Thoracic surgery
Introduction
In its broadest sense non-cardiac thoracic surgery covers the diagnosis and management of all non-cardiac disease within the chest. However, the practice varies between countries. For example, in the UK, unlike North America, most oesophageal disease is no longer managed by thoracic surgeons but by gastroenterologists and upper GI surgeons. This chapter will review the management of benign and malignant conditions of the chest and mediastinal disorders that are commonly managed by UK thoracic surgeons. The principles of oesophageal surgery are covered in Chapter 22 and chest trauma in Chapter 15.
Investigative techniques
Imaging: Chest X-ray, computerised tomography (CT), magnetic resonance imaging, ultrasound (US) and synchronized CT-positron emission tomography are the most commonly used modalities. Chest X-ray is useful as a ‘baseline’ and also for early post-procedure follow-up. CT provides the best anatomical information and is used for preoperative planning and for follow-up. low radiation-dose protocols protocols are being explored for lung cancer screening. Early evidence suggests that screening certain groups may lead to longer survival and this may become common practice. MRI is most useful to study soft tissues, and in particular in malignancy, to determine the extent of chest wall invasion, diaphragm invasion and for spread of cancers to the liver or brain.
US is useful to guide drainage of collections, siting of chest-drains and for biopsy of superficial or pleural-based lesions. Most modern thoracic surgeons can undertake basic bedside ultrasound to evaluate pleural effusions.
Ideally, before placing a non-emergency chest drain, US or preferably CT scanning should be performed to reduce the risk of complications.
Lung function tests: Lung function tests give a detailed portrait of the physiological effects of individual chest diseases and can track changes over time or as a result of treatment. When surgery is contemplated, lung function tests help assess the patient's capacity to withstand chest wall incision or lung resection. They include:
• Measurement of air flow into and out of the alveoli, i.e. FEV1, FVC, peak air flow, total lung capacity, alveolar ventilation
• Measurement of gas diffusion across the alveolar–capillary interface, usually involving measuring rates of carbon monoxide diffusion
• Assessing the amount of dead space by calculating the residual volume and total lung capacity
• Assessing exercise capacity, e.g. by the 6 minute walk test or by formal cardiopulmonary exercise testing
Bronchoscopy: Flexible bronchoscopy can be performed with minimal or no sedation and topical local anaesthesia. It is possible to examine as far as segmental bronchi, obtain small biopsies and clear secretions.
For cancer staging, transbronchial ultrasound (endobronchial US, EBUS) allows biopsy of lymph nodes close to the airway. This can be combined with transoesophageal ultrasound (EUS) to allow more lymph node stations and the left adrenal gland to be biopsied. Rigid bronchoscopy is performed under general anaesthesia and allows passage of larger instruments, including a flexible bronchoscope, for removal of foreign bodies, obtaining large biopsies and control of bleeding.
Pleural aspiration and percutaneous biopsy: Aspiration of pleural effusions for cytological examination can be performed using a standard wide-bore needle and syringe. Blind pleural biopsy is now discouraged because of its relatively low yield rate and high complication rate. Many thoracic masses are amenable to percutaneous biopsy under US or CT guidance, although thoracoscopy or direct surgical cut-down to the lesion has the best yield.
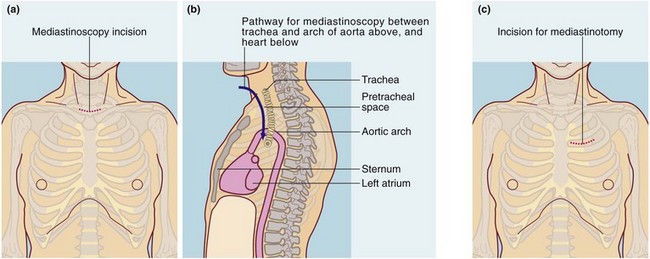
Video-mediastinoscopy: A mediastinoscope is used to biopsy paratracheal and subcarinal lymph nodes. The instrument is a rigid tube incorporating fibreoptic light guides; it is inserted via a skin incision above the suprasternal notch and passed caudally along the plane of the pretracheal fascia (see Fig. 31.1a and b). The route passes close to the azygos vein, superior vena cava, innominate artery, arch of the aorta, pulmonary artery and the recurrent laryngeal nerves posterolaterally on each side. These structures and the oesophagus are at risk of damage and, although rare, this must be explained to the patient. Mediastinoscopy gives access to the mediastinum except for the subaortic fossa (below the aortic arch and often containing lymph nodes). Access to this area is obtained by anterior mediastinotomy or video-assisted thoracic surgery.
Thoracoscopy: This is the thoracic equivalent of laparoscopy and is sometimes known as video-assisted thoracoscopic surgery or VATS. It is usually performed under general anaesthesia but basic procedures use local anaesthesia with sedation. Instruments for viewing and operating are inserted through small incisions in the chest wall.
