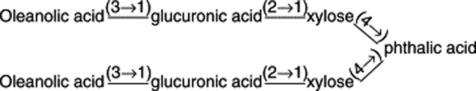Chapter 23 Saponins, cardioactive drugs and other steroids
Plant materials containing saponins have long been used in many parts of the world for their detergent properties. For example, in Europe the root of Saponaria officinalis (Caryophyllaceae) and in South America the bark of Quillaja saponaria (Rosaceae). Such plants contain a high percentage of glycosides known as saponins (Latin sapo, soap) which are characterized by their property of producing a frothing aqueous solution. They also have haemolytic properties, and when injected into the blood stream, are highly toxic. The fact that a plant contains haemolytic substances is not proof that it contains saponins, and in the species examined by Wall (1961) only about half of those containing haemolytic substances actually contained saponins. When taken by mouth, saponins are comparatively harmless. Sarsaparilla, for example, is rich in saponins but is widely used in the preparation of non-alcoholic beverages.
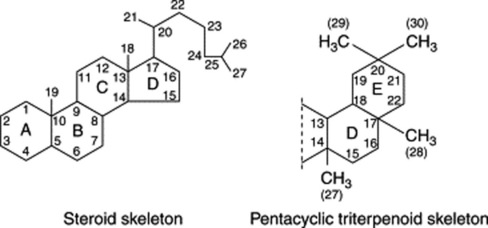
Saponins have a high molecular weight and a high polarity and their isolation in a state of purity presents some difficulties. Often they occur as complex mixtures with the components differing only slightly from one another in the nature of the sugars present, or in the structure of the aglycone. Various chromatographic techniques have been employed for their isolation. As glycosides they are hydrolysed by acids to give an aglycone (sapogenin) and various sugars and related uronic acids. According to the structure of the aglycone or sapogenin, two kinds of saponin are recognized—the steroidal (commonly tetracyclic triterpenoids) and the pentacyclic triterpenoid types (see formulae below). Both of these have a glycosidal linkage at C-3 and have a common biogenetic origin via mevalonic acid and isoprenoid units.
A distinct subgroup of the steroidal saponins is that of the steroidal alkaloids which characterize many members of the Solanaceae. They possess a heterocyclic nitrogen-containing ring, giving the compounds basic properties (as an example see solasodine, Fig. 23.5).
STEROIDAL SAPONINS
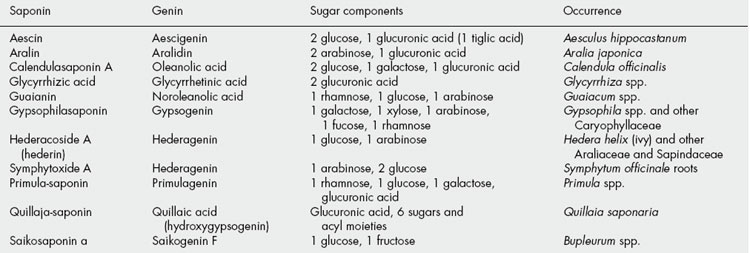
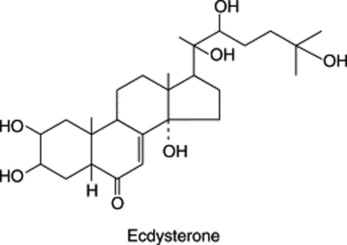
The steroidal saponins are less widely distributed in nature than the pentacyclic triterpenoid type. Phytochemical surveys have shown their presence in many monocotyledonous families, particularly the Dioscoreaceae (e.g. Dioscorea spp.), Agavaceae (e.g. Agave and Yucca spp.) and Smilacaceae (Smilax spp.). In the dicotyledons the occurrence of diosgenin in fenugreek (Leguminosae) and of steroidal alkaloids in Solanum (Solanaceae) is of potential importance. Some species of Strophanthus and Digitalis contain both steroidal saponins and cardiac glycosides (q.v.). Examples of saponins and their constituent sugars are given in Table 23.1.
Table 23.1 Examples of steroidal saponins.
| Steroidal saponin | Sugar components | Occurrence |
|---|---|---|
| Sarsaponin (Parillin) | 3 glucose, 1 rhamnose | Smilax spp. |
| Digitonin | 2 glucose, 2 galactose, 1 xylose | Seeds of Digitalis purpurea and D. lanata |
| Gitonin | 1 glucose, 2 galactose, 1 xylose | Seeds and leaves of D. purpurea and seeds of D. lanata |
| Dioscin | 1 glucose, 2 rhamnose | Dioscorea spp. |
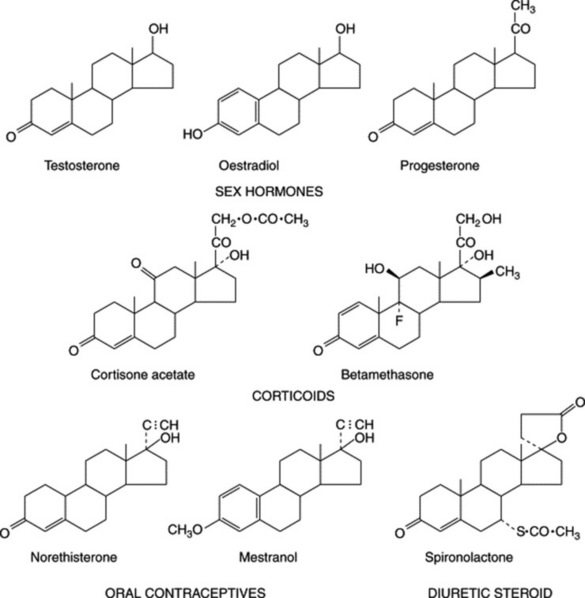
Steroidal saponins are of great pharmaceutical importance because of their relationship to compounds such as the sex hormones, cortisone, diuretic steroids, vitamin D and the cardiac glycosides. Some are used as starting materials for the synthesis of these compounds. Diosgenin is the principal sapogenin used by industry but most yams, from which it is isolated, contain a mixture of sapogenins in the glycosidic form.
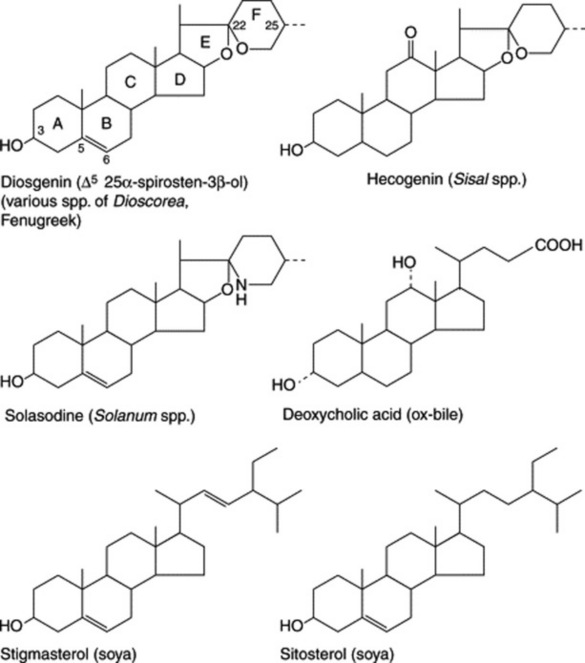
As with cardiac glycosides, the stereochemistry of the molecule is of some importance, although not so much so for cortisone manufacture. Natural sapogenins differ only in their configuration at carbon atoms 3, 5 and 25, and in the spirostane series the orientation at C-22 need not be specified (cf. steroidal alkaloids). Mixtures of the C-25 epimers—for example, diosgenin (Δ5,25α-spirosten-3β-ol) and yamogenin (Δ5,25β- spirosten-3β-ol)—are of normal occurrence and their ratio, one to the other, is dependent upon factors such as morphological part and stage of development of the plant. In some instances in the plant, the side-chain which forms ring F of the sapogenin is kept open by glycoside formation as in the bisdesmosidic saponin sarsaparilloside of Smilax aristolochiaefolia.
BIOGENESIS OF STEROIDAL SAPONINS
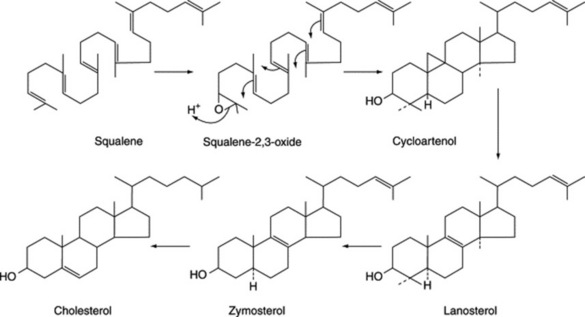
Steroidal saponins arise via the mevalonic acid pathway; the preliminary stages have been discussed in Chapter 18. A scheme for the subsequent cyclization of squalene to give cholesterol is illustrated in Fig. 23.1. Cholesterol, the wide distribution of which in plants has only relatively recently been shown, can be incorporated into a number of C27 sapogenins without side-chain cleavage (Fig. 23.2), although it is not necessarily an obligatory precursor. Extensive investigations involving whole plants, homogenates and cell cultures have been performed to elucidate these detailed pathways, including the origin of the 25-epimers (e.g. diosgenin and yamogenin).
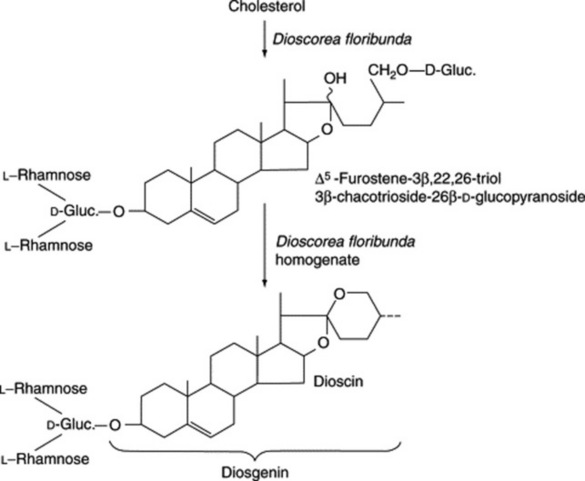
As early as 1947 Marker and Lopez had postulated that steroidal saponins exist in plants in a form where the side-chain is held open by glycoside formation. However, direct evidence for the natural occurrence of these compounds was not forthcoming for another 20 years. It has been shown that such open-chain saponins are, like the more common ones, formed from cholesterol. In Dioscorea homogenates one such compound has been converted to dioscin (a diosgenin glycoside) (Fig. 23.3).
NATURAL STEROIDS FOR THE PRODUCTION OF PHARMACEUTICALS
Although total synthesis of some medicinal steroids is employed commercially, there is also a great demand for natural products which will serve as starting materials for their partial synthesis.
As indicated in Fig. 23.4, which illustrates the range of steroids required medicinally, cortisone and its derivatives are 11-oxosteroids, whereas the sex hormones, including the oral contraceptives, and the diuretic steroids have no oxygen substitution in the C-ring. Fig. 23.5 shows some of the more important natural derivatives which are available in sufficient quantity for synthetic purposes. Hecogenin with C-ring substitution provides a practical starting material for the synthesis of the corticosteroids, whereas diosgenin is suitable for the manufacture of oral contraceptives and the sex hormones. Diosgenin, however, can also be used for corticosteroid synthesis by the employment, at a suitable stage in the synthesis, of a microbiological fermentation to introduce oxygen into the 11α-position of the pregnene nucleus.
Efforts are constantly being made to discover new high-yielding strains of plants and to assure a regular supply of raw material by the cultivation of good-quality plants. Hardman in a review on steroids (Planta Med., 1987, 53, 233) recorded that, annually, the American Chemical Abstracts contained some 3000 references pertinent to plant steroids or related compounds. Some of the better-known examples of steroidal sapogenins and their sources are given in Table 23.2. (For a review, tabulating over 200 sapogenins, see A. V. Patel et al., Fitoterapia, 1987, 58, 67.)
Table 23.2 Some steroidal sapogenins and their sources.
| Sapogenin | Species | Location |
|---|---|---|
| Diosgenin | Dioscorea sylvatica | Transvaal and Natal |
| D. mexicana and D. composita | Mexico and Central America | |
| D. collettii, D. pathaica and D. nipponica | China | |
| D. floribunda | Guatemala and cultivated in India | |
| D. deltoidea and D. prazeri | India | |
| D. tokoro | Japan | |
| Costus speciosus | India | |
| Kallstroemia pubescens | Tropical America; introduced into West Brazil | |
| Trillium spp. | North America | |
| Trigonella foenum-graecum | India, Egypt, Morocco | |
| Hecogenin | Agave sisalana | Subtropical America and cultivated in Kenya for sisal and saponin |
| A. rigida | Mexico | |
| Hechtia texensis | Central America | |
| Sarsapogenin | Yucca spp., Smilax spp. | Central America |
| Sarmentogenin | Strophanthus spp. | Africa |
Dioscorea species
Tubers of many of the dioscoreas (yams) have long been used for food, as they are rich in starch. In addition to starch, some species contain steroidal saponins, others alkaloids. From a suitable source the sapogenins are isolated by acid hydrolysis of the saponin. Previous fermentation of the material for some 4–10 days often gives a better yield. The water-insoluble sapogenin is then extracted with a suitable organic solvent. Both wild and cultivated plants are used. Cultivation requires attention to correct soil and drainage, support for the vines and freedom from weeds, virus, fungus and insect attack. According to the species, the tubers reach maturity in 3–5 years and on average, yield 1–8% of total sapogenin.
Until 1970 diosgenin isolated from the Mexican yam was the sole source for steroidal contraceptive manufacture. With the nationalization of the Mexican industry, however, prices were increased to such an extent that manufacturers switched to hecogenin for corticosteroids, to other sources of diosgenin and to the use of the steroidal alkaloids of Solanum species. Total synthesis also became economically feasible and is now much used. More recently, the economics of steroid production have again changed in that China is now exporting large quantities of diosgenin; it is of high quality, being free of the 25β-isomer yamogenin, although this is of no commercial significance, and is reasonably priced. Three of the many Dioscorea spp. found in China and used commercially are given in Table 23.2; the tubers of these yield 2% of diosgenin, with the average content of diosgenin for the main areas of production (Yunnan Province and south of the Yangtze River) being 1%.
Sisal
Hecogenin is obtained commercially as the acetate in about 0.01% yield from sisal leaves (Agave sisalana). In East Africa, from leaf ‘waste’ stripped from the leaves during removal of the fibre, a hecogenin-containing ‘sisal concentrate’ is produced. From this the ‘juice’ is separated and allowed to ferment for 7 days. The sludge produced contains about 80% of the hecogenin originally present in the leaves; steam at 1380 kPa pressure is employed to complete the hydrolysis of the original glycosides. By filtration and drying a concentrate containing about 12% hecogenin and varying amounts of other sapogenins is produced. This crude material is shipped for further processing and cortisone manufacture. Hecogenin is also produced in Israel and China. A number of new steroidal saponins have been isolated from the dried fermented residues of Chinese A. sisalana forma Dong No. 1 (see Yi Ding et al., Chem. Pharm. Bull., 1993, 41, 557).
A survey of 34 species of Agave by Blunden and colleagues in 1978 showed that the extracts of most yielded steroidal sapogenins. Previously it had been shown that certain commercial samples of crude sapogenins from A. sisalana also contained the dihydroxy steroid rockogenin, sometimes in appreciable quantity; this compound appears to be an artefact formed during processing and should be avoided. A dihydroxyspirostane, barbourgenin, has been described (G. Blunden et al., J. Nat. Prod., 1986, 49, 687). Agave hybrids with a high hecogenin content and relatively free of tigogenin, with which it is usually associated, have been developed. Gbolade et al. (Fitoterapia, 1992, 63, 45) reported on factors (season, geographical location) affecting steroidal sapogenin levels in Nigerian Agave and Furcraea species.
