Chapter 24 Local Control of Blood Flow
1. Vascular resistance is affected by intrinsic and extrinsic control mechanisms.
2. Metabolic control of blood flow is a local mechanism that matches the blood flow of a tissue to its metabolic rate.
3. Autoregulation is a relative constancy of blood flow in an organ despite changes in perfusion pressure.
4. Many chemical signals act locally (as paracrines) to exert important control on vascular resistance.
5. Regardless of the status of arterioles, mechanical compression can reduce blood flow to a tissue.
Vascular Resistance Is Affected by Intrinsic and Extrinsic Control Mechanisms
As described in Chapter 22, the blood flow through any organ or tissue is determined by the perfusion pressure (arterial pressure minus venous pressure) and by the resistance of the blood vessels of the organ (and by no other factors), as follows:
All the organs of the systemic circulation are exposed to the same perfusion pressure. Therefore, differences in blood flow to the various organs result solely from their different vascular resistances. The vascular resistance of an organ is determined mainly by the diameter of its arterioles. Thus, arteriolar vasodilation and vasoconstriction are the mechanisms that increase or decrease the blood flow in one organ relative to another organ.
In general, the factors that affect arteriolar resistance can be divided into intrinsic and extrinsic factors. Extrinsic control involves mechanisms that act from outside a tissue, through nerves or hormones, to alter arteriolar resistance. Intrinsic control is exerted by local mechanisms within a tissue. For example, as described in Chapter 23, histamine is released from mast cells of a tissue in response to injury or during an allergic reaction. Histamine acts locally on the arteriolar smooth muscle to relax it. Dilation of the arterioles decreases arteriolar resistance and therefore increases blood flow to the tissue. Histamine is an example of a paracrine: a substance released from one type of cell in a tissue that acts on another cell type in the same tissue to alter its function. A second example of intrinsic control is the arteriolar dilation and increased blood flow during exercise in skeletal muscle. This example illustrates the general phenomenon of metabolic control of blood flow: tissues tend to increase their blood flow whenever their metabolic rate increases.
Although the arterioles in all tissues are affected by both intrinsic and extrinsic mechanisms, intrinsic mechanisms predominate over extrinsic mechanisms in the control of arterioles in the coronary circulation, brain, and working skeletal muscle. By contrast, extrinsic mechanisms predominate over intrinsic mechanisms in the control of blood flow to the kidneys, splanchnic organs, and resting skeletal muscle. Skin is an example of a tissue in which both intrinsic and extrinsic control mechanisms have strong influences. In general, local (intrinsic) control dominates extrinsic control in the critical tissues: those that must have sufficient blood flow to meet their metabolic needs on a second-by-second basis for an animal to survive. Extrinsic control dominates intrinsic control in tissues that can withstand temporary reductions in blood flow (and metabolism) to make extra blood available for the critical tissues.
Metabolic Control of Blood Flow Is a Local Mechanism That Matches the Blood Flow of a Tissue to Its Metabolic Rate
Metabolic control of blood flow is the most important local control mechanism. For example, metabolic control accounts for the huge increase in blood flow through a skeletal muscle as it goes from rest to maximal exercise. The functional significance of metabolic control of blood flow is that it matches the blood flow in a tissue to the metabolic rate of the tissue. An increase in tissue blood flow in response to increased metabolic rate is called active hyperemia (hyper means “elevated,” emia refers to blood, and “active” implies an increased metabolic rate).
Metabolic control of blood flow works by means of chemical changes in the tissue. When the metabolic rate of a tissue increases, its consumption of oxygen increases, with an increased rate of production of metabolic products, including carbon dioxide, adenosine, and lactic acid. Also, some potassium ions (K+) escape from cells and end up in the interstitial fluid. Therefore, as the metabolism of a tissue increases, the interstitial concentration of oxygen decreases, and the interstitial concentrations of metabolic products and K+ increase. All these changes have the same effect on arteriolar smooth muscle: they relax it (Table 24-1). The arterioles dilate, and vascular resistance decreases; more blood flows through the tissue.
