Chapter 13 Cardiovascular System
The cardiovascular system is the first major system to function in the embryo. The primordial heart and vascular system appear in the middle of the third week. This precocious cardiac development occurs because the rapidly growing embryo can no longer satisfy its nutritional and oxygen requirements by diffusion alone. Consequently, there is a need for an efficient method of acquiring oxygen and nutrients from the maternal blood and disposing of carbon dioxide and waste products. The cardiovascular system is derived mainly from:
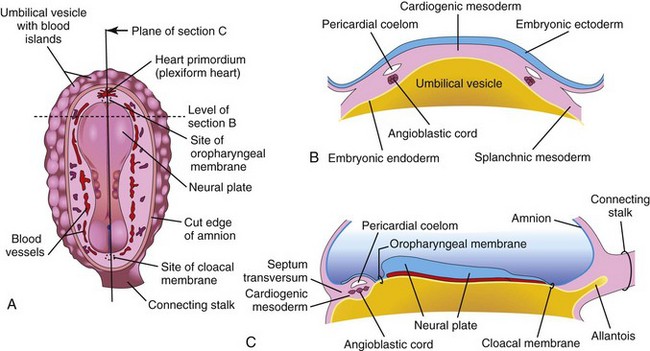
FIGURE 13–1 Early development of the heart. A, Drawing of a dorsal view of an embryo (approximately 18 days). B, Transverse section of the embryo showing the angioblastic cords in the cardiogenic mesoderm and their relationship to the pericardial coelom. C, Longitudinal section of the embryo illustrating the relationship of the angioblastic cords to the oropharyngeal membrane, pericardial coelom, and septum transversum.
Blood vessel development—angiogenesis—is described in Chapter 4. Primordial blood vessels cannot be distinguished structurally as arteries or veins, but they are named according to their future fates and relationship to the heart.
 Early Development Of The Heart And Blood Vessels
Early Development Of The Heart And Blood Vessels
Inductive signals from the anterior endoderm stimulates early formation of the heart. The earliest sign of the heart is the appearance of paired endothelial strands—angioblastic cords—in the cardiogenic mesoderm during the third week (Fig. 13-1B and C). These cords canalize to form two thin heart tubes. As lateral embryonic folding occurs, the endocardial heart tubes approach each other and fuse to form a single heart tube (see Figs. 13-7C and 13-9C). Fusion of the heart tubes begins at the cranial end of the developing heart and extends caudally. The heart begins to beat at 22 to 23 days (Fig. 13-2). Blood flow begins during the fourth week and can be visualized by Doppler ultrasonography (Fig. 13-3).
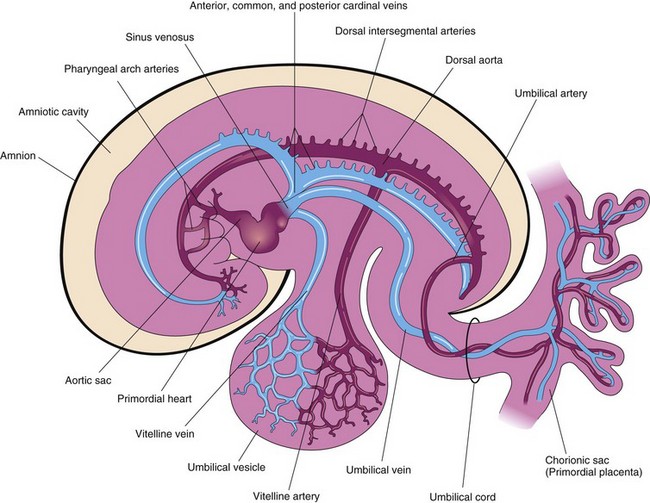
FIGURE 13–2 Drawing of the embryonic cardiovascular system (approximately 26 days), showing vessels on the left side. The umbilical vein carries well-oxygenated blood and nutrients from the chorionic sac to the embryo. The umbilical arteries carry poorly oxygenated blood and waste products from the embryo to the chorionic sac (the outermost embryonic membrane).
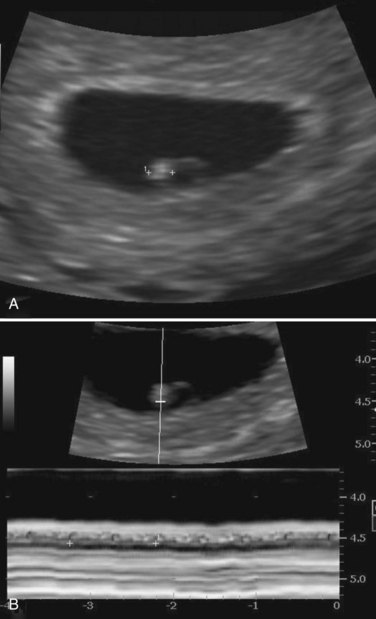
FIGURE 13–3 Endovaginal scan of a 4-week embryo. A, Bright (echogenic) 2.4-mm embryo (calipers). B, Cardiac activity of 116 beats per minute demonstrated with motion mode. Calipers used to encompass two beats.
(Courtesy of Dr. E.A. Lyons, Professor of Radiology, Obstetrics and Gynecology, and Human Anatomy, University of Manitoba and Health Sciences Centre, Winnipeg, Manitoba, Canada.)
Molecular studies show that more than 500 genes are involved in the development of the mammalian heart. Several members of the T-box family of genes play an essential role in lineage determination, specification of the cardiac chambers, valvuloseptal development, and the formation of the conducting system.
Gene expression analysis and lineage tracing experiments suggest that progenitor cells from the pharyngeal mesoderm, located anterior to the early heart tube (anterior heart field), gives rise to ventricular myocardium and the myocardial wall of the outflow tract. Moreover, another wave of progenitor cells from the pharyngeal mesoderm (second heart field) also contributes to the rapid growth and elongation of the heart tube. The myocardium of the left ventricle and the anterior pole of the heart tube are derived mostly from the second field. Expression of Hes-1, expressed in pharyngeal endoderm and mesoderm (second heart field) plays an essential role for the development of the outflow tract.
The basic helix-loop-helix genes, dHAND and eHAND, are expressed in the paired primordial endocardial tubes and in later stages of cardiac morphogenesis. The MEF2C and Pitx-2 genes, which are expressed in cardiogenic precursor cells emerging from the primitive streak before formation of the heart tubes (Wnt 3a–mediated), also appear to be essential regulators in early cardiac development.
Development of Veins Associated with Embryonic Heart
Three paired veins drain into the tubular heart of a 4-week embryo (Fig. 13-2):
The vitelline veins follow the omphaloenteric duct into the embryo. This duct is the narrow tube connecting the umbilical vesicle with the midgut (see Fig. 11-1). After passing through the septum transversum, the vitelline veins enter the venous end of the heart—the sinus venosus (Figs. 13-2 and 13-4A). The left vitelline vein regresses while the right vitelline vein forms most of the hepatic portal system (Fig. 13-5B and C), as well as a portion of the inferior vena cava. As the liver primordium grows into the septum transversum (see Chapter 11), the hepatic cords anastomose around preexisting endothelium-lined spaces. These spaces, the primordia of the hepatic sinusoids, later become linked to the vitelline veins.
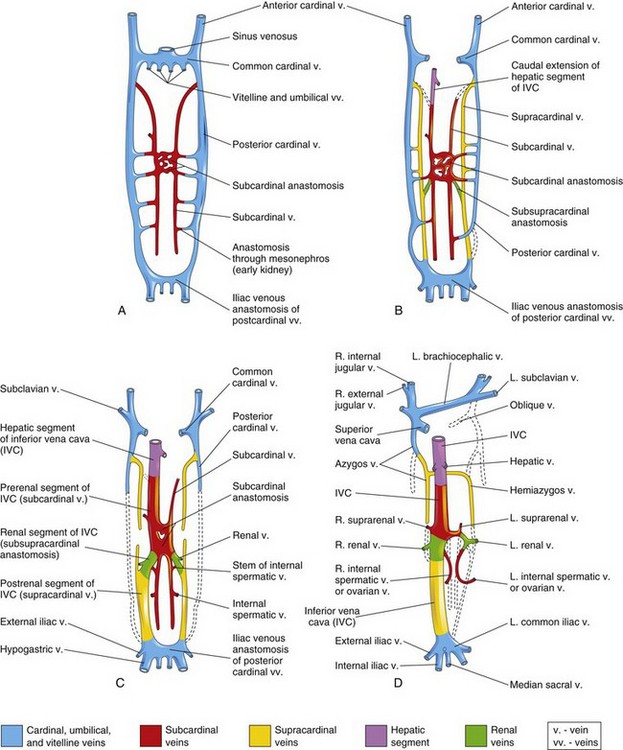
FIGURE 13–4 Illustrations of the primordial veins of embryo bodies (ventral views). Initially, three systems of veins are present: the umbilical veins from the chorion, the vitelline veins from the umbilical vesicle, and the cardinal veins from the body of the embryos. Next the subcardinal veins appear, and finally the supracardinal veins develop. A, At 6 weeks. B, At 7 weeks. C, At 8 weeks. D, Adult. This drawing illustrates the transformations that produce the adult venous pattern.
(Modified from Arey LB: Developmental Anatomy, revised 7th ed. Philadelphia, WB Saunders, 1974.)
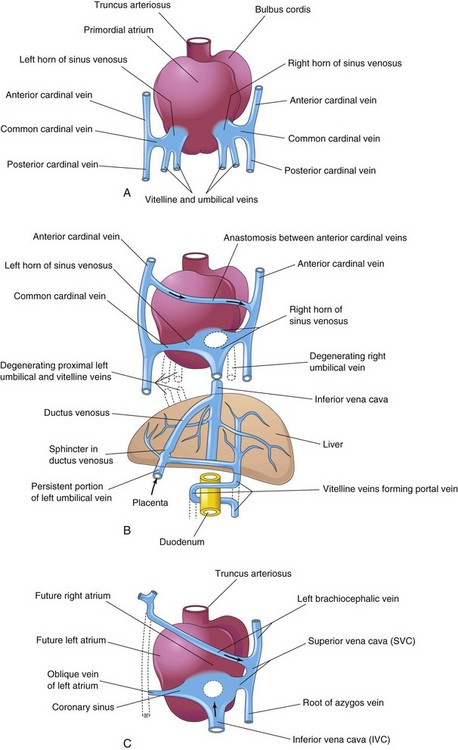
FIGURE 13–5 Dorsal views of the developing heart. A, During the fourth week (approximately 24 days), showing the primordial atrium, sinus venosus and veins draining into them. B, At 7 weeks, showing the enlarged right sinus horn and venous circulation through the liver. The organs are not drawn to scale. C, At 8 weeks, indicating the adult derivatives of the cardinal veins shown in A and B.
The umbilical veins run on each side of the liver and carry well-oxygenated blood from the placenta to the sinus venosus. As the liver develops, the umbilical veins lose their connection with the heart and empty into the liver. The right umbilical vein disappears during the seventh week, leaving the left umbilical vein as the only vessel carrying well-oxygenated blood from the placenta to the embryo.
Transformation of the umbilical veins may be summarized as follows (Fig. 13-5):
The cardinal veins (Figs. 13-2 and 13-4A) constitute the main venous drainage system of the embryo. The anterior and posterior cardinal veins, the earliest veins to develop, drain cranial and caudal parts of the embryo, respectively. They join the common cardinal veins, which enter the sinus venosus (Fig. 13-2). During the eighth week, the anterior cardinal veins become connected by an anastomosis (Fig. 13-5A and B), which shunts blood from the left to the right anterior cardinal vein. This anastomotic shunt becomes the left brachiocephalic vein when the caudal part of the left anterior cardinal vein degenerates (Figs. 13-4D and 13-5C). The superior vena cava (SVC) forms from the right anterior cardinal vein and the right common cardinal vein.
The posterior cardinal veins develop primarily as the vessels of the mesonephroi (interim kidneys) and largely disappear with these transitory kidneys (see Chapter 12). The only adult derivatives of the posterior cardinal veins are the root of the azygos vein and the common iliac veins (Fig. 13-4D). The subcardinal and supracardinal veins gradually develop and replace and supplement the posterior cardinal veins.
The subcardinal veins appear first (Fig. 13-4A). They are connected with each other through the subcardinal anastomosis and with the posterior cardinal veins through the mesonephric sinusoids. The subcardinal veins form the stem of the left renal vein, the suprarenal veins, the gonadal veins (testicular and ovarian), and a segment of the inferior vena cava (IVC) (Fig. 13-4D). The subcardinal veins become disrupted in the region of the kidneys (Fig. 13-4C). Cranial to this, they become united by an anastomosis that is represented in the adult by the azygos and hemiazygos veins (Figs. 13-4D and 13-5C). Caudal to the kidneys, the left supracardinal vein degenerates, but the right supracardinal vein becomes the inferior part of the IVC (Fig. 13-4D).
Development of Inferior Vena Cava
The IVC forms during a series of changes in the primordial veins of the trunk that occur as blood, returning from the caudal part of the embryo, is shifted from the left to the right side of the body. The IVC is composed of four main segments (Fig. 13-4C):
Anomalies of Venae Cavae
Because of the many transformations that occur during the formation of the SVC and IVC, variations in their adult form may occur. The most common anomaly of the IVC is for its abdominal course to be interrupted; as a result, blood drains from the lower limbs, abdomen, and pelvis to the heart through the azygos system of veins.
Double Superior Venae Cavae
Persistence of the left anterior cardinal vein results in a persistent left SVC; hence, there are two superior venae cavae (Fig. 13-6). This condition causes a right to left shunting of blood. The anastomosis that usually forms the left brachiocephalic vein is small or absent. The abnormal left SVC, derived from the left anterior cardinal and common cardinal veins, opens into the right atrium through the coronary sinus.
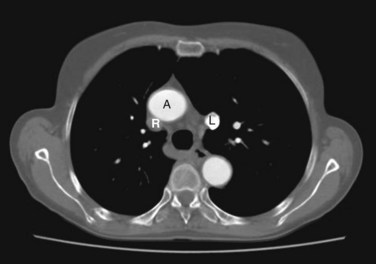
FIGURE 13–6 CT scan showing a duplicated superior vena cava. Note the aorta (A), the right superior vena cava (R, unopacified), and the left superior vena cava (L, with contrast from left arm injection).
(Courtesy of Dr. Blair Henderson, Department of Radiology, Health Sciences Centre, University of Manitoba, Winnipeg, Manitoba, Canada.)
Left Superior Vena Cava
The left anterior cardinal vein and common cardinal vein may form a left SVC, and the right anterior cardinal vein and common cardinal vein, which usually form the SVC, degenerate. As a result, blood from the right side is carried by the brachiocephalic vein to the unusual left SVC, which empties into the coronary sinus.
Absence of Hepatic Segment of Inferior Vena Cava
Occasionally the hepatic segment of the IVC fails to form. As a result, blood from inferior parts of the body drains into the right atrium through the azygos and hemiazygos veins. The hepatic veins open separately into the right atrium.
Double Inferior Venae Cavae
In unusual cases, the IVC inferior to the renal veins is represented by two vessels. Usually the left one is much smaller. This condition probably results from failure of an anastomosis to develop between the veins of the trunk (Fig. 13-4B). As a result, the inferior part of the left supracardinal vein persists as a second IVC.
Pharyngeal Arch Arteries and Other Branches of Dorsal Aortae
As the pharyngeal arches form during the fourth and fifth weeks, they are supplied by arteries—the pharyngeal arch arteries—that arise from the aortic sac and terminate in the dorsal aortae (Fig. 13-2). Initially, the paired dorsal aortae run through the entire length of the embryo. Later, the caudal portions of the aortae fuse to form a single lower thoracic/abdominal aorta. Of the remaining paired dorsal aortae, the right one regresses and the left becomes the primordial aorta.
Intersegmental Arteries
Thirty or so branches of the dorsal aorta, the intersegmental arteries, pass between and carry blood to the somites and their derivatives (Fig. 13-2). The intersegmental arteries in the neck join to form a longitudinal artery on each side, the vertebral artery. Most of the original connections of the intersegmental arteries to the dorsal aorta disappear.
In the thorax, the intersegmental arteries persist as intercostal arteries. Most of the intersegmental arteries in the abdomen become lumbar arteries, but the fifth pair of lumbar intersegmental arteries remains as the common iliac arteries. In the sacral region, the intersegmental arteries form the lateral sacral arteries.
Fate of Vitelline and Umbilical Arteries
The unpaired ventral branches of the dorsal aorta supply the umbilical vesicle, allantois, and chorion (Fig. 13-2). The vitelline arteries pass to the vesicle and later the primordial gut, which forms from the incorporated part of the umbilical vesicle. Only three vitelline artery derivatives remain: celiac arterial trunk to foregut, superior mesenteric artery to midgut, and inferior mesenteric artery to hindgut.
The paired umbilical arteries pass through the connecting stalk (primordial umbilical cord) and become continuous with vessels in the chorion, the embryonic part of the placenta (Chapter 7). The umbilical arteries carry poorly oxygenated blood to the placenta (Fig. 13-2). Proximal parts of the umbilical arteries become the internal iliac arteries and superior vesical arteries, whereas distal parts obliterate after birth and become medial umbilical ligaments.
Later Development Of The Heart
The external layer of the embryonic heart tube—the myocardium—is formed from splanchnic mesoderm surrounding the pericardial cavity (Figs. 13-7A and B and 13-8B). At this stage, the heart is composed of a thin endothelial tube, separated from a thick myocardium by gelatinous connective tissue, known as cardiac jelly (Fig. 13-8C and D).
