Cast and Wrought Base-Metal Alloys
GENERAL REQUIREMENTS OF A DENTAL ALLOY
COBALT-CHROMIUM AND NICKEL-CHROMIUM CASTING ALLOYS
MICROSTRUCTURE OF CAST BASE-METAL ALLOYS
HEAT TREATMENT OF BASE-METAL ALLOYS
WROUGHT STAINLESS STEEL ALLOYS
FUNCTION OF ALLOYING ELEMENTS AND CHEMICAL RESISTANCE
STAINLESS STEEL ORTHODONTIC WIRES
STAINLESS STEEL ENDODONTIC INSTRUMENTS
Base-metal alloys are used extensively in dentistry for a variety of appliances and instruments, as shown in Box 16-1. Cast cobalt-chromium and nickelchromium alloys have been used for many years for fabricating removable partial-denture frameworks and have replaced Type IV gold alloys almost completely for this application. Cast nickel-chromium alloys are used very sparingly in fabricating crowns and bridges. These alloys were developed as substitutes for Type III gold alloys. Nickel-chromium and cobalt-chromium alloys are used in ceramic-metal restorations. Nickel-chromium alloys containing beryllium are still very popular. However, the dental laboratories using them must follow OSHA (Occupational Safety and Health Administration) guidelines for handling an occupational hazard. Standards organizations (ISO, ADA) are promoting the reduction of beryllium content to less than 0.02%. For these reasons, cobalt-chromium alloys hold better promise and future for all-metal and ceramic-metal restorations as well. These alloys are discussed in greater detail in Chapter 19. Titanium and titanium alloys are used in cast and wrought forms for crowns, bridges, implants, orthodontic wires, and endodontic files. Stainless steel alloys are used principally for orthodontic wires, in fabricating endodontic instruments, and for preformed crowns.
Dental applications of cast and wrought base-metal alloys are summarized in Box 16-1.
GENERAL REQUIREMENTS OF A DENTAL ALLOY
The metals and alloys used as substitutes for gold alloys in dental appliances must possess certain minimal fundamental characteristics:
1. The alloy’s chemical nature should not produce harmful toxicologic or allergic effects in the patient or the operator.
2. The chemical properties of the appliance should provide resistance to corrosion and physical changes when in the oral fluids.
3. The physical and mechanical properties, such as thermal conductivity, melting temperature, coefficient of thermal expansion, and strength, should all be satisfactory, meeting certain minimum values and being variable for various appliances.
4. The technical expertise needed for fabrication and use should be feasible for the average dentist and skilled technician.
5. The metals, alloys, and companion materials for fabrication should be plentiful, relatively inexpensive, and readily available, even in periods of emergency.
This list of requirements for the ideal substitute for dental gold alloys calls attention to the fact that a combination of chemical, physical, mechanical, and biological qualities is involved in the evaluation of each alloy. Properties depend on material, compositional, and processing factors.
Cast and wrought base-metal alloys, including cobalt-chromium-nickel, nickel-chromium-iron, commercially pure titanium, titanium-aluminumvanadium, stainless steel, nickel-titanium, and titanium-molybdenum (beta-titanium) alloys are discussed in this chapter. The discussion is based on the synergistic relationship between processing, composition, structure, and properties of the materials.
COBALT-CHROMIUM AND NICKEL-CHROMIUM CASTING ALLOYS
Ever since cobalt-chromium casting alloys became available for cast removable partial-denture restorations, they have continued to increase in popularity. It was estimated as early as 1949 that more than 80% of all removable partial-denture appliances were cast from cobalt-chromium alloys. By 1969, more than 87% of all removable partial-denture appliances made in this country were cast from some type of base-metal alloy. Currently, almost all the metal frameworks of removable partial-denture appliances are made from cobalt-chromium or nickel-chromium alloys.
ANSI/ADA SPECIFICATION NO. 14
According to ANSI/ADA Specification No. 14 (ISO 6871), the weight of chromium should be no less than 20%, and the total weight of chromium, cobalt, and nickel should be no less than 85%. Alloys having other compositions may also be accepted by the ADA, provided the alloys comply satisfactorily with requirements on toxicity, hypersensitivity, and corrosion. Elemental composition to the nearest 0.5% must be marked on the package, along with the presence and percentage of hazardous elements and recommendations for processing the materials. The specification also requires minimum values for elongation (1.5%), yield strength (500 MPa), and elastic modulus (170 GPa).
An important feature of this specification is that it made available a standardized method of testing, which has, in turn, made it possible to compare results from one investigation with those of another.
COMPOSITION
The principal elements present in cast base metals for partial dentures are chromium, cobalt, and nickel, which together account for 82 to 92 wt% of most alloys used for dental restorations. Representative compositions of six commercial dental casting alloys, including four that are used for ceramic-metal restorations, are listed in Table 16-1. Chromium, cobalt, and nickel compose about 85% of the total weight of these alloys, yet their effect on the physical properties is rather limited. As discussed in this chapter, the physical properties of these alloys are controlled by the presence of minor alloying elements such as carbon, molybdenum, beryllium, tungsten, manganese, nitrogen, tantalum, gallium, and aluminum.
TABLE 16-1
Composition of Major Cast Base-Metal Alloys Used in Dentistry
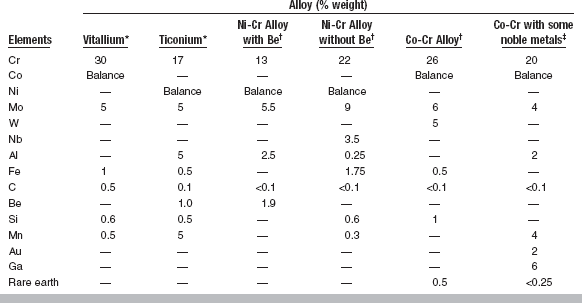
*Data from Asgar K: An overall study of partial dentures, USPHS Research Grant DE-02017, NIH; and Baran G: The metallurgy of Ni-Cr alloys for fixed prosthodontics, J Prosthet Dent 50:539, 1983.
†Typical alloy compositions for ceramic-metal restorations with conventional porcelains.
‡Alloy suitable for high-expansion porcelain.
Function of Various Alloying Elements
Chromium is responsible for the tarnish and corrosion resistance of these alloys. When the chromium content of an alloy is higher than 30%, the alloy is more difficult to cast. With this percentage of chromium, the alloy also forms a brittle phase, known as the sigma (σ) phase. Therefore, cast base-metal dental alloys should not contain more than 28% or 29% chromium. In general, cobalt and nickel, up to a certain percentage, are interchangeable elements. Cobalt increases the elastic modulus, strength, and hardness of the alloy more than does nickel.
The effect of other alloying elements on the properties of these alloys is much more pronounced. One of the most effective ways of increasing the hardness of cobalt-based alloys is by increasing their carbon content. A change in the carbon content of approximately 0.2% changes the properties to such an extent that the alloy would no longer be usable in dentistry. For example, if the carbon content is increased by 0.2% over the desired amount, the alloy becomes too hard and brittle and should not be used for making any dental appliances. Conversely, a reduction of 0.2% in the carbon content would reduce the alloy’s yield and ultimate tensile strengths to such low values that, once again, the alloy would not be usable in dentistry. Furthermore, almost all elements in these alloys, such as chromium, silicon, molybdenum, cobalt, and nickel, react with carbon to form carbides, which change the properties of the alloys. Note that, as shown in Table 16-1, the nickel-chromium alloys used with ceramic contain significantly less carbon than the alloys used for removable partial dentures. The presence of 3% to 6% molybdenum contributes to the strength of the alloys.
Aluminum in nickel-containing alloys forms a compound of nickel and aluminum (Ni3Al). This compound increases the ultimate tensile and yield strengths of the alloy considerably. The addition of as little as 1% to 2% beryllium to nickel-based alloys lowers the fusion range by about 100° C. However, recent studies suggest that this concentration of beryllium may adversely affect ductility. Corrosion resistance is also compromised, as corrosion occurs preferentially in the Ni-Be eutectic phase, presumably releasing quantities of beryllium greater than the nominal alloy composition of 1% to 2%. Silicon and manganese are added to increase the fluidity and castability of these alloys. Nitrogen, which cannot be controlled unless the castings are made in a controlled atmosphere, such as in a vacuum or under argon, also contributes to the overall qualities of these cast alloys. Numerous other modifications in composition are being proposed to develop more ductile and stronger alloys. There is a remarkable similarity in properties of different alloys having a relatively wide variation in composition. However, as discussed later, it is primarily the minor alloying elements of carbon, nitrogen, silicon, manganese, beryllium, and oxygen that influence casting and the properties of a final casting.
MICROSTRUCTURE OF CAST BASE-METAL ALLOYS
The microstructure of any substance is the basic factor that controls properties. In other words, a change in the physical properties of a material is a strong indication that there must have been some alteration in its microstructure. Sometimes this variation in microstructure cannot be distinguished by ordinary means. Neither cobalt-chromium nor nickel-chromium alloys have simple microstructures, and their microstructures change with slight alterations of manipulative conditions.
The microstructure of cobalt-chromium alloys in the cast condition is inhomogeneous, consisting of an austenitic matrix composed of a solid solution of cobalt and chromium in a cored dendritic structure. The dendritic regions are cobalt-rich, whereas the interdendritic regions can be a quaternary mixture consisting of a cobalt-rich γ-phase; a chromium-rich M23C6 phase, where M is Co, Cr, or Mo; an M7C3 carbide phase; and a chromium- and molybdenum-rich σ-phase. Interdendritic casting porosity is also associated with this structure.
Many elements present in cast base-metal alloys, such as chromium, cobalt, and molybdenum, are carbide-forming elements. Depending on the composition of a cast base-metal alloy and its manipulative condition, it may form many types of carbide. Furthermore, the arrangement of these carbides may also vary depending on the manipulative condition.
