Chapter 8 Body Cavities and Diaphragm
Early in the fourth week, the intraembryonic coelom appears as a horseshoe-shaped cavity (Fig. 8-1A). The bend in this cavity at the cranial end of the embryo represents the future pericardial cavity, and its limbs (lateral extensions) indicate the future pleural and peritoneal cavities. The distal part of each limb of the intraembryonic coelom is continuous with the extraembryonic coelom at the lateral edges of the embryonic disc (Fig. 8-1B). This communication is important because most of the midgut herniates through this communication into the umbilical cord (Fig. 8-2E) (Chapter 11). The intraembryonic coelom provides room for the organs to develop and move. During embryonic folding in the horizontal plane, the limbs of the coelom are brought together on the ventral aspect of the embryo (Fig. 8-2C). The ventral mesentery degenerates in the region of the future peritoneal cavity (Fig. 8-2F), resulting in a large embryonic peritoneal cavity extending from the heart to the pelvic region.
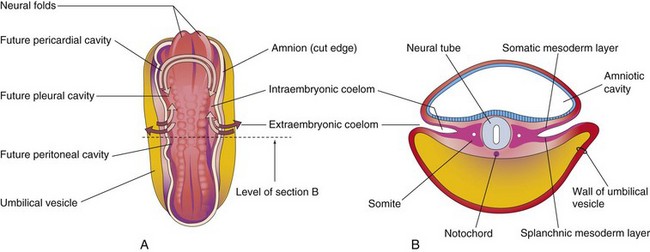
FIGURE 8–1 A, Drawing of a dorsal view of a 22-day embryo showing the outline of the horseshoe-shaped intraembryonic coelom. The amnion has been removed and the coelom is shown as if the embryo were translucent. The continuity of the intraembryonic coelom, as well as the communication of its right and left limbs with the extraembryonic coelom, is indicated by arrows. B, Transverse section through the embryo at the level shown in A.
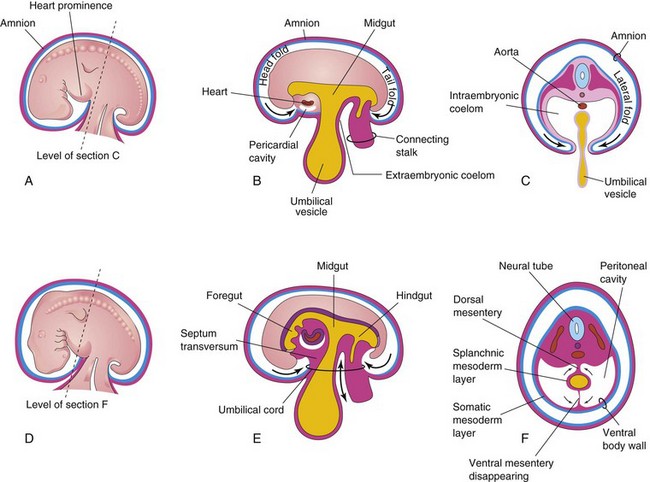
FIGURE 8–2 Illustrations of embryonic folding and its effects on the intraembryonic coelom and other structures. A, Lateral view of an embryo (approximately 26 days). B, Schematic sagittal section of this embryo showing the head and tail folds. C, Transverse section at the level shown in A, indicating how fusion of the lateral folds gives the embryo a cylindrical form. D, Lateral view of an embryo (approximately 28 days). E, Schematic sagittal section of this embryo showing the reduced communication between the intraembryonic and extraembryonic coeloms (double-headed arrow). F, Transverse section as indicated in D, illustrating formation of the ventral body wall and disappearance of the ventral mesentery. The arrows indicate the junction of the somatic and splanchnic layers of mesoderm. The somatic mesoderm will become the parietal peritoneum lining the abdominal wall, and the splanchnic mesoderm will become the visceral peritoneum covering the organs (e.g., the stomach).
