Chapter 17 Nervous System
The nervous system consists of three main regions:
Development of Nervous System
The first indications of the developing nervous system appear during the third week as the neural plate and neural groove develop on the posterior aspect of the trilaminar embryo (Fig. 17-1A). It is the notochord and paraxial mesenchyme that induce the overlying ectoderm to differentiate into the neural plate. Signaling molecules involve members of the transforming growth factor β family, Shh, and BMPs. Formation of the neural folds, neural crest, and neural tube is illustrated in Figs. 17-1B to F and 17-2.
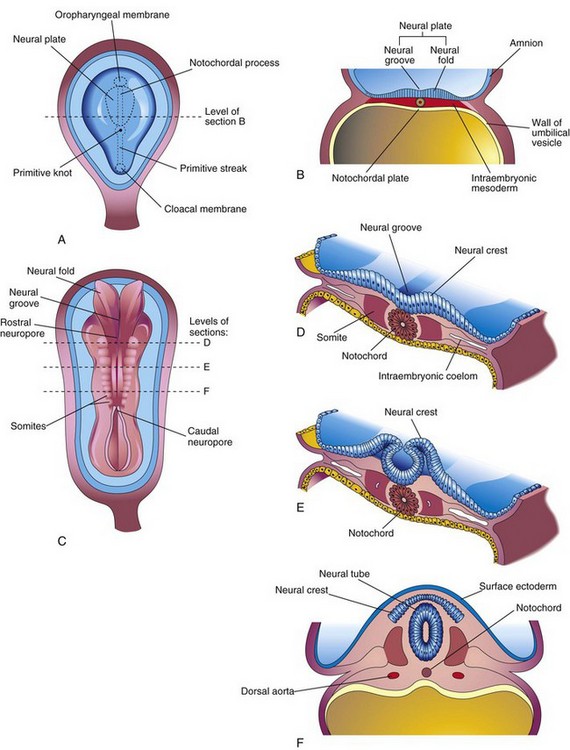
FIGURE 17–1 Illustrations of the neural plate and folding of it to form the neural tube. A, Dorsal view of an embryo of approximately 17 days, exposed by removing the amnion. B, Transverse section of the embryo showing the neural plate and early development of the neural groove and neural folds. C, Dorsal view of an embryo of approximately 22 days. The neural folds have fused opposite the fourth to sixth somites, but are spread apart at both ends. D to F, Transverse sections of this embryo at the levels shown in C illustrating formation of the neural tube and its detachment from the surface ectoderm. Note that some neuroectodermal cells are not included in the neural tube, but remain between it and the surface ectoderm as the neural crest.
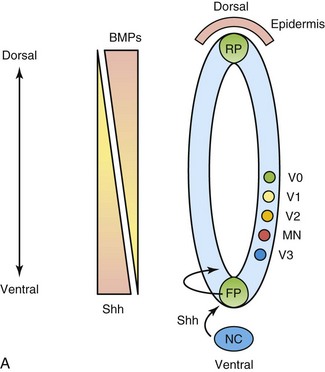
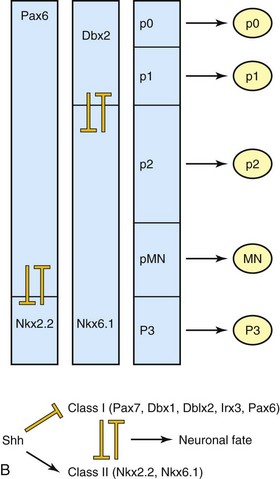
FIGURE 17–2 Morphogens and transcription factors specify the fate of progenitors in the ventral neural tube. A, Sonic hedgehog (Shh) is secreted by the notochord (NC) and the floor plate (FP) of the neural tube in a ventral to dorsal gradient. Similarly, bone morphogenetic proteins (BMPs), members of the transforming growth factor β superfamily, are secreted by the roof plate (RP) of the neural tube and the overlying epidermis, in a dorsal to ventral gradient. These opposing morphogen gradients determine dorsal-ventral cell fates. B, Shh concentration gradients define the ventral expression domains of class I (repressed) and class II (activated) homeobox transcription factors. Reciprocal negative interactions assist to establish boundaries of gene expression in the embryonic ventral spinal cord. P, progenitor; MN, motor neuron; V, ventral interneuron.
(Courtesy of Dr. David Eisenstat, Manitoba Institute of Cell Biology, and Department of Human Anatomy and Cell Science; and Dr. Jeffrey T. Wigle, Department of Biochemistry and Medical Genetics, University of Manitoba, Winnipeg, Manitoba, Canada. Adapted by permission from Macmillan Publishers Ltd.: Nature Reviews Genetics. Jessel TM: Neuronal specification in the spinal cord: Inductive signals and transcription codes. Nat Rev Genet 1:20, 2000.)
Neurulation—formation of the neural plate and neural tube—begins during the fourth week (22–23 days) in the region of the fourth to sixth pairs of somites (Fig. 17-1C and D). At this stage, the cranial two thirds of the neural plate and tube, as far caudal as the fourth pair of somites, represent the future brain, and the caudal one third of the plate and tube represents the future spinal cord.
Fusion of the neural folds and formation of the neural tube begins at the fifth somite and proceeds in both cranial and caudal directions until only small areas of the tube remain open at both ends (Fig. 17-3A and B). The lumen of the neural tube becomes the neural canal, which communicates freely with the amniotic cavity (Fig. 17-3C). The cranial opening, the rostral neuropore, closes at approximately the 25th day and the caudal neuropore closes at approximately the 27th day (Fig. 17-3D).
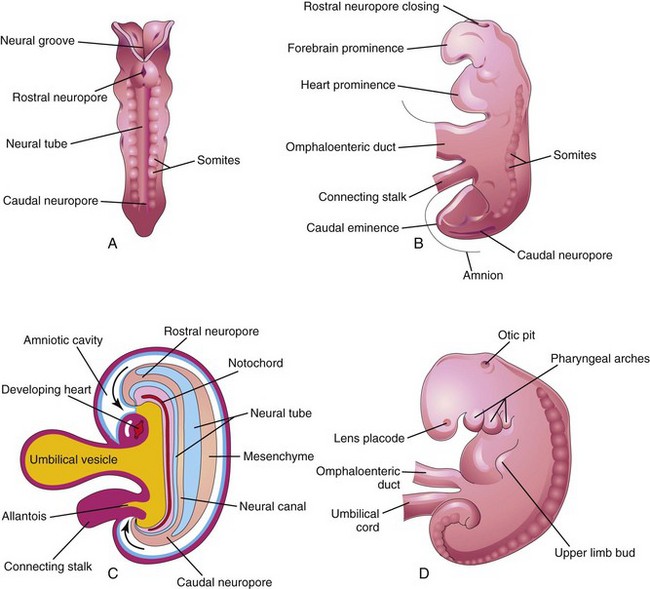
FIGURE 17–3 A, Dorsal view of an embryo of approximately 23 days showing fusion of the neural folds, which forms the neural tube. B, Lateral view of an embryo of approximately 24 days showing the forebrain prominence and closing of the rostral neuropore. C, Diagrammatic sagittal section of the embryo showing the transitory communication of the neural canal with the amniotic cavity (arrows). D, Lateral view of an embryo of approximately 27 days. Note that the neuropores shown in B are closed.
Closure of the neuropores coincides with the establishment of a vascular circulation for the neural tube. The walls of the neural tube thicken to form the brain and spinal cord (Fig. 17-4). The neural canal forms the ventricular system of the brain and the central canal of the spinal cord.

FIGURE 17–4 A, Schematic lateral view of an embryo of approximately 28 days showing the three primary brain vesicles: forebrain, midbrain, and hindbrain. Two flexures demarcate the primary divisions of the brain. B, Transverse section of the embryo showing the neural tube that will develop into the spinal cord in this region. The spinal ganglia derived from the neural crest are also shown. C, Schematic lateral view of the central nervous system (CNS) of a 6-week embryo showing the secondary brain vesicles and pontine flexure, which occurs as the brain grows rapidly.
Nonclosure of Neural Tube
The current hypothesis is that there are multiple, possibly five, closure sites involved in the formation of the neural tube. Failure of closure of site 1 results in spina bifida cystica (see Fig. 17-15); meroencephaly (anencephaly) results from failure of closure of site 2 (see Fig. 17-13); craniorachischisis results from failure of sites 2, 4, and 1 to close; site 3 nonfusion is rare.
Descriptions of these neural tube defects (NTDs) are given later (see Fig. 17-19). It has been suggested that the most caudal region may have a fifth closure site from the second lumbar vertebra to the second sacral vertebra, and that closure inferior to the second sacral vertebra is by secondary neurulation. Epidemiologic analysis of neonates with NTD supports the concept that there are multiple closure of the neural tube in humans.
Development of Spinal Cord
The spinal cord develops from the caudal part of the neural plate and the caudal eminence. The neural tube caudal to the fourth pair of somites develops into the spinal cord (Figs. 17-3, 17-4, and 17-5). The lateral walls of the neural tube thicken, gradually reducing the size of the neural canal until only a minute central canal of the spinal cord is present at 9 to 10 weeks (Fig. 17-5C). Initially, the wall of the neural tube is composed of a thick, pseudostratified, columnar neuroepithelium (Fig. 17-5D).
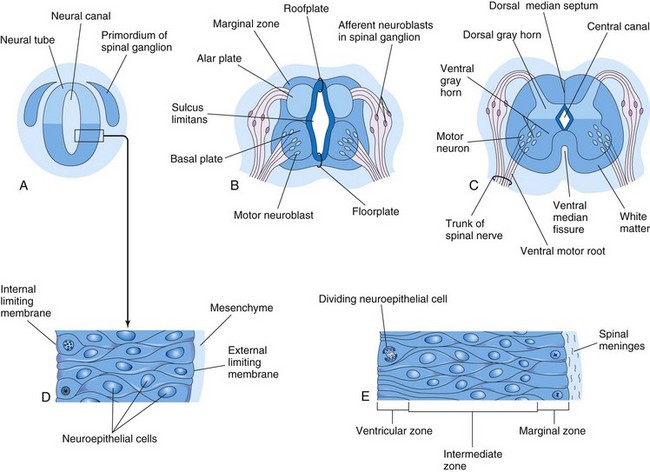
FIGURE 17–5 Illustrations of the development of the spinal cord. A, Transverse section of the neural tube of an embryo of approximately 23 days. B and C, Similar sections at 6 and 9 weeks, respectively. D, Section of the wall of the neural tube shown in A. E, Section of the wall of the developing spinal cord showing its three zones. In A to C, note that the neural canal of the neural tube is converted into the central canal of the spinal cord.
These neuroepithelial cells constitute the ventricular zone (ependymal layer), which gives rise to all neurons and macroglial cells (macroglia) in the spinal cord (Figs. 17-5E and 17-6). Macroglial cells are the larger members of the neuroglial family of cells, which includes astrocytes and oligodendrocytes. Soon a marginal zone composed of the outer parts of the neuroepithelial cells becomes recognizable (Fig. 17-5E). This zone gradually becomes the white matter of the spinal cord as axons grow into it from nerve cell bodies in the spinal cord, spinal ganglia, and brain.
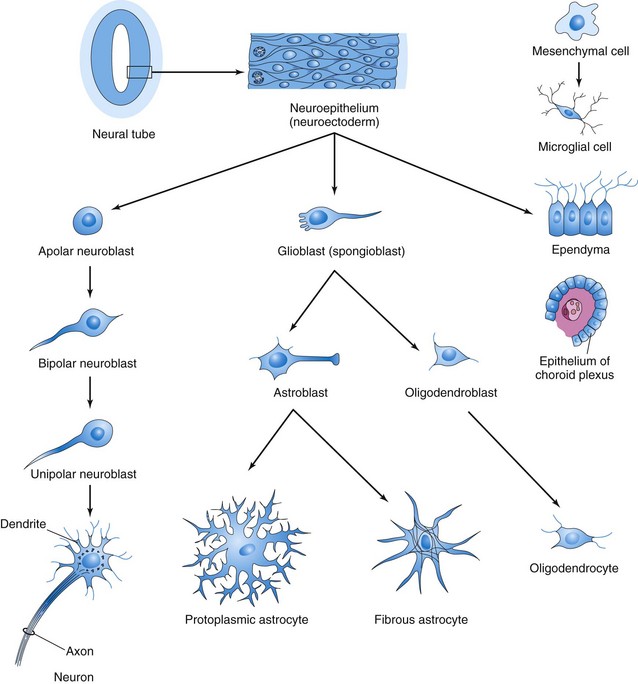
FIGURE 17–6 Histogenesis of cells in the central nervous system. After further development, the multipolar neuroblast (lower left) becomes a nerve cell or neuron. Neuroepithelial cells give rise to all neurons and macroglial cells. Microglial cells are derived from mesenchymal cells that invade the developing nervous system with the blood vessels.
Some dividing neuroepithelial cells in the ventricular zone differentiate into primordial neurons—neuroblasts. These embryonic cells form an intermediate zone (mantle layer) between the ventricular and marginal zones. Neuroblasts become neurons as they develop cytoplasmic processes (Fig. 17-6).
The supporting cells of the CNS—glioblasts (spongioblasts)—differentiate from neuroepithelial cells, mainly after neuroblast formation has ceased. The glioblasts migrate from the ventricular zone into the intermediate and marginal zones. Some glioblasts become astroblasts and later astrocytes, whereas others become oligodendroblasts and eventually oligodendrocytes (Fig. 17-6). When the neuroepithelial cells cease producing neuroblasts and glioblasts, they differentiate into ependymal cells, which form the ependyma (ependymal epithelium) lining the central canal of the spinal cord.
Sonic hedgehog signaling controls the proliferation, survival, and patterning of neuroepithelial progenitor cells by regulating Gli transcription factors (Fig. 17-2).
Microglia (microglial cells), which are scattered throughout the gray and white matter, are small cells that are derived from mesenchymal cells (Fig. 17-6). Microglia invade the CNS rather late in the fetal period after it has been penetrated by blood vessels. Microglia originate in the bone marrow and are part of the mononuclear phagocytic cell population.
