Hyperuricaemia and gout
The pathophysiology of gout
Gout is an inflammatory arthritis produced when monosodium urate crystals are deposited in synovial fluid. It is associated with a raised plasma uric acid (urate) concentration. Uric acid is a relatively insoluble product of catabolism of the nucleic acid purine bases guanine and adenine (Fig. 31.1). The immediate precursors of uric acid are xanthine and hypoxanthine, which are more water soluble. Uric acid is normally eliminated by the kidney. It is filtered at the glomerulus and then reabsorbed from the proximal tubule, and subsequently there is net secretion into the late proximal tubule.
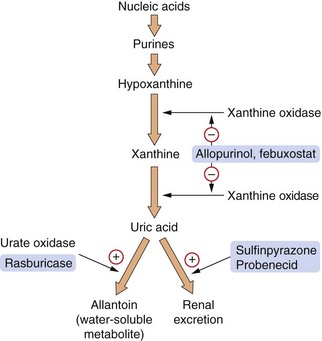
Fig. 31.1 The pathway for production of uric acid from purines and the sites of action of some of the drugs used in gout and hyperuricaemia.
Hyperuricaemia results from the following factors.
 Overproduction of uric acid, which this can arise from:
Overproduction of uric acid, which this can arise from:
 excessive cell destruction (e.g. lymphoproliferative or myeloproliferative disorders, especially during treatment for cancer, Ch. 52),
excessive cell destruction (e.g. lymphoproliferative or myeloproliferative disorders, especially during treatment for cancer, Ch. 52),
 inherited defects that increase purine synthesis,
inherited defects that increase purine synthesis,
 Reduced renal excretion of uric acid: more than 90% of filtered uric acid is reabsorbed in the early proximal tubule, but an amount equivalent to 6–10% of the filtered load is secreted by an active organic acid transporter in the second part of the proximal tubule (Ch. 14). Renal failure and certain drugs (e.g. loop and thiazide-type diuretics, low-dose aspirin, ciclosporin and lactate formed from excess alcohol) will reduce the tubular secretion of uric acid. Reduced uric acid excretion accounts for at least 80% of cases of gout.
Reduced renal excretion of uric acid: more than 90% of filtered uric acid is reabsorbed in the early proximal tubule, but an amount equivalent to 6–10% of the filtered load is secreted by an active organic acid transporter in the second part of the proximal tubule (Ch. 14). Renal failure and certain drugs (e.g. loop and thiazide-type diuretics, low-dose aspirin, ciclosporin and lactate formed from excess alcohol) will reduce the tubular secretion of uric acid. Reduced uric acid excretion accounts for at least 80% of cases of gout.
A high plasma concentration of uric acid is often an incidental finding and does not lead to symptoms, but when the plasma concentration exceeds about 0.42 mmol⋅L−1 monosodium urate crystals can be deposited in tissues, forming a tophus. If these crystals are shed from a tophus in the synovial membrane or cartilage of a joint they produce an extremely painful acute arthritis that presents with the clinical syndrome of gout. In brief, the crystals are phagocytosed by macrophage cells within the synovium, which release mediators such as interleukin-1β. These mediators activate mast cells and endothelial cells with expression of adhesion molecules and chemokines. Uric acid crystals also provide a surface on which complement C5 is cleaved, with formation of complement membrane attack complex. The activated endothelial cells and complement membrane attack complex attract neutrophil leucocytes which release proteolytic and lysosomal enzymes that enhance tissue inflammation, destroy cartilage and damage the joint. Most attacks of gout are self-limiting, probably in part due to coating of the uric acid crystals with protein, which reduces their irritant properties. Acute gout usually presents with rapid onset of severe joint pain that reaches maximum intensity within 24 h. Pseudogout, due to deposition of calcium pyrophosphate crystals, has a similar clinical presentation.
Gout in younger people usually affects a single joint, with repeated acute attacks if the underlying cause is not treated. In the elderly, a chronic arthritis affecting multiple joints can occur. The diagnosis of gout is confirmed by the finding of monosodium urate crystals in the affected joint. With persistent hyperuricaemia, chronic urate deposits are sometimes found in tendon sheaths and soft tissues. Excess uric acid can also be deposited in the interstitium of the kidney or form stones in the renal calyces, both of which can produce progressive renal damage.
