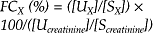Chapter 11 Diseases of the urinary system
INTRODUCTION 543
PRINCIPLES OF RENAL INSUFFICIENCY 543
CLINICAL FEATURES OF URINARY TRACT DISEASE 545
SPECIAL EXAMINATION OF THE URINARY SYSTEM 548
PRINCIPLES OF TREATMENT OF URINARY TRACT DISEASE 553
DISEASES OF THE KIDNEY 555
DISEASES OF THE BLADDER, URETERS AND URETHRA 561
CONGENITAL DEFECTS OF THE URINARY TRACT 571
Diseases of the bladder and urethra are more common and more important than diseases of the kidneys in farm animals. Occasionally, renal insufficiency develops as a sequel to diseases such as pyelonephritis, embolic nephritis, amyloidosis and nephrosis. A knowledge of the physiology of urinary secretion and excretion is required to properly understand disease processes in the urinary tract. The principles of renal insufficiency presented here are primarily extrapolated from research in other species, particularly human medicine. Although, in general, these principles probably apply to farm animals, the details of renal function and renal failure in farm animals have received only limited study.
Diseases of the reproductive tract are not presented in this book and the reader is referred to textbooks on the subject. Inevitably, some of the reproductive diseases are mentioned in the differential diagnosis of the medical conditions presented here and in circumstances in which the reproductive tract is affected coincidentally. Reference to these entries in the text can be made through the index.
Principles of renal insufficiency
The kidneys excrete the end-products of tissue metabolism (except for carbon dioxide), and maintain fluid, electrolyte and acid–base balance, by varying the volume of water and the concentration of solutes in the urine. It is convenient to think of the kidney as composed of many similar nephrons, the basic functional units of the kidney. Each nephron is composed of blood vessels, the glomerulus and a tubular system that consists of the proximal tubule, the loop of Henle, the distal tubule and the collecting duct.
The glomerulus is a semipermeable filter that allows easy passage of water and low-molecular-weight solutes but restricts passage of high-molecular-weight substances such as plasma proteins. Glomerular filtrate is derived from plasma by simple passive filtration driven by arterial blood pressure. Glomerular filtrate is identical to plasma except that it contains little protein or lipids. The volume of filtrate, and therefore its content of metabolic end-products, depends upon the hydrostatic pressure and the plasma oncotic pressure in the glomerular capillaries and on the proportion of glomeruli which are functional. Because these factors are only partially controlled by the kidney, in the absence of disease, the rate of filtration through the glomeruli is relatively constant.
The epithelium of the tubules actively and selectively reabsorbs substances from the glomerular filtrate while permitting the excretion of waste products. Glucose is reabsorbed entirely, within the normal range of plasma concentration; phosphate is reabsorbed in varying amounts depending upon the needs of the body to conserve it; other substances such as inorganic sulfates and creatinine are not reabsorbed in appreciable amounts. The tubules also actively secrete substances, particularly electrolytes as they function to regulate acid–base balance. As a result of the balance between resorption and secretion, the concentration of solutes in the urine varies widely when the kidneys are functioning normally.
The principal mechanism that regulates water reabsorption by the renal tubules is antidiuretic hormone (ADH). Tissue dehydration and an increase in serum osmolality stimulates secretion of ADH from the posterior pituitary gland. The renal tubules respond to ADH by conserving water and returning serum osmolality to normal, thereby producing a concentrated urine.
Diseases of the kidneys, and in some instances of the ureters, bladder, and urethra, reduce the efficiency of the kidney’s functions, resulting in: disturbances in protein, acid–base, solute and water homeostasis and in the excretion of metabolic end-products. A partial loss of function is described as renal insufficiency. When the kidneys can no longer regulate body fluid and solute composition, renal failure occurs.
RENAL INSUFFICIENCY AND RENAL FAILURE
Renal function depends upon the number and functionality of the individual nephrons. Insufficiency can occur from abnormalities in:
Of these three abnormalities, the latter two are intrinsic functions of the kidney, whereas the first depends largely on vasomotor control which is markedly affected by circulatory emergencies such as shock, dehydration, and hemorrhage. Circulatory emergencies may lead to a marked reduction in glomerular filtration but they are extrarenal in origin and cannot be considered as true causes of renal insufficiency. However, prolonged circulatory disruption can cause renal ischemia and ultimately renal insufficiency.
Glomerular filtration and tubular reabsorption can be affected independently in disease states and every attempt should be made to clinically differentiate glomerular disease from tubular disease. This is because the clinical and clinicopathological signs of renal dysfunction depend on the anatomical location of the lesion and the imbalance in function between glomeruli and tubules. Renal dysfunction tends to be a dynamic process so the degree of dysfunction varies with time. If renal dysfunction is so severe that the animal’s continued existence is not possible it is said to be in a state of renal failure and the clinical syndrome of uremia will be present.
CAUSES OF RENAL INSUFFICIENCY AND UREMIA
The causes of renal insufficiency, and therefore of renal failure and uremia, can be divided into prerenal, renal, and postrenal groups.
Prerenal causes include congestive heart failure and acute circulatory failure, either cardiac or peripheral, in which acute renal ischemia occurs in response to a decrease in renal blood flow. Proximal tubular function is affected by renal ischemia to a much greater extent than the glomerulus or distal tubules; this is because of the high metabolic demands of the proximal tubules. However, those parts of the tubules within the medulla are particularly susceptible to hypoxic damage because of the low oxygen tension in this tissue, the dependency of blood flow on glomerular blood flow and the high metabolic rate of this tissue. Renal medullary necrosis is a direct consequence of these factors. In ruminants, severe bloat can interfere with cardiac output and lead to renal ischemia.
Renal causes include glomerulonephritis, interstitial nephritis, pyelonephritis, embolic nephritis and amyloidosis. Acute renal failure can be produced in any of the farm animal species by administration of a variety of toxins (see Toxic nephrosis, below). The disease can also occur secondary to sepsis and hemorrhagic shock. Experimental uremia has also been induced by surgical removal of both kidneys but the results, especially in ruminants, are quite different from those in naturally occurring renal failure. The clinical pathology is similar but there is a prolonged period of normality after the surgery.
Postrenal uremia may also occur, specifically complete obstruction of the urinary tract by vesical or urethral calculus, or more rarely by bilateral urethral obstruction. Internal rupture of any part of the urinary tract, such as the bladder, ureters, or urethra, will also cause postrenal uremia.
PATHOGENESIS OF RENAL INSUFFICIENCY AND RENAL FAILURE
Damage to the glomerular epithelium destroys its selective permeability and permits the passage of plasma proteins into the glomerular filtrate. The predominant protein is initially albumin, because of its negative charge and a lower molecular weight than globulins; however, with advanced glomerulonephritis (such as renal amyloidosis) all plasma proteins are lost. Glomerular filtration may cease completely when there is extensive damage to glomeruli, particularly if there is acute swelling of the kidney, but it is believed that anuria in the terminal stages of acute renal disease is caused by back diffusion of all glomerular filtrate through the damaged tubular epithelium rather than failure of filtration. When renal damage is less severe, the remaining nephrons compensate to maintain total glomerular filtration by increasing their filtration rates. When this occurs, the volume of glomerular filtrate may exceed the capacity of the tubular epithelium to reabsorb fluid and solutes. The tubules may be unable to achieve normal urine concentration. As a result, an increased volume of urine with a constant specific gravity is produced and solute diuresis occurs. This is exacerbated if the tubular function of the compensating nephrons is also impaired. The inability to concentrate urine is clinically evident as polyuria and is characteristic of developing renal insufficiency.
Decreased glomerular filtration also results in retention of metabolic waste products such as urea and creatinine. Although marked increases in serum urea concentration are probably not responsible for the production of clinical signs, because urea readily crosses cell membranes and is an effective osmole, the serum urea nitrogen concentration can be used to monitor glomerular filtration rate. However, the utility of serum urea nitrogen concentration as a measure of glomerular filtration rate is reduced because serum urea concentrations are influenced by the amount of protein in the diet, by hydration and by gastrointestinal metabolism of urea. Serum urea concentrations are higher in animals on high-protein diets and dehydration increases serum urea concentration by increasing resorption of urea in the loop of Henle, independent of effects of hydration of glomerular filtration rate. Urea is excreted into saliva of ruminants and metabolized by ruminal bacteria. In contrast, creatinine is excreted almost entirely by the kidney, creatine originates from breakdown of creatine phosphate in muscle, and serum concentrations of creatinine are a useful marker of glomerular filtration rate. The relationship between serum creatinine concentration and glomerular filtration rate is hyperbolic – a reduction in glomerular filtration rate by half results in a doubling of the serum creatinine concentration. Phosphate and sulfate retention also occurs when total glomerular filtration is reduced and sulfate retention contributes to metabolic acidosis in renal insufficiency. Phosphate retention also causes a secondary hypocalcemia, due in part to an increase in calcium excretion in the urine. In horses, the kidneys are an important route of excretion of calcium so the decreased glomerular filtration rate present in horses with chronic renal failure can result in hypercalcemia. Variations in serum potassium levels also occur and appear to depend on potassium intake. Hyperkalemia can be a serious complication in renal insufficiency in humans, where it is one of the principal causes of the myocardial asthenia and fatal heart failure that occur in uremia in this species.
Loss of tubular resorptive function is evidenced by a continued loss of sodium and chloride; hyponatremia and hypochloremia eventually occur in all cases of renal failure. The continuous loss of large quantities of fluid due to solute diuresis can cause clinical dehydration. More often it makes the animal particularly susceptible to dehydration when there is an interruption in water availability or when there is a sudden increase in body water loss by another route – as in diarrhea.
The terminal stage of renal insufficiency – renal failure – is the result of the cumulative effects of impaired renal excretory and homeostatic functions. Continued loss of large volumes of dilute urine causes dehydration. If other circulatory emergencies arise, acute renal ischemia might result, leading to acute renal failure. Prolonged hypoproteinemia results in rapid loss of body condition and muscle weakness. Metabolic acidosis is also a contributing factor to muscle weakness and mental attitude. Hyponatremia and hyperkalemia cause skeletal muscle weakness and myocardial asthenia. Hypocalcemia may be sufficient to contribute to circulatory failure and to nervous signs. All these factors play some part in the production of clinical signs of renal failure, which are typically manifest as weakness, lethargy, inappetence and, with extensive glomerular lesions, dependent edema due to hypoproteinemia. In some cases one or other of them might be of major importance so the clinical syndrome is variable and is rarely diagnostic for renal failure. Bleeding diathesis can also be present in severely uremic animals and has been associated with a lack of antithrombin (a small protein readily lost through the damaged glomerulus), platelet factor 3, platelet dysfunction or disseminated intravascular coagulation.
Renal failure is seen as the clinical state of uremia. It is characterized biochemically by an increase in blood levels of urea and creatinine (azotemia) and by retention of other solutes as described above. Uremia can also occur in urinary tract obstruction.
