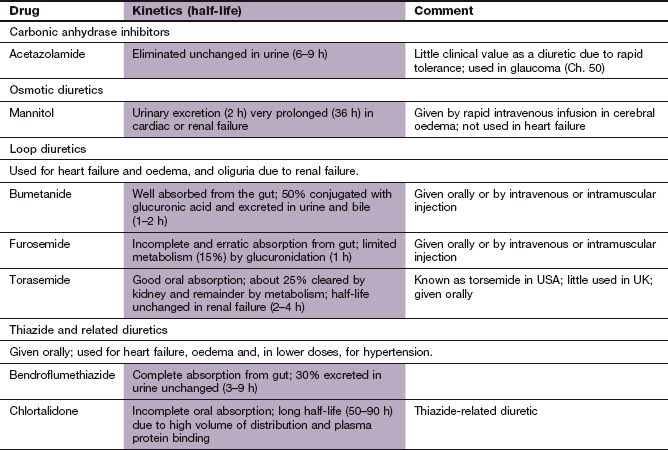
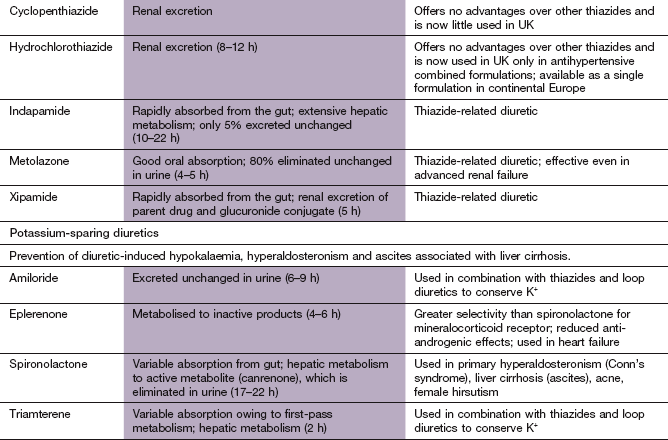
Diuretics
Almost all diuretics act on the kidney to increase the tubular concentration and elimination of Na+ ions (natriuresis), with a concurrent excretion of water. Loss of water with the Na+ ions depletes intravascular volume. Diuretics are used in the management of a wide range of conditions that produce oedema (e.g. heart failure, cirrhosis of the liver and nephrotic syndrome) and for the treatment of hypertension.
Functions of the kidney
The kidney has several important functions:
 regulation of plasma electrolyte concentrations and fluid balance,
regulation of plasma electrolyte concentrations and fluid balance,
 regulation of acid–base balance,
regulation of acid–base balance,
Of these, a basic knowledge of the mechanisms of electrolyte and fluid handling by the kidney is essential for understanding the uses and unwanted effects of diuretics.
The kidney and maintenance of salt and water balance
Each day the renal glomeruli of a healthy adult filter about 180 L of fluid (about 20% of the plasma that enters the glomerular capillaries), together with its content of ions such as Na+, K+ and Cl–. Since the urine output is only 1–2 L per day, it is clear that most of the filtered fluid is absorbed back from the tubule into the blood. Different regions of the tubule and collecting duct vary in their capacity to reabsorb water and solutes (Figs 14.1 and 14.2).
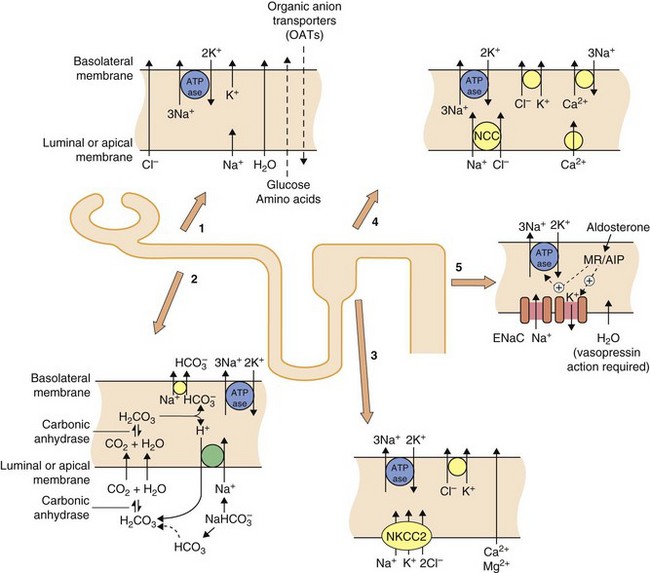
Fig. 14.1 Transport mechanisms for solutes in the kidney.
In all segments of the renal tubule there is active transport of Na+ out of and K+ into the cell across the basolateral membrane using Na+/K+-ATPase proton pumps. This sets up electrochemical gradients for the transport of other ions. In the proximal tubule (sites 1 and 2), considerable amounts of Na+, glucose and amino acids are taken up from the lumen, along with water which crosses via aquaporin channels. The principal function of the organic anion transporters (OATs) (site 1), including OAT1 and OAT3 (Ch. 2) is the elimination of metabolites of ingested xenobiotics. Transport by OATs also enables diuretic drugs such as furosemide and bendroflumethiazide to gain access to their sites of action on apical membranes in the tubule. Hydrogen ions are excreted in exchange for Na+ uptake and this, in part, depends upon the activity of carbonic anhydrase (site 2). In the ascending limb of the loop of Henle (site 3), the luminal membrane has a Na+/K+/2Cl− co-transporter (NKCC2) but is impermeable to water. In the proximal part of the distal tubule (cortical diluting segment; site 4) Na+ and Cl− ions are reabsorbed by the Na+/Cl− co-transporter (NCC), but water is not reabsorbed. Ca2+ also exchanges with three Na+ at the basolateral border at this site. In the distal part of the distal tubule and collecting duct (site 5) Na+ is reabsorbed from the lumen via an epithelial Na+ channel (ENaC), in exchange for loss of K+ into the lumen. The expression and activity of ENaC and the basolateral Na+/K+-ATPase pump are regulated by aldosterone acting via mineralocorticoid receptors (MRs) and aldosterone-induced proteins (AIPs). Water is reabsorbed in the collecting duct under the influence of antidiuretic hormone (ADH, vasopressin) acting through vasopressin receptors in the basolateral membrane.
