chapter 24 Dysphagia management
After completing this chapter, the reader will be able to accomplish the following:
1. Describe the normal anatomy and physiology of the swallowing mechanism.
2. Discuss the effects of stroke on the swallowing mechanism.
3. Describe clinical and instrumental assessment of dysphagia following stroke.
4. Describe various rehabilitative and compensatory techniques used to treat dysphagia after a stroke.
5. Discuss the efficacy of dysphagia intervention following stroke.
Dysphagia comes from the Greek prefix dys, meaning difficult, and the Greek term phagein, meaning to eat. The occurrence of dysphagia, or difficulty swallowing, immediately after stroke is common, with a reported incidence as high as 51%.82 In patients with brainstem stroke, the incidence may be as high as 81%.60 Intervention for dysphagia is a part of occupational therapy care for patients with stroke in a variety of settings. While initial evaluation and treatment for dysphagia is critical in the acute care setting, patients often require reassessment in postacute settings as well.35 See Chapter 1.
Normal anatomy and physiology of the swallowing mechanism
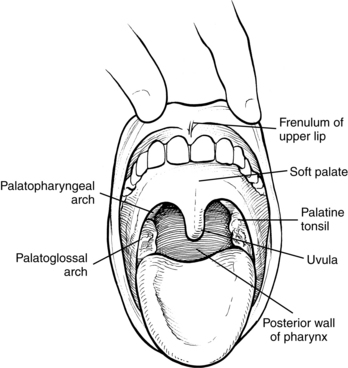
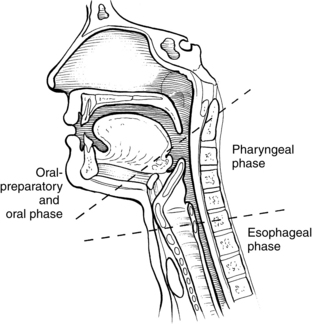
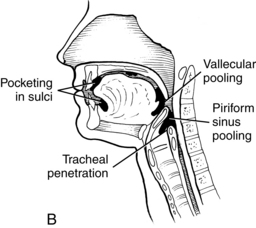
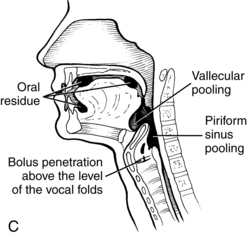
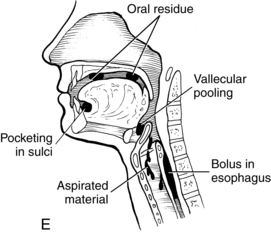
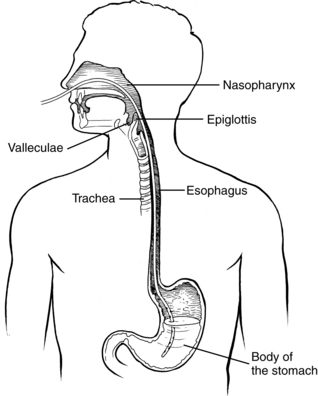
A prerequisite for successful intervention with patients with dysphagia is knowledge of the anatomy and physiology of the swallowing mechanism. Fig. 24-1 represents a midsagittal view of the anatomical landmarks of the head and neck important in swallowing. Fig. 24-2 represents anatomical landmarks of the oral cavity. The act of swallowing may be divided into five separate stages: preoral, oral-preparatory, oral, pharyngeal, and esophageal. Fig. 24-3 illustrates the anatomical division of the oral preparatory through esophageal stages.
Preoral stage
During the preoral stage, the patient engages in tray or plate setup and preparation; visual, visual-perceptual, and olfactory awareness of the food; and transportation of the food to the mouth (feeding) using a utensil, cup, or fingers. Patients with stroke often have challenges with preoral stage activities that benefit from occupational therapy interventions, even in the absence of dysphagia.
Oral-preparatory stage
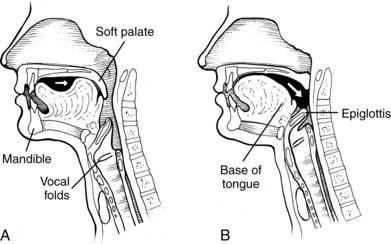
During the oral-preparatory stage (Fig. 24-4, A), the patient demonstrates adequate mouth opening, bolus reception, containment in the oral cavity, oral sensation for the bolus, and appreciation of the flavor and texture of the bolus. The muscles of mastication prepare the food, if solid, into a bolus of suitable texture for swallowing by manipulating the bolus using the muscles of mastication, the jaw, and the cheeks. During this stage, the soft palate rests on the back of the tongue to prevent food or fluid from trickling into the pharynx.
Oral stage
During the oral stage of the swallow, the prepared bolus is propelled through the oral cavity toward the pharynx (see Fig. 24-4, B). The lips and buccal muscles contract and transport the bolus posteriorly as the tongue sequentially pushes the bolus posteriorly against the hard palate, propelling it through the oral cavity, to the base of the tongue.
Pharyngeal stage
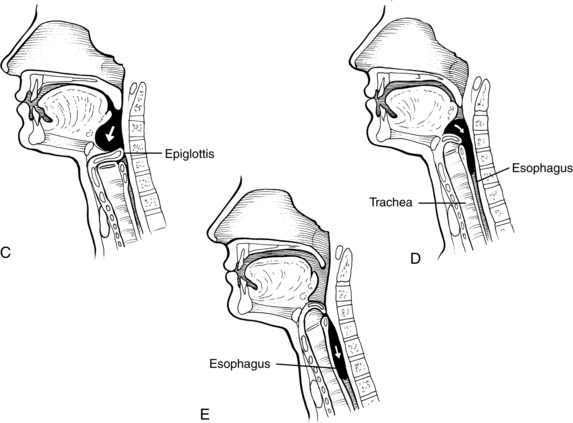
During this stage of the swallow, the following events occur in rapid sequence, producing a swallow response. The soft palate elevates, closing off the nasopharynx. Swallowing apnea, or cessation of breathing, occurs as the vocal folds close, protecting the airway from aspiration and laryngeal penetration. The epiglottis folds over the opening to the larynx (the laryngeal vestibule) (see Fig. 24-4, C), also preventing airway penetration into the larynx and directing the bolus toward the piriform sinuses. The larynx rises and tilts anteriorly, and pharyngeal peristalsis squeezes the bolus downward through the pharynx toward the cricopharyngeal sphincter (see Fig. 24-4, D). The cricopharyngeal sphincter, which is at the superior aspect of the esophagus, relaxes and allows the bolus to pass into the esophagus.
Esophageal stage
The esophageal stage begins as the bolus passes through the cricopharyngeal sphincter (see Fig. 24-4, E). The bolus is propelled through the esophagus by a sequential peristaltic “stripping wave.” The lower esophageal sphincter located at the base of the esophagus then relaxes, allowing the bolus to pass into the stomach.
Neural control of swallowing
Cortical and subcortical centers control the voluntary aspects of the swallow, particularly during the preoral, oral-preparatory, and oral stages. The swallow response, which can be initiated voluntarily or involuntarily, is controlled by cranial nerves and their nuclei in the medulla, with input from cortical and subcortical centers. Six cranial nerves are involved in the swallow process50 (Box 24-1).
Box 24-1 Cranial Nerve Functions
| STAGE | |
| Oral | Cranial nerve V (trigeminal): tactile and proprioceptive sensation and motor Cranial nerve VII (facial): taste and motor |
| Pharyngeal | Cranial nerve IX (glossopharyngeal): taste, pharyngeal peristalsis, salivation, and taste Cranial nerve X (vagus): taste and motor, intrinsic laryngeal muscles, pharyngeal peristalsis, and swallow initiation Cranial nerve XI (accessory): pharyngeal peristalsis and head and neck stability |
| Oral and pharyngeal | Cranial nerve XII (hypoglossal): lingual movement and laryngeal and hyoid movement |
Signs of dysphagia associated with stroke
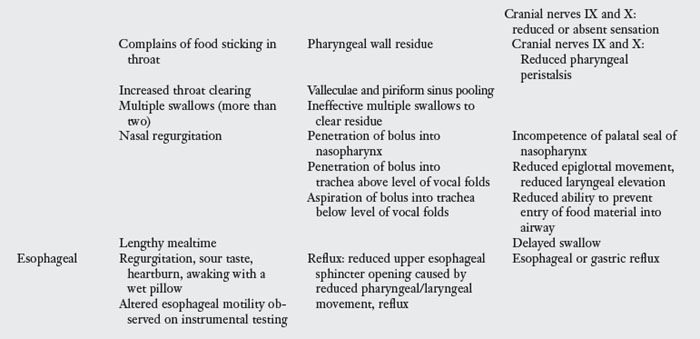
A variety of signs are observed directly or by videofluoroscopy during swallowing following stroke. Veis and Logemann91 found that 75% of patients assessed by videofluoroscopy demonstrated more than one specific sign with their swallowing. Signs and symptoms vary with location and size of the lesion or lesions caused by stroke. Table 24-1 delineates specific impairments that one may observe. Fig. 24-5 illustrates some of these impairments. Patients with dysphagia and stroke may have a tracheostomy and may require mechanical ventilation. Although this chapter does not cover these topics, the suggested reading will provide the reader with more information. Studies have observed the differences between dysphagia in stroke patients by lesion location.
