Principles and techniques of operative surgery including neurosurgery and orthopaedics
Introduction
This chapter describes the operating environment and outlines the principles of operative surgery including those employed in ‘minor’ surgical techniques. All of these should be understood by all doctors, not just by surgeons, to help them appreciate the scope of surgery, to enable them to give meaningful explanations to patients before and after surgery and to help them assist intelligently at the operating table. Furthermore, most doctors are required to perform minor operations, emergency department procedures or invasive investigations at one time or another and these require knowledge of techniques.
Various suffixes derived from Greek and Latin are used in describing certain surgical techniques; these are summarised in Box 10.1.
Principles of asepsis
Introduction
The main bacteria and viruses involved in surgical infections have been described in Chapter 3. The chief sources of infection are the patients themselves (particularly bowel flora), less commonly the hospital environment, food or cross-infection from other patients, and occasionally bacteria and viruses carried by ward staff or theatre personnel. Rare sources of infection are contaminated surgical instruments or equipment, dressings or parenteral drugs and fluids. The viruses causing hepatitis B and C and particularly human immunodeficiency virus (HIV) pose sinister risks of transmitting infection from patient to operating staff and vice versa. These risks make it mandatory to observe universal blood and body fluid precautions as described in Chapter 3.
The risk of postoperative bacterial infection depends on the extent of contamination of the wound or body cavity at operation or, in the case of intestinal perforation, before operation. Bacteria enter a wound by five possible routes:
• Direct inoculation from instruments and operating personnel
• Airborne bacteria-laden particles
• From the flora of the patient's internal viscera, especially large bowel
Modern operating theatre design and correctly observed aseptic procedures minimise wound contamination but when infections occur, results can be devastating especially in relation to artificial prostheses, skin grafts, bone and the eye. Furthermore, some patients are particularly vulnerable to infection, notably neonates, the immunosuppressed, the debilitated and the malnourished. Note that treatment of established infection is no substitute for prevention.
The use of preoperative prophylactic antibiotics began in the 1970s and has revolutionised the outcome of certain types of operative surgery in a manner comparable to the changes heralded by Lister's introduction of antisepsis in the late nineteenth century.
The operating environment
Modern operating theatre design plays a key role in control of airborne wound contamination. This is important mainly for staphylococci carried on airborne skin scales.
The main factors influencing airborne operating theatre infection rates are:
The first two are influenced mainly by theatre design and air supply, and the last can be minimised by avoiding unnecessarily long procedures. Operating theatre complexes are laid out so as to minimise introduction of infection from outside via air, personnel or patients. Air is drawn from the relatively clean external environment, filtered and then supplied to the theatres at a slightly higher pressure than outside to ensure a constant outward flow. Air turnover is the most important factor; the aim is to ensure 3–15 air changes per hour which ‘scrubs out’ the theatre air by dilution. Standard air delivery systems aim to achieve a constant flow of clean air towards the operating table, which is then exhausted from the theatre. Despite this, convection currents allow some recirculation of air, which may be contaminated, into the operation site.
The crucial importance of preventing infection in joint replacement surgery led to the development of sophisticated ultra-clean air delivery systems. These reduce postoperative infection two- to four-fold in joint replacement surgery, but the cost is very high. Enclosure of the patient in a sterile tent in which the surgeons wear space-type suits can reduce infection rates by a further 5–7.5%.
Minimising infection from operating theatre personnel: Only a modest proportion of wound infections derive from theatre personnel. Bacteria reach the wound via the air or by direct inoculation, often from viscera within the wound. About 30% of healthy people carry Staphylococcus aureus in the nose but pathogenic organisms may also be present in axillae and the perineal area, the last probably being the most important source of wound infections from theatre personnel. In addition, skin abrasions are usually infected, as are skin pustules and boils; thus personnel with these lesions must ensure that they are effectively covered with occlusive dressings or else should not enter the operating area.
Airborne, personnel-derived infection is reduced by changing from potentially contaminated day clothes to clean theatre clothes and shoes which should not be worn outside the theatre complex. Trouser cuffs should be elasticated or tucked into boots. Females should wear trousers instead of dresses, in order to reduce ‘perineal fallout’. Face masks are worn to deflect bacteria-containing droplets in expired air, but most types become ineffective after a very short period, especially when wet. With the exception of nasal Staph. aureus (particularly important in prostheses infection), bacteria derived from the head do not generally cause wound infection. The effectiveness of wearing masks and hair coverings to reduce infection is unknown.
Sterile gloves and gowns are worn by surgeons and staff directly involved in the operation to prevent inoculation of bacteria. Gloves are impermeable to bacteria but hands and forearms need washing before gloving and gowning with antiseptics that persist on the skin. This minimises bacterial contamination if a glove is punctured (as often happens) or the sleeve of the gown becomes wet. The traditional ritual of scrubbing with a brush for 3 minutes is actually less effective than washing the hands thoroughly because it causes microtrauma and brings bacteria to the surface.
Despite the protection given by wearing gloves and gown, the less a wound is handled the better. This principle applies particularly when aseptic conditions are less than ideal. On the ward, minor procedures such as bladder catheterisation or chest drain insertion should be performed using sterile precautions and a no-touch technique. Catheters, for example, should not be handled directly but only within their wrapper or with instruments.
Minimising infection from the patient's skin: The patient's skin, especially the perineal area, is the source of up to half of all wound infections. These may be minimised by the following measures:
• Removing body hair—body hair was thought to be a source of wound contamination but this is no longer believed. Hair is removed only to allow the incision site to be seen and the wound to be closed without including hair. Shaving produces abrasions which rapidly become colonised with skin commensals. Most surgeons now restrict hair removal to clipping away just enough to provide skin access.
• Painting the skin with antiseptic solutions—povidone-iodine or chlorhexidine in alcoholic or aqueous solution is applied to a wide area around the proposed operation site (‘skin prep’). In the past, patients were subjected to ineffective applications of antiseptics such as gentian violet for several days beforehand!
• Draping the patient—the operating area is isolated by placing sterile, (ideally self-adhesive) drapes made of impermeable paper or coated waterproof material over all but the immediate field of operation.
Reducing infection from internal viscera: The large bowel teems with potentially pathogenic bacteria and the peritoneal cavity inevitably becomes contaminated in any operation where large bowel is opened. Pathogenic bacteria are also found in obstructed small bowel. The same applies to the stomach and small bowel of patients on proton pump inhibitors where the normal bactericidal effect of gastric acid is lost. Great care should be taken at operation to minimise this contamination. Bowel preparation of the colon before operation may help this process. All patients having bowel operations should be given prophylactic antibiotics before operation.
Sterilisation of instruments and other supplies: (see Table 10.1)
In modern surgical practice, infection from instruments, swabs, equipment and intravenous fluids has been virtually eliminated by the use of sterile packs from central sterile supply departments (CSSD). Reusable instruments and drapes are sterilised by high-pressure steam autoclaving according to strict regulations. Most disposable items are purchased in pre-sterilised, sealed packs. Sterilisation in small autoclaves near the operating theatre should only be performed if instruments in short supply are required for successive operations. Sterilisation by any method is ineffective unless all organic material is first removed by thorough cleaning. It is also important to transport soiled instruments promptly to CSSD as proteinaceous material becomes fixed to metal and resistant to removal. This is especially true for small and microsurgical instruments (e.g. in ophthalmology), where channels easily become blocked by organic matter and prevent effective sterilisation.
Instruments which would be damaged by heat, including plastics, cystoscopic lenses, flexible endoscopes and electrical equipment, can be sterilised using a variety of chemical methods such as ethylene oxide gas. The Sterilox method is commonly used for endoscopic instruments that need to be reused several times during a session; the endoscopes are bathed in an electro-chemically activated water system through which an electrical current is passed.
World-wide, many surgical instruments are prepared by boiling water ‘sterilisers’. Boiling water is markedly inferior to other methods but is included here as it may be the only practical method in developing countries because of cost and technical difficulties. Boiling water kills most vegetative organisms within 15 minutes but spores are not killed. All organic debris should, as always, be scrupulously removed first, then the instruments immersed in visibly boiling water, returned to the boil and boiled continuously for at least 30 minutes to ensure hepatitis and human immunodeficiency viruses are destroyed.
Surgical technique
Surgical technique plays an important part in minimising the risk of operative infection. Non-vital tissue and collections of fluid and blood are vulnerable to colonisation by infecting organisms, which may enter via the bloodstream even if direct contamination has been avoided by aseptic technique. Tissue damage should be kept to a minimum by careful handling and retraction and by avoiding unnecessary diathermy coagulation. Haematoma formation is minimised by careful haemostasis and placing drains into potential sites of fluid collection; closed-drainage or suction-drainage systems reduce the risk of organisms tracking back into the wound from the ward environment.
During extensive resections of bowel, early ligation of its blood supply allows bacteria to permeate the wall (translocation) and this may contaminate the peritoneal cavity.
Faecal contamination is associated with a high risk of infection and great care needs to be taken in operations where bowel is opened. In emergency operations for large bowel perforation, free faecal matter is meticulously removed. A planned ‘second look’ laparotomy after 48 hours is advisable to deal with remaining contamination and new abscesses even if the patient appears well.
Prevention of cross-infection (nosocomial infection): Cross-infection is the term used for infection transmitted from other patients in the nearby hospital environment and should be distinguished from colonisation with other patients' bacteria. Cross-infection is mainly spread via staff, medical equipment, food or ward furnishings. Doctors are probably the worst offenders as regards transfer of infection—by removing dressings to inspect wounds in the open ward, by failing to cleanse hands between patients and by careless aseptic technique when performing ward procedures such as bladder catheterisation. Minimising patient movements between wards and hospital units also decreases cross-infection rates.
A patient with an infection that is potentially dangerous to other patients, such as meticillin-resistant Staph. aureus (MRSA), should be isolated and barrier-nursed in a single room.
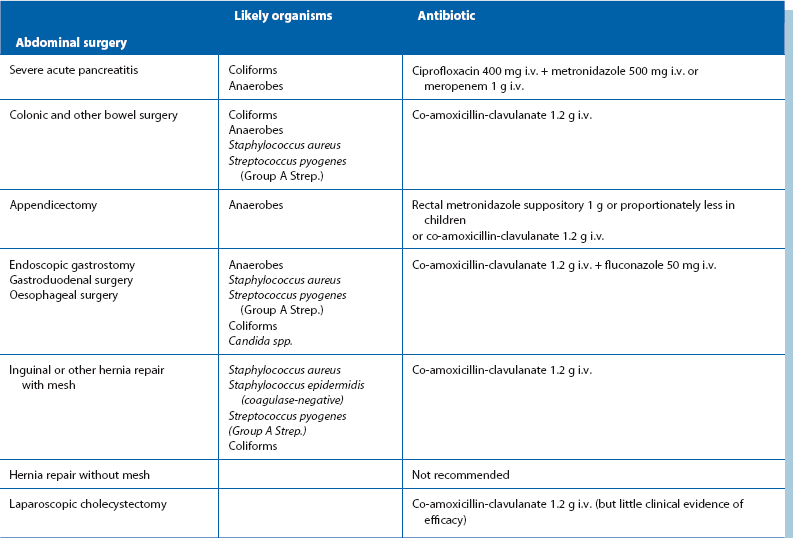
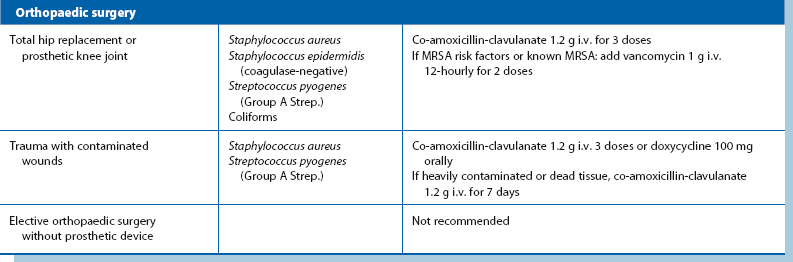
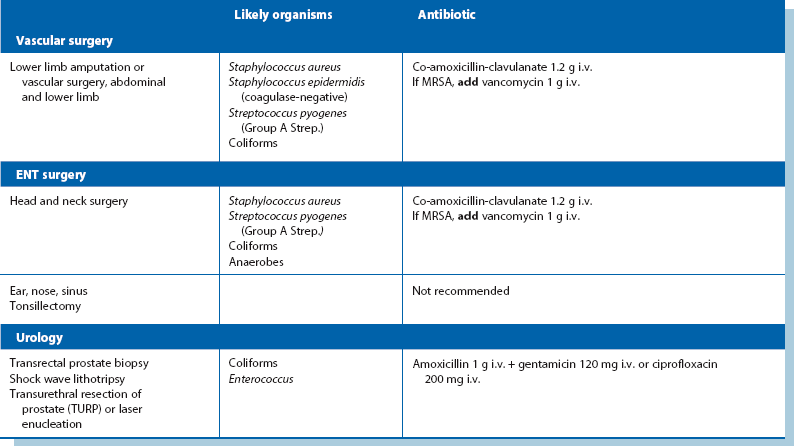
Prophylactic antibiotics: Despite using the best aseptic techniques, some operations carry a high risk of wound infection as well as other infective complications; these can be dramatically reduced by using prophylactic antibiotics. The chosen antibiotics should be matched to the organisms likely to occur in the area of the operation and should be bactericidal rather than bacteriostatic (see Table 10.2). The relative risk of postoperative infection in different types of operation is summarised in Box 10.2.
