chapter 1 Pathophysiology, medical management, and acute rehabilitation of stroke survivors
After completing this chapter, the reader will be able to accomplish the following:
1. Describe the pathophysiology of stroke.
2. Explain the diagnostic workup of stroke survivors.
3. Understand the medical management of various stroke syndromes.
4. Describe interventions to prevent the recurrence of stroke and its complications.
5. Understand normal and abnormal responses to acute stroke rehabilitation.
6. Be familiar with standardized assessments used during acute stroke rehabilitation.
7. Implement a comprehensive treatment that is safe for the acute and ICU settings.
8. Write appropriate goals for the acute and ICU settings.
9. Be able to prevent secondary complications such as skin breakdown and contracture after stroke.
Pathophysiology and medical management of stroke
Prevalence and impact of stroke
Stroke remains the third leading cause of mortality in the United States after cardiovascular disease and cancer, accounting for 10% to 12% of all deaths.15,127 Globally, stroke is the second leading cause of mortality in developed nations with 4.5 million deaths every year.109 An estimated 550,000 strokes occur each year, resulting in 150,000 deaths and more than 300,000 individuals with significant disability.119 The United States has an estimated 3 million stroke survivors today, which is double the number of survivors 25 years ago.54 The economic impact of stroke in 2007 was estimated at $62.7 billion, markedly increased from the estimate in 2001 of $30 billion, of which $17 billion were direct medical costs and $13 billion were indirect costs from lost productivity.119 Fortunately, modern medical interventions (mostly risk factor modifications) have decreased stroke mortality by approximately 7% per year in industrialized nations since 1970.15 The advances continue, but with increased cost of care for more advanced treatments.
Epidemiology of stroke
Stroke is essentially a preventable disease with known, manageable risk factors.16 The established risk factors for stroke include hypertension, cigarette smoking, obesity, elevated serum fibrinogen levels, diabetes, a sedentary lifestyle, and the use of contraceptives with high doses of estrogen.101 The most important and easily treated of these risk factors is systolic hypertension. In the Multiple Risk Factor Intervention Trial, 40% of strokes were attributed to systolic blood pressures greater than 140 mm Hg.130 Stroke incidence also increases exponentially with aging, with an increase in stroke from three in 100,000 individuals per year in the third and fourth decades of age to 300 in 100,000 individuals per year in the eighth and ninth decades of life.16 Eighty-eight percent of stroke deaths occur among persons aged 65 years or older15 Table 1-1 outlines modifiable and nonmodifiable risks.
Table 1-1 Modifiable and Nonmodifiable Risks
| TYPE OF RISK | RELATIVE RISK (PER 1000 PERSONS) |
|---|---|
| Modifiable risks | |
| Hypertension | 4.0 to 5.0 |
| Cardiac disease | 2.0 to 4.0 |
| Atrial fibrillation | 5.6 to 17.6 |
| Diabetes mellitus | 1.5 to 3.0 |
| Cigarette smoking | 1.5 to 2.9 |
| Alcohol abuse | 1.0 to 4.0 |
| Hyperlipidemia | 1.0 to 2.0 |
| Nonmodifiable risks | |
| Age | 1 to 2/1000 at age 45– to 54–years-old to 20/1000 at age 75– to 84–years-old |
| Gender | 1.2 to 2.1 |
| Race (black or Hispanic) | 2.0 |
| Heredity | 1.8 to 3.1 |
Stroke prevention interventions have reduced mortality in industrialized nations primarily through treating hypertension in the elderly. Another cause of decreased mortality has been the establishment of dedicated stroke units that can prevent acute death and later development of life-threatening complications.
Pathogenesis and pathology of stroke
Definition and description of stroke syndromes
Stroke.
Stroke is essentially a disease of the cerebral vasculature in which a failure to supply oxygen to brain cells, which are the most susceptible to ischemic damage, leads to their death. The syndromes that lead to stroke compose two broad categories: ischemic and hemorrhagic stroke. Ischemic strokes account for approximately 80% of strokes, whereas hemorrhagic strokes account for the remaining 20%.128
Transient ischemic attack.
Symptoms of a transient ischemic attack (TIA) include the focal deficits of an ischemic stroke within a clearly vascular distribution, but TIAs are reversible defects because no cerebral infarction ensues. The causes of TIAs can be thrombotic and embolic and could result from a cerebral vasospasm. By definition, the effects of TIAs must resolve in less than 24 hours. Since 35% of patients who have had a TIA will have a stroke within five years, they should have a complete evaluation for cerebrovascular disease and sources of embolism.167 The treatment of TIAs depends on the source of the emboli or thrombi and can include anticoagulation therapy and/or surgery.
Ischemic stroke
An ischemic stroke is the most common form of stroke with various causes. The one common endpoint among all the different subtypes of ischemic strokes is that injury results from tissue anoxia caused by an interruption of cerebral blood flow.
Embolic stroke.
Cerebral embolic strokes are the most common subtype of ischemic stroke. Embolic strokes usually are characterized by an abrupt onset, although they also can be associated with stuttering symptoms. Usually no heralding events occur, such as TIAs or previous small strokes evolving into larger strokes.83 A warning with microemboli that cause smaller events are uncommon, and the usual clue to a possible embolic source is a completed stroke.128 The source of approximately 40% of embolic strokes is unknown, even after the common sources have been evaluated extensively. Most embolic strokes of known cause occur after emboli that are cardiac in origin.27 The second most common sources of emboli are atherothrombotic lesions that result in artery-to-artery embolisms. These lesions can be in the aorta, the carotid and vertebrobasilar systems, and, less frequently, smaller arteries.
Sources of emboli
Cardiac sources.
Cardiac emboli can develop from numerous areas in the heart. Cardiac dysrhythmias, structural anomalies, and acute infarctions are the usual sources of emboli. The most common source of an embolism is the classical pattern of thrombosis in the left atrium of patients with atrial fibrillation. The usual mechanism of thrombus formation in atrial fibrillation is by clot formation in the left atrial appendage. This then breaks off and creates an embolus that can move through the arterial system. Patients older than 60 years are particularly prone to this type of embolization. Embolism is not limited to the brain, and infarction can occur in the kidneys, peripheral tissues, or any other location.
The most common cardiac structural cause of a cerebral embolism is due to a myocardial infarction.83 In patients with left ventricular infarcts, particularly anterior wall and apical infarctions, the endocardial damage associated with a subendocardial or transmural infarction is an excellent nidus (a focal point where bacteria or other infectious agents thrive) for thrombus formation. The emboli most often develop during the first several weeks after the infarction, although the risk for developing them can persist for much longer.
Valvular heart disease also can result in thrombi, but they more frequently develop after valve replacement rather than result directly from the native valve. More commonly the native valvular heart disease causes the patient to be in atrial fibrillation and then to develop an embolus. Mechanical heart valves (e.g., St. Jude valves) are much more likely to cause emboli than porcine (tissue) valves, so patients with the mechanical type always continue to receive anticoagulation therapy.
Much less common sources of cardiac emboli are the vegetations resulting from bacterial endocarditis. These emboli cause small septic infarcts called mycotic aneurysms, which are at high risk of conversion to hemorrhagic infarcts. Other rare causes of cardiac emboli are atrial myxomas, which are tumors of the heart endocardium. In addition, embolic infarctions also may result from cardiac and thoracic surgery.83
Cardiac emboli usually (80% of the time) occlude the middle cerebral artery, 10% of cardiac emboli occlude the posterior cerebral artery, and the rest occlude the vertebral artery or its branches.83 Anterior cerebral artery embolization from the heart is rare. The severity of the clinical syndrome is related to the size of the embolus. An embolus of 3 to 4 mm can cause a large stroke by occluding the larger brain arteries. Blood clots undergo lysis over a few days with the establishment of recanalization through the clot. Because clots naturally lyse, a stroke can convert from ischemic to hemorrhagic when reperfusion distal to the occlusion is present, because the blood vessels in the ischemic distribution may no longer be intact. This can lead to leakage from these damaged arteries, arterioles, and capillaries, leading to a phenomenon called hemorrhagic conversion. The possibility of hemorrhagic conversion contraindicates the use of anticoagulation therapy as initial treatment for large embolic strokes.
Vascular sources.
Strokes vascular in origin are far less common than cardiac strokes but are still one major type of embolic stroke. The sources of vascular emboli are usually atheromatous plaques in the walls of the aorta, carotid arteries, or smaller vessels in the cerebral circulation. Platelet activation and the formation of a fibrin clot can occur rapidly. The most common areas affected by the emboli of the vascular system are the same as those affected by cardiac sources of emboli. The most common areas for ulcerated plaques in the cerebral blood supply are the aorta and the proximal internal carotid artery. The plaques in the carotid artery can be visualized by Doppler sonography of the carotid artery system.128
Paradoxical sources.
Congenital atrial septal defects can create the opportunity for emboli to cross from the right-sided (venous) circulation to the left-sided (arterial) circulation, a rare source of cerebral emboli. A common source of paradoxical embolic material is deep venous thrombosis (DVT). The modern techniques of transesophageal echocardiography with a “bubble study” help identify patients at risk for this condition. One performs a bubble study by injecting a small bolus of air into the venous circulation while the echocardiographer observes the heart. If the air bolus, which is seen easily, has no portion cross over to the left-sided circulation, then no shunt is present. If the bubbles cross into the left-sided circulation, then a shunt is possible. One of the most common atrial shunting abnormalities is a patent foramen ovale. In young patients or patients who have had TIAs or strokes, the treatment of choice is surgical repair of the lesion.
Unknown sources.
Thrombi of unknown source often occur in patients with known hypercoagulability syndromes. These syndromes can result from acquired diseases (e.g., lupus anticoagulant and metastatic tumors) or inborn errors of the coagulation system (e.g., protein S and C deficiencies). Surgery or medication therapies such as estrogen replacement can induce iatrogenic causes of hypercoagulable states. Even when the patient is known to be in a hypercoagulable state, the source of the emboli may remain unknown. In many patients the entire workup is unrevealing.
Thrombotic stroke
A thrombotic stroke can result from a variety of causes, but most causes are related to the development of abnormalities in the arterial vessel wall. Atherosclerosis, arteritis, dissections, and external compression of the vessels are causes. In addition, some patients with hematological disorders develop thrombosis. The spectrum of disease includes stroke and TIA, and often the difference between a thrombotic and an embolic stroke may be difficult to determine. Thrombosis and embolism are often both present, especially in patients with atherosclerotic disease. The exact mechanism of infarction from thrombosis is still being debated, but atherosclerosis does play a significant role. Hypertension with associated microtrauma of the arterial intima is thought to play a role, as is hypercholesterolemia.104,128 TIAs may result from the formation of microthrombi and their embolization. Large vessel thrombosis can also occur in extracranial vessels, such as the vertebral and carotid arteries, leading to devastating strokes.117
Pathophysiology.
Atherosclerotic plaque formation is greatest at the branching points of major vessels and forms in areas of turbulent flow. Chronic hypertension is a common precursor, and damage to the intimal wall may be followed by lymphocyte infiltration. Foam cells then develop, and the first stage of atherosclerosis is formed. Calcification and narrowing with resultant turbulent flow follow. In this setting of turbulent flow, plaque ulceration can become a site for thrombus formation. If the thrombus forms and is degraded rapidly, a transient ischemic phenomenon can occur, which is the setting of a TIA. Classically, the symptoms of internal carotid disease include amaurosis fugax and monocular blindness. If the clot does not break up or lyse, a cerebral infarction can occur. The size and severity of the infarction depends on available collateral circulation and the size of the occluded vessel. In patients with extensive atherosclerotic disease, however, a limited amount of collateral circulation is available, and the sparing from collateral circulation may be limited.
Atherothrombotic disease.
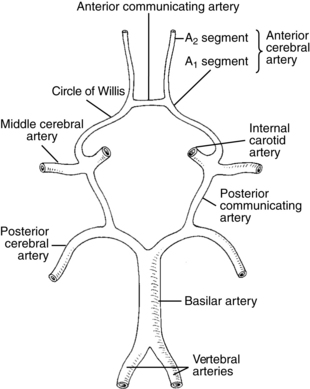
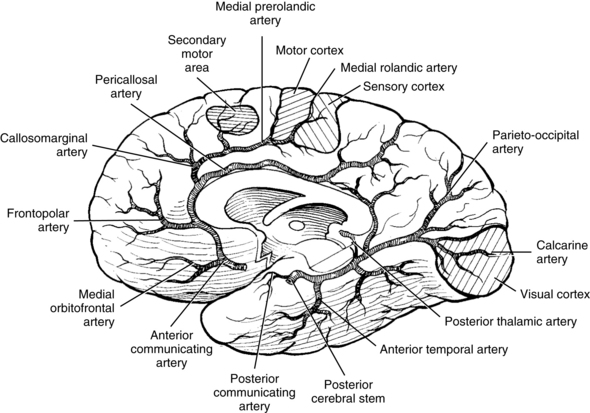
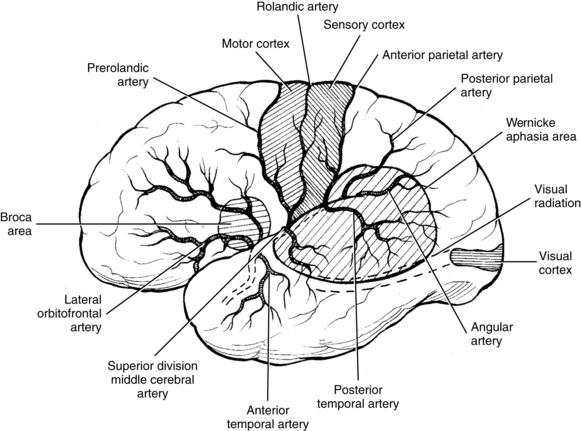
The most common site for the development of atherosclerosis and the subsequent development of atherothrombosis that leads to TIAs and stroke in the anterior circulation is the origin of the carotid artery and in the posterior circulation is the top of the basilar artery. Other sites of atherosclerosis include the carotid siphon and the stems (bases) of the middle cerebral artery, anterior cerebral artery, and origin of the basilar artery.51 The atheromatous plaques are sources of emboli that can cause distal symptoms in a TIA or stroke. These embolic events are similar events from other embolic sources. Table 1-2 lists common stroke syndromes, and Figs. 1-1 to 1-3 explain the anatomy of these strokes. Atherosclerotic disease is screened most readily by carotid Doppler ultrasonography and transcranial Doppler imaging. Magnetic resonance angiography (MRA) and carotid and cerebral angiography can further elucidate lesions, which can be treated surgically or medically.
Table 1-2 Common Stroke Syndromes
| ANATOMICAL DISTRIBUTION | STROKE SYNDROME |
|---|---|
| Common carotid artery | Often resembles middle cerebral artery (MCA) but can be asymptomatic if circle of Willis is competent |
| Internal carotid artery | Often resembles MCA but can be asymptomatic if circle of Willis is competent |
| Middle cerebral artery | |
| Main stem | Contralateral hemiplegia Contralateral hemianopia Contralateral hemianesthesia Head/eye turning toward the lesion Dysphagia Uninhibited neurogenic bladder Dominant hemisphere Global aphasia Apraxia Nondominant hemisphere Aprosody and affective agnosia Visuospatial deficit Neglect syndrome |
| Upper division | Contralateral hemiplegia; leg more spared Contralateral hemianopia Contralateral hemianesthesia Head/eye turning toward the lesion Dysphagia Uninhibited neurogenic bladder Dominant hemisphere Broca (motor) aphasia Apraxia Nondominant hemisphere Aprosody and affective agnosia Visuospatial deficit Neglect syndrome |
| Lower division | Contralateral hemianopia Dominant hemisphere Wernicke aphasia Nondominant hemisphere Affective agnosia |
| Anterior cerebral artery (ACA) | |
| Proximal (precommunal) segment (A1) | Can be asymptomatic if circle of Willis is competent, but if both ACAs arise from the same stem, then: Profound abulia (akinetic mutism) Bilateral pyramidal signs Paraplegia |
| Postcommunal segment (A2) | Contralateral hemiplegia; arm more spared Contralateral hemianesthesia Head/eye turning toward the lesion Grasp reflex, sucking reflex, gegenhalten Disconnection apraxia Abulia Gait apraxia Urinary incontinence Anterior choroidal artery Contralateral hemiplegia Hemianesthesia Homonymous hemianopsia |
| Posterior cerebral artery | |
| Proximal (precommunal) segment (P1) | Thalamic syndrome: Choreoathetosis Spontaneous pain and dysesthesias Sensory loss (all modalities) Intention tremor Mild hemiparesis Thalamoperforate syndrome: Crossed cerebellar ataxia Ipsilateral third nerve palsy Weber syndrome: Contralateral hemiplegia Ipsilateral third nerve palsy Contralateral hemiplegia Paralysis of vertical eye movement Contralateral action tremor |
| Postcommunal segment (P2) | Homonymous hemianopsia Cortical blindness Visual agnosia Prosopagnosia Dyschromatopsia Alexia without agraphia Memory deficits Complex hallucinations |
| Vertebrobasilar syndromes | |
| Superior cerebellar artery | Ipsilateral cerebellar ataxia Nausea/vomiting Dysarthria Contralateral loss of pain and temperature sensation Partial deafness Horner syndrome Ipsilateral ataxic tremor |
| Anterior inferior cerebellar artery | Ipsilateral deafness Ipsilateral facial weakness Nausea/vomiting Vertigo Nystagmus Tinnitus Cerebellar ataxia Paresis of conjugate lateral gaze Contralateral loss of pain and temperature sensation |
| Medial basal midbrain (Weber syndrome) | Contralateral hemiplegia Ipsilateral third nerve palsy |
| Tegmentum of midbrain (Benedikt syndrome) | Ipsilateral third nerve palsy Contralateral loss of pain and temperature sensation Contralateral loss of joint position sensation Contralateral ataxia Contralateral chorea |
| Bilateral basal pons (locked-in syndrome) | Bilateral hemiplegia Bilateral cranial nerve palsy (upward gaze spared) |
| Lateral pons (Millard-Gubler syndrome) | Ipsilateral sixth nerve palsy Ipsilateral facial weakness Contralateral hemiplegia |
| Lateral medulla (Wallenberg syndrome) | Ipsilateral hemiataxia Ipsilateral loss of facial pain and sensation Contralateral loss of body pain and temperature sensation Nystagmus Ipsilateral Horner syndrome Dysphagia and dysphonia |
Lacunar syndrome.
A lacunar stroke occurs in one of the perforating branches of the circle of Willis, the middle cerebral artery stem, or the vertebral or basilar arteries. The occlusion of these vessels results from the atherothrombotic or lipohyalinotic blockage of one of these arteries. The development of disease in these arteries correlates closely with the presence of chronic hypertension and diabetic microvascular disease.107,128 These are small vessels, 100 to 300 μm in diameter, that branch off the main artery and penetrate into the deep gray or white matter of the cerebrum.107 The resulting infarcts are from 2 mm to 3 cm in size and account for roughly 20% of all strokes. These types of strokes usually evolve over a few hours and sometimes can be heralded by transient symptoms in lacunar TIAs. Lacunar strokes can cause recognizable syndromes (Table 1-3). The basic lacunar syndromes are (1) pure motor hemiparesis from an infarct in the posterior limb of the interior capsule or pons, (2) pure sensory stroke from an infarct in the ventrolateral thalamus, (3) ataxic hemiparesis from an infarct in the base of the pons or the genu of the internal capsule, and (4) pure motor hemiparesis with motor apraxia resulting from an infarct in the genu of the anterior limb of the internal capsule and the adjacent white matter in the corona radiata. Recovery from a lacunar stroke often can be dramatic, and in some individuals, near complete or complete resolution of deficits can occur in several weeks or months. In patients who have had multiple lacunar infarcts, a syndrome characterized by emotional instability, slow abulia (impairment in or loss of volition), and bilateral pyramidal signs known as pseudobulbar palsy will develop. This diagnosis is based on the symptoms and the use of computerized tomography (CT) or magnetic resonance imaging (MRI). MRI is especially useful in this situation for detecting small lesions in the deep brain structures or brainstem; the ability of CT to see lesions clearly in these areas is limited.29
Table 1-3 Lacunar Stroke Syndromes and Their Anatomical Sites
| LACUNAR SYNDROME | ANATOMICAL SITES |
| Pure motor | Posterior limb of internal capsule Basis pontis Pyramids |
| Pure sensory | Ventrolateral thalamus Thalamocortical projections |
| Ataxic hemiparesis | Pons Genu of internal capsule Corona radiata Cerebellum |
| Motor hemiparesis with apraxia | Genu of the anterior limb of the internal capsule Corona radiata |
| Hemiballismus | Head of caudate Thalamus Subthalamic nucleus |
| Dysarthria/clumsy hand | Base of pons Genu of anterior limb of the internal capsule |
| Sensory/motor | Junction of the internal capsule and thalamus |
| Anarthric pseudobulbar | Bilateral internal capsule |
Hemorrhagic conversion.
