Chapter 14 Skeletal System
As the notochord and neural tube form in the third week, the intraembryonic mesoderm lateral to these structures thickens to form two longitudinal columns of paraxial mesoderm (Fig. 14-1A and B). Toward the end of the third week, these dorsolateral columns, located in the trunk, become segmented into blocks of mesoderm—somites (Fig. 14-1C). Externally the somites appear as bead-like elevations along the dorsolateral surface of the embryo (see Chapter 5). Each somite differentiates into two parts (Fig. 14-1D and E):
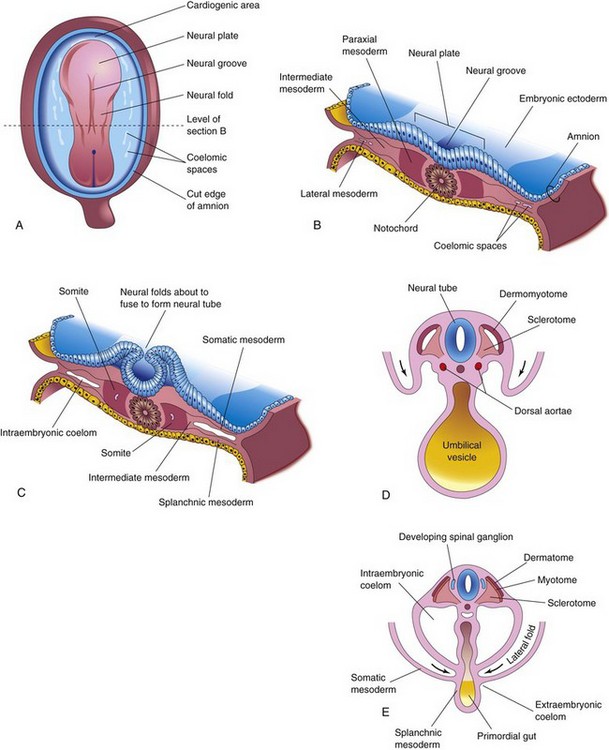
FIGURE 14–1 Illustrations of formation and early differentiation of somites. A, Dorsal view of an embryo of approximately 18 days. B, Transverse section of the embryo shown in A illustrating the paraxial mesoderm from which the somites are derived. C, Transverse section of an embryo of approximately 22 days showing the appearance of the early somites. Note that the neural folds are about to fuse to form the neural tube. D, Transverse section of an embryo of approximately 24 days showing folding of the embryo in the horizontal plane (arrows). The dermomyotome region of the somite gives rise to the dermatome and myotome. E, Transverse section of an embryo of approximately 26 days showing the dermatome, myotome, and sclerotome regions of a somite.
Development Of Bone And Cartilage
At the end of the fourth week, the sclerotome cells form a loosely woven tissue, mesenchyme (embryonic connective tissue), which has bone-forming capacity. Bones first appear as condensations of mesenchymal cells that form bone models. Condensation marks the beginning of selective gene activity, which precedes cell differentiation (Fig. 14-2). Most flat bones develop in mesenchyme within preexisting membranous sheaths; this type of osteogenesis is called membranous (intramembranous) bone formation. Mesenchymal models of most limb bones are transformed into cartilage bone models, which later become ossified by endochondral bone formation.
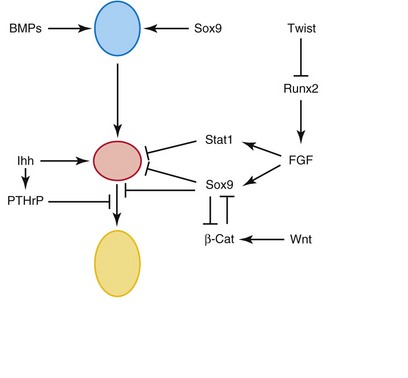
FIGURE 14–2 Schematic representation of secreted molecules and transcription factors regulating the initial differentiation, proliferation, and terminal differentiation of chondrocytes. From top to bottom: mesenchymal cells (blue), resting and proliferating (nonhypertrophic) chondrocytes (red), and hypertrophic chondrocytes (yellow). Line with arrowheads indicate a positive action, and lines with bars indicate an inhibition.
(From Karsenty G, Kronenberg HM, Settembre C: Genetic control of bone formation. Annu Rev Cell Dev Biol 25:629, 2009.)
Bone morphogenetic proteins (BMP-5 and BMP-7), the growth factor Gdf5, members of the transforming growth factor β (TGF-β) superfamily, and other signaling molecules have been implicated as endogenous regulators of chondrogenesis and skeletal development. Lineage commitment of skeletal precursor cells to chondrocytes and osteoblasts is determined by β-catenin levels. β-catenin in the canonical Wnt signaling pathway plays a critical role in the formation of cartilage and bone.
Histogenesis of Cartilage
Cartilage development begins from mesenchyme during the fifth week. In areas where cartilage is to develop, the mesenchyme condenses to form chondrification centers. The mesenchymal cells differentiate first into precondrocytes and then into chondroblasts, which secrete collagenous fibrils and ground substance (extracellular matrix). Subsequently, collagenous and/or elastic fibers are deposited in the intercellular substance or matrix. Three types of cartilage are distinguished according to the type of matrix that is formed:
Histogenesis of Bone
Bone primarily develops in two types of connective tissue, mesenchyme and cartilage, but it can also develop in other connective tissues (e.g., the patella develops in a tendon). Like cartilage, bone consists of cells and an organic intercellular substance—bone matrix—that comprises collagen fibrils embedded in an amorphous component. Studies of the cellular and molecular events during embryonic bone formation suggest that osteogenesis and chondrogenesis are programmed early in development, and are independent events under the influence of vascular changes (see Chapter 21).
Membranous Ossification
This type of bone formation occurs in mesenchyme that has formed a membranous sheath (Fig. 14-3). The mesenchyme condenses and becomes highly vascular; precursor cells differentiate into osteoblasts (bone-forming cells) and begin to deposit unmineralized matrix—osteoid. Calcium phosphate is then deposited in the osteoid tissue as it is organized into bone. Bone osteoblasts are trapped in the matrix and become osteocytes.
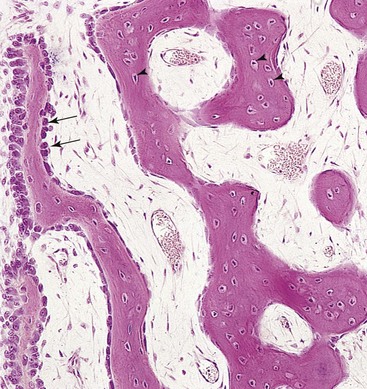
FIGURE 14–3 Light micrograph of membranous ossification (x132). Trabeculae of bone are being formed by osteoblasts lining their surface (arrows). Observe osteocytes trapped in lacunae (arrowheads) and that primordial osteons are beginning to form. The osteons (canals) contain blood capillaries.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 2nd ed. Philadelphia, WB Saunders, 2001.)
