall, leading to rupture. Regardless of the cause of furunculosis, the presence of hair fragments, keratin proteins, sebum, and possible infectious agents in the dermis leads to a suppurative inflammatory response that progresses to more long-standing chronic pyogranulomatous inflammation and scarring. Perifolliculitis, luminal folliculitis, and furunculosis often follow in sequence (Fig. 17-28). The inflammation can resolve with appropriate therapy, can extend into the deep dermis and panniculus, and/or can form sinuses that drain to the surface of the skin and are difficult to resolve. Severe inflammation can lead to complete destruction of adnexal units and replacement by scar tissue.
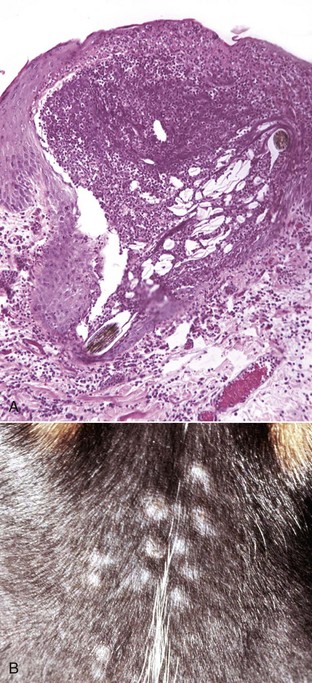
Fig. 17-26 Folliculitis, skin, haired, dog.
A, Luminal folliculitis. Note the distention of hair follicle with inflammatory cells (mostly neutrophils). H&E stain. B, Multiple alopecic papules are caused by perifollicular inflammation and edema and hair follicles distended with exudate. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
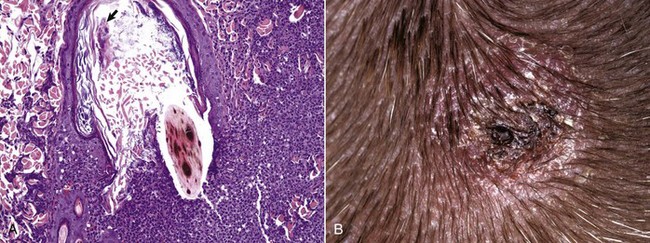
Fig. 17-27 Folliculitis and furunculosis, skin, haired, dog.
A, The wall of the follicle has become disrupted, resulting in the release of follicular contents (a hair shaft, stratum corneum of the follicle, and exudate) into the dermis. The follicular lumen also contains numerous coccoid bacteria (arrow). The hair shaft has variably sized and shaped melanin pigment granules, indicating that this dog also has coat color dilution. H&E stain. B, A circular area of erythema, scaling, and crusting is caused by follicular inflammation and rupture (furunculosis). The inflammation in the perifollicular dermis has extended into the surrounding dermis and formed a draining sinus to the epidermal surface. The exudate on the surface has dried to form a crust. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
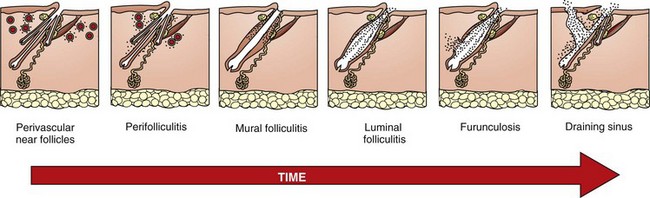
Fig. 17-28 Schematic diagram of the progression of folliculitis.
Inflammation begins with migration of leukocytes (black dots) from perifollicular dermal vessels into the perifollicular dermis (perifolliculitis). Inflammation progresses to involve the follicular wall (mural folliculitis) and then the lumen (luminal folliculitis). If the inflammation continues, the follicular wall is weakened, ruptures, and follicular contents are released into the dermis. The inflammation can extend into the deep dermis and panniculus, and/or form sinuses that drain to the surface of the skin. For purposes of simplification, a simple hair follicle is illustrated in some frames. (Redrawn from Dr. Ann M. Hargis, DermatoDiagnostics; and Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida.)
Sebaceous Adenitis: Sebaceous adenitis is a specific inflammatory reaction that targets sebaceous glands; it is largely a disease of dogs (Fig. 17-29), but rarely is seen in cats and horses. Early histologic lesions are characterized by accumulations of lymphocytes around sebaceous ducts. Fully developed lesions consist of lymphocytes, neutrophils, and macrophages that efface sebaceous glands. Chronic lesions have total loss of sebaceous glands (atrophy), scarring, and epidermal and follicular hyperkeratosis. Sebaceous gland inflammation also can occur secondary to folliculitis, demodicosis, uveodermatologic syndrome, or leishmaniasis, in which the inflammation targets other areas of the skin (follicles, epidermal cells, or dermis) and involves the neighboring sebaceous glands because of their proximity to the inflammation.
