Cell structure and function
Introduction to the Cell
Histology is the study of normal cells and tissues, mainly using microscopes. This book describes the histology of normal human tissues, although much of the material applies to other mammals and, indeed, non-mammals. Structure and function are interdependent. Histological structure determines and is determined by the functions of different organs and tissues; the study of one has enriched the understanding and study of the other. When diseases such as cancer or inflammation affect a tissue, there are often specific changes in the microscopic structure of the tissue. The microscopic study of these changes is known as histopathology, anatomical pathology or sometimes just pathology. Obviously a sound knowledge of normal structure is essential for an understanding of pathology.
The cell is the functional unit of all living organisms. The simplest organisms, such as bacteria and algae, consist of a single cell. More complex organisms consist of many cells as well as extracellular matrix (e.g. the matrix of bone). The term eukaryote refers to organisms whose cells consist of cytoplasm and a defined nucleus bounded by a nuclear membrane; this includes plants, fungi and animals, both unicellular and multicellular. The cytoplasm contains variable numbers of several different recognisable structures called organelles, each with a defined function. The nucleus may be considered to be the largest organelle. Prokaryote refers to bacteria and archaea, whose cells do not have a membrane-bound nucleus; they also have other major structural differences and will not be discussed further here.
The cells of multicellular organisms, such as humans, show a great variety of functional and morphological specialisation, with amplification of one or another of the basic functions common to all living cells. The process by which cells adopt a specialised structure and function is known as differentiation. Despite an extraordinary range of morphological forms, all eukaryotic cells conform to a basic structural model, which is the subject of this chapter.
The major tool in the study of histology is the microscope, and students will find a brief description of how microscopes work in Appendix 1 at the back of this book. Various staining procedures are used to prepare tissues to make them visible through the microscope and these are described in Appendix 2. The student is strongly urged to read these appendices first; it really will make all that follows more comprehensible. Appendix 3 is a glossary of common histological terms and, like the other appendices, can be dipped into at any time.
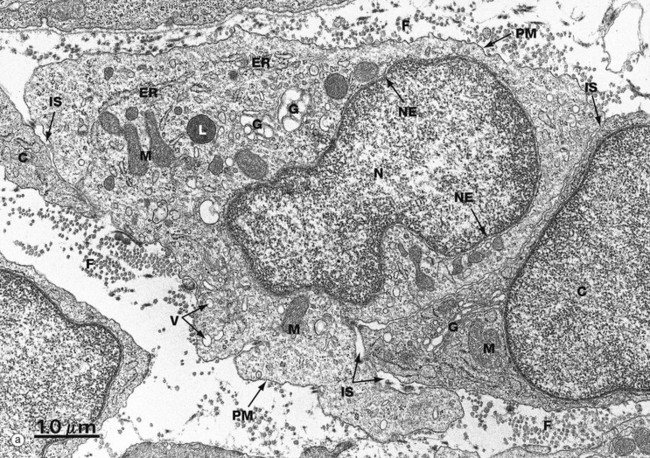
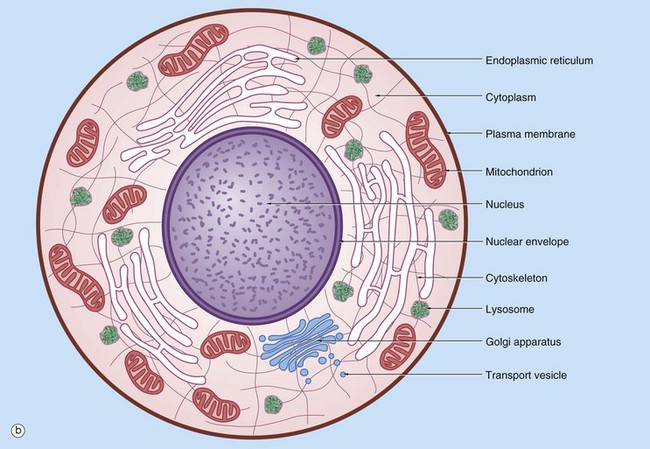
FIG. 1.1 The cell (illustration opposite)
(a) EM ×16 500 (b) Schematic diagram
The basic structural features common to all eukaryotic cells are illustrated in this electron micrograph (a) of a fibroblast and diagram (b). All cells are bounded by an external lipid membrane, called the plasma membrane or plasmalemma PM, which serves as a dynamic interface with the external environment. Most cells interact with two types of external environment: adjacent cells C and extracellular matrix as represented by collagen fibrils F. The space between cells is designated the intercellular space IS. The functions of the plasma membrane include transfer of nutrients and metabolites, attachment of the cell to adjacent cells and extracellular matrix, and communication with the external environment.
The nucleus N is the largest organelle and its substance is bounded by a membrane system called the nuclear envelope or membrane NE. The nucleus contains the genetic material of the cell in the form of deoxyribonucleic acid (DNA). The cytoplasm contains a variety of other organelles, many of which are also bounded by membranes. An extensive system of flattened membrane-bound tubules, saccules and flattened cisterns, collectively known as the endoplasmic reticulum ER, is widely distributed throughout the cytoplasm. A second discrete system of membrane-bound saccules, the Golgi apparatus G, is typically located close to the nucleus (best seen in the adjacent cell). Scattered free in the cytoplasm are a number of relatively large, elongated organelles called mitochondria M, which have a smooth outer membrane and a convoluted inner membrane system. In addition to these major organelles, the cell contains a variety of other membrane-bound structures, including intracellular transport vesicles V and lysosomes L. The cytoplasmic organelles are suspended in a gel-like medium called the cytosol, in which many metabolic reactions take place. Within the cytosol, there is a network of minute tubules and filaments, collectively known as the cytoskeleton, which provides structural support for the cell and its organelles, as well as providing a mechanism for transfer of materials within the cell and movement of the cell itself.
Thus the cell is divided into a number of membrane-bound compartments, each of which has its own particular biochemical environment. Membranes therefore serve to separate incompatible biochemical and physiological processes. In addition, enzyme systems are found anchored in membranes so that membranes are themselves the site of many specific biochemical reactions. Membrane-enclosed compartments occupy approximately half the volume of the cell.
Membrane Structure
Cell membranes, including the outer plasma membrane and the internal membranes, are composed of approximately equal amounts (by weight) of lipids and proteins. The unique properties of cell membranes allow them to separate the interior of the cell from the external milieu and the internal compartments of the cell from each other. Most of the chemical reactions of cells take place in aqueous polarised solution. The immiscibility of lipids with water leads them to form lipid bilayers, which effectively prevent passage of polarised ions and molecules; thus the contents of different compartments are kept separate and ion gradients between different compartments are maintained. Proteins embedded within the lipid bilayer act as channels to allow selective passage of particular ions and molecules. Some types of cell signalling are also mediated by membrane proteins.
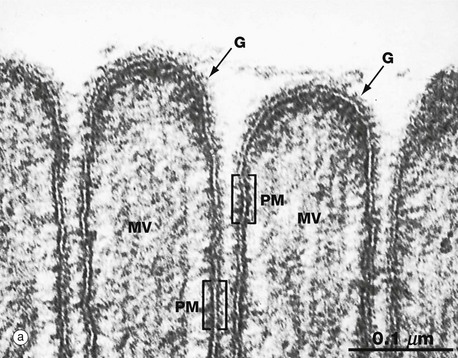
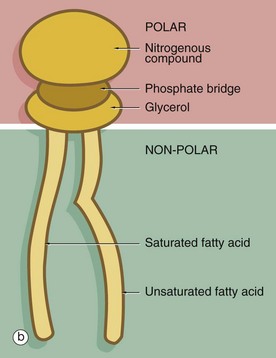
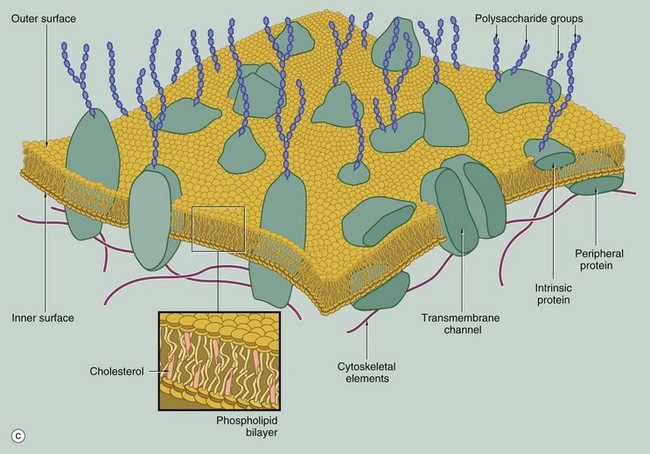
FIG. 1.2 Membrane structure (illustrations opposite)
(a) EM ×210 000 (b) Phospholipid structure (c) Membrane structure
The phospholipid molecules that make up the lipid bilayers are amphiphilic, i.e. they consist of a polar, hydrophilic (water-loving) head and a non-polar, hydrophobic (water-hating) tail. Most often, the polar heads consist of glycerol conjugated to a nitrogenous compound such as choline, ethanolamine or serine via a phosphate bridge as shown in (b). The phosphate group is negatively charged, whereas the nitrogenous group is positively charged. The non-polar tail of the phospholipid molecule consists of two long-chain fatty acids, each covalently linked to the glycerol component of the polar head. In most mammalian cell membranes, one of the fatty acids is a straight-chain saturated fatty acid, while the other is an unsaturated fatty acid which is ‘kinked’ at the position of the unsaturated bond. Sphingomyelin is another important and plentiful phospholipid in cell membranes.
Phospholipids in aqueous solution will spontaneously form a bilayer with the hydrophilic (polar) heads directed outwards and the hydrophobic (non-polar) tails forced together inwards. The weak intermolecular (non-covalent) forces that hold the bilayer together allow individual phospholipid molecules to move freely within each layer, but exchange of lipids between the two layers is uncommon. The two lipid layers of the plasma membrane have different lipid composition and the lipid composition of the cell membrane is different in different cell types. The lipid structure of membranes is not homogeneous; certain lipids, glycolipids and proteins may be transiently enriched to form a membrane or lipid ‘raft’ which is involved in various membrane functions, including the formation of caveola (see Fig. 1.11).
The fluidity and flexibility of the membrane is increased by the presence of unsaturated fatty acids, which prevent close packing of the hydrophobic tails. Cholesterol molecules are also present in the bilayer in an almost 1 : 1 ratio with phospholipids. Cholesterol molecules themselves are amphiphilic and have a kinked conformation, thus preventing overly dense packing of the phospholipid fatty acid tails while at the same time filling the gaps between the ‘kinks’ of the unsaturated fatty acid tails. Cholesterol molecules thus stabilise and regulate the fluidity of the phospholipid bilayer.
As shown in diagram (c), protein molecules are embedded within the lipid bilayer (intrinsic or integral proteins). Some of these proteins span the entire thickness of the membrane (transmembrane proteins) to be exposed to each surface, while others are embedded within the inner or outer lipid leaflet. Membrane proteins are held within the membrane by a hydrophobic zone which allows the protein to move in the plane of the membrane. The parts of these proteins protruding beyond the lipid bilayer are hydrophilic. Some membrane proteins are anchored to cytoplasmic structures by the cytoskeleton. Peripheral membrane proteins are attached to the inner or outer membrane leaflet by weak non-covalent bonds to other proteins or lipids. Membrane proteins are important in cell-cell adhesion, cell-matrix adhesion, intercellular signalling and for the formation of transmembrane channels for transport of materials into and out of the cell. In many cases, the transmembrane proteins assemble into complexes of two or more protein molecules to form a transmembrane channel or signalling complex; one example is the aquaporins which transport water molecules across the cell membrane.
On the external surface of the plasma membranes of animal cells, most of the membrane proteins and some of the membrane lipids are conjugated with short chains of polysaccharide (carbohydrate); these glycoproteins (surface mucins) and glycolipids project from the surface of the bilayer forming an outer coating, the glycocalyx, which varies in thickness in different cell types. The glycocalyx is involved in cell recognition phenomena, in the formation of intercellular adhesions and in the adsorption of molecules to the cell surface; the glycocalyx also provides mechanical and chemical protection for the plasma membrane.
The electron micrograph in (a) provides a high-magnification view of the plasma membrane PM of the minute surface projections (microvilli) MV of a lining cell from the small intestine. The characteristic trilaminar appearance is made up of two outer electron-dense layers separated by an electron-lucent layer. The outer dense layers correspond to the hydrophilic heads of phospholipid molecules, while the electron-lucent layer represents the intermediate hydrophobic layer, mainly consisting of fatty acids and cholesterol. On the external surface of the plasma membrane, the glycocalyx G is seen as a fuzzy edge to the cell membrane. This is an unusually prominent feature of small intestinal lining cells where it incorporates a variety of digestive enzymes.