At first, new bone has no organized pattern. Spicules of bone soon become organized and coalesce into lamellae (layers). Concentric lamellae develop around blood vessels, forming osteons (Haversian systems). Some osteoblasts remain at the periphery of the developing bone and continue to lay down lamellae, forming plates of compact bone on the surfaces. Between the surface plates, the intervening bone remains spiculated or spongy. This spongy environment is somewhat accentuated by the action of cells—osteoclasts—that reabsorb bone. Osteoclasts are multinucleated cells with a hematopoietic origin. In the interstices of spongy bone, the mesenchyme differentiates into bone marrow. Hormones and cytokines regulate remodeling of bone by the coordinated action of osteoclasts and osteoblasts.
Endochondral Ossification
Endochondral ossification (cartilaginous bone formation) is a type of bone formation that occurs in preexisting cartilaginous models (Fig. 14-4A to E). In a long bone, for example, the primary center of ossification appears in the diaphysis—the part of a long bone between its ends—which forms the shaft of a bone (e.g., the humerus). At this center of ossification, chondrocytes (cartilage cells) increase in size (hypertrophy), the matrix becomes calcified, and the cells die.
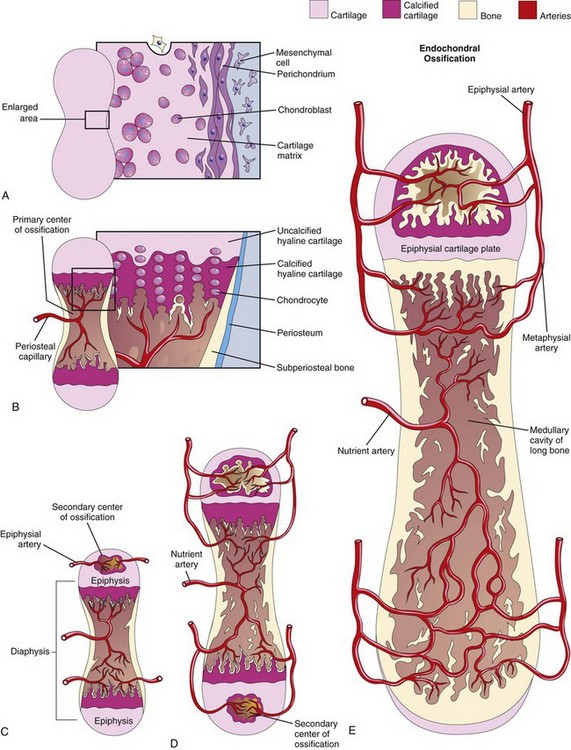
FIGURE 14–4 A to E, Schematic longitudinal sections of a 5-week embryo, illustrating endochondral ossification in a developing long bone.
Concurrently, a thin layer of bone is deposited under the perichondrium surrounding the diaphysis; thus, the perichondrium becomes the periosteum. Invasion by vascular connective tissue from blood vessels surrounding the periosteum also breaks up the cartilage. Osteoblasts reach the developing bone from these blood vessels. Some invading cells differentiate into hemopoietic cells, blood cells of the bone marrow. This process continues toward the epiphyses (ends of the bones). The spicules of bone are remodeled by the action of osteoclasts and osteoblasts. The transcription factor SOX9 and the co-activator-associated arginine methyltransferase-1 (CARM1) regulate ossochondral ossification.
Lengthening of long bones occurs at the diaphysial–epiphysial junction. Lengthening of bone depends on the epiphysial cartilage plates (growth plates), whose chondrocytes proliferate and participate in endochondral bone formation (Fig. 14-4E). Toward the diaphysis, the cartilage cells hypertrophy (increase in size) and the matrix becomes calcified. The spicules of the bone are isolated from each other by vascular invasion from the medullary (marrow) cavity of long bone (Fig. 14-4E). Bone is deposited on these spicules by osteoblasts; resorption of the bone keeps the spongy bone masses relatively constant in length and enlarges the medullary cavity.
Ossification of limb bones begins at the end of the embryonic period (56 days after fertilization). Thereafter it makes demands on the supply of calcium and phosphorus in pregnant women. Therefore, these women are advised to maintain an adequate intake of these elements to preserve healthy bones and teeth.
At birth, the diaphyses are largely ossified, but most of the epiphyses are still cartilaginous. Secondary ossification centers appear in the epiphyses in most bones during the first few years after birth. The epiphysial cartilage cells hypertrophy, and there is invasion by vascular connective tissue. Ossification spreads radially, and only the articular cartilage and a transverse plate of cartilage, the epiphysial cartilage plate, remain cartilaginous (see Fig. 14-4E). Upon completion of growth, this plate is replaced by spongy bone; the epiphyses and diaphysis are united, and no further elongation of the bone occurs.
In most bones, the epiphyses fuse with the diaphysis by the age of 20 years. Growth in the diameter of a bone results from deposition of bone at the periosteum (Fig. 14-4B), and from resorption on the internal medullary surface. The rate of deposition and resorption is balanced to regulate the thickness of the compact bone and the size of the medullary cavity. The internal reorganization of bone continues throughout life. The development of irregular bones is similar to that of the epiphyses of long bones. Ossification begins centrally and spreads in all directions.
Rickets
Rickets is a disease in children attributable to vitamin D deficiency. This vitamin is required for calcium absorption by the intestine. The resulting calcium (and also phosphorus) deficiency causes disturbances of ossification of the epiphysial cartilage plates (i.e., they are not adequately mineralized), and there is disorientation of cells at the metaphysis. The limbs are shortened and deformed, with severe bowing of the limb bones. Rickets can also delay closure of the fontanelles of the cranial bones in infants.
Development Of Joints
Joints begin to develop with the appearance of condensed mesenchyme in the joint interzone during the sixth week, and by the end of the eighth week, they resemble adult joints (Fig. 14-5). Joints are classified as fibrous joints, cartilaginous joints, and synovial joints. Joints with little or no movement are classified according to the type of material holding the bones together; for example, the bones involved in fibrous joints are joined by fibrous tissue. Molecular studies show that a distinct cohort of progenitor cells present at the prospective joint sites contribute to the formation of the synovial joints and articular cartilages.
