Chapter 30 Antibacterial and antiviral drugs
Antibacterial and antiviral compounds constitute two of the groups of antimicrobial substances; other representatives are antiprotozoal drugs (Chapter 28) and antifungal drugs. Antimicrobial agents can also be categorized according to whether they are antibiotics (derived from the growth of microorganisms), chemotherapeutic agents (synthetic compounds not found in nature) or derivatives from non-microbial natural sources (lichens, higher plants, animals).
ANTIBACTERIAL DRUGS
For the student of pharmacy, these compounds are of utmost importance and in the UK are usually studied outside the field of pharmacognosy. For this reason, and because space precludes the in-depth treatment afforded by other works available to readers, an introduction only is given below.
Sources
Waksman’s 1951 definition of antibiotics was limited to substances produced by microorganisms. The term ‘antibacterial’ is consequently used to include those active compounds prepared synthetically or isolated from higher plants. Most of the clinically used antibiotics are produced by soil microorganisms or fungi but many examples of antibacterial agents from the other groups have been recorded and are mentioned below.
History
The first scientific recording of antibiotic activity was made by Louis Pasteur, who in 1877 reported that animals injected with an inoculation containing Bacillus anthracis and certain other common bacilli failed to develop anthrax.
In 1881, Tyndal in his Essays on the Floating Matter of the Air in Relation to Putrefaction and Infection reported that in some tubes containing a nutritive infusion and bacteria which had become contaminated also with Penicillium glaucum, the bacteria lost their ‘transilatory power and fell to the bottom of the tube’. Tyndal attributed this phenomenon to the cutting off of the oxygen supply to the bacteria by the pellicles formed by the mould. Ten years after Pasteur’s discovery, Emmerich (1887) accidentally discovered that a guinea-pig which had previously been injected with Streptococcus erysipelatis failed to develop cholera when injected with virulent cultures of Vibrio cholerae. He immediately recognized the significance of the discovery and was able to prevent anthrax in experimental animals by administering S. erysipelatis prior to the injection of B. anthracis.
Bouchard (1889) noticed that Pseudomonas aeruginosa prevented the development of anthrax in the rabbit; this observation was extended in scope by Woodhead and Wood (1889), who found that sterilized cultures of P. aeruginosa exerted the same protective effect against anthrax. The lytic action of certain Actinomycetes on various microorganisms was observed by Brunel. Emmerich with his colleague Low further studied the protective action of culture filtrates of P. aeruginosa; they concentrated these cell-free filtrates to 1/10th of their original volume and demonstrated that they destroyed Corynebacterium diphtheriae, staphylococci, streptococci, the pneumococcus, the gonococcus, Vibrio cholerae and Shigella paradysenteria in vitro. The active principle present in this preparation has now been isolated and purified and its structure determined. The recognition of the phenomenon of antibiosis had now been established but in 1928 Fleming noted the inhibition of bacteria by a colony of Penicillium notatum that had developed as a contaminant on a Petri dish. He advocated in his paper (Fleming, 1929) the possible clinical use of the substance formed by the Penicillium culture.
The advent of World War II launched a large-scale programme for the production and testing of the substance now known as penicillin, andthe resources of industry and academic institutions were devoted to the study of this substance and the search for other antibiotics. This led to the discovery of streptomycin, aureomycin, chloromycetin and many other antibiotics involving notably various species of Streptomyces. High-yielding strains of Penicillium chrysogenum which produce little pigment have now replaced P. notatum for penicillin manufacture. Preferential synthesis of benzylpenicillin is achieved by the addition of phenylacetic acid to the fermentation medium.
Clinical use
Of the antibiotics in clinical use, most are of bacterial or fungal origin (Table 30.1). Among the bacteria, the genus Streptomycesis particularly noteworthy, as it produces antibiotics such as streptomycin, chloramphenicol, chlortetracycline, tetracycline, erythromycin and neomycin. The penicillins, griseofulvin (an antifungal agent) and cephalosporins are of fungal origin.