As a sequela of an embolic or ischemic infarction, a purely ischemic infarct may convert into a hemorrhagic lesion. Thrombi can migrate, lyse, and reperfuse into an ischemic area, leading to small hemorrhages (petechial hemorrhages) because the damaged capillaries and small blood vessels no longer maintain their integrity. These damaged areas then can coalesce (combine) and form a hemorrhage into ischemia.83 These conversions are more common in large infarcts, such as an occluded middle cerebral artery, or in a large infarction in the distribution of a lenticulostriate artery. In patients who have large infarcts with possibility of hemorrhage, anticoagulation therapy is not used because of the risk of hemorrhagic conversion. These types of hemorrhages have characteristics in common with hemorrhagic strokes.
Hemorrhagic stroke
Hemorrhagic strokes have numerous causes. The four most common types are deep hypertensive intracerebral hemorrhages (ICHs), ruptured saccular aneurysms, bleeding from an arteriovenous malformation (AVM), and spontaneous lobar hemorrhages.83
Hypertensive bleed.
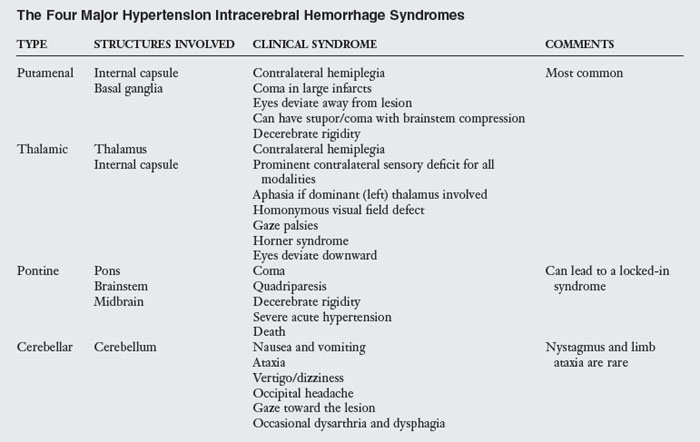
Hypertensive cerebral hemorrhages usually occur in four sites: the putamen and internal capsule, the pons, the thalamus, and the cerebellum. Usually these hemorrhages develop from small penetrating arteries in the deep brain that have had damage from hypertension. The pathological features of hypertension include lipohyalinosis (fat infiltration of pathologically degenerated tissue) and Charcot-Bouchard aneurysms.50 The usual hypertensive ICH develops over the span of a few minutes but occasionally can take as long as 60 minutes. Unlike ischemic infarcts, hemorrhagic bleeds do not follow the anatomical distribution of blood vessels but dissect through tissue planes spherically. This commonly leads to severe damage and complications, such as hydrocephalus and mass shift (movement of brain tissues to one side to accommodate the volume of the hemorrhage).83,128 Within 48 hours of the hemorrhage, macrophages begin to phagocytize the hemorrhage at its outer margins. Patients with a cerebral hemorrhage often experience a rapid recovery within the first two to three months after the hemorrhage. ICHs usually occur while patients are awake and often while they are under emotional stress. Vomiting and headache are associated commonly with ICH and are unique features that differentiate ICHs from ischemic strokes. Table 1-4 outlines the four major hypertensive ICH syndromes.
Lobar intracerebral bleed.
Lobar hemorrhages are ICHs that occur outside the basal ganglia and thalamus in the white matter of the cerebral cortex. These types of hemorrhages and hypertension are not correlated clearly; the most common underlying condition in patients with this type of ICH is the presence of AVMs.83 Other associated conditions include bleeding diatheses, tumors (e.g., melanoma or glioma), aneurysms in the circle of Willis, and a large number of idiopathic cases.49 Patients with lobar ICH initially have acute onset of symptoms, and most lobar ICHs are small enough to cause discrete clinical syndromes that may resemble focal ischemic events. Because lobar bleeds occur far from the thalamus and the brainstem, coma and stupor are much less common than they are in patients with hypertensive ICHs. Headaches are also common and can help differentiate lobar bleeds from ischemic strokes, which they can resemble so closely.126 Detection of a hemorrhage on a CT scan or MRI is the best way to distinguish these two entities.
Saccular aneurysm and subarachnoid bleed.
A saccular aneurysm rupture is the most common cause of a subarachnoid hemorrhage (SAH).150 Saccular aneurysms occur at the bifurcation (branching) points of the large arteries in the brain and are most commonly found in the anterior portion of the circle of Willis.83 An estimated 0.5% to 1% of normal individuals harbor saccular aneurysms.158 Despite the high number, bleeding from them is rare (6 to 16 per 100,000). Unlike other stroke syndromes, however, the incidence of SAH has not declined since 1970.102 The rupture risk correlates best with the size of the aneurysm. Aneurysms smaller than 3 mm have little chance of hemorrhage, whereas aneurysms 10 mm or larger have the greatest chance of rupture.95 SAH usually is characterized by acute, abrupt onset of a severe headache of atypical quality.102 These headaches are often the most severe that patients have ever experienced. A brief loss of consciousness, nausea and vomiting, focal neurological deficits, and a stiff neck at the onset of symptoms also may occur. The diagnosis is based on clinical suspicion, subarachnoid blood found on the CT scan, or blood found in the cerebrospinal fluid from a spinal tap. One determines the definitive location of the aneurysm by cerebral angiography.
The development of further delayed neurological deficits results from three major events: rerupture, hydrocephalus, and cerebral vasospasm. Rerupture occurs in 20% to 30% of cases within one month if treatment is not aggressive, and rebleeding has an associated mortality rate of up to 70%.102 Hydrocephalus occurs in up to 20% of cases, and aggressive management often is required. Chronic hydrocephalus is also common and often requires permanent cerebrospinal fluid drainage (shunting). Vasospasm also is a common problem after SAHs, occurring in approximately 30% of cases.102 The normal time course for vasospasm is an onset in three to five days, peak narrowing in five to 14 days, and resolution in two to four weeks. In half of cases, the vasospasm is severe enough to cause a cerebral infarction with resulting stroke or death. Even with modern management, 15% to 20% of patients who develop vasospasms still suffer strokes or die.96 A permanent ischemic deficit develops in approximately 50% of patients with symptomatic vasospasms after SAHs.69 Vasospasm therefore must be treated rapidly and as aggressively as possible to prevent permanent ischemic damage.
Arteriovenous malformation.
AVMs are found throughout the body and can occur in any part of the brain. They are usually congenital and consist of an abnormal tangle of blood vessels between the arterial and venous systems. They range from a few millimeters in size to large masses that can increase cardiac output because of the amount of their blood flow. The larger AVMs in the brain tend to be found in the posterior portions of the cerebral hemispheres.50 AVMs occur more frequently in men, and if found in one family member, they have a tendency to be found in other members. AVMs are present from birth, but bleeding most often occurs in the second and third decades of life. Headaches and seizures are common symptoms, as is hemiplegia. Half of AVMs initially occur as ICHs. Although rebleeding in the first month is rare, rebleeding is common in larger lesions as more time passes. Contrast CT, MRA, and MRI are useful noninvasive tests, whereas cerebral angiography is the best test for delineating the nature of the lesion. The management of these lesions is accomplished best by a team approach, a combination of surgical treatment and interventional angiography for definitive management. Treatment of hydrocephalus and increased intracranial pressure is the same as treatment for SAH and ICH.
Posttraumatic hemorrhagic stroke.
A traumatic brain injury commonly results in hemorrhagic damage to the brain in addition to ischemic and other injuries. The four major types of injury caused by traumatic brain injury include SAH and ICH, diffuse axonal injury, contusions, and anoxic injury from hypoperfusion (decreased flow in the vessels) and hypoxemia (decreased oxygen level). This combination of injuries leads to a constellation of findings that mixes the features of a number of individual ischemic and hemorrhagic injuries.
Other causes of stroke and strokelike syndromes
Arterial and medical disease.
Numerous medical conditions can result in arterial system diseases and lead to thrombosis and thromboembolism. Some conditions may cause disease in the cerebral vasculature (Table 1-5).
Table 1-5 Medical Conditions That Cause Arterial System Disease
| CONDITION | FEATURES* | TREATMENT |
|---|---|---|
| Vasculitic/inflammatory | ||
| Systemic lupus erythematosus | Most commonly associated vasculitis with stroke Vasculitic, thrombotic, and embolic events occur Greater than 50% recurrence rate Antiphospholipid antibody may play a role |
Treat lupus Anticoagulation with warfarin |
| Binswanger disease | Rare condition Diffuse subcortical infarction Diffuse lipohyalinosis of small arteries |
No clear treatment Anticoagulation |
| Scleroderma | Stroke in 6% of patients Antiphospholipid antibody may play a role |
No clear treatment Anticoagulation |
| Periarteritis nodosa | Can cause a CNS vasculitis Can cause embolic stroke |
Treat underlying condition |
| Temporal arteritis | Can cause a CNS vasculitis Can cause embolic stroke |
Treat underlying condition |
| Wegener granulomatosis | Can cause a CNS necrotizing vasculitis Can cause thrombotic stroke |
Treat underlying condition |
| Takayasu arteritis | Can cause embolic stroke | Treat underlying condition Anticoagulation |
| Isolated angiitis of the CNS | Rare primary CNS vasculitis Headache, multiinfarct dementia, lethargy |
Treat underlying condition |
| Fibromuscular dysplasia | Mostly in young women Often asymptotic Can be associated with TIA and stroke |
Anticoagulation Surgical dilation of the carotid arteries (if necessary) |
| Moyamoya disease | Vasooclusive disease of the large intracranial arteries Mainly in Asian population Cause of strokes in children and young adults |
Role of anticoagulation controversial because of risk of hemorrhage Role of surgery controversial |
| Hypercoagulable state | ||
| Antiphospholipid antibodies | Associated with recurrent thrombosis Embolic and thrombotic strokes occur |
Anticoagulation with warfarin |
| Oral contraceptive agents | Relative risk increased 4 times over controls Thought to be caused by hypercoagulability |
Stop oral contraceptives |
| Sickle cell disease | Microvascular occlusion caused by sickled cells Seen in 5% to 17% of patients with sickle cell disease |
No good treatments exist |
| Polycythemia | Vascular occlusion caused by increased viscosity and hypercoagulability | Treat underlying cause (if known) |
| Inherited thrombotic tendencies | Include many familial clotting abnormalities | Treat abnormality (if possible) Anticoagulation |
| Others | ||
| Venous thrombosis | Seen in meningitis, hypercoagulable states, and after trauma Increased intracranial pressure, headache, seizures Focal neurological signs, especially in legs more than arms Diagnosed with angiography |
Anticoagulation May need surgical decompression |
| Arterial dissection | More common in children and young adults May present with TIA Often preceded by trauma, mild to severe |
Surgical treatment as needed Anticoagulation after acute state |
* CNS, Central nervous system; TIA, transient ischemic attack.
Strokelike syndromes.
A number of conditions in addition to TIAs and cerebral infarctions can cause transient paralysis. These conditions generally resolve spontaneously with no long-term sequelae. The most common cause of transient hemiparesis is Todd paralysis, which develops postictally (after a seizure). Todd paralysis results from neurotransmitter depletion and neuronal fatigue in focal areas of the brain caused by the extremely high neuronal firing rate during a seizure.37 Patients usually regain function within 24 hours. Another common cause of focal neurological deficits is migraine headaches. These headaches are actually thought to result from cerebral vasospasms, but an actual ischemic infarct rarely if ever occurs. The deficits resolve with the resolution of the migraine and are not permanent.
Cerebral neoplasm.
Obviously, cerebral neoplasms (whether primary or metastatic) can lead to focal neurological deficits that resemble a stroke. The treatment of the sequelae and the long-term management of the deficits are the same as they are in stroke patients. Treating the primary lesions is the focus of the acute care. Often the initial symptoms are seizures and ICHs.
Stroke diagnosis
The diagnosis of stroke and differentiation of stroke from strokelike syndromes is based on the clinical presentation and physical examination of the patient. The examiner needs to differentiate a true stroke from syndromes that can mimic a stroke, such as Todd paralysis, seizures, multiple sclerosis, tumors, and metabolic syndromes. Most often, the patient’s symptoms in the emergency room include an acute onset of weakness or other neurological deficits. The patient history can help identify the risk factors for stroke and the nature of the lesion. The physical examination includes a general medical examination and a neurological examination. Only after a diagnosis of stroke based on the clinical history and examination can a further diagnostic evaluation be performed. Modern technology has improved the tools available for the accurate diagnosis of stroke and includes an armamentarium of imaging studies to identify the exact nature of the lesions that may cause neurological deficits. Each imaging study available has benefits and limitations that are useful to know for assessing a patient who has had a stroke. The stroke evaluation also should include an evaluation for the cause of the stroke.
Cerebrovascular imaging
The main tool used in stroke diagnostic evaluations is cerebral imaging, which historically included pneumoencephalography and other studies no longer performed. CT is probably the most common and the best known of the studies. MRI is now more common and has some advantages over CT, but availability and cost are still prohibitive in some areas. Positron emission tomography scans and single-photon emission CT scans are just being introduced and may have a role in stroke diagnosis.
Computerized axial tomography
CT is a readily available and useful technique that has become the standard for the evaluation of a patient experiencing an acute onset of stroke. The most important functions of CT scanning in an acute patient are ruling out other conditions (e.g., tumor or abscess) and helping identify whether evidence exists of hemorrhage into the infarction. In the acute phase of stroke, most CT scans are actually negative with no clear evidence of abnormalities. A negative immediate CT scan with an acute neurological deficit determined by physical examination actually can verify the impression of stroke because it rules out tumors, hemorrhages, and other brain lesions. The few changes seen in an acute stroke by CT are subtle and can include loss of distinction between gray and white matter and sulcal effacement. Acute bleeding, however, is visible on CT scanning and can be present in as many as 39% to 43% of patients.29 By definition, hemorrhagic infarction occurs within 24 hours of infarction, and hemorrhagic transformation occurs after 24 hours of infarction. The cause of the hemorrhagic change is thought to result from reperfusion into areas of damaged capillary endothelium and is common in large infarcts with extensive injury. Hemorrhagic transformation occurs equally in all distributions of infarcts113 and is not associated necessarily with hypertension or with older age.27 Hemorrhagic transformation can be detected in the acute phase by CT; in this case, one should not use anticoagulants because they may increase in the severity of the cerebral hemorrhage.
In the subacute phase, the findings from CT clearly show the development of cerebral edema within three days, which then fades over the next two to three weeks; then a decrease in the signal intensity occurs over the infarction. This decrease corresponds with the change from the positive mass effect (swelling) of the acute phase to the negative mass effect (shrinkage) of the chronic phase. The infarct actually may be difficult to see again in two to three weeks but is clearly visible with the addition of contrast material. Long-term parenchymal enhancement develops, which is consistent with the scar formation that becomes the permanent CT finding. The loss of tissue volume (negative mass effect) and the permanent scar tissue are the characteristic features of a chronic infarct (Figs. 1-4 to 1-8).
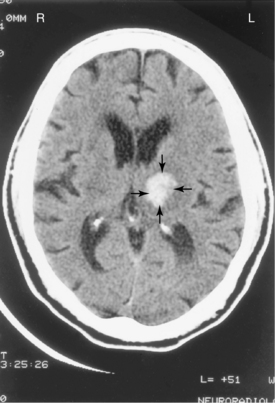
Figure 1-4 Magnetic resonance image of brain without gadolinium demonstrates an acute large left basal ganglia infarct. An acute infarct on the image appears white and is indicated by arrows.

Figure 1-5 Magnetic resonance image of the brainstem and cerebellum without gadolinium demonstrates an acute right pontine infarct. The infarct appears white and is indicated by arrows.
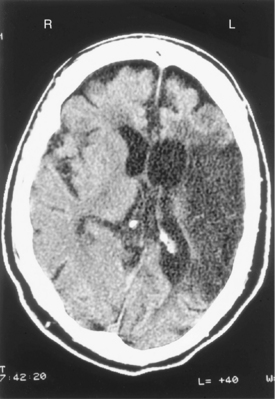
Figure 1-6 Computerized tomography scan of the brain without contrast demonstrates a large, previous, left middle cerebral artery distribution infarction. Loss of mass of brain tissue has occurred with dilated ventricles. Bleeding or acute infarction is not evident.
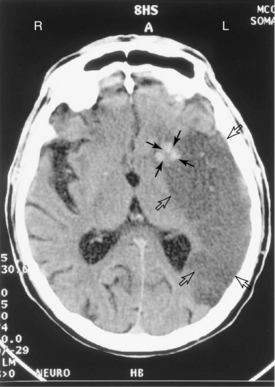
Figure 1-7 Computed tomography scan of the brain without contrast demonstrates a large subacute left middle cerebral artery distribution infarction, indicated by the hollow arrows. No loss of brain tissue mass has occurred compared with Fig. 1–6. Evidence of acute bleeding is in the basal ganglia on the left, which is white on the scan and is indicated with solid arrows.
Magnetic resonance imaging
MRI is now as commonly used in acute patients as CT, because cost and availability have improved. The MRI also has the advantage of allowing earlier detection of infarcts and, as more acute interventions have become common, allows for better evaluation of the course of acute treatment. Newer techniques such as diffusion-weighted averaging have been used to help in the identification of early infarcts.58,141 MRI also can rule out other conditions and can screen for acute bleeding. In addition, MRI can be more sensitive for detecting cerebral infarctions in acute patients. Magnetic resonance images are created by mapping out the relaxation of protons after the imposition of a strong magnetic field. These images are then taken in two ways: T1- and T2-weighted images. In T1 images, fat and tissues with similar proton densities are enhanced (bright). In T2 images, water and tissues rich in water are enhanced. As in CT scans, sulcal effacement can be seen, but hyperintensity is also evident in affected areas on the T1-weighted images. Magnetic resonance images can show meningeal enhancement over the dura, which occurs in 35% of acute stroke cases.44 MRI also can detect hemorrhage in much the same way as CT does.
The subacute changes of edema and mass effect can be seen with MRI, and use of contrast may be necessary to elucidate an infarct in the two- to three-week window. MRI has an advantage in determining a hemorrhage in a late stage because it can detect the degradation products of hemoglobin (hemosiderin deposits) and show hemorrhage areas well after CT can no longer detect a bleed. The changes on MRI in a chronic infarction are similar to those on a CT scan.
Positron emission tomography and single-photon emission computerized tomography scanning
Positron emission tomography and single-photon emission CT scanning are new techniques available only at selected centers. They have no clear role in the acute-stage evaluation of stroke.2 In the subacute and chronic stages of stroke, these techniques help to distinguish between infarcted and noninfarcted tissue and can help delineate areas of dysfunctional but potentially salvageable brain tissue. These studies can also be used to try to assess brain function in the chronic setting. However, because of cost, limited availability, and an unclear definition of their use, they are essentially only research tools and do not have a role in the routine management of stroke patients.
Workup for cause of stroke
The workup for the diagnosis of stroke is aimed at answering three main questions:
Transcranial and carotid doppler
Transcranial and carotid Doppler studies allow for noninvasive visualization of the cerebral vessels. The advantages are that they provide useful therapeutic information on the state of the cerebral vessels and the blood flow to the brain. Approximately one third of patients who have had ischemic strokes that are cardiac in origin have significant cerebrovascular disease.25 Patients with symptoms or evidence of posterior circulation disease are tested best with a transcranial Doppler study, including examination of the vertebrobasilar system. The cost is low compared with other tests such as MRA or cerebral angiography, which has significant associated morbidity and mortality. The evidence of carotid disease can help shape the patient’s treatment plan and can encourage pursuit of definitive treatments such as carotid endarterectomy.
