Diseases of the nonruminant stomach and intestines
Only those diseases that are accompanied by physical lesions, such as displacement or strangulation, or disturbances of motility, such as ileus, are presented. Diseases associated with functional disturbances of secretion are not recognized in animals. Deficiencies of biliary and pancreatic secretion are dealt with in the chapter on diseases of the liver. Those diseases of the stomach and intestines peculiar to ruminants are dealt with separately in Chapter 6.
EQUINE COLIC (ADULT HORSES)
GENERAL PRINCIPLES
Gastrointestinal disease causing signs of abdominal pain in horses is commonly referred to as colic. Colic is a frequent and important cause of death and is considered the most important disease of horses encountered by practicing veterinarians. It is estimated to cost the horse industry in the USA approximately $115 000 000 annually.1-3
ETIOLOGY
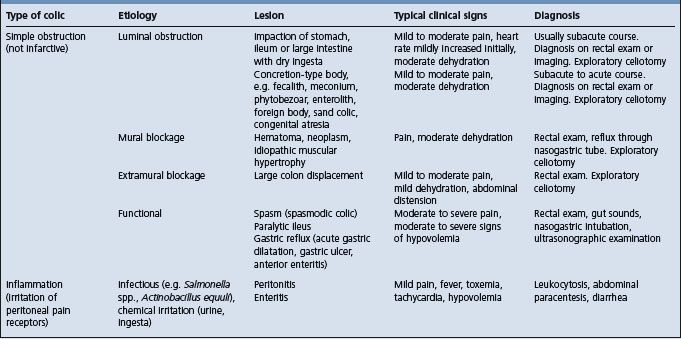
Several classification systems of equine colic have been described including a disease-based system (Table 5.3) classifying the cause of colic as:
Colic cases can also be classified on the basis of the duration of the disease: acute (< 24–36 h), chronic (> 24–36 h) and recurrent (multiple episodes separated by periods of > 2 days of normality). Another classification system is anatomically based and is listed in Table 5.4. Regardless of the classification system used, some estimates are that fewer than 20% of colic cases seen in the field have a definitive diagnosis.2,4 Horses with acute transient colic relieved by analgesics are often referred to as having ‘spasmodic colic’.5 Spasmodic or gas colic was the cause of 35% of horses with colic examined in the field by veterinarians.6 Large-colon impaction (20%) and undiagnosed (13%) were the other largest diagnostic categories.6
Table 5.4 Disorders of the equine gastrointestinal tract causing colic, by anatomical site
| Site | Disorder | |
|---|---|---|
| Stomach | Gastric dilatation | |
| Primary | ||
| Secondary to outflow obstruction, pyloric stenosis, ileus or anterior enteritis | ||
| Gastric impaction | ||
| Gastroduodenal ulceration | ||
| Small intestine | Volvulus | |
| Intussusception | ||
| Ileocecal | ||
| Jejunojejunal | ||
| Infarction or ischemia | ||
| Thromboembolic disease | ||
| Disruption of blood supply by mesenteric tear | ||
| Strangulation, including entrapment through the epiploic foramen, mesenteric rents (including cecocolic fold, splenic ligament, uterine ligaments, spermatic cord), Merkel’s diverticulum and hernias (diaphragmatic, inguinal/scrotal, umbilical). | ||
| Strangulation by pedunculated lipoma | ||
| Luminal obstruction | ||
| Foreign bodies | ||
| Ascarids | ||
| Luminal compression | ||
| Lipomas | ||
| Intramural masses such as Pythium spp. and neoplasms (adenocarcinoma, lymphoma, eosinophilic enteritis) | ||
| Adhesions | ||
| Enteritis | ||
| Cecum | Impaction | |
| Rupture and perforation | ||
| Intussusception | ||
| Cecocolic | ||
| Cecocecal | ||
| Cecal torsion | ||
| Infarction (thromboembolic disease, necrotizing enterocolitis) | ||
| Typhilitis | ||
| Tympany | ||
| Ascending (large) colon | Impaction | |
| Intestinal tympany | ||
| Volvulus | ||
| Displacement, including left dorsal (reno- or nephrosplenic), right dorsal, cranial displacement of pelvic flexure | ||
| Infarction (verminous mesenteric arteritis, necrotizing enterocolitis) | ||
| Luminal obstruction | ||
| Sand accumulation | ||
| Enterolith | ||
| Right dorsal ulcerative colitis | ||
| Colitis | ||
| Necrotizing enterocolitis | ||
| Descending (small) colon | Impaction | |
| Luminal obstruction | ||
| Fecalith | ||
| Enterolith | ||
| Luminal compression | ||
| Pedunculated lipoma | ||
| Intramural hematoma | ||
| Perirectal abscess | ||
| Perirectal tumor (melanoma) | ||
| Avulsion of mesocolon and rectal prolapse in mares at parturition | ||
| Strangulation | ||
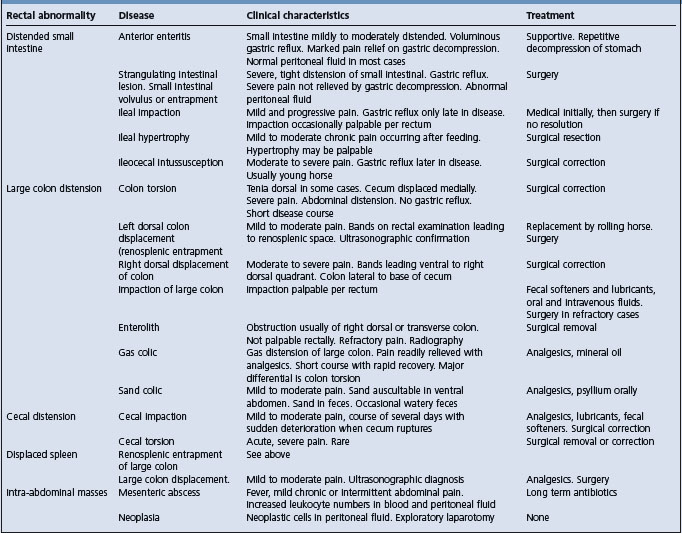
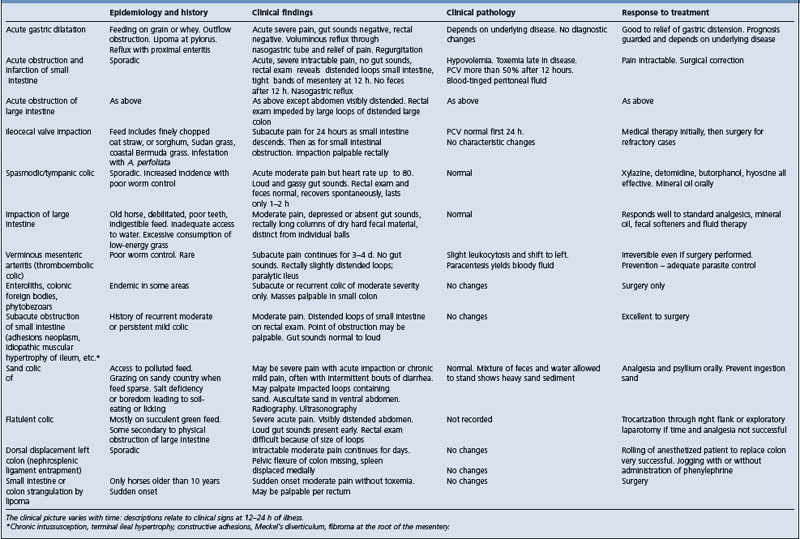
Etiology See Tables 5.4, 5.5, 5.6 and 5.7
Epidemiology Incidence of 2–30 cases per 100 horse years, mortality of 0.5–0.7 cases per 100 horse years and case fatality rate of 7–13%. Any age predisposition is weak, although certain diseases (e.g. meconium impaction, strangulation by pedunculated lipoma) have specific age distributions. Consumption of a diet high in concentrate increases the risk of colic, as does a poor parasite control program
Clinical signs Signs of abdominal pain include agitation, flank watching, flank biting, pawing, frequent lying down, kicking at the abdomen, frequent attempts to urinate or defecate, and rolling. Tachycardia is common. Normal gut sounds are absent and replaced by tympanitic sounds. Abdominal distension may develop. Reflux through a nasogastric tube may occur. Rectal examination may reveal abnormalities
Clinical pathology Few changes have diagnostic significance but many are used to monitor the severity of the disease. Hemoconcentration, azotemia and metabolic acidosis are frequent findings. Peritoneal fluid may have increased protein and leukocyte concentration
Lesions Consistent with the particular disease
Diagnostic confirmation Physical examination, exploratory laparotomy, necropsy
Treatment Analgesia (Table 5.7), correction of fluid, acid–base and electrolyte abnormalities (Ch. 2), gastric decompression via nasogastric intubation, administration of fecal softeners or lubricants (Table 5.8), surgical correction of the lesion
Control Parasite control. Ensure adequate roughage in the diet
EPIDEMIOLOGY
Most studies of the epidemiology of colic do not provide details of specific diseases but rather consider colic as one disease. This inclusion of many diseases into one category, while maximizing the statistical power of the studies, is unfortunate because it can obscure important details regarding the occurrence and risk factors of individual diseases. Furthermore, much of the information related to incidence, treatments and outcome of horses with colic is derived from studies of horses examined at referral centers. Horses examined at these centers are in all likelihood not representative of horses with colic that are not referred for examination by specialists, this being the majority of horses with colic. Details of the epidemiology of specific etiological entities are included under those headings. Only general principles are included here.
