Chapter 25 Neural and Hormonal Control of Blood Pressure and Blood Volume
1. Neurohumoral mechanisms regulate blood pressure and blood volume to ensure adequate blood flow for all body organs.
2. The autonomic nervous system affects the cardiovascular system through the release of epinephrine, norepinephrine, and acetylcholine.
3. The arterial baroreceptor reflex regulates arterial blood pressure.
4. The atrial volume receptor reflex regulates blood volume and helps to stabilize blood pressure.
5. The cardiovascular state of conscious subjects is determined by an ongoing and ever-changing mixture of reflex effects and psychogenic responses.
Neurohumoral Mechanisms Regulate Blood Pressure and Blood Volume to Ensure Adequate Blood Flow for All Body Organs
The influences of the nervous system and hormones on the cardiovascular system are referred to collectively as the neurohumoral mechanisms of cardiovascular control. The neurohumoral mechanisms are also called extrinsic control mechanisms because they act on organs from the outside. As described in Chapter 24, the mechanisms of cardiovascular control that act locally, within individual tissues, are referred to as intrinsic control mechanisms. The local, or intrinsic, mechanisms predominate over extrinsic mechanisms in the control of blood flow to the “critical” organs, which include the heart (i.e., coronary circulation), brain, and working (exercising) skeletal muscle. In contrast, neurohumoral, or extrinsic, control mechanisms predominate over the intrinsic mechanisms in the control of blood flow to the “noncritical” organs, which include the kidneys, the splanchnic organs, and resting skeletal muscle. The noncritical organs are those that can withstand temporary reductions in blood flow (and metabolism) to make extra blood flow available for the critical organs, whose optimal function may be necessary for survival in a “fight or flight” situation.
Neurohumoral mechanisms also control the heart rate and cardiac contractility. This allows cardiac output to be adjusted to provide adequate blood flow for all the systemic organs, or at least for the critical organs. An important distinction is that cardiac muscle is under neurohumoral control, whereas the coronary blood vessels are primarily under local control. When neurohumoral mechanisms increase the heart rate and cardiac contractility, the cardiac metabolic rate also increases. The increased metabolic rate works through local metabolic control mechanisms to dilate coronary arterioles, which increases coronary blood flow.
To appreciate the importance of neurohumoral control mechanisms, consider what would happen in their absence. For example, what would occur during exercise if all the body organs simply relied on local control mechanisms to adjust their blood flow? At the onset of exercise, metabolic control mechanisms would cause vasodilation in the exercising skeletal muscles. Vascular resistance would decrease in the exercising muscles, and the blood flow through the muscles would increase. However, the decrease in resistance in skeletal muscles would also lower the total peripheral resistance (TPR). As a consequence, arterial blood pressure would decrease. This would decrease the perfusion pressure for all the systemic organs, and blood flow would therefore decrease below normal levels in the brain, kidneys, splanchnic organs, and so forth.
The decreased blood flow in these organs would trigger autoregulatory responses, and these organs would vasodilate. However, the vasodilation would lower the TPR even further, which would reduce arterial pressure even more. This in turn would limit the increase in skeletal muscle blood flow. The end result would be some increase in blood flow in the exercising muscle and decreased blood flow elsewhere, but none of the organs (including skeletal muscle) would be receiving sufficient blood flow to meet their metabolic needs. Arterial pressure would be dangerously low, and the animal would exhibit profound exercise intolerance.
Neurohumoral control mechanisms allow an animal to avoid these complications. First, cardiac output is increased sufficiently to meet the increased need for blood flow in the exercising muscle (and in the coronary circulation) while keeping all the other organs supplied with a normal blood flow. If cardiac output cannot be increased sufficiently to meet all these needs, the control mechanisms take the additional step of temporarily reducing blood flow in the noncritical organs and making this extra flow available to the critical organs.
How do the neurohumoral control systems “know” when cardiac output is sufficiently high to meet the needs of all the organs and when to initiate vasoconstriction in the noncritical organs? An indirect “strategy” is used: cardiac output is increased enough to keep arterial pressure at a normal level. As long as arterial pressure is maintained at normal level, local metabolic control mechanisms can successfully match blood flow to metabolic need in each individual organ. If cardiac output cannot be sufficiently increased to keep arterial pressure from falling, vasoconstriction of the noncritical organs is initiated. Thus, neurohumoral control mechanisms deprive noncritical organs of an ideal level of blood flow if more flow is needed by the critical organs than can be supplied by the heart.
There are many important neurohumoral control mechanisms, but four are emphasized in the following presentation. Two of them are cardiovascular reflexes. The arterial baroreceptor reflex works to keep arterial pressure at its “set point” through the continual adjustment of cardiac output and vascular resistance (in the noncritical organs). The atrial volume receptor reflex works in conjunction with the arterial baroreceptor reflex to regulate arterial pressure by adjusting cardiac preload. The other two neurohumoral mechanisms described in this chapter exemplify psychogenic influences on the cardiovascular system: the defense-alarm reaction and vasovagal syncope (the “playing dead” reaction).
