Brain and Spinal Cord
The Central Nervous System – Pressing Constriction and Open Expanse
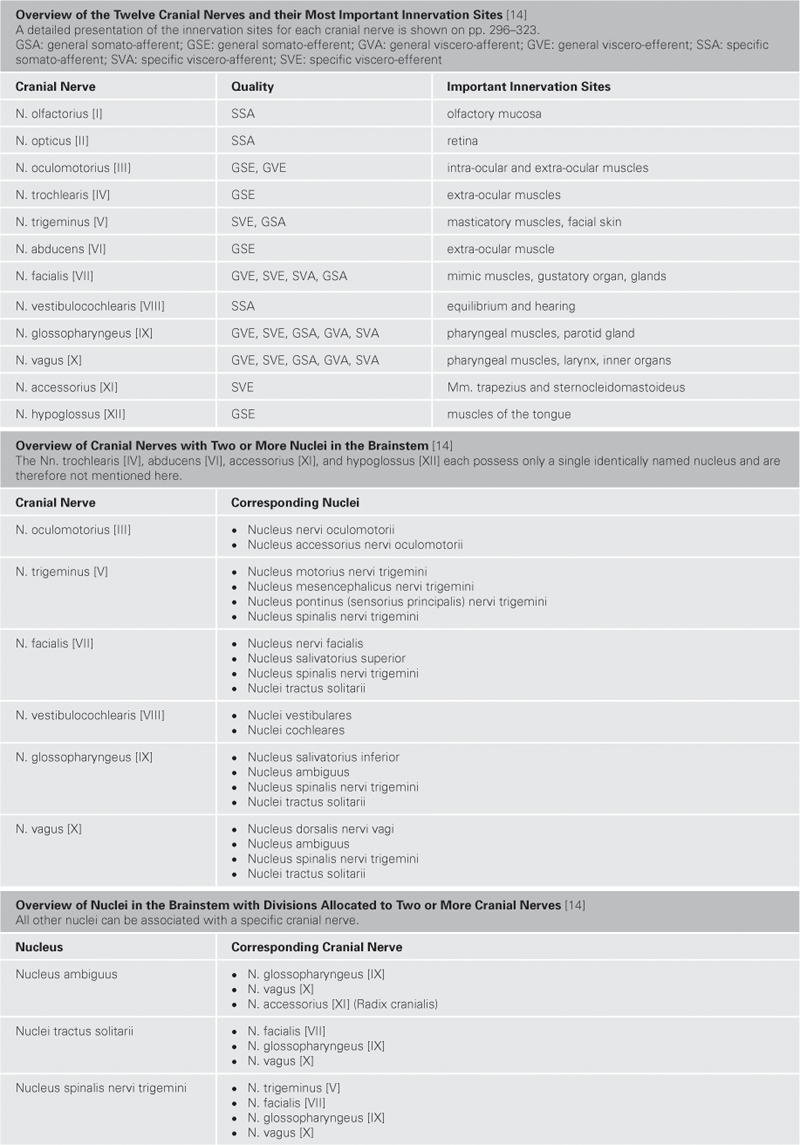
Commonly the term “central” refers to those parts of the nervous system, the brain (Encephalon) and spinal cord (Medulla spinalis), which are located within the cranial cavity (Cavitas cranii) and in the vertebral canal (Canalis vertebralis), respectively. The locations where cranial and spinal nerves (12 Nn. craniales, 31 Nn. spinales) enter and exit the CNS mark the border between the central nervous system (CNS) and the peripheral nervous system (PNS). Distal to this border in the PNS, nerve fibres are coated with an insulating sheath formed by SCHWANN’s cells; in the CNS this insulating layer is provided by oligodendrocytes.
The Maters
Three membranes, known as meninges, completely surround the brain and spinal cord. Directly beneath the outer, tough, parchment-like membrane of, the Dura mater (“tough mother”), lies a softer membrane of, the Arachnoidea mater (“spider-like mother”), from which fine and cob-webbed fibres emerge to the surface of the CNS. The narrow space between the Arachnoidea mater and Pia mater – the subarachnoid space – is filled with cerebrospinal fluid (CSF, Liquor cerebrospinalis), in which the CNS floats. Directly on the surface of the CNS lies the very delicate Pia mater (“tender mother”), which serves as an attachment site for the fibres of the arachnoid mater.
Brain …
The skull is a space of pressing constriction: the brain fills the cranial cavity almost completely, only in a few areas (especially in the area of the occipital foramen, Foramen magnum), the subarachnoid space extends beyond a few millimetres. The brain of an adult weighs on average 1300 grams. In the dissection laboratory – that is in its fixed state – the brain has a rubber-like consistency. In the natural unfixed state, its consistency is more that of a soft pudding. This consistency is due to its high moisture content: The brain consists of 85% water, whereas the rest of the body only contains about 65% water.
The embryonic brain comprises five parts and consists of five successively arranged hollow cysts. In the adult brain, only three parts are still recognisable. The brain is hollow inside. The inner cavities are called ventricles and contain cerebrospinal fluid. The largest of the three brain parts is the Cerebrum, which takes up almost the entire interior of the skull with the exception of the area above the Foramen magnum. The cerebrum consists of a right and a left hemisphere. The surface of these hemispheres is enlarged by coarse gyri (Gyri) and called the Cortex cerebri. Likewise, the Cerebellum consists of two hemispheres and lies in the “postero-inferior” region of the skull, above of and bilateral to the Foramen magnum. Its surfaces also contain folds which are much finer and more regular. These leave-resembling folds are called Folia cerebelli, encompassing the Cortex cerebelli, the cerebellum‘s own cortex. The unpaired brainstem (Truncus encephali) is about as thick as a thumb, located at the cranial base and extends through the Foramen magnum into the spinal cord. Extensive peduncles (Pedunculi) connect the brainstem to the cerebrum and cerebellum. Ten out of twelve cranial nerves emerge from the brainstem. In contrast to the cerebrum and cerebellum, its surface appears white, because it is mainly composed of nerve fibres (white matter, Substantia alba), whereas the grey cortices mainly consist of cell bodies (grey matter, Substantia grisea).
… and Spinal Cord
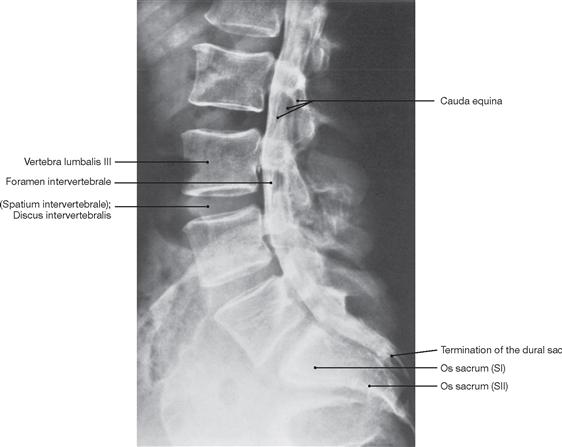
The spinal cord has a white surface and resides in a spacious spinal canal. The spinal cord is about as thick as a pencil; however, the inner diameter of the vertebral canal almost reaches the width of a thumb. More caudally towards the sacral bone the vertebral canal becomes narrower; in this lower region, it does not contain any spinal cord, but rather roots of lumbar and sacral spinal nerves, each exiting the spinal canal “much lower” through their respective intervertebral foramina. The subarachnoid space is relatively wide, and a space filled with abundant adipose tissue and veins remains in between the Dura mater and the bony wall of the Canalis vetebralis. Encompassing the Medulla spinalis, the dural sac extends downwards to the coccyx. However, the caudal tip of the spinal cord concludes at the level of the second lumbar vertebra.
The diameter of the spinal cord varies. Compared to the segments that innervate the less muscular trunk, the cervical spinal cord is thicker at the site of the motor neurons responsible for the innervation of the arm muscles. The caudal part of the spinal cord providing innervation to the lower extremities again shows an increased diameter. These two enlarged regions are termed Intumescentia cervicalis and Intumescentia lumbosacralis, respectively.
The radicular filaments (Fila radicularia) of the dorsal sensory roots of the spinal nerves enter the spinal cord bilaterally sides along two longitudinal lines at its dorsal surface. On its ventral surface, the Fila radicularia of the ventral motor roots exit in a similar manner. Five to ten Fila radicularia bundle to form the dorsal and ventral roots (Radix posterior and Radix anterior); in the foramen intervertebrale, anterior and posterior roots merge to form the spinal nerve, which passes through the intervertebral foramen and exits both the vertebral column and the dural sac.
Caveat!
“Beware!” applies to the CNS and especially to the brain. The above summary is about the surface of the organ and – deliberately – superficial in a contextual sense. Internally, no other organ is as complex as the brain: If one has seen and understood a small part of the liver, one comprehends the entire liver. However, if one has seen a part of the brain, one cannot draw conclusions about the other parts, as no two cells are identical (although they can be classified). Only a synoptic approach, involving the anatomy, physiology, and psychology/psychiatry, lets one appreciate the brain‘s complexity.
It should be noted also that the relationship of the brain to its products, the thoughts, is still a mystery. This mystery and the complexity of the brain are often exploited as an excuse to indulge in superlatives, to speak of “the miracles” of the brain, to unite human and brain, and to emphasise uniqueness by saying: “Look, this and only this is YOU!”
Sometimes, establishing an essentially sarcastic distance to this “miraculous organ” as well as to one’s own thoughts is helpful. For example, with the (slightly altered) words of the physiologist Carl Vogt (1817–1895), a notorious scoffer: “The brain treats the thought as the liver the bile and the kidney the urine: it discharges its products”.
→Dissection Link
Upon removal of the brain from the skull, the blood vessels and cranial nerves in the region of the cranial base and at the base of the brain as well as the removed brain itself are inspected. For visualisation of the superficial cerebral veins, the arachnoid mater is removed from the brain. The cerebral arterial circle (Circulus arteriosus) with adjacent vessels is dissected next. The Circulus arteriosus is detached at the branching points of the blood vessels, glued to a sheet of paper and labelled. For the dissection of the ventricles, remnants of the Leptomeninx are removed, and the remaining blood vessels are traced, studied and removed. With the brain knife, a horizontal cut above the Corpus callosum is now being conducted and the lateral ventricles are opened from cranial. Severing the two Crura fornicis and deflection of the Fornix opens the third ventricle. In the following step, the dissection of the Cornu inferius of the lateral ventricle, located in the temporal lobe, exposes the Hippocampus formation. Thereafter, the cerebellum is inspected externally, dissected, the cerebellar nuclei are examined and the Pedunculi cerebelli are removed from the brainstem, exposing the fourth ventricle. The brainstem is severed; the midbrain (Mesencephalon), Pons, and Medulla oblongata are sectioned in planes for examination. Frontal and horizontal sections through each of the brain hemispheres serve to study the basal ganglia. Finally, medial and lateral tracts (including the visualisation of the Insula, Capsula interna, and optic tract) as well as the pyramidal tract, and the middle and upper cerebellar peduncles are examined. The spinal cord is best visualised on the preserved prosected demonstration specimen, where the spinal cord, the Intumescentiae, the Cauda equina surrounded by meninges and the outgoing spinal nerve pairs are visible in the opened vertebral canal.
General
Nervous system, overview
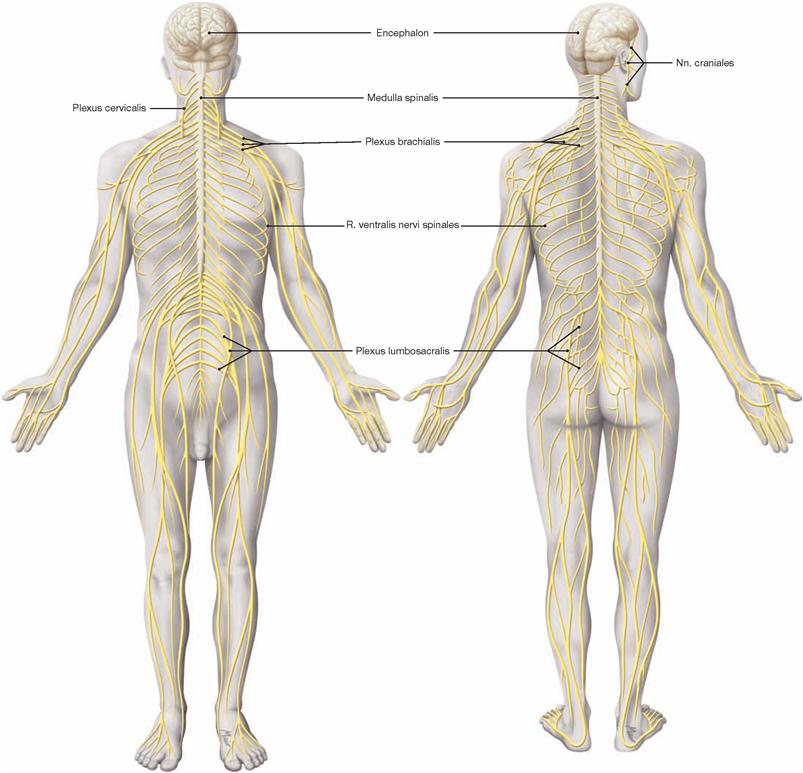
Fig. 12.1 Structure of the nervous system, Systema nervosum; ventral and dorsal views.
The nervous system is divided into a central (CNS) and a peripheral nervous system (PNS).
The brain and spinal cord constitute the CNS which regulates complex functions, including the storage of experiences (memory), the creation of imaginations (thoughts) and emotions. The CNS assists the whole body in adapting quickly to changes occurring in the environment and within the body. The PNS is mainly composed of spinal nerves (with connections to the spinal cord) and cranial nerves (with connections to the brain). Its function is to enable communication between the organs and the CNS, to control the activity of muscles and viscera, and to provide an essential link between the surrounding environment and the body interior.
Functionally, the nervous system is divided into an autonomic (vegetative visceral, control of visceral activity, mostly involuntary) and a somatic (animalic, innervation of skeletal muscles, voluntary perception of sensory input, communication with the surrounding environment) nervous system. Both systems are closely interlaced and interact with each other.
Besides the nervous system, the endocrine system also participates in the regulation of body functions.
Directional and positional informations
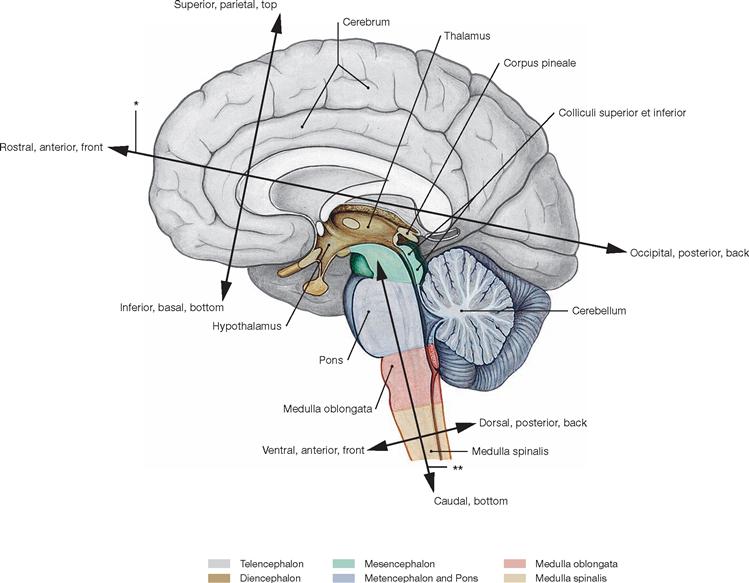
Fig. 12.2 Directional and positional informations concerning the central nervous system (CNS and spinal cord); median section.
During brain development, the neural tube bends and, thus, the longitudinal axis of the forebrain (Prosencephalon = Diencephalon and Telencephalon) tilts forward. Consequently, a unique nomenclature was generated as is shown in the figure. For example, parts formerly positioned dorsally, e.g. the Metencephalon, relocated to a parietal site, yet, their position is still referred to as dorsal.
The FOREL’s axis (*) refers to the topographic axis between the Telencephalon and Diencephalon, while the axis projecting through the centre of the brainstem (Truncus encephali) is called MEYNERT’s axis (**).
Meninges and blood supply
Arteries of the head
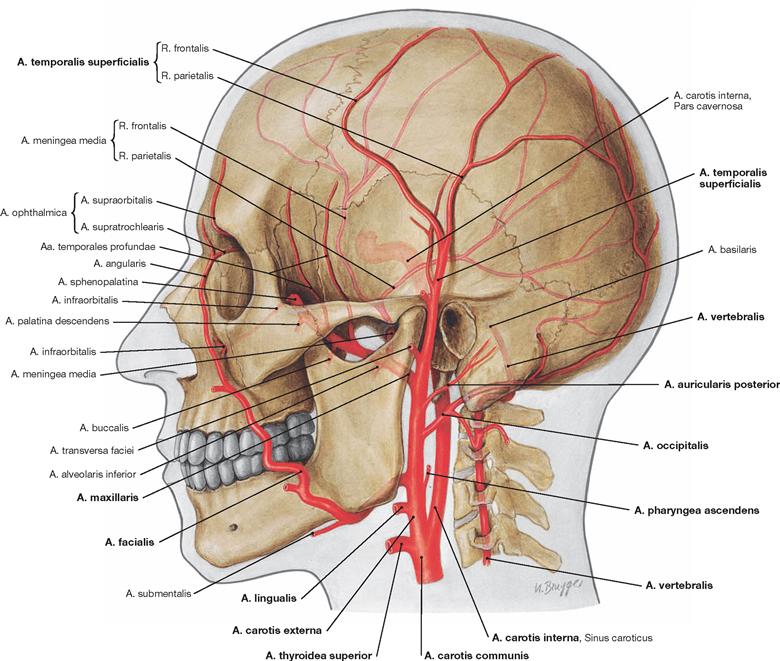
Fig. 12.3 External arteries of the head.
The A. carotis communis bifurcates (Bifurcatio carotidis) into the A. carotis externa and A. carotis interna at the level of the fourth cervical vertebra. The A. carotis externa provides the following branches: Aa. thyroidea superior, lingualis, facialis, pharyngea ascendens, occipitalis, auricularis posterior, maxillaris, and temporalis superficialis; the A. carotis interna ascends cranially without giving off branches (→ Fig. 12.15), passes through the skull base into the cranial cavity, and primarily supplies blood to the brain.
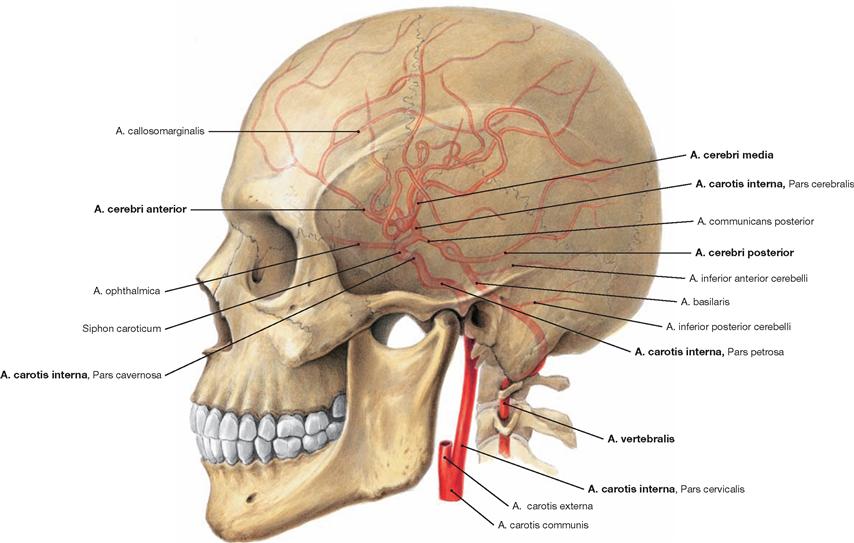
Fig. 12.4 Internal arteries of the head.
Four large arteries supply blood to the brain: the paired Aa. carotides internae and the paired Aa. vertebrales. These four blood vessels feed into the Circulus arteriosus cerebri (WILLISII, → Fig. 12.95) located at the base of the brain, which creates an anastomosis between the Aa. carotides internae and the Aa. vertebrales and releases paired branches of the cerebral arteries Aa. cerebri anterior, cerebri media, and cerebri posterior.
The anastomosing blood vessels within the Circulus arteriosus cerebri (WILLISII; circle of WILLIS) often are so narrow that they will not permit a sufficient exchange of blood.
At normal intracranial pressure, the ipsilateral A. carotis interna and the A. cerebri posterior usually supply blood to each cerebral hemisphere. In about 10% of cases, both Aa. cerebri anteriores branch off the same A. carotis interna on one side. Also, in 10% of cases the A. cerebri posterior derives from the A. communicans posterior, which, in turn, branches off the A. carotis interna.
Veins of the head
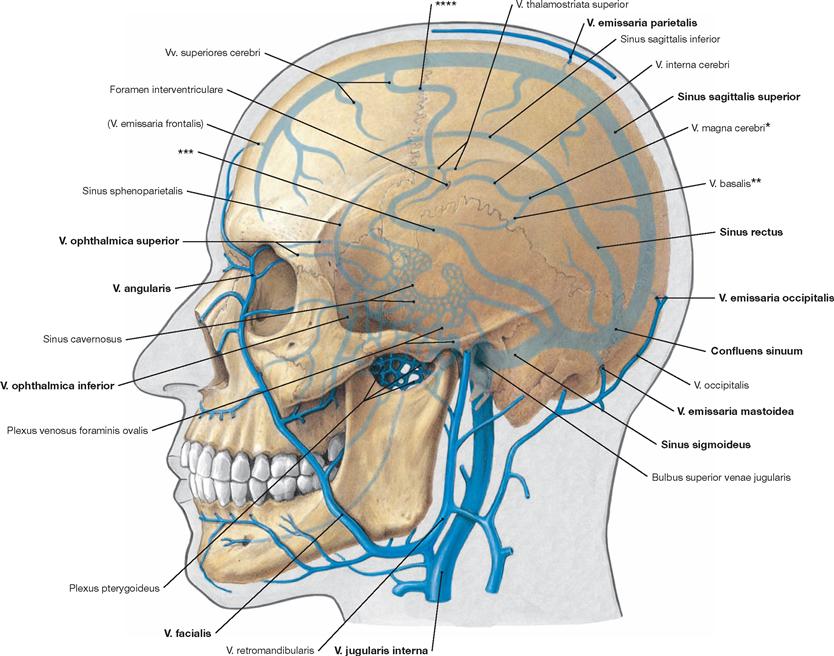
Fig. 12.6 Internal and external veins of the head.
The internal and external veins of the head communicate via by numerous anastomoses. This includes the Vv. emissariae and ophthalmicae as well as the Plexus venosi.
* vein of GALEN
** ROSENTHAL’s vein
*** vein of LABBÉ
**** TROLARD’s vein
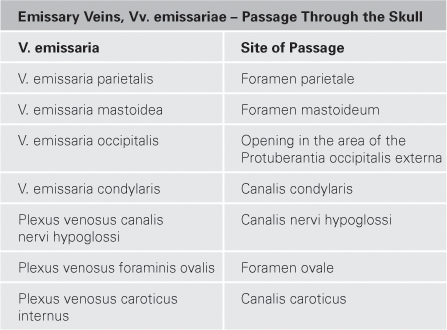
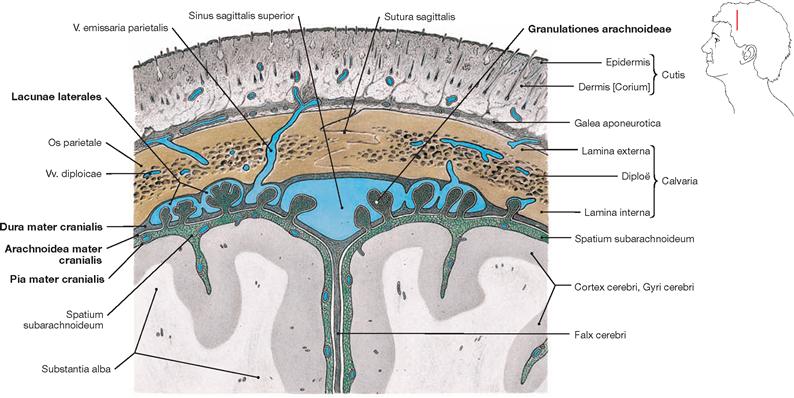
Fig. 12.7 Calvaria, Calvaria, meninges, Meninges, and dural venous sinuses, Sinus durae matris; frontal section.
In the adult, the cerebrospinal fluid is mainly reabsorbed into the venous system through the PACCHIONIAN granulations (Granulationes arachnoideae, arachnoid protrusions into the Sinus sagittalis superior or the Lacunae laterales) along the Sinus sagittalis superior. Additionally, reabsorption occurs through the lymphatic sheaths of small vessels of the cranial Pia mater and through the perineural sheaths of the cranial and the spinal nerves (not shown).
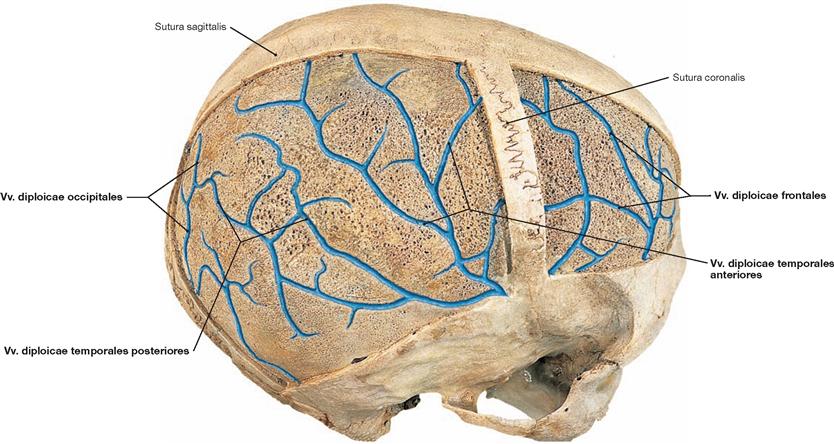
Fig. 12.8 Diploic canals, Canales diploici, and diploic veins, Vv. diploicae, of the calvaria, Calvaria, right side; superior oblique view; after the external layer of the compact bone has been removed from the Calvaria.
Passing through the diploic space are diploic canals, which harbour the Vv. diploicae. They communicate with the Vv. emissariae and the Sinus durae matris.
Blood supply of the Dura mater
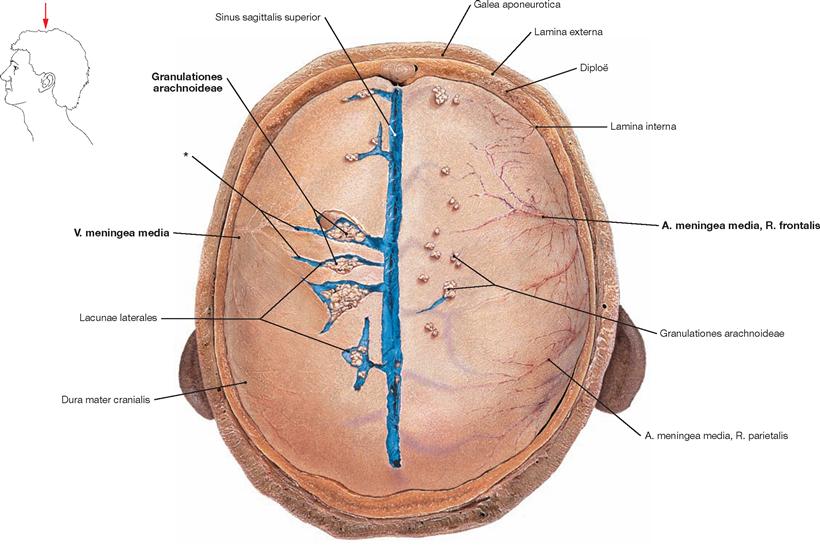
Fig. 12.9 Cranial dura mater, Dura mater cranialis, and Sinus sagittalis superior with some Lacunae laterales; superior view.
The Calvaria has been removed. On the left side of the body, the Dura mater cranialis has been opened along the Lacunae laterales and the confluence of the Vv. meningeae mediae into the lacunae is shown. The PACCHIONIAN granulations (Granulationes arachnoideae) reside within the lacunae. On the right side of the body, the Granulationes arachnoideae are visible as they rise above the level of the dura. The latter extend into the calvarian bone. Here they generate characteristic impressions and communicate with the Vv. diploicae.
* confluence of the Vv. meningeae mediae into the Lacunae laterales
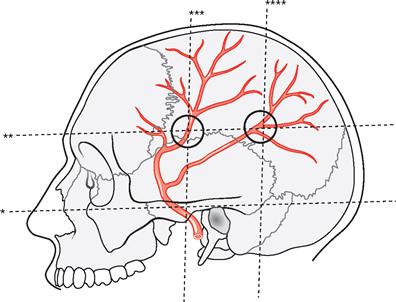
Fig. 12.10 Projection of the Rr. frontalis and parietalis of the A. meningea media onto the side of the skull. Circles mark the projections of the main branches of the A. meningea media.
The main branches of the A. meningea media are located where the upper horizontal line crosses the vertical line passing through the middle of the zygomatic arch and the vertical line passing through the posterior part of the Proc. mastoideus.
* clinical term: Linea horizontalis auriculoorbitalis (FRANKFORT horizontal line)
** clinical term: Linea horizontalis supraorbitalis
*** vertical line through the middle of the Arcus zygomaticus
**** vertical line through the posterior part of the Proc. mastoideus
Intracranial bleeding
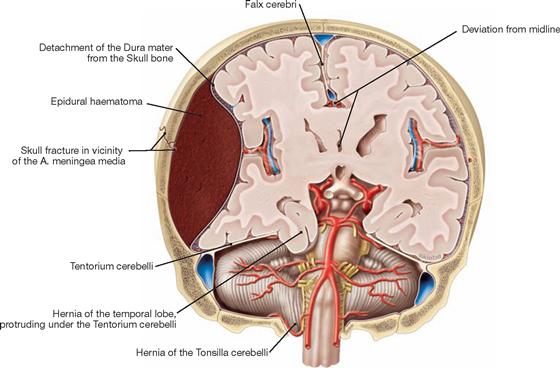
Fig. 12.11 Epidural haematoma; frontal section; frontal view.
An injury to the A. meningea media on the right side of the body has resulted in an arterial bleeding between the Calvaria and Dura mater. The pressure of the haematoma causes the midline to deviate sideways and results in parts of the temporal lobe being squeezed underneath the Tentorium cerebelli through the Incisura tentorii.
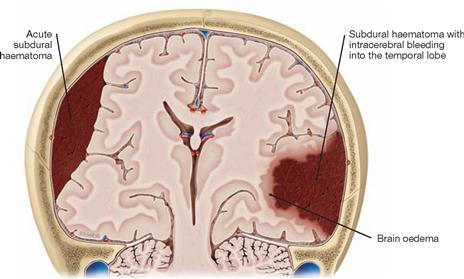
Fig. 12.12 Subdural haematoma and intracerebral bleeding; frontal section; frontal view.
Ruptures of bridging veins resulted in an acute subdural haematoma on the right side and a subdural haematoma with intracerebral bleeding into the temporal lobe on the left side.
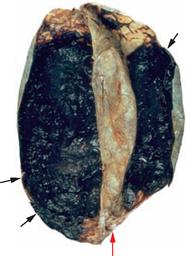
Fig. 12.13 Subdural haematoma; superior view at the brain. [5]
Large fresh bilateral traumatic subdural haematoma (arrows) on the inner aspect of the Dura mater (red arrow = Falx cerebri). The dura above the haematoma has been deflected.
Dural venous sinuses and parts of the A. carotis interna
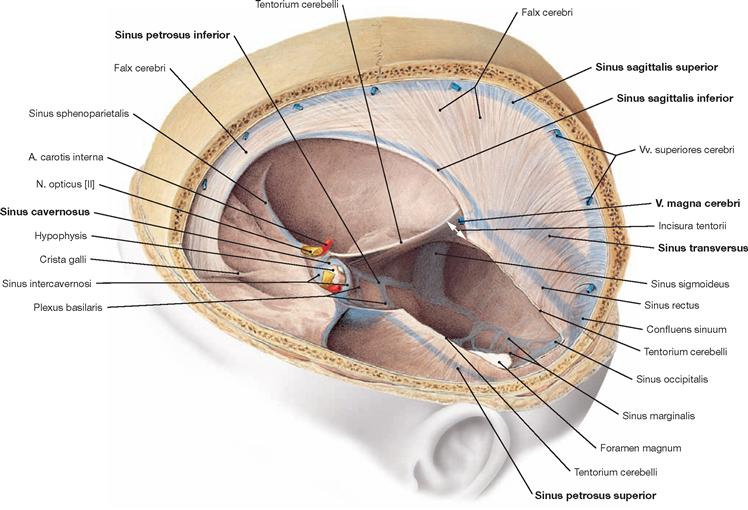
Fig. 12.14 Cranial dura mater, Dura mater cranialis, and dural venous sinus, Sinus durae matris; superior oblique view; Tentorium cerebelli partially removed.
The cranial dura mater lines the cranial cavity completely and tightly adheres to the skull bones. The Sinus durae matris course within the dura. The Falx cerebri protrudes in the sagittal plane in a sickle-like shape and stretches from the Crista galli to the ridge of the Tentorium cerebelli. This, in turn, spans the posterior cranial fossa and is attached along the Sinus transversus and the pyramidal edge. The margins of the Incisura tentorii envelope the midbrain (Mesencephalon) and taper off into the Plicae petroclinoideae which project to the Procc. clinoidei anterior and posterior. The Falx cerebri and the Tentorium cerebelli divide the cranial cavity into three spaces that are incompletely separated from one another, containing the two cerebral hemispheres and the Cerebellum.
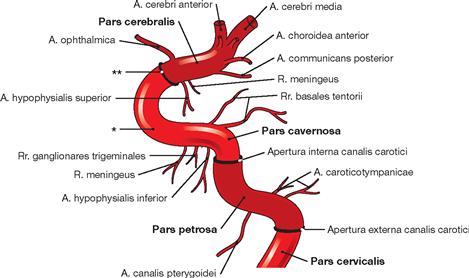
Fig. 12.15 Parts of the A. carotis interna. [8]
The A. carotis interna divides into four parts: Pars cervicalis, Pars petrosa, Pars cavernosa, and Pars cerebralis. Along its course through the base of the skull, the A. carotis interna passes through the Apertura externa canalis carotici, the Apertura interna canalis carotici, and through the Dura mater. In the Pars cervicalis small vessels branch off.
* carotid artery siphon
** passage through the Dura mater cranialis in the region of the Diaphragma sellae
Sinus cavernosus
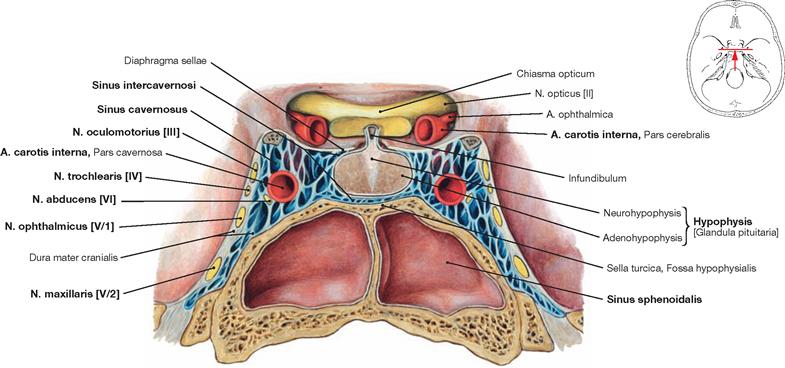
Fig. 12.16 Pituitary gland, Hypophysis [Glandula pituitaria], and Sinus cavernosus; frontal section; posterior view.
The pituitary gland is surrounded by the right and left Sinus cavernosus, which communicate via the Sinus intercavernosi. The A. carotis interna and lateral thereof the N. abducens [VI] run through the centre of the the Sinus cavernosus; the Nn. oculomotorius [III], trochlearis [IV], ophthalmicus [V/1], and maxillaris [V/2] are located in the wall of the Sinus cavernosus. The Sinus sphenoidalis is located beneath the Sella turcica which contains the pituitary gland.
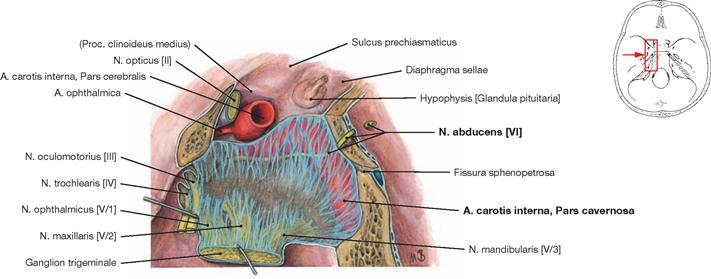
Fig. 12.17 Sinus cavernosus, left side; lateral view; the lateral part of the Dura mater contributing to the formation of the sinus wall has been removed; the Ganglion trigeminale was deflected laterally.
The course of the Pars cavernosa of the A. carotis interna and the passage of the N. abducens [VI] through the Sinus cavernosus is shown.
Dural venous sinuses
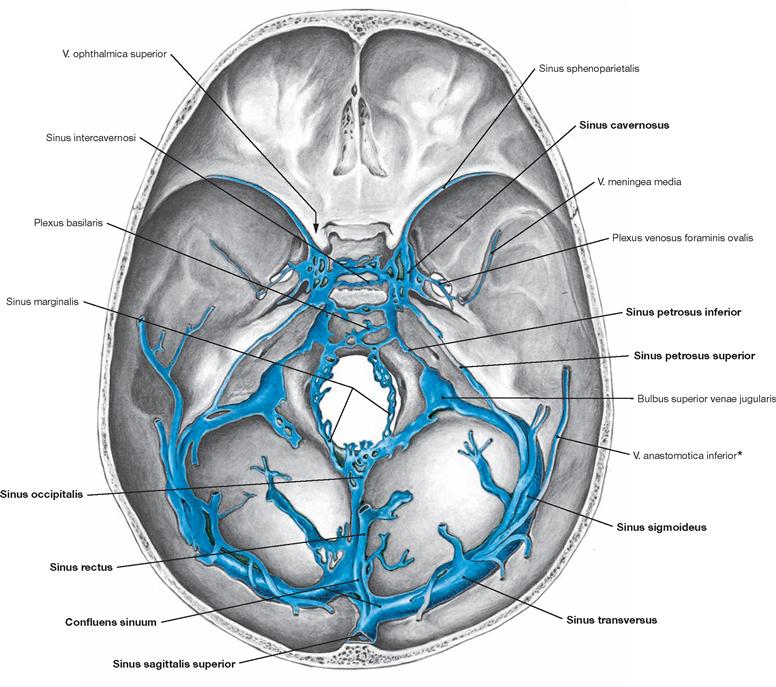
Fig. 12.18 Dural venous sinuses, Sinus durae matris; corrosion cast; superior view.
The dural venous sinuses are rigid venous canals devoid of valves that drain the venous blood from the brain via so-called bridging veins. The main drainage from within the skull occurs via the Sinus sigmoidei into the Vv. jugulares internae (initially forming the Bulbus superior venae jugularis). Additionally, the Vv. ophthalmicae superiores (in the orbit, not visible but indicated by the arrow, communication via the Fissura orbitalis superior) and the highly variable Vv. emissariae (→ Fig. 12.6) form a series of smaller, likewise valveless, venous connections between the intra- and extracranial regions.
The two Sinus cavernosi assume a central position by being situated in the middle cranial fossa to both sides of the Sella turcica. They communicate with each other through the Sinus intercavernosi and either directly or indirectly with most other sinuses and with the veins of the orbit and the infratemporal fossa.
Superficial vessels of the brain
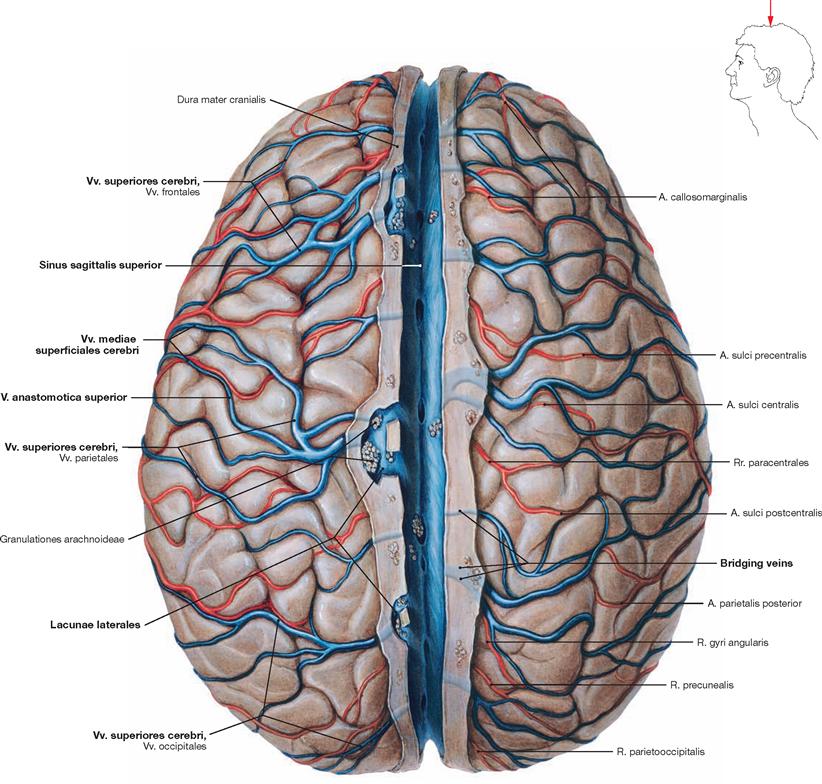
Fig. 12.19 Superficial arteries and veins of the brain; superior view; after removal of the cranial dura mater and sectioning of the Sinus sagittalis superior; cranial arachnoid mater removed.
The superficial arteries and veins supply the cerebral cortex and the subjacent basal ganglia. Superficial veins are the Vv. superiores cerebri, the V. media superficialis cerebri, and the Vv. inferiores cerebri (not shown). Anastomoses usually connect the larger veins (V. anastomotica superior [TROLARD’s vein, → Fig. 12.6] and V. anastomotica inferior [vein of LABBÉ, → Figs. 12.6 and 12.18]). The Vv. superiores cerebri drain into the Sinus sagittalis superior directly or, via dura materpiercing small bridging veins, connect with the Lacunae laterales which then drain into the Sinus sagittalis superior.
Leptomeninx
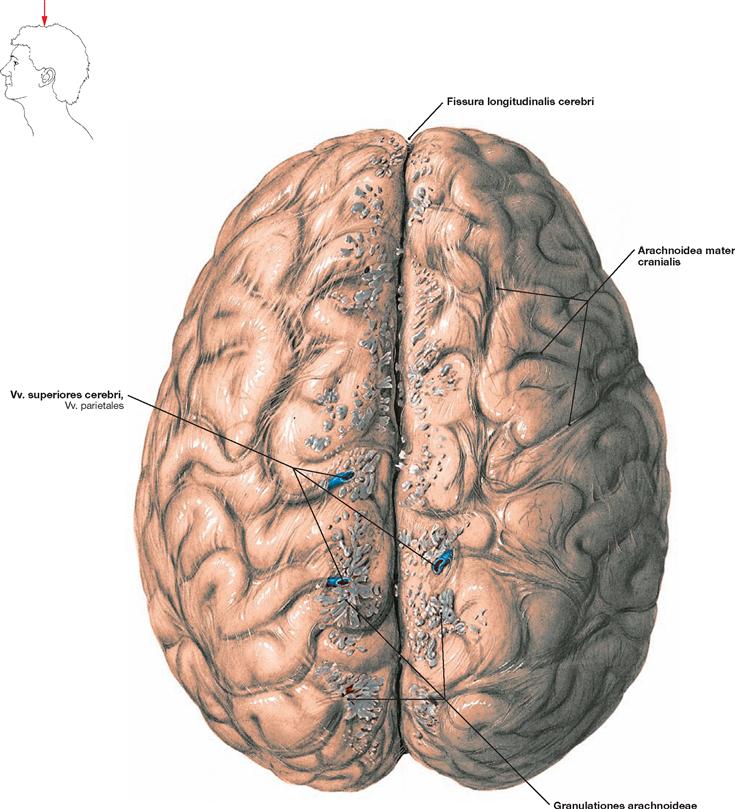
Fig. 12.20 Brain, Encephalon, with cranial arachnoid mater, Arachnoidea mater cranialis; superior view.
