Endocrine system
Introduction
The endocrine system is responsible for the synthesis and secretion of chemical messengers known as hormones. Hormones may be disseminated throughout the body by the bloodstream, where they may act on specific target organs or affect a wide range of organs and tissues. Other hormones act locally, often arriving at their site of action by way of a specialised microcirculation. In conjunction with the nervous system, hormones coordinate and integrate the functions of all the physiological systems.
As a general rule, endocrine glands are composed of islands of secretory epithelial cells with intervening supporting tissue, rich in blood and lymphatic capillaries. The secretory cells discharge hormone into the interstitial spaces and it is rapidly absorbed into the circulatory system.
Reflecting their active hormone synthesis, cells of the endocrine system have prominent nuclei and abundant mitochondria, endoplasmic reticulum, Golgi bodies and secretory vesicles. The nature of the secretory vesicles varies according to the hormone secreted. There are four main groups of chemicals which can act as hormones:
• Protein and glycoprotein molecules, e.g. insulin, growth hormone, parathyroid hormone (PTH).
• Small peptide molecules, e.g. vasopressin, products of enteroendocrine cells.
• Amino acid derivatives, e.g. thyroxine, adrenaline (epinephrine) and noradrenaline (norepinephrine).
• Steroids derived from cholesterol, e.g. adrenal cortical hormones, ovarian and testicular hormones.
Endocrine cells which produce hormones based on amino acids, peptides and proteins often have characteristic membrane-bound secretory vacuoles with electron-dense central cores (dense core granules).
The endocrine system can be divided into three parts:
• The major endocrine organs in which the sole or major function of the organ is the synthesis, storage and secretion of hormones (e.g. thyroid and adrenal glands)
• Endocrine components within other solid organs, for example, the endocrine components of the pancreas, ovary, testis and kidney, in the form of clusters of endocrine cells within other tissues
• The diffuse endocrine system, scattered individual hormone cells (or small clumps), usually within an extensive epithelium (e.g. gastrointestinal and respiratory tracts). The major function of these cells is probably paracrine (i.e. acting on adjacent non-endocrine cells, rather than entering the bloodstream and producing systemic effects).
Pituitary Gland
The pituitary gland (hypophysis) is a small bean-shaped gland, about 1 cm diameter, at the base of the brain beneath the third ventricle, sitting in a bony cavity in the base of the skull (the sella turcica). The gland is divided into anterior and posterior parts which have different embryological origins, functions and control mechanisms.
The secretion of all major pituitary hormones is controlled by the hypothalamus, which itself is under the influence of nervous stimuli from higher centres in the brain. Control is mainly by feedback from the levels of circulating hormones produced by pituitary-dependent endocrine tissues.
The pituitary hormones fall into two functional groups:
• Hormones which act directly on non-endocrine tissues: growth hormone (GH), prolactin, antidiuretic hormone (ADH, vasopressin), oxytocin and melanocyte stimulating hormone (MSH).
• Hormones which modulate the secretory activity of other endocrine glands (trophic hormones): thyroid stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH) and the gonadotrophic hormones, follicle stimulating hormone (FSH) and luteinising hormone (LH).
Thus the thyroid gland, adrenal cortex and gonads may be described as pituitary-dependent endocrine glands.
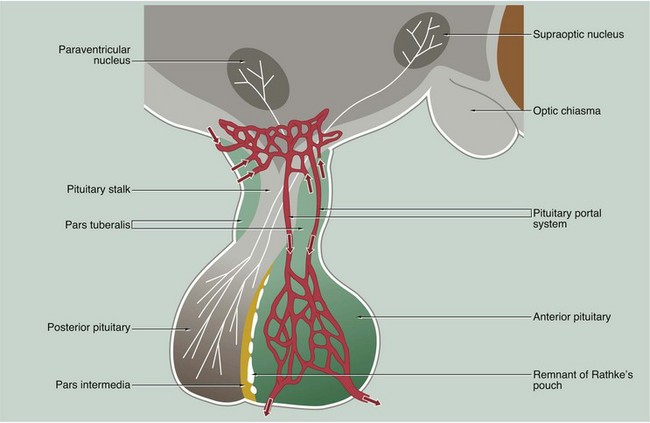
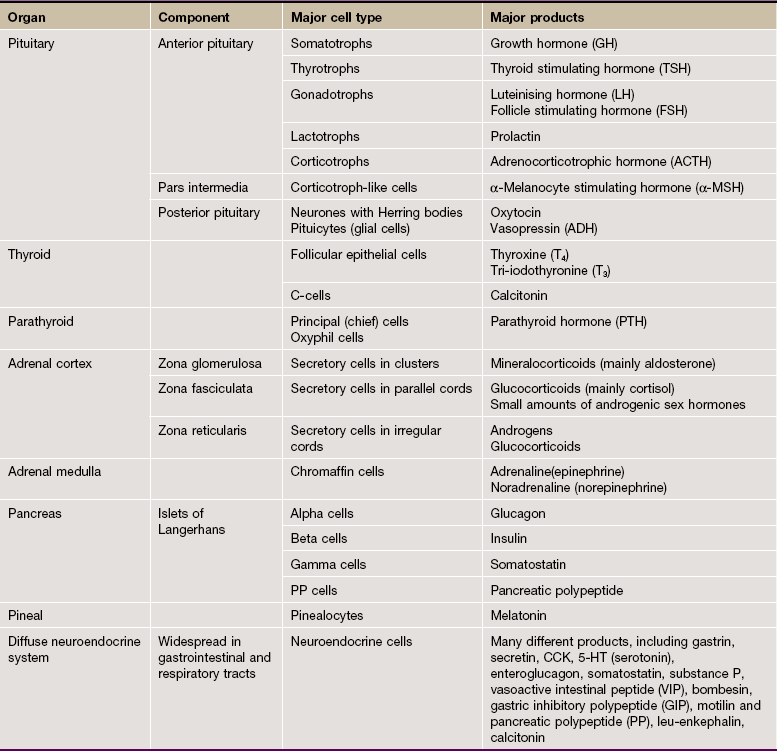
FIG. 17.1 Pituitary gland
The anterior and posterior parts of the pituitary originate from different embryological sources and this is reflected in their structure and function.
The posterior pituitary, also called the neurohypophysis or pars nervosa, is derived from a downgrowth of nervous tissue from the hypothalamus, to which it remains joined by the pituitary stalk.
The anterior pituitary arises as an epithelial upgrowth from the roof of the primitive oral cavity known as Rathke's pouch. This specialised glandular epithelium is wrapped around the anterior aspect of the posterior pituitary and is often called the adenohypophysis. The adenohypophysis may contain a cleft or group of cyst-like spaces which represent the vestigial lumen of Rathke's pouch. This vestigial cleft divides the major part of the anterior pituitary from a thin zone of tissue lying against the posterior pituitary known as the pars intermedia. An extension of the adenohypophysis surrounds the neural stalk and is known as the pars tuberalis.