Thoracoscopy is the preferred technique for pleural biopsy, pneumothorax treatment and evacuation of early empyema and is also used to sample mediastinal lymph nodes and perform cervical (thoraco-dorsal) sympathectomy.
The range of procedures is becoming increasingly complex, e.g. extensive pleurectomy for mesothelioma or lobectomy. VAT lobectomy usually involves a non rib-spreading ‘utility’ incision of 5–10 cm and two or three further ‘port’ incisions to pass a video-telescope and surgical instruments. The resected specimen is placed in an extraction bag so it can be removed intact. The ports can be used to place drains.
Anterior mediastinotomy: Anterior mediastinotomy (see Fig. 31.1c), a form of mini-thoracotomy, may be used to obtain biopsies from anterior mediastinal lesions, e.g. thymic tumours. The approach can be left or right of the sternum, either intercostally or with costal cartilage resection. Left anterior mediastinotomy affords good access for biopsy of subaortic fossa masses. Increasingly, VATS is the preferred method of accessing these sites.
Thoracotomy: Thoracotomy, described on page 400, gives full access to the paratracheal, subcarinal and hilar lymph node groups, the great vessels, oesophagus, lung and pericardium and is used when less invasive procedures are inappropriate or have failed.
Therapeutic procedures
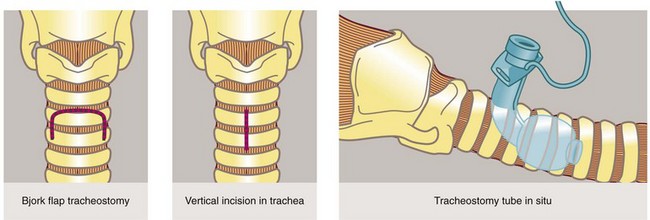
Principles of tracheostomy: A tracheostomy (see Figs 31.2, 31.3) is an artificial opening into the trachea to provide a secure airway when the pharyngeal airway or larynx needs to be bypassed. With time, an epithelialised fistula develops between the skin and trachea which allows tracheostomy tubes to be changed and the airways cleaned with ease. In many units ‘percutaneous tracheostomy’ is performed using a ‘Seldinger-type’ technique. However, the technique of ‘open’ tracheostomy should be understood by most surgeons.
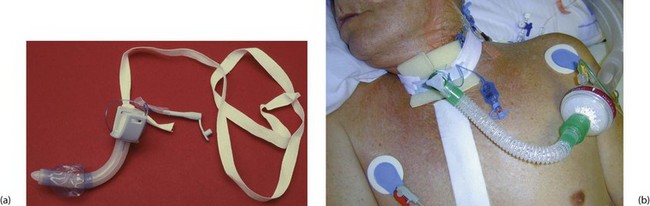
Fig. 31.3 Tracheostomy
(a) Disposable tracheostomy tube. Note the distal balloon which is inflated via the small tube to provide a snug fit inside the trachea.
(b) Patient being ventilated via an elective tracheostomy after cardiac surgery
Indications for tracheostomy include:
• Permanent functional obstruction of the upper airway, e.g. carcinoma of larynx
• Temporary or potential upper airway obstruction, e.g. facial fractures, major head and neck operations or injuries
• Long-term ventilatory support, where prolonged endotracheal intubation would otherwise be likely to cause permanent laryngeal damage and prevent swallowing and speech. Tracheostomy also provides continuous access to the lower airways for bronchial aspiration and toilet
Tracheostomy should be a planned procedure performed in the operating room under general anaesthesia. It is not an emergency procedure for patients with upper airway obstruction. For these, endotracheal intubation or cricothyroidotomy (see Fig. 31.4) should be used.
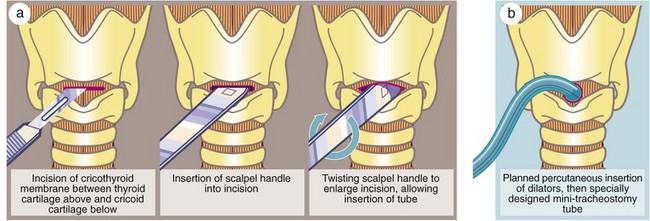
Fig. 31.4 Cricothyroidotomy
(a) In a dire emergency, this life-saving procedure can be rapidly employed to gain time. (b) Modern mini-cricothyroidotomy can be performed percutaneously by making a small incision through the cricothyroid membrane and progressively dilating it with graduated dilators before insertion of a specially constructed small-diameter tube, e.g. Minitrach or Quicktrach. These are used in accident victims and often in intensive care units
Complications of tracheostomy:
• Haemorrhage caused by erosion of the innominate (brachiocephalic) artery or vein
• Tracheo-oesophageal fistula, particularly where a nasogastric tube is in place
• Displacement of the tracheostomy tube may occur before the desired ‘fistula’ becomes established, making it difficult to reintubate the trachea
• Tracheal stenosis, usually the result of prolonged use of a high-pressure cuff (now rare due to the introduction of low-pressure cuffs)
Thoracotomy
All thoracotomies are now devised to spare at least some muscles from being divided.