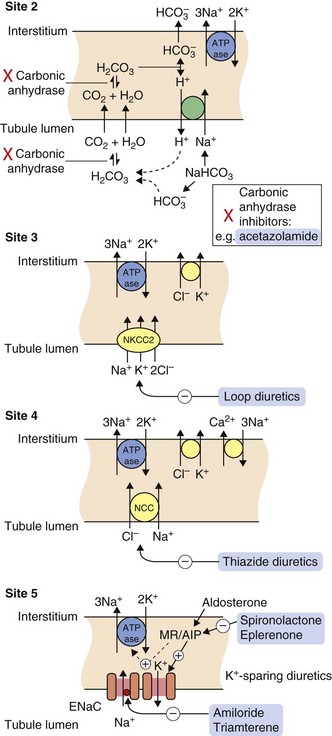
Fig. 14.2 Sites of action of diuretics.
For location of these sites in the tubule, see Fig. 14.1. Osmotic diuretics increase osmotic pressure through the tubule, reducing electrolyte reabsorption across the luminal membrane. Other drugs gain access to their sites of action after secretion into the tubule by the organic anion transporters (OATs) in the proximal tubule. Acetazolamide inhibits carbonic anhydrase (site 2) and is a weak self-limiting diuretic, now largely used for other conditions such as glaucoma. Loop diuretics such as furosemide block the luminal Na+/K+/2Cl− co-transporter (NKCC2) and inhibit up to 25% of filtered Na+ reabsorption (site 3). The thiazide diuretics inhibit the luminal Na+/Cl− co-transporter (NCC) (site 4) and can reduce reabsorption of 5–8% of filtered Na+. The potassium-sparing diuretics spironolactone and eplerenone compete with aldosterone for the mineralocorticoid receptor (MR), blocking the induction by aldosterone of the expression and activity of the epithelial Na+ channel (ENaC), the basolateral Na+/K+-ATPase pump and other aldosterone-induced proteins (AIP) (site 5). Amiloride and triamterene act directly on ENaC to block Na+ reabsorption. Potassium-sparing diuretics inhibit the reuptake of less than 3% of filtered Na+.
The proximal convoluted tubule
In the proximal convoluted tubule, about 65–70% of the filtered Na+ is reabsorbed together with an equivalent (isosmotic) amount of water. Therefore, on leaving the proximal tubule, the tubular fluid still has the same osmolarity as plasma. Reabsorption of ions from the proximal tubule into the renal tubular cells is passive (Fig. 14.1, site 1). The activity of the Na+/K+-ATPase pump on the basolateral surface of the tubular cell (transporting three Na+ out of the tubular cell in exchange for two K+) helps to establish the electrochemical gradient for passive Na+ reabsorption from the tubular lumen into the tubule cell. Water reabsorption by the proximal renal tubule is driven by the osmotic gradient across the tubular cells, which is created by the active transport of Na+ out of the cell across the basolateral membrane. Water reabsorption occurs via transmembrane aquaporin (AQP) channels. The extent of the proximal tubular reabsorption of Na+ and water is determined by two regulatory mechanisms: glomerulotubular feedback (enhanced tubular Na+ reabsorption when the glomerular filtration rate rises), and various neural and hormonal influences such as the sympathetic nervous system, angiotensin II, endothelin, dopamine and parathyroid hormone.
The proximal tubule has transporters for the secretion of organic anions into the tubular lumen (Fig. 14.1, site 1; see also Ch. 2), and the reabsorption of water-soluble essential nutrients, such as glucose and amino acids, from the lumen. The organic anion transporters (OATs) are important for the transport of many drugs and their metabolites from the blood into the tubule (e.g. see acetazolamide and loop diuretics below). The inward Na+ gradient provides the drive for several carriers, such as that for glucose. Bicarbonate is also reabsorbed from the proximal tubule by a mechanism dependent on the enzymatic activity of carbonic anhydrase (Fig. 14.1, site 2).
The loop of Henle
The descending limb of the loop of Henle is permeable to water, due to the presence of aquaporin channels, but not to Na+. Water passes from the tubule into the interstitium of the renal medulla, where the fluid is hypertonic as a result of ion transport in the thick ascending limb of the loop of Henle (see below). Therefore, tubular fluid reaching the ascending limb of the loop of Henle is hypertonic.
The thick ascending limb of the loop of Henle is impermeable to water but has an active Na+/K+/2Cl– co-transporter complex (NKCC2) in the luminal (apical) membrane (Fig. 14.1, site 3). Na+ is actively transported from the tubular cells to the interstitium by the Na+/K+-ATPase pump in the basolateral membrane. This creates a low intracellular Na+ concentration in the tubular cells and generates the Na+ ion gradient that drives the luminal NKCC2 co-transporter. The ascending limb of the loop of Henle can reabsorb up to 25% of the Na+ filtered at the glomerulus. K+ that is carried from the tubule into the cells of the loop by the NKCC2 co-transporter is recycled back into the tubular lumen, which ensures that there is always enough tubular K+ to continue to favour Na+ reabsorption. K+ recycling creates a lumen-positive transepithelial voltage gradient, which drives a paracellular ionic current that is responsible for half the total Na+ reabsorbed by this region of the kidney, along with Ca2+ and Mg2+.
The reabsorption of Na+, but not water, by the thick ascending limb of the loop of Henle establishes the hypertonicity of the medullary interstitium (the corticomedullary concentration gradient). This interstitial hypertonicity is responsible for an osmotic gradient across the collecting ducts, which permits the formation of hypertonic urine (see below). There are various hormonal regulators of Na+ reabsorption in the ascending limb of the loop of Henle, including calcitonin, parathyroid hormone and prostaglandin E2.