Another genus of the Agavaceae which has been systematically studied for the presence of steroidal compounds is Cordyline in which many sapogenins, including 1,3-dihydroxysapogenins, have been detected.
FENUGREEK
Although included in this section as a potential industrial source of diosgenin, the seeds of Trigonella foenum-graecum L. (Leguminosae) are also described in the BP and EP. However their principal current use is as a spice; India, Morocco and Egypt among others being important producers.
The very hard seeds have a strong characteristic odour and are irregularly rhomboidal and oblong or square in outline. They are somewhat flattened and are divided into two unequal parts by a groove in the widest surfaces. Their shape and size, and position of the embryo and hilum are shown in Fig. 23.6. The BP/EP describes the seeds as brown to reddish-brown but in commerce olive-green or yellow-brown samples are oftenencountered. Microscopically the testa and hypodermis are characteristic and the mucilage-containing endosperm enables the swelling index (q.v.) of the seeds (not less than six) to be used as a test of purity.
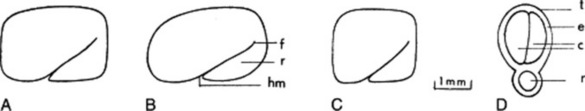
Fig. 23.6 Commercial fenugreek seed. A, Morocco, Israel; B, Ethiopia; C, India, Pakistan; D, transverse section of a seed. c, Cotyledons; e, endosperm; f, furrow; hm, hilum and micropyle region; r, radicle; t, testa.
(After Fazli and Hardman, Trop. Sci., 1968 10, 66.)
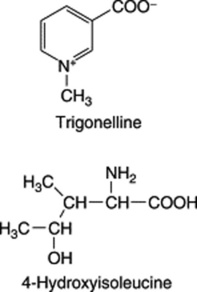
Fenugreek contains the simple pyridine-type alkaloid trigonelline; this base, also reported in garden peas, hemp seed, coffee and many other products was first isolated and described by Jahns in 1885. Today it serves as the reference substance for the BP/EP TLC identification test for the drug. Pharmaceutical manufacturing interest lies in a number of steroidal sapogenins, particularly diosgenin which is contained in the oily embryo. Fazli and Hardman investigated a number of commercial samples of seed as possible commercial sources of diosgenin (see reference in Fig. 23.6) and reported contents of 0.8–2.2% expressed on a moisture-free basis. In 1986, Gupta and colleagues isolated a series of furostanol glycosides (F-ring opened) named trigofoenocides A–G. In a series of papers, further furostanol glycosides designated trigoneosides Ia, Ib–Xlla have been reported in Egyptian seeds (T. Murakami et al., Chem. Pharm. Bull., 2000, 48, 994). As with dioscoreas, the yield of diosgenin is increased by fermentation of the seeds prior to acid hydrolysis. Although the diosgenin yield is lower than that of the dioscoreas, fenugreek is an annual plant which will also give fixed oil, mucilage, flavouring extracts and high-protein fodder as side-products. A number of Hardman’s registered varieties have been subjected to field trials in the UK. However, as long as other cheap sources of diosgenin are available commercially, fenugreek must be regarded as a fall-back source for this sapogenin.
A non-essential amino acid, 4-hydroxyisoleucine, first identified by Fowden et al. in 1973, constitutes up to 80% of the free amino acid composition of fenugreek seeds and has been shown to possess insulin-stimulating properties both in vitro and in vivo (C. Haefelé et al., Phytochemistry, 1997, 44, 563). Some 39 components of the volatile oil fraction have been identified.
Based on mucilage content, the BP/EP sets a swelling index of not less than 6.0 for the powdered seeds.
In addition to its use as a spice and potential source of diosgenin fenugreek is widely employed in traditional systems of medicine. Its antidiabetic, cholesterol-lowering, anti-inflammatory, antipyretic, antiulcer and anticancer properties have been demonstrated.
Solanum species
This large genus (over 1000 spp.) is noted for the production of C27 steroidal alkaloids in many species. Some of these alkaloids are the nitrogen analogues of the C27 sapogenins (e.g. solasodine and diosgenin: Fig. 23.5). Another series of C27 compounds contain a tertiary nitrogen in a condensed ring system (e.g. solanidine; Fig. 23.2). These compounds can also be employed in the partial synthesis of steroidal drugs, and a number of companies have devoted considerable attention to commercial production. Species so exploited are Solanum laciniatum, S. khasianum (a nearly spineless variety has been produced) and S. aviculare; trials on the production of S. marginatum have been conducted in South America. Zenk and colleagues have assayed over 250 spp. of Solanum for solasodine. A number of new glycosides have been isolated from S. dulcamara leaves and include two based on tigogenin and two on soladulcidine.
The steroidal alkaloids were reviewed in 1993 by Atta-ur-Rahman and M. I. Choudhary (Methods in Plant Biochemistry (ed. P. G. Waterman) Vol. 8. Academic Press, London, p. 451).
Soya bean sterols
The soya (soy, soja) plant, Glycine max (G. soya) (Leguminosae) is extensively cultivated for its seeds, which are rich in oil and protein. The seeds also contain appreciable quantities of the phytosterols stigmasterol and sitosterol (Fig. 23.5). Although not sapogenins, they are included here because they are now used extensively for steroid synthesis. They are obtained as byproducts of soap-making, being components of the unsaponifiable matter of the fixed oil. Pure stigmasterol, with its unsaturated side-chain is amenable to chemical conversion to suitable starting materials and can replace diosgenin. But it was more recently that sitosterol, the saturated side-chain of which could not be removed chemically without ring fragmentation, became commercially useful as the result of the discovery of a suitable microbiological side-chain removal. Both phytosterols are now processed by microorganisms. Similar phytosterols are found in other products—for example, cotton-seed oil, tall-oil (from the wood-pulp industry) and sugarcane wax.
For details of the soya isoflavones and their dietary importance, see Chapter 32: The plant nutraceuticals.
Sarsaparilla root
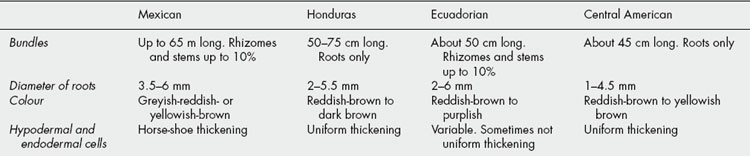
Sarsaparilla consists of the dried roots and sometimes also of the rhizomes of species of Smilax (Liliaceae, modern authors, Smilacaceae). The determination of the exact geographic and botanical sources of the numerous varieties which have from time to time been imported has been a matter of some difficulty (see Table 23.3).
Table 23.3 Varieties of sarsaparilla.
| Variety and geographic source | Synonyms | Botanical source |
|---|---|---|
| Mexican (Southern Mexico, Guatemala, British Honduras) | Vera Cruz or Grey | Smilax aristolochiaefolia |
| Honduras (Guatemala, British Honduras, Honduras, cultivated in Jamaica) | Brown | S. regelii |
| Ecuadorian and Peruvian | Guayaquil | S. febrifuga |
| Central American | Costa Rica or ‘Jamaican’ | Undetermined spp. |
The plants produce numerous roots, 3 m or so long, which are attached to a short rhizome. The roots are cut, sufficient, however, remaining in the ground for the plant to resume its growth. Sometimes the rhizomes as well as the roots are collected. After drying in the sun the drug is made into bundles and the bundles into bales.
Sarsaparilla is imported in large bales bound with wire. Each bale usually contains numerous bundles of approximately uniform size. These consist of long roots, with or without pieces of rhizome and aerial stems. The commercial varieties (Table 23.4) differ from one another in colour, ridges and furrows; in the presence or absence of rhizome and aerial stems; in the relative proportions of cortex, wood and pith, as seen in transverse section; in their microscopical structure. The drug is nearly odourless but has a somewhat sweetish and acrid taste. Owing to the presence of saponins, aqueous extractives froth readily.
Much chemical work has been done on sarsaparillas without proper botanical identification of the material. Different species contain one or more steroidal saponins. Two isomeric genins are known: smilagenin and sarsasapogenin. These differ only in their configuration at C-25 and correspond to the reduced forms of diosgenin and yamogenin respectively. The principal crystalline glycoside of Smilax aristolochiaefolia is parillin (sarsasaponin, sarsasaponoside); it was first isolated from a sample of Jamaica sarsaparilla in 1913 by Power and Salway. On hydrolysis it gives sarsasapogenin, three molecules of glucose and one of rhamnose. Sarsaparilloside, contained in the same species, is a bisdesmosidic saponin (i.e. it possesses two distinct glycosyl groupings, in this case at C-3 and C-26) and represents the parillin molecule with an opened F-ring stabilized by glucosylation.
Uses
Sarsaparilla formerly enjoyed a high reputation in the treatment of syphilis, rheumatism and certain skin diseases. It is included in the BHP (1960) where it is indicated in the treatment of psoriasis and eczema, and for rheumatism and rheumatoid arthritis. Its action would appear to arise from the steroid content of the roots. Sarsaparilla is widely used as a vehicle, and large quantities are employed in the manufacture of non-alcoholic drinks. The genins are used in the partial synthesis of cortisone and other steroids.
Black Cohosh
Black Cohosh, Cimicifuga BHP 1983, is the dried rhizome and roots of Actea racemosa L. syn. Cimicifuga racemosa [L.], Nutt., family Ranunculaceae. This perennial plant is native to Canada and the northern American states and was well-known to the native Americans of these areas. The drug contains substances with endocrine activity and extracts have been widely employed in herbal medicine to treat menopausal and other female disorders, as well as various rheumatic conditions. However, in the 1990s doubts arose over its safety following reports concerning its hepatotoxicity; restrictions on its use have now been applied in a number of countries.
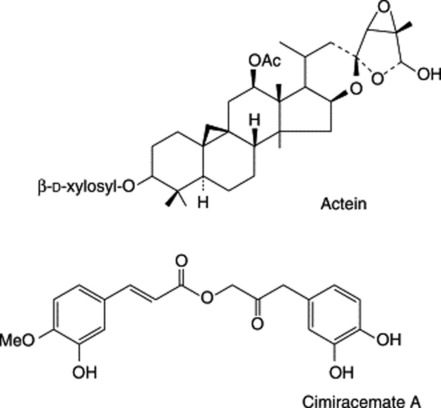
The rhizomes contain a number of triterpenoid glycosides, including actein, 23-epi-26-deoxyactein and cimiracemoside C. Cimicifugoside M and cimifugin can specifically serve as indicators for species identification (K. He et al., Planta Med., 2002, 66, 635). Phenyl propanoside esters are also present including cimiracemates A–D together with ferulic acid, isoferulic acid and methyl caffeate (S.-N. Chen et al., Phytochemistry, 2002, 61, 609). For other reports, see M. Nishida et al., Chem. Pharm. Bull., 2003, 51, 1215; H. Yoshitsu et al., Chem. Pharm. Bull., 2006, 54, 1322.
BUTCHER’S BROOM
Butcher’s broom, Ruscus aculeatus L. family Liliaceae, is a perennial, rigid, dark green much-branched bush, 2–3 feet in height. The leaf-like structures, twisted at the base and terminating in a sharp point, are actually cladodes—flattened stems or internodes that resemble and function as leaves. Small white flowers that arise in the axils of scarious bracts are followed by red berries, which appear situated on the surface of the cladodes.
The species is found in woods and dry places, extending across southern Europe to the Caucasus, and northward to Belgium. It is common in some southern areas of England.
The BP/EP drug consists of the dried, whole or broken roots and rhizomes of the plant. These are collected in autumn and dried to give yellow, knotty pieces of rhizome up to 10 cm in length, showing stem scars on the upper surface and roots and root-scars on the lower surface. A very hard, central cylinder can easily be removed from the outer layer.
Microscopical characteristics, as seen in the powder, include cells with thickened beaded walls with large oval pits, thin-walledparenchyma containing calcium oxalate, thick-walled fibres, and small vessels.
The rhizomes of this plant contain saponins related to those of Dioscorea; thus, one sapogenin is 1β-hydroxydiosgenin (ruscogenin). The plant glycosides involve up to three sugars attached at the C-1 hydroxyl with glucose terminating an uncyclized side-chain at C-26 (for detailed structures see Bombardelli et al., Fitoterapia, 1972, 43, 3). In a series of publications on Ruscus aculeatus M. Yoshikawa et al. have described many more saponins of both the spirostanol and furostanol series and recently, a new saponin which is unique in having a diglucoside unit at C-23 of the spirostanol skeleton (Phytochemistry, 1999, 51, 689). Both the alcoholic extract of the roots and the ruscogenins themselves have anti-inflammatory activity, produce diminished capillary permeability and exert a vasoconstrictor effect in the peripheral blood vessels. On the continent of Europe ointments and suppositories containing the active constituents are available for the treatment of conditions responding to the above effects.
The BP/EP TLC test for identity uses stigmasterol and ruscogenins as reference substances and a vanillin reagent for their visualization. A minimum of 1.0% total sapogenins, expressed as ruscogenins (mixture of neoruscogenin and ruscogenin) is specified; liquid chromatography is used for the assay.
ELEUTHEROCOCCUS
The drug Siberian Ginseng (BP/EP, BHP) consists of the dried, whole or cut organs of Eleutherococcus senticosus Maxim. [Acanthopanax senticosus (Rupr. et Maxim.) Harms], family Araliaceae. The plant is native to China and is now cultivated there and in Russia, Japan and Korea.
Characters
The pale brown, uneven rhizomes, up to 4 cm in diameter, bear the scars of aerial stems and on the lower surface are roots and root scars. The fracture is fibrous and the internal surface light brown to pale yellow. The cylindrical roots are of variable length, up to about 1 cm in diameter, knotty and somewhat branched. When dry the bark is not easily removed from the underlying xylem; commercially it may be removed at harvest and both wood and bark used. Features of the powder include lignified fibres, reticulate and border-pitted vessels, parenchymatous cells containing cluster crystals of calcium oxalate and secretory canals with brown contents. Odour faintly aromatic; taste bitter and persistent.
Constituents
The rhizomes and roots contain a number of constituents termed eleutherocides (A to G; M). These, however, are not all of the same chemical group but include the phenyl propane glycosides eleutheroside B (syringin) and its aglycone sinapyl alcohol; also the lignans (−)-syringaresinol diglucoside (E) and its stereoisomer (D) (formulae Table 21.7), together with caffeic acid and its ethyl ester, chlorogenicacid and coniferaldehyde. Coumarins include coumarin, isofraxidin 7-glucoside (B1) and sesamin (B4). A group of heteroglycans (eleutherans A to G) have been studied for their hypoglycaemic activity. Other constituents include hedera-saponin (M), daucosterol (A) and volatile oil, about 0.8%.
The BP/EP requires a minimum content of 0.8% for the sum of eleutheroside B and eleutheroside E determined by liquid chromatography using UV spectrophotometric absorption at 220 nm and measurement of peak areas. These eleutherosides are also characterized by the TLC test for identity.
DNA analysis has been used to authenticate Japanese and Chinese commercial samples of the drug (T. Maruyama et al., Planta Medica, 2008, 74, 787).
Uses
Eleutherococcus has been used in Chinese medicine since antiquity for the treatment of rheumatoid complaints and for its revitalization properties. For many years, its adaptogenic qualities have been utilized in former USSR countries and now in W. Europe it is employed as a tonic in states of fatigue.
