Chapter 5 Fourth to Eighth Weeks of Human Development
All major external and internal structures are established during the fourth to eighth weeks. By the end of this period, the main organ systems have started to develop. As the tissues and organs form, the shape of the embryo changes and by the eighth week, it has a distinctly human appearance.
Because the tissues and organs are differentiating rapidly, exposure of embryos to teratogens during this period may cause major birth defects. Teratogens are agents such as drugs and viruses that produce or increase the incidence of birth defects (see Chapter 20).
Phases of Embryonic Development
Human development may be divided into three phases, which to some extent are interrelated:
 Folding of Embryo
Folding of Embryo
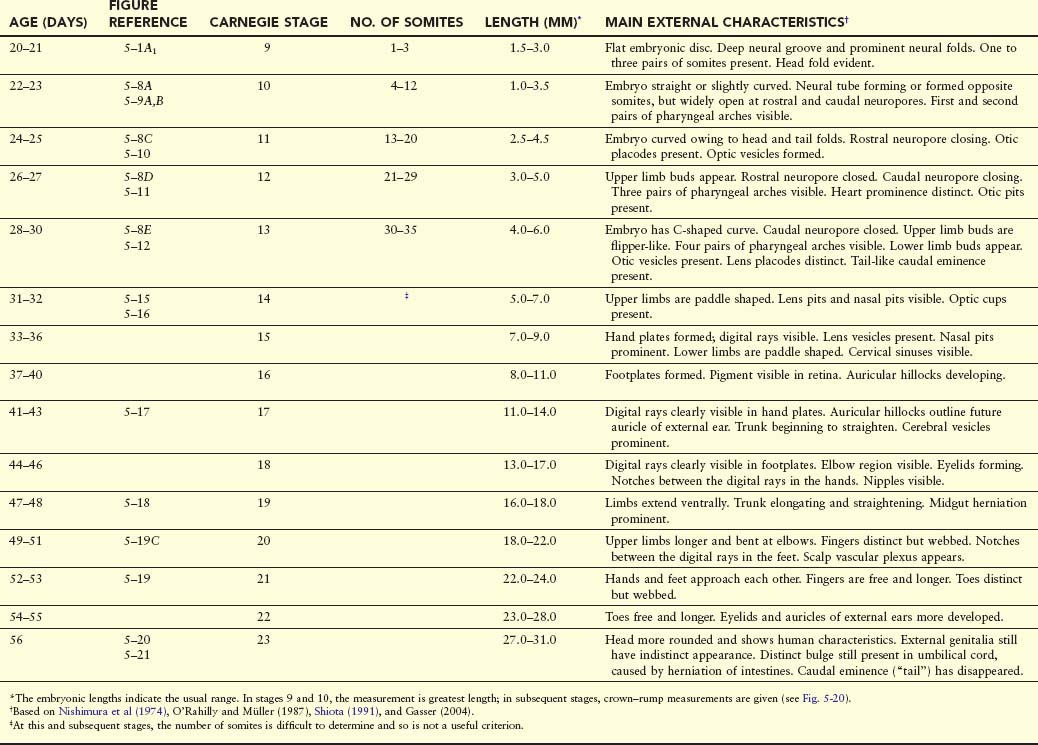
A significant event in the establishment of body form is folding of the flat trilaminar embryonic disc into a somewhat cylindrical embryo (Fig. 5-1). Folding occurs in both the median and horizontal planes and results from rapid growth of the embryo. The growth rate at the sides of the embryonic disc fails to keep pace with the rate of growth in the long axis as the embryo increases rapidly in length. Folding at the cranial and caudal ends and sides of the embryo occurs simultaneously. Concurrently, there is relative constriction at the junction of the embryo and umbilical vesicle (yolk sac).
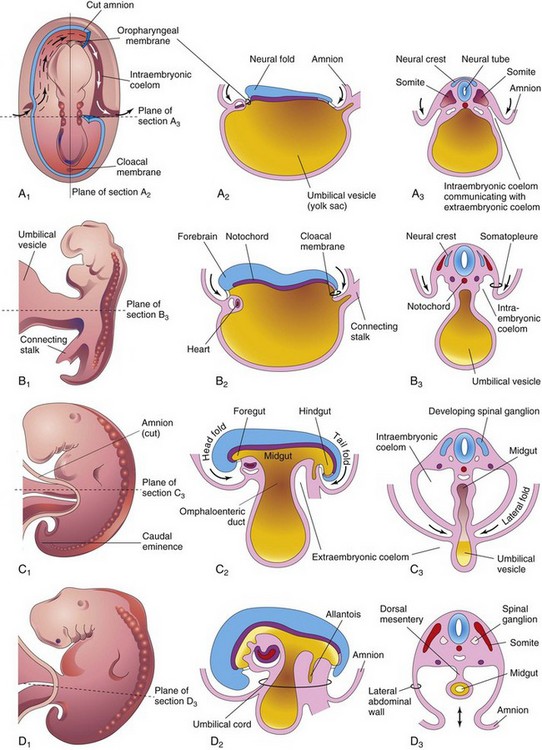
FIGURE 5–1 Drawings of folding of embryos during the fourth week. A1, Dorsal view of an embryo early in the fourth week. Three pairs of somites are visible. The continuity of the intraembryonic coelom and extraembryonic coelom is illustrated on the right side by removal of a part of the embryonic ectoderm and mesoderm. B1, C1, and D1, Lateral views of embryos at 22, 26, and 28 days, respectively. A2 to D2, Sagittal sections at the plane shown in A1. A3 to D3, Transverse sections at the levels indicated in A1 to D1.
Folding of Embryo in the Median Plane
Folding of the ends of the embryo ventrally produces head and tail folds that result in the cranial and caudal regions moving ventrally as the embryo elongates cranially and caudally (Fig. 5-1A2 to D2).
Head Fold
At the beginning of the fourth week, the neural folds in the cranial region have thickened to form the primordium of the brain. Initially, the developing brain projects dorsally into the amniotic cavity. Later, the developing forebrain grows cranially beyond the oropharyngeal membrane and overhangs the developing heart (see Fig. 5-2C). At the same time, the septum transversum, primordial heart, pericardial coelom, and oropharyngeal membrane move onto the ventral surface of the embryo (Fig. 5-2). During folding, part of the endoderm of the umbilical vesicle is incorporated into the embryo as the foregut (primordium of pharynx, esophagus, and lower respiratory system, and so on; see Chapter 11). The foregut lies between the brain and heart and the oropharyngeal membrane separates the foregut from the stomodeum, the primordium of the mouth (Fig. 5-2C). After folding of the head, the septum transversum lies caudal to the heart where it subsequently develops into the central tendon of the diaphragm (see Chapter 8). The head fold also affects the arrangement of the embryonic coelom (primordium of body cavities). Before folding, the coelom consists of a flattened, horseshoe-shaped cavity (Fig. 5-1A1). After folding, the pericardial coelom lies ventral to the heart and cranial to the septum transversum (Fig. 5-2C). At this stage, the intraembryonic coelom communicates widely on each side with the extraembryonic coelom (Figs. 5-1A3 and 5-3).