The proximal (cortical) diluting segment of the distal convoluted tubule
The filtrate leaving the loop of Henle is hypotonic and passes to the proximal part of the distal convoluted tubule (also known as the cortical diluting segment of the distal tubule). This part of the renal tubule is impermeable to water but has a luminal Na+/Cl– co-transporter (NCC) (Fig. 14.1, site 4). The driving force for this thiazide-sensitive co-transporter is again generated by the Na+/K+-ATPase pump in the basolateral membrane. About 5–8% of the filtered Na+ load can be reabsorbed at this site. The rich blood supply to this region allows rapid diffusion of the reabsorbed ions into the plasma and prevents the interstitium from becoming hypertonic. Reabsorption of Ca2+ is also regulated at this site, under the influence of parathyroid hormone and calcitriol (Ch. 42). The rate of Ca2+ transport is inversely related to that of Na+ transport; this is because Na+ inside the tubular cell either inhibits luminal voltage-gated Ca2+ channels or reduces the activity of the basolateral Na+/Ca2+ exchanger.
In the cortical diluting segment of the distal tubule the increased luminal concentration of Na+ or Cl– initiates two responses that limit Na+ loss. The first is tubuloglomerular feedback, a mechanism (possibly mediated by adenosine) that constricts the afferent glomerular arteriole to that nephron. The second is secretion of renin, which, through activation of the renin–angiotensin system, eventually enhances the release of aldosterone from the adrenal cortex and increases Na+ reabsorption at the distal part of the distal convoluted tubule (see Chs 6 and 44, and below).
The distal part of the distal convoluted tubule and the collecting duct
The tubular fluid that has become yet more hypotonic in the cortical diluting segment of the distal tubule is delivered to the distal part of the distal tubule and then to the collecting duct. There are two main cell types in this region, the principal cells and the intercalated cells.
In the principal cell, Na+ is reabsorbed through a highly specific amiloride-sensitive epithelial Na+ channel, known as ENaC, and this is accompanied by obligatory K+ loss into the urine (Fig. 14.1, site 5). Aldosterone acts at this site at cytosolic mineralocorticoid receptors (MRs), inducing transcription of genes encoding components of ENaC and the basolateral Na+/K+-ATPase pump. Other aldosterone-induced proteins (AIPs) include serum- and glucocorticoid-regulated kinases (SGK) and channel-inducing factor, which further increase the activity of ENaC and the Na+/K+-ATPase. Together, these changes increase the reabsorption of Na+ from the tubule and the concomitant loss of K+ into the lumen.
Less important hormonal regulators of Na+ reabsorption in the distal tubule and the collecting duct include calcitonin and bradykinin. The natriuretic peptides, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), also decreases Na+ reabsorption by receptor-mediated phosphorylation of ENaC. Overall, only about 3–5% of filtered Na+ is reabsorbed at the distal part of the distal tubule. However, the distal renal tubule is the primary site in the kidney responsible for maintenance of K+ homeostasis. Relatively small changes in extracellular K+ concentration can affect cardiac muscle, skeletal muscle and brain function, so the extracellular K+ concentration is closely regulated.
The principal cell is also the site of action of antidiuretic hormone (ADH, vasopressin; Ch. 43). This hormone is secreted by the posterior pituitary gland and binds to receptors in the basolateral membrane, where it increases the permeability of the cell to water by upregulating aquaporin (AQP2) channels. In the presence of ADH, water reabsorption into the hypertonic medullary interstitium is increased, which concentrates the urine as it passes through the collecting duct.
Intercalated cells are the second important cell type in the distal part of the distal tubule and the collecting duct (not illustrated in Figs 14.1 and 14.2). Two subtypes of intercalated cells express H+-ATPases that help to regulate acid–base balance by secreting or reabsorbing H+ and HCO3−.
Diuretic drugs
Mechanism of action
Acetazolamide interferes with the small proportion of Na+ that is actively reabsorbed in the proximal tubule in exchange for H+ (Fig. 14.2, site 2). This process is dependent on carbonic anhydrase, which is inhibited by acetazolamide. Acetazolamide increases HCO3−, Na+ and K+ secretion, causing alkaline urine. H+ retention produces mild acidosis in the blood, but the fall in plasma HCO3− concentration stimulates carbonic anhydrase activity, which rapidly leads to tolerance to the diuretic action of acetazolamide. In consequence, acetazolamide does not have a clinically useful diuretic action. Its clinical use is restricted to treatment of altitude sickness (Ch. 13) and glaucoma (Ch. 50).
Pharmacokinetics
Acetazolamide is secreted into the proximal renal tubule by organic acid transporters (OATs) and works at the luminal surface of the proximal tubule. It is eliminated unchanged in the urine.
Unwanted effects
 Nausea, vomiting, anorexia, taste disturbance.
Nausea, vomiting, anorexia, taste disturbance.
 Paraesthesia, dizziness, fatigue, irritability, ataxia, depression.
Paraesthesia, dizziness, fatigue, irritability, ataxia, depression.
 Hypokalaemia (see loop diuretics).
Hypokalaemia (see loop diuretics).
Osmotic diuretics
Mechanism of action
Mannitol is filtered at the glomerulus but not reabsorbed from the renal tubule. It exerts osmotic activity within the proximal renal tubule and particularly in the descending limb of the loop of Henle, and limits passive tubular reabsorption of water. Water loss produced by mannitol is accompanied by a variable natriuresis (up to 25% of filtered Na+). Unlike other diuretics, the osmotic action of mannitol produces an initial expansion of plasma and extracellular fluid volume, which limits its clinical uses.
Mannitol does not readily cross the blood–brain barrier. It is used to treat some forms of acute brain injury, when the main mechanism of action may be through haemodilution and reduced blood viscosity which may limit ischaemic damage, rather than a dehydrating action on cerebral tissues.
Pharmacokinetics
Mannitol is given by intravenous infusion and is excreted unchanged at the glomerulus. It has a half-life of 2 h, which is substantially increased in renal impairment.
Unwanted effects
 Expansion of plasma volume can precipitate heart failure.
Expansion of plasma volume can precipitate heart failure.
 Urinary K+ loss can lead to hypokalaemia (see loop diuretics).
Urinary K+ loss can lead to hypokalaemia (see loop diuretics).