The Autonomic Nervous System Affects the Cardiovascular System Through the Release of Epinephrine, Norepinephrine, and Acetylcholine
The autonomic nervous system is the “neuro” arm of neurohumoral control. Sympathetic and parasympathetic neurons influence the cardiovascular system through the release of the neurotransmitters norepinephrine and acetylcholine. In addition, sympathetic nerves affect the cardiovascular system by stimulating the release of epinephrine and norepinephrine from the adrenal medulla. The adrenal secretions enter the bloodstream as hormones and circulate throughout the body. Chapter 13 contains additional, basic information about the autonomic nervous system.
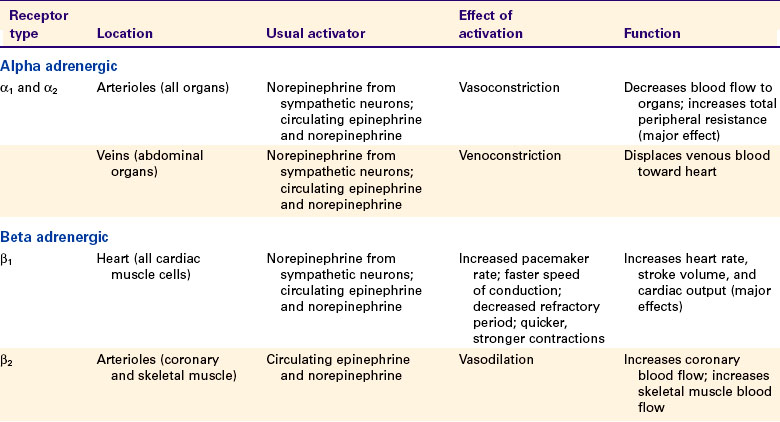
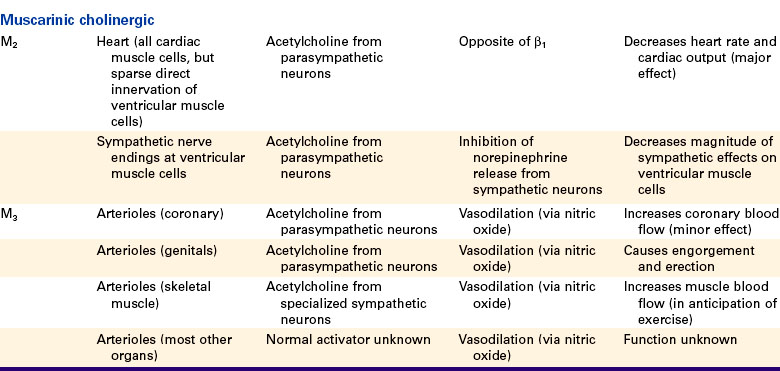
Whether acting as neurotransmitters or as hormones, epinephrine, norepinephrine, and acetylcholine exert their cardiovascular effects by activating membrane receptors on the cardiac muscle cells or on the endothelial cells or the smooth muscle cells of blood vessels. The receptors activated by epinephrine and norepinephrine are called adrenergic receptors (named after the adrenal gland). There are two major subtypes, alpha and beta: α-adrenergic receptors and β-adrenergic receptors. Each of these is subdivided into α1, α2, β1, and β2, and each of the four has important cardiovascular roles.
Acetylcholine activates cholinergic receptors. There are two major subtypes: muscarinic cholinergic receptors and nicotinic cholinergic receptors. The main cardiovascular effects of acetylcholine are mediated through muscarinic cholinergic receptors. Of five known types of muscarinic receptors, the M2 and M3 receptors have the greatest cardiovascular importance.
Table 25-1 summarizes the main cardiovascular consequences of the activation of adrenergic and cholinergic receptors. Alpha-adrenergic receptors (both α1 and α2) are located on the cell membranes of the smooth muscle cells of the arterioles in all organs of the body as well as on the smooth muscle cells of the abdominal veins. The α-adrenergic receptors on arterioles and abdominal veins are innervated by postganglionic sympathetic neurons. Activation of the α-adrenergic receptors leads to constriction of the arterioles or the veins.
Arteriolar vasoconstriction increases the resistance and decreases the blood flow through an organ. If one or more major body organs are vasoconstricted, the TPR increases. TPR (along with cardiac output) determines arterial blood pressure, so widespread α-adrenergic vasoconstriction in the body leads to an increase in arterial blood pressure. The increase in arterial pressure increases the driving force for blood flow in the nonvasoconstricted organs. In this way, the sympathetic nervous system can use vasoconstriction in some organs to direct more blood flow to other, nonvasoconstricted organs.
The major role of veins is to act as reservoirs for blood. Venoconstriction displaces venous blood toward the central circulation, which increases central venous pressure, ventricular preload, and stroke volume. Venoconstriction in the abdominal organs is particularly important in the control of central blood volume and therefore central venous pressure. Venoconstriction causes only a small increase in the resistance to blood flow through an organ because the veins, whether constricted or dilated, offer much less resistance to blood flow than do the arterioles.
