1 Some Basic Facts and Concepts
THE SCOPE OF ANATOMY
Anatomy is the branch of knowledge concerned with the form, disposition, and structure of the tissues and organs that comprise the body. The word, which is of Greek origin, literally means “cutting apart,” and the dissection of the dead is the traditional method used in anatomy. However, anatomists employ a host of other techniques to supplement the knowledge of gross anatomy obtained by use of the scalpel. Details invisible to the naked eye are revealed by light and electron microscopy and constitute the subdivision known as microscopic anatomy. The discipline is also extended by the study of the stages through which the organism evolves from conception through birth, youth, and maturity to old age; this study, known as developmental anatomy, is rather broader in scope than is classic embryology, which confines its attention to the unborn. Few anatomists are now satisfied by the mere description of the body and its parts, and most seek to understand the relationships between structure and function. The study of these relationships clearly merges into physiology, biochemistry, and other life sciences; it can be described as functional anatomy, but we prefer to regard a functional approach as one that should pervade all branches rather than constitute a quasi-independent study.
This book is mainly concerned with gross anatomy, which is a limitation justified by the general practice of presenting microscopic and developmental anatomy in separate courses. Nonetheless, we have allowed ourselves to draw on microscopic and developmental aspects when this has seemed helpful in promoting an understanding of gross anatomy or as a means of enlivening what would otherwise be a rather dry account.
The information obtained by dissection can be arranged and organized in two principal and complementary ways. In the first, systematic anatomy, attention is successively directed to groups of organs that are so closely related in their activities that they constitute body systems with an evident common function—the digestive system, the cardiovascular system, and so forth. Systematic anatomy lends itself to a comparative approach; readily combines gross, microscopic, developmental, and functional aspects; and provides the basis for the study of the other medical sciences. Moreover, for the beginner, it is easier to understand than regional anatomy. It is the approach employed in Chapter 2Chapter 3Chapter 4Chapter 5Chapter 6Chapter 7Chapter 8Chapter 9Chapter 10.
The alternative approach, regional anatomy, is used in the second and larger part of this book. Regional (or topographical) anatomy is directly concerned with the form and relationships of all the organs present in particular parts or regions of the body. It pays less attention to function, other than the simpler, mechanical functions, than does systematic anatomy but obtains a compensating importance from its immediate application to clinical work. Because matters of detail that may lack theoretical interest are often relevant to the clinician, it is necessary to give separate consideration to the regional anatomy of the different species. Regional anatomy is one of the foundations of clinical practice, and different aspects pursued with particular aims are sometimes known as surface, applied, surgical, and radiological anatomy—terms whose connotations overlap but hardly require definition.
THE LANGUAGE OF ANATOMY
Anatomical language must be precise and unambiguous. In an ideal world each term would have a single meaning, each structure a single name. Unhappily, there has long been an alarming superfluity of terms and much inconsistency in their use. In the hope of reducing this confusion, an internationally agreed-on vocabulary—Nomina Anatomica Veterinaria (NAV)*—was introduced in 1968 and has since obtained wide acceptance. It is revised periodically, most recently in 1994, and we have tried to use it consistently throughout this work. Occasionally, we have included a second, older, and unofficial alternative when such a term appears to be so deeply rooted in clinical usage that it is unlikely to be eliminated by edict. The terms of the NAV are in Latin, but it is permissible to translate them into vernacular equivalents and is usual in English-speaking countries to do so. We have given preference to translations that so closely resemble the original Latin that the equivalence is immediately recognizable. We therefore give the Latin name only when the translation could be in doubt. No handy English equivalents exist for some official terms; in these cases it is conventional to use the Latin terms, perhaps in abbreviated form, as though they were English words or phrases. The resulting mixture of languages is jarring but not easily avoided, particularly when describing groups of muscles. The names, whether in Latin or in English, are intended to be informative and an aid to comprehension. It is more sensible to look up any word whose meaning is not self-evident in an anatomical or medical dictionary than to use it “parrot fashion.”
The names that are given to particular structures will be encountered gradually, but the terms that indicate position and direction must be mastered at once. These official terms are more precise than the common alternatives because they retain their relevance regardless of the actual posture of the subject. They are defined in the following list, and their use is illustrated in Figure 1–1. We have not thought it sensible to use them pedantically when there is no reasonable prospect of misunderstanding. When we use common terms (above, behind, and so forth), we always have in mind a standard anatomical position, which, for a quadruped, is that in which the animal stands square and alert. This differs from the human anatomical position, and difficulties with terminology will be encountered when books are consulted that refer primarily to the human body. Medical anatomists make much use of the terms anterior and posterior, superior and inferior, all of which have very different connotations when applied to quadrupeds. These terms are therefore best avoided, except for a few specific applications to the anatomy of the head.
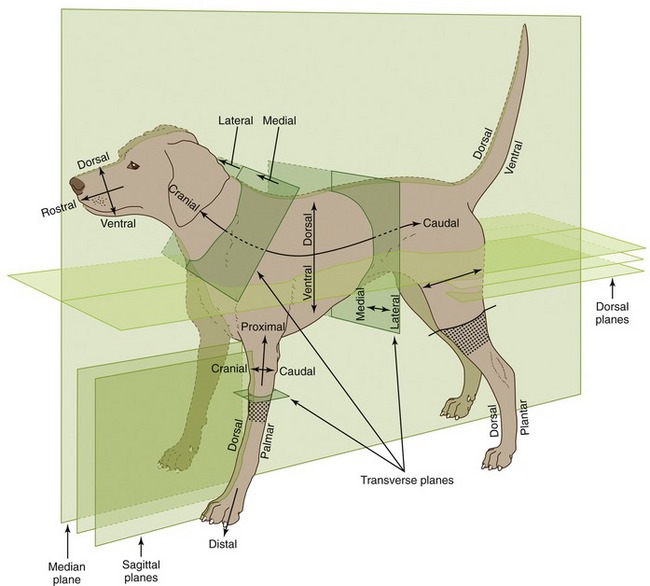
Figure 1–1 Directional terms and planes of the animal body. The stippled areas represent the carpus and tarsus on forelimbs and hindlimbs, respectively.
The principal recommended terms of position and direction are arranged in pairs, and it should be emphasized that they refer to relative, not absolute, position. Most of these adjectives form corresponding adverbs by the addition of the suffix -ly.
Dorsal structures (or positions) lie toward the back (dorsum) of the trunk or, by extension, toward the corresponding surface of the head or tail.
Ventral structures lie toward the belly (venter) or the corresponding surface of the head or tail.
Cranial structures lie toward the head (cranium, literally skull), caudal ones toward the tail (cauda). Within the head, structures toward the muzzle (rostrum) are said to be rostral; caudal remains appropriate.
Medial structures lie toward the median plane (medianus, in the middle) that divides the body into symmetrical right and left “halves.”
Lateral structures lie toward the side (latus, flank) of the animal.
Different conventions apply within the limbs. Structures that lie toward the junction with the body are proximal (proximus, near), whereas those at a greater distance are distal (distantia, distance). Within the proximal part of the limb (which is defined for this purpose as extending to the proximal limit of the carpus [wrist] or tarsus [hock, ankle]), structures that lie toward the “front” are said to be cranial, those that lie toward the “rear,” caudal. Within the remaining distal part of the limb, structures toward the “front” are dorsal (dorsum, back of the hand), and those toward the “rear” are palmar (palma, palm of the hand) in the forelimb or plantar (planta, sole of the foot) in the hindlimb. Additional terms may be applied to the anatomy of the digits. Axial structures lie close to the axis of a central digit, close to the axis of the limb if this passes between two digits; abaxial (ab, away from) positions are at a distance from the reference axis.
The terms external and internal, superficial and deep (profundus) hardly require explanation or definition.
Sometimes it is necessary to refer to a section through the body or a part of it (see Figure 1–1). The median plane divides the body into symmetrical right and left halves. Any plane parallel to this is a sagittal plane, and those close to the median are sometimes termed paramedian planes. A dorsal plane sections the trunk or other part parallel to the dorsal surface. A transverse plane transects the trunk, head, limb, or other appendage perpendicular to its own long axis.
AN INTRODUCTION TO REGIONAL ANATOMY
Although the first nine chapters that follow deal with systematic anatomy, those readers who are about to begin a laboratory course will find that they require an elementary knowledge of several systems at once. It is the principal purpose of the remainder of this chapter to supply that background. However, devoting some attention to the live animal also has benefits.
STUDY OF THE LIVE ANIMAL
Regional anatomy is conveniently studied by dissection, but this has obvious limitations if the goal is knowledge of the anatomy of the living. When embalmed, organs are uncharacteristically inert and greatly changed in color and consistency from their living state. The impressions gained in the dissection room or from prosection must therefore be modified and corrected by frequent reference to fresh material and by observation of surgical operations, whenever possible. Because most of those who study the anatomy of domestic animals do so with a future professional career in mind, they will find it both stimulating and advantageous to learn how to apply the simpler methods of clinical examination to normal animals at this stage in their training. Students in some departments receive elementary instruction in these methods; others must create their own opportunities, perhaps by enlisting the assistance of senior student colleagues. They will find a little direct experience to be far more rewarding than much unsupported reading. We merely list some methods and rely on our colleagues in the clinics to provide more adequate guidance.
The simplest method is observation of the contours, the proportions, and the posture of the body. Bony projections provide the clearest landmarks, but superficial muscles and blood vessels are also useful, if less striking; reference to these landmarks allows the positions of other structures to be deduced from their known relationships. Little experience is required to reveal the importance of breed, age, sex, and individual variation or to show that although some landmarks are fixed and reliable, others are prone to move. Some (e.g., the costal arch) move with each respiration, whereas other features change more gradually, for example, becoming more or less prominent or shifting in position with the deposition or depletion of fat or with the advance of pregnancy.
Structures that are not directly visible may be identified by touch, that is, by gentle or firmer palpation as circumstances require. Bones may be identified by their rigidity, muscles by their contraction, arteries by pulsation, veins by swelling when the blood flow is interrupted by pressure, and lymph nodes and internal organs by their size, configuration, and consistency. Nonetheless, variation is great and is affected by many factors that make it difficult to know whether one should expect to be able to identify certain organs in all normal subjects, which is, itself, another useful lesson. Palpation through the skin can be supplemented by digital or manual exploration per rectum and per vaginam.
Certain organs may be identified by percussion to elicit resonance when the overlying skin is struck a sharp blow (in a prescribed fashion). Different materials produce different notes; that from a gas-filled organ is more resonant than the duller one elicited from an organ that is solid or filled with fluid. The normal activities of certain organs produce sounds continuously or intermittently. Although the lungs and heart (not forgetting the fetal heart) are the prime examples of organs whose positions can be determined by auscultation, the movement of blood within vessels or of gas or ingesta within the stomach or intestines can also be a useful source of anatomical information. When these two techniques are applied, it must not be forgotten that the vagaries of sound conduction through materials of different densities may provide a distorted indication of the position and dimensions of the source.
The study of the anatomy of the live animal can be enlarged by other methods whose exercise requires considerable training and more elaborate apparatus than the simple stethoscope. These additional procedures have provided a variety of new illustrations scattered through later chapters but, while some elementary knowledge of how these illustrations were obtained may assist their appreciation, detailed consideration of the various technologies involved is clearly beyond the scope of this book.
Many parts and cavities that are normally out of sight can be brought into view by the use of various instruments. Perhaps the most familiar of these are the ophthalmoscope, used to study the fundus of the eye, and the otoscope, used to explore the external ear canal. Other instruments, for which the generic title “endoscope” is available, may be introduced into natural orifices and advanced to allow inspection of deeper parts, such as the nasal cavity, bronchial tree, or gastric lumen. These examples of endoscopy are noninvasive, but other examinations require preparatory surgery. Among these are arthroscopy, the inspection of the interior of synovial joints, and laparoscopy, the technique in which an endoscope is passed into the peritoneal cavity through a small opening in the abdominal wall. This last technique may be employed for diagnostic purposes or for the visual control of (“keyhole”) surgery with the use of instruments introduced through separate portals. For both purposes, moderate inflation of the abdomen creates the necessary viewing chamber.
Early endoscopes were rigid, which limited their utility, but the modern fiber-optic version is flexible and can negotiate bends while its tip may be turned, under remote control, to widen the field that may be scrutinized. The essential components of the fiber-optic instrument are two bundles of glass fibers. Such fibers, when suitably prepared and coated, conduct light from one end to the other without significant leakage to the side. One bundle is used to convey light distally, from an external source to the region to be viewed; the component fibers can be relatively coarse and randomly arranged. The second bundle conveys the image and is composed of finer fibers that maintain fixed positions in relation to each other. The image is composed of many tiny units, each corresponding to an individual fiber, and is presented to the eye (or to a camera or video system) at the proximal end of the instrument.