Loop diuretics
Mechanism of action and effects
Loop diuretics, such as furosemide, must be secreted into the proximal kidney tubule by the tubular organic anion transporters to access their site of action. The extent of the natriuresis and diuresis is dependent on the rate of delivery of the drug to the renal tubule via this secretory mechanism. Once these transporters have been saturated, increasing the dose of the diuretic will not enhance its effect. Loop diuretics bind to the Na+/K+/2Cl− co-transporter complex (NKCC2) at the luminal border of the thick ascending limb of the loop of Henle, and inhibit Cl− reabsorption. This diminishes the electrochemical gradient across the cell and reduces Na+ reabsorption from the tubular fluid (Fig. 14.2, site 3). Loop diuretics therefore reduce the ability of the kidney to generate the medullary ionic concentration gradient and impairs generation of concentrated urine in the collecting duct. Loop diuretics also inhibit tubuloglomerular feedback and the afferent artery vasoconstriction in response to the increased tubular concentrations of Na+ and Cl−. They are powerful, ‘high-ceiling’ diuretics which can inhibit reabsorption of up to 25% of the Na+ that appears in the glomerular filtrate.
The dose–response curves of loop diuretics are steep, but the doses required to achieve maximal inhibition of Na+ reabsorption show wide inter-individual variation. Because they have a short duration of action, there is partial compensation for the natriuresis by subsequent rebound Na+ uptake from the tubular fluid after their action has finished. Loop diuretics remain effective even in advanced renal failure, but larger doses are necessary to deliver an effective concentration of drug to the remaining renal tubules as the reduced tubular secretion results in greater metabolism of the drug in the liver.
When injected intravenously, furosemide releases vasodilator prostaglandins, such as prostacyclin, into the circulation and produces a short-lived venodilation. Pooling of blood in these capacitance vessels reduces central blood volume, which can be useful in the treatment of acute left ventricular failure (Ch. 7). Loop diuretics also produce arterial vasodilation (see thiazide diuretics), but because of their short duration of action they are not widely used to treat hypertension, except in renal failure when their diuretic action can be useful.
Pharmacokinetics
Furosemide is incompletely and erratically absorbed from the gut, with considerable inter-individual variation. Bumetanide is more completely and reliably absorbed. Furosemide and bumetanide can also be given intravenously. Natriuresis and diuresis begin about 30 min after an oral dose and last up to 6 h; intravenous injection produces a more rapid effect, with an onset of diuresis within minutes. Loop diuretics are partially metabolised in the liver. They are highly protein bound in plasma and little drug is filtered at the glomerulus. Renal failure impairs the delivery of drug to the tubular fluid, since the ability of the kidney to secrete organic anions is reduced and other substrates compete with the diuretic for the organic anionic transporters.
Unwanted effects
 Excessive salt and water depletion can cause intravascular volume depletion, hypotension and renal impairment.
Excessive salt and water depletion can cause intravascular volume depletion, hypotension and renal impairment.
 Dilutional hyponatraemia can arise from excessive Na+ loss that exceeds water loss. Hyponatraemia is far less common than with thiazide diuretics. Stimulation of ADH secretion in response to plasma volume contraction also contributes to hyponatraemia by promoting reabsorption of water from the collecting duct. Hyponatraemia can present with lethargy, impaired consciousness and eventually coma and seizures.
Dilutional hyponatraemia can arise from excessive Na+ loss that exceeds water loss. Hyponatraemia is far less common than with thiazide diuretics. Stimulation of ADH secretion in response to plasma volume contraction also contributes to hyponatraemia by promoting reabsorption of water from the collecting duct. Hyponatraemia can present with lethargy, impaired consciousness and eventually coma and seizures.
 Hypokalaemia is dose-related, but less severe than with diuretics such as thiazides which have a longer duration of action (see below). It arises from increased urinary K+ loss from the distal part of the distal renal tubule. There are several contributory mechanisms:
Hypokalaemia is dose-related, but less severe than with diuretics such as thiazides which have a longer duration of action (see below). It arises from increased urinary K+ loss from the distal part of the distal renal tubule. There are several contributory mechanisms:
 loop diuretics increase the delivery of Na+ to the distal convoluted tubule, so there is enhanced Na+ reabsorption at this site. This creates a negative luminal gradient that promotes K+ diffusion into the tubular lumen,
loop diuretics increase the delivery of Na+ to the distal convoluted tubule, so there is enhanced Na+ reabsorption at this site. This creates a negative luminal gradient that promotes K+ diffusion into the tubular lumen,
 the dilute urine increases the K+ gradient across the tubular membrane, which also favours K+ diffusion into the tubular lumen,
the dilute urine increases the K+ gradient across the tubular membrane, which also favours K+ diffusion into the tubular lumen,
 diuretic-induced hypovolaemia stimulates release of renin and aldosterone. Aldosterone further enhances Na+ reabsorption in the distal tubule at the expense of increased K+ excretion.
diuretic-induced hypovolaemia stimulates release of renin and aldosterone. Aldosterone further enhances Na+ reabsorption in the distal tubule at the expense of increased K+ excretion.
Obligatory urinary loss of Cl− with the K+ creates a mild metabolic alkalosis in the plasma. To counteract the alkalosis, H+ is shifted out of cells in exchange for intracellular accumulation of K+, which exacerbates the hypokalaemia.
The consequences and treatment of hypokalaemia are discussed below.
 Hypomagnesaemia can accompany hypokalaemia and makes the correction of hypokalaemia more difficult. About 70% of filtered Mg2+ is reabsorbed by paracellular diffusion in the loop of Henle, and this is impaired by loop diuretics, which inhibit the electrical gradient necessary for Mg2+ reabsorption. Hypomagnesaemia predisposes to cardiac arrhythmias.
Hypomagnesaemia can accompany hypokalaemia and makes the correction of hypokalaemia more difficult. About 70% of filtered Mg2+ is reabsorbed by paracellular diffusion in the loop of Henle, and this is impaired by loop diuretics, which inhibit the electrical gradient necessary for Mg2+ reabsorption. Hypomagnesaemia predisposes to cardiac arrhythmias.