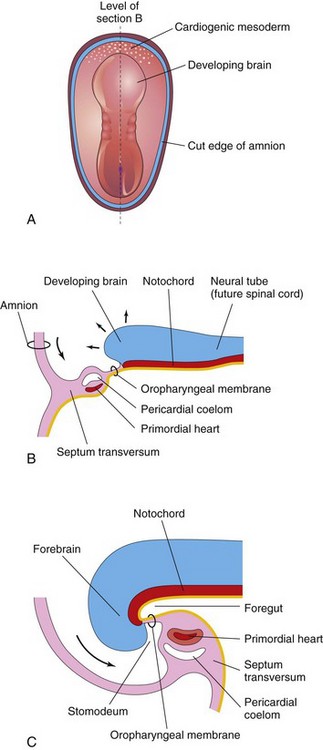
FIGURE 5–2 Folding of cranial end of embryo. A, Dorsal view of embryo at 21 days. B, Sagittal section of the cranial part of the embryo at the plane shown in A. Observe the ventral movement of the heart. C, Sagittal section of an embryo at 26 days. Note that the septum transversum, primordial heart, pericardial coelom, and oropharyngeal membrane have moved onto the ventral surface of the embryo. Observe also that part of the umbilical vesicle is incorporated into the embryo as the foregut.
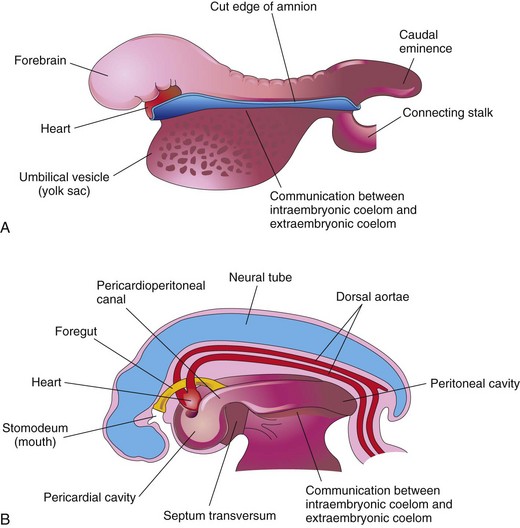
FIGURE 5–3 Drawings of the effect of the head fold on the intraembryonic coelom. A, Lateral view of an embryo (24 to 25 days) during folding, showing the large forebrain, ventral position of the heart, and communication between the intraembryonic and extraembryonic parts of the coelom. B, Schematic drawing of an embryo (26 to 27 days) after folding, showing the pericardial cavity ventrally, the pericardioperitoneal canals running dorsally on each side of the foregut, and the intraembryonic coelom in communication with the extraembryonic coelom.
Tail Fold
Folding of the caudal end of the embryo results primarily from growth of the distal part of the neural tube—the primordium of the spinal cord (Fig. 5-4). As the embryo grows, the caudal eminence (tail region) projects over the cloacal membrane (future site of anus). During folding, part of the endodermal germ layer is incorporated into the embryo as the hindgut (primordium of descending colon and rectum). The terminal part of the hindgut soon dilates slightly to form the cloaca (primordium of urinary bladder and rectum; see Chapters 11 and 12. Before folding, the primitive streak lies cranial to the cloacal membrane (Fig. 5-4A); after folding, it lies caudal to it (Fig. 5-4B). The connecting stalk (primordium of umbilical cord) is now attached to the ventral surface of the embryo, and the allantois—a diverticulum of the umbilical vesicle—is partially incorporated into the embryo.
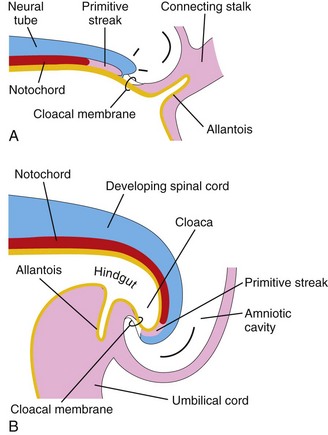
FIGURE 5–4 Folding of caudal end of the embryo. A, Sagittal section of caudal part of the embryo at the beginning of the fourth week. B, Similar section at the end of the fourth week. Note that part of the umbilical vesicle is incorporated into the embryo as the hindgut and that the terminal part of the hindgut has dilated to form the cloaca. Observe also the change in position of the primitive streak, allantois, cloacal membrane, and connecting stalk.
Folding of Embryo in the Horizontal Plane
Folding of the sides of the embryo produces right and left lateral folds (Fig. 5-1A3 to D3). Lateral folding is produced by the rapidly growing spinal cord and somites. The primordia of the ventrolateral wall fold toward the median plane, rolling the edges of the embryonic disc ventrally and forming a roughly cylindrical embryo. As the abdominal walls form, part of the endoderm germ layer is incorporated into the embryo as the midgut (primordium of small intestine; see Fig. 5-1C2 and Chapter 11). Initially, there is a wide connection between the midgut and umbilical vesicle (Fig. 5-1A2); however, after lateral folding, the connection is reduced to an omphaloenteric duct (Fig. 5-1C2). The region of attachment of the amnion to the ventral surface of the embryo is also reduced to a relatively narrow umbilical region (Fig. 5-1D2 and D3). As the umbilical cord forms from the connecting stalk, ventral fusion of the lateral folds reduces the region of communication between the intraembryonic and extraembryonic coelomic cavities to a narrow communication (Fig. 5-1C2). As the amniotic cavity expands and obliterates most of the extraembryonic coelom, the amnion forms the epithelial covering of the umbilical cord (Fig. 5-1D2).