GINSENG
For some 2000 years the roots of Panax ginseng C. A. Meyer (Araliaceae) have held an honoured place in Chinese medicine. Today it is a product of world-wide usage. Production is principally confined to China, Korea and Siberia, although it is cultivated commercially on a small scale in Holland, England, Germany and France (Champagne district).
The most expensive ginseng is that derived from Korean root. The plant, about 50 cm tall with a crown of dark green verticillate leaves and small green flowers giving rise to clusters of bright red berries, is cultivated under thatched covers and harvested when 6 years old. Sun-drying of the root, after removal of the outer layers, produces white ginseng, whereas the red ginseng is obtained by first steaming the root, followed by artificial drying and then sun-drying. Rootlets are numerous on the lower surface of white ginseng but normally absent from red ginseng. The roots are graded and packed. Nineteenth-century descriptions record the care then taken with the preparation, silk, cotton and paper wrappings being used according to the quality of the drug; the wrapped roots were finally stored in containers with quicklime. Small roots are processed separately and form a separate article of commerce. The BP/EP recognizes both red and white ginseng.
The scraping of the roots before drying would appear to be disadvantageous because histochemical tests and GLC analysis show the active saponins to be located outside the root cambium.
Constituents
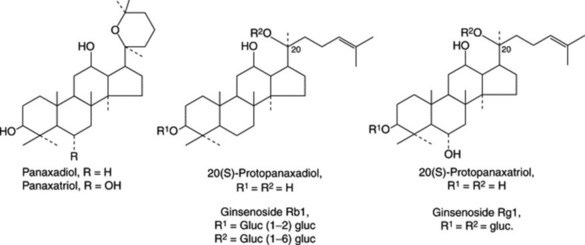
P. ginseng roots have been thoroughly studied by modern methods of analysis and, of the many compounds isolated, the medicinal activity appears to reside largely in a number of dammarane-type saponins termed ginsenosides by Japanese workers and panaxosides by Russian workers. These two series of compounds, all now generally termed ginsenosides, are glycosides respectively derived from the diol 20(S)-protopanaxadiol and the triol 20(S)-protopanaxatriol. Examples of the former are ginsenosides Rb1, Rb2 and Rb4 and of the latter ginsenosides (panaoxosides) Re, Rf, Rg1, Rg2 (see Fig. 23.7). Acid hydrolysis of these saponins involves ring closure of the aglycone giving either panaxadiol or panaxatriol (Fig. 23.7). Some 30 ginsenosides have been named, although not all fit into the above scheme, e.g. ginsenoside Ro is an oleanolic acid derivative. Glucose is the principal sugar involved with some input of arabinose and rhamnose.
The BP/EP specifies a minimum of 0.40% for the sum of ginsenosides Rg1 and Rb1; this is determined by liquid chromatography of a methanolic extract using a reference solution containing the two ginsenosides to he assayed, with absorption measurements at 203 nm. TLC is used as a test for identity and to exclude substitution with P. quinquifolium, which contains no ginsenoside Rf.
Two other groups of compounds present in the root which have known therapeutic activity are high molecular weight polysaccharides (glycans) and acetylenic compounds. The glycans of P. ginseng have been named panaxans (A–U); panaxans A and B have been shown to be constituted mainly of α-(1 → 6) linked D-glucopyranose units with C-3 branching and a small component of peptide. (For the isolation of other polysaccharides termed ginsenans see M. Tomoda et al., Biol. Pharm. Bull., 1993, 16, 1087.) Those glycans tested have hypoglycaemic, antiulcer and immunological properties. One acidic polysaccharide MW 150000, originally isolated in 1993, and composed of 3.7% protein, 47.1% hexoses and 43.1% galacturonic acid, has antineoplastic immuno-stimulant properties.
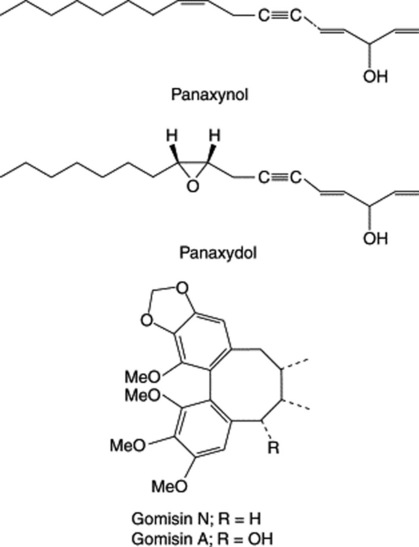
A considerable number of mainly C17, but also C14, polyacetylenic alcohols have been isolated from the roots in recent years and are typified by panaxynol and panaxydol (Fig. 23.8). These compounds have been shown to have antitumour properties and Japanese patents exist for their isolation and derivatization. The cytotoxic activity of the C17-polyacetylenes against leukaemia cells has been shown to be almost 20 times greater than that for the C14-compounds (Y. Fujimoto et al., Phytochemistry, 1992, 31, 3499). K. Hirakura et al. have characterized a series of polyacetylenes named ginsenoynes A–K (see Phytochemistry, 1992, 31, 899 and references cited therein) and subsequently (ibid., 1994, 35, 963) three new linoleoylated polyacetylenes.
Other constituents include sesquiterpenes (panacene, β-elemene, panasinsanol A and B, ginsenol, etc.) to be found in the volatile oil (0.05–0.1%), together with various monoterpenes and monoterpene alcohols. Three minor sesquiterpenes recently identified are panaxene, panaginsene and ginsinsene (R. Richter et al., Phytochemistry, 2005, 66, 2706). Lignans of the dibenzocyclooctadiene type have been isolated from Korean Red Ginseng (Fig. 23.8). Minor components isolated from ginseng roots include sterols, vitamins of the D group, flavonoids and amino acids.
Uses
In Asia the drug is held in esteem for the treatment of anaemia, diabetes, gastritis, sexual impotence and the many conditions arising from the onset of old age. In the West, too, it has in recent years become an extremely popular remedy particularly for the improvement of stamina, concentration, resistance to stress and to disease; in this sense the action of the drug is described as ‘adaptogenic’.
Many ‘ginseng’ products are available as OTC products either for oral administration or as cosmetic preparations. In the US mainstream market for herbal sales, for the first eight months of 1999 ginseng stood at third place, with retail sales valued at over $60 million. Ginseng is included in the BHP (1996) and 29 references covering its constituents and actions are given in the British Herbal Compendium, Vol. 1 (1992).
Allied species
Panax quinquefolium root is one of the major drugs of US foreign trade. It is produced in the eastern USA and Canada, 90% of the US cultivated drug coming from north-central Wisconsin. Some of the ginsenosides of this species are the same as those of the Chinese and Korean drug; others appear to differ. In addition to 14 known dammarane-type saponins, M. Yoshikawa et al. (Chem. Pharm. Bull., 1998, 46, 647) identified a further five new compounds designated quinquenosides I–V. D. Don et al. (Chem. Pharm. Bull., 2006, 54, 751) have reported on a new dammarane-type saponin, ginsenoside Rg8, together with other known ginsenosides. In 1997, Kitanaka et al. showed the hybrid P. ginseng × P. quinquefolium to be superiorto either parent in production of ginsenosides. However, as the plant is sterile, root cultures were established and these showed a comparable ginsenoside production to the field grown material (D. Washida et al., Phytochemistry, 1998, 49, 2331).
Panax pseudoginseng ssp. himalaicus var. augustifolius (Himalayan ginseng). The roots contain active saponins and ginsenosides R0 and Rb1; chikusetsusaponins IVa and VI have been recorded. Shukla and Thakur (Phytochemistry, 1990, 29, 239) characterized pseudoginsenoside-RI2 which consists of oleanolic acid, phthalic acid, glucuronic acid and xylose moieties arranged as below:
Panax notoginseng roots (Sanchi-ginseng) contain dammarane saponins, identical or similar to those of ginseng. M. Yoshikawa et al. (Chem. Pharm. Bull., 1997, 45, 1039; 1056) have isolated, in addition to 14 known saponins, nine new dammarane-type oligoglycosides named notoginsenosides A–J. The roots also contain a polysaccharide (sanchinan-A) having a branched structure with a galactose backbone and side-chains containing arabinose and galactose; this glycan contains a small amount of protein and possesses reticuloendothelial activating properties (K. Ohtani et al., Planta Med., 1987, 53, 16).
Panax japonicum and P. japonicum var. major contain chikusetsusaponins, ginsenosides and glycans.
Panax vietnamensis (Vietnamese ginseng). The roots are a secret remedy of the Sedang ethnic minority and contain a number of known and new ginsenosides (N. M. Duc et al., Chem. Pharm. Bull., 1993, 41, 2010; 1994, 42, 115, 634).
PENTACYCLIC TRITERPENOID SAPONINS
Unlike the steroidal saponins, the pentacyclic triterpenoid saponins are rare in monocotyledons. They are abundant in many dicotyledonous families, particularly the Caryophyllaceae, Sapindaceae, Polygalaceae and Sapotaceae. Among the many other dicotyledonous families in which they have been found are the Phytolaccaceae, Chenopodiaceae, Ranunculaceae, Berberidaceae, Papaveraceae, Linaceae, Zygophyllaceae, Rutaceae, Myrtaceae, Cucurbitaceae, Araliaceae, Umbelliferae, Primulaceae, Oleaceae, Lobeliaceae, Campanulaceae, Rubiaceae and Compositae. Altogether some 80 families are involved.
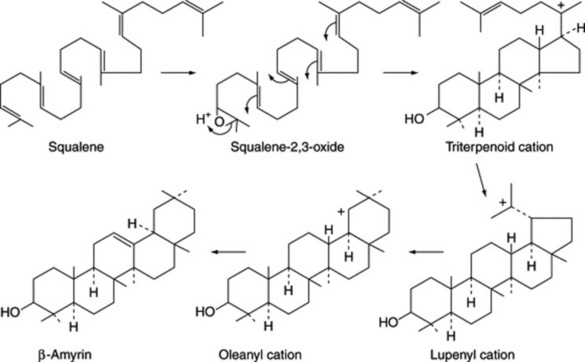
In these saponins the sapogenin is attached to a chain of sugar or uronic acid units, or both, often in the 3-position, as in the examples above. Biosynthesis, as with the steroids, involves ring-closure of squalene and is illustrated in Fig. 23.9.
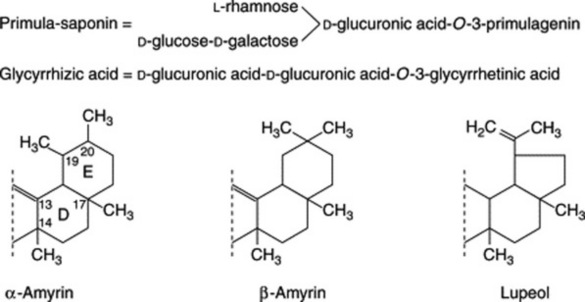
Triterpenoid saponins may be classified into three groups represented by α-amyrin, β-amyrin and lupeol.
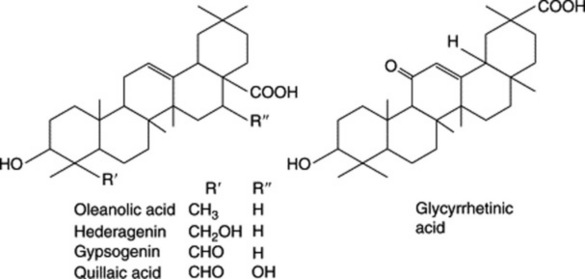
The related triterpenoid acids are formed from these by replacement of a methyl group by a carboxyl group in positions 4, 17 or 20 (Fig. 23.10).
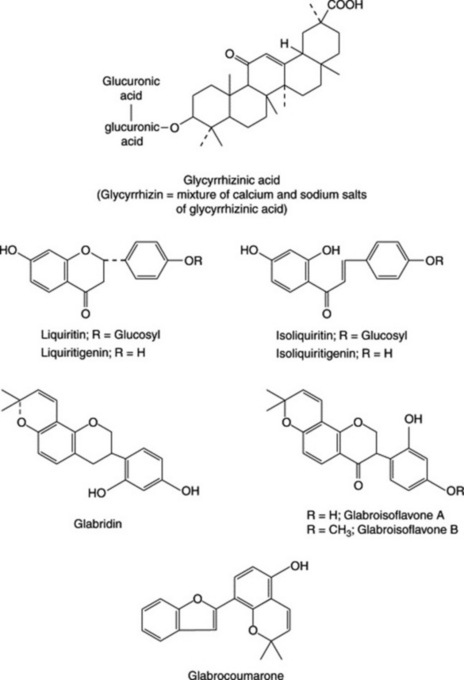
Plant materials often contain these saponins in considerable amounts. Thus, primula root contains about 5–10%; liquorice root about 2–12% of glycyrrhizic acid (and a correspondingly larger amount of glycyrrhizin, the potassium calcium salt); quillaia bark up to about 10% of the mixture known as ‘commercial saponin’; the seeds of the horse-chestnut up to 13% of aescin. As some plants contain more than one saponin and purification is often difficult, the structures of even some of the well-known saponins given in Table 23.5 have only recently been established. Oleanolic acid also occurs as a saponin in sugar beet, thyme, Guaiacum spp. (also in the nor-form), and in the free state in olive leaves and clove buds.
LIQUORICE ROOT
The pharmacopoeial drug is now defined as the dried unpeeled or peeled, whole or cut root and stolons of Glyrrhiza glabra L. and/or of G. inflata Bat. and/or G. uralensis Fisch. Varieties of G. glabra traditionally yielding the commercial drug are:
Much commercial extract is now obtained from the other species cited above.
History
Liquorice is referred to by Theophrastus. The Roman writers referred to it as Radix dulcis, but it does not appear to have been cultivated in Italy until about the thirteenth century. Its cultivation in England, now commercially ceased, has been traced back as far as the sixteenth century.
Cultivation and collection
In western Europe liquorice is cultivated, but the ‘Russian’ and ‘Persian’ drugs are obtained from wild plants. In China, large-scale cultivation is replacing collection from the wild. The plants usually grow well in deep, sandy but fertile soil, near streams. They are usually propagated by replanting young pieces of stolon but may be grown from seed. The underground organs are developed to a sufficient extent by the end of the third or fourth year, when they are dug up and washed. Some are peeled and cut up into short lengths before drying, but much is now used unpeeled. The drug is imported in bales. In southern Italy and the Levant a large proportion of the crop is made into stick or block liquorice. This is prepared by the process of decoction, the liquid being subsequently clarified and evaporated to the consistency of soft extract. The latter is made into blocks or sticks, stamped with the maker’s name (e.g. Solazzi), dried, and exported in cases, which often contain bay laurel leaves. Chinese blocks weighing 5 kg each are available.
Macroscopical characters
The Spanish and Italian drugs are derived from the variety typica. They are sold as ‘Spanish’ liquorice irrespective of their exact geographical source. Typical ‘Spanish’ liquorice occurs in straight pieces from 14 to 20 cm or more in length and from 5 to 20 mm in diameter. If unpeeled, they have a dark, reddish-brown cork, and the runners, which are more numerous than the roots, bear buds. The peeled drug has a yellow, fibrous exterior. Fracture, fibrous; odour, faint, but characteristic; taste, sweet and almost free from bitterness.
