Chapter 16 Development of Limbs
 Early Stages of Limb Development
Early Stages of Limb Development
Development of the limbs begins near the end of the fourth week (Fig. 16-1A) with the activation of a group of mesenchymal cells in the somatic lateral mesoderm. Homeobox genes regulate patterning in the formation of the limbs. The limb buds form deep to a thick band of ectoderm, the apical ectodermal ridge (AER). The buds first appear as small bulges on the ventrolateral body wall (Figs. 16-1A and 16-2). The upper limb buds are visible by day 24 and the lower limb buds appear 1 or 2 days later. Each limb bud consists of a mesenchymal core of mesoderm covered by a layer of ectoderm.
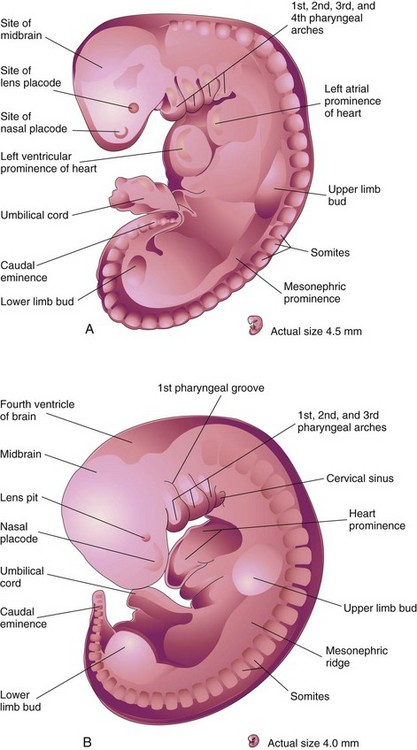
FIGURE 16–1 Drawings of human embryos showing development of the limbs. A, Lateral view of an embryo at approximately 28 days. The upper limb bud appears as a swelling or bulge on the ventrolateral body wall. The lower limb bud is smaller than the upper limb bud. B, Lateral view of an embryo at approximately 32 days. The upper limb buds are paddle-shaped and the lower limb buds are flipper-like.
(Modified from Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
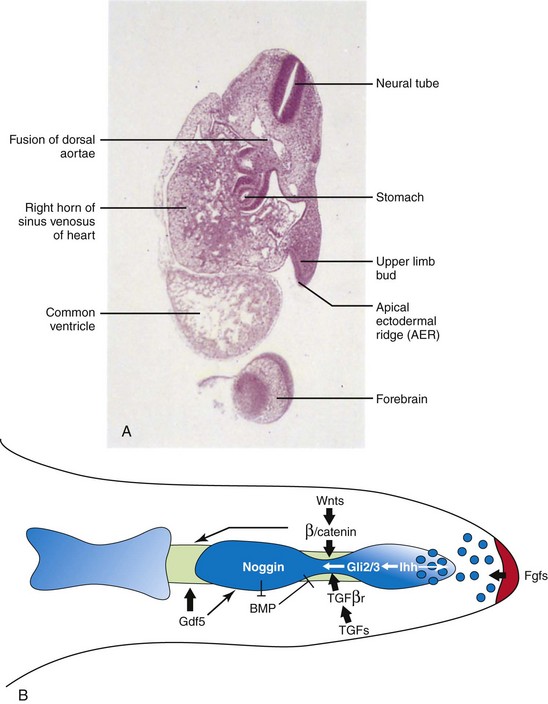
FIGURE 16–2 A, Oblique section of an embryo at approximately 28 days. Observe the paddle-like upper limb bud lateral to the embryonic heart and AER. B, Signaling pathways regulating the elongation and segmentation of the digit ray. AER-Fgf signal (red ) maintains a small population of subridge undifferentiated mesenchymal cells, which are being actively incorporated into the digital condensation (blue). At the presumptive joint site, the newly differentiated chondrogenic cells de-differentiate into the interzone fate under the regulation of multiple signaling pathways. Wnts promote chondrocyte dedifferentiation through canonical Wnt signaling. Ihh signals to the interzone region through the localized Gli2/Gli3 expression. TGFs signals to the interzone cells through the type II receptor. Gdf5 regulates the progression of the joint and skeletogenesis of the digit elements.
(A, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000. B, Reprinted from Journal of Genetics and Genomics, 35, Hu J, He L: Patterning mechanisms controlling digit development, 517-524, Copyright 2008, with permission from Elsevier.)
The limb buds elongate by proliferation of the mesenchyme. The upper limb buds appear disproportionately low on the embryo’s trunk because of the early development of the cranial half of the embryo. The earliest stages of limb development are alike for the upper and lower limbs (Figs. 16-1B and see Figure 16-4). Because of their form and function, there are distinct differences between the development of the hands and feet.
The upper limb buds develop opposite the caudal cervical segments, and the lower limb buds form opposite the lumbar and upper sacral segments. At the apex of each limb bud, the ectoderm thickens to form an apical ectodermal ridge. This ridge is a multilayered epithelial structure (Fig. 16-2) that is induced by the paracrine factor FGF-10 from the underlying mesenchyme. Bone morphogenetic protein signaling is required for its formation.