 Embryonic Body Cavity
Embryonic Body Cavity
The intraembryonic coelom becomes the embryonic body cavity, which is divided into three well-defined cavities during the fourth week (Figs. 8-2 and 8-3):
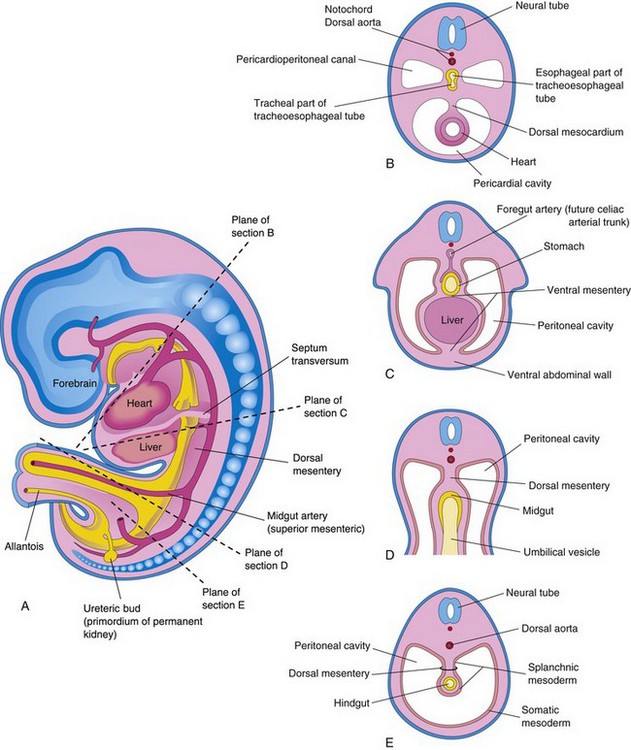
FIGURE 8–3 Illustrations of the mesenteries and body cavities at the beginning of the fifth week. A, Schematic sagittal section. Note that the dorsal mesentery serves as a pathway for the arteries supplying the developing gut. Nerves and lymphatics also pass between the layers of this mesentery. B to E, Transverse sections through the embryo at the levels indicated in A. The ventral mesentery disappears, except in the region of the terminal esophagus, stomach, and first part of the duodenum. Note that the right and left parts of the peritoneal cavity, separate in C, are continuous in E.
These body cavities have a parietal wall lined by mesothelium (future parietal layer of peritoneum) derived from somatic mesoderm and a visceral wall covered by mesothelium (future visceral layer of peritoneum) derived from splanchnic mesoderm (Fig. 8-3E). The peritoneal cavity (the major part of intraembryonic coelom) is connected with the extraembryonic coelom at the umbilicus (Fig. 8-4A and D). The peritoneal cavity loses its connection with the extraembryonic coelom during the 10th week as the intestines return to the abdomen from the umbilical cord (Chapter 11).
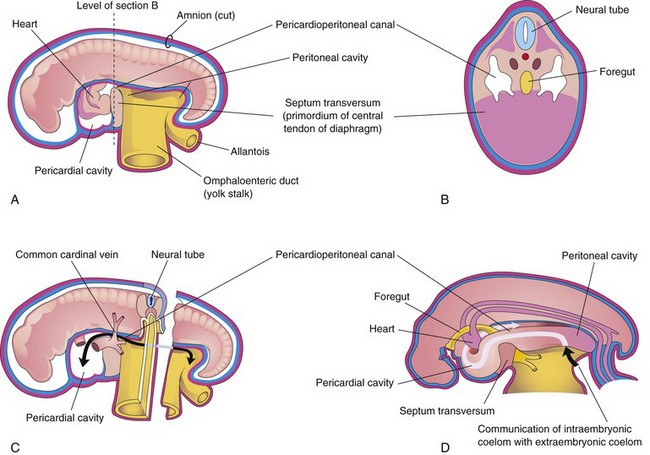
FIGURE 8–4 Schematic drawings of an embryo (approximately 24 days). A, The lateral wall of the pericardial cavity has been removed to show the primordial heart. B, Transverse section of the embryo illustrating the relationship of the pericardioperitoneal canals to the septum transversum (primordium of central tendon of diaphragm) and the foregut. C, Lateral view of the embryo with the heart removed. The embryo has also been sectioned transversely to show the continuity of the intraembryonic and extraembryonic coeloms (arrow). D, Sketch showing the pericardioperitoneal canals arising from the dorsal wall of the pericardial cavity and passing on each side of the foregut to join the peritoneal cavity. The arrow shows the communication of the extraembryonic coelom with the intraembryonic coelom and the continuity of the intraembryonic coelom at this stage.
During formation of the head fold, the heart and pericardial cavity are relocated ventrally, anterior to the foregut (Fig. 8-2B). As a result, the pericardial cavity opens into pericardioperitoneal canals, which pass dorsal to the foregut (Fig. 8-4B and D). After embryonic folding, the caudal part of the foregut, the midgut, and the hindgut are suspended in the peritoneal cavity from the dorsal abdominal wall by the dorsal mesentery (Figs. 8-2F and 8-3B, D, and E).
