Waxes
Few procedures in restorative dentistry can be completed without the use of wax in one of its many forms. Forming an inlay pattern, boxing an impression before it is poured in dental stone, and making a baseplate for a removable denture each requires a specially formulated wax. Some uses for various dental waxes are shown in Fig. 14-1. These examples display how the tasks these waxes perform, and therefore their properties, vary greatly. Accuracy is a requisite for inlay or removable denture patterns, shown in the upper left of Fig. 14-1, whereas for the boxing of an impression, shown in the upper center, the ease and convenient manipulation of the wax are essential. Other applications, such as the denture forms shown in the lower left or the corrective impression wax shown in the upper right of Fig. 14-1, require equally varying qualities in the waxes, as do the applications of wax for the border and palate of the metal tray or for attaching a plaster splint to the model, as shown in the lower right of the figure. Thus, the specific use of the dental wax determines the physical properties that are most desirable for a successful application.
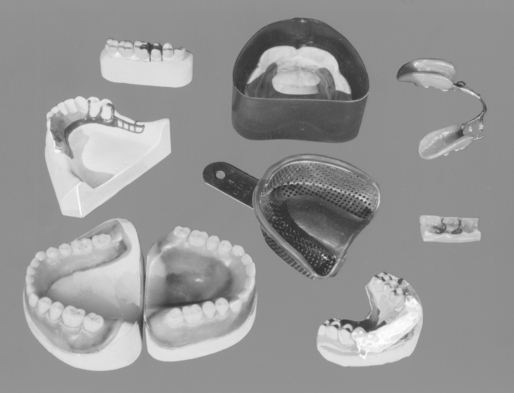
FIGURE 14-1 Applications of waxes in dentistry. Inlay pattern, upper left; boxing of an impression, upper center; corrective impression, upper right; baseplate, lower left; sticky wax, lower right; casting wax, left center; utility wax, center; and occlusal registration, right center.
WAXES, GUMS, FATS, AND RESINS
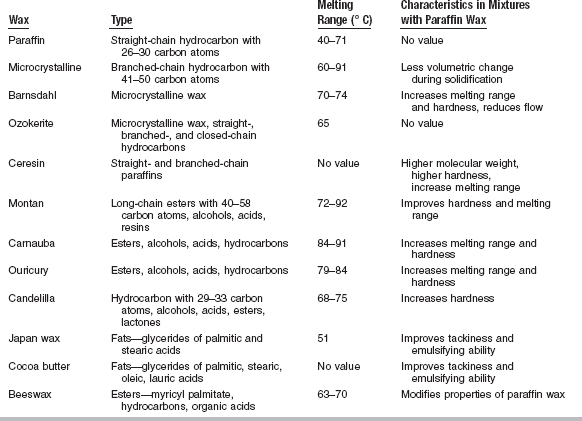
Dental waxes may be composed of natural and synthetic waxes, gums, fats, fatty acids, oils, natural and synthetic resins, and pigments. The particular working characteristics of each wax are achieved by blending the appropriate natural and synthetic waxes and resins and other additives, some of which are shown in Table 14-1.
The chemical components of both natural and synthetic waxes impart characteristic physical properties to the wax, which are of primary interest because the specific physical properties of a wax or wax blend determine its usefulness for intended applications. Natural waxes are distributed in nature, whereas synthetic waxes are produced by combination of various chemicals in the laboratory or by chemical action on natural waxes. The additives may be natural materials and synthetic products.
NATURAL WAXES
Historically, waxes have been classified according to their origin: (1) mineral, (2) plant, (3) insect, and (4) animal; however, a better classification is based on their chemical composition. The two principal groups of organic compounds contained in waxes are hydrocarbons and esters, although some waxes contain free alcohols and acids as well.
The chief constituents of most mineral waxes are hydrocarbons ranging from 17 to more than 44 carbon atoms, a fact that accounts for odd and even numbers in the chain, as shown in the following formula:
CH3—(CH2)—CH3 15 to 42 carbon atoms
The hydrocarbons in plant waxes are saturated alkanes with from 19 to 31 carbon atoms present in odd numbers. Therefore, dental waxes contain molecules having a range of molecular weights that affect the melting and flow properties of the waxes.
Plant and animal waxes contain considerable concentrations of esters, and carnauba (a plant wax) contains 85% alkyl esters of various kinds. The principal ester in beeswax is myricyl palmitate,
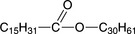
which is the reaction product of myricyl alcohol and palmitic acid. Plant and animal waxes also contain acids, alcohols, hydrocarbons, and resins; whereas Montan wax (an earth wax) contains large amounts of esters, the main compound being
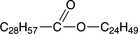
However, there are other esters composed of C20—C29 acids and C20—C30 alcohols.
This brief description of the composition of natural waxes indicates that they are complex combinations of organic compounds of reasonably high molecular weights. Also, the composition of these waxes varies, depending on the source and the time of collection; therefore, dental manufacturers must blend the particular batches of wax to obtain the properties desired for a particular application. Characteristics of various waxes used in dentistry and described here are summarized in Table 14-2.
Paraffin waxes are obtained principally from the high boiling point fractions of petroleum. The melting temperatures generally increase with increasing molecular weights. The presence of oils in the wax, however, lowers the melting temperature; paraffin waxes used in dentistry are refined waxes and have less than 0.5% oil.
Paraffin waxes produced by current refining procedures can crystallize in the form of plates, needles, or malcrystals, but are usually of the plate type. Many hydrocarbon waxes undergo crystalline changes on cooling, and a transition from needles to plates occurs about 5° to 8° C below their melting temperature. During solidification and cooling, there is a volumetric contraction that varies from 11% to 15%. This contraction is not uniform throughout the temperature range from the melting temperature to room temperature, because the wax is a mixture of hydrocarbons and the wax passes through transition points accompanied by changes in physical properties.
Microcrystalline waxes are similar to paraffin waxes, except they are obtained from the heavier oil fractions in the petroleum industry and, as a result, have higher melting points. These waxes crystallize in small plates and are tougher and more flexible than paraffin waxes. They have an affinity for oil, and their hardness and tackiness may be altered by adding oil. Microcrystalline waxes have less volumetric change during solidification than paraffin waxes.
Barnsdahl is a microcrystalline wax used to increase the melting range and hardness and reduce the flow of paraffin waxes.
Ozokerite is an earth wax found near petroleum deposits in central Europe and the western United States. Ozokerite is similar to microcrystalline wax in that it is composed of straight- and branched-chain hydrocarbons, but it also contains some closed-chain hydrocarbons. It also has great affinity for oil, and in quantities of 5% to 15% greatly improves the physical characteristics of paraffins in the melting range of 54° C.
Ceresin is a term used to describe waxes from wax-bearing distillates from natural-mineral petroleum refining or lignite refining. Like microcrystalline waxes, they are straight- and branched-chain paraffins, but they have higher molecular weights and greater hardness than hydrocarbon waxes distilled from the crude products. These waxes also may be used to increase the melting range of paraffin waxes.
