Chapter 10 Antiviral Therapy
Approximately 80 families and 4000 species of viruses are known to date, and more than 60% of illnesses afflicted humans in developed countries are caused by viruses. However, the development of drugs intended to prevent and treat viral diseases has been frustratingly protracted. Despite the long and intensive search for effective antiviral drugs, very few compounds have clinical applications. Currently, at least 23 antiviral drugs have been approved for use in human medicine; none is approved for use in animals. Unfortunately, unlike the situation with many other anti-infectious drugs, applications for human antiviral drugs in veterinary patients often have been limited because the etiologic agents of viral diseases vary so widely. In recent years, however, given the similarities between feline and human immunodeficiency viruses1 and potentially other viruses, information regarding pathophysiology and drugs with potential efficacy are increasingly applicable to veterinary use. Further, trends have developed toward development of drugs that are broadly effective against viral diseases. The development of species-specific recombinant proteins (e.g., interferons [IFNs]) has increased our knowledge base and promises to improve the therapeutic armamentarium for viral disease afflicting dogs and cats. Although these drugs are discussed in greater depth in other chapters, their support of treatment for antiviral diseases will be addressed here.
For a number of reasons, the development of effective antiviral drugs is more difficult than development of other anti-infectious agents. Drugs that target the viral processes must penetrate host cells to be effective, potentially limiting their distribution. Because the mechanisms by which viruses replicate must involve the host genome, drugs that are effective against viruses also are likely to have a negative effect on the host, with most antiviral drugs subsequently being characterized by a narrow therapeutic window. Clinical signs during the stages of infection when viruses might be most conducive to pharmacologic therapy often being mild to absent, and the need for antiviral therapy is not recognized until antiviral response is unlikely. Therapy is further complicated by viral latency, the ability of the virus to incorporate its genome into the host genome such that clinical infection becomes evident again without re-exposure to the organism. Selection of the most appropriate antiviral drugs is handicapped by the lack of broad-spectrum antivirals and the lack of rapid tests to identify the infecting virus. Newer polymerase chain reaction tests have at least helped in the more rapid diagnosis of some viral disease (e.g., parvovirus in dogs).
KEY POINT 10-1
The development of effective antiviral drugs is limited by the latent nature of disease, inherent host toxicity to viral drugs, and rapid emergence of viral resistance. Differences in viral diseases limits application of human antiviral drugs to dogs or cats.
In vitro susceptibility testing of viruses requires sophisticated and expensive techniques such as cell cultures. In vitro inhibitory testing procedures have not been standardized, and results vary with the assay system, cell type, and viral inoculum. Additionally, results may not correlate with therapeutic efficacy of antiviral drugs.2 The lack of correlation between in vitro testing and clinical efficacy reflects, in part, the requirement of some antiviral drugs for activation (i.e., metabolism of a prodrug, generally by the host).2 Not only is the spectrum of antiviral drugs narrow, but additionally, a drug often targets a specific viral protein (usually a polymerase or transcriptase enzyme) involved in viral nucleic acid synthesis.2 The limited mechanism of action tends to facilitate the development of antiviral resistance, which can occur rapidly, often reflecting substitution of only a single, although critical, amino acid in the target protein. Drugs that simply inhibit single steps in the viral replication cycle are virustatic. Consequently, viral replication is only temporarily halted, although in human medicine, chronic drug therapy may suppress reactivation of the disease caused by the virus and thus prevent clinical signs of disease. Because drugs often inhibit only active replication, viral growth often resumes once therapy is discontinued.
Antiviral drugs often cannot eliminate nonreplicating or latent viruses, and effective antiviral therapy generally also depends on an adequate host immune response. Consequently, those antiviral drugs that enhance the immune system of the host may be more likely to eradicate infection, as might combinations of antiviral and immune-enhancing drugs. Conversely, it is the overexpression of the immune response and the subsequent immune (e.g., feline immunodeficiency virus [FIV] and feline leukemia virus [FeLV]) or inflammatory response (e.g., feline infectious peritonitis [FIP]) that causes continued pathophysiology. The complex cascade of the immune system with dual pathways that balance a response renders pharmacologic management of just the right amount of immunomodulation in the right direction difficult in the face of viral diseases.
Prions (protein infectious virion) are infectious agents composed entirely of propagated misfolded protein that is resistant to endogenous straightening. The term prion refers to the unidentified unit of infection. The method of propogation is not clear but appears to involve abnormal refolding of protein such that aggregates of tightly packed beta sheets accumulate to form amyloid. Accumulation occurs only in neural tissues and is uncontrollable and invariably lethal. Among the diseases affecting dogs or cats that are thought to be associated with prions is feline spongiform encephalopathy. Because these agents are not treatable, they will not be considered further in this chapter.
Viral Replication
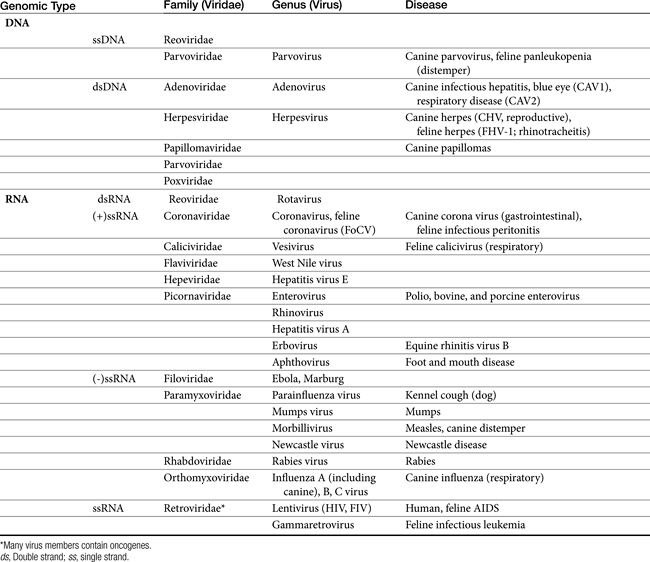
Viruses are composed of a core genome consisting of either double-stranded or single-stranded DNA or RNA surrounded by a protein shell known as a capsid. Some viruses are further surrounded by a lipoprotein membrane or envelope. Both the capsid and lipoprotein membrane may be antigenic. Viruses cannot replicate independently and must usurp the host’s metabolic machinery to replicate. Therefore viruses are obligate intracellular parasites. The host’s pathways of energy generation, protein synthesis, and DNA or RNA replication provide the virus with the means of viral replication. For some viruses, replication is initiated by viral enzymes.2 DNA viruses include poxvirus, herpesvirus, adenovirus, hepadnavirus, and papillomavirus. RNA viruses include rubella virus, rhabdovirus (rabies), picornavirus, arenaviruses, arboviruses, orthomyxovirus, and paramyxovirus (canine distemper) (Table 10-1).
Cells respond to viral infection in three ways: infection may have no impact on the cell or its function, cellular death may occur (which may preclude subsequent infection), or the cell may be transformed such that host control of cell growth is lost to viral activities. Viral replication occurs in five or six sequential steps (Figure 10-1): cell entry, including host cell attachment, generally through specific receptors, followed by host cell penetration; disassembly or uncoating resulting in release of viral genome; transcription of viral genome (or viral messenger RNA), which is dependent on virus-specified enzyme; translation of regulatory (early) or structural (late) viral proteins; post-translation modifications (including proteolytic cleavage, myristoylation, glycosylation); assembly of virion components; and release of the virus, generally by budding or cell lysis.2 For DNA viruses, viral DNA is transcribed to host mRNA by host cell mRNA polymerase (or, for poxvirus, viral RNA polymerase). Replication of RNA viruses requires virion enzymes to synthesize mRNA. Double stranded RNA (dsRNA) viruses contain RNA molecules that are transcribed into proteins. Two groups of single stranded RNA (ssRNA) viruses exist. The RNA genome of positive-sense ssRNA viruses is directly translated as mRNA by the host. In contrast, the RNA genome of negative sense ssRNA viruses must first be translated to mRNA by viral RNA-dependent RNA polymerase; host ribosomes subsequently translate to the protein.
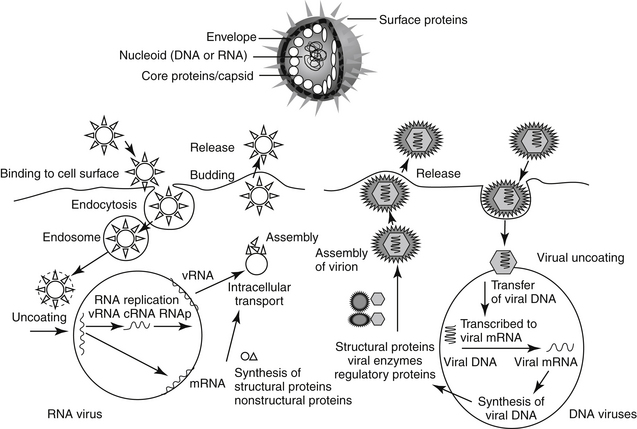
Figure 10-1 Replication of a representative RNA virus and DNA virus. Targets of antiviral drugs are presented before or during infection (including cell penetration), inside the cell (including viral replication, assembly and release), and during dissemination of progeny. The latter also includes preventing immunosuppression or the overzealous host response. cRNA, Replication intermediate; mRNA, messenger RNA; RNAp, RNA polymerase; vRNA, viral RNA.
Retrovirues are unique viruses that contain a single strand of RNA that must first be translated, via reverse transcriptase, to a DNA copy of the viral RNA template. The DNA is then incorporated into the host genome (as a provirus), duplicated, and subsequently transcribed into genomic RNA and mRNA for translation into viral proteins.2
A number of host mechanisms protect against viral infection. However, the host response not only may fail to protect but also may perpetuate the disease. Antibodies will be generated in response to viral infection, but these do little to overcome the initial infection. Rather, in part because viral activity is generally intracellular and thus inaccessible to antibodies, antibodies generally protect against subsequent infection. Unfortunately, the presence of circulating antibodies can contribute to the disease process of some infections (e.g., FIP). Cell-mediated immunity (CMI) plays a critical role in overcoming and preventing viral infection. However, viruses that can avoid an effective CMI response may cause latent or, if the cell does not result in the loss of normal cellular housekeeping activities, persistent infections. Mechanisms by which this can be accomplished included downregulation of major histocompatibility complex production such that the infected cell is not recognized by T cells; cells are generated that are not detectable by the host immune system; and infection is limited to cells located in an immunoprivileged site, such as the brain. Viruses that are particularly adept at causing persistent, chronic infections include paramyxoviruses, selected herpesviruses, and retroviruses.
KEY POINT 10-2
Host response often cannot effectively eradicate infection, even in the presence of drugs, but often contributes to the diseases process.
In addition to the directed immune response, the host will mount a number of nonspecific protective mechanisms. These include increased body temperature, activity of natural killer cells and phagocytes, and hormones. The role of IFN increasingly is being revealed as a target of pharmacotherapy. Viral infection begins with interaction between the virus and its specific host cell receptor. It is this interaction that initiates the host activation of multiple signal transduction cascades that mount a host defense. Ultimately, these mechanisms cause the nucleus to activate diverse immunoregulatory genes and proteins that cause the intracellular environment to be antagonistic toward viral replication.3 Phosphorylations activate several families of transcription factors. Among the antiviral genes regulated are those encoding interferon (IFN), including α-1 and –β regulators. Several viral cell receptor–initiated events have been identified for their ability to induce activation that ultimately involves IFN. For example, chemokine receptor binding to human immunodeficiency virus (HIV) envelope or glycoproteins, and poxvirus and measles virus binding to T and B cell membrane–bound glycoprotein each initiate such events. The 2–5A pathway is an example of an endogenous antiviral protective system that induces IFN through dependent RNase and 2’-5’ oligoadenylate synthetase (OAS). Viral infection stimulates OAS, which ultimately leads to destruction of both viral and cellular rRNA. Subsequent cell death is similar in appearance to that caused by apoptosis. Viral replication is subsequently prevented.4 Activation of IFN-based and other defense mechanisms also can occur through nonreceptor–mediated mechanisms.3 Not surprisingly, viruses have developed several mechanisms that evade IFN-mediated cell responses. An example includes production of soluble IFN receptors that preclude interaction with normal receptors (e.g., poxviruses and herpesviruses) that would otherwise activate the cascades, downregulation of IFN synthesis (adenovirus), and blocking of phosphorlyations.3
The pathophysiology of infection, including molecular mechanisms, has recently been described for FeLV5 and FIV,1 including the role of selected cytokines in the immune response.6
Antiviral Drugs
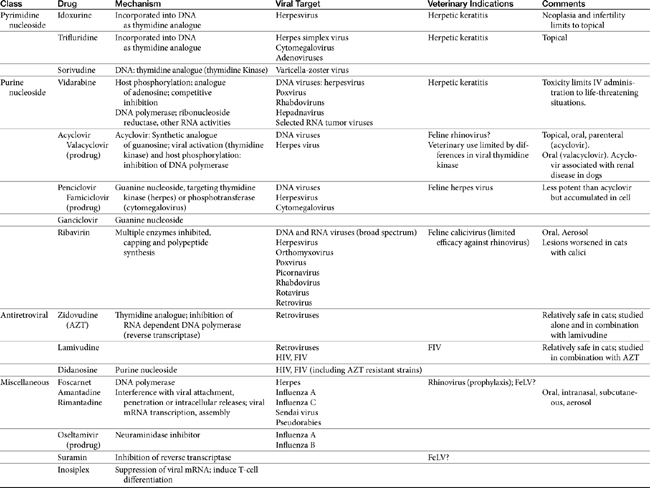
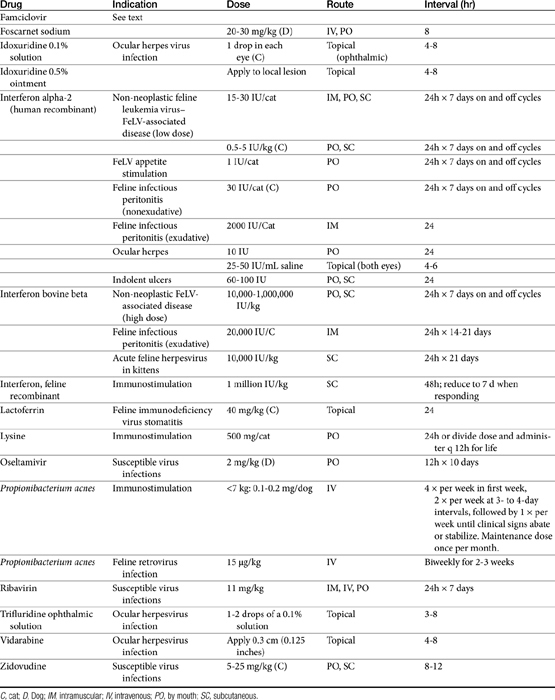
Few antiviral drugs have been studied in animals (">Tables 10-2, 10-3), and widespread clinical use of antiviral drugs is not common in veterinary medicine. Only a selection of the more promising agents and their purported attributes are briefly discussed; alternatively, when pharmacokinetic information is available, because such information is so limited, it also is provided even if the drug is not an accepted therapy for canine or feline viruses.
