8 Treatment of Bacterial Infections
This chapter focuses on the treatment of bacterial infections on a systems basis. In addition, selected organisms are discussed because of their unique nature and the difficulties encountered when treating such infections. In general, the ease with which microbes appear to become resistant requires dosing regimens that are based on scientific studies demonstrating both efficacy toward the microbe and pharmacokinetics in the target species; if the latter is not available, extrapolated doses should be promulgated by persons with expertise (e.g., a veterinary clinical pharmacologist). Because of limited evidence-based information in veterinary medicine, data from the human-medicine literature may serve as a basis for recommendations. Information supporting the judicious use of antimicrobial drugs is found in Chapters 6 and 7. This includes but is not limited to data referring to drug concentrations achieved in the plasma at recommended doses (see Table 7-7), and other pharmacokinetic data (see Table 7-1) and tissue-to-drug ratios of antimicrobial drugs (see Table 7-5). In addition, population pharmacodynamic data are available that indicate the concentration of drug necessary to inhibit the microbes (minimum inhibitory concentration [MIC]) for selected drugs: See Table 7-3 (Escherichia coli), Table 7-4 (human data for selected microbes for which veterinary information could not be consistently found), Table 7-9Table 7-10 (data for beta-lactams), and Table 7-12 (fluoroquinolones). Selected pharmodynamic data are also provided in this chapter for infections associated with specific body systems. This includes an example of regional cumulative antimicrobial susceptibility antibiograms that might help guide empirical therapy, which is increasingly discouraged as resistance emerges (Figure 8-1). The clinician is encouraged to consider the approach to individualized antimicrobial therapy presented in Chapter 6. This includes Figure 6-1, which is an algorithm for antimicrobial therapy that is intended to minimize the risk of antimicrobial resistance, particularly in the at-risk patient. Algorithms have also been offered for infections of selected body systems. Preventing resistance is among the more important considerations that must be made by veterinarians using antimicrobials. (Box 8-1). A three “Ds” approach includes “detergent” (address hygiene at multiple levels), “de-escalating” drug use, and “design” dosing optimal regimens. Hygiene, which goes beyond simple hand washing, also is a critical component of preventing emerging resistance. Resistance is also minimized by de-escalating drug use. This includes simply decreasing the number of antimicrobial drugs prescribed and dispensed. However, de-escalating also entails moving to a lower tier of drug classes (i.e., one that is less broad in its actions and less “effective” toward microbes that tend to develop multidrug resistance). Although it is critical to use the best drug possible such that the infecting population is killed, once this is accomplished, de-escalation to a less-important lower tier class of drugs should be possible. First-tier drugs might include both narrow and broader spectrum beta-lactams (amoxicillin with without clavulanic acid), clindamycin, tetracyclines, and potentiated sulfonamides. Second-tier drugs might include those drugs characterized by spectra purposefully extended to target organisms not generally susceptible to first-tier drugs. Newer drugs such as third-generation cephalosporins, extended-spectrum penicillins, and fluoroquinolones might be included in this category. Therapy should be based on culture and susceptibility testing data, whenever possible, and population pharmacodynamic statistics if patient MIC data are not available. Third-tier drugs include those that tend to be reserved for treatment of microbes associated with either inherent or acquired resistance. Their use should be based on culture and susceptibility testing data and should be de-escalated to a lower-tier drug as soon as possible. Examples might include drugs that target multidrug-resistant gram-negative (aminoglycosides, carbapenems) and gram-positive (glycopeptides, linezolids) organisms. The American College of Veterinary Internal Medicine1 and the International Society of Companion Animal Infectious Disease (publications pending) have promulgated guidelines intended to minimize emerging resistance.
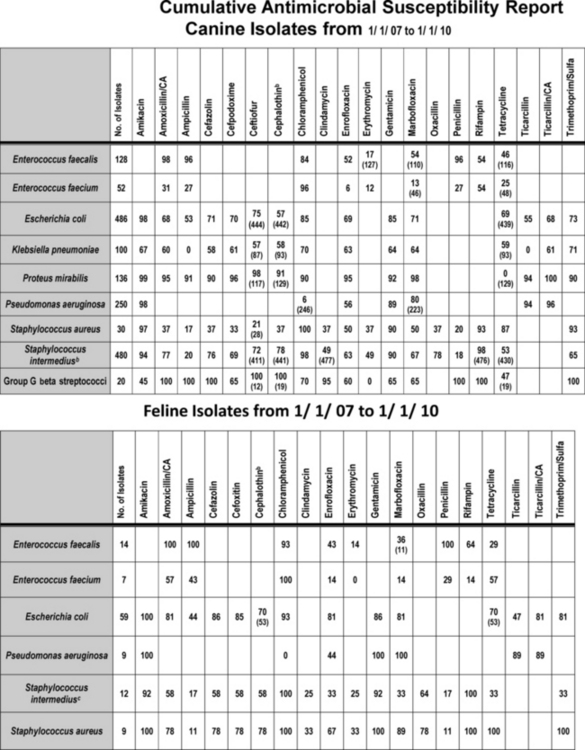
Figure 8-1 Cumulative antimicrobial susceptibility reports for canine (top) and feline (bottom) organisms isolated from samples collected during the years 2007 to 2010 at a veterinary teaching hospital. The data are geographically restricted to Alabama and are from a teaching hospital that has a wide referral base. Accordingly, the data may not be relevant to other areas of the United States nor to patients with first time infections. No attempt has been made to separate data according to history, including previous antimicrobial exposure. Ideally, each practice would generate a cumulative report at frequent intervals (e.g., yearly or every other year). The report might serve as a basis for empirical selection of antimicrobials. However, the number of isolates must be sufficient to represent the population; ideally, at least 100 isolates of each organism should be sampled. Drugs that are not included in the antibiogram for each organism generally are not included because the use of the drug for that organism is inappropriate. Each cell indicates the percentage of the tested isolates that were considered susceptible to the drug on the basis of Clinical and Laboratory Standards Institute (CLSI) guidelines promulgated in 2008. The first row lists the drugs to which susceptibility was determined for the organisms. The far left column names the genus and species of the organisms isolated and for which susceptibility was determined. The second from the left column indicates the total number of isolates tested. For some drugs not all isolates were tested; in such instances the total number of isolates tested is indicated in parentheses in that cell. New CLSI interpretive criteria for selected drugs (approved in 2010), including amoxicillin–clavulanic acid cephalexin and its model drug, cephalothin, are likely to result in a marked decrease in the percentage of susceptible isolates. Cephalothin continues to act as a class drug, representing first-generation cephalosporins (see Chapter 6 for a discussion of limitations of model drugs; e.g., cephalexin should not be used to treat E. coli, as a general rule). Staphylococcus intermedius represents what is currently referred to as S. intermedius group.
(Data provided by Terri Hathcock, MS, Diagnostic Veterinary Microbiologist, Infection Control Officer, College of Veterinary Medicine, Auburn University.)
Box 8-1 Treatment of Multidrug-Resistant Microorganisms
KEY POINT 8-1
A three-pronged approach is indicated for preventing antimicrobial resistance: escalating hygiene, de-escalating antimicrobial drug use, and optimizing dosing regimens such that the infecting inoculum is eradicated, not simply inhibited.
Infections of the Central Nervous System and Special Senses
Meningitis
Meningitis serves here as the prototypic infection of the central nervous system (CNS).
Physiology and Pathophysiology
Infections of the CNS are uniquely problematic for three reasons: cellular components reflect functional specialization, a major portion of the CNS is sequestered from the rest of the body by physiologic barriers, and tissues of the CNS are closely confined within rigid skeletal structures such that swelling cannot occur without subsequent and potentially lethal damage. Cellular specialization is of diagnostic benefit in the identification and localization of infections of the CNS because clinical signs are often referred to a specific region of the brain.
The course of CNS infection is affected by the relationship of the brain and spinal cord to the vasculature, meninges, and skeletal structure. The brain is suspended in cerebrospinal fluid (CSF) and is surrounded by the meninges (pia mater and arachnoid [together forming the leptomeninges] and the dura mater). Infections of the leptomeninges tend to involve their entire surface that surrounds the brain and spinal cord. In contrast, infections of the dura mater tend to be limited and sharply circumscribed. With persistent infection of the meninges, increased intracranial pressure results from extensive cerebral edema and hydrocephalus. Infections of the spinal cord meninges are less limited and often extend longitudinally the length of the cord.1
About 85% of CSF is produced by the choroid plexus of the lateral, third, and fourth ventricles. The CSF flows into the subarachnoid space, circulates around the brain and spinal cord by bulk flow, and is reabsorbed through the arachnoid. The CSF is totally recirculated in 3 to 4 hours. The choroid plexus is physiologically similar to renal tubules, even containing similar secretory mechanisms. Indeed, specialized transport systems allow the movement of organic acids (including many beta-lactams) against a concentration gradient out of the CSF. In cases of infections involving the ventricles, because of the flow pattern of CSF, intrathecal administration of drugs does not result in predictable drug concentrations in the ventricles. Rather, drug must be directly instilled into the ventricles. Infections of the CNS can impair CSF reabsorption across the arachnoid villi, resulting in hydrocephalus.1
Capillaries of the brain and spinal cord (with the exception of the choroid plexus) differ from other capillaries. First, the vascular endothelium is characterized by tight junctions rather than intracellular clefts. Second, they are surrounded by the foot processes of astrocytes (see Figure 29-1). Both form a barrier to passive diffusion of drugs and their compounds. Only compounds that are actively transported or are of sufficient lipid solubility can pass out of the capillaries into the brain. The barrier affects the movement of antimicrobials. In addition, impaired movement of immunoglobulins, complement, and other mediators of the immune response affects antimicrobial selection in that bacteriostatic drugs are much less desirable.1,2
Vascular damage associated with infection can affect the course of infection. Hypertrophy of the endothelium (as might occur with persistent bacterial infection) or infection of the endothelial cells (as occurs with Rocky Mountain spotted fever, for example) can cause thrombosis or embolization to arteries or veins. Loss of capillary integrity contributes to cerebral edema and movement of microorganisms into the brain. At the same time, capillary permeability facilitates movement of antimicrobials that normally cannot cross the cerebral or meningeal capillaries into the site of infection.1,2
The inflammatory response of the CNS also differs from that in other body tissues. The response tends to be less intense and is characterized by infiltration of microglial cells and proliferation of astrocytes. Abscessation is slower and involves gliosis rather than fibrosis. Host response to infection in the CNS involves antibody, cell-mediated immunity, and complement-mediated immunity. Normally excluded from the CNS, antibody in the CNS indicates damage to the blood–brain barrier or synthesis of immunoglobulin from cells that have been able to penetrate the brain parenchyma. Antibody protection is important in bacterial meningeal infections and may determine the outcome. Cell-mediated immunity, on the other hand, is the predominant host response to fungal or intracellular parasites. Infections by selected organisms, such as Mycoplasma spp., may lead to a host response to both the infecting organism and host proteins (e.g., myelin). Despite the role of the immune system in bacterial infections of the CNS, host defenses remain inadequate for control of the infection. Indeed, the relative lack of opsonization, complement, and immunoglobulins may allow bacterial survival in the subarachnoid space.1,2
Bacterial products can contribute to the development of cerebral edema. Release of cytokines and tumor necrosis factor is mediated by materials such as endotoxin of gram-negative organisms and teichoic acid produced by Staphylococcus aureus. Whereas changes in capillary permeability may increase antimicrobial movement across the blood–brain barrier, antimicrobial therapy may initially worsen cerebral edema, as bacterial death causes release of more mediators of inflammation. Inflammation, hemorrhage, hydrocephalus, and edema may cause displacement of the brain or spinal cord. Herniation may be a life-threatening sequela.1 The potential release of endotoxin may be an important consideration in the initial selection of an antimicrobial; drugs that minimize endotoxin release yet still penetrate the blood–brain barrier include meropenem and the fluoroquinolones.
Antimicrobial Selection
Successful antimicrobial therapy of CNS infections is facilitated by use of a bactericidal drug and maximization of plasma drug concentrations such that bactericidal concentrations are achieved in the CNS. The CNS is relatively immunoincompetent, thus increasing the concentration of drug necessary for effective therapy. Studies in animal models have shown that the rapid bactericidal killing in the CSF requires drug concentrations that exceed the minimum bactericidal concentration (not MIC) by tenfold to twentyfold.2 To maximize drug concentrations at the site of infection, drugs that can be given intravenously are preferred to oral preparations. Antimicrobials to treat the CNS are often selected empirically because of difficulties encountered when collecting culture and susceptibility data. Most infections reach the CNS by a hematogenous route. Organisms most likely cultured from or infecting the CNS are delineated in Table 6-1; however, the lack of predictability of the infecting organism mandates the need for a properly collected culture sample. After the most likely infecting organism and drugs effective against the organism have been selected, antibiotics should be selected next on the basis of movement into the CNS. Drug penetration of the blood–brain barrier is particularly challenging because the barrier not only prevents movement of antimicrobials into the CNS but also actively transports out or destroys some antimicrobials (i.e., selected beta-lactams) (see Table 7-5).
KEY POINT 8-2
Doses of antimicrobials, particularly those that are water soluble, may have to be increased tenfold or more to achieve effective concentrations in the brain.
To enter the CNS, antimicrobials must penetrate the epithelium of either the choroid plexus or the cerebral endothelium; both are characterized by tight junctions. Antimicrobials generally are not metabolized in the CNS; concentrations thus reflect a balance between penetration and elimination via the blood–brain or blood–CSF barrier.3 Passive diffusion is the major mechanism of drug movement. Drugs that are more likely to penetrate the barrier are characterized by high lipid solubility, small molecular weight, and low protein binding.2 Whereas lipid-soluble drugs enter the CNS through transcellular pathways, water-soluble drugs must move through paracellular pathways and thus depend on the opening of tight junctions. Several transport mechanisms facilitate influx (as well as efflux; see below and Chapter 27) of selected drugs (e.g., penicillins, ceftriaxone), but this accounts for only a low concentration of drug movement. Antimicrobial movement into the CNS thus is generally slow, with peak concentrations often not occurring until several hours after drug administration. Methods intended to increase permeability of and thus antimicrobial movement into the CSF (e.g., hyperosmotic solutions, receptor-specific antibodies, inflammatory mediators) have not been well evaluated.3 Once selected drugs successfully penetrate the CNS, drug efflux may decrease intracellular concentrations. Penicillins (but not ceftriaxone, carbapenems, nor ampicillin) are actively transported from the CNS; active transport can be inhibited by probenicid.4 Interestingly, the action of this pump is inhibited by meningeal inflammation. Therefore inflammation will increase inward movement of several antimicrobial, including many beta-lactams and vancomycin. However, as treatment is effective, increased influx declines such that within 5 days of therapy, penicillin CSF half-life and drug concentrations in humans markedly decrease. This is in contrast to drugs with a low affinity for active transport system; for such drugs concentrations tend to remain clinically relevant throughout CNS infection. Active transport mechanisms do not appear to affect CSF concentrations of fluoroquinolones or aminoglycosides.3
Antimicrobial therapy also is likely to be affected by the presence of purulent material. As in all body systems, the microenvironment can negatively affect antibacterial therapy. In the presence of meningitis, lactate accumulates in the CSF, causing the pH to decrease. Antibacterial activity of weakly basic antimicrobials may decrease, particularly for the aminoglycosides and potentially for the fluoroquinolones.2 The dynamics of CSF are altered by disease and drugs. At best, CSF production is unaltered, although several studies have demonstrated decreased CSF production. Gluocorticoids may further decrease CSF production; however, rather than prolonging the half-life of the drug in the CNS, glucocorticoids appear to decrease or not change CSF antimicrobial concentrations.5
Drug movement into the CNS was summarized in Box 6-4 and Table 7-5. Because of the impact of normal physiology and drugs, dosing regimens designed for treatment of CNS infection tend to differ from traditional therapy, in that doses often are much higher. The risk of toxicity that might accompany such increases must be weighed against the need to penetrate the CNS in concentrations sufficient to cause bactericidal effects (see Table 7-5). In general, selected beta-lactams (meropenem more than imipenem; cefoxitin, ceftazidime and cefotaxim), trimethoprim/sulfonamide, fluorinated quinolones, rifampin, and metronidazole can achieve bactericidal concentrations for some infections in the CNS; chloramphenicol and doxycycline or minocycline achieve bacteriostatic concentrations.6 Amikacin may achieve effective concentrations as well. Drugs that should be avoided or whose doses should be further markedly increased for treatment of CNS infections because of poor penetration include many beta-lactams, including carbenicillin, cephalothin, cefazolin, cefotetan; and clindamycin, erythromycin, and tetracycline. Drugs recommended for treatment of meningitis in humans are increased by at least 50% to 100% or more when safety is not an issue, with intervals being reduced for time-dependent drugs, to ensure that adequate concentrations reach the CNS. For other drugs, doses for treatment of CNS infections are increased several-fold compared with other infections. For example, beta-lactam doses in particular are increased as follows: penicillin normally dosed at 1 million U is increased 1 to 4 million U to 24 million U/person; aztreonam is increased threefold, and several third-generation cephalosporins (cefotaxime, ceftazidime, ceftriaxone) are increased sevenfold to twentyfold. Aminoglycoside doses are either not increased or increased twofold (e.g., amikacin). Doxycycline is increased twofold, and sulfonamides are increased fourfold to fivefold. Because of local immunoincompetence, duration of therapy for patients with infections of the CNS should be at least 10 to 14 days and up to 21 days.2 The need for 4 to 6 weeks of therapy, as has been suggested in dogs, is not clear; it may not be necessary.7 Intrathecal administration of antimicrobials that might be systemically toxic at concentrations necessary to be effective in the CNS might be a reasonable alternative. However, this method has not been well studied, particularly in animal patients. Further, because distribution of intrathecally administered drug into the CSF is uneven, drug concentrations may not be adequate at some sites.
Adjuvant therapy
Because of the harm associated with inflammation in a closed system, antiinflammatories should be considered when treating CNS infections (e.g., meningitis). Corticosteroid therapy may be indicated during initial stages of treatment of meningitis to minimize the effects of inflammation and loss of capillary integrity.2,7 Experimentally, methylprednisolone decreases leukocyte accumulation, CSF outflow resistance, and brain water content in animals with bacterial meningitis.2 Dexamethasone also reverses the development of brain edema and, compared with methylprednisolone, has the added advantage of decreasing CSF pressure and lactate. Note that these studies did not include comparisons with antimicrobial therapy. Nonetheless, glucocorticoid therapy may be beneficial early during the course of therapy; indeed, treatment before antibacterial therapy may minimize the effects of mediators of inflammation released by dying bacteria.2 A study of infants with bacterial meningitis treated with ceftriaxone and either a placebo or dexamethasone found the duration of infection and degree of inflammation in the latter group to be shorter and less, respectively, although mortality or long-term neurologic sequelae did not differ between the two groups.8 In an analysis of five clinical trials in adult humans with bacterial meningitis, the incidence of side effects was the same in the group treated with glucocorticoids (dexamethasone), but both mortality and persistence of clinical signs were improved in the group treated with steroids, leading the authors to conclude that a single dose is justified if given at the beginning of antimicrobial therapy.9,10 Dexamethasone can be used (0.1 to 0.15 mg/kg every 6 hours up to 4 days), particularly in the presence of cerebral edema.2
KEY POINT 8-3
A well timed but limited duration of treatment with glucocorticoids may be indicated in the patient with bacterial meningitis to prevent organ-threatening inflammation.
Treatment of cerebral edema should also include mannitol. If intensive monitoring is available, high-dose barbiturate therapy might be useful for these patients. Barbiturates decrease cerebral metabolic demands and cerebral blood flow and provide protection against oxygen radicals.