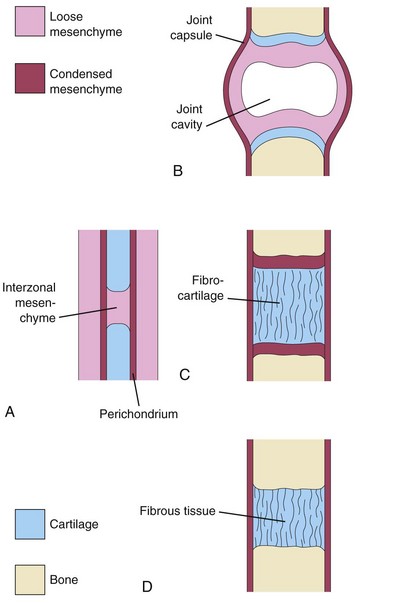
FIGURE 14–5 Development of joints during the sixth and seventh weeks. A, Condensed interzonal mesenchyme in the gap between the developing bones. This primordial joint may differentiate into a synovial joint (B), a cartilaginous joint (C), or a fibrous joint (D).
Fibrous Joints
During the development of fibrous joints, the interzonal mesenchyme between the developing bones differentiates into dense fibrous tissue (Fig. 14-5D); for example, the sutures of the cranium are fibrous joints (see Fig. 14-9).
Cartilaginous Joints
During the development of cartilaginous joints, the interzonal mesenchyme between the developing bones differentiates into hyaline cartilage (e.g., costochondral joints) or fibrocartilage (e.g., pubic symphysis) (Fig. 14-5C).
Synovial Joints
During the development of synovial joints (e.g., the knee joint), the interzonal mesenchyme between the developing bones differentiates as follows (Fig. 14-5B):
Probably as a result of joint movements, the mesenchymal cells subsequently disappear from the surfaces of the articular cartilages. An abnormal intrauterine environment restricting embryonic and fetal movements may interfere with limb development and cause joint fixation.
Development Of Axial Skeleton
The axial skeleton is composed of the cranium (skull), vertebral column, ribs, and sternum. During the fourth week, cells in the sclerotomes surround the neural tube (primordium of spinal cord) and the notochord, the structure around which the primordia of the vertebrae develop (Fig. 14-6A). This positional change of the sclerotomal cells is effected by differential growth of the surrounding structures and not by active migration of sclerotomal cells. The Hox and Pax genes regulate the patterning and regional development of the vertebrae along the anterior-posterior axis.
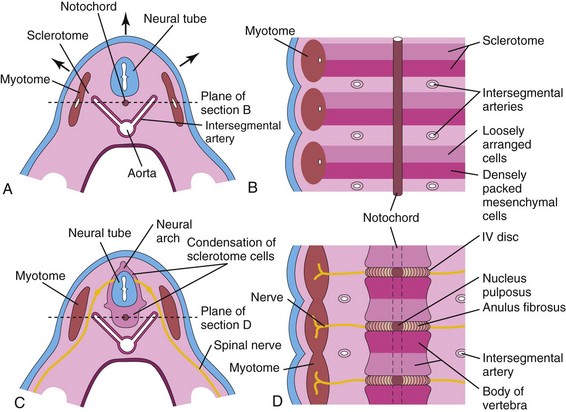
FIGURE 14–6 A, Transverse section through a 4-week embryo. The arrows indicate the dorsal growth of the neural tube and the simultaneous dorsolateral movement of the somite remnant, leaving behind a trail of sclerotomal cells. B, Diagrammatic frontal section of this embryo showing that the condensation of sclerotomal cells around the notochord consists of a cranial area of loosely packed cells and a caudal area of densely packed cells. C, Transverse section through a 5-week embryo showing the condensation of sclerotomal cells around the notochord and neural tube, which forms a mesenchymal vertebra. D, Diagrammatic frontal section illustrating that the vertebral body forms from the cranial and caudal halves of two successive sclerotomal masses. The intersegmental arteries now cross the bodies of the vertebrae, and the spinal nerves lie between the vertebrae. The notochord is degenerating except in the region of the intervertebral disc, where it forms the nucleus pulposus.
Development of Vertebral Column
During the precartilaginous or mesenchymal stage, mesenchymal cells from the sclerotomes are found in three main areas (Fig. 14-6A): around the notochord, surrounding the neural tube, and in the body wall. In a frontal section of a 4-week embryo, the sclerotomes appear as paired condensations of mesenchymal cells around the notochord (Fig. 14-6B). Each sclerotome consists of loosely arranged cells cranially and densely packed cells caudally.
Some densely packed cells move cranially, opposite the center of the myotome, where they form the intervertebral (IV) disc (Fig. 14-6C and D). The remaining densely packed cells fuse with the loosely arranged cells of the immediately caudal sclerotome to form the mesenchymal centrum, the primordium of the body of a vertebra. Thus, each centrum develops from two adjacent sclerotomes and becomes an intersegmental structure.
The nerves lie in close relationship to the IV discs, and the intersegmental arteries lie on each side of the vertebral bodies. In the thorax, the dorsal intersegmental arteries become the intercostal arteries.
The notochord degenerates and disappears where it is surrounded by the developing vertebral bodies. Between the vertebrae, the notochord expands to form the gelatinous center of the IV disc—the nucleus pulposus (Fig. 14-6D). This nucleus is later surrounded by circularly arranged fibers that form the annulus fibrosus. The nucleus pulposus and annulus fibrosus together form the IV disc. The mesenchymal cells, surrounding the neural tube, form the neural arch, that is, the primordium of vertebral arch (Fig. 14-6C). The mesenchymal cells in the body wall form costal processes, which form the ribs in the thoracic region.
Chordoma
Remnants of the notochord may persist and form a chordoma, a rare neoplasm (tumor). Approximately one third of these slow-growing malignant tumors occur at the base of the cranium and extend to the nasopharynx. Chordomas infiltrate bone and are difficult to remove. Chordomas also develop in the lumbosacral region. Surgical resection has provided long-term disease-free survival for many patients.
Cartilaginous Stage of Vertebral Development
During the sixth week, chondrification centers appear in each mesenchymal vertebra (Fig. 14-7A and B). The two centers in each centrum fuse at the end of the embryonic period to form a cartilaginous centrum. Concomitantly, the centers in the neural arches fuse with each other and the centrum. The spinous and transverse processes develop from extensions of chondrification centers in the neural arch. Chondrification spreads until a cartilaginous vertebral column is formed.
