10 The Common Integument
The term common integument comprehends ordinary skin with its covering of hair and variety of skin glands as well as more specialized parts such as claws, hoofs, and horns. The skin completely encloses the body and blends with the mucous membranes at the various natural openings. In its common form it protects against surface wear and tear and invasion by microorganisms, plays an important part in thermoregulation (p. 357), and, being practically impermeable to water, prevents the body from drying out (with the accompanying loss of electrolytes and other vital substances); conversely, it prevents excessive water uptake in aquatic mammals. Certain lipid substances can penetrate skin and are used (in the form of ointments) as vehicles for administration of medication.
The color of skin (and hair) depends partly on the presence of pigment granules in certain component cells. These protect against ultraviolet radiation and are related to the ability to reflect solar heat, which may raise body temperature; their effects partly explain why skin and coat color affects the adaptability of animals to life in sunny climates. The color of naked and nonpigmented areas is also affected in various ways by the blood in the vessels that perfuse its deeper layers; blushing in humans provides the most obvious example of such effects, but the pallor of anemia or shock, the blue tint (cyanosis) that indicates oxygen lack, and the yellow (icterus) of jaundice are of greater veterinary relevance. Very spectacular color changes, such as that for which the chameleon is famous, do not occur in mammals, although mention may be made of the garish coloration of the skin of the mask and perineum of male mandrills and related monkeys.
THE STRUCTURE OF SKIN
Some recapitulation and amplification of the earlier account (p. 8) of basic skin structure is now required. It will be recalled that skin is composed of two parts: a superficial epithelium (epidermis) and a tough fibrous layer (dermis) that rests on a stratum of loose connective tissue (subcutis) (see Figure 1–7).
The epidermis is continuously renewed. The surface cells are sloughed in flakes (e.g., dandruff) or as smaller particles (those of human skin accounting for much household dust), and this loss is made good by cell division in the deepest layer followed by migration of daughter cells toward the surface. As the epidermal cells drift superficially, they undergo a series of internal changes that gradually brings about their deaths, and when presented to the environment, they are incapable of reacting to the various influences to which they are then exposed. The sequence of changes, shown in Figure 10–1, imposes an obvious stratification. The deepest layer (stratum basale) is closely molded on the irregularities of the underlying dermis and has a considerably greater area than the surface of the body (Figure 10–1/1). As the cells move into the stratum spinosum, they shrink and draw apart, though remaining connected by intercellular bridges (desmosomes). The process of keratinization (cornification) now begins, and in the next layer (stratum granulosum) the cells contain scattered keratohyalin granules (Figure 10–1/4). In some regions this layer is followed by a narrow stratum lucidum in which the flattened cells, which have already lost their nuclei and distinct outlines, obtain a homogeneous appearance from the even dispersal of the granules. Finally, the outermost layer (stratum corneum; Figure 10–1/6) consists of squames densely packed with the fibrous protein keratin, the true horny substance, into which keratohyalin has been transformed. It is keratin that gives epidermal specializations (e.g., hair, hoof, and horn) their hardness and their strength.

Figure 10–1 Structure of the adult skin (Crossmon). A, Skin from the canine flank. B, Skin from a worn feline footpad. Note the increased keratinization and the presence of a stratum lucidum and dermal papillae. 1, Dermis; 1′, dermal papilla; 2, stratum basale; 3, stratum spinosum; 4, stratum granulosum; 5, stratum lucidum; 6, stratum corneum.
The epidermal layers are thickest and most clearly differentiated where the skin is exposed to hard usage, as on the footpads of a dog (Figure 10–2). Where abrasion is less severe, as in haired regions, the epidermis is much thinner, and neither the stratum granulosum nor the stratum lucidum may be clearly represented. The thickness of the epidermis depends on the mitotic rate within the stratum basale, which is adjusted by a substance (epidermal chalone) that inhibits cell division. Although cell production and loss normally match to maintain an even epidermal thickness, this balance may be disturbed in certain circumstances.
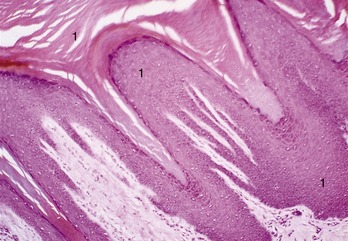
Figure 10–2 Stratified squamous epithelium of a footpad of a dog (HE) (70×). 1, Very thick stratum corneum.
There are no blood or lymphatic vessels in the epidermis, which is nourished by diffusion from the subjacent dermis.
The dermis is largely composed of collagen bundles, thickly felted together, as can be demonstrated by teasing leather (tanned dermis). Elastic fibers, which are also present, make the skin pliable and are able to restore its shape after being wrinkled or deformed. It is these fibers that draw apart the edges of a wound, making it gape (Figure 10–3). Chronic tension damages the structure of the dermis, rupturing the connective tissue bundles; subsequent repair is usually by lighter scar tissue. A physiological example of this process is provided by the white lines (striae) of abdominal skin that appear after the completion of a pregnancy, especially in women.
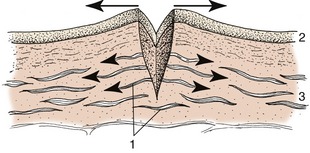
Figure 10–3 Skin incision; elastic fibers in the dermis cause the wound to gape. 1, Elastic fibers; 2, epidermis; 3, dermis.
The dermis is generously vascularized and innervated. It is also invaded by hair follicles and sweat, sebaceous, and other glands growing from the epidermis (see Figure 1–7)
The interface across which nutrients and waste substances diffuse between the epidermis and the dermis is enlarged by the complicated molding of these components. The finger- and ridgelike projections (papillae; Figure 10–1/1′) of the dermis fit closely into reciprocal depressions of the epidermis, and under normal conditions adhesion between the two structures is not easily disturbed. Trauma, such as that caused by the rubbing of an ill-fitting boot or shoe, sometimes separates them forcibly, and interstitial fluid then collects in a blister. Rupture of the blister exposes the raw surface of the dermis; normally this is quickly covered by epithelium growing inward from the margin of the sore.