The cranial arachnoid mater covers the brain. The Falx cerebri (a duplication of the Dura mater cranialis), normally residing within the Fissura longitudinalis cerebri, divides the two cerebral hemispheres into a right and a left half and extends down to the callosal commissure (Corpus callosum, not visible). To both sides of the Fissura longitudinalis cerebri, multiple PACCHIONIAN granulations (Granulationes arachnoideae) are visible. These extend above the level of the arachnoid mater and assist in the reabsorption of cerebrospinal fluid. In addition, a number of cerebral veins (Vv. superiores cerebri, Vv. parietales) are visible, which were severed from the bridging veins (small veins piercing the Dura mater cranialis on their way to the Sinus sagittalis superior) during the removal of the brain from the skull.
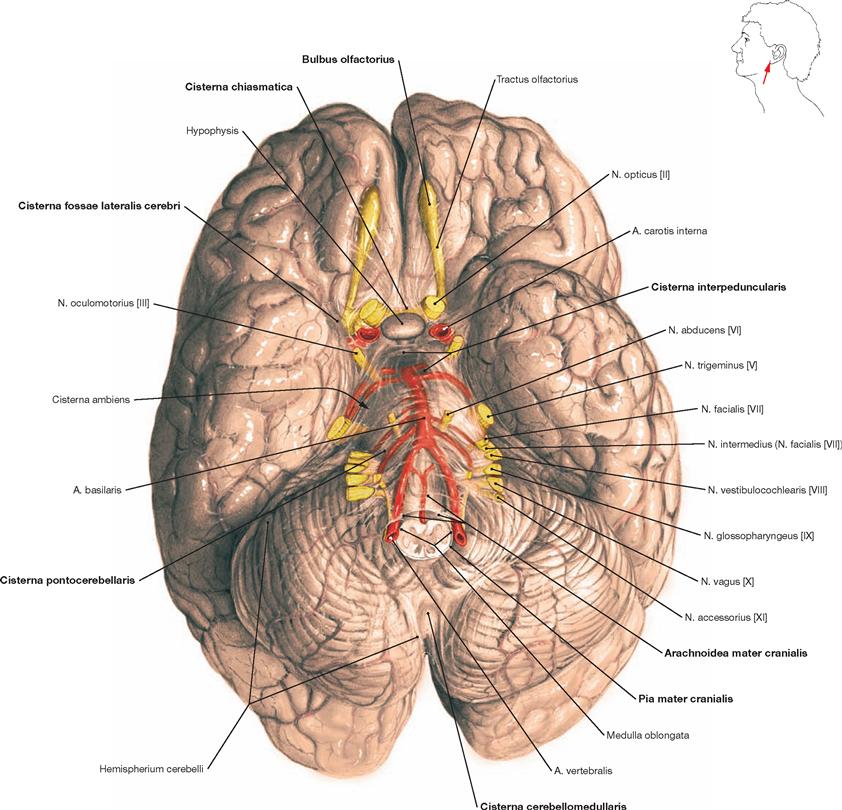
Fig. 12.21 Brain, Encephalon, with cranial arachnoid mater, Arachnoidea mater cranialis; inferior view.
Removal of the brain from the skull was accomplished by cutting the brainstem at the level of the Medulla oblongata and severing the Aa. vertebrales, the Aa. carotides, and the twelve pairs of cranial nerves (the Fila olfactoria of the first cranial nerve are teared off at the Bulbus olfactorius). The cranial arachnoid mater covers the brain. Nerves and vessels run in the Spatium subarachnoidale. The caudal part of the frontal, temporal, and occipital lobes and the Cerebellum are shown. The Circulus arteriosus cerebri (WILLISII) is preserved but only partially visible. Further, the location of the Cisternae cerebri is demonstrated.
Development of the brain
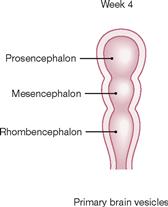
Fig. 12.22 Development of the brain: primary brain vesicles; schematic frontal section. [21]
The neural tube openings are closed in week 4. The rostral end begins to enlarge and forms the three successive primary brain vesicles: forebrain (Prosencephalon), midbrain (Mesencephalon), and hindbrain (Rhombencephalon).
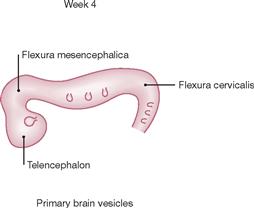
Fig. 12.23 Development of the brain: primary brain vesicles; schematic lateral view. [21]
During week 4, the midbrain flexure (Flexura mesencephalica) forms between the forebrain (Prosencephalon) and the midbrain (Mesencephalon). The cervical flexure (Flexura cervicalis) develops between the hindbrain (Rhombencephalon) and the spinal cord.
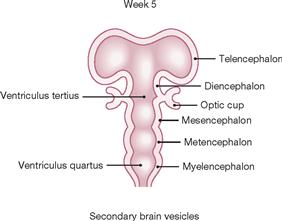
Fig. 12.24 Development of the brain: secondary brain vesicles; schematic frontal section. [21]
In week 5, parts of the Prosencephalon located on the right and left side of the midline enlarge to form the Telencephalon which generates the cerebral hemispheres. In addition, the Diencephalon derives from the Prosencephalon. The third ventricle evolves between the Diencephalon and Mesencephalon. Forming beneath the Mesencephalon is the Metencephalon with its two main components, the Pons and the Cerebellum. The Myelencephalon follows caudally; it includes the fourth ventricle and the Medulla oblongata and transitions into the spinal cord.
The three primary brain vesicles gave rise to six secondary brain vesicles (the paired vesicles of the Telencephalon and the Di-, Mes-, Met-, and Myelencephalon).
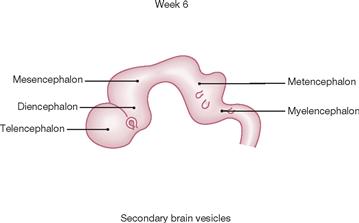
Fig. 12.25 Development of the brain: secondary brain vesicles; schematic lateral view. [21]
In week 6, the Telencephalon, Diencephalon, Mesencephalon, Metencephalon, and Myelencephalon are clearly delineated. The optic cups become visible between the Telencephalon and the Diencephalon. The development of the Cerebellum starts as a lateral extension of the Rhombencephalon. The developing Cerebellum is visible at the dorsal aspect of the Metencephalon.
Brain
Development of the brain
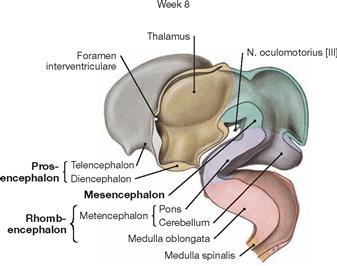
Fig. 12.26 Development of the brain; median section.
In week 8 the individual brain structures are clearly distinguishable. Telencephalon and Diencephalon derived from the Prosencephalon. The Thalamus in the Diencephalon and the N. oculomotorius [III] exiting the Mesencephalon become visible. The Rhombencephalon has differentiated into the Metencephalon and the Medulla oblongata (Myeloencephalon). Pons and Cerebellum derive from the Metencephalon. The Medulla oblongata is followed by the Medulla spinalis.
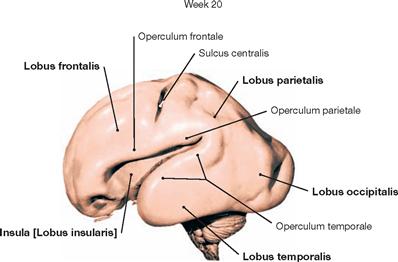
Fig. 12.27 Development of the brain; view from the left side.
At week 20 (with a foetal crown-rump length of 20 cm), the growth of the Telencephalon has progressed significantly. It is already composed of the Lobi frontalis, parietalis, occipitalis and temporalis. However, the Lobus insularis is not yet fully covered by the Lobi frontalis, parietalis, and temporalis. Of all the structures of the brainstem, only parts of the Pons, the Cerebellum, and the Medulla oblongata are still visible.
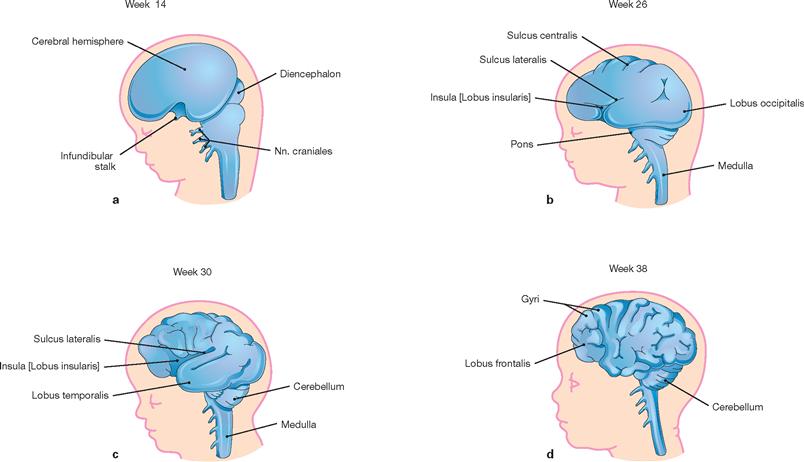
Figs. 12.28a to d Development of the left cerebral hemisphere, diencephalon and brainstem; schematic drawings; lateral view. [20]
At week 14, the surface of the Telencephalon is still smooth. Thereafter, the cerebral cortex undergoes successive stages in the development of grooves (sulci) and convolutions (gyri). In addition, the formation of the Insula becomes overlapped by the Lobi frontalis, parietalis, and temporalis.
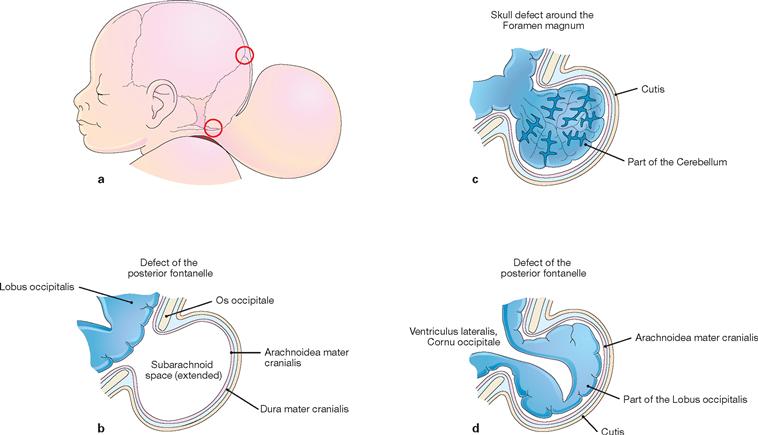
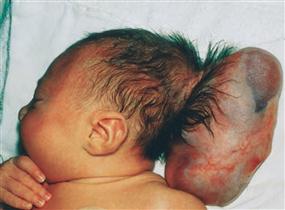
Figs. 12.29a to d Cranium bifidum formation and various types of herniation of the brain and/or meninges, schematic presentation. [20]
a. Head of a newborn with an extensive herniation in the occipital region. The upper red circle marks the defect of the small fontanelle, the lower red circle indicates the defect in the area of the Foramen magnum.
b. Meningocele: the herinal sac is formed by skin and meninges and is filled with cerebrospinal fluid.
c. Meningoencephalocele: the herinal sac comprises prolapsed parts of the Cerebellum and is covered by meninges and skin.
d. Meningohydroencephalocele: the herinal sac consists of prolapsed parts of the Lobus occipitalis and of the posterior horn of the lateral ventricle.
Organisation of the brain
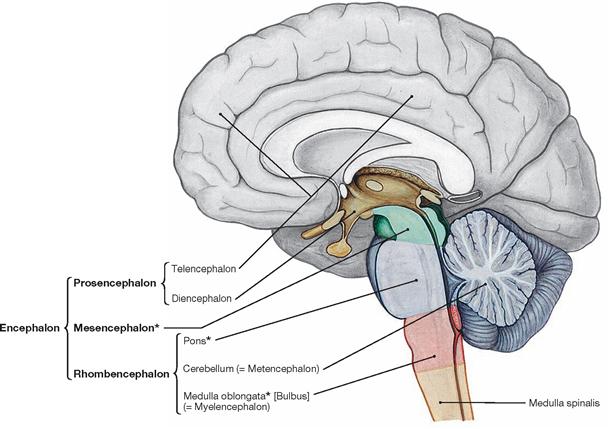
Fig. 12.31 Organisation of the central nervous system; median section; schematic drawing. The parts of the brain that constitute the brainstem, Truncus encephali, are marked by a star (*).
Based on the development of the brain from three primary brain vesicles (forebrain [Prosencephalon], midbrain [Mesencephalon], and hindbrain [Rhombencephalon]), the brain (Encephalon) divides into Telencephalon, Diencephalon, Mesencephalon, Pons, Cerebellum (Metencephalon), and Medulla oblongata.
Telencephalon, cortex
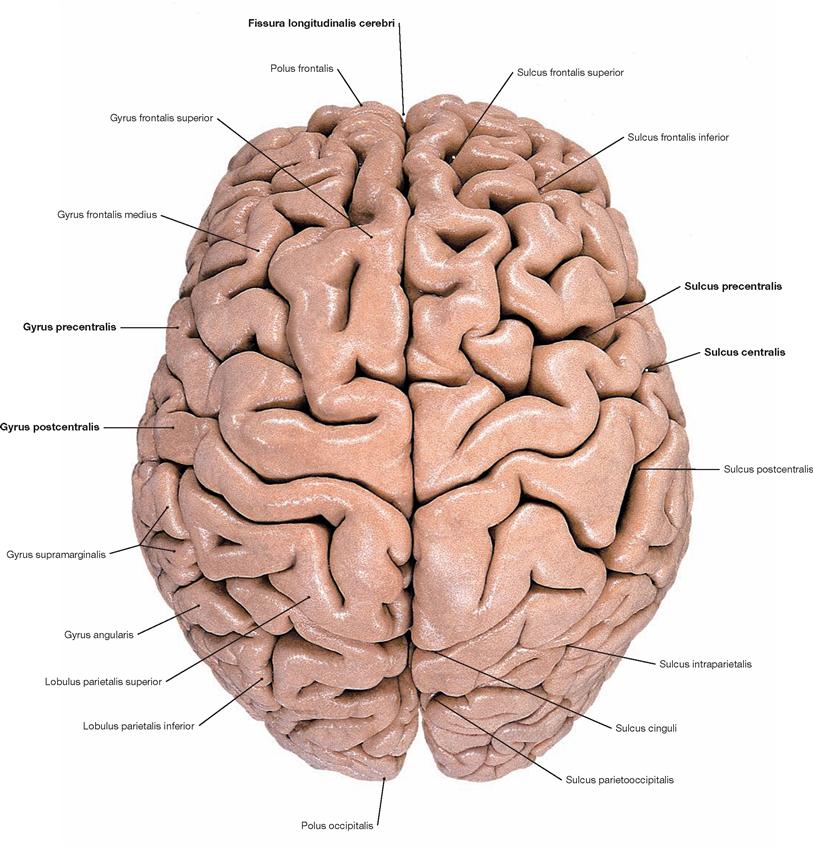
Fig. 12.32 Cerebrum, Cerebrum; superior view, after removal of the leptomeninx.
The Cerebrum constitutes the major part of the brain. It is composed of two hemispheres which are separated by the Fissura longitudinalis cerebri. During early stages of development, the surface of the Cerebrum is smooth. Strong growth results in the formation of quite variable grooves (Sulci) and convolutions (Gyri). This folding dramatically increases the cerebral surface area and, as a result, two-thirds of the cerebral surface area are invisible to the eye.
Telencephalon, organisation of the lobes
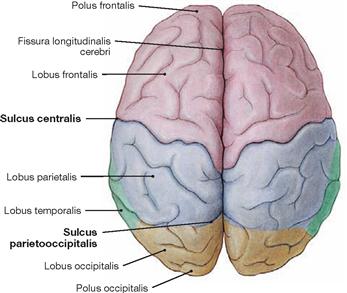
Fig. 12.33 Lobes of the Cerebrum, Lobi cerebri; superior view.
Towards the end of the 8th. month of foetal development, the primary grooves become visible (→ Table). These are regularly present in all humans. The view from the top shows the Sulcus centralis and the Sulcus parietooccipitalis.
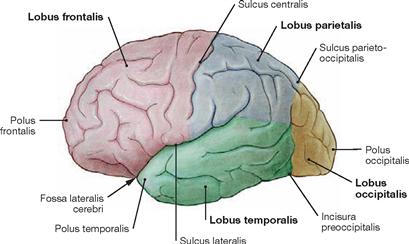
Fig. 12.34 Lobes of the Cerebrum, Lobi cerebri; view from the left side. Each cerebral hemisphere divides into four lobes:
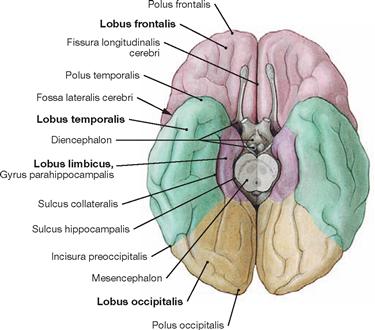
Fig. 12.35 Lobes of the Cerebrum, Lobi cerebri; inferior view.
In addition to the four lobes of the Cerebrum listed in the legend to → Figure 12.34, the Lobus limbicus (composed mainly of the Gyrus cinguli and the Gyrus parahippocampalis with the Uncus) and the Lobus insularis (Insula, not visible, since covered by the opercula of the frontal, parietal, and temporal lobes) can be distinguished.
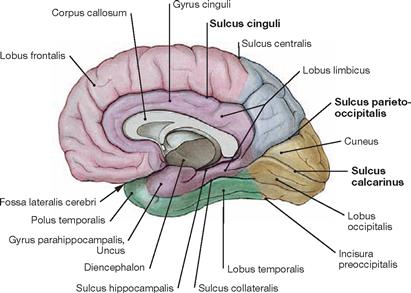
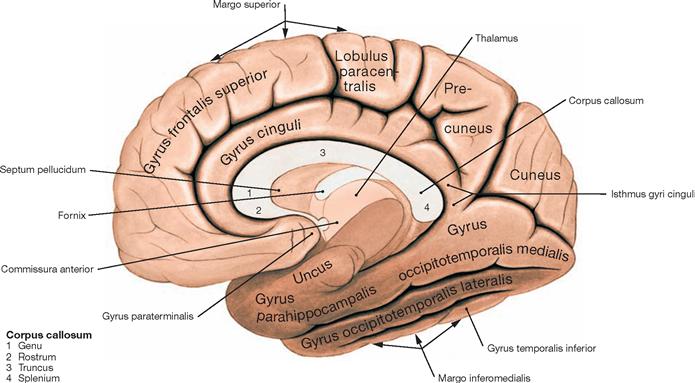
Fig. 12.36 Lobes of the Cerebrum, Lobi cerebri; medial view.
Secondary and tertiary grooves in the Telencephalon show individual variability. In many places, the margins drawn between the individual lobes are arbitrary (e.g. Incisura preoccipitalis).
| Primary Grooves of the Cerebral Cortex | |
| Sulcus | Location/Projection |
| Sulcus centralis | extends between the frontal and parietal lobes; separates the (motor) Gyrus precentralis from the (sensory) Gyrus postcentralis |
| Sulcus lateralis | separates the frontal, parietal, and temporal lobes; deep within lie the Fossa lateralis and the insula |
| Sulcus parietooccipitalis | extends from the upper rim at the medial surface of the hemisphere to the Sulcus calcarinus; separates the parietal and occipital lobes |
| Sulcus calcarinus | like the Sulcus parietooccipitalis it extends at the medial surface of the hemisphere and both enclose the Cuneus |
| Sulcus cinguli | separates the Gyrus cinguli (Lobus limbicus) from the frontal and parietal lobes |
Telencephalon, cortex
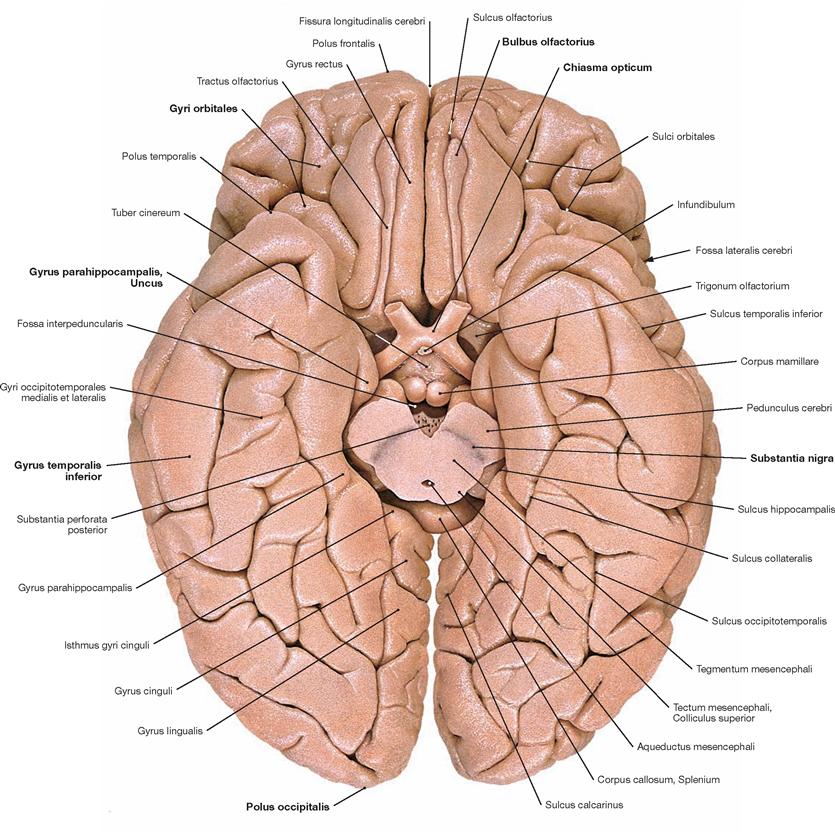
Fig. 12.37 Gyri, Gyri, and grooves, Sulci, of the cerebral hemispheres; inferior view; the midbrain has been sectioned.
The Telencephalon occupies the majority of the cerebral base. Here, the Bulbi olfactorii and Tractus olfactorii overlying the Gyri orbitales are located. In addition, the Chiasma opticum, the Gyrus parahippocampalis in the Lobus temporalis with its characteristic anterior bend, the Uncus, the Gyri temporales, and the Polus occipitalis are also visible. The dark coloured Substantia nigra is clearly visible in the Mesencephalon.
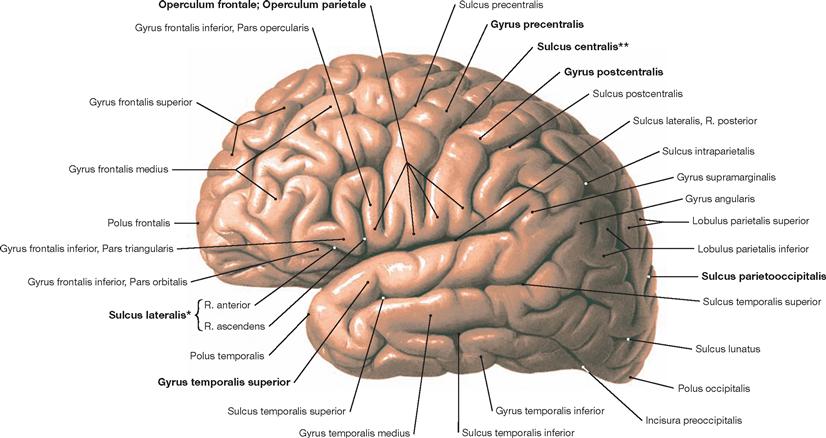
Fig. 12.38 Gyri, Gyri, and grooves, Sulci, of the cerebral hemispheres; view from the left side.
Although the indicated Gyri and Sulci are present in each human brain (e.g. Sulcus centralis, Sulcus lateralis, or Gyrus temporalis superior), no two brains or even two hemispheres of the same brain display an identical pattern of Gyri and Sulci. Similar to a fingerprint, the cerebral cortex is unique.
* SYLVIAN fissure
** fissure of ROLANDO or central fissure
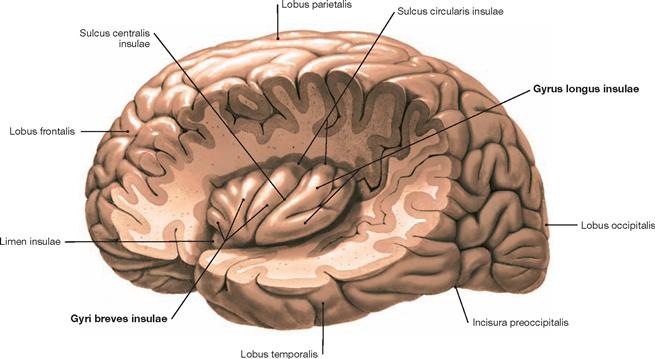
Fig. 12.39 Gyri, Gyri, and grooves, Sulci, of the cerebral hemispheres; view from the left side; after removal of the parts of the frontal, parietal, and temporal lobes covering the insula.
The cortical regions of the frontal, parietal and temporal lobes that surround the Sulcus lateralis are called the opercula and have been removed to demonstrate the Insula (→ Fig. 12.38). In the Region of the Insula olfactory, gustatory, and visceral informations are processed. In general the Insula is considered a lobe of its own.
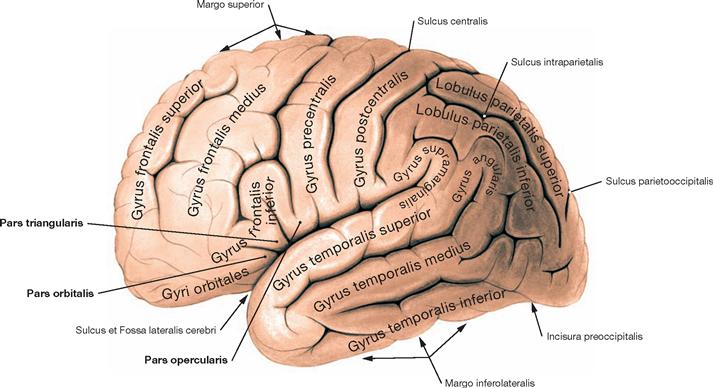
Fig. 12.40 Gyri, Gyri, of the cerebral hemispheres; view from the left side.
The Gyrus frontalis divides into a Pars orbitalis, a Pars triangularis, and a Pars opercularis.
Telencephalon, cortical areas
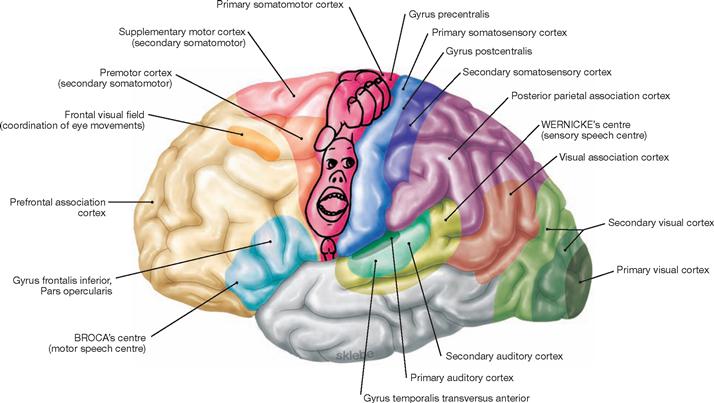
Fig. 12.42 Functional cortical areas of the cerebral hemispheres; view from the left side.
Higher cortical functions, like speech, require the cooperation of multiple different cortical areas. One can distinguish primary cortical areas (e.g. Gyrus precentralis, primary somatomotor cortex) from secondary and association areas of the cortex (e.g. premotor cortex, supplementary motor cortex). Primary and secondary cortical areas process specific sensory informations (e.g. perception and interpretation of visual impulses by the visual cortex in the occipital lobe). Cortical association areas (e.g. prefrontal association cortex) occupy most of the cortex and serve to integrate different and complex information patterns.
The outline of the human-like character (homunculus) reflects the somatotopic structure in the primary somatomotor cortex. Primary and secondary auditory cortices and the WERNICKE‘s centre extend along the upper rim and inner surface of the temporal lobe.![]()
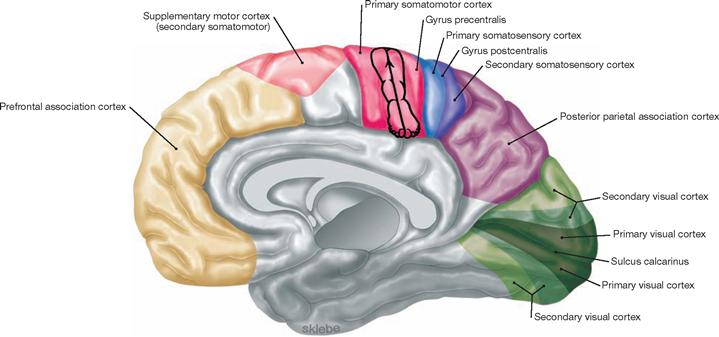
Fig. 12.43 Functional cortical areas of the cerebral hemispheres; medial view.
The schematic outline of the homunculus illustrated in this figure and in → Figure 12.42 roughly reflects the somatotopic organisation.![]()
Telencephalon, Fornix
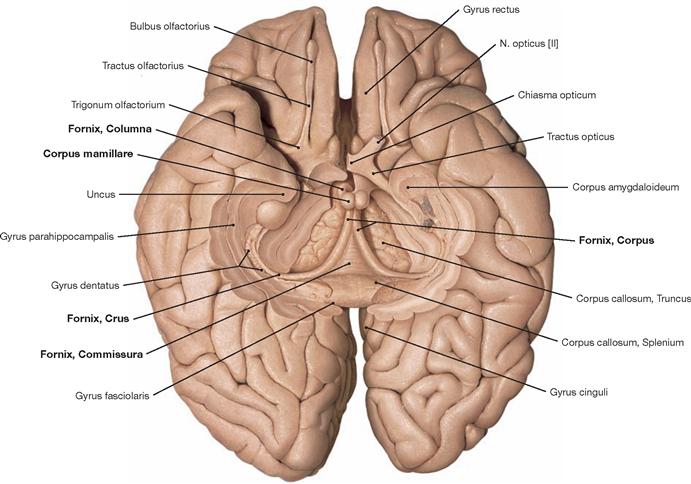
Fig. 12.44 Fornix, Fornix; inferior view; after removal of the basal parts of the brain.
The Fornix is a paired structure composed of the crus, commissure, body and column. It originates from the Hippocampus and Subiculum in the temporal lobe and arches above the third ventricle towards the Corpus mamillare. The fornices from both sides merge (Commissura fornicis) before they reach the mamillary bodies (Corpora mamillaria). At the commissure, an exchange of fibres occurs.
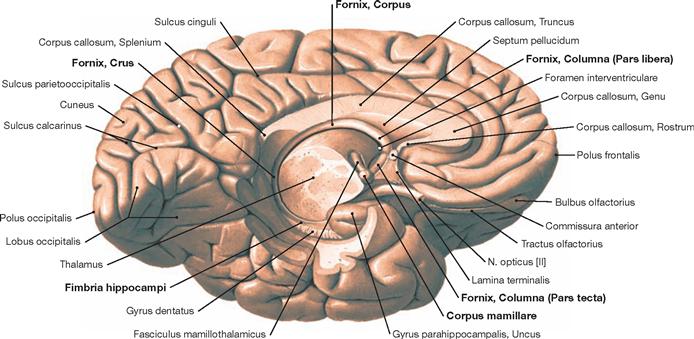
Fig. 12.45 Fornix, Fornix; inferior medial view;
The Fornix is an important tract of the limbic system. Fibre connections exist to the anterior hypothalamic nuclei, the Thalamus, and the Habenulae. The figure shows the topographic relationships of the Fornix.
Telencephalon, Fornix and anterior commissure
Fig. 12.46 Anterior commissure, Commissura anterior, and brainstem, Truncus encephali; inferior view; after partial removal of the basal parts of the Cerebrum.
The Commissura anterior is composed of commissural fibres. Located in the anterior wall of the third ventricle, it represents the commissural system of the paleocortex. The rostral part of the Commissura anterior is small and connects the two Tractus olfactorii with the olfactory cortex of both hemispheres. The much more developed dorsal part facilitates the exchange of fibres between the rostral parts of the temporal lobes (particularly the cortex and Corpora amygdaloidea).
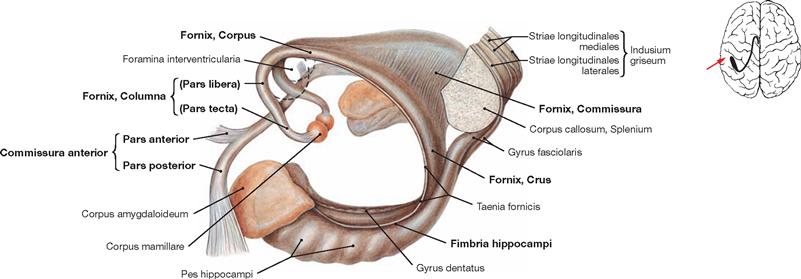
Fig. 12.47 Anterior commissure, Commissura anterior, fornix, Fornix, and Hippocampus formation, Indusium griseum; view from the left side.
All structures shown here are part of the limbic system, a functional concept with input from the Telencephalon, Diencephalon, and Mesencephalon. Relevant structures are the Hippocampi, the Corpora amygdaloidea, the Gyri cinguli, and the Nuclei septales. The limbic system regulates numerous functions, such as impulse, learning, memory, emotions, but also the regulation of food intake, digestion, and reproduction by the autonomic nervous system.
The Commissura anterior is a fibre system (commissural fibres) composed of a Pars anterior and Pars posterior. The Pars anterior connects the Tractus olfactorii and the olfactory cortices of both sides. The Pars posterior connects the rostral parts of the temporal lobes (particularly cortex and Corpora amygdaloidea). The Corpus amygdaloideum connects with the Hippocampus.
The Hippocampus displays the Digitationes hippocampi of the Pes hippocampi and the Fimbria hippocampi which transition into the crus of the Fornix. An exchange of fibres occurs in the region of the column. In its rostral part, the Columnae of the Fornix continue as Pars libera and Pars tecta and end in the Corpora mamillaria. The Pars tecta connects to the Corpus mamillare.
Telencephalon, basal ganglia
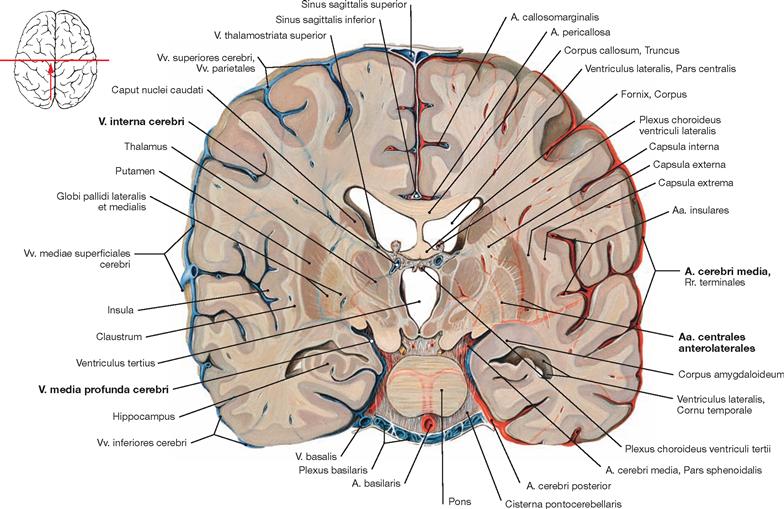
Fig. 12.48 Blood supply of the basal ganglia; frontal section; posterior view; the arteries are shown on the right side and the veins on the left side.
The basal ganglia are supplied by the branches of the A. cerebri media. On its way to the Fossa lateralis, the A. cerebri media provides the Aa. centrales anterolaterales (Aa. thalamostriatae anterolaterales, Aa. lenticulostriatae) for the basal ganglia and the Capsula interna. The venous blood is drained by the V. media profunda cerebri and the V. interna cerebri.
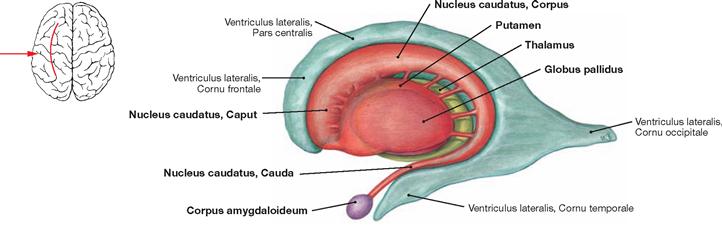
Fig. 12.49 Basal ganglia and Thalamus; view from the left side.
This figure depicts the topographic relationships between the Ventriculus lateralis, Nucleus caudatus, Corpus amygdaloideum, Putamen, Globus pallidus, and Thalamus. Many of these nuclei are collectively named basal ganglia. This includes the illustrated striatum (Nucleus caudatus und Putamen) and the Globus pallidus as well as the Nucleus subthalamicus and the Substantia nigra in the Mesencephalon (both not visible).
The basal ganglia are an integral part of different cortical feedback loops (cortex – basal ganglia – Thalamus – cortex) and participate in the motor cortical output. Their main function is to modulate the motor activity (strength, direction, range of movement). Impulses reaching the basal ganglia are modulated either to directly enhance onto indirectly inhibit motor activity.
Diencephalon
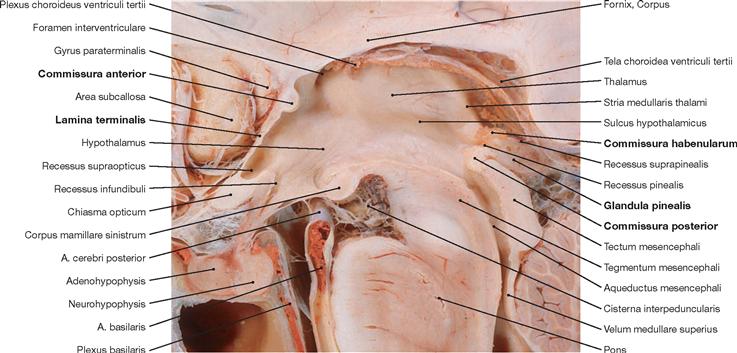
Fig. 12.50 Third ventricle, Ventriculus tertius, and diencephalon, Diencephalon; median section.
Phylogenetically, the Diencephalon derives from the Prosencephalon and is located between the Telencephalon and the Mesencephalon. The Diencephalon surrounds the third ventricle and divides into the Epithalamus, Thalamus (dorsalis), Hypothalamus, and the Subthalamus (Thalamus ventralis). The Commissura anterior and the Lamina terminalis represent the rostral margin of the Diencephalon (Commissura anterior to the Chiasma opticum). The Commissura posterior, the Commissura habenularum, and the pineal gland (Glandula pinealis) constitute the inferior margin of the Diencephalon.
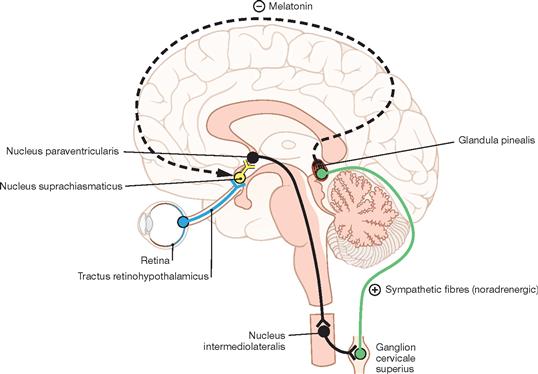
Fig. 12.51 Neural circuitry involved in the regulation of the pineal gland, Glandula pinealis; schematic median section. (according to [2])
The Epithalamus is composed of the Striae medullares thalami, the Habenulae, the Nuclei habenulares, the Commissura habenularum, the Commissura posterior (epithalamica), the Area pretectalis, and the Glandula pinealis. The production of melatonin in pinealocytes of the Glandula pinealis is light dependent. Melatonin is an important regulator of circadian rhythms and does so by affecting the function of other endocrine organs. In addition, melatonin acts on the Nucleus suprachiasmaticus and via a feedback loop modulates its role in synchronising endogenous with environmental rhythms.
The circuitry initiates at the photoreceptors of the retina which send signals to the Nucleus suprachiasmaticus in the Hypothalamus (Tractus retinohypothalamicus). This information is conveyed to the hypothalamic Nucleus paraventricularis, from here projects to the Ganglion cervicale superius of the sympathetic system, and then reaches the pinealocytes of the Glandula pinealis. The production of melatonin increases in the dark.
Diencephalon, Thalamus
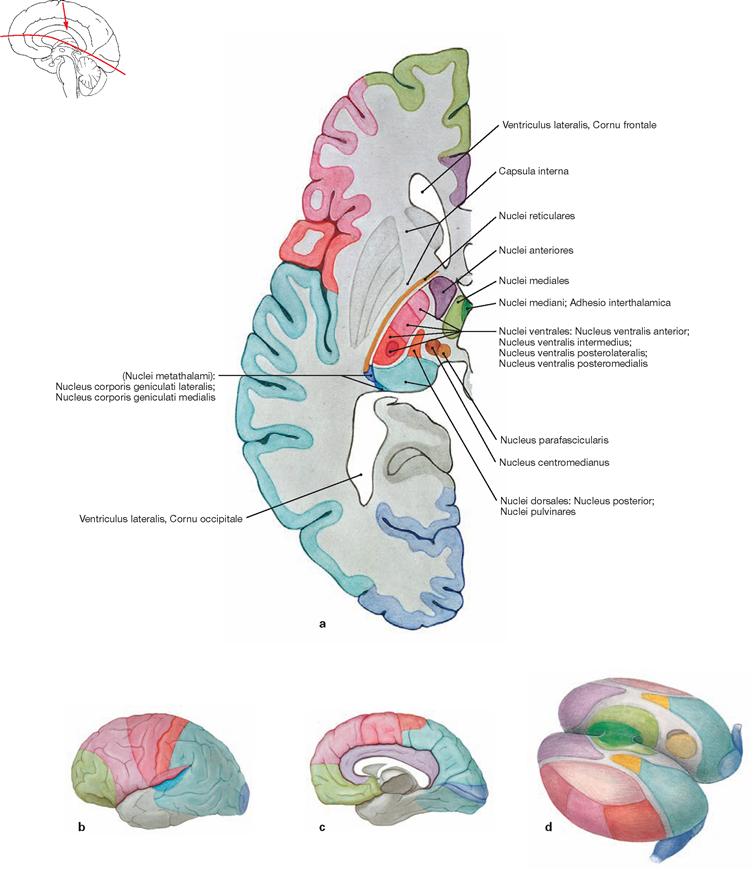
Figs. 12.52a to d Nuclei and cortical projections of the Thalamus.