Occurrence
Equine colic occurs worldwide, although there are regional differences in the types of colic, and is a common and important disease of horses. For cases of equine colic recognized in the field, as distinct from those referred for specialized treatment, the incidence rate ranges between 3.5 and 10.6 cases per 100 horse years, although individual farms may experience rates as high as 30 or more cases per 100 horse years.2,3,7,8 Mortality due to colic ranges between 0.5 and 0.7 deaths per 100 horse years, representing 28% of overall horse deaths (2.5 deaths per 100 horse years).2,4,8 The case fatality rate is 6–13% of field cases.2-48 Approximately 1–2% of colic events in the USA and the British Isles result in surgery.3,8 It should be borne in mind that these estimates of incidence and mortality are highly influenced by the population of horses studied and may be biased or unduly influenced by inclusion of farms or groups of horses with an extremely high, or low, incidence of colic.
Risk factors
Risk factors for colic can be categorized as: 1) intrinsic horse characteristics; 2) those associated with feeding practices; 3) management; 4) medical history, and; 5) parasite control.9
Horse characteristics
Age
There are conflicting results of studies that examine the association of colic and age. The conflicting results might be the result of varying study populations, study design, presence of varying confounding factors, and interpretation of data. Confounding factors are those that alter with the age of the horse, such as use, feeding and management of horses, and mask an effect of age or give the impression of an effect of age when in fact such an effect is not present.9,10 Horses 2–10 years of age are 2.8 times more likely to develop colic that horses less than 2 years.11 One large-scale study reported that foals less than 6 months of age had an incidence of 0.2 cases of colic per 100 horses per year, while horses more than 6 months of age had incidence of approximately 4–6 colic-affected horses per 100 horse years, with the incidence varying to a limited extent among older age groups.3 Other studies have not found a similar effect of age.4 However, each age group has a particular set of diseases unique or common to it. Newborn foals may have congenital colon or anal atresia, or meconium impaction (see Colic in foals), diseases that do not affect older horses, whereas strangulating or obstructive lesions caused by pedunculated lipomas are found only in older horses.12
Diet and feeding practices
Horses at pasture are at a lower risk of developing colic than are stabled horses fed concentrate feeds.11,13,14 The risk of colic increases with the amount of concentrate fed, such that a horse fed 5 kg of concentrated feed per day has 6 times as great a risk of developing colic as a horse not fed concentrate.11 However, another report did not detect an effect of diet composition on risk of colic.6 Changes to the horse’s diet through changes in quantity and quality of feed, feeding frequency, or time of feeding increase the risk of colic by 2–5 times.6,11,13,15
Management
Watering
Horses without constant access to water are at increased risk of developing colic,14 whereas horses with access to ponds or dams have a reduced risk of colic compared to horses provided with water from buckets or troughs.11,14 This might represent a confounding effect of pasturing, in that horses with access to dams are probably at pasture and benefit from the lower risk of colic associated with that management practice. Alternatively, horses provided with water from buckets may be at greater risk of having periods when water is not available.14
Housing
Increased duration of stabling per day is associated with an increased risk of colic.6,13 Horses cared for by their owner and horses in stables with large numbers of horses are less likely to develop colic.8
Exercise
Overall, there appears to be an increased risk of colic among horses that are undertaking physical activity or that have a recent change in the amount of physical activity. However, the finding of this association should be considered in the context of other differences that exist between active and inactive horses, such as in feeding practices, housing (stabling versus pasture), and transportation.
Weather and climate
Despite the widespread belief that colic is associated with changes in weather, particularly thunderstorms, there is no conclusive evidence of such an association.9,10
Medical history
Horses with a history of colic are more likely to have another episode, and horses that have had colic surgery are approximately five times more likely to have another episode of colic than are horses that have not had colic.6,15 There is no association between dental care and incidence of colic or recent vaccination and colic.6,9
Parasite control
Inadequate parasite control programs have been estimated to put horses at 2–9 times greater risk of developing colic,7 although other studies have not demonstrated a relationship between anthelmintic administration and colic.8,15 The presence of tapeworms is associated with a 3 times greater risk of ileal impaction.16 A recent large-scale study in the USA found an increased incidence of colic in horses on farms on which rotation of anthelmintics was practiced.3 This apparently paradoxical finding may be because farms with a higher incidence of colic are more likely to alter rotate anthelmintics as a result of having more horses with colic.3
The apparently conflicting results of some of the epidemiologic studies should not deter veterinarians from recommending effective parasite control programs for horses, given the clear association at an individual level of presence of tapeworms, cyathostomes and/or large strongyles and ileocecal disease, diarrhea and ill thrift, and verminous arteritis, respectively.
Importance
Losses caused by colic in horses are due almost entirely to death of the patient. However, the cost of treatment and the emotional trauma to the owners of their horse being afflicted with a potentially fatal disease are important considerations. A 1989 survey of veterinarians in the USA rated colic the most serious medical disease in horses, ahead of viral respiratory disease1 and recent studies estimated the cost of colic to the horse industry in the USA at $115000000 annually.3
PATHOGENESIS
The pathogenesis of equine colic is variable depending on the cause and severity of the inciting disease. A horse with a strangulating lesion involving 50% of its small intestine has a much more rapidly evolving disease, with severe abnormalities, than does a horse affected with mild spasmodic colic or impaction of the pelvic flexure of the large colon. While equine colic often involves changes in many body systems, notably the gastrointestinal, cardiovascular, metabolic and endocrine systems, there are several features and mechanisms that are common to most causes of colic and that depend only on the severity of the disease for the magnitude of their change. The features common to severe colic, and often present to a lesser degree in milder colics, are pain, gastrointestinal dysfunction, intestinal ischemia, endotoxemia, compromised cardiovascular function (shock) and metabolic abnormalities.
Pain
Pain is the hallmark of gastrointestinal disease in horses and is attributable to distension of the gastrointestinal tract and stimulation of stretch receptors in the bowel wall and mesentery, stretching of mesentery by displaced or entrapped bowel, and inflammation and irritation of the bowel, peritoneum or mesentery. The intensity of the pain is often, but not always, related to the severity of the inciting disease. Horses with mild impaction of the large colon of short duration (< 24 h) often have very mild pain, whereas a horse with a strangulating lesion of the small intestine will have very severe pain.
Gastrointestinal pain has an inhibitory effect on normal gastrointestinal function, causing a feedback loop in which the pain inhibits normal gut motility and function, allowing accumulation of ingesta and fluid, resulting in distension and further pain. Horses can respond very violently to abdominal pain and may injure themselves when rolling or thrashing.
Gastrointestinal dysfunction
Colic is almost invariably associated with impaired gastrointestinal function, usually alterations to motility or absorptive function. Gastrointestinal motility may be increased, as is presumed to be the case in spasmodic colic, altered in its character or coordination, as in some cases of impaction colic, or absent, such as in ileus secondary to inflammation or ischemia of the bowel or to the presence of endotoxemia. Increased or uncoordinated gastrointestinal motility probably causes pain through excessive contraction of individual segments of bowel or distension of bowel because of the loss of normal propulsive activity. Ileus is associated with fluid distension of the small intestine and stomach and fluid and gas distension of the large colon, both of which cause severe pain and can lead to gastric or colonic rupture. The absorptive function of the intestine may be decreased by inflammation or ischemia, which results in distension of the small intestine or large colon, pain and potentially rupture of the stomach or colon.