Sympathetic control of the heart is exerted through the β1-adrenergic receptors, which are found on every cardiac muscle cell. These β-adrenergic receptors are activated by norepinephrine or epinephrine. Chapters 19 and 21 discuss the effects of activation of the cardiac β-adrenergic receptors. In brief, pacemaker rate increases, cell-to-cell conduction velocity increases, and refractory period decreases. In addition, contractility is increased, so the cardiac contractions are quicker and stronger. The overall effect is increased heart rate and stroke volume.
The β2-adrenergic receptors are found on the arterioles, particularly in the coronary circulation and in skeletal muscles. The activation of arteriolar β2-adrenergic receptors causes relaxation of the vascular smooth muscle and dilation of the arterioles. However, these β2-adrenergic receptors are not innervated by the sympathetic nervous system, so they are not activated directly by sympathetic nerves. Instead, they respond to circulating epinephrine and norepinephrine (released from the adrenal medulla). The adrenal medulla releases epinephrine and norepinephrine in situations that involve trauma, fear, or anxiety. Dilation of arterioles in the coronary circulation and in skeletal muscles is appropriate in such “fear, fight, or flight” situations because the dilation results in an anticipatory increase in blood flow to the heart and skeletal muscle. Appropriately for its role in emergency situations, β2-adrenergic vasodilation can overpower α-adrenergic vasoconstriction in the coronary circulation and in skeletal muscles.
Cholinergic muscarinic receptors of the M2 type are located in the cell membranes of cardiac muscle cells. The natural stimulus for the cardiac M2 receptors is acetylcholine, released from postganglionic parasympathetic neurons. Cells of the sinoatrial and atrioventricular nodes are densely innervated by postganglionic parasympathetic neurons. Atrial cells also receive strong parasympathetic innervation. In the sinoatrial node, atria, and atrioventricular node, the activation of M2 receptors has effects basically opposite to those of the activation of β1-adrenergic receptors. Parasympathetic activation powerfully slows the pacemaker rate, decreases the cell-to-cell conduction velocity, and increases the refractory period. By contrast, few ventricular muscle cells receive direct parasympathetic innervation. Therefore, parasympathetic activation has very minor, direct effects on cardiac contractility. However, parasympathetic activation can still profoundly decrease cardiac output by decreasing the heart rate. In addition, parasympathetic neurons do exert an interesting, indirect effect on ventricular muscle cells. Most parasympathetic neurons in the ventricles release their acetylcholine onto sympathetic neuron terminals, rather than directly onto ventricular muscle cells. This acetylcholine activates muscarinic cholinergic receptors on the sympathetic neuron terminals, which inhibits the release of norepinephrine from the terminals and thus weakens the effects of sympathetic activity on ventricular cells.
Cholinergic muscarinic receptors of the M3 type are found on the endothelial cells and on the smooth muscle cells of most arteries and arterioles. Activation of M3 receptors on smooth muscle cells causes them to contract (and the blood vessels to constrict). However, this vasoconstrictor effect is usually overridden by the vasodilatory effect of activating the M3 receptors on the vascular endothelial cells. In this strange arrangement, activation of M3 receptors on endothelial cells causes the synthesis of nitric oxide, which then diffuses out of the endothelial cells and into the nearby smooth muscle cells, where it causes vasodilation. The vasodilatory effect of stimulating the M3 receptors on endothelial cells is stronger than the vasoconstrictor effect of stimulating the M3 receptors on smooth muscle cells.
The M3 receptors on vascular endothelial cells are innervated in three tissues. In the coronary circulation, parasympathetic neurons innervate vascular M3 receptors and bring about vasodilation. This vasodilatory effect is minor, however, and the function of this innervation is unclear. In the external genital organs, parasympathetic neurons release both acetylcholine and nitric oxide. The acetylcholine acts on M3 receptors to stimulate the release of additional nitric oxide from endothelial cells. The nitric oxide causes vasodilation, engorgement of the organs with blood, and erection. The third tissue in which vascular M3 receptors are innervated is skeletal muscle. In some species (e.g., cats and dogs) but not in others (e.g., primates), the M3 receptors of skeletal muscle blood vessels are innervated by special postganglionic sympathetic neurons that release acetylcholine (rather than norepinephrine) as a neurotransmitter. These sympathetic cholinergic neurons appear to be activated specifically in anticipation of muscular exercise and during the “fear, fight, or flight” (defense-alarm) reaction. The resulting vasodilation increases blood flow through the skeletal muscle just before and during the initiation of exercise. Although primates do not have sympathetic cholinergic vasodilatory nerves, they can bring about an anticipatory vasodilation of skeletal muscle arterioles in another way, through activation of β-adrenergic receptors by circulating epinephrine and norepinephrine, as mentioned earlier.
To summarize, although arteries and arterioles throughout the body dilate when exposed to acetylcholine, only the arterioles of the heart, external genitalia, and (in some species) skeletal muscle are innervated by acetylcholine-releasing autonomic neurons. The functional significance of the M3 receptors on arterioles in other organs is unclear because no neurons (either sympathetic or parasympathetic) appear to innervate them, and neither acetylcholine nor any other muscarinic receptor agonist normally circulates in the bloodstream.
