CHAPTER 93 Practical Antimicrobial Chemotherapy
Antimicrobial drugs should only be administered if the index of suspicion for an infection exists. The prescribing veterinarian should also always be cognizant of the potential for development of antimicrobial resistance, particularly when prescribing drugs also used in human beings. Veterinarians should be familiar with judicious use of antimicrobial guidelines (www.aahanet.org/About_aaha/AAFP_AAHA_AntimicrobialGuidelines.pdf).
In small animal practice, decisions to institute antimicrobial chemotherapy are almost always made without the benefit of results of culture and antimicrobial susceptibility testing. In simple, first-time infections, culture and antimicrobial susceptibility testing is often not performed. In life-threatening infections, decisions on the choice of antimicrobials must be made before obtaining the culture results; patient survival may depend on the selection of optimal treatment regimens. For some infectious agents such as Ehrlichia spp., Borrelia burgdorferi, Rickettsia rickettsii, and hemoplasmas, the organisms are not readily grown in culture and so empirical therapy is always used.
Recognition of the most common infectious agents (gram positive, gram negative, aerobic, or anaerobic) associated with infection of different organ systems or associated with different clinical syndromes is imperative in the empirical selection of antimicrobials. Cytologic findings and the results of a Gram stain can be used to identify microbes and help choose appropriate antimicrobials. The antimicrobial selected must have an appropriate mechanism of action against the suspected pathogen and must achieve an adequate concentration in infected tissues. Bacteriostatic agents may be less effective for treatment of infections in immunosuppressed animals because normal immune responses are required for the drugs to have maximal effect (Table 93-1). The owner must be willing to administer the drug in the appropriate interval, and the drug must be affordable. Whether the antimicrobial has potential for toxicity is also an important consideration (Table 93-2). In animals with simple, first-time infections or when drugs with the potential for toxicity are used, the low end of the antimicrobial dose and the longest dosage interval should be used. Intracellular pathogens, anaerobic infections, and life-threatening infections, including bacteremia and central nervous system (CNS) infections, should be treated with the high end of the dose and the shortest dosage interval. In all animals with life-threatening infections, antibiotics should be administered parenterally for at least the first 3 to 5 days. Parenteral antibiotic administration is also indicated in animals with vomiting or regurgitation. Oral administration of antibiotics can be initiated when vomiting, regurgitation, or the life-threatening condition have resolved.
 TABLE 93-1 Antibiotics Used for the Treatment of Bacterial Infections in Dogs and Cats and General Dosing Guidelines*
TABLE 93-1 Antibiotics Used for the Treatment of Bacterial Infections in Dogs and Cats and General Dosing Guidelines*
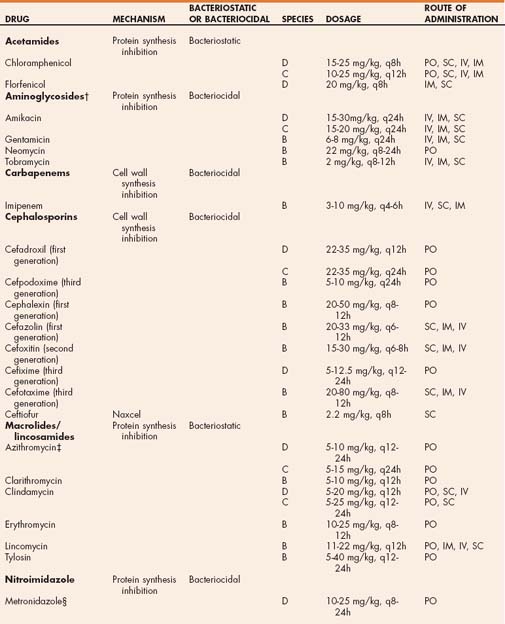
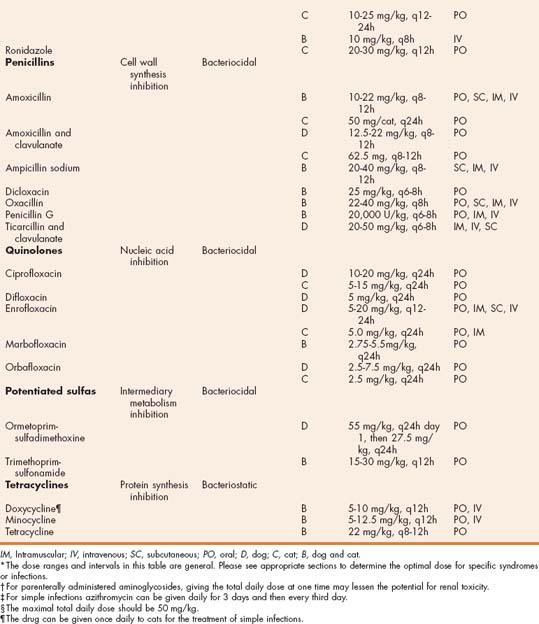
 TABLE 93-2 Common Antibiotic Toxicities
TABLE 93-2 Common Antibiotic Toxicities
| TOXICITY | ANTIBIOTIC EXAMPLES |
|---|---|
| Aminoglycosides | |
| Cephalosporins | Immune-mediated diseases |
| Chloramphenicol | |
| Doxycycline | Esophagitis or strictures in cats given tablets or capsules |
| Macrolides/lincosamides | |
| Nitroimidazoles | |
| Penicillins | Immune-mediated diseases |
| Quinolones | |
| Sulfonamides | |
| Tetracyclines |
CNS, Central nervous system.
