Chapter 12 Urogenital System
The urogenital system is divided functionally into the urinary system and the genital system. The system includes all the organs involved in reproduction and forming and voiding urine. Embryologically, the systems are closely associated, especially during their early stages of development.
The urogenital system develops from the intermediate mesenchyme (mesoderm) derived from the dorsal body wall of the embryo (Fig. 12-1A and B). This mesenchyme (primordial embryonic connective tissue) is primarily responsible for the formation of the kidneys and internal genitalia and their ducts. During folding of the embryo in the horizontal plane (see Chapter 5), this mesoderm is carried ventrally and loses its connection with the somites (Fig. 12-1C and D). A longitudinal elevation of mesoderm—the urogenital ridge—forms on each side of the dorsal aorta (Fig. 12-1F). The part of the urogenital ridge giving rise to the urinary system is the nephrogenic cord (Fig. 12-1C to F); the part giving rise to the genital system is the gonadal ridge (see Fig. 12-29C).
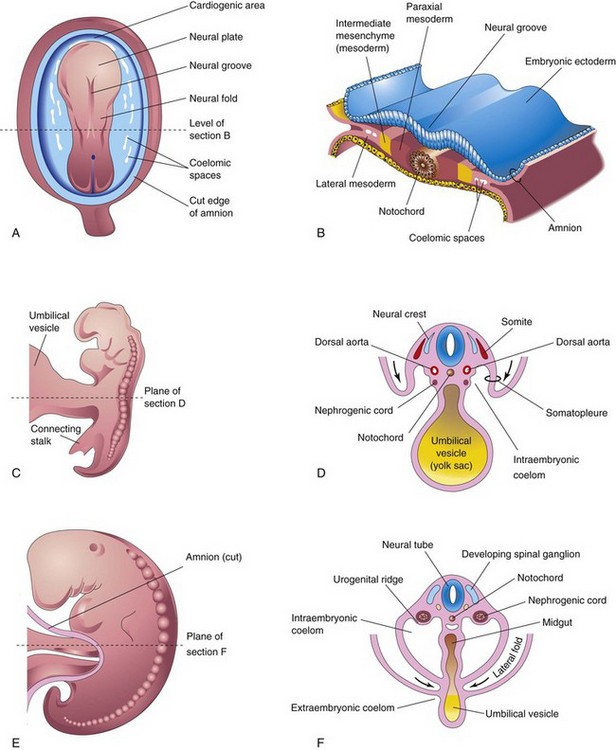
FIGURE 12–1 A, Dorsal view of an embryo during the third week (approximately 18 days). B, Transverse section of the embryo showing the position of the intermediate mesenchyme before lateral folding of the embryo. C, Lateral view of an embryo during the fourth week (approximately 24 days). D, Transverse section of the embryo after the commencement of folding, showing the nephrogenic cords. E, Lateral view of an embryo later in the fourth week (approximately 26 days). F, Transverse section of the embryo showing the lateral folds meeting each other ventrally.
Expression of the following genes is needed for the formation of the urogenital ridge: Wilms tumor suppressor 1 (WT1), steroidogenic factor 1, and DAX1 gene.
 Development of Urinary System
Development of Urinary System
The urinary system begins to develop before the genital system and consists of:
Development of Kidneys and Ureters
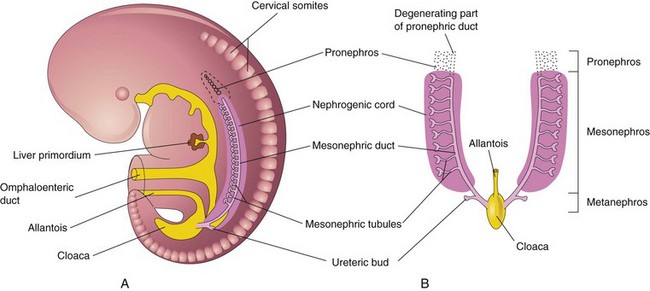
Three sets of successive kidneys develop in human embryos. The first set—pronephroi—is rudimentary and nonfunctional. The second set—mesonephroi—is well developed and functions briefly during the early fetal period. The third set—metanephroi—forms the permanent kidneys.
Pronephroi
These bilateral, transitory structures appear early in the fourth week. They are represented by a few cell clusters and tubular structures in the neck region (Fig. 12-2A). The pronephric ducts run caudally and open into the cloaca (Fig. 12-2B). The pronephroi soon degenerate; however, most parts of the pronephric ducts persist and are used by the next set of kidneys.
Mesonephroi
These large, elongated excretory organs appear late in the fourth week, caudal to the pronephroi (Fig. 12-2). The mesonephroi are well developed and function as interim kidneys for approximately 4 weeks, until the permanent kidneys develop and function (Fig. 12-3). The mesonephric kidneys consist of glomeruli (10–50 per kidney) and tubules (Figs. 12-3 to 12-5). The mesonephric tubules open into bilateral mesonephric ducts, which were originally the pronephric ducts. The mesonephric ducts open into the cloaca. The mesonephroi degenerate toward the end of the first trimester however, their metanephric tubules become the efferent ductules of the testes. The mesonephric ducts have several adult derivatives in males (Table 12-1).
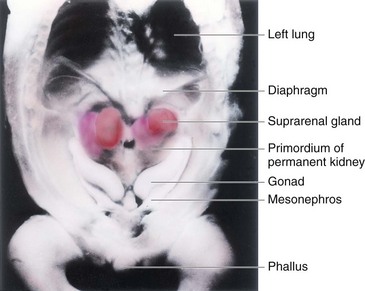
FIGURE 12–3 Dissection of the thorax, abdomen, and pelvis of an embryo at approximately 54 days. Observe the large suprarenal glands and the elongated mesonephroi (mesonephric kidneys). Also observe the gonads (testes or ovaries). The phallus will develop into a penis or clitoris depending on the genetic sex of the embryo.
(From Nishimura H [ed]: Atlas of Human Prenatal Histology. Tokyo, Igaku-Shoin, 1983, with permission.)
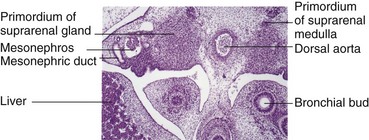
FIGURE 12–4 Photomicrograph of a transverse section of an embryo at approximately 42 days, showing the mesonephros and developing suprarenal glands.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
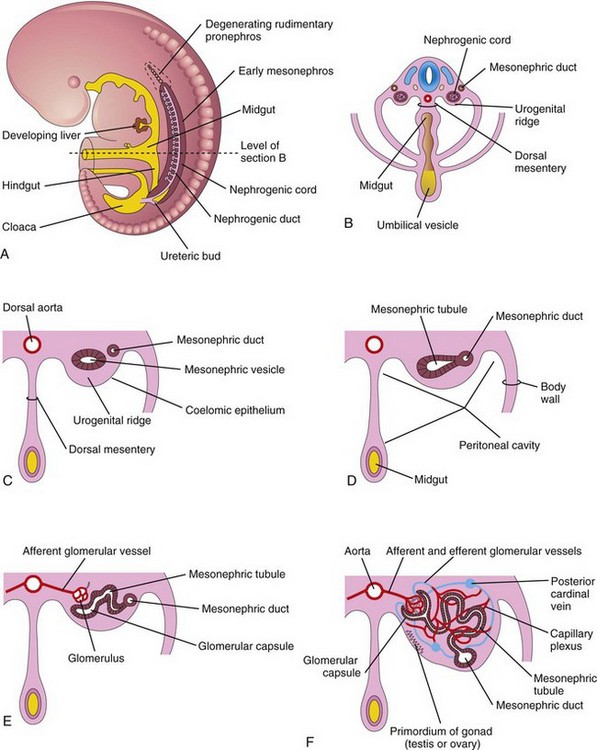
FIGURE 12–5 A, Lateral view of a 5-week embryo showing the extent of the early mesonephros and the ureteric bud, the primordium of the metanephros (primordium of the permanent kidney). B, Transverse section of the embryo showing the nephrogenic cords from which the mesonephric tubules develop. C to F, Successive stages in the development of mesonephric tubules between the 5th and 11th weeks. The expanded medial end of the mesonephric tubule is invaginated by blood vessels to form a glomerular capsule.
Table 12–1 Derivatives and Vestigial Remains of Embryonic Urogenital Structures*
| MALE | EMBRYONIC STRUCTURE | FEMALE |
|---|---|---|
| Testis | Indifferent gonad | Ovary |
| Seminiferous tubules | Cortex | Ovarian follicles |
| Rete testis | Medulla | Rete ovarii |
| Gubernaculum testis | Gubernaculum | Ovarian ligament Round ligament of uterus |
| Efferent ductules of testis | Mesonephric tubules | Epoophoron |
| Paradidymis | Paroophoron | |
| Appendix of epididymis | Mesonephric duct | Appendix vesiculosa |
| Duct of epididymis | Duct of epoophoron | |
| Ductus deferens | Longitudinal duct; Gartner duct | |
| Ureter, pelvis, calices, and collecting tubules | Ureter; pelvis, calices, and collecting tubules | |
| Ejaculatory duct and seminal gland | ||
| Appendix of testis | Paramesonephric duct | Hydatid (of Morgagni) |
| Uterine tube | ||
| Uterus | ||
| Urinary bladder | Urogenital sinus | Urinary bladder |
| Urethra (except navicular fossa) | Urethra | |
| Prostatic utricle | Vagina | |
| Prostate | Urethral and paraurethral glands | |
| Bulbourethral glands | Greater vestibular glands | |
| Seminal colliculus | Sinus tubercle | Hymen |
| Penis | Phallus | Clitoris |
| Glans of penis | Glans of clitoris | |
| Corpora cavernosa of penis | Corpora cavernosa of clitoris | |
| Corpus spongiosum of penis | Bulb of vestibule | |
| Ventral aspect of penis | Urogenital folds | Labia minora |
| Scrotum | Labioscrotal swellings | Labia majora |
Metanephroi
Metanephroi—primordia of permanent kidneys—begin to develop in the fifth week (Fig. 12-6) and become functional approximately 4 weeks later. Urine formation continues throughout fetal life. It is excreted into the amniotic cavity and mixes with the amniotic fluid.
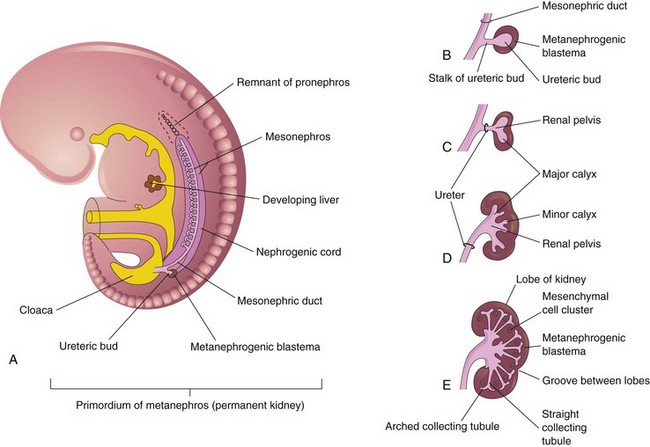
FIGURE 12–6 Development of the permanent kidney. A, Lateral view of a 5-week embryo showing the ureteric bud, the primordium of the metanephros. B to E, Successive stages in the development of the ureteric bud (fifth to eighth weeks). Observe the development of the kidney: ureter, renal pelvis, calices, and collecting tubules.
The permanent kidneys develop from two sources (Fig. 12-6):
The ureteric bud is a diverticulum (outgrowth) from the mesonephric duct near its entrance into the cloaca. The metanephrogenic blastema is derived from the caudal part of the nephrogenic cord (Fig. 12-6). As the ureteric bud elongates, it penetrates the metanephrogenic blastema—a metanephric mass of mesenchyme (Fig. 12-6B).
The stalk of the ureteric bud becomes the ureter. The cranial part of the bud undergoes repetitive branching, forming branches which differentiate into the collecting tubules of the metanephros (Figs. 12-6E and 12-7A and B). The first four generations of tubules enlarge and become confluent to form the major calices (see Fig. 12-6D), and the second four generations coalesce to form the minor calices. The end of each arched collecting tubule induces clusters of mesenchymal cells in the metanephrogenic blastema to form small metanephric vesicles (Fig. 12-7A). These vesicles elongate and become metanephric tubules (Fig. 12-7B and C).
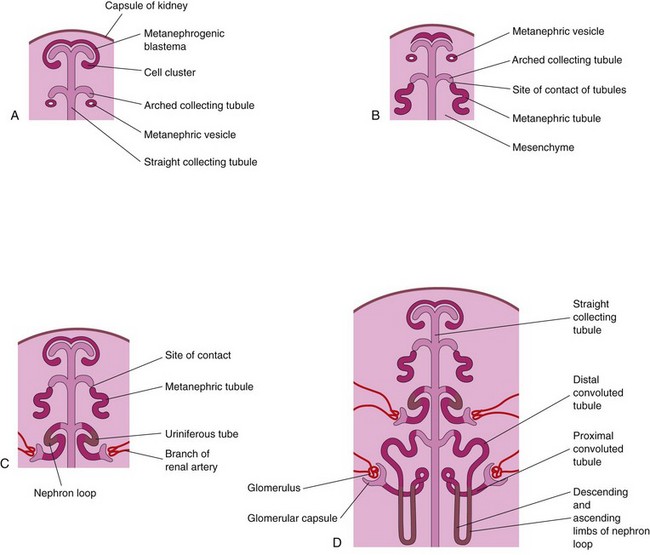
FIGURE 12–7 Development of nephrons. A, Nephrogenesis commences around the beginning of the eighth week. B and C, Note that the metanephric tubules, the primordia of the nephrons, connect with the collecting tubules to form uriniferous tubules. D, Observe that nephrons are derived from the metanephrogenic blastema and the collecting tubules are derived from the ureteric bud.
The proximal ends of these tubules are invaginated by glomeruli. The tubules differentiate into proximal and distal convoluted tubules, and the nephron loop (Henle loop), together with the glomerulus and its capsule, constitute a nephron (Fig. 12-7D). Proliferation of the nephron progenitor cells and formation of the nephrons are dependent on BMP7 and Wnt-4 (Notch)/β-catenin signaling. Each distal convoluted tubule contacts an arched collecting tubule, and the tubules become confluent.
A uriniferous tubule consists of two embryologically different parts (Figs. 12-6 and 12-7):
Between the 10th and 18th weeks, the number of glomeruli increases gradually and then increases rapidly until the 32nd week, when an upper limit is reached. At term, nephron formation is complete, with each kidney containing as many as 2 million nephrons.
The fetal kidneys are subdivided into lobes (Fig. 12-8). The lobulation usually disappears at the end of the first year of infancy as the nephrons increase and grow. The increase in kidney size after birth results mainly from the elongation of the proximal convoluted tubules as well as an increase of interstitial tissue. Nephron formation is complete at birth except in premature infants. Although glomerular filtration begins at approximately the ninth fetal week, functional maturation of the kidneys and increasing rates of filtration occur after birth.