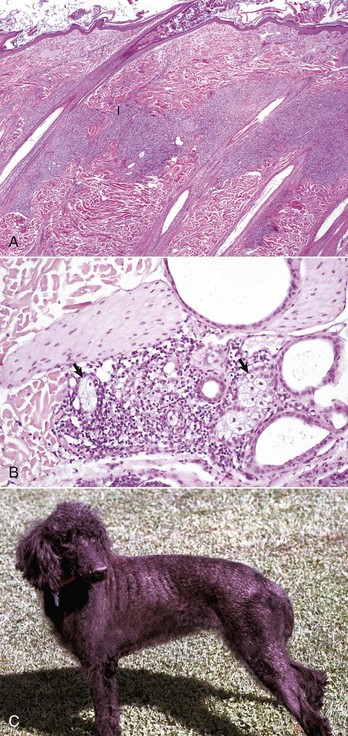
Fig. 17-29 Sebaceous adenitis, skin, haired, dog.
A, Note the inflammation (I) concentrated in and around sebaceous glands. The inflammation forms a band of inflammatory cells parallel to the epidermis at the level of the sebaceous glands. H&E stain. B, Inflammation is beginning to efface the sebaceous glands. A few sebaceous glands (arrows) are visible within the area of inflammation. H&E stain. C, Alopecia and scaling are evident. The pathogenesis of sebaceous adenitis and the alopecia and scaling is incompletely understood. (Courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
Hidradenitis: Hidradenitis, which is inflammation of apocrine glands, has rarely been studied in detail in domestic animals. Suppurative hidradenitis has been described in dogs in which most cases developed in conjunction with staphylococcal folliculitis and furunculosis, either affecting the same or other follicular units. It is speculated, because of the physical connection between the apocrine gland and hair follicle in dogs, that suppurative hidradenitis is most often an extension of hair follicle infection and is the result of the bacteria that cause the folliculitis. There are no clinical signs that are unique to or suggest the presence of hidradenitis other than the association with follicular bacterial infection. Histologically, dogs with hidradenitis associated with bacterial folliculitis and furunculosis have suppurative inflammation of the apocrine gland and surrounding dermis.
Responses of the Vessels to Injury
Vasculitis, inflammation of vessels (see Fig. 17-22, B) in which the vessels are the primary target of injury, can be the result of infection by microbes, immunologic injury, toxins, photodynamic chemicals, or disseminated intravascular coagulation (DIC) or can be idiopathic. The species most commonly presenting with vasculitis are the horse and the dog, and most cases are idiopathic in that a specific cause cannot be determined. Histologic diagnosis of vasculitis is often challenging because it is difficult to differentiate between vessels taking part in inflammation simply by providing a conduit for inflammatory cells to reach a site of injury in the epidermis or dermis from those vessels that are actually the target of injury. Also, the type of inflammatory cell that participates in the inflammatory process can vary more with the age of the vascular lesion than with the type of disease process. Histologic lesions in vasculitis include damage to the vessel wall such as the presence of few necrotic cells or foci of fibrinoid necrosis, mural infiltrates of leukocytes, and intramural or perivascular edema, hemorrhage, or fibrin exudation. Vascular injury leads to the clinical lesions of edema and hemorrhage and if severe, can include ischemic necrosis and infarction. Ulceration with or without sloughing of the skin can occur. Partial ischemia can lead to atrophic changes in the components of the skin. Classic examples include immune-complex deposition in vessel walls (systemic lupus erythematosus and equine purpura hemorrhagica), rabies vaccination–associated vasculitis and ischemic dermatopathy, infection with an endotheliotropic organism (Rickettsia rickettsii), and septicemia with bacterial embolism and infarction in pigs (Erysipelothrix rhusiopathiae).
Responses of the Panniculus to Injury
Panniculitis: Panniculitis, which is inflammation of the subcutaneous adipose tissue, can be caused by infectious agents (bacteria, fungi), immune-mediated disorders (systemic lupus erythematosus), physical injury (trauma, injection of irritant material, foreign bodies), nutritional disorders (vitamin E deficiency), or pancreatic disease (pancreatitis, pancreatic carcinoma) or can be of undetermined cause (idiopathic). Panniculitis can be primary or secondary. In primary panniculitis, the subcutaneous adipose tissue is the target of the disease process (Fig. 17-30). An example of primary panniculitis is feline pansteatitis, which occurs in cats fed diets high in polyunsaturated fats and low in antioxidants, such as vitamin E. Lack of vitamin E leads to oxidation of lipids (free radical–induced membrane lipid peroxidation) of the subcutaneous adipose tissue, inciting a pyogranulomatous inflammatory response. In secondary panniculitis, the subcutis is affected by inflammation primarily involving the contiguous dermis; the inflammation extends down into the subcutis. For example, deep bacterial folliculitis with furunculosis can lead to a secondary panniculitis, as can a penetrating wound contaminated with microbial agents or a foreign body. Animals with panniculitis clinically have palpable nodules that can ulcerate and drain an oily or hemorrhagic material. Lesions are most often on the trunk and proximal limbs and can be solitary or multifocal. Solitary lesions may be cured by excision, whereas multiple lesions may resolve with specific therapy or result in scar formation. In animals, panniculitis is subdivided based on cell type and presence or absence of microorganisms into the following basic categories: predominantly neutrophilic, predominantly lymphocytic, predominantly granulomatous to pyogranulomatous with infectious agents, predominantly granulomatous to pyogranulomatous without infectious agents, and fibrosing.