Magnetic resonance angiography
MRA is used to evaluate patients with stroke symptoms to detect any vascular abnormalities that may have caused the stroke or to look for alterations of cerebral blood flow that may have resulted from an embolic or thrombotic event. This is a very common noninvasive technique and is often done at the time of the MRI scan to assess the extent of cerebral injury; MRA is able to image vessels similarly to classical angiography.160 The newer techniques of MRA have sensitivity for detection of 86% to 90%111 for detection of severe stenosis, and the earlier issues of relatively low specificity of 64%13,79 (due to overdetection by the earlier techniques) is now in the range of 89% to 96% for studies done with contrast enhanced MRA.77 Despite these advantages, the spatial resolution is still less than traditional angiography, which may be an issue in cases where surgical management is planned. However, with constantly improving techniques and increased field strengths and parallel imaging, high resolution MRA may soon equal the resolution seen in CT angiography.65
Electrocardiography
Electrocardiography is used to evaluate patients with stroke symptoms to detect dysrhythmias (which may be a source of embolic material) or myocardial infarction or other acute cardiac events that may be related to an acute stroke.
Echocardiography
In patients with a history of cardiac disease and stroke, echocardiography usually is warranted. The types of cardiac disease that usually cause emboli and should be investigated with an echocardiograph include congestive heart failure, valvular heart disease, dysrhythmias, and a recent myocardial infarction. In some individuals, a patent foramen ovale (the fetal opening between the right and left sides of the heart) persists into adulthood and can be the source of a paradoxical embolus from the venous circulation that crosses from the right atrium into the left atrium. A transesophageal echocardiogram can then be useful in combination with a bubble study to assess for a right-to-left shunt. This specialized study also can visualize parts of the heart better in the search for emboli in areas such as the left atrial appendage when the standard transthoracic echocardiogram is inconclusive.
Blood work
The standard acute evaluation of the stroke patient includes a complete screening set of blood analyses, including hematological studies, serum electrolyte levels (ionizing substances such as sodium and potassium), and renal (e.g., serum creatinine) and hepatic chemical analyses (liver function tests). The typical hematological evaluation has a complete blood count, platelet count, prothrombin time, and partial thromboplastin time. These studies help to rule out other causes of strokelike symptoms, to diagnose complications, and to allow for a baseline analysis before the initiation of therapies such as anticoagulation. The blood chemistry analyses allow metabolic abnormalities to be ruled out, as do the renal and hepatic chemistry analyses. The latter part of the stroke evaluation can involve numerous specialized tests chosen according to the clinical symptoms and development of the differential diagnosis as the evaluation progresses (Fig. 1-9). Table 1-6 provides a sample of some of these studies and their associated conditions.
Table 1-6 Medical Studies Used to Clarify Diagnoses in Stroke Evaluation
| SPECIALIZED STUDIES TO EVALUATE STROKE | ASSOCIATED CONDITIONS |
|---|---|
| Proteins S and C | Hypercoagulable state |
| Anticardiolipin antibodies (lupus anticoagulant) | Lupus erythematosus, hypercoagulable state |
| Erythrocyte sedimentation rate | Collagen vascular disease |
| Rheumatoid factor | Lupus erythematosus, collagen vascular disease |
| Antinuclear antibody | Lupus erythematosus, collagen vascular disease |
| Hemoglobin | Polycythemia |
| Sickle cell preparation | Sickle cell disease |
| Hemoglobin electrophoresis | Sickle cell disease |
| Blood and tissue cultures | Infectious emboli |
Medical stroke management
Principal goals
As in the medical management of all patients, the care of stroke management requires good general patient care. All phases include caring for the conditions the patient may have and preventing medical complications and anticipating needs that will arise as the patient progresses through the acute phase into the convalescent, rehabilitative, and long-term maintenance phases after stroke. Care for acute patients is provided best in a specialized stroke unit that commonly deals with the issues and concerns unique to these patients.2,102 Outcome studies have demonstrated the benefit of these units in the care of stroke patients.91 Medical rehabilitation units also have been shown to be beneficial in the improvements of outcomes in the subacute and convalescent phases.
Acute stroke management
In management of acute stroke patients, basic medical needs have to be addressed and to include essentials such as airway protection, maintenance of adequate circulation, and the treatment of fractures or other injuries and conditions present at the time of admission. The neurological management of the acute stroke problems focus on identifying the cause of the stroke, preventing progression of the lesion, and treating acute neurological complications. Some specific approaches apply to treatment of each of the different types of stroke.
General principles
The general principles of acute stroke management include attempting to stop progression of the lesion to limit deficits, reducing cerebral edema, decreasing the risk of hydrocephalus, treating seizures, and preventing complications such as DVT or aspiration that may lead to severe illness. (See the previous sections for a discussion of the studies used in acute patients to diagnose stroke.) Once the type of lesion has been defined, specific treatment can be instituted. Although numerous studies have been performed and are underway on the reduction of stroke mortality or disability,136 no routine medical or surgical treatment has been shown to be effective. Currently, more aggressive methods such as angioplasty and thrombolysis are being studied, and the results of these trials are expected to lead to treatments that actually will improve the outcomes for individuals who have had strokes.
The basic principles in the approach to the treatment of acute stroke include an attempt to achieve improvement in cerebral perfusion by reestablishing blood flow, decreasing neuronal damage at the site of ischemia by modifying the pathophysiological process, and decreasing edema in the area of damaged tissue (which often can lead to secondary damage to nonischemic brain tissue). Many pharmacological and surgical treatments have been targeted toward at least one of these areas. Depending on the stroke mechanism, the agents and techniques of choice are used.
Ischemic stroke
In patients who have had ischemic strokes, the restoration of blood flow and the control of neuronal damage at the area of ischemia are of the highest priority. In large strokes, edema can play a significant role, and mass shift can even lead to hydrocephalus. The pharmacological therapies are divided broadly into antithrombotic, thrombolytic, neuroprotective, and antiedema therapies. The surgical therapies include endarterectomy, extracranial-intracranial bypass, and balloon angioplasty.
Pharmacological therapies
Antithrombotic therapy (antiplatelet and anticoagulation).
The principal rationale behind the use of antiplatelet and anticoagulation agents is that rapid recanalization and reperfusion of occluded vessels reduces the infarction area. The theoretical benefit also exists of preventing clot propagation and recurring vascular thrombosis. The risks associated with the use of these treatments includes hemorrhagic conversion, hemorrhage, and increased cerebral edema, all of which are associated with worse outcomes.90 Current research has not established a clear advantage to the use of aspirin or heparin in acute stroke patients, but these agents still are commonly used in the hope that they may decrease injury from acute stroke. Aspirin, an irreversible antiplatelet agent, is administered when symptoms appear. Heparin is administered intravenously in a continuous infusion.71 Both of these agents are started only after determination by CT or MRI that no hemorrhage is associated with the stroke. Ticlopidine, another antiplatelet agent, has been even less studied, and its role, if any, in acute stroke treatment is unclear. A recent metaanalysis of the trials of heparin and oral anticoagulation therapy in acute stroke treatment showed a marginal benefit from treatments with anticoagulation compared with no treatment at all.135 Currently, numerous large, multicentric studies in the United States and Europe are examining the best approach to the antithrombotic treatment of stroke that should provide better guidance as their results become known in the next few years.
Thrombolytic therapy.
Thrombolytic therapy is attractive as a therapy for acute stroke, because it opens up occluded cerebral vessels and immediately restores blood flow to ischemic areas. However, a problem in using these agents in stroke treatment is that the treatment must start in six hours from onset of symptoms to be therapeutic. Most patients are symptomatic at a much later stage, and even if they have symptoms early enough, a rapid workup to rule out a cerebral bleed must be performed before initiation of therapy. The successful use of these agents—primarily urokinase, streptokinase, and tissue plasminogen activator—in the treatment of myocardial ischemia has aroused interest in similar use of these agents for acute stroke treatment. The mechanism of action of these agents is to cause fibrin breakdown in the clots that have been formed and thus to lead to lysis of the occlusions in the blood vessels. Reviews of thrombolytic therapy for stroke treatment have shown some reduction in mortality, but no definitive answer is available to date concerning efficacy.163 Currently, streptokinase is out of favor because of increased mortality and morbidity from intracranial hemorrhage,123,156 but tissue plasminogen activator, a more specific thrombolytic agent, has been able to achieve favorable results. The National Institute of Neurological Disorders and Stroke trial was the cornerstone trial in approval of treatment of acute ischemic stroke with thrombolytics.3,6,103,157 The trial was a double-blind, placebo-controlled trial that revealed an improvement in early outcomes in 24 hours of treatment and demonstrated an increase in symptom-free survival from 38% (placebo) to 50% (treatment) at three months. The strict use of a three-hour window from the onset of symptoms and the rigid blood pressure guidelines of the National Institute of Neurological Disorders and Stroke trial are probably contributors to the excellent outcomes; the exact treatment protocols are still being defined. On reexamination at one year, the treated patients continued to show a benefit, and this has encouraged the use of this agent in selected groups.87 Other thrombolytic agents such as alteplase also have shown benefit and are being used routinely. The results are at the same level of effectiveness as tissue plasminogen activator.5 Unfortunately, the three-hour window of efficacy limits the number of individuals who can receive benefit, and studies to expand the window of intervention to have hours or more have not shown clear benefits.30,64 In the patient with stroke beyond three hours, the currently recommended interventions are mostly limited to the use of anticoagulants and antiplatelet agents to prevent further events.103 Further active investigation continues to search for effective treatments in this large group of individuals with late presentation of stroke.
Other treatments for altering cerebral perfusion.
A number of different treatments aimed at lowering blood viscosity or cerebral perfusion have been used, including hemodilution with agents such as dextran, albumin, and hetastarch. None of the 12 studies reviewed by Asplund demonstrated any clear benefit.9 Similarly, studies of prostacyclins and several different types of cerebral vasodilators have also shown no clear evidence of increased survival rates or improvement in outcomes after treatment.90 Research continues to be active in these areas, but so far none of these alternative treatments for increasing cerebral perfusion has yielded a favorable outcome.
Neuroprotective agents.
Neuroprotective agents are medications that can alter the course of metabolic events after the onset of ischemia and therefore have the potential to reduce stroke damage. No agent has shown clear benefits among this group of treatments. These agents include calcium channel blockers, naloxone, gangliosides, glutamate antagonists, and free-radical scavengers. Each of these agents has had promise in the theoretical or laboratory realm, but none has proved to be clinically efficacious.
The use of naloxone, a narcotic antagonist, is based on the in vitro observation that naloxone has neuroprotective effects. Unfortunately, the clinical trials to date have not demonstrated any benefit.33 The therapeutic rationale of using calcium channel blockers is that they prevent injury to ischemic neurons by preventing calcium influx, which decreases metabolic activity in the neuron.90 Initial hope was that the treatment results for SAH, in which nimodipine decreases secondary ischemia, would be similar for stroke. Unfortunately, the results of several studies have not shown any clear benefits from treatment with these agents,108 and none of them currently are used routinely for stroke treatment.
In animal experiments, glutamate antagonists decrease the size of infarction area in stroke.90 However, the few studies done in human beings have been inconclusive and have shown serious neuropsychiatric side effects.33
Gangliosides may reduce ischemic damage by counteracting toxic amino acids in ischemic tissue. Despite the many studies that have been performed, no clearly demonstrated benefits have resulted from use of these agents.33
The free-radical scavengers include 21-amino steroids (lazaroids), ascorbic acid (vitamin C), and tocopherol (vitamin E). They have not been well-evaluated, and some studies to establish their clinical use are being undertaken.90 However, vitamin E has been demonstrated clinically to reduce the risk of heart disease, so secondarily its use may decrease the risk of stroke.
Agents for cerebral edema.
Agents that reduce cerebral edema include corticosteroids, mannitol, glycerol, vinca alkaloids, and piracetam. All the studies done on persons receiving steroids122 after an acute stroke demonstrated no clear benefits, and steroid use creates a risk of diabetes and DVT.62 Use of the other agents also has no clear benefit in the treatment of acute stroke and are also not routinely used.
Cooling therapy.
An exciting new development in the treatment of acute stroke has been the initiation of cooling therapy on presentation with the induction of a medical coma to limit the extent of brain injury after stroke. In most patients who present with stroke, there is a natural tendency for the body temperature to be elevated between 4% and 25%, which is associated with increased injury and poorer outcomes.18,35 Studies have shown that injury could be slowed with supercooling, and the technique has been used in surgery to help limit injury and to prolong safe surgical time in both neurosurgical and cardiothoracic procedures.28,131,139 The pooled analysis of existing studies does not yet provide convincing evidence that death or long-term disability are significantly changed from the application of mechanical or pharmacological cooling, but the therapy is just starting to be used on a larger scale, and new research findings published in the next several years may show a benefit to routine cooling of acute stroke victims.
Surgical therapies
Endarterectomy.
A carotid endarterectomy is the surgical opening of the carotid arteries to remove plaque. This therapy has been shown to be useful in preventing recurrent strokes or development of stroke in individuals with TIAs, but it has not been used to treat acute stroke. In theory, the opening of the carotids could subject ischemic areas and their blood vessels to excessive pressure from restored blood flow and lead to hemorrhage.40 Concerns about using major anesthesia in a patient with a new stroke makes this surgery too risky to treat acute stroke.
Extracranial-intracranial bypass.
Despite the initial attraction of bringing extracranial blood flow into the intracranial vessels through the use of bypass procedures, the large trial done in the 1980s demonstrated no improvement in patient outcomes, and the procedure has been largely abandoned.47
Balloon angioplasty.
Despite its efficacy in opening blocked coronary arteries in patients with heart disease and its successful treatment of acute myocardial infarction, the use of balloon angioplasty in acute stroke has not been studied. Clinical centers are actively investigating its possible uses.
Hemorrhagic stroke
In patients who have had a hemorrhagic stroke, the size and location of the lesion determines the overall prognosis; supratentorial lesions greater than 5 cm have a poor prognosis, and brainstem lesions of 3 cm are usually fatal.49 In these cases, the control of edema is important, and the techniques previously described can be used. In patients with SAH, the treatment regimen is usually more aggressive and focuses on several issues, which include the control of intracranial pressure, prevention of rebleeding, maintenance of cerebral perfusion, and control of vasospasm.
Prevention of rebleeding.
Before 1980, six weeks of bed rest were prescribed routinely for the care of patients with acute SAH to prevent rebleeding. In 1981 a study demonstrated that bed rest was inferior to surgical treatment, lowering of blood pressure, and carotid ligation.158 Antihypertensive medications for the prevention of rebleeding are still controversial, and no consensus exists as to their use. Carotid ligation used to be popular, but more recent reevaluations of the benefits of the technique have not been as conclusive, and because of its surgical risks, direct repair of the aneurysm is a better choice. Antifibrinolytic agents have been studied and have been beneficial for low-risk patients in whom surgery must be delayed, but they seem to increase the risk of ischemic events. The placement of intraluminal coils, balloons, and polymers has shown some benefit in the short-term prevention of rebleeding, but the long-term efficacy is still unclear, and the techniques remain experimental.102 Because the risk of rebleeding is also very high in post-SAH seizures, even though the incidence of seizure is low, the recommendation is that patients receive antiseizure medications for prophylaxis.
Control of vasospasm.
The treatment of vasospasm is important for the reasons previously outlined. The current treatments include the use of orally administered nimodipine, a calcium channel blocker shown to improve outcomes of patients who have had an SAH with vasospasm. The results of using other calcium channel antagonists are unclear. The use of hypertension/hypervolemia/hemodilution has been recommended by some studies. Creating more volume than normal results in hypertension. The stretch caused by the volume stimulates the smooth muscle pressure receptors that line the vessels. These receptors inhibit muscle action by a protective response, and the blood vessel dilates to accommodate the increased volume. Hypertension/hypervolemia/hemodilution is most effective in preventing vasospasm after surgically clipping the aneurysm. Significant cardiac and hemodynamic risks are associated with this therapy, so intensive care unit (ICU) monitoring is required.102
Prevention of stroke recurrence
Ischemic stroke
In general, the strategies to prevent recurrence of ischemic stroke can be divided into two areas: risk factor modification (which also applies to primary prevention) and secondary prevention to treat the underlying cause of stroke in individuals with a history of stroke. Following is a discussion of the secondary interventions that can be used to prevent recurrence of stroke.
Hypertension.
Although the treatment of hypertension is an important primary preventive measure in the management of stroke, whether blood pressure reduction after stroke is beneficial has not been proved definitively. The transient rise in blood pressure after stroke usually settles without intervention.164 Because of the uncertainty about whether overaggressive treatment of acute elevated blood pressure is harmful, definitive antihypertensive therapy probably should be delayed for two weeks.90 At that time, one should follow the usual recommendations regarding adequate control of hypertension because some evidence indicates that it is beneficial. This seems especially appropriate in patients who have had a lacunar stroke because the development of multiple lacunae is related to uncontrolled blood pressure.
Antiplatelet medications.
In patients who have had a TIA or stroke, long-term use of aspirin has been shown to decrease the incidence of death, myocardial infarction, and recurrent events by up to 23%.7 The doses of aspirin in numerous studies have ranged from 30 mg to 600 mg; all doses resulted in a 14% to 18% reduction in recurrent cerebral events, but gastrointestinal complications increased with the higher doses.1,48,153 In general, a standard dosage of one regular adult aspirin (325 mg a day) is the usual treatment for recurrent ischemic stroke. Studies are underway that compare the efficacy of warfarin versus aspirin in treating ischemic stroke; the results of these studies are not yet available. Ticlopidine is another antiplatelet medication effective in reducing the incidence of recurrent stroke.81 Ticlopidine is most efficacious in women, patients who are not helped by aspirin therapy, and patients with vertebrobasilar symptoms, hypertension, diabetes, and no severe carotid disease.62
Anticoagulation.
The incidence of recurrent stroke and TIA in patients with atrial fibrillation is approximately 7% per year. For patients who have atrial fibrillation with cardiac sources of emboli, warfarin is the clear treatment of choice; this is true for primary and secondary prevention. Although aspirin has some preventive effects, it is not as efficacious. In the presence of structural cardiac disease or atrial fibrillation, aspirin should be used only to treat patients in whom warfarin anticoagulation is contraindicated.90
The odds ratio for recurrence is approximately 0.36 in those treated with warfarin versus control and 0.84 for those treated with aspirin versus control.45 However, problems exist with warfarin anticoagulation in the elderly. Cognitive and compliance difficulties can lead to an increase in complications. Unclear issues in anticoagulation use include when to start anticoagulants after stroke, the safety of anticoagulants in clinical practice, and the optimum anticoagulant blood level. Several studies are currently examining these questions.
Treatment of dysrhythmias or underlying disease.
Obviously, primary and secondary prevention should treat the underlying cause of the ischemic stroke. Prevention can include cardioversion to normal sinus rhythm and treatment with antidysrhythmic medications, and treatment of underlying medical conditions if they can be found. Unfortunately, only a small proportion of patients who have had TIAs and strokes can benefit from these specific treatments.
Carotid endarterectomy.
The surgical treatment of carotid artery stenosis has been shown to be beneficial in recent studies of stroke recurrence in patients with severely (greater than 70%) stenosed carotid arteries.12,46 The data on the intermediate group of patients (stenosis from 30% to 70%) are being collected. For patients with high-grade stenosis, carotid endarterectomy reduces the range of stroke risk from 22% to 26% down to 8% to 12%.
Hemorrhagic stroke
The mainstay of ICH prevention is controlling systolic and diastolic hypertension. No clear benefit exists for one group of treatment agents versus another as long as adequate hypertension control is maintained. In patients in whom the ICH follows vasculitis or the use of anticoagulants, the treatment for preventing recurrence includes treating the vasculitis or terminating anticoagulant use.128
The secondary prevention of recurrent stroke and SAH of AVMs and/or aneurysms includes surgical management of the lesions (the treatment of choice). Clipping or microsurgical dissection of the lesions is performed whenever possible and as soon as the patient is able safely to undergo the procedure.102,149 In surgically unresectable lesions, alternatives include sclerotherapy, coating, trapping, and proximal arterial occlusion.102
Prevention of complications and long-term sequelae
General principles
To prevent complications and long-term sequelae after a stroke, maximizing function, decreasing morbidity, and preventing rehospitalization from a complication are important. Prevention of these complications begins on the day the patient arrives at the hospital with symptoms of acute stroke. Many complications are associated with bed rest in general, but some are specific to stroke.
Musculoskeletal complications
Contractures.