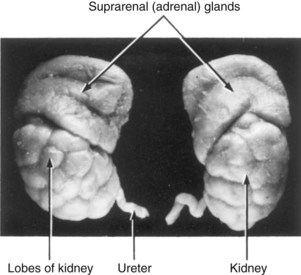
FIGURE 12–8 The kidneys and suprarenal glands of a 28-week fetus (x2). The lobes usually disappear by the end of the first postnatal year. Note the large size of the suprarenal (adrenal) glands.
Branching of the ureteric bud is dependent on induction by the metanephric mesenchyme. Differentiation of the nephrons depends on induction by the collecting tubules. The ureteric bud and the metanephrogenic blastema interact and induce each other, a process known as reciprocal induction, to form the permanent kidneys.
Molecular studies, especially knockout and transgenic analyses in the mouse, show that this process involves two principal signaling systems that use conserved molecular pathways. Recent research has provided insight into the complex interrelated molecular events regulating the development of the kidneys (Fig. 12-9). Before induction, a transcription factor WT1 is expressed in the metanephrogenic blastema supporting the survival of the as yet uninduced mesenchyme. Expression of Pax2, Eya1, and Sall1 is required for the expression of glial-derived neurotropic factor (GDNF) in the metanephric mesenchyme. The transcription factors vHNF1 (HNF1 beta) and GDNF plays an essential role in the induction and branching of the ureteric bud (branching morphogenesis). The receptor for GDNF, c-ret, is first expressed in the mesonephric duct, but later becomes localized on the tip of the ureteric bud. Subsequent branching is controlled by transcription factors, including Emx2 and Pax2 and growth factor signals of the Wnt, FGF, and BMP families. Transformation of the metanephric mesenchyme to the epithelial cells of the nephron—mesenchymal-epithelial transition—is regulated by mesenchyme factors including Wnt4. Recent studies reveal that mutation of the angiotensin-type 2 receptor gene might account for kidney and urinary tract abnormalities.
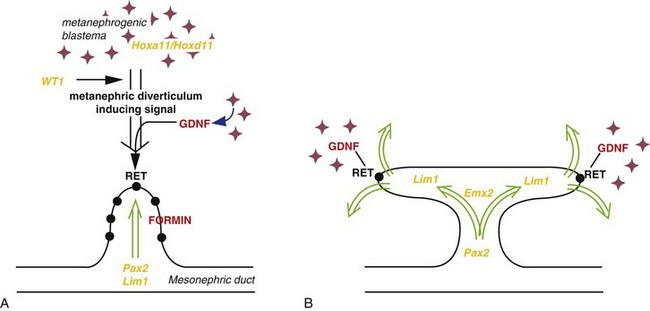
FIGURE 12–9 Molecular control of kidney development. A, The ureteric bud requires inductive signals derived from metanephrogenic blastema under control of transcription factors (orange text), such as WT1 and signaling molecules (red text) including glial-derived neurotropic factor (GDNF) and its epithelial receptor, RET. The normal ureteric bud response to these inductive signals is under the control of transcription factors such as Pax2, Lim1, and the FORMIN gene. B, Branching of the ureteric bud is initiated and maintained by interaction with the mesenchyme under the regulation of genes such as Emx2 and specified expression of GDNF and RET at the tips of the invading ureteric bud.
(From Piscione TD, Rosenblum ND: The malformed kidney: Disruption of glomerular and tubular development. Clin Genet 56:341–356, 1999.)
Positional Changes of Kidneys
Initially the primordial permanent kidneys lie close to each other in the pelvis, ventral to the sacrum (Fig. 12-10A). As the abdomen and pelvis grow, the kidneys gradually relocate to the abdomen and move farther apart (Fig. 12-10B and C). They attain their adult position by the ninth week (Fig. 12-10D). This “ascent” results mainly from the growth of the embryo’s body caudal to the kidneys. In effect, the caudal part of the embryo grows away from the kidneys so that they progressively occupy their normal position on either side of the vertebral column.
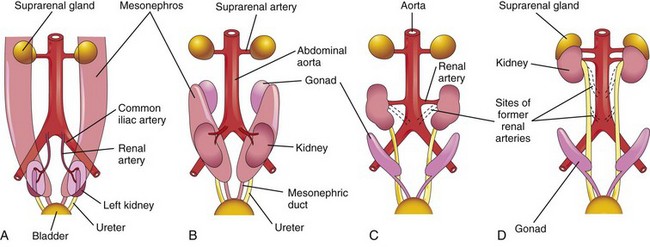
FIGURE 12–10 A to D, Diagrammatic ventral views of the abdominopelvic region of embryos and fetuses (sixth to ninth weeks), showing medial rotation and relocation of the kidneys from the pelvis to the abdomen. C and D, Note that as the kidneys relocate (ascend), they are supplied by arteries at successively higher levels and that the hila of the kidneys are directed anteromedially.
Initially the hilum of each kidney, where blood vessels, the ureter, and nerves enter and leave, face ventrally; however, as the kidneys relocate (“ascend”), they rotate medially almost 90 degrees. By the ninth week, the hila are directed anteromedially (Fig. 12-10C and D). Eventually the kidneys become retroperitoneal (external to the peritoneum) on the posterior abdominal wall.
Changes in Blood Supply of Kidneys
During the changes in the kidneys position, they receive their blood supply from vessels that are close to them. Initially, the renal arteries are branches of the common iliac arteries (Fig. 12-10A and B). Later, the kidneys receive their blood supply from the distal end of the aorta. When they are located at a higher level, they receive new branches from the aorta (Fig. 12-10C and D). Normally the caudal branches of the renal vessels undergo involution and disappear.
The position of the kidneys becomes fixed once they come into contact with the suprarenal glands in the ninth week. The kidneys receive their most cranial arterial branches from the abdominal aorta; these branches become the permanent renal arteries. The right renal artery is longer and often more superior.
Accessory Renal Arteries
The common variations in the blood supply to the kidneys reflect the manner in which the blood supply continually changed during embryonic and early fetal life (Fig. 12-10). Approximately 25% of adult kidneys have two to four renal arteries. Accessory (supernumerary) renal arteries usually arise from the aorta superior or inferior to the main renal artery and follow it to the hilum of the kidney (Fig. 12-11A, C, and D). Accessory arteries may also enter the kidneys directly, usually into the superior or inferior poles (Fig. 12-11B). An accessory artery to the inferior pole (polar renal artery) may cross anterior to the ureter and obstruct it, causing hydronephrosis—distention of the renal pelvis and calices with urine. (If the artery enters the inferior pole of the right kidney, it usually crosses anterior to the inferior vena cava and ureter.)

FIGURE 12–11 Common variations of renal vessels. A, Multiple renal arteries. B, Note the accessory vessel entering the inferior pole of the kidney and that it is obstructing the ureter and producing an enlarged renal pelvis. C and D, Supernumerary renal veins.
The accessory renal arteries are end arteries; consequently, if an accessory artery is damaged or ligated, the part of the kidney supplied by it will be ischemic (loss of blood supply). Accessory arteries are approximately twice as common as accessory veins.
Congenital Anomalies of Kidneys and Ureters
Some type of defect of the kidneys and ureters occurs in 3% to 4% of neonates. Defects in shape and position are most common. Many fetal urinary tract abnormalities can be detected before birth by ultrasonography.
Renal Agenesis
Unilateral renal agenesis (absence) occurs approximately once in every 1000 neonates. Males are affected more often than females and the left kidney is usually the one that is absent (Figs. 12-12A and B and 12-13A). Unilateral renal agenesis often causes no symptoms and is usually not discovered during infancy because the other kidney usually undergoes compensatory hypertrophy and performs the function of the missing kidney. Unilateral renal agenesis should be suspected in infants with a single umbilical artery (see Chapter 7).

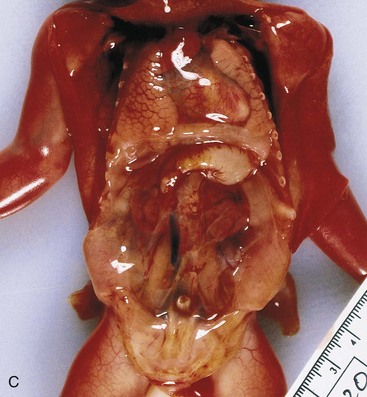
FIGURE 12–12 Sonograms of a fetus with unilateral renal agenesis. A, Transverse scan at the level of the lumbar region of the vertebral column (Sp) showing the right kidney (RK) but not the left kidney. B, Transverse scan at a slightly higher level showing the left suprarenal gland (between cursors) within the left renal fossa. C, Bilateral renal agenesis in a male fetus of 19.5 weeks.
(A and B, From Mahony BS: Ultrasound evaluation of the fetal genitourinary system. In Callen PW [ed]: Ultrasonography in Obstetrics and Gynecology, 3rd ed. Philadelphia, WB Saunders, 1994. C, Courtesy of Dr. D.K. Kalousek, Department of Pathology, University of British Columbia, Children’s Hospital, Vancouver, British Columbia, Canada.)
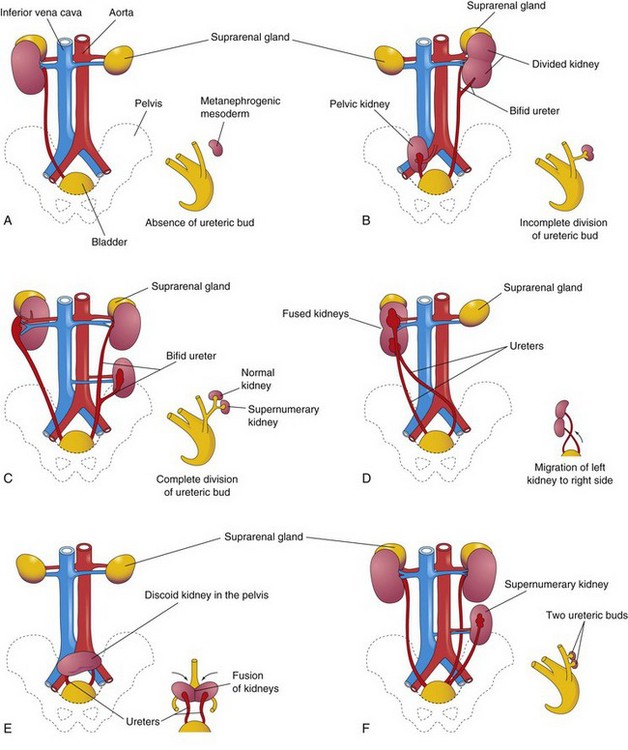
FIGURE 12–13 Illustrations of various birth defects of the urinary system. The small sketch to the lower right of each drawing illustrates the probable embryologic basis of the defect. A, Unilateral renal agenesis. B, Right side, pelvic kidney; left side, divided kidney with a bifid ureter. C, Right side, malrotation of the kidney; the hilum is facing laterally; left side, bifid ureter and supernumerary kidney. D, Crossed renal ectopia. The left kidney crossed to the right side and fused with the right kidney. E, Pelvic kidney or discoid kidney, resulting from fusion of the kidneys while they were in the pelvis. F, Supernumerary left kidney resulting from the development of two ureteric buds.
Bilateral renal agenesis (Fig. 12-12C ) is associated with oligohydramnios (small amount of amniotic fluid) because little or no urine is excreted into the amniotic cavity. This condition occurs approximately once in 3000 births, and is incompatible with postnatal life. About 20% of persons with the Potter syndrome are caused by bilateral renal agenesis. These infants have a characteristic facial appearance: the eyes are widely separated and have palpebronasal folds (epicanthic folds), the ears are low-set, the nose is broad and flat, the chin is receding, and there are limb and respiratory anomalies. Infants with bilateral renal agenesis usually die shortly after birth.
Renal agenesis results when the ureteric buds do not develop or the primordia (stalks of buds) of the ureters degenerate. Failure of the ureteric buds to penetrate the metanephrogenic blastema results in failure of kidney development because no nephrons are induced by the collecting tubules to develop from the metanephrogenic blastema. Renal agenesis probably has a multifactorial etiology. There is clinical evidence that complete in utero involution of polycystic kidneys could lead to renal agenesis with a blind ending ureter on the same side.
Malrotated Kidney
If a kidney fails to rotate, the hilum faces anteriorly; that is, the fetal kidney retains its embryonic position (Figs. 12-10A and 12-13C ). If the hilum faces posteriorly, rotation of the kidney proceeded too far; if it faces laterally, lateral instead of medial rotation occurred. Abnormal rotation of the kidneys is often associated with ectopic kidneys.
Ectopic Kidneys
One or both kidneys may be in an abnormal position (see Fig. 12-13B, E, and F ). Most ectopic kidneys are located in the pelvis (Fig. 12-14), but some lie in the inferior part of the abdomen. Pelvic kidneys and other forms of ectopia result from failure of the kidneys to ascend.
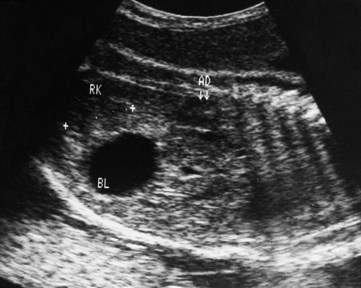
FIGURE 12–14 Sonogram of the pelvis of a fetus at 29 weeks. Observe the low position of the right kidney (RK) near the urinary bladder (BL). This pelvic kidney resulted from its failure to ascend during the sixth to ninth weeks. Observe the normal location of the right suprarenal gland (AD), which develops separately from the kidney.
(Courtesy of Dr. Lyndon M. Hill, director of ultrasound, Magee-Women’s Hospital, Pittsburgh, PA.)
Pelvic kidneys are close to each other and usually fuse to form a discoid (“pancake”) kidney (Fig. 12-13E ). Ectopic kidneys receive their blood supply from blood vessels near them (internal or external iliac arteries and/or abdominal aorta). They are often supplied by several vessels. Sometimes a kidney crosses to the other side, resulting in crossed renal ectopia with or without fusion (Fig. 12-15), showing both kidneys on the right side of the abdomen. An unusual type of abnormal kidney is unilateral fused kidney. In such cases, the developing kidneys fuse after they leave the pelvis, and one kidney attains its normal position, carrying the other kidney with it (Fig. 12-13D).
Horseshoe Kidney
In 0.2% of the population, the poles of the kidneys are fused; usually it is the inferior poles that fuse. The large U-shaped kidney usually lies in the pubic region, anterior to the inferior lumbar vertebrae (Fig. 12-16). Normal ascent of the fused kidneys is prevented because they are held down by the root of the inferior mesenteric artery.
