chapter 6 Approaches to motor control dysfunction: an evidence-based review
The therapeutic professions are in the midst of a paradigm shift with regard to stroke rehabilitation. This chapter examines the evidence for a traditional approach in stroke rehabilitation (Bobath approach or neurodevelopmental treatment [NDT]). The overwhelming lack of evidence for this approach has led to the articulation of a new clinical paradigm based on a functional task-oriented approach. Evidence for specific therapeutic applications within this task-oriented paradigm is evaluated to help determine the best (most effective) practices for rehabilitation of sensorimotor dysfunction following stroke.
Understanding evidence-based practice
Evidence-based practice has become integrated in occupational therapy (OT) and physical therapy (PT) education and practice in the past few years. Its importance stems from the need to choose the best (most effective and current) available intervention techniques, which is a fundamental ethical responsibility of clinical practice. Sackett coined the term evidence-based medicine and defined it as “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence-based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research.”72
Evidence-based rehabilitation is the application of the principles of evidence-based medicine to problems in the field of rehabilitation. According to Law,44 evidence-based rehabilitation practice is based on a self-directed learning model in which practitioners must take responsibility for continuously evaluating their techniques in an effort to improve them.
Mohide56 identified three basic components of evidence-based practice:
1. Best research evidence: A first step in evidence-based practice is to identify rigorous, clinically relevant research studies that apply to the clinical problem at hand. For this chapter, this would imply an examination of the best available evidence in the rehabilitation of sensorimotor dysfunction following stroke.
2. Clinical expertise: The second step is using one’s clinical expertise and experience to identify patients’ strengths and weaknesses and the risks and benefits of potential interventions. Once the clinician has identified the best research evidence, the next step is to determine if the techniques described in studies apply to the individual patient, given his or her strengths and weaknesses. A focus on the inclusion and exclusion criteria that the studies used is important to determine if the individual patient in question would benefit from the techniques.
3. Patient values: The final step is to incorporate a patient’s values into clinical decision-making.
This chapter examines the best research evidence for stroke rehabilitation. To do so requires first defining criteria by which clinical outcome studies were evaluated.
Criteria for evaluating research articles
For each specific intervention technique, the authors searched databases (such as Medline, Pubmed, CINAHL, PEDro) for randomized controlled trials (or high quality nonrandomized studies with low bias) published in the past decade, i.e. between 1999 and 2009. The analysis was restricted to studies published in English.
Once studies describing clinical trials on the effectiveness of specific techniques were chosen, two criteria were used to rank each study. The first ranking criteria were developed by the Centre for Evidence Based Medicine, Oxford, UK.11 The criteria are based on guidelines proposed by Sackett.72 In this framework, research articles are ranked as follows: (1a) systematic review of randomized clinical trials; (1b) individual randomized clinical trial with narrow confidence interval; (2a) systematic review of cohort studies; (2b) individual cohort study or low quality randomized clinical trial; (3a) systematic review of case-control studies; (3b) individual case-control study; (4) case series, poor quality cohort and case-control studies; (5) expert opinion.
The second criterion was the Physiotherapy Evidence Database (PEDro) score.12 In this 10-point scale, one point is scored if each of the following criteria are satisfied: (1) random allocation of subjects; (2) allocation concealment; (3) baseline similarity across groups; (4) subject blinding; (5) therapist blinding; (6) tester blinding; (7) measures of a key outcome obtained from at least 85% of subjects; (8) intention to treat analysis; (9) between-group statistical comparisons reported; (10) point estimate and measures of variability for at least one key outcome measure.
For this evidence-based review, the selected studies had a score of at least 4/10 on the PEDro score, which is used as an indicator of good quality studies. The PEDro scale was used because its validity and reliability are established.51
Before reviewing the evidence, a brief description of research designs is provided to help the reader understand the terms used in the evidence tables. For more detailed descriptions of research designs, the reader is referred to Helewa and Walker26 and Law.44
Randomized controlled trials or randomized clinical trials (RCT) are the most rigorous way of determining whether a cause-and-effect relationship exists between treatment and outcomes. Some of the important features of randomized trials are:
 Random assignment of subjects to experimental and control groups. Randomization ensures that groups are similar at the beginning of intervention.
Random assignment of subjects to experimental and control groups. Randomization ensures that groups are similar at the beginning of intervention.
 Subject, therapist, and tester blinding: Patients and experimenters should remain unaware of which treatment was given until the study is completed in order to prevent bias.
Subject, therapist, and tester blinding: Patients and experimenters should remain unaware of which treatment was given until the study is completed in order to prevent bias.
 All intervention groups are treated identically except for the experimental treatment.
All intervention groups are treated identically except for the experimental treatment.
A cohort study involves studying groups of individuals who share some common characteristics, such as positive history of stroke. In this case, subjects are not allocated to different groups at random, making them less rigorous than RCT.
The before-and-after design is a study of one group of patients without a control group. When a control group is included, the design is called case control design. Because the control group in this case consists of healthy subjects, the two groups are different at the outset of the study.
Descriptive designs are not rigorous but are useful in describing a disorder in detail.
Paradigm shifts in stroke rehabilitation
The therapeutic professions of OT and PT have witnessed two paradigm shifts related to the treatment of neurological dysfunction. According to Gordon,22 paradigm shifts within therapeutic practice can occur for two reasons: (1) because the theoretical model underlying a therapeutic approach does not fit with current knowledge, and (2) because existing approaches do not appear adequate to solve clinical problems. The past 60 years have witnessed two distinct paradigm shifts in the treatment of stroke.
The first shift occurred in the years immediately following World War II; at that time the dominant therapeutic paradigm was muscle reeducation, which was used extensively to treat peripheral nerve disorders such as poliomyelitis. Although useful for polio, muscle reeducation was not adequate to treat individuals with disorders of the upper motor neuron, such as paresis following stroke. As a result, a few therapists began studying how the nervous system controls movements and began to apply these principles into clinical practice. This approach heralded the development of techniques such as proprioceptive neuromuscular facilitation, Bobath approach or neurodevelopmental treatment (NDT), Brunnstrom movement therapy and sensory integration, to name a few of the prominent approaches.
The neurotherapeutic approaches: the first paradigm shift
Principles of neurotherapeutic approaches.
Although each neurotherapeutic approach is different from each other, all approaches share some common elements.22,23 This section and the subsequent review of the evidence focuses on the Bobath approach/NDT because this approach historically has been the most widely used in stroke rehabilitation. However, the assumptions underlying the Bobath approach also hold true for the other approaches mentioned previously. Some of the common elements of the neurotherapeutic approaches are as follows:
1. The central nervous system is organized hierarchically, with higher centers such as the cerebral cortex exerting a controlling influence over the lower centers (such as the spinal cord). When a deficit occurs in the motor system, more primitive forms of movement (such as reflexive postures and movements), controlled by the lower centers (spinal cord and brainstem), are released from their normal inhibition from the higher centers.7 Thus, treatment within this framework was aimed at reestablishing control by the higher centers.
2. Normal movement can be facilitated by providing specific patterns of sensory input, particularly through the proprioceptive and tactile sensory systems. Under this assumption, sensory stimulation was proposed to produce long-term effects of reestablishing normal sensorimotor neural connections.
3. Recovery from brain damage follows a predictable sequence that mimics normal development. Treatment used developmental postures in an effort to facilitate recovery. This principle has since been eliminated in current concepts of Bobath therapy.69
4. Reflexes were used to facilitate or inhibit motor activity. Experience of normal movement patterns must be provided so that the patient does not learn abnormal patterns of posture and movement after stroke. Reflex inhibitory movement patterns, which were opposite to the pattern of spasticity observed in patients, were used to prevent learning of abnormal movements.
5. Sequelae of stroke can be understood through a neurophysiological explanation. This assumption means that sensorimotor impairments seen after stroke result primarily from the damaged motor system.
The original principles that defined Bobath approach have been adapted and tailored to suit the current knowledge of the functioning of the central nervous system.53 Core theoretical assumptions of the Bobath approach includes an appreciation of task and context specificity of motor learning (a concept associated with a task-oriented approach), neuroplasticity, systems approach that highlights the interaction of the person, task, and environment in producing functional behavior.34,69 This amalgam of old and new conceptual principles have produced a high variability and confusion in practice patterns among therapists using the Bobath approach.53
Outcome studies on neurotherapeutic techniques.
