CHAPTER 95 Polysystemic Bacterial Diseases
CANINE BARTONELLOSIS
Etiology and Epidemiology
Bartonella vinsonii subspecies berkhoffii was initially isolated from a dog with endocarditis in North Carolina (Breitschwerdt et al., 1995). Since that time, dogs in multiple areas of the world have been shown to seroreact with B. vinsonii (berkhoffii) antigens. B. vinsonii (berkhoffii) is thought to be tickborne. Serum of some infected dogs also seroreacts with B. henselae and B. clarridgeiae antigens; these Bartonella species are transmitted by fleas. Bartonella species that have been isolated from dogs or from which DNA has been amplified from blood or tissues include B. vinsonii (berkhoffii), B. henselae, B. clarridgeiae, B. washoensis, B. quintana, and B. elizabethae. Each of these organisms potentially can induce illness in dogs. Dogs infected with a Bartonella species are commonly coinfected with other agents, such as Anaplasma spp. or Ehrlichia spp., which may play a role in the pathogenesis of disease.
Clinical Features
Clinical findings or syndromes most frequently attributed to Bartonella spp. infections of dogs include endocarditis, fever, arrhythmias, hepatitis, granulomatous lymphadenitis, cutaneous vasculitis, rhinitis, polyarthritis, meningoencephalitis, thrombocytopenia, eosinophilia, monocytosis, immune-mediated hemolytic anemia, epistaxis, and uveitis. B. vinsonii (berkhoffii) and B. henselae seem to be the most likely species to be associated with clinical disease (Breitschwerdt et al., 2004; Goodman and Breitschwerdt, 2005; Henn et al., 2005). In one study of valvular endocarditis, all dogs with Bartonella spp.–associated disease were also seropositive for Anaplasma phagocytophilum (MacDonald et al., 2004). Whether the coinfection potentiated the Bartonella-associated disease is unknown.
Diagnosis
Serum antibodies can be detected in both healthy and clinically ill dogs, so the presence of antibodies does not always correlate to illness. Some Bartonella species, in particular B. vinsonii (berkhoffii), can be difficult to culture; amplification of DNA by polymerase chain reaction (PCR) assay with or without culture is often needed to confirm infection (Duncan et al., 2007). If positive test results are detected in a clinically ill dog and no other explanation for the illness is obvious, treatment is indicated.
Treatment
Because many cases of bartonellosis in dogs have been apparently resistant to administration of doxycycline, some clinicians believe that azithromycin is the treatment of choice. Fluoroquinolones, alone or in combination with azithromycin, were apparently effective for the treatment of some dogs with suspected clinical bartonellosis. Rifampin may be required for resistant cases. No matter which drug is used, a minimum of 4 to 6 weeks of treatment is usually needed. In one study successfully treated dogs became seronegative (Breitschwerdt et al., 2004).
Zoonotic Aspects and Prevention
B. vinsonii (berkhoffii) and B. henselae have been detected in both dogs and human beings, and cat scratch disease has been documented in a human being after exposure to a dog (Chen et al., 2007). Care should be taken to avoid bites or scratches while handling or treating infected dogs. Flea and tick control is likely to lessen transmission of Bartonella species between dogs and perhaps from dogs to people.
FELINE BARTONELLOSIS
Etiology and Epidemiology
Cats have been proven to be infected by B. henselae, B. clarridgeiae, B. koehlerae, B. quintana, and B. bovis by culture or DNA amplification (Brunt et al., 2006). Antibodies against B. elizabethae have been detected in some cats, but these results should be interpreted cautiously because of the serologic cross-reactivity among Bartonella spp. Cats are the main reservoir hosts for B. henselae and B. clarridgeiae and are likely the reservoir for B. koehlerae. B. henselae is the most common cause of cat scratch disease as well as bacillary angiomatosis and peliosis hepatis, common disorders in human beings with acquired immunodeficiency syndrome. Bartonella species have both intraendothelial and intraerythrocytic phases of infection (Fig. 95-1). The intracellular location may relate to the difficulties in permanently eliminating bacteremia (Kordick and Breitschwerdt, 1995; Seubert et al., 2002).

FIG 95-1 Electron micrograph of a feline erythrocyte showing intracellular Bartonella henselae.
(Courtesy Dr. Dorsey Kordick.)
On the basis of results of seroprevalence studies, culture, or PCR assay, cats are commonly exposed to or infected by Bartonella species. The organism is transmitted between cats by Ctenocephalides felis, so prevalence is greatest in cats from regions where fleas are common. A recent study in the United States collected fleas from cats and attempted to amplify Bartonella species DNA from flea digests as well as the blood of the cat (Lappin et al., 2006). The prevalence rates for B. henselae in cats and their fleas were 34.8% and 22.8%, respectively. The prevalence rates for B. clarridgeiae in cats and their fleas were 20.7% and 19.6%, respectively. Results have been similar in other studies performed around the world. B. henselae survives in flea feces for days after being passed by infected C. felis. Infected flea feces are likely to contaminate cat claws during grooming, then Bartonella species are inoculated into the person when scratched. Open wounds also may be contaminated with infected flea feces. However, Bartonella species DNA can also be amplified from the mouths of healthy cats and those with gingivostomatitis, so bites and scratches should be avoided (Quimby et al., 2007).
Clinical Features
Most cats with serologic evidence of exposure toBartonella spp.,Bartonella spp. cultured from blood, or microbial DNA amplified from blood by PCR assay are clinically normal. However, Bartonella spp. infection of cats has also been associated directly or indirectly with a variety of clinical manifestations such as fever, lethargy, lymphadenopathy, uveitis, gingivitis, and neurologic diseases. How often cats become ill from Bartonella spp. infections is unknown, and more information is needed. For example, the association of B. henselae infection to uveitis in a cat was first made in an individual case with uveitis that ultimately responded to doxycycline therapy (Lappin and Black, 1999; Lappin et al. 2000) subsequently found Bartonella antibody production and DNA in the aqueous humor of cats previously presumed to have idiopathic uveitis. A series of clinical cases of feline ocular disease that were responsive to antibiotic therapy was recently reported (Ketring et al., 2004). Thus Bartonella species appears to cause ocular disease in some cats. However, which cats have been exposed and which cats are diseased can be difficult to determine. In one study of feral cats in North Carolina the seroprevalence rate was 93% (Nutter et al., 2005). In another study the presence of Bartonella species antibodies failed to correlate to the presence of most clinical syndromes in ill cats (Breitschwerdt et al., 2005b). In recent studies in the author’s laboratory, the prevalence rates for Bartonella species antibodies in feline sera were not significantly different for cats with or without seizures (Pearce et al., 2006) or cats with or without stomatitis (Dowers et al., 2005). Why some cats develop Bartonella-associated illness and others do not is still not clear. For example, Powell et al. (2002) failed to induce Toxoplasma gondii or Bartonella species uveitis when Bartonella was intravenously inoculated into cats with chronic toxoplasmosis.