Antiviral drugs are most practically categorized by the major viruses targeted by the drug, which tends to limit direct applicability to animal diseases. Although data specific to veterinary use are increasing, much information regarding antiviral drugs continues to reflect extrapolation from the human-medicine literature. This is particularly true for pharmacokinetics, and unless stated otherwise, such information is human in origin. Yet the disposition of antiviral drugs tends to be complex, often requiring prodrug activation, protein binding, and hepatic clearance, all of which tend to vary among species. The drugs are often characterized by a narrow therapeutic window. As such, extrapolation of dosing regimens should be done cautiously. Immune-modulating drugs in particular are not well understood, and mechanisms of action have not yet been fully elucidated. Very few human antiviral drugs have been studied or reported to be used for treatment of viral disorders in dogs or cats and the evidence provided by the few clinical trials performed in animals often is characterized by limitations in study design. Consequently, the inclusion in this chapter of information regarding antiviral drugs should not be interpreted as justification for use but rather treated as a springboard for additional studies. Two categories of drugs have been and are currently being pursued for the pharmacologic treatment of viral diseases. Antiviral chemicals directly interfere with the virus, whereas biologic response modifiers stimulate the host’s immune system, thereby increasing the host’s ability to overcome viral invasion. The latter are discussed in greater depth elsewhere in this book.
Targets of Antiviral Therapy
Potential targets in the viral life cycle that might be pharmacologically inhibited are expressed during extracellular stages of viral infection (i.e., penetration), intracellular stages (i.e., replication, assembly, and viral release), and dissemination. Those expressed during extracellular stages include specific enzymes whose release is required for skin and mucosal barrier penetration by some viruses, specific cell receptors required for penetration by other viruses, and specific precursor “fusion” proteins that must be activated before cell penetration by some viruses. Antivirals that diminish penetration of host cells by the virus are more viral specific and thus not as inherently toxic as those that prevent viral replication by interfering with viral nucleic acid, DNA, and protein synthesis. Because cell penetration is enhanced by viral-induced immunosuppression, pharmacologic immunomodulation may also help prevent viral penetration.7 Thus these drugs are inherently more useful during the early stages of infection, which are often missed because of the lack of clinical signs. Classes of antivirals that target cell entry include soluble receptor decoys and antireceptor antibodies. Uncoating of the virus can be targeted by ion channel blockers, capsid stabilizers, and fusion protein inhibitors.2
Currently, targets expressed during intracellular stages of viral infection are the most common sites of pharmacologic intervention. Drugs include antivirals as well as a number of other classes of drugs (e.g., immunomodulators). Viral replication depends on macromolecular synthesis (by the host) of viral genome and on genome replication, transcription, and translation. Classes of drugs that inhibit transcription include inhibitors of viral DNA or RNA polymerase, reverse transcriptase, helicase, primase, or integrase. Natural substances capable of inhibiting viral transcription and translation (e.g., IFN) are much more potent than synthetic compounds. Viral replication is targeted by antisense oligonucleotides and ribozymes. Many antiviral drugs are nucleoside or nucleotide analogs, whose chemical structures allow prevention of viral replication by blocking nucleic acid metabolism (Figure 10-2; see also Figure 10-1). However, viral replication is so closely connected to vital functions of the host cell that agents capable of inhibiting viral replication usually injure host cells as well. Although such drugs are more likely to be broad in their antiviral spectrum, most also are potential teratogens, mutagens, and (particularly in humans) carcinogens. Further, they are associated with a variety of other host toxicities, with bone marrow suppression a not uncommon occurrence.7
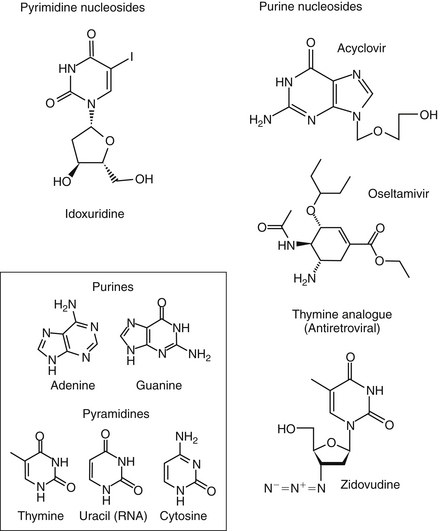
Figure 10-2 Structures of selected antiviral drugs. Structural similarity to purine or pyrimidine bases of host RNA or DNA (box) results in limited host safety.
Fewer agents have been developed that block viral translation. Classes of drugs that inhibit viral translation include IFNs, antisense oligonucleotides, and ribozymes. In addition, regulatory proteins might be inhibited. Another category of intracellular targets are specific enzymes, such as RNA or DNA polymerase, or reverse transcriptase of retroviruses, whose expression is required for the maintenance of the viral life cycle. Antiviral agents designed to block expression of these enzymes may have increased selectivity for viruses compared with the host, although their antiviral spectrum frequently is limited. Finally, assembly of synthesized viral macromolecules and release of the assembled virus may be pharmacologically inhibited. For example, IFN-induced inhibition of RNA tumor viruses occurs at assembly, although the mechanism is unknown.7
Posttranslational modifications such as proteolytic cleavage may be targeted by some drug classes (e.g., protease inhibitors). IFNs and drugs that inhibit specific proteins target viral assembly. Finally, antiviral antibodies and cytotoxic lymphocytes target the release of viruses from the host cell.
The final stage of viral infection that might be targeted pharmacologically is release of the new viral progeny from the infected host cell. Viruses can leave cells by causing cellular lysis or budding. With lysis, virus leaves in a sudden burst that kills the host cell. With budding, interaction between the virus and cell receptor induces changes that allow fusion of viral and cellular membranes, thus allowing the progeny to gradually leave the infected cell by budding. An example target for drugs during viral release is the neuraminidase in influenza viruses, a virulence factor that cleaves the sialic acid (neuraminic acid) residues from the glycan portion of cell receptors recognized by viral hemagglutinin.8
Viral infection may be also be pharmacologically inhibited during dissemination, which for some viruses appears to depend primarily on virally-induced host immunosuppression. Thus dissemination is another stage in which modulation of the immune system may help the host overcome viral infection.7 Biological response modifiers emerge with a role in modification of host response, whether it be inhibition of viral-induced immunosuppression or inhibition of an overzealous host response. The biological response modifier most commonly studied for its effect on viral infections and showing the most promise in efficacy has been IFN.
Antiviral-Induced Nephrotoxicity
Among the toxicities caused by antiviral drug is nephrotoxicity. The risk of antiviral drug–induced nephrotoxicity has increased as drugs have become more effective and novel in their action and as combination drug therapy has been implemented in response to increasing viral resistance.9 Acute tubular necrosis has been associated with a number of antiviral drugs (e.g., foscarnet, acyclovir, IFN, and cidofovir). However, variable renal lesions have been ascribed to a number of drugs, reflecting three major mechanisms: transporter defects, apoptosis, and mitochondrial injury. Glomerular disease resulting in proteinuria and, occasionally, the nephrotic syndrome has been mediated by either immune-mediated complexes (IFN) or crystal deposit (foscarnet). Crystalline deposits in the renal tubule (e.g., acyclovir, ganciclovir, and indinavir) can cause intrarenal obstruction. Isolated tubular defects may occur; examples include a Fanconi-like syndrome (cidofovir, tenofovir), distal tubular acidosis (e.g., acyclic nucleotide phosphonates, foscarnet), and nephrogenic diabetes insipidus (NDI: foscarnet).9 A major contributor to toxicity is intratubular cell drug accumulation mediated by ion-transport systems. For example, nephrotoxicity associated with cidofovir and adefovir appears to be facilitated by transport mediated by a transport protein.10 Limited use of antiviral drugs in veterinary medicine probably precludes effective evaluation of the advent of nephrotoxicity in animals with viral infections subsequently treated with antiviral drugs. However, among the clinical signs of toxicosis to accidentally ingested acyclovir in dogs were signs consistent with acute renal failure.11
Antiherpesvirus Drugs
Pyrimidine Nucleosides
A variety of pyrimidine nucleosides (both halogenated and nonhalogenated) effectively inhibit the replication of herpes simplex viruses with limited host cell toxicity. The exact mechanism of action of these compounds appears to reflect substitution of pyrimidine for thymidine, causing defective DNA molecules.
Idoxuridine
Idoxuridine (5-iodo-2-deoxyuridine, IDU; Stoxil) was the first of the nucleoside analogues to prove useful in the treatment of viral diseases. Idoxuridine resembles and is substituted for thymidine. After phosphorylation, it is incorporated into both viral and host cell DNA. Altered DNA is susceptible to breakage, resulting in faulty transcription and altered viral proteins. The spectrum of antiviral activity is limited to DNA viruses, particularly members of the herpesvirus group. Resistance to IDU develops rapidly.2 The ability of IDU to cause neoplastic changes, genetic mutation, and infertility limits its use to topical, primarily ophthalmic, infections. IDU is available as an ophthalmic ointment or solution. It is currently approved for use in the treatment of herpes keratitis in humans and has proved useful for the treatment of feline herpetic keratitis. One drop of a 0.1% solution is usually applied to the affected eye every hour; the 0.5% ointment can be applied every 2 hours.12,13 Topical application of IDU to the conjunctiva has been associated with irritation, pain, pruritus, inflammation, and edema of the conjunctiva and punctate areas on the cornea. Resistance of viruses to the drug develops readily both in vitro and in clinical cases.
Trifluridine
Trifluridine (triflurothymidine; TFT; Viroptic) is a halogenated (fluorinated) pyrimidine that is similar and often considered superior to IDU. TFT monophosphate irreversibly inhibits thymidylate synthetase, and TFT triphosphate competitively inhibits DNA polymerase incorporation of thymidine into DNA. Like IDU, it is preferentially incorporated into both viral and host DNA, and late virus-specific DNA transcription is inhibited.2 Trifluridine has in vitro inhibitory effects against herpes simplex virus (types 1 and 2), cytomegalovirus, and selected adenoviruses. Clinical resistance to TFT has been reported. As with IDU, the primary therapeutic indication for TFT is herpetic keratitis. TFT is prepared as a 0.1% ophthalmic solution and is usually applied 6 to 8 times per day. Trifluridine is frequently preferred to IDU for the treatment of human and feline herpetic keratitis in order to prevent toxicities associated with IDU.12,13 Adverse reactions include discomfort on application and palpebral edema.2
Sorivudine
Sorivudine is a pyrimidine nucleoside analog characterized by potency that results in a relative selectivity for varicella-zoster virus (VZV). The drug is initially phosphorylated by viral thymidine kinase and then metabolized to diphosphate by viral thymidylate kinase. As such, sorivudine triphosphate is a competitive inhibitor of viral DNA replication. Unlike acyclovir, however, sorivudine is not incorporated into viral DNA. Inhibitory concentrations of sorivudine are 1000-fold lower for VZV than are those of acyclovir. Cellular uptake in cells infected with herpesvirus is fortyfold greater than in uninfected cells. Clinical resistance has not yet been detected.2
Sorivudine (in humans) is well absorbed after oral administration and is characterized by 98% protein binding. The elimination half-life is 5 to 7 hours, although half-life increases with age. Elimination appears to be urinary, with minimal hepatic metabolism. Side effects are primarily gastrointestinal (nausea, vomiting, and diarrhea). Hepatic enzymes may increase. Long-term administration has caused hepatic neoplasms in rodents. Sorivudine (probably its metabolite) appears to negatively interact with 5-fluorouracil by inhibiting the enzyme responsible for fluorouracil metabolism.2 Sorivudine is available in both oral and intravenous preparations but only as investigational drugs.
Purine Nucleosides
Certain purine nucleosides have proved to be effective antivirals and are used as systemic agents. Several of these antiviral drugs deserve special mention.
Vidarabine
Vidarabine (Vira-A) was initially investigated for its efficacy as a cancer chemotherapeutic drug. An analog of adenine, vidarabine is phosphorylated by host enzymes and competitively inhibits viral DNA polymerase. It is substituted for adenine into DNA, thus inhibiting viral DNA polymerase. Mammalian DNA is also inhibited, although to a lesser extent. Ribonucleoside reductase, RNA polyadenylation, and transmethylation reactions also are inhibited.2 Vidarabine selectively inhibits DNA viruses, particularly herpesviruses. It is also effective against poxviruses, rhabdoviruses, hepadnaviruses, and selected RNA tumor viruses.2 Until recently, the drug was prepared as an injectable suspension. It is poorly water soluble, however, and must be dissolved in large volumes of fluid before intravenous use. A 3% ophthalmic ointment continues to be available. On intravenous administration, vidarabine is deaminated to hypoxanthine arabinoside which has 10% of the potency of the parent compound, but reaches concentrations that exceed the parent compound by fifteenfold after constant intravenous infusion. The drug is eliminated renally but predominantly as the hypoxanthine metabolite. The elimination half-life of the metabolite is approximately 3.5 hours. Adverse reactions are more likely with intravenous administration and include gastrointestinal upset (vomiting, diarrhea) and central nervous system (CNS) derangements (hallucinations, ataxia, tremors, and painful peripheral neuropathies with long-term use). In addition, vidarabine is probably mutagenic and carcinogenic. Phlebitis, hypokalemia, rash, elevated transaminases, and pancytopenia as well as inappropriate concentrations of antidiuretic hormone have been reported in humans. Systemic use in humans is reserved for life-threatening infections (e.g., herpes encephalitis). Although vidarabine is preferred over IDU for topical therapy of herpetic keratitis, the advent of acyclovir has reduced its use. Vidarabine can be useful for patients that have developed resistance to acyclovir or in combination with acyclovir for life-threatening infections.2 Literature regarding its use in the cat is limited, but topical administration of the 3% ointment appears to be well tolerated in cats.12,13
Acyclovir and Valacyclovir
Acyclovir is an acyclic synthetic purine nucleoside analog that substitutes for guanosine in DNA synthesis. Valacyclovir is an L-valyl ester prodrug of acyclovir. Efficacy of acyclovir depends on activation of the drug to its monophosphate derivative by viral thymidine kinase. Subsequent phosphorylation to the diphosphate and then triphosphate form is mediated selectively by cells infected with herpesvirus. The formation of acyclovir-GTP results in the inhibition of viral DNA polymerase and incorporation of acyclovir-GTP into viral DNA, which terminates viral DNA synthesis. The drug has a greater affinity for viral (versus host) thymidine synthetase. Antiviral activity of acyclovir is limited essentially to herpesviruses. The in vitro activity of acyclovir is 100 times that of vidarabine and 10 times that of IDU. Viral resistance to acyclovir results from mutation to strains that are characterized by a reduction in viral thymidine kinase (the most common mechanism), altered substrate specificity, or altered viral DNA polymerase.2
Acyclovir is available in topical, oral (capsule), and parenteral (powder to be reconstituted) preparations. The bioavailability of the oral preparations (in humans) is poor (10% to 30%) and decreases with increasing doses.2 In contrast, valacyclovir, which is rapidly and completely converted to acyclovir, increases bioavailability of acyclovir to 50% (in humans). Acyclovir distributes to all body fluids, including cerebrospinal fluid. It is eliminated primarily unchanged by glomerular filtration and tubular secretion and accumulates in patients with renal failure. The elimination half-life in adults with normal renal function is 1.5 to 6 hours; this can increase to 20 hours in anuric patients.
