CHAPTER 51 Disorders of the Thyroid Gland
HYPOTHYROIDISM IN DOGS
Etiology
Structural or functional abnormalities of the thyroid gland can lead to deficient production of thyroid hormones. A convenient classification scheme for hypothyroidism has been devised that is based on the location of the problem within the hypothalamic-pituitary-thyroid gland complex (Fig. 51-1). Primary hypothyroidism is the most common form of this disorder in dogs; it results from problems within the thyroid gland, usually destruction of the thyroid gland (Box 51-1). The two most common histologic findings in this disorder are lymphocytic thyroiditis and idiopathic atrophy of the thyroid gland (Fig. 51-2). Lymphocytic thyroiditis is an immune-mediated disorder characterized by a diffuse infiltration of lymphocytes, plasma cells, and macrophages into the thyroid gland. The factors that trigger the development of lymphocytic thyroiditis are poorly understood. Genetics undoubtedly plays a major role, especially given the increased incidence of this disorder in certain breeds and in certain lines within a breed (Table 51-1). Environmental risk factors have not been well defined in the dog. A link between infection-induced damage to the thyroid gland and development of lymphocytic thyroiditis has been the subject of speculation but has not been proved. Vaccine administration has also been hypothesized to be a contributing factor for development of lymphocytic thyroiditis but also has not been proved.
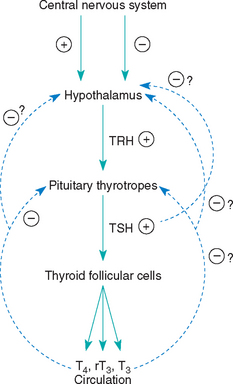
FIG 51-1 The hypothalamic-pituitary-thyroid gland axis. TRH, Thyrotropin-releasing hormone; TSH, thyrotropin; T4, thyroxine; T3, 3,5,3′-triiodothyronine; rT3, 3,3′,5′-triiodothyronine; +, stimulation; -, inhibition.
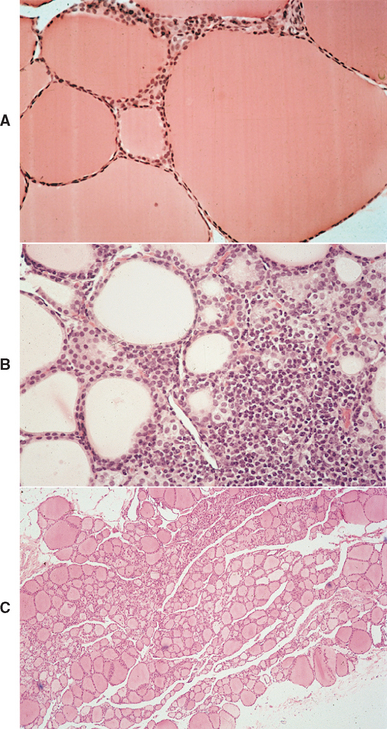
FIG 51-2 Histologic section of a thyroid gland from a healthy dog (A), from a dog with lymphocytic thyroiditis and hypothyroidism (B), and from a dog with idiopathic atrophy of the thyroid gland and hypothyroidism (C). Note the mononuclear cell infiltration, disruption of the normal architecture, and loss of colloid-containing follicles in B and the small size of the gland, decrease in follicular size and colloid content, and lack of a cellular infiltration in C, compared with A. (A and B, Hematoxylin and eosin stain; magnification ×250; C, hematoxylin and eosin stain; magnification ×40).
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
 TABLE 51-1 Dog Breeds Reported to Have an Increased Prevalence of Thyroid Hormone Autoantibodies
TABLE 51-1 Dog Breeds Reported to Have an Increased Prevalence of Thyroid Hormone Autoantibodies
| BREED | ODDS RATIO* |
|---|---|
| Pointer | 3.61 |
| English Setter | 3.44 |
| English Pointer | 3.31 |
| Skye Terrier | 3.04 |
| German Wirehaired Pointer | 2.72 |
| Old English Sheepdog | 2.65 |
| Boxer | 2.37 |
| Maltese | 2.25 |
| Kuvasz | 2.18 |
| Petit Basset Griffon Vendéen | 2.16 |
| American Staffordshire Terrier | 1.84 |
| Beagle | 1.79 |
| American Pit Bull Terrier | 1.78 |
| Dalmatian | 1.74 |
| Giant Schnauzer | 1.72 |
| Rhodesian Ridgeback | 1.72 |
| Golden Retriever | 1.70 |
| Shetland Sheepdog | 1.69 |
| Chesapeake Bay Retriever | 1.56 |
| Siberian Husky | 1.45 |
| Brittany Spaniel | 1.42 |
| Borzoi | 1.39 |
| Australian Shepherd | 1.28 |
| Doberman Pinscher | 1.24 |
| Malamute | 1.22 |
| Cocker Spaniel | 1.17 |
| Mixed | 1.05 |
* Odds of having serum thyroid hormone autoantibodies (THAA) among breeds with an increased risk of having THAA, compared with dogs of all other breeds.
From Nachreiner RF et al: Prevalence of serum thyroid hormone autoantibodies in dogs with clinical signs of hypothyroidism, J Am Vet Med Assoc 220:466, 2002.
Destruction of the thyroid gland is progressive, and clinical signs may not become evident until more than 75% of the gland is destroyed. Development of decreased serum thyroid hormone concentrations and clinical signs is usually a gradual process, often requiring 1 to 3 years to develop, which suggests that the destructive process is slow.
Idiopathic atrophy of the thyroid gland is characterized by loss of the thyroid parenchyma. There is no inflammatory infiltrate, even in areas where small follicles or follicular remnants are present in the thyroid gland. Tests for lymphocytic thyroiditis are negative. The cause of idiopathic thyroid atrophy is not known. It may be a primary degenerative disorder or represent an end stage of autoimmune lymphocytic thyroiditis.
Secondary hypothyroidism results from failure of pituitary thyrotrophs to develop (pituitary hypoplasia causing pituitary dwarfism; see Chapter 49) or from dysfunction within the pituitary thyrotropic cells causing impaired secretion of thyroid-stimulating hormone (TSH) and a “secondary” deficiency in thyroid hormone synthesis and secretion. Follicular atrophy in the thyroid gland gradually develops owing to lack of TSH. Secondary hypothyroidism could also result from destruction of pituitary thyrotrophs (e.g., pituitary neoplasia [rare]) or suppression of thyrotroph function by hormones or drugs (e.g., glucocorticoids [common]; see Box 51-1).
Tertiary hypothyroidism is a deficiency in the secretion of thyrotropin-releasing hormone (TRH) by peptidergic neurons in the supraoptic and paraventricular nuclei of the hypothalamus. Lack of TRH secretion should cause a deficiency in TSH secretion and secondary follicular atrophy in the thyroid gland. Tertiary hypothyroidism has not been reported in dogs.
Congenital primary hypothyroidism is uncommon in dogs and has been caused by deficient dietary iodine intake, dyshormonogenesis (i.e., an iodine organification defect), and thyroid dysgenesis. Secondary hypothyroidism resulting from an apparent deficiency of TSH has also been reported in a family of Giant Schnauzers and in a Boxer. Pedigree analysis showed that it may be inherited in an autosomal recessive fashion in the family of Giant Schnauzers. Development of an enlarged thyroid gland (i.e., goiter) depends on the etiology. If the hypothalamic-pituitary-thyroid gland axis is intact (e.g., as occurs with an iodine organification defect), goiter will develop, and if it is not intact (e.g., as occurs with pituitary TSH deficiency), goiter will not develop.
Clinical Features
Clinical signs of the more common forms of primary hypothyroidism usually develop during middle age (i.e., 2 to 6 years). Clinical signs tend to develop at an earlier age in breeds at increased risk than in other breeds (see Table 51-1). There is no apparent sex-related predilection.
Clinical signs are quite variable and depend in part on the age of the dog at the time a deficiency in thyroid hormone develops (Box 51-2). Clinical signs may also differ between breeds. For example, truncal alopecia may dominate in some breeds, whereas thinning of the haircoat dominates in other breeds. In adult dogs the most consistent clinical signs of hypothyroidism result from decreased cellular metabolism and its effects on the dog’s mental status and activity. Most dogs with hypothyroidism show some mental dullness, lethargy, exercise intolerance or unwillingness to exercise, and a propensity to gain weight without a corresponding increase in appetite or food intake. These signs are often gradual in onset, subtle, and not recognized by the client until after thyroid hormone supplementation has been initiated. Additional clinical signs of hypothyroidism typically involve the skin and, less commonly, the neuromuscular system.
 BOX 51-2 Clinical Manifestations of Hypothyroidism in the Adult Dog
BOX 51-2 Clinical Manifestations of Hypothyroidism in the Adult Dog
* Common.
DERMATOLOGIC SIGNS
Alterations in the skin and haircoat are the most common observable abnormalities in dogs with hypothyroidism. The classic cutaneous signs include bilaterally symmetric, nonpruritic truncal alopecia that tends to spare the head and extremities (Fig. 51-3). Alopecia may be local or generalized and symmetric or asymmetric, it may involve only the tail (i.e., “rat tail”), and it often initially starts over sites of wear and friction. Although nonpruritic endocrine alopecia is not pathognomonic for hypothyroidism (see Chapter 49), hypothyroidism is certainly the most likely diagnosis in an affected dog with lethargy, weight gain, and no polyuria-polydipsia.

FIG 51-3 A, A 6-year-old female spayed Samoyed with hypothyroidism; a dry, lusterless haircoat; hyperpigmentation; and endocrine alopecia. B and C, A 2-year-old female spayed Golden Retriever with hypothyroidism, diffuse thinning of the haircoat, and development of a “rat tail.” In both dogs note the truncal distribution of the dermatologic problem with sparing of the head and distal extremities. D, An 8-year-old male castrated Beagle with hypothyroidism, obesity, and myxedema of the face. Note the “tragic facial expression” and “mental dullness” evident from the dog’s facial expression. E, A 7-month-old female Malamute with congenital hypothyroidism. Note the retention of the puppy haircoat and small stature of the dog.
Seborrhea and pyoderma are also common signs of hypothyroidism. Depletion of thyroid hormone suppresses humoral immune reactions, impairs T-cell function, and reduces the number of circulating lymphocytes—defects that can be reversed by exogenous thyroid hormone therapy. All forms of seborrhea (i.e., sicca, oleosa, dermatitis) are possible. Seborrhea and pyoderma may be focal, multifocal, or generalized. Because both frequently result in pruritus, hypothyroid dogs with secondary pyoderma or seborrhea may initially be brought to the veterinarian because of a pruritic skin disorder.
The haircoat in dogs with hypothyroidism is often dull, dry, and easily epilated. Hair regrowth is slow. Hyperkeratosis leads to the development of scales and dandruff. Variable degrees of hyperpigmentation may also be noted. Chronic otitis externa has been noted in some dogs with hypothyroidism. In severe cases of hypothyroidism acidic and neutral mucopolysaccharides may accumulate in the dermis, bind water, and cause skin to thicken. Referred to as myxedema, the condition causes the skin to thicken predominantly in the forehead and face of dogs, resulting in rounding of the temporal region of the forehead, puffiness and thickening of the facial skin folds, and drooping of the upper eyelids.
NEUROMUSCULAR SIGNS
Neurologic signs may be the predominant problem in some dogs with hypothyroidism (see Box 51-2). Hypothyroidism-induced segmental demyelination and axonopathy may cause signs referable to the central or peripheral ner-vous system. Clinical signs referable to the central nervous system (CNS) may also appear after mucopolysaccharide accumulates in the perineurium and endoneurium or after cerebral atherosclerosis, transient ischemia or brain infarctions, or the development of severe hyperlipidemia and include seizures, ataxia, circling, weakness, and proprioceptive and postural reaction deficits. These signs are often present in conjunction with vestibular signs (e.g., head tilt, nystagmus) or facial nerve paralysis. Peripheral neuropathies include facial nerve paralysis, weakness, and knuckling or dragging of the feet, with excessive wear of the dorsal part of the toenail. Muscle wasting may also be evident, although myalgia is not common. Thyroxine-responsive unilateral forelimb lameness has also been observed in dogs. The relationship between hypothyroidism and laryngeal paralysis or esophageal hypomotility remains controversial, in part because it is difficult to prove a cause-and-effect relationship between these disorders and because treatment of hypothyroidism often does not improve the clinical signs caused by laryngeal paralysis or esophageal hypomotility.
REPRODUCTIVE SIGNS
Historically, hypothyroidism was believed to cause lack of libido, testicular atrophy, and oligospermia to azoospermia in male dogs. However, work by Johnson et al. (1999) in Beagles failed to document any deleterious effect of experimentally induced hypothyroidism on any aspect of male reproductive function. Although other classic clinical signs and clinicopathologic abnormalities of hypothyroidism developed in dogs studied, libido, testicular size, and total sperm count per ejaculate remained normal. These findings indicate that hypothyroidism may, at best, be an uncommon cause of reproductive dysfunction in male dogs, assuming that the Beagle is representative of other dog breeds.
Clinical experience has shown that hypothyroidism can cause prolonged interestrus intervals and failure to cycle in the bitch. Additional reproductive abnormalities include weak or silent estrous cycles, prolonged estrual bleeding (which may be caused by acquired problems in the coagulation system), and inappropriate galactorrhea and gynecomastia. An association between hypothyroidism and fetal resorption, abortion, and stillbirth has been suggested in the bitch; however, published documentation of this association is lacking. Maternal hypothyroidism has also been suggested to result in the birth of weak puppies that die shortly after birth.
MISCELLANEOUS CLINICAL SIGNS
Ocular, cardiovascular, gastrointestinal, and clotting abnormalities are uncommon clinical manifestations of hypothyroidism (see Box 51-2). More commonly, biochemical or functional abnormalities of these organ systems are identified in dogs exhibiting the more common clinical signs of hypothyroidism. Echocardiography may identify a decrease in cardiac contractility that is usually mild and asymptomatic but that may become relevant during a surgical procedure requiring prolonged anesthesia and aggressive fluid therapy.
A reduction in the activity of factor VIII–related antigen (von Willebrand factor) activity has been inconsistently documented in dogs with hypothyroidism, and the development of clinical signs of a bleeding disorder in hypothyroid dogs is uncommon. An evaluation of the coagulation cascade or von Willebrand factor activity is not indicated in dogs with untreated hypothyroidism unless there are concurrent bleeding problems. Thyroid hormone supplementation has a variable and sometimes deleterious effect on the blood concentration of von Willebrand factor in euthyroid dogs with von Willebrand’s disease.
A cause-and-effect relationship between hypothyroidism and behavioral problems (e.g., aggression) has not been well established in dogs. To date, most reports have been anecdotal and based on improvement in behavior following initiation of thyroid hormone treatment. An inverse relationship between development of aggression and serotonin activity in the CNS has been documented in several species, including dogs. Serotonin turnover and sympathetic activity in the CNS increase in rats made hypothyroid after surgical thyroidectomy, dopamine receptor sensitivity is affected by thyroid hormone in rats, and thyroid hormone potentiates the activity of tricyclic antidepressants in humans suffering from certain types of depression. These studies suggest that thyroid hormone may have an influence on the serotonin-dopamine pathway in the CNS, regardless of the functional status of the thyroid gland. The benefits, if any, of using thyroid hormone to treat behavioral disorders such as aggression in dogs remain to be clarified.
MYXEDEMA COMA
Myxedema coma is an uncommon syndrome of severe hypothyroidism characterized by profound weakness, hypothermia, bradycardia, and a diminished level of consciousness that can rapidly progress to stupor and then coma. Physical findings include profound weakness; hypothermia; nonpitting edema of the skin, face, and jowls (i.e., myxedema); bradycardia; hypotension; and hypoventilation. Laboratory findings may include hypoxemia, hypercarbia, hyponatremia, and hypoglycemia in addition to the typical findings of hyperlipidemia, hypercholesterolemia, and nonregenerative anemia. Serum thyroid hormone concentrations are usually extremely low or undetectable; serum TSH concentration is variable but typically increased. Treatment consists of intravenous levothyroxine (5 μg/kg q12h) and supportive care aimed at correcting hypothermia, hypovolemia, electrolyte disturbances, and hypoventilation. Once the dog has stabilized, oral levothyroxine can be started (see p. 741).
CRETINISM
Hypothyroidism in puppies is termed cretinism. As the age of onset increases, the clinical appearance of animals with cretinism merges imperceptibly with that of adult hypothyroidism. Retarded growth and impaired mental development are the hallmarks of cretinism (Box 51-3). Dogs with cretinism have a disproportionate body size, with large, broad heads; thick, protruding tongues; wide, square trunks; and short limbs (Fig. 51-4). This is in contrast to the proportionate dwarfism caused by growth hormone deficiency. Cretins are mentally dull and lethargic and do not show the typical playfulness seen in normal puppies. Persistence of the puppy haircoat, alopecia, inappetence, delayed dental eruption, and goiter are additional signs. Differential diagnoses for failure to grow include endocrine (e.g., dwarfism) and nonendo crine causes (see Box 49-4 and Fig. 49-11). The presence of goiter is variable and dependent on the underlying etiology.

FIG 51-4 A and B, Eight-month-old female Giant Schnauzer litter mates. The dog on the left is normal, whereas the smaller dog on the right has congenital hypothyroidism (cretinism). Note the small stature; disproportionate body size; large, broad head; wide, square trunk; and short limbs in the cretin.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
AUTOIMMUNE POLYENDOCRINE SYNDROMES
Because autoimmune mechanisms play an important role in the pathogenesis of lymphocytic thyroiditis, it is not surprising that lymphocytic thyroiditis may occur in conjunction with other immune-mediated endocrinopathies. Presumably, the immune-mediated attack is directed against antigens shared by the endocrine system. In human beings autoimmune polyglandular syndrome type II (Schmidt’s syndrome) is the most common of the immunoendocrinopathy syndromes, and it usually consists of primary adrenal insufficiency, autoimmune thyroid disease, and type 1 diabetes mellitus. Autoimmune polyendocrine syndromes are uncommon in dogs and should be suspected in a dog found to have multiple endocrine gland failure. Hypothyroidism; hypoadrenocorticism; and, to a lesser extent, diabetes mellitus, hypoparathyroidism, and lymphocytic orchitis are recognized combined syndromes. In most affected dogs each endocrinopathy is manifested separately, with additional disorders ensuing one by one after variable periods (months to years). Diagnostic tests and treatment are directed at each disorder as it is recognized because it is not possible to reliably predict or prevent any of these problems. Immunosuppressive drug therapy is not indicated for animals with these syndromes because the adverse effects of immunosuppressive therapy and the difficulty posed by suppression of the immune destruction of affected endocrine glands outweigh the potential benefits of such therapy.