 Increased urinary Ca2+ excretion from inhibition of paracellular reabsorption of Ca2+ at the loop of Henle. It does not produce hypocalcaemia, but this action can be helpful in the management of hypercalcaemia (Ch. 42).
Increased urinary Ca2+ excretion from inhibition of paracellular reabsorption of Ca2+ at the loop of Henle. It does not produce hypocalcaemia, but this action can be helpful in the management of hypercalcaemia (Ch. 42).
 Hyperuricaemia arises from reduced glomerular filtration of uric acid following reduction of plasma volume. There may be an additional reduction of proximal tubular urate secretion as a result of competition between uric acid and the diuretic for organic anion transporters. Clinical gout is unusual (Ch. 31) and less common with loop diuretics than with thiazide diuretics.
Hyperuricaemia arises from reduced glomerular filtration of uric acid following reduction of plasma volume. There may be an additional reduction of proximal tubular urate secretion as a result of competition between uric acid and the diuretic for organic anion transporters. Clinical gout is unusual (Ch. 31) and less common with loop diuretics than with thiazide diuretics.
 Incontinence can result from the rapid increase in urine volume. In older males with prostatic hypertrophy retention of urine can occur.
Incontinence can result from the rapid increase in urine volume. In older males with prostatic hypertrophy retention of urine can occur.
 Ototoxicity with deafness can result from cochlear damage, especially when renal failure reduces the rate of drug excretion or when very large doses of a loop diuretic are used. Tinnitus and vertigo may result from vestibular damage; both are more common with furosemide and are usually reversible.
Ototoxicity with deafness can result from cochlear damage, especially when renal failure reduces the rate of drug excretion or when very large doses of a loop diuretic are used. Tinnitus and vertigo may result from vestibular damage; both are more common with furosemide and are usually reversible.
Thiazide and related diuretics
Mechanisms of action and effects
The thiazides are structurally related to sulphonamides. They act at the luminal surface of the cortical (proximal) diluting segment of the distal convoluted tubule and inhibit the Na+/Cl− co-transporter (NCC) (Fig. 14.2, site 4). This prevents Na+ and Cl− from entering the tubular cell. Several structurally different ‘thiazide-like’ drugs, such as chlortalidone and indapamide, share this site of action. Thiazides and related diuretics have a lower efficacy than loop diuretics, achieving a maximum natriuresis of about 5–8% of the filtered Na+ load, and have shallow dose–response curves. The onset of diuresis is slow, but they have a longer duration of action than loop diuretics, which varies among the drugs; for example, bendroflumethiazide produces a natriuresis for up to 6–12 h and chlortalidone for 48–72 h. Thiazide and related diuretics are less effective in renal failure (especially when the glomerular filtration rate is below 20 mL⋅min−1). Thiazide and related diuretics, unlike the loop diuretics, reduce urinary Ca2+ loss by inhibiting Ca2+ transport in the proximal and distal tubules.
Thiazide and related diuretics produce arterial vasodilation during long-term use, which appears to be the basis of their hypotensive effect (Ch. 6), but the mechanism of vasodilation is incompletely understood. It may involve direct inhibition of agonist-induced contraction of vascular smooth muscle cells by Ca2+ desensitization linked to the Rho kinase pathway, a regulator of actin–myosin cross-bridging (Ch. 1). The vasodilator action of these drugs occurs at lower dosages than are required for significant diuresis.
Pharmacokinetics
The thiazide and related diuretics are fairly well absorbed from the gut and most are metabolised in the liver. They are highly protein-bound and therefore little is filtered at the glomerulus. Like the loop diuretics, thiazides act from within the renal tubular lumen after secretion by organic anion transporters in the proximal tubule. Thiazides generally have more prolonged durations of action than loop diuretics.
Unwanted effects
 Hypokalaemia. Clinically this is more important with thiazides than with loop diuretics. The greatest reduction in plasma K+ usually occurs within 2 weeks of starting treatment.
Hypokalaemia. Clinically this is more important with thiazides than with loop diuretics. The greatest reduction in plasma K+ usually occurs within 2 weeks of starting treatment.
 Hyponatraemia. Prolonged block by thiazides of Na+/Cl− co-transport in the distal convoluted tubule (where water cannot be reabsorbed) impairs free water clearance. The combination of a thiazide with amiloride (see below) is particularly associated with dilutional hyponatraemia (see loop diuretics).
Hyponatraemia. Prolonged block by thiazides of Na+/Cl− co-transport in the distal convoluted tubule (where water cannot be reabsorbed) impairs free water clearance. The combination of a thiazide with amiloride (see below) is particularly associated with dilutional hyponatraemia (see loop diuretics).
 Hyperuricaemia (see loop diuretics). Gout occurs infrequently and is less common in women.
Hyperuricaemia (see loop diuretics). Gout occurs infrequently and is less common in women.
 Decreased urinary Ca2+ excretion. This is in contrast to loop diuretics and the mechanism is not well understood. This action of thiazides is useful for the treatment of renal stones due to hypercalciuria. Hypercalcaemia does not usually occur unless there is another underlying disturbance of Ca2+ metabolism, such as hyperparathyroidism.
Decreased urinary Ca2+ excretion. This is in contrast to loop diuretics and the mechanism is not well understood. This action of thiazides is useful for the treatment of renal stones due to hypercalciuria. Hypercalcaemia does not usually occur unless there is another underlying disturbance of Ca2+ metabolism, such as hyperparathyroidism.
 Glucose intolerance. This is dose-related, with a progressive increase in plasma glucose over many months. The major cause is prolonged hypokalaemia and the consequent reduced intracellular K+ concentration. This inhibits insulin release and impairs tissue uptake of glucose in response to insulin. The glucose intolerance usually reverses over several months if the thiazide is stopped (see Ch. 40).
Glucose intolerance. This is dose-related, with a progressive increase in plasma glucose over many months. The major cause is prolonged hypokalaemia and the consequent reduced intracellular K+ concentration. This inhibits insulin release and impairs tissue uptake of glucose in response to insulin. The glucose intolerance usually reverses over several months if the thiazide is stopped (see Ch. 40).