The type and mode of secretion of the posterior pituitary differs greatly from that of the anterior pituitary. The posterior pituitary secretes two hormones, antidiuretic hormone (ADH), also called vasopressin or arginine vasopressin, and the hormone oxytocin, both of which act directly on non-endocrine tissues. ADH is synthesised in the neurone cell bodies of the supraoptic nucleus, and oxytocin is synthesised in those of the paraventricular nucleus of the hypothalamus. Bound to glycoproteins, the hormones pass down the axons of the hypothalamopituitary tract through the pituitary stalk to the posterior pituitary where they are stored in the distended terminal parts of the axons. Release of posterior pituitary hormones is controlled directly by nervous impulses passing down the axons from the hypothalamus, a process known as neurosecretion.
Hypothalamic control of anterior pituitary secretion is mediated by specific hypothalamic releasing hormones, such as thyroid stimulating hormone releasing hormone (TSHRH); exceptions to this rule are prolactin secretion, which is under the inhibitory control of dopamine, and secretion of growth hormone, which is controlled by both releasing and inhibitory hormones. These releasing and inhibitory hormones are conducted from the median hypothalamic eminence to the anterior pituitary by a unique system of portal veins (pituitary portal system).
The pars intermedia synthesises and secretes melanocyte-stimulating hormone (MSH); in humans, the pars intermedia is rudimentary and the physiological importance of MSH and the control of its secretion are poorly understood.
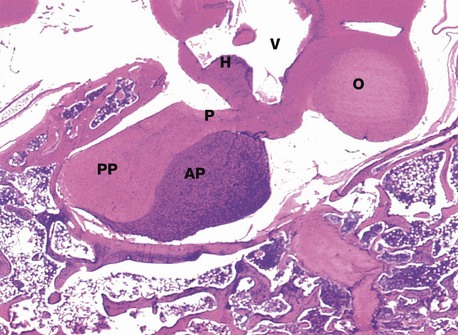
FIG. 17.2 Pituitary gland, monkey
H&E (LP)
This micrograph from a midline section through the brain and cranial floor illustrates the pituitary gland in situ. The pituitary sits in a bony depression in the sphenoid bone called the sella turcica. The two major components of the gland, the anterior pituitary AP and the posterior pituitary PP, are easily seen at this magnification. The posterior pituitary is connected to the hypothalamus H by the pituitary stalk P and, like the hypothalamus, is composed of nervous tissue. Note the close proximity of the third ventricle V above the hypothalamus and the optic chiasma O anteriorly.
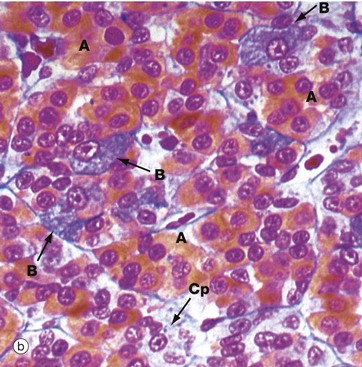
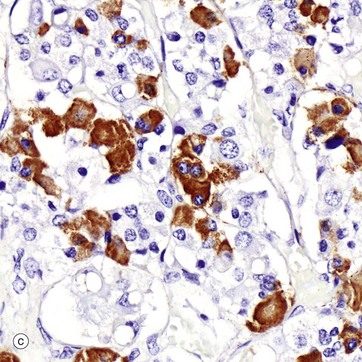
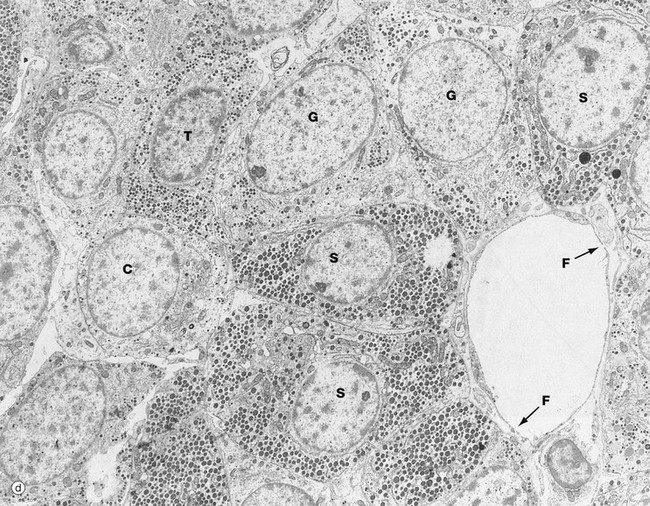
FIG. 17.3 Anterior pituitary (illustration (d) opposite)
(a) H&E (HP) (b) Azan (HP) (c) Immunohistochemical method for GH (HP) (d) EM ×4270
Micrograph (a) is an H&E-stained preparation of anterior pituitary and shows two main populations of cells, those with strongly staining cytoplasm (chromophils) and those with weakly staining cytoplasm (chromophobes Cp). The chromophils can be separated further into basophils B and acidophils A based on their cytoplasmic staining properties. This is more easily seen in micrograph (b). Note the prominent capillaries Ca lying between clumps of secretory cells. The most accurate identification of cell types is given by immunohistochemical methods and electron microscopy. The number of granules in the cytoplasm of these cells may depend on whether they are in a resting phase or actively secreting. These methods show that chromophobes have very few secretory granules but may produce small amounts of any of the hormones. Chromophobes probably represent cells at the end of a secretory phase, rather than a distinct cell.
Micrograph (c) shows a section of anterior pituitary stained by the immunohistochemical technique for growth hormone (GH). The brown-stained GH-containing cells can be seen scattered at random among the other cell types.
The different cell types are now named as follows:
• Somatotrophs, the cells responsible for growth hormone secretion, are the most numerous, making up almost 50% of the bulk of the anterior pituitary. These cells predominate in the lateral lobes of the gland and have large numbers of secretory dense granules.
• Mammotrophs (lactotrophs), the prolactin secreting cells, comprise up to 20% of the anterior pituitary, increasing in number during pregnancy; prolactin controls milk production during lactation. They are mainly situated in the postero-lateral areas of the gland.
• Corticotrophs secrete ACTH (corticotrophin) and constitute about 20% of the anterior pituitary mass. ACTH is a polypeptide which becomes split from a much larger peptide molecule known as pro-opiomelanocortin (POMC). Lipotropins (involved in regulation of lipid metabolism), endorphins (endogenous opioids) and various species of MSH can be derived from the same molecule; this explains the hyperpigmentation associated with excessive ACTH secretion. Corticotrophs are located mainly in the central part of the gland.
• Thyrotrophs, which secrete TSH (thyrotrophin), are much less numerous, making up only about 5% of the gland; they are mainly found in the central anterior area of the gland.
• Gonadotrophs, the cells responsible for the secretion of FSH and LH, make up the remaining 5% of the anterior pituitary and are widespread throughout the gland.
In general, one cell produces a single hormone, except for gonadotrophs, which mostly produce both LH and FSH. The different cell types are not evenly distributed throughout the gland, but rather particular cell types tend to congregate in particular zones of the gland.
The secretory granules of each cell type have a characteristic size, shape and electron density by which the different cell types can be recognised with electron microscopy as in micrograph (d). Somatotrophs S are packed with secretory granules of moderate size. Thyrotrophs T have smaller granules which tend to be more peripherally located. Gonadotrophs G are large cells with secretory granules of variable size. Corticotrophs C have sparse secretory granules located at the extreme periphery of the cell.
