Chapter 4 The Neuron
1. Neurons have four distinct anatomical regions.
2. Neuronal membranes contain a resting electrical membrane potential.
3. The resting membrane potential is the result of three major determinants.
4. The resting membrane potential can be changed by synaptic signals from a presynaptic cell.
5. Action potentials begin at the axon’s initial segment and spread down the entire length of the axon.
There are two classes of cells in the nervous system: the neuron and the glial cell (see Chapter 3). The neuron is the basic functional unit of the nervous system. The large number of neurons and their interconnections account for the complexity of the nervous system. The number of neurons in the vertebrate nervous system ranges from approximately 100 million in a small mammal (e.g., mouse) to 100 billion in a human to well over 200 billion in whales and elephants, far more neurons in a nervous system than people on Earth, and there are 10 to 50 times more glial cells. The structural and functional support provided to neurons by glial cells and their potential to modulate neural communication make an important contribution to the operational integrity of the nervous system. The numbers of cells in the nervous system are huge, but knowing that they have common elements makes it easier to understand them.
Neurons Have Four Distinct Anatomical Regions
A typical neuron has four morphologically defined regions (Figure 4-1): the dendrites, the cell body, the axon, and the presynaptic terminals of the axon. These four anatomical regions are important in the major electrical and chemical responsibilities of neurons: receiving signals from the presynaptic terminals of other neurons (on dendrites), integrating these often-opposing signals (on the initial segment of the axon), transmitting action potential impulses along the axon, and signaling an adjacent cell from the presynaptic terminal. These functions are collectively analogous to the general role of the nervous system: collecting information from the environment, integrating that information, and producing an output that can change the environment.
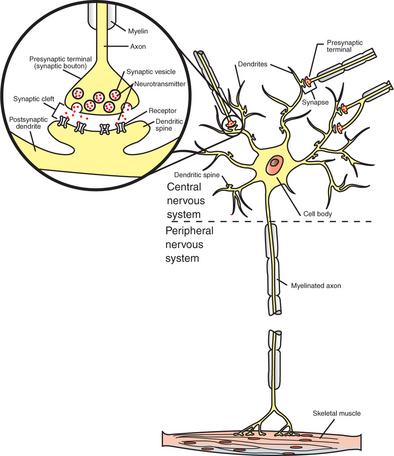
FIGURE 4-1 A typical neuron has four functionally important regions. The cell body manufactures proteins to maintain the neuron; the dendrites receive signals from neighboring neurons; the axon integrates these signals and transmits action potentials some distance along the cell; and the presynaptic terminal signals adjacent cells. The inset shows an enlargement of the circled synapse.
The cell body (also called the soma or perikaryon) plays a critical role in manufacturing proteins essential for neuronal function. Four organelles are particularly important for this purpose: the nucleus, containing the blueprint for protein assembly; the free ribosomes, which assemble cytosolic proteins; the rough endoplasmic reticulum, in which secretory and membrane proteins are assembled; and the Golgi apparatus, which further processes and sorts secretory and membrane components for transport. The cell body usually gives rise to several branchlike extensions, called dendrites, whose surface area and extent greatly exceed those of the cell body. The dendrites serve as the major receptive apparatus of the neuron, receiving signals from neighboring neurons. These signals affect specialized receptor proteins (receptors) that reside on the dendrites. The cell body also gives rise to the axon, a tubular process that is often long (>1 meter in some large animals). The axon is the conducting unit of the neuron, rapidly transmitting an electrical impulse (the action potential) from its initial segment at the cell body to the other end of the axon at the presynaptic terminal. Axons lack ribosomes and therefore cannot synthesize proteins. Instead, macromolecules are synthesized in the cell body and are carried along the axon to distant axonal regions and to the presynaptic terminals by a process called axoplasmic transport. Large axons are surrounded by a fatty, insulating coating called myelin. In the peripheral nervous system, myelin is formed by Schwann cells, specialized glial cells that wrap around the axon much like toilet paper wrapped around a broomstick. A similar function is performed by glial cells called oligodendrocytes in the central nervous system. The myelin sheath is interrupted at regular intervals by spaces called nodes of Ranvier. The myelin sheath significantly increases the speed of action potential conduction along the axon.