Germ Layer Derivatives
The three germ layers (ectoderm, mesoderm, and endoderm) formed during gastrulation (see Chapter 4) give rise to the primordia of all the tissues and organs. The specificity of the germ layers, however, is not rigidly fixed. The cells of each germ layer divide, migrate, aggregate, and differentiate in rather precise patterns as they form the various organ systems. The main germ layer derivatives are as follows (Fig. 5-5):
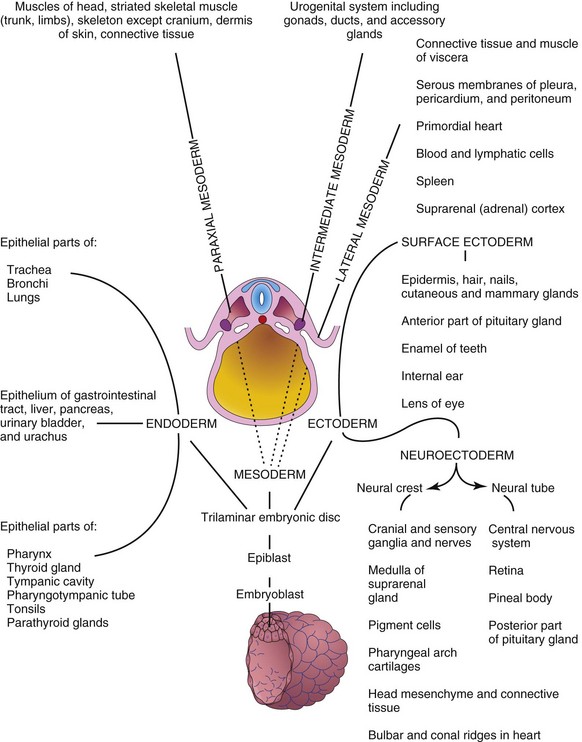
FIGURE 5–5 Schematic drawing of derivatives of the three germ layers: ectoderm, endoderm, and mesoderm. Cells from these layers contribute to the formation of different tissues and organs; for instance, the endoderm forms the epithelial lining of the gastrointestinal tract and the mesoderm gives rise to connective tissues and muscles.
Control of Embryonic Development
Embryonic development results from genetic plans in the chromosomes. Knowledge of the genes that control human development is increasing (see Chapter 21). Most information about developmental processes has come from studies in other organisms, especially Drosophila (fruit fly) and mice because of ethical problems associated with the use of human embryos for laboratory studies. Most developmental processes depend on a precisely coordinated interaction of genetic and environmental factors. Several control mechanisms guide differentiation and ensure synchronized development, such as tissue interactions, regulated migration of cells and cell colonies, controlled proliferation, and programmed cell death. Each system of the body has its own developmental pattern.
Embryonic development is essentially a process of growth and increasing complexity of structure and function. Growth is achieved by mitosis together with the production of extracellular matrices (surrounding substances), whereas complexity is achieved through morphogenesis and differentiation. The cells that make up the tissues of very early embryos are pluripotential, which under different circumstances, are able to follow more than one pathway of development. This broad developmental potential becomes progressively restricted as tissues acquire the specialized features necessary for increasing their sophistication of structure and function. Such restriction presumes that choices must be made to achieve tissue diversification. At present, most evidence indicates that these choices are determined, not as a consequence of cell lineage, but rather in response to cues from the immediate surroundings, including the adjacent tissues. As a result, the architectural precision and coordination that are often required for the normal function of an organ appears to be achieved by the interaction of its constituent parts during development.
The interaction of tissues during development is a recurring theme in embryology. The interactions that lead to a change in the course of development of at least one of the interactants are called inductions. Numerous examples of such inductive interactions can be found in the literature; for example, during development of the eye, the optic vesicle induces the development of the lens from the surface ectoderm of the head. When the optic vesicle is absent, the eye fails to develop. Moreover, if the optic vesicle is removed and placed in association with surface ectoderm that is not usually involved in eye development, lens formation can be induced. Clearly then, development of a lens is dependent on the ectoderm acquiring an association with a second tissue. In the presence of the neuroectoderm of the optic vesicle, the surface ectoderm of the head adopts a pathway of development that it would not otherwise have taken. In a similar fashion, many of the morphogenetic tissue movements that play such important roles in shaping the embryo also provide for the changing tissue associations that are fundamental to inductive tissue interactions.
The fact that one tissue can influence the developmental pathway adopted by another tissue presumes that a signal passes between the two interactants. Analysis of the molecular defects in mutant strains that show abnormal tissue interactions during embryonic development, and studies of the development of embryos with targeted gene mutations have begun to reveal the molecular mechanisms of induction. The mechanism of signal transfer appears to vary with the specific tissues involved. In some cases, the signal appears to take the form of a diffusible molecule, such as sonic hedgehog, that passes from the inductor to the reacting tissue. In others, the message appears to be mediated through a nondiffusible extracellular matrix that is secreted by the inductor and with which the reacting tissue comes into contact. In still other cases, the signal appears to require that physical contacts occur between the inducing and responding tissues. Regardless of the mechanism of intercellular transfer involved, the signal is translated into an intracellular message that influences the genetic activity of the responding cells.
The signal can be relatively nonspecific in some interactions. The role of the natural inductor in a variety of interactions has been shown to be mimicked by a number of heterologous tissue sources and, in some instances, even by a variety of cell-free preparations. Studies suggest that the specificity of a given induction is a property of the reacting tissue rather than that of the inductor. Inductions should not be thought of as isolated phenomena. Often they occur in a sequential fashion that results in the orderly development of a complex structure; for example, following induction of the lens by the optic vesicle, the lens induces the development of the cornea from the surface ectoderm and adjacent mesenchyme. This ensures the formation of component parts that are appropriate in size and relationship for the function of the organ. In other systems, there is evidence that the interactions between tissues are reciprocal. During development of the kidney, for instance, the uteric bud (metanephric diverticulum) induces the formation of tubules in the metanephric mesoderm (see Chapter 12). This mesoderm, in turn, induces branching of the diverticulum that results in the development of the collecting tubules and calices of the kidney.
To be competent to respond to an inducing stimulus, the cells of the reacting system must express the appropriate receptor for the specific inducing signal molecule, the components of the particular intracellular signal transduction pathway, and the transcription factors that will mediate the particular response. Experimental evidence suggests that the acquisition of competence by the responding tissue is often dependent on its previous interactions with other tissues. For example, the lens-forming response of head ectoderm to the stimulus provided by the optic vesicle appears to be dependent on a previous association of the head ectoderm with the anterior neural plate.
The ability of the reacting system to respond to an inducing stimulus is not unlimited. Most inducible tissues appear to pass through a transient, but more or less sharply delimited physiologic state in which they are competent to respond to an inductive signal from the neighboring tissue. Because this state of receptiveness is limited in time, a delay in the development of one or more components in an interacting system may lead to failure of an inductive interaction. Regardless of the signal mechanism employed, inductive systems seem to have the common feature of close proximity between the interacting tissues. Experimental evidence has demonstrated that interactions may fail if the interactants are too widely separated. Consequently, inductive processes appear to be limited in space as well as by time. Because tissue induction plays such a fundamental role in ensuring the orderly formation of precise structures, failed interactions can be expected to have drastic developmental consequences (e.g., birth defects such as absence of the lens).
