Chapter 5 Diseases of the alimentary tract – I
PRINCIPLES OF ALIMENTARY TRACT DYSFUNCTION 189
MANIFESTATIONS OF ALIMENTARY TRACT DYSFUNCTION 191
SPECIAL EXAMINATION 195
PRINCIPLES OF TREATMENT IN ALIMENTARY TRACT DISEASE 203
DISEASES OF THE BUCCAL CAVITY AND ASSOCIATED ORGANS 205
Diseases of the pharynx and esophagus
DISEASES OF THE NONRUMINANT STOMACH AND INTESTINES 215
CONGENITAL DEFECTS OF THE ALIMENTARY TRACT 280
NEOPLASMS OF THE ALIMENTARY TRACT 281
DISEASES OF THE PERITONEUM 282
Principles of alimentary tract dysfunction
The primary functions of the alimentary tract are the prehension, digestion and absorption of food and water and the maintenance of the internal environment by modification of the amount and nature of the materials absorbed.
The primary functions can be divided into four major modes and, correspondingly, there are four major modes of alimentary dysfunction. There may be abnormality of motility, secretion, digestion or absorption. The procedure in diagnosis should be to determine which mode or modes of function are disturbed before proceeding to the determination of the site and nature of the lesion and ultimately of the specific cause.
MOTOR FUNCTION
NORMAL GASTROINTESTINAL MOTILITY
The form and function of the small intestine of farm animals is similar between species but the stomachs and large intestines vary considerably.1 The motility patterns in both the small and large intestine are similar among the species. In the small intestine, the fundamental unit of electrical activity is the slow wave, which is a subthreshold fluctuation in membrane potential. Slow waves are constantly propagated from the stomach to the rectum. When an additional stimulus causes the membrane potential to exceed the excitation threshold, a spike or electrical response activity occurs, which is usually accompanied by contraction. Almost all spike activity in the intestine is superimposed on slow waves, which are important in controlling frequency and velocity at which spiking events occur. The spiking activity, also known as the migrating myoelectric complex, is the myoelectric pattern in the stomach and small intestine of fasted nonruminants, fed and fasted ruminants, and pigs and horses fed ad libitum.2 There are three phases of the migrating myoelectric complex:
• The quiescent phase, in which very little spike activity occurs
• The irregular phase, characterized by intermittent spike activity
• The activity front, characterized by intense, continuous spike activity.2
There is very little muscle contraction or transit of gut contents during the quiescent phase. During the irregular phase, contractions mix the intestinal contents and propel them in an aboral direction. The activity front is accompanied by intense muscular contraction that obliterates the lumen, preventing backflow of content as it propagates, or migrates, down the intestine. In nonruminants, and pigs and horses fed periodically, feeding abolishes the migrating myoelectric complex for several hours. It is replaced by the fed pattern, characterized by intermittent spike activity resembling the irregular phase.
Normal cecal and colonic myoelectric activities, like those of the small intestine, are characterized by slow waves and spikes. However, unlike the small intestine, the patterns of spikes vary greatly with the species and the area of the large intestine.2
Abnormalities of stomach and intestinal motility represent the most common consequence of gastrointestinal tract disease. Disruption in gastrointestinal tract motility can result in:
HYPERMOTILITY AND HYPOMOTILITY
The most important functions of alimentary tract motility are the peristaltic movements that move ingesta from the esophagus to the rectum, the segmentation movements that churn and mix the ingesta, and the tone of the sphincters. In ruminants these movements are of major importance in the forestomach. Prehension, mastication and swallowing are other functions of alimentary tract motility that are essential for normal function. Eructation of ruminal gases is an additional crucial function of motility in ruminants.
Abnormal motor function may take the form of increased or decreased motility. Peristalsis and segmenting movements are usually affected equally and in the same manner. Motility depends upon stimulation via the sympathetic and parasympathetic nervous systems and is thus dependent upon the activity of the central and peripheral parts of these systems, and upon the intestinal musculature and its intrinsic nervous plexuses. Autonomic imbalance, resulting in a relative dominance of one or other system, is manifested by hypermotility or hypomotility, and can arise as a result of stimulation or destruction of hypothalamic centers, the ganglia, or the efferent or afferent peripheral branches of the system. Debility, accompanied by weakness of the musculature, or severe inflammation, such as occurs in acute peritonitis or after trauma, or infarction, results in atony of the intestinal wall. Less severe inflammation, such as occurs in mild gastritis and enteritis, may result in an increase in muscular activity and increased propulsive activity. Increased motility causes diarrhea, decreased motility causes constipation, and both have deleterious effects on digestion and absorption.
Increased irritability at a particular intestinal segment increases its activity and disturbs the normal downward gradient of activity that insures that the ingesta is passed from the esophagus to the rectum. Not only is the gradient towards the rectum made steeper, thus increasing the rate of passage of ingesta in that direction, but the increased potential activity of an irritated segment may be sufficiently high to produce a reverse gradient to the oral segments so that the direction of the peristaltic waves is reversed orally to the irritated segments.
DISTENSION
One of the major results of abnormality of motility is distension of the tract. This occurs in a number of disturbances, including the rapid accumulation or inefficient expulsion of gas, complete occlusion of the lumen by intestinal accident or pyloric or ileocecal valve obstruction, and engorgement on solid or liquid feeds. Fluids, and to a lesser extent gas, accumulate because of their failure to pass along the tract. Much of the accumulated fluid represents saliva and gastric and intestinal juices secreted during normal digestion. Distension causes pain and, reflexly, increased spasm and motility of adjoining gut segments. Distension also stimulates further secretion of fluid into the lumen of the intestine and this exaggerates the distension. When the distension passes a critical point, the ability of the musculature of the wall to respond diminishes, the initial pain disappears, and a state of paralytic ileus develops in which all muscle tone is lost.
ABDOMINAL PAIN
Visceral pain may arise in any abdominal viscus or organ but the mode of its development is always the same and alimentary tract disease is the major cause of visceral and, more specifically, of abdominal pain. The most important mechanism is stretching of the wall of the viscus, which stimulates free pain endings of the autonomic nerves in the wall. Contraction does not of itself cause pain but does so by causing direct and reflex distension of neighboring segments. Thus spasm, an exaggerated segmenting contraction of one section of intestine, will result in distension of the immediately oral segment of intestine when a peristaltic wave arrives. When there is increased motility for any reason, excessive segmentation and peristalsis cause abdominal pain, and the frequent occurrence of intermittent bouts of pain depends upon the periodic increases in muscle tone that are typical of alimentary tract wall. Other factors that have some stimulating effect on the pain end organs are edema and failure of local blood supply, such as occurs in local embolism or in intestinal accidents accompanied by twisting of the mesentery. A secondary mechanism in the production of abdominal pain is the stretching and inflammation of serous membranes.
Clinically, abdominal pain can be detected by palpation and the eliciting of pain responses. However, it is unknown if the response elicited is due to involvement of underlying organs or to referred pain. It is difficult to decide if referred pain occurs in animals. In humans it is largely a subjective sensation, although often accompanied by local hyperalgesia. There are no known examples of referred pain that are of diagnostic importance in animals and a local pain response on palpation of the abdomen is accepted as evidence of pain in the serous membranes or viscera that underlie the point of palpation.
DEHYDRATION AND SHOCK
An immediate effect of distension of the stomach or small intestine by the accumulation of saliva and normal gastric and intestinal secretions is the stimulation of further secretion of fluid and electrolytes in the oral segments. The stimulation is self-perpetuating and creates a vicious cycle resulting in loss of fluid and electrolytes to the point where fatal dehydration can occur. The dehydration is accompanied by acidosis or alkalosis depending on whether the obstruction is in the intestine and accompanied by loss of alkali, or in the stomach and accompanied by a large loss of acid radicals. The net effect is the same whether the fluid is lost by vomiting or is retained in the gut.
The same cycle of events occurs in ruminants that gorge on grain but here the precipitating mechanism is not distension but a gross increase in osmotic pressure of the ingesta due to the accumulation of lactic acid. Dehydration is also of major importance in diarrhea, irrespective of the cause. An important additional factor in the production of shock, when there is distension of alimentary segments, is a marked reflex depression of vasomotor, cardiovascular and respiratory functions. In diarrhea in calves in which there is no septicemia nor toxemia associated with bacteria, the end-point in the phase of dehydration can be cardiac failure due to severe metabolic acidosis. Renal ischemia leading to uremia may result from decreased circulating blood volume and also contribute to a fatal outcome. These matters are discussed in detail in the section in Chapter 2 on disturbances of body fluids, electrolytes and acid–base balance.
SECRETORY FUNCTION
Diseases in which abnormalities of secretion occur are not generally recognized in farm animals. In humans, and to a lesser extent in small animals, defects of gastric and pancreatic secretion produce syndromes that are readily recognized, but they depend upon clinical pathological examination for diagnosis. If they do occur in farm animals, they have so far only been recognized as aberrations of motility caused by the defects of secretion. However, it is reasonable to assume that some neonates may be deficient in lactase activity, which results in dietetic diarrhea. Undigested lactose causes diarrhea by its hyperosmotic effect, and some of the lactose may be fermented in the large intestine, the products of which fermentation may exaggerate the diarrhea. A deficiency of lactase activity has been suspected in foals affected with diarrhea of undetermined origin but the definitive diagnosis has not been made. The intestinal lactase activity of foals is at its highest level at birth and gradually declines until the fourth month of age, and then disappears from adults before their fourth year.
DIGESTIVE FUNCTION
The ability of the alimentary tract to digest food depends on its motor and secretory functions and, in herbivores, on the activity of the microflora that inhabits the forestomachs of ruminants or cecum and colon of Equidae. The flora of the forestomachs of ruminants is capable of digesting cellulose, of fermenting the end-products of other carbohydrates to volatile fatty acids and converting nitrogenous substances to ammonia and protein. In a number of circumstances, the activity of the flora can be modified so that digestion is abnormal or ceases. Failure to provide the correct diet, prolonged starvation or inappetence, and hyperacidity as occurs in engorgement on grain all result in impairment of microbial digestion. The bacteria, yeasts and protozoa may also be adversely affected by the oral administration of antibiotic and sulfonamide drugs, or drugs that drastically alter the pH of the rumen content.
Diseases of the stomach of ruminants are presented in Chapter 6. Information about the digestive and absorptive capacities of the equine gut is not exhaustive but some basic data are available.1,3 The rate of passage of ingesta through the stomach and intestines is rapid but varies widely depending on the physical characteristics of the ingesta, dissolved material passaging more rapidly than particulate material; 75% of a liquid marker can be emptied from the stomach in 30 minutes and be in the cecum at 2 hours. Passage through the large bowel is much slower, especially in the latter part of the colon where much of the fluid is absorbed. There is an obvious relationship between the great activity of the small intestine and the effect of a complete obstruction of it: the pain is very severe and often uncontrollable with standard analgesics, fluid loss into the obstructed parts is rapid, and dehydration, loss of electrolytes and disturbances of acid–base balance are acute, severe and life-threatening.
ABSORPTIVE FUNCTION
Absorption of fluids and the dissolved end-products of digestion may be adversely affected by increased motility or by disease of the intestinal mucosa. In most instances, the two occur together but, occasionally, as with some helminth infestations, lesions occur in the intestinal wall without accompanying changes in motility.
Manifestations of alimentary tract dysfunction
Inanition is the major physiological effect of alimentary dysfunction when the disease is a chronic one, dehydration is the major effect in acute diseases, and shock is the important physiological disturbance in hyperacute diseases. Some degree of abdominal pain is usual in most diseases of the alimentary tract, the severity varying with the nature of the lesion. Other manifestations include abnormalities of prehension, mastication and swallowing, and vomiting, diarrhea, hemorrhage, constipation and scant feces.
ABNORMALITIES OF PREHENSION, MASTICATION AND SWALLOWING
Prehension is the act of grasping for food with the mouth (lips, tongue, teeth). It includes the ability to drink. Causes of faulty prehension include:
• Paralysis of the muscles of the jaw or tongue
• Malapposition of incisor teeth due to:
A simple examination of the mouth usually reveals the causative lesion. Paralysis is indicated by the behavior of the animal as it attempts to ingest feed without success. In all cases, unless there is anorexia due to systemic disease, the animal is hungry and attempts to feed but cannot do so.
Mastication may be painful and is manifested by slow jaw movements interrupted by pauses and expressions of pain if the cause is a bad tooth, but in a painful stomatitis there is usually complete refusal to chew. Incomplete mastication is evidenced by the dropping of food from the mouth while eating and the passage of large quantities of undigested material in the feces.
Swallowing is a complex act governed by reflexes mediated through the glossopharyngeal, trigeminal, hypoglossal and vagal nerves. It has been described endoscopically and fluoroscopically in the horse. The mechanism of the act includes closure of all exits from the pharynx, the creation of pressure to force the bolus into the esophagus, and involuntary movements of the musculature of the esophageal wall to carry the bolus to the stomach. A defect in nervous control of the reflex or a narrowing of the lumen of the pharynx or esophagus may interfere with swallowing. It is difficult to differentiate clinically between physical and functional causes of dysphagia (difficulty in eating/swallowing).
Dysphagia is manifested by forceful attempts to swallow accompanied initially by extension of the head, followed by forceful flexion and violent contractions of the muscles of the neck and abdomen. Inability to swallow is usually caused by the same lesions as dysphagia, but in a greater degree. If the animal attempts to swallow, the results depend on the site of the obstruction. Lesions in the pharynx cause regurgitation through the nostrils or coughing up of the material. In the latter instance, there is danger that some of the material may be aspirated into the lungs and cause acute respiratory and cardiac failure or aspiration pneumonia. When the obstruction is at a low level in the esophagus, a large amount of material may be swallowed and then regurgitated. It is necessary to differentiate between material regurgitated from the esophagus and vomitus: the former is usually slightly alkaline, the latter acid.
DROOLING OF SALIVA AND EXCESSIVE SALIVATION
Drooling saliva from the mouth, distinct from frothing such as occurs during convulsions, may be caused by pain in the mouth and by an inability to swallow. Excessive salivation is caused by stimulation of saliva production by systemic toxins, especially fungal toxins, or by hyperthermia. With systemic poisonings the increased salivation is often accompanied by lacrimation.
VOMITING AND REGURGITATION
VOMITING
Vomiting is the forceful ejection of contents of the stomach and the proximal small intestine through the mouth and is a complex motor disturbance of the alimentary tract. It is a vigorously active motion signaled by hypersalivation, retching and forceful contractions of the abdominal muscles and diaphragm. Vomiting is essentially a protective mechanism with the function of removing excessive quantities of ingesta or toxic materials from the stomach. It occurs in two forms: projectile and true vomiting.
Projectile vomiting
This is not accompanied by retching movements and large amounts of fluid material are ejected with little effort. It is almost always as a result of overloading of the stomach or forestomach with feed or fluid.
True vomiting
As it occurs in monogastric animals like the dog and cat, true vomiting is accompanied by retching movements including contraction of the abdominal wall and of the neck muscles and extension of the head. The movements are commonly prolonged and repeated and the vomitus is usually small in amount and of porridge-like or pasty consistency. It is most commonly a result of irritation of the gastric mucosa. Vomiting is commonly designated as being either peripheral or central in origin depending on whether the stimulation arises centrally at the vomiting center or peripherally by overloading of the stomach or inflammation of the gastric mucosa, or by the presence of foreign bodies in the pharynx, esophagus or esophageal groove. Central stimulation of vomiting by apomorphine and in nephritis and hepatitis are typical examples but vomiting occurs rarely, if at all, in these diseases in farm animals.
