9 The Sense Organs
A stabled animal, for example a dairy cow tied to a stanchion, experiences few environmental changes—and these by and large are routine. It is quite different with a wild animal, which, if it is to survive, must constantly check its environment. It must see obstacles, hear predators, smell other animals to distinguish outsiders from members of its own group, taste in order to discard harmful substances in its food, and, in a more general way, be in touch with its surroundings “through its skin” by perceiving touch, pressure, and temperature. This is made possible by those organs that represent the special senses (eye, ear, olfactory organ, and organ of taste) and those others that are widely diffused, especially in the skin, where they mediate a cutaneous sense. The former contain concentrations of highly specialized sensory cells; the latter are composed of numerous peripheral specialized endings of centrally located sensory cell bodies. Associated with the ear are sensory cells that respond to gravity and to movements of the head and so give the animal its sense of balance.
All these senses are conscious, that is to say, the animal is aware of what it has registered. However, there are other systems concerned with muscle and visceral sense of which the animal is less aware and by which it is in touch with the “internal environment” of its own body.
The organs of special sense are described first.
THE EYE
The eye, the organ of vision, consists of the eyeball and various adnexa—accessory structures such as the ocular muscles that move the eyeball, the lids that protect it, and the lacrimal apparatus that keeps its exposed parts moist. Most of these are housed in the orbit, where the eyeball is embedded in generous quantities of fat. The eyelids arise from the bony margins of the orbit and, like curtains, are intermittently drawn over the exposed part of the eye (blinking) to distribute the tears or lacrimal fluid for protection; they are kept across the eye during sleep when vision is not required.
The eyes of the domestic mammals protrude more from the surface of the face than do those of primates, ourselves included. Their position in the head is related to the animal’s environment, habits, and method of feeding. In general, predatory species (cat, dog) have eyes set well forward, whereas those that are the hunted (herbivores: horse, ruminants, rabbits) carry their eyes more laterally (Figure 9–1). The former position of the eyes provides a large field of binocular vision that allows for concentration on near objects and for the perception of depth. In the latter, the right and left fields of vision hardly overlap; consequently, though these animals are constantly aware of a large segment of their surroundings, they have little capacity for binocular vision.
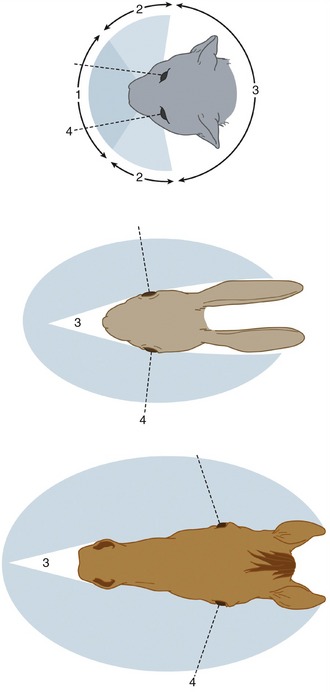
Figure 9–1 Visual fields of cat, rabbit, and horse. 1, Binocular vision; 2, monocular vision; 3, blind area; 4, visual axis of eye in central position.
When an animal is emaciated, the orbital fat is reduced and the eyes sink within the orbits, which gives the face a gaunt, suffering appearance.
THE EYEBALL
The eyeball (bulbus oculi) of the domestic mammals is nearly spherical but with some anteroposterior* compression in horses and cattle. In addition, the cornea, the transparent part of the eyeball, bulges from the anterior surface by virtue of its smaller radius of curvature (Figure 9–2).
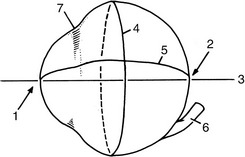
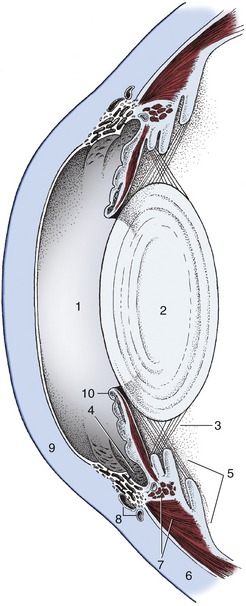
Figure 9–2 Medial view of right eyeball. 1, Anterior pole; 2, posterior pole; 3, optic axis; 4, equator; 5, a meridian; 6, optic nerve; 7, limbus.
The highest point on the cornea is the anterior pole, and the highest point on the posterior surface is the posterior pole of the eyeball; the straight line passing through both poles is the optic axis. The equator is an imaginary line about the eyeball, which, like that of the Earth, is equidistant from the poles. A meridian is one of the many lines passing from pole to pole that intersect the equator at right angles. The optic nerve (Figure 9–2/6) leaves the eyeball slightly ventral to the posterior pole.
The eyeball has three thin tunics that, being in close apposition, form a laminated sheet that surrounds the partly liquid, partly gelatinous center. The three tunics are (1) an external fibrous tunic that gives form to and protects the eyeball—it is the only complete tunic; (2) a middle vascular tunic that consists largely of blood vessels and smooth muscle and is concerned with the nutrition of the eyeball and the regulation of the shape of the lens and size of the pupil; and (3) an internal nervous tunic that consists largely of nervous tissue and is the layer most directly concerned with vision, that is, the translation of visual stimuli into nerve impulses for interpretation by the brain.
The Fibrous Tunic
The fibrous tunic of the eyeball is made up of very dense collagenous tissue, which, by resisting the internal pressure, gives the eye shape and stiffness. It consists of the sclera and cornea, which meet at the limbus (Figure 9–2/7).
The sclera is the opaque posterior part of the fibrous tunic. It consists of a dense feltwork of collagenous and elastic fibers and is generally white (“the white of the eye”), though with a bluish tinge; in some species it contains pigmented cells that render it gray. Ventral to the posterior pole it presents a small cribriform area (Figure 9–3/13) through which pass the fibers of the optic nerve. The nerve is surrounded by a connective tissue sheath that continues the dura mater to the sclera. The sclera is also pierced by several small ciliary arteries and nerves and by larger vorticose veins. It gives attachment to the tendons of the ocular muscles anterior to the equator. Posteriorly, except for the areas taken up by the retractor bulbi muscle, it is covered by a thin membrane (vagina bulbi; Figure 9–3/5) that separates it from the retrobulbar fat, which provides a socket in which the eyeball can play. Near the limbus the sclera is covered by conjunctiva (see further on), which furnishes connection to the inside of the lids (Figure 9–3/19).
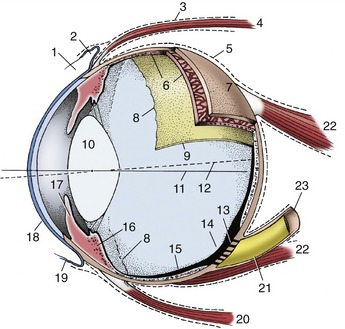
Figure 9–3 Eye opened to show the three tunics, which have been drawn thicker than they actually are. 1, Limbus; 2, upper fornix; 3, deep muscular fascia; 4, dorsal rectus muscle; 5, vagina bulbi; 6, choroid; 7, sclera; 8, ora serrata; 9, retina; 10, lens; 11, optic axis; 12, visual axis; 13, area cribrosa; 14, optic disc; 15, retina; 16, ciliary body; 17, iris; 18, cornea; 19, conjunctiva; 20, ventral rectus muscle; 21, optic nerve; 22, retractor bulbi; 23, sheath of optic nerve.
The cornea forms about one quarter of the fibrous tunic and bulges forward (Figure 9–4). It is composed of a special kind of dense connective tissue arranged in lamellar form. It is generally recognized that, despite the careful arrangement of its fibers, transparency is not only a structural but also a physiological phenomenon and depends on the continuous pumping out of interstitial fluids, which is a process that has been localized in the posterior epithelium. Its main bulk (substantia propria) is continuous with the sclera (Figure 9–5/6,9) and encased by anterior and posterior limiting membranes and epithelial layers. The anterior epithelial layer is continuous with the epithelium of the conjunctiva, while the posterior epithelial layer unites with the anterior surface of the iris across the iridocorneal angle (Figure 9–5/4). The cornea does not contain blood vessels; nutrients for its cells permeate the substantia propria from vessels in the limbus or are carried to its surfaces in the lacrimal fluid and aqueous humor. The surface of the cornea is very sensitive owing to the presence of free nerve endings near the anterior epithelium. These arise from the long ciliary nerves, which are branches of the ophthalmic nerve (see further on). Their axons form the afferent limb of the corneal reflex, which closes the lids when the cornea is touched. This reflex is employed when monitoring deep anesthesia.
The Vascular Tunic
The vascular tunic of the eye (also known as the uvea) lies deep to the sclera to which it is applied. It consists of three zones: choroid, ciliary body, and iris, given in posteroanterior sequence (see Figure 9–3). The choroid lines the sclera from the optic nerve almost to the limbus, the ciliary body follows as a thickened zone opposite the limbus, and the iris projects into the cavity of the eyeball posterior to the cornea; the iris is the only internal structure readily seen through the cornea without recourse to instruments (ophthalmoscope). Although blood supply is its principal function, the vascular tunic suspends the lens, regulates its curvature, and adjusts the size of the pupil by means of the smooth muscle in the ciliary body and iris (see Figure 9–5).
