Skeletal Muscle
Functional Anatomy And Organization
Process of muscle excitability and contractility
 Process of excitation–contraction coupling
Process of excitation–contraction coupling
 Sequence of events during muscle contraction and relaxation when stimulated by a nerve
Sequence of events during muscle contraction and relaxation when stimulated by a nerve
Introduction
The muscle cell, like the neuron, is an excitable tissue, i.e. an action potential is generated chemically stored energy which can be transformed into mechanical energy.
There are three different types of muscles in the body: skeletal muscles, cardiac muscles and smooth muscles. Based on certain distinctive features the muscles can be grouped as follows.
Striated versus non-striated muscles
Striated muscle cells show large number of cross-striations at regular intervals when seen under light microscope. Skeletal and cardiac muscles are striated.
Non-striated muscle cells do not show any striations. Smooth muscles or the so-called plain muscles are non-striated.
Voluntary versus involuntary muscles
Voluntary muscles can be made to contract under our will to perform the movements we desire. All skeletal muscles are voluntary muscles. These are supplied by the somatic motor nerves.
Involuntary muscle's activities cannot be controlled at will. Cardiac and all smooth muscles are involuntary muscles. These are innervated by the autonomic nerves.
Functional anatomy and organization
Structural organization of muscle
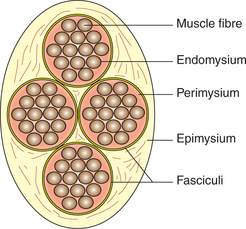
Structurally, the skeletal muscle consists of a large number of muscle fibres and a connective tissue framework organized as (Figs2.3-1 and 2.3-2):
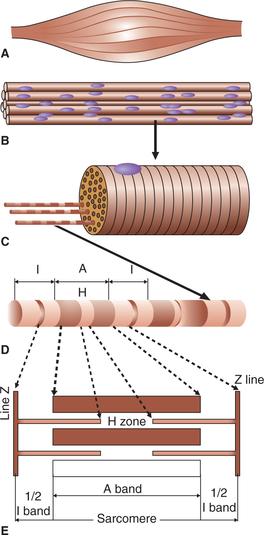
Fig. 2.3-2 Schematic diagram showing structural organization of skeletal muscle: A, muscle belly; B, muscle fibres grouped into fasciculi; C, muscle fibre; D, myofibril and E, arrangement of thick and thin filaments.
• Each muscle fibre is surrounded by a delicate connective tissue called endomysium, which contains large quantity of elastic tissue arranged longitudinally.
• The muscle fibres are grouped into a number of bundles called fasciculi. Each fasciculus is surrounded by a stronger sheath of connective tissue called perimysium.
• All the fasciculi collectively form the muscle belly. The connective tissue that surrounds the entire muscle belly is called epimysium.
• At the junction of the muscle with its tendon, the fibres of endomysium, perimysium and epimysium become continuous with the fibres of the tendon.
• Tendons are fibrous terminal ends of the muscles made up of collagen fibres.
Structure of a muscle fibre
Each muscle fibre is basically a long (1-40 mm), cylindrical (10-100 μm in diameter), multinucleated cell. Its cell membrane is called sarcolemma and the cytoplasm is called sarcoplasm. Like any other cell, in the sarcoplasm are embedded many structures, the nuclei, Golgi apparatus, mitochondria, sarcoplasmic reticulum, ribosomes, and glycogen and occasional lipid droplets. In addition, the sarcoplasm mainly contains number of myofibrils which form the main structure of a muscle fibre. The sarcolemma along with the sarcoplasmic reticulum forms the so-called sarcotubular system
Myofibril
Each muscle fibre consists of a large number of myofibrils which are arranged parallel to each other and run along the entire length of the muscle fibre.
Each myofibril consists of many thick and thin filaments (myofilaments) made up of contractile proteins. The peculiar arrangement of these myofilaments when seen under light microscope gives an appearance of alternate dark and light bands (striations) as described.
Striations of muscle fibres
The dark and light bands result from a difference in the refractive index of its different parts. The arrangement is as below (Fig. 2.3-2D):
• The dark band is called A band (anisometropic to polarized light). It is 1.5 μm in length. In the area of A band the thick (myosin) filaments line up the thin filaments.