Proliferation and differentiation of neuroepithelial cells in the developing spinal cord produce thick walls and thin roof-plates and floor-plates (Fig. 17-5B). Differential thickening of the lateral walls of the spinal cord soon produces a shallow longitudinal groove on each side—the sulcus limitans (Figs. 17-5B and 17-7). This groove separates the dorsal part, the alar plate, from the ventral part, the basal plate. The alar and basal plates produce longitudinal bulges extending through most of the length of the developing spinal cord. This regional separation is of fundamental importance because the alar and basal plates are later associated with afferent and efferent functions, respectively.
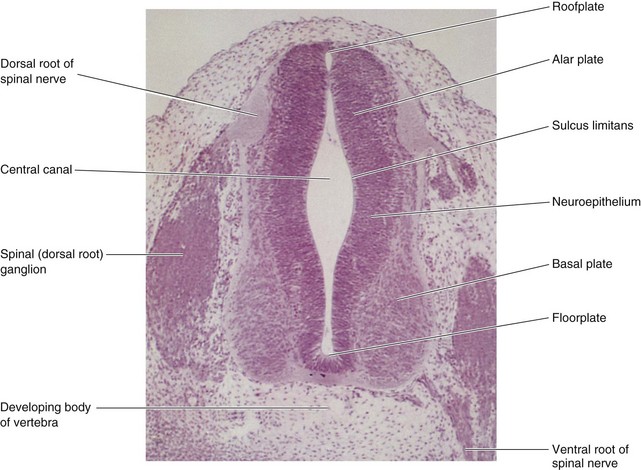
FIGURE 17–7 Transverse section of an embryo (x100) at Carnegie stage 16 at approximately 40 days. The ventral root of the spinal nerve is composed of nerve fibers arising from neuroblasts in the basal plate (developing ventral horn of spinal cord), whereas the dorsal root is formed by nerve processes arising from neuroblasts in the spinal ganglion.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
Cell bodies in the alar plates form the dorsal gray columns, which extend the length of the spinal cord. In transverse sections of the cord, these columns are the dorsal gray horns (Fig. 17-7). Neurons in these columns constitute afferent nuclei and groups of them form the dorsal gray columns. As the alar plates enlarge, the dorsal median septum forms. Cell bodies in the basal plates form the ventral and lateral gray columns.
In transverse sections of the spinal cord, these columns are the ventral gray horns and lateral gray horns, respectively (Fig. 17-5C). Axons of ventral horn cells grow out of the spinal cord and form the ventral roots of the spinal nerves. As the basal plates enlarge, they bulge ventrally on each side of the median plane. As this occurs, the ventral median septum forms and a deep longitudinal groove—the ventral median fissure—develops on the ventral surface of the spinal cord (Fig. 17-5C).
Development of Spinal Ganglia
The unipolar neurons in the spinal ganglia (dorsal root ganglia) are derived from neural crest cells (Figs. 17-8 and 17-9). The axons of cells in the spinal ganglia are at first bipolar, but the two processes soon unite in a T-shaped fashion. Both processes of spinal ganglion cells have the structural characteristics of axons, but the peripheral process is a dendrite in that there is conduction toward the cell body. The peripheral processes of spinal ganglion cells pass in the spinal nerves to sensory endings in somatic or visceral structures (Fig. 17-8). The central processes enter the spinal cord and constitute the dorsal roots of spinal nerves.

FIGURE 17–8 Diagrams showing some derivatives of the neural crest. Neural crest cells also differentiate into the cells in the afferent ganglia of cranial nerves and many other structures (see Chapter 5). The formation of a spinal nerve is also illustrated.
Development of Meninges
The meninges (membranous coverings of brain and spinal cord) develop from cells of the neural crest and mesenchyme during days 20 and 35 days, which migrate to surround the neural tube (primordium of brain and spinal cord) and form the primordial meninges (Figs. 17-1F and 17-3C). The external layer of these membranes thickens to form the dura mater (Fig. 17-10) and the internal layer, the pia arachnoid is composed of pia mater and arachnoid mater (leptomeninges). Fluid-filled spaces appear within the leptomeninges that soon coalesce to form the subarachnoid space (Fig. 17-10B). The origin of the pia mater and arachnoid from a single layer is indicated in the adult by arachnoid trabeculae—numerous delicate strands of connective tissue that pass between the pia and arachnoid. Cerebrospinal fluid (CSF) begins to form during the fifth week.
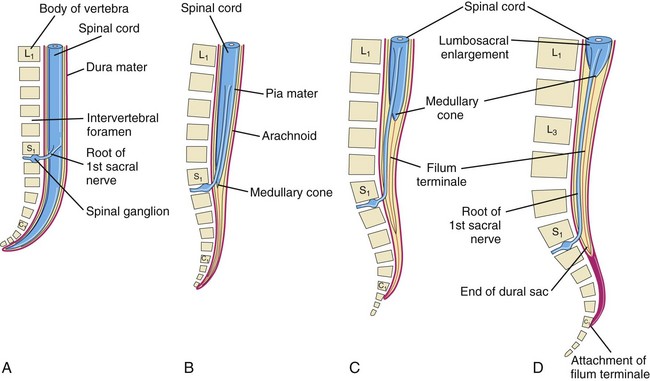
FIGURE 17–10 Diagrams showing the position of the caudal end of the spinal cord in relation to the vertebral column and meninges at various stages of development. The increasing inclination of the root of the first sacral nerve is also illustrated. A, At 8 weeks. B, At 24 weeks. C, Neonate. D, Adult.
Positional Changes of Spinal Cord
The spinal cord in the embryo extends the entire length of the vertebral canal (Fig. 17-10A). The spinal nerves pass through the intervertebral foramina opposite their levels of origin. Because the vertebral column and dura mater grow more rapidly than the spinal cord, this positional relationship of the spinal nerves does not persist. The caudal end of the spinal cord in fetuses gradually comes to lie at relatively higher levels. In a 24-week-old fetus, it lies at the level of the first sacral vertebra (Fig. 17-10B).
The spinal cord in the neonate terminates at the level of the second or third lumbar vertebra (Fig. 17-10C). In an adult, the cord usually terminates at the inferior border of the first lumbar vertebra (Fig. 17-10D). This is an average level because the caudal end of the spinal cord in adults may be as superior as the 12th thoracic vertebra or as inferior as the third lumbar vertebra. The spinal nerve roots, especially those of the lumbar and sacral segments, run obliquely from the spinal cord to the corresponding level of the vertebral column. The nerve roots inferior to the end of the cord—the medullary cone—form a bundle of spinal nerve roots—the cauda equina (horse tail)—which arises from the lumbosacral enlargement (swelling) and medullary cone of the spinal cord (Fig. 17-10D).
Although the dura mater and arachnoid mater usually end at the S2 vertebra in adults, the pia mater does not. Distal to the caudal end of the spinal cord, the pia mater forms a long fibrous thread, the filum terminale (terminal filum), which indicates the original level of the caudal end of the embryonic spinal cord (Fig. 17-10C). This filum extends from the medullary cone and attaches to the periosteum of the first coccygeal vertebra (Fig. 17-10D).
Myelination of Nerve Fibers
Myelin sheaths (membranous wrapping) around the nerve fibers within the spinal cord begin to form during the late fetal period, and continue to form during the first postnatal year (Fig. 17-11E). Myelin basic proteins, a family of related polypeptide isoforms, are essential in myelination; β1 integrins regulate this process. In general, fiber tracts become functional at approximately the time they become myelinated. Motor roots are myelinated before sensory roots. The myelin sheaths around the nerve fibers in the spinal cord are formed by oligodendrocytes that originate from the neuroepithelium. The plasma membranes of these cells wrap around the axon, forming a number of layers (Fig. 17-11F to H).
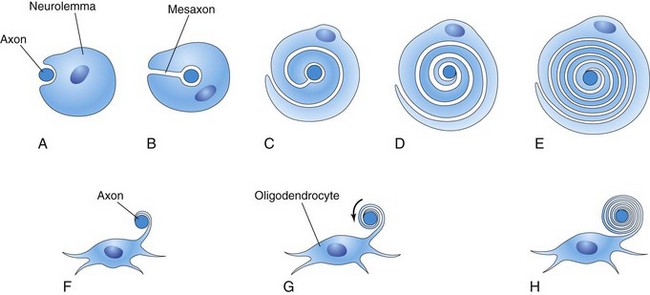
FIGURE 17–11 Diagrammatic sketches illustrating myelination of nerve fibers. A to E, Successive stages in the myelination of an axon of a peripheral nerve fiber by the neurolemma (sheath of Schwann). The axon first indents the cell; the cell then rotates around the axon as the mesaxon (site of invagination) elongates. The cytoplasm between the layers of the cell membrane gradually condenses. Cytoplasm remains on the inside of the sheath between the myelin and axon. F to H, Successive stages in the myelination of a nerve fiber in the central nervous system by an oligodendrocyte. A process of the neuroglial cell wraps itself around an axon, and the intervening layers of cytoplasm move to the body of the cell.
The myelin sheaths around the axons of peripheral nerve fibers are formed by the plasma membranes of the neurolemma (sheath of Schwann cells), which are analogous to oligodendrocytes. Neurolemma cells are derived from neural crest cells that migrate peripherally and wrap themselves around the axons of somatic motor neurons and preganglionic autonomic motor neurons as they pass out of the CNS (Figs. 17-8 and 17-11A to E). These cells also wrap themselves around both the central and peripheral processes of somatic and visceral sensory neurons, as well as around the axons of postsynaptic autonomic motor neurons. Beginning at approximately 20 weeks, peripheral nerve fibers have a whitish appearance, resulting from the deposition of myelin (layers of lipid and protein substances).
Birth Defects of Spinal Cord
Most defects result from failure of fusion of one or more neural arches of the developing vertebrae during the fourth week. Neural tube defects (NTD) affect the tissues overlying the spinal cord: meninges, vertebral arches, muscles, and skin (Fig. 17-12B to D). Birth defects involving the embryonic neural arches are referred to as spina bifida; subtypes of this defect are based on the degree and pattern of the NTD. The term—spina bifida—denotes nonfusion of the halves of the embryonic neural arches, which is common to all types of spina bifida (Fig. 17-12). Severe defects also involve the spinal cord, meninges, and neurocranium—the bones of the cranium enclosing the brain (Fig. 17-13). Spina bifida ranges from clinically significant types to minor defects that are functionally unimportant (Fig. 17-14).
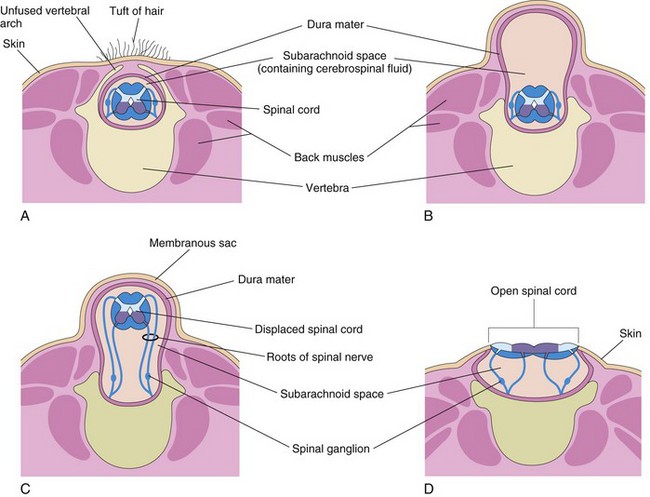
FIGURE 17–12 Diagrammatic sketches illustrating various types of spina bifida and the associated defects of the vertebral arches (one or more), spinal cord, and meninges. A, Spina bifida occulta. Observe the unfused vertebral arch. B, Spina bifida with meningocele. C, Spina bifida with meningomyelocele. D, Spina bifida with myeloschisis. The defects illustrated in B to D are referred to collectively as spina bifida cystica because of the cyst-like sac or cyst associated with them.
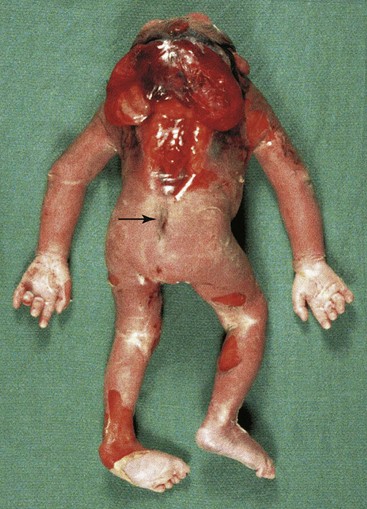
FIGURE 17–13 A fetus at 20 weeks with severe neural tube defects, including acrania, cerebral regression (meroencephaly), iniencephaly (enlargement of foramen magnum), and a sacral dimple (arrow).
(Courtesy of Dr. Marc Del Bigio, Department of Pathology [Neuropathology], University of Manitoba, Winnipeg, Manitoba, Canada.)
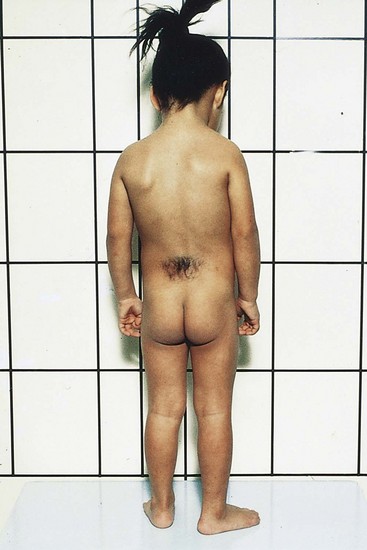
FIGURE 17–14 A female child with a hairy patch in the lumbosacral region indicating the site of a spina bifida occulta.
(Courtesy of A.E. Chudley, MD, Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Dermal Sinus
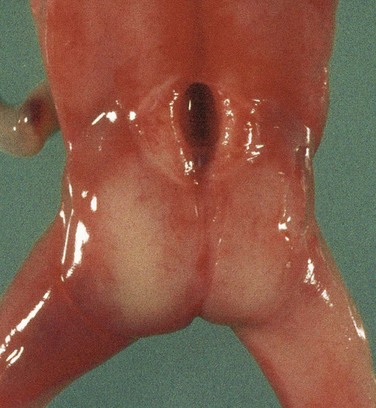
This sinus (channel) is associated with closure of the neural tube and formation of the meninges in the lumbosacral region of the spinal cord. The birth defect is caused by failure of the surface ectoderm (future skin) to detach from the neuroectoderm and meninges that envelop it. As a result, the meninges are continuous with a narrow channel that extends to a dimple in the skin of the sacral region of the back (Fig. 17-13; see arrow). The dimple indicates the region of closure of the caudal neuropore at the end of the fourth week; therefore, it represents the last place of separation between the surface ectoderm and the neural tube.