Posterolateral thoracotomy: Posterolateral thoracotomy is the standard approach for lung and oesophageal resections as well as for surgery of the descending aorta. Generally a curved incision passes below the inferior angle of the scapula, latissimus dorsi is divided and the chest is entered through the bed of the (unresected) fifth or sixth rib. If necessary, the incision can be extended into the abdomen (thoraco-abdominal incision), e.g. for oesophago-gastrectomy or thoraco-abdominal aortic aneurysm.
Lateral thoracotomy: Lateral thoracotomy involves an incision extending between anterior and posterior axillary lines.
Median sternotomy: Median sternotomy or ‘sternal split’ gives wide access to the heart and the entire anterior mediastinum including the great vessels. It is the standard incision for cardiac surgery as well as for excision of thymic lesions and large retrosternal parathyroid tumours and, occasionally, resection of a goitre with massive retrosternal extension.
Specific thoracic disorders
Problems affecting the pleural space
Introduction
The pleural cavity is a potential space between the chest wall and lung, lined by a continuous sheet of mesothelium. That lining the chest wall is parietal pleura and that covering the lung is visceral pleura. The pleural space normally contains a minute amount of serous fluid to lubricate movement of opposed pleural surfaces and it causes them to adhere by surface tension. This, and the negative pressure arising from the tendency of the chest wall to expand, keeps the lungs expanded. Disease or injury may result in accumulation of air (pneumothorax) or fluid (pleural effusion) in the pleural cavity. Pleural effusions are classified as transudates or exudates. Fluid collections can be subdivided into pus (empyema), blood (haemothorax), chyle (chylothorax) and, rarely, lymph (lymphothorax).
The pleural space is not essential to life, and the principle of managing pleural problems is often to eliminate this space. This is achieved by draining the space, ensuring the lung abuts the chest wall, and stimulating the lung to adhere to it. Such pleural adhesions are known as pleuradhesis (or pleurodesis). Pleural adhesions may occur spontaneously.
Pneumothorax
Pneumothorax is classified as primary or secondary. Primary refers to ‘spontaneous’ pneumothorax, occurring without obvious lung disease. It is most common in people between the ages of 15 and 30, and is characteristically (but not exclusively) seen in tall, thin males. Secondary pneumothorax occurs in patients with other lung disease, most often COPD. It is most common after 50 years in patients with a smoking history but can occur in a range of conditions including Pneumocystis pneumonia and collagen disorders.
Primary or ‘spontaneous’ pneumothorax most often results from rupture of a ‘bleb’ on the pleural surface of the lung, usually a small air-filled cavity that communicates with lung parenchyma. Rupture allows air to escape into the pleural space and the lung collapses. Traumatic pneumothorax usually results from blunt chest injury, often from rib fractures penetrating visceral pleura. Penetrating chest injury, e.g. stab wounds, may also be responsible. Different types of pneumothorax are illustrated in Figure 31.5.
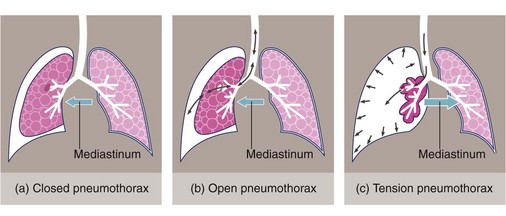
Fig. 31.5 Classification of pneumothorax
(a) In closed pneumothorax the pleural defect closes spontaneously, leaving a fixed amount of air in the pleural space. (b) In open pneumothorax there is free passage of air via an open defect in the visceral pleura. (c) In tension pneumothorax the pleural defect acts as a flap valve allowing progressive entry of air into the pleural space, collapsing the lung and pushing the mediastinum to the opposite side
Sometimes, after trauma, if the site of air leakage acts as a one-way valve, a tension pneumothorax is created. The valve effect allows air to escape into the pleural space but not to return to the airway, causing lung collapse on the affected side. Rising intrapleural pressure reduces venous return and in extreme cases can push the mediastinum towards the opposite side. Reduced venous return leads to low-output cardiac failure which in turn worsens the venous return. The physiological upset is extreme, can progress rapidly, poses an immediate threat to life and must be treated urgently.
Treatment of a pneumothorax is required under the following circumstances:
• Where there is a tension pneumothorax
• When the lung volume is compromised by more than about 25% as calculated on a PA chest X-ray
• If the pneumothorax is increasing
• When a small pneumothorax is having a disproportionate effect on lung function because of pre-existing lung disease
Aspiration: An uncomplicated pneumothorax in an otherwise fit patient can be treated by aspiration. A 50 ml syringe is connected to a three-way tap or one-way valve and a needle. The needle is inserted intercostally into the pneumothorax and air aspirated or allowed to blow out. Progress is later monitored by chest X-ray. The process can be repeated, but formal tube drainage may be needed if the pneumothorax recurs.