Of all the autonomic influences on the cardiovascular system just mentioned, three stand out as most important. The first is α1− and α2− adrenergic vasoconstriction in the arterioles of all body organs, which is brought about by the sympathetic nervous system. The second is β1-adrenergic excitation of cardiac muscle, which is brought about by the sympathetic nervous system and results in an increased heart rate and stroke volume. The third is the decrease in heart rate brought about by activation of cardiac M2 receptors.
The Arterial Baroreceptor Reflex Regulates Arterial Blood Pressure
Arterial blood pressure is monitored by pressure-sensitive nerve endings known as baroreceptors. The baroreceptors send afferent impulses to the central nervous system (CNS), which reflexively alters cardiac output and vascular resistance (in noncritical organs) to keep blood pressure at a set point. The reflex is called the arterial baroreceptor reflex.
The arterial baroreceptors are specialized nerve endings that are embedded in the walls of the carotid arteries and aortic arch (Figure 25-1). The baroreceptors are concentrated at the origin of each internal carotid artery in enlarged parts of the arteries called the carotid sinuses. Similar nerve endings are found in the wall of the aortic arch, especially at the origin of its major branches. These nerve endings are sensitive to stretch (distention) of the arterial wall. In effect, they sense arterial pressure because blood pressure is the natural force that distends these arteries. Therefore, these nerve endings are called baroreceptors (literally, “pressure sensors”), even though the actual physical factor being sensed is not pressure but rather stretch or distortion.
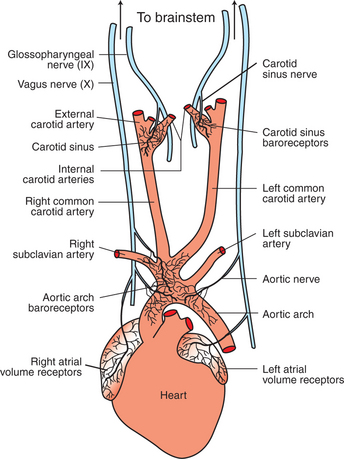
FIGURE 25-1 Arterial baroreceptors are located in the walls of the carotid sinuses and in the walls of the aortic arch and its major branches. The atrial volume receptors are located in the walls of the right and left atria. See text for a description of the neural pathways followed by the baroreceptor and volume receptor afferents.
With every systolic ejection from the heart, blood distends the aorta and arteries, including the carotid sinuses, which causes the baroreceptors to initiate neural impulses (action potentials). Figure 25-2 illustrates that the frequency of these impulses is proportional to the arterial blood pressure. The tracing on the top shows the pulsatile arterial pressure on three successive heartbeats. The mean level of arterial pressure is indicated by the dashed line. The tracings below depict the typical patterns of action potentials that would be seen in a baroreceptor afferent neuron for various levels of mean arterial pressure (MAP). When MAP is lower than normal (e.g., 50 mm Hg), there are only one or two action potentials with each heartbeat. These action potentials occur during the rapid upstroke of the pressure wave, because the baroreceptors are sensitive to the rate of change of pressure as well as to mean pressure. When MAP is at a higher level (e.g., 75 mm Hg), more action potentials are formed during each heartbeat, but the action potentials still tend to occur during the rapid pressure increase at the beginning of the cardiac ejection. The higher the MAP, the more action potentials are formed in each heartbeat. Thus the arterial baroreceptors signal increases in pressure by increasing their action potential frequency. Because the baroreceptors are active when arterial pressure is normal (MAP near 100 mm Hg), they can also signal a decrease in arterial pressure by decreasing their action potential frequency.
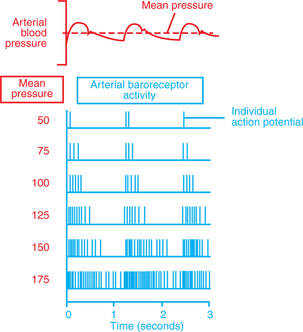
FIGURE 25-2 Each arterial pressure pulse causes action potentials to be generated in baroreceptor afferent neurons. The number of action potentials generated per heartbeat increases dramatically with increases in mean arterial pressure.
The afferent neurons from the aortic arch baroreceptors run in the vagus nerves (see Figure 25-1). In some species the aortic baroreceptor afferents form a distinct bundle within the vagal nerve sheath, called the aortic depressor nerve. The stretch receptors in the carotid sinuses have their afferents in the carotid sinus nerves (Hering’s nerves), which join into the glossopharyngeal (ninth cranial) nerve. By way of these afferent neurons, the brain receives beat-by-beat information about the level of arterial blood pressure.