Spina Bifida Occulta
This NTD is the result of failure of the embryonic halves of one or more neural arches to fuse in the median plane (Fig. 17-12A). Spina bifida occulta occurs in the L5 or S1 vertebra in approximately 10% of otherwise normal people. In its most minor form, the only evidence of its presence may be a small dimple with a tuft of hair arising from it (Fig. 17-14). An overlying lipoma, dermal sinus, or other birthmarks may also occur. Spina bifida occulta usually produces no symptoms. A small percentage of affected infants have functionally significant defects of the underlying spinal cord and dorsal roots.
Spina Bifida Cystica
Severe types of spina bifida, involving protrusion of the spinal cord and/or meninges through defects in the vertebral arches, are referred to collectively as spina bifida cystica because of the meningeal cyst (cyst-like sac) that is associated with these defects (Figs. 17-12B to D and 17-15). This NTD occurs approximately once in every 5000 births and shows considerable geographic variation in incidence. When the cyst contains meninges and CSF, the defect is called spina bifida with meningocele (Fig. 17-12B). The spinal cord and spinal roots are in the normal position, but there may be spinal cord defects. Protrusion of the meninges and CSF of the spinal cord is through a defect in the vertebral column.
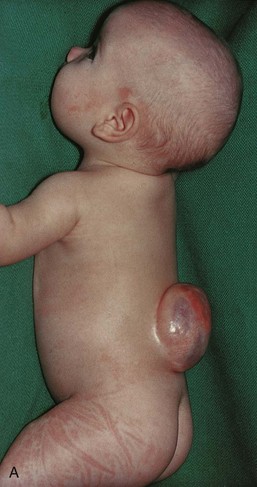
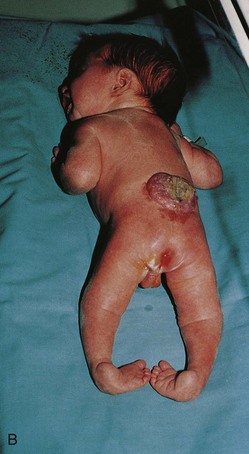
FIGURE 17–15 Infants with spina bifida cystica. A, Spina bifida with meningomyelocele in the lumbar region. B, Spina bifida with myeloschisis in the lumbar region. Note the nerve involvement has affected the lower limbs.
(Courtesy of the late Dr. Dwight Parkinson, Department of Surgery and Department of Human Anatomy and Cell Science, University of Manitoba, Winnipeg, Manitoba, Canada.)
If the spinal cord and/or nerve roots are contained within the cyst, the defect is called spina bifida with meningomyelocele (Figs. 17-12C and 17-15). Severe cases involving several vertebrae are often associated with absence of the calvaria, partial absence of the brain, and facial abnormalities, known as meroencephaly (Figs. 17-13 and 17-17). The term meroencephaly recognizes that the defects entail drastic effects in some brain areas and lesser or no effects in others. In these neonates, death is inevitable. The term anencephaly for these severe defects is inappropriate because it indicates that no part of the brain is present.
Spina bifida cystica shows varying degrees of neurologic deficit, depending on the position and extent of the lesion. There is usually a corresponding dermatome loss of sensation along with complete or partial skeletal muscle paralysis. The level of the lesion determines the area of anesthesia (area of skin without sensation) and the muscles affected. Sphincter paralysis (bladder and/or anal sphincters) is common with lumbosacral meningomyelocele (Figs. 17-12C and 17-15A). There is almost invariably a saddle anesthesia when the sphincters are involved, that is, loss of sensation in the body region that would contact a saddle.
Meroencephaly is strongly suspected in utero when there is a high level of alpha fetoprotein (AFP) in the amniotic fluid (see Chapter 6). AFP may also be elevated in the maternal blood serum. Amniocentesis is usually performed on pregnant women with high levels of serum AFP for the determination of the AFP level in the amniotic fluid (see Chapter 6, Fig. 6-13). An ultrasound scan may reveal the presence of an NTD that has resulted in spina bifida cystica. The fetal vertebral column can be detected by ultrasound at 10 to 12 weeks and if there is a defect in the vertebral arch, a cystic mass may be present in the affected area (Figs. 17-12C and 17-15A).
Menigomyelocele
Spina bifida with meningomyelocele (Fig. 17-12C ) is a more common and a more severe defect than spina bifida with meningocele (Fig. 17-12B). This NTD may occur anywhere along the vertebral column, but they are most common in the lumbar and sacral region (see Fig. 17-17). Some cases of meningomyelocele are associated with craniolacunia (defective development of calvaria); this results in depressed, nonossified areas on the inner surfaces of the flat bones of the calvaria.
Myeloschisis
This is the most severe type of spina bifida (Figs. 17-12D, 17-15B, and 17-16). In this defect, the spinal cord in the affected area is open because the neural folds failed to fuse (schisis, a cleaving). As a result, the spinal cord is represented by a flattened mass of nervous tissue. This defect usually results in permanent paralysis or weakness of the lower limbs.
Etiology of Neural Tube Defects
Nutritional and environmental factors undoubtedly play a role in the production of NTDs. Both food fortification with folic acid and folic acid supplements taken before conception and continued for at least 3 months during pregnancy reduce the incidence of NTDs. As a result, the U.S. Public Health Service recommended in 1992 that “all women of childbearing age who are capable of becoming pregnant should consume 0.4 mg, 400 µg, of folic acid daily.” Epidemiological studies have also shown that low maternal B12 levels may significantly increase the risk for NTD. Certain drugs increase the risk of meningomyelocele (e.g., valproic acid). This anticonvulsant drug causes NTDs in 1% to 2% of pregnancies if taken during early pregnancy (fourth week of development) when the neural folds are fusing (Fig. 17-17).
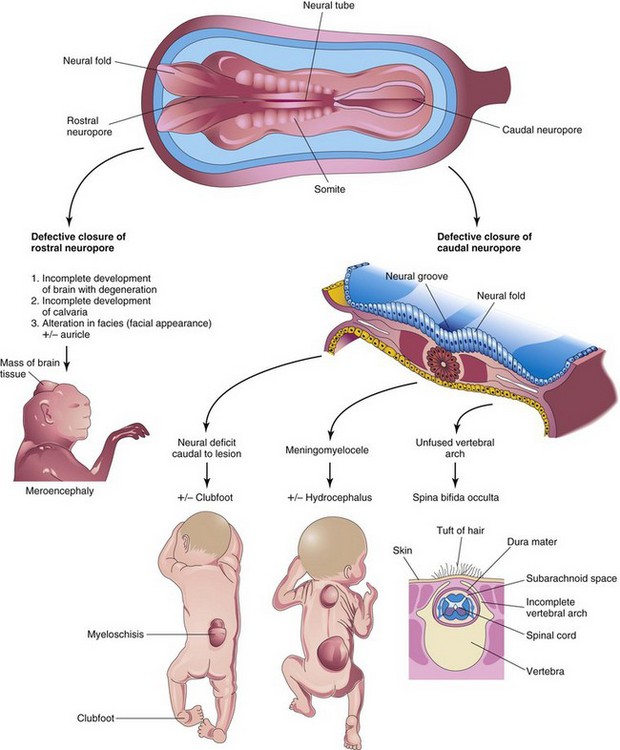
FIGURE 17–17 Schematic illustrations showing the embryologic basis of neural tube defects. Meroencephaly, partial absence of brain, results from defective closure of the rostral neuropore, and meningomyelocele results from defective closure of the caudal neuropore.
(Modified from Jones KL: Smith’s Recognizable Patterns of Human Malformations, 4th ed. Philadelphia, WB Saunders, 1988.)
 Development of Brain
Development of Brain
The brain begins to develop in the third week when the neural plate and tube are developing from the neuroectoderm (Fig. 17-1). The neural tube, cranial to the fourth pair of somites, develops into the brain. Fusion of the neural folds in the cranial region and closure of the rostral neuropore form three primary brain vesicles from which the brain develops (Fig. 17-18). The three primary brain vesicles form the:
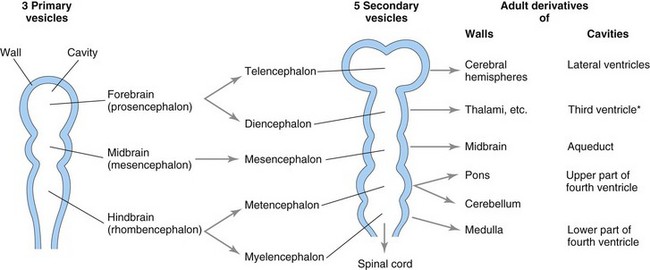
FIGURE 17–18 Diagrammatic sketches of the brain vesicles indicating the adult derivatives of their walls and cavities. The rostral part of the third ventricle (*) forms from the cavity of the telencephalon; most of this ventricle is derived from the cavity of the diencephalon.
During the fifth week, the forebrain partly divides into two secondary brain vesicles, the telencephalon and diencephalon; the midbrain does not divide; and the hindbrain partly divides into two vesicles, the metencephalon and myelencephalon. Consequently, there are five secondary brain vesicles.
 Brain Flexures
Brain Flexures
During the fifth week, the embryonic brain grows rapidly and bends ventrally with the head fold. This produces the midbrain flexure in the midbrain region and the cervical flexure at the junction of the hindbrain and spinal cord (Fig. 17-19A). Later, unequal growth of the brain between these flexures produces the pontine flexure in the opposite direction. This flexure results in thinning of the roof of the hindbrain (Fig. 17-1C).
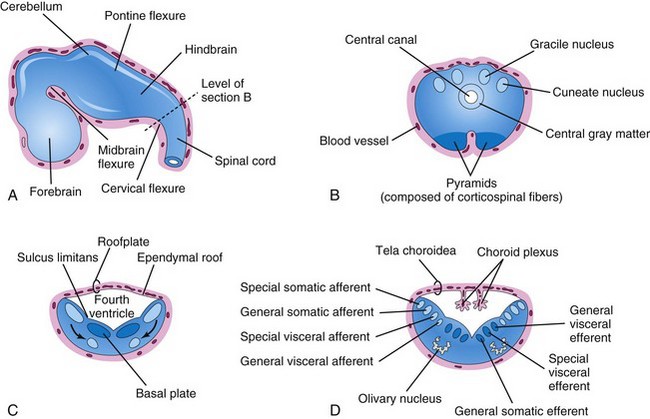
FIGURE 17–19 A, Sketch of the developing brain at the end of the fifth week showing the three primary divisions of the brain and the brain flexures. B, Transverse section of the caudal part of the myelencephalon (developing closed part of the medulla). C and D, Similar sections of the rostral part of the myelencephalon (developing open part of the medulla) showing the position and successive stages of differentiation of the alar and basal plates. The arrows in C show the pathway taken by neuroblasts from the alar plates to form the olivary nuclei.
Initially, the primordial brain has the same basic structure as the developing spinal cord; however, the brain flexures produce considerable variation in the outline of transverse sections at different levels of the brain and in the position of the gray and white matter. The sulcus limitans extends cranially to the junction of the midbrain and forebrain, and the alar and basal plates are recognizable only in the midbrain and hindbrain (Figs. 17-5C and 17-19C).
Hindbrain
The cervical flexure demarcates the hindbrain from the spinal cord (Fig. 17-19A). Later, this junction is arbitrarily defined as the level of the superior rootlet of the first cervical nerve, which is located roughly at the foramen magnum. The pontine flexure, located in the future pontine region, divides the hindbrain into caudal (myelencephalon) and rostral (metencephalon) parts. The myelencephalon becomes the medulla oblongata (commonly called the medulla), and the metencephalon becomes the pons and cerebellum. The cavity of the hindbrain becomes the fourth ventricle and the central canal in the medulla.
Myelencephalon
The caudal part of the myelencephalon (closed part of medulla) resembles the spinal cord, both developmentally and structurally (Fig. 17-19B). The neural canal of the neural tube forms the small central canal of the myelencephalon. Unlike those of the spinal cord, neuroblasts from the alar plates in the myelencephalon migrate into the marginal zone and form isolated areas of gray matter—the gracile nuclei medially and the cuneate nuclei laterally. These nuclei are associated with correspondingly named nerve tracts that enter the medulla from the spinal cord. The ventral area of the medulla contains a pair of fiber bundles—the pyramids—that consist of corticospinal fibers descending from the developing cerebral cortex (Fig. 17-19B).
The rostral part of the myelencephalon (“open” part of medulla) is wide and rather flat, especially opposite the pontine flexure (Fig. 17-19C and D). The pontine flexure causes the lateral walls of the medulla to move laterally like the pages of an open book. As a result, its roof plate is stretched and greatly thinned. In addition, the cavity of this part of the myelencephalon (part of future fourth ventricle) becomes somewhat rhomboidal (diamond shaped). As the walls of the medulla move laterally, the alar plates come to lie lateral to the basal plates. As the positions of the plates change, the motor nuclei generally develop medial to the sensory nuclei (Fig. 17-19C).
Neuroblasts in the basal plates of the medulla, like those in the spinal cord, develop into motor neurons. The neuroblasts form nuclei (groups of nerve cells) and organize into three cell columns on each side (Fig. 17-19D). From medial to lateral, the columns are the:
Neuroblasts in the alar plates of the medulla form neurons that are arranged in four columns on each side. From medial to lateral, the columns are the:
Some neuroblasts from the alar plates migrate ventrally and form the neurons in the olivary nuclei (Fig. 17-19C and D).
Metencephalon
The walls of the metencephalon form the pons and cerebellum, and the cavity of the metencephalon forms the superior part of the fourth ventricle (Fig. 17-20A). As in the rostral part of the myelencephalon, the pontine flexure causes divergence of the lateral walls of the pons, which spreads the gray matter in the floor of the fourth ventricle (Fig. 17-20B). As in the myelencephalon, neuroblasts in each basal plate develop into motor nuclei and organize into three columns on each side.