Contractures are periarticular motion impairments that result from loss of elasticity in the periarticular tissues, which include muscles, tendons, and ligaments. Contractures can occur in any immobilized joint but are particularly prevalent in the paretic limbs after a stroke. In fact, only 10% of stroke patients recover limb strength and mobility rapidly enough to avoid developing contractures.63 Shoulder pain, contractures, and muscle pain occur in 70% to 80% of patients who have had a hemiplegic stroke.128 Chapter 10 addresses the management and related issues of the hemiplegic shoulder. Contractures also occur in other areas and begin to be problematic within a few days of onset or several days after the stroke when symptoms of immobility and spasticity may begin to develop. Usually contractures occur in a pattern of flexion, adduction, and internal rotation; muscles that span two joints are more susceptible to contracture formation.66 To prevent shortening of the connective tissue in muscles and joints, an active range of motion (ROM) program must be initiated. Because certain muscles span two joints, joints must be positioned to allow full physiological stretch of the muscles involved. Once a contracture is present, the mainstay of treatment is gradual, prolonged stretch. The minimal treatment is a sustained stretch greater than 30 minutes.84 Other treatments include splinting, deep-heating modalities,23 and possible surgical release for long-standing, tight contractures66 (see Chapter 13).
Osteoporosis.
Bone is a metabolically active tissue normally in a state of equilibrium between active bone resorption and deposition. The ratio of bone formation to bone resorption is influenced by the stressors to which the bone is subjected, a relationship known as Wolff law.23 The lack of weight-bearing and normal stress on long bones on the hemiplegic side of a stroke patient leads to a predominance of bone resorption. This loss of bone mass can start as early as 30 hours after the beginning of immobility155 and with bed rest can be as high as 25% to 45% in 30 to 36 weeks.39 In patients who have had a stroke, osteoporosis is often worse, and the rate of hip fracture is far higher on the side of the hemiplegia.67
Osteoporosis prevention is accomplished best with measures that include active weight-bearing exercise and active muscle contraction. Medical therapies for individuals at risk for osteoporosis should be initiated. Therapies include bone-forming agents, calcium and vitamin D supplementation, hormone replacement, and other measures as needed. Box 1-1 shows some of the medical treatments available for osteoporosis.
Heterotopical ossification.
Heterotopical ossification is the deposition of calcium in the form of mature bone in the soft tissues. The condition is not particularly common after stroke but occurs with increased incidence after traumatic brain injury. The incidence ranges from 11% to 76% in various studies.17 Spasticity is associated with the development of heterotopical ossification as are long-bone fractures and a prolonged coma. Symptoms of heterotopical ossification usually develop one to three months after injury with pain and limited ROM.24 The diagnosis is based on clinical examination, elevated alkaline phosphatase levels in the serum, and a positive bone scan.
Treatment for heterotopical ossification includes active ROM; no studies indicate that the condition is caused or worsened by active ROM exercises.17 Pharmacological treatment options include the use of etidronate disodium and nonsteroidal antiinflammatory drugs.24 Other treatments include radiation therapy and, for refractory cases after the lesion has matured, surgical excision of the heterotopical ossification. Performance of ROM exercises after surgery is particularly important. Low-dose radiation or etidronate disodium can also be used to prevent recurrence.34
Falls.
Falls are of particular concern in survivors of stroke. These patients are at increased risk of hip fracture because of developed osteoporosis, and the acuity of their balance, visual perceptions, and spatial perceptions is decreased. The increased risk of falls has been documented in several studies and is greater in patients who have had a right hemispheric stroke.36,106,118 Fall prevention should emphasize balance and cognitive training, removing environmental hazards, and using adaptive devices. (These measures are reviewed in Chapters 8, 14, 15, 19, 27, and 28.)
Neurological complications
Seizures.
Seizures after strokes have been documented since the nineteenth century. The incidence of late-onset seizures (epilepsy) in the individuals who have had strokes ranges from 6% to 18%,59,162 whereas the incidence of early seizures is approximately 10%, with reports ranging from 3% to 38%.14,168 The risk for seizures is highest right after stroke; 57% of seizures occur in the first week, and 88% of all seizures after strokes occur in the first year.14 Seizures are more common in patients who have had an SAH; 85% of these seizures are early seizures.148 The timing of seizures that occur after stroke varies according to the mechanism of injury. The timing of seizures after thrombotic and embolic strokes appears about equal. Patients with SAH have more seizures soon after the stroke, whereas patients with ICH are more similar to patients with ischemic stroke and may have more late-onset seizures.168
The treatment and management of seizures associated with stroke are usually straightforward, and monotherapy often produces adequate results. If the patient only has acute-onset seizures in the setting of his or her stroke, the patient often does not require long-term antiseizure medication. A single, brief seizure or a nongeneralizing local seizure also can often be managed conservatively. If seizures do require treatment, a single agent usually suffices and is beneficial, because the drug interactions are fewer, and the compliance is better with monotherapy. Carbamazepine and phenytoin are the preferred agents for treating epilepsy after stroke. Management of the medication requires close follow-up to ensure that the desired outcome is achieved: an asymptomatic, seizure-free patient. Excessive medication can lead to a number of symptoms (Box 1-2). Inadequate control of the condition leads to additional seizures. For situations in which seizures become refractory to treatment, one must remember several factors.168 Intercurrent illness or metabolic disarray that lowers the seizure threshold may make the seizures more frequent and difficult to treat. Patient compliance may be a problem, especially if the stroke created cognitive and behavioral deficits. Progressive lesions or new infarcts are also causes of increasing seizure frequency. Finally, a stroke that occurs in highly epileptogenic areas—such as the hippocampus, the parietooccipital cortex surrounding the rolandic fissure, and calcarine cortex—may engender refractory epilepsy and require combination therapy. Table 1-7 lists the common seizure medications and their side effects.
Table 1-7 Medical Management of Seizures: Drug Therapy
| MEDICATION | SIDE EFFECTS | PRINCIPAL USES |
|---|---|---|
| Phenytoin | Ataxia Incoordination Confusion Rash Gum hyperplasia Hirsutism Osteomalacia |
Tonic-clonic (grand mal) Partial |
| Carbamazepine | Ataxia Dizziness Diplopia Vertigo Bone marrow suppression Hepatotoxicity |
Tonic-clonic (grand mal) Partial |
| Phenobarbital | Sedation Ataxia Confusion Dizziness Depression Decreased libido Rash |
Tonic-clonic (grand mal) Partial |
| Primidone | Same as phenobarbital | Tonic-clonic (grand mal) Partial |
| Valproic acid | Ataxia Sedation Tremor Bone marrow suppression Hepatotoxicity Weight gain Transient alopecia |
Absence (petit mal) Atypical absence Myoclonic Tonic-clonic (grand mal) |
| Clonazepam | Ataxia Sedation Lethargy Anorexia |
Absence (petit mal) Atypical absence Myoclonic |
| Ethosuximide | Ataxia Lethargy Rash Bone marrow suppression |
Absence (petit mal) |
Hydrocephalus.
Hydrocephalus can occur acutely, especially in patients with SAH and ICH as discussed previously, or it can develop symptoms insidiously later. Hydrocephalus is usually heralded by the gradual onset of a triad of symptoms, including lethargy with decreased mental function, ataxia, and urinary incontinence. Once hydrocephalus is suspected, one should perform a CT scan promptly because the increasing size of the ventricles is readily visible. Once diagnosed, one should surgically place a ventricular shunt. The procedure is well-tolerated and can lead to resolution of all the symptoms of hydrocephalus if performed promptly. Patients with an occluded shunt have symptoms that mimic the initial symptoms of hydrocephalus.
Spasticity.
Spasticity is defined as a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks. Spasticity results from hyperexcitability of the stretch reflex (which is one component of the upper motor neuron syndrome).89 In a normal recovery after a flaccid stroke, an initial period occurs with little resistance to passive motion of the muscles and joints. Approximately 48 hours after the stroke, tendon reflexes and muscle resistance to passive motion begin to return.66 Spasticity is most pronounced in the flexor muscles and occurs throughout the hemiplegic side. The lower extremity later develops a component of extensor spasticity that can assist with function, whereas the upper extremity spasticity is usually in a flexor pattern.10
The management of spasticity includes encouraging voluntary movement, ROM exercises, and a functional rehabilitative approach.66 The research data on the different neurorehabilitative treatment approaches do not define clearly which approach is most effective, so an individualized approach to treating each patient is the best course. Pharmacological treatments for spasticity are numerous, and they need to be tailored to each patient to find the best balance of side effects and efficacy. The most commonly used agents are baclofen, dantrolene sodium, and diazepam. These medications and a representative sample of the other medications used to treat patients who have had a stroke are presented in the table of medications and their side effects on the inside cover of the book. Other treatments for severe spasticity that are more invasive include phenol blocks and neurolysis, botulinum toxin (Botox) injections, and implantable baclofen pumps. Botox injections and baclofen pumps are still experimental approaches, and ongoing studies will elucidate their future roles (see Chapter 10).
Other complications
Deconditioning.
Physiological deconditioning in patients after a stroke results from the acute medical illness and the associated bed rest and immobility that may result. Table 1-8 lists some of the effects of deconditioning. All of these factors can alter the ability of the patient to recover. Therefore, to get the patient out of bed and to increase activity as early and aggressively as possible is important.
Table 1-8 Deconditioning Effects of Stroke
| Musculoskeletal | Atrophy ↓ Strength of tendons, ligaments, bones, and muscles Depression Anxiety Sleep disturbance |
| Cardiovascular | ↓ Stroke volume ↑ Heart rate ↓ VO2 max ↑ Respiratory rate ↓ Lean body mass ↑ Body fat Orthostatic hypotension |
| Neurological/emotional | Sensory deprivation ↓ Balance ↓ Coordination Fatigue |
| Genitourinary | Diuresis Difficulty voiding |
| Endocrine | Impaired glucose tolerance Altered regulation of hormones |
| Body composition and metabolism | Nitrogen loss Calcium loss Potassium loss Phosphorus loss Sulfur loss |
Psychological complications.
Stroke is a major life event and is associated with significant alterations in the individual’s well-being and independence. Negative emotional reactions are common in patients following a stroke152 and can have a significant effect on the patient’s eventual outcome. After a stroke, patients may go through the four stages of bereavement described by Worden.172 These include accepting the loss, experiencing the pain of the loss, adjusting to a new environment in which previous abilities are missing, and investing in new activities. Not all patients become depressed, and this lack of depression does not necessarily mean the patient is in denial.173 Denial is a normal defense mechanism, and as long as it does not interfere with the rehabilitative process, it is not a concern.152 The indifference reaction, a persistent denial reaction, is more common in patients who have had a right-sided stroke than a left-sided stroke.53
Another common consequence of stroke is emotional lability, which is rapidly shifting from one extreme emotion to another. Approximately 20% of patients have emotional lability six months after a stroke, and up to 10% have lability for one year. Emotional lability is more common in patients with pseudobulbar palsy and right hemispheric strokes, particularly if the patient is depressed.74
Anxiety is also common after stroke and is more frequent in patients with left hemispheric strokes94 and cortical lesions.144 Many sources of anxiety exist, including financial affairs, family issues, and a fear of dying or recurrent stroke. Reassurance and constant positive feedback during rehabilitation can help, and in severe cases, treatment with anxiolytics and psychological support may be needed.
Fortunately, outbursts and aggressive behavior are rare after a stroke, but when they occur, they are more common in patients with left-sided infarcts who are more aware of their deficits. The approach to management of these outbursts should not include restraints and threats but should be based on avoiding excessive frustration in the patient by removing emotional triggers and alternating easy and difficult tasks.152
Depression is common after stroke, developing in 20% to 50% of stroke survivors, with 30% being the most commonly accepted figure.152 The depression can be a reaction to the stroke or a neuropsychological sequela of the stroke. The consequences of depression after stroke are numerous: hospital stays are longer,42 cognitive impairment is greater,125 and motivation decreases.140 Depression is more common in patients with left cortical lesions145 and lesions close to the frontal poles and is shorter in patients with subcortical and brainstem lesions. Depression after stroke often is treated best with antidepressant medications.152 In patients who are unable to tolerate antidepressants, are unresponsive to therapy, or have active suicidal ideation, electroconvulsive therapy can be a last resort.110 (See Chapter 2 for more information about the psychological effects of stroke.)
Urinary tract dysfunction.
Urinary incontinence is common after stroke, affecting 51% to 60% of patients,20 and can cause difficulties with rehabilitation, influence eventual discharge location, and place stress on caregivers.43 One month and six months after stroke, 29% and 14% of patients, respectively, still have urinary incontinence.11 The usual pathophysiology of incontinence is detrusor hyperreflexia, which is common in patients with cortical lesions. The incontinence assessment includes a thorough history of the urinary symptoms and can include urodynamic studies to help define the problem. Incontinence treatment includes timed voiding and use of pharmacological agents and intermittent catheterization. If these treatments do not work, incontinence may need to be treated by indwelling catheterization. This is performed on patients who cannot independently self-catheterize and do not have caretakers who can provide this care or on patients who have physical barriers such as urethral strictures that prevent regular catheterizations. Unfortunately, indwelling catheters have a high incidence of associated urinary tract infections. Male patients also may use external condom catheters, which can provide socially acceptable continence when the individual is traveling or physically active. Patients with continuous dribbling also benefit from condom catheters. The goal of all of these therapies is to maintain continence and prevent urinary tract infections and other complications such as skin breakdown from skin maceration.
Skin breakdown and decubitus ulcers.
Pressure ulcer formation is a serious health problem in debilitated and immobilized patients. After a stroke, patients are at particular risk for pressure ulcers because they have numerous factors contributing to skin breakdown. Abnormal sensation, contracture, malnutrition, immobility, and muscle and soft-tissue atrophy often develop and may be complicated by advanced age. Prevention of pressure ulcers, rather than treatment of developing ulcers, should be the focus of care. Preventive measures include frequent repositioning, keeping skin clean and dry, maintaining an adequate level of nutrition, and, especially in high-risk patients, using pressure-relief mattresses.132 Once pressure ulcers have formed, in addition to strictly observing the preventive and pressure relieving measures previously noted, treatments include meticulous wound care with a variety of agents and possibly surgical reconstruction.
Dysphagia.
Swallowing disorders are common after a stroke. Dysphagia is more common in the elderly, with an incidence of 25% to 45%.59,61 Aspiration can lead to pneumonia, and a decreased eating ability can lead to dehydration and malnutrition. Chapter 24 covers the details of the pathology of aspiration and the methods of its treatment.
Aspiration.
Aspiration causes chemical pneumonitis that can lead to a secondary bacterial infection. Because numerous anaerobic organisms are in the mouth, aspiration pneumonia can develop into an anaerobic abscess.92 Such abscesses occur less frequently in edentulous individuals because they have less oral flora and can occur in up to a third of cases in hospitalized patients.97 The treatment of choice is to reduce the risk of aspiration and to administer antibiotics. Examining a radiographic film for evidence of abscess cavities and the sputum for organisms can help one develop a specific medical treatment. Sputum culture growth often requires up to three or four days, so initial treatment is often empirical and should be the administration of a wide-spectrum antibiotic that is effective against hospital-acquired organisms (which are often resistant to certain antibiotics) and anaerobic bacteria.92 The usual course of antibiotics is seven to 10 days, but cavitary pneumonia may require far longer treatment for eradication of the organism.93 Determination of which specific antibacterial agents to use depends on the resistance patterns in the institution in which the aspiration takes place; the infectious disease team at that institution should make the decision about which antibiotics to use.
Deep venous thrombosis.
DVT is a common problem after stroke and has an incidence of 23% to 75% depending on the severity of the stroke. Most of the morbidity and mortality associated with DVT results from venous thromboembolism (VTE). Pulmonary embolism after stroke has an incidence of 10% to 29% and a mortality rate of 10%.19 The formation of DVT is caused by the triad of risk factors outlined by Virchow postulates: altered blood flow, damage to the blood vessel wall, and altered blood coagulability. Box 1-3 lists the common risk factors for DVT. Of the risk factors for DVT, stasis is one of the most important. After a stroke, DVT is 10 times more common in the paretic leg.165 DVT usually begins in the calf, and although the emboli from calf thrombi are not dangerous, these thrombi propagate in about 20% of cases, and about 50% of the proximal deep venous thrombi embolize. About 20% of symptomatic pulmonary emboli are fatal.134 After a stroke, ambulation in itself is not preventive in the subacute setting: pulmonary embolism occurred in 57% of ambulatory patients in the rehabilitation setting.147 Lower extremity and pelvic DVT are the most common, but proximal upper extremity DVT also can occur, although it is rare. All of the diagnostic and management issues discussed in the section on VTE that follows applies to this condition as well.
The diagnosis of DVT in the clinical setting is unreliable,19 and many patients with life-threatening embolism and thrombosis have no clinical symptoms of DVT. Other patients with swelling and tenderness may not have DVT at all and may have any of a number of other diagnoses. The differential diagnosis of lower extremity pain and swelling includes trauma, fracture, gout, cellulitis, and superficial phlebitis. The usual clinical signs of DVT include pain and tenderness, swelling, the presence of Homans sign (elicited by dorsiflexion of the ankle while the knee is flexed resulting in pain in the calf), superficial venous distention, a palpable cord, and fever. Some of these signs, such as Homans, are unreliable indicators. Homans sign is present in less than one third of patients with DVT and is present in half of patients without DVT.73 Objective testing for DVT has venography as the gold standard, but this procedure is associated with significant risks, including anaphylaxis and causing DVT. More commonly used risk-free procedures are impedance plethysmography, which is a noninvasive test that measures volume changes in the leg with circumferential calf electrodes,75 and Doppler ultrasound, which is also a noninvasive test that uses a handheld probe to detect blood flow in deep leg veins.166 Doppler ultrasound and impedance plethysmography have similar sensitivities and specificities for DVT detection, but Doppler ultrasound is not as portable and has a higher cost than impedance plethysmography.19
The clinical diagnosis of pulmonary embolism is also unreliable, and only 30% of patients with pulmonary embolism have clinical DVT, even though 70% have venographic evidence of DVT.19 The symptoms of submassive pulmonary embolism overlap with the symptoms of many other pulmonary conditions, including tachypnea, tachycardia, rales, hemoptysis, pleuritic chest pain, pleural effusion, general malaise, bronchospasm, and fever. In patients with massive pulmonary embolism with greater than 60% of the pulmonary circulation obstructed, patients are critically ill and develop heart failure, circulatory collapse, hypotension, and coma and can die suddenly.147 The gold standard for testing for pulmonary embolism is the pulmonary angiogram, but its use is associated with significant morbidity and mortality. The preferred noninvasive test is the ventilation/perfusion scan.105
The best approach to VTE is to prevent DVT. The National Institutes of Health Consensus Conference on the Prevention of Venous Thrombosis and Pulmonary Embolism recommends using low doses of subcutaneously administered heparin in all stroke patients with no hemorrhagic components.121 In all other patients, external pneumatic calf compression is recommended. More recently, low-molecular-weight heparin has been introduced and actually may be more effective than standard heparin for DVT prophylaxis.72 Low doses of warfarin for DVT prophylaxis in stroke patients has not been well-studied, but its use in other conditions has proved its effectiveness in DVT reduction. Dextran, aspirin, and static compression stockings are not effective for preventing DVT.19 Physical treatments alone, such as ROM exercises, have not been studied. Ambulatory patients must be able to walk at least 50 feet to have a reduction in risk of DVT,21 but as previously stated, the risk of pulmonary embolism in ambulatory patients is still significant.147 The length of time prophylaxis should continue is still not definite, but evidence shows that continuing prophylaxis well into the subacute phase is warranted.19
The treatment of VTE (DVT and pulmonary embolism) is based on preventing pulmonary embolism, which can be fatal. A patient who is identified with acute VTE is started on intravenous (IV) heparin as long as no contraindications to anticoagulation exist.70 The effectiveness of the heparin is determined by monitoring the partial thromboplastin time, and the heparin is adjusted to a dose between 1.5 and 2.5 times control. In a patient with only DVT, warfarin can be started on the first day, and the heparin can be discontinued when the warfarin dose is therapeutic as measured by the increase in the prothrombin time or international normalized ratio. Targets are a prothrombin time of 1.25 to 1.5 times control or an international normalized ratio of 2 to 3.19 In patients with pulmonary embolism, warfarin may be started a few days later, and after management of the acute stage, the patient keeps receiving it longer; patients with DVT receive warfarin for approximately three months, and patients with pulmonary embolism, for six months.72 All patients who recently have been diagnosed with VTE are placed on bed rest initially and usually are allowed to become mobile two days after the partial thromboplastin time has become therapeutic.76 The rehabilitation of patients with VTE who are beginning treatment should continue at the bed side, and, in the case of patients with lower extremity DVT, the rehabilitation program should include activity of daily living (ADL) training, upper extremity programs, communication work, and dysphagia treatments.
