Fluid, Electrolyte, and Acid-Base Balance
Upon completing this chapter, you should be able to:
1 List the various functions water performs in the body.
2 List the major electrolytes and the function of each.
3 Describe three ways in which body fluids are continually being distributed among the fluid compartments.
4 Identify the signs and symptoms of the common fluid and electrolyte imbalances.
5 State the main signs and symptoms of acid–base imbalances.
1 Assess an assigned patient for signs of fluid and electrolyte imbalance.
2 From patient laboratory results, identify electrolyte values that are abnormal.
3 Implement teaching for the patient with hypokalemia.
4 Develop a plan of care for a patient who has a fluid and electrolyte imbalance.
5 Identify patients who might be at risk for an acid–base imbalance.
acidosis (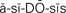 , p. 441)
, p. 441)
active transport (p. 439)
alkalosis (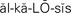 ,p. 444)
,p. 444)
ascites (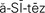 ,p. 441)
,p. 441)
dehydration ( ,p. 437)
,p. 437)
diffusion (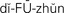 ,p. 438)
,p. 438)
edema (p. 441)
electrolytes (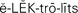 ,p. 436)
,p. 436)
extracellular (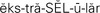 ,p. 437)
,p. 437)
filtration (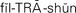 ,p. 439)
,p. 439)
hydrostatic pressure (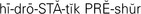 ,p. 439)
,p. 439)
hypercalcemia (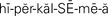 ,p. 444)
,p. 444)
hyperchloremia (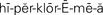 ,p. 445)
,p. 445)
hyperkalemia (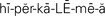 ,p. 442)
,p. 442)
hypermagnesemia ( ,p. 445)
,p. 445)
hypernatremia ( ,p. 441)
,p. 441)
hyperphosphatemia (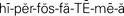 ,p. 445)
,p. 445)
hypertonic ( ,p. 439)
,p. 439)
hyperventilation (p. 447)
hypervolemia ( ,p. 441)
,p. 441)
hypocalcemia ( ,p. 444)
,p. 444)
hypochloremia ( ,p. 445)
,p. 445)
hypokalemia ( ,p. 442)
,p. 442)
hypomagnesemia ( ,p. 444)
,p. 444)
hyponatremia ( ,p. 441)
,p. 441)
hypophosphatemia ( ,p. 445)
,p. 445)
hypotonic (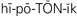 ,p. 439)
,p. 439)
hypovolemia (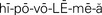 ,p. 437)
,p. 437)
interstitial ( ,p. 437)
,p. 437)
intracellular (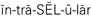 ,p. 437)
,p. 437)
intravascular (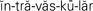 ,p. 437)
,p. 437)
isotonic (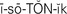 ,p. 439)
,p. 439)
osmosis (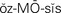 ,p. 438)
,p. 438)
tetany (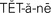 ,p. 447)
,p. 447)
transcellular ( ,p. 437)
,p. 437)
turgor (p. 440)
COMPOSITION OF BODY FLUIDS
The two largest constituents of the body fluids are water and electrolytes. Water is present in greater proportion than electrolytes. Water serves many functions, but the four main functions of water in the body are (1) as a vehicle for the transportation of substances to and from the cells; (2) to aid heat regulation by providing perspiration, which evaporates; (3) to assist maintenance of hydrogen (H+) balance in the body; and (4) to serve as a medium for the enzymatic action of digestion.
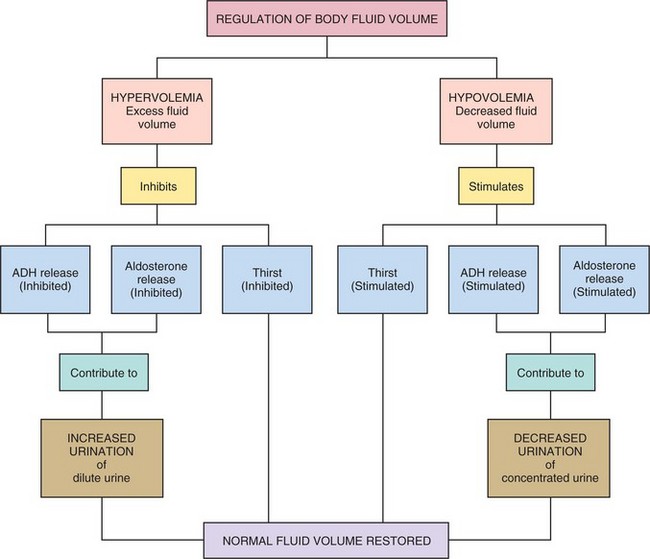
Over half of the body’s weight is water. The amount varies by age, sex, and health status. The adult male body contains about 60% water; the adult female body, because of more fat tissue, contains about 50% water. The greater the amount of fat the body contains, the less the percentage of water it has because fat contains less water than other tissue. The infant and the elderly person are more quickly and seriously affected by minor changes in their fluid balance and can become rapidly dehydrated. The infant, because of its large body surface compared with body weight, loses more fluid through the skin than the adult. The kidneys of the infant are not as efficient as the adult’s and less fluid is reabsorbed. The elderly person has an age-related decline in total body water, diminished thirst sensation, a decrease in urine-concentrating ability of the kidney, and a decrease in effectiveness of antidiuretic hormone. These factors cause dehydration to occur more quickly than in the younger adult. Dehydration may cause hypovolemia (Concept Map 25-1). If an excess of fluid volume is present in the body, hypervolemia occurs.
Water is critical to maintaining a state of homeostasis because water is the medium in which most metabolic and chemical reactions in the body take place. Without sufficient water, cells cannot function and death results.
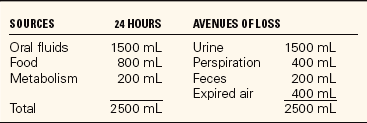
Water is the avenue for transportation within the body. It carries nutrients to the cells and transports wastes for excretion. Body water continually moves in and out of the blood, through the lymph vessels, between the cells, and in and out of the cells. Table 25-1 shows sources of water and avenues of water loss.
Electrolytes
Electrolytes are minerals or salts that are dissolved in body fluid. When in solution, they break up into particles known as ions that have a tiny electrical charge. The ions develop a positive electrical charge and are then known as cations, or they develop a negative electrical charge and are anions. For each positively charged cation in a fluid compartment, there must be a negatively charged anion so that a balance is maintained. As fluids move from compartment to compartment, the body works to maintain homeostasis in each compartment by balancing the anions and cations so that there is electrical neutrality. Electrolytes move within the body freely, but each has a primary location. Because disturbances in homeostasis upset the normal balance of electrolytes, the location and function of each electrolyte become important in understanding what is occurring in the body. The major source of electrolytes is from the diet. Table 25-2 presents the electrolytes, their normal ranges, and functions.
