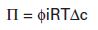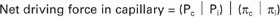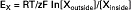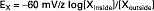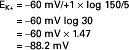Chapter 1 The Molecular and Cellular Basis of Physiological Regulation
1. All physiological change is mediated by proteins.
2. Protein function depends on protein shape and shape changes.
3. A series of enzymatic reactions converts tyrosine into the signaling molecules dopamine, norepinephrine, and epinephrine.
4. Muscle contraction and its initiation and cessation depend on the binding specificity and allosteric properties of proteins.
5. Biological membranes are a mosaic of proteins embedded in a phospholipid bilayer.
1. Only small, uncharged molecules and oily molecules can penetrate biomembranes without the aid of proteins.
2. Molecules move spontaneously from regions of high free energy to regions of lower free energy.
3. Important transport equations summarize the contributions of the various driving forces.
4. Starling’s hypothesis relates fluid flow across the capillaries to hydrostatic pressure and osmotic pressure.
5. Membrane proteins that serve the triple functions of selective transport, catalysis, and coupling can pump ions and molecules to regions of higher free energy.
6. Many membrane proteins selectively facilitate the transport of ions/molecules from high to low electrochemical potential.
7. Passive transport of K+ across the plasma membrane creates an electrical potential.
8. Spatial organization of active and passive transport proteins enables material to pass completely through the cell.
9. Membrane fusion allows for a combination of compartmentalization and transport of material.
Information Transmission and Transduction
1. Cell signaling often occurs by a lengthy chain of sequential molecular interactions.
2. Signaling pathways begin with the binding of an extracellular molecule to a receptor.
3. Specific physiological information is inherent in the receptor/ligand complex, not in the hormone/neurotransmitter molecule.
4. G-protein–coupled receptors are the largest family (a “superfamily”) of receptors and help regulate almost all physiological processes.
5. Most G-protein–linked information is sent to the cytoplasm by “second messengers.”
6. Ca2+ transport across plasma and intracellular membranes is an important second messenger.
7. Cyclic AMP is produced by activation of a membrane-bound enzyme in response to hormone/neurotransmitter binding to receptors.
8. The receptor-mediated hydrolysis of a rare phospholipid of the plasma membrane produces two different second messengers with different actions.
9. Steroid hormones and other lipid signals interact with nuclear receptors, which are transcription factors within the cell.
Physiology is the study of the regulation of change within organisms, in this case higher animals. Our understanding of physiology has changed dramatically in the past 30 years as a result of insight into the molecular basis of biological regulation. This chapter summarizes (and simplifies considerably) our current understanding of the molecular and cellular basis of that regulation. Most of the principles in this chapter apply to all animal cells. The approach taken is one of functional molecular anatomy. That is, the molecular structure of the cell is examined with particular emphasis on the physiological function, in the intact animal, of the molecules and supramolecular structures responsible for the function. Only those aspects of cell function that illuminate the medical physiology of the higher animals are discussed; the reader is referred to the Bibliography for more complete coverage of the cell. Some review of basic concepts and vocabulary is presented. However, the discussion assumes that the reader is familiar with the cell and its constituent molecules, as presented in courses in general biology and an undergraduate course in biochemistry.
All Physiological Change Is Mediated by Proteins
All physiological change is mediated by a single class of polymeric macromolecules (large molecules), the proteins. Protein function can be subdivided into a number of categories: catalysis, reaction coupling, transport, structure, and signaling.
Catalysis is the ability to increase greatly the rate of a chemical reaction without altering the equilibrium of the reaction. The vast majority of biochemical reactions occur at a physiologically useful rate only because of protein catalysts, called enzymes. Examples of enzymatic catalysis in the synthesis of a class of physiological regulator molecules, catecholamines, are given later in this chapter.
In reaction coupling, two reactions are joined together with the transfer of energy. Energy from a spontaneous reaction (similar to water flowing downhill) is funneled to a nonspontaneous reaction (e.g., sawing wood) so that the sum of the two reactions is spontaneous. That is, the energy liberated by the “downhill” reaction is used to drive the “uphill” reaction. This is the basic function of a motor; the “downhill” burning of gasoline is coupled with the “uphill” movement of the car. The ability of proteins to couple spontaneous and nonspontaneous reactions allows cells to be chemical motors, using chemical energy to do various jobs of work. One such job of work, the contraction of striated muscle, is discussed later with particular emphasis on the proteins involved.
Proteins provide a pathway for the transport of most molecules and all ions into and out of the cell. Transport and transport proteins are discussed more fully after a discussion of the lipid bilayer membrane, the major obstacle to transport.
Proteins that form filaments and that glue cells to each other and to their environment are responsible for the structure and organization of cells and of multicellular assemblies (i.e., the tissues and organs of animals). The internal structure of the muscle cell, as well as its ability to do work, is a result of the properties of the muscle proteins discussed later.
At its most basic level, signaling requires only a controlled change or difference. Human signaling occurs by way of open and closed electrical circuits (telegraphy), puffs of smoke in the air, and complex black marks on a contrasting background (numbers and letters). As discussed next, a fundamental property of proteins is the ability to change shape. The cell can use changes of protein shape directly to send signals, and the function of some proteins is purely informational. That is, all that some proteins do by changing shape is transmit and transduce information. Information can be defined as “any difference that makes a difference,” or more simply, “any difference that regulates something.” Catalysis, coupling, transport, structural, and signaling functions can be combined on individual protein molecules. As will become apparent, such multifunctional proteins carry out many important physiological functions. Also important is that a change in one or more of these protein functions can be used to carry information, to serve as a signal within the cell. Thus, in addition to proteins specialized exclusively to carry information, changes in enzymatic activity or ion transport can also “make a difference,” transmitting information and triggering an appropriate response.
Protein Function Depends on Protein Shape and Shape Changes
Protein function is founded on two molecular characteristics: (1) proteins can bind to other molecules very specifically; and (2) proteins change shape, which in turn alters their binding properties and their function. The binding specificity of proteins is the result of their complex three-dimensional structure. Grooves or indentations on the surface of protein molecules, called binding sites, permit specific interactions with a molecule of a complementary shape, called the ligand. This complementary-shape mechanism underlying binding is similar to the shape interaction between a lock and key.
Several aspects of the lock-and-key analogy are worth noting. As with a lock, only a small part of the protein is engaged in binding. The binding is very specific; and small changes in the shape of the binding site (keyhole) or the shape of the ligand (key) can cause major changes in protein (lock) behavior. Similar to the lock and key, the complementary-shape interaction serves a recognition function; only those molecules with the right shape affect protein function. This recognition function plays a primary role in information transfer. The protein recognizes a particular signal by binding to it, thus changing the protein’s shape and thus its function. Unlike the majority of locks, however, proteins frequently have multiple binding sites for multiple ligands.
Thus the three-dimensional shape of a protein, its conformation, determines protein function. A major force that stabilizes protein conformation is the hydrophobic interaction. Oily, “water-fearing” amino acids tend to congregate in the middle of a protein away from water, whereas hydrophilic (“water-loving”) amino acids tend to be found on the protein’s outer surface interacting with the abundant cellular water. The hydrophobic interaction is also important in stabilizing the interaction of proteins with the lipids of biological membranes, as discussed shortly. Protein shape is also stabilized by hydrogen bonding between polar amino acid pairs in the polypeptide (protein) chain.
The same weak forces responsible for protein conformation are also used to hold the ligand in the protein-binding site. The position of the ligand in the binding site is stabilized by hydrogen bonds between the polar groups of the ligand and polar, amino acid side groups lining the binding site, just as hydrogen bonds within the polypeptide chain stabilize the shape of the polypeptide. Precisely because the same forces are responsible for the shape of the protein and for its binding properties, shape influences binding, and in turn, binding can influence protein shape. The ability of proteins to change shape is called allostery (Greek, “other shape”).
Allosteric changes in protein conformation arise in four general ways. One way, just mentioned, is that most proteins change shape depending on which ligands are bound at particular binding sites (Figure 1-1, A). The sequence—specific ligand binding → protein shape change → change in protein-binding properties and protein function → this change regulates something—is a common molecular mechanism underlying physiological control. This method involves no alteration in the covalent structure of the protein.
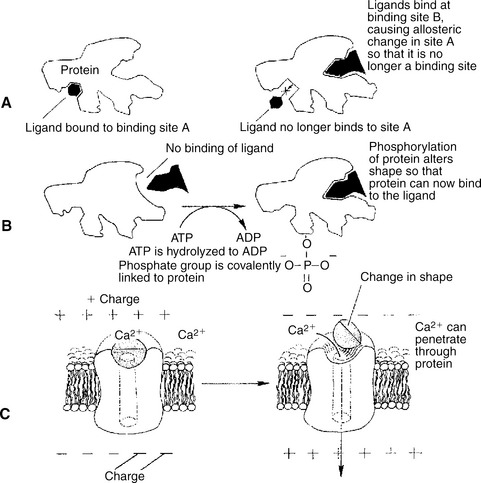
FIGURE 1-1 Three common mechanisms of allosteric shape change in proteins. A, Ligand binding. Ligand binding to an allosteric site (site B) on a protein changes the protein’s conformation such that binding site A is altered; ligand no longer binds at site A because of the binding event at site B. B, Phosphorylation. Addition of a phosphate group to a serine, threonine, or tyrosine residue of a protein alters the protein’s conformation, changing its binding characteristics. In this hypothetical example, phosphorylation activates an otherwise inactive protein. Some proteins inactivate by this mechanism. ATP, Adenosine triphosphate; ADP, adenosine diphosphate. C, Voltage-dependent proteins. The conformation of some proteins, particularly ion channels, is altered by the electrical field surrounding the protein. Shown here is the opening (activation) of a voltage-dependent, gated Ca2+ channel when the membrane depolarizes.
A second method of producing conformational change, however, occurs as a result of the covalent modification of one or more of the amino acid side groups of the protein (Figure 1-1, B). By far the most common such change is the covalent addition of a phosphate group to the hydroxyl (—OH) group on the side chain of serine, threonine, or tyrosine residues in the protein. Because the phosphate group is highly charged, phosphorylation of a protein alters hydrogen bonding and other electrostatic interactions within the protein chain, altering its conformation and functional properties.
In a third method, some physiologically important proteins change shape in response to the electrical field surrounding the protein (Figure 1-1, C). These respond to a voltage change by altering the position of charged amino acids, thus altering protein shape.
The fourth method of protein shape change is the least well understood (not shown). Some proteins change shape in a controlled manner in response to mechanical forces. Although this is not surprising, because all solids and solidlike substances change shape at least slightly in response to force, we know relatively little about mechanosensitive proteins. The best current example is a protein involved in the very early events of hearing that changes its transport of ions in response to the mechanical stimulation of sound (small changes of air pressure in waves).
The significance of binding specificity and allostery can be better appreciated with two examples of their role in physiological function. The first example is the role of enzymes in synthesizing three small, structurally similar, nonprotein signaling molecules. This example shows how binding specificity is important in catalytic function and how allostery underlies the regulation of the synthesis. The second example is more complex: the role of proteins in the contraction of muscle. The contraction of muscle shows how proteins can exploit the basic properties of specific binding and allosteric shape change to do more than one job of work at the same time; muscle proteins serve a structural role, serve a catalytic function, and couple the “downhill” hydrolysis of adenosine triphosphate (ATP) to do mechanical work, the “uphill” lifting of weight.
A Series of Enzymatic Reactions Converts Tyrosine into the Signaling Molecules Dopamine, Norepinephrine, and Epinephrine
Figure 1-2 is a diagram of the series of reactions by which the amino acid tyrosine is converted into three different signaling molecules: dopamine, a brain neurotransmitter; norepinephrine, a neurotransmitter of the peripheral autonomic nervous system; and epinephrine, an autonomic neurotransmitter and hormone. Dopamine, norepinephrine, and epinephrine share a similar structure. All contain a phenyl (benzene) ring with two hydroxyl groups (i.e., catechol) and an amine group (thus catecholamines). They are among the large number of molecules that function as neurotransmitters. That is, the electrically coded information sent along nerve cells causes the release of a chemical, the neurotransmitter, at the terminal of the neuron, which is next to a target cell, such as another nerve, a muscle, or an endocrine cell. The electrically encoded information of the nerve is transmitted to the target cell by the binding of the neurotransmitter to proteins on the surface of the target cell. proper neurotransmitter synthesis is crucial to nervous function and physiological regulation.
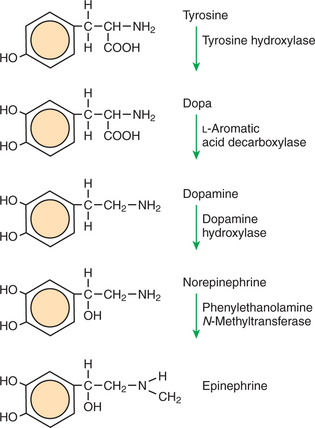
FIGURE 1-2 Epinephrine biosynthetic pathway. The amino acid tyrosine is metabolized to the neurotransmitters dopamine, norepinephrine, and epinephrine. The diagram shows the names and structural formulas for each compound in the path and the names of the enzymes that catalyze each reaction.
In the first step of catecholamine biosynthesis, tyrosine binds to the enzyme tyrosine hydroxylase, which catalyzes the addition of another hydroxyl group to the phenyl group to form dihydroxyphenylalanine, almost always called dopa. This hydroxyl group alters the enzyme-ligand interaction; the key no longer fits the keyhole. Dopa is released from the tyrosine hydroxylase and is then bound by another enzyme, L-aromatic amino acid decarboxylase. As the name implies, this enzyme catalyzes the removal of the carboxyl group, converting dopa to dopamine. Dopamine is converted into norepinephrine by the activity of dopamine hydroxylase, which adds yet another hydroxyl group, this time to the two-carbon tail of dopamine. Finally, addition of a methyl group to the amino nitrogen by phenylethanolamine N-methyltransferase gives rise to epinephrine (also called adrenalin). Note the binding specificity of the enzymes: whereas the catecholamine structures are all similar to one another, different enzymes bind each one (e.g., epinephrine does not bind to dopamine hydroxylase).
The allosteric properties of one enzyme in this pathway provide an example of physiological regulation. Certain hormones and neurotransmitters cause the phosphorylation of tyrosine hydroxylase, the first enzyme in the pathway, increasing its activity. That is, phosphorylation of the enzyme increases the rate at which it catalyzes the conversion of tyrosine to dopa. Because this step is the slowest in the pathway, an increase in the activity of this protein increases the net rate of synthesis of all the catecholamines. Regulated decreases in the rate of catecholamine synthesis are achieved by a different allosteric mechanism: binding of end products to the enzyme. Dopamine, norepinephrine, and epinephrine can all bind to tyrosine hydroxylase at a site different than the site for tyrosine. These binding events inhibit the enzymatic activity. The inhibition of the pathway by its own end products makes this a classic case of allosteric control called end-product inhibition. Many substances regulate their own synthesis by inhibiting an initial enzyme in the pathway. If the cell has enough end products, these products inhibit further synthesis by allosteric changes in the enzyme. This is an example of the following sequence: specific binding → protein shape change → change in protein-binding properties and protein function → this change regulates something.