The larger dermal ridges and papillae, generally developed where the covering epithelium is thickest, are reflected by corresponding epidermal contours. These are permanent and individually distinct and provide a means of identification, widely used in ourselves (fingerprinting) and less commonly used in other species (noseprinting of dogs and cattle; Figure 10–4).
The subcutis consists of loose connective tissue interspersed with fat. It varies in amount according to situation and is thin or even absent where movement is undesirable (e.g., over the lips, eyelids, and teats). It is particularly ample in dogs and cats, whose easily shifted skin can be grasped in large folds over much of the body (Figure 10–5). In the pig and ourselves, the subcutis contains more substantial accumulations of fat, even in relatively ill-nourished individuals; this constitutes the panniculus adiposus familiar in sliced bacon.
The clinical significance of the effects of dehydration or edema of the subcutis has been mentioned (p. 9).
The cutaneous blood vessels come from those that supply the fasciae and superficial muscles. The arteries form a series of networks within the dermis. The most superficial network lies at the bases of the papillae and provides end-arteries that enter the papillae to release numerous capillaries from which fluid passes to nourish the basal epidermal cells. Other capillary plexuses surround the hair follicles and associated glands (see Figure 1–7). Variation in flow through the superficial vessels plays an important role in temperature regulation. When the body temperature is raised, vasodilation promotes heat loss—directly by surface radiation and indirectly by favoring the activity of the glands that produce sweat, which then evaporates. Conversely, the surface vessels constrict in cold environments or when the internal temperature drops. The regulation of flow is in part achieved by opening or closing numerous anastomoses connecting the cutaneous arteries with veins. The skin vessels normally contain a considerable volume of blood, but much can be recalled to the musculature and internal organs after hemorrhage or shock.
Skin has a rich sensory innervation. The nerves accompany the vessels through the fasciae and form networks within the dermis. From these, fibers disperse to a variety of sensory receptors; some even penetrate a little way into the epidermis (see Figure 9–33). Other (autonomic) fibers regulate the caliber of the smaller vessels, control the activity of skin glands, and excite the arrector pili muscles that attach to the hair follicles.
The epidermis develops from the embryonic ectoderm. This is initially a single layer of cells lying on a bed of mesenchyme that in time gives rise to the dermis (Figure 10–6, A). Long before birth the ectodermal cells begin to proliferate, pushing new cells toward the surface to produce a multilayered epithelium, while local condensations grow into the mesenchyme as the epithelial buds from which hair and glands differentiate. By the time of birth the skin of domestic mammals has a basically adult character, unlike that of many rodents and other small mammals that are born naked.

Figure 10–6 Development of skin, schematic. A, Skin of an early embryo. B, Differentiation of epidermis and dermis. C, Further differentiation of the epidermis. D, Complete differentiation of the epidermis and dermis. 1, Ectoderm; 2, mesoderm (mesenchyme); 3, primitive stratum basale; 4, dermis; 5, stratum basale; 6, stratum spinosum; 7, stratum granulosum; 8, stratum corneum.
HAIR
Hair is a mammalian feature, diagnostic of the class. In most species a thick haircoat is spread over the body, except about the mouth and other openings and on the surfaces of the feet; in a few, including the domestic pig (though not its ancestors), the covering is sparse (Figure 10–10, E). The individual hairs take a variety of intergrading forms, but only three need be distinguished here: straight, rather stiff guard hairs provide a “topcoat”; fine, wavy wool hairs provide an “undercoat”; and stout tactile hairs of restricted distribution are associated with touch receptors.
Guard hairs mostly lie close against the skin and sweep uniformly in broad tracts, giving the coat a smooth appearance disturbed only by the whorls, crests, and partings formed where different streams converge and combine or diverge from one another. The regularity of the arrangement is significant because it promotes the runoff of rain, preventing the chilling that would occur if water were allowed to penetrate the pile to reach the skin. Occasionally, animals are born with a disturbed coat pattern, which may seriously impair their ability to withstand severe weather. However, as with so many other features, breeders have chosen to promote deviant mutant arrangements as attributes of particular breeds, particularly of dogs, cats, and rabbits.
Each hair grows from a tiny pit or follicle from which it protrudes above the surface of the skin. The follicle develops from an ectodermal bud that grows into the underlying mesenchyme in the embryonic stage of life. In addition to forming the hair, the bud branches give rise to skin glands (Figure 10–7). The distal end of the bud forms a bulbous enlargement, which is then indented by a mesenchymal (dermal) papilla to form a primitive hair follicle. The epithelial cells lying against the papilla multiply, forming a hair matrix; the cells produced here keratinize and combine to form a primitive hair that grows through the center of the bud until it rises above the epidermis on the surface of the skin. Its passage takes it past the sebaceous glands that develop to the side of the follicle, and this arrangement allows the hair to obtain the oily coating so important for its health. While the ectoderm differentiates in this way, the mesoderm also condenses so that the tiny sheath around the embedded part of the hair acquires an outer mesodermal component.
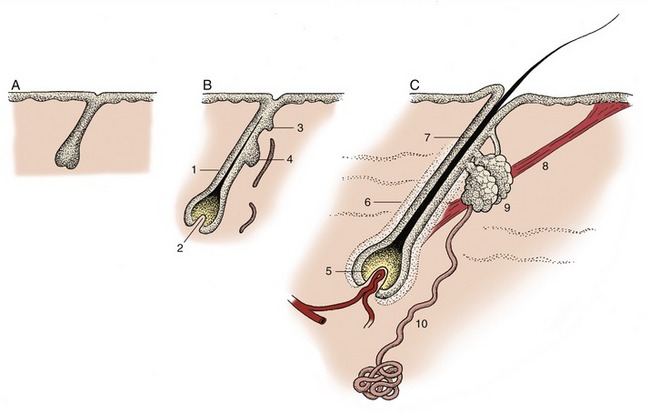
Figure 10–7 Development of hair and associated sebaceous and sweat glands, schematic. A, Ectodermal bud growing into mesenchyme. B, Differentiation of the bud; indications of glands appear. C, Hair follicle with accessory structures. 1, Primitive hair follicle; 2, dermal papilla; 3, bud of sweat gland; 4, bud of sebaceous gland; 5, bulb (hair matrix) of hair; 6, hair follicle; 7, root of hair; 8, arrector pili muscle; 9, sebaceous gland; 10, sweat gland. In the adult, many glands open independently, not into hair follicles.