Table 24-1 Chemical Signals Important in Local Control of Systemic Arterioles
| Chemical signal | Source | Effect |
|---|---|---|
| Signals related to metabolism | ||
| Oxygen | Delivered by arterial blood; consumed in aerobic metabolism | Vasoconstriction. (Rapid metabolism depletes O2, which causes vasodilation.) |
| Carbon dioxide | Produced by aerobic metabolism | Vasodilation |
| Potassium ions (K+) | Released from rapidly metabolizing cells | Vasodilation |
| Adenosine | Released from rapidly metabolizing cells | Vasodilation |
| Metabolic acids (e.g., lactic acid) | Produced by anaerobic metabolism | Vasodilation |
| Other local chemical signals (paracrines) | ||
| Endothelin-1 (ET1) | Endothelial cells | Vasoconstriction |
| Nitric oxide (NO) | Endothelial cells and some parasympathetic nerve endings | Vasodilation |
| Thromboxane A2 (TXA2) | Platelets | Vasoconstriction (also, increases platelet aggregation) |
| Prostacyclin (PGI2) | Endothelial cells | Vasodilation (also, decreases platelet aggregation) |
| Histamine | Mast cells | Vasodilation (also, increases capillary permeability) |
| Bradykinin | Globulins in blood or tissue fluid | Vasodilation (also, increases capillary permeability) |
Low levels of oxygen and high concentrations of metabolic products and K+ also cause relaxation of the precapillary sphincters (in the tissues that have them), and this opens more of the capillaries in the tissue to blood flow. As explained in Chapter 23, the opening of more capillaries decreases the diffusion distance between fresh, oxygenated blood and the metabolizing cells of the tissue. Opening more capillaries also increases the total capillary surface area for diffusional exchange. The net result of the increased blood flow, the decreased diffusion distance, and the increased total capillary surface area is a more rapid delivery of oxygen and other metabolic substrates to the tissue cells and a more rapid removal of metabolic waste products from the tissue.
Metabolic control of blood flow involves negative feedback. The accumulation of metabolic products and the lack of oxygen initiate vasodilation, which increases blood flow. The increased blood flow removes the accumulating metabolic products and delivers additional oxygen. A new balance is reached when the increased blood flow closely matches the increased metabolic needs of the tissue. Figure 24-1 summarizes the major features of metabolic control of blood flow.
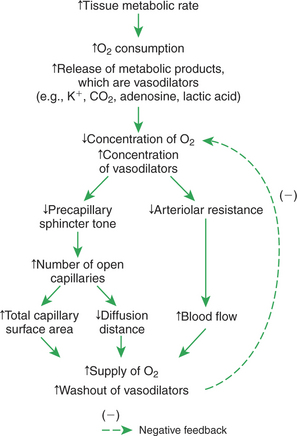
FIGURE 24-1 Metabolic control of blood flow is a local (intrinsic) mechanism that acts within a tissue to match blood flow to the metabolic rate. As a tissue becomes more active metabolically, the metabolic control mechanism increases blood flow and thereby regulates the concentration of oxygen and metabolic products in the tissue.
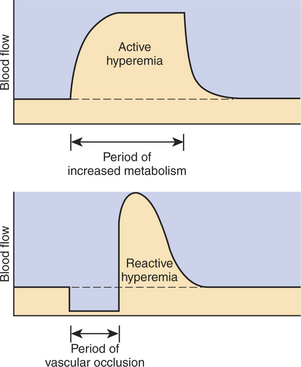
Reactive hyperemia is a temporary increase above normal in the flow of blood to a tissue after a period when blood flow was restricted. In this case the hyperemia (increased flow) is a response (reaction) to a period of inadequate blood flow. Mechanical compression of blood vessels is one cause of inadequate blood flow. One can easily demonstrate reactive hyperemia in nonpigmented epithelial tissue; for example, press a finger against nonpigmented skin hard enough to occlude the blood flow; maintain the pressure for about 1 minute, and then release. The previously compressed area of skin looks darker (redder) for a short time because blood flow becomes greater than normal when the compression is released.
