Skeletal tissues
Introduction
The skeletal system is formed from highly specialised types of supporting/connective tissue. The tissues are made up of collagen and acellular matrix, as well as the cells which synthesise them. Bone provides a rigid protective and supporting framework, the rigidity resulting from the deposition of calcium salts within the collagen and matrix. Cartilage occurs in different forms and provides a smooth articular surface at bone ends, as well as structural support in special areas (e.g. trachea, pinna). It is also important in one form of new bone formation.
Joints are composite structures which join the bones of the skeleton and, depending on the function and structure of individual joints, permit varying degrees of movement. Ligaments are robust but flexible bands of collagenous tissue which contribute to the stability of joints. Tendons provide strong, pliable connections between muscles and their points of insertion into bones.
The functional differences between the various tissues of the skeletal system relate principally to the different nature and proportion of the ground substance and fibrous elements of the extracellular matrix. The cells of all the skeletal tissues, like the cells of the less specialised supporting/connective tissues, have close structural and functional relationships and a common origin from primitive mesenchymal cells (see Ch. 4).
Cartilage
The semi-rigid nature of cartilage stems from the predominance of proteoglycan ground substance in the extracellular matrix.
Proteoglycans (see Ch. 4), disposed in proteoglycan aggregates of 100 or more molecules, make up the ground substance and account for the solid, yet flexible, consistency of cartilage. Sulphated glycosaminoglycans (GAGs, chondroitin sulphate and keratan sulphate) predominate in the proteoglycan aggregates, with molecules of the non-sulphated GAG hyaluronic acid forming the central backbone of the complex. The different types of cartilage vary in the amount and nature of fibres in the ground substance: hyaline cartilage contains few fibres, fibrocartilage contains abundant collagen fibres and elastic cartilage contains elastin fibres.
Cartilage formation commences with the differentiation of stellate-shaped primitive mesenchymal cells (see Fig. 4.2) to form rounded cartilage precursor cells called chondroblasts. Subsequent mitotic divisions give rise to aggregations of closely packed chondroblasts which grow and begin synthesis of ground substance and fibrous extracellular material. Secretion of extracellular material traps each chondroblast within the cartilaginous matrix, thereby separating the chondroblasts from one another. Each chondroblast then undergoes one or two further mitotic divisions to form a small cluster of mature cells separated by a small amount of extracellular material.
Mature cartilage cells, known as chondrocytes, maintain the integrity of the cartilage matrix. Most mature cartilage masses acquire a surrounding layer called the perichondrium, composed of collagen fibres and spindle-shaped cells which resemble fibroblasts. These have the capacity to transform into chondroblasts and form new cartilage by appositional growth. There is also very limited capacity in mature cartilages masses for interstitial growth. This occurs by further division of chondrocytes trapped within the previously formed matrix and subsequent deposition of more matrix material. The hyaline cartilage of the articular surfaces of joints does not have perichondrium on the surface and has no capacity to regenerate new cartilage after damage. In general, mature cartilage has a very limited capacity to repair and regenerate, partly because of its poor blood supply.
Most cartilage is devoid of blood vessels, and consequently the exchange of metabolites between chondrocytes and surrounding tissues depends on diffusion through the water of the ground substance. This limits the thickness to which cartilage may develop while maintaining viability of the innermost cells. In sites where cartilage is particularly thick (e.g. costal cartilage), cartilage canals convey small vessels into the centre of the cartilage mass.
The role of cartilage in bone formation is discussed in Figs 10.18 to 10.21.
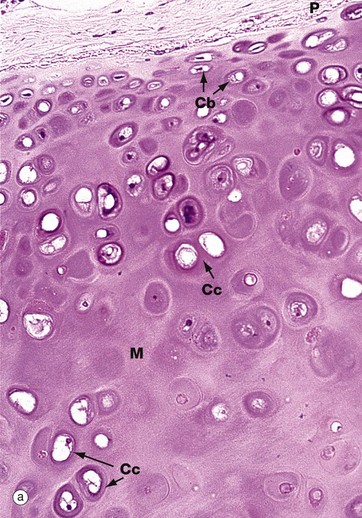
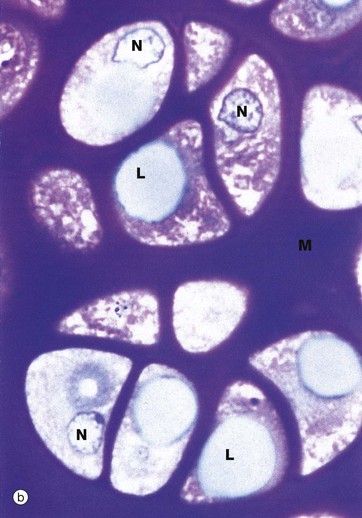
FIG. 10.1 Hyaline cartilage
(a) H&E (MP) (b) Thin epoxy resin section, toluidine blue (HP)
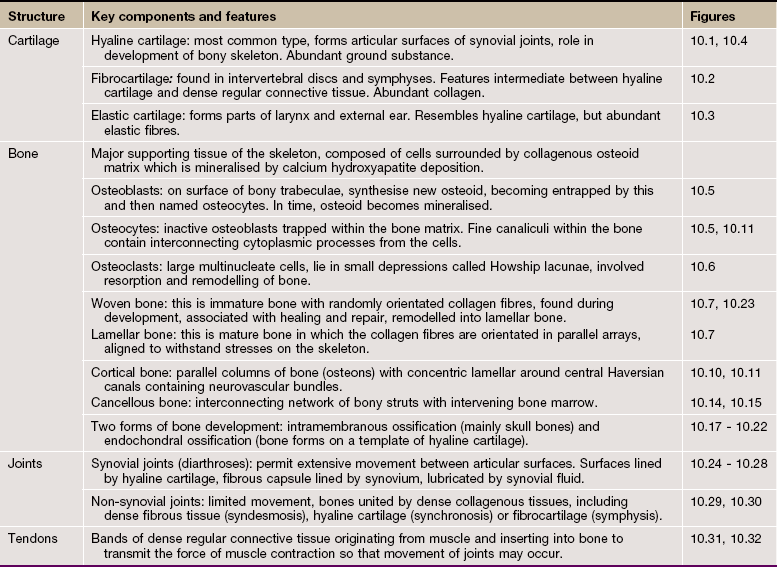
Hyaline cartilage is the most common type of cartilage. It is found in the nasal septum, larynx, tracheal rings, most articular surfaces and the sternal ends of the ribs. It also forms the precursor of bone in the developing skeleton. Mature hyaline cartilage is characterised by small aggregates of chondrocytes embedded in an amorphous matrix of ground substance, reinforced by collagen fibres.
Micrograph (a) shows a hyaline cartilage mass with its outer perichondrium P. The chondrocytes of the formed cartilage Cc are arranged in clusters, usually of 2 to 4 cells, each cluster being separated from its neighbours by amorphous cartilage matrix M. The perichondrium is composed of parallel collagen fibres containing a few spindle-shaped nuclei of inactive fibrocytes but, on its inner surface, these cells are transforming into small chondroblasts Cb which are in the process of enlarging, dividing and synthesising new cartilage matrix.
The matrix of hyaline cartilage appears fairly amorphous, since the ground substance and collagen have similar refractive properties. With the exception of articular cartilage, the collagen of hyaline cartilage, designated as type II collagen (see Ch. 4), is not cross-banded and is arranged in an interlacing network of fine fibrils. This collagen cannot be demonstrated by light microscopy.