Drugs for the treatment of gout and prevention of hyperuricaemia
Mechanism of action
Colchicine interferes with several steps in the inflammatory cascade, particularly inhibiting recruitment and actions of neutrophil leucocytes in the gouty joint:
 it reduces the production of inflammatory mediators by macrophages and downregulates their receptors on synovial and endothelial cells. Colchicine also inhibits the production of chemotaxins, which attract leucocytes into inflamed tissue,
it reduces the production of inflammatory mediators by macrophages and downregulates their receptors on synovial and endothelial cells. Colchicine also inhibits the production of chemotaxins, which attract leucocytes into inflamed tissue,
 colchicine disrupts the assembly of microtubules in neutrophil leucocytes by forming a complex with tubulin in the cell. This impairs the adhesion of neutrophils to endothelial cells, which reduces their recruitment into the inflamed joint, and also impairs phagocytosis of crystals if the neutrophil does enter the joint. In addition, if crystals are phagocytosed into the neutrophil colchicine inhibits the subsequent release of enzymes and free radicals that damage the joint.
colchicine disrupts the assembly of microtubules in neutrophil leucocytes by forming a complex with tubulin in the cell. This impairs the adhesion of neutrophils to endothelial cells, which reduces their recruitment into the inflamed joint, and also impairs phagocytosis of crystals if the neutrophil does enter the joint. In addition, if crystals are phagocytosed into the neutrophil colchicine inhibits the subsequent release of enzymes and free radicals that damage the joint.
All of these actions give colchicine a specific anti-inflammatory effect in the gouty joint; it is ineffective in other forms of inflammatory arthritis. Other uses of colchicine include the management of recurrent pericarditis and familial Mediterranean fever.
Xanthine oxidase inhibitors
Mechanism of action
Allopurinol is an analogue of hypoxanthine, which is an intermediate in the pathway that generates uric acid. Both allopurinol and its major metabolite competitively inhibit the enzyme xanthine oxidase for which hypoxanthine is the natural substrate, thereby reducing uric acid formation (Fig. 31.1). Febuxostat is a non-purine selective xanthine oxidase inhibitor. Although plasma xanthine and hypoxanthine concentrations increase when these drugs are given, they do not crystallize. Because of their greater water solubility, their concentrations remain well below saturation levels even with maximal xanthine oxidase inhibition. Xanthine and hypoxanthine are reincorporated into the purine synthetic cycle, and this decreases the need for de novo purine formation.
Pharmacokinetics
Allopurinol is well absorbed from the gut and converted in the liver to an active metabolite with a long half-life, oxipurinol (alloxanthine). Febuxostat is well absorbed from the gut; it is eliminated by both metabolism and renal excretion and has a variable half-life (1–15 h).
Unwanted effects
 An increased risk of acute gout during the first few weeks of treatment; this may be caused by fluctuations in plasma uric acid, possibly through uric acid release from tissue deposits.
An increased risk of acute gout during the first few weeks of treatment; this may be caused by fluctuations in plasma uric acid, possibly through uric acid release from tissue deposits.
 Hypersensitivity reactions with allopurinol, especially in people with renal impairment. These reactions include serious rashes such as Stevens–Johnson syndrome or toxic epidermal necrolysis.
Hypersensitivity reactions with allopurinol, especially in people with renal impairment. These reactions include serious rashes such as Stevens–Johnson syndrome or toxic epidermal necrolysis.
 Drug interactions: allopurinol and febuxostat inhibit the metabolism of the cytotoxic drugs mercaptopurine and azathioprine (Ch. 52) because these are also metabolised by xanthine oxidase.
Drug interactions: allopurinol and febuxostat inhibit the metabolism of the cytotoxic drugs mercaptopurine and azathioprine (Ch. 52) because these are also metabolised by xanthine oxidase.
Rasburicase
Rasburicase is a recombinant version of the enzyme urate oxidase which catalyses the oxidation of uric acid to a soluble metabolite, allantoin. This enzyme is present in mammals other than humans; the recombinant version is produced by a genetically modified strain of the fungus Aspergillus flavus. Rasburicase is used for prophylaxis of hyperuricaemia during treatment of malignancies with chemotherapy.
Pharmacokinetics
Rasburicase is given intravenously and is metabolised by peptide hydrolysis in plasma.