 Mesenteries
Mesenteries
A mesentery is a double layer of peritoneum that begins as an extension of the visceral peritoneum covering an organ. The mesentery connects the organ to the body wall and conveys vessels and nerves to it. Transiently, the dorsal and ventral mesenteries divide the peritoneal cavity into right and left halves (Fig. 8-3C). The ventral mesentery soon disappears (Fig. 8-3E), except where it is attached to the caudal part of the foregut (primordium of stomach and proximal part of duodenum). The peritoneal cavity then becomes a continuous space (Fig. 8-4D). The arteries supplying the primordial gut—celiac arterial trunk (foregut), superior mesenteric artery (midgut), and inferior mesenteric artery (hindgut)—pass between the layers of the dorsal mesentery (Fig. 8-3C).
Division of Embryonic Body Cavity
Each pericardioperitoneal canal lies lateral to the proximal part of the foregut (future esophagus) and dorsal to the septum transversum—a plate of mesodermal tissue that occupies the space between the thoracic cavity and omphaloenteric duct (Fig. 8-4A and B).
The septum transversum is the primordium of the central tendon of the diaphragm. Partitions form in each pericardioperitoneal canal that separate the pericardial cavity from the pleural cavities, and the pleural cavities from the peritoneal cavity. Because of the growth of the bronchial buds (primordia of bronchi and lungs) into the pericardioperitoneal canals (Fig. 8-5A), a pair of membranous ridges is produced in the lateral wall of each canal:
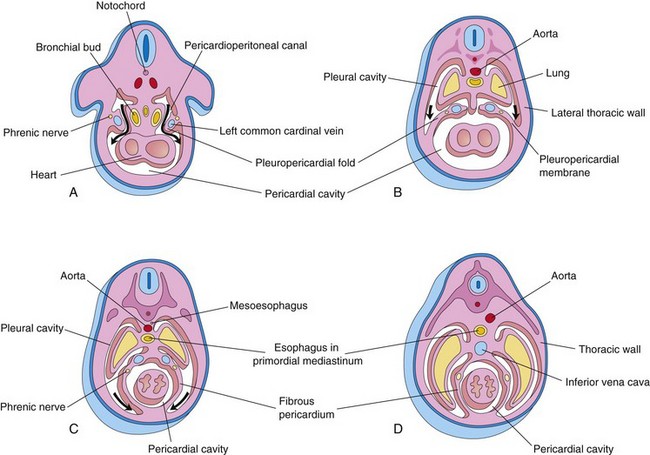
FIGURE 8–5 Drawings of transverse sections through embryos cranial to the septum transversum, illustrating successive stages in the separation of the pleural cavities from the pericardial cavity. Growth and development of the lungs, expansion of the pleural cavities, and formation of the fibrous pericardium are also shown. A, At 5 weeks. The arrows indicate the communications between the pericardioperitoneal canals and the pericardial cavity. B, At 6 weeks. The arrows indicate development of the pleural cavities as they expand into the body wall. C, At 7 weeks. Expansion of the pleural cavities ventrally around the heart is shown. The pleuropericardial membranes are now fused in the median plane with each other and with the mesoderm ventral to the esophagus. D, At 8 weeks. Continued expansion of the lungs and pleural cavities and formation of the fibrous pericardium and thoracic wall are illustrated.
Congenital Pericardial Defects
Defective formation and/or fusion of the pleuropericardial membranes separating the pericardial and pleural cavities is uncommon. This anomaly results in a congenital defect of the pericardium, usually on the left side. Consequently, the pericardial cavity communicates with the pleural cavity. In very unusual cases, part of the left atrium of the heart herniates into the pleural cavity at each heartbeat.