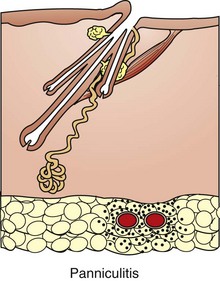
Fig. 17-30 Schematic diagram of inflammation in the panniculus.
Note the leukocytic inflammation (black dots) in the subcutaneous adipose tissue (panniculitis). In addition to spreading locally, the inflammation can form a sinus through the dermis and epidermis to the surface. The exudate can have an oily composition as a result of the fat content of the panniculus. (Redrawn from Dr. Ann M. Hargis, DermatoDiagnostics; and Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida.)
Pathologic Reactions of the Entire Cutaneous Unit
Disease processes involving the skin uncommonly affect just one component of the skin (i.e., only the epidermis or only the lumens of hair follicles). More often, multiple components of the skin are involved in the disease process. In addition, lesions evolve through different stages; some lesions can resolve, and there can be secondary lesions, such as self-induced trauma, complicating the initial lesions. Therefore multiple biopsy samples collected from different areas of the skin are often necessary to help illustrate the range of lesions necessary to reach a diagnosis. Multiple biopsy samples provide a more representative picture of the disease process than could a single biopsy sample. Even so, evaluation of multiple biopsy samples does not always lead to a specific diagnosis, but the pattern of lesions identified often suggests categories of disease and rules out other differential diagnoses.
The involvement of the multiple components of the skin in a disease process can be illustrated by a poxvirus infection (Fig. 17-31). When a poxvirus invades the epidermis, the virus replicates in the cells of the stratum spinosum and causes cytoplasmic swelling (ballooning degeneration) and rupture (reticular degeneration) of some of the epidermal cells. Cytoplasmic viral inclusions form in some cells. Cellular constituents released from damaged epidermal cells act as chemical mediators of the acute inflammatory response and are chemotactic for leukocytes. These chemical mediators and chemotactic factors (1) increase blood flow to the site of viral invasion by dilation of arterioles, (2) cause margination of leukocytes in capillaries and postcapillary venules in the dermis, (3) increase vascular permeability (dermal edema), and (4) cause migration of leukocytes out of vessels into the tissue, creating the formation of macular lesions. The epidermal degeneration, dermal edema, and perivascular inflammation can progress to exudative lesions. Ballooning and reticular degeneration of keratinocytes result in the formation of intraepidermal vesicles. Leukocytes in perivascular sites under the influence of inflammatory mediators from the epidermis migrate to the epidermis and enter the vesicle to form pustules. Some poxviruses also cause epidermal hyperplasia by stimulating host cell DNA synthesis, presumably by a viral gene product similar to epidermal growth factor, resulting in pseudocarcinomatous hyperplasia. The pustule enlarges and eventually ruptures, releasing the exudates onto the skin surface. The exudates dry and form a crust (scab). The primary lesions of vesicles and pustules are fragile and often transient, lasting only hours, and so are difficult to identify and collect in biopsy samples. The secondary lesions of crusting and scarring are more long-standing. In this way, multiple components of the skin participate in the development of the lesions and are responsible for the clinical stages of macule, papule, vesicle, pustule, crust, and scar.
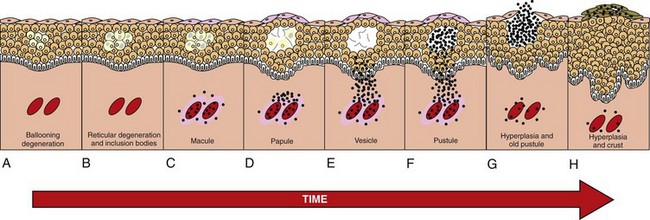
Fig. 17-31 Schematic diagram of the development of a poxvirus lesion over time.
A, Ballooning degeneration. B, Reticular degeneration with cytoplasmic inclusion bodies of keratinocytes. Both A and B are subclinical stages. C, Congestion, edema, margination, and migration of leukocytes (black dots) form the macule stage. D, Continued epidermal reticular degeneration, epidermal hyperplasia (acanthosis), dermal edema, and perivascular inflammation form the papule stage. E, The vesicle stage develops by coalescing of areas of reticular degeneration (disruption of swollen keratinocytes). F, Inflammatory cells migrate from the dermal vessels into the vesicle and accumulate in the vesicle to form the pustule stage. G, The epidermis begins to proliferate and becomes more acanthotic, and the old pustule is moved toward the epidermal surface. H, Epidermal hyperplasia progresses with the formation of elongated epidermal dermal interdigitations, and the old pustule ruptures to form a crust. Larger pustules can be umbilicated, involve the dermis, and result in scarring. (Redrawn from Dr. Ann M. Hargis, DermatoDiagnostics; and Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida.)