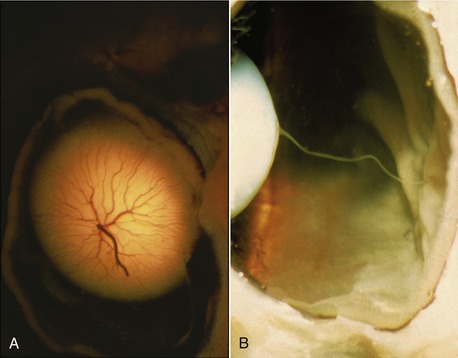
The choroid contains a dense network of blood vessels embedded in heavily pigmented connective tissue. The network is supplied by the posterior ciliary arteries and is drained by the vorticose veins. A flat sheet of capillaries on the internal surface is responsible for the nutrition of the external layers of the nervous tunic (retina), which lies deep (internal) to it. The blood in these capillaries produces the redness of the fundus (interior surface of the posterior hemisphere) seen when the eye is examined with an ophthalmoscope. In the dorsal part of the fundus the choroid forms a variously colored, light-reflecting area known as the tapetum lucidum (Figure 9–6, A-F). This is an avascular layer (cellular in carnivores, fibrous in ruminants and horses) between the capillaries and the network of larger vessels. The tapetal cells contain crystalline rods arranged in such a way that light striking them is split into its components, which results in the characteristic iridescence. The packaging of the collagen in the fibrous tapetum has the same effect. The tapetum makes the eyes of animals “shine” when they look toward a light, such as the headlights of an oncoming car. Our eyes, and those of the pig, do not have a tapetum and therefore do not give this effect. It is believed that the tapetum is a nocturnal adaptation: by reflecting incident light, it increases the stimulation of the light-sensitive receptor cells in the overlying retina and thus aids vision in dark places. The choroid adheres so closely to the pigmented external layer of the retina that the latter remains when the bulk of the retina is removed during dissection. The retina is without pigment where it overlies the tapetum lucidum.

Figure 9–6 A to F, Fundus of eye. A, Dutch sheepdog. B, Old English sheepdog. C, Cat. D, Cow. E, Goat. F, Horse.
Toward the limbus the choroid thickens to form the ciliary body (Figure 9–5/5). This is a raised ring with ridges converging toward the lens in the center; anteriorly the ring is continued by the iris. The ciliary body is best comprehended when seen in its entirety by looking into the anterior part of the eye from behind (Figure 9–7/2; Figure 9–8). The radial ridges, known as the ciliary processes, extend zonular fibers (Figure 9–5/3) to the equator of the lens, suspending it around its periphery. Between the ciliary body and the sclera is the smooth ciliary muscle (Figure 9–5/7), which functions in accommodation (the ability of the eye to focus on near or distant objects by changing the shape of the lens) (see further on).
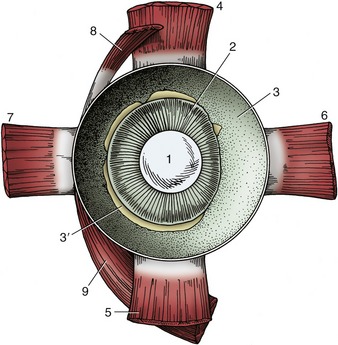
Figure 9–7 Anterior half of the left equine eye, viewed from behind. 1, Lens; 2, ciliary body; 3, choroid covered by pigmented outer layer of retina; 3′, remnants of inner nervous layer of retina, which has been removed; 4–7, dorsal, ventral, medial, and lateral rectus muscles; 8, 9, dorsal and ventral oblique muscles.
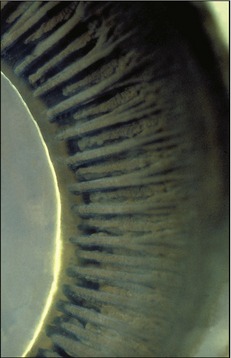
The third and smallest part of the vascular tunic is the iris (Figure 9–5/10), which is suspended between the cornea and lens. It is a flat ring of tissue attached at its periphery to the sclera (by the pectinate ligament; Figure 9–12/7) and to the ciliary body; the opening in the center is the pupil (Figure 9–9) through which light enters the posterior part of the eye. The size of the pupil and therefore the amount of light reaching the retina are regulated by smooth sphincter and dilator muscles in the iris. The sphincter lies near the pupillary margin, while the fibers of the dilator are arranged radially and, on contraction, enlarge the pupil. Irregular outgrowths (iridic granules; Figure 9–9) containing coils of capillaries are often seen on the upper and lower pupillary margins of ungulates; their significance is not known, though there are suggestions that they act as “shades.”
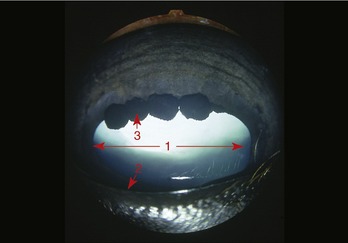
Figure 9–9 Anterior surface of the equine iris with characteristic iridic granules. 1, Pupil; 2, pupillary margin; 3, iridic granule.
The iris divides the space between the lens and cornea into anterior and posterior chambers that communicate through the pupil (see Figure 9–9). Both are filled with aqueous humor, a clear watery fluid (see further on).
The iris consists of three layers: an anterior epithelial layer continues across the iridocorneal angle and blends with the posterior epithelium of the cornea, a middle layer of connective tissue stroma contains the two smooth muscles, and the posterior layer of pigmented epithelium is the forward extension of the pigmented layer of the retina mentioned when we described the choroid; it is known as the iridic part of the retina and is closely related to the dilator pupillae (Figure 9–5/10).
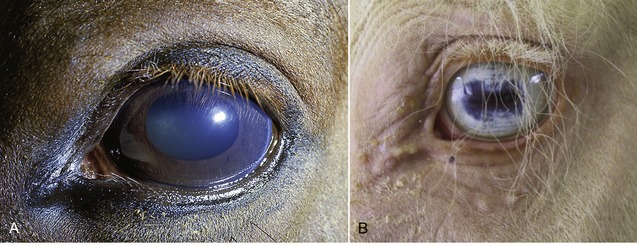
The color of the iris determines the “color of the eye” and depends on the number of pigmented cells present in its stroma and on the type of pigment in the cells. If the pigmented (melanin) cells are tightly packed the iris is dark brown, with fewer cells the iris is lighter and yellowish (Figure 9–10), and with a minimum of pigmented cells the iris appears bluish. In albinos pigment is also absent from the iridic part of the retina, that is, the iris is totally devoid of pigment; their eyes appear red because the blood in the capillaries is not obscured.
The Internal Tunic
The internal or nervous tunic of the eyeball contains the light-sensitive receptor cells and is known as the retina (Figure 9–3/9,15). It is an extension of the brain to which it remains connected by the optic nerve. The retina begins where the nerve penetrates the choroid; shaped like a hollow cup, it lines this and ends at the pupillary margin. Only the posterior two thirds or so of the retina can be reached by light entering the pupil. Consequently, only that part (pars optica retinae) is provided with receptor cells; it is relatively thick. The remaining third is “blind” (pars ceca retinae) and is mainly represented by the thin pigmented layer that continues on to the ciliary body and the back of the iris. The edge caused by the abrupt decrease in thickness at the junction of optic and blind parts is the ora serrata (Figure 9–3/8); it also demarcates the choroid from the ciliary body. The two layers of the retina develop from the inner and outer layers of the optic cup with which the eye makes its appearance in the embryo. The gap between the layers of the optic cup, though obliterated postnatally, remains a weakness where delamination produces “detachment of the retina.”
The presence of so much retinal and choroidal pigment makes the interior of the posterior part of the eye dark like the inside of a camera so that the pupil appears black. The black walls absorb scattered and reflected light and prevent it from striking the retina a second time, which would contribute to blurred vision.
The layers in the pars optica retinae are as follows, beginning at the choroid: a single layer of pigmented cells; a neuroepithelial layer containing the receptor cells, rods and cones and their nuclei (the rods, so far as we know, are concerned with black and white [night] and the cones with color [day] vision); a layer of bipolar ganglion cells; and a layer of multipolar ganglion cells whose nonmyelinated axons, lying internal (deep) to the cells, pass to the optic disc, where they aggregate to form the optic nerve. It will be clear from this arrangement that light passes through all layers except the first before reaching and stimulating the rods and cones (Figure 9–11).

Figure 9–11 Outer pigmented layer (A) and inner neuroepithelial layer (B) of retina. 1, Pigmented cells; 2, receptor cells (rods and cones); 3, bipolar ganglion cells; 4, multipolar ganglion cells; 5, incoming light (arrows).
The area where the axons of the fourth layer concentrate to leave the eye, the optic disc, can easily be seen when examining the fundus with an ophthalmoscope (see Figure 9–6). Because the axons here turn in toward the cribriform area of the sclera, there is no room for receptor cells; the optic disc, therefore, is a blind spot. In contrast, an area of maximum optical resolution (macula) is located a short distance dorsolateral to the optic disc. It is believed that when we examine objects intently, we focus them on the macula. It is not known whether animals do the same. In some species the macula is faintly visible with the ophthalmoscope. The visual axis is the line connecting the macula, the center of the lens, and the object viewed. It does not quite coincide with the optic axis because the macula is slightly dorsal to the posterior pole of the eyeball (see Figure 9–3).
Arterioles and venules emerging from the optic disc spread out in various species-specific patterns to nourish and drain the retina (see Figure 9–6). The arterioles are branches of the central artery of the retina, which arrives at the optic disc in the center of the optic nerve.
The anteroposterior compression of the equine eyeball has led to the assumption that the horse has a ramp retina. A ramp retina is one in which all parts are not equidistant from the posterior pole of the lens; the distance from the lens becomes progressively greater as the retina is followed dorsally. Presumably, as increasingly closer objects are viewed, they are focused on the more dorsal parts of the retina; focal length is automatically increased, and little accommodation of the lens is required (p. 528).