Adverse reactions
Because of altered permeability of the blood–brain barrier, the infected CNS is more likely than the normal CNS to respond adversely to antimicrobials. Seizures are the most likely manifestation. The antimicrobial most likely to cause seizures include selected beta-lactams (see Chapters 6 and 7), most notably imipenem (but not meropenem), metronidazole, and fluoroquinolones, particularly in patients also receiving nonsteroidal antiinflammatories. In general, seizures that develop as a result of drug therapy should be treated as with any acute seizural manifestation, with diazepam the preferred anticonvulsant of choice.
Ocular Infections
The principles of ocular therapy are discussed in Chapter 27.
Microbial Targets
Whitley11 has provided a review of isolates cultured from ocular tissues of clinically normal dogs and dogs with ocular disease (Table 8-1). The most common organism associated with bacterial conjunctivitis in dogs is Staphylococcus spp. (aureus [68% of infections] or epidermidis [27% of infections]). A variety of other organisms, including Corynebacterium spp. and gram-negative rods, make up the remaining infections. Most of the infecting organisms are considered normal ocular flora.11 Bacterial infection complicating keratoconjunctivitis sicca generally is caused by Staphylococcus spp. (32% to 69% of dogs) and Streptococcus spp. (9% to 25%); Pseudomonas spp. (5% to 18% of infections) is the most common gram-negative isolate. In cats Mycoplasma felis and gatea are the more common isolates associated with conjunctivitis; however, they also are commonly isolated in normal cats, calling into question the role of the organism in disease. Chlamydia (Chlamydophila felis)12 may be a concurrent pathogen, although both it and Mycoplasma spp. may reflect infection secondary to primary feline herpesvirus infection.
Table 8-1 Microbial Flora Associated with Infections at Selected Tissue Sites
| Site | Percent (%)∗ | |
|---|---|---|
| Eye11 | Staphylococcus spp. (coagulase −) | 0-11 |
| Staphylococcus spp. (coagulase +) | 16-42 | |
| Streptococcus spp. (beta hem) | 17-22 | |
| Streptococcus spp. (alpha hem) | 2-9 | |
| Streptococcus canis | 16.5 | |
| Corynebacterium spp. | 3.5 | |
| Enterococcus spp. | 0-5.6 | |
| Escherichia coli | 4-17 | |
| Proteus spp. | 0-2.6 | |
| Proprionobacterium | 0-2.6 | |
| Pseudomonas aeruginosas | 0-9.5 | |
| Wound92 | No growth | 16 |
| (n = 213) | Gram-positive | 53 |
| Staphylococcus spp. (coagulase−) | 5 | |
| Staphylococcus intermedius | 12 | |
| Oral and other Streptococcus spp. | 6 | |
| Streptococcus canis | 7 | |
| Bacillus spp.† | 6 | |
| Actinomyces spp. | 3 | |
| Corynebacterium spp. | 2 | |
| Gram-negative | 47 | |
| Pasteurella multocida | 15 | |
| Pasteurella canis | 5 | |
| Other Pasteurellaceae | 2 | |
| Enterobacteraceae | 7 | |
| Vibrionaceae | 4 | |
| Non–glucose fermenters | 16 | |
| Urine105 | Escherichia coli | 45 |
| (n = 8354) | Staphylococcus spp. total | 12 |
| Proteus mirabilis | 6-12 | |
| Klebsiella pneumoniae | 7-12 | |
| Enterococcus spp.† | 6-9 | |
| Enterococcus faecalis | (2-4) | |
| Enterococcus faecium | (1-3) | |
| Streptococcus spp. | 5 | |
| Pseudomonas aeuruginosa | 3 | |
| Mycoplasma spp. | 2-3 | |
| Enterobacter spp. | 2-3 |
∗ Proportions are approximate.
Like conjunctival infections, corneal infections (including ulceration) are most commonly caused by Staphylococcus spp., followed by Streptococcus spp. (together making up approximately 65% of infections). Other causes include Corynebacterium spp. and gram-negative rods (including Pseudomonas spp.).
Drug Preparations
Characteristics of topical ophthalmic antibacterial drugs to consider are the spectrum, and their lipid versus water solubility, with lipid solubility being more important if tissue penetration is of importance. Because of the ability to administer high concentrations of antimicrobials with topical administration, traditional classification of bacteriostatic versus bactericidal (fungal or viral -static or -cidal) may not be relevant to antiinfective drugs, and susceptibility data might underestimate topical efficacy. Ophthalmic drugs indicated for treatment of ocular infections are available as single or multiple antimicrobial agents (Table 8-2a and Table 8-2b). Generally, either gram-negative or gram-positive organisms are targeted using individual agents; mixed infections can be targeted with combination products or drugs characterized by a broad spectrum. Whereas few drugs are approved for use in animals, multiple human products are commercially available. Others can be compounded, which might include “fortification” of commercial products. However, the nuances of ocular preparations mandate that extreme caution be taken when formulating or modifying a product intended for topical ocular therapy (see Chapter 23).11
Table 8-2b Subconjunctival Doses of Drugs Also Used Systemically
| Drug | Dose (mg) | Target |
|---|---|---|
| Bacitracin | 5000-10,000 U | Gram-positive |
| Clindamycin | 15 to 50 | Gram-positive |
| Erythromycin | 100 | Gram-positive |
| Amikacin | 25-100 | Gram-negative |
| Gentamicin | 10 to 20 | Gram-negative |
| Polymyxin B | 100000 U | Gram-negative |
| Streptomycin | 40-100 | Gram-negative |
| Neomycin | 100-500 | Gram-negative>positive |
| Penicillin | 500,000-1,000,000U | Gram-positive>gram-negative |
| Ampicillin | 50-150 | Mixed |
| Carbenicillin | 100-250 | Mixed |
| Cefazolin | 50-100 | Mixed |
| Ceftazidime | 100 | Mixed |
| Ticarcillin | 1000 | Mixed |
| Chloramphenicol | 40-100 | Mixed |
KEY POINT 8-4
Whereas high concentrations of drugs might be achieved with topical ophthalmic drug administration, lipid solubility remains important if deeper tissues are to be penetrated with systemic antimicrobials.
Drugs that target gram-negative organisms include the water-soluble, weakly basic aminoglycosides. At the high concentrations achieved topically, they are generally also effective against Staphylococcus spp. and include tobramycin (0.3%; drug of choice for treatment of Pseudomonas spp.), gentamicin (available with the glucocorticoid betamethasone), and neomycin (less effective toward Pseudomonas spp. and generally available only in combination with other antimicrobials). Polymyxin B, whose systemic use is precluded by nephrotoxicity, is a water-soluble drug, characterized by limited intraocular distribution. The fluoroquinolones also target gram-negative organisms, including Pseuodmonas spp. as well as Staphyloccoccus spp. In contrast to the aminoglycosides, the fluoroquinolones are lipid soluble. Drugs include ciprofloxacin, and ofloxacin and its L-isomer, levofloxacin. Systemic fluoroquinolones have been associated with retinal degeneration in cats (see Chapter 7). The adversity reflects accumulation due to a missing transport pump in the blood-retinal barrier (personal communication, Katrina Mealey, Washington State University). It is not clear if the adversity may occur with topical use, but prudence suggests that safety not be assumed.13 Drugs that target gram-positive organisms include the lipid-soluble erythromycin (0.5%; bacteriostatic), whose efficacy is limited by resistance, and the water-soluble bacitracin, which, like polymyxin B, is most known for its nephrotoxicity associated with systemic therapy. An example of a triple-antibiotic combination is one containing neomycin, polymyxin B, and bacitracin (see Table 8-2a). Broad-spectrum topical antimicrobials include chloramphenicol (prohibited for use in food animals), tetracyclines (drugs of choice for ocular Mycoplasma or Chlamydia), and the sulfonamides. Each is lipid soluble. Whitley11 has described a preparation of vancomycin (3.1%) and cefazolin (3.3%) compounded from injectable products., although the combination might be improved by replacing cefazolin with a drug with a broader gram-negative spectrum.
Topical antifungal are limited and include the polyene natamycin. Its spectrum includes all fungal agents except dermatophytes. The imidazoles must be compounded from intravenous solutions (i.e., miconazole, fluconazole, and itraconazole). Their spectrum includes opportunistic, dimorphic fungi and dermatophytes.
Treatment of Selected Infections
Use of systemic antimicrobials for treatment of ocular infections is complicated by poor drug penetration. Therefore topical therapy is indicated for external ocular infections, and subconjunctival administration is recommended for serious corneal or anterior chamber infections. For bacterial endophthalmitis, intravitreal antimicrobial therapy is indicated, although extreme caution is indicated for this route.13 Treatment of intraocular infections can be supported with systemic therapy of lipid-soluble drugs that can achieve bactericidal concentrations. In addition to antibacterials, collagenase inhibitors such as N-acetylcysteine, sodium citrate, bacitracin, and tetracycline compounds have been recommended for treatment of corneal infections.
Treatment with tetracycline is indicated for control of Mycoplasma and Chlamydia spp. Cats may develop hypersensitivity manifested as acute conjunctivitis with topical treatment.14 Oral therapy with tetracycline (5 mg/kg bid) stops shedding within 6 days. Azithromycin starting at 7 to 10 mg/kg qd for 2 weeks, followed by 5 mg/kg qd for 1 week, followed by 5 mg/kg qod for 14 days has been advocated, although the need for the complicated dosing regimen or the long duration is not clear. The presence of sneezing may indicate infection with feline calicivirus (FCV), for which supportive therapy is indicated. Conjunctivitis, often accompanied by ulcerative keratitis, can be a manifestation of feline herpesvirus (FHV-1), In addition to supportive therapy, topical preparations are available for its treatment (see also Chapter 10).15 Antivirals include vidarabine, trifluridine, and idoxuridine (the latter must be compounded). These are generally virustatic, requiring frequent application (every 1 to 2 hours is preferred, but every 4 to 6 hours is acceptable).
Systemic treatment of experimentally induced Chlamydia psittaci with 19 days of amoxicillin–clavulanic acid (12.5 to 25 mg/kg bid) has been compared with doxycycline (10 to 15 mg/kg qd; positive control) in 5-month-old cats (n = 24; 8 per group) using a randomized, placebo-controlled, blinded design.15a Outcome was based on clinical scoring, including respiratory signs or corneal changes; other clinical signs were not described. Both treatments were associated with rapid clinical improvement and reduced chlamydial isolation, with amoxicillin–clavulanic acid being associated with less isolation. However, five of eight of the cats treated with amoxicillin–clavulanic acid, but no doxycycline-treated cats, became positive 3 weeks after treatment. These cats became negative again with 4 weeks of retreatment with amoxicillin–clavulanic acid and remained negative 6 months later.
Systemic therapy was described in cats experimentally infected with C. felis. Once the organisms was detected, cats were treated with azithromycin (10 mg/kg qd for 3 days followed by twice weekly) (n = 9; two untreated negative controls, two doxycycline-treated positive controls [10-15 mg/kg qd]).16 At the end of the 21-day treatment period, untreated control cats were also treated. Despite an initial response to treatment (negative isolation to day 14), infection was eradicated in only one of five cats treated with azithromycin compared with both cats receiving doxycycline. However, it is not clear if the dosing regimen for azithromycin resulted in effective drug concentrations.
Otic Infections
Otitis Externa
Pathophysiology
Inflammation of the ear canal and the proximal pinna affects up to 20% of dogs, whereas fewer (up to 6% of) cats are affected.17 A number of causes can be identified in otitis externa, and their resolution is paramount to successful therapy. Possible causes include foreign bodies, allergies, parasites (e.g., mites, chiggers), skin disorders of a keratinous or sebaceous origin, and autoimmune disorders. Structural characteristics of the ear also can predispose the animal to otitis externa, including pendulous ears, higher number of ceruminous glands, the vertical and horizontal paths of the canal, hair in the ear canal, stenotic ear canals, or neoplasm. Environmental factors also can contribute to the difficulty in treating otitis externa, including external conditions that perpetuate excessive moisture (e.g., humidity, bathing) or heat or irritants (e.g., irritating medicaments or shampoos). The presence of yeast or bacteria that are part of the normal flora perpetuates the inflammatory process, and, with time, the inflammatory process itself will cause proliferative changes that complicate therapy. One of the more important predisposing factors to otitis externa is inappropriate treatment, including undertreatment and overtreatment.17 Ultimately, in some animals surgical treatment will be indicated (Figure 8-2).
Microbial Targets
Bacterial organisms found in normal ears include S. epidermidis and Staphylococcus intermedius (Staphylococcus pseudintermedius; see Chapter 6) and Micrococcus spp. Coliforms are found less commonly. Infection usually can be distinguished from colonization by the presence of large numbers of organisms, particularly if the culture is pure. Infection also is indicated by the presence of inflammation and phagocytized bacteria. Inflammation reflects, in part, the breakdown of fatty materials by organisms into irritating by-products. S. intermedius is the most common organism (30% to 50%) associated with otitis externa, followed by Pseudomonas aeruginosa, Proteus spp., Streptococcus spp., E. coli, and Corynebacterium spp. The infecting organism also appears to be time dependent in that acute otitis is generally associated with Staphylococcus, whereas chronic otitis more commonly involves Pseudomonas spp. This may refelect, however response to chronic antimicrobial therapy.
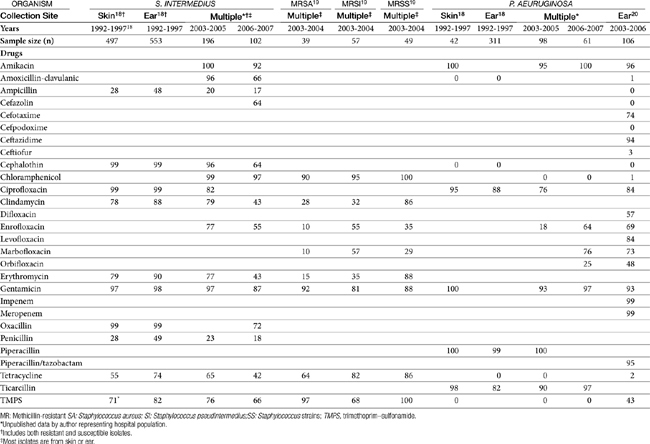
Petersen18 reported on the frequency and susceptibility of S. intermedius and P. aeruginosa in canine ear samples (n = 553) submitted to a state diagnostic laboratory between 1992 and 1997. Sampling methods varied. S. intermedius was isolated from 50% of ear samples but was the sole isolate in only 32% of the samples positive for S. intermedius. P. aeruginosa was isolated from 28% of ear cultures and was the sole isolate in 33% of P. aeruginosa samples.
The susceptibility of both S. intermedius and P. aeruginosa isolated from canine skin and ears appears to be changing across time (Table 8-3). A number of investigators have described their susceptibility. A review of the data suggests that the incidence of methicillin resistance is increasing, with a decrease in susceptibility of Staphylococcus spp. in general emerging in isolates collected during the period between 2003 to 2006. Morris19 focused on the susceptibility of methicillin-resistant isolates, including both coagulase-positive (S. aureus, and S. intermedius), as well as Staphylococcus schleiferi (including both coagulase-negative and positive subspecies; see also the discussion of Pyoderma). Of the total number of resistant isolates found in skin or ear, 28% of the methicillin-resistant S. aureus (MRSA), 40% of the methicillin-resistant S. intermedius (MRSI), and 33% of the methicillin-resistant Staphylococcus schleiferi (MRSS; presumed to be coagulase-positive subspecies schleiferi) were in the skin, and 5% MRSA, 26% MRSI, and 47% MRSS were located in the ear. Rubin20 described the susceptibility of 106 canine P. aeurginosa isolates cultured from soft tissue infections, including otitis externa and interna (see Table 8-3). No information was available regarding previous antimicrobial therapy in these animals. The limited amount of data precludes assessment across time; however, Pseudomonas is an organism recognized for its inherent resistance to multiple drugs, with an increasing tendency of resistance toward drugs to which it is normally considered susceptible (e.g., fluoroquinolones).
The combined data, particularly from Staphylococcus spp. isolates, demonstrate the limited susceptibility of these organisms to drugs most often used systemically or topically to treat organisms infecting the skin or ear. Culture and susceptibility data might increasingly become the basis for drug selection and dose design. Although the applicability of culture and susceptibility testing to the topical treatment of otitis externa may be controversial, the incidence of resistance is sufficient to justify culturing even in the earliest stages of infection, particularly in those patients at risk for recurrence, such that emergent resistance can be detected. Susceptibility testing is clearly recommended if gram-negative organisms are expected or in the face of severe or chronic otitis, failed antimicrobial therapy, and the presence of inflammatory cells. Testing is also recommended if systemic therapy is anticipated. Care should be taken to appropriately prepare and collect cultures from infected ears. Frustratingly, the accuracy of susceptibility testing may be questioned simply because of differences in laboratories. Schick21 compared the results of cultures simultaneously submitted from dogs with otitis. Swabs were rubbed together to ensure similar samplings. Labs agreed regarding the presence of Pseudomonas spp. only 83% of the time. Susceptibility of the isolates to amikacin varied in 13 of 16 samples, for gentamicin 10 of 16, and for enrofloxacin 9 of 16.
In cats Pasteurella multocida joins Staphylococcus and Streptococcus spp. as a commonly infecting organism; the coliforms and Pseudomonas spp. are less common causes. Malassezia pachydermatis is an opportunistic yeast that occurs in up to 49% of normal dogs and 23% of normal cats. The numbers increase in dogs with otitis, however, being present in 80% or more of dogs. Although it is likely that Malassezia spp. contributes to otitis externa, its role is not clear. Nonetheless, therapy should target this organism as well.
Antimicrobial and Adjuvant Options
The public health implications of treating methicillin-resistant Staphylococcus spp. in dogs or cats are addressed in the discussion of Pyoderma. Because successful treatment of otitis externa is critically dependent on proper cleaning, it is difficult to separate antimicrobial therapy from adjuvant therapy. The goals of therapy for otitis externa are to identify and resolve the primary factors, reduce inflammation, and control or eradicate infection. Sedation or anesthesia may be necessary for proper cleansing. Cleansing should remove irritating oils, waxes, and other debris that might serve as a nidus of infection by providing a microenvironment favorable to the growth of microorganisms. Ear cleaning should be less aggressive in the presence of severely swollen or proliferative canals; in such patients initial therapy might begin with antiinflammatory doses of glucocorticoids and antimicrobials, both systemically and topically.
KEY POINT 8-5
The goals of therapy for otitis externa are to identify and resolve the primary factors, reduce inflammation, and control or eradicate infection. Repetitive sedation or anesthesia may be necessary for proper cleansing.