Highlights of Fourth to Eighth Weeks
The following descriptions summarize the main developmental events and changes in external form of the embryo during the fourth to eighth weeks. The main criteria for estimating developmental stages in human embryos are listed in Table 5-1.
Fourth Week
Major changes in body form occur during the fourth week. At the beginning, the embryo is almost straight and has four to 12 somites that produce conspicuous surface elevations. The neural tube is formed opposite the somites, but it is widely open at the rostral and caudal neuropores (Fig. 5-6). By 24 days, the first two pharyngeal arches are visible. The first pharyngeal arch (mandibular arch) is distinct (Fig. 5-7). The major part of the first arch gives rise to the mandible (lower jaw), and a rostral extension of the arch, the maxillary prominence, contributes to the maxilla (upper jaw). The embryo is now slightly curved because of the head and tail folds. The heart produces a large ventral prominence and pumps blood.
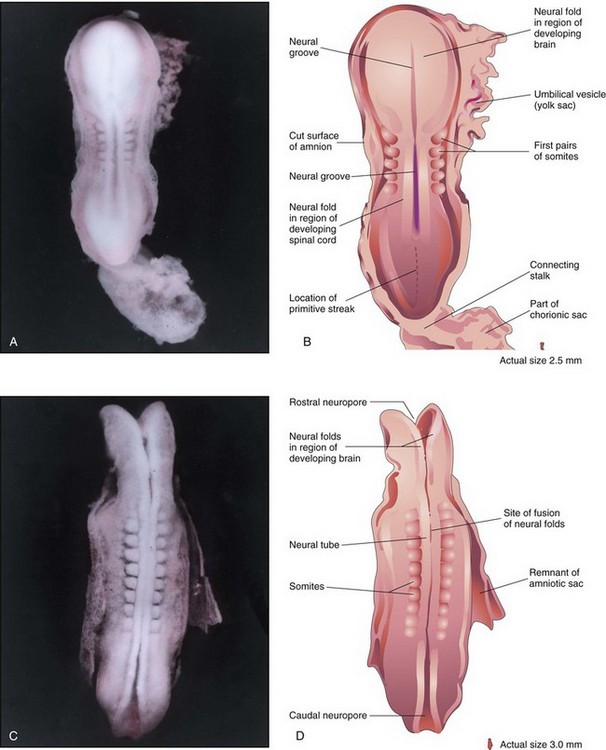
FIGURE 5–6 A, Dorsal view of a five-somite embryo at Carnegie stage 10, approximately 22 days. Observe the neural folds and deep neural groove. The neural folds in the cranial region have thickened to form the primordium of the brain. B, Drawing of the structures shown in A. Most of the amniotic and chorionic sacs have been cut away to expose the embryo. C, Dorsal view of an older eight-somite embryo at Carnegie stage 10. The neural tube is in open communication with the amniotic cavity at the cranial and caudal ends through the rostral and caudal neuropores, respectively. D, Diagram of the structures shown in C. The neural folds have fused opposite the somites to form the neural tube (primordium of spinal cord in this region).
(A and C, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
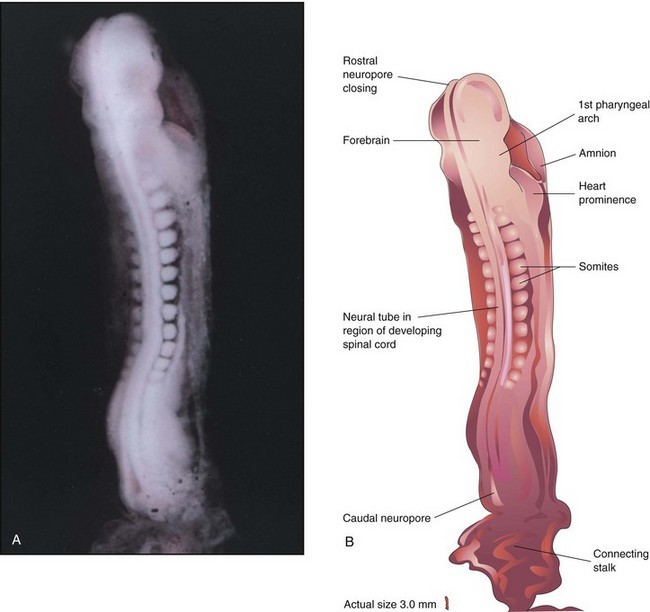
FIGURE 5–7 A, Dorsal view of a 13-somite embryo at Carnegie stage 11, approximately 24 days. The rostral neuropore is closing, but the caudal neuropore is wide open. B, Illustration of the structures shown in A. The embryo is lightly curved because of folding at the cranial and caudal ends.
(A, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
Three pairs of pharyngeal arches are visible by 26 days (Fig. 5-8), and the rostral neuropore is closed. The forebrain produces a prominent elevation of the head, and folding of the embryo has given the embryo a C-shaped curvature. Upper limb buds are recognizable by day 26 or 27 as small swellings on the ventrolateral body walls. The otic pits, the primordia of the internal ears, are also visible. Ectodermal thickenings (lens placodes) indicating the future lenses of the eyes are visible on the sides of the head. The fourth pair of pharyngeal arches and the lower limb buds are visible by the end of the fourth week. Toward the end of the fourth week, a long tail-like caudal eminence is a characteristic feature (Figs. 5-8 to 5-10). Rudiments of many of the organ systems, especially the cardiovascular system, are established (Fig. 5-11). By the end of the fourth week, the caudal neuropore is usually closed.
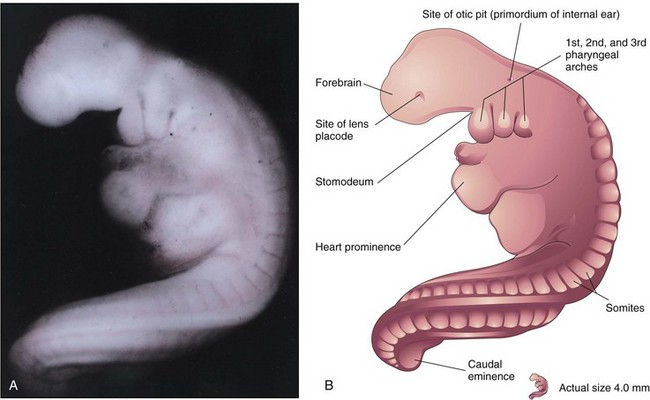
FIGURE 5–8 A, Lateral view of a 27-somite embryo at Carnegie stage 12, approximately 26 days. The embryo is curved, especially its tail-like caudal eminence. Observe the lens placode (primordium of lens of eye) and the otic pit indicating early development of internal ear. B, Illustration of the structures shown in A. The rostral neuropore is closed and three pairs of pharyngeal arches are present.