Montan waxes are obtained by extraction from various lignites, and although they are mineral waxes, their composition and properties are similar to those of the plant waxes. Montan waxes are hard, brittle, and lustrous; they blend well with other waxes, and therefore are often substituted for plant waxes to improve the hardness and melting range of paraffin waxes.
Carnauba and ouricury waxes are composed of straight-chain esters, alcohols, acids, and hydrocarbons. They are characterized by high hardness, brittleness, and high melting temperatures. Both possess the outstanding quality of increasing the melting range and hardness of paraffin waxes; for example, adding 10% of carnauba wax to paraffin wax with a melting range of 20° C increases the melting range to 46° C. Adding ouricury waxes produces a similar effect, but they are less effective than carnauba wax.
Candelilla waxes consist of 40% to 60% paraffin hydrocarbons containing 29 to 33 carbon atoms, accompanied by free alcohols, acids, esters, and lactones. Like carnauba and ouricury wax, they harden paraffin waxes but are not so effective for increasing the melting range.
Japan wax and cocoa butter are not true waxes; they are chiefly fats. Japan wax contains the glycerides of palmitic and stearic acids and highermolecular-weight acids; cocoa butter is completely fat and composed of glycerides of stearic, palmitic, oleic, lauric, and lower fatty acids. Japan wax is a tough, malleable, and sticky material that melts at about 51° C, whereas cocoa butter is a brittle substance at room temperatures. Japan wax may be mixed with paraffin to improve tackiness and emulsifying ability, and cocoa butter is used to protect against dehydration of soft tissues and to protect glass ionomer products temporarily from moisture during setting or from dehydrating after they are set.
Beeswax is the primary insect wax used in dentistry. It is a complex mixture of esters plus saturated and unsaturated hydrocarbons and high-molecular-weight organic acids. It is a brittle material at room temperature but becomes plastic at body temperature. It is used to modify the properties of paraffin waxes, and is the main component in sticky wax.
Animal waxes such as spermaceti wax, obtained from the sperm whale, are not used extensively in dentistry; like beeswax, they are mainly ester waxes. Spermaceti wax has been used as a coating in the manufacture of dental floss.
SYNTHETIC WAXES
In recent years synthetic waxes and resins have become available. Although the use of synthetic waxes and resins is increasing, it is still limited in dental formulations, and the natural waxes continue to be the primary components.
Synthetic waxes are complex organic compounds of varied chemical compositions. Although they differ chemically from natural waxes, they possess certain physical properties, such as melting temperature or hardness, which are akin to those of the natural waxes. They may differ from natural waxes in certain characteristics because of their high degree of refinement, in contrast with the contamination that is common in natural waxes.
Synthetic waxes include (1) polyethylene waxes, (2) polyoxyethylene glycol waxes, (3) halogenated hydrocarbon waxes, (4) hydrogenated waxes, and (5) wax esters from the reaction of fatty alcohols and acids. Polyethylene polymers having molecular weights from 2000 to 4000 are waxes melting at 100° to 105° C. These waxes possess properties similar to high-molecular-weight paraffin waxes obtained from petroleum. Polyoxyethylene waxes are polymers of ethylene glycols and have melting temperatures from 37° to 63° C. They have limited compatibility with other waxes but do function as plasticizers and tend to toughen films of wax. The remaining synthetic waxes are prepared by reactions with natural waxes or wax products, as with chlorine in the preparation of halogenated waxes and hydrogen in the manufacture of hydrogenated waxes. The variability of various batches of synthetic wax is similar to that of natural waxes.
GUMS
Many waxes obtained from plants and animals resemble in appearance a group of substances described as gums. Many plants produce a variety of gums that are viscous, amorphous exudates that harden on exposure to air. Most gums are complicated substances. Many are mixtures containing largely carbohydrates; when they are mixed with water, they either dissolve or form sticky, viscous liquids. Gum arabic and tragacanth are two natural gums that do not resemble waxes in either their properties or composition.
FATS
As a class of substances, waxes are harder and have higher melting temperatures than fats, but in some ways they resemble fat. Both are tasteless, odorless, and colorless in the pure form, and they usually feel greasy. Chemically, fats are composed of esters of various fatty acids with glycerol and are known as glycerides, which distinguish them from waxes. Some examples of fats are glycerides of stearic acid or tristearate, found in tallow, and the mixed glyceride of oleic, palmitic, and butyric acids, found in butter.
Glyceryl tristearate, the chief ingredient of beef tallow, is a fat with a melting temperature of about 43° C. It has a lustrous appearance and is a firm, slightly greasy solid that bears a resemblance to waxes. The fat may be used to increase the melting range and hardness of compounded wax. Oils have a pronounced effect on the properties of waxes, as mentioned earlier in connection with the discussion of paraffin waxes. Hydrocarbon oils may be used to soften mixtures of waxes, and small quantities of silicone oils may be added to improve the ease of polishing with waxes.
RESINS
In some respects natural resins resemble waxes in appearance and properties, although they form a distinct classification of substances. Many species of trees and other plants produce exudates of natural resins, such as dammar, rosin, or sandarac. Natural resins are relatively insoluble in water, but vary in solubility in certain organic liquids. Generally, resins are complex, amorphous mixtures of organic substances that are characterized by specific physical behavior rather than by any definite chemical composition. Most natural resins are obtained from trees and plants; shellac, however, is produced by insects. Numerous natural resins are blended with waxes to develop waxes for dental applications.
Natural resins such as dammar and kauri may be mixed with waxes. They are compatible with most natural waxes and produce harder products. Synthetic resins, such as polyethylene and vinyl resins of various types, may be added to paraffin waxes to improve their toughness, film-forming characteristics, and melting ranges.
Natural and synthetic resins may also be used in organic solvents to produce film-forming materials that may be used as a cavity liner. Copal is a natural, brittle resin that has a melting range well above 149° C, but when deposited as a film from an organic solvent, it serves as a liner for prepared cavities. Polystyrene is a synthetic resin that may be used in a similar manner.
CHARACTERISTIC PROPERTIES OF WAXES
Useful and important properties of waxes include melting range, thermal expansion, mechanical properties, flow, residual stress, and ductility.
MELTING RANGE
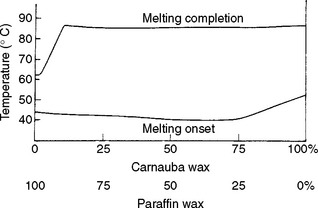
Because waxes may contain several types of molecules, each having a range of molecular weights, they have melting ranges rather than melting points. The melting ranges of a paraffin wax, a carnauba wax, and a mixture of these two waxes are illustrated in Fig. 14-2. The curves are differential thermograms obtained in the manner described in the Thermal Properties section of Chapter 3. The melting range for paraffin wax is from 44° to 62° C, and for carnauba wax, from 50° to 90° C. When a mixture of 75% paraffin and 25% carnauba wax was prepared, the paraffin component melted at essentially the same temperatures, but the melting temperature of the carnauba wax was decreased slightly. Note that adding carnauba to paraffin wax dramatically increased the melting range to 44° C, compared with 18° C for paraffin alone.
