CHAPTER 1 Clinical Manifestations of Cardiac Disease
SIGNS OF HEART DISEASE
Signs of heart disease can be apparent even if the animal is not clinically in “heart failure.” Objective signs of heart disease include cardiac murmurs, rhythm disturbances, jugular pulsations, and cardiac enlargement. Other clinical signs that can result from heart disease include syncope, excessively weak or strong arterial pulses, cough or respiratory difficulty, exercise intolerance, abdominal distention, and cyanosis. However, noncardiac diseases can cause these signs as well. Further evaluation using thoracic radiography, electrocardiography (ECG), echocardiography, and sometimes other tests is usually indicated when signs suggestive of cardiovascular disease are present.
SIGNS OF HEART FAILURE
Cardiac failure occurs when the heart cannot adequately meet the body’s circulatory needs or can do so only with high filling (venous) pressures. Most clinical signs of heart failure (Box 1-1) relate to high venous pressure behind the heart (congestive signs) or inadequate blood flow out of the heart (low output signs). Congestive signs associated with right-sided heart failure stem from systemic venous hypertension and the resulting increases in systemic capillary pressure. Congestion behind the left side of the heart produces pulmonary venous hypertension and edema. Biventricular failure develops in some animals. Chronic left-sided congestive heart failure can facilitate the development of right-sided signs when pulmonary arterial pressure rises secondary to pulmonary venous hypertension. Signs of low cardiac output are similar regardless of which ventricle is primarily affected, because output from the left heart is coupled to that from the right heart. Heart failure is discussed further in Chapter 3 and within the context of specific diseases.
 BOX 1-1 Clinical Signs of Heart Failure
BOX 1-1 Clinical Signs of Heart Failure
Congestive Signs—Left (↑Left Heart Filling Pressure)
WEAKNESS AND EXERCISE INTOLERANCE
Cardiac output often becomes inadequate for the level of activity in animals with heart disease or failure. Impaired skeletal muscle perfusion during exercise, related to vascular and metabolic changes that occur over time, can reduce exercise tolerance. Increased pulmonary vascular pressures and edema also lead to poor exercise tolerance. Episodes of exertional weakness or collapse can relate to these changes or to an acute decrease in cardiac output caused by arrhythmias (Box 1-2).
 BOX 1-2 Causes of Syncope or Intermittent Weakness
BOX 1-2 Causes of Syncope or Intermittent Weakness
AV, Atrioventricular.
Cardiovascular Causes
SYNCOPE
Transient unconsciousness associated with loss of postural tone (collapse) from insufficient oxygen or glucose delivery to the brain characterizes the clinical sign of syncope. Various cardiac and noncardiac abnormalities can cause syncope, as well as intermittent weakness (see Box 1-2). Syncope can be confused with seizure episodes (Fig. 1-1). A careful description of the animal’s behavior or activity before the collapse event, during the event itself, and following the collapse, as well as a drug history, helps the clinician differentiate among syncopal attacks, episodic weakness, and true seizures. Syncope is often associated with exertion or excitement. The actual event may be characterized by rear limb weakness or sudden collapse, lateral recumbency, stiffening of the forelimbs and opisthotonos, and micturition (Fig. 1-2). Vocalization is common; however, tonic/clonic motion, facial fits, and defecation are not. An aura (which often occurs before seizure activity), postictal dementia, and neurologic deficits are generally not seen in dogs and cats with cardiovascular syncope. Sometimes profound hypotension or asystole causes hypoxic “convulsive syncope,” with seizurelike activity or twitching; these convulsive syncopal episodes are preceded by loss of muscle tone. Presyncope, wherein reduced brain perfusion (or substrate delivery) is not severe enough to cause unconsciousness, may appear as transient “wobbliness” or weakness, especially in the rear limbs.

FIG 1-1 Syncope in a cat with intermittent complete AV block and delayed onset of ventricular escape rhythm. During these episodes the cat initially appeared dazed, then fell to her side and stiffened briefly. Within a few seconds she would regain consciousness and resume normal activity.

FIG 1-2 Syncope in a Doberman Pinscher with paroxysmal ventricular tachycardia. Note the extended head and neck with stiffened forelimbs. Involuntary micturition also occurred, followed shortly by return of consciousness and normal activity.
Testing to determine the cause of intermittent weakness or syncope usually includes ECG recordings (during rest, exercise, and/or after exercise or a vagal maneuver), complete blood count (CBC), serum biochemical analysis (including electrolytes and glucose), neurologic examination, thoracic radiographs, heartworm testing, and echocardiography. Other studies for neuromuscular or neurologic disease may also be valuable. Intermittent cardiac arrhythmias not apparent on resting ECG may be uncovered by 24-hour ambulatory ECG (Holter monitor), event monitoring, or in-hospital continuous ECG monitoring.
