chapter 11 Rehabilitation technologies to promote upper limb recovery after stroke
After completing this chapter, the reader will be able to:
1. Discuss the rationale for using rehabilitation technologies and theories that have guided their development.
2. Describe similarities and differences among different technology devices, specifically how they work and what they might add to the therapist’s repertoire of treatment tools.
3. Evaluate results of empirical studies on the use of rehabilitation technologies for the paretic upper limb after stroke.
4. Identify considerations for choosing rehabilitation technologies for clinical use, including their potential benefits and limitations.
Rehabilitation technologies to improve motor control after neurological injury have undergone tremendous growth during the past 15 years. Two forces in rehabilitation medicine have provided impetus for this technology development. The first is evidence of cortical reorganization in response to movement therapy after stroke. The second is the high cost of health care and significant reductions in rehabilitation services that have occurred in recent years.38 This chapter provides an overview of rehabilitation technologies, ranging from complex robotic devices to relatively simple spring-driven wrist/hand orthoses. Theories guiding technology development, research findings, and considerations for technology use in clinical practice are discussed.
Rationale for development
Rehabilitation scientists are concerned about the lack of empirical evidence on treatment efficacy and its impact on motor recovery and functional outcomes after stroke. At present, they do not have the knowledge needed to predict which treatment interventions, dose, or intensity elicit the best functional outcomes for a particular patient. Rehabilitation scientists are working diligently to quantify the “active ingredients” of various treatments, so they can make use of the most effective and efficient therapy methods when delivering clinical care to patients.
Rehabilitation technologies can provide quantifiable and repeatable treatment interventions and allow us to better measure the import of our interventions on impairements of motor function.18 For example, robotic devices can quantify changes in motor functions during stroke rehabilitation by gathering kinematic and kinetic data related to variables such as speed and accuracy of task completion, smoothness of reach, or forces exerted during training. Movement scientists continue to explore these data to further the understanding of how changes in motor control may contribute to increased functional use of a paretic limb after stroke.
Rehabilitation technologies are not expected to replace occupational or physical therapists, but they will become part of their treatment arsenal to optimize functional performance after a disabling event. Although the present cost of these novel technologies is high, larger scale production is expected to lower costs for clinical use in the future. Proponents of rehabilitation technologies predict that these tools will help to reduce or control rehabilitation costs by providing intensive movement therapies with minimal supervision by a therapist, which is important at a time when patients receive less therapy after neurological injuries, such as stroke, despite research evidence that more therapy is better.38 The use of this technology may help to shorten inpatient hospitalizations and enhance outpatient services, which hopefully will lead to improved long-term functional outcomes.
Theories guiding technology development
Rehabilitation technology is a new and growing field, experiencing some of the same developmental challenges seen during the history of conventional rehabilitation practice. Its development has been strongly influenced by motor learning principles, in particular massed practice and explicit learning paradigms.6 For example, rehabilitation robotics are designed to produce highly intensive upper limb training that is quantifiable, easily graded, cognitively challenging, and goal-directed. Motor learning principles guide their delivery of feedback with regard to knowledge of performance (e.g., via haptics), and knowledge of results, via graphs, changes in the virtual tasks and environment, and other forms of feedback. To date, robotic therapy trials have focused on improving motor performance at the International Classification of Functioning, Disability, and Health (ICF) level aimed at body structures and functions, rather than the activity level, aimed at task execution and functional performance. The state of robotic development has dictated this focus, as robots used in clinical trials have primarily exercised the paretic shoulder and elbow during reaching movements while the wrist and hand are supported by the device.
Motor learning approaches to conventional stroke rehabilitation have evolved to emphasize task-oriented training aimed at increasing upper limb function and patient participation in valued roles and routines.48 This task-oriented approach, in which skill-related tasks are practiced in natural contexts, has resulted in faster and better treatment outcomes than traditional methods, such as Bobath’s neurodevelopmental therapy.27 See Chapters 4 and 6. Recent trials of robot-assisted therapies have incorporated a task-oriented training approach in conjunction with other motor learning principles. For example, the Armeo and HOWARD devices provide task-oriented training via virtual games during upper limb therapy, and can allow interaction with real and virtual objects during repetitive, robot-assisted therapy. Studies have shown that the training of virtual tasks can lead to significant gains in motor performance during real world activities (see Brewer and colleagues6 for review). As technological advances continue, rehabilitation technologies will be better equipped to deliver task-oriented therapies more aligned with current rehabilitation practice and directed toward the ICF activity level of performance.
Robot assisted therapy
Two main classes of rehabilitation robots have been developed. Robots such as the Assistive Robotic Manipulator (ARM) (www.exactdynamics.nl) allow the user to compensate for lost skills when the potential for motor recovery is poor. The purpose of this chapter is to review a second class of robots, which provide repetitive, task-specific training to help restore lost motor function. Unlike constraint-induced movement therapy (CIMT), robot-assisted technologies are appropriate for persons with moderate to severe motor impairments.
Rehabilitation robots to restore lost motor function can be categorized by how the device is controlled or activated and how the user interface is designed. Robots for the upper limb can be broadly classified into three types: active systems, with actuators that provide movement assistance along a defined trajectory; passive systems that support the limb during movement attempts; and interactive systems in which actuators or motors are combined with impedance and control strategies that allow the robot to react to the patient’s movement attempts.
The way in which the robot assists with movement affects how the robot “feels” to the user during therapy. Low impedance interactive robots such as the MIT-MANUS are highly “back-drivable” and compliant to a client’s attempts to move, allowing precise and objective measures of motor performance. Active robots that use pneumatic actuators or “muscles” to power the device (e.g., Hand Mentor) are not as responsive to the patient’s movement attempts, because the mechanics of the robot create a more viscous response, similar to moving through honey. Passive robotic systems offer varied forms of nonpowered assistance with elastic bands or springs that support the limb against gravity during movement attempts. Examples include the Therapy Wilmington Robotic Exoskeleton (T-WREX) and Armeo devices described later.
The user/robot interface is another consideration when selecting rehabilitation robots for clinical use. End-effector robots, such as the MIT-MANUS and Mirror Image Motion Enabler (MIME), are typically attached to the person’s hand or forearm at a single point of contact. These robots are easily adjusted to different arm lengths, but do not control movement torques at individual joints. In contrast, the structure of exoskeletal robots more closely resembles human anatomy and allows separate control of torques applied to each joint. Exoskeletal robots, such as the interactive ARMin, require more effort when adapting them to different body sizes because each robot link must be adjusted to match the length of the user’s upper and lower arm.34 The therapeutic games used to visually direct the patient’s movement attempts during robot-assisted therapy also vary in their degree of complexity, ranging from simple stimuli to virtual environments designed to simulate functional task performance.
The discussion that follows is arranged from high to low tech, starting with more complex, low impedance robots to simpler wrist hand orthoses. Proximal devices are presented before distal technologies. During this review, readers are encouraged to consider needs specific to their patient mix and clinical setting, and potential goals for intervention. Controlled studies that compare rehabilitation technologies to other forms of therapy are highlighted in Table 11-1.