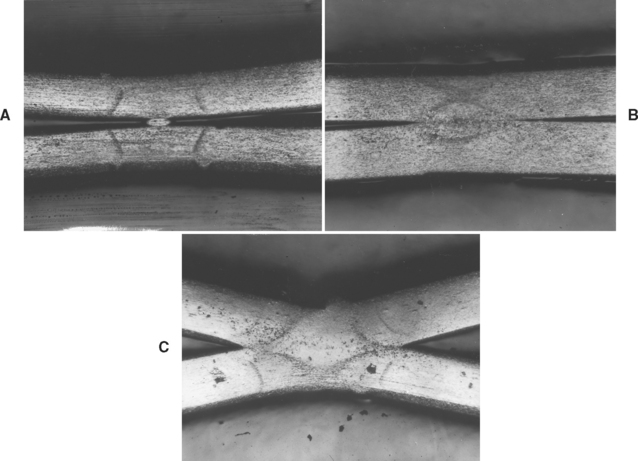
The microstructure of a commercial cobaltchromium alloy is illustrated in Fig. 16-1. In Fig. 16-1, A. the carbides are continuous along the grain boundaries. Such a structure is obtained when the metal is cast as soon as it is completely melted. In this condition, the cast alloy possesses low elongation values with a good and clean surface. Carbides that are spherical and discontinuous, like islands, are shown in Fig. 16-1, B. Such a structure can be obtained if the alloy is heated about 100° C above its normal melting temperature; this results in a casting with good elongation values but with a very poor surface because of an increased reaction with the investment. The surface is so poor that the casting cannot be used in dentistry.
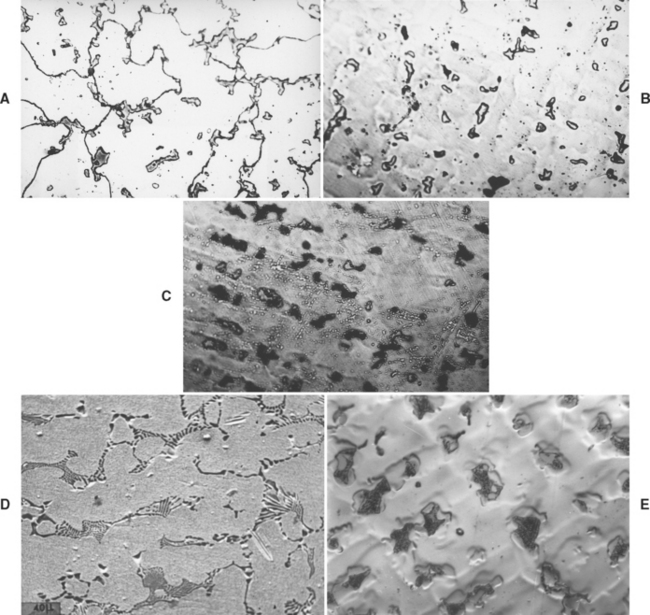
FIGURE 16-1 Microstructure of cast cobalt-chromium alloy, A, where the carbides are continuous around the grain boundaries. B, The islandlike structures are carbides, which are dispersed throughout the entire area. C, The dark areas are eutectoid, which are lamellar in nature. D, The microstructure of a beryllium-containing nickel-chromium alloy. E, The microstructure of a boron- and silicon-containing nickel-chromium alloy. (A, B, and C from Asgar K, Peyton FA: Effect of casting conditions on some mechanical properties of cobalt-base alloys, J Dent Res 40:68, 1961; D and E courtesy G Baran, Temple University.)
Dark eutectoid areas, which are lamellar in nature, are shown in Fig. 16-1, C. Such a structure is responsible for very low elongation values but a good and clean casting. From these three examples, it is clear that microstructure can strongly affect physical and mechanical properties. The microstructure of Ni-Cr alloys is strongly dependent on alloy composition. Alloys containing Be form an interdendritic NiBe phase, as shown in Fig 16-1, D. In fact, during normal metallographic procedures involving acid etching of alloy specimens, the NiBe phase is dissolved; what is seen in the figure is the void area left behind. The susceptibility of the NiBe phase to acid attack has been taken advantage of in developing resin-bonded retainers. The retainer may be etched in selected areas, where a composite-like luting agent can then mechanically adhere to the retainer after curing.
Alloys not containing Be have complicated, multiphase microstructures such as that shown in Figure 16-1, E. The precipitates dispersed within the matrix include complex carbides, and, in alloys where niobium (Nb) is present, Mo-Nb-Si compounds. All these precipitates are relatively unaffected by the short heat-treatment cycles that the alloys are subjected to during the ceramic firing procedures.
HEAT TREATMENT OF BASE-METAL ALLOYS
The early base-metal alloys used in removable partial-denture prostheses were primarily cobalt-chromium and were relatively simple. Heat treating these alloys up to 1 hour at 1000° C did not change their mechanical properties appreciably. Base-metal alloys available today for removable partial-denture prostheses, however, are more complex. Presently, complex cobalt-chromium alloys, nickel-chromium, and iron-chromium alloys are used for this purpose.
Studies have shown that many heat treatments of cobalt-based alloys reduce both the yield strength and elongation. If for any reason some soldering or welding must be performed on these partial dentures, the lowest possible temperature should be used with the shortest possible time of heating to the elevated temperature.
PHYSICAL PROPERTIES
The melting temperature of base-metal alloys differs significantly from that of dental gold casting alloys. Most base-metal alloys melt at temperatures of 1150° to 1500° C, as compared with cast gold alloy Types I to IV, which have a melting range of 800° to 1050° C. The addition of 1% to 2% beryllium lowers the melting temperature of Ni-Cr alloys about 100° C. The melting temperature is important in the selection of casting equipment, investment, and control of the casting technique.
Density
The average density of cast base-metal alloys is between 7 and 8 g/cm3, which is about half the density of most dental gold alloys. Density is of some importance in bulky maxillary appliances, in which the force of gravity causes the relative weight of the casting to place additional forces on the supporting teeth. With certain appliances, therefore, the reduction of weight resulting from the lower density of the cast base-metal alloys can be considered an advantage.
MECHANICAL PROPERTIES
Typical mechanical properties of the partial-denture alloys listed in Table 16-1 have been assembled in Table 16-2, together with a representative range of values for Type IV casting gold alloys subjected to a hardening heat treatment.
TABLE 16-2
Mechanical Properties of Alloys Used in Partial Dentures
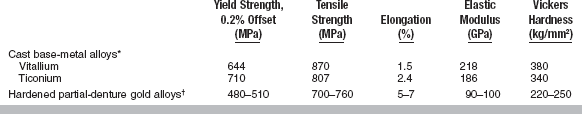
*Data from Asgar K, Techow BO, Jacobson JM: J Prosthet Dent 23:36, 1970; Morris HF, Asgar K: J Prosthet Dent 33:36, 1975; Moffa JP, Lugassy AA, Guckes AD, Gettleman L: J Prosthet Dent 30:424, 1973.
†Data from Oilo G, Gjerdet NR: Acta Odontal Scand 41:111, 1983.
Yield Strength
The yield strength gives an indication when a permanent deformation of a device or part of a device, such as a clasp, will occur. As such, it is one of the important properties of alloys intended for removable partial-denture restorations. It is believed that dental alloys should have yield strengths of at least 415 MPa to withstand permanent deformation when used as partial-denture clasps. It may be seen from Table 16-2 that base-metal dental alloys have yield strengths greater than 600 MPa.
Tensile Strength
The ultimate tensile strength of cast base-metal alloys is less influenced by variations in specimen preparation and test conditions than are some other properties, such as elongation. Table 16-2 shows that the ultimate tensile strength of cast base-metal dental alloys is greater than 800 MPa. Table 16-2 also demonstrates that hardened partial-denture gold alloys can have ultimate tensile strengths almost equal to those of cast base-metal alloys.
Elongation
The percent elongation of an alloy is important as an indication of the relative brittleness or ductility a restoration will exhibit. There are many occasions, therefore, when elongation is an important property for comparison of alloys for removable partial-denture appliances. For example, as described in Chapter 4, the combined effect of elongation and ultimate tensile strength is an indication of toughness of a material. Because of their toughness, partial-denture clasps cast of alloys with a high elongation and tensile strength do not fracture in service as often as do those with low elongation.
The percent elongation is one of the properties critical to accurate testing and to proper control during test preparation. For example, a small amount of microporosity in the test specimen will decrease the elongation considerably, whereas its effect on yield strength, elastic modulus, and tensile strength is rather limited. One can therefore assume that practical castings may exhibit similar variations in elongation from one casting to another. To some degree this is borne out in practice, with some castings from the same product showing a greater tendency toward brittleness than others. This observation indicates that control of the melting and casting variables is of extreme importance if reproducible results are to be obtained.
Although nickel and cobalt are interchangeable in cobalt-nickel-chromium alloys, increasing the nickel content with a corresponding reduction in cobalt generally increases the ductility and elongation. High values of elongation are obtained by casting at the normal melting temperature and by not heating the alloy 100° C above its normal casting temperature. High elongation is achieved without sacrificing strength and is the result of the precise and proper combination of carbon, nitrogen, silicon, manganese, and molybdenum content.
Elastic Modulus
The higher the elastic modulus, the more rigid a structure can be expected, provided the dimensions of the casting are the same in both instances. Some dental professionals recommend the use of a well-designed, rigid appliance because it properly distributes forces on the supporting tissues when in service. With a greater elastic modulus, one can design the restoration with slightly reduced dimensions. From Table 16-2, it can be seen that the elastic modulus of base-metal alloys is approximately double the modulus of Type IV cast dental gold alloys.
Hardness
Differences in composition of the cast base-metal alloys have some effect on their hardness, as indicated by the values given in Table 16-2. In general, cast base-metal alloys have a hardness about one third greater than gold alloys used for the same purpose.