Radiographic anatomy has for some time been an indispensable component of every course of anatomy influenced by clinical considerations. Most departments routinely display previously prepared radiographs and, although students are unlikely to be involved in their production, it is prudent to remind them that considerable risks are associated with x-radiation—risks that must always be assessed for those conducting and those subjected to these procedures.
X rays are produced by bombarding with electrons a tungsten target (focus) housed within a shielded tube. Only a narrow x-ray beam is permitted to escape, and this is directed toward the relevant region of the subject. The passage of the rays through the body is affected by the tissues they encounter; tissues substantially composed of elements of high atomic weight tend to scatter or absorb the rays; tissues substantially composed of elements of low atomic weight have proportionately less effect. Because of its calcium content, bone clearly belongs to the first (radiopaque) category, whereas soft tissues generally belong to the second (radiolucent) category. Those rays that succeed in passing through the subject are allowed to impinge on a sensitive film (or other detector), which responds to the radiation received. When the film is developed, those areas that were overlain by soft tissues (or gas-filled spaces) appear dark, even black, while those areas that were overlain by bone (or other radiopaque material) appear lighter, even white. The distinction between tissues of similar radiodensity may be enhanced by introducing an appropriate contrast agent to coat a surface or fill a space. Specific methods, utilizing various materials, are available to depict such different features as the gastric lumen, urinary tract, and subarachnoid space.
Radiographic views are appropriately identified by reference to the direction taken by the x-ray beam in its passage through the subject. Thus a radiograph of a supine animal, presenting its belly toward the x-ray source, is described as a ventrodorsal film; that obtained with the animal turned over, with its belly now facing the film, is described as a dorsoventral film. The convention provides little scope for confusion but occasionally produces an awkward term, such as dorsolateral-plantaromedial which specifies a particular, oblique view of the hock.
Awareness of certain general principles will help in the avoidance of some common misinterpretations: the image of any structure is always magnified to the degree determined by the ratio focus-film/focus-object; the divergence of the x rays produces an apparent shift in position of any object not directly below the focus. Two simple diagrams (Figure 1–2) will make these points clear. A less easily resolved difficulty results from the superimposition of the images of structures that lie over each other. An ingenious, only partly successful, solution to this problem was sought in the coordinated movement—in opposite directions—of tube and film during the period of the exposure (Figure 1–3, A). In this technique, known as tomography, the axis about which tube and film travel coincides with the plane of the horizontal slice of the subject that is of current interest. Structures contained within this slice remain more or less in focus throughout the exposure, while the images produced by structures at other levels are blurred or subsumed within the general background. Such tomograms never found much employment in veterinary radiology. The more recently developed and more sophisticated technique known as computed tomography (CT) has a different basis but retains the aim of clearly depicting the parts within one particular body slice while excluding extraneous images. Despite the considerable cost of the apparatus and its limited suitability for use with large animals, the technique is now widely offered by veterinary referral centers.
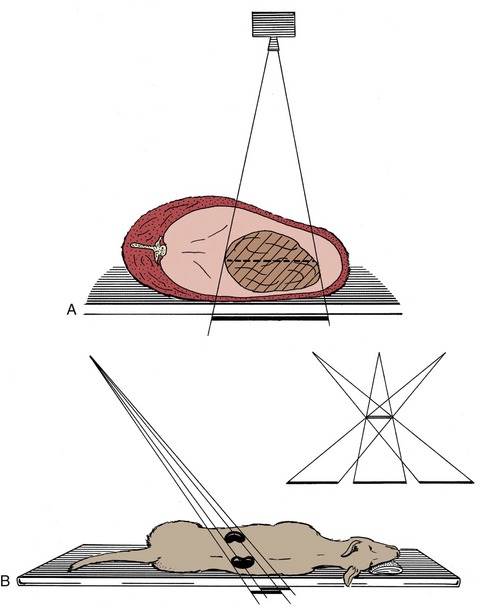
Figure 1–2 A, Schematic drawing illustrating the magnifying effect caused by the divergence of the x rays. B, Schematic drawing illustrating the apparent shift in position of an organ that is not directly below the focus.
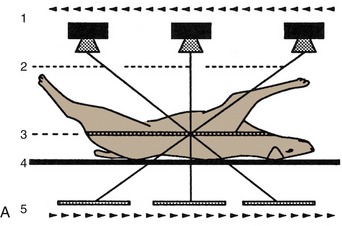
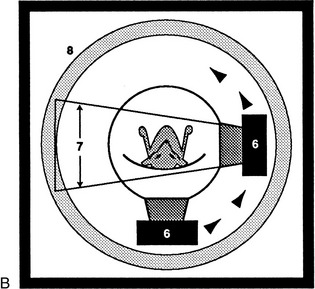
Figure 1–3 Diagrams of a basic (noncomputed) x-ray tomographic apparatus (A), and of a fourth-generation computed tomographic (CT) scanner (B).1, Movement of x-ray source during exposure; 2, lines indicating mechanical connection between x-ray source and radiation detector (i.e., film); 3, plane of focus; 4, supine patient on stationary table; 5, movement (in the opposite direction) of detector during exposure; 6, movement of x-ray source around stationary patient; 7, x-ray beam during exposure; 8, ring of fixed detectors surrounding the rotating x-ray tube mechanism.
In the modern CT scanner, the x-ray source is moved in a circle that is centered on the longitudinal axis of the subject during the procedure, which takes from one to several seconds for its completion (see Figure 1–3, B). During this time the movement of the tube is repeatedly arrested for very short periods; during each of these, a burst of radiation is directed through the subject along a different radius. The beams that penetrate the selected, very narrow slice of the subject impinge on a series of discrete detectors or, in some designs, on portions of a continuous circumferential detector and are photomultiplied. After the procedure is completed, these records are analyzed, compared, and combined according to complex formulae (algorithms*); from these computations, a single cross-sectional image is constructed in which the forms, locations, and comparative radiodensities of all the tissues within the selected body slice are represented (Figure 1–4). In more complex settings, multiple overlapping or adjacent slices can be imaged in an extended, continuous process. With the amount of information the extended process supplies, it is possible by even more elaborate computation to construct images in other than transverse planes. The data may also be manipulated to enhance subtle differences in contrast presented by tissues of very similar radiodensity.
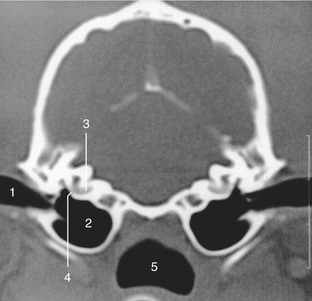
Figure 1–4 Transverse image of a 2-mm-thick computed tomographic slice of the canine tympanic bullae and petrous temporal bones. (Bone settings were used.) 1, External acoustic meatus; 2, tympanic bulla; 3, cochlea; 4, round window; 5, nasopharynx.
CT is, of course, not free from all drawbacks: subjects must be strictly immobilized during the exposure procedure; the total radiation dose may be quite considerable, even though individual exposures are very short and the resulting images amplified; artifacts may produce deceptive images; current apparatus designed for medical use is suitable for small animals but must be adapted for application with large animals and is then limited to the investigation of the head and limbs. One by-product of CT is the revival of interest in cross-sectional anatomy, an approach to the discipline that was, until recently, regarded as irretrievably passé but is now clearly indispensable for CT interpretation.
Familiarity with cross-sectional anatomy is also required for the practice of ultrasonography. This technique depends on the capacity of a piezoelectric crystal to convert electrical energy into sound waves and vice versa. When stimulated, a suitably housed crystal transducer, coupled to the appropriate area of skin, directs a narrow beam of sound waves of uniform frequency into the body. The waves are propagated through the tissues with decaying intensity, and a fraction is directed back toward the source at each encounter with an interface between tissues offering different resistance (acoustical impedance). Reconverted into electrical energy, the echoes generate a visible image on a screen. This image, which can be “frozen” or recorded in various ways, represents the thin body slice directly below the transducer. The sound wave is not produced continuously but in very short bursts, perhaps lasting for no more than one-millionth part of a second. The longer silences that alternate with these bursts allow the time necessary for the receipt of echoes bounced back from interfaces at different depths.
The frequency and the wavelength of sound waves are inversely related. The first variable determines the depth to which waves will penetrate, the second, the resolution that may be obtained (i.e., the detail that may be distinguished). Because waves of high frequency penetrate less deeply but record more detail, a compromise is involved in the selection of the appropriate crystal to deploy for a specific examination: several crystals are normally at hand, and each has its own inherent and invariable oscillation frequency. The maximum depth from which it is possible to obtain useful images is about 25 cm, and this limits the application of ultrasonography in horses and cattle. In these large species its use is more or less restricted to the examination of the distal parts of the limbs and of the genital apparatus (when the transducer may be applied to the rectal mucosa). Ultrasonography is also widely used in the diagnosis of pregnancy in sows (although here a transabdominal approach is employed).
Water, blood, and most soft tissues offer very similar acoustical impedance, and interfaces between these substances are, at best, only moderately reflective; they are hypoechoic in ultrasonographers’ jargon. In contrast, the difference in impedance between soft tissue and bone, or between soft tissue and a gas-filled cavity, is very large, and the reflection of sound waves is almost total; the interface is hyperechoic. This makes it impossible to image tissues and organs that, like the brain within the skull, lie deep to bone; such parts are said to be within acoustical shadow. Conversely, a distended bladder, or other large volume of uniform impedance, may be used as a window through which deeper structures may be approached.
There are many differences in transducer design and usage. Some transducers contain multiple crystals arranged in line; when these are activated sequentially, the resulting image is rectangular and represents the thin slice of tissue situated deep to the transducer. More often a single crystal is employed but so arranged that the narrow beam that it generates swings repetitively in an arc, producing a wedge- or sector-shaped image (Figure 1–5). In these B (or brightness) settings, the image represents a cross section through the field surveyed. In the alternative M (or motion) setting, the beam is only emitted at one fixed point in the crystal’s oscillation, and the recording is therefore limited to the structures penetrated along a single axis. If the parts are moving, successive images reveal their changing shapes, and the changes are emphasized if successive images are recorded side by side. M-mode recordings are especially useful for demonstrating the movements of the walls of the heart chambers and valves.
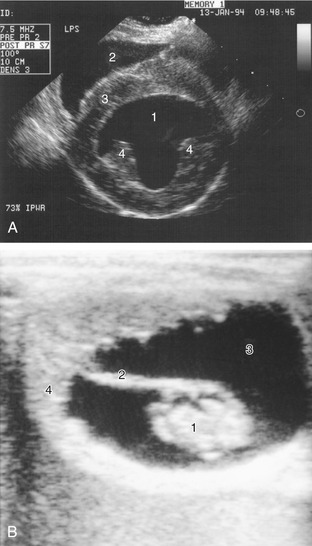
Figure 1–5 A, Ultrasonographic transverse (short-axis) view of the canine heart. 1, Left ventricle; 2, right ventricle; 3, septum; 4, papillary muscles. B, Ultrasonographic view of a 42-day-old equine embryo. 1, Embryo, about 2 cm in length; 2, umbilical cord; 3, allantoic fluid; 4, uterine wall.
Ultrasonograms are, in general, less easy for the novice to interpret than radiographs. Reverberations occur when the waves bounce back and forth, often because of defective coupling of the transducer to the skin, and this may produce what appear to be multiple parallel interfaces within an organ. Small interfaces between the parenchyma and fibrous scaffolding of certain tissues produce diffuse scattering, or a stippled effect. Despite these (and other) drawbacks, ultrasonography possesses very considerable advantages, not the least being its freedom from the risks inescapably associated with ionizing radiation.
Magnetic resonance imaging (MRI) requires less extensive consideration because the expenses of the installation and operation of the equipment make it presently available in only a few veterinary centers. The theoretical basis of MRI lies in changes in the structure of hydrogen atoms induced by strong magnetic fields and radio waves. Weak radio signals are subsequently produced when the subatomic structure returns to its normal configuration. These signals may be amplified, and their origins within the body may be precisely fixed in three dimensions. Because different tissues contain different concentrations of hydrogen atoms, their different responses can be used for their distinction. Tissues such as fat that are rich in hydrogen produce bright images in contrast to the black images of hydrogen-poor tissues such as bone (Figure 1–6). Extremely high resolution is possible. There appear to be no health risks associated with the MRI scanner. Both CT and MRI are especially useful in the study of intracranial structures.