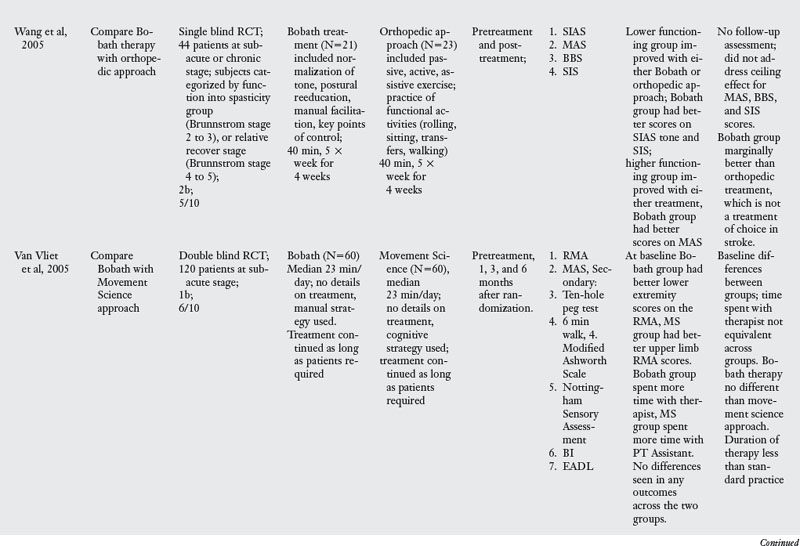
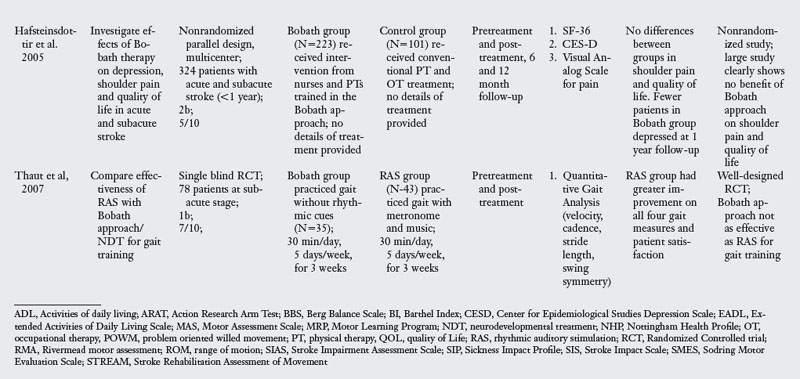
This section evaluates the evidence for the effectiveness of the Bobath approach in stroke rehabilitation. Table 6-1 presents the details of seven RCT and two high quality nonrandomized trials that compared the effectiveness of the Bobath approach with usual care, task-oriented therapy, or orthopedic approach. The studies in the evidence table are listed chronologically.
Timing of therapy.
Of the nine studies related to NDT, two included patients in the acute stage,41,42 three included patients in the subacute stage,67,86,91 two studies included patients in the acute and subacute stages,24,25 one study included patients in the subacute or chronic stage,97 and one study included patients from acute, subacute, and chronic stages.81
Outcomes measures.
Three of the nine studies on the Bobath approach measured outcomes at the impairment level only.67,81,86 Given the importance of testing outcomes at multiple levels of the International Classification of Function (ICF) model, it is important that a majority of studies (six) included outcomes at the impairments and activity levels.24,25,41,42,91,97 Outcome measures at the impairment level ranged from quantitative analysis of gait, Arm Research Action Test, Nine or Ten Hole Peg Test, Stroke Impairment Assessment Scale, Berg Balance Scale, Modified Ashworth Scale, six-minute walk test, and Stroke Rehabilitation Assessment of Movement. The most common outcome measures at the activity limitation level were the Barthel index, Extended ADL Scale, Motor Assessment Scale, and Rivermead Motor Assessment.
Study designs.
The designs included in this review were seven RCT and two high-quality nonrandomized parallel design studies with a large number of subjects. All the studies were classified as either 1b or 2b on the Oxford levels of evidence scale.
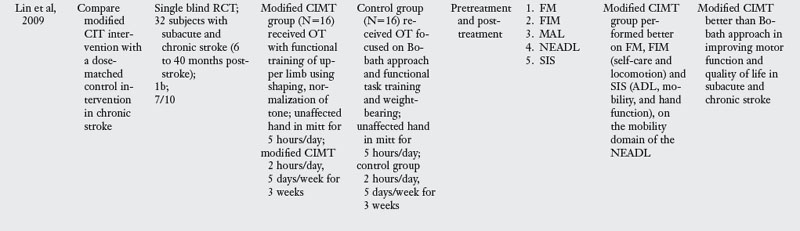
Results of the review.
Of the nine trials examining the effect of Bobath approach, one compared Bobath to an orthopedic approach to stroke rehabilitation,97 two studies compared Bobath with usual care including conventional PT and OT24,25 and the other five compared Bobath approach with the task-oriented approach41,42,67,86,91 or a variant of a task-oriented approach called the problem oriented willed movement therapy.81
The Bobath approach was marginally better than an orthopedic approach,97 which is not the therapy of choice in stroke rehabilitation. When compared with conventional PT and OT, Bobath approach was no better for impairment or activity limitation outcomes.24,25 When compared with a task-oriented approach, which represents a novel approach to stroke rehabilitation, Bobath approach was clearly less effective in four of the six studies.41,67,81,86 There were two exceptions to this pattern: one study42 found no differences in outcomes evaluated at one and four years after the initial therapy was administered, perhaps because patients did not receive therapy in the interim period. The other study91 had methodological limitations that may explain the lack of differences. For instance, at baseline testing, there were differences across the two groups, the amount of time patients spent with the therapist was not the same, and finally the duration of therapy was much less compared with all other studies. Despite these two studies, the evidence overwhelmingly points to the lack of effectiveness of the Bobath approach when compared with a task-oriented approach.
Implications for practice.
Three recent systematic reviews have reported no evidence for the superiority of the Bobath approach.34,48,62 The present review extends the results of the previous systematic reviews to demonstrate that the use of the Bobath approach needs to be reconsidered in stroke rehabilitation.
In the past few years, an attempt has been made among proponents of neurofacilitation approaches to integrate established techniques of NDT with the language of newly emerging knowledge in motor control and motor learning. This is readily seen in a recent text describing the theoretic basis of NDT.30,34 Although this is typical during paradigm shifts, the amalgamation of old techniques with new theoretical knowledge is not useful either theoretically (since established Bobath techniques are not consistent within the new paradigm of motor control and learning) or for clinical practice (since numerous studies have demonstrated that there is indeed little evidence). The challenge for therapists is to design and evaluate techniques within the newly emerging paradigm of task-oriented training.
Functional task-oriented training: the second paradigm shift
The second paradigm shift in the treatment of neurological disorders began in the 1990s. Therapists began to regard neurotherapeutic approaches with less optimism. The dissatisfaction with the neurotherapeutic approaches is due, in part, to the fact that retraining normal movement patterns do not carry over into the performance of functional daily living skills, which is the ultimate goal of rehabilitation. In addition, there is a greater demand on therapists to use interventions that have demonstrated effectiveness. Evidence that demonstrates a lack of effectiveness of neurotherapeutic approaches, particularly the Bobath approach, has led to the development of novel training regimens based on what has been termed the task-oriented approach.75
Principles of the functional task-oriented approach.
The task-oriented approach is based on a systems model of motor control and theories of motor learning. The approach attempts to understand the problems faced by the nervous system to control movements. This field of motor neuroscience represents a multidisciplinary approach to understanding motor control and learning from the perspectives of neurophysiology, biomechanics, and behavioral sciences. Within this framework, motor control is understood as an attempt by the nervous system to adapt movements to constraints imposed by the mechanics of the motor apparatus (including length, mass of limbs, and intersegmental dynamics of moving segments), constraints imposed by the environment (open or closed environment), and constraints imposed by the behavioral context. Studies on motor control often analyze movements at the biomechanical and behavior levels. See Chapters 4 and 5 for a detailed description.
