Chapter 6 The Physiology of Muscle
1. All movement is the result of contraction of skeletal muscle across a movable joint.
2. There are several levels of organization in any skeletal muscle.
3. Action potentials on the sarcolemma spread to the interior of the cell along the transverse tubules.
4. The action potential on the sarcolemma is indirectly coupled to the contraction mechanism through the release of Ca2+ from the sarcoplasmic reticulum.
5. The sliding of actin along the myosin molecule results in physical shortening of the sarcomere.
6. Most skeletal muscle fibers can be classified as either fast-contracting or slow-contracting fibers.
7. Muscles change their strength of contraction by varying the number of active motor units or the rate of motor unit activation.
8. The electromyogram is the clinical measurement of the electrical behavior within a skeletal muscle.
9. The structure of cardiac and smooth muscle differs from that of skeletal muscle.
10. The role of Ca2+ ions in excitation-contraction coupling in cardiac and smooth muscle differs from that in skeletal muscle.
There are three types of muscle in the body: skeletal, cardiac, and smooth muscle. Skeletal muscle makes up about 40% of the body, and smooth muscle and cardiac muscle make up almost 10% more. Because most veterinary patients with disease of the neuromuscular system exhibit abnormalities of movement, it is important to understand how skeletal muscle functions and how it is controlled by the nervous system. Abnormalities of cardiac muscle and smooth muscle feature prominently in many other clinical disorders (e.g., dilated cardiomyopathy, hypertension, detrusor hypertrophy), and such muscle is often the target of pharmacological clinical intervention (e.g., sympathomimetic drugs, adrenergic receptor antagonists).
This chapter explains the physiology of skeletal muscle and includes brief comparisons with cardiac and smooth muscle. Cardiac muscle is discussed more extensively in Section III chapters, and the role of smooth muscle in other body systems is mentioned throughout this book.
All Movement Is the Result of Contraction of Skeletal Muscle Across a Movable Joint
All body movement is the result of contraction of skeletal muscle. Skeletal muscle consists of a central, fleshy, contractile muscle “belly” and two tendons, one on each end of the muscle. The muscle and its tendons are arranged in the body so that they originate on one bone and insert on a different bone while spanning a joint. As the muscle contracts, shortening the distance between the origin and insertion tendons, the bones move in relation to each other, bending at the joint (Figure 6-1). When activated by a motor nerve, a skeletal muscle can only shorten. Most joints have one or more muscles on both sides, either to decrease its angle (flexion) or to increase its angle (extension).
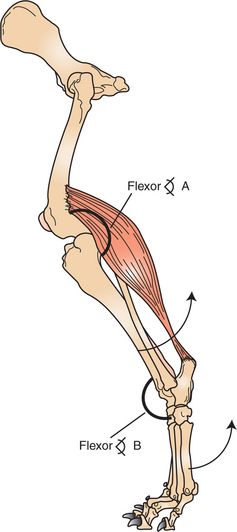
FIGURE 6-1 All noticeable movement is the result of contraction (shortening) of a skeletal muscle attached across a movable joint. Contraction of the muscle will decrease the flexor angle at joint A (the stifle joint) and increase the flexor angle at joint B (the tarsal joint). This will produce the respective movements about the joints indicated by the arrows.
All movement performed by an animal is the result of contraction of skeletal muscle across a movable joint. It is therefore important to understand the anatomy and physiology of skeletal muscle before discussing how the nervous system choreographs the contraction of groups of muscle cells to perform an impressive array of body movements.
There Are Several Levels of Organization in Any Skeletal Muscle
Figure 6-2 illustrates the levels of organization in a typical skeletal muscle. Each muscle belly seen during dissection is made up of differing numbers of muscle cells (usually called muscle fibers) that span the several inches between the origin and insertion tendons. The fibers range between 5 and 100 μmm in diameter and contain multiple nuclei, multiple mitochondria, and other intracellular organelles. The outer limiting membrane of the fiber is called the sarcolemma. It consists of a true cell membrane, called the plasma membrane, and an outer polysaccharide layer that attaches to the tendons at the cells’ extremities. Each muscle fiber is innervated by only one motor neuron, with the neuromuscular junction region located near the middle of the fiber, relative to the ends.