 Hyperlipidaemia. There is a dose-related increase in low-density lipoprotein cholesterol and triglycerides. The long-term effects (>1 year) are small, but there is a theoretical increased atherogenic risk if high doses of thiazides are used (Ch. 48).
Hyperlipidaemia. There is a dose-related increase in low-density lipoprotein cholesterol and triglycerides. The long-term effects (>1 year) are small, but there is a theoretical increased atherogenic risk if high doses of thiazides are used (Ch. 48).
 Impotence. This is reported by up to 10% of middle-aged hypertensive men treated with high doses of thiazides (Ch. 16).
Impotence. This is reported by up to 10% of middle-aged hypertensive men treated with high doses of thiazides (Ch. 16).
 Nocturia and urinary frequency can result from prolonged diuresis.
Nocturia and urinary frequency can result from prolonged diuresis.
Potassium-sparing diuretics
Mechanism of action and effects
Drugs in this class produce a diuresis while preventing urinary K+ loss. All potassium-sparing diuretics act at the late distal convoluted tubule and cortical collecting duct. Spironolactone, its active metabolite canrenone, and eplerenone are the only diuretics that do not act at the luminal membrane of the tubular cells. They compete with aldosterone for the cytoplasmic MR in the distal convoluted tubular cells and block transcriptional upregulation of the ENaC, the basolateral Na+/K+-ATPase pump and AIPs. They therefore antagonise the effects of aldosterone on Na+ reabsorption and K+ excretion (Fig. 14.2, site 5). Spironolactone and eplerenone only work in the presence of aldosterone so their effect is enhanced in hyperaldosteronism.
Amiloride and triamterene have a different mechanism of action: they directly block the epithelial Na+ channel (ENaC) at the luminal surface of the renal tubule (Fig. 14.2). Their action is independent of the presence of aldosterone.
The maximum natriuresis achieved by potassium-sparing diuretics is small (usually less than 2–3% of filtered Na+) unless there is marked secondary hyperaldosteronism, when spironolactone and eplerenone are much more effective. With potassium-sparing diuretics the Na+ and water loss is accompanied by preservation of plasma K+, because the reduced Na+ reabsorption limits ATP-dependent Na+ exchange with K+ at the basolateral membrane (Fig. 14.2, site 5). When used together with thiazide or loop diuretics, potassium-sparing diuretics reduce or eliminate the excess urinary K+ loss.
Pharmacokinetics
All potassium-sparing diuretics are given orally. Spironolactone is metabolised in the wall of the gut and the liver to canrenone, which has a much longer half-life and is probably responsible for most of the diuretic effect. The onset of action of spironolactone or eplerenone is slow, starting after 1 day and becoming maximal by 3–4 days, largely a consequence of their transcriptional mechanism of action.
Triamterene is extensively metabolised in the liver, and tubular secretion of the sulphate ester metabolite is responsible for the diuretic action. Amiloride is secreted unchanged into the proximal renal tubule. The onset of action of both drugs is rapid.
Unwanted effects
 Hyperkalaemia. This is more common in the presence of pre-existing renal disease, in the elderly and during combination treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists (Ch. 6). Retention of Mg2+ also occurs, in contrast to the loss of Mg2+ with the thiazides and loop diuretics.
Hyperkalaemia. This is more common in the presence of pre-existing renal disease, in the elderly and during combination treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists (Ch. 6). Retention of Mg2+ also occurs, in contrast to the loss of Mg2+ with the thiazides and loop diuretics.
 Hyponatraemia. This is more common with thiazide/amiloride combinations.
Hyponatraemia. This is more common with thiazide/amiloride combinations.
 Spironolactone has an anti-androgenic effect, a consequence of its ability to bind to androgen receptors and prevent their interaction with dihydrotestosterone. This causes gynaecomastia and impotence in males, and menstrual irregularities in women. The anti-androgenic effect is sometimes used in women to treat hirsutism (such as in polycystic ovary syndrome; Ch. 43), male-pattern hair loss and acne. Eplerenone has greater aldosterone receptor selectivity and does not have anti-androgenic actions.
Spironolactone has an anti-androgenic effect, a consequence of its ability to bind to androgen receptors and prevent their interaction with dihydrotestosterone. This causes gynaecomastia and impotence in males, and menstrual irregularities in women. The anti-androgenic effect is sometimes used in women to treat hirsutism (such as in polycystic ovary syndrome; Ch. 43), male-pattern hair loss and acne. Eplerenone has greater aldosterone receptor selectivity and does not have anti-androgenic actions.
Management of diuretic-induced hypokalaemia
A modest reduction in plasma K+ concentration is common during treatment with loop or thiazide diuretics. Marked hypokalaemia (below 3.0 mmol⋅L−1) predisposes to cardiac rhythm disturbances (including ventricular arrhythmias), particularly in the presence of acute myocardial ischaemia, during treatment with digitalis glycosides (Ch. 7), or with antiarrhythmic agents that prolong the Q–T interval on the electrocardiogram (Ch. 8). It may also precipitate encephalopathy in people with liver failure. The risk of hypokalaemia is greatest with:
 thiazide rather than loop diuretics, because of their longer duration of action,
thiazide rather than loop diuretics, because of their longer duration of action,
 hyperaldosteronism, for example in hepatic cirrhosis or nephrotic syndrome.
hyperaldosteronism, for example in hepatic cirrhosis or nephrotic syndrome.
Both treatment and prevention of diuretic-induced hypokalaemia can be achieved with the addition of a potassium-sparing diuretic. It is unnecessary to routinely prescribe a potassium-sparing diuretic with a thiazide or loop diuretic. A pragmatic approach would be to reserve the use of combination treatment for those at high risk from hypokalaemia, or those who develop significant hypokalaemia during regular diuretic treatment.
Alternatively, oral K+ supplements can be given to correct hypokalaemia, but these are less effective unless used in large quantities (greater than 30 mmol daily), which often cause gastric irritation. Modified-release tablets and effervescent formulations of K+ are available to improve tolerability. Oral K+ supplements should not be used together with potassium-sparing diuretics.