Table 25-2
The Major Electrolytes: Normal Range and Function
| ELECTROLYTE | NORMAL RANGE | FUNCTION |
| Sodium (Na+) | 135-145 mEq/L | Major cation of the extracellular fluid. Major role in regulation of water balance. Regulates extracellular fluid volume through osmotic pressure. Water follows sodium concentration in the body. Essential to the transmission of nerve impulses and helps maintain neuromuscular irritability. Important in controlling contractility of the heart. Helps maintain acid–base balance. Aids in maintenance of electroneutrality. |
| Potassium (K+) | 3.5-5.0 mEq/L | Major intracellular cation. Important to nerve transmission and muscle contraction. Helps maintain normal heart rhythm. Helps maintain plasma acid–base balance. |
| Calcium (Ca2+) | 8.4-10.6 mg/dL | Involved in formation of bone and teeth. Necessary for blood coagulation. Essential for normal nerve and muscle activity. |
| Magnesium (Mg2+) | 1.3-2.1 mg/dL | Necessary for building bones and teeth. Necessary for nerve transmission and is involved in muscle contraction. Plays an important role in many metabolic reactions, where it acts as a cofactor to cellular enzymes. |
| Phosphate (PO43−) | 2.7-4.5 mg/dL | Necessary for formation of adenosine triphosphate (ATP). Cofactor in carbohydrate, protein, and lipid metabolism. Activates B-complex vitamins. |
| Chloride (Cl−) | 96-106 mEq/L | Helps maintain acid–base balance. Important to formation of hydrochloric acid for secretion to the stomach. Aids in maintaining plasma electroneutrality. |
| Bicarbonate (HCO3−) | 22-26 mEq/L | A buffer that neutralizes excess acids in the body. Helps regulate acid–base balance. |
NONELECTROLYTES
The intermediate products of metabolism’amino acids (proteins), glucose, and fatty acids’are nonelectrolytes. They remain bound together when dissolved in body fluid. In the healthy individual who is eating normally, the nonelectrolytes circulating in the body fluid remain stable.
BLOOD
There are 4 to 6 L of circulating blood volume in the body, depending on body size and sex. Erythrocytes (red cells), leukocytes (white cells), and platelets (thrombocytes) are the blood cells that are carried in the plasma. Any condition that alters body fluid volume also alters the plasma volume of the blood, and can affect blood pressure and circulation. The plasma proteins and colloids contribute to plasma colloid osmotic pressure, which helps keep fluid in the vascular compartment.
DISTRIBUTION OF BODY FLUIDS
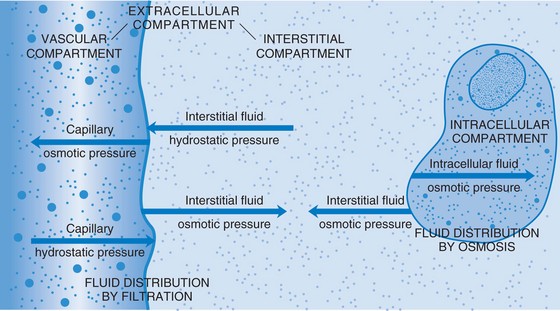
Body fluids are either intracellular (within the cell) or extracellular (outside of the cell). Extracellular fluid (ECF) is of three types: intravascular, interstitial, and transcellular. Table 25-3 describes these fluids. When fluid shifts from the plasma in the vascular space out to the interstitial space, blood volume drops and dehydration (removal of water from a tissue) and hypovolemia (decreased volume of plasma) may occur.
Table 25-3
| BODY FLUID | DISTRIBUTION |
| Extracellular fluid | Approximately ⅓ of total body water. Transports water, nutrients, oxygen, waste, etc., to and from the cells. Regulated by renal, metabolic, and neurologic factors. High in sodium (Na+) content. |
| Intravascular fluid | Fluid within the blood vessels. Consists of plasma and fluid within blood cells. Contains large amounts of protein and electrolytes. |
| Interstitial fluid | Fluid in the spaces surrounding the cells. High in sodium (Na+) content. |
| Transcellular fluid | Includes aqueous humor; saliva; cerebrospinal, pleural, peritoneal, synovial, and pericardial fluids; gastrointestinal secretions; and fluid in urinary system and lymphatics. |
| Intracellular fluid | About ⅔ of total body fluid. Fluid contained within the cell walls. Most cell walls are permeable to water. High in potassium (K+) content. |
MOVEMENT OF FLUID AND ELECTROLYTES
The amount of fluid leaving the body should be balanced by water entering it. Water is taken in through the ingestion of fluids and food and is produced by cell metabolism. The thirst mechanism located in the hypothalamus helps control fluid balance in the body. It monitors fluid volume and concentration. Hypothalamic receptors sense when fluid is more concentrated and stimulate nerve impulses that are interpreted in the brain as thirst, which motivates the person to drink. Intake of sufficient water lowers the concentration and the receptors are no longer stimulated.
Fluid is lost in the urine and feces and through insensible (invisible) losses via exhaled air and through the skin as perspiration. The kidney is the main organ through which fluid excretion is achieved. Several hormones affect urine output, particularly antidiuretic hormone (ADH), aldosterone, and atrial natriuretic peptide (ANP). ADH is secreted by the posterior pituitary. More ADH is released when the blood becomes more concentrated or there is decreased circulating blood volume, and during times of pain, nausea, and stress. When this occurs, the renal tubules reabsorb more water, and urine output decreases. Aldosterone is released by the adrenal cortex when ECF volume is low or when sodium concentration is elevated, causing reabsorption of sodium from kidney tubules. This creates an osmotic gradient by which more water is retained. The release of aldosterone is stimulated by the renin-angiotensin-aldosterone system. ANP acts to protect the body from fluid overload and is released from sites in the myocardium and the brain. Blood volume and pressure also affect the glomerular filtration rate and urine output.
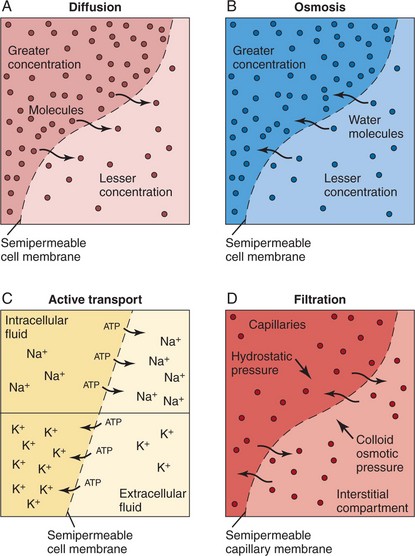
Water and the substances suspended or dissolved in it must move from compartment to compartment so that they are normally distributed within the body. They must pass through the semipermeable membranes of the body’s cells to do this. The heart circulates blood throughout the body. As the blood flows through the capillaries, fluid and solutes can move into the interstitial spaces, where substances in every cell of the body can be exchanged. Several processes move fluids, electrolytes, nutrients, and waste products back and forth across the cell membranes (Figure 25-1).
