CHAPTER 85 Leukopenia and Leukocytosis
GENERAL CONSIDERATIONS
The leukogram, evaluated as part of the complete blood count (CBC), includes a quantification of the total number of white blood cells (WBCs) and the differential WBC count. Although a specific disorder is rarely diagnosed on the basis of a leukogram, the information obtained may be useful in limiting the number of differential diagnoses or in predicting the severity of the disease and its prognosis. Sequential leukograms may also be helpful in monitoring a patient’s response to therapy.
According to standard laboratory techniques, all nucleated cells are counted during a WBC count, including nucleated red blood cells (nRBCs). Differential leukograms determined by particle counters used at human referral laboratories are not valid for cats and dogs. New veterinary benchtop analyzers (LaserCyte, IDEXX, Westbrook, Maine; and CBC-Diff, Heska Corporation, Fribourg, Switzerland) provide reliable WBC total and differential counts. The LaserCyte provides a five-part differential WBC count (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), whereas the CBC-Diff provides a three-part differential count. As a general rule, when a benchtop hematology analyzer yields values outside the reference range or the values are flagged, the clinician or a technician should carefully examine a blood smear.
Leukocytosis occurs if the WBC count exceeds the upper limit of normal for the species; leukopenia occurs if the WBC count is below the reference range. In some breeds of dogs (Belgian Tervuren, Greyhound) the WBC and neutrophil counts are frequently below the reference range for the species, thus resulting in an erroneous diagnosis of leukopenia and neutropenia in an otherwise healthy dog.
A differential WBC count may be reported in either relative (percentages) or absolute numbers (number of cells per microliter). However, the absolute leukocyte numbers, not the percentages, should always be evaluated because the latter may be misleading, particularly if the WBC count is very high or very low. For example, a total WBC of 3000/μL (or 3 × 109/L) and a differential WBC count of 90% lymphocytes and 10% neutrophils can lead to one of the following two conclusions:
The latter obviously reflects the actual clinical situation. The clinician should then concentrate on determining the cause of the neutropenia and ignore the normal lymphocyte count.
NORMAL LEUKOCYTE MORPHOLOGY AND PHYSIOLOGY
From a morphologic standpoint, leukocytes can be classified as either polymorphonuclear or mononuclear. Polymorphonuclear cells include the neutrophils, eosinophils, and basophils; the mononuclear cells include the monocytes and lymphocytes. Their basic morphologic and physiologic characteristics are reviewed elsewhere (Feldman et al., 2000).
The following morphologic changes have important clinical implications and should thus be recognized:
Other neutrophil morphologic abnormalities recognized during a careful examination of blood smears include the Pelger-Huët anomaly (cats and dogs) and Chédiak-Higashi syndrome (cats). The Pelger-Huët anomaly occurs when the nucleus of polymorphonuclear leukocytes fails to divide, but the nuclear chromatin and cytoplasm maturation is complete (i.e., the nucleus has a bandlike appearance with mature, clumped chromatin). Cats and dogs with this anomaly typically have profound left shifts in the absence of clinical signs. On careful examination of the smear, however, the cells in the left shift are mature cells with nuclear hyposegmentation and not immature neutrophils. This anomaly may be acquired or inherited (autosomal dominant) and is usually considered of minimal clinical relevance. We have seen it primarily in Australian Cattle dogs and in dogs undergoing chemotherapy.
Chédiak-Higashi syndrome, a lethal autosomal recessive condition of Persian cats with smoke-colored haircoats and yellow eyes, is characterized by enlarged neutrophilic and eosinophilic granules in association with partial albinism, photophobia, increased susceptibility to infections, bleeding tendencies, and abnormal melanocytes.
Nuclear hypersegmentation (i.e., four or more distinct nuclear lobes) may result from a prolonged neutrophil transit time (“old” neutrophils). It occurs in dogs with hyperadrenocorticism, cats and dogs receiving corticosteroid therapy, and cats and dogs with chronic inflammatory disorders.