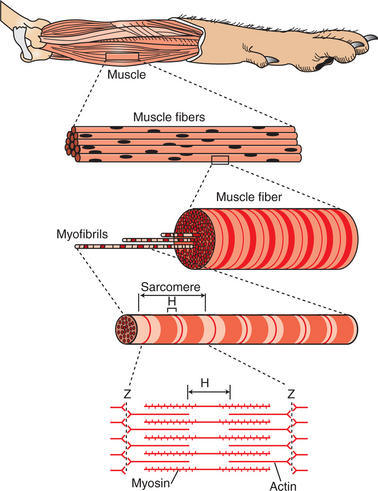
FIGURE 6-2 A typical skeletal muscle has several levels of organization. H and Z are letters assigned to stripes seen during microscopic examination of skeletal muscle.
Each muscle fiber is made up of successively smaller subunits (see Figure 6-2). Each fiber contains several hundred to several thousand myofibrils arranged in parallel along its length, like a handful of spaghetti. Each myofibril is made up of a linear series of repeating sarcomeres, the basic contractile units of the muscle fiber, which can number in the tens of thousands.
The sarcomere has a disk at each end called the Z disk. The sarcomere contains four types of large protein molecules responsible for muscular contraction, three of which are polymerized. Numerous thin protein filaments, called actin, are attached to the Z disks and extend toward the center of the sarcomere, similar to parallel fingers pointing at each other. Each actin filament consists of two intertwined, helical strands of actin protein and two such strands of tropomyosin protein, all wound together as a larger helical complex (see Chapter 1 and Figure 1-5). Also located intermittently along the tropomyosin-actin strand are complex globular protein molecules called troponin that can bind tropomyosin and actin and that have an affinity for calcium (Ca2+) ions. Suspended between and parallel to the actin thin filaments are thicker filaments of myosin protein polymers. A myosin molecule contains a tail of intertwined helices and two globular heads that can bind both adenosine triphosphate (ATP) and actin (see Figure Figure 1-3 and Figure 1-4). Approximately 500 myosin heads of a thick myosin filament form cross-bridges that interact with actin to shorten the sarcomere as the myosin heads flex and relax.
Beneath the plasma membrane of the muscle cell lies the sarcoplasmic reticulum, an intracellular storage organelle that forms a reticulated network around the myofibrils (Figure 6-3). This extensive storage sac sequesters Ca2+ ions in relaxed muscle and is analogous to the smooth endoplasmic reticulum in other cells.
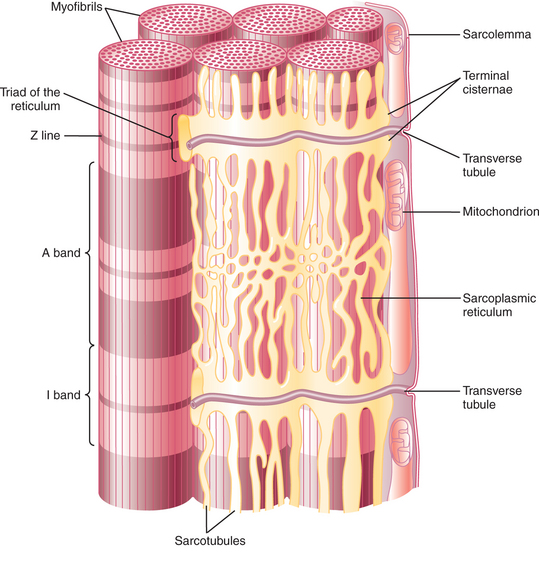
FIGURE 6-3 Diagram of skeletal muscle showing the juxtaposition of myofibrils, transverse (T) tubules, and sarcoplasmic reticula.
(From Guyton AC, Hall JE: Textbook of medical physiology, ed 11, Philadelphia, 2006, Saunders.)