Impairment of the barrier function of the gastrointestinal mucosa by inflammation or ischemia can result in leakage of endotoxin into peritoneal fluid and endotoxemia17 (see Endotoxemia).
Ischemia of the intestinal wall
Ultimately, most forms of lethal colic involve some degree of ischemia of the intestine, with subsequent loss of barrier function, evident in its most extreme form as rupture of the viscus, endotoxemia, bacteremia, cardiovascular collapse and death. Ischemia may be the result of impaired blood flow to or from the intestine because of torsion or volvulus of the intestine, entrapment of the intestine and associated mesentery in rents or hernias, strangulation such as by a pedunculated lipoma, or thromboembolic disease. Ischemia may also result from severe gastrointestinal distension, such as occurs in the terminal stage of severe colon impaction. Mild ischemia probably impairs normal intestinal motility and function. The role of reperfusion injury in pathogenesis of ischemic disease is uncertain at this time.
Endotoxemia
Death in fatal cases of colic in which the affected viscus ruptures secondary to distension, or when ischemia and/or infarction damages a segment of bowel wall, is due to the absorption of endotoxins from the gut lumen into the systemic circulation.17 (See Endotoxemia). Endotoxin absorption causes increased concentrations of tumor necrosis factor and interleukin 6 in peritoneal fluid and blood concentrations.17
Rupture of the stomach or intestine is also a characteristic termination of distension of the intestine in the horse. The resulting deposition of large quantities of highly toxic ingesta or fecal contents into the peritoneal cavity causes profound shock and death within a few hours.
Shock
The usual cause of death in severe colic is cardiovascular collapse secondary to endotoxemia and hypovolemia. In less severe colic, hypovolemia and cardiovascular dysfunction may contribute to the development of the disease, and rapid correction of hypovolemia is central to the effective treatment of colic.
Hypovolemia is due to the loss of fluid and electrolytes into the lumen of the gastrointestinal tract or loss of protein from the vascular space with subsequent reduction in the circulating blood volume. Hypovolemia impairs venous return to heart and therefore cardiac output, arterial blood pressure and oxygen delivery to tissues. Not surprisingly, measures of circulatory status are good predictors of the outcome of colic (see Prognosis, below).
Cardiorespiratory function is impaired if there is severe distension of gut, such as in large-colon torsion, because of restricted respiration by pressure on the diaphragm and reduced venous return to the heart because of pressure on the caudal vena cava.
Coagulation and fibrinolysis
Severe colic, especially that involving ischemia or necrosis of intestine, is associated with abnormalities in coagulation and fibrinolysis characterized by hypercoagulation of blood and decreases in rate of fibrinolysis.18-21 Disseminated intravascular coagulation is common among horses with ischemia or necrosis of the gut and is a good prognostic indicator of survival.19,20 Changes in coagulation and fibrinolysis include decreases in antithrombin activity and fibrinogen concentration and increases in prothrombin time, activated partial thromboplastin time and concentration of thrombin– antithrombin complexes in plasma.19-21
Overview of the pathogenesis of common colics
Simple obstructive
Simple obstructive colics are those in which there is obstruction to the aboral passage of ingesta but no ischemia or strangulation of bowel. In the terminal stages there is often ischemia caused by distension of the intestine.
Small-intestinal obstructive lesions include ileal hypertrophy, ileocecal intussusception and foreign-body obstruction of the lumen. The course of the disease is often 24–72 hours, and sometimes longer depending on the extent of the obstruction, partial obstructions having much less severe signs and disease of longer duration. The principal abnormality is reduced aboral flow of ingesta, with subsequent distension of intestine cranial to the obstruction, causing pain and, if the distension is severe, gastric rupture.
Large intestinal obstructive lesions include impaction and simple (nonstrangulating) displacements of the large colon. The course of disease is prolonged, often more than 72 hours. Signs of abdominal pain are due to distension of the bowel. There is progressive distension with fluid and gas and ultimately ischemia of the bowel and rupture.
Obstructive and strangulating
Diseases that cause both obstruction and strangulation as an initial event, such as torsion of the small intestine or volvulus of the large colon, result in severe and unrelenting pain that is little relieved with analgesics. Obstruction causes distension and strangulation causes ischemia, loss of barrier function and endotoxemia. These diseases have a short course, usually less than 24 hours and sometimes as short as 6 hours, and profound clinical signs. Endotoxemia and cardiovascular collapse are characteristic of these diseases.
Infarctive
Infarctive diseases, such as thromboembolic colic, are characterized by ischemia of the intestinal wall with subsequent alterations in motility and absorptive and barrier functions. Ileus causes distension of the intestines and stomach and altered barrier function causes endotoxemia. The course of the disease is usually less than 48 hours and is terminated by cardiovascular collapse and death.
CLINICAL FINDINGS
The bulk of the following description is generally applicable to severe acute colic. Clinical findings characteristic of each etiological type of colic are dealt with under their individual headings. The purposes of the clinical examination are diagnostic – to determine whether the pain is due to gastrointestinal tract disease and, if so, to determine the nature of the lesion – and prognostic, to provide some estimate of the likely outcome of the disease. Veterinary clinicians are able to accurately predict the site of lesions (small versus large intestine), type of lesion (simple obstructive versus strangulating or infarctive) and outcome.22 The ability to predict these events increases with training and experience.22
Accurate diagnosis of the cause of the colic has some prognostic usefulness, but assessment of the horse’s physiological state by measurement of heart and respiratory rates, mucous membrane color and refill time, arterial blood pressure, hematocrit and serum total protein concentration, and other measures, allows more accurate prognostication. Furthermore, the cause of colic is determined in only approximately 20% of field cases.
Visual examination
Behavior
Pain is manifested by pawing, stamping or kicking at the belly or by restlessness evident as pacing in small circles and repeatedly getting up and lying down, often with exaggerated care. Other signs are looking or nipping at the flank, rolling, and lying on the back. Often the penis is protruded without urinating or with frequent urination of small volumes. Continuous playing with water without actually drinking (sham drinking) is common.
Pain may be continuous or, more commonly, intermittent with bouts of pain lasting as long as 10 minutes interspersed with similar periods of relaxation. In general the intensity of the pain is of about the same severity for the duration of the illness; sudden exacerbations may indicate a change in the disease status or the development of another abnormality, such as a horse with impaction of the large colon developing a displacement of the colon or horses with diarrhea developing necrotizing enteritis. Horses in the terminal phase of the disease may have a marked diminution of pain associated with relief of pressure after rupture of distended bowel and depression caused by toxemia and shock. Pain responses in colic may be so severe, and uncontrolled movements so violent, that the horse may do itself serious injury. Other causes of pain, such as pleuritis or rhabdomyositis, can be confused with colic, although a horse that goes down and rolls almost certainly has alimentary tract colic.
Posture
The posture is often abnormal, with the horse standing stretched out with the forefeet more cranial and the hindfeet more caudal than normal – the so-called ‘saw-horse’ stance. Some horses lie down on their backs with their legs in the air, suggesting a need to relieve tension on the mesentery.
Abdomen size
Distension of the abdomen is an uncommon but important diagnostic sign. Symmetrical, severe distension is usually caused by distension of the colon, sometimes including the cecum, secondary to colon torsion, or impaction of the large or small colon and subsequent fluid and gas accumulation. If only the cecum is distended the abdomen may show an asymmetrical enlargement in the right sublumbar fossa. Maximum distension of stomach or small intestines does not cause appreciable distension of the abdomen.
Vomiting
Projectile vomiting or regurgitation of intestinal contents through the nose is very unusual in the horse and is a serious sign suggesting severe gastric distension and impending rupture.
Defecation and feces
Defecation patterns can be misleading. It is often mistakenly assumed that there is no complete obstruction because feces are still being passed. But in the very early stages of acute intestinal obstruction there may be normal feces in the rectum, and the animal may defecate several times before the more usual sign of an empty rectum with a sticky mucosa is observed.
Physical examination
Heart and respiratory rates
The heart rate is a useful indicator of the severity of the disease and its progression but has little diagnostic usefulness. Horses with heart rates less than 40/min usually have mild disease whereas horses with heart rates above 120/min are usually in the terminal stages of severe disease. Horses with obstructive, nonstrangulating disease often have heart rates between 40 and 60/min, whereas horses with strangulating disease or necrotic bowel will usually have heart rates over 80/min. However, heart rate is not an infallible indicator of disease severity, as horses with torsion of the colon can have heart rates of 40–50/min.