Muscle Contraction and Its Initiation and Cessation Depend on the Binding Specificity and Allosteric Properties of Proteins
There are three types of muscle tissue in vertebrates: skeletal muscle, responsible for the animal’s ability to move; cardiac muscle, a muscle type found only in the heart but structurally similar to skeletal muscle; and smooth muscle, which surrounds hollow organs such as blood vessels, gut, and uterus. All three produce tensile force by contracting and shortening the length of the muscle. All muscle contraction occurs by the binding and the allosteric properties of two proteins, actin and myosin. Starting and stopping the contraction process depends on two additional proteins in skeletal and cardiac muscle, troponin and tropomyosin. Contraction initiation and cessation in smooth muscle depend on a different system with different proteins and are discussed later in this chapter.
Myosin is a large protein whose shape resembles a two-headed golf club. The elongated tail of the myosin molecule corresponds to the shaft of the golf club, and there are two knobs at one end of the tail that, as with golf clubs, are called heads. Myosin tails bind specifically to other myosin tails, forming bipolar aggregates called thick filaments (Figure 1-3). Myosin heads specifically bind ATP and another muscle protein, actin. Actin binds to itself to form long, thin filaments, called thin filaments in muscle and called microfilaments or F-actin (filamentous actin) in other cell types. Actin filaments play an important architectural role in all animal cells. Although actin is best understood in muscle cells, all animal cells depend on actin filaments for their shape and for their capacity to migrate in their environment. Actin filaments can be “woven” in various ways to produce different structures, such as ropelike bundles and clothlike networks. These actin bundles and actin networks are used to support the cell in particular shapes, similar to ropes holding up the woven cloth of a tent.
FIGURE 1-3 Assembly of myosin and actin to form filamentous structure. Myosin tails aggregate with one another to form a thick filament, a substructure of striated muscle. Actin monomers (G-actin) are a single polypeptide chain forming a globular protein that can bind to other actin monomers to form actin filament, also called microfilaments. The actin filament is the basic structure of striated muscle thin filaments; thin filaments also have troponin and tropomyosin as part of their structure.
In muscle, the interaction of myosin, ATP, and actin produces contraction and force, as shown in Figure 1-4:
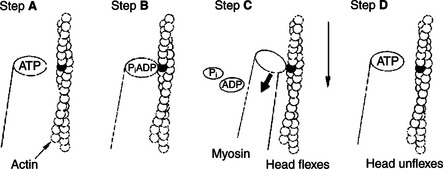
FIGURE 1-4 Power stroke of actomyosin. A, The myosin head has bound to adenosine triphosphate (ATP). In this conformation, myosin has little affinity to bind to actin. B, ATP is partially hydrolyzed to adenosine diphosphate (ADP) and inorganic phosphate (Pi); the hydrolysis is partial because the products remain bound to the myosin head. The change in what is bound to the myosin (ADP and Pi, not ATP) has the conformation of myosin so that it binds to actin with high affinity. C, Hydrolysis is complete; myosin releases ADP and Pi. This change in what is bound at the myosin head causes an allosteric change in the head; it flexes. Because the myosin head is still bound to the thin filament, the flexion causes the thin filament to slide past the thick filament. D, New ATP molecule binds to the myosin head; as for step A, myosin had little affinity for actin in this state, and the head releases from the thin filament and unflexes.
Step A: ATP binds to a myosin head; in this conformation, myosin has little ability to bind to actin.
Step B: Enzymatic activity associated with the myosin head, an adenosinetriphosphatase (ATPase), rapidly causes a partial hydrolysis of ATP to adenosine diphosphate (ADP) and inorganic phosphate (Pi), both of which stay bound to the myosin. With ADP and Pi bound, myosin has a slightly different shape that binds avidly to nearby actin filaments.
Step C: When myosin binds to actin, called cross-bridging, the myosin head couples the complete hydrolysis of ATP to a forceful flexing of the myosin head. This allosteric change causes the actin filament to slide past the thick filament. This sliding puts the actin filament under tension, which in turn causes the muscle to contract (shorten) against the load of the muscle (i.e., lifting a weight or pumping out blood). All muscle contraction depends on sliding of actin and myosin filaments. This same allosteric change of myosin also alters myosin-binding properties so that it releases the ADP and Pi.
Step D: The binding of a new ATP molecule to the myosin head again causes myosin to change shape; the head unflexes and loses its affinity for actin, releasing the cross-bridge, and the cycle can start over. Rigor mortis of dead animals is caused by a lack of new ATP to bind to myosin heads. In the absence of ATP, myosin heads remain in Step C (i.e., bound to actin). The muscle is stiff because it is completely cross-bridged together.
The actomyosin motor uses the binding and allosteric properties of proteins to (1) create structural filaments capable of withstanding and transmitting mechanical force, (2) catalyze the hydrolysis of ATP, and (3) couple the “downhill” ATP hydrolysis to the “uphill” contraction to produce force. For just the one protein, myosin, there are a number of examples of the characteristic sequence described earlier: specific binding → protein shape change → change in protein-binding properties and protein function → this change makes a difference.
This system of contractile proteins requires some control so that, for example, the heart beats rhythmically and skeletal muscle contraction is coordinated. At the organismal level, skeletal and cardiac muscle contraction is primarily under control by electrical stimulation from nerves or other electrically active cells (see Chapter 6). The transmission of electrical excitation to the actomyosin system is called excitation-contraction coupling. Excitation-contraction coupling in all types of muscle depends on changes in intracellular calcium ion (Ca2+) concentration. In skeletal and cardiac muscle, but not smooth muscle, two additional thin-filament proteins, troponin and tropomyosin, are required for this coupling. (Excitation-contraction coupling for smooth muscle is discussed later in this chapter.) In striated muscles, troponin binds to tropomyosin and to Ca2+. Tropomyosin is a long, thin protein that binds in the groove of the actin filament in such a way that its positions, high in the groove or snuggled down deep in the groove, allow or prevent the myosin head access to the thin filament (Figure 1-5). Excitation-contraction coupling of striated muscle works as follows:
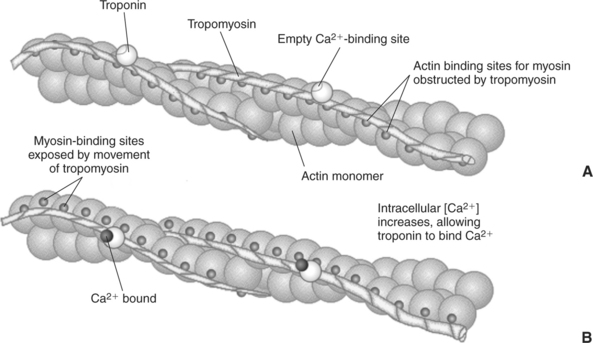
FIGURE 1-5 Regulation of the actomyosin ATPase and striated muscle contraction by Ca2+. A, In the absence of high concentrations of Ca2+, tropomyosin sits in the groove of the actin filament to obstruct the binding sites on actin for myosin. B, In the presence of higher Ca2+ concentrations, the ion binds to troponin, causing an allosteric change in the interaction of troponin with tropomyosin. This allosteric change in turn changes the interaction of tropomyosin with the actin filament to expose the myosin-binding sites on actin.
Step A: Electrical excitation of a striated muscle cell causes an increase in the intracellular concentration of Ca2+.
Step B: The additional Ca2+ binds to troponin, causing an allosteric change in troponin.
Step C: Because Ca2+ is bound to troponin, which in turn is bound to tropomyosin, the Ca2+-induced change in troponin conformation is transmitted to the tropomyosin molecule. When troponin binds Ca2+, tropomyosin changes its binding to actin in such a way that it exposes the actin site for myosin cross-bridging. (Tropomyosin snuggles down deeper in its actin groove, revealing actin to the myosin head.) As long as troponin binds Ca2+, the muscle contracts by the actomyosin cycle outlined earlier.
Step D: When the Ca2+ concentration drops to normal, however, troponin no longer binds Ca2+. This causes tropomyosin to move up in the thin filament groove so that it again blocks the myosin-binding sites on actin. Myosin heads can no longer cross-bridge, and muscle contraction stops.
As with the actomyosin force generation itself, its regulation also shows many examples of the specific binding function. The specific binding of Ca2+ to troponin is a purely informational use of protein binding and shape change; that is, troponin has no catalytic, transport, or structural function, but transmits the “on” signal to the next protein. The binding of tropomyosin to actin serves not only a regulatory role but also a structural role; the actin filament is stabilized by tropomyosin, making it less likely to disassemble into actin subunits. The change in the binding geometry of tropomyosin that directly regulates myosin access to actin is a good example of the importance of allosteric change and the following sequence: specific binding (troponin to tropomyosin) → protein (tropomyosin) shape change → change in protein-binding properties (tropomyosin to actin) → a difference in the position of tropomyosin, which in turn regulates the actomyosin motor.
Biological Membranes Are a Mosaic of Proteins Embedded in a Phospholipid Bilayer
Before continuing the discussion of the cellular basis of physiological control, an additional basic structure must be introduced. This is the phospholipid bilayer of the biomembranes of cells. Phospholipids are molecules that have two long tails of hydrophobic fatty acid and a head containing a charged, hydrophilic phosphate group. Under appropriate aqueous conditions, these molecules spontaneously form an organized membrane structure, similar to the film of a soap bubble. This filmy layer is composed of two layers (a bilayer) of phospholipid molecules. In both layers the hydrophilic heads point outward to hydrogen bond with water, and the oily, fatty-acid tails point inward, toward one another and away from the water. Proteins embedded in this lipid bilayer, called intrinsic membrane proteins or just membrane proteins, produce the fluid mosaic structure of biomembranes shown in Figure 1-6. All biological membranes share this fluid mosaic structure, whether the membrane is the outer plasma membrane separating cytoplasm from extracellular fluid or the membrane surrounding intracellular membranous organelles such as endoplasmic reticulum or lysosomes. It is called a “fluid mosaic” because of the mosaic of proteins among phospholipids, and because the phospholipid layer is fluid; proteins can move around and diffuse within the plane of the bilayer “like icebergs floating in a phospholipid sea” (the apt phrase of S.J. Singer, one of the originators of the model).
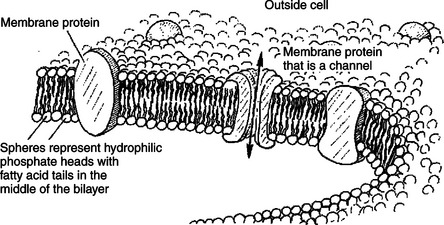
FIGURE 1-6 Fluid mosaic model for biomembranes. Biomembranes consist of a lipid bilayer in which membrane proteins are embedded.
Biological membranes are another crucial molecular structure underlying physiological control. The basic fluid mosaic structure serves four broad functions: (1) compartmentalization, (2) selective transport, (3) information processing and transmission, and (4) organizing biochemical reactions in space.
Compartmentalization is the ability to separate and segregate different regions by composition and function. For example, the lysosome is a membranous organelle within cells that contains hydrolytic (digestive) enzymes that can potentially digest the cell. The lysosomal membrane compartmentalizes these potentially harmful enzymes, segregating them from the bulk cytoplasm. The rigor mortis, mentioned earlier, that begins shortly after death is transitory because on death the lysosomes begin to break open, releasing their enzymes, and the actomyosin cross-bridges are eventually digested apart.
Clearly, the membrane cannot keep a compartment perfectly sealed; material must enter and leave the cell and its internal compartments. Selective transport results partly from the properties of the phospholipid bilayer but mostly from transport proteins embedded in the membrane. These proteins are characteristically selective in their transport functions; for example, the protein that is the specialized ion channel underlying neuronal signaling is 15 times more permeable to sodium ions (Na+) than to potassium ions (K+). Transport is a major topic of cell physiology and is discussed in more detail later.
If the cells of an organism are to respond to external changes, they must receive information about the state of the outside world. Just as we higher animals have our sensory organs—eyes, ears, nose, and so forth—arrayed on our outside surface, so too cells have most of their environmental information processing and transmission apparatus on their external surfaces. These are intrinsic membrane proteins of the plasma membrane, called receptors, that serve a purely informational function, as discussed earlier.
At first glance it might seem odd that a fluid membrane could provide spatial organization for biochemical reactions. However, returning to the “icebergs in a phospholipid sea” analogy, random collisions are much more likely for material in the two-dimensional membrane surface than for material moving through the three-dimensional volume of the cytoplasm. (If the Titanic had been able to dive or fly, it would have had additional ways to avoid the iceberg!) This much larger collision probability is exploited by the cell in a number of physiological processes. Membranes can also be fenced off into distinct regions, across which there is limited diffusion of membrane proteins. For example, certain cells in the kidney have two membrane regions that are quite distinct with respect to transport proteins, which is important in the regulation of salt and water balance by the animal.
TRANSPORT
Only Small, Uncharged Molecules and Oily Molecules Can Penetrate Biomembranes Without the Aid of Proteins
Charged particles (i.e., ions) do not pass through a pure phospholipid bilayer because of the inner, hydrophobic region of bilayer. Polar molecules (molecules with no net charge but with electrical imbalances) with a molecular weight greater than about 100 daltons are also unable to pass readily through a pure lipid bilayer, thus excluding all sugar molecules (monosaccharides), amino acids, nucleosides, as well as their polymers (polysaccharide, proteins, nucleic acids). On the other hand, some crucially important polar molecules (e.g., water, urea) are small enough to pass through the lipid bilayer. Small, moderate-size, and large molecules that are soluble in oily solvents readily pass through a pure lipid bilayer. Physiologically important molecules in this class include O2, N2, and the steroid hormones (see Chapters 33 and 34). However, many toxic, synthetic molecules, such as insecticides, are also in this category.
Molecules Move Spontaneously from Regions of High Free Energy to Regions of Lower Free Energy
The majority of biochemicals do not pass readily through a phospholipid bilayer. Transport of this molecular majority requires a protein pathway across the biomembrane. Also needed is a force causing movement along the pathway. Before elaborating on membrane proteins as pathways through the lipid bilayer, the energy factors that drive the transport are considered.