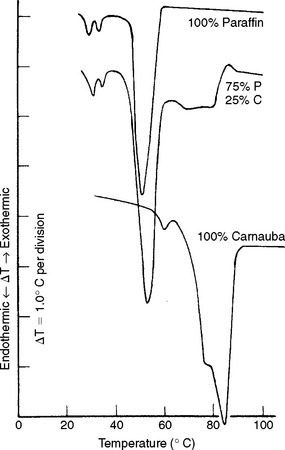
FIGURE 14-2 Differential thermograms of paraffin, carnauba, and a 75% paraffin–25% carnauba wax mixture.
The effect of the composition of paraffincarnauba mixtures on the melting range is shown in Fig. 14-3. The presence of 2.5% carnauba wax had little effect on the melting range, but the range increased rapidly as the concentration of carnauba wax was increased to 10%. Although concentrations of carnauba wax greater than 10% had no further effect on the melting range, higher amounts are necessary for certain applications to control the flow and mechanical properties.
THERMAL EXPANSION
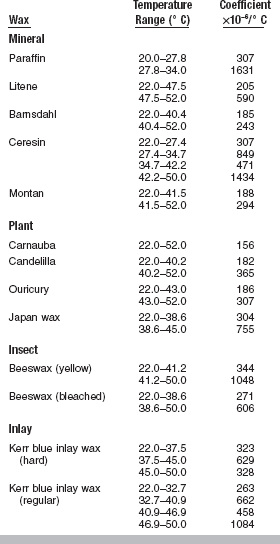
Like other materials, waxes expand when subjected to a rise in temperature and contract as the temperature is decreased. This fundamental property may be altered slightly when various waxes are blended (Fig. 14-4), but the response to thermal changes cannot be reduced to negligible values. The expansion and contraction of dental waxes with changes in temperature are pronounced, as is illustrated in Table 14-3. In general, dental waxes and their components have the largest coefficient of thermal expansion of any material used in restorative dentistry.
The linear thermal expansion properties of waxes may be explained on the basis of the strength of secondary valence forces and the transition points. Mineral waxes generally have higher coefficients of linear thermal expansion than plant waxes. Mineral waxes expand more because they have weak secondary valence forces, which are easily overcome by the energy absorbed during a rise in temperature. This permits more movement of the wax components, thus allowing a greater amount of thermal expansion.
Plant waxes, on the other hand, have high secondary valence forces because of their high concentrations of esters. Because the secondary valence forces restrict the movement of the wax components, small coefficients of thermal expansion are observed until the melting range of the wax is approached. This phenomenon is illustrated by beeswax; yellow beeswax has much higher coefficients of linear thermal expansion than bleached beeswax.
Many waxes exhibit at least two rates of expansion between 22° and 52° C. These changes in rate of expansion occur at transition points. At these points the internal structural parts become freer to move. For example, during this transition hydrocarbon chains of a mineral wax become free to rotate; consequently, after a wax has been heated through a transition point, it is freer to expand. Because the ingredient waxes are undergoing transitions that do not coincide with one another, certain inlay waxes exhibit more than two changes in rate of expansion.
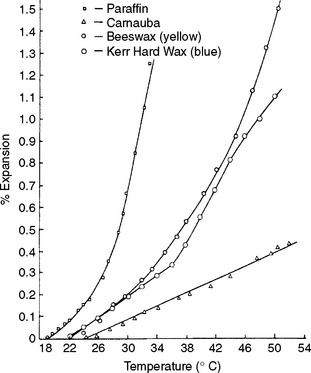
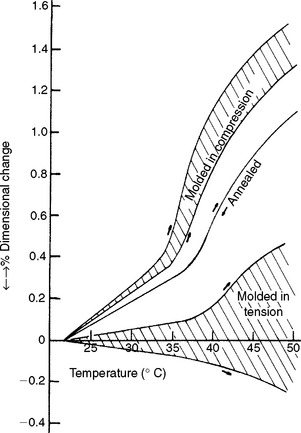
Some waxes have different rates of expansion in different temperature ranges, as seen by the change of shape of the curves for paraffin, beeswax, and an inlay wax in Fig. 14-4. Because the coefficient of thermal expansion of inlay wax is so great, temperature changes in wax patterns after the critical dimensional relationships are finalized during fabrication may be a major contributing factor in inaccuracy of the finished restoration.
MECHANICAL PROPERTIES
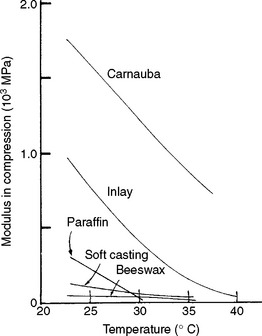
The elastic modulus, proportional limit, and compressive strength of waxes are low compared with those of other materials, and these properties depend strongly on the temperature. The elastic moduli of various waxes between 23° and 40° C are shown in Fig. 14-5, with carnauba wax having the highest values and beeswax the lowest. For example, the elastic modulus of inlay wax, which simulates a mixture of 75% paraffin and 25% carnauba wax, showed a sharp decrease in modulus from 760 to 48 MPa between 23° and 40° C.
The proportional limits and the compressive strengths of the waxes shown in Fig. 14-5 exhibit the same trends as their elastic moduli. The proportional limit of inlay casting wax experienced a decrease in proportional limit of 4.8 to 0.2 MPa from 23° to 40° C. The compressive strength of inlay wax decreased from 83 to 0.5 MPa over the same temperature range, and the percent compression at rupture varied from 2.7% to 4.3%. Hence the inlay wax would be considered a brittle material, although it possesses flow or viscous properties at stresses below its proportional limit.
FLOW
The property of flow results from the slippage of molecules over each other. A measure of flow in the liquid state of wax would be synonymous with viscosity. Below the melting point of the wax, however, a measure of the flow actually would be a measure of the degree of permanent deformation of the material at a given temperature. Flow is decidedly dependent on the temperature of the wax, the force bringing about the deformation, and the time the force is applied, as shown in Fig. 14-6. Flow greatly increases as the melting point of the wax is approached. Although a high percentage of flow at a given temperature may be required for a specific wax, it may be extremely deleterious at a temperature a few degrees lower. This is especially true for direct inlay wax (although the technique is no longer common). This material must have a relatively high flow a few degrees above mouth temperature so it is workable but not uncomfortably warm when placed in the mouth of the patient. At mouth temperature, an inlay wax to be used for a direct pattern must have essentially no flow to minimize the possibility of distortion of the pattern during removal from the tooth cavity.
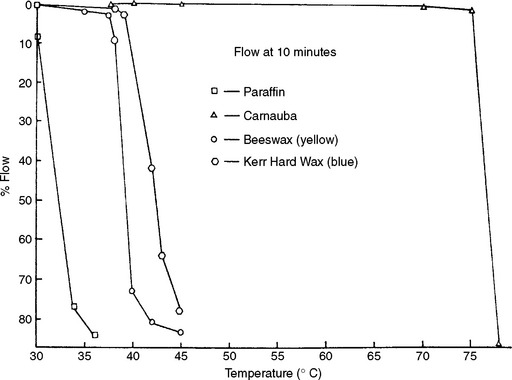
The flow of various waxes at different temperatures is shown in Fig. 14-6. Yellow beeswax does not flow extensively until it reaches 38° C, and at 40° C it flows about 7%. From these data it is easy to understand why beeswax has been used as a major ingredient in dental impression wax. Many mineral waxes have about a 10° C range between 1% and 70% flow, which indicates that these waxes soften gradually over a broad temperature range. The secondary valence forces in these waxes, which are straight- or branched-chain hydrocarbons, are rather weak and are gradually dissipated as the temperature is increased.