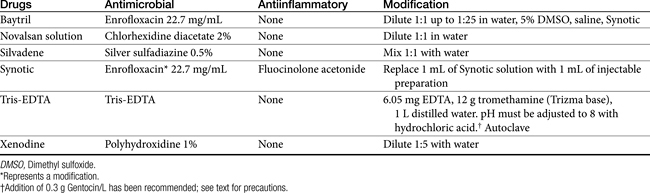
Ceruminolytics contain various surface active agents or emulsifiers (dioctyl sodium sulfosuccinate, carbamide peroxide, squalene, propylene glycol, glycerin, oil) that dissolve wax accumulation and associated debris. They should be used as an initial flush. Less messy water-soluble agents (dioctyl sodium sulfosuccinate, propylene glycol) are often preferred.22 Carbamide peroxide, a common ingredient of human preparations that is seldom found in veterinary preparations, releases nascent oxygen, causing a bubbling action that softens and removes debris. Combination products may contain drying agents such as isopropyl alcohol, silicone dioxide, and alpha hydroxy acids (lactic, malic, or salicylic acid). In addition to their drying effects, these materials may also have mild antimicrobial effects.22
Flushing solutions facilitate removal of debris. Although water and saline are the safest, some products contain germicidal ingredients. Chlorhexidine can be diluted to a 0.05% solution and used topically. Although ototoxicity should lead to cautious use in the presence of a perforated eardrum, one study failed to provide evidence of damage after 21 days of therapy in dogs with experimentally traumatized membranes; the power of the study to detect a significant difference is not stated.22 Povidone–iodine at 0.1% to 1% can provide bactericidal activity in flushing solutions, although it can cause contact irritation. Because of ototoxicity, solutions with less than 0.5% iodine are preferred if the eardrum is ruptured. Iodine might also be administered as a polyhydroxidine complex (Xenodine). Because it contains 0.5% of titratable iodine, it is less irritating and provides a longer duration of activity. Efficacy against Pseudomonas spp. has been established with this product. It must be used in an aqueous environment and therefore should be used within 2 hours after cleaning to maximize its effects.22 Acetic acid is a relatively inexpensive agent, available as white or brown vinegar in a 5% solution. Antimicrobial effects occur because of direct damage as well as acidification of the local environment. The acidic pH may also facilitate removal of necrotic debris. Pseudomonas spp. succumbs to a 2% solution within 1 minute of contact,22 whereas a 5% solution is effective against Staphylococcus spp. Streptococcus spp., E. coli, and Proteus spp. The preparation is irritating at 2% to 5% concentrations, however, and inhibition of wound healing and ototoxicity may occur at concentrations of 2.5%. Dilutions of 1:1, 1:2, or 1:3 in water daily or every other day have been recommended.
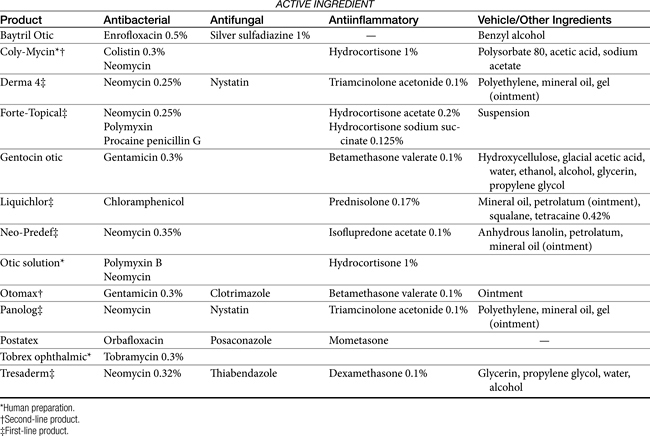
Topical antimicrobial treatment of otitis externa is generally indicated regardless of whether systemic therapy is implemented. Topical products intended specifically for treatment of otitis externa (Tables 8-4 and 8-5) generally contain various drugs intended to target bacteria, yeast, and inflammation. Vehicles are designed to maximize drug solubility (only dissolved drug will passively diffuse) but must also allow drug movement from the vehicle into tissues. Antibacterial agents often include products that are associated with severe systemic toxicity when given orally or parenterally; topical administration generally is not associated with systemic adversities.
On the basis of a MEDLINE search and review of the literature in human medicine, the ototopic use of antimicrobials does not appear to be associated with an increased risk of antimicrobial resistance.23 However, the relevance to canine or feline ear, for which the pathophysiology is substantially different, is not clear. Ototoxicity is, however, likely for many antimicrobial drugs when administered in the ear canal, especially in the presence of a perforated tympanic membrane (see later discussion).
KEY POINT 8-6
Topical treatment of otitis externa is generally indicated regardless of whether systemic therapy is implemented.
Topical antimicrobials generally are selected on the basis of cytology and potentially Gram staining. A number of products are commercially available (see Table 8-4). For selected drugs, ophthalmic (directly or diluted) or injectable (diluted) products may also be used. Vehicles used in topical products represent a balance between drug solubility and drug delivery.It may be necessary for vehicles to be pH balanced to maximize solubility. Demulcents (polyethylene or propylene glycol or glycerin) act to suspend non–water-soluble drugs; the glycol agents may irritate an already irritated ear. DMSO is included in some otic preparations as a vehicle, although it also has significant antiinflammatory effects. The agent is very hygroscopic and as such is an effective carrier agent for other drugs included in an otic preparation. In addition, DMSO provides mild antibacterial and antifungal actions as well as antiinflammatory and antifibroplastic effects. Other vehicles used to administer drugs useful for otic disorders include solutions, lotions, ointments, and other oil-based products. Occlusive oils may be undesirable for exudative lesions. Ointments and oil-based products can, however, be used in cases of chronic otitis externa that are dry. Generally, topical preparations should be applied twice daily. A number of home remedies have been recommended for treatment of otitis externa (see Table 8-5). Caution is recommended when making new preparations or modifying old preparations. A number of drug interactions between drugs and their vehicles can inhibit the efficacy of any of the drugs. In addition, drugs must be dissolved to be effective, and the addition of solid drugs (i.e., as powders or crushed tablets) to ointment vehicles (e.g., petrolatum jelly) is likely to yield an occlusive but minimally effective agent. Some acceptable modifications of commercially available products are noted in Table 8-5.
Note that modification of a product may alter the stability, and a shelf-life of 1 week or less might be prudent unless data exist to support a longer beyond-use date. Several in-practice compounded preparations have been described elsewhere.24
Examples of antibacterials used in otic preparations to target gram-negative organisms include the aminoglycosides gentamicin (0.3%), tobramycin (0.3%), and neomycin (0.33%) and the cell membrane–active agents colistin (0.3%) and polymyxin B (5000 IU/mL). Topical aminoglycosides also target Staphylococcus spp. whereas systemically, amikacin is more effective toward Pseudomonas spp., and gentamicin is more effective toward Staphylococcus spp., systemic use of an aminoglycoside as sole therapy for treatment of Staphylococcus spp. is discouraged. An otic preparation for amikacin can be compounded from the commercially available injectable product by adding 30 to 50 mg/mL to sterile saline or Tris-EDTA. Because aminoglycosides are more effective in an alkaline environment, they should be used either before or 1 or more hours after acidifying agents. Silver sulfadiazine (0.5%) is an ointment approved for humans to prevent or treat infection in burn patients. Its spectrum also includes Pseudomonas spp. However, the product, as approved in humans, may cause skin irritation. Silver sulfadiazine (1%) is available in commercial products combined with enrofloxacin (0.5%) or as a micronized powder (e.g., www.spectrumrx.com) that can be reconstituted in sterile water to a 0.5% to 1% solution (5 and 10 mg/mL, respectively). An otic preparation containing orbafloxacin and posaconazole as an antifungal drug with mometasone as an antiinflammatory has recently been approved for dogs (Table 8-4). A number of other antimicrobials have been administered topically, including the narrow-spectrum fusidic acid for treatment of Staphylococcus spp., antipseudomonadals such as colistin or polymyxin B (10,000 U/mL of Neosporin GU,24 fluoroquinolones (discussed later), and ticarcillin (which also targets Staphylococcus spp.). Ciprofloxacin and ofloxacin are available as human otic preparations. Ciprofloxacin is generally more potent than enrofloxacin against Pseudomonas spp.
Trothmethamineethylenediamine-tetraacetate (EDTA, Tris-EDTA, or commercial Triz/EDTA) is a topically applied compound that chelates cations, thus rendering the cell wall and membrane of both gram-positive and gram-negative organisms more permeable to antimicrobials. Penetration of antibacterials subsequently is facilitated. Treatment should be timed such that microorganisms are exposed to Tris-EDTA before peak concentrations of antimicrobials reach the site of infection. Direct addition of antibiotics such as gentamicin to the buffer solution may be less desirable than pretreatment with Tris-EDTA, followed later by antimicrobials (to allow time for chelation of cations). Because cations are also removed from the lipopolysaccharide covering, EDTA may be particularly effective for treatment of gram-negative organisms. Aminoglycosides in particular are facilitated because cations that otherwise would repel positively charged drugs such as aminoglycosides are removed. Synergisim has been demonstrated with Tris-EDTA and aminoglycosides (amikacin and neomycin) against S. intermedius, Proteus mirabilis, P. aeruginosa, and E. coli. Combination solutions appear to be stable for at least 3 months.25
KEY POINT 8-7
Pretreatment of an infected site with tris-EDTA has the potential to enhance efficacy of many antimicrobials against many bacteria.
However Tris-EDTA synergisim also has been demonstrated, albeit in vitro, for other drugs. These include fluoroquinolones and amoxicillin. Tris-EDTA also appeared to have a synergistic effect with 0.15% chlorhexidine digluconate in an open, unblinded clinical trial in dogs (n = 11) with chronic otitis externa associated with both gram-negative and gram-positive infections. Enrofloxacin was also used in these dogs.26
A common otic preparation compounded by veterinarians for treatment of otitis externa combines 12 mL of 100 mg/mL enrofloxacin in 8 oz (240 mL) of T8 (alcohol combined with the spermicide nonoxynol-9), 4-8 mg of dexamethasone phosphate, and 1 to 2 mL of DMSO, yielding a final product of 0.5% enrofloxacin and 0.0016 to 0.0032% dexamethasone. Because T8 can be irritating, Tris-EDTA might be substituted, although the advantages of pretreatment with Tris-EDTA will be lost.
Topically applied antifungal otic agents generally target M. pachydermatis and include the imidazoles clotrimazole (1%), miconazole (1%), and the benzimidazole thiabendazole (1%) and the polyene macrolides nystatin and amphotericin B. Among these, those containing thiabendazole might be considered first line.17,22 Ketoconazole is available as a topical preparation that can be reformulated into solutions that can be used topically.24 The allylamine, terbinafine, is also effective against Malassezia spp. and is available as a 1% solution.
Antiinflammatories often are included in topical otic solutions. Examples include DMSO (60%) and glucocorticoids. Among the glucocorticoids used topically, the order of potency (which should not be confused with efficacy) is fluocinolone > betamethasone or dexamethasone > isoflupredone > triamcinolone > prednisolone (prednisone should not be administered topically) > hydrocortisone. Note that sufficient drug may be absorbed with topical therapy that the hypothalamic–pituitary–adrenal axis will be affected.27
Several clinical trials have addressed the topical treatment of otitis externa. A preparation containing marbofloxacin (0.3%), clotrimazole (1%), and dexamethasone (0.09%) (10 gtt/ear/day) was prospectively compared with one containing polymyxin B (5.5 IU/mL), miconazole (2.3%), and prednisolone acetate (0.5%) (5 gtt/ear/bid) in dogs (n = 140; ages 4 months to 16 years) with clinical signs indicative of acute or subacute otitis externa.28 Treatments were randomized. Exclusion criteria included treatment within the previous 10 days with topical or systemic antimicrobials (including antifungals), nonsteroidal antiinflammatories, glucocorticoids (previous 14 days), or a long-acting steroidal antiinflammatory drugs (previous 60 days). The presence of concurrent auricular disease, including ear parasites, foreign bodies, and neoplasia or hyperplasia were also a basis for exclusion. Pregnant or nursing dogs were excluded. Culture (swab) revealed disease was associated with Staphylococcus spp. (39.5%; 82% S. intermedius), Pseudomonas spp. (12.9%; P. aeruginosa predominant), Enterobacteraceae spp. (17%; Proteus, spp. E. coli), and Malassezia spp. (58%). Streptococcus spp. and other gram-positive isolates represented another 11.4%, whereas 10.5% were infected with other gram-negatives; 8.6% of ears were cultured as no growth. Susceptibility testing indicated that 85% of isolates were susceptible to marbofloxacin. Interestingly, 43.5% were susceptible to polymyxin B, a drug generally considered ineffective against Staphylococcus spp. and other gram-positive as well as selective gram-negative organisms (e.g., Proteus spp.). Ears were scored for severity of otitis (0-3, with 3 most severe) and cultured (in addition to baseline) before, in the event of failure, or 2 weeks into therapy if the cause was Pseudomonas spp. Approximately 75% of yeast were susceptible to both antifungals studied. Owners and investigators were blinded to the treatment groups. Ears were cleaned according to a predefined schedule, based on the underlying classification scheme (erythematous [EO] or suppurative [SO]) approximately 2 to 3 times per week (with physiologic saline on days 0, 2, 4 (EO) or days 1 and 6 (SO). Response was assessed on days 7 and 14, with treatment continued to day 14 if indicated on day 7 assessment. The preparations were found to be equivalent with regard to success (58% for the marbofloxacin preparation compared to 41% for the polymyxin-based preparation; failure to identify this difference as significant may have reflected the sample size), but the marbofloxacin preparation was considerd superior in terms of control of pain, pus, appearance, and odor on day 14. The differential response apparently was not statistically significant, although better efficacy would be expected for marbofloxacin based on the limited spectrum of polymyxin B.
Systemic therapy is indicated for severe infections but should be coupled with topical therapy and should be based on culture and susceptibility testing. Doses should be designed with the site of infection in mind; distribution is likely to be limited to the site, particularly with marked inflammation. Water-soluble drugs might be administered topically, and lipid-soluble drugs systemically. No data support selection of the same versus different but complementary antimicrobials when both topical and systemic therapy are used. The advantage of using the same antimicrobial topically as administered systemically is increased likelihood of effective drug concentrations at the site of infection. The advantages of combination therapy have been discussed previously (see Chapter 6) and are particularly appealing for the treatment of chronic, minimally responsive otitis externa. Oral antifungals such as ketoconazole or itraconazole may be indicated for control of infection caused by Malassezia spp. (see Chapter 9). Glucocorticoids may be necessary for rapid control of inflammation regardless of the cause or duration of infection. General principles of glucocorticoid therapy should be followed (see Chapter 30).
Treatment of Specific Causes of Otitis Externa
Acute otitis externa
Although ultimately the normal ear is clean and dry, severe swelling or proliferation indicates less aggressive therapy initially and the potential need for glucocorticoid therapy (topical or systemic). Once swelling is decreased, cleaning will be more effective. If the tympanic membrane is intact, initial cleaning begins with an application of a ceruminolytic, which can then be rinsed with water, or an antimicrobial solution such as chlorhexidine, povidone, polyhydroxidine iodine, or acetic acid. If the tympanic membrane is ruptured, only water or saline should be used for cleansing because many of the cleansing agents are ototoxic. Cleansing can be accomplished at home, although initial cleaning might be more thorough if performed under general anesthesia, particularly in intractable patients. For ears in which a large amount of debris has accumulated, removal of material may require alligator forceps through an otoscope and flushing with either an open-ended Tomcat catheter or a 3.5 to 5 French feeding tube and syringe or an in-house vacuum system. Hair that might obstruct drainage of the ear canal or facilitate collection of debris should be removed.
Because otitis externa tends to be associated with similar pathogens, regardless of the underlying cause, topical medication applied after cleaning generally contains an antimicrobial, antifungal, and glucocorticoid. Glucocorticoids decrease not only swelling and proliferation but also apocrine and sebaceous secretions. Note that topical administration does not preclude suppression of the hypothalamic–pituitary–adrenal axis, and the long-term use of these products is discouraged. In the event of moderate to marked swelling of the ear canal and pinna, short-term use of systemic glucocorticoids may be indicated. Note that care should be taken not to use glucocorticoids in combination with nonsteroidal antiinflammatories (including treatment intended for control of osteoarthritis). To minimize the risk of antimicrobial resistance, products that contain commonly used first-line antimicrobial and antifungals directed toward Malassezia spp. might be chosen for acute (nonrecurring) otitis externa. Culture and susceptibility data are the best basis for selection of the most appropriate second-line antimicrobial. In the event of moderate swelling of the ear canal and pinna, topical antimicrobial therapy probably should be accompanied by systemic therapy. Therapy for acute otitis externa should be followed through rechecks at 10- to 14-day intervals. Resolution generally requires 2 to 4 weeks.
Chronic otitis
Resolution of chronic or recurring otitis includes treatment of specific diseases of the ear that allow perpetuation of infection or inflammation. Culture and susceptibility data collected during cleansing of the ears under general anesthesia should be the basis of antimicrobial selection, particularly systemic. Thorough visual examination accompanied by cytologic examination can help identify other underlying causes. Antiinflammatories (e.g., oral prednisolone at 0.5 mg/kg every 12 hours, tapered when indicated) are indicated in the presence of marked inflammation and when proliferation precludes effective examination. Topical therapy with intense cleansing should be implemented; several months of topical therapy may be indicated. Systemic therapy should accompany topical therapy if deeper infection is suspected (i.e., marked proliferation or ulceration).23
Bacterial otitis
The need for antimicrobial therapy for bacterial otitis externa should be based on cytologic evidence of inflammatory debris and intracellular organisms supports bacterial infection. A first-line product containing neomycin or chloramphenicol might be appropriate. Severe inflammation may indicate the need for a lipid soluble systemic antimicrobial therapy. Among the more commonly selected systemic antimicrobials are amoxicillin–clavulanic acid, a fluorinated quinolone, or a first-generation cephalosporin.22 Failure to respond to first-line medicaments may indicate the need for culture. Some authors recommend discontinuing antimicrobial therapy for 3 to 5 days before culture; however, while a false negative might be ignored, a false positive is a clear indicator that therapy may fail. Otic preparations containing gentamicin or tobramycin might represent the second line of medicaments for infections resistant to neomycin or chloramphenicol.
Otitis caused by Pseudomonas spp. can be among the more frustrating problems to treat and often is the incentive behind the formation of homemade otic products. Flushing with an acetic acid–based solution is indicated. Commercially available otic products have been modified by the addition of drugs effective against P. aeruginosa (amikacin, enrofloxacin, polymyxin B, or colistin sulfate) or the addition of antiinflammatory drugs. Several alternatives can be considered for refractory cases. Enrofloxacin has been added to commercially available otic solutions, but it (or ciprofloxacin or marbofloxacin) also may be the oral antimicrobial of choice. Note that ciprofloxacin is more potent against Pseudomonas spp. and may be preferred topically; oral enrofloxacin will result in ciprofloxacin exposure, as well as enrofloxacin. The maximum end of the fluoroquinolone dosing regimen should be considered when treating a refractory case of Pseudomonas-induced otitis externa (i.e., 20 mg/kg once daily for enrofloxacin, 30 to 40 mg/kg orally for ciprofloxacin in dogs). Other products to be used in resistant cases include silver sulfadiazine, xenodine, and chlorhexidine (1.5%).
Malassezia pachydermatis
Malassezia pachydermatis is an opportunistic organism that, in large numbers, causes proliferatory changes in the ear. Its presence may contribute to bacterial otitis, and thus its control may be important in the treatment of otitis. Because control of inflammation may be paramount to controlling infection by Malassezia, spp. glucocorticoids such as those provided in otic preparations may be indicated. Ketoconazole appears to be among the most efficacious antifungal drugs, followed by miconazole, nystatin, clotrimazole, and amphotericin B.22 Thiabendazole (the active antifungal in Tresaderm), however, may be sufficiently effective, allowing other antifungals to be reserved for resistant cases. Routinely used cleaning and drying solutions should be used on a daily to alternate-day basis to facilitate control.
Ear mites generally occur in young animals, and their presence is determined by direct examination. Carbaryl and pyrethrin-based products should be used for at least a 3-week cycle to ensure killing of adults and immature mites. Products containing thiabendazole should be selected because the product probably kills all stages of mites, including eggs. Polyhydroxidine iodine may also be effective when administered once weekly for 4 weeks. Otodectes can be treated with ivermectin given orally once a week for 4 weeks or subcutaneously every 10 to 14 days. With severe infestations ivermectin should be combined with other topical miticides. Because other portions of the body may harbor mites that infest the ear, affected animals should be dipped.