Clinical Pathology
The most consistent clinicopathologic findings in dogs with hypothyroidism are hypercholesterolemia and hypertriglyceridemia; the latter is identified as lipemia. Hypercholesterolemia is identified in approximately 75% of hypothyroid dogs, and the cholesterol concentration can exceed 1000 mg/ dl. Although fasting hypercholesterolemia and hypertriglyceridemia can be associated with several other disorders (see Chapter 54), their presence in a dog with appropriate clinical signs is strong evidence for hypothyroidism.
A mild normocytic, normochromic, nonregenerative anemia (packed cell volume [PCV] of 28% to 35%) is a less consistent finding. Evaluation of red blood cell morphology may reveal an increase in the numbers of leptocytes (target cells), which develop as a result of increased erythrocyte membrane cholesterol loading. The white blood cell count is typically normal, and platelet counts are normal to increased.
A mild to moderate increase in lactate dehydrogenase; aspartate aminotransferase; alanine transaminase; alkaline phosphatase; and, rarely, creatine kinase activities may be identified but are extremely inconsistent findings and may not be directly related to the hypothyroid state. Mild hypercalcemia may be found in some dogs with congenital hypothyroidism. Results of urinalysis are usually normal. Polyuria, hyposthenuria, and urinary tract infections are not typical of hypothyroidism.
DERMATOHISTOPATHOLOGIC FINDINGS
Skin biopsies are often performed in dogs with suspected endocrine alopecia, especially if screening diagnostic tests (including tests to assess thyroid gland function) have failed to identify the cause. Nonspecific histologic changes are associated with various endocrinopathies, including hypothyroidism (see Table 49-5); histologic alterations that are claimed to be specific to hypothyroidism may also be seen, including vacuolated and/or hypertrophied arrector pili muscles, increased dermal mucin content, and thickened dermis. A variable inflammatory cell infiltrate may be present if a secondary pyoderma has developed.
ULTRASONOGRAPHIC FINDINGS
Ultrasound evaluation of the thyroid lobe may be helpful in differentiating dogs with hypothyroidism from euthyroid dogs with nonthyroidal illness causing low thyroid hormone test results. Lymphocytic thyroiditis and idiopathic atrophy eventually cause a decrease in the size and alterations in the echogenicity of the thyroid lobe. The thyroid lobe in euthyroid dogs is usually fusiform and triangular to oval in shape on longitudinal and transverse views, respectively; has a homogeneous echogenic pattern; is hyperechoic to isoechoic, compared with the echogenicity of the surrounding musculature; and has a hyperechoic capsule (Fig. 51-5). Although thyroid lobe shape is often similar between euthyroid and hypothyroid dogs, there is often a significant reduction in size and volume of the thyroid lobe in hypothyroid versus euthyroid dogs. In addition, the echogenicity of the thyroid lobe in hypothyroid dogs tends to be isoechoic to hypoechoic with hyperechoic foci, and the echogenic pattern often differs between thyroid lobes in the same dog. A direct correlation between size of the dog and size and volume of the normal thyroid gland may exist; the smaller the dog, the smaller the size and volume of the thyroid lobe (Fig. 51-6). This must be considered when evaluating thyroid lobe size in a dog with suspected hypothyroidism.
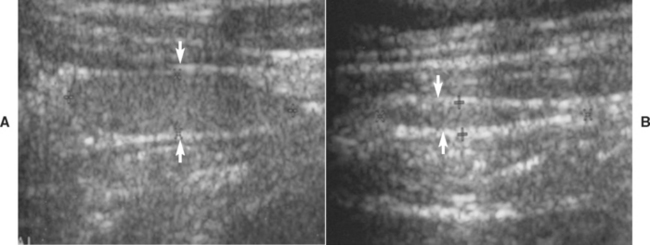
FIG 51-5 A, Ultrasound image of the normal-appearing left thyroid lobe (arrows) of a healthy adult Golden Retriever. B, Ultrasound image of the left thyroid lobe (arrows) of an adult Golden Retriever dog with primary hypothyroidism. Note the significant reduction in the size of the thyroid lobe in the dog with hypothyroidism, compared with the thyroid lobe image from the healthy dog.
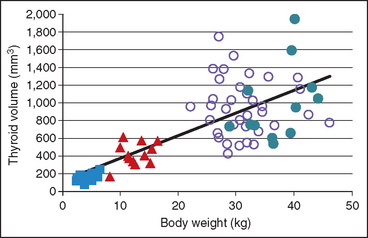
FIG 51-6 The relationship between total thyroid gland volume determined by ultrasound and body weight in 12 healthy Akitas (closed circles), 36 Golden Retrievers (open circles), 12 Beagles (triangles), and 12 Miniature and Toy Poodles (squares). Notice the positive correlation between body weight and size of the thyroid gland.
(From Brömel C et al: Comparison of ultrasonographic characteristics of the thyroid gland in healthy small-, medium-, and large-breed dogs, Am J Vet Res 67:70, 2006.)
TESTS OF THYROID GLAND FUNCTION
Overview
Function of the thyroid gland is typically assessed by measuring baseline serum thyroid hormone concentrations. 3,5,3′5′-tetraiodothyronine (thyroxine [T4]) accounts for most of the thyroid hormone secreted by the thyroid gland, with only small quantities of 3,5,3′-triiodothyronine (T3) and minor amounts of 3,3′,5′-triiodothyronine (reverse T3 [rT3]) released. Once secreted into the circulation, more than 99% of T4 is bound to plasma proteins, which serves as a reservoir and buffer to maintain a steady concentration of free T4 (fT4) in the plasma. The unbound, or free, T4 is biologically active, exerts negative feedback inhibition on pituitary TSH secretion (see Fig. 51-1), and is capable of entering cells throughout the body (Fig. 51-7). Within the cell fT4 is deiodinated to form either T3 or rT3, depending on the metabolic demands of the tissues at that particular time. T3 is preferentially produced during normal metabolic states; rT3, is biologically inactive. T3 is believed to be the primary hormone that induces physiologic effects.
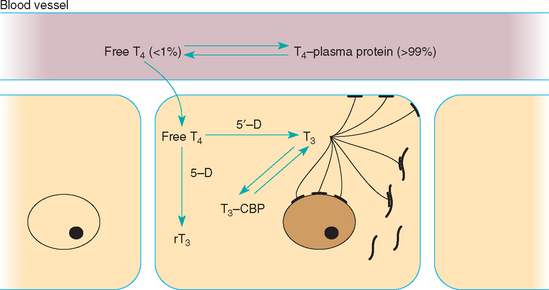
FIG 51-7 Intracellular metabolism of free T4 to either T3 or reverse T3 by 5′- or 5-monodeiodinase, respectively. Intracellular T3 formed from monodeiodination of free T4 can interact with T3 receptors on the cell membrane, mitochondria, or nucleus of the cell and stimulate the physiologic actions of thyroid hormone or bind to cytoplasmic binding proteins (CBP). The latter form an intracellular storage pool for T3.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
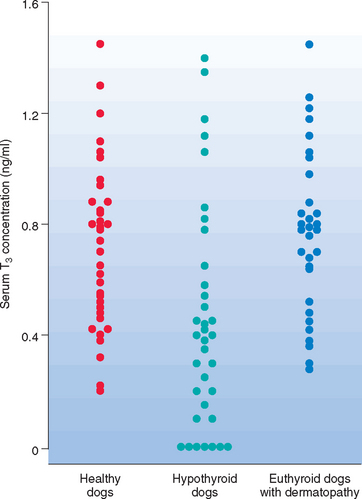
All serum T4, both protein bound and free, comes from the thyroid gland. Therefore tests that measure the serum total and fT4 concentrations, in conjunction with the serum TSH concentration, are currently recommended for the assessment of thyroid gland function in dogs suspected of having hypothyroidism. Serum T3 concentration is a poor gauge of thyroid gland function because of its predominant location within cells and the minimal amount secreted by the thyroid gland in comparison with the amount of T4 secreted (Fig. 51-8). Thus measurement of serum T3, free T3, and rT3 concentration is not recommended for assessing thyroid gland function in dogs.
Baseline Serum T4 Concentration
The baseline serum T4 concentration is the sum of the protein-bound and free levels circulating in the blood. Measurement of serum T4 concentration can be the initial screening test for hypothyroidism or be part of a thyroid panel containing T4, fT4, TSH, an antibody test for lymphocytic thyroiditis, or some combination of these tests (Box 51-4).
 BOX 51-4 Diagnostic Tests for Evaluating Thyroid Gland Function in the Dog
BOX 51-4 Diagnostic Tests for Evaluating Thyroid Gland Function in the Dog
The decision to assess thyroid gland function should be based on results of the history, physical examination, and results of routine blood work (complete blood count, serum biochemistry panel, urinalysis).
Serum Thyroxine (T4)
Most commonly used initial screening test for hypothyroidism
Normal serum T4 rules out hypothyroidism
Exception: T4 autoantibodies that interfere with T4 assay and cause spuriously high results (uncommon)
Low serum T4 does not, by itself, confirm hypothyroidism
Serum T4 commonly suppressed below the reference range by nonthyroidal illness, drugs, and other factors in dogs with normal thyroid gland function
Serum Free Thyroxine (FT4) By Dialysis
Usually measured in dogs with nondiagnostic serum T4 test results, severe nonthyroidal illness, or both; common component of canine thyroid panels
Normal serum fT4 rules out hypothyroidism
Low serum fT4 does not, by itself, confirm hypothyroidism; severe nonthyroidal illness and drugs can suppress serum fT4 to below the reference range
Serum Thyrotropin (TSH)
Usually measured in dogs with nondiagnostic serum T4 test results, severe nonthyroidal illness, or both; common component of canine thyroid panels
Provides additional evidence for or against the diagnosis of hypothyroidism
False positive and false negative serum TSH test results are common
Serum TSH should not be used, by itself, to diagnose hypothyroidism
Serum 3,5,3′-Triiodothyronine (T3)
May be a component of canine thyroid panels
Not the primary hormone secreted by the thyroid gland; T3 is primarily produced from deiodination of fT4 within cells of the body
T3 is a poor gauge of thyroid gland function and should not be used, by itself, to diagnose hypothyroidism
Clinical chemistry laboratories currently use a radioimmunoassay (RIA) technique or enzyme immunoassay for measuring serum T4. Point-of-care ELISAs for measuring serum T4 are also available, are economical, quick, and easy to perform, and allow the clinician to make recommendations the same day the dog (or cat) is evaluated. In a recent study serum T4 concentrations determined in dogs and cats by RIA, chemiluminescent enzyme immunoassay, and a point-of-care ELISA provided similar and consistent results (Kemppainen and Birchfield, 2006). For most laboratories the lower limit of the reference range for serum T4 in dogs is approximately 0.8 to 1.0 μg/dl (10 to 13 nmol/L), although in some breeds the normal range may extend to as low as 0.5 μg/dl (6 nmol/L) (see the discussion of breed variations, p. 740).
Theoretically, the interpretation of baseline serum T4 concentration should be straightforward in that dogs with hypothyroidism should have low values compared with the values in healthy dogs. Unfortunately, the serum T4 concentration range in hypothyroid dogs overlaps with that in healthy dogs and the serum T4 concentration can be suppressed by a variety of factors, most notably nonthyroidal illness and medications (Table 51-2). Clinicians often find it difficult to judge the effect that extraneous factors, especially concurrent illness, have on the serum T4 concentration. Because these variables can suppress a baseline serum T4 concentration to less than 0.5 μg/dl in a euthyroid dog and hypothyroid dogs rarely have a serum T4 concentration greater than 1.5 μg/dl, the serum T4 concentration should be used to confirm normal thyroid gland function, not hypothyroidism per se (Table 51-3). A serum T4 concentration greater than 1.5 μg/dl establishes normal thyroid gland function. The exception is a very small number (<1%) of hypothyroid dogs with lymphocytic thyroiditis that have serum T4 autoantibodies that interfere with the RIA used to measure T4. A serum T4 concentration less than 0.5 μg/dl (6 nmol/L) suggests hypothyroidism, especially if the clinical signs, physical findings, and results of routine blood tests support the diagnosis and systemic illness is not present. The definitive diagnosis relies on response to trial therapy with levothyroxine in these dogs. Additional diagnostic tests of thyroid gland function are indicated if the serum T4 concentration is between 0.5 and 1.5 μg/dl; if the clinical signs, physical examination findings, and results of routine blood work are not strongly supportive of the disease; if severe systemic illness is present and the potential for the euthyroid sick syndrome is high; or if medications known to decrease serum T4 concentration are being administered.
 TABLE 51-2 Variables that May Affect Baseline Serum Thyroid Hormone Function Test Results in the Dog
TABLE 51-2 Variables that May Affect Baseline Serum Thyroid Hormone Function Test Results in the Dog
| FACTOR | EFFECT |
|---|---|
| Age | Inversely proportional effect |
| Neonate (<3 mo) | Increased T4 |
| Aged (>6 yr) | Decreased T4 |
| Body size | Inversely proportional effect |
| Small (<10 kg) | Increased T4 |
| Large (>30 kg) | Decreased T4 |
| Breed | |
| Sight hounds (e.g., Greyhounds) | T4 and free T4 lower than normal range established for dogs; no difference for TSH |
| Nordic breeds (e.g., Huskies) | |
| Other breeds? | |
| Gender | No effect |
| Time of day | No effect |
| Weight gain/obesity | Increased |
| Weight loss/fasting | Decreased T4, no effect on free T4 |
| Strenuous exercise | Increased T4, decreased TSH, no effect on free T4 |
| Estrus (estrogen) | No effect on T4 |
| Pregnancy (progesterone) | Increased T4 |
| Surgery/anesthesia | Decreased T4 |
| Concurrent illness* | Decreased T4 and free T4; depending on illness, TSH may increase, decrease or not change |
| Moderate/severe osteoarthritis | No effect on T4, free T4, or TSH |
| Drugs | See Table 51-4 |
| Dietary iodine intake | If excessive, decreased T4 and free T4; increased TSH |
| Thyroid hormone autoantibodies | Increased or decreased T4; no effect on free T4 or TSH |
TSH, Thyroid-stimulating hormone.
* There is a direct correlation between the severity and systemic nature of the illness and suppression of serum T4 and free T4 concentrations.
 TABLE 51-3 Interpretation of Baseline Serum Thyroxine (T4) and Free Thyroxine (fT4) Concentration in Dogs with Suspected Hypothyroidism*
TABLE 51-3 Interpretation of Baseline Serum Thyroxine (T4) and Free Thyroxine (fT4) Concentration in Dogs with Suspected Hypothyroidism*
| SERUM T4 CONCENTRATION | SERUM FT4 CONCENTRATION | PROBABILITY OF HYPOTHYROIDISM |
|---|---|---|
| >2.0 μg/dl | >2.0 ng/dl | Very unlikely |
| 1.5 to 2.0 μg/dl | 1.5 to 2.0 ng/dl | Unlikely |
| 0.8 to 1.5 μg/dl | 0.8 to 1.5 ng/dl | Unknown |
| 0.5 to 0.8 μg/dl | 0.5 to 0.8 ng/dl | Possible |
| <0.5 μg/dl | <0.5 ng/dl | Very likely† |
* Interpretation based on lower end of the reference range for serum T4 and fT4 being 0.8 μg/dl and 0.8 ng/dl, respectively, without regard for breed of dog. The lower end of the reference range for serum T4 and fT4 may be as low as 0.5 μg/dl and 0.5 ng/dl, respectively, for some breeds such as sight hounds (e.g., Greyhounds) and Nordic breeds (e.g., Siberian Huskies).
Baseline Serum fT4 Concentration
Free T4 is the nonprotein-bound fraction of T4 circulating in blood and accounts for less than 1% of circulating T4. Currently, the most commonly used assays for measuring fT4 in dogs are the Nichol’s modified equilibrium dialysis assay (Antech Diagnostics, Inc.) and the Diasorin 2-step assay (Diasorin, Stillwater, Minn.). The modified equilibrium dialysis (ED) assay utilizes a short ED step to separate free from protein-bound T4 followed by measurement of the free T4 fraction by RIA. The Diasorin 2-step fT4 assay uses two incubation temperatures (37° C for 20 minutes, then room temperature for 1 hour), not ED, to separate free and protein-bound T4 followed by RIA to measure fT4. Preliminary studies suggest that results using the Diasorin 2-step RIA method are similar to results using the more traditional ED method. For most laboratories the lower limit of the reference range for serum fT4 measured by ED and the 2-step RIA is approximately 0.5 to 0.8 ng/dl (6 to 10 pmol/L) in dogs.
Measurement of serum fT4 is usually reserved for those dogs with suspected hypothyroidism and a nondiagnostic serum T4 test result, severe concurrent illness, or both. ED assays for serum fT4 concentration have comparable sensitivity but higher specificity than assays for serum T4 concentration. Similar studies have not been reported for the 2-step RIA. Serum fT4 is more resistant to the suppressive effects of nonthyroidal illness and medications than serum T4, although severe illness can cause serum fT4 concentrations to decrease below 0.5 ng/dl. In addition, serum T4 autoantibodies do not affect serum fT4 results determined by ED. Interpretation of serum fT4 test results is similar to that used to interpret serum T4 test results (see Table 51-3). Serum fT4 values greater than 1.5 ng/dl (20 pmol/L) are consistent with euthyroidism; values less than 0.5 ng/dl (6.5 pmol/L) are supportive of hypothyroidism, assuming the history, physical examination, and results of routine blood work are consistent with hypothyroidism and severe systemic illness is not present; and values between 0.5 and 1.5 ng/dl are not diagnostic.