Diagnosis
Blood culture, PCR assay on blood, and serologic testing can be used to assess individual cats for Bartonella spp. infection. Cats that are culture negative or PCR negative and antibody negative and cats that are culture negative or PCR negative and antibody positive are probably not a source of flea, cat, or human infection. However, bacteremia can be intermittent and false-negative culture or PCR results can occur, limiting the predictive value of a single battery of tests. False-positive results can occur with PCR, and positive results do not necessarily indicate that the organism is alive. Although serologic testing can be used to determine whether an individual cat has been exposed, both seropositive and seronegative cats can be bacteremic, limiting the diagnostic utility of serologic testing. Thus testing healthy cats for Bartonella spp. infection is not currently recommended (Brunt et al., 2006). Testing should be reserved for cats with suspected clinical bartonellosis. If the results of Bartonella tests are negative in a clinically ill cat, the organism is not likely the cause of the clinical syndrome unless the infection was peracute and serologic testing was used as the diagnostic test. If the results of Bartonella tests are positive, the agent remains on the list of differential diagnoses, but other causes of the clinical syndrome must also be excluded. The American Association of Feline Practitioners (AAFP) Bartonella Panel Report suggests that the diagnosis of clinical bartonellosis include the following combination of findings (Brunt et al., 2006):
However, fulfillment of these criteria does not always prove a definitive diagnosis. The antibiotics used for the treatment of bartonellosis in cats generally have a broad spectrum, are effective for other infecting organisms that can cause syndromes resembling bartonellosis, and can also have antiinflammatory properties.
Treatment
In experimental studies administration of doxycycline, tetracycline, erythromycin, amoxicillin-clavulanate, and enrofloxacin can limit bacteremia but does not cure infection in all cats. To date, use of antibiotics in healthy cats has not been shown to lessen the risk of cat scratch disease (Brunt et al., 2006). In addition, treating healthy cats with antibiotics that do not eliminate infection may predispose to resistant stains of the organism. Thus in the United States treatment is generally recommended for clinically ill cats. If clinical bartonellosis is suspected, the AAFP Panel Report recommends doxycycline at 10 mg/kg PO daily for 7 days as the initial therapeutic trial (Brunt et al., 2006). Doxycycline should be formulated into a flavored suspension or water should be administered after pilling to avoid esophageal strictures. If a beneficial response is achieved, continue treatment for 2 weeks past clinical resolution of the disease or for a minimum of 28 days. If a poor response is achieved by day 7 or doxycycline is not tolerated and bartonellosis is still a valid differential diagnosis, azithromycin or a fluoroquinolone is a good second choice. Other differential diagnoses should be considered for Bartonella spp.–positive cats that have not responded after administration of two different drugs with presumed anti-Bartonella activity. Because cats can be reinfected with Bartonella spp., clinical value to following results of Bartonella spp. tests seems to be minimal if the cat is clinically normal.
Zoonotic Aspects and Prevention
Bartonella spp. infections are an occupational risk for veterinary health care providers (Breitschwerdt et al., 2007). To lessen the likelihood of acquiring a Bartonella species infection from a cat, the following adaptations of recommendations to HIV-infected people and other cat owners by the Centers for Disease Control and Prevention and the American Association of Feline Practitioners have been developed:
The Centers for Disease Control and Prevention do not recommend testing or treating healthy cats for Bartonella spp. infections.
FELINE PLAGUE
Etiology and Epidemiology
Yersinia pestis is the facultatively anaerobic gram-negative coccobacillus that causes plague. The organism is maintained in a sylvan life cycle between rodent fleas and infected rodents, including rock squirrels, ground squirrels, and prairie dogs. Cats are susceptible to infection and can die after natural or experimental infection; dogs are highly resistant to infection. Antibodies against Y. pestis are also detected in serum of nondomestic felids (Biek et al., 2006). Clinical disease is recognized most frequently from spring through early fall, when rodents and rodent fleas are most active. Most of the cases in human beings and cats have been documented in Colorado, New Mexico, Arizona, California, and Texas (Undisclosed authors, 2006). Of the cases of human plague diagnosed from 1977 to 1998, 23 (7.7%) resulted from contact with infected cats (Gage et al., 2000).
Cats are infected after being bitten by infected rodent fleas, after ingestion of bacteremic rodents, or after inhalation of the organism. After ingestion, the organism replicates in the tonsils and pharyngeal lymph nodes, disseminates in the blood, and results in a neutrophilic inflammatory response and abscess formation in infected tissues. The incubation period is 2 to 6 days after a flea bite and 1 to 3 days after ingestion or inhalation of the organism. Outcomes in experimentally infected cats include death (six of 16 cats; 38%), transient febrile illness with lymphadenopathy (seven of 16 cats; 44%), or inapparent infection (three of 16 cats; 18%) (Gasper et al., 1993).
Clinical Features
Bubonic, septicemic, and pneumonic plague develop in infected human beings and cats (Box 95-1); clinical disease is extremely rare in dogs (Orloski et al., 1995). Bubonic plague is the most common form of the disease in cats, but individual cats can show clinical signs of all three syndromes. Most infected cats are housed outdoors and have a history of hunting. Anorexia, depression, cervical swelling, dyspnea, and cough are common presenting complaints; fever is detected in most infected cats. Unilateral or bilateral enlarged tonsils, mandibular lymph nodes, and anterior cervical lymph nodes are detected in approximately 50% of infected cats. Cats with pneumonic plague commonly have respiratory signs and may cough.
 BOX 95-1 Clinical Findings in Cats with Yersinia pestis Infection (Plague)
BOX 95-1 Clinical Findings in Cats with Yersinia pestis Infection (Plague)
History and Physical Examination
Hunting of rodents or exposure to rodent fleas
Diagnosis
Hematologic and serum biochemical abnormalities reflect bacteremia and are not specific for Y. pestis infection. Neutrophilic leukocytosis, left shift and lymphopenia, hypoalbuminemia, hyperglobulinemia, hyperglycemia, azotemia, hypokalemia, hypochloremia, hyperbilirubinemia, and increased activities of alkaline phosphatase and alanine transaminase are common. Pneumonic plague causes increased alveolar and diffuse interstitial densities on thoracic radiographs. Cytologic examination of lymph node aspirates reveals lymphoid hyperplasia, neutrophilic infiltrates, and bipolar rods (Fig. 95-2).