Figure 10–8 shows the essential features; other texts must be consulted for the histological details. It must suffice here to say that, in essence, a hair consists of a flexible column of closely consolidated and heavily keratinized, and hence dead, epithelial cells. Their arrangement permits the distinction of a medulla or core, a cortex, and an outer “scaly” cuticle. The proportions of the parts and the details of their arrangement vary and permit the microscopic determination of the origin of a hair sample. In general, hairs with a thick medulla are straight and rather brittle, whereas those in which the cortex predominates are stronger and more pliable.
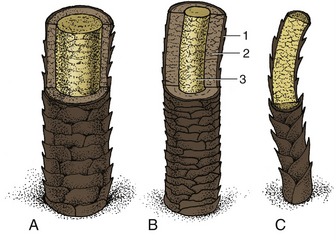
Figure 10–8 Schematic representation of three kinds of hair. A, Guard hair with thick medulla. B, Guard hair with thick cortex and thin medulla. C, Wool hair; the cortex is absent. 1, Cuticle; 2, cortex; 3, medulla.
The proximal end of the follicle is joined by a tiny arrector pili muscle passing from an attachment near the dermal papillae (Figure 10–7/8). Contraction of this muscle is involuntary and may be stimulated by a low ambient temperature. It results in erection of the hair from its normally oblique posture; when this happens to hairs en masse, the thickened pile traps more air and so improves the insulation of the body. Although functionally unimportant in the human species, the effect is very obvious in our relatively naked skin when little mounds (goose pimples) appear over the courses of the arrector muscles. A similar effect occurs in the fight-or-flight reaction mediated by the sympathetic nervous system; the pronounced response by the hairs of the neck and back raises the hackles that give an animal a threatening appearance.
There are many local variations in the form and development of guard hairs. Familiar examples are the stiff, sparsely scattered bristles of pigs (Figure 10–10, E), the coarse hair of the mane and tail of horses, the long tail hairs of cattle, the fetlock tufts of horses, and the feathering of the tail and limbs of certain breeds of dogs. Local variations that are hormone dependent are particularly evident in the human species; they include the male beard and the sexually dimorphic distribution of body hair. Baldness as an accompaniment of advancing age is especially a problem of the human male. Its causation is complicated and in part obscure. Testosterone, which is responsible for the growth of the beard and coarse body hair, paradoxically seems to trigger early baldness in genetically predisposed individuals; a reduced blood level of thyroxine, which initiates and controls hair growth, also plays some part.
Hairs have restricted lives and are discarded sooner or later. In humans this shedding is a continuous process that involves only a few hairs at a time; in most other species it is intermittent, related to the season, and affects many hairs together (though never so many that the animal is denuded). Seasonal shedding is most pronounced in wild species, but even domesticated animals protected from the more extreme climatic changes show a recurrent pattern with peaks in the spring and fall. Shedding is obviously most noticeable in animals that are not regularly groomed to remove dead hair. Information on these matters is not abundant, and most accounts rely heavily on casual observation. This is particularly so where companion animals are concerned, and veterinarians are frequently embarrassed by too-penetrating inquiry from owners. Although there seems to be much variation, most dogs molt most heavily in the spring and fall; the spring shedding is more pronounced and lasts about 5 weeks. Cats also molt most heavily in spring, but this is followed by a less substantial loss that continues through the summer and fall; it is not until winter that shedding ceases and the coat attains its prime condition. For the same reason, the pelts of furbearing species are harvested in winter, although the number of harvested pelts has been reduced as the trade in furs is regarded with increasing disfavor.
The seasonal replacement begins with a slowing of the growth of existing hair; although this appears to be mainly conditioned by a rise in temperature, other factors, including nutrition and day length, play their parts. As growth slows (in the so-called catagen phase) the hair matrix and covering papilla both atrophy (Figure 10–9, B). No growth occurs in the ensuing (telogen) phase when the follicle, including the papilla, shortens, which causes a larger part of the hair to project above the skin in simulation of growth (Figure 10–9, D). When growth resumes, the follicle, with its matrix now reactivated, lengthens, and as it again extends away from the surface, it loses its grip on the old hair, which falls out. A replacement hair then forms in the active growth (anagen) phase that follows; this new hair gradually grows from the depth of the follicle until it emerges on the surface of the skin.

Figure 10–9 Phases of the hair cycle. A, Fully functional hair follicle; anagen phase. B, Follicle begins to atrophy; early catagen phase. C, Further atrophy of follicle; late catagen phase. D, Atrophied follicle. Hair is displaced distally and new hair matrix begins to form; telogen phase. E, New hair matrix established and new hair begins to grow; early anagen phase. 1, Hair follicle; 2, root of hair; 3, sebaceous gland; 4, arrector pili muscle; 5, new hair matrix; 6, new hair.
Wool hairs provide the soft undercoat. They are thin, wavy, and in most species, shorter and more numerous than the guard hairs by which they are concealed. The distinction between hair fiber types is not always clear-cut, and intermediate forms exist to complicate description. The sheep fleece presents particular problems as well as obvious interest.* Wool is not, of course, confined to sheep among domestic animals. Cashmere and Angora goats, Angora rabbits, and alpacas all produce wools of distinctive quality that are utilized in the production of luxury yarns and textiles.
In many species, including mature dogs and cats, several hairs share a single follicle opening (Figure 10–10, B-D). The central (primary) hair is longest and of the guard type, while the surrounding (secondary) hairs are shorter and softer; they provide the undercoat and may be designated wool hairs as they have little medulla.


Figure 10–10 A–C, Hair follicles of the dog. A, Simple follicle present shortly after birth. B, Follicle present during the first few months after birth. C, Complex adult follicle; the primary hair is surrounded by several secondary hairs. D, Scanning electron micrograph of adult canine skin; note one or two follicles without primary (guard) hairs. E, “Naked” skin of a pig with sparse primary hairs (bristles) and surface debris. 1, Primary hair follicle; 2, sebaceous gland; 3, duct of sweat gland; 4, secondary hair follicle; 5, arrector pili muscle.