Hemispheric stroke
In general, patients with hemispheric stroke have difficulty with voluntary triggering of the swallow.6 Patients with right hemispheric middle cerebral artery stroke tend to have greater incidence of laryngeal penetration and aspiration than those with left hemispheric middle cerebral artery stroke. Patients with right hemispheric stroke take longer to initiate a swallow response than those with a left hemispheric stroke. Oral and pharyngeal bolus mobilization is slower in persons with right hemispheric stroke than in healthy individuals. Patients with left hemispheric stroke experience slower bolus mobilization through the pharynx compared with healthy individuals. Oral time transit time may also be delayed in those with left hemispheric stroke. Apraxia is present in those with left hemispheric stroke.85 A study by Irie and Lu42 suggested that, in general, patients with left hemispheric stroke tended to have primarily oral phase impairments and those with right strokes tended to have impairment of oral and pharyngeal phases. Patients with left hemispheric stroke tended to require fewer dysphagia interventions and to require alternative nutrition less than those with a right sided stroke did. Pharyngeal and laryngeal sensory loss may play a role in reduced ability to respond to the presence of a bolus in some stroke patients.5
Brainstem stroke
Patients with brainstem stroke have greater occurrence of persistent dysphagia than those with hemispheric stroke.6,59 With lateral medullary infarction (Wallenberg syndrome), oral control may be near intact, but the ability to trigger and achieve an effective swallow is weak bilaterally, despite a unilateral lesion.6 Reduced laryngeal elevation, unilateral pharyngeal weakness, and reduced adduction of the vocal cords may be seen, resulting in aspiration.91 A delayed or absent swallow response may be seen.55,60 Recovery does occur in 88% of patients; however, it takes longer than in those with hemispheric stroke.60
Pseudobulbar or suprabulbar palsy
Pseudobulbar or suprabulbar palsy refers to stroke that causes dysphagia affecting the lower motor neuron. The corticospinal pathways are spared. Lesions may be located in the corona radiata, internal capsule, or lenticular hemorrhage.12 Symptoms may disappear within several weeks.12 Lacunar infarcts with suprabulbar palsy demonstrate delayed trigger, absent trigger, and/or slow swallow.30
Lacunar infarcts
Lacunar infarcts, often occurring in the periventricular areas, are not always associated with specific dysphagic signs.
Multiple strokes
Patients with multiple strokes may demonstrate slow oral movements and a delayed swallow response.55 Often multiple deficits exist, resulting in a greater risk of aspiration. Patients with bilateral stroke are more likely to have sensory deficits in the pharynx and larynx.5
Resolution of dysphagia following stroke
Dysphagia clinicians and researchers have noted that difficulty with swallowing lessens in the seven days following acute stroke, although in one study 27% of patients still were considered to be at risk by the physician. After six months, only 8% retained dysphagia; however, 3% had developed new difficulty with swallowing.82 Logemann noted that 95% of patients with a single, uncomplicated stroke returned to full oral intake after nine weeks, regardless of the location of the stroke.55 However, among that 95%, pharyngeal function was not completely normal and possibly contributed to even more severe dysphagia with a subsequent stroke.55
Medical complications associated with dysphagia in stroke
Medical complications associated with dysphagia following stroke include aspiration pneumonia, dehydration, compromised nutrition, and death.81
Aspiration
Aspiration refers to the penetration of food or liquid into the airway, below the level of the vocal folds, before, during, or after the swallow. Laryngeal penetration refers to the entrance of food or liquid into the larynx, above the level of the vocal folds.55 Silent aspiration is defined as the entrance of saliva, food, or liquid below the level of the true vocal folds without a cough or any clinical signs of difficulty.38 Aspiration and laryngeal penetration occur when the ability of the swallowing mechanism to prevent material from entering the airway is impaired.
Aspiration is common in the acute phase following stroke, with a greater incidence in severe strokes and in patients with pharyngeal sensory loss.33 Approximately 40% of stroke patients with dysphagia who aspirate do not exhibit symptoms of aspiration during the bedside evaluation (silent aspiration).38 Of stroke patients selected for a videofluoroscopic study, 48% to 55% were shown actually to aspirate.28 Veis and Logemann91 found that 32% of the subjects assessed by videofluoroscopy aspirated from pharyngeal stage problems, which the bedside evaluation cannot detect. Mann and Hankey58 found that aspiration was correlated with delayed oral transit and incomplete oral clearance of the bolus. Sensory deficits in the larynx and pharynx may be associated with aspiration.5 Patients with brainstem, subcortical, or bilateral stroke are at greater risk for aspiration.26
Tolerance for aspiration appears to be individual and may depend on the frequency, volume, and content of what is aspirated. Tolerance may also depend on the overall health of the individual patient. Information regarding who may tolerate aspiration and in what parameters is scarce.
Aspiration pneumonia
Aspiration can lead to aspiration pneumonia in patients with stroke,56,59,63,76,81 which may lead to hospitalization or death.63 Pneumonia is particularly common in stroke patients with multiple-location strokes, a history of airway disease, hypertension, diabetes, and aspiration during modified barium swallow (MBS).26,59 As aspiration may occur with greater frequency in brainstem stroke, and it may occur in 11% of those with brainstem stroke.89 It occurs primarily the first few days after stroke.27 Saliva contains pathogens that may be causative factors for pneumonia when saliva is aspirated.44,45
Dehydration and compromised nutrition
Dehydration is another possible consequence of dysphagia. Schmidt and colleagues76 were unable to identify an increased risk of dehydration for patients with aspiration compared with those who did not aspirate. Dehydration may be caused by the use of dysphagia diets that provide only thickened liquids to avoid aspiration.31,95 Dehydration also may be caused by the patient’s inability to recognize thirst or to request a drink when thirsty. Nutritional status also may be compromised by stroke81 for a variety of reasons, including dysphagia, loss of appetite, decreased mental status, depression and other psychosocial factors, and medication interactions.
Aspiration and site of lesion
Teasell, Bach, and McRae88 reported that aspiration occurred in at least 9.9% of all patients who had unilateral right hemispheric strokes, 12.1% of those who had unilateral left hemispheric strokes, 24% of those who had bilateral hemispheric strokes, and 39.5% of those who had brainstem strokes. Horner, Massey, and Brazer39 reported that aspiration occurred twice as often in those with bilateral stroke compared with those with unilateral stroke. Aspiration after bilateral stroke may be caused primarily by incomplete laryngeal elevation and closure, which encourages aspiration during the swallow and reduces pharyngeal peristalsis after the swallow, causing aspiration of residue. Alberts and colleagues2 reported that patients with only small vessel infarcts had a decreased incidence of aspiration versus those with large and small vessel infarcts. Aspiration may be correlated with pharyngeal transit time, swallow response time, and duration of laryngeal closure.69
Role of the swallowing team
In inpatient settings, optimal management of dysphagia is performed by a multidisciplinary team. The team is responsible for identification, evaluation, diagnosis, treatment, and overall management of patients with dysphagia.
The multidisciplinary team includes a designated primary dysphagia therapist, usually the occupational therapist or speech-language pathologist, and the nurse, physician, respiratory therapist, dietitian, and the patient, who plays an active role in decision-making. For management of the dysphagic patient to be successful, all persons involved in the patient’s care should understand the swallowing impairment and the management techniques used. Ongoing education and follow-up are often necessary.
Evaluation of swallowing
Evaluation is the process of gathering and interpreting information needed for intervention.37 Assessment refers to use of specific standardized tools or tests used as part of overall evaluation.37 Dysphagia can be evaluated clinically and instrumentally. Clinical evaluation, which cannot rule out aspiration in those with stroke,83 usually precedes instrumental evaluation. Instrumental evaluation is better at determining aspiration risk, and clinical evaluation helps to determine whether instrumental evaluation is needed.
Dysphagia screening tools identify patients in need of a complete clinical evaluation. Screening has been shown to reduce the incidence of pneumonia, regardless of severity of the stroke.36 Several screenings are available in the literature, including the 3 ounce water test,24 the Burke Dysphagia Screening Test,25 and the Gugging Swallow Screen,90 which was developed for those with acute stroke. Facilities may also develop their own screening tests. Screening is least likely to identify the presence of dysphagia following stroke, clinical assessment is more sensitive, and MBS is most sensitive.59
Identifying those at risk for aspiration and reducing possibility of severe medical consequences is a critical purpose of evaluation. However, a complete evaluation of swallowing addresses many other important issues for those with stroke. The swallow may be simply mildly impaired; however, that may lead to a more seriously decompensated swallow in the future as illness proceeds. Mild challenges with swallowing may lead to inadequate nutrition. Pleasure, enjoyment, and socialization at meals may be severely impacted by mild impairments and may gravely affect quality of life.