The respiratory rate is variable and may be as high as 80/min during periods of severe pain.
Mucous membranes and extremities
Mucous membranes of normal horses and of horses without significantly impaired cardiovascular function are pink, moist and regain their normal color within 2 seconds after firm digital pressure is removed. Dehydrated horses have dry mucous membranes, although the capillary refill time and color are normal. Horses with impaired cardiovascular function have pale, dry mucous membranes with delayed capillary refill (> 2 s). Endotoxemic horses will often have bright red mucous membranes with normal or delayed capillary refill. As the disease becomes more severe the mucous membranes develop a bluish tint and capillary refill is longer than 3 seconds. Terminal stages of disease are associated with cold, purple, dry mucous membranes with a capillary refill time of more than 3 seconds; necrosis of the mucosa of the gingival margins of the gums, the so-called ‘toxic line’, is often seen.
Cool extremities may be indicative of compromised cardiovascular function but should be interpreted with caution and only in the context of the rest of the clinical examination. Sweating is common in horses with severe abdominal pain and, when present in a horse with cool extremities and signs of cardiovascular collapse, is indicative of a poor prognosis.
Auscultation; percussion
Auscultation of the abdomen can provide useful diagnostic and prognostic information and should be performed thoroughly and without haste. All four quadrants (dorsal and ventral, left and right sides) of the abdomen should be examined for at least 1 minute at each site. Attention should be paid to the intensity, frequency and characteristics of the spontaneous gut sounds (borborygmi). Repeated observations are often necessary to detect intermittent or rapid changes in the character of the borborygmi.
Continuous, loud borborygmi distributed in all or most quadrants are indicative of intestinal hypermotility and consistent with spasmodic colic, impending diarrhea or the very early stages of a small-intestinal obstructive/strangulating lesion. The absence of sounds, or the presence of occasional high-pitched, brief sounds, sometimes with a splashing character, is consistent with ileus. These sounds should not be mistaken for the rolling, prolonged sounds of normal peristalsis.
Combined percussion and auscultation is a valuable procedure for defining the presence of extensive gas caps; a flick or abrupt tap with a finger while auscultating with a stethoscope will elicit a ‘pinging’ sound similar to that made by flicking an inflated balloon. The detection of such sounds indicates the presence of tightly gas-distended bowel near the body wall. Such bowel is almost always large colon or cecum and is consistent with gas distension secondary to ileus, small or large colon impaction, gas colic or colon displacement, including torsion.
Rectal examination
A careful rectal examination is probably the most important part of the clinical examination in colic and should not be neglected. The examiner must know the anatomy of the posterior abdomen in order to make reasonably accurate decisions about the location of various organs. Recognition that an important abnormality exists is a critical factor in the decision to refer the horse for specialized evaluation and care.
Normal anatomy
The horse should be restrained so that the examination can be performed with minimal risk to both the examiner and patient. Fractious or painful horses should be tranquilized. A twitch should be applied to all but the most cooperative horses to minimize straining and the chance of kicking. Rectal examination in small or unruly horses should be approached with caution.
Only approximately 40% of the abdomen can be examined in a mature horse, the cranial and ventral structures being outside the reach of the examiner. In the normal 425 kg (1000lb) horse there should not be any distended intestine nor should the small intestine be palpable. The cecum is readily palpable in the right caudal abdomen, with its ventral band running from the dorsal right quadrant ventrally and slightly to the left. The base of the cecum may be palpable as a soft, compressible structure containing fluid and gas. The caudal border of the spleen is readily palpable as it lies on the left side of the abdomen against the body wall. There should be no bowel between the spleen and the body wall although occasionally small colon can be detected dorsal to the spleen. Dorsal and medial to the spleen the left kidney should be readily palpable, as should the nephrosplenic ligament and space. There should be no bowel in the nephrosplenic space, although some horses have portions of small colon in the region of the nephrosplenic space. Portions of large colon, especially the pelvic flexure, can be palpated in the caudal ventral abdomen if they contain ingesta. The inguinal rings may be palpated in males. The ovaries and uterus can be palpated in mares. The bladder can be palpated if it contains urine.
Abnormal findings
Abnormalities associated with specific diseases are discussed under those headings (Table 5.5). One should be able to recognize gas and fluid distension of the cecum and colon, fluid distension of the small intestine, impaction of the large and small colon, and displacement of the large colon.
Small intestinal distension is evident as loops of tubular structures of up to 10–15 cm diameter that may extend as far caudally as the pelvic canal. The structure is often compressible, akin to squeezing a fluid-filled tubular balloon, and slightly moveable. The presence of distended small intestine is an important sign suggestive of a small-intestinal obstructive lesion or anterior enteritis.
Colonic distension, impaction and displacement. Gas and fluid distension of the large colon is evident as large (> 20 cm) taut structures often extending into the pelvic canal. Tenial bands are often not palpable because of the distension. The distended bowel may extend into the pelvic canal, preventing examination of the caudal abdomen. Impaction is evident as columns of firm ingesta in the large or small colon. The most common site is the pelvic flexure in the caudoventral abdomen and the inlet to the pelvic canal. The impacted material remains indented when pressed with the finger tips.
Distension of the small colon is detectable as loops of tubular structures in the caudal abdomen. The loops of intestine have a prominent antimesenteric band, a feature not present on small intestine.
Displacement of the large colon is evident rectally as tight bands extending from the ventral abdomen cranially, dorsally and to the left or cranially, dorsally and to the right in left and right displacements of the colon, respectively. Displacement of the colon, if it obstructs aboral flow of ingesta and gas, may cause distension.
Nasogastric intubation
Passage of a nasogastric tube is an essential part of the examination of a horse with colic because of the diagnostic information it provides and because relief of gastric distension may be life-saving.
The nasogastric tube must be passed into the stomach. This is usually evident by the release of a small amount of sweet-smelling gas as the stomach is entered. The tube should then be advanced further into the stomach and, if reflux of material does not occur spontaneously, a siphon should be established by filling the tube with approximately 500 mL of water and rapidly dropping the end of the tube below the level of the horse’s stomach. This procedure should be repeated at least three or four times if reflux is not obtained. If reflux is obtained, its volume and character should be noted. The volume should be measured – anything more than 2 L of net reflux is likely important. If reflux is obtained, the nasogastric tube should be left in place or replaced frequently (1 h intervals) until the colic resolves. If there is no reflux but the horse remains colicky, then repeated attempts should be made to obtain reflux. Oral medications, such as mineral oil, should not be given to horses with nasogastric reflux.
Ancillary diagnostic techniques
Ultrasonography
Ultrasonographic examination of the abdomen of adult horses is useful in identifying a number of abnormalities, including small-intestinal distension, ileocecal intussusception, gastric distension, gastric squamous cell carcinoma, diaphragmatic hernia, peritoneal effusion and other conditions.23 The abdomen should be examined in a systematic fashion with a 2.0–3.5 mHz transducer. Ultrasonographic examination is useful to detect small-intestinal distension (such as occurs with anterior enteritis or small intestinal accidents), reduced motility (anterior enteritis, enteritis, obstruction), thickening of intestinal wall (>4 mm, enteritis, right dorsal colitis), volume and characteristics of peritoneal fluid (peritonitis, hemoperitoneum), abnormalities in intestinal contents (such as presence of sand or excessively fluid ingesta), presence of sacculations of the ventral colon (absence indicates distension), abnormalities in intestinal architecture (intussusceptions) and presence of abnormal structures (neoplasia, abscess). Ultrasonographic detection of small-intestinal distension is more sensitive than rectal examination.24 Ultrasonographic examination reveals colon with a mural thickness of 9 mm or greater in horses with colon torsion. The test has a sensitivity of approximately 67% (i.e. correctly predicts the presence of colon torsion in two-thirds of horses that have the disease) and specificity of 100% (correctly rules out the diagnosis in 100% of horses that do not have the disease).25
Radiology
The large size of the adult horse precludes detailed radiographic examination of intra-abdominal structures. However, enteroliths and sand accumulation can be detected with reasonable certainty provided suitable radiographic equipment is available.18 Diaphragmatic hernias can be detected on radiographic examination of the thorax.