The thin epoxy resin section of hyaline cartilage in micrograph (b) shows the cellular details of mature chondrocytes. Note that the chondrocytes fully occupy the spaces in the matrix M, each space containing a single chondrocyte. Mature chondrocytes are characterised by small nuclei N with dispersed chromatin and basophilic granular cytoplasm, reflecting a well-developed rough endoplasmic reticulum. Lipid droplets L, often larger than the nuclei, are a prominent feature of larger chondrocytes. The cytoplasm is also rich in glycogen. These characteristics reflect the active role of chondrocytes in synthesis of both the ground substance and fibrous elements of the cartilage matrix. In fully formed cartilage, the constituents of the extracellular matrix are continuously turned over, the integrity of the matrix being thus absolutely dependent on the viability of the chondrocytes.
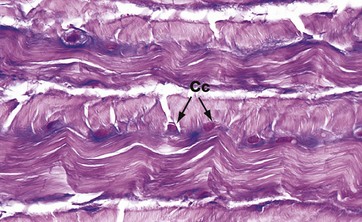
FIG. 10.2 Fibrocartilage
H&E/Alcian blue (HP)
Fibrocartilage has features intermediate between cartilage and dense fibrous supporting tissue. It is found in intervertebral discs, some articular cartilages, the pubic symphysis, in association with dense collagenous tissue in joint capsules, in ligaments and in the connections of some tendons to bone. It consists of alternating layers of hyaline cartilage matrix with thick layers of dense collagen fibres, orientated in the direction of the functional stresses.
This micrograph is taken from the same specimen of intervertebral disc illustrated in Fig. 10.30. Pink-stained collagen characteristically permeates the blue-stained cartilage ground substance. Chondrocytes Cc are arranged in rows between the dense collagen layers within lacunae in the glycoprotein matrix.
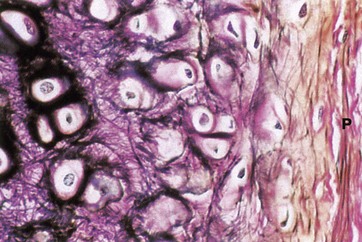
FIG. 10.3 Elastic cartilage
Elastic van Gieson (HP)
Elastic cartilage occurs in the external ear and external auditory canal, the epiglottis, parts of the laryngeal cartilages and in the walls of the Eustachian tubes.
The histological structure of elastic cartilage is similar to that of hyaline cartilage. Its elasticity is derived from the presence of numerous bundles of branching elastin fibres in the cartilage matrix. This network of elastin fibres (stained black in this preparation) is particularly dense in the immediate vicinity of the chondrocytes. Collagen (stained red) is also a major constituent of the cartilage matrix and makes up the bulk of the perichondrium P, intermingled with a few elastic fibres. Development and growth of elastic cartilage occurs by both interstitial and appositional growth, in the same manner as for hyaline cartilage.
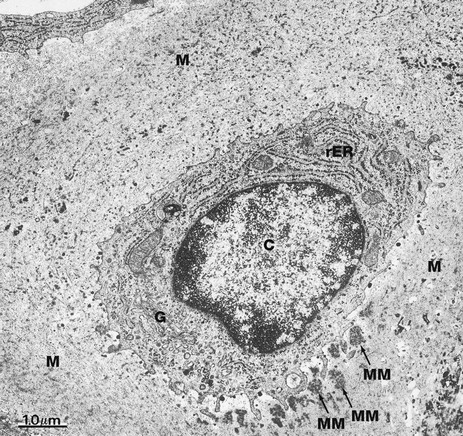
FIG. 10.4 Chondrocyte
EM ×16 000
This electron micrograph illustrates a single chondrocyte C lying within its lacuna and surrounded by abundant cartilaginous matrix material M. As is typical of cells active in protein synthesis (in this case matrix turnover), chondrocytes have very prominent rough endoplasmic reticulum rER which is distended with secretory material. A well-developed Golgi apparatus G is present. Some glycogen granules are scattered in the cytoplasm. Note that the chondrocyte completely fills its lacuna within the matrix. Small cytoplasmic extensions mediate the constant interaction between chondrocytes and the surrounding extracellular matrix material.
At this magnification, the fibrous elements of the extracellular matrix can just be discerned. The deposits of electron-dense material which can be seen lying adjacent to the deep aspect of the cell represent some recently secreted extracellular matrix material MM.
Bone
Bone is composed of cells and a predominantly collagenous extracellular matrix (type I collagen) called osteoid which becomes mineralised by the deposition of calcium hydroxyapatite, thus giving the bone considerable rigidity and strength. The cells of bone are:
• Osteoblasts which synthesise osteoid and mediate its mineralization. These are found lined up along bone surfaces.
• Osteocytes which represent largely inactive osteoblasts trapped within formed bone. These may assist in the nutrition of bone.
• Osteoclasts are phagocytic cells which are capable of eroding bone. These are important, along with osteoblasts, in the constant turnover and refashioning of bone.
Osteoblasts and osteocytes are derived from a primitive mesenchymal (stem) cell called the osteoprogenitor cell. Osteoclasts are multinucleate phagocytic cells derived from the macrophage-monocyte cell line.
Bone forms the strong and rigid endoskeleton to which skeletal muscles are attached to permit movement. It also acts as a calcium reservoir and is important in calcium homeostasis. Bone is heavy and its architecture is optimally arranged to provide maximum strength for the least weight. Most bones have a dense, rigid outer shell of compact bone, the cortex, and a central medullary or cancellous zone of thin, interconnecting narrow bone trabeculae. The number, thickness and orientation of these bone trabeculae is dependent upon the stresses to which the particular bone is exposed. For example, there are many thick intersecting trabeculae in the constantly weight-bearing vertebrae, but very few in the centre of the ribs, which are not subjected to constant stress. The space in the medullary bone between trabeculae is occupied by haematopoietic bone marrow (see Fig. 3.3).
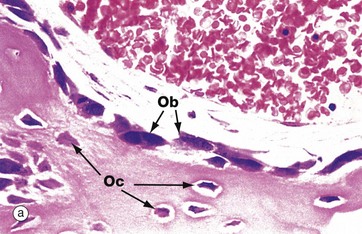
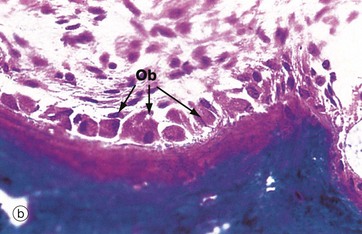
FIG. 10.5 Active osteoblasts and osteoid
(a) H&E (HP) (b) Undecalcified resin section, Goldner trichrome stain (HP)
These micrographs illustrate osteoblasts actively depositing new osteoid on a bone surface. When active, the osteoblasts Ob are large, broad, spindle-shaped or cuboidal cells with abundant basophilic cytoplasm containing much rough endoplasmic reticulum and a large Golgi apparatus. These features reflect a high rate of protein (type I collagen) and proteoglycan synthesis.
In micrograph (a), the tissue has been decalcified before sectioning and staining, so the distinction between mineralised bone and the newly formed unmineralised osteoid cannot be seen. In micrograph (b), which has not been decalcified, the mineralised bone (blue) can easily be distinguished from the new osteoid (red) which is being produced by the row of cuboidal osteoblasts. There is always a short delay between osteoid production and its mineralisation.
When inactive, osteoblasts are narrow, attenuated, spindle-shaped cells lying on the bone surface. In (a) the burst of new bone formation is nearly over, and the osteoblasts are becoming spindle-shaped again and will soon become virtually undetectable, only the long, narrow nucleus being visible histologically. A few cells are being incorporated in the newly formed bone as osteocytes Oc.