Clinical features of urinary tract disease
The major clinical manifestations of urinary tract disease are:
• Abnormal constituents of urine
• Variations in daily urine flow
• Abdominal pain, painful urination (dysuria) and difficult urination (dysuria and stranguria)
ABNORMAL CONSTITUENTS OF THE URINE
Proteinuria
Proteinuria can be prerenal, renal, or post renal in origin. Prerenal proteinuria is due to an abnormal plasma content of proteins that traverse glomerular capillary walls, with the proteins having normal permselectivity properties (such as hemoglobin, myoglobin, immunoglobulin light chains). Renal proteinuria is due to abnormal renal handling of normal plasma proteins, and is functional or pathological. Functional renal proteinuria is mild and transient as a result of altered renal physiology during or in response to a transient phenomenon, such as high-intensity exercise or fever. Pathological renal proteinuria is due to structural or functional lesions within the kidney, regardless of their magnitude or duration. There are three subcategories of pathological renal proteinuria: glomerular, which is due to lesions altering the permselectivity properties of the glomerular capillary wall; tubular, which is due to lesions that impair tubular recovery of plasma proteins that ordinarily traverse glomerular capillary walls having normal permselectivity properties (typically low-molecular-weight proteins); and interstitial, which is due to inflammatory lesions or disease processes (such as acute interstitial nephritis) that result in exudation of proteins from the peritubular capillaries into the urine. Postrenal proteinuria is due to entry of protein into the urine after it enters the renal pelvis, and is urinary or extraurinary. Urinary postrenal proteinuria is due to the entry of proteins derived from hemorrhagic or exudative processes affecting the renal pelvis, ureter, urinary bladder, and urethra. Extraurinary postrenal proteinuria is due to entry of proteins derived from the genital tract or external genitalia during voiding or in the process of collecting urine for analysis.
Normal urine contains only small amounts of protein that are insufficient to be detected using standard tests. It should be noted that the highly alkaline urine produced by herbivores produces a false-positive reaction (trace or 1+) for protein on urine dipstick tests. Prerenal proteinuria may be present in hemoglobinuria and myoglobinuria. Functional renal proteinuria is observed in normal foals, calves, kids, and lambs in the first 40 hours after they receive colostrum. Pathological renal or postrenal proteinuria and hematuria may be present when urinary tract infections are present. Postparturient cows usually have protein present in a free-catch urine sample as a result of washout of uterine fluids; this is a classic example of extraurinary postrenal proteinuria. Demonstration that proteinuria originates in the kidney is easier if elements that form in the kidney, such as tubular casts, are also present in the urine, or morphological abnormalities of the kidneys are palpable per rectum or identified ultrasonographically.
Proteinuria is most accurately quantified by determining the amount of protein passed in a 24-hour period, which is impractical in clinical cases. Proteinuria is more easily quantified by indexing the protein concentration to creatinine concentration in single urine sample; this has been shown to provide an accurate representation of 24-hour protein loss in the urine.
Chronic pathological renal proteinuria may cause hypoproteinemia as in chronic glomerulonephritis and acute tubular nephrosis in horses and in amyloidosis of cattle. When proteinuria originates from pyelonephritis or cystitis other clinical and clinicopathological evidence of these diseases is usually present.
Casts and cells
Casts are organized, tubular structures that vary in appearance depending on their composition. They occur only when the kidney is involved in the disease process. Casts are present as an indication of inflammatory or degenerative changes in the kidney, where they form by agglomeration of desquamated cells and Tamm–Horsfall protein. Casts may not form in all cases of renal disease. In addition, casts readily dissolve in alkaline urine and are best detected in fresh urine samples.
Erythrocytes, leukocytes, and epithelial cells in urine may originate in any part of the urinary tract.
Hematuria
Hematuria can result from prerenal causes when vascular damage occurs, such as trauma to the kidney, septicemia and purpura hemorrhagica. Renal causes include acute glomerulonephritis, renal infarction, embolism of the renal artery, tubular damage as caused by toxic insult, and pyelonephritis. Postrenal hematuria occurs particularly in urolithiasis and cystitis. A special instance of hematuria is enzootic hematuria of cattle when hemorrhage originates from tumors of the urinary bladder. Hematomas of the bladder wall (cystic hematoma) cause hematuria in neonatal foals.1 Typically, lesions of the kidney, bladder, and proximal urethra cause hemorrhage throughout or towards the end of urination, whereas lesions of the middle and distal urethra are responsible for bleeding at the beginning of urination.2
In severe cases of hematuria blood may be voided as grossly visible clots but more commonly it causes a deep red to brown coloration of the urine. Less severe cases may show only cloudiness that settles to form a red deposit on standing. The hematuria may be so slight that it is detectable only on microscopic examination of a centrifuged sediment. In females, free-flow urine samples may be contaminated by blood from the reproductive tract; it may therefore be necessary to collect a sample by catheterization to avoid the chance of contamination of the urine occurring in the vagina.
Blood in urine gives positive results on biochemical tests for hemoglobin and myoglobin. Because red blood cells can be lysed in dilute urine, red-colored urine should be examined microscopically for the presence of erythrocytes. The presence of a heavy brown deposit is not sufficient basis for a diagnosis of hematuria as this may also occur in hemoglobinuria. If the bladder or urethra are involved in the process that causes hematuria, abnormalities may be detectable on physical examination. Gross hematuria persisting for long periods may result in severe blood loss anemia. Severe urinary tract hemorrhage of undetermined origin in aged mares has been recorded.3 The syndrome is widely recognized, though not well documented, in Arabian mares. Endoscopic examination reveals hemorrhage in one ureter but ultrasonographic examination of the kidneys does not reveal any significant abnormalities. Surgical removal of the affected kidney is not recommended, as the hemorrhage sometimes recurs in the remaining kidney. Treatment is nonspecific. Severe hematuria can also occur in horses with pyelonephritis.4
Hemoglobinuria
False hemoglobinuria can occur in hematuria when erythrocytes are lysed and release their hemoglobin. In this case, erythrocytes can be detected only by microscopically examining urine sediment for cellular debris.
True hemoglobinuria causes a deep red to brown coloration of urine and gives a positive reaction to biochemical tests for hemoglobin. There is no erythrocyte debris in sediment. Dipstick tests for proteinuria may not be positive unless the concentration of hemoglobin is very high.5 There are many causes of intravascular hemolysis, the source of hemoglobinuria. The specific causes are listed under Hemolytic anemia.
Normally, hemoglobin liberated from circulating erythrocytes is converted to bile pigments in the cells of the reticuloendothelial system. If hemolysis exceeds the capacity of this system to remove the hemoglobin, it accumulates in the blood until it exceeds a certain renal threshold and then passes into the urine. Some hemoglobin is reabsorbed from the glomerular filtrate by the tubular epithelium, but probably not in sufficient amounts to appreciably affect the hemoglobin content of the urine. Hemoglobinuria will only be present when the plasma concentration exceeds the renal threshold. Consequently hemoglobin is grossly visible in plasma by the time hemoglobinuria is visible. Hemoglobin precipitates to form casts in the tubules, especially if the urine is acidic, and as a result some plugging of tubules occurs, but the chief cause of uremia in hemolytic anemia is ischemic tubular nephrosis.
Myoglobinuria
The presence of myoglobin (myohemoglobin) in the urine is evidence of severe muscle damage. The only notable occurrence in animals is azoturia of horses. Myoglobinuria does not occur commonly in enzootic muscular dystrophy, possibly because there is insufficient myoglobin in the muscles of young animals. The myoglobin molecule (molecular weight 16 500) is much smaller than hemoglobin (molecular weight 64000) and passes the glomerulus much more readily, so a detectable dark brown staining of the urine occurs without very high plasma levels of myoglobin. Detectable discoloration of the serum does not occur as in hemoglobinemia. Inherited congenital porphyria is the other disease that causes a red-brown discoloration of urine. In porphyria, the plasma is also normal in color, but urine porphyria is differentiated from myoglobinuria on the basis of a negative reaction to the guaiac test and the characteristic spectrograph. The porphyrins in inherited congenital porphyria are the only pigments that fluoresce under ultraviolet light.
The presence and type of pigment in the urine can be determined accurately by spectrographic examination, but this is rarely clinically available. Myoglobinuria is usually accompanied by clinical signs and clinical biochemistry abnormalities of acute myopathy, and clinical differentiation of myoglobinuria from hemoglobinuria is usually made on the basis of the clinical signs and serum biochemical findings, including measurement of muscle-derived enzymes such as creatine kinase. As with hemoglobin, myoglobin can precipitate in tubules and may contribute to uremia.
Pyuria
Leukocytes or pus in urine indicates inflammatory exudation at some point in the urinary tract, usually the renal pelvis or bladder. Pyuria may occur as grossly visible clots or shreds, but is often detectable only by microscopic examination of urine sediment. Individual cells and leukocytic casts may be present. Pyuria is usually accompanied by the presence of bacteria in urine.
Bacteriuria
Diagnosis of urinary tract infection is based on finding a clinically relevant bacteriuria in urine collected by free catch (midstream collection into a sterile container), catheterization or cystocentesis.6 In horses and adult cattle, collection of urine is limited to free catch and catheterization, because the size of the animal and intrapelvic position of the bladder prevent cystocentesis. In contrast, cystocentesis can be performed under ultrasonographic guidance in calves, small ruminants, and pigs. When culturing a urine sample obtained by catheterization, the first 20 mL or so should be discarded because of the potential for contamination from vaginal or distal urethral flora.
Reference values for urine bacterial concentrations are available for the horse; marked bacteriuria suggestive of bacterial infection may be defined as more than 40000 colony forming units (cfu)/mL from free-catch specimens, and more than 1000 cfu/mL from catheterized specimens.6
Crystalluria
Crystalluria should not be overinterpreted in farm animals. Crystals in the urine of herbivorous animals have no special significance unless they occur in very large numbers and are associated with clinical signs of irritation of the urinary tract. Calcium carbonate and triple phosphate crystals are commonly present in normal urine. If they occur in large numbers, it may suggest that the urine is concentrated and indicate the possible future development of urolithiasis. The presence of calcium carbonate crystals in the peritoneal fluid of a neonatal foal has been used to confirm a diagnosis of ruptured bladder.7
Glucosuria
Glucosuria in combination with ketonuria occurs only in diabetes mellitus, an extremely rare disease in ruminants. Glucosuria might occur in association with enterotoxemia due to Clostridium perfringens type D and can occur after parenteral treatment with dextrose solutions, adrenocorticotropic hormones or glucocorticoid analogs. Horses with tumor of the pars intermedia of the pituitary gland often have glucosuria. Glucosuria occurs also in acute tubular nephrosis as a result of failure of tubular resorption.
Ketonuria
Ketonuria is a more common finding in ruminants, occurring in starvation, acetonemia of cattle and pregnancy toxemia of ewes and does. A small amount of ketonuria is normally present in dairy cows in early lactation. As a result, it is important that the assay method used to demonstrate ketonuria is appropriate for urine, since there may be a risk for false-positive reactions on some tests. The standard test is sodium nitroprusside, which turns an intense purple color in the presence of acetoacetate, one of the three ketoacids.
VARIATIONS IN DAILY URINE FLOW
An increase or decrease in urine flow is often described in animals, but accuracy demands physical measurement of the amount of urine voided over a 24-hour period. This is not usually practicable in large-animal practice and it is often necessary to guess whether the flow is increased or decreased. Accurate measurement of the amount of water consumed is often easier. Care should be taken to differentiate between increased daily flow and increased frequency without increased flow. The latter is much more common. Decreased urine output rarely if ever presents as a clinical problem in agricultural animals.
Normal urine production is highly variable in large animals and is dependent to a large extent on diet, watering systems and the palatability of the water. Pregnant mares housed in tie stalls consume approximately 53 ± 6.2 (SD) mL of water per kilogram body weight (BW) per day, of which 50 ± 8 mL/kg is from drinking water with the remainder being water in feed.8 However, most of this water is excreted in the feces with fecal and urinary water excretion being 33.5 ± 8 (mL/kg)/d and 7.6 ± 2 (mL/kg)/d, respectively.8 Neonatal foals produce urine at an average rate of 150 (mL/kg)/d.9
Polyuria
Polyuria occurs when there is an increase in the volume of urine produced. Polyuria can result from extrarenal causes as when horses habitually drink excessive quantities of water (psychogenic polydipsia) and, much less commonly, in central diabetes insipidus, when there is inappropriate secretion of antidiuretic hormone (ADH) from the pituitary. Polyuria occurs in horses with tumors of the pars intermedia of the pituitary gland. Although the cause of the polyuria is not known it might be secondary to osmotic diuresis associated with the glucosuria, or to central diabetes insipidus. Central diabetes insipidus is reported in sibling colts.10 It is extremely rare in other species but has been reported in a ram11 and a cow. Another extrarenal cause is administration of diuretic drugs including corticosteroids.