Located perpendicular to the long axis of the muscle fiber are tubes of plasma membrane formed by periodic invaginations of the sarcolemma. These transverse tubules, or T tubules, traverse the diameter of the muscle fiber, similar to a flexible drinking straw passing perpendicularly through the handful of spaghetti (myofibrils) noted earlier. The T tubules snake around the myofibrils, forming junctions with the network of sarcoplasmic reticulum that surrounds the myofibrils (Figure 6-4). These tubules are filled with extracellular fluid and are important because they allow the electrically excitable plasma membrane of the muscle fiber to carry the depolarization of the action potential to the interior of the fiber.
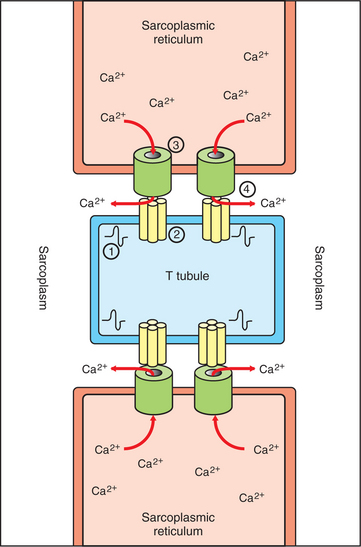
FIGURE 6-4 Relationship between the T tubules (TT) and sarcoplasmic reticulum (SR) during excitation-contraction coupling. 1, Propagation of action potential produces depolarization of the TT membrane. 2, Depolarization induces opening of voltage-gated Ca2+ channel aggregates in the TT membrane. 3, Opening of Ca2+ release channels on the SR membrane results from mechanical coupling with opening of voltage-gated Ca2+ channels on the TT. 4, Ca2+ is released from the SR into the sarcoplasm, where it can bathe the sarcomeres (not shown) to induce contraction.
(Modified from Boron WF, Boulpaep EL: Medical physiology: a cellular and molecular approach, updated edition, Philadelphia, 2005, Saunders.)
Action Potentials on the Sarcolemma Spread to the Interior of the Cell Along the Transverse Tubules
Skeletal muscle cells have a resting membrane potential, as do neurons, and the muscle cell membrane can be depolarized by synaptic transmission at the neuromuscular junction (see Chapter 5). At this junction, the acetylcholine released by the motor neuron activates nicotinic acetylcholine receptors on the sarcolemma of the muscle cell. The resulting depolarization is sufficient to open enough voltage-gated sodium (Na+) ion channels, also found at the junctional sarcolemma (see Figure 5-1), to trigger a muscle fiber action potential. Thus, it is at the sarcolemma of the neuromuscular junction that muscle fiber action potentials are generated.
Once an action potential is generated near the midpoint of the muscle fiber, it spreads in both directions along the length of the fiber by mechanisms similar to action potential spread in unmyelinated neuronal axons. In contrast to those on axons, however, action potentials on the sarcolemma are also transmitted to the interior of the muscle fiber along the T tubules. This allows the action potential to reach the location of the sarcoplasmic reticulum even in the innermost regions of the muscle fiber. The consequences of the action potential’s arrival at the location of the sarcoplasmic reticulum are critical for the coupling of excitation (the action potential) with contraction (shortening) of the sarcomeres of the myofibrils.
The Action Potential on the Sarcolemma Is Indirectly Coupled to the Contraction Mechanism Through the Release of Ca2+ from the Sarcoplasmic Reticulum
Whereas in the neuron a rise in cytoplasmic Ca2+ at the terminal is critical for initiating the process of transmitter release, a rise in Ca2+ in the muscle cell sarcoplasm (cytoplasm of a muscle cell) is critical for initiating contraction. At rest, Ca2+ ions are pumped out of the sarcoplasm and stored in the sarcoplasmic reticulum using an energy-dependent pump. This leaves too low a concentration of Ca2+ in the sarcoplasm to trigger contraction. However, as an action potential spreads along the muscle fiber surface and into the fiber’s core along the T tubules, the depolarization arrives at the junction between the tubules and the sarcoplasmic reticulum (Figure 6-4). The arrival of the action potential at this junction leads to the release of stored Ca2+ ions from the sarcoplasmic reticulum. These Ca2+ ions diffuse down their concentration gradient into the sarcoplasm, bathe the sarcomere, then trigger contraction. As the action potential passes, Ca2+ is pumped again into the sarcoplasmic reticulum, and relaxation results. This cycle of events is known as excitation-contraction coupling.