Toxicity of acyclovir, regardless of the preparation, is limited. Oral administration (of both acyclovir and valacyclovir) is associated with gastrointestinal upset. Intravenous administration may cause renal insufficiency and (rarely) CNS side effects. Cats experimentally infected with feline herpesvirus type 1 (FHV-1) that received 60 mg/kg daily of valacyclovir orally became ill within 6 to 9 days of therapy, necessitating termination of the study in 12 days. White blood cell counts declined, yet no difference was found in viral pathology in treated versus untreated cats, leading the authors to assess valacylovir as an unlikely option for treatment of FHV-1.14 Renal dysfunction is reversible and may reflect concentration in urine to the point that crystallization occurs2 (see previous discussion). Rapid infusion, dehydration, and inappropriate urine flow increase the risk of renal damage. Phlebitis also may accompany intravenous administration. A retrospective review (January 1995 through March 2000) of acyclovir toxicoses in dogs (n = 105) following accidental ingestion reported by The American Society for Prevention of Cruelty to Animals National Animal Poison Control Center found clinical signs developing in 6 of 10 dogs within 3 hours of ingestion of doses ranging from 40 to 2195 mg/kg. 11 The most common clinical signs were vomiting, diarrhea, anorexia, and lethargy, with polyuria and polydipsia reported in only 1 dog. Treatment included standard decontamination procedures, (i.e., induction of emesis, administration of activated charcoal), diuresis, and supportive care.
Veterinary use of acyclovir may be limited, probably because of differences among infecting viruses in viral thymidine kinase for acyclovir. In addition, antiviral resistance is increasing. Acyclovir is unable to eliminate latent infections. It is available as an ophthalmic ointment, a topical ointment and cream, an intravenous preparation, and various oral formulations.
Penciclovir and Famciclovir
Like acyclovir, penciclovir is an acyclic guanine nucleoside. Its spectrum of activity (herpes simplex virus and VZV) is also similar to that of acyclovir. Penciclovir (up to 77% bioavailable) is formed from its prodrug famciclovir. Penciclovir is a hundredfold less potent than acyclovir but is accumulated to higher concentrations than is acyclovir in infected cells.2 Plasma elimination half-life in humans is approximately 2 hours, and elimination is renal. Although, like acyclovir, the drug is well tolerated orally, chronic administration appears to be tumorigenic, causing testicular toxicity in animals.
The pharmacokinetics and safety of penciclovir resulting from oral administration of famciclovir have been reported in cats (n = 8) at 62.5 mg (half of a 125-mg tablet; 9 to 18 mg/kg) orally after single and multiple dosing (every 8 or 12 hours; 4 cats per group) for 3 days.15 The maximum drug (Cmax; ng/mL) concentration following a single dose was 330 ± 120, and the elimination (disappearance) half-life was 3.1 ± 0.9 hr. Using a multiple-dosing, 12-hour dosing regimen, the Cmax of penciclovir (ng/mL) did not change significantly from single dosing (multiple 12-hour dosing Cmax [ng/mL] of 330 ± 180 at a Tmax of 5 hours) but did with 8-hour dosing (multiple 8-hour dosing Cmax of 680 ± 290) µg/mL. This increase is practically expected with a 3-hour half-life in the face of an 8-hour dosing interval (accumulation ratio of 1.4), but altered clearance cannot be ruled out without intravenous pharmacokinetic studies. The half-life did not change with multiple dosing, although the duration of dosing may not have been enough to allow emergence of drug interactions and the sample size may not have been sufficient to detect a difference. Dose normalization of Cmax and area under the curve (AUC; 24 hours) yielded no significant differences between the two dosing intervals; again, limitations in sample size may have precluded detection of differences. Adverse effects may have included decreases in packed cell volume (by 27%) and total protein (by 10%). However, these changes may have reflected frequent blood draws during the study. White blood cell counts increased (neutrophils and monocytes) by 54% after the last dose. Although the cats tolerated dosing well, the target concentration suggested for treatment of FHV-1 is 3500 ng/m (based on the review of Thomasy et al.15), which was approximately tenfold higher than the Cmax achieved in this study. Further studies must verify the safety of the drug at doses necessary to achieve this concentration.
Ganciclovir
Ganciclovir is structurally similar to acyclovir, with the addition of a hydroxymethyl group on the acyclic side chain. Its spectrum includes herpesvirus, with particular efficacy against cytomegalovirus; effective concentrations are tenfold to a hundredfold lower than the concentrations effective against other herpesviruses.2
Unfortunately, similar concentrations are inhibitory to bone marrow progenitor cells. As with other guanine nucleosides, ganciclovir inhibits viral DNA synthesis after monophosphorylation mediated by viral thymidine kinase (herpes) or phosphotransferase (cytomegalovirus). Diphosphates and triphosphates of ganciclovir are formed by cellular enzymes. The triphosphate competitively inhibits deoxyguanosine triphosphate incorporation into both viral and host DNA, with preferential inhibition of viral over host DNA polymerase. Intracellular concentrations (which exceed acyclovir concentrations by at least tenfold) decline much more slowly than those of acyclovir, resulting in a cellular elimination half-life of approximately 24 hours. Hence the drug is given once daily in humans.2 Resistance most commonly reflects point mutations or deletions in viral DNA, resulting in reduced formation of viral phosphotransferase.
Ganciclovir is poorly bioavailable in humans (9%, with food). More than 90% of the absorbed drug is eliminated renally, with an (plasma as opposed to cell) elimination half-life of 2 hours. Elimination half-life increases proportionately with creatinine clearance. The primary adverse effect is myelosuppression, with neutropenia occurring in up to 40% and thrombocytopenia in 5% to 20% of patients. Myelosuppression more commonly occurs with intravenous administration and is generally reversible by 1 week after discontinuation of therapy, but it can be persistent and fatal. Treatment with granulocyte colony-stimulating factor may minimize neutropenia.2 The risk of myelosuppression is increased when ganciclovir is combined with other cytotoxic drugs. Side effects in the CNS also are frequent, occurring in up to 15% of human patients. Clinical signs include convulsions and coma. Other adverse effects include infusion-related phlebitis, azotemia, anemia, fever, hepatic dysfunction, nausea, vomiting, and eosinophilia. Therapeutic use of ganciclovir includes cytomegalovirus retinitis, particularly in humans with acquired immune-deficiency syndrome–induced immunodeficiency. Ganciclovir is also used for treatment of any infection or prevention of infection (particularly in transplant recipients) associated with cytomegalovirus.
Ribavirin
Ribavirin (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide; Virazole) is a purine nucleoside analog that is activated by viral phosphorylation and subsequently prevents the formation of mRNA and translation of viral genome.12,13 The action of ribavirin involves specific inhibition of virus-associated enzymes, inhibition of the capping of viral mRNA, and inhibition of viral polypeptide synthesis. Thus it is effective against both DNA and RNA viruses and is a broad-spectrum antiviral drug. Susceptible viruses include adenoviruses, herpesviruses, orthomyxoviruses, poxviruses, picornaviruses, rhabdoviruses, rotaviruses, and retroviruses. Viral resistance to ribavirin is rare. Ribavirin is well absorbed in humans, widely distributed in the body, and eliminated by both renal and biliary routes as both parent drug and metabolites; it has a plasma half-life of 24 hours in humans. It does not have a wide margin of safety in domestic animals. Toxicity is manifested by anorexia, weight loss, bone marrow depression, anemia, and gastrointestinal disturbances. It has been successfully administered by topical, parenteral, oral, and aerosol routes. Ribavirin is administered as an aerosol to human patients afflicted with respiratory viral infections, thus avoiding the hematopoietic toxicities associated with systemic use of the drug. In the cat, in vitro investigations revealed marked antiviral activity against a strain of calicivirus but little efficacy for rhinotracheitis.12,13 However, Povey16 studied the oral administration of 25 mg/kg every 8 hours for 10 days in cats that had been experimentally infected with calicivirus. Pathologic lesions in infected cats worsened, primarily because of severe thrombocytopenia that presumably was drug induced. Cats also developed liver disease, although clinical signs resolved 1 week after the drug was discontinued.
Miscellaneous Antiherpes Drugs
Foscarnet
Foscarnet (phosphonoformic acid) is an inorganic trisodium salt that interferes directly with herpes viral DNA polymerase. The drug may also be effective in treating retroviral infections owing to similar interference with reverse transcriptase. Direct actions preclude the need for intracellular activation. Foscarnet has a hundredfold greater affinity for viral as opposed to host DNA polymerase-α.2 Point mutations in DNA polymerase are responsible for resistance. Foscarnet is poorly bioavailable after oral administration. The drug is concentrated in bones, resulting in complicated plasma elimination. Elimination (in humans) is bimodal with an initial 4- to 8-hour elimination half-life followed by a 3- to 4-day half-life. It is eliminated primarily by the kidneys, with clearance decreasing proportionately with creatine clearance.
Major side effects in human patients include nephrotoxicity and hypocalcemia, which can become symptomatic. Serum creatinine increases in up to 50% of patients but decreases after therapy is stopped. Acute tubular necrosis, crystalluria, and interstitial nephritis have occurred. Sodium loading before therapy may decrease the risk of renal toxicity. Because foscarnet is highly ionized at physiologic pH, metabolic abnormalities are common in human patients. Calcium and phosphorus may decrease or increase. Decreases in ionized calcium may be sufficient to cause clinical signs consistent with tetany. Other CNS side effects reported in human patients (up to 25%) include tremors, irritability, seizures, and hallucinations. Fever, nausea, vomiting, anemia, leukopenia, and hepatic dysfunction also have been reported. Indications in human patients include cytomegalovirus retinitis and herpes infections that are resistant to acyclovir. The disposition of foscarnet and its precursor, thiophosphonoformate (TPFA), have been studied in normal cats. Whereas foscarnet was only 8% bioavailable, TPFA was 44%; however, only 14% of the drug is converted to foscarnet.16a The half-life of foscarnet was approximately 3 hrs with clearance of 1.88 ml/min/kg, being similar to renal clearance. Foscarnet has been shown to be effective prophylactically in the treatment of feline rhinotracheitis. Its efficacy against retroviruses warrants further investigation for the treatment of FeLV. Currently, foscarnet is given to immunocompromised human patients and is being studied in the cat for treatment of retroviral infections.
Antiretroviral Drugs
Zidovudine
All clinically used (human-approved) antiretroviral agents are 2ʹ,3ʹ– dideoxynucleoside analogs. Zidovudine (AZT; Retrovir) is a thymidine analog. Within the virus-infected cell, the 3ʹ-azido group substitutes for the 3’-hydroxy group of thymidine. The azido group is then converted to a triphosphate form, which is used by retroviral reverse transcriptase and incorporated into DNA transcript.12,17 The 3’ substitution prevents DNA chain elongation and insertion of viral DNA into the host cell’s genome, preventing viral replication. Thus the shared mechanism of action of these drugs is inhibition of RNA-dependent DNA polymerase (reverse transcriptase). This enzyme is responsible for conversion of the viral RNA genome into double-stranded DNA before it is integrated into the cell genome. Because these actions occur early in replication, the drugs tend to be effective for acute infections but relatively ineffective for chronically infected cells.2 Cellular α-DNA polymerases are inhibited only at concentrations a hundredfold greater than those necessary to inhibit reverse transcriptase, thus rendering this drug relatively safe to host cells. Cellular γ-DNA polymerase, however, is inhibited at lower concentrations. Zidovudine is effective against a variety of retroviruses at low concentrations (<0.001 to 0.04 μg/mL. The intracellular elimination time of AZT is 3 to 4 hours.2
In human patients AZT is rapidly absorbed, with a bioavailability of 60% to 70%. Food impairs absorption. Concentrations in the cerebrospinal fluid are approximately 50% of those in plasma. The plasma elimination half-life is 1 to 1.5 hours. In human patients AZT undergoes first-pass metabolism.
Among the toxicities caused by AZT is myeloid suppression. The concentration necessary to suppress (human) myeloid cells is higher than that associated with antiviral activity, but nonetheless is still relatively low at 0.3 to 0.6 μg/mL.2 Although metabolites appear to be void of antiviral toxicity, at least one may contribute to myeloid toxicity. Granulocytopenia and anemia are the major adverse effects of AZT in human patients. The risk of toxicity increases in human patients with low (CD4+) lymphocyte counts, high doses, and prolonged therapy. Idovudinzidovudine may cause Heinz body anemia in cats,21 suggesting that complete blood counts should be performed on cats receiving AZT. Granulocyte colony-stimulating factor is indicated for management of granulocytopenia. CNS side effects are more likely as therapy is begun. Other side effects reported in humans include myopathy (characterized by weakness and pain), neurotoxicities, hepatitis (uncommon), and esophageal ulceration. Resolution of myopathy occurs slowly after drug therapy is discontinued. The risk of myelosuppression is increased by drugs that inhibit glucuronidation or renal excretion. Therapeutic indications for AZT in humans include treatment of HIV infections. Treatment with AZT prolongs survival, decreases the incidence of opportunistic infections, increases measures of immune function, and decreases HIV antigens and RNA. Zidovudine has been combined with didanosine or zalcitabine for more sustained CD4+ lymphocyte response.
The disposition of AZT has been studied in cats. It is rapidly absorbed in cats after intragastric or oral administration. Administration of a single dose of 25 mg/kg in normal cats by either route generates maximum serum concentrations of 28 ± 7 and 29 ± 15 μg/mL, respectively. Bioavailability for the intragastric route is 70 ± 24%, and for the oral route 95 ± 23%. The elimination half-life is approximately 1.5 hours and volume of distribution 0.82 L/kg. Drug concentrations were above the effective concentration 50 (EC50) of 0.19 μg/mL for FIV for at least 24 hours after either intravenous or oral administration.18 The drug appears safe at this dose despite drug concentrations being well above that associated with myeloid suppression of human cells. Side effects in cats at 25 mg/kg (administered intravenously) were limited to transient restlessness, mild anxiety, and hemolysis.18
Lamivudine
Lamivudine is among the more potent drugs against human immunodeficiency virus (HIV) and also has been studied in cats. As with AZT, it is rapidly absorbed in cats after intragastric or oral administration. Administration of a single dose of 25 mg/kg in normal cats by either route generates maximum serum concentrations of 50 ± 38.5 and 40 ± 40 μg/mL, respectively, with bioavailability for the intragastric route being 88 ± 45%, and for the oral route 80 ± 52%. The elimination half-life is approximately 2 hours and volume of distribution is 0.6 L/kg. The effective concentration 50 (EC50) for lamivudine ranges from 0.8.7 (mutant) to 11 μg/mL (wild type) for FIV and is present for at least 24 hours after either intravenous or oral administration.19 As with AZT, lamivudine appears safe in cats at 25 mg/kg with similar side effects.19
The disposition of the combination of AZT (5 mg/kg) and lamivudine (3 mg/kg) has also been studied in normal and FIV-infected cats after single and multiple (7-day) dosing.20 Median AZT concentrations (ng/mL) were (median followed by range) 3.67 (2.67 to 4.66) at first dose and 3.65 (3.54 to 3.76) at steady state for AZT and 2.86 (2.62 to 3.08) at first dose and 3.89 (3.27 to 3.08) ng/mL (31.7 to 102.4 ng/mL) at steady state. These concentrations were comparable to those achieved in humans, although direct comparisons are precluded by different doses. Volume of distribution, clearance, and half-life were similar with combined therapy to that reported for individual therapy by Zhang and coworkers.18,19
Didanosine
Didanosine is a purine nucleoside effective against HIV, including strains that have developed resistance to AZT. Although it is tenfold to a hundredfold less potent than AZT, it is more active in quiescent cells and in nondividing (human) monocytes and macrophages. It also is not toxic for hematopoietic precursor cells or lymphocytes at clinically relevant concentrations. It is metabolized inside the cell to its active derivative (ddATP), which competitively inhibits virus preferentially to host reverse transcriptase. Oral bioavailability of didanosine is about 40% in humans. Because it is very acid labile, food decreases absorption by 50% or more. Didanosine is available as both tablets and powder, with the tablet being 20% to 25% more bioavailable than the powder. Only about 20% of drug in plasma distributes to the cerebrospinal fluid. Up to 60% of the drug is excreted unchanged through the kidneys, with a plasma elimination half-life of up to 1.5 hours. Intracellular metabolism may be responsible for some plasma elimination. Side effects include painful peripheral neuropathy and pancreatitis. High doses increase the risk for both. A history of pancreatitis predisposes the patient to this side effect. Up to 70% of human patients develop pancreatitis, although hyperamylasemia will occur in up to 20%. Other adverse effects include diarrhea; rashes; CNS signs, including insomnia and seizures; optic neuritis; and, rarely, hepatic failure or cardiac dysfunction. Animal studies also have found gastrointestinal, bone marrow, hepatic, and renal dysfunction. Didanosine (33 mg/kg orally per day for 6 weeks) was used to develop a model of antiretroviral peripheral neuropathy in normal and infected SPF kittens.21a Didanosine is approved for treatment of advanced HIV infections in human patients intolerant of or resistant to AZT. Stavudine, a thymidine nucleoside, and zalcitabine, a cytosine nucleoside, are alternatives to AZT therapy for human patients.2
Miscellaneous Antiviral Drugs
Amantadine
Amantadine and its derivative rimantadine are synthetic antiviral agents that appear to act on an early step of viral replication after attachment of virus to cell receptors. Interference of release of infectious viral nucleic acid into the host cell through the transmembrane domain of the viral M2 protein is proposed. The effect seems to lead to inhibition or delay of the uncoating process that precedes primary transcription. Amantadine may also interfere with the early stages of viral mRNA transcription. Amantadine also prevents virus assembly during virus replication. Viruses affected at usual concentrations include different strains of influenza A and C (but not B) virus, Sendai virus, and pseudorabies virus. It is almost completely absorbed from the gastrointestinal tract, and about 90% of a dose administered orally is excreted unchanged in the urine over several days (according to data for humans). The main clinical use has been to prevent infection with various strains of influenza A viruses. In humans, however, it also has been found to produce some therapeutic benefit if taken within 48 hours after the onset of illness. Amantadine and its derivatives may be given by the oral, intranasal, subcutaneous, intraperitoneal, or aerosol routes. It produces few side effects, most of which are CNS related; stimulation of the CNS is evident at very high doses. Acute toxicity generally reflects its anticholinergic effects and includes cardiac, respiratory, renal, or CNS toxicity.