Unpeeled ‘Russian’ liquorice occurs in somewhat tapering pieces up to 30 cm long and 5 cm in diameter. It is of less regular appearance than the Spanish and consists of rootstock and roots. The surface is covered with a somewhat scaly, purplish cork. The pieces of rootstock often bear buds and have a pith, but the roots may be distinguished from the stolons of the Spanish drug by the absence of buds. Fracture, very fibrous, the strands of fibres tending to separate from one another. This variety is sometimes peeled. The taste is sweet but usually not entirely free from bitterness or acridity. ‘Persian’ liquorice from Iran closely resembles the Russian variety and is generally unpeeled. Anatolian or Turkish liquorice may be peeled or unpeeled and some pieces may have a diameter of up to 8 cm.
Much commercial liquorice root currently available in Britain is of Chinese origin. It is imported in bundles of stolons each bundle being about 15 cm long, 15 cm diameter, and bound with wire. The bundles are packed in plaited wood containers. Generally, the stolons have a smaller diameter than the European drug.
Microscopical characters
Both roots and runners show secondary thickening—the absence of a medulla in the root and its tetrarch structure (Fig. 41.8A–C) serving to distinguish the sections. The epidermis and most of the cortex are absent, being thrown off by the development of cork. The outer surface of the unpeeled drug is bounded by some 10 rows of narrow cork cells. Within the cork is a phelloderm or secondary cortex composed of parenchymatous cells, some of which may become collenchymatous. These cells contain simple starch grains about 10 μm diameter; a few contain prisms of calcium oxalate. The secondary phloem is composed of alternating zones of groups of fibres and sieve tissue. The phloem fibres are very thick-walled, are lignified and occur in cylindrical bundles surrounded by a sheath of parenchymatous cells each of which contains a single prism of calcium oxalate 10–35 μm in length. The sieve-tube tissue suffers partial obliteration but remains functional in the cambial region. The cambium is an incomplete line composed of about three layers of flattened cells. The secondary xylem is composed of large vessels, wood fibres and wood parenchyma. The vessels are 80–200 μm in diameter and show reticulate or pitted walls. The pits are slit-like bordered pits.
The vessels occur singly in small groups and alternate with bundles of wood fibres resembling the phloem fibres in form and in being enclosed in a sheath of parenchyma containing calcium oxalate. The parenchyma of the xylem has lignified pitted walls. The secondary tissues are divided by radial medullary rays 3–5 cells wide in the xylem and funnel-shaped in the phloem. These rays are up to 100 cells high (Fig. 23.11).
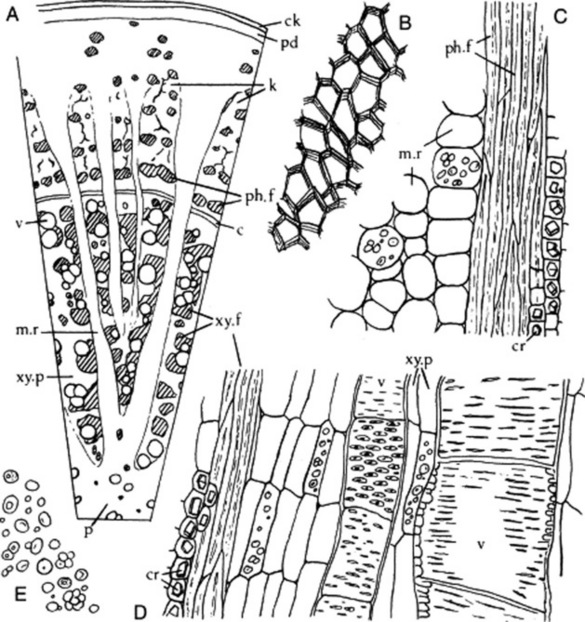
Fig. 23.11 Glycyrrhiza glabra. A, Transverse section of stolon (×25); B, fragment of cork layer from powder, in surface view; C, portion of longitudinal section through phloem; D, longitudinal section of wood; E, starch granules (all ×200). c, Cambial zone; ck, cork layer; cr, calcium oxalate crystals; k, non-functional sieve tissue (keratenchyma); m.r, medullary ray; p, pith; pd, phelloderm; ph.f, phloem fibres; v, vessel; xy. f, xylem fibres; xy. p, xylem parenchyma.
The characters of G. uralensis are more fully discussed in the next monograph.
Constituents
Since 1990 considerable research has been published on the constituents of liquorice mainly by Japanese workers in whose country the drug, imported from China, is an important traditional medicine. Unfortunately, the Chinese commercial drug, as investigated, may be derived from a number of species, e.g. Glycyrrhiza uralensis, G. inflata and G. glabra, so that it is not always possible to assign particular reported constituents to a specific source.
Liquorice owes most of its sweet taste to glycyrrhizin, the potassium and calcium salts of glycyrrhizinic acid. Glycyrrhizinic acid is the diglucopyranosiduronic acid of glycyrrhetic (glycyrrhetinic) acid, which has a triterpenoid structure (Fig. 23.12). Other hydroxy- and deoxy-triterpenoid acids related to glycyrrhetic acid have been isolated; the C-20 epimer of glycyrrhetic acid is named liquiritic acid.
The yellow colour of liquorice is due to flavonoids, which received further considerable study when in 1978 the antigastric effect of flavonoid-rich fractions was recognized. They include liquiritin, isoliquiritin (a chalcone, which occurs as a glycoside), liquiritigenin, isoliquiritigenin (chalcone form) and other compounds. Isoliquiritigenin is reported to be an aldose-reductase inhibitor and may be effective in preventing diabetic complications. Rhamnoliquiritin was isolated from the roots in 1968. Many flavonoids and isoprenoid-substituted flavonoids from G. glabra of various origins have since been reported. These include the pyranoisoflavan, glabridin (Fig. 23.12) and, more recently, two minor isoflavones, glabriso flavone A and B, and glabrocoumarone (Fig. 23.12) (T. Kinoshita et al., Chem. Pharm. Bull., 2005, 53, 847). Various 2-methylisoflavones have been isolated from indigenous Indian roots together with an unusual coumarin (liqcoumarin), 6-acetyl-5-hydroxy-4-methyl-coumarin. An examination of liquorice from five countries has shown the flavonoid content to be geographically consistent, varying only in the relative proportions of constituents. Japanese traditional (kampo) extracts prepared by boiling show a high content of flavonoid aglycones which may be pharmacologically more active than the parent glycosides.
Other active constituents of liquorice are polysaccharides with a pronounced activity on the reticuloendothelial system. Research on these, at first devoted to G. uralensis, has been extended to G. glabra var. glandulifera from which glycyrrhizan GA has been characterized as the representative polysaccharide with immunological activity (K. Takada et al., Chem. Pharm. Bull., 1992, 40, 2487). It has an estimated mass of 85 000 with a core structure which includes a backbone chain of β-1,3-linked D-galactose residues 60% of the units carrying side chains (composed of β-1,3- and β-1,6-linked D-galactosyl residues) at position 6.
The roots also contain about 5–15% of sugars (glucose, sucrose); about 1–2% of asparagine (amide of aspartic or aminosuccinic acid); 0.04–0.06% volatile compounds; β-sitosterol; starch; protein; bitter principles (glycyramarin). The latter are particularly abundant in the outer tissues and are therefore largely removed in the peeled variety of liquorice.
Published analytical figures for the amount of glycyrrhizin in liquorice vary considerably. These differences are due partly to different analytical methods and partly to actual variations in the percentages present in different commercial varieties and samples; 6–13% is the usual range. Glycyrrhizin is confined mainly to the roots but falls rapidly in concentration in organs above soil level. In a field study on G. glabra growing in Uzbekistan, H. Hayashi et al., (Chem. Pharm. Bull., 2003, 51, 1338) found that, based on the relative occurrence of rutin and isoquercetin, the populations could be divided into two distinct types.
Cell cultures
Suspension cell cultures of liquorice do not appear to produce either glycyrrhizin or glycyrrhetic acid but soyasaponins I and II, the amounts depending on culture strain and influence of plant hormones. The principal isoflavonoid produced is formononetin (C. Arias-Castro et al., Plant Cell Tissue and Org. Cult., 1993, 34, 63). Glycyrrhetic acid added to the cell culture undergoes a different mode of glycosylation to that normally occurring in the roots.
From hairy root cultures, initiated by Agrobacterium rhizogenes, two new prenylated flavonoids (licoagrochalcone A and licoagrocarpin) together with eight known flavonoids, have been isolated (Y. Asada et al., Phytochemistry, 1998, 47, 389).
Allied drug
Other species which have been investigated, some of which find domestic use, include G. aspera, G. echinata, G. hirsuta, G. inflata, G. macedonia, G. pallidiflora and G. yunnanensis. (For research papers on these species see K. Ohtani et al., Phytochemistry, 1994, 36, 139; T. Fukai et al., ibid., 1994, 36, 233.)
Standardization
The BP/EP requires a minimum content of 4.0% glycyrrhizic acid determined by liquid chromatography using monoammonium glycyrrhizate as a reference and absorption measurements at 254 nm. The total ash should not exceed 10.0% (unpeeled drug) or 6.0% (peeled drug) and the ash insoluble in hydrochloric acid similarly 2.0% (unpeeled drug), 0.5% (peeled drug).
Action and uses
Liquorice has long been employed in pharmacy as a flavouring agent, demulcent and mild expectorant. Gibson (Lloydia, 1978, 41, 348) in a summary of the uses of liquorice from 2100 BC, pointed out that many of the early claims for a broad spectrum of uses for this drug appear to be borne out by modern pharmacological research; a view that has been further substantiated during the last decade. The recognition of the deoxycorticosterone effects of liquorice extracts and glycyrrhetinic acid has led to its use for the treatment of rheumatoid arthritis, Addison’s disease and various inflammatory conditions. Interestingly, the flavonoid component of the root, which possesses antimicrobial properties, also exerts spasmolytic and antiulcerogenic activity. A Japanese patent (Chem. Abs., 1992, 117, 55948) describes the formulation of a liquiritin cream as beneficial, with no adverse effects, for the removal of skin stains in patients with chloasma, senile melanoderma, etc.
Unlike cortisone, liquorice may give symptomatic relief from peptic ulcer pain. It has been reported that glycyrrhizin gel can act as a useful vehicle for various drugs used topically; not only are the anti-inflammatory and antiviral effects relevant but also glycyrrhizin enhances skin penetration by the drug. Excessive consumption of liquorice leads to hypertension and hypokalaemic alkosis; the hospitalization of an individual taking 200 g liquorice daily was reported in 1998. Most of the liquorice imported is used in the tobacco trade and in confectionery.
LIQUORICE ROOT FOR USE IN TRADITIONAL CHINESE MEDICINE
The BP 2007 included the above as a monograph involving the dried unpeeled roots and rhizomes of Glycyrrhiza uralensis Fisch., G. inflata Bat. or G. glabra L.
Unlike Liquorice Root BP/EP, the commercial drug consists of the roots and rhizomes sliced transversely or longitudinally giving irregularly circular to ovoid pieces up to about 3 mm thick. The outer surface is dark reddish-brown and longitudinally wrinkled. The general microscopical features of the powder resemble those for G. glabra.
G. uralensis, Manchurian liquorice, as present in a commercial sample bears a chocolate-brown, exfoliating cork and differs from G. glabra in internal structure, the medullary rays being curved and lacunae being present in the wood. It appears to contain about as much glycyrrhizin as the other varieties, together with a number of new oleane-type triterpene oligosaccharides called licorice saponins (I. Kitagawa et al., Chem. Pharm. Bull., 1993, 41, 1567). Only traces of sugars are present and it gives an unpleasantly pungent extract. As with G. glabra, the yellow colouring matter contains the flavonoid glycoside liquiritin, a glycoside involving liquiritigenin, apiose and glucose, and a new chalcone oligoglycoside isoliquiritin apioside. Like G. glabra, G. uralensis contains polysaccharides showing immunological activities; glycyrrhizan UA is composed of L-arabinose, D-galactose, L-rhamnose and D-galacturonic acid.
As the supply of wild plants of G. uralensis is practically exhausted there is now large scale cultivation of the drug in the Inner Mongolian area of China. A new biflavonoid named lichobichalcone and twelve known flavonoids from the cultivated roots have been reported (H. Bal et al., Chem. Pharm. Bull., 2003, 51, 1095).
Actions
In common with G. glabra, many pharmacological activities have been cited for G. uralensis; these include: antihepatotoxic, antimutagenic, antimicrobial, antitumour and antiulcer. Glycyrrhisoflavone and glyasperin C have been reported as tyrosinase inhibitors (H. J. Kim et al., Planta Medica, 2005, 71, 785).
PRIMULA ROOT
Primula root BP/EP consists of the dried rhizome and root of Primula veris (L.) (cowslip) or P. elatior Hill. (oxlip), Primulaceae. These species occur wild throughout Europe with Bulgaria and Turkey the principal commercial sources. British herbal medicine has however traditionally used the leaves, flowers and roots of P. vulgaris (the common primrose).
The greyish-brown drug may be whole or cut with pieces of rhizome up to 5 cm in length and bearing the remains of stems and leaves together with numerous roots. Microscopical features include parenchymatous cells, reticulately thickened vessels and simple and compound starch granules. P. elatior is differentiated by the possession of groups of pitted sclereids.
Constituents include a mixture of triterpenoid saponins of the oleane type (5–10%) and phenolic glycosides such as primulaverin (primulaveroside). The latter, by enzyme hydrolysis during the drying process, forms the disaccharide primeverose and methyl 5-methoxysalicylate, the latter being responsible for the odour of the drug.
The pharmacopoeia includes a chromatographic test to detect Vincetoxicum hirundinaria, Aesclepiadaceae, a poisonous plant with similar-looking roots to those of Primula spp. The substitute also differs microscopically in its vascular structure and possesses numerous calcium oxalate crystals.
Primula root, like senega, is used as an expectorant for the treatment of bronchial conditions.
QUILLAIA BARK
Quillaia bark (Soap Bark, Panama Wood, Quillaia) is the dried inner bark of Quillaja saponaria Molina and of other species of Quillaja (Rosaceae). Quillaja saponaria is a tree about 18 m high found in Chile, Peru and Bolivia. It has been introduced into India and California. The generic name is derived from the Chilean word quillean, to wash, from the use made of the bark.
Macroscopical characters
Quillaia bark occurs in flat strips about 1 m long, 20 cm broad and 3–10 mm thick. It consists almost entirely of phloem, the outer region in which successive cork cambia develop having been more or less completely removed. A few, reddish- or blackish-brown patches of rhytidome adhere to the outer surface, which is otherwise of a brownish-white colour and reticulated. The rhytidome consists of dead portions of secondary phloem enclosed by secondary cork layers. The inner surface is yellowish-white and fairly smooth. The bark breaks with a splintery fracture and is inclined to laminate (between the zones of hard and soft phloem). Large crystals of calcium oxalate may be seen with the naked eye. The powdered drug is very sternutatory and produces an abundant froth when shaken with water. Taste, acrid and astringent.
Microscopical characters
A transverse section of quillaia bark has a chequered appearance which is caused by the crossing of the medullary rays by alternating bands of lignified and non-lignified phloem. The medullary rays are usually 2–4 cells wide. The phloem fibres are tortuous and often accompanied by small groups of rectangular sclereids. The parenchyma contains numerous starch grains up to 20 μm diameter and single prisms of calcium oxalate up to 20 μm long.