Future trends in medical stroke management
Improved primary stroke prevention
Because the treatments for stroke are so limited and the deficits that can result are so devastating, the primary prevention of stroke has to be the essential strategy to decrease morbidity and mortality from stroke. With a good understanding of the risk factors for stroke, risk factor modification can be targeted at groups and individuals who are at risk. Table 1-1 lists the preventable and nonpreventable risk factors for stroke. Fortunately, many of the risk factors are the same as those for myocardial infarction and vascular disease leading to death, so the modification of stroke risk factors also decreases the risk of cardiac-related morbidity and mortality. Due to greater awareness and risk factor modification and largely through the treatment of blood pressure, a decline of greater than 50% in the stroke mortality rate has occurred in the past 20 years.169 Each of the modifiable risk factors are considered separately.
Hypertension
Diastolic and systolic hypertension are each independently and strongly implicated in causing stroke. Hypertension increases the risk of stroke in all age groups of men and women.169 In fact, no threshold level of blood pressure exists below which the risk curve plateaus.98 For every 7.5 mm Hg increase in diastolic pressure is a 46% increase in stroke incidence and a 29% increase in coronary heart disease (CHD). Reducing blood pressure in hypertensive patients has been shown to decrease the risk of stroke significantly, with an average reduction of 5.8 mm Hg leading to a reduction in stroke incidence of 42% but only a 14% reduction in CHD incidence.32 Because these trials only spanned two to five years, the reduction in stroke incidence is a direct result of decreased blood pressure and not an alteration in atherogenesis (production of plaque in the arteries), which would take longer to develop.169 Systolic blood pressure is also a factor; the treatment of isolated systolic hypertension (>160 mm Hg) has been shown to reduce the incidence of stroke by 36% and CHD by 27% over 4.5 years.120 Treating all forms of hypertension in the older age groups is therefore essential because they are at increased risk for stroke, and most strokes occur in this age group. Screening for hypertension and aggressively treating systolic and diastolic hypertension should be the cornerstone of any primary prevention program for stroke.
Cigarette smoking
The results of the Framingham Study and the Nurses’ Health Study demonstrate that the cessation of cigarette smoking should lead to a prompt reduction in stroke mortality.31,171 Risk of CHD decreases by 50% in one year and reaches the level of a nonsmoker’s risk in five years. Smoking increases stroke risk by 40% in men and 60% in women (with no other risk factors being considered), and it seems to follow that smoking cessation leads to a reduction in stroke risk similar to the reduction in CHD incidence.
Cardiac dysrhythmia and myocardial infarction
CHD, atrial fibrillation, and congestive heart failure lead to an increased incidence of stroke.169 Preventing these conditions by modifying their associated risk factors leads to a reduction in incidence of stroke. In addition, treating patients who have established dysrhythmias and congestive heart failure with anticoagulants such as warfarin decreases the incidence of stroke (as explained previously).
Blood lipids
The development of carotid artery atherosclerotic disease has been shown to be related to the levels of serum lipids.133 However, to relate accelerated atherosclerosis clearly to an increase in the incidence of stroke has been difficult because other pathologies related to serum lipids have been observed. Levels of total serum cholesterol less than 160 mg/dL seem to be associated with ICH and SAH, whereas higher levels of serum cholesterol are associated with atherothrombosis. No relationship has been demonstrated between cholesterol and lacunar strokes.169 This unusual relationship of low serum lipids and higher hemorrhagic infarct has been demonstrated in Japan and also recently in the United States in the group of patients studied in the Multiple Risk Factor Intervention Trial.78,124 Because of the ambiguity of these data, a clear statement of guidelines for the management of cholesterol to reduce incidence is difficult to make.
Diabetes
The rate of atherosclerosis development in coronary, femoral, and cerebral vessels is increased in diabetics. Stroke is increased 2.5 to 4 times in diabetics compared with nondiabetics.86 In the Framingham Study, glucose intolerance (a blood sugar greater than 150 mg/mL) is only a significant, independent contributor to stroke in older women and is greater for women than men at any age.80 Because of the associated risk of stroke, careful management of diabetes in addition to all other risk factors is prudent.
Oral contraceptives
In female patients over the age of 35 who have other stroke risk factors, oral contraceptive use is associated with increased incidence of stroke.142 The relative risk for oral contraceptive users is approximately five times greater if they are already in the high-risk group. With the use of lower estrogen formulation oral contraceptives, the risk has decreased substantially in recent years.143 That the incidence of fatal SAH increased in oral contraceptive-using women with concomitant smoking is noteworthy; in the group over age 35 the incidence is four times higher.52 Therefore, the recommendation is that women over the age of 35 avoid using oral contraceptives, and younger women who smoke should be advised of the increased risks associated with concurrent oral contraceptive use.
Alcohol
Heavy alcohol consumption is related to an increase in stroke and stroke deaths, whereas light to moderate alcohol consumption is associated with a reduced incidence of CHD.38,85 Alcohol is clearly related to hemorrhagic stroke events, but the association with thromboembolic events is not definite. Regardless, patients at risk for stroke should avoid heavy alcohol consumption.
Physical activity
Despite the clear benefits of physical activity in the reduction of CHD morbidity and mortality, no clear association exists between physical activity and the incidence of stroke.114,115
Public education
The primary goal of primary and secondary prevention programs should be to educate individuals about risk factors and then to teach them the way to modify their risks. During routine visits, a physician should be able to identify at-risk patients through a combination of a history and physical. Routine blood pressure screening should be included in all evaluations, and patients who have hypertension should be treated. A stroke risk profile has been assembled from the Framingham Study data and can be used by physicians170 (e.g., to help a physician decide which borderline hypertensive patients to treat). Education can start in the physician’s office and be continued by all the other health professionals with whom the patient comes into contact. If the community at large is educated about the risk factors of stroke, those individuals who are at highest risk can seek out the attention they require. This model has been implemented and supported through research such as the Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guidelines.116
Part two: introduction to acute stroke rehabilitation
The neuro-ICU may be the starting point of occupational therapy (OT) evaluation and treatment. Many patients are evaluated, by an occupational therapist, within 48 hours of a stroke. The ICU environment is often fast paced with the focus on monitoring the individual patient’s medical status. The primary goals of any neuro-ICU are to stabilize the patient medically, progress the patient neurologically, and support the patient and family through this neurological crisis.137 Medical testing and procedures take precedence over any OT treatment. Scheduling OT services may be difficult, treatments may be interrupted, and flexibility is necessary.
The importance of early intervention
There are many common complications associated with a prolonged ICU stay, which include but are not limited to deconditioning, muscle weakness, contractures, skin impairments, depression, anxiety, and reduced quality of life.60 Early OT, engaging in ADL and mobilization, can increase a patient’s level of consciousness, enhance overall mental well-being, and foster functional independence.129,146 Occupational therapists provide a variety of treatments in the ICU, including, but not limited to, evaluations, splinting, positioning, cognitive retraining, self-care, and functional mobility training.
Team approach
There are many members of the neuro-ICU/acute care team, and the team may vary among settings. They include a primary team of physicians led by an attending neurologist specializing in critical care. Depending on each case, there may be neurosurgeons also involved in patient care. At teaching hospitals, a team of residents may also make medical decisions regarding the patients. Along with the occupational therapist, the ancillary team consists of nursing, including the primary nurse and nurse practitioner, social workers, nutritionist, speech and language pathologist, and physical therapist (Table 1-9). An occupational therapist treating patients in this environment must foster these relationships to safely treat patients.
Table 1-9 Members of the ICU/Acute Team
| MEMBER | ROLE |
|---|---|
| Attending physician | Leads the medical team is medical decision-making. May lead team rounds. Usually interacts with patient at least once a day. |
| Resident | At a teaching hospital, residents are responsible for the day to day, hour to hour care of patients. May be on the unit at all times to answer clinical questions regarding patients. |
| Nursing | Multiple responsibilities include but are not limited to: administering medications, ADL assist, education, positioning, and monitoring neurological status. |
| Nurse practitioner | In some facilities, nursing practitioners take the place of residents, writing orders and providing medical decision-making when needed. |
| Nutritionist | Usually the nutritionist evaluates the patient on a PRN (as needed) basis. Most patients in the ICU receive a nutrition consult when they are placed on tube feedings. The nutritionist, along with the physicians, will determine which type of tube feeding a patient should receive, along with the speed at which the feedings should be administered. |
| Social worker | In the ICU, the social workers are also usually a PRN service providing support to family members and beginning the discussion of discharge planning. |
| Speech and language pathologist | Speech and language pathologists can provide a twofold service in the ICU setting. They may provide therapy services in the form of language and communication evaluation and treatment. They may also provide bed side swallowing evaluations, along with the occupational therapist. See Chapters 20 and 24. |
| Physical therapist | The physical therapist provides bed side physical therapy services in the form of therapeutic exercise, mobility, and gait training if appropriate. Along with the occupational therapist, he or she also contributes to discharge planning. See Chapter 15. |
The relationship between the primary physician, nurse, and the occupational therapist is particular important. Daily communication with the physicians, residents, and primary nurse is necessary prior to initiating an evaluation or treatment session due to the fluctuating physical condition in the ICU phase of hospitalization.4,137 Physicians, nursing, or the occupational therapist, using their own clinical judgment will determine if intervention should be delayed should a patient’s neurological status deteriorate. Once the patient has been medically cleared for OT evaluation, a review of the patient’s medical chart should be completed. The therapist can glean information relating to any precautions and complications that may interfere with the OT treatment (Box 1-4).
1. Check to make sure occupational therapy orders are active. This should be done prior to each and every treatment session
2. Review the patient’s medical record. The therapist should evaluate the medical record for potential reasons to hold a patient from therapy. Such reasons may be a change in mental status, development of a deep vein thrombosis or pulmonary embolism, or expansion of the stroke. Every facility has different standards for when therapy is to be held.
3. Review the patient’s current status with the medical team. Using clinical reasoning the therapist will determine if the patient is appropriate for an OT session. The therapist should clear any treatment with the patient’s nurse to determine if all medical information reviewed from the medical record is most current.
4. Begin evaluation and treatment with a gross assessment of mental status, strength, and vital signs. Great discrepancies from what is reported in the medical record should be reported to the nurse and treatment suspended. Proceed with therapy as indicated.
Monitoring the icu/acute stroke survivor
Any therapist treating in the ICU should not only be aware of the medical and nursing priorities in the ICU, but also of how to monitor the patient during OT treatment. The therapist needs to be competent in reading ICU monitors and handling ICU related drains and lines, so that appropriate parameters and precautions are adhered to during the treatment session. Common monitors, drains, lines, and clinical implications are listed later.
Basic icu monitor
Most ICU patients are connected to a monitor that allows constant display of all vital signs (Fig. 1-10). These include blood pressure, telemetry reading (which include heart rate and rhythm), respiratory rate, and oxygen saturation percentages. For normal versus abnormal vital sign responses to exercises, refer to Table 1-10. Blood pressure can be monitored either noninvasively (automated pressure cuff) or by invasive measures, such as an arterial line reading (also referred to as an A-line). A common insertion site for an A-line is either the radial or femoral artery (Fig. 1-11). With radial artery placement, passive ROM of the wrist should be avoided; with femoral artery placement, no hip ROM is allowed, resulting in bed rest.
Telemetry
Telemetry detects both the heart rate and rhythm and displays this reading on the monitor. Bed side telemetry is similar to an electrocardiogram (ECG). An ECG is read by placing 12 electrical leads to read heart rate and rhythm while the bed side telemetry uses either three or five leads. The primary nurse will set both heart rate and rhythm parameters on the monitor. Should the rate and rhythm become abnormal, an alarm will sound. Physical activity should be monitored accordingly.
Common lines and drains
Foley catheter.
A Foley catheter is indwelling and is used to drain urine from the bladder. The therapist should avoid clamping the catheter; doing so could result in a backup of urine in the bladder. The bag, which collects the urine, needs to be at a lower level than the patient’s bladder for the urine to flow in the correct direction.
External ventricular drain.
The external ventricular drain (EVD) is a small tube surgically inserted into the ventricles of the brain, which drains cerebral spinal fluid (CSF) (Fig. 1-12). The tube is connected to a device that measures the amount of this fluid. This procedure is used when the intracranial pressure is elevated, and the drain may be clamped for short periods of time by nursing only. Due to specific calibration, function of the drain, and accuracy in measurement the head of the bed must be elevated to a specific level. Unless the drain is clamped, the head of the bed may not be changed, and patients should not be mobilized.
Intracranial pressure monitoring catheter.
The intracranial pressure monitoring catheter (ICP) is a catheter passed through a burr hole and placed in the ventricles of the brain. It is used with injuries such as hemorrhages, aneurysms, or head trauma that may lead to brain swelling and elevation of the intracranial pressure. This monitor measures any changes in intracranial pressure. The head of the bed is elevated to a set point (usually 30 to 45 degrees), as the intracranial pressure will increase when the head of the bed is lowered. Passive therapy, such as splinting or positioning, may be implemented with physician approval. Generally, ADL treatment and mobilization is held at this time.
Spinal drain.
A spinal drain is a catheter placed in the lumbar spine to drain CSF. It can be used for the treatment of CSF leak or to drain excess CSF fluid. The lumbar drain should be set to drain below the level of the leak. When the drain is open and is draining CSF, the spinal drain is set at a determined level next to the bed. At this time, when the drain is opened, patients are placed flat on their back to allow for drainage. Patients with this drain may get up and out of bed and may engage in ADL treatment only when the drain has been clamped by the nurse. While the drain is open to drain CSF, the patient must remain on bed rest.
Intravenous line.
IV lines are inserted into the peripheral veins and are generally used to administer IV fluids and medications. Because these lines are superficial, care should be taken not to place pressure from the positioning materials or splints directly over the area in order to avoid obstructed or dislodgment.
Feeding tubes
In the event that a stroke patient is unable to swallow effectively or appears to be a high aspiration risk, alternate methods are used for nutrition intake.
Nasogastric tube.
A nasogastric tube (NGT) is placed through the nostril down the esophagus to the stomach for liquid feeds to pass. It is generally used as a short-term alternative for nutritional intake.
Percutaneous endoscopic gastrostomy.
A percutaneous endoscopic gastrostomy is a tube inserted surgically with an endoscope through the mouth and into the stomach, exiting out through the stomach wall and dermis (Fig. 1-13).
Figure 1-13 Percutaneous endoscopic gastrostomy in abdomen.
(Photo courtesy of Millie Hepburn Smith.)
Precautions for both feeding tubes include elevating the head of bed to 30 degrees or greater while administering the tubes to prevent aspiration. Depending upon the hospital guidelines, the therapist may be allowed to turn off the feeding prior to the therapy session, but it is recommended that the primary care nurse be consulted prior to doing so, for patient safety (see Chapter 24).
Ventilator
At times stroke can result in respiratory failure. When this is the case, patients often require a ventilator to assist them with or to perform the act of breathing for them (Figs. 1-14 and 1-15). When a ventilator is used, the patient also requires an artificial airway. In the first few days after acute stroke, a ventilator can be connected to the patient via an endotracheal tube. A breathing tube is then placed into the patient’s mouth and positioned down into the patient’s lung systems. If a patient is unable to be weaned from the ventilator, a tracheotomy will be performed. In this procedure, an opening is cut in the patient’s trachea and a small endotracheal tube is placed in the opening, which is then attached to the vent via long tubing. Early mobilization of patients on ventilators is encouraged.112 A recent randomized controlled trial138 emphasized that early OT/physical therapy (PT) for those ventilated and critically ill is both beneficial and safe, resulting in better functional outcomes, decreased delirium, and more ventilator-free days.
Figure 1-14 This is a commonly used ventilator in the ICU setting. The occupational therapist needs to be aware of the vent setting and alarms while working with the patient.
Figure 1-15 The patient is properly positioned on a trach collar and is currently being weaned from the ventilator.
Once the therapist is confident to handle the lines, leads, and monitors in the ICU, the patient’s tolerance of the OT intervention should be monitored carefully. Vital signs should be observed during the entire treatment session and should be documented at the beginning, at mid-portion, and at end of treatment. In addition to vital signs, the therapist must also watch for changes in the patient’s neurological status during treatment, which may include changes in decorticate or decerebrate posturing, tone, pupils, and/or in speech.137 Patient subjective complaints must be considered. If any changes in the patient’s status occur, terminate treatment and inform the medical team immediately.
Assessments used in acute stroke rehabilitation
There are a variety of standardized assessments available82 to the occupational therapist in the hospital setting. In the acute/ICU setting, it is imperative for the occupational therapist to evaluate motor skills, cognitive function, and ADL. At times it may not be feasible for a patient to engage in ADL tasks secondary to medical status or sedation. Table 1-11 outlines some of the standardized assessments used during acute rehabilitation.
Interventions for acute stroke rehabilitation
The following sections will describe potential interventions for those in the ICU/acute stage of stroke rehabilitation.
Splinting
The primary goals at this early phase of splinting are to:
1. Correct any biomechanical malalignment and protect joint integrity.
2. Prevent shortening of soft tissues and development of contractures.
Develop an appropriate wearing schedule to prevent learned nonuse behavior patterns. Splint-wearing at night may be more appropriate than day use, particularly if the patient has begun to initiate movement or attempts to incorporate the hand or upper extremity in functional activities. A wearing schedule should be practical to achieve compliance (Box 1-5; See Chapter 13).
Box 1-5 Common Splints Used in Acute Stroke Rehabilitation
| Resting hand splint | May be fabricated for the individual but also are available prefabricated. |
| Cone splint | May prevent long finger flexor tightness when used in conjunction with a wrist extension device and also maintain skin integrity (preventing skin maceration). |
| Adjustable inflatable hand splint | Contains an air bladder in the palmar surface, which can be adjusted to achieve the level of stretch placed on the long finger flexors. It may be an appropriate choice for the patient who has had more than one stroke and demonstrates increased muscle tone. This type of splint is prefabricated. |
| Blanket/towel roll | An alternative to a thermoplastic elbow extension or drop arm splint. It is rolled around the patient’s arm to help prevent elbow flexion contractures. See Chapter 13 and Fig. 1–16. |
Positioning
Because of the medical complexity of the ICU/acute stroke survivor, many of these patients spend most, if not all, of their time confined to bed. Therefore, positioning has because an integral part of OT treatment plan. The occupational therapist will work to develop a positioning schedule for each individual positioning. The occupational therapist must rely on other members of the interdisciplinary team, including nursing and physical therapists, and the patient’s family members, if able, to carry out this portion of the treatment plan (Figs. 1-16 and 1-17).

Figure 1-17 Side lying position, with patient positioned on the affected side. Pillow placed under affected upper extremity to maintain proper alignment of the head of the humerus.
Different members of the interdisciplinary team have different priorities when it relates to positioning. A primary goal of the team in regards to positioning is to prevent skin breakdown. The occupational therapist is encouraged to teach the team how to position the patient not only to prevent skin breakdown but also to reduce the risk of contractures and encourage joint alignment, and comfort. The occupational therapist should develop a turning schedule for each patient. Patients should alternately be positioned on the affected side, the nonaffected side, and supine. A clock drawn with specific positions can be used as a reminder for the nursing team. See Chapter 10.
When the patient is being positioned, the patient’s lines and leads should be carefully observed for they provide vital medications and monitoring of each patient. Careful adjustments need to be made for head of the bed restrictions from feeding tubes or ICP/EVD. When a patient is being positioned with femoral arterial lines, care should be taken to avoid hip flexion, and the wrists of patients with radial A-lines should be maintained in a neutral position. Foley and rectal tubes should be moved to the same side to which the patient is positioned.
While in the ICU, many patients require a ventilator to provide respiratory assistance. These patients can also be positioned side to side and supine. Care should be taken when moving ventilation tubes. There are many extra articular handles that allow for addition mobility of the patient on a ventilator. If these articular handles do not provide enough length to position a patient in the proper alignment, discuss with the respiratory therapist regarding switching the ventilator from side to side every other day or so.
Functional activity suggestions during the acute phase
Bed mobility
Rolling to the affected side.
Rolling to the affected side promotes early active trunk control and may increase awareness of the weaker side.
Rolling to the unaffected side.
Rolling to the unaffected side promotes awareness and initial management of the weak upper extremity by teaching the patient to passively guide the arm across the trunk (Fig. 1-18).
Maintaining side lying.
A rolled pillow placed at the midthoracic spine to the lumbar area may assist the patient in maintaining the side-lying position. A towel roll can be placed under the patient’s waist to provide a stretch to the shortened trunk. A primary goal is to assure proper spine alignment, to avoid pressure build up over the bony prominences in the lower extremities (knees and ankles), and to position the scapula in protraction if the patient is positioned on the weakened side.
Bridging.
Bridging strengthens the back and hip extensors. From a functional perspective, this movement aids in getting on and off the bed pan, can be used during lower body dressing, and also assists moving the lower body toward the side of the bed in anticipation of assuming a sitting position.