The clumps and cords of cells have a rich capillary network. The endothelial lining of capillaries in endocrine tissue is characteristically fenestrated (see Fig. 8.16), facilitating the passage of hormones into the sinusoids. Note the fenestrations F in the sinusoid seen in micrograph (d).
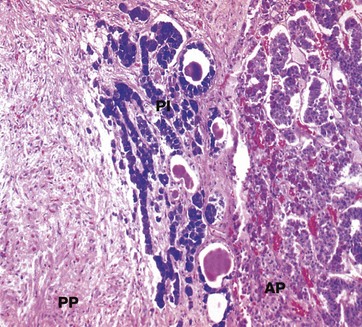
FIG. 17.4 Pituitary, pars intermedia
Isamine blue/eosin (MP)
The pars intermedia PI, like the anterior pituitary, is derived embryologically from Rathke's pouch. The cells are basophilic (stained blue here), lying in irregular clusters between the anterior AP and posterior PP pituitary. The pars intermedia also contains small cystic spaces filled with eosinophilic material.
Ultrastructurally, the cells of the pars intermedia contain secretory granules similar to those of corticotrophs. These cells produce α-MSH from pro-opiomelanocortin, usually at low levels.
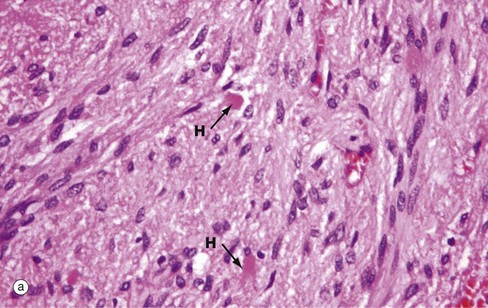
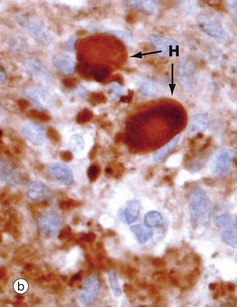
FIG. 17.5 Posterior pituitary
(a) H&E (MP) (b) Immunohistochemical method for synaptophysin (HP)
The posterior pituitary is largely composed of the non-myelinated axons of specialised neurones which have considerable neurosecretory activity. The cell bodies of these neurones are located in the supraoptic and paraventricular nuclei of the hypothalamus and it is here that the posterior pituitary peptide hormones oxytocin and ADH are produced. They are passed down the axons in neurosecretory granules which accumulate in the distended terminations of the axons where they contact capillaries. These distensions are called Herring bodies H. The axons are supported by specialised highly branched glial cells called pituicytes, the cytoplasm of which sometimes contains small amounts of yellowish-brown pigment.
Micrograph (a) shows the structure of posterior pituitary; the fibrillar structures are the axons of the hypothalamic neurones with distended terminal Herring bodies H. The nuclei are those of supporting pituicytes. Micrograph (b) is an immunohistochemical preparation for neurosecretory granules (synaptophysin). Although granules are scattered in the axons, they are particularly concentrated in the round Herring bodies H.
Thyroid Gland
The thyroid gland is a butterfly-shaped endocrine gland lying in the neck in front of the upper part of the trachea. The thyroid gland produces hormones of two types:
• Iodine-containing hormones tri-iodothyronine (T3), and thyroxine (tetra-iodothyronine, T4); T4 is converted to T3 in the general circulation by removal of one iodothyronine unit, although a small amount of T3 is secreted directly. T3 is much more potent than T4 and appears to be the metabolically active form of the hormone. Thyroid hormone regulates the basal metabolic rate and has an important influence on growth and maturation, particularly of nerve tissue. The secretion of these hormones is regulated by TSH secreted by the anterior pituitary.
• The polypeptide hormone calcitonin; this hormone regulates blood calcium levels in conjunction with parathyroid hormone. Calcitonin lowers blood calcium levels by inhibiting the rate of decalcification of bone by osteoclastic resorption and by stimulating osteoblastic activity. Control of calcitonin secretion is dependent only on blood calcium levels and is independent of pituitary and parathyroid hormone levels.
The thyroid gland is unique among the human endocrine glands in that it stores large amounts of hormone in an inactive form within extracellular compartments in the centre of follicles; in contrast, other endocrine glands store only small quantities of hormones in intracellular sites.
The main bulk of the gland develops from an epithelial downgrowth from the fetal tongue, whereas the calcitonin-secreting cells are derived from the ultimobranchial element of the fourth branchial pouch.
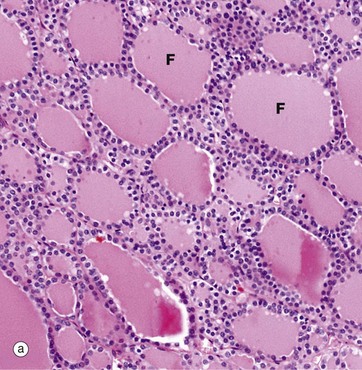
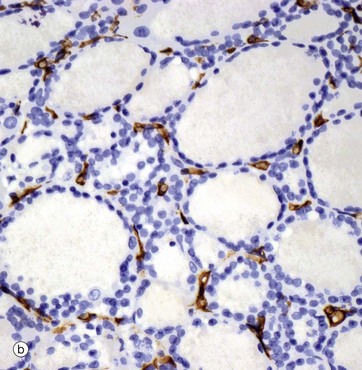
FIG. 17.6 Thyroid gland
(a) H&E (LP) (b) Immunohistochemical method for CD34
The functional units of the thyroid gland are the thyroid follicles F, spheroidal structures composed of a single layer of cuboidal epithelial cells, bounded by a basement membrane (see also Fig. 5.27). As seen in this micrograph of a normal thyroid, the follicles are variable in size and contain a homogeneous colloid, which is stained pink in this preparation.
The thyroid gland is enveloped by a fibrous capsule from which fine collagenous septa (not shown in this micrograph) extend into the gland, dividing it into lobules. The septa convey a rich blood supply, together with lymphatics and nerves. Tiny capillaries percolate through the thyroid tissue and surround the follicles and, although these are difficult to see in an H&E preparation, they can be highlighted using an immunohistochemical method for an endothelial marker (CD34), as seen in micrograph (b).
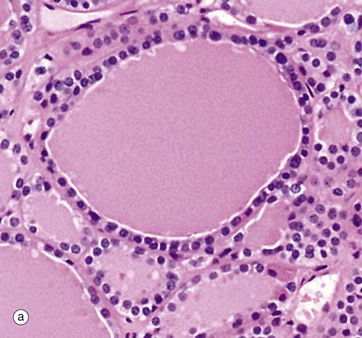
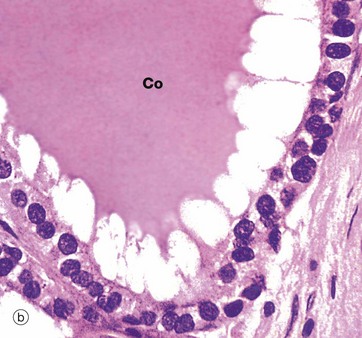
FIG. 17.7 Thyroid follicle
(a) Inactive, H&E (HP) (b) Active, H&E (HP)
Thyroid follicles store thyroglobulin, an iodinated glycoprotein, the storage form of thyroxine (T4) and tri-iodothyronine (T3). The follicles are lined by epithelial cells which are initially responsible for the synthesis of the glycoprotein component of thyroglobulin and for the conversion of iodide to iodine, the iodine linking to the glycoprotein in the follicle lumen. When active thyroid hormone is required, the same thyroid epithelial cells remove some of the stored thyroid colloid and detach T3 and T4, which then pass through the cell into an adjacent capillary. When inactive, thyroid epithelial cells are simple flat or cuboidal cells, as in micrograph (a) which shows inactive epithelial cells lining follicles filled with stored thyroglobulin (colloid Co). Micrograph (b), in contrast, shows cells actively synthesising or secreting thyroid hormone; they are tall and columnar. In this follicle, they are extracting stored thyroid colloid Co from the lumen and converting it into active thyroid hormones. The ‘scalloped’ pale edge of the colloid indicates where the colloid has been removed from the follicle lumen.