The AER secretes FGF-8, which exerts an inductive influence on the limb mesenchyme that initiates growth and development of the limbs in a proximodistal axis. Retinoic acid promotes formation of the limb bud by inhibiting FGF signaling. Mesenchymal cells aggregate at the posterior margin of the limb bud to form the zone of polarizing activity, an important signaling center in limb development. Fibroblast growth factors from the AER activate the zone of polarizing activity, which causes expression of the sonic hedgehog (Shh) genes. Shh secretions (morphogens) control the normal patterning of the limbs along the anterior-posterior axis. Expression of Wnt7a from the dorsal non-AER ectoderm of the limb bud and engrailed-1 (EN-1) from the ventral aspect are involved in specifying the dorsal-ventral axis. The AER itself is maintained by inductive signals from Shh and Wnt7. It has been suggested that epiprofin, a zinc-finger transcription factor, regulates Wnt signaling in the limb bud (see Fig. 16-2B).
The mesenchyme adjacent to the AER consists of undifferentiated, rapidly proliferating cells, whereas mesenchymal cells proximal to it differentiate into blood vessels and cartilage bone models. The distal ends of the limb buds flatten into paddle-like hand plates and flipper-like foot plates (Fig. 16-3). Studies have shown that endogenous retinoic acid is also involved in limb development and pattern formation.
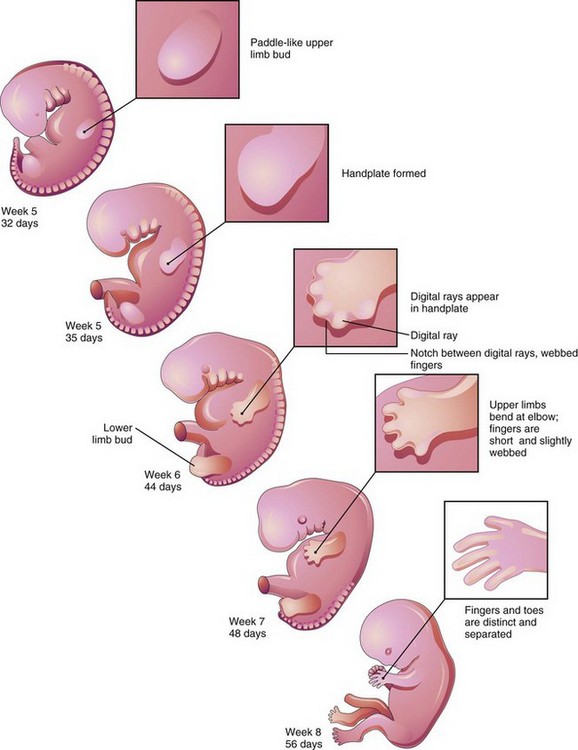
FIGURE 16–3 Illustrations of development of the limbs (32–56 days). The upper limbs develop earlier than the lower limbs.
By the end of the sixth week, mesenchymal tissue in the hand plates has condensed to form digital rays (Figs. 16-3 and 16-4A to C). These mesenchymal condensations outline the pattern of the digits (fingers) in the hand plates. During the seventh week, similar condensations of mesenchyme condense to form digital rays and digits (toes) in the foot plates (Fig. 16-4G to I).
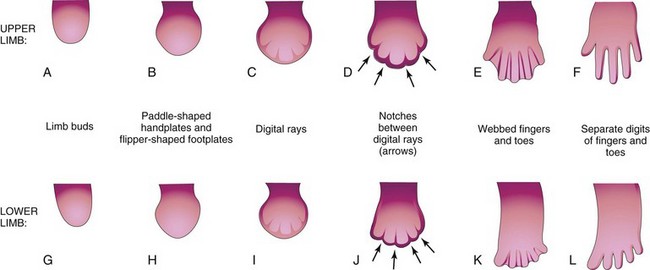
FIGURE 16–4 Illustrations of development of the hands and feet between the fourth and eighth weeks. The early stages of limb development are alike, except that development of the hands precedes that of the feet by a day or two. A, At 27 days. B, At 32 days. C, At 41 days. D, At 46 days. E, At 50 days. F, At 52 days. G, At 28 days. H, At 36 days. I, At 46 days. J, At 49 days. K, At 52 days. L, At 56 days. The arrows in D and J indicate the tissue breakdown process (apoptosis) that separates the fingers and toes.
At the tip of each digital ray, a part of the AER induces development of the mesenchyme into the mesenchymal primordia of the bones (phalanges) in the digits (see Fig. 16-6C and D). The intervals between the digital rays are occupied by loose mesenchyme. Soon the intervening regions of mesenchyme break down, forming notches between the digital rays (Figs. 16-3, 16-4D and F, and 16-5A to D). As the tissue breakdown progresses, separate digits (fingers and toes) are formed by the end of the eighth week (Fig. 16-4E, F, K, and L).