Axons branch near their ends into several specialized endings called presynaptic terminals (or “synaptic boutons”). When the action potential rapidly arrives, these presynaptic terminals transmit a chemical signal to an adjacent cell, usually another neuron or a muscle cell. The site of contact of the presynaptic terminal with the adjacent cell is called the synapse, shown in the inset in Figure 4-1. It is formed by the presynaptic terminal of one cell (presynaptic cell), the receptive surface of the adjacent cell (postsynaptic cell), and the space between these two cells (the synaptic cleft). Presynaptic terminals contain chemical transmitter–filled synaptic vesicles that can release their contents into the synaptic cleft. The presynaptic terminals of an axon usually contact the receptive surface of an adjacent neuron or muscle cell, usually on the neuron’s dendrites, but sometimes this contact is made on the cell body or, occasionally, on the presynaptic terminals of another cell (e.g., for presynaptic inhibition). On many neurons, presynaptic terminals often synapse on small protrusions of the dendritic membrane called dendritic spines. The receptive surface of the postsynaptic cell contains specialized receptors for the chemical transmitter released from the presynaptic terminal.
Neuronal Membranes Contain a Resting Electrical Membrane Potential
Neurons, as with other cells of the body, have an electrical potential, or voltage, that can be measured across their cell membrane (resting membrane potential). However, the electrical membrane potential in neurons and muscle cells is unique in that its magnitude and sign can be changed as the result of synaptic signaling from neighboring cells, or it can change within a sensory organ receptor as a response to transduction of some environmental energy. When the change in membrane potential of a neuron or muscle cell reaches a threshold value, a further and dramatic change in the membrane potential, called an action potential, occurs; this action potential spreads along the entire length of the neuronal axon (see later discussion).
The origins of the resting electrical membrane potential are complicated, particularly in a quantitative way. In qualitative terms, however, the resting membrane potential is the result of the differential separation of charged ions, especially sodium (Na+) and potassium (K+), across the membrane and the resting membrane’s differential permeability to these ions as they attempt to move back down their concentration and electrical gradients (see Chapter 1). Even though the net concentration of positive and negative charges is similar in the intracellular and extracellular fluid, an excess of positive charges accumulates immediately outside the cell membrane, and an excess of negative charges accumulates immediately inside the cell membrane (Figure 4-2). This makes the inside of the cell negatively charged with regard to the outside of the cell. The magnitude of the resulting electrical difference (or voltage) across the membrane varies from cell to cell, ranging from about 40 to 90 millivolts (mV), and is usually about 70 mV in mammalian neurons. Because the extracellular fluid is arbitrarily considered to be 0 mV, the resting membrane potential is −70 mV, more negative on the inside than on the outside.
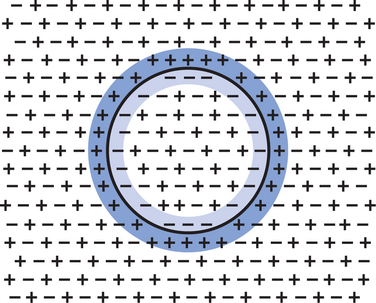
FIGURE 4-2 Concentrations of positively and negatively charged ions are similar in both the intracellular space and the extracellular space. However, more positively charged ions accumulate immediately outside the cell membrane (blue), and more negatively charged ions accumulate immediately inside the cell membrane (lighter blue).
The Resting Membrane Potential Is the Result of Three Major Determinants
Three major factors cause the resting membrane potential.
1. The Na+,K+ pump. Cell membranes have an energy-dependent pump that pumps Na+ ions out of the cell and draws K+ ions into the cell against their concentration gradients. This maintains the differential distribution of each of these charged ion species across the membrane that underlies their ability to produce a voltage across the membrane. The pump itself makes a small, direct contribution to the resting membrane potential because it pushes three molecules of Na+ out for every two molecules of K+ drawn into the cell, thus concentrating positive charges outside the cell.
2. An ion species will move toward a dynamic equilibrium if it can flow across the membrane. Using K+ as an example, the concentration difference across the membrane actively maintained by the Na+,K+ pump produces a concentration gradient, or “chemical driving force,” that attempts to push the ion passively across the membrane from high concentration inside the cell toward low concentration outside. If K+ can flow across ion channels in the membrane, exiting K+ leaves behind unopposed negative charge (often from negatively charged protein macromolecules trapped inside the cell) that builds an electrical gradient, or “electrical driving force,” pulling K+ back inside the cell. These opposing gradients eventually produce a dynamic equilibrium, even though there may still be more K+ inside than outside, as well as a charge imbalance across the membrane. This uneven distribution of charge at dynamic equilibrium produces a voltage across the membrane called the equilibrium potential for that ion. When an ion species can flow across a channel in the membrane, it flows toward its equilibrium state, and it drives the voltage across the membrane toward its equilibrium potential.