Objects fall spontaneously because of gravity. This is a manifestation of the principle that movement occurs to minimize the potential energy of the object. Indeed, all change in the universe (at scales greater than the subatomic particles) occurs to minimize the potential energy, also called the free energy, of the system. The movement of molecules is strongly affected by forces such as concentration, pressure (both part of chemical potential), and voltage (electrical potential). Molecules move spontaneously from a region of higher concentration to lower concentration, from higher to lower pressure, and from higher to lower electrical potential. Each of these factors—concentration, pressure, and electrical potential—is a source of free energy. The transport of a molecule does not depend necessarily on any one factor; rather, the sum of all the free energy contributions is the determinant of transport. The sum of all the free energy contributions on a substance is usually expressed on a per-mole basis as the electrochemical potential. The electrochemical potential is the free energy of the substance, from all sources, per mole of the substance.
In order for spontaneous transport to occur, there must be a difference in the electrochemical potential of the substance between two regions. The two regions are usually two compartments separated by a membrane. This difference in electrochemical potential is called the driving force. Typically, students have little difficulty understanding the direction of spontaneous flow as long as only one factor contributes to the electrochemical potential, pressure, or concentration or the voltage. However, understanding physiological transport, both across cells and across tissues, requires an understanding of the contribution of each factor to the driving force. For example, the flow of fluid from the capillaries of the vascular system depends on the balance between both the hydrostatic pressure difference and the concentration difference of solutes (osmotic pressure) across the capillary. Similarly, movement of Na+ and K+ ions across the plasma membrane of nerve cells depends on the driving forces contributed by both voltage differences and ion concentration differences across the membrane.
Material moves spontaneously from regions of high electrochemical potential to low electrochemical potential. Such transport is called diffusion or passive transport. Net movement of material (i.e., diffusion) stops when the electrochemical difference between regions equals zero. The state at which the free energy or the electrochemical potential difference is zero is called equilibrium. Equilibrium means “balance,” not equality. Equilibrium is reached when the free energy (electrochemical potential) is balanced; the value on one side is the same as the other. In most cases the source of the free energies on the two sides never becomes equal; the concentrations, the pressure, and the voltages remain different, but their differences “balance out” so that the sum of the free energy differences is zero.
Equilibrium is a particularly important concept because it describes the state toward which change occurs if no work is put into the system. Once the system reaches equilibrium, no further net change occurs unless some work is done on the system. The words “net change” are important. Molecules at equilibrium still move and exchange places, but as much goes in one direction as in the other, so there is no net flow of material.
If the cell requires material to move from low to high electrochemical potential (i.e., in the direction away from equilibrium), thus increasing the difference in free energy between two regions, then some driving force, some work, most be provided by some other decrease in free energy. This type of transport is active transport. Active transport uses proteins that combine transport and reaction coupling functions; the protein couples the “uphill” movement of material to a “downhill” reaction such as ATP hydrolysis.
Important Transport Equations Summarize the Contributions of the Various Driving Forces
It is worthwhile developing some quantitative aspects of transport, beginning with simple examples and developing equations for the effect of more than one driving force. These equations can be seen as summaries of the physical laws. In most cases the equations describe phenomena with which we have experience by living in a technological society. In these equations, c stands for concentration, V for volume, P for pressure, and so forth; these are common concepts. It is important, however, to think about these equations in real-life terms, not as abstract symbols.
One of these equations relates a hydrostatic (pressure) driving force for water movement that just balances a driving force caused by a chemical potential difference. Osmosis is the movement of water across a semipermeable membrane in response to the difference in the electrochemical potential of water on the two sides of the membrane (Figure 1-7). The chemical potential of water is lower in 1 liter (L) of water (H2O) in which is dissolved 2 millimoles (mmol) of sodium chloride (NaCl) than in 1L of H2O in which is dissolved 1 mmol of NaCl. If these two solutions are separated by a pure lipid bilayer, Na+ and Cl− ions cannot move to equilibrate the concentration. Rather, the freely permeable water moves from the side with the higher water potential (low concentration of solute) to the side with the lower water potential (higher concentration of solute). Thus, water follows solute (a good summary of osmosis), and this water movement dilutes the 2-mmol solution. However, water movement never produces equal concentrations of salt. Rather, another driving force appears as the water moves. The hydrostatic pressure of water increases on the side to which the water moves, increasing the electrochemical potential of the water on that side. Net water movement stops when the increase in water potential from hydrostatic pressure exactly balances the decrease in water potential from the dissolved salt, so that the electrochemical potential becomes equal on both sides of the membrane.
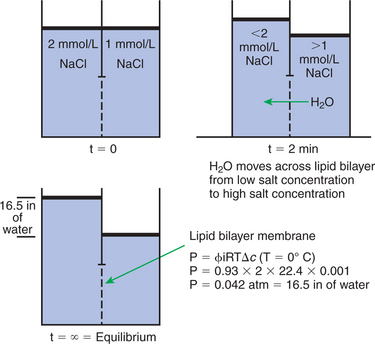
FIGURE 1-7 Osmosis. At time (t) = 0, two compartments are separated by a lipid bilayer membrane (no transport proteins) that contains salt solutions of differing concentrations. At t = 2 minutes, the salt ions cannot move across the membrane to equilibrate their concentration, but water can move. Water moves from the region of higher water potential (low salt) to the region of lower water potential (high salt). Water continues to pass the lipid bilayer until at t = equilibrium; the difference in the height of water between the two sides creates a difference in pressure that is equal but opposite to the difference in the water potential between the two sides. That is, the free energy difference resulting from differing salt concentrations is equilibrated by an equal but opposite free energy difference caused by pressure.
The initial potential difference of water in Figure 1-7 is caused by the difference in the concentration of material dissolved in the water. A proper explanation of why the water in a solution has a lower chemical potential than pure water (and why water in a concentrated solution has a lower potential than in a dilute solution) is beyond the scope of this chapter. However, readers familiar with the concept of entropy will realize that the disorder of a system increases with the introduction of different particles into a pure substance and with the number of different particles introduced. An analogy would be that a canister with mixed sugar and salt is more disordered, and therefore at higher entropy, than a canister with only pure salt or pure sugar. Also, the disorder of the system increases as more sugar is added to salt (up to 50:50); a pinch of sugar in a canister of salt only increases the disorder slightly. Because an increase in entropy causes a decrease in free energy, the free energy of a solution is decreased as the mole fraction of solute increases.
Osmosis is important to cells and tissues because, generally, water can move freely across them, whereas much of the dissolved material cannot. Given a concentration difference of some nonpermeable substances, van’t Hoff’s equation relates how much water pressure is required to bring the system to equilibrium, that is, the free energy contributed by a pressure difference across the membrane that exactly balances an opposing free energy contribution caused by a concentration difference.
Π = Osmotic pressure, the driving force for water movement expressed as an equivalent hydrostatic pressure in atmospheres (1 atm = 15.2 lb/in2 = 760 mm Hg). Osmotic pressure is symbolized by Π to distinguish it from other types of pressure terms.
i = Number of ions formed by dissociating solutes (e.g., 2 for NaCl, 3 for CaCl2).
R = Gas constant = 0.082 L atm/mol degree.
T = Temperature on the Kelvin scale; 0°C = 273°K.
(RT is a measure of the free energy of 1 mol of material because of its temperature. At 0°C, RT = 22.4 L atm/mol.)
Δc = Difference in the molar concentration of the impermeable substance across the membrane.
This equation summarizes a balance of driving forces; Π amount of hydrostatic (osmotic) pressure is the same driving force as a particular concentration difference, Δc. The osmotic pressure depends only on the concentration difference of the substance; no other property of the substance need be taken into account. Those phenomena that depend only on concentration, such as osmotic pressure, freezing-point depression, and boiling-point elevation, are called colligative properties. Van’t Hoff’s law is strictly true only for ideal solutions that are approximated in our less-than-ideal world only by very dilute solutions. Real solutions require a “fudge factor,” called the osmotic coefficient, symbolized by φ. The osmotic coefficient can be looked up in a table, then plugged into the equation as follows:
The term φic for a given substance represents the osmotically effective concentration of that substance and is often called the osmolar or osmotic concentration, measured in osmoles per liter (Osm/L). In general, the osmolar concentration of a substance is approximated by the usual concentration times the number of ions formed by the substance; the osmotic coefficient provides a small correction. The osmolarity of a 100-mmol NaCl solution (0.1 mol) is then 0.93 (φ for NaCl) × 2 (NaCl → Na+ + Cl−) × 0.1 mol = 0.186 Osm = 186 mOsm.
The previous equation summarizes a phenomenon crucial for physiological function. The greater the concentration difference of an impermeable substance across a membrane, the greater is the tendency for water to move to the side of high concentration. (Water follows solute.) Indeed, if you plug some numbers into this equation, you may be surprised at the large pressures required to balance modest concentration differences. For example, an NaCl concentration difference of 0.1 mol (5.8 g/L) is equilibrated by a pressure (4.2 atm) equal to a column of water 141 ft high (divers must be wary of “the bends” when ascending from below 70 ft of water). The importance of this is that a small concentration difference can produce a strong force for moving water. The body makes effective use of this to transport water in many tissues: ions/ molecules are transported into or out of a compartment → and water follows by osmosis.
Starling’s Hypothesis Relates Fluid Flow Across the Capillaries to Hydrostatic Pressure and Osmotic Pressure
An excellent practical example of how a balance of driving forces is responsible for the flow of water and permeable substances across a semipermeable membrane is the movement of water and ions across the single layer of cells (endothelial cells) that compose blood capillaries. The single cell layer composes, in effect, a semipermeable membrane with different transport qualities than that of a simple lipid-bilayer membrane. The junctions between cells have holes large enough for small molecules and ions to diffuse between compartments. Only large molecules, most importantly proteins, are unable to move through the holes. The difference in protein concentration between the blood and the water solution surrounding tissue cells, called the extracellular fluid (ECF) or interstitial fluid, creates an osmotic pressure for the movement of water with all its dissolved small molecules and ions. This osmotic pressure resulting from dissolved proteins has a special name: colloid osmotic pressure or oncotic pressure. Protein is more concentrated in the blood than in the interstitial fluid, producing an oncotic pressure of about 0.02 to 0.03 atm = 15 to 25 mm Hg, driving water into the capillary. On the basis of this driving force alone, one would expect the capillaries to fill up with water, thus dehydrating the tissue spaces. However, the heart is a pump that exerts a true hydrostatic pressure on the blood, tending to drive the water (and other permeable molecules) out of the capillaries. The net driving force is the algebraic sum of the oncotic pressure difference and hydrostatic pressure difference between the capillaries and the interstitial fluid, as follows:
Pc = Hydrostatic pressure in the capillary.
Pi = Hydrostatic pressure in the interstitial space (usually anear 0).
πc = Oncotic pressure of blood plasma in capillary (∼28 mm Hg).
πi = Oncotic pressure of interstitial fluid (∼5 mm Hg, but depends on the particular tissue).
This equation has enormous relevance to the function of the circulatory system. On the arterial end of capillaries the hydrostatic pressure (Pc) is high, about 35 mm Hg. Plugging this number into the equation along with the others, the net pressure in the capillary is +12 mm Hg; fluid is being driven out of the capillary on the arterial side (capillary filtration). The flow of fluid through the resistance of the capillary causes a decline in pressure so that the hydrostatic pressure on the venous side is low, Pc = 15 mm Hg. The oncotic pressures have not changed, so the net driving force on the venous side is −8 mm Hg; there is a net absorption of fluid into the capillary on the venous side (capillary reabsorption). This arrangement achieves a major function of the circulatory system; in this way the fluid of the blood circulates among the cells and is then recycled back into the circulatory system.
Pathological alterations in this system emphasize the physiological importance of balance of driving forces for transport. Chronic liver disease occurs with some frequency in horses and dogs, among other mammals. The liver is compromised in its ability to synthesize and secrete a major blood protein, serum albumin. The decline in the concentration of serum albumin lowers the oncotic pressure of the blood. As a result, there is more force to drive fluid out of the capillaries on the arterial side and less driving force for net absorption of fluid on the venous side of capillaries. This causes the tissue spaces of the diseased animals to fill with fluid, a painful and visually obvious symptom called edema. The Clinical correlations section at the end of the chapter provides another example of edema in which increased hydrostatic pressure in the veins and capillaries causes increased capillary filtration and less capillary reabsorption.
Membrane Proteins That Serve the Triple Functions of Selective Transport, Catalysis, and Coupling Can Pump Ions and Molecules to Regions of Higher Free Energy
Van’t Hoff’s law and Starling’s hypothesis deal with passive transport (i.e., movement of material in the direction of lower electrochemical potential). However, the cell moves many ions/molecules against their electrochemical potential. That is, this selective transport requires the expenditure of energy by the cell. Transport in a direction requiring an expenditure of energy (i.e., input of work) is called active transport. Active transport depends on intrinsic membrane proteins that use specific binding and allostery to achieve the dual functions of selective transport and reaction coupling. Many (but not all) active transport proteins obtain the energy for transport from ATP hydrolysis; these proteins must function also as enzymes (ATPases).
An important example of active transport is the Na+,K+ pump (also known as Na+,K+-ATPase). This intrinsic membrane protein consists of four polypeptide chains (2 α + 2 β) and has a mass of approximately 300,000 daltons. This molecule catalyzes the hydrolysis of ATP and couples the hydrolysis energy to the movement of Na+ out of the cell and K+ into the cell. This ion pump creates and maintains a considerable concentration gradient across the cell membrane for both ions (see Table 1-1).
Table 1-1 Concentrations of Various Substances in the Intracellular, Extracellular, and Plasma Fluids
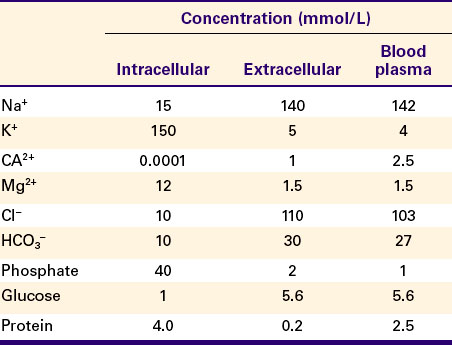
Figure 1-8 shows our current understanding of this protein’s structure and outlines the cycle of binding and conformational changes underlying its transport function. The Na+,K+-ATPase pumps three Na+ ions out of the cell and two K+ ions into the cell for each ATP molecule hydrolyzed. These directions of ion pumping cause a high Na+ concentration outside the cell and a low concentration inside, whereas K+ concentration is high inside and low outside the cell. The different directions of pumping for the two ions depend on differing binding specificity of the pump protein in the two conformational states. The ability of the protein to couple this transport to the enzymatic breakdown of ATP allows the transport to occur against the concentration gradients, from lower to higher electrochemical potentials for both ions. In the particular case of the Na+,K+ pump, the number of transported electrical charges is asymmetrical; three positive charges leave for each two positive charges that enter. This asymmetry of electrical charge transport means that the Na+,K+ pump is electrogenic, making a minor contribution to the electrical potential (voltage) across cell membranes, as discussed later.
