Chapter 3 Second Week of Human Development
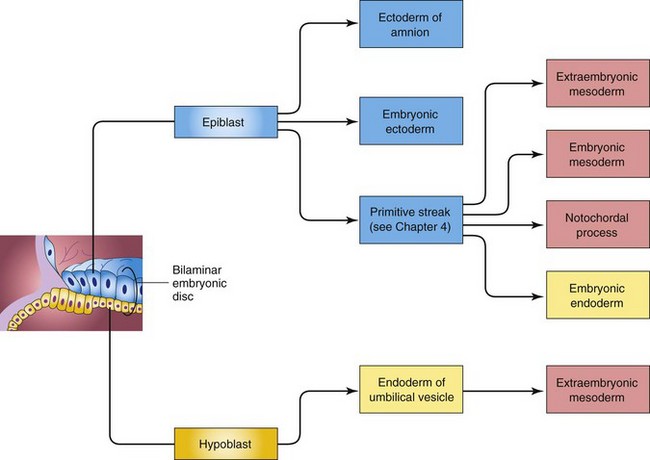
As implantation of the blastocyst occurs, morphologic changes in the embryoblast produce a bilaminar embryonic disc composed of epiblast and hypoblast (Fig. 3-1A). The embryonic disc gives rise to the germ layers that form all the tissues and organs of the embryo. Extraembryonic structures forming during the second week are the amniotic cavity, amnion, umbilical vesicle (yolk sac), connecting stalk, and chorionic sac.
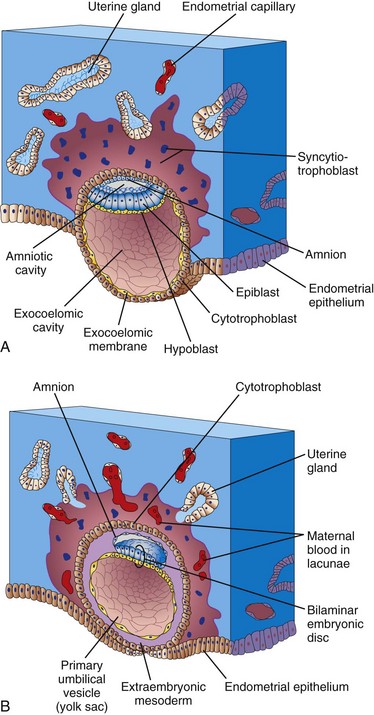
FIGURE 3–1 Implantation of a blastocyst in the endometrium. The actual size of the conceptus is 0.1 mm, approximately the size of the period at the end of this sentence. A, Drawing of a section through a blastocyst partially embedded in the uterine endometrium (approximately 8 days). Note the slit-like amniotic cavity. B, Drawing of a section through a blastocyst of approximately 9 days implanted in the endometrium. Note the lacunae appearing in the syncytiotrophoblast.
Completion of Implantation of Blastocyst
Implantation of the blastocyst is completed during the second week. It occurs during a restricted time period 6 to 10 days after ovulation. As the blastocyst implants (Fig. 3-1), more trophoblast contacts the endometrium and differentiates into two layers:
The erosive syncytiotrophoblast invades the endometrial connective tissue and the blastocyst slowly becomes imbedded in the endometrium (Fig. 3-2). Syncytiotrophoblastic cells displace endometrial cells at the implantation site. The endometrial cells undergo apoptosis (programmed cell death), which facilitates the invasion.
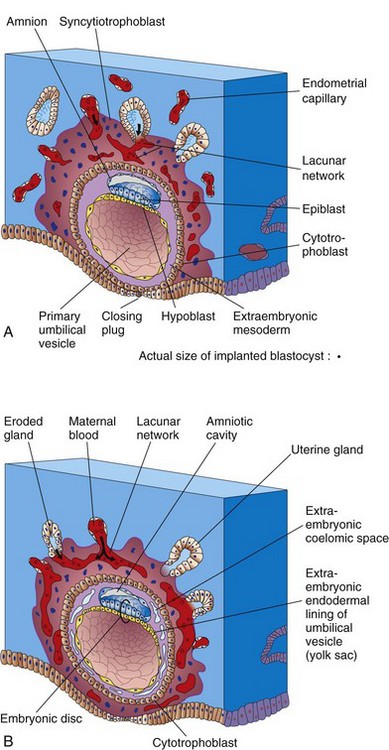
FIGURE 3–2 Embedded blastocysts. A, 10 days; B, 12 days. This stage of development is characterized by communication of the blood-filled lacunar networks. Note in B that coelomic spaces have appeared in the extraembryonic mesoderm, forming the beginning of the extraembryonic coelom (cavity).