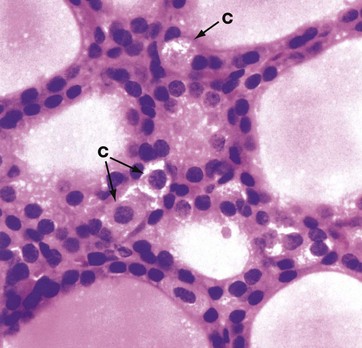
FIG. 17.8 Thyroid C cell
H&E (HP)
A second type of endocrine cell with the ultrastructural characteristics of neuroendocrine cells, the C cell or parafollicular cell C, is found in the thyroid gland as individual scattered cells in the follicle lining or as small clumps in the interstices between follicles. They are particularly prominent in dogs, where they are identifiable in H&E sections as pale-staining cells with granular cytoplasm. In humans they are much less prominent and can usually only be identified ultrastructurally (see Fig. 17.9) and by immunohistochemical methods. These cells secrete calcitonin, which is a physiological antagonist to parathyroid hormone and therefore lowers blood calcium levels by suppressing the osteoclastic resorption of bone.
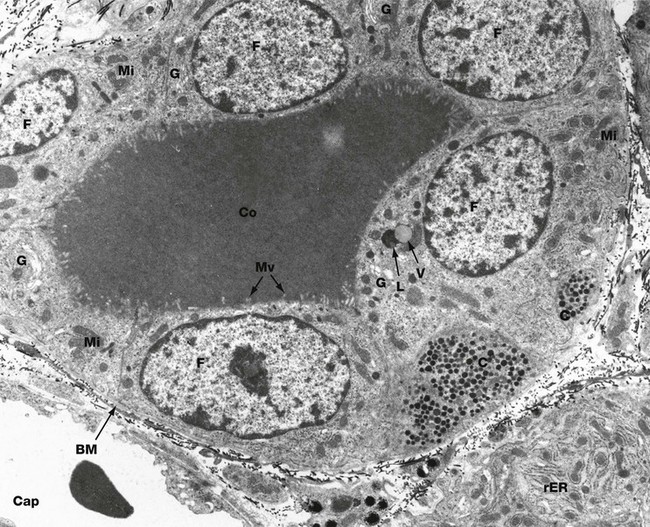
FIG. 17.9 Thyroid follicle, rat
EM ×6800
This micrograph demonstrates a thyroid follicle composed of cuboidal follicular cells F surrounding a lumen containing the homogeneous colloid, thyroglobulin Co. A basement membrane BM delineates the follicle. Two portions of the cytoplasm of a C cell C are seen within the follicular epithelium, typically located on the basement membrane and not exposed to the follicular lumen. The cytoplasm contains numerous electron-dense secretory granules of the hormone calcitonin. A fenestrated capillary Cap containing an erythrocyte is closely applied to the follicular basement membrane.
Follicular cells concentrate iodide from the blood by means of an iodide pump in the basal plasma membrane. Within the cell, iodide is oxidised to iodine and transported to the follicular plasma membrane where it is released into the follicular lumen. The glycoprotein thyroglobulin is synthesised in the rough endoplasmic reticulum, glycosylated and packaged by the Golgi apparatus, then released into the follicular lumen by exocytosis. Within the follicular lumen (not within the follicular cells), iodine combines with tyrosine residues of the thyroglobulin to form the hormones tri-iodothyronine (T3) and tetra-iodothyronine (thyroxine, T4) which remain bound to the glycoprotein in an inactive form.
Secretion of these hormones involves pinocytosis of the thyroglobulin-hormone complex to form cytoplasmic vacuoles. The vacuoles then fuse with lysosomes of the follicular cell cytoplasm, and hydrolytic enzymes cleave the hormone from the thyroglobulin. The hormones are released in the basal cytoplasm, from which they diffuse into the bloodstream. The synthetic and secretory activity of the thyroid gland is dependent on thyroid stimulating hormone (TSH), secreted by the anterior pituitary.
In this micrograph, rough endoplasmic reticulum rER is best demonstrated in the basal aspect of a secretory cell of an adjacent follicle. Mitochondria Mi are closely associated with the endoplasmic reticulum and are also scattered throughout the cytoplasm. Golgi complexes G are a prominent feature. Small microvilli Mv, associated with the exocytosis of thyroglobulin and the endocytosis of the thyroglobulin-hormone complex, protrude into the follicular lumen. In one cell a vacuole V of thyroglobulin-hormone is seen about to fuse with a large lysosome L. Electron-dense lysosomes are also seen scattered throughout the cytoplasm.
Parathyroid Gland
The parathyroid glands are small, oval endocrine glands which are closely associated with the thyroid gland. In mammals, there are usually two pairs of glands, one pair situated on the posterior surface of the thyroid gland on each side, although occasional individuals possess five or even six parathyroids. The embryological origins of the parathyroid glands are from the third and fourth branchial (pharyngeal) pouches. The parathyroid glands regulate serum calcium and phosphate levels via parathyroid hormone (PTH).
Parathyroid hormone raises serum calcium levels in three ways:
• Direct action on bone, increasing the rate of osteoclastic resorption and promoting breakdown of the bone matrix
• Direct action on the kidney, increasing the renal tubular reabsorption of calcium ions and inhibiting the reabsorption of phosphate ions from the glomerular filtrate
• Promotion of the absorption of calcium from the small intestine; this effect involves vitamin D.
Secretion of parathyroid hormone is stimulated by a decrease in blood calcium levels. In conjunction with calcitonin secreted by the C cells of the thyroid gland, blood calcium levels are maintained within narrow limits. Parathyroid hormone is the most important regulator of blood calcium levels and is essential to life, whereas calcitonin appears to provide a complementary mechanism for fine adjustment and is not essential to life.