Plasma membranes mediate the flow of both materials and information into and out of the cell, a function of vital importance to the cell. This topic is dealt with in detail in the section ‘Import, export and intracellular transport’ later in this chapter.
The Nucleus
The nucleus is the largest organelle in the cell and is usually the most obvious feature of the cell seen by light microscopy. The nucleus contains the genetic material of the cell, deoxyribonucleic acid (DNA), arranged in the form of chromosomes. Each chromosome contains a number of genes joined end to end, with each gene encoding the structure of a single protein according to the sequence of nucleotides along the length of the gene (see Fig. 1.6). Thus the genetic blueprint for all proteins, whether structural or enzymes, is contained within the nucleus of every cell in the body except for red blood cells, which have no nucleus (see Ch. 3). The substance of the nucleus is known as the nucleoplasm and it is surrounded by the nuclear membrane. The first step in cell division is replication of the DNA so that a copy of the genome goes to each of the daughter cells. Cell division is the subject of Ch. 2.
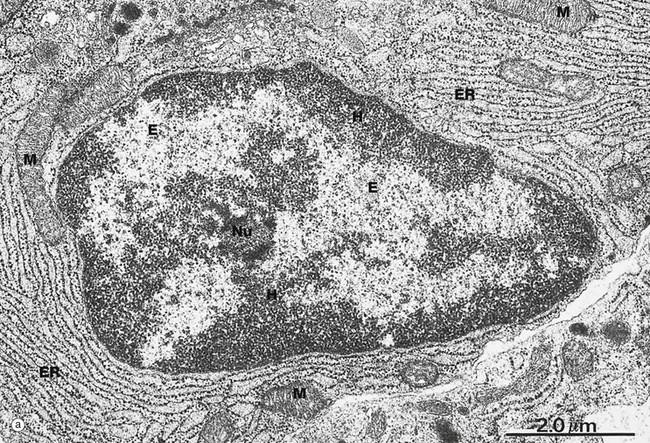
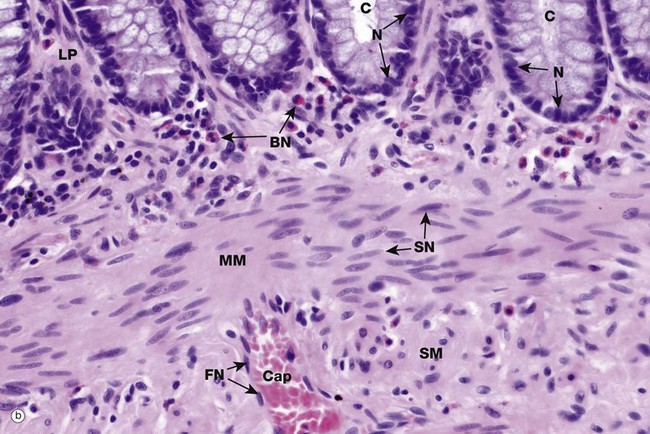
FIG. 1.3 Nucleus (illustrations opposite)
(a) EM ×15 000 (b) H&E (HP)
Micrograph (a) illustrates the nucleus of a plasma cell, a type of cell that secretes large amounts of a protein called antibody. Typical of protein-secreting cells, the cytoplasm contains plentiful ribosome-studded (rough) endoplasmic reticulum ER as well as mitochondria M, which produce the energy required for such a metabolically active cell.
The nucleus contains DNA (making up less than 20% of its mass), protein called nucleoprotein and some ribonucleic acid (RNA). Nucleoprotein is of two major types: low molecular weight, positively charged histone proteins and non-histone proteins. Histone proteins form a protein core around which the chromosome is coiled to form nucleosomes and control the uncoiling and expression of the genes encoded by the DNA strand. Non-histone proteins include all the enzymes for the synthesis of DNA and RNA and other regulatory proteins. All nucleoproteins are synthesised in the cytoplasm and imported into the nucleus. Nuclear RNA includes newly synthesised messenger, transfer and ribosomal RNA (mRNA, tRNA and rRNA, respectively) that has not yet passed into the cytoplasm. Control of DNA transcription is mediated by a variety of small RNA molecules including micro RNA (miRNA), small nuclear RNA (snRNA) and small interfering RNA (siRNA).
Except during cell division, the chromosomes, each a discrete length of DNA with bound histone proteins, exist as coiled and supercoiled strands that cannot be visualised individually. Nuclei are heterogeneous structures with electron-dense (dark, see App. 1) and electron-lucent (light) areas. The dense areas, called heterochromatin H, consist of tightly coiled inactive chromatin found in irregular clumps, often around the periphery of the nucleus. In females, the inactivated X-chromosome may form a small discrete mass, the Barr body. Barr bodies are occasionally seen at the edge of the nucleus in female cells when cut in a favourable plane of section. The electron-lucent nuclear material, called euchromatin E, represents that part of the DNA that is active in RNA synthesis. The nucleolus Nu is also evident (see Fig. 1.5). The name chromatin is derived from the strong colour of nuclei when stained for light microscopy. The chromatin is a highly organised but dynamic structure, with individual chromosomes tending to clump in particular areas of the nucleus, known as chromosome territories. Segments of the chromosome are coiled and uncoiled as different genes are brought into contact with the enzymes that make the RNA copy of the DNA, i.e. transcription. Histone proteins also exist as variant forms or can be chemically modified in ways that promote or suppress expression of a particular gene. Permanent switching on or off of a particular set of genes leads to differentiation of the cell.
The shape, appearance and position of cell nuclei can be very helpful in identifying particular cell types. Micrograph (b) shows part of the wall of the colon (see also Fig. 14.29), which has been stained with haematoxylin and eosin (H&E, see Appendix 2), the ‘standard’ histological staining method. Haematoxylin is blue in colour and eosin is pink. Haematoxylin, a basic dye which binds to negatively charged DNA and RNA, stains nuclei dark blue. Eosin, an acidic dye, has affinity for positively charged structures such as mitochondria and many other cytoplasmic constituents and so the cytoplasm is stained pink. This micrograph shows the characteristic appearances of the nuclei of various cell types. At the top of the image, the bases of the colonic crypts C can be seen. The epithelial cells forming the crypts have round to ovoid nuclei N, typical of epithelial cells. Note also that the nuclei are positioned at the bases of the cells while the superficial cytoplasm is filled with mucin; the position of the nucleus within a cell, the polarity, may also be highly characteristic. Deep to the crypts, running across the centre of the image, is the muscularis mucosae MM, which is composed of smooth muscle cells. Smooth muscle cell nuclei SN are elongated with rounded ends, often called spindle shaped. This is of course only apparent if they are cut in the right plane of section; if cut perpendicular to the long axis of the cell, they appear rounded (see also Fig. 6.15). The nuclei are typically placed in the centre of the cell, although this is not always easy to see as the cell borders are indistinct. Eosinophils scattered within the lamina propria LP have a unique bilobed nuclear form BN which, together with the prominent coral red granules in the cytoplasm, makes identification easy. Note again that if the plane of section is unfavourable, the bilobed structure of the nucleus is not apparent. A very small blood vessel, a capillary Cap, is seen in the submucosa SM and the flattened nuclei FN of the endothelial cells can be identified; the cytoplasm of these cells is so thin that it cannot be seen at this magnification.
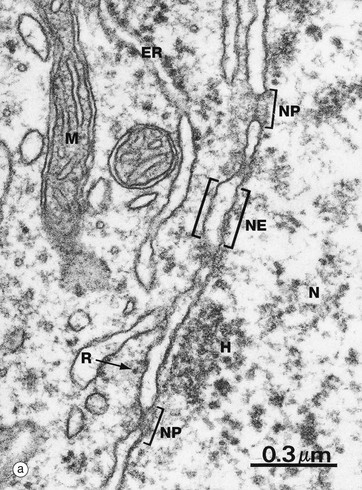
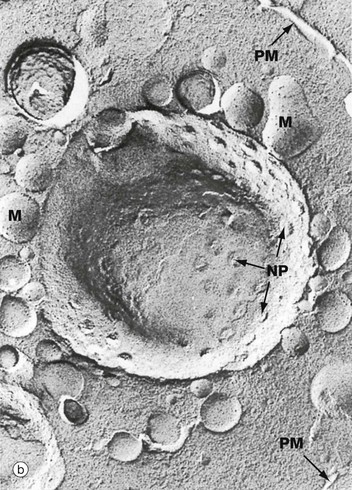
FIG. 1.4 Nuclear envelope
(a) EM ×59 000 (b) Freeze-etched preparation, SEM ×34 000
The nuclear envelope NE, which encloses the nucleus N, consists of two lipid bilayers with the intermembranous or perinuclear space between. The inner and outer nuclear membranes have the typical phospholipid bilayer structure but contain different integral proteins. The outer lipid bilayer is continuous with the endoplasmic reticulum ER and has ribosomes R on its cytoplasmic face. The intermembranous space is continuous with the lumen of the endoplasmic reticulum. On the inner aspect of the inner nuclear membrane, there is an electron-dense layer of intermediate filaments, the nuclear lamina, consisting of intermediate filaments called lamins that link inner membrane proteins and heterochromatin H.
The nuclear envelope contains numerous nuclear pores NP, at the margins of which the inner and outer membranes become continuous. Each pore contains a nuclear pore complex, an elaborate cylindrical structure consisting of approximately 30 proteins known as nucleoporins, forming a central pore approximately 125 nm in diameter. Nuclear pores permit and regulate the exchange of metabolites, macromolecules and ribosomal subunits between nucleus and cytoplasm. Ions and small molecules diffuse freely through the nuclear pore. Larger molecules, such as mRNA moving from nucleus to cytoplasm and histones from cytoplasm to nucleus, dock to the nuclear pore complex by means of a targeting sequence and are moved through the pore by an energy-dependent process. The nuclear pore complex may also hold together the two lipid bilayers of the nuclear envelope. Note that mitochondria M are also identifiable in the cytoplasm.
Micrograph (b) shows an example of a technique called freeze-etching. Briefly, this method involves the rapid freezing of cells which are then fractured. Internal surfaces of the cell are exposed at random, the fracture lines tending to follow natural planes of weakness. Surface detail is obtained by ‘etching’ or sublimating excess water molecules from the specimen at low temperature. A thin carbon impression is then made of the surface and this mirror image is viewed by scanning electron microscopy. Freeze-etching provides a valuable tool for studying internal cell surfaces at high resolution. In this preparation, the plane of cleavage has included part of the nuclear envelope in which nuclear pores NP are clearly demonstrated. Note also the outline of the plasma membrane PM and mitochondria M.
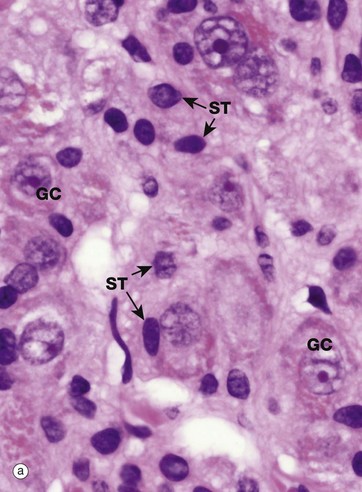
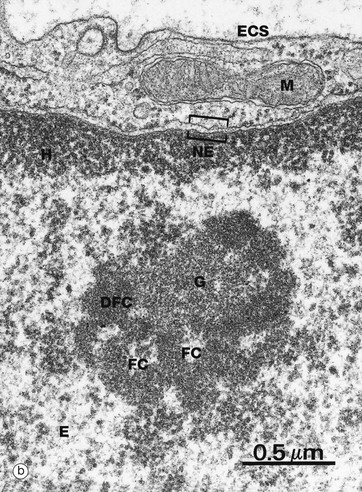
FIG. 1.5 The nucleolus
(a) H&E (HP) (b) EM ×37 000
Most nuclei contain a dense structure called the nucleolus, which is the site of ribosomal RNA (rRNA) synthesis and ribosome assembly. Transfer RNA (tRNA) is also processed in the nucleolus. More recently discovered roles include control of the cell cycle and stress responses. The nucleolus may be very prominent in some cell types and quite inconspicuous in others, as shown in micrograph (a) which is a high-power photomicrograph of an autonomic ganglion (see also Figs 7.21 and 7.22). In this micrograph, the nuclei of the ganglion cells GC contain large purple nucleoli, while the smaller sustentacular cell nuclei ST have small nucleoli that are only just visible at this magnification.