Kidney disease results in polyuria when the resorptive capacity of the remaining tubules is exceeded. Polyuria can also occur when the osmotic gradient in the renal medulla is not adequate to produce concentrated urine. Nephrogenic diabetes insipidus causes polyuria because the tubules fail to respond to ADH.
When polyuria is suspected, a urine sample should be collected to determine specific gravity or osmolality. If urine is isosthenuric with a constant specific gravity of 1.008–1.012 (the specific gravity of plasma), then the presence of renal disease should be considered. Serum urea and creatinine concentrations should be determined to evaluate glomerular filtration. If serum urea and creatinine concentrations are within normal limits, a water deprivation test can be performed to assess the animal’s ability to produce concentrated urine.12
Oliguria and anuria
Reduction in the daily output (oliguria) and complete absence of urine (anuria) occur under the same conditions and vary only in degree. In dehydrated animals, urine flow naturally decreases in an effort to conserve water as plasma osmolality pressure increases. Congestive heart failure and peripheral circulatory failure may cause such a reduction in renal blood flow that oliguria follows. Complete anuria occurs most commonly in urethral obstruction, although it can also result from acute tubular nephrosis. Oliguria occurs in the terminal stages of all forms of nephritis. Anuria and polyuria lead to retention of solutes and disturbances of acid–base balance that contribute to the pathogenesis of uremia.
Pollakiuria
This is an abnormally frequent passage of urine. Pollakiuria may occur with or without an increase in the volume of urine excreted and is commonly associated with disease of the lower urinary tract such as cystitis, the presence of calculi in the bladder, urethritis and partial obstruction of the urethra. Other causes of pollakiuria include equine herpesvirus infection, sorghum cystitis and neuritis of the cauda equina in horses, neoplasia, obstructive lesions and trauma to the urethra, abnormal vaginal conformation and urachal infection.
Dribbling is a steady, intermittent passage of small volumes of urine, sometimes precipitated by a change in posture or increase in intra-abdominal pressure, reflecting inadequate or lack of sphincter control. Dribbling occurs in large animals with incomplete obstructive urolithiasis and from persistent urachus.
Persistent urachus (also called pervious or patent urachus). Failure of the urachus to obliterate at birth causes urine to dribble from the urachus continuously. Urine may also pass from the urethra. Retrograde infection from omphalitis is common, resulting in cystitis.
Abnormalities of micturition are classified as neurogenic or non-neurogenic. Micturition is mediated principally by the pelvic and pudendal nerves through lumbosacral spinal cord segments under the involuntary control of centers in the brain stem and voluntary control of the cerebrum and cerebellum. Reported neurogenic causes of urinary incontinence in horses include cauda equine neuritis, herpesvirus 1 myelitis, Sudan grass toxicosis, sorghum poisoning, trauma, and neoplasia. Non-neurogenic causes of urinary incontinence in horses include ectopic ureter, cystitis, urolithiasis, hypoestrogenism, and abnormal vaginal conformation.13
ABDOMINAL PAIN, PAINFUL AND DIFFICULT URINATION (DYSURIA AND STRANGURIA)
Abdominal pain and painful urination (dysuria) and difficult and slow urination (stranguria) are manifestations of discomfort caused by disease of the urinary tract. Acute abdominal pain from urinary tract disease occurs only rarely and is usually associated with sudden distension of the renal pelvis or ureter, or infarction of the kidney. None of these conditions is common in animals, but occasionally cattle affected with pyelonephritis may have short episodes of acute abdominal pain due to either renal infarction or obstruction of the pelvis by necrotic debris. During these acute attacks of pain, the cow may exhibit downward arching of the back, paddling with the hind feet, rolling and bellowing. Abdominal pain from urethral obstruction and distension of the bladder is manifested by tail-switching, kicking at the belly and repeated straining efforts at urination accompanied by grunting. Horses with acute tubular nephrosis following vitamin K3 administration might show renal colic with arching of the back, backing into corners and rubbing of the perineum and tail head.14
Dysuria or painful/difficult urination occurs in cystitis, vesical calculus, and urethritis and is manifested by the frequent passage of small amounts of urine. Grunting may occur with painful urination and the animal may remain in the typical posture after urination is completed. Differentiating pain caused by urinary disease from pain due to other causes depends largely upon the presence of other signs indicating urinary tract involvement.
Stranguria is slow and painful urination associated with disease of the lower urinary tract including cystitis, vesical calculus, urethral obstruction, and urethritis. The animal strains to pass each drop of urine. Groaning and straining may precede and accompany urination when there is urethral obstruction. In urethritis, groaning and straining occur immediately after urination has ceased and gradually disappear and do not recur until urination has been repeated.
Urine scalding of the perineum or urinary burn is caused by frequent wetting of the skin with urine. It may be the result of urinary incontinence or the animal’s inability to assume normal posture when urinating.
MORPHOLOGICAL ABNORMALITIES OF KIDNEYS AND URETERS
Enlargement or decreased size of kidneys may be palpable on rectal examination or detected by ultrasonography. In cattle, gross enlargement of the posterior aspect of the left kidney may be palpable in the right upper flank. Abnormalities of the kidneys such as hydronephrosis in cattle may also be palpable on rectal examination. Increases in the size of the ureter may be palpable on rectal examination and indicate ureteritis or hydroureter.
PALPABLE ABNORMALITIES OF THE BLADDER AND URETHRA
Abnormalities of the bladder that may be palpable by rectal examination include: gross enlargement of the bladder, rupture of the bladder, a shrunken bladder following rupture, and palpable abnormalities in the bladder such as cystic calculi. Abnormalities of the urethra include: enlargement and pain of the pelvic urethra and its external aspects in male cattle with obstructive urolithiasis, and obstruction of the urethral process of male sheep with obstructive urolithiasis.
ACUTE AND CHRONIC RENAL FAILURE
The clinical findings of urinary tract disease vary with the rate of development and stage of the disease. In most cases, the clinical signs are those of the initiating cause. In horses, mental depression, colic, and diarrhea are common with oliguria or polyuria. Cattle with uremia are similar and in addition are frequently recumbent and in severe and terminal cases may have a bleeding diathesis. In chronic renal disease of all species, there is a severe loss of body weight, weakness, anorexia, polyuria, polydipsia, and ventral edema.
UREMIA
Uremia is the systemic state that occurs in the terminal stages of renal insufficiency.
Anuria or oliguria may occur with uremia. Oliguria is more common unless there is complete obstruction of the urinary tract. Chronic renal disease is usually manifested by polyuria, but oliguria appears in the terminal stages when clinical uremia develops. The uremic animal is depressed and anorexic with muscular weakness and tremor. In chronic uremia, the body condition is poor, probably as a result of continued loss of protein in the urine, dehydration and anorexia. The respiration is usually increased in rate and depth but is not dyspneic; in the terminal stages it may become periodic in character. The heart rate is markedly increased because of terminal dehydration and myocardial asthenia but the temperature remains normal except in infectious processes and some cases of acute tubular nephrosis. An ammoniacal or uriniferous smell on the breath is often described but is usually undetectable. Uremic encephalopathy occurs in a small proportion of cattle and horses with chronic renal failure.15
The animal becomes recumbent and comatose in the terminal stages. The temperature falls to below normal and death occurs quietly, the whole course of the disease having been one of gradual intoxication. Necropsy findings, apart from those of the primary disease, are nonspecific and include degeneration of parenchymatous organs, sometimes accompanied by emaciation and moderate gastroenteritis. There are rare reports of encephalopathy caused by renal insufficiency.16
Uremia has been produced experimentally in cattle by bilateral nephrectomy and urethral ligation.17,18 There is a progressive increase in serum urea concentration (mean daily increase of 53 mg/dL), serum creatinine concentration (mean daily increase of approximately 3.5 mg/dL), and serum uric acid concentration. Similar findings are reported in prerenal uremia in cattle. Interestingly, serum phosphate and potassium concentrations were for the most part unchanged because of increased salivary secretion, and metabolic acidosis was not evident. Serum potassium concentrations were mildly increased after 5–7 days of bilateral nephrectomy.
Special examination of the urinary system
Lack of accessibility limits the value of physical examination of the urinary tract in farm animals. Rectal examination can be carried out on horses and cattle and is described in chapter 1. In small ruminants and calves, the urinary system is largely inaccessible to physical examination although the kidneys may be palpated transabdominally.
Urinalysis and determination of blood urea and creatinine should be components of any examination of the urinary system.
URINALYSIS
Urinalysis is an essential component of the examination of the urinary system. The reader is referred to a textbook of veterinary clinical pathology for details of the biochemical and microscopic examination of the urine. The common abnormalities of urine are discussed under manifestations of diseases of the urinary system.
COLLECTION OF URINE SAMPLES
Collection of urine samples can be difficult. Free flow and catheterized samples are equally useful for routine urinalysis. Horses will often urinate a short time after they are walked into a freshly bedded box stall. Cows urinate if they are relaxed and have their perineum and vulval tip massaged upwards very gently, without touching the tail. Steers and bulls may urinate if the preputial orifice is massaged and splashed with warm water. Ewes often urinate immediately after rising if they have been recumbent for some time. Occluding their nostrils and threatening asphyxia may also induce urination just as they are released and allowed to breathe again; however, this is a stressful procedure and should not be performed in sick or debilitated sheep. An intravenous injection of furosemide (0.5–1.0 mg/kg BW) produces urination in most animals in about 20 minutes. The sample is useful for microbiological examination but its composition has been drastically altered by the diuretic. Diuretics should be used with extreme caution in dehydrated animals.
CATHETERIZATION OF THE BLADDER
Urine samples obtained by catheterization are preferred for microbiological examination. Rams, boars, and young calves usually cannot be catheterized because of the presence of a suburethral diverticulum6 and the small diameter of the urethra. A precurved catheter and fluoroscopic guidance can be used to facilitate catheterization of rams and bucks.19 Ewes and sows have vulvas that are too small to allow access to the urethra. Cows can be catheterized relatively simply provided that a fairly rigid, small-diameter (0.5 cm) catheter is used. A finger can be inserted into the suburethral diverticulum to direct the tip of the catheter over the diverticulum and into the external urethral orifice. Mares can be catheterized easily, either by blindly passing a rigid catheter into the external urethral orifice or by using a finger as a guide for a flexible catheter. Male horses can also be catheterized easily if the penis is relaxed. When urethral obstruction is present the penis is usually relaxed, but administration of an ataractic drug (acepromazine is often used) makes manipulation of the penis easier and often results in its complete relaxation. Because of the long urethra, the catheter must be well lubricated. The catheter should be rigid enough to pass through the long urethra but flexible enough to pass around the ischial arch. In all species, catheterization overcomes the natural defense mechanisms that prevent infectious organisms from ascending the urinary tract. As a result, attention to hygiene during catheterization is essential.
TESTS OF RENAL FUNCTION AND DETECTION OF RENAL INJURY
The simplest and most important test of urinary function is the determination of whether or not urine is being voided. This can be accomplished in large animals by restraining them on a clean, dry floor which is examined periodically. Placing an absorbent cloth under recumbent foals and calves will also help determine if urine is being passed.