FIGURE 17–20 A, Sketch of the developing brain at the end of the fifth week. B, Transverse section of the metencephalon (developing pons and cerebellum) showing the derivatives of the alar and basal plates. C and D, Sagittal sections of the hindbrain at 6 and 17 weeks, respectively, showing successive stages in the development of the pons and cerebellum.
The cerebellum develops from thickenings of dorsal parts of the alar plates. Initially, the cerebellar swellings project into the fourth ventricle (Fig. 17-20B). As the swellings enlarge and fuse in the median plane, they overgrow the rostral half of the fourth ventricle and overlap the pons and medulla (Fig. 17-20D).
Some neuroblasts in the intermediate zone of the alar plates migrate to the marginal zone and differentiate into the neurons of the cerebellar cortex. Other neuroblasts from these plates give rise to the central nuclei, the largest of which is the dentate nucleus (Fig. 17-20D). Cells from the alar plates also give rise to the pontine nuclei, the cochlear and vestibular nuclei, and the sensory nuclei of the trigeminal nerve.
The structure of the cerebellum reflects its phylogenetic (evolutionary) development (Fig. 17-20C and D):
Nerve fibers connecting the cerebral and cerebellar cortices with the spinal cord pass through the marginal layer of the ventral region of the metencephalon. This region of the brainstem is the pons (bridge) because of the robust band of nerve fibers that crosses the median plane and forms a bulky ridge on its anterior and lateral aspects (Fig. 17-20C and D).
 Choroid Plexuses and Cerebrospinal Fluid
Choroid Plexuses and Cerebrospinal Fluid
The thin ependymal roof of the fourth ventricle is covered externally by pia mater, derived from mesenchyme associated with the hindbrain (Fig. 17-20B, C, and D). This vascular membrane, together with the ependymal roof, forms the tela choroidea of the fourth ventricle (Fig. 17-19D). Because of the active proliferation of the pia mater, the tela choroidea invaginates the fourth ventricle, where it differentiates into the choroid plexus, infoldings of choroidal arteries of the pia mater (Figs. 17-19C and 17-20C and D). Similar plexuses develop in the roof of the third ventricle and in the medial walls of the lateral ventricles.
The choroid plexuses secrete ventricular fluid, which becomes cerebrospinal fluid (CSF) when additions are made to it from the surfaces of the brain and spinal cord, and from the pia-arachnoid layer of the meninges. The thin roof of the fourth ventricle evaginates in three locations. These outpouchings rupture to form openings, median and lateral apertures (foramen of Magendie and foramina of Luschka, respectively), which permit the CSF to enter the subarachnoid space from the fourth ventricle.
The main site of absorption of CSF into the venous system is through the arachnoid villi, which are protrusions of arachnoid mater into the dural venous sinuses (large venous channels between the layers of the dura mater). The arachnoid villi consist of a thin, cellular layer derived from the epithelium of the arachnoid and the endothelium of the sinus.
Midbrain
The midbrain (mesencephalon) undergoes less change than any other part of the developing brain (Fig. 17-21A), except for the caudal part of the hindbrain. The neural canal narrows and becomes the cerebral aqueduct (Figs. 17-20D and 17-21D), a channel that connects the third and fourth ventricles.
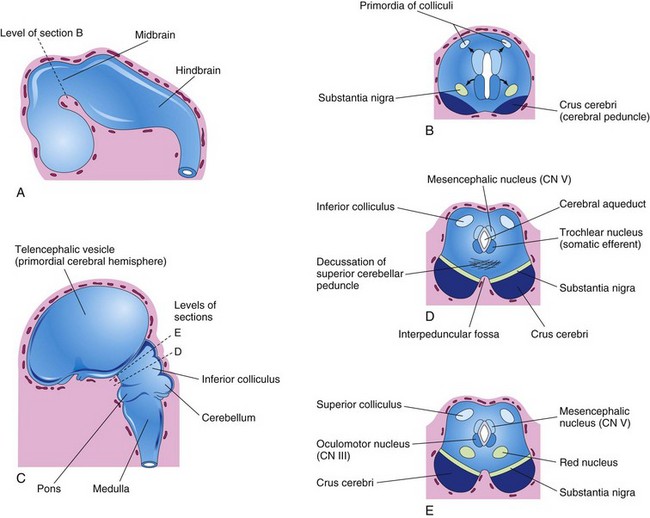
FIGURE 17–21 A, Sketch of the developing brain at the end of the fifth week. B, Transverse section of the developing midbrain showing the early migration of cells from the basal and alar plates. C, Sketch of the developing brain at 11 weeks. D and E, Transverse sections of the developing midbrain at the level of the inferior and superior colliculi, respectively. CN, cranial nerve.
Neuroblasts migrate from the alar plates of the midbrain into the tectum (roof) and aggregate to form four large groups of neurons, the paired superior and inferior colliculi (Fig. 17-21D and E), which are concerned with visual and auditory reflexes, respectively. Neuroblasts from the basal plates may give rise to groups of neurons in the tegmentum of midbrain (red nuclei, nuclei of third and fourth cranial nerves, and the reticular nuclei). The substantia nigra, a broad layer of gray matter adjacent to the cerebral peduncle (see Fig. 17-21D and E), may also differentiate from the basal plate, but some authorities believe that it is derived from cells in the alar plate that migrate ventrally.
Fibers growing from the cerebrum (principal part of brain, including the diencephalon and cerebral hemispheres) form the crus cerebri or cerebral peduncles anteriorly (Fig. 17-21B). The peduncles become progressively more prominent as more descending fiber groups (corticopontine, corticobulbar, and corticospinal) pass through the developing midbrain on their way to the brainstem and spinal cord.
Forebrain
As closure of the rostral neuropore occurs, two lateral outgrowths—optic vesicles—appear (Fig. 17-4A), one on each side of the forebrain. The optic vesicles are the primordia of the retinae and optic nerves (see Chapter 18). A second pair of diverticula, the telencephalic vesicles, soon arise more dorsally and rostrally (Fig. 17-21C). They are the primordia of the cerebral hemispheres, and their cavities become the lateral ventricles (see Fig. 17-26B).
The rostral (anterior) part of the forebrain, including the primordia of the cerebral hemispheres, is the telencephalon; the caudal (posterior) part of the forebrain is the diencephalon. The cavities of the telencephalon and diencephalon contribute to the formation of the third ventricle, although the cavity of the diencephalon contributes more (Fig. 17-22E).
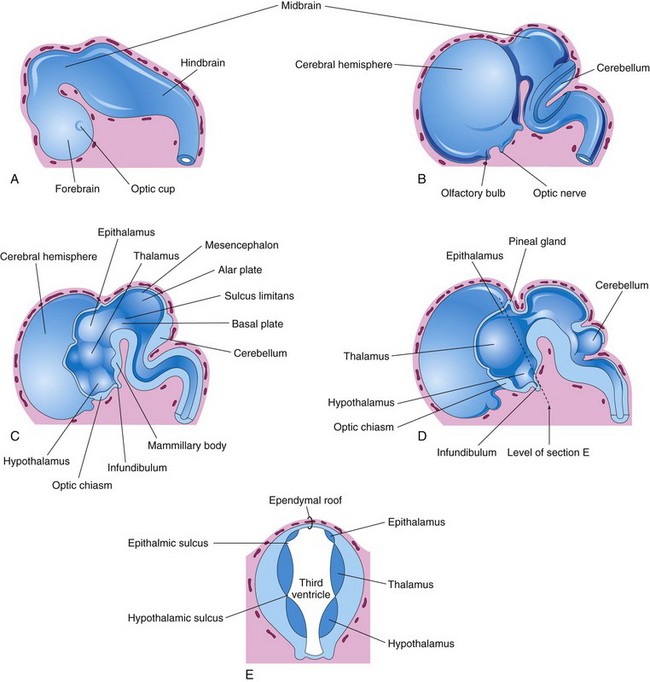
FIGURE 17–22 A, External view of the brain at the end of the fifth week. B, Similar view at 7 weeks. C, Median section of this brain showing the medial surface of the forebrain and midbrain. D, Similar section at 8 weeks. E, Transverse section of the diencephalon showing the epithalamus dorsally, the thalamus laterally, and the hypothalamus ventrally.
Diencephalon
Three swellings develop in the lateral walls of the third ventricle, which later become the thalamus, hypothalamus, and epithalamus (see Fig. 17-22C to E). The thalamus is separated from the epithalamus by the epithalamic sulcus and from the hypothalamus by the hypothalamic sulcus (Fig. 17-22E). The latter sulcus is not a continuation of the sulcus limitans into the forebrain and does not, like the sulcus limitans, divide sensory and motor areas (Fig. 17-22C).
The thalamus develops rapidly on each side of the third ventricle and bulges into its cavity (Fig. 17-22E). The thalami meet and fuse in the midline in approximately 70% of brains, forming a bridge of gray matter across the third ventricle—the interthalamic adhesion.
The hypothalamus arises by proliferation of neuroblasts in the intermediate zone of the diencephalic walls, ventral to the hypothalamic sulci. Differential expression of Wnt/beta-catenin signaling is involved in the patterning of the hypothalamus. Later, a number of nuclei concerned with endocrine activities and homeostasis develop. A pair of nuclei, the mammillary bodies, form pea-sized swellings on the ventral surface of the hypothalamus (Fig. 17-22C).
The epithalamus develops from the roof and dorsal portion of the lateral wall of the diencephalons (Fig. 17-22C to E). Initially, the epithalamic swellings are large, but later they become relatively small.
The pineal gland (body) develops as a median diverticulum of the caudal part of the roof of the diencephalon (Fig. 17-22D). Proliferation of cells in its walls soon converts it into a solid cone-shaped gland.
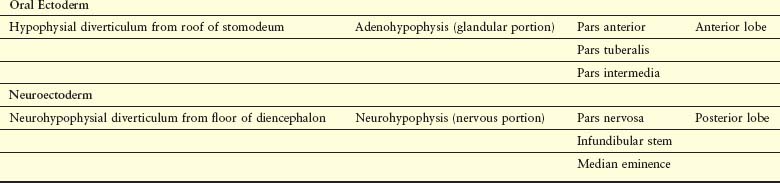
The pituitary gland (hypophysis) is ectodermal in origin (Fig. 17-23 and Table 17-1). It develops from two sources:
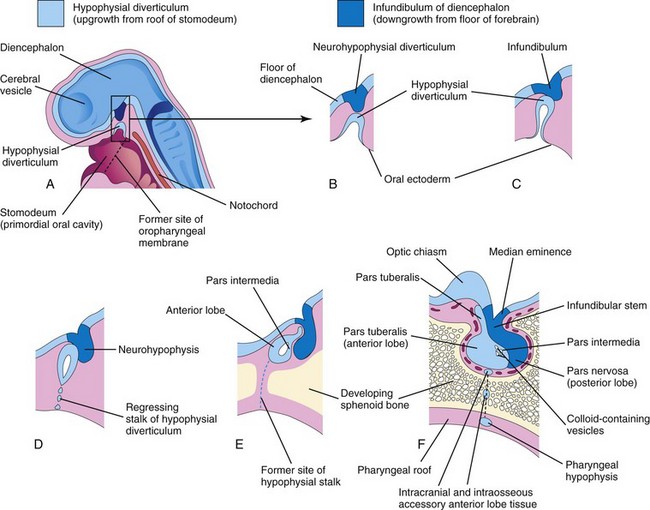
FIGURE 17–23 Diagrammatic sketches illustrating the development of the pituitary gland. A, Sagittal section of the cranial end of an embryo at approximately 36 days showing the hypophysial diverticulum, an upgrowth from the stomodeum, and the neurohypophysial diverticulum, a downgrowth from the forebrain. B to D, Successive stages of the developing pituitary gland. By 8 weeks, the diverticulum loses its connection with the oral cavity and is in close contact with the infundibulum and the posterior lobe (neurohypophysis) of the pituitary gland. E and F, Later stages showing proliferation of the anterior wall of the hypophysial diverticulum to form the anterior lobe (adenohypophysis) of the pituitary gland.
This double origin explains why the pituitary gland is composed of two completely different types of tissue:
By the third week, the hypophysial diverticulum projects from the roof of the stomodeum and lies adjacent to the floor (ventral wall) of the diencephalon (Fig. 17-23C). By the fifth week, this diverticulum has elongated and constricted at its attachment to the oral epithelium, giving it a nipple-like appearance. By this stage, it has come into contact with the infundibulum (derived from the neurohypophysial diverticulum), a ventral downgrowth of the diencephalon (Figs. 17-22C and D and 17-23).
The stalk of the hypophysial diverticulum passes between the chondrification centers of the developing presphenoid and basisphenoid bones of the cranium (Fig. 17-23E). During the sixth week, the connection of the diverticulum with the oral cavity degenerates (Fig. 17-23D and E). Cells of the anterior wall of the hypophysial diverticulum proliferate and give rise to the pars anterior of the pituitary gland (Table 17-1). Later an extension, the pars tuberalis, grows around the infundibular stem (Fig. 17-23E). The extensive proliferation of the anterior wall of the hypophysial diverticulum reduces its lumen to a narrow cleft (Fig. 17-23E). This residual cleft is usually not recognizable in the adult pituitary gland, but it may be represented by a zone of cysts. Cells in the posterior wall of the hypophysial pouch do not proliferate; they give rise to the thin, poorly defined pars intermedia (Fig. 17-23F).
The part of the pituitary gland that develops from the neuroectoderm (neurohypophysial diverticulum) is the neurohypophysis (Fig. 17-23B to F and Table 17-1). The infundibulum gives rise to the median eminence, infundibular stem, and pars nervosa. Initially, the walls of the infundibulum are thin, but the distal end of the infundibulum soon becomes solid as the neuroepithelial cells proliferate. These cells later differentiate into pituicytes, the primary cells of the posterior lobe of the pituitary gland, which are closely related to neuroglial cells. Nerve fibers grow into the pars nervosa from the hypothalamic area, to which the infundibular stem is attached (Fig. 17-23F).
Recent studies indicate that secreted inductive molecules (e.g., FGF8, BMP4, and Wnt5A) from the diencephalon are involved in the formation of the anterior and intermediate lobes of the pituitary gland; the LIM-homeobox gene Lhx2 appears to control development of the posterior lobe.