Arterial blood pressure
Arterial blood pressure is a very good indicator of the degree of shock in colic, and the availability of a simple technique makes it a practical aid in assessing prognosis in a clinical case. If normal systolic pressure is about 100 mmHg (13.3 kPa), a pressure below 80 mmHg (10.6 kPa) indicates a critical situation (it can be as low as 50 mmHg, 6.6 kPa). In horses with very severe pain but not shock, the systolic pressure is likely to be very high, up to 250 mmHg (33.3 kPa).
Course of the disease
The course of the disease depends upon its cause and the severity of the associated lesions. Spasmodic and gas colic usually resolves within hours of onset. Horses with strangulating lesions have severe clinical signs and usually die within 24 hours of the onset of signs. Horses with nonstrangulating obstructive lesions have longer courses, often 48 hours to 1 week, and die when distension causes bowel to become devitalized and rupture.
When intestinal rupture does occur, there is a sudden onset of shock and toxemia, the acute pain that preceded it disappears and the horse becomes quiet and immobile. The terminal stages after rupture of the intestine or stomach, or due to profound endotoxemia, are very distressing. The horse may be recumbent but most continue to stand until the last few minutes, when they literally drop dead. The respiration is sobbing and there is gross muscle tremor and profuse sweating, and there is often a delirious, staggering wandering. Euthanasia should be performed before this stage is reached.
CLINICAL PATHOLOGY
Examination of various clinical pathology variables is useful in assessing the severity of the changes occurring as a consequence of the disease rather than in providing a definitive diagnosis. Therefore, some of these variables have prognostic significance (Prognostication) and should be monitored repeatedly in severe cases.
Hematology and serum biochemistry
Measurement of hematocrit and plasma total protein concentration is useful in assessing hydration status (see Chapter 2). Hematocrit increases as a consequence of splenic contraction or dehydration, making the use of this variable as a sole indicator of hydration status unreliable. However, increases in both hematocrit and total protein concentration indicate dehydration, and these variables can be used as crude estimates of response to fluid therapy. Plasma total protein concentrations may decline if there is significant loss of protein into the gut lumen or peritoneal space.
Measurement of the blood leukocyte count has little diagnostic significance, with the exception that the combination of leukopenia and a left shift are consistent with the endotoxemia that accompanies devitalized bowel, enteritis or peritonitis.
Horses with severe colic often have abnormalities in coagulation, with nonsurviving horses and horses with strangulating lesions having the most severe changes, characterized by low antithrombin activity and prolonged prothrombin and activated partial thromboplastin times.18,26
Measures of serum electrolyte concentration are important in providing an assessment of the horse’s electrolyte status and in tailoring fluid therapy (see Chapter 2). The nature of the abnormalities depends to some extent on the cause of the disease, but is more markedly affected by the severity of the disease. Mild hyponatremia is not uncommon but is clinically insignificant. Hyperkalemia is common in horses with severe acidosis and large sections of devitalized intestine. Hypokalemia is common in horses with more long-standing colic, for instance impaction of the large colon, that have not eaten for several days. Hypocalcemia and hypomagnesemia are common in horses with colic, especially horses with severe colic. Measurement of total concentrations (ionized plus non-ionized) can be misleading in that reductions in concentration of the physiologically important ionized component can be present in horses with normal concentrations of the total ion.27,28 Hospitalized horses with colic or diarrhea are more likely to have hypomagnesemia than are horses with other diagnoses.29
Serum enzyme activities are rarely useful in aiding diagnosis or treatment of horses with colic, with the exception that serum gamma glutamyl transferase (GGT) activity is elevated in approximately 50% of horses with right dorsal displacement of the colon, whereas such elevations are rare in horses with left dorsal displacement.30 The elevated GGT, and less commonly serum bilirubin concentration, in horses with right dorsal displacement is attributable to compression of the common bile duct in the hepatoduodenal ligament by the displaced colon.30 Serum and peritoneal alkaline phosphatase activities are higher in horses with ischemic or inflammatory bowel disease than in horses with other forms of colic, although the differences are not sufficiently large as to be useful diagnostically.31 Serum creatine kinase activity above the normal range (385 U/L) is associated with a fourfold increase in the likelihood that a horse with colic has small intestinal ischemia.32
Serum urea nitrogen and creatinine concentrations are useful indicators of hydration status and renal function. Prerenal azotemia is common in horses with colic, and may progress to acute renal failure in severe cases of colic.
High plasma concentrations of intestinal fatty acid binding protein (> 100 pg/mL) are associated with increased need for surgery in horses with colic.32
Horses that die of colic have higher circulating concentrations of epinephrine, cortisol and lactate than do horses that survive, indicating the greater degree of sympathetic and adrenal cortical activation in these horses.33
Acid–base status
Most horses with severe colic have metabolic acidosis, although respiratory acidosis and metabolic alkalosis also occur. Horses with less severe disease, such as simple obstructive disease or spasmodic colic, might not have abnormalities in acid–base status. Metabolic acidosis, when severe, is attributable to l-lactic acidosis.34 An estimate of the plasma lactate concentration can be obtained by calculating the anion gap:
If bicarbonate concentrations are not available, total serum carbon dioxide can be substituted. Anion gaps of less than 20 mEq/L (mmol/L) are associated with 81% survival, 20–24.9 mEq/L (mmol/L) with 47% survival, and 25 mEq/L (mmol/L) or more with 0% survival.35
Abdominocentesis
Analysis of peritoneal fluid is an important component of the complete examination of a horse with colic.36 Details of the technique and interpretation of the results were discussed previously but, briefly, if there is an increase in the total protein concentration, a change in the color to red or blood-tinged, and an increase in the leukocyte count in peritoneal fluid, it is likely that there is some insult to intra-abdominal structures.32,36 Total protein concentration increases when there is an insult to the gastrointestinal tract that compromises the serosal surface of the bowel, for instance strangulating lesions of the small intestine or in the terminal stages of an impaction colic in which the bowel wall is devitalized.32,36 The presence of intracellular bacteria, plant material and degenerate neutrophils is indicative of gastrointestinal rupture provided that one is certain that the sample came from the peritoneal space and not from the bowel lumen (by inadvertent enterocentesis).
PROTOCOL FOR EVALUATING A COLIC PATIENT
When evaluating a horse with colic the aims are:
• Determine the nature and cause of the lesion
• Determine the most appropriate therapy, including consideration of euthanasia
• Determine the need for referral for specialized care, including surgery.
The suggested protocol for evaluating a horse with colic is set down below. The time intervals between repeated examinations depend on a number of factors, including severity of the disease and the accessibility of the horse. For a horse with a possible intestinal obstruction this should be every hour; for a horse with probable colonic impaction examinations every 4 hours are adequate; for a chronic colic with ileal hypertrophy an examination every day is usual. The following observations should be made.
Behavior
The following should be assessed: severity of pain, frequency and duration of attacks, whether food is taken, amount and character of feces, and frequency of urination.