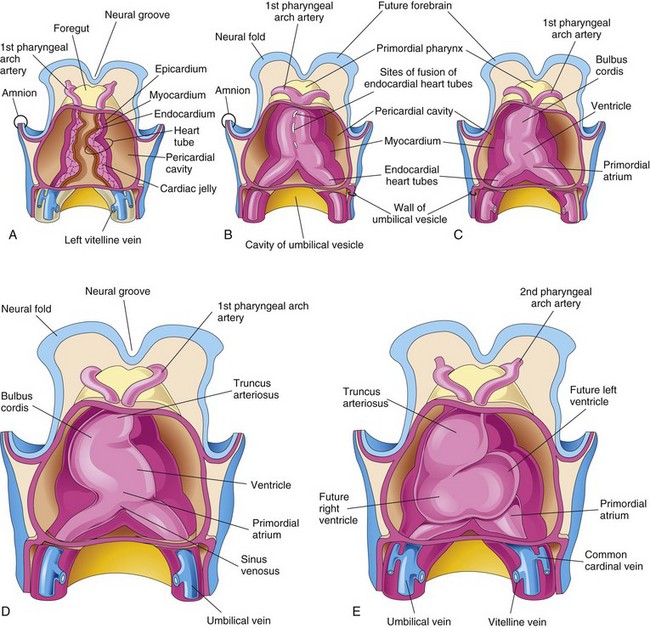
FIGURE 13–7 Drawings showing fusion of the heart tubes and looping of the tubular heart. A to C, Ventral views of the developing heart and pericardial region (22–35 days). The ventral pericardial wall has been removed to show the developing myocardium and fusion of the two heart tubes to form a tubular heart. The endothelium of the heart tube forms the endocardium of the heart. D and E, As the straight tubular heart elongates, it bends and undergoes looping, which forms a D-loop that produces a S-shaped heart.
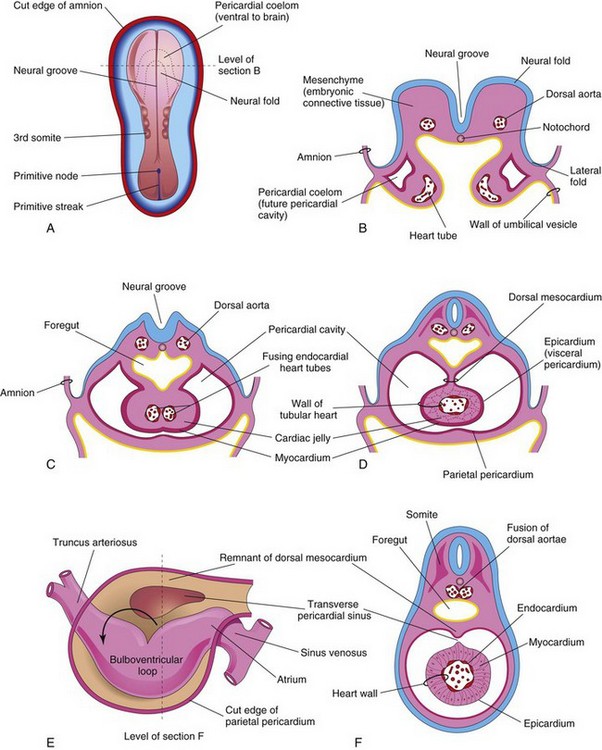
FIGURE 13–8 A, Dorsal view of an embryo (approximately 20 days). B, Schematic transverse section of the heart region of the embryo illustrated in A, showing the two heart tubes and the lateral folds of the body. C, Transverse section of a slightly older embryo showing the formation of the pericardial cavity and fusion of the heart tubes. D, Similar section (approximately 22 days) showing the tubular heart suspended by the dorsal mesocardium. E, Schematic drawing of the heart (approximately 28 days) showing degeneration of the central part of the dorsal mesocardium and formation of the transverse pericardial sinus. The tubular heart now has a D-loop. F, Transverse section of the embryo at the level indicated in E, showing the layers of the heart wall.
The endothelial tube becomes the internal endothelial lining of the heart—endocardium—and the primordial myocardium becomes the muscular wall of the heart or myocardium. The visceral pericardium or epicardium is derived from mesothelial cells that arise from the external surface of the sinus venosus and spread over the myocardium (Fig. 13-7D and F).
Prior to the formation of the heart tube, the homeobox transcription factor (Pitx2c) is expressed in the left heart forming field and plays an important role in the left-right patterning of the heart tube during formation of the cardiac loop. Progenitor cells added to the rostral and caudal poles of the heart tube from a proliferative pool of mesodermal cells located in the dorsal wall of the pericardial cavity and the pharyngeal arches.
As folding of the head region occurs, the heart and pericardial cavity come to lie ventral to the foregut and caudal to the oropharyngeal membrane (Fig. 13-9). Concurrently, the tubular heart elongates and develops alternate dilations and constrictions (Fig. 13-7C to E): the bulbus cordis (composed of the truncus arteriosus [TA], the conus arteriosus, and the conus cordis), ventricle, atrium, and sinus venosus.
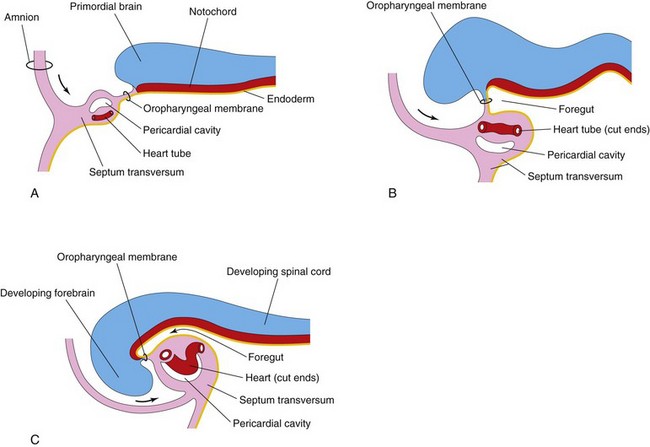
FIGURE 13–9 Longitudinal sections through the cranial half of embryos during the fourth week, showing the effect of the head fold (arrows) on the position of the heart and other structures. A and B, As the head fold develops, the tubular heart and pericardial cavity move ventral to the foregut and caudal to the oropharyngeal membrane. C, Note that the positions of the pericardial cavity and septum transversum have reversed with respect to each other. The septum transversum now lies posterior to the pericardial cavity, where it will form the central tendon of the diaphragm.
The TA is continuous cranially with the aortic sac (Fig. 13-10A), from which the pharyngeal arch arteries arise. The sinus venosus receives the umbilical, vitelline, and common cardinal veins from the chorion, umbilical vesicle, and embryo, respectively (Fig. 13-10B). The arterial and venous ends of the heart are fixed by the pharyngeal arches and septum transversum, respectively. The tubular heart undergoes a dextral (right-handed) looping at approximately 23 to 28 days, forming a U-shaped D-loop that results in a heart with the apex pointing to the left (Fig. 13-7D and E and 13-8E).
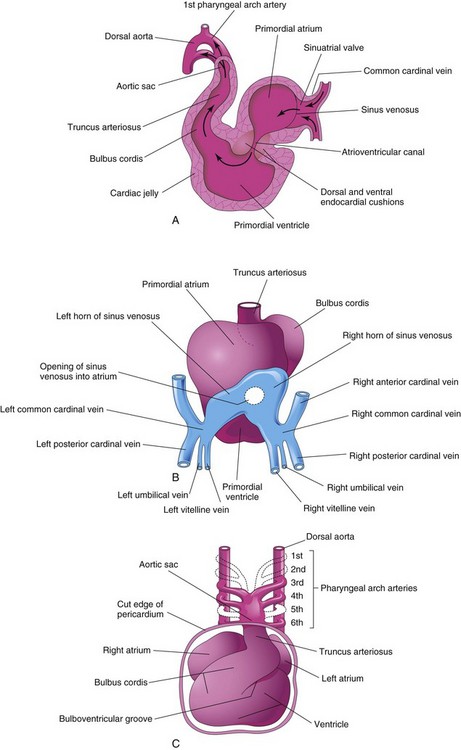
FIGURE 13–10 A, Sagittal section of the heart at approximately 24 days, showing blood flow through it (arrows). B, Dorsal view of the heart at approximately 26 days, showing the horns of the sinus venosus and the dorsal location of the primordial atrium. C, Ventral view of the heart and pharyngeal arch arteries (approximately 35 days). The ventral wall of the pericardial sac has been removed to show the heart in the pericardial cavity.
The signaling molecule(s) and cellular mechanisms responsible for cardiac looping are largely unknown. As the primordial heart bends, the atrium and sinus venosus come to lie dorsal to the TA, bulbus cordis, and ventricle (Fig. 13-10B and C). By this stage, the sinus venosus has developed lateral expansions, the right and left sinus horns (Fig. 13-5A).
As the heart elongates and bends, it gradually invaginates into the pericardial cavity (Figs. 13-7B to D and 13-8C). The heart is initially suspended from the dorsal wall by a mesentery, the dorsal mesocardium, but the central part of this mesentery soon degenerates, forming a communication, the transverse pericardial sinus, between the right and left sides of the pericardial cavity (Fig. 13-8E and F). The heart is now attached only at its cranial and caudal ends.
Circulation through the Primordial Heart
The initial contractions of the heart are of myogenic origin (in or starting from muscle). The muscle layers of the atrium and ventricle outflow tract are continuous, and contractions occur in peristalsis-like waves that begin in the sinus venosus. At first, circulation through the primordial heart is an ebb-and-flow type; however, by the end of the fourth week, coordinated contractions of the heart result in unidirectional flow. Blood enters the sinus venosus (Fig. 13-10A and B) from the:
Blood from the sinus venosus enters the primordial atrium; flow from it is controlled by sinuatrial (SA) valves (Fig. 13-11A to D). The blood then passes through the atrioventricular (AV) canal into the primordial ventricle. When the ventricle contracts, blood is pumped through the bulbus cordis and truncus arteriosus (TA) into the aortic sac, from which it is distributed to the pharyngeal arch arteries in the pharyngeal arches (Fig. 13-10C). The blood then passes into the dorsal aortae for distribution to the embryo, umbilical vesicle, and placenta (see Fig. 13-2).
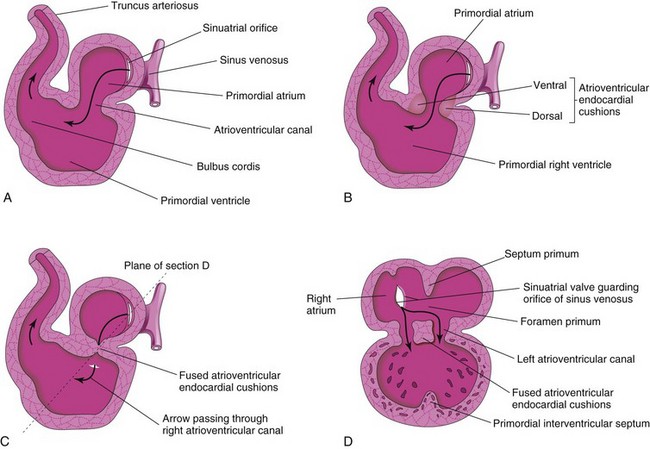
FIGURE 13–11 A and B, Sagittal sections of the heart during the fourth and fifth weeks, illustrating blood flow through the heart and division of the atrioventricular canal. The arrows are passing through the sinuatrial (SA) orifice. C, Fusion of the atrioventricular endocardial cushions. D, Coronal section of the heart at the plane shown in C. Note that the septum primum and interventricular septa have started to develop.
 Partitioning of Primordial Heart
Partitioning of Primordial Heart
Partitioning of the atrioventricular canal, primordial atrium, ventricle, and outflow tract begins around the middle of the fourth week and is essentially completed by the end of the eighth week. Although described separately, these processes occur concurrently.
Partitioning of Atrioventricular Canal
Toward the end of the fourth week, atrioventricular endocardial cushions form on the dorsal and ventral walls of the atrioventricular (AV) canal. As these masses of tissue are invaded by mesenchymal cells during the fifth week (Fig. 13-11B), the AV endocardial cushions approach each other and fuse, dividing the AV canal into right and left AV canals (Fig. 13-11C and D). These canals partially separate the primordial atrium from the primordial ventricle, and the endocardial cushions function as AV valves. The septal valves are derived from the fused superior and inferior endocardial cushions. The mural leaflets are mesenchymal in origin.
The AV endocardial cushions develop from a specialized extracellular matrix (cardiac jelly). After inductive signals from the myocardium of the AV canal, a segment of the inner endocardial cells undergoes epithelial–mesenchymal transformation, which then invades the extracellular matrix. The transformed AV endocardial cushions contribute to the formation of the valves and membranous septa of the heart.
Transforming growth factor β (TGF-β1 and TGF-β2), bone morphogenetic proteins (BMP-2A and BMP-4), the zinc finger protein Slug, and an activin receptor–like kinase (ChALK2) have been reported to be involved in the epithelial–mesenchymal transformation and formation of the endocardial cushions.
Partitioning of Primordial Atrium
Beginning at the end of the fourth week, the primordial atrium is divided into right and left atria by the formation and subsequent modification and fusion of two septa: the septum primum and septum secundum (Figs. 13-12 and 13-13).
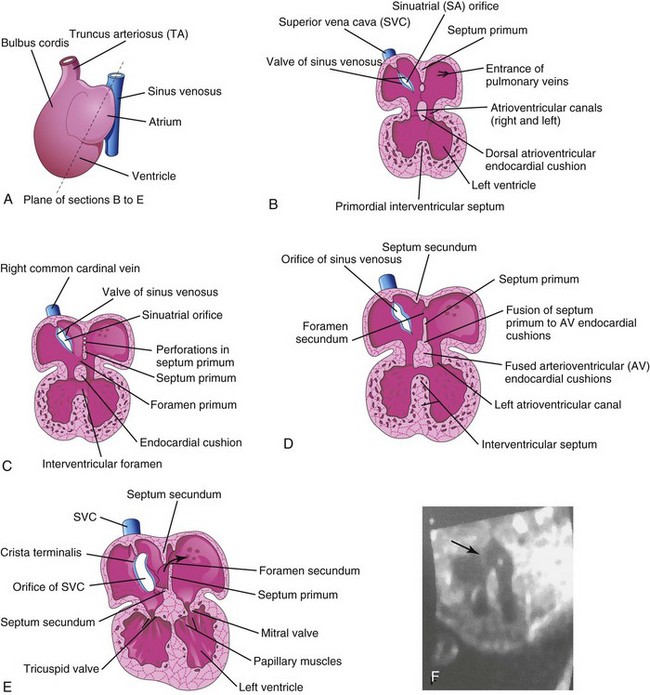
FIGURE 13–12 Drawings of the heart showing partitioning of the atrioventricular (AV) canal, primordial atrium, and ventricle. A, Sketch showing the plane of the sections. B, Frontal section of the heart during the fourth week (approximately 28 days) showing the early appearance of the septum primum, interventricular septum, and dorsal atrioventricular endocardial cushion. C, Similar section of the heart (approximately 32 days) showing perforations in the dorsal part of the septum primum. D, Section of the heart (approximately 35 days) showing the foramen secundum. E, Section of the heart (at approximately 8 weeks, showing the heart after it is partitioned into four chambers. The arrow indicates the flow of well-oxygenated blood from the right into the left atrium. F, Sonogram of a second trimester fetus showing the four chambers of the heart. Note the septum secundum (arrow).
(Courtesy of Dr. G.J. Reid, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Manitoba, Women’s Hospital, Winnipeg, Manitoba, Canada.)
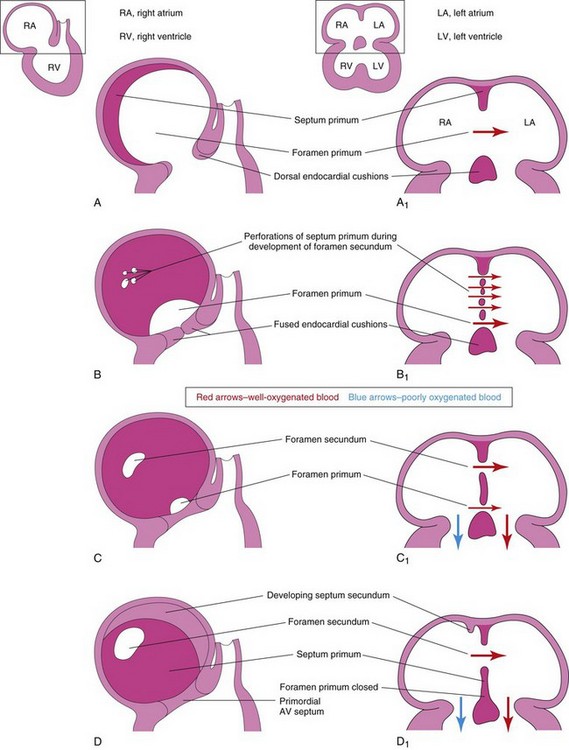
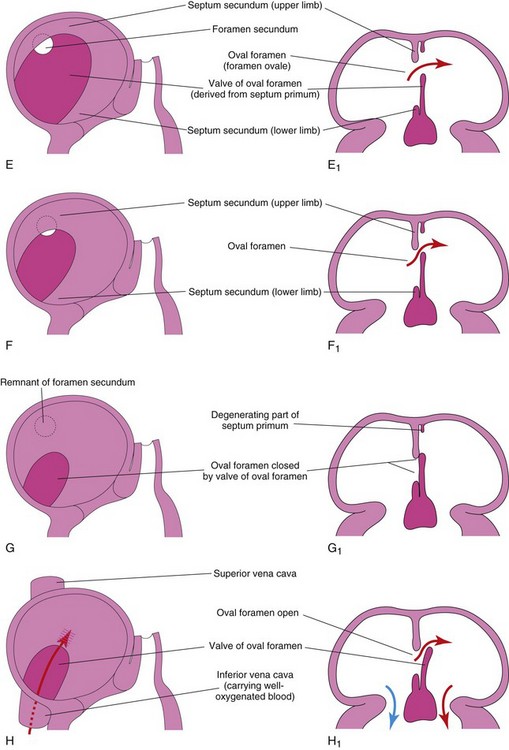
FIGURE 13–13 Diagrammatic sketches illustrating progressive stages in partitioning of the primordial atrium. A to H, Sketches of the developing interatrial septum as viewed from the right side. A1 to H1 are coronal sections of the developing interatrial septum. As the septum secundum grows, note that it overlaps the opening in the septum primum, the foramen secundum. Observe the valve of the oval foramen in G1 and H1. When pressure in the right atrium exceeds that in the left atrium, blood passes from the right to the left side of the heart. When the pressures are equal or higher in the left atrium, the valve closes the oval foramen (G1).