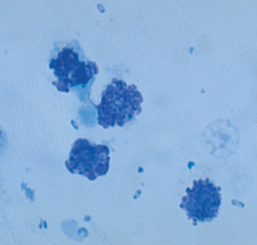
FIG 95-2 Lymph node aspirate from a cat with bubonic plague stained with Wright’s stain. Bipolar rods are scattered throughout the field.
Cytologic demonstration of bipolar rods on examination of lymph node aspirates, exudates from draining abscesses, or airway washings combined with a history of potential exposure, the presence of rodent fleas, and appropriate clinical signs lead to a presumptive diagnosis of feline plague. Because some cats survive infection and antibodies can be detected in serum for at least 300 days, detection of antibodies alone may indicate only exposure, not clinical infection. However, demonstration of a fourfold increase in antibody titer is consistent with recent infection. A definitive diagnosis is made by culture or fluorescent antibody demonstration of Y. pestis in smears of the tonsillar region, lymph node aspirates, exudates from draining abscesses, airway washings, or blood.
Treatment
Supportive care should be administered as indicated for any bacteremic animal (see Chapter 93). Cervical lymph node abscesses should be drained and flushed with the clinician wearing gloves, a mask, and a gown. Parenteral antibiotics should be administered until anorexia and fever resolve. Optimal antibiotics for treatment of plague in infected cats in the United States are unknown. Streptomycin administered intramuscularly at 5 mg/kg q12h was used historically but is not widely available. Cats treated with gentamicin intramuscularly or intravenously at 2 to 4 mg/kg q12-24h, or enrofloxacin intramuscularly or intravenously at 5.0 mg/kg q24h, has resolved clinical signs. Chloramphenicol administered orally or intravenously at 15 mg/kg q12h can be used in cats with central nervous system signs. Antibiotics should be administered orally for 21 days after the cat has survived the bacteremic phase; doxycycline at 5 mg/kg q12-24h or tetracycline at 20 mg/kg q8h is an appropriate choice. Care should be taken to avoid tetracycline-associated esophageal strictures by giving water after drug administration or liquefying the product. In one study 90.9% of cats treated with antibiotics survived, whereas only 23.8% of untreated cats survived (Eidson et al., 1991). The prognosis is poor for cats with pneumonic or septicemic plague.
Zoonotic Aspects and Prevention
Cats should be housed indoors and not allowed to hunt. Flea control should be used and the rodent population should be controlled if possible. Tetracycline or doxycycline at the doses listed for therapy should be administered for 7 days to animals with potential exposure. Human infection occurs after contact with infected fleas; contact with the tissues or exudates from infected animals, including cats; and from bites and scratches from infected cats. Even though fomite transmission is unlikely, because the organism is sensitive to drying it can survive for weeks to months in infected carcasses and for up to 1 year in infected fleas. Cats from endemic areas with clinical signs of bacteremia, respiratory tract disease, or cervical draining areas or masses in the spring, summer, and early fall months should immediately be treated for fleas and handled with the clinician wearing gloves, a mask, and a gown until the diagnosis is made or discarded. While hospitalized, infected cats should be handled by as few personnel as possible while in isolation. Exposed people should see their physicians to discuss prophylactic antibiotic therapy; antimicrobial-resistant strains of Y. pestis are uncommon (Welch et al., 2007). Cats are not infectious to human beings after 3 days of antibiotic therapy. Areas where infected cats are handled should be thoroughly cleaned with routine disinfectants (see Chapter 94).
LEPTOSPIROSIS
Etiology and Epidemiology
Leptospires are 0.1 to 0.2 μm wide by 6 to 12 μm long, motile, filamentous spirochetes that infect animals and human beings. Leptospirosis can be caused by many different serovars of Leptospira interrogans and L. kirschneri (Table 95-1). Seropositive dogs have been detected in many countries, and the most prevalent serovars vary by country and regions within countries. In the United States antibodies against L. autumnalis, L. bratislava, L. canicola, L. grippotyphosa, L. hardjo, L. icterohaemorrhagiae, and L. pomona have been detected most commonly. Cats are infected by L. bratislava, L. canicola, L. grippotyphosa, and L. pomona but appear to be resistant to clinical disease.
 TABLE 95-1 Reservoirs for Leptospira Serovars Known to Infect Dogs
TABLE 95-1 Reservoirs for Leptospira Serovars Known to Infect Dogs
| SEROVAR | PRIMARY RESERVOIR |
|---|---|
| L bataviae | Dog, rat, mouse |
| L bratislava | Pig, horse, dog |
| L canicola | Dog |
| L grippotyphosa | Vole, raccoon, skunk, opossum |
| L harp | Cow |
| L icterohaemorrhagiae | Rat |
| L pomona | Pig, skunk, opossum |
| L tarassovi | Cow, pig |
Prevalence and risk factors for cases of canine leptospirosis have been evaluated in several studies in the last few years (Ward et al., 2002; Boutilier et al., 2003; Ward et al., 2004ab; Goldstein et al., 2006; Ghneim et al., 2007; Stokes et al., 2007). In the United States the number of seropositive dogs increased between 2002 and 2004 (Moore et al., 2006). Infection by leptospires occurs in both rural and suburban environments in semitropical areas of the world with alkaline soil conditions. Exposure to water outdoors, wetlands, and public open spaces were identified as risk factors in one case-control study (Ghneim et al., 2006). Clinical cases are most commonly diagnosed in the summer and early fall, and numbers of cases often increase in years with heavy rainfall. Infection by host-adapted species results in subclinical infection; the host acts as a reservoir, shedding the organism intermittently. Infection by non–host-adapted species results in clinical illness. Leptospires are passed in urine and enter the body through abraded skin or intact mucous membranes. Transmission also occurs through bite wounds; by venereal contact; transplacentally; and by ingestion of contaminated tissues, soil, water, bedding, food, and other fomites. In a recent experimental study L. pomona but not L. bratislava was successfully transmitted by conjunctival inoculation and resulted in fever and lethargy starting within 7 days (Greenlee et al., 2005). Hosts with preexisting antibody titers usually eliminate the organism quickly and remain subclinically infected. Leptospires replicate in multiple tissues of nonimmune hosts or hosts infected by a non–host-adapted species; in the dog the liver and kidneys develop the highest levels of infection. Inflammation induced by organism replication and production of toxins leads to renal, hepatic, or pulmonary disease. Dogs that are treated or develop appropriate immune responses usually survive. Some animals clear the infection 2 to 3 weeks after exposure without treatment but develop chronic active hepatitis or chronic renal disease. Cats are generally subclinically affected but may shed the organism into the environment for variable periods after exposure.