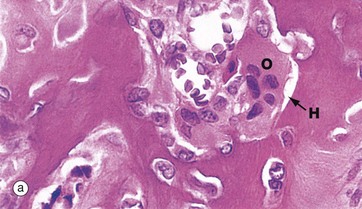
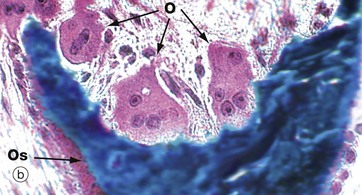
FIG. 10.6 Osteoclasts
(a) H&E (HP) (b) Undecalcified resin section, Goldner trichrome (HP)
Resorption of bone is performed by large multinucleate cells called osteoclasts O, which are often seen lying in depressions resorbed from the bone surface called Howship lacunae H. The aspect of the osteoclast in apposition to bone is characterised by fine microvilli which form a ruffled border that is readily visible with the electron microscope. The ruffled border secretes several organic acids which dissolve the mineral component, while lysosomal proteolytic enzymes are employed to destroy the organic osteoid matrix.
Osteoclastic resorption contributes to bone remodelling in response to growth or due to changing mechanical stresses upon the skeleton. Osteoclasts also participate in the long-term maintenance of blood calcium homeostasis by their response to parathyroid hormone and calcitonin (see Ch. 17). Parathyroid hormone stimulates osteoclastic resorption and so increases the release of calcium ions from bone, whereas calcitonin inhibits osteoclastic activity.
Micrographs (a) and (b) are taken from bone showing excessive osteoclastic activity due to the effects of Paget disease of bone, a disorder characterised by continuous disorganised bone resorption and associated new bone formation (see textbox). Micrograph (b) shows uncoordinated new osteoid formation by a row of cuboidal osteoblasts Ob.
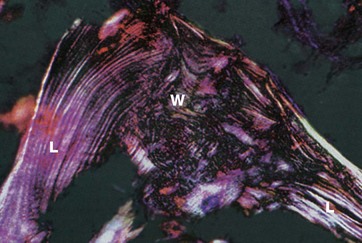
FIG. 10.7 Woven and lamellar bone, polarising microscopy
H&E (MP)
Bone exists in two main forms, woven bone W and lamellar bone L. Woven bone is an immature form with randomly arranged collagen fibres in the osteoid. Lamellar bone is composed of regular parallel bands of collagen arranged in sheets.
Woven bone is produced when osteoblasts synthesise osteoid rapidly, as in fetal bone development. It can also occur in adults when there is pathological rapid new bone formation e.g. at the site of a healing fracture and in Paget disease (see textbox).
The rapidly formed woven bone is eventually remodelled to form lamellar bone, which is physically stronger and more resilient. Virtually all bone in a healthy adult is lamellar.
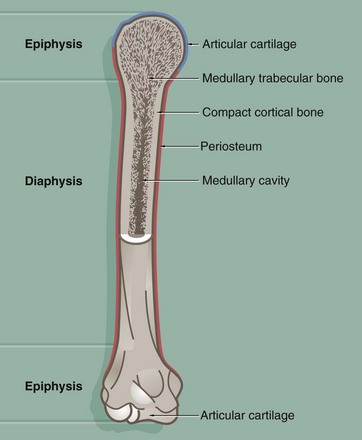
FIG. 10.8 Long bone
This diagram illustrates the general structure of long bones in the mature skeleton and the gross morphological appearance of the two types of lamellar bone found in the mature skeleton, i.e. compact (cortical) bone and cancellous (medullary) bone.
Compact bone forms the dense walls of the shaft or diaphysis, while cancellous bone occupies part of the large central medullary cavity. Cancellous bone consists of a network of fine, irregular plates called trabeculae separated by intercommunicating spaces.
The articular (joint) surfaces of the expanded ends, or epiphyses, of long bones are protected by a layer of specialised hyaline cartilage called articular cartilage. The external surface of the bone is invested in a dense fibrous layer called the periosteum, into which are inserted muscles, tendons and ligaments. The inner surfaces of the bone, including the trabeculae of cancellous bone, are invested by a delicate layer called the endosteum. The endosteum and periosteum contain cells of the osteogenic series which are responsible for growth, continuous remodelling and repair of bone fractures (see textbox).
Prior to the attainment of skeletal maturity, the long bones grow in length by the process of endochondral ossification which occurs at a growth or epiphysial plate situated at each end of the bone at the junction of the diaphysis (shaft) and epiphysis.
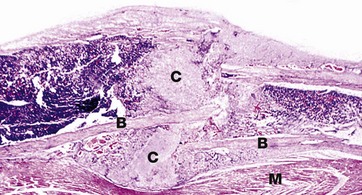
FIG. 10.9 Fracture callus
H&E (LP)
This image shows fracture callus C around the site of a rib fracture. This mass of healing tissue acts to stabilise the broken ends of the bone B so that repair can occur. There is new bone formation within the callus and, over time, this will become organised into mature lamellar bone. Striated muscle M is present at the lower border of the bone.
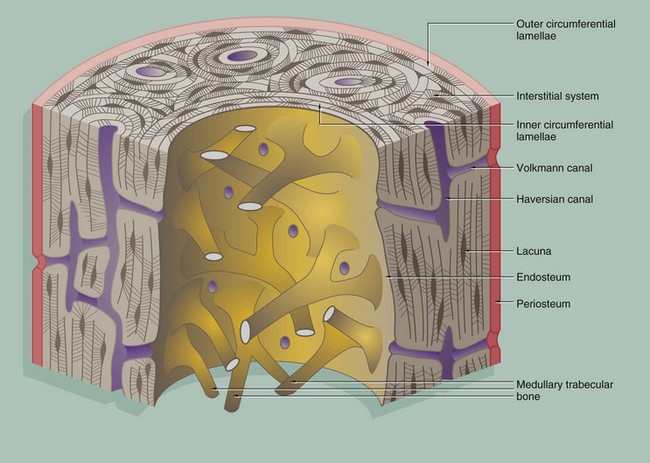
FIG. 10.10 Cortical (compact) bone
Compact bone is made up of parallel bony columns which, in long bones, are disposed parallel to the long axis, i.e. in the line of stress exerted on the bone. Each column is made up of concentric bony layers or lamellae arranged around a central channel containing blood vessels, lymphatics and nerves. These neurovascular channels are known as canals of Havers or Haversian canals and, with their concentric lamellae, form Haversian systems. The neurovascular bundles interconnect with one another and with the endosteum and periosteum via Volkmann canals which pierce the columns at right angles (or obliquely) to the Haversian canals.
Each Haversian system (osteon) develops by osteoclastic tunnelling of a mass of compact bone to form a broad channel into which blood vessels and nerves grow, after which it becomes lined internally by active osteoblasts which lay down concentric lamellae of bone.
With the deposition of successive lamellae, the diameter of the Haversian canal decreases and osteoblasts are trapped as osteocytes in spaces called lacunae in the matrix. The osteocytes are thus arranged in concentric rings within the lamellae. Between adjacent lacunae and the central canal, there are numerous minute interconnecting canals called canaliculi which contain fine cytoplasmic extensions of the entrapped osteocytes.
As a result of the continuous resorption and redeposition of bone, complete newly formed Haversian systems are disposed between partly resorbed systems formed earlier. The remnants of lamellae no longer surrounding Haversian canals form irregular interstitial systems between intact Haversian systems.
At the outermost aspect of compact bone, Haversian systems give way to concentric lamellae of dense cortical bone, laid down partly by the osteoblasts of the periosteum (outer circumferential lamellae). Similar circumferential lamellae line the inside of the cortical bone (inner circumferential lamellae) where it abuts the marrow cavity.
The inner surface of cortical bone (endosteum) is composed of the innermost layer of the inner circumferential lamellae, with a layer of inactive flat osteoblasts on its surface. When activated, these cells enlarge to become active cuboidal osteoblasts and synthesise new lamellar osteoid which, on mineralisation, forms another layer of inner circumferential lamella. This occurs regularly as part of the constant dynamic refashioning of bone and is particularly prominent during bone growth. It is also seen in response to increased or altered stress on the cortical bone, for example in the leg bones during periods of increased physical training for running and other sports.