The Refractive Media of the Eyeball
Now that the laminated wall has been described, it remains to say something of the interior of the eyeball, which is concerned with the manipulation of the light rays that enter it. It is best to do this by following the path taken by the light. Several interior structures have already been mentioned and require little further description.
The cornea is an integral part of the supporting fibrous tunic. Although dense and tough, it has the quality of being transparent and thus enables light to enter the eye. The cornea plays a major role in refraction; that is, it is capable, as is the lens, of bending light so that what is seen by the animal is miniaturized sufficiently to be focused on the retina.
The rays next encounter the aqueous humor filling the space between cornea and lens. The aqueous humor is a clear watery fluid that, apart from its refractive properties, plays an important role in the maintenance of intraocular pressure. It is continuously produced by cells of the ciliary processes and enters the system in the posterior chamber. From here it passes through the pupil into the anterior chamber and thence through the spaces in the trabecular tissue (pectinate ligament) at the iridocorneal angle. These spaces convey it to venous sinuses in the sclera and thus into the bloodstream (Figure 9–12). In the healthy eye the rate of production balances the rate of drainage, maintaining a constant pressure. Interference with drainage allows excess fluid to accumulate, causing the intraocular pressure to rise (glaucoma). This serious condition, common in humans, is rarely seen in animals.
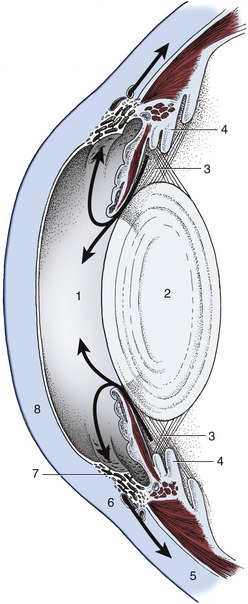
Figure 9–12 The flow (arrows) of aqueous humor. 1, Anterior chamber; 2, lens; 3, posterior chamber; 4, ciliary body; 5, sclera; 6, venous plexus; 7, pectinate ligament; 8, cornea.
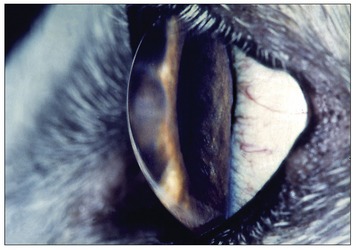
The lens (Figure 9–13), in contrast to its liquid neighbors, is a solid structure, though sufficiently elastic to be able to change in shape. It is biconvex and has anterior and posterior poles, an equator, and a central axis that coincides with the optic axis of the eye. The posterior surface is usually more convex than the anterior. The lens has an outer capsule that is thicker anteriorly and thickest at the equator, where the zonular fibers of the ciliary body are secured. The capsule of the lens is elastic and is permanently under tension, which, if unopposed by the pull exerted at the periphery, would cause the lens to assume a more spherical shape. The substance of the lens consists of very regularly arranged fibers. These form concentric sheets that can be peeled off like the layers of an onion. Within each sheet the fibers are so arranged that they loop from a point on the anterior surface to one on the posterior surface. Their ends are cemented to the ends of other fibers, forming visible sutures shaped like little three-pointed stars (radii lentis; Figure 9–13/1,2). In the peripheral, or cortical, part of the lens the fibers are relatively soft; they are firmer and thinner toward the center where they form a harder nucleus. Owing to its elastic properties the cortex can be molded so that the lens changes shape during accommodation. In many older animals the lens becomes cloudy, impairing vision; the condition is known as cataract (Figure 9–14).
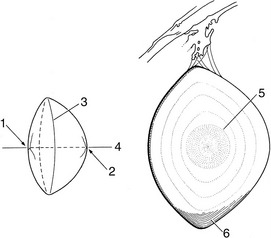
Figure 9–13 Bovine lens; on the right, a meridional section. 1, Anterior pole with lens star; 2, posterior pole with lens star; 3, equator; 4, optic axis; 5, nucleus; 6, layers of lens fibers, shown only in part.
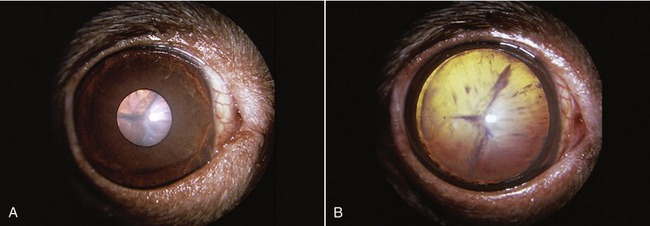
Figure 9–14 A, Slightly constricted canine pupil. Cataract of lens visible. B, Canine pupil in mydriasis (enlarged pupil): Lens is now totally visible; opacity is seen to affect the entire lens.
Accommodation
As we have said, the elastic capsule of the lens would squeeze the relatively soft cortex into a rounder shape unless opposed by the zonular fibers that arise from the ciliary processes and exert a constant radial pull on the equator. This pull flattens the lens into the shape described; this is the resting shape of the lens adapted for far vision and is present during sleep. When the animal wants to focus on a near object the muscle on the surface of the ciliary body contracts, thickening the ciliary body. This displaces the processes toward the lens and thus relaxes the zonular fibers. The lens, released from the tension at its equator, rounds out and brings the object into focus. Compared with the muscle in humans, the ciliary muscle and, therefore, the ability to accommodate are poorly developed in domestic animals.
After passing through the lens the light rays enter the vitreous body. This is a gel-like mass consisting mainly of water (vitreous humor) but with a stroma of fine transparent fibers that condenses into a membrane at the surface. The vitreous body occupies the space between lens and retina and holds the latter against the choroid. In the embryo the lens is nourished by the hyaloid artery, a branch of the central retinal artery that passes through the vitreous body. The artery usually degenerates after birth, and the lens is then nourished by diffusion (Figure 9–15, A-B). Unlike the aqueous humor, the vitreous humor is not continuously replaced; it is therefore constant in volume.
THE ADNEXA OF THE EYE
The structures that protect and move the eyeball include the orbital fasciae, the ocular muscles, the eyelids and tunica conjunctiva, and the lacrimal apparatus; most are contained within the orbit. This is a cone-shaped cavity on the lateral surface of the skull that is delimited externally by a bony margin (base of cone). In the carnivores and pig the bone is deficient laterally but the ring is completed by the orbital ligament (see Figure 2–31/1). The wall of the human orbit is entirely osseous, but in the domestic mammals the lateral and ventral parts are formed by the fibrous periorbita, one of the orbital fasciae (see further on).
The Orbital Fasciae
The eyeball is surrounded by three roughly conical fascial layers. The most external of these is the periorbita, which has just been mentioned; internal to the periorbita are superficial and deep muscular fasciae (Figure 9–16).
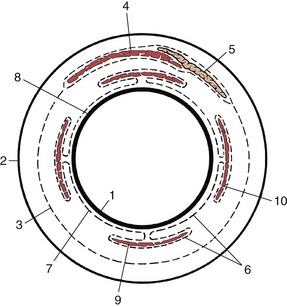
Figure 9–16 Schematic representation of the orbital fasciae: transection of orbital structures at the level of the eyeball. Part of the deep fascia (6) forms the vagina bulbi (7). 1, Eyeball; 2, periorbita; 3, superficial muscular fascia; 4, levator palpebrae; 5, lacrimal gland; 6, deep muscular fascia; 7, vagina bulbi; 8, episcleral space; 9, ventral rectus muscle; 10, lateral rectus muscle.
The periorbita is attached near the optic foramen at the apex of the cone. It blends with the periosteum at the orbital margin and on the medial and dorsal walls of the orbit. Elsewhere (mainly laterally and ventrally) it is free and forms a substantial fibrous partition between orbital and extraorbital structures (Figure 9–17/11). The periorbita splits at the orbital margin. One part is continued as the periosteum of the facial bones; the other, the orbital septum (Figure 9–17/2), forms two semilunar folds with thickened free margins (tarsi) that stiffen the edges of the upper and lower eyelids. The trochlea (Figure 9–17/6), a flat piece of cartilage embedded in the dorsomedial wall close to the orbital margin, provides a pulley around which the dorsal oblique muscle winds to change direction by nearly 90°.

Figure 9–17 Right bovine eye cut along orbital axis, rostromedial surface. 1, Tarsus; 2, orbital septum; 3, orbital margin; 4, dorsal oblique muscle; 5, periosteum of face; 6, trochlea; 7, dorsal rectus muscle; 8, levator palpebrae superioris; 9, optic nerve in optic foramen; 10, ventral rectus muscle; 11, periorbita; 12, extraperiorbital fat; 13, lacrimal bulla, a caudal recess of the maxillary sinus; 14, retractor bulbi; 15, intraperiorbital fat; 16, zygomatic arch; 17, orbicularis.
The superficial muscular fascia lies within the periorbita; it is loose and fatty and envelops the levator palpebrae superioris and the lacrimal gland (Figure 9–16/3). The deep muscular fascia is more fibrous; it arises from the eyelids and from the limbus of the eyeball, which it closely invests. It is reflected around the muscles attaching to the eyeball, providing each (and also the optic nerve) with a fascial envelope. It is known as the vagina bulbi (Figure 9–16/7) where it is applied to the eyeball, although it is separated by a narrow episcleral space. The presence of this space facilitates the movement of the eyeball against the retrobulbar fat. In enucleation (removal of the eye), advantage is usually taken of this arrangement; the eyeball is freed, and the vagina bulbi and the retrobulbar structures it covers are left in place.
The Muscles of the Eyeball
The muscles that move the eye are located behind the eyeball. All except one originate in the vicinity of the optic foramen at the apex of the orbital cone. There are four rectus muscles, two oblique muscles, and a retractor.