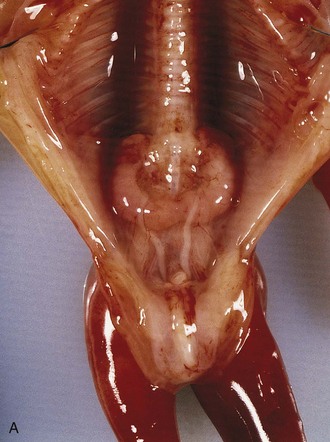
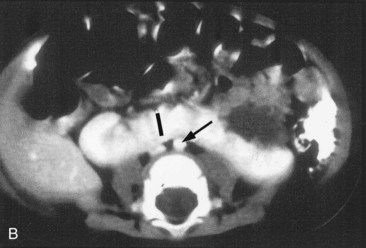
FIGURE 12–16 A, Horseshoe kidney in the lower abdomen of a 13-week female fetus. B, Contrast-enhanced computed tomography scan of the abdomen of an infant with a horseshoe kidney. Note the isthmus (vascular) of renal tissue (I) connecting the right and left kidneys just anterior to the aorta (arrow) and inferior vena cava.
(A, Courtesy of Dr. D.K. Kalousek, Department of Pathology, University of British Columbia, Children’s Hospital, Vancouver, British Columbia, Canada; B, Courtesy of Dr. Prem S. Sahni, formerly of the Department of Radiology, Children’s Hospital, Winnipeg, Manitoba, Canada.)
A horseshoe kidney usually produces no symptoms because its collecting system develops normally and the ureters enter the bladder. If urinary flow is impeded, signs and symptoms of obstruction and/or infection may appear. Approximately 7% of persons with Turner syndrome have horseshoe kidneys.
Duplications of Urinary Tract
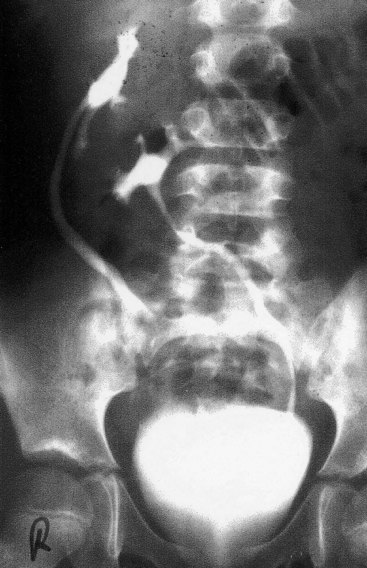
Duplications of the abdominal part of the ureter and the renal pelvis are common (Fig. 12-13F ). These defects result from abnormal division of the ureteric bud. Incomplete division of the metanephric diverticulum results in a divided kidney with a bifid ureter (Fig. 12-13B). Complete division results in a double kidney with a bifid ureter (Fig. 12-13C ) or separate ureters (Fig. 12-17).

FIGURE 12–17 A duplex kidney with two ureters and renal pelves. A, Longitudinal section through the kidney showing two renal pelves and calices. B, Anterior surface of the kidney. C, Intravenous urography showing duplication of the right kidney and ureter in a 10-year-old male. The distal ends of the right ureter are fused at the level of the first sacral vertebra.
(Courtesy of Dr. Prem S. Sahni, formerly of the Department of Radiology, Children’s Hospital, Winnipeg, Manitoba, Canada.)
A supernumerary kidney (Fig. 12-13F ) with its own ureter, which is rare, probably results from the formation of two ureteric buds.
Ectopic Ureter
An ectopic ureter does not enter the urinary bladder. In males, the ureter usually opens into the neck of the bladder or the prostatic part of the urethra. They may also enter the ductus deferens, prostatic utricle, or seminal gland. In females, the ureter opens into the neck of the bladder, or the urethra, vagina, or vestibule of vagina (Fig. 12-18). Incontinence is the common complaint resulting from an ectopic ureter because the urine flowing from the orifice does not enter the bladder; instead it continually dribbles from the urethra in males and the urethra and/or vagina in females.
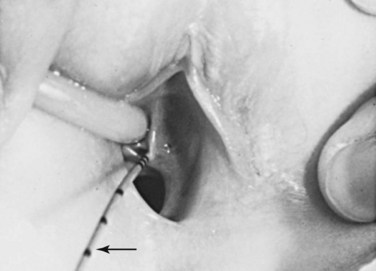
FIGURE 12–18 Ectopic ureter in a young girl. This ureter enters the vestibule of the vagina near the external urethral orifice. A thin ureteral catheter with transverse marks has been introduced through the ureteric orifice into the ectopic ureter. This girl had a normal voiding pattern and constant urinary dribbling.
(From Behrman RE, Kliegman RM, Arvin AM [eds]: Nelson Textbook of Pediatrics, 15th ed. Philadelphia, WB Saunders, 1996.)
An ectopic ureter results when the ureter is not incorporated into the trigone in the posterior part of the urinary bladder. Instead it is carried caudally with the mesonephric duct and is incorporated into the middle pelvic portion of the vesical part of the urogenital sinus. Because this part of the sinus becomes the prostatic urethra in males and the urethra in females, the location of ectopic ureteric orifices is understandable. When two ureters form on one side (Fig. 12-17), they usually open into the urinary bladder (Fig. 12-13F ).
Cystic Kidney Diseases
In autosomal recessive polycystic kidney disease, diagnosed at birth or in utero by ultrasonography, both kidneys contain many small cysts (Fig. 12-19A), which result in renal insufficiency. Death of the infant usually occurs shortly after birth; however, an increasing number of these infants are surviving because of postnatal dialysis and kidney transplantation.
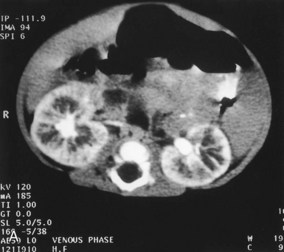
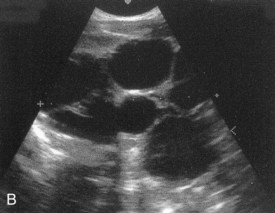
FIGURE 12–19 Cystic kidney disease. A, Computed tomography (CT) scan (contrast enhanced) of the abdomen of a 5-month-old male infant with AR polycystic kidney disease. Note the linear ectasia (cysts) of collecting tubules. B, Ultrasound scan of the left kidney of a 15-day-old male infant showing multiple noncommunicating cysts with no renal tissue (unilateral multicystic dysplastic kidney).
(Courtesy of Dr. Prem S. Sahni, formerly of the Department of Radiology, Children’s Hospital, Winnipeg, Manitoba, Canada.)
Multicystic dysplastic kidney disease results from dysmorphology during development of the renal system (Fig. 12-19B). The outcome for most children with multicystic dysplastic kidney disease is generally good because the disease is unilateral in 75% of the cases. In this kidney disease, fewer cysts are seen than in autosomal recessive polycystic kidney disease and they range in size from a few millimeters to many centimeters in the same kidney. It was thought that the cysts were the result of failure of the ureteric bud derivatives to join the tubules derived from the metanephrogenic blastema. It is now believed that the cystic structures are wide dilations of parts of the otherwise continuous nephrons, particularly the nephron loops (of Henle).
Development of Urinary Bladder
For descriptive purposes, the urogenital sinus is divided into three parts (Fig. 12-20C):
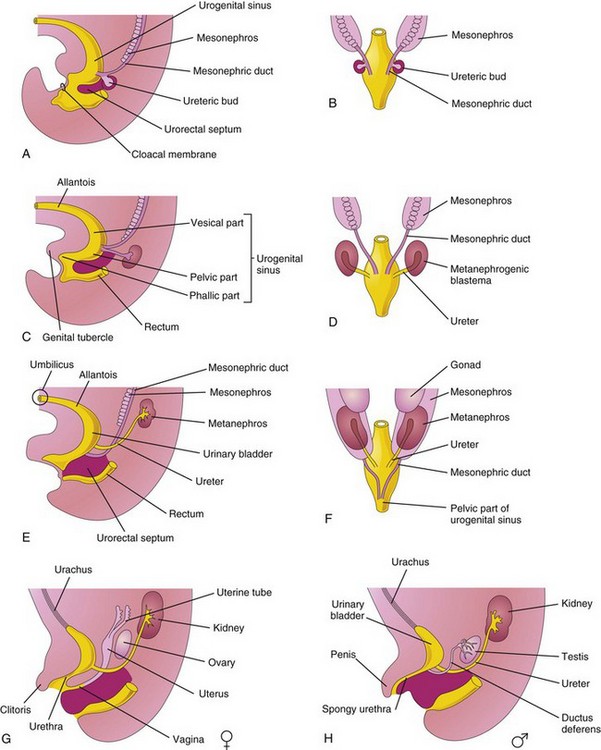
FIGURE 12–20 A, Lateral view of 5-week embryo, showing division of the cloaca by the urorectal septum into the urogenital sinus and rectum. B, D, and F, Dorsal views, showing the development of the kidneys and bladder, and changes in the location of the kidneys. C, E, G, and H, Lateral views. The stages shown in G and H are reached by the 12th week.
The bladder develops mainly from the vesical part of the urogenital sinus (Fig. 12-20C), but its trigone (triangular area at the base of the bladder between the openings of the ureters) is derived from the caudal ends of the mesonephric ducts (Fig. 12-20A and B). The entire epithelium of the bladder is derived from the endoderm of the vesical part of the urogenital sinus. The other layers of its wall develop from adjacent splanchnic mesenchyme.
Initially the bladder is continuous with the allantois (Fig. 12-20C). The allantois soon constricts and becomes a thick fibrous cord, the urachus. It extends from the apex of the bladder to the umbilicus (Figs. 12-20G and H and 12-21). In adults, the urachus is represented by the median umbilical ligament. As the bladder enlarges, distal parts of the mesonephric ducts are incorporated into its dorsal wall (Fig. 12-20B to H). These ducts contribute to the formation of the connective tissue in the trigone of the bladder. As the mesonephric ducts are absorbed, the ureters open separately into the urinary bladder (Fig. 12-20C to H). Partly because of traction exerted by the kidneys as they ascend, the orifices of the ureters move superolaterally and enter obliquely through the base of the bladder. In males, the orifices of the mesonephric ducts move close together and enter the prostatic part of the urethra as the caudal ends of these ducts develop into the ejaculatory ducts. In females, the distal ends of the mesonephric ducts degenerate.
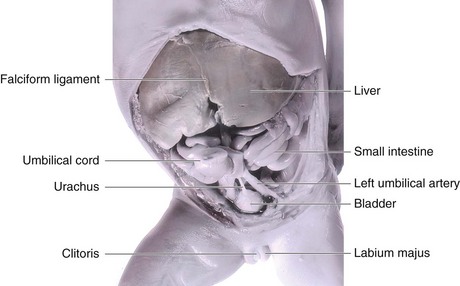
FIGURE 12–21 Dissection of the abdomen and pelvis of an 18-week female fetus showing the relation of the urachus to the urinary bladder and umbilical arteries.
In infants and children, the urinary bladder, even when empty, is in the abdomen. It begins to enter the greater pelvis at approximately 6 years of age, but it does not enter the lesser pelvis and become a pelvic organ until after puberty.
The apex of the urinary bladder in adults is continuous with the median umbilical ligament, which extends posteriorly along the posterior surface of the anterior abdominal wall.
Urachal Anomalies
In infants, a remnant of the urachal lumen may persist in the inferior part of the urachus. In approximately 50% of cases, the lumen is continuous with the cavity of the bladder. Remnants of the epithelial lining of the urachus may give rise to urachal cysts (Fig. 12-22A), which are not usually detected except during a postmortem unless the cysts become infected and enlarge. The patent inferior end of the urachus may dilate to form a urachal sinus that opens into the bladder. The lumen in the superior part of the urachus may also remain patent and form a urachal sinus that opens at the umbilicus (Fig. 12-22B). Very rarely the entire urachus remains patent and forms a urachal fistula that allows urine to escape from its umbilical orifice (Fig. 12-22C ).
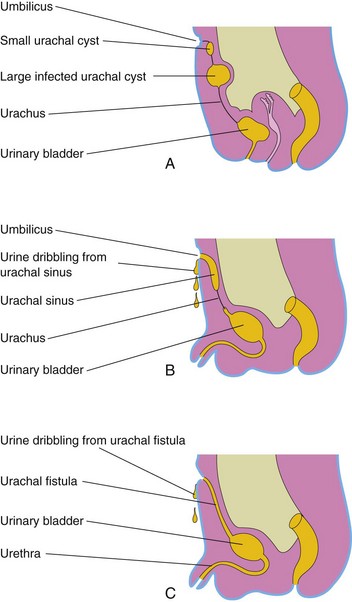
FIGURE 12–22 Urachal anomalies. A, Urachal cysts; the common site for them is in the superior end of the urachus just inferior to the umbilicus. B, Two types of urachal sinus are shown: one opens into the bladder and the other opens at the umbilicus. C, A urachal fistula connects the bladder and umbilicus.
Congenital Megacystis
A pathologically large urinary bladder—megacystis or megalocystis—may result from a congenital disorder of the ureteric bud, which may be associated with dilation of the renal pelvis. The large bladder may result from posterior urethral valves (Fig. 12-23). Many infants die from this disorder or suffer from renal failure in early childhood.
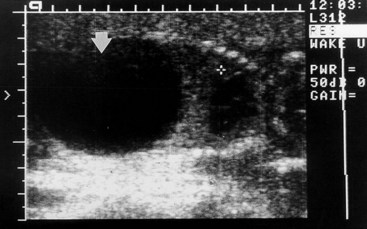
FIGURE 12–23 Sonogram of an 18-week male fetus with megacystis (enlarged bladder) caused by posterior urethral valves. The cross is placed on the fourth intercostal space, the level to which the diaphragm has been elevated by this very large fetal bladder (arrow; black = urine). In this case, the fetus survived because of the placement of a pigtail catheter within the fetal bladder, allowing drainage of urine into the amniotic cavity.
(Courtesy of Dr. C.R. Harman, Department of Obstetrics and Gynecology and Reproductive Health, University of Maryland Medical Centre, Baltimore, MD.)
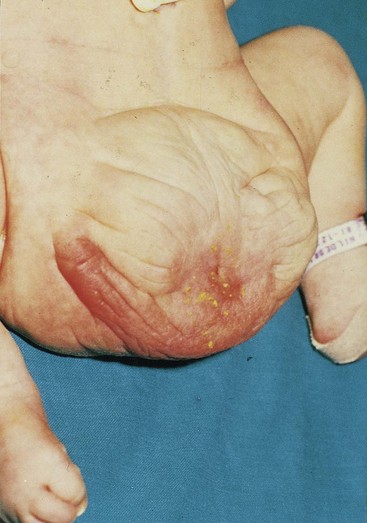
Exstrophy of Bladder
This rare birth defect occurs approximately once in every 10,000 to 40,000 births. Exstrophy (eversion) of the bladder (Fig. 12-24) usually occurs in males. Exposure and protrusion of the mucosal surface of the posterior wall of the bladder characterize this defect. The trigone of the bladder and the ureteric orifices are exposed, and urine dribbles intermittently from the everted bladder. Epispadias (urethra opens on dorsum of penis) and wide separation of the pubic bones are associated with complete exstrophy of the bladder. In some cases, the penis is divided into two parts and the halves of the scrotum are widely separated (Figs. 12-24 and 12-25).