3. Differential permeability of the membrane to diffusion of ions. The resting membrane is much more permeable to K+ than to Na+ ions because there are vastly more K+ leak channels than Na+ leak channels in the membrane. This greater membrane permeability to K+ means that K+ ions can more closely approach their dynamic equilibrium state, and equilibrium potential, compared with Na+ ions, which have difficulty moving across the membrane. Therefore the equilibrium potential for the more permeant K+ ions (about −90 mV in many mammalian neurons) will have the predominant influence on the value of the resting membrane potential compared with the equilibrium potential of the vastly less permeant Na+ ions (about +70 mV in many mammalian neurons). Therefore, as noted earlier, the resting membrane potential of many mammalian neurons is about −70 mV, close to the equilibrium potential for K+.
These three determinants—the Na+,K+ pump, the movement of a permeant ion toward dynamic equilibrium, and the differentially permeable membrane—are the primary source of the resting membrane potential. The value of this potential can be predicted by the Nernst and Goldman equations, and the reader is referred to Chapter 1 and to the bibliography for a more quantitative understanding of the resting membrane potential.
This discussion of the resting membrane potential has a number of important clinical implications. The Na+,K+ pump requires energy in the form of adenosine triphosphate (ATP), which is derived from the intracellular metabolism of glucose and oxygen. In fact, it has been estimated that 50% to 70% of the brain’s ATP-derived energy is expended on the pump. Because the neuron cannot store either glucose or oxygen, anything that deprives the nervous system of either substrate can lead to impairment of the pump and serious clinical neurological deficits. Fortunately, hormones and other factors normally maintain serum glucose and oxygen levels within narrow limits. Because Na+ and K+ are important ions involved in establishing the resting membrane potential, it is essential that serum levels of Na+ and K+ be regulated carefully. The endocrine system (Chapter 33) and kidney (Chapter 41) maintain serum Na+ and K+ levels within narrow limits. Anything altering serum levels of either ion beyond normal limits also leads to potentially severe neurological deficits.
The Resting Membrane Potential Can Be Changed by Synaptic Signals from a Presynaptic Cell
Although most cells of the body have a resting membrane potential, neurons and muscle cells are unique in that their membrane potential can be altered by a synaptic signal from another cell. Neurotransmitter released from a presynaptic axon terminal binds with receptors on the postsynaptic membrane, resulting in the opening or closing of ion selective channels and changing the membrane potential of the postsynaptic cell. Even though there are trillions of synapses in the nervous system, a presynaptic signal can alter the postsynaptic membrane potential in basically only two ways: by making it more negative or more positive (less negative). The particular change depends on the nature of the receptor activated by the chemical transmitter that is released from the synaptic vesicles of the presynaptic axon terminal. The change in postsynaptic membrane potential is called a postsynaptic potential.
If a chemical synaptic transmission leads to a postsynaptic potential that is more positive in comparison with the resting level (e.g., from −75 to −65 mV), this is said to be an excitatory postsynaptic potential (EPSP) (Figure 4-3, A). It is called “excitatory” because each such synaptic transmission increases the chances that an action potential will originate at the initial segment of the postsynaptic cell’s axon. When an EPSP changes the postsynaptic membrane potential to a more positive value, the membrane is said to be depolarized. Depolarization of the postsynaptic membrane can result if the interaction of the chemical transmitter and its appropriate receptor on the postsynaptic membrane cause (ligand-gated) Na+ channels to open. This allows Na+ ions to diffuse into the neuron as they begin to flow toward equilibrium across the membrane, moving the membrane potential toward the more positive sodium equilibrium potential. The ion channels that usually change their conductivity as a result of neurotransmitter binding with a receptor are the ligand-gated or chemically gated ion channels (see Chapter 1).
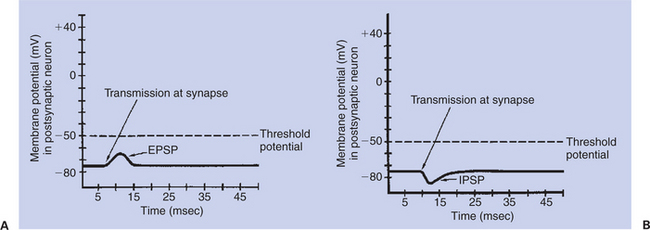
FIGURE 4-3 Postsynaptic potentials. A, excitatory postsynaptic potential (EPSP) drives the membrane potential toward threshold.