Figure 25-3 summarizes the reflex consequences of a decrease in blood pressure, which decreases afferent baroreceptor activity. The brain responds to a decrease in the afferent activity from the baroreceptors by increasing sympathetic activity. In the heart, sympathetic activation results in increased stroke volume and heart rate, which increases cardiac output. The increase in cardiac output helps to restore blood pressure toward normal. The sympathetically driven increase in heart rate is augmented by a simultaneous reduction in parasympathetic activity to the sinoatrial node. Thus the baroreceptor reflex uses reciprocal changes in sympathetic and parasympathetic activity to control heart rate. Sympathetic activity is also increased to the arterioles of all organs, but particularly to the arterioles of noncritical organs (kidney, splanchnic organs, and resting skeletal muscle). Sympathetic activation causes vasoconstriction of these arterioles, which increases the resistance to blood flow through these organs and therefore increases TPR. The increase in peripheral resistance helps to restore arterial blood pressure back toward its normal level and directs blood flow to the critical organs.
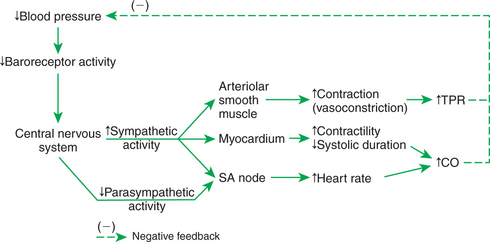
FIGURE 25-3 The arterial baroreceptor reflex responds to decreases in blood pressure (top left) by increasing cardiac output (CO), total peripheral resistance (TPR), or both (far right). These reflex effects offset the initial fall in blood pressure (dashed line). SA, Sinoatrial.
To understand fully the function of the baroreceptor reflex, it is important to recognize that the reflex does not reverse disturbances in blood pressure but only minimizes them. Also, it is important to distinguish between cause and effect when thinking about the baroreflex. For example, what causes blood pressure to decrease below normal is a decrease below normal in cardiac output, TPR, or both. There is no other way to lower blood pressure. When TPR falls below normal and causes blood pressure to decrease below normal, the compensatory response of the baroreceptor reflex is (1) to increase cardiac output above normal through increased sympathetic (and decreased parasympathetic) activation of the heart and (2) to minimize the decrease in peripheral resistance by initiating a sympathetic vasoconstriction in the noncritical organs. After compensation by the baroreceptor reflex, cardiac output is above normal. TPR is still below normal, but not as far below normal as in the uncompensated state. Blood pressure is still below normal, but not as far below normal as in the uncompensated state.
All the reflex responses just described for a decrease in arterial blood pressure occur in reverse in response to an increase in arterial blood pressure above its normal level. Thus the baroreceptor reflex acts to counteract and minimize both decreases and increases in blood pressure.
As a regulator of arterial blood pressure, the baroreflex is both rapid and powerful. It can initiate compensations for changes in blood pressure within 1 second. A hemorrhage that would decrease blood pressure by 40 to 50 mm Hg if there were no baroreflex decreases blood pressure by only 10 to 15 mm Hg in an animal with intact baroreflexes. The baroreflex also acts to maintain blood pressure close to normal during changes in posture or activity. In a dog without baroreflexes, changes in posture are accompanied by large, uncontrolled variations in blood pressure, as shown in Figure 25-4. By minimizing fluctuations in blood pressure, the baroreflex functions to ensure an adequate blood flow to the critical organs.
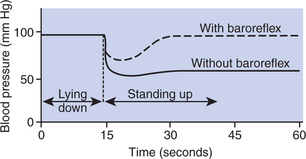
FIGURE 25-4 The baroreflex is essential for normal, moment-to-moment stability of blood pressure. Dogs in which baroreflexes are eliminated exhibit much larger swings in blood pressure in response to postural changes than do dogs with intact baroreflexes.
Although the baroreceptor reflex is essential for the moment-to-moment stability of blood pressure, it does not appear to be the major mechanism responsible for setting the long-term level of arterial blood pressure, because baroreceptors adapt slowly or reset to the prevailing level of arterial pressure. In other words, the baroreceptors come to accept whatever the prevailing blood pressure is as if it were the normal pressure. Baroreceptor resetting causes the baroreflex to “lose track of” normal blood pressure. For example, in an animal or a human who has been hypertensive for a few days or weeks, the baroreflex functions to regulate blood pressure at the elevated level rather than to restore blood pressure toward its normal level. Also, the baroreflex can become reset in a downward direction during a period of sustained hypotension. For example, in chronic heart failure, in which arterial pressure may be below normal for days or weeks, the baroreflex appears to regulate blood pressure at a depressed level rather than to push it back toward its normal level.
In summary, the baroreflex responds quickly and powerfully to counteract sudden changes in blood pressure, but it has little influence on the long-term level of blood pressure over days or weeks.
The Atrial Volume Receptor Reflex Regulates Blood Volume and Helps to Stabilize Blood Pressure
The atrial volume receptor reflex is initiated by specialized sensory nerve endings that are located in the walls of the left and right atria (see Figure 25-1). These nerve endings are activated by stretch, but they are called volume receptors because the volume of blood in each atrium determines how much the atrial wall is stretched. For example, a decrease in the total blood volume of an animal (e.g., hemorrhage) results in a decrease in the amount of blood in the major veins and in the atria. When atrial volume decreases, atrial pressure decreases, as does the stretch on the atrial walls. This decreases the frequency of action potentials generated in atrial stretch receptors. Conversely, increases in blood volume result in increased atrial stretch and an increased frequency of action potentials generated by the atrial stretch receptors. Therefore, these atrial stretch receptors are sensitive detectors of atrial blood volume and, indirectly, of total blood volume.
