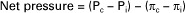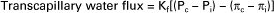Chapter 23 Capillaries and Fluid Exchange
1. Capillaries, the smallest blood vessels, are the sites for the exchange of water and solutes between the bloodstream and the interstitial fluid.
2. Lipid-soluble substances diffuse readily through capillary walls, whereas lipid-insoluble substances must pass through capillary pores.
3. Fick’s law of diffusion is a simple mathematical accounting of the physical factors that affect the rate of diffusion.
4. Water moves across capillary walls both by diffusion (osmosis) and by bulk flow.
5. The Starling equation quantifies the interaction of oncotic and hydrostatic forces acting on water.
6. Several common physiological changes alter the normal balance of Starling forces and increase the filtration of water out of capillaries.
7. Edema is a clinically noticeable excess of interstitial fluid.
Capillaries, the Smallest Blood Vessels, Are the Sites for the Exchange of Water and Solutes Between the Bloodstream and the Interstitial Fluid
Because of their small size, the capillaries are sometimes called the microcirculation. They are also called the exchange vessels, because the exchange of water and solutes between the bloodstream and the interstitial fluid takes place across the walls of the capillaries. Each type of blood vessel in the body is structurally suited for its primary function, and the walls of the capillaries are especially well adapted for their exchange function.
Figure 23-1 shows the contrasting features of the walls of the various types of blood vessels. The distinguishing feature of the walls of the aorta and large arteries is the presence of a large amount of elastic material along with smooth muscle. These vessels are called the elastic vessels; elasticity is necessary because the aorta and large arteries must distend with each pulsatile ejection of blood from the heart. The arterial walls are also strong and quite stiff (low compliance). There is no contradiction in saying that the arteries are elastic and have low compliance. Elasticity denotes distensibility and an ability to return to the original shape after the distending force or pressure is removed. Compliance is a measure of how much force or pressure is required to achieve distention. The arteries are elastic, but a high pressure (systolic pressure) is required to distend them.
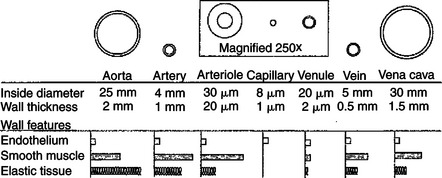
FIGURE 23-1 Each type of blood vessel in the systemic circulation is specifically suited to its particular function by its size, wall thickness, and wall composition. In this drawing, each type of vessel is shown in cross section. The drawings are to scale (the arteriole, capillary, and venule are magnified 250 times to make them visible). Also shown are the relative proportions of the three most important types of tissue found in blood vessel walls.
Small arteries, and particularly arterioles, have relatively thick walls with less elastic tissue and a predominance of smooth muscle, so they are called the muscular vessels. The muscle enables these vessels to constrict or dilate, which varies their resistance to blood flow. The muscular vessels vary the total peripheral resistance and direct blood flow toward or away from particular organs or particular regions within an organ.
Capillaries are the smallest vessels, being about 8 μm in diameter and about 0.5mm long. Capillaries are so small that red blood cells (7.5 μm in diameter) must squeeze through in single file. Capillary walls consist of a single layer of endothelial cells. The small diameter of the capillaries and the thinness of their walls facilitate the exchange of water and solutes between the blood within capillaries and the interstitial fluid immediately outside the capillaries.
Venules and veins are larger than capillaries, and they have thicker walls. Venules and veins have both elastic tissue and smooth muscle in their walls. However, the walls of veins are not as thick or as muscular as the walls of arteries or arterioles. The primary role of veins is to serve as reservoir vessels. Veins are very compliant. Furthermore, it is normal for many veins in the body to be in a state of partial collapse. Therefore, substantial changes in venous blood volume can occur without much change in venous pressure.
Capillaries form a network (see Figure 18-4). In most tissues the capillary network is so dense that each cell of the tissue is within 100 μm of a capillary. However, not all the capillaries of a tissue carry blood at all times. In most tissues the arterioles alternate between constriction and dilation, so blood flow is periodically reduced or even stopped in most capillaries. Also, in some tissues (e.g., intestinal circulation), tiny cuffs of smooth muscle encircle capillaries at the points where they branch off from arterioles. Contraction of these precapillary sphincters can reduce or stop the flow of blood in individual capillaries. When the metabolic rate of a tissue increases (and therefore its need for blood flow increases), the arterioles and precapillary sphincters still constrict periodically, but they spend more time in the dilated (relaxed) state. This increases the fraction of capillaries in which blood is flowing at any one time. At maximal metabolic rate (e.g., maximal exercise in a skeletal muscle), blood flows through all the capillaries all the time. Sending blood flow to all the capillaries not only increases the total blood flow through a tissue but also minimizes the distance between each cell of the tissue and the nearest capillary carrying blood by bulk flow. Both these effects speed up diffusional exchange between the capillary blood and the tissue cells.
Lipid-Soluble Substances Diffuse Readily Through Capillary Walls, Whereas Lipid-Insoluble Substances Must Pass Through Capillary Pores
The rate of diffusional exchange between capillary blood and the surrounding interstitial fluid depends both on the features of the capillary wall and on the properties of the substance being exchanged. In most tissues there are water-filled pores, or clefts, between the endothelial cells that form capillary walls (Figure 23-2). These pores provide channels through which water and lipid-insoluble substances can move from the capillary lumen to the interstitial space, or vice versa. Plasma electrolytes, glucose, and amino acids are among the lipid-insoluble substances that must pass through these water-filled channels. By contrast, lipid-soluble substances in blood, such as dissolved oxygen and carbon dioxide, fatty acids, ethanol, and some hormones, can dissolve in and diffuse through the endothelial cells that form the capillary wall. For a lipid-soluble substance, this process takes only a fraction of a second. In fact, the diffusional exchange of lipid-soluble substances is much more rapid than that of lipid-insoluble substances, because the lipid-insoluble substances are restricted to passage through the capillary pores, which constitute only about 1% of the total wall surface area of a typical capillary.