Because the chemical transmitter is quickly removed from the synapse, the postsynaptic potential change is transient, lasting only a few milliseconds. Furthermore, because the change in ion flow resulting from receptor activation is limited, the magnitude of a postsynaptic potential is often quite small (e.g., 2-3 mV). However, it is greatest at the synapse. Although the depolarization spreads over the postsynaptic membrane, it decreases with the distance from the originating synapse, much as the waves created by throwing a stone into a lake decrease in size with the distance from where the stone fell.
If instead the presynaptic neurotransmitter’s interaction with the postsynaptic receptor results in opening of the membrane’s chemically gated K+ channels, then K+ ions diffuse out, moving the membrane potential even closer to the equilibrium potential for K+ (–90 mV). This change from the resting potential to a more negative membrane potential is called hyperpolarization. Such hyperpolarization of the postsynaptic membrane is called an inhibitory postsynaptic potential (IPSP) (Figure 4-3, B), because each such transmission makes it less likely that an action potential will result at the axon’s initial segment. As with EPSPs, IPSPs spread over the neuron’s membrane, and the hyperpolarization decreases with the distance from the originating synapse.
Action Potentials Begin at the Axon’s Initial Segment and Spread Down the Entire Length of the Axon
Both EPSPs and IPSPs on the postsynaptic membrane are ultimately the result of an action potential and synaptic transmission from a presynaptic cell. However, these postsynaptic potentials decrease in magnitude as they spread along the postsynaptic cell membrane. Because many neurons and muscle cells are long, the cell needs a mechanism for sending an electrical signal from its information-receiving end on the postsynaptic dendritic and soma membrane to the information-transmitting zone at the terminals of the often-lengthy axon. This is accomplished by an explosive event called an action potential, a regenerative electrical signal that begins at the axon’s initial segment, results from competing EPSP and IPSP membrane potential changes, and spreads down the length of the axon without decreasing in magnitude.
EPSPs and IPSPs can respectively summate on the postsynaptic membrane to produce larger changes in membrane potential than either signal alone. At the axon’s initial segment, the arriving EPSPs and IPSPs are integrated. If only a few EPSPs arrive at the axon’s initial segment, its membrane potential is not made sufficiently positive to reach its threshold potential (often 10-20 mV more positive than the resting potential) for triggering an action potential. However, if many more EPSPs than IPSPs arrive, the initial segment’s membrane potential is made positive enough to reach its threshold potential, and an action potential is created. This action potential is the result of the sequential opening of voltage-gated membrane channels first to sodium and shortly thereafter to potassium.
The action potential is characterized by explosive changes in the membrane potential when this value reaches the threshold potential: First, a dramatic and swift depolarization of the membrane potential occurs, in which the inside of the cell actually becomes more positively charged than the outside, followed by a repolarization of the membrane, in which the membrane potential falls back toward the resting potential. The depolarization phase of the action potential is caused by the immediate and extensive opening of voltage-gated Na+ channels and the consequent influx of Na+ ions as they attempt to flow toward their equilibrium. As the action potential’s depolarization phase continues, the voltage-gated Na+ channels are spontaneously inactivated, and the voltage-gated K+ channels, which open with a longer delay than the Na+ channels, begin to allow even more K+ ions to exit as they move closer to their equilibrium state. This brings depolarization to a halt and allows repolarization to occur. As repolarization continues, the membrane potential moves temporarily beyond its resting level to a hyperpolarized state. This hyperpolarization is attributable to the flow of K+ ions out through the voltage-gated K+ channels, in addition to the flow out through the K+ leak channels, bringing the membrane potential even closer to the K+ equilibrium potential (–90 mV) than at rest. The membrane potential eventually returns to its resting state as the K+ voltage-gated channels gradually close. The whole action potential takes about 2 to 3 msec in many neurons but longer in many muscle cells. Figure 4-4 illustrates this sequence of events in a neuron.
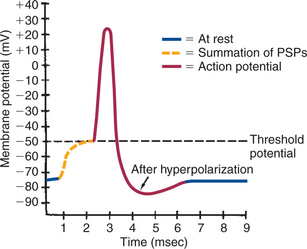
FIGURE 4-4 Axon’s membrane potential changes dramatically during an action potential. After threshold is reached by summating postsynaptic potentials, the axonal membrane depolarizes, repolarizes, hyperpolarizes, and then returns to its original resting potential.