Hardness is an indication of the ease of finishing the structure and its resistance to scratching in service. The higher hardness of the cast base-metal alloys as compared with gold alloys requires the use of different polishing equipment and compounds, which may be considered a disadvantage, but the finishing operation can be completed without difficulty by experienced operators. It is a common practice to use electrolytic polishing for a portion of the finishing operation, which reduces the time and effort necessary for mechanical finishing operations. With an electrolytic polishing procedure, cast base-metal restorations are deplated, and only a very small amount of alloy (a few angstroms) is removed from the surface. Electrolytic polishing works on the reverse principle of electroplating, with the restoration serving as the anode. The deplating exposes a new surface, which is smoother than the cast surface because the rough areas are deplated more readily than the smooth ones. The cast base-metal appliances retain their polish well in service. Deplating such a small amount of alloy from the tissue-bearing side of the prosthesis produces a clean, shiny surface, but does not alter the fit. The non-tissue-bearing side of the partial denture can be further polished on a high-speed lathe.
Fatigue
The fatigue resistance of alloys used for partial dentures is important when it is considered that these appliances are placed and removed daily. At these times, the clasps are strained as they slide around the retaining tooth, and the alloy undergoes fatigue. Comparisons among cobalt-chromium, titanium, and gold alloys shows that cobalt-chromium alloys possess superior fatigue resistance, as indicated by a higher number of cycles required to fracture a clasp. Any procedures that result in increasing the porosity or carbide content of the alloy will reduce fatigue resistance. Also, soldered joints, which often contain inclusions or pores, represent weak links in the fatigue resistance of the appliance.
CORROSION
Recent research on dental casting alloys has been dominated by studies on corrosion and potential biological effects of metal ion release. In general, in vitro corrosion tests have evaluated a number of important variables, including effects of electrolytic media and artificial saliva, alloy composition, alloy microstructure, and surface state of the metal. These variables account for 2 to 4 orders of magnitude variation in the amount of species released. The surface state of the metal is an extremely important factor influencing corrosion, because the surface composition is almost always different from that of the bulk alloy. Another important consideration is corrosion coupled with wear. Up to three times the mass of metal ions, such as Ni and Be, is released during occlusal rubbing in combination with corrosion than during corrosion alone for Ni-Cr alloys. Nickel-chromium alloys with beryllium fail to satisfy the ISO requirements for release of metal ions. No long-term studies have been performed to monitor the impact of the release of such large concentrations of metal ions on the overall health of patients.
CROWN AND BRIDGE CASTING ALLOYS
The nickel-chromium alloys can be divided into those containing or not containing beryllium. Most of the alloys contain 60% to 80% nickel, 10% to 27% chromium, and 2% to 14% molybdenum. As a comparison, cobalt-chromium alloys contain 53% to 67% cobalt, 25% to 32% chromium, and 2% to 6% molybdenum. Those alloys that contain beryllium contain 1.6% to 2.0% of the element. They may also contain small amounts of aluminum, carbon, cobalt, copper, cerium, gallium, iron, manganese, niobium, silicon, tin, titanium, and zirconium. The low atomic weight of about 9 for beryllium, compared with about 59 for nickel and 52 for chromium, results in atomic percentages of beryllium in these alloys of about 11%. OSHA requirements and recent attempts from standards organizations to lower beryllium content below 0.02% may drastically impact the marketability of beryllium-containing alloys.
Properties of these alloys are similar to those reported in Table 16-2 for the cobalt-chromium alloys. Crown and bridge casting alloys exhibit a higher hardness and elastic modulus than do noble alloys, but they require a slightly different approach in casting and soldering to accommodate their higher solidification shrinkage.
Precautions should be taken to avoid exposure to metallic vapor, dust, or grindings containing beryllium and nickel. The safety standard for pure beryllium dust is 2 μg/m3 of air for a time-weighted, 8-hour day. A higher limit of 25 μg/m3 is allowed for a minimum exposure time of less than 30 minutes. Physiological responses may range from contact dermatitis to severe chemical pneumonitis. Therefore efficient local exhaust and filtration systems as well as adequate general ventilation should be used when casting, finishing, and polishing these beryllium-containing alloys.
The presence of nickel is of greater importance because it is a known allergen. The incidence of allergic sensitivity to nickel has been reported to be from 5 to 10 times higher for females than for males, with 5% to 8% of females showing sensitivity. However, no correlation has been found between the presence of intraoral nickel-based restorations and sensitivity. A cobalt-chromium alloy without nickel or other non-nickel-containing alloy should be used on patients with a medical history indicating an allergic response to nickel. The safety standard for pure nickel is 15 μg/m3 of air for a 40-hour week. To minimize exposure of patients to metallic dust containing nickel or beryllium, intraoral finishing should be done with a high-speed evacuation system and preferably in a wet environment.
OTHER APPLICATIONS OF CAST BASE-METAL ALLOYS
Cast cobalt-chromium alloys serve a useful purpose in appliances other than removable partial-denture restorations. In the surgical repair of bone fractures, alloys of this type have been used for bone plates, screws, various fracture appliances, and splints. Metallic obturators and implants for various purposes can be formed from cast base-metal alloys. The use of cobalt-chromium alloys for surgical purposes is well established, and these alloys have numerous oral surgical uses. They can be implanted directly into the bone structure for long periods without harmful reactions. This favorable response of the tissue is probably attributable to the low solubility and electrogalvanic action of the alloy; the metal is inert and produces no inflammatory response. The product known as surgical Vitallium is used extensively for this purpose. The primary metal used in oral implantology today is titanium (see Chapter 22).
TITANIUM AND TITANIUM ALLOYS
Titanium’s resistance to electrochemical degradation; benign biological response elicited; relatively light weight; and low density, low modulus, and high strength make titanium-based materials attractive for use in dentistry. Titanium forms a very stable oxide layer with a thickness on the order of angstroms, and it repassivates in a time on the order of nanoseconds (10−9 second). This oxide formation is the basis for the corrosion resistance and biocompatibility of titanium. Titanium has therefore been called the “material of choice” in dentistry.
Commercially pure titanium (cp Ti) is used for dental implants, surface coatings, and more recently, for crowns, partial and complete dentures, and orthodontic wires. Several titanium alloys are also used. Of these alloys, Ti-6Al-4V is the most widely used. Wrought alloys of titanium and nickel and titanium and molybdenum are used for orthodontic wires. The term titanium is often used to include all types of pure and alloyed titanium. However, it should be noted that the processing, composition, structure, and properties of the various titanium alloys are quite different, and also that differences exist between the wrought and cast forms of a given type of titanium.
COMMERCIALLY PURE TITANIUM
Commercially pure Ti (CP Ti) is available in four grades, which vary according to the oxygen (0.18 to 0.40 wt%) and iron (0.20 to 0.50 wt%) content. These apparently slight concentration differences have a substantial effect on the physical and mechanical properties.
At room temperature, CP Ti has a hexagonal close-packed (HCP) crystal lattice, which is denoted as the alpha (α) phase. On heating, an allotropic phase transformation occurs. At 883° C, a bodycentered cubic (BCC) phase, which is denoted as the beta (β) phase, forms. A component with predominantly β phase is stronger but more brittle than a component with α-phase microstructure. As with other metals, the temperature and time of processing and heat treatment dictate the amount, ratio, and distribution of phases, overall composition and microstructure, and resultant properties. As a result, casting temperature and cooling procedure are critical factors in ensuring a successful casting.
The density of CP Ti (4.5 g/cm3) is about half the value of many of the other base metals. The modulus (100 GPa) is also about half the value of the other base metals. The yield and ultimate strengths vary, respectively, from 170 to 480 MPa and 240 to 550 MPa, depending on the grade of titanium.
TITANIUM ALLOYS: GENERAL
Alloying elements are added to stabilize either the α or the β phase, by changing the β-transformation temperature. For example, in Ti-6Al-4V, aluminum is an α stabilizer, which expands the α-phase field by increasing the (α + β) to β-transformation temperature, whereas vanadium, as well as copper and palladium, are β stabilizers, which expand the β-phase field by decreasing the (α + β) to β-transformation temperature.
In general, alpha-titanium is weldable, but difficult to form or work with at room temperature. Beta-titanium, however, is malleable at room temperature and is thus used in orthodontics. The (α + β) alloys are strong and formable but difficult to weld. Thermal and thermochemical treatments can refine the postcast microstructures and improve properties.
Ti-6AL-4V
At room temperature, Ti-6Al-4V is a two-phase (α + β) alloy. At about 975° C, an allotropic phase transformation takes place, transforming the microstructure to a single-phase BCC β alloy. Thermal treatments dictate the relative amounts of the α and β phases and the phase morphologies and yield a variety of microstructures and a range of mechanical properties. Microstructural variations depend on whether working and heat treatments were performed above or below the β-transition temperature and on the cooling rate.
Following forging at temperatures in the range of 700° to 950° C, thermal treatments below the β-transition temperature (typically performed at approximately 700° C) produce recrystallized microstructures having fine equiaxed α grains (Fig. 16-2). Equiaxed microstructures are characterized by small (3 to 10 μm), rounded grains that have aspect ratios near unity. This class of microstructure is recommended for Ti-6Al-4V surgical implants.

FIGURE 16-2 Microstructure of equiaxed Ti-6Al-4V (×200). Equiaxed microstructures are characterized by small, rounded α-grains, with aspect ratios near unity.
The mechanical properties of (α + β) titanium alloys are dictated by the amount, size, shape, and morphology of the α phase and the density of α/β interfaces. The tensile and fatigue properties of Ti-6Al-4V have been studied extensively. Microstructures with a small (<20 μm) α-grain size, a well-dispersed β phase, and a small α/β interface area, such as in equiaxed microstructures, resist fatigue crack initiation best and have the best high-cycle fatigue strength (approximately 500 to 700 MPa). Lamellar microstructures, which have a greater α/β surface area and more oriented colonies, have lower fatigue strengths (approximately 300 to 500 MPa) than do equiaxed microstructures.
CAST TITANIUM
Based on the attributes, extensive knowledge, and clinical success of wrought titanium implants, interest has developed in cast titanium for dental applications. Although titanium has been cast for more than 50 years, only recently have nearly precision castings been attainable. For aerospace and medical components, hot isostatic pressing and specific finishing techniques are routinely practiced. However, these techniques are beyond the capabilities and affordability of most dental laboratories.