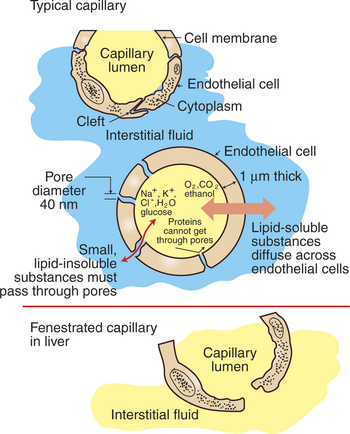
FIGURE 23-2 Capillaries in cross section. Most capillaries have pores, or clefts, between endothelial cells (top). Water and lipid-insoluble compounds move between the capillary plasma (yellow) and the interstitial fluid (blue) through these water-filled channels (center). Lipid-soluble substances diffuse directly through the capillary endothelial cells. The size of the capillary pores varies greatly from tissue to tissue, with the largest capillary pores found in the liver capillaries (liver sinusoids) (bottom).
The characteristics of the capillary pores vary from tissue to tissue. Two extremes are found in the liver and the brain. The pores or clefts in the liver capillaries are so large that even plasma proteins such as albumin and globulin can pass through them. This is an appropriate feature for the liver capillaries (hepatic sinusoids) because the plasma proteins are produced in the liver. The large clefts permit the newly synthesized protein molecules to enter the bloodstream. The large pores in liver capillaries are also appropriate for the role of the liver in detoxification. Some toxins that become bound to plasma proteins are removed from the bloodstream by the liver and chemically changed into less toxic substances. Because of their large pores, capillaries (sinusoids) in the liver are called fenestrated capillaries (“capillaries with windows”) (Figure 23-2, bottom).
The brain capillaries represent the other extreme in pore size. The pores of brain capillaries are so small that only water and electrolyte molecules can pass through them; not even glucose and amino acid molecules can pass through these tiny pores. The tight barrier provided between the bloodstream and the brain tissue by the small pores is called the blood-brain barrier (see Chapter 15). One function of the blood-brain barrier is to protect brain neurons from exposure to toxic substances. Glucose is moved across the brain capillary endothelial cells by means of specialized protein carrier molecules that are embedded in the cell membranes of the endothelial cells. The energy to drive this facilitated diffusion comes from the glucose concentration difference between the blood and the brain interstitial fluid. Brain neurons require glucose to carry out their normal metabolism.
Fick’s Law of Diffusion Is a Simple Mathematical Accounting of the Physical Factors That Affect the Rate of Diffusion
Most of the factors that affect the rate of diffusional exchange between capillary blood and interstitial fluid have been mentioned. These factors include the distance involved, the size of the capillary pores, and the properties of the diffusing substance (i.e., lipid soluble vs. lipid insoluble). The German physiologist Adoph Fick incorporated all these factors into an equation: Fick’s law of diffusion. Figure 23-3 shows how Fick’s law applies to the diffusional exchange between capillary fluid and interstitial fluid. The rate of diffusion of any substance S depends, first, on the concentration difference, that is, the difference between the concentration of the substance in capillary fluid and its concentration in interstitial fluid. Diffusion is driven by this concentration difference, and diffusion always proceeds from the area of higher concentration toward the area of lower concentration. Next, the rate of diffusion is determined by the area available for diffusion, the term A in the equation. For lipid-soluble substances, this area is equivalent to the total surface area of the capillaries. For lipid-insoluble substances, this area is much smaller, being equal to the area of the capillary pores or clefts.
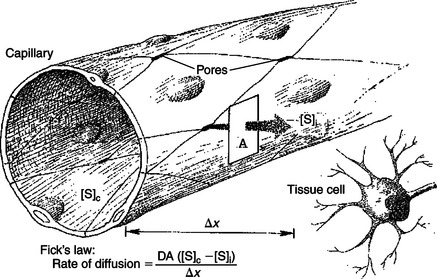
FIGURE 23-3 According to Fick’s law, the four factors that affect the rate of diffusion of a substance S from the capillary plasma to the interstitial fluid next to a tissue cell are [S]c − [S]i, the concentration difference between the capillary plasma and interstitial fluid. A, Area available for diffusion; Δx, distance involved; D, diffusion coefficient.
The term Δx in the equation represents the distance over which diffusion must occur (Figure 23-3). Functionally, Δx equals the distance from a tissue cell to the nearest capillary that is carrying blood by bulk flow. The greater the distance from the tissue cells to the capillaries, the slower is the rate of diffusional exchange of substances between that cell and the capillary blood; therefore, Δx appears in the denominator in the equation.
The term D in the equation is a diffusion coefficient. The value of D increases with temperature because diffusion depends on the random brownian motion of particles in solution, and the velocity of that motion increases with temperature. D is different for different substances. For example, D for carbon dioxide is about 20 times greater than D for oxygen. As a result, carbon dioxide diffuses much more rapidly than does oxygen for a given concentration difference, area, and diffusion distance. This difference is inconsequential under normal physiological conditions. In certain disease states, however, the area available for diffusion decreases, and the diffusion distance increases. Under these conditions, the delivery of oxygen to the metabolizing cells of a tissue generally becomes critically impaired before the removal of carbon dioxide from the cells becomes inadequate. In other words, physiological diffusion limitations generally cause bodily tissues to become hypoxic (inadequate oxygen) before they become hypercapnic (excess carbon dioxide).