Vomiting may have serious effects on fluid and electrolyte balance because of the losses of gastric and intestinal contents during vomiting. Aspiration pneumonia or laryngeal obstruction are potential serious consequences of vomiting. Examination of any suspected vomitus to determine its site of origin should always be carried out.
True vomiting is rare in farm animals except in pigs with gastroenteritis and some systemic diseases. True vomiting does not occur in ruminants but abnormal regurgitation does occur (see below under Regurgitation). True vomiting is not a feature of gastric disease in the horse for two reasons. First, the strong cardiac sphincter inhibits the release of stomach contents; in horses rupture of the stomach is more likely to occur before vomiting takes place. Secondly, the soft palate and epiglottis combine to effect a seal between the oral and nasal parts of the pharynx so that any vomited stomach contents must be discharged through the nasal cavities and not through the mouth. Spontaneous nasal regurgitation or vomiting does occur occasionally, as manifested by the production of green stomach contents at the nostrils. This suggests extreme gastric distension or a dilated esophagus and cardiac sphincter and perhaps some underlying neurological deficit. Thus vomiting of large quantities of material in the horse is usually a terminal event and suggests gastric rupture.
REGURGITATION
Regurgitation is the expulsion through the mouth or nasal cavities of feed, saliva and other substances that have not yet reached the stomach. In most cases it is due to abnormalities of the esophagus that interfere with swallowing. A common example in large animals is the regurgitation of feed, saliva, and perhaps blood-stained fluid from the esophagus of the horse with esophageal obstruction. Esophagitis is also a common cause of regurgitation.
Ruminants regurgitate rumen contents as part of rumination but the material is not expelled from the mouth nor into the nasal cavities. The regurgitation of rumen contents through the mouth does occur in cattle occasionally, is abnormal, and is a dramatic event. It is most commonly associated with loss of tone of the cardia or inflammation of the cardia (see examples below).
Nasogastric regurgitation or gastric reflux occurs in the horse. Stomach contents flow into the esophagus, and usually into the nasopharynx and nasal cavities, as a result of distension of the stomach with fluid (which usually originates in the small intestine). This involuntary process is usually slow and gradual, unlike true vomiting. Gastric reflux in the horse can be elicited by nasogastric intubation. Spontaneous efflux of stomach contents is indicative of high-volume and high-pressure fluid distension of the stomach. On other occasions the presence of sequestrated gastric fluids can be confirmed only by the creation of a siphon, using the nasogastric tube to infuse a volume of fluid then disconnecting its supply in order to retrieve the nasogastric reflux.
Causes of vomiting and regurgitation include:
• Terminal vomiting in horses with acute gastric dilatation
• ‘Vomiting’ in cattle is really regurgitation of large quantities of rumen contents through the mouth. Causes include:
• Vomiting in pigs may be due to:
• Regurgitation – in all diseases causing dysphagia or paralysis of swallowing.
DIARRHEA, CONSTIPATION AND SCANT FECES
Diarrhea and constipation are the most commonly observed abnormalities in fecal consistency, composition and frequency of defecation.
DIARRHEA
Diarrhea is the increased frequency of defecation accompanied by feces that contain an increased concentration of water and decrease in dry matter content. The consistency of the feces varies from soft to liquid.
Abnormalities of peristalsis and segmentation usually occur together and when there is a general increase in peristaltic activity there is increased caudal flow, resulting in a decrease in intestinal transit time and diarrhea. Because of a lack of absorption of fluid the feces are usually softer than normal, the dry matter content is below the normal range, and the total amount of feces passed per day is increased. The frequency of defecation is usually also increased. Common causes of diarrhea are:
• Enteritis, including secretory enteropathy
• Malabsorption, e.g. due to villous atrophy and in hypocuprosis (due to molybdenum excess)
• Neurogenic diarrhea as in excitement
• Local structural lesions of the stomach or intestine, including:
Malabsorption syndromes
Malabsorption syndromes are being recognized with increased frequency in monogastric farm animals. For example, in recently weaned pigs, there is villous atrophy with a resulting loss in secretory and absorptive function. Inefficient digestion originating in this way may or may not be manifested by diarrhea, but in malabsorption there is usually diarrhea. There is always failure to grow or maintain body weight, in spite of an apparently normal appetite and an adequate diet. In horses, the lesions associated with malabsorption, which may be with or without diarrhea, include villous atrophy, edema and/or necrosis of the lamina propria of the gut wall, and nodular tracts and aggregations of eosinophils indicating damage by migrating strongyle larvae. It is possible also that some cases are caused by an atypical reaction of tissue to unknown allergens (possibly helminths) and are probably an abnormal immunological response. A common accompaniment in the horse is thin hair coat, patchy alopecia and focal areas of scaling and crusting. The pathogenesis is unknown. Special tests are now detailed for the examination of digestive efficiency in the horse. These are listed in the next section under special tests. Increased venous pressure in the portal circuit caused by congestive heart failure or hepatic fibrosis also causes diarrhea.
The question of whether or not enteritis in animals causes intestinal hypermotility and increased peristalsis, resulting in diarrhea, remains unresolved. If hypermotility and increased peristalsis cause diarrhea, antimotility drugs may be indicated in some causes of acute infectious diarrhea. Current concepts on the pathophysiology of the common diarrheas associated with infectious agents (such as enterotoxigenic Escherichia coli) indicate that there is a net increase in the flow of intestinal fluid into the lumen and a decrease in outflow back into the systemic circulation, which causes distension of the intestine with fluid. The hydraulic effect of the distension can cause diarrhea and hypermotility is probably not necessary. In addition, because of the temporary malabsorption that exists in infectious enteritides, and the presence of infectious agents and enterotoxins in the lumen of the intestine, the emphasis should be on evacuation of the intestinal contents and not on the use of anticholinergic drugs to inhibit evacuation. Furthermore, it is unlikely that the anticholinergics will have any significant effect on the secretory-absorptive mechanisms that have been altered by an enteropathogen.
CONSTIPATION
Constipation is the decreased frequency of defecation accompanied by feces that contain a decreased concentration of water. The feces vary in consistency from being hard to dry and of small bulk. True constipation as it occurs in humans is usually characterized by failure to defecate and impaction of the rectum with feces. When the motility of the intestine is reduced, the alimentary transit time is prolonged and constipation or scant feces occurs. Because of the increased time afforded for fluid absorption, the feces are dry, hard and of small bulk and are passed at infrequent intervals. Constipation may also occur when defecation is painful, as in cattle with acute traumatic reticuloperitonitis.
SCANT FECES
Scant feces are small quantities of feces, which may be dry or soft. Scant feces occur most commonly in cattle with abnormalities of the forestomach or abomasum resulting in the movement of only small quantities of ingesta into the small and large intestines (an outflow abnormality). The details are available in Chapter 6. When there is complete intestinal stasis the rectum may be empty except for blood-tinged, thick, pasty material.
Common causes of constipation or scant feces are:
• Diseases of the forestomach and abomasum causing failure of outflow
• Impaction of the large intestine in the horse and the sow
• Severe debility, as in old age
• Deficient dietary bulk, usually fiber
• Partial obstruction of large intestine
• Painful conditions of the anus
ILEUS (ADYNAMIC AND DYNAMIC ILEUS)
Ileus is a state of functional obstruction of the intestines or failure of peristalsis. It is also known as paralytic ileus or adynamic ileus. Dynamic or mechanical ileus is a state of physical obstruction. In paralytic ileus there is loss of intestinal tone and motility as a result of reflex inhibition. This can occur in acute peritonitis, excessive handling of viscera during surgery, and prolonged and severe distension of the intestines as in intestinal obstruction or enteritis. Ileus can also be caused by acid–base imbalance, dehydration, electrolyte imbalances such as hypocalcemia and hypokalemia, and toxemia. Ileus can affect the stomach, causing delayed gastric emptying and subsequent dilatation with fluid and gas. The effect of ileus on the intestines is to cause failure of orocaudal movement of fluid, gas and ingesta and accumulation of these substances, which results in intestinal distension and varying degrees of abdominal pain, dehydration and a marked reduction in the amount of feces. Distension of the abdomen, fluid-tinkling, fluid-splashing sounds, and pings on percussion of the abdomen are common clinical findings. Impaction of the large intestine of horses is a form of ileus.
Postoperative ileus of the large intestine is a common complication of surgical treatment for colic in the horse. The clinical findings include gastric reflux because of gastric distension with fluid, absence of or minimal intestinal peristaltic sounds, an absence of feces, abdominal pain, distended loops of intestine palpable per rectum, and varying degrees of shock and dehydration as a result of intestinal fluid sequestration and a decrease in fluid absorption. Infarction of the intestinal wall associated with an acute mechanical obstruction of the intestine also results in ileus. In thromboembolic colic due to verminous mesenteric arteritis in the horse, large segments of the large colon and cecum can become infarcted, resulting in irreversible ileus.
The etiology and pathogenesis of ileus in farm animals are not well understood. Sympathetic hyperactivity is thought to be a factor. The gastroileal reflex is one example of the influence of the activity of one part of the digestive tract on that of another; inhibition of gastric motility when the ileum is distended is called ileogastric reflex. Immediate cessation of all intestinal movement (adynamic ileus) follows distension of an intestinal segment, rough handling of the intestine during abdominal surgery or peritoneal irritation. Adynamic ileus operates through three pathways: general sympathetic discharge of the peripheral reflex pathway through the iliac and mesenteric plexuses, and the intramural plexuses. The treatment of ileus depends on the original cause. Physical obstruction of the intestines and torsion of the stomach must be corrected surgically. Postoperative ileus in the horse is difficult to manage and the case fatality rate is high.2 Fluid therapy and gastric reflux decompression using a nasogastric tube are standard recommendations. Nonsteroidal anti-inflammatory drugs (NSAIDs) are used to control abdominal pain. Xylazine is contraindicated because of its depressant effect on gastric and intestinal motility.
ALIMENTARY TRACT HEMORRHAGE
Hemorrhage into the stomach or intestine is a common occurrence in farm animals. The main causes are:
• Gastric or abomasal (rarely duodenal) ulcers
• Severe hemorrhagic enteritis
• Structural lesions of the intestinal wall, e.g. adenomatosis, neoplasia
• Infestation with blood-sucking nematodes, e.g. bunostomiasis
• Local vascular engorgement or obstruction as in intussusception and verminous thrombosis.
Hemorrhage into the stomach results in the formation of acid hematin, which makes vomitus a dark brown color like coffee grounds, and feces have a black or very dark brown, tarry appearance (melena). The change in appearance of the feces caused by hemorrhage into the intestine varies with the level at which the hemorrhage occurs. If the blood originates in the small intestine, the feces may be brown-black, but if it originates in the colon or cecum, the blood is unchanged and gives the feces an even red color. Hemorrhage into the lower colon and rectum may cause the voiding of feces containing or consisting entirely of clots of whole blood.
Hemorrhage into the pharynx is unusual, but when it occurs the blood may be swallowed and appear in the feces or vomitus. If there is any doubt about the presence of blood in the feces or vomitus, biochemical tests should be performed. The hemorrhage may be sufficiently severe to cause anemia and, in particularly severe cases, acute peripheral circulatory failure. In cattle the most sensitive test is one using a dilute alcoholic solution of guaiac as the test reagent. It is capable of detecting a daily blood loss into the abomasum of as small a volume as 70 mL. Transit time of blood from abomasum to rectum in normal cows varies from 7–19 hours.
ABDOMINAL PAIN
The pain associated with diseases of the abdominal viscera causes similar signs regardless of the viscus or organ involved and careful clinical examination is necessary to locate the site of the lesion. The manifestations of abdominal pain vary with the species, horses being particularly sensitive, but comprise largely of abnormalities of behavior and posture. Pain as a systemic state is presented in general terms in Chapter 2, including its effects on body systems and methods for its detection.
Readily identifiable syndromes of abdominal pain referable to the alimentary tract include the following.
Horses
• Acute pain: Pawing, flank-watching, rolling
• Subacute pain: Lesser degree of flank-watching, often excessive pawing, lying down frequently without rolling, stretching out as if to urinate, males may extrude the penis, walking backwards, dog-sitting posture, lying on back, impulsive walking
• Peritoneal pain: Rigidity of the abdominal wall, pain on palpation.
Cattle
• Acute pain: Downward arching of back with treading of the hind feet, lying down (rolling is uncommon). Calves will lie down and bellow with severe abdominal pain, as in abomasal torsion
• Subacute pain, including peritoneal pain: Back arched upwards, grunting on walking or lying down, grunting on deep palpation of the abdomen, immobility.
The disease states likely to be mistaken for the above categories of alimentary tract pain are:
• Acute pain: Paresthesia, e.g. in photosensitive dermatitis of cows; pleuropneumonia in the horse; uterine torsion in the mare and cow; snakebite in horses; urticaria as in milk allergy in cows; renal and urethral colic; compulsive walking, e.g. in hepatic disease; lead poisoning; dysuria or obstruction of urinary tract generally; laminitis and lactation tetany in mares
• Subacute pain: Encephalopathy, possibly hepatic insufficiency
TENESMUS
Tenesmus, or persistent straining, is common in many diseases of the organs of the pelvic cavity; therefore it is not necessarily a diagnostic sign of disease in the lower alimentary tract. It is sometimes associated with frequent defecation caused by neurological stimulation of peristalsis. Common causes of tenesmus are listed by species below.
Cattle
• Lower alimentary tract disease, e.g. colitis and proctitis caused by coccidiosis
• Genital tract disease, e.g. severe vaginitis, retained placenta
• Estrogen toxicity in steers, e.g. estrogen implantation, fusariotoxicosis
• 4-aminopyridine poisoning, methiocarb poisoning
SHOCK AND DEHYDRATION
Acute rapid distension of the intestine or stomach causes reflex effects on the heart, lungs and blood vessels. The blood pressure falls abruptly, the temperature falls below normal and there is a marked increase in heart rate. In acute intestinal accidents in horses that terminate fatally in 6–12 hours, shock is the major cause of death. There appears to be some species difference in the susceptibility to shock because similar accidents in cattle rarely cause death in less than 3–4 days; acute ruminal tympany is an exception and may exert its effects rapidly, causing death in a very short time after its onset. Less severe distension, vomiting and diarrhea cause clinically recognizable dehydration and abnormalities of electrolyte concentration and acid–base balance. Determination of the relative importance of shock and dehydration in a particular case at a particular time is one of the challenges in gastroenterology. The subject is considered in detail under the heading of equine colic and under enteritis.
ABDOMINAL DISTENSION
Distension of the abdomen is a common manifestation of disease of the alimentary tract. Generally, abdominal distension associated with the alimentary tract is caused by distension of viscera with gas or fluid. The degree of abdominal distension depends on the viscera that are distended, the species involved and the age of the animal. Abdominal distension is most pronounced when large viscera of adult cattle and horses are distended. Distension of the small intestines in adult cattle and horses may not be detectable clinically. On the other hand, distension of the small intestine with fluid in calves and foals often causes noticeable abdominal distension.