The two most important factors in casting titanium-based materials are its high melting point (≈1700° C for CP Ti) and chemical reactivity. Because of the high melting point, special melting procedures, cooling cycles, mold material, and casting equipment to prevent metal contamination are required. Titanium readily reacts with gaseous elements such as hydrogen, oxygen, and nitrogen, particularly at high temperatures (>600° C). As a result, any manipulation of titanium at elevated temperatures must be performed in a well-controlled vacuum or inert atmosphere. Without such controls, titanium surfaces will be contaminated with α case, an oxygen-enriched and hardened surface layer, which can be as thick as 100 μm. Surface layers of this thickness reduce strength and ductility and promote cracking because of the embrittling effect of the oxygen. The technology required to overcome these factors is what makes casting titanium relatively more expensive.
Because of the high affinity titanium has for hydrogen, oxygen, and nitrogen, standard crucibles and investment materials cannot be used. Investment materials or face coats for the wax patterns must have oxides that are more stable than the very stable titanium oxide, and must also be able to withstand a temperature sufficient to melt titanium. If this is not the case, oxygen is likely to diffuse into the molten metal. Investment materials using a combination of ZrO2 type face coat that is backed up a phosphate-bonded silica investment or phosphate investment materials involving inert fillers (ZrO2, Al2O3, MgO) achieve this goal.
Because of the low density of titanium, it is difficult to cast in conventional, centrifugal-force casting machines. In the last 10 to 15 years, advanced casting techniques, which combine centrifugal, vacuum, pressure, and gravity casting, new investment materials, and advanced melting techniques (e.g., electric arc melting) have been developed. These advances have improved the feasibility of casting titanium-based materials in the dental laboratory.
Pure titanium and Ti-alloys such as Ti-6Al-4V have been cast into crowns, removable partial-denture frameworks, and complete denture bases. Titanium alloys have a lower melting point than pure titanium. By alloying titanium, the melting temperature can be lowered to the same temperature as that of nickel-chromium and cobalt-chromium alloys. For example, the Ti-Pd and Ti-Cu alloys have melting points of 1350° C. Lower casting temperatures may also reduce the reactivity of titanium with oxygen and other gases. Other titanium alloys, such as Ti-6Al-4V, Ti-15V, Ti-20Cu, Ti-30Pd, Ti-Co, Ti-Cu, and Ti-Cu-Ni, are still in the experimental stages and have not yet been evaluated in any large clinical studies.
Microstructures of cast titanium materials are similar to those described previously, namely coarse lamellar grains, a result of slow cooling through the β to α or β to (α + β) transformation temperature (Fig. 16-3).
The mechanical properties of cast CP Ti are similar to those of Types III and IV gold alloy, whereas cast Ti-6Al-4V and Ti-15V exhibit properties, except for modulus, similar to those of nickel-chromium and cobalt-chromium alloys.
Recently, cast Ti-6Al-4V microstructures have been refined by temporary alloying with hydrogen. The resulting microstructures (Fig. 16-4) can have α-grain sizes less than 1 μm, aspect ratios near unity, and discontinuous grain-boundary α, microstructural attributes that increase tensile and fatigue strength. These changes in microstructural form and structure result in significant increases in yield strength (974 to 1119 MPa), ultimate strength (1025 to 1152 MPa), and fatigue strength (643 to 669 MPa) as compared with respective values for lamellar (902, 994, and 497 MPa) and equiaxed microstructures (914, 1000, and 590 MPa).
Pure titanium has been cast with a pressure-vacuum casting machine. Other manufacturers have developed centrifugal casting machines that use an electric arc to melt the titanium in an argon or helium atmosphere. Melting is performed in a copper crucible, followed by centrifugal casting into a mold that uses investment. Such machines provide a relatively oxygen-free environment and, with the use of a tungsten arc, can reach temperatures of 2000° C. This latter casting regime has been used to cast CP Ti crowns and full-denture bases. Crowns cast in this manner have been evaluated clinically, and results revealed that, although fitness was inferior to that of silver-palladium alloy, it was superior to that of nickel-chromium. Occlusal adjustment was no more difficult than with conventional crowns, and discoloration, occlusal wear, and plaque retention were similar to other metals. The clinical results of full-denture bases cast in this manner have been acceptable.
Observations of randomly chosen cast crowns using old machines and silica-containing investments have revealed gross surface porosities, to a depth of 75 μm, on both the inside and outside of the surfaces. Mechanical polishing is insufficient to remove this porosity. Internal porosities, sometimes measuring up to 30% of the cross-sectional area, are also readily observed. Surfaces of castings can also be contaminated with α case. The cause of the α case is probably poor atmosphere control or contamination from crucible and mold materials. For optimum functionality of the final casting, the surface layer must be removed during finishing. However, even after the α case is removed, internal oxidation can remain and compromise the mechanical properties of the final appliance. Further examination of such castings has also revealed multiple microcracks at the edges of the margins. Some cracks are as long as 100 μm. Cracks of this length are catastrophic to a notch-sensitive material such as titanium.
As outlined, the difficulties with cast titanium for dental purposes include high melting point and high reactivity, low casting efficiency, inadequate expansion of investment, casting porosity, and difficulty in finishing this metal. From a technical standpoint, titanium is difficult to weld, solder, machine, finish, and adjust. Casting titanium requires expensive equipment.
OTHER APPLICATIONS OF WROUGHT TITANIUM
Based on clinical experience with wrought titanium dental implants, wrought titanium crown and bridge applications have been pursued. With the advent of reproducible, high-tolerance machining and processing techniques, such as spark erosion, laser welding and micromachining, and computer-aided design/computer-aided manufacturing, wrought titanium crowns are now possible. Future applications are likely to include partial-denture work, other precision work, implant-supported restorations, and orthodontic components.
WROUGHT STAINLESS STEEL ALLOYS
Steel is an iron-carbon alloy. The term stainless steel is applied to alloys of iron and carbon that contain chromium, nickel, manganese, and perhaps other metals to improve properties and give the stainless quality to the steel. These alloys differ in composition from the cobalt-chromium, nickel-chromium, and titanium casting alloys. Usually, stainless steel alloys are not cast, but instead are used in the wrought form in dentistry, which represents the second way that stainless steel differs from the cast base-metal alloys. As a result, the types of appliances formed from these two materials differ. The most common applications of stainless steel for dental purposes at present are in the preparation of orthodontic appliances and fabrication of endodontic instruments, such as files and reamers. Some specialized applications of stainless steel exist for temporary space maintainers, prefabricated crowns, or other appliances placed in the mouth, and for various clinical and laboratory instruments.
COMPOSITION
Several broad classifications of stainless steel are generally recognized. The various groups are referred to as ferritic, martensitic, and austenitic, and they have different compositions, properties, and applications. The ferritic stainless steels are chromium steels employed in the manufacture of instruments or equipment parts in which some degree of tarnish resistance is desirable. A wide range of compositions is available in this group, in which amounts of chromium, the principal element contributing to stainless qualities, may vary from 15% to 25%. Elements such as carbon, silicon, and molybdenum are included but are all held within narrow limits.
The martensitic steels also are primarily chromium steels with a lower chromium content (about 12% to 18%). These steels can be hardened to some degree by heat treatment, and they have a moderate resistance to tarnish. They are used chiefly in the manufacture of instruments and, to a limited degree, for orthodontic appliances.
The austenitic steels represent the alloys used most extensively for dental appliances. The most common austenitic steel used in dentistry is 18–8 stainless steel, so named because it contains approximately 18% chromium and 8% nickel. The carbon content is between 0.08% and 0.20%, and titanium, manganese, silicon, molybdenum, niobium, and tantalum are present in minor amounts to give important modifications to the properties. The balance (≈72%) is iron.
FUNCTION OF ALLOYING ELEMENTS AND CHEMICAL RESISTANCE
The corrosion resistance of stainless steel is attributed largely to the presence of chromium in the alloy, and no other element added to iron is as effective in producing resistance to corrosion. Iron cannot be used without chromium additions because iron oxide (Fe2O3), or rust, is not adherent to the bulk metal. About 11% chromium is needed to produce corrosion resistance in pure iron, and the necessary proportion is increased with the addition of carbon to form steel. Chromium resists corrosion well because of the formation of a strongly adherent coating of oxide on the surface, which prevents further reaction with the metal below the surface. The formation of such an oxide layer is called passivation. The surface coating is not visible, even at high magnification, but the film adds to the metallic luster of the metal. The degree of passivity is influenced by a number of factors, such as alloy composition, heat treatment, surface condition, stress in the appliance, and the environment in which the appliance is placed. In dental applications, the stainless characteristics of the alloys can therefore be altered or lost by excessive heating during assembly or adaptation; using abrasives or reactive cleaning agents, which can alter the surface conditions of the appliance; and even by poor oral hygiene practices over prolonged periods.
Of the stainless steel alloys in general use, the austenitic type of 18–8 stainless steel shows the greatest resistance to corrosion and tarnish. In these alloys, chromium and nickel form solid solutions with the iron, which gives corrosion protection. The chromium composition in these alloys must be between 13% and 28% for optimal corrosion resistance. If the chromium content is less than 13%, the adherent chromium oxide layer does not form. If there is more than 28% chromium, chromium carbides form at the grain boundaries, embrittling the steel. The amount of carbon must also be tightly controlled. If not, carbon will react with chromium, forming these grain-boundary chromium-carbides, which lead to depletion of grain-boundary chromium and decrease corrosion resistance in a process known as sensitization. Molybdenum increases the resistance to pitting corrosion.
The elements present in small amounts tend to prevent the formation of carbides between the carbon present in the alloy and the iron or chromium and, as a result, often are described as stabilizing elements. Some steels, termed stabilized stainless steels, contain titanium, niobium, or tantalum, so the carbides that do form are titanium carbides rather than chromium carbides.
The chemical resistance of stainless steel alloys is improved if the surface is clean, smooth, and polished. Irregularities promote electrochemical action on the surface of the alloy. Soldering operations on stainless steel with gold and silver solder may contribute to a reduction in stainless qualities because of electrogalvanic action between dissimilar metals or because of localized, improper composition of the stainless steel wire.