The same metabolic control mechanisms that account for active hyperemia also explain reactive hyperemia. During the period of restricted blood flow, metabolism continues in the compressed tissue, so metabolic products accumulate, and the local concentration of oxygen decreases. These metabolic effects cause dilation of the arterioles and a decrease in arteriolar resistance. When the mechanical obstruction to flow is removed, blood flow increases above normal until the “oxygen debt” is repaid and the excess metabolic products have been removed from the compressed tissue. Figure 24-2 compares active and reactive hyperemia.
Autoregulation Is a Relative Constancy of Blood Flow in an Organ Despite Changes in Perfusion Pressure
Metabolic control mechanisms also help to account for the phenomenon known as blood flow autoregulation. Autoregulation is evident in denervated organs and organs in which local control of blood flow is predominant over neural and humoral control (e.g., in coronary circulation, brain, and working skeletal muscle).
Figure 24-3 summarizes an experiment that demonstrates autoregulation in the brain. Initially, the perfusion pressure (arterial pressure minus venous pressure) in this animal is 100 mm Hg, and the blood flow to the brain is 100 milliliters per minute (mL/min) (point A). When perfusion pressure is increased suddenly to 140 mm Hg, brain blood flow rises initially to 140 mL/min but returns toward its initial level over the next 20 to 30 seconds. Eventually, blood flow reaches a stable level of about 110 mL/min (point B). Conversely, if the perfusion pressure is decreased suddenly from 100 to 60 mm Hg, blood flow in the brain decreases initially to 60mL/min but returns toward its initial level over the next 20 to 30 seconds (dashed lines in the top and middle graphs of Figure 24-3). Eventually, blood flow reaches a stable level of about 90mL/min (point C). These stable responses are plotted in the bottom graph. The remainder of the bottom graph is obtained in a similar way; that is, perfusion pressure is set artificially to various levels, ranging from 40 to 220 mm Hg, and the resulting steady-state levels of blood flow are plotted.
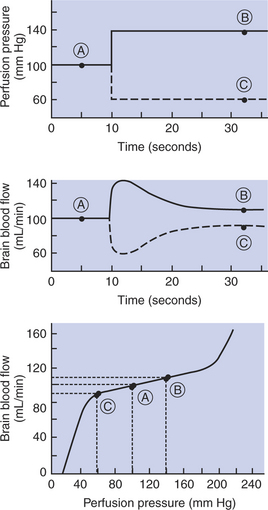
FIGURE 24-3 The experiment summarized here demonstrates autoregulation of blood flow in the brain. Perfusion pressure was artificially set to various levels (top), and the resulting steady-state values of blood flow were measured (middle). The steady-state values of brain blood flow were then plotted against the perfusion pressure (bottom). Circled points A, B, and C are discussed in the text.
Over a considerable range of perfusion pressure (about 60-190 mm Hg), relatively little change occurs in steady-state blood flow to the brain; that is, brain blood flow is autoregulated. The range of perfusion pressures over which flow remains relatively constant is called the autoregulatory range. Autoregulation fails at very high and very low perfusion pressures. Extremely high pressures result in marked increases in blood flow, and extremely low pressures result in marked decreases in blood flow. Nevertheless, over a considerable range of perfusion pressure, autoregulation keeps blood flow in the brain relatively constant.
Figure 24-4 shows how the metabolic control mechanisms previously described can account for the phenomenon of autoregulation. If the metabolic rate of an organ does not change but perfusion pressure is increased above normal, the increased pressure forces additional blood flow through the organ. The additional blood flow accelerates the removal of metabolic products from the interstitial fluid and increases the rate of oxygen delivery to the interstitial fluid. Therefore the concentration of vasodilating metabolic products in the interstitial fluid decreases, and the concentration of oxygen in the interstitial fluid increases. These changes cause the arterioles of the tissue to constrict, which increases the resistance to blood flow above normal and decreases blood flow back toward its initial level, despite the continuation of the elevated perfusion pressure.