Corresponding nuclei and cortical projections are indicated by the same colour.
a. horizontal section through the left cerebral hemisphere
b. left cerebral hemisphere from the left (lateral) side
The Thalamus is regarded as the “gateway to consciousness”. All sensory input to the body is synapsed and integrated in the Thalamus (with the exception of olfactory sensations) prior to this information reaching the cortex. In addition, the Thalamus participates in the modulation of autonomic and motor activities. The Thalamus is composed of specific and nonspecific groups of nuclei (more than 100 nuclear regions, including the Corpora geniculata laterale and mediale [see visual and auditory pathways; → Fig. 12.59]). Specific thalamic nuclei (Palliothalamus) connect with defined cortical regions (primary cortical projection and association fields); nonspecific thalamic nuclei (Truncothalamus) project broadly into the brainstem and diffusely into some cortical areas.![]()
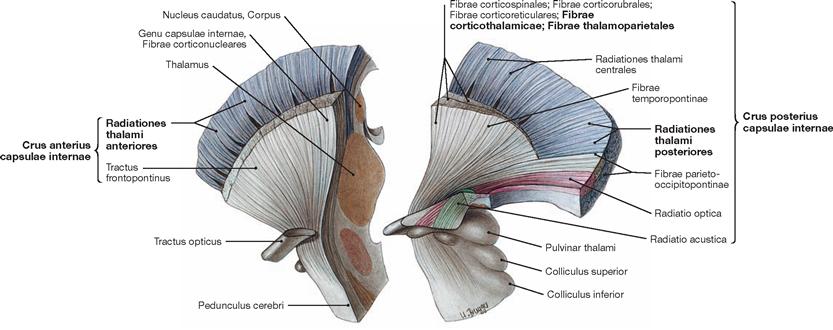
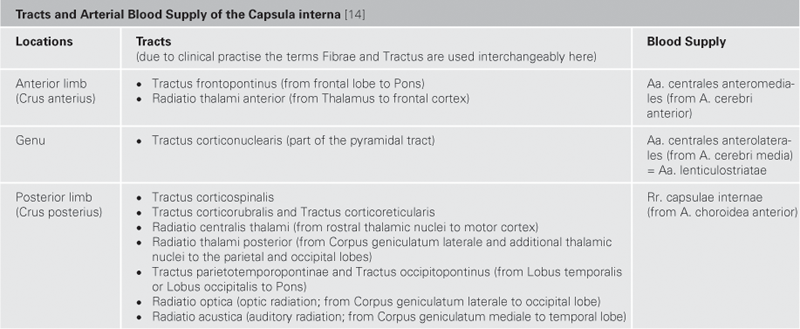
Fig. 12.53 Thalamic radiation, Radiationes thalami, and internal capsule, Capsula interna; view from the left side; divided into two parts by a frontal section.
Thalamic nuclei mainly project into the cortex. Their projections contribute to the formation of the Crus anterius and the Crus posterius of the Capsula interna. The Radiationes thalami anteriores and posteriores are part of these projections as are the Fibrae corticothalamicae and the Fibrae thalamoparietales.
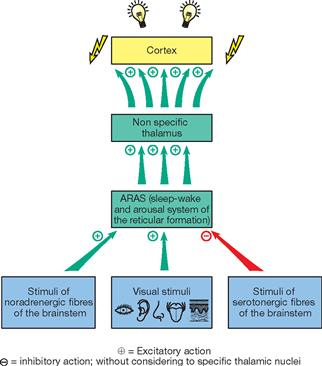
Fig. 12.54 Ascending reticular activation system (ARAS); specific thalamic nuclei have been excluded. [23]
The Nuclei mediani and the intralaminar group of nuclei, of which the Nucleus centromedianus is the largest nucleus, belong to the group of nonspecific thalamic nuclei. Corresponding to the broad and diffuse connections with the cortex, the intralaminar group of nuclei is involved in the nonspecific and general excitation of the cortex. This puts the body in a state of alertness and readiness. This state of arousal is controlled by signals from the ARAS of the Formatio reticularis reaching the intralaminar thalamic nuclei which then activate the entire cortex via the nonspecific connections.
Diencephalon, hypothalamus and pituitary gland
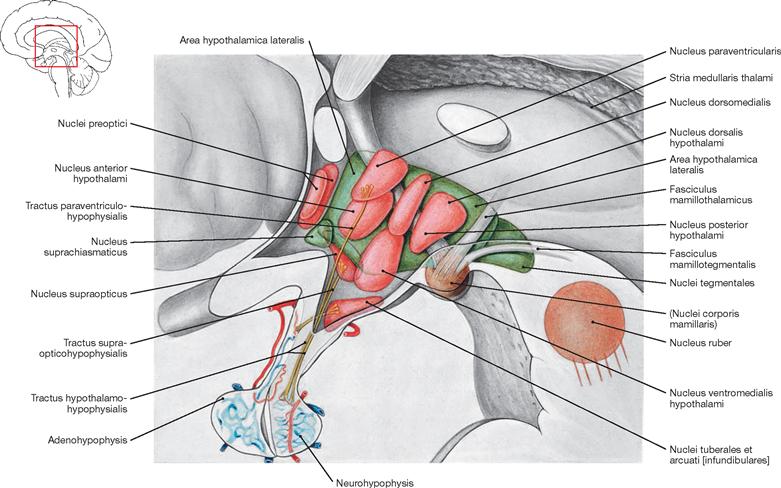
Fig. 12.55 Hypothalamus; medial view; overview, nuclei illustrated translucently.
Forming the floor of the diencephalon, the hypothalamus is the supervisory regulatory centre of the autonomic nervous system. The hypothalamus is composed of multiple groups of nuclei, which, according to their location, divide into the anterior, middle, and posterior groups of hypothalamic nuclei:
• The anterior group of hypothalamic nuclei comprises the Nucleus suprachiasmaticus (central pacemaker of the circadian rhythm, sleep-wake cycle, body temperature, blood pressure), the Nuclei paraventricularis and supraopticus (production of antidiuretic hormone [ADH] and oxytocin and axonal transport [Tractus hypothalamohypophysialis] to the Neurohypophysis), and the Nuclei preoptici (participation in the regulation of blood pressure, body temperature, sexual behaviour, menstrual cycle, gonadotropin).
• The middle group of hypothalamic nuclei comprises the Nuclei tuberales, dorsomedialis, ventromedialis, and arcuatus [infundibularis = semilunaris] (production and secretion of releasing and release-inhibiting hormones, participation in the regulation of water and food intake).
• The posterior group of hypothalamic nuclei comprises the Nuclei corporis mamillaris in the Corpora mamillaria which are integrated into the limbic system by receiving afferent fibres from the Fornix and projecting efferent fibres to the Thalamus (Fasciculus mamillothalamicus). They modulate sexual functions and play an important role in activities related to memory and emotions. These nuclei connect to the Tegmentum mesencephali via the Fasciculus mamillotegmentalis.
In the caudal aspect of the hypothalamus, the Infundibulum (pituitary stalk) connects the pituitary gland to the rest of the hypothalamus. The pituitary gland divides into the anterior (Adenohypophysis) and posterior (Neurohypophysis) lobes.
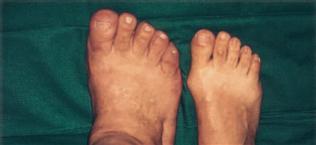
Fig. 12.56 The foot of a patient with acromegaly (left side) compared to a foot of a healthy person of similar height. [7]
Acromegaly is the result of an overproduction of the growth hormone somatotropin (STH) in the adenohypophysis caused by a benign tumour in the anterior lobe of the pituitary gland, a part of the Diencephalon.
Mesencephalon
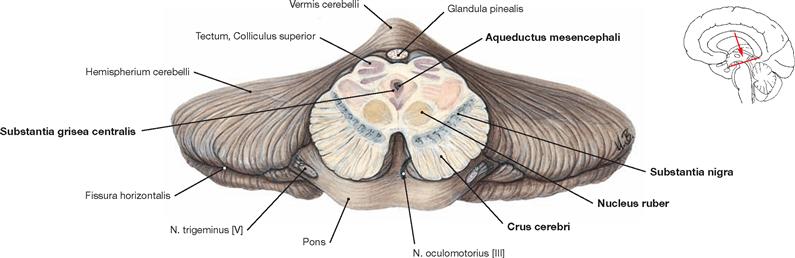
Fig. 12.57 Midbrain, Mesencephalon; cross-section at the level of the Colliculi superiores; anterior view.
The Mesencephalon is composed of the base, the tegmentum, and the tectum. Both, tegmentum and base are collectively named Pedunculus cerebri.
The Basis mesencephali comprises the cerebral crura (Crura cerebri) which contain of different fibre tracts (e.g. Fibrae corticonucleares).
The Tegmentum mesencephali comprises the Substantia grisea centralis surrounding the Aqueductus mesencephali (participates in the central suppression of pain, facilitates fear and flight reflexes, regulates autonomic nervous processes) and the Substantia nigra as part of the basal ganglia. Additional structures of the Tegmentum mesencephali include the Nucleus ruber, an important relay station of the motor system, the mesencephalic parts of the Formatio reticularis, the nuclei of the cranial nerves II and IV, as well as ascending and descending tracts.
The Tectum mesencephali (Lamina tecti [Lamina quadrigemina]) includes the Colliculi superiores and inferiores. These are important relay stations for visual reflexes (Colliculi superiores) and the central auditory pathway (Colliculi inferiores).
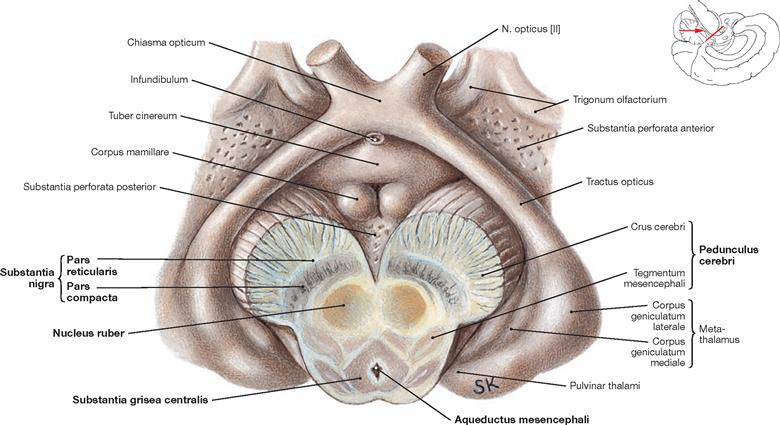
Fig. 12.58 Midbrain, mesencephalon, and diencephalon, Diencephalon; inferior view; after oblique section of the midbrain.
The illustration demonstrates the division of the Mesencephalon into basis, Tegmentum, and Tectum. Structures distinctly separate of the midbrain are the Substantia nigra, the Nucleus ruber, and the Aqueductus mesencephali with the surrounding Substantia grisea centralis. The Substantia nigra subdivides into the Pars reticularis and Pars compacta.
Mesencephalon and brainstem
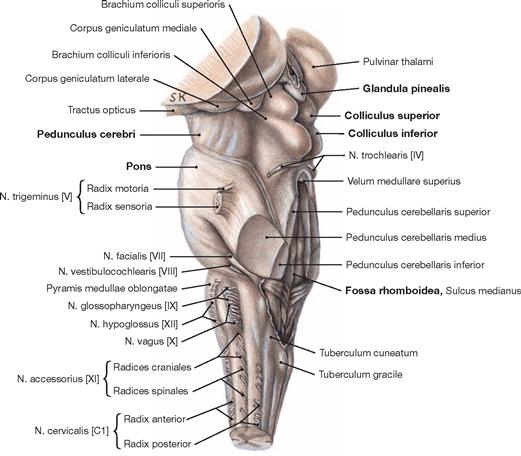
Fig. 12.59 Brainstem, Truncus encephali; lateral view; oblique view on the floor of the fourth ventricle after sectioning of the cerebellar peduncles.
The brainstem consists of the midbrain (Mesencephalon), Pons, and the Medulla oblongata. The Mesencephalon extends from the Diencephalon to the upper margin of the Pons. The Pedunculus cerebri is located at its anterior side. The Colliculi superiores and inferiores of the Tectum mesencephali form the dorsal side and create the particular shape of the quadrigeminal plate (Lamina tecti [Lamina quadrigemina]). The Glandula pinealis and the fourth ventricle are positioned superior and inferior to the quadrigeminal plate, respectively.
The Cerebellum has been sectioned at the cerebellar peduncles (Pedunculi cerebellares). Visible are the cranial nerves IV, V, and VII to XII exiting the brainstem. Their nuclei are located in the brainstem. The nuclei of the cranial nerves III and VI are also located in the brainstem but these nerves exit at the anterior side and, thus, are not visible in this figure.
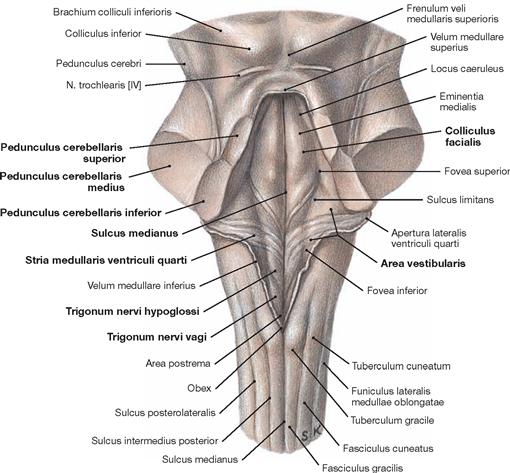
Fig. 12.60 Rhomboid fossa, Fossa rhombiodea; posterior view; view onto the floor of the fourth ventricle after dissection of the cerebellar peduncles.
The Fossa rhomboidea forms the floor of the fourth ventricle. The cerebellar peduncles (Pedunculi cerebellares), the Pons, and the Medulla oblongata provide the margins of the rhomboid fossa. As part of the area of the Fossa rhomboidea, important nuclei responsible for the regulation of the systemic circulation and the nuclei of the cranial nerves V to X, and partially cranial nerves XI and XII, are located in the Pons and Medulla oblongata. In the Fossa rhomboidea one can distinguish the Sulcus medianus, the Colliculus facialis (fibres of the N. facialis [VII]), the Striae medullares ventriculi quarti as part of the central auditory pathway, the Area vestibularis (vestibular nuclei), the Trigonum nervi hypoglossi (nucleus of the N. hypoglossus [XII]), the Trigonum nervi vagi (nuclei of the N. vagus [X] and N. glossopharyngeus [IX]), and the Area postrema (vomiting centre, see circumventricular organs, → Fig. 12.91).
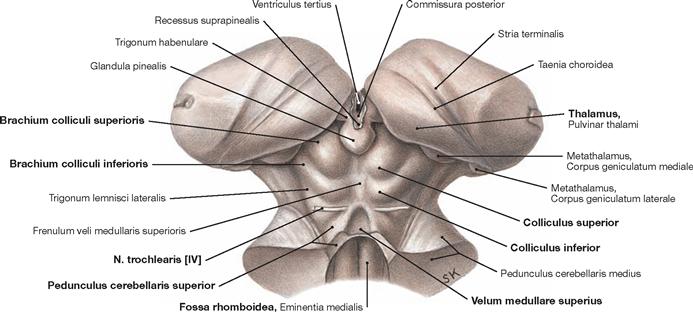
Fig. 12.61 Midbrain, Mesencephalon, and pineal gland, Glandula pinealis; posterior superior view.
At the dorsal side of the brainstem, the midbrain extends from the Diencephalon to the Pedunculi cerebellares, the Velum medullare superius, and the Fossa rhomboidea. The quadrigeminal plate (Lamina tecti [Lamina quadrigemina]) is the characteristic feature of the dorsal side. It is composed of the Colliculi superiores and the Colliculi inferiores and forms the Tectum mesencephali. To each side, the corresponding colliculi connect with the Diencephalon (Corpora geniculata mediale and laterale) through fibre bundles (Brachia colliculi superioris and inferioris). Below the Colliculi inferiores, the N. trochlearis [VI] is the only cranial nerve to exit the brainstem at its dorsal side.
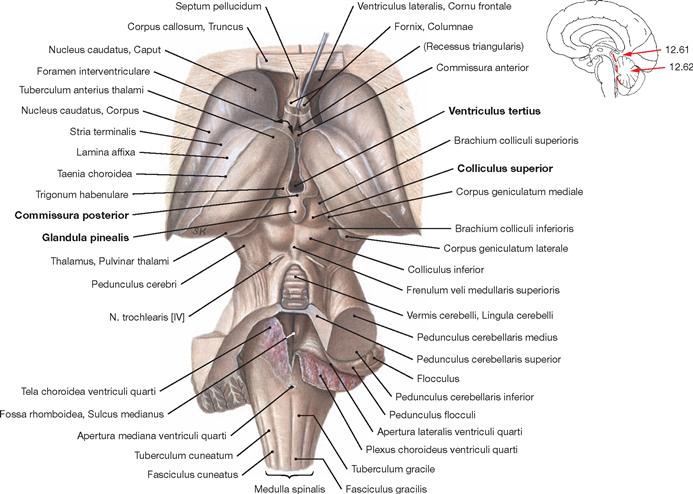
Fig. 12.62 Brainstem, Truncus encephali; posterior superior view; the Pons and major parts of the Cerebellum have been removed, the Tela choroidea of the fourth ventricle has been sectioned in the median plane and reflected to the right side. The Glandula pinealis attaches to the Commissura posterior and is located between the two Colliculi superiores. The third ventricle lies above. The brainstem contains important centres (Nuclei ruber, pontis, olivares inferiores, vestibulares and the Formatio reticularis) which coordinate critical life-saving functions, including circulation, breathing, and consciousness (ARAS → Fig. 12.54).
Brainstem and Cerebellum
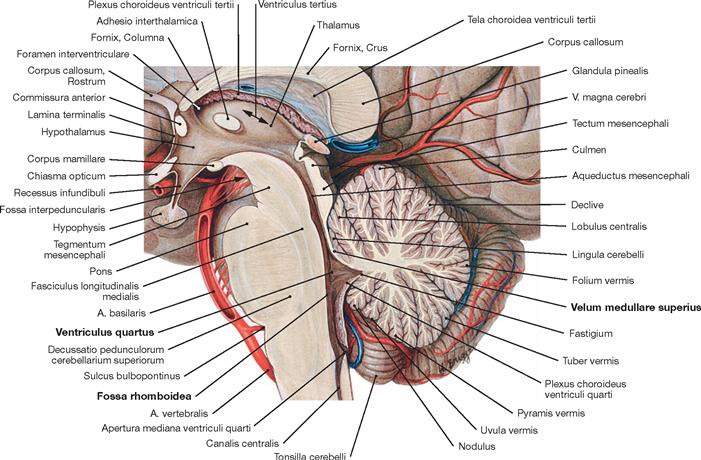
Fig.12.63 Brainstem, Truncus encephali, with fourth ventricle, Ventriculus quartus, and cerebellum, Cerebellum; median section.
The median section reveals the characteristic structure of the so-called tree of life (Arbor vitae) of the Cerebellum created by the distinct grooves (surface enlargement) of the cerebellar cortex.
The Fossa rhomboidea lies anterior to the Cerebellum and forms the floor of the fourth ventricle. The brainstem with Mesencephalon, Pons, and Medulla oblongata are positioned anterior to the fourth ventricle and even further anterior the A. basilaris runs alongside the brainstem. In the median section, the Velum medullare superius constitutes the rostral wall of the fourth ventricle and stretches from the Cerebellum to the quadrigeminal plate (Lamina tecti [Lamina quadrigemina]). The pineal gland (Glandula pinealis) and the Corpus callosum are located on top.
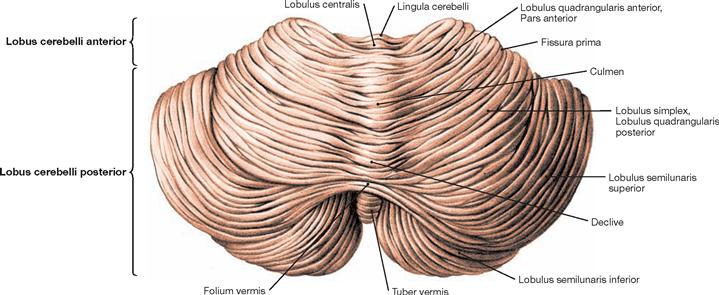
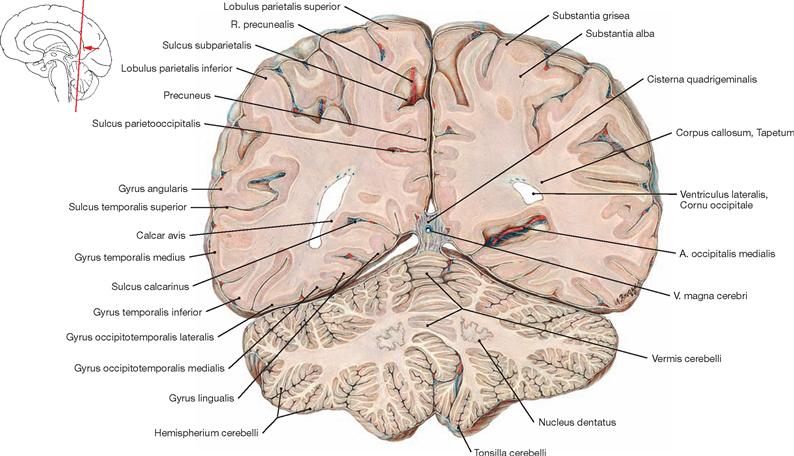
Fig. 12.64 Cerebellum, Cerebellum; posterior superior view.
The Cerebellum divides into the vermis (Vermis cerebelli) and two hemispheres. The Tuber vermis, folium, declive, culmen, as well as the Lobulus centralis and the Lingula cerebelli are shown. The cerebellar hemispheres divide into three lobes (→ Fig. 12.71):
• Lobus flocculonodularis (nodule + flocculus → Figs. 12.65 and 12.66)
The lobes subdivide further into lobuli, such as Lobulus quadrangularis anterior, Lobulus quadrangularis posterior (Lobulus simplex), and the Lobuli semilunares superior and inferior.
Cerebellum, cortex
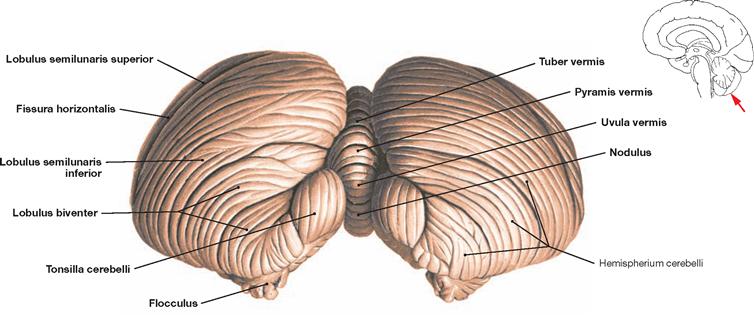
Fig. 12.65 Cerebellum, Cerebellum; posterior inferior view.
The tuber of vermis, pyramis, uvula, and nodule become visible from this angle. Visible are also the paired tonsil of the Cerebellum (Tonsilla cerebelli) as well as the Lobuli semilunares superior and inferior, separated by the Fissura horizontalis. The Lobulus biventer is located below the Lobulus semilunaris inferior and above the flocculus.
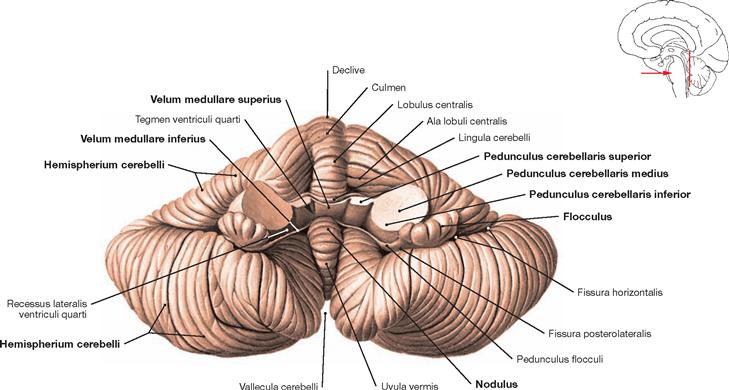
Fig. 12.66 Cerebellum, Cerebellum; anterior view; after dissection of the cerebellar peduncles.
The anterior surface depicts the cerebellar peduncles which connect the Cerebellum to the brainstem: Pedunculi cerebellares superior, medius, and inferior. The Velum medullare superius divides the vermis (Vermis cerebelli) and connects both cerebellar peduncles. The paired Velum medullare inferius located on the left and right side of the nodule continues bilaterally towards the flocculus. The cerebellar hemispheres constitute the outer parts.
Nuclei of the cerebellum
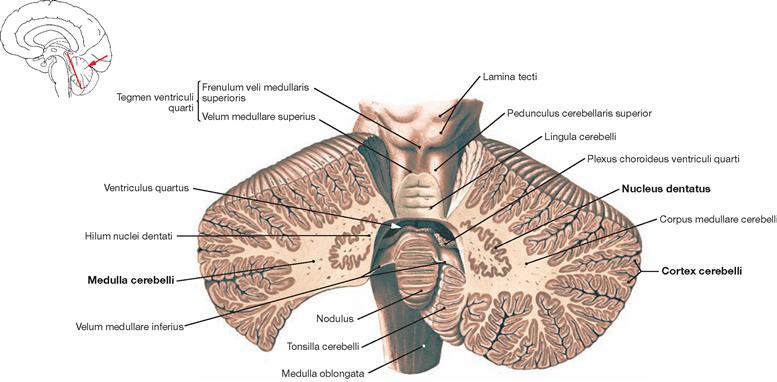
Fig. 12.67 Cerebellum, Cerebellum; oblique section; posterior view.
An oblique section through the Cerebellum reveals the structure of the grey substance which consists of the cortex (Cortex cerebelli) and medulla (Medulla cerebelli). Visible in the medulla is the biggest of the four cerebellar nuclei, the Nucleus dentatus, with its grey substance showing a jagged and gyral configuration. This nucleus is not only located in both cerebellar hemispheres (Pontocerebellum) but also has multiple close functional connections with the cerebellar cortex.
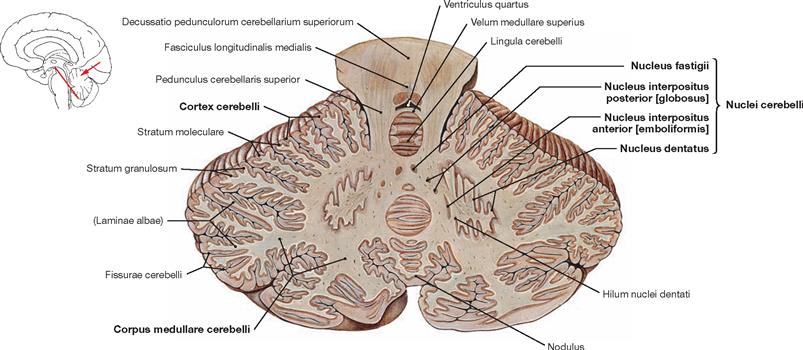
Fig. 12.68 Cerebellum, Cerebellum, with cerebellar nuclei, Nuclei cerebelli; oblique section through the upper cerebellar peduncles; posterior view.
The Cerebellum is composed of the medullary centre (Corpus medullare cerebelli) with embedded cerebellar nuclei and the surrounding cerebellar cortex (Cortex cerebelli). The oblique section reveals all four cerebellar nuclei in both hemispheres (Pontocerebellum). The Nucleus dentatus is U-shaped and jagged. Medial to the Nucleus dentatus lies the Nucleus interpositus anterior (emboliformis) and even further medial the Nucleus interpositus posterior (globosus), both collectively named Nucleus interpositus. Both nuclei share functional similarities and connect with the paravermal and vermal zone of the Cerebellum (Spinocerebellum). Located in the medulla of the vermis are the right and left Nucleus fastigii which have close functional connections with the cortex of the Lobus flocculonodularis (Vestibulocerebellum) (→ Figs. 12.65 and 12.66).
Cerebellar connections
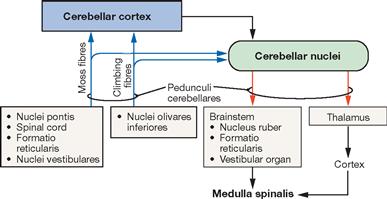
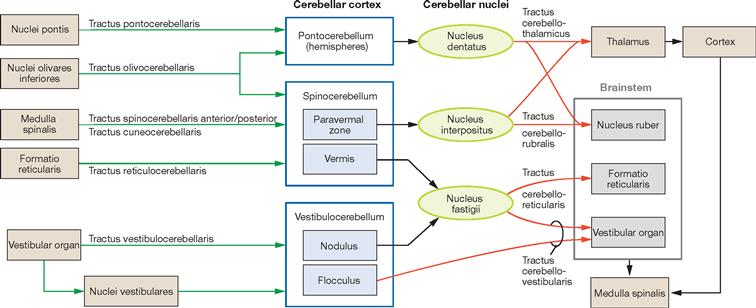
Fig. 12.69 Schematic structure of the basic flow of information from and to the Cerebellum. [14]
Blue arrows indicate the systems providing input for the Cerebellum, red arrows demonstrate the parts of the CNS receiving output information from the Cerebellum.
Cerebellum, organisation
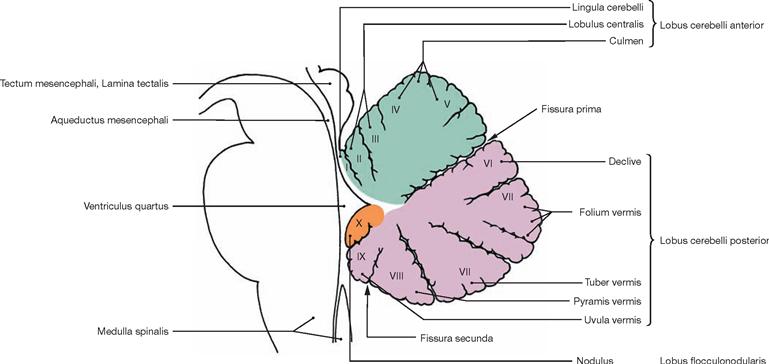
Fig. 12.71 Parts of the cerebellar vermis, Vermis cerebelli, I to X; median section; overview.
The Spinocerebellum consists of the vermis, the bilateral paravermal zone and the major part of the Lobus cerebelli anterior with the exception of the nodule. Functionally, it controls the muscular tonus and regulates body and limb movements. The Spinocerebellum receives the majority of its afferent proprioception input from the spinal cord (Tractus spinocerebellares anterior and posterior, Tractus cuneocerebellaris). Additional afferent fibers come from the Formatio reticularis and the Nuclei olivares inferiores. The nodule is part of the Vestibulocerebellum.
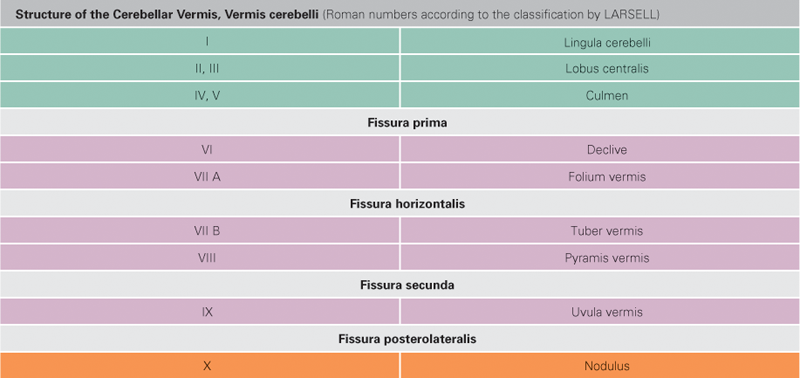
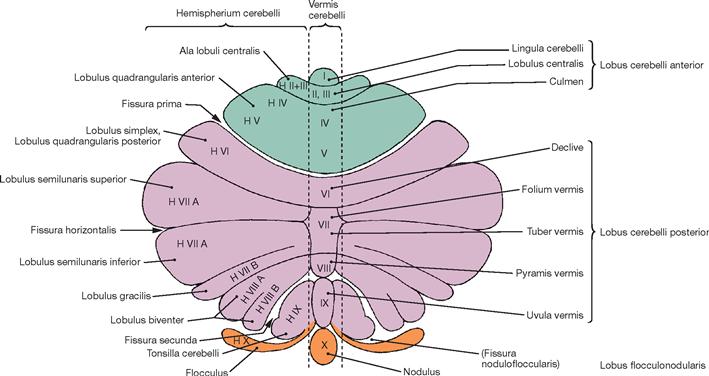
Fig. 12.72 Cerebellar cortex, Cortex cerebelli, and cerebellar vermis, Vermis cerebelli; diagram of the cerebellar cortex outstretched; overview.
With the exception of the Lobus cerebelli anterior, the hemispheres are separated by the vermis and include the areas H II to H IX of LARSELL’s classification. They constitute the Pontocerebellum (Cerebrocerebellum). The Pontocerebellum receives its afferent fibres primarily from the pontine nuclei (Nuclei pontis). This part of the Cerebellum has close connections with the cerebral cortex via the Pons and participates in the planning of voluntary movements. Collectively named the Lobulus flocculonodularis, the nodule and flocculus (X and H X) are the essential components of the Vestibulocerebellum. The extensive connections with the vestibular system of the inner ear provide the majority of afferent fibres to the Vestibulocerebellum. The main function of the Vestibulocerebellum is to regulate balance.
Association and commissural tracts
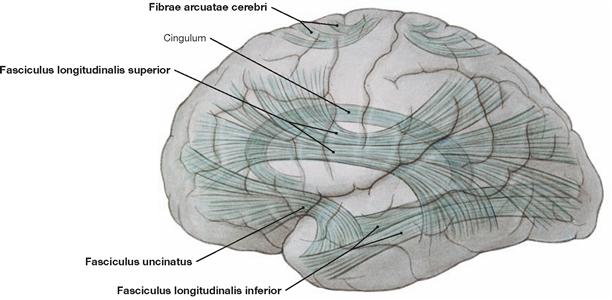
Fig. 12.73 Association tracts, Neurofibrae associationes, and arcuate fibres, Fibrae arcuatae; overview; view from the left side.
The majority of fibres in the white matter are association fibres. They connect different regions within one hemisphere and facilitate association and integrative functions by linking functionally distinct areas.
Short association fibres, known as Fibrae arcuatae cerebri, are located near the cortex and their U-shaped structure is ideally suited in connecting neighbouring gyri. Long association fibres located deeper in the medulla interconnect the lobes.
Functionally important association tracts are the Fasciculi longitudinalis superior, longitudinalis inferior, and uncinatus as well as the Fibrae arcuatae cerebri and the Cingulum.
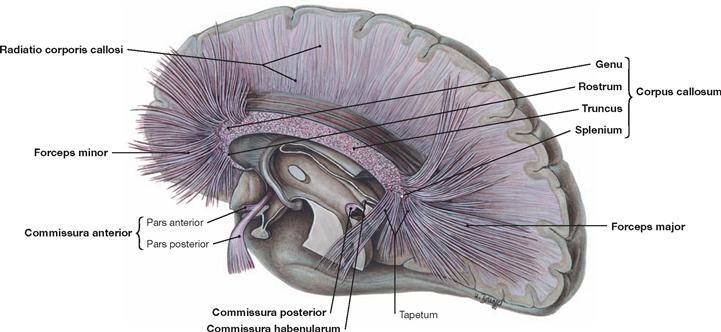
Fig. 12.74 Commissural tracts, Neurofibrae commissurales; topographic overview; view from the left side; after extensive removal of the corpus callosum in the paramedian plane, single fibres of the corpus callosum are shown.
Commissural (transverse) fibres facilitate the information exchange between the two cerebral hemispheres, e.g. to generate a complete visual image composed of the visual input to each cerebral hemisphere. Homotopic commissural fibres connect corresponding cerebral areas, heterotopic commissural fibres facilitate the exchange between non-corresponding cerebral areas.
Each phylogenetic cerebral part has its own commissure: for the paleocortex, this is the Commissura anterior, for the archicortex, it is the Commissura fornicis, and the Corpus callosum serves this function in the neocortex. The latter consists of the Rostrum, Genu, Truncus, and Splenium. The Corpus callosum is shorter than the cerebral hemispheres and, thus, the rostral and occipital fibers create fan-shaped projections into the corresponding lobes (Radiatio corporis callosi, projections of the Corpus callosum with Forceps minor and Forceps major). However, some homotopic cerebral areas do not connect via commissural fibers. These include the primary visual cortex, the primary auditory cortex, and the somatosensory areas for hand and foot.
Projection tracts
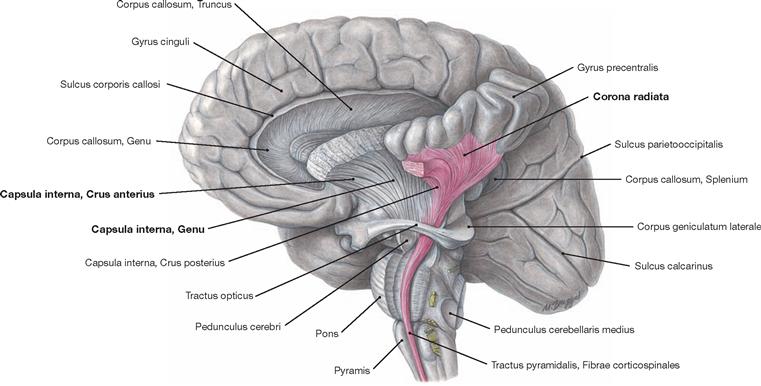
Fig. 12.75 Projection tracts, Neurofibrae projectiones; view from the left side; the internal capsule and the pyramidal tract have been exposed.
Projection tracts consist of projection fibres which connect the cortex with subjacent structures of the CNS (e.g. thalamus, brainstem). In the area of the striatum and pallidum, these fibres have to pass through narrow spaces where all fibres converge. These bottleneck areas are the Capsula interna and the Capsula externa between the Nucleus lentiformis and Claustrum as well as the Capsula extrema between the insular cortex and the Claustrum. The Capsula interna is the main passageway for projection fibres. The Capsula externa and the Capsula extrema mainly contain long association fibres. The Corona radiata describes the radial arrangement of projection fibres between the cerebral cortex and the Capsula interna.
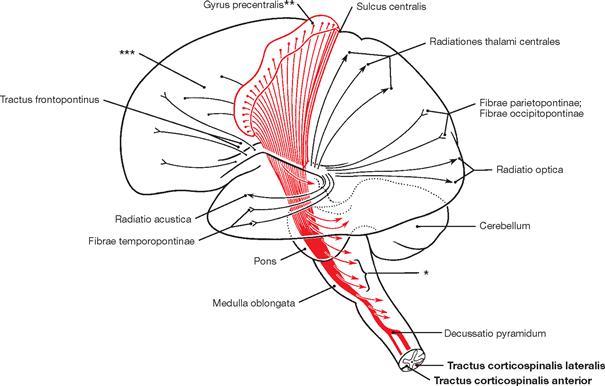
Fig. 12.76 Internal capsule, Capsula interna, and pyramidal tract, Tractus pyramidalis; functional overview; view from the left side.
At the Capsula interna, almost all cortical projection tracts converge in a narrow space. This is examplified with the pyramidal tract derived from the Gyrus precentralis shown in red, which continues as Tractus corticospinales lateralis and anterior into the spinal cord.
* fibres to the quadrigeminal plate and to the nuclei of the Rhombencephalon
** perikarya of the pyramidal tract
*** perikarya of area 6 and 8 (premotor cortical field)
Internal capsule
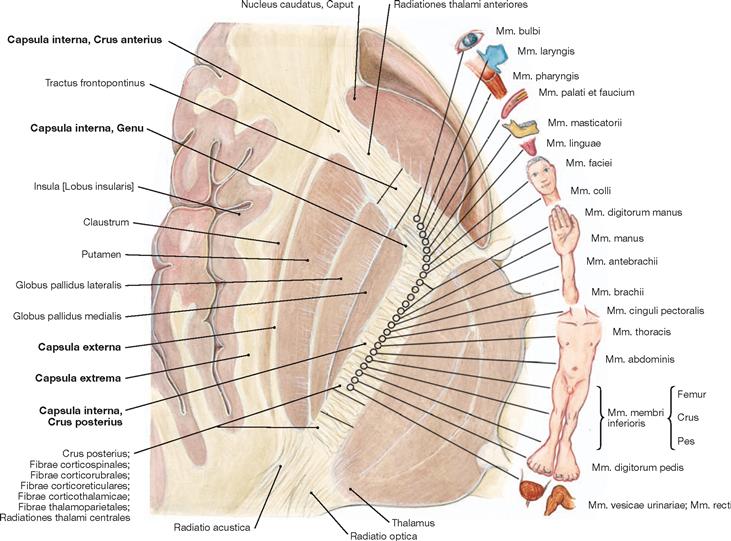
Fig. 12.77 Internal capsule, Capsula interna; functional structure.
The Capsula interna is clinically highly relevant because it contains almost all cortical projection tracts concentrated in a small space. The margins of the Capsula interna are formed by the Nucleus caudatus in the anterior medial part, the Thalamus in the posterior medial section, and the Globus pallidus and Putamen laterally. In the horizontal section, the Capsula interna has a bend shape. An anterior limb (Crus anterius), a genu (Genu), and a posterior limb (Crus posterius) are distinguishable. The descending tracts within the Capsula interna have a somatotopic arrangement. The corticonuclear fibres run in the genu, while the corticospinal fibres for the upper extremity, torso, and lower extremity are somatotopically arranged in an anterior to posterior direction in the Crus posterius.
Pyramidal tract
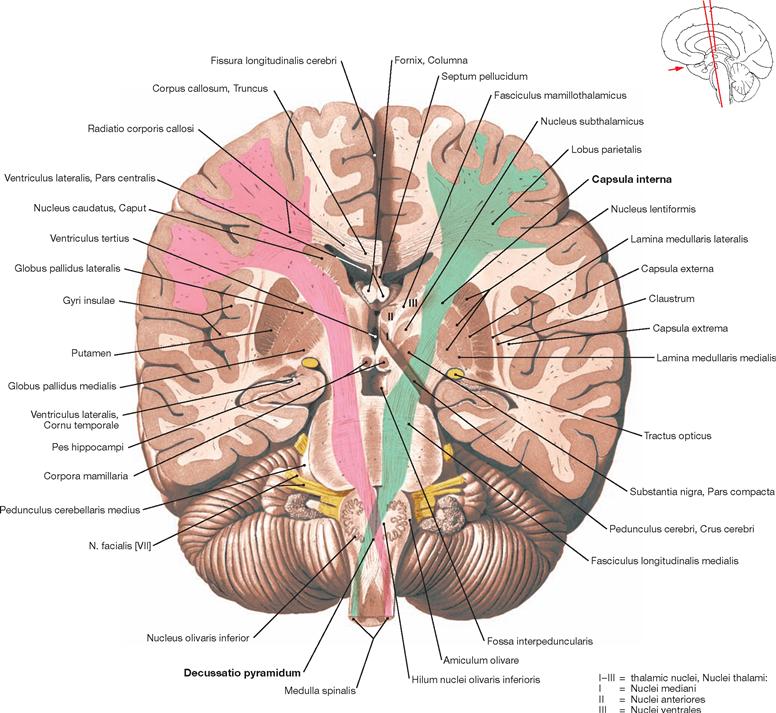
Fig. 12.78 Pyramidal tract, Tractus pyramidalis, and basal ganglia, Nuclei basales; oblique staggered section through the posterior limb of the internal capsule, the cerebral peduncles, and the medulla oblongata; anterior view; pyramidal tracts shown in colour, right: pink, left: green.
The pyramidal tract transmits motor impulses from the motor cortex to the motor efferent nuclei of the cranial nerves (Fibrae corticonucleares) and the motor neurons in the anterior horn of the spinal cord (Fibrae corticospinales). The fibres originate in the gyrus precentralis, in secondary motor fields, and in somatosensory cortical areas. The converging fibres create the Corona radiata. Somatotopically arranged, the fibres pass through the genu and posterior limb of the Capsula interna (→ Fig. 12.77). In the Mesencephalon, the fibres enter the Crura cerebri. Along the way through the brainstem, the Fibrae corticonucleares exit the pyramidal tract at different levels. At the decussation of pyramids (Decussatio pyramidum), the major part of the remaining fibres (Fibrae corticospinales) cross to the opposite side, a smaller fraction courses on the ipsilateral side downwards and crosses to the opposite side only within the spinal cord.