• In the centre of each A band there is a lighter H zone where thin filaments do not overlap the thick filaments. (The word H either represents the discoverer, Henson or the hell, which in German means light.)
• In the centre of each H zone is seen an M line, which is more pronounced during muscle contraction.
• The light band is called I band because it is isotropic to polarized light. It is about 1 μm in length. This area contains only thin (actin) filaments.
• Each I band is bisected by a narrow dark Z line (the word Z has been taken from Z Wischenscheibe, which in German means between discs).
• The portion of myofibril between two successive Z lines is called a sarcomere. Thus a sarcomere includes 1/2 I band, +1A band and 1/2 I band, and is about 2.5 μm in length at rest. The sarcomere is the structural and functional unit of the muscle fibre. During muscle contraction the sarcomere reduces in length to 1.5 μm and during stretching of the muscle it increases in length to 3.5 μm.
Thick and thin filaments
The thick and thin filaments (Fig. 2.3-2E) form the contractile apparatus of a striated muscle.
Thick filament
Thick filaments are about twice the diameter of thin filaments. Each thick filament is surrounded by six thin filaments arranged in a regular hexagonal manner. A thick filament is made up of hundreds of molecules of a complex actin-binding contractile protein called myosin.
Structure of myosin molecule. The myosin molecule has a molecular weight of 4,80,000 and is made up of six poly-peptide chains, two heavy chains and four light chains. The two heavy chains wrap around each other to form a double helix, which constitutes the tail and body of the myosin molecule (Fig. 2.3-3B). The light chains combine with the terminal part of the heavy chains to form the globular head of myosin molecule. The myosin molecule present in the skeletal muscle has two heads and is called myosin II (Fig. 2.3-3C). During muscle contraction, the head forms the cross bridging (as described later).
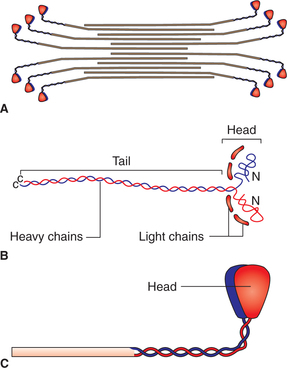
Fig. 2.3-3 Myosin molecules: A, arrangement of myosin molecules in thick filament; B, structure of myosin molecule and C, molecule of myosin II (with two heads).
Arrangement of myosin molecules in a thick filament. In a thick filament, half of the myosin molecules are oriented with their heads in one direction and the remaining half in opposite direction (Fig. 2.3-3A). Because of this arrangement the central portion of a thick filament is devoid of the head portions of the myosin molecules. This accounts for the comparatively lighter H zone seen in the centre of dark A band.
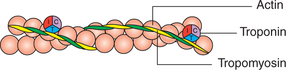
Thin filament
Each thin actin filament is made up of contractile protein molecules (actin) and two types of regulatory protein molecules (tropomyosin and troponin) (Fig .2.3-4).
Actin. About 300-400 actin molecules are present in each thin filament. The actin molecules form a long double helix consisting of two chains of globular units. The globular molecules are G-actin, and the chain formed by them is designated as F-actin.
Tropomyosin. About 40-60 tropomyosin molecules are present in each thin filament. The tropomyosin molecules are long filaments which lie in the groove between the two chains of actin molecules. It covers the binding site of actin where myosin head comes in contact with actin. Thus, it is a regulatory protein which prevents the interaction between actin and myosin filaments.
Troponin. Troponin molecules are small globular units located at intervals along the tropomyosin molecule. The troponin molecule has three subunits:
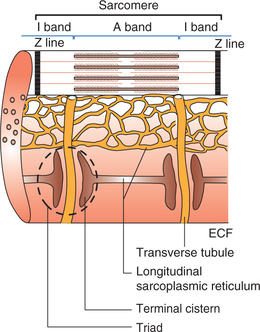
Sarcotubular system
The sarcolemma (cell membrane of muscle cell) along with the sarcoplasmic reticulum (the endoplasmic reticulum of muscle cell) forms a highly specialized system called sarcotubular system. This plays an important role in the internal conduction of depolarization within the muscle fibre. The sarcotubular system is primarily formed by a transverse tubular system (T-system) and a longitudinal sarcoplasmic reticulum (Fig. 2.3-5).