Clinical evaluation and assessment
When the physician suspects dysphagia, the physician orders a dysphagia evaluation. The physician, patient, nursing staff, and family also may identify the need for dysphagia evaluation. For patients who are NPO (not eating food by mouth), the physician must stipulate whether evaluation will include attempting trials of food by mouth with the patient. The evaluation examines factors that interfere with feeding and swallowing function, the patient’s risk for aspiration, and factors that may contribute to a decrease in oral intake. The evaluation includes observational and direct examination components: chart review, patient and caregiver interview, functional status, oral motor examination, abnormal reflexes, pharyngeal examination, feeding trial, and a statement of impression and recommendations.
Specific assessment tools may be developed by facilities or a standardized assessment may be used. Appropriate dysphagia standardized assessments for patients with stroke include the Dysphagia Evaluation Protocol,4 the Mann Assessment of Swallowing Ability,57 and the Functional Oral Intake Scale.21 The latter two were standardized on stroke populations. All of these assessments demonstrate a high degree of reliability.
Chart review
The therapist first must review the patient’s chart carefully to ascertain pertinent facts from the medical and feeding history. Pertinent information includes the following:
 Age52
Age52
 Previous evaluations and tests indicating current status (positive infiltrate on chest x-ray examination; ear, nose, and throat evaluation)
Previous evaluations and tests indicating current status (positive infiltrate on chest x-ray examination; ear, nose, and throat evaluation)
 Primary diagnosis and date of onset
Primary diagnosis and date of onset
 History of present illness, secondary diagnoses, and medical history, including history of dysphagia due to conditions other than stroke
History of present illness, secondary diagnoses, and medical history, including history of dysphagia due to conditions other than stroke
 History of aspiration pneumonia
History of aspiration pneumonia
 History of weight loss, appetite, and nutrition, especially with current inpatient admission
History of weight loss, appetite, and nutrition, especially with current inpatient admission
 Reduced oral intake and its possible relation to depression, pain, feeding dependence, and food preferences or dislikes
Reduced oral intake and its possible relation to depression, pain, feeding dependence, and food preferences or dislikes
 Dietitian, chest physical therapy, and/or respiratory therapy evaluations
Dietitian, chest physical therapy, and/or respiratory therapy evaluations
 Current method of nutritional intake
Current method of nutritional intake
 Whether calorie counts are in place
Whether calorie counts are in place
 Length of time on current diet
Length of time on current diet
 Dietary restrictions (diabetic: no concentrated sugars; cardiac: low sodium or low fat)
Dietary restrictions (diabetic: no concentrated sugars; cardiac: low sodium or low fat)
When reviewing the chart, the therapist must consider the patient’s ability to participate in the evaluation, which contributes to the ability to feed and swallow safely. Factors to consider for mental status include primary language spoken, level of alertness, ability to follow directions, insight into swallowing difficulty, cognitive and perceptual status, and ability to communicate needs. Because eating requires a coordination of breathing and swallowing, respiratory problems may affect a person’s ability to eat safely. The therapist should consider the following factors when evaluating the patient’s ability to eat orally: excessive oral secretions, presence of tracheostomy, ventilator dependence and ability to wean, and frequency and route of suctioning.
Patient/caregiver interview
Initial contact begins with medical nursing staff and in the patient’s room, where the occupational therapist may ask questions of the patient, family, and caregivers regarding the patient’s past and present eating function. This information may expand on that obtained during the chart review.
Observation begins as soon as the practitioner enters the patient’s room. The therapist should observe the room for any types of food that may indicate the patient’s recent diet. Details to observe include the presence of an untouched meal tray; residual food on the patient’s face, clothing, bed, or tray; and wet or hoarse breath sounds and abnormal vocal quality. The patient’s positioning in the bed or chair is also relevant.
Functional status
Functional status refers to the patient’s ability to move in space and interact in the environment. Some functional interventions may be needed during evaluation to elicit optimal feeding and swallowing.
If a patient is unable to self-position to achieve an upright sitting position, this may interfere with feeding and swallowing. The occupational therapist should determine the amount of assistance required to position the patient in the bed or chair and whether the patient is able to maintain the position independently. Ideally the patient should sit upright in a chair with the pelvis in a slight anterior tilt, forearms weight-bearing on the tabletop, and the head and neck at midline and upright. The therapist also evaluates upper extremity and hand function as they relate to feeding.
Adaptive equipment or environmental adaptations may enable patients to feed themselves if possible. Adaptations for positioning include supporting feet that do not reach the floor with a telephone book or foot rest, using wheelchair cushions and other devices to improve upright posture, and adjusting the table height as needed. Wheelchairs with removable or swing-away armrests allow the patient to eat at the table. Alternatively, a full lap tray can be used with a wheelchair.
The therapist should assess the patient’s ability to initiate and complete oral hygiene. A clean mouth is necessary for sensory appreciation of food, and good oral hygiene has been shown to reduce rates of pneumonia in an elderly populations.97 One-handed techniques and equipment create independence with oral care. See Chapter 28.
For feeding, helpful items include Dycem to prevent the plate from slipping, a rocker knife and plate guard for one-handed eating, a covered cup or straw for bringing beverages to the mouth without spilling, and built-up utensils for weak or poorly controlled grasp to encourage use of a hemiplegic dominant arm. Bent spoons for using a nondominant upper extremity to feed also may be helpful. Adapted cups with lids reduce spilling and provide handles for easy manipulation with a gross grasp; lids may have holes for straws, if appropriate. Specially angled dysphagia cups allow sipping without tilting the neck into extension.
Adaptations for reduced visual acuity, perception, and cognition may be useful at the table. The patient should wear eyeglasses if they usually are used at mealtime. A colorful piece of paper or “anchor” may be needed to draw the patient’s attention or vision to the neglected side of the food array. A simplified presentation of one food item at a time can help to focus visual and general attention to the eating task. For stroke patients who are distractible, eating in a quiet, reduced-distraction setting promotes attention. Safety and pacing cues and supervision may be needed, especially for those with left hemiplegia. For right hemiplegic patients with aphasia and apraxia, minimal use of verbal directions and setup of the eating environment that makes the activity obvious are helpful.
Oral examination
The therapist must administer an oral motor examination of the lips, cheeks, tongue, jaw, and palate before presenting food to the patient. The occupational therapist determines whether range of motion, muscle tone, and sensation (intraorally and extraorally) are decreased, increased, or within normal limits. Strength of oral structures is observed but may not be appropriate to assess because of the presence of abnormal muscle tone, which may invalidate strength testing.
Abnormal reflexes
If present, abnormal “primitive” reflexes can interfere with feeding. Primitive reflexes include the bite reflex, rooting reflex, and the jaw jerk. The gag reflex may be hypersensitive, and hypersensitivity of internal and external oral structures also may be present.
Pharyngeal examination
Although unseen, the therapist may assess aspects of pharyngeal function. Clinical features associated with dysphagia severity include dysphonia, dysarthria, abnormal volitional cough, abnormal gag reflex, coughing after swallowing, and voice change after swallowing.22
 Dry swallow. The ability to “dry” swallow (without food) provides information on the patient’s ability to initiate a swallow response.
Dry swallow. The ability to “dry” swallow (without food) provides information on the patient’s ability to initiate a swallow response.
 Vocal quality. A wet, gurgly vocal quality can indicate pooling of secretions above the vocal cords, which normally are cleared by coughing or throat clearing. The patient may not perceive the presence of pooled secretions or may be unable to cough them up and clear the throat. Voice hoarseness or weakness may be due to unilateral or bilateral weakness of the vocal cords. Wet voice or a weak-hoarse voice suggests that weakness of the laryngeal structures may compromise the protection of the airway during swallow.74
Vocal quality. A wet, gurgly vocal quality can indicate pooling of secretions above the vocal cords, which normally are cleared by coughing or throat clearing. The patient may not perceive the presence of pooled secretions or may be unable to cough them up and clear the throat. Voice hoarseness or weakness may be due to unilateral or bilateral weakness of the vocal cords. Wet voice or a weak-hoarse voice suggests that weakness of the laryngeal structures may compromise the protection of the airway during swallow.74
 Volitional or reflexive cough. A volitional cough provides information about the strength of the vocal cords and breath support for coughing. Presence of reflexive cough indicates a lower risk of aspiration and pneumonia.1
Volitional or reflexive cough. A volitional cough provides information about the strength of the vocal cords and breath support for coughing. Presence of reflexive cough indicates a lower risk of aspiration and pneumonia.1
 The gag reflex. In normal individuals, the presence or absence of a gag reflex can vary. Horner and Massey38 noted that a poor gag reflex proved to be a poor indicator of prognosis for safer swallowing. Triggering of the gag reflex with a tongue depressor is different from triggering the gag reflex by a misdirected bolus. Food does not (normally) trigger a gag, because it is not a foreign substance or a noxious stimulus. The presence or absence of a gag reflex in patients with neurological impairments is not an accurate indicator of the patient’s ability to swallow safely.55 However, presence of a gag reflex does indicate some level of sensory and motor function of the tenth cranial nerve, which is responsible for innervating many structures that contribute to sensory and motor aspects of the swallow.