The molecular mechanisms of implantation involve synchronization between the invading blastocyst and a receptive endometrium. The microvilli of endometrial cells, cell adhesion molecules (integrins), cytokines, prostaglandins, hormones (hCG and progesterone) growth factors, and extracellular matrix and enzymes (matrix metalloproteinase and protein kinase A) play a role in making the endometrium receptive. The connective tissue cells around the implantation site accumulate glycogen and lipids and assume a polyhedral appearance. Some of these cells—decidual cells—degenerate adjacent to the penetrating syncytiotrophoblast. The syncytiotrophoblast engulfs these cells, providing a rich source of embryonic nutrition.
The syncytiotrophoblast produces a glycoprotein hormone, human chorionic gonadotrophin (hCG), which enters the maternal blood via isolated cavities (lacunae) in the syncytiotrophoblast (Fig. 3-1B). hCG maintains the hormonal activity of the corpus luteum in the ovary during pregnancy. The corpus luteum is an endocrine glandular structure that secretes estrogen and progesterone to maintain pregnancy. Highly sensitive radioimmunoassays are available for detecting hCG and forms the basis for pregnancy tests. Enough hCG is produced by the syncytiotrophoblast at the end of the second week to give a positive pregnancy test, even though the woman is probably unaware that she is pregnant.
 Formation of Amniotic Cavity, Embryonic Disc, and Umbilical Vesicle
Formation of Amniotic Cavity, Embryonic Disc, and Umbilical Vesicle
As implantation of the blastocyst progresses, a small space appears in the embryoblast. This space is the primordium of the amniotic cavity (Figs. 3-1A and 3-2B). Soon amniogenic (amnion-forming) cells—amnioblasts—separate from the epiblast and form the amnion, which encloses the amniotic cavity. Concurrently, morphologic changes occur in the embryoblast (cluster of cells from which the embryo develops) that result in the formation of a flat, almost circular bilaminar plate of cells, the embryonic disc, consisting of two layers (Fig. 3-2A and B):
The epiblast forms the floor of the amniotic cavity and is continuous peripherally with the amnion. The hypoblast forms the roof of the exocoelomic cavity (Fig. 3-1A) and is continuous with the thin exocoelomic membrane. This membrane, together with the hypoblast, lines the primary umbilical vesicle (yolk sac). The embryonic disc now lies between the amniotic cavity and the vesicle (Fig. 3-1B). Cells from the vesicle endoderm form a layer of connective tissue, the extraembryonic mesoderm (Fig. 3-2A), which surrounds the amnion and umbilical vesicle. The umbilical vesicle and amniotic cavities make morphogenetic movements of the cells of the embryonic disc possible.
As the amnion, embryonic disc, and primary umbilical vesicle form, lacunae (small spaces) appear in the syncytiotrophoblast (Figs. 3-1A and 3-2). The lacunae become filled with a mixture of maternal blood from ruptured endometrial capillaries and cellular debris from eroded uterine glands. The fluid in the lacunar spaces—embryotroph—passes to the embryonic disc by diffusion and provides nutritive material to the embryo.
The communication of the eroded endometrial capillaries with the lacunae in the syncytiotrophoblast establishes the primordial uteroplacental circulation. When maternal blood flows into the lacunar networks, oxygen and nutritive substances pass to the embryo. Oxygenated blood passes into the lacunae from the spiral endometrial arteries, and poorly oxygenated blood is removed from them through the endometrial veins.
