CHAPTER 4 Cardiac Arrhythmias and Antiarrhythmic Therapy
GENERAL CONSIDERATIONS
Cardiac arrhythmias occur for many reasons. Although some arrhythmias are of no clinical consequence, others cause serious hemodynamic compromise and sudden death, especially in animals with underlying heart disease. It is important to make an accurate electrocardiographic diagnosis, as well as to consider the arrhythmia’s clinical context, before deciding whether to use antiarrhythmic therapy. In people the risk of death associated with ventricular tachyarrhythmias is higher when myocardial function is impaired. Dogs with cardiomyopathy also have increased risk for sudden death, especially Doberman Pinschers and Boxers. An inherited disorder predisposing to sudden death has also been identified in young German Shepherds. On the other hand, in previously healthy animals the ventricular premature activity that occurs commonly after thoracic trauma or splenectomy (see p. 139) is usually benign and resolves without therapy.
Occasional ventricular premature complexes occur without consequence in many animals. However, arrhythmias that compromise cardiac output and coronary perfusion can lead to myocardial ischemia, deterioration of cardiac pump function, and sometimes sudden death. These arrhythmias tend to be either very rapid (e.g., sustained ventricular or supraventricular tachyarrhythmias) or very slow (e.g., advanced atrioventricular [AV] block with a slow or unstable ventricular escape rhythm). Sometimes, however, a lethal arrhythmia such as ventricular fibrillation (VF) occurs without antecedent sustained arrhythmia. Rapid sustained tachycardia of either supraventricular or ventricular origin reduces cardiac output acutely and eventually leads to myocardial dysfunction and congestive heart failure (CHF).
DEVELOPMENT OF ARRHYTHMIAS
Multiple factors underlie disturbances in cardiac rhythm. Abnormalities of conduction or automaticity caused by cardiac structural or pathophysiologic remodeling can predispose to arrhythmias, even in the absence of overt cardiac disease. Genetic factors and environmental stresses contribute to this. However, additional triggering (e.g., premature stimulus or abrupt change in heart rate) and/or modulating factors (e.g., changes in autonomic tone, circulating catecholamines, ischemia, or electrolyte disturbances) are thought to be necessary to provoke and sustain a rhythm disturbance. For example, episodes of anger or aggressive behavior have been linked to increased susceptibility to ischemic arrhythmias and sudden arrhythmic death in both dogs and people. Various stresses that lead to cardiac remodeling changes also may play a role in the development of arrhythmias. Remodeling can involve myocyte hypertrophy, changes in the structure or function of ion channels, tissue fibrosis, or the activity of the autonomic nervous system (see Chapter 3). Although some of these changes act as beneficial compensatory mechanisms in the short term, they can have harmful and arrhythmogenic long-term effects. It is thought that if such underlying arrhythmogenic modulators could be controlled, arrhythmias would be lessened. Such modulators include catecholamines, free radicals, angiotensin II, cytokines, and nitric oxide (NO). The higher survival in human patients with heart failure treated with angiotensin converting enzyme (ACE) inhibitors, spironolactone, and/or some β-blockers supports this approach. There is similar evidence for ACE inhibitors in dogs with dilated cardiomyopathy and reason to suspect that other therapies might be beneficial as well.
APPROACH TO ARRHYTHMIA MANAGEMENT
If antiarrhythmic drug therapy is considered, its goals should be defined. An immediate goal is to restore hemodynamic stability. Although ideal goals include conversion to sinus rhythm, correction of underlying cause, and prevention of further arrhythmia and sudden death, suppression of all abnormal beats is generally not a realistic goal. Successful therapy may mean sufficient reduction in frequency (e.g., by ≥70-80%) or repetitive rate of ectopic beats to promote normal hemodynamics and eliminate clinical signs. However, even with apparently complete conversion to sinus rhythm, the risk of sudden death from a lethal arrhythmia may remain.
Various arrhythmias and their ECG characteristics are described in Chapter 2. This section provides a general approach to managing cardiac rhythm disturbances. Nevertheless, much remains to be learned about effective arrhythmia management and the prevention of sudden death.
 BOX 4-1 ECG Interpretation Guide
BOX 4-1 ECG Interpretation Guide
See Chapter 2 for more specific information.
DIAGNOSIS AND MANAGEMENT OF COMMON ARRHYTHMIAS
Cardiac arrhythmias in a given animal often occur inconsistently and are influenced by drug therapy, prevailing autonomic tone, baroreceptor reflexes, and variations in heart rate. Treatment decisions are based on consideration of the origin (supraventricular or ventricular), timing (premature or escape), and severity of the rhythm disturbance, as well as the clinical context. Accurate ECG interpretation is important. Although a routine (resting) ECG documents arrhythmias present during the recording period, it provides only a glimpse of the cardiac rhythms occurring over time. Because marked variation in frequency and severity of arrhythmias may occur over time, potentially critical arrhythmias are easily missed. For this reason, Holter monitoring or other forms of extended ECG acquisition are useful in assessing the severity and frequency of arrhythmias and monitoring treatment efficacy. Some rhythm abnormalities do not require therapy, whereas others demand immediate aggressive treatment. Close patient monitoring is especially important in patients with more serious arrhythmias.
Supraventricular tachyarrhythmias occur from various mechanisms, including reentry involving the AV node, accessory pathways, or sinoatrial (SA) node, as well as abnormal automaticity within atrial or junctional tissue. Many patients have atrial enlargement. Common underlying heart diseases include chronic mitral or tricuspid valve degeneration with regurgitation, dilated cardiomyopathy, congenital malformations, and cardiac neoplasia. Other factors also may predispose to atrial tachyarrhythmias (Box 4-2).
Ventricular premature contractions (VPCs) occur in association with disorders that affect cardiac tissue directly or indirectly through neurohormonal effects (see Box 4-2). For instance, disorders of the central nervous system can produce abnormal neural effects on the heart that cause ventricular or supraventricular arrhythmias (brain-heart syndrome). When VPCs are infrequent or underlying cardiac function is normal, adverse hemodynamic effects may be negligible. However, hemodynamic impairment can be severe in dogs or cats with underlying heart disease, rapid ventricular rates, or myocardial depression stemming from a systemic disease.
Factors such as underlying hypoxia, electrolyte or acid-base imbalances, and abnormal hormone concentrations (e.g., thyroid) can exacerbate arrhythmias. Therefore correcting these is usually important for arrhythmia control. Because some drugs can provoke arrhythmias, reducing dosage or discontinuing the medication may be useful.
CLINICAL PRESENTATION
Box 4-3 lists common arrhythmias according to a clinical description of the heartbeat.
TACHYARRHYTNMIAS
Rapid Irregular Rhythms
Irregular heart rhythms are common, and the ECG is important for differentiating abnormal rhythms as well as sinus arrhythmia. Pulse deficits (see p. 6) and an irregular, weak pulse with heart sounds of varying intensity and regularity may be detected on physical examination. Premature contractions interrupt ventricular filling and reduce stroke volume, sometimes to the extent that there is no ejection at all for that cycle (Fig. 4-1). Rapid atrial fibrillation (AF) and premature contractions of any origin often cause pulse deficits. Ventricular premature complexes can cause audible splitting of the heart sounds because of asynchronous ven tricular activation. Ventricular and supraventricular tachycardias and AF cause more severe hemodynamic compromise than do isolated premature contractions, especially in patients with underlying heart disease.
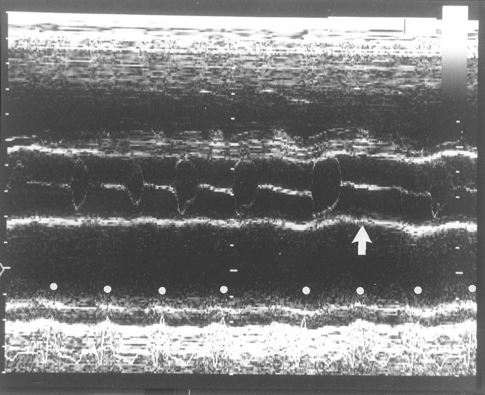
FIG 4-1 M-mode echocardiogram at the aortic root level in a Doberman Pinscher with atrial fibrillation and dilated cardiomyopathy. Pulse deficits and variable-intensity pulses occurred secondary to the variable (or absent) aortic valve opening caused by the arrhythmia and illustrated in this echocardiogram. The motion of two aortic valve leaflets is seen within the parallel aortic root echocardiograms. Most cycles are associated with variable and poor stroke volume and with abbreviated aortic valve opening, but there is no opening at all after the sixth electrocardiogram complex from the left (arrow). R waves are indicated by white dots.
Rapid Regular Rhythms
Rapid regular rhythms include sinus tachycardia, sustained supraventricular tachycardia (SVT), and sustained ventricular tachycardia. Sinus tachycardia is caused by high sympathetic tone or drug-induced vagal blockade. Underlying causes include anxiety, pain, fever, thyrotoxicosis, heart failure, hypotension, shock, the ingestion of stimulants or toxins (e.g., chocolate, caffeine), or drugs (e.g., catecholamines, anticholinergics, theophylline, and related agents). The heart rate in dogs and cats with sinus tachycardia is usually <300 beats/min, although it can be higher in those with thyrotoxicosis or in those that have ingested exogenous stimulants or drugs (particularly cats). Alleviation of the underlying cause and intravenous (IV) administration of fluids to reverse hypotension (in animals without edema) should cause the sympathetic tone and sinus rate to decrease.
SVT of varying causes can be difficult to differentiate from sinus tachycardia. The heart rate in patients with SVT is often >300 beats/min, but it is rare for the sinus rate to be this rapid. Patients with SVTs, such as sinus tachycardia, usually have a normal QRS configuration (narrow and upright in lead II). However, if an intraventricular conduction disturbance is present, SVT may resemble ventricular tachycardia. A vagal maneuver can be useful in differentiating among narrow QRS complex tachycardias.
Sustained, rapid arrhythmias lead to decrease in cardiac output, arterial blood pressure, and coronary perfusion. CHF eventually may result. Signs of poor cardiac output and hypotension include weakness, depression, pallor, prolonged capillary refill time, exercise intolerance, syncope, dyspnea, prerenal azotemia, worsening rhythm disturbances, and sometimes altered mentation, seizure activity, and sudden death.
Supraventricular Tachyarrhythmias
Occasional premature beats do not require specific therapy. Factors that predispose to these arrhythmias should be minimized as much as possible (e.g., discontinue or reduce dosage of suspected drugs, manage heart failure if present, and treat metabolic or endocrine abnormalities).
Oral therapy for frequent supraventricular premature beats and paroxysmal tachycardia.
Initial oral therapy for frequent atrial premature complexes (APCs) or paroxysmal SVT usually involves either digoxin, diltiazem, a β-blocker, or a combination of these. Digoxin (see Table 3-3) is the initial oral drug of choice in dogs with heart failure and cats with dilated cardiomyopathy (Fig. 4-2). A β-blocker or the calcium entry blocker diltiazem may be added to the regimen if the arrhythmia is not controlled with digoxin, along with other therapy (including an ACE inhibitor) indicated for heart failure. Cats with hypertrophic cardiomyopathy or hyperthyroidism are usually treated with a β-blocker such as atenolol, although diltiazem is an alternative. Refractory intermittent supraventricular tachyarrhythmias may respond to amiodarone, sotalol, procainamide, quinidine, or a class IC agent.