An interconnecting network of trabecular or cancellous bone occupies the central marrow cavity of the bone, and the ends of these bony trabeculae are attached to the inner circumferential lamellae of the cortical bone (see Fig. 10.13). The inactive osteoblasts of the endosteum also extend onto the surface of the trabecular bone and similarly deposit new osteoid when required for strengthening or remodelling.
Small blood vessels and nerves enter the cortical bone from the marrow space through defects in the endosteum and inner circumferential lamellae. These connect with the Volkmann canals, which in turn connect with Haversian canals.
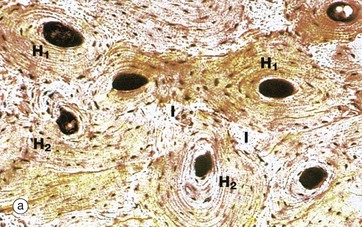
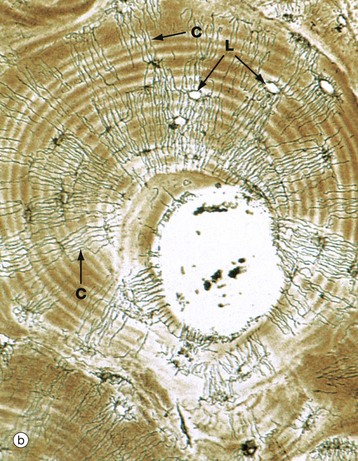
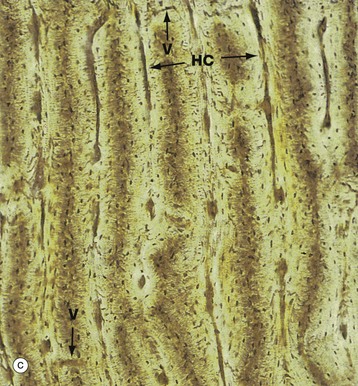
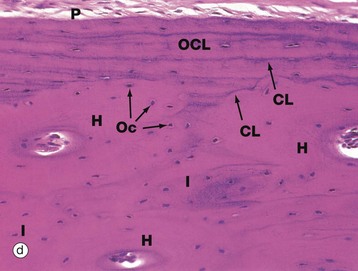
FIG. 10.11 Cortical (compact) bone
(a) Ground section, TS, unstained (LP) (b) Ground section, TS, unstained (HP) (c) Ground section, LS, unstained (LP) (d) H&E (MP)
These three ground sections illustrate many of the features described in the preceding figure. In micrograph (a), the bone has been cut transversely, demonstrating newly formed Haversian systems H1 and older, partly resorbed Haversian systems H2. Irregular interstitial systems I, representing the remnants of former Haversian systems, fill the intervening spaces. Concentric rings of flattened lacunae can be seen to surround the central Haversian canals, which appear as very dark areas in this preparation.
Micrograph (b) focuses on a single Haversian system, the central canal being surrounded by concentric lamellae of bone matrix containing empty lacunae L. Fine canaliculi C radiate from each lacuna to anastomose with those of adjacent lacunae. In life, osteocytes do not completely fill the lacunae, the remaining narrow space being filled with extracellular bone fluid. Fine cytoplasmic processes of the osteocytes pass in the canaliculi to communicate via gap junctions with the processes of osteocytes in adjacent lamellae. These canaliculi provide passages for circulation of extracellular fluid and for diffusion of metabolites between the lacunae and vessels of the Haversian canals.
Osteocytes maintain the structural integrity of the mineralised matrix and mediate release or deposition of calcium for the purpose of calcium homeostasis in the body as a whole. The activity of osteocytes in calcium regulation is controlled directly by plasma calcium concentration and indirectly by parathyroid hormone and calcitonin, secreted by the parathyroid and thyroid glands, respectively (see Ch. 17). Osteoblasts and osteocytes also appear to respond to minute piezo-electric currents induced by bone deformation, increasing or decreasing local bone formation as appropriate and inducing complementary activity in local osteoclasts via the secretion of local humoral factors. Thus bone is remodelled to adapt to mechanical stresses.
Micrograph (c) shows compact bone cut in longitudinal section, the plane of section including some Haversian canals HC and traces of the interconnecting Volkmann canal system V. These appear dark in colour as in micrograph (a) due to an optical artefact related to air trapped in the section. For the same reason, the tiny osteocyte lacunae appear as brown elongated specks which are arranged in concentric layers around the Haversian canals.
Micrograph (d) is an H&E-stained section of decalcified bone. It shows compact cortical bone with periosteum P on its outer surface. The outer circumferential lamellae OCL lie between the periosteum and three Haversian systems H, seen here in transverse section. At the centre of each Haversian system is a canal containing blood vessels. Between adjacent systems, there are irregular interstitial lamellae I. The lamellae of the Haversian systems are not clearly seen in this section, but fine basophilic cement lines CL, rich in proteoglycan ground substance, can be seen in the outer circumferential lamellae and defining the outer limits of each Haversian system. Osteocytes Oc have dark-staining nuclei.
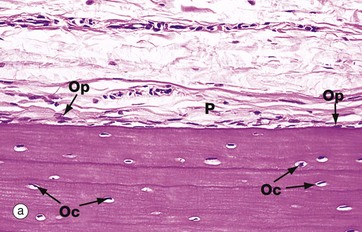
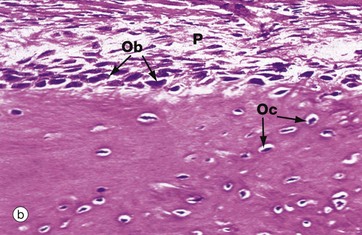
FIG. 10.12 Periosteum
(a) Inactive, H&E (MP) (b) Active, H&E (HP)
The outer surface of most bone is covered by a layer of condensed fibrous tissue, the periosteum P, which contains cells capable of converting into osteoprogenitor cells and osteoblasts. When no new bone is being formed on the bone surface, these cells are insignificant flattened cells with spindle-shaped nuclei but, when there is active new bone formation at the periosteal surface, these cells proliferate and increase in size to become osteoblasts. Micrograph (a) shows inactive periosteum with barely detectable inactive osteoprogenitor cells Op and mature formed bone containing established osteocytes Oc. Micrograph (b) shows active periosteum with new bone being formed by active periosteal osteoblasts Ob, some of which are being incorporated into newly formed bone to become osteocytes Oc.
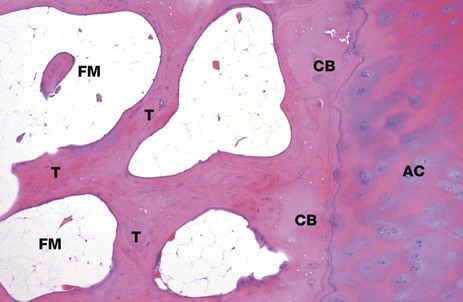
FIG. 10.13 Bone, cortical and trabecular
H&E (LP)
This micrograph shows bone from the head of the femur. It illustrates the origin of the trabecular (cancellous) bone T from the compact cortical bone CB. As this end of the bone forms part of a synovial joint (see Fig. 10.25), the outer cortical plate consists of articular hyaline cartilage AC. On the shaft of this long bone, the outer layer would be formed from fibrous periosteum. Between the bony trabeculae, there are intervening spaces. Note that these marrow spaces FM are filled with adipose tissue (fatty or yellow marrow).
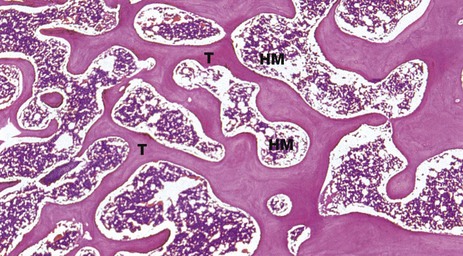
FIG. 10.14 Bone, trabecular
H&E (LP)
Trabecular (cancellous) bone consists of a network of interconnecting struts which are orientated in positions that provide the maximum strength for the minimum mass. They are composed of lamellar bone with scanty lacunae containing osteocytes. These exchange metabolites via canaliculi which communicate with each other and with blood sinusoids in the haematopoietic (red) marrow spaces HM. The trabeculae T have a thin external coating of endosteum with flat inactive osteoblasts.