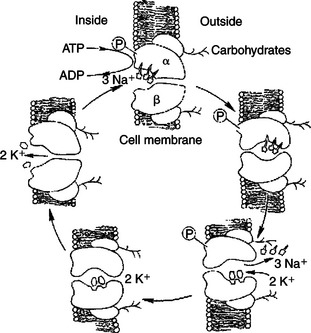
FIGURE 1-8 Hypothetical transport cycle for Na+,K+-ATPase. Changes in the conformation of this transport protein driven by ATP hydrolysis and ion-binding events cause three Na+ ions to be moved out of the cell against a concentration gradient and two K+ ions to be moved into the cell, also against a concentration gradient, for each ATP hydrolyzed.
(Redrawn from a diagram by Dr. Seth Hootman.)
Many different intrinsic membrane proteins actively transport a wide variety of ions and molecules against the transported molecules’ electrochemical gradient. Many, such as the Na+,K+ pump, couple the energy-requiring “uphill” transport with the “downhill” hydrolysis of ATP. However, any potential source of free energy can be coupled to the energy-requiring transport. Indeed, the gradient of Na+ set up by the Na+,K+ pump is itself used frequently as a source of energy. That is, the “downhill” flow of Na+ from outside the cell to the inside is a spontaneous reaction whose energy can be coupled to some “uphill” reaction (Figure 1-9). For example, the transport of glucose and many amino acids from the food mass in the small intestine into the cells lining the gut is an active transport process and requires a Na+ concentration gradient. Transport proteins in the plasma membrane of intestinal epithelial cells couple the spontaneous diffusion of Na+ into the cell to the inward, energy-requiring transport of the sugar or amino acids. These nutrients are at higher concentration inside the cell than outside, so they must be actively transported into the cell at the expense of the energy stored in the Na+ electrochemical gradient. That is, the energy from the “downhill” diffusion of Na+ into the cell is coupled to the “uphill” transport of the nutrient into the cell. Such active transport coupled to Na+ diffusion across the cell membrane is called secondary active transport because of its dependence on the Na+ concentration gradient established by the primary active transport of the Na+,K+ pump.
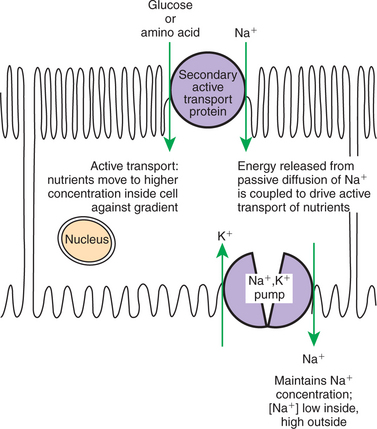
FIGURE 1-9 Secondary active transport as exemplified by uptake of nutrients by gut epithelia. Nutrients such as glucose and amino acids must be actively transported from relatively low concentration in the gut lumen toward higher concentrations within the cells lining the gut. This active transport process uses the concentration gradient of Na+ ions set up by Na+,K+-ATPase (see Figure 1-8) as the source of energy for the active transport process. In other words, the energy released by the passive diffusion of Na+ into the cell along its concentration gradient is coupled to the energy-requiring transport of glucose or amino acids against their concentration gradients. Thus the secondary active transport protein both serves a transport function and couples the “downhill” transport of Na+ to the “uphill” transport of nutrients. There are many such secondary active transport processes in the body. For example, the same mechanism shown here is used to reabsorb nutrients from blood filtrate in the kidney.
Examples of transport can be identified in a number of ways. Our examples have been instances in which two ions/ molecules must be transported together or not at all, and such transport is called co-transport. Co-transport can involve one process of passive transport (diffusion) with an active transport process, as in the two previous examples; it can involve two active transport processes (e.g., Na+,K+-ATPase) or two diffusion processes. In the first case, the need for co-transport is energetic; the flow of one ion is needed to drive the other. In the two latter cases, the need for co-transport is a restriction based on the binding properties of the transport protein; it cannot bind one without the other. Co-transport proteins that transport both substances in the same direction are called symports or symporters. The Na+/sugar co-transporter in the gut is a symport. Co-transport proteins that transport the two substances in opposite directions (e.g., Na+,K+-ATPase) are called antiports or antiporters. Parenthetically, proteins that transport only a single ion or molecule are called uniports or uniporters.
Many Membrane Proteins Selectively Facilitate the Transport of Ions/Molecules from High to Low Electrochemical Potential
The movement of ions and of medium and large polar molecules requires a protein molecule to serve as a pathway through the obstruction of the phospholipid bilayer. If the movement of the substance is in the natural direction of its electrochemical gradient (movement from high to low), the transport process is called facilitated diffusion. The membrane proteins mediating this transport process through the phospholipid bilayer are channels or carriers (Figure 1-10). These are distinguished by the extent to which the protein interacts with the transported substance.
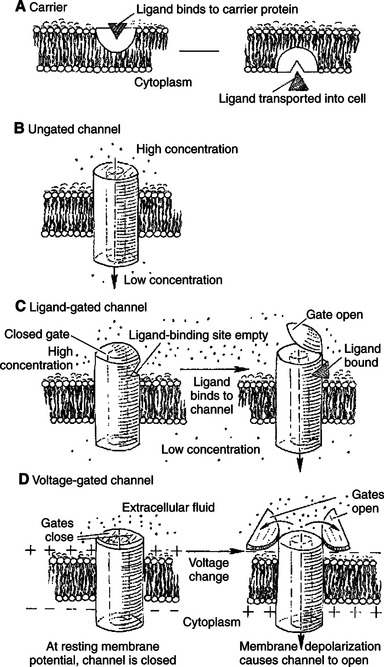
FIGURE 1-10 Types of transport proteins mediating facilitated diffusion. In all cases the ion moves from a region of high potential (shown here as high concentration) to a region of low potential. A, Carriers. In a few cases, material is carried by a transport protein that binds tightly to the material, and the complex moves through the lipid bilayer. B, Leak channels. These channels are thought not to open and close as do gated channels, and thus they support a small but persistent leak of a particular ion through the pore. Although their existence was long postulated, distinct, ungated leak channels have only recently been identified and isolated, as opposed to leaks through normally gated channels. Selectivity of these and other channels is based on the size of the pore and the weak interactions of ions with the atoms lining the pore. C, Ligand-gated channels. The transport protein again forms a pore through the membrane. In the case of gated channels, access of the ion to the pore is controlled by a gate, a substructure of the transport protein that can open and close the pore. In ligand-gated channels the opening and closing of the gate are controlled by the binding of a ligand to the channel. DM, Voltage-gated channels are similar to ligand-gated channels, except the opening and closing of the gate are controlled by the electrical field around the channel.
Carriers bind the transported substance in the lock- and-key manner, so there is a site-specific binding of the transported substance to the transport protein (Figure 1-10, A). Carrier-mediated transport is typically much slower than channel-mediated diffusion because of the relatively slow binding and unbinding processes. The Na+,K+ pump and the Na+/glucose symport are both examples of carriers.
Channels can be thought of as “protein donuts” embedded in the phospholipid bilayer. The hole in the donut is a pore in the membrane through which small ions such as Na+, K+, Ca+, Cl−, and H+ are transported. Although most channels transport ions, a class of channels called aquaporins comprises channels for water flow. (Although water will flow through a pure lipid bilayer, this transport is too slow for some functions. Kidney cells, for example, are particularly rich in aquaporins, which are required for the water balance function of the kidney.) For all channels, the pore size and the interaction of the transported material with the amino acid side groups lining the pore allow membrane channels to be selective. Only specific molecules or ions can move through a particular channel. Movement of material through channels is almost as rapid as simple diffusion through a water-filled space of the same area as the channel pore.
The plasma membranes of most cells have passive leaks of ions, particularly K+. These ionic leaks are typically ascribed to leak channels, which are open at all times (Figure 1-10, B). However, most ion channels open or close in response to signals. These latter types are called gated channels. The opening and closing of the gates are examples of the allosteric property of proteins. The same signals responsible for allosteric changes in general—ligand binding, phosphorylation, and voltage differences—also control the opening and closing of gated channels, as shown in Figure 1-10. (Because mechanically gated channels are so poorly understood, these are not discussed here.)
Channels that open in response to ligand binding are called ligand-gated channels (Figure 1-10, C). The nicotinic acetylcholine receptor is a ligand-gated channel found in skeletal muscle membrane directly beneath incoming neurons (nerve cells). This channel is found also in the membrane of neurons in autonomic ganglia and in the brain. As the name implies, the nicotinic acetylcholine receptor binds to the drug nicotine and the neurotransmitter acetylcholine. In both cases the channel opens in response to ligand binding.
This nicotinic acetylcholine channel plays a key role in transmitting electrical stimulation from neurons to skeletal muscle cells. Briefly, motor neurons release the neurotransmitter acetylcholine in response to the electrical signal coming down the neuron. This acetylcholine binds to and opens the ligand-gated channel on the skeletal muscle. The influx of Na+ into the muscle cell initiates an electrical response in the muscle, causing the release of Ca2+ (through gated channels in the endoplasmic reticulum), in turn causing contraction. (This brief account of neuromuscular transmission, presented only to provide orientation to the function of the acetylcholine channel, is expanded in Chapters 5 and 6.) In the case of the nicotinic acetylcholine receptor/channel, the specific binding and allosteric properties of the protein serve the dual functions of selective transport across the membrane and information reception and transmission to the muscle cell.
Channels that open in response to voltage changes across the membrane are called voltage-gated or voltage-dependent channels (Figure 1-10, D). This type of channel is largely responsible for the neurons’ ability to transmit information along their length and to release neurotransmitter. All voltage-gated channels have a range of membrane potentials that cause them to open; this is the channel’s activation range. The minimum membrane potential that causes opening is the channel’s threshold. The activation range and threshold vary from channel to channel, depending on the conformation of the protein and the electrical properties of the amino acid side groups that form the gate of the channel. In addition to an open and closed configuration, many voltage-dependent channels have a third conformation, called inactivated. Like the closed configuration, the inactivated conformation prohibits the diffusion of ions through the channel. Unlike the closed configuration, it does not open immediately in response to changes in membrane potential. Inactivation can be regarded as an enforced rest period for the channel. Voltage-dependent channels that do not inactivate have only open and closed conformations, and they take up one or the other conformation, depending on the membrane potential.
As previously discussed, any of the functions of proteins can be used to transmit information if a difference in the protein function changed the cell. Gated channels, both ligand and voltage gated, are ideal candidates for information transmission because they change their function: opening and closing, permitting or stopping transport. Indeed, the sole physiological function of the nicotinic acetylcholine receptor/ channel, as described earlier, is the transmission of information: turning the chemical stimulation by the neuron of the muscle into electrical stimulation (see following discussion) of the muscle membrane, leading to muscle contraction.
Passive Transport of K+ Across the Plasma Membrane Creates an Electrical Potential
As just discussed, gated ion channels can convert chemical information into electrical information. Electrical signaling in the animal body is the result of electrical imbalances maintained across the plasma membrane of virtually all cells: cells maintain an electrical potential difference across their plasma membrane. That is, the cell membrane is a battery; if one attaches electrodes to the two ends of a battery, or to the inside and outside of a cell, one finds a voltage difference between the two ends or sides. If one provides a path for electrical charges to move—a metal wire containing free electrons in the case of a battery, or a membrane channel through which ions can move in the case of the cell—an electrical current flows from higher to lower electrical potential. The diversity of battery-powered devices in our society suggests how many ways this electrical potential can be exploited. The physiology of animals also exploits the baseline electrical potential across the plasma membrane, called the resting membrane potential. The word “resting” is added to distinguish the baseline potential from the instantaneous values of membrane potential during the passage of membrane currents.
The resting membrane potential is the indirect result of the concentration gradients of ions across the plasma membrane caused by the activity of the Na+,K+-ATPase. Partly, this membrane potential is a result of the asymmetry in numbers of ions pumped by the Na+,K+-ATPase. However, most of the membrane potential is caused by the passive flow of K+ through K+ leak channels in response to the concentration gradient of K+ (high inside, low outside). This concentration gradient sets up an electrical driving force (voltage) that exactly balances the concentration driving force. The concentration of K+ inside a mammalian cell is about 150 mmol; outside in the interstitial fluid, it is about 5 mmol. As a result, K+ tends to diffuse from the cytoplasm through the leak channel to the interstitial fluid. However, when K+ alone leaves the cytoplasm without an accompanying negative ion, it causes an electrical imbalance. The exit of K+ ions leaves the inside of the cell with negative charges not neutralized by positive potassium ions, and the interstitial fluid now has positive K+ ions not balanced by negative charges. The cell is building an electrical potential difference across the plasma membrane, with the cytoplasm being negative relative to the interstitial fluid.
This electrical potential driving force increases until it balances the concentration driving force for K+. This situation is analogous to osmosis: the concentration-driven flow of water across a semipermeable membrane creates a different driving force, pressure, that eventually balances the concentration driving force. Similarly, for the resting membrane potential, the concentration-driven flow of K+ across the semipermeable membrane (semipermeable in the sense that negative ions do not accompany the K+) creates a different driving force, an electrical voltage, that eventually balances the concentration force. As in the case of osmosis, an equation is used to relate the size of the concentration gradient to the size of the electrical potential that provides an exact balance. This equation is called the Nernst equation, as follows:
EX = Equilibrium potential for ion X.
RT = Gas constant μ absolute temperature.
z = Electrical valence for the ion, +1 for Na+ and K+, −1 for Cl−, and so forth.
F = Faraday constant = number of coulombs of electrical charge in a mole of ions = 96,500 coulombs/mol.
A simpler form of this equation can be written by taking advantage of the fact that R and F are constants, T is almost constant under physiological conditions, and the natural log of a number is 2.3 times the common log (log10), as follows (mV, millivolts):
Because the state of balance between the electrical driving force and the concentration driving force is equilibrium, the value of the electrical potential is called the equilibrium potential of the ion. Given the concentrations above for K+ inside (150 mmol) and outside (5 mmol) the cell, the equilibrium potential for K+ is:
Indeed, the measured resting membrane potential across a human muscle cell is −90 mV.