Occasional cases of abdominal distension are due to pneumoperitoneum, which usually follows abdominal surgery. In ruminants the most common causes are distension of the rumen, abomasum, cecum and large intestine, the details of which are presented in Chapter 6. Abdominal distension in horses and pigs is usually due to distension of the large intestine. Gastric dilatation of the horse does not cause abdominal distension. Ascites is a cause in all species.
Abdominal distension may be symmetrical, asymmetrical or more pronounced dorsally or ventrally on one or both sides. The severity can vary from mild and barely detectable to so severe that the skin over the abdominal wall has sufficient tension that it cannot be picked up or ‘tented’. Determination of the cause of the distension requires careful examination of the abdomen by inspection, palpation, percussion and simultaneous auscultation. Rectal palpation is used to determine the location and nature of distended viscera. Diseases of other body systems that cause abdominal distension and must be considered in the differential diagnosis include advanced pregnancy and hydrops allantois.
The alimentary tract diseases of simple-stomached animals in which abdominal distension may be a manifestation are:
• Intestinal tympany – due to excessive gas production caused by abnormal fermentation in the large intestine of horses and pigs
• Obstruction of the large intestine – in horses and pigs as a result of their torsion or miscellaneous constrictions caused by adhesions, usually as a result of peritonitis
• Retention of the meconium – in foals. This is often accompanied by severe distension of the colon and abdomen.
Obstruction of the small intestine may cause abdominal distension but not to the degree that occurs in distension of the large intestine. In all the above diseases, acute abdominal pain is common.
ABNORMAL NUTRITION
Failure of normal motor, secretory, digestive or absorptive functions causes impairment of nutrient supply to body tissues. Inanition or partial starvation results and the animal fails to grow, loses body weight or shows other signs of specific nutritional deficiencies. Ancillary effects include decreased appetite when gut motility is decreased; in many cases where motility is increased and there is no toxemia, the appetite is increased and may be voracious.
Special examination
The general aspects of the clinical examination of the alimentary tract and abdomen of farm animals are described in Chapter 1 under Clinical examination. Some additional or special examination techniques and procedures are included here.
NASOGASTRIC INTUBATION
RUMEN OF CATTLE
Examination of the rumen contents is often essential to assist in determination of the state of the rumen environment and digesta. Passage of a stomach tube into the rumen will determine the patency of the esophagus and if there is increased intraruminal pressure associated with a frothy or free-gas bloat. In a free-gas bloat, large quantities of gas are usually released within a minute. In a frothy bloat, the ruminal end of the tube may become occluded by the froth and very little if any gas is released. Moving the tube back and forth within the rumen and blowing air into the tube to clear the ruminal end may result in the release of some gas.
When the tube is in the rumen, some rumen juice can be siphoned or pumped out and collected in an open beaker for field and laboratory analysis. The color, depending on the feed to a limited extent, will be green, olive-green or brown-green. In cattle on pasture or being fed good quality hay, the color is dark green. When silage or straw is the diet the color is yellow-brown. In grain overload the color is milky-gray, and in rumen stasis of long duration with putrefaction, the color is greenish-black. The consistency of the rumen contents is normally slightly viscid, and watery rumen content is indicative of inactive bacteria and protozoa. Excess froth is associated with frothy bloat as in primary ruminal tympany or vagus indigestion. The odor of the rumen contents is normally aromatic and, although somewhat pungent, not objectionable to the nose. A moldy, rotting odor usually indicates protein putrefaction, and an intensely sour odor indicates an excess of lactic acid formation, due to grain or carbohydrate engorgement. The pH of the rumen juice varies according to the type of feed and the time interval between the last feeding and taking a sample for pH examination. The normal range, however, is between 6.2 and 7.2. The pH of rumen juice should be examined immediately after the sample is obtained, using a wide range pH (1–11) paper. High pH values (8–10) will be observed when putrefaction of protein is occurring in the rumen or if the sample is mixed with saliva. Low pH values (4–5) are found after the feeding of carbohydrates. In general, a pH below 5 indicates carbohydrate engorgement; this pH level will be maintained for between 6–24 hours after the animal has actually consumed the carbohydrate diet. Microscopic examination of a few drops of rumen fluid on a glass slide with a low-power field will reveal the level of protozoon activity. Normally 5–7 protozoons are active per low-power field. In lactic acidosis the protozoa are usually absent or a few dead ones are visible.
DECOMPRESSION OF DISTENDED RUMEN
In adult cattle with severe abdominal distension due to gross distension of the rumen it is difficult, if not impossible, to assess the status of the abdomen. To determine if the rumen is distended and/or to relieve the pressure a large-bore stomach tube should be passed (Colorado Kingman Tube: 2 m long and 3 cm inside diameter). In vagus indigestion, the rumen may be grossly distended with fluid contents, which will gush out through a large-bore tube. In some cases 100–150 L of rumen contents may be released. If no contents are released the contents may be frothy or mushy and the rumen end of the tube will plug almost instantly. Rumen lavage may then be attempted using a water hose to deliver 20–40 L of water at a time followed by back drainage using gravity flow. After the rumen is partially emptied it is usually possible to more accurately assess the rumen and the abdomen.
DECOMPRESSION OF THE HORSE’S STOMACH
Attempts to pass a nasogastric tube in the horse will usually detect complete or partial obstruction of the esophagus. In gross distension of the stomach in the horse, there is an immediate rush of fluid contents as soon as the cardia is passed (gastric reflux). The technique of gastric decompression is therapeutic and diagnostic. Gastric distension is a highly distressing feature of some colic cases and the mere pain relief of gastric decompression facilitates the clinical examination. The retrieval of significant volumes (2 L or more) of sequestrated gastric fluid is also an extremely specific indicator of intestinal obstruction, especially small intestinal obstruction, and a reasonably specific indicator that surgical intervention is necessary.
MEDICAL IMAGING
RADIOGRAPHY
Because of their large size, and the presence of substantial amounts of gas in the large intestine, abdominal radiography has not been used routinely as a diagnostic aid in mature horses with abdominal pain. Similarly, in mature cattle the sheer size of the abdomen and the gas in the rumen has not favored abdominal radiography except for identifying the presence of metal objects in the reticulum. Esophageal radiography is, however, useful for the diagnosis of disorders of swallowing in horses.
Foals, calves and small horses are too small to be palpated per rectum, and abdominal radiography, with and without contrast media, has been used diagnostically in colic of foals. A standard lateral abdominal radiography is a valuable diagnostic aid in the foal with colic.4 The site of the lesion, whether gastric, small or large intestinal, or a combination of all three, can be determined from the radiographs. The sensitivity of radiography in detecting gastrointestinal lesions in neonatal foals was found to be 96%; the specificity was 71%.4
Knowledge of the radiographic appearance of the normal neonatal abdomen is important before lesions can be reliably detected. The standing lateral radiographic of the normal abdomen of the neonatal foal is characterized by:
• A gas cap over fluid and ingesta in the stomach
• Small collections of gas in the small intestine in the cranial and mid-central abdomen
• Gas caps over fluid and ingesta in the cecum and large colon, seen in the caudodorsal abdomen
• Small amounts of gas in the small colon and inconsistent gas in the rectum, seen at the pelvic inlet.
Abdominal radiography has also been used for the diagnosis of enterolithiasis and sand accumulation as causes of colic.3 The technique provides a high positive-predictive value and is cost-effective in high-prevalence areas.
ABDOMINAL ULTRASONOGRAPHY
Abdominal ultrasonography has been used to identify small intestine intussusceptions, large colon displacements, abdominal viscera and neoplasms. The technique may require only several minutes in the hands of an experienced clinician.
Horse
Abdominal ultrasonography is a diagnostic aid that is used for evaluation of equine colic and to assist in differentiation of medical from surgical colics.
It is accurate in identifying horses with abnormal small intestines.5 Ultrasonographic findings of edematous small intestine without motility provides an indication of primary small-intestine disease (obstruction or strangulation) and justifies surgical intervention. Detecting increased thickness of the wall of the large intestine during ultrasonography is a reproducible and accurate preoperative test for large-colon torsion in horses with surgical colic localized to the large colon.6 Strangulating lipomas and epiploic foramen entrapments were diagnosed more often than any other primary small intestine lesion. Detection of distended or edematous small intestine by rectal palpation provided a sensitivity of 50%, a specificity of 98% and a positive predictive value of 89% for small intestine strangulation obstructions.5 The duodenum of the horse can be evaluated by ultrasonography.7 Normally it does not contain any gas and the accumulation of fluid and gas associated with colic may be useful. The technique has been used to detect intestinal sand accumulations.8 Gastrointestinal activity patterns have been evaluated in healthy horses using B mode and Doppler ultrasonography.9 The anatomy and biometric analysis of the thoracic and abdominal organs in healthy foals from birth to age 6 months have been evaluated with ultrasonography.10
Cattle
Abdominal ultrasonography is an ideal diagnostic aid for the investigation of gastrointestinal diseases, the most common of which include traumatic reticuloperitonitis, left and right displacement of the abomasum, ileus of the small intestine, and dilatation and displacement of the cecum.11,12 The various divisions of the small intestine can be differentiated from one another with the exception that the ileum cannot be differentiated from jejunum.13 In normal cows, in which the intestine is full of ingesta, all parts of the intestine have a relatively large diameter. In cows with ileus, the loops of intestine proximal to the ileus are distended and those distal to the ileus are empty.
ENDOSCOPY
GASTROENTEROSCOPY
Fiberoptic gastroduodenoscopy is a practicable procedure in a sedated horse that has had no feed for 12–24 hours. A 275 × 13.5 cm fiberoptic instrument is passed via a nostril to the stomach, which is then distended with air. The control of the objective of the endoscope is quite difficult and entry into the pylorus particularly so, so that examination of the duodenum is not possible in all horses.
LAPAROSCOPY
In this procedure a laparoscope is passed through an incision in the abdominal wall of either the left or right paralumbar fossa.14 Feed must be withheld for 36 hours, analgesia is provided during the procedure, and abdominal insufflation with carbon dioxide is required in order to separate the viscera for viewing. Laparoscopy in standing horses is a valuable diagnostic aid for examination of the structures in the dorsal regions of the abdomen. In the standing horse, the anatomic structures of importance that can be viewed in the left half of abdomen are the hepatic duct, left lateral and quadrate lobes of the liver, stomach, left kidney with associated nephrosplenic ligament, segments of the jejunum, descending colon and ascending colon, left side of the male and female reproductive tracts, urinary bladder, vaginal ring and mesorchium. The important structures observable in the right side of the abdomen are the common hepatic duct, left lateral, quadrate and right lobes of the liver, caudate process of the liver, stomach, duodenum, right dorsal colon, epiploic foramen, omental bursa, right kidney, base of the cecum, segments of jejunum, descending colon and ascending colon, urinary bladder, right half of the male and female reproductive tracts, and rectum.14
In the dorsally recumbent horse under general anesthesia, with laparoscopy the main structures of diagnostic relevance in the caudal region of the abdomen are the urinary bladder, mesorchium, ductus deferens (left and right), left and right vaginal rings, insertion of the prepubic tendon, random segments of jejunum and descending colon, the pelvic flexure of the ascending colon, body of the cecum and cecocolic fold. The main structures observed in the cranial region of the abdomen are the ventral surface of the diaphragm, falciform ligament and round ligaments of the liver, ventral portion of the left lateral, left medial, quadrate and right lateral lobes of the liver, spleen, right and left ventral colons, sternal flexure of the ascending colon, apex of the cecum, and stomach.15 Alterations in cardiovascular and respiratory functions in response to the pneumoperitoneum and various positional changes indicated a need for continuous and thorough anesthetic monitoring and support.
EXPLORATORY LAPAROTOMY (CELIOTOMY)
An exploratory laparotomy is useful for palpating and inspecting the abdominal viscera as a diagnostic aid in cattle, sheep and horses of all ages. Cost and time are important factors but if abdominal disease is suspected and other diagnostic techniques cannot identify the location and nature of the abnormality, a laparotomy is highly desirable.
TESTS OF DIGESTION AND ABSORPTION
Digestion and absorption of nutrients are complex, interrelated functions of the gastrointestinal tract. Failure in one or more of normal motility, enzymic digestion of food and absorption of simple sugars, fat and protein by the small intestine can result in inadequate assimilation of nutrients from the gastrointestinal tract. Tests of small intestinal digestion, absorption or both have been devised for use in monogastrics. These tests take advantage of the rapid appearance in blood of products of digestion, or of compounds that are readily absorbed without digestion.
Indications for these tests include:
• Weight loss of undetermined cause that is suspected to be due to failure of absorption of food by the small intestine
• Diarrhea of suckling foals that is suspected to be due to failure of the foal to digest lactose (lactase deficiency)
• Suspected protein-losing enteropathy of older foals and adult horses.
Low serum protein and albumin concentrations with small intestinal disease can be due to failure of digestion of proteins and absorption of amino acids or leakage of plasma proteins into the intestine. Regardless of the mechanism, some horses with protein-losing enteropathy have abnormal tests of intestinal digestion and absorption of sugars. Contraindications include the presence of obstructive lesions of the gastrointestinal tract, risk of worsening the disease process by the period of fasting required for most of the tests (such as in ponies with hyperlipemia), or known adverse reactions of the animal to any of the test substances.
Interpretation of the test is based on the concentration of the variable of interest (usually glucose or xylose) in blood over a period of time after administration of the test meal (usually by nasogastric intubation). Concentration of the metabolite or marker of interest in blood is plotted against time and the shape of the curve, highest concentration attained, time to attain the highest concentration, and elevation over baseline values (i.e. those measured immediately before administration of the test meal) is compared against values obtained from clinically normal horses or foals. Blood concentrations of glucose or xylose that are lower than expected (so called ‘flat curve’) can be indicative of alterations in gastrointestinal function that hinder propulsion, digestion or absorption of nutrients. Thus, tests of digestion and absorption alone rarely provided sufficient information to make a definitive diagnosis of the functional disorder. The exception to this rule is the modified lactose tolerance test in foals (see below). Interpretation of the results of oral tests of absorption is often confounded by factors that alter gastrointestinal function, such as feed withholding or enteritis, or conditions that alter removal of the test compound from blood, such as reduced insulin sensitivity. This is particularly the case for tests that depend on measurement of blood glucose concentration. Blood glucose concentrations are determined in the absorptive state by the difference in rates of absorption of glucose from the small intestine into blood and removal of glucose from blood by uptake into muscle, adipose tissue and metabolically active tissues. Conditions that enhance glucose uptake from the blood can result in low peak blood glucose concentrations, and conditions that decrease insulin sensitivity (as is seen in fat horses) can result in high blood glucose concentrations. The use of d-xylose as an indicator of small intestinal absorption is intended to avoid these effects of variable glucose disposal. Therefore, the values obtained with oral tests of absorption and digestion should be interpreted with caution and should be considered in light of all clinical and laboratory data available for the animal.