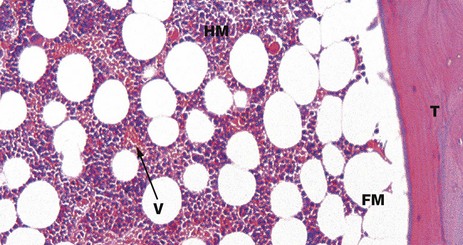
FIG. 10.15 Bone marrow
H&E (HP)
This micrograph shows part of a bone trabecula T of lamellar bone and the adjacent marrow space. In this micrograph, as in Fig. 10.14, the marrow space contains a mixture of fatty marrow FM, composed of adipose tissue, and haematopoietic (‘red’) marrow HM, composed of red and white blood cell precursors. These haematopoietic cells lie in intimate contact with numerous thin-walled blood vessels (sinusoids) V.
The various cell types and processes involved in haematopoiesis are described in Ch. 3.
Bone Matrix and Mineralisation
Mature compact bone is made up of about 70% inorganic salts and 30% organic matrix by weight. Collagen makes up over 90% of the organic component, the remainder being ground substance proteoglycans and non-collagen molecules which appear to regulate bone mineralisation. The collagen of bone is almost exclusively in the form of type I fibres. Spaces within this three-dimensional structure, called hole zones, are the initial site of mineralisation.
Ground substance proteoglycans contribute a much smaller proportion of the matrix than in cartilage and mainly consist of chondroitin sulphate and hyaluronic acid in the form of proteoglycan aggregates. As well as controlling the water content of bones, ground substance is probably involved in regulating formation of collagen fibres. The remaining non-collagen organic material includes osteocalcin (Gla protein), involved in binding calcium during the mineralisation process, osteonectin, which may serve some bridging function between collagen and the mineral component, and sialoproteins.
The mineral component of bone mainly consists of calcium and phosphate in the form of hydroxyapatite crystals. These are conjugated to a small proportion of magnesium carbonate, sodium and potassium ions but also have affinity for heavy metals and radioactive pollutants.
Collagen and the other organic matrix constituents are synthesised by the rough endoplasmic reticulum of osteoblasts, packaged by the Golgi apparatus and then secreted from the cell surface, resulting in the production of osteoid. After a maturation phase lasting several days, amorphous (non-crystalline) calcium phosphate salts begin to precipitate in the hole zones of the collagen. These mineralisation foci expand and coalesce into hydroxyapatite crystals by further remodelling. Around 20% the mineral component remains in the amorphous form, as a readily available buffer in calcium homeostasis. The concentration of calcium and phosphate ions in bony extracellular fluid is greater than that required for spontaneous deposition of calcium salts and a variety of inhibitors, including pyrophosphate, play a crucial role in controlling bone mineralisation. Deposition of calcium appears to be associated with membrane-bound vesicles derived from osteoblast plasma membrane called matrix vesicles. These contain the enzyme alkaline phosphatase and other phosphatases which may neutralise the inhibitory effect of pyrophosphate.
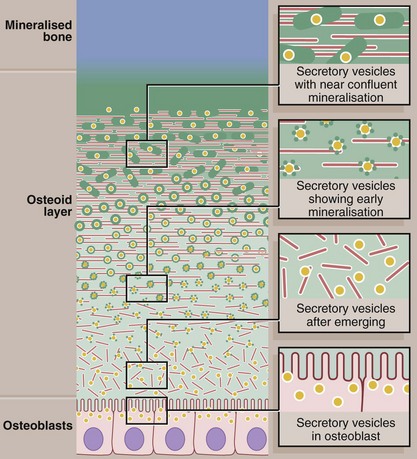
FIG. 10.16 Mineralisation of bone
This diagram shows the events believed to occur in the mineralisation of osteoid to form mineralised bone. The active cuboidal osteoblasts secrete osteoid collagen (red) but also matrix vesicles (yellow). The matrix vesicles are the focus for deposition of hydroxyapatite crystals (green), the first step in mineralisation. Continued accretion of mineral on these early foci leads eventually to confluent mineralisation of the osteoid collagen and supporting glycosaminoglycan matrix.
The matrix vesicles are rich in the enzymes alkaline phosphatase and pyrophosphatase, which can both produce phosphate ions from a range of molecules. The phosphate ions accumulate in the matrix vesicles with calcium ions and form the raw material for production of hydroxyapatite.
Bone Development and Growth
The fetal development of bone occurs in two ways, both of which involve replacement of primitive collagenous supporting tissue by bone. The resulting woven bone is then extensively remodelled by resorption and appositional growth to form the mature adult skeleton, which is made up of lamellar bone. Thereafter, resorption and deposition of bone occur at a much reduced rate to accommodate changing functional stresses and to effect calcium homeostasis.
The long bones, vertebrae, pelvis and bones of the base of the skull are preceded by the formation of a continuously growing cartilage model which is progressively replaced by bone. This process is called endochondral ossification and the bones so formed are called cartilage bones.
In contrast, the bones of the vault of the skull, the maxilla and most of the mandible are formed by the deposition of bone within primitive mesenchymal tissue. This process of direct replacement of mesenchyme by bone is known as intramembranous ossification and the bones so formed are called membrane bones. Bone development is controlled by growth hormone, thyroid hormone and the sex hormones.
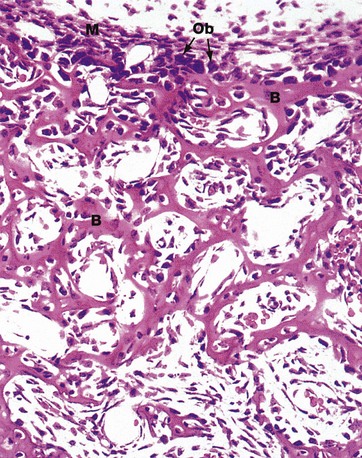
FIG. 10.17 Intramembranous ossification
H&E (HP)
Intramembranous bone formation occurs within ‘membranes’ of condensed primitive mesenchymal tissue. Mesenchymal cells differentiate into osteoblasts Ob which begin synthesis and secretion of osteoid at multiple centres of ossification. Mineralisation of osteoid follows closely.
As osteoid is laid down, osteoblasts are trapped in lacunae to become osteocytes and their fine cytoplasmic extensions shrink to form the fine processes contained within the canaliculi. Osteoprogenitor cells at the surface of the centres of ossification undergo mitotic division to produce further osteoblasts which then lay down more bone. This progressive bone formation results in the eventual fusion of adjacent ossification centres to form bone which is spongy in gross appearance.
The collagen fibres of developing bone are randomly arranged in interlacing bundles, giving rise to the term woven bone. The woven bone then undergoes progressive remodelling into lamellar bone by osteoclastic resorption and osteoblastic deposition to form mature compact or trabecular bone. The primitive mesenchyme remaining in the network of developing bone differentiates into bone marrow.
This preparation from the developing skull vault of a cat fetus illustrates spicules of woven bone B separated by primitive mesenchymal tissue. Note the condensed primitive mesenchyme M which delineates the outer margin of the developing bone. This will eventually develop into the periosteum.