Cardiovascular Causes of Syncope
Various arrhythmias, ventricular outflow obstructions, cyanotic congenital heart defects, and acquired diseases leading to poor cardiac output are the usual cardiac causes of syncope. Activation of vasodepressor reflexes and excessive dosages of cardiovascular drugs can also induce syncope. Arrhythmias that provoke syncope are usually associated with very fast or very slow heart rate and can occur with or without identifiable underlying organic heart disease. Ventricular outflow obstructions provoke syncope or sudden weakness if cardiac output becomes inadequate during exercise or if high systolic pressures activate ventricular mechanoreceptors, causing inappropriate reflex bradycardia and hypotension. Both dilated cardiomyopathy and severe mitral insufficiency can cause inadequate forward cardiac output, especially during exertion. Vasodilators and diuretics may induce syncope if given in excess. Syncope caused by abnormal peripheral vascular and/or neurologic reflex responses is not well defined in animals but is thought to occur in some patients. Syncope during sudden bradycardia after a burst of sinus tachycardia has been documented, especially in small breed dogs with advanced atrioventricular (AV) valve disease; excitement often precipitates such an episode. Doberman Pinschers and Boxers may experience a similar syndrome. Postural hypotension and hypersensitivity of carotid sinus receptors may infrequently provoke syncope by inappropriate peripheral vasodilation and bradycardia.
Fainting associated with a coughing fit (cough syncope or “cough-drop”) occurs in some dogs with marked left atrial enlargement and bronchial compression, as well as in dogs with primary respiratory disease. Several mechanisms have been proposed, including an acute decrease in cardiac filling and output during the cough, peripheral vasodilation after the cough, and increased cerebrospinal fluid pressure with intracranial venous compression. Severe pulmonary diseases, anemia, certain metabolic abnormalities, and primary neurologic diseases can also cause collapse resembling cardiovascular syncope.
COUGH AND OTHER RESPIRATORY SIGNS
Congestive heart failure (CHF) in dogs results in cough, tachypnea, and dyspnea. These signs also can be associated with the pulmonary vascular disease and pneumonitis of heartworm disease in both dogs and cats. Noncardiac conditions, including diseases of the upper and lower airways, pulmonary parenchyma (including noncardiogenic pulmonary edema), pulmonary vasculature, and pleural space, as well as certain nonrespiratory conditions, also should be considered in patients with cough, tachypnea, or dyspnea (see Chapter 19).
The cough caused by cardiogenic pulmonary edema in dogs is often soft and moist, but it sometimes sounds like gagging. In contrast, cough is an unusual sign of pulmonary edema in cats. Tachypnea progressing to dyspnea occurs in both species. Pleural and pericardial effusions occasionally are associated with coughing as well. Mainstem bronchus compression caused by severe left atrial enlargement can stimulate a cough (often described as dry or hacking) in dogs with chronic mitral insufficiency, even in the absence of pulmonary edema or congestion. A heartbase tumor, enlarged hilar lymph nodes, or other masses that impinge on an airway can also mechanically stimulate coughing.
When respiratory signs are caused by heart disease, other evidence, such as generalized cardiomegaly, left atrial enlargement, pulmonary venous congestion, lung infiltrates that resolve with diuretic therapy, and/or a positive heartworm test, is usually present. The findings on physical examination, thoracic radiographs, an echocardiogram if possible, and sometimes electrocardiography help the clinician differentiate cardiac from noncardiac causes of respiratory signs.
THE CARDIOVASCULAR EXAMINATION
The medical history (Box 1-3) is an important part of the cardiovascular evaluation that helps guide the choice of diagnostic tests by suggesting various cardiac or noncardiac diseases. The signalment is useful because some congenital and acquired abnormalities are more prevalent in certain breeds or life stages or because specific findings are common in individuals of a given breed (e.g., soft cardiac ejection murmur in normal Greyhounds).
Physical evaluation of the dog or cat with suspected heart disease includes observation (e.g., attitude, posture, body condition, level of anxiety, respiratory pattern) and a general physical examination. The cardiovascular examination itself consists of evaluating the peripheral circulation (mucous membranes), systemic veins (especially the jugular veins), systemic arterial pulses (usually the femoral arteries), and the precordium (left and right chest walls over the heart); palpating or percussing for abnormal fluid accumulation (e.g., ascites, subcutaneous edema, pleural effusion); and auscultating the heart and lungs. Proficiency in the cardiovascular examination requires practice but is important for accurate patient assessment and monitoring.