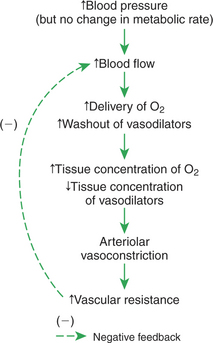
FIGURE 24-4 The same metabolic mechanism that is responsible for active hyperemia and reactive hyperemia can also account for autoregulation.
To summarize, metabolic control mechanisms bring about active hyperemia (the increase in blood flow in an organ in response to an increased metabolic rate, in the absence of any blood pressure change). The same metabolic mechanisms can also account for reactive hyperemia (the increase in blood flow above normal in an organ after a period of flow restriction). In addition, the same metabolic mechanisms can account for autoregulation (the relative constancy of blood flow in an organ when there has been no change in metabolic rate but blood pressure has either increased or decreased). Other mechanisms contribute to autoregulation, and the reader may encounter discussions of these under the terms myogenic hypothesis and tissue pressure hypothesis. However, metabolic control is the most likely explanation for autoregulation of blood flow in the critical tissues of a body (brain, coronary vessels, and exercising skeletal muscle).
Many Chemical Signals Act Locally (as Paracrines) to Exert Important Control on Vascular Resistance
As already described, metabolic control of blood flow is mediated by chemical changes that occur when tissue metabolism increases. In addition to metabolic controls on blood flow, there are many other chemicals that act locally, within a tissue, to affect vascular resistance and therefore blood flow. Some of these local (paracrine) chemical signals are listed in Table 24-1.
Endothelin-1 (ET-1) is released from endothelial cells in response to a variety of mechanical or chemical stimuli, especially those that traumatize the endothelium. Endothelin-1 causes vascular smooth muscle to contract, which results in vasoconstriction and a decrease in blood flow. Nitric oxide (NO), another signaling molecule released from endothelial cells, has the opposite effect. NO relaxes vascular smooth muscle, which results in vasodilation. One stimulus for NO release is an increase in blood flow velocity past the endothelium. The NO acts locally to dilate vessels, especially small arteries, which allows them to accommodate an increased blood flow without such high flow velocities. In some tissues, most notably the erectile tissues of the external genital organs (penis and clitoris), parasympathetic nerve endings release both NO and acetylcholine. The acetylcholine stimulates endothelial cells to release additional NO. The NO dilates local blood vessels, which causes engorgement of the tissues with blood, and therefore erection.
Thromboxane A2 (TXA2) and prostacyclin (PGI2) act antagonistically in the control of vascular smooth muscle and also in the control of platelet aggregation. Thus the relative balance between TXA2 and PGI2 is more important than the absolute level of either chemical alone. Under normal conditions the balance ensures adequate blood flow to tissues and prevents platelet aggregation. If blood vessels become traumatized or rupture, the balance shifts in favor of TXA2. The resulting vasoconstriction and platelet aggregation are critical in minimizing blood loss. In some pathological states, imbalances develop between TXA2 and PGI2. Depending on the direction of the imbalance, the result is either excessive vasoconstriction and blood coagulation or excessive vasodilation and bleeding.
The role of histamine in the vascular responses to tissue injury or antigen challenge is described in Chapter 23 (see Figure 23-8). Another signaling chemical, bradykinin, plays a similar role. Bradykinin is a small polypeptide that is split away by the proteolytic enzyme kallikrein from globulin proteins that are present in plasma or tissue fluid. Bradykinin may also be formed in sweat glands when they are activated by acetylcholine released from sympathetic nerve endings. The resulting vasodilation of skin blood vessels, together with the evaporation of sweat, promotes heat loss from the skin. Both histamine and bradykinin exert their vasodilating effects, at least in part, by stimulating the formation of NO.