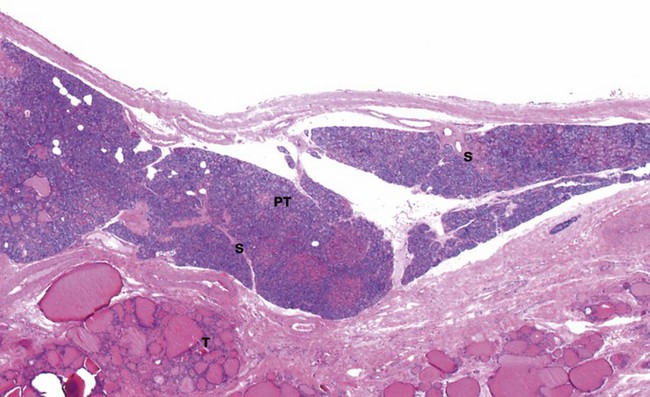
FIG. 17.10 Parathyroid gland
H&E (LP)
This micrograph shows a parathyroid gland PT, embedded under the capsule of a thyroid gland T. Some parathyroids are actually found embedded in the thyroid tissue proper. The thin fibrous capsule of the parathyroid gland gives rise to delicate septa S which divide the parenchyma into nodules of secretory cells; as seen here, they are very prone to shrinkage artefact during histological preparation. The septa carry blood vessels, lymphatics and nerves.
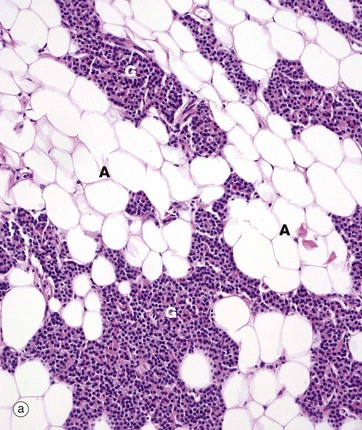
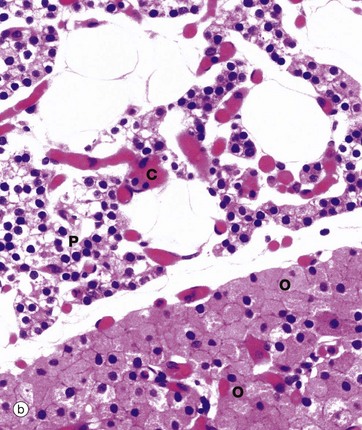
FIG. 17.11 Parathyroid gland
(a) H&E (MP) (b) H&E (HP)
Micrograph (a) is a medium-power view of normal adult parathyroid, showing the glandular elements G intermixed with adipose tissue A, which begins to accumulate after puberty and makes up 25% to 40% of the total tissue in normal adults. The glandular cells are of two types: chief or principal cells and oxyphil cells. The glandular cells are arranged as clusters, ribbons or glands.
At higher power in micrograph (b), the chief cells P are small, with round central nuclei and pale eosinophilic or clear cytoplasm. These are the cells which synthesise and secrete PTH. The staining intensity of the cytoplasm depends on whether the cells are actively secreting PTH, in which case the cytoplasm contains plentiful rough endoplasmic reticulum and stains strongly. On the other hand, resting cells have pale cytoplasm and make up about 80% of the total in normal adults.
Oxyphil cells O, which tend to occur in nodules, have copious eosinophilic cytoplasm that ultrastructurally is seen to be packed with mitochondria. These cells do not secrete PTH and increase in number with age.
Note the many delicate capillaries C between the nests of endocrine cells.
Adrenal Gland
The adrenal (suprarenal) glands are small, flattened endocrine glands which are closely applied to the upper pole of each kidney. In mammals, the adrenal gland contains two functionally different types of endocrine tissue which have distinctly different embryological origins; in some lower animals, these two components exist as separate endocrine glands. The two components of the adrenal gland are the adrenal cortex and adrenal medulla.
The adrenal cortex has a similar embryological origin to the gonads and, like them, secretes a variety of steroid hormones, all structurally related to their common precursor cholesterol. The adrenal steroids may be divided into three functional classes, mineralocorticoids, glucocorticoids and sex hormones. The mineralocorticoids are concerned with electrolyte and fluid homeostasis. The glucocorticoids have a wide range of effects on carbohydrate, protein and lipid metabolism. Small quantities of sex hormones are secreted by the adrenal cortex and supplement gonadal sex hormone secretion.
Embryologically, the adrenal medulla has a similar origin to that of the sympathetic nervous system and may be considered as a highly specialised adjunct of this system. The adrenal medulla secretes the catecholamine hormones, adrenaline (epinephrine) and noradrenaline (norepinephrine).
The control of hormone secretion differs markedly between the cortex and medulla. Glucocorticoid secretion is mainly regulated by the pituitary trophic hormone ACTH, while mineralocorticoid secretion is under the control of the renin-angiotensin aldosterone system (see Ch. 16). In contrast, the secretion of adrenal medullary catecholamines is directly controlled by the sympathetic nervous system. The function of the adrenal medulla is to reinforce the action of the sympathetic nervous system under conditions of stress, the direct nervous control of adrenal medullary secretion permitting a rapid response.
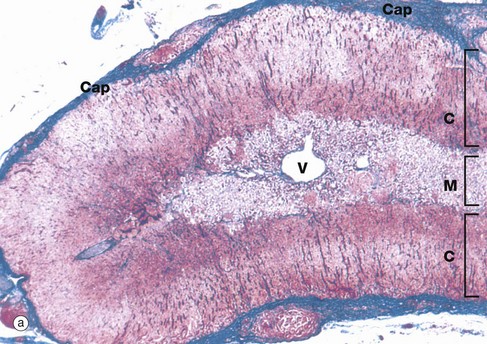
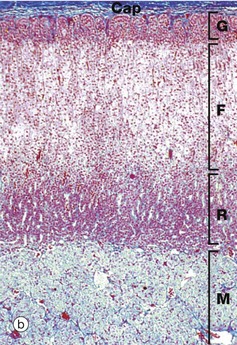
FIG. 17.12 Adrenal gland
(a) Azan (LP) (b) Azan (LP)
At low magnification, the adrenal gland is seen to be divided into an outer cortex C and a pale-stained inner medulla M. A dense fibrous tissue capsule Cap, stained blue in this preparation, invests the gland and provides external support for a delicate collagenous framework supporting the secretory cells. A prominent vein V is characteristically located in the centre of the medulla.
At higher magnification in micrograph (b), the adrenal cortex can be seen to consist of three histological zones which are named according to the arrangement of the secretory cells: zona glomerulosa, zona fasciculata and zona reticularis.
The zona glomerulosa G, lying beneath the capsule, contains secretory cells arranged in rounded clusters. The intermediate zona fasciculata F consists of parallel cords of secretory cells disposed at right angles to the capsule. The zona reticularis R, which lies adjacent to the medulla M, consists of small closely packed cells arranged in irregular cords. Often the borders of the zones are less regular and less easily recognised than in this specimen.
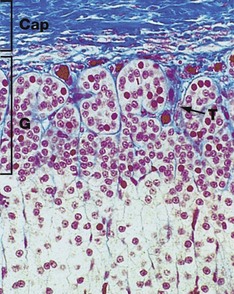
FIG. 17.13 Adrenal cortex, zona glomerulosa
Azan (MP)
The zona glomerulosa G is composed of cells arranged in irregular ovoid clusters separated by delicate fibrous trabeculae T, which are continuous with the fibrocollagenous capsule Cap; both the trabeculae and inner capsule contain prominent capillaries. The cells have round nuclei and less cytoplasm than the cells in the adjacent zona fasciculata. The cytoplasm contains plentiful smooth endoplasmic reticulum and numerous mitochondria, but with only scanty lipid droplets.