STRESS-RELIEVING TREATMENTS
The 18-8 alloys are not subject to an increase in properties by heat treatment, but they do respond to strain hardening as a result of cold work during adjustment or adaptation of the alloy to form the appliance. Heat treatment above 650° C results in recrystallization of the microstructure, compositional changes, and formation of chromium-carbides, three factors that can reduce mechanical properties and corrosion resistance.
Appliances formed from these alloys may, however, be subjected to a stress-relieving operation to remove the effects of cold working during fabrication, increase ductility, or produce some degree of hardening with some alloys. If heat treatment is to be performed, it should be held to temperatures between 400° and 500° C for 5 to 120 seconds, depending on the temperature, type of appliance, and alloy being heated. A time of 1 minute at 450° C would represent an average treatment to be used on an orthodontic appliance. Keep in mind that temperatures above 650° C will soften or anneal the alloy, and the properties cannot be restored by further treatment. The main advantage of a low-temperature heat-treating operation is that it establishes a uniformity of properties throughout the appliance after adaptation and fabrication, which may reduce the tendency toward breakage in service. Factors affecting an alloy’s ability to be heat-treated and stress-relieved include alloy composition, working history (i.e., fabrication procedure), and the duration, temperature, and atmosphere of the heat treatment.
STAINLESS STEEL ORTHODONTIC WIRES
Stainless steel wires are processed in the wrought form, through rolling or drawing. Wrought means that the near net shape is formed from the material in a solid state. Stainless steel wires can be formed easily into appliances with special orthodontic pliers and instruments. Once an appliance is fabricated, it should be heat-treated for 1 minute at 450° C to relieve stresses created during fabrication.
The soldering of stainless steel appliances requires skill, and the use of suitable materials is essential. Borax fluxes are not satisfactory, and the use of fluoride-containing flux is required for a successful solder joint. Gold and silver solders may be employed to form the union. Silver solders are considered easier to use than are most gold solders, and they provide a stronger solder joint. Silver solders also have the advantage of having slightly lower melting temperatures, 600° to 650° C, than most gold solders, which reduces the hazard of overheating the steel during the soldering operation and thus reducing properties.
The strength of a wire in the vicinity of the soldered joint formed with gold solder is lower than that of one formed with silver solder. Many operators therefore use silver solder with stainless steel or rely entirely on spot welding to assemble the appliance. In one study, which used a standard orthodontic blowtorch as a source of heat with a low-fineness gold solder and a fluoride type of flux, the temperature recorded in the center of a joint was 700° C when the least possible amount of heat was used and 800° C when the maximum amount was applied. The temperature was 650° C at 1 mm from the joint and 630° C at 3 mm, but at 5 mm the temperature in the wire was only 440° C. These observations indicate that higher temperatures are being developed during soldering than were believed to exist, and are no doubt responsible for some of the reduction in strength of stainless steel adjacent to the solder joint.
Cleaning and polishing stainless steel appliances are troublesome operations that become necessary after soldering, heat treating, or periods of service in the mouth. The appliance may be pickled in warmed nitric acid, but a gray satin finish will result that requires buffing or mechanical brushing with a fine abrasive to restore the luster of the original material. An electrolytic polishing bath, also known as an anodic polisher, is useful for restoring the surface appearance to stainless steel appliances, the same as for the cast cobalt-chromium structures mentioned previously.
Properties
ANSI/ADA Specification No. 32 (ISO 15841) for orthodontic wires not containing precious metals describes Type I (low-resilience) and Type II (high-resilience) wires. The requirements for flexure yield strength and number of bend cycles are listed in Table 16-3.
TABLE 16-3
Requirements of ANSI/ADA Specification No. 32 for Orthodontic Wires Not Containing Precious Metals

Some properties of stainless steel wires when tension, bending, and torsion are applied are listed in Table 16-4 and compared with the properties of nickel-titanium and beta-titanium wires. Of the three types of wires, the stainless steel wire has the highest values of yield strength, elastic modulus, and spring rate and the lowest springback (yield strength/elastic modulus).
TABLE 16-4
Properties of Orthodontic Wires in Tension, Bending, and Torsion
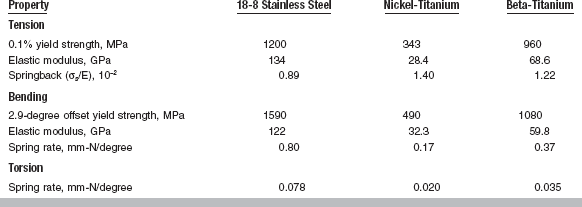
Adapted from Drake SR, Wayne DM, Powers JM, Asgar K: Am J Orthod 82:206, 1982. Values are for a 0.43 × 0.64-mm rectangular wire.
Mechanical properties of stainless steel orthodontic wires are listed in Table 16-5 for two sizes of wires in as-received and stress-relieved conditions. The higher values of proportional limit and yield strength of the 0.36-mm diameter wire reflect the increased amount of cold working used to fabricate this size of wire as compared with that required for 0.56-mm diameter wire. The properties of these wires (except tensile strength) are improved by the stress-relieving heat treatment.
TABLE 16-5
Mechanical Properties of 18-8 Stainless Steel Wires
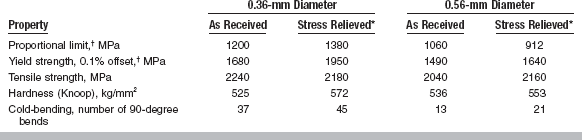
*Heated at 482° C for 3 min.
†Properties are measured in tension.
Adapted from Craig RG, editor: Dental materials: a problem oriented approach. St Louis, 1978, Mosby.
Curves of bending moment versus angular deflection are strongly affected by three factors: (1) the geometry of the wire; (2) the direction of loading, with respect to the wire orientation; and (3) the thermal history of the wire. Curves of bending moment versus angular deflection are shown in Fig. 16-5 for stainless steel wires having square and rectangular cross-sections. The direction of bending of the rectangular wire has an important effect on the bending moment because the wire is much stiffer in bending in the larger dimension (upper curve versus the middle curve). The curves for the square wire are nearly the same for bending in the direction of the square or the diagonal (bottom curve). The effect of heating on the bending moment—angular deflection curves for round stainless steel wires is shown in Fig. 16-6. Heating the wire even for a short time (15 seconds) causes a decrease in the stiffness in bending and in the angle at which permanent deformation starts as compared with that of the as-received wire. Increased temperature also leads to recrystallization and reduced properties.
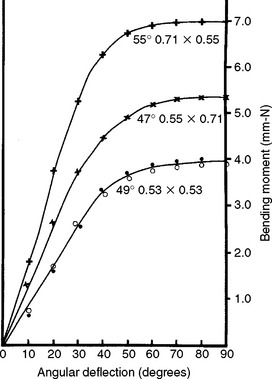
FIGURE 16-5 Bending moment—angular deflection curves for square and rectangular 18–8 stainless steel wires. The dimensions of the wires are in millimeters. (From Craig RG, editor: Dental materials: a problem-oriented approach, St. Louis, 1978, Mosby.)

FIGURE 16-6 Bending moment—angular deflection curves for 0.46-mm diameter 18-8 stainless steel wire in the as-received condition (•••) and after 15 seconds (

 ), 60 seconds (×××), and 120 seconds (+++) at 816° C. (From Craig RG, editor: Dental materials: a problem-oriented approach, St. Louis, 1978, Mosby.)
), 60 seconds (×××), and 120 seconds (+++) at 816° C. (From Craig RG, editor: Dental materials: a problem-oriented approach, St. Louis, 1978, Mosby.)
Soldering and spot welding can cause a deterioration in properties if the wire is overheated or underheated. Cross-sections of a spot-welded wire are shown in Fig. 16-7. The low setting of the spot welder (underheating) produced an inadequate joint, whereas the high setting (overheating) caused excessive melting and recrystallization of the wrought structure of the wire.
STAINLESS STEEL ENDODONTIC INSTRUMENTS
Numerous endodontic instruments are classified for hand use or as motor-driven instruments. The most common stainless steel instruments are the K type of root canal files and reamers. These are manufactured by machining a stainless steel wire into a pyramidal blank, either square or triangular in cross-section, and then twisting the blank to form a spiral cutting edge. A file with a rhombohedral cross-section has also been introduced. Examples of a K file, a K reamer, and a rhombohedral file are shown in Fig. 16-8.
Properties
Root canals are rarely straight. Therefore, endodontic files need to be able to follow a curved path. As such, bending and torsional properties of files are important. Similar to orthodontic wires, mechanical properties of endodontic files are dependent upon file geometry, direction of loading, and material composition. An example of this is illustrated by the bending moment—angular deflection curves of K and rhombohedral endodontic files (sizes 10 to 35) shown in Fig. 16-9. In bending, the rhombohedral files are less stiff than are the K files, and the increase in stiffness for increasing sizes is smaller and more uniform for the rhombohedral files.
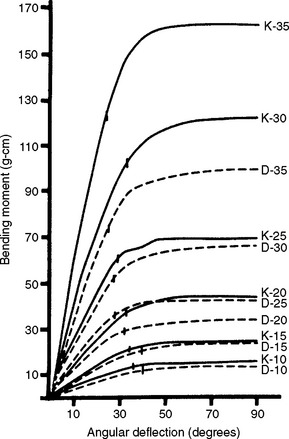
FIGURE 16-9 Bending moment—angular deflection curves of endodontic files, sizes 10 to 35. Vertical marks on curves indicate permanent deformation after 90-degree bending. (From Dolan DW, Craig RG: J Endod 8:260, 1982.)
Torsional moment-angular rotation curves of K and rhombohedral endodontic files (sizes 20 and 25) are shown for clockwise and counterclockwise rotation in Fig. 16-10. The rhombohedral files are also less stiff than are the K files in torsion, in both directions of rotation. Both types of files fail at lower angular rotation when tested in the counterclockwise direction because counterclockwise rotation increases the tightness of the twist, resulting in brittle fracture, as shown in Fig. 16-11, A. Clockwise rotation causes untwisting of the instrument, thereby allowing more rotation before failing in a ductile manner (Fig. 16-11, B). Operators are therefore cautioned against twisting a file more than a fourth turn in the counterclockwise direction when a file binds in a canal.