In tension pneumothorax, rapid emergency relief can be obtained by passing a large needle into the pleural space. A relatively safe point is just lateral to the mid-clavicular line in the 2nd intercostal space. A formal chest drain must be inserted soon afterwards.
Intercostal tube drainage: The technique of intercostal chest drainage is described in Figure 31.6; see also Figure 31.7. If a pneumothorax needs a drain, a single small apical drain is used, e.g. 16 F gauge. Smaller drains are used by non-surgeons but these have a high failure rate due to kinking and blockage.
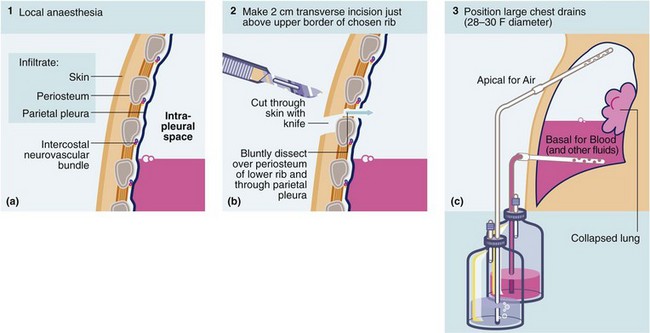
Fig. 31.6 Technique of intercostal tube drainage of chest (tube thoracostomy)
•Inject local anaesthetics to block sensitive structures and intercostal nerve and give time for this to take effect
•Make 2 cm incision near upper border of rib and parallel to it
•Bluntly dissect intercostal muscles down to parietal pleura with artery forceps. Stay near upper border of rib to avoid intercostal vessels
•Palpate lung with gloved index finger to free adhesions and ensure free entry for the drain
•Remove trocar from large-bore chest drain tube (at least 28 F gauge); 16 F can be used for pneumothorax alone. Grasp distal end with artery forceps and guide drain into chest in an apical or a basal direction according to purpose. Never insert a chest drain with the trocar in position as this is highly dangerous
• Attach drain to an underwater seal and suture drain to chest wall. Snug the skin around the drain with a purse string suture. Apply airtight dressing around the tube and tape tube to chest wall. Sit patient up to 45°
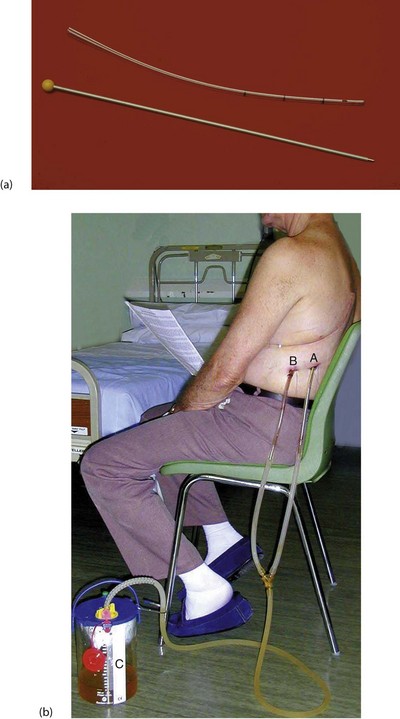
Fig. 31.7 Chest drain
(a) 30 F gauge tube (10 mm) with stylet removed (and thrown away!). Note the radiopaque line and the side holes near the tip. (b) Post thoracotomy and lobar resection of lung for cancer. This patient has two chest drains in situ, an Apical drain for Air and a Basal drain for Blood. Both are connected to an underwater seal, C, to prevent inflow of air that would cause a pneumothorax
Any intercostal tube drain must be connected to an apparatus to prevent lung collapse from air being drawn into the chest by negative intrapleural pressure. The usual arrangement is to connect the chest drain to a rigid tube secured below the water level in a bottle to form an underwater seal. This allows air and fluid to leave the chest cavity but not return. The bottle is kept below patient level so gravity assists. As the patient breathes, excess pleural air and fluid are gradually expelled into the bottle, and air bubbles from the exhaust tube. If there is a lung air leak via a breach in the visceral pleura, this is manifest by continued bubbling in the bottle and failure of the lung to expand. Continuous suction should then be applied to the underwater seal outlet for a few days; this usually helps the lung to expand and adhere to the chest wall, thereby remaining inflated and blocking the air leak.
Patients with intercostal drains must be transported with care to ensure the drainage bottle does not tip over and always remains below patient level to prevent fluid reflux into the chest. It is no longer recommended that chest tubes be clamped when patients are moved as this is dangerous (one exception to this is after pneumonectomy).