Constituents
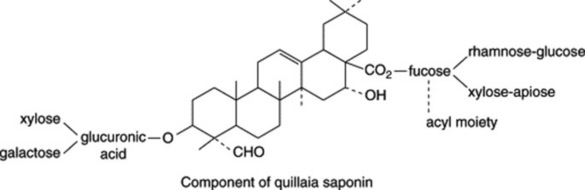
The bark contains a mixture of saponins which on hydrolysis yield the principal sapogenin quillaic acid (hydroxygypsogenin) and gypsogenin (Fig. 23.10) together with sugars, uronic acids and acyl moieties. It is little wonder that the constitution of quillaia saponin proved difficult to unravel by classical procedures as it has now been shown to contain at least 60 different saponins (D. C. van Setten et al., Anal. Chem., 1998, 70, 4401). Di- and tri-saccharides are attached at C-3 and various complex oligosaccharides at C-28, the fucosyl moiety of which may be substituted with C9-aliphatic acids or an O-acetyl group (S. Guo et al., Phytochemistry, 2000, 53, 861; 54, 615). A typical structure is shown below.
Quillaia contains about 10% of saponins, BP ethanol (45%)-soluble extractive not less than 22.0%, and also sugars, starch and calcium oxalate.
SENEGA ROOT
Senega of the BP and EP consists of the dried root crown and root of Polygala senega L. (Polygalaceae) or of closely related species of Polygala or a mixture of these. A variety found in N. America and now cultivated in Japan is P. senega var. latifolia Torr. et Gray, a robust perennial herb some 20–30 cm tall. Formerly abundant in eastern Canada and eastern USA it is now collected further westward.
History
Senega was used by the North American Indians as a snake-bite remedy. It was employed by Tennent in 1734 for pleurisy and pneumonia, and its value was made known in London in 1738.
Macroscopical characters
Senega occurs in pieces 5–10 cm long and 2–12 mm diameter. The lower part is yellowish but the crown is somewhat darker. The latter is knotty and bears numerous, often purplish buds and the remains of aerial stems, which should not exceed about 2%. The tapering and often curved root frequently divides into two or more branches. Some, but by no means all, of the pieces bear a keel or ridge in the form of a rapidly descending spiral. The drug frequently has a marked odour of methyl salicylate. Taste, at first sweet, afterwards acrid. The saponins present cause the drug to have sternutatory properties and to froth when shaken with water.
Transverse sections of different senega roots, or the same root cut at different levels, have widely different appearances. Some have a normal bark, which occupies nearly half the radius, and a uniform central wood with narrow medullary rays. In others, however, an abnormal local development of phloem gives rise to the keel, while one or more exceptionally wide, V-shaped, medullary rays give a very characteristic appearance to the wood. This is well seen in sections stained with phloroglucinol and hydrochloric acid.
For illustrations, see the 14th edition of this book, p. 309.
Constituents
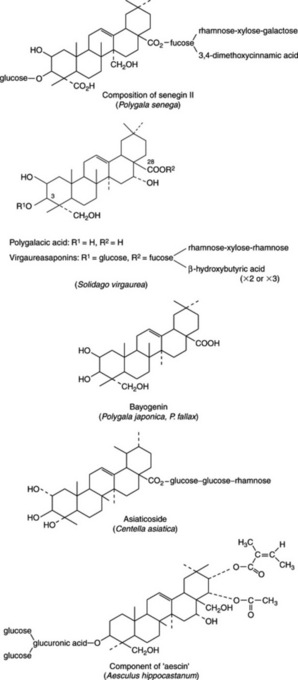
Senega contains 6–12% of triterpenoid saponins. Earlier work based on the hydrolysis of the crude saponin mixture (senegin) produced a variety of products but during the 1970s Japanese workers characterized a number of individual saponins based on the aglycone presenegenin with glucose at C-3 and a number of sugars and a cinnamic acid derivative forming a branched chain at C-28; the units comprising the principal glycoside seneginin II are shown in Fig. 23.13. It is interesting to note the involvement of fucose, a deoxy sugar also found in certain cardioactive glycosides (Fig. 23.15) and in the saikosaponins (q.v.).
A number of oligosaccharide multi-esters have recently been identified in the roots (see H. Saitoh et al., Chem. Pharm. Bull., 1993, 41, 1127, 2125) and named senegoses A–I. They appear to be di-and tetra-saccharides involving glucose and fructose esterified with acetic, benzoic, and trans- and cis-ferulic acids. A further series, senegoses J–O, are pentasaccharides (Idem., ibid., 1994, 42, 641). These compounds are structurally similar to the tenuifolioses previously isolated by the same group of workers from P. tenuifolia (vide infra).
Allied species and substitutes
P. tenuifolia is used in China and Japan as an expectorant, tonic and sedative. It contains constituents similar to those of P. senega, including saponins, xanthones, phenolic glycosides (tenuifolisides A–D) and oligosaccharides (tenuifolioses A–F), see Y. Ikeya et al., Chem. Pharm. Bull., 1994, 42, 2305). The bark, also used medicinally in China, Korea and Japan, has sedative, expectorant and anti-inflammatory properties. A number new phenones, tenuiphenones A–D, have recently been reported (Y. Jiang and P. Tu, Chem. Pharm. Bull., 2005, 53, 1164).
Many new triterpene saponins of P. japonica and P. fallax named polygalasaponins have been reported. The aglycone of a number of these saponins is bayogenin (Fig. 23.13). For recent new isolations, see H. Wang et al., Chem. Pharm. Bull., 2006, 54, 1739; W. D. Zhang et al., Fitoterapia, 2006, 77, 336.
Southern or White senega is collected in the southern USA from P. alba and P. boykini. The roots are smaller than those of P. senega and have a normal wood.
EUROPEAN GOLDENROD
It is necessary to distinguish between two commercial sources of goldenrod used in medicine: one is native to Europe and Asia and the other involves two species of Solidago native to N. America, which are now naturalized and cultivated in Europe. Although generally similar in morphological form and constituents, there is variation between the two sources and the BP/EP includes them in separate monographs.
European Goldenrod BP/EP, Goldenrod BHP 1996 consists of the whole or cut, dried flowering aerial parts of Solidago virgaurea L. It is common in Britain, preferring dry woods and grassland, rocky areas, dunes, etc. The species is polymorphic with many named varieties.
A perennial herb, it is up to 1 m in height with stems, often reddish-brown at the base, somewhat branched and leafy. The lower leaves are up to 10 cm in length, obovate to lanceolate, toothed and narrowing at the base to a winged petiole. The stem leaves are alternate becoming progressively smaller, entire, inconspicuously toothed, sessile to amplexicaul, glabrous or slightly pubescent. The short-stalked yellow flowerheads are arranged around the stem in a terminal raceme or panicle. The involucre of greenish-yellow imbricate bracts is arranged in two to four rows; each capitulum possesses six to twelve ray florets and many tubular florets, both yellow. The fruits are brown, ribbed achenes 2–4 mm in length.
Features observable in the powdered drug include epidermal leaf fragments with striated cuticle and anomocytic stomata, various uniseriate clothing trichomes with some having an extended pennant-like terminal cell, glandular trichomes, cluster crystals of calcium oxalate, pappus hairs; compositae pollen grains.
Constituents
The principal constituents of European goldenrod are oleane-type saponins based on polygalacic acid-3-glucoside (Fig. 23.13). Four such compounds, subsequently designated virgaurea-saponins B, C, D and E were 3, 28-bisdesmosidic glycosides involving glucose, rhamnose, xylose and fucose with acylation of the fucose with a chain of two or three β-hydroxybutyric acid moieties (G. Bader et al., Planta Med., 1995, 61, 158); see Fig. 23.13 and cf Senega. Compounds B and C were identical with solidagosaponins XIV and XVIII belonging to a series of saponins (solidagosaponins I–XXIX) isolated from fresh plants of the Asian variety of S. virgaurea (Y. Inose et al., Chem. Pharm. Bull., 1991, 39, 2037; 1992, 40, 946; T. Miyase et al., Chem. Pharm. Bull., 1994, 42, 617).
A number of flavonoids have been identified in the drug including chlorogenic acid and rutin, which are characterized in the pharmacopoeial TLC test; no quercitrin is evident, distinguishing this species from S. gigantea and S. canadensis. Other flavonoids include kaempferol, hyperoside and isoquercitrin. The presence of a diglucoside, leiocarposide (0.4–0.8%) is a further distinction from the above two species. Caffeic acid derivatives and phenolic acids in small amount are also present.
GOLDENROD
Goldenrod BP/EP consists of the whole or cut, dried, flowering parts of Solidago gigantea Ait or S. canadensis L., their varieties or hybrids and/or mixtures of these. Family Compositae.
These species are two of some 100 spp. of Solidago native to America and were introduced into Europe as ornamentals, being much larger and showier than the native S. virgaurea; as garden escapes they have become naturalized along streams, rivers, lakes and waste places.
S. canadensis (S. altissima), tall goldenrod, is a native of eastern N. America. It is a vigorous erect plant 1–2.5 m with a spread of about 1 m. The stems are pubescent throughout with lanceolate, sharply pointed, three-veined leaves, roughish above, pubescent below. Broadly plumose heads of yellow flowers form a large pyrimidal panicle from August to November making for an attractive autumn border plant.
S. gigantea (S. serotina) resembles the above but below 1 m the stems are glabrous and the flowers are larger in compact erect corymbose panicles.
The pharmacopoeial description relates to both species and their hybrids. In the powder the absence of multicellular trichomes with a bent-over terminal cell should be noted, cf. European goldenrod.
Constituents
As with European goldenrod, bidesmosidic saponins are present in both S. gigantea and S. canadensis. Bayogenin (Fig. 23.13) is the aglycone common to both. The canadensis and gigantia saponins differ from one another in the length and structure of the saccharide chains, particularly in those units bonded to the C-3 position of bayogenin. Unlike S. virgaurea, there is no acylation of the sugar chains. Eight such saponins were isolated from S. canadensis and four from S. gigantea (M. Sovova et al., Ceska Slov. Farm., 1999, 48, 113; Chem. Abs., 1999, 131, 240355 m).
Other constituents of S. canadensis include sesquiterpenes, di- and tri-terpenes and a very low content (1 mg/kg) of a new phenolic diglucoside related to that found in S. virgaurea (J. S. Zhang et al., Fitoterapia, 2007, 78, 69). Two new quercetin and kaempferol glycosides have recently been reported (B. Wu et al., Chem. Pharm. Bull., 2007, 55, 815).
In common with European goldenrod the drug is assayed for its flavonoid content, a minimum of 2.5% flavonoids, expressed as hyperoside (dried drug), being required by the BP/EP. This high value may reflect the large proportion of flowers in collections of these plants. Quercitrin, chlorogenic acid and rutin are detected in the official TLC test (quercitrin is absent in the same test involving S. virgaurea).
Saikosaponins
This group of oleanene saponins occurs in the roots of Bupleurum falcatum (Umbelliferae), a drug long-used in Chinese medicine for the treatment of hepato-biliary disorders and known to possess anti-inflammatory properties. For a fuller description and formulae see Chapter 29.
Horse chestnut seed
Horse chestnut seeds and the tree Aesculus hippocastanum (Hippocastanaceae) need no description; native to western Asia the species is now widely distributed over the world as an ornamental.
Medicinally the seeds have long been used for their saponin content, the principal component being aescin (in recent publications termed ‘escin’) which occurs in concentrations of up to 20% in the dried seeds. As with a number of these well-known triterpenoid saponins it is only recently that it has been possible to elucidate completely their chemical structures and, as with other crude saponins, aescin itself has been shown to be a mixture of many closely related compounds. Acid hydrolysis of the aescin complex gives the saponin aescigenin and the sugars glucose, xylose, galactose and glucuronic acid together with esterifying acetic, butyric, isobutyric, angelic and tiglic acids. From 1996–1998 M. Yoshikawa et al. described nine new such acylated polyhydroxyoleane triterpene oligoglycosides (escins Ia, Ib, IIa, etc.) together with three isoescins (Chem. Pharm. Bull., 1998, 46, 1764). As an example of one such structure see Fig. 23.13.
The seeds also contain flavones (quercitin, kaempferol and their glycosyl derivatives, Fig. 23.13), coumarins and tannins.
Extracts of horse chestnut have been traditionally employed both in the West and East for the treatment of peripheral vascular disorders including haemorrhoids, varicose veins, leg ulcers and bruises. OTC products are now available, their efficacy supported by a number of scientific reports. Thus some of the escins are anti-inflammatory, inhibiting the activity of lysosomal enzymes that damage capillary walls; coumarins cause a thinning of the blood, so much so that horse chestnut is contraindicated with anticoagulants such as warfarin; tannins tone the blood vessel walls and flavonoids are anti-inflammatory.
CENTELLA
Centella (Indian pennywort, gotu kola, Indian water navelwort, tiger grass) consists of the dried fragmented aerial parts of Centella asiatica (L.) Urban, family Umbelliferae syn. Hydrocotyle asiatica. It is typically found in moist situations throughout the pantropics including Pakistan, India, S.E. Asia and Africa. It has important traditional uses in India and Africa for the treatment of leprosy, and in the former for meditation purposes under the name brahmi.
The plant resembles the European marsh pennywort as a creeping perennial with kidney-shaped leaves, grouped at the stem nodes, up to 5 cm broad with small pink flowers giving fruits in umbels of two to four bicarpellate schizocarps.
The drug consists of a grey–green, compressed mass involving stems, leaves, flowers and fruits. Microscopical examination shows polygonal leaf epidermal cells with a striated cuticle, paracytic stomata and long, often twisted unicellular trichomes on the petiole epidermis. Also prisms of calcium oxalate crystals, bundles of septate fibres and fragments of the fruit with a parquetry arrangement of some cells.
Constituents
As an important Ayurvedic drug, Centella has been investigated over a long period of time and numerous constituents have been recorded. Among the most active are triterpenoid saponins, principally asiaticoside (Fig. 23.13), together with brahmoside, brahminoside, centelloside and medecasside, based on corresponding terpinic acids that also occur in the free state.
A minimal content of volatile oil consists principally of sesquiterpenes. O. A. Oyedej and A. J. Afolayan (Pharm. Bull., 2005, 43, 249), in a comparison of the Japanese oil with that from plants grown in S. Africa, have identified by GC-MS some 40 constituents, the chief of which are α-humulene (21.06%), β-carophyllene, myrcene, germacrene B and bicyclogermacrene. Samples varied in composition and showed broad-spectrum antibacterial activity.
Among other constituents of centella are flavonoids (quercetin, kaempferol, etc.) phytosterols, amino acids and a bitter principle, vallerin.
Uses
The BHP (1983) lists centella as a mild diuretic, anti-rheumatic, dermatological agent and peripheral vasodilator; topically as vulnerary. As such, it is used for rheumatic conditions and as a skin tonic in wound healing. Employed for indolent wounds it is also included in medicinal creams and some cosmetic preparations. These uses have largely been supported by pharmacological data.
IVY
Ivy does not feature strongly in British herbal medicine but, with a considerable number of other European drugs, it is now included in the BP as a result of its EP status. The drug consists of the whole or cut aerial leaves of Hedera helix L. family Araliaceae collected in the spring. This familiar climber and creeper is widely distributed throughout Europe and Asia. Non-flowering shoots produce alternate palmate three- to five-lobed leaves with conspicuous pale veins; leaves of the flowering shoots are often larger, ovate or rhombic and entire. Microscopical features include: epidermal cells with wavy anticlinal walls, occasionally anisocytic but mainly anomocytic stomata on the lower epidermis, mucilage cells of the mesophyll and cluster crystals of calcium oxalate about 40 μm in diameter.