The 10-day human conceptus (embryo and extraembryonic membranes) is completely embedded in the uterine endometrium (Fig. 3-2A). Initially there is a surface defect in the endometrial epithelium that is soon closed by a closing plug of a fibrin coagulum of blood. By day 12, an almost completely regenerated uterine epithelium covers the closing plug (see Fig. 3-3B). This partially results from signaling by cAMP and progesterone. As the conceptus implants, the endometrial connective tissue cells undergo a transformation, the decidual reaction. After the cells swell because of the accumulation of glycogen and lipid in their cytoplasm, they are known as decidual cells. The primary function of the decidual reaction is to provide nutrition for the early embryo and an immunologically privileged site for the conceptus.
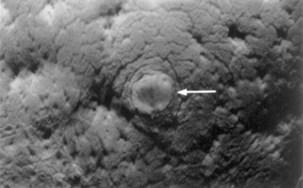
FIGURE 3–3 Photograph of the endometrial surface of the body of the uterus, showing the implantation site of the 12-day embryo shown in Figure 3-4. The implanted conceptus produces a small elevation (arrow) (×8).
(From Hertig AT, Rock J: Contrib Embryol Carnegie Inst 29:127, 1941. Courtesy of the Carnegie Institution of Washington, DC.)
In a 12-day embryo, adjacent syncytiotrophoblastic lacunae have fused to form lacunar networks (Fig. 3-2B), giving the syncytiotrophoblast a sponge-like appearance. The networks, particularly obvious around the embryonic pole, are the primordia of the intervillous spaces of the placenta (see Chapter 7). The endometrial capillaries around the implanted embryo become congested and dilated to form sinusoids, thin-walled terminal vessels that are larger than ordinary capillaries. The formation of blood vessels in the endometrial stroma is under the influence of estrogen and progesterone. Expression of connexin 43 (Cx43), a gap junction protein, plays a critical role in angiogenesis at the implantation site and in maintenance of pregnancy.
The syncytiotrophoblast erodes the sinusoids and maternal blood flows freely into the lacunar networks. The trophoblast absorbs nutritive fluid from the lacunar networks, which is transferred to the embryo. Growth of the bilaminar embryonic disc is slow compared with growth of the trophoblast (Figs. 3-1 and 3-2). The implanted 12-day embryo produces a minute elevation on the endometrial surface that protrudes into the uterine cavity (Figs. 3-3 and 3-4).
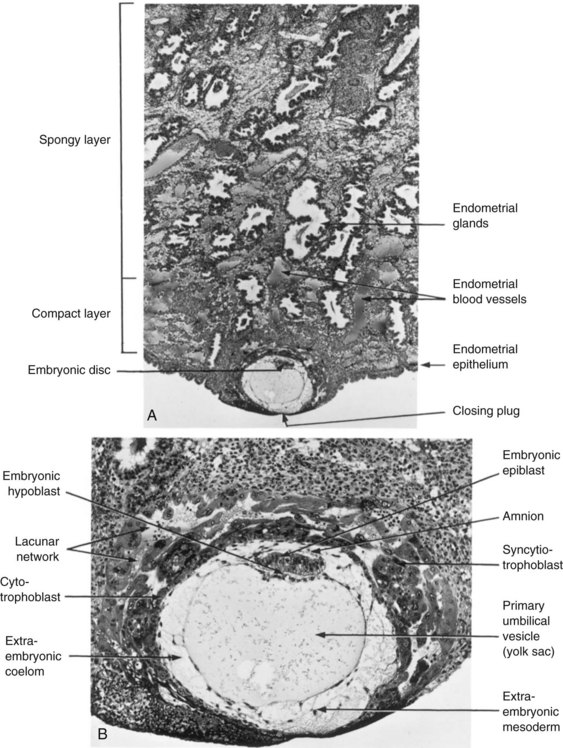
FIGURE 3–4 Embedded blastocyst. A, Section through the implantation site of a 12-day embryo described in Figure 3-3. The embryo is embedded superficially in the compact layer of the endometrium (×30). B, Higher magnification of the conceptus (embryo and associated membranes) and uterine endometrium surrounding it (×100). Lacunae containing maternal blood are visible in the syncytiotrophoblast.
(From Hertig AT, Rock J: Contrib Embryol Carnegie Inst 29:127, 1941. Courtesy of the Carnegie Institution of Washington, DC.)