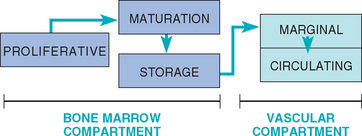
A basic review of neutrophil physiology follows. Three theoretical physiologic neutrophil compartments exist in the bone marrow (Fig. 85-1). The proliferative compartment is composed of dividing cells (myeloblasts, progranulocytes, and myelocytes); myeloblasts take approximately 48 to 60 hours to mature into metamyelocytes. The maturation compartment consists of metamyelocytes and band neutrophils; the transit time through this compartment is 46 to 70 hours. The storage compartment is composed of mature neutrophils; the transit time in this compartment is approximately 50 hours, and it contains an estimated 5-day supply of neutrophils. Mature neutrophils leave the bone marrow by a random process that involves changes in cell deformability and adhesiveness.
Two neutrophil pools are present in the vascular compartment (see Fig. 85-1). The marginal neutrophil pool (MNP) consists of neutrophils that are adhered to the vascular endothelium (and are thus not counted during a CBC). The circulating neutrophil pool (CNP) consists of the neutrophils circulating in the blood (i.e., the cells counted during a differential WBC count). The total blood neutrophil pool is composed of the MNP plus the CNP. In dogs the CNP is approximately equal in size to that of the MNP. However, in cats the MNP is approximately two to three times the size of the CNP. The neutrophil has an average blood transit time of approximately 6 to 8 hours in dogs and 10 to 12 hours in cats, with all blood neutrophils replaced every 2 to 2.5 days. Once the neutrophils leave the blood vessel (by diapedesis), they normally do not return to the circulation and are lost in the lungs, gut, other tissues, urine, or saliva.
LEUKOCYTE CHANGES IN DISEASE
Because the lower limit for the reference range for basophil and monocyte counts is 0, basopenia and monocytopenia are not discussed.
NEUTROPENIA
Neutropenia is defined as an absolute decrease in the number of circulating neutrophils. It can result from decreased (or impaired) cell production within the bone marrow or from the increased margination or destruction of circulating neutrophils (Box 85-1). Neutropenia is relatively common in cats and dogs. The clinician should keep in mind, however, that normal cats may have neutrophil counts of 1800 to 2300/μL; this reference range is also true for Greyhounds.
 BOX 85-1 Causes of Neutropenia in Cats and Dogs
BOX 85-1 Causes of Neutropenia in Cats and Dogs
Common; relatively common; uncommon; D, dog; C; cat;  , poorly documented.
, poorly documented.
Decreased or Ineffective Production of Cells in the Proliferating Pool
Myelophthisis (neoplastic infiltration of the bone marrow)
Myeloproliferative disorders (D, C)
Lymphoproliferative disorders (D, C)
Systemic mast cell disease (D, C)
Malignant histiocytosis (D, C )
)
Infectious diseases
Retrovirus infection (feline leukemia virus, feline immunode ficiency virus) (C)
Other
Idiopathic bone marrow hypoplasia-aplasia (D, C)
Cyclic neutropenia of gray Collies (D)
Acquired cyclic neutropenia (D, C)
Steroid-responsive neutropenia (D, C)
Sequestration of Neutrophils in the Marginating Pool
Sudden, Excessive Tissue Demand, Destruction, or Consumption
Peracute, overwhelming bacterial infection (e.g., peritonitis, aspiration pneumonia, salmonellosis, metritis, pyothorax) (D, C)
Viral infection (e.g., canine distemper or hepatitis, preclinical stage) (D)
Drug-induced disorders (D, C) (see above)
In a recent study of 232 dogs and 29 cats evaluated in a teaching hospital (Brown & Rogers, 2001), infectious diseases (feline leukemia virus, feline immunodeficiency virus, parvovirus) were the most common comorbid conditions, accounting for almost 52% of the cases of neutropenia. Sepsis or endotoxemia accounted for 11% of the cases, as did drug-associated neutropenia (e.g., chemotherapy, phenobar bital, antibacterials); primary bone marrow disease was found in 4% of the patients. The cause of the neutropenia was unclear in 21% of the patients.