The grouping of the hair follicles shows considerable interspecific and intraspecific variation. This may be revealed in products prepared from animal skin. The study of vellum of different periods has been used to trace the evolution of the fleece of modern breeds of sheep from the haircoat of their wild ancestors. Fragments of the Dead Sea Scrolls are among the materials utilized.
Tactile hairs are substantially thicker and generally protrude beyond the neighboring guard hairs. Most are found on the face, principally on the upper lip and about the eyes, although others are scattered (in species-variable fashion) on the lower lip, the chin, and elsewhere on the head. The cat, whose whiskers are particularly good examples (Figure 10–11), also possesses a cluster of similar hairs at the carpus. Tactile hair follicles reach deeply into the subcutis or even the superficial muscles. They are characterized by the presence of a venous sinus filled with blood and located between inner and outer layers of the dermal sheath (Figure 10–12). The nerve endings responsive to mechanical stimulation are also contained within the dermal sheath (Figure 10–12, A). The stimulus provided by disturbance of the hair is amplified by wave motion in the blood. The follicles of tactile hairs appear early in development, before those of the coat hairs, and their staged appearances provide useful criteria for aging embryos.

Figure 10–11 Tactile hairs on the head of the cat. The dots on the lips show the position of the circumoral glands. The arrows point to the buccal (tactile) hairs.
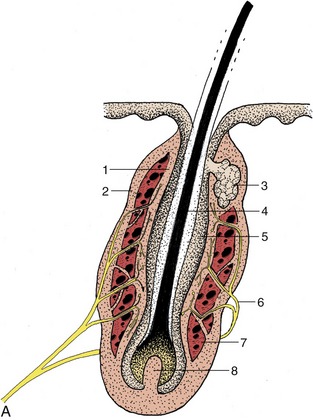

Figure 10–12 A, Schematic longitudinal section of a tactile hair follicle. 1, 2, Internal and external walls of blood sinus; 3, sebaceous gland; 4, root of hair; 5, epidermal wall of hair follicle; 6, nerve ending in wall of blood sinus; 7, blood sinus; 8, dermal papilla. B, Tactile hair follicle calf (Crossmon). 1, Epidermis; 2, sebaceous gland; 3, hair; 4, 5, inner and outer hair root sheath; 6, 7, trabeculated blood sinus; 8, inner and outer layer dermal sheath; 9, nerve ending; 10, trabecula.
The skin of dogs and cats presents minute scattered tactile elevations (toruli tactiles) usually associated with special (tylotrich) guard hairs; the roots of these are surrounded by venous sinuses similar to, though smaller than, those of true tactile hairs. These elevations are also sensitive to touch (Figure 10–13).
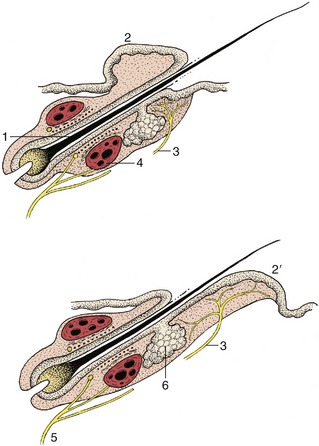
Figure 10–13 Tylotrich hairs below (top) and above (bottom) tactile elevations (2,2′). 1, Root of hair; 2, 2′, tactile elevations; 3, nerve endings associated with tactile elevations; 4, blood sinus; 5, nerve endings associated with blood sinus; 6, sebaceous gland.
Many breeds of domestic animals, such as Holstein cattle and Dalmatian dogs, are immediately recognizable from the distinctive patterns of their coats. These patterns are created by the restricted distribution of various pigments: polymers of melanin ranging from black, through brown and red, to lighter shades that are present in granule form* within cells of the epidermis, hair follicles, and hair. The pigments protect the skin from potentially harmful ultraviolet radiation and are unnecessary within those epidermal regions that are covered by a dense coat of hair. In most mammals, unlike humankind, skin pigmentation is therefore restricted to a few exposed parts that include the modified area associated with the external nose. It may be lacking here in white-coated individuals that obtain equivalent protection from a thickened stratum corneum.
FOOTPADS
The footpads (tori) are the cushions on which animals walk. They are covered by a naked, densely cornified epidermis (see Figure 10–2). The dermis is unremarkable, and the bulk of their substance is provided by a thick, resilient subcutis, an admixture of collagenous and elastic fibers interspersed with adipose tissue.
Footpads are best developed in plantigrade mammals (e.g., bears), in which digital, metacarpal (metatarsal), and carpal (tarsal) pads are all present (Figure 10–14). In the digitigrade dog and cat, only digital and metacarpal (metatarsal) pads make ground contact; there is a carpal pad of no obvious use but no corresponding tarsal pad (Figure 10–15).

Figure 10–14 Footpads of a bear: forelimb (left), hindlimb (right). 1, Digital pads; 2, metacarpal pad; 3, metatarsal pad; 4, carpal pads; 5, tarsal pad, fused with the metatarsal pad.
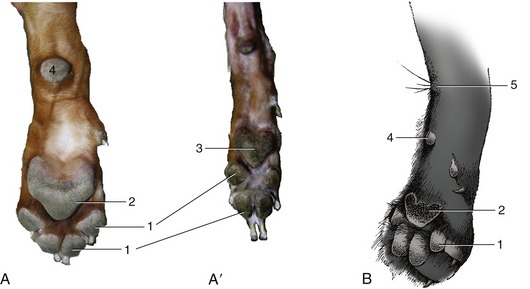
Figure 10–15 Footpads of canine forelimbs and hindlimbs (A, A′) and of feline forelimb (B). 1, Digital pads; 2, metacarpal pad; 3, metatarsal pad; 4, carpal pad; 5, carpal gland and associated tactile hairs.