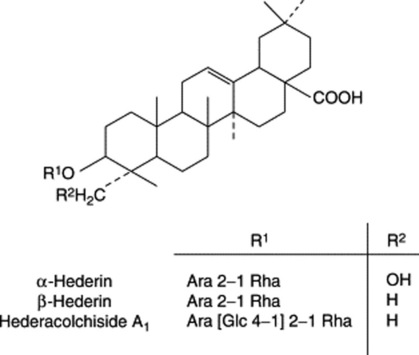
Important constituents of ivy are saponins involving the pentacyclic triterpenoid genins hederagenin, bayogenin and oleanolic acid. Examples are given in Fig. 23.14; see also F. Delmas et al., Planta Medica, 2000, 66, 343. Other constituents are flavonoids (rutin, quercetin), caffeic acid derivatives (chlorogenic, rosmarinic acid, etc.), sterols, polyacetylenes and volatile oil.
The BP/EP TLC test for identity indicates the presence of α-hederin and heteracoside C; a minimum concentration of 3.0% heteracoside C determined using liquid chromatography is specified.
Ivy-leaf extracts have been traditionally used as an expectorant for the treatment of various chest conditions, such as bronchitis and whooping cough; also for gout and rheumatic pains. Like most saponins, those of ivy are toxic in excess causing diarrhoea, vomiting and allergy. Externally, ivy is used cosmetically and for a variety of skin conditions. Molluscidal, antibacterial and antileishmanial properties have been reported for the saponins.
CARDIOACTIVE DRUGS
A considerable number of plants scattered throughout the plant kingdom contain C23 or C24 steroidal glycosides which exert on the failing heart a slowing and strengthening effect. In Western medicine it is the glycosides of various Digitalis species that are extensively employed. The introduction of the foxglove (D. purpurea) into British medicine for treatment of dropsy by the Birmingham physician and botanist William Withering, in 1785, constitutes one of the fascinating stories of medicine. It was not realized at that time, however, that dropsy could be the result of a heart condition. Before the introduction of digitalis it was treated by the oral administration of dried and powdered toad-skins; later investigations were to show that this treatment too, did not lack a pharmacological basis. The action of the digitalis glycosides on the heart is discussed in Chapter 6.
The heart-arresting properties of these glycosides also render them most effective as arrow poisons and a number of tropical plants are better-known in this respect than for their medicinal use.
Distribution in nature
In plants cardiac glycosides appear to be confined to the Angiosperms. Cardenolides (see below) are the more common and are particularly abundant in the Apocynaceae and Asclepiadaceae, but are also found in some Liliaceae (e.g. Convallaria), and in the Ranunculaceae, Moraceae, Cruciferae, Sterculiaceae, Euphorbiaceae, Tiliaceae, Celastraceae, Leguminosae and Scrophulariaceae. The bufanolides occur in some Liliaceae (e.g. Urginea) and in some Ranunculaceae (e.g. Helleborus). In toad venoms the genins are partly free and partly conjugated with suberylarginine.
Some of the main genera containing cardiac glycosides are as follows: Apocynaceae: Adenium, Acokanthera, Strophanthus, Apocynum, Cerbera, Tanghinia, Thevetia, Nerium, Carissa and Urechites; Asclepiadaceae: Gomphocarpus, Calotropis, Pachycarpus, Asclepias, Xysmalobium, Cryptostegia, Menabea and Periploca; Liliaceae: Urginea, Bowiea, Convallaria, Ornithogalum and Rohdea; Ranunculaceae: Adonis and Helleborus; Moraceae: Antiaris, Antiaropsis, Naucleopsis, Maquira and Castilla; Cruciferae: Erysimum and Cheiranthus; Sterculiaceae: Mansonia; Tiliaceae; seeds of Corchorus spp.; Celastraceae: Euonymus and Lophopetalum; Leguminosae: Coronilla; and Scrophulariaceae. In the latter family cardiac glycosides have been found only in the genus Digitalis if we include in this the plant which some botanists call Digitalis canareniensis and others place in the genus Isoplexis.
Structure of glycosides
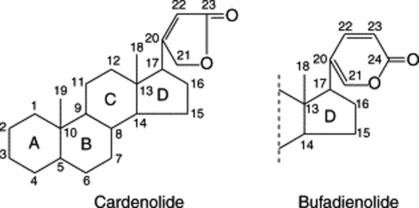
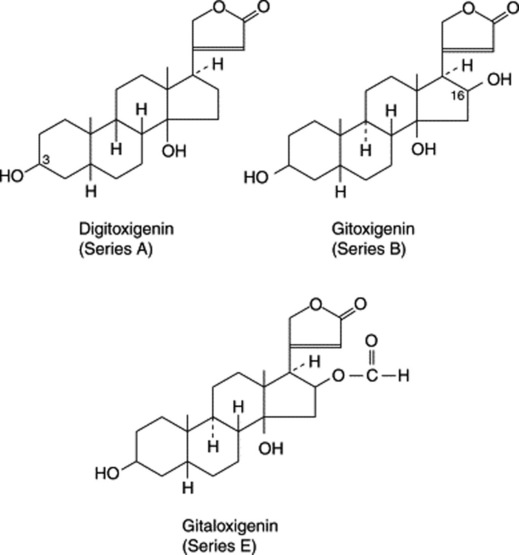
Two types of genin may be distinguished according to whether there is a five-or six-membered lactone ring. These types are known respectively as cardenolides (e.g. digitoxigenin) and bufanolides or bufadienolides (e.g. scillarenin). The following formulae indicate their structure and ring numbering:
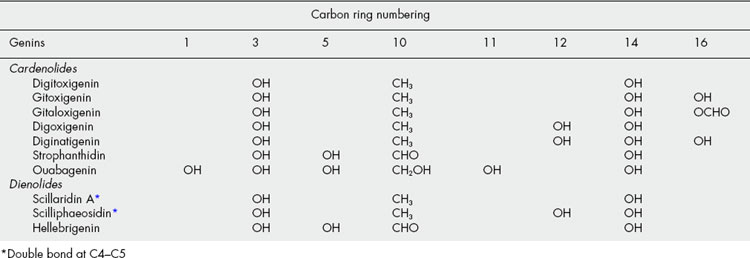
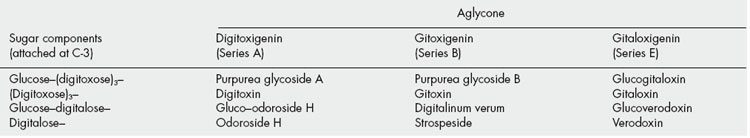
Substitution patterns for some typical genins are given in Table 23.6.
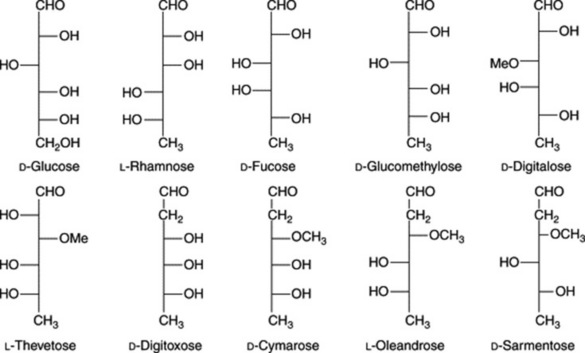
The sugar moieties, attached to the aglycone by a C-3,β-linkage, are composed of up to four sugar units which may include glucose or rhamnose together with other deoxy-sugars whose natural occurrence is, to date, known only in association with cardiac glycosides. A number of the deoxy-sugars are 2,6-dideoxyhexoses (e.g. digitoxose) or their 3-O-methyl ethers (e.g. cymarose). In addition to rhamnose and fucose, a number of other 6-deoxyhexose derivatives have more recently been discovered together with 2-O-methyl and 2-O-acetyl sugars. In the case of fucose, the D-form is known only in cardiac glycosides, whereas the L-form is widely distributed in nature. Cardiac glycosides involving cyclic sugars are known in Calotropis spp. and probably occur in other members of the Asclepiadaceae.
A characteristic arrangement of the carbohydrate side-chain at C-3 is: aglycone-(characteristic cardiacglycoside sugars, or rhamnose)x-(glucose)y; X and Y may = 0 and there are some modifications of this general pattern. Some examples of the sugars found in these glycosides are given in Fig. 23.15.
Biogenesis of cardiac glycosides
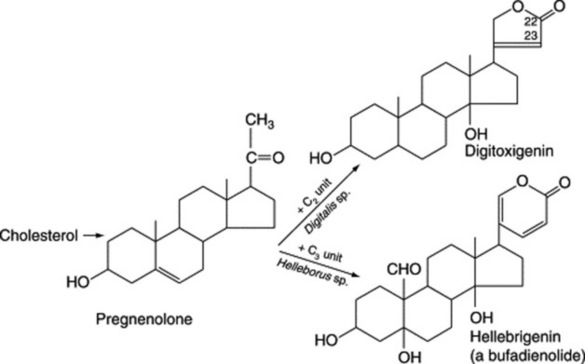
Aglycones of the cardiac glycosides are derived from mevalonic acid but the final molecules arise from a condensation of a C21 steroid with a C2 unit (the source of C-22 and C-23). Bufadienolides are condensation products of a C21 steroid and a C3 unit (Fig. 23.16).
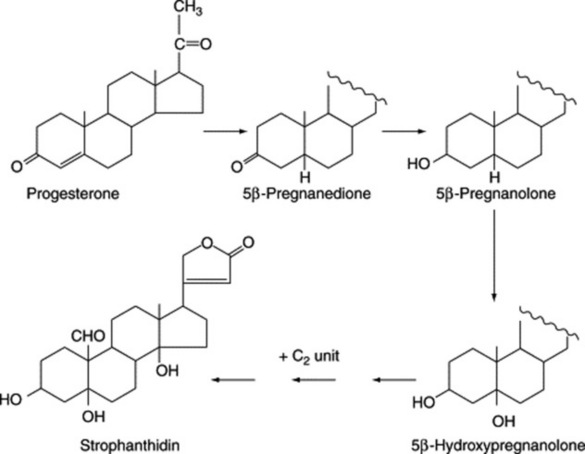
Progesterone, which is formed with cardiac glycosides, in Digitalis lanata as a result of feeding pregnenolone, is itself a precursor of the cardiac glycosides. Work, involving Strophanthus kombé, on the intermediates between progesterone and the cardenolides affords evidence consistent with the pathway: progesterone → 5β-pregnanolone → 5β-hydroxypregnanolone → cardenolides (Fig. 23.17). Similar transformations have also been demonstrated in Digitalis purpurea cultures but alternative pathways exist depending on whether hydroxylation of the nucleus occurs before or after the essential acetate condensation for the butenolide ring formation. Indeed, work involving some nine enzymes has helped to demonstrate that cardenolide genins arise as a result of a complex interlinking multi-dimensional system of pathways rather than by a single route leading directly to the end product.
As is evident from Fig. 23.17, a key enzyme in the biosynthesis of cardiac glycosides is progesterone 5β-reductase. Such an enzyme is also involved in the production of animal steroids so it is of interest to note that I. Gavidia et al. (Phytochemistry, 2007, 68, 853) have shown that with Digitalis purpurea this enzyme is not homologous with the corresponding enzyme in animals, suggesting that the steroid pathway evolved independently in plants and animals. Apparently, a similar situation occurs with 3β-hydroxy-Δ5-steroid dehydrogenase, an enzyme preceding the above in the cardenolide pathway.
Biogenetic studies involving the side-chain indicate that glucose is the most effective precursor of digitoxose and of the sugar side-chain of the Nerium oleander glycosides. Some ten enzymes have now been shown to be involved in the sugar side-chain biosynthesis in Digitalis species.
Tests and assays
The tests and assays available for cardioactive medicinals depend on biological activity, reactions of the sugar side-chain of the glycosides, and properties of the butenolide side-chain. Traditionally, the BP employed a biological assay for Digitalis (and formerly Strophanthus) on the basis that, compared with chemical and physical assays, it offered the best indication of the combined activity of the complex mixture of glycosides present. Now no biological assays are included for cardioactive drugs.
For digitalis leaves, the EP and BP utilize the red-violet colour (λmax 540 nm) produced by the interaction of cardenolides and 3,5-dinitrobenzoic acid. Other colour reactions based on the butenolide moiety are the red-orange (λmax 495 nm) given with an alkaline sodium picrate reagent (EP assay for digitoxin and digoxin), the red colour with xanthydrol reagent (EP test for digitalis leaf) and the red colour (λmax about 470 nm) produced with sodium dinitroprusside. In the ultraviolet region the butenolide side-chain exhibits λmax 217 nm and with purified substances, for example the eluted glycoside zones produced by TLC which contain little extraneous material, this can be used for rapid evaluation. These spectroscopic tests do not, in themselves, distinguish between glycosides and their corresponding aglycones.
A colour test specific for the digitoxose moiety is the Keller–Kiliani test; for details see ‘Digitalis Leaf’. The test is employed by the EP for the identification of digitoxin and digoxin and by the BP as an assay for digoxin injection and tablets (λmax 590 nm).
The separation and determination of individual glycosides by TLC, electrophoresis and GLC (derivatized glycosides) has received much attention over the years.
CARDENOLIDES
Of the cardioactive glycosides, those of the cardenolide group (Table 23.6) are the most important medicinally. Synthesis of these compounds has presented obvious difficulties but in recent years a large number of synthetic monosides have been prepared; one of these, a mannoside of strophanthidin, has proved extremely potent. All the medicinal preparations are derived from natural sources.
DIGITALIS LEAF
Digitalis (Purple Foxglove Leaves) consists of the dried leaves of Digitalis purpurea L. (Scrophulariaceae). It is required to contain not less than 0.3% of total cardenolides calculated as digitoxin.
History
Foxglove leaves appear to have been used externally by the Welsh ‘Physicians of Myddfai’ but the plant had no name in Greek or Latin until named digitalis by Fuchs (1542). The poisonous nature of the leaves was well known, and the drug was recommended by Parkinson in 1640; it was introduced into the London Pharmacopoeia of 1650. William Withering published his work on the clinical use of foxglove in 1785. For further reading, of largely historical interest, see J. K. Aronson (1985), An Account of the Foxglove and its Medical Uses 1785–1985, Oxford, Oxford University Press. Groves and Bisset have reviewed the topical use of Digitalis prior to William Withering pointing out the evidence for effective transdermal passage of the glycosides (J. Ethnopharmacol., 1991, 35, 99).
Plant
The foxglove is a biennial or perennial herb which is very common in the UK and most of Europe, including some Mediterranean regions of Italy, and is naturalized in North America. It is produced commercially in Holland and Eastern Europe. In the first year the plant forms a rosette of leaves and in the second year an aerial stem about 1–1.5 m in height. The inflorescence is a raceme of bell-shaped flowers of the floral formula K(5), C(5), A4 didynamous, G(2). The common wild form of the plant has a purple corolla about 4 cm long, the ventral side of which is whitish but bears deep purple eyespots on its inner surface. Many horticultural varieties exist, but these are of low therapeutic potency. The fruit is a bilocular capsule which contains numerous seeds attached to axile placentae.
Digitalis grows readily from seed. In the wild state it is usually found in semi-shady positions. It grows well in sandy soil, provided that a certain amount of manganese is present, this element being apparently essential and is always to be found in the ash.
Collection
Either first- or second-year leaves are permitted by the pharmacopoeias.
There has been a long-standing general belief that the pharmacological activity of leaves increases during the course of a day to reach a maximum in the early afternoon. Biological assays have given some support to this supposition and variations involving individual glycosides have also been reported.
After collection the leaves should be dried as rapidly as possible at a temperature of about 60 °C and subsequently stored in airtight containers protected from light. Their moisture content should not be more than about 6%.