Pharyngeal Hypophysis and Craniopharyngioma
A remnant of the stalk of the hypophysial diverticulum may persist and form a pharyngeal hypophysis in the roof of the oropharynx (Fig. 17-23F ). Rarely, masses of anterior lobe tissue develop outside the capsule of the pituitary gland, within the sella turcica of the sphenoid bone. A remnant of the hypophysial diverticulum, the basipharyngeal canal, is visible in sections of the neonate’s sphenoid bone in approximately 1% of cases. It can also be identified in a small number of radiographs of crania of neonates (usually those with cranial defects).
Occasionally, a craniopharyngioma develops in the pharynx or basisphenoid (posterior part of sphenoid) from remnants of the stalk of the hypophysial diverticulum (Fig. 17-24), but most often they form in and/or superior to the sella turcica. These tumors arise along the path of the hypophysial diverticulum from epithelial remnants.
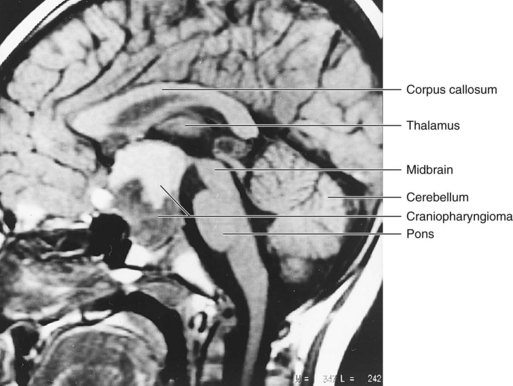
FIGURE 17–24 Sagittal magnetic resonance image of a 4-year-old boy who presented with a headache and optic atrophy. A large mass (4 cm) occupies an enlarged sella turcica, expanding inferiorly into the sphenoid bone and superiorly into the suprasellar cistern. A craniopharyngioma was confirmed by surgery. The inferior half of the mass is solid and appears dark, whereas the superior half is cystic and appears brighter.
(Courtesy of Dr. Gerald S. Smyser, Altru Health System, Grand Forks, North Dakota.)
Telencephalon
The telencephalon consists of a median part and two lateral diverticula, the cerebral vesicles (Fig. 17-23A). These vesicles are the primordia of the cerebral hemispheres (Figs. 17-22B and 17-23A). The cavity of the median portion of the telencephalon forms the extreme anterior part of the third ventricle (Fig. 17-25). At first, the cerebral hemispheres are in wide communication with the cavity of the third ventricle through the interventricular foramina (Figs. 17-25 and 17-26B).
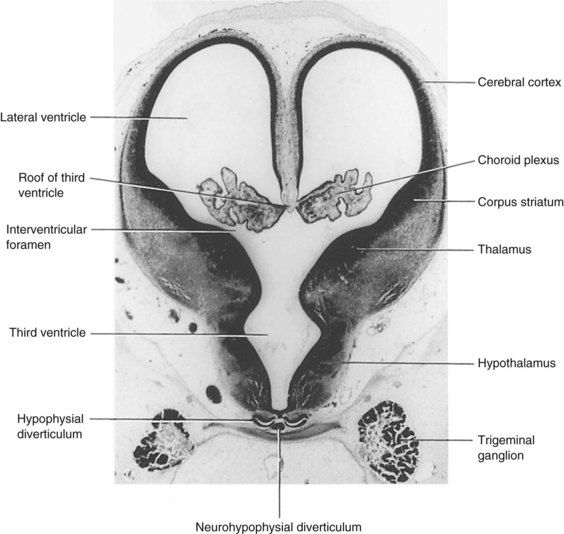
FIGURE 17–25 Photomicrograph of a transverse section through the diencephalon and cerebral vesicles of a human embryo (approximately 50 days) at the level of the interventricular foramina (×20). The choroid fissure is located at the junction of the choroid plexus and the medial wall of the lateral ventricle.
(Courtesy of Professor Jean Hay [retired], Department of Anatomy, University of Manitoba, Winnipeg, Manitoba, Canada.)
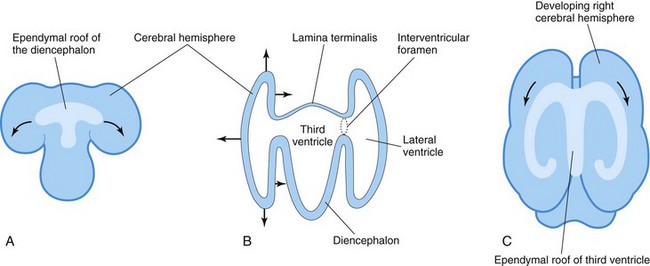
FIGURE 17–26 A, Sketch of the dorsal surface of the forebrain indicating how the ependymal roof of the diencephalon is carried out to the dorsomedial surface of the cerebral hemispheres. B, Diagrammatic section of the forebrain showing how the developing cerebral hemispheres grow from the lateral walls of the forebrain and expand in all directions until they cover the diencephalon. The arrows indicate some directions in which the hemispheres expand. The rostral wall of the forebrain, the lamina terminalis, is very thin. C, Sketch of the forebrain showing how the ependymal roof is finally carried into the temporal lobes as a result of the C-shaped growth pattern of the cerebral hemispheres.
Along the choroid fissure, part of the medial wall of the developing cerebral hemisphere becomes very thin (Figs. 17-25 and 17-26). Initially, this ependymal portion lies in the roof of the hemisphere and is continuous with the ependymal roof of the third ventricle (Fig. 17-26A). The choroid plexus of the lateral ventricle later forms at this site (Figs. 17-25 and 17-27).
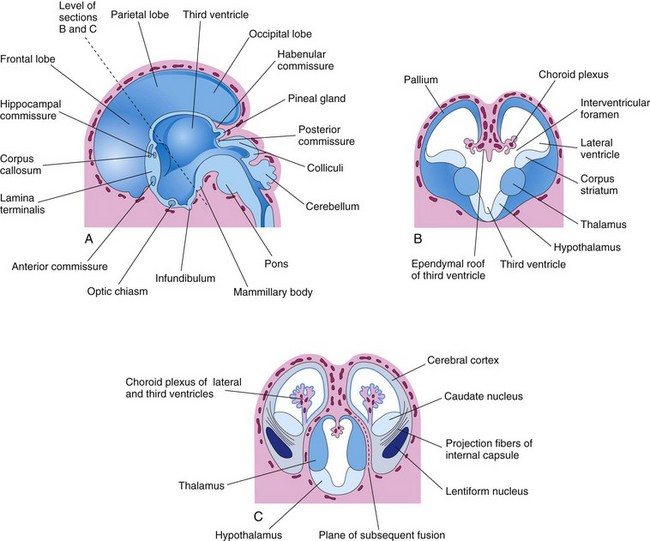
FIGURE 17–27 A, Drawing of the medial surface of the forebrain of a 10-week embryo showing the diencephalic derivatives, the main commissures, and the expanding cerebral hemispheres. B, Transverse section of the forebrain at the level of the interventricular foramina showing the corpus striatum and choroid plexuses of the lateral ventricles. C, Similar section at approximately 11 weeks showing division of the corpus striatum into the caudate and lentiform nuclei by the internal capsule. The developing relationship of the cerebral hemispheres to the diencephalon is also illustrated.
As the cerebral hemispheres expand, they cover successively the diencephalon, midbrain, and hindbrain. The hemispheres eventually meet each other in the midline, flattening their medial surfaces. The mesenchyme trapped in the longitudinal fissure between them gives rise to the cerebral falx (falx cerebri), a median fold of dura mater.
The corpus striatum appears during the sixth week as a prominent swelling in the floor of each cerebral hemisphere (Fig. 17-27B). The floor of each hemisphere expands more slowly than its thin cortical walls because it contains the rather large corpus striatum; consequently, the cerebral hemispheres become C-shaped (Fig. 17-28).
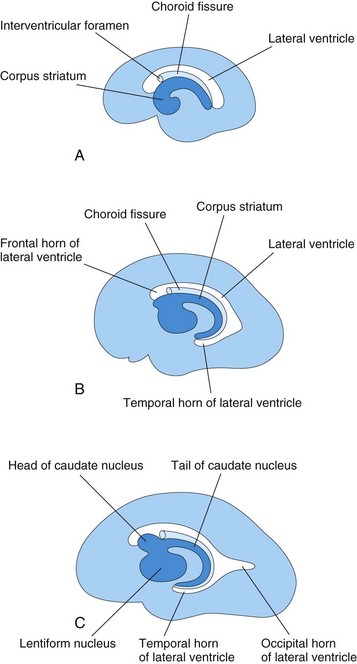
FIGURE 17–28 Schematic diagrams of the medial surface of the developing right cerebral hemisphere, showing the development of the lateral ventricle, choroid fissure, and corpus striatum. A, At 13 weeks. B, At 21 weeks. C, At 32 weeks.
The growth and curvature of the hemispheres also affect the shape of the lateral ventricles. They become roughly C-shaped cavities filled with CSF. The caudal end of each cerebral hemisphere turns ventrally and then rostrally, forming the temporal lobe; in so doing, it carries the lateral ventricle (forming its temporal horn) and choroid fissure with it (Fig. 17-28). Here, the thin medial wall of the hemisphere is invaginated along the choroid fissure by vascular pia mater to form the choroid plexus of the temporal horn (Fig. 17-27B).
As the cerebral cortex differentiates, fibers passing to and from it pass through the corpus striatum and divide it into caudate and lentiform nuclei. This fiber pathway—the internal capsule (Fig. 17-27C)—becomes C shaped as the hemisphere assumes this form. The caudate nucleus becomes elongated and C shaped, conforming to the outline of the lateral ventricle (Fig. 17-28). Its pear-shaped head and elongated body lie in the floor of the frontal horn and body of the lateral ventricle, whereas its tail makes a U-shaped turn to gain the roof of the temporal or inferior horn.
Cerebral Commissures
As the cerebral cortex develops, groups of nerve fibers—commissures—connect corresponding areas of the cerebral hemispheres with one another (Fig. 17-27A). The most important of these commissures cross in the lamina terminalis, the rostral (anterior) end of the forebrain. This lamina extends from the roof plate of the diencephalon to the optic chiasm (decussation or crossing of the fibers of the optic nerve). This lamina is the natural pathway from one hemisphere to the other.
The first commissures to form, the anterior commissure and hippocampal commissure, are small fiber bundles that connect phylogenetically older parts of the brain. The anterior commissure connects the olfactory bulb and related areas of one hemisphere with those of the opposite side. The hippocampal commissure connects the hippocampal formations.
The largest cerebral commissure is the corpus callosum (Figs. 17-27A and 17-28A), connecting neocortical areas. The corpus callosum initially lies in the lamina terminalis, but fibers are added to it as the cortex enlarges; as a result, it gradually extends beyond the lamina terminalis. The rest of the lamina terminalis lies between the corpus callosum and the fornix. It becomes stretched to form the thin septum pellucidum, a thin plate of brain tissue.
At birth, the corpus callosum extends over the roof of the diencephalon. The optic chiasm, which develops in the ventral part of the lamina terminalis (Fig. 17-27A), consists of fibers from the medial halves of the retinae, which cross to join the optic tract of the opposite side.
The walls of the developing cerebral hemispheres initially show the three typical zones of the neural tube (ventricular, intermediate, and marginal); later a fourth one, the subventricular zone, appears. Cells of the intermediate zone migrate into the marginal zone and give rise to the cortical layers. The gray matter is thus located peripherally, and axons from its cell bodies pass centrally to form the large volume of white matter—the medullary center.
Initially, the surface of the cerebral hemispheres is smooth (Fig. 17-29A); however, as growth proceeds, sulci (grooves between the gyri) and gyri (tortuous convolutions) develop (Figs. 17-29B and C). The gyri are caused by infolding of the cerebral cortex. The sulci and gyri permit a considerable increase in the surface area of the cerebral cortex without requiring an extensive increase in the size of the neurocranium (Fig. 17-30B and C). As each cerebral hemisphere grows, the cortex covering the external surface of the corpus striatum grows relatively slowly and is soon overgrown (Fig. 17-29D). This buried cortex, hidden from view in the depths of the lateral sulcus of the cerebral hemisphere (Fig. 17-30), is the insula (island).
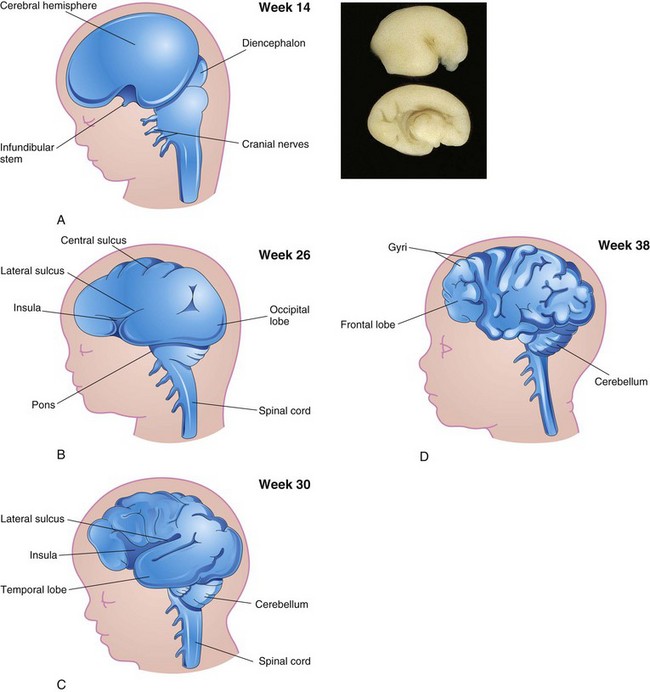
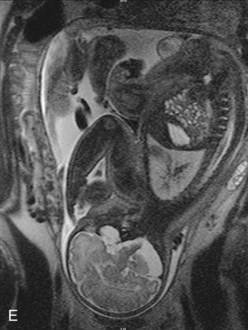
FIGURE 17–29 Sketches of lateral views of the left cerebral hemisphere, diencephalon, and brainstem, showing successive stages in the development of the sulci and gyri in the cerebral cortex. Note the gradual narrowing of the lateral sulcus and burying of the insula (island), an area of cerebral cortex that is concealed from surface view. Note that the surface of the cerebral hemispheres grows rapidly during the fetal period, forming many gyri (convolutions), which are separated by many sulci (grooves). A, At 14 weeks. B, At 26 weeks. C, At 30 weeks. D, At 38 weeks. E, Magnetic resonance image (MRI) of a pregnant woman showing a mature fetus. Observe the brain and spinal cord. Inset, The smooth lateral (top) and medial (bottom) surfaces of a human fetal brain (14 weeks).