Table 30.1 Some clinically important antibiotics
| Types and examples | Sources | Notes |
|---|---|---|
| Penicillins | Various Penicillium spp. | Based on β-lactam structure with various side-chains mainly at position 6 |
| Benzylpenicillin (Penicillin G) | Suitable strains of P. notatum | Acts by interfering with the synthesis of bacterial cell membranes. Inactivated by bacterially produced penicillinases |
| Phenoxymethyl penicillin (Penicillin V) | As above, with phenoxyacetic acid added to the culture medium | Unlike benzyl penicillin it is resistant to acid gastric juice |
| Semisynthetic penicillins | By enzymatic removal of side-chain of penicillin and re-esterification with other acids | Have modified properties of penicillin G such as acid resistance, penicillinase resistance (flucloxacillin), broad-spectrum activity (ampicillin) and antipseudomonal activity (ureidopenicillins) |
| Cephalosporins | Core structure similar to that of the penicillins and based on 7-aminocephalosporic acid | |
| Cephalosporin C | Cephalosporium acremonium | Has only moderate antibacterial activity |
| Modified cephalosporins | Side-chain substitution—see text | They have a higher degree of resistance to staphylococcal penicillinase compared with the penicillins. Wide spectrum of activity against Gram-negative bacteria |
| Tetracyclines | ||
| Tetracycline, chlortetracycline, oxytetracycline and others | Streptomyces spp., S. aureofaciens, S. rimosus | Broad-spectrum antibiotics to which bacterial resistance has greatly increased. Bacteriostatic rather than bactericidal. Most commonly prescribed for chronic bronchitis |
| Chloramphenicol | S. venezuelae: now by synthesis | Because of its toxicity chloramphenicol should be reserved for life-threatening diseases such as typhoid fever, meningitis, infections of Haemophilus influenzae and other conditions where no other antibiotic is effective. Widely used as eye drops |
| Aminoglycosides | Various soil organisms | All are bactericidal and active against many Gram-negative and some Gram-positive organisms |
| Streptomycin | Strains of Streptomyces griseus and other spp. | Discovered shortly after penicillin, streptomycin was used for the treatment of tuberculosis. Resistance was rapidly developed by the tubercle bacilli and it is now little used |
| Gentamicin | Micromonospora purpurea | The most widely used of the aminoglycosides; BP drug is a mixture of various gentamicin sulphates. Used in a variety of preparations |
| Neomycin | Selected strains of Streptomyces fradiae | Not used systemically. Administered prior to colonic surgery to suppress bowel flora. Topical applications. Eye preparations |
| Macrolides | ||
| Erythromycin | Certain strains of Streptomyces erythreus which produce principally erythromycin A | An alternative therapy for penicillin-hypersensitive patients |
| Nystatin | Streptomyces spp. | A polyene macrolide used for local treatment of the fungus Candida albicans |
| Peptides | Principally Bacillus spp. | Composed of a polypeptide chain linked to another group such as a long-chain fatty acid |
| Bacitracin | B. subtilis, B. licheniformis | In combination with other antibiotics it is used principally to treat skin infections |
| Polymyxin B | B. polymyxa | Effective against Gram-negative organisms particularly Pseudomonas aeruginosa. Included in bladder instillations, eye and ear drops and as other topical preparations |
| Colistan (Polymyxin E) | B. colistinus | Uses include bowel sterilization regimens |
| Cytotoxic antibiotics | ||
| Actinomycin D, daunorubicin and others | Various Streptomyces spp. | Anticancer therapy |
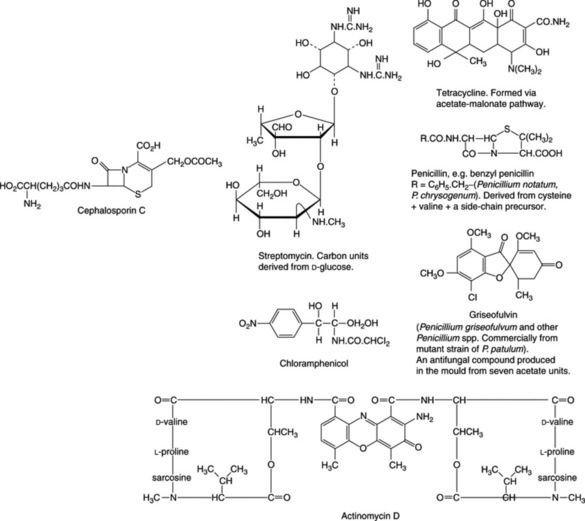
The cephalosporins (cefalosporins) are broad-spectrum antibiotics related both structurally and clinically to the penicillins. Being stable in acid solutions, some can be administered orally. Cephalosporin C arises by fermentation utilizing Cephalosporium acrimonium. As shown in Fig. 30.1, cephalosporin possesses two side-chains and substitution of these with a variety of groups has given rise to a considerable number of clinically effective drugs; some twelve, with theirpreparations, are listed in the current British National Formulary. They are usually graded, somewhat arbitrarily into first-, second- and third-generation cephalosporins, which roughly conform to the dates they were introduced and the particular type of derivative. Drugs from all generations are currently in use.