Regardless of the Status of Arterioles, Mechanical Compression Can Reduce Blood Flow to a Tissue
Mechanical compression can reduce blood flow in a tissue by literally squeezing down on all its blood vessels. The example of brief mechanical compression of the epithelial blood vessels has been mentioned as a way to trigger a readily visible reactive hyperemia. Long-term mechanical pressure on the skin must be avoided, however, because a prolonged period of subnormal blood flow (ischemia) leads to irreversible tissue damage and cell death (infarction). Pressure sores are an unfortunate and common example. Three other specific instances of mechanical compression are also described because of their clinical importance.
Figure 24-5 illustrates the effect of mechanical compression on coronary blood flow. The top tracing shows the changes in arterial (aortic) blood pressure during one complete cardiac cycle and the beginning of a second one. The periods of ventricular systole and ventricular diastole are labeled at the bottom of the figure. One would expect that left coronary blood flow would be highest during ventricular systole (when the aortic pressure is highest) and lowest during diastole (when the aortic pressure is lowest). However, the tracings of left coronary blood flow indicate that blood flow is actually depressed during systole and much higher during diastole. Flow even reverses (blood flows backward, toward the aorta) momentarily near the beginning of systole. The fact that coronary blood flow is much lower during systole, even though the perfusion pressure is higher, implies that coronary resistance must be substantially higher during systole than during diastole.
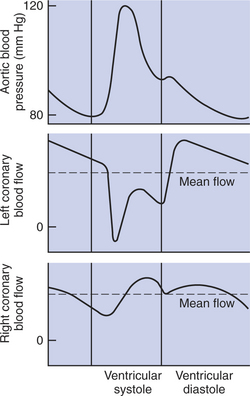
FIGURE 24-5 Coronary blood flow to the left ventricular muscle is greatly reduced during ventricular systole by mechanical compression of the left ventricular blood vessels. Coronary blood flow to the right ventricular muscle is less affected by mechanical compression.
Coronary resistance is high during systole because the contracting ventricular muscle squeezes down on the coronary blood vessels, which increases their resistance to blood flow. The coronary vessels are not constricted in this way during diastole because the ventricular muscle is relaxed. Therefore, coronary vascular resistance decreases dramatically (and blood flow increases) during diastole. The bottom tracing in Figure 24-5 indicates that mechanical compression has relatively little influence on right coronary blood flow. That is, the magnitude of right coronary blood flow closely follows the changes in arterial pressure (being highest during systole and lowest during diastole). The right ventricle contracts with much less force than the left ventricle, which explains why right coronary flow is not restricted by mechanical compression during systole. The right ventricle simply does not develop sufficient compressive force to constrict its own blood vessels.
Most of the blood that is needed to support left ventricular metabolism must be delivered during ventricular diastole, when the vessels are not compressed. This fact has great clinical significance. In a resting animal with a low heart rate, there is adequate time during diastole for the coronary vessels to supply the amount of blood needed by the ventricular tissue. During exercise, heart rate and cardiac contractility both increase, which greatly increases the metabolic rate of the ventricular muscle cells. To support the increased metabolic rate, the ventricular tissue needs much more blood flow than at rest. However, the duration of diastole is reduced during exercise, so there is less time available for delivery of this increased flow. Nevertheless, normal, healthy coronary vessels have a sufficiently low resistance during diastole to supply the needed blood flow, even during maximal exercise. The situation is different, however, in animals with coronary artery disease. In animals whose coronary vessels are narrowed because of atherosclerosis, blood flow cannot increase enough to supply the needs of the vigorously working ventricular muscles. This is why ventricular ischemia develops during exercise in patients with coronary artery disease. Ischemic areas of the ventricle fail to contract normally. Ischemia can also cause arrhythmias or even ventricular fibrillation (sudden death). Coronary artery disease is more common in humans than in veterinary species, so this scenario is more likely to occur in the veterinarian than in the veterinarian’s patients.