Mit manus and inmotion2 robots
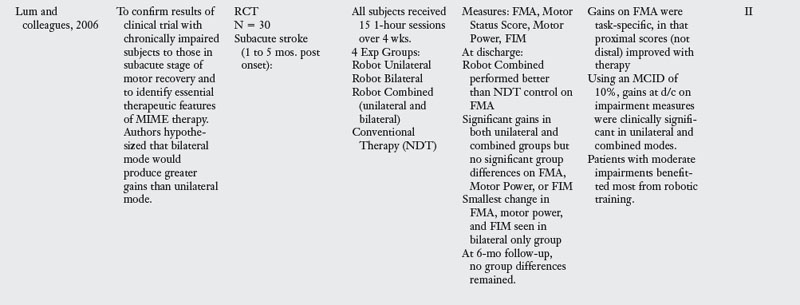
The most widely studied rehabilitation robot is the MIT-MANUS and its successor the InMotion2 (Interactive Motion Technologies, Watertown, MA). During therapy, the client is seated at the robot workstation and the paretic hand is positioned in a customized arm support attached to the end-effector (i.e., handle) of the robot arm. Therapy involves repetitive goal-directed, planar reaching tasks that emphasize shoulder and elbow movements. As clients attempt to move the robot’s handle toward designated targets, the computer screen in front of them gives visual feedback of the target location and movement of the robot handle (Fig. 11-1).

Figure 11-1 InMotion2 planar robot to exercise the paretic shoulder and elbow with wrist and hand supported.
(Courtesy of Hermano Igo Krebs.)
The low impedance controller of the MIT-MANUS is highly compliant when interacting with the client’s arm, similar to hand-over-hand assistance from a therapist during conventional therapy. Although MIT-MANUS is capable of providing passive, active-assistive, and active and resistive modes of therapy, the majority of studies have investigated the effects of active-assistive robotic therapy on motor recovery after stroke. The adaptive algorithm used in recent studies allows the robot to adjust the amount of guidance or assist provided to the patient based on his/her individual needs.
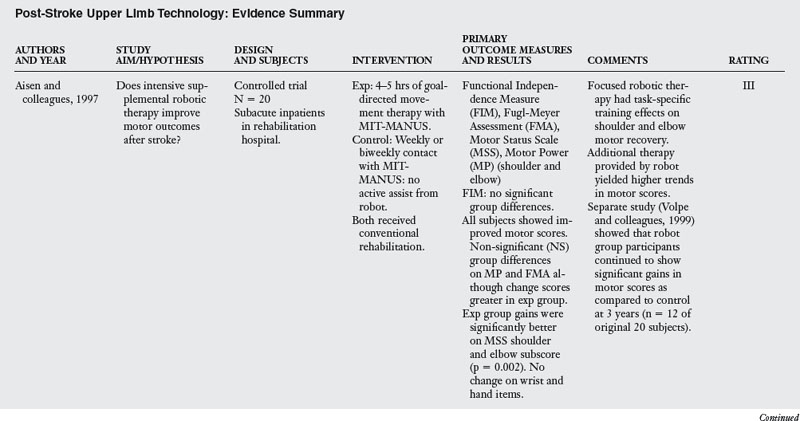
Proof of concept studies began in the mid-1990s, with a focus on the effects of intensive robot-assisted sensorimotor therapy for individuals in inpatient rehabilitation during the first weeks poststroke.1 Since then, investigations have primarily included persons with chronic and moderate to severe motor impairments more than six months after stroke. In this research, participants typically received one hour of robotic therapy three times per week for six weeks, performing approximately 18,000 repetitive reaching movements over the course of therapy.
As a whole, these studies indicate that treatment intensity and task specificity play a critical role in upper limb robot-assisted therapy. Reductions in motor impairment after MIT-MANUS training were task-specific in that the largest gains were observed in the exercised shoulder and elbow vs. the unexercised wrist and hand.12,50 Comparisons of robot vs. therapist directed therapy of equal intensity by Volpe and colleagues51 revealed no significant group differences in motor outcomes (see Table 11-1).
Stein and colleagues44 revealed that patients engaged in active-assistive or progressive-resistive training with the MIT-MANUS robot had similar gains in motor performance over the course of treatment (see Table 11-1). In this study, the level of initial severity vs. type of robotic therapy had a differential effect on motor outcomes. Individuals who were better able to reach the robotic therapy targets at study admission had larger gains in motor control on the Fugl-Meyer Assessment (FMA), regardless of treatment group. Although prior investigations have supported the use of compensatory strategies for persons with severe motor impairments after stroke,4 gains observed across robotic therapy studies indicate a potential for improvement in persons with moderate to severe motor impairments.
A report of two pilot studies with the MIT-MANUS compared robot-assisted therapy (as described previously) to “functionally-based” robotic therapy in persons with moderate to severe motor impairments. This functionally-based therapy trained both reach and grasp/release during virtual or object present tasks. Although greater gains were reported for the robot-assisted therapy group, participants who received “functionally-based” therapy improved more on wrist and hand items of the Fugl-Meyer Assessment.24 Study limitations include fewer movement repetitions and the treatment context during “functionally-based” robotic therapy (i.e., training occurred within the confines of the robot’s workspace). The authors proposed that persons with moderate to severe motor impairments after stroke may benefit more from robotic therapy focused on motor functions vs. activity-based skills training. Future studies on the relationship between stroke severity, focus of robot-assisted therapy (e.g., ICF impairment vs. activity level), and functional outcomes will both inform clinical practice patterns and guide insurance resource allocation for therapy practice.
Finally, a comparison of proximal robot-assisted therapy with the MIT-MANUS to distal training via a three degree of freedom (DOF) forearm and wrist robot showed that distal robot assisted therapy led to a greater transfer of skill to proximal limb segments than vice-versa.25 This small study implies that the sequence of treatment tasks may also be important, a finding that challenges conventional theories that support proximal to distal training after stroke.
Mirror image motion enabler
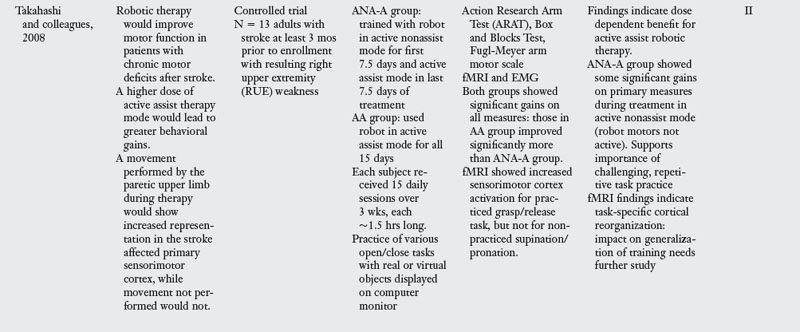
The MIME is an industrial PUMA robot reconfigured for rehabilitation that provides passive, active-assistive, active-resisted, and bimanual training of the upper limb. Its controller is not as compliant to a patient’s weak attempts to move as the MIT-MANUS described previously, so the MIME is not as sensitive for recording changes in motor performance over the course of treatment. During MIME therapy, the patient sits at the robot workstation, and his or her forearm and hand are supported in a splint attached to the robot manipulator (Fig. 11-2). The training exercises include a core set of 12 targeted reaching motions that emphasize shoulder and elbow movements in three-dimensional space.

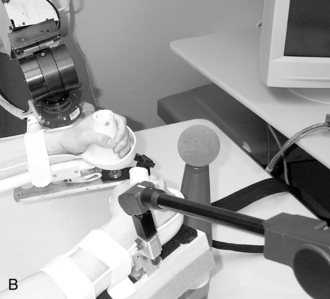
Figure 11-2 The Mirror Image Motion Enabler (MIME) robot can be used for unilateral or bilateral movement therapy.
