Diagnosis and care of recumbent adult horses
Diagnosis and management of adult horses that are recumbent can be challenging. The large size of adult horses, the variety of conditions that can cause recumbency, the difficulty in performing a thorough clinical examination and the need for prolonged and intensive care all present formidable obstacles to management of recumbent horses. Causes of prolonged (>8 h) recumbency in horses are listed in Table 2.7. Other causes of acute recumbency of shorter duration are usually obvious on initial examination and include septic or hemorrhagic shock, such as occurs in horses with colic or internal or external hemorrhage.
Table 2.7 Causes and diagnostic features of recumbency of more than 8 hours duration in adult horses
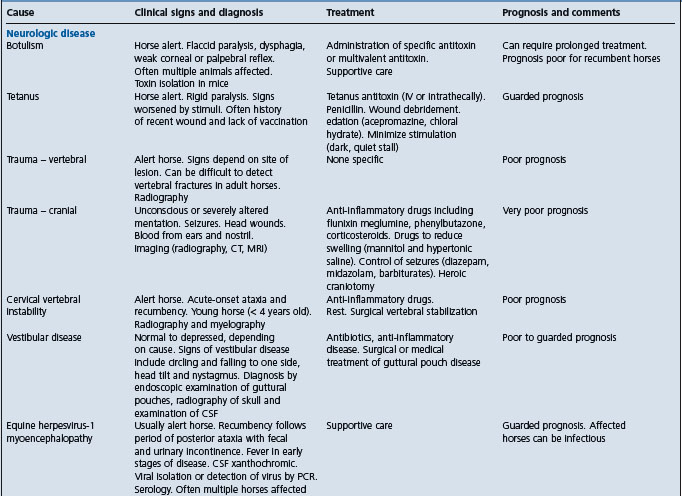
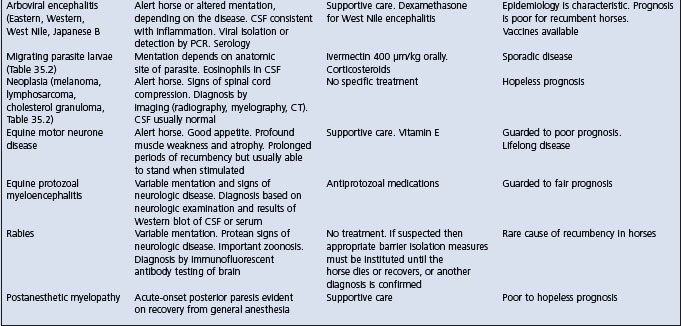
EXAMINATION OF THE RECUMBENT HORSE
History
Careful questioning of the horse’s attendants can reveal valuable information regarding the cause of recumbency. Causes such as observed trauma, foaling and excessive unaccustomed exercise are readily determined from the history. In addition to inquiries about the cause of the recumbency, estimates of the duration of recumbency should be obtained from the attendants. This can often be best elicited by asking when the horse was last observed to be standing. A history of recent illness, abnormal behavior or unusual use immediately before the horse became recumbent is useful. The horse’s age, sex, breed and use should be determined. Information regarding management, vaccination and deworming status, feeding and health of other horses can be revealing. Outbreaks of recumbency suggest either an infectious (equine herpesvirus-1) or toxic (botulism, ionophore) cause. Questions should be directed toward discerning the cause of the horse’s recumbency rather than collecting information.
Physical examination
Physical examination of recumbent horses is challenging but should be as complete as practical and safe. The examination should begin with a general assessment of the horse and its surroundings and can be directed at answering a series of questions:
• Are the surrounding conditions safe for the horse and people? Is the footing sound?
• Is there evidence of the horse struggling or thrashing?
• Has the horse defecated and urinated recently?
• Is there evidence of exposure to toxins or physical evidence of the reason for recumbency?