Montan wax, another mineral wax, requires a temperature of 71° C, or 8° C below its melting range, to flow 50%. However, this wax is similar to the plant waxes in that it is composed mainly of esters formed in nature by the union of higher alcohols with higher fatty acids. The plant waxes likewise require temperatures close to their melting range to produce 50% flow. As a result of the presence of ester groups in these waxes, the secondary valence forces are rather strong, and a high temperature is necessary to overcome these forces. Once the secondary valence forces are overcome, these waxes flow rapidly. Below this point they often appear to fracture in a manner similar to a brittle material.
Yellow beeswax, which is also primarily an ester wax, flows extensively 24° C below its melting range (61° to 63° C) and displays an 8° C temperature difference between 1% and 70% flow. This wax contains a large number of impurities, which interfere with the secondary valence forces. As beeswax goes through the bleaching process and some of these impurities are removed, the secondary valence forces increase, and the temperature difference between 1% and 70% flow is only 4° C. Note that the flow of various batches of yellow beeswax shows that significant differences may exist between batches. A similar observation is seen with paraffin and carnauba wax.
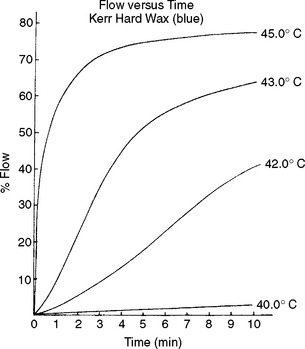
A plot of percent flow versus time for a hard inlay wax shows that at 40° C the amount of flow in relation to time is linear (Fig. 14-7). The total amount of flow after 10 minutes at this temperature is only 2%. At 42° C, the flow increases enough to cause an increase in the rate of flow. At 43° and 45° C, the rate of flow is very large at the beginning of the test, and the rate decreases rapidly as a result of the increase in diameter of the specimen.
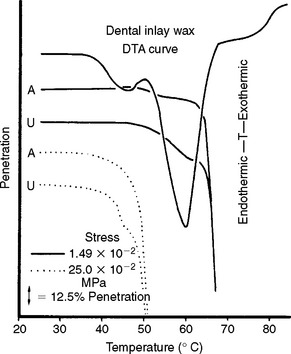
The flow of dental waxes is influenced by the presence of solid-solid and melting transformations that occur in the component waxes. The transformation temperatures can be related to flow indirectly by studying the resistance of the wax to penetration as a function of temperature. In Fig. 14-8, penetration thermograms are compared with a differential thermal analysis curve for an inlay casting wax for annealed, A, and unannealed, U, specimens tested at two stress levels. At the lower stress level, the high-melting point ester component of the wax influenced penetration. However, at a high stress level the temperature of the solid-solid transformation associated with the hydrocarbon component of the wax determined the resistance to penetration. Annealing the wax in an oven at 50° C for 24 hours before testing had the effect of increasing the resistance of the wax to penetration.
RESIDUAL STRESS
Regardless of the method used to prepare a wax pattern, residual stress exists in the completed pattern. The presence of residual stress can be demonstrated by comparing the thermal expansion curves of annealed wax with wax that has been cooled under compression or tension. The thermal expansion of an annealed inlay wax is shown in Fig. 14-9, in which the same curve is obtained on heating or cooling. When the wax specimen is prepared by holding the softened wax under compression during cooling, followed by the determination of the thermal expansion, the thermal expansion is greater than for the annealed specimen. The extent of the deviation from the curve for the annealed wax is a function of the magnitude of the residual internal stress and the time and temperature of storage of the specimen before the thermal expansion curve is determined. Therefore, a shaded area is shown rather than a specific curve. When the wax specimen is cooled while being subjected to tensile stress and the thermal expansion is determined, the curve for the wax specimen is lower than that for the annealed specimen. If sufficient residual stress is introduced, a thermal contraction may result on heating; again a shaded area indicates the direction of the effect.
DUCTILITY
Like flow, ductility increases as the temperature of a wax specimen is increased. In general, waxes with lower melting temperatures have a greater ductility at any given temperature than those with higher melting temperatures.
The ductility of a blended wax is greatly influenced by the distribution of the melting temperatures of the component waxes. A blended wax with components that have wide melting ranges generally has greater ductility than blended waxes that have a narrow range. Whenever a wide range of melting temperatures is present, the softening point of the lowest component is approached first. A further temperature rise begins to liquefy this component and approach still closer to the softening points of the higher softening point components. This tends to plasticize the entire wax mass, thereby enhancing ductility.
Generally, highly refined waxes are quite brittle. With their lower melting point, microcrystalline mineral waxes, which contain appreciable amounts of occluded oil, are moderately soft and exhibit a high degree of plasticity or ductility, even with their comparatively high melting temperatures.
DENTAL WAXES
A variety of natural waxes and resins have been used in dentistry for specific and well-defined applications. In some instances, the most favorable qualities can be obtained from a single wax, such as beeswax, but more often a blend of several waxes is necessary to develop the most desirable qualities.
A classification of dental waxes according to their use and application is given in Box 14-1. Pattern waxes are used to form the general predetermined size and contour of an artificial dental restoration, which is to be constructed of a more durable material such as cast gold alloys, cobalt-chromium alloys, or acrylic resin. All pattern waxes have two major qualities, thermal change in dimension and tendency to warp or distort on standing, which create serious problems in their use whether an inlay pattern, crown, or complete denture is being constructed.
Processing waxes are used primarily as auxiliary aids in constructing a variety of restorations and appliances, either clinically or in the laboratory. Processing waxes perform numerous tasks that simplify many dental procedures in such operations as denture construction or soldering.
One of the oldest recorded uses of wax in dentistry is for taking impressions within the mouth. Because a wax formulated for use as an impression material exhibits high flow and ductility, it distorts readily when withdrawn from undercut areas. Therefore, the use of wax has been limited to the non-undercut edentulous portions of the mouth. Recently, specially formulated addition silicone and polyether impression materials have replaced wax as an occlusal registration material.
INLAY PATTERN WAX
Gold inlays, crowns, and bridge units are formed by a casting process that uses the lost-wax pattern technique. A pattern of wax is first constructed that duplicates the shape and contour of the desired gold casting. The carved wax pattern is then embedded in a gypsum-silica investment material to form a mold with a sprue leading from the outer surface of the investment mold to the pattern, as described in Chapter 17. The wax is subsequently eliminated by heating, and the mold is further conditioned to receive the molten gold by controlled heating in a furnace.