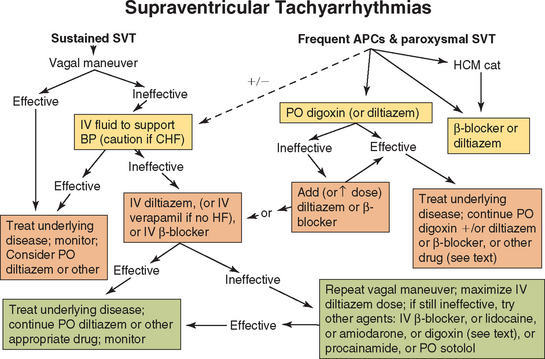
FIG 4-2 A therapeutic approach to supraventricular tachyarrhythmias. See Table 4-2 for drug doses and text for more information. APCs, Atrial premature contractions; BP, blood pressure; CHF, congestive heart failure; HCM, hypertrophic cardiomyopathy; HF, heart failure or myocardial dysfunction; SVT, supraventricular tachycardia.
Acute therapy for supraventricular tachycardia.
More aggressive therapy is warranted for rapid and persistent supraventricular tachyarrhythmias, especially in the face of hemodynamic impairment. A vagal maneuver can be tried first (discussed in more detail in the following section). IV access is secured, and fluids are administered to maintain blood pressure and enhance endogenous vagal tone. However, patients with known or suspected heart failure should receive a small volume slowly, if at all. If a vagal maneuver does not terminate the arrhythmia, diltiazem IV (or oral loading) is often chosen first because of its lesser negative inotropic effects. Although verapamil (IV) is equally effective against SVTs, it is not recommended for dogs with myocardial dysfunction or heart failure because of its greater negative inotropic effects. A slowly administered IV β-blocker (e.g., propranolol, esmolol) is an alternative therapy but also has negative inotropic effects in animals with high underlying sympathetic tone. Some cases of reentrant SVT or automatic atrial tachycardia respond to IV lidocaine. IV digoxin also may be tried, but this has been less effective than the calcium channel blockers. Digoxin has a slower onset of action, and although it increases vagal tone, IV administration can also increase central sympathetic output. IV amiodarone is an alternative agent in refractory cases. Sotalol or a class IA or IC drug might be tried if the arrhythmia is unremitting. Adenosine appears to be ineffective in dogs for terminating SVTs. Further cardiac diagnostic tests are indicated once conversion is achieved or the ventricular rate has decreased to <200 beats/min. Once the rhythm is better controlled, oral diltiazem, digoxin, amiodarone, or β-blockers are options for chronic therapy; combinations of these agents can be used.
Paroxysmal AV reciprocating tachycardia is a reentrant tachycardia involving an accessory pathway and the AV node (see p. 27). It is interrupted by slowing conduction or prolonging the refractory period of either or both tissues. A vagal maneuver may slow AV conduction enough to terminate the rhythm. Diltiazem and β-blockers slow AV conduction and increase refractoriness. Another approach is IV amiodarone or procainamide. Digoxin slows AV conduction but has variable effects on the accessory pathway; its use is usually discouraged in people with preexcitation syndromes. Procainamide and quinidine may prevent AV reciprocating tachycardia because they lengthen the refractory period of the accessory pathway. High-dose procainamide, with or without a β-blocker or diltiazem, has been successful in preventing the recurrence of tachycardia in some cases. Intracardiac electrophysiologic mapping with radiofrequency catheter ablation of accessory pathways has been used successfully to abolish refractory SVT associated with preexcitation in dogs, although this technique is not widely available yet.
Atrial tachycardia caused by a persistent automatic ectopic focus may be particularly difficult to suppress. When the antiarrhythmic strategies outlined in the preceding paragraphs are unsuccessful, the goal of therapy shifts to ventricular rate control. By prolonging AV conduction time and refractoriness, fewer atrial impulses are then conducted and ventricular rate is slowed (and usually irregular). Therapy with combinations of diltiazem or a β-blocker and digoxin, sotalol, or amiodarone can be effective. The animal with persistent automatic atrial tachycardia could be a candidate for intracardiac electrophysiologic mapping and catheter ablation when such tools are available. Alternatively, heart rate control could be achieved by AV node ablation with permanent pacemaker implantation.
Vagal maneuver.
A vagal maneuver can help the clinician differentiate among tachycardias caused by an ectopic automatic focus, those dependent on a reentrant circuit involving the AV node, or excessively rapid sinus node activation. The vagal maneuver may transiently slow or intermittently block AV conduction, exposing abnormal atrial P′ waves, and allow an ectopic atrial focus to be identified. Vagal maneuvers can terminate reentrant SVTs involving the AV node by interrupting the reentrant circuit. The maneuver tends to temporarily slow the rate of sinus tachycardia.
Vagal maneuvers are performed by massaging the area over the carotid sinuses (below the mandible in the jugular furrows) or by applying firm bilateral ocular pressure for 15 to 20 seconds. Although initial attempts are often unsuccessful, repeating the vagal maneuver after antiarrhythmic drug injection may be useful. β-blockers, Ca++ entry-blockers, digoxin, and other agents can increase the effectiveness of vagal maneuvers. The vagal maneuver can be further potentiated in dogs by administering intramuscular (IM) morphine sulfate (0.2 mg/kg) or IV edrophonium chloride (1 to 4 mg; atropine and an endotracheal tube should be readily available).
Ventricular Tachyarrhythmias
Occasional VPCs in an otherwise asymptomatic animal should not be treated. Moderately frequent single VPCs of uniform configuration may not require antiarrhythmic drug treatment either, especially if underlying heart function is normal. Nevertheless, guidelines as to whether, when, and how best to treat intermittent ventricular tachyarrhythmias remain undefined. Besides being expensive, antiarrhythmic drugs can have serious adverse effects, can provoke additional arrhythmias (proarrhythmic effects), and may not be efficacious. Pretreatment and posttreatment 24- to 48-hour ambulatory ECG recordings showing at least a 70% to 80% reduction in arrhythmia frequency provide the best indicator of drug arrhythmia-suppression efficacy. Intermittent ECG recordings cannot truly differentiate between drug effect (or lack thereof) and the spontaneous, marked variability of arrhythmia frequency that occurs in any individual. However, in-hospital ECG recordings of 15 seconds to several minutes in duration are often the most practical attempt to monitor arrhythmias.
Several factors influence the decision to use ventricular antiarrhythmic drug therapy. These factors include the nature of the animal’s underlying disease, the perceived severity of the arrhythmia, and the presence or absence of hemodynamic compromise. Diseases such as dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy in Boxers, hypertrophic cardiomyopathy, and subaortic stenosis, among others, are frequently associated with sudden death from arrhythmias. Therefore ventricular antiarrhythmic therapy would appear most urgent in animals with these diseases. However, the efficacy of a particular therapy to prolong survival as well as suppress the arrhythmia is difficult to accurately assess. Traditional guidelines for instituting ventricular antiarrhythmic therapy have been based on frequency, prematurity, and variability of the QRS configuration of the arrhythmia. Characteristics thought to imply increased electrical instability include rapid paroxysmal or sustained ventricular tachycardia (e.g., >130 beats/min), multiform (polymorphic) VPC configuration, or close coupling of VPCs to preceding complexes (R-on-T phenomenon). However, clear evidence that these guidelines predict greater risk of sudden death in all patients is lacking. It is probably more important to consider the animal’s underlying heart disease and whether the arrhythmia is causing signs of hypotension or low cardiac output. Animals that are hemodynamically unstable or have a disease associated with sudden cardiac death are treated earlier and more aggressively.
Acute therapy for ventricular tachycardia.
Sustained ventricular tachycardia is treated aggressively because it can result in marked decreases in arterial blood pressure, especially at faster rates. Lidocaine (IV) is usually the first-choice drug for controlling serious ventricular tachyarrhythmias in hospitalized dogs. It is effective against arrhythmias of several underlying mechanisms and has minimal adverse hemodynamic effects. Because the effects of IV boluses last only about 10 to 15 minutes, a constant rate infusion (CRI) is warranted if the drug is effective. Small supplemental IV boluses can be given in addition to the CRI to maintain therapeutic drug concentrations until a steady state is achieved. IV infusion can be continued for several days, if needed. If lidocaine is ineffective after maximal recommended doses, several other strategies can be tried (Fig. 4-3).
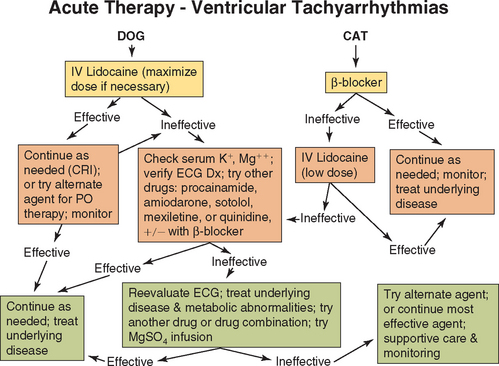
FIG 4-3 A therapeutic approach to ventricular tachyarrhythmias. See Table 4-2 for drug doses and text for more information. CRI, Constant-rate infusion; Dx, diagnosis; ECG, electrocardiogram.
IV amiodarone or oral mexiletine or sotalol can be more effective in some cases. With IV amiodarone, slow injection of conservative doses and blood pressure monitoring are recommended because marked hypotension can occur. Alternatively, procainamide (given intravenously, intramuscularly, or orally) or quinidine (given intramuscularly or orally) can be tried next. Effects of a single IM or oral loading dose of either drug should occur within 2 hours. If this is effective, lower doses can be given every 4 to 6 hours intramuscularly or orally. If ineffective, the dose can be increased or another antiarrhythmic drug chosen. Quinidine is not given intravenously because of its hypotensive effects. This drug is also not recommended in patients on digoxin or that have prolonged QT intervals. If the arrhythmia has not been controlled, a β-blocker can be added.
Cats with frequent ventricular tachyarrhythmias are usually given a β-blocker first. Alternatively, low doses of lidocaine can be administered. However, cats, especially if not anesthetized, can be quite sensitive to the neurotoxic effects of this drug. Procainamide or sotalol can also be used.
Digoxin is not used specifically for treating ventricular tachyarrhythmias, although it may be indicated for patients with concurrent heart failure and supraventricular arrhythmias. Digoxin can also predispose to the development of ventricular arrhythmias. Another antiarrhythmic drug may be necessary in animals with preexisting frequent or repetitive VPCs. Phenytoin is used only in dogs for digitalis-induced ventricular tachyarrhythmias that are refractory to lidocaine. Ancillary KCl supplementation (if serum K+ ≤4 mEq/L) with or without MgSO4 can increase antiarrhythmic efficacy.