Chapter 4 provides the reader with a more comprehensive description of the task-oriented approach. What follows is a brief description of some of the incipient principles of treatment, based on suggestions by Carr and Shepherd10 and Gentile.21 Within this framework, the responsibility of the therapist as a teacher of motor skills is to select contextually appropriate functional tasks, vary task parameters to ensure greater transfer of learning, structure practice schedules to encourage active participation of the patients, structure the environment so that all regulatory conditions of a given task are present, and provide feedback. To apply a task-oriented approach to treatment successfully, therapists need to become familiar with analyzing tasks and the processes underlying skill acquisition. The following two sections evaluate the literature on task-oriented approach to stroke rehabilitation.
Outcome studies using a task-oriented approach.
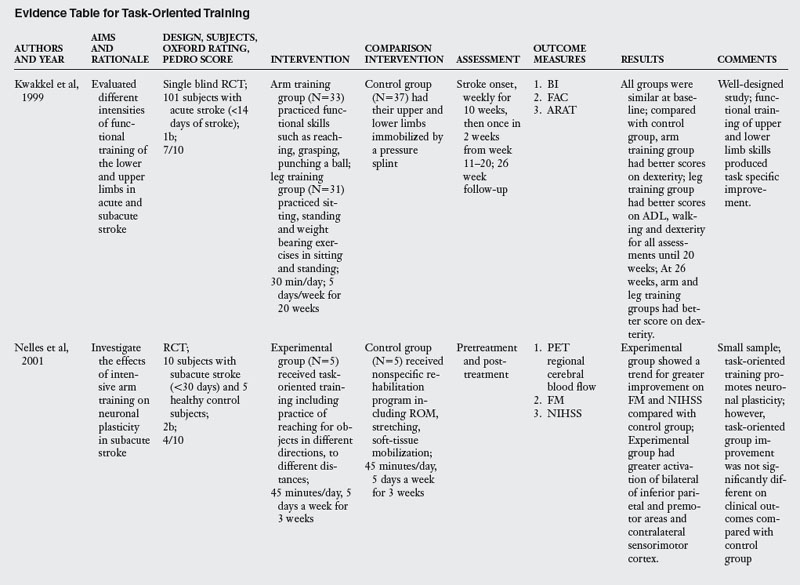
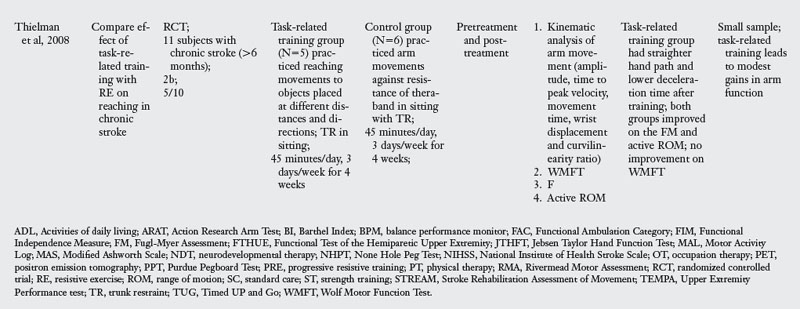
For the purpose of this review, the author chose eleven RCT that explicitly tested a task-oriented intervention for rehabilitation of upper limb function (Table 6-2). Length of the training programs across the studies varied from two to six weeks, the number of sessions ranging from 10 to 20.
Timing of intervention.
Of the eleven trials, one study tested patients in the acute stage,58 five tested patients in the subacute stage,5,16,17,60,100 one study trained patients in the acute and subacute stages,38 and four tested patients in the chronic stage.29,54,87,88
Outcome measures.
Four of the eleven studies only measured outcomes at the impairment level,16,54,60,88 whereas six studies measured outcomes at the impairment and activity limitation levels.5,17,38,58,87,100 Only one study measured outcomes at all three levels of the ICF model.29 Variables at the impairment level commonly tested were gait velocity, endurance, ground reaction forces, kinematic variables in reaching, Action Research Arm Test, and positron emission tomography (PET) scan. Variables related to activity limitation were measured using the 36-item Short-Form Health Survey, Barthel index, and the Functional Ambulation Classification. The only outcome at the participation level was the OARS-IADL and SF-36.
Study designs used.
All eleven studies included in the review were RCT, of which three were rated as 1b and the other eight rated as 2b, according to the Oxford criteria. The PEDro score ranged from 4 to 8, indicating that all were high quality studies.
Results of the review.
Task-oriented training demonstrated positive outcome when compared with immobilization,38 resistance training,87,88 Bobath approach,58,100 and usual PT and OT.16,60 Task-oriented therapy was effective in the acute (one study) and subacute stages of stroke (five out of six studies). In the chronic stage, two studies documented effectiveness primarily for lower functioning subjects.54,87 One study did not find arm training better than lower limb training,29 and one study found modest gains.88
Table 6-2 shows substantial evidence to suggest that task-oriented training leads to improvement of outcomes at the impairment and activity limitation levels. However, since some studies showing a positive effect of task-oriented therapy tested outcomes at the impairment level only, it will be useful for future randomized trials to include outcome measures at multiple levels of the ICF model.
Clinical implications.
The present review of task-oriented training studies confirms the results of two recent reviews,31,70 there is very good evidence for the effectiveness of this approach in comparison with traditional therapy, Bobath approach, or immobilization. These studies demonstrate that improvement in motor skills and function depends on contextually appropriate task-specific practice of functional skills. Given that this approach is relatively new, additional RCT with larger number of subjects are needed. It will also be important to have control groups that receive dose equivalent standard care.
A number of studies were not included under the general category of task-oriented approach because these studies tested a specific type of task-oriented therapy called constraint-induced movement therapy (CIMT), described in detail in the subsequent section.
Constraint-induced movement therapy
Rationale and principles.
Constraint-induced movement therapy is a term used for a family of intervention techniques that aim to decrease the effects of learned nonuse of a paretic limb. This family of techniques involves two basic features: (1) discouraging the use of the unaffected or less affected limb through verbal prompt but more often by applying some form of restraint to the unaffected limb with a sling, splint, or a mitten, and (2) intensive training of the paretic arm through active participation in functional activities.8,89,90
Some authors have proposed that the inability to move the paretic limb may arise, at least in part, from a phenomenon termed learned nonuse. The proposal is based on experiments in which deafferentation was performed in one limb in primates through dorsal rhizotomy.82 Following surgery, monkeys did not use their affected limbs because of the lack of sensory feedback, and they preferentially used their unaffected limbs. When the monkeys were forced to use their affected limbs, greater recovery of movement was seen. This indicates that the inability to use the affected limb may be a behavioral learned response to paresis. See Chapters 4, 5, and 10.
Outcome studies.