Pleuropericardial Membranes
As the pleuropericardial folds enlarge, they form partitions that separate the pericardial cavity from the pleural cavities. These partitions—pleuropericardial membranes—contain the common cardinal veins (Figs. 8-4C and 8-5A), which drain the venous system into the sinus venosus of the heart (see Chapter 13). Initially the bronchial buds are small relative to the heart and pericardial cavity (Fig. 8-5A). They soon grow laterally from the caudal end of the trachea into the pericardioperitoneal canals (future pleural canals). As the primordial pleural cavities expand ventrally around the heart, they extend into the body wall, splitting the mesenchyme into:
The pleuropericardial membranes project into the cranial ends of the pericardioperitoneal canals (Fig. 8-5B). With subsequent growth of the common cardinal veins, positional displacement of the heart, and expansion of the pleural cavities, the pleuropericardial membranes become mesentery-like folds extending from the lateral thoracic wall. By the seventh week, the pleuropericardial membranes fuse with the mesenchyme ventral to the esophagus, separating the pericardial cavity from the pleural cavities (Fig. 8-5C). This primordial mediastinum consists of a mass of mesenchyme that extends from the sternum to the vertebral column, separating the developing lungs (Fig. 8-5D). The right pleuropericardial opening closes slightly earlier than the left one and produces a larger pleuropericardial membrane.
Pleuroperitoneal Membranes
As the pleuroperitoneal folds enlarge, they project into the pericardioperitoneal canals. Gradually the folds become membranous, forming the pleuroperitoneal membranes (Figs. 8-6 and 8-7). Eventually these membranes separate the pleural cavities from the peritoneal cavity. The pleuroperitoneal membranes are produced as the developing lungs and pleural cavities expand and invade the body wall. They are attached dorsolaterally to the abdominal wall and initially their crescentic free edges project into the caudal ends of the pericardioperitoneal canals.
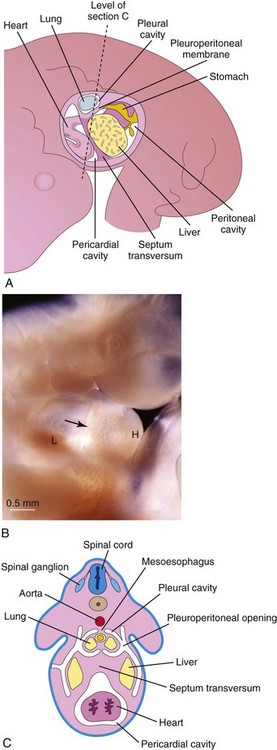
FIGURE 8–6 A, The primordial body cavities are viewed from the left side after removal of the lateral body wall. B, Photograph of a 5-week-old embryo showing the developing septum transversum (arrow), heart tube (H), and liver (L). C, Transverse section through the embryo at the level shown in A.
(B, Courtesy of Dr. Bradley R. Smith, University of Michigan, Ann Arbor, MI.)
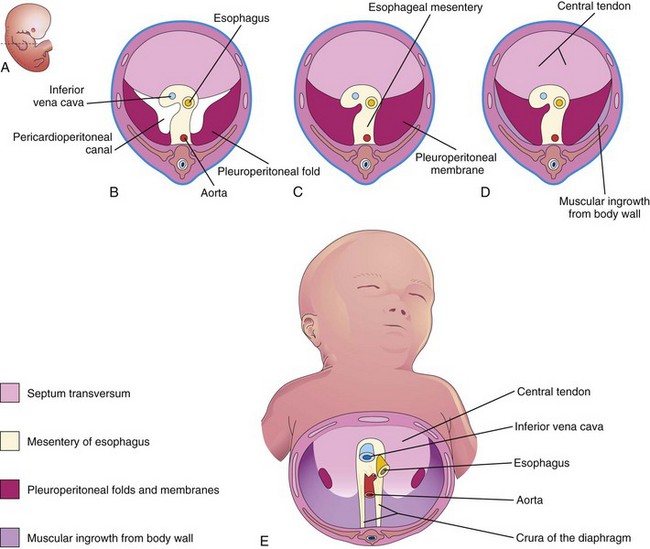
FIGURE 8–7 Illustrations of the development of the diaphragm. A, Lateral view of an embryo at the end of the fifth week (actual size) indicating the level of sections in B to D. B, Transverse section showing the unfused pleuroperitoneal membranes. C, Similar section at the end of the sixth week after fusion of the pleuroperitoneal membranes with the other two diaphragmatic components. D, Transverse section of a 12-week fetus after ingrowth of the fourth diaphragmatic component from the body wall. E, Inferior view of the diaphragm of a neonate indicating the embryologic origin of its components.