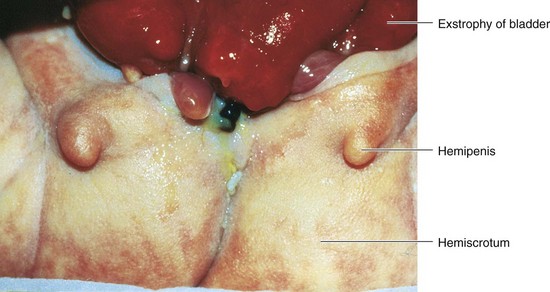
FIGURE 12–24 Exstrophy (eversion) of the bladder and bifid penis in a male infant. The red bladder mucosa is visible and the halves of the penis and scrotum are widely separated.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
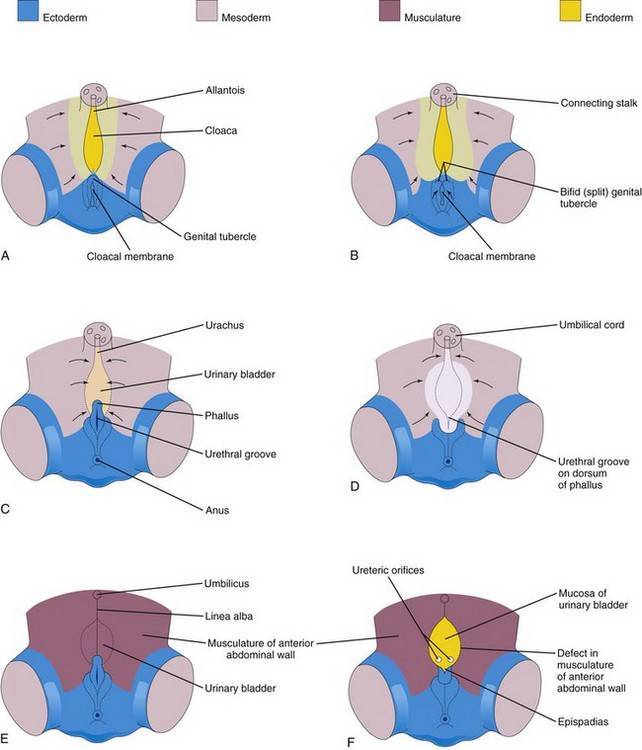
FIGURE 12–25 A, C, and E, Normal stages in the development of the infraumbilical abdominal wall and the penis during the fourth to eighth weeks. B, D, and F, Probable stages in the development of epispadias and exstrophy of the bladder. B and D, Note that the mesoderm fails to extend into the anterior abdominal wall anterior to the urinary bladder. Also note that the genital tubercle is located in a more caudal position than usual and that the urethral groove has formed on the dorsal surface of the penis. F, The surface ectoderm and anterior wall of the bladder have ruptured, resulting in exposure of the posterior wall of the bladder. Note that the musculature of the anterior abdominal wall is present on each side of the defect.
(Based on Patten BM, Barry A: The genesis of exstrophy of the bladder and epispadias. Am J Anat 90:35, 1952.)
Exstrophy of the bladder, a deficiency of the anterior abdominal wall, is caused by incomplete median closure of the inferior part of the wall (Fig. 12-25). The defect involves both the abdominal wall and the anterior wall of the urinary bladder; it results from failure of mesoderm to migrate between the ectoderm and endoderm of the abdominal wall (Fig. 12-25B and C ). As a result, the inferior parts of the rectus muscles are absent and the external and internal oblique and the transversus abdominus muscles are deficient.
No muscle and connective tissue form in the anterior abdominal wall over the urinary bladder. Rupture of the cloacal membrane results in wide communication between the exterior and the mucous membrane of the bladder. Rupture of the membrane before rupture of the cloacal membrane produces exstrophy of the cloaca, resulting in exposure of the posterior wall of the bladder (Fig. 12-25F ).
Development of Urethra
The epithelium of most of the male urethra and the entire female urethra is derived from endoderm of the urogenital sinus (Figs. 12-20E and H and 12-26). In males, the distal part of the urethra in the glans of the penis is derived from a solid cord of ectodermal cells that grows inward from the tip of the glans and joins the rest of the spongy urethra (Fig. 12-26A to C). Consequently, the epithelium of the terminal part of the urethra is derived from the surface ectoderm. The connective tissue and smooth muscle of the urethra in both sexes are derived from splanchnic mesenchyme.
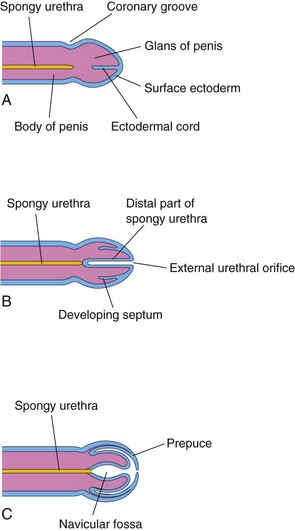
FIGURE 12–26 Schematic longitudinal sections of the developing penis illustrating development of the prepuce (foreskin) and the distal part of the spongy urethra. A, At 11 weeks. B, At 12 weeks. C, At 14 weeks. The epithelium of the spongy urethra has a dual origin; most of it is derived from endoderm of the phallic part of the urogenital sinus; the distal part of the urethra lining the navicular fossa is derived from surface ectoderm.
 Development Of Suprarenal Glands
Development Of Suprarenal Glands
The cortex and medulla of the suprarenal glands (adrenal glands) have different origins (Fig. 12-27). The cortex develops from mesenchyme and the medulla from neural crest cells. During the sixth week, the cortex begins as an aggregation of mesenchymal cells on each side of the embryo between the root of the dorsal mesentery and the developing gonad (Fig. 12-29C). The cells that form the medulla are derived from an adjacent sympathetic ganglion, which is derived from neural crest cells.
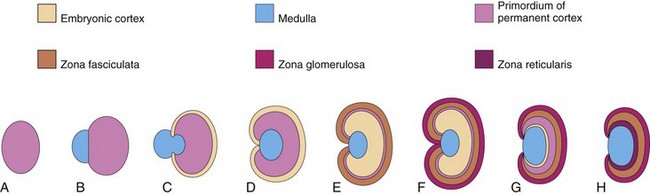
FIGURE 12–27 Schematic drawings illustrating development of the suprarenal glands. A, At 6 weeks, showing the mesodermal primordium of the fetal cortex. B, At 7 weeks, showing the addition of neural crest cells. C, At 8 weeks, showing the fetal cortex and early permanent cortex beginning to encapsulate the medulla. D and E, Later stages of encapsulation of the medulla by the cortex. F, Gland of a neonate showing the fetal cortex and two zones of the permanent cortex. G, At 1 year, the fetal cortex has almost disappeared. H, At 4 years, showing the adult pattern of cortical zones. Note that the fetal cortex has disappeared and that the gland is much smaller than it was at birth (F).
Initially the neural crest cells form a mass on the medial side of the embryonic cortex (Fig. 12-27B). As they are surrounded by the cortex, the cells differentiate into the secretory cells of the suprarenal medulla. Later, more mesenchymal cells arise from the mesothelium and enclose the cortex. These cells give rise to the permanent cortex of the suprarenal gland (Fig. 12-27C).
Immunohistochemical studies identify a “transitional zone” that is located between the permanent cortex and the fetal cortex. It has been suggested that the zona fasciculata is derived from this third layer. The zona glomerulosa and zona fasciculata are present at birth, but the zona reticularis is not recognizable until the end of the third year (Fig. 12-27H).
Relative to body weight, the suprarenal glands of the fetus are 10 to 20 times larger than in the adult and are large compared with the kidneys (Figs. 12-3 and 12-8). These large glands result from the extensive size of the fetal cortex, which produces steroid precursors that are used by the placenta for the synthesis of estrogen. The suprarenal medulla remains relatively small until after birth. The suprarenal glands rapidly become smaller as the fetal cortex regresses during the first year of infancy (Fig. 12-27). The glands lose approximately one third of their weight during the first 2 or 3 weeks after birth and do not regain their original weight until the end of the second year.
Congenital Adrenal Hyperplasia and Adrenogenital Syndrome
An abnormal increase in the cells of the suprarenal cortex results in excessive androgen production during the fetal period. In females, this usually causes masculinization of the external genitalia (Fig. 12-28). Affected male infants have normal external genitalia, and the syndrome may go undetected in early infancy. Later in childhood in both sexes, androgen excess leads to rapid growth and accelerated skeletal maturation.
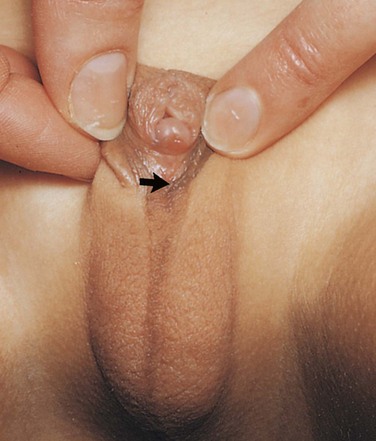
FIGURE 12–28 External genitalia of a 6-year-old girl showing an enlarged clitoris and fused labia majora that have formed a scrotum-like structure. The arrow indicates the opening into the urogenital sinus. This extreme masculinization is the result of congenital adrenal hyperplasia (CAH).
(Courtesy of Dr. Heather Dean, Department of Pediatric and Child Health, University of Manitoba, Winnipeg, Manitoba, Canada.)
The adrenogenital syndrome associated with congenital adrenal hyperplasia (CAH) manifests itself in various forms that can be correlated with enzymatic deficiencies of cortisol biosynthesis. CAH is a group of autosomal recessive disorders that result in virilization of female fetuses. CAH is caused by a genetically determined mutation in the cytochrome P450c21-steroid 21-hydroxylase gene, which causes a deficiency of suprarenal cortical enzymes that are necessary for the biosynthesis of various steroid hormones. The reduced hormone output results in an increased release of adrenocorticotropin from the anterior pituitary gland, which causes CAH and overproduction of androgens. Mutations of DAX1 result in X-linked adrenal hypoplasia congenita.
 Development Of Genital System
Development Of Genital System
Chromosomal sex of an embryo is determined at fertilization by the kind of sperm (X or Y) that fertilizes the oocyte Male and female morphologic characteristics do not begin to develop until the seventh week. The early genital systems in the two sexes are similar; therefore, the initial period of genital development is an indifferent stage of sexual development.
 Development of Gonads
Development of Gonads
The gonads (testes or ovaries) are the organs that produce sex cells (a sperm or an oocyte). The gonads are derived from three sources (Fig. 12-29):
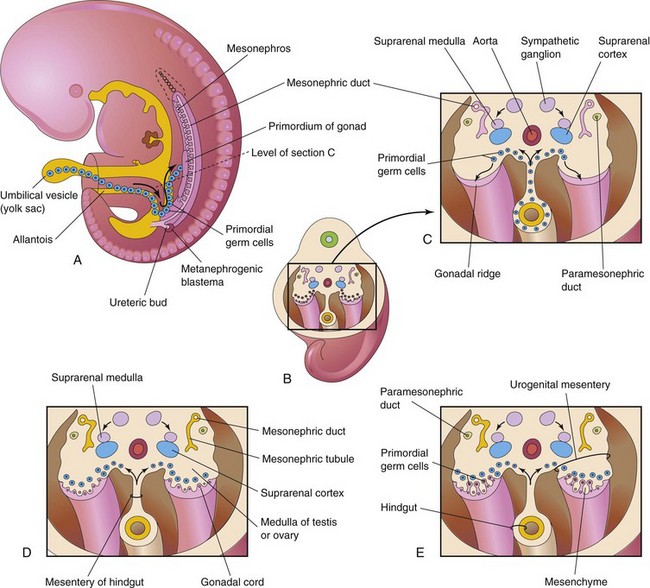
FIGURE 12–29 A, Sketch of a 5-week embryo illustrating the migration of primordial germ cells from the umbilical vesicle into the embryo. B, Three-dimensional sketch of the caudal region of a 5-week embryo showing the location and extent of the gonadal ridges. C, Transverse section showing the primordium of the suprarenal glands, the gonadal ridges, and the migration of primordial germ cells into the developing gonads. D, Transverse section of a 6-week embryo showing the gonadal cords. E, Similar section at a later stage showing the indifferent gonads and paramesonephric ducts.
Indifferent Gonads
The initial stages of gonadal development occur during the fifth week when a thickened area of mesothelium develops on the medial side of the mesonephros, a primitive kidney (Fig. 12-29A). Proliferation of this epithelium and the underlying mesenchyme produces a bulge on the medial side of the mesonephros—the gonadal ridge (Fig. 12-30). Fingerlike epithelial cords—gonadal cords—soon grow into the underlying mesenchyme (Fig. 12-29D). The indifferent gonad now consists of an external cortex and an internal medulla.
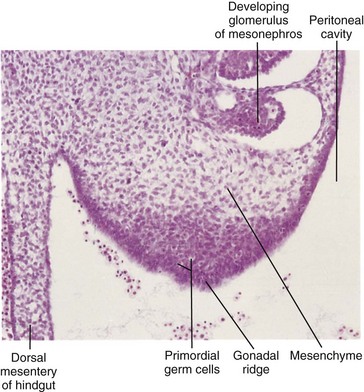
FIGURE 12–30 Photomicrograph of a transverse section of the abdomen of an embryo at approximately 40 days, showing the gonadal ridge, which will develop into a testis or ovary depending on the chromosomal sex of the embryo. Most of the developing gonad is composed of mesenchyme derived from the coelomic epithelium of the gonadal ridge. The large round cells in the gonad are primordial germ cells.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
In embryos with an XX sex chromosome complex, the cortex of the indifferent gonad differentiates into an ovary, and the medulla regresses. In embryos with an XY sex chromosome complex, the medulla differentiates into a testis, and the cortex regresses.
Primordial Germ Cells
These large, spherical sex cells are first recognizable at 24 days after fertilization among the endodermal cells of the umbilical vesicle near the origin of the allantois (Figs. 12-29A and 12-30). During folding of the embryo (see Chapter 5), the dorsal part of the umbilical vesicle is incorporated into the embryo. As this occurs, the primordial germ cells migrate along the dorsal mesentery of the hindgut to the gonadal ridges (Fig. 12-29C). During the sixth week, the primordial germ cells enter the underlying mesenchyme and are incorporated in the gonadal cords (Fig. 12-29D). The migration of the primordial germ cells is regulated by the genes stella, fragilis, and BMP-4.
Chromosomal Basis of Sex Determination
Chromosomal sex depends on whether an X-bearing sperm or a Y-bearing sperm fertilizes the X-bearing oocyte. Before the seventh week, the gonads of the two sexes are identical in appearance and are called indifferent gonads (Figs. 12-29E and 12-30).