Clinical and clinicopathological observations
• Elevated pulse rate with a fall in pulse amplitude are among the most reliable indicators of the state of dehydration or shock. They can be temporarily misleading in a horse that is excited because it is in strange surroundings, or separated from its dam, foal or close companion. They may also be marginally influenced by a bout of pain. A rate of more than 60/min and a steady climb in heart rate of about 20 beats/min at each hour in a series of monitoring examinations signal a deterioration in prognosis. A high rate that continues to worsen during a period of analgesia as a result of medication also indicates a bad outcome. A small-amplitude, ‘thready’ pulse characterizes severe shock
• Mucous membrane color and capillary refill time are assessed. Deep congestion (dark red) or cyanosis (purple) and capillary refill times much longer than 2 seconds are indicators of peripheral circulatory failure
• Temperature is infrequently taken unless there is some positive indication, such as suspicion of peritonitis, to do so
• Respiratory rate, also of minor importance except as an indicator of severity of pain, or in terminal stages of endotoxic shock or dehydration, when it becomes gasping
• Intestinal sounds. The disappearance of intestinal sounds indicates ileus. Hypermotility is usually a sign of less serious disease, except in the very early stages of a small intestinal accident. The development of a ‘ping’ on auscultation–percussion indicates accumulation of gas under some pressure
• Rectal findings. The development of palpable abnormalities is an ominous finding. A decision to intervene surgically is often made at this point. The inherent inadequacy of the rectal examination is that only the caudal half of the abdominal cavity can be reached. Therefore large bowel and terminal ileal problems are more easily detected. With anterior abdomen small-intestinal lesions, distended loops do not usually come into reach until 6 hours after colic commences. They may reach back as far as the pelvis by 18 hours
• Amount and nature of feces is important. Failure to defecate within 12 hours of treatment is a bad sign. The empty rectum with a dry, tacky feel, or with a smear of mucus and degenerated blood some hours after the last defecation, presages a completely blocked intestine. The passage of oil but no feces suggests a partial blockage of large bowel that will permit the passage of oil but not fecal balls
• Reflux through a nasogastric tube. Acute gastric dilatation or small intestinal regurgitation of fluid sufficient to cause reflux of fluid via the stomach tube is a grim development. Large-bowel distension is also associated with fluid accumulations in the stomach. A negative test in a case suggestive of small intestinal obstruction should be followed by repeated tests; reflux from a lesion well down in the small intestine may take some hours to reach the stomach. In ileocecal valve impaction gastric reflux may not develop until 24 hours after the commencement of the colic
• Abdominal paracentesis. Repeated examinations are without serious risk and can herald the development of infarction and necrosis of gut wall, leakage and the development of peritonitis, or rupture and death due to endotoxic shock
• Visible distension of the abdomen
• PCV and plasma protein. A rise in PCV of 5% (i.e. from 55 to 60%) in an hour is a serious sign. A rise in PCV with a stable or declining serum protein concentration is often indicative of loss of capillary integrity and leakage of vascular proteins into extravascular spaces, such as the intestinal lumen. This is a sign of a poor prognosis
• Skin tenting on its own can be a very misleading indicator of the state of a horse’s dehydration, but significant changes from one examination to another are likely to confirm deductions made on the basis of heart rate and mucosal color
• Arterial blood pressure is one of the most reliable prognostic indicators in cases of colic
• Response to analgesics. Diminution in the relief of pain after administration of detomidine, xylazine, butorphanol or flunixin meglumine can be interpreted as a serious decline in the status of the affected intestine.
When to refer the patient
The decision to refer a horse for specialist care and evaluation is often difficult. Most referrals occur because of the need for specialized medical or surgical treatment and therefore involve considerable expense and inconvenience to the owner. However, early referral is critical because of the improved chances of survival associated with early medical and surgical therapy of horses with severe colic.
The criteria for referral include:
• Severe persistent pain without identifiable cause for more than 24 hours. Referral should be sooner if there is evidence of compromised cardiovascular function, or any of the signs described below
• Recurrent attacks of colic over a period as long as several months
• Failure of an efficient analgesic to provide analgesia or relief for at least 20 minutes
• A rectally palpable lesion including distended small intestine, large colon, or small colon, or impaction of the large colon that does not resolve in 24 hours
• Reflux of more than 4 L of fluid through a nasogastric tube
• Blood-tinged, high-protein peritoneal fluid with a high white cell count
• A rapid worsening of the pain and vital signs during a period of 2–4 hours.
Not all of these criteria need to be fulfilled to warrant a decision to refer and in most cases the presence of one of these findings is sufficient to justify a recommendation to the owner to refer the horse for further evaluation and specialized care.
Important in the decision to refer, or to perform a laparotomy, is the client’s understanding of the costs involved and the likely outcomes. Because decisions to refer are often complicated by the emotional pressures on the owner and the need to make a decision quickly, it is important to take the time to fully inform the owner of the likely costs and outcomes before a final commitment is made to refer.
Surgery
The decision to perform surgery is best made by trained specialists and is usually based on a variety of clinical and clinicopathological findings with most weight given to the presence of severe unrelenting or intermittent pain, severe abdominal distension, large quantities of reflux through a nasogastric tube, intestinal distension palpable per rectum, serosanguinous peritoneal fluid, evidence of cardiovascular compromise including a high (> 60/min) and increasing heart rate, poor capillary refill, discolored mucous membranes and the absence of borborygmi.37,38 Presence of abnormal abdominal fluid (turbid or serosanguinous) and peritoneal fluid with an elevated total protein concentration has good sensitivity (92%) and moderate specificity (74%) for the need for surgery.36 Formal modeling of the need for surgery in horses with colic at referral institutions provides a numerical estimate of the need for surgery, but is seldom used in most referral practices.39,40
Prognosis
Given the enormous emotional and financial costs of having a severely ill horse with colic, there is an obvious need for accurate prognostication. Overall best predictors of survival are those clinical and clinicopathological factors that assess cardiovascular and metabolic status. The important factors include arterial blood pressure or its clinical correlates, pulse pressure and/or capillary refill time, pulse rate, mucous membrane color, indicators of hydration status (hematocrit, serum urea nitrogen concentration), blood lactate concentration and anion gap.33,41-44
Arterial systolic blood pressure is one of the best predictors of survival, with horses with systolic pressures of 90 mmHg (12 kPa) having a 50% chance of survival while fewer than 20% of horses with a pressure below 80 mmHg (10.6 kPa) survive.
Capillary refill time, the clinical manifestation of arterial blood pressure, is also a good predictor of the probability of survival. Capillary refill times of 3 seconds or more are associated with a survival rate of 30%. Similarly, increasing heart rate is associated with diminishing chances of survival – a horse with a heart rate of 80/min has a 50% chance of survival whereas one with a heart rate of 50/min has a 90% chance of surviving. Increasing blood lactate concentration and anion gap (see under Clinical pathology, above) are associated with increased chance of death. Measures of hydration status are also good indicators of prognosis. A hematocrit of 50% (0.50 L/L) is associated with a 50% chance of survival, while the chance of surviving drops to 15% when the hematocrit is 60% (0.60 L/L). Horses with high circulating epinephrine, cortisol or lactate concentrations are at greater risk of death.33
While individual variables may be good prognostic indicators, their predictive utility improves when they are combined40,43,44 although this introduces the need for either remembering models or keeping the model close at hand, something often not easily accomplished in the field. Furthermore, these models have been developed from cases at specific referral institutions and may not be applicable to field cases or even cases at other referral sites. However, the general principles probably apply in all circumstances even if the precise weighting appropriate for each variable does not.
NECROPSY FINDINGS
The nature of the necropsy findings depends on the underlying disease.
The following diseases may be mistaken for colic:
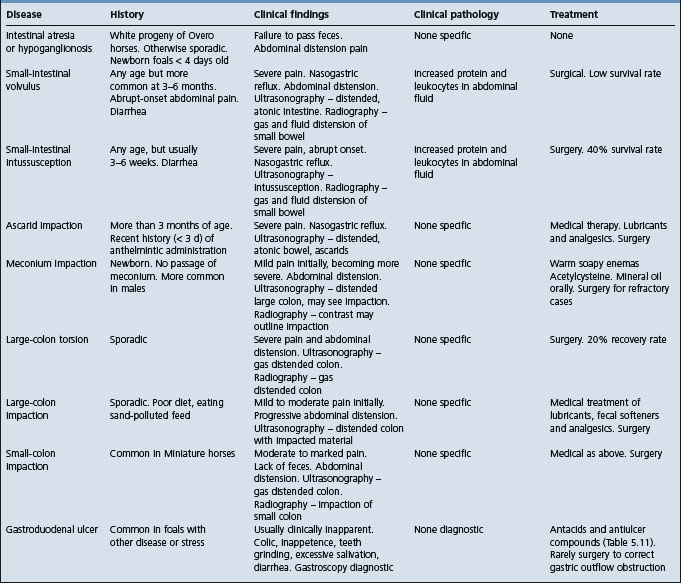
The clinical characteristics of common causes of equine colic are summarized in Table 5.6.