Furthermore, the nucleolus may change appearance depending on the state of the cell, so that an inactive fibroblast usually has a very small nucleolus while an activated fibroblast, for instance in a healing wound, has a prominent nucleolus. Remember that this is a thin slice of the tissue and that the plane of section does not go through the nucleolus of every cell, so that some nuclei appear to lack nucleoli.
The nucleolus is not membrane bound but consists of an aggregate of ribosomal genes, newly synthesised rRNA, ribosomal proteins and ribonucleoproteins. The ribosomal genes are found on five chromosomes and are called the nucleolar organiser regions (NORs). The rRNA is transcribed from the DNA template and then modified in the nucleolus and combined with ribosomal proteins. The subunits then pass back to the cytoplasm through the nuclear pore complex (NPC) to aggregate into complete ribosomes when bound to an mRNA molecule. Micrograph (b) is a high-power electron micrograph of a typical nucleolus. Nucleoli can be variable in appearance, but most contain dense fibrillar components DFC and paler fibrillar centres FC surrounded by the granular component G. The fibrillar components are the sites of ribosomal RNA synthesis, while ribosome assembly takes place in the granular components. Note also euchromatin E and heterochromatin H within the nucleus, which is bounded by the nuclear envelope NE. A thin rim of cytoplasm containing a mitochondrion M separates the nucleus from the extracellular space ECS.
Protein Synthesis
Proteins are not only a major structural component of cells, but, as enzymes, transport and regulatory proteins, they mediate many metabolic processes. The nature and quantity of proteins within a cell determine its activity and thus the study of proteomics can be very informative about the functions of a particular cell. All cellular proteins are replaced continuously. Many cells also synthesise proteins for export, including glandular secretions and extracellular structural proteins like collagen. Protein synthesis is therefore an essential and continuous activity of all cells and the major function of some cells. The ribosome is the protein factory of the cell. Every cell contains within its DNA the code for every protein that individual could produce. Production or expression of selected proteins only is characteristic of differentiated cells.
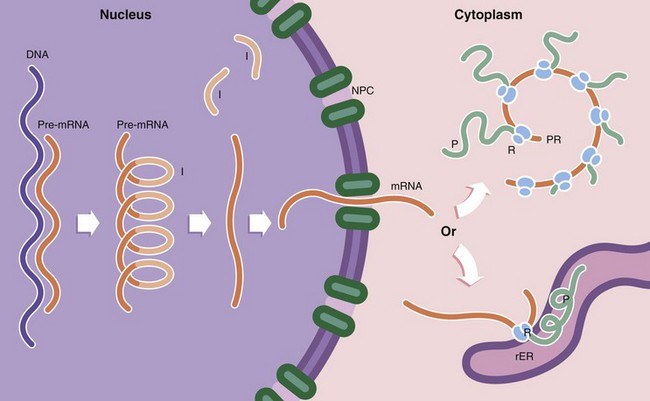
FIG. 1.6 Protein synthesis and degradation
Protein synthesis occurs in several steps. First, the DNA template (the gene) of a particular protein is copied to form a complementary pre–messenger RNA (pre-mRNA) copy, a process known as transcription. Post-transcriptional processing of the mRNA results in excision of introns I (non-coding regions of the mRNA strand). This step is controlled by small nuclear RNAs (snRNA) which in combination with various proteins form the spliceosome. The messenger RNA (mRNA) then passes through the nuclear pore complex NPC into the cytoplasm. Here the mRNA binds to ribosomes R, organelles that synthesise proteins using the mRNA strand as a template to determine the specific amino acid sequence of the protein; this is known as translation. Ribosomes, which are synthesised in the nucleolus, comprise two subunits of unequal size. Each subunit consists of a strand of RNA, ribosomal RNA (rRNA) molecules, with associated ribosomal proteins forming a globular structure. Ribosomes align mRNA strands so that transfer RNA (tRNA) molecules may be brought into position and their amino acids added sequentially to the growing polypeptide chain P. Some of the RNA molecules in ribosomes catalyse peptide bond formation between amino acids and are sometimes called ribozymes to indicate this enzymatic function. Most enzymes are proteins. Thus the DNA code is converted first into RNA and then into a specific protein. Ribosomes are often found attached to mRNA molecules in small circular aggregations called polyribosomes or polysomes PR, formed by a single strand of mRNA with ribosomes attached along its length. Each ribosome in a polyribosome is making a separate molecule of the protein.
Ribosomes and polyribosomes may also be attached to the surface of endoplasmic reticulum. The ER consists of an interconnecting network of membranous tubules, vesicles and flattened sacs (cisternae) which ramifies throughout the cytoplasm. Much of its surface is studded with ribosomes, giving a ‘rough’ appearance, leading to the name rough endoplasmic reticulum rER. Proteins destined for export, as well as lysosomal proteins, are synthesised by ribosomes attached to the surface of the rER and pass through the membrane into its lumen. Integral membrane proteins are also synthesised on rER and inserted into the membrane at this point, the extracellular part of the protein protruding into the lumen of the rER and the intramembranous part held firmly in place by hydrophobic attraction. It is within the rER that many proteins are folded to form their tertiary structure, intrachain disulphide bonds are formed and the first steps of glycosylation take place. In contrast, proteins destined for the cytoplasm, nucleus and mitochondria are synthesised on free ribosomes and folding and other post-translational modifications take place there.
Proteins that are damaged or no longer required by the cell are degraded to short peptides. The first step in this process is binding of the protein ubiquitin to the damaged protein. This acts as a signal that allows the protein to be taken up by a proteasome. Proteasomes are non–membrane bound arrays of proteolytic enzymes that are plentiful in all cells. Other proteins are degraded by proteolytic enzymes within lysosomes.
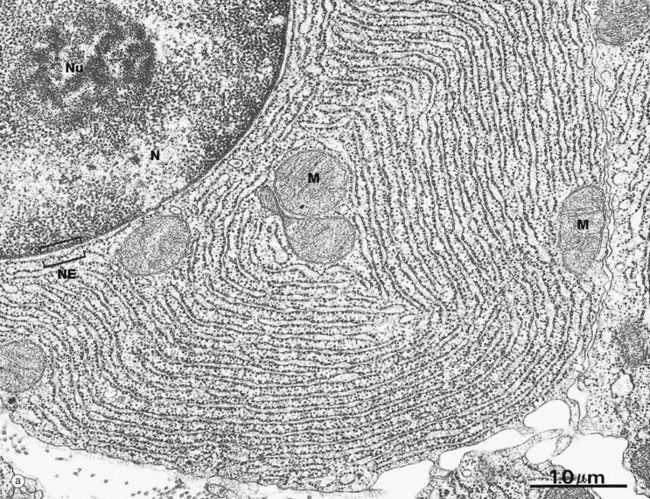
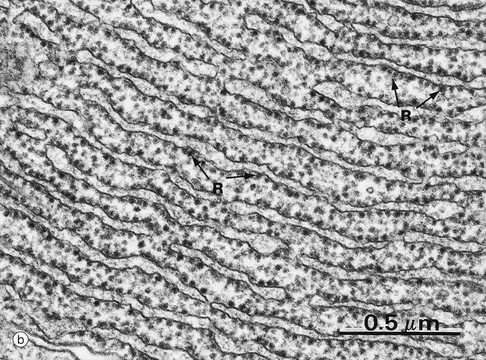
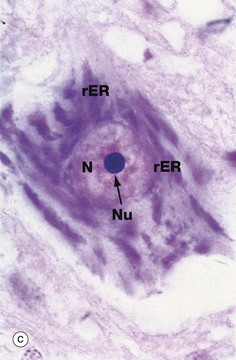
FIG. 1.7 Rough endoplasmic reticulum
(a) EM ×23 000 (b) EM ×50 000 (c) Cresyl violet (HP)
These micrographs illustrate rough endoplasmic reticulum rER in a cell specialised for the synthesis and secretion of protein; in such cells, rER tends to be profuse and to form closely packed parallel laminae of flattened cisternae. In micrograph (a), the dimensions of the rER can be compared with that of mitochondria M and the nucleus N. The nucleus typically contains a prominent nucleolus Nu. Note the close association between the rER and the outer lipid bilayer of the nuclear envelope NE with which it is in continuity. The chromatin in the nucleus is mainly dispersed (euchromatin), consistent with prolific protein synthesis.
Micrograph (b) shows part of the rER at high magnification. Numerous ribosomes R stud the surface of the membrane system and there are plentiful ribosomes lying free in the intervening cytosol. Micrograph (c) shows a nerve cell at high magnification stained by the basophilic dye cresyl violet. The basophilic clumps in the cytoplasm represent areas of plentiful rER. The nuclear envelope can be distinguished due to the basophilia of the numerous ribosomes that stud its outer surface. The nucleus N contains a prominent nucleolus Nu and dispersed chromatin.
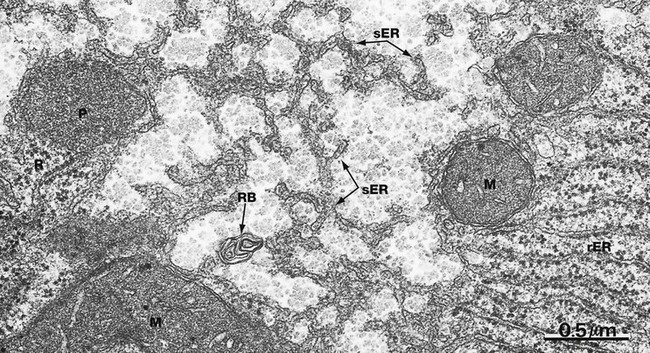
FIG. 1.8 Smooth endoplasmic reticulum
EM ×40 000
Smooth endoplasmic reticulum sER is continuous with and similar to rER except that it lacks ribosomes. The principal functions of smooth endoplasmic reticulum are lipid biosynthesis and membrane synthesis and repair. Fatty acids and triglycerides are mostly synthesised within the cytosol, whereas cholesterol and phospholipids are synthesised in sER. In liver cells, smooth endoplasmic reticulum is rich in cytochrome P450 and plays a major role in the metabolism of glycogen and detoxification of various noxious metabolic by-products, drugs and alcohol. In most cells, sER is involved in the storage and release of Ca2+ ions, an important mechanism of cell signalling. In muscle cells, where it is called sarcoplasmic reticulum, release and reuptake of Ca2+ ions activates the contractile mechanism (see Ch. 6).
Most cells contain only scattered elements of sER interspersed with the other organelles. Cell types with prominent sER include liver cells and those cells specialised for lipid biosynthesis, such as the steroid hormone-secreting cells of the adrenal glands and the gonads. In this micrograph from the liver, most of the membranous elements are sER, but it is continuous with rough endoplasmic reticulum rER in the lower right of the field. This field also includes several mitochondria M, a peroxisome P (see Fig. 1.24), free ribosomes and polyribosomes R and a whorl of membrane in a residual body RB (see Fig. 1.11).
Import, Export and Intracellular Transportation
Movement of materials into and out of cells and between separate compartments of a cell involves crossing lipid membranes. The plasma membrane thus controls the interaction of the cell with the external environment, mediating the exchange of nutrients and waste products, secretions and signalling mechanisms. Lipid membranes also separate different compartments within the cell, many of which contain mutually incompatible biochemical reactions. For instance, the process of protein synthesis and export which takes place in the rough endoplasmic reticulum and Golgi apparatus must be kept separate from the garbage disposal and recycling plant, the lysosome. Likewise, microorganisms phagocytosed by cells must be killed and disposed of without damage to normal structures and mechanisms.
Information must also cross membranes, telling the cell when to divide, release secretions, contract or perform many other functions. Many of the mechanisms used for transport of cargo also serve to transmit messages to the interior of the cell. There are also dedicated mechanisms for the transfer of information, such as the transient depolarisation of the plasma membrane along the length of a nerve axon in the conduction of a nerve impulse. Histologically, these transport processes can only be observed indirectly: for example, cells suspended in hypotonic solutions swell due to passive uptake of water, whereas cells placed in hypertonic solutions tend to shrink due to outflow of water. Radioisotope labelling techniques can be used to follow active transport processes. Bulk transport, however, is readily observable by microscopy (see Fig. 1.12). Both active and passive transport processes are enhanced if the area of the plasma membrane is increased by folds or projections of the cell surface, as exemplified by the absorptive cells lining the small intestine (see Fig. 1.2).
The main mechanisms by which materials and information are transported across cell membranes are:
• Passive diffusion. This type of transport is dependent on the presence of a concentration gradient across the membrane and also on the size and polarity of the molecule. Lipids and lipid-soluble molecules such as the hormones oestrogen and testosterone pass freely through lipid membranes, as do gases such as oxygen, nitrogen and carbon dioxide. Uncharged but polar small molecules such as water and urea diffuse through lipid membranes slowly, but charged molecules such as sodium (Na+) and potassium (K+) ions diffuse through very slowly indeed.