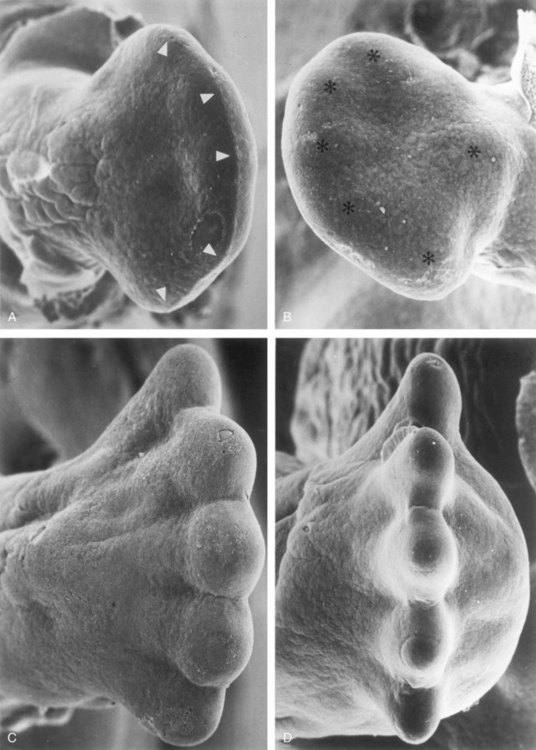
FIGURE 16–5 Scanning electron micrographs showing dorsal (A) and plantar (B) views of the right foot of an embryo at approximately 48 days. The toe buds (arrowheads in A) and the heel cushion and metatarsal tactile elevation (asterisks in B) have just appeared. Dorsal (C) and distal (D) views of the right foot of embryos at approximately 55 days. The tips of the toes are separated and interdigital degeneration has begun. Note the dorsiflexion of the metatarsus and toes (C) as well as the thickened heel cushion (D).
(From Hinrichsen KV, Jacob HJ, Jacob M, et al: Principles of ontogenesis of leg and foot in man. Ann Anat 176:121, 1994.)
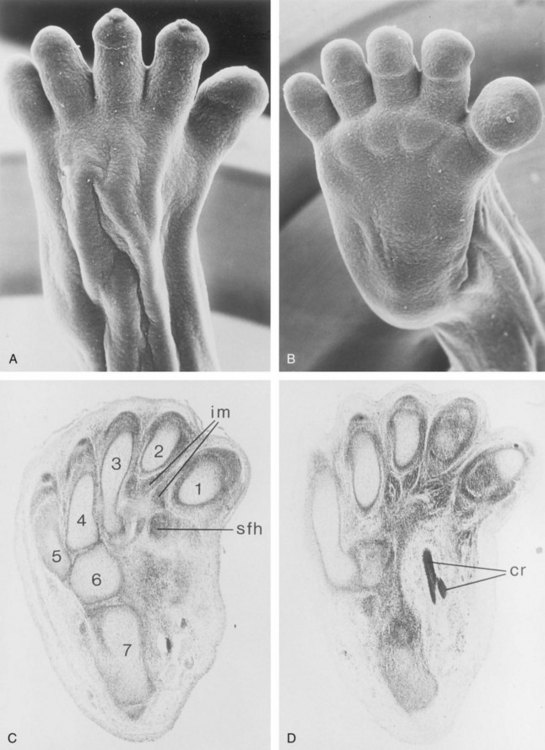
FIGURE 16–6 A and B, Scanning electron micrographs. A, Dorsal view of the right foot of an embryo at 8 weeks. B, Plantar view of the left foot of this embryo. Although supinated, dorsiflexion of the foot is distinct. C and D, Paraffin sections of the tarsus and metatarsus of a young fetus, stained with hematoxylin and eosin: 1–5, metatarsal cartilages; 6, cubital cartilage; 7, calcaneus. The separation of the interosseous muscles (im) and short flexor muscles of the big toe (sfh) is clearly seen. The plantar crossing (cr) of the tendons of the long flexors of the digits and hallux (great toe) is shown in D.
(From Hinrichsen KV, Jacob HJ, Jacob M, et al: Principles of ontogenesis of leg and foot in man. Ann Anat 176:121, 1994.)
Molecular studies indicate that the earliest stages of limb patterning and digit formation involve expression of patched 1 (Ptc 1) gene which is essential for the downstream regulation of the Shh pathway. Programmed cell death (apoptosis) is responsible for the tissue breakdown in the interdigital regions, which is probably mediated by bone morphogenetic proteins (signaling molecules of the transforming growth factor β superfamily). Blocking these cellular and molecular events could account for syndactyly or webbing of the fingers or toes (see Fig. 16-14D).
 Final Stages of Limb Development
Final Stages of Limb Development
As the limbs elongate, mesenchymal models of the bones are formed by cellular aggregations (Fig. 16-7B). Chondrification centers appear in the fifth week. By the end of the sixth week, the entire limb skeleton is cartilaginous (Figs. 16-6A to D and 16-7C and D). Osteogenesis of long bones begins in the seventh week from primary ossification centers in the middle of the cartilaginous models of the long bones. Ossification centers are present in all long bones by the 12th week (see Chapter 14). Ossification of the carpal (wrist) bones begins during the first year after birth.