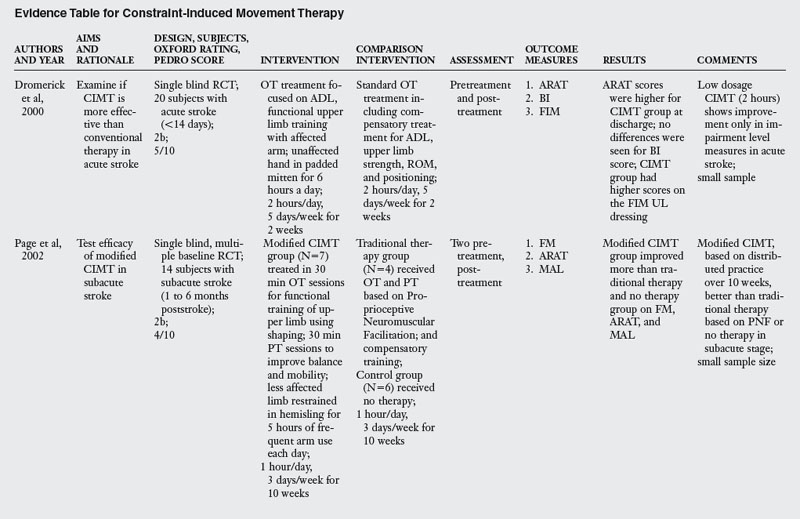
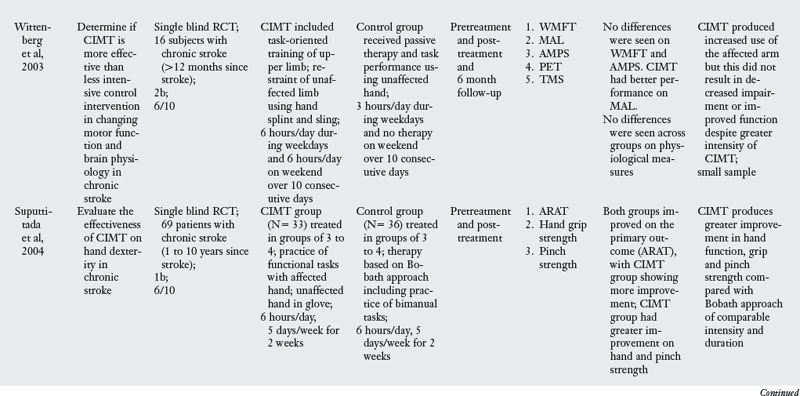
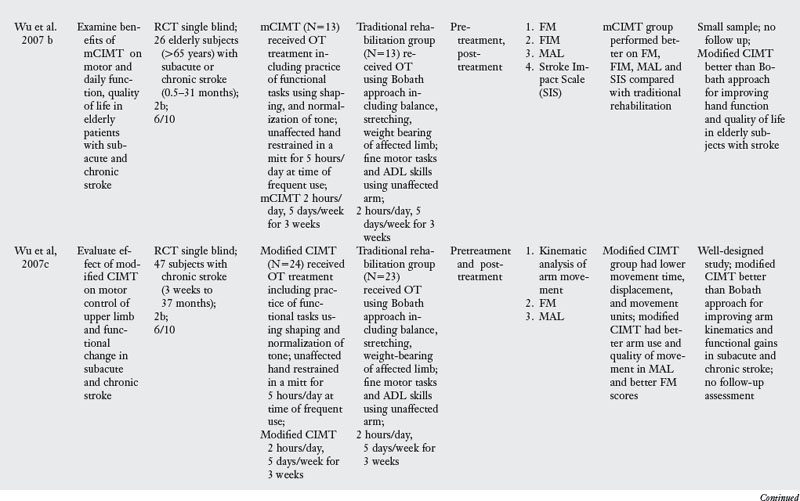
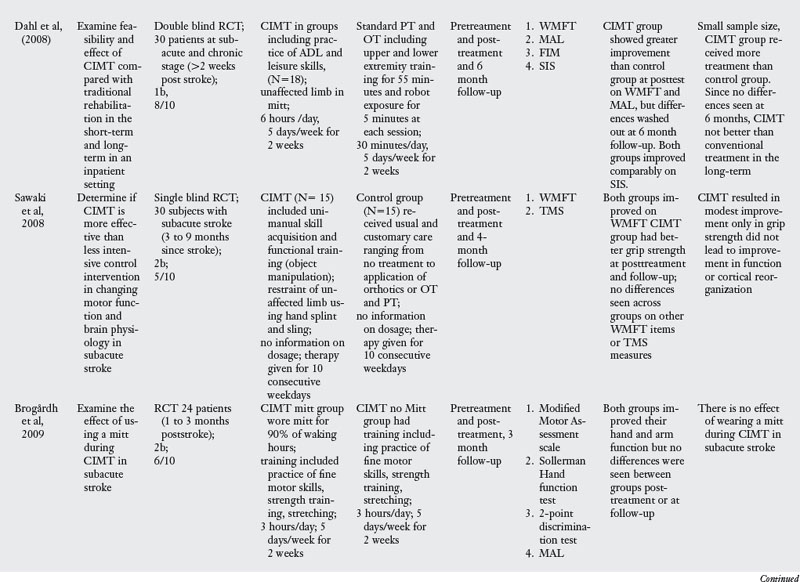
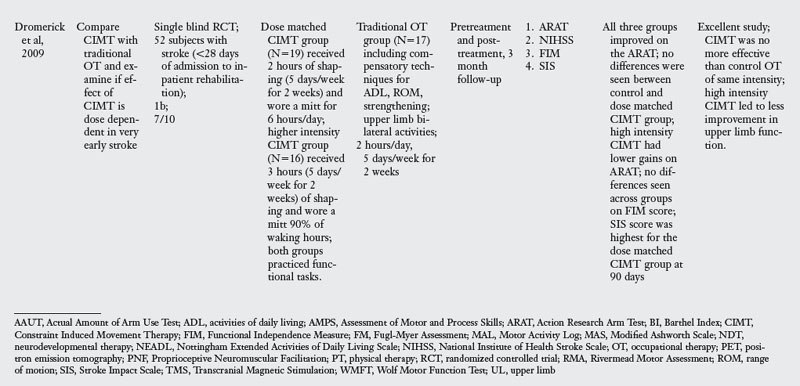
In the past decade, a large number of studies have been conducted on the effects of CIMT. As seen in Table 6-3, eighteen RCT and one dose-equivalent placebo-controlled trial were identified. All nineteen studies scored either 1b or 2b on the Oxford levels of evidence scale, and the PEDro score ranged from 4 to 8 out of a score of 10. This indicates that all studies were high quality trials. CIMT studies are unique because all studies were careful in subject selection: all subjects included required a minimum of 10-degrees of active extension at the metacarpophalangeal joint and 20-degrees of active extension at the wrist. In addition, a number of studies excluded patients with excessive spasticity and sensory deficits. The narrow specification of inclusion and exclusion criteria may have led to the selection of a highly homogeneous group of patients most likely to recover from stroke based on spontaneous recovery.32,36
There was tremendous variability in the form of CIMT and the dosage of intervention. Seven of the nineteen trials tested the standard version of CIMT, which included six hours of practice in each session. The other three trials of standard CIMT included either two hours,18 three hours,9 or four hours of training in each session.59 However, all the ten trials of standard CIMT provided massed practice over 10 sessions across two weeks. Nine trials tested a modified version of CIMT, in which practice was distributed over sessions ranging from 12 to 30. Modified CIMT trials have been designed to replicate therapeutic dosage similar to standard practice. However, as Table 6-3 demonstrates, there is tremendous variability in dosage, ranging from 30 minutes to six hours of practice per session.
Since one of the major principles of CIMT is constraint of the unaffected hand, all studies included some form of constraint using a mitt, sling, hemisling, or splint. There was a large variability across studies in terms of the hours of restraint (from five hours to 90% of waking hours).
Timing of intervention.
Four CIMT trials were conducted in the acute stage,6,18,19,66 four trials were conducted in the subacute stage,9,59,63,73 and six trials were conducted in the chronic stage.46,65,80,85,101,104 Five trials included subjects both in the subacute and the chronic stage.14,46a,103,105,106
Outcome measures.
Most of the studies reviewed measured outcomes at the impairment and activity limitation level. Typical instruments used to measure impairment level measures were the Action Research Arm Test (which measures upper limb dexterity), the Fugl-Meyer Assessment (which measures the ability of the arm to move against the typical synergistic pattern), the Wolf Motor Function Test (which quantifies motor function after stroke), PET scan, and Transcranial Magnetic Stimulation. Activity limitation was measured by measures such as the Rehabilitation Activities Profile (based on the ICF and, which assesses disability and handicap), Motor Activity Log (which measures actual amount of use and quality of movement), Barthel index, and the Functional Independence Measure (which measures activity limitation). Participation restriction was measured by administration of the Stroke Impact Scale in a few studies.
Results of the review.
The results are equivocal at present. CIMT is clearly better than the Bobath approach either in the subacute or chronic stages. When compared with usual care or conventional functional OT and PT, CIMT is more beneficial in studies where the CIMT group received a higher dosage of therapeutic intervention. When compared with dose equivalent functional training, CIMT does not seem more effective. The one exception was the study by Taub and colleagues85 who found CIMT to be more effective than general fitness. However, the results of this study have to interpreted with caution as there were differences between groups at baseline, and subjects were not randomized. In the acute stage, CIMT is no more effective than dose equivalent functional OT treatment. Higher dose CIMT was less beneficial as compared with low dose CIMT.19 In the subacute stage, when CIMT is compared with dose equivalent functional training, both interventions result in similar improvement (see Table 6-3).
Clinical implications.