(From Kahn LE, Lum PS, Rymer WZ, et al: Robot-assisted movement training for the stroke-imparied arm: does it matter what the robot does? J Rehabil Res Dev 43(5) 631–642, Aug/Sept 2006.)
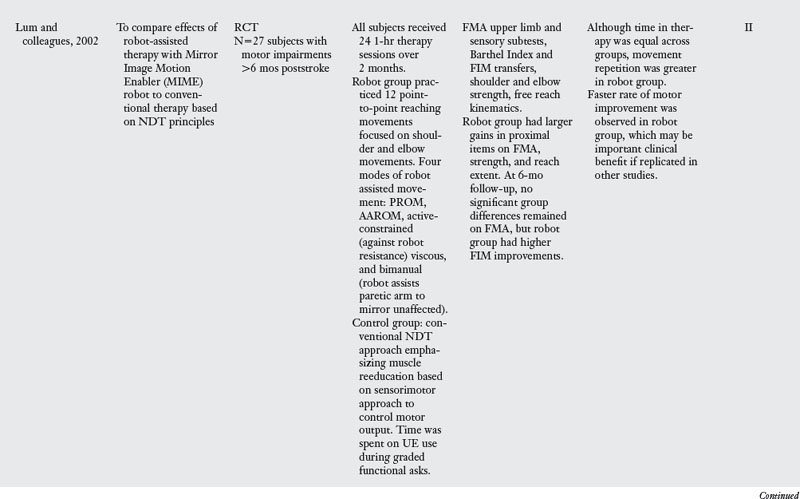
In two influential studies, Lum and colleagues29,30 compared the effects of MIME upper limb robotic therapy to conventional treatment based on Bobath’s neurodevelopmental approach. Persons in both chronic and subacute stages of motor recovery after stroke were examined (see Table 11-1). Lum found that subjects who received robotic therapy had statistically greater gains on shoulder and elbow items of the FMA during the two month treatment period. However, no between-group differences were found in FMA scores for the unexercised wrist and hand during this time. In both studies, gains in the robot and conventional therapy groups were equivalent at the six month follow-up. The authors proposed that conventional therapy led to greater carryover of home exercise programs, which resulted in continued gains in this group after the intervention trial.
In those who received robot assisted therapy, the type of robot intervention produced different motor outcomes. Subacute patients who received bilateral training did not improve as much as those who had unilateral or combined unilateral and bilateral training. Persons who received this combined robot training in the subacute and chronic studies showed an accelerated rate of motor recovery on clinical scales. The increased effort required during the combined treatment likely contributed to these gains. Although further research is needed to verify this effect, the accelerated motor recovery is an important consideration during clinical practice.
Arm guide
The Assisted Rehabilitation and Measurement (ARM) Guide is a robotic device that applies and measures assistive or resistive forces while the user performs reaching movements along a linear track.39 The patient’s paretic arm is supported in a forearm trough that is attached to the track and actuated or controlled by the robot motors. The patient is asked to initiate reaching movements with the paretic arm, and the robot motors provide assistance when the person is unable to move along the desired trajectory. The device is statically counterbalanced, so it reduces gravitational forces on the arm during movement attempts, and a small margin of error is allowed before the robot provides assistance. The track can be oriented at various angles to allow reaching into different regions of the workspace. Targets are located at the limit of the patient’s reach with elbow extended and shoulder flexed as much as possible without pain.22
Kahn and colleagues compared active assistive reaching exercises with the ARM Guide to free, unsupported reach to the same target locations.22 The research aimed to compare the effects of robot-assisted movement therapy with the ARM Guide to free reaching voluntary exercises in persons with chronic upper limb paresis after stroke (see Table 11-1). The frequency, dose, and duration of practice were identical across treatment groups. Participants in both groups showed similar gains in range of motion (ROM) and speed of supported reaching and in the time needed to complete functional tasks on the Rancho Los Amigos Functional Test of Upper Extremity Function. This indicated that repetitive movement training, regardless of how it was administered, was a key stimulus for the observed motor recovery. The only significant group difference in this experiment was that greater improvement in the smoothness of unsupported reaching movements occurred in free reaching group. One possible explanation is that the unsupported reaching exercise involved a greater degree of error correction as subjects practiced moving toward targets, supporting motor learning principles that emphasize the importance of error detection and recognition.
The authors proposed that the type of assistance provided by the ARM Guide may not have been optimal and have since begun testing a novel guided-force training program. When this training program detects misdirected forces during reach (e.g., from a strong flexor synergy), it stops the movement, provides visual feedback of the misdirection, and guides the person to activate muscle groups in appropriate combinations to reach the desired target.21 This guided-force training is also capable of adapting the amount of assistance or resistance offered, based on the person’s performance. Testing is underway to compare motor outcomes of subjects who participate in free reaching tasks vs. guided-force training with the ARM Guide. As rehabilitation technologies continue to develop, scientists expect to learn much more about the types of training that best meet an individual’s needs for motor recovery.
When thinking about clinical use of robotic devices, it is important to consider the degree to which successful practice of arm exercises influences adherence to exercise programs and contributes to gains in functional use. A home exercise program in which the patient is asked to repeatedly reach toward challenging target locations is likely to be met with frustration and limited engagement. Rehabilitation technologies can offer exercise programs that motivate, offer a relatively high degree of success and reinforcement, and give feedback concerning gains in motor performance.
Haptic master
The Haptic Master is a three DOF robot that provides gravity assistance for the paretic arm while the user sits at a workstation. A free moving elbow splint attached to an overhead frame supports the arm, and a passive mechanism supports the hand and allows for supination and pronation and wrist flexion and extension. All exercises occur in a virtual environment while force and position sensors enable interaction with virtual tasks such as reaching to a supermarket shelf or pouring a drink. The Haptic Master has been used for task-oriented training in a three dimensional virtual environment as in the GENTLE/S project3,10 or with real object manipulation as done with the Activity of Daily Living Exercise Robot (ADLER).20 Depending on the user’s movement abilities, three different therapy modes can be selected: passive, active assisted, or active. In addition to visual feedback, haptic feedback from the robot provides the user with the feeling of increased resistance when movements stray from the programmed trajectory.
Two clinical trials with the Haptic Master have shown modest reductions in upper limb impairment on the FMA following 30 minutes of therapy three times a week.10,42 The authors attributed greater gains following robotic therapy to the repetitive practice of task-oriented movements with performance feedback from the Haptic Master (see Coote and colleages10 and Table 11-1). Their assertion that cortical reorganization and movement kinematics are optimized when persons are engaged in challenging and meaningful tasks is supported by prior research and is an important consideration when using rehabilitation technologies during clinical practice. The use of individualized task-oriented training in natural environments, as emphasized in occupational therapy practice, is gaining greater attention in the rehabilitation technology literature.48
Reo
The Reo Therapy System (Motorika Ltd., Israel) is a widely marketed upper limb robot with little supporting research evidence published in peer reviewed journals. During Reo therapy the user performs computer generated games with the paretic hand attached to the robot arm. The robot provides upper limb assistance and feedback while the user performs reaching movements from a seated position at the work station. Patients have reported satisfaction with the Reo therapy program49 when combined with conventional inpatient therapies. A pilot study with 10 outpatient participants reported gains in Fugl-Meyer scores between 2 to 11 points following two to three one-hour sessions a week, with decreased perceived exertion and reductions in shoulder pain and upper limb spasticity as measured by the Modified Ashworth Score.35 Time poststroke and initial level of severity were not reported. Although additional studies are needed, initial work indicates that the Reo is well tolerated and may contribute to positive motor outcomes poststroke.