Most simple, first-time infections in immunocompetent animals respond adequately to 7 to 10 days of antibiotic therapy. Therapy is generally continued for no more than 1 to 2 days past resolution of clinical signs. Chronic infections, bone infections, infections in immunosuppressed animals, infections resulting in granulomatous reactions, and those caused by intracellular pathogens are generally treated for a minimum of 1 to 2 weeks beyond resolution of clinical or radiographic signs of disease; the duration of therapy commonly exceeds 4 to 6 weeks.
When the results of antimicrobial susceptibility tests become available, the antibiotic choice is changed if indicated. If therapeutic response to an antibiotic in 72 hours is poor and an antibiotic-responsive infectious disease is still likely, an alternative treatment should be considered. Veterinarians should always know at least two classic drugs for each infectious agent (Tables 93-3 to 93-7).
 TABLE 93-3 Empirical Antibiotic Choices for Dogs and Cats with Cutaneous and Soft Tissue Infections
TABLE 93-3 Empirical Antibiotic Choices for Dogs and Cats with Cutaneous and Soft Tissue Infections
| INFECTIOUS AGENT | ANTIBIOTIC CHOICES |
|---|---|
| Abscesses (anaerobes) | |
| Actinomyces | |
| Gram-negative or resistant pyoderma | 1. Quinolones |
| L-form bacteria | |
| Nocardia | |
| Rapidly growing Mycobacterium | |
| Staphylococcal pyoderma |
 TABLE 93-4 Empirical Antibiotic Choices for Dogs and Cats with Cardiopulmonary Infections
TABLE 93-4 Empirical Antibiotic Choices for Dogs and Cats with Cardiopulmonary Infections
| ORGAN SYSTEM OR INFECTIOUS AGENT | ANTIBIOTIC CHOICES |
|---|---|
| Bacterial pneumonia | |
| Bacterial pneumonia with bacteremia* | |
| Bacteremia, sepsis, and bacterial endocarditis |
1. Enrofloxacin and penicillin (or ampicillin or amoxicillin or clindamycin or first- generation cephalosporin)
|
| Pyothorax* | |
| Toxoplasmosis/neosporosis | |
| Upper respiratory |
* Generally mixed infections, often with gram-negative, gram-positive, aerobic, and anaerobic combinations. If signs of bacteremia or sepsis are present, use a four-quadrant antibiotic choice administered parenterally as discussed for sepsis until culture and antimicrobial susceptibility testing results return.
† Should be used if Bordetella, Mycoplasma, or Chlamydophila are suspected.
 TABLE 93-5 Empirical Antibiotic Choices for Dogs and Cats with Hepatic and Gastrointestinal Infections
TABLE 93-5 Empirical Antibiotic Choices for Dogs and Cats with Hepatic and Gastrointestinal Infections
| INFECTIOUS AGENT | ANTIBIOTIC CHOICES |
|---|---|
| Bacterial cholangiohepatitis | |
| Bacterial overgrowth | |
| Campylobacter spp. | |
| Clostridium perfringens | |
| Helicobacter spp. | |
| Hepatic encephalopathy | |
| Salmonella spp.* |
* Usually administered parenterally for the treatment of bacteremia.
 TABLE 93-6 Empirical Antibiotic Choices for Dogs and Cats with CNS and Musculoskeletal Infections
TABLE 93-6 Empirical Antibiotic Choices for Dogs and Cats with CNS and Musculoskeletal Infections
| ORGAN SYSTEM OR INFECTIOUS AGENT | ANTIBIOTIC CHOICES |
|---|---|
| CNS | |
| Encephalitis | |
| Otitis media/interna | |
| Toxoplasmosis/neosporosis | |
| Musculoskeletal | |
| Discospondylitis | |
| Hepatozoonosis | |
| Osteomyelitis | |
| Toxoplasmosis/neosporosis | |
| Polyarthritis | |
| Bartonella vinsonii | |
| Borrelia burgdorferi | |
| Ehrlichia/Anaplasma | |
| L-form bacteria or Mycoplasma | |
| Rickettsia rickettsii |
 TABLE 93-7 Empirical Antibiotic Choices for Dogs and Cats with Urogenital Infections
TABLE 93-7 Empirical Antibiotic Choices for Dogs and Cats with Urogenital Infections
| ORGAN SYSTEM OR INFECTIOUS AGENT | ANTIBIOTIC CHOICES |
|---|---|
| Aerobic urinary tract infections | |
| Bruceila canis | |
| Leptospira spp. | |
| Mastitis | |
| Mycoplasma/Ureaplasma | |
| Prostatitis | |
| Pyometra |
IV, Intravenous; PO, oral.
