Chapter 5 The Synapse
1. The anatomy of the neuromuscular junction is specialized for one-way communication.
2. An action potential on the presynaptic neuron triggers an action potential on the muscle cell through the release of acetylcholine.
3. There is greater variety in the specifics of neuron-to-neuron synaptic transmission than in transmission at the neuromuscular junction.
Neurons communicate with each other and with other cells of the body, such as muscle and secretory cells. In Chapter 4 the generation of the action potential and its rapid conduction down the axon, to the presynaptic terminal, was discussed. Using these processes, the neuron can rapidly notify its presynaptic terminals, often located far from its cell body, to initiate the transfer of information to other cells. Such communication occurs between cells rapidly, and often focally, at specialized junctions called synapses (Greek, “junction” or “to bind tightly”). Synaptic transmission between cells can be either electrical or chemical. At electrical synapses, ionic current flows directly between presynaptic and postsynaptic cells as the mediator for signal transmission. Although electrical synapses in the mammalian nervous system appear to be more widespread than originally thought, synaptic transmission is more frequently mediated by a chemical messenger. Released from the presynaptic terminals on arrival of the action potential, this chemical messenger rapidly diffuses to the postsynaptic cell membrane, where it binds with receptors. This binding initiates a postsynaptic change in function, often generating a postsynaptic potential.
The best-understood chemical synapse is that between a motor neuron and a skeletal muscle cell (fiber): the neuromuscular synapse, also known as the neuromuscular junction (Figure 5-1). Given the emphasis in Section II of this text on posture and locomotion, this synapse is the focus of this chapter. Synaptic communication at the neuromuscular junction is fundamentally similar to that between neurons, although there is greater variety in the specifics of neuron-to-neuron synaptic transmission, as also discussed.
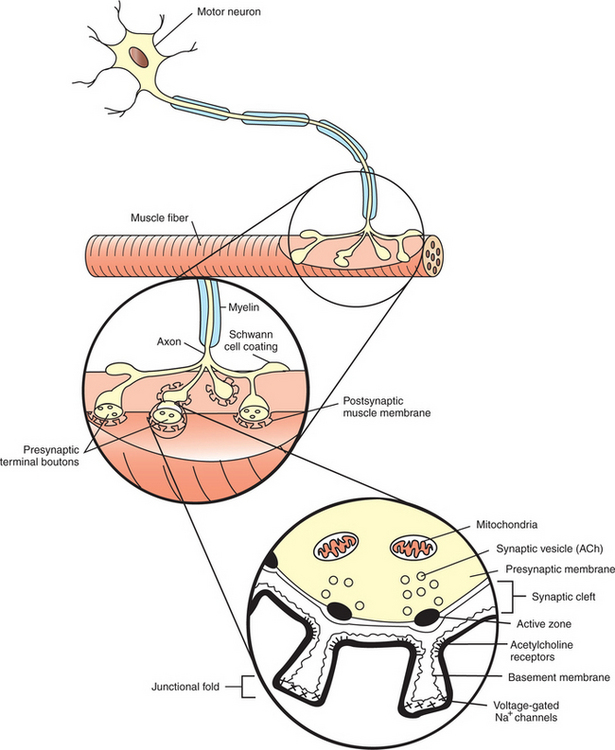
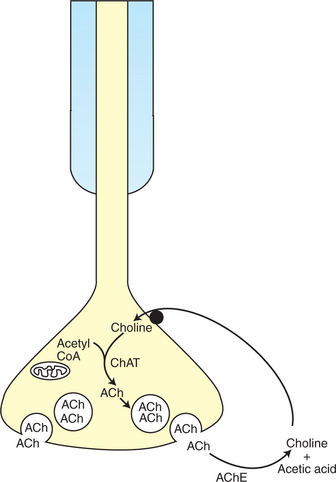
FIGURE 5-1 Synapse between a motor neuron and a skeletal muscle fiber. The neuromuscular junction has a presynaptic (neuronal) side, a narrow space between the neuron and muscle fiber called the synaptic cleft, and a postsynaptic (muscle) side. ACh, Acetylcholine.
The Anatomy of the Neuromuscular Junction Is Specialized for One-Way Communication
Motor neurons that synapse on skeletal muscles have their cell bodies located within the central nervous system (CNS), either within the spinal cord or the brainstem. The axons of these motor neurons travel within peripheral nerves, out to the muscle, where each motor neuron synapses on several individual fibers (cells) of the muscle. However, an individual skeletal muscle fiber receives synaptic input from, and therefore its contraction is controlled by, only one motor neuron.