Passive Transport
Diffusion.: Diffusion is the process by which substances move back and forth across the membrane until they are evenly distributed throughout the available space. Substances will move from a high to a low concentration until the concentration on both sides of the membrane is equal. This is called movement down a concentration gradient. Glucose, oxygen, carbon dioxide, water, and other small ions and molecules move by diffusion. It is a process of equalization.
Diffusion may occur by movement along an electrical gradient as well. The attraction between particles of opposite charge and the repellent action between particles of like charge comprise an electrical gradient. Many intracellular proteins have a negative charge that tends to attract the positively charged sodium and potassium ions from the ECF.
Osmosis.: Osmosis refers to the movement of pure solvent (liquid) across a membrane. Water diffuses by osmosis. When there are differences in concentration of fluids in the various compartments, water will be moved from the area of less solute concentration to the area of greater concentration until the solutions in the compartments are of equal concentration. The process takes place via a semipermeable membrane’a membrane that allows some substances to pass through but prevents the passage of other substances. Fluid moves between the interstitial and intracellular and the interstitial and intravascular compartments by osmosis. Cell wall membranes are semipermeable, as are the walls of blood vessels.
When living cells are surrounded by a solution that has the same concentration of particles, the water concentration of the intracellular and extracellular fluids will be equal. Such a solution is termed isotonic (of equal solute concentration). If cells are surrounded by a solution that has a greater concentration of solute than the cells, the water in the cells will move to the more concentrated solution and the cells will dehydrate and shrink. The solution is hypertonic (of greater concentration) in relation to the cells. If the cells are surrounded by a solution that has less solute than the cells, the solution is hypotonic (of less concentration) in relation to the cells. The particles within the cells exert an osmotic pressure, drawing water inward through the semipermeable membrane. The cells swell from the extra fluid (overhydrate). These concepts are important to the administration of intravenous fluids (see Chapter 36). Solutions are classified as isotonic, hypertonic, or hypotonic according to their concentration of electrolytes and other solutes. So, by osmosis water passes rather freely across cell membranes. The process of osmosis is essential to the life of the cells and to the balance of water and electrolytes in the body. Osmotic pressure within vessels helps to keep fluid from leaking out into the interstitial spaces (Figure 25-2).
Filtration.: Filtration is the movement of water and suspended substances outward through a semipermeable membrane. The pumping action of the heart creates hydrostatic pressure (pressure exerted by fluid) within the capillaries. Hydrostatic pressure causes fluid to press outward on the vessel. That force promotes filtration, forcing movement of water and electrolytes through the capillary wall to the interstitial fluid.
Active Transport
Active transport, contrary to diffusion, osmosis, and filtration, requires cellular energy. This force can move molecules into cells regardless of their electrical charge or the concentrations already in the cell. Active transport may move substances from an area of lower concentration to an area of higher concentration. The energy source for the process is adenosine triphosphate (ATP). ATP is produced during the complex metabolic processes in the body’s cells. Enzyme reactions metabolize carbon chains of sugars, fatty acids, and amino acids, yielding carbon dioxide, water, and high-energy phosphate bonds. Active transport can move amino acids, glucose, iron, hydrogen, sodium, potassium, and calcium through the cell membrane.
FLUID AND ELECTROLYTE IMBALANCES
Healthy people maintain intake and output balance by drinking sufficient fluids and eating a balanced diet each day. The healthy kidney regulates fluid and electrolyte balance by regulating the volume and composition of extracellular fluid. Illness affects fluid balance in many ways. The patient may be unable to ingest food or liquids, there may be a problem with absorption from the intestinal tract, or there may be a kidney impairment that affects excretion or reabsorption of water and electrolytes. Any disease that affects circulation (e.g., congestive heart failure) will ultimately affect the distribution and composition of body fluids. Burns, in which large amounts of body fluid may be lost through open wounds, also present problems of fluid balance. In fact, any seriously ill patient is at risk for a fluid and electrolyte imbalance.
A fluid imbalance exists when there is an excess (too much) or a deficit (too little) of water in the body. When this occurs, there will be an accompanying imbalance in the substances dissolved in it. When considering sodium imbalances, it is important to remember that water follows sodium in the body. The sodium concentration causes an osmotic pull, and water will go to where that concentration is highest.
DEFICIENT FLUID VOLUME
Those at risk for deficient fluid volume are (1) patients unable to take in sufficient quantities of fluid because of impaired swallowing, extreme weakness, disorientation or coma, or the unavailability of water; and (2) patients who lose excessive amounts of fluid through prolonged vomiting, diarrhea, hemorrhage, diaphoresis (sweating), or excessive wound drainage. Treatments that can cause a fluid deficit are diuretic therapy and gastrointestinal suction without fluid replacement.
Burns and drainage from large wounds or fistulas can deplete the fluid volume. Treatment of fluid volume deficit involves remedying the underlying cause and replacing fluids.
Dehydration
When a fluid deficit occurs, it causes loss of water from the cells (dehydration); when there is too little water in the plasma, water is drawn out of the cells by osmosis to equalize the concentration, and the cells shrivel. Dehydration is treated by fluid administration, either orally or intravenously.
Signs and symptoms of dehydration are listed in Box 25-1. Tissue turgor (degree of elasticity) is checked by gently pinching up the skin over the abdomen, forearm, sternum, forehead, or thigh (Figure 25-3). In a person with normal fluid balance, the skin when pinched will immediately fall back to normal when released. If a fluid deficit is present, the skin may remain elevated or tented for several seconds after the pinch. This test measures skin elasticity as well and is not a valid indicator of fluid status in the elderly. Weight loss is another sign of dehydration. In the infant, severe fluid deficit is evident by sunken eyeballs and depression of the anterior fontanel.
EXCESS FLUID VOLUME
Healthy people do not ordinarily drink too much water. When people become ill they may take in more water than they excrete. This can happen if they receive intravenous fluid too quickly, are given tap water enemas, or are persuaded to drink more fluids than they can eliminate. If these events happen, the patient will suffer a fluid volume excess. Impaired elimination, such as occurs in renal failure, is an important cause of fluid volume excess. Signs of overhydration are weight gain, crackles in the lungs, slow bounding pulse, elevated blood pressure, and possibly edema. When fluid volume excess occurs, hypervolemia (excessive blood volume) may also occur. Hypervolemia causes an elevation of blood pressure.