The gag reflex. In normal individuals, the presence or absence of a gag reflex can vary. Horner and Massey38 noted that a poor gag reflex proved to be a poor indicator of prognosis for safer swallowing. Triggering of the gag reflex with a tongue depressor is different from triggering the gag reflex by a misdirected bolus. Food does not (normally) trigger a gag, because it is not a foreign substance or a noxious stimulus. The presence or absence of a gag reflex in patients with neurological impairments is not an accurate indicator of the patient’s ability to swallow safely.55 However, presence of a gag reflex does indicate some level of sensory and motor function of the tenth cranial nerve, which is responsible for innervating many structures that contribute to sensory and motor aspects of the swallow.
Feeding trial
Feeding trials are appropriate for patients who are alert, able to follow commands, and medically stable. Factors that may contraindicate feeding trials include absence of or significantly reduced laryngeal elevation during dry swallows, moderate to severe dysarthria, lethargy or severely impaired mental status, and severe pulmonary compromise.4,68
Therapists may observe patients in a formal evaluation setting or informally at mealtime. Informal mealtime observation provides an efficient indication of the patient’s eating ability and allows the evaluator to assess the patient’s ability to concentrate despite distractions and interruptions. An informal evaluation allows for observation of the rate of intake and the patient’s reaction to the presentation of the meal.68 If the evaluation takes place in a formal setting, or if this is the patient’s first attempt at eating following a stroke, trials should begin with foods that are less likely to be aspirated, such as thick purees, which do not require much oral manipulation, since thin liquids are more difficult to control in the oral cavity and pharynx. The evaluation then progresses to include foods of more difficult consistencies, depending on the patient’s tolerance and medical status. Box 24-2 shows the usual progression of consistencies (from easiest to most difficult) as standardized in the National Dysphagia Diet (NDD). The NDD is the American Dietetic Association’s recommended diet level hierarchy, developed in an attempt to standardize dysphagia diets offered in hospitals in the United States.3
Box 24-2 Bolus Consistency Progression: The National Dysphagia Diet
Solid foods
Level 1: Dysphagia-Pureed: homogenous, cohesive, and puddinglike; little chewing required; examples: applesauce, pudding
Level 2: Dysphagia Mechanical-Altered: cohesive, moist, semisolid foods requiring some chewing; examples: soft macaroni and cheese, soft cooked vegetables
Level 3: Dysphagia-Advanced: Soft foods requiring more chewing
Regular: all foods allowed, including foods requiring chewing (meat) and mixed textures (cereal and milk; pills and water)
The therapist may evaluate all the food and fluid consistencies shown in Box 24-2 or begin at the consistencies the patient currently tolerates. During the feeding trial, the occupational therapist should pay close attention to the nature and quality of oral manipulation of food and to the following indicators of laryngeal function.
An automatic cough occurs under many conditions, including a dry throat, or when secretions have accumulated around the vocal cords even before eating begins. To some extent, coughing occurs with normal breathing and at times when swallowing. Although an automatic cough may not be heard during a meal or feeding trial, its presence may signal that the patient is making efforts to clear the airway of food or secretions and that there is difficulty with airway protection or aspiration of a particular texture or textures. In normal swallowing, laryngeal penetration occurs occasionally; material that is penetrated is cleared from the larynx with throat clearing and reswallowing and often does not result in a cough. However, laryngeal reaction to aspirated material below the true vocal folds is normally a cough, which ideally expels the aspirated material.80 A strong cough is necessary to protect the airway well. Horner, Massey, and Brazer39 reported that a weak cough is more likely to occur in aspirating patients than in nonaspirating patients. As with the gag reflex, the presence of a reflexive cough indicates that the structures of the larynx and pharynx innervated by cranial nerve X have sensory and motor function to some extent and protect the airway during meals.1
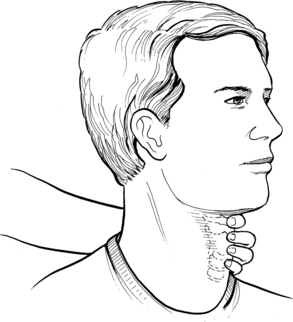
Full laryngeal elevation and depression indicates that a swallow has occurred. Perlman and colleagues68 concluded that reduced hyoid elevation impairs the pharyngeal stage of the swallow, thereby increasing the risk of vallecular residue and pharyngeal stasis. These factors may result in aspiration. Fig. 24-6 demonstrates the proper positioning of the examiner’s hand and digits on the patient’s neck for palpation of the larynx to assess laryngeal elevation.
The therapist may assess breath and voice quality by the ear and by cervical auscultation with a stethoscope. Cervical auscultation is accomplished by placing the diaphragm of the stethoscope lateral to the trachea and inferior to the cricoid cartilage.87 The therapist may adjust placement until hearing cervical breath sounds. The normal pharyngeal stage includes swallow initiation promptly after oral transit, an apneic period during the swallow, and exhalation immediately after the swallow, with clear breath sounds and vocal quality.98 Breath and vocal quality differ in patients with dysphagia and often are characterized by gurgling sounds, increased throat clearing, and a “wet” vocal quality, which may indicate pooling. The therapist also may assess voice quality with the naked ear. Although cervical auscultation is an imprecise clinical method for the evaluation of aspiration, it has some correlation with aspiration found on an MBS,98 and may be helpful in quickly identifying those at high risk for aspiration.14
Research of usefulness of pulse oximetry to detect aspiration has shown mixed results and may not be particularly useful.93
Common dysphagia signs and symptoms in stroke are compiled in Table 24-1. The therapist should make observations relating to these signs and symptoms for the oral-preparatory, oral, and pharyngeal stages for each food and fluid consistency presented. Recommendations and intervention goals are based on these observations, medical history, prognosis, and instrumental assessment results.
Instrumental assessment of dysphagia
Instrumental evaluation refers to diagnostic testing using instrumentation, the most important of which are MBS (sometimes referred to as videofluoroscopy) and fiberoptic endoscopic evaluation of swallowing (FEES) examinations.11,21,48 These evaluations use diagnostic imaging techniques and provide information about the anatomy and physiology of the swallow, including aspiration, which cannot be determined during a clinical assessment.70 They also may be rehabilitative procedures to assess efficacy and progress of compensatory techniques. The MBS and FEES provide information regarding the oral stage and the unseen pharyngeal stage of the swallow and can provide information about the patient’s ability to protect the airway during swallow, which clinical evaluation cannot. Other instrumental evaluations commonly used to assess dysphagic patients with stroke include ultrasound and electromyography.
Modified barium swallow
The MBS or videofluoroscopic evaluation of swallowing allows the clinician to directly view the oral, pharyngeal, and esophageal aspects of the swallow. The MBS also allows the clinician to observe aspiration before, during, and after the swallow.51 MBS allows for greater accuracy in identifying dysphagia in stroke patients compared with clinical evaluation or screening.59 The MBS ideally is performed jointly by the radiologist and the occupational therapist. Food and liquid boluses are mixed with barium, which is radiopaque. Alternatively, plain barium may be used, which is available in different thicknesses. The patient must be positioned in an upright position and preferably feeds himself or herself. The swallows are noted by a fluoroscopy unit and are recorded onto videotape or DVD. Thus, each stage of the swallow may be viewed during the assessment and reviewed later. The MBS not only allows the clinician to view swallow function and rule out aspiration but also provides useful information regarding compensatory swallowing strategies, discussed later in this chapter, and provides a determination of the amount, frequency, and quality of aspiration. The MBS also can assess how well the patient is able to deal with aspirated or penetrated material (e.g., his or her ability to clear aspirated material back into the pharynx). One study indicated that three specific observable aspects are related to aspiration in those with stroke: pharyngeal transit time, swallow response time, and duration of closure of the larynx.69
The MBS does expose the patient to some levels of radiation, and the ability of the patient to cooperate and follow directions is important for the success of information gathering and for minimizing radiation exposure. The MBS is difficult to achieve with patients who are in the intensive care unit, are difficult to position, and/or are difficult to transport to a radiology suite, although newer technology available at some medical centers permits MBS at the bedside. Naturally, MBS presents function at a specific moment in time, and reliability with real world swallowing function is not guaranteed, which therapists who use MBS results must consider. Additionally, interrater reliability of MBS performance assessment may vary.86
Fiberoptic endoscopic evaluation of swallowing
FEES involves passing an endoscope with a light and camera through one of the patient’s nares, down to the level of the valleculae. Before the assessment, lidocaine spray is used to numb the nares. Liquid and solid boluses are dyed with green food coloring for easy visualization. Images of the pharynx and larynx then are visualized and can be videotaped. This assessment is performed by an otolaryngologist, a trained occupational therapist, or a speech-language pathologist. The FEES allows the examiner to evaluate pharyngeal and laryngeal function and to assess the amount of residue present on the vocal cords or pooled in the valleculae or pyriform sinuses after a swallow. Thus, one can assess aspiration and competence in protecting the airway. One study suggested that FEES may be more sensitive in detecting aspiration than MBS.46 However, FEES cannot always explain the reason that aspiration occurs, and the presence of the endoscopy tube inhibits a completely normal swallow. The FEES is minimally invasive, and the patient must be able to tolerate the procedure. This procedure is contraindicated for patients with cardiac dysrhythmias, respiratory distress, bleeding disorders, anatomical deviations (narrow nasal passage), agitated or hostile patients, or patients with movement disorders.84 The FEES is particularly useful for patients who cannot undergo a MBS for the foregoing reasons or who require frequent reassessment. Clinical benefits of FEES include assessment of airway protection when vocal cord involvement or impaired adduction is suspected, assessment of laryngeal/pharyngeal sensation, and direct visualization of anatomy when it is believed to be a contributing factor in dysphagia.