The neuromuscular junction, as with most chemical synapses, has a presynaptic side; a narrow space between the neuron and muscle fiber, called the synaptic cleft; and a postsynaptic side (see Figure 5-1). The presynaptic side of the synapse is made up of the terminal (transmitting) portion of the motor neuron. This presynaptic terminal has a swelled, buttonlike appearance and is also called a synaptic bouton. The terminal (or synaptic bouton) contains a large number of membranous storage vesicles, called synaptic vesicles, which contain the chemical neurotransmitter substance, in this case acetylcholine. These synaptic vesicles are lined up in rows along the inner surface of the terminal membrane (Figure 5-2). The presynaptic membrane region associated with each double row of vesicles is called an active zone and is the site where the synaptic vesicles will eventually release acetylcholine into the synaptic cleft. The presynaptic terminal also contains mitochondria, an indication of active metabolism in the cytoplasm. Some mitochondrial products (e.g., acetyl-CoA, ATP) play a role in the local synthesis of acetylcholine and in its movement into the synaptic vesicles.
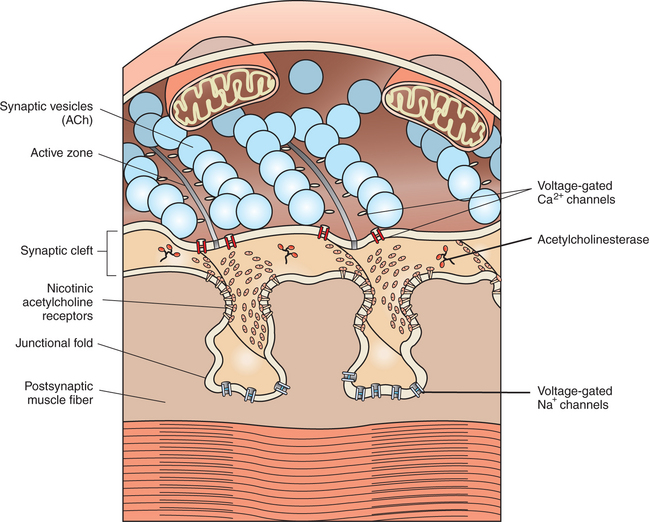
FIGURE 5-2 Presynaptic acetylcholine-filled synaptic vesicles line up at active zones, near voltage-gated Ca2+ channels. Released acetylcholine binds with nicotinic acetylcholine receptors at junctional folds on the postsynaptic muscle fiber membrane.
(Redrawn and modified from Bear MF, Connors BW, Paradiso MA: Neuroscience: exploring the brain, ed 3, Philadelphia, 2007, Lippincott Williams & Wilkins.)
The presynaptic (neural) and postsynaptic (muscle) cell membranes are separated by a narrow space, the synaptic cleft, that is about 50 nm wide (see Figure 5-1 and Figure 5-2). The cleft contains extracellular fluid and a basal lamina, composed of a matrix of molecules, that is a specialized region of the muscle basement membrane. Some of these matrix molecules mediate synaptic adhesion between neuron and muscle.
The postsynaptic muscle cell membrane has several specialized features that facilitate synaptic transmission. Directly opposite the face of the presynaptic terminal, the postsynaptic muscle cell membrane contains receptors for the acetylcholine transmitter (see Figure 5-1 and Figure 5-2). In this focal region the membrane has a series of invaginations, called junctional folds, that increase the surface area where acetylcholine receptors can reside. The acetylcholine receptors are most densely packed at the mouth of these junctional folds, and these mouths are closely aligned with the active zones of the presynaptic terminals from which the acetylcholine is released. Thus, there is a good match between the focal region of transmitter release from the neuron and the focal location of the receptors on the muscle fiber. Because the neurotransmitter is found only on the presynaptic neural side of the neuromuscular junction, transmission can go only from neuron to muscle, not in the reverse direction. Also, it should be noted that a motor neuron gives off several presynaptic terminals (synaptic boutons) to an individual muscle fiber. Together, this cluster of terminals is localized to a restricted region of the muscle fiber.
An Action Potential on the Presynaptic Neuron Triggers an Action Potential on the Muscle Cell Through the Release of Acetylcholine
The function of the neuromuscular junction is to transmit a chemical message unidirectionally between a motor neuron and a skeletal muscle cell (fiber) with a frequency established by the CNS. The arrival of an action potential at the motor neuron terminal triggers the release of the acetylcholine transmitter, which then binds with acetylcholine receptors on the postsynaptic muscle fiber membrane. This leads to the genesis of an action potential along the muscle fiber membrane that ultimately leads to contraction of the fiber.