CNS, Central nervous system.
Conditions resulting in devitalized, granulomatous, or consolidated tissues, such as aspiration pneumonia, may not show radiographic signs of improvement before 7 days. Devitalized tissues should be debrided, if possible, to aid in the resolution of infection.
The following is a brief discussion of the empirical antimicrobial choices for treatment of infections of various body systems or types of infections. The reader is referred to individual chapters for further information concerning adjunct treatments.
ANAEROBIC INFECTIONS
The anaerobic bacteria of clinical significance in dog and cats are Actinomyces spp., Bacteroides spp., Clostridium spp., Eubacterium spp., Fusobacterium spp., Peptostreptococcus spp., and Porphyromonas spp. Actinomyces is a facultative anaerobe; the other organisms are obligate anaerobes, which cannot use oxygen metabolically and die in its presence. Anaerobic bacteria are part of the normal flora in areas with low oxygen tension and low oxygen-reduction potential, such as the mucous membranes of the oral cavity and vagina. The origin of most anaerobic infections is the animal’s own flora. Anaerobic infections are potentiated by poor blood supply, tissue necrosis, prior infection, or immunosuppression. Anaerobic bacteria produce a number of enzymes and factors that induce tissue injury and promote colonization. Most infections involving anaerobes usually have coexisting aerobic bacterial infection, which should be considered when selecting antimicrobial agents.
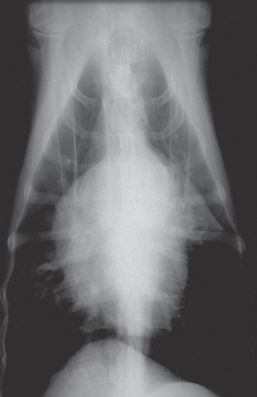
Anaerobic infections are commonly associated with infections of the oropharynx, CNS, subcutaneous space, musculoskeletal system, gastrointestinal tract, liver, and female genital tract, and they are relatively common in animals with aspiration pneumonia or consolidated lung lobes (Fig. 93-1). Dogs and cats with gingivitis or stomatitis, rhinitis, retrobulbar abscesses, retropharyngeal abscesses, aspiration pneumonia, pyothorax, otitis media or interna, CNS infection, bite wounds, open wounds, open fractures, osteomyelitis, peritonitis, bacterial hepatitis, pyometra, vaginitis, bacteremia, and valvular endocarditis should be suspected to be infected with anaerobes. Anaerobic infections also should be considered in animals with a history of fighting, a foreign body, recent surgery, recent dental procedures, a history of immunosuppressive drugs or diseases, infections resistant to aminoglycosides or fluoroquinolones, lesions with a putrid odor or black discharge, a painful lesion with a serosanguineous discharge, neutrophilic inflammation with cytologically evident bacteria but negative aerobic culture, and the presence of “sulfur granules” on cytology. The reader is referred to Chapter 92 for a discussion of the cytologic and cultural characteristics of anaerobic infections. Flaccid paralysis (Clostridium botulinum), rigid paralysis and trismus (Clostridium tetani) and subcutaneous gas production occur in association with some anaerobic infections.
Improving the blood supply and oxygenation of the infected area is the primary goal for treatment of anaerobic infections. Antibiotic therapy should be used concurrently with drainage or debridement. Parenteral antibiotics should be administered for several days in dogs or cats with pyothorax, pneumonia, peritonitis, or clinical signs consistent with bacteremia. Ampicillin, amoxicillin, amoxicillin-clavulanate, clindamycin, metronidazole, cephalosporins (first and second generation), potentiated sulfas, and chloramphenicol are commonly used for the treatment of anaerobic infections (see Table 93-3). Bacteroides spp. are commonly resistant to ampicillin and clindamycin, so if gram-negative coccobacilli are detected cytologically in a neutrophilic exudate—particularly if associated with the oral cavity—metronidazole, a first-generation cephalosporin, or amoxicillin-clavulanate should be administered instead of a penicillin derivative. Because concurrent anaerobic and aerobic infections occur frequently, combination antimicrobial treatment is often indicated, particularly if life-threatening signs of bacteremia exist.
BACTEREMIA AND BACTERIAL ENDOCARDITIS
Bacteremia can be transient, intermittent, or continuous. Routine dentistry is a common cause of transient bacteremia. Immunosuppressed or critically ill animals commonly develop intermittent bacteremia; the source of infection is commonly the genitourinary or gastrointestinal systems. Continuous bacteremia occurs most frequently in association with bacterial endocarditis. Bacteremic animals have intermittent fever, depression, and clinical signs associated with the primary organ system infected. Sepsis is the systemic response to infection and is manifested by peripheral circulatory failure (septic shock).