Several of the factors that affect the rate of diffusion are physiologically adjustable. For example, in skeletal muscle at rest, the arterioles cycle between open and closed, and even when open, their diameter is small. Consequently, at any one moment, blood flows through only about one fourth of the skeletal muscle capillaries. Blood sits still in the remainder of them. Nevertheless, this low and “part-time” blood flow through capillaries is adequate to deliver oxygen and nutrients to the resting skeletal muscle cells and to remove the small amounts of carbon dioxide and other waste products being produced by those cells. In contrast, during exercise, the metabolic rate of the skeletal muscle cells increases several-fold, as does their need for blood flow. During exercise, skeletal muscle arterioles dilate. Increasingly more of them remain open on a “full-time” basis as the level of exercise increases. Consequently, blood flow through the capillaries increases and becomes more continuous.
These changes act in three ways to speed the delivery of oxygen and metabolic substrates to the exercising muscle cells and to facilitate the removal of carbon dioxide and other metabolic waste products. First, when more capillaries carry blood, the area available for diffusion (A in Fick’s diffusion equation) is increased. Second, because more capillaries carry blood, the distance between each exercising skeletal muscle cell and the nearest open capillary (Δx in the diffusion equation) is decreased. Third, the driving force for diffusion of oxygen (the oxygen concentration difference between the capillary blood and the interstitial fluid) is increased. The concentration difference is increased because (1) the greater blood flow brings more freshly oxygenated blood into the capillaries, and (2) the rapid utilization of oxygen by the exercising skeletal muscle cells decreases the concentration of oxygen within these cells and therefore within the surrounding interstitial fluid.
The same factors that increase the rate of oxygen diffusion during exercise increase the rate of delivery of glucose and other nutrients. Furthermore, the same factors act to increase the rate at which carbon dioxide and other metabolic products are removed from the tissue cells and into the bloodstream. In the case of carbon dioxide and other metabolic products, the concentration is highest in the cells and lowest in the capillary plasma, so diffusional movement is from the cells toward the bloodstream.
Water Moves Across Capillary Walls Both by Diffusion (Osmosis) and by Bulk Flow
The exchange of water between the capillary plasma and the interstitial fluid merits special consideration for two reasons. First, the forces that govern water movement are more complicated than the simple diffusive forces that affect solute movement. Second, a particular imbalance in these forces causes an excessive amount of water to accumulate in the interstitial space, which leads to the important clinical sign, edema.
As the preceding discussion emphasized, solutes such as oxygen, carbon dioxide, glucose, electrolytes, and fatty acids move between the capillary plasma and the interstitial fluid by diffusion. Water also moves by diffusion; the diffusional movement of water is called osmosis. The physical prerequisites for osmosis are (1) the presence of a semipermeable membrane (a membrane that is permeable to water but not to specific solutes) and (2) a difference in the total concentration of impermeable solutes on the two sides of the membrane.
The capillary wall constitutes a semipermeable membrane. Water readily passes through the capillary pores; however, in most organs the capillary walls are impermeable to the plasma proteins. (The molecules of albumin, globulin, and the other plasma proteins are slightly too large to pass through the pores in most capillaries.) The normal concentration of plasma proteins is 7 grams per deciliter (g/dL) within the capillary plasma but only 0.2 g/dL in the interstitial fluid. The higher protein concentration within the capillaries creates an osmotic imbalance. Water molecules tend to move through osmosis from the interstitial fluid into the capillary blood plasma. (Remember, when water moves through osmosis, it moves toward the side of the membrane with the higher concentration of impermeable solute.)
The tendency for water to move through diffusion is quantified by osmotic pressure (see Chapter 1). The normal osmotic pressure created by the proteins in the plasma is 25 mm Hg; that is, the osmotic effect of the plasma proteins is equivalent to a pressure of 25 mm Hg driving water into the capillaries. The osmotic pressure created by the plasma proteins is also called plasma oncotic pressure or colloid osmotic pressure. (The term colloid is used because the plasma proteins are not in a true solution but rather in a colloidal suspension.)
Recall that a low concentration of plasma proteins is normally found in the interstitial fluid. The oncotic pressure created in the interstitial fluid by these proteins is normally only about 1 mm Hg. On balance, the osmotic effect of the capillary fluid is much greater than the osmotic effect of the interstitial fluid, so water would move through osmosis from the interstitial fluid into the capillaries. The movement of water in this direction is called reabsorption. The movement of water in the opposite direction, from the capillary plasma into the interstitial fluid, is called filtration.
The net oncotic pressure difference favors reabsorption. Net oncotic pressure difference is calculated by subtracting the oncotic pressure of interstitial fluid from the oncotic pressure of capillary blood (e.g., 25 mm Hg − 1 mm Hg = 24 mm Hg).