Mechanical compression caused by muscle contraction can also restrict blood flow through skeletal muscles. The blood vessels within skeletal muscles become compressed during strenuous, sustained contractions of the muscle. The compression reduces blood flow and can create ischemia. Ischemic muscles cannot contract with normal vigor. Ischemia also activates sensory nerve endings in the muscle, which causes pain. Activation of these muscle ischemia receptors also triggers a reflex increase in arterial pressure. The high arterial pressure is advantageous because it partially overcomes the effects of mechanical compression on blood flow. In other words, a high arterial pressure helps to force blood flow through the skeletal muscle blood vessels, despite the compressive effects of the muscle contraction. The high arterial pressure of ischemic exercise is risky for patients with coronary artery disease, however, because high arterial pressure causes a tremendous increase in the cardiac workload. This is why patients with coronary artery disease are cautioned against types of exercise that involve strenuous, sustained muscle contractions, such as weightlifting.
Mechanical compression has important effects on the pulmonary circulation. Pulmonary arterial pressure is much lower than systemic arterial pressure, so mechanical compression can easily reduce pulmonary blood flow, as illustrated in Figure 24-6. This diagram shows a pulmonary blood vessel passing between two alveoli. Under normal conditions the arterial pressure is 13 mm Hg and the venous pressure 5 mm Hg (Figure 24-6, A). The pressure just outside the vessels (i.e., in the alveolar air spaces) is even lower; alveolar pressures typically vary between −1mm Hg (during inspiration) and +1 mm Hg (during expiration). Because the pressure inside pulmonary vessels is greater than the pressure outside, the vessels are not compressed.
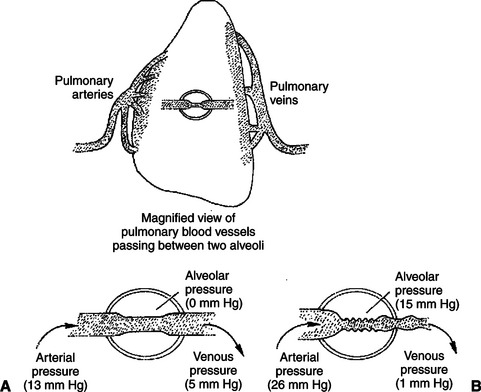
FIGURE 24-6 Pulmonary vessels are susceptible to mechanical compression. A, Normally, pulmonary arterial and venous pressures are both higher than alveolar pressure, so pulmonary vessels stay open. B, If alveolar pressure increases to 15 mm Hg or higher, however, the pulmonary blood vessels are compressed. The resulting increase in pulmonary vascular resistance causes pulmonary blood flow to decrease, pulmonary arterial pressure to increase, and pulmonary venous pressure to decrease.
Figure 24-6, B, depicts a compression of pulmonary blood vessels resulting from an abnormally high alveolar pressure. This could happen during surgery if a patient has a tracheal tube inserted into the airway and the tracheal tube is attached to a source of elevated pressure. The elevated pressure could be generated by a mechanical respirator that is not adjusted properly or by an anesthetist when he or she squeezes the bag that is attached to the tracheal tube. In either case, the pressures generated in the tracheal tube are transmitted to the alveoli. An increase in alveolar pressure increases the external compressing pressures applied around the pulmonary blood vessels.
Alveolar pressures exceeding 10 to 15 mm Hg compress pulmonary blood vessels sufficiently to raise the resistance to blood flow through the lungs. Pulmonary blood flow decreases. Two events occur as a result. First, less blood reaches the left atrium and left ventricle, which causes left ventricular stroke volume and aortic blood pressure to decrease. Second, blood ejected by the right ventricle dams up in the pulmonary arteries. This causes pulmonary arterial pressure to increase. An elevated pulmonary arterial pressure helps force blood through the compressed vessels. However, the increased pulmonary artery pressure also places an increased workload on the right ventricle. If the alveolar pressure is not excessively high, the right ventricle generates sufficient pulmonary arterial pressure to restore pulmonary blood flow almost to normal. However, with extremely high alveolar pressures, the right ventricle is unable to raise pulmonary arterial pressure high enough to sustain flow. Under these conditions, pulmonary blood flow and thus systemic blood flow fall substantially below normal, and systemic hypotension and ischemia develop in the patient. The consequences can be fatal. The veterinary clinician must be mindful of the risks of high airway pressures whenever a patient is intubated and attached to a mechanical respiratory device.