OBSERVATION OF RESPIRATORY PATTERN
Respiratory difficulty (dyspnea) usually causes the animal to appear anxious. Increased respiratory effort, flared nostrils, and often a rapid rate of breathing are evident (Fig. 1-3). Increased depth of respiration (hyperpnea) frequently results from hypoxemia, hypercarbia, or acidosis. Pulmonary edema (as well as other pulmonary infiltrates) increases lung stiffness; rapid and shallow breathing (tachypnea) results as an attempt to minimize the work of breathing. An increased resting respiratory rate is an early indicator of pulmonary edema in the absence of primary lung disease. Large-volume pleural effusion or other pleural space disease (e.g., pneumothorax) generally causes exaggerated respiratory motions as an effort to expand the collapsed lungs. It is important to note whether the respiratory difficulty is more intense during a particular phase of respiration. Prolonged, labored inspiration is usually associated with upper airway disorders (obstruction), whereas prolonged expiration occurs with lower airway obstruction or pulmonary infiltrative disease (including edema). Animals with severely compromised ventilation may refuse to lie down; they stand or sit with elbows abducted to allow maximal rib expansion, and they resist being positioned in lateral or dorsal recumbency (orthopnea). Cats with dyspnea often crouch in a sternal position with elbows abducted. Open-mouth breathing is usually a sign of severe respiratory distress in cats (Fig. 1-4). The increased respiratory rate associated with excitement, fever, fear, or pain can usually be differentiated from dyspnea by careful observation and physical examination.

FIG 1-3 Dyspnea in an older male Golden Retriever with advanced dilated cardiomyopathy and fulminant pulmonary edema. The dog appeared highly anxious, with rapid labored respirations and hypersalivation. Within minutes after this photograph, respiratory arrest occurred, but the dog was resuscitated and lived another 9 months with therapy for heart failure.
MUCOUS MEMBRANES
Mucous membrane color and capillary refill time (CRT) are used to evaluate peripheral perfusion. The oral mucosa is usually assessed, but caudal mucous membranes (prepuce or vagina) also can be evaluated. The CRT is assessed by applying digital pressure to blanch the membrane; color should return within 2 seconds. Slower refill times occur as a result of dehydration and other causes of decreased cardiac output because of high peripheral sympathetic tone and vasoconstriction. Pale mucous membranes result from anemia or peripheral vasoconstriction. The CRT is normal in anemic animals unless hypoperfusion is also present. However, the CRT can be difficult to assess in severely anemic animals because of the lack of color contrast. The color of the caudal membranes should be compared with that of the oral membranes in polycythemic cats and dogs for evidence of differential cyanosis. If the oral membranes are pigmented, the ocular conjunctiva can be evaluated. Box 1-4 outlines causes for abnormal mucous membrane color. Petechiae in the mucous membranes may be noticed in dogs and cats with platelet disorders (see Chapter 87). In addition, oral and ocular mucous membranes are often areas where icterus (jaundice) is first detected. A yellowish cast to these membranes should prompt further evaluation for hemolysis (see Chapter 83) or hepatobiliary disease (see Chapter 35).
JUGULAR VEINS
Systemic venous and right heart filling pressures are reflected at the jugular veins. These veins should not be distended when the animal is standing with its head in a normal position (jaw parallel to the floor). Persistent jugular vein distention occurs in patients with right-sided CHF (because of high right heart filling pressure), external compression of the cranial vena cava, or jugular vein or cranial vena cava thrombosis (Fig. 1-5).
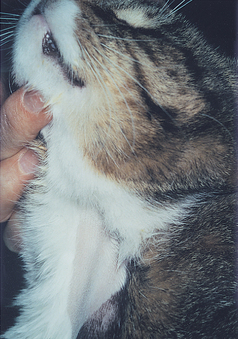
FIG 1-5 Prominent jugular vein distention is seen in this cat with signs of right-sided congestive heart failure from dilated cardiomyopathy.
Jugular pulsations extending higher than one third of the way up the neck from the thoracic inlet also are abnormal. Sometimes the carotid pulse wave is transmitted through adjacent soft tissues, mimicking a jugular pulse in thin or excited animals. To differentiate a true jugular pulse from carotid transmission, the jugular vein is occluded lightly below the area of the visible pulse. If the pulse disappears, it is a true jugular pulsation; if the pulse continues, it is being transmitted from the carotid artery. Jugular pulse waves are related to atrial contraction and filling. Visible pulsations occur in animals with tricuspid insufficiency (after the first heart sound, during ventricular contraction), conditions causing a stiff and hypertrophied right ventricle (just before the first heart sound, during atrial contraction), or arrhythmias that cause the atria to contract against closed AV valves (so-called cannon “a” waves). Specific causes of jugular vein distention and/or pulsations are listed in Box 1-5. Impaired right ventricular filling, reduced pulmonary blood flow, or tricuspid regurgitation can cause a positive hepatojugular reflux even in the absence of jugular distension or pulsations at rest. To test for this reflux, firm pressure is applied to the cranial abdomen while the animal stands quietly. This transiently increases venous return. Jugular distention that persists while abdominal pressure is applied constitutes a positive (abnormal) tests. Normal animals have little to no change in the jugular vein.