Ventricles of the brain
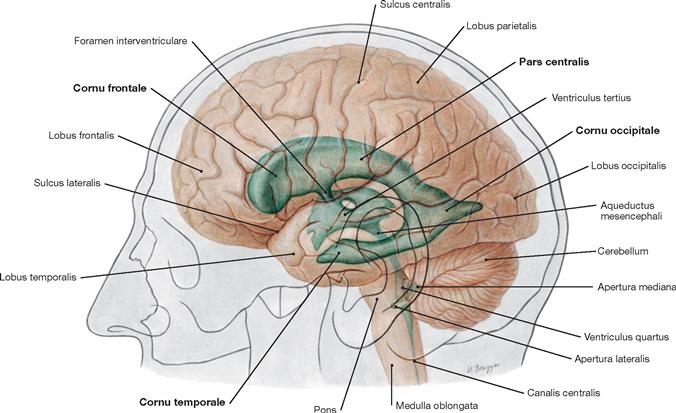
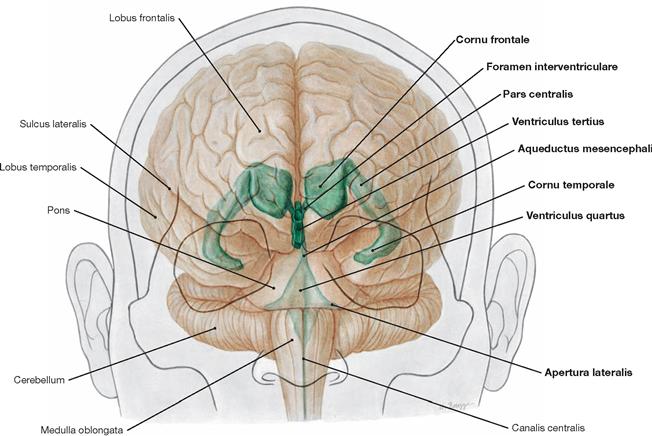
Fig. 12.79 Ventricles of the brain, Ventriculi encephali; view from the left side.
The inner subarachnoid space consists of the ventricular system and the central canal (Canalis centralis) of the spinal cord. The ventricular system is composed of the paired lateral ventricles (Ventriculi laterales) with Cornu frontale, Pars centralis, Cornu occipitale, and Cornu temporale, the third ventricle (Ventriculus tertius), the Aqueductus mesencephali, and the fourth ventricle (Ventriculus quartus).
Inner and outer subarachnoid spaces
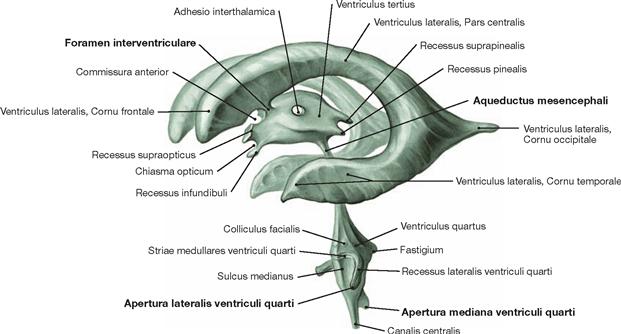
Fig. 12.81 Inner subarachnoid spaces, Ventriculi encephali; corrosion cast specimen; oblique view from the left side.
Each of the lateral ventricles connects with the third ventricle by a separate Foramen interventriculare (foramen of MONRO). The third ventricle communicates with the fourth ventricle through the Aqueductus mesencephali. The fourth ventricle contains three openings (Aperturae) to the outer subarachnoid space: the Apertura mediana (foramen of MAGENDIE) and the paired Aperturae laterales (foramina of LUSCHKA).
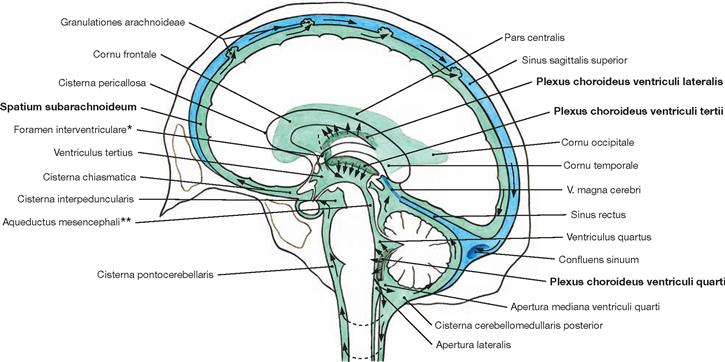
Fig. 12.82 Ventricles of the brain, Ventriculi encephali, and subarachnoid space, Spatium subarachnoideum; schematic drawing of the circulation (arrows) of the cerebrospinal fluid from the inner to the outer subarachnoid space.
The space in between the arachnoid and pia mater constitutes the outer subarachnoid space. It surrounds the brain as well as the spinal cord. The Plexus choroidei in the ventricles produce the major part of the cerebrospinal fluid (Liquor cerebrospinalis).
The circulating fluid volume (150 ml) is exchanged permanently (daily production volume approx. 500 ml).
The cerebrospinal fluid (CSF) has multiple functions. It serves as a cushion to protect the CNS from mechanical forces, reduces the weight of the CNS (the CSF creates buoyancy which causes a 97% weight reduction from 1400 g to 45 g), supports the metabolism of the CNS, removes toxic substances, and transports hormones (e.g. leptin).
* clinical term: foramen of MONRO
** clinical term: aqueduct of SYLVIUS
Ventricles
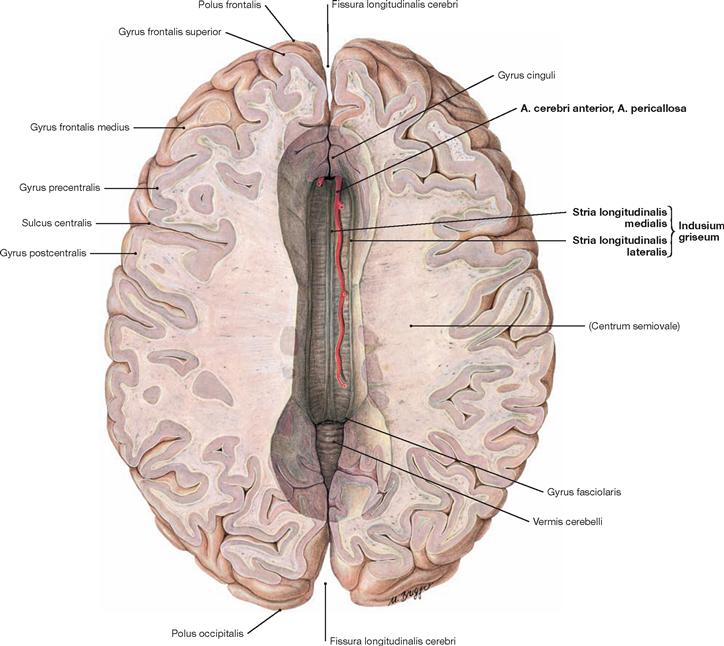
Fig. 12.83 Corpus callosum; superior view; after removal of the upper parts of the cerebral hemispheres.
A superior view onto the Corpus callosum reveals the rostral to occipital orientation of the Striae longitudinales mediales and laterales of the Indusium griseum (considered a cortical part of the limbic system) as well as the A. pericallosa (A. cerebri anterior). The Corpus callosum consists of the Rostrum, Genu, Truncus, and a thickened posterior end (Splenium; → Fig. 12.127). It creates the roof of the lateral ventricles and is composed of commissural fibres connecting one hemisphere with the other. It contains approximately 200 million axones.
The function of the Corpus callosum involves the information exchange and coordination between the two hemispheres, with each hemisphere having partially different tasks in the processing of information.
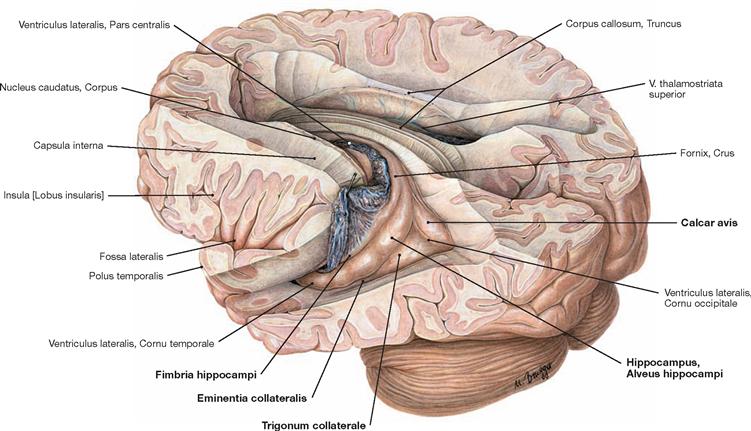
Fig. 12.84 Lateral ventricles, Ventriculi laterales; posterior superior view from the left side; after removal of the upper parts of the cerebral hemispheres.
View into both lateral ventricles. The course of the Plexus choroideus is visible in the left lateral ventricle. The Plexus choroideus has been lifted up with a probe at the transition from the Pars centralis to the Pars temporalis of the lateral ventricle. The Plexus choroideus produces cerebrospinal fluid.
The roof and the lateral wall of the Cornu occipitale are formed by the tapetum (Radiatio corporis callosi, Radiatio optica) (not visible). The calcar avis forms the medial wall, and the Trigonum collaterale and the Eminentia collateralis create the floor. The roof and lateral wall of the Cornu temporale are part of the Cauda nuclei caudati and the tapetum (not visible), the Fimbria hippocampi and the Plexus choroideus form the medial wall, and the floor consists of the Eminentia collateralis and the Alveus hippocampi (→ Figs. 12.87, 12.123 to 12.126).
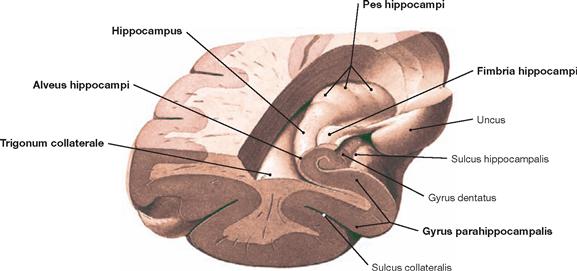
Fig. 12.85 Left temporal horn, Cornu temporale, of the lateral ventricle, Ventriculus lateralis; frontal section after removal of the temporal wall; posterior superior view.
The Hippocampus, the Alveus hippocampi, the Fimbriae hippocampi, and the Pes hippocampi form parts of the floor of the Cornu temporale of the lateral ventricle. The Trigonum collaterale is also visible. The Hippocampus formation with the Gyrus parahippocampalis is visible in the frontal section. The Hippocampus is a central element of the limbic system (→ Fig. 12.47) and is involved in processes of learning, memory, and emotions.
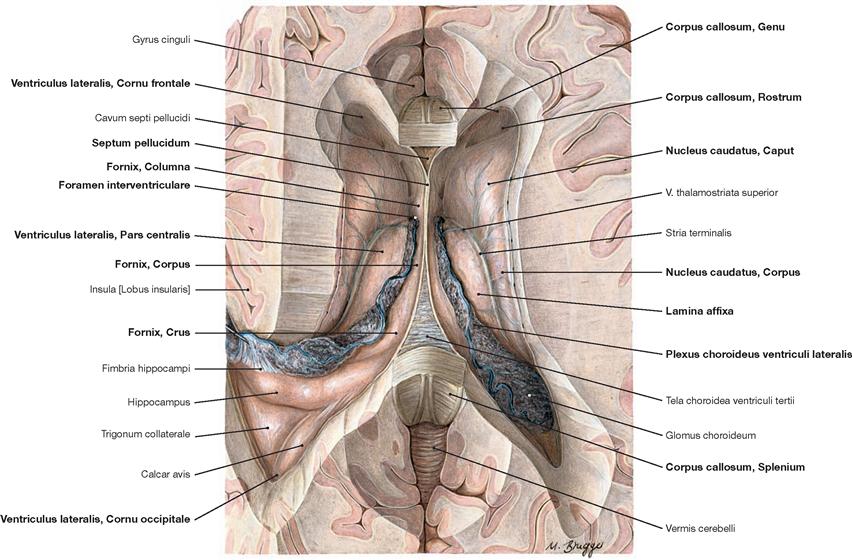
Fig. 12.86 Lateral ventricles, Ventriculi laterales; superior view; after removal of the upper part of the cerebral hemispheres and the central part of the Corpus callosum.
This view shows the Cornu frontale, the Pars centralis and the Cornu occipitale as well as the transition of both lateral ventricles to the Cornu temporale. The margins of the Cornu frontale are the Genu of the Corpus callosum (anterior wall), the Truncus of the Corpus callosum (roof, not visible, because the Corpus callosum was detached at the genu and the splenium), the Septum pellucidum (medial wall), the caput of the Nucleus caudatus (lateral wall), as well as the rostrum of the Corpus callosum (floor). The Foramina interventricularia (foramina of MONRO) in the Cornu frontale are also visible. Like the Pars frontalis, the roof of the Pars centralis is formed by the Truncus of the Corpus callosum (removed). The crus of the fornix and the Septum pellucidum create the medial wall, the Corpus of the Nucleus caudatus forms the lateral wall, and the floor consists of the Lamina affixa of the Plexus choroideus and the crus of the fornix.
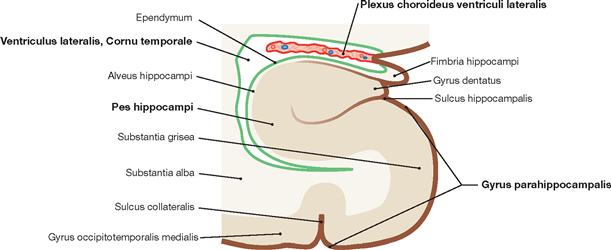
Fig. 12.87 Temporal horn, Cornu temporale, of the lateral ventricle, Ventriculus lateralis; schematic frontal section.
The scheme demonstrates the topographic relationsship of the lateral ventricle and the Hippocampus formation. The Plexus choroideus protrudes into the lateral ventricle. The walls of the ventricle are coloured in bright green, while the cerebrospinal fluid and the internal ventricular space are shown in white.
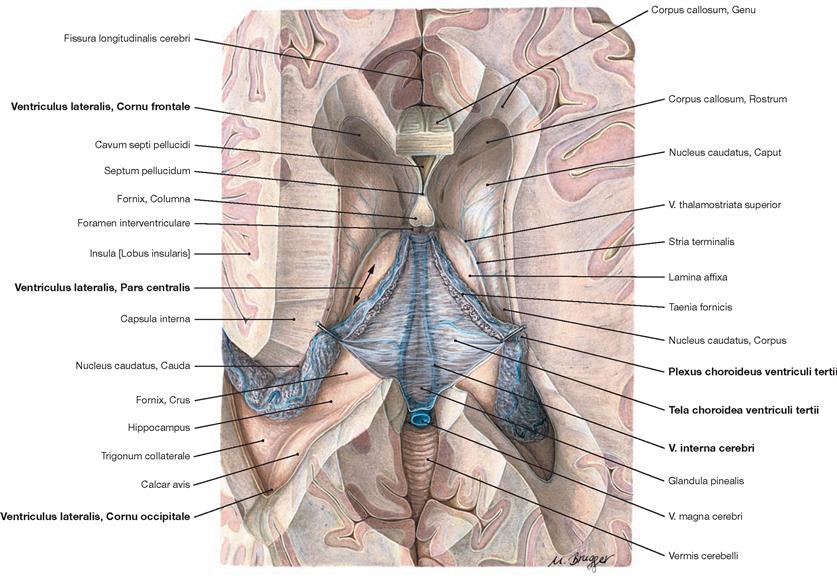
Fig. 12.88 Lateral ventricles, Ventriculi laterales; superior view; after removal of the central part of the corpus callosum and the columns of the fornix.
Shown is the Tela choroidea overarching the third ventricle. The Vv. internae cerebri gleam through and drain into the V. magna cerebri. The Cornu frontale, Pars centralis, and the Cornu occipitale of the lateral ventricles are visible. Laterally, the Plexus choroideus continues alongside the Hippocampus into the Cornu temporale.
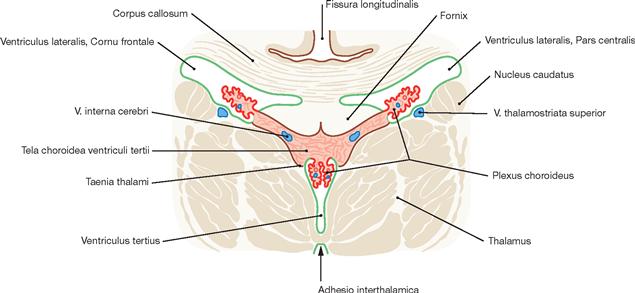
Fig. 12.89 Plexus choroideus in the lateral ventricles, Ventriculi laterales, and in the third ventricle, Ventriculus tertius; schematic frontal section. (according to [2])
The Plexus choroideus produces cerebrospinal fluid (CSF) and is present in the paired lateral ventricles (left first and right second lateral ventricle) as well as in the third and the fourth ventricles (not shown). In the Plexus choroideus, capillary blood and CSF space are separated by a blood-CSF barrier.
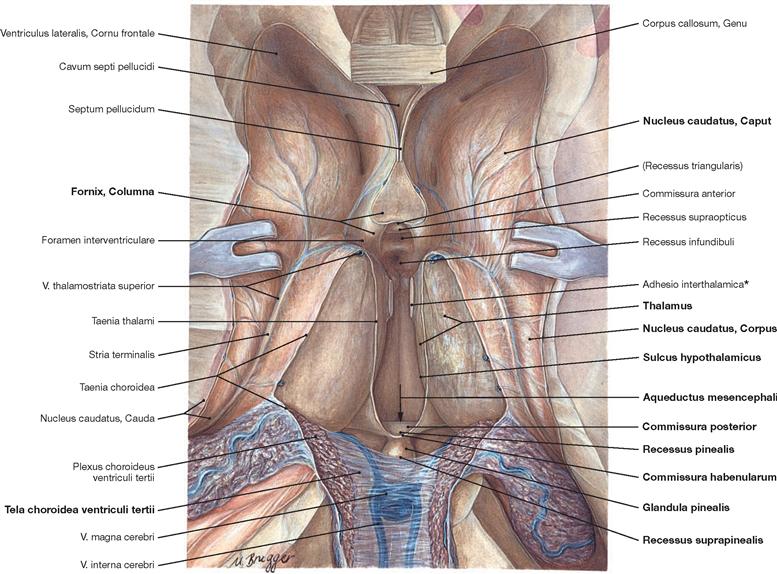
Fig. 12.90 Lateral ventricles, Ventriculi laterales, and third ventricle, Ventriculus tertius; superior view; parts of the cerebral hemispheres, the central part of the Corpus callosum as well as the Fornix and the Plexus choroideus have been removed, the Tela choroidea of the third ventricle has been reflected.
The margins of the third ventricle are:
• roof: Tela choroidea and Plexus choroideus
• anterior wall: Columnae fornicis, Commissura anterior, Lamina terminalis, Recessus triangularis, and Recessus supraopticus
• lateral wall: Thalamus, Stria medullaris thalami, Sulcus hypothalamicus, and Hypothalamus (wall)
• posterior wall: Commissura habenularum, Commissura posterior, Recessus suprapinealis, and Recessus pinealis
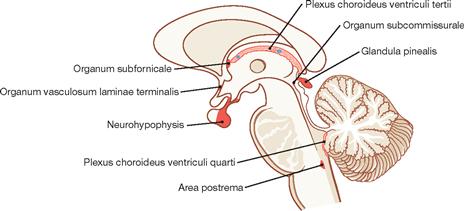
Fig. 12.91 Circumventricular organs, median sagittal section.
Characteristic features of the circumventricular organs are strong vascularisation, a modified ependyme (tanycytes with tight junctions), and the formation of a blood-CSF barrier instead of a blood-brain barrier.
Circumventricular organs include the neurohypophysis, the Eminentia mediana, the pineal gland (Glandula pinealis) as well as the Organum vasculosum laminae terminalis and the Organum subfornicale (both: regulation of blood volume and blood pressure, secretion of hormones like angiotensin, somatostatin, inducing fever), the Organum subcommissurale (present only in the foetus and newborn, secretion of a glycoprotein-rich product), and the Area postrema (triggers vomiting).
Clinics
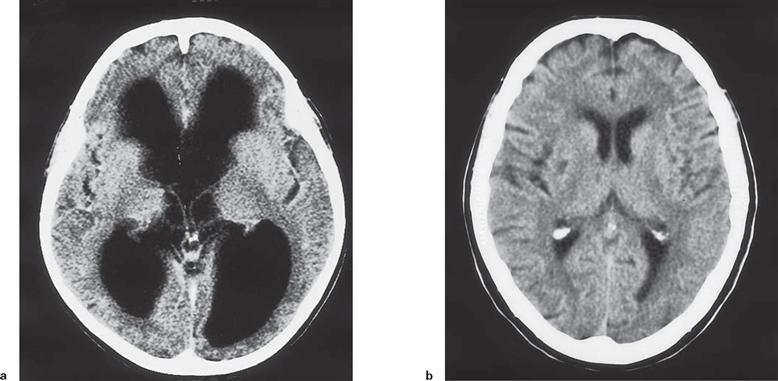
Figs. 12.92a and b Computed tomographic (CT) cross-sections of the head. [23]
a. CT scan of a patient with a cerebrospinal fluid block caused by obstruction in the cerebral aqueduct (Aqueductus mesencephali). The cerebral ventricles are greatly enlarged (hydrocephalus) at the expense of the cerebral parenchyma. The patient showed massive mental disabilities and significantly impaired gait.
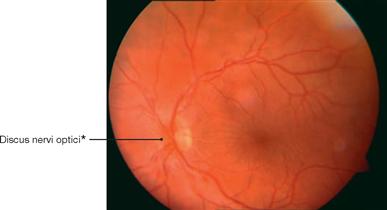
Fig. 12.93 Ocular fundus, Fundus oculi; left side; anterior view; ophthalmoscopic image of the central area with papilloedema caused by increased intracranial pressure.
The examination of the ocular fundus reveals a swelling of the Papilla nervi optici resulting from an intraventricular neurocytoma WHO grade II. As the N. opticus [II] is surrounded by meninges and cerebrospinal fluid, the optic disc bulges out into the bulbus of the eye.
* clinical term: optic disc or blind spot (discus = Papilla nervi optici)
Arteries at the cranial base
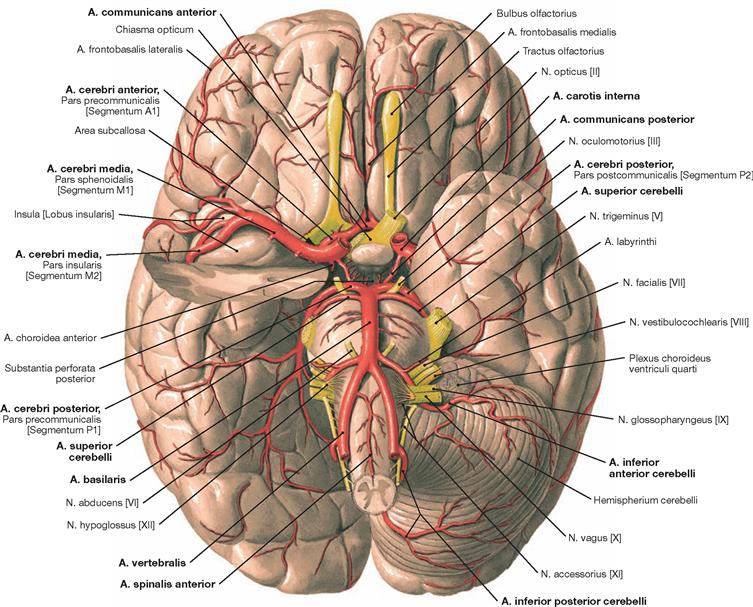
Fig. 12.94 Arteries of the brain; inferior view.
The figure demonstrates the location of the arteries at the cranial base. The Aa. vertebrales converge to form the A. basilaris which releases the Aa. cerebri posteriores and branches for the brainstem, the Cerebellum, and the inner ear (so-called vertebralis tributary). Small connecting arteries (Aa. communicantes posteriores) provide the link between the Aa. cerebri posteriores and the two Aa. carotides internae. Each of the latter contributes one A. cerebri media and one A. cerebri anterior which collectively provide the major part of the blood for the hemispheres (so-called carotis tributary). The A. communicans anterior connects both Aa. cerebri anteriores.
Clinically, the Aa. cerebri anterior, media, and posterior are divided into segments. The A1 segment (Pars precommunicalis) corresponds to the part of the A. cerebri anterior proximal to the A. communicans anterior and the part distal of the A. communicans anterior is the A2 segment (Pars infracallosa). The A3 segment (Pars precallosa) describes the part of the A. cerebri anterior located in front of the Corpus callosum and the part located on top of the corpus callosum constitutes the A4 segment (Pars supracallosa). Clinicians call the part of the A. cerebri anterior distal to the A. communicans anterior the A. pericallosa. The A. cerebri media is composed of the segments M1 (Pars sphenoidalis), M2 (Pars insularis), M3 (Pars opercularis), and M4 (Pars terminalis). The A. cerebri posterior divides into four segments: P1 (Pars precommunicalis; proximal to the A. communicans posterior), P2 (Pars postcommunicalis; up to the posterior rim of the brainstem), P3 (Pars quadrigemina; up to the point where the A. cerebri posterior enters the Fissura calcarina), and P4 (no Latin term; division into two arterial branches). Some segments are visible in the figure.
Arteries at the cranial base, Circulus arteriosus
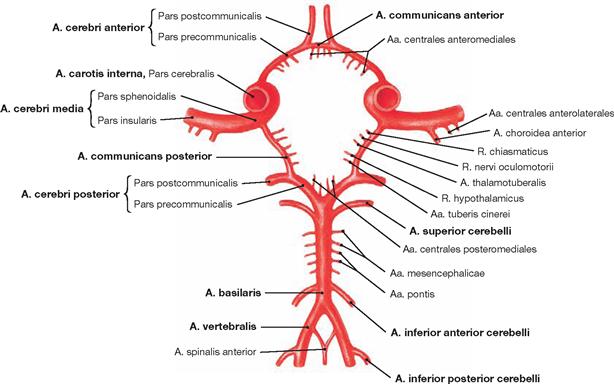
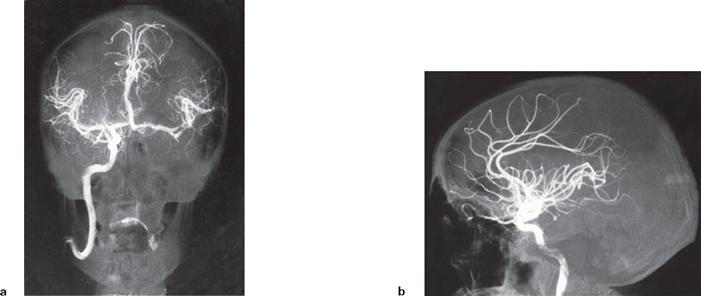
Fig. 12.95 Arterial circle of the brain, Circulus arteriosus cerebri (circle of WILLIS); superior view.
The Aa. communicantes posteriores on both sides connect the Aa. cerebri posteriores with the Partes cerebrales of the Aa. carotides internae. In front, the A. communicans anterior connects the two Aa. cerebri anteriores. This generates a closed arterial circle which provides an anastomosis between the two Aa. carotides internae and the vertebralis tributary.
Vessels and nerves at the cranial base
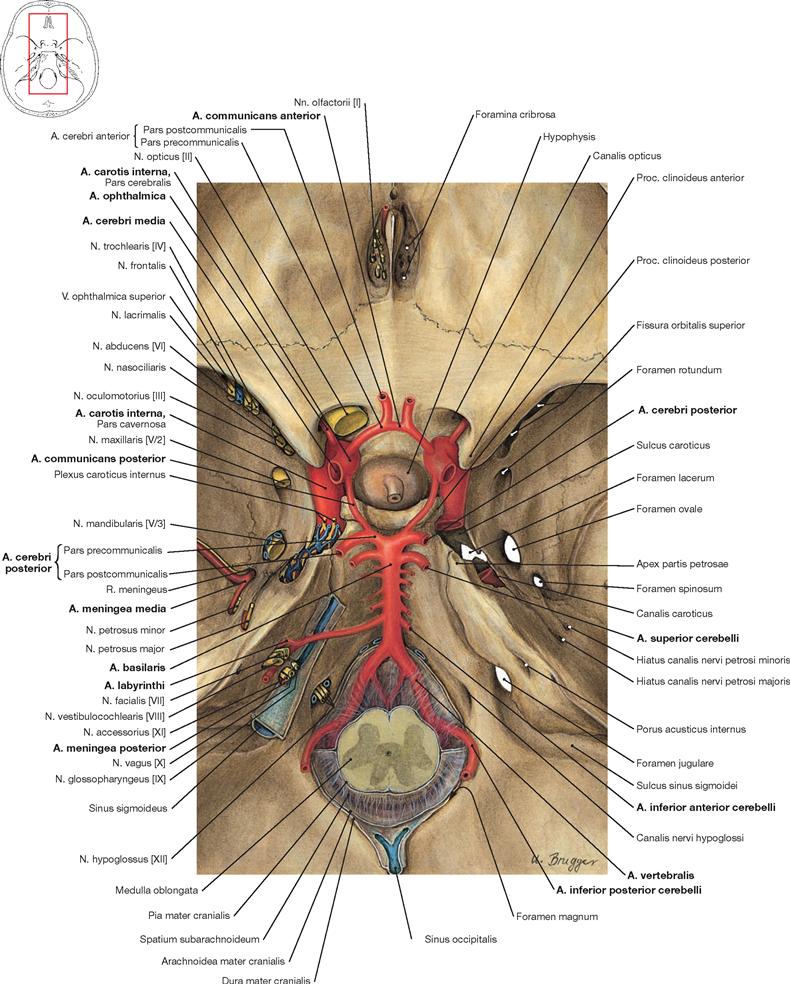
Fig. 12.96 Passageways of vessels and nerves through the internal surface of the cranial base, Basis cranii interna, and the cerebral arterial circle, Circulus arteriosus cerebri (circle of WILLIS); superior view.
From a superior view, the Circulus arteriosus cerebri projects onto the Fossa hypophysialis. The A. ophthalmica branches off the A. carotis interna at the Canalis nervi optici and, together with the N. opticus [II], enters the orbit through this bony canal. The A. basilaris runs on top of the clivus. The A. inferior anterior cerebelli derives from the A. basilaris and releases the A. labyrinthi while passing the Porus acusticus internus or entering it in an S-shaped detour.
For an overview of the passageways through the internal surface of the cranial base → Figures 8.16 and 8.17.
Arteries of the brain
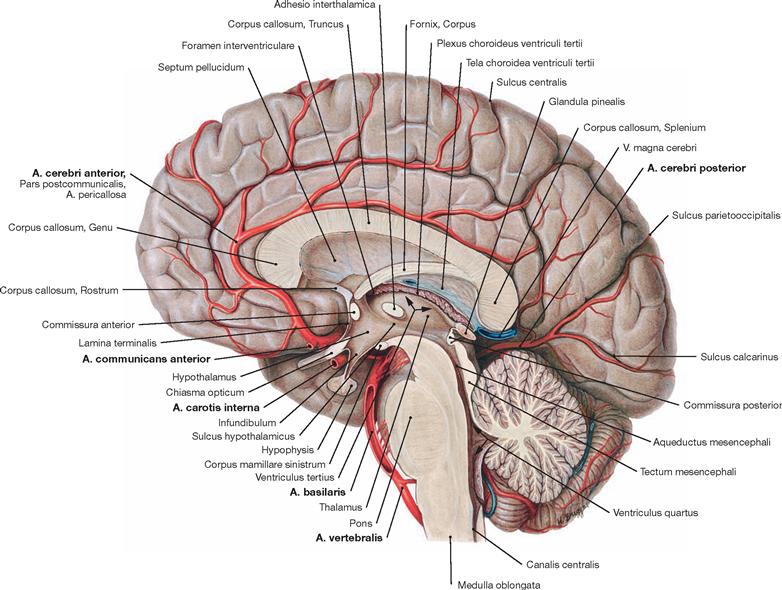
Fig. 12.97 Medial surface of the brain, Facies medialis hemispherii cerebri, diencephalon, Diencephalon, and brainstem, Truncus encephali; staggered median section; view from the left side.
Once the A. communicans anterior has branched off the A. cerebri anterior, the Pars postcommunicalis (A. pericallosa) of the latter passes around the rostrum and genu of the Corpus callosum and runs alongside the upper surface of the Corpus callosum. Its extensions reach the Sulcus parietooccipitalis. The A. cerebri anterior supplies blood to the medial area of the frontal and parietal lobes as well as the hemispheral rim and a small area alongside thereof at the cerebral convexity (→ p. 271).
The A. cerebri posterior courses to the occipital lobe, the basal part of the temporal lobe, the lower part of the striatum (not visible), and to the Thalamus.
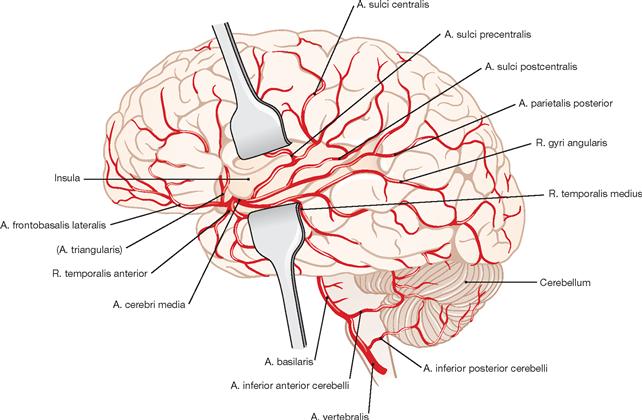
Fig. 12.98 Branches of the A. cerebri media in the insular region, and at the outer cerebral cortex; view from the left side. (according to [2])
The A. cerebri media enters the Fossa lateralis from the lateral side and divides into four parts (→ Fig. 12.94):
• Pars sphenoidalis (not visible; M1)
• Pars insularis with short branches for the insular cortex (M2)
• Pars opercularis for the cortex of the temporal lobe (A. frontobasalis lateralis and Aa. temporales; M3)
• Rr. terminales inferiores and superiores (Pars terminalis; M4) for the cortex in the area of the Sulcus centralis and the parietal lobe
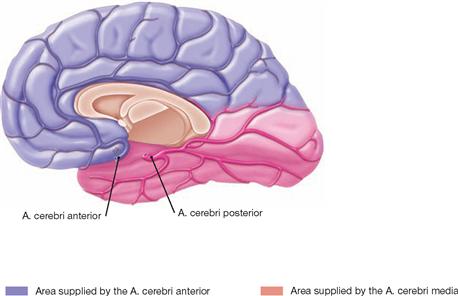
Fig. 12.99 Arteries of the right hemisphere of the brain; view from the left side.
The A. cerebri anterior supplies the medial side of the frontal and parietal lobes extending past the hemispheral rim and up to the Sulcus parietooccipitalis. The occipital lobe and the base of the temporal lobe receive their blood supply from the A. cerebri posterior.
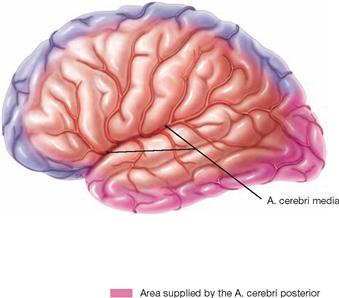
Fig. 12.100 Arteries of the left hemisphere of the brain; view from the left side.
The A. cerebri anterior supplies blood to an area of the frontal and parietal cerebral cortex extending approximately 1 cm past the hemispheral rim onto the cortex convexity. The A. cerebri posterior supplies blood to the occipital pole and the inferior rim of the temporal lobe. The remaining outer cortical area receives blood from the A. cerebri media. The area of the Gyri precentralis and postcentralis receives blood via both the A. cerebri anterior and the A. cerebri media.
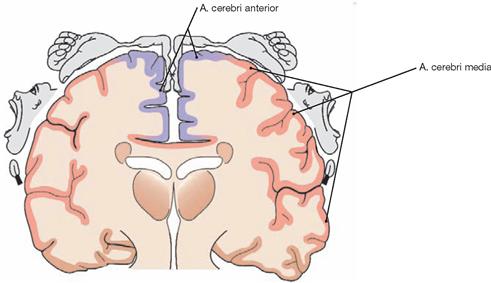
Fig. 12.101 Arteries in the region of the Gyrus precentralis and their tributary in relation to the homunculus of the primary motor cortex.
The A. cerebri anterior supplies blood to the cortex of the Gyrus precentralis up to approximately 1 cm past the hemispheral rim onto the cortical convexity. It supplies those precentral cortical areas representing the lower extremity, the pelvis, and the thorax as depicted by the homunculus. The A. cerebri media supplies the representational cortex areas representing the upper extremity and the entire head.
Arteries and veins of the brain
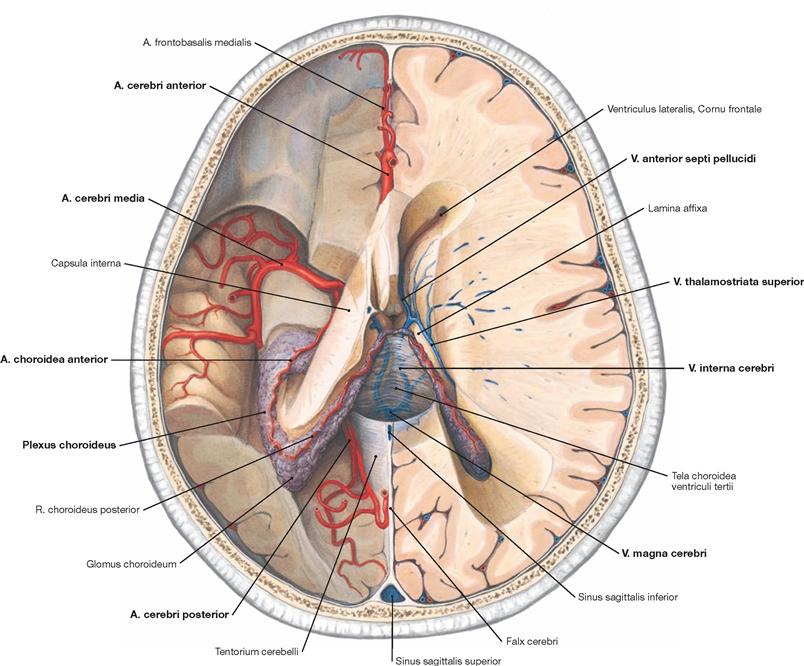
Fig. 12.102 Arteries and veins of the brain, Aa. und Vv. cerebri; superior view.
Upon removal of the parietal parts of the brain the otherwise hidden courses of the Aa. cerebri anterior, media, and posterior become visible on the left side of the body. The A. choroidea anterior derives from the A. cerebri media and supplies the Plexus choroideus of the lateral ventricle. The A. choroidea anterior continues as a R. choroideus posterior which extends into the tip of the Plexus choroideus of the Cornu frontale in the lateral ventricle.
On the right side of the body at the floor of the Cornu frontale of the lateral ventricle lies the V. anterior septi pellucidi and further posterior the V. thalamostriata superior. Both drain blood into the V. interna cerebri which drains into the V. magna cerebri (vein of GALEN). This group of veins drains the venous blood from the ventricular system, the basal ganglia, and the internal capsule.
Veins of the brain
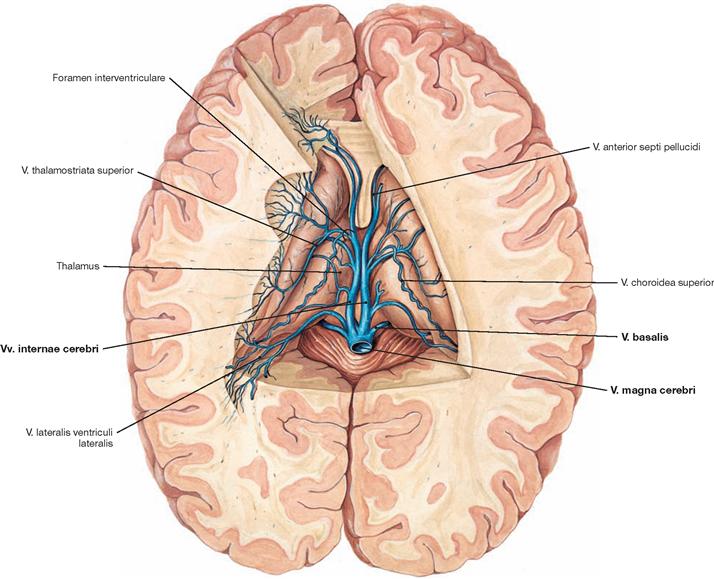
Fig. 12.103 Deep veins of the brain, Vv. profundae cerebri; superior view.
The Vv. internae cerebri run in the Tela choroidea ventriculi tertii. The veins of the ventricular system, the basal ganglia, and the internal capsule belong to the deep veins of the brain. The blood from these structures is drained through the Vv. thalamostriatae superiores into the Vv. cerebri internae and from here into the V. magna cerebri (vein of GALEN).
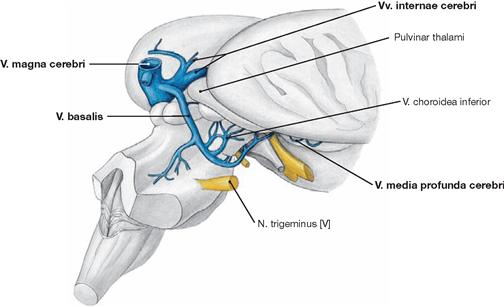
Fig. 12.104 Deep veins of the brain, Vv. profundae cerebri; posterior view from the right side.
After removal of the Cerebellum, the basal veins draining the Rhombencephalon, Mesencephalon, and Insula become visible. Like the Vv. internae cerebri, the venous blood vessels of this region, the paired V. media profunda cerebri and the V. basalis (ROSENTHAL’s vein), drain into the V. magna cerebri (vein of GALEN).
Brain, MRI
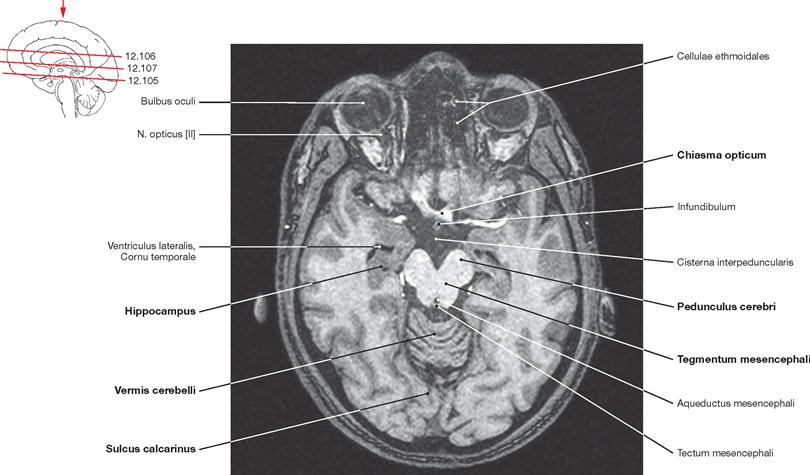
Fig. 12.105 Brain, Encephalon; magnetic resonance tomographic image (MRI); horizontal section at the level of the Mesencephalon and the temporal horns of the lateral ventricle; superior view.
The Chiasma opticum and the Pedunculi cerebri of the Mesencephalon are visible. In addition, the cerebellar vermis (Vermis cerebelli) appears in this sectional plane. The Sulcus calcarinus is discernible in the occipital lobe.
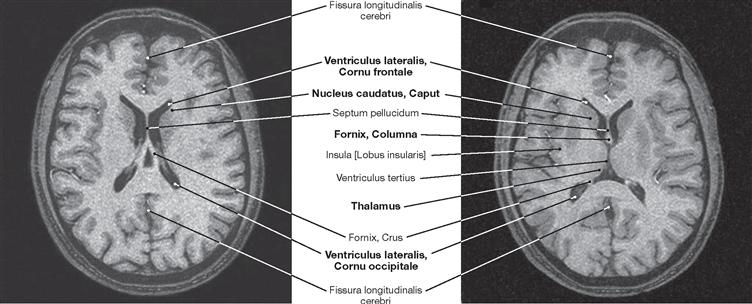
Fig. 12.106 Brain, Encephalon; magnetic resonance tomographic image (MRI); horizontal section at the level of the central parts of the lateral ventricles; superior view.
Visible are the Cornua frontale and occipitale, the Septum pellucidum, and the crus of fornix. The left side of the image also shows the Lobus insularis.