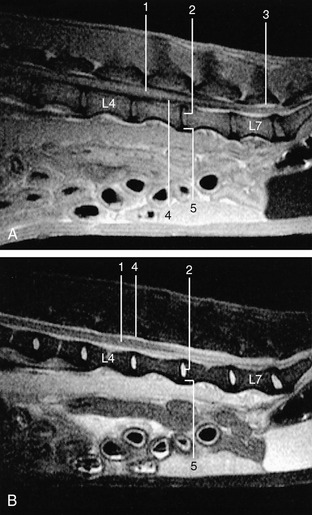
Figure 1–6 Midsagittal images of 3-mm-thick spin-echo magnetic resonance slices of the canine lumbar vertebral column. A, Tl-weighted (fat appears white, fluids dark). B, T2-weighted (fluids appear white, fat darker than on Tl-weighted images). 1, Spinal cord; 2, nucleus pulposus; 3, epidural fat; 4, cerebrospinal fluid; 5, annulus fibrosus.
SKIN
The skin covers the body and protects it against injury; it plays an important part in temperature control and enables the animal to respond to various external stimuli by virtue of its many nerve endings. There are numerous local modifications of skin (Chapter 10), but at present, we are concerned only with its more general properties.
The skin varies greatly in thickness and flexibility, both among species and locally. It is naturally thicker in larger animals (though not in constant proportion to their size) and in more exposed areas; these inequalities are obviously important to the surgeon. Although the skin is generally closely molded to the underlying structures, it appears redundant in some areas, forming folds and creases; some folding allows for change in posture, some is an adaptation to increase the area through which heat may be dissipated to the environment, and some is no more than the expression of breeders’ whims, grotesquely illustrated by the Shar-Pei breed of dog.
Skin consists of two layers, an outer epidermis and an inner dermis, and in most situations it rests on a looser connective tissue variously known as the subcutis, hypodermis, or superficial fascia (Figure 1–7). The epidermis is a stratified squamous epithelium whose thickness is adapted to the treatment it receives; it responds to rough usage, as exemplified by the footpads of dogs and cats. Numerous modifications of this layer exist, the most common being the occurrence of sweat and sebaceous glands and of hair. Sweat glands are most important as a provision for heat loss by surface evaporation but also play a subsidiary role in the excretion of waste. The sebaceous glands produce an oily secretion that waterproofs the surface and provides certain relatively naked areas, such as the groin of horses, with a characteristic sheen. Both types of gland are usually widely, though not ubiquitously, spread. The haircoat, which is a uniquely mammalian feature, is a mechanical protection and a thermal insulator, the latter property depending on the entrapment of air within the pile. The haircoat is also usually widespread. Among the more familiar species, only the human and the pig are relatively naked, although naked individuals may appear in other species as occasional “sports,” which is the origin, for example, of the Sphynx breed of cat. Some aquatic mammals, such as whales, are wholly naked.
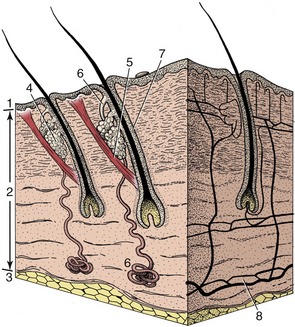
Figure 1–7 A block of skin. 1, Epidermis; 2, dermis; 3, subcutis; 4, sebaceous gland; 5, arrector pili muscle; 6, sweat gland; 7, hair follicle; 8, arterial networks.
The dermis, which consists essentially of felted connective tissue fibers, is the raw material of leather. It is secured to the epidermis by interlocking papillae, which are most pronounced where normal wear might risk separation. In most situations, the skin moves easily over the underlying tissues, and this looseness facilitates the flaying of a carcass. It is more tightly bound down in a few places where it grades into a tougher-than-usual underlying fascia; good examples of this binding are provided by the scrotum and the lips. Some risk of pressure injury is present where the dermis is molded over bony prominences, and synovial bursae (p. 24) often develop adventitiously in such sites. Unlike the epidermis, the dermis is well supplied with blood vessels (see Figure 1–7) and cutaneous nerves.
The superficial fascia is considered in the following section.
FASCIA AND FAT
The connective tissue that separates and surrounds the more obviously important structures is generically known as fascia, a term of rather elastic usage; many of its larger accumulations, particularly those of a sheetlike nature, have specific names. This tissue frequently receives scant notice, which is unwise, as it has significant functions to perform. Moreover, fascia is encountered in surgery, when it is necessary to predict its nature and extent in different situations.
The superficial fascia (subcutis) is a loose (areolar) tissue extensively spread below the skin of animals that possess a hairy coat. A similar tissue surrounds many deeper organs, and in both situations, the loose fascia allows neighboring structures to change in shape and to move easily against each other. Its looseness varies with the amount of fluid it contains and may provide an indication of ill health. The superficial fascia is one of the principal sites for the storage of fat. In naked species, the fat forms a continuous layer, the panniculus adiposus.
The deep fascia is generally organized into much tougher fibrous sheets. A layer beneath the superficial fascia extends over most of the body and fuses to bony prominences. In many places it detaches septa that penetrate between the muscles, enclosing them individually or in groups (Figure 1–8); sometimes the periosteum, the fibrous covering of the bones, participates in outlining the enclosures. This division into fascial or osteofascial compartments is very prominent in the forearm and leg and plays a part in the circulation, assisting the return of blood and lymph to the heart. Muscles thicken when they contract, and when they are contained within unyielding walls, they compress any other structures that share the space. If these are valved tubes (veins and lymphatic vessels), their contents are squeezed in one direction, toward the heart. Because of this, muscular paralysis or prolonged inactivity may lead to stasis of blood or lymph flow. Arteries and nerves whose functions would not be assisted by compression often travel in small tunnels within the septa.
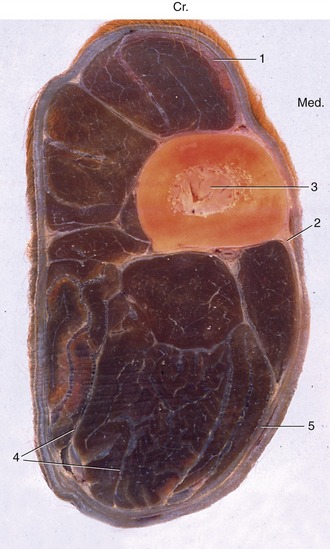
Figure 1–8 Osteofascial compartments in the forearm of a horse. 1, Superficial fascia; 2, cephalic vein; 3, radius; 4, septa of deep fascia enclosing individual muscles or groups of muscles; 5, deep fascia. (In transverse sections of the limbs, cranial [Cr.] and medial [Med.] are identified.)
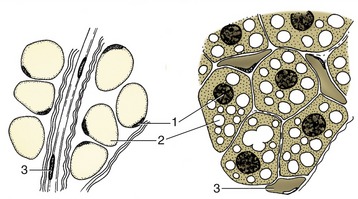
More specific functions can be assigned to localized thickenings (e.g., retinacula: tethers) of deep fascia, which hold tendons in place and sometimes provide pulleys around which the tendons wind to change direction. Good examples are provided by the retinacula on the dorsal aspect of the hock and the palmar aspect of the digits (Figure 1–9/9).
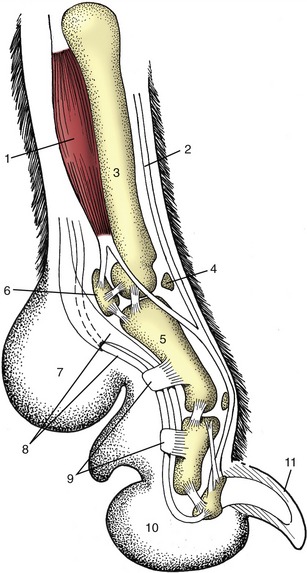
Figure 1–9 Axial section of a dog’s paw; the metacarpal pad (7) is in contact with the ground during standing. 1, Interosseous; 2, extensor tendon; 3, metacarpal bone; 4, dorsal sesamoid bone; 5, proximal phalanx; 6, proximal sesamoid bone; 7, metacarpal pad; 8, flexor tendons; 9, retinacula; 10, digital pad; 11, claw.
Because dense fascia is relatively impermeable, it determines the direction taken by spreading fluids, such as pus that sometimes tracks below a fascial sheet before breaking through far from its source. This is one reason why some knowledge of the deep fascia is useful to the surgeon. Its toughness enables it to hold sutures securely while it also provides cleavage planes, which allow relatively bloodless access to deeper parts during surgery.
Most deposits of fat (adipose tissue) may be regarded primarily as food reserves. Small amounts of fat are widely distributed, but the bulk is contained in three or four places: in the superficial fascia (Figure 1–10/2); between and within muscles; below the peritoneum (the delicate membrane lining the abdominal cavity); and in the marrow cavities of long bones. Subcutaneous fat deposits help mold the body contours and often show specific and gender differences in localization and development. Animals that are adapted to torrid habitats often develop localized depots (e.g., humped zebu cattle, camels, fat-tailed sheep), as a more even distribution might interfere with heat loss to the environment. Some of the differences in the body form of men and women that become accentuated at puberty are produced by the deposition of fat in the breasts and over the hips and lower abdomen of females. In many male animals, much fat is deposited in the tissues of the dorsal part of the neck: the thickened crest of stallions is a good example.
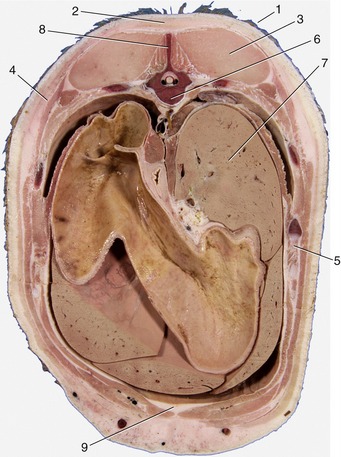
Figure 1–10 Transverse section of the back of a pig. 1, Skin; 2, fat (panniculus adiposus) associated with the superficial fascia; 3, back muscles; 4, cutaneous muscle enclosed within superficial fascia; 5, rib; 6, thoracic vertebra; 7, liver; 8, spinous process of vertebra; 9, additional fat deposited between muscles.
Some fat deposits, like that enclosed within a fibrous lattice in the footpad of the dog, function as mechanical buffers (see Figure 1–9/7,10). Fat with a mechanical function is usually resistant to mobilization in starvation.
Differences in the chemical and physical nature of fat can be pronounced but may reflect diet as much as specific genetic factors. When the origin of a specimen is being determined, it is certainly often useful to know that the fat of horses and of Channel Island breeds of cattle is yellow, that of sheep hard and white, and that of pigs soft and grayish. It should also be remembered that fat at body temperature is softer (semifluid) than that exposed in a colder environment. Certain procedures—liposuction and lipofixation—employed by the cosmetic surgeon depend on this fortunate circumstance.
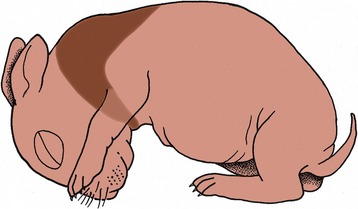
All these remarks refer to the common sort of fat. A second variety, brown fat, is of much more restricted distribution in time and place. Brown fat differs in structure (Figure 1–11) and function as well as in color. In domestic species, it is especially found during the fetal and neonatal periods; in wild species, it is especially prominent in those that hibernate (Figure 1–12). The brown adipocyte contains numerous smaller droplets and a much higher number of mitochondria. It is richly vascularized. It provides both groups with a readily available source of heat, equally useful in newborn animals with imperfect thermoregulation and in hibernators required to awaken rapidly from a deep winter sleep.
BONES
The primary functions of the skeleton are to support the body, to provide the system of levers used in locomotion, and to protect soft parts. Therefore, biomechanical factors are most important in shaping the bones and in determining their microscopic design. The major skeletal tissue, bone, has a secondary role in mineral homeostasis, supplying a reserve of calcium, phosphate, and other ions.