The four rectus muscles—dorsal, ventral, medial, and lateral—are inserted anterior to the equator by wide but very thin tendons (see Figure 9–7). The dorsal and ventral oblique muscles attach to the eyeball near the equator and tend to rotate the eyeball around the visual axis on contraction (Figure 9–18/1,7). The dorsal oblique muscle also arises close to the optic foramen and passes forward on the dorsomedial wall of the orbit before it is deflected around the trochlea to end on the dorsolateral surface of the eyeball beneath the tendon of the dorsal rectus muscle. A small synovial sheath protects the muscle as it passes around the trochlea, which in fact is its functional origin. If this muscle were to contract by itself, it would pull the dorsal part of the eyeball medially.
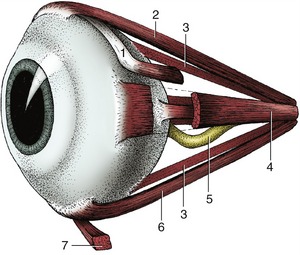
Figure 9–18 Ocular muscles. 1, Dorsal oblique m.; 2, dorsal rectus m.; 3, retractor bulbi; 4, medial rectus m.; 5, optic nerve; 6, ventral rectus m.; 7, ventral oblique m.
The ventral oblique muscle, uniquely, does not arise from the vicinity of the optic foramen. Instead, it takes its origin from a depression in the ventromedial wall of the orbit, passing laterally below the eyeball and the tendon of the ventral rectus muscle before inserting on the ventrolateral part of the eyeball. Its contraction, if isolated from the action of the other muscles, would rotate the eyeball around the visual axis so that the dorsal portion of the eyeball would move laterally. The retractor bulbi (Figure 9–17/14) arises from the vicinity of the optic foramen but is inserted on the eyeball posterior to the equator. It forms a nearly complete muscular cone about the optic nerve (Figure 9–19/7). The retractor is not present in ourselves, and it is still a matter of conjecture why we do not possess the ability to retract our eyes; perhaps we do not need the additional protection provided to the more protruding eyes of animals.
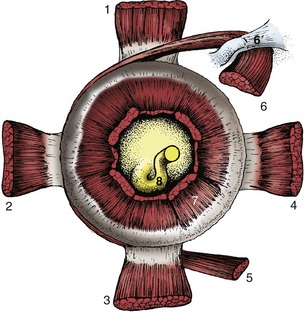
Figure 9–19 Stumps of ocular muscles viewed from behind the left eyeball. 1, Dorsal rectus m.; 2, lateral rectus m.; 3, ventral rectus m.; 4, medial rectus m.; 5, ventral oblique m.; 6, dorsal oblique m.; 6′, trochlea; 7, retractor bulbi; 8, optic nerve.
The movements of the eyes are much more complex than the origins and insertions of the individual muscles suggest. As far as we know, none ever acts singly. It seems that the tonus is increased or decreased in opposing groups for smooth transition from one eye position to another. The most difficult actions to explain are those of the oblique muscles because there is no significant rotation around the visual axis in any usual movement. Their participation is required for the following reason. The rectus muscles arise slightly medioventral to the point where the visual axis, if extended caudally, would strike the skull. That is, the visual axis does not coincide with the axis of the orbital cone. As a result, the dorsal rectus muscle, as one example, would not simply elevate the cranial pole of the eyeball but would also rotate the eyeball so that its dorsal part moved slightly medially. This slight intorsion is reflexively resisted by the ventral oblique, and the result is a smooth elevation of the anterior pole. The reverse happens in depression of the eyeball when the ventral rectus and the dorsal oblique muscles are involved.
An additional striated muscle within the orbit is conveniently considered here. This is the levator palpebrae superioris (Figure 9–17/8). It does not attach to the eyeball but passes over it to enter and elevate the upper eyelid.
In addition to these striated muscles there are three sheets of smooth muscle, although they are rarely observed during routine dissection. One (m. orbitalis) consists of a sheet of circular (with regard to the visual axis) fibers applied to the internal surface of the periorbita. A ventral longitudinal sheet extends from the sheath of the ventral rectus muscle into the lower lid (as the m. tarsalis inferior) and into the third eyelid (see further on). A medial longitudinal sheet extends from the sheath of the medial rectus muscle and from the trochlea into the upper eyelid (as the m. tarsalis superior) and into the third eyelid. Tonus in these sheets maintains the normal protruded position of the eye and retracted position of the eyelids.
The Eyelids and Conjunctiva
The eyelids (palpebrae) are two musculofibrous folds of which the upper is the more extensive and more mobile. The free margins of the lids meet at the medial and lateral angles of the eye and bound an opening known as the palpebral fissure.
The eyelids consist of three layers: skin, a middle musculofibrous layer, and a mucous membrane, known as the palpebral conjunctiva, facing the eye (Figure 9–20).
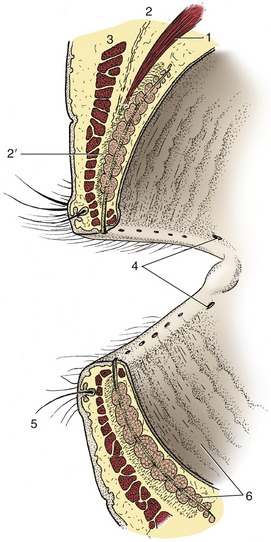
Figure 9–20 Eyelids, sectioned and viewed obliquely from behind. 1, Levator palpebrae superioris; 2, orbital septum; 2′, tarsus; 3, orbicularis oculi; 4, puncta lacrimalia; 5, cilium with associated ciliary and sebaceous glands; 6, tarsal glands.
The skin of the lids is thin and delicate and is covered with short hairs; it may also carry a few prominent tactile hairs.
The musculofibrous layer is formed by the orbicularis oculi, the orbital septum, the aponeurosis of the levator muscle, and the smooth tarsal muscle. The orbital septum arises from the margin of the orbit; the aponeurosis of the levator and the tarsal muscle originate in the orbit. Except for the orbicularis oculi, which lies directly under the skin and can be dissected away, the components of this layer intermingle inseparably. Toward the free margin these components are succeeded by the tarsus (Figure 9–20/2′), a platelike fibrous condensation that stabilizes the edge of the lid. The ends of the two tarsi are anchored to the orbital margin by medial and lateral palpebral ligaments that assure an elongated palpebral fissure when the eye is closed (by the orbicularis oculi). Deep to the tarsus and opening onto the edge of the lid by a row of tiny openings is a series of tarsal glands (Figure 9–20/6) that secrete a fatty material. Just in front of these glandular openings are the cilia (eyelashes), which are usually more prominent and numerous on the upper than on the lower lid; conspicuous cilia are absent from the lower lid of carnivores. Small ciliary and sebaceous glands are associated with the roots of the cilia; the common stye (hordeolum) is an inflammation of one of these glands.
The posterior surface of the lid is lined with conjunctiva, a thin, transparent mucous membrane. The palpebral conjunctiva is reflected at the base of the lids to continue on the sclera as the bulbar conjunctiva, which ends at the limbus, although the epithelium continues as the anterior epithelium of the cornea. The potential space between the lids and the eyeball is known as the conjunctival sac, and their dorsal and ventral extremities are the fornices (Figure 9–3/2). The transparency of the conjunctiva renders the smaller blood vessels visible, especially when they are congested in infections. Those in the bulbar conjunctiva move with this loosely attached layer; the deeper scleral vessels do not. Advantage is taken of this arrangement to distinguish inflammation of the conjunctiva from that of deeper structures. A pale conjunctiva suggests anemia, shock, or internal hemorrhage.
A slight mucosal elevation, the lacrimal caruncle, is present in the medial angle of the eye; it bears a few fine hairs in the large species (Figure 9–21/2).
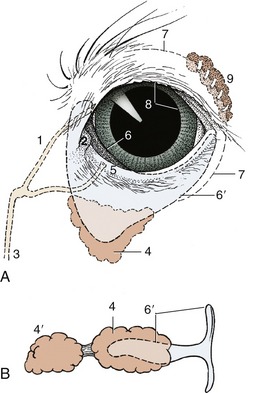
Figure 9–21 A, Left eye of dog showing third eyelid and lacrimal apparatus. B, Isolated cartilage of the third eyelid and associated glands of a pig. 1, Upper canaliculus; 2, lacrimal caruncle; 3, nasolacrimal duct; 4, gland of third eyelid; 4′, deep gland of third eyelid; 5, punctum lacrimale; 6, third eyelid; 6′, cartilage of third eyelid; 7, position of conjunctival fornix; 8, pupil; 9, lacrimal gland.
Between the lacrimal caruncle and the eyeball is a dorsoventrally oriented conjunctival fold known as the third eyelid (Figure 9–21/6). Unlike a true lid, it is covered with conjunctiva on both sides and is invisible when the eye is closed. The third eyelid is supported by a T-shaped piece of cartilage (Figure 9–21/6′) whose bar lies in the free edge of the fold and whose stem points backward into the orbit medial to the eyeball. The stem of the cartilage is surrounded by an additional lacrimal gland, the gland of the third eyelid; pigs and cattle also have a second, deeper gland. The secretion of these glands enters the conjunctival sac on the bulbar surface of the third eyelid. The third eyelid is kept retracted by smooth muscle (m. orbitalis) under sympathetic influence. It slides over the eyeball when the latter is retracted or pushed into the orbit. The lid, in conjunction with the retractor bulbi muscle, is thought to provide added protection to the protruding eyes of animals.