In addition to being affected by diffusional (osmotic) forces, water responds to hydrostatic pressure differences across the capillary wall. Hydrostatic pressure differences cause water to move by bulk flow; in this case the bulk flow occurs through the capillary pores. The hydrostatic pressure within the capillaries (capillary blood pressure) is higher at the arteriolar end of capillaries than at the venous end (see Figure 22-1). However, a representative average capillary hydrostatic pressure would be about 18 mm Hg. Interstitial fluid hydrostatic pressure is normally about −7 mm Hg. (The negative sign simply means that interstitial fluid pressure is less, but only slightly less, than atmospheric pressure.) The negative interstitial fluid pressure (−7 mm Hg) together with the positive capillary hydrostatic pressure (18 mm Hg) creates a hydrostatic pressure difference of 25 mm Hg across the wall of a typical capillary. This hydrostatic pressure difference tends to force water out of the capillaries and into the interstitial spaces; that is, the net hydrostatic pressure difference favors filtration.
The net hydrostatic pressure difference (which favors filtration) almost balances the net oncotic pressure difference (which favors reabsorption). However, the balance is rarely perfect. Usually, the hydrostatic pressure difference slightly exceeds the oncotic pressure difference, so there is a small, net filtration of water out of the capillaries. This water would simply accumulate in the interstitial spaces and cause swelling there if not for the lymph vessels, which collect excess interstitial fluid and return it to the bloodstream through the subclavian veins (Figure 23-4).
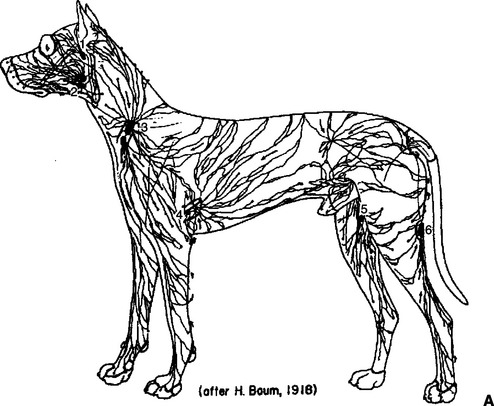
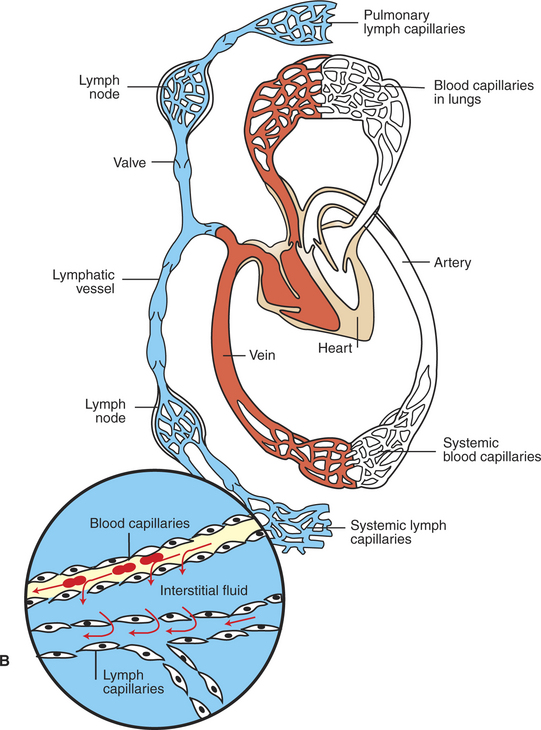
FIGURE 23-4 Anatomical (A) and schematic (B) overviews of the lymphatic system. The lymphatic vessels collect excess interstitial fluid from tissues throughout the body (including the lungs) and carry it to the subclavian veins, where the lymph reenters the bloodstream. Lymph moves through lymph vessels via bulk flow. The driving force for this flow is interstitial fluid hydrostatic pressure minus subclavian vein pressure. Lymph flow is also promoted by the massaging action exerted on lymph vessels by contraction and relaxation of skeletal muscles and (in the lungs) by the pressure variations accompanying inspiration and expiration. The lymph vessels contain one-way valves, which prevent the backflow of lymph. Thus, massaging actions propel lymph in one direction only: toward the subclavian vein. In addition, some lymph vessels have smooth muscle in their walls, and the alternating contraction and relaxation of this smooth muscle also propels lymph flow toward the subclavian veins. The numbers in A identify the major lymph nodes. The magnified inset in B depicts the typical, net filtration of fluid out of a blood capillary and into the interstitial space. This excess interstitial fluid is collected and carried away by the lymph capillaries. Three red blood cells are depicted in the blood capillary. Plasma is indicated in yellow, interstitial fluid and lymph in blue.
A from Getty R: Sisson and Grossman’s the anatomy of the domestic animal, vol 2, Philadelphia, 1975, Saunders.
The hydrostatic pressures in the capillaries and in the interstitial fluid are, by convention, always measured relative to atmospheric pressure. Thus, to say that interstitial pressure is normally “negative” does not imply that a vacuum exists but only that the interstitial pressure is slightly below atmospheric pressure. If the interstitial spaces of the body were always pressurized more than atmospheric pressure, all parts of the body would bulge outward. The subatmospheric interstitial fluid pressure probably accounts for the fact that the skin normally stays snug against the underlying tissue and that some body surfaces normally have a concave shape (e.g., axillary space, orbits of the eyes).