Clinical signs in neutropenic cats and dogs are usually vague and nonspecific; they include anorexia, lethargy, pyrexia, and mild gastrointestinal tract signs. Oral ulceration, a common feature of neutropenia in human beings, does not seem to occur in small animals. Neutropenia is frequently an incidental finding in an otherwise healthy dog or cat (i.e., the patient is asymptomatic). If the neutropenia is caused by peripheral neutrophil consumption (i.e., a septic process), most animals exhibit clinical signs. Dogs and cats with parvoviral enteritis have neutropenia in association with severe vomiting or diarrhea or both. Cats and dogs with neutropenia can occasionally present in septic shock (pale, hypoperfused, hypothermic) and should be treated aggressively.
The evaluation of neutropenic cats and dogs should include a detailed drug history (e.g., estrogen or phenylbutazone in dogs, griseofulvin in cats; see Box 85-1); vaccination history (e.g., was the cat vaccinated against panleukopenia or the dog against parvoviral enteritis?); a complete physical examination and imaging in search of a septic focus; serologic, virologic, or molecular tests for infectious diseases (e.g., feline leukemia virus, feline immunodeficiency virus, canine ehrlichiosis, parvoviral enteritis); and, if necessary, bone marrow cytologic or histopathologic studies. Evaluation of changes in a blood smear is important in establishing the pathogenesis of the neutropenia. Benchtop hematology analyzers provide total neutrophil counts and do not distinguish mature neutrophils from bands, reemphasizing the value of evaluating the blood smear. If a dog or cat has anemia and/or thrombocytopenia in association with the neutropenia, particularly if the anemia is nonregenerative, a primary bone marrow disorder should be strongly suspected. If a dog or cat has regenerative anemia and spherocytosis in association with neutropenia, an immune-mediated disease should be considered a likely diagnosis.
The presence of toxic changes in the neutrophils or a left shift (see below) tend to suggest infection (i.e., toxic changes and left shifts are typically absent in dogs and cats with steroid-responsive neutropenia or primary bone marrow disorders). In a recent study of 248 dogs with toxic neutrophil changes conducted in Israel (Aroch et al., 2005) dogs with pyometra, parvoviral infection, peritonitis, pancreatitis, and septicemia were significantly, and not surprisingly, more likely to have toxic changes than those in the control group. Interestingly, toxic neutrophil changes were also significantly associated with acute renal failure, immune-mediated hemolytic anemia, and disseminated intravascular coagulation.
Evaluation of sequential leukograms in neutropenic dogs and cats is helpful in excluding transient or cyclic neutropenia (or cyclic hematopoiesis).
If the pathogenesis of neutropenia cannot be ascertained in an animal, sophisticated diagnostic techniques such as testing for antineutrophil antibodies, leukocyte nuclear scanning, or leukocyte kinetic studies can be performed. As previously noted, normal cats and Greyhounds can have low neutrophil counts. Therefore if a cat or a Greyhound with a neutrophil count of 1800 to 2300/μL is brought in for evaluation (or, more likely, if the “neutropenia” is detected during a routine hematologic evaluation), a conservative approach (e.g., repeat the CBC in 2 to 3 weeks) is indicated as long as no other clinical or hematologic abnormalities are found (e.g., left shift, toxic changes).
Because corticosteroid-responsive neutropenia has been well characterized in cats and dogs, if most infectious and neoplastic causes of neutropenia have been ruled out in an asymptomatic neutropenic animal, an in-hospital therapeutic trial of immunosuppressive doses of corticosteroids (prednisone, 2 to 4 mg/kg/day PO for dogs; 4 to 8 mg/kg/day PO for cats) can be instituted. Responses are usually observed within 24 to 96 hours of the start of treatment in such patients. Treatment is continued as it is for dogs with immune hemolytic anemia and other immune-mediated disorders (see Chapter 93) (Fig. 85-2).
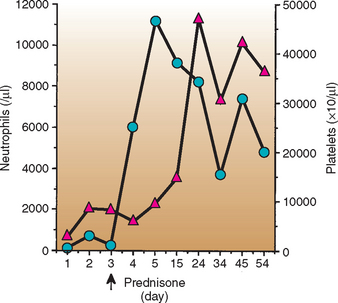
FIG 85-2 Response to therapy in a 6-year-old, female, spayed Airedale Terrier with steroid-responsive neutropenia and thrombocytopenia. Note the rapid response to immunosuppressive doses of prednisone.  , Polymorphonuclear neutrophils (in microliters);
, Polymorphonuclear neutrophils (in microliters);  , platelets (×103/μL).