Baseline Serum TSH Concentration
Measurement of serum TSH provides information on the interaction between the pituitary and thyroid gland. In theory, serum TSH concentration should be increased in dogs with hypothyroidism. In dogs serum TSH can be measured using immunoradiometric, chemiluminescent immunometric, and enzyme immunometric assays. In one study the highest precision for canine TSH analysis was obtained with the chemiluminescent assay, although the correlation between the three assays for measuring canine serum TSH was satisfactory (Marca et al., 2001). Most clinical laboratories use a serum TSH concentration of 0.6 ng/ml as the upper limit of the reference range. The lower limit of the reference range is currently below the sensitivity of these assays; differentiation between low and normal serum TSH concentrations is not possible.
Measurement of serum TSH concentration is usually reserved for dogs with suspected hypothyroidism and nondiagnostic serum T4 test results. A serum TSH concentration greater than 0.6 ng/ml is consistent with hypothyroidism. Unfortunately, serum TSH concentrations can be normal in dogs with histologically confirmed hypothyroidism and increased in euthyroid dogs with concurrent nonthyroidal illness or dogs receiving drugs such as phenobarbital (Fig. 51-9). In most studies the sensitivity and specificity of the TSH assay has ranged from 63% to 87% and 82% to 93%, respectively. Serum TSH test results should always be interpreted in conjunction with results of serum T4, fT4, or both and should not be used alone in the diagnosis of hypothyroidism. Serum TSH test results increase the likelihood of euthyroidism or hypothyroidism when results are consistent with results of serum T4 and fT4 tests. A normal serum T4 and fT4 concentration and increased serum TSH concentration occur in the early stages of primary hypothyroidism in humans. Although similar thyroid hormone and TSH test results have been identified in dogs, it is not known what percentage of these dogs progress to clinical hypothyroidism. Clinical signs of hypothyroidism are usually not evident in these dogs, presumably because serum T4 and fT4 concentrations are in the reference range. Treatment with levothyroxine is not indicated. Rather, assessment of thyroid gland function should be repeated in 3 to 6 months, especially if antibody tests for lymphocytic thyroiditis are positive. If progressive destruction of the thyroid gland is occurring, serum T4 and fT4 concentrations will gradually decrease and clinical signs will eventually develop.
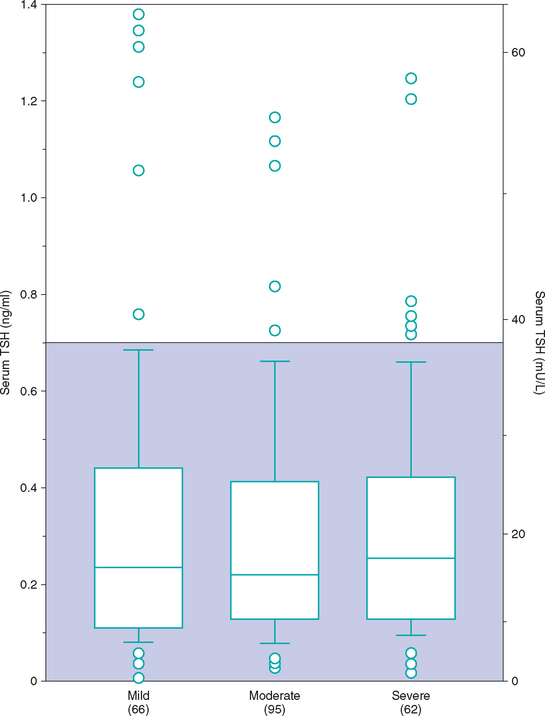
FIG 51-9 Box plots of serum concentrations of thyrotropin (TSH) in 223 dogs with nonthyroidal disease stratified according to severity of disease. For each box plot T-bars represent the main body of data, which in most instances is equal to the range. Each box represents an interquartile range (twenty-fifth to seventy-fifth percentile). The horizontal bar in each box is the median. Open circles represent outlying data points. Numbers in parentheses indicate the numbers of dogs in each group. Shaded area is the normal range.
(From Kantrowitz LB et al: Serum total thyroxine, total triiodothyronine, free thyroxine, and thyrotropin concentrations in dogs with nonthyroidal disease, J Am Vet Med Assoc 219:765, 2001.)
TSH and TRH Stimulation Tests
TSH and TRH stimulation tests evaluate the thyroid gland’s responsiveness to exogenous TSH and TRH administration, respectively. The primary advantage of these tests is that they help differentiate between hypothyroidism and nonthyroidal illness in dogs with low serum T4 and fT4 concentrations. Unfortunately, TRH for injection is currently not available. Recombinant human TSH (rhTSH) for injection is effective in stimulating thyroid hormone secretion in dogs but is not available at a reasonable cost. The current TSH stimulation protocol for dogs is 75 μg of rhTSH per dog administered intravenously or intramuscularly and blood for serum T4 concentration obtained before and 6 hours after rhTSH administration. In a euthyroid dog serum T4 concentration should be ≥ 2.5 μg/dl (30 nmol/L) 6 hours after rhTSH administration and the 6-hour post-rhTSH serum T4 concentration should be ≥1.5 times the baseline serum T4 concentration. Reconstituted rhTSH can be stored at 4° C for 4 weeks and at −20° C for 8 weeks without loss of biological activity.
Antibody Tests for Lymphocytic Thyroiditis
Circulating thyroglobulin (Tg) and thyroid hormone (T3 and T4) autoantibodies correlate with the presence of lymphocytic thyroiditis in dogs. Tests for the presence of Tg, T3, and T4 autoantibodies in the serum of dogs can be used to identify lymphocytic thyroiditis, to explain unusual serum T4 test results, and possibly to serve as a genetic screening test for hypothyroidism caused by lymphocytic thyroiditis. Autoantibodies predominantly develop against Tg. T3 and T4 are haptens and not antigenic by themselves. Tg is the protein that provides the antigenic stimulus. Because T3 and T4 are attached to the Tg molecule, autoantibodies develop against them as well. Dogs with T3 and T4 autoantibodies typically have autoantibodies against Tg, but the converse is not true. As such, the better screening test for lymphocytic thyroiditis is the Tg autoantibody test. ELISAs for detection of Tg autoantibodies are sensitive and specific for identification of Tg autoantibodies in dogs and are commercially available. Results are reported as negative, positive, and inconclusive.
A positive Tg autoantibody test suggests the possibility of lymphocytic thyroiditis but does not provide information on the severity or progressive nature of the inflammatory process. Tg autoantibody is not a thyroid function test. Positive results increase the suspicion for hypothyroidism if serum T4 and fT4 concentrations are low but have no bearing on generation of clinical signs if serum T4 and fT4 concentrations are normal. Tg autoantibodies should not be used alone in the diagnosis of hypothyroidism. Dogs with confirmed hypothyroidism can be negative and euthyroid dogs can be positive for Tg autoantibodies. Identification of Tg autoantibodies would support hypothyroidism caused by lymphocytic thyroiditis if the dog has clinical signs, physical findings, and thyroid hormone test results consistent with the disorder. Positive serum T4 and T3 autoantibody test results are interpreted in a similar manner.
The value of serum Tg autoantibodies as a marker for eventual development of hypothyroidism remains to be clarified. A 1-year prospective study found that approximately 20% of 171 dogs with positive Tg autoantibody and normal fT4 and TSH test results developed changes in fT4, TSH, or both test results consistent with hypothyroidism; 15% reverted to a negative Tg autoantibody test with no change in fT4 and TSH test results; and 65% remained Tg autoantibody positive or had an inconclusive result with no change in fT4 and TSH test results 1 year later (Graham et al., 2001). Currently, a positive Tg autoantibody test is considered suggestive of lymphocytic thyroiditis and supports retesting thyroid gland function in 3 to 6 months.
Testing for serum T4 or Tg autoantibodies is indicated in dogs with unusual serum T4 values. T4 autoantibodies may interfere with the RIAs used to measure serum T4 concentrations, which thereby yield spurious and thus unreliable values. The type of interference depends on the separation system used in the RIA. Falsely low results are obtained if nonspecific separation methods are used (e.g., ammonium sulfate, activated charcoal); falsely increased values are obtained if single-step separation systems using antibody-coated tubes are used. Fortunately, spurious T4 values resulting from clinically relevant concentrations of thyroid hormone antibody account for less than 1% of such results from commercial endocrine laboratories. Serum fT4 measured using an ED technique is not affected by T4 autoantibodies and should be evaluated in lieu of serum T4 in dogs suspected of having T4 autoantibodies.
FACTORS AFFECTING THYROID GLAND FUNCTION TESTS
There are many factors that affect baseline thyroid hormone and endogenous TSH concentrations (see Table 51-2). Unfortunately, most of these factors decrease baseline thyroid hormone concentrations and may increase endogenous TSH in euthyroid dogs, potentially causing misdiagnosis of hypothyroidism if the clinician accepts the results out of context. The most common factors that result in lower baseline thyroid hormone concentrations in euthyroid dogs are nonthyroidal illness (i.e., euthyroid sick syndrome), drugs (especially glucocorticoids, phenobarbital, and sulfonamide antibiotics; see Table 51-2), and variation in the reference range between breeds (most notably sight hounds).
Nonthyroidal Illness (Euthyroid Sick Syndrome)
Euthyroid sick syndrome refers to suppression of serum thyroid hormone concentrations in euthyroid dogs in response to concurrent illness. A decrease in serum thyroid hormone concentrations may result from a decline in TSH secretion secondary to suppression of the hypothalamus or pituitary gland, from decreased synthesis of T4, from decreased concentration or binding affinity of circulating binding proteins (e.g., thyroid binding globulin), from inhibition of the deiodination of T4 to T3, or any combination of these factors. The subsequent decrease in serum total T4 and, in many cases, fT4 concentrations is believed to represent a physiologic adaptation by the body, with the purpose being to decrease cellular metabolism during periods of illness. It is not indicative of hypothyroidism, per se. Generally, the type and magnitude of most alterations in serum thyroid hormone concentrations are not unique to a specific disorder but reflect the severity of the illness or the catabolic state and appear to represent a continuum of changes. Systemic illness has more of an effect in lowering serum thyroid hormone concentrations than do, for example, dermatologic disorders. In addition, the more severe the systemic illness, the more suppressive the effect on the serum thyroid hormone concentration (Fig. 51-10).
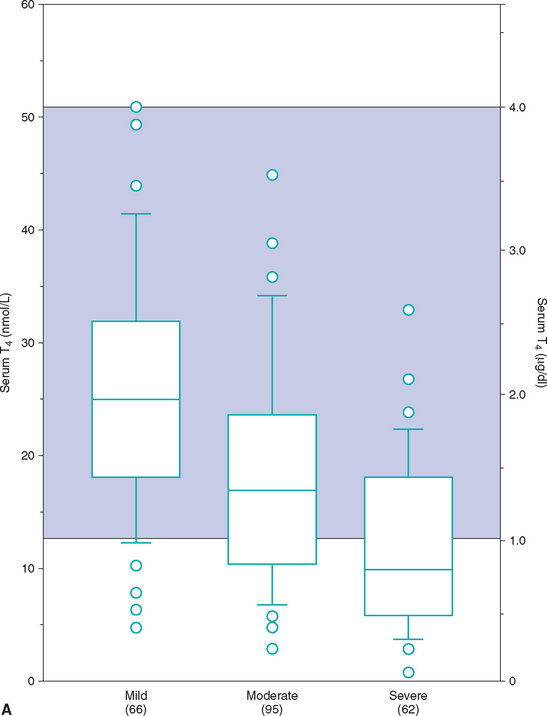
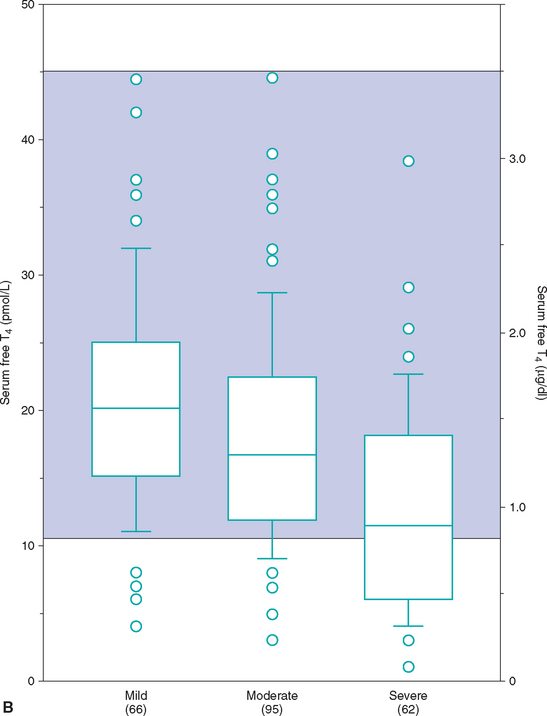
FIG 51-10 Box plots of serum total T4 (A) and free T4 (B) concentrations in 223 dogs with nonthyroidal disease stratified according to severity of disease. See Fig. 51-9 for explanation.
(From Kantrowitz LB et al: Serum total thyroxine, total triiodothyronine, free thyroxine, and thyrotropin concentrations in dogs with nonthyroidal disease, J Am Vet Med Assoc 219:765, 2001.)
Unfortunately, euthyroid dogs with concurrent illness can have serum T4 concentrations that often fall between 0.5 and 1.0 μg/dl, and with severe illness (e.g., cardiomyopathy, severe anemia) these concentrations can be less than 0.5 μg/dl. Alterations in serum concentrations of fT4 and TSH are more variable and probably depend in part on the pathophysiologic mechanisms involved in the illness. In general, serum fT4 concentrations tend to be decreased in dogs with concurrent illness but to a lesser extent than total T4 concentrations. However, fT4 concentrations can be less than 0.5 ng/dl if severe illness is present. TSH concentrations may be normal or increased depending, in part, on the effect of the concurrent illness on fT4 concentrations and on pituitary function. If pituitary function is suppressed, TSH concentrations will be in the normal range or undetectable. If pituitary response to changes in fT4 concentration is not affected by the concurrent illness, TSH concentrations will increase in response to a decrease in fT4. Serum TSH concentrations can easily exceed 1.0 ng/ml in dogs with euthyroid sick syndrome.
Treatment of euthyroid sick syndrome should be aimed at the concurrent illness. The serum thyroid hormone concentrations return to normal once the concurrent illness is eliminated. Treatment of euthyroid sick syndrome with sodium levothyroxine is not recommended.
Drugs
Clinical knowledge of the effect, if any, of various drugs and hormones on serum thyroid hormone and TSH concentrations in dogs is expanding as investigators continue to examine the interplay between medications and thyroid hormone test results (Table 51-4). As a general rule, any drug should be suspected of affecting thyroid hormone test results, especially if the history, clinical signs, and clinicopathologic abnormalities do not support a diagnosis of hypothyroidism. Glucocorticoids, phenobarbital and sulfonamides are the most commonly used drugs known to affect serum thyroid hormone test results.
 TABLE 51-4 Drugs that May Affect Baseline Serum Thyroid Hormone Function Test Results in the Dog
TABLE 51-4 Drugs that May Affect Baseline Serum Thyroid Hormone Function Test Results in the Dog
| DRUG | POSSIBLE IMPACT ON TEST RESULTS |
|---|---|
| Aspirin | Decreased T4, free T4; No effect on TSH |
| Clomipramine | Decreased T4, free T4; No effect on TSH |
| Carprofen | Decreased T4, free T4 and TSH |
| Deracoxib | No effect on T4, free T4 or TSH |
| Etodolac | No effect on T4, free T4 or TSH |
| Glucocorticoids | Decreased T4 and free T4; decreased or no effect on TSH |
| Furosemide | Decreased T4 |
| Methimazole | Decreased T4 and free T4; increased TSH |
| Phenobarbital | Decreased T4 and free T4; Delayed increase in TSH |
| Phenylbutazone | Decreased T4 |
| Potassium bromide | No effect on T4, free T4 or TSH |
| Progestagens | Decreased T4 |
| Propylthiouracil | Decreased T4 and free T4; increased TSH |
| Cephalexine | No effect on T4, free T4, or TSH |
| Sulfonamides | Decreased T4 and free T4; increased TSH |
| Ipodate | Increased T4, decreased T3 |
TSH, Thyroid-stimulating hormone.
Glucocorticoids.
Glucocorticoids cause a decrease in serum T4 and fT4 concentrations. Serum TSH concentration is variable but usually within the reference range. The magnitude and duration of suppression of serum thyroid hormone concentrations depend on the type of glucocorticoid, dosage, route of administration, and duration of glucocorticoid administration. The higher the dosage, the longer the administration, and the more potent the glucocorticoid administered, the more severe the suppression of serum thyroid hormone concentrations. If glucocorticoids have been administered in the recent past, assay of serum thyroid hormone concentrations should be delayed or must be interpreted carefully. Ideally, glucocorticoids should be discontinued and serum thyroid hormone and TSH concentrations assessed 4 to 8 weeks later.
Typically, the administration of exogenous glucocorticoids does not result in clinical signs of hypothyroidism. The exception are dogs receiving relatively high dosages of glucocorticoids for prolonged periods to treat chronic steroid-responsive disorders (e.g., immune-mediated diseases). In these dogs glucocorticoid-induced secondary hypothyroidism may become clinical and require treatment with synthetic levothyroxine.
Phenobarbital.
In dogs phenobarbital treatment at therapeutic dosages decreases serum T4 and fT4 concentrations into the range consistent with hypothyroidism. A delayed increase in the serum TSH concentration may occur secondary to loss of negative feedback as serum T4 and fT4 concentrations decline. Increased serum TSH concentrations quickly return to the reference range after discontinu ation of phenobarbital treatment, whereas serum T4 and fT4 concentrations may take up to 4 weeks to return to pretreatment values. Potassium bromide treatment does not seem to have a significant effect on serum T4, fT4 and TSH concentrations in dogs.