As changes occur in the trophoblast and endometrium, the extraembryonic mesoderm increases and isolated extraembryonic coelomic spaces appear within it (Figs. 3-2 and 3-4). These spaces rapidly fuse to form a large isolated cavity, the extraembryonic coelom (Fig. 3-5A). This fluid-filled cavity surrounds the amnion and umbilical vesicle, except where they are attached to the chorion by the connecting stalk. As the extraembryonic coelom forms, the primary umbilical vesicle decreases in size and a smaller secondary umbilical vesicle forms (see Fig. 3-5B). This smaller vesicle is formed by extraembryonic endodermal cells that migrate from the hypoblast inside the primary umbilical vesicle (Fig. 3-6). During formation of the secondary umbilical vesicle, a large part of the primary umbilical vesicle is pinched off (Fig. 3-5B). The umbilical vesicle contains no yolk; however, it has important functions (e.g., it is the site of origin of primordial germ cells (see Chapter 12)). It may have a role in the selective transfer of nutrients to the embryo.
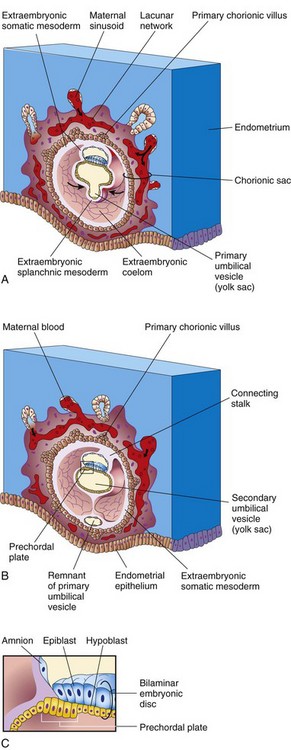
FIGURE 3–5 Drawings of sections of implanted human embryos, based mainly on Hertig and colleagues (1956). Observe (1) the defect in the endometrial epithelium has disappeared; (2) a small secondary umbilical vesicle has formed; (3) a large cavity, the extraembryonic coelom, now surrounds the umbilical vesicle and amnion, except where the amnion is attached to the chorion by the connecting stalk; and (4) the extraembryonic coelom splits the extraembryonic mesoderm into two layers: extraembryonic somatic mesoderm lining the trophoblast and covering the amnion, and the extraembryonic splanchnic mesoderm around the umbilical vesicle. A, 13-day embryo, illustrating the decrease in relative size of the primary umbilical vesicle and the early appearance of primary chorionic villi. B, 14-day embryo, showing the newly formed secondary umbilical vesicle and the location of the prechordal plate in its roof. C, Detail of the prechordal plate outlined in B.
 Development of Chorionic Sac
Development of Chorionic Sac
The end of the second week is characterized by the appearance of primary chorionic villi (Fig. 3-5). The villi form columns with syncitial coverings. The cellular extensions grow into the syncytiotrophoblast. The growth of these extensions is thought to be induced by the underlying extraembryonic somatic mesoderm. The cellular projections form primary chorionic villi, the first stage in the development of the chorionic villi of the placenta.
The extraembryonic coelom splits the extraembryonic mesoderm into two layers (Fig. 3-5A and B):
The extraembryonic somatic mesoderm and the two layers of trophoblast form the chorion. The chorion forms the wall of the chorionic sac (Fig. 3-5A and B), within which the embryo, amniotic sac, and umbilical vesicle (yolk sac) are suspended by the connecting stalk. The term umbilical vesicle is preferred because yolk is not present in the human vesicle. The extraembryonic coelom is now called the chorionic cavity.
Transvaginal ultrasonography (endovaginal sonography) is used for measuring the chorionic sac diameter (Fig. 3-7). This measurement is valuable for evaluating early embryonic development and pregnancy outcome.
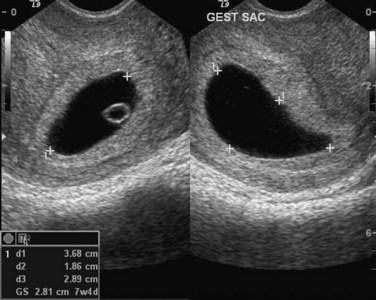
FIGURE 3–7 Endovaginal sonogram (sagittal and axial) of an early chorionic (gestational) sac (5 weeks) (+). The mean chorionic sac diameter is calculated from the three orthogonal measurements (d1, d2, d3) and dividing by 3. The secondary umbilical vesicle (yolk sac) can also be seen.