(A, From Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
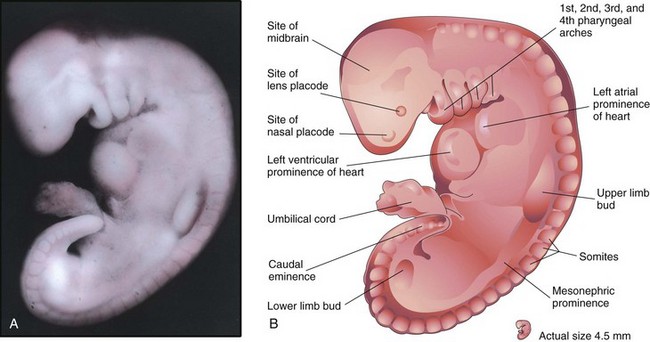
FIGURE 5–9 A, Lateral view of an embryo at Carnegie stage 13, approximately 28 days. The primordial heart is large, and its division into a primordial atrium and ventricle is visible. The rostral and caudal neuropores are closed. B, Drawing indicating the structures shown in A. The embryo has a characteristic C-shaped curvature, four pharyngeal arches, and upper and lower limb buds.
(A, From Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
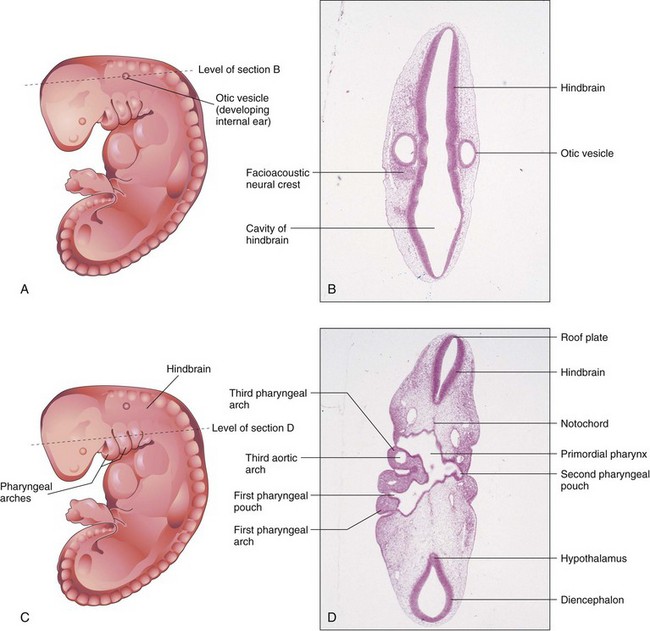
FIGURE 5–10 A, Drawing of an embryo at Carnegie stage 13, approximately 28 days. B, Photomicrograph of a section of the embryo at the level shown in A. Observe the hindbrain and otic vesicle (primordium of internal ear). C, Drawing of same embryo showing the level of the section in D. Observe the primordial pharynx and pharyngeal arches.
(B and D, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
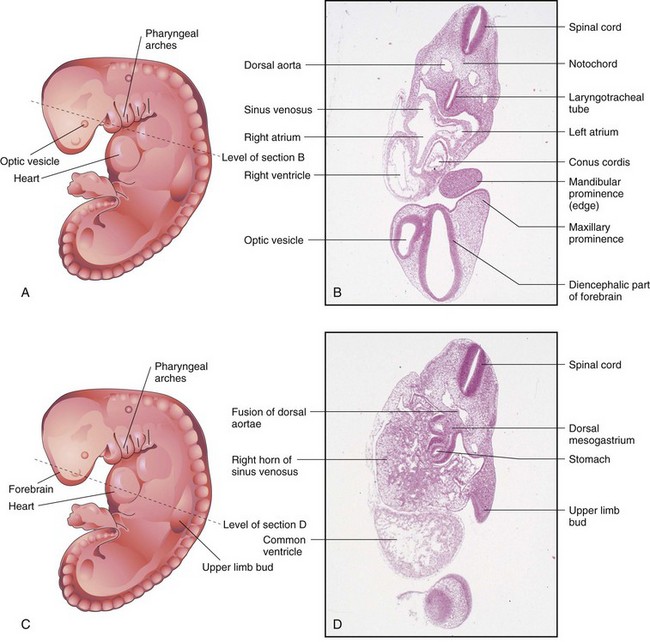
FIGURE 5–11 A, Drawing of an embryo at Carnegie stage 13, approximately 28 days. B, Photomicrograph of a section of the embryo at the level shown in A. Observe the parts of the primordial heart. C, Drawing of the same embryo showing the level of section in D. Observe the primordial heart and stomach.
(B and D, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
Fifth Week
Changes in body form are minor during the fifth week compared with those that occurred during the fourth week, but growth of the head exceeds that of other regions (Figs. 5-12 and 5-13). Enlargement of the head is caused mainly by the rapid development of the brain and facial prominences. The face soon contacts the heart prominence. The rapidly growing second pharyngeal arch overgrows the third and fourth arches, forming a lateral depression on each side—the cervical sinus. Mesonephric ridges indicate the site of the mesonephric kidneys, which are interim excretory organs in humans (Fig. 5-13B).

FIGURE 5–12 A, Scanning electron micrograph of the craniofacial region of a human embryo of approximately 32 days (Carnegie stage 14, 6.8 mm). Three pairs of pharyngeal arches are present. The maxillary and mandibular prominences of the first arch are clearly delineated. Observe the large mouth located between the maxillary prominences and the fused mandibular prominences. B, Drawing of the scanning electron micrograph illustrating the structures shown in A.
(A, Courtesy of the late Professor K. Hinrichsen, Ruhr-Universität Bochum, Bochum, Germany.)
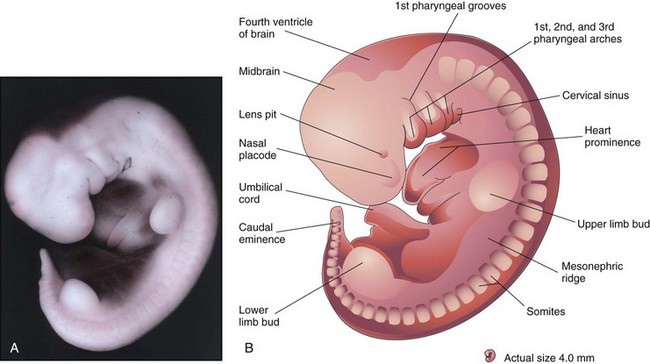
FIGURE 5–13 A, Lateral view of an embryo at Carnegie stage 14, approximately 32 days. The second pharyngeal arch has overgrown the third arch, forming the cervical sinus. The mesonephric ridge indicates the site of the mesonephric kidney, an interim kidney (see Chapter 12). B, Illustration of the structures shown in A. The upper limb buds are paddle shaped and the lower limb buds are flipper-like.