Edema
Edema is an excessive accumulation of interstitial (tissue) fluid. It is often a sign of fluid overload, but may be from other causes. In ambulatory patients, the excessive fluid tends to accumulate in the lower extremities (Figure 25-4). In the bedridden patient, the fluid accumulates in the sacral region. These types of fluid accumulations are termed dependent edema. Generalized edema occurs when excess interstitial fluid is spread throughout the body. It is most visible in the hands and face, where swelling is detectable. Causes of generalized edema are (1) kidney failure, (2) heart failure, (3) liver failure, and (4) hormonal disorders involving the overproduction of aldosterone and ADH (Figure 25-5). Local edema may be caused by infection or injury and the resulting inflammation.
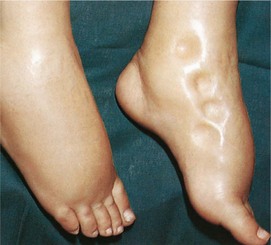
FIGURE 25-4 Example of pitting edema from fluid excess. The finger depressions do not refill quickly after pressure has been exerted.
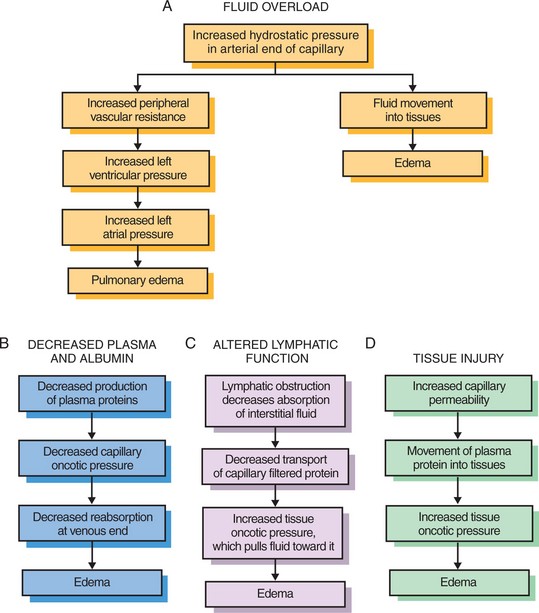
FIGURE 25-5 Mechanisms of edema formation. A, Fluid overload; B, decreased plasma and albumin; C, altered lymphatic function; and D, tissue injury.
Treatment involves correcting the underlying cause and assisting the body to rebalance fluid content. Fluid intake may be restricted or diuretic drugs may be administered to cause excretion of the excess fluid. A diuretic is a drug that causes the kidneys to increase the excretion of fluid.
ELECTROLYTE IMBALANCES
A summary of the normal ranges of the major electrolytes, the causes of imbalances, the signs and symptoms of imbalances, and nursing interventions is provided in Table 25-4.
Sodium Imbalances
Hyponatremia.: A deficit of sodium in the blood is called hyponatremia (Na+<135 mEq/L). This can occur from a sodium loss or an excess of water. This is the most common electrolyte imbalance patients experience. Sodium loss may occur from excessive vomiting or diarrhea, in which fluid loss is replaced with plain water. Decreased secretion of aldosterone can result in sodium loss. Congestive heart failure, liver disease with ascites (abnormal accumulation of fluid within the peritoneal cavity), and sometimes chronic renal failure result in excessive water retention without concurrent sodium retention. This results in a hypervolemia combined with hyponatremia. The average intake of sodium is 6 to 12 g/day. If there is a problem with water balance, sodium may be restricted in the diet.
Hypernatremia.: When the serum sodium concentration rises above 145 mEq/L, a state of hypernatremia exists. This occurs when there is an excess of sodium or a loss of body water. Excessive administration of sodium bicarbonate for the treatment of acidosis (excess of acid or depletion of alkaline substances in the blood and body tissues) is one cause. More commonly, water loss from fever, respiratory infection, or watery diarrhea is the cause.
Decreased water intake may occur in immobile, confused, or dependent patients or in those who have sustained damage to the thirst center in the hypothalamus. The body tries to correct the situation by conserving water through reabsorption in the renal tubules. Hypernatremia causes an osmotic shift of fluid from the cells to the interstitial spaces, causing a cellular dehydration and interruption of normal cell processes. Sodium intake is restricted for the patient with hypernatremia (Patient Teaching 25-1, p. 445).
Potassium Imbalances
Hypokalemia.: When the potassium level falls below 3.5 mEq/L, hypokalemia exists. Extra potassium must be given to help correct the imbalance. Hypokalemia may occur due to poor diet, illness causing a shift of potassium from ECF to intracellular fluid (ICF), or increased potassium loss. Vomiting, diarrhea, gastrointestinal (GI) suction, excessive sweating, and diuretic therapy may deplete potassium levels. The patient is encouraged to eat foods high in potassium (Patient Teaching 25-2,p. 445), and intravenous replacement may be necessary (Safety Alert 25-1, p. 445).
Hyperkalemia.: When serum potassium level rises above 5.0 mEq/L, a state of hyperkalemia exists. Patients with severe burns or crush injuries and those undergoing major surgery are at risk for hyperkalemia. The mechanical disruption of cell membranes causes a shift of potassium from the ICF to the ECF. Hyperkalemia occurs in renal failure, overuse of potassium-sparing diuretics, digitalis toxicity, overuse of potassium-containing salt substitutes, uncontrolled diabetes mellitus, and a variety of other illnesses.
Calcium Imbalances
Hypocalcemia.: When the calcium level drops below 8.4 mg/dL, hypocalcemia occurs. This can occur from nutritional deficiency of calcium or vitamin D. Hypocalcemia occurs in disorders in which there is a shift of calcium into the bone. Metastatic cancer invading bone is one such cause. Removal or injury of the parathyroid glands during thyroidectomy causes parathyroid hormone deficiency and consequent hypocalcemia. Excessive infusion of bicarbonate solution, alkalosis (excess of alkaline or decrease of acid substances in the blood and body fluids), blood transfusions, and hypoparathyroidism may cause hypocalcemia.
Hypercalcemia.: Hypercalcemia, a serum calcium level above 10.6 mg/dL, can occur during periods of lengthy immobilization when calcium is mobilized from the bone or when an excess of calcium or vitamin D is taken into the body. Most cases are related to hyperparathyroidism or malignancy in which there is metastasis with bone resorption. Such malignancies include multiple myeloma and lung or renal cancers.