CIMT appears to be a beneficial approach, but future studies need to compare CIMT with dose equivalent functional task-oriented training, which is shown to be effective. Such a study may address the criticism that the improvements demonstrated are due to a nonspecific effect of increased intensity of treatment rather than to a specific effect of constrained-induced training. Most of the studies reported in Table 6-3 used a standard CIMT training protocol in which training was massed over a period of two weeks and was compared with a control group that received less intense conventional training or ineffective traditional approaches, such as the Bobath approach. Studies using a modified CIMT protocol, while demonstrating some benefits, had the limitation of small sample size or comparison with traditional therapy known to be less effective (Bobath approach).
According to Taub and Uswatte,84 the improvements seen with CIMT could be a result of massing of practice. Given that similar positive results have been obtained by increasing the intensity of traditional therapy, van der Lee90 argues that using traditional therapeutic procedures that often may be less frustrating to patients than CIMT may be just as effective.
Robot-aided motor training for upper limb function
Rationale and principles.
A recent addition to the arsenal of techniques for stroke rehabilitation is the use of robotic manipulators for providing training of arm movements. Robot manipulators have been used successfully in experimental paradigms that attempted to elucidate the mechanisms underlying normal motor control and learning74 and also to clarify mechanisms underlying disorders of upper limb movements in patients with movement disorders.76
The rationale for using a robotic device in rehabilitation is to decrease the labor-intensive nature of therapy and to provide a device that could be used for quantitative evaluation and treatment.40 Proponents of this approach contend that current therapeutic evaluations are usually subjective and that therapists spend much time on one-on-one interaction with patients. The idea is to have devices available at rehabilitation centers for use when the patient is not in therapy sessions. Given that patients spend a large percentage of time outside therapist interaction, an attempt at facilitating practice during this time should be beneficial. Robot-assisted training attempts to provide intensive practice of repetitive and stereotyped movements. See Chapter 11 for a full discussion of this topic.
Outcome studies.
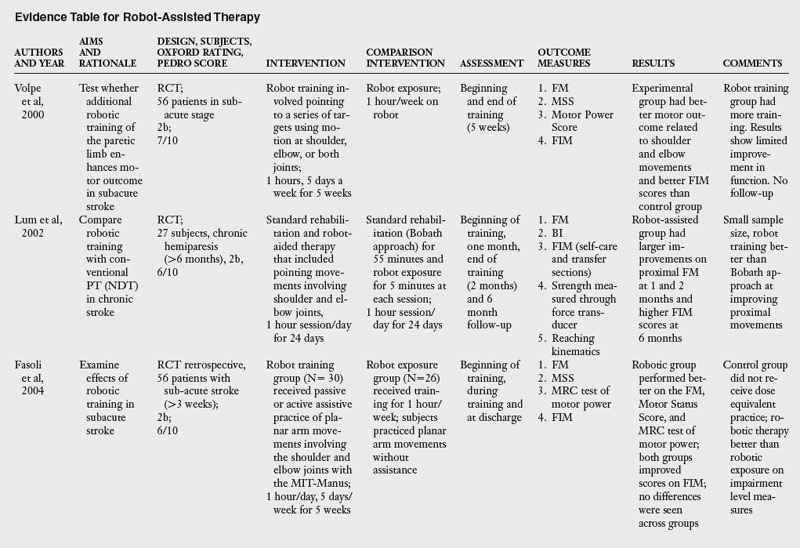
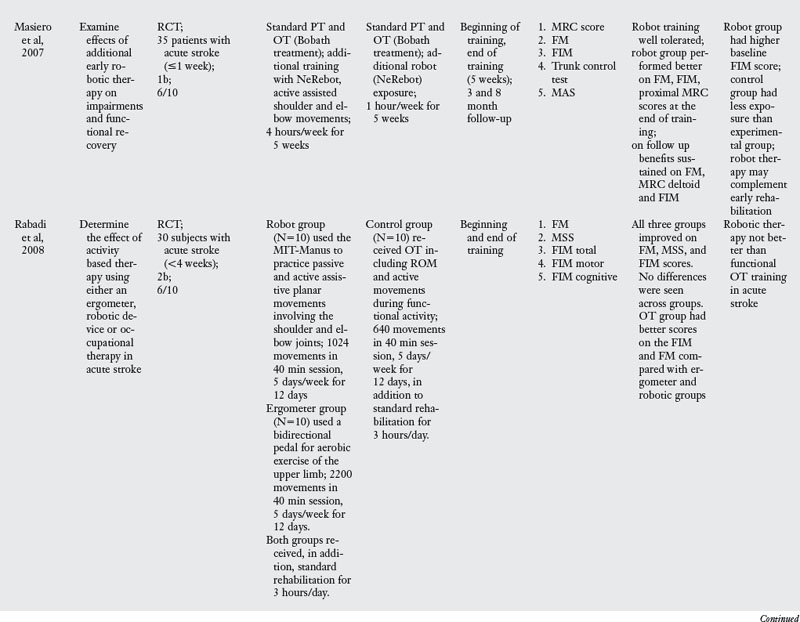
A review of studies testing the effectiveness of robot assisted training revealed ten RCT, as listed in Table 6-4. Typical training with this approach involves the patient making horizontal plane movements while grasping the handle of the robot manipulator. Target locations and patient movement are displayed on a computer screen in front of the patient. Typically, patients are trained to produce movements of the shoulder and elbow joints while the wrist and hand joints and the trunk are immobilized with restraints. The robot is typically programmed to either passively move the paretic limb or produce an assistive force during movements. The number of sessions (12 to 60) and the total training time (eight hours to 300 hours) varied tremendously across studies.
Timing of intervention.
Two studies tested patients in the acute stage,52,68 four studied patients in the subacute stage,20,28,50,93 and four studies tested patients at the chronic stage.15,33,49,95
Outcome measures.
Most of the studies reviewed measured outcome variables at the impairment and activity limitation levels. The exceptions were studies that tested outcomes only at the impairment level.15,28 Typical instruments used to measure impairments included the Fugl-Meyer Assessment, Action Research Arm Test, Trunk Control Test, and kinematic analysis of arm movement. Instruments used to measure activity limitation were the Functional Independence Measure, Chedoke-McMaster Stroke Scale, and the Barthel index.
Results of the review.
The results of effectiveness of robot assisted training are fairly clear; when compared with robot exposure,20,92 traditional therapy using the Bobath approach49,50,52 or neuromuscular facilitation,15,28 robot training is more effective in improving function. This result can be explained by the fact that subjects in the robot groups received more training of upper limb movements compared with the control groups. However, when robot assisted training is compared with dose equivalent functional training, robot assisted training offers no additional benefits.33,68,95
Clinical implications.
The results highlight that robot assisted training offers no advantage to functional training with a therapist. Its effectiveness is limited to studies where robot assisted training was compared with traditional approaches that have been shown to be ineffective (such as the Bobath approach). Given the expense and extensive training of personnel to use the robot device, and its limited effectiveness, it may be beneficial to think of testing robotic devices as an adjunct to therapy rather than as a primary method of therapy delivery. Before additional RCT are implemented, the rationale and experimental procedures need to be clarified. For instance, at present, robot training provides practice of pointing movements (movements of the shoulder and elbow) on the horizontal plane. In an effort to isolate movements to these two joints, the trunk and distal extremities are often stabilized by constraints producing rather unnatural conditions for practice of arm movements. Functional reaching movements involve coordinated movement of the trunk-arm complex and of the wrist-hand complex. Whether practice of isolated components of the shoulder-elbow complex would transfer to real-world situations is unclear, given the task-specific nature of transfer of training. The responsibility of therapists is to select appropriate, challenging functional tasks, vary task parameters, progress to more difficult tasks, and test for transfer. Given the complexity of therapeutic training, robot manipulators can perhaps serve best by providing quantitative evaluation of impairments rather than as a therapeutic tool.
Body weight support and treadmill training to improve gait
Rationale and principles.
Approximately half the individuals who suffer a stroke do not recover their ability to walk independently.32 Given that independent walking is a necessary prerequisite to successful community reintegration, not surprisingly gait training has occupied an important role in therapeutic practice following stroke. Gait training following stroke involves practice of individual segments of walking, practice of walking over ground with assistance of therapists and/or assistive devices, or more recently, practice of walking on a treadmill with partial body weight support.