Ultrasound
Ultrasound is the method of choice if only oral function is to be assessed. Ultrasound is a noninvasive, dynamical evaluation of swallowing that shows the anatomy. This procedure uses normal foods and liquids and is safe to use with patients who are unable to follow directions.84 The disadvantage of ultrasound is that it can visualize only the oral preparatory and oral stages of the swallow.
Electromyography
Surface electromyography measures myoelectrical impulses resulting from the firing of motor units. Surface electrodes are applied to the skin over specific muscles or muscle groups, producing a line tracing representing amplitude or strength of a contraction. Targeting of one muscle or the pharyngeal constrictor muscles is not possible. Placement of electrodes under the chin is used to detect motion of the suprahyoid muscles to assess whether a swallow has occurred.40
Outcome scales
Outcome scales are useful in categorizing dysphagia once evaluation is completed. The Dysphagia Outcome and Severity Scale is a seven category scale;66 the Functional Outcome Swallowing Scale is a five point scale.75 The Functional Oral Intake Scale, a seven point scale, was developed for patients with stroke.21
Evaluation impressions and recommendations
After gathering information from all aspects of the clinical assessment and instrumental evaluations, the therapist must determine whether further instrumental evaluation of swallowing, discussed subsequently, is warranted. Often concerns about unseen pharyngeal function determine whether a referral for instrumental assessment is appropriate, important since pharyngeal stage deficits are common in acute stroke. Whether feeding should be oral or nonoral is a decision to be made by the team.32 If, following a compete assessment, a patient clearly is aspirating or is at high risk for aspiration, NPO is recommended.
In acute care settings, NPO is often a short-term situation for stroke patients until swallowing improves, which it often does. For patients with complex medical conditions including stroke and resulting long-term dysphagia, for whom NPO may be a longer situation, the team should consider the impact of such a decision on the patient and family.47 The caregivers and patient provide information about the patient’s quality of life and preferences regarding medical intervention. If oral feeding is initiated against medical advice, mealtime management guidelines should be provided to optimize safety and emphasize food consistencies least likely to be aspirated.
Alternative means of nutrition
Following evaluation, some patients may not be deemed candidates for oral feeding. They require alternative means of nutrition19 unless they or their designated surrogate have made a purposeful choice not be given artificial feedings. The medical team must determine the length of time the patient will be NPO and the optimal nutritional route. One study has suggested that stroke patients who are not tolerating spoon-fed thick fluids or purees by 14 days following their stroke will need an alternative nutritional route such as a percutaneous endoscopic gastrostomy, defined later.96 Two primary feeding routes generally are used: enteral, which uses a gastrointestinal route, and parenteral, which uses an intravenous route. Table 24-2 summarizes the risks and benefits of alternative feeding routes.
Table 24-2 Risks and Benefits Associated with Oral, Enteral, and Parenteral Nutritional Support
| TYPE OF NUTRITIONAL SUPPORT | POSSIBLE RISKS AND DRAWBACKS | BENEFITS |
|---|---|---|
| Oral | Possible tracheal aspiration Possible inability to ingest sufficient calories Poor patient satisfaction (with limited dysphagia diet) |
Psychologically pleasurable Allows occupational performance of eating and feeding Provides socialization experience Promotes normal digestion |
| Nasogastric | Ulceration Bleeding Fistula Gastroesophageal reflux, aspiration Oropharyngeal discomfort Poor patient satisfaction and compliance |
Routine procedure Affordable Begins immediately Easily reversible |
| Surgical gastrostomy | Requires general anesthesia Bleeding Gastroesophageal reflux, aspiration Diarrhea Stomal irritation |
Common procedure Good for long-term care if gastrointestinal tract is inaccessible Easily replaceable Removes tube from head/neck region Nonsurgical placement available (PEG) |
| Jejunostomy | Peritonitis Diarrhea Difficult to replace |
Minimizes gastroesophageal reflux Can be used when stomach cannot tolerate diet Nonsurgical placement available (PEJ) |
| TPN | Sepsis Infection at site Short-term alimentation Pneumothorax Expensive |
Fewer complications in patients with dysphagia and malnutrition For use in nonfunctioning gastrointestinal tract Minimizes risk of aspirating stomach contents |
Adapted from Groher ME: Formulating feeding decisions for acute dysphagic patients, Occup Ther Pract 3:27, 1992. PEG, Percutaneous endoscopic gastrostomy; PEJ, percutaneous endoscopic jejunostomy; TPN, total parenteral nutrition.
Enteral feedings
Noninvasive tube feedings
Noninvasive tube feedings are most appropriate for short periods. A nasogastric tube is placed through the nose. Food in the form of an enteric feeding formula and water pass through the tube into the stomach (Fig. 24-7). Feedings may be given intermittently via boluses with a large syringe or constantly using a pump. Nasogastric tubes do not prevent pneumonia, however.27
Invasive feeding methods
Invasive feeding methods are used when a patient is activity aspirating, for whom prolonged and severe dysphagia is expected. Tube feeding may be considered a rehabilitative technique when recovery is anticipated.43 Percutaneous gastrostomy tubes are most often used and are placed with the patient under local anesthesia. The surgeon inserts an endoscope through the mouth into the stomach, makes a small incision in the stomach, and then threads a tube through the endoscope out through the abdominal wall. Special enteric formulas and water are administered for tube feeding. A percutaneous endoscopic gastrostomy may be “advanced” into the jejunum, creating a percutaneous endoscopic jejunostomy to help avoid reflux.
Occasionally, a patient will require a surgical gastrostomy. Often this is the case if there is a history of gastric disease and/or scarring. With the patient under general anesthesia, a surgeon makes an incision in the abdomen and then places a gastrostomy tube directly into the stomach. Occasionally, a tube is placed into the jejunum to reduce the reflux of stomach material into the esophagus, which gastrostomy tubes may cause. Food passes through the tube into the stomach.
Dysphagia intervention in stroke
Following evaluation, the patient and occupational therapist jointly determine specific swallowing goals. Family members and other caregivers may be involved in this process. For some patients, an initial goal is developing insight into their dysphagia, lack of which is commonly seen in patients with stroke.67 Development of insight is associated with better swallowing outcomes as patients understand and follow intervention strategies.67
Interventions for dysphagia caused by stroke may be remedial (rehabilitative), compensatory, or a combination of both. Whether remedial or compensatory, the goals of intervention include reduction of aspiration risk, improving the quality of the swallow, and developing independence in feedings skills and behaviors at mealtime. In the acute phase after a stroke, patients may require daily reevaluation and adjustment in the intervention plan because their status may change daily.
Intervention techniques
Treatments for dysphagia include positioning, feeding techniques, improvement of oral responses, facilitation of pharyngeal and laryngeal movements, facilitation of swallowing, therapeutic swallowing techniques, and diet modification. Assuring good nutrition and hydration, maintenance of eating by mouth, oral hygiene programs, and use of oral, pharyngeal and laryngeal structures in conversation are critical.
Positioning
An upright seated position allows optimal function of the muscles of swallowing, maximizes alertness for the fatigued or somewhat lethargic patient, and minimizes reflux. It aids in optimizing expiration during cough,28 which is an important safety reflex. An upright seated position can be achieved in a chair or wheelchair, at the edge of the bed if balance allows, or in bed if necessary.
Feeding
Feeding oneself allows the optimal coordination of upper extremity and oral motor responses and the best awareness of bolus approach. Awareness of the bolus, via visual and olfactory appreciation, provides oral readiness for the bolus.54 Manual guiding for stroke survivors with partial dominant upper extremity movement, particularly those with left cerebrovascular accident and apraxia, is a useful way to facilitate feeding in concert with upper extremity functional goals. Constraint induced therapy may encourage use of the affected dominant arm for eating. See Chapter 10.