Composition
The principal waxes used to formulate inlay waxes are paraffin, microcrystalline wax, ceresin, carnauba, candelilla, and beeswax. For example, an inlay wax may contain 60% paraffin, 25% carnauba, 10% ceresin, and 5% beeswax. Therefore, hydrocarbon waxes constitute the major portion of this formulation. Some inlay waxes are described as hard, regular (medium), or soft, which is a general indication of their flow. The flow can be reduced by adding more carnauba wax or by selecting a higher-melting point paraffin wax. An interesting example is that a hard inlay wax may contain a lower percentage of carnauba wax than a regular inlay wax, but the flow of the hard inlay wax is less than the regular wax because of the selection of a higher-melting-point paraffin in the formulation of the hard wax. Resins in small amounts, such as 1%, also affect the flow of inlay waxes. Inlay waxes are usually produced in deep blue, green, or purple rods or sticks about 7.5 cm long and 0.64 cm in diameter. Some manufacturers supply the wax in the form of small pellets or cones or in small, metal ointment jars or even in bulk.
Properties
The accuracy and ultimate usefulness of the resulting gold casting depend largely on the accuracy and fine detail of the wax pattern. A wax that is able to function well in the gold casting technique must possess certain physical properties.
ANSI/ADA Specification No. 4 (ISO 15854) for dental inlay casting wax has been formulated for waxes used in direct and indirect waxing techniques. A summary of flow requirements of this specification is given in Table 14-4. Because the wax patterns are to be melted and vaporized from the investment mold, it is essential that no excessive residue remain in the mold because of incomplete wax burnout. Excess residue may result in the incomplete casting of inlay margins. The specification therefore limits the nonvolatile residue of these waxes to a maximum of 0.10% or within ±20% of the manufacturer’s stated amount at an ignition temperature of 700° C.
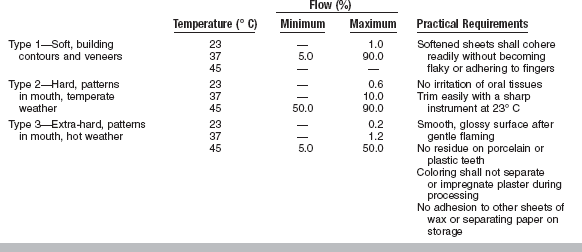
Types 1 (soft) and 2 (hard) dental inlay casting waxes are recognized by ANSI/ADA Specification No. 4. Type 1 wax is a soft wax used as an indirect technique wax. Type 2 wax is a harder wax prescribed for forming direct patterns in the mouth, where lower flow values at 37° C tend to minimize any tendency for distortion of the pattern on its removal from the cavity preparation. Type 1 wax shows greater flow than Type 2 wax at temperatures both below and above mouth temperature. The lower flow of Type 2 wax and the greater ease of carving the softer Type 1 waxes are desirable working characteristics for the techniques associated with each.
The specification also requires the manufacturers to include instructions regarding the method of softening and the working temperature for the wax preparatory to forming a direct pattern. Both types should soften without becoming flaky, and when trimmed to a fine margin during the pattern-carving operation, they should not chip or flake. Thermal expansion data for the Type 2 wax are no longer required by the specification but are sometimes provided by the manufacturer.
Flow
When forming a wax pattern directly in the mouth, the wax must be heated to a temperature at which it has sufficient flow under compression to reproduce the prepared cavity walls in great detail. The working temperature, suggested by the manufacturer, which should be satisfactory for making direct wax patterns, must not be so high as to cause damage to the vital tooth structure or be uncomfortable to the patient. Insufficient flow of the wax caused by insufficient heating results in the lack of cavity detail and introduces excess stress within the pattern. An overabundant amount of flow resulting from excessive heating makes compression of the wax difficult because of a lack of “body” in the material.
The values listed in Table 14-4 represent minimum or maximum values of percent flow that occur at various temperatures when Types 1 and 2 wax specimens are subjected to a 19.6 N load for 10 minutes. The temperature that the Type 2 wax must attain to register cavity detail is usually somewhat above 45° C. As seen from these values, the flow of the hard wax is no more than 1% at body temperature. The flow of the Type 1 wax is about 9% at this temperature. Low flow at this temperature tends to minimize distortion of a well-carved pattern as it is withdrawn from an adequately tapered cavity in the tooth.
Thermal Coefficient of Expansion
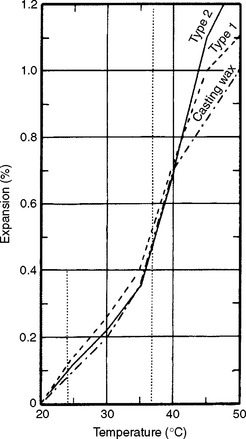
The curve in Fig. 14-10 shows that the rate of expansion of the Type I inlay wax is greatest from just below mouth temperature to just above 45° C. Knowing the amount of wax expansion or contraction allows one to judge the compensation necessary to produce an accurate casting. Data sufficient to show the thermal contraction of the wax from its working temperature to room temperature may be included in each package of inlay wax. Once the wax pattern is carved, its removal from the tooth cavity and transfer to the laboratory bring about a reduction in temperature and subsequent thermal contraction. A decrease of 12° to 13° C in temperature, from mouth temperature to a room temperature of about 24° C, causes a 0.4% linear contraction of the wax, or about 0.04% change for each degree change in temperature.
Warpage of Wax Patterns
Inlay pattern wax has a high coefficient of expansion and tends to warp or distort when allowed to stand unrestrained. The distortion is increased generally as the temperature and time of storage are increased. This quality of wax patterns is related to the release of residual stress developed in the pattern during the process of formation. This characteristic of stress release and warpage is present in all dental waxes, but is particularly troublesome in inlay patterns because of the critical dimensional relations that must be maintained in inlay castings.
Because warpage of the pattern is related to the temperature during pattern formation and storage, the rules related to the pattern temperature must be understood. In general, the higher the temperature of the wax at the time the pattern was adapted and shaped, the less is the tendency for distortion in the prepared pattern. This is reasonable, because the residual stress in the pattern causing the distortion is associated with the forces necessary to shape the wax originally. The incorporation of residual stress can be minimized by softening a wax uniformly by heating at 50° C for at least 15 minutes before use, by using warmed carving instruments and a warmed die, and by adding wax to the die in small amounts.
Because the release of internal stress and subsequent warpage are associated with the storage temperature, it follows that greater warpage results at higher storage temperatures. Lower temperature does not completely prevent distortion, but generally the amount is reduced when the storage temperature is kept to a minimum. If inlay wax patterns must be allowed to stand uninvested for a time longer than 30 minutes, they should be kept in a refrigerator. Although some distortion may take place at this temperature, it will be less than at normal room temperature. Such a practice of storage for long periods is not recommended if freedom from warpage is desired. The best way to minimize the warpage of inlay wax patterns is to invest the pattern immediately after it is completely shaped. A refrigerated wax pattern should be allowed to warm to room temperature before it is invested. During spruing, distortion can be reduced by use of a solid wax sprue or a hollow metal sprue filled with sticky wax. If the pattern was stored, the margins should be readapted. Temperature of formation, time and condition of storage, and promptness of investing the pattern are major factors related to all techniques of pattern formation.