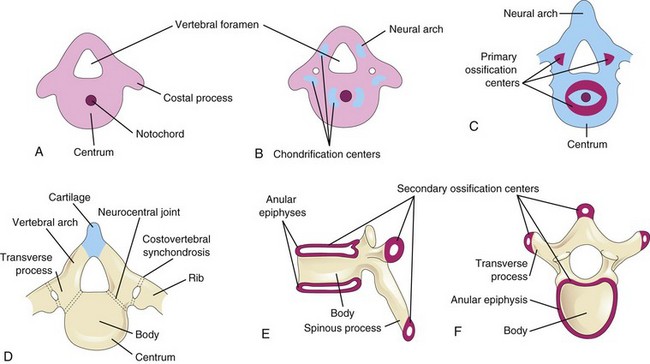
FIGURE 14–7 Stages of vertebral development. A, Mesenchymal vertebra at 5 weeks. B, Chondrification centers in a mesenchymal vertebra at 6 weeks. The neural arch is the primordium of the vertebral arch. C, Primary ossification centers in a cartilaginous vertebra at 7 weeks. D, Thoracic vertebra at birth consisting of three bony parts: vertebral arch, body of vertebra and transverse processes. Note the cartilage between the halves of the vertebral arch and between the arch and the centrum (neurocentral joint). E and F, Two views of a typical thoracic vertebra at puberty showing the location of the secondary centers of ossification.
Bony Stage of Vertebral Development
Ossification of typical vertebrae begins during the seventh week and ends by the 25th year. There are two primary ossification centers, ventral and dorsal, for the centrum (see Fig. 14-7C). These centers soon fuse to form one center. Three primary centers are present by the eighth week: one in the centrum and one in each half of the neural arch.
Ossification becomes evident in the neural arches during the eighth week. Each typical vertebra consists of three bony parts connected by cartilage: a vertebral arch, a body, and transverse processes (Fig. 14-7D). The bony halves of the vertebral arch usually fuse during the first 3 to 5 years. The arches first unite in the lumbar region, and union progresses cranially. The vertebral arch articulates with the centrum at cartilaginous neurocentral joints, which permit the vertebral arches to grow as the spinal cord enlarges. These joints disappear when the vertebral arch fuses with the centrum during the third to sixth years.
Five secondary ossification centers appear in the vertebrae after puberty:
The vertebral body is a composite of the anular epiphyses and the mass of bone between them. The vertebral body includes the centrum, parts of the vertebral arch, and the facets for the heads of the ribs. All secondary centers unite with the rest of the vertebrae at approximately 25 years of age. Exceptions to the typical ossification of vertebrae occur in the atlas or C1 vertebra, axis or C2 vertebra, C7 vertebra, lumbar vertebrae, sacrum, and coccyx.
The Notch signaling pathways are involved in the patterning of the vertebral column. Severe congenital birth defects, including: VACTERL Syndrome (Vertebral, Anal, Cardiac, Tracheal, Esophageal, Renal, and Limb birth defects), and CHARGE Association (coloboma of eye, heart defects: tetralogy of Fallot, patent ductus arteriosus, ventricular or atrial septal defect) are associated with mutation in Notch pathway genes. Minor defects of the vertebrae are common, but usually they are of little clinical importance.
Variation in the Number of Vertebrae
Most people have 7 cervical, 12 thoracic, 5 lumbar, and 5 sacral vertebrae. A few have one or two additional vertebrae or one fewer. To determine the number of vertebrae, it is necessary to examine the entire vertebral column because an apparent extra (or absent) vertebra in one segment of the column may be compensated for by an absent (or extra) vertebra in an adjacent segment; for example, 11 thoracic-type vertebrae with 6 lumbar-type vertebrae.
Development of Ribs
The ribs develop from the mesenchymal costal processes of the thoracic vertebrae (Fig. 14-7A). They become cartilaginous during the embryonic period and ossify during the fetal period. The original site of union of the costal processes with the vertebra is replaced by costovertebral synovial joints (Fig. 14-7D). Seven pairs of ribs (1–7)—true ribs—attach through their own cartilages to the sternum. Five pairs of ribs (8–12)—false ribs—attach to the sternum through the cartilage of another rib or ribs. The last two pairs of ribs (11 and 12)—floating ribs—do not attach to the sternum.
Development of Sternum
A pair of vertical mesenchymal bands, sternal bars, develop ventrolaterally in the body wall. Chondrification occurs in these bars as they move medially. By 10 weeks, they fuse craniocaudally in the median plane to form cartilaginous models of the manubrium, sternebrae (segments of sternal body), and xiphoid process. Centers of ossification appear craniocaudally in the sternum before birth, except that for the xiphoid process, which appears during childhood.
Development of Cranium
The cranium (skull) develops from mesenchyme around the developing brain. The growth of the neurocranium (bones of cranium enclosing the brain) is initiated from ossification centers within the desmocranium mesenchyme, which is the primordium of the cranium. Transforming growth factors beta (TGF-β) plays a critical role in the development of the cranium by regulating osteoblast differentiation. The cranium consists of the:
Cartilaginous Neurocranium
Initially, the cartilaginous neurocranium or chondrocranium consists of the cartilaginous base of the developing cranium, which forms by fusion of several cartilages (Fig. 14-8A to D). Later, endochondral ossification of the chondrocranium forms the bones in the base of the cranium. The ossification pattern of these bones has a definite sequence, beginning with the occipital bone, body of sphenoid, and ethmoid bone.
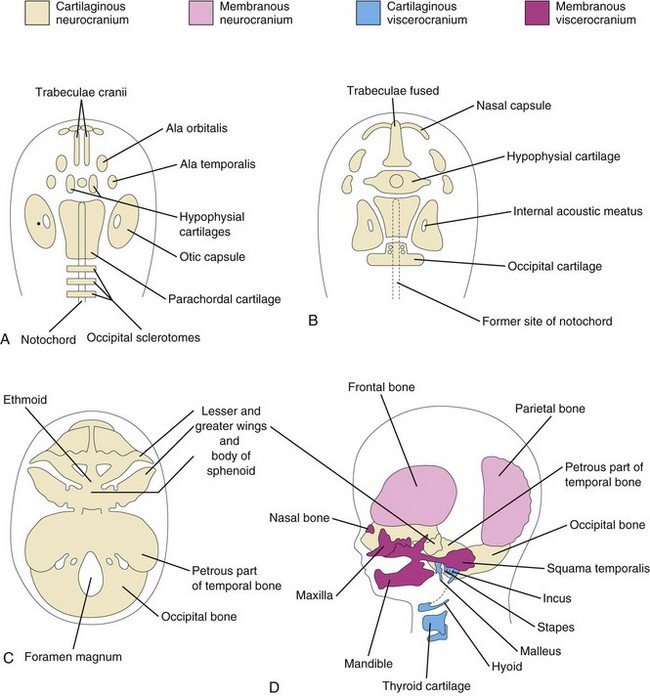
FIGURE 14–8 Stages in the development of the cranium. A to C, Views of the base of the developing cranium (viewed superiorly). D, A lateral view. A, At 6 weeks showing the various cartilages that will fuse to form the chondrocranium. B, At 7 weeks, after fusion of some of the paired cartilages. C, At 12 weeks showing the cartilaginous base of the cranium formed by the fusion of various cartilages. D, At 20 weeks indicating the derivation of the bones of the fetal cranium.