Close ECG monitoring and further diagnostic testing should follow initial therapy. Total suppression of persistent ventricular tachyarrhythmias is not expected. The patient’s clinical status, the underlying disease(s), the success of the drug in suppressing the arrhythmia, and the drug dosage (e.g., whether it could be increased) all influence the decision whether to continue or discontinue current treatment or to use a different drug. Clinical status and results of diagnostic testing also guide decisions about chronic oral therapy.
If the ventricular tachyarrhythmia appears refractory to initial treatment attempts, one or more of the following considerations may be helpful:
Chronic oral therapy for ventricular tachyarrhythmias.
The same drug that was most effective during acute therapy, or a similar one, is often continued orally when long-term therapy is thought to be needed. Although suppression of ventricular ectopy is one aim, reducing the risk of sudden arrhythmic death is the real issue for long-term therapy. Whereas the Class IB drugs (lidocaine and mexiletine) appear to raise the fibrillation threshold more than the Class IA agents (procainamide and quinidine), Class III agents appear to have much greater antifibrillatory effects than the Class I drugs. Concurrent disease should be treated if possible. It is likely that animals with arrhythmias associated with underlying heart disease also benefit from the use of β-blockers, ACE inhibitors, and some other therapies, as do people. However, β-blockers alone do not appear effective in suppressing ventricular tachyarrhythmias in Doberman Pinschers with cardiomyopathy.
Several strategies are available for long-term oral therapy of patients with ventricular tachyarrhythmias:
Presently, the three most favored options are sotalol; amiodarone; or mexiletine or sustained-release procainamide with atenolol, because they are likely to provide a greater antifibrillatory effect.
Frequent reevaluation is important for patients on long-term antiarrhythmic therapy (for any rhythm disturbance). Patients’ owners can be shown how to use a stethoscope or palpate the chest wall to count the number of “skipped” beats per minute at home; this may yield an approximation of the frequency of arrhythmic events (either single or paroxysms). However, continuous 24- to 48-hour ambulatory ECG recordings are more accurate. The decision to continue or discontinue successful antiarrhythmic therapy is also based on consideration of the clinical situation and any underlying cardiac disease.
Atrial Fibrillation
AF most often develops when there is marked atrial enlargement. It is a serious arrhythmia, especially when the ventricular response rate is high. Predisposing conditions include dilated cardiomyopathy, chronic degenerative AV valve disease, congenital malformations that cause atrial enlargement, and hypertrophic or restrictive cardiomyopathy in cats. Clinical heart failure is common in these animals. AF is characterized by an irregular and usually rapid ventricular response rate. When little time is available for ventricular filling, stroke volume is compromised. Furthermore, atrial contraction (the “atrial kick”), which is especially important to ventricular filling at faster heart rates, is lost. Cardiac output tends to decrease considerably when AF develops; poor myocardial function exacerbates this decrease.
Long-lasting conversion to sinus rhythm is rare in the face of marked underlying cardiac disease, even after successful electrical cardioversion. Therefore treatment in most cases is directed at reducing the ventricular response rate by slowing AV conduction (Fig. 4-4). A slower heart rate allows more time for ventricular filling and lessens the relative importance of atrial contraction. In-hospital heart rates <150 (or 180 in cats) beats/min are desirable. Heart rate should be documented by ECG; counting the ventricular rate by auscultation or palpation is often highly inaccurate. Resting heart rate at home, which can be monitored by the owner, is a better indicator of drug effectiveness. Heart rates of 70 to 120 beats/min in dogs and 80 to 140 beats/min in cats are probably acceptable.
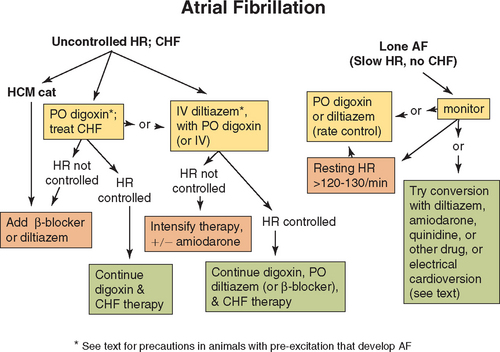
FIG 4-4 A therapeutic approach to atrial fibrillation. See Table 4-2 for drug doses and text for more information. AF, atrial fibrillation; CHF, congestive heart failure; HCM, hypertrophic cardiomyopathy; HR, heart rate.
Therapy for atrial fibrillation.
The oral drug of first choice for most dogs with AF is digoxin (see Table 3-3). If the heart rate exceeds 200 to 220 beats/min at rest, twice the eventual oral maintenance dosage can be given for 1 to 2 days. When more immediate heart rate reduction is indicated, IV diltiazem is recommended. This has less negative inotropic effect than verapamil or an IV β-blocker, although esmolol could be cautiously tried because of its short half-life. If dobutamine or dopamine infusion is needed to support myocardial function (see p. 60 and Box 3-1), IV diltiazem or an IV loading dose of digoxin (cautiously) can be used, but a β-blocker should be avoided.
Digoxin alone does not adequately reduce the heart rate in many animals. Increases in sympathetic tone from CHF, exercise, or excitement can override the vagal effect of digoxin on AV conduction. Either a β-blocker or diltiazem can be added and titrated upward as needed to further slow AV conduction and ventricular rate. Because of their potential to depress myocardial function, the agent chosen is usually added 1 to 2 days after starting oral digoxin in most patients with reduced myocardial contractililty. Amiodarone can be added (or substituted) for additional rate control. An occasional dog will revert to sinus rhythm in response to diltiazem or amiodarone therapy. Digoxin is not used in cats with hypertrophic cardiomyopathy that develop AF; a β-blocker or diltiazem is used instead.
When AF develops in patients that also have ventricular preexcitation, AV nodal blocking drugs (Ca++ blockers, digoxin, and possibly β-blockers) should not be used because they can paradoxically increase the ventricular response rate. Amiodarone is recommended in these cases. Sotalol or procainamide can also be used.
Electrical cardioversion of AF has been of limited success in animals; most revert to AF. Newer methods, including biphasic current delivery combined with amiodarone (or other drug) therapy, may be more successful. Nevertheless, experience with AF in people suggests that heart rate control provides similar survival benefit (and fewer adverse effects) than conversion to sinus rhythm.
Lone atrial fibrillation
AF sometimes develops in large or giant-breed dogs without cardiomegaly or other evidence of structural heart disease. This can occur transiently, usually in association with trauma or surgery. AF with a slow ventricular response rate can also be an incidental finding in such dogs. This is known as “lone AF”. Acute AF without signs of heart disease or failure may convert to sinus rhythm spontaneously or in response to drug therapy, such as with diltiazem (e.g. PO for ∼3 days), amiodarone, or possibly sotalol or other Class III or IC agents. Acute onset AF associated with high vagal tone may convert with IV lidocaine. Quinidine PO or IM has been used for acute AF conversion in large dogs without signs of heart disease; but adverse effects can include increased ventricular response rate from the drug’s vagolytic effects, ataxia, and most seriously, seizures or polymorphic ventricular tachycardia. If effective, the drug is discontinued after sinus rhythm is achieved. Dogs that do not convert to sinus rhythm are either given digoxin or continued on diltiazem for rate control. Alternatively, if the ventricular rate is consistently low at rest, dogs can be monitored periodically without therapy; but rapid heart rates still are likely with exercise or excitement.
BRADYARRHYTHMIAS
Sinus Bradycardia
Slow sinus rhythm (or arrhythmia) can be a normal finding, especially in athletic dogs. Sinus bradycardia has also been associated with the administration of various drugs (e.g., xylazine, thorazine tranquilizers, some anesthetic agents, medetomidine, digoxin, calcium entry blockers, β-blockers, parasympathomimetic drugs), trauma or diseases of the central nervous system, organic disease of the sinus node, hypothermia, hyperkalemia, and hypothyroidism, among other disorders. Conditions that increase vagal tone (e.g., respiratory or gastrointestinal tract disease or a mass involving the vagosympathetic trunk) may induce sinus bradycardia. Chronic pulmonary disease often is associated with pronounced respiratory sinus arrhythmia.
In most cases of sinus bradycardia, the heart rate increases in response to exercise or atropine administration, and no clinical signs are associated with the slow heart rate. Symptomatic dogs usually have a heart rate slower than 50 beats/min and/or pronounced underlying disease. Because sinus bradycardia and sinus bradyarrhythmia are extremely rare in cats, a search for underlying cardiac or systemic disease (e.g., hyperkalemia) is warranted in any cat with a slow heart rate.
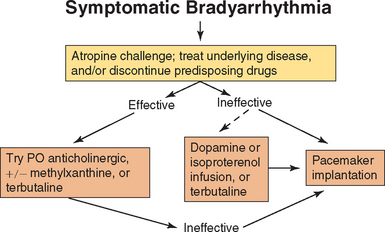
When sinus bradycardia is associated with signs of weakness, exercise intolerance, syncope, or worsening underlying disease, an anticholinergic (or adrenergic) agent is given (Fig. 4-5). If sinus bradycardia is the result of a drug effect, discontinuation, dosage reduction, or other therapy should be used, as appropriate (e.g., reversal of anesthesia or medetomidine, calcium salts for calcium entry blocker overdose, dopamine or atropine for β-blocker toxicity). If there is inadequate increase in heart rate with medical therapy, temporary or permanent pacing is indicated (see Suggested Readings).
Sick Sinus Syndrome
Sick sinus syndrome is a condition of erratic sinoatrial function characterized by episodic weakness, syncope, and Stokes-Adams seizures. Older female Miniature Schnauzers and West Highland White Terriers are commonly affected in different regions, but the syndrome is also seen in Dachshunds, Cocker Spaniels, Pugs, and mixed-breed dogs. Affected dogs have episodes of marked sinus bradycardia with sinus arrest (or sinoatrial block). Sick sinus syndrome is extremely rare in cats.
Abnormalities of the AV conduction system may coexist, causing the activity of subsidiary pacemakers to be depressed and leading to prolonged periods of asystole. Some affected dogs also have paroxysmal SVTs, prompting the name bradycardia-tachycardia syndrome (Fig. 4-6). Premature complexes may be followed by long pauses before sinus node activity resumes, indicating a prolonged sinus node recovery time. Intermittent periods of accelerated junctional rhythms and variable junctional or ventricular escape rhythms may also occur.
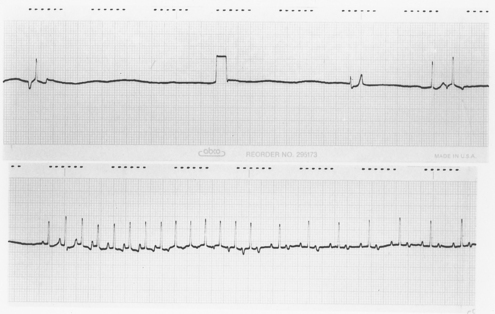
FIG 4-6 Continuous electrocardiogram from an 11-year-old female Miniature Schnauzer with sick sinus syndrome, illustrating a combination of bradycardia and tachycardia. The top portion shows persistent sinus arrest with three different escape complexes, followed by an atrial premature complex. There is a 1-mV calibration mark in the middle of the top strip. The bradycardia is interrupted by a run of atrial tachycardia at a rate of 250 beats/min, with 1 : 1 atrioventricular conduction initially; but starting in the middle of the bottom strip, every other P’ wave is blocked (2 : 1 atrioventricular conduction).