ARTERIAL PULSES
The strength and regularity of the peripheral arterial pressure waves and the pulse rate are assessed by palpating the femoral or other peripheral arteries (Box 1-6). Subjective evaluation of pulse strength is based on the difference between the systolic and diastolic arterial pressures (the pulse pressure). When the difference is wide, the pulse feels strong on palpation; abnormally strong pulses are termed hyperkinetic. When the pressure difference is small, the pulse feels weak (hypokinetic). If the rise to maximum systolic arterial pressure is prolonged, as with severe subaortic stenosis, the pulse also feels weak (pulsus parvus et tardus). Both femoral pulses should be palpated and compared; absence of pulse or a weaker pulse on one side may be caused by thromboembolism. Femoral pulses can be difficult to palpate in cats, even when normal. Often an elusive pulse can be found by gently working a fingertip toward the cat’s femur in the area of the femoral triangle, where the femoral artery enters the leg between the dorsomedial thigh muscles.
The femoral arterial pulse rate should be evaluated simultaneously with the direct heart rate, which is obtained by chest wall palpation or auscultation. Fewer femoral pulses than heartbeats constitutes a pulse deficit. Various cardiac arrhythmias induce pulse deficits by causing the heart to beat before adequate ventricular filling has occurred. Consequently, minimal or even no blood is ejected for those beats and a palpable pulse is absent. Other arterial pulse variations occur occasionally. Alternately weak then strong pulsations can result from severe myocardial failure (pulsus alternans) or from a normal heartbeat alternating with a premature beat (bigeminy), which causes reduced ventricular filling and ejection. An exaggerated decrease in systolic arterial pressure during inspiration occurs in association with cardiac tamponade; a weak arterial pulse strength (pulsus paradoxus) may be detected during inspiration in those patients.
PRECORDIUM
The precordium is palpated by placing the palm and fingers of each hand on the corresponding side of the animal’s chest wall over the heart. Normally the strongest impulse is felt during systole over the area of the left apex (located at approximately the fifth intercostal space near the costochondral junction). Cardiomegaly or a space-occupying mass within the chest can shift the precordial impulse to an abnormal location. Decreased intensity of the precordial impulse can be caused by obesity, weak cardiac contractions, pericardial effusion, intrathoracic masses, pleural effusion, or pneumothorax. The precordial impulse should be stronger on the left chest wall than on the right. A stronger right precordial impulse can result from right ventricular hypertrophy or displacement of the heart into the right hemithorax by a mass lesion, lung atelectasis, or chest deformity. Very loud cardiac murmurs cause palpable vibrations on the chest wall known as a precordial thrill. This feels like a buzzing sensation on the hand. A precordial thrill is usually localized to the area of maximal intensity of the murmur.
EVALUATION FOR FLUID ACCUMULATION
Right-sided CHF promotes abnormal fluid accumulation within body cavities (Fig. 1-6; see also Fig. 9-3) or, usually less noticeably, in the subcutis of dependent areas. Palpation and ballottement of the abdomen, palpation of dependent areas, and percussion of the chest in the standing animal are used to detect effusions and subcutaneous edema. Fluid accumulation secondary to right-sided heart failure is usually accompanied by abnormal jugular vein distention and/or pulsations, unless the animal’s circulating blood volume is diminished by diuretic use or other cause. Hepatomegaly and/or splenomegaly may also be noted in cats and dogs with right-sided heart failure.
AUSCULTATION
Thoracic auscultation is used to identify normal heart sounds, determine the presence or absence of abnormal sounds, assess heart rhythm and rate, and evaluate pulmonary sounds. Heart sounds are created by turbulent blood flow and associated vibrations in adjacent tissue during the cardiac cycle. Although many of these sounds are too low in frequency and/or intensity to be audible, others can be heard with the stethoscope or even palpated. Heart sounds are classified as transient sounds (those of short duration) and cardiac murmurs (longer sounds occurring during a normally silent part of the cardiac cycle). Cardiac murmurs and transient sounds are described using general characteristics of sound: frequency (pitch), amplitude of vibrations (intensity/loudness), duration, and quality (timbre). Sound quality is affected by the physical characteristics of the vibrating structures.
Because many heart sounds are difficult to hear, a cooperative animal and a quiet room are important during auscultation. The animal should be standing, if possible, so that the heart is in its normal position. Panting in dogs is discouraged by holding the animal’s mouth shut. Respiratory noise can be decreased further by placing a finger over one or both nostrils for a short time. Purring in cats may be stopped by holding a finger over one or both nostrils (Fig. 1-7), waving an alcohol-soaked cotton ball near the cat’s nose, or turning on a water faucet near the animal. Various other artifacts can interfere with auscultation, including respiratory clicks, air movement sounds, shivering, muscle twitching, hair rubbing against the stethoscope (crackling sounds), gastrointestinal sounds, and extraneous room noises.