Seborrheic otitis or ceruminous otitis usually accompanies endocrinopathies. Secondary bacterial and Malassezia spp. infections are not uncommon with this disorder. The first-line medications should be effective for management of most cases, but longer-term management may require a combination of cleansing, drying, and glucocorticoid products applied once to thrice weekly. Cocker Spaniels and other breeds occasionally develop what appears to be a local hypersensitivity to cerumen, resulting in a progressively inflammatory, proliferative disease that ultimately may result in calcification of auricular cartilages. Control of inflammation initially may require oral glucocorticoids accompanied by topical application of a potent (e.g., dexamethasone or fluocinolone) glucocorticoid. Topical antimicrobials (antibacterial and antifungal) probably are also indicated; systemic antimicrobial therapy may also be necessary. The ears should be frequently flushed with cleansing and drying agents. For some animals long-term, low-dose glucocorticoid therapy may be necessary.
Swimmer’s ear generally occurs because frequent swimming encourages low-grade inflammation and subsequent maceration. Ears should be kept clean and dry. Topical antiinflammatory therapy and, in some cases, topical antimicrobial therapy may be necessary. Products should be used on the day of swimming and for several days after.
Otitis Media
Otitis media associated with infection of the middle ear generally results from extension of otitis externa through a perforated eardrum.17,29 A perforated eardrum may be hard to detect and must be distinguished from a “false” eardrum. Indications that infection of the external ear has extended into the middle ear include persistence of otitis externa, pain, swelling or narrowing of the ear canal, and evidence of neurologic involvement such as facial palsy (ptosis or paralysis of the lip or ear) or vestibular abnormalities. Less commonly, infection may follow extension from the pharynx by way of the auditory tube or hematogenous spread. Radiographs and surgical exploration may be necessary for both diagnosis and treatment. Debris should be cytologically examined for evidence of predisposing conditions. Fungal infections (e.g., aspergillosis, infection caused by Malassezia) spp., foreign bodies such as grass awns, neoplasia, inflammatory polyps, and tumors, including cholesteatoma, are among the more common causes of otitis externa. Calcification of auricular cartilage may also predispose subjects to the development of infection. Breeds apparently predisposed to otitis media include Cocker Spaniels and German Shepherd Dogs.
Medical management of otitis media is often unsuccessful unless accompanied by surgical management, particularly if inflammation is severe and chronic or if the ear canal is stenotic. Surgical intervention may be the most cost-effective means of management and should provide the most accurate diagnosis. Treatment includes removal of debris and topical application of an antimicrobial with or without an antifungal preparation until the infection appears resolved. After debris is cleaned from the external canal, myringotomy (under general anesthesia) may be necessary to clean debris, collect a sample for culture and cytologic examination, and relieve pain associated with the infection. Intraoperative infusion of the bulla may be an effective means of providing higher concentrations of an antimicrobial,29 but duration of exposure is not clear and residual effects should not be anticipated. However, flushing the bulla is an effective means of reducing the microbial flora.30 In a retrospective study of 34 dogs afflicted with otitis media, the most likely causative organisms were Enterococcus and Streptococcus spp., Pseudomonas spp., Proteus spp., and E. coli.30 The varied population among animals limits empirical prediction of either the organism or, as was demonstrated in the study, susceptibility. Disconcertingly, empirical antimicrobial selection was inappropriate on the basis of culture and susceptiblitity data in nearly 60% of the patients. This probably reflects, in part, previous antimicrobial therapy.30 This study also demonstrated that the preflushing and postflushing cultures often did not match, and postflushing culture was recommended.
Systemic antibacterial therapy should begin in conjunction with topical therapy; current recommendations are to continue therapy for at least 4 to 6 weeks. For fungal infections (e.g., one caused by Malassezia spp.), oral therapy with an imidazole antifungal (e.g., thiabendazole, ketoconazole, itraconazole) should be implemented. For severe inflammation glucocorticoids may be given topically or, if indicated, systemically for the first 1 to 3 weeks of treatment. Topical glucocorticoid therapy can be continued if inflammation persists after resolution of infection. When used as a vehicle, DMSO may also impart antiinflammatory effects. Daily flushes of 5% acetic acid in water (1:1 to 1:3) may further control symptoms. Therapy must be continued until the tympanic membrane is repaired (generally 21 to 35 days). Control of the inflammatory process may be necessary before tympanic healing is complete. Should the tympanum not heal, debris may once again accumulate, and the ear must be flushed again.
Topical therapy of otitis media is complicated by the risk of ototoxicity, which is complicated by inflammation.29 Although response to inflammation may decrease the risk of ototoxins reaching the inner ear, assessing the degree of protection is difficult. Ototoxins can affect either the vestibular apparatus or the cochlea; subtle changes in response to ototoxins may be difficult to detect. Either the active ingredient or the vehicle of a topical preparation may be ototoxic. Among the known ototoxins that might be used to treat the ear topically are chlorhexidine, fluoroquinolones, aminoglycosides, polymyxins, eruthromycin, detergents, and alcohols.32 The lack of data regarding the ototoxic potential of a given product does not necessarily mean that the product is safe. In general, direct application of any antimicrobial to the external ear in the presence of a perforated eardrum is discouraged.
Otitis interna
Otitis interna or labyrinthitis resulting from infection of the inner ear also occurs as a result of extension from otitis externa and media (see discussion of otitis externa), movement of organisms through the auditory tube, or hematogenous spread. Foreign bodies, tumors (including cholesteatoma), or other occlusive or inflammatory objects may predispose the subject to infection.31 Clinical signs vary with the extent of vestibular dysfunction, which in turn reflects the extent of infection and accompanying inflammation. Continuation of infection into the meninges is more likely in cats than in dogs.
Cultures that reflect infecting organisms might be obtained by sampling the middle ear; alternatively, myringotomy may be necessary. Both topical and systemic antimicrobial therapy should be implemented. Because yeast is often present, topical therapy should include antifungal drugs (e.g., nystatin). A number of antimicrobials can be used for treatment of otitis interna, although distribution is likely to be limited for many (see Table 7-5). A lipid-soluble drug is preferred, and combination therapy should be considered to minimize the risk of resistance. Examples include the fluoroquinolones and chloramphenicol; the latter, however, is not likely to reach killing concentrations at the site of infection. Ototoxicity of antimicrobial drugs must be considered.32
Infections of the Skin
Pyoderma
Pathophysiology
Pyoderma is defined as a bacterial infection in skin associated with pus. Both the normal organ (skin) and the diseased local environment (pus) present barriers to drug movement and therefore efficacy.33 As such, pyoderma is a complex disease that may be difficult to treat effectively. The location of the infection in the skin, and particularly the depth (surface infection to cellulitis), confounds therapy.34,35 Consequently, one of the goals of therapy for pyoderma is to prevent the progression of clinical signs. Whereas a surface infection (e.g., intertrigo) is generally amenable to topical antimicrobial therapy, superficial skin structures (e.g., impetigo, superficial folliculitis) may be less responsive; however, even these infections may yet respond to proper topical therapy in lieu of systemic therapy. Certainly, as infection becomes more deep-seated, systemic antimicrobial therapy becomes more important (Figure 8-3, A).33-35 Lesions of deep pyoderma begin in the distal portion of the hair follicle, often extend below the follicle, and may be accompanied by furunculosis and a granulomatous response. As the disease worsens, antimicrobial penetration becomes more limited, and successful therapy is more difficult to achieve, requiring higher doses and, for time-dependent drugs, shorter intervals.33-35 Cellulitis is the most severe manifestation of pyoderma and involves infection of the dermis and adjacent subcutaneous tissues. Although uncommon, infection at this depth can become life threatening if sepsis develops.
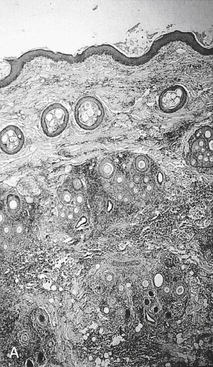
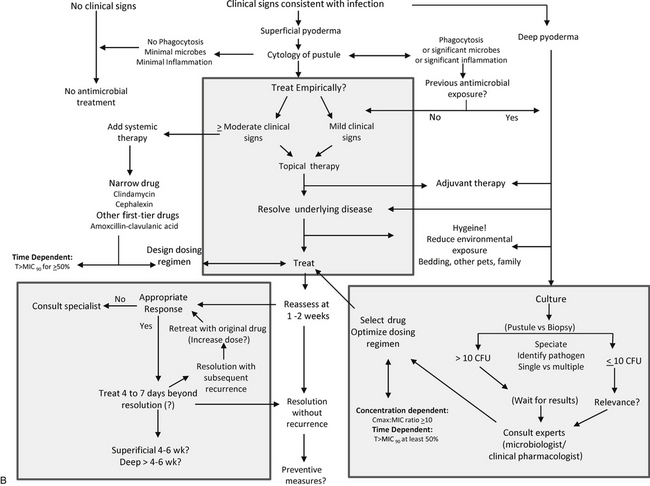
Figure 8-3 A, Histologic section of deep pyoderma. Scarring and accumulation of inflammatory debris present both mechanical and functional barriers to drug penetration. B, A suggested algorithm for treatment of canine pyoderma. Note empirical therapy of superficial pyoderma begins with topical rather than systemic therapy (center); treatment of deep pyoderma begins with culture and susceptibility testing (lower right). Once the decision is made to use systemic therapy, narrow spectrum, first-tier drugs should be chosen first (upper left). Topical therapy is a part of all therapies. Removal of underlying disease may be paramount to therapeutic success. Dosing regimens should be designed to minimize the advent of resistance; if successful, subsequent recurrences may continue to respond to first-tier drugs. Initial therapy should be reassessed within 7 to 10 days; failure to respond at this time should lead to alternate therapies, which may involve reculture and consultation with specialist. In responders, evidence regarding the duration of therapy is not clear and until such data exist, current recommendations focus on 4 to 6 weeks. (A, Photograph courtesy Bayer Animal Health.)
KEY POINT 8-8
Focusing on topical therapy as the sole method of delivery of antmicrobial therapy should minimize the need for systemic antimicrobial therapy, which might contribute to resistance.
Not all pyodermas are difficult to treat; indeed, most will respond to initial therapy. Therapy with superficial pyodermas should begin with topical therapy only (see Figure 8-3, B) However, some patients that initially respond to antimicrobial therapy experience recrudescence after therapy is discontinued. The most likely cause for recrudescence is failure or inability to control underlying skin diseases that predispose the skin to persistent or recurrent infections. Examples include ectoparasitism, seborrhea (cornification disorders), allergies (atopic dermatitis, flea or food allergies), and endocrinopathies (hyperadrenocorticism or hypothyroidism).33-35 Anatomic abnormalities such as skin folds (intertrigo) also predispose the patient to bacterial infection.33-35 Failure or inability to correct these underlying factors increases the risk of therapeutic failure, and emergent resistance if multiple courses of antimicrobials are implemented. In addition to the physiologic barriers presented by the normal animal, a number of other factors complicate treatment of pyoderma. These include the effects of the infecting microbe; mechanical, functional, and structural sequelae of progressive disease; and limitations of the drugs themselves. Although the sequelae of these interacting factors are difficult to predict in the individual patient, their impact can be minimized by decision making that is based on these interactions.
The surface of the skin normally presents several barriers to bacterial invasion and colonization. Cells of the stratum corneum desquamate from the surface of the skin and hair follicles, and the lipid-rich environment between the cells impedes bacterial movement.34,35 Epithelial proliferation follows injury to the skin, decreasing the likelihood of bacterial invasion. Sebum and sweat contain antibacterial chemicals such as inorganic salts34,35 and lipids.36 Finally, resident microflora may be important by helping to keep invading microflora in check. Differences in the incidence and ease of treatment of pyoderma in dogs versus cats and other species may reflect anatomic and physiologic differences in their skin.34,35 Canine skin may be predisposed to pyoderma because the stratum corneum is thin and compact and contains less lipid material. Therefore canine stratum corneum may present a less efficient barrier to bacterial invasion compared with that of other species. Canine hair follicles lack a lipid–squamous “plug,” which may facilitate bacterial penetration into the hair follicle.34,35,37 Finally, the pH of canine skin is higher than that of other species, perhaps providing an environment more conducive to bacterial proliferation.34,35
Skin also has a well-developed immune response, composed of proteins, immunoglobulins located in the basement membrane, cells of the immune system located in the dermis and epidermis, and regional lymphoid tissue.34,35 However, materials released from microbes facilitate invasion, impair cellular phagocytosis, and damage host tissues. Under normal circumstances components of bacteria that penetrate the skin stimulate a humoral response (immunoglobulins G and M), which in turn activates effector mechanisms leading to an acute inflammatory response.34,35 Soluble mediators released by organisms (e.g., hemolysin, epidermolytic toxin, leukocidin) may damage host tissues or alter host response.34,35 Staphylococcus spp. in particular plays a role in perpetuating the inflammatory response. Cutaneous mast cells triggered by antistaphylococcal immunoglobulin E may increase epidermal permeability and facilitate bacteria or bacterial antigen penetration in patients with allergic skin disease.34-36 Most staphylococci associated with canine pyoderma produce “slime,” a material that facilitates bacterial adhesion to cells. Staphylococcal organisms contain protein A, which impairs antibody response, activates complement, and causes chemotaxis.38 Some staphylococcal organisms release superantigens (enterotoxin), which may cause interleukin release and an inappropriately large T-lymphocyte response in the skin.34-3639 Finally, selected Staphylococcus spp. suppress or prevent intracellular killing once phagocytized by leukocytes.40 Viability of the organisms will be maintained inside the phagocyte, allowing not only survival but perhaps also continued replication inside the cell. Subsequent release of the organism on the death of the phagocyte allows reinfection, leading to persistent or recurrent infections.
The phagocytic white blood cells (initially neutrophils) that respond to inflammatory mediators use a number of oxidative (e.g., myeloperoxidase) and nonoxidative (e.g., bactericidal permeability-increasing protein) mechanisms to kill phagocytized invading bacteria.41 Unfortunately, host responses to bacterial invasion may become deleterious and can negatively affect therapy. The inflammatory response to infection may facilitate bacterial penetration, is responsible for clinical signs associated with the disease, and may preclude antimicrobial penetration of the skin. The severe and persistent inflammatory response leads to pruritus, and self-trauma further aggravates bacterial penetration. The disease may develop an autoimmune component.34,35 These long-term changes, in conjunction with inflammatory disease, predispose the patient to recurrent pyoderma.
As bacterial skin disease progresses, pathologic changes become barriers to drug distribution at the site of infection, and dosing regimens should be modified accordingly. The barriers vary with the site of infection, ranging from very superficial structures (epidermal and hair follicle) to deep structures below the hair follicle. Hair follicles may rupture, leading to furunculosis. Granulomatous changes and keratinaceous debris may act as foreign bodies, perpetuating the inflammatory response.34,35 These deeper structures may become isolated by fibrous tissue as disease progresses unchecked. Foci of organisms located in these isolated beds of scar tissue are a likely cause of recurrent infections with deep pyoderma (see Figure 8-3, A), as can intracellular survival of phagocytized organisms (see Figure 6-17Figure 6-18).34,35
Microbial Flora
The microflora of the skin is composed of both resident and transient organisms. Resident organisms in the skin of dogs include Micrococcus spp. beta-hemolytic streptococci, aerobic diphtheroids, Propionibacterium acnes, and Staphylococcus spp. Pseudomonas, Proteus, and Corynebacterium organisms have also been cultured from normal dogs. Among the resident organisms cultured from the skin of normal dogs, only S. intermedius is a likely initial cause of pyoderma. S. intermedius has recently been reclassified to Staphylococcus pseudintermedius; however, the original terminology will be used throughout this text until the newer terminology has been accepted and is in general use. S. aureus and other Staphylococcus spp.are much rarer causes of superficial pyodermas in dogs.34,35,37 S. intermedius is involved in approximately 90% of canine pyodermas.33-35 The resident status of S. intermedius in the skin is debatable. Although cultured frequently from either the skin or hair of normal dogs, S. intermedius may simply be a contaminant or a transient organism, acting as a “nomad,” proliferating only when environmental circumstances are supportive of growth.42 The microflora of dogs with pyoderma differs from the microflora of the skin in normal dogs. Even skin not clinically affected by pyoderma is characterized by increased staphylococcal colonization.34,35 Eventually, Staphylococcus spp. become the predominant organism in the skin. It can change the local environment, allowing proliferation of other organisms. Thus, even when disease has progressed to the point that gram-negative infections (e.g., E. coli, Proteus spp., and Pseudomonas spp.)34,35 are involved (generally deep pyodermas), S. intermedius is likely to be involved. In fact, if S. intermedius is not cultured from the skin lesion, the laboratory or culture technique may be questioned.33 When pyoderma is treated, concomitant infection with S. intermedius should be assumed, and control of infection by S. intermedius in such cases should facilitate treatment of other organisms, including gram-negative bacteria.
Several studies have addressed microbes causing skin infections, including prevelance of methicillin-resistant Staphylococcus spp. (MRS) in dogs and cats. Among the caveats to the studies will be differences in methodology, particularly as it pertains to sample collection. Ideally, samples are based on tissue samples following adequate cleansing; more often than not, however, samples are collected by swabs. Diagnostic aids can be useful to discriminate infection from colonization in the skin. Gram staining and cytology are particularly helpful. The presence of rods indicates a mixed infection (with gram-negative organisms); intracellular organisms indicate phagocytosis and thus infection rather than colonization. Cytology may help stage or identify the cause of disease: Mononuclear infiltrations indicate deep, chronic infections; large numbers of Staphylococcus spp. in pustules may indicate hyperadrenocorticism.34,35
Petersen and coworkers18 reported on the frequency and susceptibility of S. intermedius and P. aeruginosa in samples collected between 1992 and 1997 from canine skin and ears (n = 497) and submitted to a state diagnostic laboratory (see Table 8-3). The method of sample collection was not provided but reflects methods used by practitioners. S. intermedius was isolated from 89% of skin samples and was the only isolate cultured from 47% of samples. P. aeruginosa was isolated from 7.5% of skin samples, with the frequency of collection from the skin increasing during the 6-year period of the study. Of the isolates yielding P. aeruginosa, it was the only isolate in 33% of samples. Although Staphylococcus isolates were susceptible to drugs generally chosen empirically as first-choice treatment for pyoderma, as in the author’s institution, MRS appears to have begun increasing in the early 2000s. This is indicated by the difference in isolates collected from 2003 to 2005, versus 2006 and 2007 (Table 8-3). These data demonstrate that initially the proportion of susceptible isolates collected from skin,and other tissues was nearly 100% for drugs traditionally selected empirically, only to decrease to nearly 65% susceptible during this period. These data underscore the limitations that appear to be emerging regarding empirical selection of antimicrobials even for those conditions traditionally considered safe, such as pyoderma. They also underscore the importance of appropriate dosing regimens to minimize the advent of resistance.
KEY POINT 8-10
Methicillin-resistant Staphylococcus increasingly is being identified in association with pyoderma.
The public health significance of treating MRS is addressed in greater depth in Chapter 7. The role of previous antimicrobial therapy facilitating multidrug-resistant Staphylococcus spp. has been well established (see Chapter 6). A report from Sweden demonstrated that S. intermedius isolates from dogs with recurring pyoderma were more likely to be characterized by resistance than isolates of first-time infections.43 Approval and subsequent use of betalactamase inhibitors and cephalosporins are particularly well associated with MRSA and MRSI. Overproduction of beta-lactamases coupled with changes in penicllin-binding proteins contribute to oxacillin/methicillin-resistant Staphylococcus spp., whether aureus (MRSA) or intermedius (MRSI).