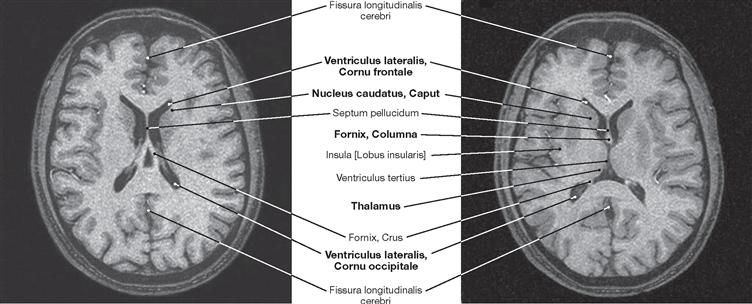
Fig. 12.107 Brain, Encephalon; magnetic resonance tomographic image (MRI); horizontal section at the level of the third ventricle and the temporal horns of the lateral ventricles; superior view.
In addition to the Lobuli insulares and the structures shown in → Figure 12.106, the Thalamus and the column of the fornix are visible.
Sections
Brain, MRI
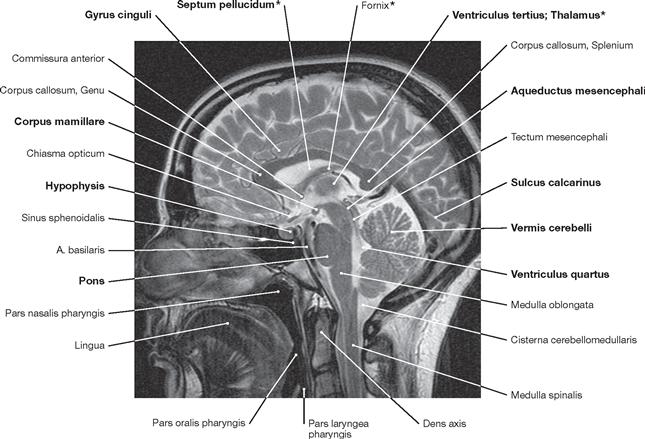
Fig. 12.108 Brain, Encephalon; magnetic resonance tomographic image (MRI); median section.
This MRI scan clearly delineates all brain structures, for example the Gyrus cinguli, Septum pellucidum, Ventriculus tertius, Thalamus, Aqueductus mesencephali, Corpus mamillare, Hypothalamus, Hypophysis, Mesencephalon, Pons, Cerebellum, and Medulla oblongata.
The structures marked with a star (*) appear partly falsified as a consequence of the “partial volume effect”.
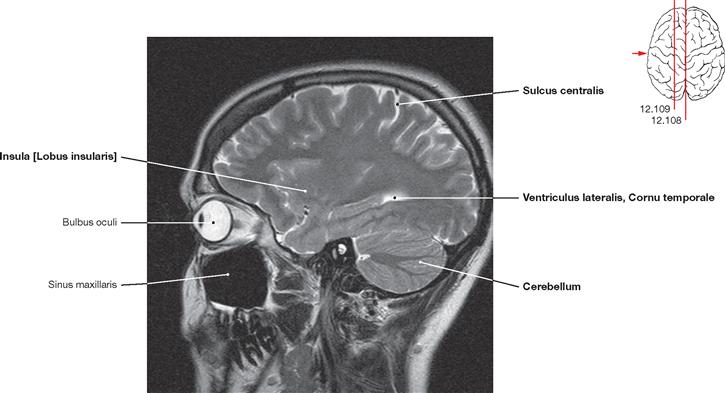
Fig. 12.109 Brain, Encephalon; magnetic resonance tomographic image (MRI); sagittal section at the level of the Mesencephalon and the temporal horns of the lateral ventricle; view from the left side.
The sagittal section includes the Cerebellum and the Sulcus centralis. A small part of the Cornu temporale of the lateral ventricle also lies within this sectional plane.
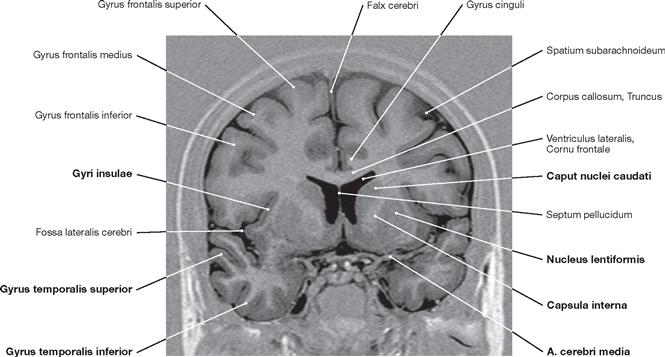
Fig. 12.110 Brain, Encephalon; magnetic resonance tomographic image (MRI); frontal section at the level of the anterior part of the third ventricle; anterior view.
On the right side, the course of the A. cerebri media projecting towards the Sulcus lateralis is visible. On both sides, the large gyri of the Lobus frontalis and Lobus temporalis are shown. Among the basal ganglia, this imaging technique allows the Nucleus caudatus, the Capsula interna, and the Nucleus lentiformis to be distinguished.
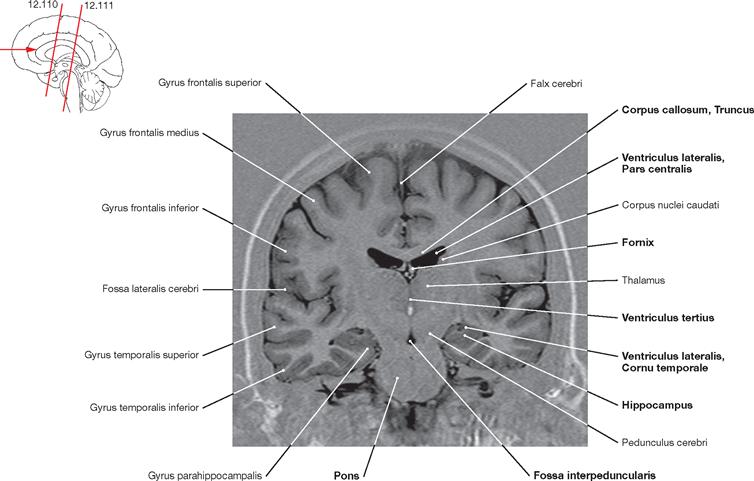
Fig. 12.111 Brain, Encephalon; magnetic resonance tomographic image (MRI); frontal section at the level of the Thalamus; anterior view.
This image shows the Cornu temporale of the lateral ventricles and the Hippocampus. Further cranial, the Pars centralis of the lateral ventricle is imaged. In the midline from cranial to caudal, the Truncus corporis callosi, the Fornix, the Ventriculus tertius, the Fossa interpeduncularis of the brainstem, and the Pons can be distinguished.
Brain, frontal sections
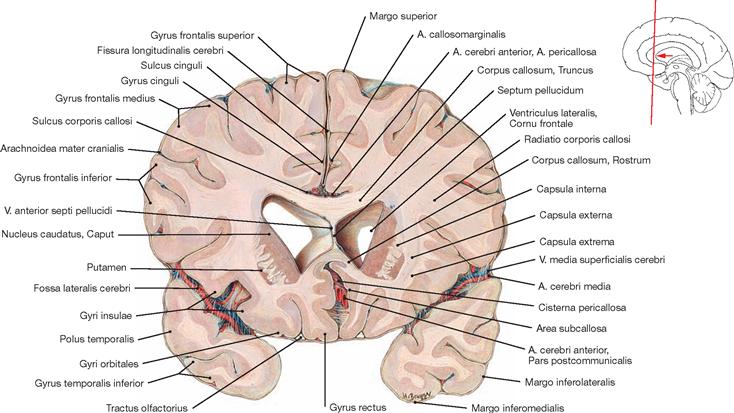
Fig. 12.112 Brain, Encephalon; frontal section at the level of the anterior parts of the frontal horns of the lateral ventricles; posterior view.
Visible are the two Ventriculi laterales, above them the Corpus callosum, and lateral to them the Nucleus caudatus and the Putamen.
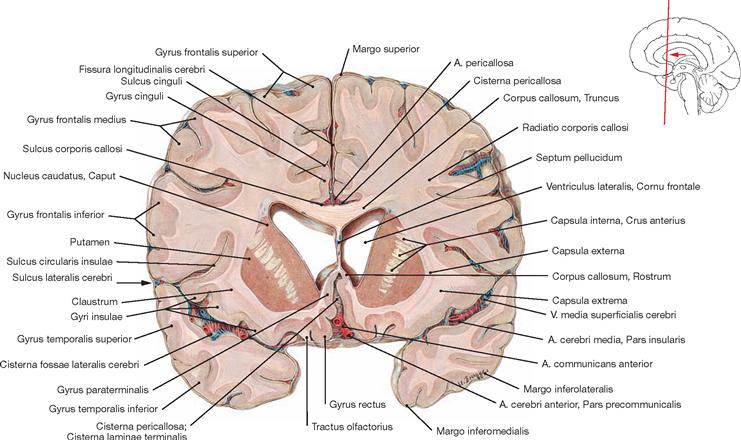
Fig. 12.113 Brain, Encephalon; frontal section at the level of the posterior parts of the frontal horns of the lateral ventricles; posterior view.
Above the Ventriculi laterales the Truncus of the Corpus callosum is discernible, lateral to the Ventriculi laterales lies the caput of the Nucleus caudatus and the Putamen, and between them the Crus anterius of the Capsula interna is visible.
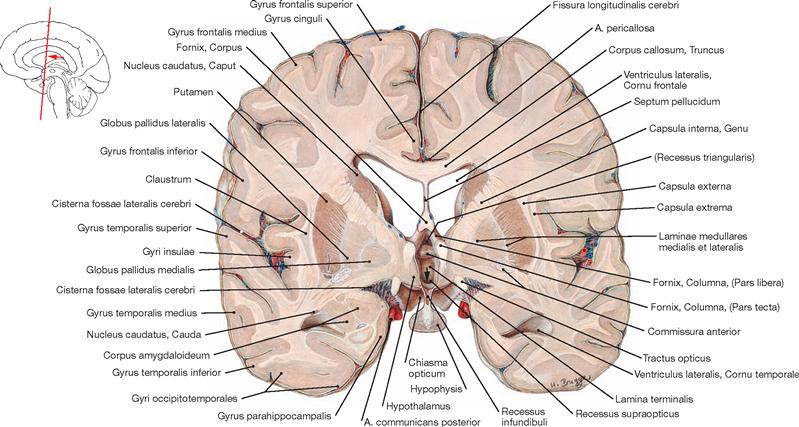
Fig. 12.114 Brain, Encephalon; frontal section at the level of the Foramina interventricularia; posterior view.
The pituitary gland is sectioned in the centre. Inferior to the Ventriculi laterales the Caput of the Nucleus caudatus, the Capsula interna, the Globus pallidus, the Putamen, the Claustrum, and some Gyri insulae are visible.
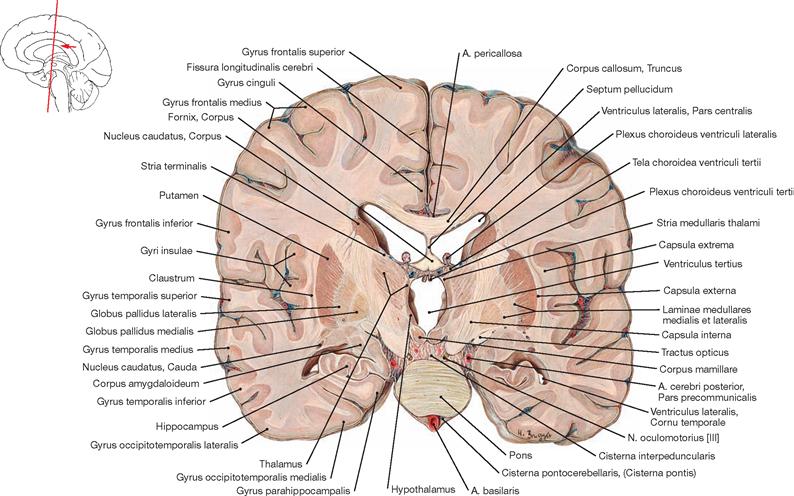
Fig. 12.115 Brain, Encephalon; frontal section at the level of the Corpora mamillaria; posterior view.
At the level of the Corpora mamillaria, the lumen of the third ventricle is located below the Ventriculi laterales. Lateral thereof from inside to outside lie the Thalamus, Capsula interna, Globus pallidus, Putamen, Capsula externa, Claustrum, Capsula extrema, and Gyri insulae.
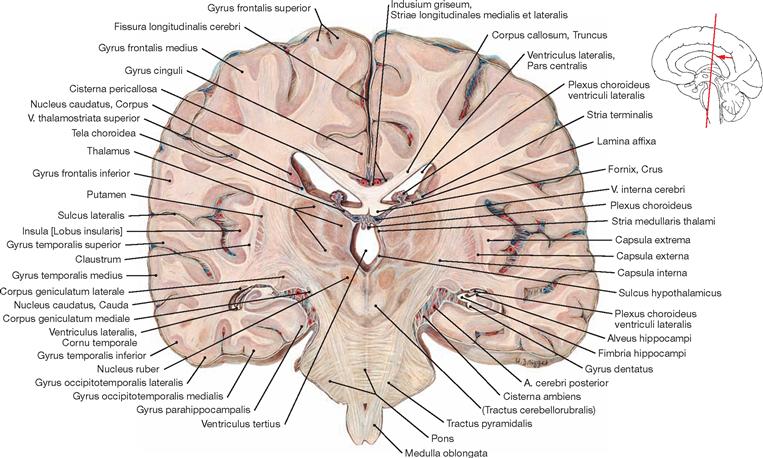
Fig. 12.116 Brain, Encephalon; frontal section at the level of the central part of the third ventricle; posterior view.
At this level, the right and left Thalamus are often cross-connected by the Adhesio interthalamica. Inferior to the Thalamus the Nucleus ruber is visible. The Pons and the Tractus pyramidalis present prominently in the brainstem.
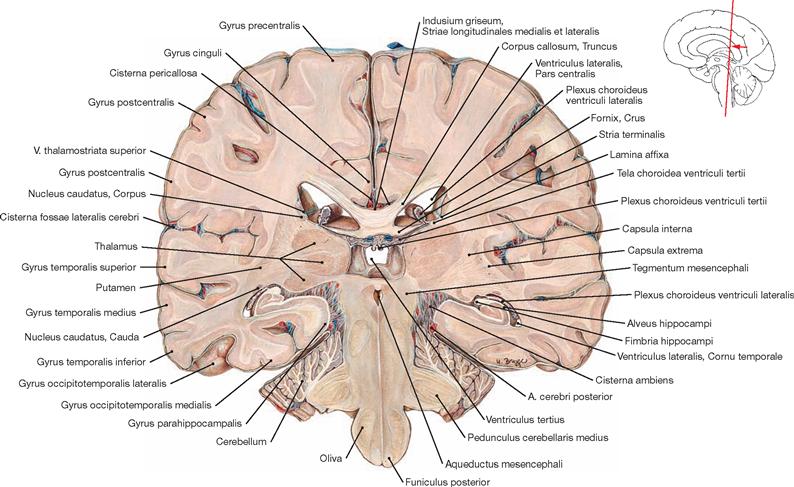
Fig. 12.117 Brain, Encephalon; frontal section at the level of the posterior wall of the third ventricle; posterior view.
Inferior to the Ventriculi laterales a number of thalamic nuclei are visible and further caudal the occipital part of the Hippocampus is shown. The brainstem has been sectioned at the level of the Aqueductus mesencephali.
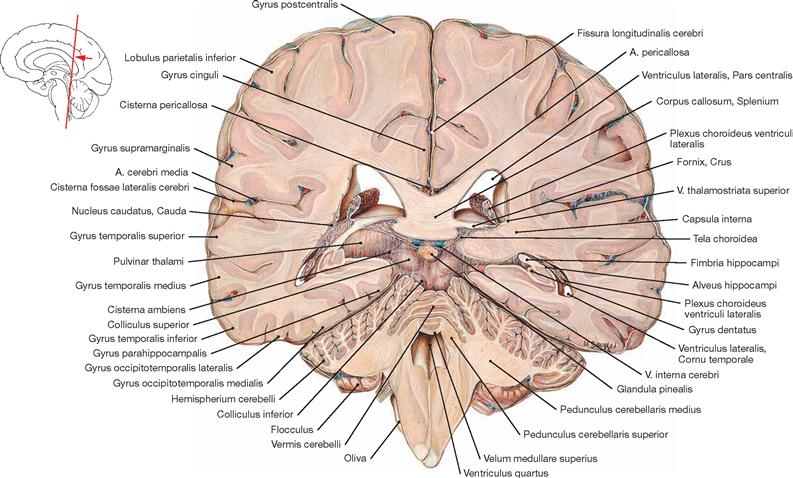
Fig. 12.118 Brain, Encephalon; frontal section at the level of the pineal gland and the fourth ventricle; posterior view.
The Splenium of the Corpus callosum and the Glandula pinealis are displayed in the centre of the image. Lateral thereof the Colliculi superiores and the Pulvinar thalami are shown. The Pedunculi cerebellares superiores are visible in the lateral brainstem slightly above the Ventriculus quartus.
Brain, horizontal sections
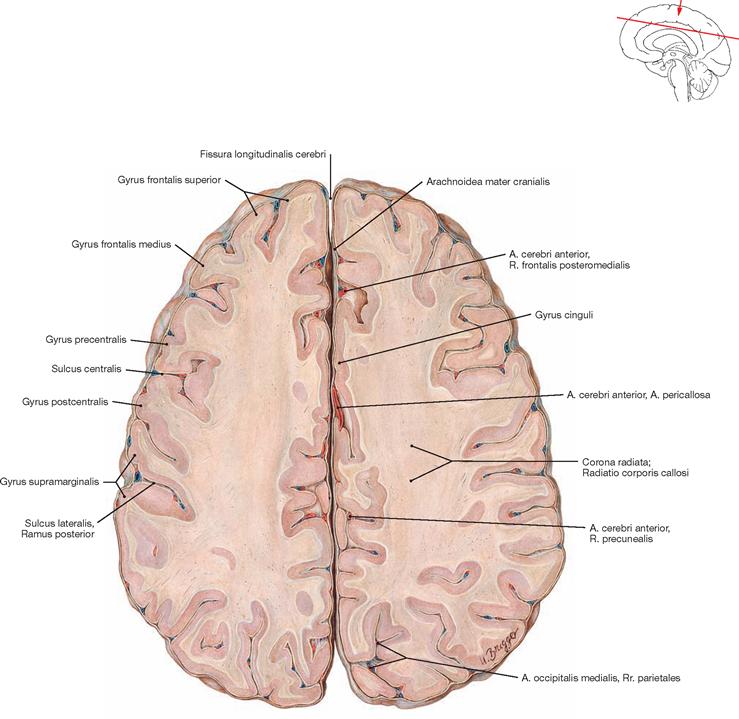
Fig. 12.120 Brain, Encephalon; horizontal section just above the Corpus callosum; superior view.
The brain has been sectioned immediately above the Corpus callosum. At this level, there are still no nuclei visible. In the broad band of white matter, fibres projecting from the Thalamus to the Cortex (Corona radiata) mix with commissural fibres of the Corpus callosum connecting the two hemispheres (Radiatio corporis callosi). In addition, fibre tracts converging on the Capsula interna contribute to the white matter (→ Figs. 12.74 to 12.76). Age-related atrophy of the brain makes the subarachnoid space appear wider (→ Figs. 12.121 to 12.130).
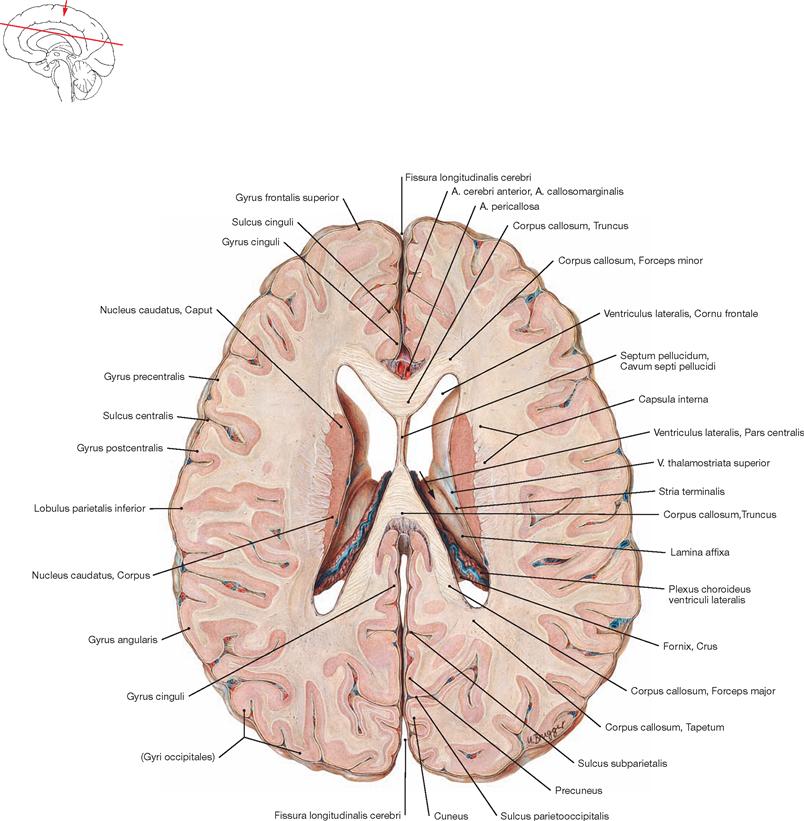
Fig. 12.121 Brain, Encephalon; horizontal section at the level of the central part of the lateral ventricles; superior view.
The Septum pellucidum extends between the body and fornix (not visible) of the Corpus callosum and separates the Ventriculi laterales. Lateral to the Ventriculi laterales, the head and body of the Nucleus caudatus are sectioned. The Capsula interna is located lateral to the nucleus.
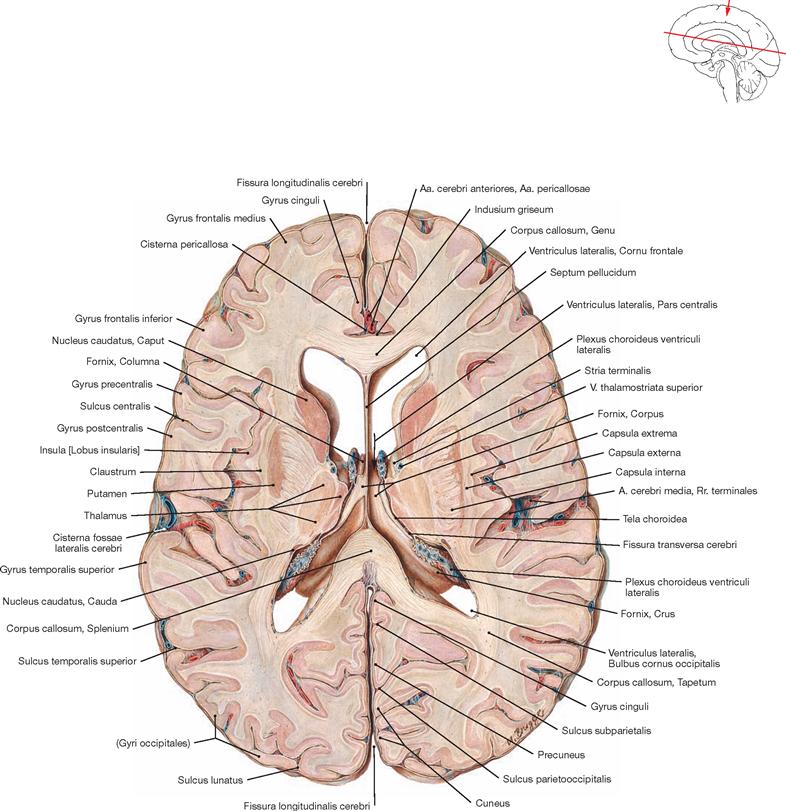
Fig. 12.122 Brain, Encephalon; horizontal section at the level of the floor of the central part of the lateral ventricles; superior view.
This central section shows parts of the Thalamus lateral to the Ventriculi laterales. Anterior and posterior to the Thalamus, the head and tail of the Nucleus caudatus are visible, respectively. Lateral to the Thalamus, the Capsula interna, Putamen, Capsula externa, Claustrum, Capsula extrema, and Gyri insulae are arranged from medial to lateral. The genu of the Corpus callosum locates to the anterior midline and its splenium is visible in the posterior midline.
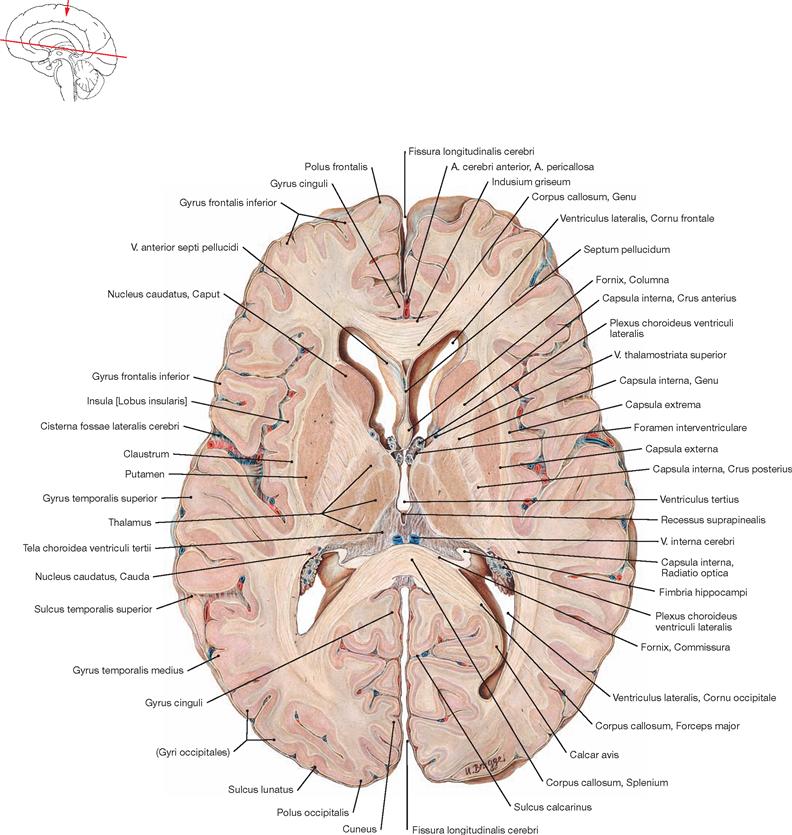
Fig. 12.123 Brain, Encephalon; horizontal section at the level of the upper part of the third ventricle; superior view.
In the central part of the image, the Ventriculus tertius is shown, with parts of the Ventriculi laterales as well as the Genu and Splenium of the Corpus callosum depicted anterior and posterior of the Ventriculus tertius. Head and tail of the Nucleus caudatus, Thalamus, Putamen, and Claustrum constitute the cerebral nuclei. The Capsula interna with its characteristic genu runs in between the large nuclei. In addition, the Radiatio optica of the Capsula interna is visible.
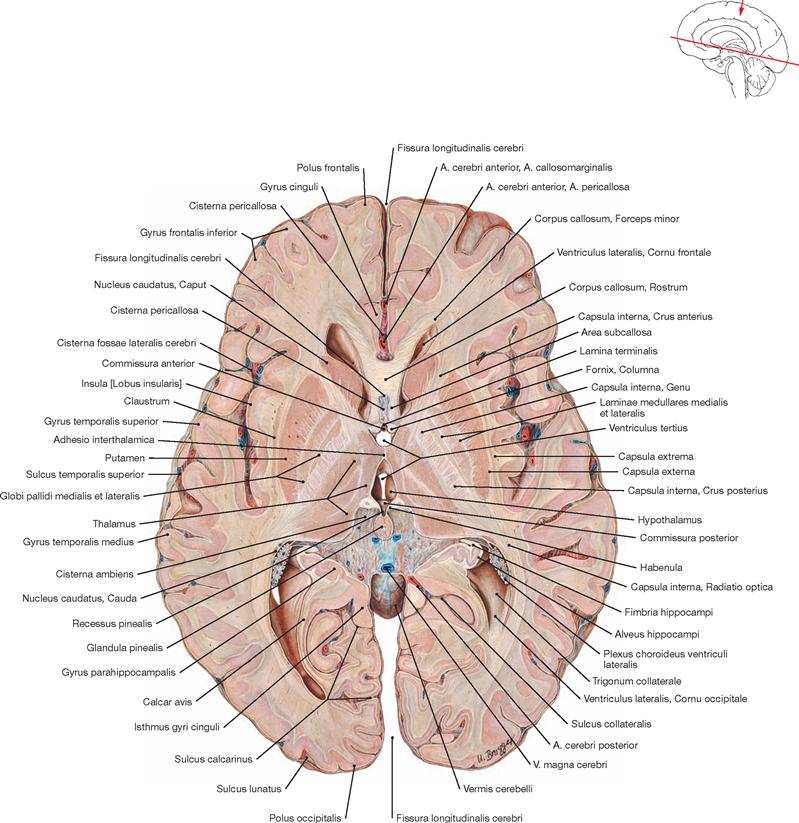
Fig. 12.124 Brain, Encephalon; horizontal section through the centre of the third ventricle at the level of the Adhesio interthalamica; superior view.
The section is centered through the Glandula pinealis and the Adhesio interthalamica. Lateral thereof the Thalamus, Capsula interna, Globus pallidus, Putamen, Capsula externa, Claustrum, Capsula extrema, and the Lobus insularis are located. The Fimbria hippocampi, the Alveus hippocampi, and the Gyrus parahippocampalis are also discernible.
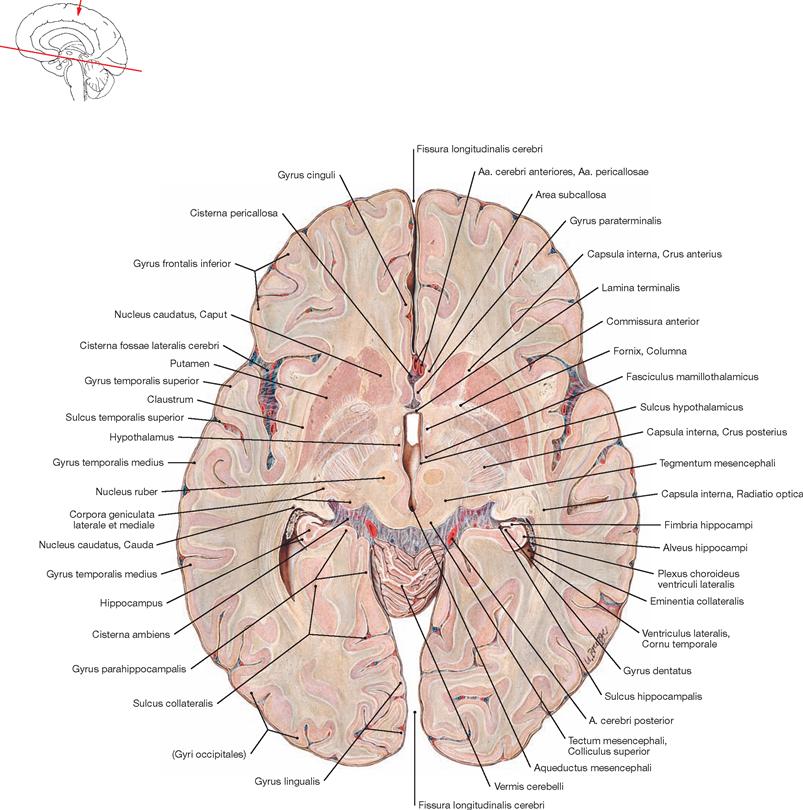
Fig. 12.125 Brain, Encephalon; horizontal section through the third ventricle at the level of the opening of the cerebral aqueduct; superior view.
Due to its reddish colouration, the Nucleus ruber prominently figures in this section plane. The close relationship between the Nucleus caudatus and Putamen also becomes obvious. The Crus anterius of the Capsula interna runs between both nuclear structures. The section is located at the transition from the third ventricle to the Aqueductus mesencephali, with both structures being sectioned. The upper rim of the Vermis cerebelli is sectioned as well.
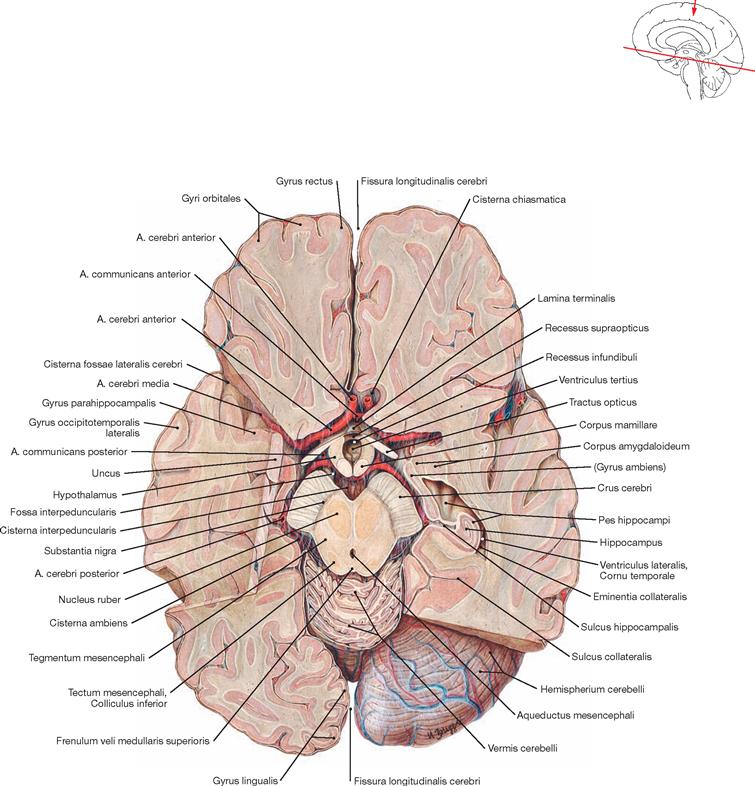
Fig. 12.126 Brain, Encephalon; staggered horizontal section through the floor of the third ventricle at the level of the Corpora mamillaria; superior view.
The Tractus optici, the Hypothalami, the Corpora mamillaria, the Crura cerebri, the Nuclei rubri, and the Colliculi inferiores of the Tectum mesencephali are sectioned. On the right side, the Hippocampus is visible, on the left side the grey and white matter of the temporal and occipital lobes are shown. Removal of the occipital pole on the right side allows an unobstructed view on the Hemispherium cerebelli.
Brain, sagittal sections
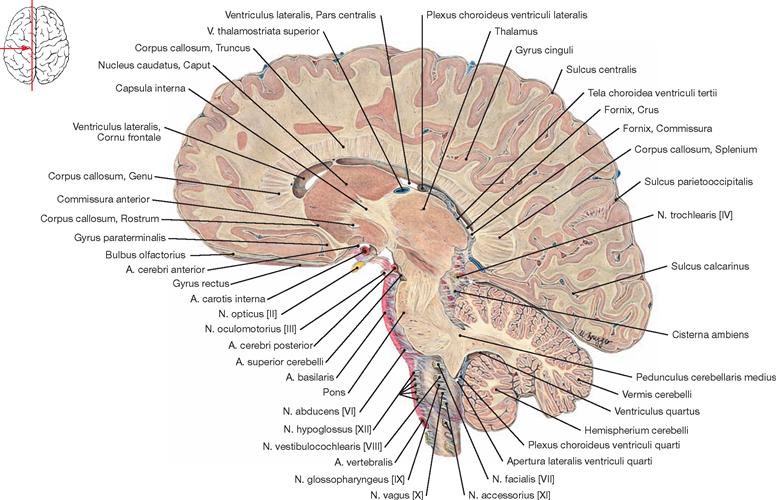
Fig. 12.127 Brain, Encephalon; sagittal section through the left hemisphere at the level of the head of the Nucleus caudatus; view from the left side.
A paramedian section shows the Corpus callosum in its entire rostro-occipital dimension. The Ventriculus lateralis positions below the Corpus callosum and, further below, the Nucleus caudatus, Thalamus, Capsula interna, and N. opticus [II] are located. The A. basilaris runs in front of the brainstem. The Pedunculus cerebellaris medius marks the transition from the Pons to the Cerebellum.
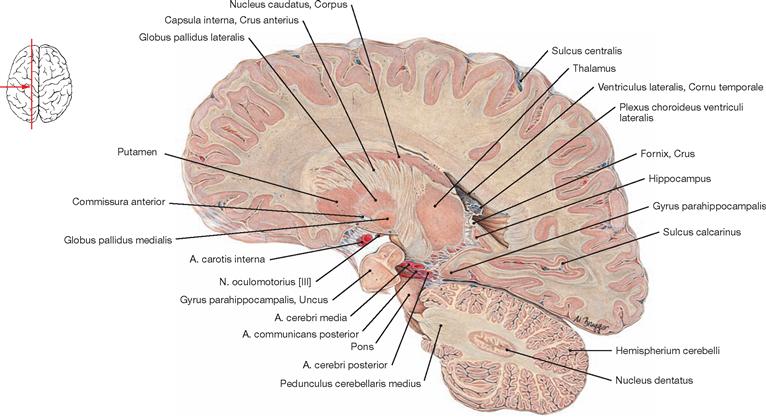
Fig. 12.128 Brain, Encephalon; sagittal section through the left hemisphere at the level of the body of the Nucleus caudatus; view from the left side.
Apart from the body of the Nucleus caudatus, the Crus anterius of the Capsula interna, the Thalamus, the Putamen, the Globus pallidus, and the Uncus of the Gyrus parahippocampalis have been sectioned. The Nucleus dentatus visualises prominently in the section plane through the Cerebellum.
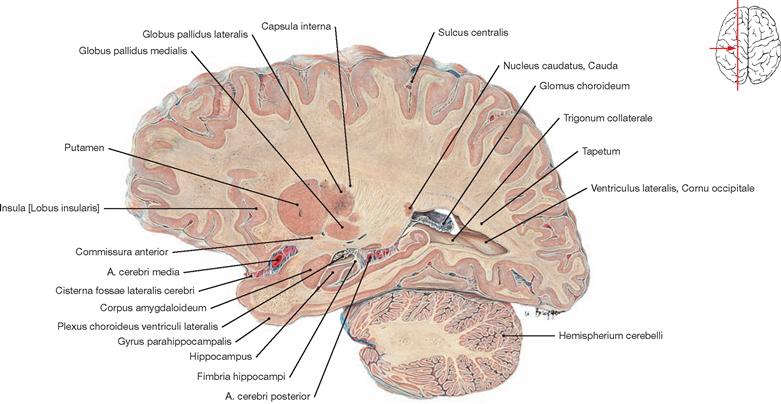
Fig. 12.129 Brain, Encephalon; sagittal section through the left hemisphere at the level of the Corpus amygdaloideum; view from the left side.
This section reveals the Hippocampus, the Fimbria hippocampi, and the tail of the Nucleus caudatus posterior to the Corpus amygdaloideum. In addition, the Putamen, the Globus pallidus, and the Capsula interna are discernible. The inferior part of this section shows the Hemispherium cerebelli.
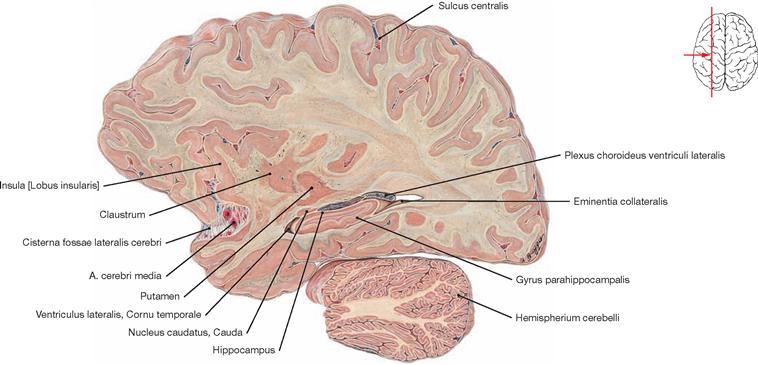
Fig. 12.130 Brain, Encephalon; sagittal section through the left hemisphere at the level of the apex of the Cornu temporale of the Ventriculus lateralis; view from the left side.
This lateral section shows the Lobus insularis and includes the Hippocampus with the Gyrus parahippocampalis, the Claustrum, and the Putamen.
Overview
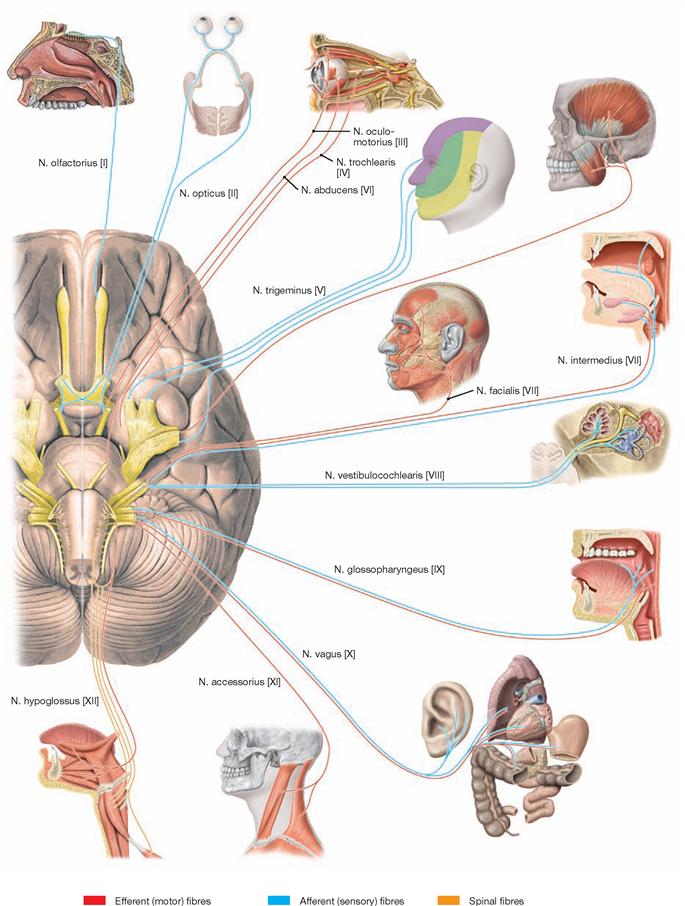
Fig. 12.131 Cranial nerves, Nn. craniales; functional overview of the Telencephalon, Cerebrum, brainstem, Truncus encephali, and Cerebellum; inferior view.
Twelve pairs of cranial nerves exit the cranial base. They are numbered in Roman digits (I–XII) according to the order in which they exit the brainstem from anterior to posterior. The Fila olfactoria constitute the first cranial nerve, collectively named N. olfactorius [I]. Through the fila, the bipolar olfactory neurons (an unnamed sensory ganglion is located within the olfactory mucosa) project into the Bulbus olfactorius, a part of the Telencephalon that was relocated cranially during development. Thus, the bulbus is the Nucleus terminationis for the N. olfactorius [I], with the exception that this nucleus does not reside in the brainstem but locates outside on the Lamina cribrosa. The fact that the neurons of the first cranial nerve are very short and the Nucleus terminationis resides outside of the brainstem are distinguishing features, separating the first from the other cranial nerves. The N. opticus [II] is exceptional as it includes the 3rd and possibly 4th neuron of the visual pathway. Contrary to all other cranial nerves, the optic nerve is a protrusion of the Diencephalon and not actually a peripheral nerve.![]()
Cranial nerves
Topography
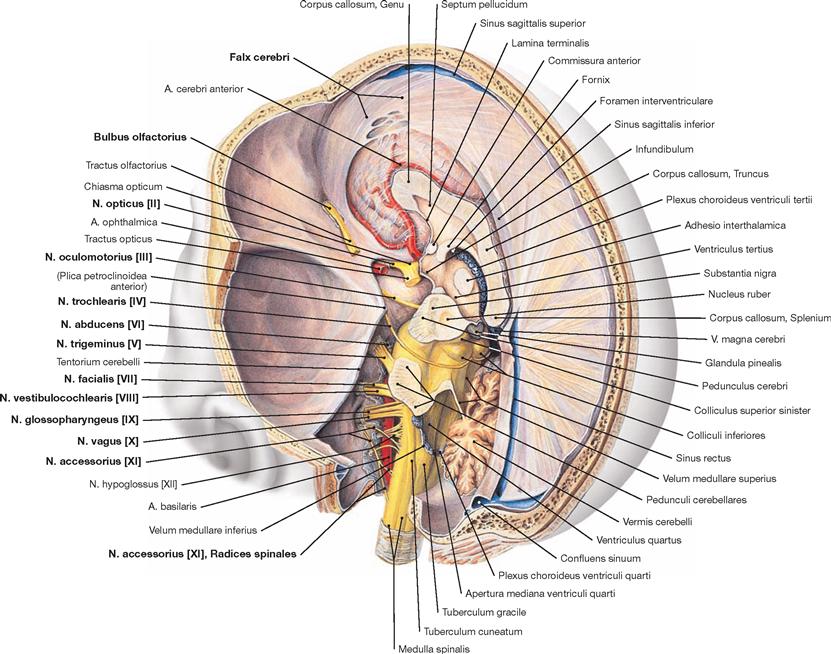
Fig. 12.132 Course of the cranial nerves, Nn. craniales, in the subarachnoid space; posterior superior view from the left side; the left hemisphere of the Cerebrum and the Cerebellum as well as the Tentorium cerebelli have been removed.