(Modified from Sherwood L: Human physiology: from cells to systems, St Paul, 1989, Wadsworth.)
An analogy may be helpful for understanding these difficult concepts. Imagine the resting neural membrane as a toilet. The toilet has stored potential energy by filling its water tank. (The neuron has done so by generating the resting membrane potential.) If the handle of the toilet is pushed down only briefly, for a short distance, some water runs into the toilet, but the flush cycle is not initiated. (This is similar to an EPSP without the action potential.) However, if the handle is pushed down far enough and held down long enough, a critical threshold is reached, the flush cycle is triggered, and it must run its course, including the refilling of the tank, before another flush cycle can be started. The action potential is analogous to this flush cycle. It is triggered once a critical depolarization threshold is reached. It usually must run its course, including reestablishing the resting membrane potential, before another action potential can be initiated. Because the flush cycle takes a finite amount of time, only a limited number of flush cycles could be completed in an hour, even if the toilet were flushed again each time the tank refilled. Similarly, because the action potential also has a finite duration, there is a limit to the number of action potentials per second that can be generated on an axon. (However, for both toilets and neurons, strategies can be employed to produce a flush or an action potential before the tank is completely refilled or before the membrane completely returns to the resting potential.)
Certain animal toxins, such as tetrodotoxin from the Japanese puffer fish, can block voltage-gated Na+ channels and therefore interfere with the generation of action potentials on axons. Many local anesthetics (e.g., lidocaine), which are used in a controlled, clinically efficacious manner, work by a similar mechanism.
The action potential actively propagates from its origin at the initial segment down the axon. The dramatic influx of Na+ ions that accompanies action potential depolarization of the initial segment’s membrane results in the passive spread of these positive charges toward the adjacent resting segment of membrane. This migration of positive charge on the inner surface of the membrane, called an electrotonic current, depolarizes this adjacent segment to threshold, causing voltage-gated Na+ channels to open. This causes an action potential to develop there, which triggers a similar cycle in its adjacent membrane, and so on down the axon. In this way an action potential spreads from the axon’s initial segment down to the presynaptic terminal at the axon’s far end (Figure 4-5).
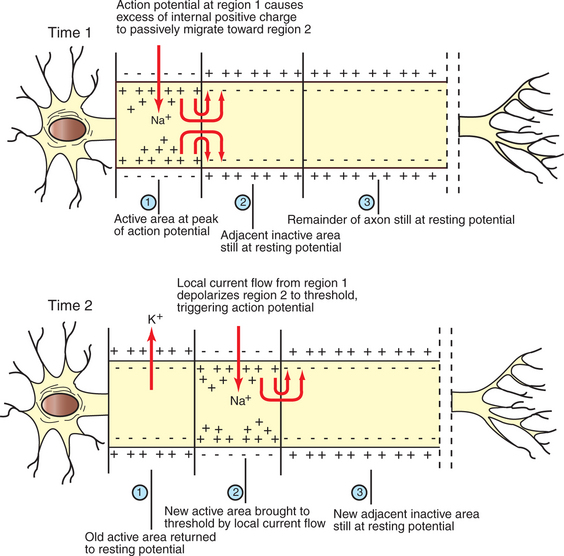
FIGURE 4-5 Action potential, first generated in the axon’s initial segment (Time 1, region 1), moves down the unmyelinated axon as positive charges passively migrate to the immediately adjacent membrane to trigger an action potential there (Time 2, region 2).
(Redrawn from Sherwood L: Human physiology: from cells to systems, St Paul, 1989, Wadsworth.)
The speed with which the action potential is conducted down the axon varies. The internal diameter and the degree of myelination of an axon play a critical role in determining this action-potential conduction velocity. In a small-diameter, unmyelinated axon, the conduction velocity is relatively slow (e.g., 0.5 meter/second); conduction velocities of greater than 90 m/sec (so that a distance as long as a football field is traveled in 1 second), however, are known to occur in large-diameter, heavily myelinated axons. This occurs because the passive electrotonic current, responsible for triggering the action potential at the next adjacent patch of axonal membrane, travels faster and farther along wider axons or along myelinated patches of axon. In myelinated axons, exchange of ions across the membrane, and thus generation of the action potential, can only occur at the bare nodes of Ranvier, where a high density of voltage-gated Na+ channels is found. Given the rapid spread of electrotonic current along the myelinated patches (internodes) and the comparatively slow process of ion exchange at the nodes, the action potential functionally jumps from node to node (saltatory conduction) in myelinated axons (Figure 4-6).