KEY POINT 8-11
The role of previous antimicrobial therapy facilitating emergence of multidrug resistant Staphylococcus has been well established.
Abraham44 prospectively studied the frequency of Staphylococcus spp. as a cause of infection in cats. No cat had received antimicrobials within the previous 7 days. The prevalance of coagulase-negative isolates was higher than coagulase-positive isolates in all cats, and the proportion of each was similar in healthy cats compared to cats with inflammatory skin disease (ISD). For healthy cats (n = 50); 21 were coagulase-positive isolates (n = 17/50, or 34% of all healthy cats) and 49 were coagulase-negative (n = 49/50 cats or (98% of all cats). This compares to ISD cats (n = 48) of which 25 were coagulase-positive isolates (n = 24/48 or 50% of cats) compared to 46 coagulase-negative isolates (n = 46/48 or 96% of cats). Of the coagulase-positive isolates, approximately 50% were S. aureus and S. intermedius, respectively, in both groups. Specifically, in healthy cats 10 and 11 of 21 coagulase-positive (48% and 52%) isolates were S. aureus and S. intermedius, respectively. From the ISD cats, 14 and 11 of 25 (56% and 44%) were S. aureus and S. intermedius, respectively. The proportions of staphylococci infections differ between dogs and cats.45 In a separate study by the same group, 74% of healthy dogs (n = 37/50) were positive for growth compared with 88% of dogs with ISD (n = 52/59).45 The total number of isolates (from different body regions) cultured was greater in ISD dogs compared with healthy dogs. For both healthy and ISD groups, coagulase-positive isolates far outweighed coagulase-negative isolates, and S. intermedius outnumbered S. aureus. For coagulase-positive isolates in the 52 ISD dogs, the frequency of isolation of S. aureus, intermedius, and schleiferi subsp. coagulans (SSc) (all coagulase positive isolates) was, respectively, 10, 134, and 6 isolates each, compared with 8, 69, and 0 isolates, each respectively, in the 37 healthy dogs (2 coagulase-negative isolates). In the healthy dogs, 9 isolates of coagulase-negative S. schleiferi subsp. schleiferi [SSs] were identified, indicating the possible importance of subspeciated S. schleiferi. Methicillin resistance occurred in both coagulase-positive (MRSA, MRSI, MRSSc) and -negative (MRSSs) isolates and was more frequent for MRSI and in ISD (n = 4 MRSI, n = 1 MRSA, MRSC = 1, MRSS = 1) compared with healthy (MRSI = 1, MRSA = 0, MRSC = 1, MRSS = 0) dogs.
Other staphylococci are being recognized for their increasing role in canine pyoderma. S. schleiferi has recently been recognized for its increasing role, particularly in recurrent infections;45,47,48 it was the second most common Staphylococcus organism cultured in one study. The increasing recognition of this organism is important because multiple antimicrobial resistances can occur with S. schleiferi, 45,49 whereas the incidence of multiple drug resistance (meaning resistance that includes drug classes other than beta-lactams) by S. intermedius remains low (see the discussion of antimicrobial resistance below). Concern might heighten if laboratories cannot or do not distinguish between S. schleiferi and S. intermedius, much as S. intermedius was confused with S. aureus in the 1970s. Further S. schleiferi exists as either coagulase-negative (schleiferi subspecies), which generally indicates contaminants, or coagulase-positive (coagulans subspecies), which has a greater clinical significance. The increasing prevalence of S. aureus infections coupled with the emergence of S. schleiferi warrants more caution and vigilance in the prevention of zoonosis.50
The role of P. aeruginosa as the sole infectious organism in canine pyoderma (n = 20) was retrospectively studied in 66 dogs with mixed or pure infections associated with pyoderma.46 As with Petersen,18 the incidence of Pseudomonas as the sole agent of pyoderma was observed to be increasing. Seven of the dogs presenting with deep pyoderma had not been previously presented for skin disease; these dogs responded well to 3 to 4 weeks of fluoroquinolone therapy. The remaining 13 dogs had a protracted history of skin disease that had been treated with antimicrobials, immunomodulatory drugs, or both. Of these dogs, 11 had been previously treated with antimicrobials effective against Staphylococcus spp. Antimicrobials to which isolates should potentially have been susceptible but were resistant included enrofloxacin (40%; half of these dogs had previously been treated with a fluoroquinolone), ticarcillin (36%), ciprofloxacin (25%), and gentamicin or amikacin and norfloxacin (5%). Interestingly, all isolates were susceptible to marbofloxacin, a questionable finding based on likely cross-resistance among fluoroquinolones. Doses of drugs used to treat the infection ranged from 6 to 13 mg/kg for enrofloxacin qd, or 5 to 12 mg/kg bid; norfloxacin at 18 to 23 mg/kg qd, marbofloxacin at 3 to 5 mg/kg qd, and cephalexin 20 to 25 mg/kg bid. All but two dogs responded after treatment for 3 to 12 weeks (mean 4.8 weeks). However, a potentially important observation that some dogs responded to cephalexin was interpreted to potentially imply that Pseudomonas was only a secondary pathogen in some of the dogs.
Considerations in Drug Selection
For most antimicrobials used to treat canine pyoderma, adverse events or side effects become less important to the selection and other considerations can take precedence. Exceptions might occur for erythromycin; up to 50% of animals should be expected to develop gastrointestinal upset when treated with erythromycin.51 Gastrointestinal side effects in particular are likely to increase if higher doses of antimicrobials are used. Immune-mediated side effects caused by potentiated sulfonamides may limit their long-term use.52 Sulfonamides are also able to decrease thyroid hormone synthesis at high doses that might otherwise be indicated to effectively treat pyoderma.
Drug Movement to the Site of Infection
Distribution of antimicrobials applied topically is limited by the presence of the stratum corneum, although distribution of topically applied drugs should be be enhanced in the presence of inflammation. Accumulation of inflammatory debris, however, also impedes drug movement after either topical or systemic administration. Shampooing not only removes inflammatory debris but also softens the stratum corneum, thus facilitating movement of topically applied antimicrobials to deeper tissues. Distribution of systemically administered antimicrobials to the skin is somewhat limited even with normal conditions. Despite the skin being the largest organ of the body, blood flow to the skin represents only 9% of the cardiac output. Drug distribution to the skin takes longer than to tissues with greater blood flow. Although skin is well perfused, blood supply to the epidermis consists of capillaries that lie under the epidermis, and drugs must passively diffuse to the epidermis and, with folliculitis, through the hair follicle. The plexi of arteries and veins that supply the skin include arteriovenous anastomoses that allow blood to bypass capillary beds. Blood supply to the skin can be altered easily in disease, particularly in the extremities and deep dermis.
Many antimicrobials used to treat pyoderma are lipid soluble, although the degree varies with each drug. Drugs that are characterized by a favorable distribution pattern to the skin include the fluoroquinolones, the sulfonamides (including those potentiated), the macrolides (erythromycin), the lincosamides (clindamycin and lincomycin), and chloramphenicol.53 The distribution of water-soluble drugs (beta-lactams, aminoglycosides) to interstitial fluid of skin should be anticipated to be less than that of plasma, particularly in the presence of physical barriers presented by fibrosis. Note that the poorer distribution of beta-lactams coupled with their time dependence indicates that those drugs with longer elimination half-lives are preferred. The presence of marked inflammatory cells may be taken advantage of with use of antimicrobials that accumulate in white blood cells (e.g., fluoroquinolones, clindamycin, macrolides, rifampin); higher intracellular concentrations may also facilitate intracellular killing of microorganisms that may survive phagocytosis.
Antimicrobial Resistance
Antimicrobial resistance may affect antimicrobial therapy of pyoderma or other infections associated with staphyloccocci. Resistance to penicillins by S. aureus or S. intermedius generally reflects an increase in chromosomally mediated beta-lactamase production.49 However, use of beta-lactamase protectors (e.g., beta-lactams coupled with clavulanic acid or sulbactam) or beta-lactamase–resistant drugs (e.g., cephalosporins) has led to adaptation by Staphylocccus spp. through mutations in the penicillin-binding proteins (PBP-2) encoded by the mecA gene, located on the staphylococcal chromosomal cassette (SCCmec). The expression of this gene prevents efficacy of any beta-lactam antibiotic.
S. aureus has developed an increasing pattern of resistance against many antimicrobials (discussed in depth in Chapter 6). Indeed, the proportion of S. aureus susceptible to drugs generally chosen to treat pyodermas is consistently less than that of S. intermedius. In contrast, S. intermedius has not appeared to be as adaptable; antimicrobial resistance in S. intermedius has been slow to emerge, and the impact of previous antimicrobial use on the susceptibility pattern of the microbe to other drugs has been controversial.34,35 However, although S. intermedius is a species distinct from S. aureus, transmission between these organisms of plasmids responsible for multiple antimicrobial resistance may be a factor that appears to be contributing to antimicrobial failure in canine pyoderma.34,35 In humans antimicrobial resistance is a rapidly emerging concern in dermatology.54 In patients with skin wounds, S. aureus (44% methicillin resistant) and P. aeruginosa were the most common bacteria isolated. The incidence of MRSA increased from 26% in 1992 to 75% in 2001. Resistance was not limited to Staphylocccus spp.; P. aeruginosa resistance to quinolones increased in deep wounds from 19% in 1992 to 56% in 2001, and in superficial wounds from 0 to 18% by 2001. Although these statistics have been generated from human medicine, their changing pattern mimics what is occuring in veterinary medicine. Several studies have demonstrated the advent of MRS in veterinary patients (see previous discussion).
The frequency of methicillin resistance in coagulase-positive and -negative Staphylococcus spp. in dogs with and without skin disease was previously cited.45 As early as 1998, in a retrospective study of 131 S. intermedius strains isolated from apparently healthy dogs and another 187 S. intermedius strains isolated from dog pyodermas, the proportion of multidrug-resistant (three or more drug classes) strains increased from 10.8% in 1986 to 1987 to 28% in 1995 to 1996.55 In dogs with pyoderma, 26% of the isolates in one study were associated with multidrug resistance, including resistance to methicillin, albeit in only 2 of 57 cases. However, although not revealed in the phenotype, close to 50% of the organisms studied carried the gene (mecA) responsible for formation of PBP2a, the penicillin-binding protein that confers resistance to methicillin. S. schleiferi was the second most common Staphylococcus organism cultured; most were resistant to methicillin (coagulase-negative versus positive subspeciation not provided). 49 That previous antimicrobial therapy may have been associated with Staphyloccoccus antimicrobial resistance was supported by the more common isolation of S. schleiferi in dogs currently receiving antimicrobials (10 of 12) compared with dogs that were not (5 of 28).47 A multiclinic prospective study that compared susceptibility in organisms isolated from samples from first-time and recurrent cases of canine pyoderma (n = 394 Staphylococci) found that resistance to macrolides, lincosamides, fusidic acid, tetracycline, and streptomycin was significantly greater in recurrent cases (45%) compared with the first-time cases (20%). Identification of S. schleiferi was not addressed. Resistance was not detected in penicillinase-stable beta-lactams.43
More recently, Morris19 retrospectively determined the proportion of Staphyloccoccus isolates expressing methicillin resistance in dogs and cats. Samples were collected from a variety of tissues; the method varied with the clinician; isolates were processed at a veterinary teaching hospital according to Clinical and Laboratory Standards Institute (CLSI) guidelines. Isolates included S. aureus (n = 139; resistant isolates = MRSA), S. intermedius (n = 463; MRSI), and S schleiferi (n = 148; MRSSs vs MRSSc not distinguished). The frequency of resistance for each Staphylococcus spp. was as follows: MRSA 35% (more commonly associated with deep infections), MRSS and MRSSc, together, 40% (more commonly associated with superficial infections), and MRSI, 17%. The higher percentage of resistance in this hospital population probably reflects the referral nature of the patients and the likelihood that patients had previously received antimicrobials. The frequency of MRSA was the same in both dogs and cats, whereas MRSI and MRSS were higher in dogs compared with cats. Of the resistant isolates, 28% of MRSA, 40% of MRSI, and 33% of MRSS were in the skin, and 5% of MRSA, 26% of MRSI, and 47% of MRSS were located in the ear. Among the organisms, MRSS remained most susceptible to all drugs save the fluoroquinolones, but susceptibility to the fluoroquinolones was ≤35% (see Table 8-3). Although chloramphenicol was the most consistently effective drug, its use as the sole agent should be questioned.
A study of healthy cats (n = 50) and cats with ISD (n = 48) found that four healthy cats and one ISD cat harbored a coagulase-positive MRS.44 In healthy dogs (n = 50), zero of six S. aureus isolates were MRSA, whereas one S. intermedius (n = 34) isolate was MRSI. In dogs with ISD (n = 48), one of six S. aureus isolates were MRSA, whereas 4 of 52 S. intermedius isolates were MRSI.45
Note that not all multidrug-resistant Staphylococcus organisms are methicillin resistant. Disconcertingly, MRS is expanding to include drug classes other than beta-lactams.56 Fluoroquinolone resistance may be associated with MRSA (ciprofloxacin and levofloxacin) and also has been associated with emergent MRSA,57 although it is not clear if the resistant isolates emerge once other microbes are inhibited by drugs, or if fluoroquinolones facilitate infection by increasing adherence of MRSA at the site of infection. On the other hand, multidrug resistance not in association with methicillin resistance (e.g., to clindamycin, chloramphenicol, doxycycline) also has emerged. Finally, resistance to vancomycin occurs with acquisition of the vanA resistance gene, usually from enterococci, which also prevents the drug from binding to the target peptidoglycan. Resistance to linezolid also has been documented.
The prudent clinician will take a proactive approach to prevent the development of antimicrobial resistance among organisms that cause canine pyoderma. Previous antimicrobial use should be considered in the selection of an antimicrobial without the benefit of culture and susceptibility data. Susceptibility data should be considered as a basis for selection of antimicrobials for patients with a history of recent antimicrobial use. Persistent infections imply therapy has failed; recurrent infections may have previously been successfully treated, but underlying disease or other factors that as yet are not identified or cannot be corrected, have facilitated return. Certainly, previously used drugs that failed should be avoided if the infection is considered persistent rather than recurrent, with selection best based on susceptibility testing for both. Perhaps more so than gram-positive isolates, resistance more likely may be encountered in gram-negative organisms associated with deep pyoderma,34,35 and culture and susceptibility data become increasingly important for antimicrobial selection with these infections. Resistance is more likely to develop with long-term therapy (as must occur for deep pyoderma) and conditions that are likely to result in subtherapeutic concentrations at the site of infection (e.g., beta-lactam antibiotics, particularly those with short half-lives compared with the dosing interval). Repetitive cultures that yield increasing MICs for the same drug may indicate development of resistance, and alternative drugs or combination antimicrobial therapy should be considered in such cases.53
Antimicrobial Selection
The number of antimicrobials recommended for initial therapy of pyoderma increases with the number of papers published regarding therapy. Clinician preferences vary, appropriately so, with experience. Although clinicians might not agree on their first-choice antimicrobial, there is little disagreement regarding the target organism. Initial therapy should be topical. If a decision is made that systemic drugs are indicated, therapy should begin with an orally bioavailable drug effective against S. intermedius. Ideally, the dosing interval should be 12 hours or longer to facilitate owner compliance; as such, time-dependent drugs with half-lives of less than 4 hours (e.g., amoxicillin, and potentially cephalexin) should be avoided in clients unwilling to dose every 8 hours. Subsequent considerations regarding drug selection vary with the severity of the disease. The more severe the disease, the greater the care that must be taken with antimicrobial selection. Tissue penetration should be increasingly predictable as the infection deepens. Detection and avoidance of resistance become increasingly important as the duration of therapy and the risk of subtherapeutic drug concentrations at the site of infection increase. With few exceptions, adverse effects are not a common cause of therapeutic failure and, as such, may not have a major impact on antimicrobial selection. Cost often has a major impact on selection. Clients may be reminded, however, that they might as well spend their money on an appropriate drug and dosing regimen rather than on prolonged therapy with an inappropriate drug or dosing regimen.
A number of drugs are indicated for initial therapy of pyoderma. Among the most frequent first-choice drugs for first time infections are beta-lactam antibiotics that tend to be resistant to Staphylococcus beta-lactamases. Cephalexin has been the treatment of choice for many clinicians. It and cefadroxil were among the earliest studied.58 As time-dependent drugs, based on integration of maximum drug concentrations and MIC50 or MIC90, high doses and frequent intervals may be indicated (Tables 8-6 and 8-7). The approval of cefpodoxime, a third-generation cephalosporin, has been a potentially important addition to the armamentarium of drug therapy for treatment of canine pyoderma. Attributes include an MIC90 for S. intermedius of 0.5 μg/mL and plasma drug concentrations that remain above this MIC for longer than 24 hours, with a dose of 10 mg/kg (but not 5 mg/kg if the 95th percentile of animals is targeted). The large structure of cefpodoxime appears to render it less susceptible to the development of resistance due to production of cephalosporinases. Another recently approved drug that will facilitate compliance is cefovecin. Based on plasma concentrations of unbound drug or interstitial concentrations,59 concentrations will remain above the MIC90 of S. intermedius, as reported on the package insert for at least 13 (plasma) to 18 days (interstitial fluid); the duration, however, will be much less if the 95th percentile is used. The advantages of this drug mandate its judicious use such that emergent methicillin resistance is minimized.
Table 8-6 Pharmacokinetic and Pharmacodynamic Indices for Treatment of Canine Staphylococcus pseudintermedius∗
Amoxicillin–clavulanic acid also is chosen as an initial drug for treatment of canine pyoderma. Its efficacy is similar to the first-generation drugs cephalexin and cefadroxil, although cephalexin is probably the most cost effective. Amoxicillin–clavulanic acid may be less likely than either first-generation cephalosporin to induce resistant bacteria at subinhibitory concentrations.34,35 However, its short half-life and low Cmax at recommended dose compared with the MIC50 or MIC90 for S. intermedius mandate an unacceptably short dosing interval if the targeted pharmacodynamic indices are to be achieved. The interpretive standards for amoxicillin have recently been updated by CLSI, causing many isolates previously considered susceptible now to be classified as resistant. Alternatively, beta–lactamase–resistant beta-lactams such as oxacillin and dicloxacillin might be used for initial or long-term therapy, although the cost may preclude their selection until initial therapy fails. Data regarding the current state of susceptibility of Staphylococcus spp. to these drugs is not available. A disadvantage of the beta-lactams is their failure to accumulate in phagocytic white blood cells. Further, distribution into tissues characterized by marked inflammation and fibrosis may be limited.
KEY POINT 8-12
A beta-lactam with a longer half-life should be selected for treatment of pyoderma; doses may need to be increased to adjust for reduced tissue distribution.
Chloramphenicol may also be an effective first-choice antimicrobial, although concerns for human exposure and its bacteriostatic nature to the drug may preclude its use. Decreased use of this drug because of toxicity concerns has probably contributed to its consistent efficacy against Staphylococcus spp., including methicillin resistance. If chloramphenicol is chosen, care must be taken to use it at appropriate dosing intervals. MIC90 data for S. intermedius was not available, but doses can be designed around MIC (generally 8 μg/mL in our hospital) from the patient’s data when available. Its use as sole agent for treatment of methicillin resistance should be avoided if possible because of its bacteriostatic nature. In our hospital, it has proven largely ineffective for treatment of MRS or multidrug resistant organisms. Combination with rifampin might be considered.