(Inset, Courtesy of Dr. Marc Del Bigio, Department of Pathology [Neuropathology], University of Manitoba, Winnipeg, Manitoba, Canada. E, Courtesy of Dr. Stuart C. Morrison, Division of Radiology [Pediatric Radiology], The Children’s Hospital, Cleveland, Ohio.)

FIGURE 17–30 A, Lateral view of the brain of a stillborn fetus (25 weeks). B, The medial (top) and lateral (bottom) surfaces of the fetal brain (week 25). C, The lateral (top) and medial (bottom) surfaces of the fetal brain (week 38). Note that as the brain enlarges, the gyral pattern of the cerebral hemispheres becomes more complex; compare with Figure 17-29.
(A, From Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. U.S. Department of Health, Education, and Welfare, National Institutes of Health, Bethesda, Maryland, 1977. B and C, Courtesy of Dr. Marc Del Bigio, Department of Pathology [Neuropathology], University of Manitoba, Winnipeg, Manitoba, Canada.)
Birth Defects of Brain
Because of the complexity of its embryologic history, abnormal development of the brain is common (approximately 3 per 1000 births). Most major birth defects, such as meroencephaly and meningoencephalocele, result from defective closure of the rostral neuropore (NTD) during the fourth week (Fig. 17-31C) and involve the overlying tissues (meninges and calvaria). The factors causing NTD are genetic, nutritional, and/or environmental in nature. Birth defects of the brain can be caused by alterations in the morphogenesis or histogenesis of the nervous tissue, or can result from developmental failures occurring in associated structures (notochord, somites, mesenchyme, and cranium).
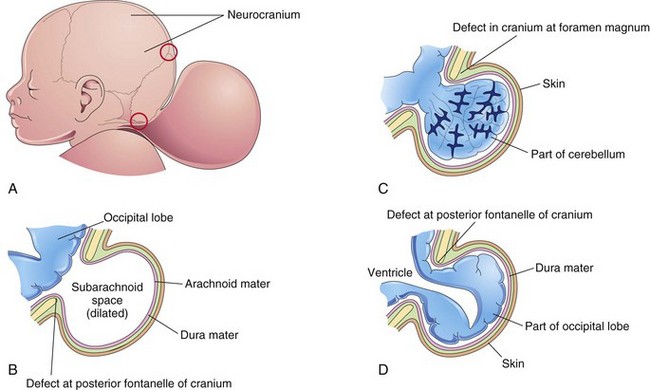
FIGURE 17–31 Schematic drawings illustrating encephalocele (cranium bifidum) and various types of herniation of the brain and/or meninges. A, Sketch of the head of a newborn infant with a large protrusion from the occipital region of the cranium. The upper red circle indicates a cranial defect at the posterior fontanelle (membranous interval between cranial bones). The lower red circle indicates a cranial defect near the foramen magnum. B, Meningocele consisting of a protrusion of the cranial meninges that is filled with cerebrospinal fluid (CSF). C, Meningoencephalocele consisting of a protrusion of part of the cerebellum that is covered by meninges and skin. D, Meningohydroencephalocele consisting of a protrusion of part of the occipital lobe that contains part of the posterior horn of a lateral ventricle.
Abnormal histogenesis of the cerebral cortex can result in seizures (Fig. 17-32) and various types of mental deficiency. Subnormal intellectual development may result from exposure of the embryo/fetus during the 8- to 16-week period to certain viruses and high levels of radiation (see Chapter 20). Prenatal factors (e.g., risk factors include maternal infection or thyroid disorder, Rh factor incompatibility, some hereditary and genetic conditions) result in the majority of cases of cerebral palsy; however, this central motor deficit may result from events during birth.
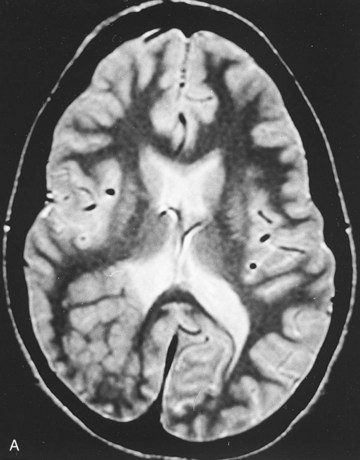
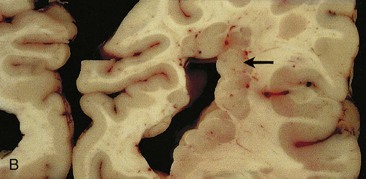
FIGURE 17–32 A, Focal heterotopic cerebral cortex. Magnetic resonance image of a 19-year-old woman with seizures, showing a focal heterotopic cortex of the right parietal lobe, indenting the right lateral ventricle; note the lack of organized cortex at the overlying surface of the brain. Heterotopic cortex is the result of an arrest of centrifugal migration of neuroblasts along the radial processes of glial cells. B, A coronal section of an adult brain with periventricular heterotopia (arrow) in the parietal cerebrum. The lobulated gray matter structures along the ventricle represent cells that failed to migrate but nevertheless differentiated into neurons.
(A, Courtesy of Dr. Gerald Smyser, Altru Health System, Grand Forks, North Dakota. B, Courtesy of Dr. Marc R. Del Bigio, Department of Pathology [Neuropathology], University of Manitoba, Winnipeg, Manitoba, Canada.)
Encephalocele
Encephalocele is a herniation of intracranial contents through a defect in the cranium (cranium bifidum). Encephaloceles are most common in the occipital region (Figs. 17-31A to D, 17-33, and 17-34). The hernia may contain meninges (menigocele), meninges and part of the brain (meningoencephalocele), or meninges, part of the brain, and part of the ventricular system (meningohydroencephalocele). Encephalocele occurs approximately once in every 2000 births.

FIGURE 17–33 An infant with a large meningoencephalocele in the occipital area.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
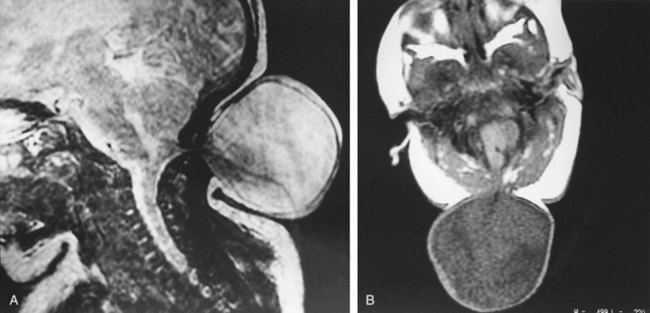
FIGURE 17–34 Magnetic resonance images (MRIs) of a 1-day-old infant, showing a menigocele. A, Sagittal MRI taken so that the cerebrospinal fluid (CSF) is bright. The image is blurred because of movement of the infant. B, Axial image located at the cranial defect near the foramen magnum and taken so that CSF appears dark.
(Compare with Figure 17-31C.) (Courtesy of Dr. Gerald S. Smyser, Altru Health System, Grand Forks, North Dakota.)
Meroencephaly
Meroencephaly (anencephaly, an inappropriate term) is a severe defect of the calvaria and brain that results from failure of the rostral neuropore to close during the fourth week. As a result, the forebrain, midbrain, most of the hindbrain and calvaria are absent (Figs. 17-13, 17-17, and 17-35). Most of the embryo’s brain is exposed or extruding from the cranium—exencephaly. Because of the abnormal structure and vascularization of the embryonic exencephalic brain, the nervous tissue undergoes degeneration. The remains of the brain appear as a spongy, vascular mass consisting mostly of hindbrain structures.
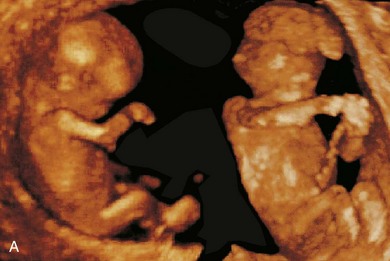
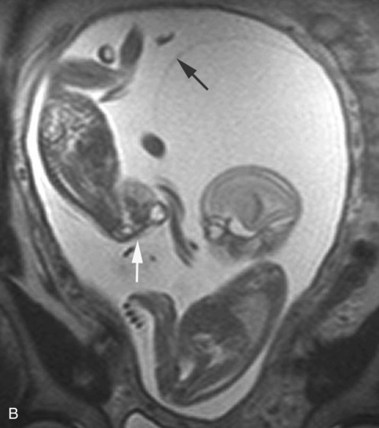
FIGURE 17–35 A, Sonogram of a normal fetus at 12 weeks (left) and a fetus at 14 weeks, showing acrania and meroencephaly (right). B, Magnetic resonance image (MRI) of diamniotic-monochorionic twins, one with meroencephaly. Note the absent calvaria (white arrow) of the abnormal twin and the amnion of the normal twin (black arrow).
(A, From Pooh RK, Pooh KH: Transvaginal 3D and Doppler ultrasonography of the fetal brain. Semin Perinatol 25:38, 2001. B, Courtesy of Deborah Levine, M.D., director, Obstetric and Gynecologic Ultrasound, Beth Israel Deaconess Medical Center, Boston, Massachusetts.)
Meroencephaly is a common lethal defect, occurring at least once in every 1000 births. It is two to four times more common in females than in males and it is always associated with acrania (complete or partial absence of the neurocranium). It may be associated with rachischisis when defective neural tube closure is extensive (Figs. 17-13 and 17-35). Meroencephaly is the most common serious defect seen in stillborn fetuses. Infants with this severe NTD may survive after birth, but only for a short period. Meroencephaly can be easily diagnosed by ultrasonography and MRI (Fig. 17-35), fetoscopy, and radiography because extensive parts of the brain and calvaria are absent.
Meroencephaly usually has a multifactorial mode of inheritance. An excess of amniotic fluid (polyhydramnios) is often associated with meroencephaly, possibly because the fetus lacks the neural control for swallowing amniotic fluid; thus, the fluid does not pass into the intestines for absorption and subsequent transfer to the placenta for disposal.
Microcephaly
Microcephaly is a neurodevelopmental disorder. The calvaria and brain are small, but the face is normal size (Fig. 17-36). These infants are grossly mentally deficient because the brain is underdeveloped. Microcephaly is the result of a reduction in brain growth. Inadequate pressure from the growing brain leads to the small size of the neurocranium. In the United States, about 25,000 infants are diagnosed annually.
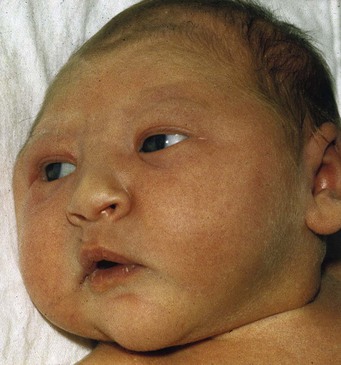
FIGURE 17–36 An infant with microcephaly showing the typical normal-sized face and small neurocranium. Usually this defect is associated with mental deficiency.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital, University of Manitoba, Winnipeg, Manitoba, Canada.)
Some cases appear to be genetic in origin. In autosomal recessive primary microcephaly, embryonic brain growth is reduced without affecting the structure of the brain. Exposure to large amounts of ionizing radiation, infectious agents (e.g., cytomegalovirus, rubella virus, and Toxoplasma gondii [see Chapter 20]), and certain drugs (maternal alcohol abuse) during the fetal period are contributing factors in some cases.
Microcephaly can be detected in utero by ultrasound scans carried out over the period of gestation. A small head may result from premature synostosis (osseous union) of all the cranial sutures (see Chapter 14); however, the neurocranium is thin with exaggerated convolutional markings.
Agenesis of Corpus Callosum
In this condition, there is a complete or partial absence of the corpus callosum, the main neocortical commissure of the cerebral hemispheres (Fig. 17-37A and B). The condition may be asymptomatic, but seizures and mental deficiency are common. Agenesis of the corpus callosum is associated with more than 50 different human congenital syndromes.
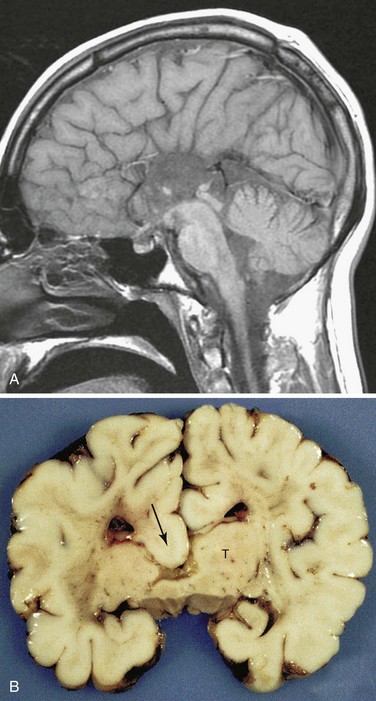
FIGURE 17–37 A, Sagittal magnetic resonance imaging of the brain of a 22-year-old normal functioning male. There is complete absence of the corpus callosum. B, A coronal slice through a child’s brain showing agenesis of the corpus callosum, which would normally cross the midline to connect the two cerebral hemispheres. Note the thalamus (T) and the downward displacement of the cingulum into the lateral and third ventricles (arrow).
(A, Courtesy of Dr. Gerald S. Smyser, Altru Health System, Grand Forks, North Dakota. B, Courtesy of Dr. Marc R. Del Bigio, Department of Pathology [Neuropathology], University of Manitoba, Winnipeg, Manitoba, Canada.)
Hydrocephalus
Significant enlargement of the head results from an imbalance between the production and absorption of CSF; as a result, there is an excess of CSF in the ventricular system of the brain (Fig. 17-38). Hydrocephalus results from impaired circulation and absorption of CSF and, in rare cases, from increased production of CSF by a choroid plexus adenoma (benign tumor). Impaired circulation of CSF often results from congenital aqueductal stenosis (Figs. 17-38 and 17-39). The cerebral aqueduct is narrow or consists of several minute channels. In a few cases, aqueductal stenosis is transmitted by an X-linked recessive trait, but most cases appear to result from a fetal viral infection (e.g., cytomegalovirus or Toxoplasma gondii [see Chapter 20]), or prematurity associated with intraventricular hemorrhage. Blood in the subarachnoid space may cause obliteration of the cisterns or arachnoid villi.