Magnesium Imbalances
Hypomagnesemia.: Hypomagnesemia, a serum level below 1.3 mEq/L, results from malabsorption, malnutrition, or increased loss from renal tubular dysfunction or thiazide diuretic use. Extensive gastric suction or diarrhea can also cause it. Hypomagnesemia usually is present when hypokalemia and hypocalcemia occur.
Anion Imbalances
Imbalances of chloride, phosphate, and bicarbonate accompany cation imbalances because of the principle of electroneutrality. Hypochloremia, a chloride level below 96 mEq/L, is associated with hyponatremia. It can also occur with severe vomiting and is seen as a compensatory decrease in acid–base disorders. Hyperchloremia, a chloride level above 106 mEq/L, occurs along with hypernatremia and a form of metabolic acidosis. Hypophosphatemia occurs when the level of phosphate falls below 3.0 mg/dL. It may result from use of aluminum-containing antacids that bind phosphate, from vitamin D deficiency, or from hyperparathyroidism. Hyperphosphatemia, a phosphate level above 4.5 mg/dL, commonly occurs in renal failure.
ACID–BASE BALANCE
Acid–base balance is very important to maintaining homeostasis in the body because cell enzymes can function only within a very narrow range of pH.
pH
pH is a measure of the degree to which a solution is acidic or alkaline. Cell metabolism constantly produces carbon dioxide, which combines with water to form carbonic acid (H2CO3), which immediately breaks down into hydrogen ions and bicarbonate ions. The concentration of hydrogen ions (H+) determines the pH reading. The normal serum pH is 7.35 to 7.45. Death may occur at a serum pH below 6.8 or above 7.8. Because of the production of acids by the body’s metabolic systems, there is a tendency for the body to become acidic if homeostasis is upset.
BICARBONATE
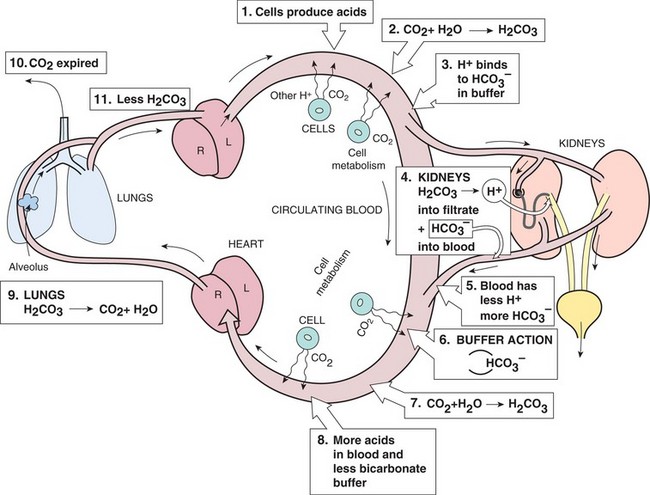
Bicarbonate is a very important substance in maintaining acid–base balance. The normal range of bicarbonate (HCO3-) is 22 to 26 mEq/L. The major function of this alkaline electrolyte is the regulation of the acid–base balance in the body. Bicarbonate acts as a buffer to neutralize the excess acids in the body and maintain the bicarbonate-to–carbonic acid ratio at 20:1, whichis needed for homeostasis. The kidneys selectivelyreabsorb or excrete bicarbonate to regulate serum levels and help maintain acid–base balance.
CONTROL MECHANISMS
For the serum pH to remain within the normal range of 7.35 to 7.45, the ratio of bicarbonate ion to carbonic acid must be 20:1. If one component of the ratio changes, the other must change proportionately to maintain the proper balance for serum pH to be within the normal range. There are three control mechanisms for pH. The first is the blood buffer system. The blood buffer system consists of weak acids and weak bases. These buffer pairs can act quickly to stabilize the serum pH. The buffer pair that is monitored in clinical settings is the sodium bicarbonate–carbonic acid buffer system. When an acid is added to the blood, it combines with the base (bicarbonate) component of the buffer, forming a weaker acid. Because weak acids do not readily release free H+, changes in serum pH are minimized. When a base is added to the blood, it combines with the acid component of the buffer to form a weaker base. The other blood buffer systems are protein buffers and phosphate buffers. They minimize pH changes but do not remove acid or base from the body.
The second control mechanism for pH is the lungs. In the lungs, the H+ ion and the HCO3− ion dissociation reaction can be reversed, and water and carbon dioxide (CO2) are reformed. The carbon dioxide and water are expired from the lungs, decreasing the amount of acid in the body. The lungs can either expel more CO2 or conserve it to help balance the pH. The respiratory system can readjust quickly to help control serum pH.
The third control mechanism for pH is the urinary system. In the kidney, enzymes promote the dissociation of carbonic acid to free hydrogen ions, which can be excreted in the urine. The bicarbonate ions are returned to the blood to restore the levels of buffer. The kidneys reduce the acid content of the serum by exchanging hydrogen for sodium with the help of aldosterone, and can neutralize acids by combining them with ammonia and other chemicals. When there is excess alkali (base), the kidney can also excrete excess bicarbonate. This compensatory ability of the kidney takes more time to work than the compensatory action in the lungs. Figure 25-6 shows the interaction of these control mechanisms.
ACID–BASE IMBALANCES
There are four types of acid–base imbalances, as shown in Table 25-5. To determine if an acid–base imbalance exists, the pH, arterial CO2 partial pressure (PaCO2), and HCO3- are measured by arterial blood gas analysis performed on a sample of arterial blood. An increase in hydrogen ions results in acidosis (decrease in pH). A decrease in hydrogen ions results in alkalosis (increase in pH). Imbalances may be acute or chronic. An initial change in carbon dioxide is nearly always due to a respiratory disorder. Disorders that show an initial change in bicarbonate ions are metabolic. The three control mechanisms continually work together to maintain acid–base balance. When an imbalance occurs, the lungs and kidneys try to compensate by working to bring the pH back toward normal limits.
Table 25-5
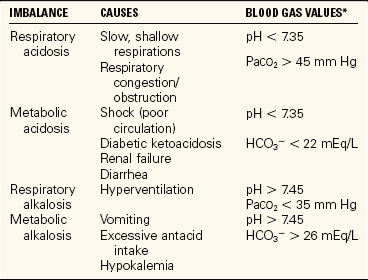
*Normal blood gas values: pH: 7.35-7.45; PaO2: 80–100 mm Hg; PaCO2: 3-45 mm Hg; HCO3−: 22–26 mEq/L.