An action potential on a motor neuron arises at its initial axon segment and then spreads along the entire axon, eventually arriving at the presynaptic terminal (see Chapter 4). As previously noted, the exchange of Na+ and K+ ions, across axonal voltage-gated Na+ and K+ channels, is responsible for the generation of the action potential and its conduction to the terminal. However, as the action potential arrives at the presynaptic membrane, the wave of depolarization opens voltage-gated Ca2+ channels located in this region; as Ca2+ flows toward equilibrium across the membrane, the Ca2+ enters the presynaptic terminal. This increase in the intracellular Ca2+ level is critical for the release of neurotransmitter from the terminal.
Recall that the acetylcholine-containing synaptic vesicles are lined up at the active zones of the presynaptic terminal. They are docked there by the intertwining of binding proteins that respectively reside on the vesicle membrane and on the inner surface of the terminal membrane. This holds the vesicles near the location of Ca2+ entry because the voltage-gated Ca2+ channels are efficiently located in the vicinity of these active zones. When Ca2+ flows into the terminal, the ion binds with yet another protein on the synaptic vesicle membrane. This triggers fusion of the vesicle with the presynaptic membrane, opening of the vesicle, and release of acetylcholine into the synaptic cleft. After transmitter release, the vesicle membrane is retrieved back into the presynaptic terminal and can be recycled to re-form a vesicle that is then refilled with acetylcholine synthesized in the cytoplasm. Certain bacterial toxins (e.g., botulinum, tetanus) can destroy the binding proteins involved in vesicle docking, ultimately interfering with the ability of the vesicle to release its contents into the synaptic cleft.
After release, acetylcholine then diffuses across the synaptic cleft and binds with transmitter-specific receptors, the nicotinic acetylcholine receptors, in the postsynaptic muscle membrane. This specific subtype of acetylcholine receptor, found at the neuromuscular junction, is so named because it can also bind the alkaloid drug nicotine. The nicotinic acetylcholine receptor is actually a ligand-gated ion channel (see Chapter 1), permeable to small cations, with two binding sites for the acetylcholine molecule. As acetylcholine binds at these two loci, the channel opens and, among other ionic movements, Na+ ions diffuse into the muscle cell as they attempt to flow toward equilibrium. This contributes to a depolarization of the postsynaptic muscle cell membrane analogous to an excitatory postsynaptic potential (EPSP). However, at the neuromuscular junction, the unitary postsynaptic potential is sufficient to open voltage-gated Na+ channels deep within the junctional folds and leads to the generation of an action potential on the muscle cell membrane.
Acetylcholine binds with its receptor only briefly (∼1 msec). Once free, it is destroyed by the enzyme acetylcholinesterase. This enzyme, anchored to the basal lamina of the synaptic cleft, inactivates acetylcholine by breaking it down into acetic acid and choline molecules (Figure 5-3). The choline, a precursor of acetylcholine synthesis, can then be transported back into the presynaptic terminal and recycled in acetylcholine synthesis. Chemicals that inhibit acetylcholinesterase, such as some organophosphate insecticides and nerve gases (e.g., sarin), can abnormally prolong the presence of acetylcholine at the synapse, often with disastrous physiological consequences. Because the neurotransmitter is normally destroyed soon after its binding with the muscle membrane receptor, and because more transmitter is not available to attach to the receptors in sufficient quantities until another motor neuron action potential occurs, there is approximately a 1:1 ratio between action potentials on the neuronal and muscle cell membranes.
There Is Greater Variety in the Specifics of Neuron-to-Neuron Synaptic Transmission Than in Transmission at the Neuromuscular Junction
As mentioned earlier, some noteworthy differences exist between synaptic transmission at the neuromuscular junction and neuron-to-neuron synaptic transmission. Although acetylcholine is the neurotransmitter responsible for the primary postsynaptic effect at the neuromuscular junction, a variety of neurotransmitters, in addition to acetylcholine, can be used to produce the principal postsynaptic effect at neuron-to-neuron synapses (Box 5-1). Furthermore, not all of these transmitters are released from morphologically distinct active zones, although their release still appears to depend on Ca2+ influx. In such cases, release from the terminal may not always occur directly at the synaptic cleft, resulting in a wider postsynaptic distribution of transmitter.
Box 5-1 Members of the Major Neurotransmitter Classes
* Only a partial list of peptide neurotransmitters.
The postsynaptic membrane of a neuron-to-neuron synapse can be the soma, dendrites, or even the terminals of the postsynaptic neuron, and junctional folds are not seen at these synapses. However, the dendritic postsynaptic membrane often possesses small protrusions called dendritic spines (see Chapter 4). As with the junctional folds on muscle cells, these spines increase the surface area of the postsynaptic membrane and, because of their narrow necks, are also thought to provide a means for biochemical isolation between nearby synapses. Further, spines can change size and shape over an animal’s lifetime, modulating the functional effectiveness of the synapse. Whereas transmitter release at the neuromuscular junction always produces postsynaptic excitation (membrane depolarization), release at synapses between neurons can produce excitation or inhibition (membrane hyperpolarization). However, synapses on dendritic spines are almost always excitatory.