The Lacrimal Apparatus
This consists of the lacrimal gland proper, the gland(s) associated with the third eyelid, several small accessory glands, and a duct system that conveys the lacrimal fluid (tears), after it has washed over the eye, into the nasal cavity for evaporation. The lacrimal gland is flat and lies between the eyeball and the dorsolateral wall of the orbit (Figure 9–21/9). Its secretion is drained by many minute ducts into the dorsal fornix of the conjunctival sac, where it mixes with the secretions of the lesser glands. Blinking movements distribute the lacrimal fluid over the exposed part of the eye, which is thus kept moist; the tears carry away foreign material and supply the cornea with some nutriment. The fluid, being repelled by the fatty secretion of the tarsal glands along the edge of the lids, is normally pooled at the medial angle of the eye in the so-called lacrimal lake, a shallow depression surrounding the lacrimal caruncle, before being drawn by capillary action into the duct system through the puncta lacrimalia (Figure 9–20/4). Lacrimal fluid escapes onto the face only when produced in excessive amounts or when normal drainage is impaired.
The puncta lacrimalia are minute slits, one on the edge of each lid next to the caruncle. Each punctum leads to a short, narrow canaliculus through which the fluid flows to the much longer nasolacrimal duct (Figure 9–21/3). The beginning of the nasolacrimal duct is slightly enlarged, forming the lacrimal sac, which occupies a funnel-shaped fossa near the bony margin of the orbit. The nasolacrimal duct runs rostrally, at first within the thickness of the maxilla, then on its internal surface where it is covered by nasal mucosa. In some species it ends at the nostril, in others more deeply in the nasal cavity.
The tear film washing the eye consists of three layers. The outermost lipid layer is derived from the secretion of the tarsal glands; it helps spread the tears evenly and retards the breakup of the film. The thick middle aqueous layer is derived from the lacrimal glands; it moistens and nourishes the cornea. The innermost mucinous layer is produced by goblet cells in the conjunctiva and holds the tear film intimately to the cornea. Tear flow can be increased by drugs or reflexively after stimulation of the conjunctiva, cornea, or nasal mucosa. Weeping as an expression of emotion is a purely human phenomenon.
THE BLOOD SUPPLY OF THE EYE
The blood supply to the eyeball and its adnexa is complex (Figure 9–22). The blood supply to the human eye enters the orbit with the optic nerve. This route is represented in the domestic mammals by the rudimentary internal ophthalmic artery (Figure 9–22/2), which loses its identity when joined by a sizable anastomosis (Figure 9–22/4) from the external ophthalmic. The principal supply is carried by the external ophthalmic artery (Figure 9–22/3), a branch detached from the maxillary as this passes ventral to the orbit to supply more rostral structures of the face. The arteries arising from the external ophthalmic and malar arteries (a further, smaller branch of the maxillary) can be divided into three groups: (1) those supplying the eyeball, (2) those supplying ocular muscles, and (3) those leaving the orbit to supply adjacent structures, regardless of whether these are associated with the eye.
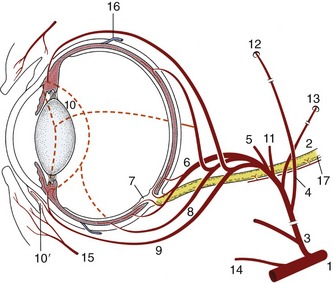
Figure 9–22 The principal arteries supplying the eye. 1, Maxillary a.; 2, rudimentary internal ophthalmic a.; 3, external ophthalmic a.; 4, anastomosis between external and internal ophthalmic aa.; 5, lacrimal a. to lacrimal gland and upper lid; 6, short posterior ciliary aa.; 7, retinal aa.; 8, long posterior ciliary aa.; 9, anterior ciliary aa., substantial branches to 10 in horse, lesser branches in the other domestic species; 10, greater arterial circle of the iris; 10′, annular pericorneal network; 11, muscular branches; 12, supraorbital a. and foramen; 13, external ethmoidal a. and foramen; 14, malar a.; 15, palpebral branches; 16, vorticose veins; 17, optic nerve.
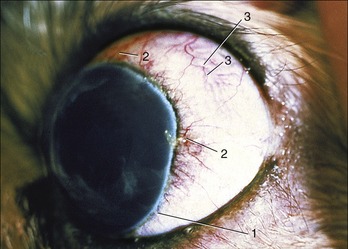
Figure 9–23 Exophthalmic canine eyeball and associated vascularization of bulbar conjunctiva and anterior sclera.
Most of the arteries described also take part in supplying the fat, fascia, and nerves within the orbit. There is some interspecific variation, but this is rarely of practical concern. However, it may be noted that the external ophthalmic artery of the ruminants breaks up and forms a small arterial network (rete mirabile ophthalmicum) on entering the orbit. The various arteries, except the malar, arise from this.
THE NERVE SUPPLY OF THE EYE
The nerve supply to the eye and its accessory structures is derived from no fewer than six cranial nerves. Most of these enter the orbital cone, but some reach accessory structures directly.
The optic nerve (II) enters the orbit through the optic foramen and passes to the light receptor cells in the retina. It is rather slack in order to allow for the movements of the eye and is covered by meninges that it acquired during its development as the stalk of the optic cup.
Though the name of the oculomotor nerve (III) implies that it controls movement of the eyeball, it does not innervate all the ocular muscles. It enters the orbit through the orbital foramen (fissure; foramen orbitorotundum in ruminants and the pig) and sends branches to the levator palpebrae; the dorsal, medial, and ventral recti; the ventral oblique; and part of the retractor muscles.
The trochlear nerve (IV) accompanies the third nerve and innervates the dorsal oblique muscle.
The ophthalmic and maxillary divisions of the trigeminal nerve (V) send branches to the eye. The ophthalmic nerve passes through the orbital foramen and supplies the following sensory branches: long ciliary nerves to the eyeball, especially the cornea; a lacrimal nerve to the eyelids and conjunctiva of the lateral angle; a supraorbital nerve that accompanies the like-named artery through the supraorbital foramen to supply the upper eyelid and skin medial to the orbit; an infratrochlear nerve (not present in all species) sensory to structures near the medial angle of the eye; and an ethmoidal nerve that follows the ethmoidal artery to innervate the caudal part of the nasal cavity. The maxillary nerve has only one relevant branch; this, the zygomatic nerve, supplies the lateroventral segment of the eyelids and conjunctiva by a zygomaticofacial branch and skin caudal to the orbit by a zygomaticotemporal branch. In horned cattle the zygomaticotemporal branch furnishes the clinically important cornual nerve to the horn. These sensory nerves to the orbit provide the afferent limbs of the palpebral and corneal reflexes that stimulate the orbicularis oculi to close the eye when the lids or cornea are touched.
The abducent nerve (VI) enters through the orbital foramen. It innervates most of the retractor bulbi and the lateral rectus muscles.
The auriculopalpebral branch of the facial nerve (VII) passes between the eye and ear and thus approaches the eyelids from behind. It innervates the orbicularis oculi. It may be blocked to immobilize the lids or to relieve the “pressure” that the tonus of the muscle may exert on a painful globe. (The levator palpebrae is not immobilized by this block.)
Sympathetic nerve fibers arising from the cranial cervical ganglion follow arteries or the ophthalmic nerve to the orbit, where they innervate the orbital muscle and dilator of the pupil. Tonus in the orbital muscle keeps the eyeball protruded, the third eyelid retracted, and the palpebral fissure open. Loss of sympathetic innervation results in a sunken eye, protrusion of the third eyelid, and constriction of the pupil (Horner’s syndrome). Dilation of the pupil (mydriasis) is initiated by fear, excitement, or pain.
Parasympathetic presynaptic nerve fibers enter the orbit within the oculomotor nerve. They synapse in the ciliary ganglion, and the postsynaptic fibers (which form the short ciliary nerves) innervate the ciliary muscle and the constrictor of the pupil. They control both the accommodation of the lens and the pupillary contraction (miosis) response to light.
THE EAR
The ear is appropriately called the vestibulocochlear organ because it not only enables the animal to hear but also provides it with a sense of balance. The mechanical stimuli produced by sound waves are transformed into nerve impulses in the cochlea, and the action of small amounts of fluid and microscopic crystals on neuroreceptors within the vestibule provides the animal with a perception of the attitude and movement of its head with respect to gravity. Both functions are performed in the internal ear, the most medial of the three subdivisions of the ear as a whole. The other subdivisions are the middle ear and the external ear. Only the external ear is visible in the intact animal; the other two are housed in the temporal bone (Figure 9–24/24).
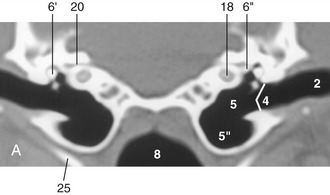
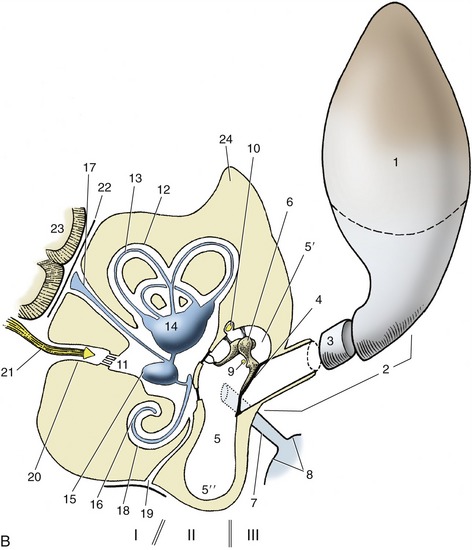
Figure 9–24 A, Transverse image of a 2-mm-thick computed tomographic slice of the canine tympanic bullae and petrous temporal bones. (Bone settings were used.) B, Schema of the right ear, caudal view. Note that the sizes of the structures shown are out of proportion to each other. I, Internal ear; II, middle ear; III, external ear. 1, Auricle; 2, external acoustic meatus; 3, annular cartilage; 4, tympanic membrane; 5, tympanic cavity; 5′, epitympanic recess; 5″, tympanic bulla; 6, auditory ossicles; 6′, malleus; 6″, base of stapes in vestibular window; 7, auditory tube; 8, nasopharynx; 9, chorda tympani; 10, facial nerve; 11, vestibule; 12, semicircular canals; 13, semicircular ducts; 14, utriculus; 15, sacculus; 16, cochlear duct; 17, endolymphatic duct; 18, cochlea; 19, perilymphatic duct; 20, internal acoustic meatus; 21, vestibulocochlear nerve in internal acoustic meatus; 22, meninges; 23, brain; 24, petrous temporal bone; 25, stylohyoid bone.