The septum primum, a thin crescent-shaped membrane, grows toward the fusing endocardial cushions from the roof of the primordial atrium, partially dividing the common atrium into right and left halves. As the curtain-like septum primum grows, a large opening, the foramen primum, is located between its crescentic free edge and the endocardial cushions (Figs. 13-12C and 13-13A to C). This foramen serves as a shunt, enabling oxygenated blood to pass from the right to the left atrium. The foramen primum becomes progressively smaller and disappears as the septum primum fuses with the fused AV endocardial cushions to form a primordial AV septum (Fig. 13-13D and D1).
Before the foramen primum disappears, perforations, produced by apoptosis, appear in the central part of the septum primum. As the septum fuses with the fused endocardial cushions, these perforations coalesce to form another opening in the septum primum, the foramen secundum. Concurrently, the free edge of the septum primum fuses with the left side of the fused endocardial cushions, obliterating the foramen primum (Figs. 13-12D and 13-13D). The foramen secundum ensures continued shunting of oxygenated blood from the right to the left atrium.
The septum secundum, a thick crescentic muscular fold, grows from the ventrocranial wall of the right atrium, immediately adjacent to the septum primum (see Fig. 13-13D1). As this thick septum grows during the fifth and sixth weeks, it gradually overlaps the foramen secundum in the septum primum (see Fig. 13-13E). The septum secundum forms an incomplete partition between the atria; consequently, an oval foramen (foramen ovale) forms. The cranial part of the septum primum, initially attached to the roof of the left atrium, gradually disappears (Fig. 13-13G1 and H1). The remaining part of the septum primum, attached to the fused endocardial cushions, forms the flap-like valve of the oval foramen.
Before birth, the oval foramen allows most of the oxygenated blood entering the right atrium from the IVC to pass into the left atrium (Fig. 13-14A). This foramen prevents the passage of blood in the opposite direction because the septum primum closes against the relatively rigid septum secundum (Fig. 13-14B).
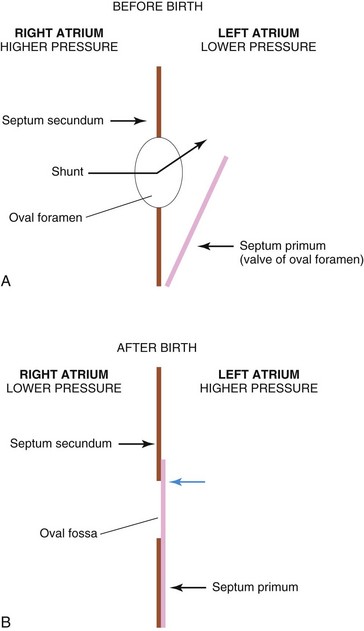
FIGURE 13–14 Diagrams illustrating the relationship of the septum primum to the oval foramen and septum secundum. A, Before birth, well-oxygenated blood is shunted from the right atrium through the oval foramen into the left atrium when the pressure increases. When the pressure decreases in the right atrium, the flap-like valve of the oval foramen is pressed against the relatively rigid septum secundum. This closes the oval foramen. B, After birth, the pressure in the left atrium increases as the blood returns from the lungs. Eventually the septum primum is pressed against the septum secundum and adheres to it, permanently closing the oval foramen and forming the oval fossa.
After birth, the oval foramen functionally closes due to higher pressure in the left atrium than in the right atrium. Approximately at 3 months of age, the valve of the oval foramen fuses with the septum secundum, forming the oval fossa (fossa ovalis).
Changes in Sinus Venosus
Initially, the sinus venosus opens into the center of the dorsal wall of the primordial atrium, and its right and left horns are approximately the same size (Fig. 13-5A). Progressive enlargement of the right horn results from two left-to-right shunts of blood:
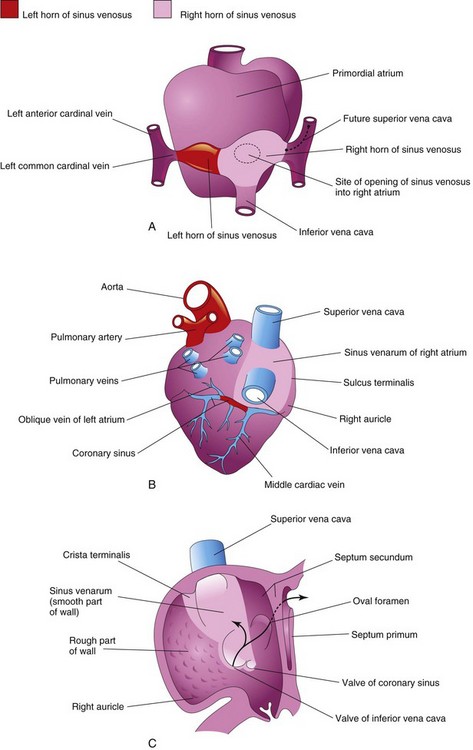
FIGURE 13–15 Diagrams illustrating the fate of the sinus venosus. A, Dorsal view of the heart (approximately 26 days) showing the primordial atrium and sinus venosus. B, Dorsal view at 8 weeks after incorporation of the right horn of the sinus venosus into the right atrium. The left horn of the sinus horn becomes the coronary sinus. C, Internal view of the fetal right atrium showing: (1) the smooth part of the wall of the right atrium (sinus venarum) that is derived from the right horn of the sinus venosus and (2) the crista terminalis and valves of the inferior vena cava and coronary sinus that are derived from the right sinuatrial valve. The primordial right atrium becomes the right auricle, a conical muscular pouch.
By the end of the fourth week, the right horn of the sinus venosus is noticeably larger than the left horn (Fig. 13-15A). As this occurs, the sinuatrial (SA) orifice moves to the right and opens in the part of the primordial atrium that will become the adult right atrium (Figs. 13-11 and 13-15C). As the right horn of the sinus venosus enlarges, it receives all the blood from the head and neck through the SVC and from the placenta and caudal regions of the body through the IVC. Initially, the sinus venosus is a separate chamber of the heart and opens into the dorsal wall of the right atrium (Fig. 13-10A and B). The left horn becomes the coronary sinus, and the right horn is incorporated into the wall of the right atrium (Fig. 13-15B and C).
Because it is derived from the sinus venosus, the smooth part of the wall of the right atrium is called the sinus venarum (Fig. 13-15B and C). The remainder of the anterior internal surface of the atrial wall and the conical muscular pouch, the right auricle, have a rough trabeculated appearance. These two parts are derived from the primordial atrium. The smooth part and the rough part are demarcated internally in the right atrium by a vertical ridge, the crista terminalis, and externally by a shallow groove, the sulcus terminalis (Fig. 13-15B and C).
The crista terminalis represents the cranial part of the right SA valve (Fig. 13-15C); the caudal part of this valve forms the valves of the IVC and coronary sinus. The left SA valve fuses with the septum secundum and is incorporated with it into the interatrial septum.
Primordial Pulmonary Vein and Formation of Left Atrium
Most of the wall of the left atrium is smooth because it is formed by incorporation of the primordial pulmonary vein (Fig. 13-16). This vein develops as an outgrowth of the dorsal atrial wall, just to the left of the septum primum. As the atrium expands, the primordial pulmonary vein and its main branches are incorporated into the wall of the left atrium. As a result, four pulmonary veins are formed (Fig. 13-16C and D).
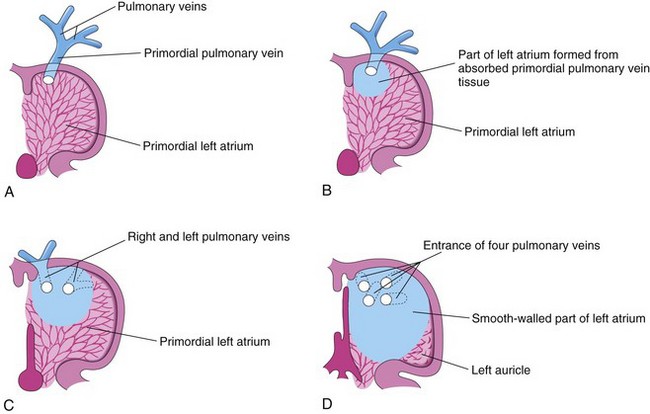
FIGURE 13–16 Diagrammatic sketches illustrating absorption of the pulmonary vein into the left atrium. A, At 5 weeks, showing the primordial pulmonary vein opening into the primordial left atrium. B, Later stage showing partial absorption of the primordial pulmonary vein. C, At 6 weeks, showing the openings of two pulmonary veins into the left atrium resulting from absorption of the primordial pulmonary vein. D, At 8 weeks, showing four pulmonary veins with separate atrial orifices. The primordial left atrium becomes the left auricle, a tubular appendage of the atrium. Most of the left atrium is formed by absorption of the primordial pulmonary vein and its branches.
Molecular studies have confirmed that atrial myoblasts migrate into the walls of the pulmonary veins. The functional significance of this pulmonary cardiac muscle (pulmonary myocardium) is uncertain. The small left auricle is derived from the primordial atrium; its internal surface has a rough trabeculated appearance.
Anomalous Pulmonary Venous Connections
In total anomalous pulmonary venous connections, none of the pulmonary veins connects with the left atrium. They open into the right atrium or into one of the systemic veins or both. In partial anomalous pulmonary venous connections, one or more pulmonary veins have similar anomalous connections; the others have normal connections.
Partitioning of Primordial Ventricle
Division of the primordial ventricle is first indicated by a median ridge—the muscular interventricular (IV) septum—in the floor of the ventricle near its apex (Fig. 13-12B). Myocytes from both the left and right primordial ventricles contribute to the formation of the muscular part of the IV septum. The IV septum has a concave free edge (Fig. 13-17A). Initially, the IV septum attains most of its height from dilation of the ventricles on each side of the muscular IV septum (Fig. 13-17B). Later, there is active proliferation of myoblasts in the septum, which increase its size.
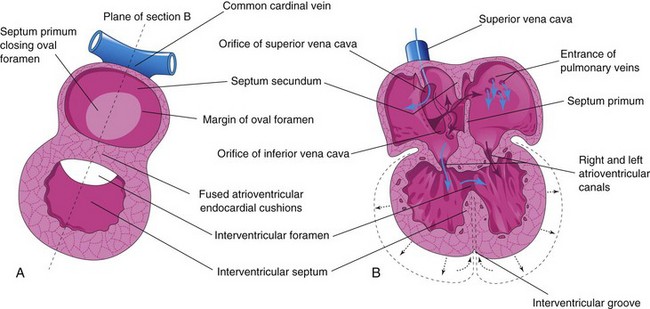
FIGURE 13–17 Schematic diagrams illustrating partitioning of the primordial heart. A, Sagittal section late in the fifth week showing the cardiac septa and foramina. B, Coronal section at a slightly later stage illustrating the directions of blood flow through the heart (blue arrows) and expansion of the ventricles (black arrows).
Until the seventh week, there is a crescent-shaped IV foramen between the free edge of the IV septum and the fused endocardial cushions. The IV foramen permits communication between the right and left ventricles (Figs. 13-17 and 13-18B). The IV foramen usually closes by the end of the seventh week as the bulbar ridges fuse with the endocardial cushion (Fig. 13-18C to E).
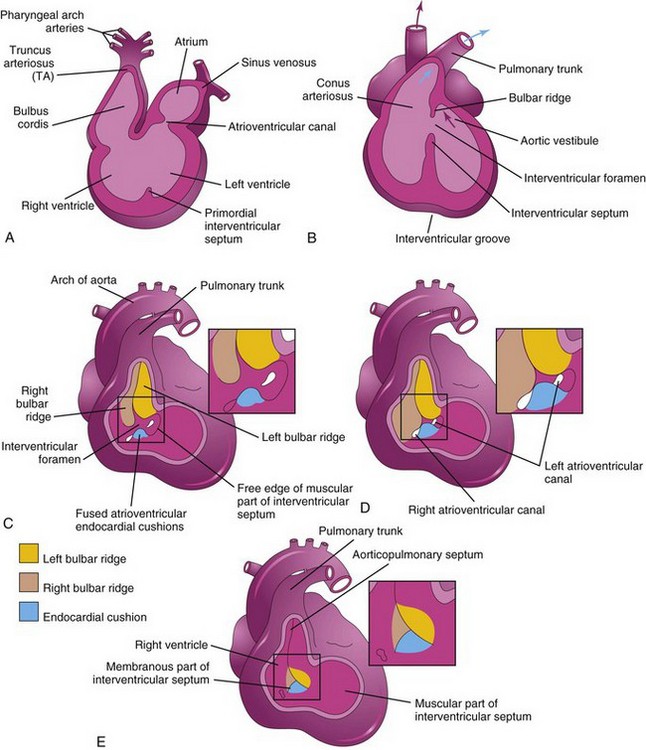
FIGURE 13–18 Sketches illustrating incorporation of the bulbus cordis into the ventricles and partitioning of the bulbus cordis and truncus arteriosus into the aorta and pulmonary trunk. A, Sagittal section at 5 weeks showing the bulbus cordis as one of the chambers of the primordial heart. B, Schematic coronal section at 6 weeks, after the bulbus cordis has been incorporated into the ventricles to become the conus arteriosus of the right ventricle, which gives origin to the pulmonary trunk and the aortic vestibule of the left ventricle. The arrow indicates blood flow. C to E, Schematic drawings illustrating closure of the interventricular foramen and formation of the membranous part of the interventricular septum. The walls of the truncus arteriosus, bulbus cordis, and right ventricle have been removed. C, At 5 weeks, showing the bulbar ridges and fused atrioventicular endocardial cushions. D, At 6 weeks, showing how proliferation of subendocardial tissue diminishes the interventricular foramen. E, At 7 weeks, showing the fused bulbar ridges, the membranous part of the interventricular septum formed by extensions of tissue from the right side of the atrioventricular endocardial cushions, and closure of the interventricular foramen.
Closure of the IV foramen and formation of the membranous part of the IV septum result from the fusion of tissues from three sources: the right bulbar ridge, the left bulbar ridge, and the endocardial cushion. The membranous part of the IV septum is derived from an extension of tissue from the right side of the endocardial cushion to the muscular part of the IV septum. This tissue merges with the aorticopulmonary septum and the thick muscular part of the IV septum (Figs. 13-18E and 13-19C). After closure of the IV foramen and formation of the membranous part of the IV septum, the pulmonary trunk is in communication with the right ventricle and the aorta communicates with the left ventricle (Fig. 13-18E).
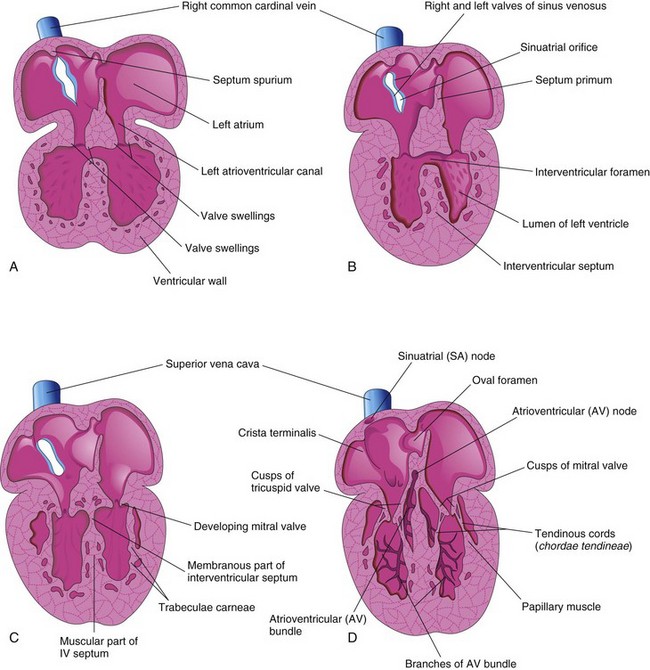
FIGURE 13–19 Schematic sections of the heart illustrating successive stages in the development of the atrioventricular valves, tendinous cords, and papillary muscles. A, At 5 weeks. B, At 6 weeks. C, At 7 weeks. D, At 20 weeks, showing the conducting system of the heart.
Cavitation of the ventricular walls forms a sponge-work of muscular bundles—trabeculae carneae. Some of these bundles become the papillary muscles and tendinous cords (chordae tendineae). The tendinous cords run from the papillary muscles to the AV valves (Fig. 13-19C and D).
Fetal Cardiac Ultrasonography
Cardiac screening using high-resolution real-time ultrasonography is usually performed between 18 and 22 weeks of gestation (Fig. 13-20) because the heart is large enough to examine.
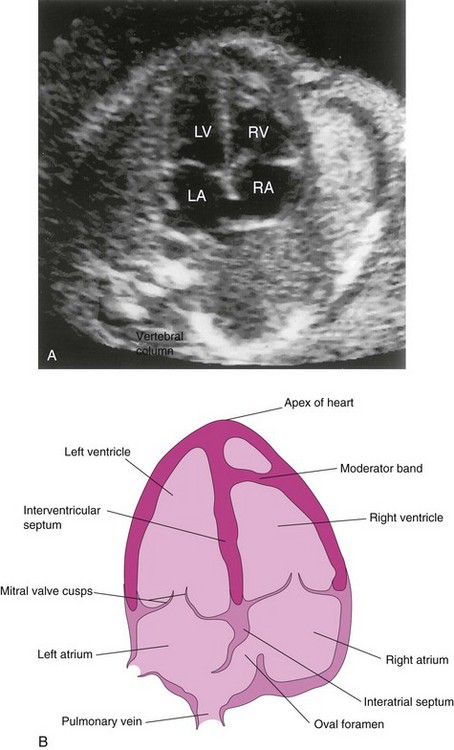
FIGURE 13–20 A, Ultrasound image showing the four-chamber view of the heart in a fetus of approximately 20 weeks gestation. B, Orientation sketch (modified from the AIUM Technical Bulletin, Performance of the Basic Fetal Cardiac Ultrasound Examination). The scan was obtained across the fetal thorax. The ventricles and atria are well formed and two atrioventricular (AV) valves are present. The moderator band is one of the trabeculae carneae that carries part of the right branch of the AV bundle. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
(Courtesy of Dr. Wesley Lee, Division of Fetal Imaging, William Beaumont Hospital, Royal Oak, Michigan.)