Clinical signs can result from bradycardia and sinus arrest, paroxysmal tachycardia, or both. Signs can mimic seizures stemming from neurologic or metabolic disorders. Concurrent degenerative AV valve disease is also often present. Some dogs have evidence of CHF, usually secondary to AV valve regurgitation, although the arrhythmias may be a complicating factor.
ECG abnormalities are frequently pronounced in dogs with long-standing sick sinus syndrome. Nevertheless, some dogs have one or more normal resting ECGs. Prolonged visual ECG monitoring or 24-hour ambulatory ECG can help establish a definitive diagnosis. An atropine challenge test is done in dogs with persistent bradycardia (see p. 93). The normal response is an increase in the heart rate of 150% or to >130 to 150 beats/min. Dogs with sick sinus syndrome generally have a subnormal response.
Therapy with an anticholinergic agent, methylxanthine bronchodilator, or terbutaline given orally may temporarily help some animals that have a positive response to atropine challenge. However, anticholinergic or sympathomimetic drugs used to accelerate the sinus rate can also exacerbate tachyarrhythmias. Conversely, drugs used to suppress these supraventricular tachyarrhythmias can magnify the brady cardia, although digoxin or diltiazem is helpful in some dogs if used cautiously. Sick sinus syndrome with frequent or severe clinical signs is best managed by permanent artificial pacing. The Suggested Readings list includes sources of further details on pacing. Dogs that remain symptomatic because of paroxysmal SVTs can safely be given appropriate antiarrhythmic therapy once a normally functioning pacemaker is in place.
Atrial Standstill
Persistent atrial standstill is a rhythm disturbance characterized by lack of effective atrial electrical activity (i.e., no P waves and a flat baseline) in which a junctional or ventricular escape rhythm controls the heart. This bradyarrhythmia is rare in dogs and extremely rare in cats; most cases have occurred in English Springer Spaniels with muscular dystrophy of the fascioscapulohumeral type, although infiltrative and inflammatory diseases of the atrial myocardium can also result in atrial standstill. Because organic disease of the atrial myocardium may also involve the ventricular myocardium, persistent atrial standstill may be a harbinger of a serious and progressive cardiac disorder.
Medical treatment for persistent atrial standstill is rarely rewarding; however, an anticholinergic drug or an infusion of dopamine or isoproterenol can sometimes temporarily accelerate the escape rhythm. If ventricular tachyarrhythmias result from this treatment, the drug should be discontinued or the dose reduced. Oral terbutaline may also have some beneficial effect. Antiarrhythmic agents are contraindicated in these animals because they may suppress the escape focus, as well as the tachyarrhythmia. Permanent pacemaker implantation is the treatment of choice, although the prognosis is poor in dogs with concurrent ventricular myocardial dysfunction.
Hyperkalemia should be ruled out in animals without P waves. The apparent lack of atrial electrical and mechanical activity (“silent atrium”) caused by hyperkalemia will resolve with treatment. Sinus node activity (and P waves) become evident as the serum K+ concentration returns to normal.
Atrioventricular Conduction Block
Second-degree, or intermittent, AV block usually causes an irregular heartbeat. In contrast, the ventricular escape rhythm that occurs with a third-degree, or complete, AV block is regular, although premature contractions or shifts in the escape focus may cause some irregularities. AV conduction disturbances may result from therapy with certain drugs (e.g., α2 agonists, opioids, digoxin), high vagal tone, or organic disease of the AV node. Diseases that have been associated with AV conduction disturbances include bacterial endocarditis (of the aortic valve), hypertrophic cardiomyopathy, infiltrative myocardial disease, and myocarditis. Idiopathic heart block may occur in middle-aged to older dogs; congenital third-degree heart block has also been seen in dogs. Symptomatic heart block is less common in cats, but evidence of any AV conduction disturbance should prompt further diagnostic evaluation. Most cases have been associated with hypertrophic cardiomyopathy. Heart block is occasionally found in old cats without detectable organic heart disease.
Type I second-degree AV block and first-degree AV block are frequently associated with high vagal tone or drug effects in dogs. These animals are often asymptomatic; exercise or injection of an anticholinergic drug (atropine or glycopyrrolate) usually abolishes the conduction disturbance. High-grade (many blocked P waves) second-degree AV block and complete heart block usually cause lethargy, exercise intolerance, weakness, syncope, and other signs of low cardiac output. These signs become severe when the heart rate is consistently <40 beats/min. CHF develops secondary to chronic bradycardia in some dogs, especially if other cardiac disease is present.
An atropine challenge test (p. 93) is used to determine the degree of vagal influence on the AV block. Long-term oral anticholinergic therapy (e.g., propantheline bromide) can be attempted in symptomatic animals that are atropine-responsive (see Fig. 4-5). Atropine or subsequent oral anticholinergic therapy is often ineffective, however, so artificial pacing is usually indicated. An emergency infusion of dopamine (see Box 3-1) or isoproterenol may increase the ventricular escape rate in animals with high-grade second- or third-degree block, although ventricular tachyarrhythmias may also be provoked. Oral isoproterenol is usually ineffective. A thorough cardiac workup is indicated before permanent artificial pacemaker implantation because some underlying diseases (e.g., myocardial disease, endocarditis) are associated with a poor prognosis, even after pacing. Temporary transvenous pacing is sometimes used for 1 to 2 days to assess the animal’s response to a normal heart rate before permanent pacemaker surgery is performed.
ANTIARRHYTHMIC AGENTS
Antiarrhythmic drugs can act by slowing the rate of a tachycardia, terminating a reentrant arrhythmia, or preventing abnormal impulse formation or conduction. These effects occur through modulation of tissue electrophysiologic properties and/or autonomic nervous system effects. The traditional (Vaughan-Williams) antiarrhythmic drugs are classified according to their main electrophysiologic effects on cardiac cells (Table 4-1). Although this classification system has several shortcomings (e.g., some drugs having antiarrhythmic effects are excluded, several drugs have the multiclass effects, and focus on ion channel mechanisms is lacking), clinical reference to this classification persists. See Table 4-2 and Box 4-4 for antiarrhythmic drug dosages and CRI calculation methods.
 TABLE 4-1 Classification and Effects of Antiarrhythmic Drugs
TABLE 4-1 Classification and Effects of Antiarrhythmic Drugs
| CLASSIFICATION | DRUG | MECHANISM AND ECG EFFECTS |
|---|---|---|
| Class I | Decreases fast inward Na+ current; membrane-stabilizing effects (decreased conductivity, excitability, and automaticity) | |
| IA | Moderately decreases conductivity, increases action potential duration; can prolong QRS complex and Q-T interval | |
| IB | Little change in conductivity, decreases action potential duration; QRS complex and Q-T interval unchanged | |
| IC | Markedly decreases conductivity without change in action potential duration | |
| Class II | β-adrenergic blockade—reduces effects of sympathetic stimulation (no direct myocardial effects at clinical doses) | |
| Class III | Selectively prolongs action potential duration and refractory period; antiadrenergic effects; Q-T interval prolonged | |
| Class IV | Decreases slow inward Ca++ current (greatest effects on sinoatrial and AV nodes) | |
| Other Antiarrhythmic Agents | Antiarrhythmic action results mainly from indirect autonomic effects (especially increased vagal tone) | |
| Anticholinergic agents oppose vagal effects on SA and AV nodes (glycopyrrolate and other drugs also have this effect) | ||
| Briefly opens K+ channels and indirectly slows Ca++ current (greatest effects on sinoatrial and AV nodes); may transiently block AV conduction, but ineffective in dogs | ||
AV, atrioventricular; SA, sinoatrial.
 TABLE 4-2 Dosage of Antiarrhythmic Drugs
TABLE 4-2 Dosage of Antiarrhythmic Drugs
| AGENT | DOSAGE |
|---|---|
| Class I | |
| Lidocaine | |
| Procainamide | |
| Quinidine | |
| Mexiletine | |
| Phenytoin | |
| Propafenone | |
| Flecainide | Dog: (?) 1-5 mg/kg by mouth q8-12h Cat: — |
| Class II | |
| Atenolol | |
| Propranolol | |
| Esmolol | |
| Metoprolol | Dog: initial dose, 0.2 mg/kg by mouth q8h, up to 1 mg/kg q8(−12)h Cat: — |
| Class III | |
| Sotalol | |
| Amiodarone | |
| Class IV | |
| Diltiazem |
Dog: Oral maintenance: initial dose 0.5 mg/kg (up to 2+ mg/kg) by mouth q8h; acute IV for supraventricular tachycardia: 0.15-0.25 mg/kg over 2-3 min IV, can repeat every 15 minutes until conversion or maximum 0.75 mg/kg; CRI: 5-15 mg/kg/hr; oral loading dose: 0.5 mg/kg followed by 0.25 mg/kg by mouth q1h to a total of 1.5(−2.0) mg/kg or conversion
Cat: Same?; for HCM: 1.5-2.5 mg/kg (or 7.5-10 mg/cat) by mouth q8h; sustained-release preparations: Cardizem-CD, 10 mg/kg/day (45 mg/cat is about 105 mg of Cardizem-CD, or the amount that fits into the small end of a No. 4 gelatin capsule); Diltiazem (Dilacor) XR, 30 mg/cat/day (one half of a 60-mg controlled-release tablet within the 240-mg gelatin capsule), can increase to 60 mg/day in some cats if necessary
|
| Verapamil | |
| Anticholinergic | |
| Atropine | |
| Glycopyrrolate | |
| Propantheline | |
| Hyoscyamine | |
| Sympathomimetic | |
| Isoproterenol | |
| Terbutaline | |
| Other Agents | |
| Digoxin | See Table 3-3 |
| Adenosine | |
| Edrophonium | |
| Phenylephrine | |
CRI, Constant rate infusion; CPR, cardiopulmonary resuscitation; —, effective dosage not known.
Class I agents tend to slow conduction and decrease automaticity and excitability by means of their membrane-stabilizing effects; traditional ventricular antiarrhythmic drugs belong to this class. Class II drugs include the β-adrenergic antagonists (β-blockers), which act by inhibiting the effects of catecholamines on the heart. Class III drugs prolong the effective refractory period of cardiac action potentials without decreasing conduction velocity; they may be most effective in suppressing reentrant arrhythmias and in preventing VF. Class IV drugs are the calcium entry blockers; ventricular arrhythmias are usually not responsive to these agents, but they are important against supraventricular tachyarrhythmias. Antiarrhythmic agents within this classification scheme are contraindicated in animals with complete heart block and should be used only cautiously in animals with sinus bradycardia, sick sinus syndrome, and first- or second-degree AV block.
CLASS I ANTIARRHYTHMIC DRUGS
Class I antiarrhythmic drugs block membrane Na+ channels and depress the action potential upstroke (phase 0), which slows conduction velocity along the cardiac cells. They have been subclassified according to differences in other electrophysiologic characteristics. These differences (see Table 4-1) may influence their efficacy against particular arrhythmias. Most of the Class I agents depend on extracellular K+ concentration for their effects, and they lose effectiveness in patients with hypokalemia.