Side lying to sitting toward the affected side.
Side lying to sitting toward the affected side promotes early stage weight-bearing on the weak upper extremity. The therapist needs to ensure that the shoulder is properly aligned, and the patient will usually require assistance with initiation of the movement.
Weight-bearing for function
Upper extremity weight-bearing activities may be done while the patient is side lying as mentioned previously, during bed mobility, or for stabilizing items. It can also be accomplished using the bed side table during meals or grooming tasks. The arm or back rest of a chair can be incorporated in the treatment plan for positioning and setup for weight-bearing (Figs. 1-19 and 1-20). The patient should be taught to push off with both upper extremities when moving from sit to stand. Weight-bearing as a postural support can reverse or prevent tissue shortening of the elbow, wrist, and finger flexors. It can also be used to strengthen the scapula musculature and the triceps. Arm extended weight-bearing can be done in front of the sink during grooming or be done in front of the bed side table while reaching for items nearby (Fig. 1-21).
Figure 1-19 While the patient sits on the edge of the bed, a bed side chair is used to facilitate upper extremity weight-bearing activities.
Figure 1-21 Supported standing with bed side table to facilitate upper extremity involvement in activity. Early upright ADL training can be initiated, and weight shifting through the lower extremities is encouraged.
For the lower extremity, bed level activities include: bridging, sitting at the edge of the bed with both feet on the floor, and early transfer training once patients are medically stable.
Graded sitting and standing activities
Supported sitting in bed.
For the supported sitting in bed position, the head of the bed should gradually be raised in approximately 30- to 40-degree increments to avoid an orthostatic hypotensive response. As the patient tolerates the change in degrees of elevation, the therapist should continue to monitor vital signs. If there appears to be no change in the patient’s blood pressure, the therapist should continue to elevate the head of the bed to approximately 80 degrees. Sitting at a slightly reclined position is less taxing on the patient’s energy and requires less recruitment of the neck, trunk, and back musculature to maintain an upright position. At this point, the patient should be engaged in functional activities, such as feeding, light grooming, upper body bathing and dressing, and leisure activities.
Supported sitting in a chair.
If the patient is well-supported and can endure sitting in a chair at the bed side, “sitting tolerance” or “out of bed tolerance” can be increased. Pillows may be useful at this early stage to support the lumbar spine and weaker upper extremity. When a therapist is placing a pillow under the upper extremity, he or she should make sure the shoulder alignment is in neutral. Adequate postural support may reduce pain and fatigue. Focus of treatment can include but is not limited to the patient performing self-care tasks, visual scanning activities, and weight-bearing through the upper and lower extremity.
Unsupported sitting.
Unsupported sitting may be done in the bed in a “tailor” (crossed legged) position, depending on the amount of ROM the patient has in the lower extremities. The head of the bed can be elevated, but should not touch the back of the patient. It is used as a safety catch should the patient lose his or her balance in a posterior direction. Pillows may be propped against the bed rails to protect the patient if he or she leans or falls laterally to the weaker side. While seated in this position, the patient can practice righting himself or herself or maintaining a midline position, and the patient should then be engaged in functional activities as tolerated.
Unsupported sitting at the edge of the bed with feet dangling.
In this position, the patient can be challenged with increased demands on alignment, trunk control, and forward and lateral weight shifts. Scooting to the edge of the bed can be introduced in anticipation of progressing to sit to stand. Postural control may be noticeably improved once the patient’s feet contact the floor. The therapist should ensure equal weight-bearing on both lower extremities. See Chapter 7.
Sit to stand: pretransfer phase.
To prepare for the sit-to-stand pretransfer phase, therapists should ensure that all lines and IVs have enough length to eliminate pulling or tension. Increasing the surface height the patient rises from will require less work. This transition may require the assistance of more than one person to gain the patient’s confidence and safety. The therapist should assure appropriate alignment of both lower extremities with feet placed firmly on the floor and then have the patient begin with several partial sit-to-stand trials. Assess how the weaker lower extremity reacts to weight-bearing, provide appropriate blocking or support to prevent collapse, and check vital signs while the patient is upright.
Supported standing in front of a raised bed.
To initiate supported standing in front of a raised bed, the therapist should position the patient in a chair that faces the side of the bed. With appropriate assistance, the therapist should stand the patient and sit in a chair on the patient’s weakened side to support the hip and knee extensors. In this standing position, the patient may practice early weight shifting through the lower extremities and bear weight on the upper extremities in either forearm or arm extended positions (see Fig. 1-21).
Edema management
Evaluate the potential cause if edema is present. Discuss with nursing whether the swelling may be associated with the presence of a blood clot or an IV infiltrate. Check to see if the patient’s limb is cool or warm to the touch, observe the skin color, and assess the firmness of the swelling (soft, fluidlike, or pitting).
In the ICU, the preferred method for treating edema is positional elevation, as compression garments or ace wraps may not be appropriate due to various IVs and line access needed by nursing. The extended limb should be positioned above the heart. Active or active assistive ROM should be encouraged and followed by manual massage (Fig. 1-22). See Chapter 12.
Shoulder management
Many patients may experience upper extremity edema, pain, humeral head subluxation, and/or impingement after a stroke. Many of the upper extremity interventions provided in the ICU/acute stage are prophylactic measures to prevent these problems.
To protect the shoulder against potential pain and subluxation, the team should be educated in proper rolling techniques and bed mobility, so they can avoid pulling on the extremity. The team should be instructed to roll the patient by placing the hands on the trunk rather than pulling on the extremity. Signage can be hung behind the patient’s bed indicating the patient may have shoulder subluxation and informing the team to not pull on the patient’s arm (Box 1-6).
Box 1-6 Patient with Right Shoulder Subluxation
Please do not pull on patient’s arm. Please contact occupational therapy at 555–8724 with questions or concerns.
Due to the medical complexity of the ICU/acute patient, most are not getting out of bed to the chair for prolonged periods or engaging in prolonged upright activities. While supine, out of bed in a chair, or dangling at the bed side, support for a weak shoulder can be provided via proper positioning.
Supine
Provide support to the affected upper extremity with pillows and/or towels. The occupational therapist must use clinical judgment to determine proper positioning for each patient. However, as a general rule, the affected scapula should be protracted, the arm in a forward position, with the wrist neutral and fingers extended.26
Out of bed in a chair
The affected upper extremity is supported on the bed side table, on numerous pillows, or on the arm support of the chair.
Most ICU/acute patients do not require supplemental shoulder supports such as sling, clavicle strap, and/or taping. These supports may be used once patients are performing ADL upright and are spending more time out of bed. See Chapter 10.
In addition to positioning, the occupational therapist will provide the ICU patient with passive and active ROM and will engage the affected upper extremity in functional tasks. The therapist should mind lines and leads while providing these services. When an A-line is present in the radial artery, wrist flexion/extension should be avoided.
Increasing spatial awareness by arranging the environment
Although the ICU environment may be more restrictive than a rehabilitation setting, there are subtle yet important interventions that can be implemented to increase spatial awareness. Strategically place items of common use, such as the television remote control, on the involved side while providing cues to assist the patient in locating them. Strategically place food items on the meal tray during feeding to encourage scanning and locating desired items to eat. Verbal cues should be diminished as the patient’s awareness increases. Reverse the position of the bed, if able, so that the patient’s involved space is stimulated (e.g., facing the hallway instead of facing a blank wall). Position the bed side table and phone on the neglected or weaker side of the patient. Use brightly colored bands tied to the bed side rails on the involved side as cues to attend to this side. Hang pictures of family and friends on the involved side while providing cues for the patient to locate them.
Early cognitive management
Patients may spend numerous days to weeks in the ICU. A well-known phenomenon called ICU psychosis can develop within days of being admitted to the ICU.55,99 ICU psychosis has been defined as a fluctuating state of consciousness characterized by fatigue, distraction, confusion, disorientation, restlessness, clouding of consciousness, incoherence, fear, anxiety, excitement, hallucinations, and delusions.41 Many factors related to the ICU environment can contribute to the development of ICU psychosis. Some include psychosocial stress, sleep deprivation, sensory overload or underload, and immobilization.41 Many patients are unable to differentiate between day and night secondary to lighting in most ICU.41
The occupational therapist can assist the primary nursing team in a variety of ways to help lessen the effects of ICU psychosis. Some measures that nursing may implement are providing tactile and verbal stimulation, involvement of the patient in his or her care, and supplying effective rest periods.99 The occupational therapist can minimize environmental monotony and mobilize and engage the patient in familiar self-care tasks. When providing a patient with OT services, communication with patient via gentle touch and voices can help calm patients. Incorporating music and massage into OT treatments can also help reduce anxiety, fear, and depression.99 See Box 1-7 for treatment ideas.
Box 1-7 Treatment Ideas to Manage ICU Psychosis
 Mobilize and engage in self-care.
Mobilize and engage in self-care.
 Engage patient in time appropriate tasks (if it is 8 am complete oral care with window shades open and lights on).
Engage patient in time appropriate tasks (if it is 8 am complete oral care with window shades open and lights on).
 Use a calm gentle voice and touch when engaging patients.
Use a calm gentle voice and touch when engaging patients.
 Decrease or increase sensory stimulation during OT treatment session depending on patient’s needs.
Decrease or increase sensory stimulation during OT treatment session depending on patient’s needs.
 Educate patient’s family in orientating patient not only to date and place but also to time of day.
Educate patient’s family in orientating patient not only to date and place but also to time of day.
Skin protection and prevention of breakdown
Skin breakdown and development of pressure ulcers are common complications associated with an ICU/acute admission. After stroke, patients are at risk for developing pressure ulcers due to prolonged bed rest and immobility. Other risk factors include poor circulation, poor nutrition, edema, low level of arousal, confusion, and incontinence.8 Pressure management and skin protection should become a part of each treatment session. See Table 1-12 for a review of the stages of pressure ulcers.
Table 1-12 Pressure Ulcer Stages
| STAGE | DESCRIPTION | ADDITIONAL INFORMATION |
| Stage I | Intact skin with nonblanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its color may differ from the surrounding area. | The area may be painful, firm, soft, warmer, or cooler as compared to adjacent tissue. Stage I may be difficult to detect in individuals with dark skin tones. May indicate “at risk” persons (a heralding sign of risk). |
| Stage II | Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum-filled blister. | Presents as a shiny or dry shallow ulcer without slough or bruising. This stage should not be used to describe skin tears, tape burns, perineal dermatitis, maceration, or excoriation. Bruising indicates suspected deep tissue injury. |
| Stage III | Full thickness tissue loss. Subcutaneous fat may be visible, but bone, tendon, or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunneling. | The depth of a stage III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput, and malleolus do not have subcutaneous tissue, and stage III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep stage III pressure ulcers. Bone/tendon is not visible or directly palpable. |
| Stage IV | Full thickness tissue loss with exposed bone, tendon, or muscle. Slough or eschar may be present on some parts of the wound bed. Often include undermining and tunneling. | The depth of a stage IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput, and malleolus do not have subcutaneous tissue, and these ulcers can be shallow. Stage IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon, or joint capsule), making osteomyelitis possible. Exposed bone/tendon is visible or directly palpable. |
| Unstageable | Full thickness tissue loss in which the base of the ulcer is covered by slough (yellow, tan, gray, green, or brown) and/or eschar (tan, brown, or black) in the wound bed. | Until enough slough and/or eschar is removed to expose the base of the wound, the true depth, and therefore stage, cannot be determined. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as “the body’s natural (biological) cover” and should not be removed. |
Courtesy of National Pressure Ulcer Advisory Panel
Prevention of skin breakdown is a team responsibility. The occupational therapist has a unique set of skills to assist the team in protecting the patient’s skin. The occupational therapist is often the first team member to mobilize patient and can observe the entire body for signs of skin breakdown. Areas of concern for the ICU patient include sacrum, occiput, heels, greater trochanter, and elbows. Therapist can suggest elbow and heel pads to protect these areas from pressure and friction. Heels can also be floated via positioning or multipodis boots (Fig. 1-23). The therapist can develop positioning devices to assist the nurse with elevating pressure on the occiput (Fig. 1-24) and the sacrum. The occupational therapist can also recommend specialized mattresses to best serve the patient’s needs.
Communication
For the patient unable to communicate verbally, whether due to mechanical ventilation or aphasia, alternative methods of communication will be necessary. Options may include use of a communication board. Single word choice or pictures that represent feelings or needs can be placed strategically on a small poster board. Examples may include Nurse, Doctor, Pain, Thirst, etc., to which the patient can then point. Alphabet boards are generally not used, as they require energy and time for the patient to “spell” words. For the aphasic patient, words might be eliminated altogether. Other alternatives may include signals for Yes/No questions, such as head nodding or thumbs up or down, and an eye blink system. Working in conjunction with the speech-language pathologist, the occupational therapist may assist with facilitating a communication system that is consistently used by other staff and family members (Box 1-8; see Chapter 20).
Box 1-8 Communication Keypoints
 Use a normal tone and volume of voice. Avoid shouting at the patient or talking to them in an infantile manner.
Use a normal tone and volume of voice. Avoid shouting at the patient or talking to them in an infantile manner.
 Give the patient enough time to respond to the question.
Give the patient enough time to respond to the question.
 Try to stay on the same subject.
Try to stay on the same subject.
 Gesture whenever possible and provide tactile cues as appropriate.
Gesture whenever possible and provide tactile cues as appropriate.
 Speak slowly and directly to the patient’s face.
Speak slowly and directly to the patient’s face.
 Try to reduce background noise to eliminate distraction. Close the door and turn off the radio or television.
Try to reduce background noise to eliminate distraction. Close the door and turn off the radio or television.
 Only one person should communicate with the patient at one time.
Only one person should communicate with the patient at one time.
 Be aware of signs of frustration by observing facial expressions.
Be aware of signs of frustration by observing facial expressions.
Dysphagia screening
Acute swallowing difficulties or dysphagia are often associated with stroke.159 The risk of aspiration is high and often leads to pneumonia. Other medical complications associated with dysphagia include malnutrition and dehydration.
During the initial admission to the hospital, patients may be placed on “NPO” (nothing by mouth) precautions. Under these circumstances an NGT is usually inserted through the nose and down the esophagus to the stomach. If the patient is conscious, the occupational therapist may initiate a swallowing or dysphagia screening at the bed side.
Before beginning the assessment, the therapist should be aware of the patient’s level of alertness, fatigue, and ability to follow commands, as these factors may significantly influence the ability to participate safely. An oral motor examination should precede administration of foods and liquids. The assessment should begin with the patient seated with the head of the bed elevated. If an oral suction device is available at the bed side, it should be turned on (Box 1-9; Fig. 1-25).
 Observe for the presence of facial asymmetry. Facial drooping or weakness is common in association with the weaker extremities. Foods can pocket in the cheek of the weakened side.
Observe for the presence of facial asymmetry. Facial drooping or weakness is common in association with the weaker extremities. Foods can pocket in the cheek of the weakened side.
 Observe mouth and lip closure. Can the patient purse his or her lips? Have him or her attempt to blow air into his or her cheeks while keeping his or her lips pursed. Observe if air escapes through one side of the mouth.
Observe mouth and lip closure. Can the patient purse his or her lips? Have him or her attempt to blow air into his or her cheeks while keeping his or her lips pursed. Observe if air escapes through one side of the mouth.
 Request the patient to stick out his or her tongue. Does it drift or deviate to one side? Can he or she lick his or her lips and perform lateral movements with the tongue?
Request the patient to stick out his or her tongue. Does it drift or deviate to one side? Can he or she lick his or her lips and perform lateral movements with the tongue?
 Use a long stick swab to assess the patient’s sensation both extra- and intra-orally.
Use a long stick swab to assess the patient’s sensation both extra- and intra-orally.
 Use a tongue depressor to assess the patient’s gag reflex. Is it present, absent, or delayed?
Use a tongue depressor to assess the patient’s gag reflex. Is it present, absent, or delayed?
 Check the soft palate. Use a flashlight to ask the patient to open mouth and say the word “AH.” Observe for soft palate elevation.
Check the soft palate. Use a flashlight to ask the patient to open mouth and say the word “AH.” Observe for soft palate elevation.
 Assess the patient’s vocal quality. Is it gurgly or wet? Can the patient “clear” his or her voice? Secretions may pool or linger around the vocal cords. Is there hoarseness of the voice? If so, it may be due to inadequate closure of the vocal cords.
Assess the patient’s vocal quality. Is it gurgly or wet? Can the patient “clear” his or her voice? Secretions may pool or linger around the vocal cords. Is there hoarseness of the voice? If so, it may be due to inadequate closure of the vocal cords.
 Can the patient demonstrate a volitional cough? Assess the strength of the cough. Is it adequate to clear the airway?
Can the patient demonstrate a volitional cough? Assess the strength of the cough. Is it adequate to clear the airway?
 Is the patient managing his or her own secretions? Does he or she choke or cough on his or her own secretions? Observe whether the swallow is present or delayed.
Is the patient managing his or her own secretions? Does he or she choke or cough on his or her own secretions? Observe whether the swallow is present or delayed.
 A standardized bed side swallowing assessment is recommended (Fig. 1-25).
A standardized bed side swallowing assessment is recommended (Fig. 1-25).
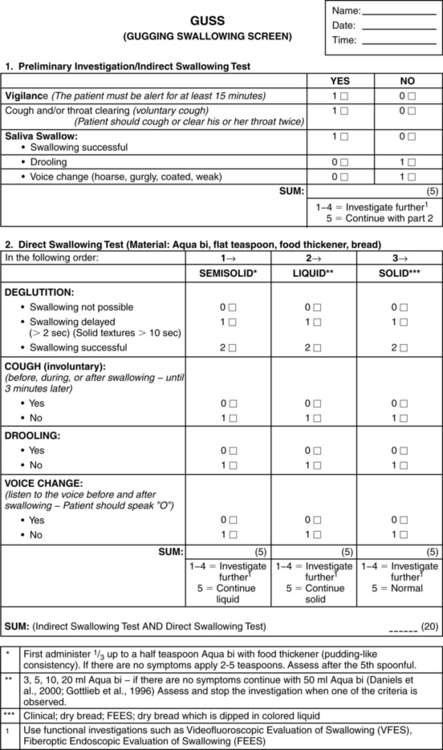
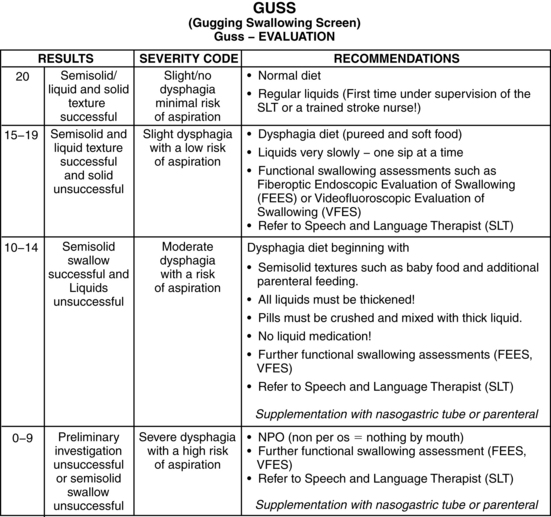
Figure 1-25 The Gugging Swallowing Screen. (From Trapl M, Enderle P, Nowotny M, et al: Stroke 38 (11):2948–2952, 2007.)
Based on the results of the bed side assessment, instrumental testing may be necessary to further evaluate the phases of swallowing that cannot be seen at a bed side oral motor examination. If the patient appears to have adequate oral and swallowing function and a physician’s order has been obtained, a feeding trial may be initiated using graded food textures and liquids of various thickness (Box 1-10; see Chapter 24).
Self-care training
Training in ADL is an integral part of OT treatment. It is important to engage the patient in self-care tasks as soon as they are medically stable.
Energy expenditure is often an issue for the low level patient, so grading the self-care task is as important as the choice of activity. The acute patient may also be limited by IVs, lines, and artificial ventilation. If the patient is having difficulty managing secretions, begin by teaching them how to use an oral suctioning device. Using an adapted call light to request assistance from nursing is also an appropriate goal.
For those with limited motor return, the upper extremity should at least be used as a stabilizer. ADL compensatory strategies can be initiated. If the patient demonstrates active movement, the upper extremity should be incorporated into the self-care task (see Chapter 28).