Several aspects of this important equation are worth discussing. If the equilibrium potential for a particular ion is the same as the measured membrane potential, the net driving force for the ion is zero. In this case, there is no net movement, even in the presence of wide-open channels, to provide a path through the membrane. However, for any gradient of a specific ion, if the measured membrane potential is not the equilibrium potential of that ion, there is a driving force for the transport of that ion. That is, when the membrane potential is anything other than the equilibrium potential, that ion will flow across the membrane if an appropriate channel is open. Thus the equlibrium potential for an ion provides a “baseline” for comparison with the actual membrane potential to determine whether an ion will tend to move across the plasma membrane. If the measured membrane potential has the same sign but is larger in magnitude than the equilibrium potential, the ion flows in the direction of the electrical potential. If the sign is the same but the magnitude lower, the concentration driving force determines the direction of flow of the ion. If the measured potential has the opposite sign of the equilibrium potential, both electrical and concentration forces are acting on the ion in the same direction. Flow of ions across the plasma membrane (i.e., electrical current) in response to the balance of force between concentration and voltage produces the electrical changes in neurons that underlie the nervous system, as discussed in Chapter 4.
It would be reasonable but incorrect to assume that the transport of ions required to set up the electrical potential measurably alters the concentration gradient. This is untrue because of the large amount of energy required to separate electrical charges. The separation of charge arising from the transport of a few ions balances the energy of quite substantial concentration gradients. Indeed, so few ions move that they cannot be measured by chemical means. Thus, electrical, not chemical, measurements are used routinely to assess transport of ions in cells. The measurable voltage changes caused by immeasurably small concentration changes of ions means also that the electrical phenomena at the membrane persist for many hours, even if the Na+,K+-ATPase is inactivated by a toxin. That is, an existing concentration gradient of K+ would require hours to dissipate at the rate of K+ leakage characteristic of the plasma membrane. Using the membrane battery analogy, the Na+,K+-ATPase is a battery recharger. A portable radio does not require the minute-to-minute services of a battery recharger. Enough energy is stored in the battery to operate the radio for an appreciable period, although the battery recharger is needed eventually. Similarly, enough energy is stored in the K+ concentration gradient to maintain the membrane potential for a period of time. The Na+,K+-ATPase is not required on a minute-to-minute basis, although it is needed ultimately to maintain the concentration gradient on which the resting membrane potential depends.
Spatial Organization of Active and Passive Transport Proteins Enables Material to Pass Completely Through the Cell
Although macromolecules and biomembranes clearly underlie physiological function, many phenomena of the intact animal emerge that are not initially apparent as a simple sum of parts. One interesting example is the spatial organization of plasma membrane transport proteins so that ions move across the cell from one ECF compartment to another. This transcellular transport is important in the kidney (see Chapter 41). The plasma membrane of the epithelial cells in the proximal tubules of the kidney contains two distinct regions. The apical membrane regions face the lumen of the tubule and the fluid that will become urine, and the basolateral regions are near the capillaries and the blood. The apical surface contains ungated leak channels for Na+, whereas the basolateral surface contains Na+,K+-ATPase molecules. The membrane proteins in one region are prevented from diffusing into the other by membrane structures called tight junctions. Na+ diffuses into the cell on the apical surface from the urinelike fluid driven by both the concentration gradient and the resting membrane potential. Once inside the cell, the Na+ is pumped from the basolateral surface, essentially into the blood, by the Na+,K+-ATPase. This allows the kidney to reabsorb and thus conserve Na+. As long as the Na+,K+-ATPase remains restricted to the basolateral surface and the passive channel to the apical membrane, Na+ can move through the cell from the urinelike fluid in the tubule to the blood in the capillaries. If either protein should lose its spatial restriction, Na+ would be transported into and out of the cell on the same surface, merely consuming ATP, with no net transport of Na+ from lumen to capillary.
Membrane Fusion Allows for a Combination of Compartmentalization and Transport of Material
Impermeable molecules can be transported across the cell membrane by means other than membrane proteins. This method involves using membrane itself as a carrier compartment. The lipid bilayer of biological membranes has a structure similar to soap bubbles. As with soap bubbles, small vesicles of biomembrane (essentially “membrane bubbles”) can fuse to form larger membrane surfaces. A large membrane surface can also pinch off (requiring fusion of two membrane surfaces) into small vesicles. When these processes occur at the plasma membrane, they are called exocytosis and endocytosis, respectively (Figure 1-11). When these processes occur at internal membrane sites, the process is referred to as membrane fusion, whatever the direction. Membrane fusion underlies much of membrane vesicle traffic around the cell. This traffic creates intracellular vesicles, renews plasma membrane by adding newly synthesized membrane, and transports material within the cell and across the plasma membrane. Because the transport is compartmentalized within a membrane bubble, the transported material can be targeted specifically to one or another region of the cell. Also, changes to the “cargo” can occur within a particular membrane compartment, as seen in cholesterol transport.
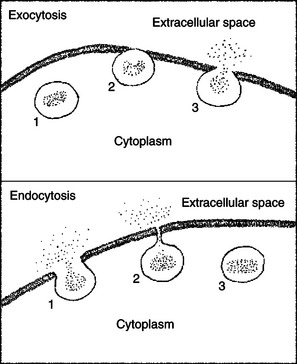
FIGURE 1-11 Two membrane fusion processes: exocytosis and endocytosis. Top, In exocytosis, a membrane-bound vesicle from the cytoplasm (1) makes contact and fuses with the plasma membrane (2). As the vesicle membrane becomes continuous with the plasma membrane, the contents of the vesicle are released to the extracellular space (3). Bottom, In endocytosis, some material from the extracellular space is surrounded by plasma membrane (1), which continues to invaginate until the edges are able to fuse (2), thus pinching off a vesicle from the plasma membrane (3). Membrane fusion can occur between any two compartments within cells separated by lipid bilayer membrane, not only between the cytoplasm and extracellular space, as shown here.
Exocytosis and endocytosis are crucial in the transport of cholesterol (Figure 1-12). Cholesterol is an essential lipid component of many animal biomembranes; the plasma membrane lipids of animals are about 15% cholesterol and 60% phospholipids. Cholesterol is also the starting material for the synthesis of the entire group of hormones called steroids (see Chapter 33). Cholesterol can be synthesized by animals and is also absorbed by meat-eating animals from their diet. Because cholesterol is soluble in oil, it passes from food through the plasma membrane without protein mediation into the cells of the gut lining. However, transport of dietary cholesterol through the circulatory system requires that cholesterol molecules form a complex with a protein molecule to create low-density lipoproteins (LDLs). To take up cholesterol from the circulation, cells bind the LDLs to intrinsic membrane proteins that act as LDL receptors, as shown in Figure 1-12. The receptor/LDL complex then diffuses in the plane of the membrane into specific regions to form coated pits. The coated pit is taken into the cytoplasm by endocytosis. In addition to the transport function, receptor-mediated endocytosis functions also to concentrate extracellular material before internalization. The coated pit is not taken into the cell until it has collected the LDLs from a much larger volume of ECF than the cell could “drink.” The membrane vesicles formed by this endocytosis fuse subsequently to become an endosome. The endosome compartment becomes acidic, which causes dissociation of the LDL and the receptor. Through unknown means, the endosome is then able to further separate and compartmentalize the receptor from the LDL. Membrane vesicles containing the now-vacated LDL receptors return to the plasma membrane and fuse by exocytosis. The LDL receptor is recycled to the plasma membrane to pick up more LDL. Experimental evidence suggests that a single LDL receptor molecule can cycle between the plasma membrane and endosomal vesicles more than 100 times before losing its activity. Meanwhile, the LDL moiety is segregated to another endosomal vesicle, which fuses with the lysosome. The lysosome contains hydrolytic enzymes, thus allowing the internalized LDL to be digested. The cholesterol is now available to the cell for steroid synthesis or incorporation into membrane.
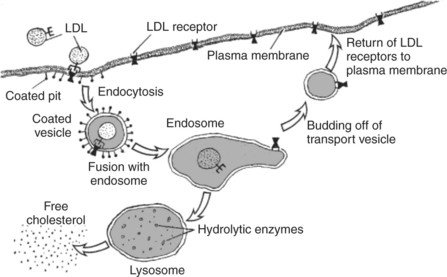
FIGURE 1-12 Processes of membrane fusion involved in cholesterol uptake by cells. Starting at the left, a low-density lipoprotein (LDL)--containing cholesterol binds to an LDL receptor protein of the plasma membrane and undergoes endocytosis, forming an endosome. The receptor is detached from its LDL ligand in the endosome. The LDL portion of the endosome fuses with a lysosome to digest the LDL and produce free cholesterol, while the receptor-containing portion of the endosome pinches off a vesicle to return to the plasma membrane, thus recycling the receptor.
(Redrawn from Alberts B, Bray D, Johnson A, et al: Molecular biology of the cell, New York, 1983, Garland.)
Other molecules are also recycled by endocytosis. As with the LDL receptor, for example, many signal receptors, discussed in the next section, are endocytosed back into the cell that released them, saving the cell the effort of synthesizing new receptors. Not all endocytosed molecules are recycled. Many are broken down after their endosome fuses with a lysosome. Indeed, as described later, this is one method of regulating receptor number on the plasma membrane.
INFORMATION TRANSMISSION AND TRANSDUCTION
Cell Signaling Usually Occurs by a Lengthy Chain of Sequential Molecular Interactions
One of the areas of most rapid progress in cellular physiology has been our understanding of the mechanisms by which extracellular signals, such as hormones, growth factors, and neurotransmitters, alter cellular function, which in turn alters tissue, organ, and animal function. At the molecular level, almost all chemical signaling shares a common “strategy” of mechanism: signals are sent as a long chain of chemical cause-and-effect interactions transmitted between many sequential chemical steps. Indeed, chemical signaling pathways are structured similar to the whimsical “machines” in the cartoons of Rube Goldberg (1883–1970), a famous American newspaper cartoonist. Figure 1-13 shows one of his cartoons from 1928 illustrating an outlandish contraption (a “Rube Goldberg device”) to serve as an automatic garage door opener, realistic versions of which had not yet been invented. The automobile (A) drives in, causing hammer (B) to ignite a toy cap gun (C), which frightens rabbit (D) with string (F) tied to its leg, thus firing pistol (G), and so forth, until a connection to a rotating water sprinkler causes the carriage-house door to slide open (overhead doors also had not yet been invented). Although much of the humor of this parody of a machine is lost on us (our attitudes about machines have changed greatly since Goldberg’s heyday), Rube Goldberg devices are a surprisingly useful analogy to the overall mechanism of cellular chemical signaling.
FIGURE 1-13 Rube Goldberg device (garage door opener, circa 1928) as an analogy for the complex cause-and-effect sequence characteristic of cellular chemical signaling. Automobile (A) drives into driveway, causing hammer (B) to ignite toy cap (C), frightening rabbit (D) into its burrow (E) and causing a pistol (G) to fire and so forth, ultimately leading to the opening of the garage door (R). As explained in the text, this whimsical “machine” serves as an analogy for chemical signaling within cells because of the multiple control elements, their connection as a cause-and-effect sequence, and the use of household items, similar to the use of evolutionarily conserved proteins of cells in signaling.
Just as the garage door opener of Figure 1-13 depends on a series of sequential cause-and-effect interactions, so chemical signaling occurs by a series of cause-and-effect changes in protein shape and binding. Just as the complex events of Goldberg’s device are linked to signal and to actuate garage door opening, so a cascade of changes in protein shape and function are linked to signal and actuate physiological events. Our earlier example of muscle contraction illustrates such a pathway of cause and effect and the analogy to Rube Goldberg devices. Electrical excitation (A) of a muscle cell increases intracellular Ca2+ concentration (B), causing Ca2+ to bind to troponin (C). This in turn alters the binding of tropomyosin (D) to actin (E) allowing the myosin heads (F) to bind to actin, thus leading to cross-bridging (G) and hydrolysis of ATP and contraction.
As this example indicates, the sequence of cause and effect for both chemical signaling and Rube Goldberg devices is complex. Both involve many different elements, none of which can be identified as the controller; all the elements are involved in control. Importantly, this creates multiple sites for regulation and for therapeutic drug action. Just as increasing the caliber of the pistol in the garage door opener would change the response time for opening, so a drug that bound to an element in the middle of a signaling pathway in a cell could increase or decrease the final physiological change in response to a particular hormone, for example. Also related to complexity is that the chain of cause and effect is not obvious; the particular sequence connecting a particular signal (adrenaline binding to a receptor on heart muscle) to a particular outcome (increased cardiac output) must be memorized. However, once the sequence is understood, you can predict from the state of one element in the chain what should happen next. Finally, Rube Goldberg devices were cobbled together from reasonably common household items, such as the bucket, fish tank, sprinkler, and even pistols. Similarly, the elements of chemical-signaling pathways are often highly conserved, and you will see throughout your studies that the same molecules or same basic types of molecules are used in a wide variety of different stimulus-response pathways.
Signaling Pathways Begin with the Binding of an Extracellular Molecule to a Receptor
In addition to the Rube Goldberg–like sequence of signal pathways, another aspect of the overall “strategy” of cellular information transmission is that signaling pathways almost always begin with the environmental signal molecule binding to a protein molecule specialized for information transfer, called a receptor. The LDL receptor discussed earlier is involved in the transport of material into cells (see Figure 1-12). However, most other receptors are proteins whose task is to transmit and transduce information to the cell from the extracellular environment. Receptors distinguish among the large number of external signaling molecules (e.g., various hormones, neurotransmitters, growth factors) through the usual protein mechanism of highly specific binding.
Three broad classes of receptors, called receptor families, are particularly important in physiological function and are discussed in this chapter and Chapter 2. Two of these families, the G-protein–coupled receptors and the receptor tyrosine kinases, are intrinsic membrane proteins of the plasma membrane. These membrane receptors bind the signal molecule in the extracellular environment, and the signal is then communicated intracellularly through a Rube Goldberg sequence of “differences that make a difference.” The third class of receptors is the nuclear receptor family. These intracellular proteins transduce signals from oily, lipidic molecules that can easily enter the cell, such as steroid and thyroid hormones, fat molecules in the diet, and derivatives of vitamins A and D. The information transduction pathway of nuclear receptors is simpler than that of the membrane receptors in that nuclear receptors are themselves direct regulators of gene transcription; that is, nuclear receptors are transcription factors. Binding of the signal molecule activates the nuclear receptor so that it is then able to bind directly to specific regions of deoxyribonucleic acid (DNA) and stimulate the binding of ribonucleic acid (RNA) polymerase to, and thus production of, messenger RNA from the particular gene or genes in that region of DNA.
Specific Physiological Information Is Inherent in the Receptor/Ligand Complex, Not in the Hormone/ Neurotransmitter Molecule
Before discussing specific receptors, it is useful to elaborate on some important points about the nature and regulation of the information transfer between the external signal molecule and receptor. This text provides ample evidence that the same hormone and especially the same neurotransmitter molecule can bind to different receptors. These different receptor-binding events send different information to the cell from the same external signal molecule. For example, acetylcholine is bound by two different receptors, the nicotinic ion channel described earlier and the muscarinic receptor, which is not an ion channel and sends completely different information to the cell. The hormone/neurotransmitter itself does not contain any specific information; rather, it is a simple signal, such as a phone ringing. One must answer the phone to receive the information. The information content of the hormone/neurotransmitter is really contained in the three-dimensional shape of the receptor molecule. The change in the shape of the receptor on binding the hormone/ neurotransmitter is the specific message to the cell.