Intercostal drains are removed when the lung is fully expanded. For pneumothorax, cessation of bubbling in the bottle for 24 hours is an indication for removal; for fluid drainage, the duration varies with the underlying problem.
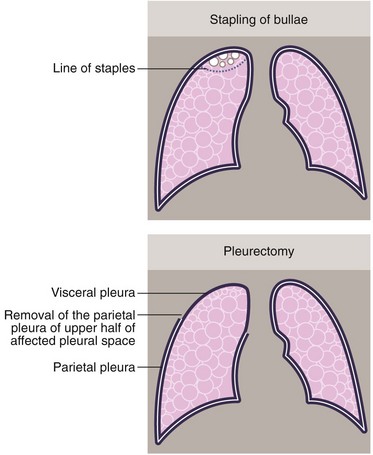
Treatment of persistent or recurrent pneumothorax: More extensive surgical intervention may be required for persistent or recurrent pneumothorax. The general rule is that surgery is offered for an unresolving pneumothorax, two pneumothoraces on the same side or one on each side. Patients in high-risk occupations, such as pilots, may be offered surgery after a single pneumothorax. Approaches include stapling of bullae to prevent air leakage and pleural abrasion or pleurectomy. In patients with fragile lungs, as in secondary pneumothorax, lung resection is sometimes avoided by insufflating talc to encourage a chemical pleurodesis (see Fig. 31.8). Most of this surgery is now performed by VATS.
Excess pleural fluid
Excess pleural fluid may be a watery transudate (e.g. in heart failure) or an exudate of variable viscosity (e.g. due to pleural infection).
Indications for draining a pleural effusion include:
Uncomplicated pleural effusions are usually drained via a large-bore tube (28 F gauge or larger) inserted towards the base of the pleural cavity in the most practicable dependent position. More than one drain may be required if fluid collections are loculated.
Malignant effusions: Malignant effusions (e.g. from breast cancer) usually recur after simple drainage and need to be treated in other ways. Methods include:
• Stimulating adhesion formation between visceral and parietal pleura (pleurodesis). This can be achieved by aspirating the fluid, injecting an irritant such as sterile talc and maintaining tube drainage until permanent adhesions develop. An alternative is to perform pleural abrasion. Via a small thoracotomy, the parietal pleura is widely abraded using a surgical swab. Again, chest drainage allows adhesions to form between the layers of pleura with permanent prevention of effusion
• Parietal pleurectomy. Traditionally performed by thoracotomy but now usually by VATS, this involves stripping the parietal pleura to cause diffuse adhesion of the lung surface to the chest wall (see Fig. 31.8b)
• Pleuro-peritoneal shunting using a tubular device connecting the two cavities. This is implanted beneath the skin and incorporates a one-way valve. Excess pleural fluid is manually ‘pumped’ from the pleura into the peritoneum by the patient several times a day where it is resorbed. Unfortunately the manual pumping can be uncomfortable and devices can become blocked
• Tunnelled intrapleural catheter. A small catheter is tunnelled a few centimetres from the skin to the pleural space and can be opened to intermittently drain the pleural space. The catheter can be inserted under local anaesthesia. Tunnelling reduces the infection risk
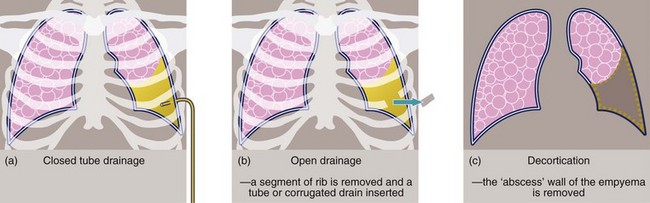
Empyema: When pleural fluid becomes infected, pus accumulates in the pleural cavity and is known as an empyema. Early on, an empyema can be treated by dependent intercostal tube drainage with irrigation if necessary; some empyemas respond to treatment with fibrinolytics. In chronic cases, a thick fibrous wall or cortex gradually forms around the pus-filled space. Treatment options then include prolonged closed tube drainage, open tube drainage (sometimes involving removing a segment of rib), and surgical ‘decortication’ (see Figs 31.9 and 31.10), which releases entrapped lung and tethered chest wall and diaphragm, allowing the lung to re-expand.