The Starling Equation Quantifies the Interaction of Oncotic and Hydrostatic Forces Acting on Water
The following equation expresses mathematically the interaction between osmotic pressures and hydrostatic pressures in determination of the net force (net pressure) acting on water. Nominal values for each pressure are also listed:
where Pc is capillary hydrostatic pressure, Pi is interstitial fluid hydrostatic pressure, πc is capillary plasma oncotic pressure, and πi is interstitial fluid oncotic pressure. Nominal values for systemic capillaries are as follows:
The solution of this equation, with nominal values inserted for each term, follows:
A positive net pressure favors filtration (a negative net pressure would indicate that reabsorption is favored). The small magnitude of the net pressure (1 mm Hg) indicates that the hydrostatic and osmotic forces that affect water are almost in balance (i.e., there is only a slight tendency for filtration). The quantitative analysis of how oncotic and hydrostatic pressures affect water movement across capillary walls was first derived by Ernest Henry Starling (the same scientist for whom Starling’s law of the heart is named). Therefore the oncotic and hydrostatic pressures that act on water are often called Starling forces. Furthermore, the tendency for the net oncotic effect to be closely balanced by the net hydrostatic effect is often referred to as the balance of Starling forces. Starling realized that the actual rate of water movement across capillary walls is affected both by the magnitude of the imbalance between hydrostatic and oncotic forces and by the permeability of the capillary wall to water. These ideas are expressed in the following equation, which indicates that the movement of water is equal to the permeability of the capillary wall (given as the filtration coefficient Kf) multiplied by the net difference between the hydrostatic and oncotic pressures:
Examination of this equation reveals that the tendency for the filtration of water out of capillaries can be enhanced by (1) increasing the hydrostatic difference between capillary blood and interstitial fluid, (2) decreasing the osmotic tendency for water to be reabsorbed, or (3) increasing the permeability of the capillary to water (i.e., increasing the filtration coefficient).
Several Common Physiological Changes Alter the Normal Balance of Starling Forces and Increase the Filtration of Water Out of Capillaries
An increase in capillary hydrostatic pressure (Pc) favors the greater filtration of water. Capillary hydrostatic pressure can be increased by an increase in arterial blood pressure or by a decrease in arteriolar resistance. An increase in arterial pressure causes more pressure to be transmitted down through the arterioles and into the capillaries. Likewise, a decrease in arteriolar resistance (e.g., a dilation of the arterioles) allows a greater portion of the arterial pressure to be transmitted into the capillaries. Capillary hydrostatic pressure can also be increased by a “backing up” (“damming up”) of venous blood. For example, an increase in central venous pressure causes blood to accumulate in the capillaries and raises capillary pressure. An obstruction to venous outflow (e.g., too tight a dressing on a limb) also causes blood to back up in the capillaries, which increases capillary hydrostatic pressure.
Although several factors typically affect capillary hydrostatic pressure, the only important determinant of interstitial fluid hydrostatic pressure is the volume of fluid present in the interstitial space. An accumulation of interstitial fluid increases interstitial hydrostatic pressure. Removal of interstitial fluid decreases the pressure. As stated earlier, interstitial fluid hydrostatic pressure is usually slightly subatmospheric (e.g., −7 mm Hg). When interstitial fluid hydrostatic pressure rises above atmospheric pressure, the accumulation of interstitial fluid becomes clinically noticeable as a swelling, or edema.
The net oncotic pressure depends on the concentrations of proteins in the capillary plasma and in the interstitial fluid. The normal protein concentration in plasma is 7 g/dL, which results in a plasma oncotic pressure of 25 mm Hg. Any alteration in the concentration of proteins in the capillary plasma alters the plasma oncotic pressure.
Similarly, changes in the interstitial protein concentration alter interstitial fluid oncotic pressure. Under normal circumstances, protein molecules are slightly too large to pass through the capillary pores or clefts. The main route for the delivery of plasma proteins into the interstitial fluid is through the three-step process of transcytosis. The first step is pinocytosis (a form of endocytosis), which involves the invagination of the capillary endothelial cell membrane to form an intracellular vesicle that contains plasma, including plasma proteins. Second, some of these vesicles cross the capillary endothelial cell from the side facing the bloodstream to the side facing the interstitial fluid. In the third step, some of these vesicles fuse with the outer membrane of the capillary endothelial cells; the vesicles discharge their contents into the interstitial space. This third step is called exocytosis. An increase in transcytotic activity increases the delivery of plasma proteins into the interstitial space and therefore increases interstitial fluid oncotic pressure. In addition, abnormal circumstances (e.g., tissue inflammation) can cause the capillary pores to open wide enough that plasma proteins can pass through.
Plasma proteins are removed from the interstitial space through lymph flow. The clefts or pores in the lymphatic capillaries are large enough to accommodate the passage of plasma protein molecules (in contrast to the smaller clefts or pores in blood capillaries). Therefore, when excess interstitial fluid flows into lymph capillaries, it carries with it any plasma proteins that are present in the interstitial fluid. The lymphatic fluid, containing these plasma proteins, flows to the thorax, where the fluid reenters the bloodstream at the subclavian veins.
The role of lymphatic flow in counteracting the accumulation of excessive interstitial fluid is especially important in the lungs. Lung capillaries are more permeable to plasma proteins than are most capillaries in the systemic circulation. As a result, the oncotic pressure of interstitial fluid in the lungs is normally about 18 mm Hg. Capillary hydrostatic pressure in the lungs is generally about 12 mm Hg. (This value is lower than the capillary hydrostatic pressure for systemic capillaries because pulmonary arterial pressure is so much lower than systemic arterial pressure.) Interstitial hydrostatic pressure in the lungs is generally about −4 mm Hg (the same as intrapleural pressure). Summation of these Starling forces for lung capillaries yields the following:
A net pressure of 9 mm Hg indicates that there is a substantial driving force for filtration of fluid out of the capillaries and into the lung interstitial spaces. The lung interstitial spaces would fill rapidly with water, and pulmonary edema would develop, were it not for the well-developed system of lymph vessels in the lungs. These vessels continuously remove interstitial fluid and prevent its excessive accumulation.