As a general principle, pre or peroperative prophylactic antibiotics are indicated if the anticipated risk of infection exceeds 10%, e.g. all emergency abdominal surgery and all elective colonic operations. Prophylactic antibiotics are also used by many surgeons for operations in the 5–10% risk category, e.g. cholecystectomy. Prophylactic antibiotics are also indicated for inherently low-risk cases where the consequences of infection would be catastrophic, e.g. operations employing prosthetic implants, or in patients with mitral stenosis or other cardiac defects at risk of infective endocarditis. Prophylactic antibiotics can reduce postoperative infection rates in high-risk cases by 75%, and may almost entirely eliminate infection where the risk is lower.
In most wound-related infections, the organisms are introduced during the operation and become established during the next 24 hours. Thus, for prophylactic antibiotics to be effective, high blood levels must be achieved during the operation when contamination occurs. To achieve this, the first dose should be given 1 hour before operation or preferably intravenously at induction of anaesthesia; prophylactic antibiotics should not be given earlier as this may encourage resistant organisms to proliferate (Box 10.3). A single preoperative dose of antibiotic is generally sufficient if it is rapidly bactericidal and the inoculum of bacteria is small; long operations with heavy blood loss, e.g. ruptured abdominal aortic aneurysm, merit a second perioperative dose of antibiotics later in the operation. Longer courses of prophylactic antibiotics are of no advantage.
In general, intravenous antibiotics give the most predictable blood levels and peak tissue levels are achieved within 1 hour of injection. However, for prophylaxis against anaerobes, metronidazole administered rectally gives blood and tissue levels equivalent to intravenous administration but later, 2–4 hours after administration.
Operations involving bowel and biliary system: Patients having these operations are at risk mainly from a mixture of Gram-negative bacilli (Enterobacteriaceae family), faecal anaerobes (Bacteroides fragilis) and Staph. aureus. Less commonly, enterococci cause surgical infection, notably E. faecalis (formerly Strep. faecalis).
The most commonly used prophylactic antibiotic regimens are shown below. A more comprehensive list is given in Table 10.2:
• For biliary surgery—co-amoxicillin-clavulanate
• For colonic and other bowel surgery—either co-amoxicillin-clavulanate or a combination of gentamicin, benzylpenicillin (or ampicillin) and metronidazole
• For appendicectomy—rectal metronidazole alone, given 2 hours before operation; this has proved as effective as any other regimen. The evidence for the beneficial effect of metronidazole in preventing infection after appendicectomy is now so strong that it may be negligent not to use it
The choice of antibiotics for prophylaxis must be kept under review because organisms change their sensitivities. Aminoglycosides such as gentamicin have the important advantage do not alter the bowel flora because their concentration in the lumen is low; this is in contrast to the cephalosporins and ampicillin, which have caused a rising tide of beta-lactam-resistant bowel organisms insensitive to cephalosporins and ampicillin but sensitive to aminoglycosides. If resistant staphylococci are a problem, vancomycin or other newer antibiotics may become necessary for prophylaxis.
Operations involving implantation of prostheses: Vascular grafts and joint replacements are at particular risk from Staph. aureus infection. Coagulase-negative slime-forming staphylococci (e.g. Staph. epidermidis) are a common source of chronic infection. Coliforms are a very rare cause. Flucloxacillin is the agent of first choice for prophylaxis but gentamicin is usually added for extra protection. Rifampicin can be used to soak vascular grafts as it is effective against Staph. epidermidis. Meticillin-resistant Staph. aureus (MRSA) is becoming a common cause of prosthetic infection. In areas where the risk is substantial, prophylaxis with vancomycin is appropriate.
Operations where ischaemic or necrotic muscle may remain: Lower limb amputations for arterial insufficiency and major traumatic injuries involving muscle are susceptible to gas gangrene and tetanus. Clostridia are highly sensitive to benzylpenicillin and metronidazole, one of which should be given in high dose as early as possible after major trauma, and before major amputations for ischaemia. Co-amoxicillin-clavulanate is a good alternative.
Basic surgical techniques
General principles: Some form of anaesthesia is needed for almost every surgical procedure, with the aim of preventing pain in all cases, minimising stress for the patient in most, and providing special conditions for some operations, e.g. muscular relaxation in abdominal surgery. The choice of anaesthetic techniques includes topical (surface) anaesthesia, local anaesthetic infiltration or peripheral nerve block, spinal or epidural anaesthesia and general anaesthesia. Methods other than general anaesthesia may be supplemented with intravenous sedation if the patient is anxious or agitated (e.g. with benzodiazepines). Intravenous sedation with these drugs produces relaxation, anxiolysis and amnesia whilst retaining protective reflexes. However, these drugs can also cause unconsciousness and they must be carefully titrated to produce just the desired effects. Intravenous sedation of this type does not provide pain relief; if needed, this is achieved with local anaesthesia or intravenous analgesics.
Choice of anaesthetic technique: Combining local or regional anaesthesia (for pain relief) with general anaesthesia can minimise postoperative respiratory and cardiovascular depression compared with general anaesthesia alone, reducing morbidity. An example is the use of caudal anaesthesia in perineal operations. Local or regional anaesthesia with bupivacaine or levobupivacaine can also be administered during an operation to provide postoperative pain relief; for example, intercostal nerve blocks during an abdominal operation allow more comfortable breathing and coughing, reducing respiratory complications. Another common example is wound infiltration with the same long-acting local anaesthetics. The main factors influencing choice of anaesthesia are summarised in Box 10.4.
Incision technique
Choice of incision: The purpose of most skin incisions is to gain access to underlying tissues or body cavities. When planning an incision, the first concern is to achieve good access and also allow it to be extended if necessary. It must also be sited in such a way that it can be effectively closed to give the best chance of primary healing and the lowest chance of an incisional hernia later. Despite patients' impressions, the length of an incision (and the number of sutures) has little bearing on the rate of healing, and the success of an operation should not be put at risk by inadequate access.
Secondary considerations in the choice of incision are as follows:
• Orientation of skin tension lines (based on Langer's lines) and skin creases—where possible, incisions should be made parallel to the lines of skin tension determined by the orientation of dermal collagen (e.g. a ‘collar’ incision for thyroid operations) as the wound is less likely to break down, there is minimal distortion, and healing occurs with little scar tissue to give the best cosmetic result
• Strength and healing potential of the tissues—the nature and distribution of muscle and fascia influences the strength of the repair, particularly in different parts of the abdominal wall. For example, a vertical lower midline incision along the linea alba, a strong layer of fascia, is less prone to incisional herniation than a paramedian incision lateral to the midline
• The anatomy of underlying structures, particularly nerves—the incision line should run parallel to, but some distance away from the expected course of underlying structures, reducing the risk of damage. For example, to gain access to the submandibular gland, the incision is made 2 cm below the lower border of the mandible to avoid the mandibular branch of the facial nerve
• Cosmetic considerations—wherever possible, incisions should be placed in the least conspicuous position, such as in a skin crease or a site that will later be concealed by clothing or hair, e.g. a transverse suprapubic (Pfannenstiel or bucket-handle) incision below the ‘bikini’ line for operations on the bladder, uterus or ovary, or a peri-areolar incision for breast biopsy
Dissection and handling of deeper tissues: The skin consists of thin epidermis and dense, somewhat thicker dermis, as well as the underlying fatty hypodermis which may be 10 cm or more thick in an obese individual.
Once the skin incision has been made, the scalpel is mainly reserved for incising fascia and other fibrous structures such as breast tissue and very fine dissection. Anatomical detail is exposed and displayed by a combination of blunt and sharp dissection. Blunt dissection involves teasing or stripping tissues apart using fingers, swabs or blunt instruments, following natural tissue planes. Sharp dissection with scissors and forceps or scalpel is used where tissues have to be cut and also to display small structures. Some surgeons prefer sharp to blunt dissection in general, believing it causes less tissue trauma. Most dissection, however, involves a combination of both.
Principles of haemostasis
Bleeding is an unavoidable part of surgery. Blood loss should be minimised because bleeding obscures the operative field and hampers operative technique (the finer the surgery, the more bleeding affects visibility and quality of outcome), and because the loss has to be made up later. Excessive bleeding can be averted by judicious dissection with control of bleeding as the operation proceeds, and by minimising the area of raw tissue exposed at the operation site by accurately siting the incision and by avoiding opening unnecessary tissue planes.
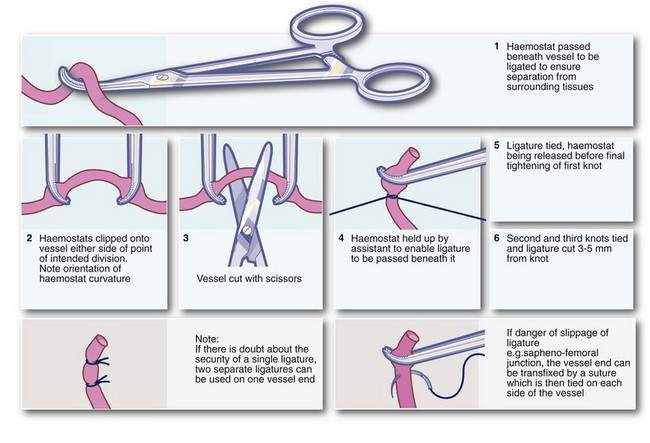
Clipping, ligation and under-running: Ligation or specialised bipolar diathermy is obligatory when large vessels are divided and is desirable for vessels larger than about 1 mm calibre (see Fig. 10.1). If the end of a bleeding vessel cannot be grasped by haemostat forceps, a suture can be used to encircle the vessel and its surrounding tissues, a technique often described as under-running. It is particularly useful for a bleeding artery in the fibrous base of a peptic ulcer.