At the neuromuscular junction, the postsynaptic receptor is almost exclusively the nicotinic acetylcholine receptor, a ligand-gated ion channel. At synapses between neurons, a much greater variety of receptors is available. These may differ from the nicotinic acetylcholine receptor with respect not only to the binding transmitter, but to the receptor mechanism as well (see Chapter 1). Also, several different types of neurotransmitter receptor are often found on a single neuron.
When transmitters other than acetylcholine are employed at neuron-to-neuron synapses, depending on the transmitter, the termination of action of that transmitter may be accomplished by (1) transporter-mediated reuptake of the transmitter itself into the terminal of release or (2) a less specific and somewhat slower form of enzymatic degradation than with acetylcholinesterase. Finally, at neuron-to-neuron synapses, a single action potential on a presynaptic neuron rarely results in a full-blown action potential on the postsynaptic neuron. Some form of summation of presynaptic inputs is required to generate a postsynaptic action potential.
As discussed in Chapter 6, action potentials on the muscle cell membrane lead to contraction, or mechanical shortening, of the muscle cell. When this contraction is combined with the shortening of many muscle cells, movement of the body occurs.
CLINICAL CORRELATIONS
Myasthenia Gravis
History.
You examine a 5-year-old female German shepherd whose owner states that the dog becomes progressively weaker with exercise. The owner also states that recently, just after eating, the dog has begun to vomit food in formed, cylinder-shaped boluses.
Clinical Examination.
All abnormalities found on physical examination were referable to the neuromuscular system. After resting, the dog’s neurological examination findings were within normal limits. with even moderate exercise, however, the dog became progressively weaker, particularly in the front legs. Intravenous injection of an acetylcholinesterase inhibitor, edrophonium (Tensilon), eliminated all clinical signs of weakness. Radiographs of the chest revealed an enlarged esophagus and thymus.
Comment.
The history of an enlarged esophagus (megaesophagus) and the response to an acetylcholinesterase inhibitor confirm the diagnosis of myasthenia gravis (“grave muscle weakness”). This is caused by a failure of transmission of acetylcholine at the neuromuscular synapse. This transmission failure is caused by antibodies produced by the body against its own acetylcholine receptors. The abnormal antibodies bind with the receptors to form complexes, which prevents acetylcholine from binding to the acetylcholine receptors. As a result, no depolarization occurs on the postsynaptic membrane of the cells. Antibodies also alter the junctional folds and number of acetylcholine receptors available to bind with the transmitter. Acetylcholinesterase inhibitors prevent the metabolism of acetylcholine, allowing acetylcholine to remain longer at the synapse, with additional time for binding to the receptors, and thus facilitating normal transmission.
The large amount of skeletal muscle in the dog’s esophagus explains its enlargement from paralysis. These patients often regurgitate formed boluses of food shortly after eating.
Myasthenia gravis can be associated with mediastinal masses, usually of the thymus. The autoantibodies that the body makes are often against antigens from the thymus or acetylcholine receptors. In addition to this cause of myasthenia gravis, idiopathic myasthenia gravis is also common.
Bear MF, Connors BW, Paradiso MA. Neuroscience: exploring the brain, ed 3. Philadelphia: Lippincott, Williams & Wilkins, 2007.
Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach, updated edition. Philadelphia: Saunders, 2005.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell. 1993;72(suppl):99.
Hughes BW, Kushner LL, Kaminski HJ. Molecular architecture of the neuromuscular junction. Muscle Nerve. 2006;33(4):445.
Kandel ER, Schwartz JH, Jessell TM. Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
Klein BG. Synaptic transmission and the neurotransmitter life cycle. Reece WO, ed. Duke’s physiology of domestic animals, ed 12, Ithaca, NY: Comstock Cornell University Press, 2004.
Meyer JS, Quenzer LF. Psychopharmacology: drugs, the brain, and behavior. Sunderland, Mass: Sinauer, 2005.
PRACTICE QUESTIONS
1. At the neuromuscular junction, Ca2+ ions are necessary for:
2. A drug that would prevent the release of acetylcholine at the neuromuscular junction would cause what, if any, clinical signs?
3. Which one of the following is true with regard to the termination of synaptic action at the neuromuscular junction?
4. Several drugs compete with acetylcholine for the postsynaptic receptor at the neuromuscular junction. If you overdosed your patient with one of these competitive drugs, what would the antidote need to do at the synapse?
5. Which of the following statements regarding neuron-to-neuron synapses is false?