Definitions of Clinical Terms
Knowledge of the clinical appearance of skin lesions, distribution of lesions, and correlation between the gross and histologic lesions is often critical in formulating differential and final diagnoses. In skin diseases, the clinical lesions represent the gross lesions and are typically examined by a practitioner, not the pathologist; thus the practitioner essentially serves as the eyes for the pathologist. It is therefore important for the practitioner to develop the ability to accurately recognize clinical lesion morphology and translate that information to the pathologist. To facilitate this process, Table 17-3 is provided to illustrate the morphology of the various clinical lesions and provide examples of the disease processes in which those lesions occur.
TABLE 17-3
Definition and Morphology of Primary and Secondary Skin Lesions
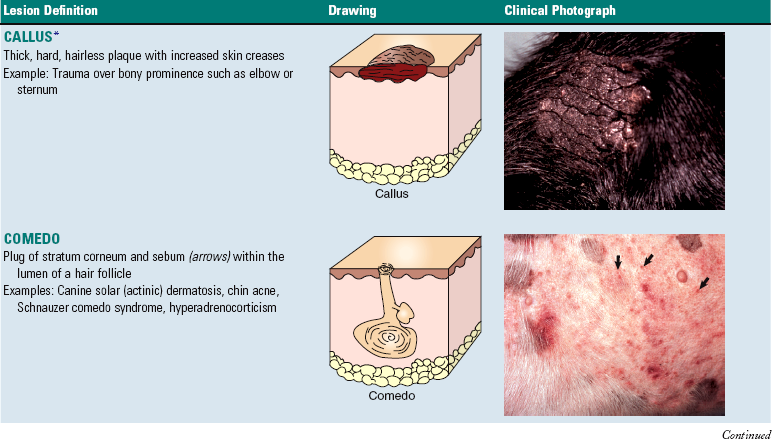
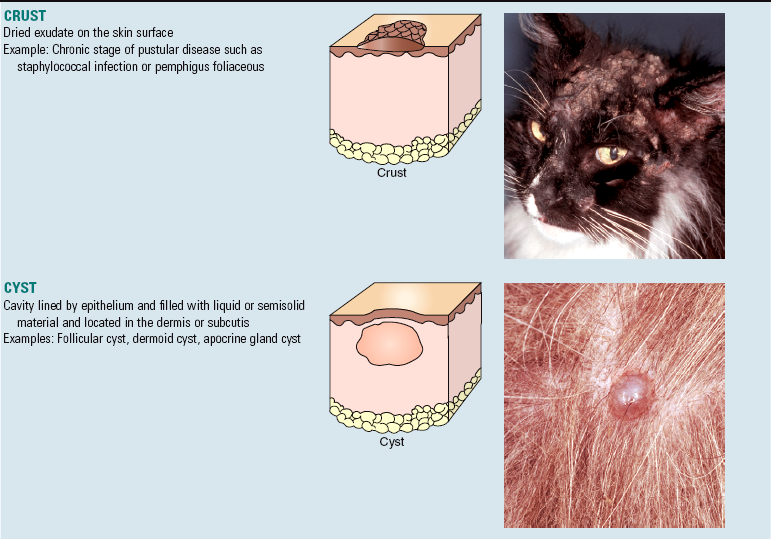
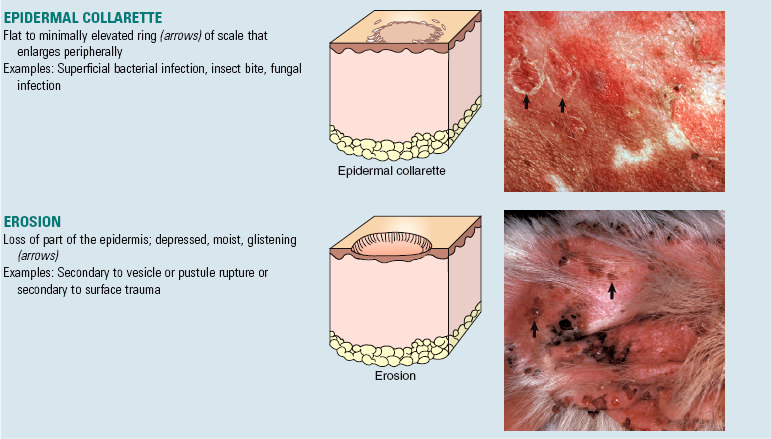
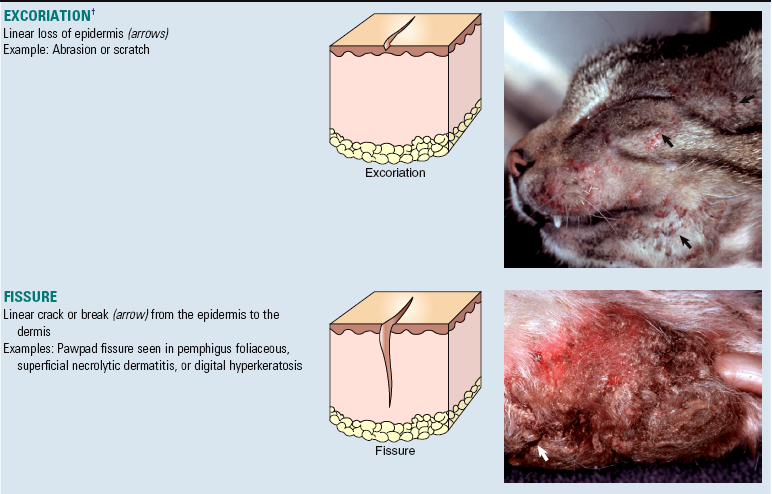
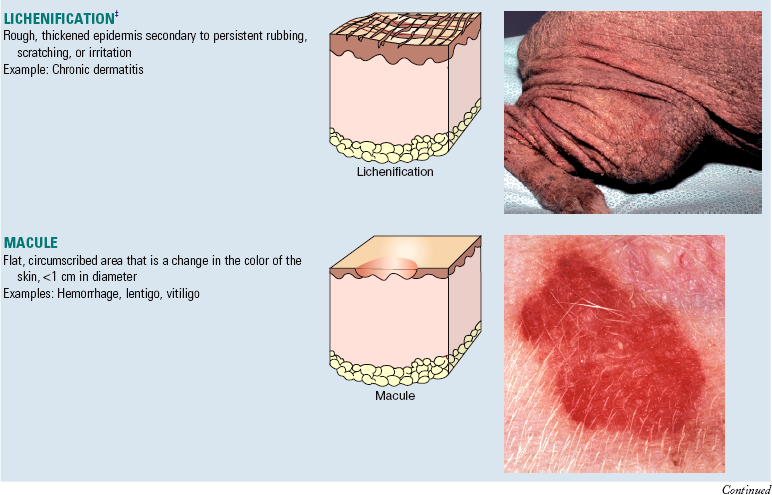
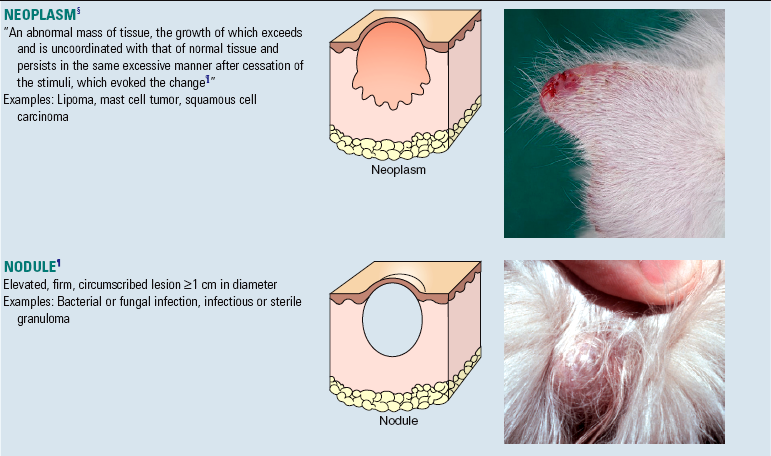
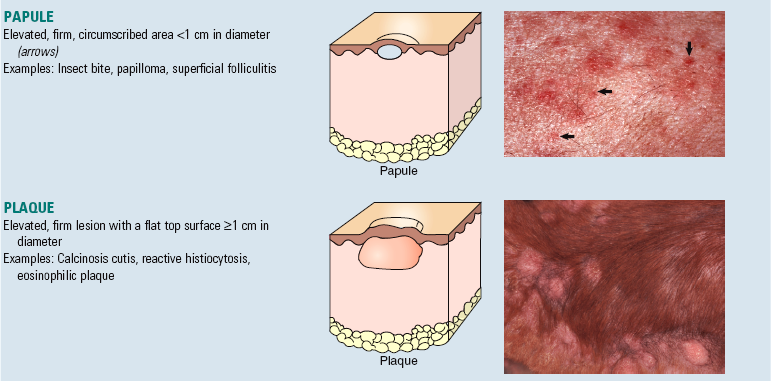
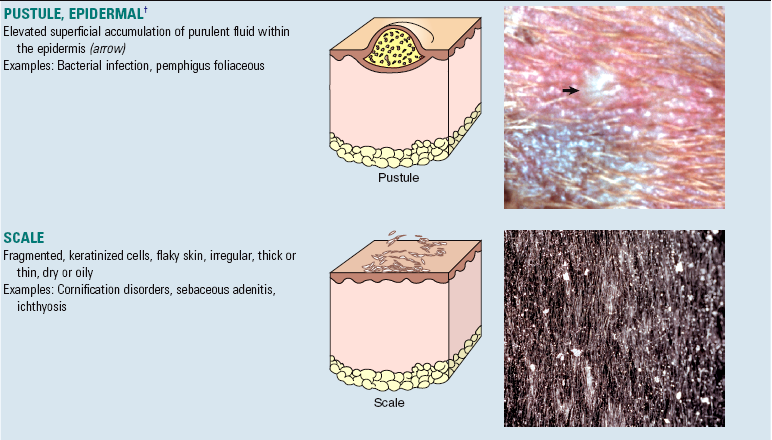
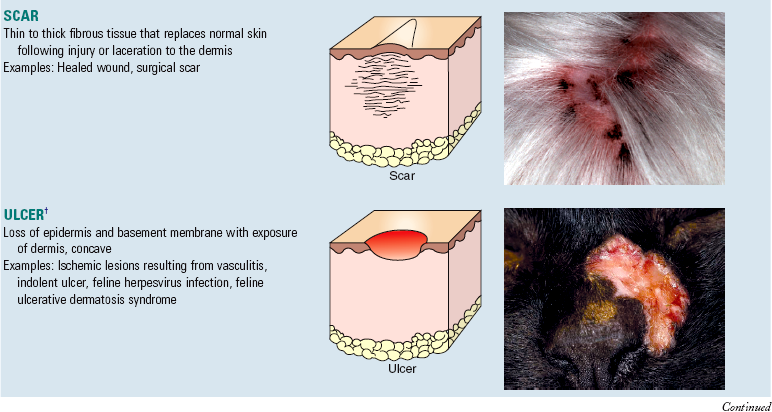
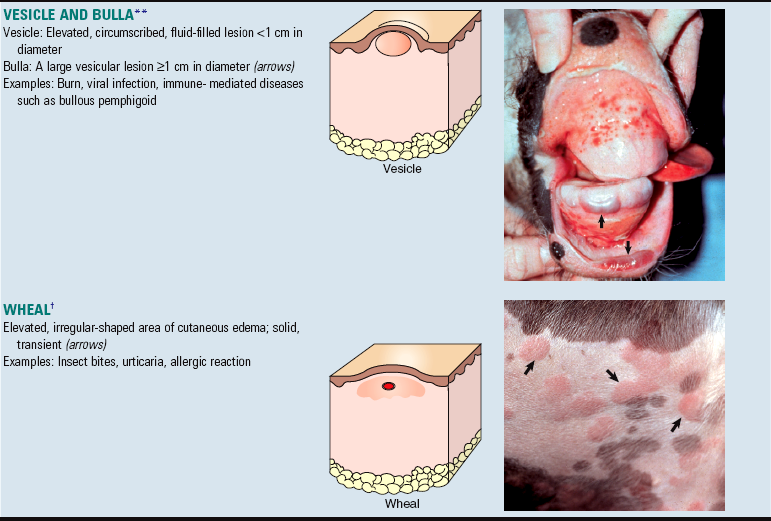