Sulfonamide antibiotics.
A decrease in serum T4 and fT4 and an increase in TSH concentrations have been documented in dogs treated with sulfonamides (e.g., sulfamethoxazole, sulfadiazine). Serum T4 concentrations can decrease into the hypothyroid range within 1 to 2 weeks and serum TSH concentrations can increase above the reference range within 2 to 3 weeks after initiating sulfonamide therapy. Clinical signs of hypothyroidism can develop with chronic sulfonamide administration. The increase in the serum TSH concentration occurs secondary to loss of negative feedback as serum T4 and fT4 concentrations decline and can lead to thyroid hyperplasia and goiter. Alterations in results of thyroid gland function tests may resolve within 1 to 2 weeks or last as long as 8 to 12 weeks after cessation of the antibiotic.
Breed Variations
Current reference ranges were established in large populations of dogs without regard for breed. It is now recognized that the reference range for serum T4 and fT4 concentration but not TSH concentration is lower in sight hounds, most notably Greyhounds, and Northern breeds such as the Siberian Husky and may be lower in other breeds as well. The lower end of the reference range for serum T4 and fT4 in these breeds may be as low as 0.4 μg/dl and 0.4 ng/dl, respectively. Serum T4 and fT4 concentrations that are consistent with hypothyroidism according to standard reference ranges may actually be normal in these breeds. Differences in the reference range between breeds emphasizes the importance of clinical signs, physical examination findings, and results of routine blood work when establishing the diagnosis of hypothyroidism in dogs.
Diagnosis
The diagnosis of hypothyroidism is based on a combination of clinical signs; findings on physical examination; and results of complete blood count (CBC), serum biochemistry panel, and tests of thyroid gland function. The presence of appropriate clinical signs is imperative, especially when relying on baseline thyroid hormone concentrations for a diagnosis. In the adult dog the most consistent clinical signs include lethargy, weight gain, and abnormalities affecting the skin (e.g., alopecia, seborrhea, pyoderma) and neuromuscular system (e.g., weakness). Other organ systems may be affected by thyroid hormone deficiency, but clinical signs related to these other systems are rarely the reason for presentation of the dog to the veterinarian. Identification of a mild nonregenerative anemia on the CBC and especially lipemia (hypertriglyceridemia) in the blood sample and an increased serum cholesterol concentration on a serum biochemistry panel adds further evidence for hypothyroidism.
Baseline serum T4 concentration is often used as the initial screening test for thyroid gland function. It is important to remember that serum T4 concentrations can be suppressed by a variety of factors, most notably nonthyroidal illness and medications such as prednisone and phenobarbital. As such, measurement of the serum T4 concentration should be used to confirm normal thyroid gland function, not hypothyroidism per se. A normal serum T4 concentration establishes normal thyroid gland function unless serum T4 autoantibodies are present and interfering with the assay. A low serum T4 concentration (ideally less than 0.5 μg/dl [6 nmol/L]) in conjunction with hypercholesterolemia and clinical signs strongly suggestive of the disease supports the diagnosis of hypothyroidism, especially if systemic illness is not present. The definitive diagnosis must then rely on response to trial therapy with synthetic levothyroxine. Additional tests of thyroid gland function are warranted if the serum T4 concentration is less than 0.8 to 1.0 μg/dl but clinical signs and physical examination findings are not strongly supportive of the disease and hypercholesterolemia is not present, if severe systemic illness is present and the potential for the euthyroid sick syndrome is high, or if medications known to decrease serum T4 concentration are being administered.
Evaluation of a thyroid panel that includes serum T4, fT4, TSH, and Tg autoantibody provides a more informative analysis of the pituitary-thyroid axis and thyroid gland function, can be used as the initial screening test for hypothyroidism, and should be used when serum T4 concentration alone fails to establish the diagnosis. Low serum T4 and fT4, and increased serum TSH concentrations in a dog with appropriate clinical signs and clinicopathologic abnormalities strongly support the diagnosis of hypothyroidism. Concurrent presence of Tg autoantibodies suggests lymphocytic thyroiditis as the underlying etiology.
Unfortunately, discordant test results are common. When this occurs, the appropriateness of clinical signs, clinicopathologic abnormalities, and clinician index of suspicion become the most important parameters when determining whether to treat the dog with levothyroxine. Serum fT4 concentration measured using ED or the 2-step RIA is the most accurate test of thyroid gland function and carries the highest priority, followed by serum T4 concentration. Results of TSH concentration increase the likelihood of euthyroidism or hypothyroidism when TSH test results are consistent with results of serum fT4, but TSH test results should not be used as the sole indicator of hypothyroidism. Low serum fT4 and normal TSH test results occur in approximately 20% of dogs with hypothyroidism, and high TSH test results occur in euthyroid dogs with nonthyroidal illness and with medications such as phenobarbital and sulfonamides (see Tables 51-2 and 51-4). Normal serum fT4 and high TSH may suggest early compensated hypothyroidism, but one has to wonder why clinical signs would develop when the serum fT4 concentration is normal. Positive Tg autoantibody findings merely suggest the possibility of lymphocytic thyroiditis; Tg autoantibody determination is not a thyroid function test. Positive results increase the suspicion for hypothyroidism if serum T4 or fT4 concentrations are low but have no bearing on the generation of clinical signs if serum T4 and fT4 concentrations are normal. When faced with discordant test results, the clinician must decide whether to initiate trial therapy with synthetic levothyroxine or repeat the tests sometime in the future—a decision that I usually base on the appropriateness of clinical signs and results of the fT4 measured using ED or the 2-step RIA.
Admittedly, interpretation of serum T4, fT4, and TSH concentrations is not always simple. Because of the expense and frustration of working with tests that are not always reliable, many veterinarians and some clients prefer trial therapy as a diagnostic test. Trial therapy should be done only when thyroid hormone supplementation does not pose a risk to the patient. Response to trial therapy with sodium levothyroxine is nonspecific. A dog that has a positive response to therapy either has hypothyroidism or “thyroid-responsive disease.” Because of its anabolic nature, thyroid supplementation can create an effect in a dog without thyroid dysfunction, especially regarding quality of the haircoat. Therefore, if a positive response to trial therapy is observed, thyroid supplementation should be gradually discontinued once clinical signs have resolved. If clinical signs recur, hypothyroidism is confirmed and the supplement should be reinitiated. If clinical signs do not recur, a thyroid-responsive disorder or a beneficial response to concurrent therapy (e.g., antibiotics, flea control) should be suspected.
DIAGNOSIS IN A PREVIOUSLY TREATED DOG
Occasionally, a clinician wants to determine if a dog receiving thyroid hormone supplementation is in fact hypothyroid. The exogenous administration of thyroid hormone, either T4 or T3, will suppress pituitary TSH secretion and cause pituitary thyrotroph atrophy and subsequently thyroid gland atrophy in a healthy euthyroid dog. Serum T4, fT4, and TSH concentrations are decreased or undetectable; the severity of the decrease is dependent on the severity of thyroid gland atrophy induced by the thyroid supplement. Serum T4 and fT4 results are often suggestive of hypothyroidism, even in a previously euthyroid dog, if testing is performed within a month of discontinuing treatment. Thyroid hormone supplementation must be discontinued and the pituitary-thyroid axis allowed to regain function before meaningful baseline serum thyroid hormone concentrations can be obtained. The time between discontinuation of thyroid hormone supplementation and acquisition of meaningful test results depends on the duration of treatment, the dose and frequency of administration of the thyroid hormone supplement, and individual variability. As a general rule, thyroid hormone supplements should be discontinued for a minimum of 4 weeks, preferably 6 to 8 weeks, before thyroid gland function is critically assessed.
DIAGNOSIS IN PUPPIES
An approach similar to that discussed in the previous section is used to diagnose congenital hypothyroidism. However, serum TSH concentrations are dependent on the etiology. TSH concentrations will be increased in dogs with primary dysfunction of the thyroid gland (e.g., iodine organification defect) and an intact hypothalamic-pituitary-thyroid gland axis. TSH concentrations will be within the normal range or undetectable in dogs with pituitary or hypothalamic dysfunction as the cause of the hypothyroidism.
THERAPY WITH SODIUM LEVOTHYROXINE (Synthetic T4)
The initial treatment and monitoring recommendations are summarized in Box 51-5. Synthetic levothyroxine is the treatment of choice for hypothyroidism. Its administration orally should result in normal serum concentrations of T4, T3, and TSH, which attests to the fact that these products can be converted to the more metabolically active T3 by peripheral tissues. A sodium levothyroxine product approved for use in dogs is recommended. Liquid and tablet formulations are effective. The initial dosage is 0.02 mg/kg body weight (0.1 mg/10 lb) with a maximum initial dose of 0.8 mg. Twice-daily administration is recommended initially unless the levothyroxine product has been specifically formulated for once-daily administration. Because of the variability in its absorption and metabolism, the dose and frequency may have to be adjusted before a satisfactory clinical response is observed; this variability is one reason for monitoring therapy in dogs.
 BOX 51-5 Recommendations for the Initial Treatment and Monitoring of Hypothyroidism in Dogs
BOX 51-5 Recommendations for the Initial Treatment and Monitoring of Hypothyroidism in Dogs
TSH, Thyroid-stimulating hormone.
Initial Treatment
Use a synthetic levothyroxine product approved for use in dogs.
Tablet and liquid formulations of levothyroxine are effective.
The initial dosage per administration should be 0.02 mg/kg (20 μg/kg) of body weight, with a maximum initial dose of 0.8 mg.
The initial frequency of administration is every 12 hours unless the levothyroxine product has been specifically formulated for once-daily administration.
Initial Monitoring
Response to treatment should be critically evaluated 4 to 8 weeks after initiating treatment.
Serum T4 and TSH concentrations should be measured 4 to 6 hours after administration of levothyroxine.
Serum T4 should be in the reference range or increased.
Serum TSH concentration should be in the reference range.
Measuring serum T4 concentration immediately before levothyroxine administration (i.e., trough level) is optional but is recommended if levothyroxine is being given once a day.
The trough concentration of serum T4 should be in the reference range.
RESPONSE TO SODIUM LEVOTHYROXINE THERAPY
Thyroid hormone supplementation should be continued for a minimum of 4 weeks before critically evaluating the effectiveness of treatment. With appropriate therapy all clinical signs and clinicopathologic abnormalities associated with hypothyroidism are reversible. Improvement in mental alertness and activity usually occurs within the first week of treatment; this is an important early indicator that the diagnosis of hypothyroidism was correct. Although some hair regrowth usually occurs within the first month in dogs with endocrine alopecia, it may take several months for complete regrowth and a marked reduction in hyperpigmentation of the skin to occur. Initially, the haircoat may worsen as large amounts of hair in the telogen stage of the hair cycle are shed. Improvement in neurologic manifestations is usually evident within days of initiating treatment; complete resolution of neurologic signs is unpredictable and may take 4 to 8 weeks or longer of treatment before it occurs.
FAILURE TO RESPOND TO SODIUM LEVOTHYROXINE THERAPY
Problems with levothyroxine therapy should be suspected if clinical improvement is not seen by 8 weeks after initiating therapy. An inappropriate diagnosis of hypothyroidism is the most obvious. Hyperadrenocorticism can be mistaken for hypothyroidism if other clinical signs (e.g., polyuria, polydipsia) commonly associated with hyperadrenocorticism are not present because of the suppressive effects of cortisol on serum thyroid hormone concentrations (see p. 738). Failure to recognize the impact of concurrent illness on thyroid hormone test results is another common reason for misdiagnosing hypothyroidism. Concurrent disease (e.g., allergic skin disease, flea hypersensitivity) is common in dogs with hypothyroidism and may affect the clinical impression of response to levothyroxine therapy if the disease is not recognized. Other possible reasons for a poor response to therapy are listed in Box 51-6. Whenever a dog shows a poor response to levothyroxine therapy, the history, physical examination findings, and diagnostic test results that prompted the initiation of levothyroxine therapy should be critically reevaluated and serum thyroid hormone concentrations measured.
 BOX 51-6 Potential Reasons for Poor Clinical Response to Treatment with Sodium Levothyroxine (Synthetic T4)
BOX 51-6 Potential Reasons for Poor Clinical Response to Treatment with Sodium Levothyroxine (Synthetic T4)
Use of inactivated or outdated product
Inappropriate levothyroxine dose
Inappropriate frequency of administration
Low tablet strength*
Poor bioavailability (e.g., poor gastrotintestinal tract absorption)
Inadequate time for clinical response to occur
Incorrect diagnosis of hypothyroidism
* Tablet strength refers to actual amount of active drug in tablet, as opposed to the stated amount.
THERAPEUTIC MONITORING
Therapeutic monitoring includes evaluation of the clinical response to levothyroxine treatment, measurement of serum T4 and TSH concentrations before or after levothyroxine administration, or both. These concentrations should be measured 4 weeks after initiating therapy, whenever signs of thyrotoxicosis develop, or in the event that there has been minimal or no response to therapy. Concentrations should also be measured 2 to 4 weeks after an adjustment in levothyroxine therapy in dogs showing a poor response to treatment.
Serum T4 and TSH concentrations are typically evaluated 4 to 6 hours after the administration of levothyroxine in dogs receiving the medication twice daily and just before and 4 to 6 hours after administration in dogs receiving it once a day. Measurement of serum fT4 can be done in lieu of measuring T4 but is more expensive and probably does not offer additional information except in dogs with T4 autoantibodies. The presence of thyroid hormone autoantibodies does not interfere with the physiologic actions of levothyroxine.
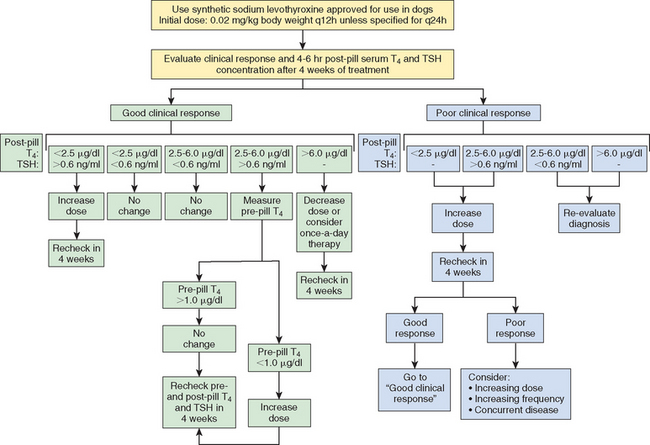
Ideally, the serum T4 concentration should be between 1.5 and 4.5 μg/dl when measured 4 to 6 hours after thyroid hormone administration and the TSH concentration should be in the reference range. Postdosing serum T4 concentrations are frequently above the reference range. The finding of an increased postdosing serum T4 concentration is not an absolute indication to reduce the dose of levothyroxine, especially if there are no clinical signs of thyrotoxicosis. However, a reduction in the dose is recommended whenever serum T4 concentrations exceed 6.0 μg/dl. Postdosing serum T4 concentrations may also be less than 1.5 μg/dl. An increase in the dose or frequency of administration of levothyroxine is indicated if clinical manifestations of hypothyroidism persist, the serum TSH concentration remains increased, or both, but it is not necessarily indicated if the clinical response to treatment is good and the serum TSH concentration is in the reference range. Postdosing serum T4 and TSH concentrations and recommendations for changes in therapy are given in Fig. 51-11.
THYROTOXICOSIS
Thyrotoxicosis may develop in dogs receiving excessive amounts of levothyroxine; in dogs in which the plasma half-life for levothyroxine is inherently prolonged, especially in those receiving levothyroxine twice daily; and in dogs with impaired metabolism of levothyroxine (e.g., concurrent renal or hepatic insufficiency). Rarely, thyrotoxicosis develops in a dog given minute amounts of levothyroxine. The reason for this marked sensitivity to the hormone is not known. Diagnosis of thyrotoxicosis is based primarily on presence of clinical signs, which include panting, nervousness, aggressive behavior, polyuria, polydipsia, polyphagia, and weight loss. Documenting increased serum T4 and fT4 and undetectable serum TSH concentrations supports the diagnosis. However, serum T4 and fT4 concentrations can occasionally be within the reference range in a dog with signs of thyrotoxicosis and are commonly increased in dogs with no signs of thyrotoxicosis. Adjustments in the dose or fre quency of administration of levothyroxine, or both measures, are indicated if clinical signs of thyrotoxicosis develop in a dog receiving thyroid hormone supplements. Supplementation should be discontinued for a few days if clinical signs are severe. Signs of thyrotoxicosis should resolve within 1 to 3 days if they are due to the thyroid medication and the adjustment in treatment has been appropriate.
Prognosis
The prognosis for adult dogs with primary hypothyroidism that are receiving appropriate therapy is excellent. The prognosis for puppies with hypothyroidism (i.e., cretinism) is guarded and depends on the severity of skeletal and joint abnormalities at the time treatment is initiated. Although many of the clinical signs resolve with therapy, musculoskeletal problems, especially degenerative osteoarthritis, may develop owing to abnormal bone and joint development. The prognosis for dogs with secondary hypothyroidism caused by congenital malformation of the pituitary gland (i.e., pituitary dwarfism) is guarded to poor because of the multiple problems that develop in early life (see Chapter 49). The prognosis for dogs with acquired secondary hypothyroidism caused by suppression of pituitary function by medications (e.g., glucocorticoids) is excellent, although treatment with levothyroxine may be necessary if the medication can not be discontinued. The prognosis for dogs with acquired secondary hypothyroidism caused by destruction of the region by a space-occupying mass is grave.