Lidocaine
Lidocaine HCl is usually the first-choice IV ventricular antiarrhythmic agent in dogs. It is often ineffective against supraventricular arrhythmias. It has little effect on sinus node rate, AV conduction rate, and refractoriness. Lidocaine suppresses automaticity in normal Purkinje fibers and diseased myocardial tissue, slows conduction, and reduces the supernormal period (during which the cell can be reexcited before complete repolarization occurs). It has greater effects on diseased and hypoxic cardiac cells and at faster stimulation rates. The electrophysiologic effects of lidocaine are dependent on the extracellular potassium concentration. Hypokalemia may render the drug ineffective, but hyperkalemia intensifies its depressant effects on cardiac membranes. Lidocaine produces little or no depression of contractility at therapeutic doses when administered slowly IV; this is useful in dogs with heart failure. The lidocaine congeners tocainide and mexiletine similarly produce minimal negative inotropic and hypotensive effects. Toxic concentrations of lidocaine can cause hypotension.
Lidocaine undergoes rapid hepatic metabolism; some metabolites may contribute to its antiarrhythmic and toxic effects. Lidocaine is not effective orally because of its almost complete first-pass hepatic elimination. IV administration, usually as slow boluses followed by CRI, is most effective. Antiarrhythmic effects after IV bolus occur within 2 minutes and abate within 10 to 20 minutes. CRI without a loading dose produces steady-state concentrations in 4 to 6 hours. The half-life is <1 hour in the dog. An initial bolus of 2 mg/kg is used in dogs and can be repeated two to three times if necessary. Lower doses should be used in cats to avoid toxicity (loading dose of 0.25 to 0.5 mg/kg). The half-life in cats is 1 to 2 hours. Therapeutic plasma concentrations are thought to range from 1.5 to 6 μg/ml in dogs. Only lidocaine without epinephrine should be used for antiarrhythmic therapy. If IV access is not possible, IM administration could be used, but IV is much preferred.
The most common toxic effect of lidocaine is central nervous system excitation. Signs include agitation, disorientation, muscle twitches, nystagmus, and generalized seizures. The latter may require diazepam (0.25 to 0.5 mg/kg IV) or a short-acting barbiturate. Nausea can also occur. Worsening of arrhythmias (a proarrhythmic effect) is seen occasionally, as it is with any drug having cardiac electrophysiologic effects. Cats are particularly sensitive to the drug’s toxic effects and may undergo respiratory arrest along with seizures. In the event of toxicity, lidocaine should be discontinued until the signs of toxicity disappear; a lower infusion rate may then be instituted. IV diazepam (0.25 to 0.5 mg/kg) is used to control lidocaine-induced seizures. There are anecdotal reports of respiratory depression and arrest after the administration of lidocaine in unconscious dogs and cats. Propranolol, cimetidine, and other drugs that decrease liver blood flow slow the metabolism of lidocaine and predispose to the development of toxicity. Animals with heart failure may also have reduced hepatic blood flow and may require a lower dosage of the drug. Hepatic disease can delay elimination as well.
Procainamide
Procainamide HCl has electrophysiologic effects similar to those of quinidine. Procainamide has both direct and indirect (vagolytic) effects; it is indicated for the treatment of premature ventricular (and sometimes atrial) depolarizations and tachycardias. It is less effective than quinidine in managing atrial arrhythmias and is usually not effective in converting chronic atrial flutter-fibrillation to sinus rhythm. Procainamide should be used only with caution in animals with hypotension.
Orally administered procainamide is well absorbed in the dog but has a half-life of only 2.5 to 4 hours. The sustained-release preparation has a slightly longer half-life of 3 to 6 hours. Food may delay the absorption of procainamide. The drug undergoes hepatic metabolism and renal excretion in proportion to the creatinine clearance. The metabolite N-acetylprocainamide is not clinically important in dogs and cats. Procainamide can be given orally or intramuscularly without marked hemodynamic effects, but rapid IV injection can cause hypotension and cardiac depression, although to a much lesser degree than IV quinidine. Administration by CRI can be useful if the arrhythmia responds to an IV bolus; a steady state is reached in 12 to 22 hours. Therapeutic plasma concentrations are thought to be 4 to 10 μg/ml.
The toxic effects of procainamide are similar to those of quinidine (discussed in the following section) but are usually milder. Gastrointestinal upset and prolongation of the QRS or QT intervals may occur. Procainamide can enhance the ventricular response rate to AF if used without digoxin or a β- or Ca++ blocker. More serious toxic effects include hypotension, depressed AV conduction (sometimes causing second- or third-degree heart block), and proarrhythmia. The latter can cause syncope or VF. Hypotension responds to IV fluids, catecholamines, or calcium-containing solutions. Gastrointestinal signs associated with oral therapy may respond to dosage reduction. High-dose oral procainamide therapy in people has been associated with a reversible lupuslike syndrome characterized by neutropenia, fever, depression, and hepatomegaly, but this has not been documented in dogs. Long-term use can cause brown discoloration of the haircoat in black Doberman Pinschers.
Quinidine
Quinidine has been used to treat ventricular and, occasionally, supraventricular tachyarrhythmias. In large dogs with recent-onset AF and normal ventricular function, quinidine may cause conversion to sinus rhythm. This drug must be used cautiously in animals with heart failure or hyperkalemia. The characteristic electrophysiologic effects of quinidine are depression of automaticity and conduction velocity and prolongation of the effective refractory period. Corresponding dose-dependent ECG changes (e.g., PR, QRS, and QT prolongation) result from direct electrophysiologic and vagolytic effects. At low doses, quinidine’s vagolytic effects may increase the sinus rate or the ventricular response rate to AF by antagonizing the drug’s direct effects. As with other class I agents, hypokalemia reduces quinidine’s antiarrhythmic effectiveness.
The drug is well-absorbed orally but has fallen out of favor for chronic oral therapy because of its frequent adverse effects and its interference with digoxin pharmacokinetics. Quinidine is metabolized extensively by the liver, with little dependence on liver blood flow. The half-life is about 6 hours in dogs and 2 hours in cats. Quinidine is highly protein-bound; severe hypoalbuminemia can predispose to toxicity. Cimetidine can also predispose to toxicity by slowing the drug’s elimination. Quinidine can precipitate digoxin toxicity (when used concurrently) by displacing digoxin from skeletal muscle binding sites and reducing its renal clearance. Anticonvulsants and other drugs that induce hepatic microsomal enzymes can speed quinidine’s metabolism. IV administration is not recommended because of quinidine’s propensity to cause vasodilation (by means of nonspecific α-adrenergic receptor blockade), cardiac depression, and hypotension. The oral and IM routes usually do not cause adverse hemodynamic effects, but close monitoring is warranted initially, especially in animals with underlying cardiac disease. Therapeutic blood concentrations are thought to be 2.5 to 5 μg/ml and are usually achieved in 12 to 24 hours after oral and IM administration. Slow-release sulfate (83% active drug), gluconate (62% active drug), and polygalacturonate (80% active drug) salts of quinidine prolong the drug’s absorption and elimination. The sulfate salt is more rapidly absorbed than the gluconate; peak effect is usually achieved 1 to 2 hours after oral administration.
Quinidine toxicity occurs as an extension of the drug’s electrophysiologic and hemodynamic actions. As the plasma concentration increases, the PR interval and QRS duration lengthen. Marked QT prolongation, right bundle-branch block, or QRS widening >25% of pretreatment value suggests drug toxicity; various conduction blocks and ventricular tachyarrhythmias are other manifestations. Marked QT prolongation implies increased temporal dispersion of myocardial refractoriness; this predisposes to torsades de pointes (see p. 25) and VF. Transient episodes of these serious arrhythmias can be a cause of syncopal attacks in people taking quinidine. Lethargy, weakness, and CHF can result from the negative inotropic and vasodilatory effects of the drug and subsequent hypotension. Cardiotoxicity and hypotension can be partially reversed by sodium bicarbonate (1 mEq/kg IV), which temporarily decreases serum K+ concentration, enhances quinidine’s binding to albumin, and reduces its cardiac electrophysiologic effects. Gastrointestinal signs (e.g., nausea, vomiting, diarrhea) are common with orally administered quinidine. Thrombocytopenia (reversible after quinidine discontinuation) can occur in people and possibly in dogs and cats.
Mexiletine
Mexiletine HCl is similar to lidocaine in its electrophysiologic, hemodynamic, toxic, and antiarrhythmic properties. It can be effective in suppressing ventricular tachyarrhythmias in dogs. The combination of a β-blocker (or procainamide or quinidine) with mexiletine may be more efficacious and associated with fewer adverse effects than mexiletine alone. The drug is easily absorbed when administered orally, but antacids, cimetidine, and narcotics reportedly slow its absorption in people. Mexiletine undergoes hepatic metabolism (influenced by liver blood flow) and some renal excretion (which is slower if the urine is alkaline). Hepatic microsomal enzyme inducers may accelerate its clearance. The half-life in dogs is from 4.5 to 7 hours (depending to some degree on the urine pH). Approximately 70% of the drug is protein bound. The therapeutic serum concentration is thought to range from 0.5 to 2.0 μg/ml (as in people). The effects of this drug in cats are not known. Adverse effects have included vomiting, anorexia, tremor, disorientation, sinus bradycardia, and thrombocytopenia. Overall, mexiletine appears to produce fewer adverse effects than tocainide.
Phenytoin
Phenytoin’s electrophysiologic effects are similar to those of lidocaine. It also has some slow-calcium channel inhibitory and central nervous system effects that may contribute to its effectiveness against digitalis-induced arrhythmias. This drug is currently used only for digitalis-induced ventricular arrhythmias that have not responded to lidocaine in dogs. Its contraindications are the same as for lidocaine. Slow IV infusion and oral administration do not cause relevant hemodynamic disturbances; however, the oral bioavailability of phenytoin is poor. Rapid IV injection should be avoided because the propylene glycol vehicle can depress myocardial contractility, exacerbate arrhythmias, and cause vasodilation, hypotension, or respiratory arrest. The half-life of phenytoin in the dog is about 3 hours. The drug is metabolized in the liver, and it may speed up its own elimination by stimulating hepatic microsomal enzymes. Co-administration of cimetidine, chloramphenicol, and other drugs that inhibit microsomal enzyme activity increases phenytoin’s serum concentration. The IV administration of phenytoin has been associated with bradycardia, AV blocks, ventricular tachycardia, and cardiac arrest. Other manifestations of phenytoin toxicity include central nervous system signs (e.g., depression, nystagmus, disorientation, ataxia). The drug is not used in cats because its half-life is >40 hours, and even low doses produce toxic serum concentrations in this species.