Clinical Findings
Dogs of any age or breed or either gender can develop leptospirosis if not previously immune. Male, middle-aged, herding dogs; hounds; working dogs; and mixed breeds are at greater risk than companion dogs younger than 1 year (Ward et al., 2002). Most dogs have subclinical infection. Dogs with peracute clinical disease are usually presented for evaluation of anorexia, depression, generalized muscle hyperesthesia, tachypnea, and vomiting (Box 95-2). Fever, pale mucous membranes, and tachycardia are usually present. Petechiae, ecchymoses, melena, and epistaxis occur frequently from thrombocytopenia and disseminated intravascular coagulation. Peracute infections may rapidly progress to death before marked renal or hepatic disease is recognized.
 BOX 95-2 Clinical Findings in Dogs with Leptospirosis
BOX 95-2 Clinical Findings in Dogs with Leptospirosis
Physical Examination
Hemorrhagic tendencies, including melena, epistaxis, petechiae, and ecchymoses
Clinicopathologic and Radiographic Evaluation
Suboptimal urine concentrating ability
Pyuria and hematuria without obvious bacteriuria
Hyperbilirubinemia and bilirubinuria
Increased activities of alanine transaminase, aspartate transaminase, alkaline phosphatase, and creatine kinase
Fever, depression, and clinical signs or physical examination findings consistent with hemorrhagic syndromes, hepatic disease, renal disease, or a combination of hepatic and renal disease are common in subacutely infected dogs. Conjunctivitis, panuveitis, rhinitis, tonsillitis, cough, and dyspnea occur occasionally. Oliguric or anuric renal failure can develop during the subacute phase. Clinical findings can vary based on the infecting serovar (Goldstein et al., 2006).
Some dogs that survive peracute or subacute infection develop chronic interstitial nephritis or chronic active hepatitis. Polyuria, polydipsia, weight loss, ascites, and signs of hepatic encephalopathy secondary to hepatic insufficiency are the most common manifestations of chronic leptospirosis.
Diagnosis
Multiple nonspecific clinicopathologic and radiographic abnormalities occur in dogs with leptospirosis and vary depending on the host, the serovar, and whether the disease was peracute, subacute, or chronic. Leukopenia (peracute leptospiremic phase), leukocytosis with or without a left shift, thrombocytopenia, regenerative anemia (from blood loss), or nonregenerative anemia (from chronic renal or hepatic disease) are common hematologic abnormalities. Hyponatremia; hypokalemia; hyperphosphatemia; hypoalbuminemia; hypocalcemia; azotemia; hyperbilirubinemia; decreased total carbon dioxide concentrations; and increased activities of alanine transaminase, alkaline phosphatase, and aspartate transaminase are common serum biochemi-cal abnormalities that develop from renal disease, hepatic disease, gastrointestinal losses, or acidosis. Hyperglobulinemia is detected in some dogs with chronic leptospirosis. Dogs with myositis may have increased creatine kinase activity. Urinalysis abnormalities include bilirubinuria, suboptimal urine specific gravity in the face of azotemia, granular casts, and increased numbers of granulocytes and erythrocytes. The organism is not seen in the urine sediment by light microscopy. Renomegaly, hepatomegaly, and interstitial or alveolar pulmonary infiltrates are common radiographic abnormalities. Mineralization of the renal pelves and cortices can occur with chronic leptospirosis.
Detection of anti-Leptospira antibodies is commonly performed by a microscopic agglutination test. Because of the wide range of leptospires infecting dogs, as many serovars as possible should be used for screening. L. bratislava, L. canicola, L. grippotyphosa, L. hardjo, L. icterohaemorrhagiae, and L. pomona are commonly used. Positive titers can result from active infection, previous infection, or vaccination. Antibody titers can be negative in animals with peracute disease; seronegative dogs with classic clinical disease should be retested in 2 to 4 weeks. The serovar with the highest titer is usually considered the infecting serovar, but this should be interpreted cautiously. When the same sera were sent to different laboratories, the results were not always in agreement for the serovar giving the highest titer (Miller et al., 2007).
Documentation of seroconversion (negative result becoming positive over time), a single microscopic agglutination test titer greater than 1 : 3200, or a fourfold increase in antibody titers combined with appropriate clinicopathologic abnormalities and clinical findings, are suggestive of clinical leptospirosis. A definitive diagnosis is made by demonstrating the organism in urine, blood, or tissues. The organism can be seen in urine using darkfield or phasecontrast microscopy, but because of intermittent shedding of small numbers of organisms these procedures can be falsely negative. The organism can be cultured from urine collected by cystocentesis, blood, or renal or hepatic tissue. Materials for culture should be collected before administration of antibiotics, placed in transport media immediately after collection, and transported to the laboratory as quickly as possible. Leptospiremia can be of short duration, and urine shedding of the organism can be intermittent, giving false-negative results. PCR can be used to demonstrate the organism in urine, blood, or tissues (Harkin et al., 2003a, 2003b). In one study of 500 dogs, 41 (8.2%) were PCR positive for a Leptospira spp. in urine, and some of these dogs were clinically normal (Harkin et al., 2003a). None of the PCR-positive dogs was culture positive, and titers were not always elevated.
Treatment
Fluid therapy is required for most dogs; intense diuresis for renal involvement may be required (see Chapter 44). Hemodialysis may increase the probability of survival in dogs with oliguric or anuric renal failure. Dogs should be treated during the initial treatment period with ampicillin administered intravenously at 22 mg/kg q8h or penicillin G administered intramuscularly or intravenously at 25,000 to 40,000 U/kg q12h. Some quinolones have an effect against leptospires and can be used in combination with penicillins during the acute phase of infection. Ampicillin and enrofloxacin were used concurrently in one study; 83% of infected dogs survived (Adin et al., 2000). Penicillins such as amoxicillin or amoxicillin clavulanate should be administered for 2 weeks. Doxycycline administered orally at 2.5 to 5.0 mg/kg q12h for 2 weeks after penicillin therapy should be used to eliminate the renal carrier phase.