Cells can make themselves more or less sensitive to the signal of the hormone/neurotransmitter. For example, most cells respond to a prolonged period of exposure to hormone/ neurotransmitter by reducing their sensitivity to that molecule. For membrane receptors, one way is to internalize the receptors by endocytosis, fuse the endosome with a lysosome, and digest the receptor. Typically, membrane receptor number is decreased by endocytosis in response to a sustained high concentration of ligand. This is called downregulation of the receptor. This process allows the cell to adapt to high ligand concentrations. Receptor-ligand interaction is a true chemical equilibrium, so the proportion of receptor-ligand complexes, which determines physiological response, depends on the concentration of both receptors and ligands. In the presence of a high ligand concentration, a decrease in receptor number returns the binding equilibrium to the normal proportion of bound/unbound receptors. This allows the cell to respond to increases and decreases in ligand, even at high concentrations of ligand. Another way of regulating the response to a hormone/ neurotransmitter is to alter the binding function of the receptor, e.g., by phosphorylating it, so that its affinity for the ligand is reduced (desensitization) or increased (hypersensitization). Nuclear receptors appear to be less subject to short-term regulation of responsiveness, but at least some nuclear receptors require constant turnover by proteolytic breakdown and new synthesis in order to function.
G-Protein–Coupled Receptors Are the Largest Family (a “Superfamily”) of Receptors and Help Regulate Almost All Physiological Processes
It would be difficult to exaggerate the importance and versatility of information processing that begins with a signal molecule binding to G-protein–coupled receptors (GPCRs). There are approximately 900 GPCRs in humans (Table 1-2). There are an even greater number in animals that depend more on olfaction, with about 1300 in rodents, because smell is mediated by different odorants binding to different GPCRs. An estimated 40% to 50% of all commercial drugs act in a GPCR pathway, exemplifying the importance of GPCRs to medicine. All GPCRs share a similar molecular shape; they are an integral membrane protein composed of a single polypeptide chain that passes in and out of the plasma membrane seven times, resembling a snake (Figure 1-14). As a result, two other names for GPCRs are seven-transmembrane receptors and serpentine receptors. However, the name GPCRs is more revealing about their mechanism because all also share the same “next step” in their Rube Goldberg signal sequence: they activate a molecular “on-off switch” known as a G protein, so called because it is a guanosinetriphosphatase (GTPase).
Table 1-2 Partial List of G-Protein–Coupled Receptors (GPCRs)
| Receptor/receptor family* | Example of function | Drug ligands |
|---|---|---|
| α-Adrenergic | Regulates vasculature | Phenylephrine, oxymetazoline |
| β-Adrenergic | Regulates heart and vasculature | Atenolol, propranolol |
| Angiotensin | Principal regulator of blood pressure | Losartan |
| Calcitonin | Regulates bone resorption | † |
| Cannabinoid | Unknown but found widely in brain | Marijuana and derivatives |
| Dopamine | Movement, cognition, and emotions | Chlorpromazine, bromocriptine |
| Frizzled | Regulates proliferation and differentiation, particularly in stem cells | † |
| Gastrin | Regulates acid secretion in stomach | Pentagastrin |
| Glucagon | Regulates “starvation” response | Exendin-4 |
| Histamine | Mediates inflammation and allergy | Diphenhydramine, chlorpheniramine |
| Muscarinic | Secretion of hormones and neurotransmitters | Atropine, carbachol |
| Olfactory | Mediates smell | † |
| Opiod | Mediates analgesia | Morphine, codeine, heroin |
| Opsins | Mediates light transduction in retina | † |
| Prostaglandin | Vasodilation | Sulprostone |
| Serotonin‡ | Regulates gut motility, behavioral arousal, feeding, circadian rhythms | Sumatriptan, ketanserin |
| Vasopressin | Regulates water balance of body | Terlipressin, desmopressin |
* In most cases, receptor is named for its ligand.
‡ One member of the serotonin receptor family is not G-protein–coupled.
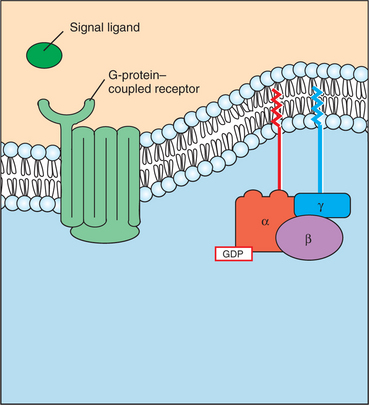
FIGURE 1-14 G-protein--coupled receptor (GPCR) and the heterotrimeric G protein. The hundreds of GPCRs share a similar protein shape, snaking in and out of the membrane seven times. Thus, GPCRs are also called “serpentine receptors” and “heptahelical receptors.” These receptors interact with a membrane-associated guanosinetriphosphatase (GTPase) molecule composed of three different polypeptide subunits (“heterotrimeric”). The heterotrimeric G protein is not an intrinsic membrane protein, but rather associates with the membrane through lipid tails inserted into the membrane.
GPCRs bind to a particular type of G protein (another of the many “families” of informational proteins), which is a membrane-associated trimeric protein composed of α, β, and γ subunits. Thus, this type of G protein is called the heterotrimeric G protein (“three different subunits”). The heterotrimeric G proteins bind directly to the cytoplasmic domain of a GPCR. Although not intrinsic membrane proteins, heterotrimeric G proteins are closely associated with the plasma membrane through lipid molecules that are posttranslationally added to the subunits and that insert into the lipid bilayer (see Figure 1-14).
As noted, G proteins are molecular “on-off switches” that are also GTPases activated by the binding of a signal molecule to its cognate GPCR. That is, in addition to binding to GPCRs, G proteins also bind guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The binding and hydrolysis of the GTP to GDP underlie the biochemical mechanism of the on-off switch. In Figure 1-15, A, the unstimulated GPCR does not bind to the heterotrimeric G protein, which is in its “off” state by virtue of the α subunit having GDP and the β and γ subunits bound to it. In Figure 1-15, B, a signal ligand binds to its GPCR, activating the receptor and the G protein. The activation of the G protein takes the form of dissociation of the β/γ complex from the α subunit, which allows the α subunit to exchange GDP for GTP. The principal “on” activity of the G protein is represented by the Gα subunit with GTP bound to it. GTP-bound Gα stimulates a variety of enzymes and ion channels that send the signal into the cytoplasm (Figure 1-15, C), as discussed in the next section. However, the Gβγ complex, once thought to be only an inhibitory factor for the Gα subunit, is now known to activate certain K+ channels itself and inhibit certain voltage-dependent Ca2+ channels. After stimulating the next element in the signal pathway, the activated GTP-bound Gα subunit returns to an inactive, “off” state as a result of its intrinsic GTPase activity (Figure 1-15, D). That is, the bound GTP is hydrolyzed to GDP, and the Gβγ complex rebinds to the Gα subunit, returning it (and the Gβγ complex) to its inactive state, awaiting the next ligand-receptor–binding event.
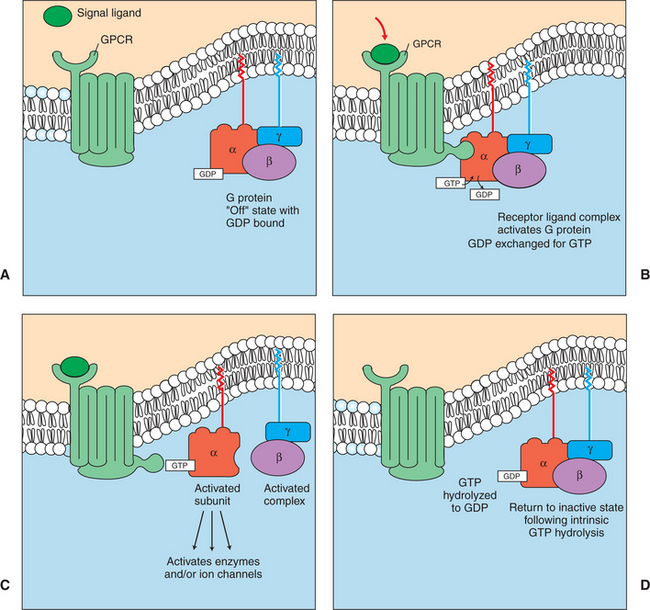
FIGURE 1-15 Duty cycle of heterotrimeric G-protein, a GTPase that acts as a molecular “on-off switch.” See text for further details. A, Unstimulated GPCR is not bound to the heterotrimeric G protein. B, Signal ligand binds to its GPCR, activating receptor and G protein. C, GTP-bound Gα subunit stimulates a variety of enzymes and ion channels that send the signal into the cytoplasm. D, After stimulating next element in signal pathway, the activated GTP-bound Gα subunit returns to inactive, “off” state because of its intrinsic GTPase activity.
As noted earlier, one of the aspects of the Rube Goldberg analogy is that the same conserved types of molecules are often used in many different pathways. Among the many protein “differences that make a difference” to transmit information, one of the most widely used is a GTPase that has on-off states based on whether GTP or GDP is bound to it. Thus the heterotrimeric G proteins that couple to GPCRs are only one type of GTPase protein acting as an on-off switch in signaling pathways. Most other members of the G-protein (GTPase) superfamily are simpler and resemble the Gα subunit alone. For example, one such class of these “small G proteins,” called Rabs, helps mediate the membrane fusion processes that underlie exocytosis and endocyotosis, discussed earlier. All G proteins share evolutionarily conserved GTP binding and enzymatic hydrolysis sites and a similar on-off mechanism: when GTP is bound, the protein is “on,” and when GDP is bound, the protein is “off.” Chapter 2 discusses a particular small G-protein, Ras, that plays a crucial role in regulating cell division and whose dysfunction plays a major role in cancer. Consequently, G proteins in general are discussed in Chapter 2, and this discussion focuses on signaling mechanisms linked to the heterotrimeric G protein specifically.
Most G-Protein–Linked Information Is Sent to the Cytoplasm by “Second Messengers”
As previously noted, the active (heterotrimeric) G protein stimulates an enzyme or ion channel that is associated with the plasma membrane. The ensuing change in ion channel or enzyme function can alter the membrane potential or cause certain molecules/ions to change their concentration in the cytoplasm. Those ions and molecules that are linked to receptor-ligand binding are called second messengers. A second messenger is an ion or molecule that carries the information within the cytoplasm of a cell in response to a signal on the outside surface of a cell (the first message), such as the binding of a hormone or neurotransmitter, or to an electrical event. Most G-protein–linked information is transduced into the cytoplasm in this manner. One of the major advances in our understanding of the molecular basis of physiological signaling is the realization that there are only a few second-messenger systems within animal cells. The most important include the following (Figure 1-16):
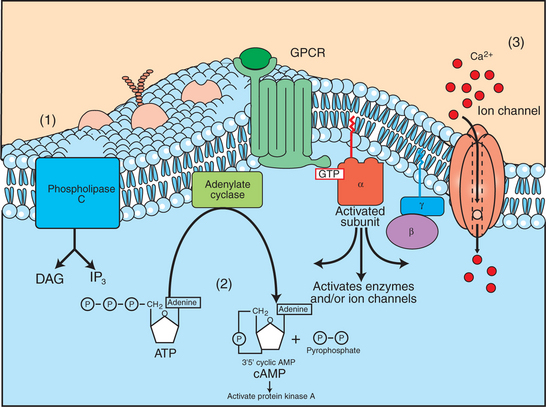
FIGURE 1-16 Activated α subunit of the G protein (Gα) can activate enzymes and ion channels, leading to second-messenger signaling within the cytoplasm. Three principal second messengers send the GPCR information to the cytoplasm. These arise from the activation of ion channels and enzymes stimulated by Gα. The second messengers are (1) increases in the concentration of inositol 1,4,5-trisphosphate (IP3) in the cytoplasm and increases in the concentration of diacylglycerol (DAG) in the plasma membrane, both as a result of the breakdown of a rare membrane phospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2) by phospholipase C, another Gα-stimulated enzyme; (2) changes in the concentration of cyclic AMP (cAMP), a special hydrolytic breakdown product of ATP created by the enzyme adenylyl cyclase, which can be activated or inhibited by α subunits; and (3) changes in Ca2+ concentration within the cytoplasm resulting from transport of Ca2+ through gated channels stimulated by Gα.
1. Two second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), are produced by G-protein activation of an enzyme phospholipase C (PLC) (see Figure 1-19 and later discussion).
2. Changes in the concentration of cyclic adenosine monophosphate (cAMP).
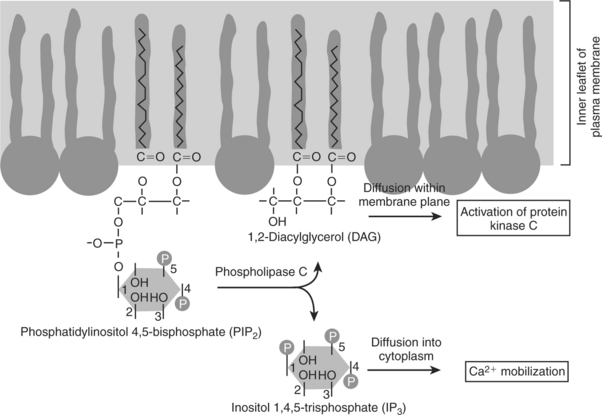
FIGURE 1-19 Hydrolysis of a membrane lipid to produce two second messengers. After appropriate receptor and G-protein activation, the rare membrane phospholipid shown to the left, phosphatidylinositol 4,5-bisphosphate (PIP2), is hydrolyzed into two separate second messengers by phospholipase C. The phosphate “head” of the PIP2 molecule is cleaved to produce the soluble messenger inositol 1,4,5-trisphosphate (IP3), which mobilizes intracellular Ca2+, as well as the lipidic messenger diacylglycerol (DAG), which remains in the membrane and activates protein kinase C.
Clearly, there are many more GPCRs than second messengers. This means that several receptor-mediated events are converted into the same intracellular signal. How does the cell sort out this information? Different cells respond differently to the same second-messenger ion/molecule as a result of the specialized function and makeup of that cell (i.e., the differentiated state it achieved during the development of the animal). For example, smooth muscle cells respond differently to activation of muscarinic acetylcholine receptors (see Table 1-2) than do nerve cells because the two cells have different proteins that are responsible for their specialized tasks. However, this is only part of the answer, and the specificity of response to the same second messenger and to activation of similar or identical receptors remains an important open question in physiology.