During the sixth week, the pleuroperitoneal membranes extend ventromedially until their free edges fuse with the dorsal mesentery of the esophagus and septum transversum (Fig. 8-7C). This separates the pleural cavities from the peritoneal cavity. Closure of the pleuroperitoneal openings is assisted by the migration of myoblasts (primordial muscle cells) into the pleuroperitoneal membranes (Fig. 8-7E). The pleuroperitoneal opening on the right side closes slightly before the left one. The reason for this is uncertain, but it may be related to the relatively large size of the right lobe of the liver at this stage of development.
 Development of the Diaphragm
Development of the Diaphragm
The diaphragm is a dome-shaped, musculotendinous partition that separates the thoracic and abdominal cavities. It is a composite structure that develops from four embryonic components (Fig. 8-7):
Several candidate genes on the long arm of chromosome 15 (15q) play a critical role in the development of the diaphragm.
Septum Transversum
The septum transversum grows dorsally from the ventrolateral body wall and forms a semicircular shelf, which separates the heart from the liver (Fig. 8-6). This transverse septum, composed of mesodermal tissue, forms the central tendon of the diaphragm (Fig. 8-7D and E). After the head folds ventrally during the fourth week, the septum transversum forms a thick incomplete partition between the pericardial and abdominal cavities (Fig. 8-4). The septum transversum does not separate the thoracic and abdominal cavities completely.
During its early development, a large part of the liver is embedded in the septum transversum. There are large openings, the pericardioperitoneal canals, along the sides of the esophagus (Fig. 8-7B). The septum transversum expands and fuses with the dorsal mesentery of the esophagus and the pleuroperitoneal membranes (Fig. 8-7C).
Pleuroperitoneal Membranes
These membranes fuse with the dorsal mesentery of the esophagus and the septum transversum (Fig. 8-7C). This completes the partition between the thoracic and abdominal cavities and forms the primordial diaphragm. Although the pleuroperitoneal membranes form large portions of the early fetal diaphragm, they represent relatively small portions of the newborn’s diaphragm (Fig. 8-7E).
Dorsal Mesentery of the Esophagus
As previously described, the septum transversum and pleuroperitoneal membranes fuse with the dorsal mesentery of the esophagus. This mesentery constitutes the median portion of the diaphragm. The crura of the diaphragm, a leg-like pair of diverging muscle bundles that cross in the median plane anterior to the aorta (Fig. 8-7E), develop from myoblasts that grow into the dorsal mesentery of the esophagus.
Muscular Ingrowth from the Lateral Body Walls
During the 9th to 12th weeks, the lungs and pleural cavities enlarge, “burrowing” into the lateral body walls (Fig. 8-5). During this process, the body-wall tissue is split into two layers:
Further extension of the developing pleural cavities into the lateral body walls forms the costodiaphragmatic recesses (Fig. 8-8), establishing the characteristic dome-shaped configuration of the diaphragm. After birth, the costodiaphragmatic recesses become alternately smaller and larger as the lungs move in and out of them during inspiration and expiration.
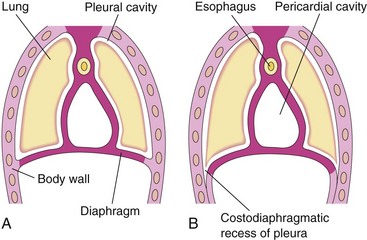
FIGURE 8–8 Illustrations of extension of the pleural cavities into the body walls to form peripheral parts of the diaphragm, the costodiaphragmatic recesses, and the establishment of the characteristic dome-shaped configuration of the diaphragm. Note that body wall tissue is added peripherally to the diaphragm as the lungs and pleural cavities enlarge.
Positional Changes and Innervation of the Diaphragm
During the fourth week, the septum transversum, before relocation of the heart, lies opposite the third to fifth cervical somites. During the fifth week, myoblasts from these somites migrate into the developing diaphragm, bringing their nerve fibers with them. Consequently, the phrenic nerves that supply motor innervation to the diaphragm arise from the ventral primary rami of the third, fourth, and fifth cervical spinal nerves. The three twigs on each side join together to form a phrenic nerve. The phrenic nerves also supply sensory fibers to the superior and inferior surfaces of the right and left domes of the diaphragm.
Rapid growth of the dorsal part of the embryo’s body results in an apparent descent of the diaphragm. By the sixth week, the developing diaphragm is at the level of the thoracic somites. The phrenic nerves now have a descending course. As the diaphragm appears relatively farther caudally in the body, the nerves are correspondingly lengthened. By the beginning of the eighth week, the dorsal part of the diaphragm lies at the level of the first lumbar vertebra. Because of the cervical origin of the phrenic nerves, they are approximately 30 cm long in adults.