The Classification of Bones
Bones may be classified in various ways. A topographical classification recognizes a cranial skeleton (of the head) and a postcranial skeleton consisting of two divisions: the axial skeleton of the trunk and the appendicular skeleton of the limbs. A second classification based on ontogeny distinguishes the somatic skeleton, formed in the body wall, from the visceral skeleton, derived from the pharyngeal (branchial) arches. A third system is also based on development and distinguishes parts preformed in cartilage (and later largely replaced by bone) from those that ossify directly in fibrous connective tissue. This classification reflects the phylogeny, as bones that develop in membrane are homologous with dermal bones of lower vertebrates.
Individual bones are classified by shape according to a rather naive system (Figure 1–13). Long bones, which are typical of the limbs, are broadly cylindrical and are clearly adapted to perform as levers. It is perhaps more important to know that they develop from at least three centers of ossification: one for the shaft (diaphysis), and one for each extremity (epiphysis) (p. 72).
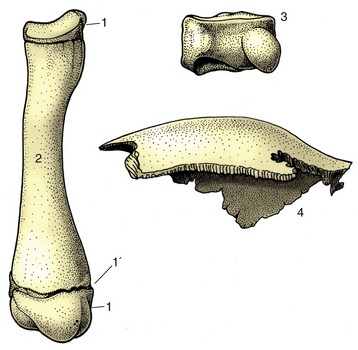
Figure 1–13 Long, short, and flat bones. 1, Proximal and distal epiphyses; 1′, epiphysial cartilage; 2, diaphysis of a young dog’s radius; 3, carpal bone of a horse; 4, parietal bone from the skull of a dog.
Short bones have no dimension that greatly exceeds the others. Many are grouped together at the carpus and tarsus, where the multiplication of articulations provides for complex movements and may also diminish concussion. The majority of short bones develop from a single center of ossification; replication of centers generally indicates that the bone represents the fusion of elements distinct in ancestral forms.
Flat bones are expanded in two directions. The category includes the scapula, the bones of the pelvic girdle, and many of those of the skull. Their broad surfaces afford attachment to large muscle masses and protection to underlying soft parts.
The remaining bones are too irregular in form to be grouped in clearly defined categories. Neither flat nor irregular bones exhibit uniformity in development.
The Organization of a Long Bone
Many features of bone construction are conveniently approached through the examination of a longitudinal section of a long bone (Figure 1–14, A). The form of the bone is determined by a sheath or cortex of solid (compact) bone that is composed of thin lamellae arranged mainly in series of concentric tubes about small central canals. Each such system is known as an osteone (Figure 1–14, B). The cortex is thick toward the middle of the shaft but thins as it flares toward each extremity, over which it continues as a crust. The external surface is smooth except where irregularities serve as the attachment sites for muscles or ligaments; these irregularities may be raised or depressed and, in both cases, permit a concentration of the attachment. These features are generally most pronounced in larger, older males. They are given a variety of descriptive names of conventional significance; most elevations are known as lines, crests, tubercles, tuberosities, or spines; most depressions, are known as fossae or grooves (sulci).
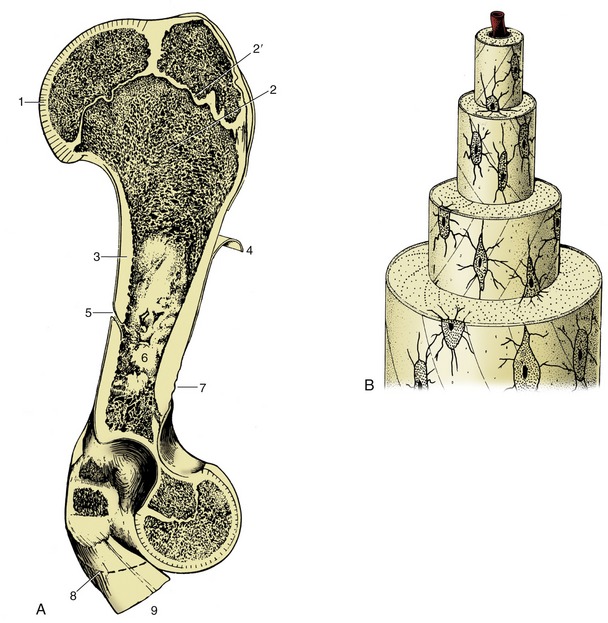
Figure 1–14 A, A long bone (bovine humerus) sectioned longitudinally. B, Osteone with central (haversian) canal. 1, Articular cartilage; 2, spongy bone; 2′, epiphysial cartilage; 3, compact bone; 4, periosteum, partly reflected; 5, nutrient foramen; 6, marrow cavity; 7, roughened area for attachment of muscle or ligament; 8, distal extent of medial epicondyle; 9, tendons of origin of carpal and digital flexors.
The inner surface of the shaft bounds a central medullary (marrow) cavity and is rough; the irregularities are low, indiscriminate, and without apparent significance.
The extremities are occupied by cancellous or spongy bone, which forms a three-dimensional lattice of interlacing spicules, plates, and tubes of varying density.
The medullary cavity and the interstitial spaces of the spongy bone are occupied by bone marrow, which occurs in two intergrading forms. Red bone marrow is a richly vascularized, gelatinous tissue with hemopoietic properties; it produces the red and granular white corpuscles of the blood. Although all marrow is of this type in the young animal, most is later infiltrated with fat and converted into waxy yellow marrow whose hemopoietic potential is dormant. It is the marrow in the larger spaces that first becomes inactive, then that of the spongy bone of the distal limb bones, until finally active marrow is confined to the proximal extremities of the humerus and femur, the bones of the limb girdles, and those of the axial skeleton. The chronology of these events for domestic animals is uncertain.
The parts that articulate with neighboring bones are smooth. These articular surfaces are more extensive than the areas in contact in any position of the joint and provide a range of movement. They are clothed in hyaline articular cartilage. The cartilage is not uniform in structure; it is calcified in its deepest layer, which is firmly attached to the underlying cortex, and becomes fibrous toward the periphery, where it blends with the periosteum and joint capsule.
A tough fibrous membrane, the periosteum, ensheathes the remainder of the outer surface, from which it can be readily stripped, except where it is penetrated by tendons and ligaments proceeding to anchor in the compacta. Its appearance is rather misleading because the deeper layer is cellular and, even in adults, retains the bone-forming capacity that it exercised during development (p. 72). This osteogenic function is reactivated in the healing of a fracture.
Bones have a generous blood supply, perhaps amounting to 5% to 10% of the cardiac output. Several sets of vessels exist; the so-called nutrient artery, though generally the largest single source, probably contributes less than do the others in the aggregate. The nutrient artery penetrates toward the middle of the shaft in a position that is fairly constant for each bone. It is usually directed toward one extremity, and the foramen through which it passes may simulate an oblique fracture when depicted in radiographs. The artery divides into two divergent branches within the marrow; these and the later divisions pursue very tortuous courses, which may have the purpose of reducing the pressure within the vessels of the delicate marrow (Figure 1–15). The smaller branches supply the sinusoids of the marrow tissue and also the arterioles and capillaries that permeate a system of tiny central channels (haversian canals) within the osteones of compact bone. A further supply to the cortex arises from the medullary sinusoids. Branches of the nutrient artery that reach the metaphysial region (the part of the shaft adjacent to the epiphysis) anastomose there with branches of metaphysial and epiphysial vessels that enter the bone toward its extremity. The central region of this part of the shaft probably relies mainly on the nutrient artery, whereas the peripheral part relies on metaphysial arteries. The anastomoses are of varying efficiency, but the collateral circulation is generally sufficient to allow a bone to survive deprivation of part of its usual supply when fractured. One technique (intramedullary pinning) employed in fracture repair is possibly even more damaging to the vessels than is the initial injury, and its success serves to emphasize the value of the anastomoses. Some authors have described an additional supply entering the cortex from numerous small periosteal arteries. The weight of opinion denies their presence in healthy young bones.

Figure 1–15 The blood supply of a long bone, schematic. The supply of the cortex is shown (enlarged) in the center. 1, Epiphysial arteries; 2, metaphysial arteries; 3, nutrient artery; 4, 4′, artery and vein of the bone marrow; 5, periosteal arteries; 5′, periosteal vein; 6, anastomosis between periosteal and bone marrow arteries; 7, capillaries of the cortex; 8, sinusoids in the bone marrow; 9, growth cartilage; 10, cortex.
The main drainage of the marrow is effected by large, thin-walled veins that accompany the major arteries and emerge through the nutrient, epiphysial, and metaphysial foramina. The capillaries within cortical tissue drain into venules within the periosteum. The normal cortical circulation is therefore centrifugal—from within outward. No lymphatic vessels are present within bone, although infections of bone may spread to the lymphatics that drain neighboring tissues.
One important difference is exhibited by the circulation in young growing bones. In these, the circulation within the epiphyses forms separate and independent compartments, as (with few exceptions) arteries do not penetrate the growth (epiphysial) cartilage.
Nerves accompany the larger vessels, and their branches are to be found within the central canals of the osteones. Some (vasomotor) fibers pass to the vessels, some are sensory to the bone tissues (especially the periosteum), and the destination of others remains unclear. It is no longer believed that nerves exert a trophic influence on bone.
Biomechanical Aspects
It has long been the convention to explain the tubular construction of long bones by drawing the comparison with a loaded beam of some stiff, homogeneous material supported at both ends (Figure 1–16). In this construction the tensile forces that tend to disrupt the material are concentrated toward the lower surface while the compressive forces that tend to crush and compact the material are concentrated toward the upper surface. These forces tend to neutralize each other along, and close to, the axis, and the material here is more or less redundant. It can be dispensed with or replaced by some weaker but lighter material, as in a long bone. The analogy is not exact—for a start, bone is a composite material—but it is useful as a first approach. The diagram (see Figure 1–16) shows that the lines of principal compressive and tensile stress intersect in orthogonal fashion toward the extremities of the model; the spongy architecture of a bone closely mimics the theoretical pattern. Indeed, the pattern of trabecular bone has been described as the crystallization of the lines of stress, which is an attractive if faulty metaphor. Because the more detailed analysis of the spongy architecture (Figure 1–17) introduces matters that are both complicated and controversial, it is probably wiser to leave discussion to the specialist.
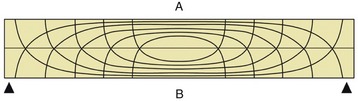
Figure 1–16 Pattern of compressive (A) and tensile (B) stress lines in a beam supported at both ends. The greatest stresses (closeness of lines) occur in the middle of the beam toward the surfaces.
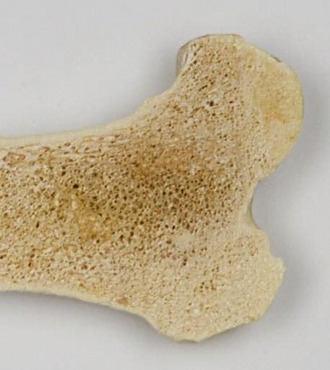
Figure 1–17 Proximal end of the humerus of a cow, sectioned sagittally, as an example of the architecture of spongy bone.
Compact bone is a plastic, composite material of considerable strength, capable of sustaining and recovering from considerable deformation. When bent, the lamellae and osteones of which compact bone is constructed first shear past each other; if bent too far, a crack appears at right angles to the line of shear and then quickly spreads to create a brittle fracture. Most fractures are caused by excessive bending, which stresses both aspects of the bone approximately equally. Since the side under tensile stress generally fails first, this indicates that compact bone is better able to resist compression. However, spongy bone is commonly crushed and impacted by compression.
Some Specialized Varieties of Bones
Bones are often found within tendons (rarely within ligaments) where they change direction over prominences that would expose them to excessive pressure and friction. These bones, known as sesamoid bones, form regular synovial joints with the major bones with which they are in contact. In addition to preventing tendon wear, a sesamoid bone displaces the tendon farther from the axis of the adjacent joint and so serves to increase the leverage exerted by the muscle. The best-known example is the patella (kneecap) that forms in the principal muscle that extends the stifle joint (the name given to the knee of quadrupeds) (see Figures 2–63 and 17–3). In the dog, smaller sesamoids also develop in muscles behind the stifle, in the tendons passing behind the metacarpophalangeal joints (at the bases of the digits), and in the extensor tendons within the digits (see Figure 1–9). The chief practical importance of these and other lesser sesamoids lies in the risk of their being wrongly identified as chip fractures when they are depicted in radiographs. In large animals, one or more additional sesamoids form dorsal to the deep flexor tendon shortly before its insertion on the distal phalanx (or phalanges). In the dog the reaction is limited to the development of a nubbin of cartilage in each branch of the tendon.