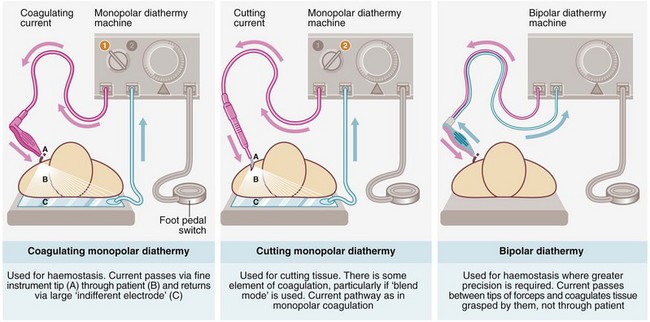
Diathermy: Diathermy achieves haemostasis by local intravascular coagulation and contraction of the vessel wall caused by heating (-thermy), generated by particular electrical waveforms. However, enough heat is also produced to burn the tissues and these may be needlessly damaged by careless use, particularly near the skin or nerves. Ordinary diathermy is ineffective for large vessels, which should be ligated. There are three main variants of diathermy, illustrated in Figure 10.2, and all three modes are available on modern diathermy machines.
Monopolar diathermy is the most widely used for operative haemostasis but there is wide dispersion of coagulating and heating effects, making it unsuitable for use near nerves and other delicate structures. Since the current passes through the patient's body, there is a risk of coagulating vessels en passant (e.g. monopolar diathermy used in circumcision may cause penile thrombosis), as well as provoking arrhythmias in patients with cardiac pacemakers. Monopolar diathermy may also result in skin burns at the indifferent electrode plate if skin contact is poor or if the plate becomes wet during operation. To improve contact, hair should be shaved from the skin where the plate is placed.
Bipolar diathermy is used mainly for finer surgery. The current passes only between the blades of the forceps and it requires fairly accurate grasping of the bleeding vessel. It uses low levels of electrical power, there is almost no electrical dispersion from the tip of the forceps and much less heat is generated. The main advantages are minimal tissue damage around the point of coagulation and safety in relation to nearby nerves, blood vessels and cardiac pacemakers. Specialised computer controlled bipolar diathermy is often used for larger vessels during laparoscopic surgery.
Cutting diathermy is mainly used for dividing large masses of muscle (e.g. during thoracotomy or access to the hip joint) and cutting vascular tissues (e.g. breast). The intention is a form of sharp dissection, at the same time coagulating the numerous small blood vessels as the tissue is cut; unfortunately this is not always wholly effective. A blend of cutting and coagulation is sometimes used.
Tourniquet and exsanguination: This technique is used in surgery of the limbs and hands where a bloodless field is desirable. For the whole limb, a pneumatic tourniquet is placed proximally. The limb is exsanguinated by elevation and spiral application of a rubber bandage (Esmark) or a ring exsanguinator from the periphery; the tourniquet is then inflated. Upper limb tourniquets must not be left inflated for more than 30 minutes and lower limb tourniquets for more than about 1 hour to avoid the risk of necrosis.
Pressure: Pressure is a useful means of controlling bleeding until platelet aggregation, reactive vasoconstriction and blood coagulation take over. It can be used for emergency temporary control of severe arterial or venous bleeding but is equally useful for controlling diffuse small-vessel bleeding from a raw area, e.g. liver bed after cholecystectomy. Pressure is usually applied with gauze swabs which must be kept in position for at least 10 minutes. Even if bleeding is not arrested completely, this process usually allows a clearer view and allows haemostasis by standard means.
For intractable bleeding which is not amenable to ligature, diathermy or suture, various resorbable packing materials, e.g. oxidised cellulose, can be left in position until haemostasis occurs, allowing the wound to be closed. If bleeding simply cannot be controlled—for example, after liver injury—the bleeding cavity can be packed with gauze swabs which are left in situ and removed 48–72 hours later at a further operation. Bleeding, once controlled by this method, rarely recurs.
When a raw cavity has been created beneath the skin, external pressure dressings are sometimes a useful method of controlling potential superficial postoperative oozing and minimising haematoma formation.
Suturing and surgical repair
Types of suture material and needles: Numerous types of suture are available (see Box 10.5), with the most important distinction being between absorbable and non-absorbable materials. The groups can be subdivided into natural and synthetic materials (although natural materials are being phased out) and further subdivided into monofilament and polyfilament (braided) materials. The choice of suture material depends upon the task at hand, the handling qualities and personal preference.
Absorbable versus non-absorbable materials: The strength of absorbable sutures declines at a predictable rate for each type of material, although the suture material remains in the wound long after it has any useful ability to hold tissues together.
In increasing duration of useful strength, the main absorbable materials are:
• Plain catgut and chromic catgut—useful strength 3 and 5 days respectively (no longer available in many countries)
• Modified polyglactin (Vicryl Rapide)—useful strength about 6 days
• Polyglycolic acid (Dexon) and polyglactin (Vicryl)—useful strength about 10 days
• Poliglecaprone 25 (Monocryl)—useful strength about 20 days
• Polydioxanone (PDS)—retains its strength for at least 28 days
The eventual elimination of absorbable materials from the body overcomes the problem of a permanent foreign body which can harbour infection. Absorbable sutures are often used in skin to avoid the need for removal; typical applications are minor skin operations, median sternotomies, surgery in children, circumcisions and vasectomies. Polyglycolic acid/polyglactin (undyed) gives good results as the sutures are removed by hydrolysis without inflammation. The modified short-lived polyglactin has ideal properties for skin closure where short suture life is required, e.g. inguinal hernia repair, and the monofilament poliglecaprone 25 (Monocryl) is ideal for situations when longer wound support is required.
Non-absorbable sutures retain most of their strength indefinitely. They are used where the repaired tissues take a long time to reach full strength (e.g. abdominal wall closure) or will be inherently weak (e.g. inguinal and incisional hernia repairs, arterial anastomoses). Non-absorbable sutures are also widely used for skin closure; synthetic monofilament sutures give reasonable cosmetic results and are easily and painlessly removed. Subcuticular sutures, which do not penetrate the epidermis, give excellent cosmetic results.
Natural versus synthetic materials: Catgut has been used as a suture and ligature material since before Roman times, derived from the material used for musical instrument strings. It consists mainly of collagen and is actually made from the dried small bowel submucosa of sheep or cattle. Silk and linen also have a long and distinguished history. Many surgeons believe that silk has the best handling and knotting properties of any material, but it provokes a strong inflammatory response exceeded only by linen. Silk was sometimes used for skin sutures, where its softness means there are no sharp ends to prick nearby skin. In general, natural materials are cheaper than synthetics, a factor of importance in developing countries.
The main advantages of synthetic absorbable suture materials are that they are stronger and provoke little or no inflammatory reaction. There is no risk of biological contamination with prions or viruses and they can be designed to meet specific requirements of absorbability, period of retention of strength, and handling properties.
Non-absorbable synthetic materials, similarly, do not provoke inflammatory reactions. Polyesters, nylon and polypropylene all retain virtually all of their strength over long periods in the tissues; this is particularly important when they are used to suture arterial prostheses where healing alone would not retain the prosthesis.
Monofilament versus polyfilament sutures: Monofilament materials have a smooth surface and can be pulled through the tissues with minimal friction; this makes them easier to insert and remove than polyfilament braided materials. On the other hand, monofilament materials are stiff, springy and more difficult to knot. Braided materials have the best handling qualities, but their interstices are a haven for bacteria. When used at a surface (e.g. skin or bowel wall) they tend to act as a ‘wick’, drawing infected material in. This problem is partly overcome by the manufacturers' application of surface coatings.
Wire sutures: Metal wire sutures have largely been displaced by non-absorbable synthetics. Stainless steel wire is, however, extensively used in orthopaedic surgery for bone fixation and sometimes for closure of sternotomy wounds in cardiac surgery. It is virtually inert but its main disadvantages are high rates of glove penetration and late breakage due to metal fatigue.
Gauge of suture material: The gauge of suture chosen for a particular task depends largely on practical experience. This takes into account the following factors:
• Number of sutures to be placed—the greater the number, the finer can be the gauge
• Type of suture material used—for a given gauge, the various materials have different strengths
• Cosmetic requirements—multiple fine sutures give a better cosmetic result than fewer heavier sutures
The traditional method of describing suture gauge (US Pharmacopoeia) is confusing for the newcomer and derives from the time when sutures were much thicker than those used today. The finest suture then was designated gauge 1, with gauge 2 and upwards applying to heavier sutures. As finer and finer sutures came into use, the scale had to be taken progressively backwards from 1, i.e. gauges 0, 00 (i.e. 2/0), 000 (3/0) and so on. Nowadays, the finest suture is 11/0, used for extremely delicate surgery such as in the eye. A more rational metric gauge, based on suture diameter, is in use but the traditional gauge is still widely used. A simple guide to the use of different gauges is outlined in Box 10.6.
Types of suture needle: Vast ranges of needles have been designed to accommodate the breadth of different demands of general and specialist surgery and the stringent requirements of microsurgery. Characteristics of needles and broad indications for their use are summarised in Box 10.7 and illustrated in Figure 10.3.
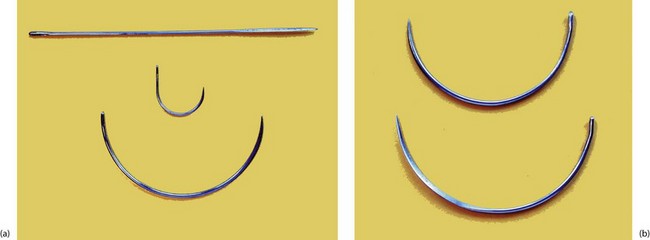
Fig. 10.3 Various types of surgical needles
(a) Three shapes of needle. The straight needle has a cutting point and is used for skin suturing. The J-shaped needle is used mainly for femoral hernia repairs, and the large half-circle needle is for abdominal wall closure. (b) Two large needles showing the difference between ‘round-bodied’ (above) and ‘cutting’ ends (below).
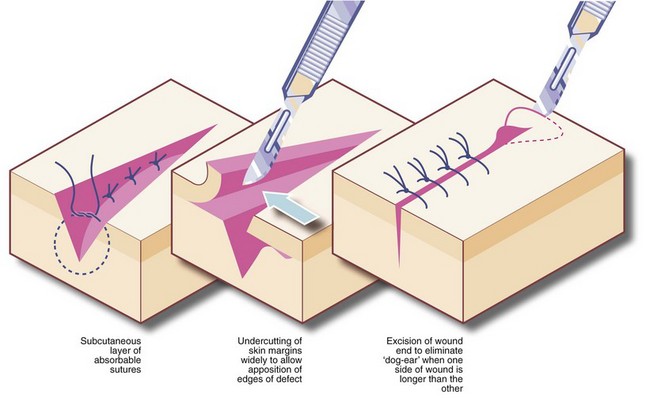
Methods of skin suturing: The objective of skin suturing is to approximate the cut edges so they will heal rapidly, leaving a minimal scar. Edges to be apposed should have been cut in a clean line and perpendicular to the skin surface; ragged or angled edges should be trimmed. The cut edges should be capable of being brought together neatly and without tension; otherwise the wound may break down or the scar slowly stretches, giving an ugly result. To achieve this, it may be necessary to insert a layer of subcutaneous sutures or even mobilise the skin by undercutting in the fatty layer (see Fig. 10.4). Undue laxity should also be avoided by trimming excess skin.
There are many techniques of skin closure, the choice being governed by the nature and site of the operation and by the surgeon's preference. In general, facial wounds are closed with multiple fine sutures removed after 4 or 5 days. Abdominal and chest wound sutures are generally removed after 7 days, while sutures for wounds on the back are best left for 14 days to minimise wound stretching.