• Facilitated diffusion. This type of transport involves the movement of hydrophilic molecules such as water, ions, glucose and amino acids. This process is strictly passive, moving polar or charged substances along an electrochemical gradient, but requires the presence of protein carrier molecules. There are two types of protein carrier molecule: the first type (known as pores or channels) form a water-filled channel across the membrane through which selected molecules or ions can pass depending on concentration, size and electrical charge, while the second type binds a particular molecule or ion and then undergoes a change in conformation, moving the substrate to the other side of the membrane (a transporter or carrier). Aquaporins, which allow water to cross membranes at a much faster rate than by diffusion alone, are an important and common example of a transmembrane channel. There are many different aquaporin molecules, some of which are highly specific for water molecules, while others allow the passage of other small molecules such as urea or glycerol. Some facilitated diffusion pores are gated, which means that the pore is open or closed depending on different physiological conditions (e.g. open only at a particular pH).
• Active transport. This mode of transport is not only independent of electrochemical gradients, but also often operates against extreme electrochemical gradients. The classic example of active transport is the continuous movement of Na+ out of the cell and K+ into the cell by the Na+-K+ pump, which moves Na+ ions out of the cell and K+ ions into the cell across the plasma membrane. Adenosine triphosphate (ATP) is converted to adenosine diphosphate (ADP) in the process to generate the energy required, hence the name Na+-K+ ATPase.
• Bulk transport. Transport of large molecules or small particles into, out of or between compartments within the cell is mediated by subcellular, transient membrane-bound vesicles. These vesicles transport proteins embedded in the membrane of the vesicle (e.g. proteins destined for the plasma membrane) and soluble cargo within the lumen. Transport vesicles are formed by the assembly of a protein ‘coat,’ leading to budding of a section of membrane which is pinched off to form a vesicle. At their destination, the reverse process takes place when the transport vesicle fuses with the target membrane, incorporating into it and releasing its contents. These mechanisms are dependent on the fluidity and deformability of lipid membranes and the mobility of intrinsic membrane proteins within the plane of the membrane. Specific examples such as endocytosis, exocytosis and intracellular transport vesicles are given in Figs 1.9–1.12.
• Transmembrane signalling. There are various ways in which signals may cross a plasma membrane to deliver information to a cell. One example is lipid-soluble molecules, such as the hormone oestrogen, which diffuse through the plasma membrane to bind to an intracellular receptor. Non–lipid soluble molecules, such as the hormone insulin, bind to a protein receptor embedded in the plasma membrane, which is thus activated, and pass the signal on to an intracellular signalling pathway. Other signalling molecules, such as neurotransmitters at nerve synapses (see Ch. 7), bind to an ion channel in the postsynaptic membrane, allowing ions to enter the cell and initiating depolarisation of the membrane.
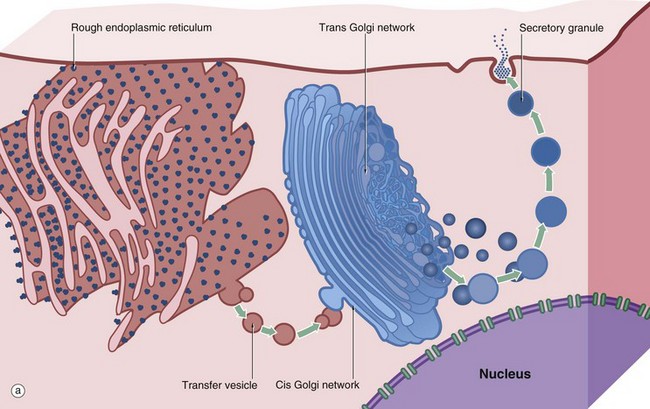
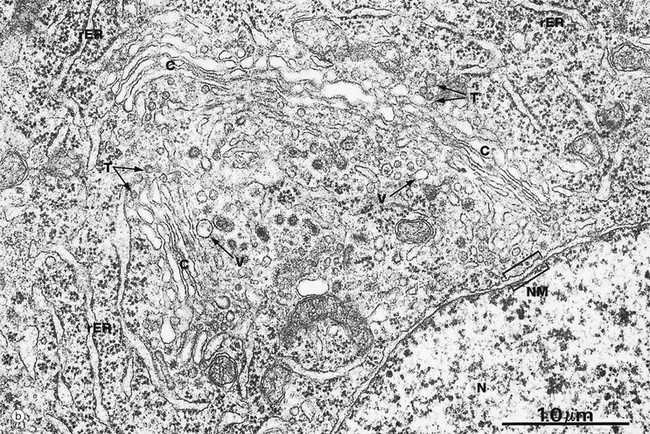
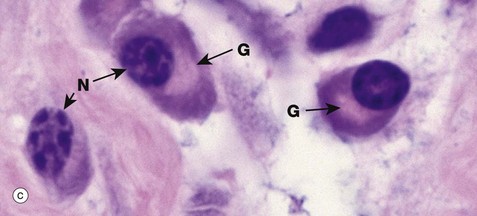
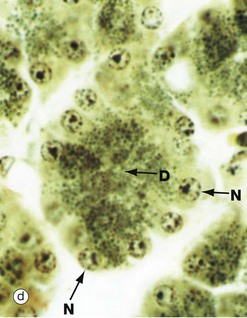
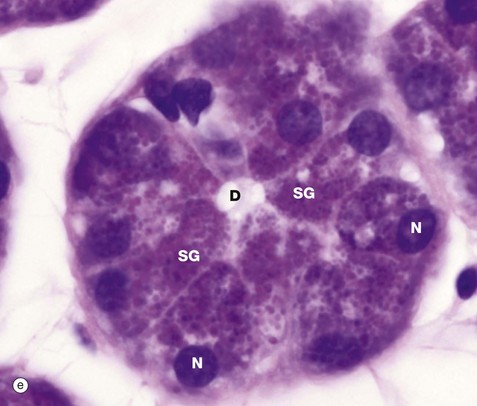
FIG. 1.9 Golgi apparatus (illustrations (b) to (e) opposite)
(a) Schematic diagram (b) EM ×30 000 (c) H&E (HP) (d) Iron haematoxylin (HP) (e) H&E (HP)
The Golgi apparatus, complex or stack is an important site of protein and lipid glycosylation, as well as the site of synthesis of many glycosaminoglycans that form the extracellular matrix. Diagram (a) illustrates the main structural features of the Golgi apparatus and summarises the mechanism by which secretory products are packaged within membrane-bound vesicles. A cell may contain one or more Golgi stacks, and these may break up and reform during different phases of the cell cycle or in different physiological states. The Golgi apparatus consists of 4 to 6 saucer-shaped membrane-bound cisternae. The outermost cisternae take the form of a network of tubules known as the cis and trans Golgi network (CGN and TGN, respectively). Proteins synthesised in the rER are transported to the Golgi apparatus in coated vesicles (see also Fig. 1.10); the coat protein in this case is known as coat protein complex II (COP II). Soon after the coated vesicles bud off from the rER, the coat proteins disengage and are recycled. On arrival at the convex forming face or CGN, the vesicles fuse with the CGN. In the Golgi apparatus the glycosylation of proteins, begun in the rER, is completed by sequential addition of sugar residues and the proteins are packaged for transport to their final destination. Each cisterna is enriched for the specific enzyme to add a specific sugar.
There appear to be two mechanisms by which proteins pass through the Golgi apparatus. In the first, proteins are passed from cisterna to cisterna in coated vesicles (COP I in this instance). However, for very large proteins such as collagen rods, the medial cisternae mature, with specific enzymes being moved backwards to less mature cisternae by coated vesicles. On arrival at the concave maturing face or TGN, the proteins are accurately sorted into secretory vesicles destined for the extracellular space (e.g. hormones, neurotransmitters, collagen) or the plasma membrane (e.g. cell surface receptors, adhesion molecules) or intracellular organelles such as lysosomes. The sorting of cargo into secretory vesicles is dependent on binding of specific adapter molecules to the cargo, which then bind to specific coat proteins. Secretory vesicles become increasingly condensed as they migrate through the cytoplasm to form mature secretory granules, which are then liberated at the cell surface by exocytosis. A group of membrane proteins called SNAREs regulate docking and fusion of coated vesicles to their target membrane.
Micrograph (b) illustrates a particularly well-developed Golgi apparatus. Transfer vesicles T and elements of the rough endoplasmic reticulum rER are seen adjacent to the forming face. A variety of larger vesicles V can be seen in the concavity of the maturing face, some of which appear to be budding from the Golgi cisternae C; such vesicles could be either secretory granules or lysosomes. Note the proximity of the Golgi apparatus to the nucleus N. The nuclear membrane NM is particularly well demonstrated in this micrograph.
Micrograph (c) illustrates a group of plasma cells from inflamed tissue; these cells are responsible for antibody production as part of the body's immune defences (see Ch. 11). The plentiful rER is strongly basophilic and the protein is acidophilic so that there is staining with both eosin and haematoxylin, giving a purple or amphiphilic colour to the cytoplasm. The well-developed Golgi complex G consists of lipid (membranes), which is dissolved out during preparation. Thus the Golgi is unstained and appears as a pale area (negative image) adjacent to the nucleus N.
Micrograph (d) demonstrates secretory granules in the acinar cells of the pancreas, which secretes digestive enzymes. The secretory cells are grouped around a minute central duct D and the secretory granules, which are stained black, are concentrated towards the luminal aspect of the cell. The nuclei N are arranged around the periphery of the secretory unit. Micrograph (e) demonstrates a very similar secretory acinus in a salivary gland. The purple-stained secretory granules SG are seen in the superficial cytoplasm of the cells towards the central duct D. The nuclei N are pushed towards the periphery of the cells.
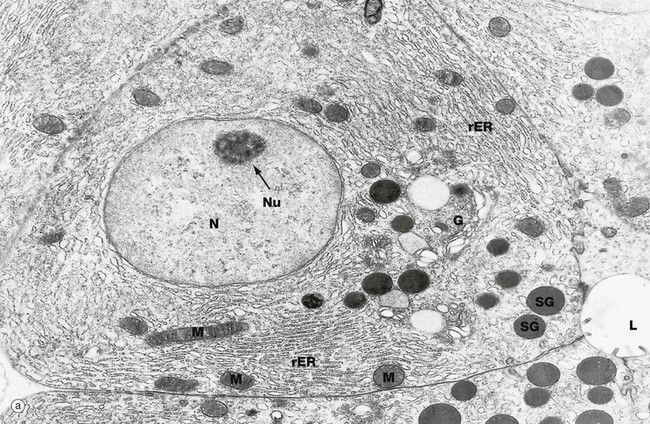

FIG. 1.10 Exocytosis
(a) EM ×14 000 (b) EM ×41 500
Micrographs (a) and (b) illustrate typical protein-secreting cells from the pancreas, which produces digestive enzymes. All cells undergo continuous exocytosis (constitutive secretion) but, in specialised secretory cells, there is also signal-dependent exocytosis (regulated secretion), as in this case where digestive enzymes are secreted in response to food in the duodenum. In micrograph (a) the nucleus N has dispersed chromatin and a prominent nucleolus Nu. The rough endoplasmic reticulum rER and Golgi apparatus G are prominent. Mitochondria M supply energy. Small secretory vesicles leave the Golgi apparatus as coated vesicles (clathrin-coated in this case) but soon uncoat and may fuse together to form larger vesicles. Vesicles are moved towards the plasma membrane of the cell along microtubules (see Fig. 1.16). Immature secretory vesicles (or granules) SG become increasingly electron dense as they approach the glandular lumen L, due to concentration of their contents and recycling of membrane back to the Golgi apparatus. When the cell receives a signal to secrete, the secretory granules dock with the plasma membrane, forming a transient opening (porosome) through which the secretory product is discharged. The vesicle membrane is merged into the plasma membrane but is later recycled by endocytosis to maintain the normal cell size.
Micrograph (b) shows secretory granules SG approaching the apices of two pancreatic secretory cells and converging on a tiny excretory duct formed by junctional complexes JC (see Fig. 5.9) joining adjacent cells. Short microvilli MV protrude into the excretory duct.