GLUCOSE ABSORPTION TEST
The oral glucose tolerance test is one of the simplest tests of small intestinal absorptive capacity to perform. However, because of the many factors that affect blood glucose concentration, including factors not related to small-intestinal absorptive capacity, results of the test can on occasion be difficult to interpret.16 Oral glucose tolerance testing can produce abnormal results in horses with diseases that do not involve the small intestine, such as lower motor neurone disease or polysaccharide storage myopathy. On the other hand, the oral glucose tolerance test is often used because of the ready availability of glucose for oral administration and routine nature of measurement of blood glucose concentrations.
The main indications for performing oral glucose tolerance testing include unexplained weight loss believed to be associated with gastrointestinal disease, and suspected protein-losing enteropathy. Contraindications are those listed above. In addition, care should be exercised in performing the test in horses at increased risk of laminitis, as rapid passage of unabsorbed glucose into the large colon and cecum can cause laminitis.
Horses for oral glucose tolerance testing are first fasted for 12–18 hours. Access to water should be provided. Glucose is given by stomach tube at 1 g/kg body weight (BW) of anhydrous glucose (or comparable) as a 10–20% solution in water. Blood for measurement of glucose concentration is collected immediately before, and every 30 minutes for 4–6 hours after glucose administration. Some protocols involve less frequent (hourly) collection of blood. One protocol requires collection of blood samples before and 120 minutes after administration of glucose. This last protocol is not recommended as early or delayed peaks in blood concentration are not detected. The blood glucose concentration in the normal horse increases by at least 85% (from 90 up to 180 mg/dL (5.0 to 10.0 mmol/L)) with peak blood concentrations attained 90–150 minutes after administration of glucose. Horses with partial malabsorption have increases in blood glucose concentration of 15–85% of baseline values, and horses with complete malabsorption have no increase or less than 15% increase in blood glucose concentration by 2 hours.17 Blood concentrations of glucose in normal horses return to resting values in approximately 6 hours. The shape of the curve is affected by the horse’s previous diet, the curve being much lower in horses fed on stored feeds such as hay and grain compared to horses eating pasture of clover and grass.
Horses with weight loss and complete failure of absorption of glucose are likely to have extensive infiltrative disease of the small intestine such as lymphosarcoma or granulomatous enteritis.17 Of 25 horses with partial failure of glucose absorption, 18 (62%) had structural abnormalities of the small intestine. Clearly abnormal results of the oral glucose tolerance test therefore appear to be fairly specific for severe and widespread small-intestinal disease. Care should be taken when interpreting results that deviate only marginally from normal values.
STARCH DIGESTION TEST
A suitable test for the evaluation of gastric, small-intestinal and pancreatic function is the starch digestion test. The test relies on the presence of amylase in the small intestine with subsequent cleavage of starch into glucose, which is then absorbed into the blood. The horse is fasted for 18 hours and then given corn starch (1 kg in 4 L of water or 2 g/kg BW) by stomach tube. A pretreatment blood sample is matched with others taken at 15, 30, 60, 90 and 120 minutes and then hourly to 6 hours.
In the normal horse there is an increase in blood glucose levels of about 30 mg/dL (1.7 mmol/L) (from 90 up to 120 mg/dL (5.0–6.7 mmol/L)), with the peak occurring at 1–2 hours and the curve returned to pretreatment level at 3 hours.18 The test can be affected by the diet of the horse prior to testing.
LACTOSE DIGESTION TEST
Newborn animals rely on ingestion of milk sugar (lactose) as an important source of energy until weaning. Lactose is digested in the proximal small intestine by lactase, a disaccharidase present in the brush border of intestinal epithelial cells that cleaves lactose into glucose and galactose. Loss of small-intestinal production of lactase, such as occurs in some bacterial and viral enteritides including rotavirus infection, results in failure to cleave lactose and passage of the sugar to the hind gut. Fermentation of lactose in the hind gut causes acute and sometimes severe osmotic diarrhea. A prime indication for the oral lactose tolerance test is therefore acute diarrhea in neonates being fed milk. The test not only has diagnostic usefulness because a positive test (i.e. demonstration of lactose intolerance) provides a clear indication for feeding lactose-free milk or providing supplemental lactase in the animal’s diet.
An oral lactose digestion test has been devised for foals. Lactose (1 g/kg BW) is given by stomach tube in a 20% solution to a foal that has been fasted for 2–4 hours. In foals and young horses up to 3 years of age there is a rise in blood glucose levels from 86 ± 11 mg/dL (4.8 ± 0.1 mmol/L) up to 153 ± 24 mg/dL (8.5 ± 1.3 mmol/L), with a peak achieved in 90 minutes, and the level returns to pretreatment levels in 5 hours. In foals of 1–12 weeks of age the plasma glucose concentration should rise by at least 35 mg/dL (1.9 mmol/L) and peak within 40 minutes of the administration of the lactose. With this test no changes in blood sugar levels occur in horses over 4 years of age. Instead there is abdominal discomfort followed by diarrhea, with feces the consistency of cow feces for the next 24 hours. Sucrose and maltose are readily digested by the intestine of the adult horse, but not by newborn foals. Maximum levels of the relevant intestinal disaccharidases (sucrase and maltase) are not achieved until 7 months of age. The oral lactose digestion test is likely to be of value as a monitor of epithelial damage in young horses. In humans the ability to hydrolyze lactose is one of the first functions of the intestinal mucosa to be lost where there is epithelial damage in the gut. It is also one of the last functions to return in the recovering patient. The loss of intestinal lactase may be the pathogenetic basis of the diarrhea that occurs in rotavirus infections in neonates. Lactase digestion is impaired in calves with mild diarrhea.19 Calves with acute diarrhea are in a catabolic state and respond with a larger increase in plasma glucose concentration to a given amount of glucose than do healthy calves.
A modification of the oral lactose tolerance test in foals includes a second evaluation in foals in which there is failure of blood glucose concentrations to increase by the appropriate amount after oral administration of lactose. At least 8 hours after the first test, foals are fed a meal of lactose-free milk, or of milk to which lactase has been added. Blood glucose concentrations are measured and an increase of at least 35 mg/dL (1.9 mmol/l) is interpreted as evidence of lactase deficiency. Such animals can then be maintained on a diet of lactose-free milk. Diarrhea usually resolves in 24 hours, but returns within hours of feeding milk containing lactose.
XYLOSE ABSORPTION TEST
D-xylose is used to evaluate small intestinal absorptive function because it is not metabolized by tissues, which is an advantage over the oral glucose tolerance test. d-xylose absorbed from the intestinal tract is excreted unchanged in the urine within 15 hours of dosing.20 Concentrations of d-xylose in blood are therefore dependent only upon the rate of absorption from the intestine and rate of excretion into the urine. However, the compound is more expensive than glucose and measurement of d-xylose in blood requires a particular analysis that might not be readily available. Indications for the test are the same as those for the oral glucose tolerance test described above.
D-xylose, at a dose rate of 0.5 g/kg BW as a 10% solution, is administered by stomach tube after a starve of 18 hours.21 A maximum blood xylose level of 30 mg/dL (2.0 mmol/L) at 1.5 hours is a normal result in adult horses. In normal foals the peak blood concentration of xylose is reached in 30–60 minutes and the level attained varies with age, being highest (47 mg/dL (3.14 mmol/L)) at 1 month of age and lowest (19 mg/dL (1.25 mmol/L)) at 3 months (the pretreatment reading should be zero). In abnormal horses the xylose curve is flat (a peak of 7–13 mg/dL (0.5 mmol/L) at 60–210 minutes) contrasted with a peak of 20 mg/dL (1.3 mmol/L) at 60 minutes in normal horses. As an initial checking test, one postdosing sample at 2 hours is recommended.
Interpretation of the test is influenced by the customary diet of tested animals and feed deprivation. Horses receiving a high energy diet have a lower absorption curve than horses on a low energy diet. The test is also affected by the duration of deprivation of feed.20 In mares deprived of feed for 72 and 96 hours, the rate of d-xylose absorption and the maximum concentrations of d-xylose in plasma were reduced.22 For example, apparent low absorption can be caused by increased transit time through the gut, due perhaps to excitement.
Low blood concentrations of xylose occur in horses with small intestinal infiltrative disease, such as lymphosarcoma or granulomatous enteritis.20 The test appears to be quite specific (low false-positive rate) for small intestinal disease, but the sensitivity (false-negative rate) is unknown.
A d-xylose absorption curve has been determined for cattle. The xylose (0.5 g/kg BW) is deposited in the abomasum by abomasocentesis, and a peak of blood glucose is attained in about 90 minutes.
SUCROSE ABSORPTION TEST
The sucrose absorption test differs from the other tests in this section in that abnormal results are associated with detection of sucrose in blood or urine of horses. Sucrose is not normally absorbed intact – it is usually cleaved by disaccharidases in the small intestine into glucose and fructose, which are then absorbed. Intact sucrose is absorbed across compromised gastric mucosa and detection of sucrose in blood or urine indicates the presence of gastric ulceration, as mammals neither synthesize nor metabolize sucrose.23,24 The sucrose absorption test involves administration of 250 g of sucrose to an adult horse that has been fasted overnight. Blood samples for measurement of serum sucrose concentration are collected at 0, 15, 30, 45, 60 and 90 minutes after dosing. Alternatively, a urine sample is collected 2 hours after dosing (the bladder must be emptied immediately before dosing). Peak serum sucrose concentrations occur 45 minutes after administration and peak values correlate with the severity of gastric ulceration. Horses with minimal lesions have serum sucrose concentrations of 103 pg/μL, whereas horses with the most severe lesions have concentrations of 3400 pg/μL.24
RADIOACTIVE ISOTOPES
A technique used for determining whether a protein-losing enteropathy is present is based on the examination of feces for radioactivity after the intravenous administration of a radioactive agent. 51Cr13C-labeled plasma protein has been used for this purpose. Similarly, administration of radioactively labeled leukocytes reveals the presence of small-intestinal inflammatory disease in horses.4,25 The test is quite specific, in that false-positive tests are uncommon, but not very sensitive.
ABDOMINOCENTESIS FOR PERITONEAL FLUID
Peritoneal fluid reflects the pathophysiological state of the parietal and visceral mesothelial surfaces of the peritoneum. Collection of a sample of peritoneal fluid is a useful aid in the diagnosis of diseases of the peritoneum and the abdominal segment of the alimentary tract.22 It is of vital importance in horses in the differential diagnosis and prognosis of colic and in cattle in the diagnosis of peritonitis.
EQUINE AND BOVINE PERITONEAL FLUID
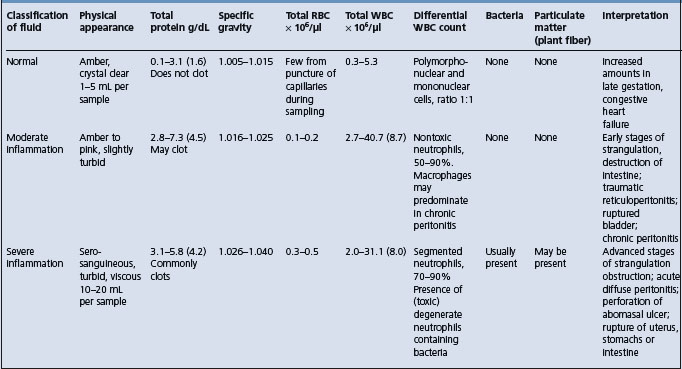
Normal peritoneal fluid is a transudate with properties as summarized in Tables 5.1 and 5.2. It has functions similar to those of other tissue fluids. It contains mesothelial cells, lymphocytes, neutrophils, a few erythrocytes and occasional monocytes and eosinophils. The following general comments apply:
• It can be examined in terms of physical characteristics, especially color, translucence, specific gravity, clotting time, biochemical composition, cell volume, cell morphology and cell type
• Examination of the fluid may help in determining the presence in the peritoneal cavity of:
• The reaction of the peritoneum varies with time and a single examination can be dangerously misleading. A series of examinations may be necessary, in acute cases at intervals of as short as an hour
• A significant reaction in a peritoneal cavity may be quite localized, so a normal sample of fluid collected at one point in the cavity may not be representative of the entire cavity
• Changes in peritoneal fluid, especially its chemical composition, e.g. lactate level, may be a reflection of a systemic change. The examination of a concurrently collected peripheral blood sample will make it possible to determine whether the changes are in fact restricted to the peritoneal cavity
• As in any clinicopathological examination the results must be interpreted with caution and only in conjunction with the history and clinical findings.
Specific properties of peritoneal fluid (normal and abnormal)
Color
Normal fluid is crystal clear, straw-colored to yellow. Turbidity indicates the presence of increased leukocytes and protein, which may include fine strands of fibrin.
A green color suggests food material; intense orange-green indicates rupture of the biliary system. A pink-red color indicates presence of hemoglobin, degenerated erythrocytes, entire erythrocytes and damage to vascular system by infarction, perforation or hydrostatic pressure. A red-brown color indicates the late stages of necrosis of the gut wall, the presence of degenerated blood and hemoglobin and damage to gut wall with hemorrhage.
Whole blood, clear fluid streaked with blood or heavily bloodstained fluid indicate that the sample has been collected from the spleen or a blood vessel or that there is hemoperitoneum. Rupture of the uterus or bladder or dicoumarol poisoning are also possibilities.
A dark green sample containing motile protozoa with very few leukocytes and no mesothelial cells indicates that the sample has been collected from the gut lumen. Enterocentesis has little apparent clinical affect in normal horses, although an occasional horse will show a transient fever. However, puncture of a devitalized loop of intestine may lead to extensive leakage of gut contents and a fatal peritonitis. The effect of enterocentesis of normal gut on peritoneal fluid is consistently to increase the neutrophilic count, which persists for several days.
Cellular and other properties
Surgical manipulation of the intestinal tract during exploratory laparotomy or intestinal resection and anastomosis in the horse results in a significant and rapid postoperative peritoneal inflammatory reaction.26 Manipulation of the viscera causes injury to the mesothelial surfaces. Total and differential nucleated cell counts, red blood cell numbers, and total protein and fibrinogen concentrations were all elevated on the first day after the surgery and remained elevated for up to 7 days in a study of this phenomenon.26
In cattle, exploratory celiotomy and omentopexy results in an increase in the total nucleated cell count by a factor of 5–8, minor increases in specific gravity and increases in total protein concentration by a factor of up to 2. These changes appear by two days after surgery and continue to increase through to day 6.27,28
Particulate matter in peritoneal fluid suggests either fibrin clots/strands or gut contents caused by leakage from a perforated or ruptured gut wall.
High specific gravity and high protein content are indicative of vascular damage and leakage of plasma protein, as in peritonitis or mural infarction.
The volume and viscosity of fluid varies. A normal flow is 1–5 mL per sample. A continuous flow with 10–20 mL per sample indicates excess fluid due to ruptured bladder or ascites (clear yellow), acute diffuse peritonitis (yellow, turbid), infarction or necrosis of gut wall (thin, red-tinged). The higher the protein content, as the peritoneal fluid shifts from being a transudate to an inflammatory exudate, the higher the viscosity becomes. Highly viscous fluid may clot.
Cells
A rapid staining method, using a modified Wright’s stain, gives a stained slide ready for examination within 5 minutes. The value of the technique is in indicating the number of leukocytes and other cells present, and in differentiating the types of cell.