Partitioning of Bulbus Cordis and Truncus Arteriosus
During the fifth week, active proliferation of mesenchymal cells in the walls of the bulbus cordis results in the formation of bulbar ridges (conotruncal ridges) (Figs. 13-18C and D and 13-21B and C). Similar ridges that are continuous with the bulbar ridges form in the truncus arterious (TA). The bulbar and truncal ridges are derived largely from neural crest mesenchyme.
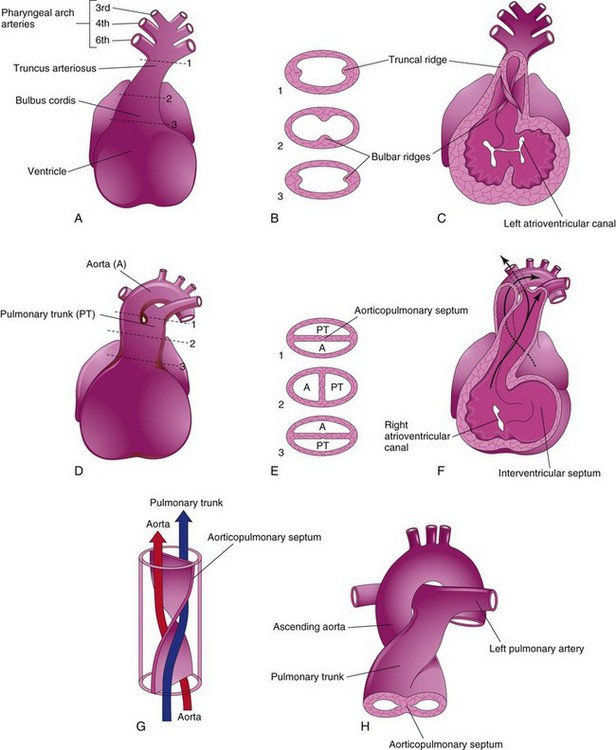
FIGURE 13–21 Partitioning of the bulbus cordis and truncus arteriosus. A, Ventral aspect of heart at 5 weeks. The broken lines and arrows indicate the levels of the sections shown in A. B, Transverse sections of the truncus arteriosus and bulbus cordis, illustrating the truncal and bulbar ridges. C, The ventral wall of the heart and truncus arteriosus has been removed to demonstrate these ridges. D, Ventral aspect of heart after partitioning of the truncus arteriosus. The broken lines and arrows indicate the levels of the sections shown in E. E, Sections through the newly formed aorta (A) and pulmonary trunk (PT), showing the aorticopulmonary septum. F, 6 weeks. The ventral wall of the heart and pulmonary trunk have been removed to show the aorticopulmonary septum. G, Diagram illustrating the spiral form of the aorticopulmonary septum. H, Drawing showing the great arteries (ascending aorta and pulmonary trunk) twisting around each other as they leave the heart.
Neural crest cells migrate through the primordial pharynx and pharyngeal arches to reach the ridges. As this occurs, the bulbar and truncal ridges undergo a 180-degree spiraling.
The spiral orientation of the bulbar and truncal ridges, caused in part by streaming of blood from the ventricles, results in the formation of a spiral aorticopulmonary septum when the ridges fuse (Fig. 13-21D to G).
This septum divides the bulbus cordis and TA into two arterial channels, the ascending aorta and pulmonary trunk. Because of the spiraling of the aorticopulmonary septum, the pulmonary trunk twists around the ascending aorta (Fig. 13-21H). The bulbus cordis is incorporated into the walls of the definitive ventricles (see Fig. 13-18A and B):
Development of Cardiac Valves
When partitioning of the truncus arteriosus (TA) is nearly completed (Fig. 13-21A to C), the semilunar valves begin to develop from three swellings of subendocardial tissue around the orifices of the aorta and pulmonary trunk. These swellings are hollowed out and reshaped to form three thin-walled cusps (Figs. 13-19C and D and 13-22). The atrioventricular (AV) valves (tricuspid and mitral valves) develop similarly from localized proliferations of tissue around the AV canals.
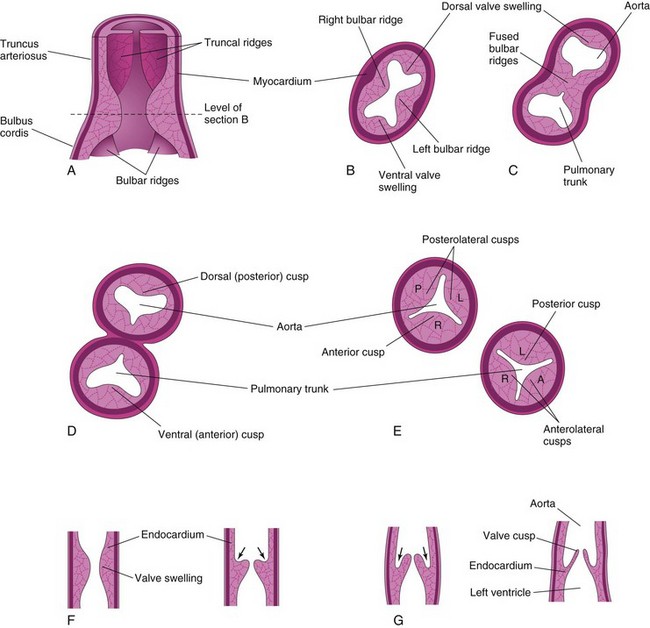
FIGURE 13–22 Development of the semilunar valves of the aorta and pulmonary trunk. A, Sketch of a section of the truncus arteriosus and bulbus cordis showing the valve swellings. B, Transverse section of the bulbus cordis. C, Similar section after fusion of the bulbar ridges. D, Formation of the walls and valves of the aorta and pulmonary trunk. E, Rotation of the vessels has established the adult relations of the valves. F and G, Longitudinal sections of the aorticoventricular junction illustrating successive stages in the hollowing (arrows) and thinning of the valve swellings to form the valve cusps.
Conducting System of Heart
Initially, the muscle in the primordial atrium and ventricle is continuous. The atrium acts as the interim pacemaker of the heart, but the sinus venosus soon takes over this function. The sinuatrial (SA) node develops during the fifth week. It is originally in the right wall of the sinus venosus, but it becomes incorporated into the wall of the right atrium with the sinus venosus (Fig. 13-19A and D). The SA node is located high in the right atrium, near the entrance of the superior vena cava (SVC).
After incorporation of the sinus venosus, cells from its left wall are found in the base of the interatrial septum just anterior to the opening of the coronary sinus. Together with cells from the AV region, they form the AV node and bundle, which are located just superior to the endocardial cushions. The fibers arising from the AV bundle pass from the atrium into the ventricle and split into right and left bundle branches. These branches are distributed throughout the ventricular myocardium (Fig. 13-19D).
The SA node, AV node, and AV bundle are richly supplied by nerves; however, the conducting system is well developed before these nerves enter the heart. This specialized tissue is normally the only signal pathway from the atria to the ventricles. As the four chambers of the heart develop, a band of connective tissue grows in from the epicardium, subsequently separating the muscle of the atria from that of the ventricles. This connective tissue forms part of the cardiac skeleton (fibrous skeleton of heart).
Sudden Infant Death Syndrome
Conducting tissue abnormalities have been observed in the hearts of several infants who died unexpectedly from a disorder known as sudden infant death syndrome (SIDS). This is the most common cause of deaths among healthy infants (0.6 per 1000 live births).
Most likely, no single mechanism is responsible for the sudden and unexpected deaths of these apparently healthy infants. Some people suggest that they have a defect in the autonomic nervous system. A brainstem developmental abnormality or maturational delay related to neuroregulation of cardiorespiratory control appears to be the most compelling hypothesis.
Birth Defects Of The Heart And Great Vessels
Congenital heart defects (CHDs) are common, with a frequency of six to eight cases per 1000 births. Some CHDs are caused by single-gene or chromosomal mechanisms. Other defects result from exposure to teratogens such as the rubella virus (see Chapter 20); however, in many cases, the cause is unknown. Most CHDs are thought to be caused by multiple factors, genetic and environmental (i.e., multifactorial inheritance), each of which has a minor effect.
The molecular aspects of abnormal cardiac development are poorly understood, and gene therapy for infants with CHDs is at present a remote prospect. Imaging technology, such as real-time two-dimensional echocardiography, permits detection of fetal CHDs as early as the 17th or 18th week.
Most CHDs are well tolerated during fetal life; however, at birth, when the fetus loses contact with the maternal circulation, the impact of CHDs becomes apparent. Some types of CHD cause very little disability; others are incompatible with extrauterine life. Because of recent advances in cardiovascular surgery, many types of CHD can be palliated or corrected surgically, and fetal cardiac surgery may soon be possible for complex CHDs.
Dextrocardia
If the embryonic heart tube bends to the left instead of to the right (Fig. 13-23), the heart is displaced to the right and the heart and its vessels are reversed left to right as in a mirror image of their normal configuration. Dextrocardia is the most frequent positional defect of the heart. In dextrocardia with situs inversus (transposition of abdominal viscera), the incidence of accompanying cardiac defects is low. If there is no other associated vascular abnormality, these hearts function normally.
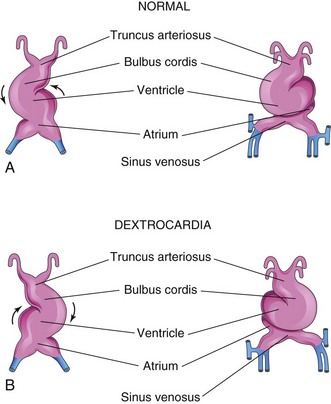
FIGURE 13–23 The embryonic heart tube during the fourth week. A, Normal looping of the tubular heart to the right. B, Abnormal looping of the tubular heart to the left.
In isolated dextrocardia, the abnormal position of the heart is not accompanied by displacement of other viscera. This defect is usually complicated by severe cardiac anomalies (e.g., single ventricle and transposition of the great vessels). The TGF-β factor Nodal is involved in looping of the heart tube, but its role in dextrocardia is unclear.
Ectopia Cordis
In ectopia cordis, a rare condition, the heart is in an abnormal location (Fig. 13-24). In the thoracic form of ectopia cordis, the heart is partly or completely exposed on the surface of the thorax. It is usually associated with widely separated halves of the sternum (nonfusion) and an open pericardial sac. Death occurs in most cases during the first few days after birth, usually from infection, cardiac failure, or hypoxemia. If there are not severe cardiac defects, surgical therapy usually consists of covering the heart with skin. In some cases of ectopia cordis, the heart protrudes through the diaphragm into the abdomen.
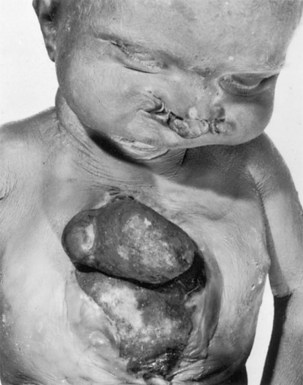
FIGURE 13–24 Neonate with ectopia cordis, cleft sternum, and bilateral cleft lip. Death occurred in the first days of life from infection, cardiac failure, and hypoxia.
The clinical outcome for patients with ectopia cordis has improved and many children have survived to adulthood. The most common thoracic form of ectopia cordis results from faulty development of the sternum and pericardium because of failure of complete fusion of the lateral folds in the formation of the thoracic wall during the fourth week (see Chapter 5, Fig. 5-1).
Atrial Septal Defects
An atrial septal defect (ASD) is a common congenital heart defect and occurs more frequently in females than in males. The most common form of ASD is patent oval foramen (Fig. 13-25B).
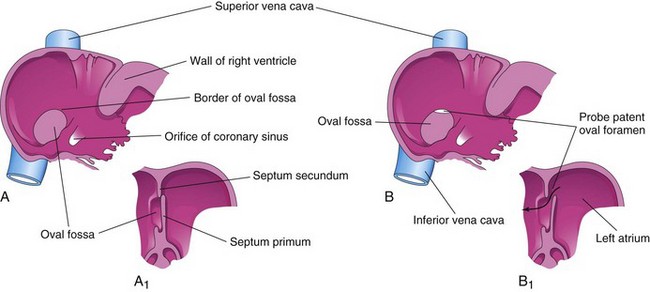
FIGURE 13–25 A, Normal postnatal appearance of the right side of the interatrial septum after adhesion of the septum primum to the septum secundum. A1, Sketch of a section of the interatrial septum illustrating formation of the oval fossa in the right atrium. Note that the floor of the oval fossa is formed by the septum primum. B and B1, Similar views of a probe patent oval foramen resulting from incomplete adhesion of the septum primum to the septum secundum. Some well-oxygenated blood can enter the right atrium via a patent oval foramen; however, if the opening is small it usually of no hemodynamic significance.
A probe patent oval foramen is present in up to 25% of people (Fig. 13-25B). In this circumstance, a probe can be passed from one atrium to the other through the superior part of the floor of the oval fossa. This defect is not clinically significant, but a probe patent oval foramen may be forced open because of other cardiac defects and contribute to the functional pathology of the heart. Probe patent oval foramen results from incomplete adhesion between the flap-like valve of the oval foramen and the septum secundum after birth.
There are four clinically significant types of ASD (Figs. 13-26 and 13-27): ostium secundum defect, endocardial cushion defect with ostium primum defect, sinus venosus defect, and common atrium. The first two types of ASD are relatively common.
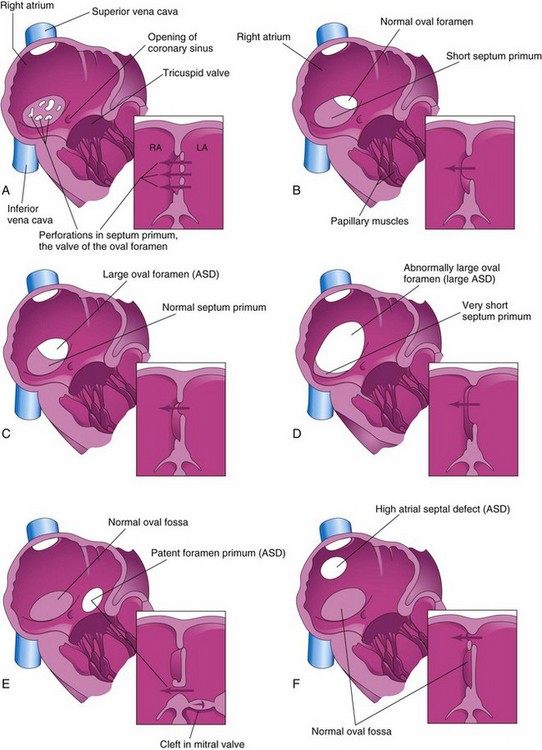
FIGURE 13–26 Drawings of the right aspect of the interatrial septum. The adjacent sketches of sections of the septa illustrate various types of atrial septal defect (ASD). A, Patent oval foramen resulting from resorption of the septum primum in abnormal locations. B, Patent oval foramen caused by excessive resorption of the septum primum (short flap defect). C, Patent oval foramen resulting from an abnormally large oval foramen. D, Patent oval foramen resulting from an abnormally large oval foramen and excessive resorption of the septum primum. E, Endocardial cushion defect with primum-type ASD. The adjacent section shows the cleft in the anterior cusp of the mitral valve. F, Sinus venosus ASD. The high septal defect resulted from abnormal absorption of the sinus venosus into the right atrium. In E and F, note that the oval fossa has formed normally.
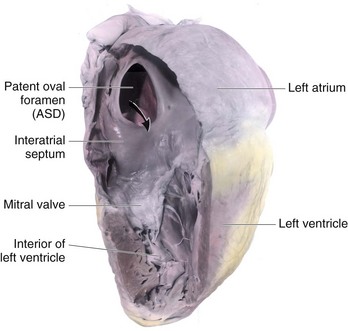
FIGURE 13–27 Dissection of an adult heart with a large patent oval foramen. The arrow passes through a large atrial septal defect (ASD), which resulted from an abnormally large oval foramen and excessive resorption of the septum primum. This is referred to as a secundum-type ASD and is one of the most common types of congenital heart disease.
Ostium secundum ASDs (Figs. 13-26A to D and 13-27) are in the area of the oval fossa and include defects of the septum primum and septum secundum. Ostium secundum ASDs are well tolerated during childhood; symptoms such as pulmonary hypertension usually appear in the 30s or later. Closure of the ASD is carried out at open heart surgery, and the mortality rate is less than 1%. The defects may be multiple and, in symptomatic older children, defects of 2 cm or more in diameter are not unusual. Females with ASD outnumber males 3:1. Ostium secundum ASDs are one of the most common types of CHD, yet are the least severe.
The patent oval foramen usually results from abnormal resorption of the septum primum during the formation of the foramen secundum. If resorption occurs in abnormal locations, the septum primum is fenestrated or net-like (Fig. 13-26A). If excessive resorption of the septum primum occurs, the resulting short septum primum will not close the oval foramen (Fig. 13-26B). If an abnormally large oval foramen occurs because of defective development of the septum secundum, a normal septum primum will not close the abnormal oval foramen at birth (Fig. 13-26C ). A small isolated patent oval foramen is of no hemodynamic significance; however, if there are other defects (e.g., pulmonary stenosis or atresia), blood is shunted through the oval foramen into the left atrium and produces cyanosis (deficient oxygenation of blood). Large ostium secundum ASDs may also occur because of a combination of excessive resorption of the septum primum and a large oval foramen (Figs. 13-26D and 13-27).