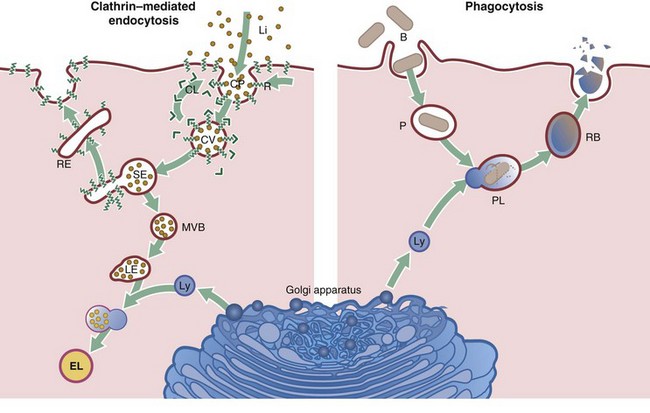
FIG. 1.11 Endocytosis
Cells take up particulate matter and large macromolecules by a variety of processes collectively known as endocytosis. The best known of these mechanisms is phagocytosis, which is used by specialised phagocytic cells to ingest particulate matter, usually larger than 0.5 µm, such as bacteria, fungi and apoptotic cells. Pinocytosis is used by all cells to take up fluid and solutes. Several mechanisms of pinocytosis are known, including clathrin-mediated pinocytosis, caveolae-mediated pinocytosis (see Fig. 6.19) and macropinocytosis. This diagram summarises the main steps of clathrin-mediated pinocytosis and phagocytosis.
Clathrin-mediated endocytosis
Clathrin-mediated endocytosis takes place continuously, with clathrin-coated pits CP constantly forming and pinching off to form coated vesicles. Clathrin CL binds to specialised areas of the plasma membrane and shapes them into vesicles. Thus the cell takes up extracellular fluid and molecules. In addition, specific molecules (ligands Li) that bind to cell surface receptors R are taken into the cell by this mechanism. A well-known example is the low-density lipoprotein (LDL) receptor, an intrinsic membrane protein with extracellular and cytoplasmic domains. Receptors with bound ligand concentrate into the coated pit by diffusion in the plane of the membrane. The coated pit then buds off to form a coated vesicle CV. Many different types of receptors may be found in a single clathrin-coated pit, although only one type is shown here for simplicity. The vesicles very quickly lose their coat and fuse with sorting endosomes SE, which are dynamic tubulovesicular structures, usually found close to the plasma membrane. The acid pH in the lumen of sorting endosomes encourages dissociation of receptor and ligand; these are then quickly separated so that most of the membrane and its intrinsic receptors are shuttled to recycling endosomes RE and from there back to the cell surface. Some membrane receptors may go through this cycle up to 300 times and the expression of receptors on the cell surface can be regulated by this mechanism. The remaining part of the sorting endosome, which contains the unbound LDL, converts into a multivesicular body MVB. Multivesicular bodies are moved towards the Golgi apparatus where they become late endosomes LE and fuse with lysosomes Ly. Degradative enzymes within the lysosomes, now called endolysosomes EL, digest the protein component of the LDL, freeing cholesterol for incorporation into membranes.
Vesicles leaving the sorting endosome may also migrate to another part of the cell membrane, such as the basal membrane of an epithelial cell. There, the vesicle fuses with the plasma membrane, releasing its contents to the extracellular space; this process is called transcytosis and is important, for instance, in the gastrointestinal tract for absorption of nutrients from food.
Phagocytosis
Bacteria B are taken up by specialised phagocytic cells, such as neutrophils and macrophages, by phagocytosis. The bacterium binds to cell surface receptors, triggering the formation of pseudopodia that extend around the organism until they fuse, leaving the engulfed bacterium in a membrane-bound phagosome P within the cytoplasm. At this stage, recycling of membrane and receptors back to the plasma membrane takes place. The phagosome then fuses with a lysosome Ly to become a phagolysosome PL (or secondary lysosome) and the bacterium is subjected to the toxic activities of the lysosomal enzymes. These enzymes also break down the components of the dead bacteria, which may be released into the cytoplasm, expelled from the cell by exocytosis or remain in the cytoplasm as a residual body RB.
Lysosomes are also involved in the degradation of cellular organelles, many of which have only a finite lifespan and are therefore replaced continuously; this lysosomal function is termed autophagy. Most autophagocytic degradation products accumulate and become indistinguishable from the residual bodies of phagocytosis. With advancing age, residual bodies accumulate in the cells of some tissues and appear as brown lipofuscin granules (see Fig. 1.25).
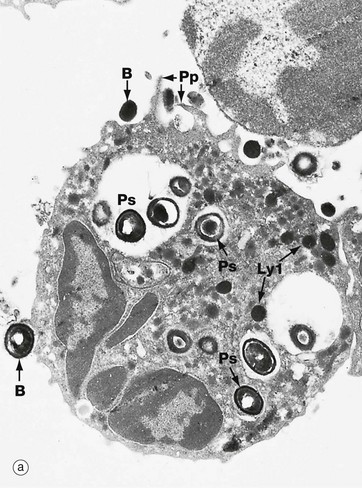
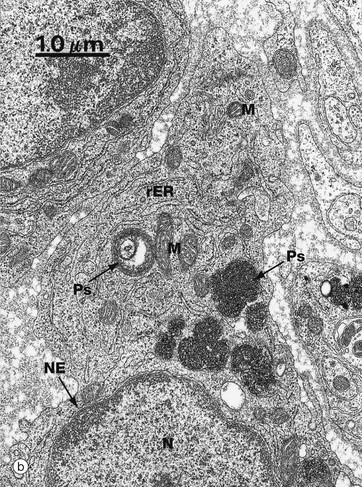
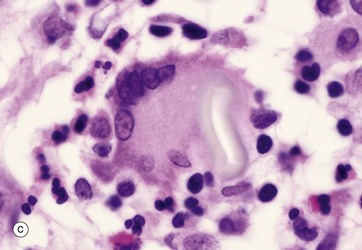
FIG. 1.12 Phagocytosis
(a) EM ×11 750 (b) EM ×14 000 (c) H&E (HP)
Micrograph (a) illustrates a professional phagocytic white blood cell, a neutrophil polymorph (see Ch. 3), in the process of engulfing and destroying bacteria B. Note the manner in which pseudopodia Pp embrace the bacteria before engulfment. Note also phagosomes Ps containing bacteria in various stages of degradation. Several lysosomes Ly1 are also visible.
Micrograph (b) is a high-power view of phagosomes Ps in the cytoplasm of a macrophage, another professional phagocyte found in almost all tissues. The large, irregularly shaped membrane-bound phagosomes contain coiled fragments of plasma membrane and other cellular constituents derived from damaged cells. This macrophage is performing its function as a scavenger cell by phagocytosing dead and damaged cells and recycling their components. Note also the cell nucleus N with its easily identified nuclear envelope NE, as well as mitochondria M and rough endoplasmic reticulum rER.
Micrograph (c) shows a light micrograph of a similar process at a site of inflammation (a healing scar in this case). The large cell in the centre is a multinucleate giant cell GC (a modified macrophage) that has phagocytosed a fragment of suture material used to suture the wound. The fragment of suture material S is easily seen as a pale, elongated shape within the giant cell. Collections of such multinucleate giant cells are often seen at sites where foreign material has entered the tissues.
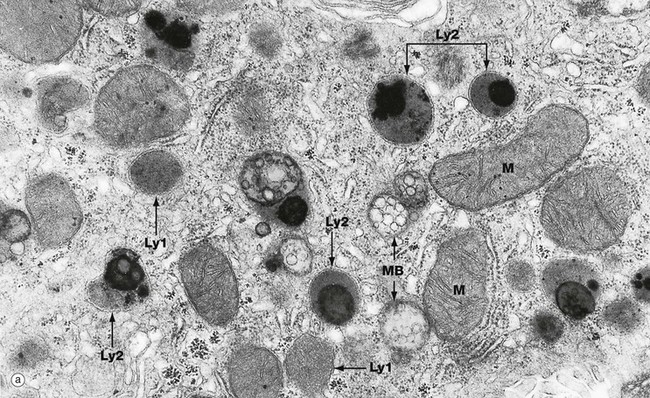
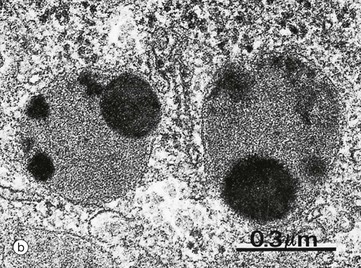
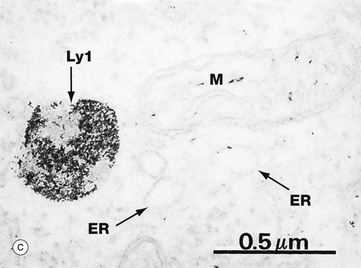
FIG. 1.13 Lysosomes
(a) EM ×27 000 (b) EM ×60 000 (c) Histochemical method for acid phosphatase, EM ×50 000
Lysosomes are the site of degradation of material taken up into the cells by phagocytosis or endocytosis and of old or unnecessary cellular constituents (autophagy). These micrographs show the typical features of lysosomes and residual bodies. Micrograph (a) shows part of the cytoplasm of a liver cell. Lysosomes Ly1 vary greatly in size and appearance but are recognised as membrane-bound organelles containing an amorphous granular material. Phagolysosomes or secondary lysosomes Ly2 are even more variable in appearance but are recognisable by their diverse particulate content, some of which is extremely electron-dense. The distinction between residual bodies and secondary lysosomes is often difficult. Late endosomes or multivesicular bodies MB are also seen in this micrograph. Note the size of lysosomes relative to mitochondria M.
Micrograph (b) shows two phagolysosomes at higher magnification, allowing the limiting membrane to be visualised. Both contain electron-dense particulate material and amorphous granular material.
The lysosomal enzymes comprise more than 40 different degradative enzymes including proteases, lipases and nucleases. These are collectively known as acid hydrolases because they are optimally active at a pH of about 5.0. This protects the cell should lysosomal enzymes escape into the cytosol where they would be inactive at the higher pH. Histochemical methods can be used to demonstrate sites of enzyme activity within cells and thus act as markers for organelles that contain these enzymes. Such a method has been used in micrograph (c) to demonstrate the presence of acid phosphatase, a typical lysosomal enzyme; enzyme activity is represented by the electron-dense area within a lysosome Ly1. Other organelles remain unstained, but the outline of a mitochondrion M and profiles of endoplasmic reticulum ER can nevertheless be identified.
The Cytoskeleton and Cell Movement
Every cell has a supporting framework of minute filaments and tubules, the cytoskeleton, which maintains the shape and polarity of the cell. Nevertheless, the cell membrane and intracellular organelles are not rigid or static structures but are in a constant state of movement to accommodate processes such as endocytosis, phagocytosis and secretion. Some cells (e.g. white blood cells) propel themselves about by amoeboid movement; other cells have actively motile membrane specialisations such as cilia and flagella (see Ch. 5); while other cells (e.g. muscle cells) are highly specialised for contractility. In addition, cell division is a process that involves extensive reorganisation of cellular constituents. The cytoskeleton incorporates features that accommodate all these dynamic functions.
The cytoskeleton of each cell contains structural elements of three main types: microfilaments, microtubules and intermediate filaments. The cytoskeleton structures are made up of protein subunits (monomers) that are non-covalently bound together into filaments (polymers). Many accessory proteins link these structures to one another, to the plasma membrane and to the membranes of intracellular organelles. Other associated proteins are the motor proteins responsible for movement, the best known of which are the myosin, dynein and kinesin protein families.
• Microfilaments. Microfilaments are extremely fine strands (5 to 9 nm in diameter) of the protein actin. Each actin filament (F-actin) consists of two protofilaments twisted together to form a helix. The protofilaments are made up of multiple globular actin monomers (G-actin) joined together head to tail and associated with ATP molecules to provide energy for contraction. The actin filament is then assembled into larger filaments, networks and 3-dimensional structures. Actin filaments are best demonstrated histologically in skeletal muscle cells where they form a stable arrangement of bundles with the motor protein myosin. Contraction occurs when the actin and myosin filaments slide relative to each other due to the rearrangement of intermolecular bonds, fuelled by the release of energy from associated ATP molecules (see Ch. 6). However, all eukaryotic cells contain a dynamic actin network. Beneath the plasma membrane, actin, in association with various transmembrane and linking proteins, forms a robust supporting meshwork called the cell cortex, which protects against deformation and yet can be rearranged to accommodate changes in cell morphology. Membrane specialisations such as microvilli (see Fig. 5.14) also contain a skeleton of actin filaments. Actin plays a central role in cell movement, pinocytosis and phagocytosis. Actin may also bind to intrinsic plasma membrane proteins to anchor them in position.