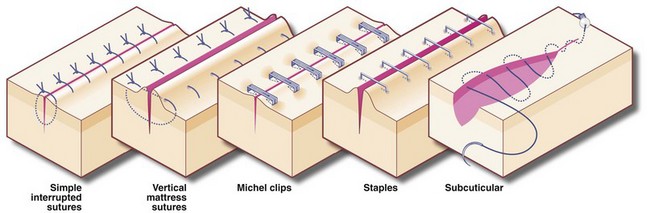
Subcuticular sutures, either non-absorbable or absorbable, are often used for longer wounds in cosmetically sensitive areas, provided the risk of infection is low. Elsewhere, the choice is between interrupted and continuous suture techniques. Interrupted sutures are indicated if there is a risk of infection; if infection develops, some sutures can be removed early to facilitate drainage. If the risk of infection is high, e.g. large bowel perforation, skin wounds are better left open and closed 48–72 hours later by delayed primary closure. The commonly used methods of skin suturing are illustrated in Figure 10.5.
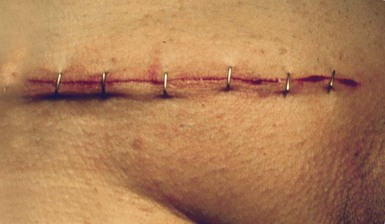
Clips and staples: Stainless steel clips (e.g. Michel clips) have been used for decades for closing skin wounds and are popular for neck incisions after thyroidectomy as they are haemostatic. As the clips do not penetrate skin yet give good edge apposition, the cosmetic result is excellent. Staples are used for both skin closure and bowel surgery. The skin closure devices are similar in concept to ordinary paper staplers, with staples stored in a magazine and applied singly instead of sutures (Fig. 10.6). More complex devices, which apply multiple staples simultaneously, either in a linear or circular fashion, are available for bowel anastomoses and closure of tubular viscera. Some devices have revolutionised surgical practice, e.g. re-anastomosis of colon to rectum after Hartmann's resection or bronchial stump closure. When used for internal viscera, the staples remain in place indefinitely.
Postoperative wound management
Once a wound has been closed, the doctor has three main responsibilities: choosing the dressing, monitoring the progress of healing and deciding when to remove the sutures.
The purposes of dressings for surgical wounds are as follows:
• To maintain the wound in a warm and moist state most conducive to healing
• To absorb or contain any superficial bleeding or inflammatory exudate
• To protect the delicate healing tissue from trauma, bacterial contamination and interference
• To prevent sutures catching on clothing or other objects
• To conceal the wound from view
• To apply pressure to the wound if haematoma formation is likely
Types of wound dressing: For small surgical wounds, particularly on the face, a dressing is often unnecessary as the linear crust of inflammatory exudate performs this task very well. For other simple wounds, plastic spray dressing is suitable, e.g. Opsite. In most other cases, prepacked adhesive dressings are used, incorporating an absorbent pad with non-stick film in contact with the wound surface. While convenient, these dressings may conceal accumulations of blood, inflammatory exudate or infected discharge. Wound inspection then requires painful removal of the dressing which can be an opportunity for infection to enter. Transparent semipermeable plastic film dressings neatly overcome this problem but are unsuitable for discharging wounds.
A flexible polyamide net coated with soft silicone (e.g. Mepitel) is useful for covering raw areas. This has very low wound adherence and is usually easy to remove. Other dressings include calcium alginate (seaweed origin), hydrocolloids and hydrogels. These dressings offer a better wound environment with increased hydration, fewer dressing changes, easier dressing removal and greater comfort.
Gamgee is a thick cotton wool dressing material enveloped in a thin layer of gauze; this is variously used for padding sites vulnerable to trauma (e.g. amputation stumps) or as pads beneath pressure bandages or as absorbent dressings for leaking wounds. Dry dressings are wads of dressing material (e.g. cotton gauze), usually taped or bandaged in place. Dry dressings need regular replacement and may be prevented from sticking by placing a piece of non-adherent dressing (e.g. Melolin, N/A dressing) against the wound.
Removal of dressings and sutures: Provided the dressing remains clean and dry and the patient afebrile and generally well, there is no need to inspect clean surgical wounds until the time of suture removal. If wound complications are suspected, the dressing should be removed and the wound checked and redressed. If infection is apparent, a wound swab should be taken for culture and sensitivity. Localised abscess formation requires suture removal and probing to effect drainage, whilst spreading cellulitis requires antibiotic therapy in addition.
Skin sutures should be removed as soon as the wound is strong enough to remain intact without support. On the back and around joints, this can take 14 days; on the abdomen, it takes about 7 days (longer in the case of steroid therapy or infection). On the face and neck, healing is rapid and less influenced by functional stresses. Here, sutures can be safely removed after 3–5 days, giving a better cosmetic result.
Management of drains in the postoperative period: Abdominal drains provide a potential route for infection to enter, even though the intra-abdominal pressure nearly always exceeds external pressure. The risk can be minimised by ensuring the drain opens into a sterile environment such as a drainage bag (closed drainage) and by removing the drain as soon as its task is completed. Decisions about removal of drains should rest with the operating surgeon who will undoubtedly have personal preferences.
The general principles of drain management are as follows:
• Suction drains help to collapse down spaces left in the tissues at operation as well as to drain blood and inflammatory exudate. These are mainly used after extensive surgery where a large covered raw surface remains, e.g. after mastectomy, thyroidectomy or large incisional hernia repair. Suction drains should not be used near bowel for fear of suction perforation. A suction drain is usually retained for only 24 hours unless substantial drainage persists (e.g. > 30 ml/24 h)
• Non-suction drains (e.g. large-bore silicone or rubber tubes or corrugated drains) are mainly used for bowel and biliary anastomoses and for abscess drainage. In this case, the drain is left in place for about 5 days. Some surgeons prefer to withdraw the drain in stages so that the deep part of the drainage tract can collapse progressively, reducing the risk of leaving a deep pool of fluid
Soft tissue surgery
Methods of obtaining tissue for diagnosis
Open or endoscopic biopsy: If major surgery or other therapy is being considered for a suspected malignant lesion, an accurate tissue diagnosis should be made.
Skin lesions can be biopsied by incision under local anaesthesia. Rectal lesions can be biopsied without anaesthesia using forceps through a rigid or flexible sigmoidoscope or colonoscope, while gastric and colonic lesions can be biopsied via a flexible endoscope. Breast lumps or suspicious mammographic lesions can be sampled using percutaneous core needle biopsy or by fine needle aspiration cytology (see below). Gastroscopy allows biopsy of upper GI mucosal lesions as well as lesion of the pancreatic head.
Enlarged lymph nodes can often be diagnosed by incision biopsy or by removing one or more completely for histological examination (excision biopsy), although many units can now obtain satisfactory results by needle biopsy. Lymphadenectomy often requires general anaesthesia, particularly for lumps in the neck.
Biopsy guided by ultrasound or CT scanning: Abdominal masses such as liver metastases or pancreatic lesions can be biopsied percutaneously with the aid of ultrasound or CT scanning. The suspicious lesion is first located as an image and then a biopsy needle is guided into it with the help of further imaging. The technique can be used to sample abdominal masses or suspicious para-aortic lymph nodes in staging lymphomas or following treatment for testicular germ cell tumours.
Cytology: Special staining techniques for malignant cells can be applied to material obtained by fine needle aspiration. Cytological diagnosis requires particular laboratory skills but often permits accurate diagnosis (e.g. of malignancy), which renders more invasive investigations unnecessary. A negative cytological result, however, must be interpreted with great caution because it may be due to sampling error.
Cytological diagnosis can be useful for the following:
• Examining cells aspirated from solid masses. This is particularly useful for thyroid nodules (see Ch. 5, p. 75 and Ch. 49), breast lumps, mammographically detected lesions and pancreatic masses
• Examining ascitic fluid obtained from the abdomen by paracentesis, or pleural effusions aspirated from the chest. Fluid may also be sent for microbiological analysis
• Examining cellular material scraped from surfaces, e.g. uterine cervical smears, or fluid obtained from within hollow viscera, e.g. urine, pancreatic secretions, sputum
‘Minor’ operative procedures
Many skin lesions are amenable to simple excision or biopsy, often under local anaesthesia. These may be performed in general practice or in dermatological or surgical clinics. Aseptic technique must be employed.
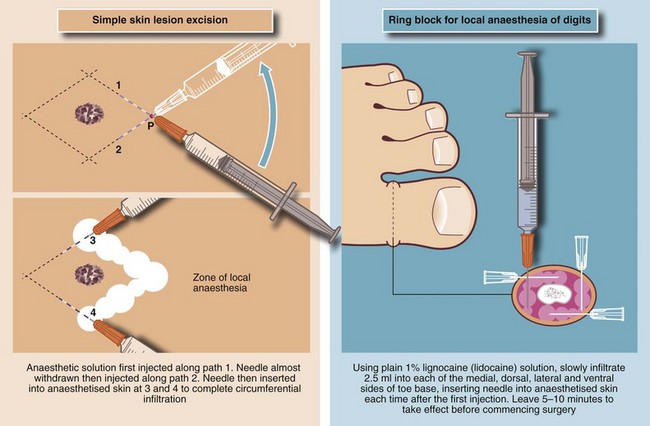
Local anaesthesia for skin lesions: The usual method of administration is by infiltration (injection) of local anaesthetic agents (e.g. lidocaine 0.5% or 1%) into the skin surrounding the lesion. Between 1 and 10 ml of solution is usually required, but care must be taken to remain within maximum safe dosages (see Table 10.3). A vasoconstrictor (e.g. adrenaline (epinephrine) 1 in 200 000) may be incorporated to reduce vascularity in the operative field, but this must never be used on the extreme peripheries, i.e. fingers, toes, penis or nose, because of the risk of ischaemic necrosis. The injecting needle should be as fine as possible and inserted into the skin as few times as possible to avoid causing unnecessary pain. Before each injection, aspiration is attempted to ensure that the solution is not injected directly into a blood vessel as intravascular injection may cause systemic toxicity. Methods of infiltration of local anaesthetic are illustrated in Figure 10.7.
Table 10.3
Maximum safe doses of local anaesthetic agents for infiltration in fit patients
| Lidocaine | Bupivacaine (Marcain) or levobupivacaine (Chirocaine) |
| 2% lidocaine is probably no more effective for achieving anaesthesia than 1% or even 0.5% so use lowest concentration needed; 10 ml of 1% lidocaine contains 100 mg | 0.5% bupivacaine is probably no more effective than 0.25%; 10 ml of 0.5% bupivacaine contains 50 mg |
| The maximum safe dose of plain lidocaine is 4 mg/kg body weight | The maximum safe dose of plain bupivacaine is 2 mg/kg body weight |
| For a fit 60 kg adult, the maximum safe dose of 1% plain lidocaine is 16–24 ml | For a fit 60 kg adult, the maximum safe dose of 0.5% bupivacaine is 24 ml |
| With adrenaline (epinephrine), this dose can be increased to 7 mg/kg body weight | Addition of adrenaline (epinephrine) does not increase the safe dose of bupivacaine and there is little point in using it for infiltration except to provide vasoconstriction |
| For a fit 60 kg adult, the maximum safe dose of 1% lidocaine with adrenaline (epinephrine) is 30–40 ml | No increase |
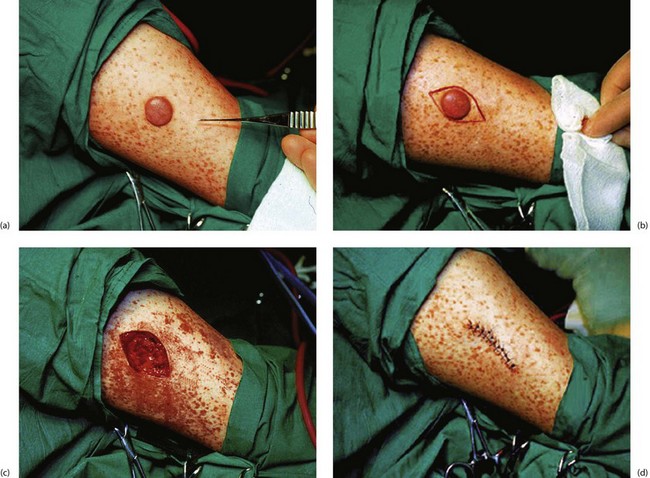
Excision biopsy: The technique of excision skin or mucosal biopsy illustrated in Figure 10.8 is appropriate for most small lesions not thought to be malignant. The lesion is removed with a fusiform piece of normal skin, with the long axis orientated along skin creases and tension lines. The specimen should include a millimetre or two of normal skin on either side of the lesion and should include the full depth of the dermis down to the subcutaneous fat.