Figure 25-5 summarizes the reflex effects of a change in the activity of the atrial volume receptors. If blood volume decreases, the result is a decrease in the afferent activity from the atrial volume receptors. The CNS responds reflexively to this decreased afferent activity by increasing sympathetic efferent activity to the heart and systemic arterioles and decreasing parasympathetic efferent activity to the heart. In this respect, the atrial volume receptor reflex and the baroreceptor reflex exert synergistic effects; that is, through the atrial volume receptor reflex a decrease in blood volume leads to the same responses that are triggered by the baroreflex in response to a decrease in arterial blood pressure. In both cases the reflex responses include an increase in cardiac contractility, a decrease in systolic duration, and an increase in heart rate as well as arteriolar vasoconstriction in the noncritical organs. By initiating these responses, the atrial volume receptor reflex helps to combat the decrease in arterial blood pressure that would otherwise result from a decreased blood volume. In effect, the atrial volume receptor reflex augments the effectiveness of the baroreceptor reflex as a regulator of blood pressure.
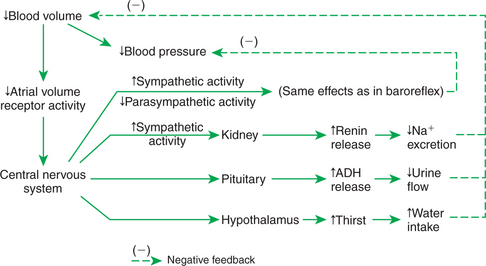
FIGURE 25-5 The atrial volume receptor reflex responds to a decrease in blood volume by decreasing sodium and water loss in the urine and by increasing oral water intake. The reflex also helps support blood pressure by increasing cardiac output and total peripheral resistance (similar to baroreflex). ADH, Antidiuretic hormone.
The volume receptor reflex also acts in three additional ways to help restore lost blood volume (Figure 25-5). First, the reflex acts through the hypothalamus to increase the sensation of thirst. If water is available, the animal drinks. This provides the fluid necessary to increase blood volume back toward normal. Second, the atrial volume receptor reflex acts through the hypothalamus and pituitary gland to increase the release of antidiuretic hormone (ADH). ADH is synthesized in hypothalamic neurons, which transport it to the posterior pituitary gland. From there, ADH is released into the bloodstream (see Chapter 33). ADH acts on the kidneys to decrease urine production. Another name for ADH is arginine vasopressin (AVP). The third effect of the atrial volume receptor reflex on blood volume is to increase the release of the hormone renin from the kidneys. Renin acts to increase the production of the hormone angiotensin II, which acts to increase production of the hormone aldosterone, which acts to decrease the amount of sodium excreted by the kidneys; that is, activation of the renin-angiotensin-aldosterone system causes the body to conserve available sodium.
The combination of decreased sodium excretion (by the actions of renin) and decreased urine flow (by the actions of ADH) results in the conservation of body fluid. The conservation of body fluid, combined with an increased water intake, eventually restores blood volume back toward normal.
Although not diagrammed in Figure 25-3, the arterial baroreceptor reflex also responds to decreases in arterial pressure by increasing thirst, ADH release, and renin release. An increase in arterial pressure above normal initiates the opposite effects. Thus the arterial baroreceptor reflex and the atrial volume receptor reflex are synergistic partners in the interrelated tasks of regulating arterial pressure and blood volume.
The Cardiovascular State of Conscious Subjects Is Determined by an Ongoing and Ever-Changing Mixture of Reflex Effects and Psychogenic Responses
The baroreceptor reflex and the atrial volume receptor reflex are only two of several important cardiovascular reflexes. They are primarily responsible for the regulation of blood pressure and blood volume, and they illustrate several properties common to all cardiovascular reflexes. First, these reflexes originate from changes detected by peripheral sensory receptors. Second, the reflexes occur at a subconscious level, through neural pathways that primarily involve cardiovascular centers in the brainstem and midbrain. Consequently, cardiovascular reflexes persist in unconscious and anesthetized subjects, although the strength and character of the reflexes are altered by anesthesia. Finally, the reflexes use sympathetic and parasympathetic neurons as well as hormonal and behavioral responses to bring about cardiovascular changes.
In conscious subjects, neurohumoral control of the cardiovascular system involves both cardiovascular reflexes and psychogenic effects. Psychogenic responses originate from conscious perceptions or emotional reactions. They are eliminated by unconsciousness or general anesthesia. They involve neural pathways of the midbrain and forebrain, including the limbic system and cerebral cortex. Psychogenic responses are often triggered by sensory stimuli. For example, the sights, sounds, and smells of a veterinary clinic may trigger perceptions and emotions that cause increases in heart rate and blood pressure in both animal patients and their human companions. Psychogenic responses can also occur without any obvious sensory triggers. For example, anxiety about a future event can increase heart rate and blood pressure, at least in humans. Cardiovascular reflexes and psychogenic reactions use the same sympathetic and parasympathetic neurons and some of the same hormonal responses to bring about cardiovascular changes.