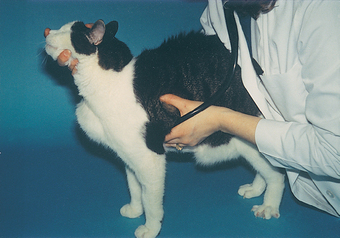
FIG 1-7 During cardiac auscultation, respiratory noise and purring can be decreased or eliminated by gently placing a finger over one or both nostrils for brief periods of time.
The traditional stethoscope has both a stiff, flat diaphragm and a bell on the chestpiece. The diaphragm, when applied firmly to the chest wall, allows better auscultation of higher-frequency heart sounds than those of low frequency. The bell, applied lightly to the chest wall, facilitates auscultation of lower-frequency sounds such as S3 and S4 (see the following section on Gallop Sounds). Some stethoscopes have a single-sided chestpiece that is designed to function as a diaphragm when used with firm pressure and as a bell when used with light pressure. Ideally the stethoscope should have short double tubing and comfortable eartips. The binaural eartubes should be angled rostrally to align with the examiner’s ear canals (Fig. 1-8).
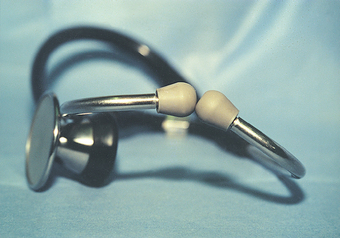
FIG 1-8 Note the angulation of the stethoscope binaurals for optimal alignment with the clinician’s ear canals (Top of picture is rostral). The flat diaphragm of the chestpiece is facing left, and the concave bell is facing right.
Both sides of the chest should be carefully auscultated, with special attention to the valve areas (Fig. 1-9). The stethoscope is moved gradually to all areas of the chest. The examiner should concentrate on the various heart sounds, correlating them to the events of the cardiac cycle, and listen for any abnormal sounds in systole and diastole successively. The normal heart sounds (S1 and S2) are used as a framework for timing abnormal sounds. The point of maximal intensity (PMI) of any abnormal sounds should be located. The examiner should focus on cardiac auscultation separately from pulmonary auscultation because full assimilation of sounds from both systems simultaneously is unlikely. Pulmonary auscultation is described further in Chapter 20.
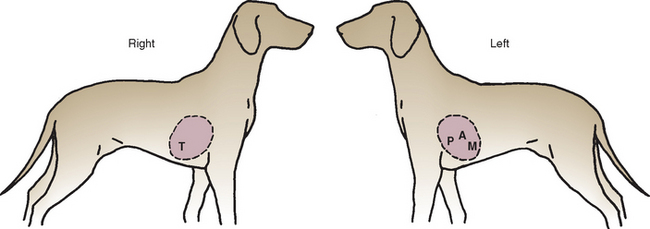
FIG 1-9 Approximate locations of various valve areas on chest wall. T, Tricuspid; P, pulmonic; A, aortic; M, mitral.
Transient Heart Sounds
The heart sounds normally heard in dogs and cats are S1 (associated with closure and tensing of the AV valves and associated structures at the onset of systole) and S2 (associated with closure of the aortic and pulmonic valves following ejection). The diastolic sounds (S3 and S4) are not audible in normal dogs and cats. Fig. 1-10 correlates the hemodynamic events of the cardiac cycle with the ECG and timing of the heart sounds. It is important to understand these events and identify the timing of systole (between S1 and S2) and diastole (after S2 until the next S1) in the animal. The precordial impulse occurs just after S1 (systole), and the arterial pulse between S1 and S2.
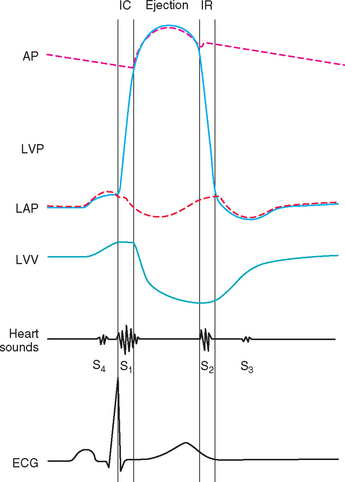
FIG 1-10 Cardiac cycle diagram depicting relationships among great vessel, ventricular and atrial pressures, ventricular volume, heart sounds, and electrical activation. AP, Aortic pressure; ECG, electrocardiogram; IC, isovolumic contraction; IR, isovolumic relaxation; LVP, left ventricular pressure; LAP, left atrial pressure; LVV, left ventricular volume.