Development of the male phenotype (characteristics of an individual) requires a Y chromosome. The SRY gene (sex-determining region on the Y) for a testis-determining factor (TDF) has been localized in the short arm region of the Y chromosome. It is the TDF regulated by the Y chromosome that determines testicular differentiation (Fig. 12-31). Under the influence of this organizing factor, the gonadal cords differentiate into seminiferous cords (primordia of seminiferous tubules). Expression of the Sox9 and Fgf9 genes is involved in the formation of the seminiferous cords. The absence of a Y chromosome results in the formation of an ovary.
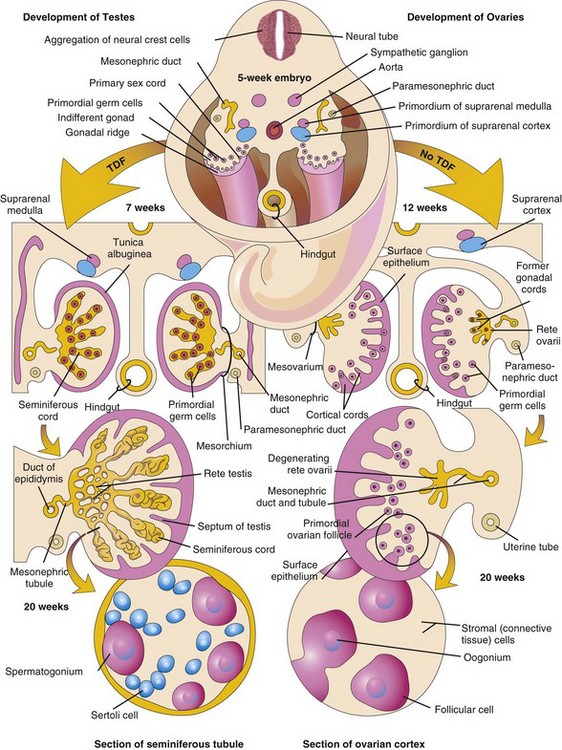
FIGURE 12–31 Schematic illustrations showing differentiation of the indifferent gonads in a 5-week embryo (top) into ovaries or testes. The left side of the drawing shows the development of testes resulting from the effects of the testis-determining factor (TDF) located on the Y chromosome. Note that the gonadal cords become seminiferous cords, the primordia of the seminiferous tubules. The parts of the gonadal cords that enter the medulla of the testis form the rete testis. In the section of the testis at the bottom left, observe that there are two kinds of cells: spermatogonia, derived from the primordial germ cells, and sustentacular or Sertoli cells, derived from mesenchyme. The right side shows the development of ovaries in the absence of TDF. Cortical cords have extended from the surface epithelium of the gonad and primordial germ cells have entered them. They are the primordia of the oogonia. Follicular cells are derived from the surface epithelium of the ovary.
Development of the female phenotype requires two X chromosomes. A number of genes and regions of the X chromosome have special roles in sex determination. Consequently, the type of sex chromosome complex established at fertilization of the oocyte determines the type of gonad that differentiates from the indifferent gonad. The type of gonad then determines the type of sexual differentiation that occurs in the genital ducts and external genitalia.
Testosterone, produced by the fetal testes, dihydrotestosterone (a metabolite of testosterone) and the antimüllerian hormone (AMH), determine normal male sexual differentiation, which begins during the 7th week. Ovarian development occurs when no Y chromosome is present, which begins about the 12th week. Primary female sexual differentiation does not depend on hormones; it occurs even if the ovaries are absent.
Development of Testes
TDF induces the seminiferous cords to condense and extend into the medulla of the indifferent gonad, where they branch and anastomose to form the rete testis, a network of canals (Fig. 12-31). The connection of the seminiferous cords—with the surface epithelium is lost when a thick fibrous capsule, the tunica albuginea, develops. The development of the dense tunica albuginea is the characteristic feature of testicular development. Gradually the enlarging testis separates from the degenerating mesonephros and is suspended by its own mesentery, the mesorchium. The seminiferous cords develop into the seminiferous tubules, tubuli recti, and rete testis.
The seminiferous tubules are separated by mesenchyme that gives rise to the interstitial cells (Leydig cells). By the eighth week, these cells begin to secrete androgenic hormones—testosterone and androstenedione, which induce masculine differentiation of the mesonephric ducts and external genitalia.
Testosterone production is stimulated by human chorionic gonadotropin, which reaches peak amounts during the 8th- to 12th-week period. In addition to testosterone, the fetal testes produce a glycoprotein, antimüllerian hormone (AMH) or müllerian-inhibiting substance (MIS). AMH is produced by the sustentacular cells (Sertoli cells), which continues to puberty after which the levels of the hormone decrease. AMH suppresses development of the paramesonephric ducts, which form the uterus and uterine tubes.
The seminiferous tubules have no lumina until puberty. The walls of the seminiferous tubules are composed of two types of cell (Fig. 12-31):
Sertoli cells constitute most of the seminiferous epithelium in the fetal testis (Figs. 12-31 and 12-32A). During later fetal development, the surface epithelium of the testis flattens to form the mesothelium on the external surface of the testis. The rete testis becomes continuous with 15 to 20 mesonephric tubules that become efferent ductules. These ductules are connected with the mesonephric duct, which becomes the duct of the epididymis (Figs. 12-31 and 12-33A).
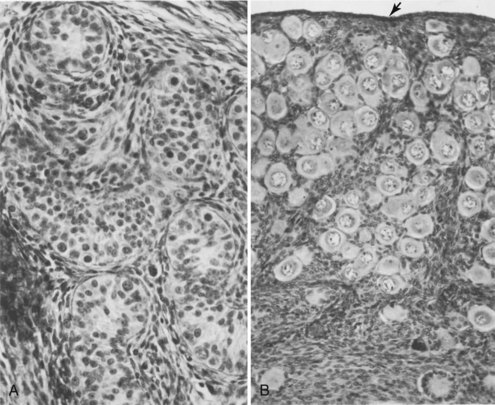
FIGURE 12–32 Transverse sections of gonads of human fetuses. A, Section of a testis from a male fetus born prematurely at 21 weeks, showing seminiferous tubules. B, Section of an ovary from a 14-day-old female infant that died. Observe the numerous primordial follicles in the cortex, each of which contains a primary oocyte. The arrow indicates the relatively thin surface epithelium of the ovary (x275).
(Reprinted with permission from van Wagenen G, Simpson ME: Embryology of the Ovary and Testis: Homo sapiens and Macaca mulatta. New Haven, CT, Yale University Press, 1965. Copyright © Yale University Press.)
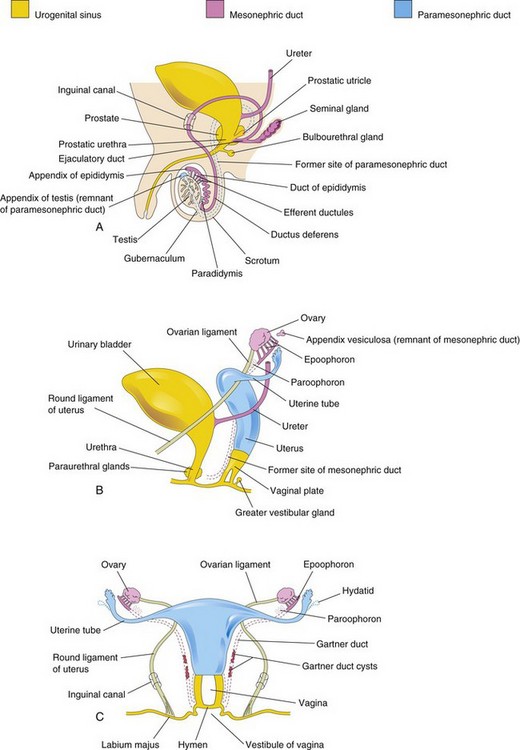
FIGURE 12–33 Schematic drawings illustrating development of the male and female reproductive systems from the genital ducts and urogenital sinus. Vestigial structures are also shown. A, Reproductive system in a male neonate. B, Female reproductive system in a 12-week fetus. C, Reproductive system in a female neonate.
Development of Ovaries
Gonadal development occurs slowly in female embryos (Fig. 12-32). The XX chromosomes have genes for ovarian development and an autosomal gene also appears to play a role in ovarian organogenesis. The ovary is not identifiable histologically until approximately the 10th week. Gonadal cords are not prominent in the developing ovary, but they extend into the medulla and form a rudimentary rete ovarii (Fig. 12-31). This network of canals and the gonadal cords normally degenerate and disappear (Fig. 12-31).
Cortical cords extend from the surface epithelium of the developing ovary into the underlying mesenchyme during the early fetal period. This epithelium is derived from the mesothelium of the peritoneum. As the cortical cords increase in size, primordial germ cells are incorporated in them. At approximately 16 weeks, these cords begin to break up into isolated cell clusters—primordial follicles—each of which contains an oogonium, derived from a primordial germ cell. The follicles are surrounded by a single layer of flattened follicular cells derived from the surface epithelium (Fig. 12-31). Active mitosis of oogonia occurs during fetal life producing primordial follicles (Fig. 12-32B).
No oogonia form postnatally. Although many oogonia degenerate before birth, the 2 million or so that remain enlarge to become primary oocytes. After birth, the surface epithelium of the ovary flattens to a single layer of cells continuous with the mesothelium of the peritoneum at the hilum of the ovary. The surface epithelium becomes separated from the follicles in the cortex by a thin fibrous capsule, the tunica albuginea. As the ovary separates from the regressing mesonephros, it is suspended by a mesentery—the mesovarium (Fig. 12-31).
Development of Genital Ducts
During the fifth and sixth weeks, the genital system is in an indifferent state, and two pairs of genital ducts are present. The mesonephric ducts (Wolffian ducts) play an important part in the development of the male reproductive system (Fig. 12-33A), and the paramesonephric ducts (müllerian ducts) have a leading role in the development of the female reproductive system.
The paramesonephric ducts develop lateral to the gonads and mesonephric ducts (Fig. 12-31) on each side from longitudinal invaginations of the mesothelium on the lateral aspects of the mesonephroi (primordial kidney). The edges of these grooves approach each other and fuse to form the paramesonephric ducts (Fig. 12-29C and E). The cranial ends of these ducts open into the peritoneal cavity (Fig. 12-33B and C). Caudally, the paramesonephric ducts run parallel to the mesonephric ducts until they reach the future pelvic region of the embryo. Here they cross ventral to the mesonephric ducts, approach each other in the median plane, and fuse to form a Y-shaped uterovaginal primordium (Fig. 12-34A). This tubular structure projects into the dorsal wall of the urogenital sinus and produces an elevation—the sinus tubercle (Fig. 12-34B).
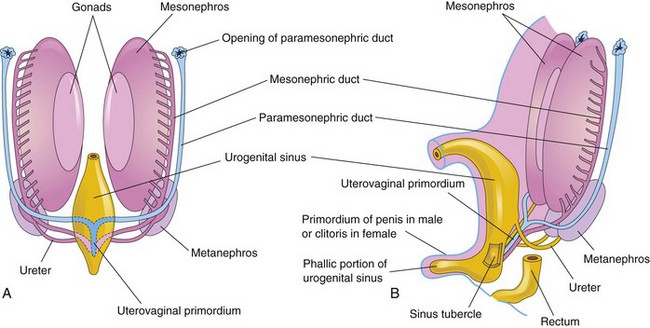
FIGURE 12–34 A, Sketch of a ventral view of the posterior abdominal wall of a 7-week embryo showing the two pairs of genital ducts present during the indifferent stage of sexual development. B, Lateral view of a 9-week fetus showing the sinus tubercle on the posterior wall of the urogenital sinus. It becomes the hymen in females and the seminal colliculus in males. The colliculus is an elevated part of the urethral crest on the posterior wall of the prostatic urethra.
 Development of Male Genital Ducts and Glands
Development of Male Genital Ducts and Glands
The fetal testes produce masculinizing hormones (e.g., testosterone) and Mullerian-inhibiting substance (MIS). The Sertoli cells produce MIS at 6 to 7 weeks. The interstitial cells begin producing testosterone in the eighth week. Testosterone stimulates the mesonephric ducts to form male genital ducts, whereas MIS causes the paramesonephric ducts to regress. Under the influence of testosterone produced by the fetal testes in the eighth week, the proximal part of each mesonephric duct becomes highly convoluted to form the epididymis. As the mesonephros degenerates, some mesonephric tubules persist and are transformed into efferent ductules (Fig. 12-33A). These ductules open into the duct of the epididymis in this region. Distal to the epididymis, the mesonephric duct acquires a thick investment of smooth muscle and becomes the ductus deferens.
Seminal Glands
Lateral outgrowths from the caudal end of each mesonephric duct become seminal glands (vesicles), which produce a secretion that makes up the majority of the fluid in ejaculate and nourishes the sperms. The part of the mesonephric duct between the duct of this gland and the urethra becomes the ejaculatory duct (Fig. 12-33A).
Prostate
Multiple endodermal outgrowths arise from the prostatic part of the urethra and grow into the surrounding mesenchyme (Fig. 12-35A to C). The glandular epithelium of the prostate differentiates from these endodermal cells, and the associated mesenchyme differentiates into the dense stroma and smooth muscle of the prostate.
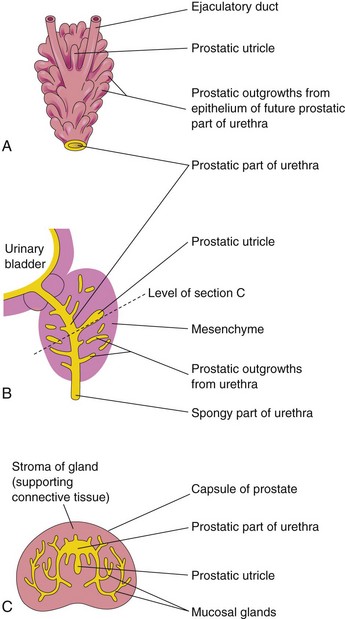
FIGURE 12–35 A, Dorsal view of the developing prostate in an 11-week fetus. B, Sketch of a median section of the developing urethra and prostate showing numerous endodermal outgrowths from the prostatic urethra. The vestigial prostatic utricle is also shown. C, Section of the prostate (16 weeks) at the level shown in B.
Bulbourethral Glands
These pea-sized glands develop from paired outgrowths derived from the spongy part of the urethra (Fig. 12-33A). The smooth muscle fibers and the stroma differentiate from the adjacent mesenchyme. The secretions of these glands contribute to the semen.
 Development of Female Genital Ducts and Glands
Development of Female Genital Ducts and Glands
The mesonephric ducts of female embryos regress because of the absence of testosterone; only a few nonfunctional remnants persist (Fig. 12-33B and C, Table 12-1). The paramesonephric ducts develop because of the absence of MIS. Female sexual development during the fetal period does not depend on the presence of ovaries or hormones. Later, estrogens produced by the maternal ovaries and the placenta stimulate development of the uterine tube, uterus, and the superior part of the vagina. The paramesonephric ducts form most of the female genital tract. The uterine tubes develop from the unfused cranial parts of these ducts (Figs. 12-33B and C and 12-34). The caudal fused portions of these ducts form the uterovaginal primordium, which gives rise to the uterus and the superior part of the vagina. The endometrial stroma and myometrium are derived from splanchnic mesenchyme. Uterine development is regulated by the homeobox gene HOXA10.