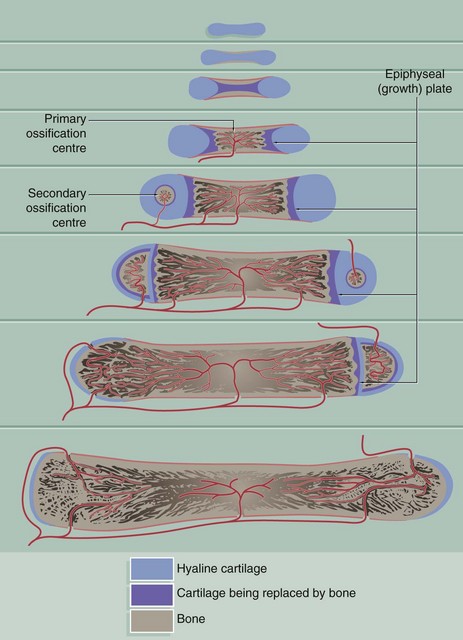
FIG. 10.18 Endochondral ossification
Endochondral ossification is a method of bone formation that permits functional stresses to be sustained during skeletal growth. It is well demonstrated in the development of the long bones.
A small model of the long bone is first formed in solid hyaline cartilage. This undergoes mainly appositional growth to form an elongated dumbbell-shaped mass of cartilage consisting of a shaft (diaphysis) and future articular portions (epiphyses), surrounded by perichondrium.
Within the shaft of the cartilage model, the chondrocytes enlarge greatly, resorbing the surrounding cartilage so as to leave only slender, perforated trabeculae of cartilaginous matrix. This cartilage matrix then becomes calcified and the chondrocytes degenerate, leaving large interconnecting spaces. During this period, the perichondrium of the shaft develops osteogenic potential and assumes the role of periosteum. The periosteum then lays down a thin layer of bone around the surface of the shaft.
At the same time, primitive mesenchymal cells and blood vessels invade the spaces left within the shaft after degeneration of the chondrocytes. These primitive mesenchymal cells differentiate into osteoblasts and blood-forming cells of the bone marrow. The osteoblasts form a layer of cells on the surface of the calcified remnants of the cartilage matrix and commence the formation of irregular woven bone.
A large site of primary ossification in the shaft has by now separated the ends of the original cartilage model. The cartilaginous ends of the model, however, continue to grow in diameter. Meanwhile, the cartilage at the ends of the shaft continues to undergo regressive changes followed by ossification, so that the developing bone now consists of an elongated bony diaphysial shaft with a semilunar cartilage epiphysis at each end. The interface between the shaft and each epiphysis constitutes a growth or epiphysial plate. Within the growth plate, the cartilage proliferates continuously, resulting in progressive elongation of the bone. At the diaphysial aspect of each growth plate, the chondrocytes mature and then die, the degenerating zone of cartilage being replaced by bone. Thus the bony diaphysis lengthens and the growth plates are pushed further and further apart.
On reaching maturity, hormonal changes inhibit further cartilage proliferation and the growth plates are replaced by bone, causing fusion of the diaphysis and epiphyses. In the meantime, in the centre of the mass of cartilage of each developing epiphysis, regressive changes and bone formation similar to that in the diaphysial cartilage occur, along with appositional growth of cartilage over the whole external surface of the epiphysis. This conversion of central epiphysial cartilage to bone is known as secondary ossification. A thin zone of hyaline cartilage always remains at the surface as the articular cartilage.
Under the influence of functional stresses, the calcified cartilage remnants and the surrounding irregular woven bone are completely remodelled so that the bone ultimately consists of a compact outer layer with a central medulla of cancellous bone.
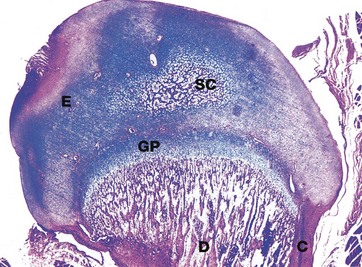
FIG. 10.19 Epiphysis
H&E/Alcian blue (LP)
This micrograph illustrates the head of a kitten femur at an advanced stage of development. The cartilaginous epiphysis E is separated from the diaphysis D by the epiphysial growth plate GP. Note the thickening cortical bone C at the outer aspect of the diaphysis and the network of trabecular of bone in the medulla of the diaphysis. Note also the centre of secondary ossification SC in the epiphysial cartilage.
Epiphysial growth plates provide for growth in length of long bones while accommodating functional stresses in the growing skeleton. The subsequent series of three micrographs illustrates particular areas of the epiphyseal plate at higher magnification.
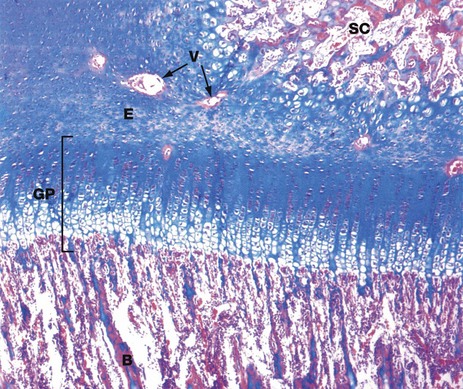
FIG. 10.20 Epiphyseal growth plate
H&E/Alcian blue (MP)
At higher magnification, the epiphysial growth plate GP shows a distinct progression of morphological changes between the epiphysial cartilage E and the newly forming bone B of the diaphysis. Similar but less stratified morphological changes are seen between the epiphysial cartilage and the centre of secondary ossification SC within the epiphysis, although this does not represent a growth plate. The Alcian blue counter-stain has been employed here as it has particular affinity for the ground substance of cartilage. Note the presence of blood vessels V which are cut in transverse section, passing into the secondary ossification centre of the epiphysis via cartilage canals.
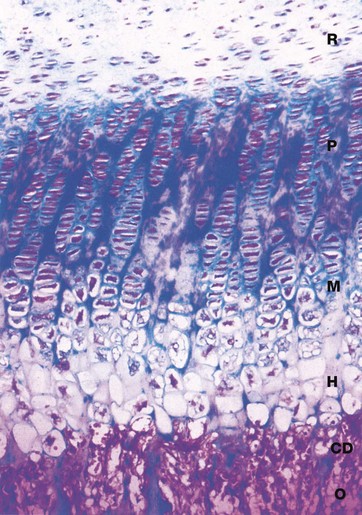
FIG. 10.21 Epiphyseal growth plate
H&E/Alcian blue (HP)
The dynamic process of endochondral ossification is summarised in this micrograph of the epiphysial growth plate at high magnification. The transition between epiphysial cartilage and new bone occurs in six functional and morphological stages:
• Zone of reserve cartilage R. This consists of typical hyaline cartilage (see Fig. 10.1) with the chondrocytes arranged in small clusters surrounded by a large amount of moderately stained matrix.
• Zone of proliferation P. The clusters of cartilage cells undergo successive mitotic divisions to form columns of chondrocytes separated by strongly stained matrix, rich in proteoglycans.
• Zone of maturation M. Cell division has ceased and the chondrocytes increase in size.
• Zone of hypertrophy and calcification H. The chondrocytes become greatly enlarged and vacuolated and the matrix becomes calcified.
• Zone of cartilage degeneration CD. The chondrocytes degenerate and the lacunae of the calcified matrix are invaded by osteogenic cells and capillaries from the marrow cavity of the diaphysis.
• Osteogenic zone O. The osteogenic cells differentiate into osteoblasts which congregate on the surface of the spicules of calcified cartilage matrix where they commence bone formation. This transitional zone is known as the metaphysis.
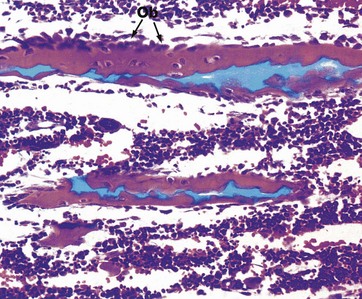
FIG. 10.22 Endochondral ossification, metaphysis
H&E/Alcian blue (HP)
The metaphysis is the name given to the area where the shaft of a long bone joins the epiphyseal growth plate. Here, the blue-stained spicules of calcified cartilage matrix are surrounded by active osteoblasts Ob and newly formed woven bone, stained pink. Further growth of metaphyseal woven bone is followed by extensive remodelling to produce mature trabecular bone.