Oseltamivir
Oseltamivir is prepared as an ester prodrug. On release by esterases in the gastrointestinal tract, the carboxylate form acts as a selective inhibitor of influenza A and B viral neuraminidases by inducing a conformation change at the enzymatic active site. The viruses cannot leave the infected cell and therefore aggregate at the cell surface and are unable to spread. Concentrations achieved in humans after oral administration of a therapeutic dose is 0.35 μg/mL of the carboxylate form. In humans the half-life is approximately 6 to 10 hours. It is excreted by renal tubular excretion; probenecid prolongs the half-life by twofold. The drug has not yet been studied in dogs or cats despite its anecdotal use for treatment of parvovirus in dogs.
Suramin
Suramin is a polysulfonate hexasodium salt capable of inhibiting reverse transcriptase; it has been studied for use in treating FeLV (discussed later).
Inosiplex
Inosiplex (Isoprinosine) is a compound formed from inosine and the para-acetamidobenzoate salt of 1-dimethylamino-2-propanol. Inosiplex can inhibit cytopathic effects of several viruses in culture. In vivo experiments, however, suggest that optimal activity of inosiplex occurs with therapeutic administration after viral infection and requires an adequate host immune response. The mechanism of antiviral activity appears to involve specific suppression of viral mRNA. Inosiplex does not appear to be as efficacious as several antimetabolite antiviral compounds. Inosiplex can also induce T-cell differentiation similar to that induced by thymic hormones, apparently by augmenting RNA synthesis. Thus inosiplex may be more useful as an immunopotentiator in immunodeficient patients (see earlier discussion of biologic response modifiers).12,17,22
Interferon and Its Inducers
Interferons (IFNs) are addressed in greater depth in Chapter 3. They are a group of multiple-gene inducible cellular glycoproteins that interact with cells and render them resistant to infection by a wide variety of RNA-containing and DNA-containing viruses. In addition, IFNs have numerous other effects on target cells, including a reduction in the rate of cell proliferation and alterations in the structure and function of the cell surface, the distribution of cytoskeletal elements, and the expression of several differentiated cellular functions. Interferons induce the synthesis of new proteins that are responsible for the activation of cellular endonucleases that degrade viral mRNA. Human IFNs are classified as α, β, or γ, depending on their physical stability, immunologic neutralization properties, host range, and homology in amino acid sequence. Viral infections generally are associated with the expression of IFN α and β genes. Those used in clinical trials have been produced by induction of synthesis by human white blood cells; fibroblasts; lymphoblasts; and, more recently, recombinant DNA techniques in bacteria. Numerous modes of antiviral action have been proposed. In addition to their ability to establish an antiviral state in host cells, they also appear to modulate the immune system of the host.
Interferons inhibit the replication of a wide variety of viruses. Among the RNA-containing viruses, the togaviruses, rhabdoviruses, orthomyxoviruses, paramyxoviruses, reoviruses, and several strains of picornaviruses and oncornaviruses are sensitive to inhibition by IFNs. Among the DNA-containing viruses, the poxviruses and several strains of herpes simplex types 1 and 2 viruses, as well as cytomegalovirus, are inhibited by IFNs. Adenoviruses are generally resistant. There are extreme variations in sensitivity to IFNs among different types and even strains of virus. In addition, the responses in different model and test systems can be extraordinarily variable. Interferons appear not to be as useful in the therapy of viral infections as was hoped initially. Interferons are usually administered parenterally but recently also have been used orally with some success. Although rare at recommended dosages, side effects may occur at higher levels.
To date, at least five feline IFN-alpha (feIFN) subtypes have been encoded,23 each of which (1, 2, 3, 5, and 6), when expressed in a Chinese hamster ovary cell line, has exhibited antiviral activity against vesicular stomatitis virus– and feline calicivirus–infected cells.24A recombinant feline IFN omega (rFeIFN-ω) is an insect- (silkworm-) generated (rather than microbial-based) product (Virbagen Omega) that is licensed by the U.S. Department of Agriculture for the treatment of retrovirus infections in cats.
Several substances induce IFN and have been tested for the prevention and treatment of viral infections and for treatment of neoplastic diseases. Although effective in some model systems, IFN inducers have not yet been found to be clinically useful because of their toxicity. High-molecular-weight inducers include polyriboinosinic acid/polyribocytidylic acid or poly(I)/poly(C); low-molecular-weight inducers include tilorone, aminobromophenyl-pyrimidinone, and aminoiodophenylpyrimidinone.
Miscellaneous Alternatives or Adjuncts to Antiviral Drugs
Lymphocyte T-Cell Immune modulator is an immune-regulating single polypeptide extracted and purified from bovine-derived stromal cells (ProLab manufacturers). The product package insert indicates that lymphocyte counts rapidly increased in cats (n = 23) with FIV or FeLV after treatment (1 mL) at 0, 7, and 14 days followed by monthly doses. Clinical scores became significantly better after the third dose. Red blood cell counts also increased in severely anemic cats. A clinical trial was not available for review, and no information was provided regarding control animals. As a biologic, the product has not been considered for approval by the Food and Drug Administration but had received conditional licensing by the United States Department of Agriculture as of November 2009.24a
Several drug classes continue to be investigated mainly because of their in vitro antiviral activities. Their potential clinical usefulness remains obscure in most instances. Included among these agents are thiosemicarbazones, guanidine, benzimidazoles, arildone, phosphonoacetic acid, rifamycins and other antibiotics, and several natural products.
Lysine is an essential amino acid. Herpes simplex viral proteins are rich in L–arginine, and in vitro tissue culture studies suggest that viral replication is enhanced in the presence of a high L-arginine to L-lysine ratio. In contrast, when the ratio is low (i.e., lysine > arginine), viral replication and the cytopathogenicity of herpes simplex virus have been found to be inhibited. The effects of lysine may reflect antagonism of arginine-growth promoting effects, although the site of interaction is not known. Replacement of viral arginine with lysine-yielding nonfunctional proteins also has been proposed.
Mycophenolic acid (MPA) is a non-nucleoside noncompetitive, reversible inhibitor of eukaryotic inosine monophosphate dehydrogenase. Inhibition of lymphocyte proliferation has led to its use to treat host versus graft rejection in human transplant recipients. Because microbial RNA and/or DNA synthesis also is inhibited, MPA has the potential for inhibiting infecting parasites and microbes, the latter including viruses. Mycophenolic acid appears to impair viral replication of a variety of viruses, including Sindbis virus, HIV herpesvirus, hepatitis B virus, orthopoxviruses, dengue virus, West Nile virus, and double-stranded RNA avian reoviruses.25 It also appears to potentiate the inhibitory effects of cyclic guanosine analogs (e.g., acyclovir, penciclovir, and ganciclovir) against herpesviruses, the nucleoside analogs against HIV. However, its use as a broad-spectrum antiviral agent against positive- and negative-stranded RNA viruses has not been established.25
Treatment of Selected Viral Infections
Treatment of viral diseases in small animals is nonspecific and seldom includes antiviral drugs. Therapy tends to be supportive, focusing on fluid and electrolyte supplementation, prevention or treatment of secondary bacterial infection, and treatments that support the function and structure of the organ targeted by the infection. By far, the most important approach to management of viral diseases in dogs and cats is prevention and, in particular, an effective vaccination program. In addition, isolation of infected animals and cleansing of environments contaminated with potentially infecting viruses are important ways to limit the spread of viral infections.
Treatment of Selected Canine Viral Infections
Canine Parvoviral Enteritis
Parvoviral enteritis, caused by canine parvovirus-2 (CPV-2), is among the most common and fatal viral infections afflicting dogs, including most members of the family Canidae. Infection by this highly contagious virus generally reflects contact with infected feces. Animals, humans, and objects can serve as vectors. After exposure viral replication begins in the lymphoid tissue of the gastrointestinal tract, from where it disseminates to the intestinal crypts of the small intestine. The virus localizes in the epithelium of the tongue, oral and esophageal mucosa, small intestine, and lymphoid tissue. Because CPV-2 infects the germinal cells of the intestinal crypt, cell turnover is impaired and villi shorten. Mitotically active myeloid cells and lymphoid cells are also targeted, leading to neutropenia and lymphopenia. Complications of intestinal damage include bacteremia, endotoxemia, and disseminated intravascular coagulation (DIC). Infections are most severe in puppies younger than 12 weeks of age because of their immature immune system. Clinical signs include vomiting (which can be severe), diarrhea, and anorexia. Animals may be febrile. Clinical pathology may reveal leukopenia. Myocarditis can develop in patients infected in utero or less than 8 weeks of age. Diagnosis is based on clinical signs, leukopenia (generally proportional to the severity of illness), and enzyme-linked immunoassay (ELISA) antigen testing.
The clinical efficacy of rfeIFN-omega has been evaluated for the treatment of dogs with experimental and spontaneous parvoviral enteritis. Martin and coworkers26 treated experimentally infected Beagle puppies with rFeIFN-omega (2.5 MU/kg) for 3 consecutive days and reported 1 of 5 deaths compared with 5 of 5 in untreated controls. De Mari and coworkers27 studied spontaneous disease using a multicentric, double-blind, placebo-controlled study. Clinical signs of the IFN-treated (2.5 million units/kg/day for 3 consecutive days) animals (n = 43) improved significantly during the 10-day study period compared with those of control animals (n = 49). Only 3 deaths occurred in the IFN group compared with 14 deaths in the placebo group, resulting in a 4.4 reduction in mortality. This increased to a 6.4–fold reduction in mortality in unvaccinated dogs.
Oseltamivir has been used to treat parvovirus, although this use is not based on a randomized clinical trial. One (abstract form) study reports a decrease in mortality of parvovirus by 75% to 100% (2 mg/kg orally every 12 hours); cost will be approximately $0.25/kg. However, neuraminidase does not appear to be a virulence factor for parvovirus, including the release of viruses from the cell. In contrast, bacterial neuraminidases are also produced by a large number of respiratory mucosal pathogens and are necessary for biofilm formation by Pseudomonas aeruginosa. Use of viral neuraminidase inhibitors prevents biofilm formation by microbial organisms. Accordingly, the use of oseltamivir for its nonviral indications warrants further consideration for its potential impact on intestinal bacterial translocation; well-designed clinical trials are needed to demonstrate efficacy.
KEY POINT 10-5
Efficacy of oseltamivir for treatment of parvovirus, if it is scientifically demonstrated, may reflect its impact on bacterial translocation rather than viral inhibition.
Symptomatic therapy for canine parvoviral enteritis focuses on restoration of fluids and electrolytes and on prevention or treatment of bacteremia or endotoxemia. Fluid therapy is the single most important treatment and should be aggressive and continued as long as the patient is vomiting or diarrhea is present. Among the antiemetics, metoclopramide originally was among the most successful, with ondansetron considered for animals that fail to respond. Maropitant may be the better choice. Treatment of diarrhea is generally not indicated. The use of narcotic motility modifiers (e.g., loperamide, diphenoxylate) has been recommended if necessary,28 but their use may prolong the presence of undesirable toxins in the gastrointestinal lumen. Thus their use is discouraged. Antimicrobial therapy should focus on both gram-negative coliforms and anaerobic organisms. In general, an injectable beta-lactam combined with an aminoglycoside has proved efficacious. Fluid therapy, once-daily dosing, and the immature nature of pediatric canine kidneys provide protection against aminoglycoside-induced renal disease. Fluorinated quinolones should not be used if possible because of the risk of cartilage damage. Ceftiofur has been used because of its potential for intravenous administration and its efficacy against Escherichia coli, one of the major contributors to secondary bacterial complications of parvovirus. Note, however, that efficacy and safety at doses necessary to control the systemic bacterial complications of parvovirus have not been documented. In addition, efficacy against anaerobic organisms of the gastrointestinal tract has not been studied. Cefazolin should be equally effective as ceftiofur against E. coli associated with translocation, although more frequent administration may be necessary and efficacy against anaerobes may be less.
Parvoviruses are extremely stable, being resistant to environmental conditions and many chemical disinfectants. Canine parvovirus is susceptible to sodium hypochlorite (1 part household bleach to 1:32 parts water). Exposure to diluted bleach must be long in duration.
Canine Distemper
Canine distemper29 spreads by aerosolization to the epithelium of the upper respiratory tract. Multiplication in tissue macrophages leads to spread to lymphatics; tonsils; bronchial lymph nodes; and ultimately to lymphatic tissues of the gastrointestinal tract, liver, and other organs. Additional spread generally is hematogenous. Leukopenia characterized by lymphopenia develops as the virus proliferates in lymphoid tissues. Animals with adequate immunity are able to clear infection within 8 to 9 days. In dogs with an insufficient immune response, the virus spreads to other tissues, including the skin and other organs. Persistent viral infection of the CNS appears to develop in dogs that are not able to generate circulating IgG antibodies to the viral envelope. Immune complex deposition in the CNS may facilitate viral infection. Lesions and their sequelae in the CNS vary with the age and immunocompetence of the dog, the pathogenicity of the virus, and the duration (acute versus chronic) of infection. Acute encephalitis is more likely in young or immunosuppressed dogs and reflects direct viral damage. Demyelinating polioencephalomalacia is characterized by minimal inflammation. Continued infection in the CNS leads to progressive increases in the immune response, ultimately contributing to continued and widespread damage. Chronic infection is associated with increased concentrations of antimyelin antibodies, activation of macrophages, and release of reactive oxygen radicals. Despite resolution of inflammation in surviving animals, canine distemper virus can persist in infected brain tissues.