For infections that do not respond to initial therapy and become increasingly complicated, a drug that targets both S. intermedius and gram-negative organisms might be selected. Because bactericidal concentrations at the site of infection are desirable,37 a drug that is likely to distribute to and through the inflammatory site is desirable. The use of beta-lactams should be de-emphasized for infections associated with marked inflammatory debris or scarring because these drugs do not penetrate these barriers well.53
Appropriate drugs should be based on susceptibility testing. Lipid-soluble drugs include the fluoroquinolones and potentiated sulfonamides (i.e., trimethoprim– or ormetoprim–sulfonamide combinations), erythromycin, azithromycin, and lincomycin or clindamycin; of these, only the potentiated sulfonamide and fluoroquinolones are classified as bactericidal. Each of these classes, except the potentiated sulfonamides, also accumulate in phagocytic WBC. Increasingly common reports of immune-mediated diseases such as keratitis sicca or impaired thyroid hormone secretion may decrease the use of potentiated sulfonamides, particularly at high doses and for long-term therapy. An advantage might, however, be once- to twice-daily therapy (sulfadimethoxine/ormetoprim), which has proved effective for some authors.34,35 A cautious reminder, however: efficacy based on resolution of clinical signs does not preclude emerging resistance.
The fluoroquinolones, such as enrofloxacin and marbofloxacin, are the drugs of choice for complicated infections. They are characterized by rapid bactericidal activity, and their spectrum includes both S. intermedius and gram-negative organisms.34,35,37 These drugs distribute very well to the skin.60 Inflammatory debris and fibrous tissue should be traversed well with little impact on antimicrobial efficacy.60 Efficacy of the fluoroquinolones is maintained despite slow growth of organisms or low oxygen tension.61 All fluoroquinolones that have been studied accumulate in white blood cells,40,62–66 thus increasing drug at the site of inflammation.67 Fluoroquionlones are able to penetrate the lipopolysaccharide covering of gram-negative organisms. Their spectrum includes Pseudomonas spp., although increasing resistance to this organism and to S. aureus is reported. In one study of 383 isolates, the MIC for enrofloxacin against S. intermedius from 1995 to 1999 ranged from 0.063 to 64 mg/mL; the MIC50 and MIC90 were 0.125 and 0.25 mg/mL, respectively. Two resistant strains were found, but only among isolates collected in 1999. However, the authors concluded that the emergence of resistant mutants following 10 in vitro passages suggests that inappropriate use might favor the development of resistant strains in vivo. Like enrofloxacin, the MICs of marbofloxacin for S. intermedius are low, ranging from 0.125 to 2 μg/mL; MIC50 and MIC90 are both 0.25 μg/mL (according to the package insert). The fluoroquinolones are concentration-dependent drugs; doses should achieve a target Cmax:MIC of greater than 8 to 10. Both enrofloxacin (with its active metabolite ciprofloxacin) and marbofloxacin achieve nearly 2 μg/mL in plasma at 5 mg/kg, yielding an inhibitory quotient of about 8. Tissue concentrations are likely to be even higher. Following administration of 5 mg/kg of enrofloxacin daily for 3 days, concentrations reached 1.9 and 4.2 μg/mL in the biopsied skin of animals with superficial and deep pyoderma, respectively, compared with 1.5 μg/mL in plasma and normal skin68; ciprofloxacin was not measured in either tissue. However, these studies are based on homogenate data, and relevancy to clinical response is not clear.
Despite favorable skin distribution of fluoroquinolones, use of high doses are recommended when possible. In one study, based on Staphylococcus organisms isolated from dogs with spontaneous disease (antimicrobial history unknown), a target Cmax:MIC90 ≥10 might be achievable based on the highest dose and the MIC50 for four of five fluoroquinolones used clinically in dogs (the exception being difloxacin), with ratios greatest for enrofloxacin and ciprofloxacin. However, no drug was able to achive the target when using the MIC90 (Table 8-6).131 However, if the isolate is known to be susceptible to fluoroquinolones, the ratio of Cmax: MIC90 can be achieved for ciprofloxacin, enrofloxacin, or marbofloxacin using the MIC90 at the high dose.131 These findings were supported by a study that found the MICs of orbifloxacin for S. intermedius isolates to be higher compared with enrofloxacin or marbofloxacin. In a study of 254 isolates (69 skin and 171 ear), the MICs for orbifloxacin ranged from 0.016 to 8 mg/mL; MIC50 and MIC90 were 0.5 and 1 μg/mL, respectively. Peak concentrations at 2.5 mg/kg achieve 2 μg/mL, yielding an inhibitory quotient at 2. Data are not available for 5 and 7.5 mg/kg. However, extrapolating doses for 7.5 mg/kg from 2.5 mg/kg, a ratio of 6 might be anticipated. Orbifloxacin exhibited a concentration-dependent, bactericidal effect against S. aureus reference strain, but a time-dependent bactericidal effect against S. intermedius.69 Tissue concentrations for comparison with MIC data are not available for orbafloxacin.
Newer third-generation fluorquinolones may have the advantage of potentially being effective toward isolates resistant to second-generation drugs. This susceptibility should not be assumed but should be based on culture data and doses (using known pharmacokinetic data) designed around MIC.
Like the fluoroquinolones, clindamycin and the macrolides (including the azalide azithromycin) accumulate in white blood cells, distribute well to skin, and target Staphyloccoccus spp.; however, neither is characterized by a gram-negative spectrum. Although these drugs are bacteriostatic in action, bactericidal concentrations may be achieved in some tissues, including phagocytic WBC. (see Chapter 7). These may be reasonable choices for treatment of canine pyoderma. Note, however, that azithromycin will not reach steady-state concentrations for 4 to 7 days after therapy is begun; accordingly, a loading dose (twice the maintenance dose) should be given the first 2 days. In an uncontrolled study of superficial pyoderma in dogs (n = 21), clindamycin at 11 mg/kg every 24 hours for 14 to 42 days (depending on response) yielded a clinical score of excellent in 71% of animals within 14 days of initiating therapy. However, the authors noted that clinical response should be evaluated at 14 and 28 days because resistance developed. Cultures should be considered to detect resistance.70 Azithromycin (10 mg/kg daily) was effective in 90% of dogs with either superficial or deep bacterial pyoderma in an uncontrolled clinical trial.71 Interestingly, rifampin as sole therapy may be effective for treatment of S. pseudintermedius (but not S. aureus), although clinical trials supporting this approach are indicated. Accumulation in phagocytic cells may facilitate its efficacy.
The use of aminoglycosides for treatment of complicated pyoderma should be limited to life-threatening conditions associated with sepsis (e.g., cellulitis) or to organisms with known (i.e., based on susceptibility data) resistance to enrofloxacin. The aminoglycosides are generally effective against Staphylococcus spp. (particularly gentamicin) and gram-negative organisms including Pseudomonas (particularly amikacin). However, aminoglycosides generally should not be used as sole agents to treat Staphyloccoccus spp. unless evidence supports such use under the intended conditions. Combination with rifampin should be considered. For gram-negative organisms, resistance is less likely to develop against amikacin than against gentamicin, particularly for Pseudomonas spp. The aminoglycosides do not distribute well through inflammatory debris and fibrous tissue, are not effective against facultative aerobes (i.e., in the presence of reduced oxygen tension), and are not accumulated in white blood cells.40 Therefore care must be taken to ensure that an adequate dose is given. Once-daily administration is encouraged, and combination antimicrobial therapy with a beta-lactam effective against the target organism should be strongly considered.
As sole therapy, rifampin is generally discouraged in part because resistance develops rapidly (indeed, resistance to rifampin is used experimentally to detect mutation frequencies). However, in an open, uncontrolled clinical trial in dogs (n = 20; all but 2 were superficial pyoderma), 40% were S. pseudintermedius positive.71a Treatment with rifampin at 5 mg/kg once daily for 10 days was clinically successful in 90% of dogs; the two dogs that failed therapy had deep pyoderma. Recurrence did not occur for at least 1 month in responders.
Designing a Dosing Regimen
Perhaps an often overlooked reason that bacterial pyoderma is difficult to resolve in dogs is use of an inappropriate dosing regimen of an otherwise appropriate drug. Although the importance of culture and susceptibility testing for chronic, recurrent, and deep pyoderma cannot be denied, it is critical to realize that in vitro conditions do not and cannot mimic the microenvironment of the host. They also do not reflect the ability of the drug to reach the site of infection, including inside white blood cells, nor do they take into account active metabolites. The effects of underlying skin disease including changes in the patient’s immune status (local or systemic) also cannot be taken into account by the in vitro system. Thus it is critical that both the dose and interval of a dosing regimen be designed such that drug concentrations are maximized at the site of infection.
In general, the dose of an antimicrobial should be as great as possible when treating complicated pyoderma to ensure effective concentrations at the site of infection. Twice the recommended dose has been suggested by some authors, and this is a reasonable starting point, but it is likely to be insufficient for some drugs in some patients, particularly if the drug is time dependent and characterized by a short half-life.34,35 Doses up to fourfold higher than recommended may be necessary for organisms whose MIC for the drug is close to breakpoint or in the presence of factors that will decrease the movement or efficacy of antimicrobial at the site of infection. Higher doses are critical for concentration-dependent drugs (see Table 8-6). The greater the inflammatory response at the site (and particularly fibrosis) and the deeper the infection, the more important the need for increasing the dose. If necessary, selection of an antimicrobial might be made with an emphasis on safety so that doses can be increased with minimal risk of side effects. High tissue concentrations in relation to the MIC of the infecting organism are particularly important to the antimicrobial efficacy of selected drugs, most notably the aminoglycosides and the fluoroquinolones. For concentration-dependent drugs (aminoglycosides and fluoroquinolones), antimicrobial efficacy is enhanced in the presence of a high Cmax:MIC ratio in part because of an enhanced postantibiotic effect. In addition, both aminoglycoside efficacy and safety are facilitated by a moderately long drug-free period during a dosing interval. Thus for aminoglycosides once-daily administration of the total daily recommended dose should be considered for pyoderma. With the fluoroquinolones, the dose should likewise be modified to maximize plasma (and thus tissue) drug concentration; this is particularly important for organisms whose MIC approaches the breakpoint for enrofloxacin (4 μg/mL). Once-daily dosing with enrofloxacin at a high dose (15 to 20 mg/kg) is recommended. However, because efficacy of fluoroquinolones also is based on area under the curve (see Chapter 6), the addition of a second dose may also enhance efficacy for complicated pyoderma.
For time-dependent antimicrobials, the interval of drug administration must be considered. These include the beta-lactams and bacteriostatic antimicrobials. For these drugs the duration that the drug concentration at the site is above the MIC is more important to antimicrobial efficacy. Thus for beta-lactams, antimicrobial efficacy is likely to be facilitated more by a shorter dosing interval than by increasing the dose. For example, to improve the efficacy of amoxicillin–clavulanic acid or cephalexin, the dose should be increased such that 2 to 4 times the MIC is reached and then an 8-hour dosing interval should be used in lieu of 12-hour intervals when treating cases at risk for development of resistance. Unfortunately, owner compliance is less likely with this regimen and may be a reason for considering an alternative class of drugs for treatment of complicated pyodermas. The long half-life of cefpodoxime supports its use at 10 mg/kg at 24-hour dosing intervals for many susceptible organisms. Cefovecin is particularly appealing; it should be effective with a single dose, particularly if the underlying cause of the disease can be identified and corrected. If healing is critical to resolution of infection (to be differentiated from resolution of clinical signs), a second dose may be indicated.
Duration of Therapy
An insufficient duration of therapy is another common cause of therapeutic failure in the treatment of pyoderma with an otherwise appropriate antimicrobial. However, clinical trials are indicated to establish if the issue may also be a less-than-ideal dose (i.e., a dose that fails to achieve the highest MIC of the infecting colony) that would allow a shorter duration. Recommendations have varied with authors, although the consensus is that the duration of therapy for pyoderma increases with the severity of infection.34,35 Historically, therapy has extended 7 to 21 days (depending on the severity of infection) after surface healing has occurred. However, whether this is based on resolution of infection versus resolution of clinical signs has not been established. Superficial infections should resolve within 3 to 4 weeks of antimicrobial therapy if the patient is immunologically normal. Recommendations for deep pyoderma or the presence of immune compromise have suggested at least 4 to 6 weeks of therapy. Treatment for 12 weeks or longer is not unusual. Evaluation by the clinician at 7 days and then at 14-day intervals may be prudent, and reculture might be considered to reconfirm infection and should be implemented to detect resistance. Clinical evaluation should continue for several more weeks after therapy has been discontinued to ensure resolution.
Several authors have suggested alternative dosing regimens for antimicrobials for patients whose disease will not resolve. Examples include dosing once daily, every other day, or pulse dosing. Pulse dosing involves administration of a drug using full dosing regimens either 2 days a week or every other week. With the every-other-week approach, if recurrence is prevented, the duration of the “off” week can be gradually increased. Intervals of greater than 3 weeks are, however, likely to result in recrudescence.34,35 Conceivably, the risk of resistance should be increased if infecting microorganisms are exposed to intermittent concentrations of drugs. Clinically, however, this does not appear to happen, although clinical trials that focus on emerging resistance (rather than resolution of infection) are lacking. It is possible that the impact of prolonged antimicrobial therapy on normal skin microflora might be minimized by pulse dosing, particularly if dosing occurs at levels high enough to target those colony-forming units (CFUs) with the highest MIC whose survival otherwise would facilitate emergence of a resistant population. If pulse dosing is to be implemented, a drug with a narrow spectrum (i.e., oxacillin, clindamycin, erythromycin) should be used to minimally affect the normal flora; further, high doses should be used to ensure efficacy against targeted organisms. Compliance may be an issue with pulse dosing, and care must be taken to ensure that drug therapy is maintained. Pulse dosing with cephalexin on the weekend (15 mg/kg every 12 hours) decreased the number of relapses compared with placebo (n = 13) after 1 year of therapy for prevention of relapse of idiopathic superficial or deep pyodermas in dogs (n = 28). The time to relapse also was greater in the treatment group (6.6 months) compared with the placebo group (2.5 months). Dogs were assigned to either group after clinical resolution was acheived following cephalexin at 15 mg/kg twice daily until 2 weeks beyond clinical cure. Dogs did not receive other systemic antibacterial drugs or any antiinflammatory.
Other Clinical Trials
Efficacy for treatment of pyoderma exists for both enrofloxacin72,73 and marbofloxacin.74 In an open (uncontrolled) study of dogs with superficial pyoderma (n = 66) caused predominantly by S. intermedius, marbofloxacin was effective in 86% and improved in another 8% after 21 to 28 days of therapy.74 In another study 81% of dogs with recurrent superficial or deep pyoderma (n = 228) associated primarily with S. intermedius receiving marbofloxacin (2 mg/kg) for 3 to 16 weeks were classified as having responded at 1 week after cessation of therapy; at 1 month 70% of the dogs were still classified as responders, whereas 11% were classified as having relapsed.75
The efficacy of azithromycin for treatment of canine pyoderma (n = 26; 8 superficial and 18 deep) was studied prospectively using open uncontrolled design and was reported as an abstract.71 Underlying disease was accepted and diagnosis was based on cytologic examination. The duration of azithromycin (10 mg/kg qd) varied with the deepness of the lesions ranging from 5 to 10 days. Eighty-eight percent of dogs recovered, with dogs that had superficial lesions requiring 5 to 7 days and dogs that had deep lesions requiring 7 to 10 days. Side effects occurred in only 24% of dogs, these included intense salivation, anorexia, and vomiting. One dog required antiemetic therapy (metaclopramide).
Stegemann and others76 reported the efficacy of cefovecin for treatment of canine pyoderma and skin wounds. Dogs (n = 354) were from the United Kingdom and were evaluated in three studies, each using a randomized parallel design such that dogs received either amoxicillin–clavulanic acid (12.5 mg/kg bid for 2 weeks; n = 112) as positive control or cefovecin (8 mg/kg, administered subcutaneously; n = 242). The blinding method varied for each study, with a double-dummy design (each dog received placebo) implemented in one of the studies (Study B). Exclusion criteria included antimicrobial use within the past 14 days, short-acting glucocorticoids in the previous 10 days, or long-acting corticosteroids for 30 days. Up to 0.5 mg/kg/day of methylprednisolone or prednisone was allowed to control intense pruritis. Concurrent otitis was treated topically. Parasitic and fungal diseases were ruled out, and bacteria were confirmed by culture (sampling technique not delineated). Animals were evaluated 28 days after the final treatment. The most common organism cultured was S. intermedius (data are included in Table 8-7) (n = 223), with the MIC50 and MIC90 being 0.12 and 0.25 μg/mL, respectively. The next most common isolate was E. coli (MIC50 and MIC90 of 0.1 and 1 μg/mL, respectively). The number of treatments did not differ between groups and depended on clinical response, with nearly 50% of animals with superficial pyoderma in both groups responding after a single 14-day course of therapy; for animals with deep pyoderma, the numbers approximated 30% for both groups. For animals with deep pyoderma, 89% of animals enrolled in two of the studies had responded by the end of the third course of treatment (42) days, which is consistent with the recommendation to treat deep pyoderma for 4 to 6 weeks. Cefovecin was considered numerically more effective in treatment of superficial pyoderma. Criteria for success varied with the study and ranged from total absence of clinical signs to mild clinical signs. The study targeted a “noninferiority” classification of cefovecin, demonstrating that it was not worse than the positive control. Clinical success with cefovecin averaged 97% (study A & C) and 87% (study B) for all indications, compared with 92.5% and 80%, respectively, for amoxicillin–clavulanic acid. For cefovecin the lowest proportion of responders was for deep pyoderma (n = 13), at 77% for the double-dummy study versus 96% for the other two studies; only one animal with deep pyoderma was treated with amoxicillin–clavulanic acid. The incidence of side effects was similar in both groups, except for vomiting, which occurred more commonly in the group receiving amoxicillin–clavulanic acid.
KEY POINT 8-13
The long half-life of cefovecin will facilitate appropriate dosing. However, the impact of persistent drug concentrations must be monitored, and indiscriminate use of this potentially important drug must be avoided.
The efficacy of pradofloxacin (n = 56, 3 mg/kg by mouth qd) was compared with that of amoxicillin–clavulanic acid (positive control; n = 51, 12.5 mg/kg by mouth bid) for the treatment of deep pyoderma in dogs (n = 56) using a multicenter, randomized blinded controlled clinical trial.77 Treatment continued until 2 weeks past remission, for a maxium treatment period of 9 weeks; final assessment took place 2 weeks later. Exclusion criteria included antiinfective or antiinflammtory drugs in the previous 14 days. Inclusion criteria required a positive culture (swabs; two attempts made before exclusion); positive cultures were found in only 71% (n = 92) of the 130 dogs studied. Bacteria isolated were predominantly Staphylococcus spp. (n = 115), with other organisms including but not limited to Enterococcous spp. (n = 5), Pseudomonas spp. (2), and E. coli (2). For the pradofloxacin group, 86% achieved clinical remission and recurrence did not occur during the 2 week posttreatment period. For the amoxicillin–clavulanic acid group, 73% (n = 37) achieved clinical remission; for the remaining dogs, 3 improved, 5 did not respond, and 6 had recurrence of clinical signs within 2 weeks of remission.