At physical maturity, endochondral ossification ceases and the diaphysis fuses with the epiphysis, obliterating the growth plates. From this point, no further endochondral ossification or bone lengthening are possible. Although bones grow in length by endochondral ossification, growth in diameter of the shaft occurs by appositional growth at the periosteal surface and complementary osteoclastic resorption at the endosteal (medullary) aspect. Note that the marrow spaces between the developing trabeculae are already populated by numerous small haematopoietic cells (red marrow).
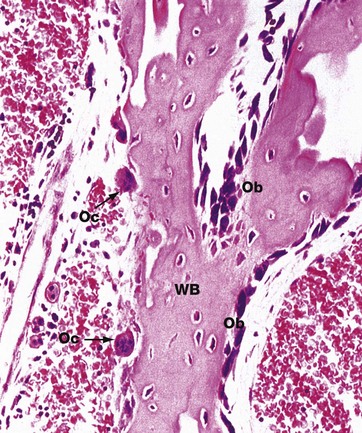
FIG. 10.23 Bone remodelling and repair
H&E (HP)
This micrograph illustrates an irregular spicule of woven bone WB from a fetus. Some of the surfaces of the spicule exhibit osteoblastic proliferation and activity Ob, whereas other surfaces are in the process of being resorbed by osteoclasts Oc.
Woven bone is not only the first type of bone to be formed during skeletal development but is also the first bone to be laid down during the repair of a fracture (see textbox and Fig. 10.9). At the fracture site, a blood clot initially forms, later being replaced by highly vascular collagenous tissue (granulation tissue) which becomes progressively more fibrous.
Mesenchymal cells then differentiate into chondroblasts and progressively replace this fibrous granulation tissue with hyaline cartilage. This firm but still flexible bridge is known as the provisional callus. The provisional callus is then strengthened by deposition of calcium salts within the cartilage matrix.
Meanwhile, osteoprogenitor cells in the endosteum and periosteum are activated and lay down a meshwork of woven bone within and around the provisional callus. The provisional callus thus becomes transformed into the bony callus.
Bony union is achieved when the fracture site is completely bridged by woven bone. Under the influence of functional stresses, the bony callus is then slowly remodelled to form mature lamellar bone.
Joints
Joints may be classified into two main functional groups, synovial and non-synovial, both of which may show wide morphological variations.
Synovial joints
In this type of joint, there is extensive movement of the bones upon one another at the articular surfaces. The articular surfaces are maintained in apposition by a fibrous capsule and ligaments and the surfaces are lubricated by synovial fluid. Synovial joints are known as diarthroses.
In some diarthroses such as the temporomandibular and knee joints, plates of fibrocartilage may be completely or partially interposed between the articular surfaces but remain unattached to the articular surfaces.
Non-synovial joints
These joints have limited movement, the articulating bones having no free articular surfaces, instead being joined by dense collagenous tissue. This may be of three types:
• Dense fibrous tissue. This forms the sutures between the bones of the skull and permits moulding of the fetal skull during its passage through the birth canal. The sutures are progressively replaced by bone with advancing age. Such fibrous tissue joints are called syndesmoses, and, when replaced by bone, are called synostoses.
• Hyaline cartilage. This type of joint, called a synchondrosis or primary cartilaginous joint, unites the first rib with the sternum and is the only synchondrosis found in the human adult.
• Fibrocartilage. The opposing surfaces of some bones are covered by hyaline cartilage but, instead of a synovial space, are directly connected to each other by a plate of fibrocartilage. Such fibrocartilaginous joints are called symphyses or secondary cartilaginous joints and occur in the pubic symphysis and at the intervertebral discs. The fibrocartilage disc of the pubic symphysis develops a central cavity and the intervertebral discs have a fluid-filled central cavity.
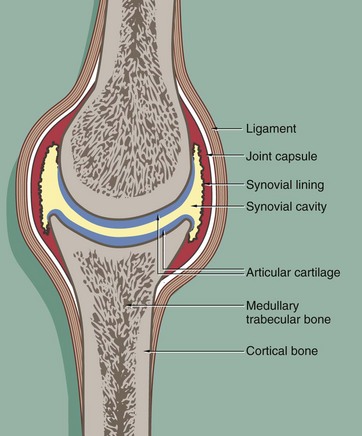
FIG. 10.24 Typical synovial joint
In synovial joints, the articulating bone surfaces are covered by a thick layer of hyaline cartilage (articular cartilage). This provides smooth, low-friction surfaces and also offers a degree of resistance to compressive forces, acting act as shock-absorbers in weight-bearing joints.
The joint is enclosed within a fibrocollagenous joint capsule which is lined internally by a specialised secretory cell layer, the synovium. The synovium secretes a small amount of lubricant fluid into the synovial cavity, aiding the smooth articulation of the cartilage-covered bone surfaces.
Excessive movement at the joint is limited by the fibrous joint capsule and by external fibro-elastic ligaments which prevent over-flexion and over-extension. In some joints such as the knee, there are internal ligaments (the cruciate ligaments) which prevent excessive joint movement, particularly excessive twisting rotation. Damage to these ligaments may occur, often during sporting pursuits, and this can lead to instability of the knee joint.
Muscles attach to bones via tendons (see Fig. 10.32b), and these may also play a role in stabilising synovial joints.
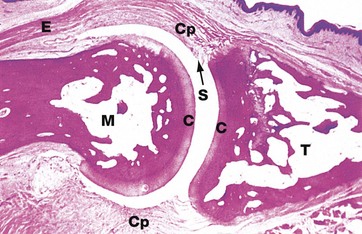
FIG. 10.25 Synovial joint
H&E, monkey (LP)
This micrograph illustrates a typical synovial joint, in this case a distal interphalangeal joint from a monkey. The articular surfaces of the terminal phalanx T and the middle phalanx M are covered by hyaline cartilage C. The joint space is artefactually widened. In vivo, the articular surfaces are maintained in close contact by a fibrous capsule Cp which is inserted into the articulating bones at some distance beyond the articular cartilages. The synovium S is a specialised layer of collagenous tissue which lines the inner aspect of the capsule. Note the extensor tendon E which inserts into the base of terminal phalanx.
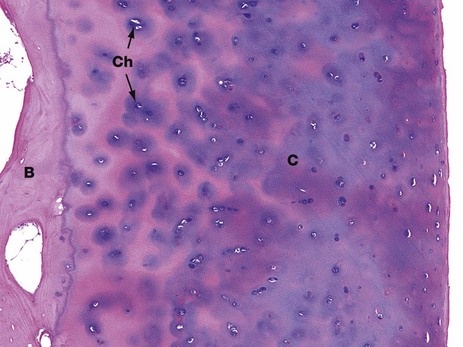
FIG. 10.26 Articular cartilage
H&E (LP)
This photomicrograph shows the articular cartilage on the surface of the head of the femur of a young adult. It is composed of hyaline cartilage C and is attached to the cortical bone B of the head of the femur. The bluish colour of the cartilage on H&E staining is due to the presence of glycosaminoglycans in the matrix. It is these, together with the collagen of the matrix, which provides the resistance to compression that is such an important property of hyaline cartilage. Both the glycosaminoglycans and collagen are synthesised and maintained by the chondrocytes Ch (see Fig. 10.1).
In this young person, the articular cartilage layer is thick and healthy. In older people, the cartilage near the surface undergoes degenerative changes as a result of wear and tear, eventually leading to arthritis (see textbox).