Clinical signs vary with the extent of infection and include general listlessness; fever; upper respiratory tract infection (similar to kennel cough); keratoconjunctivitis sicca; serous to mucopurulent discharge; and vomiting and diarrhea, often associated with tenesmus. Animals may become severely dehydrated. Neurologic signs generally develop after recovery (generally at 1 to 3 weeks but potentially up to several months) and tend to be progressive. Mature animals can abruptly develop neurologic signs despite prior vaccination and no previous evidence of disease. Clinical signs of CNS involvement vary with the area of the CNS affected and with the magnitude of damage and include hyperesthesia, cervical rigidity, seizures, cerebellar signs, paraparesis or tetraparesis, and myoclonus. Diagnosis is based on immunologic testing of IgM (ELISA). Measurements of IgG in both serum and cerebrospinal fluid may be useful for detecting chronic CNS infections. Immunocytology may also be helpful in the diagnosis of canine distemper, although the need for special equipment renders this aid less practical.
The most appropriate approach for limiting morbidity and mortality associated with canine distemper is proper vaccination.29 Treatment continues to be largely supportive and focuses on prevention or treatment of bronchopneumonia (usually caused by Bordetella bronchiseptica), fluid and electrolyte support with supplementation of B vitamins as needed, and treatment of neurologic signs. Progression of neurologic signs may provide justification for treatment of cerebral edema (e.g., single administration of dexamethasone).29 Seizures should be treated with anticonvulsant medications (diazepam for immediate control, phenobarbital or bromide for long-term control). Myoclonus is not treatable. Chronic inflammatory forms of distemper (including optic neuritis, encephalitis) may require long-term glucocorticoid therapy. Glucocorticoids that are more effective in their ability to control oxygen radicals (e.g., methylprednisolone) may offer an advantage, although this has not been clinically addressed with controlled studies. Therapy with ascorbic acid intravenously has not been proved to be clinically useful but nonetheless has been recommended.29 Infections associated with measles in children apparently have responded favorably to two treatments with vitamin A (200,000 IU or 60 mg) if given within 5 days of the onset of clinical signs.29 Canine distemper virus is extremely susceptible to common disinfectants.
Infectious Canine Hepatitis
Infectious canine hepatitis30 initially localizes in the tonsils and spreads to regional lymph nodes and then to the bloodstream. The virus rapidly disseminates to all tissues, with hepatic parenchymal cells and vascular endothelial cells serving as the primary targets. Cytotoxic effects of the virus cause injury to the liver, kidney, and eye. In immunocompetent animals, infection is cleared within 7 days. Acute hepatic necrosis tends to develop in immunoincompetent animals. Although acute necrosis is the most common cause of death in animals surviving the initial phases of infection, it also can be self-limiting. Animals that respond with a partial neutralizing antibody tend to develop chronic active hepatitis, which can progress to fibrosis. Although renal lesions may develop with acute infection, progression to chronic renal disease apparently does not occur. Animals, however, remain prone to pyelonephritis. Ocular location of the virus occurs in about 20% of animals and can cause severe anterior uveitis and corneal edema. Ocular lesions tend to be self-limiting unless complications develop. DIC is a frequent acute complication of infectious canine hepatitis, probably triggered by widespread endothelial damage and activation of the clotting cascade. Decreased hepatic function and inability to clear products of degradation and to synthesize clotting factors contribute to DIC. Clinical signs in the acute stages of infectious canine hepatitis include enlargement of lymphoreticular tissues, fever, coughing, abdominal tenderness associated with hepatomegaly, and hemorrhagic diathesis. Less commonly, icterus and CNS signs may develop. Ocular lesions may be associated with blepharospasm, photophobia, cloudiness of the cornea, and ocular discharge. Diagnosis is based on clinical laboratory changes consistent with damage caused by infectious canine hepatitis and serologic testing.
Therapy is supportive and should continue until the liver has adequately healed from acute damage. Among the alternative therapies that might be considered is lactoferrin. Inhibition of growth has been demonstrated toward a number of viruses by the iron-binding protein lactoferrin. This endogenous compound is found in mucosal membranes, milk, and other tissues where it imparts other antimicrobial effects. Among the viruses targeted is canine herpesvirus, as has been demonstrated using in vitro techniques (canine kidney cells). The effects targeted viral replication and were independent of the iron-binding effects of the drug.31 Impaired interaction between the virus and cell receptors leading to altered viral–host cell attachment was a suggested mechanism.
Therapy focuses on fluid and electrolyte support, treatment as indicated for DIC (including both replacement therapy and anticoagulant therapy), and treatment for hepatic encephalopathy as needed in acute stages. Hypertonic glucose (0.5 mL/kg of a 50% solution given intravenously over 5 minutes) may be helpful in the presence of hypoglycemia. Polyinosinic–polycytidylic acid, an IFN inducer, has been used experimentally but is not a practical therapy. Persistence of chronic liver disease should be treated appropriately.
Infectious canine hepatitis is very resistant to many chemical disinfectants, including chloroform, ether, acid, and formalin. Chemical disinfectants that appear to be useful include iodine, phenol, and sodium hydroxide. The application of steam (5 minutes at 50° to 60° F) may be a reasonable method of disinfection for instruments.
Canine Infectious Tracheobronchitis (Kennel Cough)
The most common causative organisms of kennel cough are canine parainfluenza virus, a single-stranded RNA virus, and B. bronchiseptica.32 Other viruses and bacterial infections are also associated with the syndrome. Bacterial causes of tracheobronchitis are discussed in Chapter 8. Viral transmission occurs primarily by aerosol or, for some viruses, oronasal contact. The lack of viral replication in macrophages limits infection of the virus to the upper respiratory tract, although it is the viral-induced damage to the respiratory epithelium that allows secondary bacterial infection. B. bronchiseptica preferentially attaches to the respiratory epithelium, replicates on respiratory cilia, and releases potent toxins that impair phagocytosis and cause ciliostasis, allowing infection by opportunistic organisms. The most common clinical signs associated with canine infectious tracheobronchitis (ITB) is paroxysmal nonproductive coughing, often associated with retching. Edema of the vocal folds is responsible for the characteristic honking sound of the cough. History includes exposure to other dogs, often at a boarding facility. Diagnosis is based on history and clinical signs. Culture of the upper airways (by bronchoscopy or transtracheal wash) can support diagnosis of a bacterial component. Rising antibody titers may be helpful in identifying a specific viral etiology. Therapy focuses on control of cough and, in cases complicated by persistent bacterial infection, (as evidenced by mucopurulent discharge that emerges after viral phase) antimicrobials. Glucocorticoids may be helpful for controlling cough but do not appear to shorten the clinical outcome. Antitussive therapy should include both peripheral bronchodilators and centrally active drugs. Narcotic derivatives are more likely than non-narcotics to control cough associated with ITB. Aerosol therapy may be helpful in cases associated with marked accumulation of respiratory secretions or pneumonia. Mucolytics, such as N–acetylcysteine, may be very irritating to the respiratory tract and can be given orally or parenterally.
Parainfluenza virus is susceptible to sodium hypochlorite, chlorhexidine, and benzalkonium solution. Control of outbreaks in a kennel may require isolation of the entire facility for up to 2 weeks. Vaccines are available; intranasal vaccination may lead to clinical signs typical of ITB.
Canine Papillovirus
Papillovirus is a largely self-limiting infection. However, antiviral therapy might be considered in nonresponders or in the interest of improving the comfort of animals. One uncontrolled clinical trial reported response of infection with nonspecific immunomodulation. Dogs (n = 16) presenting with papillomas in the oral mucosae and palate were treated with 2 mg Propionibacterium acnes intramuscularly once per week. Response was realized in 2 weeks, with resolution of lesions occurring within 5 weeks in younger animals. However, in older animals response required treatment 3 times per week, with regression of lesions beginning at week 3 and completed by week 6. No significant side effects of therapy were reported, leading the authors to conclude that P. acnes would be a reasonable alternative for treatment of canine papillomas that have not naturally regressed.33 Other anecdotal treatments have included IFN–alpha–2a, 1–3 million IU/dog, orally 3 times per week.
Yagci and coworkers34 prospectively studied the positive effects of azithromycin (10 mg/kg once daily for 10 days) for treatment of canine oral (n = 12) or cutaneous (n = 5) papillomatosis using a double-blinded controlled design. Dogs were assigned to treatment groups based on entry into the study; 10 dogs (7 oral and 3 cutaneous) received treatment, whereas 7 did not. Cutaneous lesions on 1 dog in the placebo group spontaneously resolved at day 41. However, skin lesions in the 10 dogs with cutaneous lesions in the treatment group resolved in 10 to 15 days (although not stated in the report, it is assumed that all dogs with oral lesions also had skin lesions). The number of animals with oral lesions that responded was not provided. Recurrence of lesions was not evident during the 8-month follow-up period of the study.
Treatment of Selected Feline Viral Infections
Feline Panleukopenia
Feline panleukopenia35 is caused by parvovirus transmitted by direct contact between cats or between cats and vehicles acting as vectors. As with other parvoviruses, cells that are rapidly dividing are particularly susceptible to infection, including bone marrow, lymphoid tissue, and intestinal mucosal crypt cells. In utero infection can cause a number of reproductive disorders in the pregnant cat, ranging from loss of fetuses if infection occurs early in the pregnancy to birth of affected kittens. Injuries in kittens occur in the CNS, particularly the cerebellum, optic nerve, and retina. Panleukopenia causes acute signs, including fever; depression; anorexia; and, less frequently, vomiting. Dehydration can be extreme. Other potential clinical signs include ulceration, bloody diarrhea and icterus, and signs indicative of DIC. Queens infected during pregnancy may be diagnosed with infertility, and dead fetuses may mummify. Kittens affected in utero present with classic signs of cerebellar hypoplasia.
Diagnosis generally is based on a complete blood count. Therapy is symptomatic and focuses on fluid and electrolyte replacement (with vitamin B) and maintenance, antiemetics (generally metaclopramide), and broad-spectrum antimicrobials to control secondary infection. The use of antivirals has not been established. Diazepam or other appetite stimulants can be attempted in anorectic cats that are not vomiting. Blood transfusions may be indicated in the presence of severe anemia.
Feline Infectious Peritonitis (FIP)∗
The treatment of FIP has recently been reviewed.36 Although caused by a coronavirus, the pathophysiology of infection is complex, in part because a variety of coronaviruses are capable of infecting cats. Further, cat susceptibility to infection and subsequent development of FIP vary unpredictably.37 The underlying relationship between FIP virus and non-FIP feline corona virus (FeCoV) cannot yet be discriminated based on current serology methods alone. The role of polymerase chain reaction based assays has to be defined; the ABCD Guidelines for FIA indicates that it cannot diagnose FIP in that positive results have been obtained in healthy carriers and negative results may occur in cats with FIP. Rivalta’s test—based on high protein content, inflammatory mediators, and fibrinogen present in fluids of the effusive form—is characterized by a high predictive value.37a Among the feline corona viruses are strains whose pathogenicity and virulence vary from minimal, with replication limited to the gastrointestinal epithelium, to the virulent strains causing (FIP). Variability in virulence exists even within strains causing FIP. Cats infected with non-FIP corona virus are at risk to develop FIP as some strains appear to rapidly mutate to the virulent form. Virulence may be related to the ability of the virus to infect and replicate within macrophages. The “S” protein on the viral envelope appears to be responsible for viral attachment, membrane fusion, and virus-neutralizing antibody production. Infection and subsequent reinfection among carrier cats (e.g., in catteries or multiple-cat households) probably facilitates mutation. Cats do not develop FIP unless preexisting corona antibodies exist. Antibodies to the virus facilitate monocytes and macrophage infection (antibody-dependent enhancement), leading to dissemination. Cats that develop antibodies before mounting an effective cell-mediated response to FeCoV appear to develop the effusive form of FIP on reinfection; a partial cell-mediated response may result in the noneffusive form of the disease.
Clinical signs of FIP reflect immune-complex deposition (Arthus-type reaction) in smaller vessels. Clinical signs of FIP vary with the site of virus and immune complex deposition and generally reflect either an effusive or noneffusive form. Immune complexes that form in response to the virus or the specific viral antigen include both antiviral antibodies and complement. Activation of complement leads to the release of vasoactive amines, endothelial retraction, and increased epithelial permeability, which in turn allows exudation of the protein-rich exudate typical of FIP. If vascular permeability is the predominant effect, the effusive form develops. Less severe permeability with subsequent recruitment of inflammatory cells appears to lead to the pyogranulomatous inflammation characteristic of the noneffusive form. Neutrophil accumulation and subsequent release of lysozymes cause vascular necrosis. Systemic involvement may reflect spread of viral-infected macrophages and subsequent complement activation or deposition of immune complexes from circulation into tissues. Pyogranulomata develop, the magnitude of which reflects the size, number, and amount of antibody and antigens. Regions of high blood pressure and turbulence appear to be more common sites of deposition. Effusive FIP causes ascites with or without pleural effusions. Noneffusive FIP tends to be vague in presentation and includes fever, weight loss, anorexia, and depression. Ocular lesions are common, characterized by iritis, hypopyon, and hyphema. Pyogranulomata may be present in the vitreous or the retina. Neurologic signs are not uncommon and include ataxia, nystagmus, and seizures. Meningitis may lead to tremors, hyperesthesia, behavioral changes, or cranial nerve defects. Hydrocephalus also may develop.
Investigations into treatment of FIP have focused on both antiviral therapy (particularly IFN) as well as control of the immune response. A number of drugs have been studied for potential efficacy against FIP, some with little hope of being clinically applicable. For example, in one in vitro study, the rank of CD50: ED50 (the ratio of a cytotoxic to effective dose) of drugs toward FIP was pyrazofuin > 6-azauridine > 3-deazaguanosine > hygromycin B > fusidic acid > dipyridamole. Compounds with no effect were caffeic acid, carbodine, 3-deazauridine, 5-fluoroorotic acid, 5-fluorouracil, D(+)glucosamine, indomethacin, D-penicillamine, rhodamine, and taurine.38 More recent in vitro studies have demonstrated the potential efficacy of ribavirin or adenine arabinoside, but neither acyclovir nor AZT.
A variety of studies (as reviewed by Hartmann and others36) have focused on minimizing the immune and thus inflammatory response. However, studies have been characterized by a number of limitations, including failure to accurately diagnose FIP and limited sample size. Glucocorticoids with or without cyclosphosphamide have been associated with variable success in uncontrolled studies. Despite in vitro studies, ribavirin has not been proven effective and appears to be too toxic at doses that are necessary to achieve effective concentrations. Case reports have described variable success with thromboxane synthetase inhibitors, melphalan, chlorambucil, tylosin (for its immunomodulatory effects), promodulin, human IFN, P. acnes and the “paraimmunity” inducer Baypamum have all been reported either as single cases or in clinical trials. No clear effective treatment has emerged.