Staphylococcus, Streptococcus, Enterococcus, Corynebacterium, Escherichia coli, Salmonella, Klebsiella, Enterobacter, Pseudomonas, Proteus, Pasteurella, Clostridium, Fusobacterium, and Bacteroides organisms are commonly isolated from the blood of bacteremic animals. Bacterial endocarditis is often caused by Staphylococcus aureus, E. coli, or β- hemolytic Streptococcus infection. Bartonella vinsonii (dogs) and B. quintana (cats and dogs; see Chapter 95) have recently been associated with bacterial endocarditis (Sykes et al., 2006).
If the source of bacteremia or bacterial endocarditis is from an area with mixed flora, such as the gastrointestinal tract, or if the animal has life-threatening clinical signs of disease, an antibiotic or combination of antibiotics that is effective against gram-positive, gram-negative, aerobic, and anaerobic organisms should be used (a four-quadrant approach). An aminoglycoside or quinolone for gramnegative organisms combined with ampicillin, a firstgeneration cephalosporin, metronidazole, or clindamycin for gram-positive and anaerobic organisms is a commonly prescribed combination treatment (see Table 93-4). Second- and third-generation cephalosporins, ticarcillin combined with clavulanate, and imipenem are some of the agents with a four-quadrant spectrum. After parenteral treatment with these drugs for 5 to 7 days, oral treatment is selected on the basis of culture and antimicrobial susceptibility results. Optimal treatment for valvular endocarditis from bartonellosis in dogs has not be determined, but the combination of azithromycin and a fluoroquinolone or rifampin may be required in some cases (see Chapter 95). Oral treatment is continued for at least 4 to 6 weeks, particularly in dogs or cats with bacterial endocarditis. The blood culture should be rechecked 1 and 4 weeks after discontinuation of therapy to confirm control of the infection. The prognosis in dogs and cats with bacterial endocarditis is guarded to poor because of damage to the infected heart valves (see Chapter 6).
CENTRAL NERVOUS SYSTEM INFECTIONS
Chloramphenicol, the sulfonamides, trimethoprim, metronidazole, and the quinolones penetrate the CNS and should be chosen for empirical treatment of suspected bacterial infections of this system (see Table 93-6). Anaerobic bacterial infection and rickettsial infections (Ehrlichia spp. and R. rickettsii) of the CNS occur in some cases, making chloramphenicol a logical first choice. Multiple other drugs, including penicillin derivatives, tetracyclines (doxycycline), and clindamycin, may cross into the cerebrospinal fluid when inflammation exists. Clindamycin achieves adequate brain tissue concentrations in normal cats for the treatment of toxoplasmosis. Potentiated sulfas are alternative anti-Toxoplasma drugs.
GASTROINTESTINAL TRACT AND HEPATIC INFECTIONS
Oral administration of antibiotics is indicated for the treatment of small intestinal bacterial overgrowth, hepatic encephalopathy, cholangiohepatitis, hepatic abscessation, and infection by Helicobacter spp., Campylobacter spp., Clostridium perfringens, Giardia spp., Cryptosporidium spp., Cystoisospora spp., Tritrichomonas foetus, and Toxoplasma gondii (see Table 93-5). Administration of parenteral antibiotics is indicated in dogs and cats with bacteremia from translocation of enteric flora or Salmonella infection.
Giardia spp. infections often respond clinically to the administration of metronidazole, but infection is usually not eliminated. Administration of metronidazole benzoate at 25 mg/kg q12h PO for 7 days was effective in suppressing cyst shedding to below detectable limits in 26 cats (Scorza et al., 2004). This is the maximal dose of metronidazole that should be used; CNS toxicity can be induced by overdosing or as a cumulative neurotoxin. Fenbendazole is the most commonly used alternate drug. Metronidazole also has the advantage of helping correct secondary small intestinal bacterial overgrowth. For T. foetus infections, ronidazole at 30 mg/kg PO q12h for 14 days eliminated clinical signs of disease and trophozoites from cats infected with one strain of the organism. In the United States this drug currently must be purchased from a custom pharmacy. CNS toxicity is also common with ronidazole.
Sequential administration of clindamycin followed by tylosin blocked oocyst shedding and resolved diarrhea in one cat with chronic clinical cryptosporidiosis. Tylosin (10-15 mg/kg q12h PO) has apparently been successful in lessening diarrhea and oocyst shedding in multiple other cats and dogs with diarrhea that were positive for Cryptosporidium. However, infection is not eliminated. Unfortunately, tylosin is quite bitter and usually has to be given to cats in capsules. Treatment duration may need to be weeks. Paromomycin can be effective for lessening diarrhea and oocyst shedding associated with cryptosporidiosis in cats and also is an alternate anti-Giardia drug. However, this orally administered aminoglycoside may cross the diseased intestinal wall and result in renal toxicity and should never be used in cats with bloody diarrhea. In cats with naturally occurring cryptosporidiosis, response to azithromycin has been variable (Lappin MR, unpublished data, 2005). If tried, use 10 mg/kg PO weekly for at least 10 days. If responding, continue treatment for at least 1 week past clinical resolution. The Toxoplasma gondii oocyst shedding period can be shortened by administration of clindamycin or sulfadimethoxine. Cystoisospora spp. generally respond to the administration of sulfadimethoxine or other sulfa-containing drugs.