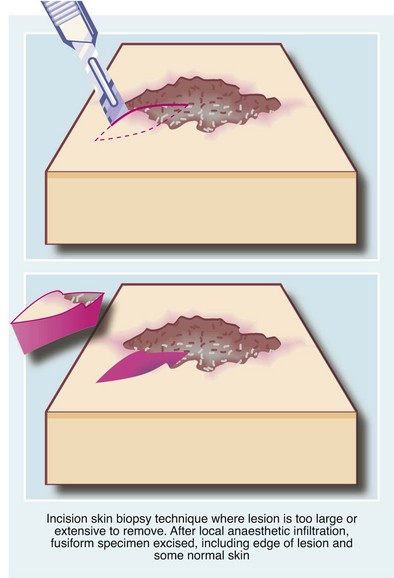
Incision biopsy: Incision biopsy is a technique of obtaining a tissue sample from a lesion that is too large or anatomically unsuitable for excision biopsy, e.g. a skin rash or a suspected malignant tumour. For the latter, major surgery or radiotherapy should not be performed without histological confirmation of the diagnosis. The biopsy objective is to obtain a representative sample of the full depth of the lesion, including an area of the margin and adjoining normal tissue (see Fig. 10.9).
Destruction of lesions by diathermy, electrocautery, cryocautery or curettage: These techniques are used for small lesions where there is no clinical suspicion of malignancy or for small basal cell carcinomas where a histological diagnosis is not required; the lesion is destroyed in the process of removal. Local anaesthesia is needed except for cryocautery, which is relatively painless.
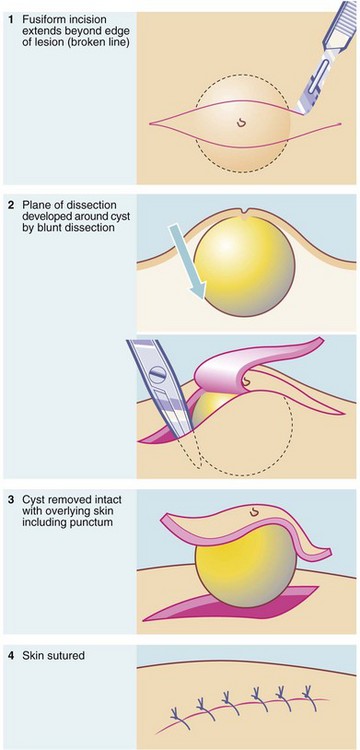
Removal of cysts: A cyst is a fluid-filled lesion, lined by epithelium and usually encapsulated by a condensation of fibrous tissue. The aim of treatment is to remove the whole epithelial lining because any remnant leads to recurrence. The technique of removal is outlined in Figure 10.10. Ideally, the cyst is dissected out intact without puncturing the cavity. If this occurs, the cyst collapses, making it difficult to identify and remove the epithelial lining. Inflamed cysts are best simply drained and excised later when the inflammation has settled.
Marsupialisation: This is usually employed for cysts or other fluid-filled lesions that are too large, inaccessible or technically difficult to remove. It is rarely appropriate for skin lesions but is often used for large salivary retention cysts in the floor of the mouth, cysts in the jaw and pancreatic pseudocysts. The surgeon usually removes a disc from the wall of the cavity and sutures the lining to the overlying epithelium around the cut edge. This leaves a pouch, which slowly fills in from below once the pressure of the cyst contents has been removed.
Surgery involving infected tissues
The first principle of managing an abscess is to establish drainage of the pus. When an abscess is ‘pointing’ to the surface, surgical drainage involves a skin incision at the site of maximum fluctuance followed by blunt probing with sinus forceps or a finger (usually under general anaesthesia) to ensure that all loculi are drained; necrotic material is removed at the same time by curettage.
After drainage, small abscesses need only a dry dressing, the cavity filling in rapidly from beneath. Larger and deeper abscesses need a method of keeping the skin opening patent until the cavity has filled with granulation tissue. A corrugated drain, which is gradually withdrawn (‘shortened’) over a few days, will achieve this or else the cavity may be packed with ribbon gauze soaked in antiseptic solution, or one of the newer absorbent dressings such as an alginate (e.g. Kaltostat); these packs are usually changed daily or every other day. Good analgesia is required in the early stages.
Management of infected surgical wounds
Grossly infected surgical wounds must be opened up to ensure free drainage, and cleaned. All necrotic tissue is excised leaving only healthy tissue, and the wound packed daily afterwards with antiseptic-soaked gauze or an alginate or hydrocolloid dressing. Wounds with large amounts of necrotic tissue may need more than one operation. Wounds are usually allowed to heal by secondary intention, although a large wound can be sutured later when infection is no longer a problem (‘delayed primary closure’).
Management of dirty or contaminated wounds: Major soft tissue injuries result in crushing and tearing of tissues, leaving devascularised areas and deep impregnation with soil, road grit or fragments of clothing. If such a wound is merely sutured, pyogenic infection is certain, and there is a serious risk of gas gangrene or tetanus.
The principles of managing these wounds were first established during the First World War, as follows:
• Thorough removal of all foreign material from the wound (sometimes called ‘debridement’)
• Excision of all non-viable tissue (‘necrosectomy’)
• Loose open packing of the wound with gauze without suturing
• Inspection of the wound under anaesthesia 2–4 days later, plus drainage of any new abscesses and removal of any newly apparent non-viable tissue
• Suturing of the wound when it looks clean and granulating, but avoiding tension, i.e. delayed primary closure. Alternatively, split skin grafting may be employed without wound closure
Principles of plastic surgery
The discipline of plastic surgery was born during the First World War in response to the appalling disfigurement caused by blast injuries, burns and facial trauma from trench warfare. Since then, specialised techniques of skin reconstruction have progressed much further, especially in the fields of axial flap design and microvascular reconstructive surgery. The scope of modern plastic surgery is outlined in Box 10.8. There is a considerable degree of overlap and collaboration between plastic surgery and other surgical specialties, especially ear, nose and throat, maxillofacial, breast and orthopaedic surgery.
Tissue transfer techniques: Obtaining satisfactory skin cover is a key problem in plastic surgery, and other tissues such as fat and muscle cannot satisfactorily be transferred without revascularisation. Free transplantation of full-thickness skin (other than tiny grafts) or any other tissue other than bone without revascularisation is usually unsuccessful.
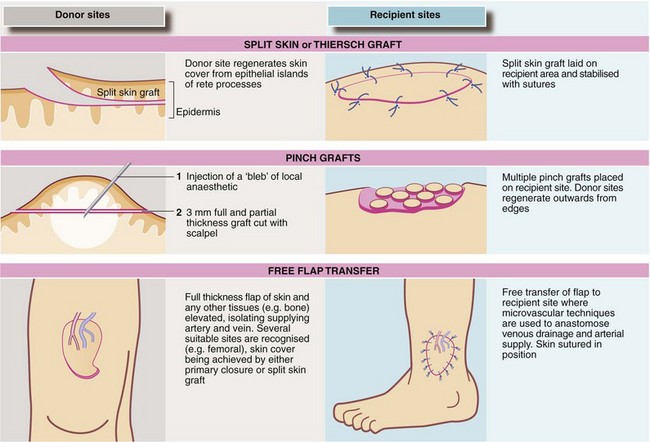
Skin grafts: Split skin (Thiersch) grafting involves transplanting a very thin layer of skin consisting of little more than epidermis, which has no blood supply of its own. It depends on nutrition from the recipient bed for its survival. Split skin grafts are commonly employed for burns and after wide excision of skin lesions, provided there is a recipient base of healthy tissue. The donor site heals rapidly since small islands of epithelium are left behind (see Fig. 10.11). The donor site is potentially more painful than the recipient but pain is well controlled if the donor site is dressed immediately with hydrogel or similar dressings. These are left in position for 7–10 days until the wound has epithelialised. Grafts fail because of sliding or shearing movement, or lifting off by haematoma or infection. Dressings exerting mild pressure such as a ‘sponge tie-over’ aim to reduce this. Creating a ‘mesh’ of the graft with a device to make multiple regular perforations helps the graft attach in certain circumstances as it allows free drainage through the perforations. Meshing also allows a graft to be expanded to cover a greater area but gives a poor cosmetic result.
Full thickness (Wolfe) grafts have some advantages for small skin grafts. They do not contract and usually have a better colour match. Donor sites include skin from behind the ear (post-auricular), where the defect can be closed primarily. This can be used for lower eyelid or finger tip reconstruction. However, these grafts pick up blood supply less readily than split skin and so are restricted to reconstructing small areas. See also pinch grafts (Fig. 10.11).
Vascularised flaps: Skin grafts cannot be used where there is a cavity, or bare bone or cartilage and these are absolute indications for flap repair. Flaps can also give good cosmesis, e.g. after skin cancer excision on the face. A flap has a blood supply of its own and is not a graft. The blood supply reaches the flap via its base, the vascular pedicle. Flaps can be advanced, rotated or transposed into the defect and this alone may provide sufficient mobility to close a moderate defect. If flap rotation produces a new defect, this clean area can usually be covered with a split skin graft.
The early random flaps, devised by Gillies, were limited in scope because there was no dominant blood supply and this prevented construction of a flap longer than 2 : 1 ratio to breadth. Later it was realised that longer flaps could be created by ‘axialising’ them. Most flaps are now axial, using recognised anatomical sites to ensure a blood supply running the full length. An early technique was the pedicle flap, a flap of skin raised and formed into a tube while initially remaining attached to its site of origin at both ends.
Axial flaps are selected according to the site of the defect and the tissues needed. A series of flaps have now been described, based on detailed anatomical studies of blood supply. Flaps may be cutaneous (e.g. forehead flap), fasciocutaneous (e.g. the ‘Chinese’ radial forearm flap), myocutaneous (e.g. latissimus dorsi, pectoralis major), TRAM (transverse rectus abdominis myocutaneous—often used for breast reconstruction), and osseous (e.g. fibula, radius, iliac crest and rib).
Free flaps: In free tissue transfer, a carefully planned piece of tissue is first dissected out, complete with at least one main artery and vein; axial flap sites are often suitable. The whole flap is then relocated to the recipient site where the vessels are anastomosed to a suitable local artery and vein by microvascular anastomoses. The donor site can often be closed primarily or else covered with a split skin graft. Examples are the radial forearm flap which supplies bone, muscle and skin (often used for mandibular and intra-oral reconstruction), and the big toe which can be used to replace a lost thumb.
Laparoscopic surgery
Introduction to minimal access surgery
The last few decades have seen an explosion of interest and rapid development of techniques to achieve accurate diagnosis and treatment with the least possible tissue injury and trauma. Laparoscopic surgery was among the first to catch surgeons' imagination but other techniques such as lithotripsy, percutaneous stone removal and angioplasty advanced earlier or more or less in parallel. Initially called minimally invasive surgery, the principle is that if less tissue is damaged by minimising surgical incisions, then less pain is likely afterwards and the physiological responses to injury which slow recovery will be reduced. The intention is to reduce patient suffering and allow more rapid return to normal life. The greatest benefits of laparoscopic surgery are evident when the amount of tissue injured in accessing the abdomen at open surgery is greater than the surgical trauma of the actual procedure. In addition, patients need to be informed that even with short-stay laparoscopic surgery, there will still be a period of physiological recovery.