The cranial nerves III–XII exit the brainstem in chronological order from cranial to caudal. Some exit as a loose bundle of nerve roots and form the actual cranial nerve (IX–XII) later. The N. trochlearis [IV] not only is the thinnest cranial nerve but also uniquely exits from the posterior side of the brainstem. The N. abducens [VI] has the longest intradural course before exiting through its opening at the cranial base.![]()
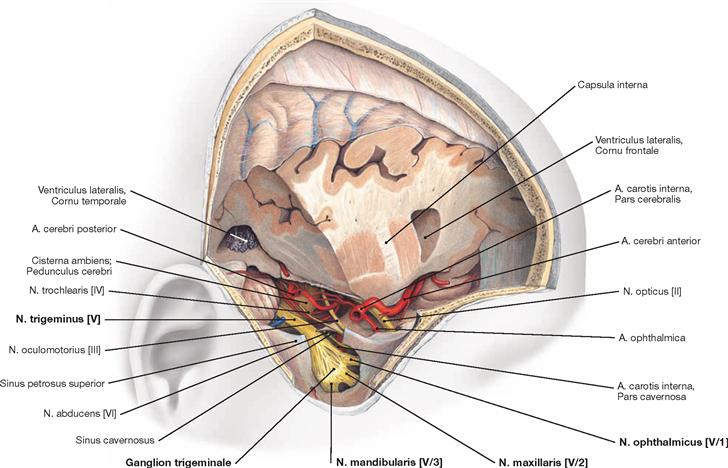
Fig. 12.133 Course of the cranial nerves, Nn. craniales, in the middle cranial fossa, Fossa cranii media; view from the right side.
Large parts of the frontal and temporal lobes were removed to allow an unobstructed view on the cranial base below. The Cavum trigeminale (MECKEL’s cave) has been opened. Located within is the Ganglion trigeminale (V, Ganglion semilunare, clinical term: Ganglion GASSERI), with the three main branches of the N. trigeminus (N. ophthalmicus [V/1], N. maxillaris [V/2], N. mandibularis [V/3]). In addition to the N. trigeminus [V], parts of the Nn. opticus [II], oculomotorius [III] and trochlearis [IV] and arteries originating from the Pars cerebralis of the A. carotis interna (A. ophthalmica, A. cerebri anterior) are visible.![]()
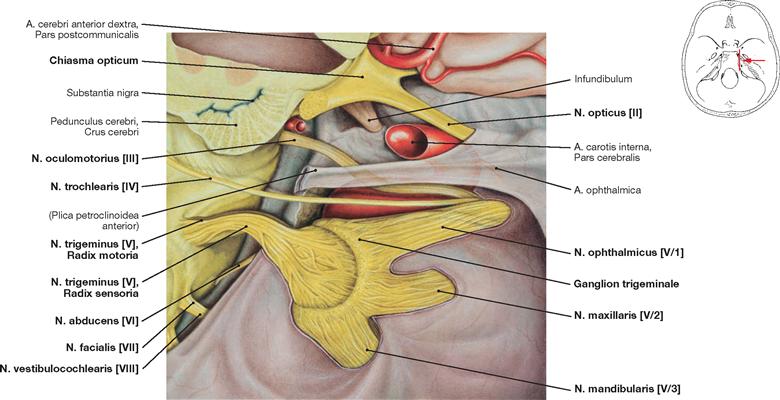
Fig. 12.134 Arteries and nerves in the region of the Sella turcica and the Sinus cavernosus; view from the right side.
The Cavum trigeminale (MECKEL’s cave) has been opened by removing the Dura mater cranialis and the Arachnoidea mater at this site. Visible is the Ganglion trigeminale (V, Ganglion semilunare, clinical term: Ganglion GASSERI) with the three trigeminal nerve branches. In addition, the course of the cranial nerves III, IV, and VI to VIII from exiting the brainstem to entering the cranial base is shown. The Pars cavernosa of the A. carotis interna transitions into the Pars cerebralis which lies close to the N. opticus [II]. The Chiasma opticum is located above the hypophyseal stalk (Infundibulum).![]()
Nuclei of the cranial nerves
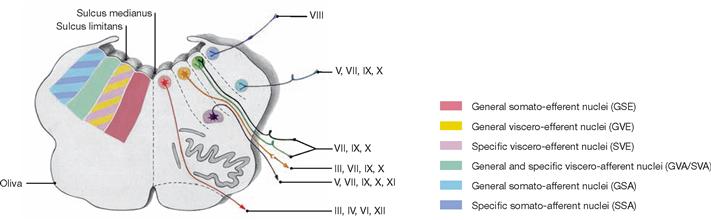
Fig. 12.135 Cranial nerves, Nn. craniales; schematic cross-section through the rhomboid fossa demonstrating the nuclei.
In the brainstem, nuclei with similar functions are arranged in a column in a cranial to caudal direction. Due to spatial restrictions, the nuclei form four longitudinal nuclear columns and are arranged alongside each other. This includes in a medial to lateral direction a somato-efferent, a viscero-efferent, a viscero-afferent, and a somato-afferent column of nuclei. Within the viscero-efferent, the viscero-afferent, and the somato-afferent columns of nuclei, one can distinguish general and specific afferent nuclei.
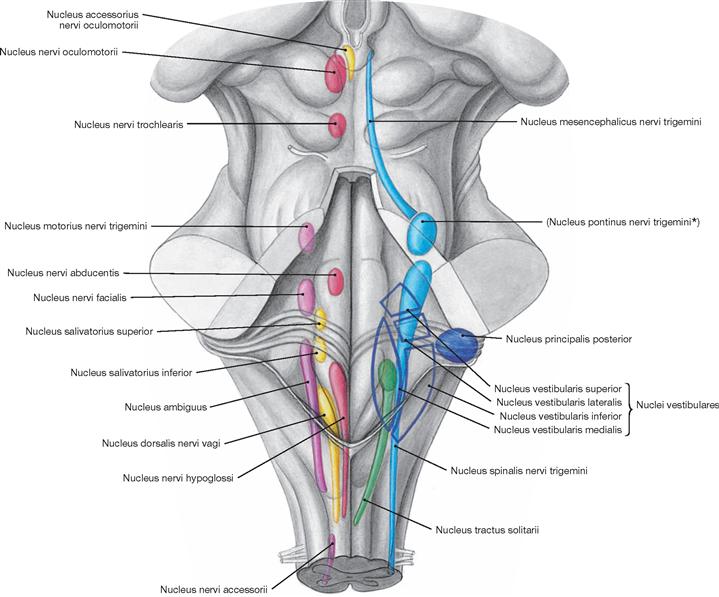
Fig. 12.136 Cranial nerves, Nn. craniales; topographic overview of the nuclei; posterior view.
With the exception of the cranial nerves I and II, all cranial nerves (III–XII) have nuclei located in the brainstem. The Mesencephalon contains the nuclei of the cranial nerves III and IV, the nuclei of the cranial nerves V to VII lie in the Pons, and the Medulla oblongata contains the nuclei of the cranial nerves VII to XII.
It is easy to understand the topographic arrangement of the nuclei of the cranial nerves if one keeps in mind the separation into functional nuclear columns (→ Fig. 12.135). On the left side are the nuclei of origin (Nuclei originis) which contain the perikarya of the efferent neurons projecting into the periphery. In the terminal nuclei (Nuclei terminationes) on the right side, the afferent fibres derived from the periphery synapse onto the 2nd neuron of the sensory tract.![]()
* clinical term: Nucleus sensorius principalis nervi trigemini
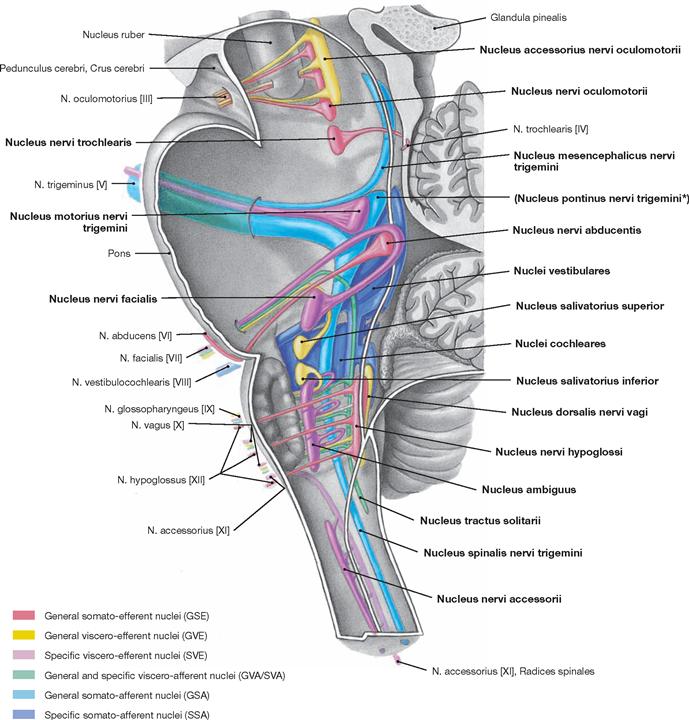
Fig. 12.137 Cranial nerves, Nn. craniales; topographic overview of the nuclei of the cranial nerves III to XII in the median plane.
Nuclei of origin (Nuclei originis) with perikarya of the efferent/motor fibres divide into:
• general somato-efferent nuclei (Nuclei nervi oculomotorii [III, extraocular muscles], trochlearis [IV, M. obliquus superior], abducens [VI, M. rectus lateralis], and hypoglossi [XII, muscles of the tongue])
• general viscero-efferent nuclei (Nuclei accessorius nervi oculomotorii [III, Mm. sphincter pupillae and ciliaris], salivatorius superior [VII, Glandulae submandibularis, sublingualis, lacrimalis, nasales and palatinales], salivatorius inferior [IX, Glandula parotidea], dorsalis nervi vagi [X, viscera])
• special viscero-efferent nuclei (Nuclei motorius nervi trigemini [V, masticatory muscles, muscles of the floor of the mouth], nervi facialis [VII, mimic muscles], ambiguus [IX, X, Radix cranialis of XI, pharyngeal and laryngeal muscles] and Nucleus nervi accessorii [XI, Radix spinalis, shoulder muscles])
Terminal nuclei (Nuclei terminationes) are targeted by afferent/sensory fibres and divide into:
• general viscero-afferent nuclei (Nuclei tractus solitarii, Pars inferior [IX, X, sensory innervation of smooth muscles (viscera)])
• special viscero-afferent nuclei (Nuclei tractus solitarii, Pars superior [VII, IX, X], taste fibres)
• general somato-afferent nuclei (Nuclei mesencephalicus nervi trigemini [V, proprioception of masticatory muscles], pontinus (sensorius principalis) nervi trigemini [V, touch, vibration, position of temporomandibular joint], spinalis nervi trigemini [V, pain and temperature sensation in the head region])
• special somato-afferent nuclei (Nuclei vestibulares superior, lateralis, medialis and inferior [VIII, vestibularis part, equilibrium] as well as Nuclei cochleares anterior and posterior [VIII, cochlear part, hearing]
* clinical term: Nucleus sensorius principalis nervi trigemini![]()
N. olfactorius [I]
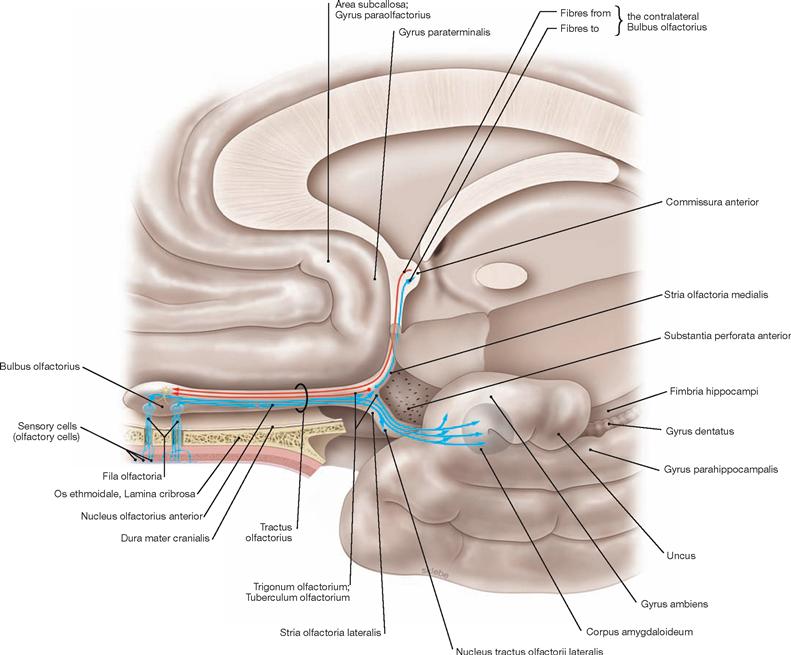
Fig. 12.138 N. olfactorius [I], with olfactory nerves, Nn. olfactorii (Fila olfactoria), and olfactory tract; view from the left side.
An area of 3 cm2 of olfactory mucosa (Regio olfactoria) locates to both sides at the roof of the nasal cavity. It contains approximately 30 million receptor cells (olfactory sensory cells) which respond to chemical signals. These are bipolar neurons (olfactory neurons, 1st neuron, SSA). On the one side, they connect with the outer environment and on the other side their axons form the Fila olfactoria. The olfactory neurons have a short life span of 30–60 days and are replaced by neuronal stem cells throughout life.
The Fila olfactoria are collectively named N. olfactorius [I]. In each bulbus, they converge onto approximately 1000 glomeruli. From here, the olfactory information reaches different areas at the cranial base and the temporal lobe (primary olfactory cortical area) and, through direct and indirect connections, projects to secondary olfactory cortical areas and other brain regions, including the Hypothalamus. That way, the conscious realisation of olfactory stimuli and the connection with other sensory perceptions is accomplished.![]()
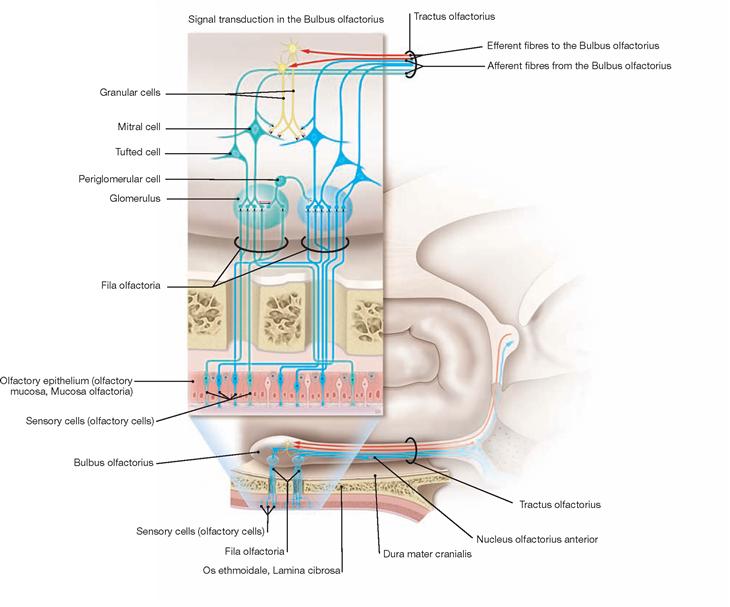
Fig. 12.139 Scheme of the projections and synaptic connections of the Fila olfactoria; view from the left side.
In each bulbus, all Fila olfactoria converge onto approximately 1000 glomeruli (in the figure two glomeruli are demonstrated as an example) which collectively form the Tractus olfactorius. Multiple synapses within the glomeruli finally converge on the mitral cells (2nd neuron). The axons of all neurons possessing the identical odorant receptor reach the glomerulus that is specific for each of the approximately 1000 different olfactory receptors. Mitral cells of the olfactory bulb project to different areas at the cranial base and the temporal lobe (→ Fig. 12.138). Feedback mechanisms increase the discrimination of odorant stimuli and involve granular cells that connect back with different mitral cells.
N. opticus [II]
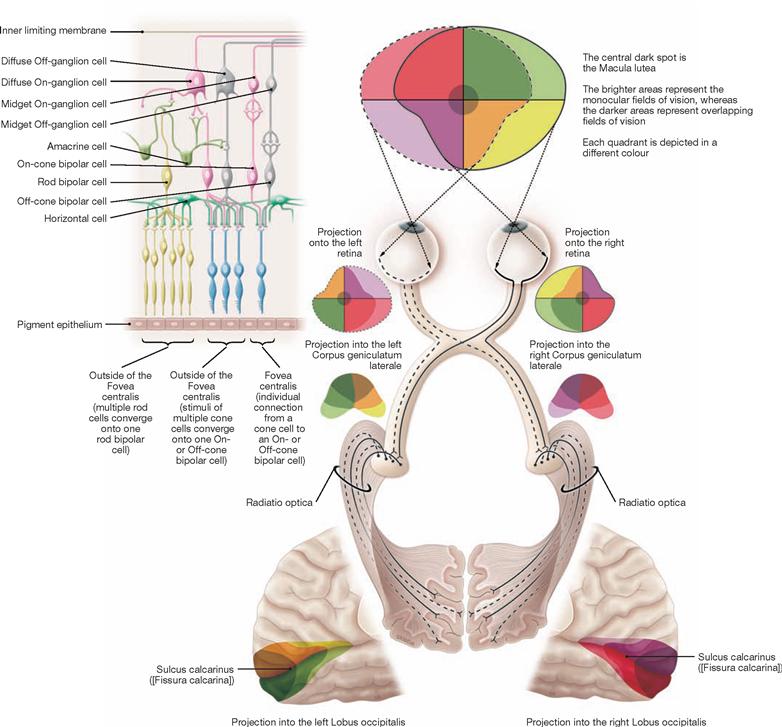
Fig. 12.140 Neuronal network in the retina, Retina, and the central visual tract; strongly simplified schematic representation.
Cone cells (1st neuron) direct the information to cone bipolar cells (2nd neuron) and ganglion cells (3rd neuron). Horizontal and amacrine cells modify the processing of information. The axons of the ganglion cells form the N. opticus [II]. The above-mentioned network of connections represented by an intraretinal chain of three neurons only applies to cone cells (for rod cells → Fig. 12.141 and textbooks of histology). For the central visual tract → pages 131 and 132.![]()
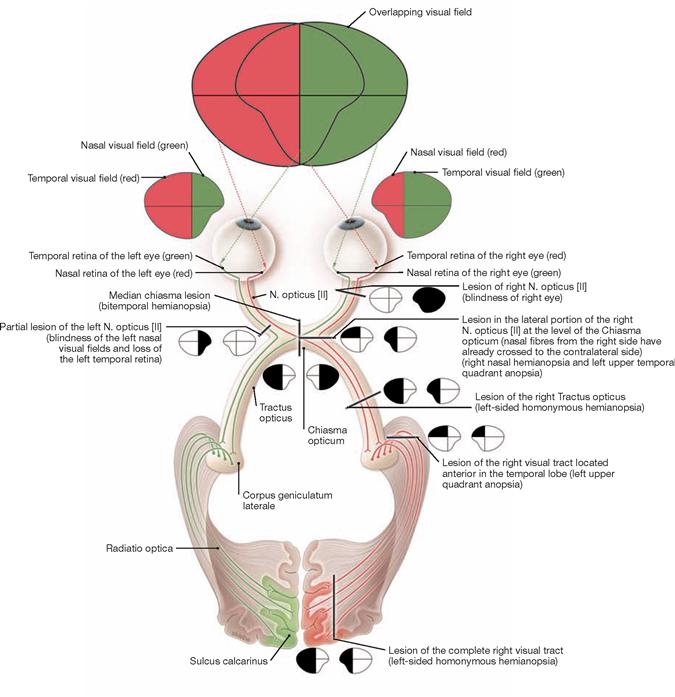
Fig. 12.141 N. opticus [II] and visual tract. [23]
The visual tract starts at the retina which contains the first three projection neurons and interneurons (horizontal cells, amacrine cells) (→ Fig. 12.140).
Up to 40 rod cells transmit their signals to a single rod bipolar cell. From here, the information is transmitted indirectly through amacrine cells (depending on the literature, today there are 20 to 50 different types described) to ganglion cells. Thus, an intraretinal chain of four neurons exists for the rod cells. The axons of the ganglion cells run in the N. opticus to the Chiasma opticum, where the fibres of the nasal part of the retina cross to the opposite side (red). The fibres of the temporal part do not cross (green).
Directly following the chiasma is the Tractus opticus which contains the fibres with visual information of the contralateral visual field. The major part of these fibres (Radix lateralis) synapse in the Corpus geniculatum laterale (CGL). Some fibres (Radix medialis) divert before reaching the CGL and project into the Area pretectalis, the Colliculus superior, and into the Hypothalamus. The GRATIOLET’s optic radiation (Radiatio optica) originates from the Corpus geniculatum laterale and projects into the region around the Sulcus calcarinus to the areas 17 and 18 of the cerebral cortex (Area striata).
N. oculomotorius [III], N. trochlearis [IV], N. abducens [VI]
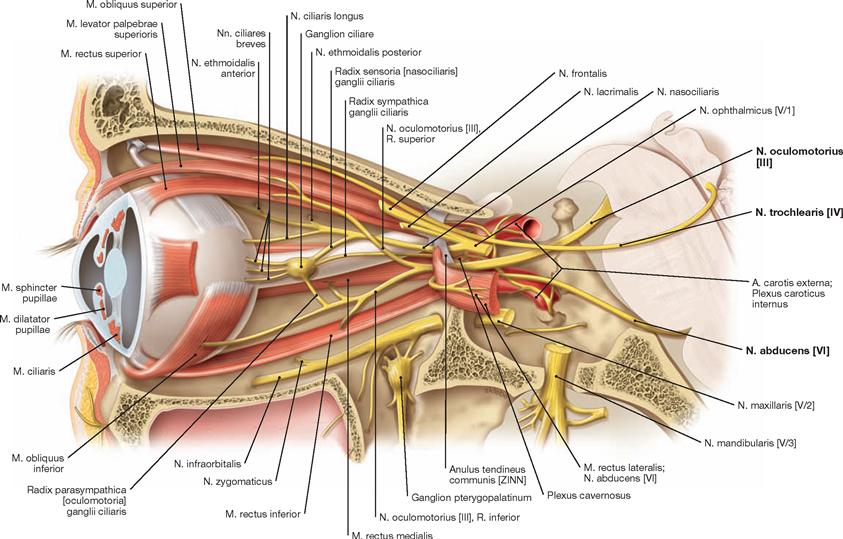
Fig. 12.142 Nn. oculomotorius [III], trochlearis [IV] and abducens [VI], left side; lateral view; orbit opened, orbital fat body removed, the M. rectus lateralis was sectioned close to its insertion and deflected.
The N. oculomotorius [III] innervates the extra-ocular muscles with the exception of the M. obliquus superior (N. trochlearis [IV]) and the M. rectus lateralis (N. abducens [VI]). The parasympathetic part of the cranial nerve III innervates the M. sphincter pupillae and the M. ciliaris (two intrao-cular muscles).![]()
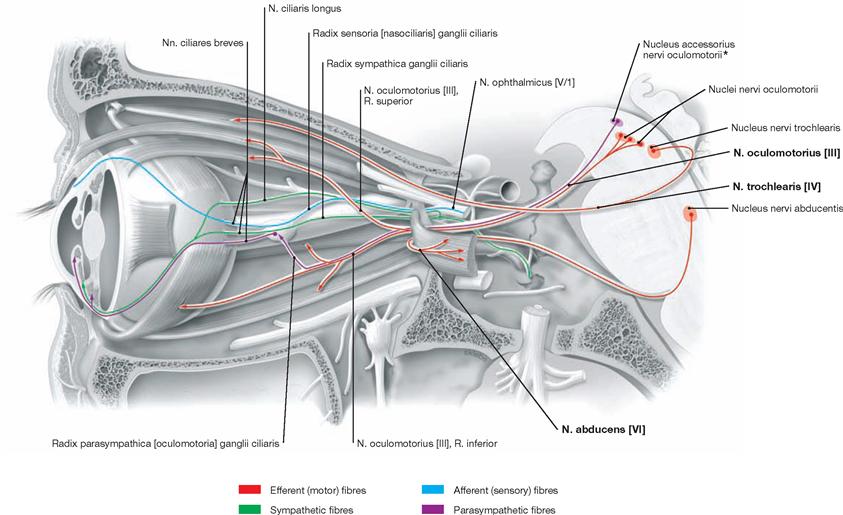
Fig. 12.143 Fibre qualities of the Nn. oculomotorius [III], trochlearis [IV], and abducens [VI], left side; lateral view.
The N. oculomotorius [III] contains motor fibres (GSE) derived from the Nucleus nervi oculomotorii for the major part of extra-ocular muscles. In the orbit, the nerve divides into a R. superior to innervate the Mm. rectus superior and levator palpebrae superioris and a R. inferior for the innervation of the Mm. rectus medialis, rectus inferior, and obliquus inferior. The Nucleus accessorius nervi oculomotorii (EDINGER-WESTPHAL) contributes parasympathetic fibres (GVE) which reach the Ganglion ciliare through the R. inferior as well as a Radix parasympathica (oculomotoria). In the Ganglion ciliare, preganglionic parasympathetic fibres synapse onto postganglionic neurons. The postganglionic fibres project alongside the Nn. ciliares breves to the Bulbus oculi, traverse its wall, and reach the intra-ocular Mm. ciliaris and sphincter pupillae.
The N. trochlearis [IV] contains motor fibres (GSE) for the M. obliquus superior from the Nucleus nervi trochlearis in the brainstem.
The N. abducens [VI] contains motor fibres (GSE) from the Nucleus nervi abducentis for the M. rectus lateralis.
* EDINGER-WESTPHAL nucleus
N. trigeminus [V]
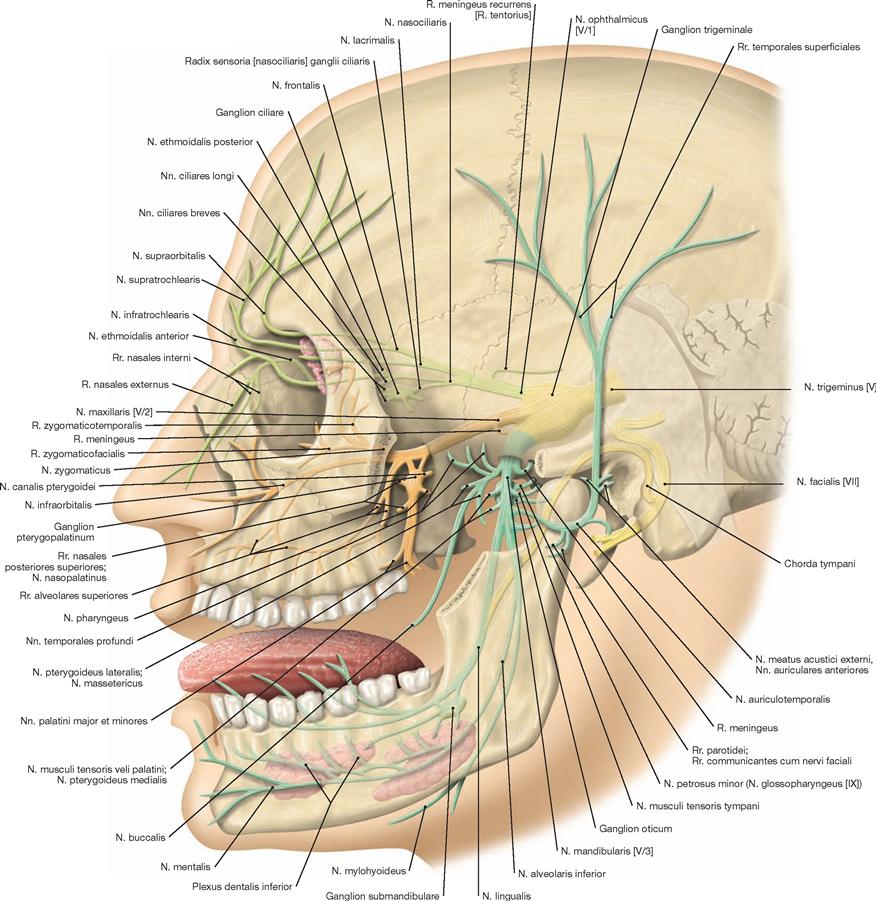
Fig. 12.144 N. trigeminus [V], left side; lateral view.
The trigeminal nerve [V] is the nerve of the first pharyngeal arch and divides into the three main branches: Nn. ophthalmicus [V/1] (bright green), maxillaris [V/2] (orange), and mandibularis [V/3] (turquoise). It mainly carries general somato-afferent (GSA) fibres, some special viscero-efferent (SVE) fibres, and motor fibres (V/3).
The N. ophthalmicus [V/1] innervates the eye (including cornea and conjunctiva), the skin of the upper eyelid, forehead, back of the nose, the nasal and paranasal mucosa. Parasympathetic fibres innervate the lacrimal gland and associate with the peripheral course of the N. ophthalmicus [V/1].
The N. maxillaris [V/2] innervates the skin of the anterior temporal region and the upper cheek as well as the skin below the eye. In addition, this nerve provides sensory fibres to the palate, the teeth of the upper jaw, the gingiva, and the mucosa of the Sinus maxillaris.
The N. mandibularis [V/3] innervates the masticatory muscles, two muscles at the floor of the mouth (M. mylohyoideus and Venter anterior of the M. digastricus), as well as the Mm. tensor veli palatini and tensor tympani. It also contributes sensory branches to the skin of the posterior temporal region, the cheek, and the chin, and innervates the teeth and gingiva of the lower jaw. Parasympathetic fibres for the large salivary glands as well as taste fibres for the tongue associate with branches of the N. mandibularis [V/3]. The latter also provides sensory fibres for the anterior two-thirds of the tongue.![]()
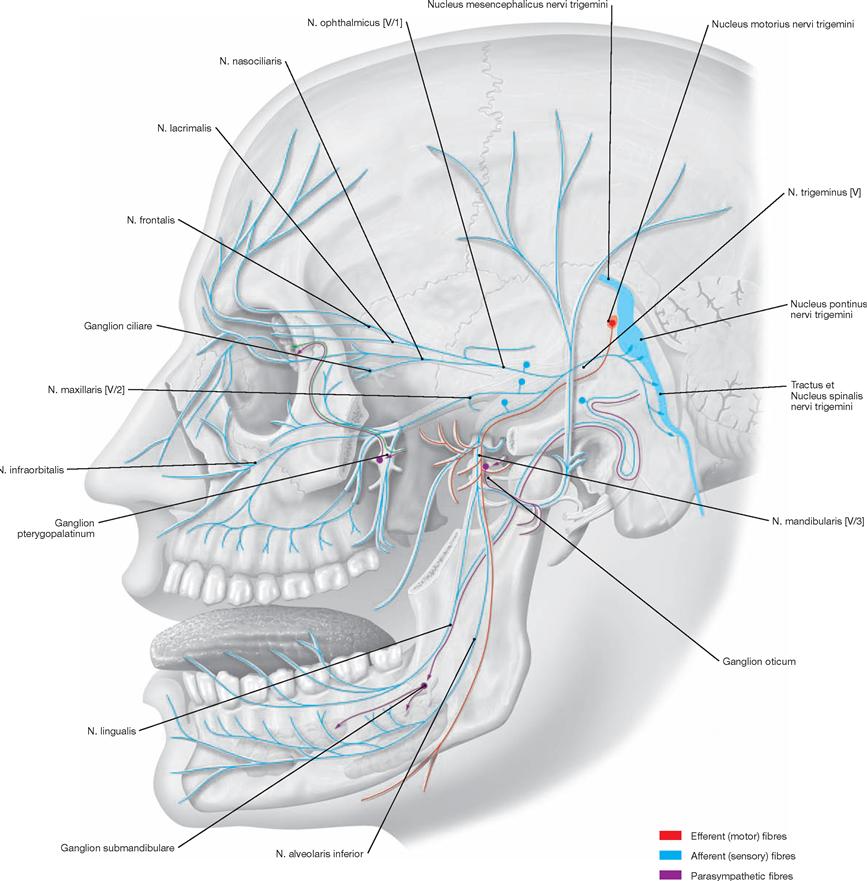
Fig. 12.145 Fibre qualities of the N. trigeminus [V], left side; lateral view.
Nuclei of origin and terminal nuclei of the N. trigeminus [V] are the Nucleus mesencephalicus nervi trigemini (somato-sensory), the Nucleus pontinus (sensorius principalis) nervi trigemini (somato-sensory), the Nucleus spinalis nervi trigemini (general somato-afferent, GSA), and the Nucleus motorius nervi trigemini (specific viscero-efferent, SVE).
The N. trigeminus [V] consists of a Radix sensoria (Portio major) and a Radix motoria (Portio minor). After exiting the Pons, the trigeminal nerve passes over the Clivus and reaches the Ganglion trigeminale (Ganglion semilunare, clinical term: Ganglion GASSERI, contains pseudo-unipolar neurons which provide the Nuclei pontinus and spinalis nervi trigemini with protopathic and epicritic sensory stimuli) and divides into its three main branches Nn. ophthalmicus [V/1], maxillaris [V/2], and mandibularis [V/3].
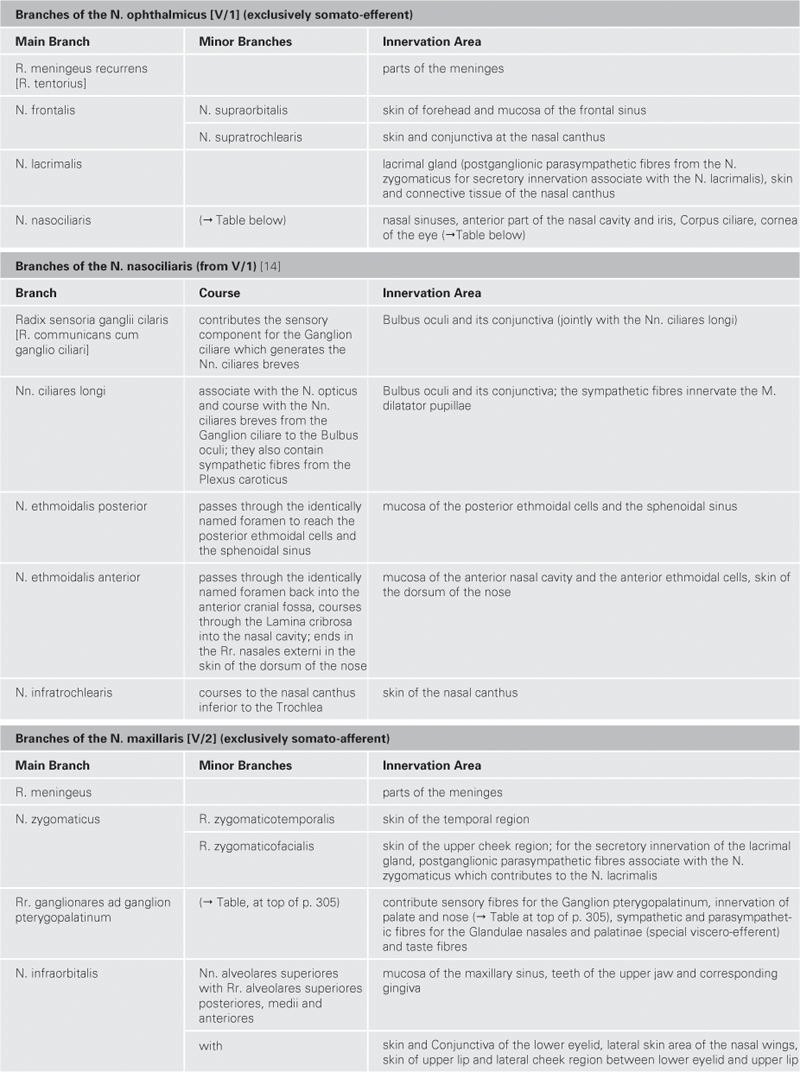
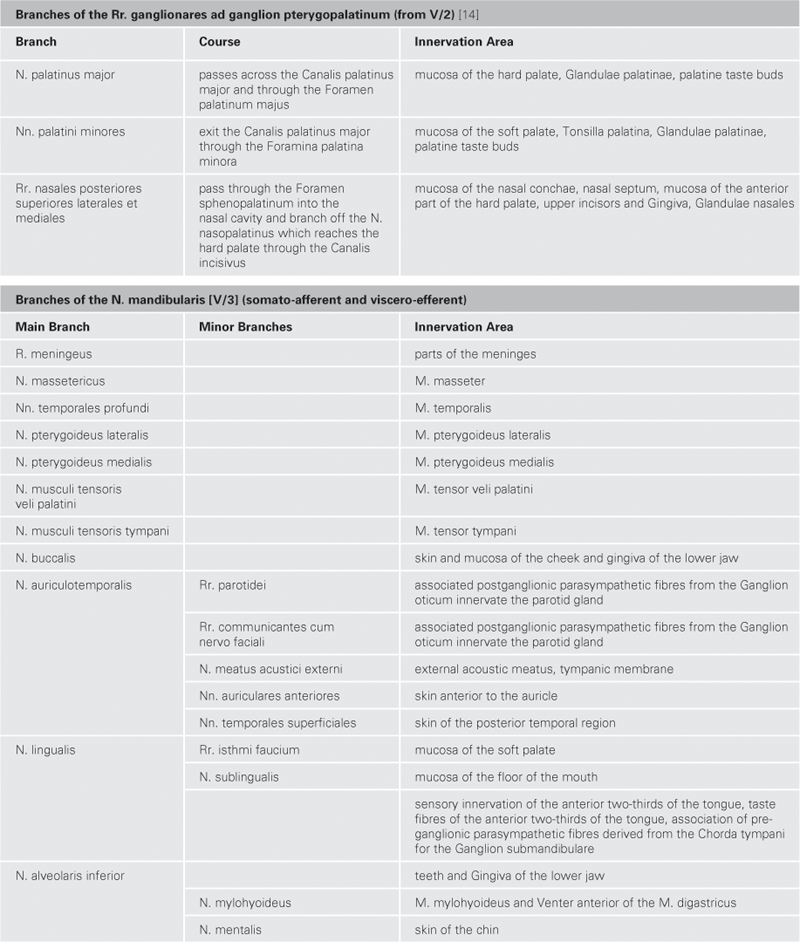
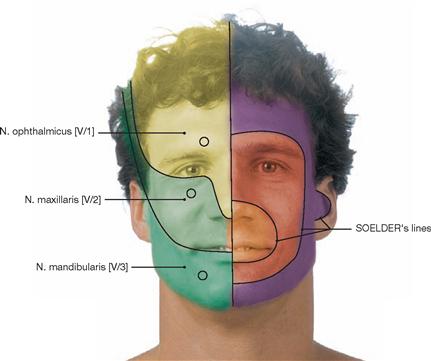
Fig. 12.146 Innervation areas of the facial skin, exit points for nerves, and protopathic sensibility.
On the left side of the face, the somatotopic order of the protopathic sensibility is demonstrated. The right side of the face shows the innervation areas and exit points for the three branches of the trigeminal nerve.
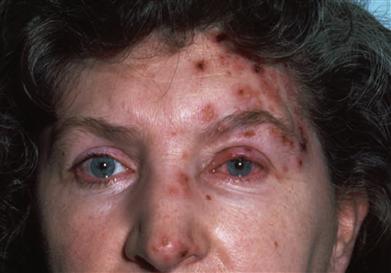
Fig. 12.147 Zoster ophthalmicus. [16]
Patient with Zoster ophthalmicus (skin in the innervation area of the first trigeminal branch is affected by the infection with varicella zoster virus, facial herpes zoster). The involvement of the surface epithelium of the eye (Cornea and Conjunctiva) is particularly dangerous (risk of blindness) and painful. The redness of the conjunctiva and the narrowing of the eyelids are clearly visible.
N. facialis [VII]
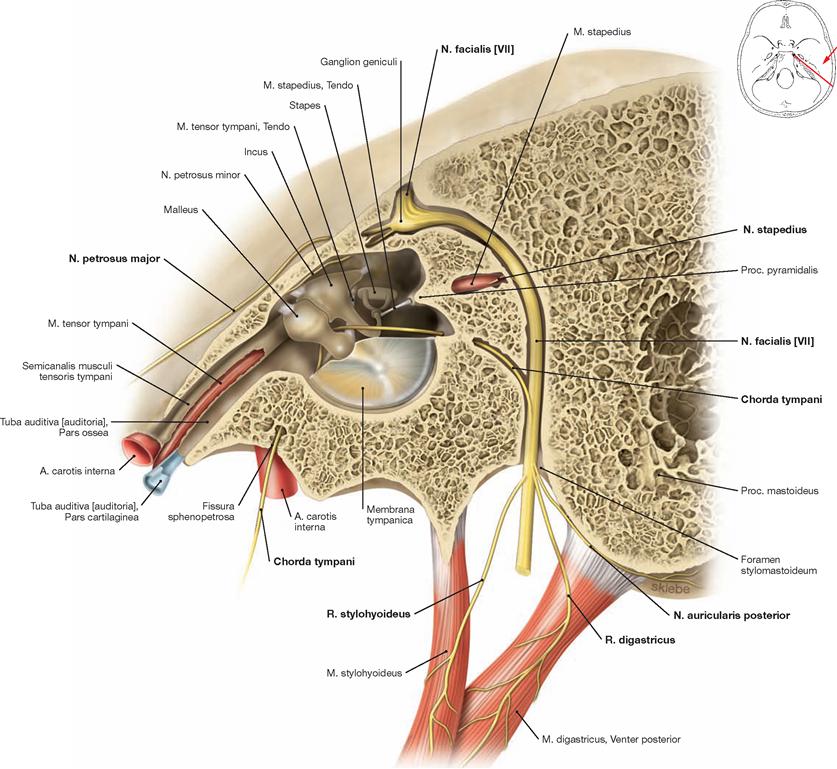
Fig. 12.148 Course of the N. facialis [VII]; vertical section through the Canalis facialis; view from the left side.