TREATMENT
Medical treatment
The specific treatment of each case of colic varies and depends on the nature of the lesion and the severity of the disease. However several principles are common to the treatment of most colic:
• Correction of fluid, electrolyte and acid–base abnormalities
• Gastrointestinal lubrication or administration of fecal softeners
Analgesia
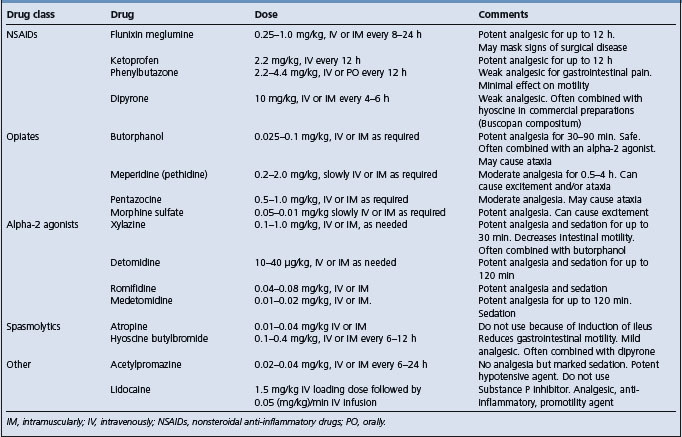
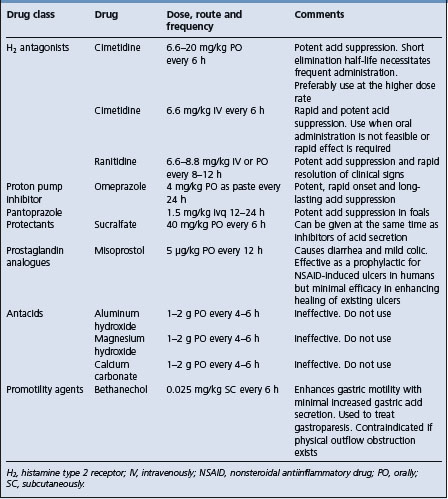
Analgesia is important in that it relieves the horse’s discomfort, minimizes the physiological consequences of pain, including the pain-induced reduction in gastrointestinal motility, permits a thorough clinical examination and reduces the likelihood of the horse injuring itself while rolling or thrashing. Analgesics can be divided into NSAIDs, sedating analgesics and spasmolytics. The doses of these drugs are provided in Table 5.7.
The analgesic and its dose rate should be chosen such that the horse’s pain is relieved but signs of progressive cardiovascular compromise indicative of the need for more aggressive therapy or surgery are not masked. Acupuncture does not provide effective analgesia in horses with colic and should not be used in these animals.45
Nonsteroidal anti-inflammatory drugs
Flunixin meglumine is a potent, long-acting analgesic with the ability to mask signs of surgical disease, with the consequence that surgery may be delayed and the chance of recovery diminished. Flunixin meglumine should only be used to control pain when the diagnosis is clear or when surgical intervention is not an option. It should not be used routinely in horses being monitored for progression of disease unless such monitoring is frequent and thorough, which may not be the situation in field colics. A horse that remains painful 30 minutes after the administration of flunixin meglumine is likely to have severe gastrointestinal disease and should be further evaluated.
Comments similar to flunixin meglumine apply to ketoprofen but not to phenylbutazone, which has relatively weak analgesic effects in colic patients (as opposed to its potent analgesic effects in musculoskeletal disease). Dipyrone is a weak analgesic that is useful in treatment of mild cases of colic.
Flunixin meglumine and etodolac retard recovery of equine jejunum and barrier function and flunixin inhibits electrical activity in the ventral colon.46,47 However, these effects detected in vitro have not been demonstrated to have practical relevance to treatment of horses with colic with NSAIDs. Horses in pain should not, based on current information, be deprived of these drugs.
Alpha-2 agonists
The alpha-2 agonists (xylazine, detomidine, romifidine) provide potent analgesia, especially when combined with the opiate butorphanol. Duration is relatively short (up to 90 min for detomidine), which means that signs of progressive disease are readily detectable. The effect of alpha-2 agonists in reducing gastrointestinal motility is not clinically important in most colic cases and should not discourage use of these very useful drugs.
Opiates
Opiates, including butorphanol, meperidine (pethidine), morphine and pentazocine, are potent analgesics useful in the management of abdominal pain in the horse. These drugs are often combined with an alpha-2 agonist. Morphine and meperidine can cause excitement or urticaria in some horses. All are drugs with the potential for human abuse and the consequent limitation on their availability limits their use in horses.
Other agents
Acetylpromazine has almost no analgesic properties, although it is a potent sedative, and should not be used in the routine treatment of colic. Acetylpromazine is a potent hypotensive agent and should not be administered to any horse that is dehydrated or has compromised cardiovascular function.
Hyoscine butylbromide, a parasympatholytic drug, is widely used in certain parts of the world as the drug of choice in the initial treatment of field cases of colic. It is often combined with dipyrone and is effective in the field treatment of mild, uncomplicated colic.
Atropine causes gastrointestinal stasis in horses and should not be used in the routine treatment of colic.48
Lidocaine (Table 5.7) is a potent analgesic when administered systemically, but must be given by constant intravenous infusion. Overdosing results in central nervous system excitement.
Prophylaxis and treatment of endotoxemia
Treatment of endolemma has been recently reviewed.49 Administration of plasma from horses hyperimmunized with Salmonella typhimurium or E. coli reduces the severity of clinical signs and shortens the duration of disease in horses with endotoxemia secondary to enterocolitis or colic.50 Polymyxin (5000 IU/kg intravenously every 8–12 h) attenuates the effect of endotoxin in experimental disease and is used for the prevention and treatment of endotoxemia in hospitalized horses.51 Its efficacy in clinical settings has not been determined. Aspirin (10 mg/kg orally every 48 h) is administered to diminish platelet aggregation around intravenous catheters. Flunixin meglumine (1 mg/kg intravenously every 8–12 h) or phenylbutazone (2.2 mg/kg intravenously every 12 h) is given for analgesia and to prevent endotoxin-induced increases in plasma prostaglandins. Pentoxifylline (8 mg/kg orally every 8 h) is administered for its putative effective in attenuating the effects of endotoxemia. The efficacy of these treatments in a clinical setting and their effect on measures of outcome of disease, such as duration of illness, case fatality rate or incidence of complications, has not been determined, with the exception of hyperimmune plasma or serum.50
Antibiotics are often administered to horses with severe colic and evidence of toxemia because of presumed bacteremia. The antibiotics of choice should have a broad spectrum including Gram-negative and positive and anaerobic bacteria. A suitable regimen includes an aminoglycoside and a penicillin, possibly combined with metronidazole. NSAIDs are administered to prevent the increased production of prostaglandins induced by endotoxin and the associated clinical abnormalities including fever, malaise and tachycardia. However, the effect of NSAIDs in improving survival or shortening the duration of treatment has not been demonstrated.
Fluid and electrolyte therapy
Horses with evidence of dehydration, compromised cardiovascular function or electrolyte imbalances should be administered fluids intravenously, preferably a balanced, isotonic, polyionic fluid such as lactated Ringer’s solution. Horses with severe colic and signs of cardiovascular collapse may require urgent resuscitation by intravenous administration of large quantities of fluids or administration of hypertonic saline followed by administration of isotonic fluids. Horses with hypoproteinemia may benefit from administration of plasma or colloidal fluids such as hetastarch. (See Chapter 2 for details on fluid therapy and the section on Shock for a discussion of the treatment of this syndrome.)
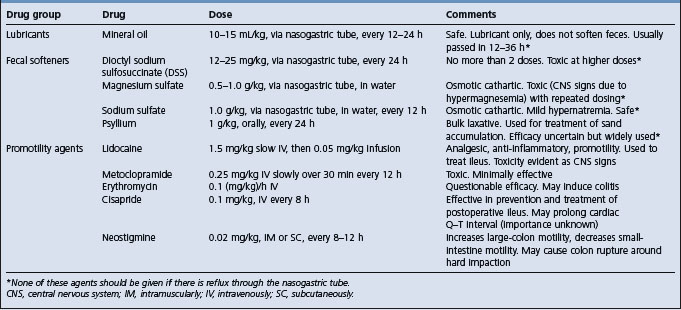
Intestinal lubricants and fecal softeners
The intestinal lubricant of choice is mineral oil (Table 5.8). It should be given only through a nasogastric tube as its aspiration is associated with severe and usually fatal pneumonia. Mineral oil is useful in cases of mild impaction colic and is often administered when the cause of the colic is not known, provided that there is no reflux of gastric contents through the nasogastric tube.
Dioctyl sodium sulfosuccinate (DSS) is a fecal softener with the potential to be toxic at therapeutic doses and its use is now not generally recommended.52 Magnesium sulfate is an effective fecal softener useful in the treatment of impaction colic.52 However, it can cause hypermagnesemia and toxicity characterized by depression and signs of central nervous system dysfunction.53 Sodium sulfate is a safe and effective fecal softener, although it may induce mild hypernatremia and hypokalemia.54
Other treatments
Promotility agents (Table 5.8) may be used in cases of ileus or large colon impaction. Postoperative ileus is a common complication of surgical colic and should be treated by maintenance of hydration and electrolyte status and administration of promotility agents.55 Cisapride is apparently effective in reducing the incidence of postoperative ileus and may be useful in the treatment of ileus of other cause.56 The clinical efficacy of other putative promotility agents has not been demonstrated.