Topical Therapy
Topical antimicrobial therapy may be the only method of antimicrobial administration needed to treat selected surface and superficial pyodermas. Antiseptic bathing (e.g., benzoyl peroxide, chlorhexidine, ethyl lactate, iodine, or triclosan) should be considered as initial sole therapy in uncomplicated pyodermas. Topical antimicrobials also might be considered, particularly for localized infections. Mupirocin might be considered for treatment of localized dermatologic problems, including interdigital abcesses, pressure point pyodermas, and secondary pyodermas associated with lick dermatitis. Early use of mupirocin, in particular, may be beneficial in limiting the recurrence of pyodermas. The efficacy of mupirocin 2% ointment (bid for 3 weeks) was reported as good to excellent in an open, uncontrolled design for treatment of chin acne in cats (n = 25). Therapy had to be discontinued in one cat because of a contact allergy.78
Topical antimicrobial therapy should also be considered an adjuvant for superficial and deep pyodermas. Shampoos remove debris that might impede drug movement or affect drug efficacy. In addition, the combined effect of a topical and systemic antimicrobial may result in additive or synergistic antibacterial effects in the patient with pyoderma. Although irritating with long-term use, products that contain benzoyl peroxide tend to be the most efficacious in controlling bacterial growth and removing accumulated debris on the skin.79 Products containing chlorhexidine, sulfur, triclosan, and ethyl lactate are also acceptable. Shampoos should be used twice weekly.34,35
KEY POINT 8-14
The combined effect of a topical and systemic antimicrobial may result in additive or synergistic antibacterial effects in the patient with pyoderma.
A human clinical trial examined the effect of terbinafine cream (1%) compared with gentamicin (0.1%) using a contralateral design in patients with pyoderma associated with S. aureus.80 After treatment, S. aureus could not be isolated in any patient treated with gentamicin and in only three patients treated with terbinafine; a negative control was not reported. Both groups markedly improved during the treatment period and treatments did not differ significantly. The authors concluded that terbinafine might be beneficial for treatment of S. aureus, particularly that associated with fungal infections. Checkerboard MIC studies have demonstrated that the combined effect of benzoyl peroxide and terbinafine is greater than that of either drug alone against Candida spp., S. aureus, and Pseudomonas spp., resulting in additive or synergistic effects against all three isolates.81
Adjuvant Therapy
The importance of adjuvant therapy should not be overlooked in the treatment of pyoderma.34,35 Adjuvant therapy may target the underlying skin disease or support antimicrobial therapy. Antihistamines (see Chapter 29) and 3-omega fatty acids should be considered when appropriate in conjunction with antimicrobial therapy to control the inflammatory response associated with infection or the underlying disease. Antimicrobial efficacy will be complicated by coadministration of drugs that alter the immune response. Most notably, glucocorticoids should not be used in patients with pyoderma. Although these drugs effectively ameliorate the inflammatory response and are useful in cases of pruritus leading to self-trauma, they also may facilitate the spread of bacterial infection.34,35 Other adjuvant therapies to consider include immunomodulatory therapy and topical antimicrobial therapy. Although immunomodulatory therapy (levamisole and cimetidine) may prove beneficial, there is often a bimodal effect with these drugs: if the proper dose is not given, immunosuppression rather than enhancement of the immune system may occur.34,35
Adjvant therapy may include immunomodulators. In a pilot study human recombinant interferon alpha-2b (1000 IU/day by mouth) was only minimally effective compared with placebo in dogs with idiopathic recurrent superficial pyoderma.82 Analgesic therapy also should be considered, particularly in animals that are experiencing pain. To prevent immunosuppression, nonsteroidal antiinflammatories should be considered. Indeed, meloxicam proved better than a placebo in control of pain associated with pyotraumatic dermatitis or folliculitis.83 Tepoxalin might be worthwhile because it targets both prostaglandins and leukotrienes, the latter implicated in perpetuation of chronic inflammatory allergic diseases such as atopy (see Chapter 31). It is reasonable to consider the use of nonsteroidals, and tepoxalin in particular for treatment of pruritis associated with pyoderma. In a report from the Czech Republic, dogs (n = 18) with recurrent pyoderma received either cephalexin or cephalexin and an immunomodulator (characterized by natural killer cell activity, lymphocyte proliferation, and enhanced macrophage activity). The study did not appear to be randomized or blinded; further, dogs had previously received glucocorticoids. The authors reported that dogs receiving the immunomodulator had a better cure rate and a shorter time to cure.84
Juvenile Cellulitis (Puppy Strangles)
Juvenile cellulitis is probably more appropriately addressed as an immune-mediated disease, but its potential microbial-based pathophysiology as well as its presentation warrant inclusion under bacterial infections. Juvenile cellulitis is an unusual but often painful nonpruritic inflammatory condition afflicting puppies generally from 4 weeks to 4 months of age.85,86 Clinical signs of inflammation generally involve the face or head. Its presentation may begin as a mild inflammation (e.g., redness and edema at tips of ears) but may rapidly become severe. Lymphadenopathly of nodes draining the inflamed areas (e.g., submandibular) has led to the misnomer “puppy strangles”; prescapular and other lymph nodes may become involved. Lesions range from (granulomatous) inflammation to pustules, ulcers, or erosions; fistulae may develop. Pustular otitis externa may be present. Less commonly, other areas of the body may be involved, including the abdomen, thorax, feet, vulva, prepuce, or anus. The extent of lesions may result in systemic illness; leukocytosis caused by neutrophilia and anemia typical of chronic disease may be present. Occasionally, other body systems will be involved (e.g., sterile suppurative arthritis)87. Cytologic examination reveals sterile pyogranulomatous inflammation; cultures generally are negative. Histopathology generally reveals granulomas and pyogranulomas consisting of clusters of large epithelioid macrophages. The cause of juvenile cellulitis is unknown, but the syndrome responds well to immunosuppressive doses (prednisolone at 2 mg/kg/day for up to 3 weeks), indicating a potential immune dysfunction. Hypersensitivity to (previously eradicated) microorganisms has been proposed. Therapy should be early and aggressive to prevent scarring. Antimicrobials are indicated only if supported by diagnostics. Although an etiologic agent has not been identified, a streptococcal-based reaction is suspected. Ideally, a lipid-soluble drug effective against Streptococcus spp. would be indicated to penetrate inflamed tissues. Fluoroquinolones are not recommended, not only because of questionable efficacy toward Streptococcus spp. but also because of age-related effects on cartilage as well as the potential induction of bacteriophage toxolysins carried by Streptococcus spp. (see Chapter 7). Assuming that there is a need for antimicrobial therapy targeting Streptococcus spp. high doses and frequent intervals of penicillin beta-lactams (amoxicillin–clavulinic acid) are indicated. Supportive therapy (e.g., cleaning of wounds, use of astringents) are indicated as needed. Analgesic therapy should be considered.
Wounds
The use of systemic antimicrobials for treatment of wounds is controversial, particularly in human medicine, for which diabetic and decubitus ulcers present a profound problem. Although the pathophysiology of these wounds may differ from that of the most common wounds treated in veterinary medicine, the principal approach should be similar. Disruption of skin or other tissue exposes the wound to infection, dehydration associated with fluid loss occurs, immunity is compromised, and scarring occurs. In contrast to the noninfected surgical wound that heals by primary intention, all open skin wounds, including those healing by secondary intention,88 will be colonized with microbial organisms. This does not, however, indicate infection or the need for antimicrobial therapy. Host factors largely will determine if growth of the microbial population will be limited to colonization, with healing proceeding normally. Chronic wounds in particular are predisposed to infection because extensive synthesis of new, primarily granulation, tissue must occur. Scar tissue, which contains no epidermal appendages, is more extensive. As such, multiple aspects of the skin immune system are absent. Chronic wounds are further complicated by the presence of fibronectin, an adhesive glycoprotein that, while intended to serve as a matrix adhesive, also binds bacteria. Neutrophil and macrophage influx is critical to keeping bacteria in check.
KEY POINT 8-15
All open skin wounds, including those healing by secondary intention,will be colonized with microbial organisms. This does not, however, indicate infection or the need for antimicrobial therapy.
Colonizing microbes generally do not penetrate to deeper tissues, whereas infecting microbes will; accordingly, swabs are undesirable culture methods. Whether colonization progresses to infection depends on the number of organisms per gram of tissue (thus tissue samples rather than swabs are critically important to proper culture) and the ability of the patient to mount an effective immune reponse. Infection generally requires greater than 100,000 CFU/g tissues in a wound. An exception occurs for beta–hemolytic Streptococcus, which is particularly virulent in humans. Successful wound healing depends on an organism load below 100,000 CFU/gm. Studies generally demonstrate that in contrast to acute wounds, systemically administered drugs generally do not reach deeper tissues where infection occurs. This reflects not only the changes associated with healing but also the formation of biofilm (see Chapter 6). The impact of biofilm on healing of chronic wounds can be profound. Infections involving biofilm are resistant to host immune responses and more resistant to antimicrobials and topical antibacterials. The moist environment generally associated with chronic wounds facilitates biofilm, as does fibroinnectin.
KEY POINT 8-16
The impact of biofilm on healing of chronic wounds can be profound, leading to resistance to host immune responses and resistance to both systemic and topical antimicrobials.
In human medicine the consensus is that wound care should be optimal. Wound care should prevent microbial growth, which will inevitably occur on the surface of the wound, from progressing from colonization to infection. Accordingly, cleansing, débridement, nutritional support, and actions that enhance oxygenation, including increased perfusion, are paramount to success. A variety of wound-assessment tools have been developed in human medicine.88 Wound care should be thoroughly reviewed before implementing systemic antimicrobial therapy. Topical antimicrobials are considered the key to successful management of wounds and are indicated if there is a risk that colonization will progress to infection. In humans wound contamination is treated simply with irrigation and cleansing with sterile water or saline. Likewise, wound colonization is treated with irrigation and cleansing of wounds, removal of necrotic tissue and foreign bodies, and nanocrystalline silver dressings. Critical colonization may require systemic antimicrobials but should be accompanied by medicated bandages and the topical application of slow-release antimicrobials (e.g., topical silver and cadexomer iodines). Wound infection is an indication that systemic antimicrobials should be added to topical therapy. Both systemic and topical medications are particularly critical in the face of poor wound perfusion.
KEY POINT 8-17
Topical antimicrobials are considered the key to the successful management of wounds and are indicated if there is a risk that colonization will progress to infection.
The most common topical antimcirobials used for wound management in humans are mupirocin, neomycin, bacitracin, polymyxin, erythromyicn, gentamicin, and silver sulfadiazine. Other topical antimicrobials to consider include fusidic acid and metronidazole. Their use for wound management was reviewed by Spann et al.89 In addition to these traditional drugs, newer antimicrobials are under development. These include protegrin-1, an antimicrobial peptide that occurs naturally in a number of mammalian tissues, including neutrophils. These are particularly effective against gram-positive organism, including MRSA and vancomycin-resistant Enterococcus spp. Other topically applied antimicrobials are under investigation (see Box 8-1).
The use of antiseptics on wounds is controversial, in part because of their impact on wound healing. This impact appears to be concentration dependent.90 Topical antiseptics include sodium chloride, (preferred) chlorhexidine, and povidone–iodine. The Food and Drug Administration has approved povidone–iodine for short-term treatment of superficial and acute wounds, indicating that its impact on wound healing is neither positive nor negative. A potential reason for using antiseptics, rather than antimicrobials, on wounds, is to prevent antimicrobial resistance. However, care should be taken not to use antiseptics and disinfectants indiscriminately. Resistance has developed toward most antiseptics or disinfectants for many microbes, with the mechanisms similar to those for which antimicrobial resistance has emerged. For example, a number of organisms, including Pseudomonas spp., have become resistant to quarternary ammonium compounds, Klebsiella and E. coli have expressed resistance to chlorine, and peroxides are among the compounds toward which the most resistance has emerged.91
KEY POINT 8-18
Resistance that has developed toward most antiseptics or disinfectants by many microbes occurs by way of mechanisms similar to those that result in antimicrobial drug resistance.
Occlusive or vapor-permeable dressings may reduce the risk of bacterial contamination. Note that for all levels of infection, appropriate cleansing, including débridement, is key to the success of antimicrobial therapy, whether topical or systemic.
Dog or cat bite wounds may be deep, penetrating wounds. As such, the risk for infection may be greater compared with more superficial skin wounds. However, particularly with cat bite wounds that are open and in which the risk of cellulitis is minimal, antimicrobial therapy may not be indicated (Figure 8-4). Hot-packing is an important component of therapy.
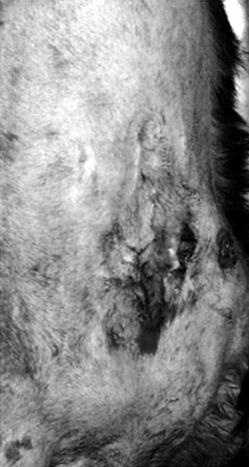
Figure 8-4 Feline abscesses are an example of an infection that may not require treatment with antimicrobials, particularly if the abscess is open and draining well. Treatment is indicated if associated with a risk of cellulitis.
The microbiology of canine bite wounds was recently reviewed prospectively.92 Wounds were clipped and cleansed with 70% alcohol. Culture samples were collected before cytologic samples, using swabs, which may limit accuracy and preclude colony counts. Swabs were inserted deep within puncture wounds or deep pockets. Both aerobic and anaerobic cultures were collected from dogs (n = 50) in which wounds were inflicted within the previous 72 hours. A total of 104 wounds were studied, with 21 assessed cytologically as infected and 83 noninfected. However, based on growth, 75% were positive. Sixty-six of the 83 noninfected (cytologically) wounds yielded positive growth, for a total of 213 isolates. Aerobic isolates were equally represented by gram-negative and gram-positive organisms (see Table 8-1). Anaerobic organisms were cultured in 17 of 50 wounds; 17 yielded anaerobic organisms. The most common anaerobic organisms (obligate) were Prevotella melaninogenica (59%) followed by Clostridium and Peptostreptococcus spp.
Cat bite wounds have also been studied (Australia).93 P. multocida historically is recognized to be a causative agent on the basis of wounds in humans. However, as in the dog, a range of aerobic and anaerobic organisms are associated with feline bite wounds. In humans the sequelae of cat bites result in infection with a variety of aerobes, including Staphylococcus spp., Streptococcus spp., including alpha-hemolytic; Moraxella spp., Enterobacteriaceae spp., Weeksella zoohelcum; and Capnocytophaga. Anaerobic organisms include a number of Fusobacterium spp., Bacteroides spp., Porphyromonas spp., Prevotella spp., Peptostreptococcus spp., and Propionobacterium spp. Bite wounds as they develop in cats include Pasteurella spp. (the most common facultative anaerobe), Bacteroides spp. (including Prevotella, Porphyromonas; 29%), Fusobacterium spp. (19%), Peptostreptococcus spp. (13%), and Actinomyces spp. Closed abscesses in cats contain an average of three isolates, although as many as eight have been identified. Isolates have been confirmed to be of oral origin. Accordingly, the role of Porphyromonas, and particularly Porphyromonas gingivalis, is increasingly recognized for its importance.
Miscellaneous Infections
Dapsone is an antimicrobial that inhibits para-aminobenzoic acid metabolism to folic acid. Selected mycobacterial strains, most notably Mycobacteria leprae or Mycobacteria lepraemurium, are exquisitively sensitive to its effects. In addition, the drug appears to be capable of some form of immunomodulation and may prove beneficial in deep skin infections associated with a pyogranulomatous response for which no organism has been identified. Because of difficulty associated with identifying infectious organisms such as Nocardia spp. and atypical mycobacterial organisms, however, use of dapsone should be reserved until a susceptible organism has been identified or other causes of inflammatory skin disease have been ruled out. The drug has not been studied in animals, and in humans, it is characterized by marked differences in disposition. Hemolytic episodes have been reported in humans; however, generally these individuals are suffering from metabolic defects of red blood cells. Dapsone also is used to treat bites caused by brown recluse spiders in humans. When Dapsone is administered within 48 hours of the bite, tissue necrosis is markedly decreased and wound healing is subsequently faster.
Infections of the Musculoskeletal System
Osteomyelitis
Because bacterial myositis is rare unless it accompanies infection of the surrounding soft tissues, this discussion is limited to bacterial infections affecting the skeletal structures.
Pathophysiology
Similar to models described in humans,94 infections of the bone occur by a hematogenous route, secondary to a contiguous focus or direct inoculation through surgery or trauma.95 The metaphyses of long bones are the most common sites of hematogenous infection.94 Blood flow becomes slow and turbulent in this region, and capillaries lack phagocytic cells. Acute infection causes local cellulitis resulting in leukocyte accumulation, increased bone pressure, decreased pH, and reduced oxygen tension. Contiguous infections generally result from direct inoculation of the bone because of trauma, extension from surrounding soft tissues, or contamination associated with surgery. Vertebral osteomyelitis most commonly reflects a hematogenous source of infection, usually from an artery. Because each artery supplies two adjacent vertebrae, the infection usually involves two vertebrae. Secondary osteomyelitis is further classified on the basis of the presence or absence of vascular insufficiency.
Both hematogenous and secondary osteomyelitis can be classified as acute or chronic. Acute osteomyelitis is a suppurative infection accompanied by edema, vascular congestion, and small vessel thrombosis. Vascular supply to the site of infection becomes compromised as the infection extends into the soft tissue. Both medullary and periosteal blood supplies can become compromised, resulting in necrotic, ischemic, and ultimately dead bone forming a sequestrum. Bacteria located in this tissue become isolated, with acute osteomyelitis progressing to chronic osteomyelitis. Chronic osteomyelitis is characterized by local bone loss, a nidus of infected dead bone or scar tissue, an area of ischemic soft tissue, persistent drainage, and sinus tracts. The clinical course can be refractory. The risk of infection continues even in apparently cured osteomyelitis because of this nidus of infection. Indeed, the term arrested is often used instead of the term cured when referring to successful therapy in human patients. The presence of foreign bodies facilitates sustained infection through formation of biofilm (see Chapter 6), promotion of virulence factors (e.g., adherence receptors), and their negative impact on local immune function.95 Successful treatment of osteomyelitis is less likely in the presence of necrotic surrounding soft tissue, foreign bodies, bone instability, nonunion, or septic joints. Consequently, surgical removal of the nidus of infection and foreign bodies often must accompany antimicrobial therapy.
KEY POINT 8-19
Successful treatment of osteomyelitis is less likely in the presence of necrotic soft tissue, foreign bodies, bone instability, nonunion, or septic joints.
In addition to the classes of osteomyelitis based on route of infection, a more clinically relevant classification system (Ciemy–Mader) developed in humans94 yields 12 distinct stages based on patient status, anatomic considerations, cause of infection, treatment factors, and prognosis. The advantages of this approach is that stages delineate factors that complicate therapy. Four stages are described on the basis of anatomic considerations, with each stage characterized by additional complications.
Stage 1 (medullary) occurs early; the primary site of infection is endosteal or may include infected intramedullary pins. Therapy may simply include antimicrobials but may also include surgical débridement or removal of the surgical foreign body. Stage 2 (superficial) osteomyelitis occurs as a result of extension of infection from soft tissue. As such, a secondary contiguous focus exists; this focus requires surgical débridement in conjunction with antimicrobial therapy. Stage 3 (localized) osteomyelitis is characterized by full-thickness infection of the cortices with sequestration that requires surgical removal. Stage 4 (diffuse) osteomyelitis involves the full diameter of the infected bone and may cross a joint. Bone instability occurs as a result of either infection or surgical treatment. Therapy includes débridement, management of dead space, and stabilization. Each of these four stages can be further categorized on the basis of the status of the host: A (normal), B (local or systemic compromise), or C (treatment of the infection is more life threatening than the osteomyelitis). Local compromise includes chronic lymphedema, venous stasis, compromise of major vessels, arteritis, and extensive scarring fibrosis. Systemic compromise includes malnutrition, evidence of metabolic disease (e.g., renal or liver disease, diabetes mellitus), malignancy, age extremes, or immunosuppression. These stages are dynamic, being affected by therapy, progression of disease, and host status.