HYPOTHYROIDISM IN CATS
Etiology
Iatrogenic hypothyroidism is the most common cause of hypothyroidism in cats and can result from bilateral thyroidectomy, radioactive iodine treatment, or an overdose of antithyroid drugs. Naturally acquired adult-onset primary hypothyroidism is rare. Congenital primary hypothyroidism causing disproportionate dwarfism is recognized more frequently in cats than adult-onset hypothyroidism. Reported causes of congenital hypothyroidism include a defect in thyroid hormone biosynthesis, most notably an iodine organification defect, and thyroid dysgenesis. Goiter is common in cats with defects in thyroid hormone biosynthesis because the hypothalamic-pituitary-thyroid gland axis remains intact. A suspected autosomal recessive inherited defect in iodine organification was documented in a family of Abyssinian cats with congenital hypothyroidism. Although rare, iodine deficiency may cause hypothyroidism in kittens fed a strict all-meat diet.
Clinical Signs
Clinical signs of feline hypothyroidism are listed in Box 51-7. The most common are lethargy, inappetence, obesity, and seborrhea sicca. Lethargy and inappetence may become severe. Additional dermatologic signs may include a dry, lusterless, unkempt haircoat; easily epilated hair; poor regrowth of hair; and alopecia. Bradycardia and mild hypothermia may be additional findings on physical examination.
The clinical signs of congenital hypothyroidism are similar to those in dogs (see p. 729). Affected kittens typically appear normal at birth, but delayed growth usually becomes evident by 8 weeks of age. Disproportionate dwarfism develops over the ensuing months, with large heads; short, broad necks; and short limbs developing in affected kittens (Fig. 51-12). Additional findings include lethargy, mental dullness, constipation, hypothermia, bradycardia, and prolonged retention of deciduous teeth. The haircoat may consist mainly of an undercoat with primary guard hairs scattered thinly throughout.

FIG 51-12 A 1-year-old domestic long-haired cat with pituitary dwarfism. A comparably aged cat is also present to illustrate the small size of the pituitary dwarf. Note the square, chunky contour of the head and the dull facial expression of the cat—findings that are suggestive of cretinism (see Fig. 49-10, for comparison). The cat had concurrent growth hormone and thyroid hormone deficiency.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
Diagnosis
Establishing a diagnosis of hypothyroidism in the cat should be based on a combination of history, clinical signs, physical examination findings, results of routine blood and urine tests, and baseline serum T4 and fT4 concentrations. Measurement of serum TSH concentration using the canine TSH assay should also be considered. Abnormalities identified on routine blood and urine tests include hypercholesterolemia and a mild nonregenerative anemia. Serum T4 concentration is often used as the initial screening test of thyroid gland function. A normal serum T4 concentration indicates that the cat is euthyroid. A low serum T4 concentration in a cat that has undergone thyroidectomy or radioactive iodine treatment or in a kitten with disproportionate dwarfism supports the diagnosis of hypothyroidism. The effect of age should be considered when interpreting serum T4 concentrations in kittens (see Table 51-2). Because naturally acquired primary hypothyroidism is rare and low serum T4 concentrations in adult cats is almost always caused by nonthyroidal illness (see Fig. 51-13) or some other nonthyroidal factor, the diagnosis of hypothyroidism should never be made solely on the basis of the serum T4 concentration in an adult cat that has not been previously treated for hyperthyroidism. Documenting a low serum fT4 and high serum TSH concentration and failure of serum T4 to increase following administration of rhTSH adds further evidence for the diagnosis of hypothyroidism. The definitive diagnosis relies on the cat’s response to trial therapy with levothyroxine.
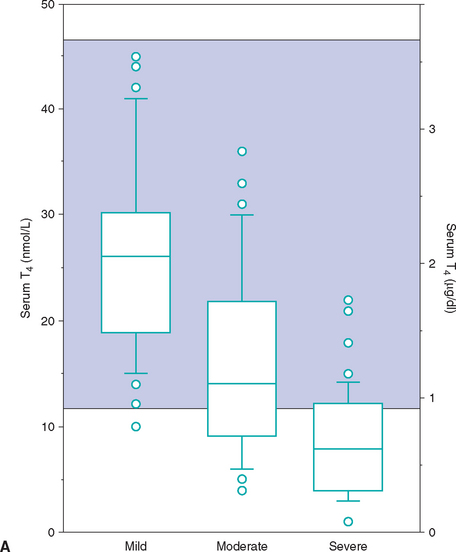
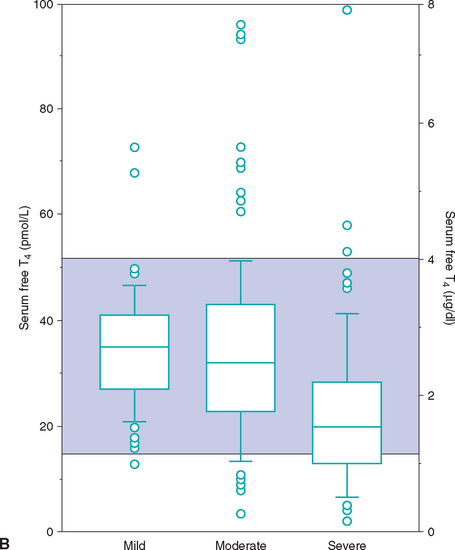
FIG 51-13 Box plots of serum total T4 (A) and free T4 (B) concentrations in 221 cats with nonthyroidal disease, grouped according to severity of illness. Of 221 cats with nonthyroidal illness 65 had mild disease, 83 had moderate disease, and 73 had severe disease. See Fig. 51-9 for explanation.
(From Peterson ME et al: Measurement of serum concentrations of free thyroxine, total thyroxine, and total triiodothyronine in cats with hyperthyroidism and cats with nonthyroidal disease, J Am Vet Med Assoc 218:529, 2001.)
Treatment
Treatment of hypothyroidism in cats is similar to that used in dogs, which is described in detail on p. 741. Treatment with levothyroxine is indicated for cats with congenital and naturally acquired adult-onset hypothyroidism and for cats with iatrogenic hypothyroidism following treatment for hyperthyroidism that are symptomatic for the disease. Asymptomatic cats with a low serum T4 concentration following treatment for hyperthyroidism should not be treated until clinical signs become evident in the hope that additional time will allow atrophied or ectopic thyroid tissue to become functional.
Synthetic levothyroxine is recommended at an initial dosage of 0.05 or 0.1 mg once or twice daily. A minimum of 4 weeks should elapse before the cat’s clinical response to treatment is critically assessed. Subsequent evaluations should include a history, physical examination, and measurement of serum T4 concentration (see the discussion of therapeutic monitoring, p. 742). The goal of therapy is to eliminate the clinical signs of hypothyroidism and prevent signs of hyperthyroidism. This can usually be accomplished by maintaining the serum T4 concentration between 1.0 and 2.5 μg/dl. The dose and frequency of levothyroxine administration should be adjusted accordingly to attain these goals. If the serum T4 concentration is within the reference range after 4 to 8 weeks of treatment but there is minimal or no clinical response, the clinician should reassess the diagnosis.
Prognosis
The prognosis for adult cats with hypothyroidism that are receiving appropriate therapy is excellent. The prognosis for kittens with congenital hypothyroidism is guarded and depends on the severity of the skeletal changes at the time treatment is initiated. Although many of the clinical signs resolve with therapy, musculoskeletal problems may persist or develop owing to abnormal bone and joint development.
HYPERTHYROIDISM IN CATS
Etiology
Hyperthyroidism is a multisystemic disorder resulting from the excessive production and secretion of T4 and T3 by the thyroid gland and is almost always a result of chronic intrinsic disease in one or both thyroid lobes. One or more usually small, discrete thyroid masses are palpable in the ventral region of the neck in most cats with hyperthyroidism. Multinodular adenomatous hyperplasia is the most common histologic finding. Less common are thyroid adenomas that cause the lobes to be enlarged and distorted; thyroid carcinoma accounts for fewer than 5% of clinical cases.
One or both thyroid lobes can be affected in thyrotoxic cats. Approximately 20% of hyperthyroid cats have involvement of a single thyroid lobe (Fig. 51-14). The nondiseased thyroid lobe is nonfunctioning and atrophied because of the suppressive effects of the hyperactive thyroid tissue on TSH secretion. More than 70% of hyperthyroid cats have involvement of both thyroid lobes (Fig. 51-15). Of these cats the thyroid lobes are symmetrically enlarged in 10% to 15% and asymmetrically enlarged in the remainder. Approximately 3% to 5% of thyrotoxic cats have hyperactive thyroid tissue in the anterior mediastinum, with or without a palpable mass in the neck (Fig. 51-16). Presumably, this tissue represents ectopic thyroid tissue. Functional thyroid carcinoma is the most likely diagnosis if more than two thyroid masses are present (see Fig. 51-16). Some of these cats initially have only one or two thyroid masses, emphasizing the importance of histologic evaluation of surgically removed tissue.
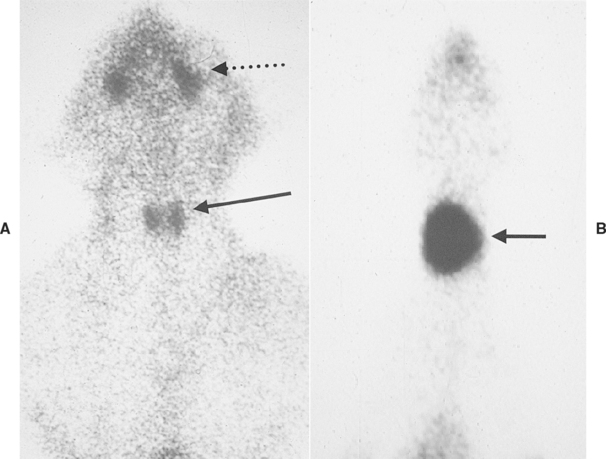
FIG 51-14 A, Sodium pertechnetate scan of the head, neck, and proximal thorax of a healthy cat. Note that the uptake of pertechnetate (i.e., darkness) is comparable between the two thyroid lobes (solid arrow) and the salivary glands (broken arrow). B, Sodium pertechnetate scan of the head, neck, and proximal thorax of a cat with hyperthyroidism caused by unilateral disease affecting the right thyroid lobe (arrow). Note the difference in uptake of pertechnetate between the hyperfunctioning thyroid lobe and the salivary glands.
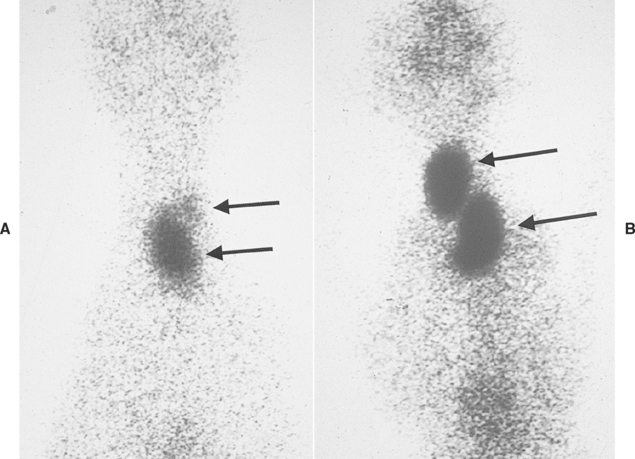
FIG 51-15 A, Sodium pertechnetate scan of the head, neck, and proximal thorax of a cat with hyperthyroidism caused by bilateral, asymmetric disease affecting both thyroid lobes (arrows), with the right lobe more severely involved. This is the most common form of the disease. B, Sodium pertechnetate scan of the head, neck, and proximal thorax of a cat with hyperthyroidism caused by bilateral, symmetric disease affecting both thyroid lobes (arrows). Hypocalcemia after bilateral thyroidectomy is a major concern.
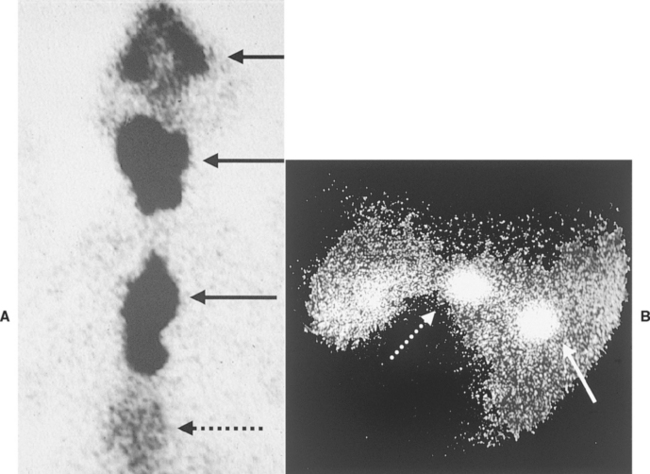
FIG 51-16 A, Sodium pertechnetate scan of the head, neck, and proximal thorax of a cat with hyperthyroidism caused by metastatic thyroid adenocarcinoma with multiple masses present in the head, neck, and anterior mediastinum (solid arrows). Heart (broken arrow). B, Sodium pertechnetate scan of the head, neck, and proximal thorax of a cat with hyperthyroidism caused by two hyperfunctioning masses: one located in the neck (broken arrow) and one in the anterior mediastinum (i.e., ectopic site) (solid arrow). Heart (broken arrow). 131I therapy is the treatment of choice for both forms of hyperthyroidism illustrated in this figure.
The pathogenesis of adenomatous hyperplastic changes of the thyroid gland remains unclear. It has been postulated that immunologic, infectious, nutritional, environmental, or genetic factors may interact to cause pathologic changes. Epidemiologic studies have identified consumption of commercial canned cat foods as a risk factor for development of hyperthyroidism, suggesting that a goitrogenic compound may be present in the diet. Excessive or deficient iodine content, isoflavones from soybeans, and chemicals lining pop-top canned foods (specifically bisphenol A) that have migrated into the food during storage have been proposed as potential dietary and chemical goitrogens. Epidemiologic studies suggest that environmental factors such as use of kitty litter may be involved. Recent studies have identified overexpression of the c-ras oncogene in areas of nodular follicular hyperplasia in feline thyroid glands, suggesting that mutations in this oncogene may play a role in the etiopathogenesis of hyperthyroidism in cats (Merryman et al., 1999). In the normal cell activation of the ras protein leads to mitosis. Mutations of the ras oncogene produce mutated ras proteins that are not subject to the normal cellular feedback mechanisms that prevent uncontrolled mitosis. Altered expression of G proteins involved in the signal transduction pathway that stimulates growth and differentiation of thyroid cells has also been identified in adenomatous thyroid glands obtained from hyperthyroid cats (Ward et al., 2005). Decreased inhibitory G protein expression has been identified, a decrease that creates a relative increase in stimulatory G protein expression that may stimulate unregulated mitogenesis and thyroid hormone production in hyperthyroid cells. Further studies are necessary to clarify the significance of these findings and the relationships among abnormalities identified in thyroid cells from hyperthyroid cats, potential dietary or chemical goitrogens identified in canned cat foods, and the development of hyperthyroidism in cats.
SIGNALMENT
Hyperthyroidism is the most common endocrine disease affecting cats older than 8 years. The average age at the time of initial presentation to the veterinarian is 13 years, with a range of 4 to 20 years. Fewer than 5% of cats with this disorder are younger than 8 years. There is no sex-related predisposition; domestic short-haired and long-haired cats are the most frequently affected breeds. Siamese and Himalayans have a decreased risk for development of hyperthyroidism.
CLINICAL SIGNS
Clinical signs are a result of excessive secretion of thyroid hormone by the thyroid mass. Rarely, a client will seek veterinary care because of an observed mass in the ventrocervical region of the neck. The classic clinical signs of hyperthyroidism are weight loss (which may progress to cachexia), polyphagia, and restlessness or hyperactivity. Additional clinical signs include haircoat changes (patchy alopecia, matted hair, minimal or excessive grooming behavior), polyuria, polydipsia, vomiting, and diarrhea (Table 51-5). Some cats show aggressive behavior that resolves in response to successful treatment of the hyperthyroid state. In some cats lethargy, weakness, and anorexia are the dominant clinical features, in addition to weight loss. Because of the multisystemic effects of hyperthyroidism, the variable clinical signs, and its resemblance to many other diseases of the cat, hyperthyroidism should be suspected in any aged cat with medical problems.
 TABLE 51-5 Clinical Signs and Physical Examination Findings in Cats with Hyperthyroidism
TABLE 51-5 Clinical Signs and Physical Examination Findings in Cats with Hyperthyroidism
| CLINICAL SIGNS | PHYSICAL EXAMINATION FINDINGS |
|---|---|
| Weight loss* | Palpable thyroid* |
| Polyphagia* | Thin* |
| Unkempt haircoat, patchy alopecia* | Hyperactive, difficult to examine* |
| Polyuria-polydipsia* | Tachycardia* |
| Vomiting* | Hair loss, unkempt hair coat* |
| Nervous, hyperactive | Small kidneys |
| Diarrhea, bulky stools | Heart murmur |
| Decreased appetite | Easily stressed |
| Tremor | Dehydrated, cachectic appearance |
| Weakness | Premature beats |
| Dyspnea, panting | Gallop rhythm |
| Decreased activity, lethargy | Aggressive |
| Anorexia | Depressed, weak |
| Ventral flexion of the neck |
PHYSICAL EXAMINATION
Physical examination findings are listed in Table 51-5. A discrete thyroid mass is palpable in approximately 90% of cats with hyperthyroidism. However, the palpation of a cervical mass is not pathognomonic for hyperthyroidism. Some cats with palpable thyroid lobes are clinically normal, and some palpable cervical masses are not thyroid in origin. It is frequently difficult to accurately assess unilateral versus bilateral thyroid lobe involvement on the basis of palpation. Two distinct masses cannot always be appreciated on palpation, even if both lobes are large. Large thyroid masses may gravitate to the region of the thoracic inlet, which can interfere with their palpation. The thyroid mass may even descend into the anterior mediastinum. This should be suspected when a thyroid mass is not palpable in a hyperthyroid cat, although a small, nonpalpable mass is also possible.