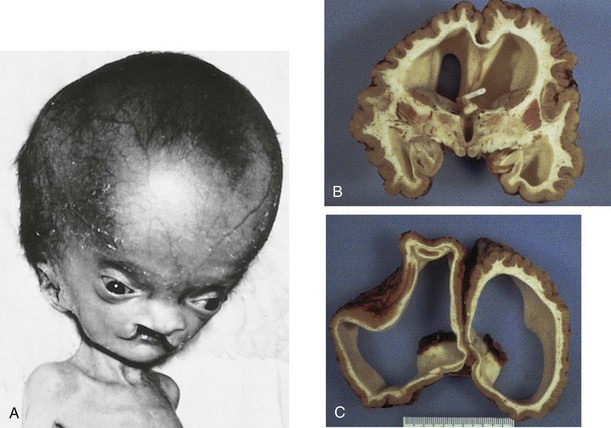
FIGURE 17–38 A, An infant with hydrocephalus and bilateral cleft palate. B and C, The brain of a 10-year-old child who had developed hydrocephalus in utero as a result of aqueductal stenosis. The thin white matter is well myelinated. A shunt tube meant to treat the hydrocephalus lies in the frontal horn of the ventricle.
(Courtesy of Dr. Marc R. Del Bigio, Department of Pathology [Neuropathology], University of Manitoba, Winnipeg, Manitoba, Canada.)
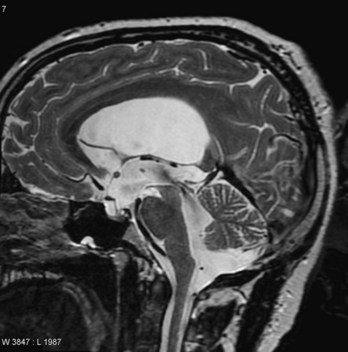
FIGURE 17–39 MRI demonstrating congenital stenosis of the cerebral aqueduct. This sagittal magnetic resonance image shows large lateral and third ventricles. The cerebrospinal fluid (CSF) appears bright in this image. There is also a marked flow void within the cerebral aqueduct.
(From Dr. Frank Gaillard, Radiopaedia.org, with permission.)
Blockage of CSF circulation results in dilation of the ventricles proximal to the obstruction, internal accumulation of CSF, and pressure on the cerebral hemispheres (Fig. 17-39). This squeezes the brain between the ventricular fluid and the neurocranium. In infants, the internal pressure results in an accelerated rate of expansion of the brain and neurocranium because most of the fibrous sutures are not fused. Hydrocephalus usually refers to obstructive or noncommunicating hydrocephalus, in which all or part of the ventricular system is enlarged. All ventricles are enlarged if the apertures of the fourth ventricle or the subarachnoid spaces are blocked, whereas the lateral and third ventricles are dilated when only the cerebral aqueduct is obstructed (Fig. 17-39). Obstruction of an interventricular foramen can produce dilation of one ventricle.
Hydrocephalus resulting from obliteration of the subarachnoid cisterns or malfunction of the arachnoid villi is called nonobstructive or communicating hydrocephalus. Although hydrocephalus may be associated with spina bifida cystica, enlargement of the head may not be obvious at birth. Hydrocephalus often produces thinning of the bones of the calvaria, prominence of the forehead, atrophy of the cerebral cortex and white matter (Fig. 17-38B and C ), and compression of the basal ganglia and diencephalon.
Holoprosencephaly
Holoprosencephalopathy (HPS) results from incomplete separation of the cerebral hemispheres and most are associated with facial abnormalities. Genetic and environmental factors have been implicated in this severe and relatively common (1 : 250 fetuses and 1 : 15,000 live births) developmental defect (Fig. 17-40). Maternal diabetes and teratogens, such as high doses of alcohol, can destroy embryonic cells in the median plane of the embryonic disc during the third week, producing a wide range of birth defects resulting from defective formation of the forebrain. In alobar holoprosencephaly, the forebrain is small and the lateral ventricles often merge to form one large ventricle.
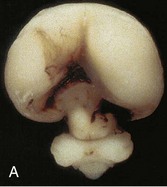
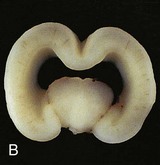
FIGURE 17–40 A frontal view of an intact (A) and coronally sectioned (B) fetal brain 21 weeks with holoprosencephaly. This defect results from failure of cleavage of the prosencephalon (rostral neural tube) into right and left cerebral hemispheres, telencephalon and diencephalon, and into olfactory bulbs and optic tracts.
(Courtesy of Dr. Marc R. Del Bigio, Department of Pathology [Neuropathology], University of Manitoba, Winnipeg, Manitoba, Canada).
Defects in forebrain development often cause facial anomalies resulting from a reduction in tissue in the frontonasal prominence (see Chapter 9). Holoprosencephaly (HPE) is often indicated when the eyes are abnormally close together (hypotelorism). Molecular studies have led to the identification of several holoprosencephaly-related genes, including sonic hedgehog (Shh).
Hydranencephaly
In this rare anomaly (Fig. 17-41), the cerebral hemispheres are absent or represented by membranous sacs with remnants of the cerebral cortex dispersed over the membranes. The brainstem (midbrain, pons, and medulla) is relatively intact. These infants generally appear normal at birth; however, the head grows excessively after birth because of the accumulation of CSF. A ventriculoperitoneal shunt is usually made to prevent further enlargement of the neurocranium. Mental development fails to occur and there is little or no cognitive development. The cause of this unusual, severe anomaly is uncertain; however, there is evidence that it may be the result of an early obstruction of blood flow to the areas supplied by the internal carotid arteries.
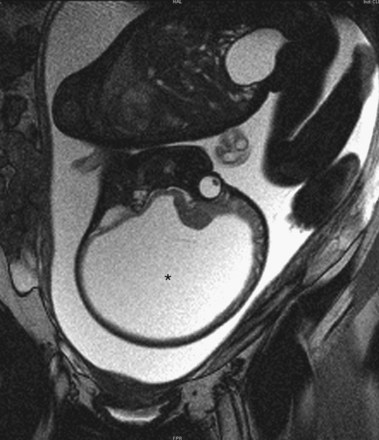
FIGURE 17–41 MRI of a fetus with massive hydrocephalus or hydrocephaly (*), showing excessive accumulation of CSF. Note the greatly reduced cerebral and displaced cerebral hemispheres and cerebellum.
(Courtesy of Dr. Stuart C. Morrison, Division of Radiology [Pediatric Radiology], The Children’s Hospital, Cleveland, Ohio.)
Arnold-Chiari Malformation
This is the most common birth defect involving the cerebellum (Fig. 17-42). It is a tongue-like projection of the medulla and inferior displacement of the vermis of the cerebellum through the foramen magnum into the cervical canal. The defect results in a type of communicating hydrocephalus in which there is interference with the absorption of CSF; as a result, the entire ventricular system is distended. The Arnold-Chiari malformation occurs once in every 1000 births and is always associated with spina bifida with meningomyelocele and hydrocephaly. The cause of the Arnold-Chiari malformation is uncertain; however, the posterior cranial fossa is abnormally small in these infants.
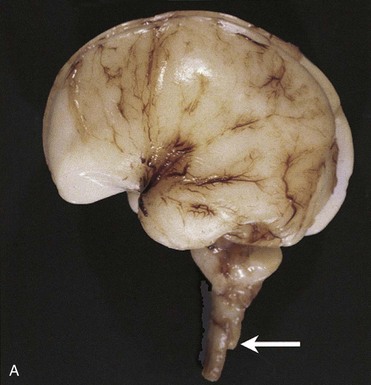
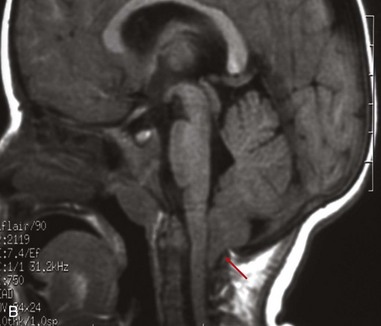
FIGURE 17–42 A, An Arnold-Chiari type II malformation in a fetus at 23 weeks. In situ exposure of the hindbrain reveals cerebellar tissue (arrow) well below the foramen magnum. B, MRI of a child with Arnold-Chiari type I malformation. Note the cerebellar tonsils lie inferior to the foramen magnum (red arrow).
(A, Courtesy of Dr. Marc R. Del Bigio, Department of Pathology [Neuropathology], University of Manitoba, Winnipeg, Manitoba, Canada. B, Courtesy of Dr. R. Shane Tubbs and Dr. W. Jerry Oakes, Children’s Hospital Birmingham, Birmingham, Alabama.)
Mental Deficiency
Congenital impairment of intelligence may result from various genetically determined conditions (e.g., Down syndrome). Mental deficiency (retardation) may also result from the action of a mutant gene or from a chromosomal abnormality (e.g., an extra chromosome 13, 17, or 21). Chromosomal abnormalities and mental deficiency are discussed in Chapter 20. Approximately 25% of cases have a demonstrable cause.
Maternal alcohol abuse is a common identifiable cause of mental deficiency. The 8th to 16th week period of human development is also the period of greatest sensitivity for fetal brain damage resulting from large doses of radiation. By the end of the 16th week, most neuronal proliferation and cell migration to the cerebral cortex are completed.
Cell depletion of sufficient degree in the cerebral cortex results in severe mental deficiency. Disorders of protein, carbohydrate, or fat metabolism may also cause mental deficiency. Maternal and fetal infections (e.g., syphilis, rubella virus, toxoplasmosis, and cytomegalovirus) and cretinism are commonly associated with mental deficiency. Deficient mental development throughout the postnatal growth period can result from birth injuries, toxins (e.g., lead), cerebral infections (e.g., meningitis), cerebral trauma from head injuries, and poisoning.
Development of Peripheral Nervous System
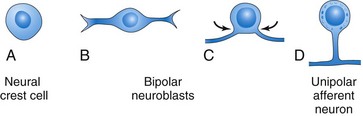
The Peripheral Nervous System (PNS) consists of cranial, spinal, and visceral nerves, and cranial, spinal, and autonomic ganglia. The PNS develops from various sources, mostly from the neural crest. All sensory cells (somatic and visceral) of the PNS are derived from neural crest cells. The cell bodies of these sensory cells are located outside the CNS. With the exception of the cells in the spiral ganglion of the cochlea and the vestibular ganglion of CN VIII (vestibulocochlear nerve), all peripheral sensory cells are at first bipolar. Later, the two processes unite to form a single process with peripheral and central components resulting in a unipolar type of neuron (Fig. 17-9D). The peripheral process terminates in a sensory ending, whereas the central process enters the spinal cord or brain (Fig. 17-8). The sensory cells in the ganglion of CN VIII remain bipolar.
The cell body of each afferent neuron is closely invested by a capsule of modified Schwann cells—satellite cells (Fig. 17-8), which are derived from neural crest cells. This capsule is continuous with the neurolemma (sheath of Schwann) that surrounds the axons of afferent neurons. External to the satellite cells is a layer of connective tissue that is continuous with the endoneurial sheath of the nerve fibers. This connective tissue and the endoneurial sheath are derived from mesenchyme.
Neural crest cells in the developing brain migrate to form sensory ganglia only in relation to the trigeminal (CN V), facial (CN VII), vestibulocochlear (CN VIII), glossopharyngeal (CN IX), and vagus (CN X) nerves. Neural crest cells also differentiate into multipolar neurons of the autonomic ganglia (Fig. 17-8), including ganglia of the sympathetic trunks that lie along the sides of the vertebral bodies; collateral, or prevertebral, ganglia in plexuses of the thorax and abdomen (e.g., cardiac, celiac, and mesenteric plexuses); and parasympathetic, or terminal, ganglia in or near the viscera (e.g., submucosal or Meissner plexus). Cells of the paraganglia—chromaffin cells—are also derived from the neural crest The term paraganglia includes several widely scattered groups of cells that are similar in many ways to medullary cells of the suprarenal glands. The cell groups largely lie retroperitoneally, often in association with sympathetic ganglia. The carotid and aortic bodies also have small islands of chromaffin cells associated with them. These widely scattered groups of cells constitute the chromaffin system. Neural crest cells also give rise to melanoblasts (precursors of melanocytes) and cells of the medulla of the suprarenal gland.
Spinal Nerves
Motor nerve fibers arising from the spinal cord begin to appear at the end of the fourth week (Figs. 17-4, 17-7, and 17-8). The nerve fibers arise from cells in the basal plates of the developing spinal cord and emerge as a continuous series of rootlets along its ventrolateral surface. The fibers destined for a particular developing muscle group become arranged in a bundle, forming a ventral nerve root. The nerve fibers of the dorsal nerve root are formed by axons derived from neural crest cells that migrate to the dorsolateral aspect of the spinal cord, where they differentiate into the cells of the spinal ganglion (Figs. 17-8 and 17-9).
The central processes of neurons in the spinal ganglion form a single bundle that grows into the spinal cord, opposite the apex of the dorsal horn of gray matter (Fig. 17-5B and C). The distal processes of spinal ganglion cells grow toward the ventral nerve root and eventually join it to form a spinal nerve.
Immediately after being formed, a mixed spinal nerve divides into dorsal and ventral primary rami (Latin, branches). The dorsal primary ramus, the smaller division, innervates the dorsal axial musculature (Fig. 15-1), vertebrae, posterior intervertebral joints, and part of the skin of the back. The ventral primary ramus, the major division of each spinal nerve, contributes to the innervation of the limbs and ventrolateral parts of the body wall. The major nerve plexuses (cervical, brachial, and lumbosacral) are formed by ventral primary rami.
As the limb buds develop, the nerves from the spinal cord segments opposite to the bud elongate and grow into the limb. The nerve fibers are distributed to its muscles, which differentiate from myogenic cells that originate from the somites (see Chapter 15).