THE EXTERNAL EAR
The external ear consists of two parts, the auricle and the external acoustic meatus (Figure 9–24/1,2). The auricle, or pinna, is the “ear” as it is understood by the layperson, the part that sticks out from the head. The external acoustic meatus is the canal that leads from the base of the auricle to the eardrum (tympanic membrane) stretched across an opening in the temporal bone.
The auricle is shaped like a funnel; distally it is wide open to receive the sound, and more proximally it is rolled up to form a tube that bends medially for connection with the external acoustic meatus. The auricle can be turned toward the source of sound; right and left auricles can move independently so that each can focus on separate sounds. The animal does not have to turn its head as we with our immobile “ears” are obliged to do.
The shape of the auricle is determined by the supporting auricular cartilage (Figure 9–25). In most domestic mammals this is sufficiently stiff to keep the auricle erect at all times. In many breeds of dogs and in certain other animals, the cartilage is relatively soft, allowing the auricle to collapse; even so, most dogs can prick their ears and make them turn when attention to sound requires it.
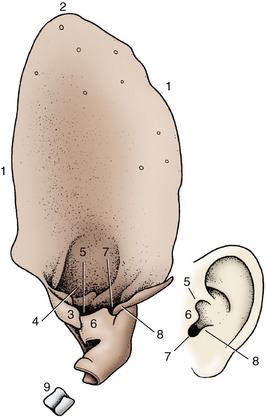
Figure 9–25 Left auricular cartilage of dog compared with human ear. 1, Helix; 2, apex; 3, medial crus of helix; 4, lateral crus of helix; 5, pretragic notch; 6, tragus; 7, intertragic notch; 8, antitragus; 9, annular cartilage.
A complex set of auricular muscles, all voluntary, is responsible for the movement of the ear. These muscles arise from various points on the skull and adjacent fasciae and attach to the base of the auricle. A flat, palpable (scutiform) cartilage rostral to the ear redirects the pull of some. The auricular muscles are innervated by branches of the facial nerve.
The external acoustic meatus begins where the rolled-up part of the auricular cartilage narrows and ends at the eardrum (Figure 9–24/2). The meatus therefore has cartilaginous and osseous parts. It is lined with skin that contains sebaceous and tubular ceruminous glands. The latter secrete the earwax (cerumen), which is thought to prevent dust from reaching the delicate tympanic membrane. The ear of the dog is of the most clinical interest. Unfortunately, its external acoustic meatus is curved, which makes the passage of the straight otoscope for the examination of the proximal part of the meatus and eardrum difficult.
THE MIDDLE EAR
The middle ear is housed in the temporal bone and is essentially the small air-filled space known as the tympanic cavity (Figure 9–24/5). It is lined with a thin mucous membrane and communicates with the nasopharynx by the auditory tube (Figure 9–24/7). The upper part of the tympanic cavity is compressed from side to side and slanted outward. The lateral wall of the cavity incorporates the tympanic membrane (Figure 9–24/4). The medial wall is formed by the petrous part of the temporal bone, which houses the internal ear. It contains two windows (fenestrae), closed in the natural state, through which the mechanical stimuli produced by sound waves enter the internal ear for translation into nerve impulses. The more dorsal vestibular window connects the tympanic cavity with the vestibule of the internal ear. In the live animal it is occupied by the stapes, the most medial of the auditory ossicles (Figure 9–24/6). The other, the cochlear window, leads to the cavity of the cochlea (Figure 9–24/18). It is closed by the thin secondary tympanic membrane. Ventral to the two windows the medial wall bulges over the cochlea, forming the promontory.
The tympanic cavity may be divided into dorsal, middle, and ventral parts. The dorsal part (epitympanic recess) is situated above the level of the tympanic membrane. It contains the chain of auditory ossicles and the two associated muscles. The middle part includes the tympanic membrane in its lateral wall and opens rostrally into the nasopharynx via the auditory tube. The ventral part is an enlarged bulbous extension of the temporal bone known as the tympanic bulla (Figure 9–24/5″). The bulla varies in prominence among species; in some it is subdivided into numerous bony cells. The function is not known with certainty, but it has been suggested that it may improve the perception of sounds of very low and very high frequencies.
The tympanic membrane (Figure 9–26) is a thin partition separating the lumen of the external acoustic meatus from that of the tympanic cavity. Like the tympanic cavity, it is slanted so that its dorsal part is more lateral than its ventral part, and its surface area is thus considerably larger than that of the transected external acoustic meatus. The dog’s eardrum on average measures 10 × 15 mm; its long axis is oriented rostrocaudally. Its lateral surface is covered with an epidermis continuous with that of the meatus, its medial surface by the mucosa lining the tympanic cavity. A layer of fibrous tissue between epidermis and mucosa firmly attaches the membrane to the osseous tympanic ring of the temporal bone. The tympanic ring is interrupted dorsally by a notch that extends onto the roof of the external acoustic meatus. The part of the tympanic membrane attached to the tympanic ring is tense; the part that closes the notch is flaccid.
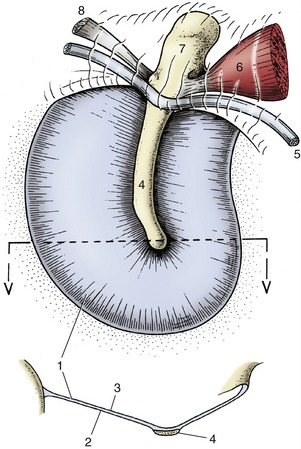
Figure 9–26 Medial surface and transverse section (below) of canine tympanic membrane. 1, Tense part of tympanic membrane; 2, medial surface; 3, lateral surface; 4, handle of malleus; 5, chorda tympani; 6, m. tensor tympani; 7, head of malleus; 8, one of the ligaments associated with the malleus.
The handle of the malleus (Figure 9–26/4), the most lateral of the ear ossicles, is embedded in the medial surface of the tympanic membrane. Tension in the chain of ossicles pulls the tympanic membrane medially, which hollows its lateral surface. The handle shines through the thin membrane and is visible as a light band (stria mallearis) when the eardrum is examined with an otoscope (see Figure 11–43, A-B).
Auditory Ossicles
The transmission of sound waves across the tympanic cavity is mediated by the three auditory ossicles (Figure 9–24/6) known, in lateromedial sequence, as malleus, incus, and stapes (Latin names for hammer, anvil, and stirrup, from their rather fanciful resemblance to these objects).
The handle (manubrium) of the malleus (Figure 9–27/3) is embedded in the tympanic membrane so that the head of the malleus protrudes above the membrane by a few millimeters. The head articulates with the body of the incus, and the latter articulates with the head of the stapes by means of its long crus. The base (footplate) of the stapes sits in the vestibular window in the medial wall of the tympanic cavity.
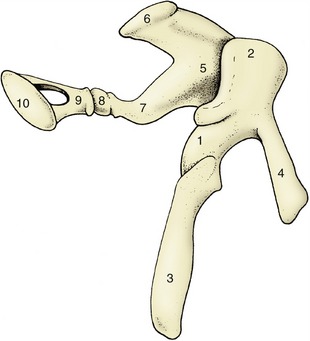
Figure 9–27 Left auditory ossicles of the horse, craniomedial view. 1, Malleus; 2, head of malleus; 3, handle of malleus; 4, rostral process; 5, incus; 6, short crus; 7, long crus; 8, os lenticulare; 9, head of stapes; 10, base (footplate) of stapes.
The oscillations of the tympanic membrane perceived by the handle of the malleus are magnified and transmitted to the vestibular window by lever action through the chain of ossicles. The base of the stapes is set in motion, which causes the fluid in the internal ear to vibrate. This stimulates the neuroreceptor cells in the membranous labyrinth, and sound is perceived.
The mechanism of sound transmission from the outside to the internal ear may not in fact be quite so simple. There is evidence that sound waves are also transmitted to the fluid through the walls of the tympanic cavity and directly through the cochlear window.
The auditory ossicles are attached to the wall of the epitympanic recess by several ligaments, and their relationships can be altered by two small muscles (tensor tympani and stapedius). These are believed to tense the tympanic membrane and the chain of ossicles in an effort to decrease the amplitude of their vibrations in the lower frequencies and to protect the system from damage caused by sudden overload (see p. 317 for their innervation).
Auditory Tube
This structure, often called the Eustachian tube, connects the tympanic cavity with the nasopharynx (Figure 9–24/8). It is short with a narrow lumen that is laterally compressed and usually collapsed. The tube is confined by an inverted cartilaginous trough except along its ventral border. The membranous wall of the horse’s auditory tube evaginates through this ventral defect in the cartilaginous support to form the large, thin-walled guttural pouch dorsolateral to the nasopharynx (see p. 522).