Only digital pads are functional and in contact with the ground in ungulates, in which they are (generally) incorporated in the hoof, providing the features known as the bulb in ruminants and pigs and the more complex frog in horses. The bulbs of the pig are soft and well set off from the sole (see further on); in ruminants they are harder, though less so than other parts of the hoof (Figure 10–16/1).
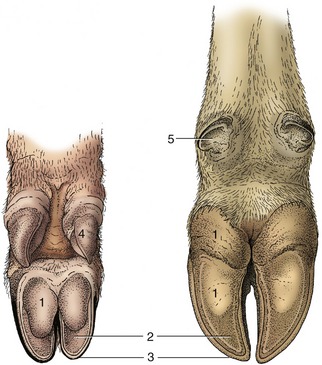
Figure 10–16 Palmar surface of foot of the pig (left) and of a cow (right). 1, Bulb (digital pad) of hoof; 2, sole of hoof; 3, wall of hoof; 4, hoof of accessory digit; 5, rudimentary hoof of dewclaw.
The digital cushion (pulvinus digitalis) deep to the frog of the horse consists of an apex and a base. The apex lies deep to the horny frog on the ground surface of the hoof (Figure 10–17/4), while the base helps shape the palmar (plantar) surface, forming the swellings at the heels. These, the bulbs of the heels (Figure 10–17/3), do not make contact with the ground and are covered by periople, the softer horn produced at the junction of the skin with the wall of the hoof. The horse, unlike the other domestic ungulates, also has rudimentary metacarpal (metatarsal) pads (“ergots”; Figure 10–17/2) embedded in a tuft of hair behind the fetlock joint and vestigial carpal (tarsal) pads (chestnuts; Fig 10–17/1,1′).
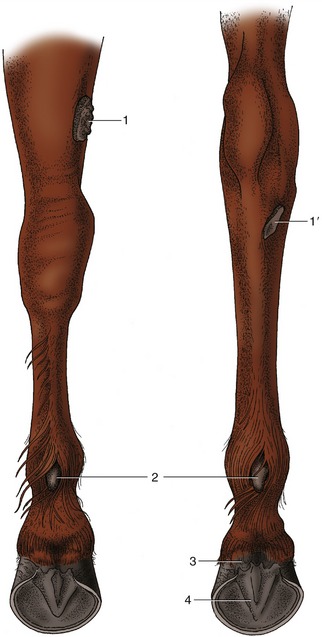
Figure 10–17 Left forelimb (on the left) and left hindlimb (on the right) of the horse, caudal view. 1, 1′, Chestnuts above carpus and below hock, respectively; 2, ergots; 3, bulbs of the heels; 4, frog.
The subcutis of the canine footpads, porcine bulbs, and equine frog contains sweat glands whose ducts channel through the thick, cornified epidermis. The secretions function as territorial or trail markers.
NAILS, CLAWS, AND HOOFS
Although these structures enclosing the distal phalanx appear strikingly different at first glance, they are in fact basically similar. Their origins as local modifications of skin are reflected in their retention of epidermal, dermal, and subcutis layers (though perhaps in greatly altered form). Nails, claws, and hoofs serve primarily to protect the underlying tissues, but each is also used for other purposes, such as scratching or digging or as a weapon. The equine hoof, the most complex, reduces concussion on foot impact, and its elastic nature also aids the return of blood to the heart. Figure 10–18 shows the correspondences between these appendages, each of which presents three parts: wall, sole, and associated pad. It is only in ungulates that the last forms part of the horny structure; it corresponds with the digital bulb of primates and the digital pad of carnivores.

Figure 10–18 Schematic representation of nail, claw, and hoof. A–C, Longitudinal section, palmar surface, and head-on view of human fingertip. D, E, Longitudinal section and palmar surface of canine claw. F, G, Longitudinal section and ground surface of equine hoof. 1, Nail (wall); 2, “sole horn” of nail; 3, bulb of finger; 4, wall of claw; 5, “sole” of claw; 6, digital pad; 7, wall of hoof; 8, sole of hoof; 9, frog.
The nail (wall) of primates grows from the epidermis covering a curved fold of dermis at its base. The epidermis under most of the nail produces a little horn that helps maintain adhesion as the nail gradually slides distally. The dermis under this rather unproductive portion of the epidermis is gathered into a few low, longitudinal folds (laminae) that interdigitate with corresponding epidermal laminae; increased dermoepidermal contact strengthens the bond between the nail and the deeper tissues. The epidermis underlying the free border of the nail produces small amounts of soft “sole horn” (Figure 10–18/2).
The wall of the claw of carnivores can be likened to a nail that has been laterally compressed and so has obtained a sharp dorsal border. Its proximal part and the germinal layer from which it is derived are similarly shaped and are lodged with the associated dermis within the unguicular crest of the distinctively shaped distal phalanx (Figure 10–18, D). The epidermis deep to the wall is minimally productive. The dermis that covers the unguicular process fuses with the periosteum, and as with the primate nail, longitudinal interdigitations between dermal and epidermal laminae strongly bond the claw to the dorsal border of the bone. The space between the free margins of the wall on the undersurface of the unguicular process is filled with flaky “sole horn” (Figure 10–18/5).
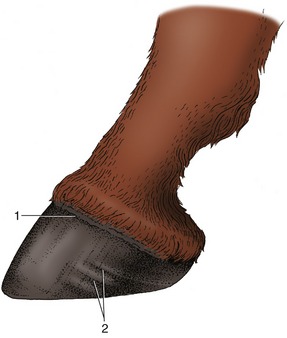
The wall of the horse’s hoof is also strongly curved, and the sides are sharply inflected to form the so-called bars (Figure 10–19, E/2″). The space between the bars is occupied by the frog, the part of the footpad that makes contact with the ground. The sole horn that fills the ground surface between wall and frog meets the wall at a junction known as the white line (zona alba; Figure 10–19/5). The wall grows distally from the epidermis over a bulging (coronary) dermis* studded with numerous papillae directed toward the ground. The epidermis covering these papillae produces horn tubules that run distally, toward the weight-bearing margin of the wall. The tubules are embedded in less structured intertubular horn formed by the epidermis over the interpapillary regions of the dermis; the combination of horn types gives the tissue a finely striated appearance. The (laminar) epidermis deep to the wall is again only minimally productive. It is arranged as several hundred well-formed laminae that tightly interdigitate with an equal number of dermal laminae (p. ••), bonding the wall to the underlying distal phalanx. One should remember that this is a living bond that allows the wall to slide gradually toward the ground where its distal border is worn away. A band of soft horn (periople) lies over the external surface of the wall near its junction with the skin (Figure 10–20/1). It descends with the wall and dries to a protective glossy layer. The band widens at the back of the hoof, where it covers the bulbs of the heels and part of the frog.