Although sesamoids are a device to protect tendons from injury, the major sesamoids develop in the embryo before movement is possible, and their origin must therefore be genetically determined. They do not reform after extirpation when the limb is immobilized but only if movement is allowed; this indicates that they can also develop in reaction to an appropriate stimulus in the lifetime of the animal.
Splanchnic bones develop in soft organs, remote from the rest of the skeleton. The most familiar, indeed the only significant, examples in veterinary anatomy are the os penis (and the female equivalent, os clitoridis) of the dog and cat and the ossa cordis found in the heart, especially in the hearts of ruminants.
Certain bones are excavated to contain air spaces. In mammals, these pneumatic bones are confined to the skull and contain the paranasal sinuses, which communicate with the nasal cavities. The sinuses principally develop after birth, when outgrowths of the nasal mucosa invade certain skull bones and replace the diploë, the spongy bone between the outer and inner layers (“tables”) of compacta. The separation of the tables can be very considerable and can lead to a remarkable postnatal remodeling of the skull, best exhibited by cattle and pigs. The postcranial skeleton of birds develops an extensive system of air-filled cavities in communication with the respiratory organs.
JOINTS
Bones meet each other at joints or articulations, some of which are designed to unite the bones firmly and others of which are designed to allow free movement. Because of this and because of differences in development, enormous variation in joint structure exists, which makes it extremely difficult to devise a suitable classification. Periodic revisions of terminology have seen new categories defined and former categories merged or renamed so that some confusion now exists and many superfluous terms circulate. The current official system recognizes three major categories, namely, fibrous joints, in which the bones are united by dense connective tissues; cartilaginous joints, in which the bones are united by cartilage; and synovial joints, in which a fluid-filled cavity intervenes between the bones. It is obvious that most joints of the first and second categories must be relatively immovable or even rigid; these classes were formerly together known as synarthroses. In contrast, most joints of the third category are freely movable; they were formerly termed diarthroses. Both of these terms, although obsolete, are likely to be encountered.
Fibrous Joints
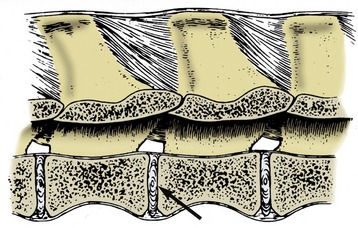
Most fibrous joints occur in the skull and are known as sutures (Figure 1–18). The narrow strips of fibrous tissue that outline and unite the margins of the bones represent the surviving part of the originally continuous membrane in which the separate ossification centers appeared. Sutures play an important role in the young animal, allowing for the growth of the skull through the extension of individual bones at their margins while proliferation of the membrane continues. Sutures are gradually eliminated when ossification extends across the membrane after it has ceased to grow. This is a slow and uneven process that is not complete even in the aged. The gradual modification of the sutural pattern is used in anthropology and forensic medicine as a guide, though not a very reliable one, to the age of the individual. Although movement between the bones of the adult skull is neither required nor allowed, the wider sutures of the fetal skull allow some useful passive deformation during birth in some species, including primates.
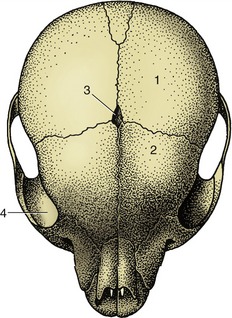
Figure 1–18 Sutures between the bones of a puppy’s skull. 1, Parietal bone; 2, frontal bone; 3, fontanelle (fonticulus); 4, orbit.
The other fibrous joints are known as syndesmoses. In these, facing areas of two bones are joined by connective tissue ligaments. In some syndesmoses, relatively broad areas of bone are united by short ligaments, and movement is inevitably very limited; examples are the joints between the major and minor bones of the horse’s metacarpus. In others the ligaments are longer and their attachments narrower so that more appreciable movement is possible; an example is the joint between the shafts of the radius and ulna in the forearm of the dog.
The attachment of a tooth to the bone of its socket may be included among the fibrous joints under the name gomphosis.
Cartilaginous Joints
Most cartilaginous joints are known as synchondroses. These include the joints between the epiphyses and diaphyses of juvenile long bones and the corresponding joints of the base of the skull. Most are temporary and disappear after growth has ceased, when the cartilage is replaced by bone. The few permanent synchondroses include the joint between the skull and hyoid apparatus (p. 65), which allows appreciable movement in some species.
In the more complicated symphysis the articulating bones are divided by a succession of tissues; usually cartilage covers the bones with fibrocartilage or fibrous tissue in the middle. The category includes the joints between the symmetrical halves of the mandible (in species such as the dog, cat, and ruminants, in which fusion is not complete) and of the pelvic girdle and the joints between the bodies of successive vertebrae (Figure 1–19). Each of these joints presents its own, sometimes specifically variable, features that are best considered later.
Synovial Joints
Structure
In synovial joints the articulating bones are separated by a fluid-filled space, the joint cavity (Figure 1–20). The boundaries of the space are completed by a sleeve of delicate connective tissue, the synovial membrane. This is attached around the periphery of the articular surfaces, which are clothed with thin layers of cartilage. No other essential features exist. However, in most synovial joints the synovial membrane is strengthened externally by a fibrous capsule, and additional fibrous bands (ligaments) are strategically placed to join the bones and to restrict movement to the required directions and extents. Each of these components is described in the detail made necessary by the prevalence of joint injuries and pathology in domestic animals. It may be stated with confidence that no branch of anatomy better rewards study.
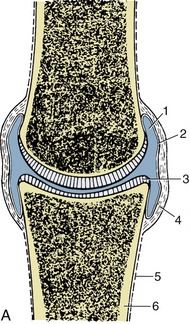

Figure 1–20 A, A synovial joint in section. B, Scanning electron micrograph of villi projecting from the synovial membrane of the equine fetlock joint; greatly enlarged. 1, Joint cavity; 2, synovial membrane; 3, articular cartilage; 4, fibrous layer of joint capsule; 5, periosteum; 6, compact bone.
The articular surface is clothed with articular cartilage that is generally of the hyaline variety, although fibrocartilage or even dense fibrous tissue is substituted in a few locations. The cartilage is only about a millimeter thick in the joints of the dog but may be several millimeters thick in the larger joints of horses and cattle. It accentuates the curvature of the underlying bone, being thickest in the center of convex surfaces and about the periphery of concave ones. It is a pliant material that is translucent and glassy in appearance and, while generally white with a blue or pink tinge in young animals, it becomes yellowish with age, a change indicating a loss of elasticity. The surface is smooth to the touch and to the naked eye but quite irregular when seen at low magnification.
The cartilage has a complex structure in which fine fibers within its matrix pass from the underlying bone to the surface, where they bend to lie closely together. Because splitting of the cartilage, common in joint disease, tends to follow the fiber course, superficial lesions lead to tangential flaking, whereas those that extend more deeply create more or less vertical cracks.
Articular cartilage is insensitive and avascular. The insensitivity explains why joint lesions may progress far before the patient becomes aware of their existence. The oxygen and nutritive requirements are met by diffusion from three sources: fluid within the joint cavity, vessels in the tissues at the periphery of the cartilage, and vessels in the subjacent marrow spaces. Diffusion is assisted by the porosity of the cartilage matrix, which soaks up and releases fluid as the cartilage is alternately unloaded and compressed during movements of the joint.
Certain large articular cartilages are interrupted by depressed areas that may indent the periphery or appear as islands. These naked areas (synovial fossae) are clothed by a thin connective tissue resting on the underlying bone; they are sometimes interpreted by the unwary as pathological lesions. Their significance is disputed, but the constancy of their occurrence as well as frequent coincidence in opposing bones in certain positions of the joint has led to the speculation that they assist in spreading synovia.*
The synovial membrane, which completes the lining of the joint, is a glistening pink connective tissue sheet. It may be left entirely unsupported, may rest directly on a tough outer fibrous capsule, or may be separated from this by the interposition of pads of fat; all three arrangements may occur in different regions of the same joint. The membrane may pouch where it is unsupported, and these diverticula may extend quite far, a point of potential significance because it explains how joints may be entered by apparently remote wounds. The inner surface of the membrane carries many projections of various sizes and degrees of permanency, which greatly increase its surface area (see Figure 1–20, B). Unlike mucous membranes, the synovial membrane has no continuous covering of cells; the more cellular parts, limited to relatively protected situations, are responsible for the production of the lubricant component (aminoglycans) of the synovial fluid. The other components are derived from the blood plasma. The membrane is both vascular and sensitive.
Synovia, the fluid within the cavity, obtains its name from its resemblance to egg white. It is a viscous, glairy fluid, whose color ranges from pale straw to medium brown. It is usually said to be present in very small amounts but is, in fact, quite copious in the larger joints; as much as 20 to 40 mL can sometimes be aspirated from limb joints of horses and cattle. The quantity is greatest in animals permitted free exercise.
Synovia has both lubricant and nutritive functions. The ways in which it acts as a lubricant are disputed, but it is certainly very efficient, the friction being such that virtually no wear occurs in healthy joints. The fluid helps to nourish the articular cartilage, any intraarticular structures, and, possibly, the surface layer of the synovial membrane itself.
An outer fibrous layer usually completes the capsule. It attaches around the margins of the articular surfaces and presents local thickenings, which are named individually as ligaments when well developed and discrete. Some, of which the cruciate ligaments of the stifle are good examples, appear to run within the joint cavity from bone to bone. Such ligaments are sometimes designated intracapsular to distinguish them from the majority in peripheral and clearly extracapsular positions; however, they are actually excluded from the cavity by a covering of synovial membrane (Figure 1–21). The fibrous layer and ligaments are supplied with proprioceptive nerve endings that register the position and the rate of change in position of the joint; other receptors register pain.

Figure 1–21 Cranial view of left stifle joint of the dog, resected to show intracapsular (1, 2) and extracapsular (6, 8) ligaments. 1, Cranial cruciate ligament; 2, caudal cruciate ligament; 3, medial meniscus; 4, lateral meniscus; 5, tendon of origin of long digital extensor; 6, lateral collateral ligament; 7, patellar ligament; 8, medial collateral ligament; 9, medial condyle, partly removed.
A few joints possess disks or menisci that are truly intracapsular (Figure 1–22, A-B). A disk, such as occurs in the temporomandibular joint formed between the mandible and the skull, fuses with the synovial membrane around its periphery and so divides the cavity into upper and lower compartments. Paired menisci, which are semilunar as the name suggests, are found within the stifle joint. They are attached only around their convex borders and therefore divide the cavity incompletely. Both of these structures are composed of hyaline cartilage, fibrocartilage, and fibrous tissue in proportions that vary with the part, the species, and the age. Menisci and disks provide congruence of incompatible articulating surfaces, but this can hardly explain their presence because congruence is achieved at other joints more simply. The most probable alternative explanation is that they are a means of resolving complicated movements into simpler components that are assigned to different levels of the articulation. Thus, in the temporomandibular joint the hinge movement involved in opening the mouth occurs at the lower level (between the disk and the mandible), while the translatory movements that protrude, retract, or slide the lower jaw sideways occur at the upper level (between the disk and the skull).

Figure 1–22 A, Synovial joint with articular disk. B, Synovial joint with meniscus. 1, Compact bone; 2, periosteum; 3, fibrous layer of joint capsule; 4, synovial membrane; 5, articular disk; 6, meniscus; 7, joint cavity.
An articular labrum is a fibrocartilaginous lip or rim placed around the circumference of certain concave articular surfaces, including the acetabulum (the deep socket at the hip). A labrum serves to extend and deepen the articular surface, increasing the load-bearing area and helping to spread the synovial fluid. Because a labrum is deformable, it allows the surface to adapt to disparities in the curvature of the bone with which it comes in contact.
Synovial pads or cushions are formed where fat masses are included between the synovial and fibrous layers of the joint capsule. They are sometimes interpreted as swabs that spread the synovia over the surface, but their main purpose is to allow the synovial membrane to accommodate its shape to the part of the bone with which it is momentarily in contact.
Movements
Although many joint movements appear to be complicated, they can always be resolved into simple components. Moreover, many activities are the result of coordinated movement at several neighboring joints; the sum of changes can be considerable even when the movement at each individual joint is modest.
The simplest type of movement is described as translation. In its pure form, translation consists of one flat surface sliding over another while the bodies to which the surfaces belong maintain their original orientation. True translatory movements probably never occur because the prerequisites are perfectly flat surfaces and the absence of spin. Nonetheless, a category of joint (plane joint) is defined in which movement is supposed to be of this kind. These joints have small articular surfaces that appear flat at first scrutiny; in reality, articular surfaces are always curved.