Uricosuric agents
Mechanism of action
Sulfinpyrazone competitively inhibits transporters responsible for reabsorption of uric acid in the proximal tubule, therefore increasing urate concentrations in urine and reducing levels in plasma. There is a risk of precipitation of uric acid crystals in the kidney, particularly during the early stages of treatment, which can be prevented by maintaining a high fluid intake and alkaline urine (using potassium citrate or sodium bicarbonate) and by slowly titrating the dose. Aspirin and other salicylates should not be given with uricosuric drugs, because low doses of salicylates inhibit tubular uric acid secretion.
Probenecid used to be used as a uricosuric agent, but is now only available in the UK by special order for prevention of nephrotoxicity caused by the antiviral drug cidofovir.
Treatment of gout
Efforts should always be made to identify and remove precipitating causes of gout, particularly enquiring about alcohol intake and reviewing concurrent drug therapy. For acute attacks, non-steroidal anti-inflammatory drugs (NSAIDs; Ch. 29) are the treatment of choice. Aspirin should be avoided because at low doses it can inhibit renal excretion of uric acid and increase plasma urate concentration. Cyclo-oxygenase 2 (COX-2)-selective anti-inflammatory drugs are as effective as classic NSAIDs (Ch. 29). Colchicine is usually reserved for people who are intolerant of NSAIDs, or who have a contraindication to their use. Intra-articular injection of corticosteroid can be very effective if a single joint is involved, especially if other treatments are contraindicated. Oral corticosteroids, for example prednisolone (Ch. 44), are reserved for resistant episodes of gout. A 5-day high-dose regimen can be used, or two days of a high dose followed by a gradual reduction over 8–12 days to minimise the risk of a rebound flare of symptoms.
Prevention of gout attacks
Allopurinol is given for prophylaxis against recurrent attacks of acute gout, for chronic tophus formation in the tissues, or for the prevention of uric acid-induced renal damage. It is also given prophylactically before cytotoxic chemotherapy, when tissue breakdown releases purines, which generate large amounts of uric acid. To prevent gout, the serum uric acid concentration should be reduced to less than 0.36 mmol⋅L−1, although it may be necessary to go below 0.3 mmol⋅L−1 to reabsorb gouty tophi.
Allopurinol should not be used during an acute attack of gout since it can prolong the attack, so waiting 1–2 weeks after symptoms have settled is advisable. There are two strategies to reduce the risk of provoking an attack when allopurinol is started in someone with hyperuricaemia. First, a low dosage of allopurinol can be given initially with slow dose titration until the target plasma uric acid concentration is achieved. Secondly, low dosage of an NSAID or colchicine can be given during the first 3 months of treatment. Febuxostat or sulfinpyrazone are reserved for people who do not tolerate allopurinol. Sulphinpyrazone is also used in combination with allopurinol for resistant hyperuricaemia. Low-dose NSAIDs or colchicine are sometimes used long term to prevent gout, although good data on their efficacy are lacking.
Prophylactic treatment should usually be life-long, since recurrence of gout or tophi frequently occurs if treatment is stopped. Short-term prophylaxis is possible when allopurinol is used during cytotoxic chemotherapy.
Rasburicase is used when intravenous prophylaxis against gout is required during cancer chemotherapy.
One-best-answer (OBA) question
Choose the most appropriate statement below concerning drugs used in the treatment of gout.
A Sodium urate is more water soluble than its precursor hypoxanthine.
B Febuxostat is a purine analogue.
C Allopurinol enhances the renal secretion of uric acid.
D Colchicine inhibits the release of neutrophil proteases that cause joint damage.
E Aspirin is safe to use in acute attacks of gout to reduce the pain and inflammation.
Case-based questions
A 56-year-old man awoke in the night with sudden severe pain in his first metatarsophalangeal joint, which lasted for a week. Over the next few months, he had similar acute episodes of pain in his ankles and knees, as well as his big toe. He had hypertension but no other vascular disease. The GP suspected gout and referred him to a specialist.
A What treatment should the GP institute for the acute attacks, prior to the specialist diagnosis?
B What test could the rheumatologist do to confirm the suspected diagnosis?
C The diagnosis of gout was confirmed. What is the cause of gout?
D What would you prescribe for prophylaxis to reduce recurrent attacks and how does this agent act?
E The chosen treatment was only partially effective; what additional treatment could you prescribe?
F What might be the consequences of inadequate treatment of this man?