• Intermediate filaments. Intermediate filaments (approximately 10 nm in diameter) are, as their name implies, intermediate in size between microfilaments and microtubules. These proteins have a purely structural function and consist of filaments that self-assemble into larger filaments and bind intracellular structures to each other and to plasma membrane proteins. In humans, there are more than 50 different types of intermediate filament, but these can be divided into different classes, with some classes characteristic of particular cell types. This feature is used in diagnostic pathology to identify different varieties of tumour. For example, the keratin (or cytokeratin) intermediate filament family is characteristic of epithelial cells, where they form a supporting network within the cytoplasm and are anchored to the plasma membrane at intercellular junctions. Specific keratin types form hair and nails. Likewise, vimentin is found in cells of mesodermal origin, desmin in muscle cells, neurofilament proteins in nerve cells and glial fibrillary acidic protein in glial cells. Lamin intermediate filaments form a structural layer on the inner side of the nuclear membrane.
• Microtubules. Microtubules (25 nm in diameter) are much larger than microfilaments but, like them, are made up of globular protein subunits which can readily be assembled and disassembled to provide for alterations in cell shape and position of organelles. The microtubule subunits are of two types, α- and β-tubulin, which polymerise to form a hollow tubule; when seen in cross-section, thirteen tubulin molecules make up a circle. Microtubules originate from a specialised microtubule organising centre, the centriole, found in the centrosome (see below), and movement may be effected by the addition or subtraction of tubulin subunits from the microtubules, making them longer or shorter. Microtubule-associated proteins (MAPs) stabilise the tubular structure and include capping proteins, which stabilise the growing ends of the tubules. The motor proteins dynein and kinesin move along the tubules towards and away from the cell centre, respectively. These motors attach to membranous organelles (e.g. mitochondria, secretory vesicles) and move them about within the cytoplasm, rather like an engine pulling cargo along a railway track. The function of the spindle during cell division is a classic example of this process on a large scale (see Fig. 2.3). The centrosome, consisting of a pair of centrioles, each of nine triplets of microtubules, organises the microtubules of the cell spindle during cell division (see Figs 1.17 and 1.18). In cilia, nine pairs of microtubules form a cylindrical structure and movement occurs by rearrangement of chemical bonds between adjacent microtubule pairs (see Fig. 5.13).
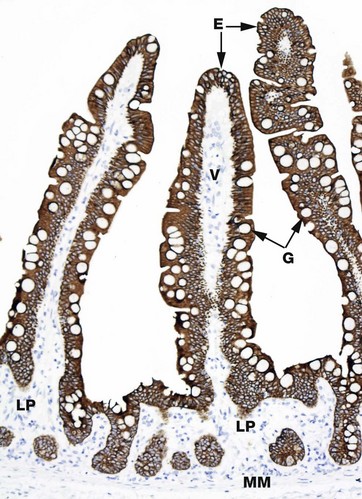
FIG. 1.14 Cytoskeleton
Immunohistochemical method for keratin (MP)
Individual elements of the cytoskeleton are not easily visualised by routine light microscopy, but immunostaining techniques can indirectly identify cellular constituents and are commonly used to do so in research and in diagnostic histopathology. The principles of the method are explained in Appendix 2. In brief, this section of small bowel mucosa (see also Fig. 14.19) has been treated with an antibody that is specific for keratin. The antibody binds to the keratin and is then further treated with a chromogen (a colour-producing chemical) which turns areas with bound antibody a different colour. In this case, the intermediate filament keratin is present in the epithelial cells E that form the surface of the villi V and line the crypts C of the small bowel mucosa. The cytoplasm of these cells is stained a strong brown colour. Notice that the mucous vacuoles of the goblet cells G are unstained as they do not contain keratin and the nuclei can be seen as a blue area in each cell (stained by the haematoxylin counterstain), as again they do not contain keratin intermediate filaments. The stromal cells of the lamina propria LP and the smooth muscle cells of the muscularis mucosae MM are also demonstrated only by the haematoxylin counterstain that highlights their nuclei.
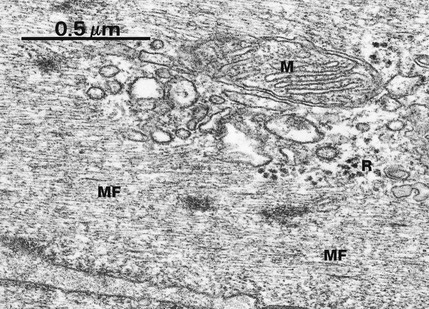
FIG. 1.15 Microfilaments
EM ×76 500
In general, individual microfilaments are difficult to demonstrate due to their small diameter (approximately 7 nm) and diffuse arrangement among other cytoplasmic components. In this example from a smooth muscle cell, a cell type in which cytoplasmic microfilaments are a predominant feature, parallel arrays of microfilaments MF are readily seen. The diameter of microfilaments may be compared with the diameter of a mitochondrion M and ribosomes R.
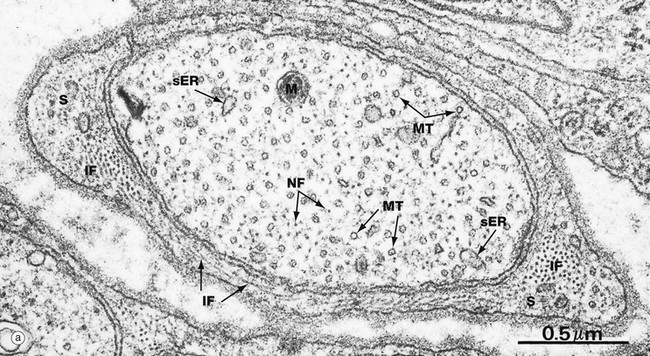
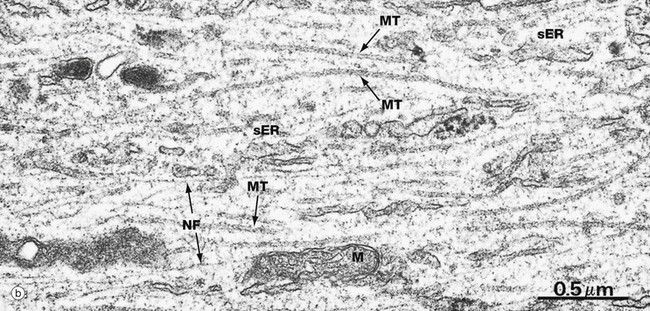
FIG. 1.16 Intermediate filaments and microtubules
(a) EM, TS ×53 000 (b) EM, LS ×40 000
These micrographs are taken from nerve tissue; nerve cells contain both intermediate filaments and microtubules, allowing comparison of size and morphology. Each nerve cell has an elongated cytoplasmic extension called an axon (see Ch. 7) which, in the peripheral nervous system, is ensheathed by a supporting Schwann cell. Micrograph (a) shows an axon in transverse section wrapped in the cytoplasm of a Schwann cell S. Micrograph (b) shows part of an axon in longitudinal section. The axonal microtubules provide structural support and transport along the axon.
In longitudinal section, microtubules MT appear as straight, unbranched structures and, in transverse section, they appear hollow. Their diameter can be compared with small mitochondria M and smooth endoplasmic reticulum sER.
Intermediate filaments (known as neurofilaments in this case) are a prominent feature of nerve cells, providing internal support for the cell by cross-linkage with microtubules and other organelles. The neurofilaments NF are dispersed among and in parallel with the microtubules, but are much smaller in diameter and are not hollow in cross-section. Intermediate filaments IF are also seen in the Schwann cell cytoplasm in micrograph (a), both in transverse and longitudinal view.
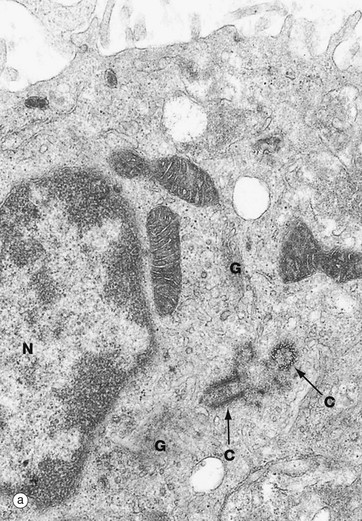
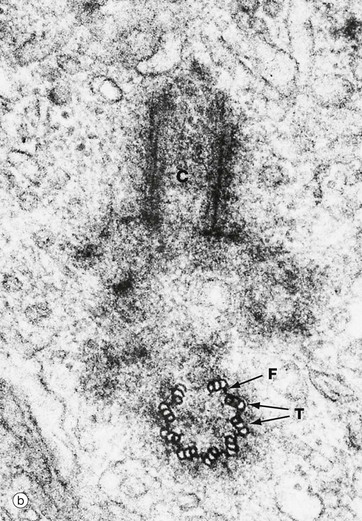
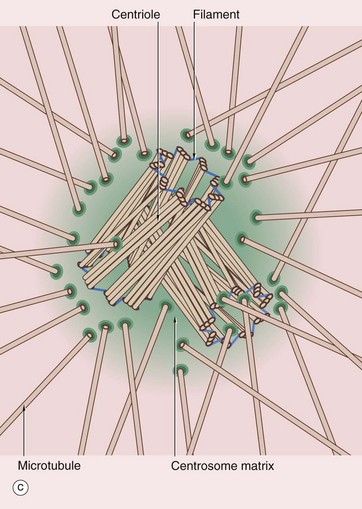
FIG. 1.17 Centrosome
(a) EM ×9200 (b) EM ×48 000 (c) Schematic diagram
The centrosome includes a pair of centrioles C and the centrosome matrix or pericentriolar material. The centrosome matrix is a zone of cytoplasm distinguishable by its different texture. It is usually centrally located in the cell, adjacent to the nucleus N and often surrounded by the Golgi apparatus G. The pair of centrioles are also known as a diplosome. There are also 50 or more δ-tubulin ring complexes, which form a nucleus for the polymerisation of microtubules. Thus the centrioles, themselves composed of microtubules, act as a microtubule organising centre. Microtubules radiate outwards from the centrioles in a star-like arrangement, often called an aster.
Each centriole is cylindrical in form, consisting of nine triplets of parallel microtubules. In transverse section, as in the lower half of micrograph (b) and in diagram (c), each triplet T is seen to consist of an inner microtubule, which is circular in cross-section, and two further microtubules, which are C-shaped in cross-section. Each of the inner microtubules is connected to the outermost microtubule of the adjacent triplet by fine filaments F, thus forming a cylinder. The two centrioles of each diplosome are arranged with their long axes at right angles to each other, as can be seen in these micrographs.
Structures apparently identical to centrioles form the basal bodies of cilia and flagella (see Figs 5.13, 18.6 and 18.7), both of which are moved by microtubules. Cilia are a cell surface specialisation, each cilium comprising a minute hair-like cytoplasmic extension containing microtubules. Cilia move in a wave-like fashion for the purpose of moving secretions across a tissue surface. Flagella are the long tails responsible for the motility of sperm.
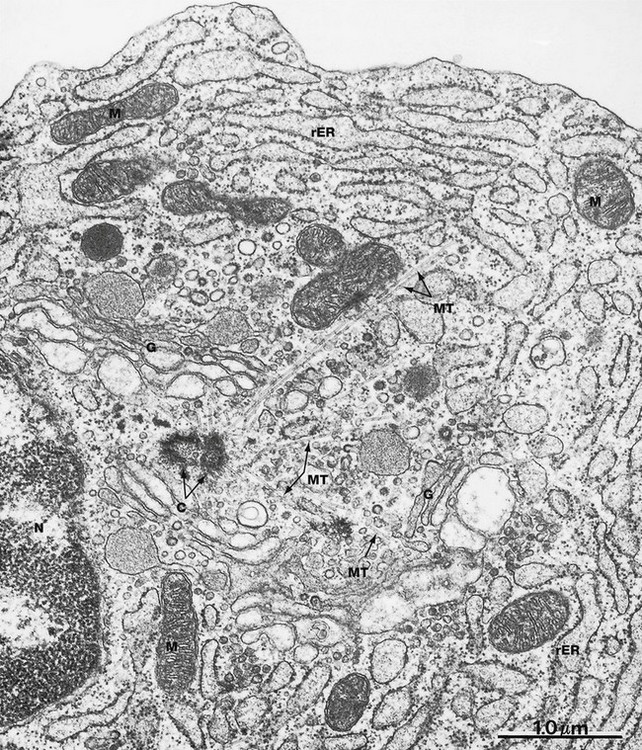
FIG. 1.18 Centrosome and microtubules
EM ×30 000
This micrograph shows the centrosome acting as an organising centre for the microtubules of the cytoskeleton. The centrosome consists of two centrioles C (both cut somewhat obliquely in this specimen), typically located at the centre of the cell close to the nucleus N. Several microtubules MT are seen radiating from the centrosome towards the cell periphery. Centrioles appear to be necessary for microtubule function. For example, prior to cell division the pair of centrioles is duplicated, the pairs migrating towards opposite ends of the cell. Here they act as organising centres for the microtubules of the spindle that controls distribution of chromosomes to the daughter cells (see Ch. 2). Likewise, a centriole known as a basal body is found attached to the microtubules at the base of cilia.