The phrenic nerves in the embryo enter the diaphragm by passing through the pleuropericardial membranes. This explains why the phrenic nerves subsequently lie on the fibrous pericardium, the adult derivative of the pleuropericardial membranes (Fig. 8-5C and D).
As the four parts of the diaphragm fuse (Fig. 8-7), mesenchyme in the septum transversum extends into the other three parts. It forms myoblasts that differentiate into the skeletal muscle of the diaphragm. The costal border receives sensory fibers from the lower intercostal nerves because of the origin of the peripheral part of the diaphragm from the lateral body walls (Fig. 8-7D and E).
Posterolateral Defect of Diaphragm
Posterolateral defect of the diaphragm is the only relatively common birth defect of the diaphragm (Figs. 8-9A and B and 8-10). This diaphragmatic defect occurs about once in 2,200 newborn infants and is associated with congenital diaphragmatic hernia (CDH); herniation of abdominal contents into the thoracic cavity.
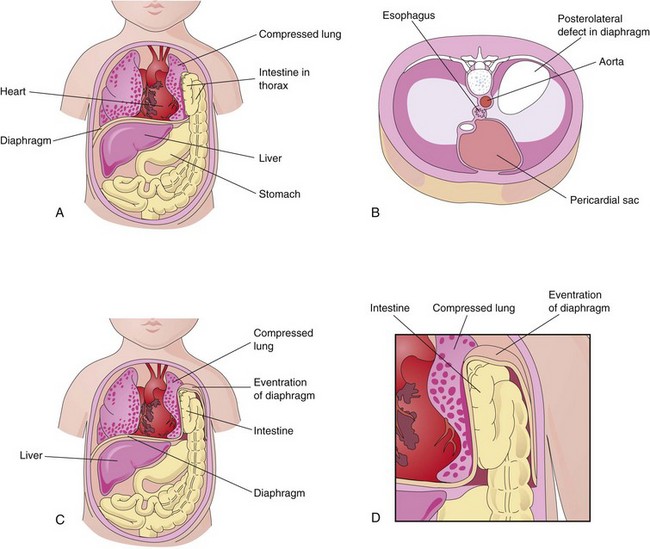
FIGURE 8–9 A, A “window” has been drawn on the thorax and abdomen to show the herniation of the intestine into the thorax through a posterolateral defect in the left side of the diaphragm. Note that the left lung is compressed and hypoplastic. B, Drawing of a diaphragm with a large posterolateral defect on the left side due to abnormal formation and/or fusion of the pleuroperitoneal membrane on the left side with the mesoesophagus and septum transversum. C and D, Eventration of the diaphragm resulting from defective muscular development of the diaphragm. The abdominal viscera are displaced into the thorax within a pouch of diaphragmatic tissue.
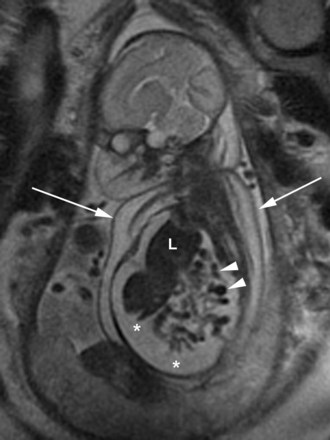
FIGURE 8–10 Coronal magnetic resonance image of a fetus with right-sided congenital diaphragmatic hernia. Note the liver (L) and loops of small intestine (arrowheads) in the thoracic cavity. There are ascites (*)—accumulation of serous fluid in the peritoneal cavity—extending into the thoracic cavity, and skin thickening (arrows).
(Courtesy of Deborah Levine, M.D., Director of Obstetric and Gynecologic Ultrasound, Beth Israel Deaconess Medical Center, Boston, MA.)
Life-threatening breathing difficulties may be associated with CDH because of inhibition of development and inflation of the lungs (Fig. 8-11). Moreover, fetal lung maturation may be delayed. Polyhydramnios (excess amniotic fluid) may also be present. CDH is the most common cause of pulmonary hypoplasia. The candidate region for CDH has been reported to be chromosome 15q26.