Armin
The ARMin is an exoskeletal, low impedance robot designed for repetitive, task-oriented upper limb therapy after stroke. The interactive assistance it provides is based on “patient-cooperative” control strategies that allow patient-driven movements while the robot gives support only as needed (vs. preprogrammed levels of assistance). This form of control is expected to increase the intensity of practice while gamelike training scenarios enhance patient motivation to engage in repetitive training. Haptic, visual, and auditory feedbacks are provided during patient use.
The increased DOFs afforded by the ARMin and other exoskeletal devices (e.g., the T-WREX and Armeo discussed later) more closely mimic task-oriented therapy provided by rehabilitation clinicians.34 Although passive nonmotorized devices (e.g., T-WREX, Armeo) support the arm against gravity and are intrinsically safer and less expensive, they cannot assist movements that the patient is unable to perform (e.g., elbow extension). However, the exoskeletal ARMin can apply and control torques at each joint individually when assistance is needed due to increased muscle tone or poor isolated movement.34
Clinical testing of the ARMin robot with patients after stroke has been reported for three individuals with moderate to severe motor impairments.33 This pilot study by Nef and colleagues found that ARMin exoskeletal therapy led to modest but significant gains in motor function as evident on the FMA, with no change on the Barthel Index or Action Research Arm Test. Nef and colleagues33 proposed that the threes DOFs allowed by the ARMin I device may have limited task-oriented training and functional outcomes. A newer model, the ARMin III (Fig. 11-3), allows six to seven DOFs during upper limb training and is presently undergoing clinical investigations. Although the evidence is pending, this robot holds promise for providing high quality, task-oriented stroke rehabilitation in the future.
T-wrex and armeo
The T-WREX is a passive, body powered orthosis for the upper limb that was based on an earlier device developed for persons with muscular dystrophy.37 It was adapted for individuals with stroke-induced motor impairments to allow a lower cost, safe option for semiautonomous upper limb training. Easily adjusted elastic bands provide a safe method of passively supporting the limb to allow greater active ROM and reach. The T-WREX enables naturalistic movement across two thirds or more of a normal workspace while the user engages in task-oriented virtual games, such as moving apples from a produce shelf to a shopping cart. Electronic sensors detect arm movement and hand grasp, allow the user to interact with the therapeutic games, and provide quantitative feedback about reach and grasp performance. A modified version of the T-WREX, the Armeo (Hocoma A.G., Switzerland) is commercially available for clinic use (Fig. 11-4).
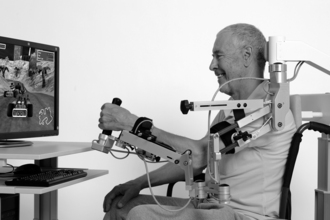
Figure 11-4 Armeo body powered orthosis with virtual training task.
(Courtesy Hocoma AG, Switzerland.)
Housman and colleagues17 reported results of a randomized control trial in which conventional table-top exercises were compared to T-WREX training in persons with chronic, moderate to severe upper limb paresis (see Table 11-1). In this study, the amount of weight support provided by the T-WREX orthosis was rarely decreased to less than 50% of the weight of the arm. Despite the high degree of gravity assist during training, effects did generalize to upper limb movements in nonweight-supported conditions, as seen by significant improvements in the FMA and Motor Activity Log scores. The amount of change measured on the FMA was comparable to that seen in studies of active devices, including the MIT-MANUS and MIME robots. This further suggests that highly repetitive movement therapy is a key stimulus to neuromotor recovery after stroke.17
The study also found that subjects could perform T-WREX exercises with only brief direct supervision from a therapist (four minutes for each hour of therapy),17 so the T-WREX has good potential for cost effective, semiautonomous practice of upper limb motor tasks within clinical and home settings. Participants in both treatment groups found the novel T-WREX intervention more enjoyable and motivating than conventional table-top exercises typically prescribed as a home program after stroke.
Hand robots
Development of robots to assist with wrist and hand retraining has lagged behind that of shoulder and elbow devices, largely due to the complexity of control needed to assist with grasp and release. Two robots that have been empirically tested are the Hand Mentor (Kinetic Muscles Inc., Tempe, AZ) and the Hand Wrist Assistive Rehabilitation Device (HOWARD) developed at the University of California, Irvine.
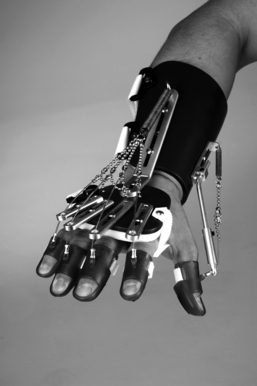
The Hand Mentor is a repetitive motion device designed for home and clinical use (Fig. 11-5). It uses a pneumatic artificial muscle to extend the wrist and fingers and provides electromyographic (EMG) biofeedback of muscle activation via light emitting diodes displayed on a small screen. Its purpose is to inhibit flexor tone of the wrist and fingers, provide neuromuscular reeducation, and increase ROM and strength of the paretic wrist and fingers.
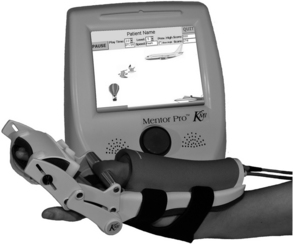
Figure 11-5 Hand Mentor device combines pneumatic “muscle” activation with electromyography to provide active assistive therapy for wrist and fingers.
(Courtesy of Kinetic Muscles, Inc.)
In two single case studies, Hand Mentor training was combined with repetitive task practice to improve upper limb function in persons seven and 11 months poststroke.14,41 Intervention occurred four hours per day, either three or five days a week, over a three-week period. During two hours of each session, Hand Mentor training included use of EMG biofeedback to reduce abnormal muscle tone in the wrist and fingers, and two active motor control modes to elicit wrist flexion and extension. If the user could not reach the target position during the active training mode, the pneumatic muscle inflated to assist wrist movements. Study participants spent up to two additional hours doing repetitive task practice of functionally oriented tasks with the paretic arm. The level of difficulty increased gradually, and participants selected activities for training. Gains reported at the end of intervention included faster speed of performance on some Wolf Motor Function Test items; increased active ROM in the shoulder, wrist, and thumb; better isolation of upper limb motions; and a slight decrease in maximum grip force.14,41 Despite limited evidence of efficacy, the Hand Mentor has been used more frequently in rehabilitation clinics in recent years. Certainly, empirical studies are needed to more closely examine the potential uses and benefits of this technology.
The Hand Wrist Assistive Rehabilitation Device (“HOWARD”) is another pneumatically actuated robot that assists with repetitive grasp and release movements.46 While seated at a computer monitor, the subject’s paretic hand is secured to the robotic device, and the forearm is supported in a padded splint. HOWARD controls flexion/extension of the four fingers about the metacarpophalangeal (MCP) joint; flexion/extension of the thumb at the MCP joint; and flexion/extension of the wrist (Fig. 11-6). Joint angle sensors allow for real time control of virtual hand movements displayed on the monitor, and the backdriveable control allows the patient to move freely when the robot is not engaged in active assistance. The palmar surface of the hand is left unobstructed to allow for grasping practice of both virtual and real objects. Takahashi and colleagues demonstrated HOWARD’s effectiveness in promoting motor recovery and cortical reorganization in persons with chronic motor impairments after stroke46 (see Table 11-1). HOWARD’s unique capability to combine repetitive robot-assisted training with the rich sensory experience of grasping and holding real objects represents another step toward more closely aligning robotic technology with current rehabilitation theories that emphasize task-oriented training.