An increase in total white cell count of the fluid including a disproportionate number of polymorphonuclear cells indicates acute inflammation, which may have an infectious origin or else be sterile. An increase in mononuclear phagocytes from the peritoneum is an indication of chronic peritonitis. Degenerate and toxic neutrophils suggest the probability of infection being present.
An increase in the number of mesothelial cells with the distinctive presence of actively dividing mitotic figures suggests neoplasia.
Bacteria found as phagocytosed inclusions in leukocytes, or by culture of fluid, indicate an infective peritonitis, which may arise by hematogenous spread, in which case the infection is likely to be a specific one. If there has been leakage from a peritoneal abscess the same comment applies, but if there is leakage through a segment of devitalized or perforated bowel wall there is likely to be a mixed infection and possibly particulate matter from bowel contents.
Entire erythrocytes, often accompanied by some hemoglobin, indicate either hemoperitoneum, in which case there should be active phagocytosis of erythrocytes, or that the sample has been inadvertently collected from the spleen. The blood is likely to be concentrated if there has been sufficient time for fluid resorption across the peritoneum. Splenic blood has a higher packed cell volume (PCV) also, but there is no erythrophagocytosis evident in the sample. A PCV of less than 5% in peritoneal fluid suggests extravasation of blood from an infarcted or inflamed gut wall; one of more than 20% suggests a significant hemorrhage.
Abdominocentesis in horses
In the horse the recommended site for paracentesis is on the ventral midline, 25 cm caudal to the xiphoid (or midway between the xiphoid and the umbilicus). Following surgical preparation and subcutaneous infiltration of an anesthetic, a stab incision is made through the skin and subcutaneous tissues and into the linea alba. A 9 cm long blunt-pointed bovine teat cannula, or similar metal catheter, with the tip wrapped in a sterile swab to avoid blood and skin contamination, is inserted into the wound and manipulated until the incision into the linea alba can be felt. With a quick thrust the cannula is pushed through the linea alba into the peritoneal cavity. A ‘pop’ is often heard on entry into the peritoneal cavity. Failure to incise into the linea alba first will cause many cannulas to bend and break.
In most horses (about 75%) a sample of fluid is readily obtained. In others it takes a moment or two before the fluid runs out, usually spurting synchronously with the respiratory movements. Applying suction with a syringe may yield some fluid if there is no spontaneous flow. Normal fluid is clear, yellow and flows easily through an 18-gauge needle. Two samples are collected, one in a plain tube and one in a tube with an anticoagulant. In case the fluid clots readily a few drops should be placed and smeared out on a glass slide and allowed to dry for staining purposes.
In peritonitis, the total leukocyte count will increase markedly, but wide variation in the total count can occur between horses with similar conditions, and in the same horse within a period of hours. Variations are due to the nature and stage of the lesion and to the total amount of exudate in the peritoneal cavity, which has a diluting effect on the total count. Total leukocyte counts ranging from 10000–150 000 μL have been recorded in peritonitis and in infarction of the intestine in horses. Experimentally, the intravenous injection of endotoxin into horses causes marked changes in the peripheral blood cellular components but there are no changes in the total white cell count of the peritoneal fluid.29
In healthy foals the reference values for peritoneal fluid are different than in adult horses.30 The maximum peritoneal fluid nucleated cell counts in foals are much lower than in adult horses (1.5 × 109/L versus 5.0 × 109/L. Nucleated cell counts greater than 1.5 × 109/L should be interpreted as elevated.
Peritoneal fluid abnormalities in mares within a week of foaling should be attributed to a systemic or gastrointestinal abnormality not due to the foaling event.31 The nucleated cell count, protein concentration, fibrinogen concentration and specific gravity of peritoneal fluid from recently foaled mares should be normal; however, differential cell counts may be abnormal for up to 1 week after foaling.
Risks
Abdominocentesis is not without some danger, especially the risk of introducing fecal contents into the peritoneal cavity and causing peritonitis. This appears to be of major importance only if there are loops of distended atonic intestine situated on the ventral abdominal wall. This is a common occurrence in the later stages of intestinal obstruction that is still amenable to surgery. Puncture of a devitalized loop of intestine may cause a leakage of intestinal contents and acute diffuse peritonitis, which is rapidly fatal. Penetration of a normal loop of intestine occurs often enough to lead to the conclusion that it appears to have no ill-effects. If a sample of peritoneal fluid is an important diagnostic need in a particular case and the first attempt at paracentesis causes penetration of the gut, it is recommended that the attempt be repeated, if necessary two or three times, at more posterior sites. Repeated abdominocentesis does not cause alterations in peritoneal fluid constituents and any significant changes are likely due to alterations in the disease state present.32 The technique most likely to cause bowel penetration is the use of a sharp needle instead of the blunt cannula recommended, and forcibly thrusting the cannula through the linea alba without a prior incision. When the suggested incision is made in the linea alba, the cannula can be pushed gently through whilst rotating it.
Abdominocentesis in cattle
The choice of sites for paracentesis is a problem because the rumen covers such a large portion of the ventral abdominal wall and avoiding penetration of it is difficult. Cattle have a low volume of peritoneal fluid, and failure to obtain a sample is not unusual.27 The most profitable sites are those that, on an anatomical basis, consist of recesses between the forestomachs, abomasum, diaphragm and liver. These are usually caudal to the xiphoid sternum and 4–10 cm lateral to the midline. Another recommended site is left of the midline, 3–4 cm medial and 5–7 cm cranial to the foramen for the left subcutaneous abdominal vein. A teat cannula similar to the one described for use in the horse is recommended but, with care and caution, a 16-gauge 5 cm hypodermic needle may also be used. The needle or cannula is pushed carefully and slowly through the abdominal wall, which will twitch when the peritoneum is punctured. When this happens the fluid will usually run out into a vial without the aid of a vacuum. However, if it does not, a syringe may be used and the needle may be moved backwards and forwards in a search for fluid, with the piston of the syringe withdrawn. A further site is the right caudoventral abdominal wall medial to the fold of the flank, using a 3.8 cm, 15-gauge needle.27
In calves, a reliable technique includes the use of sedation with intravenous xylazine hydrochloride and diazepam. The animal is placed in left lateral recumbency with the right hind limb pulled dorsally and caudally. One site slightly dorsal and caudal to the umbilicus is prepared together with another site in the center of the inguinal region. The site is prepared with local anesthetic and a 14-gauge needle is introduced and directed slightly caudally and toward the midline while keeping it parallel to the inner abdominal wall once the peritoneal cavity is entered.33 A 3.5 gauge urinary catheter (1.2 mm × 56 cm sterile feeding tube) is inserted through the needle and a 3 mL sterile syringe is attached to the catheter. Gentle suction is applied. The fluid is placed in a 2 mL tube containing tri-potassium EDTA. A 14-gauge over-the-needle catheter can also be used, followed by insertion of a 3.5 French feeding tube. If fluid cannot be obtained from the first site, the inguinal site is used using the same basic technique and with the catheter directed slightly cranially toward the midline.
Failure to obtain a sample does not preclude the possibility that peritonitis may be present: the exudate may be very thick and contain large masses of fibrin, or the peritonitis may be localized. Also, animals that are dehydrated may have less peritoneal fluid than normal. Most animals from which samples cannot be obtained, however, are in fact normal. In animals in which peritonitis is strongly suspected for clinical reasons, up to four attempts at paracentesis should be made before aborting the procedure. The fluid should be collected into an anticoagulant, preferably EDTA, to avoid clotting.
Abnormal peritoneal fluid in cattle is a highly sensitive indicator of peritoneal disease, but not a good indicator of the nature of the disease. The most pronounced abnormalities occur in acute diseases of the peritoneum; chronic peritonitis may be accompanied by peritoneal fluid which is almost normal.
Examination of the fluid should take into account the following characteristics:
• Large amounts (10–20 mL) of serosanguineous fluid suggests infarction or necrosis of the gut wall
• Heavily bloodstained fluid, whole blood or fluid with streaks of blood through it are more likely to result from puncture of a blood vessel or from bleeding into the cavity, as in dicoumarol poisoning or with a neoplasm of the vascular system
• The same sort of bloodstained fluid as above may accompany a ruptured uterus or bladder or severe congestive heart failure
• Large quantities of yellowish-colored turbid fluid suggests acute diffuse peritonitis. The degree of turbidity depends on the number of cells and the amount of fibrin present
• Particulate food material in the sample indicates perforation or rupture of the gut, except that penetration of the gut with the instrument during collection may be misleading. Such samples are usually heavily fecal in appearance and contain no mesothelial cells
• Laboratory examination is necessary to derive full benefit from the sample. This will include assessment of: the number and type of leukocytes present – the number is increased in peritonitis, neutrophils predominating in acute peritonitis and monocytes in chronic forms; the number of erythrocytes present; whether bacteria are present inside or outside the neutrophils; and total protein content.
The significant values for these items are included in Table 5.1.
Reference values for peritoneal fluid constituents of normal adult cattle may be inappropriate for interpretation of peritoneal fluid analysis in calves of up to 8 weeks of age.34 The peritoneal fluid nucleated cell count and mononuclear cell counts are higher in calves, and the eosinophil counts are lower than in adult cows.
INTESTINAL AND LIVER BIOPSY
An intestinal biopsy may be obtained from an exploratory laparotomy but is costly and time-consuming. Rectal biopsy is easily done and of low cost. It is a valuable diagnostic aid for evaluating certain intestinal diseases of the horse.35 Biopsy specimens are taken using minimal restraint and unaided by proctoscopic visualization in the standing horse. A rectal biopsy forceps is used to obtain the biopsy from the floor of the rectum approximately 30 cm proximal to the anal sphincter.
The technique for liver biopsy is presented in Chapter 7.
Principles of treatment in alimentary tract disease
Removal of the primary cause of the disease is essential but a major part of the treatment of diseases of the alimentary tract is supportive and symptomatic. This is aimed at relieving pain and distension, replacement of fluids and electrolytes, correcting abnormal motility and relieving tenesmus and reconstitution of the digestive flora if necessary. Specific treatment for individual diseases is presented with each disease throughout this book. General principles are outlined here.
RELIEF OF ABDOMINAL PAIN
The relief of abdominal pain is of prime importance from the humane aspect, to prevent the animal from self-injury associated with falling and throwing itself against a wall or other solid objects, and to allay the concerns of the owner. No single analgesic is completely satisfactory for every situation. Non-narcotic and narcotic analgesics are in general use and are discussed under the heading of pain, and under the individual diseases. The analgesics used in the important subject of equine colic are presented under that heading.
RELIEF OF DISTENSION
The relief of distension of the gastrointestinal viscera is a critical principle in order to minimize shock and to prevent rupture of the viscus. Relief of distension of the stomach of the horse with colic is accomplished by nasogastric intubation. Distension due to bloat in cattle can be relieved by stomach tube or trocarization of the rumen. Relief of distension may be possible by medical means alone with the use of laxatives and purgatives when there is accumulation of ingesta without a physical obstruction. Surgical intervention is often necessary when the distension is associated with a physical obstruction. In functional distension (paralytic ileus), relief of the atony or spasm can be effected by the use of drugs such as metoclopramide. Distension due to intestinal or gastric accidents requires surgical correction.
REPLACEMENT OF FLUIDS AND ELECTROLYTES
Replacement of fluid and electrolytes lost in gastrointestinal disease is one of the most important principles of treatment. In gastric or intestinal obstruction, or when diarrhea is severe, it is necessary to replace lost fluids and electrolytes by the parenteral administration of large quantities of isotonic glucose–saline or other physiologically normal electrolyte solutions. The amount of fluid lost may be very large and fluids must be given in quantities to replace losses and to support continuing losses and maintenance requirements. In acute, severe dehydration in horses, such as occurs in acute intestinal obstruction, the amount of fluid required before and during surgery ranges from 50–100 mL/kg BW per 24 hours. It is critical that administration of fluid be commenced at the earliest possible time because of the need to maintain homeostasis and thus ameliorate the almost impossible task of restoring animals to normal before surgery is to be attempted. Details of fluid therapy are given in the section on disturbances of water, electrolytes and acid–base balance in Chapter 2.
In young animals the need is much greater still and amounts of 100 mL/kg BW, given slowly intravenously, are commonly necessary and not excessive. The treatment of shock is also presented in Chapters 2 and 9 and includes the administration of fluids, plasma or blood and NSAIDs. The use of intravenous hypertonic saline followed by the ingestion of large quantities of water by the animal is another aspect of fluid therapy in gastrointestinal disease (see Chapter 2).
CORRECTION OF ABNORMAL MOTILITY
INCREASED MOTILITY
When motility is increased, the administration of atropine or other spasmolytics such as dipyrone or proquamezine is usually followed by disappearance of the abdominal pain and a diminution of fluid loss. Meperidine, butorphanol and pentazocine inhibit regular cyclic myoelectric activity in the jejunum.36 There is a need for some scientific clinical investigation into the desirability of treating intestinal hypermotility, if it does exist in enteritis for example, and the efficacy of anticholinergics. Loperamide has an antidiarrheal effect in experimentally induced diarrhea in calves but the mechanism of action does not involve changes in intestinal motility.
DECREASED MOTILITY
When gastrointestinal motility is decreased, the usual practice is to administer parasympathomimetic drugs or purgatives, usually combined with an analgesic. Prokinetic drugs such as metoclopramide hydrochloride and cisapride monohydrate increase the movement of ingesta through the gastrointestinal tract.37 They are useful because they induce coordinated motility patterns.
Metoclopramide
Metoclopramide, acting in the upper gastrointestinal tract, increases acetylcholine release from neurons and increases cholinergic receptor sensitivity to acetylcholine. It is a dopamine antagonist and stimulates and coordinates esophageal, gastric, pyloric and duodenal motor activity. It increases lower esophageal sphincter tone and stimulates gastric contractions, while relaxing the pylorus and duodenum. This results in accelerated gastric emptying and reduced esophageal reflux. The transit time of ingested material from the duodenum to the ileocecal valve is reduced, due to increased jejunal peristalsis. It has little or no effect on colonic motility. The pharmacokinetics of metoclopramide in cattle have been studied.38
Metoclopramide crosses the blood– brain barrier, where its dopamine antagonist activity at the chemoreceptor trigger zone can result in an antiemetic effect. It can also result in involuntary activity including tremors, restlessness and aggressive behavior characterized by charging and jumping walls. This can be reversed by the use of an anticholinergic such as diphenhydramine hydrochloride intravenously at 0.5–2.0 mg/kg BW.
Indications for metoclopramide include reflux esophagitis and gastritis, chronic gastritis associated with delayed emptying, abomasal emptying defects in ruminants, gastric stasis following gastric dilatation and volvulus surgery, and postoperative ileus. It is contraindicated in animals with physical obstruction of the gastrointestinal tract.