(Courtesy of E.A. Lyons, M.D., Professor of Radiology, Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
A 14-day embryo still has the form of a flat bilaminar embryonic disc (Fig. 3-8), but the hypoblastic cells in a localized area are now columnar and form a thickened circular area—the prechordal plate (Fig. 3-5B and C). The prechordal plate indicates the site of the mouth and is an important organizer of the head region.
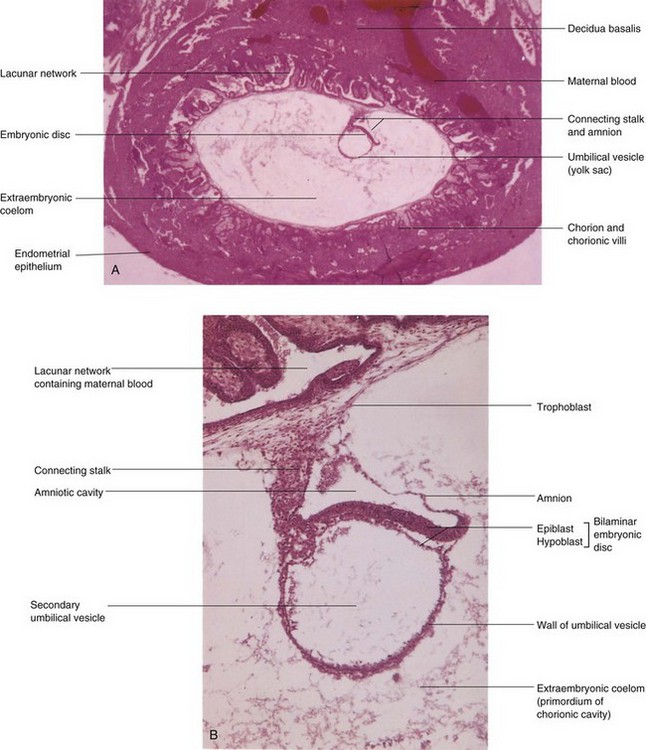
FIGURE 3–8 Photomicrographs of longitudinal sections of an embedded 14-day embryo. Note the large size of the extraembryonic coelom. A, Low-power view (×18). B, High-power view (×95). The embryo is represented by the bilaminar embryonic disc composed of epiblast and hypoblast.
(From Nishimura H (ed): Atlas of Human Prenatal Histology. Tokyo, Igaku-Shoin, 1983)
Implantation Sites of Blastocysts
Implantation of blastocysts usually occurs in the uterine endometrium in the superior part of the body of the uterus, slightly more often on the posterior than on the anterior wall of the uterus. Implantation of a blastocyst can be detected by ultrasonography and highly sensitive radioimmunoassays of hCG as early as the end of the second week (Figs. 3-9 to 3-11).
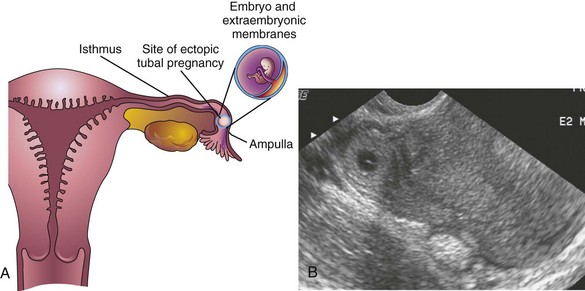
FIGURE 3–9 A, Frontal section of the uterus and left uterine tube, illustrating an ectopic pregnancy in the ampulla of the tube. B, Ectopic tubal pregnancy. Endovaginal axial sonogram of the uterine fundus and isthmic portion of the right uterine tube. The ring-like mass is a 4-week ectopic chorionic sac in the tube.