Other features of this micrograph, which is from an antibody-secreting plasma cell, include profuse rough endoplasmic reticulum rER distended with secretory product, several saccular profiles of an extensive Golgi complex G and scattered mitochondria M.
Energy Production and Storage
All cellular functions are dependent on a continuous supply of energy, which is derived from the sequential breakdown of organic molecules during the process of cellular respiration. The energy released during this process is ultimately stored in the form of ATP molecules. In all cells, ATP forms a pool of readily available energy for all the metabolic functions of the cell. The main substrates for cellular respiration are simple sugars and lipids, particularly glucose and fatty acids. Cellular respiration of glucose (glycolysis) begins in the cytosol, where it is partially degraded to form pyruvic acid, yielding a small amount of ATP. Pyruvic acid then diffuses into specialised membranous organelles called mitochondria where, in the presence of oxygen, it is degraded to carbon dioxide and water; this process yields a large quantity of ATP. In contrast, fatty acids pass directly into mitochondria where they are also degraded to carbon dioxide and water; this also generates a large amount of ATP. Glycolysis may occur in the absence of oxygen and is then termed anaerobic respiration, whereas mitochondrial respiration is dependent on a continuous supply of oxygen and is therefore termed aerobic respiration. Mitochondria are the principal organelles involved in cellular respiration in mammals and are found in large numbers in metabolically active cells, such as those of liver and skeletal muscle.
When there is excess fuel available, most cells convert glucose and fatty acids into glycogen and triglycerides, respectively, for storage. The amounts of each vary in different cell types. For example, nerve cells contain very little of either; most of the body's limited store of glycogen is found in muscle and liver cells and triglycerides can be stored in almost unlimited amounts in fat (adipose) cells.
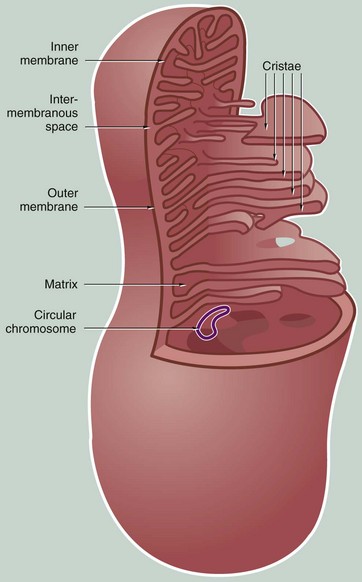
FIG. 1.19 Mitochondria
Mitochondria vary considerably in size and shape and change shape over time but are most often elongated, sausage-shaped organelles. Mitochondria are very mobile, moving around the cell by means of microtubules. They tend to localise at intracellular sites of maximum energy requirement. The number of mitochondria in cells is highly variable; liver cells contain as many as 2000 mitochondria whereas inactive cells contain very few. The number of mitochondria in a cell are modified by mitochondrial division and fusion and by autophagy. In some cells, fused mitochondria may form an interconnected network throughout the cytoplasm.
Each mitochondrion consists of four compartments:
• The outer membrane is relatively permeable as it contains a pore-forming protein known as porin, which allows free passage of small molecules. The outer membrane contains enzymes that convert certain lipid substrates into forms that can be metabolised within the mitochondrion.
• The inner membrane, which is thinner than the outer, is thrown into complex folds and tubules called cristae that project into the inner cavity. In some cell types, mitochondria typically have tubular cristae (see Fig. 17.16).
• The inner cavity filled by the mitochondrial matrix. The matrix is the site of the mitochondrial DNA and ribosomes. The matrix also contains a number of dense matrix granules, the function of which is unknown.
• The intermembranous space between the two membranes also contains a variety of enzymes.
Aerobic respiration takes place within the matrix and on the inner membrane, a process enhanced by the large surface area provided by the cristae. The matrix contains most of the enzymes involved in oxidation of fatty acids and the Krebs cycle. The inner membrane contains the cytochromes, the carrier molecules of the electron transport chain, and the enzymes involved in ATP production.
As organelles, mitochondria have several unusual features. The mitochondrial matrix contains one or more circular strands of DNA resembling the chromosomes of bacteria. The matrix also contains ribosomes with a similar structure to bacterial ribosomes. Mitochondria synthesise 13 of their own constituent proteins, others being synthesised by the usual protein synthetic mechanisms of the cell and imported into the mitochondrion. In addition, mitochondria undergo self-replication in a manner similar to bacterial cell division. Mitochondria are thought to be derived from bacteria which formed a symbiotic relationship with eukaryotic cells during the process of evolution.
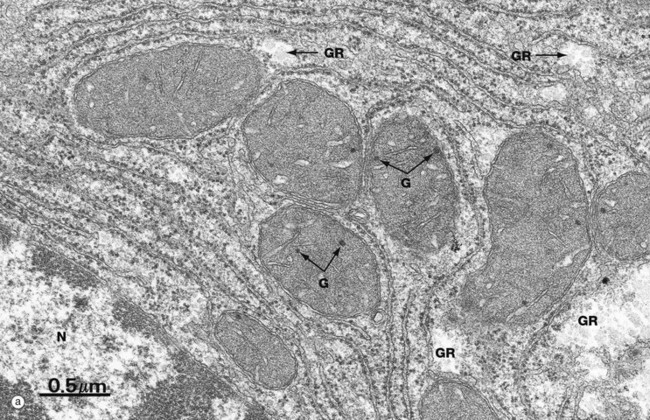
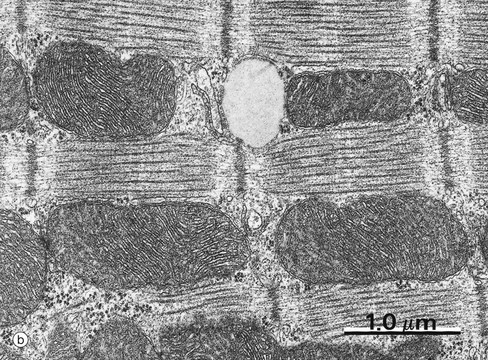
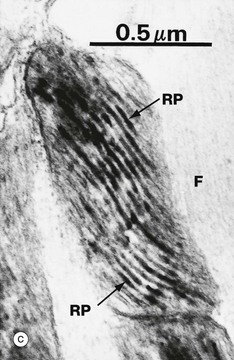
FIG. 1.20 Mitochondria
(a) EM ×34 000 (b) EM ×25 000 (c) Histochemical method for cytochrome oxidase, EM ×50 000
All mitochondria conform to the same general structure but vary greatly in size, shape and arrangement of cristae; these variations are often characteristic of the cell type. Mitochondria move freely within the cytosol and tend to aggregate in intracellular sites with high energy demands, where their shape often conforms to the available space.
Micrograph (a) of liver cell cytoplasm shows the typical appearance of mitochondria when cut in different planes of section; note their relatively dense matrix containing a few matrix granules G. Glycogen rosettes GR are also seen in this micrograph (see Fig. 1.22). Part of the nucleus N is seen in the bottom left corner.
Mitochondria from heart muscle cells can be seen in micrographs (b) and (c). The cristae are densely packed, reflecting the metabolic activity of the cell. In some cells the cristae have a characteristic shape, those of heart muscle being laminar. Micrograph (c) uses a histochemical technique to localise a mitochondrial enzyme, cytochrome oxidase. The electron-dense reaction product RP is located in the intermembranous space. The actin and myosin filaments F are essentially unstained in this preparation.
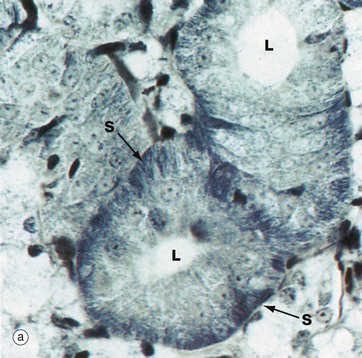
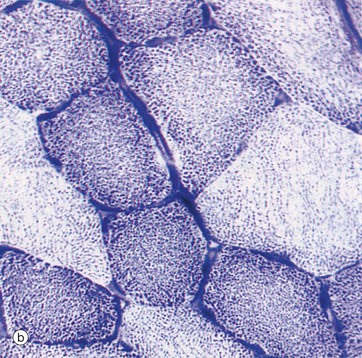
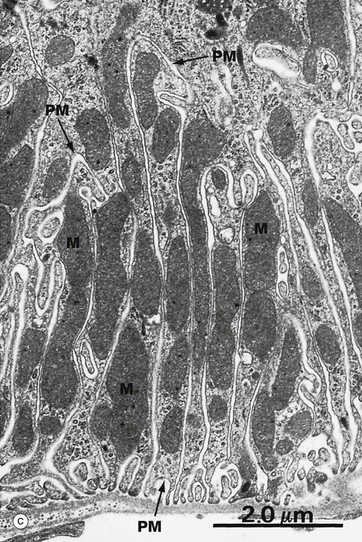
FIG. 1.21 Mitochondria
(a) Iron haematoxylin (HP) (b) Succinate dehydrogenase (HP) (c) EM ×13 000
Mitochondria are, in general, not seen individually by light microscopy. However, they are acidophilic and, with the standard H&E stain, are responsible for much of the eosinophilia (pink staining) of cytoplasm. In some cells, the mitochondria are profuse and may be concentrated in one region of the cell where they can be demonstrated directly and indirectly by various staining methods.
Micrograph (a) shows a salivary gland duct made up of cells that are extremely active in secretion and reabsorption of a variety of inorganic ions. This takes place at the base of the cells (i.e. the surface away from the lumen L) and is powered by ATP produced by elongated mitochondria associated with numerous basal plasma membrane interdigitations between adjacent cells. This strategy greatly increases the plasma membrane surface area. The cells have been stained by a modified haematoxylin method which stains not only basophilic structures (i.e. DNA and RNA) but also acidophilic structures such as mitochondria that can be seen as striations S in the basal aspect of the cells.
In specimen (b), which shows skeletal muscle cells in transverse section, an enzyme histochemical method for succinate dehydrogenase has been employed. Succinate dehydrogenase is an enzyme of the citric acid cycle that is exclusive to mitochondria and therefore provides a marker for them. In skeletal muscle there are three muscle cell types, which differ from each other in mitochondrial concentration. Such a staining method can be used to demonstrate their relative proportions (see also Fig. 6.14), as shown here by the different intensity of staining for mitochondria in different cells.
Micrograph (c) shows the base of an absorptive cell from a kidney tubule where there is intense active transport of ions. The basal plasma membranes PM of adjacent cells form interdigitations that greatly increase their surface area, and elongated mitochondria M are packed into the intervening spaces. Micrographs (a) and (c) demonstrate an example of the same structure, interdigitation of a membrane, being used for the same purpose in two different situations to maximise ion transportation.
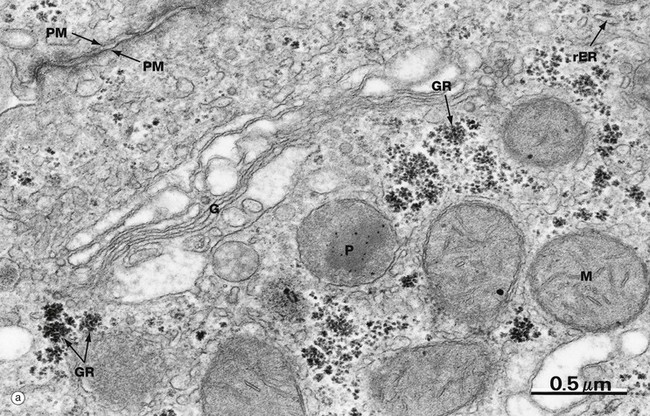
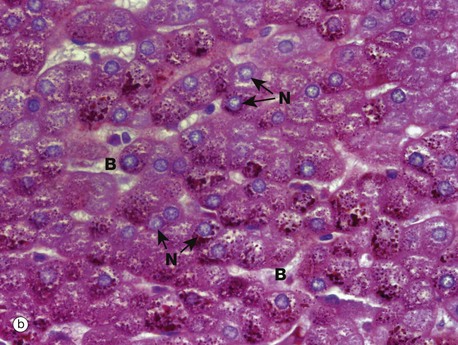
FIG. 1.22 Glycogen
(a) EM ×47 000 (b) PAS/haematoxylin (MP)
Glycogen is found in the cytoplasm of many cell types but is most prominent in muscle cells and liver cells (hepatocytes). In micrograph (a), plentiful glycogen granules are present, appearing either as irregular single granules (called β particles) or as aggregations termed glycogen rosettes GR (also called α particles). Compare the size of the ribosomes on the rough endoplasmic reticulum rER with glycogen granules, which are slightly larger on average. A prominent Golgi apparatus G can be seen near the plasma membrane PM. Note that although the Golgi apparatus is classically found near the nucleus, it is not at all unusual to find it in other areas of the cytoplasm, especially in cells like hepatocytes which contain multiple Golgi stacks. Several mitochondria M and a peroxisome P can also be seen in this field.