Clostridium perfringens and bacterial overgrowth generally respond to treatment with tylosin, metronidazole, ampicillin, amoxicillin, or tetracyclines. The drug of choice for campylobacteriosis is erythromycin; however, oral administration of quinolones or chloramphenicol are often less likely to potentiate vomiting. Salmonellosis should only be treated parenterally because of rapid resistance that occurs after oral administration of antibiotics. Appropriate antibiotics for the empirical treatment of salmonellosis while awaiting susceptibility testing results include ampicillin and trimethoprim-sulfa; quinolones are also effective. Helicobacter spp. infections are usually treated with the combination of metronidazole and tetracycline or amoxicillin and clarithromycin. In cats the use of clarithromycin alone may be logical because the species is often difficult to treat with multiple drugs.
Dogs or cats with apparent bacteremia from enteric bacteria should be treated with parenteral antibiotics with a spectrum against anaerobic and gram-negative organisms. The combination of enrofloxacin with a penicillin or first-generation cephalosporin is generally effective. Intravenous metronidazole can also be used. Second-generation cephalosporins or imipenem is also an appropriate choice.
The most common bacteria in one study of hepatic infections were E. coli, Enterococcus, Streptococcus, Clostridium, and Bacteroides (Wagner et al., 2007). Dogs or cats with hepatic infections and signs of bacteremia should be treated with antibiotics that kill gram-positive, gram-negative, and anaerobic bacteria, as previously discussed. Nonbacteremic hepatic infections generally respond to amoxicillinclavulanate, first-generation cephalosporins, or metronidazole; a fluoroquinolone should be added if signs of sepsis are present. Decreasing numbers of enteric flora by oral administration of penicillins, metronidazole, or neomycin can lessen the clinical signs of hepatic encephalopathy.
MUSCULOSKELETAL INFECTIONS
Osteomyelitis and discospondylitis are commonly associated with infections by Staphylococcus, Streptococcus, Proteus, Pseudomonas spp., E. coli, and anaerobes. First-generation cephalosporins, amoxicillin-clavulanate, and clindamycin are logical antibiotics for empirical therapy of these conditions because of their spectrum of activity against the gram-positive organisms and anaerobic bacteria and their ability to achieve high concentrations in bone (see Table 93-6). Quinolones should be used if gram-negative organisms are suspected. Antibiotic treatment should be continued for a minimum of 2 weeks beyond resolution of radiographic changes.
Dogs and cats with septic polyarthritis should be treated in the same way as those with osteomyelitis. The source of infection should be removed if possible. Bartonella vinsonii, Ehrlichia spp., Rickettsia rickettsii, Borrelia burgdorferi, Mycoplasma organisms, and l-form bacteria can induce nonseptic, suppurative polyarthritis. Occasionally, morulae of Ehrlichia spp. are identified cytologically in the joint fluid. In general, the cytologic findings in joint fluid induced by these agents are similar to those of immune-mediated polyarthritis. For this reason doxycycline is a logical empirical antibiotic choice for dogs with nonseptic, suppurative polyarthritis pending the results of further diagnostic tests. Amoxicillin is an alternative drug for the treatment of B. burgdorferi infection. Fluoroquinolones can also be used for R. rickettsii, Mycoplasma, and l-form bacteria infections. B. vinsonii infection may require the administration of azithromycin, with or without concurrent fluoroquinolones.
Muscle disease from T. gondii infection often resolves during treatment with clindamycin hydrochloride. Although many dogs with neosporosis die, some have survived after treatment with trimethoprim-sulfadiazine combined with pyrimethamine; sequential treatment with clindamycin hydrochloride, trimethoprim-sulfadiazine, and pyrimethamine; or clindamycin alone. For treatment of acute Hepatozoon americanum infection, the combination of trimethoprim-sulfadiazine, pyrimethamine, and clindamycin for 14 days is highly successful; the use of decoquinate at 10 to 20 mg/kg q12h with food lessens the likelihood of recurrence of clinical disease and prolongs survival time.