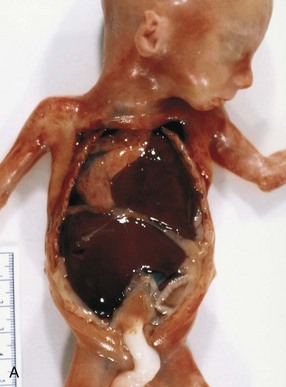
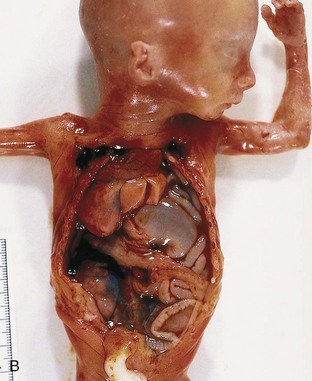
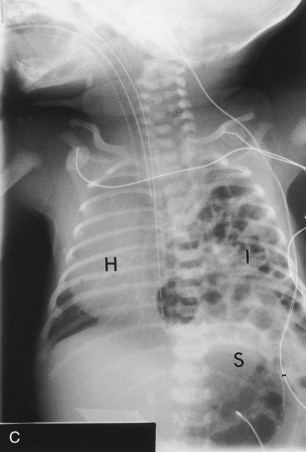
FIGURE 8–11 Diaphragmatic hernia on the left side of a fetus, showing herniation of liver (A), stomach, and bowel (B), underneath the liver into left thoracic cavity. Note the pulmonary hypoplasia visible after liver removal (female fetus at 19 to 20 weeks). C, Diaphragmatic hernia (posterolateral defect). Chest radiograph of a neonate showing herniation of intestinal loops (I) into the left side of the thorax. Note that the heart (H) is displaced to the right side and that the stomach (S) is on the left side of the upper abdominal cavity.
(A and B, Courtesy of Dr. D.K. Kalousek, Department of Pathology, University of British Columbia, Children’s Hospital, Vancouver, British Columbia, Canada. C, Courtesy of Dr. Prem S. Sahni, formerly of the Department of Radiology, Children’s Hospital, Winnipeg, Manitoba, Canada.)
CDH, usually unilateral, results from defective formation and/or fusion of the pleuroperitoneal membranes with the other three parts of the diaphragm (Fig. 8-7). This results in a large opening in the posterolateral region of the diaphragm. As a result, the peritoneal and pleural cavities are continuous with one another along the posterior body wall. This birth defect, sometimes referred to as the foramen of Bochdalek, occurs on the left side in 85% to 90% of cases.
The preponderance of left-sided defects may be related to the earlier closure of the right pleuroperitoneal opening. Prenatal diagnosis of CDH depends on ultrasound examination and magnetic resonance imaging of abdominal organs in the thorax.
The pleuroperitoneal membranes normally fuse with the other three diaphragmatic components by the end of the sixth week (Fig. 8-7C). If a pleuroperitoneal canal is still open when the intestines return to the abdomen from the physiological hernia of the umbilical cord in the 10th week (see Chapter 11), some intestine and other viscera may pass into the thorax. The presence of abdominal viscera in the thorax pushes the lungs and heart anteriorly and compression of the lungs occurs. Often the stomach, spleen, and most of the intestines herniate (Fig. 8-11). Most babies born with CDH die not because there is a defect in the diaphragm or abdominal viscera in the chest, but because the lungs are hypoplastic due to compression during development.
The severity of pulmonary developmental abnormalities depends on when and to what extent the abdominal viscera herniate into the thorax, that is, on the timing and degree of compression of the fetal lungs. The effect on the ipsilateral (same side) lung is greater, but the contralateral lung also shows morphologic changes. If the abdominal viscera are in the thoracic cavity at birth, the initiation of respiration is likely to be impaired. The intestines dilate with swallowed, which compromises the functioning of the heart and lungs. Because the abdominal organs are most often in the left side of the thorax, the heart and mediastinum are usually displaced to the right.
The lungs in infants with CDH are often hypoplastic. The growth retardation of the lungs results from the lack of room for them to develop normally. The lungs are often aerated and achieve their normal size after reduction (repositioning) of the herniated viscera and repair of the defect in the diaphragm. Prenatal detection of CDH occurs in about 50% of cases. Most infants with CDH now survive because of improvements in ventilator care.