Heparin and low-molecular-weight heparins have been recommended for the treatment and prevention of coagulopathies associated with severe colic.21 The use of heparin or low-molecular-weight heparin is associated with increased risk of hemorrhage and heparin use causes a decrease in hematocrit.21 The efficacy of this treatment in improving survival has not been demonstrated.
Trocarization
Occasionally in severe cases of flatulent (gas) colic or in cases of colon torsion in which the abdominal distension is impairing respiration, it may be necessary to relieve the gas distension of the colon or cecum by trocarization. Trocarization is usually performed through the right paralumbar fossa immediately caudal to the last rib. The exact place for trocarization can be located by simultaneous flicking the body wall with a finger and listening with a stethoscope. The area of loudest ping will indicate the point of insertion of the trocar. A suitable trocar is a 12.5–15 cm 14–16-gauge needle. The needle is inserted through the skin and advanced into the abdomen until there is an audible expulsion of gas through the trocar. The trocar should be kept in position as long as gas is escaping. It may need to be replaced as the bowel is decompressed and moves away from the trocar. The procedure is reasonably safe but will cause inflammatory changes in the peritoneal fluid. The major danger is laceration of the colon or cecum and leakage of ingesta. It is advisable to administer systemic antibiotics to horses that have been trocarized.
Management of field colic
Initial treatment of field cases of colic that do not have signs indicative of the need for referral or surgery usually includes administration of an analgesic and an intestinal lubricant. Analgesics suitable for the initial treatment of colic in the field are an alpha-2 agonist, such as xylazine, hyoscine butylbromide, dipyrone, butorphanol or phenylbutazone. If there is no reflux through the nasogastric tube, then mineral oil should be administered. Fluids should be administered intravenously if there are signs of dehydration, cardiovascular compromise or electrolyte imbalance. The response to this therapy should be monitored as described under Protocol for evaluating a colic patient. Further doses of analgesic can be given as required and the horse should be monitored for any evidence of deterioration. If referral is contemplated, the referral institution should be contacted for advice on analgesia during transportation. Horses should be transported with a nasogastric tube in place.
Surgery
The only definitive treatment for many causes of equine colic is surgical correction or removal of the lesion. The availability of surgical facilities staffed by appropriately trained personnel has increased over the past two decades and there is often the opportunity to refer horses for examination by personnel with specialist training. Gastrointestinal surgery should not be attempted by those untrained or inexperienced in the necessary techniques or without the facilities to provide postoperative care.
The decision to perform an exploratory laparotomy on a horse with colic is based on a number of factors, including the provisional diagnosis, findings on physical and laboratory examination and degree of pain. Horses with severe pain refractory to treatment with analgesics should have an exploratory laparotomy even if no other significant abnormalities can be detected. Algorithms for the decision to perform surgery have been developed, but are not perfect and do not replace the opinion of an appropriately trained and experienced examiner.40 Examination of peritoneal fluid contributes to the decision to perform surgery.36 The survival rate for horses undergoing surgical correction of lesions depends on the nature and location of the underlying disease and its duration.57 However, survival rates range from 50–75%, with approximately two thirds of horses returning to their intended use.58-60 The survival rate of horses with small-intestinal lesions is less than that of horses with large-intestinal disease, and the survival rate for horses with strangulating disease is much less than that of horses with nonstrangulating disease.58
Prevention
Minimization of colic episodes depends on management factors, including ensuring adequate parasite control, feeding large quantities of forage and minimizing the amount of concentrate fed, and providing dental care. However, most cases of colic not attributable to parasites or dietary factors cannot be prevented.
Johnston M. Equine colic — to refer or not to refer. In Pract. 1992;14:134-141.
Hay WP, Moore JN. Management of pain in horses with colic. Compend Contin Educ Pract Vet. 1997;19:987-990.
Singer ER, Smith MA. Examination of the horse with colic: is it medical or surgical? Equine Vet Educ. 2002;14:87.
1 Traub-Dargatz JL, et al. J Am Vet Med Assoc. 1991;198:1745.
2 Tinker MK, et al. Equine Vet J. 1997;29:448.
3 Traub-Dargatz JL, et al. J Am Vet Med Assoc. 2001;219:67.
4 Kaneene JB, et al. Prev Vet Med. 1997;30:23.
5 Proudman CJ. Equine Vet J. 1991;24:90.
6 Cohen ND, et al. J Am Vet Med Assoc. 1999;215:53.
7 Uhlinger C. Equine Vet J. 1990;22:251.
8 Hillyer MH, et al. Equine Vet J. 2001;33:380.
9 Goncalves S, et al. Vet Res. 2002;33:641.
10 Cohen ND. Equine Vet J. 2003;35:343.
11 Tinker MK, et al. Equine Vet J. 1997;29:454.
12 Freeman DE, et al. J Am Vet Med Assoc. 2001;219:87.
13 Hudson JM, et al. J Am Vet Med Assoc. 2001;219:1419.
14 Reeves MJ, et al. J Prevent Med. 1996;26:285.
15 Cohen ND, et al. J Am Vet Med Assoc. 1995;206:667.
16 Proudman CJ, et al. Equine Vet J. 1998;30:194.
17 Barton MH, et al. J Vet Intern Med. 1999;13:457.
18 Prasse KW, et al. J Am Vet Med Assoc. 1993;203:685.
19 Monreal L, et al. Equine Vet J Suppl. 2000;32:19.
20 Fiege K, et al. J Vet Med A. 2003;50:30.
21 Fiege K, et al. Equine Vet J. 2003;35:506.
22 Blikslager AT, Roberts MC. J Am Vet Med Assoc. 1995;207:1444.
23 Freeman S. In Pract. 2002; May:262.
24 Klohnen A, et al. J Am Vet Med Assoc. 1996;209:1597.
25 Pease AP, et al. Vet Radiol Ultrasound. 2004;45:220.
26 Collatos C, et al. J Vet Intern Med. 1995;9:18.
27 Garcia-Lopez JM, et al. Am J Vet Res. 2001;62:7.
28 Van der Kolk JH, et al. Equine Vet J. 2002;34:528.
29 Johansson AM, et al. J Vet Intern Med. 2003;17:860.
30 Gardner RB, et al. J Vet Intern Med. 2005;19:761.
31 Saulez M, et al. J Vet Intern Med. 2004;18:564.
32 Nieta JE, et al. Am J Vet Res. 2005;66:223.
33 Hinchcliff KW, et al. J Am Vet Med Assoc. 2005;227:276.
34 Nappert G, Johnson PJ. Aust Vet J. 2001;42:703.
35 Bristol DG. J Am Vet Med Assoc. 1982;181:63.
36 Matthews S, et al. Aust Vet J. 2002;80:132.
37 Edwards GB. Equine Vet Educ. 1991;3:19.
38 Baxter GM. Vet Med. 1992;87:1012.
39 Reeves MJ, et al. Am J Vet Res. 1991;52:1903.
40 Thoefner MB, et al. Can Vet J. 2003;67:20.
41 Parry BW, et al. Equine Vet J. 1983;15:211.
42 Puotunen-Reinert A. Equine Vet J. 1986;18:275.
43 Furr MO, et al. Vet Surg. 1995;24:97.
44 Reeves MJ, et al. Prev Vet Med. 1990;9:241.
45 Merritt AM, et al. Am J Vet Res. 2002;63:1006.
46 Tomlinson JE, et al. Am J Vet Res. 2004;65:761.
47 Freeman DE, et al. Am J Vet Res. 1997;58:915.
48 Sykes BW, Furr MO. Aust Vet J. 2005;83:45.
49 Ducharme NG, Fubini SL. J Am Vet Med Assoc. 1983;182:229.
50 Spier SJ, et al. Circ Shock. 1989;28:235.
51 Barton MH, et al. Equine Vet J. 2004;36:397.
52 Freeman DE, et al. Am J Vet Res. 1992;53:1347.
53 Henninger RW, Horst J. J Am Vet Med Assoc. 1997;211:82.
54 Lopes MAF. Am J Vet Res. 2004;65:695.
55 Hoogmoed LM. Vet Clin North Am Equine Pract. 2003;19:729.
56 Velden MA, Klein WR. Vet Q. 1993;15:175.
57 Van der Linden MA, et al. J Vet Intern Med. 2003;17:343.
58 Phillips TJ, Walmsley JP. Equine Vet J. 1993;25:427.