The link between the action potential on the transverse tubule and Ca2+ release from the sarcoplasmic reticulum is mediated by voltage-gated Ca2+ channels on the T tubule and Ca2+-induced Ca2+ release channels on the sarcoplasmic reticulum (see Figure 6-4). In skeletal muscle a mechanical coupling of these two types of channels is thought to exist; action potential opening of the voltage-gated Ca2+ channels of the T tubule produces a direct configurational change in the Ca2+-induced Ca2+ release channels on the sarcoplasmic reticulum, allowing the stored Ca2+ ions to escape from the sarcoplasmic reticulum into the sarcoplasm. The Ca2+ sensitivity of the Ca2+-induced Ca2+ release channels plays a more important role in excitation-contraction coupling in cardiac muscle than in skeletal muscle (see later discussion).
The Sliding of Actin Along the Myosin Molecule Results in Physical Shortening of the Sarcomere
Figure 6-5 illustrates the sarcomere in the relaxed state and in its shorter, contracted state. The sarcomere is changed from its relaxed state to the shorter, contracted state when Ca2+ ions become available to the sarcomere. In the presence of Ca2+ ions and a sufficient source of ATP, the actin thin filaments are pulled in parallel along the myosin thick filaments by the repetitive movement of the myosin molecule heads, thus shortening the sarcomere. Because each myofibril is made up of a linear series of repeating and connected sarcomeres, the net result is the physical shortening of the distance between the two ends of the muscle. A more detailed molecular explanation of this sliding filament mechanism of sarcomere shortening is provided in Chapter 1 as an example of the binding specificity and allosteric interactions of proteins. However, the events can be briefly summarized as follows.
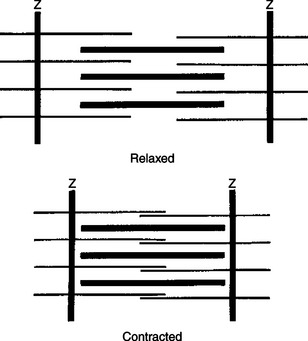
FIGURE 6-5 Sliding of actin along the myosin molecule results in the physical shortening (contraction) of the sarcomere.
At several points along the actin thin filament, there are sites that can bind with the head of the myosin molecule (see Figure 1-4 and Figure 1-5). In the absence of Ca2+ ions, these sites are either inhibited or covered by the tropomyosin molecules that are normally interwoven within the actin helix. When Ca2+ is present and binds with troponin, a regulatory molecule attached to tropomyosin, the troponin molecule undergoes a configurational change. It is thought that this then causes the tropomyosin molecule to move away from and uncover the myosin-binding site on the actin thin filament, permitting actin-myosin binding. Through a cycle that includes binding with and hydrolysis of ATP, the myosin heads alternately relax and flex while respectively detaching and attaching to the exposed binding sites on the actin thin filament. This results in the actin thin filaments sliding in parallel along the myosin thick filaments to shorten the sarcomere. In the absence of Ca2+, the myosin-binding sites on actin once again become blocked, and sarcomere relaxation results.
Most Skeletal Muscle Fibers Can Be Classified as Either Fast-Contracting or Slow-Contracting Fibers
Skeletal muscle fibers with short contraction times are sometimes called fast-twitch fibers. They tend to be larger, have extensive sarcoplasmic reticulum for rapid release of Ca2+ ions, and have less extensive blood and mitochondrial supplies because aerobic metabolism is less important. Fast-twitch fibers are fairly rapidly fatigued but are well adapted for jumping, sprinting, and other brief, powerful movements.