(Courtesy of E.A. Lyons, M.D., Professor of Radiology and Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
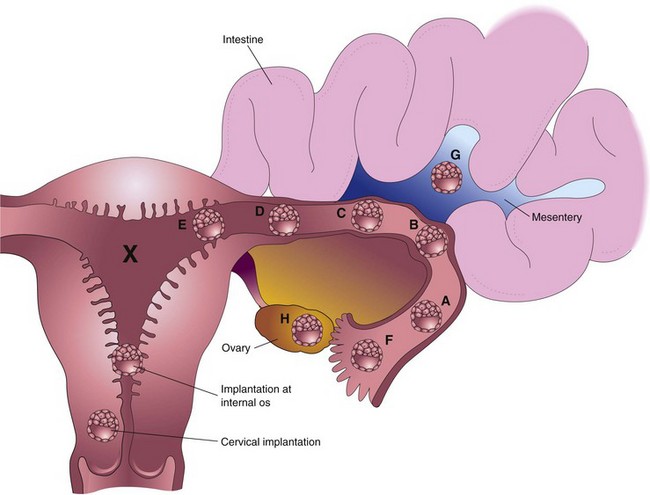
FIGURE 3–10 Implantation sites of blastocysts. The usual site in the posterior wall of the body of the uterus is indicated by an X. The approximate order of frequency of ectopic implantations is indicated alphabetically (A, most common, H, least common). A to F, tubal pregnancies; G, abdominal pregnancy; H, ovarian pregnancy. Tubal pregnancies are the most common type of ectopic pregnancy. Although appropriately included with uterine pregnancy sites, a cervical pregnancy is often considered to be an ectopic pregnancy.
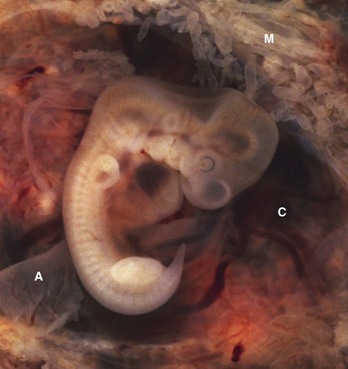
FIGURE 3–11 Tubal pregnancy. The uterine tube has been surgically removed and sectioned to show the 5-week old embryo (10 mm crown-rump length CRL) within the opened chorionic sac (C). Note the fragments of the amnion (A) and the thin mucosal folds (M) of the uterine tube projecting into the lumen of the tube.
(Courtesy of Ed Uthman, M.D., pathologist, Houston/Richmond, TX.)
Extrauterine Implantations
Blastocysts sometimes implant outside the uterus. These implantations result in ectopic pregnancies; 95% to 98% of ectopic implantations occur in the uterine tubes, most often in the ampulla and isthmus (Figs. 3-9 to 3-11). The incidence of ectopic pregnancy has increased in most countries, ranging from 1 in 80 to 1 in 250 pregnancies, depending on the socioeconomic level of the population. In the United States, the frequency of ectopic pregnancy is approximately 2% of all pregnancies; tubal pregnancy is the main cause of maternal deaths during the first trimester.
A woman with a tubal pregnancy has signs and symptoms of pregnancy (e.g., misses her menstrual period). She may also experience abdominal pain and tenderness because of distention of the uterine tube, abnormal bleeding, and irritation of the pelvic peritoneum (peritonitis). The pain may be confused with appendicitis if the pregnancy is in the right uterine tube. Ectopic pregnancies produce β-human chorionic gonadotropin at a slower rate than normal pregnancies; consequently β-human chorionic gonadotropin assays may give false-negative results if performed too early. Transvaginal ultrasonography is very helpful in the early detection of ectopic tubal pregnancies.
There are several causes of tubal pregnancy, and are often related to factors that delay or prevent transport of the cleaving zygote to the uterus, for example, by mucosal adhesions in the uterine tube or from blockage of the tube that is caused by scarring resulting from pelvic inflammatory disease. Ectopic tubal pregnancies usually result in rupture of the uterine tube and hemorrhage into the peritoneal cavity during the first 8 weeks, followed by death of the embryo. Tubal rupture and hemorrhage constitute a threat to the mother’s life. The affected tube and conceptus are usually surgically removed (Fig. 3-11).