Gynaecologists led the way with laparoscopic diagnosis and procedures, particularly tubal ligation, but little development in instrumentation occurred until laparoscopic cholecystectomy emerged as a viable and popular option. Early laparoscopic cholecystectomy was hampered by poor equipment, poor imaging and lack of experience. Now that equipment is first class, training and experience have disseminated and audit is in place, the indications, contraindications and risks have become clear; as a result, laparoscopic cholecystectomy has become the standard procedure for removal of the gall bladder.
Interest in minimal access techniques has spread through the surgical specialties, prompting surgeons to seek new ways of performing operations that cause less trauma to their patients. Thus orthopaedic surgeons perform many operations arthroscopically and joint replacements are being performed through smaller incisions, allowing rapid mobilisation. Thoracoscopy uses similar instruments to laparoscopy for diagnostic and therapeutic applications in the thorax (see details in Ch. 31, p. 398). Endocrine surgeons now perform parathyroidectomy through very small incisions using special microscopes and retractors. Breast surgeons perform sentinel lymph node biopsy for diagnostic purposes using dye or radioactive marker techniques instead of axillary clearance. Many urological procedures such as nephrectomy and ureteric operations are undertaken laparoscopically, and vascular surgeons even perform aortic aneurysm operations laparoscopically. In fact there is probably no abdominal operation that has not been attempted laparoscopically, although the difficulties of many larger procedures make them impractical for standard use. Laparoscopic techniques have proved most useful in areas of the body with limited access, such as the gastro-oesophageal junction, the adrenal gland and the pelvis, allowing delicate instruments to be used in confined spaces with excellent views unhampered by the surgeon's hands.
Laparoscopy
Laparoscopy or peritoneoscopy was in wide use clinically by gynaecologists from the early 1970s for diagnosing pelvic disorders and for sterilisation by tubal ligation. The first therapeutic gastrointestinal procedure was probably an appendicectomy performed by Semm in 1983. In 1987, Mouret first removed a diseased gall bladder laparoscopically in France. Since then, the techniques have been adopted on an increasing scale by general and thoracic surgeons for performing abdominal and thoracic operations via a series of small punctures rather than through large incisions.
Advantages of laparoscopic surgery: The advantages of laparoscopic surgery go well beyond simply avoiding large painful wounds to give better cosmesis and less postoperative pain:
• Improved access and vision in difficult areas, e.g. adrenal, pelvic organs and gastro-oesophageal junction
• Avoids handling, exposure, desiccation and cooling of abdominal tissues, which may reduce adhesion formation
• Reduced contact with patient's blood and body fluids for health care professionals
• Fewer wound infections, dehiscences and incisional hernias
Risks and complications: Certain complications are common to open and laparoscopic surgery, but laparoscopic operations carry their own particular risks. Specific problems include:
• Induction of the pneumoperitoneum (inflating the abdomen with gas) may cause subcutaneous emphysema or injury to bowel or major blood vessels and must be performed with care, preferably using an open technique rather than a Veress needle
• Trocar insertion may injure the abdominal wall (including the diaphragm), intra-abdominal organs and blood vessels. It must be performed under direct vision, being alert to structures that could be damaged
• The raised intra-abdominal pressure and head-down position mean that patients have to be ventilated at higher inflation pressures. This reduces venous return and cardiac output and may cause circulatory compromise in patients with cardiac ischaemia. To mitigate this, intra-abdominal pressures should be kept to the minimum necessary for the procedure to be performed safely, i.e. 8–12 mmHg; patients should not be placed in extreme head-up or head-down positions for prolonged periods. Intravenous infusion should be stopped whilst head-down
• The diaphragm is ‘splinted’ by the pneumoperitoneum and this can precipitate cardiorespiratory complications in patients with respiratory problems. Again, minimising inflation pressures reduces this effect
• The pneumoperitoneum compresses intra-abdominal veins and reduces venous return. This predisposes to thromboembolic complications such as deep venous thrombosis. Patients should receive appropriate prophylaxis at all times
• Inadvertent injury can occur to a range of structures during dissection, diathermy or laser instrumentation, often through excess heating. The damage may go unrecognised if the damage occurs out of the sight of the laparoscope, leading to late complications including haemorrhage and bowel perforation
• Postoperatively, bowel may strangulate through peritoneal defects, and incisional hernias can occur through port sites. The latter should be carefully closed under direct vision to minimise this risk
Technique of laparoscopy: Laparoscopy is usually performed under general anaesthesia, most often with muscle relaxation to allow full and safe insufflation of the abdominal cavity. A pneumoperitoneum is first created by introducing carbon dioxide under controlled pressure to lift the abdominal wall away from the viscera, allowing inspection of the peritoneal cavity.
Gas is most safely introduced via a blunt cannula placed by an open technique to enable direct visualisation of the peritoneal contents. The technique has largely replaced ‘blind’ insufflation via a Veress needle which has a significant risk of vascular injury. The gas pressure should be kept as low as possible while maintaining a satisfactory view, in order to minimise cardiac and respiratory risks. A 5 or 10 mm diameter laparoscope with video camera is then introduced into the abdominal cavity, displaying the image on monitors.
Most laparoscopic procedures need additional cannulae (trocars) through which to pass diathermy hooks, graspers, needle holders, clip appliers and linear staplers. These secondary trocars are inserted through new access points under direct vision from within the abdomen to prevent injury to bowel and other viscera. Different operations each require different port placements. Preferences vary from surgeon to surgeon and port siting also depends on the particular conditions of the operation; for example, cholecystectomy in an obese patient necessitates different port sites from those used in a slim patient.
The laparoscopic operation is performed by an operator and one or more assistants, all observing progress on monitors. Sharp or blunt dissection may be employed as in open surgery. Sharp dissection uses laparoscopic scissors whilst blunt dissection is carried out using fine dissecting forceps (‘Petolins’ or ‘Marylands’), laparoscopic gauze pledgets or the tip of a suction probe. Blunt dissection tears rather than cuts small vessels, deliberately causing minor tissue damage that rapidly activates the coagulation cascade and causes spontaneous cessation of bleeding. This is safer than sharp dissection which may require (potentially excessive) use of diathermy. Haemostasis still requires diathermy and this must be cautiously used to prevent arcing to adjacent organs. Increasingly, safer alternatives are being used such as bipolar diathermy forceps or the harmonic scalpel, which uses ultrasound to coagulate and cut vessels and tissue.
In laparoscopic-assisted surgery, for example colectomy, a large part of the dissection is performed laparoscopically and then a small abdominal incision is made to deliver the resected specimen.
After operation, patients experience abdominal discomfort at trocar insertion sites and shoulder discomfort from retained gas in the peritoneal cavity. Few restrictions are placed on the patient after discharge; he or she can return to work as soon as comfortable, often after a few days.
Robotic-assisted surgery: Robotically assisted surgical systems are increasingly being used for certain technically challenging operations such as laparoscopic prostatectomy and cardiothoracic operations. These robots are not autonomous but aid the surgeon who sits at a remote console (which may be close to the patient or far away). The surgeon very precisely controls the robotic manipulation of laparoscopic instruments, previously inserted into the anaesthetised patient, via the surgical arm unit. The imaging system provides three-dimensional vision. The da Vinci system allows manipulation of all instruments whilst other simpler systems just control the camera.
Potential benefits include increased precision of movement with ‘smoothing’ of tremor, prevention of fatigue in long operations and perhaps eventually the need for fewer assistants in the theatre. In the longer term, a surgeon could, at least theoretically, operate on a patient many kilometres away by tele-surgery. Disadvantages of robotic surgery include the high capital cost (currently around $1–1.5 million), complete lack of any tactile feedback for the surgeon and long set-up times for individual patients.
Applications of laparoscopy
Laparoscopy in general surgery has diagnostic and therapeutic applications. The diagnostic applications are well recognised and are increasingly employed as first-line investigative procedures.
Diagnostic laparoscopy: Diagnostic laparoscopy has long been used by surgeons for assessing chronic liver disease and ascites of unknown origin. Advances in instrumentation and imaging now allow surgeons to perform abdominal exploration almost as thoroughly as is possible via a long laparotomy incision. A key application is for staging gastric or pancreatic cancer to assess operability. This is achieved by inspection, by obtaining peritoneal washings for cytology and by performing biopsies. By this method, patients with incurable disease can be spared the trauma of exploratory open surgery (Box 10.9). Diagnostic laparoscopy is valuable for assessing right iliac fossa pain, particularly in women of menstruating age. The procedure allows more accurate assessment of the gynaecological organs, improves diagnostic accuracy and minimises negative appendicectomy rates.
Therapeutic laparoscopy: Laparoscopic cholecystectomy is already the standard operation for removal of the gall bladder, both in the elective and the emergency situation (described in Ch. 20). Other procedures described below and listed in Box 10.10 are becoming standard operations in the armoury of laparoscopic surgeons.
Laparoscopic appendicectomy: Open appendicectomy for acute appendicitis is one of the most frequently performed operations by general surgeons, and laparoscopic appendicectomy is becoming an operation within the repertoire of surgical trainees. Laparoscopic appendicectomy offers improved diagnostic accuracy with the ability to examine the entire abdomen if the appendix is normal, a lower rate of wound complications, reduced postoperative pain and hospital stay, and perhaps more rapid return to normal activities. Laparoscopic appendicectomy may offer a lower rate of pelvic adhesions because of reduced trauma; this is an advantage in young women.
Laparoscopic inguinal hernia repair: Inguinal hernias can be repaired laparoscopically using a transperitoneal or an extraperitoneal approach. Both techniques involve less dissection than the open approach and are believed to reduce the likelihood of damage to testicular vessels and the ilioinguinal nerve (particularly for recurrent hernias), resulting in a lower incidence of long-term chronic groin pain.
Learning laparoscopic inguinal hernia repair requires a great deal of supervised experience but the procedure is now widely performed, and controlled trials show that it can have results comparable with open hernia repair techniques, including term recurrence rates. Laparoscopic repair is of particular value for recurrent hernias, allowing surgery to be performed in tissue planes free from scarring. It is also recommended for bilateral hernias, when both sides can be repaired through three small incisions.
Laparoscopic fundoplication: Laparoscopic fundoplication can be employed for patients suffering from large hiatus hernias or gastro-oesophageal reflux disease (GORD) resistant to medical management. The technique involves dissecting the gastro-oesophageal junction at the hiatus, repairing the crura and wrapping the gastric fundus around the lower oesophagus (Nissen-type wrap). Clinical trials show that it offers rapid return to normal activity and results are as durable as open fundoplication.
Laparoscopic management of duodenal ulcer perforation: Duodenal ulcers usually perforate anteriorly and the perforation can readily be seen with the laparoscope. Under laparoscopic visualisation, the ulcer is closed in the same way as at open operation, with part of the greater omentum secured to the duodenum to seal the perforation with laparoscopically placed sutures. The peritoneal cavity is irrigated with a suction irrigator and the fluid aspirated.
Laparoscopic placement of enterocutaneous jejunostomy tube: In patients requiring long-term enteral feeding in whom a gastrostomy is unsuitable, a fine-bore feeding tube can be placed into the jejunum using laparoscopy, thus avoiding the need for laparotomy.
Laparoscopic splenectomy: Laparoscopic splenectomy has become the gold standard for elective splenectomy, particularly in haematological conditions. Substantially enlarged spleens can be removed laparoscopically but the risk of conversion to open operation rises steeply once the spleen weighs over 1 kg (such spleens usually reach the costal margin). At operation, the patient lies on the right side for best access and vision, and the hilar vessels are divided between clips or with vascular staplers. The spleen is placed in a laparoscopic retrieval bag and broken up or liquidised to enable removal via one of the small port incisions.