The skin of the developing limbs is also innervated in a segmental manner. Early in development, successive ventral primary rami are joined by connecting loops of nerve fibers, especially those supplying the limbs (e.g., the brachial plexus). The dorsal division of the trunks of these plexuses supplies the extensor muscles and the extensor surface of the limbs; the ventral divisions of the trunks supply the flexor muscles and the flexor surface. The dermatomes and cutaneous innervation of the limbs are described in Chapter 16.
Cranial Nerves
Twelve pairs of cranial nerves form during the fifth and sixth weeks. They are classified into three groups, according to their embryologic origins.
Somatic Efferent Cranial Nerves
The trochlear (CN IV), abducent (CN VI), hypoglossal (CN XII), and the greater part of the oculomotor (CN III) nerves are homologous with the ventral roots of spinal nerves (Fig. 17-43). The cells of origin of these nerves are located in the somatic efferent column (derived from the basal plates) of the brainstem. Their axons are distributed to muscles derived from the head myotomes (preotic and occipital; Fig. 15-4).
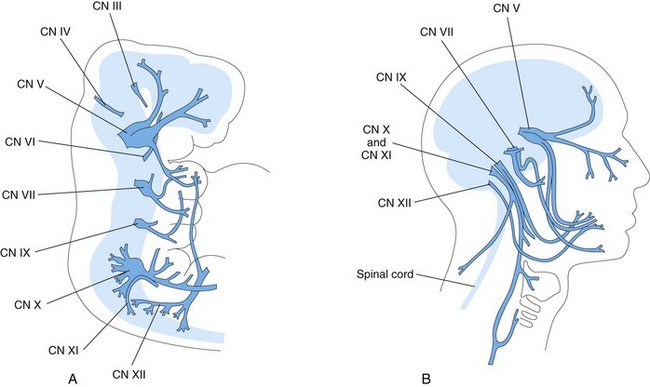
FIGURE 17–43 A, Schematic drawing of a 5-week embryo showing distribution of most of the cranial nerves, especially those supplying the pharyngeal arches. B, Schematic drawing of the head and neck of an adult showing the general distribution of most of the cranial nerves.
The hypoglossal nerve (CN XII) resembles a spinal nerve more than do the other somatic efferent CNs. CN XII develops by the fusion of the ventral root fibers of three or four occipital nerves (Fig. 17-43A). Sensory roots, corresponding to the dorsal roots of spinal nerves, are absent. The somatic motor fibers originate from the hypoglossal nucleus, consisting of motor cells resembling those of the ventral horn of the spinal cord. These fibers leave the ventrolateral wall of the medulla in several groups, the hypoglossal nerve roots, which converge to form the common trunk of CN XII (Fig. 17-43B). They grow rostrally and eventually innervate the muscles of the tongue, which are thought to be derived from occipital myotomes (see Fig. 15-4). With development of the neck, the hypoglossal nerve comes to lie at a progressively higher level.
The abducent nerve (CN VI) arises from nerve cells in the basal plates of the metencephalon. It passes from its ventral surface to the posterior of the three preotic myotomes from which the lateral rectus muscle of the eye is thought to originate.
The trochlear nerve (CN IV) arises from nerve cells in the somatic efferent column in the posterior part of the midbrain. Although a motor nerve, it emerges from the brainstem dorsally and passes ventrally to supply the superior oblique muscle of the eye.
The oculomotor nerve (CN III) supplies most muscles of the eye (i.e., the superior, inferior, and medial recti and inferior oblique muscles), which are derived from the first preotic myotomes.
Nerves of Pharyngeal Arches
CNs V, VII, IX, and X supply the embryonic pharyngeal arches; thus, the structures that develop from these arches are innervated by these CNs (Fig. 17-43A, Table 9-1).
The trigeminal nerve (CN V) is the nerve of the first pharyngeal arch, but it has an ophthalmic division that is not a pharyngeal arch component. CN V is chiefly sensory and is the principal sensory nerve for the head. The large trigeminal ganglion lies beside the rostral end of the pons, and its cells are derived from the most anterior part of the neural crest. The central processes of cells in this ganglion form the large sensory root of CN V, which enters the lateral portion of the pons. The peripheral processes of cells in this ganglion separate into three large divisions (ophthalmic, maxillary, and mandibular nerves). Their sensory fibers supply the skin of the face as well as the lining of the mouth and nose (Fig. 9-7).
The motor fibers of CN V arise from cells in the most anterior part of the special visceral efferent column in the metencephalon. The motor nucleus of CN V lies at the mid-level of the pons. The fibers leave the pons at the site of the entering sensory fibers and pass to the muscles of mastication and to other muscles that develop in the mandibular prominence of the first pharyngeal arch (Table 9-1). The mesencephalic nucleus of CN V differentiates from cells in the midbrain that extend rostrally from the metencephalon.
The facial nerve (CN VII) is the nerve of the second pharyngeal arch. It consists mostly of motor fibers that arise principally from a nuclear group in the special visceral efferent column in the caudal part of the pons. These fibers are distributed to the muscles of facial expression and to other muscles that develop in the mesenchyme of the second pharyngeal arch (Table 9-1). The small general visceral efferent component of CN VII terminates in the peripheral autonomic ganglia of the head. The sensory fibers of CN VII arise from the cells of the geniculate ganglion. The central processes of these cells enter the pons, and the peripheral processes pass to the greater superficial petrosal nerve and, via the chorda tympani nerve, to the taste buds in the anterior two thirds of the tongue.
The glossopharyngeal nerve (CN IX) is the nerve of the third pharyngeal arch. Its motor fibers arise from the special and, to a lesser extent, general visceral efferent columns of the anterior part of the myelencephalon. CN IX forms from several rootlets that arise from the medulla just caudal to the developing internal ear. All fibers from the special visceral efferent column are distributed to the stylopharyngeus muscle, which is derived from mesenchyme in the third pharyngeal arch (Table 9-1). The general efferent fibers are distributed to the otic ganglion, from which postganglionic fibers pass to the parotid and posterior lingual glands. The sensory fibers of CN IX are distributed as general sensory and special visceral afferent fibers (taste fibers) to the posterior part of the tongue.
The vagus nerve (CN X) is formed by fusion of the nerves of the fourth and sixth pharyngeal arches (Table 9-1). It has large visceral efferent and visceral afferent components that are distributed to the heart, foregut and its derivatives, and to a large part of the midgut. The nerve of the fourth pharyngeal arch becomes the superior laryngeal nerve, which supplies the cricothyroid muscle and constrictor muscles of the pharynx. The nerve of the sixth pharyngeal arch becomes the recurrent laryngeal nerve, which supplies various laryngeal muscles.
The spinal accessory nerve (CN XI) emerges as a series of rootlets from the cranial five or six cervical segments of the spinal cord (Fig. 17-43). The fibers of the traditional cranial root are now considered to be part of CN X. The fibers of the CN X supply the sternocleidomastoid and trapezius muscles.
Special Sensory Nerves
The olfactory nerve (CN I) arises from the olfactory organ. The olfactory receptor neurons differentiate from cells in the epithelial lining of the primordial nasal sac. The central processes of the bipolar olfactory neurons are collected into bundles to form approximately 20 olfactory nerves around which the cribriform plate of the ethmoid bone develops. These unmyelinated nerve fibers end in the olfactory bulb.
The optic nerve (CN II) is formed by more than a million nerve fibers that grow into the brain from neuroblasts in the primordial retina. Because the retina develops from the evaginated wall of the forebrain, the optic nerve actually represents a fiber tract of the brain. Development of the optic nerve is described in Chapter 18.
The vestibulocochlear nerve (CN VIII) consists of two kinds of sensory fiber in two bundles; these fibers are known as the vestibular and cochlear nerves. The vestibular nerve originates in the semicircular ducts, and the cochlear nerve proceeds from the cochlear duct, in which the spiral organ (of Corti) develops. The bipolar neurons of the vestibular nerve have their cell bodies in the vestibular ganglion. The central processes of these cells terminate in the vestibular nuclei in the floor of the fourth ventricle. The bipolar neurons of the cochlear nerve have their cell bodies in the spiral ganglion. The central processes of these cells end in the ventral and dorsal cochlear nuclei in the medulla.
 Development of Autonomic Nervous System
Development of Autonomic Nervous System
Functionally, the ANS can be divided into sympathetic (thoracolumbar) and parasympathetic (craniosacral) parts.
Sympathetic Nervous System
During the fifth week, neural crest cells in the thoracic region migrate along each side of the spinal cord, where they form paired cellular masses (ganglia) dorsolateral to the aorta (Fig. 17-8). All these segmentally arranged sympathetic ganglia are connected in a bilateral chain by longitudinal nerve fibers. These ganglionated cords—sympathetic trunks—are located on each side of the vertebral bodies. Some neural crest cells migrate ventral to the aorta and form neurons in the preaortic ganglia, such as the celiac and mesenteric ganglia (Fig. 17-8). Other neural crest cells migrate to the area of the heart, lungs, and gastrointestinal tract, where they form terminal ganglia in sympathetic organ plexuses, located near or within these organs.
After the sympathetic trunks have formed, axons of sympathetic neurons, located in the intermediolateral cell column (lateral horn) of the thoracolumbar segments of the spinal cord, pass through the ventral root of a spinal nerve and a white ramus communicans (communicating branch) to a paravertebral ganglion (Fig. 17-8). Here they may synapse with neurons or ascend or descend in the sympathetic trunk to synapse at other levels. Other presynaptic fibers pass through the paravertebral ganglia without synapsing, forming splanchnic nerves to the viscera. The postsynaptic fibers course through a gray communicating branch (gray ramus communicans), passing from a sympathetic ganglion into a spinal nerve; hence, the sympathetic trunks are composed of ascending and descending fibers. Bone morphogenic protein (BMP) signaling regulates the development of the sympathetic system through Smad 4 pathways.
Parasympathetic Nervous System
The presynaptic parasympathetic fibers arise from neurons in nuclei of the brainstem and in the sacral region of the spinal cord. The fibers from the brainstem leave through the oculomotor (CN III), facial (CN VII), glossopharyngeal (CN IX), and vagus (CN X) nerves. The postsynaptic neurons are located in peripheral ganglia or in plexuses near or within the structure being innervated (e.g., the pupil of the eye and salivary glands).
Summary of Nervous System
Clinically Oriented Problems
Case 17–1
A pregnant woman developed polyhydramnios over the course of a few days (acute polyhydramnios). After an ultrasound examination, a radiologist reported that the fetus had acrania and meroencephaly.
Case 17–2
A male infant was born with a large lumbar meningomyelocele that was covered with a thin membranous sac. Within a few days, the sac ulcerated and began to leak. A marked neurologic deficit was detected inferior to the level of the sac.
Case 17–3
An MRI scan of an infant with an enlarged head showed dilation of the lateral and third ventricles.
References and Suggested Reading
Amron D, Walsh CA. Genetic malformations of the human frontal lobe. Epilepsia. 2010;51(suppl 1):13.
Barros CS, Nguyen T, Spencer KS, et al. β1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development. 2009;136:2717.
Bekiesinska-Figatowska M, Herman-Sucharska I, Romaniuk-Doroszewska A, et al. Brain development of the human fetus in magnetic resonance imaging. Med Wieku Rozwoj. 2010;14:5.
Bell JE. The pathology of central nervous system defects in human fetuses of different gestational ages. In: Persaud TVN, editor. Advances in the Study of Birth Defects, Vol 7, Central Nervous System and Craniofacial Malformations. New York: Alan R. Liss, 1982.
Biencowe H, Cousens S, Modell B, et al. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol. 2010;39(Suppl 1):i110.
Cau E, Blader P. Notch activity in the nervous system: to switch or not switch. Neural Dev. 2009;4:36.
Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. 2010;220:217.
Cordero A, Mulinare J, Berry RJ, et al. CDC grand rounds: Additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010;59:980.
Davis SW, Castinetti F, Carvalho LR, et al. Molecular mechanisms of pituitary organogenesis: in search of novel regulatory genes. Mol Cell Endocrinol. 2010;323:4.
De Wals P, Tairou F, Van Allen MI, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med. 2007;357:135.
Diaz AL, Gleeson JG. The molecular and genetic mechanisms of neocortex development. Clin Perinatol. 2009;36:503.
Evans OB, Hutchins JB. Development of the nervous system. In Haines DE, editor: Fundamental Neuroscience, ed 3, New York: Churchill Livingstone, 2006.
Gressens P, Hüppi PS. Normal and abnormal brain development. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Guillemont F, Molnar Z, Tarabykin V, et al. Molecular mechanisms of cortical differentiation. Eur J Neurosci. 2006;23:857.
Haines DE. Neuroanatomy: An Atlas of Structures, Sections, and Systems, ed 8. Baltimore: Lippincott Williams & Wilkins; 2012.
Howard B, Chen Y, Zecevic N. Cortical progenitor cells in the developing human telencephalon. Glia. 2006;53:57.
Johnston MV, Kinsman S. Congenital anomalies of the central nervous system. In Behrman RE, Kliegman RM, Jenson HB, editors: Nelson Textbook of Pediatrics, ed 17, Philadelphia: WB Saunders, 2004.
Liu W, Komiya Y, Mezzacappa C, et al. MIM regulates vertebrate neural tube closure. Development. 2011;138:2035.
Lowery LA, Sive H. Totally tubular: the mystery behind function and origin of the brain ventricular system. BioEssays. 2009;31:446.
Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Nakatsu T, Uwabe C, Shiota K. Neural tube closure in humans initiates at multiple sites: Evidence from human embryos and implications for the pathogenesis of neural tube defects. Anat Embryol. 2000;201:455.
Noden DM. Spatial integration among cells forming the cranial peripheral neurons. J Neurobiol. 1993;24:248.
O’Rahilly R, Müller F. Embryonic Human Brain: An Atlas of Developmental Stages, ed 2. New York: Wiley-Liss; 1999.
ten Donkelaar HT, Lammens M. Development of the human cerebellum and its disorders. Clin Perinatol. 2009;36:513.
Thomaidou D, Politis PK, Matsas R. Neurogenesis in the central nervous system: cell cycle progression/exit and differentiation of neuronal progenitors. In: Giordano A, Galderisi U, editors. Cell Cycle Regulation and Differentiation in Cardiovascular and Neural Systems. New York: Springer, 2010.