Other Class I Agents
Disopyramide is similar to quinidine and procainamide electrophysiologically. It has a very short half-life in the dog (<2 hours), as well as marked depressive effects on the canine myocardium. Tocainide, a class IB agent similar to lidocaine, is no longer available in the United States. Flecainide and propafenone are class IC agents. They produce marked reduction in cardiac conduction velocity but have little effect on sinus rate or refractoriness. High doses depress automaticity in the sinus node and specialized conducting tissues. Vasodilation and myocardial depression can result in severe hypotension after IV injection, especially in animals with underlying cardiac disease. Proarrhythmia is a serious potential adverse effect of these agents. Bradycardia, intraventricular conduction disturbance, and consistent (although transient) hypotension, as well as nausea, vomiting, and anorexia, have occurred in dogs. Flecainide (and encainide) have been associated with increased mortality in people. These agents are rarely (and cautiously) used for treating life-threatening ventricular arrhythmias refractory to other therapy.
CLASS II ANTIARRHYTHMIC DRUGS: β-ADRENERGIC BLOCKERS
Class II antiarrhythmic drugs act by blocking catecholamine effects. They slow heart rate, reduce myocardial O2 demand, and increase AV conduction time and refractoriness. The antiarrhythmic effect of β-blockers relates to β1-receptor blockade rather than direct electrophysiologic effects. They are often used in combination with a class I agent (e.g., procainamide or mexiletine), although their negative inotropic effect demands caution when used in animals with myocardial failure. β-receptor blockers are used in animals with hypertrophic cardiomyopathy, certain congenital and acquired ventricular outflow obstructions, systemic hypertension, hyperthyroid heart disease, supraventricular and ventricular tachyarrhythmias (especially those induced by enhanced sympathetic tone), and other diseases or toxicities that cause excessive sympathetic stimulation. A β-blocker is often used in conjunction with digoxin to slow the ventricular response rate to AF. A β-blocker such as propranolol or atenolol is considered the first-line antiarrhythmic agent in cats for the treatment of both supraventricular and ventricular tachyarrhythmias. In people with stable heart failure, long-term therapy with certain β-blockers improves cardiac function and prolongs survival in those who tolerate the drug (see p. 69).
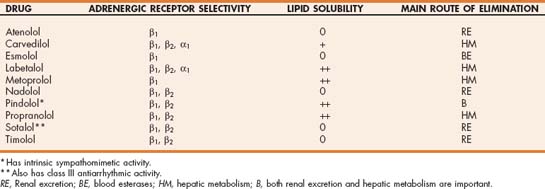
β-adrenergic receptors have been classified into subtypes. β1-receptors are located primarily in the myocardium and mediate increases in contractility, heart rate, AV conduction velocity, and automaticity in specialized fibers. Extracardiac β2-receptors mediate bronchodilation and vasodilation, as well as renin and insulin release. There are also some β2- as well as β3-receptors in the heart. “Nonselective” β-blockers inhibit catecholamine binding to both β1- and β2-adrenergic receptors. Other β-blockers are more selective; they antagonize mainly one or the other receptor subtype (Table 4-3). The first-generation β-blockers (e.g., propranolol) have nonselective β-blocking effects. Second-generation agents (e.g., atenolol, metoprolol) are relatively β1 selective. The third-generation β-blockers affect both β1 and β2 receptors but also antagonize α1 receptors and may have other effects. A few β-blockers have some degree of intrinsic sympathomimetic activity.
The clinical antiarrhythmic effect of class II drugs is thought to relate to β1-receptor blockade rather than to direct electrophysiologic mechanisms. In normal animals β-receptor blockers have little negative inotropic effect. However, they must be used cautiously in animals with underlying myocardial disease because increased sympathetic drive may be needed to maintain cardiac output. Marked depression of cardiac contractility, conduction, or heart rate can result in such cases. β-blockers are generally contraindicated in patients with sinus bradycardia, sick sinus syndrome, high-grade AV block, or severe CHF and in animals also receiving a Ca++-blocking drug. Nonselective β-blockers may increase peripheral vascular resistance (because of unopposed α-adrenergic effects) and provoke bronchoconstriction. β-blockers may also mask the early signs of acute hypoglycemia in diabetics (e.g., tachycardia and blood pressure changes), and reduce the release of insulin in response to hyperglycemia. Because the effect of β-blockers depends on the level of sympathetic activation, individual patient response is quite variable. Therefore initial dosages should be low and cautiously titrated upward as needed.
β-blockers enhance the depression of AV conduction produced by digitalis, class I antiarrhythmic drugs, and Ca++-blockers. Use of a β-blocker and a Ca++-blocker simultaneously can markedly decrease heart rate and myocardial contractility. Because of possible β-receptor upregulation (in-creased number or affinity of receptors) during long-term β-blockade, therapy should not be abruptly discontinued.
Propranolol
Propranolol HCl is a nonselective β-blocker that was widely used in dogs and cats, although atenolol is used more often now. Propranolol is not recommended for patients with pulmonary edema because of the potential for bronchoconstriction caused by β2-receptor antagonism. The β2-receptor blocking effects of propranolol also make it relatively contraindicated in patients with asthma or chronic small airway disease.
Propranolol undergoes extensive first-pass hepatic metabolism, so oral bioavailability is low; but with time and use of higher doses hepatic enzymes become saturated and bioavailability increases. Propranolol reduces hepatic blood flow, which prolongs its elimination as well as that of other drugs dependent on liver blood flow for their metabolism (e.g., lidocaine). Feeding delays oral absorption and increases drug clearance after IV dosing (by increasing hepatic blood flow). The half-life of propranolol in the dog is only about 1.5 hours (0.5 to 4.2 hours in cats). Active metabolites exist and dosing every 8 hours appears to be adequate in both species. IV propranolol is used mainly for refractory ventricular tachycardia (in conjunction with a class I drug) and for emergency treatment of atrial or junctional tachycardia.
Toxicity is most often related to excessive β-blockade; this can develop at relatively low doses in some animals. Bradycardia, heart failure, hypotension, bronchospasm, and hypoglycemia can occur. Infusion of a catecholamine (e.g., dopamine or dobutamine) will help reverse these effects. Propranolol and other lipophilic β-blockers can cause central nervous system effects such as depressed attitude and disorientation.
Atenolol
Atenolol is a selective β1-blocker. It is used commonly to slow sinus rate and AV conduction and to suppress ventricular premature beats. The half-life of atenolol is slightly more than 3 hours in dogs and about 3.5 hours in cats. Its oral bioavailability in both species is high (∼90%). Atenolol is excreted in the urine, so renal dysfunction delays its clearance. Atenolol’s β-blocking effect lasts more than 12 hours but less than 24 hours in normal cats. This drug is hydrophilic. Adverse central nervous system effects are unlikely because it does not readily cross the blood-brain barrier. As with other β-blockers, weakness or exacerbation of heart failure can occur.
Metoprolol
Metoprolol tartrate is another β1-selective agent. It is well absorbed orally, but bioavailability is reduced by a large first-pass effect. There is minimal protein-binding. The drug is metabolized in the liver and excreted in the urine. Half-life is 1.6 hours in dogs and 1.3 hours in cats. Metoprolol has been used in some dogs with dilated cardiomyopathy and chronic valvular disease. It may possibly contribute to improved cardiac function over time (see p. 69).
Esmolol
Esmolol HCl is an ultra-short acting β1-selective agent. It is rapidly metabolized by blood esterases and has a half-life of <10 minutes. Steady state occurs in 5 minutes after a loading dose or 30 minutes without. Esmolol’s effects are gone within 10 to 20 minutes after infusion is terminated. This drug is used for acute therapy of tachyarrhythmias and feline hypertrophic obstructive cardiomyopathy.
Other β-Blockers
Many other β-blocking drugs are available. Their receptor selectivity as well as their pharmacologic characteristic vary. Certain β-blockers may prove useful in patients with chronic, stable myocardial failure by reducing the cardiotoxic effects of excessive sympathetic stimulation, improving cardiac function, promoting upregulation of cardiac β-receptors, and increasing survival time (see p. 69). The third-generation β-blocker, carvedilol, and the second-generation agent, metoprolol, are effective in this regard. Nonselective (first-generation) agents, such as propranolol, and some later-generation agents do not appear to confer these survival benefits. Agents with intrinsic sympathomimetic activity appear to have deleterious effects.
CLASS III ANTIARRHYTHMIC DRUGS
Common features of class III drugs include prolongation of the cardiac action potential and effective refractory period without a decrease in conduction velocity. Their effects are mediated by inhibition of potassium channels responsible for repolarization (delayed rectifier current). These agents are useful for ventricular arrhythmias, especially those caused by reentry. Class III drugs have antifibrillatory effects as well. They share some characteristics of other antiarrhythmic drug classes in addition to their class III effects.
Sotalol
Sotalol HCl is a nonselective β-blocker that has Class III effects at higher doses. Its oral bioavailability is high, although absorption is reduced when given with food. Sotalol’s half-life is about 5 hours in dogs. It is eliminated unchanged by the kidneys, and renal dysfunction prolongs elimination. Sotalol’s β-blocking effect outlasts its plasma half-life. The drug has minimal hemodynamic effects, although it can cause slowed sinus rate, first-degree AV block, and hypotension. Proarrhythmia can occur (as with all antiarrhythmic agents), including torsades de pointes. Sotalol’s class III effects occur at higher doses in dogs than in people. Doses used clinically in dogs may be producing primarily β-blocking effects. On the other hand, a high incidence of proarrhythmia (especially torsades de pointes), of concern in people taking sotalol, has not been reported clinically in dogs. Experimentally, in dogs with hypokalemia, co-administration of mexiletine reduced the proarrhythmic potential.
Sotalol may worsen heart failure in animals with dilated cardiomyopathy. However, sotalol is thought to have less negative inotropic effect than propranolol. Other adverse effects of sotalol have included hypotension, depression, nausea, vomiting, diarrhea, and bradycardia. There are occasional anecdotal reports of aggression that resolved after sotalol was discontinued.
Amiodarone
Amiodarone HCl is thought to produce its antiarrhythmic effects by prolonging the action potential duration and effective refractory period in both atrial and ventricular tissues. Although considered a class III agent, it shares properties with all three other antiarrhythmic drug classes. Amio-darone is an iodinated compound that also has noncompetitive α1- and β-blocking effects, as well as Ca++ channel-blocking effects. The β-blocking effects occur soon after administration, but maximal class III effects (and prolongation of action potential duration and QT interval) are not achieved for weeks with chronic administration. Its Ca++ blocking effects may inhibit triggered arrhythmias by reducing afterdepolarizations. Therapeutic doses slow the sinus rate, decrease AV conduction velocity, and minimally depress myocardial contractility and blood pressure. Indications for amiodarone include refractory atrial and ventricular tachyarrhythmias, especially reentrant arrhythmias using an accessory pathway. The IV form is used in people with AF, ventricular tachycardia, and during cardiopulmonary resuscitation from recurrent ventricular tachycardia and fibrillation; similar applications are expected in dogs. However, conservative dosing with slow injection over 10 to 20 minutes is recommended, because IV use can cause hypotension and bradycardia. The drug is also given by CRI in people; 10 to 15 mg/kg/day has been used in children.