Endocardial cushion defects with ostium primum ASDs are less common forms of ASD (Fig. 13-26E ). Several cardiac defects are grouped together under this heading because they result from the same developmental defect, a deficiency of the endocardial cushions and the AV septum. The septum primum does not fuse with the endocardial cushions; as a result, there is a patent foramen primum-ostium primum defect. Usually there is also a cleft in the anterior cusp of the mitral valve. In the less common complete type of endocardial cushion and AV septal defects, fusion of the endocardial cushions fails to occur. As a result, there is a large defect in the center of the heart known as an AV septal defect (Fig. 13-28). This type of ASD occurs in approximately 20% of persons with Down syndrome; otherwise, it is a relatively uncommon cardiac defect. It consists of a continuous interatrial and interventricular defect with markedly abnormal AV valves.
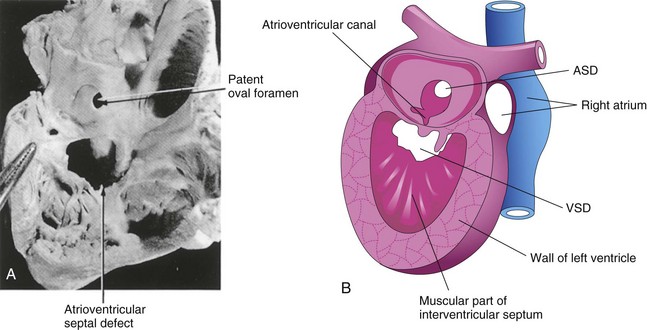
FIGURE 13–28 A, An infant’s heart, sectioned and viewed from the right side, showing a patent oval foramen and an atrioventricular septal defect. B, Schematic drawing of a heart illustrating various septal defects. ASD, atrial septal defect; VSD, and ventricular septal defect.
(A, From Lev M: Autopsy Diagnosis of Congenitally Malformed Hearts. Springfield, IL: Charles C Thomas, 1953.)
All sinus venosus ASDs (high ASDs) are located in the superior part of the interatrial septum close to the entry of the SVC (Fig. 13-26F ). A sinus venosus defect is a rare type of ASD. It results from incomplete absorption of the sinus venosus into the right atrium and/or abnormal development of the septum secundum. This type of ASD is commonly associated with partial anomalous pulmonary venous connections.
Common atrium is a rare cardiac defect in which the interatrial septum is absent. This defect is the result of failure of the septum primum and septum secundum to develop (combination of ostium secundum, ostium primum, and sinus venosus defects).
Ventricular Septal Defects
Ventricular septal defects (VSDs) are the most common type of CHD, accounting for approximately 25% of heart defects. VSDs occur more frequently in males than in females. VSDs may occur in any part of the IV septum (Fig. 13-28B), but membranous VSD is the most common type (Figs. 13-28B and 13-29A). Frequently, during the first year, 30% to 50% of small VSDs close spontaneously.
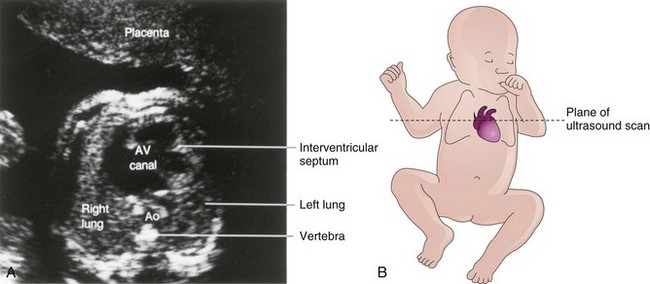
FIGURE 13–29 A, Ultrasound image of the heart of a second-trimester fetus with an atrioventricular (AV) canal (atrioventricular septal) defect. An atrial septal defect and ventricular septal defect are also present. Ao, aorta. B, Orientation drawing.
(A, Courtesy of Dr. B. Benacerraf, Diagnostic Ultrasound Associates, P.C., Boston, Massachusetts.)
Incomplete closure of the IV foramen results from failure of the membranous part of the IV septum to develop. It results from failure of an extension of subendocardial tissue to grow from the right side of the endocardial cushion and fuse with the aorticopulmonary septum and the muscular part of the IV septum (Fig. 13-18C to E). Large VSDs with excessive pulmonary blood flow (Fig. 13-30) and pulmonary hypertension result in dyspnea (difficult breathing) and cardiac failure early in infancy.
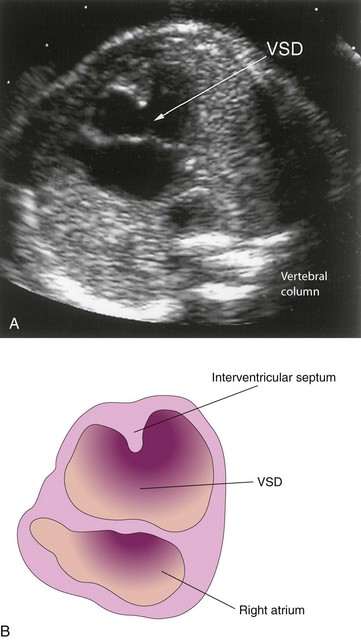
FIGURE 13–30 Ultrasound scan of a fetal heart at 23.4 weeks with an atrioventricular septal defect and a large ventricular septal defect (VSD).
(A, Courtesy of Dr. Wesley Lee, Division of Fetal Imaging, William Beaumont Hospital, Royal Oak, Michigan.)
Muscular VSD is a less common type of defect and may appear anywhere in the muscular part of the interventricular septum. Sometimes there are multiple small defects, producing what is sometimes called the “Swiss cheese” VSD. Muscular VSDs probably occur because of excessive cavitation of myocardial tissue during formation of the ventricular walls and the muscular part of the interventricular septum.
Absence of the IV septum—single ventricle or common ventricle—resulting from failure of the IV septum to form, is extremely rare and results in a three-chambered heart (Latin cor triloculare biatriatum). When there is a single ventricle, the atria empty through a single common valve or two separate AV valves into a single ventricular chamber. The aorta and pulmonary trunk arise from the ventricle. TGA (transposition of great arteries) (see Fig. 13-32) and a rudimentary outlet chamber are present in most infants with a single ventricle. Some children die during infancy from congestive heart failure.
Persistent Truncus Arteriosus
Persistent truncus arteriosus (TA) results from failure of the truncal ridges and aorticopulmonary septum to develop normally and divide the TA into the aorta and pulmonary trunk (Fig. 13-31). A single arterial trunk, the TA, arises from the heart and supplies the systemic, pulmonary, and coronary circulations. A VSD is always present with a TA defect; the TA straddles the VSD (Fig. 13-31B).
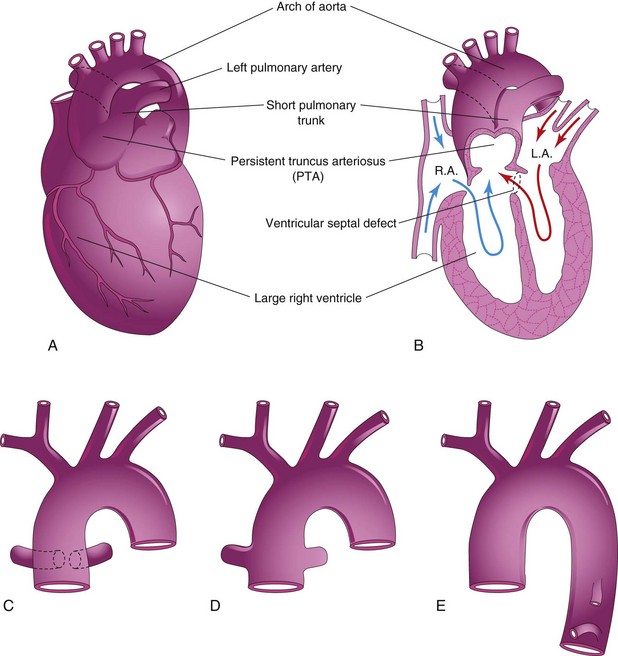
FIGURE 13–31 Illustrations of the common types of persistent truncus arteriosus (PTA). A, The common trunk divides into the aorta and a short pulmonary trunk. B, Coronal section of the heart shown in A. Observe the circulation of blood in this heart (arrows) and the ventricular septal defect. C, The right and left pulmonary arteries arise close together from the truncus arteriosus. D, The pulmonary arteries arise independently from the sides of the truncus arteriosus. E, No pulmonary arteries are present; the lungs are supplied by the bronchial arteries. LA, left atrium; RA, right atrium.
Recent studies indicate that developmental arrest of the outflow tract, semilunar valves, and aortic sac in the early embryo (days 31-32) is involved in the pathogenesis of TA defects. The common type of TA is a single arterial vessel that branches to form the pulmonary trunk and ascending aorta (Fig. 13-31A and B). In the next most common type, the right and left pulmonary arteries arise close together from the dorsal wall of the TA (Fig. 13-31C ). Less common types are illustrated in Figure 13-31D and E.
Aorticopulmonary Septal Defect
Aorticopulmonary septal defect is a rare condition in which there is an opening (aortic window) between the aorta and pulmonary trunk near the aortic valve. The aorticopulmonary defect results from localized defect in the formation of the aorticopulmonary septum. The presence of pulmonary and aortic valves and an intact IV septum distinguishes this anomaly from the persistent truncus arteriosus defect.
Transposition of the Great Arteries
Transposition of the great arteries (TGA) is the common cause of cyanotic heart disease in newborn infants (Fig. 13-32). TGA is often associated with other cardiac anomalies (e.g., ASD and VSD). In typical cases, the aorta lies anterior and to the right of the pulmonary trunk and arises from the morphologic right ventricle, whereas the pulmonary trunk arises from the morphologic left ventricle. The associated ASD and VSD defects permit some interchange between the pulmonary and systemic circulations.
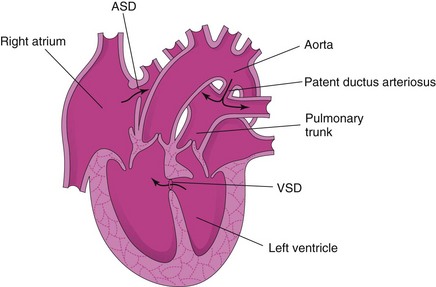
FIGURE 13–32 Drawing of a heart illustrating transposition of the great arteries (TGA). The ventricular and atrial septal defects allow mixing of the arterial and venous blood. This birth defect is often associated with other cardiac defects as shown (ventricular septal defect [VSD] and atrial septal defect [ASD]).
Because of these anatomic defects, deoxygenated systemic venous blood returning to the right atrium enters the right ventricle and then passes to the body through the aorta. Oxygenated pulmonary venous blood passes through the left ventricle back into the pulmonary circulation. Because of the patent oval foramen, there is some mixing of the blood. Without surgical correction of the TGA, these infants usually die within a few months.
Many attempts have been made to explain the basis of TGA, but the conal growth hypothesis is favored by most investigators. According to this explanation, the aorticopulmonary septum fails to pursue a spiral course during partitioning of the bulbus cordis and TA. This defect is thought to result from failure of the conus arteriosus to develop normally during incorporation of the bulbus cordis into the ventricles. Defective migration of neural crest cells is involved.
Unequal Division of the Truncus Arteriosus
Unequal division of the TA (Figs. 13-33A and 13-34B and C ) results when partitioning of the TA superior to the valves is unequal. One of the great arteries is large and the other is small. As a result, the aorticopulmonary septum is not aligned with the IV septum and a VSD results; of the two vessels, the one with the larger diameter usually straddles the VSD (Fig. 13-33B).
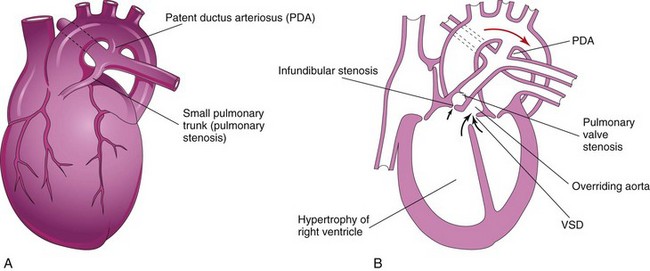
FIGURE 13–33 A, Drawing of an infant’s heart showing a small pulmonary trunk (pulmonary stenosis) and a large aorta resulting from unequal partitioning of the truncus arteriosus. There is also hypertrophy of the right ventricle and a patent ductus arteriosus (PDA). B, Frontal section of this heart illustrating the tetralogy of Fallot. Observe the four cardiac defects of this tetralogy: pulmonary valve stenosis, ventricular septal defect (VSD), overriding aorta, and hypertrophy of the right ventricle.
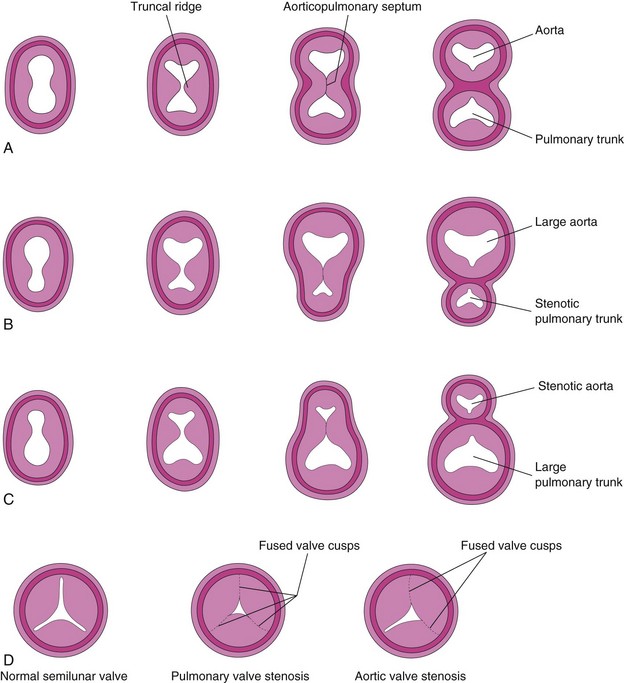
FIGURE 13–34 Abnormal division of the truncus arteriosus (TA). A to C, Sketches of transverse sections of the TA illustrating normal and abnormal partitioning of the TA. A, Normal. B, Unequal partitioning of the TA resulting in a small pulmonary trunk. C, Unequal partitioning resulting in a small aorta. D, Sketches illustrating a normal semilunar valve and stenotic pulmonary and aortic valves.
In pulmonary valve stenosis, the cusps of the pulmonary valve are fused to form a dome with a narrow central opening (Fig. 13-34D).
In infundibular stenosis, the conus arteriosus (infundibulum) of the right ventricle is underdeveloped. The two types of pulmonary stenosis may occur. Depending on the degree of obstruction to blood flow, there is a variable degree of hypertrophy of the right ventricle (Fig. 13-33A and B).
Tetralogy of Fallot
This classic group of four cardiac defects (Figs. 13-33B, 13-35, and 13-36) consists of:
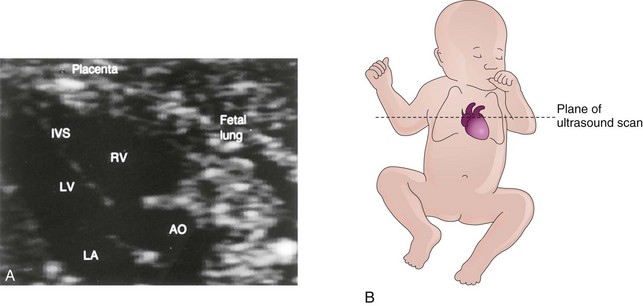
FIGURE 13–35 A, Ultrasound image of the heart of a 20-week fetus with tetralogy of Fallot. Note that the large overriding aorta (AO) straddles the interventricular septum. As a result, it receives blood from the left (LV) and right (RV) ventricles. IVS, Interventricular septum; LA, left atrium. B, Orientation drawing.
(A, Courtesy of Dr. B. Benacerraf, Diagnostic Ultrasound Associates, P.C., Boston, Massachusetts.)
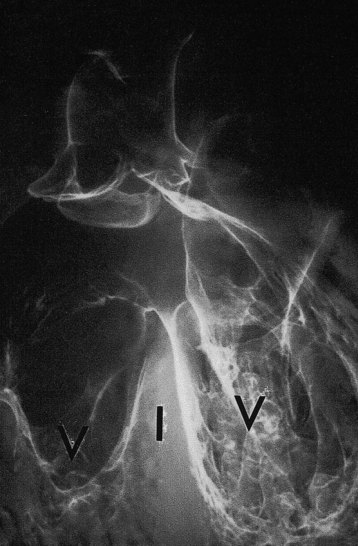
FIGURE 13–36 Tetralogy of Fallot. Fine barium powder was injected into the heart. Note the two ventricles (V), interventricular septum (I), interventricular septal defect at the superior margin, and origin of the aorta above the right ventricle (overriding aorta). The main pulmonary artery is not visualized.
(Courtesy of Dr. Joseph R. Siebert, Children’s Hospital & Regional Medical Center, Seattle, Washington.)
The pulmonary trunk is usually small (Fig. 13-33A) and there may be various degrees of pulmonary artery stenosis. Cyanosis (deficient oxygenation of blood), is an obvious sign of the tetralogy, but it is not usually present at birth.
The tetralogy results when division of the TA is unequal and the pulmonary trunk is stenotic. Pulmonary atresia with VSD is an extreme form of tetralogy of Fallot; the entire right ventricular output is through the aorta. Pulmonary blood flow is dependent on a PDA (patent ductus arteriosus) or on bronchial collateral vessels. Initial treatment may require surgical placement of a temporary shunt, but in many cases, primary surgical repair is the treatment of choice in early infancy.