In horses, the dose is 0.125–0.25 mg/kg BW diluted in multiple electrolyte solution and given intravenously over 60 minutes.37 It is used for stimulating equine gastric and small intestinal activity at dose rates of 0.25 mg/kg BW per hour when there is intestinal hypomotility.39 Given as continuous intravenous infusion of 0.04 (mg/kg)/h it can decrease the incidence and severity of persistent postoperative ileus following resection and anastomosis of the small intestine in horses without serious side effects.40
In cattle and sheep metoclopramide is used at 0.3 mg/kg BW subcutaneously every 6–8 hours. Metoclopramide did not alter cecocolic myoelectrical activity in cattle.41
Cisapride
Cisapride promotes gastrointestinal motility by enhancing the release of acetylcholine from postganglionic nerve endings of the myenteric plexus. Cisapride is more potent and has broader prokinetic activity than metoclopramide by increasing the motility of the colon as well as the esophagus, stomach and small intestine.37 It is does not have dopaminergic effects and does not have either the antiemetic or the extrapyramidal effects of metoclopramide. Cisapride is useful for the treatment of gastric stasis, gastroesophageal reflux and postoperative ileus. In horses, cisapride increases left dorsal colon motility and improves ileocecal junction coordination. The suggested dose is 0.1 mg/kg BW orally every 8 hours. Cisapride may have some value in the clinical management of cecal dilatation in cattle.42
Xylazine and naloxone
While xylazine is used for alleviation of visceral pain in horses and cattle, it is not indicated in cecal dilatation in cattle because it reduces the myoelectric activity of the cecum and proximal loop of the ascending colon.42 Naloxone, a widely used opiate antagonist with a high affinity for μ receptors, is also not indicated for medical treatment of cecal dilatation when hypomotility must be reversed.
Bethanechol, neostigmine
Bethanechol is a methyl derivative of carbachol and classified as a direct-acting cholinomimetic drug. Its action is more specific on the gastrointestinal tract and urinary bladder. Neostigmine, a cholinesterase inhibitor, is an indirect-acting cholinergic drug with motor-stimulating activities but only on the gastrointestinal tract. Bethanechol at 0.07 mg/kg BW intramuscularly may be useful for medical treatment of cecal dilatation in cattle in which hypomotility of the cecum and proximal loop of the ascending colon must be reversed.41 Neostigmine at 0.02 mg/kg BW intramuscularly increased the number of propagated spike sequences but they were uncoordinated.41
RELIEF OF TENESMUS
Tenesmus can be difficult to treat effectively. Long-acting epidural anesthesia and sedation are in common use. Combinations of xylazine and lidocaine may be used. Irrigation of the rectum with water and the application of topical anesthetic in a jelly-like base are also used.
RECONSTITUTION OF RUMEN FLORA AND CORRECTION OF ACIDITY OR ALKALINITY
When prolonged anorexia or acute indigestion occurs in ruminants, the rumen flora may be seriously reduced. In convalescence, the reconstitution of the flora can be hastened by the oral administration of a suspension of ruminal contents from a normal cow, or of dried ruminal contents, which contain viable bacteria and yeasts and the substances necessary for growth of the organisms.
The pH of the rumen affects the growth of rumen organisms, and hyperacidity, such as occurs on overeating of grain, or hyperalkalinity, such as occurs on overeating of protein-rich feeds, should be corrected by the administration of alkalinizing or acidifying drugs as the case may be.
1 Roussel AJ. Compend Contin Educ Pract Vet. 1994;16:1433.
2 Navarre CB, Roussel AJ. J Vet Intern Med. 1996;10:51.
3 Yarbrough TB, et al. J Am Vet Med Assoc. 1994;205:592.
4 Fischer AT, et al. Vet Radiol. 1987;28:42.
5 Klohnen A, et al. J Am Vet Med Assoc. 1996;209:1597.
6 Pease AP, et al. Vet Radiol Ultrasound. 2004;45:220.
7 Kirberger RM, et al. Vet Radiol Ultrasound. 1995;36:50.
8 Korolainen R, Ruohoniemi M. Equine Vet J. 2002;34:499.
9 Mitchell CF, et al. Can Vet J. 2005;46:134.
10 Aleman M, et al. Equine Vet J. 2002;34:649.
11 Braun U. Vet J. 2003;166:112.
12 Braun U. Vet J. 2002;150:75.
13 Braun U, Marmier O. Vet Rec. 1995;136:239.
14 Galuppo LD, et al. Am J Vet Res. 1995;56:518.
15 Galuppo LD, et al. Am J Vet Res. 1996;57:923.
16 Kronfeld DS, et al. J Am Vet Med Assoc. 2005;226:712.
17 Mair TS, et al. Equine Vet J. 1991;23:344.
18 van Amstel SR, et al. J S Afr Vet Assoc. 1984;55:119.
19 Gutzwiller A, Blum JW. Am J Vet Res. 1996;57:560.
20 Freeman DE, et al. Am J Vet Res. 1989;50:1609.
21 Brown CM. Br Vet J. 1992;148:41.
22 Freeman DE, et al. Am J Vet Res. 1989;50:1609.
23 O’Connor MS, et al. Am J Vet Res. 2004;65:31.
24 Hewetson M, et al. J Vet Intern Med. 2006:20.
25 Menzies-Gow NJ, et al. Vet Rec. 2003;153:457.
26 Blikslager AT, et al. J Am Vet Med Assoc. 1994;205:1748.
27 Hanson RR, et al. Am J Vet Res. 1992;53:216.
28 Anderson DE, et al. Am J Vet Res. 1994;55:1633.
29 Valadao CAA, et al. J Equine Vet Sci. 1995;15:124.
30 Grindem CB, et al. Equine Vet J. 1990;22:359.
31 Hoogmoed L, et al. J Am Vet Med Assoc. 1996;209:1280.
32 Juzwiak JS, et al. Vet Res Commun. 1991;15:177.
33 Burton S, et al. Vet Clin Pathol. 1997;26:38.
34 Anderson DE, et al. Am J Vet Res. 1995;56:973.
35 Lindberg R, et al. Equine Vet J. 1996;28:275.
36 Sojka JE, et al. Am J Vet Res. 1988;49:527.
37 Dowling PM. Can Vet J. 1995;36:115.
38 Jones RD, et al. J Vet Pharmacol Ther. 1994;17:1141.
39 Hunt JM, Gerring EL. J Vet Pharmacol Ther. 1989;9:109.
40 Dart AJ, et al. Aust Vet J. 1996;74:280.
Diseases of the buccal cavity and associated organs
DISEASES OF THE MUZZLE
The congenital defect of harelip may be contiguous with a cleft palate. Severe dermatitis with scab formation, development of fissures, and sloughing and gangrene of the skin of the muzzle are common lesions in cattle affected with photosensitive dermatitis, bovine malignant catarrh, bovine virus diarrhea and rinderpest.
In sheep severe lesions of the muzzle are less common, but occur in bluetongue and ecthyma.
In pigs, only the vesicular diseases – vesicular exanthema of swine, swine vesicular disease, and foot-and-mouth disease – cause such lesions on the snout and on other sites. The lesions are vesicular initially and confusion has arisen in recent years because of isolated incidents in Australia and New Zealand in which such outbreaks occurred but in which no pathogenic agent was identified.
STOMATITIS
Stomatitis is inflammation of the oral mucosa and includes glossitis (inflammation of the tongue), palatitis (lampas) (inflammation of the palate) and gingivitis (inflammation of the mucosa of the gums). Clinically it is characterized by partial or complete loss of appetite, smacking of the lips and profuse salivation. It is commonly an accompaniment of systemic disease.
ETIOLOGY
Stomatitis may be caused by physical, chemical or infectious agents, the last being the largest group of causes. The agents are listed under these group headings below.
Physical agents
• Trauma while dosing orally with a balling gun
• Laceration of the tongue1
• Sharp awns or spines on plants. The commonest lesions are on the gums of cattle and sheep just below the corner incisors where tough grass is pulled around the corner of the incisor arcade. In spear grass country the alveoli are often stuffed full of grass seeds. Very young animals, e.g. 1–6-week-old lambs, are particularly susceptible to traumatic injury from abrasive feed.2 Among the most dramatic lesions are those in the mouths of horses. They are large (2–3 cm long and 5 mm wide) and linear in shape. They may be caused by eating hairy caterpillars that infest pasture, or by the awns in hay or chaff made from triticale (a hybrid of wheat and rye) and a yellow bristle grass (Setaria lutescens). Foxtail awns can cause multiple painful nodules on the lips of horses that have eaten hay contaminated with the awns
• The strength and thickness of the awn in dwarf barley cultivars used to make silage fed to feedlot cattle in some regions is associated with mouth lesions. The incidence of tongue lesions in slaughter cattle in some areas can be about 19% and the incidence is higher in cattle finished on silage from semidwarf rough awn (29.3%) compared to normal-stem rough awn (13.5%) and normal-stem smooth awn barley (11.8%)3
• Eating frozen feed and drinking hot water are recorded, but seem highly improbable
• Ulcers of the soft palate of horses may be due to mechanical trauma associated with dorsal displacement of the soft palate.4
Chemical agents
• Irritant drugs, e.g. chloral hydrate, administered in overstrong concentrations
• Counterirritants applied to skin, left unprotected and licked by the animal, including mercury and cantharides compounds
• Irritant substances administered by mistake, including acids, alkalis and phenolic compounds
• Manifestation of systemic poisoning, e.g. chronic mercury poisoning. Poisoning with bracken, Heraclum mantegazzianum, furazolidone and some fungi (Stachybotrys, Fusarium spp. and mushrooms) cause a combination of focal hemorrhages and necrotic ulcers or erosions. They are a common cause of confusion with vesicular or erosive disease
Infectious agents
Cattle
• Oral necrobacillosis associated with Fusobacterium necrophorus
• Actinobacillosis of the bovine tongue is not a stomatitis, but there may be one or two ulcers on the dorsum and sides of the tongue and on the lips. Characteristically, there is initially an acute diffuse myositis of the muscle of the tongue, followed by the development of multiple granulomas and subsequently fibrosis and shrinkage
• Ulcerative, granulomatous lesions may occur on the gums in cases of actinomycosis
• Stomatitis with vesicles occurs in foot-and-mouth disease and in vesicular stomatitis
• Erosive, with some secondary ulcerative, stomatitis occurs in bovine viral diarrhea (mucosal disease), bovine malignant catarrh, rinderpest and rarely in bluetongue. Cases of infectious bovine rhinotracheitis in young calves may have similar lesions
• Proliferative lesions occur in papular stomatitis, proliferative stomatitis and rare cases of rhinosporidiosis and papillomatosis where the oral mucosa is invaded
• Oral mucosal necrosis in bovine sweating sickness
• Nondescript lesions varying from erosions to ulcers occur late in the stages of many of the above diseases when secondary bacteria have invaded the breaches in the mucosa. In some cases the involvement goes deeper still and a phlegmonous condition or a cellulitis may develop. Thus, lesions that were initially vesicular are converted to what look like bacterial ulcers. Secondary infection with fungi, especially Monilia spp., may also occur.
Sheep
• Erosive lesions in bluetongue, rinderpest and peste de petits ruminantes
• Vesicular lesions rarely in foot and mouth disease
• Granulomatous lesions due to ecthyma are not unusual in the mouth, especially in young lambs. Similarly, oral lesions occur in bad cases of sheep pox, ulcerative dermatosis, coital exanthema and mycotic dermatitis.
Horses
• Cheilitis and gingivitis (inflammatory nodules of the lips and gums caused by plant awns)
• Vesicular lesions in vesicular stomatitis
• Herpesvirus infections are commonly accompanied by small (1 mm diameter) vesicles surrounded by a zone of hyperemia. The lesions are in groups and at first glance appear to be hemorrhages
Bullous stomatitis
• Bullous stomatitis has been reported in the horse and may be associated with a paraneoplastic pemphigus syndrome.5
Many other causes of stomatitis have been suggested but the relationship of these conditions to the specific diseases listed above is unknown. It is common to find stomatitides that cannot be defined as belonging to any of these etiological groups. An example is necrotic glossitis reported in feeder steers in the USA in which the necrotic lesions are confined to the anterior part of the tongue.
PATHOGENESIS
The lesions of stomatitis are produced by the causative agents being applied directly to the mucosa, or gaining entrance to it by way of minor abrasions, or by localization in the mucosa from a viremia. In the first two instances, the stomatitis is designated as primary. In the third, it is usually described as secondary because of the common occurrence of similar lesions in other organs or on other parts of the body, and the presence of a systemic disease. The clinical signs of stomatitis are caused by the inflammation or erosion of the mucosa and the signs vary in severity with the degree of inflammation.
CLINICAL FINDINGS
There is partial or complete anorexia and slow, painful mastication. Chewing movements and smacking of the lips are accompanied by salivation, either frothy and in small amounts, or profuse and drooling if the animal does not swallow normally. The saliva may contain pus or shreds of epithelial tissue. A fetid odor is present on the breath only if bacterial invasion of the lesion has occurred. Enlargement of local lymph nodes may also occur if bacteria invade the lesions. Swelling of the face is observed only in cases where a cellulitis or phlegmon has extended to involve the soft tissues. An increased desire for water is apparent and the animal resents manipulation and examination of the mouth.
Toxemia may be present when the stomatitis is secondary to a systemic disease or where tissue necrosis occurs. This is a feature of oral necrobacillosis and many of the systemic viremias. In some of the specific diseases, lesions may be present on other parts of the body, especially at the coronets and mucocutaneous junctions.
Several different lesions of the oral cavity may be present and their characteristic appearances are as follows. The importance of vesicular diseases such as foot-and-mouth disease means that the recognition and differentiation of these lesions assumes major importance.
Erosions are shallow, usually discrete, areas of necrosis, which are not readily seen in the early stages. They tend to occur most commonly on the lingual mucosa and at the commissures of the mouth. The necrotic tissue may remain in situ but is usually shed, leaving a very shallow discontinuity of the mucosa with a dark red base that is more readily seen. If recovery occurs, these lesions heal very quickly.
Vesicles are thin-walled swellings 1–2 cm in diameter filled with clear serous fluid. They are very painful and rupture readily to leave sharp-edged, shallow ulcers.
Ulcerative lesions penetrate more deeply to the lamina propria and are painful, as in necrotic stomatitis in calves associated with F. necrophorus. In lambs the tongue may be swollen and contain many microabscesses infected with Actinomyces (Corynebacterium) pyogenes. There is an accompanying abscessation of the pharyngeal lymph nodes.2
Proliferative lesions are characterized by an abnormality raised above the surface of the mucous membrane as in oral papillomatosis. Traumatic lesions are usually solitary and characterized by a discontinuity in the mucous membrane often with evidence of healing and the presence of granulation tissue.
Catarrhal stomatitis is manifested by a diffuse inflammation of the buccal mucosa and is commonly the result of direct injury by chemical or physical agents. Mycotic stomatitis is characterized by a heavy, white, velvety deposit with little obvious inflammation or damage to the mucosa.
Deformity of or loss of tissue at the tip of the tongue may result in a chronic syndrome of chewing and swallowing food in such a way that food is always oozing from between the lips. In sheep this may cause permanent staining of the hair around the mouth, creating an appearance similar to that of a tobacco-chewer. Loss of the tip is usually the result of predator attack on a newborn or sick lamb.
Laceration of the tongue can result in complete or partial severance of the organ, with the severed portion protruding from the oral cavity. In cattle, glossectomy interferes with prehension and the animal is unable to eat. Excessive loss of saliva is common because of interference with swallowing.
Ulceration of the soft palate of horses may occur in 16% of horses with dorsal displacement of the soft palate and is characterized clinically by reduced exercise tolerance, respiratory noise during light exercise or racing, dysphagia and coughing after exercising.4 The ulcers can be viewed by upper respiratory airway video-endoscopy. Bullous stomatitis in the horse is characterized by intact or ruptured vesicles on the peripheral margin of the tongue, the sublingual region and the mucosa of the oral cavity and lips.