Bilateral arm training
A number of studies have examined the effects of repetitive bilateral training on upper limb motor recovery. This research has shown that bimanual practice can have a facilitating effect on the paretic arm after stroke, with movement of the nonaffected upper limb stimulating ipsilateral corticospinal projections to the paretic arm. Two rehabilitation devices, the BI-MANU-TRACK and Bilateral Arm Training with Rhythmic Auditory Cuing (BATRAC) trainers, have undergone a fair amount of study.
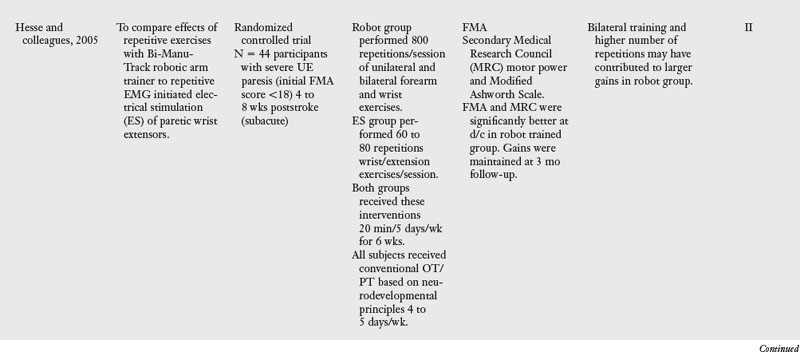
The BI-MANU-TRACK is a one DOF computer-assisted arm trainer that allows bimanual practice of supination and pronation and wrist flexion and extension (Fig. 11-7).15 Exercises can be performed passively or actively, and isometric resistance can be added at the start of active exercise, based patient’s ability level and needs. When compared to a control group that received electrical stimulation, Hesse and colleagues16 found that subjects who performed BI-MANU-TRACK training four to eight weeks poststroke had significant improvements on the FMA and in muscle power scores for the paretic arm (see Table 11-1). However, persons in the bilateral training group engaged in 10 times more movement repetitions than did those who received electrical stimulation. Follow-up research needs to examine whether the significant group differences were due to the greater number of movement repetitions or to the bilateral nature of the training. A similar BI-MANU-TRACK protocol for persons with chronic motor impairments15 revealed less change in motor performance of the paretic arm than was seen in the subacute group. This suggests that the timing of intervention is important for this form of therapy.
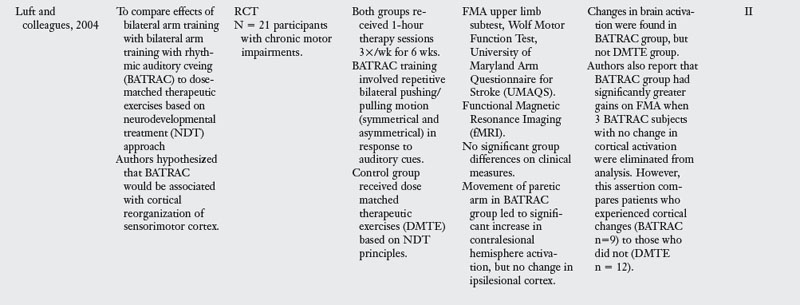
The Bilateral Arm Training with Rhythmic Auditory Cuing (BATRAC) trainer is another device used for repetitive bilateral upper limb therapy. BATRAC therapy involves moving two unyoked handles forward and backward in a reaching motion, both symmetrically and asymmetrically in response to auditory cues set at individually determined rates. A single group pilot study by Whitall and colleagues52 showed gains in Fugl-Meyer scores, speed of arm movements, and use of the paretic arm for supportive roles during bilateral tasks after 18 sessions of therapy over a six-week period. These improvements were largely sustained eight weeks posttraining. However, when Luft and colleagues28 compared subjects who received NDT-based exercises to those treated by BATRAC, they found no significant group differences on clinical measures but did see changes in brain activation in the BATRAC group (see Table 11-1).
Other BATRAC research by Richards and colleagues40 examined effects of a condensed BATRAC protocol, in which treatment was delivered to persons with mild chronic impairments during two-hour and 15-minute sessions, four times a week for two weeks. The condensed protocol led to small gains on the Motor Activity Log but no change in motor impairment or speed, as measured by the FMA and Wolf Motor Function Test. It appears that persons with severe motor impairments may benefit more from BATRAC training than those with milder impairments, and that the distribution of training sessions is an important consideration when using this device. Other studies also have reported smaller gains in motor recovery when patients engaged in a compressed treatment schedule.13 This is an important consideration for clinicians faced with limited insurance coverage for patients following stroke. Evidence concerning optimal timing and distribution of treatment is sorely needed.
Although this bilateral training research may contribute to the knowledge of neuromotor recovery mechanisms poststroke, clinically relevant limitations of these devices include the lack of patient feedback and focus on impairment (not activity level) changes in performance. The effect of this bilateral training on functional use of the paretic arm and real life outcomes is unknown. McCombe Waller and colleagues32 recommended that specific bilateral training exercises be matched to the patient’s baseline characteristics, and that the contribution of supportive role functions by the paretic arm be further examined during unilateral and bilateral tasks. Research studies that include bilateral task analysis and assessment of interlimb coordination could play an important role in clarifying ways in which motor function of the paretic arm changes during the course of intervention.32
Functional electrical stimulation
Functional electrical stimulation (FES) is another technological advancement designed to facilitate motor recovery after neurological insult. A number of studies have been published on neuromuscular electrical stimulation (NMES) and FES. FES is actually a subcategory of NMES and refers to the use of NMES to substitute for an orthosis while assisting with a functional activity, such as holding a glass to drink.5 See Chapter 10.
Myomo
The Myomo e100 NeuroRobotic system (Myomo, Inc., Boston, MA) is a wearable device that assists with elbow flexion and extension of the paretic arm. It uses a novel surface EMG control mechanism to detect and amplify signals generated by a stroke survivor’s muscles. The Myomo device includes a surface electrode that is placed over the biceps or triceps muscle and a motorized elbow brace that is powered by rechargeable batteries in a portable control pack. The treating therapist selects the appropriate location for the sensing electrode and sets a virtual spring that counterbalances the powered assistance of the device, which allows the device to assist with the desired movement (e.g., elbow flexion) and aids return to the starting position when the EMG stimulus subsides and the person relaxes. The Myomo device controls the amount of force generated based on the amplitude of the EMG signal, which results in movement assistance that is proportional to the patient’s effort.43
The Myomo e100 is a portable, exoskeletal device that can be used in different settings, unlike robotic devices limited to a fixed work station. It allows for the training of basic functional tasks, such as pushing up from an arm chair or reaching for a light switch. However, it only assists elbow motions and does not directly address impaired supination/pronation or distal function. At this time, its weight makes it inappropriate for use by persons with glenohumeral subluxation and weak shoulder control. Data from a small pilot study for patients with severe chronic upper limb paresis after stroke showed modest, but statistically significant gains in upper limb scores on the FMA.45
Handmaster
The Handmaster, marketed as the NESS H200 Hand Rehabilitation System (Bioness, Valencia, CA) is a noninvasive, advanced neuroprosthesis used for the treatment of upper limb paresis following stroke, traumatic brain injury, or C5-C6 spinal cord injury. It contains a custom-fit orthosis that uses functional electrical stimulation (FES) to provide neuromuscular reeducation, to sequentially activate muscle groups in the forearm, and to elicit active grasp and release in the paretic hand.