Renal function tests evaluate the functional capability of the kidney and, in general, assess blood flow to the kidneys, glomerular filtration and tubular function. These tests depend on whether they are based on the examination of serum, urine or both, and assess either function or the presence of injury. The most practical screening tests for the presence of decreased renal function are determination of serum creatinine concentration and urine specific gravity. Determination of both assists differentiation of renal azotemia from prerenal azotemia. In prerenal azotemia, tubular function remains intact and renal conservation of water is optimized, resulting in the production of a concentrated urine. Animals with prerenal azotemia therefore have increased serum concentrations of creatinine and urea, and increased urine specific gravity. For comparison, animals with some degree of renal azotemia have increased serum concentrations of creatinine and urea and a lower than expected value for urine specific gravity. Determination of urine specific gravity should therefore be routinely performed in all dehydrated animals before the initiation of treatment, because oral or intravenous fluid therapy will directly change urine specific gravity.
Tests of urine
Urine samples for analysis should be collected by midstream voiding, or cystocentesis in small male ruminants. Bethanechol (0.075 mg/kg subcutaneously) has occasionally been used to produce urine in reluctant individuals, but a spontaneously voided sample is preferred for initial screening. The sample should be centrifuged and the supernatant should be used for laboratory analysis and the sediment and remaining supernatant for routine urine analysis.
Specific gravity
Specific gravity of urine is the simplest test to measure the capacity of renal tubules to conserve fluid and excrete solute. For most species, the normal specific gravity range is 1.015–1.035, and in azotemic animals, specific gravity should be greater than 1.020 if the azotemia is prerenal in origin. In chronic renal disease the urine specific gravity decreases to 1.008–1.012 and is not appreciably altered by either deprivation of water for 24 hours or the administration of large quantities of water by stomach tube. It is important to recognize that a specific gravity of less than 1.008 indicates that the kidney can produce a dilute urine and, if sustained, indicates better renal function than a fixed urine specific gravity of 1.008–1.012.
Specific gravity can be inaccurate when other refractive particles are present in urine, such as glucose or protein. Urine specific gravity should therefore be used with caution in animals with proteinuria or glucosuria. As an alternative to specific gravity, osmolality of a fluid directly measures the concentration of solute in the fluid. Urine osmolality therefore provides a more accurate assessment of the tubule’s ability to conserve or excrete solute than does specific gravity, and is the preferred test of urine concentrating ability for research studies. However, urine specific gravity is sufficiently accurate for clinical use in animals without proteinuria or glucosuria,20 in that there is a linear relationship between urine specific gravity and osmolality, and that urine specific gravity explains 52% of the variation in urine osmolality, the 95% confidence interval for predicting osmolality from the specific gravity measurement being ± 157 mosmol/kg.21
Enzymuria
A clinically useful index of tubular injury is determining the gamma-glutamyltransferase (GGT) activity in urine. Most enzymes present in serum have a molecular weight greater than that of albumin and are normally not detectable in the glomerular filtrate; the presence of high-molecular-weight enzymes in urine is called parenchymatous enzymuria. For comparison, the presence of low-molecular-weight enzymes (such as lysozyme) in urine is called tubular enzymuria because damage to the proximal tubule impairs its ability to reabsorb enzymes from the glomerular filtrate.22
Most of the GGT activity in urine originates from the luminal brush border of the proximal tubular epithelial cells of the kidney. High levels of GGT activity in the urine result from an increase in the rate of proximal tubular epithelial cell destruction, with GGT being released into the urine during the active phase of tissue destruction; an increase in urine GGT activity therefore reflects parenchymatous enzymuria. The activity of GGT (or other high-molecular-weight enzymes such as β-N-acetylglucosaminidase, β-glucuronidase, N-acetyl-β-glucosaminidase) in urine can therefore be used to detect the presence of proximal renal tubular epithelial cell damage before the onset of renal dysfunction.22-24 GGT is the preferred enzyme to identify the presence of parenchymatous enzymuria because the assay is inexpensive and widely available, and the kidney has the highest content of GGT of any organ in the body, thereby increasing the sensitivity of the test.
Urine GGT activity is frequently indexed to an indicator of urine concentration, such as urine creatinine concentration,22-24 in order to correct for denominator effects induced by changes in urine volume, and a GGT:creatinine higher than 25 IU/g creatinine is considered abnormal in the horse. However, it may be more appropriate to calculate the fractional clearance of GGT (which compares the extent of tubular damage to the amount of functioning kidney mass) instead of the urinary GGT to creatinine ratio (which compares the amount of tubular damage to muscle mass).22,23 Indexing GGT to creatinine is not physiologically valid because enzymes present in urine are not filtered through the glomerulus; using the urine GGT activity alone therefore appears to be more appropriate. Interestingly, urine GGT activity appears more sensitive as an index of tubular injury than the urine GGT to creatinine ratio in horses and sheep,25-27 and appears to be the most sensitive indicator of tubular injury in animals being treated with aminoglycosides.
Glucosuria
Glucose is freely filtered by the glomerulus and reabsorbed from the filtrate in the proximal tubules. Glucosuria in the face of a normal serum glucose concentration therefore indicates the presence of abnormal proximal tubular function. Glucosuria occurs early in the development of aminoglycoside-induced proximal tubule nephropathy, and may provide a useful inexpensive and practical screening test for nephrotoxicity in animals without hyperglycemia.26
Proteinuria
Urine protein concentrations in animals without lower urinary tract disease or hematuria are normally much lower than serum protein concentrations and similar to cerebrospinal fluid protein concentration. Glomerular filtrate normally contains low concentrations of low-molecular-weight proteins such as β2-microglobulin (molecular weight 11800) and lysozyme (molecular weight 14400). This is because the healthy glomerulus excludes high-molecular-weight proteins such as albumin (molecular weight 65 000) and globulins from the glomerular filtrate; normally functioning proximal tubules reabsorb these low-molecular-weight proteins, leading to very low urine protein concentrations. Alterations in tubular function can therefore lead to proteinuria, but typically glomerular injury produces much larger increases in urine protein concentration than those produced by altered proximal tubule function.
Determination of urine protein concentrations requires a sensitive analytical test, such as the Coomassie brilliant blue method. Urinary protein concentrations may be indexed to the urine creatinine concentration in order to account for denominator effects of changes in urine volume. Dividing the urinary protein concentration (mg/dL) by the creatinine concentration (mg/dL) produces a unitless ratio, which provides a sensitive and reliable diagnostic method for the detection and quantification of proteinuria.28 In general, increased urinary concentrations of albumin and β2-microglobulin indicate glomerular proteinuria and tubular proteinuria, respectively. Proteinuria is massive and sustained in cattle and sheep with advanced renal amyloidosis and animals with advanced glomerulonephritis (glomerular proteinuria) but is mild in animals without glomerular disease but with proximal tubular injury (tubular proteinuria). A urine protein concentration to creatinine concentration ratio of less than 13 is considered to be more indicative of tubular than glomerular proteinuria.29 Microalbuminuria does not appear to have been evaluated as an early and sensitive test of glomerular disease in large animals but increases in urine albumin concentration would be expected in animals with glomerular disease.
Tests of serum
These tests depend on either the accumulation, in cases of renal insufficiency, of metabolites normally excreted by the kidney or the excretion of endogenous substances by the kidney. Determination of serum urea and creatinine concentration are essential components of an evaluation of the urinary system. These serum indices of function are simple estimates of glomerular filtration because urea and creatinine are freely filtered by the glomerulus. Serum concentrations of urea and creatinine do not rise appreciably above the normal range until 60–75% of nephrons are destroyed.
Serum urea and creatinine concentrations are influenced by blood flow to the kidneys and may be increased in prerenal uremia. They also suffer from the disadvantage that their serum concentrations can vary with the rate of protein catabolism and are not dependent only on renal function. In cattle, for example, serum urea concentrations caused by prerenal lesions may be higher than those resulting from renal disease, because salivary secretion of urea, rumen metabolism of urea and decreased feed intake (and therefore decreased protein intake) may lower serum urea concentration in chronic disease.
Creatinine in herbivores is essentially totally derived from endogenous creatine. Creatine is produced by the liver from amino acids and circulates in the plasma before being taken up by skeletal muscle, where it stores energy in the form of phosphocreatine. Creatine is converted to creatinine by a non-enzymatic irreversible process and is distributed throughout the body water. Creatinine is therefore released from skeletal muscle at a constant rate in animals without myonecrosis and is therefore an indirect index of muscle mass; this is the reason why serum creatinine concentrations are highest in intact males, intermediate in adult females and lowest in neonates and cachetic animals. Serum creatinine concentrations are constant within an animal because they reflect muscle mass, which does not change rapidly; an increase in serum creatinine concentration of more than 0.3 g/dL should be considered to be clinically significant.30
Serum creatinine concentration is routinely measured using the Jaffe reaction, in which a colored product is formed from creatinine and picrate in an alkaline solution. However, the alkaline picrate reaction has poor specificity, as it also detects a number of non-creatinine chromogens in serum, which do not appear to be present in urine. In other words, the creatinine concentration may be overestimated in serum but is accurately measured in urine. The former induces some error in the calculation of fractional clearance of electrolytes. The progression of renal failure may be monitored by plotting the reciprocal of serum creatinine concentration against time. Extrapolation of the resultant linear relationship to the x axis intercept provided some clinically useful prognostic information in a horse with advanced renal failure.31
Glomerular filtration rate
The accepted gold standard measurement for renal function is measurement of the glomerular filtration rate using inulin clearance. Inulin, a metabolically inert carbohydrate, crosses freely across the glomerulus and is neither absorbed nor secreted by renal tubules. Endogenous creatinine clearance has also been used to estimate glomerular filtration rate; however, this test suffers from inaccuracies related to the presence of noncreatinine chromogens in plasma and the tubular secretion of creatinine in some species. Although the renal clearances of inulin or creatinine are the preferred research methods for measuring renal excretory function, these techniques are impractical in clinical patients and male ruminants because they require urethral catheterization, rinsing of the bladder contents and timed urine collections.
Renal excretory function is more practically assessed in clinical patients by measuring the plasma clearance of compounds of exogenous origin (such as phenolsulfonphthalein or sodium sulfanilate), as these techniques do not require urine collection. Plasma clearance of technetium-diethyleneaminopentaacetic acid (Tc-DTPA) or technetium-mercaptoacetyltriglycine (Tc-MAG3) have also been evaluated in horses32,33 but the technique requires measurement by a gamma camera and is therefore not suitable for use in the field. Plasma clearance tests have been evaluated in cattle, goats, sheep, and horses and provide a useful clinical test to monitor renal function in an individual animal over time.30,34-36 However, the accuracy of plasma clearance techniques may not be adequate for research studies.
Tests of urine and serum
Urine osmolality to serum osmolality ratio
A urine:plasma osmolality ratio of 1 indicates isosmotic clearance of materials by the kidney. A ratio less than 1 indicates that the kidneys are diluting the urine, and a ratio more than 1 indicates that the urine is being concentrated. Because the plasma osmolality is much more constant than urine osmolality, the important clinical factor is whether urine osmolality is less than, equal to, or greater than 300 mosmol/kg, Measurement of urine osmolality requires a dedicated laboratory unit and is rarely indicated in the clinical management of renal disease because of the widespread availability of hand-held refractometers. In fact, measurement of urine osmolality is needed only in research studies.
Water deprivation test
This can be used to assess renal concentrating ability in animals that have isosthenuria with urine specific gravity of 1.008–1.012 but do not have azotemia.12 Water deprivation tests should not be performed on animals that are already azotemic and should be undertaken with extreme caution and frequent (hourly to 2-hourly) monitoring in animals that are polyuric but not azotemic. Animals that are unable to conserve water because of renal disease can rapidly become dehydrated and develop prerenal uremia as a result.