Ca2+ Transport Across Plasma and Intracellular Membranes Is an Important Second Messenger
The transport of Ca2+ ions through gated channels across the plasma membrane and across intracellular membranes (e.g., endoplasmic reticulum) is a major second messenger system for physiological information transfer. The available evidence suggests that the major role of Ca2+ within cells is as a physiological signal. In the extracellular compartment, the major physiological function of Ca2+ is as the principal mineral of bone. Ca2+ is an excellent ion for use as a second messenger because the cytoplasmic concentration of Ca2+ is extremely low, about 10−7 mol/L in a resting cell. Increases in intracellular Ca2+ concentration can be (1) detected easily because the background noise is so low and (2) achieved easily because the Ca2+ concentration [Ca2+] in the ECF and in some cellular compartments, such as the endoplasmic reticulum and mitochondria, is 104 higher than in the cytoplasm (see Table 1-1). Thus, there is an enormous driving force for Ca2+ diffusion into the cytoplasm under most conditions.
Although many GPCRs use Ca2+ as one part of their intracellular pathway, the interaction is more complex than usual, as discussed shortly. Thus, we focus here on a simpler and very important example of Ca2+ as a second messenger that has already been discussed: the role of Ca2+ in regulating the actomyosin ATPase of muscle.
Increased [Ca2+] in the cytoplasm alters cellular function by binding to any of several Ca2+-binding proteins that serve as control proteins. Troponin is one Ca2+-binding protein already mentioned. Reviewing the example of striated muscle contraction from the Ca2+ point of view, Ca2+ (second messenger) diffuses through gated channels in the endoplasmic reticulum (sarcoplasmic reticulum) of muscle in response to electrical events (first message) on the plasma membrane of the muscle cell. The diffusion of Ca2+ from the concentrated storehouse of the sarcoplasmic reticulum increases [Ca2+] in the cytoplasm of the muscle cell, where it binds to troponin. On binding Ca2+, troponin changes its interaction with tropomyosin, which now moves to allow myosin heads access to the actin of the thin filament. The actomyosin ATPase is activated, and muscle contraction ensues.
Calmodulin is a Ca2+-binding protein that plays an important control function in almost all animal cells. As with troponin, calmodulin binds Ca2+ when the cytoplasmic [Ca2+] increases. The Ca2+/calmodulin complex activates a large number of different cellular processes. In most such cases, but not all, the Ca2+/calmodulin complex binds to and activates an enzyme. One such enzyme, a protein kinase, is involved in the excitation-contraction coupling in smooth muscle (Figure 1-17), not discussed earlier with the striated muscle types. Protein kinases in general catalyze the hydrolysis of ATP and couple it to the simultaneous phosphorylation of other proteins, as follows:
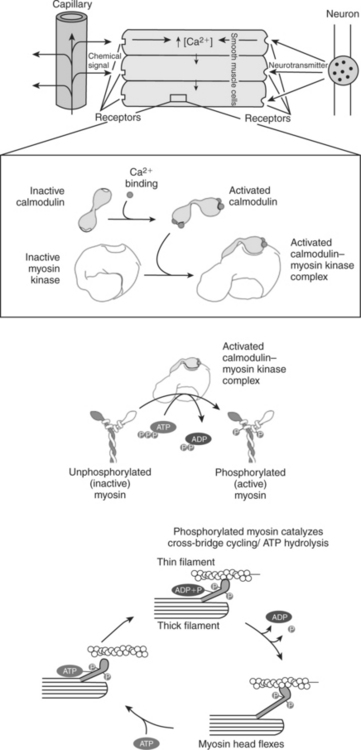
FIGURE 1-17 Role of Ca2+ and calmodulin in the regulation of smooth muscle contraction. Smooth muscle regulation is more complex than regulation of striated muscle, and the account here is a simplification. Smooth muscle can be stimulated to contract by a variety of stimuli, including neural signals and soluble chemical signals, as shown here. These external signals all stimulate increased intracellular [Ca2+], which leads to smooth muscle contraction. In the presence of increased intracellular [Ca2+], the Ca2+ ions bind to calmodulin, activating it by causing a conformational change. In smooth muscle cytoplasm, the activated Ca2+/calmodulin complex activates myosin kinase, which catalyzes the phosphorylation of myosin. Phosphorylated, activated myosin in turn catalyzes actin-dependent ATP hydrolysis (cross-bridge cycling). Thus, smooth muscle contraction is thick filament regulated, because changes in myosin activate cross-bridging, whereas striated muscle contraction is thin filament controlled, because changes in troponin and tropomyosin of the thin filament activate cross-bridging.
In the case of smooth muscle, the particular protein kinase is myosin kinase, which, as its name implies, specifically phosphorylates myosin. This phosphorylation increases the affinity of the myosin heads for actin filaments; thus allowing cross-bridging to actin. On formation of the cross-bridge, myosin strokes past the thin filament, producing filament sliding, contraction, and force production by smooth muscle. Cessation of contraction is achieved by cleavage of the phosphate from the myosin by another enzyme, myosin phosphatase.
Thus, initiating smooth muscle contraction involves a Rube Goldberg sequence in which environmental stimulation of a smooth muscle cell causes an increase in the intracellular [Ca2+], the second messenger. This in turn leads to a cascade of cause and effect. Increased intracellular [Ca2+] causes calmodulin to bind Ca2+. The Ca2+/calmodulin complex activates the myosin kinase. This enzyme phosphorylates the myosin head, allowing it to cross-bridge to actin. Cross-bridging leads to actomyosin activation, causing filament sliding that is observed as muscle contraction at the tissue level.
This discussion of Ca2+ as a second messenger emphasizes one of its major physiological functions: as the second messenger responsible for mediating contraction for all types of muscle (skeletal, cardiac, and striated), although the details of each pathway differ.
Cyclic AMP Is Produced by Activation of a Membrane-Bound Enzyme in Response to Hormone/Neurotransmitter Binding to Receptors
Changes in the activity of membrane-associated enzyme activities are an important mechanism of transmitting information across the cell membrane and are used by most GPCRs. Binding of a signaling molecule to receptors on the extracellular face of the plasma membrane changes the activity of an enzyme located on the cytoplasmic face. The enzyme catalyzes a breakdown reaction; one or more of the breakdown products released into the cytoplasm are second messengers. One important such second-messenger system, and the first to have been discovered, is the hydrolytic breakdown of ATP to 3′,5′-adenosine monophosphate, or cAMP, by the enzyme adenylyl cyclase (previously called adenyl cyclase and adenylate cyclase). Cyclic AMP is the second messenger, and adenylyl cyclase is turned on or off as a result of the binding of various hormones and neurotransmitters to cell surface receptors.
As summarized in Figure 1-18, three distinct membrane proteins interact to produce cAMP: (1) any of several receptors, including many GPCRs; (2) the heterotrimeric G protein; and (3) the catalytic protein that actually hydrolyzes ATP to cAMP. Their interaction provides an example of the ability of biomembranes to organize biochemical reactions in space. The likelihood of three proteins colliding and thus being able to interact is much greater in the two-dimensional “phospholipid sea” than in the three-dimensional cytoplasm.
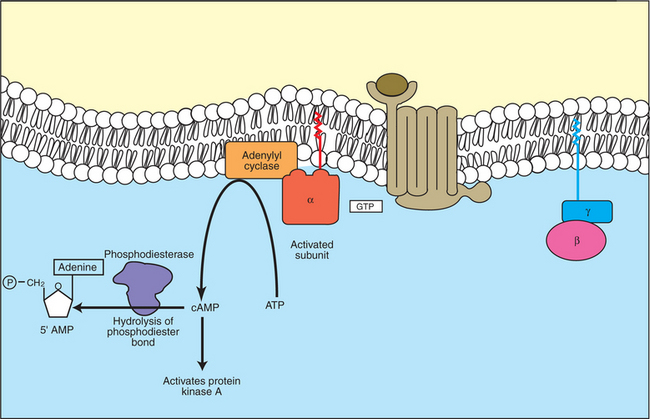
FIGURE 1-18 Activity of cyclic adenosine monophosphate (cAMP) as a second messenger. Cyclic AMP is generated through GPCR-linked activation of adenylyl cyclase, causing hydrolysis of ATP to cAMP. The cAMP thus generated binds to and activates a specific protein kinase, protein kinase A, which in turn can phosphorylate and change the activity of various cellular substrates. Once generated, cAMP is broken down by phosphodiesterase (PDE), which hydrolyzes cAMP to “normal” adenosine monophosphate (i.e., 5′ AMP).
A large number of different hormones/neurotransmitters that bind to different membrane receptors use cAMP to transmit information across the membrane. Among the GPCRs (see Table 1-2) and their hormones/neurotransmitters that use cAMP as their second messenger are β-adrenergic receptors that bind epinephrine or norepinephrine, increasing cAMP production and providing important regulation to almost all tissues. The starvation message carried by the binding of glucagon to its receptor (see Chapter 34) is carried to the cytoplasm by an increase in cAMP. Vasopressin (also called antidiuretic hormone, ADH) binding to its receptors in kidney cells uses cAMP to regulate urine production (see Chapter 33). A number of therapeutic drugs bind to these same receptors and mimic or prevent the physiological action of the hormone/ neurotransmitter that normally binds to the receptor.
After ligand binding, the ligand-receptor complex is able to bind to and activate the regulatory G protein (see Figure 1-15, B). The G protein in turn changes shape and binds to the catalytic subunit, altering its shape and regulating its ability to bind ATP, and hydrolyzes the catalytic subunit to cAMP (see Figure 1-18). There are two types of G proteins in the adenylyl cyclase system, which differ in their α subunit. The Gs (more specifically, Gαs, s for stimulatory) activates the catalytic subunit; this is the G protein shown in Figure 1-18. A different G protein, the α subunit of Gi, inhibits adenylyl cyclase when activated. Some diseases are the result of the binding of bacterial toxins to the G proteins. Cholera symptoms result in part from the binding of the toxin of the bacteria Vibrio cholerae to the Gs protein, and the irreversible activation of the Gs protein, which in turn irreversibly activates the catalytic subunit. Pertussis (whooping cough) toxin binds irreversibly to and activates Gi, thus inactivating the enzymatic activity.
As suggested by the inhibitory G protein (Gi), regulated decreases in cAMP concentrations are an important part of the cAMP second-messenger system. There are two mechanisms for such decreases: decreasing the rate of cAMP production and eliminating cAMP after formation. The former is achieved by Gi inhibiting the catalytic subunit. Certain inhibitory receptors specifically interact with Gi. Opium and drugs derived from it, such as codeine and morphine, are examples of signaling molecules that bind to inhibitory GPCR (opioid) receptors, activate Gi, and inhibit production of cAMP. Other examples are norepinephrine and epinephrine acting through α2-adrenergic receptors. Recall that these same neurotransmitters activate adenylyl cyclase when bound to β-adrenergic receptors. This is another example of the principle that the receptor/ligand complex contains the information, not the hormone/neurotransmitter itself.
The other control on cAMP levels is elimination of cAMP after formation. This is regulated by the enzyme cyclic nucleotide phosphodiesterase. This enzyme hydrolyzes the 3′ ester bond of the phosphate to the sugar to produce “plain” 5′ AMP (see Figure 1-18). As with myosin kinase discussed earlier, phosphodiesterase is a Ca2+/calmodulin-activated enzyme, so in many cells the activities of the Ca2+ and cAMP second-messenger systems antagonize one another.
The increase or decrease in cAMP concentrations most often affects cell function through cAMP’s interaction with a particular protein kinase. This protein kinase is called cAMP-dependent protein kinase, or protein kinase A. This protein kinase is distinct from the Ca2+/calmodulin-dependent protein kinase discussed earlier, although the basic outline of action is similar. Protein kinase A is activated by binding cAMP. The higher the concentration of cAMP in a cell, the greater is the number of active protein kinase A molecules. The activated kinase binds to proteins and ATP, hydrolyzing the ATP and phosphorylating the protein. As shown in previous examples, this phosphorylation alters the activity of the target protein, altering its particular characteristic function: catalysis, transport, coupling, and so forth.
Mammals respond to a stressful stimulus by increasing the force and rate of heart contraction, among other physiological effects. This increase in force demonstrates the role of cAMP as a second messenger and the role of Ca2+ in GPCR signaling, and it is another example of physiological Rube Goldberg devices based on allosteric changes in proteins. The stressful stimulus causes the adrenal medulla to release epinephrine to the blood, and sympathetic nerves release norepinephrine to the heart. Both catecholamines bind to β-adrenergic GPCRs on the cardiac muscle cells. The receptor-ligand interaction stimulates adenylyl cyclase by way of Gs, increasing intracellular [cAMP], thus increasing protein kinase A activity. Protein kinase A phosphorylates a number of substrates in the cardiac muscle cells, including voltage-dependent Ca2+ channels in the plasma membrane. In the phosphorylated state, these channels remain open somewhat longer in response to membrane potentials above threshold. Consequently, more Ca2+ enters the cell for a given electrical stimulation than at lower levels of cAMP. The increase in Ca2+ allows more troponin to bind Ca2+; more tropomyosin moves out of the way of myosin heads, causing more cross-bridging and more force production. (Rube Goldberg would have loved modern physiology!)
Another cyclic nucleotide, cyclic guanosine monophosphate (cyclic GMP), also serves as a second messenger but is not nearly as widely used as cyclic AMP. Cyclic GMP (cGMP) is the second messenger stimulated by opsins (see Table 1-2) in the rod cells of the retina underlying vision and also causes relaxation of some vascular smooth muscle, including that responsible for penile erection (i.e., blood flow into the corpus cavernosum). The role of cGMP in erections is mediated by its activation of cGMP protein kinases, similar to cAMP action via protein kinase A. Activation of cGMP-dependent protein kinase causes relaxation of certain smooth muscles, including those responsible for blood flow to the corpus cavernosum. This has an important clinical correlate: the drug Viagra (sildenafil) inhibits the breakdown of cGMP by a cyclic nucleotide phosphodiesterase, thus increasing blood flow to the penis, and aids erection, but only if neural signals (i.e., sexual stimulation) have stimulated cGMP production initially. This is a good example of how the multistep pathway of cell signaling provides multiple potential sites for appropriate therapeutic intervention; a drug that simply stimulated cGMP production would cause inappropriate erections, whereas inhibiting its breakdown aids timely erections. Although used mostly by men, sildenafil is also occasionally used for stallions to assist them in “covering” a mare.