Sometimes the first (S1) and/or second (S2) heart sounds are altered in intensity. A loud S1 may be heard in dogs and cats with a thin chest wall, high sympathetic tone, tachycardia, systemic arterial hypertension, or shortened PR intervals. A muffled S1 can result from obesity, pericardial effusion, diaphragmatic hernia, dilated cardiomyopathy, hypovolemia/poor ventricular filling, or pleural effusion. A split or sloppy-sounding S1 may be normal, especially in large dogs, or it may result from ventricular premature contractions or an intraventricular conduction delay. The intensity of S2 is increased by pulmonary hypertension (for example, from heartworm disease, a congenital shunt with Eisenmenger’s physiology, or cor pulmonale). Cardiac arrhythmias often cause variation in the intensity (or even absence) of heart sounds.
Normal physiologic splitting of S2 can be heard in some dogs because of variation in stroke volume during the respiratory cycle. During inspiration, increased venous return to the right ventricle tends to delay closure of the pulmonic valve, while reduced filling of the left ventricle accelerates aortic closure. Pathologic splitting of S2 can result from delayed ventricular activation or prolonged right ventricular ejection secondary to ventricular premature beats, right bundle branch block, a ventricular or atrial septal defect, or pulmonary hypertension.
Gallop Sounds
The third (S3) and fourth (S4) heart sounds occur during diastole (see Fig. 1-10) and are not normally audible in dogs and cats. When an S3 or S4 sound is heard, the heart may sound like a galloping horse, hence the term gallop rhythm. This term can be confusing because the presence or absence of an audible S3 or S4 has nothing to do with the heart’s rhythm (i.e., the origin of cardiac activation and the intracardiac conduction process). Gallop sounds are usually heard best with the bell of the stethoscope (or by light pressure applied to a single-sided chestpiece) because they are of lower frequency than S1 and S2. At very fast heart rates, differentiation of S3 from S4 is difficult. If both sounds are present, they may be superimposed, which is called a summation gallop.
The S3, also known as an S3gallop or ventricular gallop, is associated with low-frequency vibrations at the end of the rapid ventricular filling phase. An audible S3 in the dog or cat usually indicates ventricular dilation with myocardial failure. The extra sound can be fairly loud or very subtle and is heard best over the cardiac apex. It may be the only auscultable abnormality in an animal with dilated cardiomyopathy. An S3 gallop may also be audible in dogs with advanced valvular heart disease and congestive failure.
The S4 gallop, also called an atrial or presystolic gallop, is associated with low-frequency vibrations induced by blood flow into the ventricles during atrial contraction (just after the P wave of the ECG). An audible S4 in the dog or cat is usually associated with increased ventricular stiffness and hypertrophy, as with hypertrophic cardiomyopathy or hyperthyroidism in cats. A transient S4 gallop of unknown significance is sometimes heard in stressed or anemic cats.
Other Transient Sounds
Other brief abnormal sounds are sometimes audible. Systolic clicks are mid-to-late systolic sounds that are usually heard best over the mitral valve area. These sounds have been associated with degenerative valvular disease (endocardiosis), mitral valve prolapse, and congenital mitral dysplasia; a concurrent mitral insufficiency murmur may be present. In dogs with degenerative valvular disease, a mitral click may be the first abnormal sound noted, with a murmur developing over time. An early systolic, high-pitched ejection sound at the left base may occur in animals with valvular pulmonic stenosis or other diseases that cause dilation of a great artery. The sound is thought to arise from either the sudden checking of a fused pulmonic valve or the rapid filling of a dilated vessel during ejection. Rarely, restrictive pericardial disease causes an audible pericardial knock. This diastolic sound is caused by sudden checking of ventricular filling by the restrictive pericardium; its timing is similar to the S3.
Cardiac Murmurs
Cardiac murmurs are described by their timing within the cardiac cycle (systolic or diastolic, or portions thereof), intensity, PMI on the precordium, radiation over the chest wall, quality, and pitch. Systolic murmurs can occur in early (protosystolic), middle (mesosystolic), or late (telesystolic) systole or throughout systole (holosystolic). Diastolic murmurs generally occur in early diastole (protodiastolic) or throughout diastole (holodiastolic). Murmurs at the very end of diastole are termed presystolic. Continuous murmurs begin in systole and extend through S2 into all or part of diastole. Murmur intensity is arbitrarily graded on a I to VI scale (Table 1-1). The PMI is usually indicated by the hemithorax (right or left) and intercostal space or valve area where it is located, or by the terms apex or base. Because murmurs can radiate extensively, the entire thorax, thoracic inlet, and carotid artery areas should be auscultated. The pitch and quality of a murmur relate to its frequency and subjective assessment. “Noisy” or “harsh” murmurs contain mixed frequencies. “Musical” murmurs are of essentially one frequency with its overtones.