In brief, the water deprivation test monitors the animal’s ability to detect an increase in serum osmolality, release antidiuretic hormone and produce a concentrated urine as a result of the action of antidiuretic hormone on the kidney. The test usually requires documentation that the animal has polyuria and polydipsia, with water consumption greater than cohorts of the same age, lactation stage and diet, when housed under the same conditions. Before conducting the water deprivation test, the animal is weighed and a Foley catheter is placed in the bladder (females), or the animal is housed in a dry stall (males). Access to water is prevented and the urine and serum are tested every 1–2 hours or when voided in males. The test should be stopped when the urine specific gravity increases to more than 1.015–1.020, when there is an increase in serum creatinine concentration of 0.3 g/dL or greater, or when there has been a decrease in body weight of 5% or more.
Animals that concentrate their urine after water deprivation are diagnosed with psychogenic polydipsia and their water availability is gradually decreased. Animals that fail to concentrate their urine after water deprivation are diagnosed with diabetes insipidus; nephrogenic diabetes insipidus can be ruled out if the animal produces a concentrated urine within a few hours of an intramuscular injection of exogenous vasopressin (0.15–0.30 U/kg BW). In the latter case, the diagnosis is neurogenic diabetes insipidus as a result of inadequate release of antidiuretic hormone. Such cases are extremely rare in large animals and have been attributed to pituitary neoplasia (particularly pituitary adenoma in horses) or encephalitis.10 Determination of plasma vasopressin concentrations using a radioimmunoassay may assist in differentiation of nephrogenic from neurogenic diabetes insipidus; in the former the plasma vasopressin concentration increases during the water deprivation test. However, because the assay for plasma vasopressin concentration is not widely available and has not been validated for all large animals,10 the response to exogenous vasopressin is the preferred clinical test for differentiating nephrogenic from neurogenic diabetes insipidus. Two related horses have been diagnosed with nephrogenic diabetes insipidus,10 suggesting that this may be inherited as an X-linked disorder.
Water deprivation tests are not needed if urine specific gravity is below 1.008, because the presence of hyposthenuria indicates that tubular function is acting to conserve solute and produce dilute urine. In other words, a specific gravity below 1.008 is a better clinical sign than a constant specific gravity of 1.008–1.012, because a low specific gravity indicates the presence of some tubular function. Low specific gravity may occur in diabetes insipidus, following excessive water intake or fluid administration, or following diuretic administration. Neonatal animals on fluid diets and lactating dairy cows often produce dilute urine.
Renal clearance studies
In animals with renal disease, serum creatinine and urea nitrogen concentrations are insensitive indicators of renal dysfunction and exceed the upper limit of the reference range only after extensive loss of nephron function. Increases in serum concentrations of creatinine or urea nitrogen cannot be used to distinguish between prerenal, renal, and postrenal azotemia. Urine specific gravity can be used to differentiate prerenal from renal azotemia. However, results of urinalysis do not reflect the magnitude of the disease and they are not specific for specific renal disease.
Calculation of renal clearance of creatinine, urea nitrogen, and electrolytes, along with measurement of specific enzyme activity in the urine, is a more sensitive indicator of damage to the tubules than serum biochemical analysis.37,38 Urinary diagnostic indices have been used to evaluate renal function and to detect and estimate the extent of renal damage in adult cattle, calves, horses, and foals. For example, it can be clinically useful to determine the urine to serum concentration, the ratio of urinary creatinine to urea nitrogen, the renal clearance of creatinine and urea nitrogen, the urine to serum osmolality ratio, the urine protein concentration or urine protein to creatinine ratio, the fractional clearances of electrolytes, and urine enzyme activity. Early diagnosis of renal injury facilitates initiation of appropriate treatment and reduces the incidence of irreversible renal failure. Sequential measurement of these indices can aid in the determination of prognosis and allows monitoring and evaluation of the extent of recovery of renal function.
The tests require simultaneous sampling of blood and urine.37 Samples can also be collected daily for several days and weekly to determine any age-related changes, and these are available for calves from birth to 90 days of age.24
Fractional clearance
The fractional clearance from plasma of a given substance is calculated by comparing the amount of the substance excreted in the urine with the amount filtered through the glomerulus. The formula used to calculate fractional clearance of substance X (FCX) is:
where [UX] and [SX] are the urine and serum concentrations of X, respectively, and [Ucreatinine] and [Ucreatinine] are the urine and serum concentrations of creatinine, respectively. Fractional clearance has been erroneously called fractional excretion; the latter term is confusing, inappropriate and has no scientific basis.39 The fractional clearance provides information regarding the action of tubular transport mechanisms on the filtered substances; a value below 100% indicates net reabsorption, whereas a ratio above 100% indicates net secretion.
Sodium and inorganic phosphate are reabsorbed from the glomerular filtrate by the renal tubules; therefore, the fractional clearance of sodium and phosphate provide clinically useful indices of tubular function. Sodium retention is an important proximal tubular function and the fractional clearance of Na is usually less than 1% for animals (and often <0.2%) unless they have a high oral or intravenous sodium intake, when fractional clearance values can be increased to 4%. Renal phosphorus excretion is affected by acid–base status and body calcium and phosphate status and is therefore a less specific indicator of tubular function than fractional clearance of sodium. Values for the fractional clearance of phosphorus normally vary from 0.1–0.4%, although higher values may be seen in ruminants with high phosphate intakes. Typically, tubular function can be adequately characterized by determining the fractional clearance of sodium alone, or sodium and phosphorus; the fractional clearance of chloride rarely adds useful information in clinical cases because it is highly correlated to the fractional clearance of sodium,40 and determination of the fractional clearance of potassium is hampered by methodological limitations associated with zwitterion formation in urine. Determination of the fractional clearance of calcium can be useful when dietary intake and metabolism of calcium are being evaluated. Substantial variations in fractional clearance values are present in horses over a 24-hour period as a result of the electrolyte load ingested with feed.41 Some standardization of the time of urine collection in relationship to feeding is therefore needed in research studies, but is clearly impractical in clinical cases.
Fractional clearance values for a number of electrolytes have been determined for horses,42,43 foals,37 cattle,40,44-46 and sheep.47 Renal clearance, urinary excretion of endogenous substances and urinary diagnostic indices have been measured in healthy neonatal foals.37 The urine volume of neonatal foals is proportionately greater than that of calves and the normal neonatal foal produces a dilute urine.37 When compared with normal values in adult horses, fractional clearance of electrolytes was similar for sodium but higher for potassium, phosphorus, and calcium. Renal function in newborn calves is similar to adult cattle within 2–3 days of birth and calves can excrete large load volumes in response to water overload and conserve water in response to water deprivation as efficiently as adult cattle.
Animals with acute renal azotemia have low urinary creatinine:serum creatinine and urine nitrogen:serum nitrogen; animals with acute prerenal azotemia have normal to high urinary creatinine:serum creatinine and urinary nitrogen:serum nitrogen. However, animals with acute renal azotemia also have a low urine specific gravity relative to the serum creatinine concentration, and it remains to be determined whether measurement of urinary creatinine and urea concentrations and serum urea concentrations provide any more information in clinical cases than that provided by urine specific gravity and serum creatinine concentration.
Summary of renal function tests
In summary, the serum creatinine or urea concentration provides a useful screening test for the presence of urinary tract disease, with an increase in serum creatinine concentration of more than 0.3 mg/dL over baseline providing a useful clinical test for the presence of nephrotoxicosis in normally hydrated animals being treated with potentially nephrotoxic agents. Azotemia can be prerenal, renal, or post renal in origin; the cause is most practically differentiated in azotemic animals by measuring the specific gravity of urine before any treatment has been administered. In animals suspected of having urinary tract disease, the urinary protein concentration and protein to creatinine ratio provide clinically useful indices of glomerular and tubular function and injury, the urine specific gravity and fractional clearance of sodium and phosphorus provide clinically useful indices of tubular function in animals not on intravenous or oral fluids and consuming a normal diet, and determination of urine GGT activity and analysis of urine for the presence of casts provide clinically useful and sensitive indices of tubular injury. The results of most other laboratory tests rarely provide additional information in an animal suspected to have urinary tract disease, and are not currently recommended for routine clinical use.
DIAGNOSTIC EXAMINATION TECHNIQUES
Ultrasonography
Transcutaneous and transrectal ultrasonography is commonly used to detect and characterize anatomical abnormalities of the kidneys, ureters, bladder, and urethra in horses, cattle, and small ruminants. Ultrasonography is an effective screening test for diagnosing obstructive conditions of the urinary tract, including hydronephrosis, hydroureter, and bladder distension, and can be used to visualize the kidney and guide the biopsy needle during renal biopsy.48 Removal of the haircoat and the use of an ultrasonographic coupling gel assist in obtaining acceptable acoustic coupling, whereas saturation of a foal’s haircoat with alcohol or coupling gel may be adequate when clipping is not desirable.
Techniques for ultrasonographic49 evaluation of the urinary system of the horse have been described, and extensive information is available that documents age-related changes in renal dimensions.50,51 Ureteral tears have been identified using transrectal ultrasonography.52 Uroperitoneum is readily diagnosed in foals by ultrasonographic examination, as is the underlying lesion in the bladder or urachus. Ultrasonography has been used to visualize the renal changes in foals following administration of phenylbutazone.53
In cattle, the right kidney is easily accessible to ultrasonography from the body surface.38 Images of the right kidney are visualized best with the transducer placed in the lumbar or paralumbar region, whereas images of the left kidney are best obtained using a transrectal approach. Ultrasonographic changes in the cow with pyelonephritis include: a dilated renal collecting system, renal or ureteral calculi, echogenic material within the renal collecting system, and subjective enlargement of the kidney with acute disease or a small irregular kidney with chronic disease.54 Cattle with enzootic bovine hematuria due to chronic bracken fern ingestion have a thickened bladder wall (normally <2 mm) on transrectal ultrasonography and irregular sessile masses (transitional cell papilloma) extending into the bladder lumen.55
Techniques for ultrasonographic evaluation of the urinary system of the sheep have been described.56
Renal biopsy
Percutaneous renal biopsy can be carried out in sedated and adequately restrained cows and horses. A coagulation profile should be run before renal biopsy is attempted in animals with severe and chronic renal disease or those animals suspected to have a coagulopathy. Renal biopsy is contraindicated in animals with documented pyelonephritis because of the risk of perirenal abscessation after the biopsy procedure.
The left kidney is usually biopsied because it is more accessible. In cows, the left kidney is moved to the right paralumbar fossa and fixed in position by rectal manipulation. In horses, the left kidney is identified using transabdominal ultrasonography and fixed in position by palpation per rectum.57 The skin over the biopsy site is aseptically prepared and 5–10 mL of local anesthetic is infiltrated along the proposed track for the biopsy needle. A small stab incision is made in the skin with a scalpel and a renal biopsy sample is collected by introducing a biopsy needle through the abdominal wall and manipulating it into the caudal pole of the kidney. The renal biopsy is fixed in 10% formalin and submitted for examination and histological diagnosis. Biopsy of the caudal pole minimizes the risk of trauma to the renal pelvis, renal artery, and renal vein.
Possible complications of renal biopsy are hemorrhage or abscessation in animals with pyelonephritis. Hemorrhage after renal biopsy can be extensive, and is usually perirenal but rarely life threatening. Occasionally, severe hematuria is present for hours after the biopsy procedure, but usually resolves within a few days. Because of the potential for life-threatening sequelae, renal biopsy should only be performed when the etiology is uncertain and histologic examination will direct treatment, or when an early and accurate prognosis is desired. In animals with acute tubular injury, electron microscopic examination of the basement membrane is required to accurately prognose return to normal function.