According to the ABCD, low dose human IFN- α is contraindicated and SC high dose was ineffective in cats with FIP. Most recently, efficacy of feline recombinant-omega IFN has been studied. A series of 12 cases of spontaneous FIP treated with a combination of glucocorticoids and recombinant feline-omega IFN (106 IU/kg administered subcutaneously every 48 hours until clinical improvement followed by once weekly); complete remission (over 2 years) was reported in 4 cats and partial remission (2 to 5 months) in another 4. However, all survivors were older cats (older than 5 years), with the effusive form of the disease.39 Hartmann and coworkers Ritz and coworkers36 failed to find a treatment effect compared to placebo for rF-INFω in cats (n = 37) whose FIP was confirmed histologically and immunohistochemically (ability to detect a significant difference may have been limited by sample size). Effusive disease was also treated with dexamethasone or prednisolone. rF-INFω was administered at 106 IU/kg subcutaneously daily for 8 days and then weekly thereafter. Despite ongoing and historical studies, effective treatment of FIP remains elusive. Anecdotal reports suggest efficacy of pentoxifylline for the effusive form of FIP. Supportive therapy includes fluids as necessary, antimicrobials, ascorbic acid, vitamin B, and vitamin A.
Options for treatment of ocular FIP (Table 10-4) include topical and oral glucocorticoids (prednisolone or dexamathasone) or a combination thereof. As a primary T-cell, rather than B-cell inhibitor, cyclosporine is not recommended. As with systemic disease, anecdotal reports suggest efficacy of pentoxifylline. Intracameral tissue plasminogen activase has been anecdotally suggested if fibrin does not resolve.
Table 10-4 Summary of the European Advisory Board on Cat Disease Guidelines for Feline Viral Diseases∗37a, 40a, 40b, 69a, 73, 74
Preventive efforts toward FIP infection are also complicated by the futility of identifying and removing carriers: serologic testing will identify only previous exposure to coronavirus, which will be true for the majority of cats. Vaccines thus far have proved ineffective because antibodies sensitize to rather than protect from the disease. Strains and route of inoculation will influence outcome of vaccination. Because animals will have been exposed by the time diagnosis is made, isolation is not necessary. However, environmental cleansing should be relatively easy. Although FeCoV is relatively stable in the environment, it is easily destroyed by most common detergents and disinfectants, including diluted (1:32) sodium hypochlorite solution.
Feline Respiratory Disease
Feline rhinovirus (FRV) and calicivirus (FCV) are the major viral causes of respiratory disease in the cat,40 but a number of bacterial organisms contribute to the pathogenesis, including B. bronchiseptica, Mycoplasma spp., and Chlamydia psittaci. Other viral organisms (e.g., reovirus, poxvirus) also may contribute. Rhinovirus is a herpesvirus. Natural routes for both viral infections are by way of the nasal, oral, and conjunctival mucosae. Viral replication of rhinovirus occurs primarily in the nasal mucosal epithelium and, for calicivirus, throughout the respiratory epithelium. Growth of rhinovirus tends to be restricted to areas of lower body temperature; thus lesions tend to be limited to the nasal mucosa and the pharynx. Lesions reflect necrosis and result in the typical clinical signs of marked sneezing, pyrexia, depression, and anorexia; cats may salivate. Conjunctivitis with chemosis and hyperemia are common, with mucopurulent discharge of the nares and eyelids developing. Oral ulceration is rare. Several syndromes have been described, including the classical acute rhinosinusitis and corneal disease; an atypical disease that may be accompanied by a systemic response (including fading kitten syndrome), and chronic rhinosinusitis disease. For the latter, damage to nasal turbinates can be extensive and permanent, leaving the infected cats susceptible to lifelong chronic upper respiratory tract infections (e.g., rhinitis, sinusitis) and conjunctivitis. Rhinosinusitis may also reflect a chronic allergic inflammatory response.40b Calicivirus infection has variable clinical signs because it is more likely to affect the lungs. Oral lesions (tip of the tongue, mouth, and nose) are the most predominant sign, reflecting epithelial necrosis; fever and mild respiratory and conjunctival signs also occur. Feline calicivirus also has been associated with chronic gingivitis and stomatitis. Sneezing and ocular and nasal discharge are not as common as with rhinovirus. Pulmonary lesions begin with alveolitis. Lameness also may occasionally develop. An often lethal, highly virulent form of FVC resulting in systemic disease has been reported, with the disease more severe in adults compared to kittens.40a The syndrome is associated with a systemic inflammatory response syndrome. Diagnosis using molecular-based assays should be made only cautiously for FVC as the presence of virus and clinical signs are poorly correlated.
Similarities between human and feline herpesvirus infection justifies the potential application of human antiherpetic drugs to treatment of feline infections. Accordingly, information regarding their use for treatment of feline respiratory infections is increasing. For example, using infected feline kidney cells, the inhibitory concentration (50%) was determined for a number of antiviral drugs toward FHV-1. In vitro efficacy of IDU and that of ganciclovir were approximately equivalent and approximately twice that of cidofovir and penciclovir. Foscarnet appeared to be comparatively ineffective.41 Also using in vitro techniques, cidofovir decreases cytopathic effects and viral load FHV-1 of feline corneal epithelia at concentrations of 0.05 and 0.02 mg/mL. However, cytotoxic effects also were evident in cultured cells.42 Van der Meulen and coworkers43 reported on the in vitro efficacy of six antiviral drugs [acyclovir, ganciclovir, cidofovir, foscarnet, adefovir, and 9–(2-phosphonylmethoxyethyl)–2, 6-diaminopurine (PMEDAP)], using an in vitro plaque reduction assay (embryo-derived feline kidney cells). Of the six drugs, ganciclovir, PMEDAP, and cidofovir were most effective in reducing plaque numbers and thus were cited as potentially viable candidates for treatment. In another in vitro study penciclovir was found to be much more potent (concentration by which plaques were reduced by 50% of 1.6 μg/mL) against FHV than acyclovir (24 μg/mL) and trifluorothymidine (5.7 μg/mL).44
Although in vitro studies are useful for identifying potential drugs, clinical trials are necessary to support their use. However, well-controlled clinical trials are limited. Stiles45 retrospectively described the use of a variety of drugs for treatment of FHV-1 in cats (n = 17). Drugs included IDU (n = 7), vidarabine (n = 4), and trifluridine (n = 3) as well as recombinant human alpha-IFN (PO, n = 3) in conjunction with topical administration of antiviral agents. Williams followed up on his 2004 in vitro study46 with a clinical trial involving cats (n = 30) with clinical signs associated with and demonstrated to be positive for FHV-1. Acyclovir ointment (0.5%) was applied 5 times daily; cats had been previously treated (for 21 days) with topical chlortetracycline three times daily for treatment of Chlamydia. No placebo or other control groups were studied. Cats that were FHV-1 positive did not respond to the 21 days of chlortetracycline therapy, but did respond to acyclovir in a median of 12 days of treatment. Cats that were treated only 3 times a day with acyclovir (due to poor owner compliance) did not respond initially, but did respond when the frequency of treatment was increased. Based on these studies and anecdotal reports, ocular herpetic infections can be treated topically with, in order of efficacy, trifluridine (1%) or idoxuridine (0.1%), vidarabine (3%), and acyclovir (3%). Each must be applied at 4-hour intervals for 1 week beyond the resolution of clinical signs.47 Preference as to idoxuridine or trifulridine as first choice may depend on cost and tolerance (irritation).
Anecdotal reports suggest the potential efficacy of other drugs. For example, famciclovir (31 mg or ¼ of a 125-mg tablet orally twice daily for 10 days) has been used for acute flare-ups in cats not exhibiting systemic signs of illness associated with upper respiratory tract infections. In their review, Caney and coworkers48 report that FHV-1 might be treated with topical use of trifluorothymidine (trifluridine; most potent; hourly the first 24 hours, then hourly during the waking hours, to every 4 hours as soon as re-epithelialization has occurred), IDU (every 2 to 4 hours for the first 24 hours, then 4 to 6 times daily until 1 week beyond clinical resolution of signs) and vidarabine. It is best if treatment is started early, but the duration of therapy should be limited to 2 to 3 weeks because of the risk of epithelial toxicity. Oral administration of AZT also should begin early, although it is a hundredfold less potent against FHV than it is toward human herpes simplex.
Other drugs might be considered for treatment of feline viral respiratory infections, although evidence for use is limited. Among those most commonly cited is lysine. Using a placebo-controlled design, L-lysine administration (400 mg in food once daily) was associated with a decrease in the incidence of conjunctivitis in cats with experimentally induced viral respiratory diseases.49 Animals were infected 5 months before the study such that infections were latent. Viral expression was induced by either the stress of rehousing or administration of glucocorticoids. The drug caused a delay in onset of clinical signs (average delay of 7 days) and a decrease in episodes of viral shedding 5 months but only in rehoused cats and not glucocorticoid-treated cats. The 400-mg dose yielded a maximum plasma concentration of about 450 nmol/mL after single dosing (two cats; time not noted) and approximately 300 nmol/mL at 3 hours after 30 days of therapy.49 Stiles and colleagues50 also studied the impact of lysine (500 mg orally twice daily) beginning 6 hours before experimental infection of cats (n = 4; an additional 4 cats received placebo). Because arginine is an essential amino acid, both arginine and lysine were analyzed in plasma. Lysine did not affect arginine concentrations, supporting its safe use in cats. Despite the small number of animals, clinical scores differed between the two treatment groups between days 5 and 15 after infection; however, the scores of the two groups were similar during the final week of the 21-day study, as resolution of clinical signs caused the scores to return to baseline (preinfection).This study provides some evidence of support; failure to maintain a significant difference at 21 days may reflect sample size. However, the efficacy of once-daily L–lysine (250 mg if less than 5 months or 500 mg if greater than 5 months) also was studied for its impact on emergent upper respiratory infection in animal shelter cats (n = 144 treated and 147 nontreated cats). No difference was found in the subsequent incidence of upper respiratory infection that emerged between the two treatment groups.51
KEY POINT 10-6
A consensus cannot yet be determined from clinical trials that address the efficacy of lysine for treatment of upper respiratory infections in cats.
Interferon also has been studied. Human recombinant drug has been used at 5 to 25 U per day orally, whereas rFeIFN-ω is recommended at 2 drops of 500,000U/mL saline topically 5 times a day. Sandmeyer and coworkers52 studied the in vitro effect of rFeIFN-ω n cultured corneal epithelial cells infected with FHV-1. Concentrations of 100,000 IU/mL significantly reduced virus-induced pathology without causing cytotoxicity. Siebeck and coworkers53 also studied in vitro the impact of recombinant human IFN α-2b and rFeIFN-ω in FHV-1 using feline kidney cells. Both plaque number and size were reduced by 100,000 U/mL of both recombinant IFNs; neither product at any concentration (up to 500,000 U/mL) caused cytotoxicity. The feline product was more effective but only at higher concentrations. Ohe and coworkers53a demonstrated in vitro sensitivity to recombinant feline IFN (the IFN type was not provided but the product is produced in Japan) by 5 strains of FCV causing breakthrough disease in vaccinated cats. No well-controlled study has identified drugs useful for treatment of feline calicivirus.
Supportive therapy for respiratory disease associated with viral infections must also be directed toward the secondary bacterial infection associated with the syndrome. This includes both removing debris that might support bacterial growth at the site of infection and controlling bacterial infection and inflammation. Antimicrobial therapy is best based on an appropriately collected culture, which may be difficult. Empirical selection should target the most likely infecting organisms (e.g., fluorinated quinolones, doxycycline, azithromycin). Note that ineffectual antibacterial therapy is likely to contribute to infection by P. aeruginosa. Alternating antimicrobial therapy may reduce the development of resistance; higher doses should be used not only because penetrability is likely to be impaired but also because minimizing the advent of resistance is important. Combination therapy should be considered with acute flare-ups associated with bacteria (see Chapter 8). Because oral medication may be difficult to administer, injections and medications that can be given once daily may be preferred. Nasal decongestants may be helpful during the acute phases, but note that α–adrenergic decongestants may contribute to nasal mucosal necrosis owing to impaired blood flow. Antihistaminergic products are probably preferable; among them are newer drugs that may have better efficacy than older antihistamines if they preclude mast cell degranulation (Zyrtec [cetirizine], 2.5 mg/cat daily). However, their use should be discontinued in the presence of purulent secretions. At this point, liquefaction of secretions may be paramount to success; a dysfunctional mucociliary apparatus may contribute to infection by providing an optimal environment for microbial growth. Mucolytic drugs and mucokinetics may facilitate movement of accumulated respiratory secretions. N-acetylcysteine can be given by injection (125 mg), although oral administration (⅛ tsp sprinkled on food) might be helpful despite significant first-pass metabolism of the compound. Aerosolization or local installation of saline also may be helpful if lesions tend to predominate in upper airways.
The impact of glucocorticoids on viral-induced feline respiratory infections is not well studied. In general, immune suppression is discouraged because of the risk of recrudescence of latent infections. However, in one study, administration of methylprednisolone (5 mg/kg intramuscularly) did not statistically increase shedding of FHV in experimentally infected cats compared with pretreatment shedding, although the power of the study to detect a significant difference was low.49
Feline Viral Neoplasia: Feline Leukemia Virus
FeLV is caused by an oncornavirus subfamily of retroviruses. Its pathophysiology has been well reviewed.5 Viral replication depends on the presence of reverse transcriptase (RNA-dependent DNA polymerase). The enzyme makes a provirus (a copy of DNA) that is subsequently inserted into the host genome. The binding site of FeLV is the major envelope glycoprotein (gp70); antibodies to this envelope provide protective immunity. Malignant cells also contain the feline oncornavirus cell membrane antigen (FOCMA); high levels of FOCMA antibodies render the cat resistant to viral-induced leukemia or lymphoma. FeLV is contagious, with transmission occurring by way of the saliva after close contact between cats. Iatrogenic transmission can occur through contaminated blood or instruments that penetrate (e.g., needles). Initial infection is characterized by malaise and lymphadenopathy. Cats that mount an adequate immune response recover. FeLV spreads hematogenously to the bone marrow in cats that do not mount an immune response. Cats can become latently infected, with the virus residing undetected (by ELISA, fluorescent antibody testing, or viral culture) in the bone marrow.
Cats infected with FeLV die as a result of viral-induced neoplasia (lymphoma or leukemia), suppression of the bone marrow (anemia), or infections caused by FeLV-induced immunosuppression. Bone marrow suppression occurs because FeLV can block differentiation of erythroid progenitors. Other mechanisms may also be involved. Platelet and leukocyte abnormalities also may occur, and a panleukopenia-like syndrome induced by FeLV has been described. Immunosuppression reflects disruption of T-cell function, ultimately affecting both cellular and humoral immunity. Immune complex disease has been described and can be induced experimentally with antibodies to gp70. Glomerulonephritis may be a sequela. Other disorders include those of the reproductive tract (infertility, abortions, endometritis), lymphadenopathy (most severe in submandibular lymph nodes), osteochondromas, and olfactory neuroblastomas. Diagnosis of FeLV is based on fluorescent antibody testing and ELISA. The indications and advantages for each are described elsewhere.54
Treatment of FeLV-related diseases varies with the syndrome resulting from the infection and, for most syndromes, is discussed in other chapters. Lymphoma is generally fatal in 1 to 2 months if not treated. Prognosis for complete remission is relatively good for the otherwise healthy cat.54 Treatment focuses on combinations of chemotherapeutic drugs and, for selected cancers (e.g., nasal lymphoma), radiation therapy. Single-agent glucocorticoids are relatively ineffective and are palliative only. The most commonly used combination of chemotherapeutic agents is cyclophosphamide, vincristine, and prednisone. Other drugs that might be added to this regimen, depending on the cell type and response, include doxorubicin and, less commonly, L-asparaginase, cytosine arabinoside, and methotrexate. Antiemetics may be necessary, as might appetite stimulants (cyproheptadine, diazepam, megestrol acetate).