Transverse tubular system (T-system)
The T-system of transverse tubules is formed by the thorough and thorough invagination of sarcolemma into the muscle fibre in the region of junction of A and I bands (Fig. 2.3-5). Since the T-tubules are formed by the invagination of sarcolemma, their lumen contains ECF which surrounds the muscle cell. These T-tubules facilitate rapid transmission of the impulse in the form of action potential from sarcolemma to the myofibrils. The membrane of T-tubules contains voltage-gated Ca2+ channels called dihydropyridine (DHP) receptors (as they get blocked by the drug DHP), through which they activate the longitudinal sarcoplasmic reticulum.
Longitudinal sarcoplasmic reticulum (L-tubules)
The longitudinal sarcoplasmic reticulum is the name given to the sarcoplasmic tubules of the sarcoplasmic reticulum which run in long axis of the muscle fibre forming a closed tubular system around each myofibril. These L-tubules do not open to the exterior like T-tubules. The longitudinal sarcoplasmic tubules on either side of the T-tubule are dilated to form the so-called terminal cisterns. A single T-tubule with the two terminal cisterns lying in close proximity (contiguity) constitute a triad, which is found at the junction of A and I bands. Thus there are two triads in each sarcomere.
The longitudinal tubules store a large quantity of calcium ions. When the action potential reaches the cisterns of L-tubules, these Ca2+ ions are released into the sarcoplasm through the calcium channels present on their membrane. These are called ryanodine receptors as they are kept in open position by ryanodine alkaloid.
Process of muscle excitability and contractility
As we know, the muscle is an excitable tissue, i.e. when stimulated an action potential is produced (electrical phenomenon). The skeletal muscle responds to stimulus by contracting (mechanical phenomenon). The events which link the electrical phenomenon with the mechanical phenomenon is called excitation-contraction coupling phenomenon. These three phenomena which mark the excitability and contractility of the muscle when stimulated by the nerve innervating it are discussed.
Process of muscle excitation
As discussed in the transmission across neuromuscular junction (page 49), an end plate potential (EPP) is developed at the motor end plate by the nerve impulse travelling across the innervating axon. The EPP, as discussed earlier (page 14), is localized and non-propagated. However, when EPP reaches a threshold level it produces an action potential which propagates over muscle fibre surface and into the muscle fibre along the transverse tubules.
Essential features of electrical phenomena
Essential features of electrical phenomena which occur in the muscle fibre (resting membrane potential and action potential) are similar to those occurring in a nerve fibre. However, there are some quantitative differences between the electrical phenomenon occurring in a skeletal muscle fibre and a nerve fibre which are summarized in Table 2.3-1.
Table 2.3-1
Essential features of electrical phenomena in the skeletal muscle fibre and the nerve fibre
| Features | Skeletal muscle fibre | Nerve fibre |
| 1. Resting membrane potential | −90 mV | −70 mV |
| 2. Initial excitation threshold level | 30–40 mV | 15 mV |
| 3. Magnitude of action potential | 120–130 mV | 100–105 mV |
| 4. Duration of spike potential | 2–4 ms | 0.4–2 ms |
| 5. Absolute refractory period | 1–3 ms | 0.4–2 ms |
| 6. Maximum number of impulses conducted | Less (100–200/s) | More (1000/s) |
| 7. The excitability | Less (chronaxie is longer) | More (chronaxie is shorter) |
| 8. Conduction velocity of action potentials | Low (3–5 m/s) | Variable, directly proportional to its diameter. In amyelinated nerve fibre it is up to 120 m/s |
Process of excitation-contraction coupling
The sequence of events by which an action potential in the plasma membrane of a muscle fibre leads to cross-bridge activity (excitation-contraction coupling) is as follows:
• Action potential initiated in the plasma membrane of a muscle fibre spreads rapidly on the surface as well as into the interior of the muscle fibre through the T-tubules.
• When the action potential reaches the tip of T-tubule, it activates the voltage-gated channels called DHP receptors, which are located on the T-tubule membrane (Fig. 2.3-6).
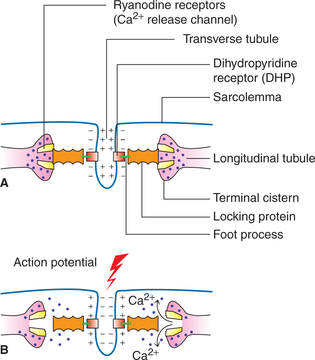
Fig. 2.3-6 Mechanism of release of calcium ions from terminal cistern of longitudinal tubules: A, in resting state Ca2+ release channels (RYR) remain closed due to mechanical interlocking between DHP and RYR and B, during activation state (depolarization of T-tubule). Conformational change in DHP results in opening of RYR and release of Ca2+.
• Activated DHP receptors in turn trigger the opening of Ca2+ release channels located on the terminal cisterns, the so-called ryanodine receptor (RYR).
• Due to opening of calcium release channels (RYR), calcium ions diffuse into the cytoplasm. The concentration of Ca2+ in the intracellular fluid is increased by some 2000 times.
• The Ca2+ ions get attached to troponin-C and start a chain of events (discussed below) which produce contraction. Thus the calcium ions act as linking or coupling material between the excitation and the contraction of the muscle. Hence, the calcium ions are said to form the basis of excitation-contraction coupling.
Process of muscle contraction
Molecular basis of muscle contraction
The process of muscle contraction is initiated by the calcium ions as discussed above. A. F. Huxley and H. E. Huxley in 1954 put forward the sliding theory or ratchet theory to explain how the actin filaments slide over myosin filaments and form the actin-myosin complex during muscular contraction. This theory explains that the sliding of filaments is brought about by a repeated cycle of formation of the cross bridges between the head of myosin and actin molecules.
1. Initiation of cross-bridge cycling
• During resting stage, troponin-I is lightly bound to actin and the tropomyosin molecules are located in the groove between the strands of actin filaments in such a way that they block the myosin binding sites on actin. Thus, during resting stage, no actin-myosin cross bridges are formed. Thus, the troponin-tropomyosin complex so-called relaxing proteins which inhibit the interaction between actin and myosin (Fig. 2.3-7A).
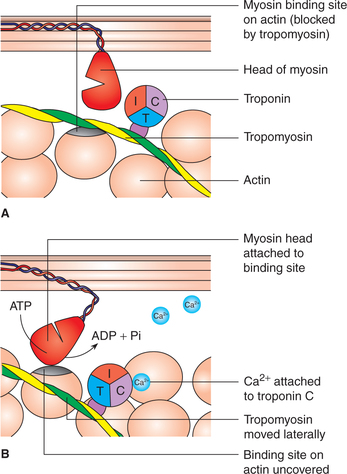
Fig. 2.3-7 Initiation of cross-bridge cycling: A, resting state (myosin binding site on actin is covered by the troponin-tropomyosin complex) and B, on activation Ca2+ binds to troponin C subunit, which results in conformational change and lateral displacement of tropomyosin causing uncovering of binding site for myosin (head of myosin) on actin (initiation of cross-bridge cycle).
• When activation takes place, the Ca2+ ions released into the cytosol from the terminal cisterns of the sarcoplasmic reticulum get attached to troponin-C subunit of the protein troponin. It results in a conformation change which causes the tropomyosin molecule to move laterally, uncovering the binding sites on the actin molecules for head of the myosin molecules. Seven myosin binding sites on the actin filament are uncovered for each molecule of troponin that binds a Ca2+ ion. Thus the cross- bridge cycle is switched on (initiated) by the lateral movement of the tropomyosin (Fig. 2.3-7B).
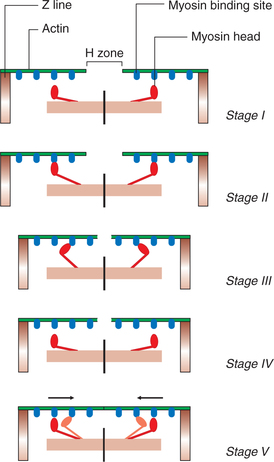
2. Formation of actin-myosin complex (i.e. attachment of myosin head to active site of actin filament)
• The head of myosin molecule binds with adenosine triphosphate (ATP). The ATPase activity of myosin head immediately causes breaking of ATP to adenosine diphosphate (ADP) and Pi cleavage products which remain bound to the myosin head. The head of myosin therefore becomes energized. The activated myosin head extends perpendicularly (at 90° conformation) towards the actin filament and gets attached to actin filament (Fig. 2.3-8) (Stages I and II).
• Formation of the actin-myosin-ADP Pi complex triggers the following events:
3. Detachment of head of a cross-bridge from active site of an actin filament. The release of ADP and Pi allows a fresh ATP molecule to bind to the myosin head. The myosin-ATP complex has a low affinity for actin and, therefore, it results in the dissociation of myosin head from the actin filament (Fig. 2.3-8) (Stage IV).
4. Reactivation of myosin head. The freshly bound ATP molecule splits again and myosin head is reactivated for the next cycle to begin.
Thus with each cross-bridge cycle, there is movement of the actin filament towards the centre of myosin to a small degree. Repeated cross-bridge cycling causes the movement of actin filaments of either side towards the centre of myosin filament of the sarcomere leading to muscle contraction (Fig. 2.3-8) (Stage V).
Changes produced by sliding of thin filaments over thick filaments during muscle contraction
Fig. 2.3-9 shows the following changes produced by sliding of thin filaments over thick filaments during muscle contraction.
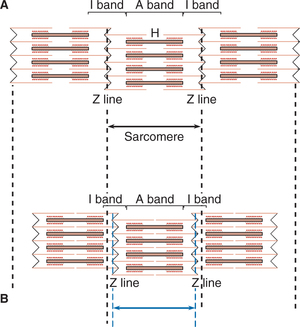
Fig. 2.3-9 Changes produced in a sarcomere by sliding of thin filament (actin) over thick filament (myosin) during muscle contraction: A, relaxed state and B, contracted state.
1. The width of A band remains constant
6. The actin filaments from the opposite end of the sarcomere approach each other and when the muscle shortening is marked, these filaments apparently overlap.
Within a few milliseconds of the action potential the calcium pump transports Ca2+ ions present in the sarcoplasm during contraction, back into the longitudinal portion of the sarcoplasmic reticulum, from where the Ca2+ ions are discharged into the terminal cistern for storage.
Removal of calcium from troponin restores blocking action of the troponin-tropomyosin complex. Myosin cross-bridge cycle closes and muscle relaxes.
Sequence of events during muscle contraction and relaxation when stimulated by a nerve
The events which occur during contraction and relaxation in a skeletal muscle when excited by a nerve are summarized sequence wise.
Nerve excitation
• Stimulation of motor neuron
↓
• Initiation of action potential in motor neuron's axon
↓
Nerve conduction
• Propagation of action potential in the motor nerve
↓
• Impulse reaching at nerve ending (at synaptic button)
↓
Neuromuscular transmission
• Increased permeability of presynaptic membrane to Ca2+ ions
↓
• Inflow of Ca2+ ions from extracellular fluid (ECF) into he nerve terminals
↓
• Release of acetylcholine (ACh) from the microvesicles resent at the nerve terminal
↓
• Diffusion of ACh into the synaptic cleft
↓
• Binding of ACh to receptors on the motor end plate postsynaptic membrane)
↓
• Opening of ACh-gated channels in the motor end plate membrane
↓
• Entry of mainly Na+ ions and to a lesser extent Ca2+ ions through these channels into the muscle fibre
↓
• Development of EPP
Muscle excitation
• Local EPP when reaches a threshold magnitude, voltagegated Na+ channels are opened up at the site
↓
• Generation of action potential (AP) in the muscle fibre by the end plate depolarization
↓
• Propagation of AP in muscle fibre along the surface and into the fibre along the T-tubules
↓
Excitation-contraction coupling
• Release of Ca2+ ions from terminal cistern
↓
• Diffusion of Ca2+ ions into the sarcoplasm
↓
• Binding of Ca2+ ions to troponin C
↓
Muscle contraction (molecular theory)
• Uncovering of binding sites for myosin on actin
↓
• Cross-bridge formation between myosin head and actin
↓