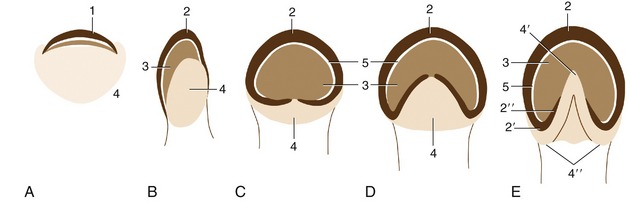
Figure 10–19 An interpretation of the phylogenetic “development” of the horn structures associated with the distal phalanx. A, A human fingertip. B, Pig. C, Rhinoceros. D, Tapir. E, Horse. 1, Nail; 2, wall of hoof; 2′, 2″, heel and bar (of horse); 3, sole; 4, footpad (bulb in human finger and pig); 4′, 4″, frog and bulbs of the heels (of horse); 5, white line.
The hoofs of ruminants and the pig, although resembling those of the horse in principle, differ in several respects: the wall is sharply bent to form a dorsal border (like that of the claw); the footpad (bulb) is relatively large and furnishes the entire caudal part of the hoof (Figure 10–19, B/4); the sole between the bulb and wall is small; and the interdigitating laminae are less developed (Figure 10–21/2).
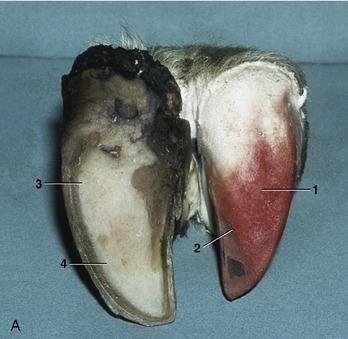

Figure 10–21 A, Bovine foot, palmer view. B, Bovine foot, dorsal view. The horn shoe (epidermis) has been pulled off one digit, exposing the dermis. 1, Dermis of bulb; 2, dermis of sole; 3, horn of bulb; 4, horn of sole; 5, dorsal border of hoof; 6, abaxial surface of hoof.
In all species, periods of disturbed or lessened horn production create ridges on the wall parallel to the formative region at the junction with the skin (Figure 10–20/2).
Fuller accounts of these specializations are found in the appropriate later chapters.
HORNS
The horns of domestic ruminants have osseous bases provided by the cornual processes of the frontal bones. Unlike antlers, which are shed and replaced yearly, horns are permanent* and grow continuously after their first appearance soon after birth.
The dermis is tightly adherent to the cornual process and bears numerous short papillae that are slanted apically, which ensures that the horn elongates as well as thickens as it grows (Figure 10–22). The horn substance resembles that of the hoof in being an admixture of tubules and intertubular horn. The horn (epiceras) produced by the epidermis at the base is soft and somewhat transparent, resembling the periople of the hoof. It gives the horn its glossy sheen.

Figure 10–22 Longitudinal section of bovine horn. 1, Caudal frontal sinus extending into horn; 2, cornual process of frontal bone; 3, combined periosteum, dermis, and noncornified stratum of epidermis; 4, horn tubules separated by intertubular horn; 5, horn tubules (inset); 6, dermal papilla; 7, hair.
In general, horns are found in both sexes, although obviously not in naturally polled breeds, but those of males are usually more massive. Their shape is strongly characteristic of the breed and reflects the shape and size of the cornual process. In cattle, these processes are invaded by the frontal sinuses (Figure 10–22/1), which are therefore opened when an adult animal is dehorned.
The horny shell separates from the bony core on maceration, and this explains the (obsolete) zoological designation Cavicornia (hollow-horned animals) sometimes given to ruminants with permanent horns. Ruminants of the deer family (Cervidae) have antlers and are specifically excluded from this grouping. Antlers are sturdy outgrowths of the skull that are initially covered with skin but become exposed when the skin dies. The dead skin, or velvet, is removed by rubbing it against trees and other objects. The osseous processes lose their blood supply when exposed, die, and are shed, and the animal is left relatively defenseless until a new set of antlers grows next season.
SKIN GLANDS
The glands of the skin develop as epidermal sprouts that invade the underlying mesoderm. They generally develop from primitive hair follicles and retain these connections; the ducts deliver the secretion into the adult follicles from which it oozes onto the skin surface beside the projecting hairs. Two basic types, sweat and sebaceous glands (Figure 10–7/9,10), are distinguished, but each occurs in various subvarieties and in more definitely specialized forms.
THE SEBACEOUS GLANDS
These produce a fatty secretion (sebum) that lubricates and waterproofs the skin and coat. It also promotes the spread of sweat, retards bacterial growth, and, in certain instances, serves as a territorial marker that is recognized by other members of the species. The odor of the wet dog is due to these glands. Certain substances (pheromones) present in sebum are known to be sexually attractive; the rate of production is controlled by steroid hormones (androgens generally promote secretion, and estrogens retard secretion). A good illustration of a selective effect of androgens is found in the reaction of the so-called acne region of the human adolescent.
The sebum of the fleece of sheep is collected and processed; known as lanolin commercially, it is used as a base for ointments, in cosmetics, and as a cleansing agent in soaps. The secretions of certain specialized glands (e.g., the preputial glands of musk deer and the anal glands of the civet) have long been collected for use by the perfume industry.
The major localized accumulations of sebaceous glands that are of a size visible to the naked eye found in domestic animals are listed; several are associated with skin pouches.