*Courtesy Washington Animal Disease Diagnostic Laboratory.
†Courtesy Dr. David Duclos, Animal Skin and Allergy Clinic.
‡Courtesy Dr. Helen Power, Dermatology for Animals
§Courtesy Dr. David Duclos, Animal Skin and Allergy Clinic.
 From Willis RA: Pathology of tumors, Philadelphia, 1948, FA Davis.
From Willis RA: Pathology of tumors, Philadelphia, 1948, FA Davis.
¶Courtesy Dr. Alan Mundell, Animal Dermatology Service.
**Courtesy Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida; and Ginn PE, Hillier A, Lester GD: Vet Dermatol 9:249-256, 1998.
All drawings modified from Copstead LC, Banasik JL: Pathophysiology: biological and behavioral perspectives, ed 3, Philadelphia, 2005, Saunders. All clinical photographs courtesy Dr. Ann M. Hargis, DermatoDiagnostics, unless otherwise noted.
Techniques for Skin Biopsy Sampling
Important tips for biopsy sampling of the skin are listed in Table 17-4. Biopsy do’s and don’ts are listed in Boxes 17-4 and 17-5, respectively.
When to Collect Biopsy Samples
Knowing when to collect biopsy samples helps obtain the most diagnostic samples; facilitates obtaining samples early so acute, serious, or neoplastic disorders are diagnosed quickly; and prevents the frustration and economic loss when samples are inappropriately collected, such as when concurrent therapy might alter diagnostic lesions, when lesions are in a quiescent stage and might not be diagnostic, and when clinical dermatologic evaluation would have been a better method of achieving a diagnosis. Biopsy sampling is recommended when the following are present:
1. The therapy for the skin disorder is associated with significant side effects (to confirm the clinical diagnosis before starting therapy).
2. A nodular lesion, ulcer, or nonhealing wound might represent a tumor (so that surgical excision of the tumor can be performed as early as possible).
3. Lesions develop suddenly, are severe, or are unusual (to help identify a serious disease so that therapy can be instituted early).
4. Lesions develop during the course of therapy (to identify a potential adverse reaction to drug therapy).
5. When lesions are active and before use of therapy that might alter the histologic appearance of the lesions and when there are multiple clinical differential diagnoses and the thorough clinical dermatologic examinations do not differentiate the conditions.
6. When a skin disorder fails to respond to apparently appropriate therapy or when the disorder responds to therapy but recurs when therapy is stopped (to establish the correct diagnosis, or evaluate for predisposing factors). Recall that antiinflammatory therapy can alter lesions.