Aortic Stenosis and Aortic Atresia
In aortic valve stenosis, the edges of the valve are usually fused to form a dome with a narrow opening (Fig. 13-34D). This defect may be congenital or develop after birth. The valvular stenosis causes extra work for the heart and results in hypertrophy of the left ventricle and abnormal heart sounds (heart murmurs).
In subaortic stenosis, there is often a band of fibrous tissue just inferior to the aortic valve. The narrowing of the aorta results from persistence of tissue that normally degenerates as the valve forms.
Aortic atresia is present when obstruction of the aorta or its valve is complete.
Hypoplastic Left Heart Syndrome
The left ventricle is small and nonfunctional (Fig. 13-37); the right ventricle maintains both pulmonary and systemic circulations. The blood passes through an atrial septal defect or a dilated oval foramen from the left to the right side of the heart, where it mixes with the systemic venous blood.
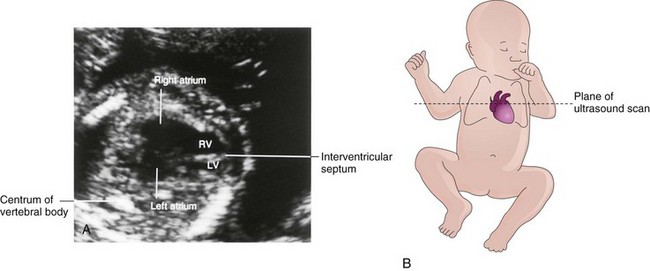
FIGURE 13–37 A, Ultrasound image of the heart of a second-trimester fetus with a hypoplastic left heart. Note that the left ventricle (LV) is much smaller than the right ventricle (RV). This is an oblique scan of the fetal thorax through the long axis of the ventricles. B, Orientation drawing.
(A, Courtesy of Dr. B. Benacerraf, Diagnostic Ultrasound Associates, P.C., Boston, Massachusetts.)
In addition to the underdeveloped left ventricle, there are atresia of the aortic or mitral orifice and hypoplasia of the ascending aorta. Infants with this severe defect usually die during the first few weeks after birth. Disturbances in the migration of neural crest cells, in hemodynamic function, in apoptosis, and in the proliferation of the extracellular matrix are likely responsible for the pathogenesis of many CHDs such as this syndrome.
 Derivatives Of Pharyngeal Arch Arteries
Derivatives Of Pharyngeal Arch Arteries
As the pharyngeal arches develop during the fourth week, they are supplied by arteries—the pharyngeal arch arteries—from the aortic sac (Fig. 13-38B). Mesodermal cells migrate from the pharyngeal arches to the aortic sac, connecting the pharyngeal arch arteries to the outflow tract. These arteries terminate in the dorsal aorta of the ipsilateral side. Although six pairs of pharyngeal arch arteries usually develop, they are not all present at the same time. By the time the sixth pair of pharyngeal arch arteries has formed, the first two pairs have disappeared (Fig. 13-38C). During the eighth week, the primordial pharyngeal arch arterial pattern is transformed into the final fetal arterial arrangement (Fig. 13-39C).
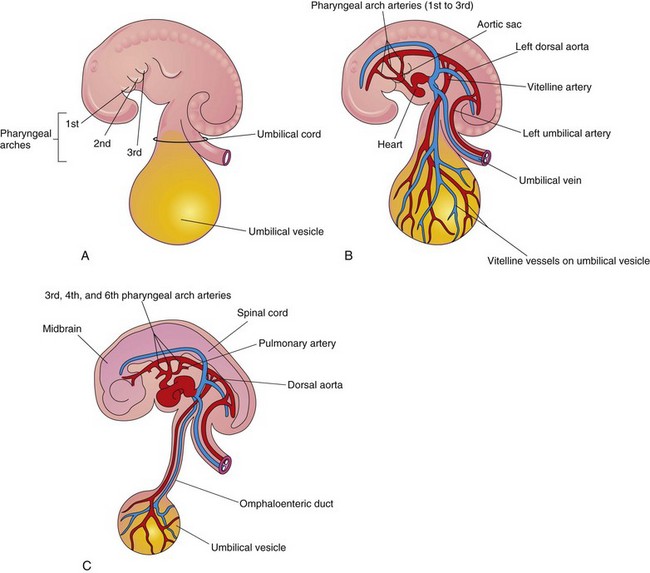
FIGURE 13–38 Pharyngeal arches and pharyngeal arch arteries. A, Left side of an embryo (approximately 26 days). B, Schematic drawing of this embryo showing the left pharyngeal arch arteries arising from the aortic sac, running through the pharyngeal arches, and terminating in the left dorsal aorta. C, An embryo (approximately 37 days) showing the single dorsal aorta and that most of the first two pairs of pharyngeal arch arteries have degenerated.
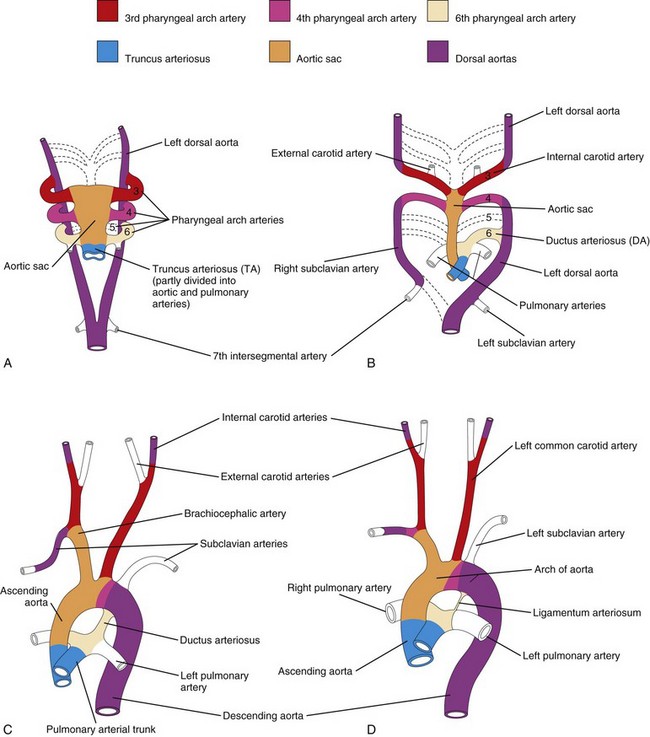
FIGURE 13–39 Schematic drawings illustrating the arterial changes that result during transformation of the truncus arteriosus, aortic sac, pharyngeal arch arteries, and dorsal aortae into the adult arterial pattern. The vessels that are not colored are not derived from these structures. A, Pharyngeal arch arteries at 6 weeks; by this stage, the first two pairs of arteries have largely disappeared. B, Pharyngeal arch arteries at 7 weeks; the parts of the dorsal aortae and pharyngeal arch arteries that normally disappear are indicated with broken lines. C, Arterial arrangement at 8 weeks. D, Sketch of the arterial vessels of a 6-month-old infant. Note that the ascending aorta and pulmonary arteries are considerably smaller in C than in D. This represents the relative flow through these vessels at the different stages of development. Observe the large size of the ductus arteriosus (DA) in C and that it is essentially a direct continuation of the pulmonary trunk. The DA normally becomes functionally closed within the first few days after birth. Eventually the DA becomes the ligamentum arteriosum, as shown in D.
Molecular studies indicate that the transcription factor Tbx1 regulates migration of the neural crest cells which contribute to the formation of the pharyngeal arch arteries.
Derivatives of First Pair of Pharyngeal Arch Arteries
Most of these pharyngeal arch arteries disappear, but remnants of them form part of the maxillary arteries, which supply the ears, teeth, and muscles of the eye and face. These arteries may also contribute to the formation of the external carotid arteries.
Derivatives of Second Pair of Pharyngeal Arch Arteries
Dorsal parts of these arteries persist and form the stems of the stapedial arteries; these small vessels run through the ring of the stapes, a small bone in the middle ear.
Derivatives of Third Pair of Pharyngeal Arch Arteries
Proximal parts of these arteries form the common carotid arteries, which supply structures in the head (Fig. 13-39D). Distal parts of the third pair of pharyngeal arch arteries join with the dorsal aortae to form the internal carotid arteries, which supply the middle ears, orbits, brain and its meninges, and the pituitary gland.
Derivatives of Fourth Pair of Pharyngeal Arch Arteries
The left fourth pharyngeal arch artery forms part of the arch of the aorta (Fig. 13-39C). The proximal part of the artery develops from the aortic sac and the distal part is derived from the left dorsal aorta.
The right fourth pharyngeal arch artery becomes the proximal part of the right subclavian artery. The distal part of the right subclavian artery forms from the right dorsal aorta and right seventh intersegmental artery. The left subclavian artery is not derived from a pharyngeal arch artery; it forms from the left seventh intersegmental artery (Fig. 13-39A). As development proceeds, differential growth shifts the origin of the left subclavian artery cranially. Consequently, it lies close to the origin of the left common carotid artery (Fig. 13-39D).
Fate of Fifth Pair of Pharyngeal Arch Arteries
Approximately 50% of the time, the fifth pair of pharyngeal arch arteries consists of rudimentary vessels that soon degenerate, leaving no vascular derivatives. In the other 50%, these arteries do not develop.
Derivatives of Sixth Pair of Pharyngeal Arch Arteries
The left sixth pharyngeal arch artery develops as follows (Fig. 13-39B and C):
The right sixth pharyngeal arch artery develops as follows:
The transformation of the sixth pair of pharyngeal arch arteries explains why the course of the recurrent laryngeal nerves differs on the two sides. These nerves supply the sixth pair of pharyngeal arches and hook around the sixth pair of pharyngeal arch arteries on their way to the developing larynx (Fig. 13-40A). On the right, because the distal part of the right sixth artery degenerates, the right recurrent laryngeal nerve moves superiorly and hooks around the proximal part of the right subclavian artery, the derivative of the fourth pharyngeal arch artery (Fig. 13-40B). On the left, the left recurrent laryngeal nerve hooks around the ductus arterious (DA) formed by the distal part of the sixth pharyngeal arch artery. When this arterial shunt involutes after birth, the nerve remains around the ligamentum arteriosum (remnant of DA), and the arch of the aorta (Fig. 13-40C).
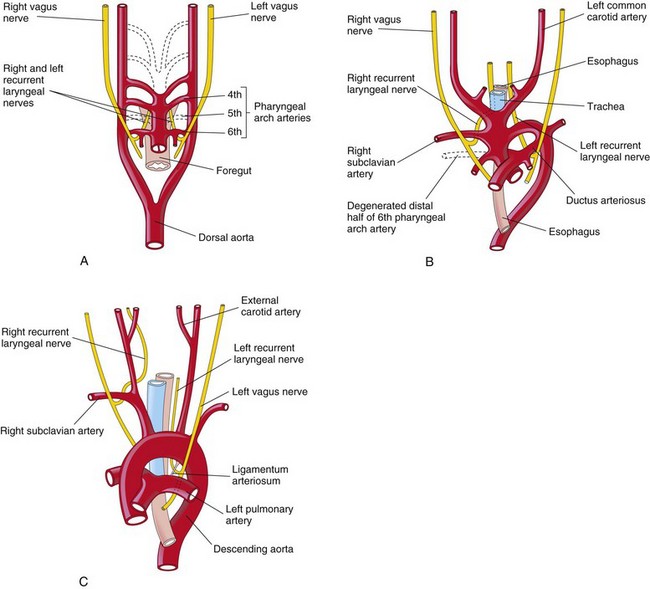
FIGURE 13–40 The relation of the recurrent laryngeal nerves to the pharyngeal arch arteries. A, At 6 weeks, showing the recurrent laryngeal nerves hooked around the sixth pair of pharyngeal arch arteries. B, At 8 weeks, showing the right recurrent laryngeal nerve hooked around the right subclavian artery, and the left recurrent laryngeal nerve hooked around the ductus arteriosus and the arch of the aorta. C, After birth, showing the left recurrent nerve hooked around the ligamentum arteriosum and the arch of the aorta.
Pharyngeal Arch Arterial Birth Defects
Because of the many changes involved in transformation of the embryonic pharyngeal arch arterial system into the adult arterial pattern, arterial birth defects may occur. Most defects result from the persistence of parts of the pharyngeal arch arteries that usually disappear, or from disappearance of parts that normally persist.
Coarctation of Aorta
Aortic coarctation (constriction) occurs in approximately 10% of children with congenital heart defects (CHDs). Coarctation is characterized by an aortic constriction of varying length (Fig. 13-41). Most coarctations occur distal to the origin of the left subclavian artery at the entrance of the ductus arteriosis (DA) (juxtaductal coarctation).
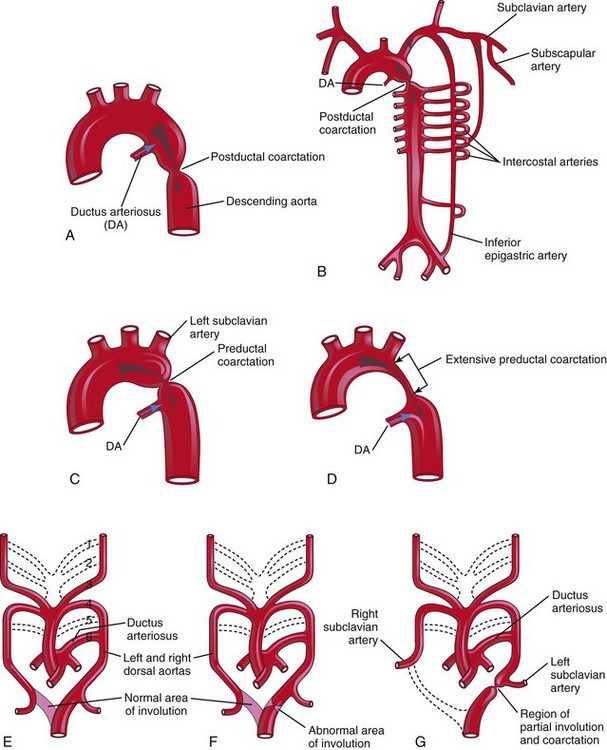
FIGURE 13–41 A, Postductal coarctation of the aorta. B, Diagrammatic representation of the common routes of collateral circulation that develop in association with postductal coarctation of the aorta. C and D, Preductal coarctation. E, Sketch of the pharyngeal arch arterial pattern in a 7-week embryo, showing the areas that normally involute (see dotted branches of arteries). Note that the distal segment of the right dorsal aorta normally involutes as the right subclavian artery develops. F, Abnormal involution of a small distal segment of the left dorsal aorta. G, Later stage showing the abnormally involuted segment appearing as a coarctation of the aorta. This moves to the region of the ductus arteriosus with the left subclavian artery. These drawings (E to G) illustrate one hypothesis about the embryologic basis of coarctation of the aorta.
The classification into preductal and postductal coarctations is commonly used; however, in 90% of instances, the coarctation is directly opposite the DA. Coarctation of the aorta occurs twice as often in males as in females and is associated with a bicuspid aortic valve in 70% of cases.
In postductal coarctation (Fig. 13-41A and B), the constriction is just distal to the DA. This permits development of a collateral circulation during the fetal period (Fig. 13-41B), thereby assisting with passage of blood to inferior parts of the body.
In preductal coarctation (Fig. 13-41C ), the constriction is proximal to the DA. The narrowed segment may be extensive (Fig. 13-41D); before birth, blood flows through the DA to the descending aorta for distribution to the lower body.
In an infant with severe aortic coarctation, closure of the DA results in hypoperfusion and rapid deterioration of the infant. These babies usually receive prostaglandin E2 (PGE2) in an attempt to reopen the DA and establish an adequate blood flow to the lower limbs. Aortic coarctation may be a feature of Turner syndrome (see Chapter 20). This and other observations suggest that genetic and/or environmental factors cause coarctation.
There are three main views about the embryologic basis of coarctation of the aorta:
Double Pharyngeal Arch Artery
This rare anomaly is characterized by a vascular ring around the trachea and esophagus (Fig. 13-42B). Varying degrees of compression of these structures may occur in infants. If the compression is significant, it causes wheezing respirations that are aggravated by crying, feeding, and flexion of the neck. The vascular ring results from failure of the distal part of the right dorsal aorta to disappear (Fig. 13-42A); as a result, right and left arches form. Usually the right arch of the aorta is larger and passes posterior to the trachea and esophagus (Fig. 13-42B).
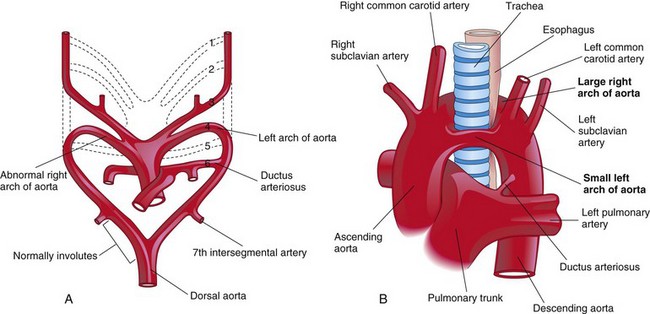
FIGURE 13–42 A, Drawing of the embryonic pharyngeal arch arteries illustrating the embryologic basis of the right and left arches of the aorta (double arch of aorta). B, A large right arch of the aorta and a small left arch of the aorta arise from the ascending aorta and form a vascular ring around the trachea and esophagus. Observe that there is compression of the esophagus and trachea. The right common carotid and subclavian arteries arise separately from the large right arch of the aorta.