Treatment of the cancer component of viral diseases is addressed in Chapter 33. Bone marrow suppressive disease has been treated with repetitive blood transfusions. Prednisolone may increase the life span of erythrocytes if immune-mediated destruction is contributing to anemia, but glucocorticoids also will contribute to immune suppression. Human recombinant (and, when available, canine recombinant) growth factors may be useful for increasing bone marrow production of precursor cells despite the fact that most animals with anemia have high endogenous concentrations. Response to human recombinant erythropoietin (100 U/kg, subcutaneously 3 times weekly) requires about 3 to 4 weeks. This product should be reviewed before its use. Likewise, recombinant granulocyte colony-stimulating factor may be of benefit in the treatment of leukopenias. Development of antibodies to both of these products may limit their use beyond several weeks.
No antiviral drug has been shown to clear a FeLV viremic cat. Drugs that target reverse transcriptase, such as AZT, offer the most promise for effective therapy. Zidovudine suppresses viral replication but will not eliminate the virus. Experimentally, the drug can prevent viremia when administered (60 mg/kg per day divided every 8 hours) within 96 hours of infection. Zidovudine (30 mg/kg per day) inhibited antigenemia in kittens and prolonged survival time from 35 to 102 weeks. Myelosuppression, however, occurred in 33% of the treated cats.
In another study AZT (10 to 20 mg/kg twice daily for 42 days) prevented retroviral infection in cats if administered immediately after virus exposure. Replication also may have been reduced when it was administered to previously infected animals.55 Serum-neutralizing antibodies developed in some of the infected cats, and the cats became resistant to subsequent viral challenge. However, progression of disease was not altered in cats if treatment was withheld until day 28 after infection, although the level of viremia was much lower than in untreated cats. Zidovudine appeared to be nontoxic in uninfected cats, although 3 of 12 infected kittens became anorectic and icteric and were vomiting after 40 days of treatment. Zidovudine may be beneficial in reducing FeLV-associated diseases; one study reported improved health status and a reduction of oral lesions in cats with FeLV-associated stomatitis.
In their review of antiviral therapy in cats, Caney and coworkers48 acknowledge the controversy regarding the efficacy of AZT for treatment of FIV and FeLV but report that positive cats with gingivitis or neurologic disorders have responded clinically to either oral or subcutaneous administration, with reversible anemia being the major side effect. Kociba and coworkers56 studied the effect of leukocyte-derived human IFN-α on FeLV-associated erythroid aplasia but found no beneficial effects. Other drugs have been studied for potential efficacy against FeLV. Phosphomethoxyethyl adenine (PMEA), another reverse transcriptase inhibitor, also has been studied in cats. Cats with stomatitis associated with FeLV responded better to PMEA (AZT was also given at 5 mg/kg every 12 hours), but adverse reactions to the drug are likely to limit its use. The combination of AZT with IFN (1.6 × 106 U/kg, SC, sid) or IFN by itself may reduce antigenemia, but antibody development may reverse the effect. Cogan55 evaluated the efficacy and safety of suramin (10 to 20 mg/kg) in two FeLV-infected cats. Although toxic signs were limited to vomiting and anorexia (both resolving between treatments), viremia in both peripheral blood cells and serum did not resolve. Serum viral infectivity transiently decreased during treatment but was significantly higher 14 days after treatment was discontinued. Previous in vitro studies by the same author revealed a 90% inhibition of infectivity at drug concentrations of 100 mg/mL. Immunomodulating therapies also have been studied or reported after empiric use in cats with FeLV-related diseases. Immunomodulators may provide the most effective means of treating or controlling FeLV-related diseases.
KEY POINT 10-7
Although the efficacy of AZT for treatment of FIV and FeLV is controversial, gingivitis or neurologic disorders associated with disease may respond clinically.
A clinical case of feline epitheliotrophic T-cell lymphoma with paraneoplastic eosinophilia that failed initial therapy responded when rhINFa2b was added. Response was based on clinical, hematogenous, and sonographic evaluation. Relapse coincided with detection of antibodies directed toward the IFN.58
Other immunomodulatory drugs have been studied for treatment of FeLV. Antibodies that target gp70 have proved useful experimentally only when given within 3 weeks of the initial infection. Immunostimulants including IFN-α, staphylococcal protein A (10 μg/kg twice weekly for 10 weeks, then monthly), P. acnes (0.5 mL intravenously twice weekly for 2 weeks, then weekly), acemannan (2 mg/kg twice weekly), and evening primrose (550 mg daily) have been used with variable success, but no well-designed study has proved efficacy. Staphylococcal protein A has reversed viremia in a few cats, but only a small number of cats have been studied. Ultimately, combinations of therapies (e.g., antiviral drugs combined with immune modulators) may prove most beneficial. For example, response to Staphylococcals protein A (intraperitoneal) and IFN (oral) or the combination thereof was studied in a clinical trial of 36 cats with spontaneous FeLV infection (animals with tumors were excluded). No differences were found in clinical scores, clinical pathology, or survival time, although the authors reported that owners reported improved health more often in those cats treated with Staphylococcals protein A, leading the authors to recommend this form of immunomodulation in conjunction with supportive care.59
Adoptive immunotherapy using autologous lymph node cells that have been activated and expanded ex vivo using interleukin-2 (IL-2) in short-term cultures resulted in clinical improvement within 2 to 4 weeks and lasting at least 13 months in 9 of 18 cats with FeLV; 4 of the 18 cats became antigen free.60
Feline Immunodeficiency Virus
Pathophysiology of infection
FIV, like HIV, is caused by a lentivirus.21 The pathophysiology of infection with FIV has been reviewed.1,6 FIV continues to serve as a model for the study of human lentiviruses, and information regarding transmission, pathogenesis, host response, and immune dysfunction antiviral strategies is often shared among the two syndromes.61 As with FeLV, transmission of FIV among cats occurs by way of saliva or blood, presumably through bite wounds. Transmission also can occur in utero or through milk ingested by nursing infants. Whereas CD4 receptors on T-cells serve as receptors for HIV, CD4 is not the receptor for FIV. One group of investigators has identified CD134 (OX40) as a primary receptor.62 However, CD4 cells decrease early in infection, and therefore FIV causes progressive disruption of normal immune function. Viral replication begins in lymphoid tissues and salivary glands and spreads to mononuclear cells and nonlymphoid organs. Clinical signs may occur during the initial phases of viremia. The cause of the decrease is not known, but the result is an inversion of the normal CD4/CD8 ratio in infected cats. CD8 cells may increase, contributing to the inversion. Formation of immunoglobulins (dysregulation may lead to hypergammaglobulinemia in some cases) and cytokines also is disrupted. Several phases of infection have been described after infection with FIV: an acute phase, followed by a clinically asymptomatic phase that varies in duration, and a terminal phase. Other phases have been described by other investigators.
As with HIV, clinical signs of FIV are highly variable, reflecting different tissues and the role of secondary pathogens. Secondary bacterial infections reflect opportunistic microflora. Infections by fungal (e.g., Cryptococcus) and protozoal (e.g., Toxoplasma) organisms also should be anticipated. Abnormal neurologic signs are not uncommon and may reflect an inflammatory response to altered astrocyte metabolism. Changes in behavior are most commonly reported, followed by seizures, paresis, motor abnormalities, and disrupted sleep patterns. Direct damage is the most common cause of neurologic signs, although secondary infection by Toxoplasma or Cryptococcus spp. should be considered. Abnormalities in renal function and wasting disease also may reflect either abnormal function or an inflammatory response in the respective organs. Ocular diseases include anterior uveitis (caused by either FIV or opportunistic secondary organisms), glaucoma, vitreal changes, retinal degeneration, and retinal hemorrhage. Respiratory disease generally reflects secondary infection. Neoplasia is a common reason for presentation. A number of tumor types have been reported in FIV-infected cats, including lymphomas (usually B cell) and leukemias. Diagnosis is based on clinical signs and serologic testing.
Treatment
Therapy of FIV has largely focused on supportive care. Antiviral therapy thus far has been unrewarding, but newer information may provide potentially effective choices. Both AZT and PMEA have been studied; although neither drug thus far has prevented infection, onset to detectable viremia and immunologic changes can be prolonged. A trend toward normalization of inverted CD4:CD8 ratios and clinical evidence of improvement in diseases such as stomatitis have occurred. Of the two drugs, AZT is most likely to improve the quality of life of a cat infected with FIV. In general, improvements in the cat’s general condition, immune status, and quality of life can be expected, along with a longer life span. Benefits of immunomodulators in cats with FIV are not clear, and assessment of scientific studies is clouded by the additional use of antimicrobials and other drugs. Immunostimulation should be avoided, however, because of the association of enhanced immune response with enhanced production of FIV experimentally.
In human patients with AIDs, highly active antiretroviral therapy has essentially revolutionized therapy, often rendering the lethal disease into a chronic but often manageable disease. The combination therapy is designed to suppress viral replication while preserving and potentially repairing the host immune response. Combination therapy generally includes two antiretroviral drugs such as AZT or lamivudine, which target early viral replication, with an HIV-1 protease inhibitor that targets later stages of replication. Other antiretroviral drugs approved for use in humans include ddi (didanosine), ddc (zalcitabine), D4T (stavudine), 3TC (lamivudine), and most recently Ziagen (abacavir). The first protease inhibitor approved in the United States was saquinavir (late 1995); others approved since then include ritonavir, indinavir, nelfinavir, and amprenavir. This approach has proved to be effective in decreasing viral loads and improving the CD4:CD8 ratio in human patients with AIDS; mortality and morbidity of HIV infection have subsequently been reduced. However, treatment paradigms have shifted from “hit hard and early” to delaying aggressive therapy until clinical signs have progressed. Whether a similar approach should be considered for treatment of FIV is not clear, although the indications and criteria for decision making have been reviewed.63 Unfortunately, although FIV, like HIV, is susceptible to nucleoside analogs, it may not be susceptible to currently prescribed HIV-1 protease inhibitors, although this may change for newer protease inhibitors.20
FIV is susceptible in vitro to a number of nucleoside analogs, including AZT, zalcitabine, didanosine, and lamivudine in vitro at concentrations similar to those necessary to inhibit HIV-1. However, identifying the proper dose will be important; for example, subinhibitory concentrations of AZT increased the (in vitro) mutation frequency of FIV in a dose-dependent manner.64
As with patients with HIV, feline patients infected with FIV and subsequently treated with AZT show delayed onset of viremia, reduced plasma virus loads, and clinical improvement. Zivudine has been successfully used at 5 to 15 mg/kg orally every 12 hours to treat FIV-induced neurologic manifestations and 5 mg/kg subcutaneously every 12 hours to treat stomatitis, conjunctivitis, and alopecia; it improved the CD4:CD8 ratio. Quality and quantity of life also improved.18 However, Hayes and coworkers65 studied the effect of AZT in kittens, focusing on pathophysiology of the disease at the thymus. The loss of thymic function in kittens infected with FIV appears to reflect an inflammatory process that continues even if viral burden is significantly reduced. In 8-week-old kittens experimentally infected with FIV, zidovudine monotherapy reduced viral load in peripheral blood lymphocytes, plasma, and thymus, compared with saline-controlled kittens. However, an impact on neither thymus lesions nor CD4 could be detected, and neither thymic involution nor CD4 cell decline were prevented.65
According to Jordan and coworkers,20 combination therapy with AZT and lamivudine demonstrated some clinical benefit in infected cats receiving experimental bone marrow transplants, although specifics were not provided. Using in vitro methods, AZT or lamivudine alone or in combination were somewhat effective in FIV-infected peripheral blood mononuclear cells, with the combination resulting in an additive or synergistic effect.66 Follow-up in vivo studies were performed in specific pathogen-free cats receiving the combination before infection, at the same time as infection, or 2 weeks after infections. Doses were high (50 to 75 mg/kg every 12 hours). The authors found that the combination was helpful in preventing (five of six cats) but not treating infection. Both infection and antibody seroconversion were delayed in all treatment groups. However, adverse drug reactions (anemia and neutropenia) occurred at either treatment dose, although these resolved when the dose was dropped to 10 mg/kg twice daily.
Interferon
Using in vitro techniques, viral replication was decreased in feline cell lines infected with FIV and subsequently treated rFeINF-ω, but not rFeIFN-γ. A similar effect was found with simultaneous infection and treatment of peripheral mononuclear blood cells: replication was decreased with both rhIFN-α2 and rFeINF–ω, but not rFeIFN-γ. Pretreating 3 days before infection did not improve efficacy of rFeIFN-γ.67 The effect of subcutaneous administration of rFeIFN-v (1 million U/kg per day) for 5 consecutive days on day 0, 14, 60 was studied in 81 cats experimentally infected with FeLV or FeLV/FIV (based on ELISA) using a multicentric double-blinded placebo controlled design.68 All cats were exhibiting clinical signs associated with infection, but the study did not include cats with malignancy. The treatment group receiving IFN therapy was associated with less mortality, improvement in hematologic indicators, and improved clinical scores compared with placebo-treated cats.68 However, in another study, although well tolerated, rf-IFN α did not significantly alter CD4:CD8 ratios or proviral load in cats with experimentally induced chronic FIV infection after treatment at either a high dose at 106 U/kg per day subcutaneously for 5 days or a lower dose of 104 U/cat orally per day for 6 weeks.48
Pedretti and coworkers57 reported on the administration of a low dose (10 IU/kg) of natural human IFN-α (a combination of at least 9 different α subtypes) in naturally infected cats (n = 24; six placebo-treated cats). The product was diluted in phosphate-buffered saline, fortified with bovine albumin, filtered, and administered over the gums. The product was administered using a 7-days-on, 7-days-off cycle for 6 months; after 2 months of no therapy, another round was instituted. Treatment was considered easy by practitioners and was well tolerated with no overt side effects in cats. All treated cats survived the treatment period except for one cat that was seriously ill at study start; in contrast, only one placebo-treated cat survived the initial 6-month treatment period. Fever and lymphadenopathy resolved in the treatment group by day 10 of therapy but persisted throughout the study in the placebo group. However, viremic counts (excluding two cats with outlying high counts) did not change among groups. Survivability of CD4 cells was better in treated cats, although CD8 counts slowly increased such that the balance between CD4 and CD8 cats was not maintained. White cell counts declined in placebo cats. The placebo group, but not the treatment group, was characterized by progressive liver disease and failure. The authors indicated that the favorable response probably reflected the downregulation of inflammatory cytokines realized only with low (1 to 10 IU/kg) doses and the loss of this control such that proinflammatory responses occur with higher doses.
In a study of 40 naturally infected FIV cats treated with AMD3100 (a bicyclam chemokine receptor inhibitor; 0.5 mg/kg every 12 hours, administered subcutaneously), PMEA (10 mg/kg twice a week), or a combination of the two drugs for 6 weeks, stomatitis improved with either PMEA or the combination therapy. Further, the provirus load decreased in the AMD3100group compared with other groups.69,69a However, treatment in either PMEA group was accompanied by decreased red blood cell, hemoglobin, and hematocrit counts; serum magnesium was decreased in the AMD3100 group. The authors concluded that the combination therapy was less effective than the use of the bicyclam alone. Guidelines of the ABCD recommend its use for treatment of FIV.
Bleomycin inhibits HIV viral replication apparent through oxygen-radical generation that also characterizes its anticancer effects. The use of bleomycin in combination with highly active antiretroviral therapy, particularly in those situations in which resistance has developed, has been recommended;70 efficacy in cats infected with FIV has not yet been reported.