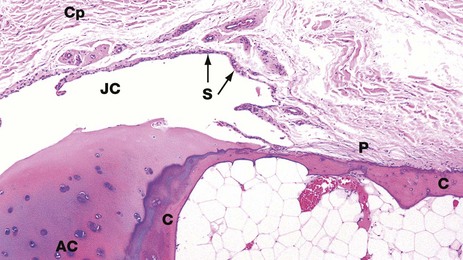
FIG. 10.27 Joint capsule and synovium
H&E (LP)
This photomicrograph shows the relationship between the articular surfaces of bone and the joint capsule. The bone end is cortical bone C, covered by a cap of articular cartilage AC. This protrudes into the joint cavity JC. The joint cavity is contained by a dense collagenous fibrous capsule Cp which is lined internally by a layer of synovium S. The synovial lining cells secrete serous fluid which lubricates the articulation of the joint. The collagen fibres of the joint capsule merge with those of the periosteum P over the shaft of the bone.
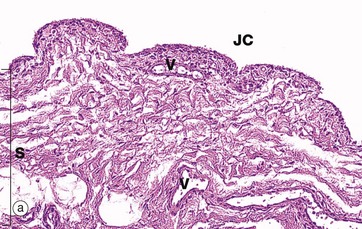
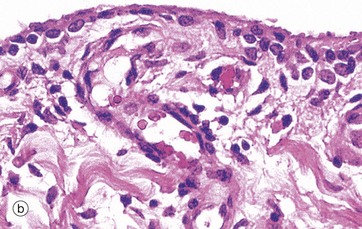
FIG. 10.28 Synovium
(a) H&E (LP) (b) H&E (HP)
The inner surface of the capsule of synovial joints and tendon sheaths is lined by a specialised collagenous tissue, the synovium, which is responsible for the elaboration of the synovial fluid that lubricates the movement of articular surfaces. Depending on the location, the bulk of the synovial tissue may be of loose collagenous type (areolar synovium), of more dense collagenous type (fibrous synovium) or predominantly composed of fat (adipose synovium), as in the case of intra-articular fat pads.
As seen in micrograph (a), the surface of the synovium S is thrown up into folds and small villi which may extend for some distance into the joint cavity JC. The synovial tissue contains numerous blood vessels V, lymphatics and nerves.
Micrograph (b) illustrates the free surface of the synovium, which is characterised by a discontinuous layer of cells up to four cells deep. These synovial cells are not connected by junctional complexes and do not rest on a basement membrane. As a result, the synovial surface does not constitute an epithelium. The synovial cells are of mesenchymal origin. The majority are plump, with an extensive Golgi complex and numerous lysosomes, features suggestive of macrophages (type A synoviocytes). The remainder have profuse rough endoplasmic reticulum and represent fibroblasts (type B synoviocytes). Also in this micrograph, note the rich network of capillaries and the thick strands of collagen which would define this as fibrous synovium.
In the normal joint, the synovial fluid is little more than a thin film covering the articular surfaces. In that the articular space is not demarcated from the synovium by an epithelium, the synovial fluid represents a highly specialised fluid form of synovial extracellular matrix rather than a secretion in the usual sense. Its major constituents are hyaluronic acid and associated glycoproteins which are secreted by the type B synoviocytes. Its fluid component is a transudate from the synovial capillaries. This arrangement facilitates the continuous exchange of oxygen, carbon dioxide and metabolites between blood and synovial fluid, which is the major source of metabolic support for articular cartilage. Normal synovial fluid also contains a small number of leucocytes (<100/mL), predominantly monocytes.
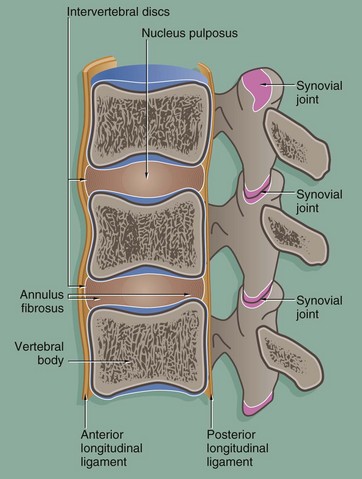
FIG. 10.29 The intervertebral joints
The vertebrae articulate by means of two different types of joints:
• The vertebral bodies are united by symphysial joints, the intervertebral discs, which permit movement between the vertebral bodies while maintaining a union of great strength. The fibrocartilage of each intervertebral disc is arranged in concentric rings, forming the annulus fibrosus. Within the disc, there is a central cavity containing a viscous fluid, the nucleus pulposus, which acts as a shock absorber. The annulus fibrosus is reinforced peripherally by circumferential ligaments. A thick ligament extending down the anterior aspect of the spinal column merges with and further reinforces the annulus fibrosus and a similar but thinner ligament reinforces the posterior aspect.
• The vertebral arches articulate with each other by pairs of synovial joints known as facet or zygapophyseal joints. Strong elastic ligaments connecting the bony processes of the vertebral arches contribute to the stability of the spinal column.
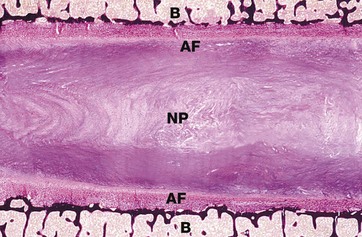
FIG. 10.30 Intervertebral disc
Haematoxylin-von Kossa (LP)
The intervertebral disc lies between the surfaces of adjacent vertebral bodies B and acts as a shock absorber. The disc is composed of an outer compact region of dense fibrocollagenous tissue containing occasional chondrocytes, the annulus fibrosus AF, with a variable thin layer of hyaline cartilage between this and the bone. The annulus fibrosus surrounds a central area of semi-fluid gelatinous matrix material known as the nucleus pulposus NP.
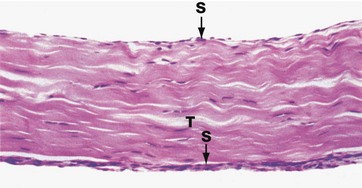
FIG. 10.31 Tendon
H&E (MP)
Tendons are tough, flexible straps or cords which connect muscles to bone. They are composed of compact linear collagen fibres with the compressed nuclei of inactive fibroblasts (tenocytes) between the collagen bundles. Tendon T is poorly vascularised and heals slowly when damaged. It also contains tiny nerve fibres and tendon stretch receptors. Some tendons have a thin outer layer of synovium S. These tendons run for part of their course through a cylindrical fibrous sheath which is lined internally by synovium. The synovia secrete lubricatory fluid.
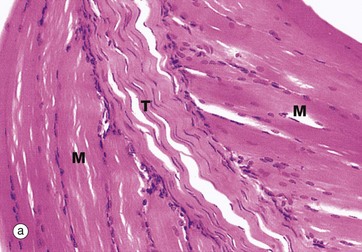
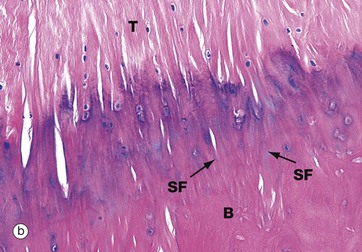
FIG. 10.32 Tendon insertions
(a) Muscle insertion, H&E (MP) (b) Insertion into bone, H&E (MP)
Micrograph (a) shows two masses of skeletal muscle M which are inserted into a common tendon T. Within the muscle close to the tendon, some of the muscle fibres show splitting of their ends. In addition, some of the collagen fibres from the tendon penetrate the muscle to form a complex interdigitation with the split muscle fibres (myotendinous junction), thus increasing the surface area for anchorage and so improving the overall strength of the attachment.
Tendons sometimes attach to bone by the tendon collagen fibres intermingling with the collagen of the periosteum, a few of the fibres penetrating into the bone. At other sites, the collagen fibres of the tendon T penetrate directly into the bone B in the form of Sharpey fibres SF. This is illustrated in micrograph (b).