Clinical Pathology
Results of a CBC are usually normal. The most common abnormalities are a mild increase in the PCV and mean corpuscular volume. Neutrophilia, lymphopenia, eosinopenia, or monocytopenia is identifed in less than 20% of hyperthyroid cats. Common serum biochemical abnormalities include an increase in serum activities of alanine aminotransferase, alkaline phosphatase, and aspartate aminotransferase; the increase is typically in the mild to moderate range (i.e., 100 to 400 IU/L). One or more of these liver enzymes are increased in approximately 90% of hyperthyroid cats. Additional evaluation of the liver should be considered if liver enzyme activities are greater than 500 IU/L. Increased serum urea nitrogen and creatinine concentrations are identified in approximately 25%, and hyperphosphatemia in 20%, of hyperthyroid cats at our clinic—findings that have important implications regarding treatment (see the discussion of renal insufficiency). Urine specific gravity ranges from 1.008 to greater than 1.050. Most hyperthyroid cats have urine specific gravities greater than 1.035. The remainder of the urinalysis is usually unremarkable unless concurrent diabetes mellitus or urinary tract infection exists.
COMMON CONCURRENT PROBLEMS
Thyrotoxic Cardiomyopathy
Hypertrophic and, less commonly, dilative thyrotoxic cardiomyopathy may develop in cats with hyperthyroidism. Cardiovascular abnormalities detectable during physical examination include tachycardia; a pounding heartbeat noted on palpation of the ventral thorax; and, less frequently, pulse deficits, gallop rhythms, cardiac murmur, and muffled heart sounds resulting from a pleural effusion. Electrocardiographic abnormalities include tachycardia; an increased R-wave amplitude in lead II; and, less commonly, a right bundle-branch block, a left anterior fascicular block, widened QRS complexes, and atrial and ventricular arrhythmias. Thoracic radiographs may reveal cardiomegaly, pulmonary edema, or a pleural effusion. Echocardiographic abnormalities identified in cats with hypertrophic thyrotoxic cardiomyopathy include left ventricular hypertrophy, thickening of the interventricular septum, left atrial and ventricular dilation, and myocardial hypercontractility. Those seen in cats with dilative thyrotoxic cardiomyopathy include subnormal myocardial contractility and marked ventricular dilation. Either form of cardiomyopathy may result in the development of congestive heart failure. Hypertrophic thyrotoxic cardiomyopathy is usually reversible once the hyperthyroid state is corrected, whereas dilative thyrotoxic cardiomyopathy is not.
Renal Insufficiency
Hyperthyroidism and renal insufficiency are common diseases of older cats and often occur concurrently. Identification of small kidneys on physical examination, increased serum urea nitrogen and creatinine concentrations, and urine specific gravity between 1.008 and 1.020 should raise suspicion for concurrent renal insufficiency in a cat with hyperthyroidism. Unfortunately, hyperthyroidism increases glomerular filtration rate (GFR), renal blood flow, and renal tubular resorptive and secretory capabilities in normal and compromised kidneys. Renal perfusion and GFR may acutely decrease and azotemia or clinical signs of renal insufficiency become apparent or significantly worsen after treatment of the hyperthyroid state. It is not easy to determine what impact the hyperthyroid state is having on renal function in cats. The clinical and biochemical manifestations of renal failure may be masked in cats with both thyroid and renal disease in which renal perfusion is enhanced by the circulatory dynamics produced by hyperthyroidism. Thomas Graves, at the University of Illinois, has recently described a group of hyperthyroid cats with urine specific gravities greater than 1.040 that developed renal failure after treatment for hyperthyroidism, suggesting that urine specific gravity is a poor predictor of renal function in cats with hyperthyroidism. For these reasons cats with hyperthyroidism should initially be given reversible therapy (i.e., oral antithyroid drugs) until the impact of establishing euthyroidism on renal function can be determined (see p. 749).
Urinary Tract Infections
Urinary tract infections are relatively common in untreated hyperthyroid cats, with a reported prevalence of 12% to 22%. The most common bacterial isolate is Escherichia coli. Urine culture is indicated in hyperthyroid cats with lower urinary tract signs or presence of bacteriuria, pyuria, or both on urinalysis. Unfortunately, most hyperthyroid cats are asymptomatic for urinary tract infection, suggesting that urine culture should be a routine part of the complete diagnostic evaluation of cats with newly diagnosed hyperthyroidism.
Systemic Hypertension
Systemic hypertension is common in cats with hyperthyroidism and results from the effects of increased β-adrenergic activity on heart rate, myocardial contractility, systemic vasodilation, and activation of the renin-angiotensin-aldosterone system. Hypertension caused by hyperthyroidism is usually clinically silent. Retinal hemorrhages and retinal detachment are the most common clinical complications of systemic hypertension in hyperthyroid cats, but in general, ocular lesions are not commonly identified.
Gastrointestinal Tract Disorders
Gastrointestinal tract signs are common in cats with hyperthyroidism and include polyphagia, weight loss, anorexia, vomiting, diarrhea, increased frequency of defecation, and increased volume of feces. Intestinal hypermotility and malassimilation have been documented in some cats with hyperthyroidism and are responsible for producing some of the gastrointestinal tract signs. Inflammatory bowel disease is a common concurrent gastrointestinal tract disorder that should be considered in any hyperthyroid cat that has persistence of gastrointestinal signs after correction of the hyperthyroid state (see Chapter 33). Intestinal neoplasia, most notably lymphoma, is perhaps the most important differential diagnosis in cats seen because of polyphagia and weight loss. The abdomen should be carefully palpated in a search for thickening of the intestinal tract and mesenteric lymphadenopathy—findings that may be the only clues for intestinal lymphoma. Abdominal ultrasonography may also provide clues to the possibility of lymphoma.
Diagnosis
The diagnosis of hyperthyroidism is based on identification of appropriate clinical signs, palpation of a thyroid nodule, and documentation of an increased serum T4 concentration.
Baseline Serum T4 Concentration
Measurement of random baseline serum T4 concentration has been extremely reliable in differentiating hyperthyroid cats from those without thyroid disease (Fig. 51-17). An abnormally high serum T4 concentration strongly supports the diagnosis of hyperthyroidism, especially if appropriate clinical signs are present, and a low serum T4 concentration rules out hyperthyroidism, except in extremely uncommon situations when severe life-threatening nonthyroidal illness is present (Table 51-6). Serum T4 concentrations that fall within the upper half of the normal range (i.e., 2.5 to 5.0 μg/dl) create a diagnostic dilemma, especially if clinical signs are suggestive of hyperthyroidism and a nodule is palpable in the ventral region of the neck. This combination of findings is referred to as occult hyperthyroidism and is most commonly identified in cats in the early stages of hyperthyroidism. Serum T4 concentrations are more likely to be influenced by nonthyroidal factors such as concurrent illness and are more likely to randomly fluctuate into the reference range in cats with mild hyperthyroidism, compared with cats with more advanced disease (Fig. 51-18; see also Fig. 51-13). The diagnosis of hyperthyroidism should not be excluded on the basis of one “normal” serum T4 test result, especially in a cat with appropriate, albeit often mild, clinical signs and a palpable mass in the neck. Additional diagnostic factors to consider include measurement of serum free T4 (fT4), the T3 suppression test, sodium pertechnetate thyroid scan, or repetition of the serum T4 test 3 to 6 months later. It is important to remember that the thyroid nodule may also be nonfunctional and the clinical signs may be the result of another disease (see Chapter 54).
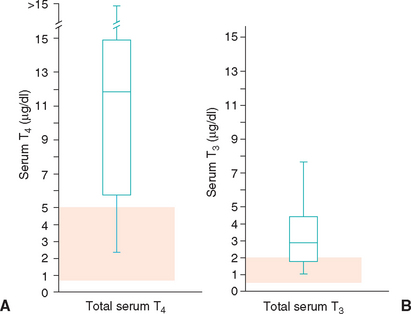
FIG 51-17 Mean and range of random total serum T4 (A) and total serum T3 (B) concentrations in hyperthyroid cats. Seventy-five percent of hyperthyroid cats have values within the box, and the balance is within the limitation bars above and below the box. Note that virtually all hyperthyroid cats have abnormal or borderline serum T4 concentrations, whereas serum T3 concentrations are less sensitive. The pink region represents the normal reference range.
 TABLE 51-6 Interpretation of Baseline Serum Thyroxine (T4) Concentration in Cats with Suspected Hyperthyroidism
TABLE 51-6 Interpretation of Baseline Serum Thyroxine (T4) Concentration in Cats with Suspected Hyperthyroidism
| SERUM T4 CONCENTRATION | PROBABILITY OF HYPERTHYROIDISM |
|---|---|
| >5.0 μg/dl | Very likely |
| 3.0-5.0 μg/dl | Possible |
| 2.5-3.0 μg/dl | Unknown |
| 2.0-2.5 μg/dl | Unlikely |
| <2.0 μg/dl | Very unlikely* |
* Assuming that a severe systemic illness is not present.
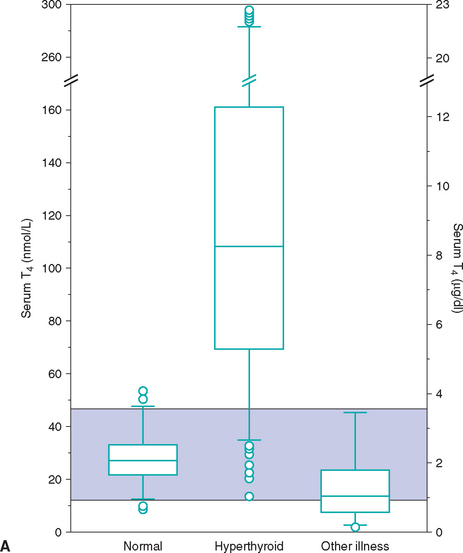
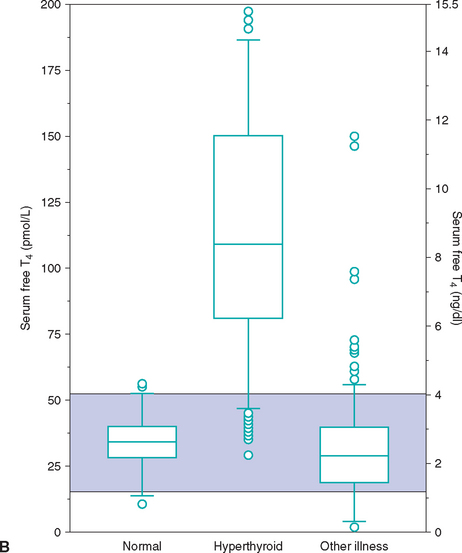
FIG 51-18 Box plots of serum total T4 (A) and free T4 (B) concentrations in 172 clinically normal cats, 917 cats with untreated hyperthyroidism, and 221 cats with nonthyroidal disease. See Fig. 51-9 for explanation.
(From Peterson ME et al: Measurement of serum concentrations of free thyroxine, total thyroxine, and total triiodothyronine in cats with hyperthyroidism and cats with nonthyroidal disease, J Am Vet Med Assoc 218:529, 2001.)
Serum Free T4 Concentration
Measurement of serum fT4 using equilibrium dialysis or the 2-step RIA (see p. 733) is the current recommendation of choice to confirm hyperthyroidism in a cat with nondiagnostic serum T4 test results. Measurement of serum fT4 is a more reliable means of assessing thyroid gland function than serum T4 concentration, in part because nonthyroidal illness has less of a suppressive effect on serum fT4 than T4 (see Fig. 51-13) and serum fT4 is increased in many cats with occult hyperthyroidism and “normal” T4 test results. Because of cost, measurement of serum fT4 is often reserved for cats with suspected hyperthyroidism in which T4 values are nondiagnostic. Concurrent illness may increase the serum fT4 concentration in cats, an increase that can exceed the reference range (see Fig. 51-18). For this reason serum fT4 concentration should always be interpreted in conjunction with a T4 concentration measured from the same blood sample. An increased serum fT4 concentration in conjunction with high-normal or increased serum T4 concentration is supportive of hyperthyroidism. An increased serum fT4 concentration in conjunction with a low-normal or low serum T4 concentration is supportive of the euthyroid sick syndrome rather than hyperthyroidism.
T3 Suppression Test
The T3 suppression test is used to distinguish euthyroid from mildly hyperthyroid cats in cases in which T4 and fT4 test results are nebulous. The T3 suppression test is based on the theory that oral administration of T3 will suppress pituitary TSH secretion in euthyroid cats, resulting in a decrease in circulating T4 (Fig. 51-19). In contrast, pituitary TSH secretion is already suppressed in cats with hyperthyroidism, oral administration of T3 will not cause further suppression, and serum T4 will not decrease following T3 administration. In this test 25 μg of T3 (e.g., Cytomel, King Pharmaceuticals) is administered orally three times per day for seven treat ments and serum T4 and T3 concentration is determined before and 8 hours after the last T3 administration. Normal cats consistently have postdosing serum T4 concentrations of less than 1.5 μg/dl, whereas hyperthyroid cats have postdosing T4 concentrations of greater than 2.0 μg/dl. Values of 1.5 to 2.0 μg/dl are nondiagnostic. The percentage decrease in the serum T4 concentration is not as reliable a gauge as the absolute value, although suppression of more than 50% below the baseline value occurs in normal but not hyperthyroid cats. Serum T3 concentrations are used to determine whether the client has successfully administered the thyroid medication to the cat. Serum T3 concentration measured in the postpill blood sample should be increased compared with results obtained before initiating the test in all cats properly tested, regardless of the status of thyroid gland function.
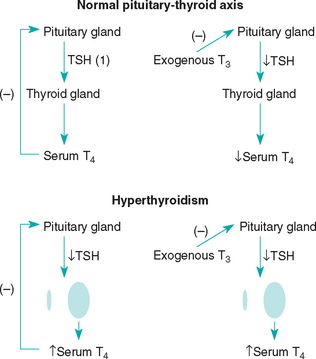
FIG 51-19 Effect of T3 supplementation on the pituitary-thyroid axis in healthy cats and cats with hyperthyroidism. Suppression of pituitary TSH secretion by the T3 supplement decreases serum T4 concentration in healthy cats. In hyperthyroid cats the serum TSH concentration is already suppressed; the T3 supplementation has no effect. The serum T4 concentration remains increased.
Radionuclide Thyroid Scanning
Radionuclide thyroid scanning identifies functional thyroid tissue and is used as a diagnostic test in cats with suspected occult hyperthyroidism; to identify ectopic thyroid tissue in cats with appropriate signs of hyperthyroidism and increased serum T4 concentrations but no palpable thyroid nodule in the neck; to identify sites of metastasis in cats with thyroid carcinoma; and to provide guidance for developing the best treatment plan, especially if thyroidectomy is being considered. Radioactive technetium 99m (pertechnetate) is used for routine imaging of the thyroid gland in cats. It has a short physical half-life (6 hours), is concentrated within functioning thyroid follicular cells, and reflects the trapping mechanism of the gland. Because antithyroid drugs do not affect the trapping mechanism of the thyroid pump, a pertechnetate scan can be done in cats being treated with antithyroid drugs. Salivary glands and the gastric mucosa also concentrate pertechnetate; it is excreted by the kidneys.
Scanning of the thyroid provides a picture of all functioning thyroid tissue and permits the delineation and localization of functioning as opposed to nonfunctioning areas of the thyroid. Fig. 51-14 shows the similarity between the size and shape of the thyroid lobes and similarity of radionuclide uptake by the thyroid and salivary glands in a normal cat. This 1 : 1 ratio of salivary gland to thyroid lobe uptake is the standard by which to judge the status of the thyroid. Findings in most hyperthyroid cats are markedly abnormal and usually easy to interpret (see Figs. 51-14 to 51-16).
Cervical Ultrasound
Ultrasonographic evaluation of the thyroid gland can be used to confirm the origin of the palpable cervical mass, differentiate unilateral versus bilateral thyroid lobe involvement, assess the size of the thyroid mass(es), and provide guidance for developing the best treatment plan (Fig. 51-20). Ultrasound does not provide information on the functional status of the thyroid mass and should not be used for establishing the diagnosis of hyperthyroidism. Rather, cervical ultrasound should be used as an adjunctive tool for locating cervical thyroid tissue.
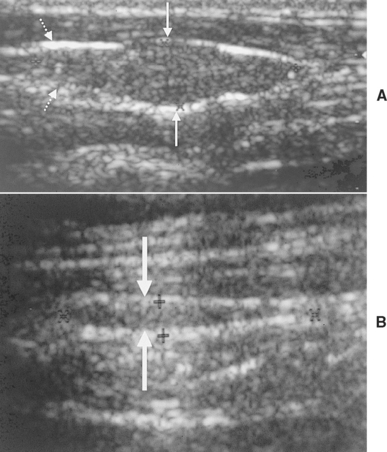
FIG 51-20 A, Ultrasound image of the right thyroid lobe of a 13-year-old domestic short-haired cat with hyperthyroidism. A mass is in the midregion of the thyroid lobe (solid arrows). Normal appearing portion of thyroid lobe (broken arrows). B, Ultrasound image of the small (atrophied) normal left thyroid lobe (solid arrows). Left thyroid lobe (small arrows). Results of the ultrasound examination supported unilateral disease affecting the right thyroid lobe, which was confirmed with a sodium pertechnetate scan.
Treatment
Hyperthyroidism in cats can be managed by thyroidectomy, oral antithyroid medications, or radioactive iodine (Table 51-7). All three modes of therapy are effective. Surgery and radioactive iodine treatments are used in the hope of providing a permanent cure for the disease; oral antithyroid drugs only control the hyperthyroidism and must be given daily to achieve and maintain their effect.
Initial Treatment Recommendation
Hyperthyroid cats should be treated initially with an oral antithyroid drug (i.e., methimazole) to reverse the hyperthyroid-induced metabolic and cardiac derangements, decrease the anesthetic risk associated with thyroidectomy, and assess the impact of treatment on renal function. Hyperthyroidism may mask renal insufficiency in some cats (see p. 749), and azotemia may develop or worsen and clinical signs of renal insufficiency may develop after treatment of the hyperthyroid state. Because it is not easy to determine what impact the hyperthyroid state is having on renal function, it is preferable to treat cats with reversible therapy (i.e., methimazole) until the impact of hyperthyroidism on renal function can be determined. If renal parameters remain static or improve after resolution of hyperthyroidism with methimazole, a more permanent treatment can be recommended. If significant azotemia or clinical signs of renal insufficiency develop during methimazole therapy, the treatment protocol for methimazole should be modified to attain the best possible control of both disorders and treatment for renal insufficiency should be instituted. Maintaining a mild hyperthyroid state may be necessary to improve renal perfusion and GFR and prevent the uremia of renal failure.