The parachordal cartilage, or basal plate, forms around the cranial end of the notochord (Fig. 14-8A), and fuses with the cartilages derived from the sclerotome regions of the occipital somites. This cartilaginous mass contributes to the base of the occipital bone; later, extensions grow around the cranial end of the spinal cord and form the boundaries of the foramen magnum (Fig. 14-8C).
The hypophysial cartilage forms around the developing pituitary gland (hypophysis cerebri) and fuses to form the body of the sphenoid bone. The trabeculae cranii fuse to form the body of the ethmoid bone, and the ala orbitalis forms the lesser wing of the sphenoid bone.
Otic capsules develop around the otic vesicles, the primordia of the internal ears (see Chapter 18), and form the petrous and mastoid parts of the temporal bone. Nasal capsules develop around the nasal sacs (Chapter 18) and contribute to the formation of the ethmoid bone.
Membranous Neurocranium
Membranous ossification (intramembranous ossification) occurs in the head mesenchyme at the sides and top of the brain, forming the calvaria (skullcap). During fetal life, the flat bones of the calvaria are separated by dense connective tissue membranes that form fibrous joints, the sutures of the calvaria (Fig. 14-9).
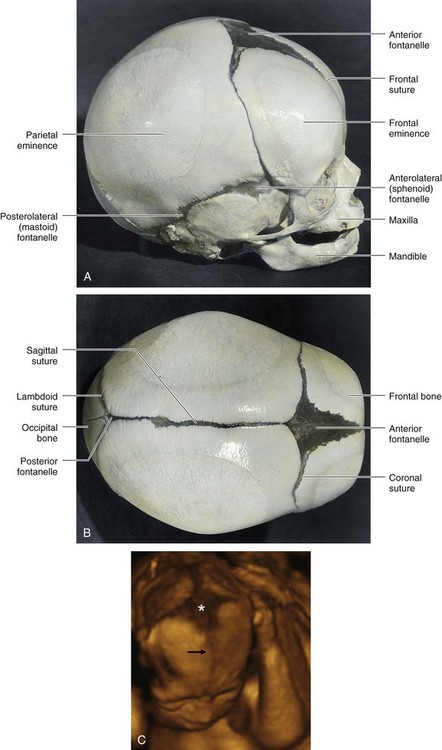
FIGURE 14–9 A fetal cranium showing the bones, fontanelles, and sutures. A, Lateral view. B, Superior view. The posterior and anterolateral fontanelles disappear because of growth of surrounding bones, within 2 or 3 months after birth, but they remain as sutures for several years. The posterolateral fontanelles disappear in a similar manner by the end of the first year and the anterior fontanelle by the end of the second year. The halves of the frontal bone normally begin to fuse during the second year, and the frontal suture is usually obliterated by the eighth year. The other sutures disappear during adult life, but the times when the sutures close are subject to wide variations. C, Three-dimensional ultrasound rendering of the fetal head at 22 weeks. Note the anterior fontanelle (*) and the frontal suture (arrow). The coronal and sagittal sutures are also shown.
(C, Courtesy of Dr. G. J. Reid, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Manitoba, Women’s Hospital, Winnipeg, Manitoba, Canada.)
Six large fibrous areas—fontanelles—are present where several sutures meet. The softness of the bones and their loose connections at the sutures enable the calvaria to undergo changes of shape during birth, called molding. During molding of the fetal cranium (adaptation of the fetus’s head to the pelvic cavity during birth), the frontal bones become flat, the occipital bone is drawn out, and one parietal bone slightly overrides the other one. Within a few days after birth, the shape of the calvaria returns to normal.
Cartilaginous Viscerocranium
Most mesenchyme in the head region is derived from the neural crest. Neural crest cells migrate into the pharyngeal arches and form the bones and connective tissue of craniofacial structures. Homeobox (Hox) genes regulate the migration and subsequent differentiation of the neural crest cells, which are crucial for the complex patterning of the head and face. These parts of the fetal cranium are derived from the cartilaginous skeleton of the first two pairs of pharyngeal arches (see Chapter 9).
Membranous Viscerocranium
Membranous ossification occurs in the maxillary prominence of the first pharyngeal arch (see Chapter 9), and subsequently forms the squamous temporal, maxillary, and zygomatic bones. The squamous temporal bones become part of the neurocranium. The mesenchyme in the mandibular prominence of the first pharyngeal arch condenses around its cartilage and undergoes membranous ossification to form the mandible. Some endochondral ossification (formation of osseous tissue by the replacement of calcified cartilage) occurs in the median plane of the chin and the mandibular condyle.
Cranium of Neonate
After recovering from molding, the neonate’s cranium is rather round and its bones are thin. Like the fetal cranium (Fig. 14-9), it is large in proportion to the rest of the skeleton, and the face is relatively small compared with the calvaria. The small facial region of the cranium results from the small size of the jaws, virtual absence of paranasal (air) sinuses, and underdevelopment of the facial bones.
Postnatal Growth of Cranium
The fibrous sutures of the neonate’s calvaria permit the brain to enlarge during infancy and childhood. The increase in the size of the calvaria is greatest during the first 2 years, the period of most rapid postnatal growth of the brain. The calvaria normally increases in capacity until approximately 16 years of age. After this, it usually increases slightly in size for 3 to 4 years because of thickening of its bones. There is also rapid growth of the face and jaws, coinciding with eruption of the primary (deciduous) teeth. These facial changes are more marked after the secondary (permanent) teeth erupt (see Chapter 19). There is concurrent enlargement of the frontal and facial regions, associated with the increase in the size of the paranasal sinuses (e.g., frontal and ethmoid sinuses). Most paranasal sinuses are rudimentary or absent at birth. Growth of these sinuses is important in altering the shape of the face and in adding resonance to the voice.
Klippel-Feil Syndrome (Brevicollis)
The main features of this syndrome are shortness of the neck, low hairline, and restricted neck movements, fusion of cervical vertebral bodies and abnormalities of the brainstem and cerebellum. In most cases, the number of cervical vertebral bodies is fewer than normal due to fusion of vertebrae before birth. In some cases, there is a lack of segmentation of several elements of the cervical region of the vertebral column. The number of cervical nerve roots may be normal but they are small, as are the intervertebral foramina. Individuals with this syndrome may have other birth defects, including scoliosis (abnormal lateral and rotational curvature of the vertebral column) and urinary tract disorders.