Right Arch of Aorta
When the entire right dorsal aorta persists (Fig. 13-43A and B) and the distal part of the left dorsal aorta involutes, a right arch of the aorta results. There are two main types:
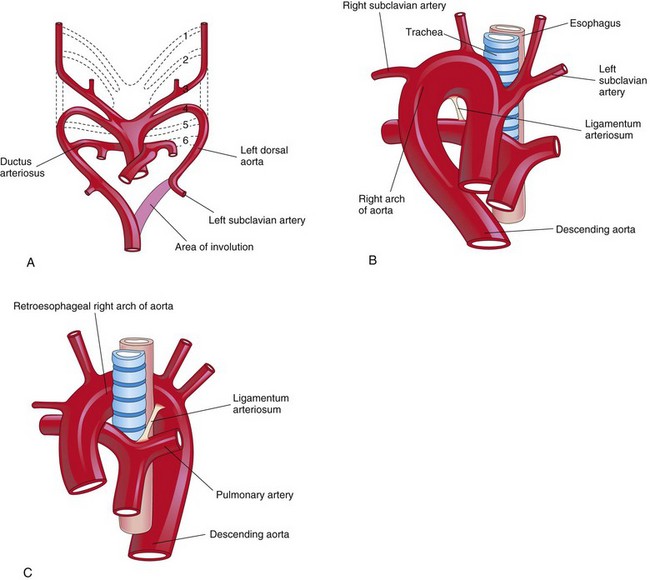
FIGURE 13–43 A, Sketch of the pharyngeal arch arteries showing the normal involution of the distal portion of the left dorsal aorta. There is also persistence of the entire right dorsal aorta and the distal part of the right sixth pharyngeal arch artery. B, Right pharyngeal arch artery without a retroesophageal component. C, Right arch of the aorta with a retroesophageal component. The abnormal right arch of the aorta and the ligamentum arteriosum (postnatal remnant of the ductus arteriosus) form a ring that compresses the esophagus and trachea.
Anomalous Right Subclavian Artery
The right subclavian artery arises from the distal part of the arch of the aorta and passes posterior to the trachea and esophagus to supply the right upper limb (Figs. 13-44 and 13-45). A retroesophageal right subclavian artery occurs when the right fourth pharyngeal arch artery and the right dorsal aorta disappear cranial to the seventh intersegmental artery. As a result, the right subclavian artery forms from the right seventh intersegmental artery and the distal part of the right dorsal aorta. As development proceeds, differential growth shifts the origin of the right subclavian artery cranially until it comes to lie close to the origin of the left subclavian artery.
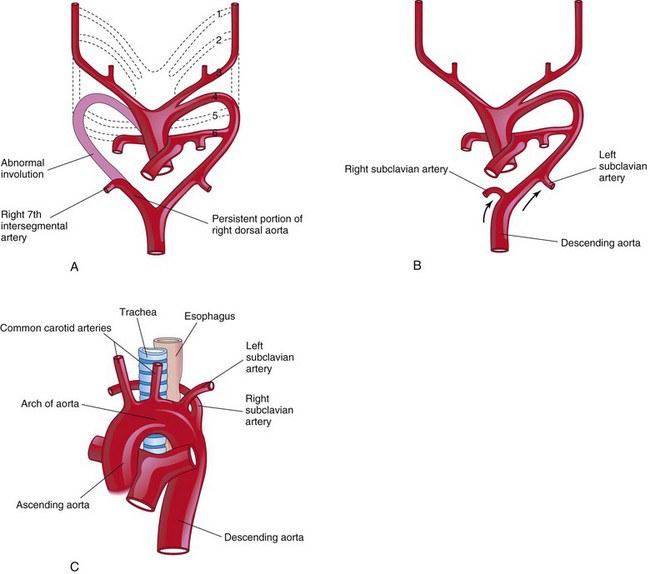
FIGURE 13–44 Sketches illustrating the possible embryologic basis of abnormal origin of the right subclavian artery. A, The right fourth pharyngeal arch artery and the cranial part of the right dorsal aorta have involuted. As a result, the right subclavian artery forms from the right seventh intersegmental artery and the distal segment of the right dorsal aorta. B, As the arch of the aorta forms, the right subclavian artery is carried cranially (arrows) with the left subclavian artery. C, The abnormal right subclavian artery arises from the aorta and passes posterior to the trachea and esophagus.
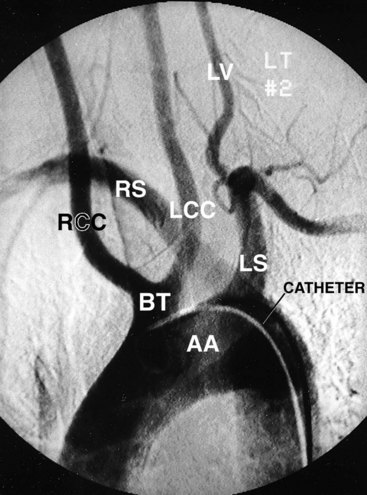
FIGURE 13–45 Abnormal origin of right subclavian artery. This left anterior oblique view of an aortic arch arteriogram shows both common carotid arteries arising from a common stem of the arch of the aorta (BT). The origin of the right subclavian artery (RS) is distal to the separate origin of the left subclavian artery (LS), but is superimposed in this view. The right subclavian artery then courses cranially and to the right, posterior to the esophagus and trachea. AA, Arch of aorta; BT, brachiocephalic trunk; RCC, right common carotid artery; LCC, left common carotid artery; LV, left vertebral artery.
(Courtesy of Dr. Gerald S. Smyser, Altru Health System, Grand Forks, ND.)
Although an anomalous right subclavian artery is fairly common and always forms a vascular ring, it is rarely clinically significant because the ring is usually not tight enough to constrict the esophagus and trachea very much.
 Fetal And Neonatal Circulation
Fetal And Neonatal Circulation
The fetal cardiovascular system (Fig. 13-46) is designed to serve prenatal needs and permit modifications at birth that establish the neonatal circulatory pattern (Fig. 13-47). Good respiration in a neonate is dependent on normal circulatory changes occurring at birth, which result in oxygenation of the blood occurring in the lungs when fetal blood flow through the placenta ceases. Prenatally, the lungs do not provide gas exchange and the pulmonary vessels are vasoconstricted. The three vascular structures most important in the transitional circulation are the DV, oval foramen, and DA.
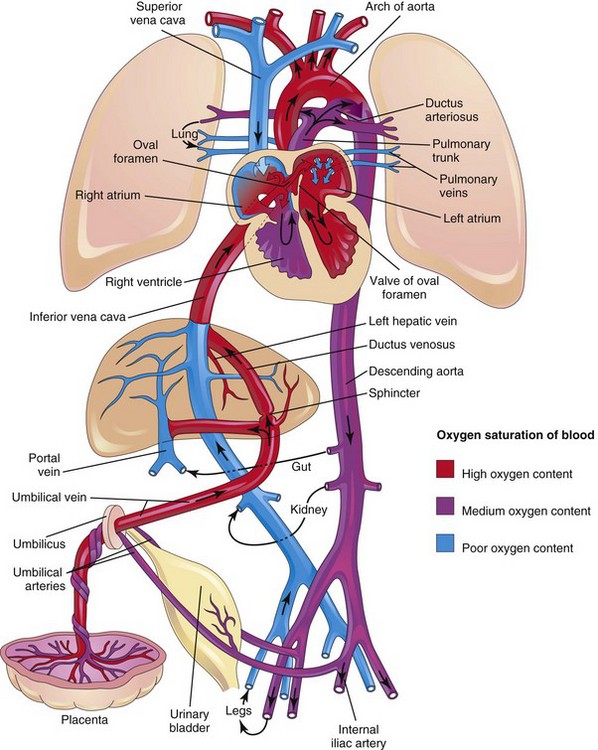
FIGURE 13–46 Fetal circulation. The colors indicate the oxygen saturation of the blood, and the arrows show the course of the blood from the placenta to the heart. The organs are not drawn to scale. A small amount of highly oxygenated blood from the inferior vena cava remains in the right atrium and mixes with poorly oxygenated blood from the superior vena cava. The medium oxygenated blood then passes into the right ventricle. Observe that three shunts permit most of the blood to bypass the liver and lungs: (1) ductus venosus, (2) oval foramen, and (3) ductus arteriosus. The poorly oxygenated blood returns to the placenta for oxygenation and nutrients through the umbilical arteries.
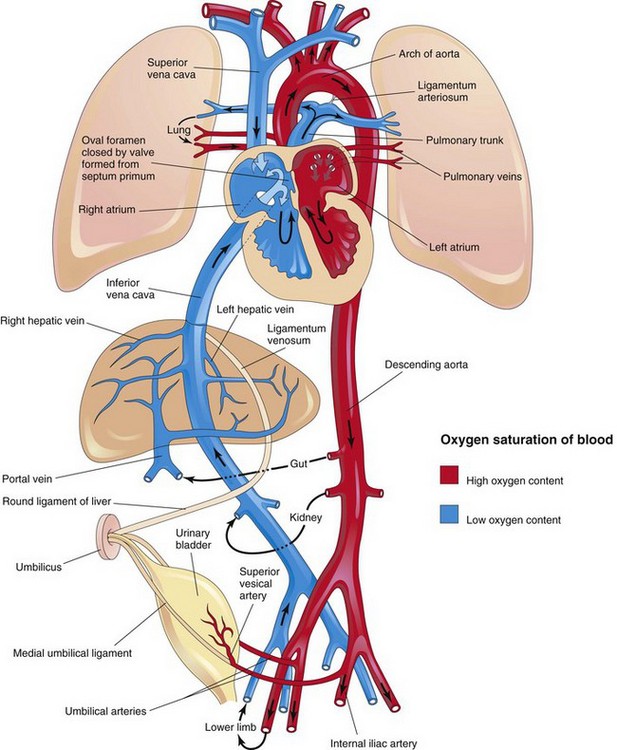
FIGURE 13–47 Neonatal circulation. The adult derivatives of the fetal vessels and structures that become nonfunctional at birth are shown. The arrows indicate the course of the blood in the infant. The organs are not drawn to scale. After birth, the three shunts that shortcircuited the blood during fetal life cease to function, and the pulmonary and systemic circulations become separated.
Fetal Circulation
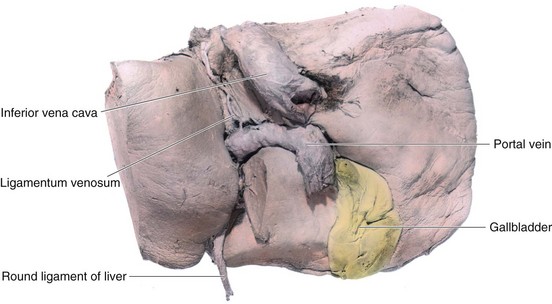
Highly oxygenated, nutrient-rich blood returns under high pressure from the placenta in the umbilical vein (Fig. 13-46). On approaching the liver, approximately half of the blood passes directly into the DV, a fetal vessel connecting the umbilical vein to the IVC (Figs. 13-48 and 13-49); consequently, this blood bypasses the liver. The other half of the blood in the umbilical vein flows into the sinusoids of the liver and enters the IVC through the hepatic veins.
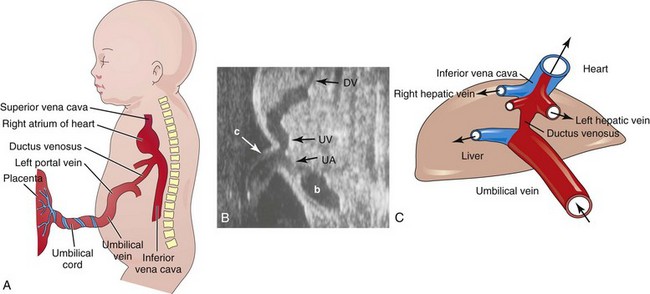
FIGURE 13–48 A, Schematic illustration of the course of the umbilical vein from the umbilical cord to the liver. B, Ultrasound scan showing the umbilical cord and the course of its vessels in the embryo, b, bladder; c, umbilical cord; DV, ductus venosus; UV, umbilical vein; UA, umbilical artery. C, Schematic presentation of the relationship among the ductus venosus, umbilical vein, hepatic veins, and inferior vena cava. The oxygenated blood is coded with red.
(B, From Goldstein RB: Ultrasound evaluation of the fetal abdomen. In Callen PW [ed]: Ultrasonography in Obstetrics and Gynecology, 3rd ed. Philadelphia, WB Saunders, 1996. C, From Tekay A, Campbell S: Doppler ultrasonography in obstetrics. In Callen PW [ed]: Ultrasonography in Obstetrics and Gynecology, 4th ed. Philadelphia, WB Saunders, 2000.)
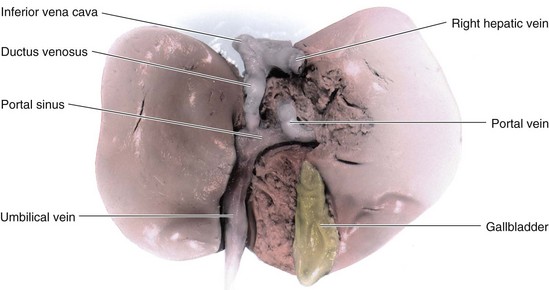
FIGURE 13–49 Dissection of the visceral surface of the fetal liver. Approximately 50% of umbilical venous blood bypasses the liver and joins the inferior vena cava through the ductus venosus.
Blood flow through the DV is regulated by a sphincter mechanism close to the umbilical vein. When the sphincter contracts, more blood is diverted to the portal vein and hepatic sinusoids and less to the DV (Fig. 13-49). Although an anatomic sphincter in the DV has been described, its presence is not universally accepted. However, it is generally agreed that there is a physiologic sphincter that prevents overloading of the heart when venous flow in the umbilical vein is high (e.g., during uterine contractions).
After a short course in the IVC, the blood enters the right atrium of the heart. Because the IVC also contains poorly oxygenated blood from the lower limbs, abdomen, and pelvis, the blood entering the right atrium is not as well oxygenated as that in the umbilical vein, but it still has a high oxygen content (Fig. 13-46). Most blood from the IVC is directed by the crista dividens (inferior border of the septum secundum), through the oval foramen into the left atrium (Fig. 13-50). Here it mixes with the relatively small amount of poorly oxygenated blood returning from the lungs through the pulmonary veins. The fetal lungs use oxygen from the blood instead of replenishing it. From the left atrium, the blood then passes to the left ventricle and leaves through the ascending aorta.
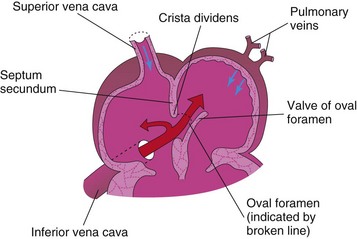
FIGURE 13–50 Schematic diagram of blood flow through the fetal atria illustrating how the crista dividens (lower edge of septum secundum) separates the blood coming from the inferior vena cava into two streams. The larger stream passes through the oval foramen into the left atrium, where it mixes with the small amount of poorly oxygenated blood coming from the lungs through the pulmonary veins. The smaller stream of blood from the inferior vena cava remains in the right atrium and mixes with poorly oxygenated blood from the superior vena cava and coronary sinus.
The arteries to the heart, neck, head, and upper limbs receive well-oxygenated blood from the ascending aorta. The liver also receives well-oxygenated blood from the umbilical vein (Figs. 13-48 and 13-49). The small amount of well-oxygenated blood from the IVC in the right atrium that does not enter the oval foramen mixes with poorly oxygenated blood from the SVC and coronary sinus and passes into the right ventricle. This blood, with a medium oxygen content, leaves through the pulmonary trunk.
Approximately 10% of this blood flow goes to the lungs; most blood passes through the ductus arteriosus (DA) into the descending aorta to the fetal body and returns to the placenta through the umbilical arteries (Fig. 13-46). The DA protects the lungs from circulatory overloading and allows the right ventricle to strengthen in preparation for functioning at full capacity at birth. Because of the high pulmonary vascular resistance in fetal life, pulmonary blood flow is low. Approximately 10% of blood from the ascending aorta enters the descending aorta; 65% of the blood in the descending aorta passes into the umbilical arteries and is returned to the placenta for reoxygenation. The remaining 35% of the blood in the descending aorta supplies the viscera and the inferior part of the body.
Transitional Neonatal Circulation
Important circulatory adjustments occur at birth when the circulation of fetal blood through the placenta ceases and the infant’s lungs expand and begin to function (Fig. 13-47).
As soon as the baby is born, the oval foramen, DA, DV, and umbilical vessels are no longer needed. The sphincter in the DV constricts, so that all blood entering the liver passes through the hepatic sinusoids. Occlusion of the placental circulation causes an immediate decrease in blood pressure in the IVC and right atrium.
Aeration of the lungs at birth is associated with a:
The thinning of the arterial walls results mainly from stretching the lungs at birth.
Because of increased pulmonary blood flow and loss of flow from the umbilical vein, the pressure in the left atrium is higher than in the right atrium. The increased left atrial pressure functionally closes the oval foramen by pressing the valve of the oval foramen against the septum secundum (Fig. 13-47). The output from the right ventricle now flows into the pulmonary trunk. Because pulmonary vascular resistance is lower than the systemic vascular resistance, blood flow in the DA reverses, passing from the descending aorta to the pulmonary trunk.
The right ventricular wall is thicker than the left ventricular wall in fetuses and newborn infants because the right ventricle has been working harder in utero. By the end of the first month, the left ventricular wall thickness is greater than the right because the left ventricle is now working harder. The right ventricular wall becomes thinner because of the atrophy associated with its lighter workload.
The DA constricts at birth but there is often a small shunt of blood via the DA from the aorta to the pulmonary trunk for 24 to 48 hours in a normal full-term infant. At the end of 24 hours, 20% of ducts are functionally closed, about 80% by 48 hours, and 100% at 96 hours. In premature infants and in those with persistent hypoxia, the DA may remain open much longer. In full-term infants, oxygen is the most important factor in controlling closure of the DA and appears to be mediated by bradykinin, a substance released from the lungs during their initial inflation. Bradykinin has potent contractile effects on smooth muscle. The action of this substance appears to be dependent on the high oxygen content of the blood in the aorta resulting from aeration of the lungs at birth. When the pO2 of the blood passing through the DA reaches approximately 50 mm Hg, the wall of the ductus constricts. The mechanisms by which oxygen causes ductal constriction are not well understood.