Site Selection
Multiple cutaneous sites representative of the range of lesions should be selected for biopsy. Fully developed nontreated primary lesions, such as macules, papules, pustules, nodules, neoplasms, vesicles, and wheals, are often the most useful for diagnosis (see Table 17-3). However, primary lesions may not be present at the time the animal is examined, so secondary lesions, such as scales, crusts, ulcers, comedones, or scars then need to be sampled and evaluated (see Table 17-3). These secondary lesions can be diagnostic or contribute substantially to the diagnosis when multiple cutaneous sites are selected for biopsy. One of the most useful secondary lesions is the crust because acantholytic cells from drying pustules in pemphigus foliaceous and organisms, such as Dermatophilus congolensis or dermatophytes, may be identified in crusts, providing the information necessary for diagnosis. Also, the margin of a chronic ulcer may represent a squamous cell carcinoma, or the scale at the edge of an epidermal collarette (i.e., peripherally expanding ring of epidermal scales) may represent superficial spreading pyoderma, thus providing the key to diagnosis.
Methods
Excisional biopsy samples (entire lesions) are recommended for large pustules or vesicles that can be damaged by use of a smaller punch biopsy instrument. Deeper excisional biopsy samples are generally necessary for diagnosis of lesions, such as panniculitis, that are deep to the epidermis and dermis. Digital amputation can be required, particularly in dogs, for the diagnosis of claw bed lesions. Electrocautery or laser should not be used for small biopsy samples because the samples can be damaged and rendered nondiagnostic. Tissue forceps (small toothed) should grasp, if at all, only one nonaffected margin, preferably in the subcutis.
Site Preparation
Generally, the skin at a punch biopsy site should not be surgically prepared because the procedure may remove a diagnostic portion of the sample. Gentle clipping of hair is acceptable, and surgical preparation of the skin is acceptable for an excisional biopsy of lesions deep to the epidermis. For collection of biopsy samples in areas of alopecia, drawing a line with a fine-tipped permanent marking pen in the direction of the hair coat helps laboratory personnel orient the sample (Fig. 17-32, A). In the laboratory, the sample is cut along the line so that the hair follicles are oriented longitudinally (Fig. 17-32, B). If the line is not drawn on the sample, the sample might be cut so that the follicles are in cross or tangential section rather than longitudinal section (Fig. 17-32, C), which reduces histologic value of the sample when evaluating for follicular disease. For collection of ulcers, depigmented lesions, or other lesions where the interface between normal and affected skin is critical to diagnosis, use of incisional or excisional samples collected from affected skin and contiguous normal skin is often preferable. However, a large (8 mm) biopsy punch instrument can be used to collect the junction between normal and affected skin, if a line with a fine-tipped permanent marking pen is drawn perpendicular to the junction between the normal and affected tissue before sample collection (Fig. 17-33). The line instructs laboratory personnel how to trim the sample to ensure that the critical areas are present for dermatopathologic evaluation. For lesions suspected of being invasive tumors, complete excision of the mass, including a 3-cm margin of clinically normal skin around all borders, is recommended (Fig. 17-34).
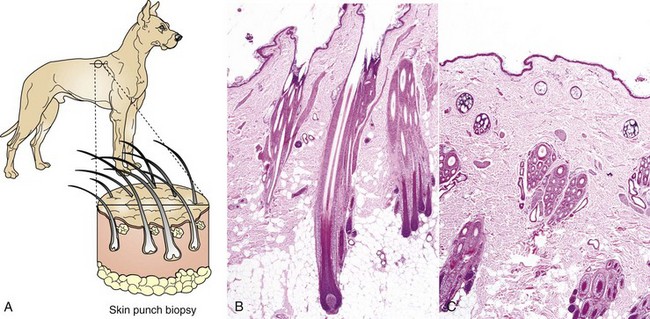
Fig. 17-32 Sampling of the skin for histopathologic evaluation, haired, dog.
A, Schematic diagram of the line drawn (with fine tipped permanent marking pen) on the skin in the direction of the hair coat. The line directs laboratory personnel to cut and embed the sample parallel to the hair follicles. Cutting the sample parallel to the hair follicles allows visualization of the entire length of the follicles during microscopic examination, which facilitates evaluation for causes of alopecia. For simplicity, simple follicles are used in this illustration. B, Section of skin trimmed parallel to the hair follicles. Note that the hair follicles are cut longitudinally and that the full length of the hair follicle is present. H&E stain. C, Section of skin trimmed in cross-section to the hair follicles. Note that the hair follicles are cut tangentially and that only portions of each follicle are present for examination, which reduces the ability to evaluate follicular morphology. H&E stain. (B and C courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
Fig. 17-33 Schematic diagram of collecting the margin of a lesion and normal skin using a large punch biopsy instrument (8 mm).
It is necessary to draw a line on the sample from normal into affected skin to direct laboratory personnel to cut and embed the sample so that the interface between the normal and affected skin is present for microscopic examination. Without this line, the sample could be cut at a right angle to the desired line and thus miss the interface between normal and affected skin essential for histologic examination of the area most likely to have diagnostic changes.