Endoscopy
Transurethral endoscopy can be easily performed in mares, stallions, geldings, and cows in order to examine the urethra and bladder, and flow of urine from both ureters. Horses and cows are sedated and adequately restrained for the procedure. Biopsy of diseased tissue or mechanical disruption of calculi can be attempted under endoscopic guidance. Identification of an ectopic ureter may be assisted by intramuscular administration of azosulfamide (1.9 mg/kg BW) or intravenous administration of sodium fluorescein (11 mg/kg BW), phenolsulfonphthalein (0.01 mg/kg BW) or indigo carmine (0.25 mg/kg BW) to color the urine being produced, 5–20 minutes before endoscopy; this assists visualization of the urine stream.58
Cystometry and urethral pressure profile
Urodynamic tests have been evaluated in the mare that allow comparison of the normal micturition reflex with that of the incontinent patient. Cystometry involves measurement of luminal pressure during inflation of the bladder with measured volumes of 0.9% NaCl or carbon dioxide. The pressure–volume relationship during filling with fluid or gas provides information on bladder capacity, maximal luminal pressure during the detrusor reflex, and stiffness of the bladder wall. The urethral pressure profile involves measurement of pressure along the urethra while withdrawing a fluid- or gas-filled catheter at constant rate. The catheter tip pressure is graphed against distance, and the maximum urethral closure pressure is determined as the maximum urethral pressure minus bladder luminal pressure. The functional urethral length is defined as the length of the urethra in which urethral pressure exceeds bladder luminal pressure.
The test can be performed in restrained mares with or without xylazine sedation (1.1 mg/kg BW, intravenously), but sedation is recommended. Values for cystometry and urethral pressure profiles in female horses and pony mares are available.59
Test of uroperitoneum and bladder rupture
Ultrasonographic examination of the abdomen is most useful in detecting the presence of excessive fluid, and this examination frequently allows visualization of the lesion in the bladder or urachus. Further testing is sometimes needed to confirm that the fluid is urine. Generally, in uroperitoneum, substantial quantities of fluid can be easily obtained by abdominocentesis. Warming the fluid may facilitate detection of the urine odor, although this is a subjective and poorly sensitive diagnostic test. If there is doubt that the fluid is urine, its creatinine concentration can be compared to the serum creatinine. If creatinine in the fluid is at least twice the serum value, the fluid is confirmed as urine, although ruptured bladder should be suspected whenever the abdominal fluid creatinine concentration exceeds that of serum. In animals with uroabdomen or suspected to have uroabdomen, the administration of 30 mL of sterile 1% methylene blue into the bladder via a urethral catheter or cystocentesis has been used to confirm that the bladder is the site of urine leakage. Abdominal paracentesis is performed some minutes after administration and the fluid examined visually for the presence of a blue tinge. Absence of a blue color suggests the presence of ureteral or renal rupture.
Radiography
Radiographic examination has limited value for the diagnosis of urinary tract disease in farm animals but contrast studies may be used to examine the lower urinary tract in neonatal animals. With the widespread availability of ultrasonography and endoscopy, the indications for radiography have become limited. A positive-contrast urethrogram was of value in diagnosing urethral recess dilatation in a bull calf,60 and intravenous urography was successful in diagnosing a dilated ureter in a 4-month-old heifer calf.61 Historically, excretory urography, positive contrast cystography and urethrography have been used, particularly in foals, but these tests are expensive, not widely available and time-consuming. Radiography is currently being performed on animals with equivocal results using other cheaper, faster and more widely available tests.
Principles of treatment of urinary tract disease
Fluid and electrolytes
Treatment of acute renal failure in all species is aimed at removing the primary cause and restoring normal fluid balance by correcting dehydration, acid–base disorders, and electrolyte abnormalities. The prognosis for acute renal failure will depend on the initiating cause and severity of the lesion. If the acute disease process can be stopped the animal may be able to survive on its remaining functional renal tissue. When toxic nephrosis is suspected, an attempt should be made to identify and remove the initiating cause or to move the animal from the suspect environment.
Ruminants with chronic renal failure typically have mild to marked hyponatremia and hypochloremia; the serum calcium and potassium concentrations may be decreased because of inappetence, serum magnesium concentration may be normal or increased, and serum phosphate concentration may be normal or increased, because urine provides a route of excretion of magnesium and phosphorus. The acid–base status is characterized by metabolic acidosis in severely affected cases to metabolic alkalosis in mildly affected cases. Ruminants with acute renal failure have similar clinicopathological changes, although the serum phosphorus concentration is usually markedly elevated in acute renal failure because many cases are initiated by decreased renal blood flow.
Horses with acute or chronic renal failure have similar electrolyte changes to those in ruminants, with the marked difference being the presence of hypercalcemia and hypophosphatemia in some horses. Hypercalcemia in horses with renal disease is suspected to be due to the relatively greater efficiency of intestinal calcium absorption in the horse, with urine being the predominant route of excretion. Decreases in the function of nephrons in the horse will therefore decrease the urinary loss of calcium and result in hypercalcemia. The hypercalcemia is marked and is thought to result directly in hypophosphatemia in horses with renal failure.
Balanced electrolyte solutions or normal saline supplemented with potassium and calcium can be used to correct fluid and electrolyte deficits. The required volume of replacement fluid can be determined on the basis of clinical signs as outlined in chapter 2. As the fluid deficit is corrected, the patient should be observed for urination. If anuria or oliguria is present, the rate of fluid administration should be monitored to prevent overhydration. If the patient has anuria or oliguria after the fluid volume deficit is corrected, a diuretic should be administered to help restore urine flow. Furosemide (1–2 mg/kg BW every 2 h) or mannitol (0.25–2.0 g/kg BW in a 20% solution) may be used, but furosemide is preferred because of its much lower cost and ease of administration. Diuretics should not be used until dehydration has been corrected. After urine flow is restored, the resulting diuresis will increase the maintenance fluid requirement. B vitamins should be frequently administered because their rate of loss in the urine is anticipated to be higher than normal in animals with renal failure. Animals nonresponsive to fluid loading and diuretics could be administered low-dose (‘renal dose’) dopamine as a continuous intravenous infusion (2–5 μg/kg BW/min) with dopamine being diluted in 0.9% NaCl, 5% dextrose or lactated Ringer’s solution. Dopamine is an α1, β1, β1, DA1 and DA2 agonist and therefore has a complex pharmacodynamic profile that is dependent upon species, organ, and cardiovascular status. Dopamine is theoretically the preferred pharmacological agent to selectively increase renal blood flow and therefore glomerular filtration rate in animals with renal failure, although low-dose dopamine infusion does not alter creatinine clearance (an index of glomerular filtration rate) in healthy adult horses and has not been shown to be of benefit in treating renal failure in humans.62 At low doses (<5 μg/kg BW/min) dopamine acts primarily as an inotropic agent and at higher doses primarily as a vasopressor. The mean arterial blood pressure and electrocardiogram should therefore be monitored during dopamine administration to insure that dopamine infusion does not lead to hypertension or clinically significant cardiac arrhythmias. Although there are good theoretical grounds for use of dopamine in animals with renal failure, this is no longer the practice in human medicine because of the lack of efficacy of the drug in preventing or treating acute renal failure. Animals that remain anuric after intravenous fluid administration of furosemide/mannitol and dopamine have a grave prognosis and can only be managed with peritoneal dialysis or hemodialysis.
Intermittent-flow peritoneal dialysis has been used successfully in a foal with a ruptured urinary bladder.63 A urinary catheter was placed in the bladder and secured to the perineal region. An area of the ventral midline was clipped and prepared for aseptic surgery. Local anesthetic was infused, and a stab incision was made in the skin with a scalpel blade. An 11 French peritoneal dialysis catheter was placed in the stab incision then forced into the abdomen. The rigid stylet was removed, the catheter was secured to the skin and the stab incision site was bandaged. Peritoneal fluid was allowed to drain; dialysis was then accomplished by infusing 2 L of a hypertonic dialysis solution, clamping the catheter for 1 hour, then opening the catheter and allowing drainage to occur for 2–3 hours. Dialysis was repeated nine times over a 36-hour period.63 Intermittent-flow peritoneal dialysis has also been used in an adult horse using a similar catheterization technique (24 French de Pezzer catheter) and infusion of 10–15 L of warmed, sterile, acetated Ringer’s solution.64 However, only 26–65% of the infused solution was recovered from the abdomen.
Continuous-flow peritoneal dialysis has been used successfully in an adult horse with azotemia refractory to intravenous fluids, furosemide, dopamine infusion and intermittent-flow peritoneal dialysis.64 A 28 French indwelling thoracic tube was placed in the ventral abdomen and a 2.2 mm diameter, 15 cm long spiral fenestrated catheter was placed in the left flank via peritoneoscopy to allow for inflow of dialysate. Acetated Ringer’s with 1.5% glucose was continuously infused through the catheter in the left flank at approximately 3 L/h, with abdominal fluid being collected into a sterile closed collection system from the catheter in the ventral midline of the abdomen.
Hemodialysis was used successfully to treat a foal with presumed oxytetracycline nephrotoxicosis.65 Hemodialysis was performed under isoflurane anesthesia after surgical placement of a Teflon/Silastic arteriovenous shunt in the median artery and vein using a dialysis delivery system, a hollow-fiber artificial kidney, and acetate-base dialysate. Anticoagulation during dialysis was accomplished with a loading dose of heparin (100 U/kg BW) and then hourly boluses of 20 U/kg BW to prolong the activated clotting time. Three dialysis treatments, lasting 4–6 hours, were administered over a 4-day period, resulting in a marked reduction in azotemia. Hemodialysis is more efficient than peritoneal dialysis and requires shorter treatment intervals, but does require vascular access and anticoagulation treatment, which may predispose to hypotension.65
The treatment of chronic renal failure will depend on the stage of disease and the value of the animal. In chronic failure, therapy is aimed at prolonging life. In food-producing animals, emergency slaughter is not recommended because the carcass is usually unsuitable for human consumption. Animals in chronic failure should have free access to water and salt, unless edema is present. Stresses such as sudden environmental and dietary changes should be avoided. The ration should be high in energy-giving food and properly balanced for protein. Acute renal failure may occur in patients in chronic failure and can be treated like other cases of acute renal failure.
Antimicrobial agents
Selection of antimicrobial agents for the treatment of urinary tract infections should be based on quantitative urine culture of a catheterized urine sample. A clinically relevant bacterial concentration indicative of cystitis or pyelonephritis is 1000 or 40 000 cfu/mL of urine from a catheterized or midstream free-catch sample, respectively.66
The ideal antimicrobial for treatment of urinary tract infections should meet several criteria. It should:
• Be active against the causal bacteria
• Be excreted and concentrated in the kidney and urine
• Be active at the pH of urine
• Have low toxicity, particularly nephrotoxicity
• Have no harmful interactions with other concurrently administered drugs.
Appropriate first-line antimicrobials include penicillin, ampicillin, amoxicillin, ceftiofur, and cefquinome in ruminants and trimethoprim–sulfa and ceftiofur in horses. Antimicrobial therapy for lower urinary tract infections should continue for at least 7 days; for upper urinary tract infections 2–4 weeks of treatment is often necessary. Success of therapy can be evaluated by repeating the urine culture 7–10 days after the last treatment.
Manipulation of urine pH should be considered as part of the treatment of bacterial urinary tract infections. In general, Escherichia coli attach best to urinary epithelial cells at pH 6.0, whereas Corynebacterium renale attaches best in alkaline urine. In other words, when treating an E. coli pyelonephritis or cystitis, the diet should be altered to ensure an alkaline urine pH. Likewise, urine pH should be acidic when treating urinary tract infections due to C. renale.
Osbaldiston GW, Moore WE. Renal function tests in cattle. J Am Vet Med Assoc. 1971;159:292-301.
Koterba AM, Coffman JR. Acute and chronic renal disease in the horse. Compend Contin Educ Pract Vet. 1981;3:S461-S469.