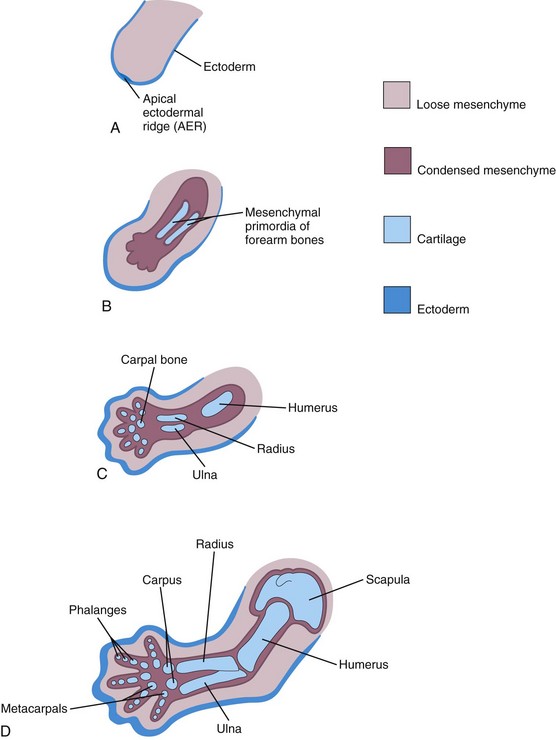
FIGURE 16–7 Schematic longitudinal sections of the upper limb of a human embryo, showing the development of cartilaginous bones. A, At 28 days. B, At 44 days. C, At 48 days. D, At 56 days.
From the dermomyotome regions of the somites, myogenic precursor cells migrate into the limb buds and later differentiate into myoblasts, precursors of muscle cells. The c-met receptor tyrosine kinase plays an essential role in regulating this process. As the long bones form, the myoblasts aggregate and form a large muscle mass in each limb bud (Fig. 16-1). In general, this muscle mass separates into dorsal (extensor) and ventral (flexor) components. The mesenchyme in the limb bud also gives rise to ligaments and blood vessels (Fig. 16-6).
Early in the seventh week, the limbs extend ventrally. Originally the flexor aspect of the limbs is ventral and the extensor aspect dorsal, and the preaxial and postaxial borders are cranial and caudal, respectively (see Fig. 16-10A and D). The developing upper and lower limbs rotate in opposite directions and to different degrees (Figs. 16-8 and 16-9):
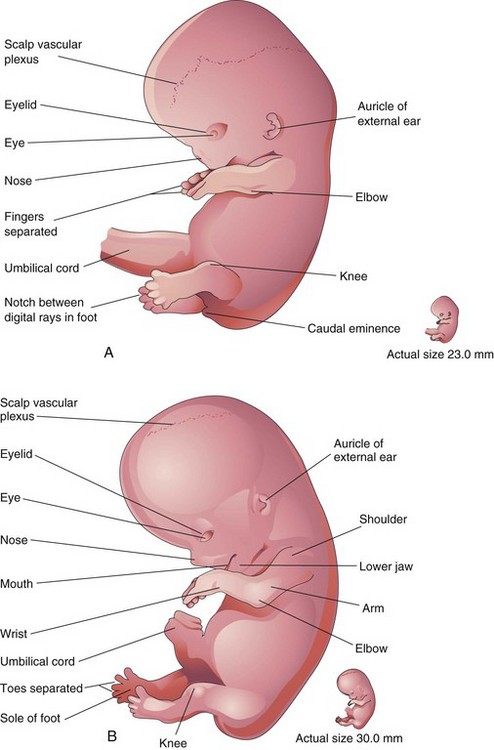
FIGURE 16–8 Drawings of lateral views of embryos. A, At approximately 52 days. The fingers are separated and the toes are beginning to separate. Note that the feet are fan shaped. B, At approximately 56 days. All regions of the limbs are apparent and the digits of the hands and feet are separated.
(Modified from Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Washington, DC, National Institutes of Health, 1977.)
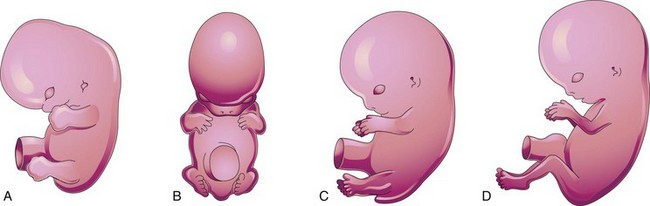
FIGURE 16–9 Illustrations of positional changes of the developing limbs of embryos. A, At approximately 48 days, showing the limbs extending ventrally and the hand plates and foot plates facing each other. B, At approximately 51 days, showing the upper limbs bent at the elbows and the hands curved over the thorax. C, At approximately 54 days, showing the soles of the feet facing medially. D, At approximately 56 days (end of embryonic stage). Note that the elbows now point caudally and the knees cranially.