When blastocysts implant in the isthmus of the uterine tube (see Fig. 3-10D), the tube tends to rupture early because this narrow part of the tube is relatively unexpandable, and often with extensive bleeding, probably because of the rich anastomoses between ovarian and uterine vessels in this area. When blastocysts implant in the uterine (intramural) part of the tube (Fig. 3-10E), they may develop beyond 8 weeks before expulsion occurs. When an intramural uterine tubal pregnancy ruptures, it usually bleeds profusely.
Blastocysts that implant in the ampulla or on fimbriae of the uterine tube may be expelled into the peritoneal cavity where they commonly implant in the rectouterine pouch (a pocket formed by the deflection of the peritoneum from the rectum to the uterus). In exceptional cases, an abdominal pregnancy may continue to full term and the fetus may be delivered alive through an abdominal incision. Usually, however, the placenta attaches to abdominal organs (Fig. 3-10G) and causes considerable intraperitoneal bleeding. Abdominal pregnancy increases the risk of maternal death from hemorrhage by a factor of 90 when compared with an intrauterine pregnancy, and seven times more than that for tubal pregnancy. In very unusual cases, an abdominal conceptus dies and is not detected; the fetus becomes calcified, forming a “stone fetus”—lithopedion (Greek lithos, stone, + paidion, child).
Simultaneous intrauterine and extrauterine pregnancies are unusual, occurring approximately 1 in 7000 pregnancies. The ectopic pregnancy is masked initially by the presence of the uterine pregnancy. Usually the ectopic pregnancy can be terminated by surgical removal of the involved uterine tube without interfering with the intrauterine pregnancy (Fig. 3-11).
Cervical implantations are unusual (Fig. 3-10); in some cases, the placenta becomes firmly attached to fibrous and muscular tissues of the cervix, often resulting in bleeding and requiring subsequent surgical intervention, such as hysterectomy (excision of uterus).
 Summary of Implantation
Summary of Implantation
Implantation of the blastocyst in the uterine endometrium begins at the end of the first week and is completed by the end of the second week. The cellular and molecular events relating to implantation are complex. Implantation may be summarized as follows:
Placenta Previa
Implantation of a blastocyst in the inferior segment of the uterus near the internal os (opening) of the cervix results in placenta previa, a placenta that partially or completely covers the os (Fig. 3-10). Placenta previa may cause bleeding because of premature separation of the placenta during pregnancy or at the time of delivery of the fetus (see Chapter 7).
Spontaneous Abortion of Embryos and fetuses
Miscarriage (early pregnancy loss) occurs within the first 12 completed weeks of pregnancy with a frequency of 10% to 20%. Most spontaneous abortions (miscarriage) of embryos occur during the first 3 weeks. Sporadic and recurrent spontaneous abortions are two of the most common gynecologic problems. The frequency of early spontaneous abortions is difficult to establish because they often occur before a woman is aware that she is pregnant. A spontaneous abortion occurring several days after the first missed period is very likely to be mistaken for a delayed menstruation.
More than 50% of all known spontaneous abortions result from chromosomal abnormalities. The higher incidence of early spontaneous abortions in older women probably results from the increasing frequency of nondisjunction during oogenesis (see Chapter 2). It has been estimated that 30% to 50% of all zygotes never develop into blastocysts and implant. Failure of blastocysts to implant may result from a poorly developed endometrium; however, in many cases, there are probably lethal chromosomal abnormalities in the embryo. There is a higher incidence of spontaneous abortion of fetuses with neural tube defects, cleft lip, and cleft palate.
Inhibition of Implantation
The administration of relatively large doses of progestins and/or estrogens (“morning-after pills”) for several days, beginning shortly after unprotected sexual intercourse, usually does not prevent fertilization but often prevents implantation of the blastocyst. A high dose of diethylstilbestrol, given daily for 5 to 6 days, may also accelerate passage of the cleaving zygote along the uterine tube. Normally, the endometrium progresses to the luteal phase of the menstrual cycle as the zygote forms, undergoes cleavage, and enters the uterus. The large amount of estrogen disturbs the normal balance between estrogen and progesterone that is necessary for preparation of the endometrium for implantation.