Circumoral Glands (Figure 10–11)
These large glands are found in the lips of cats, which use them to mark their territories. The secretion is deposited directly by the animal rubbing its head against an object or ingratiatingly against its owner and indirectly after transference to the body during grooming.
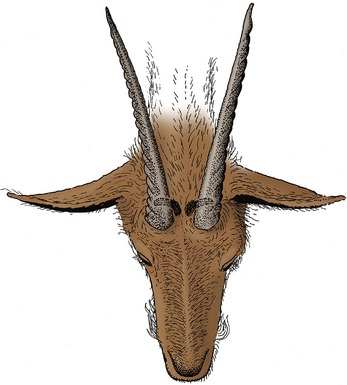
Horn Glands (Figure 10–23)
These musk or scent glands are present in goats of both sexes, caudomedial to the horn base (or at the corresponding site in polled animals). They are larger and more productive in the breeding season; stimulated by testosterone, those of males produce a secretion with an odor so pungent that some owners insist on their surgical removal.
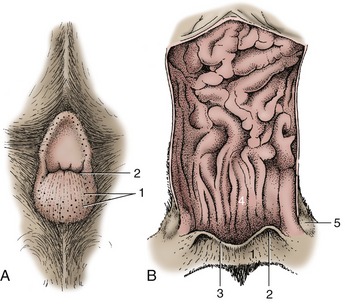
Glands of the Infraorbital Pouch (Figure 10–24)
These glands are contained in a cutaneous pouch rostral to the eye and opening ventrolaterally on the face of sheep. The pouch wall contains both sebaceous and tubular serous glands whose mixed secretion stains the skin when it escapes from the pouch. The glands, which serve as territorial markers, are larger in rams.
Carpal Glands (Figure 10–25)
These are present in pigs and cats. In pigs they surround several cutaneous invaginations on the mediopalmar aspect of the carpus. They are found in both sexes and serve to indicate territorial claims; boars are said to make particular use of them when “marking” sows during copulation.
The location of the glands in cats is marked by a tuft of a few tactile hairs proximal to the carpal pad. The site is betrayed by a palpable thickening of the skin (Figure 10–15, B/5).
Glands of the Interdigital Pouch (Figure 10–26)
Interdigital pouches are found on the forelimbs and hindlimbs of sheep of both sexes. The pouches are tubular invaginations of the skin whose walls contain branched sebaceous and serous glands. The waxy secretion is discharged through a single opening above the hoofs and serves as a “trail marker.” Many gregarious wild species have similar glands.
Glands of the Inguinal Pouch (Figure 10–27)
Inguinal pouches, found near the base of the udder or scrotum of sheep, contain both sebaceous and sweat glands. The secretion escapes as a brown waxy substance whose odor may assist the lamb to find the udder.
Preputial Glands (Figure 35–11)
Sebaceous and apocrine sweat glands within the prepuce produce secretions that combine with desquamated cells to form the crumbly substance known as smegma. They are best developed in the boar, in which they are massed within a dorsal diverticulum of the preputial cavity (see Figure 35–11/5). Their secretion gives the boar its characteristic odor. They are present but less offensive in other species (which lack the diverticulum).
Tail Glands (Figure 10–28)
Collections of large sebaceous and serous glands are found in an oval patch on the dorsal surface of the tail of certain carnivores. The skin over these glands is often defined by its sparser hair and yellowish color. Activity is greatest during the breeding season. The patch is situated more proximally in cats, toward the root of the tail, than in dogs (Figure 10–28).
Circumanal Glands (Figure 10–29)
These sebaceous glands are restricted to the perianal skin of certain carnivores, including dogs, where they drain into (and are believed to influence) special sweat glands. It is probably their secretion that excites the particular attention paid to the anal region when dogs confer. It has been suggested that some of these glands have an endocrine function.
Glands of the Anal Sacs (Figure 10–30)
Sebaceous and serous glands are found in the walls of the anal sacs, cutaneous pouches that open beside the anus of carnivores (Figure 10–29/2). The secretion, which is particularly foul-smelling, is expressed during defecation and apparently serves as a marker. It is well-known that skunks can forcefully expel the contents of the sacs to fend off aggressors.
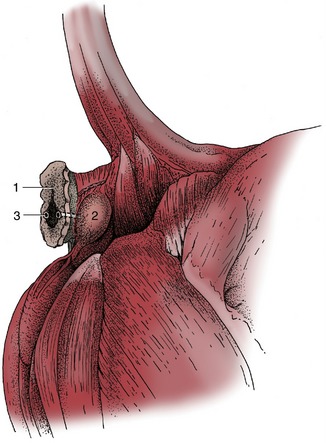
Figure 10–30 Exposed right anal sac of a dog. 1, Anus; 2, anal sac; 3, opening of excretory duct of anal sac (emphasized; see Figure 10–29, A/2).
THE SWEAT GLANDS
Sweat glands are scattered over the entire body but are somewhat sparse in carnivores and pigs. Two types are distinguished by (a probably erroneous interpretation of) the histology of the secretory process. Apocrine sweat glands discharge an albuminous sweat into hair follicles over most of the body.* Eccrine glands secrete a more watery sweat directly onto certain naked, or nearly naked, regions of the skin (e.g., the nasolabial plate of cattle and the footpads of dogs). The apocrine variety predominates, and its secretion and subsequent evaporation are important in salt metabolism and temperature regulation. The secretion is degraded by bacteria, which form substances that provide the characteristic body odor. The product of the eccrine variety is thought to play a lesser role in temperature regulation.
Most mammals possess fewer glands and sweat less profusely than humans. However, impressions can be misleading because the sweating that does occur tends to be masked by the more generous coat. The horse is an obvious exception to the general statement as it not only sweats abundantly but also produces an especially albuminous sweat that froths when worked by movement of the skin and coat (“lathering up”). Certain breeds of cattle also sweat visibly along the neck and over the flanks; in this species there are well-established differences in the number, size, and distribution of the glands between temperate and tropical breeds. Surprisingly, the Asiatic buffalo has fewer sweat glands than cattle and resorts to wallowing in water in compensation. Among domestic species, dogs and cats sweat least, although the skin of short-haired individuals sometimes feels moist. Sweat glands are present in the footpads of dogs and cats. In dogs, it is asserted that excessive activity of these glands may, in cold climates, lead to snow- or ice-balling on digital hair, making it painful for the animals to walk. Attention is apparently paid to this propensity when selecting sled dogs for breeding. It is not surprising to learn that Arctic wolves lack these glands.