(A, From Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
Sixth Week
By the sixth week, embryos show reflex response to touch. The upper limbs begin to show regional differentiation as the elbows and large hand plates develop (Fig. 5-14). The primordia of the digits (fingers), called digital rays, begin to develop in the hand plates.
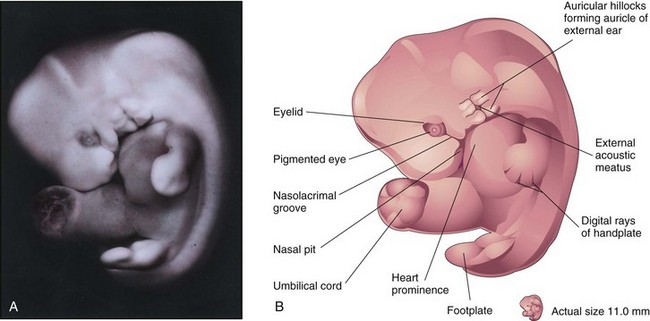
FIGURE 5–14 A, Lateral view of an embryo at Carnegie stage 17, approximately 42 days. Digital rays are visible in the hand plate, indicating the future site of the digits. B, Drawing illustrating the structures shown in A. The eye, auricular hillocks, and external acoustic meatus are now obvious.
(A, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
Embryos in the sixth week show spontaneous movements, such as twitching of the trunk and limbs. Development of the lower limbs occurs 4 to 5 days later than that of the upper limbs. Several small swellings—auricular hillocks—develop around the pharyngeal groove between the first two pharyngeal arches (Fig. 5-14B). This groove becomes the external acoustic meatus. The auricular hillocks contribute to the formation of the auricle, the shell-shaped part of the external ear. Largely because retinal pigment has formed, the eyes are now obvious. The head is now much larger relative to the trunk and is bent over the heart prominence. This head position results from bending in the cervical (neck) region. The trunk and neck have begun to straighten. The intestines enter the extraembryonic coelom in the proximal part of the umbilical cord. This umbilical herniation is a normal event in the embryo. The herniation occurs because the abdominal cavity is too small at this age to accommodate the rapidly growing intestine.
Seventh Week
The limbs undergo considerable change during the seventh week. Notches appear between the digital rays in the hand plates, clearly indicating the digits—fingers or toes (Fig. 5-15). Communication between the primordial gut and umbilical vesicle is now reduced to a relatively slender duct, the omphaloenteric duct (Fig. 5-1C2). By the end of the seventh week, ossification of the bones of the upper limbs has begun.
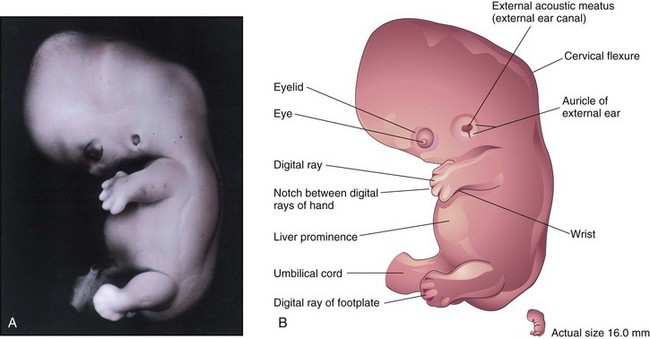
FIGURE 5–15 A, Lateral view of an embryo at Carnegie stage 19, about 48 days. The auricle and external acoustic meatus are now clearly visible. Note the relatively low position of the ear at this stage. Digital rays are now visible in the footplate. The prominence of the abdomen is caused mainly by the large size of the liver. B, Drawing indicating the structures shown in A. Observe the large hand and the notches between the digital rays, which clearly indicate the developing digits or fingers.
(A, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
Eighth Week
At the beginning of this final week of the embryonic (organogenetic) period, the digits of the hand are separated but noticeably webbed (Fig. 5-16). Notches are now clearly visible between the digital rays of the feet. The caudal eminence is still present but stubby. The scalp vascular plexus has appeared and forms a characteristic band around the head. By the end of the eighth week, all regions of the limbs are apparent, the digits have lengthened and are completely separated (Fig. 5-17). Purposeful limb movements first occur during this week. Ossification begins in the femora. All evidence of the caudal eminence has disappeared by the end of the eighth week. Both hands and feet approach each other ventrally. At the end of the eighth week, the embryo has distinct human characteristics (Fig. 5-18); however, the head is still disproportionately large, constituting almost half of the embryo. The neck region is established, and the eyelids are more obvious. The eyelids are closing, and by the end of the eighth week, they begin to unite by epithelial fusion. The intestines are still in the proximal portion of the umbilical cord.
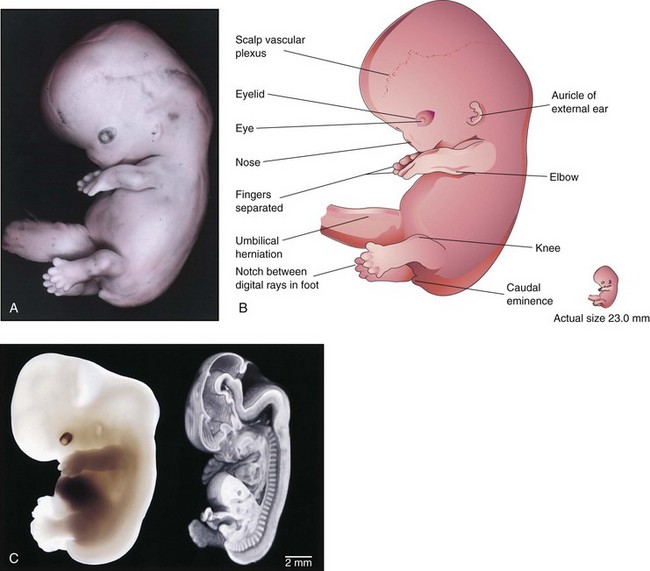
FIGURE 5–16 A, Lateral view of an embryo at Carnegie stage 21, approximately 52 days. Note that the feet are fan-shaped. The scalp vascular plexus now forms a characteristic band across the head. The nose is stubby and the eye is heavily pigmented. B, Illustration of the structures shown in A. The fingers are separated and the toes are beginning to separate. C, A Carnegie stage 20 human embryo, approximately 50 days after ovulation, imaged with optical microscopy (left) and magnetic resonance microscopy (right). The three-dimensional data set from magnetic resonance microscopy has been edited to reveal anatomic detail from a mid-sagittal plane.