Other antibiotics of bacterial origin are the actinomycin, bacitracin, tyrothrycin and polymixin groups. These are all polypeptides and although their strong antibacterial properties were recognized early in the development of antibiotics their cytotoxicity prevented clinical use as internal medicines. However, the subsequent quest for anticancer agents led to a renewed interest in their antitumour action and now some of them (e.g. doxorubicin, daunorubicin, actinomycin D) are used to treat a variety of cancers. Such cytotoxic antibiotics can completely block RNA replication and are among the most potent antitumour compounds discovered but, like others of this group, cause unwanted side-effects owing to their non-selective action.
Unfortunately, due in part to the widespread and often indiscriminate use of antibiotics, together with poor hygiene, many pathogenic organisms have acquired a resistance to specific antibiotic treatments and these strains are particularly evident in the hospital environment. The problem is further compounded by the fact that resistance to a particular antibiotic can be transferred from one organism to another (jumping genes). Thus, the clinician’s antibiotic armamentarum is being steadily eroded and research directed towards the isolation or synthesis of new drugs is still a matter of considerable urgency.
Antibiotics are employed topically, orally or as injections but in order to reduce the development of antibiotic-resistant strains of microorganisms, they are now being prescribed in a more restrictive manner than was originally the case. For this reason the use of topicalcreams and ointments has declined although topical ocular preparations remain important.
The varied chemical nature of a number of antibiotics is illustrated in Fig. 30.1.
Non-microbial sources of antibacterials
Lichens
Many of these appear to owe their bacteriostatic and antifungal properties to usnic acid or vulpinic acid.
Monocotyledons
Fresh garlic owes its antibiotic action to alliine, a sulphur-containing amino acid; ginger has antibacterial properties, so too aloe vera gel.
Dicotyledons
Examples from this group are: the sesquiterpene ketones of hops (humulene and lupulene) and those of myrrh (furanodiene-6-one and methoxyfuranoguaia-9-ene-8-one); protoanemonine, a lactone present in Anemone pulsatilla and many other Ranunculaceae; various sulphur-containing compounds found in the Cruciferae; plumbagin (2-methyl-5-hydroxyl-1,4-napthaquinone), found in Drosera; and compounds found in compositous plants such as burdock, thistle and Hieracium pilosella. The last plant, mouse-ear hawkweed, has been used clinically for the treatment of Malta fever. Mastic gum is effective in the treatment of gastric ulcers and has been shown to be active in low doses against Helicobacter pylori, an organism associated with this condition, (F. U. Huwez et al., New Engl. J. Med., 1998, 339, 1946). Cinnamon extracts have been shown to inhibit the growth and urease activity of the same organism (M. Tabek et al., J. Ethnopharm, 1999, 67, 269). Many other plants have been screened for antimicrobial activity and new reports continually appear in the literature.
ANTIVIRAL AGENTS
The success achieved in tackling bacterial infection by the use of natural antibiotics derived from microorganisms was not paralleled in the quest for antiviral agents. Virus diseases still remain an area of medicine for which specific treatments are lacking. Apart from the paucity of drugs which can prevent replication of the virus within the living cell there is also the problem that the peak rate of growth of the virus is usually over before the clinical symptoms appear. Treatment is often, of necessity, symptomatic. In some instances, e.g. for various types of influenza, vaccines are available.