Antithyroid Drugs
Oral antithyroid drugs include methimazole, propylthiouracil, and carbimazole. Oral antithyroid drugs are inexpensive, readily available, relatively safe, and effective in the treatment of hyperthyroidism in cats. They inhibit the synthesis of thyroid hormone by blocking the incorporation of iodine into the tyrosyl groups in thyroglobulin and by preventing the coupling of these iodotyrosyl groups into T3 and T4. Antithyroid drugs do not block the release of stored thyroid hormone into the circulation and do not have antitumor actions. Oral antithyroid drugs do not interfere with results of pertechnetate scanning or radioactive iodine therapy. Indications for oral antithyroid drugs include (1) test treatment to normalize serum T4 concentrations and assess the effect of resolving hyperthyroidism on renal function, (2) initial treatment to alleviate or eliminate any medical problems associated with the syndrome before thyroidectomy is performed or before the hospitalization required for radioactive iodine treatment, and (3) long-term treatment of hyperthyroidism.
Methimazole (Tapazole; Eli Lilly & Co.) is currently the antithyroid drug of choice because the incidence of adverse reactions associated with its use is lower than that associated with the use of propylthiouracil (Table 51-8). Adverse reactions are less likely to occur when the dosage of methimazole is started low (typically at subtherapeutic dose initially) and gradually increased to effect. The recommended initial dose of methimazole is 2.5 mg administered orally twice a day for 2 weeks. If adverse reactions are not observed by the client, if the physical examination reveals no new problems, if results of a CBC and platelet count are within reference limits, if the serum creatinine and urea nitrogen concentrations have not increased, and if serum T4 concentration is greater than 2 μg/dl after 2 weeks of therapy, the dose is increased by 2.5 mg per day (i.e., 5 mg in the morning and 2.5 mg in the evening) twice daily and the same parameters evaluated 2 weeks later. The dosage should continue to be increased every 2 weeks by 2.5 mg/day increments until the serum T4 concentration is between 1 and 2 μg/dl or adverse reactions develop. Serum T4 concentrations decline into the reference range within 2 weeks once the cat is receiving an effective dose of methimazole; clinical improvement is usually noted by clients within 2 to 4 weeks once good control of serum T4 concentration is achieved. Most cats respond to 5 to 7.5 mg of methimazole per day, and the drug is most effective when given twice a day. Attempts at decreasing the daily dosage, frequency of administration, or both can take place once clinical signs have resolved and a euthyroid state is attained, especially for cats receiving chronic methimazole treatment.
Rarely, cats are encountered that seem particularly resistant to methimazole, requiring as much as 20 mg/day. The most common cause for apparent resistance to methimazole is the inability of some clients to administer the drug to their cats. One alternative is to have a compounding pharmacy incorporate methimazole into tasty kitty treats. Another alternative is the topical application of methimazole to the pinna of the ear. Compounding veterinary pharmacies offer transdermal methimazole in a pluronic lecithin organogel (PLO) formulation. Creams can be made with methimazole at any concentration and are usually provided in 1-cc syringes that allow the client to place the appropriate dose on the fingertip and rub the cream into the pinna of the cat’s ear. The client must wear gloves to avoid absorption of methimazole, should alternate ears, and should wipe away any residual cream 30 to 60 minutes after each administration. The dosage and frequency of administration is as discussed with oral methimazole treatment. The bioavailability of transdermal methimazole is more variable, the overall effectiveness is not as good, and the prevalence of gastrointestinal adverse effects is lower, compared with oral methimazole. One important concern with using transdermal methimazole is the lack of regulation of compounding pharmacies; consistency between products created can vary considerably.
Adverse reactions to methimazole typically occur within the first 4 to 8 weeks of therapy (see Table 51-8). The cat should be examined every 2 weeks during the initial 3 months of methimazole treatment and a CBC, platelet count, assessment of kidney function, and serum T4 concentration evaluated at each visit. After the initial 3 months of therapy a CBC, platelet count, serum biochemistry panel, and serum T4 concentration should be evaluated every 3 to 6 months. Using the dosing protocol described above, lethargy, vomiting, and anorexia occur in fewer than 10% of cats; these mild adverse reactions are usually transient and often resolve despite continued administration of the drug. Mild methimazole-induced hematologic changes occur in fewer than 10% of cats and include eosinophilia, lymphocytosis, and transient leukopenia. More worrisome but less common (fewer than 5% of cats) alterations include facial excoriations, thrombocytopenia (platelet counts less than 75,000/mm3), leukopenia (total white blood cell counts less than 2000/mm3), and immune-mediated hemolytic anemia. Apparent hepatic toxicity or injury occurs in fewer than 2% of cats receiving methimazole and is characterized by clinical signs of liver disease (i.e., lethargy, anorexia, vomiting), icterus, and increased serum alanine transaminase and alkaline phosphatase activities. Some cats test positive for antinuclear antibodies, but the importance of this finding is not known. Development of myasthenia gravis has also been reported with methimazole treatment. If any of these serious complications develop, methimazole treatment should be discontinued and supportive care given. Adverse reactions typically resolve within 1 week after methimazole treatment is discontinued. It is common for these potentially life-threatening adverse reactions to recur, regardless of the dose or type of antithyroid drug used; thus alternative therapy (i.e., surgery, radioactive iodine) is recommended.
Carbimazole (NeoMercazole; Amdipharm) is an antithyroid drug that is converted to methimazole in vivo; it is an effective alternative treatment if methimazole is not available. The dosage and frequency of administration are the same as those in oral methimazole treatment. Long-term, twice-daily schedules are effective in controlling hyperthyroidism. Adverse reactions are similar to those seen in cats receiving methimazole, but they occur less frequently. Cats being treated with carbimazole should be monitored in the same manner as that suggested for cats receiving methimazole.
Surgery
Thyroidectomy is an effective treatment but should always be considered an elective procedure. Surgery is not indicated if the risk of anesthesia in the cat is unacceptable, its renal function is questionable, the likelihood of postoperative hypocalcemia is great, ectopic thyroid tissue is present in the thorax, or thyroid carcinoma with metastasis is suspected. Treatment with methimazole for 1 to 2 months before thyroidectomy is recommended for reasons previously discussed. If possible, an ultrasound examination of the ventral neck or a radionuclide scan should be performed before surgery to identify the location of the abnormal thyroid tissue, differentiate unilateral from bilateral lobe involv ement, and provide some insight into the probability of hypocalcemia developing postoperatively (see Fig. 51-15). Similar information can also be gained by direct visualization at the time of surgery.
Postoperative complications are listed in Box 51-8. The most worrisome is hypocalcemia. There is a direct correlation between the size of the thyroid lobes, the inability to visualize the external parathyroid glands, and the risk of hypocalcemia. Care must be taken to preserve at least one, preferably both, external parathyroid glands and their associated blood supply. A “subcapsular” thyroidectomy affords the best chance of retaining functional parathyroid glands. (See Suggested Readings for thyroidectomy procedures.) If all four parathyroid glands are inadvertently removed, the two external parathyroid glands should be removed from their respective thyroid lobes, minced, and placed within the muscle belly of one of the sternohyoideus muscles by bluntly dissecting parallel to the muscle fibers. Hypoparathyroidism usually resolves within a month of surgery if revascularization of the parathyroid autotransplant occurs.
 BOX 51-8 Complications of Thyroidectomy in Cats with Hyperthyroidism
BOX 51-8 Complications of Thyroidectomy in Cats with Hyperthyroidism
Serum calcium concentration should be assessed at least once daily for 5 to 7 days if a bilateral thyroidectomy has been performed. Clinical signs of hypocalcemia typically develop within 72 hours of surgery, although signs may not develop for 7 to 10 days. These signs include lethargy, anorexia, reluctance to move, facial twitching (especially the ears), muscle tremors and cramping, tetany, and convulsions. If all four parathyroid glands are removed at surgery, appropriate calcium and vitamin D supplementation should be initiated once the cat has recovered from anesthesia (see p. 735). If at least one parathyroid gland has been spared, transient hypocalcemia may still develop and last for several days to weeks, probably as a result of disruption of blood flow to the parathyroid gland after surgical manipulation. In these cats oral vitamin D and calcium therapy should be initiated only if clinical signs develop or if hypocalcemia becomes severe (i.e., serum total or ionized calcium concentration less than 8 mg/dl and 0.8 mmol/L, respectively). A decline in the blood calcium concentration is not an absolute indication to begin therapy because the remaining parathyroid glands may respond before clinical signs or severe hypocalcemia develop.
The persistence of hypoparathyroidism is unpredictable. Parathyroid function may recover after days, weeks, or months of vitamin D and calcium supplementation. Whenever resolution of hypoparathyroidism is observed, it is assumed that reversible parathyroid damage occurred, accessory parathyroid tissue may be starting to compensate for glands damaged or removed at surgery, or the parathyroid autotransplant (if performed at surgery) has revascularized and become functional. It is also possible that calcium-regulating mechanisms are functioning in the absence of parathyroid hormone. Because it is difficult to predict the long-term requirement for vitamin D therapy in any cat, an attempt should be made to gradually wean all treated cats off medication while monitoring the serum calcium concentration. The tapering process should extend over a period of at least 12 to 16 weeks. The goal is to maintain the serum calcium concentration between 8.5 and 10.0 mg/dl. If hypocalcemia recurs, therapy with vitamin D and calcium must be reinstituted.
Hypothyroidism may develop in some cats after bilateral thyroidectomy. The clinical signs, diagnosis, and treatment are discussed on p. 744. The decision to initiate levothyroxine treatment should be based on the presence or absence of clinical signs, not on the serum T4 concentration, per se. Serum T4 concentrations commonly decrease after surgery, often to less than 0.5 μg/dl, but thyroid function returns in most cats before clinical signs become apparent. Thyroid hormone supplementation should be initiated in cats that develop clinical signs in conjunction with a low serum T4 concentration. Because thyroid replacement therapy may not be needed long term in some of these cats, thyroid replacement therapy should be tapered slowly and then discontinued after 1 to 3 months to determine the continued need for treatment.
If clinical signs of hyperthyroidism persist despite thyroidectomy, the serum T4 concentration should be measured. If the serum T4 concentration is low-normal or low (i.e., <2.0 μg/dl), another disorder should be suspected. If the serum T4 concentration is high-normal or high (i.e.,>4.0 μg/dl), ectopic abnormal thyroid tissue, metastatic thyroid carcinoma, or, if unilateral thyroidectomy was performed, abnormal tissue in the remaining thyroid lobe should be suspected. Ectopic thyroid tissue would most likely be in the mediastinum, cranial to the heart (see Fig. 51-16). Thyroid scanning is recommended to identify ectopic or metastatic thyroid tissue. Alternatively, oral methimazole or radioactive iodine therapy can be considered. Clinical signs of hyperthyroidism may also recur months to years after thyroidectomy. The serum T4 concentration should be monitored once or twice a year in all cats successfully treated with surgery.
Radioactive Iodine
If available, radioactive iodine is the treatment of choice for hyperthyroidism because of the very low morbidity and mortality and very high success rate associated with the treatment (Fig. 51-21). Hypoparathyroidism is not a concern with radioactive iodine treatment, is effective in cats with hyperfunctioning ectopic thyroid tissue, and is the only option offering the potential for a cure in cats with metastatic or nonresectable thyroid carcinoma. Treatment with methimazole for 1 to 2 months before radioactive iodine treatment is recommended for reasons previously discussed. Prior or current treatment with methimazole does not alter the efficacy of radioactive iodine treatment.
FIG 51-21 Box plots of serum thyroxine (T4) concentrations in 524 cats before and at various times after administration of radioiodine for treatment of hyperthyroidism. The shaded area indicates the reference range for serum T4 concentration. Please see Fig. 51-9 for the key.
(From Peterson ME et al: Radioiodine treatment of 524 cats with hyperthyroidism, J Am Vet Med Assoc 207:1422, 1995.)
Iodine 131 (131I) has a half-life of 8 days and is the radionuclide of choice for treating hyperthyroidism. 131I administered intravenously or subcutaneously is concentrated within the thyroid, and the emitted radiation destroys surrounding functioning follicular cells while causing minimal radiation damage to contiguous structures. At doses of 3 to 5 mCi of 131I, the thyroid cells killed are those that are functioning. Atrophied normal thyroid cells receive a relatively small dose of radiation and are usually able to return to function, thereby preventing hypothyroidism in most cats. Depending on the dose administered, more than 80% of treated cats become euthyroid within 3 months—most within 1 week—and more than 95% of treated cats are euthyroid at 6 months. In one study by Peterson et al. (1995), clinical signs and laboratory data consistent with hypothyroidism developed in approximately 2% of 254 131I-treated cats, 2% to 4% required a second 131I treatment, and hyperthyroidism recurred in 2% within 1 to 6 years of treatment. Chun et al. (2002) found no correlation between pretreatment serum T4 concentration or thyroid to salivary gland ratios and resolution of hyperthyroidism after treatment with radioactive iodine. The most common complication following radioactive iodine treatment is hypothyroidism, which typically develops in cats with large, diffusely affected thyroid lobes receiving large doses of 131I. The duration of hospitalization following 131I administration varies depending on state regulations and the dosage of 131I administered. In our hospital the average cat is treated with 3 to 5 mCi of 131I and requires 4 to 6 days of hospitalization after therapy until the radioactivity of the cat and its excretions reach an acceptable level.
Prognosis
The prognosis is excellent for most cats with hyperthyroidism, assuming concurrent disease can be managed and thyroid carcinoma is not the etiology. Surgery and 131I therapy have the potential for cure, although hyperthyroidism may recur months to years (or not at all) after thyroidectomy or 131I treatment. Hyperthyroid cats with adenomatous hyperplasia or adenoma can potentially be treated with methimazole for years, assuming adverse reactions related to the medication are avoided. In a recent retrospective study cats with concurrent renal disease had significantly shorter survival times than cats with normal renal function and the survival time in cats treated with methimazole alone (median 2 years; interquartile range 1 to 3.9 years) was significantly shorter than cats treated with 131I alone (4.0 years; 3.0 to 4.8 years) or methimazole followed by 131I (5.3 years; 2.2 to 6.5 years; Milner et al., 2006).
CANINE THYROID NEOPLASIA
Etiology
Thyroid adenomas are usually small, nonfunctional masses that do not cause clinical signs and are usually found incidentally at necropsy. Exceptions are thyroid adenomas that are functional and cause hyperthyroidism or are unexpectedly identified during ultrasound examination of the ventral neck. Thyroid carcinomas are more commonly identified antemortem because of their large size, presence of clinical signs that can be recognized by clients, and ease of palpation by veterinarians. One or both thyroid lobes may be involved, and ectopic thyroid tissue located in the anterior mediastinum and base of the heart occasionally may become neoplastic. Thyroid carcinomas frequently infiltrate into surrounding structures such as the esophagus, trachea, and cervical musculature. Regional and distant metastasis to the retropharyngeal and cervical lymph nodes and lungs is common. Metastasis to other locations such as the liver, kidney, bone, and brain is also possible.
Most dogs with thyroid tumors are euthyroid or hypothyroid; approximately 10% of dogs have functional thyroid tumors that secrete excess thyroid hormone, causing hyperthyroidism. Clinical signs of hyperthyroidism may predominate in these dogs. Hyperthyroidism may be caused by functional thyroid adenomas and carcinomas. Adenomatous hyperplasia is the most common cause of hyperthyroidism in cats but has not been described in dogs.
Clinical Features
Thyroid tumors occur in middle-aged to older dogs, with an average age of 10 years. There is no sex-related predilection. Although any breed can be affected, Boxers, Beagles, and Golden Retrievers may be at an increased risk.
Dogs with nonfunctional thyroid tumors are usually brought to veterinarians because the client has seen or felt a mass in the ventral region of the dog’s neck (Fig. 51-22). Clinical signs may develop as a result of the mass compressing on adjacent structures (e.g., dyspnea, dysphagia) or as a result of metastasis (e.g., exercise intolerance, weight loss; Box 51-9). Clinical signs of hypothyroidism may develop with large invasive tumors that destroy both thyroid lobes. Clinical signs of hyperthyroidism occur in approximately 10% of dogs with thyroid tumors and are similar to those seen in hyperthyroid cats (see p. 748).
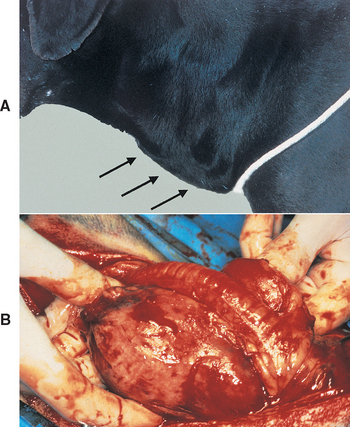
FIG 51-22 A, A 13-year-old male Labrador Retriever was presented to the veterinarian because the client noticed a mass in the neck (arrows). The mass was a thyroid adenocarcinoma. B, Thyroid adenocarcinoma in an 11-year-old mixed-breed dog. Clinical signs included dysphagia, coughing, and a visible mass in the ventral region of the neck.
Most thyroid tumors are firm, asymmetric, lobulated, and nonpainful masses located close to the typical thyroid region in the neck. The mass is usually well embedded in surrounding tissue and not freely movable. Additional physical examination findings may include dyspnea, cough, cachexia, lethargy, Horner’s syndrome, and dehydration. A dry, lusterless haircoat is common, but alopecia is rare. Mandibular or cervical lymph nodes (or both) may be enlarged as a result of tumor spread or lymphatic obstruction. Dogs with functional thyroid tumors may be restless, thin, and panting, and auscultation of the heart frequently reveals tachycardia. Surprisingly, many dogs are found to be remarkably healthy on physical examination.