 TABLE 1-1 Grading of Heart Murmurs
TABLE 1-1 Grading of Heart Murmurs
| GRADE | MURMUR |
|---|---|
| I | Very soft murmur; heard only in quiet surroundings after prolonged listening |
| II | Soft murmur but easily heard |
| III | Moderate-intensity murmur |
| IV | Loud murmur but no precordial thrill |
| V | Loud murmur with a palpable precordial thrill |
| VI | Very loud murmur with a precordial thrill; can be heard with the stethoscope lifted from the chest wall |
Murmurs are also described by phonocardiographic configuration (Fig. 1-11). A holosystolic (plateau-shaped) murmur begins at the time of S1 and is of fairly uniform intensity throughout systole. Loud holosystolic murmurs may mask the S1 and S2 sounds. AV valve insufficiency and interventricular septal defects commonly cause this type of murmur because turbulent blood flour occurs throughout ventricular systole. A crescendo-decrescendo or diamond-shaped murmur starts softly, builds intensity in midsystole, and then diminishes; S1 and S2 can usually be heard clearly before and after the murmur. This type is also called an ejection murmur because it occurs during blood ejection, usually because of ventricular outflow obstruction. A decrescendo murmur tapers from its initial intensity over time; it may occur in systole or diastole. Continuous (machinery) murmurs occur throughout systole and diastole.
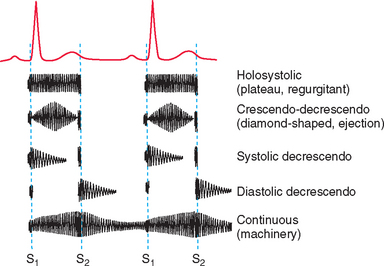
FIG 1-11 The phonocardiographic shape (configuration) as well as the timing of different murmurs are illustrated in this diagram.
Systolic murmurs.
Systolic murmurs can be decrescendo, holosystolic (plateau-shaped), or ejection (crescendo-decrescendo) in configuration. It can be difficult to differentiate these by auscultation alone. But the most important steps toward diagnosis include establishing that a murmur occurs in systole (rather than diastole), determining its PMI, and grading its intensity. Fig. 1-12 depicts the typical PMI of various murmurs over the chest wall.
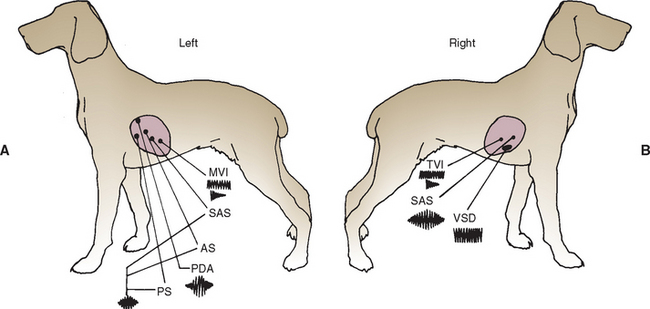
FIG 1-12 The usual point of maximal intensity (PMI) and configuration for murmurs typical of various congenital and acquired causes are depicted on left (A) and right (B) chest walls. AS, aortic (valvular) stenosis; MVI, mitral valve insufficiency; PDA, patent ductus arteriosus; PS, pulmonic stenosis; SAS, subaortic stenosis; TVI, tricuspid valve insufficiency; VSD, ventricular septal defect.
(From Bonagura JD, Berkwitt L: Cardiovascular and pulmonary disorders. In Fenner W, editor: Quick reference to veterinary medicine, ed 2, Philadelphia, 1991, JB Lippincott.)
Functional murmurs usually are heard best over the left heartbase. They are usually soft to moderate in intensity and of decrescendo (or crescendo-decrescendo) configuration. Functional murmurs may have no apparent cardiovascular cause (e.g., “innocent” puppy murmurs) or can result from an altered physiologic state (physiologic murmurs). Innocent puppy murmurs generally disappear by the time the animal is approximately 6 months of age. Physiologic murmurs have been associated with anemia, fever, high sympathetic tone, hyperthyroidism, marked bradycardia, peripheral arteriovenous fistulae, hypoproteinemia, and athletic hearts. Aortic dilation (e.g., with hypertension) and dynamic right ventricular outflow obstruction are other conditions associated with systolic murmurs in cats.
The murmur of mitral insufficiency is heard best at the left apex, in the area of the mitral valve. It radiates well dorsally and often to the left base and right chest wall. Mitral insufficiency characteristically causes a plateau-shaped murmur (holosystolic timing), but in its early stages the murmur may be protosystolic, tapering to a decrescendo configuration. Occasionally this murmur has a musical or “whoop-like” quality. With degenerative mitral valve disease, murmur intensity is related to disease severity.