Examination of the horse should begin with measurement of heart rate, respiratory rate and temperature (rectal temperature might not be accurate if there is dilation of the anus), examination of mucous membranes and an assessment of its hydration, body condition and level of consciousness. The horse should be thoroughly examined for evidence of trauma. Although the examination should be complete, initial examination of cases for which the cause of recumbency is not immediately obvious should focus on the nervous and musculoskeletal systems.
• Is the horse alert and able to sit in sternal recumbency or is it unconscious and in lateral recumbency? Can the horse rise with assistance?
• Is the horse’s mentation normal?
• Are there any spontaneous voluntary or involuntary movements?
• Can the horse eat and drink?
• Are the cranial nerves normal?
• Is there evidence of trauma to the head or neck?
• Is there evidence of paresis or paralysis? Are only the hind limbs involved or are both the hind limbs and forelimbs involved?
• Are the peripheral reflexes normal (withdrawal, patellar, cervicofacial, cutaneous, anal, penile)?
• Is cutaneous sensation present in all regions? If not, what are the anatomic boundaries of desensitized areas?
• Is the position of the limbs normal? Is there evidence of crepitus, swelling or unusual shape of the limbs or axial skeleton?
• Are the horse’s feet normal? Does it have laminitis? What is the response to application of hoof testers?
• Are abnormalities detected on rectal examination (fractured pelvis, distended bladder, fecal retention, pregnancy), provided that it is safe to perform one?
Other body systems should be evaluated as indicated or necessary. The heart and lungs should be auscultated, although detecting abnormal lung sounds in a recumbent horse is difficult. The horse should be rolled so that a complete examination can be performed. Assisting the horse to stand using a rope tied to the tail and thrown over a rafter, or preferably using a sling, can be useful in assessing the severity of the horse’s illness (can it stand at all?) and in facilitating a complete physical examination. If there is a suspicion that the horse has colic a nasogastric tube should be placed to check for accumulation of liquid gastric contents, a rectal examination performed and peritoneal fluid collected.
Ancillary diagnostic testing includes radiography of limbs and/or axial spine as indicated by the history or physical examination; myelography if a compressive lesion of the cervical spinal cord is suspected; endoscopic examination of the pharynx and guttural pouches (especially in horses with a history of falling, see Rupture of the longus capitus muscle, ultrasonography of the chest and abdomen; collection of cerebrospinal fluid; and electromyography.
Hematologic abnormalities are sometimes reflective of the causative disease. Serum biochemical abnormalities are reflective of the causative disease and in addition are influenced by muscle damage caused by the horse being recumbent (increased creatine kinase and aspartate aminotransferase activity), inappetent (increased total and indirect bilirubin, and triglyceride concentrations), and unable to drink or gain access to water (increased serum urea nitrogen, creatinine, sodium, chloride, total protein and albumin concentrations). Cerebrospinal fluid is reflective of any inciting disease but is usually normal.
MANAGEMENT AND CARE
The principles of care are treatment of the primary disease, prevention of further illness or injury, assisting the horse to stand, and provision of optimal nutrition and hydration.
Treatment of the primary disease is covered in other sections of this book. Similarly, maintenance of hydration and electrolyte status is covered elsewhere. Maintenance of normal hydration is sometimes problematic in recumbent horses because of limited access to water and unwillingness to drink. Provision of fresh, palatable water is essential. Intravenous or enteral (nasogastric intubation) administration of fluids and electrolyte solutions might be necessary in some recumbent horses, especially early in their illness.
Horses with diseases that cause recumbency often have problems with fecal and urinary incontinence or retention. Catheterization of the urinary bladder might be necessary to relieve distension in horses with neurogenic upper motor bladder or lower motor bladder dysfunction, or in male horses that are reluctant to urinate when recumbent. Catheterization of the bladder is often repeated. To minimize the risk of iatrogenic cystitis, the procedure should be performed aseptically. Administration of bethanechol might increase detrusor muscle tone and aid urination, and phenoxybenzamine (0.5 mg/kg intravenously over 15 min) might decrease sphincter tone in horses with upper motor neurone bladder.