Zoonotic Aspects and Prevention
All mammalian serovars should be considered potentially zoonotic to human beings. Some human beings have antibodies against canine serovars, suggesting the dog can be a reservoir for human infection (Brod et al., 2005). Infected urine, contaminated water, and reservoir hosts should be avoided. Infected dogs should be handled with the clinician wearing gloves. Contaminated surfaces should be cleaned with detergents and disinfected (see Chapter 94).
To lessen risk of exposure, owners should attempt to restrict dogs from drinking potentially contaminated water. Vaccines available for some serovars reduce the severity of disease. Vaccination against serovars L. canicola and L. icterohaemorrhagiae can induce serologic cross-reactivity against serovars not contained in the vaccine, but cross-protection against other serovars is not always induced (Barr et al., 2005). Products containing serovars L. canicola, L. icterohaemorrhagiae, L. grippotyphosa, and L. pomona are now available and provide the greatest spectrum of protection (see Chapter 94). Vaccination can lessen shedding of leptospires in the urine; not all vaccines perform the same (Andre-Fontaine et al., 2003; Schreiber et al., 2005). Dogs in endemic areas should receive three vaccinations 2 to 3 weeks apart. The duration of immunity is longer than 1 year in dogs receiving three vaccinations.
MYCOPLASMA AND UREAPLASMA
Etiology and Epidemiology
Mycoplasma spp. and Ureaplasma spp. are small, free-living microorganisms that lack a rigid, protective cell wall and depend on the environment for nourishment. Some Mycoplasma spp. and Ureaplasma spp. are considered normal flora of mucous membranes. For example, Mycoplasma spp. have been isolated from the vagina of 75% of healthy dogs (Doig et al., 1981), the pharynx of 100% of healthy dogs, and the pharynx of 35% of healthy cats (Randolph et al., 1993). Haemobartonella felis and H. canis were recently shown to be Mycoplasmas. Cats are infected by three species, M. haemofelis, “Candidatus M. haemominutum,” and “Candidatus M. turicensis.” Dogs are infected by two species, M. haemocanis and Candidatus M. haematoparvum. These organisms are associated with red blood cells and may result in the development of anemia. These organisms are discussed in Chapter 85.
M. felis conjunctivitis in cats, M. felis upper respiratory tract infection in cats, M. gateae polyarthritis in cats, and M. cynos pneumonia in dogs have been induced experimentally. The pathogenic potential for most Mycoplasma spp. or Ureaplasma spp. is difficult to determine because the organisms can be cultured or amplified from both healthy and sick animals. In many cases Mycoplasma spp. or Ureaplasma spp. may be colonizing diseased tissues as opportunists as a result of inflammation induced by other causes. Other bacteria are usually isolated concurrently with Mycoplasma spp. or Ureaplasma spp., making it difficult to determine which agent is inducing disease. Ureaplasma spp. have been cultured from the vagina (40%) and prepuce (10%) of healthy dogs (Doig et al., 1981).
Mycoplasma spp. were isolated in pure culture from 20 of 2900 dogs with clinical signs of urinary tract inflammation (Jang et al., 1984), M. canis was isolated from four of 100 dogs (three in pure culture) with clinical signs of lower urinary tract disease (Ulgen et al., 2006), and M. canis was isolated from nine dogs with clinical signs of urogenital disease (L’Abee-Lund et al., 2003). Some M. canis–infected dogs were azotemic, suggesting pyelonephritis (Ulgen et al., 2006), and some have been resistant to therapy (L’Abee-Lund et al., 2003). Multiple studies suggest that some Mycoplasma spp. can be primary pathogens of the respiratory tract of dogs. Mycoplasma spp. were the only organism cultured from seven of 93 dogs (Jameson et al., 1995), five of 38 dogs (Randolph et al., 1993), and 14 dogs (Chandler et al., 2002) with lower respiratory tract disease. In one study that compared Mycoplasma isolates from dogs with and without respiratory disease, M. cynos in the lower respiratory tract was statistically associated with respiratory disease (Chalker et al., 2004b). In another study, 80% of dogs that developed antibodies to M. cynos had respiratory signs of disease (Rycroft et al., 2007).
In a recent study of cats with and without conjunctivitis, the presence of Mycoplasma spp. DNA was associated with the presence of conjunctivitis (Low et al., 2007). Both M. felis and M. gateae have been associated with feline ulcerative keratitis (Gray et al., 2005). M. gateae and M. felis have been detected in cats with polyarthritis. Mycoplasma spp. have also been associated with the presence of rhinosinusitis (Johnson et al., 2005; Bannasch et al., 2005), lower respiratory disease (Randolph et al., 1993; Chandler et al., 2002; Foster et al., 2004a, 2004b), and pyothorax (Gulbahar et al., 2002; Barrs et al., 2005).
Clinical Findings
Mycoplasma spp. infection should be considered a potential differential diagnosis for cats presented for evaluation of conjunctivitis, keratitis, sneezing and mucopurulent nasal discharge, coughing, dyspnea, fever, lameness with or without swollen painful joints, subcutaneous abscessation, or abortion. Mycoplasma spp. or Ureaplasma spp. infections were not associated with lower urinary tract disease of cats in one study (Abou et al., 2006). Mycoplasma spp. or Ureaplasma spp. infection should be considered a potential differential diagnosis for dogs presented for evaluation of coughing, dyspnea, fever, pollakiuria, hematuria, azotemia, lameness with or without swollen painful joints, mucopurulent vaginal discharge, or infertility. Mycoplasma spp. and Ureaplasma spp. are generally not recognized cytologically and usually do not grow on aerobic media; infection should be suspected in animals with neutrophilic inflammation without visible bacteria or negative aerobic culture. The index of suspicion for Mycoplasma spp. or Ureaplasma spp. infection is higher if the animal has neutrophilic inflammation and has been poorly responsive to cell wall–inhibiting antibiotics such as penicillins or cephalosporins.
Diagnosis
The clinical laboratory and radiographic abnormalities associated with Mycoplasma spp. or Ureaplasma spp. infections are similar to those induced by other bacterial infections. Neutrophilia and monocytosis are common in dogs with pneumonia; pyuria and proteinuria occur in dogs with urinary tract disease.
Preputial discharges, vaginal discharges, chronic draining wounds, airway washings, and synovial fluid from animals with Mycoplasma spp. or Ureaplasma spp. infections have nondegenerate neutrophils as the most common cell type. Dogs with lower respiratory tract disease and pure Mycoplasma cultures have alveolar lung patterns that cannot be differentiated from those in dogs with mixed bacterial and Mycoplasma cultures. In some dogs and cats with small airway disease evident radiographically, Mycoplasma spp. are isolated from the airways in pure culture (Chandler et al., 2002). Joint radiographs of animals with Mycoplasmaassociated polyarthritis reveal nonerosive changes.