CASTING WAX
The patterns for the metallic framework of removable partial dentures and other similar structures are fabricated from casting waxes. These waxes are available in the form of sheets, usually of 28- and 30-gauge (0.40 and 0.32 mm) thickness, ready-made shapes, and in bulk. As shown in Fig. 14-11, the ready-made shapes are supplied as round, half-round, and half-pear-shaped rods and wires of various gauges in approximately 10-cm lengths. Although casting waxes serve the same basic purpose as inlay waxes in the formation of patterns for metallic castings, their physical properties differ slightly. Little is known of the exact composition of these sheet and shaped waxes, but they include ingredients similar to those found in inlay waxes, with various combinations and proportions of paraffin, ceresin, beeswax, resins, and other waxes being used.
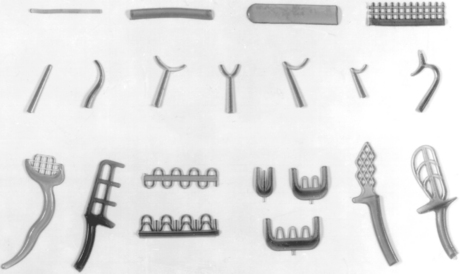
FIGURE 14-11 Wax patterns for use in fabrication of metallic framework of removable partial dentures. Preformed bars and mesh, top; clasps, center; retention forms, bottom.
The casting wax sheets are used to establish minimum thickness in certain areas of the partial denture framework, such as the palatal and lingual bar, and to produce the desired contour of the lingual bar. A partial denture framework in the process of being waxed is shown in the left center of Fig. 14-1. The physical nature and form in which the sheet casting wax is supplied result in its use for post damming of complete maxillary denture impressions, checking high points of articulation, producing wax bites of cusp tips for the articulation of stone casts, and many other uses.
Physical Characteristics
The casting sheets and ready-made shapes of certain types of casting waxes may possess a slight degree of tackiness, which helps to maintain their position on the cast and on each other during assembly of the pattern. This tackiness is not sufficient to prevent changes in position from being made with relative ease, and when the waxes are in final position, they are sealed to the investment cast with a hot spatula.
There is no ANSI/ADA specification for these casting waxes, but a federal specification has been formulated that includes values for softening temperature, amount of flow at various temperatures, general working qualities, and other characteristics. A summary of the properties included in Federal Specification No. U-W-140 is given in Table 14-5. In general, the characteristics most desired include a certain degree of toughness and strength, with a true gauge dimension, combined with a minimum of dimensional change with change in temperature, and the ability to be vaporized completely from the investment mold.
TABLE 14-5
Summary of Requirements of Federal Specifications for Dental Casting Wax

Adapted from Federal Specification No. U-W-140, March 1948, for casting wax.
Because the pattern for the removable partial denture framework is constructed on and sealed to an investment cast (from which it is not separated subsequently) at room temperature, there is little need for the casting wax to exhibit low flow at body temperature. The flow characteristics of the casting wax, when measured similarly to the inlay wax, show a maximum of 10% flow at 35° C and a minimum of 60% flow at 38° C. These characteristics are significantly different from the flow values for inlay waxes that comply with the requirements of ANSI/ADA Specification No. 4.
The requirement for ductility of the casting waxes is high. The federal specification requires that the casting wax be bent double on itself without fracture at a temperature of 23° C and that the waxes be pliable and readily adaptable at 40° to 45° C. Heating over a flame and the compression to adapt either the ready-made shapes or the sheet casting wax easily may alter their thickness and contour because of their relatively high ductility and flow.
Because these materials are casting pattern waxes for partial denture cast restorations, as is the inlay wax, they too must vaporize at about 500° C with no residue other than carbon. The mold cavity thus produced will result in more desirable casting surfaces, because it will be free of foreign materials. Pattern waxes are being replaced to some extent by preformed plastic patterns.
RESIN MODELING MATERIAL
Light-curing resins are available as low- and high-viscosity pastes and as a liquid for the fabrication of patterns for cast metal or ceramic inlays, crowns and bridges, and precision attachments (Fig. 14-12). The modeling pastes are based on diurethane dimethacrylate oligomers with 40% to 55% polyurethane dimethacrylate or poly(methyl methacrylate) fillers. The liquid consistency is mostly urethane dimethacrylate. These resins have a camphorquinone activator. Self-cured acrylic plastics used as inlay patterns are described in Chapter 21.
FIGURE 14-12 Sculpted anterior crowns made from light-cured resin modeling material. (Courtesy Heraeus Kulzer, GmbH, Wehrheim, Germany.)
Modeling resins are characterized by lower heat of polymerization and shrinkage than acrylics, higher strength and resistance to flow than waxes, good dimensional stability, and burnout without residue. Average marginal discrepancies of light-cured resin and self-cured acrylic patterns are similar to those of wax for full-crown patterns but less than those of wax for inlay patterns (Table 14-6). Dimethacrylate resin patterns do not result in cracked investment from heating during burnout, which can occur with acrylic patterns.
TABLE 14-6
Average Marginal Discrepancies (μm) for Full-Crown and Inlay Patterns Measured 1 Hour and 24 Hours after Forming
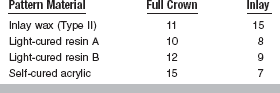
Adapted from Iglesias A, Powers JM, Pierpont HP: J Prosthodont 5:201, 1996.
Gypsum and resin dies must be treated with a separator and have undercuts blocked out. The modeling resin is applied in layers 3- to 5-mm thick, with each layer cured separately in a high-intensity, light-curing chamber for 90 seconds or by using a hand-held, light-curing unit for 20 to 40 seconds per area of irradiation. The liquid material is used first to obtain close adaptation to the die and last to provide a smooth surface. Complete elimination of modeling resins occurs between 670° to 690° C and requires about 45 minutes.
BASEPLATE WAX
Baseplate wax derives its name from its use on the baseplate tray to establish the vertical dimension, plane of occlusion, and initial arch form in the technique for the complete denture restoration. This wax also may be used to form all or a portion of the tray itself. The normally pink color provides some esthetic quality for the initial stage of construction of the denture before processing. Baseplate wax is the material used to produce the desired contour of the denture after teeth are set in position. As a result, the contour wax establishes the pattern for the final plastic denture. Patterns for orthodontic appliances and prostheses other than complete dentures, which are to be constructed of plastics, also are made of baseplate wax. Although these are the primary functions of baseplate wax, it has also been widely used in many phases of dentistry to check the various articulating relations in the mouth and to transfer them to mechanical articulators.
Composition
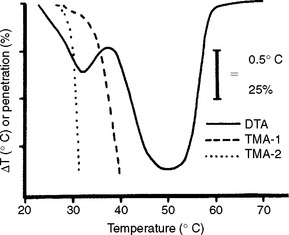
A few formulas are found in the literature for baseplate wax. Baseplate waxes may contain 70% to 80% paraffin-based waxes or commercial ceresin, with small quantities of other waxes, resins, and additives to develop the specific qualities desired in the wax. A typical composition might include 80% ceresin, 12% beeswax, 2.5% carnauba, 3% natural or synthetic resins, and 2.5% microcrystalline or synthetic waxes. Differential thermal analysis and penetration curves of a typical baseplate wax are shown in Fig. 14-13 (left).