Horses that can eat should be fed a balanced, palatable and nutritious diet. Tempting horses with reduced appetite with treats such as apples, carrots and horse treats might stimulate appetite for hay and grain. Horses that are unable to eat should be fed through a nasogastric tube. Slurries of alfalfa pellets or commercial diets can be administered through nasogastric tubes. The maintenance needs of a sedentary 425 kg horse are approximately 15–18 Mcal/d. The maintenance needs of a recumbent horse are unknown, but are probably less than that of normal sedentary horses.
COMPLICATIONS – PREVENTION
A major challenge in managing recumbent horses is preventing further injury. Recumbent horses often make repeated efforts to stand, which, while encouraging to all involved, can result in further injury. Horses attempting to stand can injure their head, especially the periorbital regions, and skin over bony prominences such as over the wing of the ilium. Minimizing further injury is achieved by use of a sling or tail rope to assist horses to stand, housing in a padded stall with deep, soft bedding (although this can interfere with the horse’s ability to stand), and protection of the head and distal limbs with a helmet and bandages, respectively. Recumbent horses kept in well-grassed pasture often do well and have minimal self-inflicted trauma.
Decubital ulcers occur over pressure points such as the wing of the ilium, point of the shoulder and zygomatic arch, and can become severe. Recumbent horses that paddle can abrade the skin over limb joints with subsequent increased risk of septic arthritis. Bandages, helmets, ointments such as silver sulfadiazine paste, and soft bedding minimize but do not eliminate these abrasions. Recumbent horses that cannot or do not voluntarily move from side to side should be rolled every 2–4 hours.
Peripheral pressure neuropathy can occur in recumbent horses. The radial nerve and facial nerve are most often affected. Prevention is achieved by use of padded bedding, slings, frequent rolling and a helmet.
Recumbent horses can sustain muscle damage from pressure on large muscle groups. For large or well-muscled horses this can result in large increases in serum creatine kinase activity and myoglobinuria. Myoglobinuria can cause acute renal failure, although this degree of myoglobinuria in recumbent horses is unusual.
Pneumonia can occur as a result of recumbency. Horses that are dysphagic are at increased risk of aspiration of feed material and saliva, and hence development of aspiration pneumonia. Horses receiving corticosteroids are at increased risk of bacterial and fungal (Aspergillus spp.,) pneumonia. While not every recumbent horse should be administered antimicrobials, this is indicated in horses at increased risk of developing pneumonia. Antimicrobials should have a broad spectrum, including activity against Streptococcus spp., such as a combination of penicillin and an aminoglycoside.
Slinging horses is labor-intensive and requires the use of a sling that is designed for use with horses. Horses should not be lifted using hip slings intended for use with cattle. Use of these slings to lift horses by grasping over the wing of each ilium is inhumane and unsuccessful. Horses in slings should be closely monitored and not allowed to hang in the sling. The horses should be assisted to stand in the sling every 6 or 8 hours. The sling should be used to help the horse to get up and provide some support while it is standing, but the horse should not have all its weight borne by the sling for more than a few minutes. Horses that have an excessive amount of weight borne by the sling for a prolonged period of time have trouble breathing and are likely to develop colic, rupture of the urinary bladder, diaphragmatic hernia or rectal prolapse.
Potentially catastrophic complications include septic arthritis, radial nerve injury, bladder rupture, diaphragmatic hernia, rectal prolapse, colon torsion and long bone fracture. The risk of these complications can be minimized by the practices detailed above, but cannot be eliminated.
Chandler K. Clinical approach to the recumbent adult horse. In Practice. June: 2000:308.
Davis EG, et al. Treatment and supportive care of recumbent horses. Compend Contin Educ Pract Vet. 2004;26:216.
Rush BR, et al. Compend Contin Educ Pract Vet. 2004;26:256.
Nout YS, Reed SM. Management and treatment of the recumbent horse. Equine Vet Educ. 2005;17:324.