Fetcher A. Renal disease in cattle. Part 1. Compend Contin Educ Pract Vet. 1985;7:S701-S707. Part 2. Compend Contin Educ Pract Vet 1986;8:S338-S345.
Kohn CW, Chew DJ. Laboratory diagnosis and characterization of renal disease in horses. Vet Clin North Am Equine Pract. 1987;3:585-615.
Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J Vet Intern Med. 2005;19:377-385.
1 Arnold CE, et al. J Am Vet Med Assoc. 2005;227:778.
2 Kent Lloyd KC, et al. J Am Vet Med Assoc. 1987;194:1324.
3 Schott II HC, Hines MT. J Am Vet Med Assoc. 1994;204:1320.
4 Kisthardt KK, et al. Can Vet J. 1999;40:571.
5 Jansen BS, Lumsden JH. Aust Vet J. 1985;26:221.
6 Garrett PD. J Am Vet Med Assoc. 1987;191:689.
7 Morley PS, Desnoyers M. J Am Vet Med Assoc. 1992;200:1515.
8 Freeman DA, et al. Am J Vet Res. 1999;60:1445.
9 Brewer B. Equine Vet Educ. 1990;2:127.
10 Schott HC, et al. J Vet Intern Med. 1993;7:68.
11 Thomson JR. J Comp Pathol. 1986;96:119.
12 Young A. Equine Vet Educ. 1990;2:130.
13 Johnson PJ, et al. J Am Vet Med Assoc. 1987;191:973.
14 Rebhun WC, et al. J Am Vet Med Assoc. 1984;184:1237.
15 Frye MA, et al. J Am Vet Med Assoc. 2001;218:560.
16 Summers BA, et al. Cornell Vet. 1985;75:524.
17 Watts C, Campbell JR. Res Vet Sci. 1970;11:508.
18 Watts C, Campbell JR. Res Vet Sci. 1970;12:234.
19 Van Weeren PR, et al. Vet Q. 1987;9:79.
20 English PB, Hogan AE. Aust Vet J. 1979;55:584.
21 Thornton JR, English PB. Aust Vet J. 1976;52:335.
22 Amodio P, et al. Enzyme. 1985;33:216.
23 Mathur S, et al. Toxicol Sci. 2001;60:385.
24 Sommardahl C, et al. J Am Vet Med Assoc. 1997;211:212.
25 Bayly WM, et al. Cornell Vet. 1986;76:306.
26 Garry F, et al. Am J Vet Res. 1990;31:420.
27 Garry F, et al. Am J Vet Res. 1990;31:428.
28 White JV. J Am Vet Med Assoc. 1984;185:882.
29 Lulich JP, Osborne CA. Compend Contin Educ Pract Vet. 1990;12:59.
30 Hinchcliff KW. Am J Vet Res. 1989;50:1848.
31 Bertone JJ, et al. J Am Vet Med Assoc. 1987;191:565.
32 Gleadhill A, et al. Vet J. 1999;158:204.
33 Woods PR, et al. Vet Radiol Ultrasound. 2000;41:85.
34 Hinchcliff KW. Am J Vet Res. 1987;46:1256.
35 Brown SA, et al. Am J Vet Res. 1990;51:581.
36 Danielson TJ, Taylor WG. Am J Vet Res. 1993;54:2179.
37 Brewer BD, et al. J Vet Intern Med. 1991;5:28.
38 Braun U. Am J Vet Res. 1991;52:1933.
39 Constable PD. J Vet Intern Med. 1991;5:357.
40 Neiger RD, Hagemoser WA. Vet Clin Pathol. 1985;14:31.
41 McKenzie EC, et al. Am J Vet Res. 2003;64:284.
42 Grossman BS, et al. J Am Vet Med Assoc. 1982;180:284.
43 Morris DD, et al. Am J Vet Res. 1984;45:2431.
44 Itoh N. Vet Clin Pathol. 1989;18:86.
45 Fleming SA, et al. Am J Vet Res. 1991;52:5.
46 Fleming SA, et al. Am J Vet Res. 1992;53:222.
47 Garry F, et al. Am J Vet Res. 1990;31:313.
48 Modransky PD. Vet Clin North Am Equine Pract. 1986;2:115.
49 Kiper ML, et al. Compend Contin Educ Pract Vet. 1990;12:993.
50 Aleman M, et al. Equine Vet J. 2002;34:649.
51 Hoffmann KL, et al. Am J Vet Res. 1995;56:1403.
52 Diaz OS, et al. Vet Radiol Ultrasound. 2004;45:73.
53 Leveille R, et al. Can Vet J. 1996;37:235.
54 Hayashi H, et al. J Am Vet Med Assoc. 1994;205:736.
55 Hoque M, et al. J Vet Med A. 2002;49:403.
56 Braun U, et al. Am J Vet Res. 1992;53:1734.
57 Bayly WM, et al. Mod Vet Pract. 1980;61:763.
58 MacAllister CG, Perdue BP. J Am Vet Med Assoc. 1990;197:617.
59 Clark ES, et al. Am J Vet Res. 1987;48:552.
60 Anderson DE, et al. Can Vet J. 1993;34:234.
61 Barclay WP. J Am Vet Med Assoc. 1978;173:485.
62 Corley KTT. Vet Clin North Am Equine Pract. 2004;20:77.
63 Kritchevsky JE, et al. J Am Vet Med Assoc. 1984;185:81.
64 Gallatin LL, et al. J Am Vet Med Assoc. 2005;226:756.
Diseases of the kidney
GLOMERULONEPHRITIS
Glomerulonephritis can occur as a primary disease or as a component of diseases affecting several body systems, such as equine infectious anemia and chronic swine fever. In primary glomerulonephritis, the disease involves only the kidney, predominantly affecting the glomeruli although the inflammatory process extends to affect the surrounding interstitial tissue and blood vessels. Primary and secondary glomerulonephritis are rare causes of clinical disease in farm animals. The disease is sometimes associated with other chronic, systemic illness such as in cows with Johne’s disease, bovine virus diarrhea, or leptospirosis; pigs with hog cholera or African swine fever; and horses with equine infectious anemia. Proliferative glomerulonephritis is reported as an incidental finding in normal sheep, cattle, goats, and pigs. Clinical disease from glomerulonephritis is rare in these species but has been reported in cattle1 and as a congenital condition in sheep, described below. Proliferative glomerulonephritis can cause chronic renal failure in horses.2 Glomerulonephritis is present is animals with amyloidosis, which is a generalized deposition of antibody–antigen complexes. Amyloidosis is discussed in detail in Chapter 9.
The immune system plays a major role in the pathogenesis of glomerular lesions. Glomerular injury can be initiated by an immune response whereby antibodies are directed against intrinsic glomerular antigens or by foreign antigens planted in the glomerulus. Alternatively, and more commonly, circulating antigen–antibody complexes may be deposited in the glomerulus. As the complexes accumulate, they stimulate an inflammatory response that damages the glomerular filtration system. Inflammatory damage to the glomerulus alters the selective permeability of the filtration system allowing plasma protein, particularly albumin, to pass into the glomerular filtrate. In horses, the glomerular lesion is thought to be caused by the deposition of circulating antigen– antibody complexes but the origin of these complexes is unknown. Infections with streptococci and equine infectious anemia virus may be involved but are not likely to be involved in all cases.
Dermatitis–nephropathy syndrome is a systemic necrotizing vasculitis and glomerulonephritis syndrome of growing pigs in the UK and Canada.3 The cause is unknown but an immune-mediated pathogenesis is suspected. Growing pigs are affected with a morbidity ranging from 1–3%. The skin is affected with a papular dermatopathy with a characteristic distribution of bluish red spots at least 1 cm in diameter beginning first in the perineal region and then extending to the pelvic limbs and along the ventral body wall to the neck and ears. In the glomeruli, there are extensive granular complement deposits with scattered immunoglobulins.
Porcine dense deposit disease, porcine membranoproliferative glomerulonephritis type II is a common cause of early loss of piglets in the Norwegian Yorkshire breed.4,5 The disease is associated with extensive complement activation due to a deficiency of factor H, a plasma protein that regulates complement. Affected piglets are clinically normal at birth and for the first few weeks of life.1 Thereafter they become unthrifty and die from renal failure within 72 days of birth. In the kidneys there is extensive glomerular proliferation and marked thickening of the glomerular capillary wall. Large amounts of dense deposits are consistently found within the glomerular basement membrane. This disease is inherited with a simple autosomal recessive pattern and complete penetrance. A pathogenetic mechanism of a defective or missing complement regulation protein is hypothesized. A spontaneous glomerulonephritis of unknown etiology and unrelated to any breed has been recorded in pigs.6 A necrotizing glomerulonephritis is listed as occurring in pigs fed a waste product from an industrial plant producing a proteolytic enzyme. Glomerulonephritis has also been recorded in pigs in the absence of clinical illness, although an association with the ‘thin sow’ syndrome is suggested.
In Finnish Landrace lambs less than 4 months of age there is an apparently inherited disease that is remarkably similar to forms of human glomerulonephritis. Affected lambs appear to absorb an agent from colostrum that induces an immunological response, followed by the granular deposition of immune complexes and complement within the glomerular capillary walls; this initiates a fatal mesangiocapillary glomerulitis. Many affected lambs are asymptomatic until found dead. Some have signs of tachycardia, edema of the conjunctiva, nystagmus, walking in circles and convulsions. The kidneys are enlarged and tender. There is severe proteinuria and low plasma albumin. Serum urea concentration is increased (>35 mmol/L) with hyperphosphatemia and hypocalcemia. At necropsy the kidneys are large and pale and have multifocal pinpoint yellow and red spots throughout the cortex. On histopathological examination there are severe vascular lesions in the choroid plexuses and the lateral ventricles of the brain. The disease is thought to be conditioned in its occurrence by inheritance and to be limited to the Finnish Landrace breed. However, cases have also occurred in crossbred lambs.7
Glomerulopathy and peripheral neuropathy in Gelbvieh calves is a familial, and probably heritable, disease causing illness in calves of this breed of less than 13 months of age. The initial physical abnormality is posterior ataxia that progresses to generalized paresis and recumbency.8 The neurological deficits include loss of conscious proprioception, diminished or absent peripheral reflexes but maintained consciousness. Affected animals continue to eat and drink normally. Serum creatinine and urea concentrations are markedly elevated. Necropsy examination reveals neuropathy, myelopathy, and glomerulopathy. The disease is terminal.
Glomerulonephritis is a common cause of chronic renal failure in horses. Several forms of glomerulonephritis are recognized in horses: membranous glomerulonephritis, post-streptococcal glomerulonephritis, membranoproliferative glomerulonephritis and focal glomerulosclerosis.9 As discussed above, most are probably immune-mediated and associated with circulating antibody– antigen complexes. Over 80% of horses with equine infectious anemia have glomerular lesions, and viral antigen– antibody complexes are present in the glomerular basement membrane. Purpura hemorrhagica is associated with glomerulonephritis.