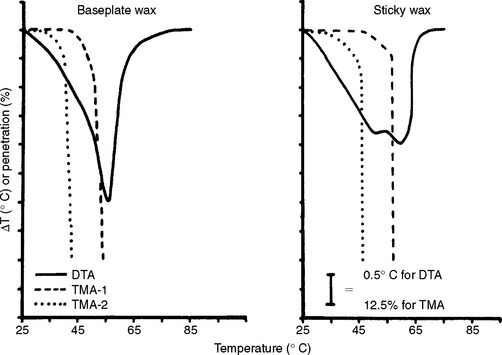
FIGURE 14-13 Differential thermal analysis and penetration curves for dental baseplate and sticky waxes. The stresses for TMA-1 and TMA-2 are 0.015 and 0.25 MPa, respectively. (Adapted from Powers JM, Craig RG: Thermomechanical analysis of dental waxes in the penetration mode. In Porter RS, Johnson JF, editors: Analytical calorimetry, vol 3, New York, 1974, Plenum.) Plenum
Physical Characteristics
Baseplate waxes are normally supplied in sheets 7.60 × 15.00 × 0.13 cm in pink or red. The manufacturer usually formulates three types of wax to accommodate the varying climates in which they will be used, because the flow of the wax is influenced greatly by the temperature.
The requirements for dental baseplate wax are listed in Table 14-7, which summarizes ANSI/ADA Specification No. 24 (ISO 15854). Three types of wax are included: Type 1 is a soft wax for building contours and veneers, Type 2 is a hard wax to be used for patterns to be tried in the mouth in temperate climates, and Type 3 is an extra-hard wax for patterns to be tried in the mouth in tropical weather. The flow values are listed for 23°, 37°, and 45° C when they are applicable. The maximum flow allowed at any given temperature decreases rapidly from Type 1 to Type 3. The flow requirements of Type 3 baseplate wax are comparable to those of the Type 2 (hard) inlay wax, with less flow allowed for the baseplate wax at 45° C.
Because baseplate wax is used both to set denture teeth and to adapt around these teeth to develop proper contour, the dimensional changes that may take place because of variations in temperature are important. Although the need for dimensional stability is not as critical as with the inlay wax, the maintenance of good tooth relationship is important. Although no shrinkage value from a molten state to room temperature is required by the specification, the linear thermal expansion from 26° to 40° C should be less than 0.8%.
A summary of practical requirements is also given in Table 14-7. Baseplate waxes should be easily trimmed with a sharp instrument at 23° C and should yield a smooth surface after gentle flaming. These waxes should not leave any residue on porcelain or plastic teeth, and the coloring agents in the wax should not separate or impregnate the plastic mold during processing.
There is residual stress within the baseplate wax that holds and surrounds the teeth of a wax denture pattern. This stress results from differential cooling, “pooling” the wax with a hot spatula, and physically manipulating the wax below its most desirable working temperature. Remember that both time and temperature affect the relief of these residual stresses; the waxed and properly articulated denture should not be allowed to stand for long periods of time, especially when subjected to elevated temperatures. Such treatment often results in distortion of the wax and movement of the teeth. The waxed denture should be flasked soon after completion to maintain the greatest accuracy of tooth relations.
BOXING WAX
To form a plaster or stone cast from an impression of the edentulous arch, first a wax box must be formed around the impression, into which the freshly mixed plaster or stone is poured and vibrated. This boxing procedure is also necessary for some other types of impressions. The boxing operation usually consists of first adapting a long, narrow stick or strip of wax around the impression below its peripheral height, followed by a wide strip of wax, producing a form around the entire impression, as seen in the upper center of Fig. 14-1.
The dental literature occasionally refers to carding wax for use in the boxing operation. Carding wax was the original material on which porcelain teeth were fixed when received from the manufacturer. The terms carding wax and boxing wax have been used interchangeably, although boxing wax is more acceptable.
The requirements of Federal Specification No. U-W-138 for boxing wax, which are summarized in Table 14-8, stipulate that this wax should be pliable at 21° C and should retain its shape at 35° C. This broadly defines its lower temperature limit of ductility and flow. Because the impression may be made from a viscoelastic material that is easily distorted, a boxing wax that is readily adaptable to the impression at room temperature is desirable. This property reduces the likelihood of distorting the impression, from the standpoint of both the temperature and stress involved in the boxing procedures. In general, boxing wax should be slightly tacky and have sufficient strength and toughness for convenient manipulation.
TABLE 14-8
Summary of Requirements of Federal Specifications for Dental Boxing, Utility, and Sticky Waxes

Adapted from Federal Specification No. A-A-51296, Dec 18, 1985, for boxing wax; Federal Specification A-A-51291, Dec 23, 1985, for utility wax; Federal Specification A-A-53639, Sep 6, 1988, for sticky wax.
UTILITY WAX
An easily workable, adhesive wax is often desired. For example, a standard perforated tray for use with hydrocolloids may easily be brought to a more desirable contour by such a wax, as shown in the center view of Fig. 14-1. This is done to prevent a sag and distortion of the impression material. A soft, pliable, adhesive wax may be used on the lingual portion of a bridge pontic to stabilize it while a labial plaster splint is poured. These and many other tasks are performed by the utility wax.
The utility wax is usually supplied in both stick and sheet form in dark red or orange. The ductility and flow of utility waxes, as indicated by the summarized requirements of Federal Specification No. U-W-156 in Table 14-8, are the highest of any of the dental waxes. The utility wax should be pliable at a temperature of 21° to 24° C, which makes it workable and easily adaptable at normal room temperature. The flow of this wax should not be less than 65% or more than 80% at 37.5° C. Because building one layer on top of another is often desirable, the specification requires a sufficient adhesiveness at 21° to 24° C. Utility wax probably consists of beeswax, petrolatum, and other soft waxes in varying proportions.
STICKY WAX
A suitable sticky wax for prosthetic dentistry is formulated from a mixture of waxes and resins or other additives. Such a material is sticky when melted and adheres closely to the surfaces on which it is applied. However, at room temperature the wax is firm, free from tackiness, and brittle. Sticky wax should fracture rather than flow if it is deformed during soldering or repair procedures. Although this wax is used to assemble metallic or resin pieces in a fixed temporary position, it is primarily used on dental stones and plasters. The lower right view of Fig. 14-1 shows an application of sticky wax to seal a plaster splint to a stone model in the process of forming ceramic facings.
According to Federal Specification No. U-W-00149a (DSA-DM), sticky wax should have a dark or vivid color so it is readily distinguishable from the light-colored gypsum materials. The specification, summarized in Table 14-8, also limits the shrinkage of sticky wax to 0.5% at temperatures between 43° and 28° C.