In contrast, slow-twitch fibers are smaller muscle fibers, have a rich blood and mitochondrial supply, and have a large amount of myoglobin, an iron-containing and oxygen-storing protein similar to hemoglobin. These fibers rely more heavily on oxidative metabolism, are less amenable to fatigue, and are better adapted for the continual contraction of antigravity extensor muscles.
Because slow-twitch fibers have more myoglobin, they are sometimes called red muscle; fast-twitch fibers are sometimes called white muscle. A third type of fiber, a subclass of fast-twitch fibers, has properties between the two types. Usually, a muscle belly is made up of a blend of these three types, the proportions varying in accordance with the muscle’s use. This blend can be changed somewhat with exercise, such as in an athlete training for a different type of sports event.
Muscles Change Their Strength of Contraction by Varying the Number of Active Motor Units or the Rate of Motor Unit Activation
Even though each muscle fiber is innervated by only one neuron, each motor neuron’s axon branches as it reaches the muscle and innervates several muscle fibers. A motor unit is defined as one alpha (α) motor neuron and all the extrafusal (force-generating, striated) muscle fibers that it innervates (Figure 6-6, A). All the muscle fibers of each motor unit are of the same functional type (e.g., fast or slow twitch), and an action potential on the motor neuron causes all the muscle fibers to contract simultaneously. In motor units a relationship exists among the functional type of muscle fiber innervated, the number of muscle fibers innervated, and motor neuron size. Small motor units tend to be made up of a motor neuron with a small cell body and a narrow, slower-conducting axon that innervates a small number of slow-twitch fibers. Large motor units have a motor neuron with a large cell body and a faster-conducting, wide axon innervating a large number of fast-twitch fibers. Activation of a small motor unit produces a smaller, slower, less fatiguable increment of contractile force in the muscle compared with a larger motor unit. The neuronal cell bodies of all the motor units from a single muscle form a cluster within the central nervous system (CNS) called the motor neuron pool of that muscle (Figure 6-6, B). Within the motor neuron pool for a given muscle, there is a range of motor unit sizes. Muscles with a larger proportion of smaller motor units tend to be amenable to finer control of contractile force.
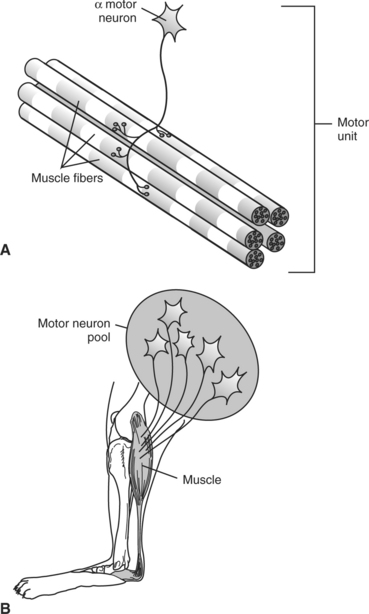
FIGURE 6-6 Innervation of skeletal muscle by α motor neurons of central nervous system (CNS). A, motor unit is an α motor neuron and all the skeletal muscle fibers it innervates. B, neuronal cell bodies of all the motor units from a single muscle form a cluster within the CNS called the motor neuron pool of that muscle.
(Redrawn from Bear MF, Connors BW, Paradiso MA: Neuroscience: exploring the brain, ed 3, Philadelphia, 2007, Lippincott Williams & Wilkins.)
Although an action potential on a motor neuron produces a simultaneous, brief twitch in all the muscle fibers of the motor unit, the pattern of excitation of the units originating from within the CNS produces the smooth, graded contraction of which most muscles are capable. The CNS can instruct a muscle to contract with greater force primarily by increasing the number of motor units that contract at any one time; this is called recruitment or spatial summation. The force of contraction can also be increased by increasing the frequency of activation of a motor unit, in which a subsequent twitch begins before relaxation of the previous twitch; this is called temporal summation. The recruitment of motor units to increase contractile force occurs in an orderly manner, according to motor unit size, with the smaller units activated first. This results in force being increased gradually in small, more precise amounts when the muscle force required is low. As the force required increases, faster and larger increases in contractile force are progressively added by orderly activation of the larger motor units. This produces an overall smoothness of contraction, keeping the movement as precise as possible until larger, grosser increments are needed, usually when significant tension has already been generated in the muscle.