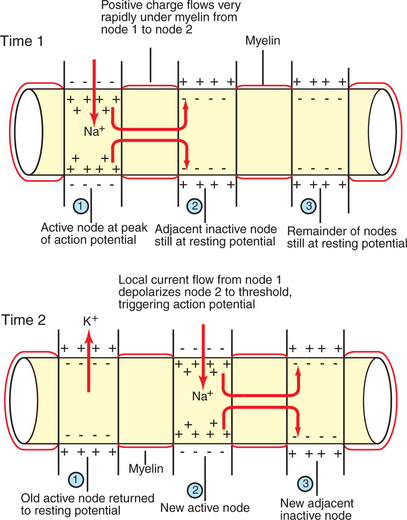
FIGURE 4-6 Saltatory conduction of action potentials in myelinated axons is faster than action-potential conduction in unmyelinated axons because the passive local current flows very rapidly under the myelin to trigger an action potential at the next node. Thus the action potential seems to jump functionally from node to node.
(Modified from Sherwood L: Human physiology: from cells to systems, St Paul, 1989, Wadsworth.)
The normal facilitation of action-potential conduction velocity by myelin can be appreciated by considering diseases that attack myelin, such as acute idiopathic polyradiculoneuritis (“coonhound paralysis”). Slowing of evoked electrical signals along sensory and motor nerves and depressed spinal reflexes are associated with this condition.
CLINICAL CORRELATIONS
Hypoglycemia
History.
You examine an 8-year-old male boxer dog whose owner complains that the dog experiences seizures, weakness, and confusion around the time he is fed.
Clinical Examination.
The findings of the dog’s physical examination, including his neurological examination, were within normal limits. His fasting serum glucose level, however, was 29 mg/dL (normal, 70-110 mg/dL), and the ratio between serum insulin and serum glucose levels was significantly elevated.
Comment
Neurons depend primarily on oxygen and glucose as metabolites for ATP energy production, and neurons cannot store appreciable quantities of glucose. ATP is needed for maintenance of the normal electrical membrane potential. When deprived of glucose and subsequently ATP, the brain does not function properly; associated clinical signs include seizures, weakness, and confusion. In this animal, these signs were more common at the time of feeding because as the dog anticipated eating or actually did begin to eat, insulin was released, causing hypoglycemia.
In this case the ratio of insulin to glucose is elevated, probably because of an insulin-secreting tumor of the pancreas. Because insulin facilitates glucose transport through cell membranes, too much insulin results in the transfer of too much serum glucose to the cytoplasm of other cells of the body, thus depriving the brain’s neurons of this essential metabolite.
Treatment.
Insulinomas can usually be found and removed from the pancreas surgically. After surgical removal of the tumor, additional medical treatment is warranted to maintain normoglycemia. Medications include glucocorticoids, to stimulate gluconeogenesis; diazoxide, to inhibit insulin secretion; streptozocin, which is toxic to the beta cells; and somatostatin, which increases gluconeogenesis. With this tumor type, there is a high rate of metastasis, which means that other tumor sites may remain, in the liver and elsewhere, to overproduce insulin.
Bear MF, Connors BW, Paradiso MA. Neuroscience: exploring the brain, ed 3. Philadelphia: Lippincott, Williams & Wilkins, 2007.
Garrett L: Insulinomas: a review and what’s new. In ACVIM 21st annual proceedings, Charlotte, NC, 2003.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Kandel ER, Schwartz JH, Jessell TM. Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
Klein BG. Membrane potentials: the generation and conduction of electrical signals in neurons. Reece WO, ed. Duke’s physiology of domestic animals, ed 12, Ithaca, NY: Comstock Cornell University Press, 2004.
PRACTICE QUESTIONS
1. In treating critically ill patients with intravenous fluids, which two ions are most important to the neuronal membrane potential?
2. The energy required by the Na+,K+ neural membrane pump is derived from ATP. In the neuron, this energy results from the nearly exclusive metabolism of oxygen and:
3. If the number of IPSPs on the dendritic membrane decreases while the number of EPSPs remains the same, what will happen to the action potentials on that neuron?
4. During an excitatory postsynaptic potential in a neural membrane, which of the following is the most important ion flow?