Intravenous K+ treatment is rarely needed unless there is severe K+ depletion. Rapid intravenous injection of K+ can produce potentially lethal hyperkalaemia (provoking asystole), and a maximum infusion rate of 10 mmol⋅h−1 is recommended, with hourly monitoring of the plasma K+ concentration if such a high infusion rate is necessary.
Major uses of diuretics
Diuretics can be used to treat a number of conditions.
Oedema in heart failure, nephrotic syndrome and hepatic cirrhosis
Mild oedema can sometimes be controlled by a thiazide diuretic, but more marked oedema usually requires the use of a loop diuretic (see Ch. 7 for oedema in heart failure). Modest doses of a loop diuretic provide a near-maximal response if renal function is normal, but large doses are sometimes necessary if there is renal failure (see above). Long-term use of a loop diuretic can occasionally produce tolerance, due to hypertrophy of epithelial cells of the cortical diluting segment of the distal convoluted tubule, which results in increased Na+ reabsorption at this site. There are various strategies that can be tried if fluid retention is resistant to oral furosemide, as follows.
 Salt restriction and avoidance of salt-retaining drugs, such as non-steroidal anti-inflammatory drugs (Ch. 29). Water restriction may be necessary if there is dilutional hyponatraemia, since raising the serum Na+ concentration improves diuretic responsiveness.
Salt restriction and avoidance of salt-retaining drugs, such as non-steroidal anti-inflammatory drugs (Ch. 29). Water restriction may be necessary if there is dilutional hyponatraemia, since raising the serum Na+ concentration improves diuretic responsiveness.
 Divided oral doses of a loop diuretic can be used to give more prolonged drug delivery to the kidney. This also reduces post-diuretic rebound Na+ retention.
Divided oral doses of a loop diuretic can be used to give more prolonged drug delivery to the kidney. This also reduces post-diuretic rebound Na+ retention.
 Oral bumetanide can be used rather than furosemide, because of its more consistent oral absorption.
Oral bumetanide can be used rather than furosemide, because of its more consistent oral absorption.
 A loop diuretic can be given by intravenous infusion (often over 24 h) to prolong the duration of action and give a more sustained natriuresis and diuresis. Slow intravenous infusion of higher drug doses will also help to avoid ototoxicity.
A loop diuretic can be given by intravenous infusion (often over 24 h) to prolong the duration of action and give a more sustained natriuresis and diuresis. Slow intravenous infusion of higher drug doses will also help to avoid ototoxicity.
 The addition of a thiazide or related diuretic to a loop diuretic. Sequential inhibition of tubular Na+ reabsorption can produce a dramatic diuresis and natriuresis. However, hyponatraemia, hypokalaemia, hypovolaemia and renal impairment are all more frequent with such combinations.
The addition of a thiazide or related diuretic to a loop diuretic. Sequential inhibition of tubular Na+ reabsorption can produce a dramatic diuresis and natriuresis. However, hyponatraemia, hypokalaemia, hypovolaemia and renal impairment are all more frequent with such combinations.
 If there is marked secondary hyperaldosteronism, for example in ascites associated with cirrhosis of the liver or caused by treatment with high doses of a loop diuretic, spironolactone or eplerenone can be particularly useful.
If there is marked secondary hyperaldosteronism, for example in ascites associated with cirrhosis of the liver or caused by treatment with high doses of a loop diuretic, spironolactone or eplerenone can be particularly useful.
Hypertension
Low doses of a thiazide or related diuretic are usually used for hypertension. A loop diuretic or spironolactone can be useful for resistant hypertension or when there is renal impairment. See also Chapter 6.
Glaucoma
Acetazolamide can be used to reduce intraocular pressure (Ch. 50). Tolerance does not occur to this effect, unlike the diuretic action.
Altitude sickness
An unlicensed use for acetazolamide is the prevention and treatment of altitude sickness (Ch. 13). It should be taken for several days before ascending to altitude, and continued until descent. The mechanism is unclear but the drug may combat respiratory alkalosis produced by hyperventilation at high altitudes.
Hypoventilation in chronic obstructive pulmonary disease
Acetazolamide creates a mild metabolic acidosis. This can stimulate respiration in the short term, and reduce carbon dioxide retention (Ch. 12).
True/false questions
1. A fall in plasma K+ concentration can affect cardiac muscle function.
2. The main renal site of K+ loss in the urine is from the proximal convoluted tubule.
3. The Na+/K+-ATPase pump is only found on the basolateral membrane of the loop of Henle.
4. The thick ascending limb of the loop of Henle is impermeable to water.
5. Osmotic diuretics are poorly reabsorbed from the renal tubule.
6. Osmotic diuretics should not be given in heart failure.
7. The carbonic anhydrase inhibitor acetazolamide is used in the treatment of glaucoma.
8. All thiazide diuretics are shorter-acting than loop diuretics.
9. Thiazide diuretics act by inhibiting Na+/Cl− co-transport in the basolateral membrane.
10. Thiazide diuretics increase urinary Ca2+ excretion.
11. Thiazide diuretics may exacerbate diabetes mellitus.
12. Spironolactone and amiloride act by the same mechanism to reduce K+ loss.
13. Potassium-sparing diuretics and angiotensin-converting enzyme (ACE) inhibitors can cause a harmful interaction.
14. Thiazide or loop diuretics should not be given together with potassium-sparing diuretics.
15. Non-steroidal anti-inflammatory drugs (NSAIDs) reduce the response to diuretics.
One-best-answer (OBA) questions
1. Which of the following best describes the mechanism of action of triamterene?
A Antagonism of a cytosolic receptor
B Blockade of a Na+/K+/2Cl− co-transporter
C Blockade of a selective Na+ channel
2. Which of the following is the most accurate statement about loop diuretics?
A Loop diuretics are useful in the treatment of acute pulmonary oedema.
B Loop diuretics and thiazide diuretics should not be taken together.
C Loop diuretics do not produce hypokalaemia.
D Loop diuretics increase the hypertonicity of the interstitium in the medullary region.