This zone secretes the mineralocorticoid hormones, principally aldosterone, the secretion of which is controlled by the renin-angiotensin system (see Ch. 16), which in turn is controlled by the macula densa of the distal renal tubule. Aldosterone acts directly on the renal tubules to increase sodium and therefore water retention. This increases extracellular fluid volume and therefore increases arterial blood pressure. Aldosterone secretion is independent of ACTH control.
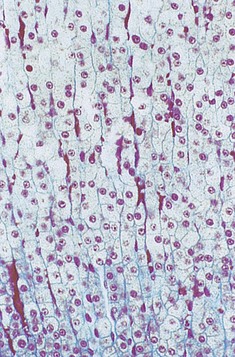
FIG. 17.14 Adrenal cortex, zona fasciculata
Azan (MP)
The zona fasciculata is the middle and broadest of the three cortical zones. It consists of narrow columns and cords of cells, often only one cell thick, separated by fine strands of collagen and wide-bore capillaries. The cell cytoplasm is abundant and pale staining due to the large number of lipid droplets present; mitochondria and smooth endoplasmic reticulum are also abundant. The zona fasciculata secretes glucocorticoid hormones, mainly cortisol, which have many metabolic effects, one of which is to raise blood glucose levels and increase cellular synthesis of glycogen. They also increase the rate of protein breakdown and the rate of liberation of lipid from tissue stores.
Cortisol secretion is controlled by the hypothalamus via the anterior pituitary trophic hormone ACTH. By this means, many stimuli, such as stress, promote glucocorticoid secretion.
The zona fasciculata is also the site of secretion of small amounts of androgenic sex hormones.
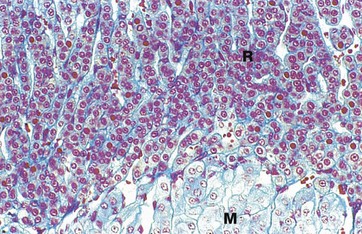
FIG. 17.15 Adrenal cortex, zona reticularis
Azan (LP)
The zona reticularis R is the thin, innermost layer of the adrenal cortex and lies next to the adrenal medulla M. It consists of an irregular network of branching cords and clusters of glandular cells, separated by numerous wide-diameter capillaries. The zona reticularis cells are much smaller than those of the adjacent zona fasciculata, with less cytoplasm. The cytoplasm is darker staining because it contains considerably fewer lipid droplets. Brown lipofuscin pigment (see Fig. 1.25) is sometimes seen in the cells of this layer. The zona reticularis secretes small quantities of androgens and glucocorticoids.
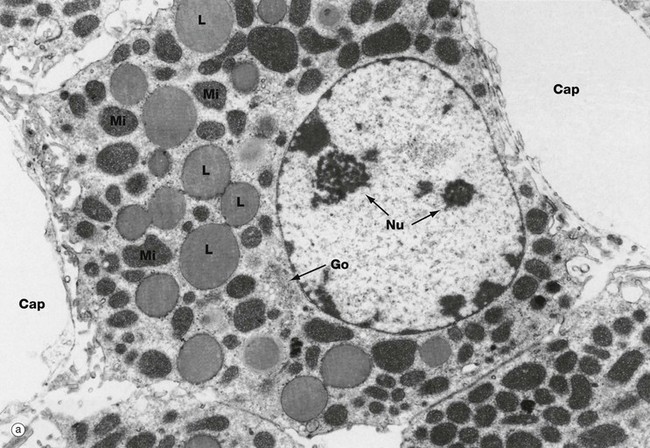
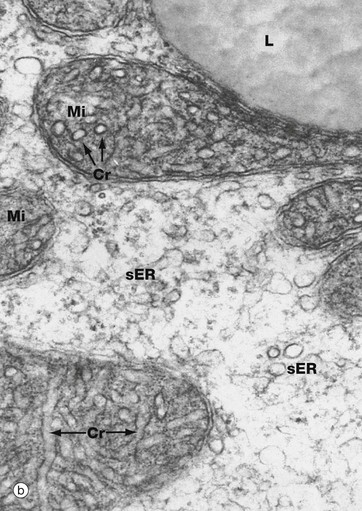
FIG. 17.16 Steroid-secreting cell
(a) EM ×8500 (b) EM ×110 500
These micrographs illustrate the typical ultrastructural features of steroid-secreting cells, which are seen not only in the cells of the adrenal cortex but also in the steroid-secreting cells of the ovaries and testes (see Chs 18 and 19). At low magnification in micrograph (a), a secretory cell is seen, intimately associated with fenestrated capillaries Cap. Note the short microvillous projections of the secretory cell plasma membrane subjacent to the capillary endothelium. The rounded secretory cell nucleus is characterised by one or more prominent nucleoli Nu.
The abundant cytoplasm contains many large lipid droplets L containing stored cholesterol esters. A small Golgi apparatus Go is seen close to the nucleus. Numerous variably shaped mitochondria Mi crowd the cytoplasm. As seen in micrograph (b) at high magnification, the mitochondria have unusual tubular cristae Cr. The cytoplasm contains a prolific system of smooth endoplasmic reticulum sER.
Synthesis of steroid hormones begins with the liberation of cholesterol esters from lipid droplets. The cholesterol molecule is modified to form a wide range of steroid hormones by enzyme systems found in the smooth endoplasmic reticulum and in the mitochondria.
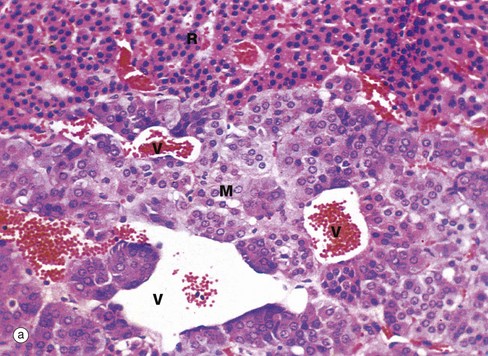
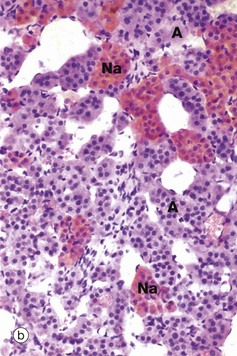
FIG. 17.17 Adrenal medulla
(a) H&E (MP) (b) Chrome salt fixation, H&E (MP)
The adrenal medulla secretes the amines adrenaline (epinephrine) and noradrenaline (norepinephrine) under the control of the sympathetic nervous system. When stained with the standard H&E method the adrenal medulla M, as shown in micrograph (a), is composed of clusters of cells with granular, faintly basophilic cytoplasm, with numerous capillaries in their fine supporting stroma. Venous channels V draining blood from the sinusoids of the cortex pass through the medulla towards the central medullary vein. This photomicrograph also shows part of the zona reticularis R of the cortex.
When fixed in chrome salts as in micrograph (b), the stored catecholamine granules of adrenal medullary cells are oxidised to a brown colour; consequently the name chromaffin cells was often applied to the secretory cells of the adrenal medulla. Some adrenal medullary cells synthesise noradrenaline; however, the majority synthesise adrenaline by the addition of a further N-methyl group to noradrenaline. Those cells containing noradrenaline Na exhibit a much more strongly positive chromaffin reaction than adrenaline-secreting cells A. Ultrastructurally, the cytoplasm of the medullary cells contains dense core granules similar to those illustrated in Fig. 17.23. The granules in adrenaline-secreting cells have a narrow clear halo surrounding the dense core, while those containing noradrenaline have a much wider clear halo around the dense core.