Improving oral responses
Interventions begin with symmetrical body position and then are directed toward the affected side of the face to try to create symmetrical movement. When increased skeletal muscle activity (hypertonicity) is present, passive stretching of tight musculature such as a tight cheek with the back of a spoon or gloved finger is useful. When patients present with hypotonicity or low-toned motion in the oral structures, the therapist encourages movement using functional speech and eating tasks; for example, using oral exercises such as blowing or sucking tasks to elicit movement. Overflow motions or increased activity of undesired motions should be discouraged. The therapist can provide sensory stimulation for reduced sensation using a gloved hand inside and outside the mouth. Having the patient accomplish regular oral hygiene helps to establish sensory awareness and motor responses. For abnormally heightened sensation, graded sensory stimulation programs help the patient tolerate stimulation of the face and oral cavity to accept food and utensils. The therapist addresses abnormal reflexes with positioning and avoiding the stimuli that trigger the response.23
Weakness (as opposed to hypotonicity) of oral structures may be an issue with the debilitated stroke patient with reduced endurance. Some dysphagia therapists find that direct oral range of motion exercises are useful and often progress patients to gentle oral progressive resistive exercises. Tongue exercises have been shown to improve swallow pressure and airway safety in patients with both acute and chronic stroke.72 Lip exercises have been found to improve lip force for eating in stroke patients.34 A new study suggests that strength training does not exacerbate spasticity, as previously thought.7
While the patient eats, alteration in bolus qualities may help to trigger oral responses to food and thus improve the ensuing pharyngeal responses. Pushing down slightly with the spoon on the tongue as the bolus is introduced into the mouth can help with sensory awareness. Presentation of a cold bolus13 or a sour bolus53 can facilitate oral and also pharyngeal responses. Alternating food textures with each mouthful—for example, alternating fluids with solids—is a way of altering sensory input with each bite.55
Facilitation of pharyngeal and laryngeal movements
Exercises involving pulling the tongue back, yawning, and gargling with saliva serve to strengthen retraction of the base of the tongue,92 which is necessary to execute a swallow. Shaker exercises strengthen laryngeal elevation.79 To accomplish Shaker exercises, the therapist has the patient perform repetitive tucking of the chin to the chest while supine. Shaker exercises have been shown to help patients with chronic dysphagia who are fed by tube to return to eating food by mouth.78 Encouraging the patient to talk, cough, and clear the throat intermittently provides functional exercise for motions of the pharynx and larynx.
As with facilitation of oral motions, pharyngeal and laryngeal, strength training may assist in improving the force with which motions can be accomplished.16
Facilitation of swallowing
Different methods are available to facilitate a swallow when its initiation is weak or delayed:
 Thermal-tactile stimulation consists of stroking the faucial arches with a chilled laryngeal mirror before eating and has been shown to speed the onset of the swallow response and the total swallow time in stroke patients.73 A study indicated that the use of citrus flavored cold stimulus was optimal; however, the effect lasted for only one swallow.77
Thermal-tactile stimulation consists of stroking the faucial arches with a chilled laryngeal mirror before eating and has been shown to speed the onset of the swallow response and the total swallow time in stroke patients.73 A study indicated that the use of citrus flavored cold stimulus was optimal; however, the effect lasted for only one swallow.77
 Surface electromyography has been used to retrain brainstem stroke patients with chronic dysphagia to eat safely by mouth20 and also has been demonstrated to be useful in providing biofeedback for relaxing high tone in laryngeal musculature, which allows an improved swallow response.40
Surface electromyography has been used to retrain brainstem stroke patients with chronic dysphagia to eat safely by mouth20 and also has been demonstrated to be useful in providing biofeedback for relaxing high tone in laryngeal musculature, which allows an improved swallow response.40
 Electrical stimulation. Neuromuscular electrical stimulation therapy is used to target specific muscle groups to strengthen the swallow response. The VitalStim unit was developed by the Chattanooga group specifically for swallowing therapy. Practitioners must be certified to perform this therapy. One metaanalysis has demonstrated that this modality is effective for strengthening the swallow.18
Electrical stimulation. Neuromuscular electrical stimulation therapy is used to target specific muscle groups to strengthen the swallow response. The VitalStim unit was developed by the Chattanooga group specifically for swallowing therapy. Practitioners must be certified to perform this therapy. One metaanalysis has demonstrated that this modality is effective for strengthening the swallow.18
 Improving quality of the swallow. Different techniques to improve the bolus direction during the swallow have been attempted with dysphagia patients. Patients with stroke often have residue in the affected cheek; using the tongue to clear the bolus or massaging the cheek with the hand are helpful to route the bolus back to the center of the tongue. Holding the affected lip closed with a finger to allow oral containment of the bolus may be necessary. Having the patient chew with the hemiparetic side of the jaw stimulates movement and function and helps the patient to practice transfer of the bolus between the two molar surfaces.
Improving quality of the swallow. Different techniques to improve the bolus direction during the swallow have been attempted with dysphagia patients. Patients with stroke often have residue in the affected cheek; using the tongue to clear the bolus or massaging the cheek with the hand are helpful to route the bolus back to the center of the tongue. Holding the affected lip closed with a finger to allow oral containment of the bolus may be necessary. Having the patient chew with the hemiparetic side of the jaw stimulates movement and function and helps the patient to practice transfer of the bolus between the two molar surfaces.
Using a chin tuck position during the swallow may be beneficial in decreasing aspiration in persons who experience a delayed pharyngeal swallow and reduced airway closure if the source of aspiration is material pooled in the valleculae.80 The study by Shanahan and colleagues80 did not find a decrease in the risk of aspiration with pooling in the piriform sinus with chin tuck. Chin tuck causes the structures of the pharynx to move posteriorly, reducing the size of the opening to the larynx.94
Full rotation of the head causes the bolus to move away from the direction of rotation and can be used to direct the bolus down the intact side of the pharynx.65
The “effortful swallow” is done by contracting the muscles of the throat hard during the swallow; this moves the base of the tongue posteriorly and helps to clear bolus from the valleculae.
The Mendelson maneuver, accomplished by pushing the tongue into the hard palate while swallowing, has been demonstrated to open the cricopharyngeal sphincter better and for a longer period, allowing the bolus to pass.10
Throat clearing and reswallowing may be useful in clearing pooled residue and can be done with other swallowing techniques.
Diet modification
Research demonstrates that stroke patients aspirate less on pureed textures compared with liquids and soft solids.28 The American Dietetic Association has standardized levels of a dysphagia diet, called the National Dysphagia Diet, which is appropriate for many diagnoses, including stroke.3 Box 24-2 presents levels of diet based on the NDD. Naturally, patients will require an individual approach to determining safe and manageable textures to swallow. For example, carbonated liquids, not noted on the NDD, have been found to reduce incidence of aspiration compared with noncarbonated thin fluids.15
Follow-up care
Follow-up dysphagia care is advised for determining whether caregivers and patients understand and are complying with recommendations; outpatient visits after acute and rehabilitative inpatient care may be needed. Diets may need to be upgraded as improvements occur, and the patient and caregiver should be reminded about safe swallowing strategies and food textures.
Patient and caregiver education
The education process begins with initial contact with the patient and caregivers and continues with follow-up visits, informational pamphlets, and referrals to other health care professionals. Patients and caregivers must understand the concept of dysphagia, including the causes and consequences of aspiration, because they cannot follow recommended treatment without knowledge of the problem and its possible consequences. Anatomical pictures, handouts, and verbal explanations are useful educational tools. Precautionary signs placed by the bed also may be helpful in reinforcing the need to follow mealtime management guidelines.
Types and efficacy of dysphagia intervention
Recovery of swallowing function is likely due to a combination of natural recovery and therapeutic effects. These effects include facilitation of available motions where structures and functions have been lost due to reduced sensation and altered muscle tone, and volitional strengthening of weak structures. Neuroplasticity is facilitated by these interventions9 in ways that rehabilitation science has yet to fully understand.71
While swallowing compensations are used initially to encourage function in early stroke recovery, the goal for many is recovery of premorbid function. In those for whom recovery of lost function cannot be achieved, compensatory strategies may be permanent.
Regardless of whether it is rehabilitative or compensatory in nature, dysphagia intervention has been shown to improve aspects of oral and pharyngeal function61 and nutritional status in patients with stroke.29 Dysphagia intervention is associated with the ultimate ability to eat by mouth in those with neurological diagnoses.17,62 Intervention has been shown in one small study to enable those with chronic dysphagia requiring alternative nutrition sources to return to eating by mouth with the use of surface electromyography biofeedback.41 Dysphagia intervention for patients with stroke has been shown to reduce the risk of aspiration pneumonia62 and thus is cost-effective.64
CASE STUDY 1 Swallowing After Right Hemispheric Stroke
Mrs. Jones was admitted to the hospital with a right middle cerebral artery stroke, resulting in a left hemiplegia with dysphagia. She had a nasogastric tube and was not referred for dysphagia evaluation until she was medically stable, a week after her admission. On evaluation, she demonstrated a left facial droop involving reduced muscle tone in the lip, cheek, and tongue. Drooling from the left side of her mouth was a problem because of reduced sensation. The gag reflex was reduced on the left side of the pharynx, although she could elicit a dry swallow with difficulty. Once her dentures were inserted and the nasogastric tube was removed, a feeding trial was done. During the feeding trial, Mrs. Jones demonstrated pocketing of food in her left cheek and in the sulcus between her lower jaw and cheek. She was able to swallow soft purees and honey-thick fluids, although thin fluids extracted a cough. An MBS further revealed pooling in the pyriform sinuses and occasional laryngeal penetration with honey-thick fluids, which was alleviated with a chin tuck and by intermittent throat clearing. At this time, she still had an intravenous line, so hydration was not a concern. She was able to feed herself with her dominant right hand once her tray was set up, with frequent cues to regard the left side of her plate because of left neglect. She also needed cues to swallow each mouthful and eat slowly because of reduced judgment and impulsivity. Mrs. Jones massaged her left cheek with tactile cues to move pocketed food back onto her tongue. Within a week, Mrs. Jones progressed to soft solids and nectar-thick fluids, and her intravenous line was discontinued. The following week, she proceeded to thin fluids and ground solids and was able to prepare her tray independently. She still needed occasional safety cues to eat slowly, to take single sips, and to look at the left side of her plate.