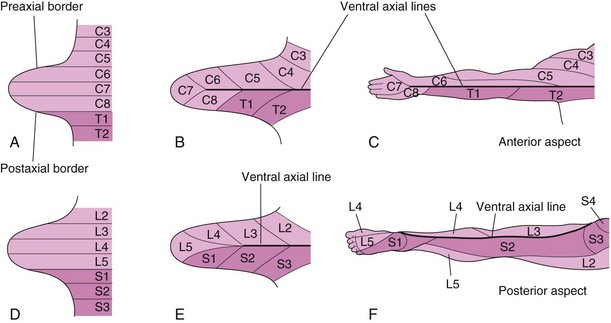
FIGURE 16–10 Illustrations of development of the dermatomal patterns of the limbs. The axial lines indicate where there is no sensory overlap. A and D, Ventral aspect of the limb buds early in the fifth week. At this stage, the dermatomal patterns show the primordial segmental arrangement. B and E, Similar views later in the fifth week showing the modified arrangement of dermatomes. C and F, The dermatomal patterns in the adult upper and lower limbs. The primordial dermatomal pattern has disappeared, but an orderly sequence of dermatomes can still be recognized. F, Note that most of the original ventral surface of the lower limb lies on the back of the adult limb. This results from the medial rotation of the lower limb that occurs toward the end of the embryonic period. In the upper limb, the ventral axial line extends along the anterior surface of the arm and forearm. In the lower limb, the ventral axial line extends along the medial side of the thigh and knee to the posteromedial aspect of the leg to the heel.
Developmentally, the radius and tibia are homologous bones, as are the ulna and fibula, just as the thumb and great toe are homologous digits. Synovial joints appear at the beginning of the fetal period (ninth week), coinciding with functional differentiation of the limb muscles and their innervation.
 Cutaneous Innervation of Limbs
Cutaneous Innervation of Limbs
There is a strong relationship between the growth and rotation of the limbs and the cutaneous segmental nerve supply of the limbs. Motor axons arising from the spinal cord enter the limb buds during the fifth week and grow into the dorsal and ventral muscle masses. Sensory axons enter the limb buds after the motor axons and use them for guidance. Neural crest cells, the precursors of Schwann cells, surround the motor and sensory nerve fibers in the limbs and form the neurolemma (sheath of Schwann) and myelin sheaths (see Chapter 17).
During the fifth week, peripheral nerves grow from the developing limb plexuses (brachial and lumbosacral) into the mesenchyme of the limbs (Fig. 16-10B and E). The spinal nerves are distributed in segmental bands, supplying both dorsal and ventral surfaces of the limbs. A dermatome is the area of skin supplied by a single spinal nerve and its spinal ganglion; however, cutaneous nerve areas and dermatomes show considerable overlapping. As the limbs elongate, the cutaneous distribution of the spinal nerves migrates along the limbs and no longer reaches the surface in the distal part of the limbs. Although the original dermatomal pattern changes during growth of the limbs, an orderly sequence of distribution can still be recognized in the adult (Fig. 16-10C and F). In the upper limb, observe that the areas supplied by C5 and C6 adjoin the areas supplied by T2, T1, and C8, but the overlap between them is minimal at the ventral axial line.
A cutaneous nerve area is the area of skin supplied by a peripheral nerve. If the dorsal root supplying the area is cut, the dermatomal patterns indicate that there may be a slight deficit in the area indicated. Because there is overlapping of dermatomes, a particular area of skin is not exclusively innervated by a single segmental nerve. The limb dermatomes may be traced progressively down the lateral aspect of the upper limbs and back up its medial aspect. A comparable distribution of dermatomes occurs in the lower limbs, which may be traced down the ventral aspect and then up the dorsal aspect of the lower limbs. As the limbs descend, they carry their nerves with them; this explains the oblique course of the nerves arising from the brachial and lumbosacral plexuses.
 Blood Supply of Limbs
Blood Supply of Limbs
The limb buds are supplied by branches of the intersegmental arteries (Fig. 16-11A), which arise from the dorsal aorta and form a fine capillary network throughout the mesenchyme. The primordial vascular pattern consists of a primary axial artery and its branches (Fig. 16-11B and C), which drain into a peripheral marginal sinus. Blood in the marginal sinus drains into a peripheral vein. The vascular patterns change as the limbs develop, chiefly by angiogenesis (sprouting from existing vessels). The new vessels coalesce with other sprouts to form new vessels.
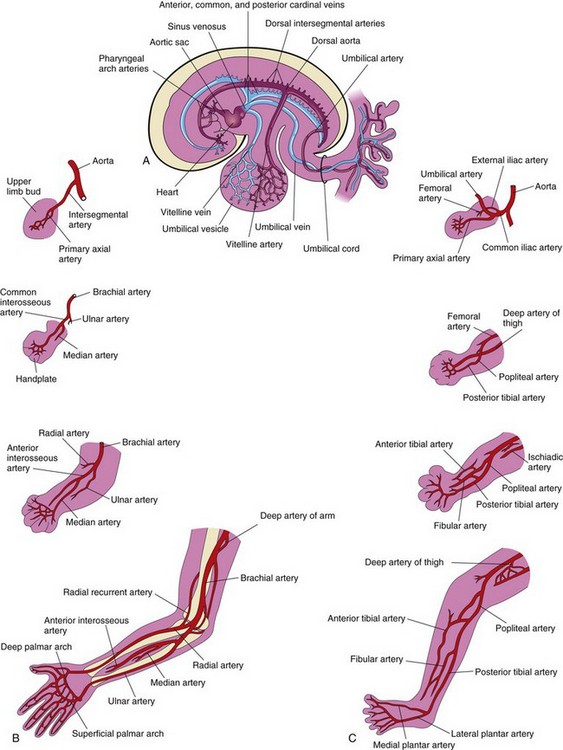
FIGURE 16–11 Development of limb arteries. A, Sketch of the primordial cardiovascular system in an embryo at approximately 26 days. B, Development of arteries in the upper limb. C, Development of arteries in the lower limb.