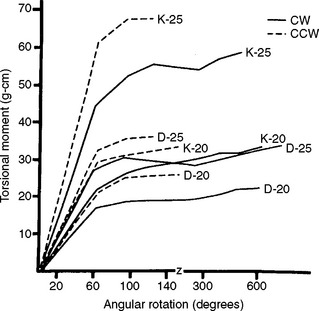
FIGURE 16-10 Torsional moment-angular rotation curves of endodontic files, sizes 20 to 25. Clockwise (CW) and counterclockwise (CCW) rotation. (From Dolan DW, Craig RG: Bending and torsion of endodontic files with rhombus cross sections, J Endod 8:260, 1982.)
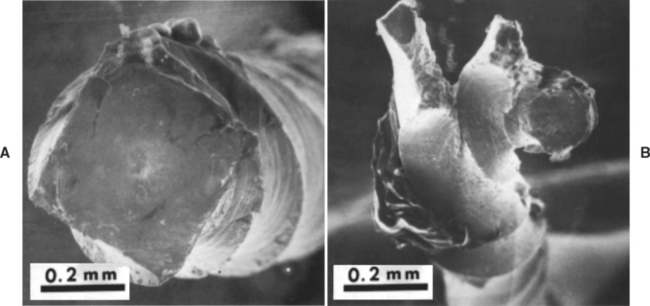
FIGURE 16-11 Fracture of K file by counterclockwise torque, A, and clockwise torque, B. (From Chernick LB, Jacobs JJ, Lautenschlager EP et al: J Endod 2:94, 1976.)
ANSI/ADA Specification No. 28 (ISO 3630) for endodontic files and reamers defines geometry, minimum values of torque and angular deflection, and maximum values of stiffness in bending for various sizes of files and reamers. Mechanical tests are made in the clockwise direction. No requirements exist for the counterclockwise direction. Stainless steel instruments are required to be resistant to corrosion when tested according to the specification.
A property not covered in the ANSI/ADA specification, but of importance clinically, is cutting ability. Measurement of cutting ability requires construction of machines that simulate the cutting motion of the instrument. Dentin substrates have been used but produced variation in the data because of biological differences in the hardness of teeth. To overcome this problem, some investigators use acrylic specimens. Cutting ability of individual files varies considerably but is reproducible for the same file with various numbers of strokes. Wear of a file does not appear to affect its cutting ability. Sterilization by dry heat or salt has no effect on the cutting ability of stainless steel files, but autoclave sterilization causes a reduction. Irrigants such as sodium hypochlorite, hydrogen peroxide, and EDTA-urea cause a reduction in cutting ability, whereas a saline irrigant does not cause a reduction. These solutions, excluding saline, also corrode stainless steel at room temperature. Therefore, irrigants should be rinsed from the instruments as soon as possible after use.
NICKEL-TITANIUM ENDODONTIC INSTRUMENTS
The alloys used in nickel-titanium root canal instruments contain about 56% Ni and 44% Ti by weight, which calculate to be 50% of each by atoms. In some instances, less than 2% of cobalt may be substituted for nickel. Of special interest for this application is that these alloys can change their structure from austenitic (body-centered cubic) to martensitic (hexagonal close-packed) as a function of stress during root canal preparation. When the instrument is used in the canal, the rate of stress levels off due to progressive deformation even if strain is increased due to the transformation to martensite. Note the modulus of Ni-Ti austenite is 120 GPa, and that of martensite is 50 GPa. This effect results in what is termed super-elasticity. When the stress decreases, springback occurs without permanent deformation and a return to the austenitic phase.
The super-elasticity of Ni-Ti permits deformations of 8% strain in endodontic files with complete recovery. This value compares to less than 1% with stainless steel instruments. In addition, the Ni-Ti alloys have higher strengths and lower moduli of elasticity than stainless steel, advantages in preparing curved root canals. Also, it has been shown that Ni-Ti and stainless steel endodontic files do not differ with respect to corrosion resistance.
These improved properties of Ni-Ti root canal instruments have permitted them to be effectively used as engine-driven, rotary instruments. In spite of these improved properties, Ni-Ti instruments can fracture. Studies of cyclic fatigue have shown that the radius of curvature of the file was the most important factor in fatigue resistance, with decreasing radius of curvature (increasing diameter) resulting in decreased fracture time. The fracture was always of a ductile type, suggesting that cyclic fatigue was the major cause of failure.
One drawback of Ni-Ti endodontic instruments is that because of their super-elastic quality they must be manufactured by machining rather than by twisting of tapered wire blanks. The instrument design must therefore be ground into the Ni-Ti tapered blanks, thus increasing their cost.
BASE-METAL PREFABRICATED CROWNS
Stainless steel crowns were introduced in 1950 and are recommended for the permanent restoration of primary teeth, particularly in children suffering from rampant caries or in situations where the crowns of the teeth are destroyed. The approximate composition of stainless steel used for crowns and its mechanical properties are listed in Table 16-6. A titanium-stabilized stainless steel may also be used. For purposes of comparison, the properties of a nickel-based alloy used for permanent prefabricated crowns and tin-based and aluminum-based alloys used for temporary prefabricated crowns are also shown. The mechanical properties of stainless steel and nickel-based materials are similar, and the high ductility is important in the clinical adaptation of the crowns. In addition, they have reasonable hardness and strength and, as a result, are classified as permanent restorations. The tin-based and aluminum-based temporary types also have high ductility, but are soft and have lower yield and tensile strengths and thus do not resist clinical wear as do the stainless steel and nickel-based types.
WROUGHT COBALT-CHROMIUM-NICKEL ALLOY
A cobalt-chromium-nickel alloy known as Elgiloy is available in wire and band form for various dental appliances. Elgiloy is nominally composed of 40% cobalt, 20% chromium, 15% nickel, 7% molybdenum, 2% manganese, 0.4% beryllium, 0.15% carbon, 15.4% iron, and 0.05% other. Of particular interest is the fact that beryllium serves to decrease the alloy’s melting point, facilitating manufacturing. In composition, this alloy resembles the cast base-metal alloys more than it does stainless steel.
PROCESSING AND MANIPULATION
The orthodontic wire is supplied in various tempers (amounts of cold work): soft, ductile, semispring temper, and spring temper. The wires are typically formed in a ductile condition, allowing them to be easily deformed and shaped into appliances and then heat-treated to maximize strength. The standard heat treatment, similar to the treatment used to relieve stress in a stainless steel wire, is 482° C for 7 minutes. Low-temperature heat treatment causes a phase change and stress relief. Excessive heat treatment can cause embrittlement.
The fabrication and soldering techniques used with Elgiloy are similar to those used with stainless steel wires. Elgiloy wires should be soldered with a silver solder in the presence of a fluoride flux or joined by spot welding.
PROPERTIES
The properties of Elgiloy as received are similar to those of stainless steel wires; however, its properties can be modified slightly by the heat treatment (7 minutes at 482° C) used to stress relieve a stainless steel wire. The mechanical properties of spring-temper Elgiloy wire are proportional limit, 1610 MPa; 0.2% yield strength, 1930 MPa; tensile strength, 2540 MPa; and Vickers hardness number, 700 kg/mm2. The number of 90-degree bends until failure for 0.36-mm and 0.46-mm wires is listed in Table 16-7 for as-received and heat-treated conditions. Note that temper, heat treatment, and wire size all affect this property. Bending moment—angular deflection curves for several Elgiloy wires are shown in Fig. 16-12. The stiffness in bending of the wires is similar; however, the angle at which permanent deformation occurs increases from soft-temper to spring-temper types. The permanent set after a 90-degree bend decreases from soft-temper to spring-temper types. Heat treatment for 7 minutes at 482° C causes an increase in the angle at which permanent deformation occurs but decreases the permanent set.
TABLE 16-7
Cold Bending of Cobalt-Chromium-Nickel (Elgiloy) Wires
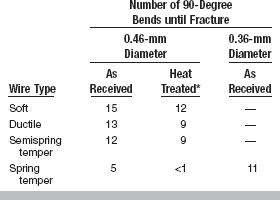
*Heated at 482° C for 7 minutes.
From Craig RG, editor: Dental materials: a problem oriented approach, St Louis, 1978, Mosby.
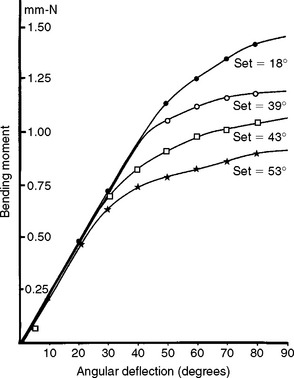
FIGURE 16-12 Bending moment—angular deflection curves for 0.46-mm diameter cobalt-chromium-nickel (Elgiloy) wires of the soft (***), ductile (

 ), ductile heat-treated for 7 minutes at 482° C (
), ductile heat-treated for 7 minutes at 482° C (

 ), and spring temper (•••) types. (From Craig RG, editor: Dental materials: a problem-oriented approach, St. Louis, 1978, Mosby.)
), and spring temper (•••) types. (From Craig RG, editor: Dental materials: a problem-oriented approach, St. Louis, 1978, Mosby.)
WROUGHT NICKEL-TITANIUM ALLOY
A wrought nickel-titanium alloy known as Nitinol was introduced as a wire for orthodontic appliances in 1972. Nitinol is characterized by its high resiliency, limited formability, and thermal memory.
COMPOSITION AND SHAPE-MEMORY EFFECT
The industrial alloy is 55% nickel and 45% titanium and possesses a temperature transition range (TTR). At temperatures below the TTR, the alloy can be deformed plastically. When the alloy is then heated from below to above the TTR, a temperature-induced crystallographic transformation from a martensitic to an austenitic microstructure occurs and the alloy will return to its original shape. Hence, nickel-titanium is called a shape-memory alloy. The orthodontic alloy contains several percent cobalt to lower the TTR. A number of variations of the Ni-Ti alloy have been developed in dentistry. Compositional variations lead to changes in the martensitic and austenitic start and finish temperatures and mechanical properties. Only those wires with austenitic finish temperatures less than 37° C exhibit super-elasticity.