, platelets (×103/μL).
Asymptomatic, afebrile neutropenic dogs and cats should be treated with broad-spectrum bactericidal antibiotics because they are at high risk for sepsis. The authors’ drug of choice in dogs is sulfamethoxazole and trimethoprim, at a dosage of 15 mg/kg PO q12h; another drug that can be used in both dogs and cats is enrofloxacin (Baytril) at a dosage of 5 mg/kg PO q12-24h. Antibiotics with an anaerobic spectrum should not be used because they deplete intestinal anaerobes, a protective bacterial population.
Neutropenic febrile (or symptomatic) cats and dogs constitute a medical emergency and should be treated with aggressive intravenous antibiotic therapy. The authors’ treatment of choice consists of a combination of ampicillin (20 mg/kg IV q8h) and enrofloxacin (5-10 mg/kg IV q24h).
Neutrophil production can be stimulated by the administration of human recombinant granulocyte colony-stimulating factor (G-CSF) (5 μg/kg SQ q24h). Although results are quite spectacular, the responses are usually short lived because of the counteractive effects of anti-CSF antibodies produced by the affected dog or cat. Lithium carbonate (10 mg/kg PO q12h) can increase the neutrophil counts in dogs; the therapeutic trough serum concentration of lithium is 0.8 to 1.5 mmol/L. This drug should be used with caution in dogs with decreased glomerular filtration rate because it is primarily excreted by the kidneys. Lithium carbonate does not appear to be effective in cats and may be toxic.
NEUTROPHILIA
Neutrophilia is defined as an absolute increase in the number of neutrophils; it is the most common cause of leukocytosis in dogs and cats. Several terms used to characterize neutrophilia are defined below.
The term mature neutrophilia refers to an increase in the number of segmented (mature) neutrophils without an increase in the number of immature forms (e.g., bands). Neutrophilia with a left shift refers to an increase in the number of both mature and immature neutrophils (more than 300/μL bands). A regenerative left shift is neutrophilia with increased numbers of immature neutrophils in which the number of immature forms does not exceed the number of mature neutrophils; most dogs and cats with a regenerative left shift have leukocytosis. A degenerative left shift occurs when the number of immature forms exceeds that of mature neutrophils; the number of the latter may be normal, low, or high. Degenerative left shifts are usually suggestive of an aggressive disease; toxic neutrophil changes (see previous section) are common in dogs and cats with degenerative left shifts. Disorders commonly associated with degenerative left shifts include pyothorax, septic peritonitis, bacterial pneumonia, pyometra, prostatitis, and acute pyelonephritis. The term extreme neutrophilia refers to situations in which the neutrophil count is above 50,000/μL; it can be associated with a left shift or mature neutrophilia. Diseases typically associated with extreme leukocytosis include septic foci (e.g., pyometra), immune-mediated diseases, hepatozoonosis, mycobacteriosis, and chronic myelogenous leukemia. A leukemoid reaction refers to a marked neutrophilia with a severe left shift, which includes metamyelocytes and myelocytes. It indicates severe inflammatory disease and may be difficult to distinguish from chronic granulocytic (myelogenous) leukemia (see Chapter 81).
Although a high percentage of cats and dogs with neutrophilia have underlying infectious disorders, neutrophilia is not synonymous with infection. Rather, neutrophilia in cats and dogs is commonly the result of inflammatory or neoplastic processes. Several disorders resulting in neutrophilia are listed in Box 85-2.
 BOX 85-2 Causes of Neutrophilia in Cats and Dogs
BOX 85-2 Causes of Neutrophilia in Cats and Dogs
Common; relatively common; uncommon; D, dog; C, cat;  , poorly documented.
, poorly documented.
Of note, neutrophilia commonly results from endogenous epinephrine release (physiologic neutrophilia). This neutrophilia, which is associated with the release of neutrophils from the MNP, is transient (lasting 20 to 30 minutes after endogenous release of catecholamines) and is commonly associated with erythrocytosis and lymphocytosis (the latter primarily in cats).