Microbial Targets
Hematogenous osteomyelitis is usually caused by a single pathogenic organism, with Staphylococcus spp. (50% to 70%) predominating and S. intermedius most common. Vertebral osteomyleitis also is most commonly caused by Staphylococcus spp. Common sites of infection in humans that lead to vertebral osteomyelitis are the genitourinary tract, skin and soft tissue, respiratory tract, mouth, endocardium, and intravenous lines.94 Multiple pathogenic organisms are usually involved with contiguous infections, and as in hematogenous infections, Staphylococcus spp. is one of the most common organisms isolated. Gram-negative and anaerobic organisms are also commonly involved.94 The most common gram-negative are E. coli, Klebsiella, Pasteurella, Serratia, and Proteus spp. The most common anaerobes are Bacteroides, Fusobacterium, (including F. necrophorum), Clostridium spp., and others. The risks of therapeutic failure associated with osteomyelitis outweigh the risks associated with culture and susceptibility testing; accordingly, drug selection and the design of the dosing regimen should be culture based. Care must be taken with sample collection to ensure that proper techniques are followed.
Antimicrobial Therapy
Clinical trials have provided only limited direction in the successful treatment of osteomyelitis, being handicapped by variations in duration (acute versus chronic), organisms studied, the model of osteomyelitis used, mode of infection (in nonspontaneous models), presence of foreign material, previous surgical procedures, and previous antimicrobial therapy. Among the critical host factors of concern when treating osteomyelitis is drug distribution to the site. Distribution may be limited because of changes in blood flow, presence of inflammation, and poor local immunity. Note that some drugs distribute to the bone only to bind to calcium or other cations (e.g., tetracyclines, potentially fluoroquinolones) (Table 8-8). As with other sites of infection, drug penetration in osteomyelitis is determined by the molecular weight and the lipid solubility of the drug. Protein-bound drugs do not distribute to the site of infection. The mechanisms of capillary transport in osteomyelitic bone are similar to those in normal bone, although permeability ratios to the site of the drugs differ. A blood-bone barrier does not appear to exist. However, the impact of the intramedullary infection on blood distribution may limit drug delivery. The impact of biofilm on successful therapy also may be profound, particularly in the presence of foreign bodies.
Table 8-8 Concentrations of Selected Antimicrobials in Bone Experimentally Infected with Staphylococcus aureus
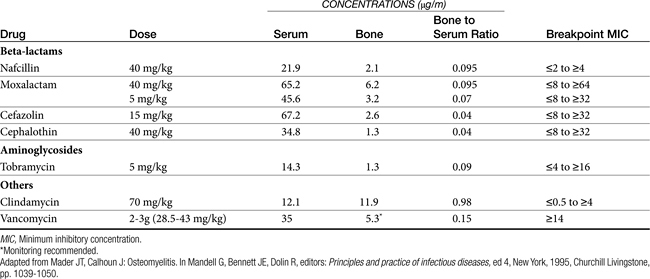
Determining the extent of drug distribution to potential sites of infection in bone is difficult. Distribution studies often are based on cannulation of nutrient artery and the ipsilateral femoral vein. Detection of drug in bone is limited by recovery of the drug, which tends to be low because tissue is lost during the extraction procedure. Volume of distribution studies generally have shown that concentrations of biologically active drug in the interstitial fluid space of normal cortical bone are equivalent to that in the serum, regardless of the lipophilicity of the drug. The characteristics of cefazolin have resulted in its use for surgical prophylaxis in patients undergoing orthopedic surgical procedures.96 Cefazolin readily traverses capillaries of both normal and osteomyelitic bone, and the pathophysiology of osteomyelitis enhances penetration. Although the volume of spaces (plasma and interstitial) increases in osteomyelitis by 330% and 941%, respectively, the distribution of cefazolin increases proportionately. Cefazolin is not as highly protein bound in dogs as it is in humans (35% versus 80%); accordingly, effective concentrations of pharmacologically active drug should be expected in normal bone tissues of dogs.96 However, other drugs appear to penetrate infected bone better than cefazolin. The prophylactic use of antimicrobials increasingly is not considered necessary (see Chapter 6). Although the long half-life of cefovecin might support its indication for treatment of osteomylitis caused by susceptible bacteria, it is not indicated for prophylaxis. Not only will maximum concentrations take up to 8 to 24 hours to achieve (with efficacy depending on the MIC90 of the target microbe), but therapy cannot be discontinued after surgery (the elimination half-life causes drug to persist for 1 to 2 weeks), as is indicated for prophylaxis.
In experimental rabbit models of osteomyelitis caused by S. aureus, drugs characterized by the best bone-to-serum ratio were, in order, clindamycin, vancomycin, moxalactam (a beta-lactam antibiotic), tobramycin, cefazolin, and cephalothin (see Table 8-8).94
Because of the potential difficulty in achieving high concentrations at the osteomyelitic site, a number of methods of local drug delivery might be considered, particularly for infections associated with multidrug-resistant organisms. Advantages include high local concentrations for a longer period of time and avoidance of systemic exposure to antimicrobials that might otherwise be toxic. Among those methods most considered are antimicrobial-impregnated beads. Two forms of beads have been used. Beads made of polymethyl methacrylate (PMMA) are nonbiodegradable; their use in horses has been reviewed, and much of the information is applicable to dogs and cats.97 A number of commercially available PMMA cements are available; additionally, compounding pharmacies are beginning to offer antimicrobial-impregnated beads. Mixing drug in PMMA results in an exothermic reaction, causing tissue damage and the potential release of toxins; bone-healing and phagocytic function may be impaired. Antimicrobials are added in powder form but must be heat stable. More recently, calcium-based (e.g., sulfate, plaster of paris) antimicrobial-impregnated beads have been studied. In contrast to the PMMA, these beads are biodegradable and their formation is not exothermic. However, beads should be sterilized using non–heat-based methods. The advantages of calcium sulfate includes osteoconductivity, potentially facilitating bone healing; the biodegradable nature often precludes the need for retrieval surgery. Even more recently, biodegradable matrices are being studied.
KEY POINT 8-20
Local drug delivery may be a viable adjuvant for treatment of problematic osteomyelitis, particularly if infection involves a multidrug-resistant organism.
Regardless of the matrix type, antimicrobial release or elution tends to be bimodal, with an initial rapid release (12 to 48 hours) followed by a slower release over weeks or months. For PMMA, elution rates (based on in vitro studies) vary with antimicrobial concentration, pore and bead size, and permeability of the cement (which, in turn, can be affected by the amount and type of antimicrobial). The form (e.g., liquid versus powder form) of the antimicrobial will also influence elution. Host factors influencing elution rates include surface area available for bead exposure, blood flow, and fluid content. In general, concentrations in the first stage of elution surrounding the area can be anticipated to exceed the MIC90 of most susceptible organisms by more than a hundredfold, increasing the likelihood of a quick kill. Concentrations during the second phase of elution will be much lower but may still surpass the MIC90 of infecting microbes by several to a hundredfold. Sayegh97 indicates that encapsulation of the beads during healing results in therapeutic concentrations being achieved up to 2 to 3 mm surrounding the beads.
Atilla and others98 have demonstrated that the combination of drugs in beads alters their elution rate, causing it to be more rapid. Atlhough concentrations are initially higher, the duration of effective concentration may be shorter. The authors also demonstrated that, interestingly, the elution rate may change even if drugs are not mixed in the same bead but are in different beads. Antimicrobial-impregnated beads can be used to treat bone, synovial structures, and other soft tissues; use of PMMA in joints is discouraged, however, because it tends to be irritating. Indications include refractory infections, particularly osteomyelitis, and prophylaxis after surgery of contaminated wounds.
A disadvantage of beads, particulary PMMA-based beads, is the foreign nature. Further, the PMMA can inhibit phagocytic function. Heat-damaged tissue may facilitate infection. Host response to the cement may also facilitate infection by S. aureus, and microorganisms can secrete materials that protect against host defense mechanisms. As such, they may be associated with risk of infection, as has been described in a series of human patients (n = 20). Cultures of gentamicin-loaded beads removed 2 weeks after implantation from prosthesis-related infections revealed 90% to be infected. Of the 28 isolates, nearly 70% were gentamicin-resistant.99 Interestingly, 12 of the 18 infected patients were considered infection free before removal of the infected beads. Most common isolates were P. aeruginosa and S. aureus. In contrast, the prosthetic devices in the area of the beads tended to not be infected.
The use of tobramycin antimicrobial-impregnated calcium sulfate beads in a series of dogs (n = 6) was recently reported.100 The beads are commercially available, approved for use as bone filler. Sites included forelimb and hindlimbs. Infecting organisms included S. intermedius, S. aureus, and P. aeruginosa. The number of beads implanted ranged from four to nine; one dog that received a total hip replacement received 24 beads. Beads generally lost their radio-opacity by 4 weeks and were no longer visible radiographically by 6 to 8 weeks. Clinical signs associated with infection resolved in all but one dog.
A case report described the successful use of a commercially available gentamicin-impregnated collagen sponge (CollaRx) in the treatment of septic arthtritis associated with MRSA in a dog.12 Other matrices are under investigation, with a focus on those that slow release or are biodegradable. However, a commonality of most of the studies addressing drug elution from foreign matrices is of questionable applicability to the patient. This includes studies that expose representative pathogens (e.g., Staphylococcus spp.) to eluent containing the antimicrobial. The accuracy of concenterations measured in vitro from eluent representing matrices incubated in buffer as predictors of interstial concentrations in a surgical patient receiving these drug delivery devices is debatable. As such, studies are needed based on concentrations measured in vivo using ultrafiltration probes that collect interstitial fluid.
Medical management of chronic osteomyelitis in humans has not been well supported by well-designed controlled clinical trials; the knowledge base in veterinary medicine is even more limited. Variability in risk factors, pathophysiology of infection, and etiologic agents and failure to identify the organism preclude the generation of consensus regarding recommendations in humans. Further, outcome measures of therapeutic success are not always identified or clear. A large trial using expensive therapies for extended periods of time is prohibitively expensive and is complicated by the ability of pathogens to cause low-grade symptoms or lie dormant for many years, requiring long-term follow-up.95 It is not surprising, therefore, that recommendations have been based on historical observations, experimental models, and nonrandomized trials.95 Retrospective studies have been helpful but generally are more representative of complicated cases.101
Although drug distribution to healthy bone is adequate, the detrimental effects of osteomyelitis minimize drug distribution to the site of infection. As such, culture-based drug selection and dosing regimen design is likely to be paramount to therapeutic success. Parenteral antimicrobial therapy should begin with drugs targeting the most likely organisms; drug therapy can be changed if indicated on the basis of susceptibility data. Duration of therapy for osteomyelitis is generally recommended for 4 to 6 weeks, in part because tissue revascularization after débridement requires at least 3 to 4 weeks.95 However, as with other therapies, the evidence for duration of therapy is limited by the lack of clinical trials. The need for longer term therapy might be anticipated for chronic osteomyelitis.
The first 2 weeks of therapy should be by parenteral administration (data from the start of therapy or after surgical débridement). For acute hematogenous osteomyelitis, the causative agent must be properly identified. Mismanagement with inappropriate antimicrobial can lead to extension of the infection, necrosis, and the formation of sequestra, as well as emergence of resistance. If the patient has not responded to specific antimicrobial therapy (i.e., based on culture) within 48 hours, surgical intervention is indicated for human patients. Bone biopsy specimens are necessary for culture for human patients94 unless the patient also has positive blood cultures. Because of its excellent distribution characteristics, oral quinolone therapy may be an acceptable alternative for gram-negative and Staphylococcus spp. infections. Note, however, that the activity of the second-generation fluoroquinolones against anaerobes and other gram-positive organisms (e.g., Streptococcus spp., some Corynebacterium spp.) is less predictable, whereas third-generation fluoroquinolones have a broader spectrum. In addition, although bone concentrations of difloxacin are high (see package insert), concentrations do not decline over time, suggesting that the drug is bound and thus inactive. Whether this is true for other fluoroquinolones is not clear. Clindamycin, metronidazole, or amoxicillin–clavulanic acid can be used in combination with the quinolones to provide gram-positive and anaerobic coverage. Intravenous therapy generally is recommended, in part because antimicrobials most commonly associated with clinical success (e.g., cefazolin and vancomycin) are characterized by poor oral bioavailability. However, because of the potential risks and inconvenience, alternative therapies, such as oral antimicrobial therapy, have been considered. The potential efficacy of oral therapy is more reasonable with the availability of several drugs that are safe and highly bioavailable after oral administration. Several oral antimicrobial agents have undergone evaluation for the treatment of acute and chronic osteomyelitis in human medicine. Oral antimicrobials are considered for treatment of acute osteomylitis in children and chronic infection caused by atypical gram-positive and selected gram-negative organisms. Choices include the fluoroquinolones, clindamycin, and linezolid, the latter being reserved for MRS or potentially enterococci.95 Retained foreign material or prosthetic devices complicate oral management of osteomyelitis, and therapy is more likely to fail with oral rather than intravenous therapy.95 Despite these drawbacks, a number of studies have supported oral therapy for chronic osteomyelitis. In general, oral therapy with ciprofloxacin as sole agent compares favorably to parenteral therapy with single or combination antimicrobials. However, resistance in gram-positive organisms appears to develop rapidly. Rifampin used in combination with other antimicrobials appears to be effective for treatment of staphylococcal orthopedic implant-related infections. Thus whereas intravenous antimicrobials for 2 weeks followed by long-term oral (ciprofloxacin) therapy appears to be effective, longer-term therapy (3 to 6 months or more) with a combination of ciprofloxacin and rifampin was associated with a 100% cure rate compared with a 58% cure rate for ciprofloxacin only.95 A meta-analysis study of 22 trials involving 927 human patients with orthopedic devices associated with S. aureus–induced osteomyelitis offers some insight into antimicrobial therapy. Disconcertingly, most of the studies were characterized as poor in quality, impaired by inappropriate methods and statistical analysis. However, a trend of improvement was detected for the combination of rifampicin–ciprofloxacin compared with ciprofloxacin alone. Use of ticarcillin for bone infections associated with Pseudomonas spp. was described as favorable. Therapeutic success with oral fluoroquinolones did not differ from success with intravenous beta-lactams. Comparison of locally placed PMMA gentamicin bead chains compared with parenteral antimicrobials could not be evaluated well because all patients received systemic therapy.102
Chronic contiguous osteomyelitis may be particularly difficult to resolve. Assessment of vascular integrity by cutaneous oxygen tension can be useful for determining the extent of damaged tissue to be removed.94 Hyperbaric oxygen therapy may facilitate healing in tissues characterized by low oxygen tension. Dead space can be managed short term with antimicrobial-impregnated acrylic beads; the beads are generally replaced with a cancellous bone graft after 2 to 4 weeks. Hyperbaric oxygen is an important adjunct therapy for human patients with osteomyelitis. It serves to restore intramedullary oxygen tension and thus facilitates phagocytic killing. Furthermore, it supports collagen production, angiogenesis, and wound healing.94
Infections associated with prosthetic devices involving cement are particularly problematic to resolve. Infections generally occur at the bone–cement interface. Infectious pathogens are numerous and, in humans, often include microorganisms considered to be contaminants of cultures (e.g., corynebacteria, propionibacteria, and Bacillus spp.). PMMA cement may predispose the site to infection, as previously discussed. Infection can be treated with removal of the prosthetic device and 6 weeks of antimicrobial therapy followed by reimplantation or surgical removal and débridement with immediate reimplantation accompanied by polymethyl methacrylate cement inpregnated with an antimicrobial (an aminoglycoside). When the prosthesis cannot be removed, lifelong antimicrobial therapy has been implemented for human patients, assuming that the microorganism is sufficiently susceptible to oral antimicrobial therapy and the patient can tolerate the therapy.
Septic Arthritis
Pathophysiology
Septic arthritis most commonly reflects hematogenous inoculation. Patients with osteoarthritis or immune-mediated arthritis, trauma, or intraarticular inflammation are predisposed to infections in the inflamed joint. Trauma may predispose to infection because of a loss of vascular integrity. Synovial tissue is very vascular, and the lack of a basement membrane facilitates bacterial penetration. Bacteria can contribute to the process of inflammation through production of tissue-damaging mediators such as proteases. S. aureus is among the organisms most commonly causing infection in humans, in part because of its ability to bind to bone sialoprotein, a glycoprotein of joints.103 It is able to contribute to destruction of cartilage because of the production of chondrocyte proteases. Infection often affects a single joint and usually is limited to the joint. Exceptions are made in the presence of predisposing factors that affect multiple joints (e.g., immune-mediated arthritis). Drug distribution into inflamed synovium occurs more rapidly and results in higher concentrations than in uninflamed joints.
Clinical signs associated with septic arthritis include fever, limited joint motion, and swelling of peripheral joints associated with joint tenderness. Synovial fluid analysis discriminates between septic and nonseptic causes of arthritis. In general, septic arthritis more commonly is associated with increased polymorphonuclear leukocytes. The presence of immunomodulating drugs (e.g., glucocorticoids) may blunt leukocyte infiltration. In human patients the presence of bacteria can be detected in approximately 33% of cases by cytologic examination of a smear of synovial fluid. Cytologic examination should include Gram stains. Synovial fluid should be cultured; for human patients blood culturing also is recommended.103 False-negative cultures may not, however, rule out bacterial arthritis; in human patients with documented infection, synovial fluid cultures were negative 10% of the time. Radiographic examination may help rule in arthritis.
Microbial Targets
The most common causes of septic arthritis reported in animals are bacterial in origin. The potential for viral causes should not be ignored. In human patients two syndromes of arthritis are described: acute and chronic, nonarticular. The syndromes are caused by different types of organisms, with mycobacterial or fungal infections of the joint largely responsible for the chronic form. According to surgical texts, organisms most commonly associated with septic joints include Staphylococcus spp., Streptococcus spp., E. coli, and Pasteurella spp.
Antimicrobial Therapy
In human patients septic arthritis is treated aggressively. Empirical therapy is begun after culture collection using an intravenously administered antimicrobial effective against S. aureus. Examples include cefuroxime, cefotaxime, and ceftriaxone; the role of extended-spectrum beta-lactamases and therapeutic success has not been addressed, and the prudent clinician might consider combining therapy with a drug that is not vulnerable to these destructive enzymes. The use of fluoroquinolones for treatment of infections associated with joints or ligaments should be done cautiously. Their impact on healing cartilage may be similar to that of cartilage in growing animals, and evidence has emerged that demonstrates their negative impact on ligaments. Use of chondroprotective disease-modifying agents (including both glucosamine and chondroitin sulfates) should be considered in any patient with joint disease receiving any fluorinated quinolone. Note that fluoroquinolones have been associated with impaired healing in tendons103a; the impact on other skeletal tissues is not clear. The use of an antimicrobial-impregnated collagen sponge was addressed previously.
KEY POINT 8-21
The use of a fluoroquinolone for treatment of infections associated with joints or ligaments requires caution.
Duration of therapy for treatment of septic arthritis in humans generally is at least 3 weeks, although these recommendations are based on older retrospective studies.101 Shorter treatment periods are being promoted, particularly in children. Similarly, a recent trend is oral treatment after a brief period of intravenous therapy. Response to antimicrobial therapy is based, in humans, on repetitive analysis of synovial fluid collected by joint aspiration. Persistence of infusion beyond 7 days is interpreted as the need for surgical drainage. Use of appropriate antimicrobials early in the course of arthritis will minimize damage to the joint.103
Adjuvant Therapy
Because septic arthritis can be accompanied by the loss of collagen and erosion of articular surfaces, therapy with disease-modifying agents should strongly be considered. Injectable products such as polysulfated glycosaminoglycans (Adequan), pentosan polysulfate, and hyaluronic acid (the latter perhaps in combination with either of the former) are more apt to act more rapidly than oral products. Oral disease-modifying agents such as chondroitin sulfates, keratan sulfates, and glucosamines (e.g., Cosequin) also should be strongly considered until the joint is healed.