Laparoscopic adrenalectomy: Laparoscopic adrenalectomy has proved a highly beneficial procedure, allowing adrenal tumours of all sizes to be removed safely and with much less trauma than the muscle-cutting flank incisions previously employed. It is particularly useful in phaeochromocytoma where very delicate handling of the tumour is needed. This includes precise delineation and clipping of the vessels in the appropriate order to avoid catecholamine surges.
Laparoscopically assisted colectomy: Laparoscopic techniques were first used to perform uncomplicated colorectal procedures such as rectopexy and formation of colostomies. Early experience with colorectal cancer showed port site metastases appearing more commonly than wound metastases at open surgery. This led to concern that the cancer was being disseminated by the pneumoperitoneum. However, further research and refinement of ‘no touch’ techniques have laid these fears to rest. Laparoscopically assisted resection of benign and malignant colonic lesions is becoming more commonplace, and all elective colonic resections can now be undertaken laparoscopically. However, substantial benefits have not yet been demonstrated and there may be a higher rate of anastomotic complications.
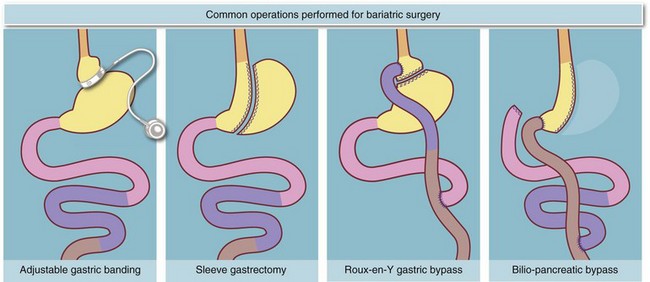
Laparoscopic surgery for obesity: (Fig. 10.12)
In recent years there has been a major expansion in laparoscopic surgery for obesity (bariatric surgery). The most common technique involves placing an adjustable restrictive silicone band around the upper part of the stomach to create a small proximal pouch that causes early satiety. Results are excellent in carefully selected, well-motivated patients who have good support and follow-up. More radical bariatric operations such as gastric bypass and biliary-pancreatic diversion (BPD) operations are also being performed laparoscopically. These operations achieve greater weight loss and more rapid regression of diabetes but may carry higher rates of morbidity and mortality, largely operator-dependent.
Principles of neurosurgery
Melanie Sharp and Robert Macfarlane
Introduction
The practice of neurosurgery is evolving rapidly, in much the same way that general surgery did in the 1980s. Neurosurgery is now subdivided into sub-specialities, including head trauma and:
• Vascular (e.g. aneurysm, arteriovenous malformation and stroke)
• Paediatric (e.g. hydrocephalus, tumours and congenital anomalies)
• Spinal (e.g. degenerative disease and tumours)
• Oncology (e.g. glioma, meningioma and metastases)
• Functional (e.g. movement disorders, chronic pain and epilepsy)
• Skull base (e.g. acoustic neuroma, meningioma and chordoma)
Neurosurgery is performed only in specialised centres because the patients present specific challenges that require a multidisciplinary team which includes neuro-anaesthesia, neuro-radiology, neuro-oncology, neuro-intensive care, neuro-rehabilitation as well as specialist nurses/ physiotherapists.
Perioperative considerations
Most neurosurgical units have procedure-specific guidelines for preoperative management including blood ordering. All neurosurgical patients should have baseline neurological assessment recorded and anticoagulants and antiplatelet therapy should be stopped preoperatively. Patients undergoing brain tumour surgery are often on steroids (e.g. dexamethasone) which may cause neutrophilia and adrenal suppression. Angiography and image-guided scanning protocols may be required in some cases.
Intraoperative considerations: Good intraoperative care and attention to detail allows the operation to proceed safely. ‘Special considerations’ for neurosurgical patients are shown in Table 10.4.
Table 10.4
Specific perioperative considerations in neurosurgical patients
| Patient group | Factors to consider |
| Patients taking anti-epileptic drugs (AEDs) | Measure drug blood levels and check electrolytes. Ensure medications are continued perioperatively |
| Cognitive impairment and confusion | Mental capacity needs to be assessed before obtaining valid informed consent (See Ethics chapter) New onset confusion needs investigation. It may indicate intracranial or biochemical abnormalities. Sedation may be dangerous. |
| Hair shaving | No evidence for increased wound infection with or without shaving. Cosmesis and surgeon preference determine the decision |
| Head positioning | Aim to achieve optimal operating position whilst aiding venous drainage. Avoid excessive neck rotation. Elevation of head reduces intracranial pressure but may risk of air embolism. 3-point clamp fixation stabilises the head and allows table-fixed retraction. During the WHO checklist, the operation side is confirmed against the imaging |
| Infection prophylaxis | Antibiotic prophylaxis on induction of anaesthetic typically with gram + coccal cover. No further doses in clean elective surgery |
| Intra-operative measures to lower ICP | Elevation of head (30–45 degrees) Mild hyperventilation to reduce cerebral blood volume Intravenous mannitol |
Postoperative considerations: Patients should be recovered in a monitored environment with regular neurological observations. The surgeon should be informed of any neurological deterioration. Depressed consciousness is usually an early sign of rising intracranial pressure. Pupillary dilatation and autonomic changes occur late (e.g. Cushing's reflex).
Postoperative seizures, reduced conscious level or altered neurology may herald intracranial complications such as oedema or bleeding. Repeat CT is needed after stabilising blood pressure and oxygenation. Sedating analgesics should generally be avoided. Paracetamol and codeine phosphate or small doses of morphine may be appropriate. NSAIDs impair platelet function and should be avoided early on in high-risk patients.
Intravenous fluid needs careful consideration; dextrose may increase cerebral oedema and 0.9% saline or Hartmann's solution is usually administered. Plasma sodium abnormalities are relatively common in neurosurgical patients and need investigation. These include the syndrome of inappropriate antidiuretic hormone hypersecretion (SIADH), which causes hyponatraemia and sometimes fluid overload, and diabetes insipidus (DI) which causes polyuria and polydipsia by diminishing the ability to concentrate urine.
Surgical management of hydrocephalus
Hydrocephalus results from a mismatch between cerebrospinal fluid (CSF) production and reabsorption, leading to raised intracranial pressure. It is classified into communicating and non-communicating types, depending on whether the obstruction lies within ventricles or the subarachnoid space. Common causes include:
• Congenital (e.g. spina bifida)
• Trauma (blood clot, brain swelling, venous sinus injury)
Hydrocephalus can be an acute emergency; patients can present to a general hospital with morning headache, vomiting, blurred or double vision and ataxia. Signs include depressed conscious level, papilloedema, cranial nerve IV or VI palsies or nystagmus. An urgent CT scan identifies hydrocephalus or shunt dysfunction. Hydrocephalus in infants may manifest with gradually enlarging head circumference or, in the elderly, with the triad of ataxia, dementia and incontinence (‘normal pressure hydrocephalus’).
Treatment of communicating hydrocephalus: This can be relieved temporarily via lumbar puncture (but never in non-communicating hydrocephalus). Long-term management requires diversion by insertion of a shunt as described below.
Treatment of non-communicating hydrocephalus: If secondary to tumour, this may respond to dexamethasone to temporise before tumour removal. Otherwise the obstruction needs to be bypassed by endoscopic third ventriculostomy, insertion of an external ventricular drain, or creation of a permanent CSF diversion with a shunt. Shunting is usually via a catheter between lateral ventricle and peritoneum with a pressure regulating valve.
Basic principles of craniotomy
Craniotomy is the standard method of accessing the cranial cavity. A bone flap is fashioned which is replaced at the end of the operation. Bevelling the bone edges and using titanium plates and/or screws facilitates closure. If bone is not replaced, this is termed craniectomy (the bone forms a template for a titanium or acrylic plate, or is stored for later reimplantation). Indications for craniectomy include raised intracranial pressure refractory to medication, bone infection or osteomyelitis, or infiltration of bone by tumour.
Preparation for operation begins with optimal patient and head positioning. Planning the incision and craniotomy needs to take the following factors into account:
• Cosmesis (e.g. avoiding forehead incisions and excessive hair shaving)
• The scalp blood supply: large arteries include occipital and superficial temporal arteries and these should be preserved. Bleeding from even small scalp arteries can lead to large blood loss
• Avoiding placing implants or foreign material (e.g. screws/plates) directly under wounds
• Location of scalp nerves: damage to temporalis and frontalis nerve branches can cause unsightly forehead muscle asymmetry
• Position of cranial landmarks and underlying structures in relation to the pathology, including major dural venous sinuses, cranial arteries and areas of special importance such as motor and visual pathways. Modern planning includes CT or MR image-guided navigation, but it cannot replace knowledge of anatomy
In elective surgery, craniotomy flaps are ‘tailored’ to access the operative area, but when an intracranial haematoma needs rapid and safe evacuation after trauma, a standard fronto-temporo-parietal craniotomy is used. Meticulous haemostasis before wound closure is critical in operative neurosurgery.
Presentation of brain tumours
Brain tumours are primary or secondary. Primary tumours are categorised by histological type, location within the brain and grade. The terms benign or malignant are not normally used because tumours rarely metastasise outside the craniospinal axis, and long-term survival depends on whether tumour growth can be controlled. Glioma is the most common primary brain tumour, whilst meningioma (see Fig. 10.13) is the most common extra-axial tumour. The WHO pathological classification provides a more precise system, encompassing the tissue of origin and its behavioural characteristics. Potential modes of tumour presentation are shown in Table 10.5.
Table 10.5
Presentation of brain tumours according to site
| Mode of presentation | Significance |
| Incidental finding | Intracranial pathology identified during imaging for another purpose (e.g. sinusitis). A period of active observation may be appropriate |
| Seizures | Generalised tonic-clonic seizures—usually only in supratentorial tumours Partial sensory or motor seizures—particularly in tumours around the sensory or motor strip Complex partial seizures—particularly in temporal lobe tumours |
| Raised intracranial pressure | Reduced conscious level, confusion, headache, vomiting, papilloedema, unilateral or bilateral pupillary dilatation |
| Altered brain function | Frontal—altered personality, contralateral face, arm or leg weakness, expressive dysphasia (dominant side), incontinence Temporal—upper homonymous quadrantanopia, receptive dysphasia (dominant hemisphere) Parietal—lower homonymous quadrantanopia Occipital—homonymous hemianopia Cerebellar—nystagmus, ataxia, speech disturbance including dysarthria and staccato speech and intention tremor Brainstem—vomiting, lower cranial nerve palsies, long tract signs, reduced consciousness, pupil changes, altered eye movements Hypothalamic/pituitary—visual deficit (bitemporal hemianopia), endocrine disturbance |
Orthopaedic surgery
Axial and appendicular skeleton
The axial skeleton comprises the skull, spine and rib cage whilst the appendicular skeleton comprises the arms and shoulder girdle, the legs and pelvis. The normal locomotor system relies on a stable skeleton to provide attachment for muscles and a base for positioning the hands and feet in space. Muscles are grouped into bundles or mass-acting units according to function and along embryological lines.
Disease of any part of the control or action mechanism leads to loss of function and requires diagnosis and reversal to restore functional ability. For example, a finger contracture due to Dupuytren's disease limits movement and small items cannot be retrieved from a pocket or purse. Contrast this local problem with a motorbike rider who injures his neck in a collision. The subsequent brachial plexus injury prevents accurate nerve signalling to the hand and he also cannot retrieve his coins. Management of these similar functional disturbances is very different but the aim for both is to recover full functional capability.
Structure of bone and articular cartilage
Bone: Bone is composed of an organic and an inorganic matrix. It undergoes continual adjustment as cells are remodelled and elements refreshed in response to external stimuli. The skeleton is a living organ providing support for muscles and tendons, it has haemopoetic capacity, and it is a calcium reservoir.