Added impetus was given to the search for antiviral drugs by the recognition that the retrovirus termed human immunodeficiency virus (HIV) was the causative agent of acquired immunodeficiency syndrome (AIDS) [a retrovirus is one which utilizes the enzyme reverse transcriptase (RT) for the conversion of its RNA into DNA, in this way enabling it to become incorporated into the DNA of the host]. This prompted the large-scale screening of natural products and synthetic compounds for anti-HIV activity by pharmaceutical companies and organizations such as the US National Cancer Institute.
It is considered that there are some ten stages in the replication of the HIV virus which could be targeted in the search for effective drugs. One such stage of critical importance is the reverse transcription step mediated by the enzyme RT and many compounds have now been shown to have HIV-RT inhibitory properties.
Few of the compounds showing activity in the initial screens reach the stage of clinical testing, most being of low potency, too cytotoxic or, as with the tannins and flavonoids, being non-specific in their action. The removal of the latter such ‘nuisance compounds’ in the testing of crude plant extracts has already been discussed in Chapter 9. Successful compounds may well serve as lead compounds for the semisynthetic preparation of less toxic derivatives.
As is often the case in science, a significant discovery came from an unrelated area of research. Bell and colleagues at London University, working on possible pesticidal non-protein amino acids, discovered in the seeds of Castanospermum australe (Leguminosae), a new alkaloid of the tetrahydroxyindolizidine group which was named castanospermine. This alkaloid had unusual solubility properties and was isolated in experiments designed to separate amino acids rather than alkaloids. Castanospermine was found to exert its biological effect on insect larvae by inhibiting the carbohydrase enzymes which are essential for the elaboration of the oligosaccharide side-chains on glycoproteins. This led to the testing of the alkaloid against HIV on the basis that as the compound inhibits α-glucosidase I and II, which control the formation of glycoproteins in the viral coat, then, without the essential envelope structure the virus would be unable to infect healthy white blood cells. The antiviral tests proved positive and various O-acyl derivatives have since been shown to be up to 20 times more active than castanospermine itself. The enzyme inhibitory properties of the alkaloid have now been considerably studied. Although the toxicity levels are unsatisfactory for clinical use it constitutes a prime lead compound for the development of other glucosidase inhibitors. Castanospermine is isolated in yields of up to 0.3% from the seeds and has also been isolated from the closely related genus Alexa. Two other inhibitors of α-glucosidase isolated from the seeds of C. australe are 6-epi-castanospermine and the tetrahydroxypyrrolizidine alkaloid australine.
One significant discovery in this field to date relates to a series of coumarins—the calanolides. In 1991 calanolide A and calanolide B were isolated in small yield from the leaves and twigs of the tropical rainforest tree Calophyllum lanigerum (Guttiferae) and shown to possess anti-HIV activity. Botanists returned to Sarawak in 1992 to discover the trees had been destroyed; other collections from the same locality proved to be inactive, demonstrating once again the problems of securing sustainable sources of raw material. However the structures of the active (+)-calanolide A (Fig. 30.2) and (−)-calanolide B were established together with some structure–activity correlations; synthesis gave a less active racemate. Although (+)-calanolide A is now unavailable as a natural product (−)-calanolide B (also known as costatolide) and the related soulattrolide are apparently available in high yield from the latex of C. teysmannii which can be collected without destruction of the tree. Other HIV-inhibitors named inophyllums, and related to the calanolides, have been isolated from C. inophyllum; in these compounds a phenyl ring replaces the propyl side-chain at C-4. By 1998 preclinical trials of calanolide A racemate were in progress.
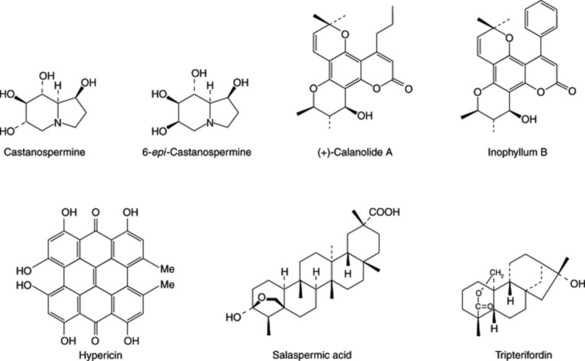
Fig. 30.2 Structures of potentially useful natural products for the treatment of AIDS. For other compounds see Table 30.2
Sumbul root (Ferula sumbul, Umbelliferae), a drug formerly used for its stimulant and antispasmodic properties, contains at least 27 coumarins and possesses anti-HIV activity (P. Zhou et al., Phytochemistry, 2000, 53, 689).