PROPERTIES AND MANIPULATION
Mechanical properties of an orthodontic nickel-titanium alloy are compared with those of stainless steel and a beta-titanium alloy in tension, bending, and torsion in Table 16-4. The nickel-titanium alloy has the lowest elastic modulus and yield strength but the highest springback (maximum elastic deflection). As shown in Figs. 16-13 and 16-14, nickel-titanium has the lowest spring rate but the highest resiliency in bending and torsion of the three alloys used for orthodontic wires. Clinically, the low elastic modulus and high resiliency mean that lower and more constant forces can be applied with activations and an increased working range. The high springback is important if large deflections are needed, such as with poorly aligned teeth. Nitinol wire requires special bending techniques and cannot be bent over a sharp edge or into a complete loop; thus the wire is more suited for use with pretorqued, preangulated brackets. The alloy is brittle and therefore cannot be soldered or welded, so wires must be joined mechanically.
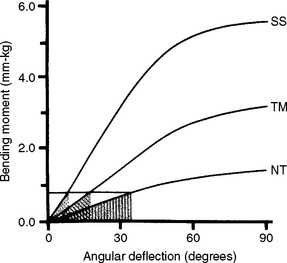
FIGURE 16-13 Stored energy at a fixed bending moment below the proportional limit for 0.48-mm by 0.64-mm wires of alloys stainless steel (SS), beta-titanium (TM), and nickel-titanium (NT). The stored energy is equal to the shaded areas under the curve for each wire. The spring rate is equal to the slope of each curve. (From Drake SR, Wayne DM, Powers JM, Asgar K: Am J Orthod 82:206, 1982.)
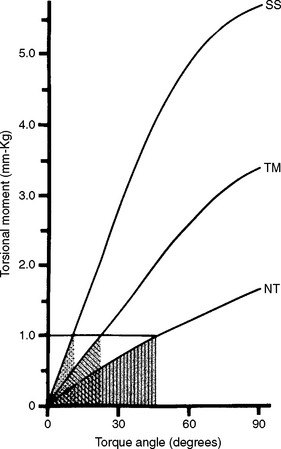
FIGURE 16-14 Stored energy at a fixed torsional moment below the proportional limit for 0.48-mm by 0.64-mm wires of alloys stainless steel (SS), beta-titanium (TM), and nickel-titanium (NT). The stored energy is equal to the shaded area under the curve for each wire. The spring rate is equal to the slope of each curve. (From Drake SR, Wayne DM, Powers JM, Asgar K: Am J Orthod 82:206, 1982.)
WROUGHT BETA-TITANIUM ALLOY
COMPOSITION AND MICROSTRUCTURE
A titanium-molybdenum alloy known as betatitanium was introduced in 1979 as a wrought orthodontic wire. As discussed previously, cp Ti exists in a hexagonal close-packed crystal lattice at temperatures below 883° C and in a body-centered cubic crystal lattice at higher temperatures. These structures are referred to as alpha-titanium and betatitanium, respectively. The beta form of Ti can be stabilized at room temperature by alloying with certain elements. Beta-titanium alloy for dental use has the composition 78% titanium, 11.5% molybdenum, 6% zirconium, and 4.5% tin and is supplied as wrought wire.
MANIPULATION
Beta-titanium wires can be shaped easily, and the wires can be soldered and welded. Joints can be made by electrical resistance welding. Under proper welding conditions, minimum distortion of the cold-worked microstructure occurs.
PROPERTIES
Compared with stainless steel and Elgiloy wires, beta-titanium wire has lower force magnitudes, a lower elastic modulus, higher springback (maximum elastic deflection), a lower yield strength, and good ductility, weldability, and corrosion resistance. The mechanical properties of betatitanium alloy in tension, bending, and torsion are compared with stainless steel and nickel-titanium alloys in Table 16-4 and Figs. 16-13 and 16-14. Beta-titanium alloy has values of yield strength, modulus of elasticity, and springback intermediate to those of stainless steel and Nitinol. Its formability and weldability are advantages over Nitinol, and it has a larger working range than do stainless steel or Elgiloy wires.
OTHER ORTHODONTIC WIRES
Recent developments in orthodontic wires include a titanium-based alloy (Ti-15V-3Cr-3Al-3Sn), which is reported to offer a yield strength/modulus ratio slightly greater than that of beta-titanium. Fiber-reinforced thermoplastic wires have also been studied. Candidate fibers include fiberglass and aramid. Candidate resins include polycarbonate and polyethylene terephthalate glycol. For each resin/fiber system, there is a heating or working range where the material can be formed without property degradation. This temperature range is primarily related to the glass transition temperature (Tg) of the resin matrix. A temperature above the Tg is necessary to allow sufficient softening. However, higher temperatures will lead to structural changes and reduction in flexural modulus.
SUMMARY OF ORTHODONTIC WIRES
Properties of stainless steel, nickel-titanium, and beta-titanium wires in tension, bending, and torsion are compared in Table 16-4. Of the three types of wires, stainless steel wire has the highest values of yield strength, elastic modulus, and spring rate and the lowest springback (elastic deflection or yield strength/elastic modulus). Elgiloy is easily deformed and shaped. Nickel-titanium alloy has the lowest elastic modulus and yield strength but the highest springback. Nickel-titanium has the lowest spring rate but highest resiliency in bending and torsion of the three alloys used for orthodontic wires. The disadvantages are that it is hard to bend and cannot be soldered, welded, or heat-treated. Beta-titanium offers an intermediate force delivery system and greater formability and weldability.
SOME OTHER ALLOYS
Certain alloys have considerable value for dental instruments and equipment because of their special properties. For example, Monel metal is an alloy of copper and nickel used for equipment parts because of its good physical properties and resistance to tarnish or corrosion. It has not been popular for constructing appliances placed in the mouth because of difficulties in manipulation. Such an alloy has a composition of approximately 28% copper, 68% nickel, 2% iron, 1.5% manganese, and 0.2% silicon.
Other stainless steels are also used, to lesser extents. A steel with 3% Al may be heat-treated and can be used in wrought condition. Following forming, heat treatment at 900° C for 1 hour leads to an increase in properties. The heat treatment forms coherent precipitate Ni3Al, which strain-hardens the lattice.
The possibility of developing satisfactory substitutes for gold in dental appliances is far from being exhausted. Numerous new alloys and technologies based on nanostructures and powder metallurgy are being developed for use by the engineering profession, some of which eventually may be found to be satisfactory for dental purposes. Many metals, such as tantalum, molybdenum, columbium, vanadium, and gallium, are becoming available in increasing quantities. These metals and their alloys, along with chromium, nickel, cobalt, titanium, stainless steel, and various copper, aluminum, or magnesium alloys, may be developed to possess physical and chemical qualities that satisfy the requirements of various dental applications.
Asgar, K, Allan, FC. Microstructure and physical properties of alloy for partial denture castings. J Dent Res. 1968;47:189.
Asgar, K, Peyton, FA. Effect of casting conditions on some mechanical properties of cobalt-base alloys. J Dent Res. 1961;40:73.
Asgar, K, Peyton, FA. Effect of microstructure on the physical properties of cobalt-based alloys. J Dent Res. 1961;40:63.
Asgar, K, Peyton, FA. Flow and fracture of dental alloys determined by a microbend tester. J Dent Res. 1962;41:142.
Asgar, K, Techow, BO, Jacobson, JM. A new alloy for partial dentures. J Prosthet Dent. 1970;23:36.
Baran, GR. The metallurgy of Ni-Cr alloys for fixed prosthodontics. J Prosthet Dent. 1983;50:639.
Bates, JF. Studies related to the fracture of partial dentures. Br Dent J. 1965;118:532.
Bumgardner, JD, Lucas, LC. Surface analysis of nickel-chromium dental alloys. Dent Mater. 1993;9:252.
Ben-Ur, Z, Patael, H, Cardash, HS, et al. The fracture of cobalt-chromium alloy removable partial dentures. Quint Int. 1986;17:797.
Bergman, M, Bergman, B, Soremark, R. Tissue accumulation of nickel released due to electrochemical corrosion of non-precious dental casting alloys. J Oral Rehabil. 1980;7:325.
Brune, D, Beltesbrekke, H. Dust in dental laboratories: types and levels in specific operations. J Prosthet Dent. 1980;43:687.
Cecconi, BT. Removable partial denture research and its clinical significance. J Prosthet Dent. 1978;39:203.
Cecconi, BT, Asgar, K, Dootz, ER. Fit of the removable partial denture base and its effect on abutment tooth movement. J Prosthet Dent. 1971;25:515.
Cheng, TP, Tsai, WT, Chern Lin, JH. The effect of beryllium on the corrosion resistance of nickel-chromium dental alloys. J Mater Sci Mater Med. 1990;1:211.
Council on Dental Materials, Instruments, and Equipment. Report on base metal alloys for crown and bridge applications: benefits and risks. J Am Dent Assoc. 1985;111:479.
Cunningham, DM. Comparison of base metal alloys and Type IV gold alloys for removable partial denture frameworks. Dent Clin North Am. 1973;17:719.
Frank, RP, Brudvik, JS, Nicholls, JI. A comparison of the flexibility of wrought wire and cast circumferential clasps. J Prosthet Dent. 1983;49:471.
Geis-Gerstorfer, J, Passler, K. Studies of the influence of Be content on corrosion behaviour and mechanical properties of Ni25Cr10Mo alloys. Dent Mater. 1993;9:177.
Hinman, RW, Lynde, TA, Pelleu, GB, Jr., et al. Factors affecting airborne beryllium concentrations in dental space. J Prosthet Dent. 1975;33:210.
Lucas, LC, Lemons, JE. Biodegradation of restorative metal systems. Adv Dent Res. 1992;65:32.
Mohammed, H, Asgar, K. A new dental super alloy system, I, II, III. J Dent Res. 1973;52:136. 145, 151
Morris, HF, Asgar, K. Physical properties and microstructure of four new commercial partial denture alloys. J Prosthet Dent. 1975;33:36.