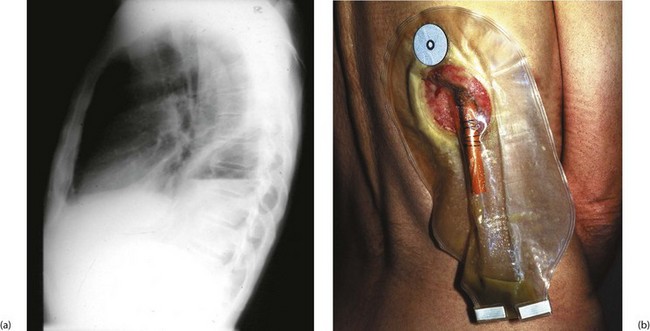
Fig. 31.10 Empyema thoracis
This 51-year-old man underwent a thoraco-abdominal oesophagogastrectomy for proximal gastric cancer. He suffered a small intrathoracic anastomotic leak and developed an intrapleural empyema, shown by the fluid level in (a). (b) Drainage of empyema. A section of rib overlying the cavity was resected and a large red rubber tube was inserted to give dependent drainage into a bag. The drain was gradually shortened as the cavity closed. This patient was alive and well 15 years later
Haemothorax: Following chest trauma, blood can accumulate in the pleural space. This usually needs draining via two large drains, one apically and one basally. A similar strategy is employed after open chest surgery. Clotted blood does not drain well and usually needs evacuation by VATS or thoracotomy. Removing blood allows the lung to expand against the chest wall and helps to arrest continuing haemorrhage from intercostal vessels. Persistent or increasing drainage of blood indicates continuing intrathoracic bleeding and requires intervention. Continued bleeding is usually from the systemic circulation (e.g. internal mammary, intercostal or great vessels) rather than from lung parenchyma.
Lung abscess
Lung abscesses have become much less common with effective antibiotic treatment of pulmonary infections. Onset of symptoms may be insidious, with clinical features including a swinging pyrexia, foul-smelling sputum and a cavitating shadow on chest X-ray. Primary lung abscess may follow bacterial lung infection, most commonly with Staphylococcus aureus, Streptococci, Pseudomonas or Klebsiella.
Secondary lung abscess may follow aspiration of gastric contents or occur in lung segments distal to bronchial obstruction by a centrally placed neoplasm or inhaled foreign body.
Lung abscesses are usually treated with antibiotics alone but occasionally a cavity requires drainage. Drains are usually placed percutaneously under ultrasound or CT guidance. Surgery by deroofing (‘marsupialisation’) or resection of the abscess may be required. Good chest drainage is vital after the procedure.
Cancer of the lung
Lung cancers are either primary tumours or metastatic deposits from elsewhere. Smoking is the commonest cause of primary lung cancer. Due to smoking control, rates of lung cancer are starting to plateau and even fall in developed countries but continue to increase elsewhere. Of primary tumours, about 80% will have metastasised by the time of presentation. Lymph nodes in the hilar area are usually involved first, followed by other mediastinal nodes. The liver, adrenal glands, bone and brain are common sites of metastatic spread.
Primary lung cancers are classified into small cell tumours (10–15%) and non-small cell lung cancers (NSCLC). Small cell cancers are usually treated non-surgically as they have a very high risk of metastases and are more responsive to chemoradiotherapy than NSCLC. Non-small cell cancers are further classified as squamous cell carcinoma, adenocarcinoma, large cell carcinoma, undifferentiated carcinoma, and broncho-alveolar cell tumours. Many lung cancers grow rapidly and assessment must not be delayed if curative treatment with surgery or chemo-/radiotherapy is contemplated. A much less aggressive form of lung cancer is typical carcinoid tumours of the lung. They are still usually removed by surgery.
Staging of lung cancer and its implications
TNM staging of lung cancer is fundamental to planning appropriate treatment. This is periodically reviewed and the most recent classification (the seventh version) is shown in Figure 31.11. In staging, contrast-enhanced CT scanning of chest and upper abdomen is the first step. The likelihood of enlarged lymph nodes being malignant increases with size. The order of further tests varies with local practice but generally includes a CT-PET scan, brain imaging with CT or MRI and evaluation of enlarged or PET-positive sites by biopsy. If histological confirmation has not been achieved by biopsy of a suspicious node, the primary site may need biopsy.
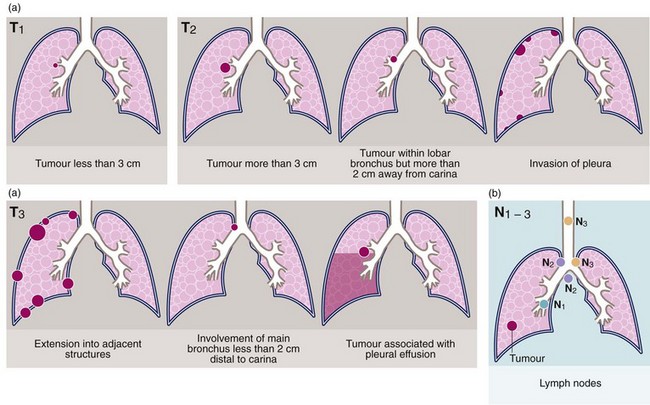
Fig. 31.11 Tumour staging using the TNM classification
(a) Tumour size and extent. (b) Lymph node involvement
If the lung cancer is confirmed to be ‘early’, i.e. stage I or II and selected patients with IIIa disease, fit patients are offered surgery. Stage 1a NSCLC can have a 5-year survival of > 70% but very few patients present this early.