(A, From Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977; B, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders 2000; C, Courtesy of Dr. Bradley R. Smith, University of Michigan, Ann Arbor, MI.)
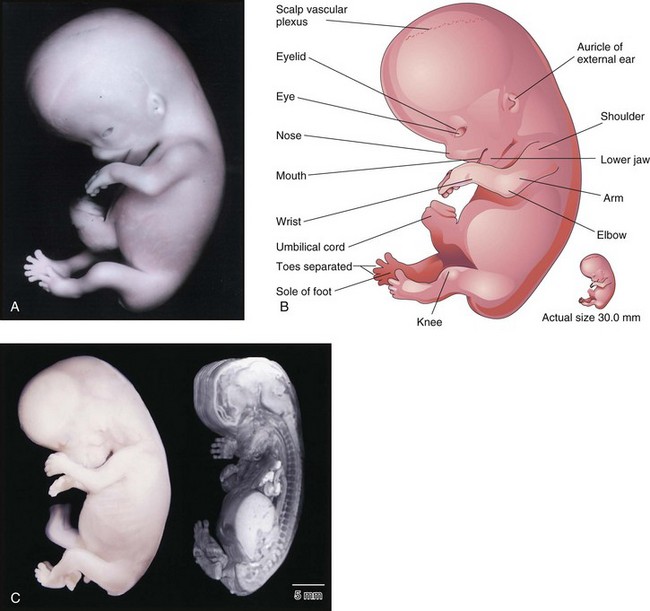
FIGURE 5–17 A, Lateral view of an embryo at Carnegie stage 23, approximately 56 days. The embryo has a distinct human appearance. B, Illustration of the structures shown in A. C, A Carnegie stage 23 embryo, approximately 56 days after ovulation, imaged with optical microscopy (left) and magnetic resonance microscopy (right).
(A, From Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977; B, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000; C, Courtesy of Dr. Bradley R. Smith, University of Michigan, Ann Arbor, MI.)
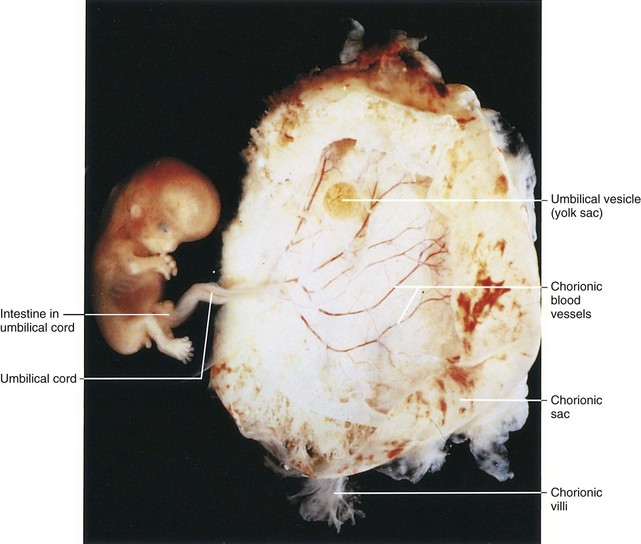
FIGURE 5–18 Lateral view of an embryo and its chorionic sac at Carnegie stage 23, approximately 56 days. Observe the human appearance of the embryo.
(From Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
Although there are sex differences in the appearance of the external genitalia, they are not distinctive enough to permit accurate sexual identification in the eighth week (see Chapter 12).
Estimation of Gestational and Embryonic Age
By convention, obstetricians date pregnancy from the first day of the LNMP (last normal menstrual period). This is the gestational age. Embryonic age begins at fertilization, approximately 2 weeks after the LNMP. Fertilization age is used in patients who have undergone in vitro fertilization or artificial insemination (see Chapter 2).
Knowledge of embryonic age is important because it affects clinical management, especially when invasive procedures such as chorionic villus sampling and amniocentesis are necessary (see Chapter 6). In some women, estimation of gestational age from the menstrual history alone may be unreliable. The probability of error in establishing the LNMP is highest in women who become pregnant after cessation of oral contraception because the interval between discontinuance of hormones and the onset of ovulation is highly variable. In others, slight uterine bleeding (“spotting”), which sometimes occurs during implantation of the blastocyst, may be incorrectly regarded by a woman as light menstruation. Other contributing factors to LNMP unreliability may include oligomenorrhea (scanty menstruation), pregnancy in the postpartum period (i.e., several weeks after childbirth), and use of intrauterine devices. Despite possible sources of error, the LNMP is a reliable criterion in most cases. Ultrasound assessment of the size of the chorionic cavity and its embryonic contents (see Fig. 5-19) enables clinicians to obtain an accurate estimate of the date of conception.
The day on which fertilization occurs is the most accurate reference point for estimating age; this is commonly calculated from the estimated time of ovulation because the oocyte is usually fertilized within 12 hours after ovulation. All statements about age should indicate the reference point used, that is, days after the LNMP or after the estimated time of fertilization.
Estimation of Embryonic Age
Estimates of the age of embryos recovered after a spontaneous abortion, for example, are determined from their external characteristics and measurements of their length (Figs. 5-19 and 5-20; see Table 5-1). Size alone may be an unreliable criterion because some embryos undergo a progressively slower rate of growth before death. The appearance of the developing limbs is a helpful criterion for estimating embryonic age. Because embryos of the third and early fourth weeks are straight (see Fig. 5-20A), measurements indicate the greatest length. The crown–rump length (CRL) is most frequently used for older embryos (see Fig. 5-20B). Because no anatomic marker clearly indicates the crown or rump, one assumes that the longest crown–rump length is the most accurate. Standing height, or crown–heel length, is sometimes measured for 8-week embryos. The length of an embryo is only one criterion for establishing age (Table 5-1). The Carnegie Embryonic Staging System is used internationally; its use enables comparisons to be made between the findings of one person and those of another.
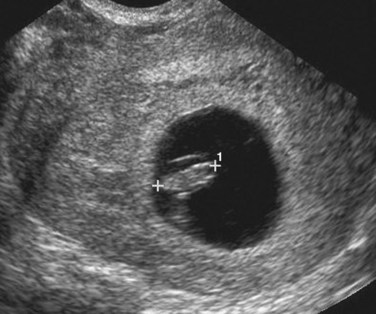
FIGURE 5–19 Transvaginal sonogram of a 7-week embryo (calipers, CRL 10 mm) surrounded by the amniotic membrane within the chorionic cavity (dark region).