Fixation
Punch biopsy specimens should be placed in 10 times the volume of 10% neutral buffered formalin (NBF). To prevent warping in the fixative, thin excisional biopsy specimens should be gently attached to a flat object, such as a piece of cardboard or tongue depressor, and permitted to dry for 20 to 30 seconds. Twenty to 30 seconds is all that is necessary for the sample to adhere to the flat object. The specimen and flat object are then immediately immersed in formalin. Care should be taken not to let the sample become dehydrated (i.e., remain unfixed for longer than 20 to 30 seconds), which could damage the morphology of small samples and the lesions. In cold climates during winter months, adding 1 part alcohol to 9 parts 10% NBF reduces the chance of freezing of specimens during transport.
History
Accurate histopathologic diagnosis and interpretation require knowledge of the gross features of the lesions. Therefore it is essential to include with biopsy samples the following information: (1) age, breed, and sex of the animal; (2) location, gross appearance, and duration of the lesions; (3) presence or absence of lesion symmetry; and (4) presence or absence of pruritus affecting the animal (Box 17-6). Clinical information, including results of laboratory evaluations (i.e., hemograms, serum biochemical analyses), results of skin cultures, scrapings, or cytology, current medications, and response to therapy, should be included along with a list of clinical differential diagnoses. History can be critical to reaching a diagnosis. For example, presence of luminal and mural folliculitis with no apparent follicular infectious agents in H&E-stained sections in conjunction with the history of lack of response to appropriate antibiotic therapy would suggest to the pathologist that a fungal stain to evaluate for occult dermatophyte infection should be performed. Without the history of lack of response to appropriate antibiotic therapy, the folliculitis could easily be presumed to be of bacterial origin, and a fungal infection missed.
Ancillary Procedures
Other diagnostic procedures can supplement information gained from histologic examination of biopsy samples. These procedures include aspiration of pustule contents for cytologic evaluation, performing touch imprints of the cut surface of suspected neoplastic or infectious lesions for cytologic evaluation, aseptically collecting a tissue sample for microbiologic culture, and collecting biopsy samples of suspected immune-mediated diseases or poorly differentiated tumors for immunostaining. Use of immunostaining for identification of cell surface or cytoplasmic proteins (to aid in diagnosis of tumors) or for identification of immunoglobulin, complement, or other antigens (to aid in the diagnosis of immune-mediated skin disease, such as pemphigus) can be helpful in some cases. The formalin-fixed tissue submitted for histopathology also can be used for most routine immunohistochemical procedures, since most employ immunoperoxidase staining rather than immunofluorescence.
Unfortunately, immunostaining techniques for immune-mediated skin diseases can give false-positive or false-negative results, thus they must be done in conjunction with standard histopathology. For autoimmune diseases, if immunofluorescence evaluation is desired, specimens can be fixed in Michel’s medium, which preserves immunoglobulin and complement. If immunohistochemistry (immunoperoxidase) staining is desired, formalin-fixed samples are used; however, for best results, samples should not remain in formalin longer than 48 hours. Prolonged fixation in formalin results in cross-linking of proteins and false-negative results. For autoimmune disease, newer techniques that detect more specific antigens, such as desmoglein (transmembrane glycoproteins found in desmosomes that provide physical connections between keratinocytes), use of “salt-split” skin sections (a technique used in immunostaining of the skin where sodium chloride splits the epidermis from the dermis through the lamina lucida, allowing better differentiation of subepidermal bullous diseases), use of better substrates for indirect immunostaining, and use of immunoblotting and enzyme-linked immunosorbent assay (ELISA) techniques may improve diagnostic accuracy of immune-mediated skin diseases in the future.
Identification of cell surface or cytoplasmic proteins can help identify the cell type in poorly differentiated tumors (Table 17-5). However, there can be anomalous expression of proteins in some tumors (e.g., anomalous expression of cytokeratin in melanoma); thus evaluation of a series (panel) of antibodies is preferred because the pattern of staining with a panel of antibodies is more reliable than staining with one or two antibodies. Formalin-fixed specimens are acceptable for most procedures, but for others, fresh or frozen specimens are better. Discussion with a pathologist is recommended regarding when to use immunostaining procedures.
Disorders of Domestic Animals
Congenital and Hereditary Disorders
The terms congenital and hereditary are not synonymous. Congenital lesions develop in the fetus (in utero), are present at birth, and have a variety of causes. An example is hypotrichosis in the fetus associated with maternal dietary iodine deficiency. Inherited conditions are transmitted genetically and are not always manifested phenotypically in utero or at birth but may develop later in life. An example is sebaceous adenitis, which may not develop until 1 to 2 years of age or later. Web Box 17-2 lists selected cutaneous inherited diseases in animals. There are many other diseases (e.g., cutaneous and renal glomerular vasculopathy in greyhounds, atopic dermatitis in selected breeds) that may also be inherited, but the mode of inheritance has not been documented.