Fusion of the paramesonephric ducts also forms a peritoneal fold that becomes the broad ligament, and forms two peritoneal compartments — the rectouterine pouch and the vesicouterine pouch (Fig. 12-36A to D). Along the sides of the uterus, between the layers of the broad ligament, the mesenchyme proliferates and differentiates into cellular tissue—the parametrium—which is composed of loose connective tissue and smooth muscle.
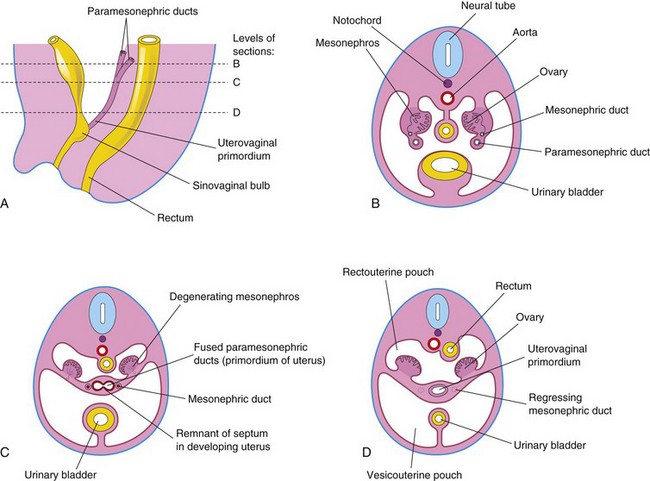
FIGURE 12–36 Early development of the ovaries and uterus. A, Schematic drawing of a sagittal section of the caudal region of an 8-week female embryo. B, Transverse section showing the paramesonephric ducts approaching each other. C, Similar section at a more caudal level illustrating fusion of the paramesonephric ducts. A remnant of the septum that separates the paramesonephric ducts is shown. D, Similar section showing the uterovaginal primordium, broad ligament, and pouches in the pelvic cavity. Note that the mesonephric ducts have regressed.
Auxiliary Genital Glands in Females
Outgrowths from the urethra into the surrounding mesenchyme form the bilateral mucus secreting urethral glands and paraurethral glands (Fig. 12-33B). Outgrowths from the urogenital sinus form the greater vestibular glands in the lower third of the labia majora. These tubuloalveolar glands also secrete mucus and are homologous to the bulbourethral glands in males (Table 12-1).
 Development of Vagina
Development of Vagina
The fibromuscular wall of the vagina develops from the surrounding mesenchyme. Contact of the uterovaginal primordium with the urogenital sinus, forming the sinus tubercle (Fig. 12-34B), induces the formation of paired endodermal outgrowths—the sinovaginal bulbs (Fig. 12-36A). They extend from the urogenital sinus to the caudal end of the uterovaginal primordium. The sinovaginal bulbs fuse to form a vaginal plate (Fig. 12-33B). Later the central cells of this plate break down, forming the lumen of the vagina. The epithelium of the vagina is derived from the peripheral cells of the vaginal plate (Fig. 12-33C).
Until late fetal life, the lumen of the vagina is separated from the cavity of the urogenital sinus by a membrane—the hymen (Figs. 12-33C and 12-37H). The membrane is formed by invagination of the posterior wall of the urogenital sinus, resulting from expansion of the caudal end of the vagina. The hymen usually ruptures during the perinatal period and remains as a thin fold of mucous membrane just within the vaginal orifice.
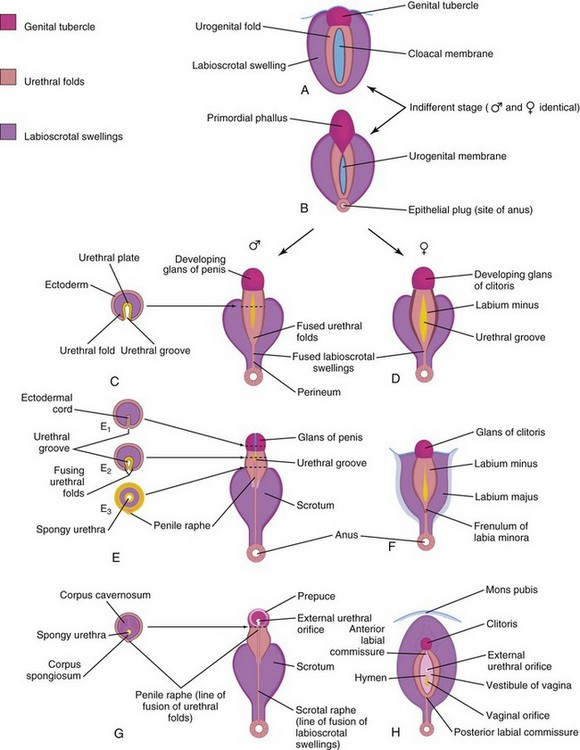
FIGURE 12–37 Development of external genitalia. A and B, Diagrams illustrating the appearance of the genitalia during the indifferent stage (fourth to seventh weeks). C, E, and G, Stages in the development of male external genitalia at 9, 11, and 12 weeks, respectively. To the left are schematic transverse sections of the developing penis illustrating formation of the spongy urethra. D, F, and H, Stages in the development of female external genitalia at 9, 11, and 12 weeks, respectively. The mons pubis is a pad of fatty tissue over the symphysis pubis.
Vestigial Remains of Embryonic Genital Ducts
During conversion of the mesonephric and paramesonephric ducts into adult structures, parts of them remain as vestigial structures (see Fig. 12-33; Table 12-1). These vestiges are rarely seen unless pathologic changes develop in them.
Mesonephric Duct Remnants in Males
The cranial end of the mesonephric duct may persist as an appendix of the epididymis, which is usually attached to the head of the epididymis (Fig. 12-33A). Caudal to the efferent ductules, some mesonephric tubules may persist as a small body, the paradidymis.
Mesonephric Duct Remnants in Females
The cranial end of the mesonephric duct may persist as an appendix vesiculosa (see Fig. 12-33B). A few blind tubules and a duct, the epoophoron may persist in the mesovarium between the ovary and uterine tube (Fig. 12-33B and C ). Closer to the uterus, some rudimentary tubules may persist as the paroophoron. Parts of the mesonephric duct, corresponding to the ductus deferens and ejaculatory duct, may persist as Gartner duct cysts between the layers of the broad ligament along the lateral wall of the uterus and in the wall of the vagina (Fig. 12-33C ).
Paramesonephric Duct Remnants in Males
The cranial end of the paramesonephric duct may persist as a vesicular appendix of the testis, which is attached to the superior pole of the testis (Fig. 12-33A). The prostatic utricle, a small saclike structure arising from the paramesonephric duct, opens into the prostatic urethra. The lining of the prostatic utricle is derived from the epithelium of the urogenital sinus. Within its epithelium, endocrine cells containing neuron-specific enolase and serotonin have been detected. The seminal colliculus, a small elevation in the posterior wall of the prostatic urethra, is the adult derivative of the sinus tubercle (Fig. 12-34B).
Paramesonephric Duct Remnants in Females
Part of the cranial end of the paramesonephric duct that does not contribute to the infundibulum of the uterine tube may persist as a vesicular appendage (Fig. 12-33C ), called a hydatid (of Morgagni).
 Development Of External Genitalia
Development Of External Genitalia
Up to the seventh week, the external genitalia are similar in both sexes (Fig. 12-37A and B). Distinguishing sexual characteristics begin to appear during the 9th week, but the external genitalia are not fully differentiated until the 12th week. Early in the fourth week, proliferating mesenchyme produces a genital tubercle (primordium of penis or clitoris) in both sexes at the cranial end of the cloacal membrane. The cloacal ectoderm is believed to be the source of the genital initiation signal which involves Fgf8 expression.
Labioscrotal swellings and urogenital folds soon develop on each side of the cloacal membrane. The genital tubercle elongates to form a primordial phallus. The urogenital membrane lies in the floor of a median cleft, the urethral groove, which is bounded by the urethral folds (Fig. 12-37B and D). In female fetuses, the urethra and vagina open into a common cavity, the vestibule of the vagina (Fig. 12-37B).
Development of Male External Genitalia
Masculinization of the indifferent external genitalia is induced by testosterone produced by the interstitial cells of the fetal testes (Fig. 12-37C, E, and G). As the phallus enlarges and elongates to form the penis, the urethral folds form the lateral walls of the urethral groove on the ventral surface of the penis (Fig. 12-38A and B). This groove is lined by a proliferation of endodermal cells, the urethral plate (Fig. 12-37C), which extends from the phallic portion of the urogenital sinus. The urethral folds fuse with each other along the ventral surface of the penis to form the spongy urethra (Figs. 12-37G and 12-38C1 and C3). The surface ectoderm fuses in the median plane of the penis, forming the penile raphe and enclosing the spongy urethra within the penis.
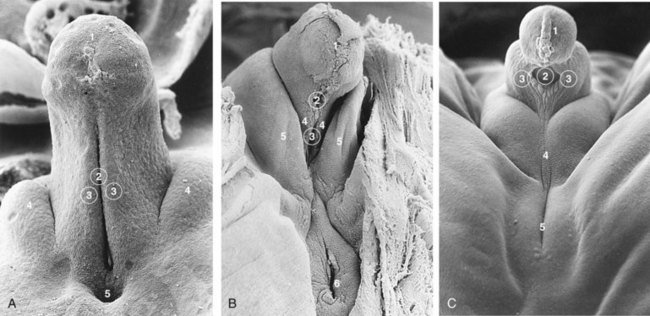
FIGURE 12–38 Scanning electron micrographs of the developing external genitalia. A, The perineum during the indifferent stage of a 17-mm, 7-week embryo (x100). 1, developing glans of penis with the ectodermal cord; 2, urethral groove continuous with the urogenital sinus; 3, urethral folds; 4, labioscrotal swellings; 5, anus. B, The external genitalia of a 7.2-cm, 10-week female fetus (x45). 1, glans of clitoris; 2, external urethral orifice; 3, opening into urogenital sinus; 4, urethral fold (primordium of labium minus); 5, labioscrotal swelling (labium majus); 6, anus. C, The external genitalia of a 5.5-cm, 10-week male fetus (x40). 1, glans of penis with ectodermal cord; 2, remains of urethral groove; 3, urethral folds in the process of closing; 4, labioscrotal swellings fusing to form the scrotal raphe; 5, anus.
(From Hinrichsen KV: Embryologische Grundlagen. In Sohn C, Holzgreve W [eds]: Ultraschall in Gynäkologie und Geburtshilfe. New York, Georg Thieme Verlag, 1995, reprinted with permission.)
At the tip of the glans penis, an ectodermal ingrowth forms a cellular ectodermal cord, which grows toward the root of the penis to meet the spongy urethra (Fig. 12-26A). As this cord canalizes, its lumen joins the previously formed spongy urethra. This completes the terminal part of the urethra and moves the external urethral orifice to the tip of the glans penis (Fig. 12-26C). HOX, FGF, and Shh genes regulate the development of the penis.
During the 12th week, a circular ingrowth of ectoderm occurs at the periphery of the glans penis (Fig. 12-26B). When this ingrowth breaks down, it forms the prepuce (foreskin)—a covering fold of skin (Fig. 12-26C). The corpora cavernosa and corpus spongiosum of the penis develop from mesenchyme in the phallus. The labioscrotal swellings grow toward each other and fuse to form the scrotum (Fig. 12-37E and G). The line of fusion of these folds is clearly visible as the scrotal raphe (Figs. 12-37G and 12-38C).
Development of Female External Genitalia
The primordial phallus in the female fetus gradually becomes the clitoris (Figs. 12-20G and 12-37D, F, and H). The clitoris is still relatively large at 18 weeks (Fig. 12-21). The urethral folds do not fuse, except posteriorly, where they join to form the frenulum of the labia minora (Fig. 12-37F). The unfused parts of the urogenital folds form the labia minora. The labioscrotal folds fuse posteriorly to form the posterior labial commissure and anteriorly to form the anterior labial commissure and mons pubis (Fig. 12-37H). Most parts of the labioscrotal folds remain unfused, but develop into two large folds of skin, the labia majora.
Determination of Fetal Sex
Visualization of the external genitalia during ultrasonography (Fig. 12-39) is clinically important for several reasons, including detection of fetuses at risk of severe X-linked disorders. Careful examination of the perineum may detect ambiguous genitalia (Fig. 12-40B). Ultrasonographic confirmation of testes in the scrotum provides the only 100% gender determination, which is not possible in utero until 22 to 36 weeks. Fetal position prevents good visualization of the perineum in 30% of fetuses.
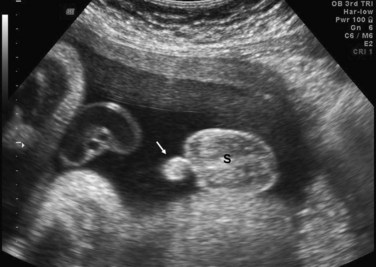
FIGURE 12–39 Sonogram of a 33-week male fetus showing normal external genitalia. Observe the penis (arrow) and scrotum (S). Also note the testes in the scrotum.
(Courtesy of Dr. G.J. Reid, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Manitoba, Women’s Hospital, Winnipeg, Manitoba, Canada.)
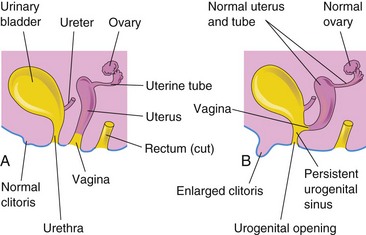
FIGURE 12–40 Schematic lateral views of the female urogenital system. A, Normal. B, Female with 46 XX, DSD caused by congenital adrenal hyperplasia (CAH). Note the enlarged clitoris and persistent urogenital sinus that were induced by androgens produced by the hyperplastic suprarenal glands.
When there is normal sexual differentiation, the appearance of the external and internal genitalia is consistent with the sex chromosome complement. Errors in sex determination and differentiation result in various degrees of intermediate sex.
Advances in molecular genetics have led to a better understanding of abnormal sexual development and ambiguous genitalia. Because of psychosocial stigma and in order to provide better clinical management for infants born with atypical chromosomal constitution or gonads, a new nomenclature has been introduced to describe these conditions that are now called disorders of sex development (DSD). The new classification avoids using the term “hermaphrodite” (see Lee PA, Houk CP, Ahmed SF, Hughes IA: Consensus Statement on Management of Intersex Disorders. Pediatrics 118:e488, 2006). DSD implies a discrepancy between the morphology of the gonads (testes/ovaries) and the appearance of the external genitalia. Intersexual conditions are classified according to the histologic appearance of the gonads:
Ovotesticular DSD
Persons with this rare intersexual condition usually have chromatin-positive nuclei (sex chromatin in cells observed in a buccal smear). Approximately 70% of them have a 46, XX chromosome constitution; approximately 20% have 46, XX/46, XY mosaicism (presence of two or more cell lines), and approximately 10% have a 46, XY chromosome constitution. The causes of ovotesticular DSD (true hermaphroditism) are still poorly understood. Most persons have both testicular and ovarian tissue or an ovotestis. These tissues are not usually functional. An ovotestis (containing both testicular and ovarian tissue) forms if both the medulla and cortex of the indifferent gonads develop. Ovotesticular DSD results from an error in sex determination. The phenotype may be male or female but the external genitalia are always ambiguous.