In addition to activating protein kinases, cAMP and cGMP can also bind directly to and cause opening of a class of ligand-gated ion channels, cyclic nucleotide–gated ion channels. These ion channels are atypical in that their structure resembles voltage-gated K+ channels, but they open by directly binding a cyclic nucleotide. These channels play an important role in smell, for which cAMP is the relevant second messenger. In vision, as noted earlier, cGMP is the second messenger, and mutations in the cyclic nucleotide–gated ion channels of cones are responsible for most forms of complete color blindness (but which is rare).
The examples of physiological control by second messengers discussed thus far are short time-scale changes (seconds to hours), which historically have been the purview of “physiologists.” It has become increasingly clear, however, that most, if not all, major signals have longer-term (days and weeks) effects based on changes of gene transcription, which in turn mediates changes in growth, differentiation, and long-term behavior. For example, cyclic AMP is now known to be an important regulator of gene transcription controlling learning, production of gametes, and cell division. The effect of cAMP on gene expression is the result of protein kinase A phosphorylating a specific transcription factor associated with cAMP signaling (“cyclic AMP response element binding protein,” or CREB). While space does not allow further discussion of the transcriptional roles of “classic” physiological signal pathways, when dealing with signal pathways it is worthwhile to keep in mind the disclaimer in the first paragraph: only a highly simplified account of cell function is presented here!
The Receptor-Mediated Hydrolysis of a Rare Phospholipid of the Plasma Membrane Produces Two Different Second Messengers with Different Actions
Another second-messenger system differs from both Ca2+ and cAMP in that two distinct second-messenger molecules are produced as a result of an enzymatic activation by a single receptor/ligand complex. Phosphatidylinositol (PI) is a membrane phospholipid that can accept additional phosphate groups by reaction with the —OH groups on the inositol (Figure 1-19). Phosphatidylinositol 4,5-bisphosphate (PIP2) is the membrane phospholipid that is broken down to produce two important second messengers. PIP2 is hydrolyzed to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) by a receptor-mediated enzyme called phospholipase C (PLC) or phosphoinositidase. Although many distinct processes are controlled through the PIP2 path, it plays a particularly important role in control of growth and of receptor-mediated secretion. The effect of acetylcholine acting through muscarinic receptors (not the nicotinic receptor/ion channel of the nerve-muscle synapse) is often transmitted and transduced through activation of the PIP2 pathway.
The events involved in the receptor-mediated production of IP3 and DAG from PIP2 are similar to those in the production of cAMP. The membrane system appears to consist of three distinct intrinsic membrane proteins: (1) any of several different GPCRs, including the muscarinic acetylcholine receptor and the receptors for some growth factors; (2) a heterotrimeric G protein, similar but not identical to Gs of the cAMP pathway; and (3) the hydrolytic enzyme PLC. A hormone/neurotransmitter or growth factor binds to the receptor, forming a receptor/ligand complex. This complex activates the G protein, which in turn activates the hydrolytic enzyme. At present, only a stimulatory G activity on PLC is known; there is no evidence for an inhibitory G activity in this system.
The activation of the hydrolytic enzyme increases the concentration of IP3, which is water soluble and thus diffuses through the cytoplasm. IP3 binds to and opens ligandgated Ca2+ channels in the endoplasmic reticulum. This releases Ca2+ from that high [Ca2+] compartment into the cytoplasm. Ca2+ thus becomes the “third messenger” in this system (although this term is not in widespread use) and is another example of a role of Ca2+ in GPCR signaling. The ensuing increase in cytoplasmic [Ca2+] affects cellular function by the same mechanisms outlined earlier for Ca2+ as a second messenger (e.g., binding to calmodulin), with the Ca2+/calmodulin complex in turn activating various enzyme activities. In receptor-mediated secretion, for example, the binding of acetylcholine to muscarinic receptors in the pancreas (the organ that secretes digestive enzymes) causes an increase in PIP2 breakdown and an increase in cytoplasmic IP3. The IP3 opens ligand-gated Ca2+ channels in the endoplasmic reticulum, and intracellular [Ca2+] increases. The process then becomes similar to smooth muscle contraction. Calmodulin binds Ca2+, and the complex activates a protein kinase. However, rather than activating myosin, as for smooth muscle, activation of this protein kinase causes exocytosis of secretory vesicles (membrane bubbles full of secretory product) with the plasma membrane, releasing the enzymes into an extracellular space that is contiguous with the gut.
DAG is also produced on activation of PLC, but it is not at all soluble. DAG diffuses in the plasma membrane, binding to and activating membrane-associated protein kinase, protein kinase C (PKC). PKC is not an intrinsic membrane protein and can bind reversibly to the cytoplasmic face of the plasma membrane. PKC phosphorylates other proteins and changes their activity. Because of the membrane-bound character of the enzyme, most evidence indicates that PKC phosphorylates membrane proteins such as receptors and ion channels, regulating their function. In the case of the secretory response to some hormone/neurotransmitter stimulus, PKC generally acts separately but additively with IP3 to produce the response. As with cAMP and protein kinase A, however, much interest focuses on longer-term effects of DAG activation of PKC, particularly its role in growth control and cancer. A class of chemicals long known to promote the onset of cancer, phorbol esters, is a potent substitute for DAG at activating PKC. It is now known that PKC indirectly activates an important transcription factor involved in cell proliferation, nuclear factor kappa B (NF-κB). Thus, as a second messenger and as with cAMP, DAG has both short-term effects and longer-term transcriptional effects.
Steroid Hormones and Other Lipid Signals Interact with Nuclear Receptors, Which Are Transcription Factors Within the Cell
Nuclear receptors are another large class of protein molecules specialized for information transmission and transduction. Nuclear receptors are sufficiently numerous and diverse that they compose a “superfamily” of evolutionarily conserved and related receptors, as with the GPCRs. All nuclear receptors are transcription factors that respond to the binding of their cognate lipid signal by regulating which genes are expressed within particular cells under particular conditions. Accordingly, one of the conserved features of nuclear receptors is their DNA-binding domain, which can bind directly to specific sequences of DNA (promoter regions) that control the expression of the neighboring gene(s) (Figure 1-20). As with all other proteins, the DNA-binding function of nuclear receptors is based on their shape. The DNA-binding domain, for example, is a part of the protein shaped into “fingers” by a zinc ion. These zinc fingers, also found in many other transcription factors, fit into the grooves of the double helix of DNA at the appropriate base-pair sequence.
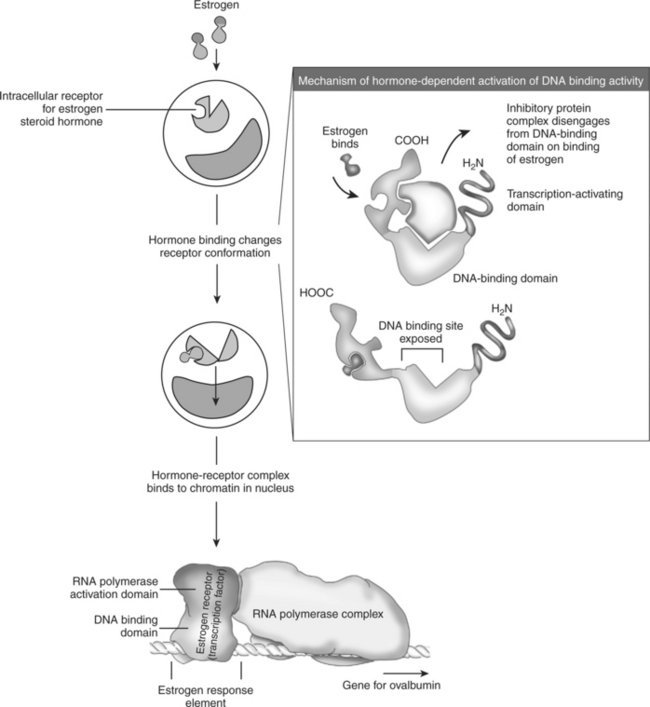
FIGURE 1-20 Steroid hormone action as illustrated by control of ovalbumin expression by estrogen in hens. The steroid hormone estrogen penetrates the lipid bilayer passively because of the oil solubility of the steroid. Inside the cell, the estrogen binds to a cytoplasmic receptor, the estrogen receptor. The binding of estrogen to its receptor causes the receptor protein to change conformation, which in turn changes the DNA-binding activity of the receptor. The hormone/receptor complex enters the nucleus and binds to regulatory sequences of DNA, the estrogen response element. This binding, in turn, activates ribonucleic acid (RNA) polymerase. This initiates transcription of the ovalbumin gene, an estrogen-responsive gene, to produce messenger RNA (mRNA), which is ultimately translated into the ovalbumin protein for secretion.
Recall that steroid hormones are soluble in oily solvents and are able to diffuse through the lipid bilayer without the mediation of transport proteins. Thyroid hormones are also lipophilic and diffuse through the lipid bilayer. Additionally, several lipid-soluble nutrients are also signaling molecules, including vitamins A and D. Vitamin A is required for vision because it is the covalently bound cofactor for the opsin GPCRs, but it also plays a role in embryonic development. Vitamin D controls Ca2+ metabolism. Similarly, saturated and unsaturated fats in the diet are also known to provide signals that control their own breakdown and metabolism and to regulate the differentiation of fat cells (adipose tissue). Consequently, the receptors for these lipid signals are soluble proteins within the target cell. The cellular location of the nuclear receptors varies. Some receptors can be found in the cytoplasm before ligand binding, whereas others are largely restricted to the nucleus (after their initial synthesis in the cytoplasm), but all are functional as transcription factors in the nucleus after activation. The lipid-soluble hormone/nutrient diffuses from the blood into the cell and binds to its receptor, and the hormone/receptor complex is, as in previous examples, the physiologically active entity that ultimately triggers a cellular response. As noted earlier, because the nuclear receptor complex is itself a transcription factor, steroid and thyroid hormones do not require a second messenger; the hormone/receptor complex is itself active within the cell, altering gene expression.
A well-studied example of nuclear receptor action as a regulated transcription factor, with some relevance to veterinary medicine, is the action of estrogen on the reproductive tracts of female chickens (see Figure 1-20). Estrogen is the principal female sex hormone of birds and mammals, and, of course, hens lay eggs whose embryo and yolk is surrounded by an “eggwhite.” The principal protein of eggwhite is ovalbumin, which is secreted by the epithelial cells of the avian oviduct as the ovum slides by. Thus, one of estrogen’s targets in female chickens is oviduct epithelial cells. Estrogen enters the cytoplasm of these cells and binds to its receptor, the estrogen receptor. The hormone/receptor complex, but not the ligand-free receptor, is able to mediate estrogen-specific, essentially female-specific, gene transcription. The estrogen receptor complex binds to a sequence of DNA, called an estrogen response element, that controls the transcription of a neighboring gene, for ovalbumin in this case. In other cells of the female, binding of the estrogen receptor to the estrogen response elements of other genes would cause these other female-specific genes to be transcribed and ultimately expressed as a protein (e.g., proteins in yolk of egg). Different steroids bind to different receptors (e.g., male sex hormone testosterone binds to testosterone receptor), which bind to different response elements, leading to different genes expressed (e.g., male-specific gene expression).
Differential gene expression and its regulation were initially pursued primarily by molecular biologists. It rapidly gained importance in physiology, however, and will do so soon in veterinary medicine. Humankind will have fewer scruples about controlling gene expression in domestic animals than for their own species. Indeed, understanding control of gene expression may prove more important to veterinary students in the near term than for students of human medicine.
CLINICAL CORRELATIONS
Peripheral Edema
History.
You examine a 2-year-old cow that has been grazing on a poor-quality pasture. The owner states that the cow seems to have a poor appetite, walks slowly, and stands apart from the rest of the herd. The cow has developed swelling beneath the skin of her brisket and ventral thorax.
Clinical Examination.
On clinical examination, you find a listless cow standing in a pasture littered with various metal objects. Examination of the cardiovascular system reveals distended jugular veins and abnormal heart sounds characterized by irregular sloshing sounds through-out the cardiac cycle that drastically muffle the first and second heart sounds. Subcutaneous edema (swelling) can be seen throughout the chest and abdomen, but most prominently in the dependent ventral areas of the thorax. Pushing on these swollen areas leaves a dent (pitting edema).
Comment.
This is a characteristic history of a cow with hardware disease. The cow, grazing on a pasture littered with metal debris, swallows nails, wire, and so forth. Because these objects are heavier than the feed, they drop into the reticulum, a stomach chamber located just caudal to the diaphragm and heart. With the contractions of the reticulum, a metal object migrates through the reticular wall, diaphragm, and pericardium, leading to an inflammatory response in the pericardium (pericarditis). The resulting process is caused by both inflammation and possible secondary bacterial infections from a contaminated metal object traversing regions of the gastrointestinal tract that contain numerous microorganisms, before the object penetrates the pericardium. An inflammatory exudate fills the pericardial sac; it muffles the heart sounds, and a sloshing sound may be heard on auscultation. As this exudative fluid fills the pericardial sac, it limits the pumping efficiency of the heart by limiting its filling during diastole and by obstructing venous return to the heart (see Chapter 21). The result is left-sided heart failure because the heart cannot circulate (pump) the blood throughout the body. This causes the blood to accumulate initially, leading to an increased hydrostatic pressure in the veins and capillaries. As the capillary hydrostatic pressure rises, capillary filtration is favored over reabsorption, and water leaves the capillary and accumulates in the interstitial space. This accumulated interstitial fluid, primarily as the result of increased capillary filtration, is seen clinically as edema. The other common cause of edema is decreased capillary colloidal osmotic pressure from low serum protein. However, this does not usually play a part in hardware disease.
Treatment.
Treatment includes surgical removal of the foreign object or objects, antiinflammatory agents, and antibiotic treatment for the pericarditis. Even though considerable inflammation is present, a secondary bacterial infection often contributes to the response. In such an advanced case, however, treatment often is not completely successful.
Alberts B, Johnson A, Lewis J, et al. Molecular biology of the cell, ed 4. New York: Garland Publishing, 2002.
Lodish H, Berk A, Matsudaira P, et al. Molecular cell biology, ed 5. San Francisco: Freeman, 2004.
McCudden C, Hains MD, Kimple RJ, et al. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551.
Novac N, Heinzel T. Nuclear receptors: overview and classification. Curr Drug Targets Inflamm Allergy. 2004;3:335.
PRACTICE QUESTIONS
1. Increasing the extracellular K+ concentration will:
2. G proteins are similar to receptors in that both:
3. Which of the following statements concerning intracellular Ca2+ is false?
4. If, in a particular capillary bed, the plasma oncotic pressure were to increase and hydrostatic pressure remained constant:
5. Substance X is found to be at a much higher concentration on the outside of a cell than in the cytoplasm, but no transport of X from the extracellular fluid to the cytoplasm occurs. Which of the following statements is inconsistent with this situation?