Research studies indicate that FES has the potential to benefit persons with subacute and chronic upper limb paresis after stroke. An evidence-based review by Chan8 revealed that patients who performed FES in conjunction with active practice of functional tasks outperformed those involved with task-oriented training alone or sham stimulation. This outcome was reinforced by Alon and colleagues2 in a study of the NESS H200 during subacute inpatient rehabilitation (see Table 11-1). Chan’s review8 also discovered that treatment protocols varied across studies, but the stimulation parameters did not appear crucial in determining motor outcomes. This is likely related to variations in residual motor abilities, degree of spasticity, and the duration and frequency of treatment across study samples. Cauraugh and Kim7 proposed that FES works to improve voluntary initiation of movements in the impaired limb by decreasing the processing time needed for stimulus identification and response initiation. FES with the NESS H200 was suitable for persons with mild to moderate upper limb dysfunction after stroke, and was reported to be well tolerated by those engaged in home programs. As with all present rehabilitation technologies, the high cost of the NESS H200 may present a barrier to its widespread use after stroke.
Other devices for repetitive task practice
Saebo
The SaeboFlex is a high-tech, dynamic orthosis developed to address the difficulty that many stroke survivors have in opening their paretic hand after stroke. This orthosis consists of a forearm cuff attached to a dorsal hand platform that anchors two spring attachments. Individual finger sleeves are placed over the distal phalanges and then are attached to the spring attachments via a high tensile line to provide assistance with finger and thumb extension (Fig. 11-8). A small phase 1 trial tested the feasibility of using this orthosis in 13 individuals with chronic upper limb motor impairments.11 The training protocol, based on systems theory and motor learning principles, emphasized repetitive practice, active problem solving and use of the hand to promote motor recovery of the upper limb. Other interventions provided during the SaeboFlex training period included strengthening exercises, ROM, and electrical stimulation to wrist and finger extensors. Significant gains in upper limb measures were found after five days of intensive treatment. Although further research on its efficacy is indicated, this trial suggests that the SaeboFlex orthosis has good potential to provide low-cost, repetitive motor training to persons with moderate motor impairments after stroke.
Autocite
The AutoCITE was developed to automate CIMT for individuals with mild to moderate motor impairments after stroke.31 It is comprised of a computer, chair, and eight task devices arranged on four work surfaces in a modified cabinet. The training tasks are derived from those used in therapist-mediated CIMT and include reaching, tracing, peg board use, supination/pronation, threading, arc and rings, finger tapping and object flipping (Fig. 11-9). While the user sits at the workspace, instructions are given via a computer monitor, and device sensors monitor performance. Several types of feedback and encouragement are provided, including the number of successful repetitions and time for task completion.
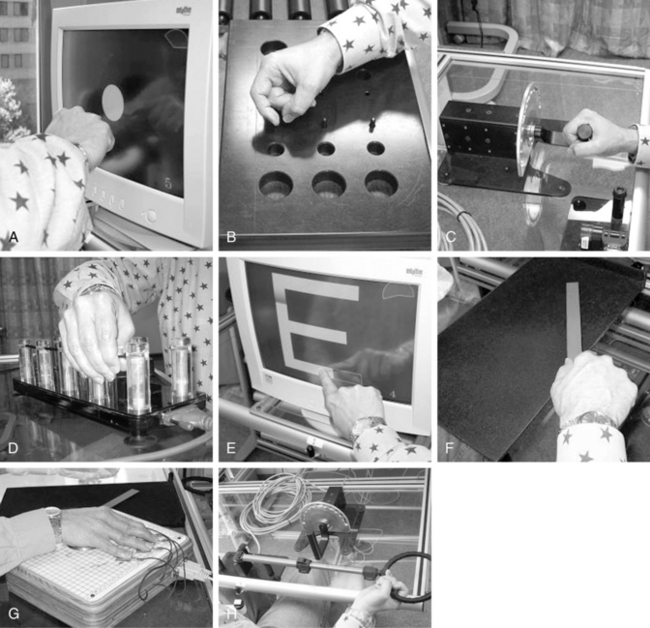
Figure 11-9 AutoCITE activities for persons with mild to moderate impairments after stroke.
(From Taub E, Lum PS, Hardin P, et al: Automated delivery of CI therapy with reduced effort by therapists. Stroke 36(9):1301-1304, 2005.)
The AutoCITE is another technological device designed to provide semiautonomous, repetitive task practice and reduce health care costs. Taub and colleagues47 reported that patients with chronic mild to moderate upper limb paresis who trained with the AutoCITE had significant gains in motor ability and real world use, as indicated by improved scores on the Wolf Motor Function Test and Motor Activity Log. The authors found no significant difference in treatment outcome among subjects who received therapist supervision for 25%, 50%, or 100% of the AutoCITE treatment time (see Table 11-1). Taub and colleagues concluded that AutoCITE training with limited therapist supervision was as effective as one-on-one CIMT.
In addition to three hours of AutoCITE training five days a week, all participants were asked to wear a padded safety mitt on their less-affected hand for 90% of waking hours during the two-week trial period. This study did not include a control group that received only the mitt constraint. It is possible that these participants improved merely because of increased hand use during waking hours vs. the time spent on AutoCITE training (supervised or unsupervised). Future research may well address this question.
Clinical use of rehabilitation technologies
The rehabilitation technologies discussed previously offer a wide range of treatment options for persons with upper limb paresis after stroke. Choosing the “right” technology for a particular patient during clinical practice involves an appreciation for key features of the device, the patient’s level of function, and therapeutic goals.
Key features to consider
Key considerations when choosing technologies for patient treatment after stroke include the type of assistance and DOFs afforded by the device, whether it is portable or stationary (e.g., requires treatment space for a workstation), and the amount of training needed to safely and effectively administer movement therapy. The device should be easily programmed to meet the patient’s needs as motor recovery occurs and allow for semiautonomous training, which can enhance therapist productivity while the patient engages in CIMT. Many robotic devices provide quantitative measures of motor functions such as movement speed, accuracy, and forces generated. These objective measures can complement activity and participation level evaluation findings, and can be used when documenting treatment plans and progress toward goals. Ease and time for treatment set-up are other important considerations when selecting these tools for the clinic.
The present rehabilitation devices are most appropriately used as an adjunct to therapist-rendered intervention. Technologies are best at providing intensive practice while the therapist emphasizes use of the paretic arm during valued everyday tasks. Because individual devices are not currently able to assist with of all DOFs needed for task-oriented training, some researchers have advocated for the use of robotic “gyms”23 or an “integrated suite of low cost robotic and computer assistive technologies.”19 The therapist’s expertise in upper limb function and task analysis is essential when selecting rehabilitation technologies and establishing treatment plans that effectively combine technology-driven and therapist-rendered interventions.