The literature contains several formulas for sticky wax, representing both high and low resin content. In addition to rosin and yellow beeswax, which are the usual major constituents, coloring matter and other natural resins such as gum dammar may be present. Differential thermal analysis and penetration curves for a typical dental sticky wax are shown in Fig. 14-13 (right).
CORRECTIVE IMPRESSION WAX
Corrective impression wax is used as a wax veneer over an original impression to contact and register the detail of the soft tissues. It is claimed that this type of impression material records the mucous membrane and underlying tissues in a functional state in which movable tissue is displaced to such a degree that functional contact with the base of the denture is obtained. Corrective waxes are formulated from hydrocarbon waxes such as paraffin, ceresin, and beeswax and may contain metal particles. There are no ANSI/ADA or federal specifications for corrective impression waxes. The flow of several corrective waxes measured by penetration at 37° C is 100%. Differential thermal analysis and penetration curves for a typical corrective impression wax are shown in Fig. 14-14. These waxes are subject to distortion during removal from the mouth.
OCCLUSAL (BITE) REGISTRATION WAX
Occlusal registration wax is used to accurately articulate certain models of opposing quadrants. Occlusal registrations are often made from 28-gauge casting wax sheets or from hard baseplate wax, but waxes identified as occlusal registration waxes seem to be formulated from beeswax or hydrocarbon waxes such as paraffin or ceresin. Certain occlusal registration waxes contain aluminum or copper particles. There are no ANSI/ADA or federal specifications for occlusal registration waxes. The flow of several occlusal registration waxes as measured by penetration at 37° C ranges from 2.5% to 22%, indicating that these waxes are susceptible to distortion on removal from the mouth. Occlusal registration materials made from addition silicone elastomers are becoming more popular because of their high elastic recovery and excellent dimensional stability.
Anderson, JN. Applied dental materials, ed 5. Oxford: Blackwell Scientific, 1976.
Bennett, H. Industrial waxes, 1 and 2 . Chemical Publishing: Brooklyn, 1963.
Coleman, RL. Physical properties of dental materials, US Bureau of Standards, Research Paper No 32. J Res Nat Bur Stand. 1928;1:867.
Craig, RG, Eick, JD, Peyton, FA. Properties of natural waxes used in dentistry. J Dent Res. 1965;44:1308.
Craig, RG, Eick, JD, Peyton, FA. Flow of binary and tertiary mixtures of waxes. J Dent Res. 1966;45:397.
Craig, RG, Eick, JD, Peyton, FA. Strength properties of waxes at various temperatures and their practical application. J Dent Res. 1967;46:300.
Craig, RG, Powers, JM, Peyton, FA. Differential thermal analysis of commercial and dental waxes. J Dent Res. 1967;46:1090.
Craig, RG, Powers, JM, Peyton, FA. Thermogravimetric analysis of waxes. J Dent Res. 1971;50:450.
Dirksen, LC. Composition and properties of a wax for lower impressions. J Am Dent Assoc. 1939;26:270.
Farah, JW, Powers, JM. Bite registration materials. Dent Advis. 1998;15(4):1.
Grajower, R. A new method for determining the thermal expansion of dental waxes. J Dent Res. 1978;57:659.
Grossman, LI. Dental formulas and aids to dental practice. Philadelphia: Lea & Febiger, 1952.
Hollenback, GM, Rhodes, JE. A study of the behavior of pattern wax. J South Calif State Dent Assoc. 1959;27:419.
Iglesias, A, Powers, JM, Pierpont, HP. Accuracy of wax, autopolymerized, and light-polymerized resin pattern materials. J Prosthodont. 1996;5:201.
Ito, M, Yamagishi, T, Oshida, Y, et al. Effect of selected physical properties of waxes on investments and casting shrinkage. J Prosthet Dent. 1999;75:211.
Lasater, RL. Control of wax distortion by manipulation. J Am Dent Assoc. 1940;27:518.
Ludwig, FJ, Jr. Modern instrumental wax analysis. Soap & Chem Spec. 1966;42:70.
Markley, MR. The wax pattern, Dental Clinics of North America, Symposium on Dental Materials. Philadelphia: WB Saunders, Nov 1958.
Maves, TW. Recent experiments demonstrating wax distortion on all wax patterns when heat is applied. J Am Dent Assoc. 1932;19:606.
McCrorie, JW. Dental modelling waxes A new approach to formulation. Br Dent J. 1972;132:189.
McCrorie, JW. Corrective impression waxes. Br Dent J. 1982;152:95.
Morrison, JT, Duncanson, MG, Jr., Shillingburg, HT, Jr. Wetting effects of surface treatments on inlay wax-investment combinations. J Dent Res. 1981;60:1858.
Nelson, EA. Practical applications of crowns and inlay casting technics. J Am Dent Assoc. 1940;27:588.
Ohashi, M, Paffenbarger, GC. Melting, flow, and thermal expansion characteristics of some dental and commercial waxes. J Am Dent Assoc. 1966;72:1141.
Ohashi, M, Paffenbarger, GC. Some flow characteristics at 37° C of ternary wax mixtures that may have possible dental uses. J Nihon Univ Sch Dent. 1969;11:109.
Phillips, RW, Biggs, DH. Distortion of wax patterns as influenced by storage time, storage temperature, and temperature of wax manipulation. J Am Dent Assoc. 1950;41:28.
Powers, JM, Craig, RG. Penetration of commercial and dental waxes. J Dent Res. 1974;53:402.
Powers, JM, Craig, RG. Thermal analysis of dental impression waxes. J Dent Res. 1978;57:37.
Powers, JM, Craig, RG, Peyton, FA. Calorimetric analysis of commercial and dental waxes. J Dent Res. 1969;48:1165.
Smith, DC, Earnshaw, R, McCrorie, JW. Some properties of modelling and base plate waxes. Br Dent J. 1965;118:437.
Taylor, NO. Progress report. Research on dental materials: the research program; cooperative work on inlay casting technic. J Am Dent Assoc. 1931;18:294.
Taylor, NO, Paffenbarger, GC. A survey of current inlay casting technics. J Am Dent Assoc. 1930;17:2058.
Taylor, PB. Inlay casting procedure—its evolution and the effect of manipulatory variables. In: Anderson GM, ed. Proceedings of the Dental Centenary Celebration. Baltimore: Maryland State Dental Association, 1940.
U.S. General Services Administration, Federal Supply Service, Federal Specification No. A-A-54966, July 15, 1994, Wax, Baseplate, Dental; No. A-A-51296, Dec 18, 1985, Wax, Dental (Boxing); No. A-A-53714, Sep 23, 1988, Wax, Dental (Casting); No. A-A-53337A, May 31, 1988, Wax, Dental (Impression); No. A-A-51889, Apr 6, 1987, Wax, Dental (Inlay); No. A-A-51495, July 21, 1986, Wax, Dental (Periphery); No. A-A-53639, Sep 6, 1988, Wax, Dental (Sticky); No. A-A-51291, Dec 23, 1985, Wax, Dental (Utility).
Warth, AH. The chemistry and technology of waxes, ed 2. New York: Reinhold, 1956.
Washburn, KC. Inlay wax and its manipulation. J Ill Dent. 1947;16:409.