1 Hogasen K, et al. J Clin Invest. 1995;95:1054.
2 Divers TJ. Compend Contin Educ Pract Vet. 1983;5:S310.
3 Helie P, et al. Can Vet J. 1995;36:150.
4 Jansen JH, et al. Vet Rec. 1995;137:240.
5 Jansen JH, et al. Am J Pathol. 1993;143:1356.
6 Bourgalt A, Drolet R. J Vet Diagn Invest. 1995;7:122.
7 Dore M, et al. Can Vet J. 1987;28:40.
8 Panciera RJ, et al. Vet Pathol. 2003;40:63.
9 Biervliet JV, et al. Compend Contin Educ Pract Vet. 2002;24:892.
HEMOLYTIC-UREMIC-LIKE SYNDROME
Glomerular and tubulointerstitial disease, consistent with profound microangiopathy and glomerular degeneration in humans with hemolytic–uremic syndrome, has been diagnosed in two horses. Both horses were in oliguric renal failure and had clinicopathological evidence of intravascular hemolysis and morphological evidence of arteriolar microangiopathy and intravascular coagulation. The mortality rate is expected to be extremely high. The pathogenesis in horses is unclear, although hemolytic–uremic syndrome in humans is due to toxins produced by E. coli O157:H7.1
NEPHROSIS
Nephrosis includes degenerative and inflammatory lesions primarily affecting the renal tubules, particularly the proximal convoluted tubules. It can occur as a sequel to renal ischemia and following toxic insult to the kidney. Nephrosis is the most common cause of acute kidney failure. Uremia from nephrosis may develop acutely or may occur in the terminal stages of chronic renal disease.
RENAL ISCHEMIA
Reduced blood flow through the kidneys usually results from general circulatory failure.1 There is transitory oliguria followed by anuria and uremia if the circulatory failure is not corrected.
ETIOLOGY
Any condition which predisposes the animal to marked hypotension and release of endogenous pressor agents potentially can initiate hemodynamically mediated acute renal ischemia and renal failure. Ischemia may be acute or chronic.
Acute renal ischemia
• General circulatory emergencies such as shock, dehydration, acute hemorrhagic anemia, acute heart failure. Renal failure secondary to calf diarrhea has been described2
PATHOGENESIS
Acute ischemia of the kidneys occurs when compensatory vasoconstriction affects the renal blood vessels in response to a sudden reduction in cardiac output. As blood pressure falls, glomerular filtration decreases and metabolites that are normally excreted accumulate in the bloodstream. The concentration of urea in the blood increases, giving rise to the name prerenal uremia. As glomerular filtration falls, tubular resorption increases, causing reduced urine flow. Up to a certain stage, the degenerative changes are reversible by restoration of renal blood flow, but if ischemia is severe enough and of sufficient duration, the renal damage is permanent. Acute circulatory disturbances are more likely to be followed by degenerative lesions than chronic congestive heart failure.
The parenchymatous lesions vary from tubular necrosis to diffuse cortical necrosis in which both tubules and glomeruli are affected. The nephrosis of hemoglobinuria appears to be caused by the vasoconstriction of renal vessels rather than a direct toxic effect of hemoglobin on renal tubules. Uremia in acute hemolytic anemia and in acute muscular dystrophy with myoglobinuria may be exacerbated by plugging of the tubules with casts of coagulated protein, but ischemia is also an important factor.
CLINICAL FINDINGS
Renal ischemia does not appear as a distinct disease and its signs are masked by the clinical signs of the primary disease. Oliguria and azotemia will go unnoticed in most cases if the circulatory defect is corrected in the early stages. However, renal insufficiency may cause a poor response to treatment with transfusion or the infusion of other fluids in hemorrhagic or hemolytic anemia, in shock or dehydration. In these cases, unexplained depression or a poor response to therapy indicates that renal involvement should be investigated. The general clinical picture is one of acute renal failure and is described under uremia.
CLINICAL PATHOLOGY
Laboratory tests can be used to evaluate renal function once the circulatory condition has been corrected. Urinalysis as well as serum urea nitrogen and creatinine concentrations are most commonly used as indices. Serum biochemistry on serially collected samples may also be used to monitor the response to therapy. On urinalysis, proteinuria is an early indication of damage to the renal parenchyma. The passage of large volumes of urine of low specific gravity after a period of oliguria is usually a good indication of a return of normal glomerular and tubular function.
NECROPSY FINDINGS
Lesions of renal ischemia are present primarily in the cortex, which is pale and swollen. There may be a distinct line of necrosis visible at the corticomedullary junction. Histologically there is necrosis of tubular epithelium and, in severe cases, of the glomeruli. In hemoglobinuria and myoglobinuria hyaline casts are present in the tubules.
TREATMENT
Treatment must be directed at correcting fluid, electrolyte and acid–base disturbance as soon as possible. If renal damage has occurred, supportive treatment as suggested for the treatment of acute renal failure should be instituted.
Evidence of oliguria and azotemia in the presence of circulatory failure suggests renal ischemia and the possibility of permanent renal damage. It is important to attempt to differentiate the early reversible prerenal stage from the stage when degeneration of renal parenchyma has occurred. When ischemic renal lesions are present, urinalysis may be helpful in diagnosis, particularly if urine is not appropriately concentrated in a dehydrated patient. After irreversible ischemic changes have occurred it is impossible to differentiate clinically between ischemia and other primary renal diseases such as glomerulonephritis and toxic nephrosis. History and clinical signs of chronic disease will help determine if the acute syndrome is superimposed on chronic renal disease.
TOXIC NEPHROSIS
The kidneys are particularly vulnerable to endogenous and exogenous toxins because they receive a large proportion of the total cardiac output and because substances are concentrated in the kidney for excretion.
ETIOLOGY
Most cases of nephrosis are caused by the direct action of toxins but hemodynamic changes may contribute to the pathogenesis.
Toxins
• Metals – mercury, arsenic, cadmium, selenium, organic copper compounds; nephrosis can be reproduced experimentally in horses by the oral administration of potassium dichromate and mercuric chloride,3,4 including topical blistering agents containing mercuric chloride
• Antimicrobials such as aminoglycosides, and overdosing with neomycin and gentamicin in treatment of calves. Treatment with tetracycline preparations accidentally contaminated by tetracycline degradation compounds and repeated daily dosing with long-acting oxytetracycline preparations may induce toxicity. Treatment with sulfonamides
• Horses treated with vitamin K3 (menadione sodium bisulfite) administered by intramuscular or intravenous injection
• Horses treated with vitamin D2 (ergocalciferol) and cholecalciferol (D3)
• Treatment of horses with nonsteroidal anti-inflammatory drugs (NSAIDs), including phenylbutazone and flunixin meglumine. Dose rates of more than 8.8 mg/kg BW of phenylbutazone per day for 4 days are likely to cause nephrosis. Doses of 4.4 mg/kg BW are considered to be safe but the toxicity is enhanced by water deprivation. More commonly, NSAID toxicity occurs as interstitial nephritis and renal crest necrosis. The usual presentation of NSAID toxicosis in horses is gastrointestinal ulceration, including right dorsal colitis
• Benzimidazole compounds used as anthelmintics; only some of them but including thiabendazole
• Low-level aldrin poisoning in goats
• Highly chlorinated naphthalenes
• Oxalate in plants, listed under the heading of oxalate poisoning
• Oxalate in fungi, e.g. Penicillium spp. and mushrooms
• Oxalate in ethylene glycol or ascorbic acid, which is a metabolic precursor to oxalate
• Primary hyperoxaluria due to inherited metabolic defect in beef master calves5
• Tannins in the foliage of oak trees and acorns6
• Unidentified toxin in Amaranthus retroflexus in pigs and cattle in Narthecium asiaticum fed to cattle and Isotropis forrestii in ruminants
• Mycotoxins: ochratoxins and citrinins, fumonisins in ruminants
• Ingestion of Lophyrotoma interrupta (sawfly) larvae by cattle
• Cantharidin in horses following ingestion of dead blister beetles in alfalfa hay and hay products
• Most nonspecific endogenous or exogenous toxemias cause some degree of temporary nephrosis.
PATHOGENESIS
In acute nephrosis there is obstruction to the flow of glomerular filtrate through the tubules as a result of interstitial edema and intraluminal casts. If there is sufficient tubular damage, there may be back leakage of glomerular filtrate into the interstitium. There may also be a direct toxic effect on glomeruli, which decreases glomerular filtration. The combined effect is oliguria and uremia. In subacute cases, impaired tubular resorption of solutes and fluids may lead to polyuria.
CLINICAL FINDINGS
Clinical signs may not be referable to the urinary system. In peracute cases, such as those caused by vitamin K3 administered by injection, there may be colic and stranguria. In acute nephrosis there is oliguria and proteinuria with clinical signs of uremia in the terminal stages. These signs include depression, dehydration, anorexia, hypothermia, a slow or an elevated heart rate, and weak pulse. Diarrhea may be present that is sufficiently intense to cause severe clinical dehydration. In cattle there is a continuous mild hypocalcemia with signs reminiscent of that disease, which responds, in a limited way, to treatment with calcium. Cattle with advanced and severe nephrosis may exhibit a bleeding diathesis. Polyuria is present in chronic cases.
Many systemic diseases such as septicemia cause temporary tubular nephrosis. The degree of renal epithelial loss is not sufficient to cause complete renal failure and, provided the degree of renal damage is small, complete function is regained.
CLINICAL PATHOLOGY
In acute tubular nephrosis, urinalysis abnormalities are usually present before serum urea and creatinine concentration are increased. Proteinuria, glucosuria, enzymuria, and hematuria are initial changes on urinalysis in experimental toxic nephrosis. The earliest indication of tubular epithelial damage in experimentally induced nephrosis is the detection of the proximal tubule enzyme GGT in urine.4 Hypoproteinemia may be present. In acute renal disease of horses, hypercalcemia and hypophosphatemia can be present,7 although this is not the usual finding. In the chronic stages the urine is isosthenuric and may or may not contain protein. Azotemia occurs when uremia is present. Ultrasonographically, renal changes are seen in foals receiving high daily doses of phenylbutazone.8
NECROPSY FINDINGS
In acute cases the kidney is swollen and wet on the cut surface and edema, especially of perirenal tissues, may be apparent. Histologically there is necrosis and desquamation of tubular epithelium, and hyaline casts are present in the dilated tubules. In phenylbutazone poisoning the renal lesion is specifically a renal medullary necrosis. There may also be ulcers in all or any part of the alimentary tract from the mouth to the colon if phenylbutazone was administered orally.
TREATMENT
Treatment should be directed at general supportive care for acute renal disease as outlined above. If the toxin can be identified, it should be removed. Treatment for specific toxins may be available, as described elsewhere in the text. Hemodialysis was used successfully to treat a foal with presumed oxytetracycline nephrotoxicosis.9
Clinical differentiation from acute glomerulonephritis is difficult but clinical signs of involvement of other organs in the toxic process may be present.
• A combination of polyuria and glycosuria is an uncommon finding in large animals and is usually caused by nephrosis
• Diabetes mellitus is rare in horses and extremely rare in ruminants
• Cushing’s syndrome (chronic hyperadrenocorticism pituitary pars intermedia dysfunction) is more common in horses but this includes characteristic signs of polyuria, glycosuria, debilitation, hirsutism, polyphagia, and hyperglycemia
• Diarrhea in terminal stages of uremia in a horse can be confused with the other causes of acute diarrhea. It requires a blood urea and creatinine estimation and a urinalysis for differentiation
Fetcher A. Renal disease in cattle, Part 1. Compend Contin Educ Pract Vet. 1985;7:S701-S707. Part 2 Compend Contin Educ Pract Vet 1986;8:S338-S345.
Schmitz DG. Toxic nephropathy in horses. Compend Contin Educ Pract Vet. 1988;10:104-111.
Angus KW. Nephropathy in young lambs. Vet Rec. 1990;126:525-528.
1 Divers TJ, et al. Equine Vet J. 1987;19:178.
2 Mechor GD, et al. Cornell Vet. 1993;83:325.
3 Bayly WM, et al. Cornell Vet. 1986;76:287.
4 Bayly WM, et al. Cornell Vet. 1986;76:306.
5 Rhyan JC, et al. J Am Vet Med Assoc. 1992;201:1907.
6 Ostrowski SR, et al. J Am Vet Med Assoc. 1989;195:481.
7 Elfers RS, et al. Cornell Vet. 1986;76:317.