CASE STUDY 2 Swallowing after Left Hemispheric Stroke
Mr. Smith was admitted to the hospital with a left middle cerebral artery stroke and was referred for dysphagia evaluation the day after admission. His oral movements and ability to follow commands were difficult to assess formally because of aphasia. Active and symmetrical motion of his lips, cheeks, and tongue were observed on attempts to speak. Mr. Smith’s dentition was intact. His gag reflex was intact, although palatal movement was not observed because of inability to phonate on command; he was unable to produce an automatic cough. On the feeding trial, he initially demonstrated slow initiation of oral and hand-to-mouth movement characteristic of apraxia, but once he had eaten several bites, he was able to manipulate foods more efficiently during the preoral and oral-preparatory stages of the swallow. Mr. Smith was able to manage soufflé textures and soft chewable solids and to drink thin fluids using a dysphagia cup to prevent tipping his head back to swallow. He required some tactile guiding to self-feed with his dominant right upper extremity, which had exhibited isolated but weak movements. Within the week, he was able to chew and swallow food with regular textures. Upper extremity function improved as well, and he could prepare his tray independently and cut solid foods using his right hand in dominant fashion.
Review questions
2. Define laryngeal penetration.
3. Describe the five stages of swallowing. Indicate three signs or symptoms of dysphagia at each stage.
4. Name the cranial nerves and identify their functions in swallowing.
5. Name 10 items important for chart review.
6. Describe the elements of a dysphagia intervention program for a stroke patient.
1. Addington WR, Stephens RE, Gilliland KA. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Stroke. 1999;30(6):1203-1207.
2. Alberts MJ, Horner J, Gray L, et al. Aspiration after stroke. lesion analysis by brain MRI. Dysphagia. 1992:7;3:170-173.
3. American Dietetic Association. National Dysphagia Diet. Standardization for Optimal Care. Chicago: American Dietetic Association. 2002.
4. Avery-Smith W, Rosen AB, Dellarosa DM. Dysphagia evaluation protocol. San Antonio, TY: Therapy Skill Builders; 1997.
5. Aviv JE, Martin JH, Sacco RL, et al. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Ann Otol Rhinol Laryngol. 1996;105(2):92-97.
6. Aydogdu I, Ertekin C, Sultan T, et al. Dysphagia in lateral medullary Infarctions (Wallenberg’s Syndrome). Stroke. 2001;32(9):2081-2087.
7. Badics E, Wittmann A, Rupp M, et al. Systematic muscle building exercises in the rehabilitation of stroke patients. Neurorehabilitation. 2002;17(3):211-214.
8. Badr C, Ekins MR, Ellis ER. The effect of body position on maximal expiratory pressure and flow. Aust J Physiother. 2002;48(2):95-102.
9. Barrett AW, Smithard DG. Role of cerebral cortex plasticity in the recovery of swallowing function following dysphagia stroke. Dysphagia. 2009;24(1):83-90.
10. Bartolome G, Neumann S. Swallowing therapy in clients with neurological disorders causing cricopharyngeal dysfunction. Dysphagia. 1993;8(2):146.
11. Bastian RW. The videoendoscopic swallowing study. an alternative and partner to the videofluoroscopic swallowing study. Dysphagia. 1993:8;4:359-367.
12. Besson G, Bogousslavsky J, Regli F, Maeder P. Acute pseudobulbar or suprabulbar palsy. Arch Neurol. 1991;8(5):501-507.
13. Bisch EM, Logemann JA, Rademaker AW, et al. Pharyngeal effects of bolus volume, viscosity, and temperature in clients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech Hear Res. 1994;37(5):1041.
14. Borr C, Hielscher-Fastabend M, Lucking A. Reliability and validity of cervical auscultation. Dysphagia. 2007;2(3):225-234.
15. Bulow M, Olsson R, Ekberg O. Videoradiographic analysis of how carbonated thin liquids and thickened liquids affect the physiology of swallowing in subjects with aspiration on thin liquids. Acta Radiologica. 2003;44(4):366-372.
16. Burkhead LM, Sapienza CM, Rosenbek JC. Strength training in dysphagia rehabilitation. principles, procedures, and directions for future research. Dysphagia. 2007:22;3:251-265.
17. Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke. a randomized controlled trial. Lancet Neurol. 2007:5;1:31-37.
18. Carnaby-Mann GD, Crary MA. Examining the evidence on neuromuscular electrical stimulation for swallowing. a meta-analysis. Arch Otol Head Neck Surg. 2007:133;6:564-571.
19. Ciocon JO. Indications for tube feedings in elderly patients. Dysphagia. 1990;5(1):1-5.
20. Crary MA. A direct intervention program for chronic neurogenic dysphagia secondary to brainstem stroke. Dysphagia. 1995;10(1):6-18.
21. Crary MA, Carnaby Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86(8):1516-1520.
22. Daniels SK, McAdam CP, Braily K, Foundas AL. Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Path. 1997;6:17-24.
23. Davies PM. Starting again. Berlin: Springer-Verlag; 1994.
24. DePippo KL, Hosas MA, Reding MJ. Validation of the 3–oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259-1261.
25. DePippo KL, Hosas MA, Reding MJ. The Burke dysphagia screening test. validation of its use in patients with stroke. Arch Phys Med Rehabil. 1994:75;12:1284-1286.
26. Ding R, Logemann JA. Pneumonia in stroke patients. a retrospective study. Dysphagia. 2000:15;2:51-57.
27. Dziewas R, Ritter M, Schilling M, et al. Pneumonia in acute stroke patients fed by nasogastric tubes. J Neurol Neurosurg Psychiatry. 2004;5(6):852-856.
28. Dziewas R, Warnecke T, Olenberg S, et al. Towards a basic endoscopic assessment of swallowing in acute stroke-development and evaluation of a simple dysphagia score. Cerebrovascular Dis. 2008;6(1):41-47.
29. Elmstahl S, Bulow M, Ekberg O, et al. Treatment of dysphagia improves nutritional conditions in stroke patients. Dysphagia. 1999;14(2):61-66.
30. Ertekin C, Aydogdu I, Tarlaci S, et al. Mechanisms of dysphagia in suprabulbar palsy with lacunar infarct. Stroke. 2000;1(6):1370-1376.
31. Finestone HM, Foley NC, Woodbury MG, et al. Quantifying fluid intake in dysphagia stroke patients. a preliminary comparison of oral and nonoral strategies. Arch Phys Med Rehabil. 2001:82;12:1744-1746.
32. Groher ME. Determination for the risks and benefits of oral feeding. Dysphagia. 1994;9(4):233-235.
33. Groher ME, Bukatman R. The prevalence of swallowing disorders in two teaching hospitals. Dysphagia. 1986;1(1):3.
34. Hagg M, Anniko M. Lip muscle training in stroke patients with dysphagia. Acta Otolaryngologica. 2008;128(9):1027-1033.
35. Heckert KD, Komaroff E, Adler U, Barrett AM. Postacute re-evaluation may prevent dysphagia-associated morbidity. Stroke. 2009;40(4):1381-1385.
36. Hinchey JA, Shephard T, Furie K, et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972-1976.
37. Hinojosa J, Kramer P, Crist P, Evaluation P. Obtaining and interpreting data, 2nd ed. Bethesda, MD: AOTA Press; 2005.
38. Horner J, Massey EW. Silent aspiration following stroke. Neurology. 1988;38(2):317-319.
39. Horner J, Massey EW, Brazer SR. Aspiration in bilateral stroke patients. Neurology. 1990;40(11):1686-1688.
40. Huckabee ML. Maximizing rehabilitation efforts for dysphagia recovery. SEMG biofeedback monitoring. Lincoln Park, NJ: Kay Elemetrics Corp. Application Note. 1997.
41. Huckabee ML, Cannito MP. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia. a retrospective evaluation. Dysphagia. 1999:14;2:93-109.
42. Irie H, Lu CC. Dynamic evaluation of swallowing in patients with cerebrovascular accident. Clin Imaging. 1995;19(4):240-243.