The initial position may be with the head of the bed elevated. This position provides support of the head and trunk. Vital signs should be monitored throughout the activity. As patients progress, they might be positioned in sitting at the edge of the bed. Demands are greater as patients must maintain their balance while performing the task. Once a patient is able to tolerate sitting at the edge of the bed, the progression should lead to performing tasks seated in a chair. If the patient is able to stand for short periods, then appropriate self-care activities should be performed in standing, such as brushing teeth at the sink or combing hair. Chaining the tasks together will demand more tolerance. Self-care tasks can be graded from simple to complex (Box 1-11).
Box 1-11 Grading ADL during Acute Stroke Rehabilitation
| SIMPLE | COMPLEX |
|---|---|
| Sitting with back supported | Sitting with back unsupported |
| Finger feeding | Feeding with utensils |
| Drinking from a cup | Pouring liquids and drinking with a straw |
| Brushing teeth with set-up | Brushing and cleaning dentures |
| Washing face with cloth | Washing face and upper body |
| Donning pullover shirt | Donning a button-down shirt |
| Donning shorts in bed with bridging | Donning pants while standing to pull up |
Family training
The primary purpose of family training in the ICU/acute setting is to allow for the patient to engage in as many therapeutic activities as possible immediately following the neurological event. Family members should be empowered to assist their loved ones to achieve their therapy goals. Occupational therapists may spend as much time educating the family as they do treating the patients. When training family members, the therapist should demonstrate the tasks and then provide an opportunity for the family member to attempt the tasks. Positive feedback should be provided with corrections given as needed. Families should be provided with written instructions for any tasks they are asked to carry out. During one OT session, no more than three tasks should be given to the family members. This will ensure greater carryover of the tasks provided. The following are suggestions for a family training scheduled in the ICU/acute setting.
Occupational therapists must use their clinical reasoning when providing family training. Many ICU/acute care patients are too medically complex for the family to provide additional therapy services. Such patients may require constant monitoring during physical activity, while other patients may have lines and leads that require a nurse or therapist to handle.
After evaluation patients, family members should be instructed in the following.
 Safely moving noncomplex lines and leads. These may be noninvasive a blood pressure cuff, an O2 monitor, an IV, and, in certain cases, A-lines.
Safely moving noncomplex lines and leads. These may be noninvasive a blood pressure cuff, an O2 monitor, an IV, and, in certain cases, A-lines.
 Positioning of affected extremities
Positioning of affected extremities
 Splint wearing schedule, donning and doffing the splint, and performing skin checks
Splint wearing schedule, donning and doffing the splint, and performing skin checks
 ROM for elbow, wrist, and hand
ROM for elbow, wrist, and hand
 Setting up environment for patient during ADL tasks supine and interacting with patient on affected side (in the case of neglect or sensory loss)
Setting up environment for patient during ADL tasks supine and interacting with patient on affected side (in the case of neglect or sensory loss)
As treatment progresses, the family can be further engaged in the treatment and trained in the following areas:
 Shoulder management: Families must be educated in positioning of the involved upper extremity in bed, during bed mobility, for transfers, during ADL activities, and while upright. Family members can be instructed to don and doff shoulder supports if needed.
Shoulder management: Families must be educated in positioning of the involved upper extremity in bed, during bed mobility, for transfers, during ADL activities, and while upright. Family members can be instructed to don and doff shoulder supports if needed.
 ADL training: Family members can be trained in setting up the environment using the bed side table, giving simple verbal cues, and providing physical cues to engage the affected upper extremity. If the patient is to go home directly from the acute care setting, family training of both compensatory and remedial techniques for ADL trainings should be initiated.
ADL training: Family members can be trained in setting up the environment using the bed side table, giving simple verbal cues, and providing physical cues to engage the affected upper extremity. If the patient is to go home directly from the acute care setting, family training of both compensatory and remedial techniques for ADL trainings should be initiated.
 Shoulder ROM: Once family members are educated on how to safely handle a subluxed shoulder, they can also be educated to passively range the affected shoulder to 90 degrees of forward flexion. In some cases, occupational therapists can use their clinical judgment and teach the family to perform over head ROM if they can maintain proper alignment of the head of the humerus.
Shoulder ROM: Once family members are educated on how to safely handle a subluxed shoulder, they can also be educated to passively range the affected shoulder to 90 degrees of forward flexion. In some cases, occupational therapists can use their clinical judgment and teach the family to perform over head ROM if they can maintain proper alignment of the head of the humerus.
 Positioning: After the family is educated in upper extremity positioning, they should be involved in the patient’s positioning schedule. A physically able family member should be trained in proper body mechanics during bed positioning. If a family member is unable to physically complete the positioning himself, he should be educated on the turning schedule and proper positioning. In addition to positioning supine, family should be educated in the proper position of the affected upper extremity while out of bed in a chair. This position should be determined on a case-by-case basis depending on the specific needs of each patient.
Positioning: After the family is educated in upper extremity positioning, they should be involved in the patient’s positioning schedule. A physically able family member should be trained in proper body mechanics during bed positioning. If a family member is unable to physically complete the positioning himself, he should be educated on the turning schedule and proper positioning. In addition to positioning supine, family should be educated in the proper position of the affected upper extremity while out of bed in a chair. This position should be determined on a case-by-case basis depending on the specific needs of each patient.
 Transfer training: If a patient is to be discharged from the acute care setting to home transfer, training may be appropriate.
Transfer training: If a patient is to be discharged from the acute care setting to home transfer, training may be appropriate.
Goal setting in acute care
Setting appropriate short-term goals can be challenging in the ICU and acute care environments. Mobility goals should not be omitted as part of the occupational therapist’s treatment plan as these mobility skills are a part of not only performing self-care activities but also of enabling the patient to participate in life. Examples of short-term goals are listed in Box 1-12.
| Samples of short-term goals for patients with low arousal or in coma | Patient will withdraw from noxious stimuli 1 out of 3 times. Patient will open eyes when name is called 1 out of 3 times. Patient will turn head away from tactile stimuli. Patient will tolerate resting hand splint schedule for 2 hours. Patient will tolerate lying on the affected side. |
| Samples of short-term goals for early stroke rehabilitation | Patient will tolerate sitting in upright in bed at a 60 degree angle for 30 minutes in preparation for engaging in self-care. Patient will roll in bed with maximum assistance. Patient will tolerate splint wearing schedule for 2-4 hour periods (if appropriate). Patient will remove a wash cloth from his or her face independently. Patient will wash face with minimal assistance. Patient will manage oral secretions with an oral suctioning device with minimal assistance. Patient will use call light for nursing attention independently. Patient will tolerate dangling at the bed side for 15 minutes with close supervision in preparation for self-care training. Patient will feed self 25% to 50% of a meal independently. Patient will brush teeth with set-up assistance. Patient will don hospital gown with moderate assistance. Patient will tolerate sitting in a chair for 60 minutes. |
Discharge planning
As part of the multidisciplinary team, the occupational therapist should assist and provide input for the patient’s discharge plan.151 The patient’s family, support system, and the patient’s ultimate destination of home and into the community should be taken into consideration. The goal is for the patient to be safe and as independently functioning as possible. There are several options available for immediate disposition from the ICU and acute care setting (Box 1-13).
| Inpatient rehabilitation | In this setting the patient must be able to tolerate a minimum 3 hours of therapy 6 days per week. The therapy is more aggressive, and length of stay is usually shorter than other settings. The patient’s length of stay is dependent upon the rate of progress and attaining established goals. |
| Subacute rehabilitation | This setting usually occurs in a skilled nursing facility. The patient may receive 90 minutes of therapy 5 times per week. The length of stay may be longer dependent upon tolerance and progress in therapy. Medical insurance coverage may also dictate how long the patient can remain in a subacute center. |
| Home care services | In some instances, a patient may recover enough function to return home with services. In this case, a referral for visiting nurse and therapy services may be recommended. |
| Outpatient therapy | If the patient has sufficient recovery to return home and can enter and exit the home with ease, outpatient therapy may be an appropriate option for discharge planning. |
Careful consideration should be taken when consulting with the physician and social worker. If the patient appears in need and could benefit from inpatient rehabilitation, the primary care physician may request a physiatry consultation. At this point, the occupational therapist may communicate his or her clinical observations on the patient’s progress since admission to the acute care setting.
Summary
In summary acute stroke rehabilitation is multifaceted. Interventions focus on prevention of secondary complications, such as learned nonuse, contracture, and aspiration, and on early attempts at remediation of impairments. Two overarching goals include maximizing participation in appropriate ADL and acting with the team to assure proper discharge planning.
CASE STUDY 1 Ischemic Stroke: Management of Acute Case and Complications with Workup
G.H. is a 76-year-old woman who has a history of hypertension and diabetes mellitus and had a myocardial infarction two years ago. She arrives at her local emergency room four hours after an acute onset of weakness in her left arm and leg. She fell at home after trying to get up, and it was only after her neighbors heard her calls for help that the emergency services rescue team came to her aid. On admission to the emergency room, she has an elevated blood pressure of 200/100 and is alert and oriented. Her initial physical examination reveals left-sided weakness and sensory loss that is greater in her arm than her leg. The emergency room team has the impression that she has an acute stroke in evolution, so an emergency CT scan is ordered. The initial blood work and electrocardiogram are unremarkable. While she is in the CT scanner, the on-call resident is paged and asked to come see her because the radiology technician notes that she has become unable to move while in the machine. She now has a dense left hemiplegia. Because of fear of stroke progression, she is admitted to the ICU.
Review of the CT scan shows some mild effacement of the sulci on the right side of the brain and no other clear abnormalities. The neurological consultant advises that G.H.’s treatment that night be conservative and supportive and recommends that G.H. be given an enteric-coated aspirin each day. By the next morning, she has had no further progression of her symptoms but has flaccid left hemiplegia and hemineglect. She remains medically stable during the next several days but is unable to achieve adequate oral intake and has to have an NGT placed for enteral feeding. A physiatric consultation is obtained, and physical and occupational therapy is started at the bed side in the ICU.
Another CT scan is performed on the third hospital day, which reveals a clear, acute infarct in the right temporoparietal area with associated edema and no mass effect or hemorrhage, so the neurologist recommends an extended workup. Carotid Doppler images are normal, and the electrocardiogram indicates stability, but the echocardiogram reveals that G.H. has a decreased ejection fraction of 25% with a visible apical thrombus in the area of her previous myocardial infarction. The neurologist and cardiologist concur on anticoagulation with heparin followed by conversion to warfarin. Anticoagulant therapy is initiated, and the aspirin is no longer administered.
On the sixth hospital day, G.H. is started successfully on warfarin, her hemiparesis has improved, and she is able to move her leg against gravity and with gravity eliminated. However, she is still unable to swallow safely and still has an NGT. G.H. is accepted for inpatient rehabilitation and is transferred to the rehabilitation service on the eighth hospital day.
G.H.’s rehabilitation course is notable because of swelling and pain in her left leg, which is found by duplex Doppler scanning to result from a DVT. Because she developed the thrombosis while receiving adequate anticoagulation medication, she has an umbrella filter placed in her inferior vena cava to prevent development of a pulmonary embolus. G.H. becomes severely depressed and after consultation with the psychiatry service begins receiving antidepressant medication, which has good results. G.H. progresses in therapy, but her left shoulder becomes painful because of a shoulder-hand syndrome, which responds well to aggressive therapeutic intervention. She also develops a progressive increase in skeletal muscle activity, particularly in her left hand, which can only be kept under control with aggressive ROM exercises. At the time of her discharge, she is able to move short distances with a hemiwalker and needs assistance with dressing her lower extremities and setting up for her basic ADL.
G.H.’s one-year follow-up is notable for the continuing intractable painful spasticity in her left arm, so treatment with Botox is instituted and results in adequate pain relief. She remains stable until five years after her stroke when she suffers a fall with a subsequent hip fracture. Evaluation of bone density shows accelerated osteoporosis in the left hip. She needs left hip hemiarthroplasty but is unable to regain her previous level of function, despite aggressive therapy, and finally has to be admitted to a nursing home when discharged from the hospital.
CASE STUDY 2 Hemorrhagic Stroke: Management of Acute Case with Workup
C.C. is a 25-year-old man who works as a sales manager in a local retail store. While dismissing a store clerk whom he caught stealing from the store safe, he suddenly complains of a severe headache, sinks to the chair in his office, and slumps over to the right. Within a few minutes, he is unconscious, and the staff calls the ambulance. C.C. is admitted to the emergency room within 20 minutes, accompanied by the fired clerk who is proclaiming loudly that she has done nothing to him. In the emergency room, C.C. is in a deep coma, breathing deeply, and has dilated pupils and absent reflexes. He is intubated immediately for airway protection and is taken for an emergency CT scan. The study is not completed because C.C. has a seizure while in the CT scanner, but the partially completed study shows a great deal of blood in the ventricles. C.C. is diagnosed with a presumed SAH, and treatment is started. Hyperventilation and treatment with mannitol begin. An intracranial pressure monitor is inserted, and C.C. is given phenytoin and nimodipine. C.C. is managed closely in the ICU and after three days comes out of the coma. He remains intubated and has an MRI/MRA performed that shows a probable berry aneurysm on the anterior communicating artery.
A cerebral angiogram is performed, and a 2-cm aneurysm is clearly visible. C.C. has a good response to the treatment and is extubated on the sixth hospital day. His neurological examination reveals mild disorientation, dysarthria, and tetraparesis more pronounced on the right than the left.
The neurological and neurosurgical team, patient, and family have a discussion and decide that surgical clipping of the aneurysm is the best approach to treating the lesion. C.C. is scheduled for operative intervention the next day. However, in the middle of the night, he suddenly loses consciousness and stops breathing. He has a cardiac arrest but is resuscitated successfully. An emergency CT scan reveals a large recurrent hemorrhage that extends into the cerebral cortex and a herniated brainstem. Aggressive treatments are instituted, but despite all measures the herniation progresses, and C.C. lapses into an irreversible coma. One week later C.C. is declared brain dead, and according to his family’s wishes, his organs are donated for transplantation.
Review questions
1. Which stroke risk factors are considered modifiable?
2. Which procedures are used to diagnose a stroke?
3. Which clinical signs indicate a patient is receiving excessive seizure medication?
4. What are the risk factors and recommended treatments for DVTs?
5. Other than neurological, what are the common complications that follow a stroke?
1. The Dutch TIA Trial Study Group. A comparison of two doses of aspirin (30 mg versus 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med. 1991;325(18):1261-1266.
2. Adams HPJr, Brott TG, Crowell RM, et al. Guidelines for the management of patients with acute ischemic stroke. a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation. 1994:90;3:1588-1601.
3. Adams HPJr, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke. a supplement to the guidelines for the management of patients with acute ischemic stroke—a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1996:94;5:1167-1174.
4. Affleck AT, Liberman S, Polon J, et al. Providing occupational therapy in the intensive care unit. Am J Occup Ther. 1986;40(5):323-332.
5. Albers GW, Bates VE, Clark WM, et al. Intravenous tissue-type plasminogen activator for treatment of acute stroke. the standard treatment with alteplase to reverse stroke (STARS) study. JAMA. 2000:283;9:1145-1150.
6. Alberts MJ. Diagnosis and treatment of ischemic stroke. Am J Med. 1999;106(2):211-221.
7. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomized trials of antiplatelet therapy. I. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994:308;6921:81-106.
8. Antle D, Leafgreen P. Reducing the incidence of pressure ulcer development in the ICU. AJN. 2001;101(5):24-26.
9. Asplund K. Hemodilution in acute stroke, (suppl). Cerebrovasc Dis. 1991;1:129.
10. Bach-y-Rita P. Process of recovery from stroke. In: Brandstser ME, Basmajian JV, editors. Stroke rehabilitation. Baltimore: Williams & Wilkins, 1987.
11. Barer DH. Continence after stroke. useful predictor or goal of therapy. Age Ageing. 1989:18;3:183-191.
12. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991:325;7:445-453.
13. Berry E, Kelly S, Westwood ME, et al. The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease. a systematic review. Health Technol Assess. 2002:6;7:1-155.
14. Black SE, Norris JW, Hachinski VC. Post stroke seizures. Stroke. 1983;14:134.
15. Bonita R. Epidemiology of stroke. Lancet. 1992;339(8789):342-344.
16. Bonita R, Beaglehole R, North JD. Event, incidence and case fatality rates of cerebrovascular disease in Auckland, New Zealand. Am J Epidemiol. 1984;120(2):236-243.
17. Bontke CF, Boake C. Principles of brain injury rehabilitation. In: Braddom RL, editor. Physical medicine and rehabilitation. Philadelphia: Saunders, 1996.
18. Boysen G, Christensen H. Stroke severity determines body temperature in acute stroke. Stroke. 2001;32(2):413-417.
19. Brandstater ME, Roth EJ, Siebens HC. Venous thromboembolism in stroke. literature review and implication for clinical practice. Arch Phys Med Rehabil. 1992:73;suppl 5:S379-S391.
20. Brockhurst JC, Andrews K, Richards B, et al. Incidence and correlates of incontinence in stroke patients. J Am Geriatr Soc. 1985;33(8):540-542.
21. Bromfield EB, Reding MJ. Relative risk of deep venous thrombosis or pulmonary embolism post-stroke based on ambulatory status. J Neurol Rehab. 1988;2(2):51.
22. Brott T, Adams HPJr, Olinger CP, et al. Measurements of acute cerebral infarction. a clinical examination scale. Stroke. 1989:20;7:864-870.
23. Bushbacher RM. Deconditioning, conditioning, and the benefits of exercise. In: Braddom RL, editor. Physical medicine and rehabilitation. Philadelphia: Saunders, 1996.
24. Bushbacher R. Heterotopic ossification. a review. Crit Rev Phys Med Rehabil. 1992;4:199.
25. Caplan LR. Diagnosis and treatment of ischemic stroke. JAMA. 1991;266(17):2413-2418.
26. Carr EK, Kenney FD. Positioning of the Stoke Patient. a review of the literature. J Nurs Stud. 1992:29;4:355-369.
27. Cerebral Embolism Study Group. Immediate anticoagulation of embolic stroke, Brain Hemorrhage and Cerebral Embolism Task Force. cardiogenic brain embolism—the second report of the Cerebral Embolism Task Force. Arch Neurol. 1989:46;7:727.
28. Chyatte D, Elefteriades J, Kim B. Profound hypothermia and circulatory arrest for aneurysm surgery, Case report. J Neurosurg. 1989:70;3:489-491.
29. Cinnamon J, Viroslav AB, Dorey JH. CT and MRI diagnosis of cerebrovascular disease. going beyond the pixels. Semin Ultrasound CT MRI. 1995:16;3:212-236.
30. Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA. 1999:282;21:2019-2026.
31. Colditz GA, Bonita R, Stampfer MJ, et al. Cigarette smoking and risk for stroke in middle-aged women. N Engl J Med. 1988;318(15):937-941.
32. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Lancet. II. Short-term reductions in blood pressure: overview of randomised drug trials in an epidemiological context. 1990:335;8693:827-838.
33. Counsel C, Sandercock P. The management of patients with acute ischemic stroke. Curr Med Lit Geriatr. 1994;7:99.
34. Coventry MB, Scanlon PW. The use of radiation to discourage ectopic bone. a nine-year study in surgery about the hip. J Bone Joint Surg Am. 1981:63;2:201-208.
35. Den Hertog HM, van der Worp HB, Tseng MC, et al. Cooling therapy for acute stroke. Cochrane Database Syst Re. 2009;21;1. CD001247
36. DeVincenzo DK, Watkins S. Accidental falls in a rehabilitation setting. Rehabil Nurs. 1987;12(5):248-252.
37. Dichter MA. The epilepsies and convulsive disorders. In: Isselbacher KJ, Braunwald E, Wilson JD, editors. Harrison’s principles of internal medicine. New York: McGraw-Hill, 1994.
38. Donahue RP, Abbott RD, Reed DM, et al. Alcohol and hemorrhagic stroke. the Honolulu Heart Program. JAMA. 1986:255;17:2311-2314.
39. Donaldson CL, Hulley SB, Vogel JM, et al. Effect of prolonged bed rest on bone mineral. Metabolism. 1970;19(12):1071-1084.
40. Dyken ML. Overview of trends in management and prognosis in stroke. Ann Epidemiol. 1993;3(5):535.
41. Dyson M. Intensive Care Unit psychosis, the therapeutic nurse-patient relationship and the influence of the intensive care setting. analyses of interrelating factors. J Clin Nurs. 1999;8:284-290.
42. Ebrahim S. Clinical epidemiology of stroke. Oxford: Oxford University Press; 1995.
43. Ebrahim S, Nouri F. Caring for stroke patients at home. Int Rehabil Med. 1987;8(4):171-173.
44. Elster AD, Moody DM. Early cerebral infarction. gadopentetate dimeglumine enhancement. Radiology. 1990:177;3:627-632.