Edema Is a Clinically Noticeable Excess of Interstitial Fluid
Edema is a common clinical problem. Edema results either from excessive filtration of fluid out of capillaries or from depressed lymphatic function. One common cause is increased venous pressure. Increased venous pressure can result from the application of a too-tight dressing on the extremity of an animal. The resulting constriction of the veins impedes the outflow of venous blood from the limb. Blood backs up in the limb veins, which increases venous pressure. Blood then backs up in the capillaries and increases capillary hydrostatic pressure. As shown in Figure 23-5, the increase in capillary hydrostatic pressure leads to an excessive filtration of capillary fluid into the interstitial space. When this accumulation of fluids becomes clinically noticeable, the patient is said to exhibit edema.
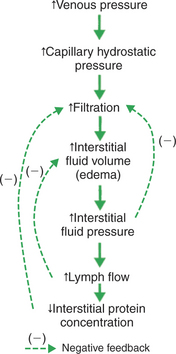
FIGURE 23-5 Increase in venous pressure leads to increase in interstitial fluid volume (edema). The dashed lines (negative feedback) indicate the counteracting effects of the three safety factors against edema. First, an increase in interstitial fluid hydrostatic pressure reduces the rate of filtration back toward normal. Second, an increase in lymph flow reduces interstitial fluid volume back toward normal. Third, a decrease in interstitial fluid protein concentration reduces the rate of filtration back toward normal.
Three factors (safety factors) limit the degree of edema. All three safety factors depend on an increased interstitial fluid volume leading to an increase in interstitial fluid hydrostatic pressure. The first safety factor is that the increased interstitial fluid pressure acts directly to oppose or limit filtration. Interstitial fluid pressure does not need to rise above capillary hydrostatic pressure to limit edema. Any increase in interstitial fluid pressure (e.g., from a normal value of −7 to +2 mm Hg) helps to change the net balance of the Starling forces in the direction of reducing excessive filtration.
The second safety factor against edema is that increased interstitial fluid pressure promotes lymph flow. Lymph flow removes edema fluid from the tissue and therefore helps to limit the degree of edema.
The third safety factor is an indirect consequence of increased lymph flow. Recall that interstitial fluid normally has a small amount of plasma protein, usually the result of transcytosis. This protein exerts a small but significant oncotic pressure that favors filtration. As lymph carries away interstitial fluid under these circumstances, the proteins originally present in the interstitial fluid are carried away by the lymph, and fluid relatively free of proteins is being added to the interstitial space by the increased filtration. Therefore the interstitial protein concentration and interstitial fluid oncotic pressures decrease, which helps reduce filtration.
To summarize, venous obstruction causes capillary hydrostatic pressure to increase, which increases filtration. Edema develops. Three safety factors then come into play to limit the degree of edema. A steady-state degree of edema is eventually reached, in which interstitial fluid is removed by lymph vessels as fast as it is filtered.
Another common clinical situation that leads to an increased venous pressure (and therefore edema) is heart failure. If the right ventricle fails (i.e., if right ventricular contractility decreases), blood backs up or pools in the right atrium and venae cavae. This increase in venous and atrial pressures is beneficial in one respect: it increases preload, which helps promote diastolic filling of the failing ventricle. An increased diastolic ventricular volume helps restore the stroke volume of the failing right ventricle back toward its normal level (recall Starling’s law of the heart). Although the increased venous pressure is beneficial in terms of restoring the stroke volume toward normal, the increased venous pressure often leads to excessive capillary filtration and edema in the systemic organs. Systemic edema therefore is a common complication of right-sided heart failure. The edema is often most noticeable in the dependent regions of the body, such as the lower extremities, particularly if the patient remains standing for a long time. Systemic edema also is often noted in the abdomen. When edema develops in the abdominal organs, excess interstitial fluid tends to ooze out and accumulate in the peritoneal space. Excessive fluid in the peritoneum is called ascites. Marked ascites is common in patients with right-sided heart failure.
Failure of the left ventricle leads to increased left atrial pressure and increased pulmonary venous pressure. The result is an increase in capillary filtration in the lungs, which leads to an increase in the amount of interstitial fluid in the lung tissue, or pulmonary edema. In extreme cases, some of the excess interstitial fluid oozes into the alveolar and bronchial air spaces of the lungs, and frank fluid is found in the airways. Such a patient typically coughs up a frothy fluid.
A decreased plasma protein concentration is another common cause of edema (Figure 23-6). One common cause of decreased plasma protein concentration is a decrease in the rate of plasma protein production by the liver. This occurs in malnutrition and leads to the clinical syndrome of kwashiorkor. Victims of kwashiorkor typically look emaciated, except that the abdomen is grossly distended by ascites (edema fluid in the peritoneum). Another cause of abnormally low plasma protein concentration is an increase in the rate of loss of proteins from the body. Protein loss occurs in kidney disease. For example, in nephrotic syndrome, the kidney glomerular capillaries become permeable to plasma proteins. Plasma proteins leave the bloodstream and enter the urinary tubules (nephrons) of the kidney. A chronic loss of proteins in the urine reduces the plasma protein concentration. Therefore the presence of substantial amounts of plasma protein in the urine is an alarming clinical sign.