The effects of oxygen on the ductal smooth muscle may be direct or may be mediated by its effects on prostaglandin E2 (PGE2) secretion. Transforming growth factor β (TGF-β) is probably involved in the anatomic closure of the DA after birth. During fetal life, the patency of the DA before birth is controlled by the lower content of oxygen in the blood passing through it and by endogenously produced prostaglandins (PGs) that act on the smooth muscle in the wall of the DA. The PGs cause the DA to relax. Hypoxia and other ill-defined influences cause the local production of PGE2 and prostacyclin (PGI2), which keep the DA open. Inhibitors of PG synthesis, such as indomethacin, can cause constriction of a patent DA (PDA) in premature infants.
The umbilical arteries constrict at birth, preventing loss of the neonate’s blood. Because the umbilical cord is not tied for a minute or so, blood flow through the umbilical vein continues, transferring well-oxygenated fetal blood from the placenta to the infant. The change from the fetal to the adult pattern of blood circulation is not a sudden occurrence. Some changes occur with the first breath; others take place over hours and days. During the transitional stage, there may be a right-to-left flow through the oval foramen. The closure of fetal vessels and the oval foramen is initially a functional change. Later, anatomic closure results from proliferation of endothelial and fibrous tissues.
 Derivatives of Fetal Vessels and Structures
Derivatives of Fetal Vessels and Structures
Because of the changes in the cardiovascular system at birth, some vessels and structures are no longer required. Over a period of months, these fetal vessels form nonfunctional ligaments. Fetal structures, such as the oval foramen, persist as anatomic vestiges (e.g., oval fossa; see Fig. 13-52).
Umbilical Vein and Round Ligament of Liver
The umbilical vein remains patent for a considerable period and may be used for exchange transfusions of blood during early infancy. These transfusions are often done to prevent brain damage and death in infants with anemia from erythroblastosis fetalis (a grave hemolytic anemia). In exchange transfusions, most of the infant’s blood is replaced with donor blood. The lumen of the umbilical vein usually does not disappear completely; in these people, the round ligament can be cannulated, if necessary, for the injection of contrast media or chemotherapeutic drugs.
The intra-abdominal part of the umbilical vein eventually becomes the round ligament of liver (ligamentum teres) (Fig. 13-47), which passes from the umbilicus to the porta hepatis; here it is attached to the left branch of the portal vein (Fig. 13-51).
Ductus Venosus and Ligamentum Venosum
The ductus venosus (DV) becomes the ligamentum venosum. This ligament passes through the liver from the left branch of the portal vein and attaches to the IVC (Fig. 13-51).
Umbilical Arteries and Abdominal Ligaments
Most of the intra-abdominal parts of the umbilical arteries become medial umbilical ligaments (Fig. 13-47); the proximal parts of these vessels persist as the superior vesical arteries, which supply the urinary bladder.
Oval Foramen and Oval Fossa
The oval foramen usually closes functionally at birth. Anatomic closure occurs by the third month and results from tissue proliferation and adhesion of the septum primum to the left margin of the septum secundum. The septum primum forms the floor of the oval fossa (Fig. 13-52). The inferior edge of the septum secundum forms a rounded fold, the border of the oval fossa (limbus fossae ovalis), which marks the former boundary of the oval foramen.
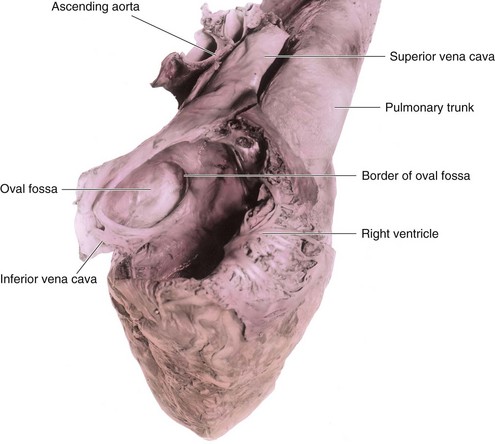
FIGURE 13–52 Dissection of the right atrial aspect of the interatrial septum of an adult heart. Observe the oval fossa and the border of the oval fossa. The floor of the oval fossa is formed by the septum primum, whereas the border of the fossa is formed by the free edge of the septum secundum. Aeration of the lungs at birth is associated with a dramatic decrease in pulmonary vascular resistance and a marked increase in pulmonary flow. Because of the increased pulmonary blood flow, the pressure in the left atrium is increased above that in the right atrium. This increased left atrial pressure closes the oval foramen by pressing the valve of the oval foramen against the septum secundum. This forms the oval fossa.
Ductus Arteriosus and Ligamentum Arteriosum
Functional closure of the ductus arteriosus (DA) in healthy term neonates is usually completed within the first few days after birth (Fig. 13-53A). Anatomic closure of the DA and formation of the ligamentum arteriosum normally occurs by the 12th postnatal week (Fig. 13-53C). The short, thick ligamentum arteriosum extends from the left pulmonary artery to the arch of the aorta.
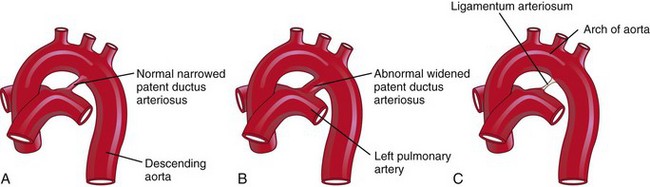
FIGURE 13–53 Closure of the ductus arteriosus (DA). A, The DA of a neonate. B, Abnormal patent DA in a 6-month-old infant. C, The ligamentum arteriosum in a 6-month-old infant.
Patent Ductus Arteriosus
Patent ductus arteriosus (PDA), a common birth defect, is two to three times more frequent in females than in males (Fig. 13-53B). Functional closure of the DA usually occurs soon after birth; however, if it remains patent, aortic blood is shunted into the pulmonary trunk. It has been suggested that persistent patency of the DA may result from failure of TGF-β induction after birth.
PDA is a common birth defect that is associated with maternal rubella infection during early pregnancy (see Chapter 20). Preterm infants and infants born at high altitude may have a PDA; the patency is the result of hypoxia and immaturity. Virtually all preterm infants (≤28 weeks) whose birth weight is less than 1750 g have a PDA in the first 24 hours of postnatal life.
The embryologic basis of PDA is failure of the DA to involute after birth and form the ligamentum arteriosum. Failure of contraction of the muscular wall of the DA after birth is the primary cause of patency. There is some evidence that low oxygen content of the blood in newborn infants with respiratory distress syndrome can adversely affect closure of the DA. For example, PDA commonly occurs in small premature infants with respiratory difficulties associated with a deficiency of surfactant (a phospholipid that reduces surface tension in alveoli in the lungs).
PDA may occur as an isolated anomaly or in infants with certain chromosomal anomalies or cardiac defects. Large differences between aortic and pulmonary blood pressures can cause a heavy flow of blood through the DA, thereby preventing normal constriction. Such pressure differences may be caused by coarctation of the aorta (Fig. 13-41C), transposition of the great arteries (TGA) (Fig. 13-32), or pulmonary stenosis and atresia (Fig. 13-34).
Development Of Lymphatic System
The lymphatic system begins to develop at the end of the sixth week, approximately 2 weeks after the primordia of the cardiovascular system are recognizable. Lymphatic vessels develop in a manner similar to that previously described for blood vessels (see Chapter 4), and make connections with the venous system. The early lymphatic capillaries join each other to form a network of lymphatics (Fig. 13-54A).
FIGURE 13–54 Development of the lymphatic system. A, Left side of a 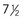 -week embryo showing the primary lymph sacs. B, Ventral view of the lymphatic system at 9 weeks showing the paired thoracic ducts. C, Later in the fetal period, illustrating formation of the thoracic duct and right lymphatic duct.
-week embryo showing the primary lymph sacs. B, Ventral view of the lymphatic system at 9 weeks showing the paired thoracic ducts. C, Later in the fetal period, illustrating formation of the thoracic duct and right lymphatic duct.
Development of Lymph Sacs and Lymphatic Ducts
There are six primary lymph sacs present at the end of the embryonic period (Fig. 13-54A):
Lymphatic vessels soon connect to the lymph sacs and pass along main veins: to the head, neck, and upper limbs from the jugular lymph sacs; to the lower trunk and lower limbs from the iliac lymph sacs; and to the primordial gut from the retroperitoneal lymph sac and the cisterna chyli. Two large channels (right and left thoracic ducts) connect the jugular lymph sacs with this cistern. Soon a large anastomosis forms between these channels (Fig. 13-54B).
Development of Thoracic Duct
The thoracic duct develops from the caudal part of the right thoracic duct, the anastomosis between the left and right thoracic ducts, and the cranial part of the left thoracic duct. As a result, there are many variations in the origin, course, and termination of the thoracic duct. The right lymphatic duct is derived from the cranial part of the right thoracic duct (Fig. 13-54C). The thoracic duct and right lymphatic duct connect with the venous system at the venous angle between the internal jugular and subclavian veins (Fig. 13-54B).
Development of Lymph Nodes
Except for the superior part of the cisterna chyli, the lymph sacs are transformed into groups of lymph nodes during the early fetal period. Mesenchymal cells invade each lymph sac and break up its cavity into a network of lymphatic channels—the primordia of the lymph sinuses. Other mesenchymal cells give rise to the capsule and connective tissue framework of the lymph nodes.
Development of Lymphocytes
The lymphocytes are derived originally from stem cells in the umbilical vesicle mesenchyme and later from the liver and spleen. These early lymphocytes eventually enter the bone marrow, where they divide to form lymphoblasts. The lymphocytes that appear in lymph nodes before birth are derived from the thymus, a derivative of the third pair of pharyngeal pouches (see Chapter 9). Small lymphocytes leave the thymus and circulate to other lymphoid organs. Later, some mesenchymal cells in the lymph nodes also differentiate into lymphocytes. Lymph nodules do not appear in the lymph nodes until just before and/or after birth.
Development of Spleen and Tonsils
The spleen develops from an aggregation of mesenchymal cells in the dorsal mesogastrium (see Chapter 11). The palatine tonsils develop from the second pair of pharyngeal pouches and nearby mesenchyme. The tubal tonsils develop from aggregations of lymph nodules around the pharyngeal openings of the pharyngotympanic tubes. The pharyngeal tonsils develop from an aggregation of lymph nodules in the wall of the nasopharynx. The lingual tonsil develops from an aggregation of lymph nodules in the root of the tongue. Lymph nodules also develop in the mucosa of the respiratory and alimentary systems.
Anomalies of Lymphatic System
Congenital anomalies of the lymphatic system are rare. There may be diffuse swelling of a part of the body—congenital lymphedema. This condition may result from dilation of primordial lymphatic channels, or from congenital hypoplasia of lymphatic vessels. More rarely, diffuse cystic dilation of lymphatic channels involves widespread portions of the body.
In cystic hygroma, large swellings usually appear in the inferolateral part of the neck and consist of large single or multilocular, fluid-filled cavities (Fig. 13-55). Hygromas may be present at birth, but they often enlarge and become evident during infancy, especially after infection or hemorrhage. Most hygromas appear to be derived from abnormal transformation of the jugular lymph sacs. Hygromas are believed to arise from parts of a jugular lymph sac that are pinched off or from lymphatic spaces that fail to establish connections with the main lymphatic channels. Hygromas diagnosed in utero in the first trimester are associated with chromosomal abnormalities in about 50% of cases. Fetal outcome in these cases is poor.
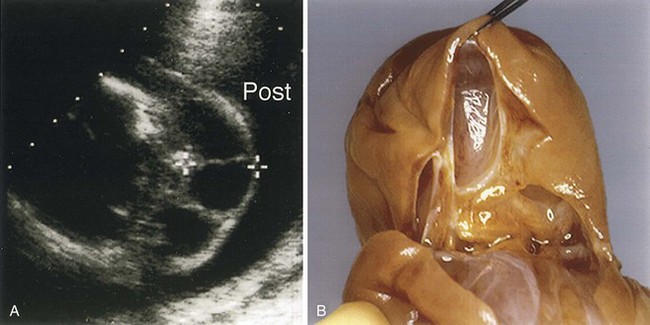
FIGURE 13–55 Cystic hygroma. A, Transverse axial sonogram of the neck of a fetus with a large cystic hygroma. B, Photograph of a neck dissection. Cystic hygroma was demonstrated from this cross-sectional view of the posterior fetal neck at 18.5 weeks. The lesion was characterized by multiple, septated cystic areas within the mass itself as shown in the pathology specimen. Post, posterior.
(Courtesy of Dr. Wesley Lee, Division of Fetal Imaging, William Beaumont Hospital, Royal Oak, Michigan.)
 Summary Of Cardiovascular System
Summary Of Cardiovascular System
Clinically Oriented Problems
Case 13–1
A pediatrician detected a congenital cardiac defect in an infant, and he explained to the baby’s mother that this is a common birth defect.
Case 13–2
A female infant was born normally after a pregnancy complicated by a rubella infection during the first trimester of pregnancy. She had congenital cataracts and congenital heart disease. A radiograph of the infant’s chest at 3 weeks showed generalized cardiac enlargement with some increase in pulmonary vascularity.
Case 13–3
A male neonate was referred to a pediatrician because of the blue color of his skin (cyanosis). An ultrasound examination was ordered to confirm the preliminary diagnosis of tetralogy of Fallot.
Case 13–4
A male neonate was born after a full-term normal pregnancy. Severe generalized cyanosis was observed on the first day. A chest radiograph revealed a slightly enlarged heart with a narrow base and increased pulmonary vascularity. A clinical diagnosis of transformation of the great arteries (TGA) was made.
Case 13–5
During an autopsy of a 72-year-old man who died from chronic heart failure, it was observed that his heart was very large and that the pulmonary artery and its main branches were dilated. Opening the heart revealed a very large atrial septal defect (ASD).
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Adams SM, Good MW, De Franco GM. Sudden infant death syndrome. Am Fam Physician. 2009;79:870.
Anderson RH, Brown NA, Moorman AFM. Development and structures of the venous pole of the heart. Dev Dyn. 2006;235:2.
Bajolle F, Zaffran S, Bonnet D. Genetics and embryological mechanisms of congenital heart disease. Arch Cardiovasc Dis. 2009;102:59.
Baschat AA. Examination of the fetal cardiovascular system. Semin Fetal Neonatal Med. 2011;16:2.
Bentham J, Bhattacharya S. Genetic mechanisms controlling cardiovascular development. Ann N Y Acad Sci. 2008;1123:10.
Bernstein E. The cardiovascular system. In Behrman RE, Kliegman RM, Jenson HB, editors: Nelson Textbook of Pediatrics, ed 17, Philadelphia: WB Saunders, 2004.
Camp E, Munsterberg A. Ingression, migration and early differentiation of cardiac progenitors. Front Biosci. 2011;17:2416.
Chappell JC, Bautch VL. Vascular development:genetic mechanisms and links to vascular disease. Curr Top Dev Biol. 2010;90:43.
Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408.
Conte G, Pellegrini A. On the development of the coronary arteries in human embryos, stages 13–19. Anat Embryol. 1984;169:209.
Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Develop Dyn. 2009;336:137.
Hildreth V, Anderson RH, Henderson DJH. Autonomic innervations of the developing heart. Origins and function. Clin Anat. 2009;22:36.
Gessert S, Kuhl M. The multiple phases and faces of Wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186.
Gloviczki P, Duncan A, Kaira M, et al. Vascular malformations: an update. Perspect Vasc Surg Endovasc Ther. 2009;21:133.
Harvey RP, Meilhac SM, Buckingham M. Landmarks and lineages in the developing heart. Circ Res. 2009;104:1235.
Horsthuis T, Christoffels VM, Anderson RH, et al. Can recent insights into cardiac development improve our understanding of congenitally malformed heart. Clin Anat. 2009;22:4.
Jones PN, Showengerdt KOJr. Prenatal diagnosis of congenital heart disease. Pediatr Clin North Am. 2009;56:709.
Kamedia Y. Hoxa3 and signaling molecules involved in aortic arch patterning and remodeling. Cell Tissue Res. 2010;336:165.
Loukas M, Groat C, Khangura R, et al. Cardiac veins: a review of the literature. Clin Anat. 2009;22:129.
Loukas M, Bilinsky C, Bilinski E, et al. The normal and abnormal anatomy of the coronary arteries. Clin Anat. 2009;22:114.
Männer J. The anatomy of cardiac looping: A step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin Anat. 2009;22:21.
Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Moorman AFM, Brown N, Anderson RH. Embryology of the heart. In Anderson RH, Baker EJ, Penny DJ, et al, editors: Pediatric Cardiology, ed 3, Philadelphia: Elsevier, 2009.
Nemer M. Genetic insights into normal and abnormal heart development. Cardiovasc Pathol. 2008;17:48.
O’Rahilly R. The timing and sequence of events in human cardiogenesis. Acta Anat. 1971;79:70.
Penny DJ, Vick GW. Ventricular septal defect. Lancet. 2011;377:1103.
Pierpont MEM, Markwald RR, Lin AE. Genetic aspects of atrioventricular septal defects. Am J Med Genet. 2000;97:289-296.
Solloway M, Harvey RP. Molecular pathways in myocardial development: a stem cell perspective. Cardiovasc Res. 2006;58:264.
Srivastava D. Genetic regulation of cardiogenesis and congenital heart disease. Ann Rev Pathol. 2006;1:199.
Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1.
Watanabe M, Schaefer KS. Cardiac embryology. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Yoo S-J, Jaeggi E. Ultrasound evaluation of the fetal heart. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Zavos PM. Stem cells and cellular therapy: Potential treatment for cardiovascular diseases. Int J Cardiol. 2006;107:1.