Macroscopical characters
Digitalis leaves (Fig. 23.18) are usually ovate-lanceolate to broadly ovate in shape, petiolate and about 10–30 cm long and 4–10 cm wide. The dried leaves are of a dark greyish-green colour. The lamina is decurrent at the base; apex subacute. The margin is crenate or dentate and most of the teeth show a large water pore. Both surfaces are hairy, particularly the lower, and a fringe of fine hairs is found on the margin. The veins are depressed on the upper surface but very prominent on the lower. The main veins leave the midrib at an acute angle, afterwards branching and anastomosing repeatedly. The drug has no marked odour, but a distinctly bitter taste.
Fig. 23.18 Digitalis purpurea leaf. A, First-year leaf (×0.25); B, transverse section midrib of first-year leaf (×15); C, upper epidermis; D, lower epidermis; E, trichomes (all ×200); F–H, scanning electron micrographs: F, lower surface or leaf and G, H ditto showing glandular trichomes. a.v, Anastomosing veins; c, collenchyma, cic, cicatrix; d.b, decurrent base; d.m, dentate margin; e, endodermis; ep, epidermis; g.t, glandular trichome; g.t1, ditto surface view; m, mesophyll; p, palisade; ph, phloem; s.m, serrate margin; st, stoma; t, trichome base; xy, xylem.
(Photos: Lorraine Seed and R. Worsley.)
Microscopical characters
A transverse section of a foxglove leaf shows a typical bifacial structure and a midrib strongly convex on the lower surface (Fig. 23.18). Stomata and hairs are present on both surfaces, but are more numerous on the lower one. Calcium oxalate is absent. The palisade tissue is interrupted at the midrib. A zone of collenchyma underlies both epidermi in the midrib region. The crescent-shaped midrib bundle is enclosed in an endodermis one or two cells thick developed as a starch sheath. The pericycle is parenchymatous above and collenchymatous below. Sclerenchymatous fibres are absent.
Surface preparations (Fig. 23.18C, D) show that the upper epidermis consists of polygonal, relatively straight-walled cells, and bears both clothing and glandular hairs. The cells of the lower epidermis are wavy, and the stomata and hairs much more numerous than on the upper surface of the leaf. The stomata are small and slightly raised above the surrounding cells. The clothing hairs are uniseriate, two- to seven-celled, bluntly pointed, smooth or finely warty, with cells often collapsed alternatively at right angles (Fig. 23.18). The glandular hairs have a unicellular or occasionally a short uniseriate pedicel, with a unicellular or bicellular terminal gland (Fig. 23.18E). The cuticle of the hairs and epidermal cells may be stained red with a solution of Sudan Red in glycerin.
Prepared digitalis
This was a standardized powder of the BP (1989); it was adjusted to strength with weaker powdered digitalis or with powdered grass.
Constituents
The chemistry of digitalis has engaged the attention of many workers since about 1820. Important progress was made by Nativelle (1868), Kiliani (1891), Stoll (1938) and Haack et al. (1956).
The primary (tetra) glycosides (purpurea glycoside A, purpurea glycoside B and glucogitaloxin) all possess at C-3 of the genin a linear chain of three digitoxose sugar moieties terminated by glucose. Purpurea glycosides A and B, first characterized by A. Stoll in 1938, constitute the principal active constituents of the fresh leaves. On drying, enzyme degradation takes place with the loss of the terminal glucose to give digitoxin, gitoxin and gitaloxin, respectively. Digitoxin and gitoxin are therefore the main active components of the dried drug. Poor storage conditions will lead to further hydrolysis and complete loss of activity. The gitaloxigenin series with its formyl group at C-16 is less stable than the other two series and was not discovered until 1956; the glycosides of this series are claimed to have similar or greater activities than those of the digitoxigenin group. The aglycones digitoxigenin and gitoxigenin are produced by acid hydrolysis of the respective glycosides but they are not found in quantity in the fresh or dried leaves. The aglycones, the formulae of which are shown in Fig. 23.19 are formed in the plant via the acetate-mevalonate pathway as already indicated in Fig. 23.16.
Other glycosides, present in small proportions, and involving the same genins contain digitalose and glucose; they exist as mono- and diglycosides. In this group verodoxin is claimed to have a toxicity of three times that of gitaloxin. These series are listed in Table 23.7. In 1961 small yields of yet other glycosides were reported from digitalis and include those with an acetylated side-chain (see Table 23.8).
Table 23.8 Acetylated side-chain glycosides of Digitalis purpurea.
| Glycoside | Aglycone | Sugar moieties |
|---|---|---|
| Acetyl glucogitoroside | Gitoxigenin | Glucose–acetyldigitoxose– |
| Acetyl digitalinum verum | Gitoxigenin | Glucose–acetyldigitalose– |
| Purlanoside A | Digitoxigenin | Glucose–(digitoxose)2–acetyldigitoxose– |
| Purlanoside B | Gitoxigenin | Glucose–(digitoxose)2–acetyldigitoxose– |
Over the years much attention has been given to the variation of glycoside content in digitalis both throughout the 2-year life cycle and during thecourse of a single day. General conclusions are not easily drawn, because of the apparently contradictory results obtained by different workers. These contradictions probably arise from the different methods of assay employed, the different environmental conditions of the plants studied, and the possible use of different chemical races. It is generally agreed that first-year leaves collected July–August have the highest content of total glycosides and that after a fall during the winter months, another peak, but not as high as the first-year one, is reached at the time of flowering.
For plants of Belgian origin, Lemli showed, in 1961, by chromatographic analysis that digitalinum verum and glucoverodoxin are formed first in the young leaves and then cease to accumulate further. At this stage small amounts only of purpurea glycoside B and glucogitaloxin are present but these steadily increase, finally to reach 40% of the total glycosides. Purpurea glycoside A is formed last and eventually becomes the major component at 50% of the total glycoside mixture.
Chemical races with respect to glycosides derived from digitoxin and gitoxin have been identified.
Digitalis purpurea leaves also contain anthraquinone derivatives, which include: 1-methoxy-2-methylanthraquinone, 3-methoxy-2-methylanthraquinone, digitolutein (3-methylalizarin-1-methylether), 3-methylalizarin, 1,4,8-trihydroxy-2-methyl-anthraquinone, etc. Saponins have also been isolated from the leaves, the sapogenins being produced more readily than cardenolides towards the end of the growing season. A number of leaf flavonoids have been described.
Keller–Kiliani test for digitoxose
Boil 1 g of powdered digitalis leaf with 10 ml of 70% alcohol for 2–3 min, filter; to 5 ml of filtrate add 10 ml of water and 0.5 ml of strong solution of lead acetate; shake and filter. Shake the filtrate with 5 ml of chloroform, allow to separate, pipette off the chloroform and remove the solvent by gentle evaporation in a porcelain dish. Dissolve the cooled residue in 3 ml of glacial acetic acid containing two drops of 5% ferric chloride solution. Carefully transfer this-solution to the surface of 2 ml of concentrated sulphuric acid; a reddish-brown layer forms at the junction of the two liquids and the upper layer slowly becomes bluish-green, darkening with standing.
Digitalis seeds
The seeds of D. purpurea contain different glycosides from those of the leaves. When extracted and standardized, they are known as ‘Digitalin’ (Digitalinum Purum Germanicum or amorphous Digitalin). This consists of the physiologically active ‘digitalinum verum’, with other water-soluble glycosides, including the saponins digitonin and gitonin.
Allied drugs
Digitalis thapsi is found in Spain and Italy. The leaves have a crenate margin and decurrent lamina. The leaves are characterized by the absence of non-glandular hairs, the presence of glandular hairs of two types, some consisting of a bicellular gland and unicellular stalk, others having a unicellular gland and a three- to four-celled stalk, the presence of a striated cuticle, pericyclic fibres and small prisms of calcium oxalate. The vein-islet number is higher than the other Digitalis species, varying from 8.5 to 16. D. lutea has a potency similar to those of D. purpurea and D. ferruginia and is cultivated in the former USSR. D. ferruginea ssp. ferruginea is the most widespread of the nine Digitalis spp. which grow in Turkey. For a report on the isolation of phenylethanoid glycosides from the aerial parts see Ihsan Çalis et al., Chem. Pharm. Bull., 1999, 47, 1305.
Adulterants
The characters described above, particularly the margin, venation and trichomes, distinguish the official leaves from all the adulterants which have been recorded. For example, mullein leaves (Verbascum thapsus) are densely covered with large branched woolly hairs (see Fig. 42.4I). Other possible adulterants are comfrey (Symphytum officinale; see Fig. 42.3G), primrose (Primula vulgaris; see Fig. 42.5G, H), elecampane (Inula helenium), ploughman’s spikenard (Inula conyza) and nettle (Urtica dioica).
DIGITALIS LANATA LEAF
The plant, Digitalis lanata (Scrophulariaceae), the leaves of which are used as a source of the glycosides digoxin and lanatoside C, is a perennial or biennial herb about 1 m high, indigenous to central and south-eastern Europe. It is also cultivated in Holland, Ecuador and the USA. Some 1000 tonnes of plant material are required annually to meet world demand.
Characters
The leaves are sessile, linear-lanceolate to oblong- lanceolate and up to about 30 cm long and 4 cm broad. The margin is entire, the apex is acuminate and the veins leave the midrib at a very acute angle. The distinctive microscopical characters are the beaded anticlinal walls of the epidermal cells, the 10–14-celled non-glandular trichomes which are confined almost exclusively to the margin of the leaf, and the glandular hairs found on both surfaces; some have bicellular heads and unicellular stalks, while others have unicellular heads and 3–10-celled, uniseriate stalks. As in D. purpurea, pericyclic fibres and calcium oxalate are absent.
Constituents
First isolated by Stoll in 1933, the primary glycosides resemble those of D. purpurea but are acetylated at the digitoxose moiety next to the terminal glucose. This confers crystalline properties on the compounds, making them more amenable to isolation. Partial hydrolysis of the glycosides occurs during the drying and storage of leaves, and deacetylation will produce products the same as in D. purpurea. In addition to the above series of glycosides, two others, involving digoxigenin and diginatigenin (Table 23.6 and Fig. 23.20), are found in the leaves.
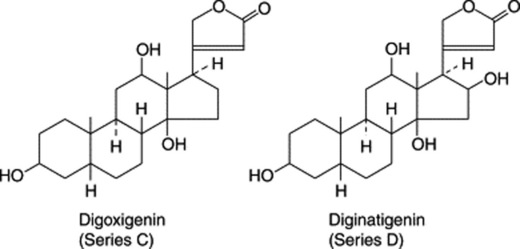
Fig. 23.20 Aglycones of Digitalis lanata cardioactive glycosides. See Fig. 23.19 for those also found in D. purpurea.
The principal glycosides of D. lanata leaves are listed in Table 23.9. In a series of papers by D. Krüger and colleagues (Planta Med., 1984, 50, 267 and references cited therein) nine other glycosides based on digitoxigenin and digoxigenin are described; two sugars involved, not listed in Table 23.9, are xylose and 2,6-dideoxyglucose.
Table 23.9 Some cardioactive glycosides of Digitalis lanata leaves.
| Glycoside | Aglycone | Sugar moieties |
|---|---|---|
| Lanatoside A | Digitoxigenin | Glucose–acetyldigitoxose–(digitoxose)2– |
| Acetyldigitoxin | Digitoxigenin | Acetyldigitoxose–(digitoxose)2– |
| Digitoxin | Digitoxigenin | (Digitoxose)3– |
| Glucoevatromonoside | Digitoxigenin | Glucose–digitoxose– |
| Digitoxigenin-O-glucosyl-6-deoxyglucoside | Digitoxigenin | Glucose–glucomethylose– |
| Glucodigifucoside | Digitoxigenin | Glucose–fucose– |
| Lanatoside B | Gitoxigenin | Glucose–acetyldigitoxose-(digitoxose)2– |
| Glucogitoroside | Gitoxigenin | Glucose–digitoxose– |
| Digitalinum verum | Gitoxigenin | Glucose–digitalose– |
| Lanatoside C | Digoxigenin | Glucose–acetyldigitoxose-(digitoxose)2– |
| Acetyldigoxin | Digoxigenin | Acetyldigitoxose–(digitoxose)2– |
| Deacetyl-lanatoside C | Digoxigenin | Glucose–(digitoxose)3– |
| Digoxin | Digoxigenin | (Digitoxose)3– |
| Digoxigenin–glucosyl–bis–digitoxoside | Digoxigenin | Glucose–(digitoxose)2– |
| Lanatoside D | Diginatigenin | Glucose–acetyldigitoxose-(digitoxose)2– |
| Lanatoside E | Gitaloxigenin | Glucose–acetyldigitoxose-(digitoxose)2– |
| Glucolanadoxin | Gitaloxigenin | Glucose–digitoxose– |
| Glucoverodoxin | Gitaloxigenin | Glucose–digitalose– |
Using radioimmunoassay techniques (q.v.), Weiler and Westerkamp have been able to assay many thousands of individual plants and by selection have obtained races yielding 2–4 times the quantity of digoxin found in normal mixed populations (see Chapter 14 for further details). Lehtola et al. (1981) found that digoxigenin glycoside levels do not appear to be influenced by collection date (June 28–September 9) or by time of collection (a.m. or p.m.). However, total glycoside levels are higher in first-year leaves but the important medicinal glycosides (e.g. lanatoside C) attain their highest levels in the second-year plants. Rather similar results have been recorded for a Brazilian cultivar (F. C. Braga et al., Phytochemistry, 1997, 45, 473). The primary glycosides appear to be stored exclusively in the vacuoles of cells.
Anthraquinone derivatives, similar to those found in D. purpurea, have been recorded in the leaves and a number of flavonoid glycosides characterized.
Cell and organ culture
Both cell and hairy root cultures of D. lanata have proved disappointing as a source of cardioactive glycosides. Green tissues appear to be a requisite and green hairy roots produced by light exposure give a 600-fold increase in cardenolide accumulation over roots cultivated in the dark. Luckner and colleagues have studied the development and cardenolide formation of somatic embryos; they established the optimum conditions for the regeneration of shoots from shoot tips and the subsequent adaptation of the regenerated D. lanata plants to open ground (Planta Med., 1990, 56, 53; 175).
Uses
The leaves are used almost exclusively for the preparation of the lanatosides and digoxin. Over the past decades digoxin has become the most widely used drug in the treatment of congestive heart failure. In long-term treatments patients require about 1 mg day−1 and the world-wide use of the drug now amounts to several thousand kilograms per year.
Proprietary preparations of the lanatoside complex, lanatoside C and lanatoside A are available in various countries but the glycoside from D. lanata most widely used is digoxin. Acting similarly to digitalis leaf, digoxin is more rapidly absorbed from the gastrointestinal tract than are the purpurea glycosides, which renders it of value for rapid digitalization in the treatment of atrial fibrillation and congestive heart failure. Lanatoside C is less well absorbed than digitoxin but it is less cumulative and for rapid digitalization the deacetyl derivative is preferable.
CARDIAC GLYCOSIDES OF THE APOCYNACEAE
At least a dozen genera of the Apocynaceae are known to contain cardioactive glycosides. Although all parts of the appropriate plants contain the glycosides, the latter often become concentrated in particular structures (e.g. seeds, barks, etc.). Extracts of a number are used as arrow poisons.
Strophanthus
Seeds of East African Strophanthus kombé were formerly official in the BP and a tincture prepared from them was used similarly to digitalis. The principal glycosides are K-strophanthoside, K-strophanthin-beta; and cymarin, all based on the genin strophanthidin (Table 23.6). Many minor glycosides have also been isolated. The seeds also contain about 30% of fixed oil, the bases trigonelline and choline, resin and mucilage.
For a full description of the seeds of this and other species, see earlier editions.