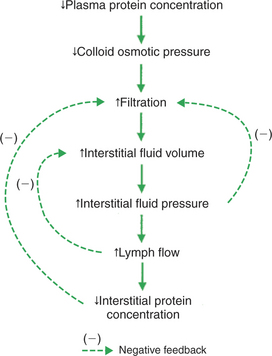
FIGURE 23-6 Decrease in plasma protein concentration leads to edema, but three safety factors limit the degree of the edema, as shown in Figure 23-5.
Severe burns are another common cause of the loss of plasma proteins from the body. The capillaries of burned skin become permeable to both fluid and proteins. Substantial amounts of plasma can leave the body through these damaged capillaries. The presence of plasma proteins in the fluid weeping from a burn site accounts for the typical yellow color of that fluid. If the water and electrolytes lost through burns are replaced through the intravenous administration of saline or lactated Ringer’s solution or through the ingestion of salt and water, and if the plasma proteins are not replaced, the plasma protein concentration decreases.
Whether it results from decreased production or increased loss, a decrease in plasma protein concentration leads to a decrease in plasma colloid osmotic pressure. This alters the balance of the Starling forces in a direction that favors excessive filtration of fluid from the capillaries (see Figure 23-6). Interstitial fluid accumulates and edema is noticed. However, the same three safety factors that limit edema in the case of increased venous pressure (see Figure 23-5) also operate in the case of decreased plasma protein concentration. The degree of edema is limited by (1) an increased interstitial fluid pressure, (2) an increased lymph flow, and (3) a decreased interstitial protein concentration.
Another cause of edema is lymphatic obstruction. Clinically, this situation is called lymphedema. Inflammatory diseases and cancers that produce obstruction of the lymphatic vessels cause lymphedema. Also, in certain parasitic diseases, microfilaria lodge in the lymph nodes and obstruct lymph flow. Filarial parasites cause the pronounced edema seen in cases of elephantiasis. Lymphedema also occurs as a secondary consequence of surgical procedures that damage lymph nodes. A common example of this in human medicine is the edema of the arm that follows radical mastectomy. The removal of axillary lymph nodes during radical mastectomy creates scar tissue that impairs lymphatic drainage from the arm.
Figure 23-7 traces the causes of edema after lymphatic obstruction and shows why lymphedema is clinically so troublesome. Lymphatic obstruction decreases lymph flow. Interstitial fluid accumulates instead of being removed, and edema results. The accumulation of edema fluid raises interstitial fluid pressure, which acts as a safety factor to limit the excess capillary filtration. However, the second and third safety factors discussed earlier are absent in the case of lymphedema because these factors depend on an increase in lymph flow. In lymphedema a decreased lymph flow is the causative problem, so there cannot be an increased lymph flow (second safety factor) to compensate for the edema. An additional complicating factor with lymphatic obstruction is that interstitial proteins accumulate instead of being carried away by the lymph. Therefore the third safety factor that protects against edema (decreased interstitial fluid oncotic pressure) is also compromised in lymphedema.
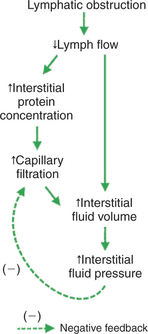
FIGURE 23-7 Lymphatic obstruction leads to edema. Lymphedema is clinically troublesome because only one of the normal three safety factors is operative to limit the degree of edema.
Another cause of edema is physical injury or an allergic reaction to antigen challenges. Physical trauma, such as a scratch or a cut on the skin, results in a localized bump or swelling. A similar swelling is observed when the skin reacts to an irritating agent or antigen challenge. An allergic swelling can also occur in bronchial tissue during an asthmatic reaction. The edema of asthma can be life threatening because it limits airflow to the lungs. As shown in Figure 23-8, an injury or antigen challenge leads to the release of the chemical histamine from mast cells in the affected tissue. Histamine has two effects that cause edema. First, histamine increases the permeability of capillaries to proteins. As proteins leave the bloodstream and accumulate in the interstitial space of the damaged tissue, they increase the interstitial fluid oncotic pressure, which promotes filtration of fluid. Second, histamine promotes filtration by relaxing arteriolar smooth muscle. The arterioles dilate, and the resulting decrease in arteriolar resistance allows more of the arterial blood pressure to impinge on the capillaries. This leads to an increase in the capillary hydrostatic pressure, which promotes filtration. Although histamine promotes excess filtration and edema through two mechanisms, all three safety factors that protect against edema are intact and act to limit the degree of edema.
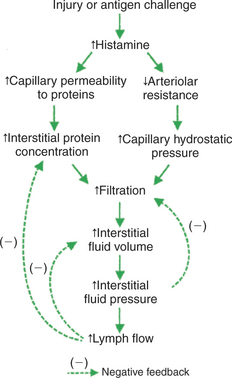
FIGURE 23-8 Histamine mediates the changes that lead to edema in response to a physical injury or an antigen challenge. The normal three safety factors against edema are intact and help to limit the degree of edema. Treatment with an antihistamine (a drug that blocks histamine receptors on arterioles and capillaries) also helps to reduce edema in these cases.
Other situations also cause edema, but the examples discussed here cover some of the most common causes of clinical edema. These examples also reinforce an understanding of the interplay of the osmotic and hydrostatic forces that act on water to govern its filtration out of capillaries or its reabsorption into capillaries.
CLINICAL CORRELATIONS
Acute Protein-Losing Enteropathy in a Horse
History.
You are called to a home a few miles from your clinic by parents who are concerned about their daughter’s 4-year-old quarterhorse mare. They report that the horse is listless and has had diarrhea for 2 days.
Clinical Examination.