46 XX, DSD
Persons with this intersexual condition have chromatin-positive nuclei and a 46, XX chromosome constitution. This anomaly results from exposure of the female fetus to excessive androgens, causing virilization of the external genitalia (clitoral enlargement and labial fusion [Figs. 12-28 and 12-40]). A common cause of 46 XX, DSD (female pseudohermaphroditism) is CAH (congenital adrenal hyperplasia). There is no ovarian abnormality, but the excessive production of androgens by the fetal suprarenal glands causes varying degrees of masculinization of the external genitalia. Commonly there is clitoral hypertrophy, partial fusion of the labia majora, and a persistent urogenital sinus (Fig. 12-40). In rare cases, the masculinization may be so intense that a complete clitoral urethra results. The administration of androgenic agents to women during early pregnancy may cause similar anomalies of the fetal external genitalia (see Chapter 20). Most cases have resulted from the use of certain progestational compounds for the treatment of threatened abortion. Masculinizing maternal tumors can also cause virilization of female fetuses.
46 XY, DSD
Persons with this intersexual condition have chromatin-negative nuclei (no sex chromatin) and a 46, XY chromosome constitution. The external genitalia are developmentally variable as is the development of the internal genitalia due to varying degrees of development of the paramesonephric ducts. These anomalies are caused by inadequate production of testosterone and MIS by the fetal testes. Testicular development in these males ranges from rudimentary to normal. Genetic defects in the enzymatic synthesis of testosterone by the fetal testes and in interstitial cells produce 46 XY, DSD (male pseudohermaphroditism) through inadequate virilization of the male fetus.
Androgen Insensitivity Syndrome
Persons with androgen insensitivity syndrome (AIS)—previously called testicular feminization syndrome—(1 in 20,000 live births) are normal-appearing females, despite the presence of testes and a 46, XY chromosome constitution (Fig. 12-41). The external genitalia are female, but the vagina usually ends in a blind pouch and the uterus and uterine tubes are absent or rudimentary. At puberty there is normal development of breasts and female characteristics, but menstruation does not occur. The testes are usually in the abdomen or the inguinal canals, but they may be within the labia majora. The failure of masculinization to occur in these individuals results from a resistance to the action of testosterone at the cellular level in the genital tubercle and labioscrotal and urethral folds.
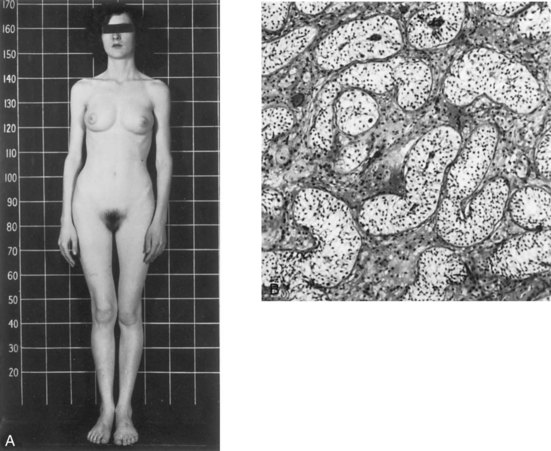
FIGURE 12–41 A, Photograph of a 17-year-old woman with androgen insensitivity syndrome (AIS). The external genitalia are female, but she has a 46, XY karyotype and testes in the inguinal region. B, Photomicrograph of a section through a testis removed from the inguinal region of this woman showing seminiferous tubules lined by Sertoli cells. There are no germ cells and the interstitial cells are hypoplastic.
(From Jones HW, Scott WW: Hermaphroditism, Genital Anomalies and Related Endocrine Disorders. Baltimore, Williams & Wilkins, 1958. Courtesy of Williams & Wilkins.)
Persons with partial AIS exhibit some masculinization at birth, such as ambiguous external genitalia, and may have an enlarged clitoris. The vagina ends blindly and the uterus is absent. Testes are in the inguinal canals or the labia majora. There are usually point mutations in the sequence that codes for the androgen receptor. Usually the testes are removed as soon as they are discovered because, in approximately one third of these individuals, malignant tumors develop by 50 years of age. AIS follows X-linked recessive inheritance and the gene encoding the androgen receptor has been localized.
Mixed Gonadal Dysgenesis
Persons with this rare condition usually have a 46, XY chromosomal complement, with a testis on one side, and an undifferentiated gonad on the other side. The internal genitalia are female, but male derivatives of the mesonephric ducts are sometimes present. The external genitalia range from normal female through intermediate states to normal male. At puberty, neither breast development nor menstruation occurs, but varying degrees of virilization are common.
Hypospadias
Hypospadias is the most common birth defect of the penis. There are four main types:
In 1 of every 125 male infants, the external urethral orifice is on the ventral surface of the glans of the penis (glanular hypospadias), or on the ventral surface of the body of the penis (penile hypospadias). Usually the penis is underdeveloped and curved ventrally—chordee.
Glanular hypospadias and penile hypospadias constitute approximately 80% of cases (Fig. 12-42). In penoscrotal hypospadias, the urethral orifice is at the junction of the penis and scrotum. In perineal hypospadias, the labioscrotal folds fail to fuse and the external urethral orifice is located between the unfused halves of the scrotum. Because the external genitalia in this severe type of hypospadias are ambiguous, persons with perineal hypospadias and cryptorchidism (undescended testes) are sometimes misdiagnosed as 46 XY, DSD.
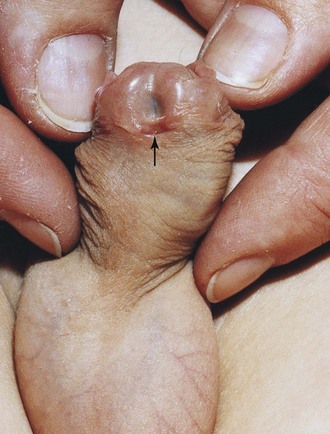
FIGURE 12–42 Glanular hypospadias in an infant. The external urethral orifice is on the ventral surface of the glans of the penis (arrow).
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, University of Manitoba, Children’s Hospital, Winnipeg, Manitoba, Canada.)
Hypospadias results from inadequate production of androgens by the fetal testes and/or inadequate receptor sites for the hormones. Most likely, both genomic and environmental factors are involved. It has been suggested that the expression of testosterone-related genes is affected. These defects result in failure of canalization of the ectodermal cord in the glans of the penis and/or failure of fusion of the urethral folds; as a consequence, there is incomplete formation of the spongy urethra.
Epispadias
In one of every 30,000 male infants, the urethra opens on the dorsal surface of the penis. Although epispadias may occur as a separate entity, it is often associated with exstrophy of the bladder (Figs. 12-24 and 12-25). Epispadias may result from inadequate ectodermal-mesenchymal interactions during development of the genital tubercle. As a consequence, the genital tubercle develops more dorsally than in normal embryos. Consequently, when the urogenital membrane ruptures, the urogenital sinus opens on the dorsal surface of the penis. Urine is expelled at the root of the malformed penis.
Agenesis of External Genitalia
Congenital absence of the penis or clitoris is an extremely rare condition (Fig. 12-43). Failure of the genital tubercle to develop may result from inadequate ectodermal-mesenchymal interactions during the seventh week. The urethra usually opens into the perineum near the anus.
Bifid Penis and Double Penis
These defects are rare. Bifid penis is usually associated with exstrophy of the bladder (Fig. 12-24). It may also be associated with urinary tract abnormalities and imperforate anus. Double penis results when two genital tubercles develop.
Micropenis
In this condition, the penis is so small that it is almost hidden by the suprapubic pad of fat. Micropenis results from fetal testicular failure and is commonly associated with hypopituitarism.
Anomalies of Uterine Tubes, Uterus, and Vagina
Defects of the uterine tubes are rare; only a few types have been reported. These include hydatid cysts, accessory ostia (openings), complete and segmental absence, duplication of a uterine tube, lack of the muscular layer, and failure of the tube to canalize. Various types of uterine duplication and vaginal anomalies result from arrests of development of the uterovaginal primordium during the eighth week (Fig. 12-44) by:
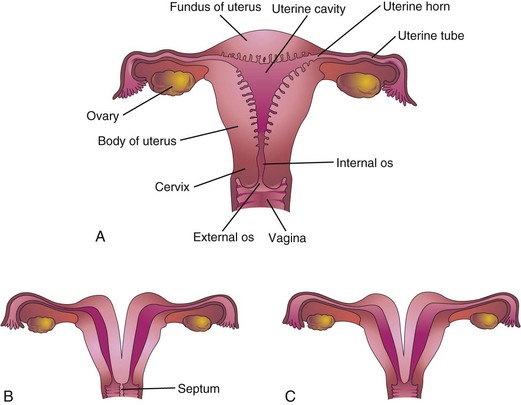
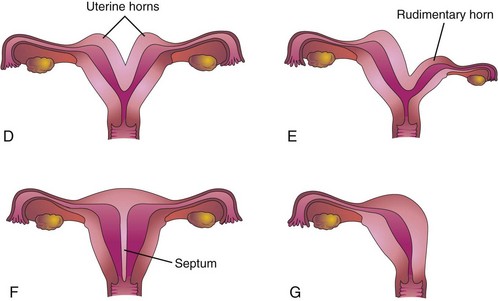
FIGURE 12–44 Uterine anomalies. A, Normal uterus and vagina. B, Double uterus (uterus didelphys) and double vagina (vagina duplex). Note the septum separating the vagina into two parts. C, Double uterus with single vagina. D, Bicornuate uterus (two uterine horns). E, Bicornuate uterus with a rudimentary left horn. F, Septate uterus; the septum separates the body of the uterus. G, Unicorn uterus; only one lateral horn exists.
Double uterus (uterus didelphys) results from failure of fusion of the inferior parts of the paramesonephric ducts. It may be associated with a double or a single vagina (Fig. 12-44B to D). In some cases, the uterus appears normal externally but is divided internally by a thin septum (Fig. 12-44F ). If the duplication involves only the superior part of the body of the uterus, the condition is called bicornuate uterus (Figs. 12-44D and E and 12-45).
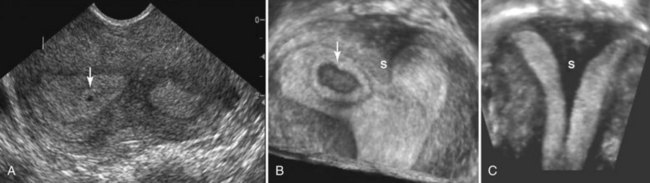
FIGURE 12–45 Sonogram of bicornuate uterus. A, Axial sonogram of the uterine fundus showing two separate endometrial canals with a 1-week chorionic (gestational) sac (arrow). B, A three-dimensional ultrasound scan of the same patient with a 4-week chorionic sac (arrow) on the right of a uterine septum (S). C, Coronal ultrasound scan of a uterus with a large septum (S) extending down to the cervix.
(Courtesy of Dr. E.A. Lyons, Department of Radiology, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
If growth of one paramesonephric duct is retarded and does not fuse with the other one, a bicornuate uterus with a rudimentary horn (cornu) develops (Fig. 12-44E ). The rudimentary horn may not communicate with the cavity of the uterus. A unicornuate uterus develops when one paramesonephric duct fails to develop; this results in a uterus with one uterine tube (Fig. 12-44G). In many cases, the individuals are fertile but may have an increased incidence of preterm delivery or recurrent pregnancy loss.
Absence of Vagina and Uterus
Once in approximately every 5000 births, absence of the vagina occurs. This results from failure of the sinovaginal bulbs to develop and form the vaginal plate (Figs. 12-33B and 12-36A). When the vagina is absent, the uterus is usually absent because the developing uterus (uterovaginal primordium) induces the formation of sinovaginal bulbs, which fuse to form the vaginal plate.
Vaginal Atresia
Failure of canalization of the vaginal plate results in atresia (blockage) of the vagina. A transverse vaginal septum occurs in approximately one in 80,000 women. Usually the septum is located at the junction of the middle and superior thirds of the vagina. Failure of the inferior end of the vaginal plate to perforate results in an imperforate hymen. Variations in the appearance of the hymen are common (Fig. 12-46). The vaginal orifice varies in diameter from very small to large, and there may be more than one orifice.
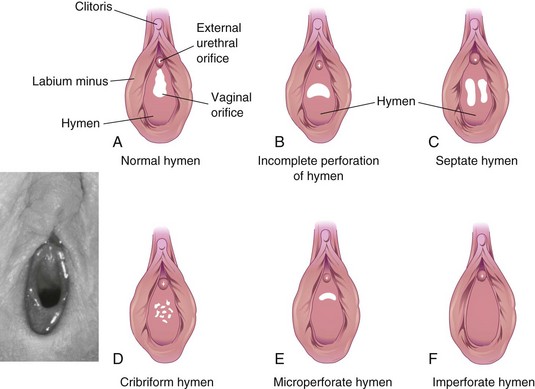
FIGURE 12–46 A to F, Congenital anomalies of the hymen. The normal appearance of the hymen is illustrated in A and in the inset photograph. Inset, Normal crescentic hymen in a prepubertal child.
(Courtesy of Dr. Margaret Morris, Professor of Obstetrics, Gynaecology and Reproductive Sciences, Women’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
 Development Of Inguinal Canals
Development Of Inguinal Canals
The inguinal canals form pathways for the testes to descend from the dorsal abdominal wall through the anterior abdominal wall into the scrotum. Inguinal canals develop in both sexes because of the morphologically indifferent stage of sexual development. As the mesonephros degenerates, a ligament—the gubernaculum—develops on each side of the abdomen from the caudal pole of the gonad (Fig. 12-47A). The gubernaculum passes obliquely through the developing anterior abdominal wall at the site of the future inguinal canal (Fig. 12-47B to D) and attaches caudally to the internal surface of the labioscrotal swellings (future halves of the scrotum or labia majora).