Two important psychogenic responses are the defense-alarm reaction and vasovagal syncope (the “playing dead” reaction). The defense-alarm reaction (“fear, fight, or flight” response) is an emotional and behavioral response to a threatening situation, physical injury, or trauma. The cardiovascular component of this reaction involves increased sympathetic activity and decreased parasympathetic activity. Typically, the sympathetic activation is sufficiently strong to cause the release of epinephrine and norepinephrine from the adrenal medulla. The cardiovascular responses during a defense-alarm reaction therefore include an increased heart rate, increased stroke volume, vasoconstriction in noncritical organs (kidneys, splanchnic organs, resting skeletal muscle), vasoconstriction in skin, vasodilation in coronary vessels and in working skeletal muscle, and increased blood pressure. The cardiovascular responses during the defense reaction are enhanced by other circulating hormones, including ADH and angiotensin II. The resulting, elevated blood pressure helps to ensure adequate blood flow for the critical organs (exercising skeletal muscles, heart, and brain).
During a defense-alarm reaction, the baroreceptor reflex is reset by the CNS so that it regulates blood pressure at an elevated level rather than acting to oppose the increased pressure. This is analogous to resetting the cruise control on a car so that it regulates speed at an elevated level rather than acting to oppose an increased speed. Thus it is more accurate to say that the baroreceptor reflex regulates blood pressure at a variable set point (set by the CNS) than to say that the baroreflex regulates arterial pressure at any single “normal” pressure.
It is important to recognize that the defense-alarm reaction is simply the extreme form of a continuum of states of emotional arousal. Sleep is at the opposite end of this cardiovascular and emotional continuum. In quiet rest or sleep, sympathetic activity is minimal and parasympathetic activity is maximal. During a full-blown defense-alarm reaction, sympathetic activity is maximal and parasympathetic activity is minimal. Between these extremes lie all the levels of emotional arousal experienced by animals and humans, from moment to moment, during ordinary and extraordinary daily activities. Cardiovascular variables, such as heart rate and blood pressure, are sensitive to these changes in emotional state (Figure 25-6). For example, a large dog may normally have a heart rate of 70 beats/min while resting at home; it would be entirely normal for the same dog to have a heart rate of 120 beats/min while “resting” in a clinic, if the dog is apprehensive in that setting. Another important point for the clinician to remember is that emotional responses are subjective. Situations that severely agitate one animal may cause only a mild alerting response in another animal. The clinician must evaluate heart rate, blood pressure, and other cardiovascular signs with respect to the particular patient’s emotional state.
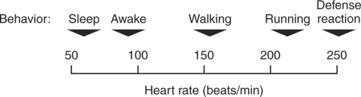
FIGURE 25-6 The defense-alarm reaction is simply the extreme on a continuum of emotional and physical arousal. The cardiovascular system (e.g., heart rate) responds sensitively to every change along this arousal scale.
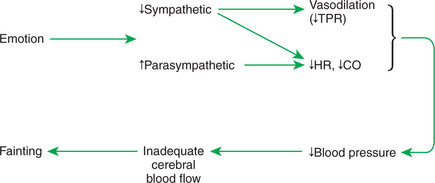
Vasovagal syncope is another psychogenic response that may be encountered in veterinary practice. This response is also called the “playing dead” reaction or “playing possum.” In response to certain threatening or emotional situations, some humans and animals experience a psychogenic decrease in blood pressure and may faint. In many ways, this response is the opposite of the defense-alarm reaction. As shown in Figure 25-7, vasovagal syncope involves a decrease in sympathetic activity and an increase in parasympathetic activity. These neural changes bring about a vasodilation in the noncritical organs and a decrease in TPR. Heart rate and cardiac output also decrease, so there is a large drop in arterial blood pressure. The expected compensatory reflex responses do not take place because emotional state appears to override the baroreceptor reflex in this case. If blood pressure falls so low that there is inadequate cerebral blood flow, the patient faints. The term vasovagal syncope denotes vaso dilation, vagal (parasympathetic) activation, and syncope (fainting). It is not clear why some animals respond to a threatening situation with a defense-alarm reaction, whereas others exhibit vasovagal syncope. Also unclear is the survival value of “playing dead,” although this response seems to have served opossums well over several million years.
CLINICAL CORRELATIONS
Intraoperative Hemorrhage
History.
Four hours after abdominal surgery for a splenic sarcoma, a 30-kg, 9-year-old male Labrador retriever is observed to be severely lethargic and recumbent. An abnormally large amount of blood had been lost during the surgical removal of the spleen because the dog had a hereditary blood-clotting defect (von Willebrand’s disease).
Clinical Examination.