Morris, HF, Asgar, K, Rowe, AP, et al. The influence of heat treatments on several types of base-metal removable partial denture alloys. J Prosthet Dent. 1979;41:388.
Rowe, AP, Bigelow, WC, Asgar, K. Effect of tantalum addition to a cobalt-chromium-nickel base alloy. J Dent Res. 1974;53:325.
Smith, DC, Tissue reaction to noble and base metal alloys. Smith, DC, William, DF, eds. Biocompatibility of dental materials, 4. Boca Raton: CRC Press, 1982.
Strandman, E. Influence of different types of acetylene-oxygen flames on the carbon content of dental Co-Cr alloy. Odontol Rev. 1976;27:223.
Vallittu, PK, Kokkonen, M. Deflection fatigue of cobalt-chromium, titanium, and gold alloy cast denture clasps. J Prosthet Dent. 1995;74:412.
Wakasa, K, Yamaki, M. Dental application of the 30Ni-30Cu-40Mn ternary alloy system. J Mater Sci: Mater Med. 1990;1:44.
Wakasa, K, Yamaki, M. Corrosive properties in experimental Ni-Cu-Mn based alloy systems for dental purposes. J Mater Sci: Mater Med. 1990;1:171.
Wakasa, K, Yamaki, M. Tensile behaviour in 30Ni-30Cu-30Mn based alloys for a dental application. J Mater Sci: Mater Med. 1991;2:71.
Wataha, JC, Craig, RG, Hanks, CT. The release of elements of dental casting alloys into cell-culture medium. J Dent Res. 1991;70:1014.
Wataha, JC, Craig, RG, Hanks, CT. The effects of cleaning on the kinetics of in vitro metal release from dental casting alloys. J Dent Res. 1992;71:1417.
Waterstrat, RM. New alloys. J Am Dent Assoc. 1992;123:33.
Yong, T, De Long, B, Goodkind, RJ, et al. Leaching of Ni, Cr and Be ions from base metal alloys in an artificial oral environment. J Prosthet Dent. 1992;68:692.
Alapati, SB, Brantley, WA, Svec, TA, et al. Observations of nickel-titanium rotary endodontic instruments that fractured during clinical use. J Endod. 2005;31:40.
Alapati, SB, Brantley, WA, Svec, TA, et al. Scanning electron microscope observations of new and used nickeltitanium rotary files. J Endod. 2003;29:667.
Andreasen, GF, Barrett, RD. An evaluation of cobaltsubstituted Nitinol wire in orthodontics. Am J Orthod. 1973;63:462.
Andreasen, GF, Brady, PR. A use hypothesis for 55 Nitinol wire for orthodontics. Angle Orthod. 1972;42:172.
Andreasen, GF, Morrow, RE. Laboratory and clinical analyses of Nitinol wire. Am J Orthod. 1978;73:142.
Andreasen, GF, Bigelow, H, Andrews, JG. 55 Nitinol wire: force developed as a function of “elastic memory,”. Aust Dent J. 1979;24:146.
Braff, MH. A comparison between stainless steel crowns and multisurface amalgams in primary molars. J Dent Child. 1975;46:474. Nov-Dec
Brantley, WA, Augat, WS, Myers, CL, et al. Bending deformation studies of orthodontic wires. J Dent Res. 1978;57:609.
Brantley, WA, Svec, TA, Iijima, M, et al. Differential scanning calorimetric studies of nickel-titanium rotary endodontic instruments after simulated clinical use. J Endod. 2002;28:774.
Brantley, WA, Svec, TA, Iijima, M, et al. Differential scanning calorimetric studies of nickel titanium rotary endodontic instruments. J Endod. 2002;28:567.
Burstone, CJ, Goldberg, AJ. Beta titanium: a new orthodontic alloy. Am J Orthod. 1980;77:121.
Chen, R, Zhi, YF, Arvy Stas, MG. Advanced Chinese NiTi alloy wire and clinical observations. Angle Orthod. 1992;62:15.
Dolan, DW, Craig, RG. Bending and torsion of endodontic files with rhombus cross sections. J Endod. 1982;8:260.
Drake, SR, Wayne, DM, Powers, JM, et al. Mechanical properties of orthodontic wires in tension, bending, and torsion. Am J Orthod. 1982;82:206.
Goldberg, J, Burstone, CJ. An evaluation of beta titanium alloys for use in orthodontic appliances. J Dent Res. 1979;58:593.
Goldberg, AJ, Burstone, CJ, Hadjinikolaoa, I, et al. Screening of matrices and fibers for reinforced thermoplastics intended for dental applications. J Biomed Mater Res. 1994;28:167.
Haïkel, Y, Serfaty, R, Bateman, G, et al. Dynamic and cyclic fatigue of engine-driven rotary nickel-titanium endodontic instruments. J Endod. 1999;25:434.
Kapila, S, Sachdeva, R. Mechanical properties and clinical applications of orthodontic wires. Am J Orthod Dentofac Orthop. 1989;96:100.
Kusy, RP. Comparison of nickel-titanium and beta titanium wire sizes to conventional orthodontic arch wire materials. Am J Orthod. 1981;79:625.
Neal, RG, Craig, RG, Powers, JM. Cutting ability of K-type endodontic files. J Endod. 1983;9:52.
Neal, RG, Craig, RG, Powers, JM. Effect of sterilization and irrigants on the cutting ability of stainless steel files. J Endod. 1983;9:93.
Newman, JG, Brantley, WA, Gorstein, H. A study of the cutting efficiency of seven brands of endodontic files in linear motion. J Endod. 1983;9:316.
Parmiter, OK. Wrought stainless steels. In ASM metals handbook. Cleveland: American Society for Metals; 1948.
Patel, AP, Goldberg, AJ, Burstone, CJ. The effect of thermoforming on the properties of fiber-reinfroced composite wires. J Appl Biomat. 1992;3:177.
Peterson, DS, Jubach, TS, Katora, M. Scanning electron microscope study of stainless steel crown margins. ASDC J Dent Child. 1978;45:376. Sept-Oct
Schwaninger, B, Sarkar, NK, Foster, BE. Effect of long-term immersion corrosion on the flexural properties of Nitinol. Am J Orthod. 1982;82:45.
Shastry, CV, Goldberg, AJ. The influence of drawing parameters on the mechanical properties of two beta-titanium alloys. J Dent Res. 1983;62:1092.
Stokes, OW, Fiore, PM, Barss, JT, et al. Corrosion in stainless-steel and nickel-titanium files. J Endod. 1999;25:17.
Svec, TA, Powers, JM. The deterioration of rotary nickel-titanium files under controlled conditions. J Endod. 2002;28:105.
Svec, TA, Powers, JM. A method to assess rotary nickel-titanium files. J Endod. 2000;26:517–518.
Thompson, SA. An overview of nickel-titanium alloys used in dentistry. Int Endod J. 2000;33:297.
Waters, NE. Superelastic nickel-titanium wires. Br J Orthod. 1992;19:319.
Wilkinson, JV. Some metallurgical aspects of orthodontic stainless steel. Am J Orthod. 1962;48:192.
Wilson, DF, Goldberg, AJ. Alternative beta-titanium alloys for orthodontic wires. Dent Mater. 1987;3:337.
Yoneyama, T, Doi, H. Superelasticity and thermal behaviour of NiTi orthodontic archwires. Dent Mater. 1992;11:1.
Ducheyne, P, Kohn, D, Smith, TS. Fatigue properties of cast and heat treated Ti-6Al-4V alloy for anatomic hip prostheses. Biomaterials. 1987;8:223.
Ida, K, Togaya, T, Tsutsumi, S, et al. Effect of magnesia investments on the dental casting of pure titanium or titanium alloys. Dent Mater J. 1982;1:8.
Ida, K, Tani, Y, Tsutsumi, S, et al. Clinical applications of pure titanium crowns. Dent Mater J. 1985;4:191.
Kimura H, Izumi O, eds. Titanium ’80 science and technology. Warrendale, PA: The Metallurgical Society of AIME, 1980.
Kohn, DH, Ducheyne, P. A parametric study of the factors affecting the fatigue strength of porous coated Ti6Al-4V implant alloy. J Biomed Mater Res. 1990;24:1483.
Kohn, DH, Ducheyne, P. Microstructural refinement of beta-sintered and porous coated Ti-6Al-4V by temporary alloying with hydrogen. J Mater Sci. 1991;26:534.
Kohn, DH, Ducheyne, P. Tensile and fatigue strength of hydrogen treated Ti-6Al-4V alloy. J Mater Sci. 1991;26:328.
Lutjering, G, Gysler, A. Critical review-fatigue. In: Lutjering G, Zwicker U, Bunk W, eds. Titanium, science and technology. Oferursel, West Germany: Deutsche Gesellschaft fur Metallkunde, 1985.
Moser, JB, Lin, JH, Taira, M, et al. Development of dental Pd-Ti alloys. Dent Mater. 1985;1:37.
Okabe, T, Hero, H. The use of titanium in dentistry. Cells Mater. 1995;5:211.
Peters, M, Gysler, A, Lutjering, G. Influence of microstructure on the fatigue behavior of Ti-6Al-4V. In: Kimura H, Izumi O, eds. Titanium ’80 science and technology. Warrendale, PA: The Metallurgical Society of AIME, 1980.
Szurgot, KC, Marker, BC, Moser, JB, et al. The casting of titanium for removable partial dentures. Dent Mater Sci QDT Yearbook. 1988:171.
Taira, M, Moser, JB, Greener, EH. Studies of Ti alloys for dental castings. Dent Mater. 1989;5:45.
Voitik, AJ. Titanium dental castings, cold worked titanium restorations—yes or no? Trends and Techniques. Dec 1991;8(10):23.
Waterstrat, RM. Comments on casting of Ti-13Cu-4.5Ni alloy Pub No (NIH) 77–1227. Washington, DC: DHEW, 1977;224.
Yamauchi, M, Sakai, M, Kawano, J. Clinical application of pure titanium for cast plate dentures. Dent Mater J. 1988;7:39.