45. European Atrial Fibrillation Trial Study Group. Secondary prevention in nonrheumatic atrial fibrillation after transient ischemic attack or minor stroke. Lancet. 1993;342(8882):1255-1262.
46. European Carotid Surgery Trial Collaborative Group. Medical research council carotid surgery trial. interim results for patients with severe (70% to 90%) or with mild (0% to 30%) carotid stenosis. Lancet. 1989:334;8655:175.
47. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. results of an international randomized trial, the EC/IC Bypass Study Group. N Engl J Med. 1985;313(19):1191-2000.
48. Farrell B, Godwin J, Richards S, et al. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial. final results. J Neurol Neurosurg Psychiatry. 1991:54;12:1044.
49. Fisher CM. Clinical syndromes in cerebral thrombosis, hypertensive hemorrhage, and ruptured saccular aneurysm. Clin Neurosurg. 1975;22:117-147.
50. Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30(3):536-550.
51. Fisher CM. Atherosclerosis of the carotid and vertebral arteries. extracranial and intracranial. J Neuropathol Exp Neurol. 1965:24;3:455.
52. Further analyses of mortality in oral contraceptive users. Royal College of General Practicioners’ Oral Contraceptive Study. Lancet. 1981;1;8219:541-546.
53. Gainotti G. Emotional behavior and hemispheric side of the lesion. Cortex. 1972;8(1):41-55.
54. Garraway WM, Whisnant JP, Drury I. The changing pattern of survival following stroke. Stroke. 1983;14(5):699.
55. Geary SM. Intensive care unit psychosis revisited. Understanding and managing delirium in the critical care setting. Crit Care Nurs Q. 1994:17;1:51-63.
56. Giancio JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised. measurement characteristic and diagnostic utility. Arch Phys Med Rehabil. 2004:85;12:2020-2029.
57. Giancino J, Kalmar K. Coma Recovery Scale-Revised,. The Center for Outcome Measurement in Brain Injury, (website). www.tbims.org/combi/crs, 2009. Accessed
58. Gonzalez RG, Schaefer PW, Buonanno FS, et al. Diffusion weighted MR imaging. diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology. 1999:210;1:155-162.
59. Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br J Med. 1987;295(6595):411-414.
60. Gosselink R, Bott J, Johnson M, et al. Physiotherapy for adult patients with critical illness. recommendations of the European Respiratory Society and the European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients. Intensive Care Med. 2008:34;7:1188-1199.
61. Groher ME, Bukatman R. The prevalence of swallowing disorders in two teaching hospitals. Dysphagia. 1986;1(1):3.
62. Grotta JC, Norris JW, Kamm B. Prevention of stroke with ticlopidine. who benefits most? TASS Baseline and Angiographic Data Subgroup. Neurology. 1992:42;1:111-115.
63. Hachinski V, Norris JW. The acute stroke. Philadelphia: FA Davis; 1985.
64. Hacke W, Kaste M, Fieschi C, et al. Randomized double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASS II), Second European-Australian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245-1251.
65. Hadizadeh DR, Gieseke J, Lohmaier SH, et al. Peripheral MR angiography with blood pool contrast agent. prospective intra individual comparative study of high-spatial-resolution steady-state MR angiography versus standard-resolution first-pass MR angiography and DSA. Radiology. 2008:249;2:701-711.
66. Harburn KL, Potter PJ. Spasticity and contractures. Phys Med Rehabil State Art Rev. 1993;7(8):113.
67. Hassenfeld M. Increased incidence of hip fracture on the hemiplegic side of post stroke patients. New York: Unpublished work presented at Columbia Presbyterian Medical Center, May; 1993.
68. Hauser WA, Ramirez-Lassepas M, Rosenstein R. Risk for seizures and epilepsy following cerebrovascular insults. Epilepsia. 1984;25(5):666.
69. Heros RC, Zervas NT, Varsos V. Cerebral vasospasm after subarachnoid hemorrhage. an update. Ann Neurol. 1983:14;6:599-608.
70. Hirsh J. Heparin. N Engl J Med. 1991;324(22):1565-1574.
71. Hirsh J. From unfractionated heparins to low molecular weight heparins. Acta Chir Scand Suppl. 1990;556:42-50.
72. Hirsh J, Genton E, Hull R. Venous thromboembolism. New York: Grune & Stratton; 1981.
73. Hirsh J, Hull R. Natural history and clinical features of venous thrombosis. In: Coleman RW, Hirsh J, Marder V, editors. Haemostasis and thrombosis: basic principles and clinical practice. Philadelphia: Lippincott, 1982.
74. House A, Dennis M, Molyneux A, et al. Emotionalism after stroke. BMJ. 1989;298(6679):991-994.
75. Hull R, Hirsh J. Diagnosis of venous thromboembolism. In: Coleman RW, Hirsh J, Marder V, editors. Haemostasis and thrombosis: basic principles and clinical practice. Philadelphia: Lippincott, 1982.
76. Hull RD, Raskob GE, Rosenbloom D, et al. Heparin for 5 days as compared with 10 days in the initial treatment of proximal venous thrombosis. N Engl J Med. 1990;322(18):1260-1264.
77. Huston J. 3rd, Fain SB, Riederer SJ, et al. Carotid arteries. maximizing arterial to venous contrast in fluoroscopically triggered contrast-enhanced MR angiography with elliptic centric view ordering. Radiology. 1999:211;1:265-273.
78. Iso H, Jacobs DRJr, Wentworth D, et al. Serum cholesterol levels and six year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med. 1989;320(14):904-910.
79. Kallmes DF, Omary RA, Dix JE, et al. Specificity of MR angiography as a confirmatory test of carotid artery stenosis. Am J Neuroradiol. 1996;17(8):1501-1506.
80. Kannel WB, McGee DL. Diabetes and cardiovascular disease. the Framingham Study. JAMA. 1979:241;19:2035-2038.
81. Kanter MC, Sherman DG. Strategies for preventing stroke. Curr Opin Neurol Neurosurg. 1993;6(1):60-65.
82. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603-612.
83. Kistler JP, Ropper AH, Martin JB. Cerebrovascular disease. In: Isselbacher KJ, Braunwald E, Wilson JD, editors. Harrison’s principles of internal medicine. New York: McGraw-Hill, 1994.
84. Kottke FJ, Pauley DL, Ptak RA. The rationale for prolonged stretching for correction of shortening of connective tissue. Arch Phys Med Rehabil. 1966;47(6):345-352.
85. Kozararevic D, McGee D, Vojvodic N, et al. Frequency of alcohol consumption and morbidity and mortality. the Yugoslavia Cardiovascular Disease Study. Lancet. 1980:1;8169:613-616.
86. Kuller LH, Dorman JS, Wolf PA. Cerebrovascular disease and diabetes. In Diabetes in America: diabetes data compiled for 1984, National Diabetes Data Group, NIH Pub No 85–1468. Bethesda, MD: Department of Health and Human Services. August 1985.
87. Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year, National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med. 1999;340(23):1781-1787.
88. Lai S, Duncan PW, Keighley J. Prediction of functional outcome after stroke comparison of the Orpington Prognostic Scale and the NIH Stroke Scale. Stroke. 1998;29(9):1838-1842.
89. Lance JW. Pathophysiology of spasticity and clinical experience with baclofen. In: Feldman RG, Young RR, Koella P, editors. Spasticity-disordered motor control. Chicago: Year Book, 1980.
90. Langhorne P, Stott DJ. Acute cerebral infarction. optimal management in older patients. Drugs Aging. 1995:6;6:445-455.
91. Langhorne P, Williams BO, Gilchrist W, et al. Do stroke units save lives. Lancet. 1993;342(8868):395-398.
92. Levison ME. Pneumonia, including necrotizing pulmonary infections (lung abscesses). In: Isselbacher KJ, Braunwald E, Wilson JD, editors. Harrison’s principles of internal medicine,. New York: McGraw-Hill, 1994.
93. Levison ME, Bush L. Pharmacodynamics of antimicrobial agents. bactericidal and postantibiotic effects. Infect Dis Clin North Am. 1989:3;3:415-421.
94. Lezak MD. Neuropsychological assessment, ed 2. New York: Oxford University Press; 1983.
95. Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms, and arteriovenous malformations. based on 6368 cases in a cooperative study. Sahs AL, Perret G, Locksley HB, editors. Intracranial aneurysms and subarachnoid hemorrhage: a cooperative study. Philadelphia: Lippincott, 1969.
96. Longstreth WTJr, Nelson LM, Koepsell TD, et al. Clinical course of spontaneous subarachnoid hemorrhage. a population based study in King County, Washington. Neurology. 1993:43;4:712-718.
97. Lorber B, Swenson RM. Bacteriology of aspiration pneumonia. a prospective study of community and hospital acquired cases. Ann Intern Med. 1974:81;3:329-331.
98. MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. I. Prolonged differences in blood pressure: prospective observational studies corrected for regression dilution bias. Lancet. 1990:335;8692:765-774.
99. Maddocks W. The role of the nurse in preventing intensive care psychosis. Nurs Prax N Z. 1995;10(3):12-15.
100. Mahoney FI, Barthel DW. Functional evaluation. the Barthel index. Maryland State Med J. 1965;14:61-65.
101. Marmot MG, Poulter NR. Primary prevention of stroke. Lancet. 1992;339(8789):344-347.
102. Mayerberg MR, Batjer HH, Dacey R, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994:25;11:2315-2328.
103. McCullough LD, Beauchamp NB, Wityk R. Recent advances in the diagnosis and treatment of stroke. Surv Ophthalmol. 2001;45(4):317-330.
104. McGill HCJr. The pathogenesis of atherosclerosis. Clin Chem. 1988;34(8B):B33-B39.
105. McNeil BJ, Bettman MA. The diagnosis of pulmonary embolism. In: Coleman RW, Hirsh J, Marder V, et al, editors. Haemostasis and thrombosis: basic principles and clinical practice,. Philadelphia: Lippincott, 1982.
106. Mion LC, Gregor S, Buettner M, et al. Falls in the rehabilitation setting. incidence and characteristics. Rehabil Nurs. 1989:14;1:17-22.
107. Mohr JP. Lacunes. Stroke. 1982;13(1):3-11.
108. Mohr JP, Orgogozo JM, Harrison MJG, et al. Meta-analysis of nimodipine trials in acute ischemic stroke. Cerebrovasc Dis. 1994;4:197.
109. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world. Global Burden of Disease study. Lancet. 1997:349;9061:1269-1276.
110. Murray GB, Shea V, Conn DK. Electroconvulsive therapy for post-stroke depression. J Clin Psychiatry. 1986;47(5):258.
111. Nederkoorn PJ, Van Der Graaf Y, Hunink MG, et al. Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis. a systematic review. Stroke. 2003:34;5:1324-1331.
112. Needham D. Mobilizing patients in the intensive care unit improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685-1689.
113. Okada Y, Yamaguchi T, Minematsu K, et al. Hemorrhagic transformation in cerebral embolism. Stroke. 1989;20(5):598-603.
114. Paffenbarger RSJr, Laughlin ME, Gima AS, et al. Work activity of longshoremen as related to death from coronary heart disease and stroke. N Engl J Med. 1970;282(20):1109-1114.
115. Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161-175.
116. Perry RF. Clinical practice guidelines for smoking cessation. JAMA. 1996;276(6):448.
117. Pessin MS, Duncan GW, Mohr JP, et al. Clinical and angiographic features of carotid transient ischemic attacks. N Engl J Med. 1977;296(7):358-362.
118. Poplingher AR, Pillar T. Hip fracture in stroke patients. epidemiology and rehabilitation. Acta Orthop Scand. 1985:56;3:226-227.
119. PORT Study (funded by the Agency for Health Care Policy and Research. Durham, NC: Duke University Medical Center, 1994.
120. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. final results of the Systolic Hypertension in the Elderly Program (SHEP), SHEP Cooperative Research Group. JAMA. 1991;265(24):3255-3264.
121. Prevention of venous thrombosis and pulmonary embolism, NIH Consensus Development. JAMA. 1986;256;6:744-749.
122. Quizilbash N, Murphy M. Meta-analysis of trials of corticosteroids in acute stroke, (suppl). Age Ageing. 1993;22:2.
123. Randomised controlled trial of streptokinase. aspirin, and combination of both in treatment of acute stroke, Multicenter Acute Stroke Trial: Italy (MAST-I) Group. Lancet. 1995;346(8989):1509-1514.
124. Reed DM. The paradox of high risk of stroke in populations with low risk of coronary heart disease. Am J Epidemiol. 1990;131(4):579-588.
125. Robinson RG, Bolla-Wilson K, Kaplan E, et al. Depression influenced intellectual impairment in stroke patients. Br J Psychiatry. 1986;148:541.
126. Ropper AH, Davis KR. Lobar cerebral hemorrhages. acute clinical syndromes in 26 patients. Ann Neurol. 1980:8;2:141-147.
127. Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update. a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007:115;5:e69-e171.
128. Roth EJ, Harvey RL. Rehabilitation of stroke syndromes. In: Braddom RL, editor. Physical medicine and rehabilitation. Philadelphia: Saunders, 1996.
129. Rowland TJ, Cooke DM, Gustafsson LA. Role of occupational therapy after stroke.. Ann Indian Acad Neurol. 2008;11;5:99-107. [serial online]
130. Rutan GH, Kuller LH, Neaton JD, et al. Mortality associated with diastolic hypertension and isolated systolic hypertension among men screened for the Multiple Risk Factor Intervention Trial. Circulation. 1988;77(3):504-514.
131. Saccani S, Beghi C, Fragnito C, et al. Carotid endarterectomy under hypothermic extracorporeal circulation. a method of brain protection for special patients. J Cardiovasc Surg (Torino). 1992:33;3:311-314.
132. Salcido R, Hart D, Smith AM. The prevention and management of pressure ulcers. In: Braddom RL, editor. Physical medicine and rehabilitation. Philadelphia: Saunders, 1996.
133. Salonen R, Seppanen K, Rauramaa R, et al. Prevalence of carotid atherosclerosis and serum cholesterol levels in eastern Finland. Atherosclerosis. 1988;8(6):788-792.
134. Salzman EW, Hirsh J, et al. Prevention of venous thromboembolism. In: Coleman RW, Hirsh J, Marder V, editors. Haemostasis and thrombosis: basic principles and clinical practice,. Philadelphia: Lippincott, 1982.
135. Sandercock PA, van den Belt AG, Lindley RI, et al. Antithrombotic therapy in acute ischemic stroke. an overview of the randomized trials. J Neurol Neurosurg Psychiatry. 1993:56;1:17-25.
136. Sandercock PAG, Willems H. Medical treatment of acute ischemic stroke. Lancet. 1992;339(8792):537-539.
137. Schwartz Cowley R, Swanson B, Chapman P, et al. The role of rehabilitation in the intensive care unit. J Head Trauma Rehabil. 1994;9(1):32-42.
138. Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients. a randomised controlled trial. Lancet. 2009:373;9678:1874-1882.
139. Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. New Engl J Med. 2005;353(15):1574-1584.
140. Sinyor D, Amato P, Kaloupek DG, et al. Post-stroke depression. relationships to functional impairment, coping strategies and rehabilitation outcome. Stroke. 1986:17;6:1102-1107.
141. Sorenson AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke. evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996:199;2:391-401.
142. Stadel BV. Oral contraceptives and cardiovascular disease. N Engl J Med. 1981;305(12):672-677.
143. Stampfer MJ, Willett WC, Colditz GA, et al. A prospective study of the past use of oral contraceptive agents and the risk of cardiovascular diseases. N Engl J Med. 1988;319(20):1313-1317.
144. Starkstein SE, Cohen BS, Fedoroff P, et al. Relationship between anxiety disorders and depressive disorders in patients with cerebrovascular injury. Arch Gen Psychiatry. 1990;47(3):246-251.
145. Starkstein S, Robinson R, Price TR. Comparison of cortical and subcortical lesions in the production of post stroke mood disorders. Brain. 1987;110(Pt 4):1045.
146. Stiller K, Phillips A. Safety aspects of mobilising acutely ill inpatients. Physiother Theory Pract. 2003;19(4):239-257.
147. Subbarao J, Smith J. Pulmonary embolism during stroke rehabilitation. Ill Med J. 1984;165(5):328-332.
148. Sundaram MB, Chow F. Seizures associated with spontaneous subarachnoid hemorrhage. Can J Neurol Sci. 1986;13(3):229-231.
149. Sundt TMJr, Kobayashi S, Fode NC, et al. Results and complications of surgical management of 809 intracranial aneurysms in 722 cases. related and unrelated to grade of patient, type of aneurysm, and timing of surgery. J Neurosurg. 1982:56;6:753-765.
150. Sundt TMJr, Whisnant JP. Subarachnoid hemorrhage from intracranial aneurysms. N Engl J Med. 1978;299(3):116-122.
151. Sutton S. An acute medical admission unit. is there a place for an occupational therapist. Br J Occup Ther. 1998:61;1:3-7.
152. Swartzman L, Teasell RW. Psychological consequences of stroke. Phys Med Rehabil State Art Rev. 1993;7(1):179.
153. Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischemic events, the SALT Collaborative Group. Lancet. 1991;338;8779:1345-1349.
154. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974:2;7872:81-84.
155. Thompson DD, Rodan GA. Indomethacin inhibition of tenotomy induced bone resorption in rats. J Bone Miner Res. 1988;3(4):409.
156. Thrombolytic therapy with streptokinase in acute ischemic stroke. the Multicenter Acute Stroke Trial—Europe Study Group. New Engl J Med. 1996;335(3):145-150.
157. Tissue plasminogen activator for acute ischemic stroke. the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. New Engl J Med. 1995:333;24:1581-1587.
158. Torner JC, Nibbelink DW, Burmeister LF. Statistical comparisons of end results of a randomized treatment study. In: Sahs AL, Nibbelink DW, Torner JC, editors. Aneurysmal subarachnoid hemorrhage: report of the cooperative study. Baltimore: Urban & Schwarzenberg, 1981.
159. Trapl M, Enderle P, Nowotny M, et al. Dysphagia bedside screening for acute stroke patients. the Gugging Swallowing Screen. Stroke. 2007:38;11:2948.
160. U-King-Im JM, Young V, Gillard JH. Carotid-artery imaging in the diagnosis and management of patients at risk of stroke. Lancet Neurol. 2009;8(6):569-580.
161. Uniform Data System for Medical Rehabilitation. The Guide for the Uniform Data Set for Medical Rehabilitation (Including the FIMTM Instrument). Version 5.1. Buffalo, NY: Research Foundation, State University of New York at Buffalo, 1997.
162. Viitanen M, Eriksson S, Asplund K. Risk of recurrent stroke, myocardial infarction and epilepsy during long-term follow-up after stroke. Eur Neurol. 1988;28(4):227-231.
163. Wardlaw JM, Warlow CP. Thrombolysis in acute ischemic stroke. does it work. Stroke. 1992:23;12:1826-1839.
164. Warlow C. Disorders of the cerebral circulation. In Walton J, editor: Brain’s disease of the nervous system, ed 10, Oxford, England: Oxford University Press, 1993.
165. Warlow C, Ogston D, Douglas AS. Deep venous thrombosis of the legs after strokes. I. Incidence and predisposing factors; II. Natural history. BMJ. 1976:1;6019:1178-1181.
166. Wheeler HB, Anderson FAJr. Diagnostic approaches for deep vein thrombosis. Chest. 1986;89(suppl 5):407S-412S.
167. Whisnant JP, Matsumotoa N, Elveback LR. The effect of anticoagulant therapy on the prognosis of patients with transient cerebral ischemic attacks in a community. Rochester, Minnesota, 1955–1969. Mayo Clinic Proc. 1973:48;12:844-848.
168. Wiebe-Velasquez S, Blume WT. Seizures. Phys Med Rehabil State Art Rev. 1993;7(1):73.
169. Wolf PA, Belanger AJ, D’Agostino RB. Management of risk factors. Neurol Clin. 1992;10:177.
170. Wolf PA, D’Agostino RB, Belanger AJ, et al. Probability of stroke. a risk profile from the Framingham Study. Stroke. 1991:22;3:312-318.
171. Wolf PA, D’Agostino RB, Kannel WB, et al. Cigarette smoking as a risk factor for stroke. the Framingham Study. JAMA. 1988:259;7:1025-1029.
172. Worden JW. Grief counseling and grief therapy. New York: Springer. 1982.
173. Wortman CB, Silver RC. The myths of coping with loss. J Consult Clin Psychol. 1989;57(3):349-357.