Spina Bifida
Failure of the halves of the embryonic cartilaginous neural arch to fuse results in major birth defects—spina bifida (see Fig. 17-12). The incidence of these vertebral defects ranges from 0.04% to 0.15%; they occur more frequently in girls than boys. Most cases of spina bifida (80%) are “open” and covered by a thin membrane. For a description of the various types of spina bifida, see Chapter 17 (Figs. 17-14 to 17-17).
Accessory Ribs
Accessory ribs, usually rudimentary, result from the development of the costal processes of cervical or lumbar vertebrae (Fig. 14-10A). These processes usually form ribs only in the thoracic region. The most common type of accessory rib is a lumbar rib, but it usually does not cause problems. A cervical rib occurs in 0.5% to 1% of individuals. This supernumerary rib is usually attached to the manubrium of the sternum (Fig. 14-10A), or the seventh cervical vertebra. Accessory ribs may be unilateral or bilateral. Pressure of a cervical rib on the brachial plexus of nerves, located partly in the neck and axilla, or on the subclavian artery often produces neurovascular symptoms (e.g., paralysis and anesthesia of the upper limb).
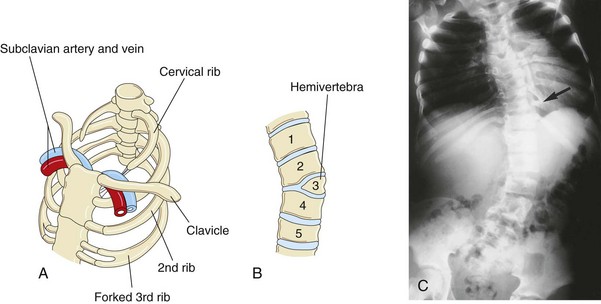
FIGURE 14–10 Vertebral and rib abnormalities. A, Cervical and forked ribs. Observe that the left cervical rib has a fibrous band that passes posterior to the subclavian vessels and attaches to the manubrium of the sternum. B, Anterior view of the vertebral column showing a hemivertebra. The right half of the third thoracic vertebra is absent. Note the associated lateral curvature (scoliosis) of the vertebral column. C, Radiograph of a child with the kyphoscoliotic deformity of the lumbar region of the vertebral column showing multiple anomalies of the vertebrae and ribs. Note the fused ribs (arrow).
(Courtesy of Dr. Prem S. Sahni, formerly of the Department of Radiology, Children’s Hospital, Winnipeg, Manitoba, Canada.)
Fused Ribs
Fusion of ribs occasionally occurs posteriorly when two or more ribs arise from a single vertebra (Fig. 14-10C ). Fused ribs are often associated with a hemivertebra.
Hemivertebra
In normal circumstances, the developing vertebral bodies have two chondrification centers that soon unite. A hemivertebra results from failure of one of the chondrification centers to appear and subsequent failure of half of the vertebra to form (see Fig. 14-10B). Hemivertebrae are the most common cause of congenital scoliosis (lateral and rotational curvature) of the vertebral column (Fig. 14-10C ). There are other less common causes of scoliosis (e.g., myopathic scoliosis resulting from weakness of the back muscles).
Rachischisis
Rachischisis (cleft vertebral column) refers to vertebral abnormalities in a complex group of anomalies (spinal dysraphism) that primarily affect axial structures (Fig. 14-11). In these infants the neural folds fail to fuse, either because of faulty induction by the underlying notochord, or from the action of teratogenic agents on the neuroepithelial cells in the neural folds. The neural and vertebral defects may be extensive or be restricted to a small area.

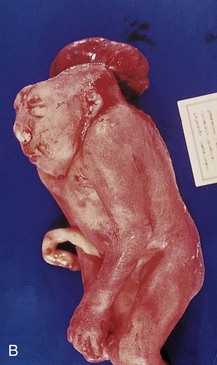
FIGURE 14–11 A, A second-trimester fetus with holoacrania (absence of the cranium, i.e., acrania). Note the cyst-like structure surrounding the intact fetal brain. B, Lateral view of a newborn infant with acrania and meroencephaly (partial absence of the brain), as well as rachischisis, which are extensive clefts in vertebral arches of the vertebral column (not clearly visible).
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, University of Manitoba, Children’s Hospital, Winnipeg, Manitoba, Canada.)
Anomalies of Sternum
A concave depression of the lower sternum—pectus excavatum—is the most common (90%) thoracic wall defect seen. Males are more often affected (1:400–1:1000 live births). It is probably due to overgrowth of the costal cartilages, which displaces the lower sternum inward. Minor sternal clefts (e.g., a notch or foramen in the xiphoid process) are common and are of no clinical concern. A sternal foramen of varying size and form occurs occasionally at the junction of the third and fourth sternebrae (segments of primordial sternum). This insignificant foramen is the result of incomplete fusion of the cartilaginous sternal bars during the embryonic period.
Cranial Birth Defects
These abnormalities range from major defects that are incompatible with life (Fig. 14-11) to those that are minor and insignificant. With large defects, there is often herniation of the meninges and/or brain (see Chapter 17).
Acrania
In this condition, there is complete or partial absence of the neurocranium (brain box); extensive defects of the vertebral column are often present (Fig. 14-11). Acrania associated with meroencephaly (partial absence of the brain) occurs approximately once in 1000 births and is incompatible with life. Meroencephaly results from failure of the cranial end of the neural tube to close during the fourth week. This birth defect causes subsequent failure of the neurocranium to form (Fig. 14-11B).
Craniosynostosis
Prenatal fusion of the cranial sutures results in several birth defects. The cause of craniosynostosis is unclear. Homeobox gene Msx2, Alx4, FGFR, TWIST, and MSX2 mutations have been implicated in the molecular mechanisms of craniosynostosis and other cranial defects. A strong association between maternal anticonvulsant use during early pregnancy and infant craniosynostosis has been reported. These birth defects are more common in males than in females and they are often associated with other skeletal anomalies.
The type of deformed cranium produced depends on which sutures close prematurely. If the sagittal suture closes early, the cranium becomes long, narrow, and wedge shaped—scaphocephaly (Fig. 14-12A and B). This type of cranial deformity constitutes about half the cases of craniosynostosis. Another 30% of cases involve premature closure of the coronal suture, which results in a high, tower-like cranium—brachycephaly (Fig. 14-12C ). If the coronal suture closes prematurely on one side only, the cranium is twisted and asymmetrical—plagiocephaly. Premature closure of the frontal (metopic) suture results in a deformity of the frontal bone and other anomalies—trigonocephaly (Fig. 14-12D).