The primary axial artery becomes the brachial artery in the arm and the common interosseous artery in the forearm (Fig. 16-11B), which has anterior and posterior interosseous branches. The ulnar and radial arteries are terminal branches of the brachial artery. As the digits (fingers) form, the marginal sinus breaks up and the final venous pattern, represented by the basilic and cephalic veins and their tributaries, develops. The primary axial artery becomes the deep artery of the thigh (profunda femoris artery), and the anterior and posterior tibial arteries in the leg.
Birth Defects of Limbs
Minor birth defects are relatively common and can usually be corrected surgically. Although these anomalies are usually of no serious medical consequence, they may serve as indicators of more serious defects, which may be part of a recognizable pattern of birth defects.
The critical period of limb development is from 24 to 36 days after fertilization. This statement is based on clinical studies of infants who were exposed in utero to the drug thalidomide, a potent human teratogen—a factor known to cause a defect—during the embryonic period. Exposure to this teratogen before day 36 may cause severe limb defects, such as amelia, the absence of limbs (Fig. 16-12A). Consequently, a teratogen that may cause amelia or meromelia (partial absence of limbs) must be taken before the end of the critical period of limb development. Many severe limb anomalies occurred from 1957 to 1962 as a result of maternal ingestion of thalidomide. This hypnotic drug, widely used as a sedative and antinauseant, was withdrawn from the market in December 1961. Since that time, similar limb defects have rarely been observed. Because thalidomide is now used for the treatment of leprosy and other disorders, it must be emphasized that thalidomide is absolutely contraindicated in women of child-bearing age.
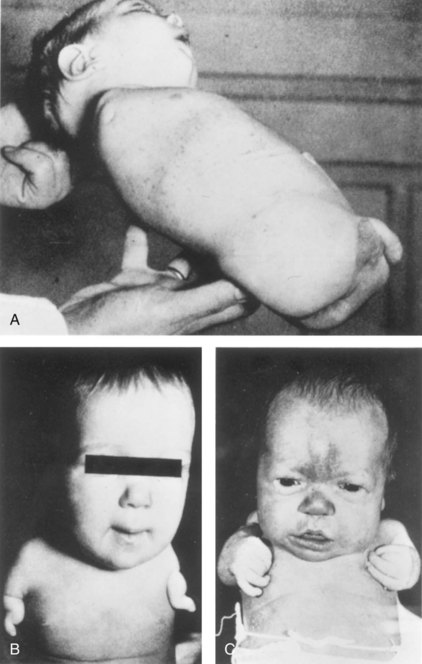
FIGURE 16–12 Birth defects of limbs caused by maternal ingestion of thalidomide. A, Quadruple amelia: absence of upper and lower limbs. B, Meromelia of the upper limbs; the limbs are represented by rudimentary stumps. C, Meromelia with the rudimentary upper limbs attached directly to the trunk.
(From Lenz W, Knapp K: Foetal malformation due to thalidomide. Geriatr Med Monthly 7:253, 1962.)
Major limb defects appear approximately twice in 1000 neonates. Most of these defects are caused by genetic factors (see Fig. 16-14). Molecular studies have implicated gene mutation (Hox gene, BMP, Shh, Wnt7, En-1, and others) in some cases of limb defects. Several unrelated birth defects of the lower limb were found to be associated with an aberrant arterial pattern, which might be of some importance in the pathogenesis of these defects. Experimental studies indicate that thalidomide affects the formation of early blood vessels in the limb buds.
Limb Anomalies
There are two main types of limb anomalies or defects:
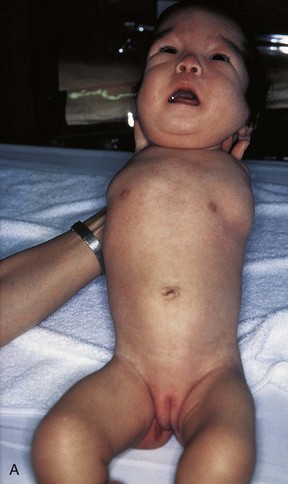
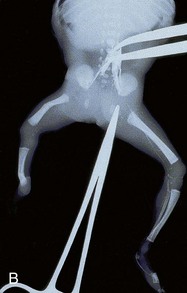
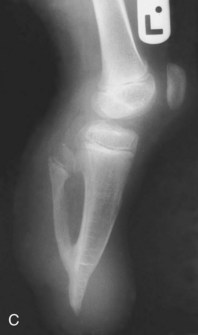
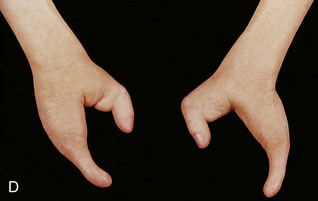
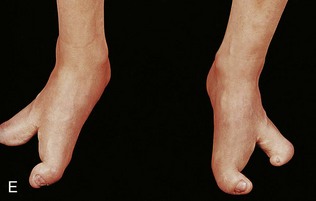
FIGURE 16–13 Various types of birth defects. A, Female neonate with amelia, complete absence of the upper limbs. B, Radiograph of a female fetus showing absence of the right fibula. Note also that the right leg is shorter and the femur and tibia are bowed and hypoplastic (underdevelopment of tissue of the limb). C, Radiograph showing partial absence and fusion of the lower ends of the tibia and fibula in a 5-year-old child. D, Absence of the central digits of the hands, resulting in a defect called bifurcate (forked) hand or split hand. E, Absence of the second to fourth toes, resulting in a bifurcate hand or split foot.