The pharyngeal openings of the auditory tubes are located in the lateral walls of the nasopharynx and are marked by accumulations of lymphoid tissue (tubal tonsils) (see Figure 18–11/8). The cartilage of the auditory tube extends into the medial wall of the pharyngeal opening and stiffens it. The auditory tubes allow equalization of the pressures on the two sides of the delicate eardrums. The pressure sometimes becomes unbalanced, for example, during a ride in an express elevator, and its sudden restoration causes our ears to pop. The auditory tubes temporarily open each time we swallow or yawn. This permits the slight secretion from the goblet cells and the glands in the lining of the tympanic cavity to escape.
THE INTERNAL EAR
The mechanical stimuli produced by sound and by the positional changes of the head are transformed into nerve impulses in the internal ear. This is a delicate mechanism, no larger than about 12 mm across in the dog, and is completely enclosed in the very hard petrous temporal bone for protection and proper functioning (Figure 9–24, A). It is exposed to sound vibrations on the lateral surface, and the impulses into which these are converted leave the bone in nerve fibers that pass through the internal acoustic meatus on the medial surface.
The internal ear consists of a closed system of tiny membranous ducts and cavities known, because of its complexity, as the membranous labyrinth (Figure 9–28, A). This contains endolymph whose movement inside the system stimulates sensory cells in the membranous wall. Two enlargements in the center of the membranous labyrinth are known as the utriculus and sacculus. From the utriculus arise three semicircular ducts concerned with balance, and from the sacculus, the spiral cochlear duct, which is concerned with hearing.
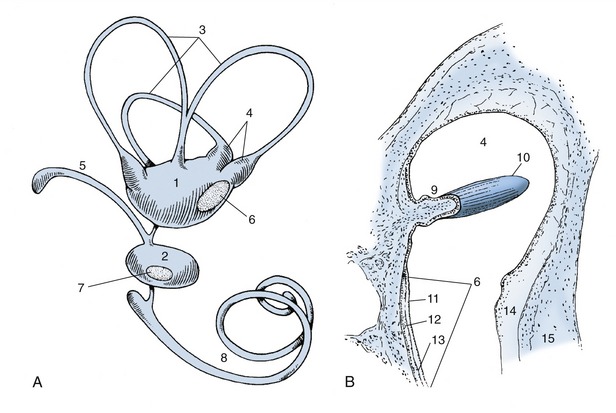
Figure 9–28 A, Membranous labyrinth. B, Section of ampulla. 1, Utriculus; 2, sacculus; 3, semicircular ducts; 4, ampullae containing ampullary crests; 5, endolymphatic duct; 6, 7, maculae; 8, cochlear duct; 9, ampullary crest; 10, cupula containing sensory hairs; 11, layer of neuroepithelial hair cells; 12, statoconia; 13, gelatinous layer of macula; 14, perilymphatic space; 15, wall of osseous labyrinth.
The semicircular ducts stand roughly at right angles to each other and are designated anterior, posterior, and lateral; one end of each duct is ampullated close to the utriculus. The endolymph within them is set in motion by movements of the head, and this results in pressure on minute barriers (cristae ampullares) present in each ampulla (Figure 9–28/9,10). These pressures deflect sensory hairs projecting from the receptor cells of the cristae, stimulating the individual cells to send impulses to the central nervous system.
Two further receptor areas called maculae (Figure 9–28/6,7) are present in the walls of the utriculus and sacculus. They monitor the position of the head with respect to gravity. Although the maculae are bathed in endolymph, they react to a layer of crystals (statoconia) adhering to a gelatinous layer that surrounds the sensory hairs of the receptor cells. When the gelatinous layer of the maculae faces toward the ground, the cells are maximally stimulated by the gravitational pull. The maculae thus record the position of the head, whereas the cristae record the movements of the head.
The sacculus gives origin to the endolymphatic duct, which ends blindly in the epidural space (Figure 9–24/17). It is thought to function in the resorption of the endolymph secreted by the epithelial lining of the membranous labyrinth.
The membranous labyrinth is housed in a similar but slightly larger osseous labyrinth, a complex excavation in the temporal bone. The central chamber of the osseous labyrinth, the vestibule, houses the utriculus and the sacculus. The semicircular ducts lie within the osseous semicircular canals. The cochlear duct passes up the spiral canal of the cochlea, which is an excavation very similar to the inside of a snail’s shell. The center of the cochlea is an osseous pyramid known as the modiolus (Figure 9–29/2). Running around the modiolus is the spiral canal, the actual lumen of the cochlea, which ends blindly at the apex of the modiolus. Projecting into the spiral canal from the modiolus is an osseous shelf, the spiral lamina (Figure 9–29/5), which terminates in the blind end of the spiral canal of the cochlea. The spiral lamina itself is hollow, forming the spiral canal of the modiolus.
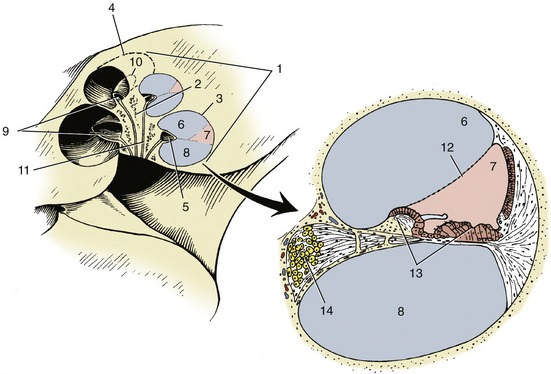
Figure 9–29 Cochlea and enlarged cochlear duct. 1, Cochlea; 2, modiolus; 3, 4, spiral canal of cochlea; 5, osseous spiral lamina; 6, scala vestibuli; 7, cochlear duct; 8, scala tympani; 9, 10, spiral canal of modiolus; 11, longitudinal canals; 12, spiral membrane; 13, spiral organ; 14, spiral ganglion.
Because the osseous labyrinth is slightly larger than the membranous labyrinth it encloses, there is a minute space between the two containing perilymph. Only the two perilymphatic spaces (scala tympani and scala vestibuli) accompanying the cochlear duct into the cochlea need be considered further.
The spiral canal of the cochlea is divided by a split longitudinal membrane into three channels (9–29/6,7,8), all running around the modiolus to the apex of the cochlea. The membrane arises centrally from the spiral lamina and, after splitting, attaches to the outside wall of the spiral canal. The uppermost channel is the scala vestibuli, the middle one is the cochlear duct, and the lowest is the scala tympani. The two scalae communicate at the apex of the cochlea around the blind end of the cochlear duct. At the base of the cochlea, the scala vestibuli communicates with the perilymphatic space in the vestibule, and the scala tympani ends at the secondary tympanic membrane (see Figure 9–24).
An enlarged transverse section of the spiral canal of the cochlea shows the composition of the split membrane, particularly the part that forms the walls of the triangular cochlear duct (Figure 9–29/7). The simplest of these walls separates the cochlear duct from the scala vestibuli; it consists of a single layer of cells and is known as the spiral membrane. The wall of the cochlear duct facing the scala tympani is complex by virtue of the large neuroreceptor and other cells found in it. Its connective tissue component is the basilar lamina, which plays an important role in the perception of sound. The cells form the spiral organ (Figure 9–29/13) in which originate the nerve impulses that are produced by the sounds received by the external ear.
The impulses travel toward the modiolus to ganglion cells housed in the spiral canal. The aggregate of these cells forms the spiral ganglion (Figure 9–29/14), which also winds around the modiolus. From the spiral ganglion the impulses travel along nerve fibers within canals to the base of the modiolus, where the fibers join to form the cochlear part of the vestibulocochlear nerve.
As the base of the stapes rocks in the vestibular window in unison with the vibrations of the tympanic membrane, it compresses the perilymph in the closed system of perilymphatic spaces. Because fluids are incompressible, the required “give” is found in similar vibrations of the secondary tympanic membrane closing the cochlear window. The way in which the mechanical stimuli in the vibrating columns of fluid within the cochlea act on the receptor cells in the spiral organ is complex and beyond the scope of this book. The width and structure of the basilar lamina suggests that, at least in humans, lower pitched sound is “read” by a relatively short stretch of the spiral organ near the apex of the cochlea. The remaining much longer stretch of the spiral organ responds to sounds of higher frequency, including those of speech.
The anatomy of the internal and middle ear is complicated by the passage of the facial nerve through this area (Figure 9–24/10). The facial nerve enters the internal acoustic meatus together with the vestibulocochlear nerve and, within an osseous facial canal, traverses the temporal bone to emerge at the stylomastoid foramen. The facial canal makes a sharp kneelike bend within the temporal bone, and at this point the nerve is enlarged by the geniculate ganglion. From this arises the major petrosal nerve, which regulates secretion of the lacrimal and nasal glands. The chorda tympani, regulating the sublingual and mandibular glands but also relaying taste from the rostral two thirds of the tongue, leaves the facial nerve a little more distally. The chorda tympani is so named because, for a short segment of its course, it lies on the upper part of the tympanic membrane (Figure 9–26/5). Both major petrosal and chorda tympani nerves leave the temporal bone through foramina on the rostroventral aspect of the bone. The facial nerve also supplies the stapedius muscle. (The tensor tympani is activated through the mandibular division of the trigeminal nerve [V3].)
The vestibulocochlear nerve (VIII) divides into vestibular and cochlear parts as it enters the internal acoustic meatus. The branches of the vestibular part pass to the neuroreceptor areas in the utriculus and sacculus, conveying impulses concerned with balance; the cochlear part passes into the base of the cochlea to mediate the impulses concerned with hearing.