The efficacy of two acyclic phosphonyl adenine nucleosides, (fluoro, or FPMPA and methoxyethyl; PMEA) ameliorated clinical symptoms of FIV. Response included the incidence and severity of stomatitis, immunologic parameters such as relative and absolute CD4+ lymphocyte counts, and virologic parameters, including proviral DNA levels in peripheral blood mononuclear cells. However, of the two FPMPA was not associated with hematologic side effects, even at 2.5-fold higher dose, compared with PMEA.71
Because of its efficacy in mice, 16alpha-bromo-epiandrosterone (epiBr), a synthetic derivative of the natural hormone dehydroepiandrosterone (DHEA), also has been evaluated for efficacy against experimentally induced FIV infection in cats.72 Two treatment regimens were studied: 5 consecutive days for weeks 0, 4, 8, and 16, or treatment 1 week before infection and continuing for 4 weeks after infection. All animals were studied for 20 weeks. For both groups, compared with control animals, CD4: CD8 T-cell ratio and total CD4 cell counts were less and CD8 cells higher from weeks 2 through 20 after infection. Although virus load was initially higher in treated cats, viremia subsequently declined to less than that of controls, and treated cats had higher FIV–p24 antibody responses.72
1. Elder J.H., Sundstrom M., de Rozieres S., et al. Molecular mechanisms of FIV infection. Vet Immunol Immunopathol. 2008;123:3-13.
2. Hayden F.G. Antiviral agents (non-retroviral). In: Hardmen J., Limbird L., editors. Goodman & Gilman’s the pharmacologic basis of therapeutics. ed 10. New York: McGraw-Hill; 2001:1313-1347.
3. Grandvaux N., tenOever B.R., Servant M.J., et al. The interferon antiviral response: from viral invasion to evasion,. Curr Opin Infect Dis. 2002;15:259-267.
4. Castelli J., Hassel B.A., Wood K.A., et al. A study of the interferon antiviral mechanism: apoptosis activation by the 2–5A system,. J Exp Med. 1997;186(6):967-972.
5. Levy L.S. Advances in understanding molecular determinants in FeLV pathology. Vet Immunol Immunopathol. 2008;123:14-22.
6. Willett B.J., Hosie M.J. Chemokine receptors and co-stimulatory molecules: unravelling feline immunodeficiency virus infection. Vet Immunol Immunopathol. 2008;123:56-64.
7. Carrasco L. The replication of animal viruses. Shugar D., editor. Viral chemotherapy. vol I. 1984. Pergamon. New York. 111-148.
8. Hayden F.G. Antiviral agents (nonretroviral). In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill, 2006.
9. Izzedine H., Launay-Vacher V., Deray G. Antiviral drug–induced nephrotoxicity. Am J Kidney Dis. 2005;45:804-817.
10. Chilar T., Lin D.C., Pritchard J.B., et al. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1,. Mol Pharmacol. 1999;56:570-580.
11. Richardson J.A. Accidental ingestion of acyclovir in dogs: 105 reports. Vet Hum Toxicol. 2000;42(6):370-371.
12. Dolin R. Antiviral chemotherapy and chemoprophylaxis. Science. 1987;227:1296-1303.
13. Gustafson D.P. Antiviral therapy. Vet Clin North Am Small Anim Pract. 1986;16:1181-1189.
14. Nasisse M.P., Dorman D.C., Jamison K.C., et al. Effects of valacyclovir in cats infected with feline herpesvirus 1. Am J Vet Res. 1997;58:1141-1144.
15. Thomasy S.M., Maggs D.J., Moulin N.K., et al. Pharmacokinetics and safety of penciclovir following oral administration of favciclovir to cats. Am J Vet Res. 2007;68:1252-1258.
16. Povey R.C. Effect of orally administered ribavirin on experimental FCV infection in cats. Am J Vet Res. 1978;39:1337-1341.
16a. Straw J.A., Loo T.L., de Vera C.C., et al. Pharmacokinetics of potential anti-AIDS agents thiofoscarnet and foscarnet in the cat,. J Acquir Immune Defic Syndr. 1992;5(9):936-942.
17. De Clercq E. New selective antiviral agents active against the AIDS virus. Trends Pharm Sci. 1987;8:339-345.
18. Zhang W., Maudin J.K., Schmidt C., et al. Pharmacokinetics of zidovudine in cats. Am J Vet Res. 2004;66:835-840.
19. Zhang W., Maudin J.K., Schmidt C.W., et al. Pharmacokinetics of lamivudine in cats. Am J Vet Res. 2004;66:841-846.
20. Jordan H.L., Pereira A.S., Cohen M.S., et al. Domestic cat model for predicting human nucleoside analogue pharmacokinetics in blood and seminal plasma. Antimicrob Agents Chemother. 2001;45(7):2173-2176.
21. Sellon R.K., Hartmann K. Feline immunodeficiency virus infection. In Greene C.E., editor: Infectious diseases of the dog and cat, ed 3, St Louis: Saunders, 2006.
21a. Zhu Y., Antony J.M., Martinez J.A., Glerum, et al. Didanosine causes sensory neuropathy in an HIV/AIDS animal model: impaired mitochondrial and neurotrophic factor gene expression,. Brain. 2007;130(Pt 8):2011-2023.
22. Tavares L., Roneker C., Johnston K., et al. 3’-Azido-3’-deoxythymidine in feline leukemia virus-infected cats: a model for therapy and prophylaxis of AIDS. Can Res. 1987;47:3190-3194.
23. Wonderling R., Powell T., Baldwin S., et al. Cloning, expression, purification and biological activity of five feline IFN-alpha subtypes. Vet Immunol Immunopathol. 2002;89:13-27.
24. Baldwin S.L., Powell T.D., Sellins K.S., et al. The biological effects of five feline IFN-alpha subtypes,. Vet Immunol Immunopathol. 2004;99(3-4):153-167.
24a. Gingerich DA: Lymphocyte T-cell immunomodulator (LTCI): Review of the immunopharmacology of a new veterinary biologic. Int J Vet Res. 2008;6(2):61-68.
25. Robertson C.M., Hermann L.L., Coombs K.M. Mycophenolic acid inhibits avian reovirus replication. Antiviral Res. 2004;64:55-61.
26. Martin V., Najbar W., Gueguen S., et al. Treatment of canine parvoviral enteritis with interferon-omega in a placebo-controlled challenge trial. Vet Microbiol. 2002;89(2–3):115-127.
27. de Mari K., Maynard L., Eun H.M., et al. Treatment of canine parvoviral enteritis with interferon-omega in a placebo-controlled field trial. Vet Rec. 2003;152(4):105-108.
28. McCaw D.L., Hoskins J.D. Canine viral enteritis. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:63-73.
29. Greene C.E., Appel M.J. Canine distemper. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:25-41.
30. Greene C.E. Infectious canine hepatitis and canine acidophil cell hepatitis. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:41-47.
31. Tanaka T., Nakatani S., Xuan X. Antiviral activity of lactoferrin against canine herpes virus. Antiviral Res. 2003;60:193-199.
32. Ford R.B. Canine infectious tracheobronchitis. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:54-61.
33. Megid J, Júnior JGD, Nardi G: Efficacy of canine papillomatosis treatment using propionibacterium acnes (P. acnes). Paper presented at the International Symposium on Predictive Oncology and Intervention Strategies, February 7-10, Nice, France, 2004, in poster session 995 (Immunotherapy).
34. Yagci B.B., Ural K., Öcal N., et al. Azithromycin therapy of papillomatosis in dogs: a prospective, randomized, double-blinded, placebo-controlled clinical trial. Vet Dermatol. 2008;19:194-198.
35. Greene C.E., Addie D.D. Feline parvovirus infections. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:78-88.
36. Hartman K., Ritz S. Treatment of cats with feline infectious peritonitis. Vet Immunol Immunopathol. 2008;123:172-175.
37. Addie D.D., Jarrett O. Feline coronavirus infection. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:88-102.
37a. Truyen U., Addie D., Belák S., et al. Feline panleukopenia. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11(7):538-546.
38. Barlough J.E., Shacklett B.L. Antiviral studies of feline infectious peritonitis virus in vitro. Vet Rec. 1994;135(8):177-179.
39. Ishida T., Shibanai A., Tanaka S., et al. Use of recombinant feline interferon and glucocorticoid in the treatment of feline infectious peritonitis. J Feline Med Surg. 2004;6(2):107-109.
39a. Ritz S., Egberink H., Hartmann K. Effect of feline interferon-omega on the survival time and quality of life of cats with feline infectious peritonitis. J Vet Intern Med. 2007;21(6):1193-1197.
40. Gaskell R.M., Dawson S., Radford A. Feline respiratory disease. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:145-154.
40a. Radford A.D., Addie D., Belák S., et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11(7):556-564.
40b. Thiry E., Addie D., Belák S. Feline herpesvirus infection. ABCD guidelines on prevention and management. J Feline Med Surg Jul;. 2009;11(7):547-555.
41. Maggs D.J., Clarke H.E. In vitro efficacy of ganciclovir, cidofovir, penciclovir, foscarnet, idoxuridine, and acyclovir against feline herpesvirus type-1. Am J Vet Res. 2004;65(4):399-403.
42. Sandmeyer L.S., Keller C.B., Bienzle D. Effects of cidofovir on cell death and replication of feline herpesvirus-1 in cultured feline corneal epithelial cells. Am J Vet Res. 2005;66(2):217-222.
43. van der Meulen K., Garre B., Croubels S., et al. In vitro comparison of antiviral drugs against feline herpesvirus 1. BMC Vet Res. 2006;2:13.
44. Williams D.L., Fitzmaurice T., Lay L., et al. Efficacy of antiviral agents in feline herpetic keratitis: results of an in vitro study. Curr Eye Res. 2004;29(2-3):215-218.
45. Stiles J. Treatment of cats with ocular disease attributable to herpesvirus infection: 17 cases (1983-1993). J Am Vet Med Assoc. 1995;207(5):599-603.
46. Williams D.L., Robinson J.C., Lay E., et al. Efficacy of topical aciclovir for the treatment of feline herpetic keratitis: results of a prospective clinical trial and data from in vitro investigations. Vet Rec. 2005;157:254-257.
47. Stiles J. Ocular infections. In: Green C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:974-991.
48. Caney S.M.A., Helps C.R., Finerty S., et al. Treatment of asymptomatic chronically FIV–infected barrier-maintained cats with recombinant feline interferon omega. J Vet Int Med. 2003;17:423.
49. Maggs D.J., Nasisse M.P., Kass P.H. Efficacy of oral supplementation with L-lysine in cats latently infected with feline herpesvirus. Am J Vet Res. 2003;64:37-42.
50. Stiles J., Townsend W.M., Rogers Q.R., Krohne S.G. Effect of oral administration of L-lysine on conjunctivitis caused by feline herpesvirus in cats. Am J Vet Res. 2002;63:99-103.
51. Rees T.M., Lubinski J.L. Oral supplementation with L-lysine did not prevent upper respiratory infection in a shelter population of cats. J Feline Med Surg. 2008;10:510-513.
52. Sandmeyer L.S., Keller C.B., Bienzle D. Effects of interferon-α on cytopathic changes and titers for feline herpesvirus-1 in primary cultures of feline corneal epithelial cells. Am J Vet Res. 2005;66:210-216.
53. Siebeck N., Hurley D.J., Garcia M., et al. Effects of human recombinant alpha-2b interferon and feline recombinant omega interferon on in vitro replication of feline herpesvirus-1. Am J Vet Res. 2006;67:1406-1411.
53a. Ohe K., Takahashi T., Hara D., et al. Sensitivity of FCV to recombinant feline interferon (rFeIFN),. Vet Res Commun. 2008;32(2):167-174.
54. Hartmann K. Feline leukemia virus infection. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:105-131.
55. Cogan D.C. Effect of suramin on serum viral replication in feline leukemia virus-infected pet cats. Am J Vet Res. 1986;47:2230-2232.
56. Kociba G.J., Garg R.C., Khan K.N.M., et al. Effects of orally administered interferon-α on the pathogenesis of feline leukaemia virus-induced erythroid aplasia. Comp Haematol Inter. 1995;5(2):79-83.
57. Pedretti E., Passeri B., Amadori M., et al. Low dose interferon-α treatment for feline immunodeficiency virus infection. Vet Immunol Immunopathol. 2006;109:245-254.
58. Cave T.A., Gault E.A., Argyle D.J. Feline epitheliotrophic T-cell lymphoma with paraneoplastic eosinophilia–immunochemotherapy with vinblastine and human recombinant interferon alpha2b. Vet Comp Oncol. 2004;2(2):91-97.
59. Macaw D.L., Boon D., Jargons A.E., et al. Immunomodulation therapy for feline leukemia virus infection. J Am Anim Hosp Assoc. 2001;37(4):356-363.
60. Blakeslee J., Noll G., Richard O. Autologous lymph node lymphocytes. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(1):1-6.
61. Giannecchini S., Di Fenze A., D’Ursi A.M., et al. Antiviral activity and conformational features of an octapeptide derived from the membrane-proximal ectodomain of the feline immunodeficiency virus transmembrane glycoprotein. J Virology. 2003;77(6):3724-3733.
62. Shimojima M., Miyazawa T., Ikeda Y., et al. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 20;. 2004;303(5661):1192-1195.
63. Louie M., Markowitz M. Goals and milestones during treatment of HIV-1 infection with antiretroviral therapy: a pathogenesis-based perspective. Antiviral Res. 2002;55:15-25.
64. LaCasse R.A., Remington K.M., North T.W. The mutation frequency of feline immunodeficiency virus enhanced by 3’-Azido-3’-deoxythymidine,. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(1):26-32.
65. Hayes K.A., Phipps A.J., Francke S., et al. Antiviral therapy reduces viral burden but does not prevent thymic involution in young cats infected with feline. Immunodefic Virus Antimicrob Agents Chemother. 2000;44(9):2399-2405.
66. Arai M., Earl D.D., Yamamoto J.K. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet Immunol Immunopathol. 2002;85(3-4):189-204.
67. Tanabe T. Feline immunodeficiency virus lacks sensitivity to the antiviral activity of feline IFN–γ. J Interferon Cytokine Res. 2001;21(12):1039-1046.
68. de Mari K., Maynard L., Sanquer A., et al. Therapeutic effects of recombinant feline interferon-v on feline leukemia virus (FeLV)-infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic. J Vet Intern Med. 2004;18:477-482.
69. Stengel C., Klein D., Egerbink H. Placebo-controlled double blind treatment study in naturally feline immunodeficiency virus infected cats using the chemokin receptor inhibitor 1,1-Bis 1,4,8,11-tetra-azacylotetradekan (AMD3100). J Vet Int Med. 2003;17:381.
69a. Gfuffydd-Jones T., Hartmann K., Hosie M.J., et al. Feline leukaemia. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11(7):565-574.
70. Georgiou N., van der Bruggen T., Oudshoorn M., et al. Mechanism of inhibition of the human immunodeficiency virus type 1 by the oxygen radical generating agent bleomycin. Antiviral Res. 2004;63:97-106.
71. Artmann K., Kuffer M., Balzarini J. Efficacy of the acyclic nucleoside phosphonates(S) -9- (3-fluoro-2- phosphonylmethoxypropyl) adenine (FPMPA) and -(2-phosphonylmethoxyethyl) adenine (PMEA) against feline immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(2):120-128.
72. Pedersen N.C., North T.W., Rigg R., et al. 10-16alpha-Bromo-epiandrosterone therapy modulates experimental feline immunodeficiency virus viremia: initial enhancement leading to long-term suppression. Vet Immunol Immunopathol. 2003;94(3-4):133-148.
73. Hosie M.J., Addie D., Belák S., et al. Feline immunodeficiency. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11(7):575-584.
74. Thiry E., Addie D., Belák S., et al. H5N1 avian influenza in cats. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11(7):615-618.