Systolic ejection murmurs are most often heard at the left base and are caused by ventricular outflow obstruction, usually from a fixed narrowing (e.g., subaortic or pulmonic valve stenosis) or dynamic muscular obstruction. Ejection murmurs become louder as cardiac output or contractile strength increases. The subaortic stenosis murmur is heard well at the low left base and also at the right base because the murmur radiates up the aortic arch, which curves toward the right. This murmur also radiates up the carotid arteries and occasionally can be heard on the calvarium. Soft (grade I-II/VI), nonpathologic systolic ejection (physiologic) murmurs are common in sight hounds and certain other large breeds; these can be related to a large stroke volume (relative aortic stenosis), as well as breed-related left ventricular outflow tract characteristics. The murmur of pulmonic stenosis is best heard high at the left base. Relative pulmonic stenosis occurs with greatly increased flow through a structurally normal valve (e.g., with a large left-to-right shunting atrial or ventricular septal defect).
Most murmurs heard on the right chest wall are holosystolic, plateau-shaped murmurs, except for the subaortic stenosis murmur (above). The tricuspid insufficiency murmur is loudest at the right apex over the tricuspid valve. Its pitch or quality may be noticeably different from a concurrent mitral insufficiency murmur, and it often is accompanied by jugular pulsations. Ventricular septal defects also cause holosystolic murmurs. The PMI is usually at the right sternal border, reflecting the direction of the intracardiac shunt. A large ventricular septal defect may also cause the murmur of relative pulmonic stenosis.
Diastolic murmurs.
Diastolic murmurs are uncommon in dogs and cats. Aortic insufficiency from bacterial endocarditis is the most common cause, although congenital malformation or degenerative aortic valve disease occasionally occurs. Clinically relevant pulmonic insufficiency is rare but would be more likely in the face of pulmonary hypertension. These diastolic murmurs begin at the time of S2 and are heard best at the left base. They are decrescendo in configuration and extend a variable time into diastole, depending on the pressure difference between the associated great vessel and ventricle. Some aortic insufficiency murmurs have a musical quality.
Continuous murmurs.
As implied by the name, continuous (machinery) murmurs occur throughout the cardiac cycle. They indicate that a substantial pressure gradient exists continuously between two connecting areas (vessels). The murmur is not interrupted at the time of S2; instead, its intensity is often greater at that time. The murmur becomes softer toward the end of diastole, and at slow heart rates it can become inaudible. Patent ductus arteriosus (PDA) is by far the most common cause of a continuous murmur. The PDA murmur is loudest high at the left base above the pulmonic valve area; it tends to radiate cranially, ventrally, and to the right. The systolic component is usually louder and heard well all over the chest. The diastolic component is more localized to the left base in many cases. The diastolic component (and the correct diagnosis) may be missed if only the cardiac apical area is auscultated.
Continuous murmurs can be confused with concurrent systolic ejection and diastolic decrescendo murmurs. But with these so-called “to-and-fro” murmurs, the ejection (systolic) component tapers in late systole and the S2 can be heard as a distinct sound. The most common cause of to-and-fro murmurs is the combination of subaortic stenosis with aortic insufficiency. Rarely, stenosis and insufficiency of the pulmonic valve cause this type of murmur. Likewise, both holosystolic and diastolic decrescendo murmurs occur occasionally (e.g., with a ventricular septal defect and aortic insufficiency from loss of aortic root support). This also is not considered a true “continuous” murmur.
Braunwald E, Perloff JK. Physical examination of the heart and circulation. In: Zipes DP, et al, editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. ed 7. Philadelphia: WB Saunders; 2005:77-106.
Davidow EB, Woodfield JA. Syncope: pathophysiology and differential diagnosis. Compend Contin Educ. 2001;23:608.
Fabrizio F, et al. Left basilar systolic murmur in retired racing greyhounds. J Vet Intern Med. 2006;20:78.
Haggstrom J, Kvart C, Hansson K. Heart sounds and murmurs: changes related to severity of chronic valvular disease in the Cavalier King Charles Spaniel. J Vet Intern Med. 1995;9:75.
Hamlin RL. Normal cardiovascular physiology. In: Fox PR, Sisson DD, Moise NS, editors. Canine and feline cardiology. ed 2. New York: WB Saunders; 1999:25-37.
Kienle R. Pulse alterations. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. ed 6. Philadelphia: WB Saunders; 2005:200-204.
Koplitz SL, Meurs KM, Bonagura JD. Echocardiographic assessment of the left ventricular outflow tract in the Boxer. J Vet Intern Med. 2006;20:904.
Pedersen HD, et al. Auscultation in mild mitral regurgitation in dogs: observer variation, effects of physical maneuvers, and agreement with color Doppler echocardiography and phonocardiography. J Vet Intern Med. 1999;13:56.
Prosek R. Abnormal heart sounds and heart murmurs. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. ed 6. Philadelphia: WB Saunders; 2005:195-200.
Rishniw M, Thomas WP. Dynamic right ventricular outflow obstruction: a new cause of systolic murmurs in cats. J Vet Intern Med. 2002;16:547.