RESPIRATORY ACIDOSIS
An increase in carbon dioxide levels occurs in a variety of disorders. It is seen in patients with acute problems such as airway obstruction, pneumonia, asthma, or chest injuries. It is also seen in patients taking opiates, which depress the respiratory rate. Chronic respiratory acidosis is prevalent among people with chronic obstructive pulmonary disease (COPD), also called chronic airflow limitation (CAL).
METABOLIC ACIDOSIS
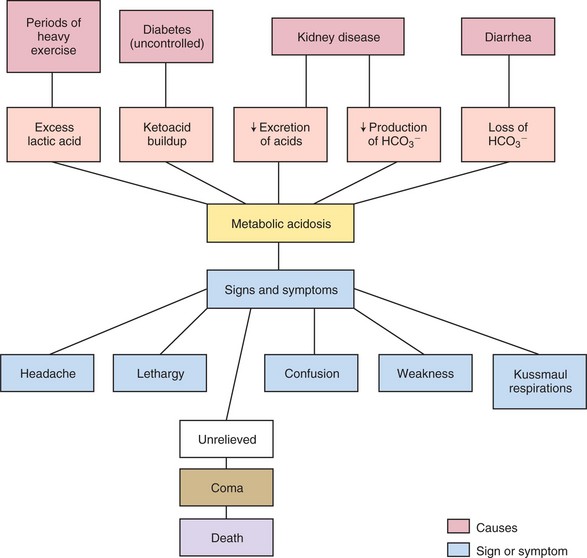
An excessive loss of bicarbonate ions or an increased production or retention of hydrogen ions leads to metabolic acidosis. The loss of bicarbonate ions with diarrhea is one cause of metabolic acidosis. Metabolic acidosis also occurs when large amounts of acid are produced within the body. This happens when more energy than usual is expended and lactic acid builds up in the body, as occurs when oxygenation of tissue falls. The faulty metabolism of a diabetic patient causes a build up of ketoacids, resulting in metabolic acidosis. The other major cause of metabolic acidosis is kidney disease, in which there is decreased excretion of acids and decreased production of bicarbonate (Concept Map 25-2). In this instance, dialysis is required to maintain the pH within life-permitting limits.
Effects of Acidosis
Acidosis depresses the nervous system, causing headache, lethargy, weakness, and confusion. If the acidosis is unrelieved, coma and death will ensue. Evidence that the compensatory mechanisms are at work in metabolic acidosis is deep rapid breathing (Kussmaul’s respirations) and secretion of urine with a low pH.
RESPIRATORY ALKALOSIS
Hyperventilation (a rapid respiratory rate) results in respiratory alkalosis. It is usually caused by anxiety, high fever, or an overdose of aspirin. Head injuries may also lead to hyperventilation. Treatment for hyperventilation is to treat the underlying disorder. The person may breathe through a re-breather mask temporarily, mixing the excessively exhaled carbon dioxide with oxygen so that carbon dioxide is inhaled.
METABOLIC ALKALOSIS
Vomiting resulting in loss of hydrochloric acid from the stomach may cause metabolic alkalosis; gastrointestinal suction can occasionally cause it also.
Effects of Alkalosis
Irritability of the nervous system occurs when the pH balance shifts to alkalosis. The patient may display restlessness, muscle twitching, and tingling and numbness of the fingers. If the alkalosis progresses, tetany will occur and seizures and coma result. Tetany is characterized by severe muscle cramps, carpopedal spasms, laryngeal spasms, and stridor (shrill harsh sound upon inspiration).
More in-depth information about acid–base imbalances will be covered in your medical-surgical nursing courses and can be found in a medical-surgical nursing textbook.
APPLICATION of the NURSING PROCESS
First, assess the patient for risk of fluid, electrolyte, or acid–base imbalance. Then assess for physical signs and symptoms of alterations in normal balance. Examine laboratory test results for electrolyte levels that are outside the normal range. Evaluate blood gas determinations to determine if an acid–base imbalance exists and, if so, what type of imbalance is present. Evaluate intake and output (I & O) records to determine if there is a fluid imbalance. The urine volume the adult usually excretes in 24 hours is approximately 1500 mL. In stressful situations, it may decrease slightly from the effects of increased aldosterone and ADH. Urine concentration provides another clue to the fluid status. Urine concentration is commonly measured by the specific gravity. The concentration of urine is compared with the specific gravity of distilled water, which is 1.000. Urine contains urea, electrolytes, and other substances, so its specific gravity will exceed 1.000. Urine specific gravity normally ranges between 1.003 and 1.030. The average range is 1.010 to 1.025. The urine specific gravity is measured with a urinometer, a refractometer, or a dipstick that contains a reagent for specific gravity.
Tracking daily weight is another way to assess for alterations in fluid balance (Assignment Considerations 25-1).
Skin turgor (elastic condition) is partially dependent on the amount of tissue fluid supporting the skin. Checking skin turgor is useful when assessing fluid balance (see Figure 25-3). The sternum is one of the most reliable places to check skin turgor, particularly in the elderly.
Edema may be an indicator of fluid volume overload. Look for puffy eyelids and swollen hands. Edema may be sometimes evidenced by a pit developing when a fingertip is pressed into the tissue over a bony prominence, such as the malleolus or tibia, and held for 5 seconds. After the finger is removed, the pit slowly disappears. A better method of assessing the course of peripheral edema is to measure the circumference of the extremity in the same location each day.
Changes in vital signs are pertinent when assessing fluid, electrolyte, and acid–base balance. Fever increases fluid loss and predisposes the patient to fluid volume deficit. A pulse rate greater than 100 beats per minute (bpm) may be an early sign of decreased vascular volume from fluid volume deficit. A weak, thready pulse accompanies fluid volume deficit, and a bounding pulse is associated with fluid volume overload. Potassium and magnesium deficits may cause an irregular pulse rate. Rapid breathing may cause an alkaline blood pH by expelling large amounts of carbon dioxide, or it may be the body’s way to compensate for an acidic blood pH. Moist respiratory sounds in the absence of cardiac or respiratory disease are a sign of excess fluid in the lungs from fluid overload. Fluid overload will cause a rise in systolic blood pressure.
Severe fluid volume deficit will decrease blood flow to the brain and result in decreased sensorium and confusion. Imbalances in sodium have direct effects on the brain volume and mental function as well.