All other movements involve angular change. In some, the moving bone turns (spins) about an axis perpendicular to its articular surface, which is a movement described as rotation. Rotation can always be reversed, and it is therefore necessary to specify its direction. According to convention, an internal rotation of a limb carries the cranial surface medially (Figure 1–23/4); an external rotation carries this surface laterally (see Figure 1-23/5).
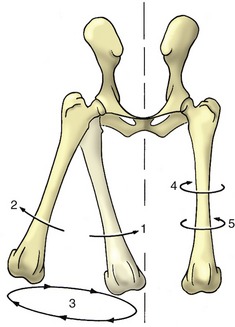
Figure 1–23 Limb movements illustrated by the femurs of the dog, cranial view. 1, Adduction; 2, abduction; 3, circumduction; 4, inward rotation; 5, outward rotation.
Other movements involve the moving bone turning about an axis parallel to its articular surface in a pendular or rolling movement (Figure 1–24/3); this is a slide between curved surfaces and may be described as a swing. Most swings are accompanied by some rotation, although this often goes undetected.
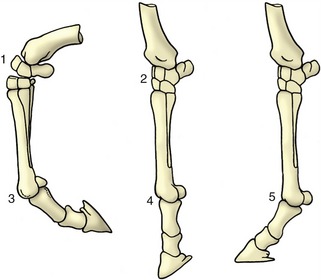
Figure 1–24 Flexion, extension, and overextension illustrated by the distal part of the horse’s forelimb. 1, Flexed carpal joint; 2, extended carpal joint; 3, flexed fetlock joint; 4, extended fetlock joint; 5, overextended fetlock joint.
Pendular movements in sagittal planes predominate in the joints of the limbs and are known as flexion and extension. Flexion reduces the angle between the two segments of the limb. The opposite movement of extension opens the angle and brings the two segments more closely into alignment (see Figure 1–24). However, the movement at some joints ranges from one flexed position through full extension (180°) to a second flexed position at the other limit. The fetlock joint of the horse is a good example of a joint with such a wide range of movement. In such cases the two terminal positions may be distinguished as overextension (or dorsal flexion), the posture of the animal standing at rest, and (palmar) flexion, the posture when the foot is passively raised. Figure 1–24 may make this rather confusing distinction plain.
Adduction and abduction are pendular movements in transverse planes (see Figure 1–23/1,2). Adduction carries the moving part toward the median plane, and abduction carries it farther from this plane. When applied to the digits, adduction and abduction describe movement with reference to the axis of the limb and indicate the convergence or the spread of the digits, respectively.
The combination of flexion and extension and adduction and abduction allows the extremity of the limb to describe a circle or ellipse, which is a movement known as circumduction.
Limitations are placed on the movements of all joints. Several potentially limiting factors exist, and it is not easy to determine their relative importance. The shape of the articular surfaces is obviously relevant. A degree of incongruence is required to maintain a wedge of the lubricant synovia between the surfaces. This wedge is reduced when the radius of curvature of the convex surface increases toward its margin to approximate to the radius of curvature of the opposing concave surface. The surfaces thus become congruent in the closely packed terminal position, and further movement is checked by their being squeezed together.
Tension in extracapsular ligaments can certainly arrest movement, although it is uncertain whether this method of braking is required in normal circumstances. Some ligaments appear to be moderately taut throughout the normal range of movement, whereas others are generally slack and become taut only when movement threatens to go beyond the normal limit.
In some situations, contact between extraarticular structures may be of importance; the olecranon obviously prevents forceful overextension of the elbow, and apposition of the caudal muscles of the thigh and calf prevents overflexion of the human knee. Tension in muscles and other soft structures in the neighborhood of a joint may first decelerate and then arrest movement; inability of the muscles of the caudal aspect of the human thigh to stretch beyond a certain limit—passive insufficiency—prohibits many people from touching their toes. The contraction of muscles that oppose a given movement may be the most important factor; its significance is discussed in the following section.
Classification
Synovial joints may be classified according to numerical and geometrical criteria. The numerical system distinguishes simple joints with one pair of articular surfaces and composite joints in which more than two opposing surfaces are involved and in which movement occurs at more than one level within a shared capsule. The shoulder joint illustrates the first and the carpal joint the second variety.
There are seven categories in the current version of the geometrical system. One, the plane joint (Figure 1–25, A), has already been mentioned (p. 20).
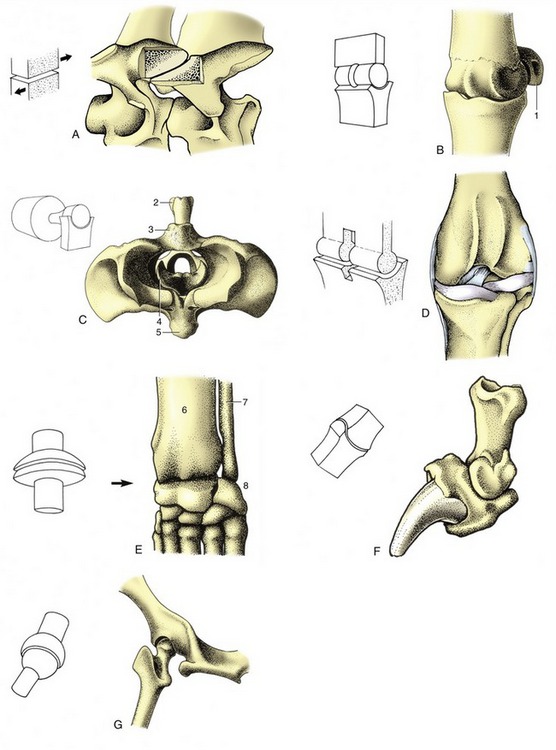
Figure 1–25 The seven types of synovial joints, with examples. A, Plane joint: articular processes of equine cervical vertebrae. B, Hinge joint: equine fetlock (metacarpophalangeal) joint. C, Pivot joint: bovine atlantoaxial joint (cranial view). D, Condylar joint: canine femorotibial joint (stifle). E, Ellipsoidal joint: canine carpus. F, Saddle joint: canine distal interphalangeal joint. G, Spheroidal joint: canine hip joint (caudodorsal view). 1, Proximal sesamoid bone; 2, spine of axis; 3, dorsal arch of atlas; 4, dens of axis; 5, ventral arch of atlas; 6, radius; 7, ulna; 8, proximal row of carpal bones.
The hinge joint (ginglymus; Figure 1–25, B) has one articular surface shaped like a segment of a cylinder and the other excavated to receive it. Pendular movement is possible in one plane only; prohibition of other movements may be reinforced by stout collateral (one to each side) ligaments and possibly by the development of matching ridges and grooves on the articular surfaces. The elbow joint between the humerus and bones of the forearm is an example.
The pivot joint (articulatio trochoidea; Figure 1–25, C) comprises a peg fitted within a ring. Movement takes place about the long axis of the peg. In some joints (e.g., the proximal radioulnar joint) the peg rotates within the fixed ring; in others (e.g., the atlantoaxial joint between the first two vertebrae) the ring rotates about the fixed peg.
The condylar joint (Figure 1–25, D) is formed by two knuckle-shaped condyles that engage with corresponding concave surfaces. The two complexes may be close together, as in the femorotibial joint, or widely separate and provided with independent joint capsules, as are the twin articulations of the mandible. In each case the whole arrangement is regarded as constituting a single condylar joint. Movement is primarily uniaxial, about a transverse axis common to the two condyles; certain amounts of rotation and slide are also permitted.
The ellipsoidal joint (Figure 1–25, E) presents an ovoid convex surface that fits into a corresponding concavity. Movements are principally in two planes at right angles to each other (flexion–extension; adduction–abduction), but a small amount of rotation may be possible. The radiocarpal joint of the dog is of this variety.
The saddle joint (articulatio sellaris; Figure 1–25, F) combines two surfaces, each maximally convex in one direction and maximally concave in a second direction at right angles to the first. These are also biaxial joints, allowing flexion–extension and adduction–abduction but with a certain amount of rotation permitted or imposed by the geometry of the surfaces. An example is the distal interphalangeal joint of the dog.
The ball-and-socket or spheroidal joint (Figure 1–25, G) consists of a portion of a sphere received within a corresponding cup. This multiaxial joint enjoys the greatest versatility of movement. The hip joint is the best example; the human shoulder joint also conforms closely to the pattern, but the shoulder of domestic species largely restricts its movement to flexion and extension.
It must be emphasized that anatomical joints correspond very imperfectly to the theoretical models. Sometimes, the departure from the ideal can be sufficiently large to make it a matter of controversy over which category best accommodates a particular articulation.
MUSCLES
Most movements of the animal body and its parts are caused by muscular contraction. The exceptions are those caused by gravity or other external forces and those, trivial in magnitude although not in importance, produced at the cellular level by cilia and flagella. Muscle is also used to prevent movement, stabilizing joints to prevent their collapse under a load and maintaining continence of bladder and bowel. A subsidiary function of the skeletal muscles is to generate heat by shivering, involuntary tremors initiated by exposure to cold.
There are three varieties of muscle tissue, but two, the specialized (cardiac) muscle that forms the bulk of the heart and the smooth (visceral) muscle of the blood vessels and viscera (internal organs), are not of present concern. The third variety is generally known as skeletal muscle because it is organized into units that are mostly attached to the bones and used to effect their movements. Skeletal muscle is also known as striated, somatic, or voluntary muscle, but these terms are less acceptable for one reason or another.
The Organization of Skeletal Muscles
Skeletal muscle is butcher’s meat and accounts for about half the weight of an animal carcass (the proportion varies with species, breed, age, sex, and method of husbandry). Each individual muscle is composed of many cells held together by connective tissue. When compared with the common run of cell, these muscle cells are giants, varying from about 10 to 100 µm in diameter and being about 5 or 10 cm in length (some are probably much longer). They are visible to the naked eye when teased apart and are also called muscle fibers because of their size and shape. The whole muscle is covered by a dense connective tissue sheet, the epimysium (Figure 1–26); below this, a looser layer, the perimysium, covers the small bundles (fasciculi) into which the fibers are grouped. Finally, each fiber is provided with its own delicate covering, the endomysium. These connective tissue components merge at each end of the muscle “belly” and continue as the tendons by which the muscle makes its attachment. The amount and quality of the connective tissue partly explain variations in the appearance and in the cooking and table qualities of different “cuts” of meat (another important factor is the degree of shortening that is allowed by hanging during postmortem rigor). The consumer is willing to pay more for some cuts than for others; much effort has been devoted to breeding animals in which the more desirable muscle groups form a larger part of the carcass but has met with limited success (except in a few breeds, e.g., Charolais and Belgian Blue, in which the natural tendency is toward muscular hypertrophy).
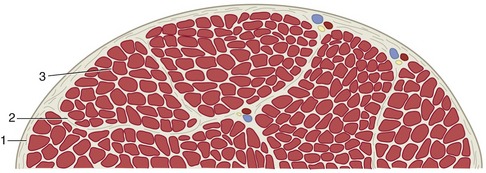
Figure 1–26 Transection of a skeletal muscle; the fibrous tissue has been emphasized. 1, Epimysium; 2, perimysium; 3, endomysium.
Variations in Muscle Architecture
The way in which the muscle fibers are arranged within the muscle belly varies greatly, which can be explained by reference to two principles. The shortening (about 50%) that a muscle may demonstrate on contraction is a function of the length of the component fibers. The power that it may develop is a function of the aggregate of their cross-sectional area. The greatest displacement is therefore produced by the so-called strap muscle (Figure 1–27), in which the fibers run parallel to the long axis and throughout the length of the muscle, which is completed by very short tendons of attachment.
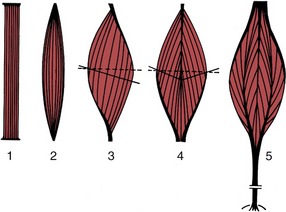
Figure 1–27 Architecture of skeletal muscles. The broken lines represent the “anatomical,” the solid lines the “physiological” transverse sections. 1, Strap muscle; 2, spindle-shaped muscle; 3, pennate muscle; 4, bipennate muscle; 5, multipennate muscle.