The dog’s gums are pale, and his capillary refilling time is abnormally prolonged (3 seconds). His extremities are cool to the touch. The femoral pulse is rapid and weak. An electrocardiogram indicates sinus tachycardia at a rate of 185 beats/min. The hematocrit (packed cell volume) is 38%, and the plasma protein concentration is 5.6g/dL; both these values are below normal. A jugular catheter is inserted, and central venous pressure is measured and found to be −1 mm Hg (normal, 0 to +3 mm Hg). Despite the intravenous administration of 600mL of lactated Ringer’s solution during surgery, the dog has not produced any urine. About 100mL of blood-tinged fluid is removed from the abdomen by abdominocentesis.
Comment.
This case illustrates the clinical signs that are typical of hemorrhage. Most of the blood in a dog is in the systemic veins, so most of the blood missing after hemorrhage is missing from the veins. The result is an abnormally low central venous pressure, as observed in this dog. The decreased central venous pressure causes a decreased ventricular preload and a decreased ventricular end-diastolic volume. This leads to decreases in stroke volume (Starling’s law of the heart), cardiac output, and arterial blood pressure. Inadequate cardiac output and blood pressure lead to behavioral depression.
Neurohumoral compensation for the hemorrhage is initiated by the atrial volume receptor reflex and the arterial baroreceptor reflex. Heart rate is increased by the combination of increased sympathetic activation and decreased parasympathetic activation. The combination of high heart rate and low stroke volume accounts for the rapid but weak (low pulse pressure) femoral pulse. Sympathetic activity also causes vasoconstriction in the mucous membranes, resting skeletal muscle, splanchnic organs, and kidneys. The reduced blood flow in these tissues accounts for the pale gums, the slow refilling of capillaries, the cool limbs, and the lack of urine production by the kidneys. Urine formation by the kidneys is also being depressed by the combined hormonal effects of ADH and the renin-angiotensin-aldosterone system.
Hemorrhage per se does not reduce either the hematocrit or the plasma protein concentration, because whole blood is being lost. However, two factors caused the hematocrit and plasma protein concentration to decrease in this dog. First, the fluid administered intravenously during surgery (lactated Ringer’s) contained neither red blood cells nor plasma proteins, so the cells and proteins remaining in the bloodstream were diluted by the addition of the fluid. Second, the hemorrhage reduced not only venous and arterial pressures but also capillary hydrostatic pressure, which changed the balance of hydrostatic and oncotic forces (“Starling forces”) across the capillary walls in favor of reabsorption. The interstitial fluid that was reabsorbed into the bloodstream contained no red blood cells and almost no plasma proteins. This caused a further dilution of the cells and proteins in the blood.
Treatment.
Therapy for this dog involves measures to stop ongoing blood loss and to restore the lost blood volume. In this dog the hemorrhage is predominantly seepage from small intraabdominal vessels as a result of the coagulation defect. Transfusions of fresh blood or plasma, or concentrated preparations of clotting proteins, would promote clotting, limit subsequent hemorrhage, and expand the intravascular fluid volume. Additional crystalloid solutions (e.g., lactated Ringer’s) also can be infused into this dog because the hematocrit and plasma protein concentration are not dangerously low. If crystalloid solutions are administered, the hematocrit and plasma protein concentration should be monitored closely to avoid the hypoxia that results from too much dilution of the red blood cells, or the edema that results from too much dilution of the plasma proteins. Renal function should be monitored closely because the combination of hypoxia and reflex vasoconstriction can lead to ischemic damage, resulting in renal failure.
Boulpaep EL. Integrated control of the cardiovascular system. In: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. Philadelphia: Saunders, 2005.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Katz AM. Physiology of the heart, ed 4. Baltimore: Lippincott Williams & Wilkins, 2005.
Levy MN, Pappano AJ. Cardiovascular physiology, ed 9. Philadelphia: Mosby, 2007.
Mohrman DE, Heller LJ. Cardiovascular physiology, ed 6. New York: Lange Medical Books/McGraw-Hill, 2006.
Reece WO. Dukes’ physiology of domestic animals, ed 12. Ithaca, NY: Comstock Cornell University Press, 2004.
Richerson GB. The autonomic nervous system. In: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. Philadelphia: Saunders, 2005.
Shepherd JT, Vatner SF. Nervous control of the heart. Amsterdam: Harwood, 1996.
Waddell LS. Hypotension. Ettinger SJ, Feldman EC. Textbook of veterinary internal medicine: diseases of the dog and cat, ed 6, St Louis: Saunders, 2005.
PRACTICE QUESTIONS
2. The dilation of arterioles that occurs during steady-state exercise in active skeletal muscles could be eliminated by:
3. A drug is injected intravenously into a dog and causes a transient increase in mean arterial pressure and a transient decrease in heart rate. The baroreceptor nerves are cut; the drug is reinjected and now causes a greater increase in blood pressure but no change in heart rate. Which choice most likely explains the results caused by the first injection of the drug?
4. A dog has had a hemorrhage. The heart rate is increased above normal, and the skin is cold. The mucous membranes are pale. In this situation (compared with normal):
5. Blood (250mL) is taken from a vein of a dog. Mean arterial pressure does not decrease measurably. Nevertheless, it is likely that: