Chapter 15 Diseases of the mammary gland
INTRODUCTION 673
BOVINE MASTITIS 673
MASTITIS PATHOGENS OF CATTLE 697
MASTITIS OF CATTLE ASSOCIATED WITH COMMON CONTAGIOUS PATHOGENS 697
MASTITIS OF CATTLE ASSOCIATED WITH TEAT SKIN OPPORTUNISTIC PATHOGENS 708
MASTITIS OF CATTLE ASSOCIATED WITH COMMON ENVIRONMENTAL PATHOGENS 709
MASTITIS OF CATTLE ASSOCIATED WITH LESS COMMON PATHOGENS 724
MISCELLANEOUS CAUSES OF BOVINE MASTITIS 726
CONTROL OF BOVINE MASTITIS 728
MISCELLANEOUS ABNORMALITIES OF THE TEATS AND UDDER 749
MASTITIS–METRITIS–AGALACTIA SYNDROME IN SOWS 754
MASTITIS OF SHEEP 759
MASTITIS OF GOATS 761
MASTITIS OF MARES 762
Mastitis is inflammation of the parenchyma of the mammary gland regardless of the cause. Mastitis is therefore characterized by a range of physical and chemical changes in the milk and pathological changes in the glandular tissue. The most important changes in the milk include discoloration, the presence of clots and the presence of large numbers of leukocytes. There is swelling, heat, pain and edema in the mammary gland in many clinical cases. However, a large proportion of mastitic glands are not readily detectable by manual palpation nor by visual examination of the milk using a strip cup; these quarters represent subclinical infections. Because of the large numbers of subclinical cases, the diagnosis of mastitis depends largely on indirect tests, which depend, in turn, on the somatic cell concentration (SCC) or electrolyte (sodium or chloride) concentration of milk. It seems practicable and reasonable to define mastitis as a disease characterized by the presence of a significantly increased SCC in milk from affected glands. The increased SCC is, in almost all cases, due to an increased neutrophil concentration, represents a reaction of glandular tissue to injury and is preceded by changes in the milk that are the direct result of damage to glandular tissue. However, the exact clinical and laboratory changes that occur in the udder as a result of infection can also be caused by other factors in the absence of infection.1 Until such time as it becomes common usage to define mastitis in terms of the sodium or chloride concentration of the milk (as measured by electrical conductivity) or increased permeability of the blood–milk barrier (as measured by albumin concentration) there appears to be no point in changing the current definition of mastitis based on an abnormal looking secretion or an increased SCC. Characterization of mastitis depends on the identification of the causative agent whether it be infectious or physical.
Most of the information presented here deals almost entirely with bovine mastitis because of its economic importance, but small sections on ovine, caprine, porcine and equine mastitis are included at the end of the chapter.
Bovine mastitis
GENERAL FEATURES
A total of about 140 microbial species, subspecies and serovars have been isolated from the bovine mammary gland. Microbiological techniques have enabled precise determination of the identity of many of the mastitis pathogens. Based on their epidemiology and pathophysiology, these pathogens have been further classified as causes of contagious, teat skin opportunistic or environmental mastitis.
Etiology
• Contagious pathogens: Staphylococcus aureus, Streptococcus agalactiae, Mycoplasma bovis and Corynebacterium bovis
• Teat skin opportunistic pathogens: coagulase-negative staphylococci
• Environmental pathogens: environmental Streptococcus spp. including Streptococcus uberis and Streptococcus dysgalactiae, which are the most prevalent; less prevalent is Streptococcus equinus (formerly referred to as Streptococcus bovis). Environmental coliforms include the Gram-negative bacteria Escherichia coli, Klebsiella spp. and Enterobacter spp., and Arcanobacterium (formerly Actinomyces) pyogenes
• Uncommon pathogens: many, including Nocardia spp., Pasteurella spp., Mycobacterium bovis, Bacillus cereus, Pseudomonas spp., Serratia marcescens, Citrobacter spp., anaerobic bacterial species, fungi and yeasts
Epidemiology
• Incidence of clinical mastitis ranges from 10–12% per 100 cows at risk per year. Prevalence of intramammary infection is about 50% of cows and 10–25% of quarters. Case fatality rate depends on cause of mastitis
• Contagious pathogens are transmitted at time of milking; teat skin opportunistic pathogens take any opportunity to induce mastitis; environmental pathogens are from the environment and induce mastitis between milkings
• Environmental pathogens are the most common cause of clinical mastitis in herds that have controlled contagious pathogens
• Prevalence of infection with contagious pathogens ranges from 7–40% of cows and 6–35% of quarters
• Prevalence of infection with environmental pathogens: coliforms 1–2% of quarters; streptococci less than 5%
Risk factors
• Animal risk factors: prevalence of infection increases with age. Most new infections occur in dry period and in early lactation. Highest rate of clinical disease occurs in herds with low somatic cell counts (SCCs). Morphology and physical condition of teat are risk factors. Selenium and vitamin E status influence incidence of clinical mastitis. High-producing cows are more susceptible
• Environmental risk factors: poor quality management of housing and bedding increases infection rate and incidence of clinical mastitis due to environmental pathogens
• Pathogen risk factors: ability to survive in environment, virulence factors (colonizing ability, toxin production), susceptibility to antimicrobial agents
• Economics: subclinical mastitis is a major cause of economic loss due to loss of milk production, costs of treatment and early culling
Clinical signs
• Gross abnormalities in milk (discoloration, clots, flakes, pus)
• Physical abnormalities of udder: acute – diffuse swelling, warmth, pain, gangrene in severe cases; chronic – local fibrosis and atrophy
• Systemic response: may be normal or mild, moderate, acute, peracute with varying degrees of anorexia, toxemia, dehydration, fever, tachycardia, ruminal stasis, and recumbency and death
Clinical pathology
• Detection at the herd level: bulk tank milk SCCs. Culture of bulk tank milk
• Detection at the individual cow level: abnormal looking milk, culture of composite or quarter milk samples. Indirect tests include SCCs of composite or quarter milk samples, California Mastitis Test (CMT) of quarter milk samples, inline milk conductivity tests of quarter milk samples
• Use of selective media to differentiate Gram-positive and Gram-negative pathogens in cases of clinical mastitis
• Differential diagnosis list: other mammary abnormalities: Periparturient udder edema, rupture of suspensory ligament, and hematomas. Blood in the milk of recently calved cows
Treatment
• Clinical mastitis in lactating cow: mild cases of clinical mastitis (abnormal secretion only) may not require treatment; however, all clinical mastitis episodes accompanied by an abnormal gland or systemic signs of illness should be treated with antimicrobial agents given by intramammary infusion (all cases) and parenterally (selected cases). Acute and peracute mastitis cases require also require supportive therapy (fluid and electrolytes) and nonsteroidal anti-inflammatory agents (NSAIDs). Culture milk of representative clinical cases but antimicrobial susceptibility testing has not been validated
• Dry cow therapy: intramammary infusion of long-acting antimicrobial agents at drying off provides the best treatment for subclinical mastitis due to contagious pathogens. Must adhere to milk withholding times after treatment with antimicrobial agents to prevent milk drug residues, which is major public health issue. Currently available cowside antimicrobial residue tests are not reliable
Contagious mastitis pathogens
There are many contagious mastitis pathogens. The most common are Staphylococcus aureus and Streptococcus agalactiae. The usual source of contagious pathogens is the infected glands of other cows in the herd; however, the hands of milkers can act as a source of S. aureus. The predominant method of transmission is from cow to cow by contaminated common udder wash cloths, residual milk in teat cups and inadequate milking equipment. Programs for the control of contagious mastitis involve improvements in hygiene and disinfection aimed at disrupting the cow-to-cow mode of transmission. In addition, methods to eliminate infected cows involve antimicrobial therapy and the culling of chronically infected cows.
In general, a conscientious mastitis control program will eradicate S. agalactiae from most dairy herds. It is much more difficult to deal with a herd that has a high prevalence of S. aureus, but S. aureus can be eradicated from low-prevalence herds.
Mycoplasma bovis is a less common cause of contagious mastitis; it causes outbreaks of clinical mastitis that do not respond to therapy and are difficult to control. Most outbreaks of M. bovis are associated with recent introductions of new animals into the herd. Characteristically, clinical mastitis occurs in more than one quarter, there is a marked drop in milk production and there is little evidence of systemic disease. The laboratory diagnosis of mycoplasmal mastitis requires specialized media and culture conditions. Antimicrobial therapy is relatively ineffective and culling is the predominant strategy.
Teat skin opportunistic mastitis pathogens
The incidence of mild clinical mastitis associated with bacterial pathogens that normally reside on the teat skin is increasing, particularly in herds that have controlled major contagious mastitis pathogens. Teat skin opportunistic pathogens have the ability to create an intramammary infection via ascending infection through the streak canal. Accordingly, their epidemiology of infections differs from those of contagious and environmental pathogens, and it is useful to consider them in a separate category. Coagulase-negative staphylococci are the most common teat skin opportunistic mastitis pathogens.
Environmental mastitis pathogens
Environmental mastitis is associated with three main groups of pathogens, the coliforms (particularly E. coli and Klebsiella spp.), environmental Streptococcus spp. and Arcanobacterium pyogenes. The source of these pathogens is the environment of the cow. The major method of transmission is from the environment to the cow by inadequate management of the environment. Examples include wet bedding, dirty lots, milking wet udders, inadequate premilking udder and teat preparation, housing systems that allow teat injuries, and poor fly control. Control strategies for environmental mastitis include improved sanitation in the barn and yard areas, good premilking udder preparation so that teats are clean and dry at milking time, and fly control. Special attention is necessary during the late dry period and in early lactation.
Coliform organisms are a common cause of clinical mastitis, occasionally in a severe peracute form. Clinical cases of coliform infection are generally found in low levels in most herds and do not routinely result in chronic infections. There is increasing evidence that, as the contagious pathogens are progressively controlled in a herd, the incidence of clinical cases associated with coliform organisms increases. The pathogenesis, epidemiology, predisposing risk factors, diagnostic problems, therapy and control methods have been the subject of extensive, worldwide research efforts.
Environmental streptococci have become a major cause of mastitis in dairy cattle. Streptococcal infections are associated with many different species, however the most prevalent species are Streptococcus uberis and Streptococcus dysgalactiae. Infections with these organisms can cause clinical mastitis that is commonly mild to moderate in nature. More frequently, these organisms cause a chronic subclinical infection with an increased milk SCC. Many herds that have implemented the five-point program for mastitis control have found that environmental streptococci represent their most common mastitis problem.
A. pyogenes is an important seasonal cause of mastitis in dry cows and late pregnant heifers in some parts of the world. Intramammary infections with A. pyogenes are severe and the gland is almost always lost to milk production.
Several other pathogens are included in the environmental class of infections. These pathogens invade the mammary gland when defense mechanisms are compromised or when they are inadvertently delivered into the gland at the time of intramammary therapy. This group of opportunistic organisms includes Pseudomonas spp., yeast agents, Prototheca spp., Serratia marcescens and Nocardia spp. Each of these agents has unique microbiological culture characteristics, mechanisms of pathogenesis and clinical outcomes. These infections usually occur sporadically. However, outbreaks can occur in herds or in an entire region and are usually the result of problems with specific management of hygiene or therapy. For example, mastitis associated with Pseudomonas aeruginosa has occurred in outbreaks associated with contaminated wash hoses in milking parlors. Iodide germicides used in wash lines are often at too low a concentration to eliminate Pseudomonas spp. Outbreaks of clinical mastitis associated with Nocardia spp. have been associated with the use of blanket dry cow therapy and the use of a specific neomycin-containing dry cow preparation.
The mastitis pathogens, and their relative importance, continue to evolve as new management methods and control practices are developed. Thus, there is an ongoing need for epidemiological studies to characterize the pathogens and describe their association with the animals and their environment. Improved control methods can develop only from investigations into the distribution and pathogenic nature of the microorganisms isolated.
ETIOLOGY
Bovine mastitis is associated with many different infectious agents, commonly divided into those causing contagious mastitis, which are spread from infected quarters to other quarters and cows, those that are normal teat skin inhabitants and cause opportunistic mastitis, and those causing environmental mastitis, which are usually present in the cow’s environment and reach the teat from that source. Pathogens causing mastitis in cattle are further divided into major pathogens (those that cause clinical mastitis) and minor pathogens (those that normally cause subclinical mastitis and less frequently cause clinical mastitis).
Major pathogens
Environmental pathogens
Environmental Streptococcus species include S. uberis and S. dysgalactiae, which are the most prevalent; less prevalent is S. equinus (formerly referred to as S. bovis). The environmental coliforms include the Gram-negative bacteria E. coli, Klebsiella spp. and Enterobacter spp. A. pyogenes mastitis can be an important problem in some herds.
Minor pathogens
Several other species of bacteria are often found colonizing the teat streak canal and mammary gland. They rarely cause clinical mastitis and are known as minor pathogens. They include the coagulase-negative Staphylococcus spp. such as Staphylococcus hyicus and Staphylococcus chromogenes, which are commonly isolated from milk samples and the teat canal. Staphylococcus xylosus and Staphylococcus sciuri are found free-living in the environment; Staphylococcus warneri, Staphylococcus simulans and Staphylococcus epidermidis are part of the normal flora of the teat skin (and therefore are teat skin opportunistic pathogens). The prevalence of coagulase-negative Staphylococcus spp. is higher in first-lactation heifers than cows, and higher immediately after calving than in the remainder of lactation. In recent studies, they have been found as teat canal and intramammary infections in nulliparous heifers.
C. bovis is also a minor pathogen; it is mildly pathogenic and the main reservoir is the infected gland or teat duct. However, in some herds, C. bovis appears to be a common cause of mild clinical mastitis. C. bovis spreads rapidly from cow to cow in the absence of adequate teat dipping. The prevalence of C. bovis is low in herds using an effective germicidal teat dip, good milking hygiene and dry cow therapy. The presence of C. bovis in a gland will reduce the likelihood of subsequent infection with S. aureus.
Uncommon mastitis pathogens
Many other bacteria can cause severe mastitis, which is usually sporadic and usually affects only one cow or a few cows in the herd. These include Nocardia asteroides, Nocardia brasiliensis and Nocardia farcinica, Histophilus somni, Pasteurella multocida, Mannheimia (formerly Pasteurella) haemolytica, Campylobacter jejuni, B. cereus and other Gram-negative bacteria including Citrobacter spp., Enterococcus faecalis, Enterococcus faecium, Proteus spp., P. aeruginosa, and Serratia spp. Anaerobic bacteria have been isolated from cases of mastitis, usually in association with other facultative bacteria, e.g. Peptostreptococcus indolicus, Prevotella melaninogenica (formerly Bacteroides melaninogenicus), Eubacterium combesii, Clostridium sporogenes and Fusobacterium necrophorum.
Fungal infections include Trichosporon spp., Aspergillus fumigatus, Aspergillus nidulans and Pichia spp.; yeast infections include Candida spp., Cryptococcus neoformans, Saccharomyces spp. and Torulopsis spp. Algal infections include Prototheca trispora and Prototheca zopfii.
Leptospiras, including Leptospira interrogans serovar. pomona, and especially Leptospira interrogans hardjo, cause damage to blood vessels in the mammary gland and gross abnormality of the milk. They are more correctly classified as systemic diseases with mammary gland manifestations, and are described under those headings in the book.
Some viruses may also cause mastitis in cattle, but they are of little importance.
EPIDEMIOLOGY
This section deals with the general aspects of epidemiology of bovine mastitis. For information about the epidemiology of mastitis in the other animal species see the appropriate sections at the end of this chapter.
Occurrence and prevalence of infection
Occurrence refers to the location of the disease and the kinds of animals affected. Prevalence is the percentage of the population affected with a specific disease in a given population at a certain point in time. The incidence is a rate, such as the total number of new cases of clinical mastitis as a percentage of the animals at risk that occur during a certain period of time. Prevalence is a function of the incidence and the duration of infection.
Prevalence
In most countries, surveys in dairy herds indicate that the prevalence of infection of mastitis pathogens is approximately 50% of cows and 10–25% of quarters. The prevalence of infection in dairy heifers of breeding age and in pregnant dairy heifers varies widely1 from 30–50% of heifers and 18% of quarters2 to as high as 97% of heifers and 75% of quarters.3
Incidence
The average annual incidence of clinical mastitis, calculated as the number of clinical quarter cases per 100 cows at risk per year, including the dry period, in individual herds ranges from 10–12% in most herds4 but higher incidences, ranging from 16–65%, occur in some herds.5,6 The greatest risk of first acquiring mastitis occurs early in lactation, usually in the first 50 days.7 The risk of clinical mastitis also increases with increasing parity.7 In beef herds, 32–37% of cows and 18% of quarters may have intramammary infection, which has a significant negative effect on calf weaning weights.8
Case fatality rates vary widely depending largely on the identity of the causative organism. For example, S. agalactiae mastitis is not a fatal disease but peracute staphylococcal mastitis in a recently calved cow often may be fatal. Details of the occurrence of the different types of mastitis are presented in their individual sections in this chapter.
Relative prevalence of infection with intramammary pathogens
The prevalence of infection with intramammary pathogens in cattle is remarkably similar in different countries. The bacteriological identification of mastitis pathogens is important because control and eradication procedures depend on the kind of infection prevalent in the herd. In addition, the validity of epidemiological investigations aiming at determining transmission patterns or the impact of environmental and managemental factors to a large extent depends on exact bacteriological diagnosis.
Contagious pathogens
The prevalence of infection with S. aureus in cows ranges widely, usually from 7–40%, but higher in some herds.9 A survey of Danish dairy herds found that 21–70% of all cows and 6–35% of all quarters were infected.10 S. aureus was isolated from 10% of quarter samples and was the most common species isolated.10 The prevalence of streptococci, including S. agalactiae, ranges from 1–8% of cows. A relative incidence of S. agalactiae, other streptococci and S. aureus of 1:1:2 is a common finding. S. aureus may still assume some importance as a cause of subclinical mastitis but its prevalence has been reduced by modern mastitis control programs, leading to a higher proportion of culture-negative mastitic quarters and a corresponding, and perhaps consequent, increase in infections by E. coli and Klebsiella spp. The prevalence of infection due to Mycoplasma spp. varies widely.9
The prevalence of infection due to an individual pathogen, and therefore the ratio between its incidence and that of other pathogens, depends on several risk factors such as size of herd and quality of management, especially milking parlor hygiene and cleanliness of accommodation, and parity of animal (heifer or cow). For example, large, zero-grazed herds kept in drylot conditions are likely to encounter more hygiene problems than conventionally housed herds mainly because of constant soiling of the udder by inadequate or improper bedding in larger units. In those circumstances there is likely to be a much higher prevalence than usual of mastitis associated with E. coli and S. uberis.
Teat skin opportunistic pathogens
Coagulase-negative staphylococcal species were found in 4.1% of samples; the most frequently isolated were S. epidermidis (1.3%), S. chromogenes (1.0%) and S. simulans (0.7%).
Environmental pathogens
The prevalence of intramammary coliform infections in a dairy herd seldom exceeds 1–2%; the prevalence of intramammary environmental streptococci is less than 5% in well managed herds but may exceed 10% in some problem herds.11 A characteristic of intramammary coliform infections is the short duration: 40–50% persist less than 7 days. The majority of environmental streptococci infections last less than 30 days. In a survey of Danish dairy herds, S. dysgalactiae (1.6%) and S. uberis (1.4%) were the second and third most common species isolated.
Heifers
Surveys of intramammary infection of heifers in regions such as Louisiana indicate variability in prevalence and duration of intramammary infection according to species of bacteria present around the time of parturition. About 20% of heifers were infected with S. aureus and 70% with coagulase-negative staphylococci, the minor pathogens that are part of the normal teat skin flora of heifers.12 S. chromogenes was isolated from 15% of all quarters of heifers before parturition but decreased shortly after parturition to 1%.13 Up to 97% of breeding age and pregnant dairy heifers and 75% of their quarters may be infected with S. aureus, S. hyicus and S. chromogenes.3 Infections with S. simulans and S. epidermidis occurred in 1–3% of quarters both before and after parturition. S. dysgalactiae was isolated from 4–6% of quarters before and immediately after parturition. Intramammary infections with S. aureus rarely occurred before parturition but increased during the first week after parturition. There was no association between the prevalence of S. aureus in heifers before parturition and the prevalence in lactating cows.
Distribution of pathogens in clinical mastitis
The distribution of pathogens isolated from cases of clinical mastitis has changed with the adoption of control programs from a high frequency of isolation of S. aureus and S. agalactiae to a higher isolation rate of other pathogens, particularly environmental pathogens. For example, in 171 randomly selected dairy herds, the average annual incidence of clinical mastitis was 12.7 quarter cases per 100 cows per year. The most frequent isolates from clinical cases were E. coli (16%), S. aureus (14%), S. uberis (11%), and S. dysgalactiae (8%).4 In another survey, the most common isolates from clinical cases were coagulase-negative staphylococci and E. coli, each at 15% of samples taken. In a 2-year observational study of 65 dairy herds in Canada, there was considerable variation in the incidence of clinical mastitis among farms.7 Overall, 20% of cows experienced one or more cases of clinical mastitis during lactation. The pathogens isolated were coliforms (17%), other Streptococcus spp. (14%), S. aureus (7%), Gram-positive bacilli (6%), C. bovis (2%), S. agalactiae (1%), and other Staphylococcus spp. (29%). There was no growth in 18% of samples and 8% were contaminated. Clearly the main difference is that the rate of S. aureus in clinical cases is higher in continental Europe4 and lower in England and North America.
Source of infection
Contagious pathogens
S. agalactiae and S. aureus reside primarily in the udder of infected cows; the source of infection is other infected cows and exposure to uninfected quarters is limited to the milking process.
Teat skin opportunistic pathogens
A number of species of coagulase-negative staphylococcus reside primarily on the teat skin of cattle.
Environmental pathogens
S. uberis, S. dysgalactiae, and coliforms are common inhabitants of the cow’s environment such as bedding. The exposure of uninfected quarters to environmental pathogens can occur at any time during the life of the cow, including milking time, between milkings, during the dry period and prior to first calving in heifers.
Methods of transmission
Infection of each mammary gland occurs via the teat canal, the infection originating from either an infected udder or the environment; in dairy cattle the infection originating from infected udders is transmitted to the teat skin of other cows by milking machine liners, milkers’ hands, wash cloths and any other material that can act as an inert carrier.
Risk factors
Risk factors that influence the prevalence of infection and the incidence of clinical mastitis are outlined here. Individual factors that are of particular importance in the individual types of mastitis are described under those headings.
Animal risk factors
Age and parity
The prevalence of infected quarters increases with age, peaking at 7 years. Surveys of the prevalence of intramammary infection in dairy heifers a few days before their first parturition reveals that 45% are infected, and the quarter infection rate may be 18%.2 Some studies found intramammary infections in 97% of heifers and 74% of quarters.3
Stage of lactation
Most new infections occur during the early part of the dry period and in the first 2 months of lactation, especially with the environmental pathogens. In heifers, the prevalence of infection is often high in the last trimester of pregnancy and several days before parturition, followed by a marked decline after parturition.13 In dairy heifers, most of these prepartum infections are associated with the minor pathogens but some surveys have found evidence of infection by the major pathogens.2,3 The mean prevalence of S. aureus intramammary infection in primiparous cows at first parturition in high prevalence herds can be as high as 30%, ranging from 13–65%, and in low prevalence herds it may as low as 2%, ranging from 0–5%.14 The overall prevalence of infection of S. aureus intramammary infection in primiparous cows at parturition was 8%, ranging from 0–27%. Of those cows with S. aureus intramammary infection at parturition, 43% had S. aureus intramammary infection at least 2 months after parturition. Primiparous cows with these infections may represent significant reservoirs of infection to uninfected animals in the herd.
Some of these differences may be related to changes in the milk as a medium for bacterial growth. For example, bacteria such as C. bovis grow best in milk secreted in the middle of lactation, whereas dry period secretion inhibits its growth.15 During the dry period the quarter’s capacity to provide phagocytic and bactericidal activities diminishes.16
Somatic cell count
The highest average incidence of clinical mastitis due to environmental bacteria may occur in herds with the lowest bulk tank milk SCC (< 150000 cells/mL) and a low prevalence of subclinical infection.17
Breed
Generally the incidence of mastitis is greater in Holstein–Friesians than in Jerseys, but this may reflect differences in management rather than a true genetic difference. Valid comparisons between breeds have not been reported.
Milking characteristics and morphology of udder and teat
High milking rate and large teat canal diameter have been associated with increased SCC or risk of intramammary infection.18 Milk leaking in cows in herds with a low bulk tank milk SCC has also been associated with an increased rate of clinical mastitis. Decreasing teat-end-to-floor distance is also a risk factor for clinical mastitis and may be associated with an increased incidence of teat lesions. Heritability estimates of teat-end-to-floor distance or udder height range from 0.2–0.7, which may be a consideration in the selection indices of bulls. Periparturient udder edema may also be a risk factor for clinical mastitis.
Physical condition of teat
The teat end is the first barrier against invading pathogens, and the efficiency of teat defense mechanisms depends on the integrity of teat tissue; its impairment leads to an increase in the risk of intramammary infection. Teat thickness is an aid to evaluating teat tissue status. Milking machine characteristics can induce a decrease or increase in teat thickness after milking compared with premilking values. Increases in teat thickness of more than 5% are significantly associated with infection and new infection, but the association was not significant when teat thickness decreased by more than 5%.19 Coagulase-negative staphylococcal infections are significantly associated with both increases and decreases in teat thickness numerically greater than 5%, but there is no association between teat thickness and S. aureus infections.
Hyperkeratosis of the teat orifice is a commonly observed condition in the dairy cow because of machine milking; the degree of hyperkeratosis may be increased by a poor milking system.20 There is wide variation in the degree of hyperkeratosis between herds; the score increases with lactational age and peaks, for any lactation, at 3–4 months after parturition, declining as the cows dry off. There is no significant relationship between mean SCC and the degree of hyperkeratosis at the herd level.
Udder hygiene
Dirty udders are associated with increased SCC and an increased prevalence of intramammary infection due to contagious pathogens, but surprisingly are not associated with intramammary infections due to environmental pathogens.21 This suggests that udder hygiene is a proxy for general mastitis management skills, in that good mastitis control programs result in low prevalence of infection with contagious pathogens.
Nutritional status
Vitamins E and A and selenium may be involved in resistance to certain types of mastitis.22 Early reports found that supplementation with antioxidants such as selenium and vitamin E had a beneficial effect on udder health in dairy cattle by decreasing the incidence and duration of clinical mastitis. An increase in selenium concentration in whole blood was associated with a decrease in all infections, including S. aureus, A. pyogenes, and C. bovis.23 There was no association between different infections or SCC and concentrations of vitamin E, vitamin A, or beta-carotene, but an association existed between vitamin A concentration and SCC. The lower selenium concentration in whole blood did not increase the incidence of clinical mastitis.
Genetic resistance to mastitis
A variety of morphological, physiological and immunological factors contribute to a cow’s resistance or susceptibility to mastitis, and each of these factors is influenced to some extent by heredity. Differences in udder depth, teat length, teat shape, and teat orifice morphology are thought to be associated with differences in mastitis. The production of keratin in the streak canal and the physical and biochemical characteristics of keratin are important contributors to mastitis resistance. Many of the defense mechanisms of the udder, including lysozyme, lactoferrin, immunoglobulins and leukocytes, are direct products of genes and have a genetic basis. For dairy cattle, heritability estimates for clinical mastitis average about 0.05. These low heritability estimates indicate that there is very little genetic influence on clinical mastitis but a very strong environmental influence.24
Differences in heritability between herds with high and low SCCs are not significant. However, differences among bulls’ daughter groups for both clinical mastitis and SCC are reasonably large, suggesting that selection of sires can be important in mastitis control.25 An analysis of the disease and breeding records of a large number of Swedish bulls siring daughters whose milk had a low SCC count found genetic correlations from 0.71–0.79 between SCC and clinical mastitis. It was concluded that it is possible to improve resistance to clinical mastitis by selecting for a low SCC.
The strong phenotypic and genetic association between SCC and mastitis indicates that breeding programs based on SCC may be effective as an indirect means of improving mastitis resistance. However, greater emphasis on SCC may decrease genetic gain in yield traits, which are economically more important.26
Milk yield
The genetic correlation between milk yield and mastitis is about 0.2–0.3, which suggests that animals genetically above average for milk yield are more susceptible to mastitis and that low-yielding cows tend to be more resistant. However, the low correlation value suggests that this relationship is not a strong tendency. The positive correlation implies that genetic improvement for milk yield is accompanied by a slow decline in genetic resistance to mastitis. Examination of the association between milk yield and disease in a large number of dairy cows found that higher milk yield was not a factor for any disease except mastitis.27 However, the association between milk yield and mastitis does not imply causation. At least two biological explanations are plausible: increased injury and leaking of milk between milkings. Improved mastitis control efforts have offset the genetic trend for increased susceptibility to mastitis. The low heritability for mastitis indicates the great importance of environmental factors in causing differences in the prevalence of infection and the incidence of clinical mastitis.
In summary, selection for milk yield alone results in increased incidence of mastitis. The positive genetic correlation between milk yield and mastitis suggests that genes that increase milk yield tend to increase susceptibility to mastitis. Selection indices that maximize genetic improvement for net economic benefit will not decrease the incidence of mastitis, but indices that include SCC, udder depth or clinical mastitis will diminish the rate of increase in mastitis by 20–25%. Using predicted transmitting ability (PTA), an estimate of genetic merit, it has been found on average that daughters of bulls with high PTAs for SCC have a higher incidence of mastitis; sires with low PTA for somatic cell scores should therefore be selected. All of the economically important traits are weighted into a selection index for the selection of bulls which will improve net income over cost of production.
Other concurrent diseases
These may be important risk factors for mastitis. Retained placentas, teat injuries and teat sores may be associated with a higher incidence of mastitis. Sole ulceration of any severity occurring in more than one digit has been associated with an approximately threefold higher risk of S. aureus infections in the first lactation.28 It is suggested that sore feet could increase the risk of teat lesions, presumably as a result of difficulty in standing.
Immunological function of mammary gland
The immune function of the mammary gland is impaired during the periparturient period; it is susceptible to mastitis during transition periods, such as drying off and colostrogenesis. As a result, the incidence of new intramammary infections is highest during the early nonlactating period and the periparturient period.
The most important components of the defense against common bacterial pathogens are blood-derived neutrophils and opsonizing antibodies. An inadequate rate of neutrophil recruitment to combat a new intramammary infection has a profound effect on the outcome of infection, in that cows with a rapid and massive recruitment of neutrophils to an infected gland clear an intramammary infection within 12–18 hours postinfection.
It is also important that an early inflammatory response in the infected mammary gland enables leakage of IgG2 (opsonizing antibodies) as this facilitates neutrophil phagocytosis of bacteria. The staggered one–two punch of peak IgG2 concentrations within 4 hours of infection and peak neutrophil response within 6–12 hours of infection greatly facilitates clearance of new intramammary infections.
Blood-derived neutrophils must undergo margination, adherence and migration in order to arrive in the mammary gland, where they perform phagocytosis, respiratory burst and degranulation. Margination is via expression of three adhesion molecules from the selectin family, specifically L-selectin (also called CD62L) on neutrophils, E-selectin (also called CD62E), and P-selectin (also called CD62P) on vascular endothelial cells. Neutrophil L-selectin makes the initial contact between ‘streaming’ neutrophils in the blood stream and the vascular wall; this contact slows neutrophil movement and allows them to ‘roll’ along the endothelium while surveying for the presence of proinflammatory mediators at the sites of tissue infection. When the rolling neutrophils detect the presence of one or more proinflammatory mediators they immediately shed surface L-selectin (CD62L) adhesion molecules and upregulate and activate Mac-1 (CD11b/CD18) adhesion molecules, thereby stopping neutrophil rolling and permitting tight adherence of the neutrophil to the endothelium. Once adhered, neutrophils commence diapedesis by migrating between endothelial cells to the site of infection. Neutrophil migration therefore has three components; hyperadherence (cessation of rolling), diapedesis and chemotaxis. Any delay or inhibition in this process can lead to peracute mastitis and severe clinical disease. This is best illustrated by bovine leukocyte adhesion deficiency (BLAD) in Holstein–Friesian cattle; affected calves cannot produce Mac-1 molecules and have a prominent neutrophilia because streaming neutrophils cannot migrate to the site of infection. Migration of neutrophils is slow during the first few weeks of lactation and this delay in neutrophil migration is believed to be responsible for the increased incidence and severity of intramammary infections during early lactation.
Previous mastitis
Cows with a history of mastitis in the preceding lactation may be almost twice as susceptible to clinical mastitis in the current lactation as those without mastitis in the preceding lactation.29
Pre-existing intramammary infections
Natural infection with minor pathogens has a protective effect against infections with major pathogens.30 The lowest rate of infection with major pathogens has been observed in quarters infected with C. bovis. Elimination of these minor pathogens with postmilking teat disinfection may result in an increase in the incidence of clinical mastitis. Discontinuation of the teat dipping may be associated with an increase in the prevalence of minor pathogens, increase in the incidence of S. aureus infections, and decrease in the incidence of E. coli infections. Thus quarters already infected with a minor or major infection are less likely to acquire a new infection than uninfected quarters.
Use of recombinant bovine somatotropin
Because the risk of clinical mastitis increases as milk production increases there has been considerable scientific and public controversy over the potential effects that the use of recombinant bovine somatotropin (bST) might have on the incidence of clinical mastitis and the subsequent use of antimicrobials from therapy. In some field trials, the use of bST did not result in an increase in the incidence of clinical mastitis compared to controls. In other trials, a significant increase in the incidence of clinical mastitis occurred in treated cows compared to controls. However, the incidence of clinical mastitis was greater in treated cows compared to controls before bST was used. In trials done on well managed farms which had controlled contagious mastitis and had low rates of clinical mastitis due to environmental pathogens, the use of bST was not associated with an increase in clinical mastitis, discarded milk because of therapy or culling for mastitis.31 Interpretation of a direct effect of bST on mastitis incidence is confounded by the higher incidence of mastitis in cows of higher milk production.
Environmental and management risk factors
Quality and management of housing
Factors such as climate, housing system, type of bedding and rainfall interact to influence the degree of exposure of teat ends to mastitis pathogens. Because dairy cattle spend 40–65% of their time lying down, the quality and management of housing for dairy cattle has a major influence on the types of mastitis pathogen that infect the mammary gland, as well as the degree of infection pressure.
The major sources of environmental pathogens are the cow’s environment, including bedding, soil, feedstuffs and water supplies. Environmental pathogens multiply in bedding materials, with which the cow’s teats are in close and prolonged contact. Bacterial growth in bedding depends on temperature, amount of moisture and nutrients available, and the pH. Fresh bedding can be a source of contamination even before it is used: Klebsiella pneumoniae can be present in green, hardwood sawdust in higher numbers than in other types of bedding and major outbreaks of environmental mastitis due to K. pneumoniae have occurred following the use of contaminated wood products bedding, described in detail in that section. Dry, unused bedding contains few pathogens but after being used it becomes contaminated and provides a source in which pathogens multiply to high numbers in 24 hours. Organic bedding materials such as straw, sawdust, wood shavings and paper support the growth of pathogens. Inorganic materials such as sand retain less moisture and do not provide a supply of nutrients for the pathogens; bacterial counts in these materials are usually lower than in organic materials. Housing lactating cattle on sawdust leads to six times more Klebsiella bacteria and twice as much coliform bacteria on the teat ends compared to housing cattle on sand. In contrast, there were 10 times more environmental streptococci bacteria on teat ends when cows were housed on sand, compared to housing on sawdust.32 Surveys indicate that herds using wood chips or sawdust as bedding material have higher rates of clinical mastitis compared to those using straw bedding.33
High humidity and high ambient temperatures favor growth of pathogens. Cows in confinement housing with organic bedding materials have the highest incidence of environmental mastitis in the warm, humid months of the year. Pasturing herds during the summer months usually reduces the incidence of coliform mastitis, although rates of environmental streptococci may remain high. In drylot systems the incidence of coliform mastitis may be associated with periods of high rainfall. Herds with more months on pasture may have a higher incidence of clinical mastitis,33 which may be associated with factors such as sanitation and the stress of transition between pasture and confinement housing.
The management and design of housing systems influence the prevalence of intramammary infection and the incidence of clinical mastitis. Any housing factor or management system that allows cows to become dirty or damage teats or that causes overcrowding will result in an increase in clinical mastitis. This includes the size and comfort of free stalls, the size of the alleyways, ease of movement of cattle and the cleaning system. Failure to keep alleyways, cow stalls and bedding clean and dry will increase the level of contamination of the teats. Overcrowding, poor ventilation, access to dirty ponds of water and muddy areas where cows congregate are major risk factors.
The size of the milking cow herd may be positively associated with an increased incidence of clinical mastitis because it can be more difficult to control contagious mastitis in a herd with a greater prevalence of infection and a larger number of cow-to-cow contacts. As herd size increases, manure disposal and sanitation problems may increase exposure to environmental pathogens. However, regional and management differences may confound the association of size with infection status. Some recent data suggest lower SCC in large herds. The use of designated maternity areas providing an isolated and clean environment for parturition33 may be associated with a lower incidence of clinical mastitis.
If hygiene and bedding maintenance are neglected in the housing accommodation the prevalence of environmental forms of mastitis may increase markedly. Periodic inspection of dry cows is an essential part of mastitis control.
Milking practices
The failure to employ established and reliable methods of mastitis control is an important risk factor. This is a major subject, which includes efficiency of milking personnel, milking machines, high milking speed and especially hygiene in the milking parlor. Wet teats and udders are a risk factor for increased SCC, especially in the presence of teat impacts from liner slippage.33 The use of a separate drying cloth for each cow is associated with a lower SCC. Effective use of a postmilking germicidal teat dip is critical for the control of contagious mastitis. Increasing person-hours spent milking per cow may be associated with a higher rate of clinical mastitis.33 Contaminated milking equipment – including milk hoses, udder wash towels and teat dip products – has been associated with outbreaks of environmental mastitis from S. marcescens and P. aeruginosa. Drying off procedures at the end of lactation and an active policy on drying off treatment are equally important.
The absence of milk quality regulations that place emphasis on SCC is also a risk factor. Conversely, the presence of strict regulations with penalties for high SCC is an important incentive to institute mastitis control programs that improve the quality of milk. The absence of a health management program consisting of regular farm visits by the veterinarian may also be a risk factor for mastitis, which may be associated with a relative lack of awareness by the producer of the importance of the principles of mastitis control.
Season of year
The relationship between the incidence of mastitis and season of the year is variable, depending on geographical and climatic conditions. In subtropical and tropical areas the incidence may be higher during winter or spring calvings from the increase in infection pressure associated with increased humidity. In temperate climates, the incidence of mastitis is higher in autumn and winter, when calving occurs along with an extended period of housing.34 Under conditions of housing for long winter periods, infectious agents are most likely to be found in higher numbers in the bedding. In the UK there is an increased frequency of mastitis when cows are housed for the winter.
Pathogen risk factors
Viability of pathogens
The ability of the pathogen to survive in the cow’s immediate environment (resistance to environmental influences including cleaning and disinfection procedures) is a characteristic of each pathogen. The causes of contagious mastitis are more susceptible to disinfection than the causes of environmental mastitis.
Virulence factors
There is a wide variety of virulence factors among the mastitis pathogens. These are described under specific mastitides. The influence of many bacterial virulence factors depends on the stage of lactation and severity of the intramammary infection and the effects elicited by the virulence factors on bovine mammary tissue. A few examples of the common virulence factors are noted here.
Colonizing ability
The ability of the pathogens to colonize the teat duct, then to adhere to mammary epithelium and to initiate mastitis is a major characteristic of the major bacterial causes of mastitis. S. aureus strains that cause mastitis can bind to ductular udder epithelial cells and to explant cultures of bovine mammary glands. There are differences in the adhesion characteristics among strains of the organism, which may explain the different epidemiological characteristics of the organisms in some herds. Comparison of strains isolated from different S. aureus mastitis cases between herds reveals that only a limited number of genotypes of S. aureus are most prevalent.34
Toxins
E. coli isolates that cause mastitis produce lipopolysaccharide endotoxin, which is responsible for many of the inflammatory and systemic changes observed during acute coliform mastitis. S. aureus isolated from intramammary infections produces many potential virulence factors, including enterotoxins, coagulase and alpha, beta, delta toxins, hemolysin, hyaluronidase and leukocidins, which are considered to be involved in the varying degrees of inflammation characteristic of staphylococcal mastitis from subclinical to peracute gangrenous mastitis. Virulence factors of S. uberis include hyaluronidase and the hyaluronic capsule.
Production and economic losses
Although mastitis occurs sporadically in all species, it assumes major economic importance in dairy cattle and may be one of the most costly diseases in dairy herds. Mastitis results in economic loss for producers by increasing the costs of production and by decreasing productivity. The premature culling of potentially profitable cows because of chronic mastitis is also a significant loss. Because of the large economic losses, there is a potential for high returns on investment in an effective control program. The component economic losses can be divided into:
• Discarded milk from cows with clinical mastitis and treated cows
• Replacement cost of culled cows
• Extra labor required for treatment and monitoring
• Veterinary service for treatment and control
• Cost of first trimester abortions due to clinical mastitis36
There are additional costs such as antimicrobial residues in milk from treated cows, milk quality control, dairy food manufacturing, nutritional quality of milk, degrading of milk supplies due to high bacteria or SCC, and interference with the genetic potential of some cows from early involuntary culling because of chronic mastitis. The total annual cost of mastitis in the dairy cattle population is estimated to be 10% of the total value of farm milk sales, and about two-thirds of this loss is due to reduced milk production in subclinically affected cows.
The production and economic losses are commonly divided into those associated with subclinical and clinical mastitis.
Subclinical mastitis
Total milk losses from quarters affected with subclinical mastitis have been estimated to range from 10–26%.37 Lower SCCs are associated with higher milk production, and rolling herd average milk production has been estimated to decrease by 190 kg per unit increase of linear somatic cell score. Most estimates indicate that on average an affected quarter results in a 30% reduction in productivity, and an affected cow is estimated to lose 15% of its production for the lactation. This loss is sometimes expressed as a loss of about 340 kg of saleable milk, due to loss of production and the value of milk that has to be withheld from sale. The loss in production by an infected quarter may be largely compensated by increased production in the other quarters so that the net loss from the cow may be less than expected. In addition to these losses, there is an added loss of about 1% of total solids by changes in composition (fat, casein, and lactose are reduced and glycogen, whey proteins, pH, and chlorides are increased), which interferes with manufacturing processes, and other losses include increased culling rates and costs of treatment. Comparisons between low- and high-prevalence herds always show a financial advantage of about 20% to the low-prevalence herds, the gain varying with the local price of milk or butter fat. In beef herds the losses are in the form of rare deaths of cows and failure of the calves to gain weight.
Approximately 75% of the economic loss from subclinical mastitis is attributable to loss of milk production. Other costs include discarding milk from treated cows, drug costs, veterinary costs, labor and loss of genetic potential of culled cows.
Clinical mastitis
Clinical mastitis results in marked decreases in milk production, which are much larger in early than late lactation. Milk production losses are also greater in cows with multiple lactations than first-lactation cows, and clinical mastitis also decreases the duration of lactation and increases the likelihood of culling. Clinical cases of brief duration that occur after the peak of lactation affect milk production very little but can induce abortion during the first 45 days of gestation.36 Clinically affected quarters may not completely recover milk production in subsequent lactations but these carry-over losses are not as large as the losses from acute mastitis. In the National Animal Health Monitoring System of dairy herds in the US, clinical mastitis alone was the most costly disease identified, at a loss to the producer of $27–50 per cow per year.37
The costs of clinical mastitis and mastitis prevention in dairy herds have been estimated, based on monitoring 50 dairy herds over 1 year.38 Mean incidence of clinical mastitis was 39 cases/100 cow-years; each clinical case cost $38/cow-year, with a mean cost per clinical episode of US$107. Prevention of mastitis cost $14.50/cow-year.39 Lost milk production was estimated at $14.85/cow-year, which does include the losses associated with subclinical mastitis.
The component causes of economic loss associated with mastitis outlined above vary according to the causative pathogen and are described under specific mastitides. In general terms S. aureus and E. coli may cause death from peracute mastitis; A. pyogenes causes complete loss of quarters; staphylococci and streptococci cause acute clinical mastitis, but their principal role is in causing subclinical mastitis, resulting in a reduction of milk produced and a downgrading of its quality. Of these, S. agalactiae causes the greatest production loss, whereas S. aureus has the higher infection rate, greater resistance to treatment and longer duration of infection. At one time S. aureus represented the impassable barrier to mastitis control programs.
Other factors that affect the magnitude of the loss associated with mastitis include age (the loss is greatest in mature cows), and when the attack occurs in the first 150 days of lactation.
Zoonotic potential
With mastitis there is the danger that the bacterial contamination of milk from affected cows may render it unsuitable for human consumption by causing food poisoning, or interfere with manufacturing processes or, in rare cases, provide a mechanism of spread of disease to humans. Tuberculosis, streptococcal sore throat and brucellosis may be spread in this way. Raw (unpasteurized) milk can be a source of food-borne pathogens, and consumption of raw milk can result in sporadic disease outbreaks. For instance, sampling bulk tank raw milk in Ontario revealed the presence of Listeria monocytogenes, Salmonella spp., Campylobacter spp. or verocytoxigenic E. coli in 2.7%, 0.2%, 0.5% and 0.9% of milk samples, respectively.40 These findings emphasize the importance of continued diligence in the application of hygiene programs within dairies and the separation of raw from pasteurized milk and milk products.
PATHOGENESIS
Infection of the mammary gland always occurs via the teat canal and on first impression the development of inflammation after infection seems a natural sequence. However, the development of mastitis is more complex than this and can be most satisfactorily explained in terms of three stages: invasion, infection, inflammation. Of the three phases, prevention of the invasion phase offers the greatest potential for reducing the incidence of mastitis by good management, notably in the use of good hygienic procedures.
Invasion is the stage at which pathogens move from the teat end to the milk inside the teat canal.
Infection is the stage in which the pathogens multiply rapidly and invade the mammary tissue. After invasion the pathogen population may be established in the teat canal and, with this as a base, a series of multiplications and extensions into mammary tissue may occur, with infection of mammary tissue occurring frequently or occasionally depending on its susceptibility. Multiplication of certain organisms may result in the release of endotoxins, as in coliform mastitis, which causes profound systemic effects with minimal inflammatory effects.
Inflammation follows infection and represents the stage at which clinical mastitis occurs with varying degrees of clinical abnormalities of the udder and variable systemic effects from mild to peracute; gross and subclinical abnormalities of the milk appear. Abnormalities of the udder include marked swelling, increased warmth and, in acute and peracute stages, gangrene in some cases and abscess formation and atrophy of glands in chronic stages. The systemic effects are due to the mediators of inflammation. Gross abnormalities of the milk include a decrease in milk yield, the presence of the products of inflammation and marked changes in the composition of the milk.
The most significant subclinical abnormality of the milk is the increase in the somatic cell count, the most common measurement of milk quality and udder health. Milk somatic cells in a healthy gland consist of several cell types, including neutrophils (<11%), macrophages (66–88%), lymphocytes (10–27%), and a smaller percentage of epithelial cells (0–7%).41 Neutrophils are the predominant cell type found in mammary tissues and secretions during inflammation, in mastitis they constitute more than 90% of total mammary gland leukocytes. Once at the site of infection, neutrophils phagocytose and kill pathogens. Neutrophils exert their bactericidal effect through a respiratory burst that produces hydroxyl and oxygen radicals, important components of the oxygen-dependent killing mechanism.
In the healthy lactating mammary gland, the SCC is less than 100000 cells/mL of milk. During intramammary infection, the glandular SCC can increase to more than 1 000000 cells/mL of milk within a few hours because of the combined effect of an increased number of neutrophils (numerator) and a decreased glandular secretion volume (denominator). The severity and duration of mastitis are critically related to the promptness of the neutrophil migratory response and their bactericidal activity at the site of infection. As they colonize and multiply in the mammary gland, some bacteria release metabolic byproducts or cell-wall components (endotoxin if a Gram-negative bacteria) that serve as chemoattractants for leukocytes. If neutrophils move rapidly from the blood stream and are able to eliminate the inflammatory stimuli (bacteria), then recruitment of neutrophils ceases and the SCC returns to normal levels. If bacteria are able to survive this immediate host response, then the inflammation continues, resulting in neutrophil migration between adjacent mammary secretory cells toward the alveolar lumen. Prolonged diapedesis of neutrophils damages mammary tissue, resulting in decreased milk production. The duration and severity of the inflammatory response therefore has a major impact on the quantity and quality of milk produced.
The major factor affecting the SCC at the herd and individual cow level is the prevalence of intramammary infection at a glandular level. Because marked increases in SCC are a result of cells being attracted to the mammary tissue because of the mediators produced during a local infection, events that do not affect udder health are unlikely to have a direct or dramatic effect on SCC. Little evidence exists that any factor other than normal diurnal variation has a major influence on SCC in the absence of intramammary infections.
The effects of mastitis on milk yield are highly variable and depend on the severity of the inflammation, the causative agents and the lesions produced, the efficiency of treatment, the production level and the stage of lactation.42 Mastitis in early lactation causes a larger decrease in milk yield with long-term effects than mastitis in late lactation. Mastitis due to S. aureus generally evolves into persistent but moderate infections, unlike those associated with coliforms. Mastitis associated with A. pyogenes results in suppurative lesions, poor response to treatment and culling. M. bovis causes chronic induration and almost complete loss of milk production without recovery.
CLINICAL FINDINGS
Details of the clinical findings are provided under each specific type of mastitis. The clinical findings should be used only as a guide because different pathogens can cause chronic, subclinical, subacute, acute and peracute forms of the disease, and clinical differentiation of the different causes of mastitis is difficult. The greatest clinical accuracy achievable, even in a specialist hospital environment and after adaptation to suit local conditions, is about 70%,43 which is not sufficiently accurate to be clinically useful. In other words, bacteriological culture of milk from an affected gland is required before specific pathogen-directed treatment can be implemented.
Clinical mastitis is detected using only the results of the physical examination, and a useful definition of clinical mastitis is a negative answer to the question ‘would you drink this?’ In other words, ‘undrinkable’ is a simple and generalizable concept for defining clinical mastitis, in that milk from cows with clinical mastitis is not suitable for drinking. New cases of clinical mastitis are defined as being separated by at least 14 days.
The clinical findings in mastitis include abnormalities of secretion, abnormalities of the size, consistency and temperature of the mammary glands and, frequently, a systemic reaction. In other words, there are three categories of clinical mastitis: abnormal milk, abnormal gland and an abnormal cow (systemic disease). Abnormal milk is visibly abnormal (i.e. is not ‘drinkable’). An abnormal gland is larger and firmer than other quarters. An abnormal cow is pyrexic, depressed or has decreased appetite or milk production. This three-part categorization scheme has excellent clinical utility, is readily understood by everyone and provides a sound pathophysiological basis for treatment. In particular, it is likely that optimal treatment protocols can be developed for the three levels of clinical mastitis. Other categorization systems have been developed, but they lack the simplicity and generalizability of the secretion, gland and cow system.
Clinical mastitis episodes are also categorized according to their severity and duration.
• Peracute: severe inflammation, with swelling, heat and pain of the quarter, with a marked systemic reaction, which may be fatal
• Acute: severe inflammation without a marked systemic reaction
• Subacute: mild inflammation with persistent abnormality of the milk.
Abnormal milk
Proper examination of the milk requires the use of a strip cup, preferably one that has a shiny, black plate, permitting the detection of discoloration as well as clots, flakes and purulent material. Milk is drawn on to the black plate in pools and comparisons are made between the milk of different quarters. Because the herdsman frequently has little time to examine milk for evidence of mastitis it is customary to milk the first few streams on to the floor; in some parlors black plates are set in the floor. The practice does not appear to be harmful if the floor is kept washed down.
Discoloration may be in the form of blood-staining or wateriness, the latter usually indicating chronic mastitis when the quarter is lactating. Little significance is attached to barely discernible wateriness in the first few streams but, if this persists for 2–3 streams or more, it is an abnormality. One of the major unresolved issues in bovine mastitis is how to treat a cow with abnormal secretion on the first 1–2 streams that subsequently has normal-looking milk. Clots or flakes are usually accompanied by discoloration and they are always significant, indicating a severe degree of inflammation, even when small and present only in the first few streams. Blood clots are of little significance in a mastitis case, neither are the small plugs of wax that are often present in the milk during the first few days after calving, especially in heifers. Flakes at the end of milking may be indicative of mammary tuberculosis in cattle.
During the dry period in normal cows, the secretion changes from normal milk to a clear watery fluid, then to a secretion the color and consistency of honey, and finally to colostrum in the last few days before parturition. Some variation may occur between individual quarters in the one cow; if this is marked, it should arouse suspicion of infection.
The strip cup provides a valuable tool to detect clinical mastitis and constitutes part of the routine physical examination of the lactating cow. The most sensitive use of the strip cup is to observe the ability of milk from one quarter to mix with milk from another quarter; incomplete mixing (evidence of ‘streaming’) indicates that secretions from the two quarters differ and suggests the presence of an intramammary infection in one of the quarters. However, it should be remembered that the strip cup can only detect clinical mastitis, and detection of subclinical mastitis requires use of indirect tests such as SCC of composite milk samples from individual cows, or application of the California Mastitis Test to quarter samples or measuring the electrical conductivity of quarter samples.
Abnormal gland
Abnormalities of size and consistency of the quarters may be seen and felt. Palpation is of greatest value when the udder has been recently milked, whereas visual examination of both the full and empty udder may be useful. The udder should be viewed from behind and the two hind quarters should be examined for symmetry. By lifting up the hind quarters, the fore quarters can be viewed. A decision on which quarter of a pair is abnormal may depend on palpation, which should be carried out simultaneously on the opposite quarter of the pair. Although in most forms of mastitis the observed abnormalities are mainly in the region of the milk cistern, the whole of the quarter must be palpated, particularly if tuberculosis is suspected. The teats should be inspected and palpated for skin lesions, especially around the teat end. The supramammary lymph nodes should also be palpated for evidence of enlargement.
Palpation and inspection of the udder are directed at the detection of fibrosis, inflammatory swelling and atrophy of mammary tissue. Fibrosis occurs in various forms. There may be a diffuse increase in connective tissue, giving the quarter a firmer feel than its opposite number and usually a more nodular surface on light palpation. Local areas of fibrosis may also occur in a quarter; these may vary in size from pealike lesions to masses as large as a fist. Acute inflammatory swelling is always diffuse and is accompanied by heat and pain and marked abnormality of the secretion. In severe cases there may be areas of gangrene, or abscesses may develop in the glandular tissue. The terminal stage of chronic mastitis is atrophy of the gland. On casual examination an atrophied quarter may be classed as normal because of its small size, while the normal quarter is judged to be hypertrophic. Careful palpation may reveal that, in the atrophic quarter, little functioning mammary tissue remains.
Abnormal cow (systemic response)
A systemic response including toxemia, fever, tachycardia, ruminal stasis, depression, recumbency and anorexia may or may not be present, depending on the type and severity of the infection. A systemic response is usually associated with severe mastitis associated with E. coli, Klebsiella spp. or A. pyogenes and occasionally with Streptococcus spp. or Staphylococcus spp. Clinical mastitis episodes due to A. pyogenes produces the greatest decrease in milk production. In contrast, clinical mastitis due to environmental streptococci and coagulase-negative staphylococci is associated with the smallest decrease in milk production.44 Clinical mastitis episodes due to S. aureus are associated with the highest risk of culling.45
DIAGNOSIS
Detection of clinical mastitis
The initial diagnosis of clinical mastitis is made during the routine physical examination. Laboratory culturing of milk samples for bacteria and Mycoplasma spp., and for determining the antimicrobial susceptibility of S. aureus (specifically whether it produces beta-lactamase), is very useful for instituting optimal treatment protocols for cows with clinical mastitis and for instituting appropriate control measures. However, because subclinical mastitis has the greatest influence on the cost of mastitis to the producer, it is advantageous to also diagnose subclinical mastitis, on a cow and quarter level.
Detection of subclinical mastitis
Culturing large numbers of milk samples, although the gold standard for intramammary infection and subclinical mastitis, is expensive and impractical for routine use. Much attention has therefore been given to the development of indirect tests to predict the presence of an intramammary infection. Currently available indirect tests detect only the presence of inflammation but are of value as screening tests; milk from quarters or cows with a positive screening test are then submitted to bacteriological culture. Subclinical mastitis can only be detected by laboratory examination and cannot, by definition, be detected during the routine physical examination. In other words, the secretion from a quarter with subclinical mastitis appears drinkable.
Detection at the herd level
The prevalence of subclinical mastitis or intramammary infection is monitored by determining the bulk tank milk SCC and the most likely mastitis pathogens are identified by culturing bulk tank milk. These two methods are recommended to diagnose the presence and prevalence of mastitis pathogens on a herd basis.
Bulk tank milk somatic cell counts
The SCC of bulk tank milk is an indirect measure of the prevalence of mastitis within a dairy herd. The SCC is increased primarily, but not exclusively, because of subclinical mastitis associated with Gram-positive bacterial intramammary infections. There is a good correlation between the number of streptococci (S. agalactiae, S. dysgalactiae, and S. uberis) colony-forming units found in bulk tank milk and its SCC. The number of colony forming units (cfu) of S. aureus is moderately correlated to the bulk tank milk SCC.46 As contagious mastitis has become more effectively controlled, environmental mastitis pathogens have become a relatively more important cause of high SCC in bulk tank milk, especially in herds with moderate (<400000 cells/mL) to low (<150000 cells/mL) bulk tank milk SCC.
The association between management practices, dairy herd characteristics and SCC of bulk tank milk has been examined in about 60000 cows in 843 herds over a 2-year period.47 Results indicated that the prevalence of S. agalactiae and S. aureus intramammary infections was associated with bulk tank SCC.47 In herds free of S. agalactiae mastitis, the prevalence of S. aureus and C. bovis intramammary infections were correlated with bulk tank SCC. For herds without S. agalactiae, use of sawdust bedding was associated with a decrease in SCC in bulk tank milk, while a dirty loose housing area was associated with an increase in SCC in bulk tank milk. Increased milk production, repeated mastitis control visits and use of particular predip compounds were significantly associated with decreased SCC in bulk tank milk in all herds, regardless of whether any cows in the herd had S. agalactiae mastitis. In herds with S. agalactiae mastitis, use of iodine, chlorhexidine, peroxide or sodium chlorite–lactic acid as a predip was associated with a decrease in SCC of bulk tank milk.47
The SCC of bulk tank milk has become a widely used test because it provides a sensitive and specific indicator of udder health and milk quality. The sample for analysis is obtained by agitating the milk for 5–10 minutes and collecting a sample from the top of the bulk tank milk using a clean dipper. The sample should not be collected near the outlet valve because this varies from that in the rest of the tank. The SCC of bulk tank milk is widely used to regulate whether milk may be legally sold and to determine the price paid for raw milk. Premium and penalty payments are calculated on the basis of 3-month geometric mean of weekly bulk milk tank SCC measurements. Milk processing plants in most developed countries use automatic electronic somatic cell counters routinely in order to provide a monthly report of the bulk tank milk SCC. The test requires only that the sample for examination be taken randomly and not frozen, that it be prepared with the correct reagent, that the laboratory counter be set at the right calibration and that the sample be examined quickly or preserved with formalin to prevent cell losses during storage. The bulk tank milk SCC is extremely useful in creating awareness of the existence of a mastitis problem, so that when the SCC of bulk tank milk exceeds permissible limits further investigation of the herd is indicated. In a seasonal herd in which all cows are at the same stage of lactation the bulk milk cell count will normally be high in early lactation and just before drying off. To overcome these and other factors that are likely to transiently influence bulk tank milk SCC, it is recommended that correction factors be introduced into the estimation or that a rolling SCC, in which monthly data are averaged for the preceding 3 months, be used. Consideration of this figure will avoid too hasty conclusions on one high count caused by an extraneous factor.
It is not possible to use the bulk tank milk SCC to determine the number of cows in a herd affected by mastitis but it is possible to estimate fairly accurately the number of infected quarters. In general, as the bulk tank milk SCC increases, the prevalence of infection increases and losses in production increase. Production losses calculated as a percentage of production expected with a count of 200000 cells/mL are shown in Table 15.1. A bulk tank milk SCC of more than 300000 cells/mL is considered to indicate a level of mastitis in the herd that warrants examination of individual cows. Herds with a high bulk tank milk SCC have significantly lower production levels and are less likely to use a postmilking teat dip or to have a regular program of milking machine maintenance or automatic cluster removal.46
Table 15.1 Estimated prevalence of infection and losses in milk production associated with bulk tank milk somatic cell count
| Bulk tank milk somatic cell count (cells/mL) | Infected quarters in herd (%) | Production loss (%) |
|---|---|---|
| 200 000 | 6 | 0 |
| 500 000 | 16 | 6 |
| 1 000 000 | 32 | 18 |
| 1 500 000 | 48 | 29 |
Culture of bulk tank milk
Bacteria present in bulk tank milk may originate from infected udders, from teat and udder surfaces or from a variety of other environmental sources; however, despite the large number of potential sources for bacteria, culture of bulk tank milk is a useful technique for screening for major mastitis pathogens.48 The culture of S. aureus and S. agalactiae from bulk tank milk is a reliable indicator of infection by those pathogens in the herd. The number of those pathogens found on culture is determined by the number of bacteria shed, the number of infected cows, the milk production level of infected cows relative to herd mates, and the severity of infection. A single culture of bulk tank milk has low sensitivity but high specificity for determining the presence of S. agalactiae or S. aureus in the herd. Thus many infected herds will be called negative but few uninfected herds will be called positive. Pathogens such as Nocardia spp. and Mycoplasma spp. have also been identified by culture of bulk tank milk. In general, the sensitivity of a single bulk tank milk culture to detect the presence of intramammary infections due to S. agalactiae ranges from 21–77%, for S. aureus it ranges from 9–58% and for M. bovis it is 33%.
Environmental bacteria such as S. uberis, S. dysgalactiae, and coliforms may enter milk from intramammary infections, but also from nonspecific contamination. The presence of these organisms in bulk tank milk may relate to the general level of environmental contamination and milking hygiene in the herd. Udder infections with these environmental pathogens are predominantly of short duration and characterized by clinical disease, which makes their inadvertent introduction to the bulk tank less likely.
String sampling or milk line sampling from the positive pressure side of the milking system is the collection of milk samples from a group of cattle instead of the entire herd, as in bulk tank milk sampling. String sampling may have some merit in identifying subgroups of cattle with the highest prevalence of infection. String sampling is thought to be more sensitive in monitoring herds for contagious pathogens than bulk tank milk sampling. If a production group tests positive on a string culture, then individual composite milk cultures can be performed to identify individual animals. Information of the culture results from string sampling should assist development of control programs; however, milk samples left in the pipeline from one string can confound the culture results of subsequent strings.
Numerous bacteriological techniques have been used to isolate and identify pathogens in bulk tank milk but none has been established as the gold standard method for the culture of milk from bulk tanks. The most suitable laboratory medium for growth and classification of the pathogens from bulk tank milk needs to be assessed. Sampling strategies have included weekly and monthly samples, but it remains to be determined which strategy is optimal relative to herd size and management, disease characteristics and practicality.
Culture of bulk tank milk has been compared to bulk tank SCC, herd summaries of SCC of individual cows and herd summaries of cultures of individual cows.48 A significant correlation was found between bulk tank SCC and the frequency of isolating S. agalactiae from monthly samples of bulk tank milk. The correlations between S. aureus from bulk tank milk and SCC are lower.
Detection at the individual cow level
Abnormalities of the udder and gross abnormalities of milk in cattle with clinical mastitis have been described above under clinical findings. In individual cows with clinical mastitis, culture of the secretion from an infected quarter can be done. In animals without clinical mastitis, culture of the secretion represents a direct test for subclinical mastitis. The objective is to identify cows with contagious mastitis so that they can be treated or culled, or to identify the nature and source of environmental mastitis pathogens. Fulfillment of these requirements requires bacteriological culture of milk samples so that the pathogens can be named; identification of mastitis pathogens is central to the development of effective treatment and control programs. Detection of infected cows requires an individual cow examination and application of an indirect (screening) test for infection, such as the SCC of a composite milk sample, followed by culture of a representative subset of cows in order to determine the most prevalent pathogen. Indirect tests estimate the prevalence of infection and microbiological examination identifies the mastitis pathogens; from this information an appropriate control plan can be formulated.
Culture of individual cow milk
Individual cow milk can be cultured as part of a herd examination for mastitis, or on individual quarter samples, or on composite samples including milk from all four quarters. An intramammary infection is defined as the presence of the same pathogen in duplicate samples collected immediately after each other, or the presence of the same pathogen in two of three consecutive cultures obtained on different sampling dates. Individual quarter samples are preferred because the costs of treatment dictate that the least possible number of quarters be treated. With this technique only affected quarters are treated; if the quarter infection rate is low the saving in treatment costs is relatively large.
Milk sampling for culture must be carried out with due attention to cleanliness since samples contaminated during collection are worthless. The technique of cleaning the teat is of considerable importance. If the teats are dirty, they must be washed and then properly dried or water will run down the teat to the teat end and infect the milk sample. The end of the teat is cleaned with a swab dipped in 70% alcohol, extruding the external sphincter by pressure to insure that dirt and wax are removed from the orifice. Brisk rubbing is advisable, especially of teats with inverted ends. The first two or three streams are rejected because their cell and bacterial counts are likely to be a reflection of the disease situation within the teat rather than within the udder as a whole. The next few streams, the premilking sample, is the approved one because of its greater accuracy. For complete accuracy a premilking and a postmilking sample are taken. If tuberculosis is suspected, the last few streams are the critical ones. Indirect and chemical tests for mastitis can be carried out as accurately on foremilk as on later milk.
If individual quarter samples are collected, screw-cap vials are most satisfactory. During collection the vial is held at an angle to the ground in order to avoid as far as possible the entrance of dust, skin scales and hair. If there is delay between the collection of samples and laboratory examinations, the specimens should be refrigerated or frozen. Freezing of milk samples appears to have variable effects on bacterial counts, depending on the bacteria. A. pyogenes and E. coli counts are decreased by freezing, coagulase-negative Staphylococcus spp. counts are increased, and Streptococcus and S. aureus counts are either unaffected or increased by about 200%.49
The laboratory techniques used vary widely and depend to a large extent on the facilities available. Incubation on blood agar is most satisfactory, selective media for S. agalactiae having the disadvantage that other pathogens may go undetected. Smears of incubated milk are generally unsatisfactory as not all bacteria grow equally well in milk. An augmented system of culturing milk samples, which has given superior results in terms of the number of infected quarters detected, includes preculture incubation, then freezing of the milk sample, then inoculation of the medium with a larger than normal inoculum of milk.50 The concern with augmented culture systems is that they may amplify contaminants obtained during sampling and therefore decrease the specificity of milk culture. Laboratory culturing techniques can be very time-consuming and expensive unless modern, prepackaged identification systems are used. They also provide the speed needed to make the examination a worthwhile one.
A milk sample is considered contaminated when more than three species of bacteria are isolated. A quarter is considered to be infected when the same bacteria is isolated in at least two out of three milk samples. A quarter is considered to be cured when bacteria, isolated at drying off, are not present in any samples 28 days after calving. An uninfected quarter at drying off that is infected at calving is considered to indicate a new intramammary infection. A quarter that is infected at drying off but infected with another bacteria at calving also indicates a new intramammary infection.
Selective culture plates, such as biplates (MacConkey agar and blood agar with 1% esculin), triplates (MacConkey agar, blood agar and TKT agar (thallium, crystal violet and staphylococcal toxin in 5% blood agar with 1% esculin)), Petrifilm® plates, which are selective culture media products,51 the MASTiK® diagnostic kit or the ColiMast® test can be used to differentiate between Gram-positive and Gram-negative pathogens and no growth,52 and may aid in the rational and targeted use of antimicrobial agents for clinical cases of mastitis. A commercially available cowside test for endotoxin (Limast-test®) is available in Scandinavia. The test takes 15 minutes to run on milk samples and is able to detect the presence of endotoxin using the Limulus amebocyte lysate assay. The test therefore can detect the presence or absence of Gram-negative bacteria but does not differentiate between E. coli and K. pneumoniae.53 At least 104–105 cfu of Gram-negative bacteria are necessary for a positive test result; this does not decrease the clinical utility of the test, because low bacterial counts in the milk from infected quarters usually indicates that antimicrobial agents are not required in order to clear the infection.
There is interest in developing other cowside tests to determine whether the causative pathogen is Gram-negative or Gram-positive. One such approach uses dilution of the milk sample, filtration through a membrane with a pore size that retains bacteria, and staining of the bacteria with specific stains.54 The filtration procedure reportedly takes 5 minutes, but the need for microscopic examination decreases the utility of this as a cowside test.
A common diagnostic problem is a bacteriologically negative culture in cows with clinical mastitis. Even when milk samples are collected appropriately and bacteriological culture is done using routine laboratory methods, 15–40% of samples from clinical mastitis episodes are bacteriologically negative (yield no growth). Failure of these samples to yield a mastitis pathogen may be the result of spontaneous elimination of infection, a low concentration of pathogens in the milk, intermittent shedding of the pathogen, intracellular location of the pathogens or the presence of inhibitory substances in the milk. Augmented culture techniques may reduce, but do not eliminate negative culture results and may facilitate growth of contaminant organisms. Dairy producers and veterinarians therefore face a dilemma when no bacteria or bacteria commonly regarded to be of minor pathogenicity, such as C. bovis or coagulase-negative Staphylococcus spp., are cultured from the milk of cows with clinical mastitis, particularly if clinical signs persist.55 Most bacteriologically negative cases of clinical mastitis appear to be caused by low-grade infections with Gram-negative bacteria.55 When no bacterial pathogen can be isolated from cases of clinical mastitis using standard culture techniques, enzyme-linked immunosorbent assays (ELISAs) may be used to detect antigens against S. aureus, E. coli, S. dysgalactiae, and S. agalactiae.56 Antigens to these pathogens may be detectable using an ELISA in up to 50% of quarter samples from cows with clinical mastitis in which no pathogens were isolated but in which the SCC was more than 500000 cells/mL. Despite these promising findings, ELISAs are not widely used in the identification of mastitis pathogens.
Indirect tests for subclinical mastitis
Indirect tests include SCCs using automated electronic counters, the California Mastitis Test, increases in electrical conductivity of milk, and increases in the activity of cell associated enzymes (such as NAGase) in milk. ELISA tests to detect neutrophil components have been developed but are not commercially available.57 Of these indirect tests, only the CMT and electrical conductivity can be used cowside, with CMT providing a more accurate screening test than electrical conductivity.
The somatic cell count of composite or quarter samples
There is a strong relationship between the SCC of quarter samples of milk and the milk yield, with SCC increasing slightly as milk production decreases but increasing markedly with intramammary infection of the quarter. The distribution of SCC in a herd therefore reflects the distribution of intramammary infections. The most important factor affecting SCC in an individual cow is the number of quarters infected with a major or minor pathogen. In most herds, the prevalence of infection will increase through a lactation and will also increase with the age of the cow. Cell counts in the first few days of the lactation are often exceptionally high and unreliable as indicators of intramammary infection, and in uninfected cows the counts will drop to a low level within 2 weeks of calving and remain low throughout the lactation unless an intramammary infection occurs. The SCC of a cow that remains free of infection throughout her life will remain very low. However, older cows may have higher counts because the prevalence of infection is higher with age and older cows are more likely to have had previous infections with residual lesions and leaking of somatic cells into the milk. There are also consistent and significant differences in actual SCC between cows, individual cows tending to maintain the same class of count throughout their lives. Cows that have consistently low SCC do not seem to be more susceptible to mastitis than others. Attempts to base a breeding program to reduce the prevalence of mastitis on the selection of cows with an innately low composite SCC have been discarded because of fluctuations in numbers within cows.
Healthy quarters have a SCC below 100000 cells/mL, and this cutpoint should be used to indicate the absence or presence of intramammary infection on a gland basis. This cutpoint looks very solid for a gland, because many milk components differ from normal values whenever the SCC exceeds 100000 cells/mL. Moreover, mean SCC counts for bacteriologically negative quarters, quarters infected with minor pathogens and quarters infected with major pathogens were 68 000, 130000, and more than 350000 cells/mL, respectively.58
Because of the time and labor saved it is now customary to do automated electronic cell counts on composite milk samples that have already been collected for butterfat testing. Regular reports of individual cow SCCs are therefore widely available in herds that routinely test production parameters of their cattle. An exciting new development in mastitis control is the portable somatic cell counter, which was designed for on farm use, thereby providing targeted and immediate SCC information for quarter or composite milk samples. Using the composite sample technique does distort the SCC; for example, the dilution of high-SCC milk from a bad quarter by low-SCC milk from three normal quarters could mean that a cow with one infected quarter might not be detected. Composite SCCs of less than 200000 cells/mL are considered to be below the limit indicative of inflammation, even though uninfected quarters have a SCC of less than 100000 cells/mL. Factors that affect the composite milk SCC include the number of infected quarters infected, the kind of infection (S. agalactiae is a more potent stimulator of cellular reaction than S. aureus), the strictness with which milk from cows with clinical mastitis is kept out of the bulk tank, the age of the cows (older cows have higher counts), the stage of lactation (counts are highest in the first days after calving and toward the end of lactation) and the herd’s average production, the cell count reducing as milk yield increases.59
A SCC scoring system that divides the SCC of composite milk into 10 categories from 0–9, known as linear score, is becoming more widely used. The linear score is a base 2 logarithm of the SCC (in cells/mL), whereby linear score = log2(SCC/100000) + 3. Likewise, to calculate SCC (in cells/mL) from the linear score (LS), the following formula is used: SCC = 100 000 × 2(LS – 3). A SCC of 100000 cells/mL therefore converts to a score of 3. Each 1-unit increase (or decrease) in linear score is associated with a doubling (or halving) of the SCC. For example, score 2 is equivalent to a SCC of 50000 cells/mL, and scores of 4 and 5 correspond to 200000 and 400000 cells/mL. Conversion of SCC to linear score values is performed as shown in Tables 15.3 and 15.4.. The principal reason for using the linear score is to achieve properties that are required in order to use conventional statistical methods: mean equal to median, normal distribution and uniform variance amongst samples within lactation, amongst cows within herd or among daughters within sire.
Table 15.3 Linear score calculation from the somatic cell count
| Example: SCC = 200 000 cells/mL |
Table 15.4 Conversion of linear scores to somatic cells counts (cells/mL) and predicted loss of milk98
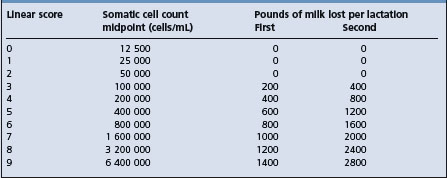
The proportion of neutrophils in the SCC is very low (<11%) in healthy quarters but is markedly increased in quarters with intramammary infection (to >90%). Accordingly, the percentage of neutrophils in the SCC may provide a useful indication of intramammary infection, but is not currently performed.58
Somatic cell counts can also be determined for colostrum, where they are useful in indicating the presence of intramammary infection (Table 15.2).60
The California Mastitis Test of quarter samples
The CMT is the most reliable and inexpensive cowside test for detecting subclinical mastitis. The CMT is also known as the Rapid Mastitis test, Schalm test or Mastitis-N-K test, was developed in 1957 and constituted a modification of the Whiteside test. The CMT reagent contains a detergent that reacts with DNA of cell nuclei, and a pH indicator (bromcresol purple) that changes color when the milk pH is increased above its normal value of approximately 6.6 (mastitis increases pH to 6.8 or above). The CMT is mixed with quarter milk samples that have been previously collected into a white container and the sample is gently swirled; the result is read within 15 seconds as a negative, trace, 1, 2, or 3 reaction depending on the amount of gel formation in the sample. Maximum gel formation actually occurs from 1–2.5 minutes, depending on the quarter SCC and continued swirling of the mixture after the time of peak viscosity produces an irreversible decrease in viscosity.61 Cows in the first week after calving or in the last stages of lactation may give a strong positive reaction.
The close relationship between the CMT reaction and the SCC of milk and the reduced productivity of affected cows is shown in Table 15.4. If the CMT is used to minimize the false negative rate (produce the highest sensitivity), then the test should be read as negative (CMT = negative) or positive (CMT = trace, 1, 2 or 3). If the CMT is used to minimize the false-positive rate (produce the highest specificity) for culling decisions, then the test should be read as negative (CMT = negative or trace) or positive (CMT = 1, 2 or 3).
CMT scores can also be determined for colostrum, where the score is useful in indicating the presence of intramammary infection (Table 15.2). The equivalent SCC for CMT scores of negative or trace are different for colostrum and milk, but the SCC for CMT scores of 1, 2 and 3 are similar for colostrum and milk.62
The NAGase test of composite or quarter samples
The NAGase test is based on the measurement of a cell-associated enzyme (N-acetyl-β-d-glucosaminidase) in the milk, a high enzyme activity indicating a high cell count. NAGase is an intracellular lysosomal enzyme derived primarily from neutrophils but also from damaged epithelial cells. The test is suited to the rapid handling of large numbers of samples because of the ease of its automation, and the test can be done on fresh milk and read on the same day.63 However, because most of the NAGase activity is intracellular, samples should be frozen and thawed before analysis to induce maximal NAGase activity.58 The NAGase test is reputed to be the most accurate of the indirect tests and as good as SCC in predicting the infected status of a quarter. The NAGase test uses a less sophisticated reading instrument than the average automatic cell counter. If all tests are available it is best to consider the NAGase test and SCC as complementary tests and carry out both of them. Milk NAGase levels are high at the beginning and the end of lactation, as with cell counts. The test has also been validated for use with goat milk.
Electrical conductivity tests of quarter samples
A test that has received a lot of attention because it can be used in robotic milking systems is based on the increase in concentration of sodium and chloride ions, and the consequent increase in electrical conductivity, in mastitic milk.64 The electrolyte changes in milk are the first to occur in mastitis and the test has attractions for this reason. A number of factors affect these characteristics, however, and to derive much benefit from the test it is necessary to examine all quarters and use differences between the quarters to indicate affected quarters. For greater accuracy all quarters need to be monitored each day. An experimental unit that takes all these factors into consideration has been fitted to a milking machine and, by a computer-prepared analysis, monitors variations in electrical conductivity in each quarter every day.65 Electrical conductivity is attractive as a test because it measures actual injury to the udder rather than the cow’s response to the damage, as SCC and NAGase activity do.66 However, a meta-analysis indicated that using an absolute threshold for conductance did not provide a suitable screening test, as both sensitivity and specificity were unacceptably low.67 The use of differential conductivity (within cow quarter comparison) results in improvement in test sensitivity and specificity, and is currently the only recommended application of this indirect test.58,68
The most commonly promoted method for measuring electrical conductivity is a hand-held device with a built-in cup into which milk is squirted (foremilk is preferred). Experimentally induced clinical mastitis due to S. aureus and S. uberis was detectable by changes in electrical conductivity of foremilk: 90% of cases were detectable when clots first appeared and 55% of cases were detectable up to two milkings prior to the appearance of clots.64 This suggests that clinical mastitis associated with these two major pathogens may be able to be detected earlier by electrical conductivity than by waiting for milkers to detect visible changes in the milk.
Comparison of indirect methods
The effects of subclinical intramammary infection on several parameters in foremilk from individual quarter milk samples have been compared.62 Somatic cell count, electrical conductivity, pH, NAGase activity and the concentrations of sodium, potassium, lactose and alpha-1-antitrypsin were measured from individual quarters. Somatic cell count, NAGase activity, electrical conductivity and concentrations of sodium, alpha-1-antitrypsin and lactose were all useful indirect indicators of infection. The SCC was better able to discriminate between infected and uninfected quarters and cows than were the electrical conductivity, pH and NAGase activity.
Hematology and serum biochemistry
In severe clinical mastitis there may be marked changes in the leukocyte count, packed cell volume and serum creatinine and urea nitrogen concentration because of the effects of severe infection and toxemia.69 In particular, clinical mastitis episodes associated with Gram-negative bacteria frequently cause a profound leukopenia, neutropenia, lymphopenia and monocytopenia as a result of the endotoxemia. as well an increased packed cell volume.70 In contrast, the leukogram in cattle with clinical mastitis associated with Gram-positive bacteria is normal or mildly increased.
Ultrasonography of the mammary gland
Two-dimensional ultrasonographic images of the gland cistern, parenchymal tissue and teat are easily obtained using a 5, 7.5, or 8.5 MHz linear array transducer, and ultrasonography is becoming more widely used to guide treatment of teat and gland cistern abnormalities. However, there are few reports of the use of ultrasonography to diagnose or prognose clinical mastitis episodes, although this is likely to be a fruitful area for investigation.
The best two-dimensional images of the udder parenchyma are obtained by clipping the hair on the udder and applying a coupling gel. This minimizes air between the transducer face and skin. Imaging the normal adjacent quarter is very helpful in identifying abnormalities. Imaging should be performed in two planes, sagittal to the teat (and therefore perpendicular to the ground), and transverse to the teat (and therefore horizontal to the ground). The injection of sterile 0.9% NaCl through a teat cannula into the gland provides a practical contrast agent that can help further define the extent of any abnormalities. The superficial supermammary lymph nodes can be ultrasounded using a 7.5 MHz linear transducer, with the lymph node being well demarcated from the surrounding tissues. Mean lymph node length was 7.4 cm (range 3.5–15.0 cm) and mean depth was 2.5 cm (range, 1.2–5.7 cm). Lymph node size increased with age but was not correlated with SCC.71
Mastitis produces an increased heterogeneous echogenicity to the milk in the gland cistern, compared to an uninfected quarter. It is important to make this visual comparison without altering the contrast and brightness setting on the ultrasonographic unit.72
Three-dimensional ultrasonography of the bovine mammary gland and teat has recently been evaluated73 and has many promising applications.
Biopsy of mammary tissue
A biopsy of mammary tissue can be used for histological and biochemical evaluation in research studies. The use of a rotating stainless steel cannula with a retractable blade at the cutting edge has been described for obtaining biopsy material from cows.74 Despite some postoperative bleeding, milk yield and composition in the biopsied gland were affected only transiently.
NECROPSY FINDINGS
Necropsy findings are not of major interest in the diagnosis of mastitis and are omitted here but included in the description of specific infections.
The diagnosis of clinical mastitis is not difficult if a careful clinical examination of the udder is carried out as part of the complete examination of a cow with systemic clinical findings. Examination of the udder is sometimes omitted in a recumbent animal only for severe mastitis to be discovered later. The diagnosis of mastitis depends largely upon the detection of clinical abnormalities of the udder and gross abnormalities of the milk or the use of an indirect test like the CMT to detect subclinical mastitis.
Other mammary abnormalities that must be differentiated from clinical mastitis include periparturient edema, rupture of the suspensory ligament, and hematoma. These are not accompanied by abnormalities of the milk unless there is hemorrhage into the udder. The presence of stray voltage in the milking plant should not be overlooked in herds where the sudden lowering of production arouses an unfounded suspicion of mastitis. Differentiation of the different causes of mastitis is difficult on the basis of clinical findings alone but must be attempted, especially in peracute cases where specific treatment must be given before results of laboratory examinations are available. A pretreatment sample of milk from the affected glands for culture and antimicrobial sensitivity may provide useful information about the health record of the cow and the need to consider alternative therapies, and could provide information on new infections in the herd.
TREATMENT
The treatment of the different causes of clinical and subclinical mastitis may require specific protocols, which are described under specific mastitis pathogens later in the chapter. The general principles of mastitis treatment are outlined here.
Historical aspects of antimicrobial therapy for clinical and subclinical mastitis
Between about 1950 and 1990, on a worldwide basis, all forms of both clinical and subclinical bovine mastitis were treated with a wide variety of antimicrobial agents either by intramammary infusions or parenterally, and commonly by both routes in acute and peracute cases. Most veterinarians treated clinical mastitis and evaluated the response on the basis of clinical outcome. In general, it was believed that antimicrobial agents were effective for the treatment of clinical and subclinical mastitis in lactating cows. However, there are very few scientific publications based on randomized clinical trials in which the efficacy of intramammary antimicrobial agents for treatment of clinical mastitis was compared to untreated controls. If antimicrobial agents were used and the animal recovered, it was assumed that treatment was efficacious. If the cow did not respond favorably, several reasons were usually enumerated for the treatment failure. However, most of these reasons, while biologically attractive, are hypothetical and have not been substantiated scientifically. Gradually, over the years, veterinarians began to doubt the efficacy of antimicrobial agents for the treatment of all cases of clinical mastitis. In addition, and of major importance, milk from treated cows had to be discarded for up to several days after the last day of treatment because of antimicrobial residues; this was a major expense. Currently, optimized treatment strategies focus on efficacy, economics, animal welfare aspects and the milk withhold time of antimicrobial treatment.
Efficacy is assessed on the basis of clinical cure or bacteriological cure. Most producers are interested in the return to normal milk (clinical cure) and are much less interested in the return to a sterile quarter (bacteriological cure). Because clinical mastitis is defined as abnormal milk, the return to normal (‘drinkable’) milk represents a clinical cure. Bacteriological cure represents the inability to isolate the initial pathogen 14–28 days after the start of treatment. Other important indicators of efficacy are milk production, dry matter intake, the amount of saleable milk and mortality or culling rates after treatment.
Some examples of the efficacy or inefficacy of antimicrobial agents illustrate the controversy. It is well accepted that the cure rate following intramammary treatment of clinical or subclinical mastitis due to S. agalactiae in the lactating cow is high (80–90%). In contrast, the cure rate of clinical and subclinical mastitis due to S. aureus in the lactating cow is considerably lower (40–50%), but certainly not 0%. In herds with a low prevalence of contagious mastitis, most cases of mild clinical mastitis (abnormal secretion only) in lactating cows are due to environmental streptococci and coliforms and may recover without antimicrobial therapy, although antimicrobial administration increases the clinical and bacteriological cure rate. Antimicrobial agents may be ineffective for the treatment of clinical mastitis associated with M. bovis, A. pyogenes, Nocardia spp., and P. aeruginosa.
In the 1970s dairy processing plants, veterinarians, consumer advocates, public health authorities and milk-quality regulating agencies began to express concern about antimicrobial residues in milk from cows treated for mastitis. The public health and milk industry concerns about residues combined with the controversy about the efficacy of antimicrobial agents for clinical mastitis has also provided a stimulus to evaluate the efficacy and consequences of using antimicrobial agents. Since the early 1990s much emphasis has been placed on alternative methods of treating clinical mastitis, leading to a reduction in the use of antimicrobial agents during the lactating period.75 Such strategies have been defended based on a lack of information concerning the efficacy and economics of antimicrobial therapy associated with pathogens other than S. agalactiae, and by the need to reduce the risk of residue violation. However, a recent study concluded that not administering antibiotics to cows with clinical mastitis was imprudent and unethical.76
There is a need for randomized controlled field trials to evaluate the use of antimicrobial agents for the treatment of clinical mastitis; the design for such trials and the statistical analysis required have been reviewed.77 Well conducted clinical mastitis treatment trials represent an invaluable, although difficult and expensive, effort to evaluate efficacy of antimicrobial agents under field conditions. The use of intramammary antimicrobial agents for the treatment of clinical mastitis in lactating cows in 40 dairy herds over 4 years found an economic benefit compared to nonmedicated controls.78 In the antimicrobial-treated group, the number of pathogens in the milk following treatment was reduced, the number of quarters returning to normal milk was increased, and the number of cured quarters was increased. In should be noted that the use of antimicrobial agents for the treatment of subclinical mastitis at the end of lactation, known as dry cow therapy, is accepted worldwide and is based on scientific evidence using randomized clinical trials. Dry cow therapy is one of the principles applied in the effective control of bovine mastitis, in which much progress has been made since the early 1970s.
Treatment strategy
The treatment strategy will depend on whether the mastitis is clinical or subclinical, and the health status of the herd, including its mastitis history. Clinical mastitis is further categorized as abnormal milk, abnormal gland or abnormal cow, as described under Diagnosis. If treatment is indicated, the major decision is whether to administer antimicrobial agents parenterally or by intramammary infusion.
An important aspect of treatment is the accurate positive identification of the animal(s) to be treated, the recording of the relevant clinical and laboratory information, and the treatments being used, and monitoring the response. Useful information would include:
• Identification of pathogen(s)
• Treatment used, including dose, route and duration
• Milk withholding time and time when returned to the milking string
Options for treating cows with clinical mastitis include treating all cows with antimicrobial agents, treating none of the cows with antimicrobial agents or treating only specific cows with antimicrobial agents.79 Treating all cows results in increased costs for those cows with clinical mastitis associated with pathogens not susceptible to the antimicrobial agent used, especially if the signs are likely to resolve before the milk withholding period has expired. Treatment of all cows is also associated with increased risk of violative residues in the bulk milk. Treating none of the cows with antimicrobial agents has animal welfare implications, in that an effective treatment is not administered to some cattle with clinical mastitis, and nontreatment allows Gram-positive pathogens to persist, increasing the probability of a recurrence of clinical mastitis or causing a herd epidemic of mastitis. Accordingly, nontreatment of all cases of mastitis is not a viable option. Treating only specific cows with antimicrobial agents requires an accurate method of determining which animals should be treated. However, clinical judgment and predictive models are too inaccurate to distinguish between clinical mastitis associated with Gram-negative and Gram-positive pathogens.79 To select cows for antimicrobial therapy on the basis of bacteriological culture is costly and delays treatment; clinical judgment would still be necessary because bacteria are not isolated from 15–40% of milk samples from cows with clinical mastitis.
Veterinarians should always ask and answer four questions related to antimicrobial therapy in bovine mastitis:
• Is antimicrobial therapy indicated?
• Which route of administration (intramammary, parenteral or both) should be used?
Is antimicrobial therapy indicated?
The first decision is whether to treat a particular case with antimicrobial agents and whether supportive therapy is required. Therapy decisions should be made in context with the overall objectives of the lactating cow treatment protocol. The availability of approved, effective treatment products is an essential component of the program. A number of factors are important in determining which cases of mastitis should be treated during lactation. These factors include the type of pathogen involved, the type and severity of the inflammatory response, the duration of infection, the stage of lactation, and the age and pregnancy status of the cow.
Type of pathogen involved
There are marked differences in the bacteriological cure rates of the various major mastitis pathogens after therapy during lactation. The outcome of treatment during lactation is poor for cases of S. aureus mastitis. On the other hand, S. agalactiae responds extremely well to lactating cow therapy and all infected cows should be treated. Cases of mastitis associated with environmental organ-isms have reasonable, but variable, cure rates.
Type and severity of the inflammatory response
The predominant type of inflammatory process involved influences the objectives of the therapy program. Herds with clinical mastitis problems will aim at reducing clinical signs, returning the milk to saleable quality and avoiding residue violations. Herds with a predominance of subclinical mastitis are concerned with avoiding the spread of infection and reducing the prevalence of the major pathogens involved. Both types of herd have the primary objective of restoring the production potential.
The severity of the inflammatory response is also important in the selection of cases for mastitis therapy during lactation. Heat, pain and swelling of the quarter (abnormal gland) are clinical signs that indicate the need for antimicrobial therapy. Many producers, however, will treat any cow that shows clots in the milk (abnormal milk). There are no reports to verify that treatment of cows exhibiting abnormal milk only is efficacious and economically justifiable, although it is probable that treatment of clinical mastitis episodes of abnormal milk but normal gland due to S. agalactiae is efficacious and economic. Treatment success is lower in cows with high NAGase concentrations in milk compared to low NAGase concentrations.80
Duration of infection
For the contagious organisms, especially S. aureus, the duration of infection is an important determinant of its susceptibility to therapy during lactation. In chronic S. aureus mastitis, the organism survives intracellularly in leukocytes, becomes walled-off in small abscesses of mammary ducts, and has the ability to exist in the L-form state. At this point S. aureus is virtually incurable during lactation. With new methods of automated detection of subclinical intramammary infection such as in-line electrical conductivity measurement, new infections may be detected much earlier. The cure rate of S. aureus during lactation needs to be re-evaluated when treatment is administered early in the course of infection.
Stage of lactation
The stage of lactation is an important determinant of the benefit:cost ratio of mastitis therapy during lactation. It may be uneconomical to treat even cases with a high probability of cure during late lactation.
Age and pregnancy status of cow
The probability of a cure is greater in young cows, and age should be considered in selecting cases for mastitis therapy during lactation.80 The economic aspects of treatment for late-lactation, nonpregnant cows are obviously different from those for midlactation pregnant cows.
A mastitis therapy program for lactating cows should be based on a complete understanding of the mastitis status of the herd, and individual cow treatment decisions should be consistent with the overall herd mastitis therapy program. A record system for treatment should be established so that it is possible to monitor the efficacy of the mastitis treatment program.
The udder health status in a particular herd will determine whether the lactating cow mastitis therapy strategy should be targeted at the individual cow level or at the herd level. The level of emphasis should clearly reflect the objectives of the therapy program. For example, a herd with low bulk tank milk SCC and sporadic cases of environmental mastitis should target the lactating cow therapy strategy at the cow level. The primary objectives would be to alleviate clinical signs, to achieve a bacteriological cure and to restore the cow’s production. On the other hand, a herd with moderate to high bulk tank milk SCCs and a significant prevalence of contagious organisms should aim the program at the herd level. In this case, the objective would be to limit the spread of infection, markedly reduce or eradicate a specific pathogen and increase herd production. A clear statement of treatment philosophy (individual cow level or herd level) in a particular herd is needed to direct establishment of well defined treatment protocols for mastitis in lactating cows.
Intramammary infection (mastitis) is identified by the presence of clinical signs or the results of a direct test (culture of milk) or indirect tests such as SCC, CMT or electrical conductivity. The detection of clinical or subclinical mastitis does not necessarily indicate that therapy should be administered, although animal welfare issues dictate that treatment must be administered to cattle with an abnormal gland or systemic signs (abnormal cow) because these animals are undergoing pain and discomfort. A decision to use treatment during lactation should be based on the likelihood of achieving the objectives of the therapy program. Several factors are important in the selection of cows for treatment. These factors can significantly influence the cure rate achieved with therapy, or the economic benefit realized.
The herd history of udder health will indicate the probable cause of clinical mastitis. Cows with mild cases of clinical mastitis (abnormal milk only) in herds with a low prevalence of contagious mastitis pathogens are likely to be affected with environmental pathogens17 and commonly return to clinically normal milk in four to six milkings.81 This has led to the development of treatment algorithms based on the results of culturing clinical cases using selective media. Using this approach, milk cultures are obtained from all cattle with clinical mastitis and plated using biplates or triplates. All cattle with abnormal glands or signs of systemic illness (abnormal cow) are immediately treated with antimicrobial agents and appropriate ancillary treatment, with subsequent antimicrobial treatment based on the preliminary culture results at 18–24 hours or the final culture results at 48 hours. In contrast, treatment is initially witheld from all cattle demonstrating abnormal secretion only; antimicrobial treatment is instituted based on the culture results. One such scheme recommends using intramammary antibiotics to treat affected quarters with S. aureus, coagulase-negative staphylococci and environmental streptococci, infusing intramammary antibiotics into all quarters of cows with one or more quarters infected with S. agalactiae and not administering antibiotics to cows with coliform bacteria or no growth.82 When using this delayed approach to antimicrobial treatment, it is important that cattle with abnormal milk only are closely monitored and that antimicrobial treatment is immediately instituted when signs of an abnormal gland or abnormal cow are present. The major difficulty with implementing the delayed approach is the difficulty in transporting the milk sample to and receiving the results from the diagnostic laboratory in a time effective manner.
Which route of administration (intramammary, parenteral or both?)
The second decision is the route of administration. The goal of antimicrobial treatment is to attain and maintain an effective concentration at the site of infection. Three pharmacological compartments are recognized for infection by mastitis pathogens:
• Milk and epithelial lining of the ducts and alveoli
• Parenchyma of the mammary gland
• The cow83 (Table 15.5).
Table 15.5 Summary of three-compartment model for anatomical location of infection due to mastitis pathogens in cattle
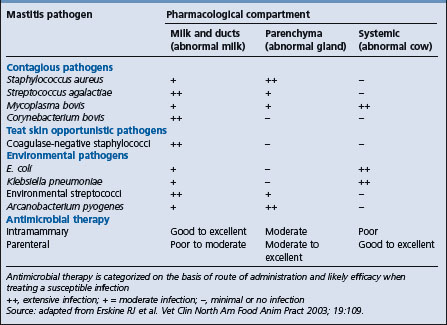
In general, infections confined primarily to the milk and ducts (such as C. bovis, coagulase-negative staphylococci) are easily treated with intramammary antibiotics. In contrast, infections due to mastitis pathogens with potential for systemic infection (such as E. coli, K. pneumoniae, M. bovis) are best treated with parenteral antibiotics. Mastitis pathogens that are the most difficult to treat are those that are principally infections of parenchymal tissue (such as S. aureus, A. pyogenes); this is because it is more difficult to attain and maintain an effective antibiotic concentration at this anatomical site when administering antibiotics by the intramammary or parenteral routes.
Which antimicrobial agent should be administered?
The third decision is the antimicrobial agent. The selection of the antimicrobial class for the particular mastitis pathogen has traditionally been based on culture and susceptibility testing and, although some in vivo data are now available, the choice is still largely dependent on case studies rather than on controlled experiments. Culture and antimicrobial susceptibility testing of the pathogen is not necessarily a justifiable basis for selecting the antimicrobial agent to be used in individual cows, and the response to treatment of clinical mastitis in two recent studies84,85 was unrelated to the results of in vitro susceptibility tests.
Antimicrobial agents are usually selected based on availability of labeled drugs, clinical signs in the cow, milk culture results for previous mastitis episodes in the herd, experience of treatment outcome in the herd, treatment cost and withdrawal times for milk and slaughter. Many veterinarians and researchers have also recommended the use of susceptibility testing to guide treatment decisions. The validity of agar diffusion susceptibility breakpoints derived from humans in the treatment of bovine mastitis has not been established and is extremely questionable because bovine mastitic milk pH, electrolyte, fat, protein and neutrophil concentrations, growth factor composition and pharmacokinetic profiles differ markedly from those of human plasma.86 Moreover, antibiotics are distributed unevenly in an inflamed gland, and high antibiotic concentrations can alter neutrophil function in vitro and therefore have the potential to inhibit bacterial clearance in vivo.
Adequate databases of in vitro minimum inhibitory concentration (MIC) values for clinical mastitis pathogens are currently unavailable, although adequate databases are available for subclinical mastitis isolates. Although we have a good knowledge of the pharmacokinetics of many parenteral antibiotics used to treat clinical mastitis, most pharmacokinetic data have been obtained in healthy cattle and it has not been determined whether pharmacokinetic values in healthy cows are the same as those in cows with clinical mastitis. In addition, pharmacokinetic values for many of the intramammary antibiotics used to treat clinical mastitis are unknown, and we have a limited understanding of the pharmacodynamics of antibiotics in treating mastitis. More importantly, the breakpoints currently recommended for all parenterally and almost all intramammarily administered antibiotics are based on achievable serum and interstitial fluid concentrations in humans after oral or intravenous antibiotic administration. The relevance of these breakpoints to achievable milk concentrations in lactating dairy cows after intramammary, subcutaneous, intramuscular or intravenous administration is dubious at best.86
Results from field studies are available to evaluate the validity of susceptibility breakpoints in guiding treatment of cows with clinical or subclinical mastitis. The results from these field studies suggest that the following antibiotics may have valid (but not necessarily optimal) breakpoints for treating clinical or subclinical mastitis associated with specific bacteria: parenteral penicillin G for subclinical S. aureus infections, intramammary cephapirin for clinical Streptococcus spp. infections, and parenteral trimethoprim– sulfadiazine for clinical E. coli infections. Of these three antibiotics, the breakpoints for penicillin G and cephapirin have only been validated for bacteriological cure, whereas the breakpoint for trimethoprim– sulfadiazine is validated for clinical cure.84 Because duration of infection before treatment, antibiotic dosage, dosage interval and duration of treatment influence treatment outcome, many more field studies must be completed to validate the currently assigned antibiotic breakpoints for pathogens causing clinical mastitis.
To properly utilize the known pharmacokinetics of parenterally and intramammarily administered drugs, it is necessary to know something about their diffusion into mammary tissue, the degree of binding of a drug to mammary tissues and secretions, the ability to pass through the lipid phase of milk and the degree of ionization. All of these factors influence the level of the antibiotic in the mammary gland. For lactating cows the preferred treatment is one that maintains a MIC for 72 hours without the need for multiple infusions and without prolongation of the withdrawal time. The most successful antimicrobial agents for dry period treatment are those that persist longest in the udder, preferably as long as 8 weeks. These characteristics depend on the release time from the transport agent in the formulation, and the particle size and diffusion capabilities of the antibiotic.
The formulation of the preparation will affect the duration of the maintenance of the MIC. The third-generation cephalosporins (such as ceftiofur) and fluoroquinolones are the drugs of choice for use in cases in which the infection may be associated with either a Gram-positive or Gram-negative organism; however, these antimicrobial agents may not be able to be used to treat mastitis in some countries. Mixtures of penicillin and an aminoglycoside are also in common use for this purpose. Penicillin G and penethamate are favored for Gram-positive infections.
Of special importance are the beta-lactamase-producing strains of S. aureus, against which beta-lactam penicillins are ineffective; cloxacillin is a commonly used and effective intramammary formulation for these strains of S. aureus. The drugs that have the best record of diffusion through the udder after intramammary infusion are penethamate, ampicillin, amoxicillin, erythromycin and tylosin. Those of medium performance are penicillin G, cloxacillin and tetracyclines. Poor diffusers, which have a longer half-life in the udder because they bind to protein, include streptomycin and neomycin. Streptomycin is not much used now because of the high level of resistance to it, especially by S. uberis and E. faecalis.
What should be the frequency and duration of treatment?
The fourth decision is the frequency and duration of treatment. The frequency of administration for parenterally administered antimicrobial agents is dependent primarily on their pharmacokinetics and pharmacodynamics. Fluoroquinolones and aminoglycosides are concentration-dependent antimicrobial agents where increasing concentrations at the site of infection increase the bacterial kill rate. Macrolides, beta-lactams, and lincosamides are time-dependent antimicrobial agents where exceeding the minimum inhibitory concentration at the site of infection for a prolonged percentage of the interdosing interval correlates with improved efficacy.87 In contrast, the frequency of administration for intramammary formulations is dependent primarily on the milking schedule, in that these agents are primarily cleared by milk removal. For example, the clearance of pirlimycin is strongly and positively correlated (r = 0.97) to 24-hour milk production at the time of dosing.88 With all intramammary formulations being licensed based on the results of studies of twice-daily milking, the recent industry trend in some parts of the world towards thrice-daily milking has created uncertainty as to whether intramammary treatment should be repeated after every milking, or even whether once-a-day intramammary administration is as efficacious as twice- or thrice-daily administration.
Recent studies have confirmed long held beliefs that appropriate antimicrobial therapy (commonly called extended or aggressive antimicrobial therapy) for 5–8 days is much more effective in treating intramammary infections than label intramammary therapy (2–3 d). In other words, increasing the duration of antimicrobial administration increases treatment efficacy.89-92 Extended antimicrobial therapy is opposed by producers because such treatment may be off-label and results in a longer milk withhold time, and consequently the amount of milk that has to be discarded. Extended therapy is opposed by dairying administrators because of the inevitable increase in the number of infringements of health regulations relating to antibiotic residues in milk. The inappropriately short treatment duration for most intramammary products has been a major hindrance to developing effective antimicrobial treatment protocols.
Intramammary antimicrobial therapy
For reasons of convenience and efficiency, antimicrobial udder infusions are in common use for the treatment of certain causes of mastitis in lactating cows, and for dry cow therapy. For example, the cure rate of S. agalactiae using intramammary infusions in lactating cows exceeds 95%. Disposable tubes containing suitable antimicrobials in a water-soluble ointment base are ideal for dispensing and for the treatment of individual cows. Multiple-dose bottles containing aqueous infusions are adequate, and much cheaper per dose when large numbers of quarters are to be treated, but repeated use of the same container increases the risk of contamination. The degree of diffusion into glandular tissue is the same when either water or ointment is used as a vehicle for infusion; the duration of retention within the gland depends on the vehicle.
Most antimicrobial agents currently available in the USA in commercial intramammary infusion products are active against the staphylococci and streptococci,93 with cephapirin (a first-generation cephalosporin) having good activity against coliform bacteria, and ceftiofur (a third-generation cephalosporin) having excellent activity against coliform bacteria.94 Until recent years the emphasis was on the elimination of Gram-positive cocci from the udder, but Gram-negative infections, especially E. coli, have increased in prevalence to the point where a broad-spectrum preparation is almost essential for both lactation and dry period treatments.
The choice of antimicrobial agents for intramammary infusion should be based on:
Strict hygiene is necessary during treatment to avoid the introduction of bacteria, yeasts and fungi into the treated quarters; the use of a short cannula that just penetrates the external sphincter is preferred as it is less likely to introduce bacteria and leaves more of the keratin plug in place in the streak canal; the keratin plug has antimicrobial properties itself. Care must be taken to insure that bulk containers of mastitis infusions are not contaminated by frequent withdrawals and that individual, sterilized teat cannulas, usually part of commercial, single-dose ointment tubes, are used for each quarter. Bulk treatments are best avoided because of the high risk of spread of pathogens.
Infusion procedure
The teats must be cleaned and sanitized before infusing the quarter in order to avoid introduction of infection. The following steps are recommended:
• Dip teats in an effective germicidal product. Allow 30 seconds contact time before wiping teats with an individual disposable towel (one towel/cow, use one corner of the towel for each teat)
• Thoroughly clean and disinfect each teat end with cotton soaked in 70% alcohol. Use a separate piece of cotton for each teat
• Prepare teats on the far side of the udder first, followed by teats on the near side
• Treat quarters in reverse order; near side first, far side last
• Insert only the tip of the cannula into the teat end (partial insertion). Do not allow the sterile cannula to touch anything prior to infusion. Most approved dry cow infusion products (and lactating tubes, too) are marketed with a dual cover that can be used for partial or full insertion
• Dip teats in a germicidal product after treatment
• Identify treated cows and remove them from the milking herd to prevent antimicrobials from entering the milk supply.
Diffusion of infused intramammary drugs is often impeded by the blockage of lactiferous ducts and alveoli with inflammatory debris. Complete emptying of the quarter by the parenteral injection of oxytocin (10–20IU intramuscularly) followed by hand-stripping of affected quarters before infusion has been recommended in cases of clinical mastitis, but efficacy studies are lacking, the volume stripped is usually small and the procedure is painful to the cow. If stripping is performed, the intramammary infusion is given after the last stripping of the day has been done, avoiding any further milking of the gland until the next milking. Our current knowledge can be summarized by the following: ‘To strip or not to strip, that is the question.’
Parenteral antimicrobial therapy
This should be considered in all cases of mastitis in which there is an abnormal gland or abnormal cow (fever, decreased appetite, or inappetence). The systemic reaction can usually be brought under control by standard doses of antimicrobial agents but a bacteriological cure of the affected glands is seldom achieved because of the relatively poor diffusion of the antimicrobial from the blood into the milk. However, the rate of diffusion is greater in affected than in normal quarters. Parenteral treatment is also recommended when the gland is markedly swollen and intramammary infusions are unlikely to diffuse to all parts of the glandular tissue. To achieve adequate therapeutic levels of an antimicrobial in the mammary gland by parenteral treatment it is necessary, for the above reasons, to use higher than normal dose rates daily for 3–5 days. Milk from treated cows must be withheld from the bulk tank for the stated period of time of that antimicrobial following the date of last treatment.
Treatment of lactating quarters
There are three situations to consider: the emergency single case of clinical mastitis requiring immediate treatment, the herd with a problem of too many clinical cases or intractable cases, but where the identity of the pathogen is known, and the cow with subclinical mastitis.
Emergency treatment when the type of infection is unknown
Cases of acute and peracute mastitis (abnormal cow) in lactating cows, and in dry cows close to calving, are serious problems for the field veterinarian. The need for treatment is urgent; it is not possible to wait for the results of laboratory tests to guide the selection of the most appropriate antibiotic. Clinical findings, season of the year and management practices may give a broad hint as to the specific bacterial cause, but in most such circumstances it is necessary to use a broad-spectrum approach to treatment.95 Parenteral therapy with oxytetracycline (administered intravenously to increase bioavailability and therefore plasma and milk concentrations), a potentiated sulfonamide or similar broad-spectrum antimicrobial agent should be supplemented with intramammary infusion with a beta-lactamase-resistant antimicrobial such as a first-generation cephalosporin (cephapirin), a third-generation cephalosporin (e.g. ceftiofur), penicillin G–neomycin combination or other approved broad-spectrum intramammary infusion.96 Parenteral ceftiofur is not effective in clinical mastitis episodes that have abnormal secretion or abnormal gland and secretion.97
Field studies show that, in herds in which clinical mastitis is often caused by environmental pathogens, intravenous administration of oxytetracycline, intramammary infusion of cephapirin and supportive therapy (including intravenous administration of flunixin meglumine or fluids) produces a higher rate of clinical and bacteriologic cure than supportive treatment alone.96 In addition, antimicrobial treatment is more effective than supportive treatment alone.86 In cows with clinical mastitis caused by E. coli the use of procaine penicillin G IM was no more effective than not using antimicrobial agents;98 this result is expected based on penicillin’s Gram-positive spectrum of activity. Knowledge of the likely causative agent is therefore helpful when making decisions about therapy of clinical mastitis episodes during lactation.
Provision of other supportive therapy such as fluids and electrolytes is also crucial to the survival of the cow and minimization of the severity of the mastitis and extent of permanent injury to the udder. The efficacy of frequent stripping, with or without intramammary infusion, is uncertain. NSAIDs decrease pain associated with an abnormal gland; in addition, they enhance recovery and reduce fever in severe cases.
Treatment when the infecting organism is known
A common situation encountered by a bovine practitioner is the dairy herd that has had an outbreak of clinical mastitis or has received a warning notice from the milk processor that the bulk milk SCC or bacterial count is above acceptable limits. The situation calls for a complete mastitis control program, including conducting an investigation to determine the causative bacteria present, the source of the infection, hygiene in the milking parlor and the importance of risk factors such as milking machine management, plus recommended antimicrobial preparations selected on the basis of the causative agent. Treatment of a number of identified subclinical cases at the commencement of the program, and of individual cases subsequently, can be based on the known common infection in the herd. Among Gram-positive cocci, the response to antimicrobial agents is excellent for streptococci. For staphylococci a cure rate of 65% is about the best that can be expected, and unless there are good reasons for doing otherwise it is recommended that treatment be postponed until the cow is dry. Standard treatments for lactating cows include penicillin alone (100000 units) or in combination with streptomycin (1 g) or neomycin (500 mg), and a combination of ampicillin (75 mg) and sodium cloxacillin (200 mg). Acid-resistant penicillins, e.g. phenoxymethylpenicillin, are probably best not used as mammary infusions because of their ability to pass through the human stomach, thus presenting a more serious potential threat to humans drinking contaminated milk. Because of the widespread and often indiscriminate use of penicillin, a large part of the mastitis that occurs is associated with penicillin-resistant bacteria, especially S. aureus. Treatment programs need to take this into account when recommendations are made about the antibiotic to be used.
Intramammary infections associated with environmental streptococci that manifest signs of clinical mastitis are usually acute but only moderately severe. In most of these cases the streptococci are sensitive to antimicrobial agents, and they often recover spontaneously with good management and nursing care. If not, they usually respond well to therapy. Bacteriological cure rates of 60–65% can be expected following a single intramammary infusion of a cephalosporin product. In one randomized controlled field trial of clinical mastitis associated with Streptococcus spp. or coliform bacteria, the clinical cure rate by the tenth milking was significantly higher when intramammary cephapirin, intravenous oxytetracycline, or both, were used along with supportive therapy (oxytocin and stripping of affected glands and, in severely affected cows, the use of flunixin meglumine and fluids) compared to supportive treatment alone.99 These results indicate that, in herds in which clinical mastitis is often associated with environmental pathogens, antimicrobial therapy and supportive therapy may result in a better outcome than supportive therapy alone.
Treatment of subclinical mastitis
It is generally considered not advisable to treat subclinical mastitis during lactation. However, it is important to consider the causative organism and the udder health status of the herd. There are several situations in which lactational therapy of subclinical mastitis is indicated; for example, herds with S. agalactiae infections should consider several approaches to therapy during lactation. S. agalactiae infections respond well to therapy during lactation, with cure rates of 80–100% expected.59 All approved intramammary therapy preparations are efficacious, including penicillin, cephalosporins, cloxacillin and erythromycin. In herds with a high prevalence of S. agalactiae mastitis, blitz therapy can be used for eradication of the pathogen, increased milk production and reduced penalties for high SCCs. There is, however, a risk of residue violation, problems with disposal of milk from treated cows and considerable costs involved. It is also important to insure that standard mastitis control procedures, such as postmilking teat disinfection and blanket dry cow therapy, have been implemented. The benefit:cost ratios for various approaches to blitz therapy of S. agalactiae infected herds have been studied.59 The prevalence of infected cows, and their stage of lactation, are important determinants of the type of program selected.
Therapy of cows with subclinical mastitis due to S. aureus during lactation is much less rewarding. Under field conditions, cases of S. aureus are difficult to cure during lactation. Reported cure rates following intramammary therapy are between 15% and 60%. Lactational therapy of subclinical S. aureus mastitis using intramuscular penicillin along with intramammary amoxicillin infusion, compared with the intramammary infusion alone, increased the cure rate to 40%, which represented a doubling of the cure rate with intramammary therapy alone.48 If treatment by this method is used in combination with data on the age of cow, stage of lactation, duration of infection and level of SCC, the economic benefit of treating some cases of S. aureus mastitis during lactation may be attractive.
Subclinical infections associated with environmental streptococci, and occasionally by coliform organisms, can be found in moderate numbers in some herds.56 Although spontaneous cure rates are higher with these environmental infections, individual cows may merit treatment during lactation. In these cases, the previously listed factors should be used, and are important in the selection of cases to be treated.
Prepartum antibiotic treatment of heifers is of benefit in herds experiencing a high incidence of clinical mastitis in recently calved heifers. Coagulase-negative staphylococci are frequently isolated from late-gestation heifers, and intramammary treatment with sodium cloxacillin (200 mg) or cephapirin sodium (200 mg) 7 days before expected parturition is highly effective and economically beneficial.100
Anti-inflammatory agents
NSAIDs have been evaluated for the treatment of field and experimental cases of acute and peracute mastitis. NSAIDs have beneficial effects on decreasing the severity of clinical signs based on changes in rectal temperature, heart rate, rumen motility and pain associated with the mastitis, and are routinely administered as part of the initial treatment of cattle with severe clinical mastitis and marked systemic signs. On the basis of one comparative study, NSAIDs appear to ameliorate systemic abnormalities to a greater degree than corticosteroids.101 The strongest evidence available to support the administration of NSAIDs is available for ketoprofen and phenylbutazone.
Ketoprofen at 2 g intramuscularly once daily combined with sulfadiazine and trimethoprim intramuscularly given daily to cows with acute clinical mastitis, and complete milking of affected quarters several times daily, significantly improved survival and milk production compared to cows not receiving the NSAID.102 A re-analysis of the published results indicated that phenylbutazone at 4 g intramuscularly once daily combined with sulfadiazine and trimethoprim intramuscularly given daily to cows with acute clinical mastitis significantly improved the percentage of cows with milk production returning to more than 75% of previous levels compared to cows not receiving the NSAID.103 However, intramuscular administration of phenylbutazone is not currently recommended because of the potential for myonecrosis. Dipyrone at 20 g intramuscularly once daily in the same study was not effective.103
There is no evidence that treatment of clinical cases with NSAIDs alters the inflammatory response in the udder,101 although pretreatment of cattle with experimentally induced mastitis does alter the local (glandular) inflammatory response to infection. Flunixin meglumine concentrations are low in milk, which is consistent with its properties as a weak acid, which has difficulty crossing the blood–milk barrier.104 Flunixin meglumine (2 mg/kg, intravenously, twice 24 h apart) did not alter the survival rate of dairy cows with severe E. coli or S. uberis mastitis compared to intravenous administration of 45 L of isotonic crystalloid fluids.105 The one-time administration of 1 g of flunixin meglumine intravenously or 4 g of phenylbutazone intravenously, along with intramammary infusion of gentamicin (150 mg) at 12-hour intervals for four treatments, had no significant beneficial effect in cows with acute toxic mastitis associated with E. coli and Klebsiella spp.106 However, the results of this study do not indicate a lack of effectiveness of flunixin meglumine or phenylbutazone because it is difficult for one dose of any NSAID to have a detectable effect on clinical signs in naturally occurring mastitis cases.
Supportive therapy
Supportive treatment, including the intravenous administration of large quantities of isotonic crystalloid fluids, is indicated in cattle with severe systemic illness. Large volumes of isotonic crystalloid fluids can be rapidly administered under pressure at 0.5 L/min through a 12-gauge catheter in the jugular vein, using a 7.5 L garden weed killer spray pump.105 The administration of hypertonic saline followed by immediate access to drinking water is a practical method of providing fluid therapy to cows with severe mastitis, especially peracute coliform mastitis.107 A dose of 4–5 mL/kg body weight (BW) of 7.5% saline is given intravenously over 4–5 minutes.108 This is usually followed by the animal consuming large quantities of water. Circulating blood volume is increased and there is mild strong ion (metabolic) acidosis, improved renal function and changes in calcium and phosphorus homeostasis when compared to cows given a similar volume of 0.9% NaCl. Fluid therapy is covered extensively in Chapter 2.
Adjunctive therapy
Cytokines may be useful as adjunctive therapy with existing antimicrobials to improve therapeutic efficacy, particularly in lactating cows.109 Cytokines are natural regulators of the host defense system in response to infectious diseases. The combination of a commercial formulation of cephapirin with recombinant interleukin-2 consistently improved the cure rate of treating S. aureus mastitis by 20–30% compared to use of the antimicrobial alone.109
Magnitude of response to therapy
The treatment of some causes of mastitis can be highly effective in removing infection from the quarter and returning the milk to normal composition. However, the yield of milk, although it can be improved by the removal of congestion in the gland and inflammatory debris from the duct system, is unlikely to be returned to normal in severe clinical cases, at least until the next lactation. The degree of response obtained depends particularly on the causative agent, the speed with which treatment is commenced, and other factors described above. A ‘cure’ may mean disappearance of clinical signs, elimination of the infectious cause, or both of those plus return to normal function and productivity. Which of these is the objective in any particular case or herd will influence the decisions to be made about treatment in an individual case of the disease.
Failure to respond to therapy of the lactating cow may be due to:
Dry cow therapy
Dry cow therapy is the use of intramammary antimicrobial therapy immediately after the last milking of lactation and is an important component of an effective mastitis control program.110 Intramammary infusions at drying off decrease the number of existing infections and prevent new infections during the early weeks of the dry period. Dry cow therapy should be routinely administered and remains one of the cornerstones of an effective mastitis control program. Blanket dry cow therapy is treatment of all four quarters at drying off, compared to selective dry cow therapy based on treatment of only those quarters that are infected. When subclinical mastitis is very low in some herds, selective dry cow therapy can be considered, but nearly all herds use blanket dry cow therapy. The problem with selective dry cow therapy is the accuracy of available indirect tests to ‘select’ cows for treatment or nontreatment. Currently available indirect tests are not sufficiently accurate (the exception being quarter milk cultures) to be used as a basis for selective dry cow therapy.
Intramammary infusions approved for dry cow therapy contain high levels of antimicrobial agents in a slow-release base that maintains therapeutic levels in the dry udder for long periods of time. Most dry cow therapy infusion products are intended to eliminate existing infections due to S. aureus and S. agalactiae at drying off and to prevent new infections due to the same pathogens and environmental streptococci in the early dry period.
In herds with a high prevalence of contagious mastitis, dry cow therapy has been efficacious and economically beneficial in reducing the prevalence of intramammary infections. The consistent application of effective mastitis control procedures has reduced the prevalence of contagious pathogens and the bulk tank milk SCC (<300000 cells/mL) and owners of these herds questioned dry cow therapy because of the economics and the concerns of residues in the milk. Field trials in herds with a low prevalence of contagious mastitis indicate that dry cow therapy at the end of lactation increased 17-week milk production during the subsequent lactation and was economically beneficial compared to not treating them.111 However, in the subsequent lactation, the incidence of clinical mastitis was not reduced nor were the SCCs significantly different from those of cows not treated at the end of lactation.
The most effective time to treat subclinical intramammary infections is at drying off. Dry cow therapy has the following advantages over lactation therapy:
• The cure rate is higher than that achieved by treatment during lactation
• A much higher dose of antimicrobial can be used safely
• Retention time of the antimicrobial in the udder is longer
• The incidence of new infections during the dry period is reduced
• Tissue damage by mastitis may be regenerated before parturition
• Clinical mastitis at calving may be reduced
• The risk of contaminating milk with antimicrobial residue is reduced.
Selection of a suitable dry period treatment should take into account the fact that Gram-negative infections are not common at that time because of the high concentration of lactoferrin in the dry secretions. Accordingly attention should be directed at the inclusion of a potent antibiotic against Streptococcus spp., beta-lactamase-producing S. aureus, and A. pyogenes. Cloxacillin, nafcillin, and cephalosporins are popular for the purpose; for example, a recommended treatment is cephapirin or sodium cloxacillin in a slow-release base with an expected cure rate of 80% against streptococci and 60% against S. aureus.
Most dry cow preparations maintain an adequate minimum concentration in the quarter for about 4 weeks, but some persist for 6 weeks. There is little, if any, value in treating cows again before the due calving date. There is always a possibility of introducing infection while infusing an intramammary preparation and farmers are reluctant to break the teat canal seal, but it may be necessary to do so if summer mastitis is prevalent in the area.
Prepartum antimicrobial therapy in heifers
Intramammary infusion of a cephapirin dry cow therapy preparation into pregnant heifers 10–12 weeks prepartum eliminated over 90% of the intramammary infection due to S. aureus, Streptococcus spp., coagulase-negative staphylococci spp. and coliforms. The SCCs of cured quarters were comparable to uninfected control quarters after parturition.112 At parturition, 24% of treated quarters were positive for the antimicrobial; however, no quarters were positive at 5 days postpartum.
Antimicrobial residues in milk and withholding times
Label instructions must be followed to insure that drug residues do not occur, especially from cows with a shorter than normal dry period. Antibiotic residue testing of the milk of a recently calved cow can be done if there is a suspicion of residues, but this is a misuse of a test designed for bulk tank milk testing and therefore suffers from problems with sensitivity and specificity.
Treatment and control of mastitis accounts for the largest percentage of antimicrobial use on dairy farms. Following treatment by the intramammary or parenteral route, the concentration of antimicrobial agents in the milk declines over time to levels that are considered safe and tolerable for humans. The duration of time for the concentrations to decline to acceptable limits is known as the withholding time or the withdrawal period during which the milk cannot be added to the bulk tank supply but must be withheld and discarded. The presence of residues in milk is a major public health concern that adversely affects the dairy industry, the practicing veterinarian and the perception the public has of the safety of milk for human consumption. The public perception of the safety of milk is crucial and veterinarians have a responsibility to respond to these concerns through public education and quality control of milk production.
Other serious consequences of antimicrobial residues in milk are their effect on the manufacture of dairy products and the potential development of antimicrobial sensitivity syndromes in humans. In most countries the maximum intramammary dose of antimicrobial agents is limited by legislation and the presence of detectable quantities of antimicrobial agents in milk constitutes adulteration. Attention has also been directed to the excretion of antimicrobial agents in milk from untreated quarters, after treatment of infected quarters and after their administration by parenteral injection or by insertion into the uterus. The degree to which this excretion occurs varies widely between animals and in the same animal at different points in the lactation period, and differs from one antibiotic to another. Milk from cows subjected to dry period treatment is usually required to be withheld for 4 days after calving. The use of any dry period treatments in lactating cows causes prolonged retention of the antimicrobial in milk and is a most serious violation of the legislation.
Veterinarians have the responsibility to warn farmers of the need to withhold milk, and both should be aware of the withholding times of each product, details of which are usually required to be included on its label. Marking the cow in some way to remind the farmer, by application of a leg band or placing dye on the udder, is advisable.
Antimicrobial residue tests
Several cowside tests are available to detect antimicrobial residues in the milk of cows that have been treated for mastitis.113 The goal of cowside testing is to assist in the production of high-quality, antimicrobial-residue-free milk from dairy herds. To be consistent with the intent of a quality assurance program, cowside testing would be used only on cows recently treated with antimicrobial agents and only after appropriate milk withholding times had been followed. The ideal test would have a high sensitivity and high specificity.
Most of the cowside screening tests for antimicrobial residues are imperfect because of a high rate of false-positive results when used on field samples. The direct costs to producers can be high because of the unnecessary disposal of milk and imposition of fines and penalties. False-positive results also cause the unnecessary culling of some cows, and concern about the interpretation of positive assay results, the appropriateness of withholding periods and the safety of milk creates mistrust among consumers, producers, veterinarians and regulatory personnel. The specificity of four commercially available tests ranged from 0.78–0.95.114 None of the test kits has been validated to meet performance standards for sensitivity and specificity. This applies to individual cow samples, bulk tank milk samples and tanker truck samples.
The presence of naturally occurring bactericidal products in the milk of cows with acute and convalescent mastitis is the most likely cause of the false-positive results of the tests (such as Delvo-test®) that are based on bacterial growth inhibition of beta-lactam antimicrobial agents.113 Immunoglobulins, complement, lysozyme, lactoferrin and phagocytic cells are products of inflammation in the milk of cows with mastitis that can inhibit bacterial growth. The milk from cows with experimental endotoxin-induced mastitis is at increased risk for false-positive assay results using commercial residue tests.114 The incidence of false-positive results is very low in milk from cows that have not had a history of mastitis or antimicrobial therapy.115 Naturally occurring bactericidal products in mastitic milk can be removed by heating at 82°C for 5 minutes; this temperature does not denature antimicrobial agents present in milk.116 Heat treatment therefore appears to provide a very practical way to reduce false-positive results on milk from individual cows.
A sample of milk can be submitted for antimicrobial residue testing up to three times. First, a producer may test a sample from a specific cow at the end of her withdrawal period. Second, milk is sampled at the tanker truck level. Third, should the tanker truck sample have positive results, bulk tank milk samples from each dairy herd that contributed to that tanker truck are tested.
There is a need for validation of the diagnostic assays used to detect antimicrobial residues in milk.117 Acceptance of assays for regulatory purposes must be based on protocols that include field estimates of assay performance before the assays are used by the public. Three strategies have been suggested to balance public health concerns with economic concerns of dairy producers caused by false-positive results:
• Retest samples that yield positive results with a confirmatory assay of specificity close to 100%. Only those samples that also yield positive results on the second assay are considered to be positive for violative residues
• Recalibrate the assay to increase specificity. This will usually result in loss of sensitivity
• Use an alternative assay of higher specificity.117
It is suggested that regulatory monitoring of residues at a national level will be best served by use of a combination of at least two assays: initial screening with a highly sensitive and inexpensive assay followed by confirmation testing with an assay of high specificity (> 99%) that can quantify the concentration of the antimicrobial residue. All tanker samples that yield positive results with a screening assay should be rechecked with a quantitative assay. If the quantitative assay detects a concentration greater than the safe level, safe concentration or tolerance level, only then would the milk be deemed to have violative residue and fines and penalties be imposed.117 The complex dynamics of current milk residue tests discourage practitioners from recommending testing procedures to dairy producers.118
As an approximate guide, the recommended periods for which milk should be withheld from sale after different methods of antimicrobial administration are (in times after last treatment):
• Udder infusion in a lactating cow – 72 hours
• Parenteral injection, one only – 36 hours
• Parenteral injections, series of – 72 hours
• Antimicrobial agents parenterally in long-acting bases – 10 days
• Intrauterine tablet – 72 hours
• Dry cow intramammary infusion – to be administered at least 4 weeks before calving and the milk withheld for at least 96 hours afterwards.
Permanently drying off chronically affected quarters
If a quarter does not respond to treatment and is classified as incurable, the affected animal should be isolated from the milking herd or the affected quarter may be permanently dried off by inducing a chemical mastitis. Historically used methods, arranged in decreasing order of severity, are infusions of:
• 30–60 mL of 3% silver nitrate solution
• 20 mL of 5% copper sulfate solution
• 100–300 mL of 1:500, or 300–500 mL of a 1:2000 acriflavine solution.
If a severe local reaction occurs, the quarter should be milked out and stripped frequently until the reaction subsides. If no reaction occurs, the quarter is stripped out 10–14 days later. Two infusions of these solutions may be necessary.
The best method for permanently drying off a quarter is infusion of 120 mL of 5% povidone–iodine solution (0.5% iodine) after complete milk-out and administration of flunixin meglumine (1 mg/kg BW, intravenously). This causes permanent cessation of lactation in the quarter but does not alter total milk production by the cow. If the goal is chemical sterilization, then three daily infusions of 60 mL of chlorhexidine suspension should be administered after complete milk-out. The majority of treated cows (5/7) returned to milk production in the quarter in the subsequent lactation.119 The infusion of 60 mL of chlorhexidine, followed by milking out at the next subsequent milking and repeat of the infusion 24 hours after the initial treatment is also effective in making infused quarters nonfunctional within 14–63 days.120 Histological evaluation of the infused quarters revealed that secretory tissues had involuted to a nonsecretory state and appeared similar to blind or nonfunctional quarters. However, as noted above, milk production may return in the gland in the subsequent lactation.
Burton JL, Erskine RJ. Immunity and mastitis: some new ideas for an old disease. Vet Clin North Am Food Anim Pract. 2003;19:1-46.
Constable PD, Morin DE. Treatment of clinical mastitis: using antimicrobial susceptibility profiles for treatment decisions. Vet Clin North Am Food Anim Pract. 2003;19:139-156.
Erskine RJ, Wagner SA, DeGraves FJ. Mastitis therapy and pharmacology. Vet Clin North Am Food Anim Pract. 2003;19:109-138.
Pyörälä S. Indicators of inflammation in the diagnosis of mastitis. Vet Res. 2003;34:565-578.
Roberson JR. Establishing treatment protocols for clinical mastitis. Vet Clin North Am Food Anim Pract. 2003;19:223-234.
Schukken YH, et al. Monitoring udder health and milk quality using somatic cell counts. Vet Res. 2003;34:579-596.
Sears PM, McCarthy KK. Diagnosis of mastitis for therapy decisions. Vet Clin North Am Food Anim Pract. 2003;19:93-108.
1 Fox LK, et al. J Dairy Sci. 1995;78:1619.
2 Pankey JW, et al. J Dairy Sci. 1991;74:1550.
3 Nickerson SC, et al. J Dairy Sci. 1995;78:1607.
4 Miltenburg JD, et al. Vet Rec. 1996;139:204.
5 Firat MZ. Livestock Prod Sci. 1993;36:311.
6 Bartlett PC, et al. Prev Vet Med. 1992;12:59.
7 Sargeant JM, et al. Can Vet J. 1998;39:33.
8 Simpson RB, et al. J Anim Sci. 1995;73:1552.
9 Fox LK, Gay JM. Vet Clin North Am Food Anim Pract. 1993;9:475.
10 Aarestrup FM, et al. Acta Vet Scand. 1995;36:475.
11 Smith KL, Hogan JS. Vet Clin North Am Food Anim Pract. 1993;9:489.
12 Trinidad P, et al. J Dairy Sci. 1990;73:107.
13 Aarestrup FM, Jensen NE. J Dairy Sci. 1997;80:312.
14 Robertson JR, et al. J Dairy Sci. 1994;77:958.
15 Oliver SP, et al. J Dairy Sci. 1990;73:351.
16 Paape MJ, et al. J Dairy Sci. 1992;75:1849.
17 Erskine RJ, et al. J Am Vet Med Assoc. 1988;192:761.
18 Slettbakk T, et al. Prev Vet Med. 1995;24:235.
19 Zecconi A, et al. J Dairy Sci. 1996;63:361.
20 Shearn MFH, Hillerton JE. J Dairy Res. 1996;63:525.
21 Schreiner DA, Ruegg PL. J Dairy Sci. 2003;86:3460.
22 Erskine RJ. Vet Clin North Am Food Anim Pract. 1993;9:551.
23 Jukola E, et al. J Dairy Sci. 1996;79:838.
24 Shook GE, Schutz MM. J Dairy Sci. 1994;77:648.
25 Philipsson J, et al. Livestock Prod Sci. 1995;41:195.
26 Schutz MM. J Dairy Sci. 1994;77:2113.
27 Grohn YT, et al. J Dairy Sci. 1995;78:1693.
28 Enevoldsen C, et al. J Dairy Res. 1995;62:69.
29 Firat MZ. Livestock Prod Sci. 1993;34:175.
30 Lam TJM, et al. Am J Vet Res. 1997;58:17.
31 Judge LJ, et al. J Dairy Sci. 1997;80:3212.
32 Zdanowicz M, et al. J Dairy Sci. 2004;87:1694.
33 Bartlett PC, et al. J Dairy Sci. 1992;14:195.
34 Lescourret F, et al. J Dairy Sci. 1995;78:2167.
35 Lipman LJA, et al. Vet Microbiol. 1995;48:51.
36 Risco CA, et al. J Dairy Sci. 1999;82:1684.
37 DeGreaves FJ, Fetrow J. Vet Clin North Am Food Anim Pract. 1993;9:421.
38 Miller GY, et al. J Am Vet Med Assoc. 1993;202:1230.
39 Hoblet KH, et al. J Am Vet Med Assoc. 1991;199:190.
40 Steele ML, et al. J Food Prot. 1997;60:1341.
41 Lee CS, et al. J Dairy Res. 1980;47:39.
42 Lescourret F, Coulon JB. J Dairy Sci. 1994;77:2289.
43 Jones GF, Ward GE. J Am Vet Med Assoc. 1990;196:597.
44 Grohn YT, et al. J Dairy Sci. 2004;87:3358.
45 Grohn YT, et al. Prev Vet Med. 2005;71:105.
46 Fenlon DR, et al. Br Vet J. 1995;151:17.
47 Wilson DJ, et al. J Am Vet Med Assoc. 1997;210:1499.
48 Godkin MA, Leslie KE. Aust Vet J. 1993;34:601.
49 Villanueva MR, et al. J Am Vet Med Assoc. 1991;198:1398.
50 Dinsmore RP, et al. J Dairy Sci. 1992;75:2706.
51 Walker M, et al. Proc Am Assoc Bovine Pract. 2005:171.
52 Leslie KE, et al. In: Proceedings of the 3rd International Mastitis Seminar, Tel Aviv, Israel 1995; (S2):66.
53 Waage S, et al. Acta Vet Scand. 1994;35:207.
54 Yazdankhah SP, et al. J Clin Microbiol. 2001;39:3228.
55 Morin DE, Constable PD. J Am Vet Med Assoc. 1998;213:855.
56 Zorah RCW, et al. Vet Rec. 1993;132:208.
57 Gooray R. Vet Microbiol. 1994;42:317.
58 Pyörälä S. Vet Res. 2003;34:565.
59 Emanuelson U, Funke H. J Dairy Sci. 1991;74:2479.
60 Maunsell FP, et al. J Am Vet Med Assoc. 1999;214:1817.
61 Whyte D, et al. J Dairy Res. 2005;72:115.
62 Holdaway RJ, et al. Aust J Dairy Technol. 1996;51:64. 72, 80
63 Ball HJ, Greer D. Vet Rec. 1991;129:507.
64 Milner P, et al. J Dairy Sci. 1996;79:83.
65 Maatje K, et al. Livestock Reprod Sci. 1992;30:239.
66 Nielen M, et al. J Dairy Sci. 1995;78:1039. 1050
67 Hamann J, Zecconi A. Int Dairy Fed Bull. 1998:334.
68 Norberg E, et al. J Dairy Sci. 2004;87:1099.
69 Sischo WM, et al. J Am Vet Med Assoc. 1997;211:470.
70 Smith GW, et al. J Vet Intern Med. 2001;15:394.
71 Bradley KJ, et al. Vet Rec. 2001;148:497.
72 Trostle SS, O’Brien RT. Compend Contin Educ Pract Vet. 1998;20:S65.
73 Franz S, et al. Am J Vet Res. 2004;65:1159.
74 Farr VC, et al. J Dairy Sci. 1996;79:543.
75 Kirk JJ, et al. J Am Vet Med Assoc. 1994;204:1152.
76 Shim EH, et al. J Dairy Sci. 2004;87:2702.
77 Shukken YH, Deluyker HA. J Vet Pharmacol Ther. 1995;18:274.
78 Hallberg JW, et al. In: Proceedings of the 33rd National Mastitis Council Annual Meeting 1994:28.
79 Morin DE, et al. J Am Vet Med Assoc. 1998;212:1423.
80 Pyörälä S, Pyörälä E. J Am Vet Med Assoc. 1998;212:407.
81 Guterbock WM. Proc Annu Conv Am Assoc Bovine Pract. 1994;26:67.
82 Sears PM, McCarthy KK. Vet Clin North Am Food Anim Pract. 2003;19:93.
83 Erskine RJ, et al. Vet Clin North Am Food Anim Pract. 2003;19:109.
84 Constable PD, Morin DE. J Am Vet Med Assoc. 2002;221:103.
85 Hoe FGH, Ruegg PL. J Am Vet Med Assoc. 2005;227:1461.
86 Constable PD, Morin DE. Vet Clin North Am Food Anim Pract. 2003;19:139.
87 McKellar QA, et al. J Vet Pharmacol Ther. 2004;27:503.
88 Whittem T. J Vet Pharmacol Ther. 1999;22:41.
89 Oliver SP, et al. J Dairy Sci. 2004;87:3322.
90 Oliver SP, et al. J Dairy Sci. 2004;87:2392.
91 Deluyker HA, et al. J Dairy Sci. 2005;88:604.
92 Hillerton JE, Kliem KE. J Dairy Sci. 2002;85:1009.
93 Watts JL, et al. J Dairy Sci. 1995;78:1637.
94 Bezek DM. J Am Vet Med Assoc. 1998;212:404.
95 Ziv G. Vet Clin North Am Food Anim Pract. 1992;8:1.
96 Morin DE, et al. J Am Vet Med Assoc. 1998;213:676.
97 Wenz JR, et al. J Dairy Sci. 2005;88:3496.
98 Pyorala SHK, Pyorala EO. J Am Vet Med Assoc. 1998;212:407.
99 Sears PM, et al. Vet Clin North Am Food Anim Pract. 1993;9:445.
100 Olover SP, et al. J Dairy Sci. 2003;86:1187.
101 Wagner SA, Apley MD. Am J Vet Res. 2004;65:64.
102 Shipgel NY, et al. Res Vet Sci. 1994;56:62.
103 Shpigel NY, et al. J Vet Med A. 1996;43:331.
104 Rantala M, et al. J Vet Pharmacol Ther. 2002;25:251.
105 Green MJ, et al. Vet Rec. 1997;140:149.
106 Dascanio JJ, et al. Am J Vet Res. 1995;56:1213.
107 Roeder BL, et al. Am J Vet Res. 1997;58:549.
108 Tyler JW, et al. Am J Vet Res. 1994;55:278.
109 Daley MJ, et al. J Dairy Sci. 1992;75:3330.
110 National Mastitis Council. Dry cow therapy. In Bulletin C-12254. Arlington, VA: National Mastitis Council; 1995.
111 Berry SL, et al. J Am Vet Med Assoc. 1997;211:207.
112 Owens WE, et al. J Vet Med B. 1994;41:90.
113 Sischo WM, Burns CM. J Am Vet Med Assoc. 1993;202:1249.
114 Tyler JW, et al. J Am Vet Med Assoc. 1992;201:1378.
115 Halbert LW, et al. J Food Prot. 1996;59:886.
116 Kang JH, et al. J Dairy Sci. 2005;88:908.
117 Gardner IA, et al. J Am Vet Med Assoc. 1996;209:46.
118 Slenning BD, Gardner IA. J Am Vet Med Assoc. 1997;211:419.