Specimens for Mycoplasma spp. or Ureaplasma spp. culture should be plated immediately or transported to the laboratory in Hayflicks broth medium, Amies medium without charcoal, or modified Stuart bacterial transport medium. Specimens should be shipped on ice packs if the transport time is expected to be less than 24 hours and on dry ice if the transport time is expected to be longer than 24 hours. Most Mycoplasma spp. require special media, but in one report M. canis grew on regular blood agar plates (L’Abee-Lund et al., 2003). Because the organisms are part of the normal flora, culture of the mucous membranes of healthy animals is never indicated. Because Mycoplasma spp. or Ureaplasma spp. can be cultured from healthy animals, interpretation of positive culture results in sick animals is difficult. Most laboratories do not report results of antibiotic susceptibility testing. The disease association is strong if Mycoplasma spp. or Ureaplasma spp. are isolated in pure culture from tissues from which isolation is unusual (lower airway, uterus, joints). Response to treatment with drugs with known activity against Mycoplasma spp. or Ureaplasma spp. may help support the diagnosis of disease induced by these agents. PCR assays are now available for detection of mycoplasmal DNA (Johnson et al., 2004; Chalker et al., 2004a; Low et al., 2007) in several diagnostic laboratories, but they have the same diagnostic limitations as culture and positive results do not prove the organism is alive.
Treatment
Tylosin, erythromycin, clindamycin, lincomycin, tetracyclines, chloramphenicol, aminoglycosides, and fluoroquinolones are effective for treatment of Mycoplasma spp. or Ureaplasma spp. infections (see Chapter 93). Doxycycline administered orally at 5-10 mg/kg q12-24h is generally effective in animals with a competent immune system or without life-threatening disease and is proposed to have the added benefit of being antiinflammatory. In animals with mixed infections with gram-negative organisms, life-threatening disease, or suspected tetracycline-resistant strains, fluoroquinolones or azithromycin are good alternate antibiotic choices. In one cat with mycoplasmal polyarthritis, enrofloxacin therapy, but not doxycycline therapy, eliminated infection. Treatment for 4 to 6 weeks is usually required for lower airway, subcutaneous, or joint infections. Erythromycin administered orally at 20 mg/kg q8-12h or lincomycin administered orally at 22 mg/kg q12h should be used in pregnant animals.
Zoonotic Aspects and Prevention
Although risk of zoonotic transfer is likely minimal, bite wound transmission of Mycoplasma spp. from an infected cat to the hand of a human being has been reported (McCabe et al., 1987). Most Mycoplasma spp. or Ureaplasma spp. infections in dogs and cats are opportunistic and associated with other causes of inflammation; thus they are not likely to be directly contagious from animal to animal. However, M. felis may be transmitted from cat to cat by conjunctival discharges. Mycoplasma spp. appear to have been associated with respiratory tract disease in dogs and cats as primary pathogens and may be spread from animal to animal, as with M. pneumoniae in human beings. Animals with conjunctivitis or respiratory tract disease should be isolated from other animals until clinical signs of disease have resolved (see Chapter 94). Mycoplasma spp. and Ureaplasma spp. are susceptible to routine disinfectants and rapidly die outside the host.
Canine and Feline Bartonellosis
Breitschwerdt EB, et al. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154.
Breitschwerdt EB, et al. Bartonella vinsonii subsp. berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J Clin Microbiol. 1999;37:3618.
Breitschewerdt EB, et al. Clinicopathological abnormalities and treatment response in 24 dogs seroreactive to Bartonella vinsonii (berkhoffii) antigens. J Am Anim Hosp Assoc. 2004;40:92.
Breitschwerdt EB, et al. Bartonella species as a potential cause of epistaxis in dogs. J Clin Microbiol. 2005;42:2529.
Breitschwerdt ED, et al. Bartonella henselae and Rickettsia seroreactivity in a sick cat population from North Carolina. Inter J Appl Res Vet Med. 2005;3:287.
Breitschwerdt EB, et al. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Inf Dis. 2007;13:938.
Breitschwerdt EB, et al. Isolation of Bartonella quintana from a woman and a cat following putative bite transmission. J Clin Microbiol. 2007;45:270.
Brunt J, et al. Association of Feline Practitioners 2006 panel report on diagnosis, treatment and prevention of Bartonella species infections. J Fel Med Surg. 2006;8:213.
Chen TC, et al. Cat scratch disease from a domestic dog. J Formos Med Assoc. 2007;106:S65.
Chomel BB, et al. Isolation of Bartonella washoensis from a dog with mitral valve endocarditis. J Clin Microbiol. 2003;41:5327.
Cockwill KR, et al. Bartonella vinsonii subsp. berkhoffii endocarditis in a dog from Saskatchewan. Can Vet J. 2007;48:839.
Dowers KL, Lappin MR. The association of Bartonella spp. infection with chronic stomatitis in cats. J Vet Int Med. 2005;19:471.
Duncan AW, et al. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre-enrichment liquid culture followed by PCR and subculture onto agar plates. J Microbiol Methods. 2007;69:273.
Goodman RA, Breitschwerdt EB. Clinicopathologic findings in dogs seroreactive to Bartonella henselae antigens. Am J Vet Res. 2005;66:2060.
Honadel TE, et al. Seroepidemiology of Bartonella vinsonii subsp berkhoffii exposure among healthy dogs. J Am Vet Med Assoc. 2001;15:219.
Human plague—four states, 2006. MMWR Morb Mortal Wkly Rep. 2006;55:940.
Kelly P, et al. Bartonella quintana endocarditis in dogs. Emerg Inf Dis. 2006;12:1869.
Ketring KL, et al. Bartonella: a new etiological agent of feline ocular disease. J Am Anim Hosp Assoc. 2004;40:6.
Kordick DL, Breitschwerdt EB. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol. 1995;33:1655.
Kordick DL, et al. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol. 1996;46:704.
Kordick DL, Breitschwerdt EB. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis. 1998;4:325.
MacDonald KA, et al. A prospective study of canine infective endocarditis in northern California (1999-2001): emergence of Bartonella as a prevalent etiologic agent. J Vet Intern Med. 2004;18:56.