The pharmacokinetics of amiodarone are complex. Chronic oral use is associated with a prolonged time to steady state (of several weeks), concentration of drug in myocardial and other tissues, and accumulation of an active metabolite (desethylamiodarone). Therapeutic serum concentration is thought to be 1 to 2.5 μg/ml. Amiodarone may have less of a proarrhythmic effect than other agents and may reduce the risk of sudden death because of uniform prolongation of repolarization throughout the ventricles, as well as suppression of Purkinje fiber automaticity. In normal dogs IV amiodarone does not adversely affect contractility at cumulative doses less than 12.5 to 15 mg/kg. However, the potential exists for more profound cardiac depression and hypotension in dogs with myocardial disease. Amiodarone use is not described in cats.
Long-term amiodarone is associated with many potential adverse effects, including depressed appetite, gastrointestinal upset, pneumonitis leading to pulmonary fibrosis, hepatopathy, thyroid dysfunction, positive Coombs test, thrombocytopenia, and neutropenia. Occasional hypersensitivity reactions (with acute angioedema formation) or of tremors have occurred in dogs. Other adverse effects observed in people have included corneal microdeposits, photosensitivity, bluish skin discoloration, and peripheral neuro-pathy. Amiodarone can increase the serum concentration of digoxin, diltiazem, and possibly procainamide and quinidine.
Other Class III Agents
Ibutilide fumarate is somewhat effective for converting recent-onset AF in people, but there is little veterinary experience with this drug. In experimental rapid-pacing–induced cardiomyopathy in dogs, ibutilide caused episodes of torsades de pointes.
Dofetilide is another drug that selectively blocks the rapid component of the K+ current responsible for repolarization. It too is used in people for the conversion of AF and to maintain sinus rhythm. Its efficacy for this appears to be comparable to that of other class III drugs, and it does not exacerbate left ventricular dysfunction. Bretylium tosylate is no longer available in the United States.
CLASS IV ANTIARRHYTHMIC DRUGS: CALCIUM ENTRY BLOCKERS
The Ca++ entry blockers are a diverse group of drugs that have the common property of decreasing cellular Ca++ influx by blocking transmembrane L-type calcium channels. As a group, these drugs can cause coronary and systemic vasodilation, enhance myocardial relaxation, and reduce cardiac contractility. Some calcium entry blockers have antiarrhythmic effects, especially on tissues dependent on the slow inward Ca++ current, such as the sinus and AV nodes. Other conditions for which calcium entry blockers are potentially useful include hypertrophic cardiomyopathy, myocardial ischemia, and hypertension.
Possible adverse effects of these agents include reduced contractility, vasodilation, hypotension, depression, anorexia, lethargy, bradycardia, and AV block. Low initial doses are used and increased as needed to effect or to maximal recommended dose. Contraindications to Ca++ channel blocker use include sinus bradycardia, AV block, sick sinus syndrome, digoxin toxicity, and myocardial failure (for agents with pronounced negative inotropic effect). They are usually not prescribed in patients receiving a β-blocker because of additive negative effects on contractility, AV conduction, and heart rate. An overdose or exaggerated response to a Ca++ blocker is treated with supportive care, including atropine for bradycardia or AV block, dopamine or dobutamine (see Box 3-1) and furosemide for heart failure, and dopamine or IV calcium salts for hypotension.
Diltiazem
Diltiazem HCl is a benzothiazepine Ca++ channel blocker. It slows AV conduction, causes potent coronary and mild peripheral vasodilation, and has a lesser negative inotropic effect than the prototypical calcium entry blocker, verapamil. Diltiazem is often combined with digoxin to further slow the ventricular response rate to AF in dogs. It is indicated for other supraventricular tachyarrhythmias as well. Diltiazem is often used in cats with hypertrophic cardiomyopathy; its beneficial effects can include enhanced myocardial relaxation and perfusion, as well as a mild decrease in heart rate, contractility, and myocardial oxygen demand (see Chapter 8). Chronic diltiazem therapy may be associated with a decrease in left ventricular wall and septal thickness in cats with hypertrophic cardiomyopathy.
Peak effects are seen within 2 hours of oral dosing, and the effects last at least 6 hours in dogs. Extensive first-pass effect limits bioavailability, especially in dogs. The half-life of diltiazem in the dog is just over 2 hours, but chronic oral treatment prolongs it because of enterohepatic circulation. In cats plasma diltiazem concentration peaks in 30 minutes, and the effects last for 8 hours. The therapeutic range is 50 to 300 ng/ml. Diltiazem is metabolized in the liver; active metabolites exist. Drugs that inhibit hepatic enzyme systems (e.g., cimetidine) decrease the metabolism of diltiazem. Propranolol and diltiazem reduce each other’s clearance when used simultaneously. A sustained-release preparation (Cardizem-CD), at 10 mg/kg daily in cats, produces plasma concentrations that peak in 6 hours and remain in the therapeutic range for 24 hours. A dose of 45 mg per cat is approximately equal to 105 mg of Cardizem-CD (or the amount that fits into the small end of a No. 4 gelatin capsule; a 300-mg capsule provides about 6.5 doses); this is given once daily. Diltiazem XR is another sustained-release diltiazem preparation. The 240-mg capsule contains four 60-mg tablets. There is much intercat variability in pharmacokinetics with this form. Higher doses are more likely to be associated with anorexia and other gastrointestinal signs.
Adverse effects of diltiazem are uncommon at therapeutic doses, although anorexia, nausea, and bradycardia may occur. Rarely, other gastrointestinal, cardiac, and neurologic adverse effects develop. High liver enzyme activities and anorexia occur sporadically in cats. Some cats have become aggressive or shown other personality change when treated with diltiazem.
Verapamil
Verapamil HCl is a phenylalkylamine and has the most potent cardiac effects of the Ca++-blockers used clinically. The drug increases the refractory period of nodal tissues and can abolish reentrant SVT as well as slow the ventricular response rate in AF. Verapamil causes dose-related slowing of the sinus rate and AV conduction. It is sometimes used for supraventricular and atrial tachycardias in animals without heart failure. Verapamil’s half-life in dogs is about 2.5 hours. It is poorly absorbed and undergoes first-pass hepatic metabolism, resulting in low bioavailability with oral use. The pharmacokinetics in cats are similar to those of dogs.
The drug has important negative inotropic and some vasodilatory effects that can cause cardiac decompensation, hypotension, and even death in the presence of underlying myocardial disease. An initially low IV dose is given very slowly; this can be repeated at 5- (or more) minute intervals if no adverse effects have occurred and the arrhythmia persists. Blood pressure monitoring is advisable because of the potential for hypotension. As discussed above, verapamil is not recommended for use in animals with heart failure. The toxic effects of verapamil include sinus bradycardia, AV block, hypotension, reduced myocardial contractility, and cardiogenic shock. Verapamil reduces the renal clearance of digoxin.
Other Calcium Channel Blockers
A number of other Ca++-blockers are available. Most (dihydropyridine group) are used as antihypertensives. Amlodipine besylate is recommended as the first-line antihypertensive agent in cats and is also used in some hypertensive dogs (see Chapter 11). Amlodipine is also used in the treatment of chronic refractory heart failure in some dogs (see Table 3-3). The drug is not useful as an antiarrhythmic agent. Nifedipine is another potent vasodilator without antiarrhythmic effects.
ANTICHOLINERGIC DRUGS
Atropine and Glycopyrrolate
Anticholinergic drugs increase sinus node rate and AV conduction when vagal tone is increased (see Table 4-2). Parenteral atropine or glycopyrrolate is indicated for bradycardia or AV block induced by anesthesia, central nervous system lesions, and certain other diseases or toxicities. Atropine is a competitive muscarinic receptor antagonist that is used to determine whether excess vagal tone is responsible for arrhythmias attributed to sinus and/or AV nodal dysfunction. This is known as the atropine challenge test (or atropine response test). Response to atropine challenge is most consistent with IV administration of 0.04 mg/kg. An ECG is recorded within 5 to 10 minutes after atropine injection. If the heart rate has not increased by at least 150%, the ECG is repeated 15 (to 20) minutes after atropine injection; sometimes, an initial vagomimetic effect on the AV node lasts longer than 5 minutes. The normal sinus node response is a rate increase to 150 to 160 beats/minute (or >135 beats/minute). A positive response may not predict response to oral anticholinergic therapy. Atropine has little to no effect on bradyarrhythmias caused by intrinsic disease of the sinus or AV node.
Atropine given by any parenteral route can transiently exacerbate vagally mediated AV block when the atrial rate increases faster than AV conduction can respond. However, IV administration causes the fastest and most consistent onset and resolution of the exacerbated block, as well as the most rapid postbradycardia heart rates, compared with the IM and subcutaneous routes. Unlike atropine, glycopyrrolate does not have centrally mediated effects, and its effects are longer-lasting than those of atropine.
Oral Anticholinergic Drugs
Some animals that respond to parenteral atropine or glycopyrrolate will also respond to an oral anticholinergic agent. Clinical signs may be relieved in these animals, at least for a time. Nevertheless, animals with symptomatic bradyarrhythmias usually require permanent pacemaker implantation to effectively control heart rate. Propantheline bromide and hyoscyamine sulfate are commonly used, but other oral anticholinergic agents are also available. Individual dosage is adjusted to effect. Oral absorption of propantheline is variable; food may decrease drug absorption.
Vagolytic drugs can aggravate paroxysmal supraventricular tachyarrhythmias (as in sick sinus syndrome) and should be used only cautiously as chronic therapy in those patients. Other adverse effects of anticholinergic therapy include vomiting, dry mouth, constipation, keratoconjunctivitis sicca, increased intraocular pressure, and drying of respiratory secretions.
SYMPATHOMIMETIC DRUGS
Isoproterenol HCl is a β-receptor agonist that has been used to treat symptomatic AV block or bradycardia refractory to atropine, although electrical pacing is safer and more effective. It also can be effective for torsades de pointes. Because of its affinity for β2-receptors, isoproterenol can cause hypotension. It is not used for treating either heart failure or cardiac arrest. Isoproterenol can be arrhythmogenic, as can other catecholamines. The lowest effective dose (see Table 4-2) is used, and the animal is monitored closely for arrhythmias. Oral administration is not effective because of marked first-pass hepatic metabolism.
Terbutaline sulfate is a β2-receptor agonist that may have a mild stimulatory effect on heart rate when given orally. Methylxanthine bronchodilators (e.g., aminophylline and theophylline) increase heart rate in some dogs with sick sinus syndrome when used at higher doses.
OTHER DRUGS
Edrophonium chloride is a short-acting anticholinesterase with nicotinic and muscarinic effects. Although mainly used for diagnosing myasthenia gravis, it slows AV conduction, which can help in the diagnosis and resolution of some cases of acute SVT. The drug’s effect begins within 1 minute and lasts up to 10 minutes after IV injection. Adverse effects are primarily cholinergic and include gastrointestinal (e.g., vomiting, diarrhea, salivation), respiratory (e.g., bronchospasm, respiratory paralysis, edema), cardiovascular (e.g., bradycardia, hypotension, cardiac arrest), and muscular (e.g., twitching, weakness) signs. Atropine and supportive care are used if necessary.
Phenylephrine HCl is an α-adrenergic agonist that increases blood pressure by peripheral vasoconstriction. A baroreflex-mediated increase in vagal tone slows AV conduction and is thought to underlie its effects on SVT. Phenylephrine’s pressor effect begins rapidly after IV injection and persists for up to 20 minutes. The drug is contraindicated in patients with hypertension or ventricular tachycardia. Extravasation can cause ischemic necrosis of surrounding tissue.