Approximately 1 cm after the N. facialis [VII] enters the petrosal part of the temporal bone through the Porus acusticus internus (not shown), the nerve makes a sharp bend, known as the external genu of the facial nerve. Here, the Ganglion geniculi is located. The main stem of the facial nerve runs within a bony canal towards the Foramen stylomastoideum. Along its way through the petrous bone, the N. facialis [VII] releases the Nn. petrosus major, stapedius, and the Chorda tympani (→ Table, p. 310).![]()
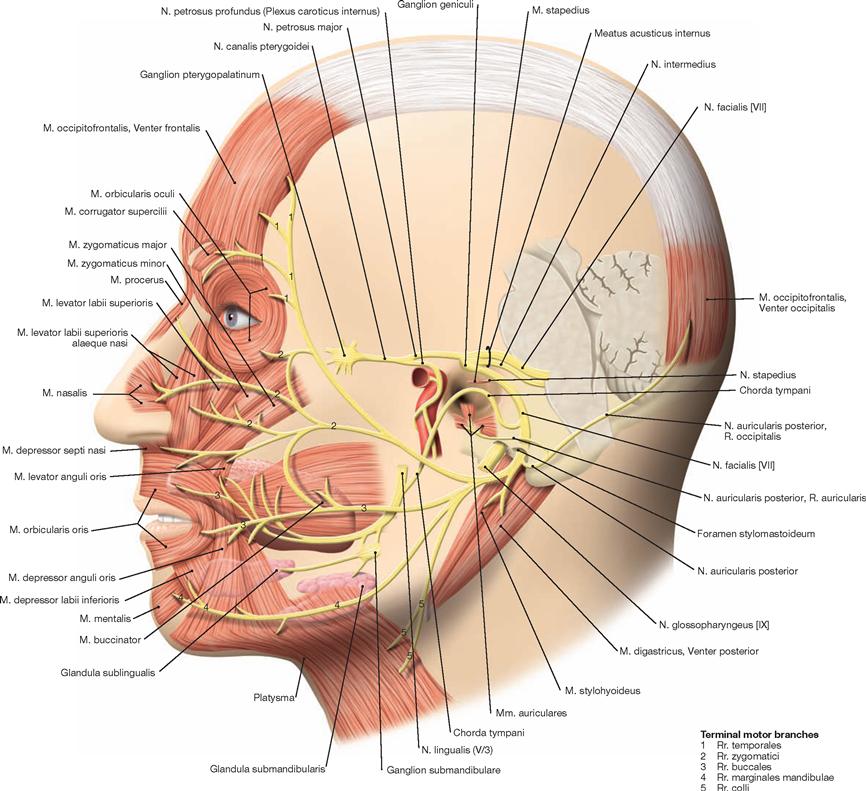
Fig. 12.149 N. facialis [VII], left side; lateral view.
The N. facialis [VII], the N. intermedius (a part of the N. facialis [VII] but often viewed as a separate nerve), and the N. vestibulocochlearis [VIII] jointly exit the cerebellopontine angle. Shortly thereafter, the N. intermedius and N. facialis [VII] unite. The N. facialis [VII] and N. vestibulocochlearis [VIII] project towards the petrous part of the temporal bone and enter the bone through the Porus and the Meatus acusticus internus. Upon release of the Nn. cochlearis and vestibularis, the N. facialis [VII] enters the Canalis facialis (→ also Fig. 12.153). Here the facial nerve makes a posterior inferior turn in an almost right angle (external genu of the facial nerve; → Fig. 12.148). The Ganglion geniculi is located just prior to the location of the turn of the facial nerve. Along its course within the Canalis facialis, this cranial nerve provides a number of branches (→ Table, p. 310). Upon exiting the cranial base through the Foramen stylomastoideum, the facial nerve turns rostral, provides additional branches, and then enters the Glandula parotidea. Here, the nerve divides into its terminal motor branches (Plexus intraparotideus; → Table, p. 310).![]()
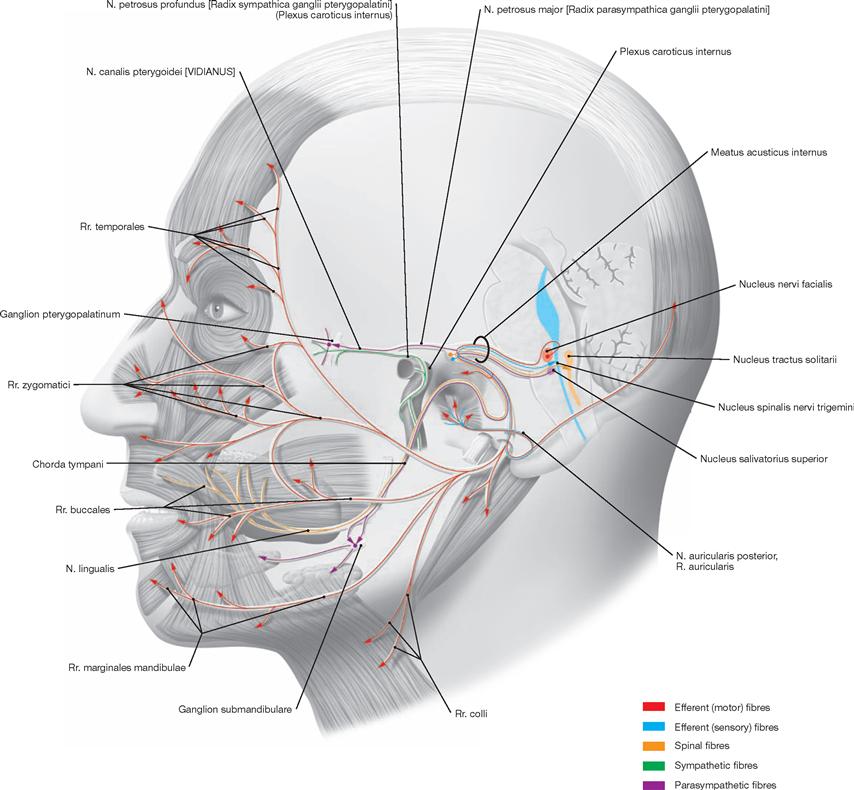
Fig. 12.150 Fibre qualities of the N. facialis [VII], left side; lateral view.
The N. facialis [VII] is the nerve of the second pharyngeal arch and has several different fibre qualities.
Its motor fibres (special viscero-efferent, SVE) derive from the Nucleus nervi facialis. These fibers course around the Nucleus nervi abducentis in a posterior arch (internal genu of the facial nerve). The upper part of the nucleus contains the neurons for the innervation of the mimetic muscles for the forehead and external orbit, whereas the lower part of the nucleus harbours the neurons innervating all mimic muscles located below the eye. The upper nuclear portion receives double innervation from both cortical hemispheres (→ Fig. 12.152). Thus, it receives corticonuclear fibres from the ipsilateral and contralateral sides. By contrast, the lower portion of the Nucleus nervi facialis exclusively receives corticonuclear fibres from the contralateral sides.
Preganglionic parasympathetic fibres derive from the Nucleus salivatorius superior (general viscero-efferent, GVE). They run with the intermedius part across the N. facialis [VII], course via the N. petrosus major to the Ganglion pterygopalatinum or associate with the Chorda tympani and reach the Ganglion submandibulare via the N. lingualis (from V/3). Synapsing onto the postganglionic fibres occurs in these ganglia. These postganglionic fibres project into the lacrimal, nasal and palatine glands, and into the Glandulae sublingualis and submandibularis (→ N. trigeminus [V], p. 302).
Special viscero-afferent (SVA) fibres of the anterior two-thirds of the tongue for the perception of taste project into the upper part of the Nucleus tractus solitarii. These fibres reach the N. facialis [VII] via the N. lingualis and Chorda tympani and then enter the brainstem. General somato-afferent fibres (GSA) from the posterior wall of the external acoustic meatus and partially from behind the ear, the auricle, and the tympanic membrane join the N. vagus [X] (R. communicans nervi vagi) for a short distance. However, these GSA fibres separate from the vagus nerve while still in the Pars petrosa and associate with the N. facialis [VII]. The perikarya of both the GSA fibres and the gustatory fibres locate in the Ganglion geniculi. They reach the Nucleus spinalis nervi trigemini via the intermedius part of the N. facialis [VII].
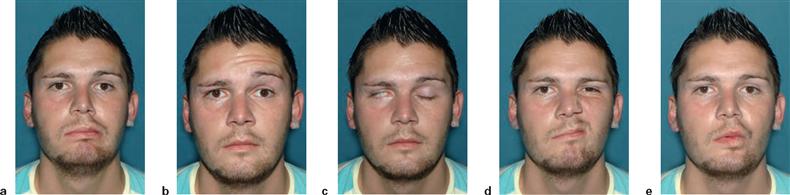
Figs. 12.151a to e Peripheral paralysis of the N. facialis [VII], right side.
a. Admission status of the patient. Skin folds on the right side of the face have disappeared.
b. When the patient is asked to raise the eyebrows, only the left side of the forehead displays wrinkles (paralysis of the M. occipitofrontalis, evidence for a peripheral facial palsy).
c. When the patient is asked to shut both eyes, this is not accomplished at the side of the damaged facial nerve (lagophthalmos). When eyelids are closed, the eyeball automatically turns upwards. Due to the inability to close the eye properly, the white sclera of the eye becomes visible at the side of facial palsy (BELL’s phenomenon).
d. When the patient is asked to wrinkle his nose this is impossible on the right side of the face.
e. When the patient is asked to whistle, no tone is produced but the air escapes through the lips at the paralysed side.
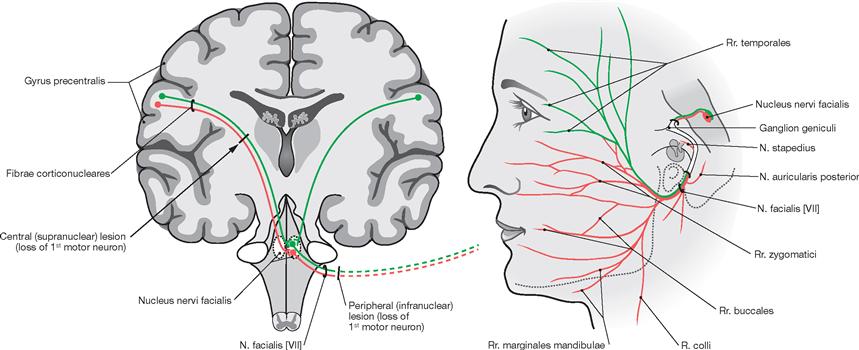
Fig. 12.152 Corticonuclear connections and peripheral course of the N. facialis [VII]. (according to [2])
On the left side, the central connections to the Nucleus nervi facialis are shown in a simplified schematic representation. The corticonuclear tracts to the upper part of the nucleus (for Rr. temporales; green) derive from both hemispheres. The lower part of the nucleus (for Rr. zygomatici, buccales, marginales mandibulae, and R. colli) connects exclusively with the contralateral hemisphere (red).
On the right side, the peripheral efferent fibres (SVE) derived from the upper and lower part of the Nucleus nervi facialis are shown.
N. vestibulocochlearis [VIII]
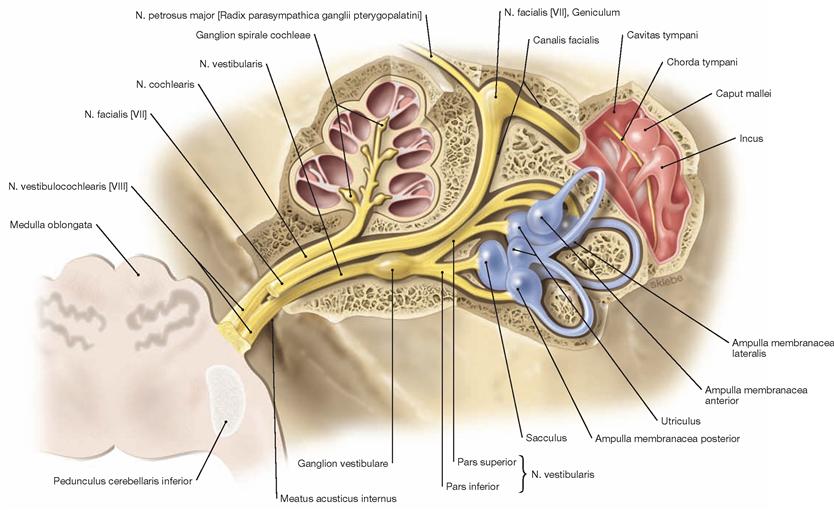
Fig. 12.153 N. vestibulocochlearis [VIII], course in the Pars petrosa of the Os temporale; superior view; the Pars petrosa has been opened.
The N. cochlearis is composed of nerve fibres generated in the organ of CORTI of the cochlea. The perikarya of these fibres are located in the Ganglion spirale cochleae within the modiolus (bipolar neurons) and the central axons form the N. cochlearis. The vestibular organ also possesses bipolar neurons. Like the cochlear neurons, they receive sensory input from hair cells. Their perikarya reside in the Ganglion vestibulare which is located at the floor of the internal acoustic meatus. The central neuronal projections form the N. vestibularis. The nerve merges with the N. cochlearis to form the N. vestibulocochlearis [VIII] (clinically frequently referred to as N. statoacusticus) at the Meatus acusticus internus and enters the brainstem at the cerebellopontine angle.
Also demonstrated is the course of the N. facialis [VII] in the internal acoustic meatus and the Canalis facialis. In addition, the Ganglion geniculi, the separation of the N. petrosus major and the course of the N. facialis [VII] in the tympanic cavity are shown. The Chorda tympani runs between the Malleus and the Incus.![]()
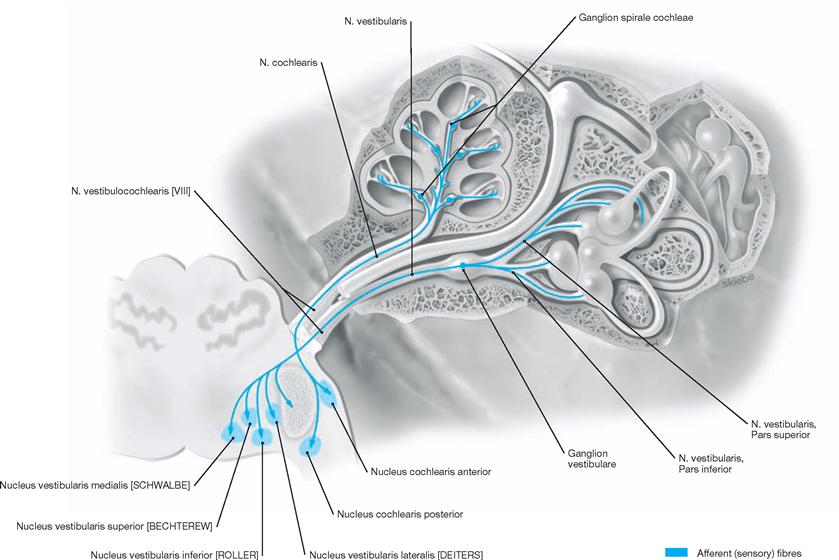
Fig. 12.154 Fibre qualities of the N. vestibulocochlearis [VIII]; superior view; Pars petrosa of the Os temporale has been opened.
The inner hair cells of the organ of CORTI and hair cells of the semicircular canals as well as utricle and saccule of the vestibular apparatus transmit sensory information to the specific somato-afferent neuronal fibres (SSA). These fibres constitute the peripheral projections of bipolar neurons (1st neuron of the central auditory and vestibular tracts). The perikarya of these bipolar neurons reside in the Ganglion spirale cochleae and the Ganglion vestibulare, respectively. The central projections of the Ganglion spirale merge to form the N. cochlearis, course through the internal acoustic meatus, and reach the brainstem via the cerebellopontine angle. Here they connect with the Nuclei cochleares anterior and posterior. The central projections of the 1st neuron of the vestibular tract (SSA) form the N. vestibularis and also pass through the cerebellopontine angle into the Medulla oblongata. Here they project to the Nuclei vestibulares medialis (SCHWALBE), superior (BECHTEREW), inferior (ROLLER), and lateralis (DEITERS).
N. glossopharyngeus [IX]
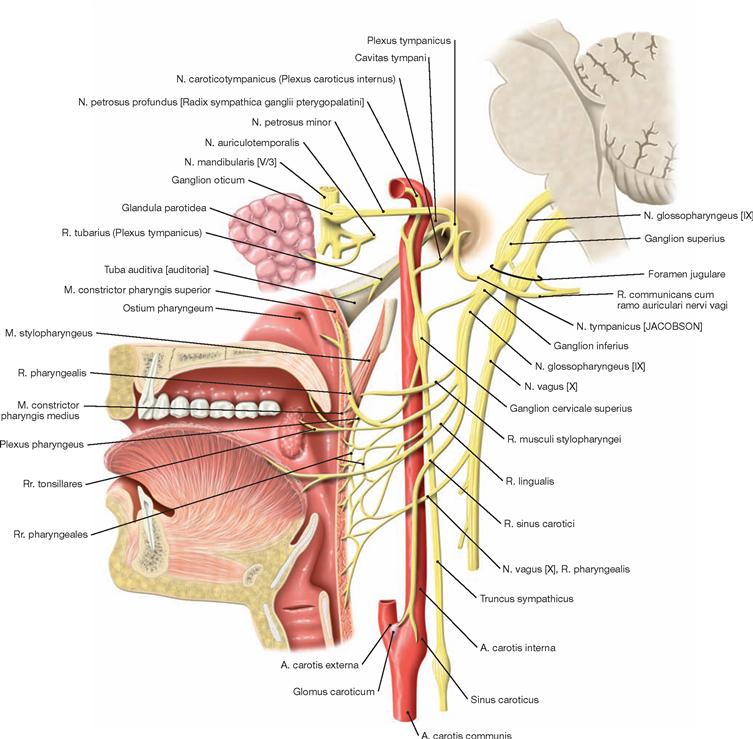
Fig. 12.155 N. glossopharyngeus [IX]; schematic median section; view from the left side.
The N. glossopharyngeus [IX], the N. vagus [X], and the N. accessorius [XI] exit the brainstem in the Sulcus retroolivaris and pass through the Foramen jugulare at the cranial base. Within the foramen lies the smaller of two ganglia, the Ganglion superius, followed immediately by the caudal Ganglion inferius. Once the glossopharyngeal nerve has passed through the cranial base, it courses caudally in between the V. jugularis interna and the A. carotis interna and by arching forward and running between the Mm. stylopharyngeus and styloglossus enters the root of the tongue. In its course, the N. tympanicus branches off and projects to the tympanic cavity. Here the tympanic nerve divides into the intramucosal Plexus tympanicus and exits the tympanic cavity as N. petrosus minor. The N. petrosus minor runs parallel to the N. petrosus major at the anterior aspect of the petrous bone and passes through the Foramen lacerum to reach the Ganglion oticum. Fibres of the N. glossopharyngeus [IX] passing through this ganglion innervate the parotid gland.
Additional branches are the R. musculi stylopharyngei to the M. stylopharyngeus and the Rr. pharyngeales to the Mm. constrictor pharyngis superior, palatoglossus, and palatopharyngeus as well as sensory fibres to the pharyngeal mucosa and to the Glandulae pharyngeales.
Together with the sympathetic trunk and the N. vagus [X], additional fibres generate the Plexus pharyngeus which innervates the Mm. constrictor pharyngis inferior, levator veli palatini, and uvulae.
The Rr. tonsillares supply sensory fibres to the Tonsilla palatina and the mucosa of the Isthmus faucium, the Rr. linguales contain gustatory (taste) fibres for the posterior third of the tongue. The R. sinus carotici transmits sensory input from mechano- and chemoreceptors at the Sinus caroticus and Glomus caroticum to the brainstem.![]()
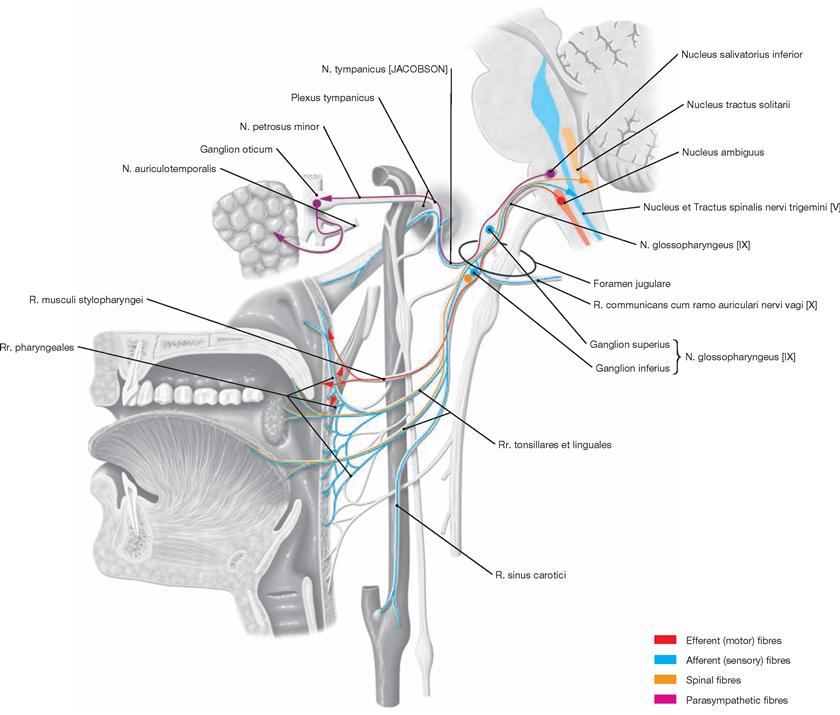
Fig. 12.156 Fibre qualities of the N. glossopharyngeus [IX]; schematic median section, view from the left side.
Motor fibres (SVE) of the N. glossopharyngeus [IX] derived from the Nucleus ambiguus and from the N. vagus [X] (also from the Nucleus ambiguus, SVE) jointly innervate the muscles of the soft palate.
Parasympathetic fibres (GVE) from the Nucleus salivatorius inferior project to the Ganglion oticum via the N. tympanicus, Plexus tympanicus, and N. petrosus minor. In the Ganglion oticum, the preganglionic fibres synapse to postganglionic neurons. The postganglionic fibres associate with the N. auriculotemporalis (from V/3) and the N. facialis [VII] to reach the Glandula parotidea. Additional parasympathetic fibres (GVE) reach the pharyngeal glands. Numerous general somato-afferent fibres (GSA) that project to the Nucleus spinalis nervi trigemini derive from the tympanic cavity, the pharyngeal mucosa, and the posterior third of the tongue.
General viscero-afferent fibres (GVA) transmit the sensory input of mechanoreceptors in the Sinus caroticus (determine blood pressure) and of chemoreceptors in the Glomus caroticus (measure partial pressure of O2 and CO2 and pH of the blood). The brainstem integrates this sensory input and issues reflexive changes in the frequency of breathing and of the central blood pressure.
Specific viscero-afferent fibres (SVA) conduct taste sensations to the Nucleus and Tractus solitarius of the posterior third of the tongue.
N. vagus [X]
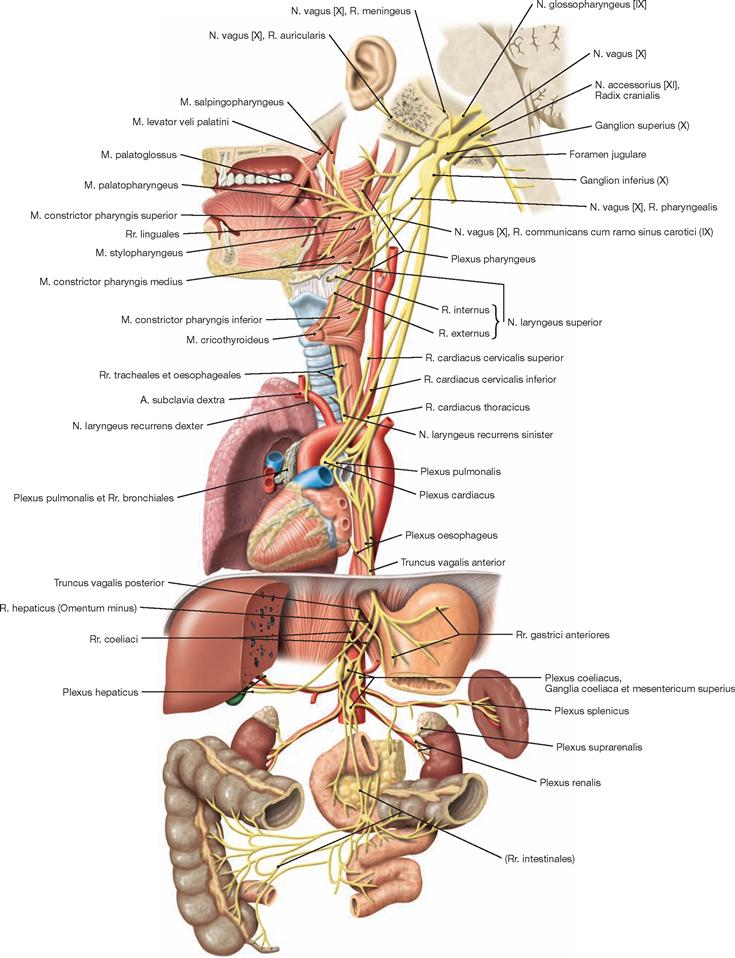
Fig. 12.157 N. vagus [X]; schematic median section in the region of the head.
For a detailed description of the course of the N. vagus [X] → page 318.![]()
Together with the Nn. glossopharyngeus [IX] and accessorius [XI], the N. vagus [X] exits the brainstem in the Sulcus retroolivaris and traverses the cranial base through the Foramen jugulare. The Ganglion superius locates in the Foramen jugulare and releases the R. meningeus which re-enters the cranial cavity to provide sensory innervation to the meninges of the posterior cranial fossa. Also branching off is the R. auricularis for the innervation of the outer wall of the external acoustic meatus. The Ganglion inferius locates slightly below the Foramen jugulare.
The N. vagus [X] crosses the neck and the thoracic cavity and enters the abdominal cavity. In its course, the N. vagus [X] progressively loses its appearance as a coherent nerve. At the level of the oesophagus, two distinct trunks can still be discerned (Trunci vagales anterior and posterior), but from the stomach onward the fibres distribute more widely and form multiple Plexus to reach the liver, pancreas, spleen, kidney, adrenal gland, small intestine, and colon. The fibres of the N. vagus [X] terminate at the level of the CANNON-BÖHM point (left colic flexure).
In its cervical passage, the N. vagus [X] provides Rr. pharyngeales to the Plexus pharyngeus. This plexus also receives contributions from the N. glossopharyngeus [IX] and from sympathetic fibres (innervation of the Mm. constrictor pharyngis medius and inferior, levator veli palatini, uvulae – motor function [SVE], Glandulae pharyngeales – parasympathetic function [GVE], and pharyngeal mucosa – sensory function [GVA]). Additional vagal branches are the R. lingualis (taste fibres from the root of the tongue and epiglottis, SVA), the N. laryngeus superior (with the R. externus for the Mm. cricothyroideus and constrictor pharyngis inferior as well as the R. internus for the sensory innervation of the laryngeal mucosa above the vocal cords) and the Rr. cardiaci cervicales superiores and inferiores to the Plexus cardiacus at the heart (which affects the regulation of the blood pressure).
In its thoracic part, the N. vagus [X] releases the N. laryngeus recurrens. The latter loops around the aortic arch on the left side and the A. subclavia on the right side and projects back cranially to the larynx. Here the N. laryngeus recurrens innervates all laryngeal muscles (with the exception of the M. cricothyroideus) and the mucosa below the vocal cords. Additional thoracic vagal branches include the Rr. cardiaci thoracici for the Plexus cardiacus at the heart. The Rr. bronchiales reach the Plexus pulmonalis and innervate muscles and glands of the bronchial tree. The pulmonary vagal innervation registers the tension within the lung tissue and adjusts breathing by a reflectory neuronal feedback loop.
Right and left N. vagus [X] form a web-like plexus (Plexus oesophageus) at the middle part of the oesophagus. The plexus eventually contributes to the formation of the Truncus vagalis anterior (mainly fibers of the left N. vagus [X]) and the Truncus vagalis posterior (mainly fibres of the right N. vagus [X]). Both Trunci accompany the Oesophagus during its passage through the diaphragm into the abdominal cavity. From the stomach onwards, the Trunci diversify further to create numerous plexuses for the above-mentioned abdominal organs.
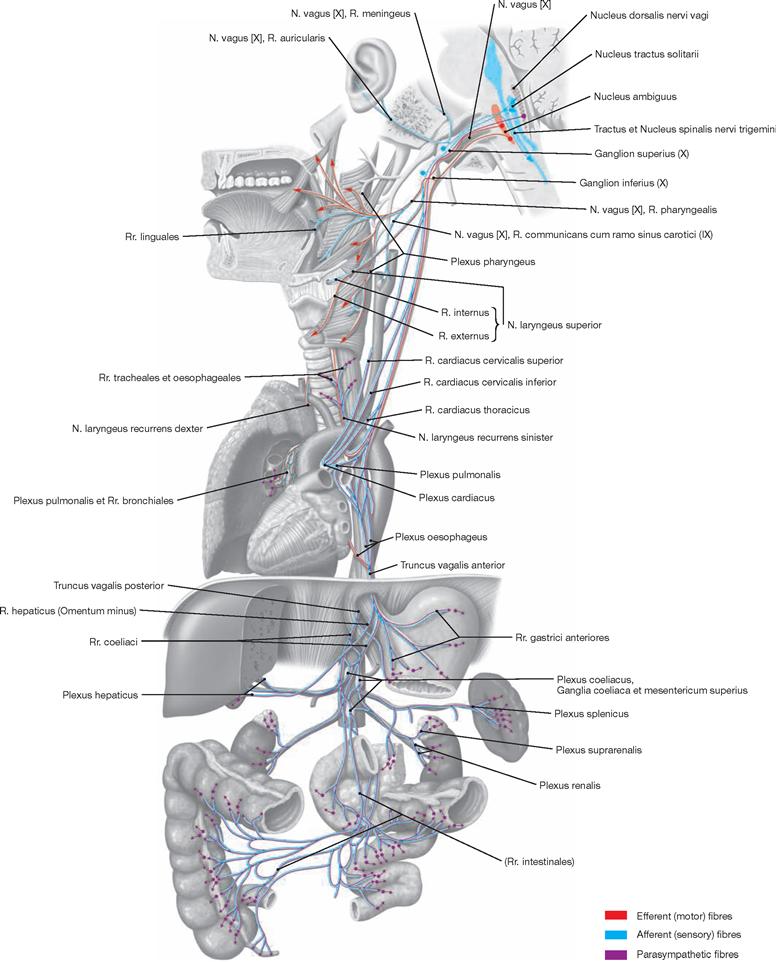
Fig. 12.158 Fibre qualities of the N. vagus [X]; schematic median section in the region of the head.
For a detailed description of the fibre qualities of the N. vagus [X] → page 318.
Parasympathetic fibres (GVE) of the N. vagus [X] originate from the Nucleus dorsalis nervi vagi in the Medulla oblongata and innervate glands and smooth muscles of the viscera.
General viscero-afferent fibres (GVA) of the same organs project into the Nucleus dorsalis nervi vagi and the Nucleus tractus solitarii.
Specific viscero-efferent fibres (SVE) originate in the Nucleus ambiguus and innervate the skeletal muscles of the palate, Pharynx, Larynx, and Oesophagus.
General viscero-afferent fibres (GVA) from the mucosa of the same structures project into the Nucleus dorsalis nervi vagi and the Nucleus tractus solitarii.
General somato-afferent fibres (GSA) of the external acoustic meatus and the meninges of the posterior cranial fossa project into the Nucleus spinalis nervi trigemini.
Gustatory fibres (SVA) at the root of the tongue and the Epiglottis connect with the Nucleus tractus solitarii.
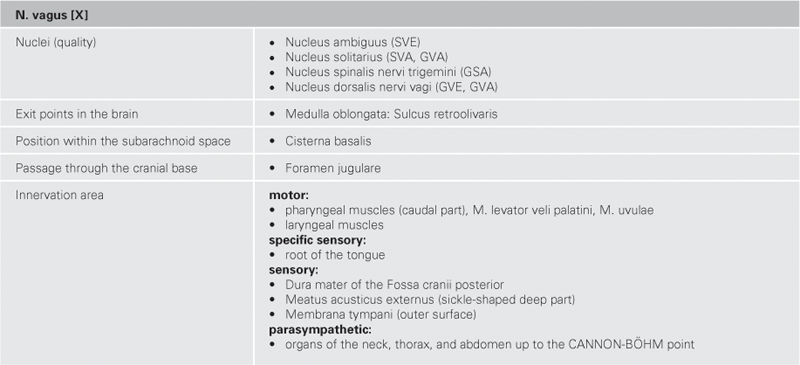
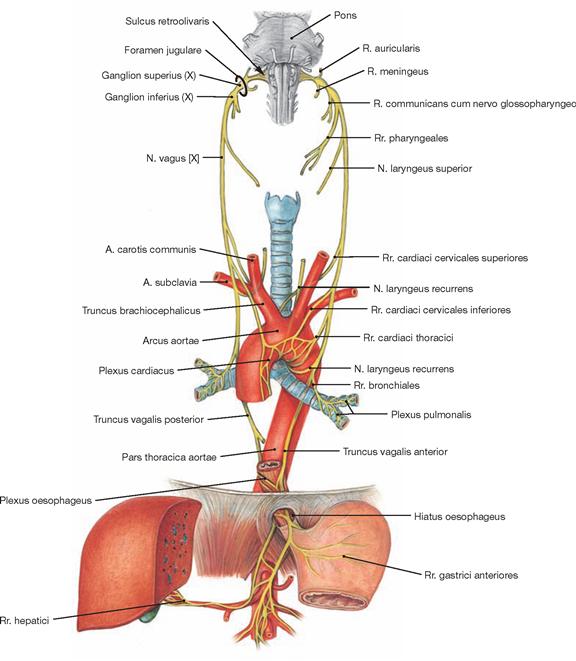
Fig. 12.159 N. vagus [X]; both nerve branches; anterior view.
The image emphasises the slightly different course of the Nn. vagi dexter and sinister and the course of their branches until the Trunci vagales anterior and posterior enter the abdominal cavity.![]()
N. accessorius [XI]
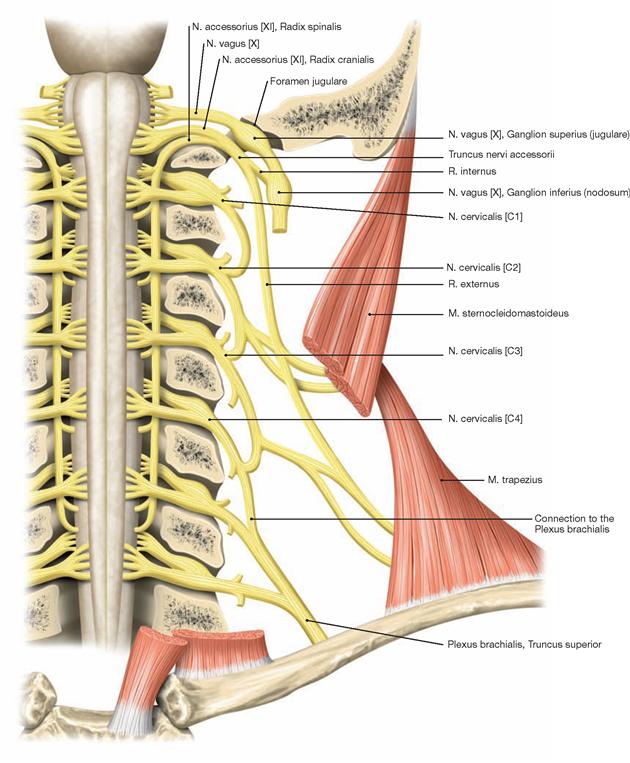
Fig. 12.160 N. accessorius [XI]; anterior view; vertebral canal and skull have been opened.
The N. accessorius [XI] exits the brainstem in the Sulcus retroolivaris together with the N. glossopharyngeus [IX] and the N. vagus [X] and all three cranial nerves traverse the cranial base through the Foramen jugulare. The N. accessorius [XI] has two different roots. The Radix cranialis of the N. accessorius [XI] originates from the Nucleus ambiguus in the Medulla oblongata. At the level of the Foramen jugulare, it joins the Radix spinalis of the N. accessorius [XI] which consists of fibres derived from the anterior and posterior segmental roots in the cervical spinal cord. According to current textbook knowledge, the fibres of the Radix cranialis form the R. internus and converge on the N. vagus [X] inferior to the Foramen jugulare (according to newer preliminary findings which require further analysis, the N. accessorius [XI] has no cranial root and no connection to the N. vagus [X]). The Radix cranialis participates in the innervation of the pharyngeal and laryngeal muscles and, strictly speaking, is not part of the N. accessorius [XI]. The fibres of the Radix spinalis project caudally to the M. sternocleidomastoideus, course through the lateral cervical triangle to the anterior margin of the M. trapezius, and innervate both muscles.![]()
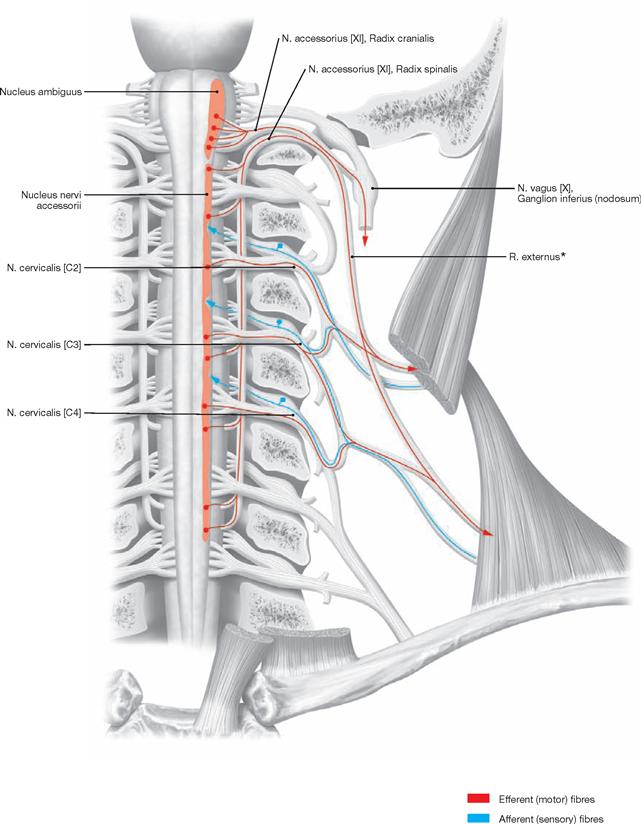
Fig. 12.161 Fibre qualities of the N. accessorius [XI]; anterior view, vertebral canal and skull have been opened.
The N. accessorius [XI] innervates the M. sternocleidomastoideus and M. trapezius with specific viscero-efferent fibres (SVE) from the Nucleus nervi accessorii.
* for M. sternocleidomastoideus and M. trapezius
N. hypoglossus [XII]
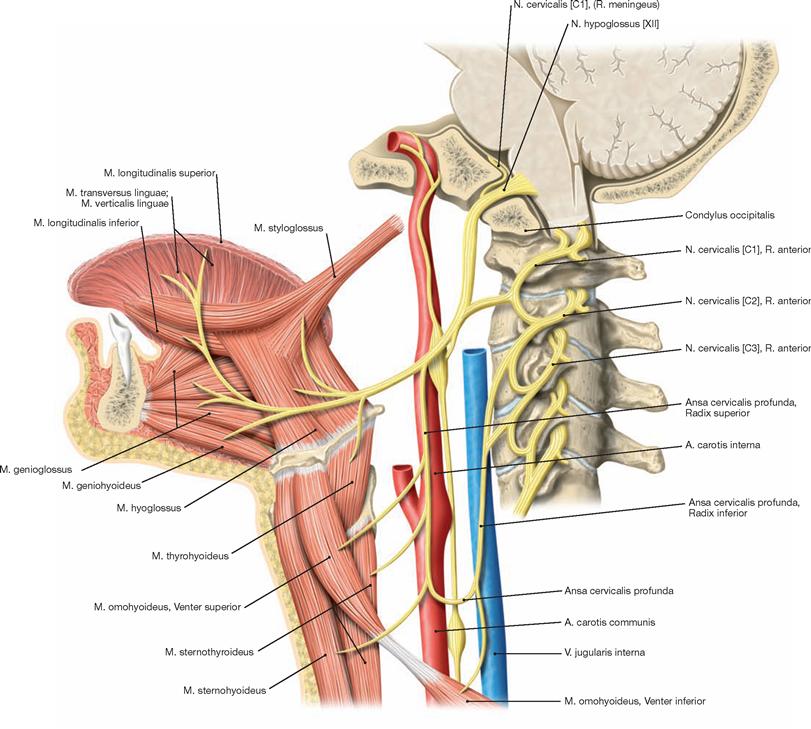
Fig. 12.162 N. hypoglossus [XII]; schematic median section; view from the left side.
The Nucleus nervi hypoglossi in the Medulla oblongata provides the fibres for the N. hypoglossus [XII]. The fibres exit the brainstem as multiple small bundles between the pyramid and olive in the Sulcus anterolateralis. They join to form the N. hypoglossus [XII] which passes through the Canalis nervi hypoglossi. Inferior to the cranial base, fibres of the spinal nerves C1 and C2 accompany the hypoglossal nerve for a short distance and part again, first as Radix superior of the Ansa cervicalis profunda and then as a branch to the M. geniohyoideus. Together with fibres from C2 and C3, these fibres form the Ansa cervicalis profunda and, in addition, innervate the M. geniohyoideus. Posterior to the N. vagus [X] in the neurovascular bundle behind the Pharynx, the N. hypoglossus [XII] passes caudally and, in an arch-shaped bend of 90°, turns rostrally and medially. It runs at the upper margin of the Trigonum caroticum, crosses the A. carotis externa at the branching point of the A. lingualis and reaches the tongue between the M. hyoglossus and M. mylohyoideus. The N. hypoglossus [XII] innervates all internal muscles of the tongue and the Mm. styloglossus, hyoglossus, and genioglossus.![]()
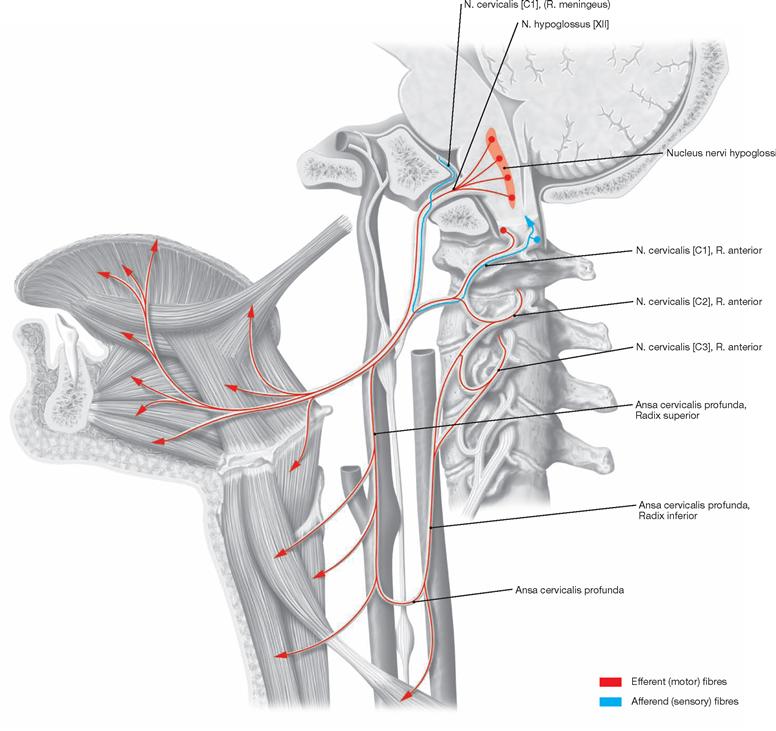
Fig. 12.163 Fiber qualities of the N. hypoglossus [XII]; schematic median section; view from the left side.
The N. hypoglossus [XII] consists of general somato-efferent fibres (GSE) from the Nucleus nervi hypoglossi and innervates the internal muscles of the tongue and the Mm. styloglossus, hyoglossus, and genioglossus.
Spinal cord segments
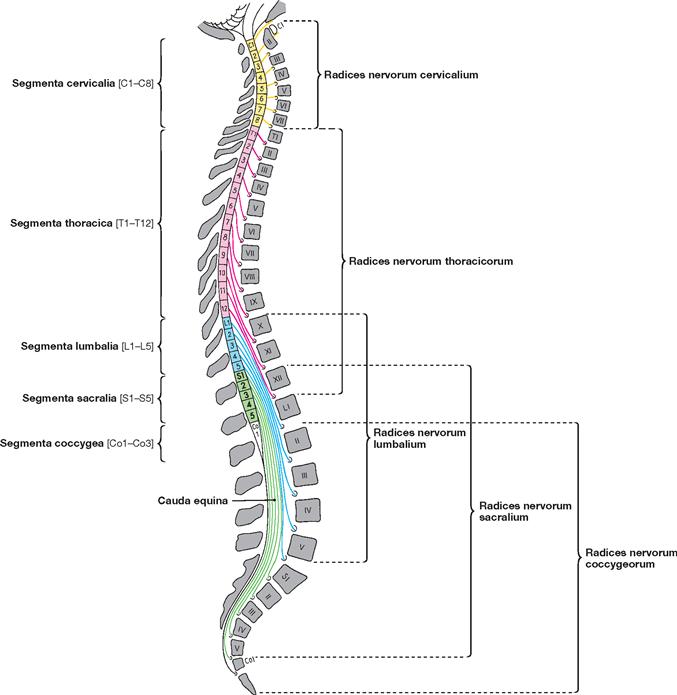
Fig. 12.164 Spinal cord segments, Segmenta medullae spinalis; schematic median section; view from the left side; regional segments highlighted in different colours.