CLINICAL CORRELATIONS
Patent Ductus Arteriosus
History.
A 3-month-old female Welsh corgi is brought to your clinic by its owner, who has noticed a “rumbling noise” in the dog’s chest. The dog is smaller than her littermates and less playful. The dog coughs occasionally, but the cough does not produce fluid.
Clinical Examination.
The dog appears to be in good health except for the occasional cough. The mucous membranes are pink, and the capillary refill time is normal (1.5 seconds). However, when you place your hand on the anterior left chest, you feel an abnormal vibration (thrill) with each heartbeat. With a stethoscope, you can auscultate a cardiac murmur that is loudest during systole but continues throughout both systole and diastole (continuous murmur). The murmur is heard most loudly at the ventral third intercostal space on the left side. Expiratory sounds are slightly louder than normal. The pulse rate is 152 beats per minute, which you consider to be above normal for a dog of this size and age. While you are listening to the heart with the stethoscope, you palpate the femoral pulses, which are very strong.
The electrocardiogram indicates that the dog has sinus tachycardia; the atrial and ventricular rates are both 152 beats per minute. The R waves are abnormally large in leads II and III (2.5 and 3.5 mV, respectively). The QRS complex in lead I shows a large negative deflection followed immediately by a slightly larger positive deflection.
Thoracic radiographs show a generalized enlargement of the heart. The initial portion of the pulmonary artery is also substantially larger than normal, and the pulmonary blood vessels appear generally to be more prominent than normal.
An echocardiogram confirms the presence of a patent ductus arteriosus.
Comment.
A murmur in a young, otherwise-healthy dog is most likely the result of a congenital cardiac abnormality. A continuous murmur can occur only if a defect causes turbulent flow throughout both diastole and systole. Because flow can occur only when there is a pressure gradient, the defect in this dog must be in a location where there is a substantial pressure gradient throughout the cardiac cycle. No single intracardiac defect meets this criterion; that is, a stenotic or regurgitant valve produces either a systolic murmur or a diastolic murmur, but not both. A valve that is both stenotic and regurgitant produces two murmurs: one in systole and one in diastole. In such a case, however, brief moments occur during the cardiac cycle when no pressure gradient exists across the valve, so there are moments of silence between the systolic murmur and the diastolic murmur. (Admittedly, if the heart rate is high, these moments of silence are very brief, and the two murmurs can be mistaken for a continuous murmur, particularly in the case of combined aortic stenosis and regurgitation.)
The most common cardiac defect that causes turbulent flow throughout both systole and diastole is a patent ductus arteriosus (PDA). This vessel is normal in the fetus but should close shortly after birth. The flow through a PDA is continuous because aortic pressure is higher than pulmonary artery pressure throughout the cardiac cycle. The resulting murmur is usually heard best in the left third intercostal space. All the other clinical signs in this dog are also consistent with the diagnosis of PDA. The prominence of the pulmonary vessels on the radiographs indicates that pressure and flow are abnormally high in the pulmonary artery and its branches. In a dog with a PDA the pulmonary artery receives blood flow from both the right ventricle and the aorta, which increases both pulmonary arterial pressure and pulmonary flow.
The radiographs and electrocardiograms indicate that this dog has both right and left ventricular hypertrophy. The large R waves in leads II and III indicate left ventricular hypertrophy, and the large negative deflection during the QRS complex in lead I suggests that the right ventricle is hypertrophic as well. The left ventricle becomes hypertrophic in a dog with PDA because it is called on to pump three to five times the normal cardiac output. (It pumps a near-normal volume to the organs of the systemic circulation and two to four times that much through the PDA.) The flow through the PDA is large because the PDA offers little resistance to flow. The demand on the left ventricle to pump so much blood (increased volume work) leads to left ventricular hypertrophy. The volume of blood pumped by the right ventricle is almost normal; it only needs to pump the blood that returns through the venae cavae from the systemic organs. However, the right ventricle must develop higher systolic pressures than normal to eject this blood into the pulmonary artery because pulmonary artery pressure is higher than normal, as explained earlier. This increase in pressure work often leads to right ventricular hypertrophy.