Muscles in which the fibers join the tendons at an angle tend to be strong in relation to their bulk because more fibers and a greater total cross section can be accommodated. Although muscles of this sort are powerful, they waste a proportion of their strength and their potential for displacement; only part, corresponding to the cosine of the angle of fiber insertion, is applied along the line of pull. In calculating the power that such muscles develop, one needs to replace the simple “anatomical” cross section by the “physiological” cross section, which is the complex plane that divides the muscle in such a way that each component fiber is cut transversely. Muscles with angled fibers can be arranged in several categories of increasing complexity of construction: pennate, bipennate, circumpennate, and multipennate (see Figure 1–27).
Many limb muscles have a pennate form and, unlike the strap muscles, are provided with long, cordlike tendons that permit the heavy bellies to be placed close to the trunk; because only the light tendons extend to the digits to operate the joints, less energy is required to swing the limb to and fro. Certain muscles of the body wall form thin flat layers that are continued by broad tendon sheets (distinguished as aponeuroses), an arrangement clearly adapted to supporting the abdominal organs. Other muscles arise by two, three, or four separate heads that join in a common tendon; these arrangements are indicated by inclusion of the descriptive terms biceps (two-headed), triceps, or quadriceps in the muscles’ names.
In another less common variety, two or more fleshy units are separated by intermediate tendon-forming digastric (two-bellied) or polygastric units. Still other muscles are arranged in rings that surround natural orifices, such as the mouth or anus, and act as sphincters to constrict or close the opening. In all these examples the construction of the muscle is clearly adapted to the functions that it is called on to perform.
Paired muscles lying against, or originating from, the midline are separated by a connective tissue strip known as a raphe.*
Muscles also vary in appearance according to color, which reflects the amount of myoglobin (a pigment related to hemoglobin) within their constituent fibers. The difference, well exemplified by the pale breast and dark leg muscles of the chicken, is generally regarded as reflecting a pale muscle’s adaptation for rapid contraction over a short period and a darker muscle’s adaptation for slower but sustained activity; the correlation does not always pertain. Most muscles are actually composed of two fiber types in varying proportion: fast twitch fibers that rely on glycolytic metabolism predominate in dark (red) muscles and slow twitch fibers that obtain their energy from aerobic metabolism predominate in pale (white) muscles. There are many other structural and physiological differences between fibers, and the suggestion that there are only these two, sharply distinguished varieties, although convenient, is a dangerous simplification.
Tendons
Muscles always attach by means of connective tissue tendons; when these are so short that they almost evade notice, muscles are loosely said to have direct attachments. Tendons consist almost entirely of collagen bundles in regular arrangement, and they possess great tensile strength. Indeed, excessive tension is more likely to rupture the muscle belly or to detach a fragment of bone at the insertion than to disrupt the tendon itself. Tendons are also more elastic than is commonly supposed and are capable of absorbing and storing energy when stretched. It is not always sufficiently appreciated that the elastic recoil of tendons makes a substantial contribution to locomotion and that a good fraction of the metabolic work performed by many muscles is devoted to stretching tendons so that the stored energy can later be released.
Although they are tough, tendons may be damaged by excessive pressure or friction, particularly when they change direction over bony prominences or are shifted over hard tissues. One form of the protection that they develop in such places, local chondrification or ossification (sesamoid bones), has been mentioned (p. 16). An alternative is provided by the development of fluid-filled cushions at the danger sites. If only one aspect of the tendon is at risk, a bag (bursa synovialis) may be interposed on that side (Figure 1–28, A); if a greater part of the circumference is vulnerable, the cushion wraps around the tendon, enclosing it within a tendon sheath (vagina synovialis; Figure 1–28, B). The walls of these bursae and sheaths and the fluid they contain resemble the similar components of synovial joints. When the tendon moves, it is the lubricated synovial layers that rub together.
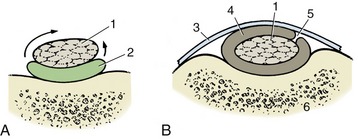
Figure 1–28 Sections of a synovial bursa (A) and a tendon sheath (B). The bursa permits frictionless movement of a tendon (1) over bone, and the sheath permits movement of a tendon over bone and under a retinaculum. The arrows show that a tendon sheath may be regarded as a large bursa that has wrapped around a tendon. 1, Tendon; 2, bursa; 3, retinaculum; 4, tendon sheath; 5, mesotendon, through which blood vessels reach the tendon; 6, bone.
Inflammation of synovial bursae and sheaths is common, and it is necessary to know their positions and extents; however, this is not difficult because they occur precisely where they can be seen to be required.
Blood and Nerve Supply of Muscles
Muscles receive a relatively generous blood supply from neighboring arteries. Sometimes, a single artery enters the muscle belly, and then the well-being of the muscle clearly depends on the integrity of that artery. Often, two or more arteries enter separately, which would appear to be a safer arrangement because the arteries form connections within the flesh. Unfortunately, these connections (anastomoses) are not always sufficient to allow the muscle to survive unscathed an interruption to one of its sources of supply. The intramuscular arteries ramify within the perimysium to open into capillaries that follow the endomysial sheaths of individual fibers.
The veins are satellite to the arteries. Normal activity, when only a fraction of the muscle fibers contract, probably promotes the circulation within the muscle by massaging the capillaries and smaller veins. Mass contractions squeeze these vessels from all sides, stopping the circulation, and are likely to be harmful if sustained.
Tendons have low metabolic needs, are poorly vascularized, and do not hemorrhage when cut. This feature, initially an apparent advantage, has its adverse side: damaged tendons are inevitably slow to heal. Lymphatic vessels are found within the larger connective tissue tracts of the muscle belly.
Most muscles are supplied by a single nerve, but those of the trunk that are formed from several somites (p. 32) retain multiple innervation. The nerve that enters a muscle, generally in company with the principal vessels, ramifies within the connective tissue septa. It consists of fibers of several types: large alpha motor fibers supply the muscle fibers of the main mass; smaller gamma motor fibers supply modified muscle cells within the muscle spindles buried in the muscle; nonmyelinated vasomotor fibers supply blood vessels; and sensory fibers supply the spindles, tendon organs, and other receptors. The ratio of motor to sensory fibers varies considerably and is one among many complications in the determination of motor unit size.
The motor neurons that supply a particular muscle are roughly grouped within the ventral horns of gray matter in the spinal cord (or within motor nuclei of the brainstem). The axon from each neuron branches repeatedly in its passage, both within the nerve trunk and within the intermuscular septa, and ultimately ends in the motor end plates of several or many muscle fibers. The single neuron, as well as the (alpha) fibers it supplies, is known as the motor unit, an important concept, as it is the physiological unit of muscular contraction. It is these groups and not individual fibers that are called up or discharged from service when a muscle varies the force of its contraction. The muscle fibers belonging to a unit are intermingled with those of other units and do not correspond with any readily identifiable portion of the muscle—they do not correspond with the fasciculi, as one might suppose. The fibers constituting a motor unit are invariably of a uniform type.
In the human species, the number of fibers within a unit varies from about 5 to 10 in the muscles that move the eyeball, around 200 in the muscles of the fingers, and around 2000 in the muscles of the limbs. The exact figures are not important, but the trend is: the muscles with the smallest units are those capable of the most delicate adjustment. Motor unit size is determined from the innervation ratio, the ratio between the number of fibers within a muscle and the number of motor neurons that supply it.
Muscle Actions
When a muscle is activated, its fibers attempt to shorten. When shortening occurs, the tension in the muscle may increase, stay the same, or decrease, according to circumstances. When external forces prevent the muscle from shortening, the tension within it increases; such activity is said to be isometric.
The usual activity of most muscles involves changes in the angle of the joint(s) bridged by that muscle. The musculoskeletal system thus operates as a system of levers in which the joints act as fulcra. The mechanical advantages of the arrangement depend on the positions (relative to the fulcrum) of the muscle attachment and the application of the load (Figure 1–29). Although a muscle attaching close to a fulcrum is less powerful than a comparable muscle attaching at a greater distance, it produces its effect more rapidly; the requirements of speed and power thus conflict. When several muscles are available to move a joint in a particular way, the attachments of some make them more suited to getting the movement started, whereas the attachments of others make them more suited to carrying the movement through to completion.
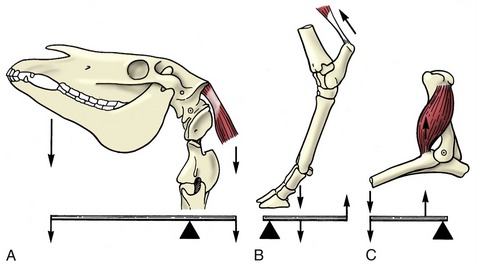
Figure 1–29 The action of muscles on the skeleton can be compared to different lever systems. A, Support of the head by dorsal neck muscles. B, Extension of the hock joint. C, Flexion of the elbow joint.
Biarticular or polyarticular muscles (those that cross two or several joints) may be incapable of shortening sufficiently to produce the full range of movement at both or all of the relevant joints at the same time. Such muscles are said to be actively insufficient.
Any muscle that produces a certain effect may be termed an agonist or prime mover; a muscle capable of actively opposing that movement is termed an antagonist. Clearly, these terms have force only in relation to a specified movement. Thus, in flexion of the elbow, the brachialis that produces the movement is agonist, and the triceps brachii that opposes the movement is antagonist; in extension of the same joint, however, the triceps is agonist, and the brachialis antagonist. Other muscles may neither facilitate nor directly oppose a movement but may modify the action of the agonist, perhaps by eliminating an unwanted side effect. Such muscles are known as synergists. When muscles are employed to stabilize joints rather than to promote their movement, they are known as fixators. Fixation or stabilization of a joint often involves the co-contraction of muscles that oppose each other when the joint is moved.
The terms origin and insertion have been left undefined until now. They are best regarded as having purely conventional meaning: origin denotes the more proximal or central attachment, and insertion the more distal or peripheral attachment. Although it is true that in their common employment most muscles draw the insertion toward the origin, the vast majority are able to shorten toward either extremity. Which attachment will maintain its position and which will be drawn toward the other depend on external circumstances. These circumstances must always be taken into account when considering the possible actions of a muscle.
Deductions about muscle action may be made from consideration of the attachments in relation to the axis (axes) of the joint(s). If these deductions are sound, they indicate what a muscle can do but not how it is habitually used in life. Direct stimulation of a muscle or of its nerve shows what that muscle can do when it acts alone. It does not show how it is used naturally, for often, several alternative muscles are available to perform a given movement but not all are normally used.
The most elegant technique for studying muscle actions is electromyography, the registration of the electrical activity that accompanies muscular contraction. Electrodes are placed over or inserted into the muscles to time the activity and crudely quantify its intensity (Figure 1–30). The use of this technique has upset many long-held beliefs concerning the actions and use of the muscles of humans; much remains to be investigated where domestic animals are concerned. Even this method demands that caution be used. It shows when a muscle is active but leaves the experimenter to interpret the activity as agonistic, antagonistic, or a mere adjustment to the alteration in joint angle brought about by other forces.
PERIPHERAL BLOOD VESSELS
The peripheral blood vessels comprise arteries that lead blood from the heart, veins that return blood to the heart, and capillaries that are the minute connections between the smallest arteries and the smallest veins within the tissues. These vessels are arranged to form two circuits (Figure 1–31). One, the greater or systemic circulation, arises from the left ventricle, conveys oxygenated (arterial) blood to all organs and parts of the body other than the exchange tissue of the lungs, and then transports the now deoxygenated (venous) blood back to the right atrium; the second, the lesser or pulmonary circulation, conveys deoxygenated blood from the right ventricle to the exchange tissue of the lungs, where it is reoxygenated before being returned to the left atrium by a special set of veins. The systemic and pulmonary circulations together with the chambers of the heart form a single complex course through which the blood circulates endlessly.
Figure 1–31 Schema of the circulation; vessels carrying oxygenated blood are shown in red, those carrying deoxygenated blood in blue. Systemic circulation: 1, Left side of the heart; 2, vessels in the cranial part of the body; 3, aorta; 4, liver; 5, intestines; 6, portal vein; 7, kidneys; 8, vessels in the caudal part of the body; 9, caudal vena cava; 10, cranial vena cava. Pulmonary circulation: a, Right side of the heart; b, pulmonary artery; c, lung; d, pulmonary vein.
Arteries
In the dissection room, the arteries may be distinguished from other vessels by their white, thick, and relatively rigid walls and their empty lumina (unless filled with an injection mass for the convenience of the dissector). The larger arteries follow a rather constant pattern, but their smaller branches show much variation—so much so that some patterns described in the textbooks, though the most common, may actually occur in only a minority of subjects. When arteries branch, the combined cross-sectional area of the daughter vessels always exceeds the cross section of the parent trunk (Figure 1–32).