Experiments on animals have shown that the basic neural circuitry for producing the rhythmic alternating movements of the lower limb is at the spinal cord level. Locomotor training with weight support of the hindlimbs has been shown to improve gait to near normal levels in cats whose spinal cords have been transected at thoracic levels, thereby isolating lower cord segments from the rest of the central nervous system.2 In fact, patients with spinal cord injury have been shown to improve after treadmill training with body weight support.99 Apart from the limited early evidence of the benefit of treadmill training in patients with spinal cord injury, the rationale for this approach is that it removes some of the biomechanical and equilibrium constraints of weight-bearing and facilitates walking by activation of spinal locomotor circuits. See Chapter 15.
Outcome studies.
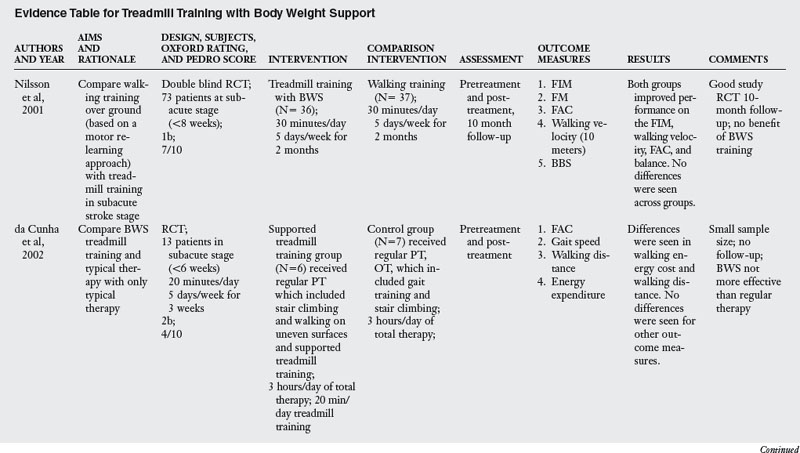
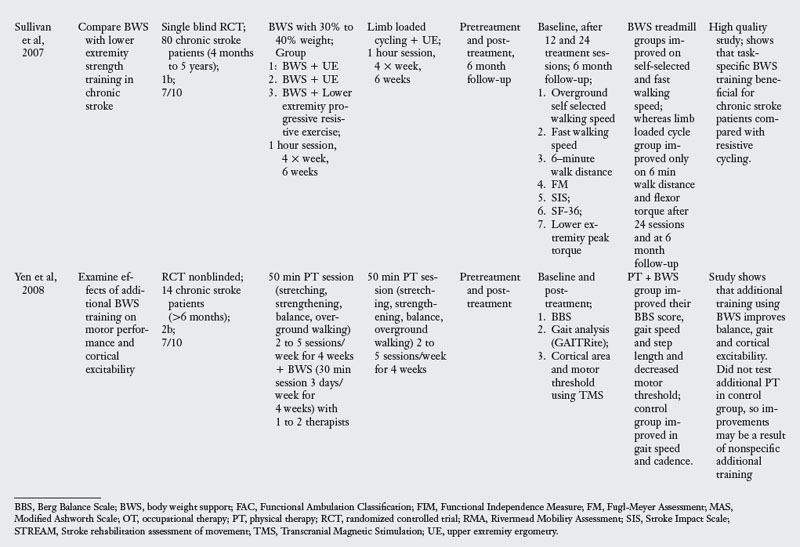
A review of studies testing the effectiveness of body weight support training revealed six RCT listed in Table 6-5. Typical training with this approach involves beginning gait training on a treadmill by supporting the body in a harness. The initial support given was generally 40% of the body weight, which is gradually decreased as the patient improves. The length of training ranged from 12 to 42 sessions conducted across four to eight weeks. In two of the studies, body weight support treadmill training was coupled with gait training.
Timing of intervention.
Treadmill training was initiated in the subacute stage in four studies3,13,61,98 and in the chronic stage in two studies.79,107
Outcome measures.
Most of the studies reviewed measured outcome variables at the impairment and activity limitation levels. Only one study measured outcomes at all three levels of the ICF.79 Typical instruments used to assess impairment level measures were the Stroke Rehabilitation Assessment of Movement (which evaluates voluntary movement of the limbs and mobility), Berg Balance Scale (which evaluates balance during sitting and standing activities), walking speed, distance and endurance, the Fugl-Meyer Assessment (which evaluates locomotor function and control, sensory quality, and balance), and kinematic analysis of walking. Instruments used to measure activity limitation were the Functional Independence Measure, Functional Ambulation Classification (which quantifies amount of assistance needed in walking), and the Rivermead Motor Assessment. Instruments used to measure participation limitation were Stroke Impact Scale and SF-36.
Results of the review.
When compared with functional training of ambulation or training with an electromechanical trainer, body weight supported treadmill training was no more effective, all interventions producing similar, but positive, outcomes. When compared with control groups that did not have training of walking, body weight support was more effective in outcomes related to walking and balance. When body weight support was added to the PT intervention, it was more effective.107 However, this benefit may be the result of additional training since the control group did not receive dose equivalent therapy. The clearest evidence for the benefit of body weight support treadmill training was seen for severely impaired patients.3
Clinical implications.
The review suggests that training of walking and balance may be task-specific, and body weight support treadmill training may not be more effective compared with functional training without body weight support. When examined in the context of the high cost associated with body weight support apparatus, and the number of therapists required to administer therapy, functional training may be more cost-effective and equally beneficial. The only indication for body weight support training may be in the case of severely impaired patients who may benefit from relearning the walking movement patterns without being encumbered with controlling their body weight and forward progression.
Summary
A challenging yet exciting period for stroke rehabilitation is occurring as occupational and physical therapists are being asked to provide training based on sound scientific principles and with demonstrated effectiveness. The lack of support for traditional neurotherapeutic approaches, such as Bobath approach, recent advances in understanding of motor control and dyscontrol, and emerging technologies have facilitated a second paradigm shift toward a functional task-oriented approach. At present, the literature suggests that task-oriented training of the upper limb and functional walking training is the most effective method in stroke rehabilitation. The challenge for the next decade is to develop more creative, functional, task-oriented intervention techniques that will maximize the independent functioning of patients within their natural contextual settings10 and to test these techniques in a systematical manner at different stages of the recovery process, in different practice settings, and at different intensities. Most likely, no one technique will offer a panacea for stroke rehabilitation given the varied nature of impairments and activity limitations.
Acknowledgments
The author dedicates this chapter to the memory of his uncle Dr. Sangameshwar who lost his battle to stroke during the writing of this chapter. The author acknowledges Glen Gillen and Clare Bassile for helpful discussions.
Review questions
1. What is evidence-based practice?
2. What are the principles of evidence-based practice?
3. What are the criteria for reviewing articles on treatment outcomes?
4. Describe the most common research designs used in outcome studies.
5. What are some of the basic principles of neurotherapeutic approaches?
6. Is there evidence to support the application of neurotherapeutic approaches?
7. What are some of the basic principles of the functional task-oriented approach?
8. Describe the evidence to support the task-oriented approach, CIMT, treadmill training and body weight support, and robot-assisted training.
1. Aisen ML, Krebs HI, Hogan N, et al. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Arch Neurol. 1997;54(4):443-446.
2. Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412(1):84-95.
3. Barbeau H, Visintin M. Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch Phys Med Rehabil. 2003;84(10):1458-1465.
4. Blanton S, Wolf SL. An application of upper-extremity constraint-induced movement therapy in a patient with subacute stroke. Phys Ther. 1999;79(9):847-853.
5. Blennerhassett J, Dite W. Additional task-related practice improves mobility and upper limb function early after stroke. a randomised controlled trial. Aust J Physiother. 2004:50;4:219-224.
6. Boake C, Noser EA, Ro T, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21(1):14-24.
7. Bobath B. Adult hemiplegia. evaluation and treatment, ed 3. Oxford: Butterworth-Heinemann. 1990.
8. Bonaiuti D, Rebasti L, Sioli P. The constraint induced movement therapy. a systematic review of randomised controlled trials on the adult stroke patients. Eura Medicophys. 2007:43;2:139-146.
9. Brogårdh C, Vestling M, Sjölund BH. Shortened constraint-induced movement therapy in subacute stroke—no effect of using a restraint. a randomized controlled study with independent observers. J Rehabil Med. 2009:41;4:231-236.