RESPIRATORY TRACT INFECTIONS
Most bacterial upper respiratory infections are secondary to other primary diseases, including foreign bodies, viral infections, tooth root abscesses, neoplasms, trauma, and fungal infections. After the epithelium of the nose and sinuses is inflamed, normal bacterial flora can colonize and perpetuate inflammation; deep infection can result in chondritis and osteomyelitis. Because the upper respiratory passageways have a normal flora, it is difficult to assess the results of culture and antimicrobial susceptibility testing in these tissues. The source of the primary insult should always be removed if possible. Broad-spectrum antibiotics with an anaerobic spectrum, including amoxicillin, amoxicillinclavulanate, potentiated sulfas, and first-generation cephalosporins, are commonly prescribed empirically to treat upper respiratory infections caused by normal flora overgrowth (see Table 93-4). Treatment duration is generally 1 to 2 weeks for acute, first-time infections. Dogs and cats with chronic rhinitis and suspected osteochondritis that respond to antibiotics should be treated for a minimum of 4 to 6 weeks or until clinical signs have been resolved for 2 weeks. Chronic rhinitis often responds to treatment with clindamycin because of the excellent anaerobic and gram-positive spectrum and its ability to penetrate cartilage and bone well. Bordetella bronchiseptica, Mycoplasma spp., and Chlamydophila felis infection of cats are primary bacterial pathogens that infect the upper respiratory tissues. If the animal responds poorly to broad-spectrum antibiotics, doxycycline, azithromycin, chloramphenicol, or quinolones can be administered; Chlamydophila, Bordetella, and Mycoplasma organisms generally respond to these drugs. Epistaxis can be caused by B. vinsonii, E. canis, and R. rickettsii. No evidence currently supports Bartonella spp. as a cause of rhinitis in cats. Canine bartonellosis often fails to respond to the administration of doxycycline but can be successfully treated with azithromycin. Canine kennel cough syndrome caused by Bordetella or Mycoplasma spp. is usually effectively treated with doxycycline, chloramphenicol, quinolones, or amoxicillin-clavulanate. Bacterial bronchitis in cats generally responds to administration of amoxicillin-clavulanate. In dogs and cats with chronic bronchitis, doxycycline, chloramphenicol, quinolones, or amoxicillin-clavulanate are rational empirical antibiotic choices.
Common bacteria associated with pneumonia in dogs include E. coli, Klebsiella spp., Pasteurella spp., Pseudomonas spp., B. bronchiseptica, Streptococcus spp., Staphylococcus spp., and Mycoplasma spp. In cats, Bordetella, Pasteurella, and Mycoplasma organisms are commonly isolated. Aspiration of gastrointestinal contents is a common cause of bacterial pneumonia with a mixed population of bacteria. Multiple species of bacteria are typically cultured from dogs and cats with bronchopneumonia. B. bronchiseptica is the most important primary pathogen in dogs and cats; most other bacteria colonize after airways have been previously damaged. If consolidated lung lobes are detected radiographically, an anaerobic infection should be assumed. Whether species of Mycoplasma infecting dogs and cats are capable of being primary respiratory pathogens is unknown. Chlamydophila infection in cats is not a common cause of lower respiratory tract infection. Yersinia pestis causes pneumonia in cats in western states (see Chapter 100); aminoglycosides, tetracycline derivatives, and quinolones can be used successfully.
In dogs and cats with bacterial pneumonia, culture and antimicrobial susceptibility testing should be performed on secretions collected by transtracheal wash or bronchoalveolar lavage. If the animal shows signs of bacteremia or if radiographic evidence of consolidated lung lobes is present, parenteral administration of a four-quadrant antibiotic choice, as previously discussed for bacteremia, should be used initially. Quinolones combined with clindamycin or azithromycin or chloramphenicol alone is a good choice for animals with consolidated lung lobes because of their broad spectrum, excellent tissue penetration, and efficacy against B. bronchiseptica (see Table 93-5). In animals with pneumonia but without clinical signs of bacteremia or consolidated lung lobes, broad-spectrum antibiotics, including amoxicillin, amoxicillin-clavulanate, potentiated sulfas, and firstgeneration cephalosporins, may be effective. Surface-dwelling organisms such as B. bronchiseptica and Mycoplasma may respond to nebulization of gentamicin diluted in sterile saline (25 to 50 mg in 3 to 5 mL saline/nebulization). Treatment for bacterial pneumonia should be continued for at least 4 weeks or for 1 to 2 weeks beyond resolution of clinical and radiographic signs of disease.
T. gondii occasionally causes pneumonia in neonatally infected, transplacentally infected, and immunosuppressed cats and dogs (see Chapter 99). Clindamycin or potentiated sulfas should be used if toxoplasmosis is suspected. Azithromycin may also be effective for the treatment of toxoplasmosis. Neospora caninum has occasionally been associated with pneumonia in dogs and should be treated with a combination of clindamycin and potentiated sulfas.
If pyothorax is attributable to penetration of foreign material from an airway or esophagus into the pleural space, thoracotomy is usually required for removal of devitalized tissue and the foreign body (see Chapter 23). Pyothorax occasionally results from hematogenous spread of bacteria to the pleural space; this may be common in cats. Pleural lavage through chest tubes is the most effective treatment for patients with pyothorax and no obvious foreign material. Most dogs and cats with pyothorax have mixed aerobic and anaerobic bacterial infections. Animals with pyothorax and clinical signs of bacteremia should initially receive parenteral four-quadrant antibiotics, as previously discussed for bacteremia.