CLINICAL PATHOLOGY
Material collected from lesions of stomatitis should be examined for the presence of pathogenic bacteria and fungi. Transmission experiments may be undertaken with filtrates of swabs or scrapings if the disease is thought to be due to a viral agent.
NECROPSY FINDINGS
The oral lesions are easily observed but complete necropsy examinations should be carried out on all fatally affected animals to determine whether the oral lesions are primary or are local manifestations of a systemic disease.
• Particularly in cattle, and to a less extent in sheep, the diagnosis of stomatitis is most important because of the occurrence of oral lesions in a number of highly infectious viral diseases. The diseases are listed under etiology and their differentiation is described under their specific headings elsewhere in this book
• Careful clinical and necropsy examinations are necessary to define the type and extent of the lesions if any attempt at field diagnosis is to be made
• In cattle, lymphoma of the ramus of the mandible may spread extensively through the submucosal tissues of the mouth causing marked swelling of the gums, spreading of the teeth, inability to close the mouth and profuse salivation. There is no discontinuity or inflammation of the buccal mucosa but gross enlargement of the cranial lymph nodes is usual
• The differentiation of causes of hypersalivation must depend on a careful examination of the mouth (the causative gingivitis is often surprisingly moderate in horses) and an awareness of the volume of increased saliva output caused by toxic hyperthermia, e.g. in fescue and ergot poisonings
• Poisoning by the mycotoxin slaframine also causes hypersalivation
TREATMENT
Affected animals should be isolated and fed and watered from separate utensils if an infectious agent is suspected. Specific treatments are described under the headings of the individual diseases. Nonspecific treatment includes frequent application of a mild antiseptic collutory such as a 2% solution of copper sulfate, a 2% suspension of borax or a 1% suspension of a sulfonamide in glycerin. Indolent ulcers require more vigorous treatment and respond well to curettage or cauterization with a silver nitrate stick or tincture of iodine.
In stomatitis due to trauma, the teeth may need attention. In all cases, soft, appetizing food should be offered and feeding by stomach tube or intravenous alimentation may be resorted to in severe, prolonged cases. If the disease is infectious, care should be exercised to insure that it is not transmitted by the hands or dosing implements.
DISEASES OF THE TEETH
Surgical diseases of the teeth of animals are presented in textbooks of surgery. Some of the medical aspects of diseases of the teeth of farm animals are described here.
ETIOLOGY
The causes may be congenital or acquired.
Congenital defects
• Malocclusion of sufficient degree to interfere with prehension and mastication
• Red-brown staining of inherited porphyrinuria of cattle
• Defective enamel formation on all teeth combined with excessive mobility of joints in inherited defect of collagen metabolism in Holstein/Friesian cattle identified as bovine osteogenesis imperfecta. The teeth are pink and obviously deficient in substance. This defect is also recorded in a foal with severe epitheliogenesis imperfecta.1
Dental fluorosis
The teeth are damaged before they erupt and show erosion of the enamel. See the section on fluorosis.
Enamel erosion
The feeding of acidic byproduct feed such as sweet potato cannery waste, which is acidic because of the presence of lactic acid, can cause erosion of the enamel of the incisors of cattle.2,3 Exposure of incisor teeth in vitro to a supernatant of cannery waste or lactic acid at pH 3.2 results in removal of calcium from the surface enamel of bovine teeth. Neutralizing the cannery waste to a pH of 5.5 does not cause detectable etching of the teeth. Feeding cattle with heavily compacted silage is also associated with loss of incisor enamel and severe incisor wear.4
Premature wear and loss of teeth in sheep (periodontal disease)
Premature loss of incisor teeth or ‘broken mouth’ causes concern because of the early age at which affected sheep have to be culled. Broken mouth is a chronic inflammatory disease of the tissue supports of the tooth.5 Between 60% and 70% of ewes sold at slaughter in England and Scotland have loose or missing incisor teeth.6 Broken mouth is geographically specific and it seems that once the disease is established on a particular farm, the animals are permanently susceptible.7 Many sheep are culled before the end of their useful reproductive life because of broken mouth. The problem is particularly severe in New Zealand and the hill country in Scotland.8 The cause is uncertain but environmental factors that result in periodontal disease are probably important. Bacteroides gingivalis, an organism that is found in plaque from sheep’s teeth, has been found with increased frequency in diseased compared to unaffected animals.8 The depths of the gingival crevice of sheep are heritable and it is possible that deeper crevices may already be harboring greater numbers of periodontally pathogenic bacteria so that when the animals are exposed to a broken-mouth environment they may be more prone to the changes.9 While nutrition and mineral deficiencies influence dental development and tooth eruption of sheep, there is no significant difference in calcium or phosphorus status between control and affected populations of sheep. Low planes of nutrition have delayed eruption of the permanent dentition and retarded mandibular growth but these changes are not seen in broken mouth in sheep. The occurrence of this periodontal disease is higher on some soil types than on others. The ingestion of irritating materials such as sand and spiny grass seeds10 has been suggested as causes, but they are considered to be secondary complications in a pre-existing disease.
Another dental disease of sheep is also recorded on an extensive scale in New Zealand.11 There is excessive wear of deciduous incisors but no change in the rate of wear of the molar teeth. The incisor wear is episodic and is not due to any change in the supportive tissues, nor is there any change in the intrinsic resistance to wear of the incisor teeth.12 The disease is not related to an inadequate dietary intake of copper or vitamin D and is thought to be caused by the ingestion of soil particles.13 The two New Zealand diseases do not occur together and have no apparent effect on body condition score.14
Dentigerous cysts have been described in ewes in the South Island of New Zealand with a prevalence of 0.91%.15
PATHOGENESIS
There are some limitations to the use of number of incisors for determining age in sheep.16 In mixed-age female sheep flocks, the median age when two, four, six and eight incisors come into wear is 15, 23, 30 and 42 months of age, respectively.16 Errors will be made by assuming that all sheep gain a pair of permanent incisors at annual intervals between 1.5 and 4.5 years of age.
In periodontal disease or broken-mouth disease of sheep the primary lesion is an acute gingivitis around permanent incisors and premolars at the time of their eruption. This subsides leaving a chronic gingivitis and an accumulation of subgingival plaque. On some farms, for reasons not understood, this gingivitis penetrates down into the alveoli, causing a severe periodontitis and eventual shedding of the teeth.17 The severity of the gingivitis can vary between farms.18 The disease is episodic in nature, with discrete acute inflammatory incidents leading to periodontal injury that may resolve by healing.8 The balance between repair and the various short- and long-term acute episodes probably accounts for the large variation in incidence and age onset of tooth loss both within and between flocks.4 The inflammatory periodontal disease markedly affects the tooth’s mobility.19 Collagen fibrils supporting the tooth become abnormal. The deepened periodontal pocket resulting from inflammation removes the major area of support for the tooth and abnormal loads are applied to fibers deeper within the tissue.20 While the incisor teeth are usually most severely affected, the cheek teeth are also involved.5 In some unusual circumstances the gingivitis appears to arise from heavy deposits of dental calculus.21 In the Scottish disease there is local alveolar bone loss but no accompanying general skeletal deficiency.6
CLINICAL FINDINGS
The most obvious evidence of broken-mouth disease is incisor tooth loss, which usually occurs when sheep are between 3.5 and 6.6 years; normal sheep without broken mouth will retain their teeth beyond 7 years of age. Several dental health indices can assist to assess the amount of gingivitis, tooth movement, gum recession and pocketing.5 Gingivitis is characterized by redness and edema of the attached gingiva. Bleeding from the gingivae is also a feature. Clinical gingivitis is evident as soon as the permanent teeth erupt. Chronic gingivitis results in a downward retreat of the gum margin, loss of its normal, scalloped shape and fibrosis of the gingiva. Within a year prior to tooth loss, tissue damage around the incisors leads to deepening of the gingival sulcus and the formation of pockets which are readily detected by the use of graduated dental measuring probes. The normal sulcus is 0.5–1.0 mm deep labially and up to 4 mm deep lingually; pockets may be over 1.0 cm in depth prior to tooth loss. Crown lengthening, protrusion, hemorrhages, loosening and lingual periodontitis are characteristic. If sheep affected with broken mouth periodontal disease are examined over a 12-month period, only a few animals undergo clinically significant destruction.8 The relationship between periodontal disease and body condition score in sheep is variable.14
Secondary starvation occurs even with a plentiful feed supply. Inspection of the mouth may reveal the worn or damaged incisor teeth but the molar teeth are not easily inspected in the living animal and tooth lesions can be missed. Since it is common to find that both incisors and molars are all affected, damage to incisors should lead the clinician to suspect that molar disease is also present.
Cattle fed sweetpotato cannery waste develop black, stained teeth with severe enamel erosion.3
An abattoir survey of dental defects in cull cows, all over 30 months of age found that 14.6% had one or more missing incisors, most of which were acquired losses.22 Rotation and overlapping of rostral teeth were common, as was attrition. Congenitally absent first lower premolars, other missing teeth, large and often multiple interdental spaces and a few cases of macrodontia, cavitation, multiple defects and fractures were observed in cheek tooth arcades. There were also some unusual patterns of premolar and molar attrition, often attributable to malocclusion, one result of which was the formation of a hook at the posterior extremity of the third maxillary molar.
TREATMENT AND CONTROL
There is no reliable treatment and control for broken mouth in sheep. The use of dental prosthetics glued to the incisors when the ewe has three pairs of incisors in place is being investigated. The use of antimicrobials has been proposed to control the gingivitis but there is no apparent effect on the periodontal disease. Cutting the incisor teeth of ewes to control premature tooth loss has been explored but the practice has been banned in the UK.23
1 Dubielzig RR, et al. Vet Pathol. 1986;23:325.
2 Rogers GM, et al. Am J Vet Res. 1997;58:498.
3 Rogers GM, et al. J Am Vet Med Assoc. 1999;214:681.
4 Smith AJ, et al. Vet Rec. 1992;130:352. 455, 479
5 Spence JA, et al. Res Vet Sci. 1988;45:324.
6 Aitchison GU, Spence JA. J Comp Pathol. 1984;94:285. 95:505
7 Frisken KW, et al. Res Vet Sci. 1989;46:147.
8 Laws AJ, et al. NZ Vet J. 1988;36:32.
9 Laws AJ, et al. Res Vet Sci. 1993;54:379.
10 Anderson BC, et al. J Am Vet Med Assoc. 1984;184:737.
11 Thurley DC. NZ Vet J. 1984;32:25. 33:25, 157
12 Erasmuson AF. NZ Vet J Agric Res. 1985;28:225.
13 Millar KR, et al. NZ Vet J. 1985;33:41.
14 Orr MB, Chalmers M. Aust Vet J. 1988;36:171.
15 Orr MB, Gardner DG. Aust Vet J. 1996;44:198.
16 Bray A, et al. Proc NZ Soc Anim Prod. 1989;49:303.
17 Morris PL, et al. NZ Vet J. 1985;33:87. 131
18 Thurley DC. J Comp Pathol. 1987;97:375.
19 Moxham BJ, et al. Res Vet Sci. 1990;48:99.
20 Shore RC, et al. Res Vet Sci. 1998;47:148.
21 Baker JR, Britt DP. Vet Rec. 1984;115:411.
PAROTITIS
Parotitis is inflammation of any of the salivary glands.
ETIOLOGY
Parotitis may be parenchymatous, when the glandular tissue is diffusely inflamed, or it may be a local suppurative process. There are no specific causes in farm animals, cases occurring only sporadically and due usually to localization of a blood-borne infection, invasion up the salivary ducts associated with stomatitis, irritation by grass awns in the duct, or salivary calculi.1 Avitaminosis A often appears to be a predisposing cause.
Local suppurative lesions are caused usually by penetrating wounds or extension from a retropharyngeal cellulitis or lymph node abscess. Neoplasia of the parotid glands of cattle examined at slaughter has been described.2
PATHOGENESIS
In most cases only one gland is involved. There is no loss of salivary function and the signs are restricted to those of inflammation of the gland.
CLINICAL FINDINGS
In the early stages, there is diffuse enlargement of the gland accompanied by warmth and pain on palpation. The pain may interfere with mastication and swallowing and induce abnormal carriage of the head and resentment when attempts are made to move the head. There may be marked local edema in severe cases. Diffuse parenchymatous parotitis usually subsides with systemic and local treatment within a few days, but suppurative lesions may discharge externally and form permanent salivary fistulae.
CLINICAL PATHOLOGY
Bacteriological examination of pus from discharging abscesses may aid the choice of a suitable antibacterial treatment.
NECROPSY FINDINGS
Death occurs rarely and necropsy findings are restricted to local involvement of the gland or to primary lesions elsewhere in the case of secondary parotitis.
• Careful palpation is necessary to differentiate the condition from lymphadenitis, abscesses of the throat region and metastases to the parotid lymph node in ocular carcinoma or mandibular lymphoma of cattle
• Acute phlegmonous inflammation of the throat is relatively common in cattle and is accompanied by high fever, severe toxemia and rapid death. It may be mistaken for an acute parotitis but the swelling is more diffuse and causes pronounced obstruction to swallowing and respiration
Diseases of the pharynx and esophagus
PHARYNGITIS
Pharyngitis is inflammation of the pharynx and is characterized clinically by coughing, painful swallowing and a variable appetite. Regurgitation through the nostrils and drooling of saliva may occur in severe cases.
ETIOLOGY
Pharyngitis in farm animals is usually traumatic. Infectious pharyngitis is often part of a syndrome with other more obvious signs.
Physical causes
• Injury while giving oral treatment with balling or drenching gun1 or following endotracheal intubation.2 The administration of intraruminal anthelmintic coils to calves under a minimum body weight have also been associated with pharyngeal and esophageal perforation3
• Improper administration of a reticular magnet, resulting in a retropharyngeal abscess
• Accidental administration or ingestion of irritant or hot or cold substances
• Foreign bodies, including grass and cereal awns, wire, bones, gelatin capsules lodged in the pharynx or suprapharyngeal diverticulum of pigs.
Infectious causes
Cattle
• Oral necrobacillosis, actinobacillosis as a granuloma rather than the more usual lymphadenitis
• Infectious bovine rhinotracheitis
• Pharyngeal phlegmon or intermandibular cellulitis is a severe, often fatal, necrosis of the wall of the pharynx and peripharyngeal tissues without actually causing pharyngitis. F. necrophorum is a common isolate from the lesions.
Horses
• As part of strangles or anthrax
• Viral infections of the upper respiratory tract, including equine herpesvirus-1, Hoppengarten cough, parainfluenza virus, adenovirus, rhinovirus, viral arteritis, influenza-1A/E1 and 1A/E2, cause pharyngitis
• Chronic follicular pharyngitis with hyperplasia of lymphoid tissue in pharyngeal mucosa giving it a granular, nodular appearance with whitish tips on the lymphoid follicles. An exaggerated form of the disease is a soft tissue mass hanging from the pharyngeal roof and composed of lymphoid tissue.