An intrauterine device inserted into the uterus through the vagina and cervix usually interferes with implantation by causing a local inflammatory reaction. Some intrauterine devices contain progesterone that is slowly released and interferes with the development of the endometrium so that implantation does not usually occur.
Summary of Second Week
Clinically Oriented Problems
Case 3–1
A 22-year-old woman who complained of a severe “chest cold” was sent for a radiograph of her thorax.
Case 3–2
A woman who was sexually assaulted during her fertile phase was given large doses of estrogen twice for 1 day to interrupt a possible pregnancy.
Case 3–3
A 23-year-old woman consulted her physician about severe right lower abdominal pain. She said that she had missed two menstrual periods. A diagnosis of ectopic pregnancy was made.
Case 3–4
A 30-year-old woman had an appendectomy toward the end of her menstrual cycle; 8- months later, she had a child with a congenital anomaly of the brain.
months later, she had a child with a congenital anomaly of the brain.
References and Suggested Reading
Bianchi DW, Wilkins-Haug LE, Enders AC, et al. Origin of extraembryonic mesoderm in experimental animals: relevance to chorionic mosaicism in humans. Am J Med Genet. 1993;46:542.
Cadmak H, Taylor HS. Implantation failure: treatment and clinical implications. Hum Reprod Update. 2011;17:242.
Callen PW. Obstetric ultrasound examination. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Cole LA. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod Biol Endocrinol. 2009;7:8.
Coulam CB, Faulk WP, McIntyre JA. Spontaneous and recurrent abortions. In: Quilligan EJ, Zuspan FP, editors. Current Therapy in Obstetrics and Gynecology, Vol 3. Philadelphia: WB Saunders; 1990.
Dickey RP, Gasser R, Olar TT, et al. Relationship of initial chorionic sac diameter to abortion and abortus karyotype based on new growth curves for the 16 to 49 post-ovulation day. Hum Reprod. 1994;9:559.
Enders AC, King BF. Formation and differentiation of extraembryonic mesoderm in the rhesus monkey. Am J Anat. 1988;181:327.
Hertig AT, Rock J. Two human ova of the pre-villous stage, having a development age of about seven and nine days respectively. Contrib Embryol Carnegie Inst. 1945;31:65.
Hertig AT, Rock J. Two human ova of the pre-villous stage, having a developmental age of about eight and nine days, respectively. Contrib Embryol Carnegie Inst. 1949;33:169.
Hertig AT, Rock J, Adams EC. A description of 34 human ova within the first seventeen days of development. Am J Anat. 1956;98:435.
Hertig AT, Rock J, Adams EC, et al. Thirty-four fertilized human ova, good, bad, and indifferent, recovered from 210 women of known fertility. Pediatrics. 1959;23:202.
Kodaman PH, Taylor HS. Hormonal regulation of implantation. Obstet Gynecol Clin North Am. 2004;31:745.
Lessey BA. The role of the endometrium during embryo implantation. Human Reprod. 2000;15(Suppl 6):39.
Levine D. Ectopic pregnancy. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Lindsay DJ, Lovett IS, Lyons EA, et al. Endovaginal sonography: Yolk sac diameter and shape as a predictor of pregnancy outcome in the first trimester. Radiology. 1992;183:115.
Lipscomb GH. Ectopic pregnancy. In Copeland LJ, Jarrell JF, editors: Textbook of Gynecology, ed 4, Philadelphia: WB Saunders, 2000.
Luckett WP. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat. 1978;152:59.
. The Human Yolk Sac and Yolk Sac Tumors. Nogales FF. Springer-Verlag, New York, 1993.
Sen C, Yayla M. Chromosomal abnormalities of the embryo. In: Kurjak A, Chervenak FA, Carrera JM, editors. The Embryo as a Patient. New York: Parthenon Publishing Group, 2001.
Staun-Ram E, Goldman S, Shalev E. Ets-2 and p53 mediate cAMP-induced MMP-2 expression, activity and trophoblast invasion. Reprod Biol Endocrinol. 2009;7:135.
Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005;3:56.
Streeter GL. Developmental horizons in human embryos. Description of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. Contrib Embryol Carnegie Inst. 1942;30:211.
Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221.