Arrhythmias and Antiarrhythmic Drugs
Awaji T, Wu ZJ, Hashimoto K. Acute antiarrhythmic effects of intravenously administered amiodarone on canine ventricular arrhythmias. J Cardiovasc Pharmacol. 1995;26:869.
Baty CJ, Sweet DC, Keene BK. Torsades de pointes-like polymorphic ventricular tachycardia in a dog. J Vet Intern Med. 1994;8:439.
Bicer S, et al. Hemodynamic and electrocardiographic effects of graded doses of amiodarone in healthy dogs anesthetized with morphine/alpha chloralose. J Vet Intern Med. 2000;14:90.
Bicer S, et al. Effects of chronic, oral amiodarone on left ventricular pressure, electrocardiograms, and action potentials from myocardium in vivo and from Purkinje fibers in vitro. Vet Therap. 2001;2:325.
Bright JM, Martin JM, Mama K. A retrospective evaluation of transthoracic biphasic electrical cardioversion for atrial fibrillation in dogs. J Vet Cardiol. 2005;7:85.
Brundel BJJM, et al. The pathology of atrial fibrillation in dogs. J Vet Cardiol. 2005;7:121.
Calvert CA, Brown J. Influence of antiarrhythmia therapy on survival times of 19 clinically healthy Doberman Pinschers with dilated cardiomyopathy that experienced syncope, ventricular tachycardia, and sudden death (1985–1998). J Am Anim Hosp Assoc. 2004;40:24.
Calvert CA, Meurs KM. CVT Update: Doberman Pinscher occult cardiomyopathy. In: Bonagura JD, editor. Kirk’s current veterinary therapy XIII. Philadelphia: WB Saunders; 2000:756-760.
Calvert CA, Sammarco C, Pickus C. Positive Coombs’ test results in two dogs treated with amiodarone. J Am Vet Med Assoc. 2000;216:1933.
Cooke KL, Snyder PS. Calcium channel blockers in veterinary medicine. J Vet Intern Med. 1998;12:123.
Cote E, et al. Atrial fibrillation in cats: 50 cases (1979-2002). J Am Vet Med Assoc. 2004;225:256.
Gelzer ARM, Kraus MS. Management of atrial fibrillation. Vet Clin North Am: Small Anim Pract. 2004;34:1127.
Hsieh MH, et al. Proarrhythmic effects of ibutilide in a canine model of pacing induced cardiomyopathy. Pacing Clin Electrophysiol. 2000;23:149.
Jacobs G, Calvert CA, Kraus M. Hepatopathy in 4 dogs treated with amiodarone. J Vet Intern Med. 2000;14:96.
Johnson LM, et al. Pharmacokinetic and pharmacodynamic properties of conventional and CD-formulated diltiazem in cats. J Vet Intern Med. 1996;10:316.
Johnson MS, Martin M, Smith P. Cardioversion of supraventricular tachycardia using lidocaine in five dogs. J Vet Intern Med. 2006;20:272.
Kellum HB, Stepien RL. Third degree atrioventricular block in 21 cats (1997-2004). J Vet Intern Med. 2006;20:97.
Kovach JA, Nearing BD, Verrier R. Anger-like behavioral state potentiates myocardial ischemia-induced T-wave alternans in canines. J Am Coll Cardiol. 2001;37:1719.
Lunney J, Ettinger SJ. Mexiletine administration for management of ventricular arrhythmia in 22 dogs. J Am Anim Hosp Assoc. 1991;27:597.
Menaut P, et al. Atrial fibrillation in dogs with and without structural or functional cardiac disease: a retrospective study of 109 cases. J Vet Cardiol. 2005;7:75-83.
Merot J, et al. Effects of chronic treatment by amiodarone on transmural heterogeneity of canine ventricular repolarization in vivo: interactions with acute sotalol. Cardiovasc Res. 1999;44:303.
Merot J, et al. Electropharmacological characterization of cardiac repolarization in German Shepherd Dogs with an inherited syndrome of sudden death: abnormal response to potassium channel blockers. J Am Coll Cardiol. 2000;36:939.
Meurs KM, et al. Use of ambulatory electrocardiography for detection of ventricular premature complexes in healthy dogs. J Am Vet Med Assoc. 2001;218:1291.
Meurs KM, et al. Comparison of the effects of four antiarrhythmic treatments for familial ventricular arrhythmias in Boxers. J Am Vet Med Assoc. 2002;221:522.
Miyamoto M, et al. Cardiovascular effects of intravenous diltiazem in dogs with iatrogenic atrial fibrillation. J Vet Intern Med. 2000;14:445.
Miyamoto M, et al. Acute cardiovascular effects of diltiazem in anesthetized dogs with induced atrial fibrillation. J Vet Intern Med. 2001;15:559.
Moise NS, et al. Diagnosis of inherited ventricular tachycardia in German Shepherd Dogs. J Am Vet Med Assoc. 1997;210:403.
Moise NS. CVT Update: ventricular arrhythmias. In: Bonagura JD, editor. Kirk’s current veterinary therapy XIII. Philadelphia: WB Saunders; 2000:733-737.
Moise NS, et al. Cardioversion with lidocaine of vagally associated atrial fibrillation in two dogs. J Vet Cardiol. 2005;7:143.
Moneva-Jordan A, et al. Sick sinus syndrome in nine West Highland White Terriers. Vet Rec. 2001;148:142.
Oyama MA, Prosek R. Acute conversion of atrial fibrillation in two dogs by intravenous amiodarone administration. J Vet Intern Med. 2006;20:1224-1227.
Pinson DM. Myocardial necrosis and sudden death after an episode of aggressive behavior in a dog. J Am Vet Med Assoc. 1997;211:1371.
Poole JE, Bardy GH. Sudden cardiac death. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: from cell to bedside. ed 3. Philadelphia: WB Saunders; 2000:615-639.
Quiñones M, Dyer DC, Ware WA. Pharmacokinetics of atenolol in clinically normal cats. Am J Vet Res. 1996;57:1050.
Rishniw M, et al. Characterization of parasympatholytic chronotropic responses following intravenous administration of atropine to clinically normal dogs. Am J Vet Res. 1999;60:1000.
Saunders AB, et al. Oral amiodarone therapy in dogs with atrial fibrillation. J Vet Intern Med. 2006;20:921.
Sawangkoon S, et al. Acute cardiovascular effects and pharmacokinetics of carvedilol in healthy dogs. Am J Vet Res. 2000;61:57.
Seidler RW, et al. Influence of sotalol on the time constant of isovolumic left ventricular relaxation in anesthetized dogs. Am J Vet Res. 1999;60:717.
Sicilian Gambit members. New approaches to antiarrhythmic therapy, Part I. Circulation. 2001;104:2865.
Sicilian Gambit members. New approaches to antiarrhythmic therapy, Part II. Circulation. 2001;104:2990.
Sicouri S, et al. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. J Cardiovasc Electrophysiol. 1997;8:1269.
Smith CE, et al. Omega-3 fatty acids in Boxer dogs with arrhythmogenic right ventricular cardiomyopathy. J Vet Intern Med. 2007;21:265.
Stafford Johnson M, Martin M, Smith P. Cardioversion of supraventricular tachycardia using lidocaine in five dogs. J Vet Intern Med. 2006;20:272.
Steinberg SF, Robinson RB, Rosen MR. Molecular and cellular bases of α-adrenergic modulation of cardiac rhythm. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: from cell to bedside. ed 3. Philadelphia: WB Saunders; 2000:283-294.
Thomasy SM, et al. Pharmacokinetics of lidocaine and its active metabolite, monoethylglycinexylidide, after intravenous administration of lidocaine to awake and isoflurane-anesthetized cats. Am J Vet Res. 2005;66:1162.
Ulloa HM, Houston BJ, Altrogge DM. Arrhythmia prevalence during ambulatory electrocardiographic monitoring of Beagles. Am J Vet Res. 1995;56:275-281.
Wall M, et al. Evaluation of extended-release diltiazem once daily in cats with hypertrophic cardiomyopathy. J Am Anim Hosp Assoc. 2005;41:98.
Ware WA. Twenty-four-hour ambulatory electrocardiography in normal cats. J Vet Intern Med. 1999;13:175.
Wright KN. Interventional catheterization for tachyarrhythmias. Vet Clin North Am: Small Anim Pract. 2004;34:1171.
Wright KN. Assessment and treatment of supraventricular tachyarrhythmias. In: Bonagura JD, editor. Kirk’s current veterinary therapy XIII. Philadelphia: WB Saunders; 2000:726-729.
Wright KN, Knilans TK, Irvin HM. When, why, and how to perform cardiac radiofrequency catheter ablation. J Vet Cardiol. 2006;8:95.
Wright KN, et al. Radiofrequency catheter ablation of atrioventricular accessory pathways in 3 dogs with subsequent resolution of tachycardia-induced cardiomyopathy. J Vet Intern Med. 1999;13:361.
Bulmer BJ, et al. Implantation of a single-lead atrioventricular synchronous (VDD) pacemaker in a dog with naturally occurring 3rd degree atrioventricular block. J Vet Intern Med. 2002;16:197.
Bulmer BJ, et al. Physiologic VDD versus nonphysiologic VVI pacing in canine 3rd degree atrioventricular block. J Vet Intern Med. 2006;20:257.
Côté E, Laste NJ. Transvenous cardiac pacing. Clin Tech Small Anim Pract. 2000;15:165. (erratum: Clin Tech Small Anim Pract 15:260).
Fine DM, Tobias AH. Cardiovascular device infections in dogs: report of 8 cases and review of the literature. J Vet Intern Med. 2007;21:1265-1271.
Flanders JA, et al. Introduction of an endocardial pacing lead through the costocervical vein in six dogs. J Am Vet Med Assoc. 1999;215:46.
Fox PR, et al. Techniques and complications of pacemaker implantation in four cats. J Am Vet Med Assoc. 1991;199:1742.
Francois L, et al. Pacemaker implantation in dogs: results of the last 30 years. Schweiz Arch Tierheilkd. 2004;146:335.
Johnson MS, Martin MWS, Henley W. Results of pacemaker implantation in 104 dogs. J Small Anim Pract. 2007;48:4.
Moise NS. Pacemaker therapy. In: Fox PR, Sisson D, Moise NS, editors. Textbook of canine and feline cardiology. ed 2. Philadelphia: WB Saunders; 1999:400-426.
Oyama MA, Sisson DD, Lehmkuhl LB. Practices and outcomes of artificial cardiac pacing in 154 dogs. J Vet Intern Med. 2001;15:229.
Wess G, et al. Applications, complications, and outcomes of transvenous pacemaker implantation in 105 dogs (1997-2002). J Vet Intern Med. 2006;20:877.
Zimmerman SA, Bright JM. Secure pacemaker fixation critical for prevention of Twiddler’s syndrome. J Vet Cardiol. 2004;6:40-44.
 BOX 4-3
BOX 4-3