In some skeletal muscles the CNS can command some percentage of motor units to be active for extensive periods (various motor units take turns), thus continually shortening the distance between the origin and insertion tendon. When contraction of a whole muscle belly occurs without relaxation, the muscle is said to be in tetany. Tetanization of cardiac muscle would be fatal, because heart muscle must relax to allow cardiac filling before it contracts to pump out the blood. Chapter 19 discusses how cardiac muscle prevents tetany.
The Electromyogram Is the Clinical Measurement of the Electrical Behavior Within a Skeletal Muscle
As an action potential spreads along a muscle fiber, a small portion of the electrical current generated spreads away from the fiber, even to the overlying skin. Electrodes placed on the skin or inserted into the muscle belly can record a summated electrical potential when the muscle contracts. Such a measurement, when displayed on an oscilloscope or computer screen, is called an electromyogram (EMG) and is for skeletal muscle what the electrocardiogram (ECG) is for cardiac muscle. The EMG, often used in conjunction with nerve conduction analysis, helps to determine whether weakness or paralysis is caused by disease in the skeletal muscle, neuromuscular junction, motor neuron, or CNS.
The Structure of Cardiac and Smooth Muscle Differs from That of Skeletal Muscle
As with skeletal muscle, cardiac muscle is striated and contains sarcoplasmic reticulum and myofibrils; the fundamental contractile component is formed by actin and myosin subunits (see Figure 19-1). Cardiac muscle also contains transverse tubules, but cardiac muscle differs from skeletal muscle in some important ways. The long skeletal muscle fibers are electrically isolated from each other, whereas the shorter cardiac muscle cells are electrically coupled to each other through end-to-end intercalated disks that contain gap junctions. Because gap junctions provide continuity between the cytoplasm of adjacent cells, action potentials can spread from one cardiac muscle cell to another, across these intercalated disks, without the need for chemical neurotransmission to each cell. The cardiac muscle cells can also possess branchlike extensions that form similar connections with some of their parallel neighbors. In fact, as explained in Chapter 19, action potentials arise spontaneously in specialized cardiac muscle cells and then spread throughout a large population of cardiac muscle cells as if they were a functional syncytium. This can result in coordinated contraction of a large region of the heart muscle. The frequency of such action potentials and the force of the resulting contraction are influenced by the autonomic nervous system, but such innervation is not necessary for action potential genesis.
Smooth muscle cells, as with cardiac myocytes, are smaller and shorter than skeletal muscle cells. They do not contain t tubules, and their sarcoplasmic reticulum is poorly developed (Figure 6-7). These cells rely primarily on the transmembrane diffusion of Ca2+ ions from the extracellular fluid to induce the actin-myosin interactions responsible for contraction (see following discussion). Although overlapping actin and myosin molecules form the contractile units of smooth muscle cells, the arrangement of these units lacks the structural regularity responsible for the striated appearance of skeletal and cardiac muscle cells. Actin filaments are anchored to dense bodies (instead of Z disks), which are found within the cytoplasm as well as in the cell membrane. Therefore, these cells can appear to wrinkle on contraction.
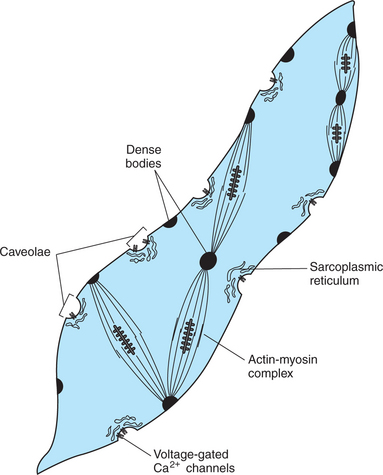
FIGURE 6-7 General organization of a smooth muscle cell. T tubules are absent, and the sarcoplasmic reticulum is poorly developed. Transmembrane diffusion of extracellular Ca2+, through voltage-gated Ca2+ channels in caveolae, plays an important role in initiating contraction. Actin and myosin are present, with actin anchored to dense bodies. Activating the actin-myosin complex can change the cell’s shape.