SKIN AND SOFT TISSUE INFECTIONS
Staphylococcus pseudointermedius is the most common cause of pyoderma in dogs and cats. Deep pyoderma can be induced by any organism, including gram-negative types. Most soft tissue infections, including open wounds and abscesses, are infected with a mixed population of bacteria; the aerobic and anaerobic flora from the mouth are often involved. Recommended empirical antibiotic choices for routine cases of pyoderma and soft tissue infections are listed in Table 93-3. Antibiotics with a broad spectrum, such as first-generation cephalosporins and amoxicillin-clavulanate, are often first choices. Other β-lactamase–resistant penicillins, such as oxacillin, dicloxacillin, and cloxacillin, also can be used. Potentiated sulfas can be used to treat dogs and cats with superficial pyoderma but should be avoided if long-term treatment is needed because bacterial resistance occurs quickly. Cutaneous and soft tissue infections that do not respond to these antibiotics may be caused by gram-negative bacteria, l-form bacteria, Mycoplasma organisms, rapidly growing Mycobacterium spp., systemic fungi, or Sporothrix schenckii. Quinolones are the antibiotic class of choice for the treatment of gram-negative infections. Animals that do not respond to empirical antibiotic treatment should undergo further diagnostic testing or be treated with antibiotics known to have an effect against the less-common pathogens (see Table 93-3). If not previously done, microscopic examination of tissue or pustule aspirates should be performed for the presence of Sporothrix organisms and bacteria morphologically similar to Mycobacterium spp. After surgical preparation of the skin, deep tissues should be obtained for aerobic, anaerobic, Mycoplasma, fungal, and atypical Mycobacterium spp. culture (see Chapter 92).
UROGENITAL TRACT INFECTIONS
Microscopic examination and Gram stain of urine sediment aids in the empirical choice of an antibiotic in dogs and cats with signs of urinary tract infection. Culture and antimicrobial susceptibility testing should always be performed if possible. Approximately 75% of urinary tract infections in dogs are caused by gram-negative organisms; E. coli, Proteus, Klebsiella, Pseudomonas, and Enterobacter infections are common. In cats that have been previously catheterized, E. coli is most common; Staphylococcus and Streptococcus organisms are common after urethrostomy (see Chapter 45).
In bitches with simple, first-time urinary tract infections, amoxicillin or amoxicillin-clavulanate should be used if cocci are observed; potentiated sulfas or first-generation cephalosporins should be used if rods are observed. Quinolones should be reserved for life-threatening or resistant infections. Many antibiotics do not penetrate the prostate unless it is markedly inflamed. Because the prostate can be a source of recurrent urinary tract infection, all male dogs with urinary tract infection should be assumed to have prostatitis, and antibiotics that penetrate the prostate should be chosen (see Table 93-7). The majority of urinary tract infections in cats respond to amoxicillin. Administration of antibiotics for 10 to 14 days is generally sufficient for simple urinary tract infections. Urinalysis, culture, and antimicrobial susceptibility testing should be performed 7 days after finishing treatment if possible.
Mycoplasma and Ureaplasma infections have been documented in dogs with clinical signs of urinary tract infections. If poor response to penicillin derivatives, cephalosporins, or potentiated sulfas is observed, further diagnostics should be performed. If empirical therapy is deemed necessary, chloramphenicol, doxycycline, or quinolone treatment can be administered and may be more effective for Mycoplasma and Ureaplasma organisms.
All dogs and cats with urinary tract infection and azotemia should be assumed to have pyelonephritis and be treated accordingly, even if further diagnostic procedures are not performed. Treatment for pyelonephritis should be based on susceptibility results if possible; potentiated sulfa combinations or quinolones are good empirical choices. If Leptospira spp. infection is suspected, intravenous administration of ampicillin is indicated (see Chapter 95). If renal insufficiency exists, the tetracyclines (except doxycycline) and aminoglycosides should be avoided, and the dosage or dosing interval of quinolones and cephalosporins should be extended proportionally to the diminution in renal function. The new dosage can be calculated by multiplying the current dosage by the result obtained when the mean normal creatinine concentration is divided by the patient’s creatinine concentration. The new dosing interval can be calculated by multiplying the current dosing interval by the result obtained when the patient’s creatinine concentration is divided by the mean normal creatinine concentration. Treatment for pyelonephritis and other chronic, complicated urinary tract infections should be continued for at least 6 weeks. Urinalysis, culture, and antimicrobial susceptibility testing should be performed 7 and 28 days after treatment. Some infections cannot be eliminated and require administration of pulse antibiotic therapy.
Most bacterial prostatic infections involve gram-negative bacteria. During acute prostatitis almost all antibiotics penetrate the prostate well because of inflammation. After reestablishment of the blood-prostate barrier in dogs with chronic prostatitis, the acidic prostatic fluid allows only the basic antibiotics (pKa less than 7) to penetrate well (see Table 93-7). Chloramphenicol, because of its high lipid solubility, also penetrates prostatic tissue well. In acute prostatitis administration of acidic antibiotics, including penicillins and first-generation cephalosporins, may initially penetrate well, lessening clinical signs of disease but not eliminating the infection. This predisposes to chronic bacterial prostatitis and prostatic abscessation. For this reason the use of penicillins and first-generation cephalosporins is contraindicated for the treatment of urinary tract infections in male dogs. In dogs with chronic prostatitis antimicrobial therapy should be continued for at least 6 weeks. Urine and prostatic fluid should be cultured 7 days and 28 days after therapy.