E Loop diuretics reduce the risk of ototoxicity with aminoglycoside antibacterial drugs.
Extended-matching-item question
Choose the most likely option (A–F) related to the case scenarios 1 to 3 below.
A Raised serum K+ concentration
B Lowered serum K+ concentration
Case 1. A 58-year-old woman was taken to the accident and emergency department with dyspnoea and bradycardia (40 beats⋅min−1). She had previously had a myocardial infarction and coronary angioplasty. She was taking the diuretics bendroflumethiazide and amiloride, and had recently had her dose of the ACE inhibitor lisinopril increased.
Case 2. A 40-year-old man with type 1 diabetes mellitus and hypertension was being treated with insulin. He had started on the thiazide-related diuretic chlortalidone 2 months previously for his hypertension and was seeking medical advice about his increased tiredness and lethargy.
Case 3. A 55-year-old man with congestive heart failure was treated with digoxin and lisinopril. Furosemide was added because of oedema and he subsequently complained of palpitations. He was admitted to hospital and the electrocardiogram showed atrial tachycardia.
1. True. Hypokalaemia can also affect brain and skeletal muscle function.
2. False. Much of the filtered K+ is reabsorbed in the proximal tubule and loop of Henle, and its loss into the urine occurs mainly in the collecting ducts.
3. False. The basolateral Na+/K+-ATPase pump is present throughout the renal tubule.
4. True. Impermeability to water and the NKCC2 transporter that co-transports Na+, K+ and Cl− ions from the lumen into the tubular cell in the thick ascending limb together generate the hyperosmotic interstitium important in concentrating urine in the collecting duct.
5. True. Osmotic diuretics are retained within the tubule where their osmotic activity reduces passive reabsorption of water in the proximal tubule and descending limb of the loop of Henle.
6. True. By extracting water from intracellular compartments and expanding extracellular and intravascular fluid volumes, osmotic diuretics can precipitate pulmonary oedema.
7. True. Acetazolamide reduces the formation of aqueous humour.
8. False. Some thiazide diuretics such as chlortalidone can produce a diuresis for 48–72 h, while most loop diuretics are relatively short-lived.
9. False. Thiazide diuretics act from within the renal tubular lumen on the thiazide-sensitive Na+/Cl− co-transporter (NCC) on the luminal (apical) membrane.
10. False. The thiazide diuretics do not increase urinary Ca2+ excretion, unlike the loop diuretics.
11. True. Thiazide diuretics may exacerbate diabetes mellitus, probably through hypokalaemia reducing insulin release from pancreatic β-cells.
12. False. Amiloride blocks the epithelial Na+ channel (ENaC) directly, whereas spironolactone competes with aldosterone at mineralocorticoid receptors, thus reducing the transcriptional expression of ENaC. The reduced Na+ reabsorption produced by both drugs in the collecting duct reduces the loss of K+ into the urine.
13. True. ACE inhibitors, by reducing angiotensin-induced aldosterone secretion, will reduce K+ excretion and hence increase plasma K+ concentration, particularly when combined with potassium-sparing diuretics.
14. False. Thiazides and loop diuretics increase Na+ concentrations in the tubular fluid reaching the collecting duct; the excessive loss of K+ that results can be reduced by combining the thiazide or loop diuretic with a potassium-sparing diuretic.
15. True. NSAIDs inhibit prostaglandin synthesis in the kidney and this reduces renal blood flow, leading to reduced natriuretic responses to thiazide and loop diuretics.
OBA answers
1. Answer C is correct. Triamterene is a potassium-sparing diuretic that directly blocks a selective Na+ channel (ENaC) on the luminal membrane of tubule cells in the collecting duct. Answer A is the mechanism of action of aldosterone (mineralocorticoid) receptor antagonists such as spironolactone. Answers B, D and E are the mechanisms of action of loop diuretics, thiazides and carbonic anhydrase inhibitors respectively.
A Correct. Loop diuretics are widely used in the control of oedema in heart failure for the elimination of excessive salt and water load. The direct venodilator activity of furosemide reduces central blood volume.
B Incorrect. A thiazide diuretic can be added to a loop diuretic to act sequentially at different sites in the nephron, thus producing a marked diuresis and natriuresis.
C Incorrect. Delivery of greater concentrations of Na+ to the collecting ducts increases the exchange for K+ at that site, thus increasing K+ loss.
D Incorrect. By inhibiting the Na+/K+/2Cl− co-transporter (NKCC2), the medullary interstitial hypertonicity falls and this reduces the reabsorption of water in the collecting ducts (in the presence of antidiuretic hormone).
E Incorrect. Loop diuretics alone can cause ototoxicity (especially at high doses or in renal impairment) and also when taken with other ototoxic drugs such as aminoglycosides.
Extended-matching-item answers
Case 1. Answer A. The combination of a potassium-sparing diuretic (amiloride) and an ACE inhibitor (lisinopril) may have raised the plasma K+ concentration and this may have been the cause of the profound bradycardia.
Case 2. Answer E. Thiazide-like diuretics can worsen insulin resistance, resulting in an increased plasma glucose concentration.
Case 3. Answer B. The loop diuretic may cause hypokalaemia. This enhances the toxicity of digoxin, resulting in arrhythmias.
Brater, DC. Pharmacology of diuretics. Am J Med Sci. 2000;319:38–50.
De Bruyne, LKM. Mechanisms and management of diuretic resistance in congestive heart failure. Postgrad Med J. 2003;79:268–271.
Duarte, JD, Cooper-DeHoff, RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Exp Rev Cardiovasc Ther. 2010;8:793–802.
Greenberg, A. Diuretic complications. Am J Med Sci. 2000;319:10–24.
Krämer, BK, Schweda, F, Riegger, GAJ. Diuretic treatment and diuretic resistance in heart failure. Am J Med. 1999;106:90–96.
Shankar, SS, Brater, DC. Loop diuretics: from the Na-K-2Cl transporter to clinical use. Am J Physiol Renal Physiol. 2003;284:F11–F21.
Wright, SH, Dantzler, WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049.