The spinal cord is composed of eight cervical segments (Segmenta cervicalia [C1–C8]), twelve thoracic segments (Segmenta thoracica [T1–T12]), five lumbar segments (Segmenta lumbalia [L1–L5]), five sacral segments (Segmenta sacralia [S1–S5]), and one to three coccygeal segments (Segmenta coccygea [Co1–Co3]). In the adult, the spinal cord extends only to the level of the lumbar vertebra LI–LII.
As the spinal cord does not follow the faster growth of the vertebral column, the course of the spinal roots (Radices nervorum) towards their corresponding segmental intervertebral foramina becomes steeper and longer from cranial to caudal within the vertebral canal. Below LI–LII the arrangement of spinal nerves in the vertebral canal resembles a horse tail, thus, the name Cauda equina.
Spinal cord
Spinal cord segments
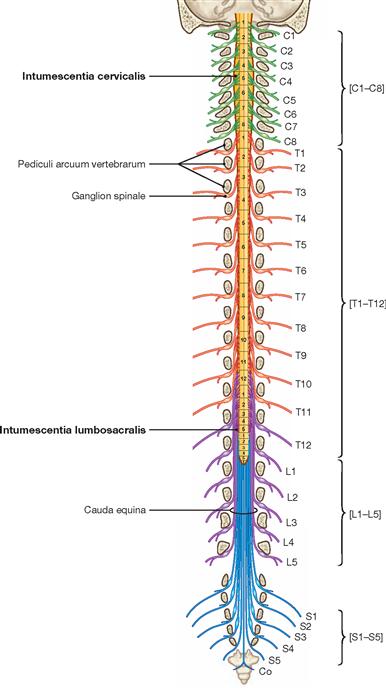
Fig. 12.165 Spinal cord segments, Segmenta medullae spinalis; schematic frontal section; ventral view. [8]
As the spinal cord does not follow the faster growth of the vertebral column and is much shorter than the vertebral column, the course of the spinal roots towards their corresponding segmental intervertebral foramina becomes steeper and longer from cranial to caudal and more oblique for those fibres located more lateral within the vertebral canal. In adults, the spinal cord ends at the level of LI–LII (ranging from TXII to LII/LIII). Therefore, the Radices anteriores and posteriores locate at higher segments of the vertebral column than the corresponding spinal nerve exiting the vertebral canal. Inferior to the Conus medullaris, the Radices anteriores and posteriores of the bundled lumbar, sacral, and coccygeal nerves extend caudally to reach their intervertebral foramina to exit the vertebral canal. This collection of nerve roots is named the Cauda equina.
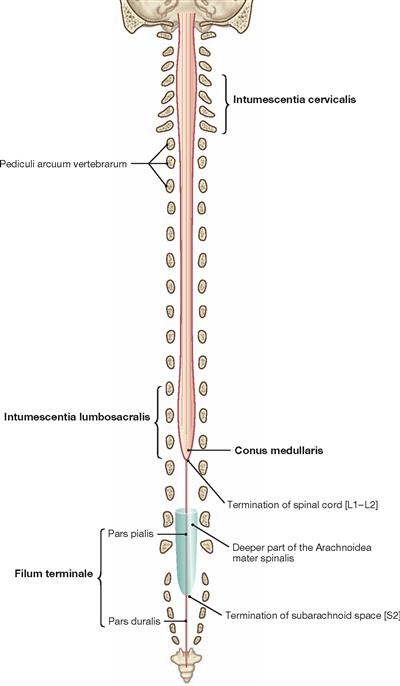
Fig. 12.166 Spinal cord, Medulla spinalis; ventral view. [8]
The spinal cord is the part of the CNS located in the upper two-thirds of the vertebral canal. In the adult, it extends from the Foramen magnum to approximately the level of LI/LII. In the newborn, the spinal cord reaches to the level of LIII or even LIV. The distal end has the shape of a conus (Conus medullaris). The Conus medullaris contains a fine network of connective tissue (Filum terminale), derived from parts of the Pia mater, which extends caudally into the vertebral canal. The diameter of the spinal cord increases in the areas with spinal nerve roots dedicated for the extremities. The upper enlargement (Intumescentia cervicalis, C5–T1) contains neurons for the innervation of the upper extremities, the lower enlargement (Intumescentia lumbosacralis) lies at the level of the spinal nerve roots L1–S3 and serves for the innervation of the lower extremities.
Somatic and visceral nerve plexuses
Fig. 12.167 Somatic and visceral nerve plexuses. [8]
The nature of nerve plexuses can be somatic (left side of the image) or visceral (right side of the image) and include fibres of different qualities and levels. Nerves that originate from a plexuses project towards different target tissues and organs. The plexuses of the enteric nervous system can generate reflex activities independent of the CNS.
The extensive somatic plexuses originate from the Rr. anteriores of the spinal nerves: Plexus cervicalis (C1–C4), Plexus brachialis (C5–T1), Plexus lumbalis (L1–L4), Plexus sacralis (L4–S4), and Plexus coccygeus (S5–Co). With the exception of the spinal nerve T1, all Rr. anteriores of the thoracic spinal nerves are independent and do not participate in the formation of the plexus.
The visceral plexuses form in conjunction with the viscera and normally contain efferent (sympathetic and parasympathetic) and afferent parts. Visceral plexuses are the Plexus cardiacus and pulmonalis in the thorax as well as the Plexus prevertebralis anterior to the aorta in the abdomen, which extends caudally to the lateral walls of the pelvis. The Plexus prevertebralis projects efferent fibres to all abdominal and pelvic organs and receives afferences from the same organs.
Spinal nerves
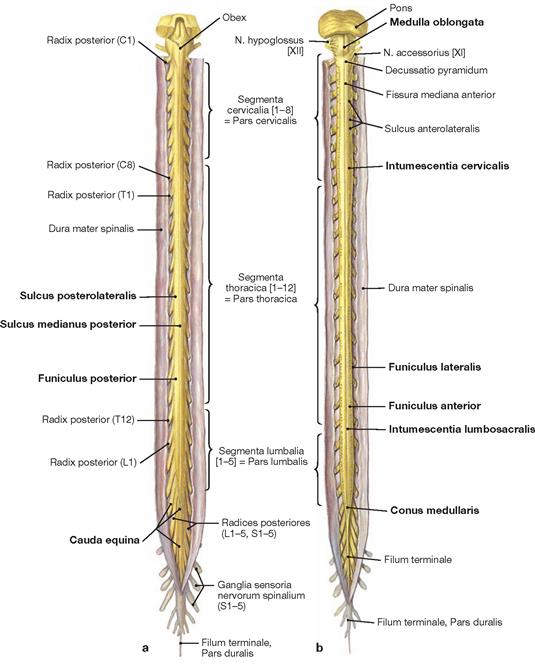
Figs. 12.168a and b Spinal cord, Medulla spinalis, and spinal nerves, Nn. spinales; the vertebral canal and the dural sac have been opened.
The spinal cord has the shape of a sword and a diameter of 1–1.5 cm. It extends from the Medulla oblongata of the brainstem. Its cervical and lumbar segments increase in diameter to form the Intumescentia cervicalis (C5–T1) and the Intumescentia lumbosacralis (L2–S3). These are the location of multiple neurons and nerve fibres concerned mainly with the innervation of the extremities. The Conus medullaris is the caudal tip of the spinal cord.
The surface of the spinal cord displays characteristic longitudinal grooves. In the midline on the ventral side this is the Fissura mediana anterior and on the posterior side the Sulcus medianus posterior. The Funiculus anterior located to both sides of the Fissura mediana anterior, is followed by the Sulcus ventrolateralis which separates the Funiculus anterior from the Funiculus lateralis. On the dorsal side and bilaterally to the Sulcus medianus posterior are the Funiculi posteriores. The latter are separated from the Funiculi laterales by the Sulci posterolaterales.
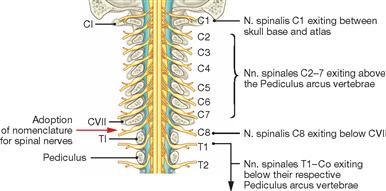
Fig. 12.169 Nomenclature of the spinal nerves. [8]
In contrast to the other spinal cord segments, the number of spinal cord segments in the cervical spinal cord is not identical with the number of vertebrae. The cervical region has eight cervical segments but only seven cervical vertebrae. The first pair of cervical nerves exits between the cranial base and the atlas (CI vertebra). The spinal nerve pairs C2–C7 each exit superior to the corresponding Pediculus arcus vertebrae. At the transition from the 7th cervical vertebra to the 1st thoracic vertebra, the nomenclature changes since the 8th spinal nerve exits inferior to the 7th cervical vertebra. All pairs of spinal nerves T1–Co that follow will always exit inferior to the corresponding vertebral arch.
Arteries of the spinal cord
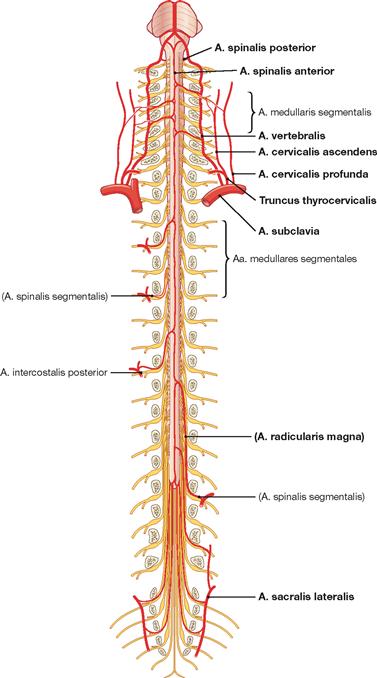
Fig. 12.170 Arteries of the spinal cord, Medulla spinalis; ventral view; not all segmental spinal arteries are shown. [8]
There are three sources of arterial supply for the spinal cord:
• through the A. subclavia (cervical) via the A. spinalis anterior and Rr. radiculares anteriores and posteriores from the Aa. vertebralis, cervicalis ascendens, and cervicalis profunda
• through the Aorta thoracica (thoracic) via the A. intercostalis suprema and Aa. intercostales posteriores
• through the Aorta abdominalis (lumbosacral) via Aa. lumbales
The A. iliaca interna supplies the Cauda equina through the A. iliolumbalis and A. sacralis lateralis. All these arteries provide Rr. spinales.
The largest R. spinalis is the A. radicularis magna (ADAMKIEWICZ; vertebra TXII–LII) which is usually found on the left side of the body.
Arteries and meninges of the spinal cord
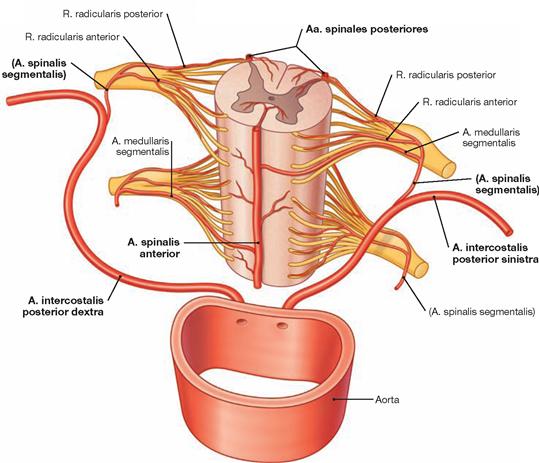
Fig. 12.171 Segmental arterial supply of the spinal cord. [8]
Blood supply to the spinal cord is achieved through the A. spinalis anterior and the Aa. spinales posteriores, longitudinal blood vessels running alongside the spinal cord which originate in the cervical region. Additional contributors are feeder arteries (spinal segmental arteries from the Aa. vertebrales, the deep cervical arteries, the Aa. intercostales and the Aa. lumbales) which enter the vertebral canal through the Foramina intervertebralia and divide into Rr. radiculares anteriores and posteriores at the level of each spinal cord plane. The Radices anteriores and posteriores follow the spinal nerves and supply them with blood. At different planes, the spinal segmental arteries release segmental Aa. medullares which project to and anastomose with the longitudinal arteries.
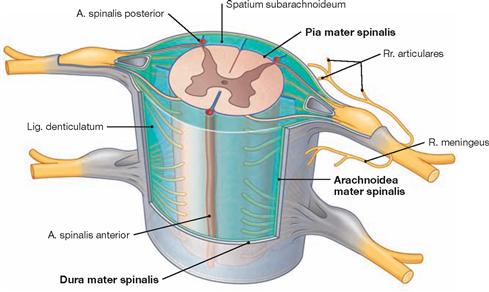
Fig. 12.172 Meninges of the spinal cord, Meninges; oblique ventral view. [8]
Like the brain, the spinal cord is surrounded by the three meninges, which provide protection and suspension of this CNS structure within the vertebral canal.
The Dura mater spinalis is the strongest of the three meninges and is located farthest to the outside. The laterally exiting spinal nerves and their roots are surrounded by a tubular dural sheath which radiates into and fuses with the nerve sheath (epineurium) of the spinal nerves. Inside the dura follows the spinal arachnoid mater which is separated from the Pia mater spinalis by the subarachnoid space filled with cerebrospinal fluid (Liquor cerebrospinalis). Delicate trabeculations (Trabeculae arachnoideae, not shown) connect the spinal arachnoid mater of one side with the Pia mater spinalis on the other side. This connective tissue also surrounds the blood vessels located within the subarachnoid space.
The Pia mater spinalis is a membrane rich in blood vessels and tightly attached to the surface of the spinal cord. It extends deeply into the Fissura mediana anterior, creates a sheath-like lining around the Radices posteriores and anteriores of the spinal nerves and accompanies them on their way through the subarachnoid space.
In the exit and entry areas of the radices, the Pia mater spinalis transitions into the Arachnoidea mater spinalis. The Ligg. denticulata are lateral extensions of the Pia mater spinalis to the spinal arachnoid and Dura mater along both sides of the spinal cord. They serve to attach the spinal cord in the centre of the subarachnoid space.
Venous plexus of the spinal cord
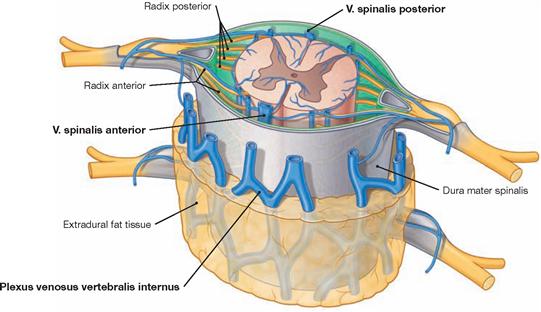
Fig. 12.173 Veins of the vertebral canal, Canalis vertebralis; oblique ventral view. [8]
The veins draining the spinal cord mainly form longitudinal collecting vessels running alongside the spinal cord. Two pairs of longitudinal veins group around the exit and entry points of the Radix anterior and Radix posterior out of and into the spinal cord, respectively. In addition, the V. spinalis anterior and V. spinalis posterior course alongside the Fissura mediana anterior and the Sulcus medianus posterior, respectively. These veins drain into the Plexus venosus vertebralis internus in the epidural space of the vertebral canal. The venous plexus connects with segmental veins which, like the azygos system, drain into the large collecting veins of the body. The Plexus venosus vertebralis internus also communicates with intracranial veins.
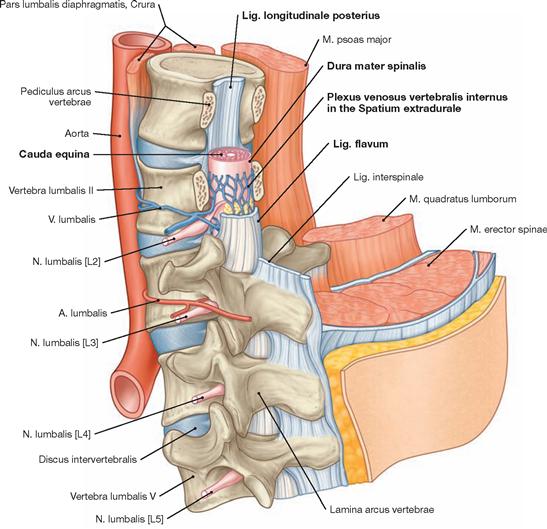
Fig. 12.174 Position of the spinal cord within the vertebral canal; dorsolateral view. [8]
The dural tube positions ventral to the Lig. longitudinale posterius and is surrounded by the Plexus venosus vertebralis internus. The vertebral arches of the first two lumbar vertebrae have been removed. The topographic relationship of the nerve root to the intervertebral disc below the spinal nerve L2 is shown. The Lig. flavum provides the dorsal cover for the dural tube.
Clinics
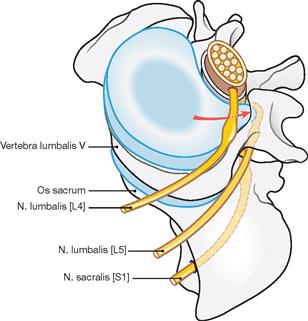
Fig. 12.175 Schematic representation of a mediolateral herniation of the intervertebral disc between the 4th and 5th lumbar vertebrae; lateroventral superior view. [23]
This disc prolapse results in the compression of the spinal nerve root L5 located one segment below; the more medially positioned L4 root exiting in the same segment remains unaffected.
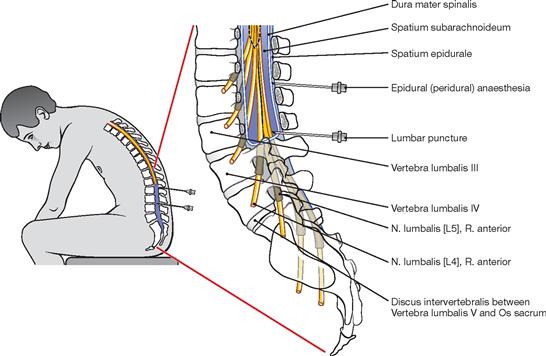
Fig. 12.176 Epidural (peridural) anaesthesia and spinal anaesthesia. [23]
Anaesthetics are injected into the epidural space (epidural or peridural anaesthesia) to anaesthetise individual spinal nerves. The local adipose tissue prevents the anaesthetic from affecting other spinal cord segments.
In contrast to the epidural anaesthesia, in spinal anaesthesia the anaesthetics are applied directly into the subarachnoid space. The medication mixes with the cerebrospinal fluid but, as a result of g-force, remains below the injection site (in an upright sitting patient) and, thus, exclusively anaesthetises nerve fibres located below the injection site.
For lumbar puncture, the back must be maximally bent forward and the needle is inserted between the spinous processes of the lumbar vertebrae III and IV or IV and V. Then, the needle is pushed forward carefully until the Dura mater spinalis is punctured and the tip of the needle rests in the subarachnoid space. Now, cerebrospinal fluid can be drawn for diagnostic purposes or an anaesthetic can be applied.
Spinal cord and vertebral canal, imaging
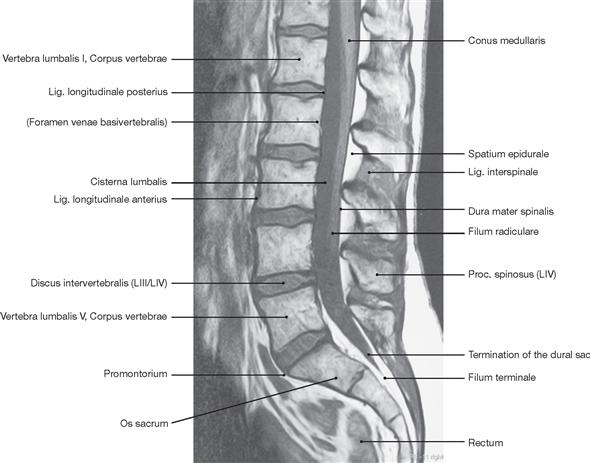
Fig. 12.177 Lumbar part of the vertebral column; magnetic resonance tomographic image (MRI), T1-weighted; median section of the lumbar and lower thoracic parts of the vertebral column. [27]
The border between the end of the spinal cord at the level of LI/LII and the beginning of the Cauda equina, which only partially occupies the vertebral canal, is clearly visible.
Clinics
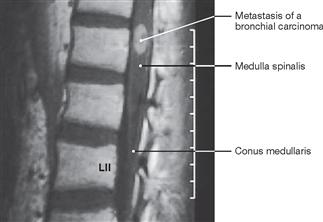
Fig. 12.179 Vertebral canal, Canalis vertebralis, with spinal cord, Medulla spinalis; magnetic resonance tomographic image (MRI); median section of the lower thoracic and lumbar parts of the vertebral column, paraplegia due to a spinal tumour. [23]
In the MRI images, the tumour presents as a white mass against the surrounding spinal cord. This is a metastasis of a known bronchial carcinoma. The patient was admitted with complete paraplegia of the lower extremities and loss of all sensory functions below dermatome L2.
Fig. 12.180 Spina bifida cystica. [20]
Infant with Spina bifida cystica (meningomyelocele) in the lumbar region.
Fig. 12.181 Spina bifida occulta. [20]
The hairy skin area in the lumbosacral region is the visible sign of the underlying Spina bifida occulta.
Spinal cord, sections
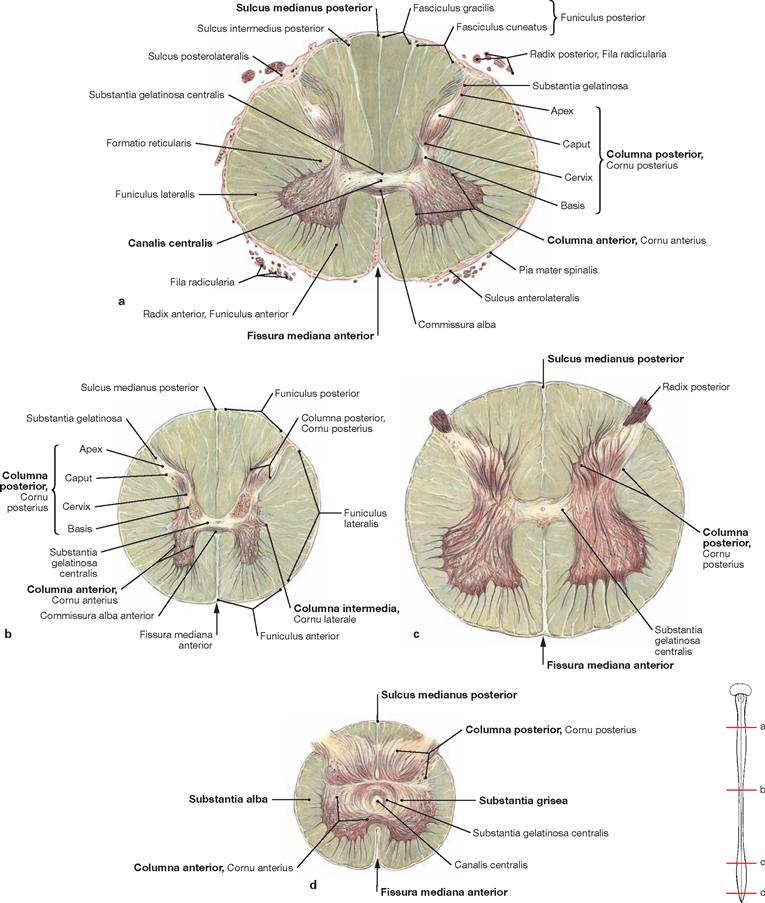
Figs. 12.182a to d Spinal cord, Medulla spinalis; cross-sections; myelin stain; approximately 500%.
The spinal cord has a symmetrical mirror-image structure and all spinal cord segments (a–d) consist of grey and white matter. The grey matter (Substantia grisea) consists mainly of the perikarya of neurons, it has the shape of a butterfly in cross-sectional images, and is surrounded by white matter (Substantia alba). The latter is mainly composed of neuronal fibres and glia cells and divides into tracts (Funiculi). The centre of the butterfly structure contains the Canalis centralis. Although part of the inner CSF space, this canal has a caudal blind end, preventing the circulation of the cerebrospinal fluid.
The wings of the butterfly represent columns: an anterior column (Columna anterior), an intermediate column (Columna intermedia), and a posterior column (Columna posterior). These columns form the anterior horn (Cornu anterius), lateral horn (Cornu laterale), and posterior horn (Cornu posterius). The Commissurae griseae (not shown) connect the intermediate columns from both sides.
Functional organisation of the spinal cord
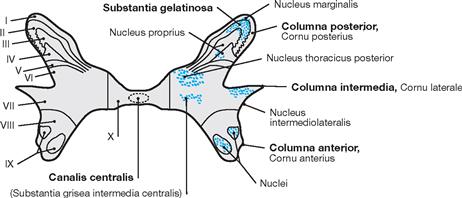
Fig. 12.183 Spinal cord, Medulla spinalis; laminar organisation of the grey matter according to its cytoarchitecture [according to REXED, 1952], exemplified by the tenth thoracic segment (T10).
Histologically (cytoarchitecturally), the grey matter (Substantia grisea) divides into a number of layers (Laminae) which are numbered I to X from dorsal to ventral (extent and number of the layers vary in different segments of the spinal cord). In addition, various nuclei are distinguished and can stretch over more than one cytoarchitectural neuronal layer. The structure of the Laminae reflects functional aspects.
The posterior horns (Laminae I–VI: Nucleus thoracicus posterior [CLARKE’s column], Nucleus proprius, Substantia gelatinosa) contain relais neurons for the transmission of afferent sensory input (sensory information from the skin, proprioceptive information, perception of pain from the periphery). The lateral horns (Lamina VII) harbour neurons (Nucleus intermediolateralis) for autonomic efferences. The anterior horns (Columna anterior, Cornu anterius; Laminae VIII, IX) contain the efferent neurons (somato-efferent root cells) for the muscles.
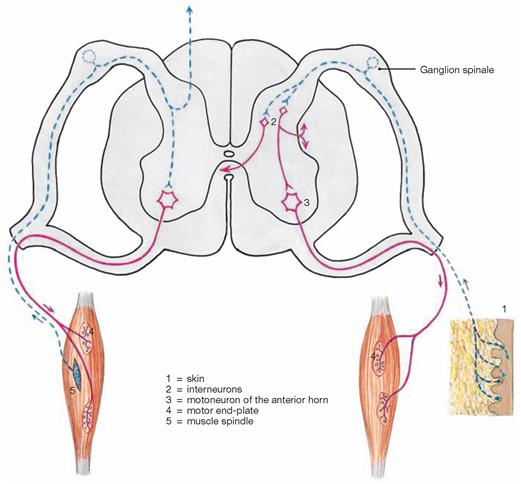
Fig. 12.184 Reflexes of the spinal cord, Medulla spinalis.
The spinal cord contains a system that connects it with supraspinal centres and a local autonomic system capable of eliciting spinal reflexes without the input from supraspinal neuronal structures. Spinal reflexes for example are important in keeping an adequate muscle tonus during different activities or to protect against harmful stimuli (e.g. withdrawal reflex from a painful stimulus).
The type of connectivity and complexity distinguishes two forms of reflex circuitry: monosynaptic and polysynaptic reflexes. Supraspinal centres can modify polysynaptic reflexes.
Left side of the image: reflex circuitry of a monosynaptic, bineuronal, proprioceptive reflex (a typical stretch reflex like the knee-jerk [(patellar)] reflex, etc., collectively named myotactic or deep tendon reflexes [DTRs]).
Right side of the image: complex reflex circuitry of a polysynaptic, polyneuronal reflex (typical flexor or withdrawal reflexes are initiated by cutaneous receptors and include the abdominal, cremaster reflex, foot sole reflex etc.).
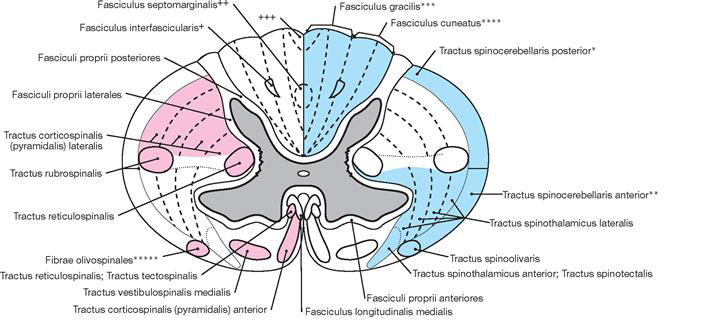
Fig. 12.185 Spinal cord, Medulla spinalis; schematic organisation of the white matter exemplified by a lower cervical segment.
Afferent (= ascending) pathways in blue; efferent (= descending) pathways in red.
The regions indicated with +, ++, and +++ designate descending collateral tracts of the posterior fasciculi.
* clinical term: FLECHSIG’s tract
** clinical term: GOWERS‘ tract
*** clinical term: GOLLS‘ tract
**** clinical term: BURDACH’s tract
***** The actual existence of these fibres has not definitely been documented.
+ SCHULTZE’s comma tract (cervical part)
++ oval bundle of FLECHSIG (thoracic part)
+++ triangle of PHILIPPE-GOMBAULT (lumbar and sacral parts)
Tracts of the spinal cord
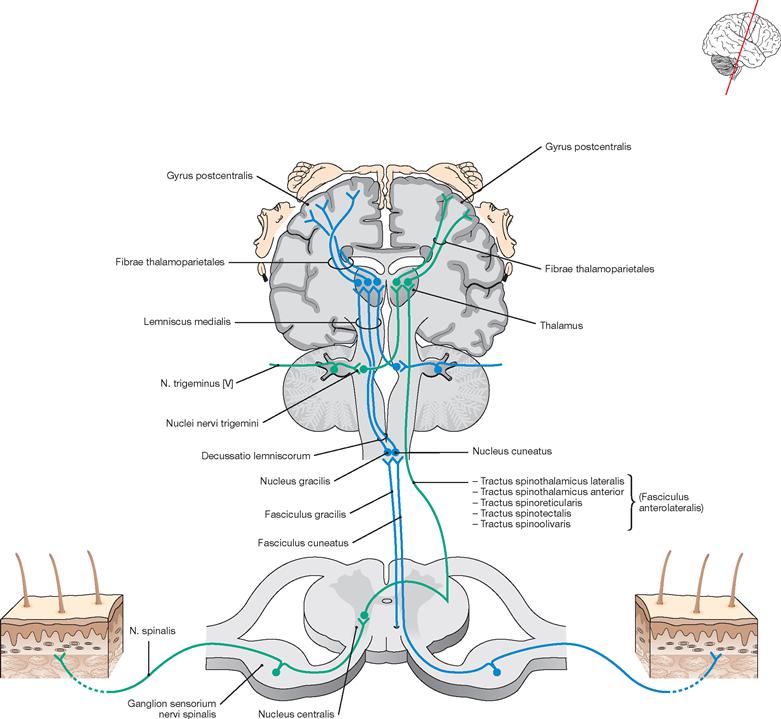
Fig. 12.186 Pathways for epicritic (blue) and protopathic (green) sensibility (afferent tracts).
Pathway of epicritic sensibility (touch pathway, serves the perception of precise differentiation of pressure and touch as well as proprioception):
• 1st neuron (uncrossed): from receptors (exteroceptors) in the skin and the mucosa, the periosteum, the joints and the muscle spindles etc., to the Nuclei gracilis and cuneatus in the Medulla oblongata via the Fasciculi gracilis and cuneatus in the Funiculus posterior (perikarya in the spinal ganglia); additional descending collaterals
• 2nd neuron (crossed): from the Medulla oblongata (Nucleus cuneatus, Nucleus gracilis) to the Thalamus (Lemniscus medialis, perikarya in Nucleus cuneatus and Nucleus gracilis)
• 3rd neuron (uncrossed): from the Thalamus (Nucleus ventralis posterolateralis) to the cerebral cortex, particularly to the Gyrus postcentralis (thalamocortical fibres, perikarya in the Thalamus)
Pathway for protopathic sensibility (pain pathway, serves the pain, temperature and general pressure sensation):
• 1st neuron (uncrossed): from receptors (exteroceptors) of the skin and the mucosa etc., to the posterior horn, Laminae I to V (perikarya in the dorsal root ganglia)
• 2nd neuron (crossed, some fibres possibly uncrossed): from the posterior horn to the Thalamus, in the Formatio reticularis and to the Tectum mesencephali (Tractus spinothalamici anterior and lateralis, Tractus spinoreticularis, Tractus spinotectalis; perikarya in the posterior column)
• 3rd neuron (uncrossed): from the Thalamus among others to the cerebral cortex, particularly to the Gyrus postcentralis (thalamocortical fibres, perikarya in the Thalamus)
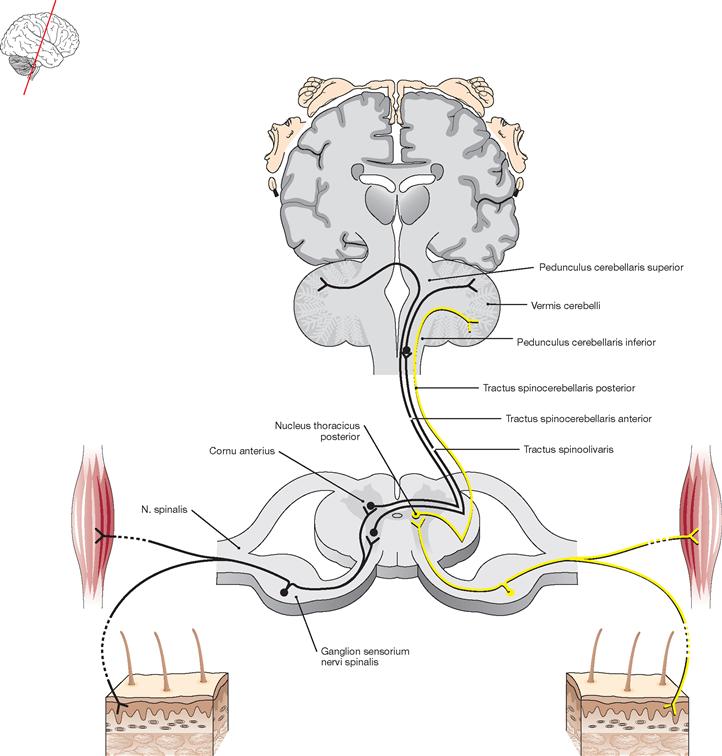
Fig. 12.187 Pathway of unconscious proprioception (afferent tract).
Pathway of unconscious proprioception (unconscious, but precise spatial differentiation as a prerequisite for movement coordination by the Cerebellum) via the anterior spinocerebellar tract (black):
• 1st neuron (uncrossed): from receptors (proprioceptors) in muscles, tendons, and in the connective tissue to the nuclei in the Zona intermedia and the anterior column (perikarya in the spinal ganglia).
• 2nd neuron (two times crossed): from the anterior horn within the anterior spinocerebellar tract of the anterolateral tract via the superior cerebellar peduncle to the Cerebellum (perikarya in the intermediate zone and the anterior horn).
Pathway of unconscious proprioception via the posterior spinocerebellar tract (yellow):
• 1st neuron (uncrossed): from end organs (proprioceptors) in muscles, tendons, and in the connective tissue to the nuclei of the posterior column and to the Nucleus thoracicus (perikarya in the dorsal root ganglia).
• 2nd neuron (uncrossed): from the posterior horn and the thoracic nucleus within the posterior spinocerebellar tract of the lateral tract via the inferior cerebellar peduncle to the Cerebellum (perikarya in the thoracic nucleus and at the base of the posterior column).
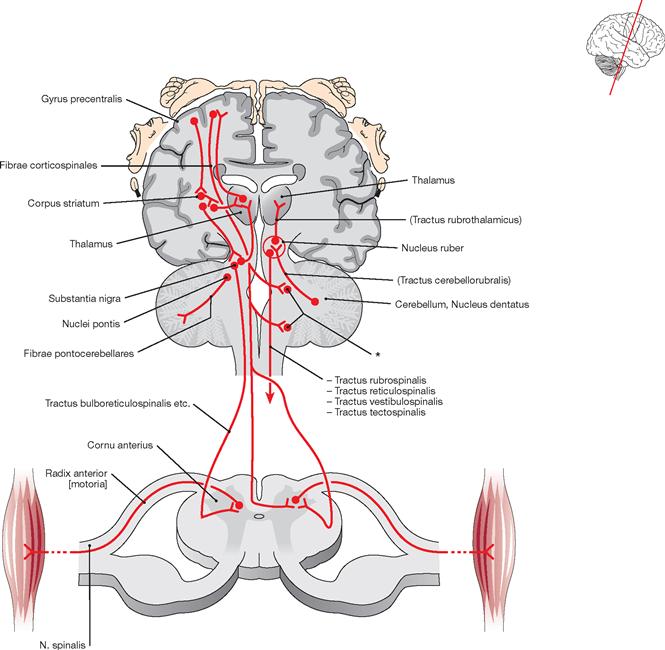
Fig. 12.188 Pathways of the motor system (efferent tracts).
The motor system comprises a large number of nuclear regions and tracts. The “final motor pathway” are the motoneurons. Despite the extraordinary complexity of these circuits, the traditional organisation will be maintained for didactic reasons.
Pyramidal tract:
• (Central) neuron (crossed): from the cerebral cortex through the internal capsule and the cerebral peduncles to interneurons within the anterior and posterior columns (Tractus corticospinalis lateralis, Tractus corticospinalis anterior, perikarya in the Gyrus precentralis).
• (Peripheral) neuron (final motor pathway, α-motoneurons): from the anterior horn to the motor end plates of the skeletal muscles (motoneurons, perikarya in the anterior horn).
• From the Tractus corticospinalis anterior of the pyramidal tract fibres branch off for the nuclei of the cranial nerves (Fibrae corticonucleares and Fibrae corticonucleares bulbi).
• Central neurons: (crossed and uncrossed): from the cerebral cortex, particularly the Gyrus precentralis and the adjacent anterior cortical areas including synapses to the basal ganglia, Thalamus, Nucleus subthalamicus, Nucleus ruber, Substantia nigra, Cerebellum, etc. and feedback loops to interneurons of the anterior column (Tractus rubrospinalis, Tractus reticulospinalis, Tractus vestibulospinales medialis and lateralis, Tractus tectospinalis).
• Peripheral neuron (motor end pathway, α-motoneurons): from the anterior horn to the motor end plates of the skeletal muscles (motoneurons, perikarya in the anterior horn).
Tracts of the spinal cord, Clinics
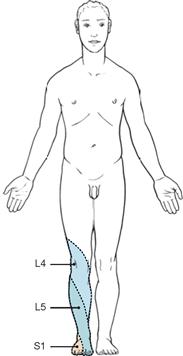
Fig. 12.189 Dysfunctional cutaneous innervation due to palsy of certain, frequently affected spinal nerves.
A disc prolapse frequently affects the spinal nerves L4, L5, and S1.
Fig. 12.190 Complete paraplegia at the level of the 11th thoracic segment (T11).
Paralysis of the whole motor and sensory system in the hatched area.
Fig. 12.192 Hemiplegia (BROWN-SÉQUARD) due to a hemilateral right-sided disruption of the spinal cord at the level of the 11th thoracic segment (T11).
On the right side (ipsilateral): loss of motor function (initially flaccid, later spastic); loss of fine discriminative tactile sensation as well as loss of postural sense and vibration (gross touch sensation remains functionally normal). On the left side (contralateral): loss of pain and temperature sensation (→ Fig. 12.186).
Autonomic nervous system, functional overview
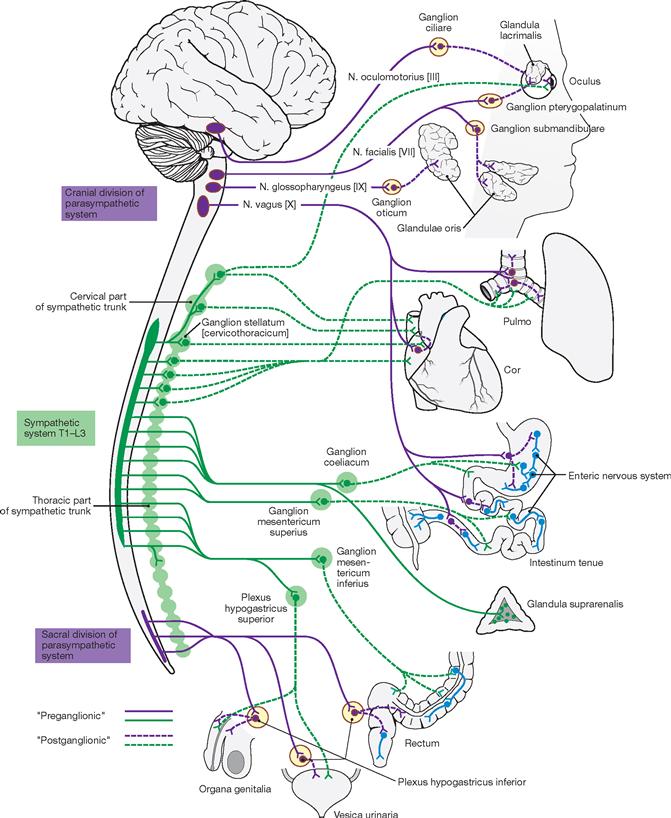
Fig. 12.193 Autonomic nervous system (Sympathicus and Parasympathicus). [22]
The autonomic nervous system comprises the sympathetic (green), parasympathetic (purple), and the enteric nervous system (blue).
The neurons of the Sympathicus locate in the intermediolateral horn of the thoracolumbar section of the spinal cord. Their axons project to the sympathetic chain of ganglia and to the ganglia of the enteric system. Here they synapse to postganglionic neurons which project to the target organs. The sympathetic activation serves to mobilise the body in case of an emergency. The adrenal medulla is part of the sympathetic system and secretes adrenaline and noradrenaline.
Nuclear areas of the Parasympathicus locate in the brainstem and the sacral part of the spinal cord. Their axons project to ganglia adjacent to the target organs which locate in the head, thorax, and the abdominal cavity. Here synapsing onto postganglionic neurons occurs, which reach the target organs via short axons. The parasympathetic system has important roles in food intake and digestion, sexual arousal, and opposes the sympathetic system.
The enteric nervous system regulates the intestinal activity and is modulated by sympathetic and parasympathetic influences.
Central motor system
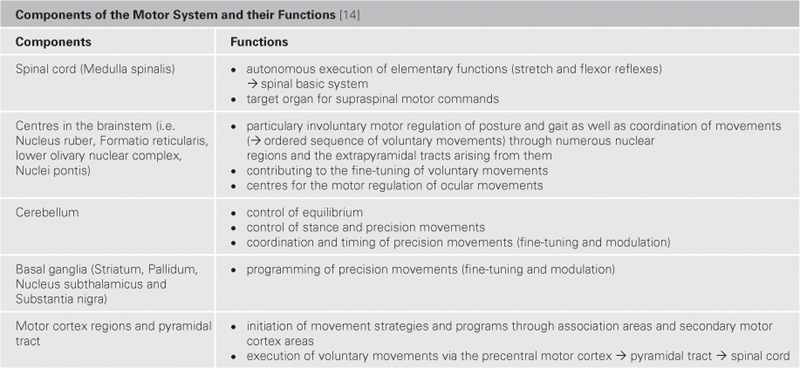
Fig. 12.194 Simplified schematic representation of the organisation of the somatomotor system. [14]
The current assumption is that the inner motivation for motor activity (the initial motor action impulse) initiates in the limbic system. From here, these impulses reach association areas (e.g. in the prefrontal cortex) and a strategic action plan for this movement is created. The realisation requires the inclusion of secondary motor areas which plan the details of the intended movement and fine-tune the motion program through feedback from the Cerebellum and the basal ganglia. Once the planning phase of the intended movement is completed, the so modulated motion program is transmitted to the Thalamus and from here to the motor areas, particularly to the motor cortex that signals the beginning of the execution phase. The pyramidal tract originates at the motor cortex and projects into the spinal cord, where the information reaches the muscles. The inferior olivary nucleus and the Cerebellum receive copies of the motion program to initiate modulations and/or corrections of the motor action in a timely fashion. In addition, extensive sensory feedback loops exist between peripheral receptor systems and all structures involved in the motion program to ensure a smooth execution of the motor action.