Mexas AM, et al. Bartonella henselae and Bartonella elizabethae as potential canine pathogens. J Clin Microbiol. 2002;40:4670.
Michau TM, et al. Bartonella vinsonii subspecies berkhoffii as a possible cause of anterior uveitis and choroiditis in a dog. Vet Ophthalmol. 2003;6:299.
Morales SC, et al. Detection of Bartonella henselae DNA in two dogs with pyogranulomatous lymphadenitis. J Am Vet Med Assoc. 2007;230:681.
Nutter FB, et al. Seroprevalences of antibodies against Bartonella henselae and Toxoplasma gondii and fecal shedding of Cryptosporidium spp., Giardia spp., and Toxocara cati in feral and domestic cats. J Am Vet Med Assoc. 2004;235:1394.
Pappalardo BL, et al. Granulomatous disease associated with Bartonella infection in 2 dogs. J Vet Intern Med. 2000;14:37.
Pappalardo BL, et al. Immunopathology of Bartonella vinsonii (berkhoffii) in experimentally infected dogs. Vet Immunol Immunopathol. 2001;83:125.
Pearce L, et al. Prevalence of Bartonella henselae specific antibodies in serum of cats with and without clinical signs of central nervous system disease. J Fel Med Surg. 2006;8:315.
Pesavento PA, et al. Pathology of Bartonella endocarditis in six dogs. 2005;42:370.
Podsiadly E, et al. Bartonella henselae in Ixodes ricinus ticks removed from dogs. Vector Borne Zoonotic Dis. 2007;7:189.
Powell CC, et al. Inoculation with Bartonella henselae followed by feline herpesvirus 1 fails to activate ocular toxoplasmosis in chronically infected cats. J Fel Med Surg. 2002;4:107.
Quimby J, et al. Evaluation of the association of Bartonella spp., feline herpesvirus-1, feline calicivirus, feline leukemia virus and feline immunodeficiency virus with chronic feline gingivostomatitis. J Fel Med Surg. 2007. Aug 31; [Epub ahead of print]
Roux V, et al. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J Clin Microbiol. 2000;38:1698.
Saunders GK, Monroe WE. Systemic granulomatous disease and sialometaplasia in a dog with Bartonella infection. Vet Pathol. 2006;43:391.
Seubert A, et al. Bacterial persistence within erythrocytes: a unique pathogenic strategy of Bartonella spp. Int J Med Microbiol. 2002;291:555.
Solano-Gallego L, et al. Bartonella henselae IgG antibodies are prevalent in dogs from southeastern USA. Vet Res. 2004;35:585.
Sykes JE, et al. Evaluation of the relationship between causative organisms and clinical characteristics of infective endocarditis in dogs: 71 cases (1992-2005). J Am Vet Med Assoc. 2006;228:1723.
Tuttle AD, et al. Concurrent bartonellosis and babesiosis in a dog with persistent thrombocytopenia. J Am Vet Med Assoc. 2003;223:1306.
Vissotto De Paiva Diniz PP, et al. Canine bartonellosis: serological and molecular prevalence in Brazil and evidence of co-infection with Bartonella henselae and Bartonella vinsonii subsp berkhoffii. Vet Res. 2007;38:697.
Windsor RC, et al. Molecular detection of microbes in nasal tissue of dogs with idiopathic lymphoplasmacytic rhinitis. J Vet Intern Med. 2006;20:250.
Biek R, et al. Factors associated with pathogen seroprevalence and infection in Rocky Mountain cougars. J Wildl Dis. 2006;42:606.
Eidson M, et al. Feline plague in New Mexico: risk factors and transmission to humans. Am J Publ Health. 1988;78:1333.
Eidson M, et al. Clinical, clinicopathologic, and pathologic features of plague in cats: 119 cases (1977–1988). J Am Vet Med Assoc. 1991;199:1191.
Gage KL, et al. Cases of cat-associated human plague in the Western US, 1977-1998. Clin Infect Dis. 2000;30:893.
Gasper PW, et al. Plague (Yersinia pestis) in cats: description of experimentally induced disease. J Med Entomol. 1993;30:20.
Kirkpatrick C. Plague. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 2. Philadelphia: WB Saunders; 1998:806.
Orloski KA, et al. Yersinia pestis infection in three dogs. J Am Vet Med Assoc. 1995;207:316.
Watson RP, et al. Histopathology of experimental plague in cats. Vet Pathol. 2001;38:165.
Welch TJ, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS ONE. 2007;2:e309.
Adin CA, et al. Treatment and outcome of dogs with leptospirosis: 36 cases (1990–1998). J Am Vet Med Assoc. 2000;216:371.
Andre-Fontaine G, et al. Comparison of the efficacy of three commercial bacterins in preventing canine leptospirosis. Vet Rec. 2003;153:165.
Barr SC, et al. Serologic responses of dogs given a commercial vaccine against Leptospira interrogans serovar pomona and Leptospira kirschneri serovar grippotyphosa. Am J Vet Res. 2005;66:1780.
Batza HJ, et al. Occurrence of Leptospira antibodies in cat serum samples. Kleintierpraxis. 1987;32:171.
Boutilier P, et al. Leptospirosis in dogs: a serologic survey and case series 1996 to 2001. Vet Ther. 2003;4:387.
Brod, et al. Evidence of dog as a reservoir for human leptospirosis: a serovar isolation, molecular characterization and its use in a serological survey. Rev Soc Bras Med Trop. 2005;38:294.
Brown CA, et al. Leptospira interrogans serovar grippotyphosa infection in dogs. J Am Vet Med Assoc. 1996;209:1265.
Geisen V, et al. Canine leptospirosis infections—clinical signs and outcome with different suspected Leptospira serogroups (42 cases). J Small Anim Pract. 2007;48:324.
Ghneim GS, et al. Use of a case-control study and geographic information systems to determine environmental and demographic risk factors for canine leptospirosis. Vet Res. 2007;38:37.
Goldstein RE, et al. Influence of infecting serogroup on clinical features of leptospirosis in dogs. J Vet Intern Med. 2006;20:489.
Greene CE. Leptospirosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 2. Philadelphia: WB Saunders; 1998:588.
Greenlee JJ, et al. Experimental canine leptospirosis caused by Leptospira interrogans serovars pomona and Bratislava. Am J Vet Res. 2005;66:1816.
Harkin KR, et al. Comparison of polymerase chain reaction assay, bacteriologic culture, and serologic testing in assessment of prevalence of urinary shedding of leptospires in dogs. J Am Vet Med Assoc. 2003;222:1230.