CBC, serum biochemistry panel, and urinalysis findings usually do not help establish the diagnosis. A mild normocytic, normochromic, nonregenerative anemia, hypercholesterolemia, and hypertriglyceridemia causing lipemia may be present in dogs with concurrent hypothyroidism. A mild increase in the blood urea nitrogen concentration and liver enzyme activities has been identified in less than 35% of dogs; however, the latter changes were not found to be indicative of hepatic metastasis. Hypercalcemia has also been noted in a few dogs.
Baseline serum T4 and fT4 concentrations are increased and serum TSH is undetectable in dogs with a functional thyroid tumor causing hyperthyroidism. However, most canine thyroid tumors are nonfunctional, and most of these dogs are found to be euthyroid when serum thyroid hormone concentrations are evaluated. Approximately 30% of dogs with thyroid tumors have serum T4 and fT4 concentrations below the reference range and suggestive of hypothyroidism resulting from destruction of normal thyroid tissue by the tumor. However, interpretation of low serum thyroid hormone concentrations must be done with caution and consideration of the suppressive effects of nonthyroidal illness on thyroid function (see p. 737).
Cervical ultrasonography will confirm the presence of a mass, regardless of its size and location; can distinguish between cavitary, cystic, and solid tumors; can identify the presence and severity of local tumor invasion; can identify the presence and location of metastatic sites in the cervical region; and improve the likelihood that representative tissue for cytologic or histologic evaluation is obtained during fine-needle aspiration or percutaneous biopsy of the mass (Fig. 51-23). Because metastasis to the lungs and base of the heart is common with thyroid carcinoma, thoracic radiographs should always be included in the diagnostic evaluation of dogs with a suspected thyroid mass. Cervical radiographs may identify a small mass that was suspected but not definitively identified on physical examination, may show the severity of the displacement of adjacent structures, and may identify local invasion of the mass into the larynx and trachea. Abdominal ultrasonography can be used to identify abdominal (most notably hepatic) metastatic lesions. Computed tomographic and magnetic resonance imaging can define the extent of tumor invasion into surrounding structures, identify distant metastasis to the lymph nodes and lung, and identify ectopic thyroid tissue in the mediastinum (Fig. 51-24)—information that is valuable if surgery or megavoltage irradiation is being considered.
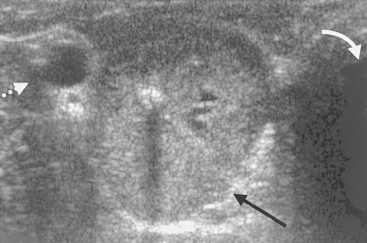
FIG 51-23 Ultrasound image of a mass in the region of the right thyroid lobe (straight arrow), the carotid artery (broken arrow), and the trachea (curved arrow) in an 11-year-old female spayed Labrador mix. A small region of mineralization causing a shadowing effect is evident within the mass. The mass was an unexpected finding during a routine physical examination. Thyroid adenocarcinoma was the histopathologic diagnosis after surgical removal of the mass.
FIG 51-24 Magnetic resonance image of a right-sided thyroid mass (solid arrow) adjacent to the trachea (broken arrow) in a 10-year-old male castrated Golden Retriever that was presented for a swelling in the neck. The histopathologic diagnosis was thyroid C-cell carcinoma with vascular invasion. The affected region of the neck was treated with radiation after thyroidectomy.
Thyroid scans using sodium pertechnetate can be used to confirm that a cervical mass is thyroid in origin; assess the degree of regional tissue invasion; and identify unusual areas of uptake in the head, neck, and thorax suggestive of metastatic sites. Most thyroid carcinomas demonstrate heterogenous uptake of pertechnetate, irregular gland shape, and evidence of regional tissue invasion. If the malignancy, especially a distant site of metastasis, does not trap iodine effectively, the scintigraphic study will fail to identify the site. Failure to identify distant metastatic sites with scintigraphy does not mean that distant metastasis does not exist. The amount of radionuclide uptake by the thyroid tumor is not a reliable indicator of its functional status (i.e., euthyroid, hypothyroid, or hyperthyroid) or the benign versus malignant nature of the tumor. Thoracic radiographs are more sensitive than a thyroid scan for identifying pulmonary metastasis.
Diagnosis
For a definitive diagnosis to be rendered, a biopsy specimen must be obtained from the tumor and evaluated histologically. Unfortunately, canine thyroid tumors are highly vascular, and it is common for hemorrhage to occur after biopsy. Fine-needle aspiration using a 21- or 23-gauge needle and cytologic examination of the mass are recommended initially to confirm that the mass is of thyroid origin. Contamination of the aspirate with blood is common, and differentiation between adenoma and carcinoma is difficult. Large-bore needle biopsy, surgical exploration, or ultrasound-guided biopsy is often required to confirm the diagnosis. Ultrasonography identifies solid areas of the mass to biopsy and large blood vessels to be avoided. This procedure is preferred if the findings yielded by needle aspiration are inconclusive.
Treatment
Treatment options for thyroid tumors in dogs include surgery, chemotherapy, megavoltage irradiation, radioactive iodine, and antithyroid drugs. The therapeutic approach is based, in part, on the size and invasiveness of the tumor and the presence of regional and distant metastasis. The functional status of the thyroid tumor does not dramatically alter the treatment approach. All thyroid tumors in dogs should be considered malignant until proved otherwise. Treatment is warranted even for large, locally invasive tumors. Many dogs with large invasive tumors appear more comfortable and have the potential for increased longevity after treatment. In addition, local control of the tumor may halt or reduce metastatic spread, and the presence of metastatic spread may not ultimately affect outcome. Local control of the thyroid carcinoma is of primary importance in managing this disease.
SURGERY
Surgical excision of thyroid adenomas and small, well-encapsulated, movable thyroid carcinomas is likely to be curative. Surgical removal of a fixed, invasive thyroid carcinoma, regardless of size, carries a guarded to poor prognosis for complete excision of the tumor. Megavoltage irradiation is the treatment of choice for these tumors. Chemotherapy is indicated if distant metastasis is identified. Surgical debulking of fixed, invasive tumors is indicated to relieve tumor-induced problems such as dysphagia or dyspnea and allow more time for other therapies to work. Surgical debulking may also be considered after megavoltage irradiation or chemotherapy has caused the size of large invasive tumors to shrink. Aggressive attempts at surgical removal, especially of bilateral tumors, threaten the integrity of recurrent laryngeal nerves, parathyroid glands, and normal thyroid tissue. It is important to monitor serum calcium concentrations before and for 7 to 10 days after surgery if there is any chance that the parathyroid glands have been excised or damaged. Vitamin D and calcium therapy should be initiated if any evidence of hypoparathyroidism is found (see p. 735). Serum T4, fT4, and TSH concentrations should be monitored 2 to 3 weeks after surgery and, depending on clinical signs, replacement therapy implemented accordingly (see p. 741). (See Slatter [2003] and Fossum [2007] for information on surgical techniques for the thyroparathyroid complex.)
MEGAVOLTAGE IRRADIATION
Megavoltage irradiation is the treatment of choice for locally advanced thyroid carcinoma. Megavoltage irradiation can be used alone or in conjunction with surgery or chemotherapy. There is a slow regression rate of thyroid carcinoma after radiation therapy in dogs. In one study involving 25 dogs with unresectable differentiated thyroid carcinoma and no evidence of metastasis, the time to attain maximum reduction in tumor size ranged from 8 to 22 months after megavoltage irradiation (Theon et al., 2000). Progression-free survival rates (defined as the time between completion of irradiation and detection of measurable local tumor recurrence or death from causes unrelated to tumor progression) were 80% at 1 year and 72% at 3 years with a mean progression-free survival time of 55 months in the 25 dogs. Acute radiation reactions to megavoltage irradiation include esophageal, tracheal, or laryngeal mucositis causing dysphagia, cough, and hoarseness. These reactions tend to be mild and self-limiting. Chronic radiation reactions include skin fibrosis, permanent alopecia, chronic tracheitis causing a dry cough, and hypothyroidism.
CHEMOTHERAPY
Chemotherapy is indicated when total surgical removal or destruction with megavoltage irradiation is not successful, if distant metastatic lesions have been identified, and if the size of the primary tumor is such that local invasion or metastasis is likely, even though it cannot be identified with diagnostic tests. Whenever the thyroid mass exceeds approximately 4 cm in diameter, the probability of metastasis becomes extremely high. Doxorubicin given at a dosage of 30 mg/m2 body surface area intravenously every 3 to 6 weeks is the historic treatment of choice. The response of canine thyroid tumors to doxorubicin is variable. In most dogs doxorubicin prevents further growth of the tumor and may cause the tumor to shrink, but total remission is uncommon. Combination chemotherapy with 5-fluorouracil, cyclophosphamide, and/or vincristine may enhance the effectiveness of doxorubicin. Cisplatin or carboplatin should be considered in dogs that fail to respond to or have recurrence of disease with doxorubicin therapy. The response to cisplatin has been reported to be similar to the response to doxorubicin, although several cisplatin-treated dogs were previously treated with doxorubicin (Fineman et al., 1998). (See Chapters 77 and 78 for a discussion of the use of these chemotherapeutic agents.)
RADIOACTIVE IODINE (131I)
Recent retrospective studies suggest that 131I therapy will prolong survival times when used as sole therapy or in combination with surgery for the treatment of thyroid tumors in dogs. Worth et al. (2005) reported a median survival time of 30 months for dogs treated with radioiodine alone, 34 months when radioiodine was combined with surgery, and 3 months for dogs that did not receive treatment. Turrell et al. (2006) reported a median survival time of 839 days for dogs with local or regional tumors (i.e., stage II and III disease) and 366 days for dogs with metastasis. Tumor site (cervical versus ectopic), age, body weight, treatment protocol (131I alone or with surgery), and serum T4 concentration were not significantly associated with survival time. Iodine 131 therapy is useful for any thyroid tumor tissue that can accumulate organic iodine, including metastatic sites. Kinetic studies to evaluate the ability of the tumor to trap iodine should be conducted before considering radioactive iodine treatment. Large doses of 131I (i.e., 30 to 150 mCi) are typically administered intravenously or subcutaneously to treat canine thyroid tumors. Potential adverse reactions include esophagitis, tracheitis, and bone marrow suppression.
ORAL ANTITHYROID DRUGS
Oral antithyroid drugs are used as palliative therapy to control the clinical signs of hyperthyroidism in dogs with functional thyroid tumors. Oral antithyroid drugs are not used as a primary treatment because they are not cytotoxic. The therapeutic approach is similar to that used in hyperthyroid cats (see p. 754), beginning with 2.5 mg of methimazole administered twice a day, with subsequent increases in the dosage and frequency of administration as needed to control clinical signs and maintain the serum T4 concentration within the reference range.
Prognosis
The prognosis for thyroid adenomas is excellent after surgical removal. The prognosis is guarded to good for dogs that undergo surgical resection of small, well-encapsulated carcinomas. Unfortunately, most dogs have relatively large thyroid masses, which have frequently invaded surrounding tissues or metastasized at the time of diagnosis. In these dogs aggressive therapy using multiple treatments can alleviate the clinical signs and in some cases dramatically reduce the tumor burden. The long-term prognosis, however, remains guarded to poor, with survival times typically ranging from 6 to 24 months, depending on the aggressiveness of treatment.
Feldman EC, Nelson RW. Canine and feline endocrinology and reproduction, ed 3. St Louis: WB Saunders, 2004.
Fossum TW. Small animal surgery, ed 3. St Louis: Mosby, 2007.
Slatter D. Textbook of small animal surgery, ed 3. Philadelphia: WB Saunders, 2003.
Canine and Feline Hypothyroidism
Bromel C, et al. Ultrasound of the thyroid gland in healthy, hypothyroid, and euthyroid Golden Retrievers with nonthyroidal illness. J Vet Intern Med. 2005;19:499.
Credille KM, et al. The effects of thyroid hormones on the skin of Beagle dogs. J Vet Intern Med. 2001;15:539.
Graham PA, et al. A 12-month prospective study of 234 thyroglobulin antibody positive dogs which had no laboratory evidence of thyroid dysfunction. J Vet Intern Med. 2001;15:298.
Higgins MA, et al. Hypothyroid-associated central vestibular disease in 10 dogs: 1999-2005. J Vet Intern Med. 2006;20:1363.
Johnson C, et al. Effect of 131I-induced hypothyroidism on indices of reproductive function in adult male dogs. J Vet Intern Med. 1999;13:104.
Kantrowitz LB, et al. Serum total thyroxine, total triiodothyronine, free thyroxine, and thyrotropin concentrations in dogs with nonthyroidal disease. J Am Vet Med Assoc. 2001;219:765.
Kemppainen RJ, Birchfield JR. Measurement of total thyroxine concentration in serum from dogs and cats by use of various methods. Am J Vet Res. 2006;67:259.
Kyfe JC, et al. Congenital hypothyroidism with goiter in Toy Fox Terriers. J Vet Intern Med. 2003;17:50.
Marca MC, et al. Evaluation of canine serum thyrotropin (TSH) concentration: comparison of three analytical procedures. J Vet Diag Invest. 2001;13:106.
Nachreiner RF, et al. Prevalence of serum thyroid hormone autoantibodies in dogs with clinical signs of hypothyroidism. J Am Vet Med Assoc. 2002;220:466.
Peterson ME, et al. Measurement of serum total thyroxine, triiodothyronine, free thyroxine, and thyrotropin concentrations for diagnosis of hypothyroidism in dogs. J Am Vet Med Assoc. 1997;211:1396.
Pullen WH, Hess RS. Hypothyroid dogs treated with intravenous levothyroxine. J Vet Intern Med. 2006;20:32.
Schachter S, et al. Comparison of serum free thyroxine concentrations determined by standard equilibrium dialysis, modified equilibrium dialysis, and 5 radioimmunoassays in dogs. J Vet Intern Med. 2004;18:259.
Scott-Moncrieff JCR, et al. Lack of association between repeated vaccination and thyroiditis in laboratory Beagles. J Vet Intern Med. 2006;20:818.
Stegeman JR, et al. Use of recombinant human thyroid-stimulating hormone for thyrotropin-stimulation testing of euthyroid cats. Am J Vet Res. 2003;64:149.
Chun R, et al. Predictors of response to radioiodine therapy in hyperthyroid cats. Vet Radiol Ultrasound. 2002;43:587.
Court MH, et al. Identification and concentration of soy isoflavones in commercial cat foods. Am J Vet Res. 2002;63:181.
Fischetti AJ, et al. Effects of methimazole on thyroid gland uptake of 99mTC-pertechnetate in 19 hyperthyroid cats. Vet Radiol Ultrasound. 2005;46:267.
Hammer KB, et al. Altered expression of G proteins in thyroid gland adenomas obtained from hyperthyroid cells. Am J Vet Res. 2000;61:874.
Hoffman SB, et al. Bioavailability of transdermal methimazole in a pluronic lecithin organogel (PLO) in healthy cats. J Vet Intern Med. 2002;16:359.
Kass PH, et al. Evaluation of environmental, nutritional, and host factors in cats with hyperthyroidism. J Vet Intern Med. 1999;13:323.
Martin KM, et al. Evaluation of dietary and environmental risk factors for hyperthyroidism in cats. J Am Vet Med Assoc. 2000;217:853.
Merryman JI, et al. Overexpression of c-ras in hyperplasia and adenomas of the feline thyroid gland: an immunohistochemical analysis of 34 cases. Vet Pathol. 1999;36:117.
Milner RJ, et al. Survival times for cats with hyperthyroidism treated with iodine 131, methimazole, or both: 167 cases (1996-2003). J Am Vet Med Assoc. 2006;228:559.
Nykamp SG, et al. Association of the risk of development of hypothyroidism after iodine 131 treatment with the pretreatment pattern of sodium pertechnetate Tc 99m uptake in the thyroid gland in cats with hyperthyroidism: 165 cases (1990-2002). J Am Vet Med Assoc. 2005;226:1671.
Padgett SL, et al. Efficacy of parathyroid gland autotransplantation in maintaining serum calcium concentrations after bilateral thyroparathyroidectomy in cats. J Am Anim Hosp Assoc. 1998;34:219.
Peterson ME, et al. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc. 1995;207:1422.
Peterson ME, et al. Measurement of serum concentrations of free thyroxine, total thyroxine, and total triiodothyronine in cats with hyperthyroidism and cats with nonthyroidal disease. J Am Vet Med Assoc. 2001;218:529.
Sartor LL, et al. Efficacy and safety of transdermal methimazole in the treatment of cats with hyperthyroidism. J Vet Intern Med. 2004;18:651.
Slater MR, et al. Long-term health and predictors of survival for hyperthyroid cats treated with iodine-131. J Vet Intern Med. 2001;15:47.
Trepanier LA, et al. Efficacy and safety of once versus twice daily administration of methimazole in cats with hyperthyroidism. J Am Vet Med Assoc. 2003;222:954.
Ward CR, et al. Expression of inhibitory G proteins in adenomatous thyroid glands obtained from hyperthyroid cats. Am J Vet Res. 2005;66:1478.
Brearley MJ, et al. Hypofractional radiation therapy for invasive thyroid carcinoma in dogs: a retrospective analysis of survival. J Small Anim Pract. 1999;40:206.
Fineman LS, et al. Cisplatin chemotherapy for treatment of thyroid carcinoma in dogs: 13 cases. J Am Anim Hosp Assoc. 1998;34:109.
Theon AP, et al. Prognostic factors and patterns of treatment failure in dogs with unresectable differentiated thyroid carcinomas treated with megavoltage irradiation. J Am Vet Med Assoc. 2000;216:1775.
Turrel JM, et al. Sodium iodide I 131 treatment of dogs with nonresectable thyroid tumors: 39 cases (1990-2003). J Am Vet Med Assoc. 2006;229:542.
Worth AJ, et al. Radioiodide (131I) therapy for treatment of canine thyroid carcinoma. Aust Vet J. 2005;83:208.
 BOX 51-1
BOX 51-1