The endogenous release or exogenous administration of corticosteroids results in stress- or corticosteroid-induced neutrophilia, which is associated with decreased neutrophil egress from the vasculature and increased bone marrow release of neutrophils from the storage pool. Other hematologic changes typical of a stress leukogram include lymphopenia, eosinopenia, and monocytosis (the latter does not occur in cats). These abnormalities are commonly seen in sick dogs and cats.
Clinical signs in cats and dogs with neutrophilia are usually secondary to the underlying disorder. Pyrexia may or may not be present. If the patient has persistent neutrophilia, if the neutrophils display toxic changes (see p. 1229), or if a degenerative left shift is present, every effort should be made to identify a septic focus or an infectious agent promptly. The workup in such animals should include a thorough physical examination (e.g., abscess); thoracic and abdominal radiography (e.g., pneumonia, pleural or abdominal effusion); abdominal ultrasonography (e.g., peritonitis, pancreatic or hepatic abscess); and the collection of blood, urine, fluid, or tissue samples for cytology and bacterial and fungal cultures. As previously discussed, autologous or allogeneic neutrophils labeled with radionuclides (i.e., technetium 99m or indium 111) can be injected intravenously and the septic focus, or foci, identified by gamma camera imaging; an inflammatory focus can also be detected by radiolabeled ciprofloxacin.
The treatment of dogs and cats with neutrophilia is aimed at the primary cause. Empiric antibiotic therapy with a broad-spectrum bactericidal antibiotic (e.g., sulfa-trimethoprim, enrofloxacin, cephalosporin, amoxicillin) is an acceptable approach if a cause for the neutrophilia cannot be identified after exhaustive clinical and clinicopathologic evaluation or as the first line of treatment in a fairly asymptomatic dog or cat.
EOSINOPENIA
Eosinopenia is defined as an absolute decrease in the number of circulating eosinophils. It is commonly seen as part of the stress leukogram or with exogenous corticosteroid administration and is usually of little clinical relevance.
EOSINOPHILIA
Eosinophilia is defined as an absolute increase in the circulating eosinophil numbers. It is relatively common in small animals and can have a variety of causes (Box 85-3). Because eosinophilia is quite common in dogs and cats with parasitic disorders, no animal should undergo a thorough evaluation for eosinophilia before parasitic causes have been ruled out. In cats, flea infestation usually results in marked increases in the eosinophil count. In dogs, eosinophilia is frequently seen in roundworm and hookworm infestations or with dirofilariasis or dipetalonemiasis. Three additional relatively common causes of eosinophilia in cats include eosinophilic granuloma complex, bronchial asthma, and eosinophilic gastroenteritis. A clinical entity resembling feline hypereosinophilic syndrome has been reported in Rottweilers (Sykes et al.); in addition, lesions compatible with oral eosinophilic granulomas have been reported in Siberian Huskies. Eosinophilia can also occur in dogs and cats with mast cell tumors, but it is rare.
 BOX 85-3 Causes of Eosinophilia in Cats and Dogs
BOX 85-3 Causes of Eosinophilia in Cats and Dogs
Common; relatively common; uncommon; D, dog; C, cat;  , poorly documented.
, poorly documented.
Clinical signs in dogs and cats with eosinophilia are related to the primary disorders rather than to the hematologic abnormality. Because eosinophilia is so commonly found in animals with parasitic diseases, clinical evaluation of these animals should be aimed mainly at excluding these disorders. Once this has been done, other causes of eosinophilia should be pursued (see Box 85-3) by using the appropriate diagnostic procedures (e.g., tracheal wash or pulmonary fine-needle aspiration for pulmonary infiltrates with eosinophils, endoscopic biopsy for eosinophilic gastroenteritis). Treatment is usually aimed at the primary disorder.
A syndrome with high eosinophil counts in peripheral blood and tissue infiltration with eosinophils has been well documented in cats, Rottweilers, and occasionally other dog breeds. This syndrome is termed hypereosinophilic syndrome and is usually indistinguishable from eosinophilic leukemia. These patients have primary gastrointestinal tract signs, although multisystemic signs are also common. In cats, treatment with immunosuppressive doses of corticosteroids, 6-thioguanine, cytosine arabinoside, cyclophosphamide, and other anticancer agents (see Chapter 79) has been unrewarding, and most affected patients die within weeks of diagnosis. Clinical response to some of these drugs has been documented in Rottweilers.