If preoperative staging of mediastinal lymph nodes is negative but N2 disease is discovered at surgery (i.e. occult N2 disease), around 50% can undergo a histologically complete resection; this subgroup would expect a 5-year survival of 30%.
Some patients with more advanced disease are also offered surgery, including patients with limited mediastinal node involvement, and very occasionally patients with a removable isolated distant metastasis.
Palliative treatment: For patients whose disease is too extensive for surgery, yet the tumour and lymph nodes can be encompassed within a radiotherapy ‘field’ with acceptable toxicity, they can be offered radiotherapy with curative intent, usually accompanied by chemotherapy. For advanced disease, radiotherapy can increase life expectancy as well as provide effective palliation for troublesome complications such as lobar collapse, haemoptysis, superior vena caval obstruction or symptomatic metastases in brain or bone. Advanced radiotherapy techniques such as stereotactic radiotherapy are under evaluation and may allow higher doses to be given with acceptable toxicity.
Chemotherapy adds around 5% on average to 5-year survivals and is offered to appropriate patients. New targeted therapies have been introduced and can have benefits. For example, patients with certain epidermal growth factor receptor mutations respond very well to modulator drugs of those pathways.
Surgical treatment of lung cancer
The role of surgery varies according to cell-type, known responsiveness to other therapies, respiratory function, age and fitness of the patient for major surgery. Surgery is usually reserved for potentially curable patients. Surgical treatment of operable lung cancer involves wide resection of the primary with sampling of loco-regional (mediastinal) lymph nodes to establish accurate pathological TNM staging which influences any decision to employ adjuvant chemotherapy or radiotherapy.
Operation involves removing one or more affected lung lobes (lobectomy) or the whole lung (pneumonectomy), sometimes with resection of involved chest wall. In patients with impaired lung function, sublobar resection (segmentectomy or non-anatomical ‘wedge’ resection) may be justified with the aim of preserving lung function.
The standard surgical approach is postero-lateral thoracotomy. The right lung has upper, middle and lower lobes, the left has upper and lower lobes. The empty thoracic space left by resection is soon taken up by hyperinflation of the remaining lung tissue, mediastinal shift and elevation of the hemidiaphragm.
After pneumonectomy there is a space which may become partially or sometimes fully occupied by mediastinal shift, fibrosis and reduced dimensions of that side of the chest.
Non-malignant indications for lung resection
• Trauma—major sharp injury to a lobe or lung, or blunt trauma where a bronchus has been ruptured
• Infection—consequences of infection including bleeding due to bronchiectasis or secondary fungal infection of a persistent, antibiotic ‘sterilised’ abscess cavity
• Benign tumours—if curative excision via bronchotomy is impracticable
• Lung transplantation—transplantation of a single lung is sometimes performed for non-malignant disease when there is minimal respiratory reserve and one lung is substantially more affected than the other. Bilateral lung transplantation is performed in patients with lung diseases involving infection, e.g. cystic fibrosis or bronchiectasis. Heart–lung transplantation is offered when both lungs and heart are irreparably damaged, e.g. Eisenmenger's syndrome (see Ch. 14)
Malignant mesothelioma
This is a malignant proliferation of mesothelial cells and can arise in pleura (or rarely in peritoneum). There is usually extensive local spread, restriction of the lung and pleural effusion. Spread is usually late and can be to local lymph nodes and eventually more distantly. There are three main histological forms somewhat correlated with disease behaviour: epithelioid is the least aggressive, biphasic or mixed (histologically a mixture of epithelioid and sarcomatoid types), and sarcomatoid which is the most aggressive.
Malignant mesothelioma is strongly associated with asbestos exposure, either direct, as in a building industry worker, or indirect, such as in a family member. There is generally a long interval between exposure and development of the disease (20–40+ years). The carcinogenic agent is the asbestos fibre that provokes chronic inflammation that may eventually become malignant. Asbestos exposure is also associated with increased rates of lung cancer. Mesothelioma is likely to peak between 2010 and 2020 in developed countries, but is still rising in other parts of the world. This is due to the time lag from asbestos exposure. Unfortunately asbestos is still widely used in less developed countries.
Presentation of malignant mesothelioma: This usually presents with chest pain due to irritation of intercostal nerves, or shortness of breath due to loss of lung volume from the tumour or the pleural effusion, or both.
Investigation: A chest X-ray is usually followed by a CT scan. A tissue diagnosis can then be obtained by percutaneous radiologically guided biopsy or thoracoscopy.
Treatment: Unfortunately mesothelioma is rarely curable. Occasionally a localised malignant mesothelioma can undergo complete resection with hope of cure, but the more usual aim of surgery is maximum debulking (‘cytoreduction’) in advance of adjuvant therapy, or pure palliation. Two operations aim to achieve maximum debulking. Extrapleural pneumonectomy (EPP) involves removing pleura, lung, usually pericardium, and diaphragm, which then requires reconstruction. The other operation is pleurectomy-decortication (PD) which aims to retain the lung but removes pleura and any lung cortex to allow reinflation. Sometimes this also involves resection of diaphragm and/or pericardium. In specialist centres, approximately 30% 5-year overall survival can be expected.