A Incorrect. Hypoxanthine is more water soluble than urate; this is the rationale for the use of allopurinol, which inhibits conversion of hypoxanthine to urate by inhibiting xanthine oxidase.
B Incorrect. Febuxostat is a non-purine inhibitor of xanthine oxidase.
C Incorrect. Allopurinol prevents uric acid formation; the renal secretion of urate is enhanced by sulfinpyrazone and other uricosuric drugs.
D Correct. Reducing neutrophil leucocyte activity is one of the anti-inflammatory mechanisms of colchicine in gout.
E Incorrect. Unlike non-steroidal anti-inflammatory drugs (NSAIDs) used in acute gout, aspirin can inhibit the renal secretion of urate, exacerbating the gout.
Case-based answers
A The treatment of choice for an acute attack is an NSAID to reduce pain and inflammation. Indometacin is often used and is effective within two days. Colchicine or glucocorticoids can be used in people intolerant to NSAIDs, but both have significant unwanted effects. Aspirin and other salicylates should be avoided as at low doses they reduce uric acid excretion, although at high doses they are uricosuric.
B Plasma uric acid will be raised. An arthrocentesis sample will show sodium urate crystals. Infection should be excluded in an acutely inflamed joint.
C Gout is caused by relatively insoluble sodium urate, a product of purine metabolism, crystallising in the joint space. People who develop gout have had hyperuricaemia for years. Overproduction of uric acid due to dietary purines (e.g. in meat and fish) or excessive alcohol consumption can contribute to gout, but in most people hyperuricaemia is caused by impaired renal clearance of uric acid.
D Hyperuricaemia is treated after resolution of the acute attack. People who overproduce uric acid are best treated with allopurinol, which reduces plasma uric acid by inhibiting xanthine oxidase. This increases concentrations of hypoxanthine and xanthine, which are more water soluble than urate.
E A low renal excretion of uric acid may be treated with a uricosuric drug (sulfinpyrazone); this inhibits the reabsorption of uric acid in the proximal convoluted tubule.
F Untreated gout can lead to chronic joint damage and formation of kidney stones. A significant number of people with gout will have hypertension and increased risk of cardiovascular and renal disease.
Compendium: drugs used for gout and hyperuricaemia
| Drug | Kinetics (half-life) | Comments |
| Allopurinol | High oral bioavailability; eliminated by metabolism to oxipurinol (0.5–2.0 h), which is less potent but has longer half-life (10–40 h) and may accumulate; both compounds are renally excreted | Xanthine oxidase inhibitor; used for prophylaxis of gout and of hyperuricaemia associated with cancer chemotherapy; given orally |
| Colchicine | Good oral absorption; rapidly eliminated from plasma (<1 h) but may be sequestered in leucocytes with a half-life of about 60 h | Anti-inflammatory drug used for acute gout and short-term prophylaxis during initial therapy with other drugs; given orally |
| Febuxostat | Eliminated by glucuronide conjugation and renal excretion (45–50%); highly variable half-life (1–15 h) | Non-purine xanthine oxidase inhibitor; used for prophylaxis in patients intolerant to allopurinol or when allupurinol is contraindicated; given orally |
| Probenecid | Complete oral bioavailability; hepatic metabolism and renal excretion (4–17 h); some active metabolites | Uricosuric drug; used to prevent nephrotoxicity associated with the use of the antiretroviral drug cidofovir; given orally; only available on special order in the UK |
| Rasburicase | Recombinant peptide eliminated by proteolysis (18–22 h) | Recombinant form of fungal urate oxidase which converts urate to soluble allantoin; used for hyperuricaemia during initial chemotherapy of haematological malignancy; given intravenously |
| Sulfinpyrazone | Good oral absorption; metabolised to an inactive sulphone and a sulphide analogue that inhibits platelet aggregation (4–5 h) | Uricosuric drug; used for gout prophylaxis and hyperuricaemia; given orally |
For non-steroidal anti-inflammatory drugs (NSAIDs), see Ch 29.
Burns, CM, Wortmann, RL. Gout therapeutics: new drugs for an old disease. Lancet. 2011;377:165–177.
Keith, MP, Gilliland, WR. Update in the management of gout. Am J Med. 2007;120:221–224.
Neogi, T. Gout. N Engl J Med. 2011;364:443–452.
Underwood, M. Diagnosis and management of gout. BMJ. 2006;332:1315–1319.