• Angular movement of cross bridges (power stroke)
↓
• Sliding of thin filaments over thick filaments
↓
• Initiation of muscle contraction
↓
Muscle relaxation
• Active transport of Ca2+ into sarcoplasmic reticulum
↓
• Decreased concentration of Ca2+ in sarcoplasm
↓
• Removal of Ca2+ ions from troponin-C
↓
• Cessation of cross-bridge cycling
↓
• Relaxation of muscle fibre
Characteristics of muscle contractility
Contractility
To understand contractile response and its characteristics, it is essential to have knowledge about the following elementary aspects in relation to skeletal muscle:
Contractile and elastic components of a muscle
To understand certain facts associated with muscle contraction such as shortening, contraction without shortening and effect of passive stretch, a three-component model has been proposed. According to this model the skeletal muscle as a whole consists of three components (Fig. 2.3-10A):
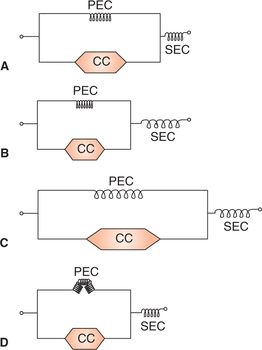
Fig. 2.3-10 Three component model of skeletal muscle consisting of contractile component (CC), series elastic component (SEC) and parallel elastic component (PEC): A, when muscle is at normal length; B, during isometric contraction; C, when muscle is passively stretched and D, in isotonic contraction.
1 Contractile component
The contractile component (CC) represents the thick (myosin) and thin (actin) filaments present in the myofibrils. It offers no resistance to stretch and is unable to return to its original length after it has shortened.
2 Series elastic component
The series elastic component (SEC) refers to that elastic tissue of the muscle which is present in series with the CC of the muscle. It consists of the elastic tendon of the muscle. In resting condition the SEC offers resistance to passive stretch and explains how muscle is able to contract even when its external length does not change, i.e. isometric contraction (Fig. 2.3-10B). It also explains how the muscle regains its original length after contracting isometrically.
3 Parallel elastic component
The parallel elastic component (PEC) refers to the elastic tissue of the muscle which is attached parallel to the CC. The PEC is represented by the structural elastic tissue of the muscle such as connective tissue sheaths of the muscle, sarcolemma and filaments. Presence of this component explains why the muscle regains its original length after it is passively stretched (Fig. 2.3-10C and D). In isotonic contraction this component gets folded up. It also offers some resistance to passive stretch.
Concepts about muscle length
Following concepts about muscle length will be useful in understanding certain characteristics about muscle contraction:
Optimum length refers to that length of the muscle at which it will develop maximum active tension.
Resting length of a muscle represents the length of the muscle during relaxed state under natural conditions in the body. The resting length of many muscles in the body is optimum length.
Equilibrium length refers to the length of a relaxed muscle cut free from its bony attachments.
Initial length is the length of the muscle before it contracts.
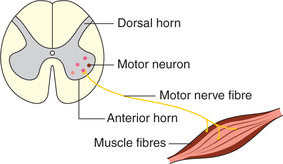
Motor unit
Motor unit is the functional unit of muscle contraction in the intact body. It consists of the single motor neuron cell body, its axon fibres and the muscle fibres innervated by it (Fig. 2.3-11). The cell bodies of the motor neurons (α motor neuron) supplying the skeletal muscle fibres lie in the ventral horn of the spinal cord or the motor cranial nerve nuclei.