Palliative procedures include thoracoscopic pleurectomy, simple talc pleurodesis, pleuro-peritoneal shunts and insertion of tunnelled intrapleural catheters.
Overall, chemotherapy has been shown to extend survival in mesothelioma by only about 3 months. The place of radiotherapy is also controversial. ‘Port-site’ radiotherapy has been used to reduce the growth of mesothelioma through previous incisions and high-dose hemithoracic radiotherapy to the operated side can be used after pneumonectomy with the aim of reducing recurrence.
Benign asbestos diseases of the chest
Asbestos exposure may be associated with pleural effusions, pleural plaques, ‘infolded lung’ and lung fibrosis. These are not of themselves premalignant, but are markers of exposure. They may cause major problems, culminating in respiratory failure.
In some countries such as the UK, mesothelioma is considered an occupational disease and financial support is available from the government or other bodies.
Disorders of the mediastinum
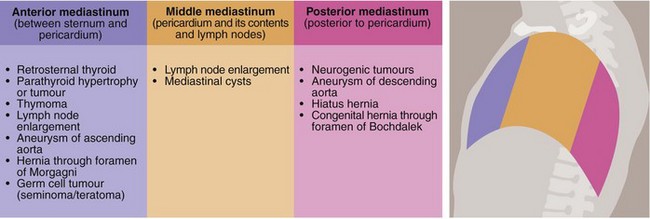
Disorders of the mediastinum of surgical importance are summarised in Figure 31.12.
Anterior mediastinum
Retrosternal thyroid: Rarely, the thyroid gland is ectopically located in the anterior mediastinum where it can become enlarged by any of the processes discussed in Chapter 49. Alternatively, an inferior extension of a normally located thyroid gland may spread retrosternally into the anterior mediastinum. The adverse effects of retrosternal thyroid enlargement are usually related to progressive displacement of the trachea. Sudden enlargement of retrosternal thyroid tissue caused by haemorrhage can threaten the airway. Most retrosternal thyroids can be removed through a standard thyroidectomy collar incision and only rarely is a median sternotomy required.
Thymus: The thymus causes few pathological problems except for uncommon benign or malignant tumours. For benign thymomas or low-grade cancers, surgery alone may be sufficient and 5-year survival can be as good as 70% or better following complete resection. Thymic cancers are also often sensitive to chemotherapy and radiotherapy, and large, aggressive or invasive thymic cancers may benefit from neoadjuvant or adjuvant treatment.
Myasthenia gravis is an unusual clinical condition associated with certain thymic tumours or thymic hyperplasia. After thymectomy, some patients with myasthenia gravis improve but this is unpredictable.
Parathyroid: Benign and malignant parathyroid tumours usually occur in the neck but sometimes occur anywhere between the retrothyroid area and the aortic arch. If retrosternal lesions are suspected, exploration of the anterior mediastinum accompanied by thymectomy usually enables the abnormal parathyroid tissue to be removed.
Middle mediastinum
This contains the pericardium and hila of both lungs. Disorders requiring surgical intervention include:
• Lymph node enlargement—lung cancer or lymphoma are the most common causes. Tumour type may be defined by biopsy, and the extent by CT scanning. Non-malignant granulomatous diseases can also manifest here (e.g. sarcoid)
• Aneurysms—those of the ascending aorta or the arch of the aorta are usually degenerative but may also appear as a late complication of aortic trauma or dissection. Syphilitic aneurysm is now rare
• Developmental cysts—these include pericardial, bronchogenic, enterogenous and cysts of uncertain origin. Mediastinal cysts occasionally become infected and even more rarely undergo malignant change
• Germ cell tumours—primary or secondary teratoma or seminoma occasionally occur. These are diagnosed by histology or raised serum markers and can usually be treated successfully with chemotherapy
Posterior mediastinum
The posterior mediastinum lies behind the pericardium. Surgery may be required for the following conditions:
• Aneurysms of the descending aorta
• Tumours of neurological origin—these may be bilobed and extend into the spinal canal. These are usually benign
• Diaphragmatic hernia—hiatus hernia is common but is not always associated with gastric reflux. Most sliding hernias can now be treated with acid-reducing drugs, weight loss and other simple advice (see Ch. 22). The more unusual para-oesophageal or rolling hiatus hernia may lead to gastric infarction and many believe its presence is an indication for surgery. Congenital herniation into the posterior mediastinum (hernia of Bochdalek) is very rare. Thoracic or abdominal approaches for hiatus hernia are performed. The commonest operation for hiatus hernia with reflux is a laparoscopic fundoplication