BASOPHILIA
Basophilia is defined as an absolute increase in the basophil numbers and is commonly associated with eosinophilia. Because basophils are similar to tissue mast cells, their numbers increase in disorders characterized by excessive immunoglobulin E production and binding and in a variety of nonspecific inflammatory disorders. Causes of basophilia are listed in Box 85-4.
MONOCYTOSIS
Monocytosis refers to an absolute increase in monocyte numbers. It can occur in response to inflammatory, neoplastic, or degenerative stimuli. Although monocytosis has traditionally been observed primarily in chronic inflammatory processes, it is also common in acute disorders. Causes of monocytosis in cats and dogs are listed in Box 85-5. The monocytosis in dogs is typically more pronounced than that in cats; monocytosis is extremely rare in Greyhounds.
 BOX 85-5 Causes of Monocytosis in Cats and Dogs
BOX 85-5 Causes of Monocytosis in Cats and Dogs
Common; relatively common; uncommon; D, dog; C, cat;  ; poorly documented.
; poorly documented.
Trauma with Severe Crushing Injuries (D, C)
Hemorrhage into Tissues or Body Cavities (D, C)
Monocytosis is part of a stress leukogram in dogs. It can result from a variety of bacterial, fungal, and protozoal diseases. In the Midwest, systemic fungal disorders (e.g., histoplasmosis and blastomycosis) are relatively common causes. Because monocytes are precursors of tissue macrophages, granulomatous and pyogranulomatous reactions commonly result in monocytosis (see Box 85-5). In addition, immune-mediated injury resulting in cell destruction (e.g., immune hemolysis, polyarthritis) and certain neoplasms (e.g., lymphomas) may cause monocytosis. Some neoplasms secrete CSFs for monocytes and can result in marked monocytosis (more than 5000/μL).
The nature of the clinical evaluation in patients with monocytosis is similar to that used with neutrophilia: it should concentrate on identifying infectious foci. If an immune-mediated disorder is suspected, arthrocentesis to obtain fluid for analysis or other immune tests (see Chapter 92) should be performed. Treatment should be aimed at the primary disorder.
LYMPHOPENIA
Lymphopenia is defined as an absolute decrease in the lymphocyte count. It constitutes one of the most common hematologic abnormalities in hospitalized or sick dogs and cats, in which it is attributed to the effects of endogenous corticosteroids (stress leukogram). Lymphopenia is also commonly identified in dogs and cats with chronic loss of lymph, such as those with chylothorax or intestinal lymphangiectasia (Box 85-6).
 BOX 85-6 Causes of Lymphopenia in Cats and Dogs
BOX 85-6 Causes of Lymphopenia in Cats and Dogs
Common; relatively common; uncommon; D, dog; C, cat;  , poorly documented.
, poorly documented.
In general, cats and dogs with lymphopenia have obvious clinical abnormalities. As a general rule, it should be “ignored” (i.e., a diagnosis should not be pursued) in sick cats and dogs and in those receiving corticosteroids. The lymphocyte count should be reevaluated after the clinical abnormalities have resolved or steroid therapy has been discontinued. Contrary to popular belief, lymphopenia does not appear to predispose to infection.
LYMPHOCYTOSIS
Lymphocytosis is defined as an absolute increase in lymphocyte numbers. It is common in several clinical situations, including fear (cats; see Neutrophilia, above), vaccination (dogs and possibly cats), chronic ehrlichiosis (dogs), Addison’s disease (hypoadrenocorticism; dogs), and chronic lymphocytic leukemia (CLL). The lymphocytes are morphologically normal in all these disorders, with the exception of vaccination reactions, in which reactive lymphocytes (larger cells with a dark blue cytoplasm) are commonly seen. High numbers of morphologically abnormal (i.e., blast) lymphoid cells are found in dogs and cats with acute lymphoblastic leukemia (see Chapter 81).
In cats with marked lymphocytosis and neutrophilia, endogenous release of catecholamines should be ruled out as the cause of these hematologic abnormalities. If the cat is fractious and blood cannot be collected without a considerable struggle, a blood sample should be collected under chemical restraint.