Neuromuscular irritability is assessed when imbalances in calcium and magnesium are suspected. Deep tendon reflexes may be tested to monitor neuromuscular irritability. Check for Chvostek’s sign and Trousseau’s sign when calcium or magnesium deficit is a possibility. Chvostek’s sign is assessed by tapping the facial nerve about an inch in front of the earlobe. A unilateral twitching of the face is a positive response. A blood pressure cuff is placed on the arm and inflated above systolic pressure for 3 minutes to test for Trousseau’s sign. If a spasm of the hand occurs, the reaction is positive. Deep tendon reflexes are tested by tapping a partially stretched muscle tendon with a percussion hammer. The extent of the reflex is scored from 0 to 4+, with 0 representing no response, 2+ a normal response, and 4+ a hyperactive response.
Nursing Diagnosis
Using critical thinking, the assessment database is analyzed, problem areas are identified, and nursing diagnoses are chosen. Nursing diagnoses commonly used for patients with fluid, electrolyte, or acid–base imbalances are as follows:
Other nursing diagnoses may be appropriate as a result of the fluid, electrolyte, or acid–base imbalance or may be related to the cause of the imbalance, for example, diarrhea.
Planning
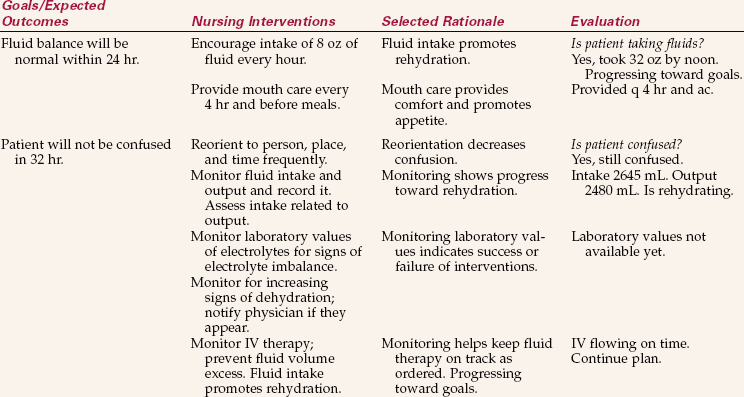
Collaboration with the patient and family or caregiver allows the best plan to be devised. Priorities of care are set. The goal is to restore the patient’s fluid, electrolyte, or acid–base balance. Individual expected outcomes are written as appropriate. Expected outcomes might be as follows:
• Patient will exhibit normal skin turgor.
• Patient’s weight will stabilize at normal baseline.
• Intake and output will be balanced.
• Blood gases will return to normal.
• Breath sounds will be clear to auscultation.
A specific time frame would be incorporated into the expected outcome. Nursing interventions are chosen to help the patient achieve the outcomes. Nursing Care Plan 25-1 presents examples of expected outcomes and nursing interventions.
Implementation
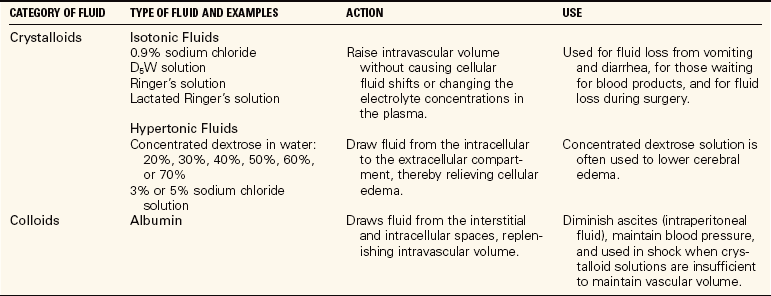
When patients are unable to take in sufficient fluids on their own, work with the physician to provide an adequate intake of fluid and electrolytes. If patients can swallow and retain fluid, assist them frequently with taking small amounts of fluid. Establish a plan for assisting with both hot and cold liquid consumption. With conscientious care, the need for intravenous feeding can be avoided. Assessment of what the patient prefers is helpful. In addition to water, fruit juices, bouillon, Popsicles, soft drinks, or gelatin can be offered. For patients who cannot drink and will need only short-term assistance, intravenous therapy is ordered (Table 25-6). See Chapter 36 for more discussion on intravenous therapies. For those who will be unable to take in fluids or food on their own for an extended period, a feeding tube must be placed or total parenteral nutrition (TPN) is started. Care of the patient with a feeding tube and TPN is discussed in Chapter 27. It is important to track the patient’s intake and output whether there is a risk of fluid volume imbalance, actual deficit, or actual overhydration (fluid volume excess).
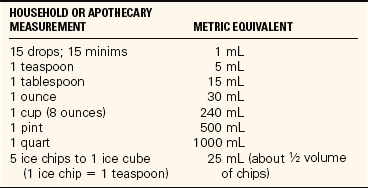
Recording Intake and Output: To check oral intake at mealtimes, look at the tray before it is removed from the room and record the intake on the shift intake and output record. Fluids that must be measured include anything that is liquid or will become liquid if left at room temperature. Foods such as ice cream, sherbet, milk shakes, gelatin, and gruel or thinned baby cereals are counted as liquids. Amounts should be converted from household measures to milliliters or cubic centimeters (Table 25-7). Most facilities provide a list of the equivalent amounts contained in the type or size of dish used by the dietary department (Table 25-8). If this information is not available, and for the home care patient, use a graduated container to measure the capacity of various dishes and glasses. The amounts the patient drinks between meals are also recorded.
Table 25-8
Common Equivalents of Food Containers*
| CONTAINER | VOLUME |
| Coffee cup | 240 mL |
| Iced tea glass | 320 mL |
| Juice glass | 120 mL |
| Wax drinking cup | 180 mL |
| Styrofoam cup | 210 mL |
| Large glass | 230 mL |
| Cream package | 15 mL |
| Sherbet | 90 mL |
| Soup, clear | 120 mL |
| Soup, thick | 180 mL |
| Gelatin | 80 mL |
| Milk carton | 240 mL |
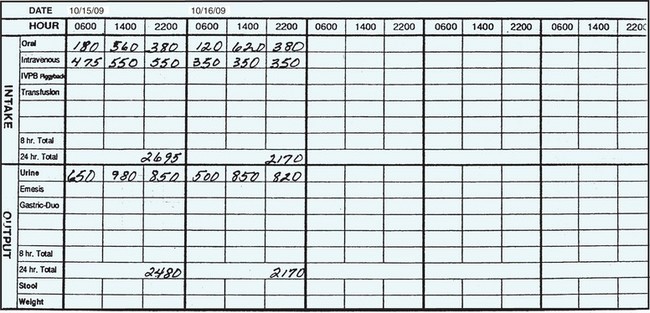
Intravenous (IV) fluid infused is added as intake. At the beginning of the shift during the report, note how much is left in an IV container that is in progress. Another IV container may be started during the shift when the old one is totally infused. By adding and subtracting, the total amount of IV intake can be calculated. For example, suppose a patient has 350 mL remaining in the IV bag at the beginning of the shift, it infuses, and a new bag is hung at 1:00 P.M. At the end of the shift, 200 mL has infused from the new bag. Add the 350 mL and the 200 mL for a total of 550 mL of IV intake for the shift. This amount is entered on the I & O record as intake under the IV column (Figure 25-7). Most fluids entering the patient’s body arerecorded as intake; blood and blood products administered are recorded separately.