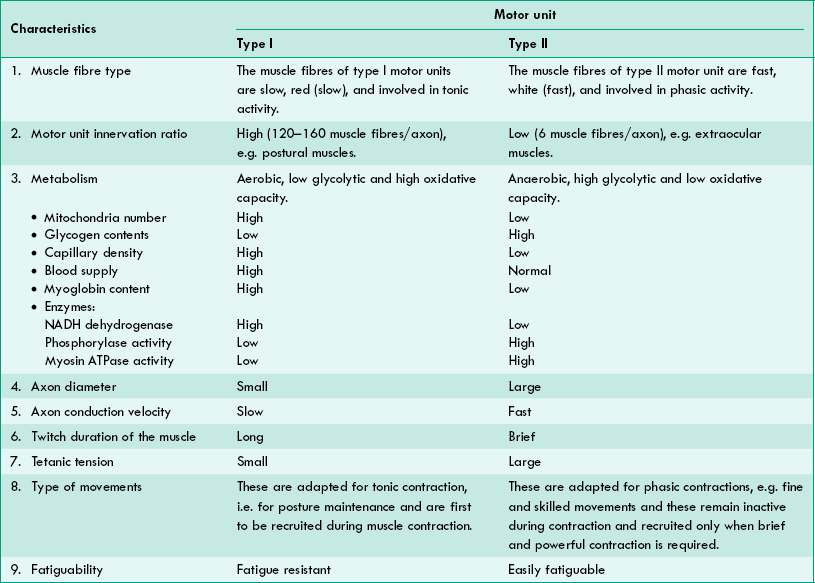
Type of motor units. Each motor neuron innervates only one type of muscle fibre. In other words, in a single motor unit the muscle fibres supplied by it are of the same type. Therefore, depending on the type of muscle fibres, the motor units are also of two types:
• Type I (Red or slow) motor units (fast or slow type) and
• Type II (White or fast) motor units. The characteristic features of each type of motor units are given in Table2.3-2.
Contractile response
Contractile response is the characteristic feature of a skeletal muscle. An action potential is developed in the muscle fibres upon stimulation, which is followed by the muscle contraction. The muscle contraction is manifested by either shortening (isotonic contraction) or development of tension (isometric contraction) or both. The contractile response of a skeletal muscle can be discussed under following headings:
Isometric versus isotonic contraction
As the name indicates (iso = same, metric = measure, i.e. length), in this type of contraction the length of muscle remains same but tension is developed in the muscle. Thus there is no movement of the object. Since work done is the product of force x distance; therefore, in isometric contraction no external work is done.
How the muscle length remains same in isometric contraction is depicted in Fig. 2.3-10B. As shown in this figure the shortening produced by the CC of the muscle is compensated by the stretching of SEC.
As the name indicates (iso = same, and tonic = tone or tension), in this type of contraction the tension in the muscle remains same whereas its length decreases. Since the muscle length is shortened, the external work is done in isotonic contraction. As shown in Fig. 2.3-10D, in isotonic contraction the CC and PEC are shortened, but the SEC does not stretch further, producing a visible shortening of the muscle.
Examples of isotonic muscle contraction
• Contraction of leg muscles during walking and running.
Length-tension relationship. When a muscle is removed from the body, it shortens because muscles in the body are in a state of slight stretch. The length of the muscle when it is detached from its bony attachments is called equilibrium length.
The length-tension relationship graph can be plotted by measuring isometric tension at different muscle lengths in an isolated muscle preparation. For this, the isolated muscle is attached to an isometric lever, which does not allow the shortening of muscle to occur. The tension developed is recorded through the force transducer.
• At each length, the passive tension is measured. The passive tension is due to stretching of PEC and SEC.
• The muscle is then electrically stimulated at each length and the tension developed is recorded. The total tension so recorded includes both the passive tension and active tension developed due to contraction of the CC of the muscle.
• The active tension is thus denoted by the difference between the total tension and passive tension at any length.
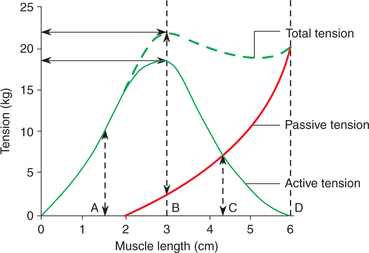
Length-tension relationship graph is then plotted. The following inferences can be drawn from this graph (Fig. 2.3-12):
• Passive tension due to stretching of elastic components (PEC and SEC) of the muscle increases with the passively increased muscle length.
• At each length the passive tension is measured.
• Active tension developed is maximum at the optimum length of the muscle (position B in Fig. 2.3-12), which is equivalent to the resting length in intact body.
• Total tension is contributed by the active tension and the passive tension at different muscle lengths (Fig. 2.3-12).