(Modified from Guyton AC, Hall JE: Textbook of medical physiology, ed 11, Philadelphia, 2006, Saunders.)
Some smooth muscle cell tissues, usually called visceral or unitary smooth muscle, have gap junctions between cells and operate similar to a functional syncytium, with cell-to-cell action potential transmission, and coordinated contraction, much as in cardiac muscle. Visceral smooth muscle is abundant in the gastrointestinal tract and other organs of the thoracic and abdominal cavities. This type of smooth muscle is described more fully in Chapter 28. Another type of smooth muscle cell tissue, usually called multiunit smooth muscle, has electrically isolated muscle cells that are capable of contracting independently of each other. Multiunit smooth muscle can be found, for example, in the iris and ciliary body of the eye, where precise control of muscular contraction is needed.
Smooth muscle tissue is innervated by neurons of the autonomic nervous system. In contrast to neuromuscular junctions at skeletal muscle, either acetylcholine or norepinephrine can be released (by different neurons) at junctions with smooth muscle, smooth muscle cells can be either excited or inhibited by their presynaptic input, and a single smooth muscle cell can receive presynaptic input from more than one neuron. Visceral smooth muscle tends to receive a more diffuse innervation from an autonomic neuron, and the neurotransmitter is released at greater distance from the smooth muscle cell, compared with a skeletal neuromuscular junction. In multiunit smooth muscle, it is more common to find synaptic input onto each cell and a synaptic cleft width similar to a skeletal neuromuscular junction. In addition to control by autonomic neurons, various types of smooth muscle tissue can contract in response to self-induced generation of electrical activity, hormonal action or stretch.
The Role of Ca2+ Ions in Excitation-Contraction Coupling in Cardiac and Smooth Muscle Differs from That in Skeletal Muscle
Contraction of both cardiac and smooth muscle cells results from the sliding together of actin and myosin protein filaments, just as in skeletal muscle. This sliding of actin over myosin requires ATP and does not occur unless Ca2+ ions are present, again as in skeletal muscle. However, the origins of the intracytoplasmic Ca2+ ions that permit contraction differ.
In skeletal muscle, Ca2+ is sequestered in the sarcoplasmic reticulum. With the arrival of the action potential along the sarcolemma and T tubule, Ca2+ is released from the sarcoplasmic reticulum and diffuses out into the cytoplasm, where it triggers contraction. With passage of the action potential, Ca2+ is pumped back into the sarcoplasmic reticulum, and the muscle relaxes. In skeletal muscle, little if any influx of extracellular Ca2+ is needed for contraction.
In cardiac muscle the sarcoplasmic reticulum is not as well developed as in skeletal muscle. Therefore the influx of extracellular Ca2+ and the release of Ca2+ from the sarcoplasmic reticulum are important in triggering contraction. In cardiac muscle the arrival of the action potential along the cell membrane and the T tubules opens voltage-gated Ca2+ channels, allowing the influx of extracellular Ca2+ ions into the cytoplasm. Some of these Ca2+ ions activate the Ca2+-induced Ca2+ release channels on the sarcoplasmic reticulum, and these combined sources of increased cytoplasmic Ca2+ trigger contraction. If antihypertensive drugs called calcium channel blockers are used to block the entry of extracellular Ca2+ ions, the force of contraction is reduced. Once the action potential has passed, muscle relaxation is accomplished primarily by pumping cytoplasmic Ca2+ back into the sarcoplasmic reticulum, although some Ca2+ is transported across the sarcolemma into the extracellular space.
In many smooth muscle cells the sarcoplasmic reticulum is poorly developed, and extracellular Ca2+ influx plays the principal role in initiating the contractile process. Even though smooth muscle cells have no T tubules, this Ca2+ influx is achieved, on membrane depolarization, through activation of voltage-gated Ca2+ channels located in shallow depressions of the membrane (caveolae) (see Figure 6-7). Calcium channel blockers interfere with this process and can relax smooth muscle in arterial walls, which dilates arteries and lowers blood pressure. Contraction is terminated in many smooth muscle cells primarily by Ca2+ transport back into the extracellular space, which is a fairly slow process.