Eventration of Diaphragm
In this uncommon condition, half the diaphragm has defective musculature and balloons into the thoracic cavity as an aponeurotic (membranous) sheet, forming a diaphragmatic pouch (Fig. 8-9C and D). Consequently, there is superior displacement of abdominal viscera into the pocket-like outpouching of the diaphragm. This birth defect results mainly from failure of muscular tissue from the body wall to extend into the pleuroperitoneal membrane on the affected side.
Eventration of the diaphragm is not a true diaphragmatic herniation; it is a superior displacement of viscera into a sac-like part of the diaphragm. However, the clinical manifestations of diaphragmatic eventration may simulate CDH.
Gastroschisis and Congenital Epigastric Hernia
Gastroschisis is a congenital fissure in the anterior abdominal wall: usually there is protrusion of viscera. This uncommon hernia occurs in the median plane between the xiphoid process and umbilicus. The defect is similar to an umbilical hernia (see Chapter 11), except for its location.
Gastroschisis and epigastric hernias result from failure of the lateral body folds to fuse completely when forming the anterior abdominal wall during folding in the fourth week (Fig. 8-2C and F). The small intestine herniates into the amniotic cavity, which can be detected prenatally by ultrasonography.
Congenital Hiatal Hernia
Herniation of part of the fetal stomach may occur through an excessively large esophageal hiatus—the opening in the diaphragm through which the esophagus and vagus nerves pass. A hiatal hernia is usually an acquired lesion occurring during adult life; a congenitally enlarged esophageal hiatus may be the predisposing factor in some cases.
Retrosternal (Parasternal) Hernia
Herniations through the sternocostal hiatus (foramen of Morgagni)—the opening for the superior epigastric vessels in the retrosternal area—may occur; however, they are uncommon. This hiatus is located between the sternal and costal parts of the diaphragm.
Herniation of intestine into the pericardial sac may occur, or conversely, part of the heart may descend into the peritoneal cavity in the epigastric region. Large defects are commonly associated with body wall defects in the umbilical region (e.g., omphalocele; see Chapter 11). Radiologists and pathologists often observe fatty herniations through the sternocostal hiatus; however, they are usually of no clinical significance.
Accessory Diaphragm
More than 30 cases of this rare anomaly have been reported. It is most often on the right side and associated with lung hypoplasia and other respiratory complications. An accessory diaphragm can be diagnosed by magnetic resonance imaging and computed tomography. It is treated by surgical excision.
 Summary of Development of the Body Cavities and Diaphragm
Summary of Development of the Body Cavities and Diaphragm
Clinically Oriented Problems
Case 8–1
A neonate has severe respiratory distress. The abdomen is unusually flat and intestinal peristaltic movements are heard over the left side of the thorax.
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Clugston RD, Greer JJ. Diaphragmatic development and congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16:94.
Clugston RD, Zhang W, Alvarez S, et al. Understanding abnormal retinoid signaling as a causative mechanism in congenital diaphragmatic hernia. Am J Res Cell Mol Biol. 2010;42:276.
Cass DL. Fetal surgery for congenital diaphragmatic hernia: The North American Experience. Semin Perinatol. 2005;29:104.
Deprest J, Jani J, Gratacos E, et al. Fetal intervention for congenital diaphragmatic hernia: The European experience. Semin Perinatol. 2005;29:94.
Groth SS, Andrade RS. Diaphragmatic eventration. Thorac Surg Clin. 2009;19:511.
Wladimiroff JW, Cohen-Overbeek TE, Laudy JAM. Ultrasound evaluation of the fetal thorax. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Graham G, Devine PC. Antenatal diagnosis of congenital diaphragmatic hernia. Semin Perinatol. 2005;29:69.
Kays DW. Congenital diaphragmatic hernia and neonatal lung lesions. Surg Clin North Am. 2006;86:329.
Mayer S, Metzger R, Kluth D. The embryology of the diaphragm. Semin Pediatr Surg. 2011;20:161.
Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Moya FR, Lally KP. Evidence-based management of infants with congenital diaphragmatic hernia. Semin Perinatol. 2005;29:112.
Rottier R, Tibboel D. Fetal lung and diaphragm development in congenital diaphragmatic hernia. Semin Perinatol. 2005;29:86.
Slavotinek AM. The genetics of congenital diaphragmatic hernia. Semin Perinatol. 2005;29:77.
Turell DC. Advances with surfactant. Emerg Med Clin North Am. 2008;26:921.
Wells LJ. Development of the human diaphragm and pleural sacs. Contr Embryol Carnegie Inst. 1954;35:107.