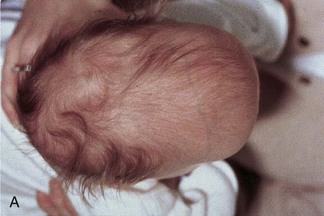
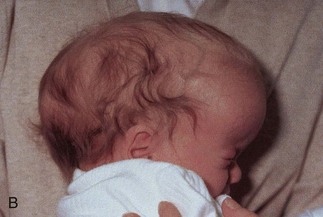
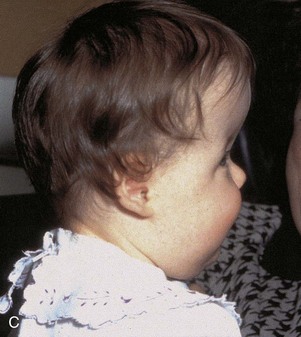
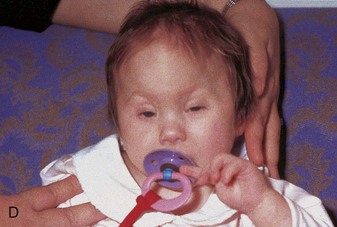
FIGURE 14–12 Craniosynostosis. A and B, An infant with scaphocephaly. This condition results from premature closure (synostosis) of the sagittal suture. Note the elongated, wedge-shaped cranium seen from above (A) and the side (B). C, An infant with bilateral premature closure of the coronal suture (brachycephaly). Note the high, markedly elevated forehead. D, An infant with premature closure of the frontal suture (trigonocephaly). Note the hypertelorism (abnormal distance between the eyes) and prominent midline ridging of the forehead.
(Courtesy of Dr. John A. Jane, Sr., David D. Weaver Professor of Neurosurgery, Department of Neurological Surgery, University of Virginia Health System, Charlottesville, Virginia.)
Microcephaly
Infants with this birth defect are born with a normal sized or slightly small calvaria. The fontanelles close during early infancy and the other sutures close during the first year. However, this defect is not caused by premature closure of sutures. Microcephaly is the result of abnormal development of the central nervous system in which the brain, and consequently the neurocranium, fail to grow. Generally, infants with microcephaly have small heads and are mentally deficient. This birth defect is also illustrated and discussed in Chapter 17.
Anomalies at Craniovertebral Junction
Congenital abnormalities at the craniovertebral junction are present in approximately 1% of neonates, but they may not produce symptoms until adult life. The following are examples of these anomalies: basilar invagination (superior displacement of bone around the foramen magnum), assimilation of the atlas (nonsegmentation at the junction of the atlas and occipital bone), atlantoaxial dislocation, Arnold-Chiari malformation (see Chapter 17), and a separate dens (failure of the centers in the dens to fuse with the centrum of the axis).
Development Of Appendicular Skeleton
The appendicular skeleton consists of the pectoral and pelvic girdles and the limb bones. Mesenchymal bones form during the fifth week as condensations of mesenchyme appear in the limb buds (Fig. 14-13A to C). During the sixth week, the mesenchymal bone models in the limbs undergo chondrification to form hyaline cartilage bone models (Fig. 14-13D and E).

FIGURE 14–13 A, Photograph of an embryo at approximately 28 days showing the early appearance of the limb buds. B, Longitudinal section through an upper limb bud showing the apical ectodermal ridge, which has an inductive influence on the mesenchyme in the limb bud. This ridge promotes growth of the mesenchyme and appears to give it the ability to form specific cartilaginous elements. C, Similar sketch of an upper limb bud at approximately 33 days showing the mesenchymal primordia of the forearm bones. The digital rays are mesenchymal condensations that undergo chondrification and ossification to form the bones of the hand. D, Upper limb at 6 weeks showing the cartilage models of the bones. E, Later in the sixth week showing the completed cartilaginous models of the bones of the upper limb.
(A, Courtesy of Dr. Brad Smith, University of Michigan, Ann Arbor, Michigan.)
The clavicle initially develops by membranous ossification and it later forms growth cartilages at both ends. The models of the pectoral girdle and upper limb bones appear slightly before those of the pelvic girdle and lower limb bones. The bone models appear in a proximodistal sequence. Patterning in the developing limbs is regulated by homeobox containing (Hox) genes (see Chapter 21).
Ossification begins in the long bones by the eighth week and initially occurs in the diaphyses of the bones from primary ossification centers (Fig. 14-4). By 12 weeks, primary ossification centers have appeared in nearly all bones of the limbs (Fig. 14-14). The clavicles begin to ossify before any other bones in the body. The femora are the next bones to show traces of ossification. The first indication of the primary center of ossification in the cartilaginous model of a long bone is visible near the center of its future shaft, the diaphysis (Fig. 14-4C). Primary centers appear at different times in different bones, but most of them appear between the 7th and 12th weeks. Virtually all primary centers of ossification are present at birth.
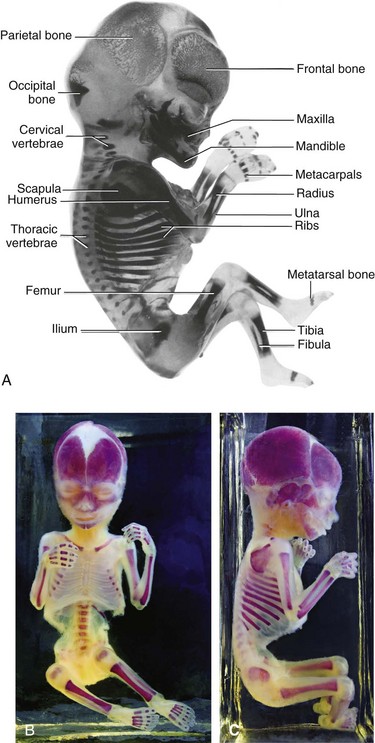
FIGURE 14–14 Alizarin-stained and cleared human fetuses. A, A 12-week fetus. Observe the degree of progression of ossification from the primary centers of ossification, which is endochondral in the appendicular and axial parts of the skeleton except for most of the cranial bones (i.e., those that form the neurocranium). Observe that the carpus and tarsus are wholly cartilaginous at this stage, as are the epiphyses of all long bones. B and C, An approximately 20-week fetus.