Fluid output is also counted and recorded. The average urinary output in a 24-hour period is 1000 to 1500 mL; an output of less than 600 mL in a 24-hour period, or less than 30 mL/hr, should be reported to the charge nurse or physician. To measure urinary output, pour the urine from the bedpan or urinal into a graduated container. (Gloves are always worn when handling urine containers.) The container is placed on a flat surface and the level of fluid is read at eye level. The amount is recorded on the I & O record under output. For the ambulatory patient, give instructions about saving urine and provide a collection container for the toilet. Ask the patient to call when the container needs emptying or show the patient how to measure and record the urine before disposing of it. Urine drainage bags are emptied before they become full or at least once a shift. The amount is measured and recorded on the I & O record.
Output from all other sources is measured, including drainage from nasogastric tubes, chest tubes, and wound drainage suction devices. If the patient has diarrhea, the liquid stool can be measured in the same manner as urine. Profuse perspiration that requiresa change of dampened linen is noted on the I & Orecord according to agency protocol. Emesis is also measured and recorded as output. At the end of each shift, the total intake and total output are tallied and entered on the 24-hour I & O record in the chart. You do not need a physician’s order for instituting therecording of I & O; this is a nursing responsibility(Skill 25-1).
The patient with a fluid volume excess may have an order for fluid restriction. This means that the patient may have only a certain amount of fluid over a 24-hour period. Work out a schedule of fluid intake so that liquids are spaced evenly and the patient does not receive all the allotted liquids in a short time. A typical schedule would be day: 600 mL; evening: 400 mL; night: 200 mL. If not prohibited, hard candies and chewing gum can help relieve thirst. Frequent oral care is essential.
Diuretics are often prescribed, particularly when there is a potential for congestive heart failure or pulmonary edema. Daily weight and electrolyte status must be monitored along with intake and output for these patients.
Skin care is particularly important in preventing a breakdown over an edematous area. The stretched skin is extremely fragile, has a decreased blood supply, and is no longer flexible. Keep bed linens dry and smooth and turn the patient frequently to relieve pressure over bony prominences. Be very gentle in repositioning and turning the patient to avoid friction on the skin; use a lift sheet. A break in edematous skin can quickly form a pressure ulcer.
The patient with a fluid volume excess may be placed on sodium restriction because sodium usually is retained along with water. Table salt is prohibited, and special attention must be paid to the foods and fluids allowed the patient. Items that should be particularly avoided are listed in Patient Teaching 25-1.
Laboratory values for electrolytes and acid–base balance are monitored to determine if treatment is effective, and imbalances are being corrected. When potassium is ordered, the level is checked before administering the next dose (Safety Alert 25-2). Urine output is assessed to ensure adequate flow. Orders for fluids are carefully checked before a new intravenous infusion is begun. Assessment for fluid imbalance is ongoing.
For the home care patient, thorough teaching is performed so that the patient can meet the requirements for fluid intake or restriction. Adherence to sodium restriction is monitored by checking the food intake of the patient periodically. Obtaining feedback regarding comprehension of instructions is a vital part of the teaching. Collaboration with the patient on the plan of care is essential to obtain patient compliance.
When acid–base imbalance occurs, control of the underlying disorder is instituted. Blood gases are monitored, and oxygen and electrolytes are administered as needed. Nursing measures to improve pulmonary function are instituted as appropriate.
Evaluation
Every 24 hours, evaluation is performed to see if the nursing interventions are assisting the patient to meet expected outcomes. If the patient is not progressing toward achievement of the outcomes, problem solving and critical thinking are used to determine why. The plan of care is altered appropriately. When outcomes are met, that portion of the plan is discontinued.
NCLEX-PN® EXAMINATION–STYLE REVIEW QUESTIONS
Choose the best answer(s) for each question.
1. Which of the following patients would be at risk for a fluid imbalance? The patient:(Select all that apply.)
1. with a history of congestive heart failure.
3. who is 5 days postoperative after abdominal surgery.
2. The elderly individual is at greater risk for dehydration than the middle-aged adult because: (Select all that apply.)
1. the elderly have a diminished sense of thirst.
2. the elderly have less muscle mass as years advance.
3. Your patient has been ordered to receive nothing by mouth today because of scheduled diagnostic tests. Even without fluid intake, what amount of fluids would be lost, if any?
1. The 200 mL normally produced by metabolism of food
3. Only the minimum amount of urine needed to excrete wastes
4. The patient who ate a serving of soup (120 mL), a container of gelatin (80 mL), a glass of iced tea (320 mL), and a serving of sherbet (90 mL) had an intake of __________ mL.
5. An 82-year-old patient is admitted with vomiting and diarrhea. On assessment, you note that he is apprehensive and his skin is cool and pale. His pulse is rapid and his blood pressure is lower. These symptoms are indicative of:
6. Your patient has been ill with pneumonia and was admitted yesterday to start IV antibiotic therapy. He has a history of congestive heart failure and is taking digitalis and furosemide. He has not been eating well the past 3 days because he was feeling so bad. The physician ordered fluids and daily lab work yesterday. The patient seems more confused this morning and is very weak, and his urinary output has fallen. In order to determine what is causing these symptoms, you would first:
7. A patient has food poisoning and has been vomiting frequently for the past 8 hours. If this continues, he will likely develop:
8. Patients who are undergoing diuretic therapy to decrease excess body fluid tend to lose potassium. If too much potassium is lost, the patient will have which of the following electrolyte and acid–base imbalances?
1. Hyperkalemia; metabolic acidosis
2. Hypokalemia; metabolic alkalosis
9. One of the best methods to assess whether peripheral edema is increasing or decreasing is to:
CRITICAL THINKING ACTIVITIES ? Read each clinical scenario and discuss the questions with your classmates.
George Torres is admitted with a head injury. He is comatose and is breathing rapidly. His blood gases show a pH of 7.37, PaCO2 of 32, and HCO3− of 26. Compare these gases to normal values. What type of acid–base imbalance does this patient have?
Scenario B
Lena Mason, who has diabetes, is admitted in a stuporous condition. Her blood gases show a pH of 7.33, PaCO2 of 40, and HCO3− of 20. What type of acid–base imbalance does this patient have?