THE MAMMARY GLANDS
Mammary glands (mammae) are greatly modified, much enlarged sweat glands whose secretion nourishes the young. The modified milk (colostrum) produced immediately after parturition has an additional role in the passive transfer of immunity to the newborn. Its importance varies among species: some correlation with the nature of the placental barrier exists. Each mammary gland is a compound tubuloalveolar gland that consists of secretory units grouped into lobules defined by intervening connective tissue septa (see Figure 29–48, B). The mammary glands develop as epithelial buds that grow into the underlying mesenchyme from linear ectodermal thickenings (mammary ridges). These ridges may extend from the axilla to the groin (as in carnivores and pigs) or may be of more limited extent, restricted to the axilla (as in elephants), the thorax (as in women), or the groin (as in ruminants and horses). Usually more buds appear than survive in the adult, and while most extra buds soon regress, some persist to give rise to supernumerary teats. These may be independent or attached to other, better developed glands (see Figure 10–33, A/7). They are unsightly, and because they may interfere with milking, they are often removed from the udders of cows and goats.
Proliferation of the mesenchyme surrounding the bud raises a teat (papilla) on the surface of the body. One or more epidermal sprouts grow from the mammary bud into the connective tissue of the teat and begin to canalize at about the time of birth. Each sprout is destined to form a separate duct system with associated glandular tissue. When there is only one sprout, the mammary gland arising from it has a single duct system leading to a single orifice on the tip of the teat (Figure 10–31, A).
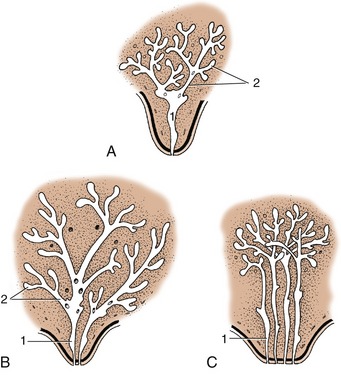
Figure 10–31 Developing duct systems growing proximally from the tip of the fetal teat. A, Cow, ewe, and goat. B, Mare and sow. C, Bitch and cat (only four primary sprouts are shown). 1, Primary sprout, which gives rise to the lactiferous sinus; 2, secondary and tertiary sprouts, which give rise to the lactiferous ducts.
When there are more, for example, two or four as in the illustration, there will be that number of separate duct systems, each with an associated glandular mass and separate orifice. The growth of the ducts and gland tissue is continued after puberty and especially during the first pregnancy, forming the swelling that pushes the teat away from the body wall. The process is controlled by the intricate interplay of several hormones from the hypophysis, ovaries, and other endocrine glands.
Examination of one of the several units formed along the trunk of a lactating sow (see Figure 10–31, B) reveals that it is composed of glandular tissue supported and enclosed by a fibrous tissue framework in which run the mammary vessels and nerves. The whole formation is pervaded with fat and covered by skin. Sometimes, as in ruminants and horses, the mammary glands are so closely placed that they appear to merge in a single consolidated complex, the udder. Although the glands of the pig, like those of the dog and cat, remain more distinctly separated, this collective term is sometimes also used in the sow. The number of mammary glands (as well as their duct systems) in the domestic species is shown schematically in Figure 10–32.

Figure 10–32 Distribution of mammary glands in certain mammals. The dots indicate the number of orifices on the teat. A, Sow. B, Bitch. C, Cat. D, Woman. E, Cow. F, Ewe and she-goat. G, Mare.
The more detailed organization is illustrated by reference to the cow. The glandular tissue is arranged in lobules, each 1 mm or perhaps a little more in diameter and consisting of about 200 alveoli. The milk drains to an intralobular duct that joins others to form a larger interlobular duct (Figure 10–33/2). Interlobular ducts lead in their turn to a system of lactiferous (milk-carrying) ducts that ultimately convey the milk to the relatively large cavity known as the lactiferous sinus (Figure 10–33/3). The lactiferous ducts of successive orders increase in diameter but diminish in number so that only 10 or so enter the sinus. Unlike most ducts, they have alternating narrow and dilated portions; contraction of the muscular wall of the narrow portions holds the milk in the expansions before it is “let down” when the cow suckles or is milked. The lactiferous sinus extends into the teat and is incompletely divided into gland and teat sinuses (Figure 10–33/3′,3″) by a constriction. The teat sinus is continued by the papillary duct (Figure 10–33/4), which opens at the tip of the teat where the orifice is surrounded by a smooth muscle sphincter (Figure 10–33/6).

Figure 10–33 A, Sagittal section of udder, showing teat and gland sinuses and lactiferous ducts filled with latex (cranial quarter, green; caudal quarter, blue). B, Section of teat. 1, Parenchyma of gland; 2, lactiferous ducts of various diameters; 3, lactiferous sinus; 3′, gland sinus; 3″, teat sinus; 4, papillary duct; 5, teat orifice; 6, teat sphincter; 7, supernumerary teat.
Corresponding parts can be identified in other species, including those in which each gland contains several small lactiferous sinuses, each served by a separate duct system and each opening independently.
It must be stressed that mammary glands are fully developed and fully functional only at the height of lactation. They are then large and show a predominance of yellow glandular tissue over the paler fibrous stroma. When the dam weans her young, involution sets in and the parenchyma regresses (see Figure 29–48, A); the connective tissues now form the bulk of the organ. However, the gland never quite reverts to its prelactation size and it grows a little more with each pregnancy.
Mammary buds also form in male embryos and persist to give rise to the rudimentary teats found on the ventral surface of the trunk (carnivores and pig) or on the cranial surface of the scrotum (ruminants). They are less common in horses but occasionally appear beside the prepuce. On the other hand, in certain species, such as rats, the male glands regress completely.