You arrive at the client’s home to find that the horse is stabled in a small barn with no access to pasture. Poor-quality grass hay is stacked in the barn. On physical examination, you find the horse to be somewhat emaciated, with dry mucous membranes, a foul-smelling diarrhea, and a fast heart rate (tachycardia). When you pinch up a section of the horse’s skin, it falls back to the normal position slowly, which indicates dehydration.
You take a blood sample and then begin an intravenous administration of polyionic fluid (lactated Ringer’s solution). You tell the clients that you will return later. Analysis of the blood sample indicates a hematocrit of 55% (normal range for the horse, 35%-45%) and a plasma protein concentration of 4.5 g/dL (normal range, 5.9-7.8 g/dL). You become concerned that the administration of fluids, without replacement of plasma proteins, will exacerbate the horse’s hypoproteinemia, so you arrange to obtain plasma from a donor horse. You return to examine the sick horse and find it is still listless. Edema is now evident along the ventral abdomen and in the limbs.
Comment.
Acute enteropathy (intestinal disorder) often causes diarrhea. The loss of water and solutes leads to dehydration. Before treatment, blood volume and interstitial fluid volume are both reduced. The hematocrit (fraction of cells in blood) is typically elevated because fluid is removed from the bloodstream but blood cells are not. In some forms of enteropathy (called protein-losing enteropathy) the capillaries in the intestine become leaky to plasma proteins. Albumin, in particular, moves from the bloodstream into the intestinal lumen and is eliminated in the feces.
This horse has a severe shortage of plasma proteins. The shortage of plasma proteins probably resulted from a combination of poor nutrition (which depresses the production of plasma proteins by the liver) and the protein-losing enteropathy. The deficit of plasma proteins in this horse is even more severe than might be suspected on the basis of the plasma protein concentration of 4.5 g/dL, because this value is the net result of two opposing processes. The loss of protein in the diarrhea lowered the plasma protein concentration, but the loss of water (dehydration) decreased plasma volume and therefore increased the plasma protein concentration.
The administration of intravenous fluids adds water and electrolytes to the circulating blood volume, but the plasma proteins remaining in the bloodstream are further diluted. As a result, plasma oncotic pressure decreases even further, and this leads to excess filtration of water out of capillaries and into the interstitial space. The result is edema, especially in the dependent regions of the body (ventral abdomen and legs). Restoration of a normal plasma protein concentration would reverse the edema.
Treatment.
The enteropathy in cases such as this one is often self-limiting. Therefore the aim of treatment should be to remedy the dehydration, the electrolyte loss, and the plasma protein deficit. Intravenous administration of both polyionic fluids and plasma is usually effective. Better nutrition, regular deworming, and improved stable management would be important steps for long-term health.
Berne RM, Levy MN. Cardiovascular physiology, ed 8. St Louis: Mosby, 2001.
Boulpaep EL. The microcirculation. In: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. Philadelphia: Saunders, 2005.
Cunningham SL. The physiology of body fluids. Patton HD, Fuchs AF, Hille B, et al, eds. Textbook of physiology, vol 2. Philadelphia: Saunders, 1989.
Eades SC. Diseases affecting plasma proteins. Colahan PT, Merritt AM, Moore JN, et al, eds. Equine medicine and surgery, ed 5, vol 1. St Louis: Mosby-Year Book, 1999.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Raffe MR, Roberts J. Edema. Ettinger SJ, Feldman EC. Textbook of veterinary internal medicine: diseases of the dog and cat, ed 6, St Louis: Saunders, 2005.
Renkin EM. Microcirculation and exchange. Patton HD, Fuchs AF, Hille B, et al, eds. Textbook of physiology, vol 2. Philadelphia: Saunders, 1989.
Taylor AE, Mortillaro NA. The pathophysiology of the microcirculation. Boca Raton, Fla: CRC Press, 1994.
Taylor FGR. Differential diagnosis of dependent edema. In: Robinson NE, ed. Current therapy in equine medicine 4. Philadelphia: Saunders, 1997.
Ware WA, Bonagura JD. Pulmonary edema. Fox RR, Sisson D, Moise NS. Textbook of canine and feline cardiology, ed 2, Philadelphia: Saunders, 1999.
PRACTICE QUESTIONS
1. Which of the following will not cause pulmonary edema?
2. A patient with a form of protein-losing kidney disease has a plasma colloid osmotic pressure of 10 mm Hg. The patient has edema but is not getting any worse. Blood pressure and heart rate are normal. Which of the following is probably preventing further edema?
3. The following parameters were measured in the microcirculation of a skeletal muscle: Pc (capillary hydrostatic pressure), 34 mm Hg; Pi (interstitial fluid hydrostatic pressure), 10 mm Hg; πc (capillary plasma oncotic pressure), 24 mm Hg; and πi (interstitial fluid oncotic pressure), 1 mm Hg. Which of the following is true?
4. The rate of diffusion of glucose molecules from capillary blood to interstitial fluid is most directly affected by the:
5. During a 30-minute hemorrhage, a horse loses a substantial volume of blood. The horse’s mean arterial pressure decreases from 90 to 75 mm Hg, and the heart rate increases from 40 to 90 beats per minute. The skin becomes cold and the mucous membranes become pale, suggesting marked vasoconstriction. Because hemorrhage involves the loss of whole blood (both plasma and cells), you might expect that, soon after such a hemorrhage, the horse’s remaining blood would still have a normal composition. However, you take a blood sample and discover that the hematocrit is abnormally low (only 28%). Which of the following would most likely account for the decrease in hematocrit observed after the hemorrhage?