Brucella canis causes a number of clinical syndromes in dogs, including epididymitis, orchitis, endometritis, stillbirths, abortion, discospondylitis, and uveitis. Ovariohysterectomy or neutering lessens contamination of the human environment. (See Chapter 100 for a discussion of the zoonotic potential.) Long-term antibiotic administration usually does not lead to a complete cure (Wanke et al., 2006). Some dogs become antibody-negative, but the organism can still be cultured from tissues. Several antibiotic protocols have been suggested for dogs with brucellosis (see Table 93-7). However, owners should be carefully counseled concerning zoonotic risks before initiating treatment.
Vaginitis generally results from overgrowth of normal flora secondary to primary diseases, including herpesvirus infection, urinary tract infection, foreign bodies, vulvar or vaginal anomalies, vaginal or vulvar masses, or urinary incontinence. In dogs and cats with bacterial vaginitis from overgrowth of flora and resolution of the primary insult, broad-spectrum antibiotics, including amoxicillin, potentiated sulfas, first-generation cephalosporins, tetracycline derivatives, and chloramphenicol, are typically successful. Because Mycoplasma and Ureaplasma organisms are part of the normal vaginal flora, providing a clinical disease association is virtually impossible; positive cultures do not confirm disease because of the organism (see Chapter 95). Hence a positive vaginal culture from an asymptomatic dog (excluding B. canis) is meaningless.
In all dogs and cats with pyometra, ovariohysterectomy or medically induced drainage of the uterus is imperative. Antibiotic treatment is for the bacteremia that commonly occurs concurrently (i.e., E. coli and anaerobes). Animals with clinical signs of bacteremia or sepsis should be treated with a four-quadrant antibiotic choice (see Table 93-5). Broad-spectrum antibiotics with efficacy against E. coli, such as potentiated sulfas or amoxicillin-clavulanate, are appropriate empirical choices pending the results of culture and antimicrobial susceptibility testing. Potentiated sulfas and the quinolones commonly are effective for E. coli but are not as effective as other drugs for the treatment of anaerobic infections in vivo.
Ampicillin, amoxicillin, and first-generation cephalosporins achieve good concentrations in milk and are relatively safe for the neonate; therefore they can be used in the empirical treatment of mastitis. Chloramphenicol, quinolones, and tetracycline derivatives should be avoided because of potential adverse effects on the neonate.
Brady CA, et al. Severe sepsis in cats: 29 cases (1986–1998). J Am Vet Med Assoc. 2000;217:531.
Breitschwerdt EB, et al. Clinicopathological abnormalities and treatment response in 24 dogs seroreactive to Bartonella vinsonii (berkhoffii) antigens. J Am Anim Hosp Assoc. 2004;40:92-101.
Chandler JC, et al. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999). J Am Anim Hosp Assoc. 2002;38:111.
Freitag T, et al. Antibiotic sensitivity profiles do not reliably distinguish relapsing or persisting infections from reinfections in cats with chronic renal failure and multiple diagnoses of Escherichia coli urinary tract infection. J Vet Intern Med. 2006;20:245.
Greiner M, et al. Bacteraemia and antimicrobial susceptibility in dogs. Vet Rec. 2007;160:529.
Jameson PH, et al. Comparison of clinical signs, diagnostic findings, organisms isolated, and clinical outcome in dogs with bacterial pneumonia: 93 cases (1986–1991). J Am Vet Med Assoc. 1995;206:206.
Jang SS, et al. Organisms isolated from dogs and cats with anaerobic infections and susceptibility to selected antimicrobial agents. J Am Vet Med Assoc. 1997;210:1610.
Johnson JR, et al. Assessment of infectious organisms associated with chronic rhinosinusitis in cats. J Am Vet Med Assoc. 2005;227:579.
Radhakrishnan A, et al. Community-acquired infectious pneumonia in puppies: 65 cases (1993-2002). J Am Vet Med Assoc. 2007;230:1493.
Scorza V, Lappin MR. Metronidazole for treatment of giardiasis in cats. J Fel Med Sung. 2004;6:157.
Sykes JE, et al. Evaluation of the relationship between causative organisms and clinical characteristics of infective endocarditis in dogs: 71 cases (1992-2005). J Am Vet Med Assoc. 2006;228:1723.
Ulgen M, et al. Urinary tract infections due to Mycoplasma canis in dogs. J Vet Med A Physiol Pathol Clin Med. 2006;53:379.
Wagner KA, et al. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998-2003. J Vet Intern Med. 2007;21:417.
Walker AL, et al. Bacteria associated with pyothorax of dogs and cats: 98 cases (1989–1998). J Am Vet Med Assoc. 2000;216:359.
Wanke MM, et al. Use of enrofloxacin in the treatment of canine brucellosis in a dog kennel (clinical trial). Theriogenology. 2006;66:1573.