THE OLFACTORY ORGAN
The sense of smell is much better developed in the domestic mammals than in ourselves; this is particularly true of the dog, which can detect airborne substances in incredibly low concentrations. Much of the “contact” with the environment and with other animals is made through this sense, and the examples given here underscore the importance of olfaction in animal life. This capability is exploited when dogs are used to “point” at game, to follow a scent in tracking fugitives (or detect drugs and explosives), and when dogs and pigs are trained to find buried truffles. Dams recognize their offspring largely by the sense of smell, wild animals identify the extent of their territory by odorants on the ground, and wild herbivores test the air for the scent of predators.
The olfactory organ is of course situated in the nose. In animals with a well-developed sense of smell, it consists of a relatively large area of olfactory mucosa covering the lateral wall and the ethmoidal conchae in the caudal part of the nasal cavity. Although claimed to be a little more yellowish than the respiratory mucosa rostral to it, the olfactory mucosa cannot convincingly be identified by gross inspection. Histological sections show the presence of olfactory cells that, like the light-receptor cells in the retina, are bipolar neurons. Their dendrites reach the surface of the epithelium, presenting several minute olfactory hairs (cilia) to the air in the nasal cavity. The axons of the cells combine to form the fascicles of the olfactory nerve (cranial nerve I) that pass through the cribriform plate to the nearby olfactory bulb. Serous olfactory glands below the olfactory epithelium moisten the surface of the epithelium, presumably to wash away previously perceived odorants no longer present in the air.
The vomeronasal organ* found in the nasal cavity is also concerned with olfaction. It consists of two narrow, parallel ducts that are embedded in the hard palate, one to each side of its junction with the nasal septum. The ducts, which are supported laterally, ventrally, and medially by thin cartilages, are lined in part with olfactory mucosa (Figure 9–30, A-B). Caudally they end blindly, but rostrally they open into the incisive ducts, which in most mammals connect the nasal and oral cavities through openings in the hard palate. The communication with the oral cavity is lacking in horses and donkeys. This organ has received considerable attention from animal behaviorists and reproductive physiologists because of its involvement in sexual activity, particularly in the lip-curl (Flehmen) reaction demonstrated by male animals aroused by the odor of vaginal secretion or urine from estrous females (Figure 9–31, A-B). Whether the Flehmen reaction as well as the accompanying extension of the head helps the odorants reach the vomeronasal organ is still a matter of speculation. Experimental blockage of the incisive ducts modifies but does not eliminate the Flehmen reaction and other responses of bulls exposed to the pheromones contained within the vaginal secretion of cows in heat.
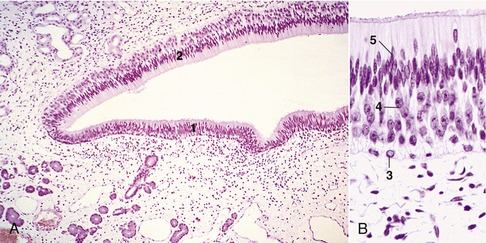
Figure 9–30 A, Vomeronasal organ (pig) (HE) (70×). B, Vomeronasal organ (pig) (HE) (279×). 1, Ciliated pseudostratified columnar respiratory epithelium; 2, pseudostratified columnar epithelium of basal; 3, ciliated sustentacular; 4, and neurosensitive; 5, cells.
THE GUSTATORY ORGAN
The receptors for the sense of taste are the taste buds (Figure 9–32), microscopic nests of cells mainly associated with the papillae of the tongue, although small numbers are also found in the soft palate and in the vicinity of the epiglottis. Taste buds are about as tall as the epithelium in which they lie and communicate with the oral cavity by taste pores through which solutions enter to stimulate the receptor cells. Taste pores cannot be seen with the naked eye.
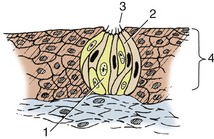
Figure 9–32 Histological section of a taste bud. 1, Sustentacular cell; 2, gustatory cells; 3, taste pore; 4, epithelium.
The taste buds consist of sustentacular or supporting cells in addition to the receptor or gustatory cells. The latter have elongated nuclei and at their free tips bear microvilli (taste hairs) that project into the taste pore. Glands deep to the papillae discharge a serous secretion on the surface of the epithelium. It is believed that the secretion cleanses the taste pores and enhances perception by the gustatory cells.
To be discerned, food substances have to be in solution. One of the reasons food is insalivated is to dissolve parts for sampling by the taste buds. The principal taste sensations are sweetness, sourness, and saltiness. In the dog it appears that sweetness and saltiness are perceived in the rostral two thirds of the tongue where taste buds are present on the fungiform papillae. Sour substances are perceived over the entire tongue. The caudal third of the tongue, which incorporates the vallate and foliate papillae, therefore seems to respond only to what tastes sour.
The afferent pathways mediating these sensations are similarly divided. In the rostral two thirds of the tongue the pathways travel at first in the lingual nerve and then in the chorda tympani, which we encountered in the description of the ear. After passing through the geniculate ganglion of the seventh cranial nerve, they enter the medulla oblongata. The afferent fibers from the caudal third of the tongue travel in the glossopharyngeal nerve (and to a small extent in the vagus) to the medulla oblongata.
THE CUTANEOUS SENSE
As mentioned at the beginning of the chapter, much of the more immediate environment is experienced by the animal through its skin. The sensations are touch, pressure, pain, heat, and cold; touch is a light stimulus such as is produced by a fly on the haircoat, and pressure is a stronger and deeper stimulus such as a horse feels from a saddle or girth. The receptors responsible for the detection of these stimuli vary considerably in structure. Unfortunately, because many intermediate forms exist, it is difficult to classify them and assign clear-cut functions to each kind. The simple classification given here is probably adequate for the purpose of this book.
The sensory receptors of the skin can be divided into free nerve endings and those that bear terminal corpuscles. The free nerve endings are tufts formed by the branches of nerve fibers that terminate either in fine points or in buttonlike swellings; they are found principally in the epidermis and are thought to be pain receptors (Figure 9–33/1). The corpuscular endings fall into three kinds: bulbous, lamellar, and meniscoid. The bulbous corpuscles, which are encapsulated terminal tufts of nerve fibers found in the dermis, are thought to respond to heat or cold (Figure 9–33/2). The lamellar corpuscles are large (2–3 mm) and consist of many concentric lamellae (flattened cells) in the center of which is the nerve fiber; they are found in the subcutis and are thought to be pressure receptors (Figure 9–33/3). Meniscoid corpuscles consist of small cup-shaped discs (menisci) at the ends of nerve fibers with which they contact “tactile” cells; they are found, usually encapsulated, both in the papillary layer of the dermis and free in the adjacent epidermis and are thought to be touch receptors (Figure 9–33/4).
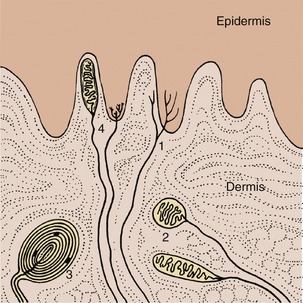
Figure 9–33 Sensory nerve endings of the skin, schematic. 1, Free nerve endings (pain); 2, bulbous corpuscles (heat or cold); 3, lamellar corpuscles (vibration); 4, meniscoid nerve endings (touch).
A special sort of cutaneous sense is mediated by the tactile hairs. These are long, protrude from the head, and are substantially thicker than the hairs forming the haircoat. The cat’s whiskers are good examples, but all domestic mammals have them, principally about their muzzle and eyes. The walls of blood spaces (sinuses) surrounding the roots of these hairs contain numerous nerve endings. When the tips of the sinus hairs are touched, these nerve endings are stimulated and an impulse is sent to the central nervous system (see also p. 360).
PROPRIOCEPTION
This depends on the action of numerous nerve endings (proprioceptors) embedded in skeletal muscle, tendons, joint capsules, and ligaments. These specialized nerve endings are not unlike some of the skin receptors. They respond to stretching or compression and inform the animal not only of the degree to which a muscle is contracted, a tendon tensed, or a joint flexed but also of the rate at which these changes occur. This information travels centrally through sensory cell bodies in the dorsal root ganglia near the spinal cord and activates reflexes indispensable for the coordination of muscle groups in maintaining posture and effecting movement. (It is proprioception that enables us to describe the exact position and attitude of our lower limbs, i.e., without having to look at them.) If for some reason proprioception is disturbed, movements become ataxic; that is, muscular coordination is lost. Receptors that mediate pain, particularly of joints, are closely associated with the proprioceptors; the impulses from these travel in pathways that accompany those of proprioception to the spinal cord and thence to higher centers.
ENTEROCEPTION
Receptors present in the hollow viscera respond to extreme dilation, to contraction or spasm (colic), and to chemical irritation. These sensations are translated as pain and, when the affected organ is in the abdominal cavity, are often accompanied by reflex contraction of the abdominal muscles and cessation of abdominal breathing. A rigid abdomen is an important accompanying diagnostic sign.
Referred pain, although important in human medicine, is of doubtful significance in animals. Pain impulses from the viscera share spinal cord pathways with sensory impulses arising from cutaneous zones that do not necessarily overlie these viscera but develop at the same embryological level. Because these pathways are much more often used by impulses from the cutaneous zones, it is not surprising that the brain misinterprets the origin of the much less frequent pain impulses from the viscera. The most widely known example is the pain referred to the presternal region, neck, shoulders, and inner aspect of the left arm in humans with angina pectoris, which is a lack of oxygen to the heart tissue due to an inadequate blood supply.
Small cutaneous zones have been identified in the cow as being related through the nervous system to certain abdominal organs. These zones become hypersensitive when the corresponding organs are diseased. It is interesting that these zones broadly coincide with the acupuncture points specified in the animal “maps” from China that have become known to the Western world in recent years.