10. Carr JH, Shepherd RB. Stroke rehabilitation. guidelines for exercise and training to optimize motor skill. Oxford: Butterworth-Heinemann. 2003.
11. Centre for Evidence Based Medicine. Levels of evidence. (website) www.cebm.net/index.aspx?o=1025, November 2009. Accessed
12. Centre of Evidence-Based Physiotherapy. Physiotherapy evidence database. (website) www.pedro.org.au/english/downloads/pedro-scale/, November 2009. Accessed
13. da Cunha ITJr, Lim PA, Qureshy H, et al. Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training. a randomized controlled pilot study. Arch Phys Med Rehabil. 2002:83;9:1258-1265.
14. Dahl AE, Askim T, Stock R, et al. Short- and long-term outcome of constraint-induced movement therapy after stroke. a randomized controlled feasibility trial. Clin Rehabil. 2008:22;5:436-447.
15. Daly JJ, Hogan N, Perepezko EM, et al. Response to upper-limb robotics and functional neuromuscular stimulation following stroke. J Rehabil Res Dev. 2005;42(6):723-736.
16. Dean CM, Channon EF, Hall JM. Sitting training early after stroke improves sitting ability and quality and carries over to standing up but not to walking. a randomised trial. Aust J Physiother. 2007:53;2:97-102.
17. Desrosiers J, D Bourbonnais D, Corriveau H, et al. Effectiveness of unilateral and symmetrical bilateral task training for arm during the subacute phase after stroke. a randomized controlled trial. Clin Rehabil. 2005:19;6:581-593.
18. Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke. Stroke. 2000;31(12):2984-2988.
19. Dromerick AW, Lang CE, Birkenmeier RL, et al. Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS). A single-center RCT. Neurology. 2009:73;3:195-201.
20. Fasoli SE, Krebs HI, Ferraro M, et al. Does shorter rehabilitation limit potential recovery poststroke. Neurorehabil Neural Repair. 2004;18(2):88-94.
21. Gentile AM. Skill acquisition. action, movement and neuromotor processes. Carr J, Shepherd RB, editors. Movement science: foundations for physical therapy in rehabilitation. Gaithersburg, MD: Aspen, 2000.
22. Gordon J. Assumptions underlying physical therapy intervention. theoretical and historical perspectives. Carr J, Shepherd RB, editors. Movement science: foundations for physical therapy in rehabilitation. Gaithersburg, MD: Aspen, 2000.
23. Hafsteinsdottir TB. Neurodevelopmental treatment. application to nursing and its effects on the hemiplegic stroke patient. J Neurosci Nurs. 1996:28;1:36-47.
24. Hafsteinsdottir TB, Algra A, Kappelle JL, Grypdonck MHF. Neurodevelopmental treatment after stroke. a comparative study. J Neurol Neurosurg Psychiatry. 2005:76;6:788-792.
25. Hafsteinsdottir TB, Kappelle JL, Grypdonck MHF, Algra A. Effects of Bobath-bed therapy on depression, shoulder pain and health-related quality of life in patients after stroke. J Rehabil Med. 2007;39(8):627-632.
26. Helewa A, Walker JM. Critical evaluation of research in physical rehabilitation. Philadelphia: Saunders; 2000.
27. Hesse SA, Bertelt C, Schaffrin A, et al. Restoration of gait in nonambulatory hemiparetic patients by treadmill training with partial body-weight support. Arch Phys Med Rehabil. 1994;75(10):1087-1093.
28. Hesse S, Werner C, Pohl M, et al. Computerized arm training improves the motor control of the severely affected arm after stroke. a single-blinded randomized trial in two centers. Stroke. 2005:36;9:1960-1966.
29. Higgins J, Salbach NM, Wood-Dauphinee S, et al. The effect of a task-oriented intervention on arm function in people with stroke: a randomized controlled trial. Clin Rehabil. 2006;20(4):296-310.
30. Howle JM. Neuro-developmental treatment approach. theoretical foundations and principles of clinical practice. Laguna Beach, CA: Neuro-Developmental Treatment Association. 2002.
31. Hubbard IJ, Parsons MW, Neilson C, Carey LM. Task-specific training. evidence for and translation to clinical practice. Occup Ther Int. 2009:16;3–4:175-189.
32. Jørgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995:76;5:399-405.
33. Kahn LE, Zygman ML, Rymer WZ, Reinkensmeyer DJ. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke. a randomized controlled pilot study. J Neuroeng Rehabil. 2006;3:12.
34. Kollen BJ, Lennon S, Lyons B, et al. The effectiveness of the Bobath concept in stroke rehabilitation. Stroke. 2009;40(4):89-97.
35. Krebs HI, Hogan N, Aisen ML, et al. Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng. 1998;6(1):75-87.
36. Kreisel SH, Hennerici MG, Bäzner H. Pathophysiology of stroke rehabilitation. the natural course of clinical recovery, use-dependent plasticity and rehabilitative outcome. Cerebrovasc Dis. 2007:23;4:243-255.
37. Kunkel A, Kopp B, Muller G, et al. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80(6):624-628.
38. Kwakkel G, RC Wagenaar RC, Twisk JW, et al. Intensity of leg and arm training after primary middle-cerebral-artery stroke. a randomised trial. Lancet. 1999:354;9174:191-196.
39. Kwakkel G, Kollen BJ, Wagenaar RC. Long term effects of intensity of upper and lower limb training after stroke. a randomised trial. J Neurol Neurosurg Psychiatry. 2002:72;4:473-479.
40. Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke. A systematic review. Neurorehabil Neural Repair. 2008:22;2:111-121.
41. Langhammer B, Stanghelle JK. Bobath or motor relearning programme. A comparison of two different approaches of physiotherapy in stroke rehabilitation: a randomized controlled study. Clin Rehabil. 2000:14;4:361-369.
42. Langhammer B, Stanghelle JK. Bobath or motor relearning programme. A follow-up one and four years post stroke. Clin Rehabil. 2003:17;7:731-734.
43. Laufer Y, Dickstein R, Chefez Y, et al. The effect of treadmill training on the ambulation of stroke survivors in the early stages of rehabilitation. a randomized study. J Rehabil Res Dev. 2001:38;1:69-78.
44. Law M. In Evidence-based rehabilitation. a guide to practice, ed 2. Thorofare, NJ: Slack. 2008.
45. Liepert J, Bauder H, Wolfgang HR, et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31(6):1210-1216.
46. Lin KC, Wu CY, Wei TH, et al. Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke. a randomized controlled study,. Clin Rehabil. 2007:21;12:1075-1086.
46a. Lin K, Wu C, Liu J, et al. Constraint-induced therapy versus dose-matched control intervention to improve motor ability, basic/extended daily functions, and quality of life in stroke. Neurorehab Neural Repair. 2009;23(2):160-165.
47. Lord JP, Hall K. Neuromuscular reeducation versus traditional programs for stroke rehabilitation. Arch Phys Med Rehabil. 1986;67(2):88-91.
48. Luke C, Dodd KJ, Brock K. Outcomes of the Bobath concept on upper limb recovery following stroke. Clin Rehabil. 2004;18(8):888-898.
49. Lum PS, Burgar CG, Shor PC, et al. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83(7):952-959.
50. Lum PS, Burgar CG, Van der Loos M, et al. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects. A follow-up study. J Rehabil Res Dev. 2006:43;5:631-642.
51. Maher CG, Sherington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713-721.
52. Masiero S, Celia A, Rosati G, Armani M. Robotic-assisted rehabilitation of the upper limb after acute stroke. Arch Phys Med Rehabil. 2007;88(2):142-149.
53. Mayston M. Bobath concept. Bobath@50mid-life crisis—what of the future. Physiother Res Int. 2008;13;3:131-136. Editorial
54. Michaelsen SM, Dannenbaum R, Levin MF. Task-specific training with trunk restraint on arm recovery in stroke. randomized control trial. Stroke. 2006:37;1:186-192.
55. Miltner WH, Bauder H, Sommer M, et al. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke. a replication. Stroke. 1999:30;3:586-592.
56. Mohide EA. What is EBP. how do we facilitate its use. Wong R, editor. Evidence-based healthcare practice: general principles and focused applications to geriatric physical therapy. Arlington, VA: Marymount University, 2002.