Harkin KR, et al. Clinical application of a polymerase chain reaction assay for diagnosis of leptospirosis in dogs. J Am Vet Med Assoc. 2003;222:1224.
Mastrorilli C, et al. Clinicopathologic features and outcome predictors of Leptospira interrogans Australis serogroup infection in dogs: a retrospective study of 20 cases (2001–2004). J Vet Intern Med. 2007;21:3.
Miller MD et al: Variability in the microscopic agglutination test for the diagnosis of leptospirosis in dogs, Proceedings of the ACVIM Forum, Seattle, June 2007.
Moore GE, et al. Canine leptospirosis, United States, 2002-2004. Emerg Infect Dis. 2006;12:501.
Rentko VT, et al. Canine leptospirosis. J Vet Intern Med. 1992;6:235.
Schreiber P, et al. Prevention of renal infection and urinary shedding in dogs by a Leptospira vaccination. Vet Microbiol. 2005;108:113.
Stokes JE, et al. Prevalence of serum antibodies against six Leptospira serovars in healthy dogs. J Am Vet Med Assoc. 2007;230:1657.
Townsend WM, et al. Leptospirosis and panuveitis in a dog. Vet Ophthalmol. 2006;9:169.
Ward MP, et al. Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998). J Am Vet Med Assoc. 2002;220:53.
Ward MR. Clustering of reported cases of leptospirosis among dogs in the United States and Canada. Prev Vet Med. 2002;56:215.
Ward MP, et al. Evaluation of environmental risk factors for leptospirosis in dogs: 36 cases (1997-2002). J Am Vet Med Assoc. 2004;225:72.
Ward MP, et al. Serovar-specific prevalence and risk factors for leptospirosis among dogs: 90 cases (1997-2002). J Am Vet Med Assoc. 2004;224:1958.
Abou N, et al. PCR-based detection reveals no causative role for Mycoplasma and Ureaplasma in feline lower urinary tract disease. Vet Microbiol. 2006;116:246.
Bannasch MJ, Foley JE. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J Feline Med Surg. 2005;7:109.
Barrs VR, et al. Feline pyothorax: a retrospective study of 27 cases in Australia. J Feline Med Surg. 2005;7:211.
Chalker VJ, et al. Development of a polymerase chain reaction for the detection of Mycoplasma felis in domestic cats. Vet Microbiol. 2004;100:77.
Chalker VJ, et al. Mycoplasmas associated with canine infectious respiratory disease. Microbiol. 2004;150:3491.
Chalker VJ. Canine mycoplasmas. Res Vet Sci. 2005;79:1.
Chandler JC, et al. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999). J Am Anim Hosp Assoc. 2002;38:111.
Doig PA, et al. The genital Mycoplasma and Ureaplasma flora of healthy and diseased dogs. Can J Comp Med. 1981;45:233.
Foster SF, et al. Pneumonia associated with Mycoplasma spp. in three cats. Aust Vet J. 1998;76:460.
Foster SF, et al. Lower respiratory tract infections in cats: 21 cases (1995-2000). J Feline Med Surg. 2004;6:167.
Foster SF, et al. A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995-2000). J Feline Med Surg. 2004;6:189.
Gray LD, et al. Clinical use of 16S rRNA gene sequencing to identify Mycoplasma felis and M. gateae associated with feline ulcerative keratitis. J Clin Microbiol. 2005;43:3431.
Greene CE. Mycoplasmal, ureaplasmal, and l-form infections. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:260.
Gulbahar MY, Gurturk K. Pyothorax associated with a Mycoplasma sp and Arcanobacterium pyogenes in a kitten. Aust Vet J. 2002;80:244.
Hooper PT, et al. Mycoplasma polyarthritis in a cat with probable severe immune deficiency. Aust Vet J. 1985;62:352.
Jameson PH, et al. Comparison of clinical signs, diagnostic findings, organisms isolated, and clinical outcome in dogs with bacterial pneumonia: 93 cases (1986–1991). J Am Vet Med Assoc. 1995;206:206.
Jang SS, et al. Mycoplasma as a cause of canine urinary tract infection. J Am Vet Med Assoc. 1984;185:45.
Johnson LR, et al. A comparison of routine culture with polymerase chain reaction technology for the detection of Mycoplasma species in feline nasal samples. J Vet Diagn Invest. 2004;16:347.
Johnson LR, et al. Assessment of infectious organisms associated with chronic rhinosinusitis in cats. J Am Vet Med Assoc. 2005;227:579.
Kirchner BK, et al. Spontaneous bronchopneumonia in laboratory dogs with untyped Mycoplasma sp. Lab Anim Sci. 1990;40:625.
L’Abee-Lund TM, et al. Mycoplasma canis and urogenital disease in dogs in Norway. Vet Rec. 2003;153:231.
Liehmann L, et al. Mycoplasma felis arthritis in two cats. J Small Anim Pract. 2006;47:476.
Low HC, et al. Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res. 2007;68:643.
McCabe SJ, et al. Mycoplasma infection of the hand acquired from a cat. J Hand Surg. 1987;12:1085.
Moise NS, et al. Mycoplasma gateae arthritis and tenosynovitis in cats: case report and experimental reproduction of the disease. Am J Vet Res. 1983;44:16.
Moise NS, et al. Clinical, radiographic, and bronchial cytologic features of cats with bronchial disease: 65 cases (1980–1986). J Am Vet Med Assoc. 1989;194:1467.
Randolph JF, et al. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and prevalence of mycoplasmal recovery from pharyngeal swab specimens in dogs with or without pulmonary disease. Am J Vet Res. 1993;54:387.
Rycroft AN, et al. Serological evidence of Mycoplasma cynos infection in canine infectious respiratory disease. Vet Microbiol. 2007;120:358.
Senior DF, et al. The role of Mycoplasma species and Ureaplasma species in feline lower urinary tract disease. Vet Clin North Am Small Anim Pract. 1996;26:305.
Slavik MF, et al. Mycoplasma infections of cats. Fel Pract. 1992;20:12.
Stenske DA, et al. Acute polyarthritis and septicemia from Mycoplasma edwardii after surgical removal of bilateral adrenal tumors in a dog. J Vet Intern Med. 2005;19:768.
Ulgen M, et al. Urinary tract infections due to Mycoplasma canis in dogs. J Vet Med Am Physiol Pathol Clin Med. 2006;53:379.
Zeugswetter F, et al. Erosive polyarthritis associated with Mycoplasma gateae in a cat. J Feline Med Surg. 2007;9:226.