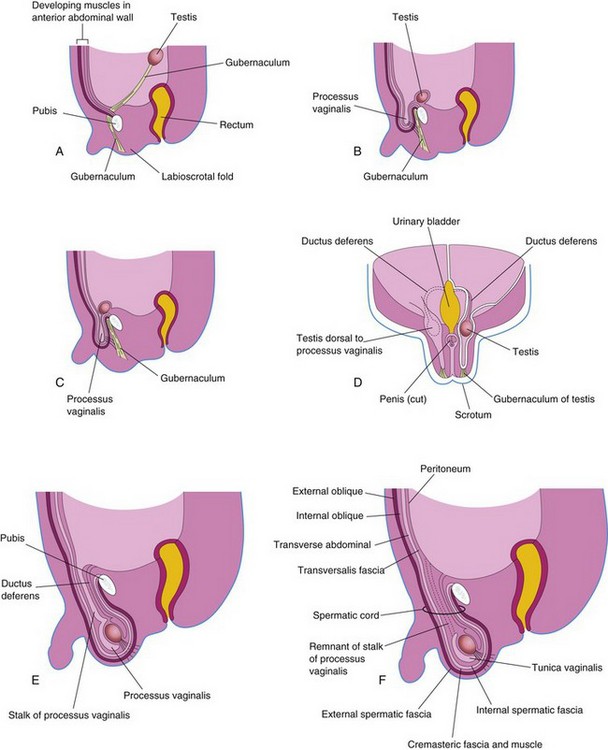
FIGURE 12–47 Formation of the inguinal canals and descent of the testes. A, Sagittal section of a 7-week embryo showing the testis before its descent from the dorsal abdominal wall. B and C, Similar sections at approximately 28 weeks showing the processus vaginalis and the testis beginning to pass through the inguinal canal. Note that the processus vaginalis carries fascial layers of the abdominal wall before it. D, Frontal section of a fetus approximately 3 days later illustrating descent of the testis posterior to the processus vaginalis. The processus has been cut away on the left side to show the testis and ductus deferens. E, Sagittal section of a male neonate showing the processus vaginalis communicating with the peritoneal cavity by a narrow stalk. F, Similar section of a 1-month-old male infant after obliteration of the stalk of the processus vaginalis. Note that the extended fascial layers of the abdominal wall now form the coverings of the spermatic cord.
The processus vaginalis, an evagination of peritoneum, develops ventral to the gubernaculum and herniates through the abdominal wall along the path formed by the gubernaculum (Fig. 12-47B). The vaginal process carries along extensions of the layers of the abdominal wall before it, which form the walls of the inguinal canal. These layers also form the coverings of the spermatic cord and testis (Fig. 12-47D to F). The opening in the transversalis fascia produced by the processus vaginalis becomes the deep inguinal ring, and the opening created in the external oblique aponeurosis forms the superficial inguinal ring.
Abnormal Sex Chromosome Complexes
In embryos with abnormal sex chromosome complexes, such as XXX or XXY, the number of X chromosomes appears to be unimportant in sex determination. If a normal Y chromosome is present, the embryo develops as a male. If no Y chromosome is present or the testis-determining region of the Y chromosome is absent, female development occurs. The loss of an X chromosome does not appear to interfere with the migration of primordial germ cells to the gonadal ridges because some germ cells have been observed in the fetal gonads of 45, XO females with the Turner syndrome. Two X chromosomes are needed, however, to bring about normal ovarian development.
 Relocation Of Testes And Ovaries
Relocation Of Testes And Ovaries
Testicular Descent
Testicular descent is associated with:
By 26 weeks, the testes have usually descended retroperitoneally (external to the peritoneum) from the superior lumbar region to the posterior abdominal wall to the deep inguinal rings (Fig. 12-47B and C). This change in position occurs as the fetal pelvis enlarges and the body or trunk of the embryo elongates. Transabdominal movement of the testes is largely a relative movement that results from growth of the cranial part of the abdomen away from the future pelvic region. Testicular descent through the inguinal canals into the scrotum is controlled by androgens (e.g., testosterone) produced by the fetal testes. The gubernaculum forms a path through the anterior abdominal wall for the processus vaginalis to follow during formation of the inguinal canal. The gubernaculum anchors the testis to the scrotum and guides its descent into the scrotum. Passage of the testis through the inguinal canal may also be aided by the increase in intra-abdominal pressure resulting from the growth of abdominal viscera.
Descent of the testes through the inguinal canals into the scrotum usually begins during the 26th week and in some fetuses takes 2 or 3 days. By 32 weeks, both testes are present in the scrotum in most cases. The testes pass external to the peritoneum and processus vaginalis. After the testes enter the scrotum, the inguinal canal contracts around the spermatic cord. More than 97% of full-term neonates have both testes in the scrotum. During the first 3 months after birth, most undescended testes descend into the scrotum.
The mode of descent of the testis explains why the ductus deferens crosses anterior to the ureter (Fig. 12-33A); it also explains the course of the testicular vessels. These vessels form when the testes are located high on the posterior abdominal wall. When the testes descend, they carry the ductus deferens and vessels with them. As the testis and ductus deferens descend, they are ensheathed by the fascial extensions of the abdominal wall (Fig. 12-47F).
Within the scrotum, the testis projects into the distal end of the processus vaginalis. During the perinatal period, the connecting stalk of the processus normally obliterates, forming a serous membrane—tunica vaginalis—which covers the front and sides of the testis (Fig. 12-47F).
Ovarian Descent
The ovaries also descend from the lumbar region of the posterior abdominal wall and relocate to the lateral wall of the pelvis; however, they do not pass from the pelvis and enter the inguinal canals. The gubernaculum is attached to the uterus near the attachment of the uterine tube. The cranial part of the gubernaculum becomes the ovarian ligament, and the caudal part forms the round ligament of the uterus (Fig. 12-33C). The round ligaments pass through the inguinal canals and terminate in the labia majora. The relatively small processus vaginalis in the female usually obliterates and disappears long before birth. A persistent processus in a female fetus is known as a vaginal process of peritoneum or a canal of Nuck.
Cryptorchidism
Cryptorchidism (hidden testes) is the most common anomaly in neonates and occurs in about 30% of premature males and in 3% to 5% of full-term males. This reflects the fact that the testes begin to descend into the scrotum by the end of the second trimester. Cryptorchidism may be unilateral or bilateral. In most cases, undescended testes descend into the scrotum by the end of the first year. If both testes remain within or just outside the abdominal cavity, they fail to mature and sterility is common. If uncorrected, these men have a significantly higher risk of developing germ cell tumors, especially in cases of abdominal cryptorchidism. Undescended testes are often histologically normal at birth, but failure of development and atrophy are detectable by the end of the first year. Cryptorchid testes may be in the abdominal cavity or anywhere along the usual path of descent of the testis, but they are usually in the inguinal canal (Fig. 12-48A). The cause of most cases of cryptorchidism is unknown, but a deficiency of androgen production by the fetal testes is an important factor.
Ectopic Testes
After traversing the inguinal canal, the testes may deviate from its usual path of descent and lodge in various abnormal locations (Fig. 12-48B):
All types of ectopic testes are rare, but interstitial ectopia occurs most frequently. An ectopic testis occurs when a part of the gubernaculum passes to an abnormal location and the testis follows it.
Congenital Inguinal Hernia
If the communication between the tunica vaginalis and the peritoneal cavity fails to close (Fig. 12-49A and B), a persistent processus vaginalis exists. A loop of intestine may herniate through it into the scrotum or labium majus (Fig. 12-49B). Embryonic remnants resembling the ductus deferens or epididymis are often found in inguinal hernial sacs. Congenital inguinal hernia is much more common in males, especially when there are undescended testes. Congenital inguinal hernias are also common with ectopic testes and in females with androgen insensitivity syndrome (Fig. 12-41).
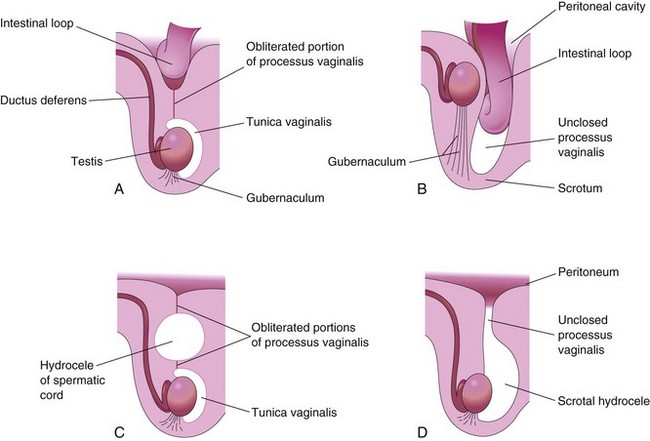
FIGURE 12–49 Diagrams of sagittal sections illustrating conditions resulting from failure of closure of the processus vaginalis. A, Incomplete congenital inguinal hernia resulting from persistence of the proximal part of the processus vaginalis. B, Complete congenital inguinal hernia into the scrotum resulting from persistence of the processus vaginalis. Cryptorchidism, a commonly associated anomaly, is also illustrated. C, Large hydrocele that resulted from an unobliterated portion of the processus vaginalis. D, Hydrocele of the testis and spermatic cord resulting from peritoneal fluid passing into an unclosed processus vaginalis.
Hydrocele
Occasionally the abdominal end of the processus vaginalis remains open but is too small to permit herniation of intestine (Fig. 12-49D). Peritoneal fluid passes into the patent processus vaginalis and forms a scrotal hydrocele. If the middle part of the processus vaginalis remains open, fluid may accumulate and give rise to a hydrocele of the spermatic cord (Fig. 12-49C ).
Summary Of Urogenital System
Clinically Oriented Problems
Case 12–1
A 4-year-old girl was still in diapers because she was continually wet. The pediatrician saw urine coming from the infant’s vagina. An intravenous urogram showed two renal pelves and two ureters on the right side. One ureter was clearly observed to enter the bladder, but the termination of the other one was not clearly seen. A pediatric urologist examined the child under general anesthesia and observed a small opening in the posterior wall of the vagina. She passed a tiny catheter into it and injected contrast media. This procedure showed that the opening in the vagina was the orifice of the second ureter.
Case 12–2
A seriously injured young man suffered a cardiac arrest. After cardiopulmonary resuscitation, his heart began to beat again, but spontaneous respirations did not occur. Artificial respiration was instituted, but there was no electroencephalographic evidence of brain activity. After 2 days, the man’s family agreed that there was no hope of his recovery and asked that his kidneys be donated for transplantation. The radiologist carried out femoral artery catheterization and aortography (radiographic visualization of the aorta and its branches). This technique showed a single large renal artery on the right, but two renal arteries on the left, one medium in size and the other small. Only the right kidney was used for transplantation because it is more difficult to implant small arteries than large ones. Grafting of the small accessory renal artery into the aorta would be difficult because of its size, and part of the kidney would die if one of the arteries was not successfully grafted.
Case 12–3
A 32-year-old woman with a short history of cramping, lower abdominal pain, and tenderness underwent a laparotomy because of a suspected ectopic pregnancy. The operation revealed a pregnancy in a rudimentary right uterine horn. The gravid (pregnant) uterine horn was totally removed.
Case 12–4
During the physical examination of a male neonate, it was observed that the urethra opened on the ventral surface of the penis at the junction of the glans of penis and the body of the penis. The penis was curved toward the undersurface of the penis.
Case 12–5
A 20-year-old woman was prevented from competing in the Olympics because her buccal smear test was chromatin negative, indicating that she had a male sex chromosome complement.
Case 12–6
A 10-year-old boy suffered pain in his left groin while attempting to lift a heavy box. Later he noticed a lump in his groin. When he told his mother about the lump, she arranged an appointment with the family physician. After a physical examination, a diagnosis of indirect inguinal hernia was made.
References and Suggested Reading
Ashley RA, Barthold JS, Kolon TF. Cryptorchidism: pathogenesis, diagnosis and prognosis. Urol Clin North Am. 2010;37:183.
Avni FE, Maugey-Laulom B, Cassart M, et al. The fetal genitourinary tract. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Bendon RW. Oligohydramnios. Front Fetal Health. 2000;2:10.
Billmire DF. Germ cell tumors. Surg Clin North Am. 2006;86:489.
Elder JS. Urologic disorders in infants and children. In Behrman RE, Kliegman RM, Jenson HB, editors: Nelson Textbook of Pediatrics, ed 17, Philadelphia: WB Saunders, 2004.
Fiegel HC, Rolle U, Metzger R, et al. Embryology of testicular descent. Semin Pediatr Surg. 2011;20:161.
Haynes JH. Inguinal and scrotal disorders. Surg Clin North Am. 2006;86:371.
Hecht NB. Molecular mechanism of male germ cell differentiation. BioEssays. 1998;20:555.
Kluth D, Fiegel HC, Geyer C. Embryology of the distal urethra and external genitals. Semin Pediatr Surg. 2011;20:176.
Kraft KH, Shukla AR, Canning DA. Hypospadia. Urol Clin North Am. 2010;37:167.
Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: cellular and molecular regulation. Mech Dev. 2000;92:19.
Lambert SM, Vilain EJ, Kolon TF. A practical approach to ambiguous genitalia in the new born period. Urol Clin North Am. 2010;37:195.
Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends Mol Med. 2010;16:349.
Lee PA, Houk CP, Ahmed SF, et al. Consensus statement on management of intersex disorders. Pediatrics. 2006;118:e4888.
Little M, Georgas K, Pennisi D, et al. Kidney development: two tales of tubulogenesis. Curr Top Dev Biol. 2010;90:193.
Meeks J, Schaeffer EM. Genetic regulation of prostate development. J Androl. 2011;32:210.
Moore KL. The development of clinical sex chromatin tests. In: Moore KL, editor. The Sex Chromatin. Philadelphia: WB Saunders, 1966.
Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Nebot-Cegarra J, Fàbregas PJ, Sánchez-Pérez I. Cellular proliferation in the urorectal septation complex of the human embryo at Carnegie stages 13–18: A nuclear area-based morphometric analysis. J Anat. 2005;207:353.
Nishida H, Miyagawa S, Matsumaru D, et al. Gene expression analyses on embryonic external genitalia: identification of regulatory genes possibly involved in masculinization process. Congenit Anom. 2008;48:63.
Palmert MR, Dahms WT. Abnormalities of sexual differentiation. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal–Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Persaud TVN. Embryology of the female genital tract and gonads. In Copeland LJ, Jarrell J, editors: Textbook of Gynecology, ed 2, Philadelphia: WB Saunders, 2000.
Poder L. Ultrasound evaluation of the uterus. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Powell DM, Newman KD, Randolph J. A proposed classification of vaginal anomalies and their surgical correction. J Pediatr Surg. 1995;30:271.
Sobel V, Zhu Y-S, Imperato-McGinley J. Fetal hormones and sexual differentiation. Obstet Gynecol Clin North Am. 2004;31:837.
Stec AA. Embryology and bony and pelvic floor anatomy in the bladder and exstrophy-epispadias complex. Sem Pediatr Surg. 2011;20:66.
Vogt BA, Dell KM, Davis ID. The kidney and urinary tract. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal–Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Witschi E. Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal folds. Contr Embryol Carnegie Inst. 1948;32:67.
Woolf AS. A molecular and genetic view of human renal and urinary tract malformations. Kidney Int. 2000;58:500.
Yiee JH, Baskin LS. Environmental factors in genitourinary development. J Urol. 2010;184:34.