Secretion of catecholamines by the adrenal medulla is controlled by preganglionic neurones of the sympathetic nervous system; thus, the secretory cells of the adrenal medulla are functionally equivalent to the postganglionic neurones of the sympathetic nervous system. Acute physical and psychological stresses initiate release of adrenal medullary hormones. The released catecholamines act on adrenergic receptors throughout the body, particularly in the heart and blood vessels, bronchioles, visceral muscle and skeletal muscle, producing physiological effects very familiar to those who have ever taken a viva voce examination. Adrenaline also has potent metabolic effects, such as the promotion of glycogenolysis in liver and skeletal muscle, thus releasing a readily available energy source during stress situations.
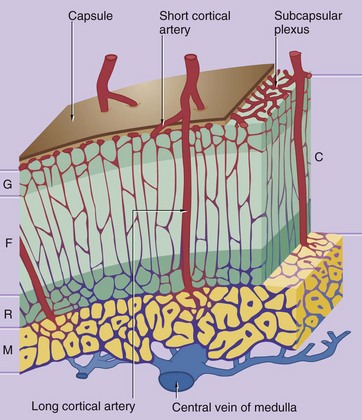
FIG. 17.18 Blood supply of the adrenal
The adrenal gland is supplied by the superior, middle and inferior suprarenal arteries, which form a plexus just under the capsule of the gland.
The vascular system of the cortex C consists of an anastomosing network of capillary sinusoids supplied by branches of the subcapsular plexus, known as short cortical arteries. The sinusoids descend between the cords of secretory cells in the zona fasciculata F into a deep plexus in the zona reticularis R before draining into small venules which converge upon the central vein of the medulla M. The central medullary veins contain longitudinal bundles of smooth muscle between which the cortical venules enter; contraction of this smooth muscle is thought to hold back cortical blood and thus regulate flow.
The medulla is supplied by long cortical arteries which descend from the subcapsular plexus through the cortex into the medulla where they ramify into a rich network of dilated capillaries surrounding the medullary secretory cells. The medullary capillaries also drain into the central vein of the medulla. Thus the secretory cells of the medulla are exposed to fresh arterial blood as well as blood rich in adrenocorticosteroids, which are believed to have an important influence on the synthesis of adrenaline by the medulla.
Endocrine Pancreas
The pancreas is not only a major exocrine gland (see Ch. 15) but also has important endocrine functions.
The embryonic epithelium of the pancreatic ducts consists of both potential exocrine and endocrine cells. During development, the endocrine cells migrate from the duct system and aggregate around capillaries to form isolated clusters of cells known as islets of Langerhans, scattered throughout the exocrine glandular tissue. The islets vary in size and are most numerous in the tail of the pancreas. The islets contain a variety of cell types, each responsible for secretion of one type of polypeptide hormone.
The main secretory products of the endocrine pancreas are insulin and glucagon, polypeptide hormones which play an important role in carbohydrate metabolism. Insulin promotes the uptake of glucose by most cells, particularly those of the liver, skeletal muscle and adipose tissue, thus lowering plasma glucose concentration. In general, glucagon has metabolic effects that oppose the actions of insulin. Apart from their role in carbohydrate metabolism, these hormones have a wide variety of other effects on energy metabolism, growth and development.
At least four other types of endocrine cells are present in the islets or else scattered singly or in small groups between the exocrine acini and along the ducts. Their secretory products include somatostatin (which has a wide variety of effects on gastrointestinal function and may also inhibit insulin and glucagon secretion), vasoactive intestinal peptide (VIP) and pancreatic polypeptide (PP). Another cell type, the enterochromaffin (EC) cell, appears to secrete several different peptides including motilin, 5-hydroxytryptamine (5-HT, serotonin) and substance P.
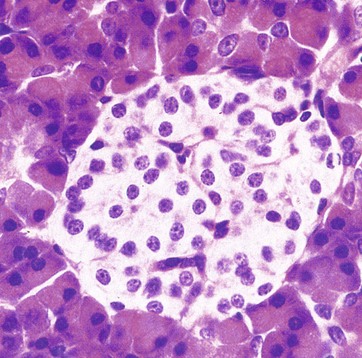
FIG. 17.19 Islet of Langerhans
H&E (HP)
The islets of Langerhans are composed of groups of up to 3000 secretory cells supported by a fine collagenous network containing numerous fenestrated capillaries. A delicate capsule surrounds each islet. The endocrine cells are small with a pale-stained granular cytoplasm: in contrast, the large cells of the surrounding exocrine pancreatic acini stain strongly.
The endocrine pancreas contains secretory cells of several types; however, in H&E stained preparations the cell types are indistinguishable from one another and special staining methods are required to differentiate between them. Traditionally, the glucagon-, insulin- and somatostatin-secreting cells have been designated as alpha, beta and delta cells, respectively. However, with the advent of immunohistochemical methods for identification of secretory products, it is most appropriate to identify cells by their products.
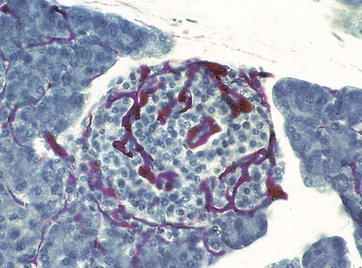
FIG. 17.20 Blood supply of the endocrine pancreas
Carmine perfused/haematoxylin (MP)
This specimen was perfused with a red dye before fixation to demonstrate the rich blood supply of the pancreatic islets. Each islet is supplied by as many as three arterioles, which ramify into a highly branched network of fenestrated capillaries, into which the hormones produced in the islet are secreted. The islet is drained by about six venules, passing between the exocrine acini to the interlobular veins.
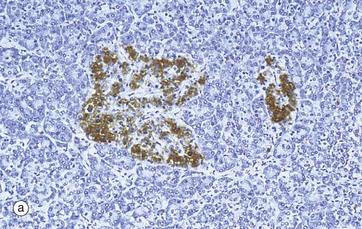
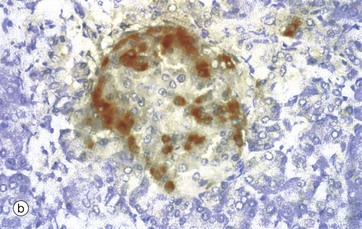
FIG. 17.21 Islet of Langerhans
(a) Immunohistochemical stain for insulin (MP) (b) Immunohistochemical stain for glucagon (HP)
In the past, empirical staining methods were used to demonstrate the different cell types in the islets of Langerhans. These have now been superseded by the immunohistochemical techniques which are able to detect specific intercellular products, in this case insulin and glucagon. The insulin-producing beta cells, which constitute over 60% of the cells in the islet, are stained brown in micrograph (a). Beta cells are distributed throughout the islet, while in contrast, glucagon-producing alpha cells (about 25% of the total) are arranged around the periphery, as in micrograph (b). Other hormone-producing cells are unstained in these micrographs. The close proximity of these cells facilitates their interaction for control of blood glucose levels and other metabolic functions.