57. Monger C, Carr JH, Fowler V. Evaluation of a home-based exercise and training programme to improve sit-to-stand in patients with chronic stroke. Clin Rehabil. 2002;16(4):361-367.
58. Mudie MH, Winzeler-Mercay U, Radwan S, Lee L. Training symmetry of weight distribution after stroke. a randomized controlled pilot study comparing task-related reach, Bobath and feedback training approaches. Clin Rehabil. 2002:16;6:582-592.
59. Myint JM, Yuen GF, Yu TK, et al. A study of constraint-induced movement therapy in subacute stroke patients in Hong Kong. Clin Rehabil. 2008;22(2):112-124.
60. Nelles G, Jentzen W, Jueptner M, et al. Arm training induced brain plasticity in stroke studied with serial positron emission tomography. Neuroimage. 2001;13(6 pt 1):1146-1154.
61. Nilsson L, Carlsson J, Danielsson A, et al. Walking training of patients with hemiparesis at an early stage after stroke. a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clin Rehabil. 2001:15;5:515-527.
62. Paci M. Physiotherapy based on the Bobath concept for adults with post-stroke hemiplegia. a review of effectiveness studies. J Rehabil Med. 2003:35;1:2-7.
63. Page SJ, Sisto SA, Johnston MV, et al. Modified constraint-induced therapy after subacute stroke. a preliminary study. Neurorehabil Neural Repair. 2002:16;3:290-295.
64. Page SJ, Sisto SA, Levine P, et al. Modified constraint induced therapy. a randomized feasibility and efficacy study. J Rehabil Res Dev. 2001:38;5:583-590.
65. Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of modified constraint-induced movement therapy in chronic stroke. a single-blinded randomized controlled trial. Arch Phys Med Rehabil. 2004:85;1:14-18.
66. Page SJ, Levine P, Leonard AC Modified constraint-induced therapy in acute stroke. a randomized controlled pilot study. Neurorehabil Neural Repair. 2005;19(1):27-32.
67. Platz T, Eickhof C, van Kaick S, et al. Impairment-oriented training or Bobath therapy for severe arm paresis after stroke. a single-blind, multicentre randomized controlled trial. Clin Rehabil. 2005:19;7:714-724.
68. Rabadi M, Galgano M, Lynch D, et al. A pilot study of activity-based therapy in the arm motor recovery post stroke. a randomized controlled trial. Clin Rehabil. 2008:22;12:1071-1082.
69. Raine S. The current theoretical assumptions of the Bobath approach as determined by the members of the BBTA. Physiother Theory Pract. 2007;23(3):137-152.
70. Rensink M, Schuurmans M, Lindeman E, Hafsteinsdóttir T. Task-oriented training in rehabilitation after stroke. systematic review. J Adv Nurs. 2009:65;4:737-754.
71. Reinkensmeyer DJ, Kahn E, Averbuch M, et al. Understanding and treating arm movement impairment after chronic brain injury. progress with the ARM guide. J Rehabil Res Dev. 2000:37;6:653-662.
72. Sackett DL. Clinical epidemiology: a basic science for clinical medicine, ed 2. Boston: Little, Brown; 1991.
73. Sawaki L, Butler AJ, Leng X, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22(5):505-513.
74. Shadmehr R, Donchin O, Hwang EJ. Learning to compensate for dynamics of reaching movements. In: Vaadia E, et al, editors. Motor cortex and voluntary movements. Boca Raton, FL: CRC Press, 2005.
75. Sumway-Cook A, Woollacott MH. Motor control. Translating research into clinical practice, ed 3. Philadelphia: Lippincott Williams & Wilkins. 2007.
76. Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature. 2000;403(6769):544-549.
77. Smith GV, Silver KHC, Goldberg AP, et al. “Task-oriented” exercise improves hamstring strength and spastic reflexes in chronic stroke patients. Stroke. 1999;30(10):2112-2118.
78. Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support. effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002:83;5:683-691.
79. Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke. results of the STEPS randomized clinical trial. Phys Ther. 2007:87;12:1580-1602.
80. Suputtitada A, Suwanwela NC, Tumvitee S. Effectiveness of constraint-induced movement therapy in chronic stroke patients. J Med Assoc Thai. 2004;87(12):1482-1490.
81. Tang QP, Yang QD, Wu YH, et al. Effects of problem-oriented willed-movement therapy on motor abilities for people with poststroke cognitive deficits. Phys Ther. 2005;85(10):1020-1033.
82. Taub E. Movement in non-human primates deprived of somatosensory feedback. Exerc Sport Sci Rev. 1977;4:335-374.
83. Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74(4):347-354.
84. Taub E, Uswatte G. Constraint-induced movement therapy and massed practice. Stroke. 2000;31(4):983-986.
85. Taub E, Uswatte G, King DK, et al. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37(4):1045-1049.
86. Thaut MH, Leins AK, Rice RR, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke. A single-blind, randomized trial. Neurorehabil Neural Repair. 2007:21;5:455-459.
87. Thielman GT, Dean CM, Gentile AM. Rehabilitation of reaching after stroke. task-related training versus progressive resistive exercise. Arch Phys Med Rehabil. 2004:85;10:1613-1618.
88. Thielman G, Kaminski T, Gentile AM. Rehabilitation of reaching after stroke. Comparing 2 training protocols utilizing trunk restraint. Neurorehabil Neural Repair. 2008:22;6:697-705.
89. Tuke A. Constraint-induced movement therapy. a narrative review. Physiotherapy. 2008:94;2:105-111.
90. van der Lee JH. Constraint-induced therapy for stroke. more of the same or something completely different. Curr Opin Neurol. 2001:14;6:741-744.
91. van Vliet PM, NB Lincoln NB, Foxall A. Comparison of Bobath based and movement science based treatment for stroke. a randomized controlled trial. J Neurol Neurosurg Psychiatry. 2005:76;4:503-508.
92. Visintin M, Barbeau H, Korner-Bitensky N, et al. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29(6):1122-1128.
93. Volpe BT, Krebs HI, Hogan N, et al. A novel approach to stroke rehabilitation. robot-aided sensorimotor stimulation. Neurology. 2000:54;10:1938-1944.
94. Volpe BT, Krebs HI, N Hogan N, et al. Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology. 1999;53(8):1874-1876.
95. Volpe BT, Lynch D, Rykman-Berland A. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22(3):305-310.
96. Wagenaar RC, Meijer OG, van Wieringen PC, et al. The functional recovery of stroke. a comparison between neuro-developmental treatment and the Brunnstrom method. Scand J Rehabil Med. 1990:22;1:1-8.
97. Wang RY, Chen HI, Yang YR. Efficacy of Bobath versus orthopedic approach on impairment and function at different motor recovery stages after stroke. a randomized controlled study. Clin Rehabil. 2005:19;2:155-164.
98. Werner C, Von Frankenberg S, Treig T, et al. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients. a randomized crossover study. Stroke. 2002:33;12:2895-2901.
99. Wernig A, Muller S. Improvement of walking in spinal cord injured persons after treadmill training. In: Wernig A, editor. Plasticity of motoneuronal connections. Berlin: Elsevier, 1991.
100. Winstein CJ, Rose DK, Tan SM, et al. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke. A pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004:85;4:620-628.
101. Wittenberg GF, R Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;1(1):48-57.
102. Wolf SL, Lecraw DE, Barton LA, et al. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104(2):125-132.
103. Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke. the EXCITE randomized clinical trial. JAMA. 2006:296;17:2095-2104.
104. Wu CY, Lin KC, Chen HC, et al. Effects of modified constraint-induced movement therapy on movement kinematics and daily function in patients with stroke. a kinematic study of motor control mechanisms. Neurorehabil Neural Repair. 2007:21;5:460-466.
105. Wu CY, Chen CL, Tsai WC, et al. A randomized controlled trial of modified constraint-induced movement therapy for elderly stroke survivors. changes in motor impairment, daily functioning, and quality of life. Arch Phys Med Rehabil. 2007:88;3:273-278.
106. Wu CY, Chen CL, Tang SF, et al. Kinematic and clinical analyses of upper-extremity movements after constraint-induced movement therapy in patients with stroke. a randomized controlled trial. Arch Phys Med Rehabil. 2007:88;8:964-970.
107. Yen CL, Wang RY, Liao KK, et al. Gait training induced change in corticomotor excitability in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22(1):22-30.