The mechanism by which Ca2+ induces actin-myosin cross-bridge cycling in smooth muscle differs from that in skeletal and cardiac muscle (see Figure 1-17). In skeletal and cardiac muscle, cross-bridge cycling relies primarily on the Ca2+-induced removal of the tropomyosin block of the actin-binding site. In smooth muscle, cycling relies on a Ca2+-induced increase in the ATPase activity of the myosin head, another slow process.
CLINICAL CORRELATIONS
Down Cow After Calving
History.
A 4-year-old Jersey cow calved earlier this morning; this was her second calf. The producer called you because the cow stood after calving but appeared uncoordinated. Now, a few hours later, the cow is just recumbent and appears dull. She has been offered water and hay but has not accepted either. No other cows are affected. This cow has no history of medical problems.
Clinical Examination.
The cow appears dull and does not pay much attention to you or the other activity in the barn. The cow’s temperature is slightly low, and her heart rate is slightly increased. Her respirations are normal. She is slightly dehydrated. Her ears are cool to the touch, peripheral pulses are weak, and her rumen contractions are decreased. On examination, she does not appear to have any injuries that would prevent her from standing. Your brief neurological examination is normal, but the cow does have an S-shaped curve to her spine.
Comment.
This cow most likely has hypocalcemia. Because of the high demands for calcium in the development of the calf in late pregnancy, combined with the production of colostrum and milk, this cow has become hypocalcemic. As reviewed in this chapter, calcium is critical for muscle contractions. Calcium also assists with membrane stabilization of peripheral nerves. Deficits can cause mild tetany, which is sometimes seen in cows with hypocalcemia. Additionally, the release of acetylcholine (ACh) at the neuromuscular junctions is mediated by calcium. Hypocalcemia causes decreased ACh release, which can cause paralysis. All the clinical signs—hypothermia, increased heart rate, weak pulses, paresis, cool extremities, S-shaped curve of the spine, and decreased rumen contractions—can be attributed to hypocalcemia. A definitive diagnosis can be made by measuring ionized calcium. However, most veterinarians and producers will treat based on clinical signs, with the diagnosis confirmed based on response to treatment.
Treatment.
Cows are treated with calcium gluconate, which is slowly given intravenously. Most cows will show improvement in clinical signs during the treatment. Cows often become brighter, their rumen contractility and peripheral circulation improve, and their core body temperature normalizes. Most cows attempt to stand after treatment, which is usually about 1 g per 100 pounds. Some cows will relapse and will need to be re-treated.
Bailey JG. Muscle physiology. Reece WO, ed. Duke’s physiology of domestic animals, ed 12, Ithaca, NY: Comstock Cornell University Press, 2004.
Bear MF, Connors BW, Paradiso MA. Neuroscience: exploring the brain, ed 3. Philadelphia: Lippincott, Williams & Wilkins, 2007.
Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach, updated edition. Philadelphia: Saunders, 2005.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Hunt E, Blackwelder JT. Disorders of calcium metabolism. Smith B, ed. Large animal internal medicine, ed 3, St Louis: Mosby, 2002.
Kandel ER, Schwartz JH, Jessell TM. Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
Matthews GG. Cellular physiology of nerve and muscle, ed 4. Malden, Mass: Blackwell, 2003.
PRACTICE QUESTIONS
1. Troponin and tropomyosin are components of which one of the following structures?
2. Action potentials in skeletal muscle cells trigger the release from the sarcoplasmic reticulum of what ion critical to the muscle’s contractile process?
3. A gross skeletal muscle belly can be instructed (by the central nervous system) to contract more forcefully by:
4. Which one of the following is not found in smooth muscle?
5. Choose the incorrect statement below:
6. Which one of the following is least likely to be significantly associated with a muscle that is primarily involved in brief, powerful movements?