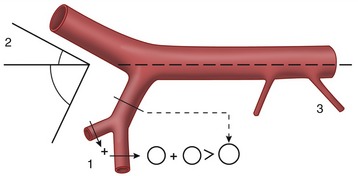
Figure 1–32 The branching of the arteries. Note that (1) the sum of the cross-sectional areas of the branches always exceeds that of the parent trunk; (2) large branches leave the trunk at more acute angles than smaller branches; and (3) the smallest branches leave erratically.
A general correspondence exists between the absolute and relative sizes of parent and daughter vessels and the angles at which the latter diverge from the main trunk. Although there are exceptions, larger branches diverge at more acute angles to minimize resistance. Hemodynamic factors are less important where small branches are concerned, and these often follow the shortest routes to their destinations (see Figure 1–32).
Another factor influencing arterial course is a preference for protected situations; this is well illustrated in the limbs, where the major vessels tend to run medially and also tend to reorient themselves to cross the flexor aspects of successive joints. In comparable fashion, arteries that supply organs that change much in size or position are protected against stretching by taking meandering courses.
Although arteries ultimately discharge into capillary beds, most also have more proximal and more substantial connections with their neighbors. These interarterial connections (anastomoses) provide alternative, collateral pathways or bypasses by which circulation can be maintained when the more direct route is blocked. Collateral circulation operates as soon as a main trunk is obstructed and becomes more efficient with the passage of time.
The possibility for collateral circulation in different regions and organs has obvious importance to the clinician and the pathologist, and more attention is given to this topic later (p. 242). Meanwhile, this possibility suggests that it may be unnecessary to know the details of all the smaller vessels.
Veins
In the dissection room, veins are distinguished by their thinner walls, their frequently collapsed appearance, and their capacity, which is invariably greater than that of the associated arteries. They appear blue when filled with clotted blood. Most veins are also distinguished by the presence of valves, which are repeated at intervals along their length; the valves ensure a unidirectional flow and prevent reflux of blood when the circulation stagnates (Figure 1–33). Each valve consists of two or three semilunar cusps facing each other. Valves are most numerous in veins that are exposed to intermittent changes in external pressure and are wholly lacking in those isolated from such influences. They are thus common in veins running between muscles and absent from those in the vertebral canal and cranial cavity; partly on this account, the veins in the latter site are known by the special term venous sinuses.
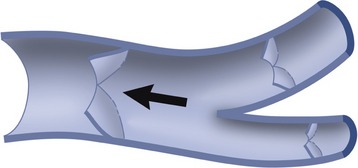
Figure 1–33 A branching vein opened to expose valves. The arrow indicates the direction of blood flow.
The very largest arteries and veins run separately, but most veins of medium and lesser size accompany the corresponding arteries to which they are said to be satellite. However, they show even more variation than do the arteries and are quite commonly duplicated, further replicated, or arranged in plexus formation.
LYMPHATIC STRUCTURES
The lymphatic system has two components. The first comprises a system of lymphatic capillaries and larger vessels that return interstitial fluid to the bloodstream. The second comprises a variety of widely scattered aggregations of lymphoid tissue, including the many lymph nodes; less discrete lymphoid aggregations, such as tonsils, are not considered until later (p. 257).
Lymphatic Vessels
A plexus of lymphatic capillaries that is spread through most tissues collects a fraction of the interstitial fluid. This fraction is disproportionately important because it includes the proteins and other large molecules that are unable to enter the less permeable blood vessels. The greater permeability of the lymphatic capillaries also allows them to take in particulate matter, including microorganisms on occasion. The lymphatic capillaries commence blindly and form plexuses from which larger lymphatic vessels take origin. These larger vessels closely resemble veins in structure but are more delicate. Because the fluid (lymph) they contain is generally pale, they are rarely conspicuous; however, they are easily identified once seen, as closely spaced valves give them a distinctive beaded appearance when they are well filled. The largest vessels take independent courses, but many of smaller size accompany blood vessels and nerves. The lymphatic vascular tree eventually converges on two or three large trunks that open in a rather erratic fashion into major veins at the junction of the neck and thorax (Figure 1–34).
Figure 1–34 Generalized schema of the lymph nodes and lymphatic vessels (dorsal view). The top of the diagram represents the neck region. 1, External and internal jugular veins; 2, lymph from the head; 3, lymph from the shoulder and forelimb; 4, tracheal duct; 5, thoracic duct; 6, lymph from the thoracic organs; 7, cisterna chyli; 8, lymph from the abdominal organs; 9, lymph from the lumbar region and kidneys; 10, lymph nodes of the pelvis; 11, lymph from the hindlimb.
Lymph Nodes
Lymph nodes, often incorrectly termed lymph glands, are placed along the lymph pathways in a pattern that shows considerable specific and some individual variation. Groups of neighboring nodes constitute lymphocenters, whose occurrence and drainage territories exhibit greater constancy than is presented by individual nodes. There are important interspecific differences in the lymphocenters: those of the domestic carnivores and ruminants each contain rather few but individually large nodes, particularly in cattle, and those of pigs and, more especially, horses each contain a great many small nodes packeted together.
Lymph nodes are firm, smooth-surfaced, and generally ovoid or bean-shaped. Some that are superficial can be identified on palpation through the skin. Naturally, they are more easily found when they are enlarged, and it is therefore a matter of importance to have a clear expectation of which nodes can usually be identified in the healthy animal. Each node is bounded by a capsule, below which runs an open space (subcapsular sinus) into which the afferent vessels open at scattered sites. Branches from the subcapsular sinus lead to a medullary sinus close to the generally indented hilus, where the few efferent vessels emerge (Figure 1–35, A; see also Figure 7–50). The tissue of the node is divided between cortical and medullary regions. The cortex contains the germinal centers in which lymphocytes are continually produced; the medulla consists of looser branching cellular cords. Both are supported by a reticular framework containing many phagocytic cells. The organization of the lymph nodes of pigs (Figure 1–35, B) shows a reversal of the usual flow pattern: the afferent vessels enter together, whereas the efferent vessels have dispersed origins (Figure 7–51, A-B).
Figure 1–35 Structure of a lymph node (A) in which the germinal centers (lymph nodules) occupy the cortical region. In the pig (B) the germinal centers lie centrally. The arrows indicate the direction of lymph flow. 1, Afferent lymphatics; 2, subcapsular sinus; 3, efferent lymphatics.
With very few exceptions (and these are disputed), all lymph passes through at least one node in its passage from the tissues to the bloodstream. As it percolates through the node, it receives a recruitment of lymphocytes and is also exposed to the activities of the phagocytes. These remove and destroy, or attempt to remove and destroy, particulate matter, including any microorganisms within the lymph. The lymph node thus provides a barrier to the spread of infection and tumors, some varieties of which favor lymphatic pathways for their dissemination. Swelling of a lymph node frequently indicates the existence of a disease process in its drainage territory. It is clear that the role of the lymphatic system in disease is equivocal. On the one hand, lymph flow facilitates the spread of microorganisms or tumor cells; on the other, the intervention of the node provides an opportunity for their containment and destruction. There are obviously weighty reasons why the position, the accessibility, the drainage territory, and the destination of the efferent flow of all major nodes must be familiar to the clinician, the pathologist, and the veterinarian engaged in meat inspection.
PERIPHERAL NERVES
The central nervous system, the brain and spinal cord, is in two-way communication with virtually all body tissues by means of a system of branching peripheral nerves. These are composed of afferent (sensory) fibers, which convey information to the central nervous system from peripheral receptors, and efferent (motor) fibers, which convey instructions from the central nervous system to peripheral effector organs. The peripheral nerves comprise the 12 pairs of cranial nerves and the considerably larger number of pairs of spinal nerves whose total varies with the vertebral formula. The dog has 8 cervical, 13 thoracic, 7 lumbar, 3 sacral, and about 5 caudal pairs. The present account is restricted to the rather uniform spinal nerves; the cranial nerves differ from these and from one another in many respects that are considered later (p. 314).
The orderly origin of the spinal nerves reveals the segmentation of the spinal cord. Each nerve is formed by the union of two roots (Figure 1–36). The dorsal root is almost exclusively composed of afferent fibers whose cell bodies are clumped together to form a visible swelling, the spinal (dorsal root) ganglion. The central processes enter the cord along a dorsolateral furrow. The peripheral processes extend from the wide variety of exteroceptive, proprioceptive, and enteroceptive endings that respond to external stimuli, changes within the muscles and other locomotor organs, and changes in the internal organs, respectively. The ventral root is exclusively composed of efferent fibers emanating from motor neurons within the ventral horn of gray matter and leaving the cord along a ventrolateral strip; they are in passage to the effector organs—muscles and glands.
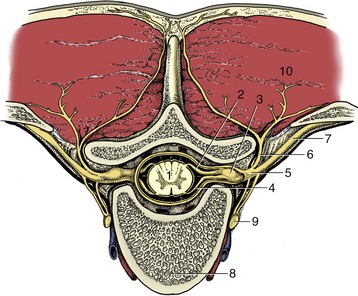
Figure 1–36 Transection of the vertebral column to show the formation of a spinal nerve. 1, Spinal cord; 2, dorsal root; 3, spinal ganglion; 4, ventral root; 5, spinal nerve; 6, dorsal branch of spinal nerve; 7, ventral branch of spinal nerve; 8, body of vertebra; 9, sympathetic trunk; 10, epaxial muscles.
The dorsal and ventral roots join peripheral to the dorsal root ganglion to form the mixed spinal nerve (Figure 1–36/5), which leaves the vertebral canal through the appropriate intervertebral foramen. In the cervical region, each nerve emerges cranial to the vertebra of the same numerical designation as the nerve, except the eighth, which emerges between the last cervical and first thoracic vertebrae. In other regions, each nerve emerges caudal to the vertebra of the same numerical designation.
The mixed trunk formed by the union of dorsal and ventral roots divides almost at once into dorsal and ventral branches (rami). The dorsal branch is distributed to dorsal structures: epaxial muscles of the trunk (broadly, those that lie dorsal to the line of transverse processes) and the skin over the back (Figure 1–37). The much larger ventral branch is distributed to hypaxial muscles of the trunk (broadly, those ventral to the transverse processes), the muscles of the limbs (with a few exceptions), and the remaining part of the skin, including that of the limbs. Both dorsal and ventral branches have connections with their neighbors that form continuous dorsal and ventral plexuses. These plexuses are generally neither obvious nor important, except for enlarged portions of the ventral plexus opposite the origins of the limbs. These, the brachial and lumbosacral plexuses, give rise to the nerves that are distributed to forelimb and hindlimb structures, respectively.
Figure 1–37 The distribution of a (lumbar) spinal nerve. 1, Epaxial muscles; 2, sublumbar muscles; 3, spinal nerve; 4, dorsal branch of spinal nerve; 5, ventral branch of spinal nerve; 6, 7, external and internal abdominal oblique muscles; 8, transversus abdominis muscle; 9, rectus abdominis muscle; 10, linea alba.
The brachial plexus (Figure 1–38) is usually formed by contributions from the last three cervical and the first two thoracic nerves, the lumbosacral plexus by contributions from the last few lumbar and the first two sacral nerves. The limb plexuses allow for regrouping and reassociation of the constituent nerve fibers, and the nerve trunks that emerge distally are each composed of fibers derived from two or three spinal segments; thus the median nerve is composed of fibers from spinal nerves C8 and T1, the femoral nerve of fibers from L4–L6.
Figure 1–38 The brachial plexus. The ventral divisions of the spinal nerves (C6–T2) contributing to the plexus are at the top of the schema, the peripheral branches (N) supplying the forelimb at the bottom. Contributions from C5, C6, and C7 form the phrenic nerve.
The courses of the major peripheral trunks must be known to avoid placing the nerves at unnecessary risk during surgery. Their central connections are important in two contexts. First, local anesthetic solutions injected near selected spinal nerves have predictable effects in paralyzing muscles and in depriving skin areas of sensation. Conversely, paralysis of particular muscles or absent or altered sensibility of specific skin areas may point to the precise location of a central lesion.
So far, reference to nerve fibers concerned with the innervation of blood vessels, glands, and internal organs has been avoided. These structures are supplied by the autonomic division of the nervous system, which is described in Chapter 8. For the present, it is sufficient to state that although autonomic fibers are not present in the roots of every spinal nerve, arrangements exist that ensure that each peripheral nerve receives its necessary quota.