Sometimes anti-AIDS agents of more than one chemical class occur in the same plant, e.g. coumarins, flavonoids and pentacyclic triterpenoids in liquorice (Table 30.2). Also salaspermic acid, a pentacyclic triterpene, from the roots of Tripterygium wilfordii (Celastraceae)shows inhibition of HIV reverse transcriptase and HIV replication in HG lymphocyte cells. Also active, from the same plant, is tripterifordin, a kaurene-type diterpene lactone. The plant is a toxic liane known for its pesticidal properties and since 1960 has been shown to possess a number of other biological actions.
Table 30.2 Plant constituents with anti-HIV activity
| Constituent | Plant source | Observations |
|---|---|---|
| Alkaloids | ||
| Castanospermine (Fig. 30.2) | See text | |
| Michellamines A–C (Naphthylisoquinoline dimers) | Ancistrocladus korupensis (Ancistrocladaceae) | Michellamine B has broad range of anti-HIV activity. Submitted for pre-clinical trials (see Chapter 38) |
| Anthraquinones | ||
| Hypericin (Fig. 30.2) | Hypericum spp. (Guttiferae) | Antiretroviral activity; clinical trials (1991) |
| Coumarins | ||
| Calanolides A and B (Fig. 30.2)LycopyranocoumarinGlycycoumarin | See textGlycyrrhiza glabra | Inhibition of giant cell formation in HIV-infected cell cultures |
| Dimeric sesquiterpenes | ||
| Gossypol (Fig. 24.3) | Seeds of Gossypium spp. | Inhibitory effect on HIV replication |
| Diterpene lactones | ||
| Tripterifordin (Fig. 30.2) | See text | |
| Flavonoids | ||
| Glycyrrhizoflavone Isolicoflavonol Licochalcone | Glycyrrhiza glabra | Similar action to liquorice coumarins |
| Lignans | ||
| (−)-Trachelogenin | Ipomoea cairica (Convolvulaceae) | Suppresses the integration of proviral DNA into cellular genome |
| Pentacyclic triterpenoids | ||
| Glycyrrhizin (Fig. 23.12) | Glycyrrhiza glabra and other spp. | Asymptomatic HIV carriers experienced delayed development of AIDS symptoms |
| Salaspermic acid (Fig. 30.2) | See text | |
| Polysaccharides | ||
| Sulphated polysaccharides | Various Chinese herbal medicines including Viola yedoensis (Violaceae); Prunella vulgaris (Labiatae); Alternanthera philoxeroides (Amaranthaceae) | In vitro inhibitory activity against HIV |
| Tannins | ||
| Tetragalloylquinic acids | Commercial tannic acid | HIV reverse transcriptase inhibitors |
Several species of a genus of gourds (Trichosanthes, Cucurbitaceae) widespread in Asia, contain a toxic protein trichosanthin. Preparations based on this compound appear to have ribosome-inactivating properties and selectively kill cells infected with HIV. Although the roots of T. kirilowii have traditional medicinal uses in China, Taiwan and Korea a number of AIDS sufferers in the US who took this preparation on their own initiative suffered severe side effects, including death, illustrating the necessity for adequate testing of such materials.
Most of the major chemical groups of natural products have yielded compounds with anti-HIV activity and more continue to appear in the literature. As an illustration, a few plants and their active consituents are given in Table 30.2 and formulae in Fig. 30.2.
Plant constituents showing activity against Herpes simplex are the podophyllotoxin lignan deoxypodophyllotoxin (from Thuja occidentalis), saponins of the oleane type which inhibit viral DNA synthesis, and ursane-type saponins which interfere with the formation of capsidal proteins. Of the numerous 3-methoxyflavones some are active against polio and rhino-viruses. A standardized extract of elderberry (Sambucus nigra) proved effective in the treatment of individuals during an outbreak of influenza B Panama; it also inhibited, in vitro, several other strains of the virus.
The use of shikimic acid as a starting material for the synthesis of oseltamivir (Tamiflu®) has been discussed elsewhere (q.v.).