(Courtesy of Dr. E.A. Lyons, Professor of Radiology, Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba.)
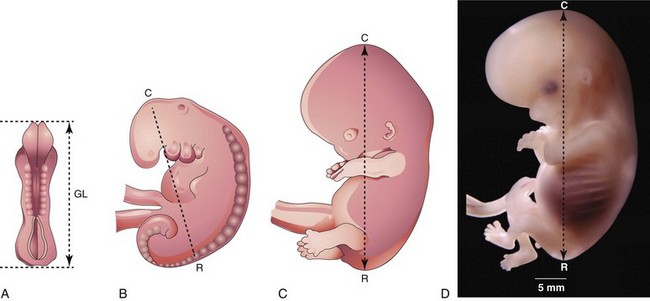
FIGURE 5–20 Illustrations of methods used to measure the length of embryos. A, Greatest length (GL). B, C, and D, Crown-rump length (CRL). D, Photograph of an 8-week-old embryo
(D, Courtesy of Dr. Bradley R. Smith, University of Michigan, Ann Arbor, MI.)
Ultrasound Examination of Embryos
Most women seeking obstetric care have at least one ultrasound examination during their pregnancy for one or more of the following reasons:
Current data indicate that there are no confirmed biologic effects of ultrasonography on embryos or fetuses from the use of diagnostic ultrasound evaluation.
The size of an embryo in a pregnant woman can be estimated using ultrasound measurements. Transvaginal sonography permits an earlier and more accurate measurement of CRL in early pregnancy. Early in the fifth week, the embryo is 4 to 7 mm long (see Fig. 5-13). During the sixth and seventh weeks, discrete embryonic structures can be visualized (e.g., parts of limbs), and crown–rump measurements are predictive of embryonic age with an accuracy of 1 to 4 days. Furthermore, after the sixth week, dimensions of the head and trunk can be obtained and used for assessment of embryonic age. There is, however, considerable variability in early embryonic growth and development. Differences are greatest before the end of the first 4 weeks of development, but less so by the end of the embryonic period.
Summary of Fourth to Eighth Weeks
Clinically Oriented Problems
Case 5–1
A 28-year-old woman who has been a heavy cigarette smoker since her teens was informed that she was in the second month of pregnancy.
Case 5–3
A patient was concerned about what she had read in the newspaper about recent effects of drugs on laboratory animals.
Case 5–4
A 30-year-old woman was unsure when her LNMP was. She stated that her periods were irregular.
Case 5–5
A woman who had just become pregnant told her doctor that she had taken a sleeping pill given to her by a friend. She wondered whether it could harm the development of her baby’s limbs.
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385.
Barnea ER, Hustin J, Jauniaux E, editors. The First Twelve Weeks of Gestation. Berlin: Springer-Verlag, 1992.
Callen PW. Obstetric ultrasound examination. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Dickey RP, Gasser RF. Computer analysis of the human embryo growth curve: Differences between published ultrasound findings on living embryos in utero and data on fixed specimens. Anat Rec. 1993;237:400.
Dickey RP, Gasser RF. Ultrasound evidence for variability in the size and development of normal human embryos before the tenth post-insemination week after assisted reproductive technologies. Hum Reprod. 1993;8:331.
Gasser R. Virtual Human Embryo DREM Project. New Orleans: Louisiana State University; 2007.
Gilbert SF. Developmental Biology, ed 9. Sunderland: Sinauer; 2010.
Hardin J, Walston T. Models of morphogenesis: The mechanisms and mechanics of cell rearrangement. Curr Opin Genet Dev. 2004;14:399.
Iffy L, Shepard TH, Jakobovits A, et al. The rate of growth in young human embryos of Streeter’s horizons XIII and XXIII. Acta Anat. 1967;66:178.
Iwarsson E, Malmgren H, Blennow E. Preimplantation genetic diagnosis: twenty years of practice. Semin Fetal Neonatal Med. 2011;16:74.
Jirásek JE. An Atlas of Human Prenatal Developmental Mechanics: Anatomy and Staging. London and New York: Taylor & Francis; 2004.
Kliegman RM. Intrauterine growth restriction. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Laing FC, Frates MC, Benson CB. Ultrasound evaluation during the first trimester. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Moore KL, Persaud TVN, Shiota K. Color Atlas of Clinical Embryology, ed 2. Philadelphia: WB Saunders; 2000.
Nishimura H, Tanimura T, Semba R, Uwabe C. Normal development of early human embryos: Observation of 90 specimens at Carnegie stages 7 to 13. Teratology. 1974;10:1.
O’Rahilly R, Müller F. Developmental Stages in Human Embryos. Washington, DC: Carnegie Institute of Washington; 1987.
Persaud TVN, Hay JC. Normal embryonic and fetal development. In Reece EA, Hobbins JC, editors: Clinical Obstetrics: The Fetus and Mother, ed 3, Oxford: Blackwell Publishing, 2006.
Pooh RK, Shiota K, Kurjak A. Imaging of the human embryo with magnetic resonance imaging microscopy and high-resolution transvaginal 3-dimensional sonography:human embryology in the 21st century. Am J Obstet Gynecol. 2011;204:77. e1
Shiota K. Development and intrauterine fate of normal and abnormal human conceptuses. Congen Anom. 1991;31:67.
Steding G. The Anatomy of the Human Embryo. A Scanning Electron-Microscopic Atlas. Basel: Karger; 2009.
Streeter GL. Developmental horizons in human embryos: Description of age groups XV, XVI, XVII, and XVIII. Contrib Embryol Carnegie Inst. 1948;32:133.
Streeter GL. Developmental horizons in human embryos: Description of age group XI, 13 to 20 somites, and age group XII, 21 to 29 somites. Contrib Embryol Carnegie Inst. 1942;30:211.
Streeter GL. Developmental horizons in human embryos: Description of age group XIII, embryos of 4 or 5 millimeters long, and age group XIV, period of identification of the lens vesicle. Contrib Embryol Carnegie Inst. 1945;31:27.
Streeter GL, Heuser CH, Corner GW. Developmental horizons in human embryos: Description of age groups XIX, XX, XXI, XXII, and XXIII. Contrib Embryol Carnegie Inst. 1951;34:165.
Yamada S, Samtani RR, Lee ES, et al. Developmental atlas of the early first trimester human embryo. Dev Dyn. 2010;239:1585.
Whitworth M, Bricker L, Neilson JP, et al: Ultrasound for fetal assessment in early pregnancy, Cochrane Database Syst Rev 4:CD007058, 2010.
Zhang J, Merialdi M, Platt LD, et al. Defining normal and abnormal fetal growth: promises and challenges. Am Obstet Gynecol. 2010;202:522.