(A, Courtesy of Dr. Gary Geddes, Lake Oswego, Oregon. B and C, Courtesy of Dr. David Bolender, Department of Cell Biology, Neurobiology, and Anatomy, Medical College of Wisconsin, Milwaukee, Wisconsin.)
The secondary ossification centers of the bones at the knee are the first to appear in utero. The centers for the distal end of the femur and the proximal end of the tibia usually appear during the last month of intrauterine life. Consequently, these centers are usually present at birth; however, most secondary centers of ossification appear after birth. The part of a bone ossified from a secondary center is the epiphysis (Fig. 14-4C). The bone formed from the primary center in the diaphysis does not fuse with that formed from the secondary centers in the epiphyses until the bone grows to its adult length. This delay enables lengthening of the bone to continue until the final size is reached. During bone growth, a plate of cartilage known as the epiphysial cartilage plate intervenes between the diaphysis and the epiphysis (see Fig. 14-4E). The epiphysial plate is eventually replaced by bone development on each of its two sides, diaphysial and epiphysial. When this occurs, growth of the bone ceases.
Bone Age
Bone age is a good index of general maturation. Determination of the number, size, and fusion of epiphysial centers from radiographs is a commonly used method. A radiologist determines the bone age of a person by assessing the ossification centers using two criteria:
Fusion of the diaphysial–epiphysial centers, which occurs at specific times for each epiphysis, happens 1 to 2 years earlier in females than in males. Individual variation also occurs. In the fetus, ultrasonography is used for the evaluation and measurement of bones as well as for determination of fertilization age.
Generalized Skeletal Malformations
Achondroplasia is the most common cause of dwarfism—shortness of stature (see Chapter 20). It occurs approximately once in 15,000 births. The limbs become bowed and short (Fig. 14-15) because of disturbance of endochondral ossification during fetal life at the epiphysial cartilage plates, particularly of long bones. The trunk (of body) is usually short and the head is enlarged with a bulging forehead and “scooped-out” nose (flat nasal bridge).

FIGURE 14–15 Radiograph of the skeletal system of a 2-year-old child with achondroplasia. Note the shortening of the humerus and femur with metaphysis flaring.
(Courtesy of Dr. Prem S. Sahni, formerly of the Department of Radiology, Children’s Hospital, Winnipeg, Manitoba, Canada.)
Achondroplasia is an autosomal dominant disorder and approximately 80% of cases arise from new mutations; the rate increases with paternal age. The majority of cases are due to a point mutation (f.1,11,12) in the FGFR3 gene, which results in magnification of the normal inhibiting effect of endochondral ossification, specifically in the zone of chondrocyte proliferation. This results in shortened bones but does not affect periosteal bone growth.
Thanatophoric dysplasia is the most common type of lethal skeletal dysplasia. It occurs approximately once in 20,000 births. The affected infants die within minutes or days of respiratory failure. This lethal disorder is associated with mutations in the fibroblast growth factor receptor 3.
Hyperpituitarism
Congenital infantile hyperpituitarism, which causes an infant to grow at an abnormally rapid rate, is rare. This may result in gigantism (excessive height and body proportions) or acromegaly in the adult (enlargement of soft tissues, visceral organs, and bones of face, hands, and feet [acral bones, limb bones]). Both gigantism and acromegaly result from an excessive secretion of growth hormone.
Hypothyroidism and Cretinism
A severe deficiency of fetal thyroid hormone production results in cretinism, a condition characterized by growth retardation, mental deficiency, skeletal abnormalities, and auditory and neurologic disorders. Bone age appears as less than chronologic age because epiphysial development is delayed. Cretinism is rare except in areas where there is a lack of iodine in the soil and water. Agenesis (absence) of the thyroid gland also results in cretinism.
Summary Of Skeletal System
Clinically Oriented Problems
Case 14–1
A neonate presented with a lesion in his lower back, which was thought to be a neural arch defect.
Case 14–2
A young girl presented with pain in her upper limb, which worsened when she lifted heavy objects. After a radiographic examination, the physician told her parents that she had an accessory rib in her neck.
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Alexander PG, Tuan RS. Role of environmental factors in axial skeletal dysmorphogenesis. Birth Defects Res C. 2010;90:118.
Bamshad M, Van Heest AE, Pleasure D. Arthrogryposis: a review and update. J Bone Joint Surg Am. 2009;91(Suppl 4):40.
Brewin J, Hill M, Ellis H. The prevalence of cervical ribs in a London population. Clin Anat. 2009;22:331.
Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16:525.
Cohen MMJr. Perspectives on craniosynostosis: sutural biology, some well-known syndromes and some unusual syndromes. J Craniofac Surg. 2009;20:646.
Cooperman DR, Thompson GH. Musculoskeletal disorders. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal–Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann NY Acad Sci. 2010;1192:437.
Dunwoodie SL. The role of Notch in patterning the human vertebral column. Curr Opin Genet Dev. 2009;19:329.
Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: How osteoblasts become osteocytes. Dev Dyn. 2006;235:176.
Gibb S, Maroto M, Dale JK. The segmentation clock mechanism moves up a notch. Trends Cell Biol. 2010;20:593.
Gardner LP, Hiatt JL. Color Textbook of Histology, ed 3. Philadelphia: Saunders; 2007.
Hall BK. Bones and Cartilage: Developmental Skeletal Biology. Philadelphia: Elsevier; 2005.
Hinrichsen KV, Jacob HJ, Jacob M, et al. Principles of ontogenesis of leg and foot in man. Ann Anat. 1994;176:121.
Iimura T, Denans N, Pourquie O. Establishment of Hox vertebral identities in the embryonic spine precursors. Curr Top Dev Biol. 2009;88:201.
Javed A, Chen H, Ghori FY. Genetic and transcriptional control of bone formation. Oral Maxillofacial Surg Clin North Am. 2010;22:283.
Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629.
Keller B, Yang T, Munivez E, et al. Interaction of TGF-beta and BMP signaling pathways during chondrogenesis. PloS ONE. 2011;6:e16421.
Kubota T, Michigami T, Ozono K. Wnt signaling in bone. Clin Pediatr Endocrinol. 2010;19:49.
Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol. 2010;90:291.
Lewis J, Hanisch A, Holder M. Notch signaling, the segmentation clock, and the patterning of vertebrate somites. J Biol. 2009;8:44.
Mackie EJ, Tatarczuch L, Mirams M. The growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011 Jun 3. [Epub ahead of print]
Seo HS, Serra R. Tgfbr2 I required for development of the skull vault. Dev Biol. 2009;334:481.
Thornton GK, Woods CG. Primary microcephaly: Do all roads lead to Rome? Trend Genet. 2009;25:501.
Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev Dyn. 2007;236:2454.