(A, Courtesy of Dr. Y. Suzuki, Achi, Japan. B, Courtesy of Dr. Joseph R. Siebert, Children’s Hospital and Regional Medical Center, Seattle, Washington. C, Courtesy of Dr. Prem S. Sahni, formerly of the Department of Radiology, Children’s Hospital, Winnipeg, Manitoba, Canada. D and E, Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, Manitoba, Canada.)
Causes of Limb Anomalies
Birth defects of the limbs originate at different stages of development. Suppression of limb bud development during the early part of the fourth week results in absence of the limbs, known as amelia. Arrest or disturbance of differentiation or growth of the limbs during the fifth week results in various types of meromelia. Like other congenital anomalies, limb defects are caused by:
Experimental studies support the suggestion that mechanical influences during intrauterine development may cause some fetal limb defects. A reduced quantity of amniotic fluid (oligohydramnios) is commonly associated with limb deformations; however, the significance of in utero mechanical influences on congenital postural deformation is still open to question.
Bifurcate Hand and Cleft Foot or Split Hand/Foot Malformations
In severe birth defects such as bifurcate (forked) hand and cleft foot, which clinically is called split hand/foot malformations (SHFM), there is absence of one or more central digits (fingers or toes), resulting from failure of development of one or more digital rays (Fig. 16-13D and E ). The hand or foot is divided into two parts that oppose and curve inward. This is a rare condition affecting approximately 1 : 20,000 live births. The split hand syndrome is an autosomal dominant abnormality with incomplete penetrance. The malformation originates in the fifth to sixth week of development, when the hands are forming. This disorder has 70% penetrance; that is, only 70% of the people who have the gene exhibit the defect.
Congenital Absence of Radius
The radius is partially or completely absent. The hand deviates laterally and the ulna bows with the concavity on the lateral side of the forearm. This defect results from failure of the mesenchymal primordium of the radius to form during the fifth week of development. Absence of the radius is usually caused by genetic factors.
Brachydactyly
Shortness of the digits (fingers or toes) is the result of reduction in the length of the phalanges. This birth defect is usually inherited as a dominant trait and is often associated with shortness of stature.
Polydactyly
Polydactyly is the presence of more than five digits on the hands or feet, that is, supernumerary digits (Figs. 16-14A and B). Often the extra digit is incompletely formed and lacks normal muscular development. If the hand is affected, the extra digit is most commonly medial or lateral rather than central. In the foot, the extra toe is usually on the lateral side. Polydactyly is inherited as a dominant trait.
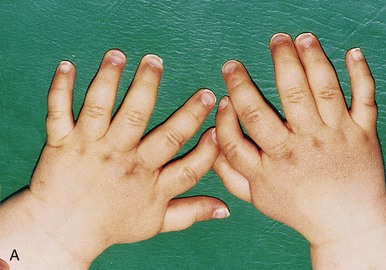

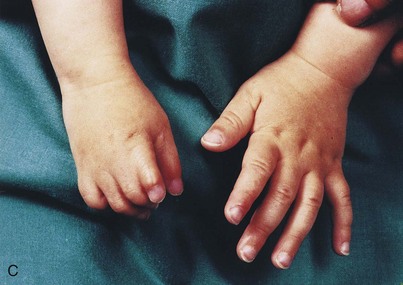
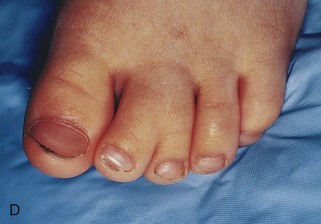
FIGURE 16–14 Types of digital birth defects. Polydactyly (more than five digits, fingers or toes, on the hands (A) or (B) feet). Syndactyly (webbing or fusion) of the fingers (C) or toes (D).
(Courtesy of Dr. A.E. Chudley, Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Syndactyly
Syndactyly is a common birth defect of the hand or foot. Cutaneous syndactyly (simple webbing between digits) is a common limb defect. It is more frequent in the foot than in the hand (Fig. 16-14C and D). Cutaneous syndactyly results from failure of the webs to degenerate between two or more digits. Programmed cell death (apoptosis) is responsible for the tissue breakdown between the digits. Blocking of the cellular and molecular events are likely responsible for the defects of the fingers.