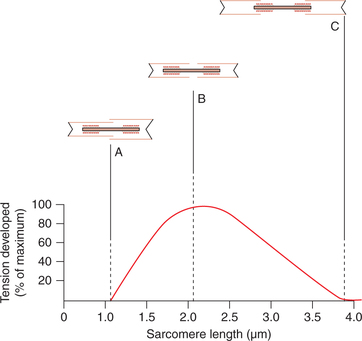
Molecular basis of length-tension relationship. The effect of muscle length on the tension produced during contraction can be explained by the sliding filament theory of muscle contraction as:
• At optimum length, there is optimum overlapping between the actin and myosin filaments, so maximum cross bridges are formed between them.
• At muscle length shorter than optimum length (at position A in Fig. 2.3-13) the thin filaments overlap each other and thus reduce the cross bridges between the actin and myosin filaments and so the active tension produced is less.
• When the muscle is overstretched (position C in Fig. 2.3-13) the Z lines are pulled apart and the overlapping between the actin and myosin filaments is markedly reduced and so no active tension is developed at this level.
Effect of afterload. Afterload refers to the load which acts on the muscle after the beginning of muscular contraction. Thus, the afterload opposes the force produced by muscle contraction. The work done in an afterloaded muscle is less than that of a free-loaded muscle. An example of afterload in an intact body is lifting any object from the ground.
Force-velocity relationship. The force-velocity curve (Fig. 2.3-14) is plotted by noting the velocity of shortening of muscle with progressively increasing load on the muscle. Following inferences can be drawn from the force-velocity curve:
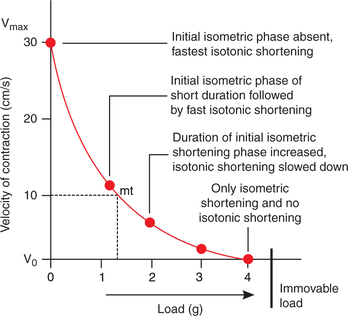
Fig. 2.3-14 Force-velocity curve plotted by recording velocity of the shortening of skeletal muscle with progressively increasing load.
• When load is zero, the muscle contracts rapidly and the velocity of muscle shortening is maximum (Vmax).
• As the load increases the velocity of shortening decreases. With further increase in the load, a stage comes when the muscle is unable to lift the load. At this point muscle contracts isometrically.
• Between the two extremes of zero load and immovable load, all contractions have variable durations of isometric and isotonic contractions.
• In the force-velocity curve, mt is the point of maximum efficiency of the muscle. It lies about one-third of the abscissa and one-third from the ordinate.
Muscle tone
Muscle tone is the state of slight contraction with certain degree of vigour and tension. All the skeletal muscles exhibit muscle tone. However, it is more marked in the anti-gravity muscles, viz. extensors of the lower limbs, trunk muscles and muscles of the neck.
Maintenance of muscle tone. Muscle tone is a state of partial tetanus of the muscle maintained by asynchronous discharge of impulses from γ motor neurons in the anterior grey horn of the spinal cord concerned with the motor nerve supply of the muscles. The γ motor neurons in turn are controlled by some higher centres in brain (see page 526).
Electromyography and common disorders of muscles
Electromyography
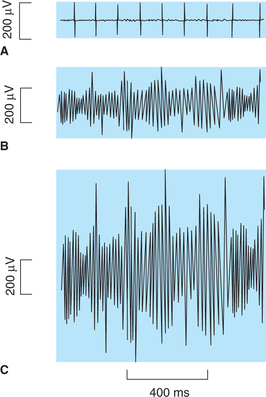
Electromyography refers to the technique of recording the total electrical activity of the motor nerve and the muscle under study. The machine used to record the said electrical activity is called electromyograph and the record obtained is called the electromyogram (Fig. 2.3-15).
Disorders of skeletal muscles
1 Muscular dystrophy
Muscular dystrophy is a syndrome which occurs due to genetic mutation and is characterized by progressive muscle weakness.
2 Myotonia
Myotonia is a disorder which occurs due to abnormalities of the sodium and chloride channels caused by abnormal genes on chromosomes 7, 17 or 19. It is characterized by an abnormally prolonged muscle relaxation after voluntary contraction.
3 Myasthenia gravis
It is a disorder of neuromuscular junction, characterized by a grave weakness of the muscles (see page 45).
4 Abnormal muscle tone
The normal muscle tone of a muscle may be increased or decreased constituting abnormal muscle tone.