Recent vaccination should be ruled out in dogs with lymphocytosis and reactive lymphocytes in the blood smear. Most dogs with lymphocyte counts of more than 10,000 cells/μL have either chronic ehrlichiosis or CLL; most dogs with monocytic ehrlichiosis have increased numbers of large granule lymphocytes (LGL), larger lymphocytes with abundant cytoplasm, and large azurophilic cytoplasmic granules. LGL lymphocytosis can also occur in dogs with CLL. Lymphocyte counts of more than 20,000 cells/μL are extremely rare in dogs with ehrlichiosis (i.e., dogs with more than 20,000 lymphocytes/μL more likely have CLL). A high proportion of these dogs also has hyperproteinemia caused by a monoclonal or polyclonal gammopathy (see Chapter 89). The clinical and hematologic features of monocytic ehrlichiosis and CLL are quite similar (e.g., cytopenia, hyperproteinemia, hepatosplenomegaly, lymphadenopathy). Serologic tests or polymerase chain reaction (PCR) testing for Ehrlichia canis, immunophenotyping of peripheral blood lymphocytes, PCR for clonality, and bone marrow aspiration findings may be helpful in differentiating these two disorders. Bone marrow cytologic findings in dogs with chronic ehrlichiosis usually consist of generalized hematopoietic hypoplasia and plasmacytosis, whereas hypoplasia with increased numbers of lymphocytes is more common in dogs with CLL. Causes of lymphocytosis in cats and dogs are listed in Box 85-7.
Aroch I, et al. Clinical, biochemical, and hematological characteristics, disease prevalence, and prognosis of dogs presenting with neutrophil cytoplasmic toxicity. J Vet Intern Med. 2005;19:64.
Avery AC, Avery PR. Determining the significance of persistent lymphocytosis. Vet Clin North Am Small Anim Pract. 2007;37:267.
Brown CD, et al. Evaluation of clinicopathologic features, response to treatment, and risk factors associated with idiopathic neutropenia in dogs: 11 cases (1990-2002). J Am Vet Med Assoc. 2006;229:87.
Brown MR, Rogers KS. Neutropenia in dogs and cats: a retrospective study of 261 cases. J Am Anim Hosp Assoc. 2001;37:131.
Carothers M, et al. Disorders of leukocytes. In: Fenner WR, editor. Quick reference to veterinary medicine. ed 3. New York: JB Lippincott; 2000:149.
Center SA, et al. Eosinophilia in the cat: a retrospective study of 312 cases (1975 to 1986). J Am Anim Hosp Assoc. 1990;26:349.
Couto CG. Immune-mediated neutropenia. In: Feldman BF, et al, editors. Schalm’s veterinary hematology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2000:815.
Couto GC, et al. Disorders of leukocytes and leukopoiesis. In Sherding RG, editor: The cat: diseases and clinical management, ed 2, New York: Churchill Livingstone, 1994.
Feldman BF, et al, editors. Schalm’s veterinary hematology, ed 5, Philadelphia: Lippincott Williams & Wilkins, 2000.
Huibregtse BA, et al. Hypereosinophilic syndrome and eosinophilic leukemia: a comparison of 22 hypereosinophilic cats. J Am Anim Hosp Assoc. 1994;30:591.
Iazbik MC, Couto CG. Morphologic characterization of specific granules in Greyhound eosinophils. Vet Clin Pathol. 2005;34:140.
Lilliehöök I, et al. Diseases associated with pronounced eosinophilia: a study of 105 dogs in Sweden. J Small Anim Pract. 2000;41:248.
Lucroy MD, Madewell BR. Clinical outcome and associated diseases in dogs with leukocytosis and neutrophilia: 118 cases (1996–1998). J Am Vet Med Assoc. 1999;214:805.
Madewell BR, et al. Oral eosinophilic granuloma in Siberian husky dogs. J Am Vet Med Assoc. 1980;177:701.
Perkins M, Watson A. Successful treatment of hypereosinophilic syndrome in a dog. Aust Vet J. 2001;79:686.
Sykes JE, et al. Idiopathic hypereosinophilic syndrome in 3 Rottweilers. J Vet Intern Med. 2001;15:162.
Weiss DJ. Evaluation of antineutrophil IgG antibodies in persistently neutropenic dogs. J Vet Intern Med. 2007;21:440.