PATHOGENESIS
Inflammation of the pharynx is attended by painful swallowing and disinclination to eat. If the swelling of the mucosa and wall is severe, there may be virtual obstruction of the pharynx. This is especially so if the retropharyngeal lymph node is enlarged, as it is likely to be in equine viral infections such as rhinovirus.
In balling-gun-induced trauma of feedlot cattle treated for respiratory disease with boluses of sulfonamides, perforations of the pharynx and esophagus may occur with the development of periesophageal diverticulations with accumulations of ruminal ingesta, and cellulitis.1 Improper administration of a magnet to a mature cow can result in a retropharyngeal abscess.4
Pharyngeal lymphoid hyperplasia in horses can be graded into four grades (I–IV) of severity based on the size of the lymphoid follicles and their distribution over the pharyngeal wall.
CLINICAL FINDINGS
The animal may refuse to eat or drink or it may swallow reluctantly and with evident pain. Opening of the jaws to examine the mouth is resented and manual compression of the throat from the exterior causes paroxysmal coughing. There may be a mucopurulent nasal discharge, sometimes containing blood, spontaneous cough and, in severe cases, regurgitation of fluid and food through the nostrils. Oral medication in such cases may be impossible. Affected animals often stand with the head extended, drool saliva and make frequent, tentative jaw movements. Severe toxemia may accompany the local lesions, especially in oral necrobacillosis and, to a less extent, in strangles. Empyema of the guttural pouches may occur in horses. If the local swelling is severe, there may be obstruction of respiration and visible swelling of the throat. The retropharyngeal and parotid lymph nodes are commonly enlarged. In ‘pharyngeal phlegmon’ in cattle there is an acute onset with high fever (41–41.5°C, 106–107°F), rapid heart rate, profound depression and severe swelling of the soft tissues within and posterior to the mandible to the point where dyspnea is pronounced. Death usually occurs 36–48 hours after the first signs of illness.
In traumatic pharyngitis in cattle, visual examination of the pharynx through the oral cavity reveals hyperemia, lymphoid hyperplasia and erosions covered by diphtheritic membranes. Pharyngeal lacerations are visible, and palpation of these reveals the presence of accumulated ruminal ingesta in diverticulae on either side of the glottis.1 External palpation of the most proximal aspect of the neck reveals firm swellings, which represent the diverticula containing rumen contents. A retropharyngeal abscess secondary to an improperly administered magnet can result in marked diffuse painful swelling of the cranial cervical region.4 Ultrasonographic examination of the swelling may reveal the magnet within the abscess.4
Palpation of the pharynx may be performed in cattle with the use of a gag if a foreign body is suspected, and endoscopic examination through the nasal cavity is possible in the horse.
Most acute cases recover in several days but chronic cases may persist for many weeks, especially if there is ulceration, a persistent foreign body or abscess formation. Pharyngitis has become one of the most commonly recognized diseases of the upper respiratory tract of the horse. Chronic pharyngitis after viral infections is relatively common and results in a break in training, which is inconvenient and costly. On endoscopic examination there may be edema in early and relatively acute cases. In long-standing cases there is lymphoid infiltration and follicular hyperplasia. This is more common and more severe in young horses, who also suffer more attacks of upper respiratory tract disease. The condition does not appear to diminish racing performance or respiratory efficiency. If secondary bacterial infection is present a purulent exudate is seen on the pharyngeal mucosa and in the nostrils. Affected horses cough persistently, especially during exercise, are dyspneic and tire easily. Guttural pouch infections may occur secondarily. An occasional sequel is aspiration pneumonia.
CLINICAL PATHOLOGY
Nasal discharge or swabs taken from accompanying oral lesions may assist in the identification of the causative agent. Moraxella spp. and Streptococcus zooepidemicus can be isolated in large numbers from horses with lymphoid follicular hyperplasia grades III and IV.
NECROPSY FINDINGS
Deaths are rare in primary pharyngitis and necropsy examinations are usually undertaken only in those animals dying of specific diseases. In ‘pharyngeal phlegmon’ there is edema, hemorrhage and abscessation of the affected area and on incision of the area a foul-smelling liquid and some gas usually escape.
• Pharyngitis is manifested by an acute onset and local pain
• In pharyngeal paralysis, the onset is usually slow
• Acute obstruction by a foreign body may occur rapidly and cause severe distress and continuous, expulsive coughing – but there are no systemic signs
• Endoscopic examination of the pharyngeal mucous membranes is often of diagnostic value
TREATMENT
The primary disease must be treated, usually parenterally, by the use of antimicrobials. ‘Pharyngeal phlegmon’ in cattle is frequently fatal and early treatment, repeated at short intervals, with a broad-spectrum antimicrobial is necessary.
Pharyngeal lymphoid hyperplasia is not generally susceptible to antimicrobials or medical therapy. Surgical therapy including electrical and chemical cautery is indicated and has been successfully applied.
PHARYNGEAL OBSTRUCTION
Obstruction of the pharynx is accompanied by stertorous respiration, coughing and difficult swallowing.
ETIOLOGY
Foreign bodies or tissue swellings are the usual causes.
Foreign bodies
These include bones, corn cobs and pieces of wire. While horses are considered discriminating eaters in comparison to cattle, they will occasionally pick up pieces of metal while eating.1
Tissue swellings
Horses
• Retropharyngeal lymph node hyperplasia and lymphoid granulomas as part of chronic follicular pharyngitis syndrome
• Retropharyngeal abscess and cellulitis
• Retropharyngeal lymphadenitis caused by strangles
• Pharyngeal cysts in the subepiglottic area of the pharynx, probably of thyroglossal duct origin, and fibroma; also similar cysts on the soft palate and pharyngeal dorsum, the latter probably being remnants of the craniopharyngeal ducts
PATHOGENESIS
Reduction in caliber of the pharyngeal lumen interferes with swallowing and respiration.
CLINICAL FINDINGS
There is difficulty in swallowing and animals may be hungry enough to eat but, when they attempt to swallow, cannot do so and the food is coughed up through the mouth. Drinking is usually managed successfully. There is no dilatation of the esophagus and usually little or no regurgitation through the nostrils. An obvious sign is a snoring inspiration, often loud enough to be heard some yards away. The inspiration is prolonged and accompanied by marked abdominal effort. Auscultation over the pharynx reveals loud inspiratory stertor. Manual examination of the pharynx may reveal the nature of the lesion but an examination with a fiberoptic endoscope is likely to be much more informative. When the disease runs a long course, emaciation usually follows. Rupture of abscessed lymph nodes may occur when a nasal tube is passed, and can result in aspiration pneumonia.
In horses with metallic foreign bodies in the oral cavity or pharynx, the clinical findings include purulent nasal discharge, dysphagia, halitosis, changes in phonation, laceration of the tongue and stertorous breathing.1 In case studies, most horses were affected with clinical signs for more than 2 weeks and had been treated with antimicrobials with only temporary improvement.1
CLINICAL PATHOLOGY
A tuberculin test may be advisable in bovine cases. Nasal swabs may contain S. equi when there is streptococcal lymphadenitis in horses.
NECROPSY FINDINGS
Death occurs rarely and in fatal cases the physical lesion is apparent.
• Signs of the primary disease may aid in the diagnosis in tuberculosis, actinobacillosis and strangles
• Pharyngitis is accompanied by severe pain and commonly by systemic signs and there is usually stertor
• It is of particular importance to differentiate between obstruction and pharyngeal paralysis when rabies occurs in the area. Esophageal obstruction is also accompanied by the rejection of ingested food but there is no respiratory distress. Laryngeal stenosis may cause a comparable stertor but swallowing is not impeded. Nasal obstruction is manifested by noisy breathing but the volume of breath from one or both nostrils is reduced and the respiratory noise is more wheezing than snoring
• Radiography is useful for the identification of metallic foreign bodies1
TREATMENT
Removal of a foreign body may be accomplished through the mouth. Treatment of actinobacillary lymphadenitis with iodides is usually successful and some reduction in size often occurs in tuberculous enlargement of the glands but complete recovery is unlikely to occur. Parenteral treatment of strangles abscesses with penicillin may effect a cure. Surgical treatment has been highly successful in cases caused by medial retropharyngeal abscess.
PHARYNGEAL PARALYSIS
Pharyngeal paralysis is manifested by inability to swallow and an absence of signs of pain and respiratory obstruction.
ETIOLOGY
Pharyngeal paralysis occurs sporadically, due to peripheral nerve injury, and in some encephalitides with central lesions.
PATHOGENESIS
Inability to swallow and regurgitation are the major manifestations of the disease. There may be an associated laryngeal paralysis, accompanied by ‘roaring’. The condition known as ‘cud-dropping’ in cattle may be a partial pharyngeal paralysis as there is difficulty in controlling the regurgitated bolus, which is often dropped from the mouth. In these circumstances, aspiration pneumonia is likely to develop.
CLINICAL FINDINGS
The animal is usually hungry but, on prehension of food or water, attempts at swallowing are followed by dropping of the food from the mouth, coughing and the expulsion of food or regurgitation through the nostrils. Salivation occurs constantly and swallowing cannot be stimulated by external compression of the pharynx. The swallowing reflex is a complex one controlled by a number of nerves and the signs can be expected to vary greatly depending on which nerves are involved and to what degree. There is rapid loss of condition and dehydration. Clinical signs of the primary disease may be evident but, in cases of primary pharyngeal paralysis, there is no systemic reaction. Pneumonia may follow aspiration of food material into the lungs and produces loud gurgling sounds on auscultation.
In ‘cud-dropping’ in cattle, the animals are normal except that regurgitated boluses are dropped from the mouth, usually in the form of flattened disks of fibrous food material. Affected animals may lose weight but the condition is usually transient, lasting for only a few days. On the other hand, complete pharyngeal paralysis is usually permanent and fatal.
CLINICAL PATHOLOGY
The use of clinicopathological examinations is restricted to the identification of the primary specific diseases.
NECROPSY FINDINGS
If the primary lesion is physical, it may be detected on gross examination.
• In all species, often the first clinical impression is the presence of a foreign body in the mouth or pharynx and this can only be determined by physical examination
• Pharyngeal paralysis is a typical sign in rabies and botulism but there are other clinical findings that suggest the presence of these diseases
• Absence of pain and respiratory obstruction are usually sufficient evidence to eliminate the possibility of pharyngitis or pharyngeal obstruction
• Endoscopic examination of the guttural pouch is a useful diagnostic aid in the horse
ESOPHAGITIS
Inflammation of the esophagus is accompanied initially by clinical findings of spasm and obstruction, pain on swallowing and palpation, and regurgitation of bloodstained, slimy material.
ETIOLOGY
Primary esophagitis caused by the ingestion of chemical or physical irritants is usually accompanied by stomatitis and pharyngitis. Laceration of the mucosa by a foreign body or complications of nasogastric intubation may occur.1 Nasogastric intubation is associated with a higher risk of pharyngeal and esophageal injury when performed in horses examined for colic.1 This may be related to the use of larger-diameter nasogastric tubes to provide more effective gastric decompression, the longer duration of intubation in some horses, or the presence of gastric distension resulting in increased resistance to tube passage at the cardia.1 In a series of six horses with esophageal trauma the lesions were detected 5 and 20 cm from the cranial esophageal opening.1
The administration of sustained-release anthelmintic boluses to young calves that are not large enough for the size of the bolus used may cause esophageal injury and perforation.2,3 The boluses are 8.5 cm in length and 2.5 cm in diameter and the calves 100–150 kg. The minimum body weight for these boluses is 100 kg but in the study some calves were younger than the recommended age and were also fractious when handled, which may have contributed to the injury.2 Death of Hypoderma lineatum larvae in the submucosa of the esophagus of cattle may cause acute local inflammation and subsequent gangrene.
Inflammation of the esophagus occurs commonly in many specific diseases, particularly those that cause stomatitis, but the other clinical signs of these diseases dominate those of esophagitis.
PATHOGENESIS
Inflammation of the esophagus combined with local edema and swelling results in a functional obstruction and difficulty in swallowing. Traumatic injury to the esophagus results in edema, hemorrhage, laceration of the mucosa and possible perforation of the esophagus, resulting in periesophageal cellulitis, which spreads proximally and distally along the esophagus in fascial planes from the site of perforation. Perforation of the thoracic esophagus can result in severe and fatal pleuritis. There is extensive edema and accumulation of swallowed or regurgitated ingesta along with gas. The extensive cellulitis and the presence of ingesta results in severe toxemia, and dysphagia may cause aspiration pneumonia.
CLINICAL FINDINGS
In the acute injury of the esophagus, there is salivation and attempts to swallow, which cause severe pain, particularly in horses. In some cases, attempts at swallowing are followed by regurgitation and coughing, pain, retching activities and vigorous contractions of the cervical and abdominal muscles. Marked drooling of saliva, grinding of the teeth, coughing and profuse nasal discharge are common in the horse with esophageal trauma with complications following nasogastric intubation.1 Regurgitation may occur and the regurgitus contains mucus and some fresh blood. If the esophagitis is in the cervical region, palpation in the jugular furrow causes pain and edematous tissues around the esophagus may be palpable. If perforation has occurred, there is local pain and swelling and often crepitus. Local cervical cellulitis may cause rupture to the exterior and development of an esophageal fistula, or infiltration along fascial planes with resulting compression obstruction of the esophagus, and toxemia. Perforation of the thoracic esophagus may lead to fatal pleurisy. Animals that recover from esophageal traumatic injury are commonly affected by chronic esophageal stenosis with distension above the stenosis. Fistulae are usually persistent but spontaneous healing may occur. In specific diseases such as mucosal disease and bovine malignant catarrh, there are no obvious clinical findings of esophagitis, the lesions being mainly erosive.
Endoscopy of the esophagus will usually reveal the location and severity of the lesion. Lateral cervical radiographs may reveal foreign bodies and extensive soft tissue swelling with pockets of gas.
CLINICAL PATHOLOGY
In severe esophagitis of traumatic origin a marked neutrophilia may occur, suggesting active inflammation.
NECROPSY FINDINGS
Pathological findings are restricted to those pertaining to the various specific diseases in which esophagitis occurs. In traumatic lesions or those caused by irritant substances, there is gross edema, inflammation and, in some cases, perforation.
• Esophagitis must be differentiated from pharyngitis, in which attempted swallowing is not as marked and coughing is more likely to occur. Palpation may also help to localize the lesion; however, pharyngitis and esophagitis commonly occur together
• When the injury is caused by a foreign body, it may still be in the esophagus and, if suitable restraint and anesthesia can be arranged, the passage of a nasogastric tube or endoscope may locate it. Complete esophageal obstruction is accompanied by bloat in ruminants, by palpable enlargement of the esophagus and by less pain on swallowing than in esophagitis, although horses may show a great deal of discomfort
• In cattle perforation of the esophagus is not uncommon. There is a persistent, moderate toxemia, a moderate fever and a leukocytosis. Edema and swelling are prominent in surrounding fascial planes, but may cause only slight physical enlargement, which is easily missed on a routine examination
TREATMENT
Feed should be withheld for 2–3 days and fluid and electrolyte therapy may be necessary for several days. Parenteral antimicrobials are indicated, especially if laceration or perforation has occurred. Reintroduction to feed should be monitored carefully and all feed should be moistened to avoid the possible accumulation of dry feed in the esophagus, which may not be fully functional.