Osseous syndactyly (fusion of bones, synostosis) occurs when the notches between the digital rays fail to develop; as a result, separation of the digits does not occur. Syndactyly is most frequently observed between the third and fourth fingers and between the second and third toes (SD type I). It is inherited as a simple autosomal dominant. A case of synpolydactyly (SD type II) (syndactyly and polydactyly), caused by mutations in the NH2-terminal, non–DNA binding part of HoxD13, has been reported.
Congenital Clubfoot
Talipes equinovarus (clubfoot) is a relatively common birth defect, occurring approximately once in 1000 births. It is the most common musculoskeletal defect. It is characterized by multiple components that lead to an abnormal position of the foot that prevents normal weight bearing. The sole of the foot is turned medially and the foot is inverted (Fig. 16-15). Clubfoot is bilateral in approximately 50% of cases and it occurs approximately twice as frequently in males.

FIGURE 16–15 Neonate with bilateral talipes equinovarus (clubfeet). Observe the hyperextension and incurving of the feet.
(Courtesy of Dr. A.E. Chudley, Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Although it is commonly stated that clubfoot results from abnormal positioning or restricted movement of the fetus’s lower limbs in utero, the evidence of this is inconclusive. Clubfoot appears to be caused by multifactorial inheritance (genetic and environmental factors acting together). In this condition all anatomical structures are present so the majority of cases can be treated with casting or taping.
Developmental Dysplasia of the Hip
This birth defect occurs approximately once in 1500 neonates, and is more common in females than in males. The joint capsule is very relaxed at birth and there is underdevelopment of the acetabulum of the hip bone and the head of femur. Dislocation almost always occurs after birth. There are two causative factors:
 Summary of Limb Development
Summary of Limb Development
Clinically Oriented Problems
Case 16–1
A mother consulted her pediatrician after noticing that when her 11-month-old daughter began to stand independently, her legs seemed to be of different lengths.
Case 16–2
A male infant was born with limb defects. His mother said that one of her relatives had similar defects.
Case 16–3
A neonate presented with clubfeet. The physician explained that this is a common birth defect.
References and Suggested Reading
Ambler CA, Nowicki JL, Burke AC, et al. Assembly of trunk and limb blood vessels involves extensive migration and vasculogenesis of somite-derived angioblasts. Dev Biol. 2001;234:352.
Butterfield NC, McGlinn E, Wicking C. The molecular regulation of vertebrate limb patterning. Curr Top Dev Biol. 2010;90:319.
Cole P, Kaufman Y, Hatef DA, et al. Embryology of the hand and upper extremity. J Craniofac Surg. 2009;20:992.
Cooperman DR, Thompson GH. Congenital abnormalities of the upper and lower extremities and spine. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Dahn RD, Fallon JF. Limiting outgrowth: BMPs as negative regulators in limb development. BioEssays. 1999;21:721.
Elliott AM, Evans JA, Chudley AE. Split hand foot malformation (SHFM). Clin Genet. 2005;68:501.
Gold NB, Westgate MN, Holmes LB. Anatomic and etiological classification of congenital limb deficiencies. Am J Med Genet A. 2011;155:1225.
Goncalves LF, Kusanovic JP, Gotsch F, et al. The fetal musculoskeletal system. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: Elsevier, 2008.
Grzeschik K-H. Human limb malformations: An approach to the molecular basis of development. Int J Dev Biol. 2002;46:983.
Hall BK. Bones and Cartilage: Developmental Skeletal Biology. Philadelphia: Elsevier; 2005.
Hinrichsen KV, Jacob HJ, Jacob M, et al. Principles of ontogenesis of leg and foot in man. Ann Anat. 1994;176:121.
Kabak S, Boizow L. Organogenese des Extremitätenskeletts und der Extremitätengelenke beim Menschenembryo. Anat Anz. 1990;170:349.
Logan M. Finger or toe: The molecular basis of limb identity. Development. 2003;130:6401.
Manske PR, Oberg KC. Classification and developmental biology of congenital anomalies of the hand and upper extremity. J Bone Joint Surg Am. 2009;91:3.
Marini JC, Gerber NL. Osteogenesis imperfecta. JAMA. 1997;277:746.
Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Muragaki Y, Mundlos S, Upton J, et al. Altered growth and branching patterns in synpolydactyly caused by mutations in HoxD13. Science. 1996;272:548.
O’Rahilly R, Müller F. Developmental Stages in Human Embryos. Washington, DC: Carnegie Institution of Washington; 1987.
Robertson WWJr, Corbett D. Congenital clubfoot. Clin Orthop. 1997;338:14.
Sammer DM, Chung KC. Congenital hand differences: embryology and classification. Hand Clin. 2009;25:151.
Talamillo A, Delgado I, Nakamura T, et al. Role of Epiprofin, a zinc-finger transcription factor in limb development. Dev Biol. 2010;337:363.
Towers M, Tickle C. Generation of pattern and form in the developing limb. Int J Dev Biol. 2009;53:805.
Van Allen MI. Structural anomalies resulting from vascular disruption. Pediatr Clin North Am. 1992;39:255.
Van Heest AE. Congenital disorders of the hand and upper extremity. Pediatr Clin North Am. 1996;43:1113.
Zuniga A. Globalisation reaches gene regulation: The case for vertebrate limb development. Curr Opin Genet Dev. 2005;15:403.