Micrograph (b) has been stained by a histochemical method to demonstrate the presence of glycogen, which is stained magenta (see Appendix 2). The specimen is of liver, the cytoplasm of each liver cell being packed with glycogen which is easily identified as granules. The section has been counterstained (i.e. stained with a second dye) to demonstrate the liver cell nuclei N (blue). It also stains the nuclei of the cells lining the blood channels B (sinusoids) between the rows of liver cells; these nuclei are smaller and more condensed and hence stain more intensely.
Lipid Biosynthesis
Lipids are synthesised by all cells in order to maintain the constant turnover of cell membranes. Cells may also synthesise lipid as a means of storing excess energy as cytoplasmic droplets, for lipid transport, e.g. chylomicron production by cells of the small intestine, and in the form of steroid hormones, for sending information to other cells. The precursor molecules (fatty acids, triglycerides and cholesterol) are available to the cell from dietary sources, from mobilisation of lipid stored in other cells or can be synthesised by most cells using simple sources of carbon such as acetyl CoA and other intermediates of glucose catabolism. Fatty acids and triglycerides are mostly synthesised within the cytosol, whereas cholesterol and phospholipids are synthesised in areas of smooth endoplasmic reticulum (see Fig. 1.8).
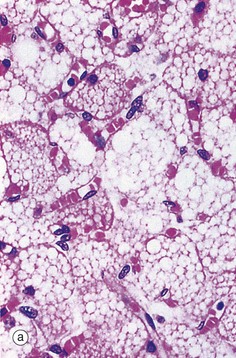
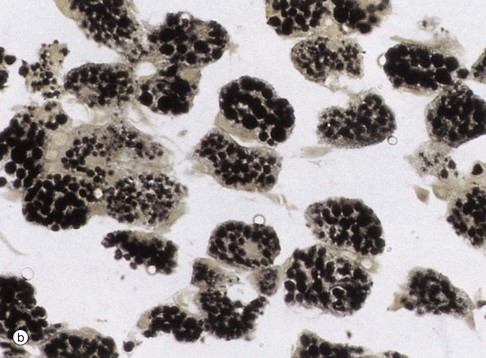
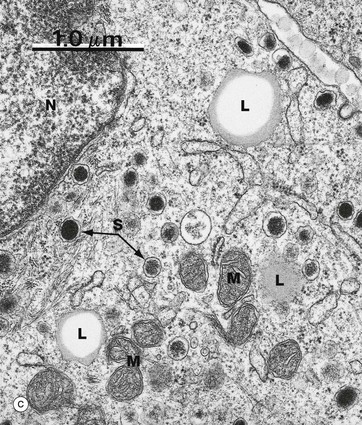
FIG. 1.23 Lipid
(a) H&E (HP) (b) Osmium (HP) (c) EM ×24 000
Routine processing methods for microscopy generally extract lipid from tissues. Therefore lipid droplets within cells appear as unstained vacuoles, as in micrograph (a). Lipids are therefore best demonstrated in frozen sections stained by specific lipid methods such as osmium tetroxide, as in micrograph (b), which stains lipid droplets black. Both micrographs show brown adipose tissue at similar magnifications (see also Fig. 4.17). Lipid droplets L, as seen in electron micrograph (c) of the cytoplasm of a neuroendocrine cell, can be identified as homogeneous rounded (really spherical in three dimensions) globules of lipid. The lipid forms droplets for the same reason that lipid membranes separate themselves from the cytoplasm, i.e. hydrophobic forces tend to exclude water as in the old saying ‘oil and water don’t mix’. As lipid droplets accumulate and enlarge, the same hydrophobic forces tend to make them fuse together to form larger droplets which may eventually occupy the entire cytoplasm and push the nucleus to the edge of the cell, as in adipocytes (fat cells) (see Fig. 4.15). Note also that lipid droplets do not have a limiting membrane in contrast to mitochondria M, the nucleus N and secretory granules S of the ‘dense core’ type that are characteristic of neuroendocrine cells (see Ch. 17).
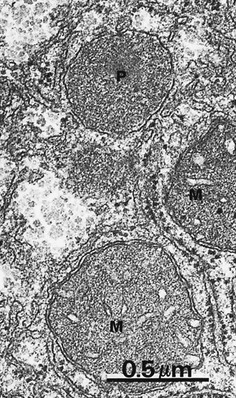
FIG. 1.24 Peroxisomes
EM ×40 000
Peroxisomes (sometimes called microbodies) are unusual organelles: they are present in all cells but have different functions in different cell types and in the same cell type in different species. Broadly speaking, peroxisomes perform enzymatic oxidation and contain a range of oxidative enzymes. Functions include β-oxidation of certain long chain fatty acids, some steps in the synthesis of plasmalogens (lipids found in the myelin sheath of nerve axons) and some steps in the synthesis of bile acids (in the liver). Hydrogen peroxide is a by-product in some of these reactions and peroxisomes commonly contain catalase which utilises the hydrogen peroxide to detoxify other substances such as phenols and alcohol by peroxidation.
Peroxisomes appear somewhat similar to lysosomes by electron microscopy but vary greatly in size, ranging from 0.1 to 0.9 µm in different tissues. The peroxisomes in the liver and kidney are particularly large and abundant. In this micrograph, note the fine, granular, electron-dense contents of a peroxisome P, the size of which can be compared to that of adjacent mitochondria M. Like mitochondria, peroxisomes self-replicate, and the numbers of peroxisomes within a cell may change over the life-time of the cell.
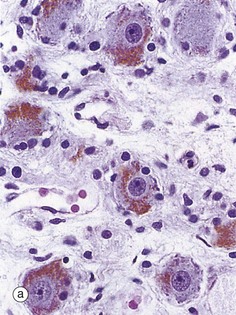
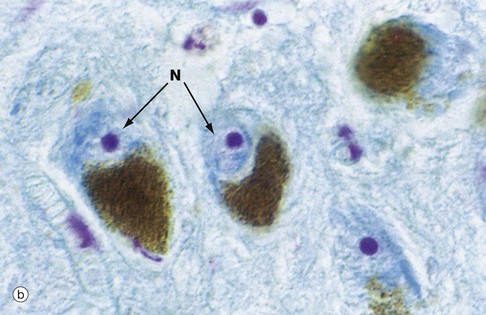
FIG. 1.25 Cellular pigments: lipofuscin and melanin
(a) H&E (HP) (b) Modified Azan (HP)
Most mammalian tissues have minimal intrinsic colour, thus the need for staining for microscopy. A few tissues, however, contain intracellular pigments such as lipofuscin, which probably represents an insoluble degradation product of organelle turnover. With increasing age, it accumulates as brown, granular material in the cytoplasm, particularly of sympathetic ganglion cells as seen in micrograph (a), other neurones and cardiac muscle cells; it is thus sometimes referred to as ‘age pigment’. Another natural pigment is melanin, which is mainly responsible for skin colour (see Ch. 9). This brown pigment is also present in nerve cells in certain brain regions such as the substantia nigra, shown in micrograph (b), where the cell cytoplasm is largely obscured by its content of brown melanin pigment. This specimen has been stained by the Azan method to pick out the nuclei N which are stained pale blue with prominent magenta nucleoli.
The Integrated Function of Cells in Tissues, Organs and Organ Systems
As mentioned at the outset, cells are the functional units of all living organisms; indeed the most primitive organisms merely consist of single cells. In multicellular organisms, however, individual cells become specialised (differentiated) and grouped together to perform specific functions. Multicellular organisms would obviously disintegrate if the cells were not organised into specific structures and held together by intercellular junctions (see Ch. 5) and extracellular matrix (see Ch. 4). Cells of similar morphology and function along with extracellular matrix form tissues, which are relatively homogeneous in overall structure; examples include cartilage, bone and muscle. Extracellular matrix, such as the calcified matrix of bone and the fibrous matrix of the deep layer of the skin (the dermis), can impart great strength to tissues. Other cell types, such as blood cells and tissue macrophages, migrate throughout the body either in the blood or other body fluids or through the extracellular matrix. Organs are anatomically discrete collections of tissues that together perform certain specific functions (e.g. liver, kidney, eye and ovary). Tissues and organs may constitute integrated functional systems forming major anatomical entities (e.g. central nervous system, female reproductive tract, gastrointestinal tract, urinary system) or be more diffusely arranged (e.g. immune defence system, diffuse neuroendocrine system). Despite the foregoing, the terms tissue, organ and system are not necessarily mutually exclusive and may in some cases be used interchangeably, depending on the functional implications. Part 2 of this book describes five basic tissues: blood, supporting/connective tissue, epithelia, muscle and nervous tissues. These are constituents of all organs and organ systems. In tissues and organs, the functionally specialised cells are often called the parenchyma and the less specialised supporting tissue, the stroma.
Within tissues and organs, cells interact with one another in numerous ways during embryological development and growth, maintenance of structural integrity, response to injury (inflammation and repair), integration and control of tissue and organ functions and the maintenance of overall biochemical and metabolic integrity (homeostasis). Various types of intercellular junctions also serve as conduits for information exchange in the form of electrical excitation or chemical messengers. Within tissues, cellular functions are integrated by a great variety of local chemical mediators (paracrine signalling) or by direct contact between cells and interaction between messaging molecules bound to the cell surface. At the level of systems and of the whole body, functions are coordinated via circulating chemical messengers (hormones) and/or via the nervous system (synaptic signalling). The great pleasure to be derived from the study of histology is that all structures, from the subcellular to organ systems, reflect these functional requirements and interrelationships; it really is all very cleverly arranged.
Review
TABLE 1.1
Review of cell structure and function
| Organelle | Brief description | Functions |
| Nucleus | Double membrane-bound large structure containing chromatin | Chromosomes (DNA) contain the genetic blueprint for every protein in the body |
| Nuclear envelope/membrane | Double lipid bilayer with nuclear pore complexes | Separates and mediates transport between nucleus and cytoplasm |
| Nucleolus | Dense non-membrane-bound structure in nucleus | Ribosomal RNA synthesis and ribosome assembly |
| Ribosomes | Small structures free in cytoplasm or bound to endoplasmic reticulum. Consist of two subunits of ribosomal RNA. | Protein synthesis—formation of peptide bonds between amino acids to make polypeptide chains using messenger RNA as template |
| Endoplasmic reticulum | Extensive membrane system within the cell; may be rough (rER) with associated ribosomes, or smooth (sER) | Modification and folding of proteins synthesised on ribosomes (rER), synthesis of some lipids (sER) |
| Golgi apparatus/stack | Stacks of flattened membrane-bound cisternae | Final assembly and glycosylation of proteins and dispatch to their ultimate destination |
| Mitochondria | Double membrane-bound organelles with folded inner membrane | Energy production, mainly in the form of ATP |
| Plasma membrane | Lipid bilayer containing intrinsic proteins and with an external coat of carbohydrate | Divides cell from external environment and mediates interactions with external environment |
| Cytoskeleton | Microfilaments, intermediate filaments and microtubules | Maintain cell shape and orientation, cell movement, movement of organelles around the cell, movement of chromosomes during cell division |
| Transport/secretory vesicles | Membrane-bound vesicles often with a protein coat, e.g. COP I, clathrin | Transport materials between different cell compartments and to plasma membrane for export |
| Phagosomes/endosomes—including sorting and recycling endosomes | Membrane-bound vesicles containing material imported into cell | Phagocytosis/endocytosis and transport of cargo to intracellular destination, e.g. lysosome |
| Lysosomes | Membrane-bound vesicles containing hydrolytic enzymes | Killing of pathogenic organisms (in phagocytic cells) and degradation of waste products |
| Peroxisomes | Membrane-bound vesicle containing oxidases and catalase | Production of hydrogen peroxide for killing pathogens, detoxification of certain toxic materials, β-oxidation of long chain fatty acids, synthesis of bile acids (in liver) |
| Lipid droplets | Non-membrane-bound spherical aggregates of lipid of variable size | Energy storage |
| Glycogen granules | Non-membrane-bound granules and aggregates of granules (rosettes) | Energy storage |
| Lipofuscin | Brown pigment in cytoplasm | Waste product |
| Melanin | Brown pigment in cytoplasm | Skin pigmentation |