43. James R, Gines D, Menlove A, et al. Nutrition support (tube feeding) as a rehabilitation intervention. Arch Phys Med Rehabil. 2005;86(12):82-92.
44. Johnson ER, McKenzie SW, Sievers A. Aspiration pneumonia in stroke. Arch Phys Med Rehabil. 1993;74(9):973-976.
45. Kalra L, Yu G, Wilson K, et al. Medical complications during stroke rehabilitation. Stroke. 1995;26(6):990-994.
46. Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration. how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare. Laryngoscope. 2007:117;10:1723-1727.
47. Kidd D, Lawson J, Nesbitt R, et al. The natural history and clinical consequences of aspiration in acute stroke. Q J Med. 1995;88(6):409-413.
48. Kidder TM, Langmore SE, Martin BJW. Indications and techniques of endoscopy in evaluation of cervical dysphagia. comparison with radiographic techniques. Dysphagia. 1994:9;4:256-261.
50. Linden-Castelli P. Treatment strategies for adult neurogenic dysphagia. Semin Speech Lang. 1991;12(3):255.
51. Logemann JA. Criteria for studies of the treatment for oral-pharyngeal dysphagia. Dysphagia. 1987;1(4):193.
52. Logemann JA. Effects of aging on the swallowing mechanism. Otolaryngol Clin North Am. 1990;23(6):1045-1056.
53. Logemann JA, Pauloski BR, Colangelo L, et al. Effects of a sour bolus on oropharyngeal swallowing measures in clients with neurogenic dysphagia. J Speech Hear Res. 1995;38(3):556-563.
54. Logemann JA. Preswallow sensory input. its potential importance to dysphagic patients and normal individuals. Dysphagia. 1996:11;1:9-10.
55. Logemann JA. Evaluation and treatment of swallowing disorders. Austin, TX: Pro-Ed; 1997.
56. Lorish TR, Sandin KJ, Roth EJ, et al. Stroke rehabilitation evaluation and management. Arch Phys Med Rehabil. 1994;75(5 Spec No):S47-S51.
57. Mann G. MASA. The Mann assessment of swallowing ability. Clifton Park, NY: Singular. 2000.
58. Mann G, Hankey GJ. Initial clinical and demographic predictors of swallowing impairment following acute stroke. Dysphagia. 2001;16(3):208-215.
59. Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke. incidence, diagnosis, and pulmonary complications. Stroke. 2005:36;12:2756-2763.
60. Meng NH, Wang TG, Lien IN. Dysphagia in patients with brainstem stroke. incidence and outcome. Am J Phys Med Rehabil. 2000:79;2:170-175.
61. Neumann S. Swallowing therapy with neurologic patients. results of direct and indirect therapy methods in 66 patients suffering from neurologic disorders. Dysphagia. 1993:8;2:150-153.
62. Neumann S, Bartolome G, Buchholz D, et al. Swallowing therapy of neurologic patients. correlation of outcome with pretreatment variables and therapeutic methods. Dysphagia. 1995:10;1:1-5.
63. Noll SF, Roth EJ. Stroke rehabilitation. I. Epidemiologic aspects and acute management. Arch Phys Med Rehabil. 1994;75(5 Spec No):S38-S41.
64. Odderson IR, Keaton JC, McKenna BS. Swallow management in patients on an acute stroke pathway. quality is cost effective. Arch Phys Med Rehabil. 1995:76;12:1130-1133.
65. Ohmae Y, Ogura M, Kitahara S, et al. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol, Laryngol. 1998;107(4):344-348.
66. O’Neil KH, Purdy M, Falk J, Gallo L. The Dysphagia Outcome and Severity Scale. Dysphagia. 1999;14(3):139.
67. Parker C, Power ML, Hamdy S, et al. Awareness of dysphagia by patients following stroke predicts swallowing performance. Dysphagia. 2004;19(1):28-35.
68. Perlman AL, Langmore SE, Milianti FJ, et al. Comprehensive clinical examination of oropharyngeal swallowing function. Veteran’s Administration procedure. Semin Speech Lang. 1991:12;3:246.
69. Power ML, Hamdy S, Goulermas JY, et al. Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia. 2009;4(3):257-264.
70. Ramsey DJC, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34(5):1252-1257.
71. Robbins J, Butler SG, Daniels SK, et al. Swallowing and dysphagia rehabilitation. translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res. 2008:51;1:S276-S300.
72. Robbins J, Kays SA, Gangnon RE, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):8-150.
73. Rosenbek JC, Roecker EB, Wood JL, et al. Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia. 1996;11(4):225-233.
74. Ryu JY, Park SR, Choi KH. Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753-757.
75. Salassa J. A Functional Outcome Swallowing Scale for staging oropharyngeal dysphagia. Dig Dis. 1999;7(4):230-234.
76. Schmidt J, Holas M, Halvorson K, et al. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia. 1994;9(1):7.
77. Sciortino KF, Liss JM, Case JL, et al. Effect of mechanical, cold, gustatory, and combined stimulation to the human anterior faucial pillars. Dysphagia. 2003;18(1):16-26.
78. Shaker R, Easterling C, Kern M, et al. Rehabilitation of swallowing by exercise in tube-fed clients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122(5):1314-1321.
79. Shaker R, Kern M, Bardan E, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol. 1997;272(6 pt 1):G1518-G1522.
80. Shanahan TK, Logemann JA, Rademaker AW, et al. Chin-down posture effect on aspiration in dysphagic patients. Arch Phys Med Rehabil. 1993;74(7):9-736.
81. Smithard DG, O’Neill PA, Parks C, et al. Complications and outcome after acute stroke. does dysphagia matter?. Stroke. 1996:27;7:1200-1204.
82. Smithard DG, O’Neill PA, England RE, et al. The natural history of dysphagia following stroke. Dysphagia. 1997;12(4):188-193.
83. Smithard DG, O’Neill PA, Park C, et al. Can bedside assessment reliably exclude aspiration following acute stroke. Age Ageing. 1998;27(2):99-106.
84. Sonies BC. Instrumental procedures for dysphagia diagnosis. Semin Speech Lang. 1991;12(3):186.
85. Steinhagen V, Grossmann A, Benecke R, Walter U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke. 2009;40(5):1903-1906.
86. Stoeckli S, Huisman TA, Seifert B, et al. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia. 1991;18(1):53-57.
87. Takahashi K, Groher ME, Michi K. Methodology for detecting swallowing sounds. Dysphagia. 1994;9(1):54-62.
88. Teasell RW, Bach DB, McRae M. Prevalence and recovery of aspiration poststroke. a retrospective analysis. Dysphagia. 1994:9;1:35-39.
89. Teasell RW, Foley N, Doherty T, et al. Clinical characteristics of patients with brainstem stroke admitted to a rehabilitation unit. Arch Phys Med Rehabil. 2002;83(7):1013-1016.
90. Trapl M, Enderle P, Nowotny M, et al. Dysphagia bedside screening for acute-stroke patients. The Gugging Swallowing Screen. Stroke. 2007:38;11:2948-2952.
91. Veis SL, Logemann JA. Swallowing disorders in persons with cerebrovascular accident. Arch Phys Med Rehabil. 1985;66(6):372-375.
92. Veis SL, Logemann JA, Colangelo L. Effects of three techniques on maximum posterior movement of the tongue base. Dysphagia. 2002;15(3):142-145.
93. Wang T-G, Chang Y-C, Chen S-Y, Hsiao T-Y. Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730-734.
94. Welch MV, Logemann JA, Rademaker AW, et al. Changes in pharyngeal dimensions effected by chin tuck. Arch Phys Med Rehabil. 1993;74(2):178-181.
95. Whelan K. Inadequate fluid intakes in dysphagic acute stroke. Clin Nutr. 2001;20(5):423-428.
96. Wilkinson TJ, Thomas K, MacGregor S, et al. Tolerance of early diet textures as indicators of recovery from dysphagia after stroke. Dysphagia. 2002;17(3):227-232.
97. Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50(3):430-433.
98. Zenner PM, Losinski DS, Mills RH. Using cervical auscultation in the clinical dysphagia examination in long-term care. Dysphagia. 1995;10(1):27-31.
Carnaby-Mann K, Lenius G, Crary MA. Update on assessment and management of dysphagia post stroke. Northeast Florida Medicine. 2007;58(2):31-34.
Clark HM. Neuromuscular treatments for speech and swallowing. A tutorial. Am J Speech Lang Pathol. 2003:12;4:400-415.
Clark HM. Clinical decision making and oral motor treatments. ASHA Leader; June 8-9, 2005.
Crary MA, Groher ME. Adult swallowing disorders. Philadelphia: Elsevier, 2003.
Davies P. The neglected face. In Steps to follow. New York: Springer-Verlag. 2000.
Fornataro-Clerici L, Roop TA. Clinical management of adults requiring tracheostomy tubes and ventilators. Gaylord, MI: Northern Speech Services, 1997.
Groher ME. Dysphagia. diagnosis and management. Boston: Butterworth-Heinemann. 1997.
Ramsey DJC, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34(5):1252-1257.