Strophanthus gratus seeds contain 4–8% of ouabain (G-strophanthin), a rhamnose glycoside more stable than those present in other species. It can be isolated in a pure crystalline form, and has been used as a standard in biological assays and for the preparation of ouabain injections. Ouabain is also the principal glycoside of the wood of the African Acokanthera schimperi (A. ouabaio). For the structure of the aglycone see Table 23.6.
Strophanthus sarmentosus seeds yield a number of glycosides with sarmentogenin as the aglycone. See also Chapter 14 for other information on these seeds.
The oleander glycosides
Nerium oleander, the oleander plant, and related species contain glycosides having a similar action to that of digitalis. Of Mediterranean origin, this evergreen flowering tree is widely cultivated in Japan and other countries as a garden and roadside ornamental.
The principal constituents of the leaves are oleandrin and digitalinum verum. Oleandrin is the monoside, comprising oleandrigenin (16-acetylgitoxigenin) and L-oleandrose. Other components are interesting, as they demonstrate modification of the cardiac activity with change of structure. Thus, the uzarigenin glycosides have a trans-fusion of the A/B rings at C-5 (uzarigenin = 5α-digitoxigenin) and have a lowered activity. And the adynerigenin and presumably also the Δ16-dehydroadynerigenin glycosides involving diginose and digitalose are inactive. Adynerigenin is digitoxigenin in which the 14-OH group has been replaced with an 8,14-ββ-epoxy group.
The leaves also contain gitoxigenin and digitoxigenin glycosides. A new glycoside, neridiginoside, has recently been obtained by activity directed isolation using the CNS depressant effect of a methanolic extract of the leaves on mice; it is the 3β-O-(D-diginosyl)-glycoside of a 5β,14β-cardenolide. Three other known constituents nerizoside, neritaloside and odoroside-H were also obtained (S. Begum et al., Phytochemistry, 1999, 50, 435 and references cited therein). For the isolation of three new triterpenes, see L. Fu et al., J. Nat. Prod., 2005, 68, 198. From the bark and twigs, new pregnanes in addition to the known neridienone have been described and their anti-inflammatory and cytotoxic properties studied (L. Bai et al., J. Nat. Prod., 2007, 70, 14).
Two glycosides isolated from N. odorum, in addition to others, are oleandrigenin-β-D-glucosyl-β-D-diginoside and gentiobiosyloleandrin.
The seeds of Thevetia peruviana (T. neriifolia) the yellow oleander, are a rich source of the glycoside thevetin A, which by partial hydrolysis and the loss of two glucose units yields peruvoside, the therapeutic cardioactive properties of which are well-known. Peruvoside consists of L-thevetose linked to the aglycone cannogenin. Thevetin has found use in continental Europe, and is considered particularly useful in cases of mild myocardial insufficiency and where digitalis intolerance exists.
Oleander ingestion causes many cases of poisoning world-wide; in 1994, 303 cases were reported in Texas, and in Australia during 1972–8 it was responsible for 27% of paediatric plant poisonings. Fatal cases have been reported elsewhere and in Sri Lanka the use of the
seeds in suicide attempts, particularly among teenagers, poses a problem (see M. Eddleston et al., Lancet, 2000, 355, 967 and references cited therein).
MISCELLANEOUS SOURCES
Plants which contain medicinally useful cardenolides have been obtained from other families (Asclepiadaceae, Cruciferae, Euphorbiaceae, Liliaceae, Moraceae, Leguminosae, Ranunculaceae, Sterculiaceae).
Convallaria
The lily of the valley, Convallaria majalis (Liliaceae) is much used on the continent of Europe and in herbal medicine for its cardioactive properties which are similar to those of digitalis but much less cumulative. Either the aerial parts, collected when the flowers are beginning to open, or the rhizomes and roots, are used.
The principal glycoside is convallatoxin which on hydrolysis gives strophanthidin (Fig. 23.17) and (−)-rhamnose. The plant contains many minor cardenolides, about 40 glycosides associated with nine different aglycones having been identified. Sugars not recorded elsewhere for cardiac glycosides are allose and the disaccharide rhamnosido-6-deoxyallose. (For the isolation of novel cardenolides see V. K. Saxena et al., J. Nat. Prod., 1992, 55, 39.)
The glycosides appear to be formed in the leaves and a turnover apparently takes place towards the end of the vegetative period. Bioconversions which lead to the formation of minor glycosides have been studied, along with the utilization of digitoxigenin and digitoxin by the plant for production of convallatoxin.
Convalloside, a glycoside of the seeds, when acted on by strophanthobiase yields convallatoxin and D-glucose. A number of flavonoid glycosides are also present in the leaves, and the roots contain a saponin, convallamaroside, which is a 22-hydroxyfuranostanol saponin with three independent sugar chains at C-1, C-3 and C-7.
Japanese lily of the valley, Convallaria keiskei, contains glycosides of convallagenin.
The dried aerial roots of Adonis vernalis (Ranunculaceae) contain more than 30 cardenolides, acting similarly to those of strophanthus; cymarin is the major constituent. (See B. Kopp et al., Phytochemistry, 1992, 31, 3195.)
The aerial parts of Erysimum canescens and other species of Erysimum (Cruciferae) are used in the former USSR, and contain glycosides based on strophanthidin. Erysimin, for instance, gives on hydrolysis strophanthidin and digitoxose. Another drug, used in Russia similarly to digitalis, is derived from the bark of the silk-vine Periploca graeca (Asclepiadaceae). Its principal glycoside, periplocin, affords on hydrolysis glucose, cymarose and periplogenin.
BUFADIENOLIDES
The bufadienolides (Table 23.6) are less widely distributed in nature than are the cardenolides; they are found in some Liliaceae and Ranunculaceae, and in the toad venoms the genins are partly free and partly combined with suberyl arginine. Therapeutically they find little use as cardioactive drugs because of their low therapeutic index and their production of side-effects. However squill (q.v.) has a time-honoured place as an expectorant and has been widely used in the treatment of cough.
In a review (see ‘Further reading’) Krenn and Kopp have listed 267 bufadienolides for the period 1967–1995 found in six plant families—Crassulaceae, Hycinthaceae (Liliaceae), Iridaceae, Melianthaceae, Ranunculaceae and Santalaceae—and in animals in the Bufonidae, Cilucridae and Lampyridae.
SQUILL
Squill BP consists of the dried sliced bulbs of Drimia maritima (L.) Stearn [Urginea maritima (L.) Baker], Liliaceae collected after the plant has flowered and from which the membranous outer scales have been removed. D. maritima occurs wild as an aggregate of at least six species of varying chromosome number, not all giving a bulb with an acceptable glycoside content. D. maritima (L.) Baker sens. str. is hexaploid and is a variety from which the commercial drug can be obtained and which has recently been the most studied. It is known in commerce as white squill. That grown in the Mediterranean area (Italy, Malta) is now almost unobtainable and the principal source is Indian squill (see below). Red squill, which is also derived from a variety of U. maritima, is collected in Algiers and Cyprus, and differs from the white in containing red anthocyanin pigment and the glycoside scilliroside.
Collection and preparation
The bulbs are collected in August, a month in which the plant has finished flowering and is without aerial leaves. After the dry outer scales have been removed, the bulbs are cut transversely into thin slices. These are dried in the sun or by stove heat, when they lose about 80% of their weight. The dried slices are packed in bags (containing about 50 kg) or in barrels.
History
Squill was well known to the early Greek physicians and to the Egyptians. A vinegar of squills was known to Dioskurides and an oxymel of squills to the Arabian physicians.
Macroscopical characters
Squill bulbs are pear-shaped and about 15–30 cm diameter. Whole bulbs are rarely imported because they tend to start growing unless stored in a refrigerator.
The dried drug occurs in yellowish-white, translucent strips about 0.5–5 cm in length and tapering at both ends. The drug is brittle when perfectly dry, but it readily absorbs moisture and becomes tough and flexible. The hygroscopic nature is particularly noticeable in the powdered drug, which, if carelessly stored, tends to cake into solid masses and develop mould. It should be stored in an atmosphere free from moisture. Odour, slight; taste, bitter and acrid.
Microscopical characters
Under the microscope squill shows abundant large polygonal parenchymatous cells of the mesophyll, many of which contain mucilage, surrounding a bundle of many raphides of calcium oxalate (see Fig. 42.9G). The individual crystals are 50–250–500–900 μm long and 1–6 μm diameter. The mucilage sheath is stained by corallin soda. Very occasional small, rounded, starch grains, about 10 μm diameter, are present in the mesophyll cells. The epidermis is composed of rectangular cuticularized cells, in surface view polygonal and usually axially elongated. Stomata are absent or very rare on the adaxial surface; a few are constantly present on the abaxial surface. They are anomocytic, circular in outline, with wide guard cells. The mesophyll is traversed by numerous small vascular bundles, which account for the presence in the powdered drug of occasional small spiral and annular xylem vessels.
Constituents
Pure glycosides were not isolated until in 1923 Stoll separated a crystalline glycoside, scillaren A, and an amorphous mixture of glycosides, scillaren B. Scillaren A, the most important constituent of squill, is readily hydrolysed by an enzyme scillarenase or by acids as shown at top of p. 331.
Small quantities of glucoscillaren A (a triglycoside) also occur in the bulb. Other glycosides with 12α- and 12β-OH substitution have similar sugar side-chains at C-3. Other minor glycosides and the isolation of new bufadienolides are described by B. Kopp and L. Krenn (Phytochemistry, 1996, 42, 513; J. Nat. Prod., 1996, 59, 612). D. maritima collected
in Egypt is markedly different in chemical constitution to bulbs of U. aphylla from Greece and Turkey, and U. numidica from Tunisia.
Many flavonoids have been detected in extracts of the bulb of U. maritima; they include quercetin derivatives and kaempferol polyglycosides together with C-glycosides such as vitexin and isovitexin. The drug also contains sinistrin, a fructan resembling inulin; it is composed largely of β-D-fructofuranosyl residues. M. Iizuka et al. have recorded 33 compounds isolated from the squill bulb—ten were new natural compounds, nine were bufadienolides and one was a lignan (Chem. Pharm. Bull., 2001, 49, 282). Idioblasts (see above) contain calcium oxalate crystals embedded in mucilage consisting mainly of glucogalactans. Anthocyanins are present in small amount.
Tests
The BP specifies an ethanol (90%) extractive of not less than 68.0%, an acid-insoluble ash limit of not more than 1.5%, mucilage staining red with alkaline corallin solution and no purple stain with 0.01-M iodine solution.
Action and uses
The glycosides are poorly absorbed from the gastrointestinal tract, they are of short-action duration and they are not cumulative. In small doses the drug promotes mild gastric irritation causing a reflex secretion from the bronchioles. It is for this expectorant action that it is widely used; in larger doses it causes vomiting.
INDIAN SQUILL OR URGINEA
This consists of the dried, usually longitudinally sliced, bulb of Drimia indica (Roxb.) J. P. Jessop [Urginea indica (Roxb.) Kunth.]. The drug is used in India and has now been reintroduced into the BP and is described in the BHP. It occurs in curved pieces of a slightly darker colour than the European squill, which it closely resembles. The strips are sometimes united in groups of about four to eight to a portion of the axis, such pieces being seldom found in the European drug. Also in contrast to the latter, the mucilage of the mesophyll cells stains red with alkaline corallin solution and reddish-purple with iodine solution.
The constituents appear to be similar to those of white squill.
Black hellebore rhizome
Black hellebore rhizome is obtained from Helleborus niger (Ranunculaceae), a perennial herb indigenous to Central Europe. It contains three crystalline cardiac glycosides: helleborin, helleborein and hellebrin. Of these, the last two have a digitalis-like action, hellebrin being approximately 20 times more powerful than helleborein. The aglycone hellebrigenin (Fig. 23.16) is the bufadienolide analogue of strophanthidin. The drug which has abortifacient as well as cardiotonic properties is considered dangerous and is now obsolete in ordinary medicine.
OTHER STEROIDS
There are few other types of steroidal compounds in addition to those already discussed that have, at the moment, any pharmaceutical significance.
The phytosterols (e.g. stigmasterol and sitosterol) have been mentioned in connection with steroid synthesis, and the role of cholesterol in the biosynthesis of other steroids has been noted (Figs 23.2, 23.16). Steroidal alkaloids in which the nitrogen may be either cyclic or noncyclic are considered in Chapter 26.
WITHANOLIDES
This class of steroidal lactones involves an ergostane-type framework in which C–22 and C–26 are appropriately oxidized to form a δ-lactone ring.
Chemists had long been interested in the constituents of Withania somnifera (Solanaceae), an Indian plant well-known in traditional medicine, and in 1968 Israeli workers determined the structure of a constituent lactone (withaferin A) of the plant; since then many more compounds of this now large class, have been characterized. They are subdivided into nine groups: withanolides, withaphysalins, physalins, nicandrenones, jaborols, ixocarpalactones, perulactones, acnistins and miscellaneous withasteroids (see M. Leopoldina et al., Planta Medica, 2004, 70, 551).
In addition to their potential pharmacological value as sedatives, hypnotics, antiseptics and antimitotics, some withanolides are cell differentiation inducers—compounds of a new type of antitumour agent (M. Kuroyanagi et al., Chem. Pharm. Bull., 1999, 47, 1646). Withanolides have proved of interest because of their occurrence as chemical races of Withania and because of their structural variation in hybrids of different races (q.v.). Other genera of the family in which they occur include Acnistus, Datura, Deprea, Hyoscyamus, Iochroma, Jaborosa, Lycium, Nicandra, Physalis and Solandra.
The first report of a withanolide from the Labiatae concerns the characterization of a new compound, ajugin, from Ajuga parviflora whole plants (P. M. Khan et al., Phytochemistry, 1999, 51, 669). Previously the steroidal lactone, ajugalactone, had been isolated from A. decumbens; this compound has an α,β-unsaturated carbonyl group and inhibits insect metamorphosis (contrast the ecdysones below). A number of other species have been used by various cultures to treat a variety of ailments. There are rare reports of withanolides of the Taccaceae and Leguminosae.
The isolation of new withanolides continues to be an active area of research.
Ecdysones
The ecdysis of insects relates to the moults which larvae undergo during their transformation into an adult, a process controlled by complex hormonal mechanisms. Ecdysones, or insect-moulting hormones, are substances which stimulate these changes, and one example is ecdysone itself, which was first isolated from silk-worm pupae.
Only a few such compounds have been isolated from arthropods, but in plants they occur in much greater variety and abundance. Ecdysterone (20-hydroxyecdysone) is also an example of one which has been obtained from both plant and insect sources; whether a plant–insect relationship exists with regard to this substance and whether such compounds have a function in the plant is at present not known. It is perhaps
significant, however, that insects do not themselves biosynthesize steroids de novo and rely on plant materials for suitable precursors.
In the plant, cholesterol is a precursor of insect-moulting hormones, and in one morphological group of Helleborus they are formed together with bufadienolides (q.v.) and saponins.
Cucurbitacins
These tetracyclic triterpenoids are of interest because of their cytotoxic and antitumour properties. They occur in the Cucurbitaceae and other families such as the Euphorbiaceae and Cruciferae. Cucurbitacin A was isolated in 1953 and its structure determined in 1963. They may occur in the glucosidic form and are hydrolysed by the enzyme elaterase. The formulae of two examples are given below.
Cycloartanes
As was indicated previously (see Fig. 23.1) the ring closure of squalene 2,3-oxide yields cycloartenol as an intermediate in plant sterol biosynthesis. However, these phytosterols are also found in the free state in a wide range of plants; medicinal examples include the neem and olive plants, Euphorbia spp., Hypericum, the woody nightshade and a number of members of the Cucurbitaceae. The formula of one such compound is given below.