Certainly the organization’s rehabilitation agenda, patient caseload, and cost strongly influence decisions to purchase rehabilitation technologies for clinical use. In addition, barriers to therapist and physician acceptance deserve attention: specific concerns include treatment efficacy, equipment expense, and lack of time to evaluate technology options for stroke rehabilitation.6 In terms of efficacy, research studies have begun to show that rehabilitation technologies can offer benefits not easily achieved by additional conventional therapy. For example, patients who had robot-assisted therapy early after stroke showed an accelerated rate of motor recovery when compared to a control group that received conventional therapy.29 Although this study showed no group differences after six months, it is likely that accelerated motor recovery during inpatient rehabilitation could contribute to improved functional use of the upper limb and positively impact self-care performance and patient satisfaction at hospital discharge.
Level of function and therapeutic goals
Many factors influence a patient’s ability to benefit from rehabilitation of the paretic arm after stroke. Critical factors include the level of neurological damage and resulting motor impairment, and the individual’s ability to engage successfully in therapeutic activities aimed at improving motor function. Rehabilitation technologies can be easily programmed to ensure a certain level of success for patients with a wide range motor abilities. This feature can enhance patient motivation and independent carryover of exercise programs. Although researchers are working to identify “active ingredients” needed for the learning and acquisition of motor functions after stroke, the “best” rehabilitation and technology choice for a given level of motor function is not well-established. It is not safe to assume that one treatment approach, or one form of rehabilitation technology, is optimal for all patients with hemiparesis.
Clinical practice illustrates the need for different techniques and treatment strategies for patients with minimal vs. moderate to severe levels of motor impairment. Unfortunately, therapist-rendered interventions are difficult to quantify or reproduce across treatment sessions. Rehabilitation robots are able to objectively measure the amount and type of assistance provided during therapy, and to track changes in motor functions that occur during the course of treatment. Clinicians can use these measures to judge the effectiveness of treatment, and to learn more about how changes in motor functions might translate to activity level performance.
Although many advocate for rehabilitation technologies able to deliver task-oriented training across multiple DOFs,48 it is not known whether this approach would be as effective for persons with mild or with moderate to severe motor impairments after stroke. Krebs and colleagues23 proposed that modular robotic systems may be particularly well-suited for addressing this question. Modular systems can deliver training to individual limb segments or can combine robot components to allow practice of tasks that involve greater DOFs. However, current modular systems are limited in their ability to provide true “task-oriented” training because they do not allow the practice of contextually-rich virtual or actual tasks. While these modular tools may be key to identifying what form of movement therapy is best for which patient, clinicians should use a combination of empirical evidence, clinical experience, and practice theory when deciding which technology features are best suited for their rehabilitation patients.
When selecting rehabilitation technologies, the therapist also should assure that the feedback provided by the device is clear, easily interpreted by the patient and clinician, and pertinent to the patient’s goals for improved motor function. The therapeutic device should offer a variety of metrics relevant to functional task performance. Colombo9 asserted that patients with greater motor impairments could benefit more from feedback regarding the efficacy of movement attempts. Conversely, patients with higher level motor functioning could expect to benefit more from feedback concerning movement accuracy or force control.9 A patient’s therapeutic goals may be better addressed when the clinician has a clear understanding of the types of feedback and forms of intervention that best promote functional motor recovery.
As research unfolds, specific treatment protocols to more efficiently and effectively address patient needs across levels of function will help to guide the integration of technology-driven and therapist-rendered rehabilitation. Thoughtful treatment planning for technology driven rehabilitation is no different from that of conventional therapies. It just requires an understanding of the therapy options made available by these technologies.
Summary
The focus of conventional therapies during recent years has shifted from analytical training methods directed at impairments in motor function to an emphasis on task-oriented training for the upper limb.48 The development of rehabilitation technologies, although still in its infancy, is following a similar trend. Potential benefits include controllable treatment intensity, repetition, task-specific practice, and sensory-motor feedback to enhance knowledge of performance and results.
The research presented in this chapter generally supports the use of rehabilitation technologies to improve upper limb motor functions after stroke. Although systematic reviews of robot-assisted therapies have substantiated task-specific training effects at the ICF impairment level, these have not generalized to arm and hand use during activities of daily living.26-36 As technology-aided distal training and task-oriented interventions are further developed and therapists become more experienced with integrating technology-driven and therapist-rendered interventions, the effects of the activity and participation level functions are expected to improve. Ultimately, rehabilitation technologies are anticipated to provide cost effective treatment options and to help to inform clinicians about the “active ingredients” key to effective and efficient rehabilitation for persons after stroke.
Review questions
1. Describe the difference between active and passive rehabilitation robots and provide an example of each.
2. List the hand robots reviewed in this chapter and discuss research findings. Why has the development of shoulder/elbow robots exceeded that of distal robots for the wrist and hand?
3. Which rehabilitation technology would you choose for a patient with mild motor impairments after stroke? Explain the therapy approach you would take and why.
4. The studies in Table 11-1 compared the effectiveness of rehabilitation technologies with conventional therapy methods. Discuss one or two ways that you might use this evidence to guide your therapy for persons with moderate upper limb impairments after stroke.
5. There are many factors to consider when choosing rehabilitation technologies for use in the clinic. What considerations are especially important for your setting and what device(s) would you select based on these factors?
1. Aisen ML, Krebs HI, Hogan N, et al. The effect of robot assisted therapy and rehabilitative training on motor recovery following stroke. Arch Neurol. 1997;54(4):6-443.
2. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation. A pilot study. Neurorehabil Neural Repair. 2007:21;3:207-215.
3. Amirabdollahian F, Loureiro R, Gradwell E, et al. Multivariate analysis of the Fugl-Meyer outcome measures assessing the effectiveness of GENTLE/S robot-mediated stroke therapy. J Neuroeng -Rehabil. 4(4), 19 February 2007. Published online
4. Barreca S, Wolf S, Fasoli S, et al. Treatment interventions for the paretic upper limb of stroke survivors. a critical review. Neurorehabil Neural Repair. 2003:17;4:220-226.
5. Bracciano AG. Physical Agent Modalities. In Radomski MV, Latham CAT, editors: Occupational therapy for physical dysfunction, ed. 6, Baltimore: Lippincott Williams & Wilkins, 2008.
6. Brewer BR, McDowell SK, Worthen-Chaudhari LC. Poststroke upper extremity rehabilitation. A review of robotic systems and clinical results. Top Stroke Rehabil. 2007:14;6:22-44.
7. Cauraugh JH, Kim SB. Stroke motor recovery. Active neuromuscular stimulation and repetitive practice schedules. J Neurol Neurosurg Psychiatry. 2003:74;11:1562-1566.
8. Chan CKL. A preliminary study of functional electrical stimulation in the upper limb rehabilitation after stroke. An evidence-based review. HKJOT. 2008:18;2:52-58.
9. Colombo R, Pisano FM Micera S, et al. Assessing mechanisms of recovery during robot-aided neurorehabilitation of the upper limb. Neurorehabil Neural Repair. 2008;22(1):50-63.
10. Coote S, Murphy B, Harwin W, et al. The effect of the GENTLE/S robot-mediated therapy system in arm function after stroke. Clin Rehabil. 2008;22(5):395-405.
11. Farrell JF, Hoffman HB, Snyder JL, et al. Orthotic aided training of the paretic upper limb in chronic stroke. Results of a phase I trial. NeuroRehabilitation. 2007:22;2:99-103.