Because the PDA carries so much blood away from the aorta, dogs with PDA tend to have an abnormally low aortic pressure. Diastolic pressure is particularly reduced because of the rapid outflow of blood from the aorta during ventricular diastole. Therefore, PDA is typically associated with low mean aortic pressure but elevated pulse pressure (review Figure 22-9, G).
Two mechanisms work together to keep blood flow to the systemic organs almost normal despite the fact that a large fraction of cardiac output is “lost” through the PDA. First, reflex mechanisms (discussed in Chapter 25) increase sympathetic activity to the heart, which increases heart rate and contractility above normal. These sympathetic effects keep left ventricular output (and aortic pressure) sufficiently high to supply blood to the systemic organs, despite the PDA. Second, metabolic control mechanisms cause the systemic organs to vasodilate, which keeps their blood flow almost normal despite the subnormal aortic pressure.
The compensatory mechanisms just described allow most dogs with a PDA to maintain a nearly normal blood flow to the systemic organs at rest. Several months may pass before the dog’s owner notices limitations in the dog’s activity or growth. Eventually, however, the heart cannot increase its output sufficiently to supply the systemic blood flow needed by the muscles during exercise, so a puppy with a PDA becomes less playful and energetic than its normal littermates. Also, if the heart is unable to supply the blood flow needed by metabolically active tissues, the owner may notice some stunting of growth. In any case, a dog with a widely open ductus has a poor long-term prognosis, unless treated.
Treatment.
You show the dog’s owner a diagram of the fetal circulation and explain that the ductus arteriosus normally closes and seals itself within 1 to 6 weeks after birth, but that the ductus fails to close spontaneously in about 1 of every 700 newborns (the condition is four times more common in female pups than in male pups). Treatment involves closure of the ductus, either by ligation during open-chest surgery or by insertion of a specially designed plug during a cardiac catheterization procedure. Most dogs treated before age 6 months go on to lead completely normal lives. However, you inform the owner that PDA is hereditary and that this puppy should probably not be used for breeding.
The owner elects to have the dog treated surgically, and the surgery is successful. The murmur and cough disappear immediately. Within 1 week the dog is noticeably more energetic. At age 6 months, the dog has “grown into” her enlarged heart, and all physical findings are within normal limits.
Boulpaep EL. The microcirculation. In: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. Philadelphia: Saunders, 2005.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Kittleson MD, Kienle RD. Small animal cardiovascular medicine. St Louis: Mosby-Year Book, 1998.
Levy MN, Pappano AJ. Cardiovascular physiology, ed 9. Philadelphia: Mosby, 2007.
Mohrman DE, Heller LJ. Cardiovascular physiology, ed 6. New York: Lange Medical Books/McGraw-Hill, 2006.
Oyama MA, Sisson DD, Thomas WP, Bonagura JD. Congenital heart disease: edema. Ettinger SJ, Feldman EC. Textbook of veterinary internal medicine: diseases of the dog and cat, ed 6, St Louis: Saunders, 2005.
PRACTICE QUESTIONS
1. The increase in coronary blood flow during exercise is:
2. A dog with an arterial blood pressure of 120/80 mm Hg has a cerebral blood flow of 100 mL/min. When blood pressure is increased to 130/100 mm Hg, the cerebral blood flow increases to 105 mL/min. This is an example of:
3. Local, metabolic control of blood flow through skeletal muscle:
4. In response to an increase in perfusion pressure, the arterioles of an autoregulating organ _________________, and the vascular resistance of the organ _______________.