Bone structure includes cortical and trabecular components. A solid shell of cortical bone is strong in compression and resists bending forces. Mature cortical bone is arranged in lamellae or layers with vascular channels running through. Its precursor is immature woven bone, seen in healing and in the growing skeleton. Its organisation is random without heed to external stresses.
Bone metabolism involves complicated homeostasis of its matrix (governed by physical stresses), plus regulation of minerals and ions, principally calcium and phosphate. The inorganic component is largely crystalline calcium phosphate and hydroxyapatite, and a feedback loop controls mineralisation and turnover, involving serum ion levels, vitamin D3, parathormone and calcitonin (see Fig. 49.10, p. 611).
The organic matrix of bone is largely composed of type I collagen, with types V and XI in small amounts contributing to flexibility and strength. Also found are bone morphogenic proteins (BMP 1–17), and cytokines and signallers (interleukin 1 and 6 and insulin-like growth factor).
Articular cartilage: Articular cartilage provides a near frictionless interface between bones to facilitate movement. Synovial joints are covered with hyaline cartilage, a special tissue composed of chondrocytes bound in an extracellular matrix. The matrix is layered tangentially and radially throughout the cartilage; 90% of this collagen is type II.
Hyaline cartilage has very poor healing ability. It is aneural, avascular and alymphatic, and derives nourishment by osmosis from synovial fluid. Superficial abrasions or injuries provoke no healing response and result in permanent damage. Deeper injuries that reach the capillary bed of the bone cause clot formation and later repair with fibrocartilage. Healing never perfectly restores the initial hyaline cartilage or its specialised smooth surface.
Limb compartments
Limb muscles are arranged into compartments during embryological life; grouping those with similar actions limiting friction between them. The compartments have inflexible fascial walls restricting expansion beyond a limited volume so if traumatic swelling occurs, there is a risk of compartment syndrome causing ischaemia (see Ch. 17, p. 235).
Orthopaedic disorders
Arthritis
The arthritides are joint disorders leading to pain and dysfunction. They can be grouped into acquired and congenital, inflammatory, infective, autoimmune and degenerative. Anything that alters the low friction surface of a synovial joint leads to pain on movement, and diseases that alter the surface or the synovial envelope can do the same.
Osteoarthritis: Osteoarthritis (OA) is an idiopathic disorder of joint wear. Over the lifetime of a joint, the hyaline cartilage tends to wear out causing pain and stiffness. The process is governed by genetic, developmental and other factors that are not yet completely understood. Some joints such as hip and knee are often affected, whereas others (e.g. ankle) rarely wear out. Some hips wear out because of imperfect articulation or development from birth. It is not yet known why some individuals are plagued by widespread osteoarthritis in their 50s and others have little or none in their 80s. Treatment includes palliative analgesia and often, ultimately surgery.
Post-traumatic osteoarthritis: This is linked to trauma sustained at the joint surface. This may be an intra-articular fracture, a cartilage tear or anything that increases friction at the bearing surface. Post-traumatic OA occurs between 5 and 20 years after the trauma. Heavy impact sport in early life can play a part.
Inflammatory arthropathies: This group includes rheumatoid arthritis (RA) and other joint diseases characterised by a chronic nature and an inflammatory component. Autoimmunity is often involved, and the group should be considered as systemic diseases which often lead to multijoint involvement with symmetrical patterns.
In most inflammatory arthropathies the pathology is synovial, with chronic inflammation and thickening of epithelial layers leading to pain, stiffness and altered blood chemistry. Low-level joint destruction occurs over many years, and secondary osteoarthritis is eventually superimposed. Treatment aims to control or modify the inflammation to prevent progression.
Crystal arthropathies: A subgroup of inflammatory joint diseases occurs in acute episodes and causes painful joints. The most common is gout, where monosodium urate crystals enter the synovium and synovial fluid causing profound inflammation. The joint becomes hot, swollen and painful. Initial treatment is analgesia and rehydration with joint splintage. In pseudogout, similar problems are caused by calcium pyrophosphate dehydrate.
Infection
Orthopaedic infections involve soft tissue and/or bone. Soft tissue infections include those of bursae adjoining joints, in tendons or tendon sheaths of hands and feet, and cellulitis. These are often easy to diagnose, but may be difficult to treat without leaving a functional deficit or stiffness; outcome often depends on the pathogen responsible. Fibrosis of upper limb tendons and sheaths develops quickly even after eradication of infection and can severely limit hand function.
Bone infection is termed osteomyelitis and affects cortex or cancellous bone. Joint infection is termed septic arthritis. Areas with poor blood supply cannot eliminate infection effectively and antibiotic delivery to these areas is also poor.
Osteomyelitis: Bone infection passes through several stages: an acute inflammatory response with soft tissue swelling, demineralisation and death of bone (sequestrum formation), then, if infection is controlled, new bone (involucrum) formation. Identifying the infecting organism by biopsy, aspirate and culture is critically important before targeted antibiotic therapy. Long courses may be needed to eradicate infection from sites with poor blood supply such as the tibia. Surgical debridement of abscesses and removal of dead bone is also an important step.
Septic arthritis: (Fig. 10.14)
This is an orthopaedic emergency and occurs by traumatic penetration of a joint or by spread from metaphyseal osteomyelitis. Articular cartilage in synovial joints is very easily damaged when a joint fills with pus. Hyaline cartilage breaks down in hours, so treatment is urgent. This means early removal of dead tissue, joint irrigation and targeted antibiotics. Without this, secondary osteoarthritis is inevitable within very few years.
In adults, the usual infecting organism in primary osteomyelitis and septic arthritis is Staphylococcus spp., although Streptococcus spp., Salmonella, Pseudomonas and other rarities occur, usually in immunocompromised patients.
Children also suffer predominantly staphylococcal infection. Widespread immunisation against Haemophilus has largely banished it as a cause of bone infection. Yeasts and fungi sometimes infect the musculoskeletal system but viruses do not, although viral influenza can cause multiple painful joint effusions and synovitis.
Tumours
Primary bone tumours are rare. Malignant tumours are classified by primary cell type and are staged using the T-N-M system. The neoplastic cell grade gives an indication of aggressiveness.
Bony metastases are much more common than primary malignancies and spring from solid primary tumours or diffuse diseases such as lymphoma or myeloma. The classic primary sources are breast, lung and prostate cancers and the usual presentation is bone pain before pathological fracture. After fracture, the abnormal bone is unlikely to heal and often requires surgical stabilisation.
Diagnosis and management of primary bone tumours follows the usual pattern of detailed history and systematic examination, biopsy for histological diagnosis, and staging. Because of rarity this is usually undertaken in tertiary referral centres. Neoplasms are treated with various combinations of radiotherapy, chemotherapy and surgery depending on cell type and stage. Some primaries, for example osteosarcoma, still carry a very poor prognosis.
Paediatric orthopaedics and growth disorders
The skeleton begins to form in utero and bones ossify through childhood and teenage years to reach a stable state usually between 15 and 18 years. Most long bones originate with a cartilage shape that gradually transforms to adult-type bone as osteoblasts infiltrate and lay down calcified bone by the process known as enchondral ossification. By contrast, intramembranous ossification occurs without a cartilage model; examples include clavicle and the flat skull bones.
Enchondral ossification proceeds from several ossification centres at varying speeds and with predictable onset. Long bones have one centre in the middle of the diaphysis (main shaft) from where ossification spreads longitudinally, plus centres in the epiphyses (near the ends). The physis or growth plate at the bone end is where new bone forms to cause longitudinal growth. Diseases that affect the physis or epiphysis can disrupt bone growth and lead to a range of abnormalities. The Salter-Harris classification (Fig. 10.15) describes fractures at the physis and/or through the epiphysis in children's bones.
Children's bones contain less calcified osteoid (bone matrix) than those of adults; they flex more before they fracture and demonstrate more plastic fracture patterns when they do. Bone healing after fracture is also different: bones heal with callus and re-ossify, but remodel quickly, often smoothing out deformity or malunion. As the bone grows longer, it attempts to return (remodel) to its normal shape; the more growth years left, the more scope there is for remodelling.
Primary bone tumours in children are rare although Ewing's sarcoma must be considered during teenage years. Bone cysts and remnants of ossification errors such as cortical fibromas are often seen incidentally on X-ray. They usually cause no symptoms and need no treatment.
Disorders affecting epiphyseal bone growth can cause dwarfism or short stature and many are effectively uncorrectable. Faults in calcification or generation of bone matrix can lead to weak bone. This is seen in rickets or osteogenesis imperfecta. Cooperative management between endocrinologist, paediatrician and orthopaedic surgeon can limit the functional disturbances.
Elective orthopaedics
Plain radiology: This is the chief orthopaedic investigation. X-rays have been used for decades and doses and techniques are now refined to minimise ionising radiation. Bones are shown in great detail because of the high calcium content. The usual model is two X-rays at right angles and including nearby joints; both views must be examined for abnormality, fracture or disruption before reaching a diagnosis. Radiology of the skeleton in its loaded state can give more information, e.g. standing or flexed, and X-rays of an arthritic joint are often taken with knees and hips in a standing position.
Ultrasound: Ultrasound is widely used in orthopaedics. It gives a dynamic view of soft tissues and is good at examining liquid filled structures; bones are not seen in useful detail. Cysts, ganglia and tendons are well evaluated and a dynamic picture of a joint is valuable in motion disorders or impingement syndromes.
Magnetic resonance imaging (MRI): This modality is risk free and is increasingly used to give detail of soft tissues and three-dimensional architecture. Some examples include: nerve roots exiting the spinal cord, menisci and ligaments in the knee joint (with contrast); wrist ligaments and carpal bones can also be examined.
Computed tomography (CT): CT scans can give detail of bones down to millimetre level. Images can be reformatted into three-dimensional virtual models, e.g. for fractures or to plan complex anatomical reconstructions of acetabulum or spine.
Arthroscopy: Accurate diagnosis of a joint injury often requires internal inspection. Despite accurate history, examination and even MRI, the problem can be missed if it is transient, or only reproduced under conditions of load.
Arthroscopy is often employed for shoulder or knee, less often in the elbow and rarely in the hip. Under anaesthesia, a 5 mm arthroscope (rigid telescope with video camera) is inserted into the joint enabling inspection and, if necessary, probing with another instrument. Abnormalities can often be repaired or material removed at the same time and most procedures take less than 30 minutes so patients can go home the same day and usually recover rapidly. Arthroscopic techniques have been extended over the years to include ligament reconstruction, rotator cuff repair and cartilage repair.
Joint replacement surgery
Joint replacement is advocated when pain plus major damage to articular cartilage has exhausted non-surgical options. Replacement of synovial joints can restore anatomical alignment and provide pain-free mobility. Hip replacement was pioneered in Wrightington Hospital, Lancashire, in the 1960s and has taken off world-wide; progress in replacing other large joints proceeds apace. End-stage osteoarthritis remains the most common indication, but replacement is also performed for malalignment, post-traumatic arthritis and revision replacement when primary replacement fails. Joint replacement surgery is a major undertaking; patients are usually in their 60s or 70s and complication rates of 5% are currently unavoidable.
Replacement of the knee, hip, ankle, shoulder, elbow joints and most others relies on surgically excising the diseased, high-friction articular cartilage and resurfacing the bone ends with metal, plastic or ceramic bearing surfaces. Various techniques are used to secure the implant components. Bone cement works very reliably but an alternative method of fixation uses osseointegration, encouraging bone growth into a hydroxyapatite-coated metal implant. After joint replacement surgery, pain is markedly reduced but the articulation continues to wear and usually has a finite life with 10–15 years or even more expected for 90% of patients with hip or knee replacement. Revision joint replacement is well established, but success rates drop and functional results are rarely as good.