12. Fasoli SE, Krebs HI, Stein J, et al. Robotic therapy for chronic motor impairments after stroke. follow-up results. Arch Phys Med Rehabil. 2004:85;7:11-1106.
13. Finley MA, Fasoli SE, Dipietro L, et al. Short duration upper extremity robotic therapy in stroke patients with severe upper extremity motor impairment. J Rehabil Res Dev. 2005;42(5):683-692.
14. Frick EM, Alberts JL. Combined use of repetitive task practice and an assistive robotic device in a patient with subacute stroke. Phys Ther. 2006;86(10):1378-1386.
15. Hesse S, Schulte-Tigges G, Konrad M, et al. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic stroke. Arch Phys Med Rehabil. 2003;84(6):915-920.
16. Hesse S, Werner C, Pohl M, et al. Computerized arm training improves the motor control of the severely affected arm after stroke. Stroke. 2005;36(9):1960-1966.
17. Housman SJ, Scott KM, Reinkensmeyer DJ. A randomized controlled trial of gravity-supported, computer-enhanced arm exercise for individuals with severe hemiparesis. Neurorehabil Neural Repair. 2009;23(5):505-514.
18. International classification of functioning. disability and health. ICF. Geneva: World Health Organization. 2001.
19. Johnson MJ, Feng X, Johnson LM, et al. Potential of a suite of robot/computer-assisted motivating systems for personalized, home based stroke rehabilitation. J Neuroeng Rehabil. 4(6), 2007.
20. Johnson MJ, Wisneski KJ, Anderson J, et al. Development of ADLER. The activities of daily living exercise robot, BioRob 2006. IEEE NY, NY: The First IEEE/RAS-EMBS International Conference on Biomedical Robotics & Biomechatronics. 2006.
21. Kahn LE, Lum PS, Rymer WZ, et al. Robot-assisted movement training for the stroke-impaired arm. Does it matter what the robot does. J Rehabil Res Dev. 2006:43;5:619-630.
22. Kahn LE, Zygman ML, Rymer WZ, et al. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke. a randomized controlled pilot study. J Neuroeng Rehabil. 2006;3:12.
23. Krebs HI, DiPietro L, Levy-Tzedek S, et al. A paradigm shift for rehabilitation robotics. Engineering in Medicine & Biology Magazine, IEEE. 2008;27(4):61-70.
24. Krebs HI, Mernoff S, Fasoli S, et al. A Comparison of Functional and Impairment-Based Robotic Training in Severe to Moderate Chronic Stroke. A Pilot Study. NeuroRehabilitation. 2008:23;1:81-87.
25. Krebs HI, Volpe BT, Williams D, et al. Robot-aided neurorehabilitation. A robot for wrist rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2007:15;3:327-335.
26. Kwakkel G, Kollen BJ, Krebs BI. Effects of robot-assisted therapy on upper limb recovery after stroke. A systematic review, of gravity-supported, computer-enhanced arm exercise for individuals with severe hemiparesis. Neurorehabil Neural Repair. 2008:22;2:111-121.
27. Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation. a randomized controlled study. Clin Rehabil. 2000:12;4:361-369.
28. Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke. JAMA. 2004;292(15):1853-1861.
29. Lum PS, Burgar CG, van der Loos M, et al. MIME robotic device for the upper-limb neurorehabilitation in subacute stroke subjects. A follow up study. J Rehabil Res Dev. 2006:43;5:631-642.
30. Lum PS, Burgar CG, Shor PC, et al. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83(7):952-959.
31. Lum PS, Taub E, Schwandt D, et al. Automated constraint-induced therapy extension (AutoCITE) for movement deficits after stroke. J Rehabil Res Dev. 2004;41(3A):249-258.
32. McCombe Waller S, Whitall J. Bilateral arm training. Why and who benefits. NeuroRehabilitation. 2008:23;1:29-41.
33. Nef T, Quinter G, Muller R, et al. Effects of arm training with the robotic device ARMin I in chronic stroke. Three single cases. Neurodegener Dis. 2009:6;5–6:240-251.
34. Nef T, Guidali M, Riener R. ARMin III – arm therapy exoskeleton with an ergonomic shoulder actuation. Appl Bionics Biomech. 2009;6(2):127-142.
35. Padova J, Werner L, Mahoney R. Pilot trial of a robot-assisted upper limb therapy system. Arch Phys Med Rehabil. 2007;88(9):E106.
36. Prange GB, Jannink MJA, Groothuis-Oudshoorn CGM, et al. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J Rehabil Res Dev. 2006;43(2):171-184.
37. Rahman T, Sample W, Selliktar R, et al. A body-powered functional upper limb orthosis. J Rehabil Res Dev. 2000;37(6):675-680.
38. Reinkensmeyer DJ, Emken JL, Cramer SC. Robotics, motor learning and neurologic recovery. Annu Rev Biomed Eng. 2004;6:497-525.
39. Reinkensmeyer DJ, Kahn LE, Averbuch M, et al. Understanding and treating arm movement impairment after chronic brain injury. Progress with the ARM guide. J Rehabil Res Dev. 2000:37;6:653-662.
40. Richards LG, Senesac CR, Davis SB, et al. Bilateral arm training with rhythmic auditory cueing in chronic stroke. Not always efficacious. Neurorehabil Neural Repair. 2008:22;2:180-184.
41. Rosenstein L, Ridgel AL, Thota A, et al. Effects of combined robotic therapy and repetitive task practice on upper extremity function in a patient with chronic stroke. Am J Occup Ther. 2008;62(1):28-35.
42. Seelen HAM, Geers RPJ, Soede M, et al. Training of arm-hand kinaesthetics in subacute stroke patients using robotics. J Biomech. 2007;40(Supplement 2):S645.
43. Stein J. e100 NeuroRobotic system. Expert Rev Med Devices. 2009;6(1):15-19.
44. Stein J, Krebs HI, Frontera WR, et al. Comparison of two techniques of robot-aided upper limb exercise training after stroke. Am J Phys Med Rehabil. 2004;83(9):720-728.
45. Stein J, Narendran K, McBean J, et al. Electromyography controlled exoskeletal upper-limb–powered orthosis for exercise training after stroke. Am J Phys Med Rehabil. 2007;86(4):255-261.
46. Takahashi CD, Der-Yeghiaian L, Le V, et al. Robot-based hand motor therapy after stroke. Brain. 2008;131(Pt 2):425-437.
47. Taub E, Lum PS, Hardin P, et al. AutoCITE Automated delivery of CI therapy with reduced effort by therapists. Stroke. 2005;36(6):1301-1304.
48. Timmermans AAA, Seelen HAM, Willmann RD, et al. Technology assisted training of arm-hand skills in stroke. concepts on reacquisition of motor control and therapist guidelines for rehabilitation technology design. J Neuroeng Rehabil. 2009;6:1.
49. Treger I, Faran S, Ring H. Robot-assisted therapy for neuromuscular training of sub-acute stroke patients. A feasibility study. Eur J Phys Rehabil Med. 2008:44;4:431-435.
50. Volpe BT, Krebs HI, Hogan N, et al. A novel approach to stroke rehabilitation. Robot-aided sensorimotor stimulation. Neurology. 2000:54;10:1938-1944.
51. Volpe BT, Lynch D, Rykman-Berland A, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22(3):305-310.
52. Whitall J, McCombe Waller S, Silver KHC. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic stroke. Stroke. 2000;31(10):2390-2395.