Insulin, a small protein, is synthesised in the rough endoplasmic reticulum as preproinsulin which is then cleaved to form proinsulin. Proinsulin is cleaved again, this time in the Golgi apparatus, to form insulin, which is then packaged with a small amount of uncleaved proinsulin into membrane-bound secretory granules which remain in the cytoplasm until insulin secretion is triggered.
Pineal Gland
The pineal gland is a small, roughly spherical gland 6 to 10 mm in diameter which lies in the midline of the brain, just below the posterior end of the corpus callosum. It represents an evagination of the posterior part of the roof of the third ventricle. It is connected to the brain by a short stalk containing nerve fibres, some of which communicate with the hippocampus. The pineal gland synthesises the hormone melatonin which acts as an endocrine transducer, inducing rhythmical changes in the endocrine activity of the hypothalamus, pituitary, ovaries and testes in response to changes in light received by the retina. Melatonin production by the pineal is induced by darkness and inhibited by light, probably via sympathetic nerves transmitting messages from the eye through the suprachiasmatic nucleus, central sympathetic pathways and the superior cervical ganglion.
Effects of melatonin in man include an influence on the onset of puberty and body biorhythms. In other animals, it plays a role in the timing of seasonal reproductive cycles and, in reptiles and other lower vertebrates, is responsible for changing skin colour through its action on melanophores, pigmented cells analogous to melanocytes in mammals. Melatonin is also synthesised in many peripheral tissues, where it appears to have paracrine effects. In recent years, melatonin has been used as a treatment for sleep disturbance (e.g. jet lag) and melatonin analogues are also used to treat depression.
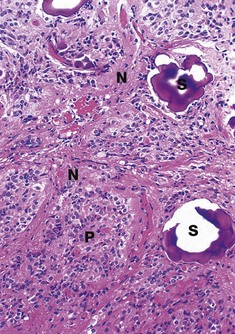
FIG. 17.22 Pineal gland
H&E (MP)
The pineal consists of two main cell types: pinealocytes (pineal chief cells) and neuroglial cells. Pinealocytes P are highly modified neurones, arranged in clusters and cords surrounded by a rich network of fenestrated capillaries. Pinealocytes have round nuclei with prominent nucleoli and granular cytoplasm and many highly branched processes, some of which terminate near or upon blood vessels. The cytoplasmic granules of pinealocytes contain melatonin and its precursor, 5-HT.
The neuroglial cells N, which are similar to the astrocytes of the rest of the CNS, are dispersed between the clusters of pinealocytes and in association with capillaries.
A characteristic feature of the ageing pineal is the presence of basophilic extracellular bodies called pineal sand S, consisting of concentric layers of calcium and magnesium phosphate in an organic matrix. The calcified pineal can be seen on X-rays of the skull and its position can be a useful guide to pathological conditions causing the midline to be displaced to one side.
Diffuse Neuroendocrine System
This is the name given to a scattered system of neuroendocrine cells which secrete hormones and active peptides. Neuroendocrine cells possess characteristic membrane-bound neurosecretory vesicles, usually spherical, with an electron-dense central core (dense core vesicles). Although neuroendocrine-type cells form part of other endocrine organs (e.g. adrenal medulla) and some non-endocrine organs (e.g. islets of Langerhans in the pancreas, juxtaglomerular apparatus in kidney), they are particularly important in the diffuse neuroendocrine systems in the gastrointestinal and respiratory tracts.
Gastrointestinal neuroendocrine cells are found scattered in the mucosa of the gastrointestinal tract and in the pancreatic and biliary ducts. These cells secrete more than 20 different peptide and amine hormones, including gastrin, secretin, CCK, 5-HT, enteroglucagon, somatostatin, substance P, vasoactive intestinal peptide (VIP), bombesin, gastric inhibitory polypeptide (GIP), motilin and pancreatic polypeptide (PP). These hormones constitute a system of interacting mediators which collectively regulate and coordinate most aspects of gastrointestinal activity, in concert with the autonomic nervous system.
While some of these substances are true endocrine hormones, acting at a distance from their site of origin, others are locally acting mediators known as paracrine hormones. A third mechanism of action (neurocrine) is by neurotransmitter activity and indeed some of these substances also act as neurotransmitters within the central nervous system (gastrin, VIP, CCK and many others).
The lower respiratory tract contains scattered peptide- and amine-secreting endocrine cells, analogous to those in the gastrointestinal tract, which are probably involved in local and autonomically mediated regulation of respiratory tract function, particularly in early childhood. The endocrine cells are scattered individually in the epithelium or in clumps (see Fig. 12.11) protruding into the airway and have a variety of secretory products including 5-HT, calcitonin, bombesin and leu-enkephalin.
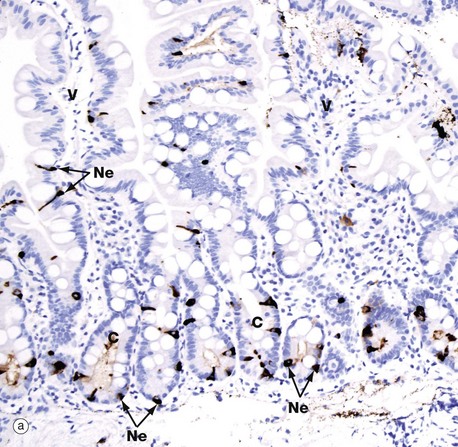
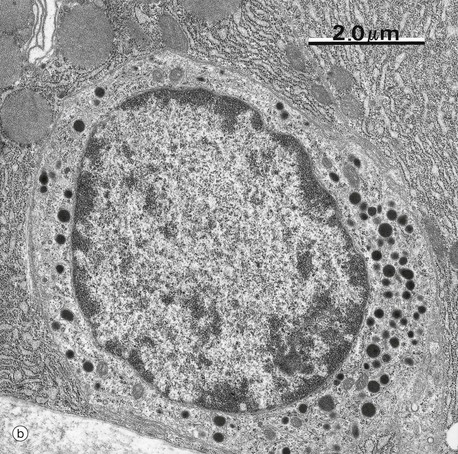
FIG. 17.23 Gastrointestinal neuroendocrine cells
(a) Immunohistochemical stain for chromogranin A (MP) (b) EM ×12 500
Neuroendocrine cells are very difficult to identify on H&E stained sections, but immunohistochemistry and electron microscopy make the task easy. Micrograph (a) shows duodenal mucosa with the crypts C as well as the lower segments of the villi V stained by an immunohistochemical method for chromogranin A. Chromogranin A is a component of all neurosecretory granules and so merely identifies the cells as neuroendocrine cells, without giving any clue as to their secretory product. The neuroendocrine cells Ne (dark brown) are pyramidal-shaped, with a broad base sitting on the basement membrane of the epithelium and a narrower part approaching the lumen of the gland. In so-called open-type mucosal neuroendocrine cells, the narrow end of the cell is in contact with the lumen and its contents. Note that the neuroendocrine cells are concentrated in the crypts, but occasional cells are also seen within the villous epithelium.
Micrograph (b) shows a somatostatin-secreting neuroendocrine cell in the gastric mucosa. The cytoplasm contains large numbers of electron-dense neurosecretory vacuoles (dense core granules); they are mainly spherical, of various sizes, and are particularly concentrated towards the base of the cell.